- 1College of Food and Bioengineering, Henan University of Science and Technology, Luoyang, China
- 2Henan International Joint Laboratory of Food Green Processing and Quality Safety Control, Henan University of Science and Technology, Luoyang, China
- 3School of Zhang Zhongjing Health Care and Food, Nanyang Institute of Technology, Nanyang, China
- 4Key Lab of Dairy Science, Ministry of Education, College of Food Science, Northeast Agricultural University, Harbin, China
- 5Department of Agro-Processing, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
The research was conducted to elucidate the antibacterial performance and mode of action of Eucommia ulmoides male flower extract (EUMFE) against Staphylococcus aureus and its application as a natural preservative in cooked beef. The antibacterial activity was evaluated by determining the diameter of inhibition zone (DIZ), minimum inhibitory concentration (MIC), and minimum bactericide concentration (MBC). The changes in membrane potential, contents of bacterial DNA and protein, integrity and permeability of the cell membrane, and cell morphology were analyzed to reveal the possible mode of action. The effect of EUMFE on the counts of S. aureus, pH, color, total volatile basic nitrogen (TVB-N), and thiobarbituric acid reactive substances (TBARS) of the cooked beef stored at 4°C for 9 days were studied. The results showed that the DIZ, MIC, and MBC of EUMFE against S. aureus were 12.58 ± 0.23 mm, 40 mg/mL, and 80 mg/mL, respectively. The mode of action of EUMFE against S. aureus included hyperpolarization of cell membrane, decrease in bacterial DNA and protein contents, destruction of cell membrane integrity, increase in cell membrane permeability, and damage of cell morphology. After treatments with EUMFE, the growth of S. aureus and lipid oxidation in cooked beef were significantly inhibited (P < 0.05). The pH and TVB-N values of cooked beef treated with EUMFE were significantly reduced as compared to control group (P < 0.05). The color of cooked beef samples containing EUMFE showed decreased L* and b* values, and increased a* and ΔE* values. Therefore, our findings showed that EUMFE had a good antibacterial effect on S. aureus, and provided a theoretical basis for the application of EUMFE as a natural preservative in the preservation of cooked beef.
Introduction
Staphylococcus aureus, a facultative anaerobe and gram-positive food-borne pathogen, is abundantly distributed in water, air, dust, and animal feces. The Staphylococcal enterotoxins produced by it are associated with food spoilage and food poisoning (Wang et al., 2018; Liang et al., 2020). In addition, S. aureus can cause human infectious diseases including suppurative skin infections, endocarditis, pneumonia, and bacteremia, with a higher mortality rate (Wang et al., 2020).
In recent years, the contamination of cooked meat products with S. aureus has been concerned as severe health problems faced by food industry and consumers (Ifesan et al., 2009). Chemical preservatives have commonly been used to inhibit S. aureus growth as well as prolong the shelf life of food products due to their lower cost and better effects (Ghabraie et al., 2016). However, some studies have suggested that the long-term use and consequent residual toxicity of chemical preservatives has carcinogenic and teratogenic effects which pose a significant risk to consumers’ health (Diao et al., 2018). Therefore, more attention has been paid to natural preservatives for their green, safe, and non-polluting characteristics.
Eucommia ulmoides Oliver, belonging to monotypic family Eucommiaceae, is a woody perennial dioecious plant native to China and its male flower has favorable prospects (Ding et al., 2015). The male flowers of E. ulmoides are available in relatively large yields and are easy to harvest (Ding et al., 2020). Moreover, E. ulmoides male flower is abundant in phytonutrients and various biologically active substances including phenols, flavonoids, chlorogenic acid, and aucubin, which possess stronger antioxidant, anti-inflammatory, antihypertensive, and analgesic effects (Xing et al., 2019). For decades, E. ulmoides male flower has been widely used as healthcare tea and herbal medicine in China (Dong et al., 2011). However, the research on the antibacterial effect of E. ulmoides male flower against food-borne pathogens has not yet been progressed which limits its application in food preservation to a certain extent.
Therefore, the present study aimed to assess the antibacterial activity and mode of action of E. ulmoides male flower extract (EUMFE) against S. aureus in terms of changes in membrane potential, contents of bacterial DNA and protein, integrity and permeability of cell membrane, and cell morphology. Furthermore, the application feasibility of the EUMFE as a natural preservative in cooked beef was also investigated.
Materials and Methods
Main Reagents
Propidium iodide (PI) was purchased from Beijing Solarbio Technology Co., Ltd. (Beijing, China). All media were obtained from Shanghai SIG Biotechnology Co., Ltd. (Shanghai, China). All other chemicals and reagents used in this study were of analytical grade and provided by Xi’an Jinyuan Biotechnology Co., Ltd. (Xi’an, China).
Bacterial Strain and Culture Condition
Staphylococcus aureus (ATCC 25923) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, United States) and stored at −80°C in Luria-Bertani (LB) broth with 20% glycerol (v/v). Before each experiment, the strain was re-cultured in LB broth at 37°C for 20 h and streaked onto Tryptone soy agar (TSA) plate followed by incubating at 37°C for 24 h to get individual colonies. A typical colony was transferred into LB broth followed by incubating at 37°C for 24 h for further use.
Preparation of Eucommia ulmoides Male Flower Extract
The E. ulmoides male flower were first crushed to a fine powder to pass 60-mesh sieve, and then extracted by ultrasound assistance for 2 h using 70% ethanol (v/v) as an extraction solvent at a ratio of 1:30 (g/mL). The extracted suspension was filtered, and two extractions for the filter residues were performed by following the same procedure. The collected filtrate was evaporated at 40°C in a rotary-evaporator (Shanghai Kexiao Scientific Instrument Co., Ltd., Shanghai, China) to remove ethanol and concentrated until a “paste like form” was obtained. Finally, the paste was freeze-dried (−80°C) for 24 h to obtain EUMFE powder. The images of E. ulmoides male flower and EUMFE powders were shown in Supplementary Figure 1. The major chemical components in EUMFE were tabulated in Supplementary Table 1.
Determination of Diameter of Inhibition Zone
The diameter of inhibition zone (DIZ) of EUMFE against S. aureus was determined using the Oxford cup method as described by Chang et al. (2021). TSA plates were prepared by pouring 20 mL of TSA medium onto sterile glass petri dishes. 100 μL of S. aureus suspension (approximately 107 CFU/mL) was uniformly spread on TSA plates. Three sterile oxford cups (8 mm in diameter) were placed on the surface of each TSA plate followed by addition of 200 μL EUMFE (160 mg/mL). After incubation at 37°C for 24 h, the DIZ was measured with a vernier caliper and recorded in mm.
Determination of Minimum Inhibitory Concentration and Minimum Bactericide Concentration
The minimum inhibitory concentration (MIC) and minimum bactericide concentration (MBC) of EUMFE against S. aureus was determined by agar dilution method (Fei et al., 2020). In brief, EUMFE was thoroughly mixed with sterilized TSA medium (at about 50°C) in a 24-well plate to obtain the final concentrations of 5, 10, 20, 40, 80, and 160 mg/mL. After solidifying, each well was inoculated with 2 μL of bacterial suspension of S. aureus (106 CFU/mL) and incubated at 37°C for 24 h. The MIC was defined as the lowest concentration of EUMFE with no visual bacterial growth. Further, after treatments with ≥ 1 MIC of EUMFE for 30 min, 100 μL of S. aureus suspension (106 CFU/mL) was spread on TSA plate and incubated at 37°C for 24 h. The MBC was interpreted as the lowest concentration of EUMFE inhibiting growth of colonies of S. aureus. As a validation experiment, the growth curves of S. aureus treated with different concentrations (0 MIC, 1/4 MIC, 1/2 MIC, 1 MIC, and 2 MIC) of EUMFE were measured by the method of Shi et al. (2016).
Measurement of Membrane Potential
The effect of EUMFE on the membrane potential of S. aureus was analyzed as described by Guo et al. (2019a). Briefly, 0.5 μL of bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3); Beijing Solarbio Science and Technology Co. Ltd., Beijing, China] was added to a 96-well microtiter plate containing 125 μL of S. aureus suspension (107 CFU/mL) and cultured at 37°C for 24 h, followed by addition of EUMFE at final concentrations of 0 MIC (control), 1 MIC, and 2 MIC. The fluorescence intensities of the experimental samples were measured by a multifunctional microplate reader (Nanjing Junwei Biotechnology Co. Ltd., Jiangsu, China) with excitation/emission wavelengths of 492/515 nm at 37°C.
Agarose Gel Electrophoresis for DNA Fragmentation
The effect of EUMFE on the genomic DNA of S. aureus was analyzed using agarose gel electrophoresis according to the report of Guo et al. (2020). Approximately 108 CFU/mL of S. aureus culture were treated with 0 MIC (control), 1 MIC and 2 MIC of EUMFE at 37°C for 1, 3, 5, 7, and 9 h. The genomic DNA was extracted using a bacterial genomic DNA extraction kit (Tiangen Biotechnology Co., Ltd., Beijing, China). After electrophoresis with 1.5% agarose gel at 100 V for 30 min, the gels were stained with 10 mg/mL of ethidium bromide for 15 min and visualized through the gel imaging system (Bio-Rad Laboratories, Hercules, CA, United States).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) can separate the protein into several bands in electrophoretic gel according to the different molecular weight and charge of the protein, and the bands with high protein content are deep. SDS-PAGE analysis of bacterial proteins was performed following the procedure as described by Fei et al. (2019). In brief, S. aureus culture (107 CFU/mL) was treated with 0 MIC (control), 1 MIC, and 2 MIC of EUMFE for 1, 3, 5, 7, and 9 h. The cell pellets were prepared by centrifugation at 8000 × g for 10 min at 4°C, then resuspended in normal saline followed by mixing with SDS-PAGE loading buffer. After heating at 95°C for 10 min, the samples were analyzed by SDS-PAGE with 5% stacking gel and 15% separating gel, and the protein bands were stained with Coomassie brilliant blue R-250 (Beijing Zhongsheng Ruitai Technology Co. Ltd., Beijing, China). Finally, the image was taken with the HP scanner (HP 1000, Hewlett-Packard Co. Ltd., Beijing, China).
Fluorescence Microscope
The fluorescence microscope observation was used to evaluate the changes in membrane integrity of S. aureus according to the previously reported method (Han et al., 2021). Briefly, after treatments with 0 MIC (control), 1 MIC, and 2 MIC of EUMFE at 37°C for 3 h, the S. aureus culture (108 CFU/mL) was harvested by centrifugation at 8000 × g for 10 min and then washed three times followed by re-suspension in 0.01 M sterilized phosphate buffered solution (PBS). The bacterial suspension was incubated with PI dye (2 μg/mL) at room temperature (25 ± 2°C) in the dark for 30 min. Finally, 10 μL from aforementioned culture was observed and imaged under the fluorescence microscopy (Hunan Andao Technology Co. Ltd, Hunan, China) using excitation and emission wavelengths of 536 and 617 nm respectively.
Flow Cytometry
The effect of EUMFE on membrane permeability of S. aureus was assessed by the procedure as described by Kang et al. (2020). S. aureus suspension (108 CFU/mL) was treated with 0 MIC (control), 1 MIC and 2 MIC of EUMFE and incubated at 37°C for 3 h followed by centrifugation at 5000 × g for 10 min. The collected cells were stained with 10 μg/mL PI dye for 30 min at room temperature (25 ± 2°C) in the dark, and detected by a flow cytometer (Shenzhen Mindray Bio-Medical Electronics Co. Ltd., Guangdong, China).
Observation of Morphological Changes of Staphylococcus aureus
According to the report of Wang et al. (2018), the morphological changes of S. aureus cells treated with different concentrations of EUMFE were observed under Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM). After treatments with EUMFE of 0 MIC (control), 1 MIC, and 2 MIC for 3 h, the suspensions of S. aureus (107 CFU/mL) were centrifuged at 4000 × g for 10 min at 4°C to get the cell pellets, which were washed three times with 0.01 M PBS and fixed in 2.5% glutaraldehyde at 4°C for 12 h. The cultures were dehydrated with a series of dilutions of ethanol including 30, 50, 70, 90, and 100% ethanol for 15 min. Finally, the experimental samples were dried in freeze dryer, coated with gold and subjected to observations under the SEM (TM3030, Hitachi, Tokyo, Japan).
The pretreatment of S. aureus culture for TEM was performed by following the similar procedure as mentioned above for SEM. The prefixed cells were fixed up by 1% osmic acid for 2 h and washed three times with PBS. After dehydrating with different concentrations of ethanol, the cells were embedded by Epon Lx-112 (Ladd Research, Williston, VT, United States) and double-stained with uranyl acetate and lead citrate. Eventually, all samples were observed under the TEM (Hitachi, Tokyo, Japan).
Preparation of Cooked Beef Samples
The cooked beef samples were prepared according to the procedure as reported by Gong et al. (2021). Fresh beef purchased from the local supermarket was chopped into small pieces keeping a weight around 1.5 g and sterilized at 121°C for 15 min to obtain sterile cooked beef samples. These sterile samples were dipped into EUMFE solutions with the concentrations 0 MIC (control), 1 MIC, and 2 MIC for 10 s and placed in petri dishes for 30 min followed by packaging in sterile polythene bags. Finally, all samples were stored at 4°C for further analyses.
Microbiological Analysis
Cooked beef samples were inoculated with S. aureus to approximately 103 CFU/g and stored at 4°C for 0, 3, 6, and 9 days. The inoculated samples (1 g) were thoroughly mixed with 9 mL of sterile normal saline (NS), and a 10-fold serial dilution was prepared. 100 μL from each dilution was evenly spread onto TSA plate individually and incubated at 37°C for 24 h (Guo et al., 2020).
pH Measurement
The pH values of cooked beef samples were determined using the method of Carpenter et al. (2007). On days 0, 3, 6, and 9, cooked beef samples (1 g) were homogenized in 9 mL of distilled water for 1 min. The pH of the homogenate was measured using a portable pH-meter (Radiometer, Copenhagen, Denmark).
Color Measurement
According the report of Pissaia et al. (2015), a CR-300 Chroma Meter (Minolta Co., Osaka, Japan) was used to measure the color parameters including L*(lightness), a*(redness), and b*(yellowness) of cooked beef samples after 0, 3, 6, and 9 days of storage at 4°C. In addition, the total color difference (ΔE*) was calculated by the following formula: ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2.
Where ΔL*, Δa*, and Δb* are the difference of L*, a*, and b* of the samples between control and the EUMFE treatments.
Determination of Total Volatile Basic Nitrogen
The total volatile basic nitrogen (TVB-N) value of cooked beef samples was measured according to the method as mentioned by Zhang et al. (2011). On days 0, 3, 6, and 9, each sample (1 g) was homogenized in 10 mL distilled water for 30 min and then filtered. 5 mL of filtrate and 5 mL of MgO solution was distilled through Kjeldahl Apparatus (Shanghai Yihong Analytical Instrument Co. Ltd., Shanghai, China). The distillate was collected in a flask containing 10 mL of aqueous solution of boric acid (20 g/L) and a mixed indicator prepared by dissolving equal weight (0.1 g) of methyl red and methylene blue in 100 mL ethanol (w/v). The flask’s content was then titrated against 0.01 mol/L hydrochloric acid. The TVB-N value was determined according to the consumption of hydrochloric acid and expressed as mg per 100 g cooked beef.
Determination of Thiobarbituric Acid Reactive Substances
The thiobarbituric acid reactive substances (TBARS) value of cooked beef sample was determined by following the method as stated by Yarnpakdee et al. (2012). Every 3 days during storage, the cooked beef sample (1 g) was mixed with 5 mL of thiobarbituric acid (TBA) reagent consisting of 0.375% thiobarbituric acid, 15% trichloroacetic acid, and 0.25 M HCl. The mixture was heated in a boiling water bath for 10 min to develop a pink color. After cooling with running tap water, the mixture was centrifuged at 3600 × g for 20 min to obtain supernatant. Finally, the absorbance of obtained supernatant was measured at a wavelength 532 nm. 1,1,3,3-tetramethox-ypropane was used to build the standard curve, and the TBARS value was calculated and expressed as mg malonaldehyde per kg of sample.
Statistical Analysis
All experiments were conducted in triplicate and results were expressed as mean ± standard deviation (SD). The data were analyzed using analysis of variance (ANOVA) in the SPSS 20.0 software. The difference was considered statistically significant when P-values less than 0.05.
Results
Diameter of Inhibition Zone, Minimum Inhibitory Concentration, and Minimum Bactericide Concentration of Eucommia ulmoides Male Flower Extract Against Staphylococcus aureus
The results showed that the mean DIZ value of EUMFE against S. aureus was 12.58 ± 0.23 mm. Meanwhile, the MIC and MBC of EUMFE against S. aureus were 40 and 80 mg/mL, respectively. In addition, the growth curve of S. aureus under action of 1 MIC of EUMFE was almost stagnant (Supplementary Figure 2), which indicated that the result of MIC value was valid.
Changes in Membrane Potential
As shown in Figure 1, the fluorescence intensities of S. aureus cells treated with 1 and 2 MIC of EUMFE were significantly reduced as compared to the control group (0 MIC) (P < 0.05), which indicated that treatment with EUMFE resulted in hyperpolarization of S. aureus cell membrane. Besides, the results obtained in this study (Figure 1) showed no significant difference in fluorescence intensity between the cells treated with 1 and 2 MIC of EUMFE.
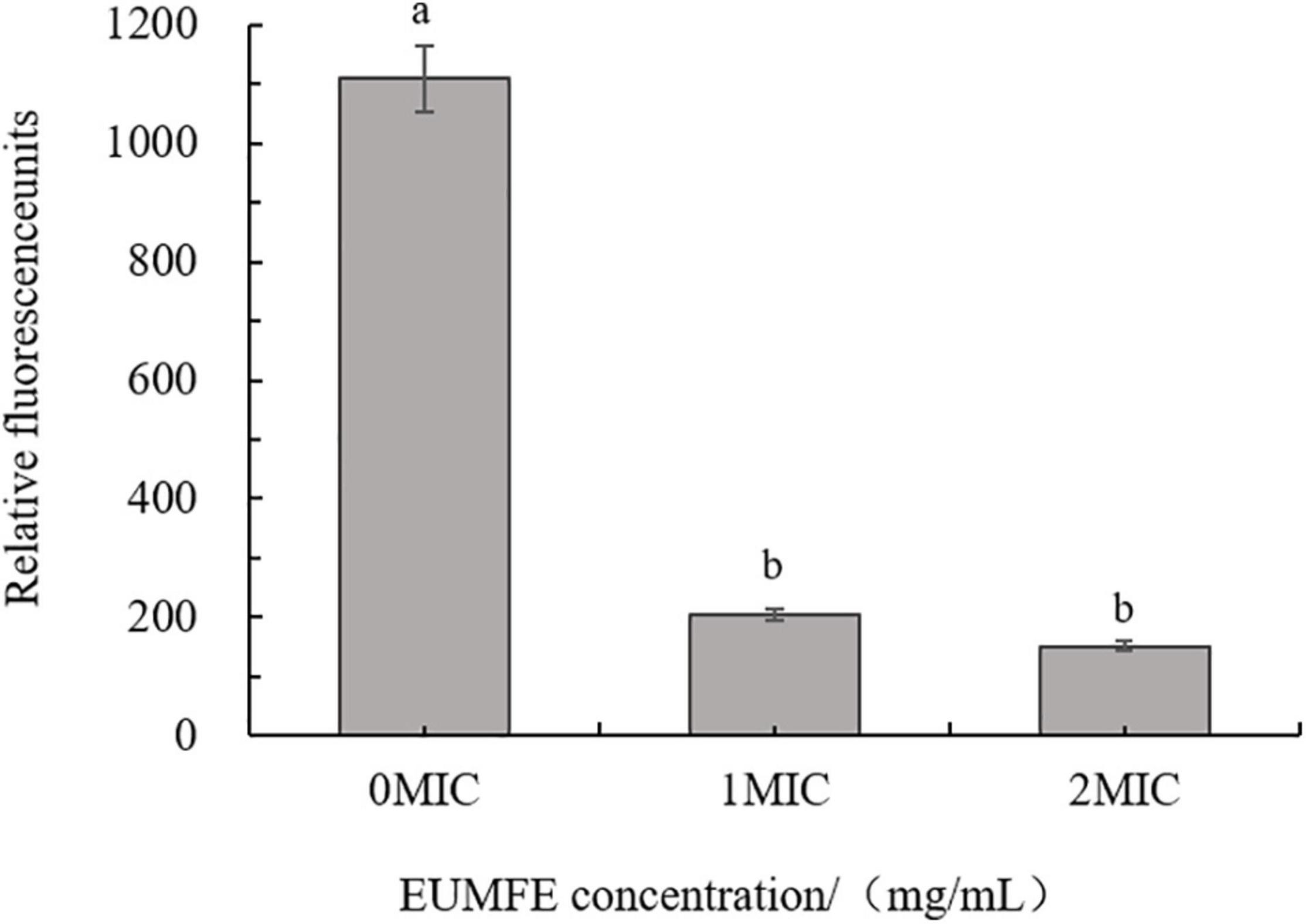
Figure 1. Effect of Eucommia ulmoides male flower extract (EUMFE) on the membrane potential of Staphylococcus aureus. Each bar represents the mean ± SD of three independent experiments. Different lowercase letters indicate the significant difference at P < 0.05.
DNA Fragmentation Analysis
Figure 2 showed that the DNA bands of S. aureus cells treated with 1 and 2 MIC of EUMFE in genomic DNA electrophoretogram were faded in comparison with untreated cells. The bacterial DNA bands got much fainter with increase in treatment time and concentration, and almost disappeared after 9 h of treatment with 2 MIC of EUMFE.
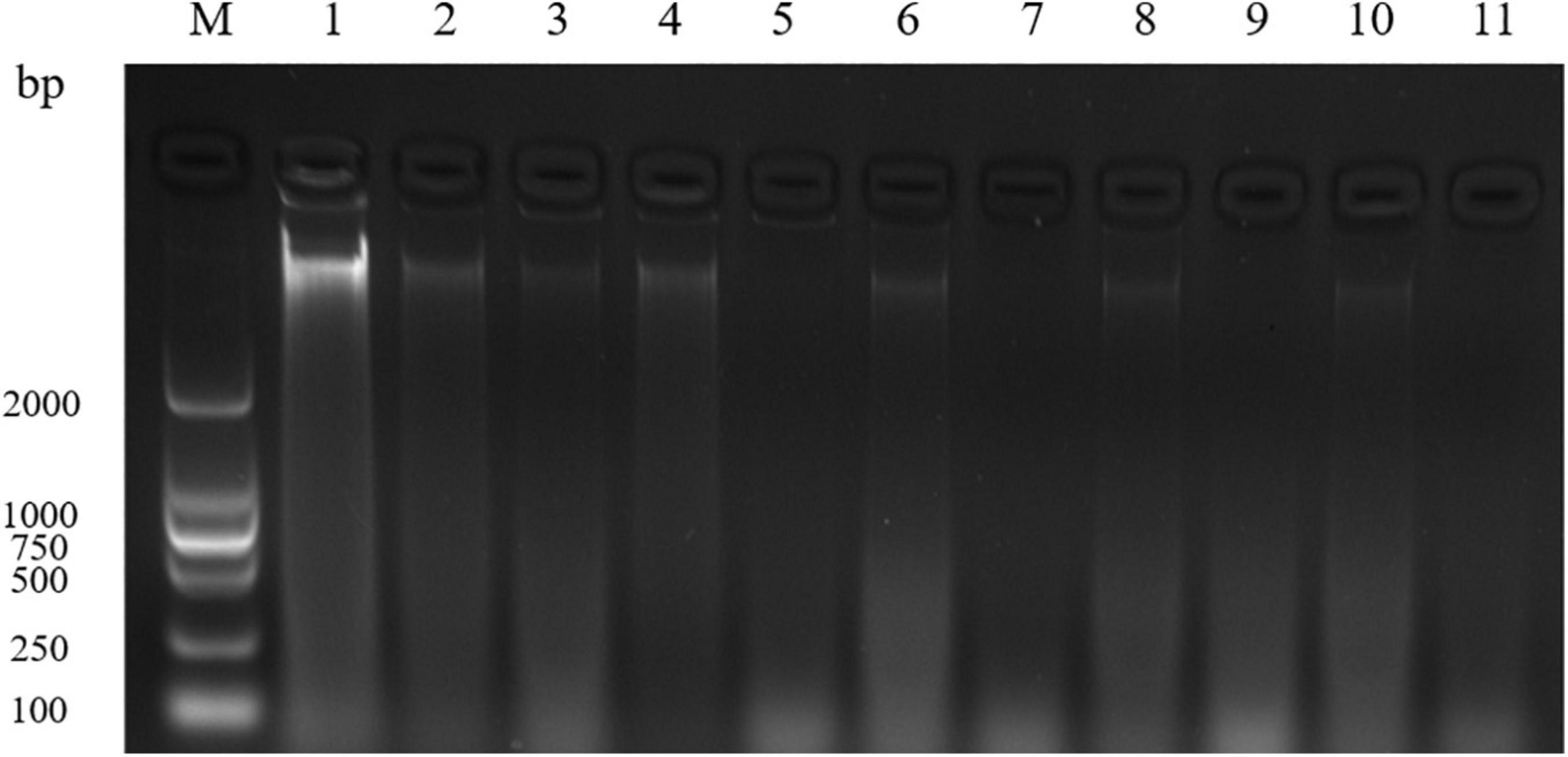
Figure 2. Agarose gel electrophoretogram of DNA in Staphylococcus aureus treated with 1 and 2 MIC of Eucommia ulmoides male flower extract (EUMFE). Lane M: marker; Lane 1: control; Lane 2, 4, 6, 8, and 10: treatment with 1 MIC of EUMFE for 1, 3, 5, 7, and 9 h, respectively. Lane 3, 5, 7, 9, and 11: treatment with 2 MIC of EUMFE for 1, 3, 5, 7, and 9 h, respectively.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis Analysis
The SDS-PAGE profiles (Figure 3) showed that protein bands of S. aureus cells treated with 1 MIC and 2 MIC of EUMFE got weaker as compared to those of control group (0 MIC), and the degree of protein bands weakening became more pronounced as the treatment time and concentration increased. Furthermore, the protein bands of S. aureus completely disappeared after treatments with 1 and 2 MIC of EUMFE for 9 h.
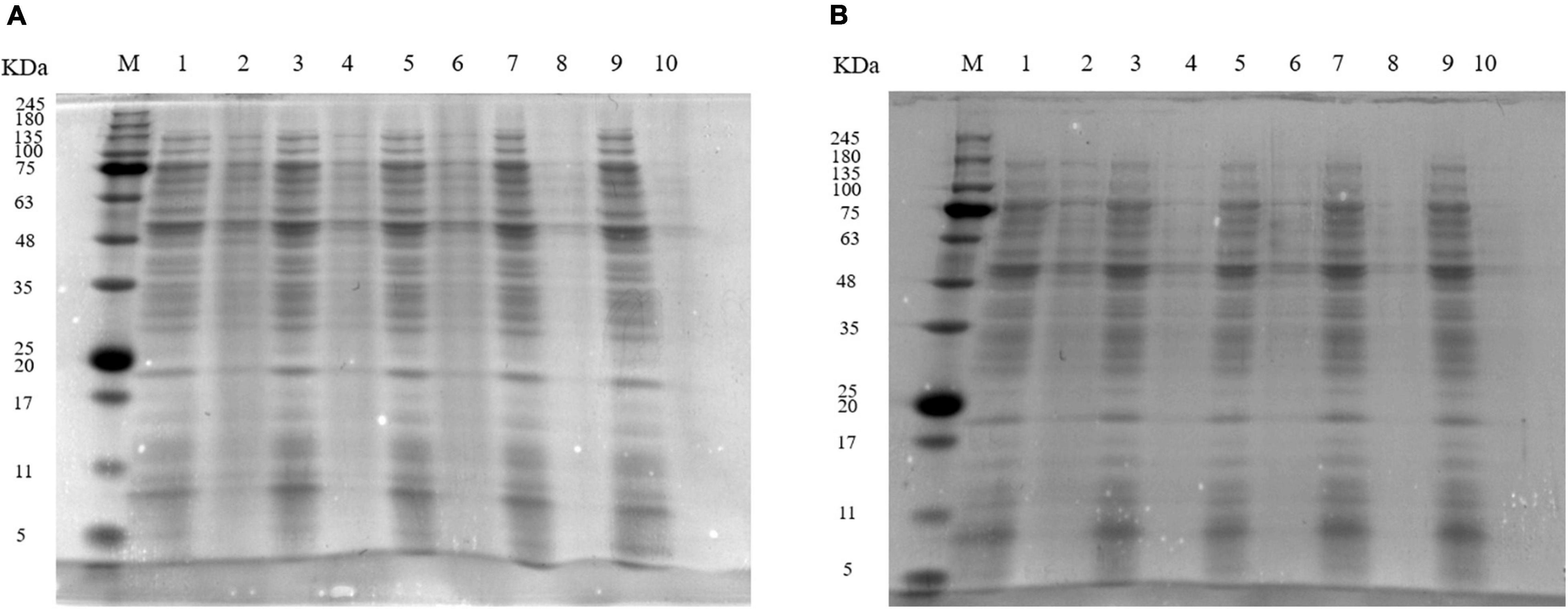
Figure 3. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profiles of proteins in Staphylococcus aureus treated with (A) 1 MIC and (B) 2 MIC of Eucommia ulmoides male flower extract (EUMFE). Lane M: marker; Lane 1, 3, 5, 7, and 9: control; lane 2, 4, 6, 8, and 10: treatment with 1 MIC or 2 MIC of EUMFE for 3, 6, 9, 12 h, respectively.
Fluorescence Microscope Observation
The results of fluorescence microscope images showed that only very few cells were red for the control group (0 MIC) (Figure 4A). After treatments with 1 MIC of EUMFE for 3 h, a little red fluorescence in the field of vision was observed (Figure 4B). On the other hand, the red fluorescence appeared increasingly against an increase in concentration of EUMFE (Figure 4C).
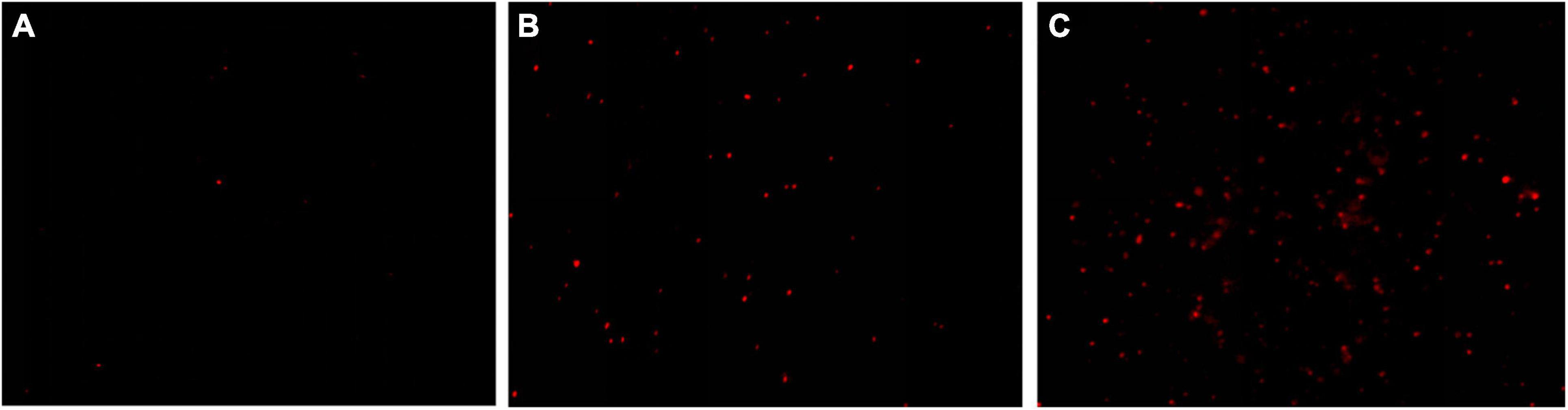
Figure 4. Fluorescence microscope images of Staphylococcus aureus cells stained with propidium iodide (PI) after treatments with Eucommia ulmoides male flower extract (EUMFE) for 3 h. (A) Untreated cells; (B) cells treated with EUMFE at 1 MIC; (C) cells treated with EUMFE at 2 MIC.
Flow Cytometer Analysis
The effect of EUMFE on membrane permeability of S. aureus was shown in Figure 5. Compared to the control group (0 MIC) (Figure 5A), the percentage of S. aureus cells stained with PI (in the area to the right of the vertical line) was significantly increased by following the exposure to 1 and 2 MIC of EUMFE for 3 h (Figures 5B,C). The results obtained in this study (Figure 5) indicated that EUMFE treatment increased the cell membrane permeability of S. aureus.
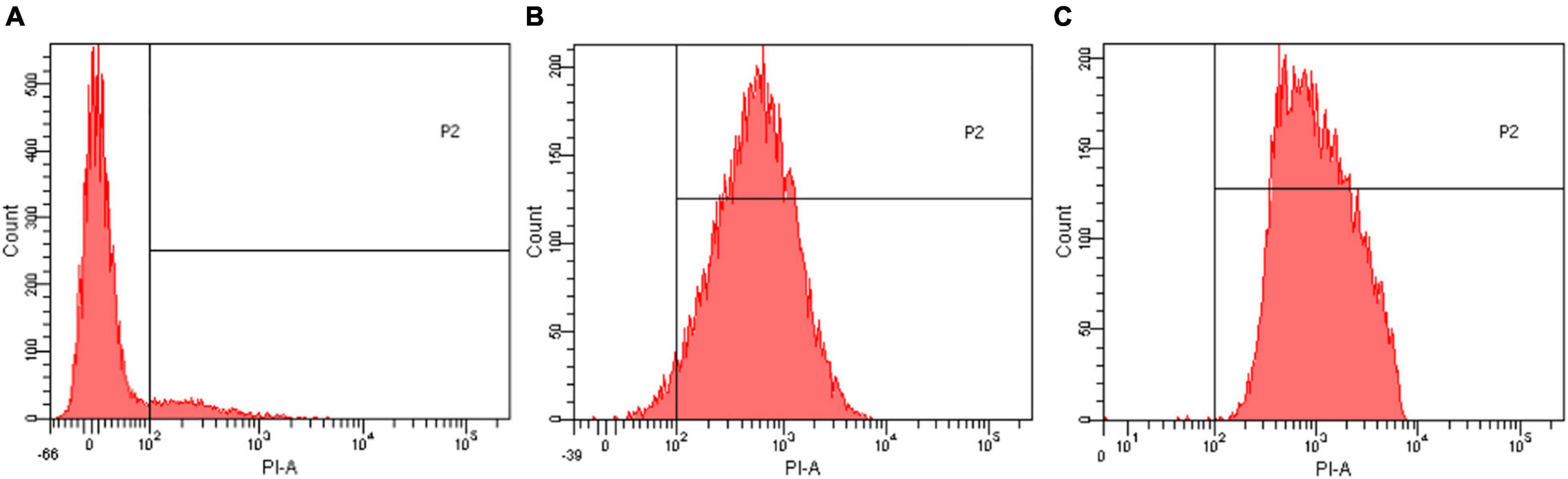
Figure 5. Flow cytometric histograms of Staphylococcus aureus stained with propidium iodide (PI) after treatments with Eucommia ulmoides male flower extract (EUMFE) for 3 h. (A) Untreated cells; (B) cells treated with EUMFE at 1 MIC; (C) cells treated with EUMFE at 2 MIC.
Scanning Electron Microscopy and Transmission Electron Microscopy Observations
The morphological changes of S. aureus cells after treatments with EUMFE for 3 h were observed by SEM and TEM (Figure 6). Untreated S. aureus cells remained the normal cell morphology of typical spherical shape, intact and smooth surfaces as well as homogeneous electron density in the cytoplasm (Figures 6A,D). In contrast, S. aureus treated with 1 (Figures 6B,E) and 2 MIC (Figures 6C,F) of EUMFE exhibited severe morphological damages including the surface depression, cell distortion, and leakage of intracellular materials. Moreover, the damages were obviously enhanced with increasing EUMFE concentration.
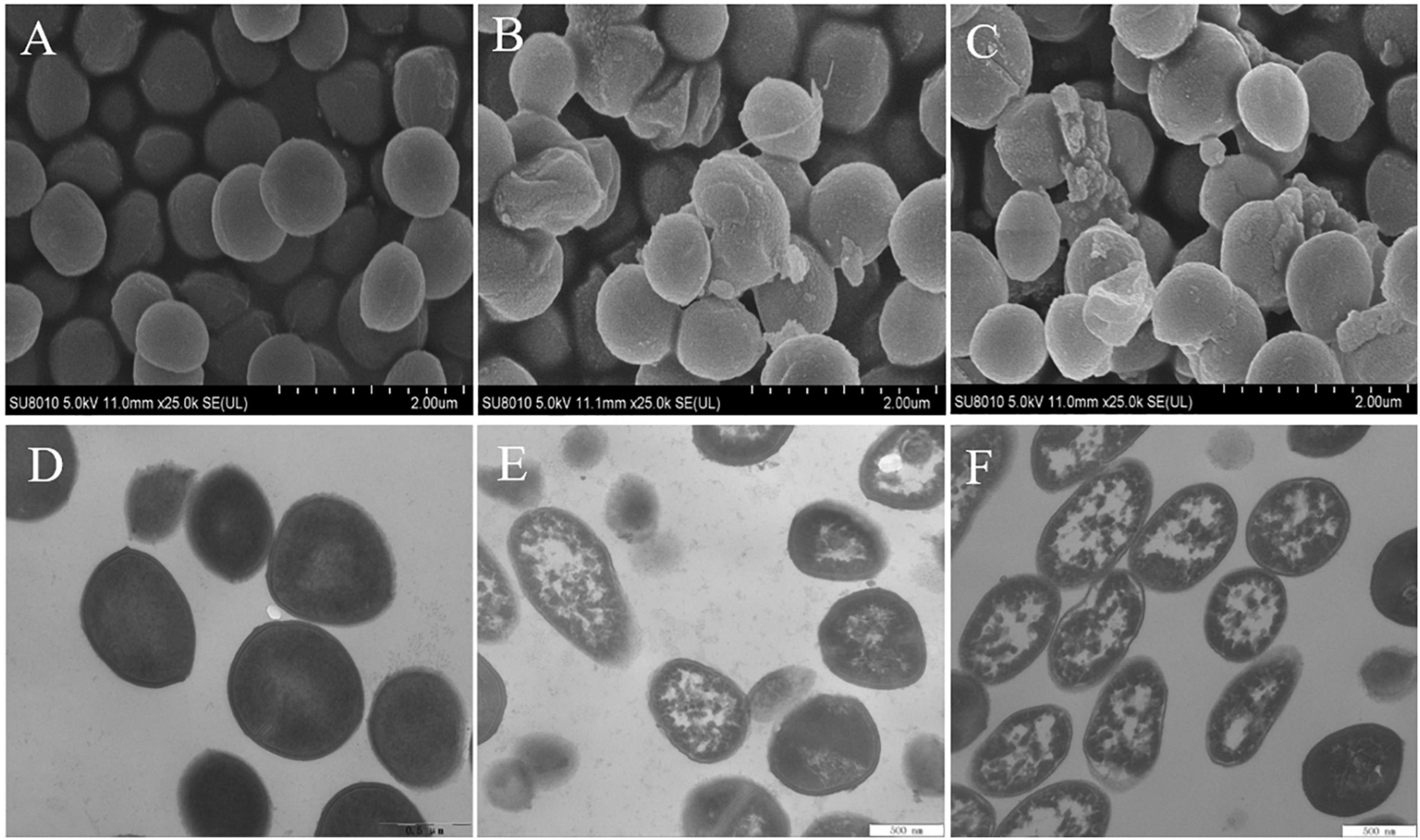
Figure 6. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) images of Staphylococcus aureus after treatments with Eucommia ulmoides male flower extract (EUMFE) for 3 h. Panels (A–C) are SEM images of the cells untreated, treated with 1 and 2 MIC of EUMFE, respectively; Panels (D–F) are TEM images of the untreated, treated with 1 and 2 MIC of EUMFE, respectively.
Application in Cooked Beef
As shown in Figure 7, compared to the control group (0 MIC), the number of S. aureus strains in cooked beef after treatments with EUMFE were reduced significantly (P < 0.05) on days 3, 6, and 9 of storage, and decreased significantly (P < 0.05) with an increase in concentration of EUMFE in the treatment medium. In addition, the number of S. aureus treated with EUMFE was slightly higher than that of initial populations on day 9.
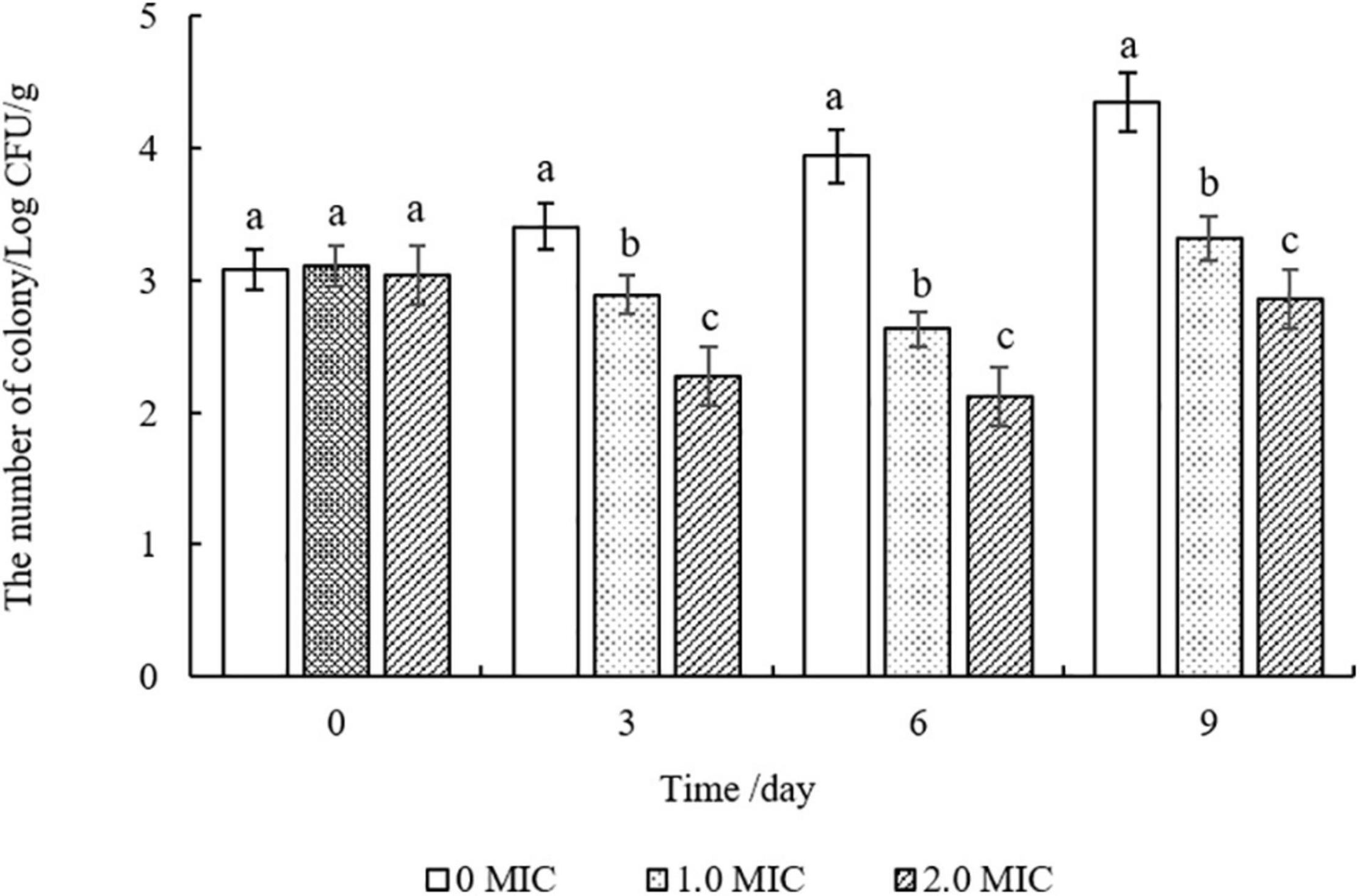
Figure 7. Inhibitory effect of Eucommia ulmoides male flower extract (EUMFE) on Staphylococcus aureus in cooked beef samples during storage at 4°C. Each bar represents the mean ± SD of three independent experiments. Different lowercase letters indicate the significant difference at P < 0.05.
pH Changes
The changes in pH values of cooked beef samples during storage were shown in Table 1. After treatments with different concentrations of EUMFE, significantly lower pH values were measured for cooked beef than that of control group (0 MIC) (P < 0.05). However, no significant changes in pH value in cooked beef samples, treated with EUMFE of similar concentration, were observed by 9 days of storage (P > 0.05).
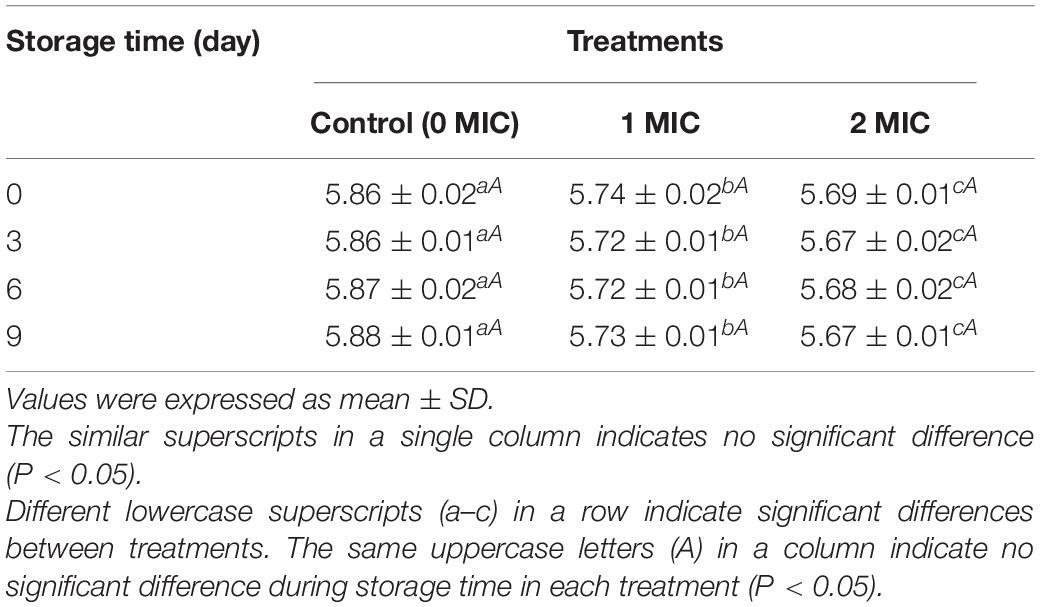
Table 1. The pH of cooked beef after treatments with Eucommia ulmoides male flower extract (EUMFE) of different concentrations during storage for 9 days.
Color Changes
As shown in Table 2, compared to the control group (0 MIC), the cooked beef samples treated with EUMFE showed significantly lower L* and b* values, and higher a* and ΔE* values (P < 0.05). With an extension of storage time, there was significant decreases in L* values and increases in b* values (P < 0.05) of all samples. In addition, the a* values of control samples (0 MIC) significantly decreased with an increase in storage time (P < 0.05) while no significant differences in a* values were found in cooked beef treated with EUMFE by first 6 days of storage (P > 0.05).
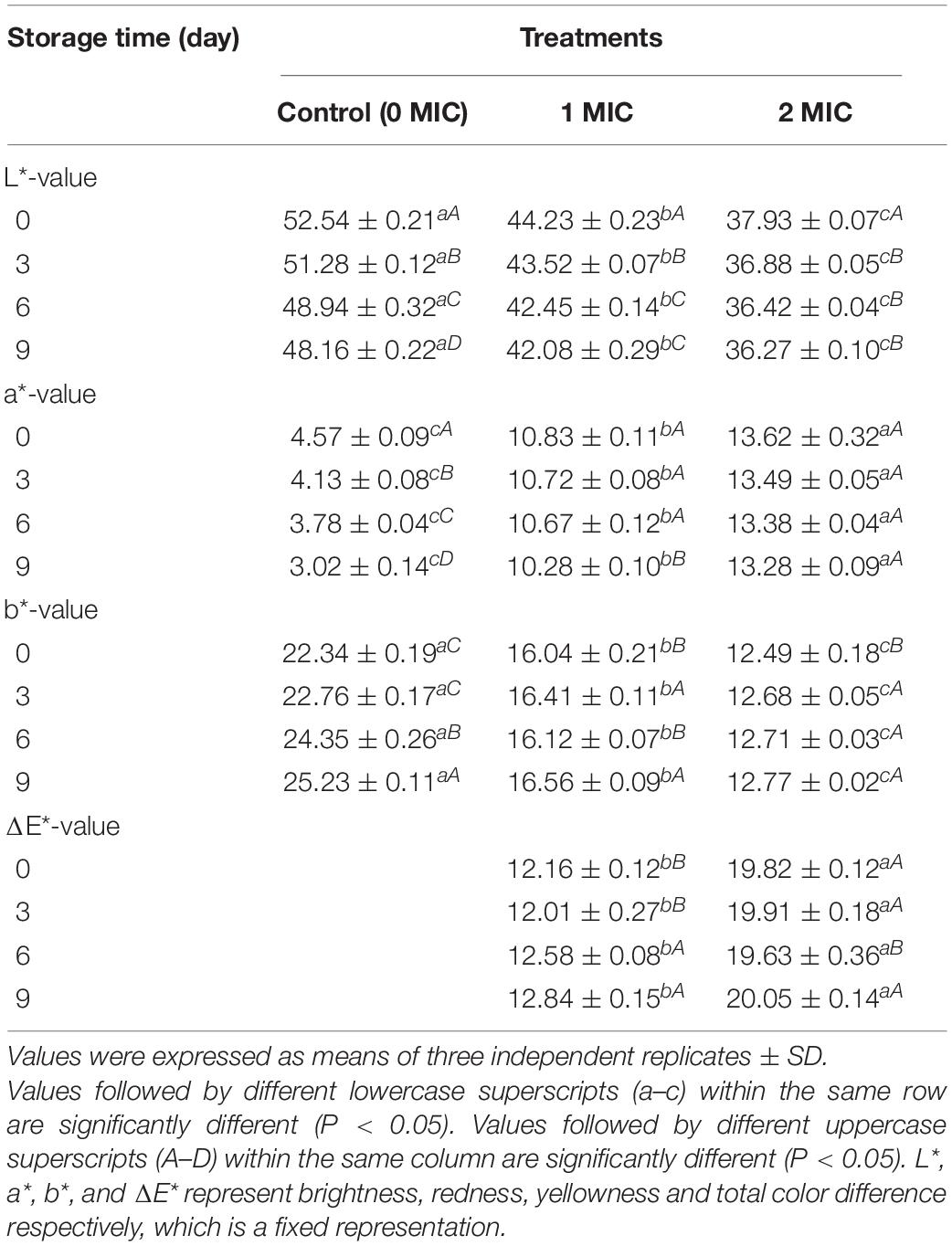
Table 2. The color of cooked beef treated with different concentrations of Eucommia ulmoides male flower extract (EUMFE) during storage for 9 days.
Changes in Total Volatile Basic Nitrogen and Thiobarbituric Acid Reactive Substances of Cooked Beef
Figure 8 showed that the TVB-N and TBARS values of all cooked beef samples continuously increased during the storage of 9 days. The TVB-N values of the samples treated with EUMFE were significantly lower than those for untreated samples from the third day (P < 0.05), and no significant difference (P > 0.05) was found in TVB-N values between 1 and 2 MIC treated samples by 3 days (Figure 8A).
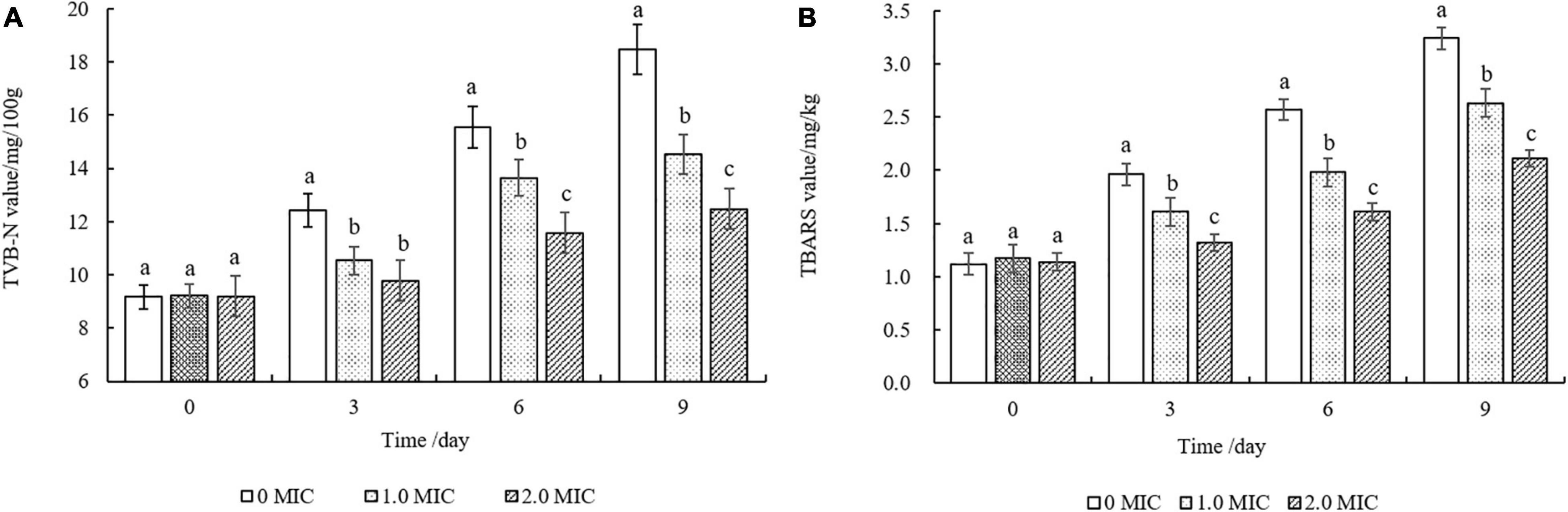
Figure 8. Changes in TVB-N (A) and TBARS (B) values of cooked beef treated with different concentrations of Eucommia ulmoides male flower extract (EUMFE) during storage at 4°C. Each bar represents the mean ± SD of three independent experiments. Different lowercase letters indicate the significant difference at P < 0.05.
In addition, cooked beef samples treated with 1 and 2 MIC of EUMFE exhibited significantly lower lipid oxidation in comparison with control group (0 MIC) after 3 days of storage (P < 0.05) (Figure 8B).
Discussion
Plant extracts and their biologically active components are considered as potential natural antimicrobials and preservatives because of their ideal antibacterial roles against food-borne pathogens (Liu et al., 2020). In our study, the MIC of EUMFE against S. aureus was 40 mg/mL, which is higher than those of dihydromyricetin (1.25 mg/mL), vine tea extract (6.3 mg/mL), and aqueous garlic extract (24 mg/mL) (Lee S.Y. et al., 2015; Liang et al., 2020). In the context of this study, it is obvious that EUMFE is significantly effective in inhibition of S. aureus, and exhibits a stronger antibacterial effect against S. aureus as compared to Amaranthus tricolor crude extract (ATCE) (80 mg/mL) (Guo et al., 2020). Of course, it is not enough to only study the antibacterial effect of EUMFE on S. aureus. In the future, we will continue to study the antibacterial effect of EUMFE on other food-borne pathogens to determine whether EUMFE has a broad-spectrum antibacterial effect.
Membrane potential refers to the potential differences between two sides of a cell membrane during the resting state of the cells, as it closely relates to antibiotic uptake and bactericidal actions by the cells (Shi et al., 2016). Our results showed that EUMFE caused hyperpolarization of cell membranes of S. aureus which might be occurred primarily in response to pH change and K+ efflux (Bot and Prodan, 2009). Similarly, cell membrane hyperpolarization was found when S. aureus exposed to 2R,3R-dihydromyricetin, shikimic acid, and chlorogenic acid (Li et al., 2014; Bai et al., 2015; Wu et al., 2017). As a contrast, cell membrane depolarization associated with migration of Na+ was observed in S. aureus while treated with olive oil polyphenols extract (OOPE) and fermented Inula britannica extract (Bae et al., 2019; Guo et al., 2019b).
DNA is vital for survival of bacterial cells because it is an important genetic material that control bacterial growth, development, and inheritance (Kang et al., 2020). In the present study, after treatments with EUMFE, the DNA fragmentation appeared in S. aureus cells, which is consistent with the changes in bacterial DNA in the inhibition of ATCE against S. aureus (Guo et al., 2020). Similarly, a study of Liu et al. (2011) indicated cajanol inhibited the growth of Escherichia coli and S. aureus through DNA cleavage. Wu et al. (2017) reported that 2R,3R-dihydromyricetin can cause the nucleic acid leakage from S. aureus through a destruction of cell membrane and interact with genomic DNA by the groove binding mode, and thereby reduce the bacterial DNA contents.
Proteins are essential biological macromolecules of bacterial cells and they are closely related to the bacterial metabolism and physiological functions (Fei et al., 2019). SDS-PAGE analysis results indicated that after treatments with EUMFE, the protein levels in S. aureus were obviously reduced. Similar findings have been reported in the action process of sugar beet molasses polyphenols on S. aureus, E. coli, Listeria monocytogenes, and Salmonella typhimurium (Chen et al., 2017). In addition, Song et al. (2020) suggested that mandarin essential oil treatment affects the protein synthesis and related gene expression in S. aureus. Yoo et al. (2021) believed that the reduction in protein contents of S. aureus cells treated with clove oil might be associated with the protein leakage caused by an increase of cell membrane permeability.
Propidium iodide is a DNA stain reagent that can penetrate membranes of damaged cells and embed in double-stranded DNA to exhibit red fluorescence. Therefore, PI staining could frequently be used to assess the extent of damage to the cell membrane (Yang et al., 2020). In this study, after a treatment with EUMFE, the percentage of staining of S. aureus cells with PI as well as red fluorescence under fluorescence microscope increased significantly, which revealed that EUMFE induced cell membrane damage in S. aureus, destroyed membrane integrity and increased membrane permeability. Han et al. (2021) found the similar results where the limonene significantly destroyed cell membrane integrity of S. aureus. Besides, Shi et al. (2017) reported that severe membrane damage of S. aureus was induced while treated with a combination of nisin and p-Anisaldehyde for 3 h.
Scanning electron microscopy and transmission electron microscopy observations can reflect the changes in cell morphology and ultrastructure of the tested bacteria caused by natural products on the whole. Kang et al. (2019) reported that peppermint essential oil irreversibly damages the cell membranes of S. aureus, causing leakage of intracellular substances. Zhang et al. (2016) indicated that the antibacterial effect of cinnamon essential oil against S. aureus is achieved by a direct change in the structure of cell wall and cell membrane. In the current study, severe cell morphological damage and cytoplasmic content leakage were observed in S. aureus while exposed to EUMFE for 3 h. These findings further support the other results, obtained in this study, including cell membrane hyperpolarization, DNA fragmentation, bacterial protein reduction and membrane damage in S. aureus cells being induced by EUMFE treatment. Therefore, we speculate that EUMFE damages the membrane of S. aureus cell, reducing the content of intracellular materials and eventually resulting in cell death.
Although the individually cooked meat products are commercially sterile, there is a risk of possible contamination by food-borne pathogens during transportation, storage, and other processes. Meanwhile, cooked meat produced in small scale industries are usually sold and stored in unhygienic even poor conditions being more likely to be contaminated by food-borne pathogens (Guo et al., 2020). Previous studies reported that natural antibacterial products can inhibit the growth of food-borne pathogens in cooked meat products, preventing them from being contaminated by microorganisms. For instance, Gong et al. (2021) found a significant reduction in S. aureus count in cooked pork and beef treated with cranberry anthocyanin. Apostolidis et al. (2008) reported that oregano, cranberry, and sodium lactate combination possessed obvious antibacterial effect on L. monocytogenes in cooked beef. Similarly, the addition of EUMFE reduced the number of S. aureus in cooked beef, indicating that EUMFE could effectively inhibit the growth of S. aureus in cooked beef. Besides, because it is the preliminary study to evaluate the behavior of EUMFE inside a food matrix, a small sample size (1 g) was used in this research. In future studies, commercial hunk of cooked beef will be used as samples to evaluate the potential of EUMFE as a natural preservative.
The decrease of pH due to the addition of natural antibacterials in cooked meat products is helpful to inhibit the growth of undesirable bacteria and extend the shelf life of products (Ozaki et al., 2020). According to the report of Lucas and Were (2009), who found the addition of lyophilized pomegranate juice could effectively reduce the pH of cooked ground beef during storage. Ahn et al. (2007) reported that the efficacy of the pycnogenol treatment for inhibiting microbial growth in cooked beef attributes to the lower pH of cooked meat by gallic acid, vanillic acid, caffeic acid, and ferulic acid in pycnogenol. Therefore, in this study, the reduction in pH values of cooked beef samples treated with different concentrations of EUMFE is beneficial to the preservation of cooked beef.
Color of meat and meat products is an important factor, affecting its marketability and consumer’s purchase preferences (Carpenter et al., 2007). In this study, the addition of EUMFE decreased the L* and b* values, and increased b* and ΔE* values of cooked beef samples which might be due to the red-brown color of the EUMFE. The color of the cooked beef would gradually darken during storage since myoglobin was oxidized to metmyoglobin along with formation of hemichrome in the meat (Verma et al., 2019). Therefore, the change in L* and a* values of cooked beef treated with EUMFE over storage period might be related to retardation of oxidation reaction by EUMFE. In agreement with our results, it has been shown that treatment of cooked beef with cranberry anthocyanin increased ΔE* value as an indicator of total color change (Gong et al., 2021). Moreover, addition of tea catechins, Perilla frutescens var. acuta water extract and pycnogenol resulted in color changes of cooked beef, and played roles in stability of meat color during the storage period (Mitsumoto et al., 2005; Ahn et al., 2007; Lee C.W. et al., 2015).
Total volatile basic nitrogen is a commonly used parameter to characterize the freshness of meat products and represent the total content of ammonia, dimethylamine, trimethylamine, and other volatile basic nitrogenous compounds (Li et al., 2016). In this study, the addition of EUMFE significantly decreased TVB-N values during storage as compared to control group (0 MIC), showing a protective effect to reduce the deterioration of quality. In general, cooked beef have a shelf life of about 3–7 days under refrigeration, while the TVB-N value of cooked beef treated with 2 MIC on day 9 was still less than 15 mg/kg, indicating that cooked beef was not spoiled. Therefore, the reduction of TVB-N value in cooked beef samples treated with EUMFE should be related to the antibacterial activity of EUMFE. Similar reduction in TVB-N value of minced beef packaged with polylactic acid film containing propolis ethanolic extract and Ziziphora clinopodioides essential oil have been reported (Shavisi et al., 2017). Besides, a combined effect of some natural preservatives produced by mixing clove cinnamon extracts with tea polyphenol, chitosan, propolis, nisin, and lysozyme could slow the development of TVB-N in refrigerated beef (Hao et al., 2013).
Thiobarbituric acid reactive substances values are, generally, considered as an important quality parameter of meat products because they can reflect the degree of lipid peroxidation (Ifesan et al., 2009). Our results showed that the addition of EUMFE can significantly reduce TBARS values in cooked beef as compared to control sample (0 MIC), indicating an inhibition of lipid oxidation. Similarly, Ye et al. (2015) have explained the ability of dihydromyricetin to reduce TBARS in cooked ground beef. As reported by Rojas and Brewer (2010) the grape seed extract reduces the oxidative rancidity and thus improves shelf life of refrigerated cooked beef and pork patties while incorporated to these products at 0.02%. Moreover, previous studies have reported that cooked meat is more sensitive to lipid oxidation than raw meat because of thermal damage in membrane phospholipids along with protein denaturation due to heating (Dai et al., 2014).
Conclusion
The EUMFE exerts a potential antibacterial effect on S. aureus by inducing cell membrane hyperpolarization, decreasing bacterial DNA and protein content, increasing cell membrane permeability, and destroying cell membrane integrity and cell morphology. Moreover, the addition of EUMFE effectively inhibits the growth of S. aureus, lowers L*, and b* values, increases a* and ΔE* values, decreases the pH and TVB-N values, and retards the lipid oxidation in cooked beef. Therefore, EUMFE has potential as a food preservative to control S. aureus contamination and to improve quality of cooked beef. However, the dose optimization of the natural preservative EUMFE and its application to other food system remains to be further studied.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
PF, MX, HK, and MA conceived and designed the experiments. MX, SL, YY, and YF performed the experiments. PF, LG, and YW supervised the project. MX, YY, PF, and LG analyzed the data. MX, MA, and PF wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Natural Science Foundation of Henan Province (212300410137), Key Scientific Research Projects of Institutions of Higher Learning of Henan (21A550007), Youth Talent Support Project of Henan Province (2020HYTP029), Key Science and Technology Program of Henan Province (202102110290), and Youth Talent Support Project of Henan Province (2020HYTP029).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.846622/full#supplementary-material
Supplementary Figure 1 | The images of (A) initial Eucommia ulmoides male flowers and (B) experimental Eucommia ulmoides male flower extract (EUMFE) powders in this study.
Supplementary Figure 2 | Growth curves of Staphylococcus aureus treated with different concentrations of Eucommia ulmoides male flower extract (EUMFE). Each bar represents the mean ± SD of three independent experiments.
References
Ahn, J., Grün, I. U., and Mustapha, A. (2007). Effects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiol. 24, 7–14. doi: 10.1016/j.fm.2006.04.006
Apostolidis, E., Kwon, Y. I., and Shetty, K. (2008). Inhibition of Listeria monocytogenes by oregano, cranberry and sodium lactate combination in broth and cooked ground beef systems and likely mode of action through proline metabolism. Int. J. Food Microbiol. 128, 317–324. doi: 10.1016/j.ijfoodmicro.2008.09.012
Bae, W. Y., Kim, H. Y., Kim, K. T., and Paik, H. D. (2019). Inhibitory effects of Inula britannica extract fermented by Lactobacillus plantarum KCCM 11613P on coagulase activity and growth of Staphylococcus aureus including methicillin-resistant strains. J. Food Biochem. 43:e12785. doi: 10.1111/jfbc.12785
Bai, J. R., Wu, Y. P., Liu, X. Y., Zhong, K., Huang, Y. N., and Gao, H. (2015). Antibacterial activity of shikimic acid from pine needles of Cedrus deodara against Staphylococcus aureus through damage to cell membrane. Int. J. Mol. Sci. 16, 27145–27155. doi: 10.3390/ijms161126015
Bot, C., and Prodan, C. (2009). Probing the membrane potential of living cells by dielectric spectroscopy. Eur. Biophys. J. 38, 1049–1059. doi: 10.1007/s00249-009-0507-0
Carpenter, R., O’Grady, M. N., O’Callaghan, Y. C., O’Brien, N. M., and Kerry, J. P. (2007). Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 76, 604–610. doi: 10.1016/j.meatsci.2007.01.021
Chang, Y. H., Xing, M., Hu, X. Y., Feng, H. X., Wang, Y., Guo, B. R., et al. (2021). Antibacterial activity of Chrysanthemum buds crude extract against Cronobacter sakazakii and its application as a natural disinfectant. Front. Microbiol. 11:632177. doi: 10.3389/fmicb.2020.632177
Chen, M., Zhao, Z., Meng, H., and Yu, S. (2017). The antibiotic activity and mechanisms of sugar beet (Beta vulgaris) molasses polyphenols against selected food-borne pathogens. LWT Food Sci. Technol. 82, 760–773. doi: 10.1016/j.lwt.2017.04.063
Dai, Y., Lu, Y., Wu, W., Lu, X. M., Han, Z. P., Liu, Y., et al. (2014). Changes in oxidation, color and texture deteriorations during refrigerated storage of ohmically and water bath-cooked pork meat. Innov. Food Sci. Emerg. Technol. 26, 341–346. doi: 10.1016/j.ifset.2014.06.009
Diao, M., Qi, D., Xu, M., Lu, Z., Lv, F., Bie, X., et al. (2018). Antibacterial activity and mechanism of monolauroyl-galactosylglycerol against Bacillus cereus. Food Control 85, 339–344. doi: 10.1016/j.foodcont.2017.10.019
Ding, Y. X., Qin, L., Zhang, H., Kim, Y. H., and Dou, D. Q. (2015). Iridoid constituents from the male flower of Eucommia ulmoides and their promotion proliferation on ESF-1. J. Asian Nat. Prod. Res. 17, 867–875. doi: 10.1080/10286020.2015.1039999
Ding, Z. J., Liang, C., Wang, X., Yao, X., and Li, Q. (2020). Antihypertensive activity of Eucommia ulmoides oliv: male flower extract in spontaneously hypertensive rats. Evid. Based Complement. Alternat. Med. 2020:6432173. doi: 10.1155/2020/6432173
Dong, J., Ma, X., Fu, Z., and Ying, G. (2011). Effects of microwave drying on the contents of functional constituents of Eucommia ulmoides flower tea. Ind. Crops Prod. 34, 1102–1110. doi: 10.1016/j.indcrop.2011.03.026
Fei, P., Feng, H., Wang, Y., Kang, H., and Chen, J. (2020). Amaranthus tricolor crude extract inhibits Cronobacter sakazakii isolated from powdered infant formula. J. Dairy Sci. 10, 9969–9979. doi: 10.3168/jds.2020-18480
Fei, P., Xu, Y., Zhao, S., Gong, S., and Guo, L. (2019). Olive oil polyphenol extract inhibits vegetative cells of Bacillus cereus isolated from raw milk. J. Dairy Sci. 102, 3894–3902. doi: 10.3168/jds.2018-15184
Ghabraie, M., Vu, K. D., Tata, L., Salmieri, S., and Lacroix, M. (2016). Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT Food Sci. Technol. 66, 332–339. doi: 10.1016/j.lwt.2015.10.055
Gong, S. Y., Fei, P., Sun, Q., Guo, L., Liang, L. G., Duo, K., et al. (2021). Action mode of cranberry anthocyanin on physiological and morphological properties of Staphylococcus aureus and its application in cooked meat. Food Microbiol. 94:103632. doi: 10.1016/j.fm.2020.103632
Guo, L., Bi, X., Wang, Y. Y., Duo, K., Sun, Q., Yun, X. Q., et al. (2020). Antimicrobial activity and mechanism of action of the Amaranthus tricolor crude extract against Staphylococcus aureus and potential application in cooked meat. Foods 9:359. doi: 10.3390/foods9030359
Guo, L., Sun, Q., Gong, S., Bi, X., Jiang, W., Xue, W., et al. (2019a). Antimicrobial activity and action approach of the olive oil polyphenol extract against Listeria monocytogenes. Front. Microbiol. 10:1586. doi: 10.3389/fmicb.2019.01586
Guo, L., Gong, S. Y., Wang, Y. Y., Sun, Q., Duo, K., and Fei, P. (2019b). Antibacterial activity of olive oil polyphenol extract against Salmonella Typhimurium and Staphylococcus aureus: possible mechanisms. Foodborne Pathog. Dis. 17, 396–403. doi: 10.1089/fpd.2019.2713
Han, Y. J., Chen, W. X., and Sun, Z. C. (2021). Antimicrobial activity and mechanism of limonene against Staphylococcus aureus. J. Food Saf. 41:e12918. doi: 10.1111/jfs.12918
Hao, J. M., Yang, W. P., Yang, H., and Ma, L. Z. (2013). The application of a compound natural preservative solution to chilled beef and mutton under vacuum packaging during refrigerated storage. Food Sci. Technol. Res. 19, 591–599. doi: 10.3136/fstr.19.591
Ifesan, B. O. T., Siripongvutikorn, S., Hutadilok-Towatana, N., and Voravuthikunchai, S. P. (2009). Evaluation of the ability of Eleutherine americana crude extract as natural food additive in cooked pork. J. Food Sci. 74, 352–357. doi: 10.1111/j.1750-3841.2009.01254.x
Kang, J., Jin, W., Wang, J., Sun, Y., Wu, X., and Liu, L. (2019). Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 101, 639–645. doi: 10.1016/j.lwt.2018.11.093
Kang, S. M., Kong, F. H., Shi, X. Y., Han, H. J., Li, M. H., Guang, B. Y., et al. (2020). Antibacterial activity and mechanism of lactobionic acid against Pseudomonas fluorescens and Methicillin-resistant Staphylococcus aureus and its application on whole milk. Food Control 108:106876. doi: 10.1016/j.foodcont.2019.106876
Lee, S. Y., Nam, S. H., Lee, H. J., Son, S. E., and Lee, H. J. (2015). Antibacterial activity of aqueous garlic extract against Escherichia coli O157:H7, Salmonella Typhimurium and Staphylococcus aureus. J. Food Hyg. Safe 30, 210–216. doi: 10.13103/JFHS.2015.30.2.210
Lee, C. W., Choi, H. M., Kim, S. Y., Lee, J. R., Kim, H. J., Jo, C., et al. (2015). Influence of Perilla frutescens var. acuta water extract on the shelf life and physicochemical qualities of cooked beef patties. Korean J Food Sci An. 35, 389–397. doi: 10.5851/kosfa.2015.35.3.389
Li, G. H., Wang, X., Xu, Y. F., Zhang, B. G., and Xia, X. D. (2014). Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 238, 589–596. doi: 10.1007/s00217-013-2140-5
Li, Y., Yang, Z., and Li, J. (2016). Shelf-life extension of pacific white shrimp using algae extracts during refrigerated storage. J. Sci. Food Agric. 97, 291–298. doi: 10.1002/jsfa.7730
Liang, H. Y., He, K. K., Li, T., Cui, S. M., Tang, M., Kang, S. Y., et al. (2020). Mechanism and antibacterial activity of vine tea extract and dihydromyricetin against Staphylococcus aureus. Sci. Rep. 10:21416. doi: 10.1038/s41598-020-78379-y
Liu, X., Cai, J. X., Chen, H. M., Zhong, Q. P., Hou, Y. Q., Chen, W. J., et al. (2020). Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 141:103980. doi: 10.1016/j.micpath.2020.103980
Liu, X. L., Zhang, X. J., Fu, Y. J., Zu, Y. G., Wu, N., and Liu, L. (2011). Cajanol inhibits the growth of Escherichia coli and Staphylococcus aureus by acting on membrane and DNA damage. Planta Med. 77, 158–163. doi: 10.1055/s-0030-1250146
Lucas, D. L., and Were, L. M. (2009). Anti-Listeria monocytogenes activity of heat-treated lyophilized pomegranate juice in media and in ground top round beef. J. Food Prot. 72, 2508–2516. doi: 10.1111/j.1745-4549.2008.00362.x
Mitsumoto, M., O’Grady, M. N., Kerry, J. P., and Joe, B. D. (2005). Addition of tea catechins and vitamin C on sensory evaluation, colour and lipid stability during chilled storage in cooked or raw beef and chicken patties. Meat Sci. 69, 773–779. doi: 10.1016/j.meatsci.2004.11.010
Ozaki, M. M., Munekata, P., Lopes, A., Nascimento, M., and Pollonio, M. (2020). Using chitosan and radish powder to improve stability of fermented cooked sausages. Meat Sci. 167:108165. doi: 10.1016/j.meatsci.2020.108165
Pissaia, J. F., Correr, G. M., Gonzaga, C. C., and da Cunha, L. F. (2015). Influence of shade, curing mode, and aging on the color stability of resin cements. Braz. J. Pharm. Sci. 14, 272–275. doi: 10.1590/1677-3225v14n4a04
Rojas, M. C., and Brewer, M. S. (2010). Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. J. Food Sci. 72, S282–S288. doi: 10.1111/j.1750-3841.2007.00335.x
Shavisi, N., Khanjari, A., Basti, A. A., Misaghi, A., and Shahbazi, Y. (2017). Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 124, 95–104. doi: 10.1016/j.meatsci.2016.10.015
Shi, C., Song, K. K., Zhang, X. R., Song, Y., Sui, Y., Chen, Y. F., et al. (2016). Antimicrobial activity and possible mechanism of action of citral against Cronobacter sakazakii. PLoS One 11:e0159006. doi: 10.1371/journal.pone.0159006
Shi, C., Zhao, X., Meng, R., Liu, Z., Zhang, G., and Zhang, N. (2017). Synergistic antimicrobial effects of nisin and p-Anisaldehyde on Staphylococcus aureus in pasteurized milk. LWT Food Sci. Technol. 84, 222–230. doi: 10.1016/j.lwt.2017.05.056
Song, X. Y., Liu, T., Wang, L., Liu, L., and Wu, X. X. (2020). Antibacterial effects and mechanism of Mandarin (Citrus reticulata L.) essential oil against Staphylococcus aureus. Molecules 25:4956. doi: 10.3390/molecules25214956
Verma, A. K., Chatli, M. K., Kumar, P., and Mehta, N. (2019). Antioxidant and antimicrobial activity of porcine liver hydrolysate in meat emulsion and their influence on physico-chemical and color deterioration during refrigeration storage. J. Food Sci. 84, 1844–1853. doi: 10.1111/1750-3841.14683
Wang, J. Y., Ma, M. M., Yang, J., Chen, L., and Yu, P. (2018). In vitro antibacterial activity and mechanism of monocaprylin against Escherichia coli and Staphylococcus aureus. J. Food Prot. 81, 1988–1996. doi: 10.4315/0362-028X.JFP-18-248
Wang, Z. C., Zhu, J. F., Li, W. T., Li, R. F., Wang, X. Q., Qiao, H. Z., et al. (2020). Antibacterial mechanism of the polysaccharide produced by Chaetomium globosum CGMCC 6882 against Staphylococcus aureus. Int. J. Biol. Macromol. 159, 231–235. doi: 10.1016/j.ijbiomac.2020.04.269
Wu, Y. P., Bai, J. R., Zhong, K., Huang, Y. N., and Gao, H. (2017). A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 218, 463–470. doi: 10.1016/j.foodchem.2016.07.090
Xing, Y. F., He, D., Wang, Y., Zeng, W., Zhang, C., Lu, Y., et al. (2019). Chemical constituents, biological functions and pharmacological effects for comprehensive utilization of Eucommia ulmoides Oliver. Food Sci. Hum. Well. 9, 177–188. doi: 10.1016/j.fshw.2019.03.013
Yang, Y. J., Lin, M. Y., Feng, S. Y., Gu, Q., Chen, Y. C., Wang, Y. D., et al. (2020). Chemical composition, antibacterial activity, and mechanism of action of essential oil from Litsea cubeba against foodborne bacteria. J. Food Process. Preserv. 44:e14724. doi: 10.1111/jfpp.14724
Yarnpakdee, S., Benjakul, S., Nalinanon, S., and Kristinsson, H. G. (2012). Lipid oxidation and fishy odour development in protein hydrolysate from Nile tilapia (Oreochromis niloticus) muscle as affected by freshness and antioxidants. Food Chem. 132, 1781–1788. doi: 10.1016/j.foodchem.2011.11.139
Ye, L., Wang, H., Duncan, S. E., Eigel, W. N., and O’Keefe, S. F. (2015). Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 172, 416–422. doi: 10.1016/j.foodchem.2014.09.090
Yoo, J. H., Baek, K. H., Heo, Y. S., Yong, H. I., and Jo, C. (2021). Synergistic bactericidal effect of clove oil and encapsulated atmospheric pressure plasma against Escherichia coli O157:H7 and Staphylococcus aureus and its mechanism of action. Food Microbiol. 93:103611. doi: 10.1016/j.fm.2020.103611
Zhang, L., Xue, L., Wei, L., Shen, H., and Luo, Y. (2011). Quality predictive models of grass carp (Ctenopharyngodon idellus) at different temperatures during storage. Food Control 22, 1197–1202. doi: 10.1016/j.foodcont.2011.01.017
Keywords: Eucommia ulmoides male flower extract, Staphylococcus aureus, antibacterial mode, natural preservative, cooked beef
Citation: Xing M, Liu S, Yu Y, Guo L, Wang Y, Feng Y, Fei P, Kang H and Ali MA (2022) Antibacterial Mode of Eucommia ulmoides Male Flower Extract Against Staphylococcus aureus and Its Application as a Natural Preservative in Cooked Beef. Front. Microbiol. 13:846622. doi: 10.3389/fmicb.2022.846622
Received: 31 December 2021; Accepted: 10 February 2022;
Published: 08 March 2022.
Edited by:
Mehran Moradi, Urmia University, IranReviewed by:
Yugang Shi, Zhejiang Gongshang University, ChinaLuxin Wang, University of California, Davis, United States
Copyright © 2022 Xing, Liu, Yu, Guo, Wang, Feng, Fei, Kang and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Fei, ZmVpcGVuZzAyMjBAMTI2LmNvbQ==; Huaibing Kang, a2hiaW4wMDFAMTYzLmNvbQ==; Md. Aslam Ali, YXNsYW1fYWdwQGJzbXJhdS5lZHUuYmQ=
 Min Xing
Min Xing Shun Liu
Shun Liu Yaping Yu
Yaping Yu Ling Guo
Ling Guo Yao Wang
Yao Wang Yage Feng
Yage Feng Peng Fei
Peng Fei Huaibing Kang
Huaibing Kang Md. Aslam Ali
Md. Aslam Ali