- Department of Clinical Laboratory, Children’s Hospital of Soochow University, Suzhou, China
Introduction: To analyze the pathogen distribution and drug resistance of newborns with bloodstream infection (BSI) to help clinicians choose the appropriate empirical antibiotic therapy for clinical infection control.
Methods: A total of 707 neonatal BSI cases were retrospectively analyzed. The bacteria in blood culture-positive samples were cultured, identified, and analyzed for drug sensitivity by routine methods. Statistical software was used to compare and analyze the basic data, pathogenic information, and drug resistance of the main bacteria.
Results: The 5-year average positive rate of neonatal blood culture was 2.50%. The number of specimens submitted for inspection in 2020 significantly decreased. The top five infectious pathogens with the highest proportion were coagulase-negative Staphylococcus (67.35%), of which Staphylococcus epidermidis had the highest proportion (31.26%), followed by Escherichia coli (12.87%), Klebsiella pneumoniae (9.05%), Streptococcus agalactiae (8.63%), and Staphylococcus aureus (3.25%). Gram-positive (G+) bacteria were dominant, accounting for 69.45%. The main G+ bacteria had a higher rate of resistance to erythromycin and penicillin G. The main Gram-negative (G–) bacteria had a high resistance rate to a variety of antibacterial drugs, especially cephalosporin antibiotics. The overall resistance of K. pneumoniae was higher than that of E. coli. The top two fungi detected were Candida parapsilosis and Candida albicans. C. parapsilosis did not appear to be resistant to antibiotics, while C. albicans was resistant to multiple antibiotics. The type of microbial infection had a statistically significant difference in the positive rate among the age at delivery and wards (p < 0.05). There were significant differences in the detection of fungi among these groups (p < 0.05). The positive rate of G+ bacteria in the term newborns was significantly higher than that in the preterm newborns (p < 0.05). Preterm newborns are more susceptible to pneumonia.
Conclusion: G+ bacteria are the main pathogens of neonatal BSI. Preterm newborns are more likely to be infected with G– bacteria. E. coli and K. pneumoniae are the most common G– bacteria, and both have a high resistance rate to a variety of antibacterial drugs. According to the distribution characteristics and drug resistance, it is very important to select antibiotics reasonably.
Introduction
Infection is the main cause of morbidity in infancy, accounting for 15% of global neonatal deaths (Lucia Hug and You, 2017). Among them, bloodstream infection (BSI) is a common nosocomial type of neonatal death (Yuan et al., 2015). In 2017, the National Bacterial Drug Resistance Monitoring Network reported that 15.2% of bacterial infections in China came from blood samples (Hu et al., 2018). The immune function of newborns is underdeveloped, and resistance is poor. It is very easy to cause sepsis when blood flow infection occurs. The incidence rate of neonatal septicemia among the surviving newborns was 4.5–9.7% (FLeischmann-Struzek et al., 2018). However, due to the clinical use of unilateral blood culture for examination, fewer bacteria, and the use of antibiotics during delivery, blood culture results are often false negative (Klingenberg et al., 2018). The treatment and survival of newborns, especially premature babies, often rely on effective antibiotics, but due to the delay in laboratory tests, empirical medication is often given before the results are available (Puopolo et al., 2018). For neonates, especially premature infants, the use of antibiotics for more than 5 days in infants with negative blood cultures will increase the risk of necrotizing enterocolitis, bronchopulmonary dysplasia, and invasive fungal infections (Ting et al., 2016; Esaiassen et al., 2017). Therefore, the use of big data analysis to explore the results of neonatal drug susceptibility is very important to guide the clinical selection of appropriate antibiotics. At present, there have been research reports on BSI (Spaulding et al., 2019; Johnson et al., 2020; Liu et al., 2020), but due to the influence of factors such as different subjects and regions, the infection characteristics are also different. There are few research papers and comments on the correlation of neonatal BSI in East China. Grasping the distribution characteristics of BSI pathogens in a certain area and performing empirical treatment for the first time are of great significance to saving the lives of newborns. Therefore, a retrospective study of 707 clinical cases of neonatal BSI in East China was performed to understand the composition of pathogenic bacteria and bacterial resistance. The report is as follows.
Materials and Methods
General Information
During January 1, 2016, to December 31, 2020, 28,287 blood culture specimens were collected from the Children’s Hospital of Soochow University. A total of 707 newborns with BSI were selected as the research subjects. The inclusion criteria were as follows: (1) newborns; (2) positive blood culture; and (3) increased inflammatory indexes with fever and other blood flow infection symptoms. Among them, 16,040 were male newborns, and 12,244 were female newborns, with a male–female ratio of 1.31:1. According to the age at delivery, the term newborns (11,822 cases) have gestational ages of ≥ 37 weeks, and the preterm newborns (16,465 cases) have gestational ages of < 37 weeks. According to the different admission wards, newborns who have been assessed by the doctor in serious condition will be admitted to the neonatal intensive care unit (NICU) (6,628 cases), and other newborns will be admitted to the general neonatology unit (21,659 cases).
Instruments and Reagents
The blood culture instrument was purchased from BD (BACTEC FX, United States). The carbon dioxide incubator was purchased from Panasonic (MCO-18AC, Japan). Mass spectrometry was purchased from Bruker (Microflex LT/SH, Germany). The automatic bacterial detection and analysis system was purchased from BioMerieux (VITEK2® compact, France). Drug-sensitive paper was purchased from Oxoid (Basingstoke, Britain). All kinds of culture plates were purchased from Antu (Zhenzhou, China).
Strain Identification and Drug Sensitivity Test
Blood culture bottles were placed into the instrument for incubation. The positive samples were transferred to the culture plate and incubated at 37°C for 18–24 h (5% CO2). The colonies were identified by using a mass spectrometer. The automatic bacterial detection and analysis system and Kirby–Bauer (KB) method were used for the drug sensitivity test. The results were judged according to the latest standards of the Clinical Laboratory Standardization Association (Clinical and Laboratory Standards Institute, 2020). Extended-spectrum β-lactamases (ESBLs) were determined by the automatic bacterial detection and analysis system. The judgment results were obtained according to its own expert system. The quality control strains were Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 25923 and ATCC 29213), Enterococcus faecalis (ATCC 29212), and Streptococcus pneumoniae (49619), which were purchased from the clinical testing center of the National Health Commission.
Statistical Analysis
SPSS 20.0 and WHONET 5.6 were used to analyze data. The counting data were expressed as the number of cases (n) and rate (%). The χ2-test was used in univariate analysis. The comparison between groups was carried out by the χ2-test, with p < 0.05 as the difference, which was statistically significant.
Results
Annual Distribution of Pathogenic Bacteria [n (%)]
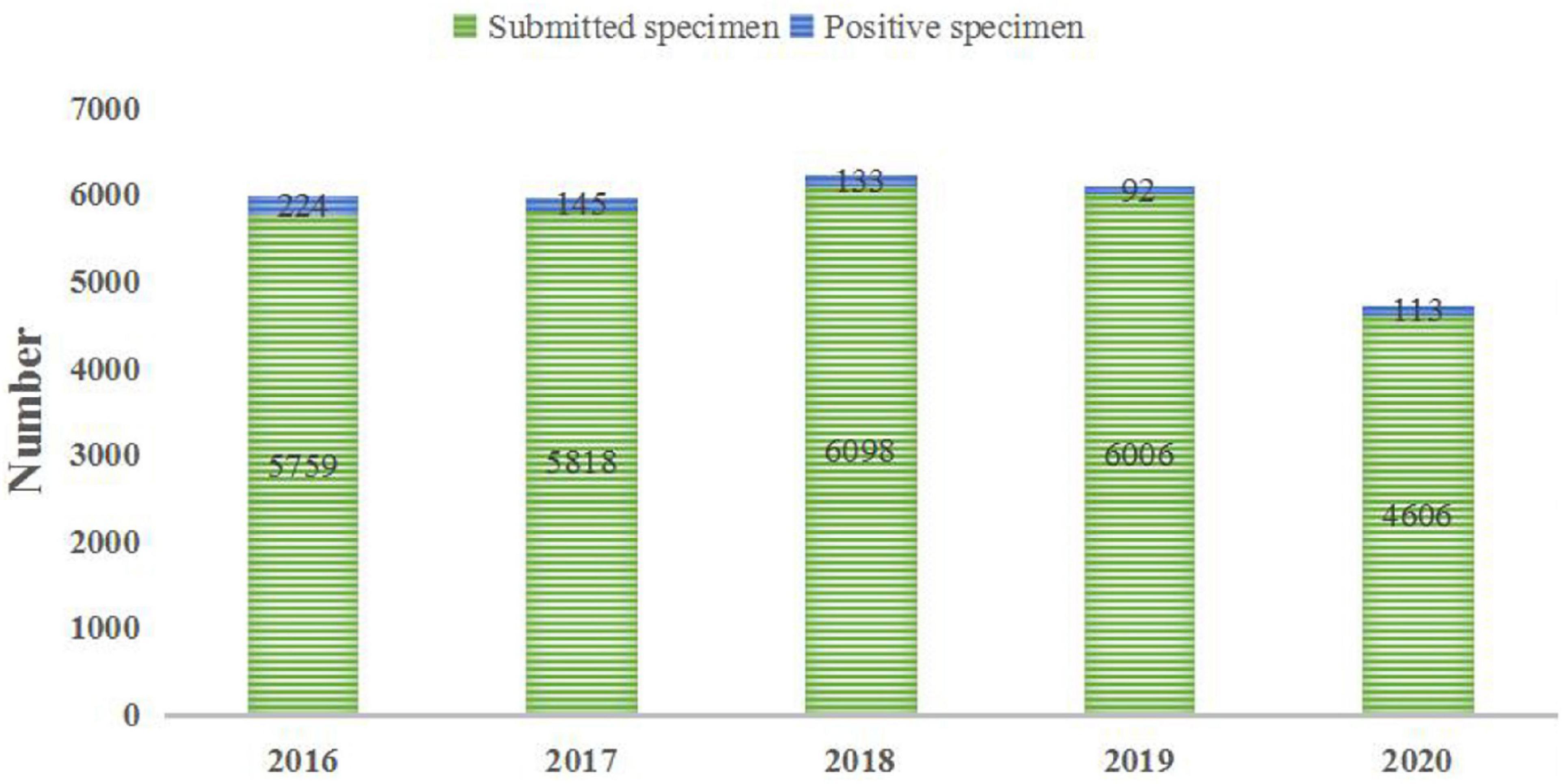
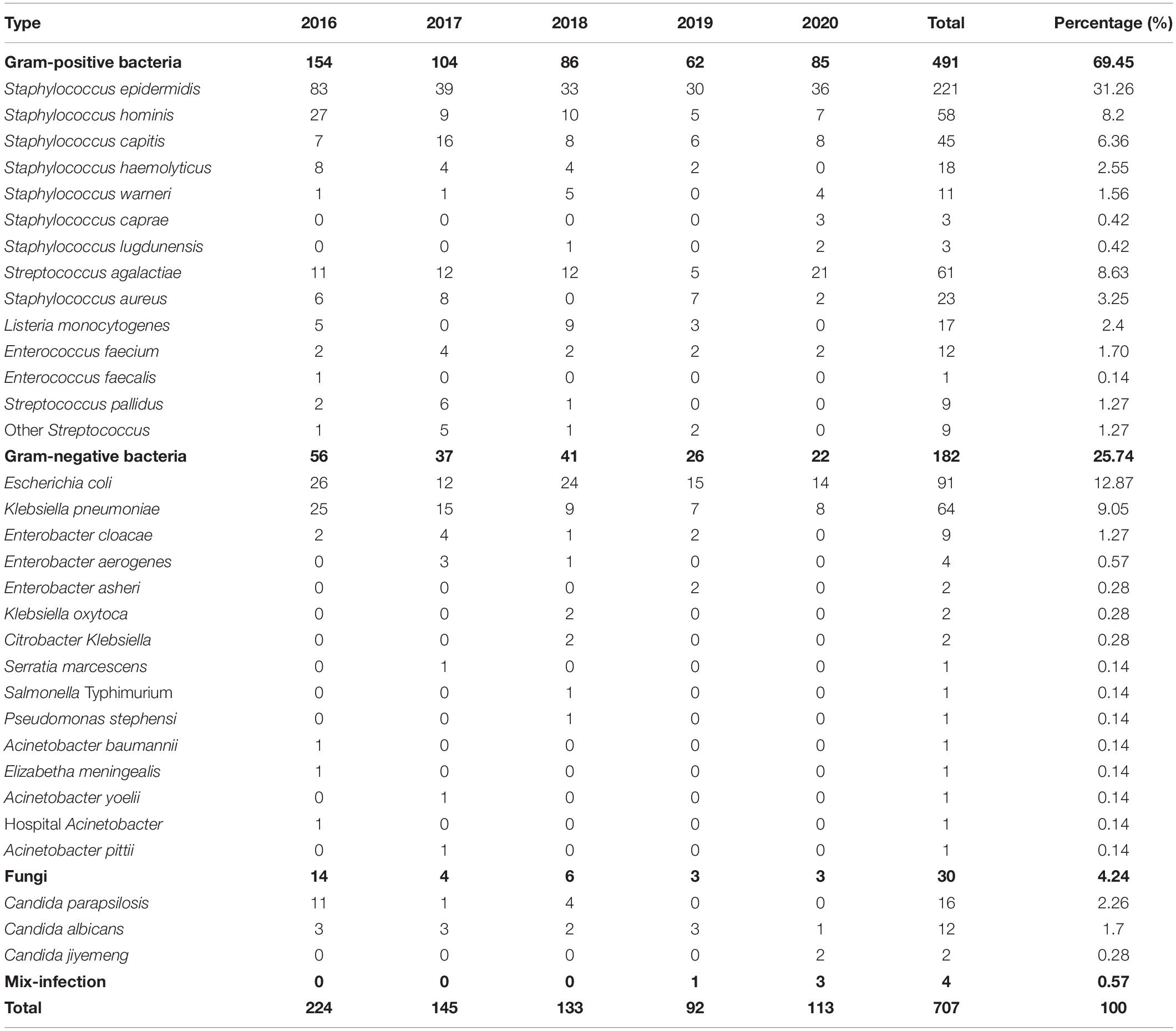
The positive rates in the 5 years from 2016 to 2020 were 3.89, 2.49, 2.18, 1.53, and 2.45%, respectively. In 707 cases of neonatal BSI, 491 strains of Gram-positive (G+) bacteria were isolated, accounting for 69.45%, and among them were Staphylococcus epidermidis (31.26%), Streptococcus agalactiae (8.63%), and Staphylococcus hominis (8.20%). Strains of Gram-negative (G–) bacteria (182) were isolated, accounting for 25.74%, and among them were E. coli (12.87%) and Klebsiella pneumoniae (9.05%). Strains of fungi (Akbarian-Rad et al., 2020) were isolated, accounting for 4.24% (see Figure 1 and Table 1 for details).
Resistance Rate of the Main G+ Bacteria to Common Antibiotics (%)
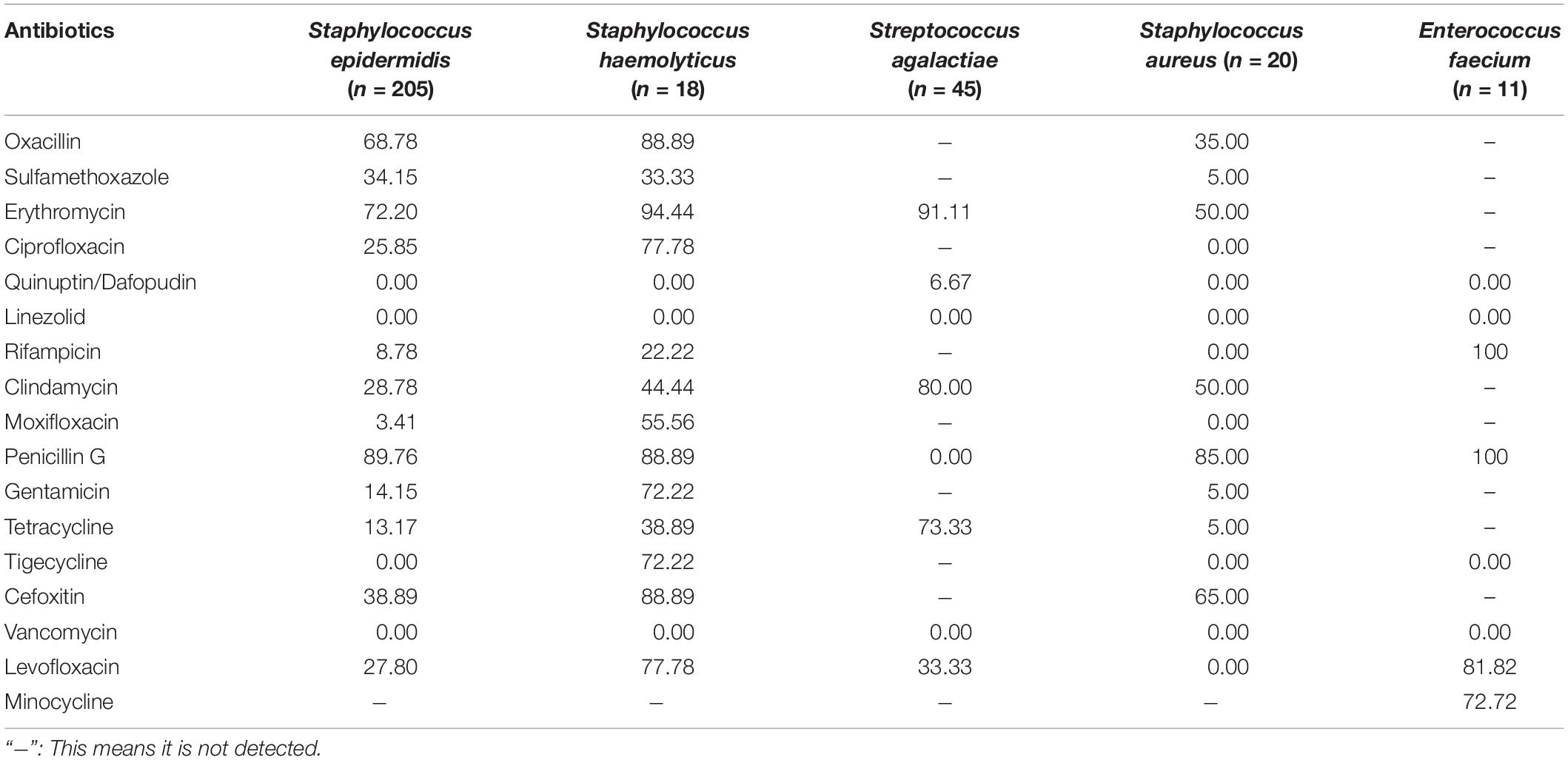
Coagulase-negative Staphylococcus (CNS), S. agalactiae, S. aureus, and Enterococcus are the main G+ bacteria. Table 2 shows that the above bacteria are resistant to various antibiotics to varying degrees. They have a higher rate of resistance to erythromycin. Staphylococcus has over 80% resistance to penicillin G, and S. agalactiae is 100% sensitive to penicillin G (see Table 2 for details).
Resistance Rate of the Main G– Bacteria to Common Antibiotics (%)
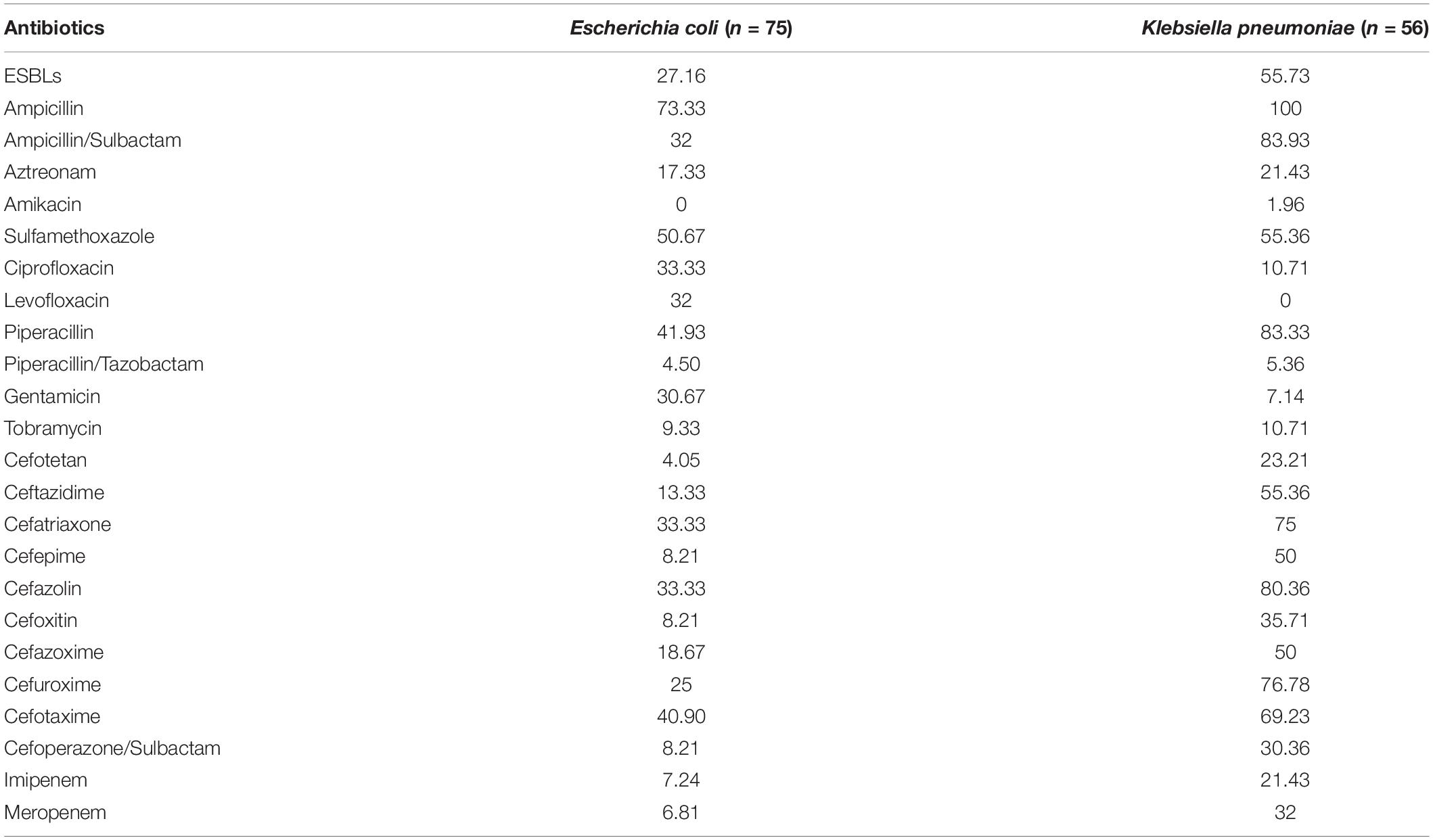
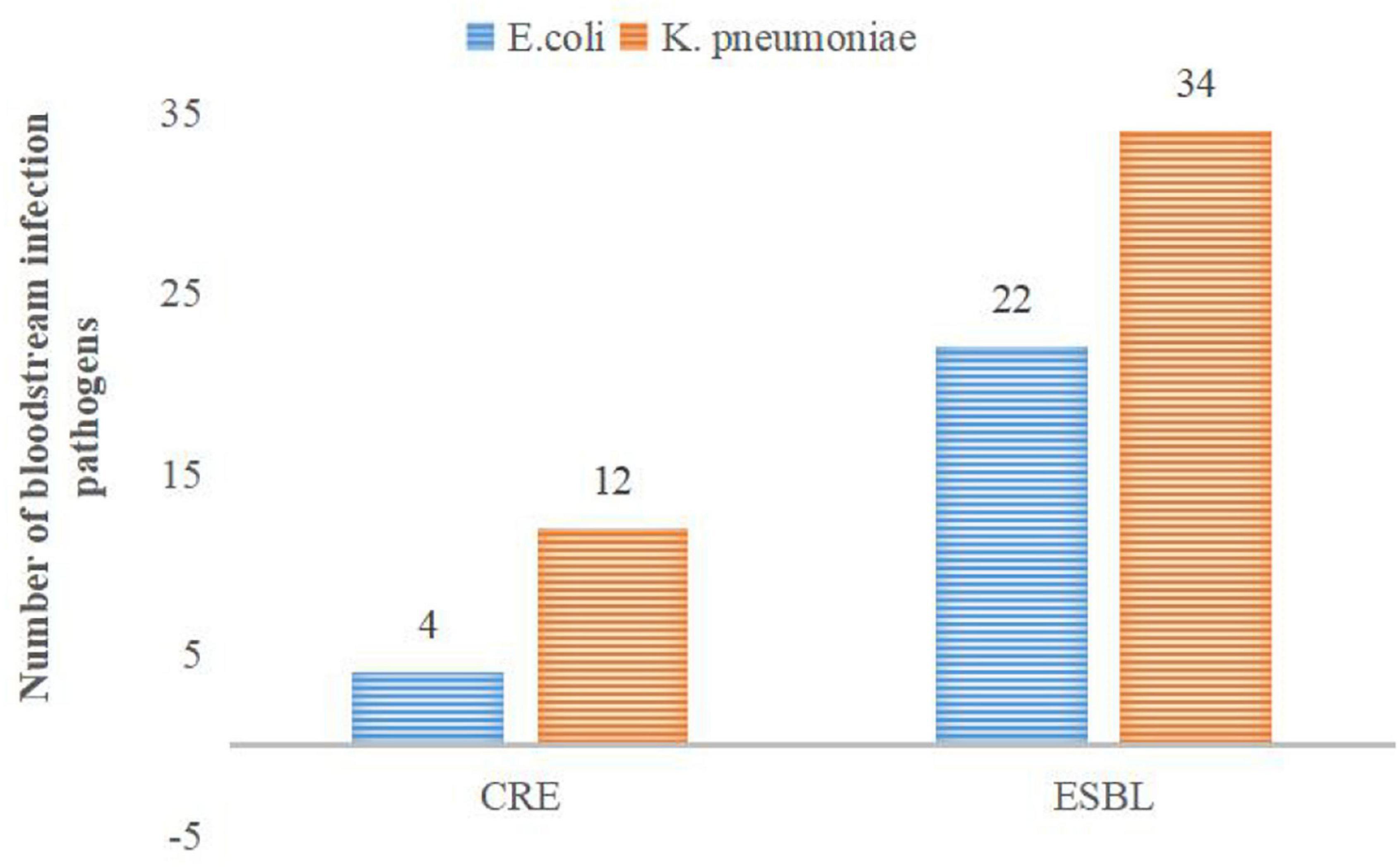
E. coli and K. pneumoniae are the main G– bacteria. K. pneumoniae produces ESBLs, accounting for up to 55.73%. The overall resistance of K. pneumoniae is higher than that of E. coli (see Table 3 for details). Subsequently, the multidrug resistances of K. pneumoniae and E. coli were analyzed. The results are shown in Figure 2.
Comparison of Positive Rates of Bloodstream Infection in Newborns of Different Groups [n (%)]
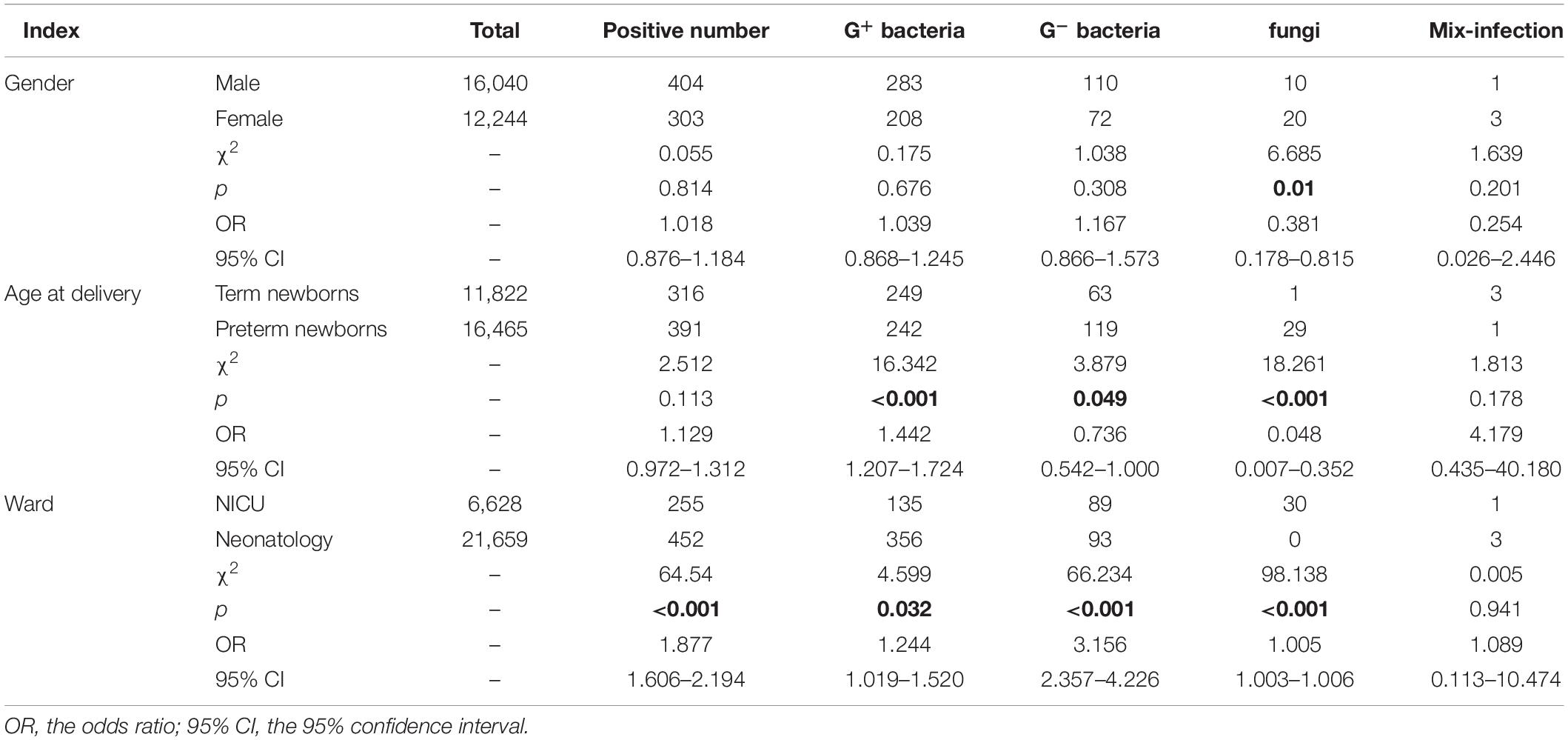
The type of microbial infection had a statistically significant difference in the positive rate among the age at delivery and the ward (p < 0.05). There were significant differences in the detection of fungi among these groups (p < 0.05). Details are shown in Table 4.
Clinical Diagnoses
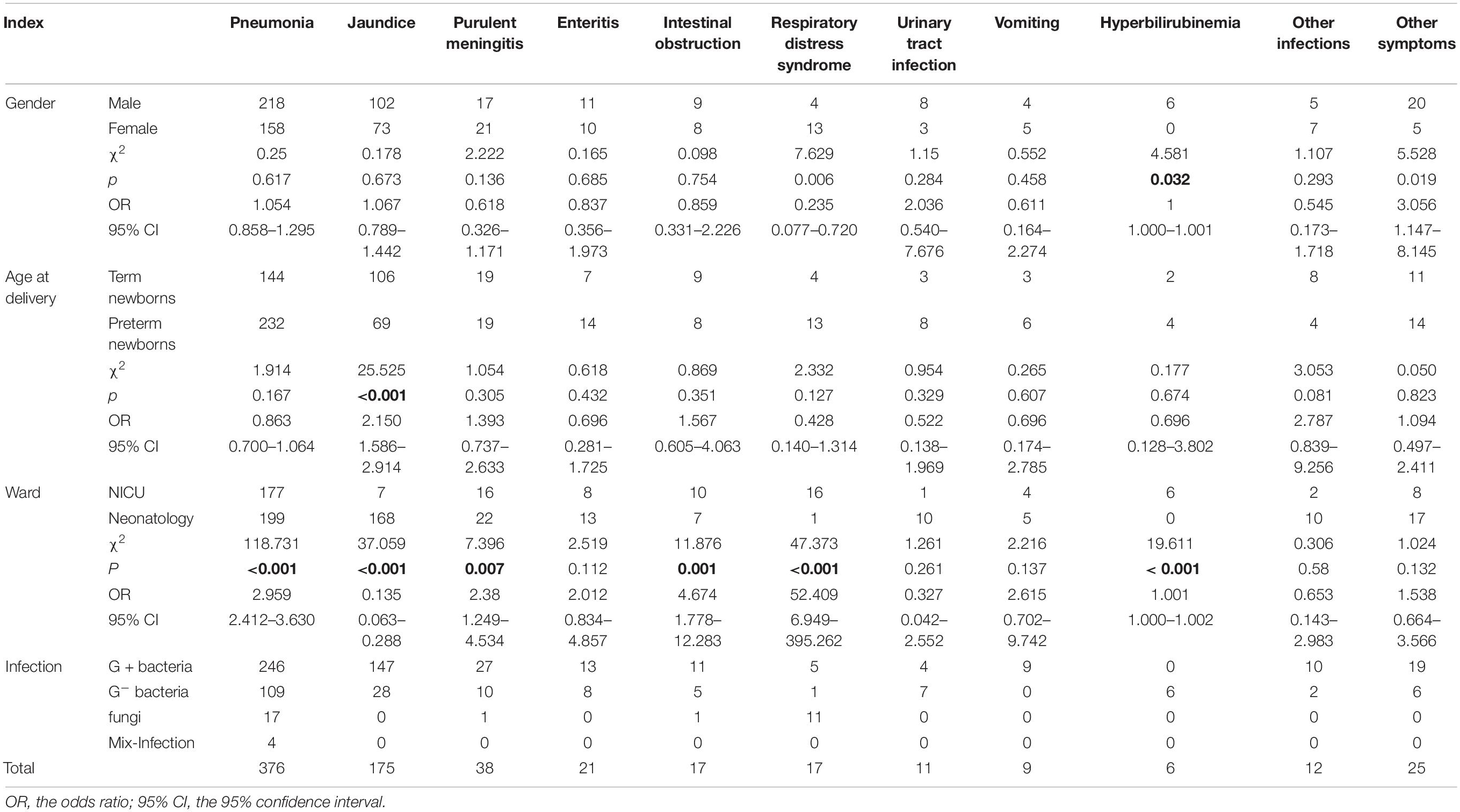
Among the clinical diagnoses, pneumonia, jaundice, purulent meningitis, newborn enteritis, and newborn intestinal obstruction accounted for the top five, of which pneumonia accounted for 53.18%, followed by jaundice, accounting for 24.75% (see more in Table 5). Pathogens detected in the different groups are further analyzed in Table 6.
Discussion
BSI has a high incidence and accounts for a high proportion of nosocomial infections, and its incidence has been increasing in recent years. It is complicated and poorly effective. According to reports, the in-hospital mortality rate of sepsis is as high as 30–60% (Bouza et al., 2015), exceeding the sum of mortalities due to acquired immune deficiency syndrome, breast cancer, and prostate cancer. For every hour of delay in treatment, the patient’s mortality rate will increase by 7.6% (Kumar et al., 2006). International guidelines recommend that effective antibiotics should be used intravenously within 1 h after sepsis is diagnosed (Dellinger et al., 2013).
In this study, the 5-year average positive rate of neonatal blood culture was 2.50%. As shown in Figure 1, the positive rates of blood culture from 2016 to 2020 were 3.89, 2.49, 2.18, 1.53, and 2.45%, respectively. Even during the COVID-19 pandemic, the total number of specimens submitted for clinical examination decreased, but it did not affect the detection of positive specimens. The 5-year average distribution of pathogens was mainly G+ bacteria (69.45%), which is consistent with previous similar research results (Jing and Big, 2019). However, a survey of pathogens of neonatal sepsis from Nigeria showed that the infection was mainly G– bacteria (Pius et al., 2016). The above differences suggest that the distribution of common pathogens of neonatal BSI may have differences in research time, research locations, or research objects, which only represent the situation of the research institution at a certain time. CNS is the main pathogen of neonatal blood flow infection in the hospital, accounting for 50.77%, but the CNS isolated from a single blood culture may also be contaminated (García-Gudiño et al., 2017). Therefore, blood collection personnel should pay special attention to aseptic operation and hand hygiene. Nevertheless, the positive rate of CNS is still very high in neonatal blood culture, as reported by Siti et al. (2020). The detection of Streptococcus is very important for perinatal pregnant women, especially those with premature rupture of membranes. Previous studies have found that the colonization rate of S. agalactiae in pregnant women is 19% (Lixiang et al., 2018). S. agalactiae was the second most susceptible G+ bacteria to neonatal BSI in this study, accounting for 8.63%, which is consistent with the results reported in other literature (Lili et al., 2017). It is worth noting that the ratio of S. agalactiae among the detected G+ bacteria was quite different in 2020 (∼25%) compared with other years (7–14%). Recent studies have shown that S. agalactiae is closely related to COVID-19 (Soto et al., 2021; Xiong et al., 2021). The high ratio of S. agalactiae among the detected G+ bacteria in 2020 may be related to the environment of COVID-19 infection, but further studies are needed. There are relatively few cases of neonatal BSI caused by S. aureus. Only 23 strains were found in this study, of which 8 strains were methicillin-resistant S. aureus (MRSA). The positive rate of MRSA is significantly lower in neonates than that in older children (Seas et al., 2018; Fang et al., 2020). The drug sensitivity results showed that the drug resistance rates of the main G+ bacteria to erythromycin and penicillin G were high, while they were 100% sensitive to vancomycin and linezolid. The resistance rates of CNS represented by S. epidermidis to penicillin G, erythromycin, and oxacillin were 89.76, 72.20, and 68.78%, respectively, and it is 100% sensitive to quinuptin/dafopudin, linezolid, and vancomycin. The resistance rate to moxifloxacin was 3.41% based on the overall antimicrobial susceptibility testing profile. It should be noted that the resistance rates of Staphylococcus epidermidis to moxifloxacin from 2016 to 2020 were 3.7, 7.69, 2.94%, 0, and 0, respectively. This suggests that in the case of poor efficacy of conventional drugs, moxifloxacin can be selected according to the situation of patients. In this study, Staphylococcus haemolyticus had high resistance to most antibiotics. There were 16 strains of methicillin-resistant S. haemolyticus. According to Takeuchi et al. (2005) S. haemolyticus has the maximum level of antimicrobial resistance among all CNS species. In recent years, linezolid-resistant S. haemolyticus has been reported (Rajan et al., 2017; Ahmed et al., 2019), and no quinuptin/dafopudin-, linezolid-, or vancomycin-resistant S. haemolyticus were reported in the present study. S. agalactiae has different degrees of resistance to common antibiotics, and the rate of resistance to erythromycin is the highest, up to 91.11%. However, it is 100% sensitive to penicillin G, so penicillin G is also the first choice for the treatment of neonatal S. agalactiae infection. The resistance rate of S. aureus to penicillin G was as high as 85%, but to macrolide antibiotics represented by erythromycin, it was 50%. Therefore, macrolides have been widely used in neonatal and perinatal diseases in recent years (Wang et al., 2015). Enterococcus faecium has a high resistance rate to a variety of antibiotics, such as 100% resistance to rifampicin and penicillin G, 81.82% resistance to levofloxacin, and 72.72% resistance to minocycline. However, it was 100% sensitive to tigecycline and linezolid. There were no vancomycin-resistant E. faecium strains. See Supplementary Material 1 for more antimicrobial susceptibility testing results of pathogen.
In the present research, 182 G– bacterial strains were detected, accounting for 25.74%. The proportion of the positive rate of G– bacteria was lower than that in previous similar literature reports (Pius et al., 2016; Akbarian-Rad et al., 2020). This may be related to the high positive rate of CNS (accounting for 50.77%) in the study. The main G– bacteria in neonatal BSI were E. coli and K. pneumoniae, which is consistent with the study by other developing countries (Dat et al., 2017). The detected proportions of E. coli and K. pneumoniae were 12.87 and 9.05%, respectively. Interestingly, there was a little difference in the rate of E. coli and K. pneumoniae among the detected G– bacteria from 2016 to 2017. However, after 2018, the proportion of E. coli significantly increased and was nearly twice that of K. pneumoniae. Nevertheless, due to the small number of positive specimens every year, it is necessary to continue to track the changes in detected bacteria in follow-up studies. Both K. pneumoniae and E. coli have high resistance to ampicillin, trimethoprim, and cefotaxime, which is similar to the results of Michael et al. (2017). However, the resistance rate to piperacillin/tazobactam, ciprofloxacin, aztreonam, and so on is low. As shown in Table 3, K. pneumoniae was 100% resistant to ampicillin, and the resistance rate to ampicillin/sulbactam reached 83.93%. The resistance rate to second- and third-generation cephalosporins is very high, and the resistance rate to some antibiotics is as high as 50%. The resistance rate of E. coli to cefuroxime was 25%, the resistance rate to ceftriaxone was 33.33%, and the resistance rate to fourth-generation cefotaxime was as high as 40.90%. The above results indicate that the resistance rate of K. pneumoniae to multiple antibacterial drugs is significantly higher than that of E. coli. The high resistance of bacteria to a variety of cephalosporin antibiotics indicates that the proportion of bacteria producing ESBLs is relatively high. In this study, the positive rates of ESBLs-producing K. pneumoniae and E. coli were 55.73 and 27.16%, respectively, which are lower than the results reported in previous studies (Wang et al., 2020). In recent years, the clinical isolation rate of carbapenem-resistant Enterobacter (CRE), especially carbapenem-resistant K. pneumoniae (CRKP), has increased (Stein et al., 2019). According to data from the China Antibiotic Resistance Surveillance Network, the isolation rate of CRKP among Chinese children rose from 3.0 to 20.9% from 2005 to 2017, which was significantly higher than that of adults (Wang et al., 2020). As shown in Figure 2, there were 12 strains of CRKP and 4 strains of carbapenem-resistant E. coli, which accounted for 18.75 and 4.40% of their respective strains. Carbapenem-resistant strains are resistant to most antibacterial drugs, and clinical treatment measures are limited. Therefore, it is difficult to control infection, and the mortality rate is high (Gu et al., 2018). Special attention should be given to the abovementioned carbapenem-resistant strains to actively prevent infection.
In recent years, BSI caused by fungi has increased significantly, and Candida is the most common fungi (Dilhari et al., 2016). Previous studies reported that the neonatal BSI caused by fungi was mainly by Candida albicans (Ting et al., 2018), which is somewhat different from the results of this study. In this study, a total of 30 strains of fungi were detected, including 16 strains of Candida parapsilosis and 12 strains of C. albicans. This may be related to the low number of fungi detected in this study. C. parapsilosis is 100% sensitive to 5-fluorocytosine, voriconazole, fluconazol, itraconazole, and amphotericin B, which is also consistent with the conclusion reported by Van Asbeck et al. (2009). Drug resistance was concentrated on C. albicans, and two strains of resistant C. albicans were obtained. One strain was only resistant to 5-fluorocytosine, and the other was only sensitive to amphotericin B. The low resistance rate to amphotericin B may be related to the greater side effects of the drug.
The differences in pathogen detection between genders, age at delivery, and ward were further analyzed. In Table 4, fungi were a risk factor for BSI in female newborns. The positive rate of G+ bacteria in term newborns was 2.11% (249/11,822), which was higher than the 1.47% (242/11,465) of preterm newborns, while the positive rate of G– bacteria was the opposite. The above differences were statistically significant (p < 0.05). These results are consistent with the finding that preterm newborns are more susceptible to G– bacteria (Zhang, 2020). The study also found that 30 patients with fungal infections were all preterm newborns, and the difference in fungal detection between preterm newborns and term newborns was statistically significant (χ2 = 18.261, p < 0.001). Studies have shown that the immune function of preterm newborns is relatively weak, and they are more prone to fungal infections (Manzoni et al., 2015). Most of the newborns admitted to the NICU have more serious underlying diseases, may undergo more invasive procedures, and have a significantly increased risk of infection. Statistics found that the positive rates of G± bacteria and fungi in newborns in the NICU were significantly higher than those in neonatology, and the difference was statistically significant (p < 0.05). Therefore, the ICU should pay special attention to aseptic operation and hand hygiene and reduce cross-infection among newborns. In the main clinical diagnosis, the top five diseases were pneumonia (53.18%), jaundice (24.75%), purulent meningitis (5.37%), and neonatal enteritis (2.97%), the intestinal obstruction and respiratory distress syndrome ranked fifth (2.40%). Statistical analysis of the prevalence of pneumonia and jaundice found that compared with term newborns, preterm newborns are more susceptible to pneumonia and have a lower risk of jaundice, which is consistent with the results reported by Chen et al. (2017). The above results may be related to premature newborns due to immature lung development and more mechanical ventilation, so they are prone to respiratory tract infections which induce severe pneumonia. The study also found that the positive rate of pneumonia among newborns in the NICU was 2.67%, which was significantly higher than that of newborns in neonatology (0.92%), and the difference in detection was statistically significant (χ2 = 118.731, p < 0.001). Among the 255 patients with BSI in the NICU, there were 181 preterm newborns, of which 141 were clinically diagnosed with pneumonia, accounting for 77.91%. Therefore, it is necessary to pay special attention to the correlation between bloodstream infection and pneumonia in premature infants to be vigilant and take preventive measures as early as possible.
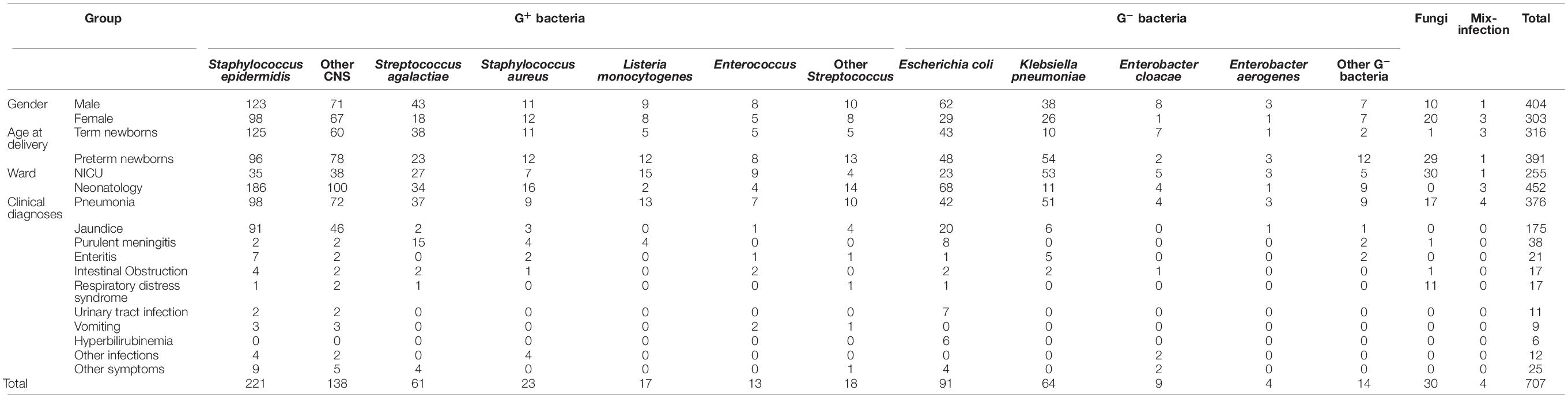
Subsequently, the types and quantities of bacteria among different genders, age at delivery, wards, and clinical diagnoses were determined. As shown in Table 6, many clinical diagnoses were closely related to the detection of G+ bacteria, except urinary tract infection and hyperbilirubinemia. It is worth noting that 17 strains of Listeria monocytogenes were detected in the present study. Among them, 6 strains of L. monocytogenes entered the cerebrospinal fluid and caused meningitis. L. monocytogenes is a pathogen of sepsis and meningitis in children. A study on L. monocytogenes infection shows that infection in children is common in newborns, especially in preterm newborns, which easily causes suppurative meningitis (Cai et al., 2020). The detection of L. monocytogenes is closely related to preterm newborns admitted to the NICU with purulent meningitis and pneumonia in this study. Therefore, the neonatal ward should attach great importance to the spread of bacterial infection and do a good job in the prevention and control measures of cross infection.
In summary, regular monitoring of bacterial resistance and understanding of changes in pathogen spectrum and antimicrobial resistance patterns will help clinicians use drugs rationally and better prevent and control the occurrence of infectious diseases. Corresponding wards should pay attention to the inspection rate of blood cultures, consider the trend of drug resistance in the hospital, adjust medications, and reduce infection mortality. However, there are some limitations in this study. The overall positive rate and drug resistance of pathogens in the last 5 years were analyzed. There is a lack of further exploration of the resistance changes of various antimicrobials. It is also significant to analyze the change in the positive rate and drug resistance rate of each pathogen during the epidemic period of COVID-19. Therefore, we will pay more attention to the above limitations in a follow-up study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Children’s Hospital of Soochow University (ethics batch number: 2021CS158). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XZ and WL conceived the study and designed the experiments. YL and XS provided financial support. XS, YD, and YT collected, analyzed the data, and interpreted the results. XZ drafted the manuscript. All authors critically revised the manuscript for intellectual content and read and approved the final manuscript.
Funding
This study was supported by grants from the Special Foundation for National Science and Technology Basic Research Program of China (2019FY101200), the High-level Innovative and Entrepreneurial Talents Introduction Program of Jiangsu Province (2020-30191), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (20KJB310012), the Medical Research Project of Jiangsu Commission of Health (M2020027), the Science and Technology Program of Suzhou (SYS2020163 and SYSD2019120), and the Science and Technology Program of Suzhou (SLC201904).
Author Disclaimer
The views, opinions, assumptions, or any other information set out in this article are solely those of the authors and should not be attributed to the funders or any other person connected with the funders.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the staff from the Department of Clinical Laboratory, Children’s Hospital of Soochow University, who took part in the study. We thank YT for the great help offered in manuscript revision.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.820577/full#supplementary-material
References
Ahmed, A., Satti, L., Zaman, G., Gardezi, A., Sabir, N., and Khadim, M. T. (2019). Catheter related recurrent blood stream infection caused by linezolid-resistant, methicillin resistant Staphylococcus haemolyticus; an emerging super bug. J. Pak. Med. Assoc. 69, 261–263.
Akbarian-Rad, Z., Riahi, S. M., Abdollahi, A., Sabbagh, P., Ebrahimpour, S., Javanian, M., et al. (2020). Neonatal sepsis in iran: a systematic review and meta-analysis on national prevalence and causative pathogens. PLoS One 15:e0227570. doi: 10.1371/journal.pone.0227570
Bouza, C., Cuadrado, T. L., Parkinson, Z. S., and Amate-Blanco, J. M. (2015). Epidemiology and recent trends of severe sepsis in Spain: a nationwide population-based analysis 2006∼2011. BMC Infect. Dis. 14:717. doi: 10.1186/s12879-014-0717-7
Cai, Z. Q., Yang, J. Y., Jiang, X. Y., Huang, Y. C., Lin, T. Y., and Chiu, C. H. (2020). Clinical characteristics of Listeria monocytogenes infection. Chin. J. Infect. Control 19, 900–903.
Chen, D. X., Wang, X. J., Lu, H. R., Zhang, Q., and Chen, F. N. (2017). Comparative study on the clinical characteristics of sepsis in preterm infants and term infants. J. Shanxi Med. Univ. 48, 1291–1294.
Clinical and Laboratory Standards Institute (2020). Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Clinical And Laboratory Standards Institute.
Dat, V. Q., Vu, H. N., Nguyen, T. H., Nguyen, H. T., Hoang, L. B., Vu Tien Viet, D., et al. (2017). Bacterial bloodstream infections in a tertiary infectious diseases hospital in Northern Vietnam: aetiology, drug resistance, and treatment outcome. BMC Infect. Dis. 17:493. doi: 10.1186/s12879-017-2582-7
Dellinger, R. P., Levy, M. M., Rhodes, A., Annane, D., Gerlach, H., Opal, S. M., et al. (2013). Surviving sepsis campaign: international guidelines for the treatment of severe sepsis and septic shock 2012. Crit. Care Med. 41:580.
Dilhari, A., Weerasekera, M. M., Siriwardhana, A., Maheshika, O., Gunasekara, C., Karunathilaka, S., et al. (2016). Candida infection in oral leukoplakia:an unprceived public health problem. Acta. Odontol. Scand. 74, 565–569.
Esaiassen, E., Fjalstad, J. W., Juvet, L. K., van den Anker, J. N., and Klingenberg, C. (2017). Antibiotic exposure in neonates and early adverse outcomes:a systematic review and meta-analysis. J Antimicrob. Chemother. 72, 1858–1870.
Fang, P. P., Yang, J. W., Gao, K. J., Yang, J. M., Sun, H. Q., and Wang, Y. Y. (2020). Distribution and drug resistance analysis of pathogenic bacteria from bloodstream infection in a children’s hospital in Zhengzhou from 2014 to 2019. China Pharm. 31, 98–103.
FLeischmann-Struzek, C., GoLdfarb, D. M., SchLattmann, P., Schlapbach, L. J., Reinhart, K., and Kissoon, N. (2018). The global burden of paediatric and neonataL sepsis: a systematic review. Lancet Respir. Med. 6, 223–230.
García-Gudiño, I., Yllescas-Medrano, E., Maida-Claros, R., Soriano-Becerril, D., Díaz, N. F., García-López, G., et al. (2017). Microbiological comparison of blood culture and amplification of 16S rDNA methods in combination with DGGE for detection of neonatal sepsis in blood samples. Eur. J. Pediatr. 177, 85–93. doi: 10.1007/s00431-017-3036-3
Gu, D., Dong, N., Zheng, Z., Lin, D., Huang, M., Wang, L., et al. (2018). A fatal outbreak of ST11 carbapenem-resistant hyper virulent Klebsiella pneumoniaeina Chinese hospital: a molecular epidemiological study. Lancet Infect. Dis. 18, 37–46. doi: 10.1016/s1473-3099(17)30489-9
Hu, F. P., Guo, Y., Zhu, D. M., Wang, F., Jiang, X. F., Xu, Y. C., et al. (2018). 2017 China bacterial drug resistance monitoring. Chin. J. Infect. Chemother. 18, 241–251.
Jing, Z., and Big, L. (2019). Analysis on the applicability of neonatal blood flow infection and antimicrobial regimen in a third class hospital. Chin. Med. Rec. 20, 96–101.
Johnson, A., Watson, D., Dreyfus, J., Heaton, P., Lampland, A., and Spaulding, A. B. (2020). Epidemiology of serratia bloodstream infections among hospitalized children in the united states, 2009-2016. Pediatr. Infect. Dis. J. 39, e71–e73. doi: 10.1097/INF.0000000000002618
Klingenberg, C., Kornelisse, R. F., Buonocore, G., Maier, R. F., and Stocker, M. (2018). Culture-negative early onset neonatal sepsis:at the crossroad between efficient sepsis care and antimicrobial stewardship. Front. Pediatr. 6:285. doi: 10.3389/fped.2018.00285
Kumar, A., Roberts, D., Wood, K. E., Light, B., Parrillo, J. E., Sharma, S., et al. (2006). Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34, 1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9
Lili, L., Da, W., and Lifan, D. (2017). Correlation analysis between colonization of Streptococcus agalactiae in the birth canal of pregnant women with premature rupture of membranes and neonatal infection in the third trimester. Chin. J. Microecol. 29, 1330–1332.
Liu, M. X., Huang, L. Y., Liang, J. H., Wang, S. J., Cen, Z. J., Zeng, S. J., et al. (2020). Analysis of pathogen distribution and drug resistance of blood flow infection in children in Nanning from 2017 to 2018. J. Pract. Med. 36, 527–531.
Lixiang, Y., Huimin, L., and Xiaoqiong, Z. (2018). Clinical characteristics and drug sensitivity analysis of Streptococcus agalactiae infection in pregnant women and neonatal patients in Heyuan area. Mod. Med. Health 34, 710–712.
Manzoni, P., Mostert, M., and Castagnola, E. (2015). Update on the management of Candida infections in preterm neonates. Arch. Dis. Child Fetal Neonatal Ed. 100, 454–459.
Michael, G. B., Kaspar, H., Siqueira, A. K., de Freitas Costa, E., Corbellini, L. G., Kadlec, K., et al. (2017). Extendedspectrum β-lactamase (ESBL)-producing Escherichia coli isolates collected from diseased food-producing animals in the GERM-Vet monitoring program 2008-2014. Vet. Microbiol. 200, 142–150. doi: 10.1016/j.vetmic.2016.08.023
Pius, S., Bello, M., Galadima, G. B., Ibrahim, H. A., Yerima, S. T., and Ambe, J. P. (2016). Neonatal septicaemia, bacterial isolates and antibiogram sensitivity in Maiduguri North-Eastern Nigeria. Niger. Postgrad. Med. J. 23, 146–151. doi: 10.4103/1117-1936.190340
Puopolo, K. M., Benitz, W. E., Zaoutis, T. E., and Committee On Fetus And Newborn; Committee On Infectious Diseases. (2018). Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven early-on set bacterial sepsis. Pediatrics 142:e20182896.
Rajan, V., Haleebedo, P., and Gopal, S. (2017). Occurrence of linezolid-resistant Staphylococcus haemolyticus in two tertiary care hospitals in Mysuru, South India. J. Glob. Antimicrob. Resist. 8, 140–141. doi: 10.1016/j.jgar.2016.12.005
Seas, C., Garcia, C., Salles, M. J., Labarca, J., Luna, C., Alvarez-Moreno, C., et al. (2018). Staphylococcus aureus bloodstream infections in Latin America:results of a multinational prospective cohort study. J. Antimicrob. Chemother. 73, 212–222. doi: 10.1093/jac/dkx350
Siti, N. M., Wan Nazirah, W. A. B., Rosni, I., Mohamed, A. N., and Salbiah, N. (2020). Species distribution and clinical profiles of coagulase-negative staphylococci (CoNS) isolated from blood cultures among paediatric patients in Hospital Kuala Lumpur. Med. J. Malaysia 75, 266–273.
Soto, A., Quiñones-Laveriano, D. M., Valdivia, F., Juscamayta-López, E., Azañero-Haro, J., Chambi, L., et al. (2021). Detection of viral and bacterial respiratory pathogens identified by molecular methods in COVID-19 hospitalized patients and its impact on mortality and unfavorable outcomes. Infect. Drug Resist. 21, 2795–2807. doi: 10.2147/IDR.S306439
Spaulding, A. B., Watson, D., Dreyfus, J., Heaton, P., Grapentine, S., Bendel-Stenzel, E., et al. (2019). Epidemiology of bloodstream infections in hospitalized children in the United States, 2009-2016. Clin. Infect. Dis. 69, 995–1002.
Stein, C., Vincze, S., Kipp, F., Makarewicz, O., Al Dahouk, S., and Pletz, M. W. (2019). Carbapenem-resistant Klebsiella pneumoniae with low chlorhexidine susceptibility. Lancet Infect. Dis. 19, 932–933. doi: 10.1016/S1473-3099(19)30427-X
Takeuchi, F., Watanabe, S., Baba, T., Yuzawa, H., Ito, T., Morimoto, Y., et al. (2005). Whole-genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of humancolonizing staphylococcal species. J. Bacteriol. 187, 7292–7308. doi: 10.1128/JB.187.21.7292-7308.2005
Ting, J. Y., Roberts, A., Synnes, A., Canning, R., Bodani, J., Monterossa, L., et al. (2018). Canadian neonatal network investigators. invasive fungal infections in neonates in Canada: epidemiology and outcomes. Pediatr. Infect. Dis. J. 37, 1154–1159. doi: 10.1097/INF.0000000000001968
Ting, J. Y., Synnes, A., Roberts, A., Deshpandey, A., Dow, K., Yoon, E. W., et al. (2016). Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr. 70, 1181–1187. doi: 10.1001/jamapediatrics.2016.2132
Van Asbeck, E. C., Clemons, K. V., and Stevens, D. A. (2009). Candida parapsilosis: a review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit. Rev. Microbiol. 35, 283–309. doi: 10.3109/10408410903213393
Wang, B., Pan, F., Wang, C., Zhao, W., Sun, Y., Zhang, T., et al. (2020). Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in a paediatric hospital in China. Int. J. Infect. Dis. 93, 311–319. doi: 10.1016/j.ijid.2020.02.009
Wang, H. M., Jiang, Y. Q., Huang, B. X., Zhao, R. Z., Cheng, H. Y., and Ma, D. L. (2015). Analysis of pathogen distribution and drug resistance of neonatal infectious pneumonia from 2010 to 2013. Chin. J. Infect. Control 13, 411–412.
Xiong, D., Muema, C., Zhang, X., Pan, X., Xiong, J., Yang, H., et al. (2021). Enriched opportunistic pathogens revealed by metagenomic sequencing hint potential linkages between pharyngeal microbiota and COVID-19. Virol. Sin. 36, 924–933. doi: 10.1007/s12250-021-00391-x
Yuan, Y., Zhou, W., Rong, X., Lu, W. N., and Zhang, Z. (2015). Incidence and factors associated with nosocomial infections in a neonatal intensive care unit (NICU) of an urban children’ s hospital in China. Clin. Exp. Obstet. Gynecol. 42, 619–628.
Keywords: newborns, bloodstream infection, resistance, antibacterial drugs, epidemiology
Citation: Zhang X, Li Y, Tao Y, Ding Y, Shao X and Li W (2022) Epidemiology and Drug Resistance of Neonatal Bloodstream Infection Pathogens in East China Children’s Medical Center From 2016 to 2020. Front. Microbiol. 13:820577. doi: 10.3389/fmicb.2022.820577
Received: 23 November 2021; Accepted: 24 January 2022;
Published: 10 March 2022.
Edited by:
Fang He, Zhejiang Provincial People’s Hospital, ChinaReviewed by:
Soojin Jang, Korea Pasteur Institute, South KoreaAnusak Kerdsin, Kasetsart University, Thailand
Copyright © 2022 Zhang, Li, Tao, Ding, Shao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuejun Shao, eGpzaGFvQHN1ZGEuZWR1LmNu; Wei Li, MjI0ODc5NzkwN0BxcS5jb20=
†These authors have contributed equally to this work
 Xin Zhang
Xin Zhang Yang Li
Yang Li Yunzhen Tao
Yunzhen Tao Yu Ding
Yu Ding Xuejun Shao
Xuejun Shao Wei Li
Wei Li