- Department of Food Hygiene and Environmental Health, Faculty of Veterinary Medicine, University of Helsinki, Helsinki, Finland
Yersinia enterocolitica is a psychrotrophic zoonotic foodborne pathogen. Pigs are considered the main reservoir of Y. enterocolitica 4/O:3, which is the most commonly isolated bioserotype in many European countries. Consuming pork contaminated with Y. enterocolitica can be a health threat, and antimicrobial-resistant strains may complicate the treatment of the most severe forms of yersiniosis. We analyzed the antimicrobial resistance of 1,016 pathogenic porcine Y. enterocolitica 4/O:3 strains originating from Belgium, Estonia, Finland, Germany, Italy, Latvia, Russia, Spain, and the United Kingdom. Based on available reports, we also compared antimicrobial sales for food production animals in these countries, excluding Russia. Antimicrobial resistance profiles were determined using a broth microdilution method with VetMIC plates for 13 antimicrobial agents: ampicillin, cefotaxime, ceftiofur (CTF), chloramphenicol (CHL), ciprofloxacin, florfenicol, gentamicin, kanamycin, nalidixic acid (NAL), streptomycin (STR), sulfamethoxazole (SME), tetracycline (TET), and trimethoprim (TMP). The antimicrobial resistance of Y. enterocolitica 4/O:3 strains varied widely between the countries. Strains resistant to antimicrobial agents other than ampicillin were rare in Estonia, Finland, Latvia, and Russia, with prevalence of 0.7, 0.4, 0, and 8.3%, respectively. The highest prevalence of antimicrobial resistance was found in Spanish and Italian strains, with 98 and 61% of the strains being resistant to at least two antimicrobial agents, respectively. Resistance to at least four antimicrobial agents was found in 34% of Spanish, 19% of Italian, and 7.1% of English strains. Antimicrobial resistance was more common in countries where the total sales of antimicrobials for food production animals are high and orally administered medications are common. Our results indicate that antimicrobials should be used responsibly to treat infections, and parenteral medications should be preferred to orally administered mass medications.
Introduction
Yersinia enterocolitica is a foodborne pathogen capable of causing yersiniosis, the fourth most reported bacterial zoonosis in the European Union [European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC), 2021]. Pigs are the main reservoir of pathogenic Y. enterocolitica, especially bioserotype 4/O:3, and these bacteria have frequently been isolated from the tonsils and feces of clinically healthy pigs (Gürtler et al., 2005; Fredriksson-Ahomaa et al., 2007; Laukkanen et al., 2009; Ortiz Martínez et al., 2009, 2011; Virtanen et al., 2014; Koskinen et al., 2019). Consequently, pork products are important sources of human yersiniosis (Tauxe et al., 1987; Ostroff et al., 1994; Fredriksson-Ahomaa et al., 2006, 2010a; Virtanen et al., 2013).
Yersiniosis usually manifests as gastroenteritis, but the infection may, for example, cause pseudoappendicitis or sepsis or lead to immunological sequelae such as reactive arthritis or erythema nodosum (Bottone, 1999; Fredriksson-Ahomaa et al., 2010a). Most infections are self-limiting, and antimicrobial therapy is therefore not needed. Antimicrobial agents, such as fluoroquinolones, trimethoprim-sulfamethoxazole (SME), tetracycline (TET), and third-generation cephalosporins, are warranted for more severe infections, in which mortality can rise to 50%, and for severe postinfectious complications (Crowe et al., 1996; Jiménez-Valera et al., 1998; Hoogkamp-Korstanje et al., 2000; Guinet et al., 2011; Fàbrega and Vila, 2012). Y. enterocolitica has intrinsic resistance to many β-lactam antibiotics, such as penicillin, ampicillin, and first-generation cephalosporins, due to the presence of two β-lactamase genes blaA and blaB (Cornelis and Abraham, 1975; Bent and Young, 2010; Bonke et al., 2011).
Antimicrobial resistance is a concerning global health threat [World Health Organization (WHO), 2015]. If necessary actions are not taken, an estimated 10 million people could die in 2050 due to antimicrobial resistance with massive consequences for patients, healthcare systems, and economies (Dadgostar, 2019). Using antimicrobial agents for livestock is a significant part of this multifactorial problem and may increase the antimicrobial resistance of foodborne pathogens. These pathogens may transmit from production animals to humans via food products, water, or by direct contact (Collignon, 2012). The transmission of antimicrobial-resistant porcine Y. enterocolitica strains to humans may complicate the treatment of the most severe forms of yersiniosis or other bacterial infections. To control this public health threat, regular and comprehensive monitoring of antimicrobial resistance is required worldwide, so that necessary actions can be taken.
The aim of our study was to assess the antimicrobial resistance of 1,016 Y. enterocolitica bioserotype 4/O:3 strains isolated from porcine origin in nine European countries, and to compare the use of antimicrobial agents in these countries. We observed wide variation in antimicrobial resistance. Concerning levels of antimicrobial resistance were found in countries where the total use of antimicrobial agents is high, especially Spain and Italy. Our results indicate that prudent use of antimicrobials is essential to control antimicrobial resistance already at the farm level.
Materials and Methods
Strains
A total of 1,016 strains of Y. enterocolitica pathogenic bioserotype 4/O:3 from nine European countries (Belgium, Estonia, Finland, Germany, Italy, Latvia, Russia, Spain, and the United Kingdom) were studied. Yersinia enterocolitica strains originated from pork and were chosen from the culture collection of the Department of Food Hygiene and Environmental Health (University of Helsinki, Helsinki, Finland). The strains had been isolated between 1999 and 2007 (Supplementary Table 1) from pig tonsils, except for eight of the German strains that were isolated from the tongue (n = 4), surface samples of pig carcasses (n = 3), and head meat (n = 1).
Antimicrobial Resistance Testing
Antimicrobial resistance was tested using a broth microdilution method according to the standards of the Clinical and Laboratory Standards Institute (CLSI) (2017). The following 13 antimicrobials were tested: ampicillin (concentration range 0.25–32 mg/L), cefotaxime (0.06–2 mg/L), ceftiofur (CTF; 0.12–16 mg/L), chloramphenicol (CHL; 1–128 mg/L), ciprofloxacin (0.008–1 mg/L), florfenicol (4–32 mg/L), gentamicin (0.5–64 mg/L), kanamycin (2–16 mg/L), nalidixic acid (NAL; 1–128 mg/L), streptomycin (STR; 2–256 mg/L), sulfamethoxazole (16–2,048 mg/L), tetracycline (0.5–64 mg/L), and trimethoprim (TMP; 0.25–32 mg/L). Susceptibility monitoring was performed on VetMIC plates (SVA, Uppsala, Sweden). The strains were grown on Yersinia selective agar base (Oxoid Ltd., Basingstoke, New Hampshire, United Kingdom) at 30°C for 24 h. Four or five colonies were transferred into 5 ml of Müller-Hinton II broth (BBL, Müller-Hinton II broth, cation adjusted; Beckton, Dickinson and Company, Sparks, MD, United States) and incubated at 30°C until the absorbance of the broth was 0.08–0.1, to obtain an inoculum size of 108 CFU/ml. The inoculums for broth microdilution were prepared by mixing 10 μl of the inoculum to 10 ml of Müller-Hinton II broth. Each well of the VetMIC plate was filled with 50 μl of the inoculum and sealed with covering tape. The plates were incubated for 1 h in a shaker (150 rpm) and 16–18 h at 30°C. Escherichia coli ATCC 25922 was used as a control strain and incubated at 37°C.
The plates were evaluated with visual examination using a magnifying mirror. The minimum inhibitory concentration (MIC) was defined as the lowest antimicrobial concentration that inhibited bacterial growth. The strains were categorized as susceptible (S), intermediately resistant (I), or resistant (R). Clinical breakpoints for Enterobacteriaceae from the Clinical and Laboratory Standards Institute (CLSI) (2017) were used, except for ceftiofur, florfenicol, and streptomycin, for which we used the breakpoints for Salmonella spp. of Danish Integrated Antimicrobial Resistance Monitoring and Research Program (DANMAP) (2015). The results were analyzed using WHONET 5.6 software (Stelling and O'Brien, 1997). In our study, a strain was classified as multiresistant if it was resistant to at least two of the antimicrobial agents tested, excluding ampicillin, which shows frequent intrinsic resistance.
Estimations of Antimicrobial Use in European Countries
We evaluated the antimicrobial use and policies of eight countries (Belgium, Estonia, Finland, Germany, Italy, Latvia, Spain, and the United Kingdom) based on the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) sales report by the European Medicines Agency (EMA) for year 2011 [European Medicines Agency (EMA), 2013], as no direct data are available for the actual use of antimicrobial agents. This report was the first to cover all eight countries. Respective data were not available from Russia.
In the ESVAC reports, sales are measured in relation to the estimated quantity of animal biomass, i.e., the antimicrobials used for food production animals in milligrams per population correction unit (mg/PCU). The PCU is a technical unit of measurement, which is used to estimate the mass of treated livestock and slaughtered animals during a year, and animals exported or imported for slaughter or fattening in another member country are also taken into account [European Medicines Agency (EMA), 2013]. In addition, we compared the use of orally administered and injectable veterinary antimicrobials based on the corresponding proportions of the sales in each country. Data from the first Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) report by the European Centre for Disease Prevention and Control, European Food Safety Authority, and European Medicines Agency (ECDC, EFSA, and EMA) (2015) were also used to measure antimicrobial use for food production animals and as a baseline for humans.
A report by the European Agency for the Evaluation of Medicinal Products (EMEA) (1999) was used to estimate the use of all antimicrobials, therapeutic antimicrobials, and growth promoter antimicrobials for animals in the mid-1990s. Antimicrobial use in Belgium and Luxemburg, Finland, Germany, Italy, Spain, and the United Kingdom in 1997 was made proportional to the number of animals slaughtered (mg/kg) in these countries in 1996. These data represent antimicrobial use in the countries in the mid-1990s, a decade before antimicrobial growth promoters were banned in the European Union (EU) in 2006 [European Union (EU), 2005]. The data regarding Belgium include Luxemburg, and in Finland, growth promoter antimicrobial levels were less than 2 mg/kg, but 2 mg/kg was used for the analyses.
Statistical Analyses
Statistical analyses were performed in IBM SPSS Statistics 27 (IBM, Armonk, NY, United States). One-tailed Pearson’s correlation was used to measure correlations between the observed prevalence of antimicrobial resistance and the different estimations of antimicrobial use, that is, total, oral, and injectable antimicrobial sales in mg/PCU, growth promoter sales, as well as proportions of antimicrobial sales by administration route. In addition, countries were categorized into two groups based on whether more than two thirds of antimicrobial sales were oral medications instead of parenteral medications such as injections, and antimicrobial resistance between the two groups was compared with the Student’s t test with unequal variances.
Results
Antimicrobial Resistance of Yersinia enterocolitica
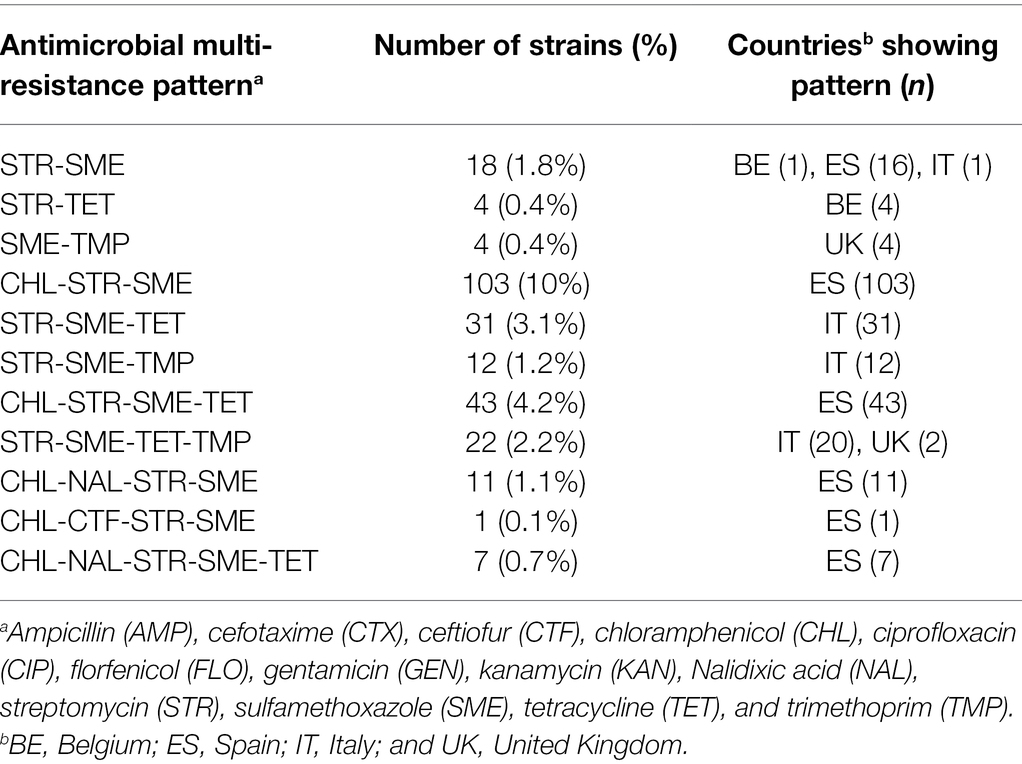
All strains were susceptible to cefotaxime, ceftiofur, ciprofloxacin, florfenicol, gentamicin, and kanamycin (Table 1). The MICs are shown in Supplementary Table 2. Estonian, Finnish, and Latvian strains were susceptible to all other antimicrobials than ampicillin, excluding one Estonian strain resistant to trimethoprim and one Finnish strain resistant to sulfamethoxazole. Resistance to streptomycin was found frequently, with highest resistance levels in Spain (98%), Italy (62%), and Belgium (55%). Resistance to sulfamethoxazole was also common, with highest prevalence of resistance in Spanish (99%), English (82%), and Italian (61%) strains. Tetracycline resistance was found in 49% of Italian and 27% of Spanish strains. Trimethoprim resistance was most common among Italian (30%) and English (21%) strains. Resistance to chloramphenicol and nalidixic acid was found in Spanish strains only.
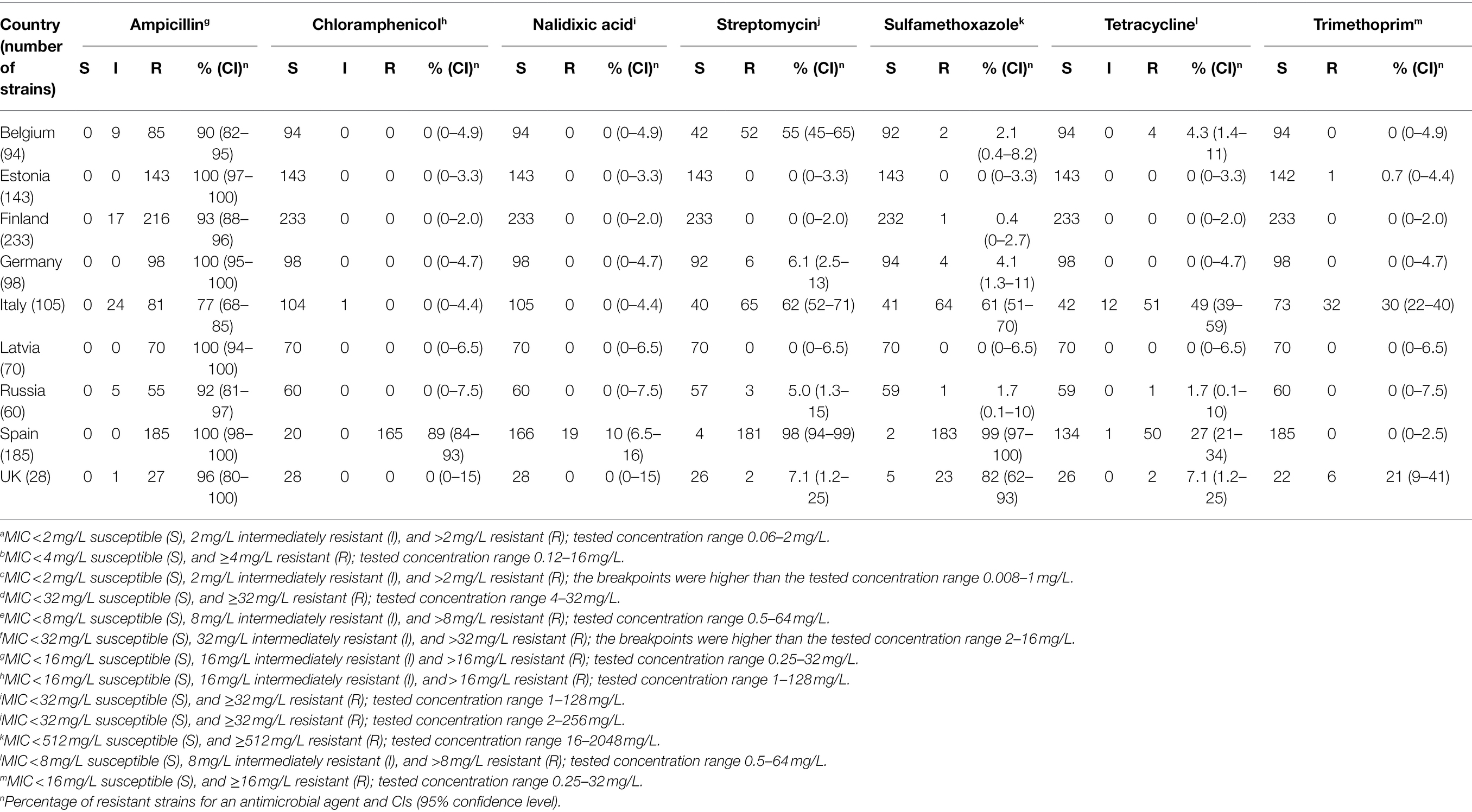
Table 1. Number of porcine Yersinia enterocolitica 4/O:3 strains resistant to antimicrobials. All strains were susceptible to cefotaximea, ceftiofurb, ciprofloxacinc, florfenicold, gentamicine, and kanamycinf.
Antimicrobial Resistance to at Least Two Antimicrobial Agents
Resistance to multiple antimicrobials was found especially in Spanish, Italian, and English Y. enterocolitica 4/O:3 strains, and in a few Belgian strains (Table 2). The majority of the Spanish strains (98%) were multiresistant, and the most common antimicrobial resistance pattern was CHL-STR-SME (Table 3). Multiresistance patterns CHL-STR-SME-TET and CHL-NAL-STR-SME were found in 23 and 6.0% of Spanish strains, respectively. More than half of the Italian strains (61%) were multiresistant, while 21, and 5.3% of the English, and Belgian strains were resistant to at least two antimicrobials, respectively. Resistance to four or five antimicrobials was observed in Spanish (n = 62), Italian (n = 20), and English (n = 2) strains.
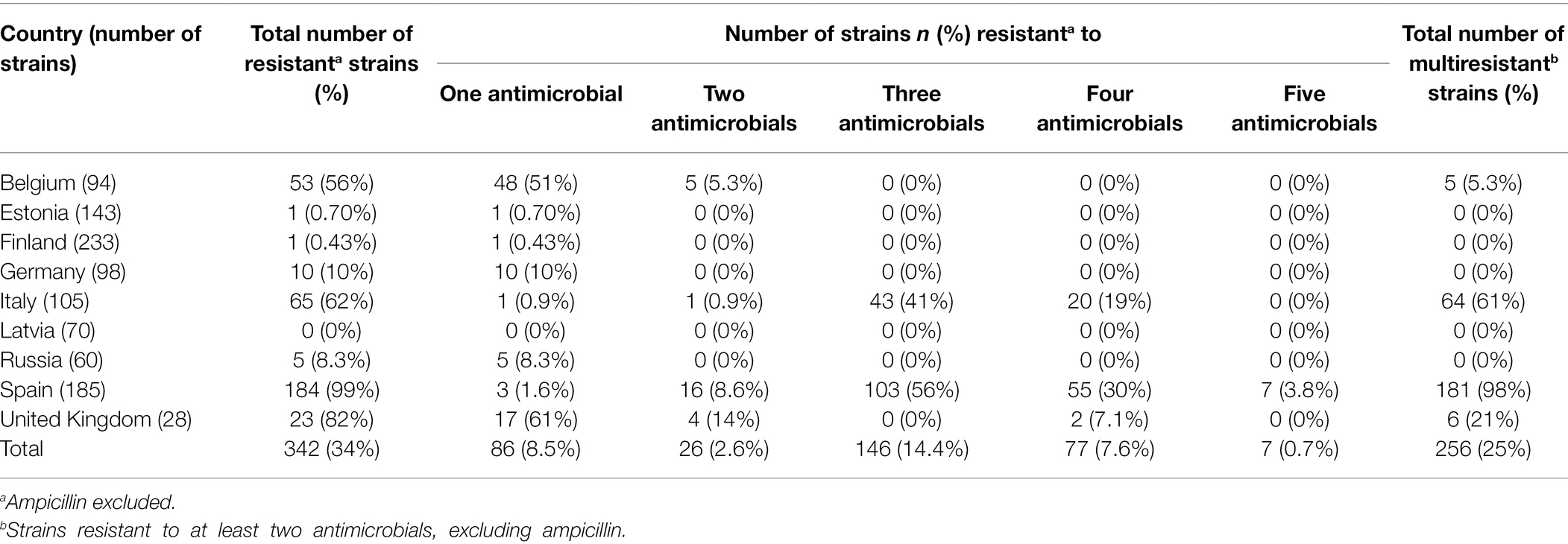
Table 2. Antimicrobial resistance of porcine Yersinia enterocolitica 4/O:3 strains in nine European countries.
Use of Antimicrobial Agents and Its Correlation With Antimicrobial Resistance
Based on sales data, we observed vast differences in the use of antimicrobial agents in the eight countries (Table 4). In general, higher levels of antimicrobials were used in countries, where antimicrobial resistance and multiresistance were frequent (Figures 1, 2). The prevalence of antimicrobial resistance of Y. enterocolitica in the countries significantly correlated with antimicrobial multiresistance (Table 5).
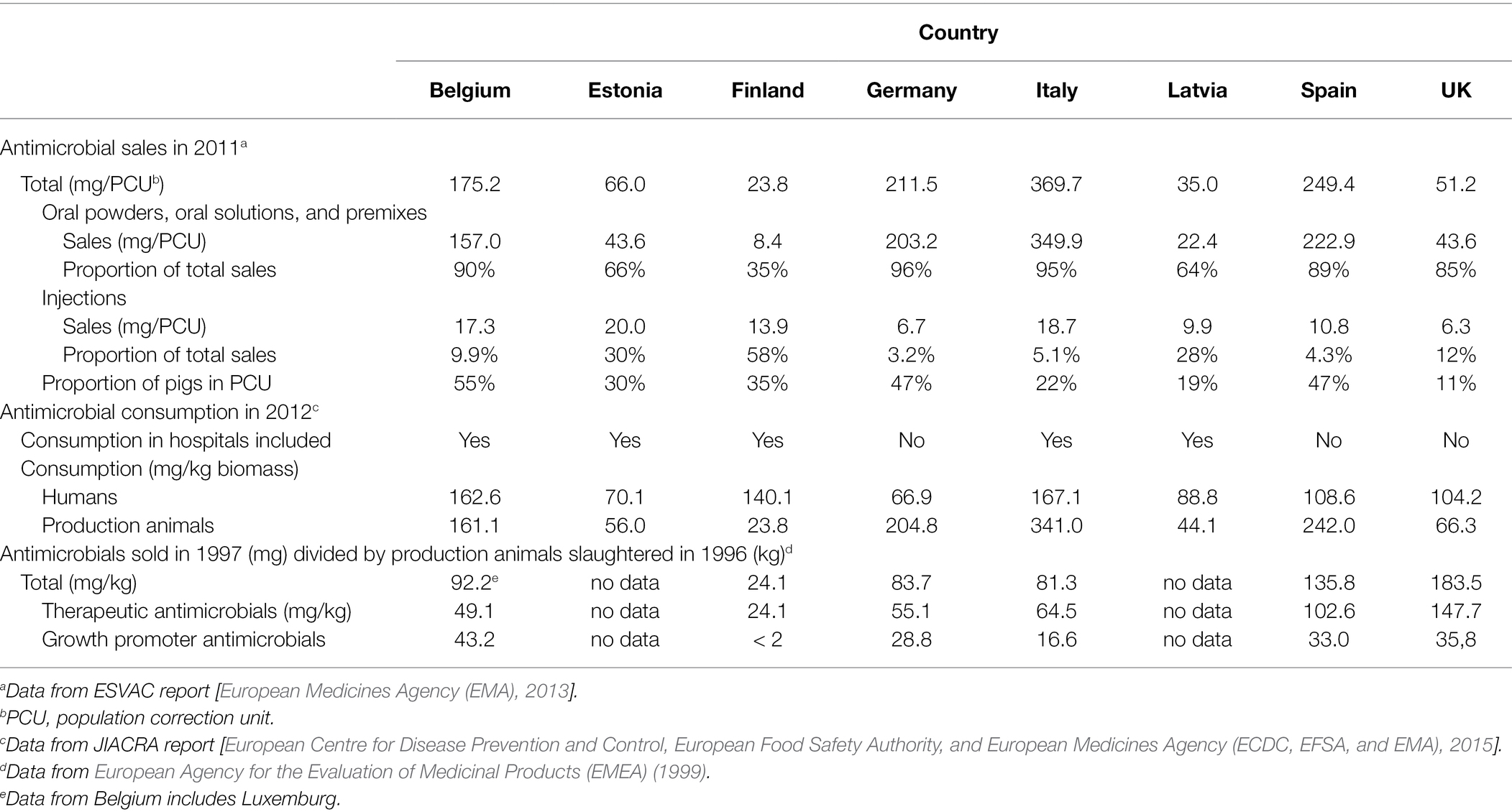
Table 4. Estimated antimicrobial use for food production animals, and for humans as a baseline, in eight European countries based on available reports.
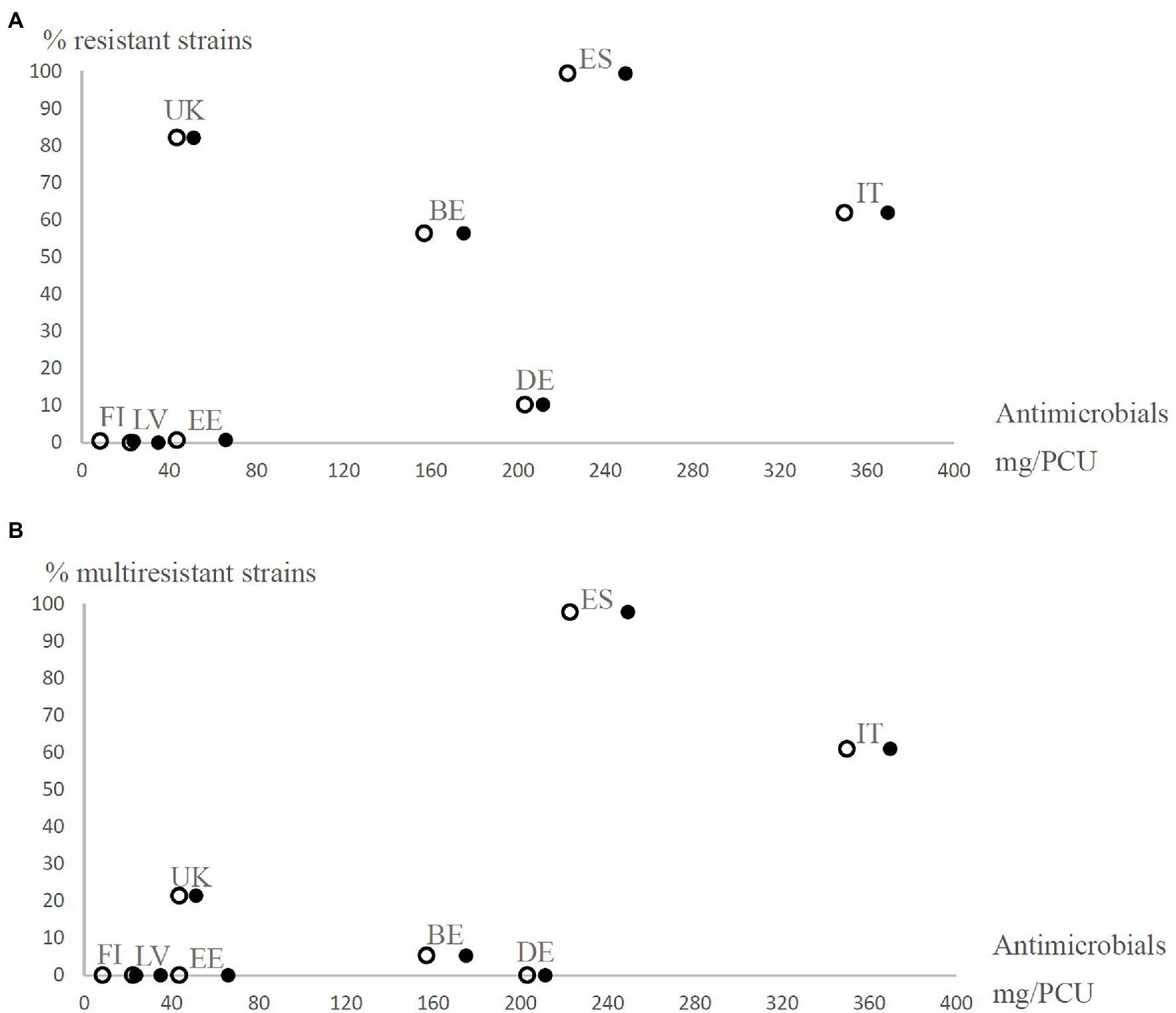
Figure 1. Prevalence of resistant (resistance to at least one antimicrobial agent excluding ampicillin; A) and multiresistant (resistance to at least two antimicrobial agents excluding ampicillin; B) Yersinia enterocolitica 4/O:3 strains from different European countries (BE, Belgium; DE, Germany; EE, Estonia; ES, Spain; FI, Finland; IT, Italy; LV, Latvia; and UK=United Kingdom) observed in the present study in relation to the estimated number of antimicrobials sold for treating production animals in mg per population correction unit (mg/PCU) in 2011. Open circles represent orally administered antimicrobials and closed circles represent all antimicrobials. The proportions of oral solutions, oral powders, and premixes as percentages of total sales of antimicrobial agents in each country [European Medicines Agency (EMA), 2013] were used as coefficients to estimate how many mg/PCU of antimicrobials were administered orally for production animals.
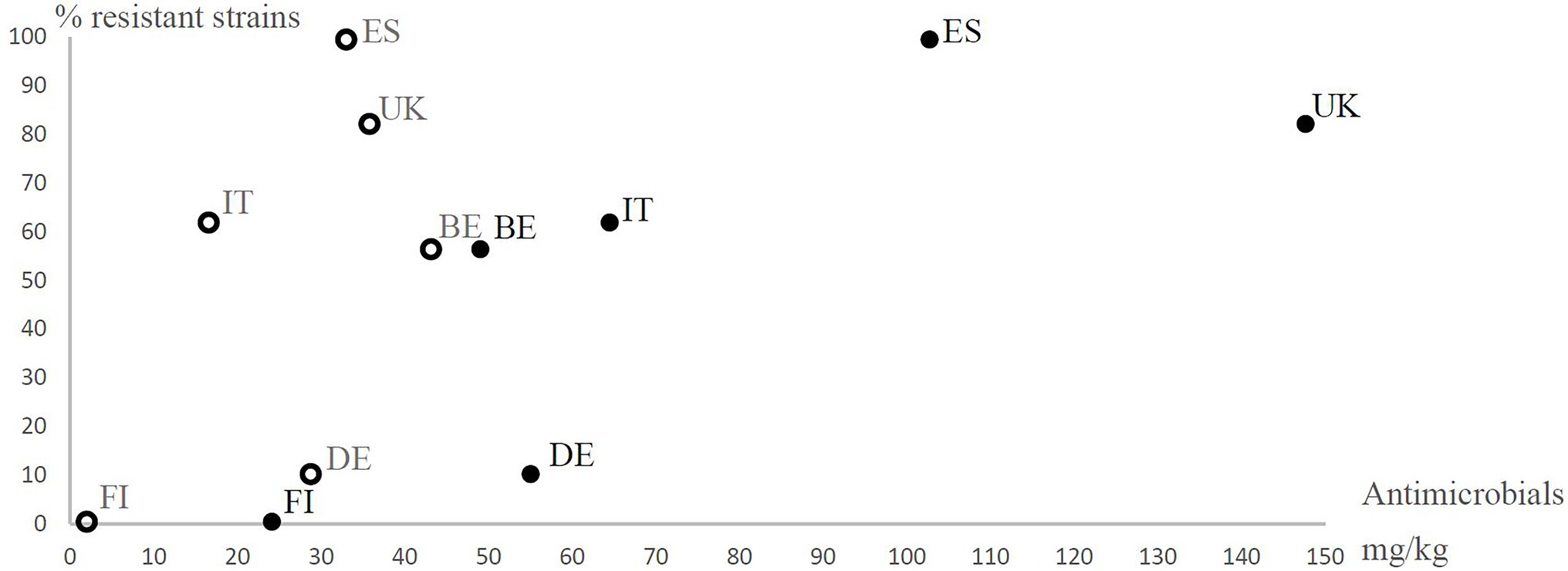
Figure 2. Prevalence of antimicrobial resistant (resistant to at least one antimicrobial agent excluding ampicillin) Yersinia enterocolitica 4/O:3 strains in different European countries (BE, Belgium; DE, Germany; ES, Spain; FI, Finland; IT, Italy; and UK, United Kingdom) observed in the present study in relation to the estimated number of growth promoter antimicrobials used (open circles) and the therapeutic antimicrobials used (closed circles) for animals (mg/kg) in six European countries in the mid-1990s. The use of antimicrobial agents for animals in mg per kg was calculated from the estimated numbers of antimicrobial agents sold for the treatment of animals in 1997 and the estimated weights of slaughtered animals in 1996 in each country. The data are from European Agency for the Evaluation of Medicinal Products (EMEA) (1999). The data regarding Belgium include Luxemburg. In Finland, growth promoter antimicrobial levels were less than 2 mg/kg, but 2 mg/kg was used for the analyses.
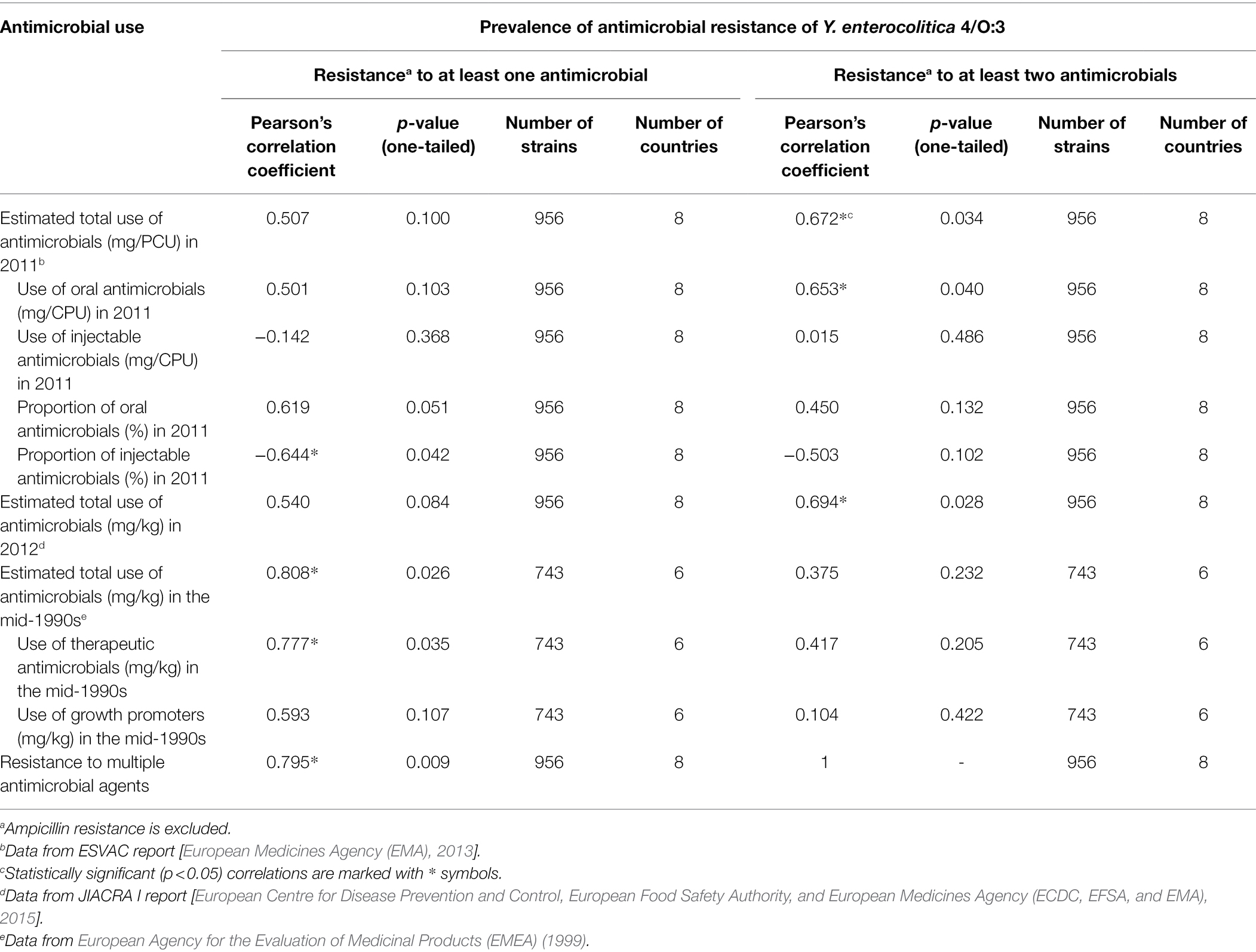
Table 5. Correlation between antimicrobial use and resistance of porcine Yersinia enterocolitica 4/O:3 strains in Europe.
Correlations between estimated antimicrobial use and observed antimicrobial resistance are summarized in Table 5. Total sales of antimicrobial agents and oral antimicrobial agents in the countries in 2011, calculated from the ESVAC report (European Medicines Agency (EMA), 2013), positively correlated with antimicrobial multiresistance of the Y. enterocolitica strains. By contrast, the proportion of injectable antimicrobial agents sold in the countries correlated negatively with antimicrobial resistance. In addition, resistance levels were statistically significantly higher in countries where more than two thirds of antimicrobial sales were orally administered products than in countries where less than two thirds were oral antimicrobials (p = 0.015). Total use of antimicrobial agents in 2012, obtained from the JIACRA I report [European Centre for Disease Prevention and Control, European Food Safety Authority, and European Medicines Agency (ECDC, EFSA, and EMA), 2015], did not statistically significantly correlate with antimicrobial resistance but did correlate with antimicrobial multiresistance.
Prevalence of antimicrobial resistance in the studied strains positively correlated with the total sales of antimicrobials and the sales of therapeutic antimicrobials in the mid-1990s, calculated from the report of European Agency for the Evaluation of Medicinal Products (EMEA) (1999). However, the use of growth promoter antimicrobials in the mid-1990s did not significantly correlate with either antimicrobial resistance or multiresistance (Table 5).
Discussion
We observed wide variation in the antimicrobial resistance profiles of Y. enterocolitica 4/O:3 strains in the nine European countries. Alarming antimicrobial resistance levels were found in Spain and Italy, where most strains were resistant to two or more antimicrobials. Similar concerning levels of antimicrobial resistance of Y. enterocolitica, including multiresistant strains, have also previously been reported in Italy (Bonardi et al., 2013, 2014, 2016) and Greece (Gousia et al., 2011). Gkouletsos et al. (2019) studied the antimicrobial resistance of Greek Y. enterocolitica O:3 strains isolated from production animals, companion animals, and humans, and found no statistically significant differences in the overall levels of antimicrobial resistance between the three groups. As Y. enterocolitica strains can spread between production animals, companion animals, and humans, antimicrobial-resistant strains may also spread between these groups. In Spain, trends of increasing antimicrobial resistance to various antimicrobials, including streptomycin, sulfonamides, trimethoprime-sulfamethoxazole, chloramphenicol, and nalidixic acid, have been observed in Y. enterocolitica strains isolated from human patients (Prats et al., 2000; Marimon et al., 2017).
According to the ESVAC report [European Medicines Agency (EMA), 2013], tetracyclines were the most common veterinary antimicrobial agents sold for treating food production animals in both Italy and Spain in 2011. The common tetracycline use is also likely to explain the high tetracycline resistance observed in our present study. Tetracyclines were the most sold antimicrobials in Italy in 2018 and, bypassed only by penicillin preparations, the second most sold antimicrobial agents in Spain [European Medicines Agency (EMA), 2020]. Of our study countries, Spain and Italy showed the highest antimicrobial sales for treating food production animals per population correction unit (mg/CFU). Multiresistant strains were frequent in these countries and the observed antimicrobial resistance levels the highest in the present study.
The English Y. enterocolitica strains were mainly resistant to sulfamethoxazole, but resistance to trimethoprim, streptomycin, and tetracycline was also observed. According to the ESVAC reports [European Medicines Agency (EMA), 2013, 2020], sulfonamides, following tetracyclines and penicillins, were the third most common veterinary antimicrobial agents sold in the United Kingdom in 2011 and 2018, which may partly explain the relatively high resistance rates to sulfamethoxazole. Sulfamethoxazole is commonly used with trimethoprim, against which antimicrobial resistance was also observed.
Over half of the Belgian strains were resistant to streptomycin, but otherwise antimicrobial resistance levels were moderate in Belgium and Germany. Interestingly, aminoglycosides are not particularly commonly used in Belgium or Germany, yet streptomycin resistance was frequent in Belgium but not in Germany. Low or moderate resistance levels of Y. enterocolitica have been reported in Austria, Germany, and Switzerland (Mayrhofer et al., 2004; Baumgartner et al., 2007; Fredriksson-Ahomaa et al., 2007, 2010b; Bucher et al., 2008; von Altrock et al., 2010; Bonke et al., 2011; Meyer et al., 2011; Schneeberger et al., 2015).
The low antimicrobial resistance levels of Y. enterocolitica 4/O:3 strains detected in Latvia, Estonia, Finland, and Russia, are in accordance with earlier studies available from these countries (Kontiainen et al., 1994; Terentjeva and Bērziņs, 2010; Bonke et al., 2011). However, Terentjeva and Bērziņs (2010) found high resistance (100%) to sulfamethoxazole in Latvian Y. enterocolitica strains and concluded that extensive sulfonamide use for livestock during the time when Latvia belonged to the Soviet Union could have affected the development of such resistance. In our study, resistance to sulfamethoxazole was not observed in the Latvian strains. However, we used the broth microdilution method while Terentjeva and Bērziņs (2010) utilized the disc diffusion method. Different breakpoints and methods complicate the comparison of antimicrobial susceptibility results gained from various studies, and disagreement between different tests is relatively frequent, especially with sulfamethoxazole (Meyer et al., 2011), which may partly explain this difference in results.
The multiresistant Y. enterocolitica 4/O:3 strains were most commonly resistant to streptomycin, sulfamethoxazole, and chloramphenicol. This pattern appears to be common in Y. enterocolitica strains in several European countries (Baumgartner et al., 2007; Sihvonen et al., 2011; Bonardi et al., 2014, 2016). Karlsson et al. (2021) reported a chromosomally encoded multi-drug resistance cassette containing resistance genes to these three types of antimicrobials and mercury.
Antimicrobial resistance in our study positively and statistically significantly correlated with the total sales of antimicrobials and the sales of therapeutic antimicrobials in the mid-1990s, but no statistically significant correlation was found with growth promoter sales. However, the EU banned the use of antimicrobials as growth promoters in 2006 [European Union (EU), 2005], and most of the antimicrobial growth promoters, such as vancomycin and avoparcin, used in the EU in the past are mainly active against Gram-positive bacteria (Wegener et al., 1999). Despite veterinary antimicrobials currently being prescription-only medicines in the member countries of the ESVAC reports [European Medicines Agency (EMA), 2013, 2020], preventative medications are still commonly used. For example, prophylactic medications are given to healthy animals with no clinical symptoms but with a high risk of disease, while metaphylactic medications are given to healthy animals living in the same group as symptomatic animals. Callens et al. (2012) studied 50 Belgian herds of fattening pigs and found that antimicrobials, including critical and broad-spectrum ones, had been used preventatively in 98% of the herds, and 93% of the group treatments were prophylactic while only 7% were metaphylactic. Along with the therapeutic use of antimicrobials, prophylaxis, and metaphylaxis may be needed in certain situations, for example, if a serious disease is threatening an entire group of animals. However, the benefits of preventative medications should always be considered in relation to the risk of developing antimicrobial resistance.
In the present study, the antimicrobial resistance levels were higher in countries where more than two thirds of antimicrobials were sold in enteral forms, which reflect the importance of using parenteral medications for individual animals rather than enteral mass medications via feed. The overall sales data collected by EMA and summarized in Table 4 show that most veterinary antimicrobials in Belgium, Germany, Italy, Spain, and the United Kingdom were sold in enteral forms, such as premixes, oral powders, and oral solutions, while parenteral medications were preferred in Finland, and both enteral and parenteral forms were commonly used in Estonia and Latvia. This finding is supported by Sjölund et al. (2016), who compared antimicrobial use in Belgium, France, Germany, and Sweden and found that the overall use of antimicrobials was highest in German pig herds and lowest in Swedish herds, and antimicrobials were usually given in enteral forms, except in Sweden, where parenteral forms were preferred. According to European Medicines Agency (EMA) (2013), the sales of orally administered antimicrobials are a reasonable estimate of group treatments, because premixes and the majority of oral powders and oral solutions are applicable for group treatment while the sales of small packages of oral powders and oral solutions sufficient for treatment of only a single or a few animals are very low. Frequent use of oral antimicrobials indicates that mass medications are common in certain countries. By contrast, parenteral forms are preferred in Northern Europe [European Medicines Agency (EMA), 2013], which indicates more prudent use of antimicrobial agents mostly targeting individual animals rather than groups of animals.
We observed major differences in veterinary antimicrobial use between the countries. This variation cannot solely be explained by the different proportions of production animal species in European countries, because the antimicrobial use also depends on several other factors, such as the infectious disease situation, economic incentives, and the culture of prescribing antimicrobials [Grave et al., 2012; European Medicines Agency (EMA), 2013, 2020; Speksnijder et al., 2015]. For example, veterinarians in Finland are not allowed to financially profit from selling antimicrobials or other prescription medications. According to guidelines by the European Commission (2015), one key factor in prudent antimicrobial use is to avoid any financial or material benefits for the suppliers or prescribers of medicines.
Some limitations of our study should be considered when interpreting the results. Sales data are an indirect way to simulate the use of antimicrobial agents, as there are no available data on actual antimicrobial use. Dosages vary between and within the classes of antimicrobial agents and between animal species, the proportions of domestic animal species differ by country, and the population correction unit represents all animals, not only pigs. In addition, the population correction unit is a mathematical unit of measurement only and does not represent any actual animal population possibly treated with antimicrobials. Hence, a detailed comparison is difficult, and ESVAC reports should be interpreted carefully [European Medicines Agency (EMA), 2013, 2020]. Despite the limitations of our study and the multifactorial nature of antimicrobial resistance as a phenomenon, the present study shows that the use of antimicrobial agents is a key factor in the emergence of antimicrobial resistance. Our study also shows differences in general antimicrobial policies between the countries. Similarly, Chantziaras et al. (2013) found that antimicrobial use positively correlated with antimicrobial resistance of E. coli isolates.
According to the first JIACRA report by European Centre for Disease Prevention and Control, European Food Safety Authority, and European Medicines Agency (ECDC, EFSA, and EMA) (2015), antimicrobials were used more for production animals than for humans in 2011 and 2012 in Europe. However, according to the newest JIACRA report, the situation was reversed in 2016 [European Centre for Disease Prevention and Control, European Food Safety Authority, and European Medicines Agency (ECDC, EFSA, and EMA), 2021]. Despite increasing efforts to reduce antimicrobial use in the EU, the global trends are concerning. For example, Van Boeckel et al. (2015) estimated that antimicrobial consumption in livestock will increase by 67% from 2010 to 2030, mainly because intensive farming is becoming more common in middle-income countries.
To conclude, the antimicrobial resistance of Y. enterocolitica 4/O:3 strains of porcine origin varied widely between European countries. Resistance was most frequent in countries where antimicrobials, especially enteral medications, are used in large quantities. The antimicrobial resistance of numerous pathogens, including Y. enterocolitica, is considered one of the most severe global health threats. Despite encouraging news that antimicrobial use has generally decreased in Europe during recent years, much work is required globally. We recommend that antimicrobial resistance control should begin already at the farm level. This can be achieved through the strict control of prescriptions, sales, and use of antimicrobial agents. When antimicrobial agents are needed, treating individual animals should be preferred to mass medications whenever possible. Regular antimicrobial susceptibility monitoring and data on actual antimicrobial use are also needed in the battle against antimicrobial resistance.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
HK and RK-T designed the study. PO-M, SJ, JK, and MF-A performed the laboratory work and analyzed the antimicrobial resistance data. JK performed the analysis on antimicrobial use. JK and SJ drafted the manuscript. HK, RK-T, MF-A, and PO-M contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by the Finnish Ministry of Agriculture and Forestry (4877/501/2005) and by the Walter Ehrström Foundation and performed in the Finnish Centre of Excellence in Microbial Food Safety, Academy of Finland (grant numbers 118602 and 141140).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Sonja Alaskewicz, Vilja Kajanti, Erika Pitkänen, and Johanna Ranta for laboratory assistance. We also thank Anna-Liisa Myllyniemi and Riikka Laukkanen-Ninios for methodological discussions, and Henriette Helin-Soilevaara and Katariina Kivilahti-Mäntylä for discussions regarding the interpretation of antimicrobial sales data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.841841/full#supplementary-material
References
Baumgartner, A., Küffer, M., Suter, D., Jemmi, T., and Rohner, P. (2007). Antimicrobial resistance of Yersinia enterocolitica strains from human patients, pigs and retail pork in Switzerland. Int. J. Food Microbiol. 115, 110–114. doi: 10.1016/j.ijfoodmicro.2006.10.008
Bent, Z. W., and Young, G. M. (2010). Contribution of BlaA and BlaB beta-lactamases to antibiotic susceptibility of Yersinia enterocolitica biovar 1B. Antimicrob. Agents Chemother. 54, 4000–4002. doi: 10.1128/AAC.01754-09
Bonardi, S., Alpigiani, I., Pongolini, S., Morganti, M., Tagliabue, S., Bacci, C., et al. (2014). Detection, enumeration and characterization of Yersinia enterocolitica 4/O:3 in pig tonsils at slaughter in Northern Italy. Int. J. Food Microbiol. 177, 9–15. doi: 10.1016/j.ijfoodmicro.2014.02.005
Bonardi, S., Bassi, L., Brindani, F., D'Incau, M., Barco, L., Carra, E., et al. (2013). Prevalence, characterization and antimicrobial susceptibility of Salmonella enterica and Yersinia enterocolitica in pigs at slaughter in Italy. Int. J. Food Microbiol. 163, 248–257. doi: 10.1016/j.ijfoodmicro.2013.02.012
Bonardi, S., Bruini, I., D’Incau, M., Van Damme, I., Carniel, E., Brémont, S., et al. (2016). Detection, seroprevalence and antimicrobial resistance of Yersinia enterocolitica and Yersinia pseudotuberculosis in pig tonsils in northern Italy. Int. J. Food Microbiol. 235, 125–132. doi: 10.1016/j.ijfoodmicro.2016.07.033
Bonke, R., Wacheck, S., Stüber, E., Meyer, C., Märtlbauer, E., and Fredriksson-Ahomaa, M. (2011). Antimicrobial susceptibility and distribution of β-lactamase A (blaA) and β-lactamase B (blaB) genes in enteropathogenic Yersinia species. Microb. Drug Resist. 17, 575–581. doi: 10.1089/mdr.2011.0098
Bottone, E. J. (1999). Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1, 323–333. doi: 10.1016/s1286-4579(99)80028-8
Bucher, M., Meyer, C., Grötzbach, B., Wacheck, S., Stolle, A., and Fredriksson-Ahomaa, M. (2008). Epidemiological data on pathogenic Yersinia enterocolitica in southern Germany during 2000-2006. Foodborne Pathog. Dis. 5, 273–280. doi: 10.1089/fpd.2007.0076
Callens, B., Persoons, D., Maes, D., Laanen, M., Postma, M., Boyen, F., et al. (2012). Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Vet. Med. 106, 53–62. doi: 10.1016/j.prevetmed.2012.03.001
Chantziaras, I., Boyen, F., Callens, B., and Dewulf, J. (2013). Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J. Antimicrob. Chemother. 69, 827–834. doi: 10.1093/jac/dkt443
Clinical and Laboratory Standards Institute (CLSI) (2017). Performance Standards for Antimicrobial Susceptibility Testing; 27th Edn. CLSI Supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute.
Collignon, P. (2012). Clinical impact of antimicrobial resistance in humans. Rev. Sci. Tech. 31, 211–220. doi: 10.20506/rst.31.1.2111
Cornelis, G., and Abraham, E. P. (1975). ß-lactamases from Yersinia enterocolitica. J. Gen. Microbiol. 87, 273–284. doi: 10.1099/00221287-87-2-273
Crowe, M., Ashford, K., and Ispahani, P. (1996). Clinical features and antibiotic treatment of septic arthritis and osteomyelitis due to Yersinia enterocolitica. J. Med. Microbiol. 45, 302–309. doi: 10.1099/00222615-45-4-302
Dadgostar, P. (2019). Antimicrobial resistance: implications and costs. Infect. Drug Resist. 12, 3903–3910. doi: 10.2147/IDR.S234610
Danish Integrated Antimicrobial Resistance Monitoring and Research Program (DANMAP) (2015). DANMAP 2014—Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600-2032.
European Agency for the Evaluation of Medicinal Products (EMEA) (1999). Final report: Antibiotic resistance in the European Union associated with therapeutic use of veterinary medicines – report and qualitative risk assessment by the Committee for Veterinary Medicinal Products. EMEA/CVMP/342/99.
European Centre for Disease Prevention and Control, European Food Safety Authority, and European Medicines Agency (ECDC, EFSA, and EMA) (2015). First joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J. 13:4006. doi: 10.2903/j.efsa.2015.4006
European Centre for Disease Prevention and Control, European Food Safety Authority, and European Medicines Agency (ECDC, EFSA, and EMA) (2021). Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA, JIACRA III. 2016–2018. EFSA J. 19:6712. doi: 10.2903/j.efsa.2021.6712
European Commission (2015). Commission notice: guidelines for the prudent use of antimicrobials in veterinary medicine (2015/C 299/04). Off. J. Eur. Union 58, 7–26.
European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC) (2021). The European Union one health 2019 zoonoses report. EFSA J. 19:6406. doi: 10.2903/j.efsa.2021.6406
European Medicines Agency (EMA) (2013). European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Sales of veterinary antimicrobial agents in 25 European countries in 2011. EMA/236501/2013.
European Medicines Agency (EMA) (2020). European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Sales of veterinary antimicrobial agents in 31 European countries in 2018. EMA/24309/2020.
European Union (EU) (2005). Ban on Antibiotics as Growth Promoters in Animal Feed Enters Into Effect. European Union. Available at: http://europa.eu/rapid/press-release_IP-05-1687_en.htm (Accessed October 12, 2021).
Fàbrega, A., and Vila, J. (2012). Yersinia enterocolitica: pathogenesis, virulence and antimicrobial resistance. Enferm. Infecc. Microbiol. Clin. 30, 24–32. doi: 10.1016/j.eimc.2011.07.017
Fredriksson-Ahomaa, M., Lindström, M., and Korkeala, H. (2010a). “Yersinia enterocolitica and Yersinia pseudotuberculosis,” in Pathogens and Toxins in Foods: Challenges and Interventions. eds. V. K. Juneja and J. N. Sofos (Washington, DC: ASM Press), 164–180.
Fredriksson-Ahomaa, M., Meyer, C., Bonke, R., Stűber, E., and Wacheck, S. (2010b). Characterization of Yersinia enterocolitica 4/O:3 isolates from tonsils of Bavarian slaughter pigs. Lett. Appl. Microbiol. 50, 412–418. doi: 10.1111/j.1472-765X.2010.02816.x
Fredriksson-Ahomaa, M., Stolle, A., Siitonen, A., and Korkeala, H. (2006). Sporadic human Yersinia enterocolitica infections by bioserotype 4/O:3 originate mainly from pigs. J. Med. Microbiol. 55, 747–749. doi: 10.1099/jmm.0.46523-0
Fredriksson-Ahomaa, M., Stolle, A., and Stephan, R. (2007). Prevalence of pathogenic Yersinia enterocolitica in pigs slaughtered at a Swiss abattoir. Int. J. Food Microbiol. 119, 207–212. doi: 10.1016/j.ijfoodmicro.2007.07.050
Gkouletsos, T., Patas, K., Lambrinidis, G., Neubauer, H., Sprague, L. D., Ioannidis, A., et al. (2019). Antimicrobial resistance of Yersinia enterocolitica and presence of plasmid pYV virulence genes in human and animal isolates. New Microbe. New Infect. 32:100604. doi: 10.1016/j.nmni.2019.100604
Gousia, P., Economou, V., Sakkas, H., Leveidiotou, S., and Papadopoulou, C. (2011). Antimicrobial resistance of major foodborne pathogens from major meat products. Foodborne Pathog. Dis. 8, 27–38. doi: 10.1089/fpd.2010.0577
Grave, K., Greko, C., Kvaale, M. K., Torren-Edo, J., Mackay, D., Muller, A., et al. (2012). Sales of veterinary antibacterial agents in nine European countries during 2005–09: trends and patterns. J. Antimicrob. Chemother. 67, 3001–3008. doi: 10.1093/jac/dks298
Guinet, F., Carniel, E., and Leclercq, A. (2011). Transfusion-transmitted Yersinia enterocolitica sepsis. Clin. Infect. Dis. 53, 583–591. doi: 10.1093/cid/cir452
Gürtler, M., Alter, T., Kasimir, S., Linnebur, M., and Fehlhaber, K. (2005). Prevalence of Yersinia enterocolitica in fattening pigs. J. Food Prot. 68, 850–854. doi: 10.4315/0362-028x-68.4.850
Hoogkamp-Korstanje, J. A., Moesker, H., and Bruyn, G. A. (2000). Ciprofloxacin v placebo for treatment of Yersinia enterocolitica triggered reactive arthritis. Ann. Rheum. Dis. 59, 914–917. doi: 10.1136/ard.59.11.914
Jiménez-Valera, M., Gonzalez-Torres, C., Moreno, E., and Ruiz-Bravo, A. (1998). Comparison of ceftriaxone, amikacin, and ciprofloxacin in treatment of experimental Yersinia enterocolitica O9 infection in mice. Antimicrob. Agents Chemother. 42, 3009–3011. doi: 10.1128/AAC.42.11.3009
Karlsson, P. A., Tano, E., Jernbergm, C., Hickman, R. A., Guy, L., Järhult, J. D., et al. (2021). Molecular characterization of multidrug-resistant Yersinia enterocolitica from foodborne outbreaks in Sweden. Front. Microbiol. 12:664665. doi: 10.3389/fmicb.2021.664665
Kontiainen, S., Sivonen, A., and Renkonen, O.-V. (1994). Increased yields of pathogenic Yersinia enterocolitica strains by cold enrichment. Scand. J. Infect. Dis. 26, 685–691. doi: 10.3109/00365549409008636
Koskinen, J., Keto-Timonen, R., Virtanen, S., Vilar, M. J., and Korkeala, H. (2019). Prevalence and dynamics of pathogenic Yersinia enterocolitica 4/O:3 among Finnish piglets, fattening pigs, and sows. Foodborne Pathog. Dis. 16, 831–839. doi: 10.1089/fpd.2019.2632
Laukkanen, R., Ortiz Martínez, P., Siekkinen, K.-M., Ranta, J., Maijala, R., and Korkeala, H. (2009). Contamination of carcasses with human pathogenic Yersinia enterocolitica 4/O:3 originates from pigs infected on farms. Foodborne Pathog. Dis. 6, 681–688. doi: 10.1089/fpd.2009.0265
Marimon, J. M., Figueroa, R., Idigoras, P., Gomariz, M., Alkorta, M., Cilla, G., et al. (2017). Thirty years of human infections caused by Yersinia enterocolitica in northern Spain: 1985-2014. Epidemiol. Infect. 145, 2197–2203. doi: 10.1017/S095026881700108X
Mayrhofer, S., Paulsen, P., Smulders, F. J., and Hilbert, F. (2004). Antimicrobial resistance profile of five major food-borne pathogens isolated from beef, pork and poultry. Int. J. Food Microbiol. 97, 23–29. doi: 10.1016/j.ijfoodmicro.2004.04.006
Meyer, C., Stolle, A., and Fredriksson-Ahomaa, M. (2011). Comparison of broth microdilution and disk diffusion test for antimicrobial resistance testing in Yersinia enterocolitica 4/O:3 strains. Microbial. Drug. Res. 17, 479–484. doi: 10.1089/mdr.2011.0012
Ortiz Martínez, P., Fredriksson-Ahomaa, M., Pallotti, A., Rosmini, R., Houf, K., and Korkeala, H. (2011). Variation in the prevalence of enteropathogenic Yersinia in slaughter pigs from Belgium, Italy, and Spain. Foodborne Pathog. Dis. 8, 445–450. doi: 10.1089/fpd.2009.0461
Ortiz Martínez, P., Fredriksson-Ahomaa, M., Sokolova, J., Roasto, M., Bérziņš, A., and Korkeala, H. (2009). Prevalence of enteropathogenic Yersinia in Estonian, Latvian and Russian (Leningrad region) pigs. Foodborne Pathog. Dis. 6, 719–724. doi: 10.1089/fpd.2008.0251
Ostroff, S. M., Kapperud, G., Hutwagner, L. C., Nesbakken, T., Bean, N. H., Lassen, J., et al. (1994). Sources of sporadic Yersinia enterocolitica infections in Norway: a prospective case-control study. Epidemiol. Infect. 112, 133–141. doi: 10.1017/s0950268800057496
Prats, G., Mirelis, B., Llovet, T., Muñoz, C., Miró, E., and Navarro, F. (2000). Antibiotic resistance trends in enteropathogenic bacteria isolated in 1985-1987 and 1995-1998 in Barcelona. Antimicrob. Agents Chemother. 44, 1140–1145. doi: 10.1128/AAC.44.5.1140-1145.2000
Schneeberger, M., Brodard, I., and Overesch, G. (2015). Virulence-associated gene pattern of porcine and human Yersinia enterocolitica biotype 4 isolates. Int. J. Food Microbiol. 198, 70–74. doi: 10.1016/j.ijfoodmicro.2014.12.029
Sihvonen, L. M., Toivonen, S., Haukka, K., Kuusi, M., Skurnik, M., and Siitonen, A. (2011). Multilocus variable-number tandem-repeat analysis, pulsed-field gel electrophoresis, and antimicrobial susceptibility patterns in discrimination of sporadic and outbreak-related strains of Yersinia enterocolitica. BMC Microbiol. 11:42. doi: 10.1186/1471-2180-11-42
Sjölund, M., Postma, M., Collineau, L., Lösken, S., Backhans, A., Belloc, C., et al. (2016). Quantitative and qualitative antimicrobial usage patterns in farrow-to-finish pig herds in Belgium France, Germany and Sweden. Prev. Vet. Med. 130, 41–50. doi: 10.1016/j.prevetmed.2016.06.003
Speksnijder, D. C., Jaarsma, A. D. C., van der Gugten, A. C., Verheij, T. J. M., and Wagenaar, J. A. (2015). Determinants associated with veterinary antimicrobial prescribing in farm animals in the Netherlands: a qualitative study. Zoonoses Public Health 62, 39–51. doi: 10.1111/zph.12168
Stelling, J. M., and O'Brien, T. F. (1997). Surveillance of antimicrobial resistance: the WHONET program. Clin. Infect. Dis. 24, S157–S168. doi: 10.1093/clinids/24.Supplement_1.S157
Tauxe, R. V., Vandepitte, J., Wauters, G., Martin, S. M., Goossens, V., De Mol, P., et al. (1987). Yersinia enterocolitica infections and pork: the missing link. Lancet 329, 1129–1132. doi: 10.1016/s0140-6736(87)91683-7
Terentjeva, M., and Bērziņs, A. (2010). Prevalence and antimicrobial resistance of Yersinia enterocolitica and Yersinia pseudotuberculosis in slaughter pigs in Latvia. J. Food Prot. 73, 1335–1338. doi: 10.4315/0362-028x-73.7.1335
Van Boeckel, T. P., Brower, C., Gilbert, M., Grenfell, B. T., Levin, S. A., Robinson, T. P., et al. (2015). Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U. S. A. 112, 5649–5654. doi: 10.1073/pnas.1503141112
Virtanen, S., Laukkanen-Ninios, R., Ortiz Martínez, P., Siitonen, A., Fredriksson-Ahomaa, M., and Korkeala, H. (2013). Multiple-locus variable-number tandem-repeat analysis in genotyping Yersinia enterocolitica strains from human and porcine origins. J. Clin. Microbiol. 51, 2154–2159. doi: 10.1128/JCM.00710-13
Virtanen, S., Nikunen, S., and Korkeala, H. (2014). Introduction of infected animals to herds is an important route for the spread of Yersinia enterocolitica infection between pig farms. J. Food Prot. 77, 116–121. doi: 10.4315/0362-028X.JFP-13-144
von Altrock, A., Roesler, U., Merle, R., and Waldmann, K. H. (2010). Prevalence of pathogenic Yersinia enterocolitica strains on liver surfaces of pigs and their antimicrobial susceptibility. J. Food Prot. 73, 1680–1683. doi: 10.4315/0362-028x-73.9.1680
Wegener, H. C., Aarestrup, F. M., Jensen, L. B., Hammerum, A. M., and Bager, F. (1999). Use of antimicrobial growth promoters in food animals and Enterococcus faecium resistance to therapeutic antimicrobial drugs in Europe. Emerg. Infect. Dis. 5, 329–335. doi: 10.3201/eid0503.990303
Keywords: antibiotic, antimicrobial, antimicrobial policy, control, foodborne pathogen, pig, resistance, Yersinia enterocolitica
Citation: Koskinen J, Ortiz-Martínez P, Keto-Timonen R, Joutsen S, Fredriksson-Ahomaa M and Korkeala H (2022) Prudent Antimicrobial Use Is Essential to Prevent the Emergence of Antimicrobial Resistance in Yersinia enterocolitica 4/O:3 Strains in Pigs. Front. Microbiol. 13:841841. doi: 10.3389/fmicb.2022.841841
Edited by:
Dario De Medici, National Institute of Health (ISS), ItalyReviewed by:
Thilo M. Fuchs, Friedrich-Loeffler-Institute, GermanyHuaiqi Jing, Chinese Center for Disease Control and Prevention, China
Copyright © 2022 Koskinen, Ortiz-Martínez, Keto-Timonen, Joutsen, Fredriksson-Ahomaa and Korkeala. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juho Koskinen, anVoby5rb3NraW5lbkBoZWxzaW5raS5maQ==
 Juho Koskinen
Juho Koskinen Pilar Ortiz-Martínez
Pilar Ortiz-Martínez Riikka Keto-Timonen
Riikka Keto-Timonen Maria Fredriksson-Ahomaa
Maria Fredriksson-Ahomaa Hannu Korkeala
Hannu Korkeala