- 1Department of Botany, Government College Women University, Faisalabad, Pakistan
- 2Department of Biology, Al Khumra University College, Taif University, Taif, Saudi Arabia
- 3Department of Plant Protection, College of Agriculture Engineering Science, University of Baghdad, Baghdad, Iraq
- 4School of Biology Science, Universiti Sains Malaysia, George Town, Malaysia
- 5Department of Horticultural Sciences, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
- 6Department of Biology, Faculty of Science, University of Tabuk, Tabuk, Saudi Arabia
- 7Department of Botany, University of Agriculture Faisalabad, Faisalabad, Pakistan
- 8Department of Soils and Water, Faculty of Agriculture, Benha University, Benha, Egypt
- 9Department of Soils and Water, Faculty of Agriculture, New Valley University, Kharga, Egypt
- 10National Committee of Soils Science, Academy of Scientific Research and Technology, Cairo, Egypt
- 11Botany Unit, Finnish Museum of Natural History, University of Helsinki, Helsinki, Finland
- 12Department of Microbiology, College of Basic Sciences and Humanities, Dr. Rajendra Prasad Central Agricultural University, Pusa, India
- 13Department of Botany and Microbiology, Faculty of Science, Helwan University, Cairo, Egypt
- 14Department of Biology, College of Sciences, Taif University, Taif, Saudi Arabia
Less nutrient availability and drought stress are some serious concerns of agriculture. Both biotic and abiotic stress factors have the potential to limit crop productivity. However, several organic extracts obtained from moringa leaves may induce immunity in plants under nutritional and drought stress for increasing their survival. Additionally, some rhizobacterial strains have the ability to enhance root growth for better nutrient and water uptake in stress conditions. To cover the knowledge gap on the interactive effects of beneficial rhizobacteria and moringa leaf extracts (MLEs), this study was conducted. The aim of this experimental study was to investigate the effectiveness of sole and combined use of rhizobacteria and MLEs against nutritional and drought stress in wheat. Nitrogen-fixing bacteria Pseudomonas aeruginosa (Pa) (108 CFU ml–1) was inoculated to wheat plants with and without foliar-applied MLEs at two different concentrations (MLE 1 = 1:15 v/v and MLE 2 = 1:30 v/v) twice at 25 and 35 days after seed sowing (50 ml per plant) after the establishment of drought stress. Results revealed that Pa + MLE 2 significantly increased fresh weight (FW), dry weight (DW), lengths of roots and shoot and photosynthetic contents of wheat. A significant enhancement in total soluble sugars, total soluble proteins, calcium, potassium, phosphate, and nitrate contents validated the efficacious effect of Pa + MLE 2 over control-treated plants. Significant decrease in sodium, proline, glycine betaine, electrolyte leakage, malondialdehyde, hydrogen peroxide, superoxide dismutase (SOD), and peroxide (POD) concentrations in wheat cultivated under drought stress conditions also represents the imperative role of Pa + MLE 2 over control. In conclusion, Pa + MLE 2 can alleviate nutritional stress and drought effects in wheat. More research in this field is required to proclaim Pa + MLE 2 as the most effective amendment against drought stress in distinct agroecological zones, different soil types, and contrasting wheat cultivars worldwide.
Introduction
Crop production is always under pressure to increase and sustain the food demands, considering the estimated increase in global population that might increase from the current 7.7 billion to approximately 9.6 billion in the year 2050 (Zulfiqar et al., 2020). Moreover, constantly changing climatic conditions are the other major challenge for sustainable crop production (Wheeler and Von Braun, 2013). Several abiotic and biotic stress factors such as temperature, water-logging, salinity, drought, weed, and pest infestations critically limit crop production (Parajuli et al., 2019). Climate change leads to widely drought-affected areas, such as tropics (Grover et al., 2011).
Water constitutes approximately 80–90% of the total biomass of herbaceous plants and is crucial in almost all plant physiological processes, a principal means of nutrients and metabolite transport (Lisar et al., 2012). Water scarcity is one of the leading plant stress factors. A drought effect reduces cell turgor and water potential causing adversities in carrying out plant’s normal physiological functions (Lisar et al., 2012). In fact, water is the key determining factor for crop growth and productivity, thus playing an essential role in species distribution and evolution (Ngumbi and Kloepper, 2016).
The induction of drought stress tolerance has been described by several physiological and biochemical changes (Shukla et al., 2012). In spite of these tolerance strategies including elaborated antioxidative activities, losses to crop production are increasing rapidly. Chemical fertilizers are largely being used to increase agricultural production, which is a matter of concern due to their improper use and potential adverse effects on human health and environment (Ruzzi and Aroca, 2015; Jiménez-Gómez et al., 2018). Expensive agrochemicals and synthetic fertilizers are commercially undesirable (Colla et al., 2015). Hence, to maintain and increase the yield, it is crucial to devise some efficient, low-cost, less time taking, and environment-friendly techniques to cope with drought conditions and achieve a sustainable agricultural system (Venkateswarlu and Shanker, 2009; Zulfiqar et al., 2019).
Several microorganisms, mainly bacteria, colonize the plant root zone (Kaushal and Wani, 2016; Barnawal et al., 2019). The beneficial associations between microbes and roots are an important determinant of soil texture, water conservation, and plant health. Plant growth-promoting rhizobacteria (PGPR) improve nutrient uptake by roots, growth, and consequently crop yield by causing biochemical changes in the plant body, meanwhile protecting from a variety of other plant diseases (Khan et al., 2020; Singh et al., 2021; Soumare et al., 2021). Furthermore, improvement in soil texture and fertility enhances crop production (Nardi et al., 2009).
Moringa leaf extract (MLE), as a plant biostimulant in the foliar application, enhances the growth of plants that are grown even under abiotic stress conditions (Semida and Rady, 2014). Moringa oleifera Lam belongs to the Moringaceae family and native to the subcontinent (Shahzad et al., 2013). Moringa leaves are rich in both micronutrients and macronutrients, i.e., N, P, Ca, K, Na, Zn, Mg, B, Cu, Mn, and Fe (Yasmeen et al., 2014). These mineral nutrients can supplement the nutritional demands of stressed grown crops and help in decreasing the use of agrochemicals and synthetic fertilizers (Zulfiqar et al., 2020). Spraying MLE diluted solution on plant leaves seems to have a considerable positive impact, i.e., delay in senescence, higher sugar contents, increased plant height, and bigger fruits and seeds (Yasmeen et al., 2013a; Nasir et al., 2014). This validates the potential effects of MLE to be used to improve plant vigor specifically in suboptimal environmental situations like drought stress as a foliar application. MLE foliar spray increased approximately 20–35% yield of several plants (Yasmeen et al., 2012).
Wheat (Triticum aestivum L.) is one of the most important cereal crops worldwide, considering its production and human consumption. Wheat supplements almost one-third of the total global population. Harvesting more yields to meet the future food demands of the increasing population is a major agricultural concern at all times. Different environmental factors contribute to crop yields (Yasmeen et al., 2012; Fasiha Amjad et al., 2021). Wheat in Pakistan experiences severe abiotic constraints during all developmental stages (Rashid et al., 2018), and drought stress is the main limiting factor among abiotic stresses for wheat yield (Budak et al., 2013; Mutumba et al., 2018).
Previously, the effects of exogenous applied MLE and treating plants with nitrogen-fixing bacteria (NFB) were studied solely on wheat grown at high temperatures. This study covers the knowledge gap in the combined use of NFB and MLE under normal and heat stress situations. In earlier studies, treating plants with rhizobacteria and the effects of foliar sprayed MLE were assessed solely on wheat grown under drought stress conditions by considering their ameliorative implications on various physiological and biochemical attributes but their combined implications are still not confirmed.
Therefore, this study aimed to investigate the best treatment combination for alleviation of heat stress in wheat. Anaj 17 genotype was selected because it is one of the latest genotypes developed in the past 5 years in Pakistan and it performed well under abiotic stress conditions so we wanted to check its response to the application of MLE and rhizobacteria. It is hypothesized that the combined use of MLE and NFB might be a better approach than the sole application to improve wheat growth attributes under heat stress. This study was conducted to analyze the ameliorative and best co-application of applied amendments in alleviating the drought stress effects on wheat plants. It is hypothesized that MLE and rhizobacterial combined application might be an effective approach to improve wheat attributes than their sole applications in drought effects. In earlier studies, treating plants with rhizobacteria and the effects of foliar sprayed MLE were assessed solely on wheat grown under drought stress conditions by considering their ameliorative implications on various physiological and biochemical attributes but their combined implications are still not confirmed. Therefore, this study is conducted to analyze the ameliorative and best co-application of applied amendments in alleviating the drought stress effects on wheat plants. It is hypothesized that MLE and rhizobacterial combined application might be an effective approach to improve wheat attributes than their sole applications in drought effects.
Materials and Methods
Experimental Site and Design
The wheat crop was sown at the experimental site of the Government College University Faisalabad (30°–31.5° N and 73°–74° E, 184.4 m above sea level). A greenhouse pot experiment was carried out from November to January. The experimental layout was a randomized complete block design (RCBD) that was replicated three times.
Soil Characteristics
The clay loam soil (8–12 inches in depth) collected from the experimental site of the Government College University Faisalabad was air-dried and sieved through a 2-mm sieve. The collected soils were sterilized through solarization, by covering the soil with a thin layer of plastic sheet. The heat from the sun builds up the temperature of the soil to kill most of the bacteria, weeds, and pests (Stapleton et al., 2008). Some soil chemical characteristics that were analyzed before the experiment include EC 7.78 (d Sm–1), pH (water) 7.3; organic matter contents 1.38%; available N 0.032 ppm, available P 5.93 ppm, and available K 32.3 ppm.
Seed Collection and Sterilization
A well-adapted wheat genotype Anaj 17, which performs well under abiotic stress conditions, was selected. Seeds were disinfected using 95% ethanol and washed using 70% sodium hypochlorite solution followed by rinsing with distilled water three times.
Treatments
The treatments (two sets of pots) were as follows: (i) control (untreated with foliar spray, no bacterial inoculation], (ii) Pseudomonas aeruginosa (Pa) (strain) [inoculation with Pa], (iii) MLE 1 [foliar sprayed MLE at 1:15], (iv) MLE 2 [foliar sprayed MLE at 1:30], (v) Pa + MLE 1 [inoculation with Pa bacteria + foliar sprayed MLE at 1:15], and (vi) Pa + MLE 2 [inoculation with Pa bacteria + foliar sprayed MLE at 1:30].
Fertilizer and Seed Sowing
At the start of the experiment, the soil was fertilized with a basal dose of N–P–K fertilizer (0.51–0.45–0.38 N–P–K g) using urea (46% N), sulfate of potash (50% K2O), and diammonium phosphate (46% P2O5, 18% N) [24]. Initially, ten seeds were sown in each pot containing 12 kg clay loamy soil (25 cm diameter × 30 cm height), and after complete emergence, they were thinned to six plants per pot.
Moringa Leaf Extract, Pa (Strain), and Establishment of Drought Stress
An aerobic PGPR strain of free-living soil nitrogen-fixing bacteria; Pa (Pa strain) isolated from the rhizosphere of wheat roots growing in local field areas by serial dilution method was used in this study. The serial dilutions up to 10–7 were spread on Luria-Bertani (LB) agar plates at 37°C temperature and inoculated overnight. Bacterial growth was determined by measuring optical density at 600 nm using a spectrophotometer (Gontia-Mishra et al., 2016). Before inoculation, the seeds were coated with 20% gum Arabic as an adhesive and immersed in a bacterial suspension of 108 CFU ml–1 strength just before 15 min of sowing. After 20 days of seed sowing, one set of pots (half number) was shifted to canopies for the imposition of drought stress. Well-watered conditions were maintained at 70 FC (70% field capacity), while drought stress was maintained at 45 FC (45% field capacity). The plants that remained were kept at different water regimes until harvesting.
Fresh and disease-free moringa leaves were collected and rinsed with water. Notably, a 100-g leaf sample was extracted in 1 L distilled water (1:10 w/v) for 15 min (Yasmeen et al., 2013b). Later, MLE was filtered and diluted with distilled water at two concentrations: MLE 1 = 1:20 v/v and MLE 2 = 1:30 v/v. Some chemical properties of moringa leaves are total chlorophyll = 3.79 mg g–1 FW, carotenoids = 1.58 mg g–1 FW, total phenolics = 1.68 μmol g–1 FW, mg g–1 FW, nitrogen = 14.13 mg g–1 DW, phosphorous = 2.98 mg g–1 DW, potassium = 11.97 mg g–1 DW, and calcium = 16.8 mg g–1 DW. The extracts were freshly prepared before their application. Distilled water was sprayed on control plants in both applications. MLE was sprayed to a pot-grown wheat twice after 25 and 35 days of sowing date (50 ml per plant) after the establishment of drought stress. Tween-20 surfactant was used in a foliar spray (0.1% v/v).
Harvesting and Measurement of Growth Attributes
Harvest was done after 45 days of planting. Root and shoot fresh weights (FWs) and lengths were measured immediately after harvest at the experimental site. Fresh samples were stored at -30°C in a biomedical refrigerator for fresh analysis. Three samples per treatment were oven-dried (65°C) for 3 days to determine their dry weights (DWs) and ionic content analysis using the acid digestion method.
Photosynthetic Pigments
The chlorophyll contents of wheat leaves were determined as described by Dere et al. (1998). Notably, 0.2 g of leaves (randomly collected) were extracted in 10 ml methanol (96%) using a homogenizer at 1,000 rpm for 1 min, then filtered, and centrifuged for 10 min at 2,500 rpm. The supernatant was separated and used to determine chlorophyll contents at 666 (chlorophyll a), 653 (chlorophyll b), and 470 nm (total carotenoids) wavelengths using a spectrophotometer (Model SM1200; Randolph, NJ, United States).
Measurements of Stomatal Conductance (gs)
Stomatal conductance (gs) of fully developed leaves (three plants per treatment) was measured by putting them in a portable infrared gas analyzer chamber (Analytical Development Company, Hoddeson, United Kingdom). The measurements were made 6 days after the first MLE foliar spray.
Leaf Biochemical Analysis
Fully expanded leaves from each replicate were taken, wrapped in aluminum foils, immersed in liquid nitrogen, and transferred into plastic zipper bags. These samples were stored at –80°C for further analysis. Following, biochemical analysis was performed using a spectrophotometer (Model SM1200; Randolph, NJ, United States).
To determine osmolytes as sugars and non-enzymatic antioxidants, 50 mg of dried leaves were homogenized in 10 ml of 80% ethanol and filtered followed by the re-extraction in 10 ml ethanol, and a 20 ml of the final volume was maintained. This obtained solution was used to evaluate flavonoids (Lewis et al., 1999), soluble sugars (Dubois et al., 1956), proteins (Bradford, 1976), and proline (Bates et al., 1973) contents. Glycine betaine (GB) was assessed by following the method of Holmström et al. (2000).
Mineral Content
For the determination of P contents in wheat molybdate/ascorbic acid, the blue technique was used, and nitrate contents were assessed by Kowalenko and Lowe (1973) method using a spectrophotometer (Model SM1200; Randolph, NJ, United States). K concentration was determined using a flame photometer. Ca and Na ions were evaluated by Atomic Absorption Spectrum (AAS; Shimadzu instruments, Inc., Spectra AA-220, Kyoto, Japan).
Statistical Analysis
Statistical analysis of data was performed using a post-hoc test, which was performed to measure specific differences between treatments using the Duncan’s Multiple Range Test (DMRT) in a completely randomized block design. The significant diffidences between treatment means were determined using analysis of variance and mean separation at a 5% significance level (p ≤ 0.05). In addition, the Pearson correlation of different wheat attributes under drought stress and well-watered conditions was performed. Logarithmic data transformation to obtain near-normal distribution was implemented before analysis, where required.
Results
Length, Fresh Weight, and Dry Weight of Roots and Shoot
All applied treatments had a significant positive effect on root and shoot FWs, DWs, and lengths under control and drought stress conditions (Figure 1). In this regard, Pa + MLE 1 and Pa + MLE 2 were proved significantly efficient amendments in increasing root FW and shoot FW of wheat plants during drought conditions. Furthermore, MLE 2 enhanced root DW and shoot DW at 45 FC compared with control (70 FC). The treatments MLE 2, Pa + MLE 1, and Pa + MLE 2 had a more significant effect on root length and shoot length as compared with the control under drought stress. A significant difference was observed between the given amendments and control plants which signify their effectiveness in drought stress environments.

Figure 1. Impact of sole and combined applications of Pa and moringa leaf extract (MLE) on different growth attributes of wheat plants under 45 FC and 70 FC irrigation levels. Different bars represent the mean values of three replicates. Error bars represent standard error (SE). Different letters on bars indicate significant difference at p ≤ 0.05 [70 FC = well-irrigated, 45 FC = drought stress condition; C = control, Pa (strain) = Pseudomonas aeruginosa inoculated plants, MLE 1 = foliar-applied MLE at 1:15, and MLE 2 = foliar-applied MLE at 1:30]. Means with the same letters within the column are not significantly different.
Photosynthetic Pigments
All photosynthetic pigments such as chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids were significantly affected when subjected to drought stress conditions (45 FC) as compared with well-irrigated conditions (70 FC) (Figure 2). All applied amendments significantly improved plant photosynthetic pigments over the control. Pa + MLE 1 and Pa + MLE 2 treatments were remained efficient in increasing chlorophyll a and chlorophyll b at 45 FC over control-treated wheat plants (70 FC). Furthermore, MLE 2 differed significantly better for the enhancement of total chlorophyll contents at 45 FC than 70 FC. Treatments MLE 2, Pa + MLE 1, and Pa + MLE 2 were significantly different for wheat carotenoid contents under drought stress as compared with the control. A significant difference was observed in MLE at both concentrations and Pa for all studied parameters in all treatment levels. The above results signify the value of given amendments in enhancing the efficiency of water uptake and use by the grown plants.
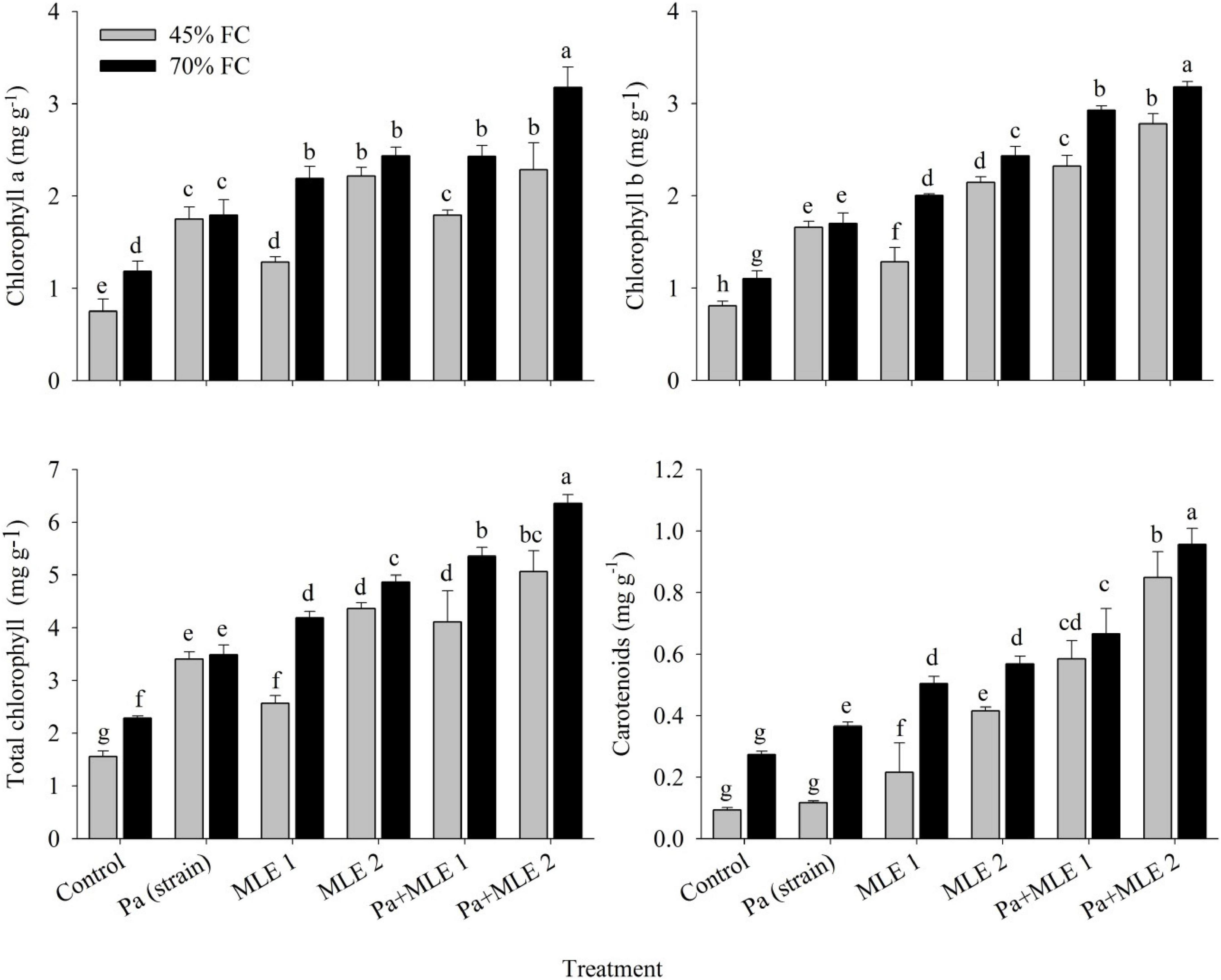
Figure 2. Impact of sole and combined applications of Pa and MLE on different photosynthetic pigments of wheat plants under 45 FC and 70 FC irrigation levels. Different bars represent the mean values of three replicates. Error bars represent SE. Different letters on bars indicate significant difference at p ≤ 0.05 [70 FC = well-irrigated, 45 FC = drought stress condition; C = control, Pa (strain) = P. aeruginosa inoculated plants, MLE 1 = foliar-applied MLE at 1:15, and MLE 2 = foliar-applied MLE at 1:30]. Means with the same letters within the column are not significantly different.
Flavonoids, Phenolics, Total Soluble Sugars, Total Soluble Proteins, and Stomatal Conductance
Flavonoids, phenolics, total soluble sugars, total soluble proteins, and stomatal conductance of wheat plants were significantly affected in drought stress (45 FC) compared with well-watered plants (70 FC) (Figure 3). Both sole and combined applications of Pa and MLE significantly increased plant total soluble sugars, total soluble proteins, and stomatal conductance over the control. Pa + MLE 1 and Pa + MLE 2 treatments were remained efficient in decreasing flavonoids and phenolics at 45 FC over 70 FC. Moreover, MLE 2 differed significantly better than MLE 1 and Pa either in sole or combined applications at 45 FC than 70 FC, while, MLE 2, Pa + MLE 1, and Pa + MLE 2 were significantly efficient in decreasing flavonoids and phenolics of wheat plants under drought stress as compared with the control. These results prove the effectiveness of these amendments in increasing the water uptake efficiency of wheat plants.
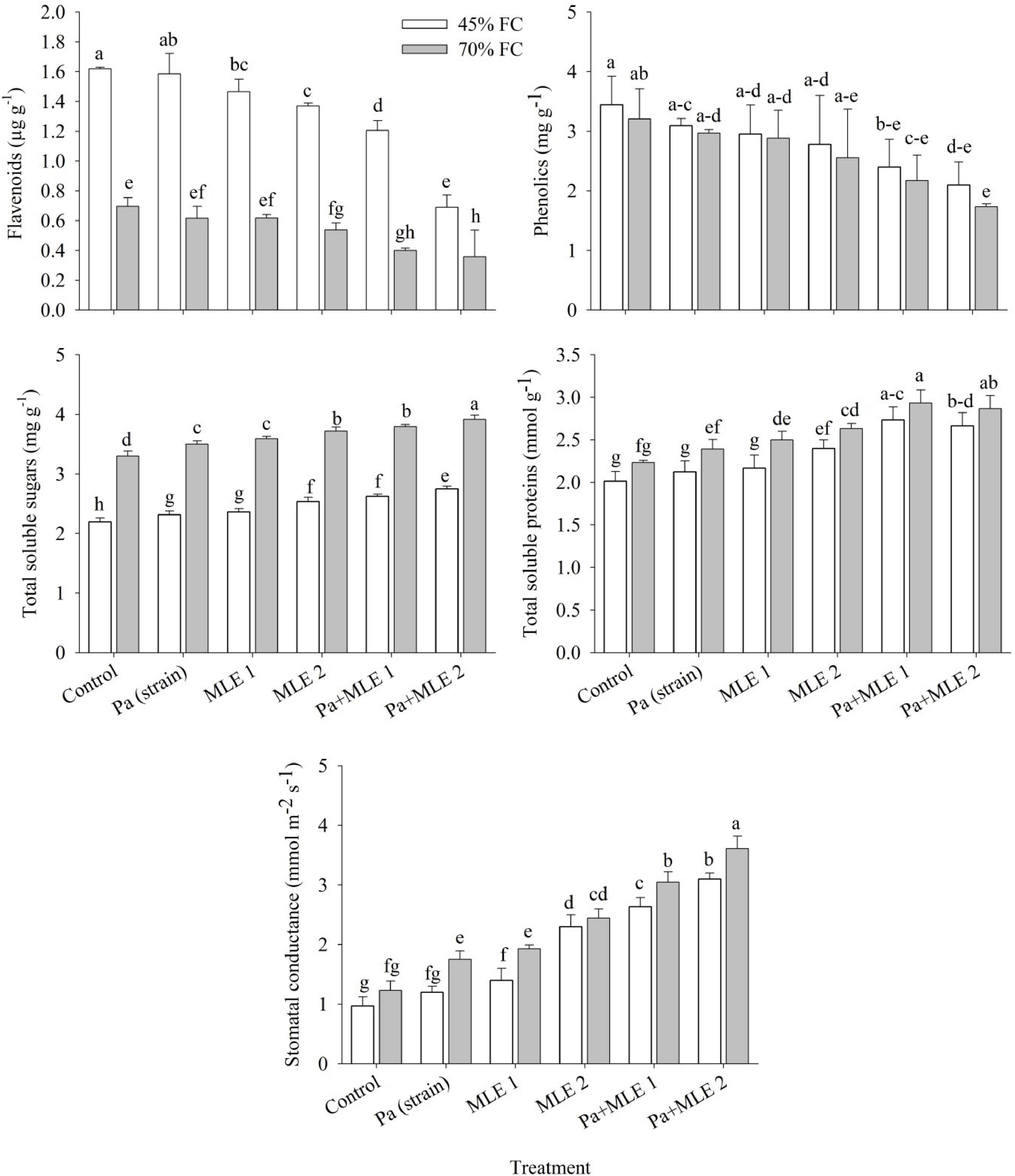
Figure 3. Impact of sole and combined applications of Pa and MLE on flavonoids, phenolics, total soluble sugars, total soluble proteins, and stomatal conductance of wheat plants under 45 FC and 70 FC irrigation levels. Different bars represent the mean values of three replicates. Error bars represent SE. Different letters on bars indicate significant difference at p ≤ 0.05 [70 FC = well-irrigated, 45 FC = drought stress condition; C = control, Pa (strain) = P. aeruginosa inoculated plants, MLE 1 = foliar-applied MLE at 1:15, MLE 2 = foliar-applied MLE at 1:30, and S.C. = stomatal conductance]. Means with the same letters within the column are not significantly different.
Oxidative Stress Indicators, Enzymatic Antioxidants, and Ionic Contents
Ionic nutrient contents increased significantly in plants that were irrigated at 70 FC compared with plants irrigated with water to reach only 45 FC (Table 1). However, in contrast, different oxidative stress indicators such as sodium, proline, GB, electrolyte leakage (EL), malondialdehyde (MDA), and hydrogen peroxide (H2O2) increased significantly in plants subjected to 45 FC water conditions. Furthermore, antioxidant enzyme [superoxide dismutase (SOD) and peroxide (POD)] activities were increased in drought stress.
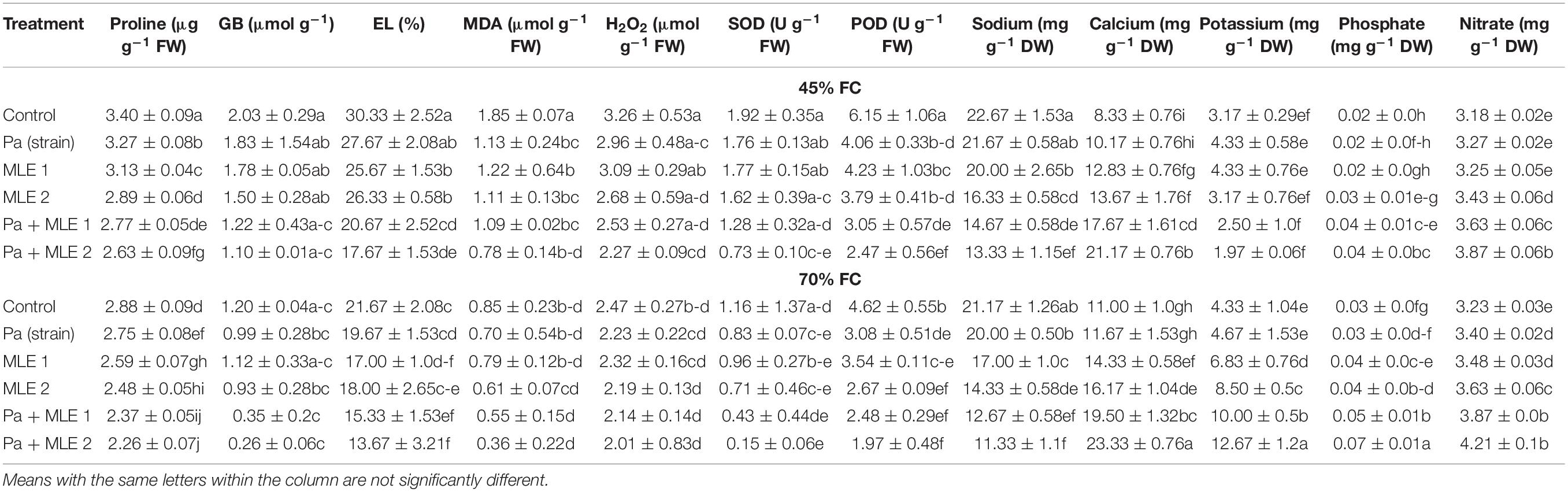
Table 1. Oxidative stress indicators, enzymatic antioxidants, and ionic contents of wheat grown in well-watered and drought stress conditions.
All additives had significant positive improvements in ameliorating drought effects in wheat. These amendments were more detectable when the dose of MLE foliar application was increased. Pa and MLE decreased sodium, proline, GB, EL, MDA, H2O2, and SOD and POD contents significantly. Also, they increased calcium, potassium, phosphate, and nitrate concentrations in plant tissues. It is worth mentioning in this study that sole and combined applications of either MLE or Pa were better in decreasing the oxidative stress indicators and increasing nutrient contents of wheat, and this signifies the success of these ameliorating treatments for drought stress. Pa + MLE 1 and Pa + MLE 2 recorded the least oxidative stress indicators and enzymatic antioxidant activities during more nutrient assimilation in plants.
All values are the means of three replicates ± SD. Different labels represent significant different alphabets using the least significant difference (LSD) test. [70 FC = well-irrigated conditions, 45 FC = drought stress conditions; C = control, Pa (strain) = P. aeruginosa inoculated plants, MLE 1 = foliar applied MLE at 1:15, and MLE 2 = foliar applied MLE at 1:30] [GB = glycine betaine, EL = electrolyte leakage, MDA = malondialdehyde, H2O2 = hydrogen peroxide, SOD = superoxide dismutase, and POD = peroxide].
Pearson Correlation
A significant positive correlation exists between plant morphological attributes and photosynthetic pigments (Figure 4). This might, in turn, improve plant vigor and consequently improved plant growth. Also, the total soluble sugars, total soluble proteins, stomatal conductance, and ionic contents were increased with the given amendments. However, oxidative stress indicators, enzymatic antioxidants, flavonoids, and phenolics increased under drought stress conditions recorded significant negative correlations with plant growth parameters.
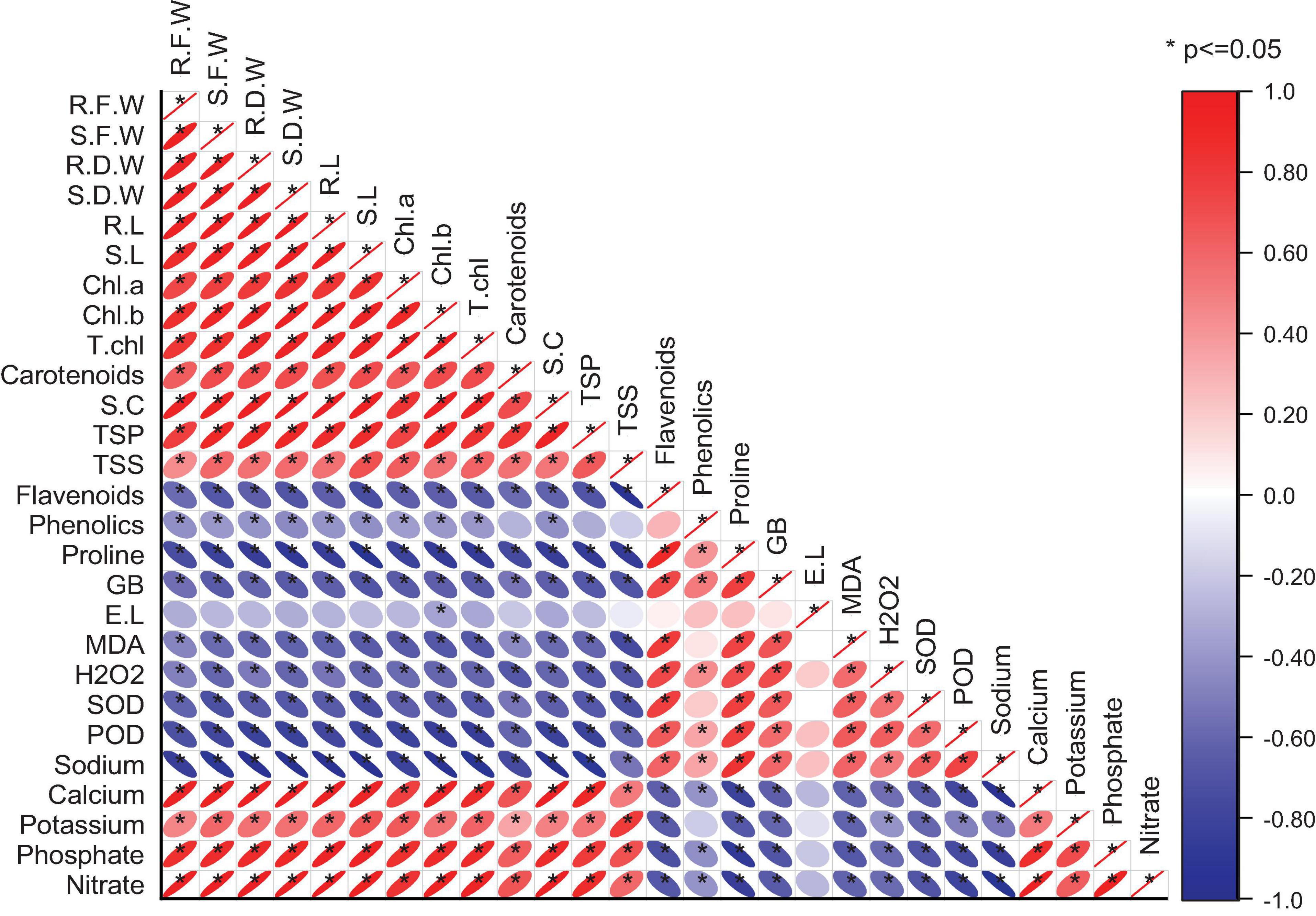
Figure 4. Pearson correlation of different wheat attributes under drought stress and well-watered conditions.
Discussion
This study evaluates the effect of MLE (MLE 1 = 1:20 v/v and MLE 2 = 1:30 v/v) and PGPR strain (Pa) on drought stress tolerance ability of wheat (Anaj 17 genotype) under two irrigation regimes (45 FC and 70 FC). The use of organic or biofertilizers as a global initiative instead of costly chemical fertilizers is addressed by several researchers (Talaei et al., 2014; El-Naggar et al., 2015; El-Serafy, 2018; Rahimi et al., 2019). Plant-derived biostimulants that trigger abiotic stress tolerance to ameliorate stress-induced yield reduction involve many different mechanisms, and most of these involve phytohormones and upregulated antioxidant defense systems (Bulgari et al., 2017; Sadasivam et al., 2017).
The highest dry matter contents in both root and shoot were obtained with the combined application of Pa + MLE 2 mixture that agrees with previous findings describing that soil beneficial bacteria can promote plant growth in abiotic stress conditions. Durán et al. (2016) had similar results on lettuce inoculated with Bacillus sp. and Klebsiella sp. and concluded that inoculated plants have more DW than non-inoculated ones. In this sense, Glick et al. (2007) said that this increase in the dry matter might be the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzyme activity of bacteria to lower the stress-induced ethylene levels, leaving α-ketobutyrate and NH4+ available to the plants. Moreover, according to the study by Sánchez López (2011), auxins, i.e., indole-3-acetic acid (IAA), have a stimulatory effect on secondary root development that contributes to the total biomass of the plant by increasing nutrient and water absorption in moisture deficit conditions. The results from this study are in accordance with those claimed by Mishra et al. (2012) that an increase in auxin concentration stimulates adventitious root formation and thus increases root surface area and total plant biomass, proclaiming a morphological strategy of plants to tolerate drought with the help of bacterial strains.
Photosynthetic pigment contents are considered a valuable physiological indicator for evaluating the damage caused by stress intensity. The wheat genotype in this study had more chlorophyll contents when supplemented with Pa and MLE with the highest value recorded in their combined treatment at both water regimes. This alteration is directly related to plant macronutrient contents such as nitrogen. The reactive oxygen species (ROS) produced during abiotic stress cause the reduction of chlorophyll contents by damaging photosynthetic machinery (Manivannan et al., 2007). Similar to our results, Ahmadi et al. (2013) also found a significant increase in chlorophyll contents by inoculating wheat with different bacterial strains (Pseudomonas sp. and Azospirillum sp.).
This study indicates that the increase in mineral nutrient contents of wheat is due to the use of biostimulants that enhanced drought stress tolerance. The inoculation with P. aeruginosa and MLE applied separately and in combination increased levels of sodium, calcium, potassium, phosphate, and nitrate in wheat submitted to drought. This might be due to the nutrient solubilization by added microorganisms due to the production of ACC deaminase (Mutumba et al., 2018). In addition, Römheld and Kirkby (2010) considered potassium as a key element in reducing water stress by adjusting osmoregulation in cells as a compatible solute. Zulfiqar et al. (2020) reported enhancement in soil nutrient availability and improvement in fruit quality induced by plant-derived biostimulants in stress conditions. The increase in plant nutritional status and vigor is due to improved soil microbial activity (Colla et al., 2015) and the presence of some chelating agents that enhance nutrient solubility in soil (Abou Chehade et al., 2018).
Induced tolerance by plant growth regulators is triggered by external stress factors and enhancement in plant sterols by reducing EL and regulating membrane stability (Lucini et al., 2015), thus providing an antioxidant defense system. Results of this experimental study indicate that Pa and MLE significantly improve growth, biochemical, antioxidant, and ionic attributes of wheat. Rhizobacteria potentially increase plant vigor by increasing atmospheric nitrogen fixation ability of roots and mineral absorption.
In accordance with our findings, previous studies also validate drought stress effects that consequently increase ROS that activate plant enzymatic and non-enzymatic antioxidant systems in order to balance the cellular redox state (Zulfiqar et al., 2020). Kumar et al. (2019) reported seed priming that involves plant growth regulators inducing sugar-triggered plant immunity (called “sweet immunity”—immunity by sugar compounds) having a positive priming effect on antioxidant systems. Results of our study validate that the antioxidant contents were improved using MLEs as a bioactive compound. Furthermore, a study by Rashid et al. (2018) confirmed the presence of a primary growth regulator, zeatin (a cytokinin derivative) that provided abiotic stress tolerance. Moringa extract constitutes considerable quantities of secondary metabolites, antioxidants, and osmoprotectants (Rehman et al., 2015). MLE’s growth-promoting and abiotic stress tolerance inducing effects justify its use as a potential alternative to synthetic chemical fertilizers for improving wheat productivity (Nasir et al., 2016; Brockman and Brennan, 2017; Merwad, 2018; Younis et al., 2018). MLE aqueous solutions are very easy to make, cost-effective, and environment-friendly and can be used by the farmers effectively for increasing their crop yields. This study clearly reveals that all studied growth, biochemical, ionic, and antioxidant constituents of wheat plants were improved by using either of the two given amendments. Pa and MLE reduced all investigated oxidants and oxidative stress indicators. It is worth mentioning in this study that these supplementary amendments might antagonize each other’s effects to some extent in their combined applications, as their sole applications had lowered physiological, biochemical, and ionic attributes. Thus, constitutive effects, i.e., Pa and MLE, at both concentration levels were more elaborated, thus inducing drought tolerance in wheat.
Conclusion
The application of both nitrogen-fixing bacteria (Pa strain) and foliar sprayed MLE either in sole or combined treatments can alleviate nutrient and drought stress effects. Nevertheless, the combined application of Pa and MLE 2 (Pa + MLE 2) can efficiently improve wheat growth attributes, photosynthetic pigment contents, and nutrient uptake under drought stress. The combined supplementation of Pa and MLE had more significant positive effects compared with their sole applications. Pa + MLE 2 was also efficient in decreasing oxidative stress indicators and enzymatic antioxidants of wheat under drought stress. There is a need for more investigations at field levels by considering the effect of other environmental constraints to effectively validate these findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
IL, SA-D, LA-A, RH, KAM, NM, SA, MA, AA, PP, KM, and TG: researching and writing. AA, SA, and LA-A: writing. All authors contributed to the article and approved the submitted version.
Funding
The research team and authors are thankful to Taif University Research Supporting project number TURSP-2020/315, Taif University, Taif, Saudi Arabia, for providing the research and lab facilities and financial support throughout the research experiment.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FZ declared a shared affiliation with one of the author, RH, to the handling editor at the time of the review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to the Taif University Research Supporting project number (TURSP-2020/315) Taif University, Taif, Saudi Arabia for providing the research and lab facilities and financial support throughout the research experiment. We also thank the support of the iASK Research Grant and Eötvös Research Grant (MAEÖ-00074-002/2021) for their help in publishing this research.
References
Abou Chehade, L., Al Chami, Z., De Pascali, S. A., Cavoski, I., and Fanizzi, F. P. (2018). Biostimulants from food processing by-products: agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J. Sci. Food Agric. 98, 1426–1436. doi: 10.1002/jsfa.8610
Ahmadi, J., Asgharzadeh, A., and Bakhtiari, S. (2013). The effect of microbial inoculants on physiological responses of two wheat cultivars under salt stress. Int. J. Adv. Biol. 4, 364–371.
Barnawal, D., Singh, R., and Singh, R. P. (2019). “Role of plant growth promoting rhizobacteria in drought tolerance: regulating growth hormones and osmolytes,” in PGPR Amelioration in Sustainable Agriculture, eds A. K. Singh, A. Kumar, and P. K. Singh (Amsterdam: Elsevier), 107–128. doi: 10.1016/b978-0-12-815879-1.00006-9
Bates, L. S., Waldren, R. P., and Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1007/BF00018060
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Brockman, H. G., and Brennan, R. F. (2017). The effect of foliar application of Moringa leaf extract on biomass, grain yield of wheat and applied nutrient efficiency. J. Plant Nutr. 40, 2728–2736. doi: 10.1080/01904167.2017.1381723
Budak, H., Kantar, M., and Yucebilgili Kurtoglu, K. (2013). Drought tolerance in modern and wild wheat. Sci. World J. 2013:548246. doi: 10.1155/2013/548246
Bulgari, R., Morgutti, S., Cocetta, G., Negrini, N., Farris, S., Calcante, A., et al. (2017). Evaluation of borage extracts as potential biostimulant using a phenomic, agronomic, physiological, and biochemical approach. Front. Plant Sci. 8:935. doi: 10.3389/fpls.2017.00935
Colla, G., Nardi, S., Cardarelli, M., Ertani, A., Lucini, L., Canaguier, R., et al. (2015). Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 196, 28–38. doi: 10.1016/j.scienta.2015.08.037
Dere, S., Güneş, T., and Sivaci, R. (1998). Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk. J. Bot. 22, 13–18.
Dubois, M., Gilles, K., Hamilton, J., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Durán, P., Acuña, J. J., Armada, E., López-Castillo, O. M., Cornejo, P., Mora, M. L., et al. (2016). Inoculation with selenobacteria and arbuscular mycorrhizal fungi to enhance selenium content in lettuce plants and improve tolerance against drought stress. J. Plant. Nutr. Soil Sci. 16, 211–225.
El-Naggar, A. H. M., Hassan, M. R. A., Shaban, E. H., and Mohamed, M. E. A. (2015). Effect of organic and biofertilizers on growth, oil yield and chemical composition of the essential oil of Ocimum basillicum L. plants. Alex. J. Agric. Res. 60, 1–16.
El-Serafy, R. S. (2018). Growth and productivity of roselle (Hibiscus sabdariffa L.) as affected by yeast and humic acid. Sci. J. Flowers Ornam. Plants 5, 195–203. doi: 10.21608/sjfop.2018.18129
Fasiha Amjad, S., Mansoora, N., Yaseen, S., Kamal, A., Butt, B., Matloob, H., et al. (2021). Combined use of endophytic bacteria and pre-sowing treatment of thiamine mitigates the adverse effects of drought stress in wheat (Triticum aestivum L.) cultivars. Sustainability 13:6582. doi: 10.3390/su13126582
Glick, B. R., Cheng, Z., Czarny, J., and Duan, J. (2007). “Promotion of plant growth by ACC deaminase-producing soil bacteria,” in New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research, eds P. A. H. M. Bakker, J. M. Raaijmakers, G. Bloemberg, M. Höfte, P. Lemanceau, and B. M. Cooke (Dordrecht: Springer), 329–339. doi: 10.1007/978-1-4020-6776-1_8
Gontia-Mishra, I., Sapre, S., Sharma, A., and Tiwari, S. (2016). Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 18, 992–1000. doi: 10.1111/plb.12505
Grover, M., Ali, S. Z., Sandhya, V., Rasul, A., and Venkateswarlu, B. (2011). Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 27, 1231–1240. doi: 10.1007/s11274-010-0572-7
Holmström, K.-O., Somersalo, S., Mandal, A., Palva, T. E., and Welin, B. (2000). Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 2000, 177–185. doi: 10.1093/jexbot/51.343.177
Jiménez-Gómez, A., Flores-Félix, J. D., Garcia-Fraile, P., Mateos, P. F., Menéndez, E., and Velázquez, E. (2018). Probiotic activities of Rhizobium laguerreae on growth and quality of spinach. Sci. Rep. 8:295. doi: 10.1038/s41598-017-18632-z
Kaushal, M., and Wani, S. P. (2016). Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 66, 35–42. doi: 10.1007/s13213-015-1112-3
Khan, N., Ali, S., Tariq, H., Latif, S., Yasmin, H., and Mehmood, A. (2020). Water conservation and plant survival strategies of rhizobacteria under drought stress. Agronomy 10:1683. doi: 10.1002/jobm.201800309
Kowalenko, C. G., and Lowe, L. E. (1973). Determination of nitrates in soil extracts. Soil Sci. Soc. Am. J. 37:660. doi: 10.2136/sssaj1973.03615995003700040051x
Kumar, S., Chinnannan, K., Thamilarasan, S. K., Seralathan, M., Shanmuganathan, R., and Padikasan, I. A. (2019). Enzymatically hydrolysed sago bagasse improves physiological, biochemical and molecular attributes of Solanum lycopersicum. Biocatal. Agric. Biotechnol. 17, 499–506. doi: 10.1016/j.bcab.2019.01.005
Lewis, C. E., Walker, J. R. L., Lancaster, J. E., and Sutton, K. H. (1999). Determination of anthocyanins, flavonoids and determination phenolic acids in coloured potatoes. i: cultivars of Solanum tuberosum L. J. Sci. Food Agricult. 77, 45–57. doi: 10.1002/(sici)1097-0010(199805)77:1<45::aid-jsfa1>3.0.co;2-s
Lisar, S. Y. S., Motafakkerazad, R., Hossain, M. M., and Rahman, I. M. M. (2012). “Water stress in plants: causes, effects and responses,” in Water Stress, eds I. M. M. Rahman and H. Hasegawa (Rijeka: InTech), 1–14. doi: 10.1007/978-1-4614-4747-4_1
Lucini, L., Rouphael, Y., Cardarelli, M., Canaguier, R., Kumar, P., and Colla, G. (2015). The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 182, 124–133. doi: 10.1016/j.scienta.2014.11.022
Manivannan, P., Jaleel, C. A., Sankar, B., Kishorekumar, A., Somasundaram, R., Lakshmanan, G. A., et al. (2007). Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf. B. 59, 141–149. doi: 10.1016/j.colsurfb.2007.05.002
Merwad, A.-R. M. A. (2018). Using Moringa oleifera extract as biostimulant enhancing the growth, yield and nutrients accumulation of pea plants. J. Plant Nutr. 41, 425–431. doi: 10.1080/01904167.2017.1384012
Mishra, P. K., Bisht, S. C., Bisht, J. K., and Bhatt, J. C. (2012). “Cold-tolerant PGPRs as bioinoculants for stress management,” in Bacteria in Agrobiology: Stress Management, ed. D. Maheshwari (Berlin: Springer), 95–118. doi: 10.1007/978-3-662-45795-5_6
Mutumba, F. A., Zagal, E., Gerding, M., Castillo-Rosales, D., Paulino, L., and Schoebitz, M. (2018). Plant growth promoting rhizobacteria for improved water stress tolerance in wheat genotypes. J. Soil Sci. Plant Nutr. 18, 1080–1096.
Nardi, S., Carletti, P., Pizzeghello, D., and Muscolo, A. (2009). “Biological activities of humic substances,” in Biophysico-chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems. Vol 2, Part 1: Fundamentals and Impact of Mineral-organic Biota Interactions on the Formation, Transformation, Turnover, and Storage of Natural Nonliving Organic Matter (NOM), eds N. Senesi, B. Xing, and P. M. Huang (Hoboken: Wiley), 305–340. doi: 10.1002/9780470494950.ch8
Nasir, M., Khan, A. S., Basra, S. M. A., and Malik, A. U. (2014). Foliar application of moringa leaf extract, potassium and zinc influence yield and fruit quality of Kinnow mandarin. Sci. Hortic. 210, 227–235. doi: 10.1016/j.scienta.2016.07.032
Nasir, M., Khan, A. S. Ahmad Basra, S. M., and Malik, A. U. (2016). Foliar application of moringa leaf extract, potassium and zinc influence yield and fruit quality of ‘Kinnow’ mandarin. Scientia Horticulturae 210, 227–235. doi: 10.1016/j.scienta.2016.07.032
Ngumbi, E., and Kloepper, J. (2016). Bacterial-mediated drought tolerance: current and future prospects. Appl. Soil Ecol. 105, 109–125. doi: 10.1016/j.apsoil.2016.04.009
Parajuli, R., Thoma, G., and Matlock, M. D. (2019). Environmental sustainability of fruit and vegetable production supply chains in the face of climate change: a review. Sci. Total Environ. 650, 2863–2879. doi: 10.1016/j.scitotenv.2018.10.019
Rahimi, A., Siavash Moghaddam, S., Ghiyasi, M., Heydarzadeh, S., Ghazizadeh, K., and Popović-Djordjević, J. (2019). The influence of chemical, organic and biological fertilizers on agrobiological and antioxidant properties of Syrian Cephalaria (Cephalaria syriaca L.). Agriculture 9:122. doi: 10.3390/agriculture9060122
Rashid, N., Basra, S. M. A., Shahbaz, M., Iqbal, S., and Hafeez, M. B. (2018). Foliar applied moringa leaf extract induces terminal heat tolerance in quinoa. Int. J. Agric. Biol. 20, 157–164.
Rehman, H., Kamran, M., Basra, S. M. A., Afzal, I., and Farooq, M. (2015). Influence of seed priming on performance and water productivity of direct seeded rice in alternating wetting and drying. Rice Sci. 22, 189–196. doi: 10.1016/j.rsci.2015.03.001
Römheld, V., and Kirkby, E. A. (2010). Research on potassium in agriculture: needs and prospects. Plant Soil 335, 155–180. doi: 10.1007/s11104-010-0520-1
Ruzzi, M., and Aroca, R. (2015). Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 196, 124–134. doi: 10.1016/j.scienta.2015.08.042
Sánchez López, D. B. (2011). Efecto de la Inoculación con Bacterias Promotoras de Crecimiento Vegetal Sobre el Cultivo de Tomate (Solanum Lycopersicum var. Sofía) bajo Invernadero. Master’s thesis. Pontificia Universidad Javeriana, Bogotá.
Sadasivam, V., Packiaraj, G., Subiramani, S., Govindarajan, S., Kumar, G. P., Kalamani, V., et al. (2017). Evaluation of seagrass liquid extract on salt stress alleviation in tomato plants. Asian J. Plant Sci. 16, 172–183. doi: 10.3923/ajps.2017.172.183
Semida, W. M., and Rady, M. M. (2014). Pre-soaking in 24-epibrassinolide or salicylic acid improves seed germination, seedling growth, and anti-oxidant capacity in Phaseolus vulgaris L. grown under NaCl stress. J. Hortic. Sci. Biotechnol. 89, 338–344. doi: 10.1080/14620316.2014.11513088
Shahzad, U., Khan, M. A., Jaskani, M. J., Khan, I. A., and Korban, S. S. (2013). Genetic diversity and population structure of Moringa oleifera. Conserv. Genet. 14, 1161–1172. doi: 10.1007/s10592-013-0503-x
Shukla, N., Awasthi, R. P., Rawat, L., and Kumar, J. (2012). Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 54, 78–88. doi: 10.1016/j.plaphy.2012.02.001
Singh, S., Kumar, V., Dhanjal, D. S., Sonali, Dhaka, V., Thotapalli, S., et al. (2021). “Rhizosphere biology: a key to agricultural sustainability,” in Current Trends in Microbial Biotechnology for Sustainable Agriculture, eds A. N. Yadav, J. Singh, C. Singh, and N. Yadav (Singapore: Springer Nature), 161–182. doi: 10.1007/978-981-15-6949-4_7
Soumare, A., Diédhiou, A. G., Arora, N. K., Al-Ani, L. K. T., Ngom, M., Fall, S., et al. (2021). Potential role and utilization of plant growth promoting microbes in plant tissue culture. Front. Microbiol. 12:649878. doi: 10.3389/fmicb.2021.649878
Stapleton, J. J., Wilen, C. A., and Molinar, R. H. (2008). Soil Solarization for Gardens and Landscapes. Pest Note Publication, 74145. Davis, CA: University of California Agriculture and Natural Resources.
Talaei, G. H., Vazirimehr, M. R., Shahgholi, H., Shirmohammadi, E., Sabbagh, E., and Rigi, K. (2014). Influence of biological and chemical nitrogen fertilizers on grain yield and yield components of Fennel (Foeniculum vulgare Mill.). Int. J. Biosci. 4, 206–211. doi: 10.12692/ijb/4.9.206-211
Venkateswarlu, B., and Shanker, A. K. (2009). Climate change and agriculture: adaptation and mitigation stategies. Indian J. Agron 54, 226–230.
Wheeler, T., and Von Braun, J. (2013). Climate change impacts on global food security. Science 341, 508–513.
Yasmeen, A., Basra, S. M. A., Ahmad, R., and Wahid, A. (2012). Performance of late sown wheat in response to foliar application of Moringa oleifera Lam. leaf extract. Chil. J. Agric. Res. 72:92. doi: 10.4067/s0718-58392012000100015
Yasmeen, A., Basra, S. M. A., Farooq, M., ur Rehman, H., Hussain, N., and Athar, H. R. (2013a). Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul. 69, 225–233. doi: 10.1007/s10725-012-9764-5
Yasmeen, A., Basra, S. M. A., Wahid, A., Nouman, W., and Rehman, H. U. R. (2013b). Exploring the potential of Moringa oleifera leaf extract (MLE) as a seed priming agent in improving wheat performance. Turk. J. Bot. 37, 512–520.
Yasmeen, A., Nouman, W., Basra, S. M. A., Wahid, A., and Hussain, N. (2014). Morphological and physiological response of tomato (Solanum lycopersicum L.) to natural and synthetic cytokinin sources: a comparative study. Acta Physiol. Plant. 36, 3147–3155. doi: 10.1007/s11738-014-1662-1
Younis, A., Akhtar, M. S., Riaz, A., Zulfiqar, F., Qasim, M., Farooq, A., et al. (2018). Improved cut flower and corm production by exogenous moringa leaf extract application on gladiolus cultivars. Acta Sci. Pol. Hortorum Cultus 17, 25–38. doi: 10.24326/asphc.2018.4.3
Zulfiqar, F., Allaire, S. E., Akram, N. A., Méndez, A., Younis, A., Peerzada, A. M., et al. (2019). Challenges in organic component selection and biochar as an opportunity in potting substrates: a review. J. Plant Nutr. 42, 1386–1401. doi: 10.1080/01904167.2019.1617310
Keywords: PGPR, agroecological zones, drought stress, Pseudomonas, nitrogen-fixing bacteria, biofertilizers, bioinnoculants
Citation: Lalarukh I, Al-Dhumri SA, Al-Ani LKT, Hussain R, Al Mutairi KA, Mansoora N, Amjad SF, Abbas MHH, Abdelhafez AA, Poczai P, Meena KR and Galal TM (2022) A Combined Use of Rhizobacteria and Moringa Leaf Extract Mitigates the Adverse Effects of Drought Stress in Wheat (Triticum aestivum L.). Front. Microbiol. 13:813415. doi: 10.3389/fmicb.2022.813415
Received: 11 November 2021; Accepted: 11 March 2022;
Published: 21 June 2022.
Edited by:
Giuseppe Colla, University of Tuscia, ItalyReviewed by:
Kamlesh Kumar Meena, National Institute of Abiotic Stress Management (ICAR), IndiaFaisal Zulfiqar, Islamia University of Bahawalpur, Pakistan
Olubukola Oluranti Babalola, North-West University, South Africa
Copyright © 2022 Lalarukh, Al-Dhumri, Al-Ani, Hussain, Al Mutairi, Mansoora, Amjad, Abbas, Abdelhafez, Poczai, Meena and Galal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syeda Fasiha Amjad, ZmFzaWhhbXVzaGFkaTc1QGdtYWlsLmNvbQ==; Laith Khalil Tawfeeq Al-Ani, Y212X3ZpcnVzMjAwMkB5YWhvby5jb20=, bGFpdGgua3Q3N0BnbWFpbC5jb20=; Ahmed A. Abdelhafez, YWhtZWQuYXppekBhZ3IubnZ1LmVkdS5lZw==; Peter Poczai, cGV0ZXIucG9jemFpQGhlbHNpbmtpLmZp
 Irfana Lalarukh
Irfana Lalarukh Sami A. Al-Dhumri2
Sami A. Al-Dhumri2 Laith Khalil Tawfeeq Al-Ani
Laith Khalil Tawfeeq Al-Ani Rashid Hussain
Rashid Hussain Syeda Fasiha Amjad
Syeda Fasiha Amjad Mohamed H. H. Abbas
Mohamed H. H. Abbas Ahmed A. Abdelhafez
Ahmed A. Abdelhafez Tarek M. Galal
Tarek M. Galal