
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 28 February 2022
Sec. Microbe and Virus Interactions with Plants
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.792876
This article is part of the Research Topic Rhizosphere Microbiology: Toward a Clean and Healthy Soil Environment View all 25 articles
One of the major mechanisms underlying plant growth-promoting rhizobacteria (PGPR) is the lowering of ethylene level in plants by deamination of 1-aminocyclopropane-1-carboxylic acid (ACC) in the environment. In the present study, using ACC as the sole nitrogen source, we screened seven ACC deaminase-producing bacterial strains from rhizosphere soils of tea plants. The strain with the highest ACC deaminase activity was identified as Serratia marcescens strain JW-CZ2. Inoculation of this strain significantly increased shoot height and stem diameter of tea seedlings, displaying significant promotive effects. Besides, S. marcescens strain JW-CZ2 displayed high ACC deaminase activities in wide ranges of ACC concentration, pH, and temperature, suggesting the applicable potential of JW-CZ2 as a biofertilizer. Genome sequencing indicated that clusters of orthologous groups of proteins (COG) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of JW-CZ2 mainly included amino acid transport and metabolism, transcription, carbohydrate transport and metabolism, inorganic ion transport and metabolism, and membrane transport. Moreover, genes in relation to phosphate solubilization, indole acetic acid (IAA) production, and siderophore were observed in the genome of JW-CZ2, and further experimental evidence demonstrated JW-CZ2 could promote solubilization of inorganic phosphate, inhibit growth of pathogenic fungi, and produce IAA and siderophore. These aspects might be major reasons underlying the plant growth-promoting function of JW-CZ2. Overall, this study provides a new S. marcescens strain, which has applicable potential as a promising biofertilizer.
The tea plant (Camellia sinensis L. O. Kuntze) is an evergreen perennial shrub and is broadly cultivated in the tropical and subtropical regions across the world (Wang et al., 2016). The young leaves of the tea plant are processed to prepare tea for drinking. China is the source of indigenous tea plants and the birthplace of cultivated tea plantation (Liu et al., 2019). However, the productivity of tea is decreasing in recent years. The underlying reasons include nutrient deficiency and abiotic stresses. The annual plucking of new shoots easily induces nutrient deficiency of soils (Upadhyaya and Panda, 2013). To supplement this, more chemical fertilizers are applied, which causes nutrient imbalance and soil pollution and finally negatively affects tea plantations. Thus, alternative environmentally friendly fertilizer or technology is required.
In the rhizosphere of plants, a broad spectrum of bacteria reveal promotive effects on plant growth, yield, and resistance to environmental stresses, which are named plant growth-promoting rhizobacteria (PGPR) (Numan et al., 2018). As reported, PGPR could promote plant growth and increase the tolerance to stresses in plants. In addition, the application of PGPR generally does not cause environmental pollution, contributing significantly to sustainable agriculture (Figueiredo et al., 2016; Ramakrishna et al., 2019). Thus, PGPR are more environmental friendly and an ideal alternative to replace chemical fertilizers.
Various mechanisms underlying the promotion of PGPR for plant growth have been reported, depending on the species of the plant and the bacterium, including enhancement of nitrogen fixation (James and Baldani, 2012), phosphate solubilization (Calvo et al., 2017), induction of siderophore (Ramakrishna et al., 2019), production of indole acetic acid (IAA) (Pérez-Flores et al., 2017), competitive exclusion of pathogens (Ahemad and Kibret, 2014), stimulation of mycorrhizae development, and removal of phytotoxic substances (Bashan and de-Bashan, 2010). More specifically, ethylene is a ubiquitous plant hormone and plays multiple roles in the regulation of plant growth and development. Under adverse environment, the production of ethylene was stimulated to increase plant resistance but restrain the growth and the final biomass of plants (Tiwari et al., 2018). Some PGPR revealed a high activity of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, which can hydrolyze ACC to α-ketobutyrate (α-KB) and ammonia and then further utilize them as a carbon and nitrogen source (Penrose and Glick, 2003; Tiwari et al., 2018; Gupta and Pandey, 2019; Nascimento et al., 2019). This kind of PGPR is called ACC deaminase-producing bacterium (ADB), which has been reported in Agrobacterium, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Erwinia, Flavobacterium, Micrococcous, Pseudomonas, and Serratia (Bhattacharyya and Jha, 2012). Since ACC is an immediate precursor of ethylene, reduction of ACC could inhibit the production of ethylene and finally alleviate the inhibitory effects of ethylene on plant growth (Li et al., 2000; Glick, 2014; Gupta and Pandey, 2019). Moreover, utilization of ADB revealed enhancive effects on the growth of many crops and reduced susceptibility to diseases caused by plant pathogens under either stressful or normal conditions, displaying applicable and commercial values of PGPR as biofertilizer on crop plantation. For example, field application of Bacillus PSB12, PSB5, and Enterobacter 77-NS5 significantly increased dry weight and length of root and shoot, as well as seed weight in wheat (Mukhtar et al., 2017). Inoculation of Sinoorhizobium meliloti, Bacillus flexus and Bacillus megaterium separately or together in P-deficient soils improved plant height, shoot and root dry weight, as well as P nutrition in maize (Ibarra-Galeana et al., 2017). Fertilization of Bacillus subtilis increased growth and salt tolerance in Arabidopsis (Cabrefiga et al., 2007; Han et al., 2014), Bacillus amyloliquefaciens could effectively prevent bottom rot in lettuce (Chowdhury et al., 2013), and Pseudomonas fluorescens A506 could control fire blight in pome fruits by competing with the pathogen Erwinia amylovora (Cabrefiga et al., 2007).
Generally, the promotive effects of PGPR on plant growth are affected by plant species, cultivar, genotype, and soil type (Lucy et al., 2004). However, some reports also indicated that PGPR strains isolated from one plant species could excellently promote growth of another plant species (Aggangan and Anarna, 2019; Mayer et al., 2019; Gou et al., 2020). Theoretically, native PGPR strains may be more adaptative to the local environment than non-native strains, and thus may show better promotive functions on local target plants (Fischer et al., 2007). In the rhizosphere soil of tea plants, abundant microbial resources have been identified (Chahboune et al., 2011; Zhao et al., 2012; Wang and Han, 2019). However, up to date, investigations of ADB from rhizosphere of tea plants are still limited. In the present study, we collected rhizosphere soils from seven tea plantation gardens in the Anhui province, China. From these samples, ADB were screened and their ACC deaminase activities were compared. The strain with the highest activity was then applied on tea plants to evaluate their effects on tea growth. Afterward, the effects of culture conditions on ACC deaminase activity were assayed to evaluate its applicability on fields. To further explore the mechanisms underlying its plant growth-promoting functions, the whole genome of this strain was sequenced, and the predicted potential mechanisms were further validated by experimental evidence. Overall, these results may contribute a novel plant growth-promoting bacterial strain to tea plantation and enrich our understandings in the mechanisms underlying the promotion of PGPR on plant growth.
The rhizosphere soil samples of tea plants were collected from seven different tea gardens in Anhui Province, the south of Yangtze river region, China (Supplementary Table 1). ADB were enriched and then isolated as described in Penrose and Glick (2003) with modifications. Briefly, 1 g of rhizosphere soil was suspended in 50 ml of sterile Dworkin and Foster (DF) salts minimal medium (Dworkin and Foster, 1958) and then cultured in a shaker at 200 rpm and 28°C. After 24 h, 1 ml of the liquid was transferred to a 250-ml flask containing 50 ml of sterile DF salts minimal medium, in which 3.0 mM ACC was supplied instead of (NH4)2SO4 as the sole nitrogen source. After being further cultured for 24 h, the medium was diluted, spread on DF salts minimal medium agar plates, and cultured for 48 h at 28°C. The bacterial colonies were picked and stored in 20% glycerol (v/v) solution at –80°C for further experiments.
Selected bacterial strains were cultured in tryptic soybean broth (TSB) medium at 28°C and 200 rpm for 12 h (approximately 3 × 108–5 × 108 CFU/ml) and washed twice with sterile DF salts minimal medium. The harvested cells were transferred to the DF salt minimal medium containing 3 mM ACC [instead of (NH4)2SO4] and cultured at 28°C and 200 rpm for 24 h to induce the production of ACC deaminase. The bacterial culture was collected to determine the ACC deaminase activity by measuring the production of α-KB (the cleavage product of ACC) according Penrose and Glick (2003). Total protein concentrations were determined using a Bradford protein assay kit (Tiangen, Beijing, China). The ACC deaminase activity was expressed as the amount of α-KB produced per milligram of total protein per hour. The bacterial strain displaying the highest ACC deaminase activity was named JW-CZ2 and subjected to further investigations.
Genomic DNA of JW-CZ2 was extracted using a Genomic DNA Extraction Kit (Sangon Biotech, Shanghai, China) following the manufacturer’s protocol. The universal forward primer (5′-CAGAGTTTGATCCTGGCT-3′) and reverse primer (5′-AGGAGGTGATCCAGCCGCA-3′) were used to amplify the 16S rDNA gene. PCR cycling was performed under the following conditions: 95°C for 5 min, 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 90 s, with the final extension at 72°C for 8 min. The PCR product was sequenced by the Shanghai Sangon Biotech Co. Ltd. (Shanghai, China), and the data were deposited in the GenBank with the access number KP903465.
The 16S sequence was blasted against the GenBank database using the NCBI-BLASTn tool for species identification. Next, a phylogenic tree was constructed using the neighbor-joining method using MEGA 11 software with 1,000 bootstraps.
C. sinensis seeds were obtained from a farm located in Henan Province, China. The seeds were surface-sterilized in 70% ethanol for 5 min, washed three times using sterile water, immersed in 0.5% NaClO solution for 10 min, and further washed five times in sterile water. One seed was sown in each plot (6 cm diameter, 12 cm height) filled with 500 g of acid-washed and autoclaved vermiculite (granule diameter 0.1–0.5 mm). The seeds were germinated, and then the seedlings were cultured in a plant growth chamber at 25 ± 2°C and 60–80% relative humidity. The photoperiod was 14 h:10 h with the light intensity of 4,000 lx. During the culture period, the seedlings were irrigated with sterile distilled water appropriately. After cultivation for 90 days, seedlings of similar shoot height and stem diameter were selected for inoculation of JW-CZ2.
JW-CZ2 strain was cultured in Luria–Bertani (LB) medium at 30°C and 200 rpm for 48 h. After centrifugation at 6,757 rpm for 10 min at 4°C, the cell pellets were washed and re-suspended in sterile saline with a bacterial density of approximately 3–5 × 108 CFU/ml. For each seedling, 5 ml of bacterial suspension was inoculated using the root-drenching method (Ge et al., 2004), and the control seedlings received an equal volume of sterile saline. The treatment and the control group were repeated eight times independently. Afterward, plants were cultured in the growth chamber under the same condition for 180 days. Finally, seedlings were collected carefully from the pots and washed with tap water to remove soil particles. The shoot length and stem diameter were measured. After drying at 60°C until constant dry weight, the dry weights were determined.
Six plant pathogens were selected for the tests, including Fusarium oxysporum f. sp. cubense (stored in Nanjing Agricultural University, China), Diaporthe biconispora, Rhizoctonia solani, Fusarium oxysporum, Phomopsis macrospore, and Sphaeropsis sapinea (stored in the Laboratory of Forest Pathology, Nanjing Forestry University, China). The growth-inhibition effects of JW-CZ2 on these pathogens were determined using the plate-confrontation method (Xie and Wu, 2021). First, a 5 mm hole was punched on the center of potato dextrose agar (PDA) agar plates using a sterile perforator. Second, the pathogens were inoculated in these holes, and JW-CZ2 was inoculated as streaks at 3 cm from the center of the plate. After being cultured at 28°C until the pathogens covered the whole dish in the control group, the width of inhibition zone in the experimental groups was measured using a vernier caliper. The width of inhibition zone is defined as the distance between the edge of the pathogen colony and the edge of the JW-CZ2 colony (mm). Each treatment was repeated four times.
To test the effects of single inducing factors on ACC deaminase activity, JW-CZ2 was cultured in 250 ml flasks containing 50 ml media. (1) JW-CZ2 was inoculated into DF salt minimal medium containing different ACC concentrations (0, 0.3, 3, 30, 300, and 3,000 μM) and then cultured at 28°C for 24 h; (2) JW-CZ2 was inoculated into DF salt minimal medium containing 3 mM ACC and then cultured at 20°C, 25°C, 28°C, 30°C, and 35°C for 24 h; (3) JW-CZ2 was inoculated into DF salt minimal medium containing 3 mM ACC at pH 6.5, 7.0, 7.5, 8.0, and 8.5 and then cultured at 28°C for 24 h; (4) JW-CZ2 was inoculated into DF salt minimal medium containing 3 mM ACC and then cultured at 28°C for 4, 8, 12, 16, 20, 24, 28, 36, and 40 h. At each time point or in each treatment, an aliquot was sampled from each flask to determine the ACC activity as described above. All experiments were performed in triplicates.
To further explore the mechanisms underlying the tea plant growth-promoting effects of JW-CZ2, the whole genome of JW-CZ2 was sequenced. Briefly, the genomic DNA of JW-CZ2 was isolated using the Wizard genomic DNA purification kit (Promega, Madison, WI). DNA quality and concentration were evaluated using the NanoDrop (Thermo Fisher Scientific, Wilmington, DE) and Qubit assay (Thermo Fisher Scientific, Wilmington, DE). The sequencing library was prepared using the SMRTbell Express Template Prep Kit 2.0 and then sequenced on a PacBio RS II sequencer following the manufacturer’s protocol (Pacific Biosciences, Menlo Park, CA, United States). Two single-molecule real-time (SMRT) cells were performed with a 120-min movie time per SMRT cell (Eid et al., 2009; Faino et al., 2015). SMRT Analysis portal version 2.1 was used for read filtering and adapter trimming with the default parameters, and 400 Mb of postfiltered data (approximately 80 × coverage) with an average read length of 7 kb were used for genome assembly. The genome was then de novo assembled using the Hierarchical Genome Assembly Process V3 (HGAP) incorporated in the SMRT Analysis package version 2.1 (Chin et al., 2013).
The gene structure of JW-CZ2 genome was predicted using the Glimmer Version 3.02 software, and the rRNA sequences were obtained using RNAmmer Version 1.2. To predict the biological functions, all genes were annotated by blasting against Non-Redundant (NR), Swiss-Prot, COG (clusters of orthologous groups of proteins), and KEGG (Kyoto Encyclopedia of Genes and Genomes) databases using the RAST annotation system (Tatusov et al., 2000; Moriya et al., 2007; Aziz et al., 2008; Overbeek et al., 2014; Brettin et al., 2015) with the-value cutoff of 1E–5.
JW-CZ2 was inoculated in National Botanical Research Institute’s Phosphate (NBRIP) medium containing 0.5% Ca3(PO4)2, FePO4, or Mg3(PO4)2 as sole phosphate source. After incubation at 28°C and 180 rpm for 72 h, the culture liquid was centrifuged at 10,000 rpm at 4°C for 10 min, and the concentrations of soluble phosphate in the supernatant were determined using the molybdenum blue method following Watanabe and Olsen (1965).
JW-CZ2 was inoculated in King’s broth with or without 100 mg/L tryptophan and then cultured at 28°C and 200 rpm for 3 days. Each assay included 50 ml of culture media and was repeated three times. Next, the OD600 values of culture media were determined to evaluate cell densities. After centrifugation at 11,261 rpm for 10 min, 50 μl of supernatant was used to colorimetrically determine the production of IAA using the Salkowsky’s reagent (2% of 0.5 M FeCl3 in 35% HClO4 solution) (Benizri et al., 1998; Rahman et al., 2010). The data were normalized by setting OD600 as 1.
To determine the production of siderophore, 1 μl of bacterial culture grown overnight in King’s broth was spotted on Chrome Azurol S agar plates (Ames-Gottfred et al., 1989). After incubation for 48 h at 28°C, the appearance of plates was observed by eye. The formation of an orange halo around bacterial colonies is a sign of siderophore production (Ali et al., 2014).
All data were analyzed using the SPSS 19.0 software for Windows (IBM Corporation, United States). Results are expressed as mean values ± standard deviation (SD). Effects of JW-CZ2 on the growth parameters of tea plants were compared between treatment and control using the Student’s t-test. The statistical significance between treatments and control in other experiments was assessed by one-way analysis of variance and Turkey’s test. p < 0.05 was considered statistically significant.
Since ACC deaminase was first isolated from Pseudomonas sp. strain ACP in 1978 (Honma and Shimomura, 1978), the production of ACC deaminase became a key trait for screening PGPR that facilitate plant growth under normal and/or adverse environmental conditions (Glick, 1995; Chandra et al., 2018; Gupta and Pandey, 2019). In this study, from the rhizosphere soils of tea plants, seven bacterial strains displayed significant ACC deaminase activity, which were named JW-WH8, JW-CZ2, JW-XC1, JW-LA10, JW-AQ7, JW-HS2, and JW-HS3. Among them, JW-CZ2 revealed the highest ACC deaminase activity (1,077.94 nmol α-KB mg–1 h–1), followed by JW-XC1 (922.65 nmol α-KB mg–1 h–1) and JW-AQ10 (813.70 nmol α-KB mg–1 h–1) (Figure 1). The strain JW-CZ2 was isolated from Qingyang County, Chizhou City, Anhui Province, China. The higher the ACC deaminase activity displays, the more valuable the strain is. Thus, we chose JW-CZ2 for further investigations.
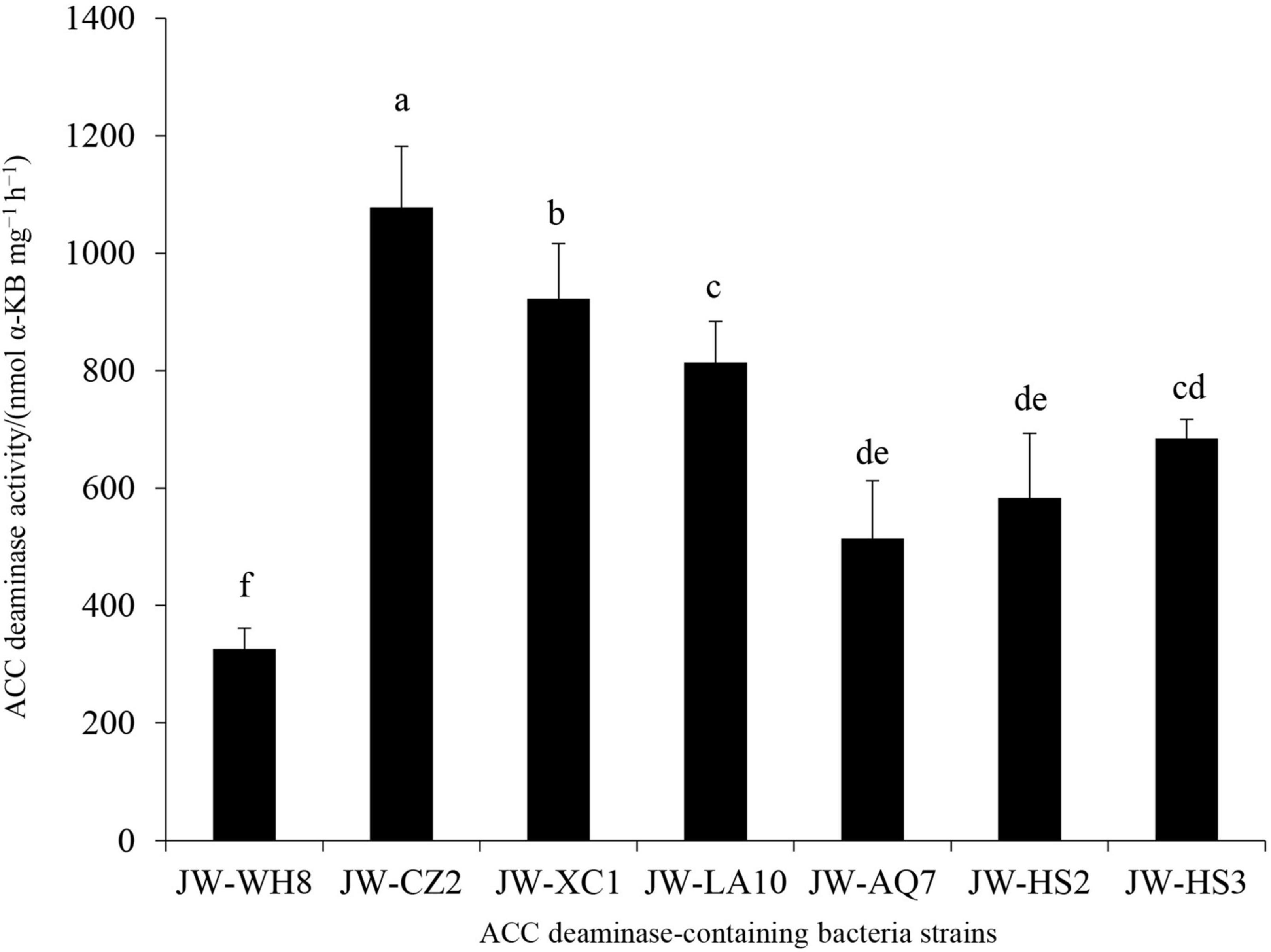
Figure 1. ACC deaminase activities of seven isolates from rhizosphere soil of C. sinensis. Data represent mean and SD (n = 3). Different letters indicate statistically significant differences (p < 0.05).
Blasting against the GenBank database showed that the 1,503 bp partial 16S rDNA gene of JW-CZ2 was highly consistent to that of Serratia marcescens (KY379045) with an identity percentage of 99.93%. The phylogenetic tree also revealed that JW-CZ2 was clustered with two other S. marcescens strains (Figure 2). Thus, these results indicated that JW-CZ2 was a S. marcescens strain.
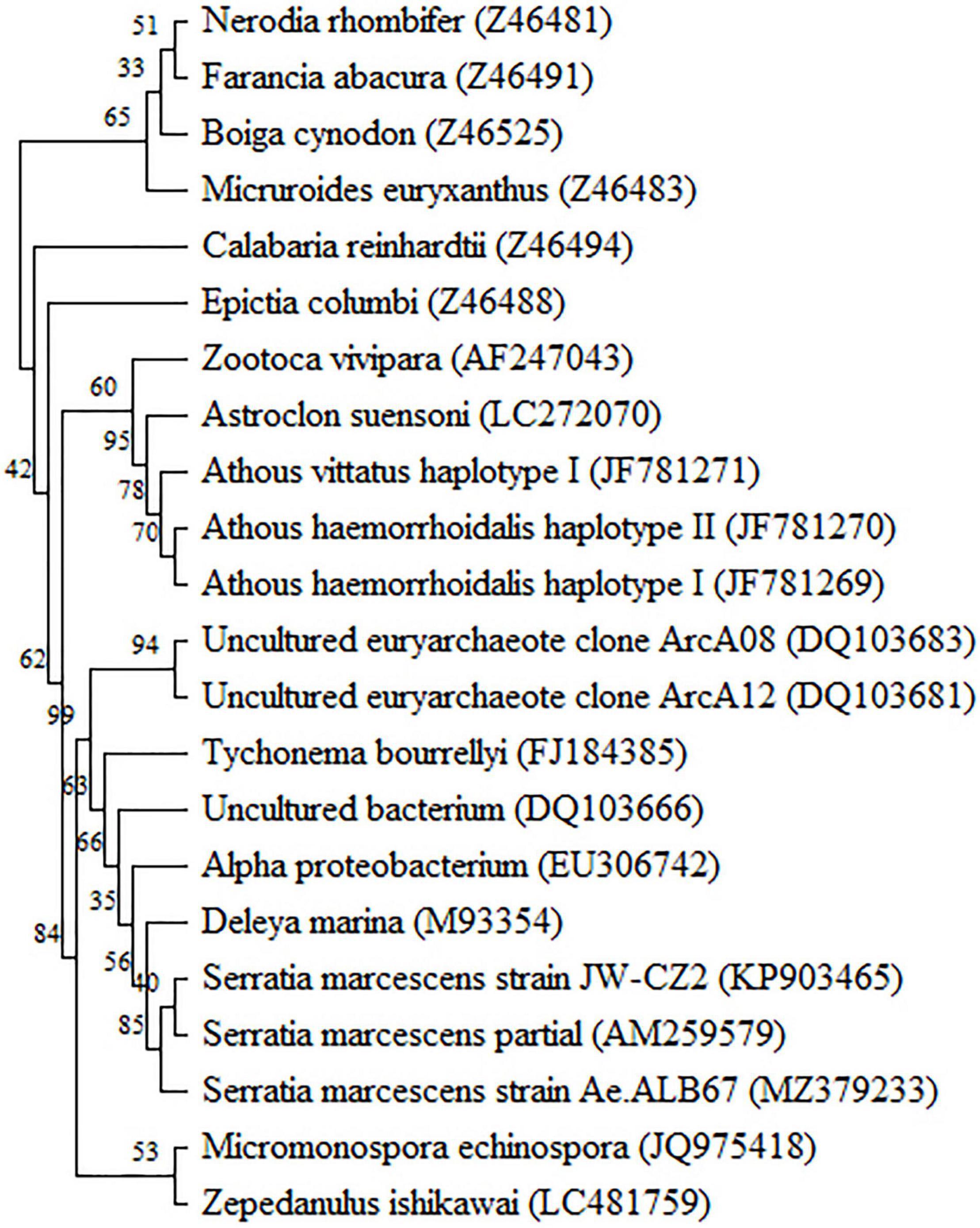
Figure 2. Phylogenetic tree based on the 16S rRNA sequences of J W-CZ2 and other bacterial strains. The GenBank accession numbers for nucleotide sequence data are shown at the beginning of each species.
In the present study, JW-CZ2 displayed a high ACC deaminase activity. Inoculation with JW-CZ2 for 180 days significantly increased shoot height, stem diameter, and dry weight of tea plants, with increasing rates of 53.44, 11.37, and 29.34%, respectively, in comparison to the control, indicating significant growth-promoting effects of S. marcescens JW-CZ2 on tea plants (Table 1). Similarly, Dhar Purkayastha et al. (2018) isolated S. marcescens strain ETR17 from the tea gardens in West Bengal and Assam, which also displayed promotive effects on tea plants. Forty-five days after inoculation, shoot length and root length increased approximately 20 and 32% compared to the control, respectively, which were lower than those of JW-CZ2 in the present study. Moreover, Chakraborty et al. (2013) reported the promotive effects of S. marcescens on tea plant growth with an increasing rate of approximately 50% for seedling height. Although other strains of S. marcescens have been reported, JW-CZ2 is a native strain to the Yangtze River region and might show better adaptability to the local environment. Taking these results together, S. marcescens is a plant growth-promoting bacterium (PGPB) to tea plants and displayed applicable values for tea plantation.
The major mechanism underlying the promotion of PGPR with ACC deaminase activity on plant growth is the repression of ethylene production in plants (Penrose and Glick, 2003). ACC deaminase is an inducible enzyme. To further explore the applicable potential of JW-CZ2 as a biofertilizer, the effects of inducing time, ACC concentration, pH, and temperature on ACC deaminase activity were investigated (Figure 3).
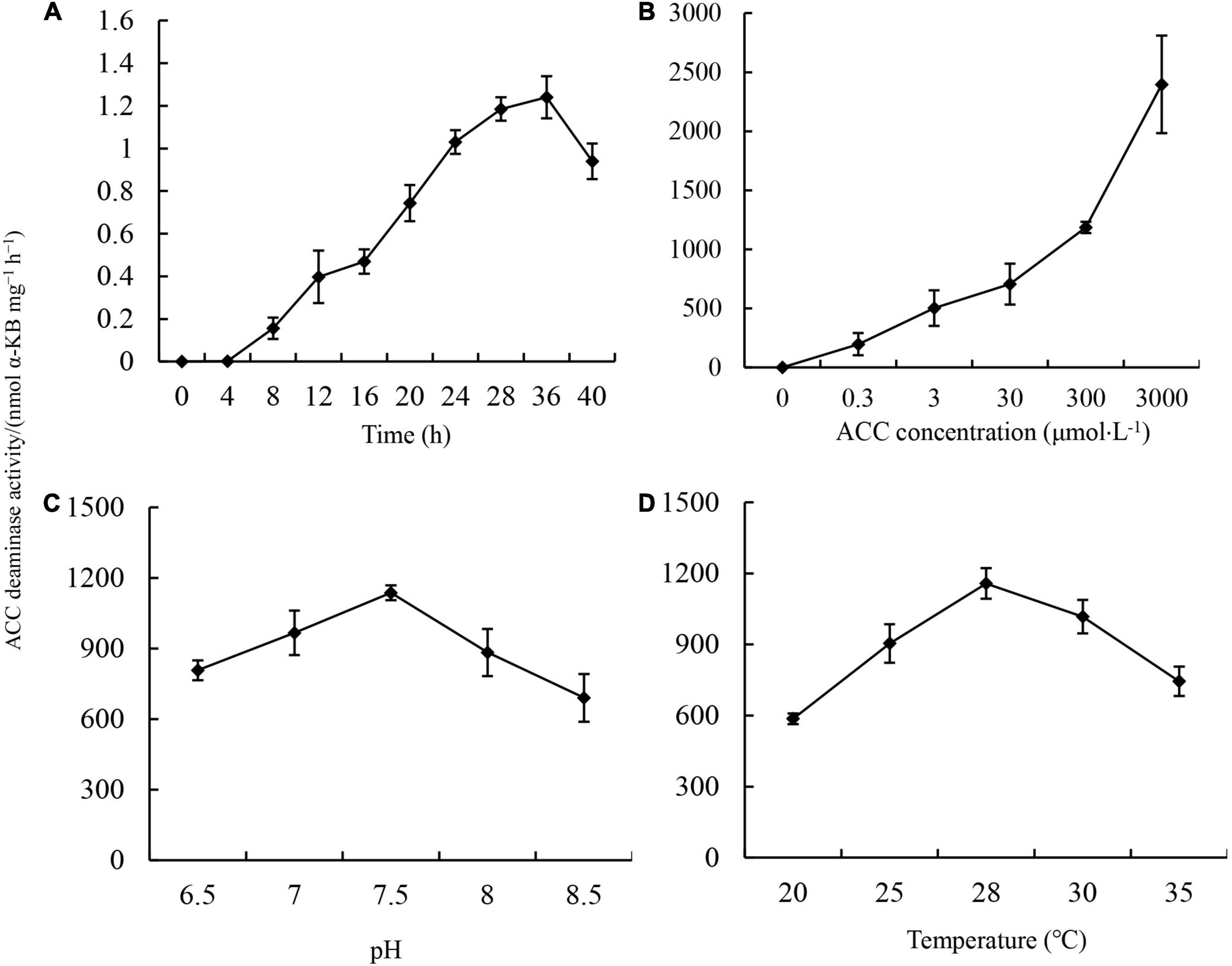
Figure 3. Effects of inducing time (A), ACC concentration (B), pH (C), and inducing temperature (D) on ACC deaminase activity in S. marcescens JW-CZ2. Data indicate mean and standard deviation (n = 3). Different letters indicate significant differences (p < 0.05).
In the absence of ACC, ACC deaminase activity was almost not detected in the JW-CZ2. However, after addition of 3 mM ACC, ACC deaminase activity increased from 4 to 36 h and then started to decrease at 40 h. The highest activity was 1,240.38 nmol α-KB mg–1 h–1, which was observed at 36 h (Figure 3A). With increasing ACC concentrations from 0.3 to 3,000 μM in culture media, ACC activity increased greatly and significantly, suggesting that JW-CZ2 could adapt to various concentrations of ACC (Figure 3B). JW-CZ2 showed significant ACC deaminase activity from pH 6.5 to 8.5 and with the highest ACC activity at pH 7.5 (Figure 3C). From 20 to 35°C, JW-CZ2 functioned well to produce ACC deaminase, and the maximum activity was observed at 28°C (Figure 3D). Similarly, in vitro expressed ACC deaminase from Enterobacter cloacae and Enterobacter cancerogenus also revealed activity at pH ranging from 5.5 to 8.5 and temperature ranging from 4 to 50°C, with the highest ACC activity at pH 7.5 and at 28°C (Jha et al., 2012). The bacterial strain XG32 revealed the highest ACC activity at 25°C, pH from 7.0 to 8.0, and inducing time of 24 h (Shen et al., 2008). Overall, these data indicated that JW-CZ2 could grow well and produce ACC deaminase activity at conditions with a broad range of ACC concentration, pH, and temperature, which increased the applicable potential of JW-CZ2 in fields as a biofertilizer.
The genome of JW-CZ2 consisted of 4,925,622 bp with Guanine-Cytosine (GC) content of 59.82%. The DNA coding sequences were 4,268,136 bp in total, which contained approximately 4,670 coding sequences (CDS), including 4,547 proteins, 96 tRNA genes, and 22 rRNA genes. Among the CDS, 4,197 (89.83%) were successfully annotated to at least one database, while 475 (10.17%) were unknown (Table 2). On the other hand, Khan et al. (2017) reported that the genome of S. marcescens RSC-14 was 5.12 Mbp in length and included 4,593 CDS, 22 rRNA, and 88 tRNA genes. Obviously, the strain JW-CZ2 identified in the present study was different from RSC-14.
Blasting against the COG database revealed that 4,274 of the 4,670 predicted CDS (91.52%) could match to at least one COG category. Among them, the top four were metabolism of amino acid transport and metabolism (9.54%), transcription (9.52%), carbohydrate transport and metabolism (7.81%), and inorganic ion transport and metabolism (7.37%). In addition, 27.94% of the predicted CDS were still functionally unknown (Figure 4).
Characterization of KEGG pathways showed that 2,937 genes (62.89% of all CDS) were involved in at least one pathway (Figure 5). Among them, the top four pathways were carbohydrate metabolism, membrane transport, amino acid metabolism, and global and overview maps, in total representing 23.62% of the predicted CDS. In addition, metabolism of cofactors and vitamins, energy metabolism, and signal transduction included 4.11, 3.34, and 3.28% of CDS, respectively.
The protein sequence of ACC deaminase (GenBank access number: WP_195315725.1) was used to blast against the genome of JW-CZ2, and the most similar sequence was D-cysteine desulfhydrase (SC_2041, Table 3). Todorovic and Glick (2008) reported that ACC deaminase and D-cysteine desulfhydrase could interconvert after directed mutagenesis. Thus, we speculated that D-cysteine desulfhydrase in JW-CZ2 might function as an ACC deaminase.
As reported, genes in relation to phosphate solubilization and IAA production were observed in the genome of S. marcescens RSC-14, which were believed to be major reasons for the plant growth-promoting function of RSC-14 (Khan et al., 2017). In the genome of JW-CZ2, similar results were found. Gluconic acid is an important molecule contributing to dissolving inorganic phosphate (Bagchi et al., 1997; Holguin and Glick, 2001). Gluconic acid synthesis requires pyrroloquinoline quinone (PQQ) as cofactor, which is produced by PQQ biosynthesis proteins (Vessey, 2003). In the present study, four genes encoding PQQ biosynthesis proteins B, C, D, and E (pqqB, pqqC, pqqD, and pqqE) were found in the genome of JW-CZ2 (Table 3). In addition, phosphate transporters are specific proteins responsible for inorganic phosphate uptake (Hoffer et al., 2001). In the present study, six genes encoding four phosphate transporters (pstB, pstA, pstC, and pstS) were observed in the JW-CZ2 genome (Table 3), suggesting an enhancive functional basis of phosphate uptake in JW-CZ2. These results predicted that JW-CZ2 might display a strong capacity of phosphate solubilization and transportation, which might be reasons to explain its plant growth-promoting function. In addition, two genes encoding indolepyruvate decarboxylase (ipdC) were observed in the genome of JW-CZ2 (Table 3), which are key genes for IAA biosynthesis, converting indole-3-pyruvate to indole-3-ethanol (Brandl and Lindow, 1996). These results suggested that JW-CZ2 might be able to synthesize IAA to stimulate plant growth.
Another S. marcescens strain was reported to produce siderophore, which is a typical plant growth-promoting trait (Baruah and Handique, 2014). Ferrochelatase (hemH) is an enzyme to sequestrate iron (Cornelis et al., 2011), and bacterioferritin (bfr) incorporates iron to form siderophore (Expert et al., 2008). In the present study, both hemH and bfr were observed in the JW-CZ2 genome, suggesting its potential to produce siderophore. In comparison, genes associated with siderophore was not reported in the genome of S. marcescens RSC-14 (Overbeek et al., 2014).
Based on the genome sequencing, we speculated that the capacity of phosphate solubilization and production of IAA and siderophore might be potential mechanisms underlying the promotion of S. marcescens JW-CZ2 on tea plant growth. In the present study, after addition of Ca3(PO4)2, FePO4, and Mg3(PO4)2 in the culture media for 72 h, 806.06 ± 24.15, 42.89 ± 3.27, and 48.84 ± 2.96 mg/L soluble phosphate was detected in the culture media (Table 4), demonstrating the phosphate solubilization capacity of JW-CZ2, which was consistent with the prediction from genome sequencing of both S. marcescens JW-CZ2 in the present study and S. marcescens RSC-14 in Khan et al. (2017) and experimental evidence for S. marcescens PH1 and PH2 in Mohamed et al. (2018). In comparison, the phosphate solubilization capacity of JW-CZ2 was higher than that of Bacillus sp. TRSB16, which could solubilize 239 mg/L phosphate in the presence of Ca3(PO4)2 (Banerjee, 2010), but lower than that of S. marcescens GPS-5 mutants [higher than 1,500 mg/L phosphate in the presence of Ca3(PO4)2; Tripura et al., 2007]. P is a limiting element for plants. The phosphate solubilization capacity of S. marcescens JW-CZ2 could convert insoluble inorganic P to soluble status, which should increase P availability and promote plant growth.
After culture in the presence of tryptophan for 3 days, 7.27 ± 0.24 μg/ml IAA was detected in the culture media. In addition, on the Chrome Azurol S agar plate, JW-CZ2 formed an orange halo around the colonies, indicating the production of siderophore (Figure 6). These results were consistent with the genome sequencing of JW-CZ2. Partially similarly, IAA production was observed in S. marcescens AEP5, AEN38, and AEP66 strains, while siderophore was detected in S. marcescens AEP85 strain (Aktan and Soylu, 2020). IAA is a phytohormone and promotes plant growth at certain concentrations. Bacterial siderophore is an important mechanism for iron uptake in plants (Yehuda et al., 1996), which could convert biologically unavailable Fe element to available form adsorbed by plants (Katiyar and Goel, 2004). Thus, production of IAA and siderophore might also contribute to the tea plant-promoting effects of S. marcescens JW-CZ2.
As shown in Table 5 and Figure 7, JW-CZ2 showed significant inhibitory effects on all the six tested plant pathogens, and the inhibitory effect was the most significant on P. macrospore and S. sapinea, with width of inhibition zones of 3.22 ± 0.81 and 3.17 ± 0.98 mm, respectively. P. macrospore is an important pathogenic fungus and displayed significant inhibition to tea plant growth (Ponmurugan and Baby, 2007). S. sapinea causes tip blight and death in Pinus radiata (Corrêa et al., 2012). These results indicated that inhibition by JW-CZ2 of plant pathogenic fungi might be a potential reason to explain its plant growth-promoting effects.
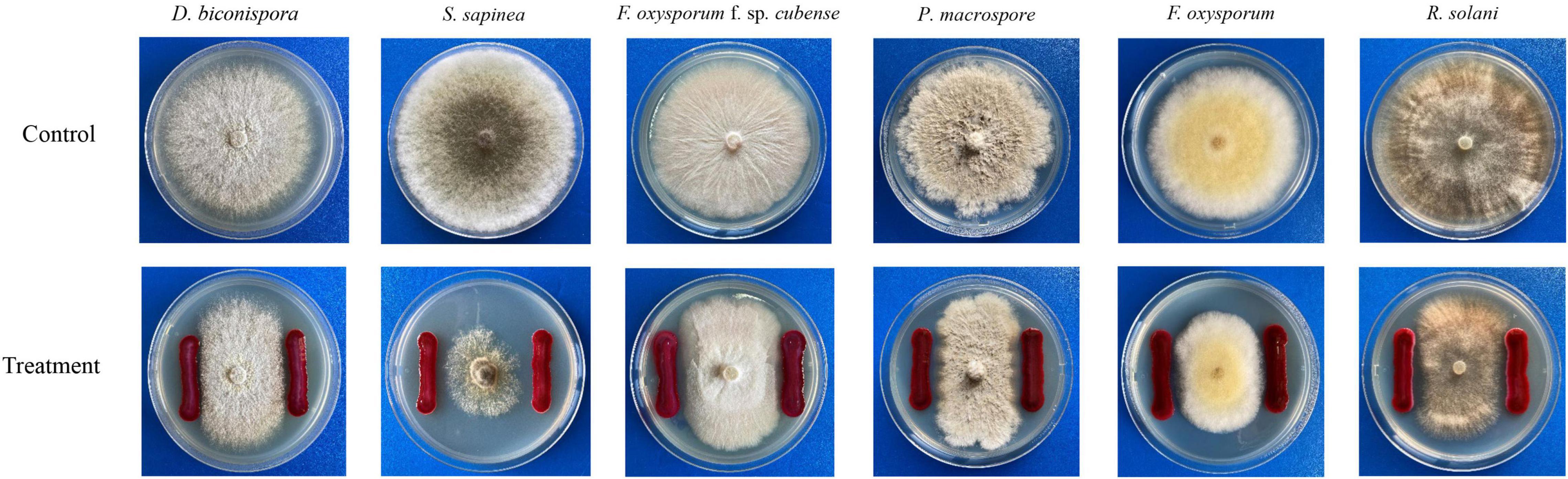
Figure 7. The results of confrontation culture between S. marcescens JW-CZ and six different plant pathogens.
From rhizosphere soils of tea plants, we identified S. marcescens strain JW-CZ2 which displayed high ACC deaminase activities at various ACC concentrations, pH, and temperature. Application of this strain significantly promoted the growth of tea seedlings. Genome sequencing and experimental evidences indicated that JW-CZ2 could produce IAA and siderophore, enhance the solubilization of inorganic phosphate, and inhibit the growth of pathogenic fungi, which all contributed to its promotive functions of plant growth. Overall, S. marcescens JW-CZ2 is a promising tea plant growth-promoting bacterium.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
HL and J-HR designed the experiments. G-HC, J-JS, and SC collected the samples. HL and YF performed the genomic analysis and analyzed the data. HL drafted the manuscript. All authors revised the manuscript.
This work was funded by the Natural Science Foundation of China (31400589) and the Program in the Youth Elite Support Plan in Universities of Anhui Province (gxyq2019006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.792876/full#supplementary-material
Aggangan, N. S., and Anarna, J. A. (2019). Plant growth promoting potential of indigenous mine tailing nitrogen fixing bacteria and commercial biofertilizers on narra (Pterocarpus indicus). Philipp. J. Crop Sci. 44, 39–47.
Ahemad, M., and Kibret, M. (2014). Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J. King Saud Univ. Sci. 26, 1–20. doi: 10.1016/j.jksus.2013.05.001
Aktan, Z. C., and Soylu, S. (2020). Prevalence and characterization of plant growth promoting mechanisms of endophytic and epiphytic bacterial species isolated from almond trees growing in Diyarbakır province of turkey. Ksu. J. Agric. Nat. 23, 641–654.
Ali, S. Z., Sandhya, V., and Venkateswar Rao, L. (2014). Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann. Microbiol. 64, 493–502. doi: 10.1007/s13213-013-0680-3
Ames-Gottfred, N. P., Christie, B. R., and Jordan, D. C. (1989). Use of the chrome azurol s agar plate technique to differentiate strains and field isolates of rhizobium leguminosarum biovar trifolii. Appl. Environ. Microbiol. 55, 707–710. doi: 10.1128/aem.55.3.707-710.1989
Aziz, R. K., Bartels, D., Best, A. A., DeJongh, M., Disz, T., Edwards, R. A., et al. (2008). The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75
Bagchi, D., Vuchetich, P. J., Bagchi, M., Hassoun, E. A., Tran, M. X., Tang, L., et al. (1997). Induction of oxidative stress by chronic administration of sodium dichromate [chromium VI] and cadmium chloride [cadmium II] to rats. Free Radic. Biol. Med. 22, 471–478. doi: 10.1016/S0891-5849(96)00352-8
Banerjee, S. (2010). Stress induced phosphate solubilization by Arthrobacter sp. and Bacillus sp. isolated from tomato rhizosphere. Aust. J. Crop Sci. 4, 378–383.
Baruah, D. K., and Handique, P. J. (2014). Estimation of the efficiency of siderophore production in pathogenic bacteria Bacillus anthracis and Serratia marcescens. South Asian J. Exp. Biol. 4, 91–95. doi: 10.38150/sajeb.4(3).p91-95
Bashan, Y., and de-Bashan, L. E. (2010). “Chapter two - how the plant growth-promoting bacterium azospirillum promotes plant growth—a critical assessment,” in Advances in Agronomy, ed. D. L. Sparks (Cambridge: Academic Press), 77–136. doi: 10.1016/S0065-2113(10)08002-8
Benizri, E., Courtade, A., Picard, C., and Guckert, A. (1998). Role of maize root exudates in the production of auxins by Pseudomonas fluorescens M.3.1. Soil Biol. Biochem. 30, 1481–1484. doi: 10.1016/S0038-0717(98)00006-6
Bhattacharyya, P. N., and Jha, D. K. (2012). Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 28, 1327–1350. doi: 10.1007/s11274-011-0979-9
Brandl, M. T., and Lindow, S. E. (1996). Cloning and characterization of a locus encoding an indolepyruvate decarboxylase involved in indole-3-acetic acid synthesis in Erwinia herbicola. Appl. Environ. Microbiol. 62, 4121–4128. doi: 10.1128/aem.62.11.4121-4128.1996
Brettin, T., Davis, J. J., Disz, T., Edwards, R. A., Gerdes, S., Olsen, G. J., et al. (2015). RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 5:8365. doi: 10.1038/srep08365
Cabrefiga, J., Bonaterra, A., and Montesinos, E. (2007). Mechanisms of antagonism of Pseudomonas fluorescens EPS62e against Erwinia amylovora, the causal agent of fire blight. Int. Microbiol. 10, 123–132.
Calvo, P., Watts, D. B., Kloepper, J. W., and Torbert, H. A. (2017). Effect of microbial-based inoculants on nutrient concentrations and early root morphology of corn (Zea mays). J. Plant Nutr. Soil Sci. 180, 56–70. doi: 10.1002/jpln.201500616
Chahboune, R., Barrijal, S., Moreno, S., and Bedmar, E. J. (2011). Characterization of Bradyrhizobium species isolated from root nodules of Cytisus villosus grown in Morocco. Syst. Appl. Microbiol. 34, 440–445. doi: 10.1016/j.syapm.2011.04.008
Chakraborty, U., Chakraborty, B. N., Chakraborty, A. P., Sunar, K., and Dey, P. (2013). Plant growth promoting rhizobacteria mediated improvement of health status of tea plants. Indian J. Biotechnol. 12, 20–31.
Chandra, D., Srivastava, R., Glick, B. R., and Sharma, A. K. (2018). Drought-Tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana (L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere 28, 227–240. doi: 10.1016/S1002-0160(18)60013-X
Chin, C. S., Alexander, D. H., Marks, P., Klammer, A. A., Drake, J., Heiner, C., et al. (2013). Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 10, 563–569. doi: 10.1038/nmeth.2474
Chowdhury, S. P., Dietel, K., Rändler, M., Schmid, M., Junge, H., Borriss, R., et al. (2013). Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS One 8:e68818. doi: 10.1371/journal.pone.0068818
Cornelis, P., Wei, Q., Andrews, S. C., and Vinckx, T. (2011). Iron homeostasis and management of oxidative stress response in bacteria. Metallomics 3, 540–549. doi: 10.1039/c1mt00022e
Corrêa, P. R. R., Auer, C. G., dos Santos, A. F., and Higa, A. R. (2012). Early selection of Pinus radiata progenies to Sphaeropsis sapinea. Cienc. Florest. 22, 275–281. doi: 10.5902/198050985734
Dhar Purkayastha, G., Mangar, P., Saha, A., and Saha, D. (2018). Evaluation of the biocontrol efficacy of a Serratia marcescens strain indigenous to tea rhizosphere for the management of root rot disease in tea. PLoS One 13:e0191761. doi: 10.1371/journal.pone.0191761
Dworkin, M., and Foster, J. W. (1958). Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 75, 592–603. doi: 10.1128/jb.75.5.592-603.1958
Eid, J., Fehr, A., Gray, J., Luong, K., Lyle, J., Otto, G., et al. (2009). Real-time DNA sequencing from single polymerase molecules. Science 323, 133–138. doi: 10.1126/science.1162986
Expert, D., Boughammoura, A., and Franza, T. (2008). Siderophore-controlled iron assimilation in the enterobacterium Erwinia chrysanthemi: evidence for the involvement of bacterioferritin and the Suf iron-sulfur cluster assembly machinery. J. Biol. Chem. 283, 36564–36572. doi: 10.1074/jbc.M807749200
Faino, L., Seidl, M. F., Datema, E., van den Berg, G. C., Janssen, A., Wittenberg, A. H., et al. (2015). Single-molecule real-time sequencing combined with optical mapping yields completely finished fungal genome. mBio 6:e936-15. doi: 10.1128/mBio.00936-15
Figueiredo, M. D. V. B., Bonifacio, A., Rodrigues, A. C., and de Araujo, F. F. (2016). “Plant growth-promoting rhizobacteria: key mechanisms of action,” in Microbial-mediated Induced Systemic Resistance in Plants, eds D. K. Choudhary and A. Varma (Singapore: Springer), 23–37. doi: 10.1007/978-981-10-0388-2_3
Fischer, S. E., Fischer, S. I., Magris, S., and Mori, G. B. (2007). Isolation and characterization of bacteria from the rhizosphere of wheat. World J. Microbiol. Biotechnol. 23, 895–903. doi: 10.1007/s11274-006-9312-4
Ge, H. L., Guo, J. H., Qi, H. Y., Guo, Y., and Huang, Y. (2004). Biological control of Capsicum bacterial wilt by compound bacterial mixture AR99. Acta Phytopathol. Sin. 34, 162–165.
Glick, B. R. (1995). The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 41, 109–117. doi: 10.1139/m95-015
Glick, B. R. (2014). Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 169, 30–39. doi: 10.1016/j.micres.2013.09.009
Gou, J. Y., Suo, S. Z., Shao, K. Z., Zhao, Q., Yao, D., Li, H. P., et al. (2020). Biofertilizers with beneficial rhizobacteria improved plant growth and yield in chili (Capsicum annuum L.). World J. Microbiol. Biotechnol. 36:86. doi: 10.1007/s11274-020-02863-w
Gupta, S., and Pandey, S. (2019). ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in french bean (Phaseolus vulgaris) plants. Front. Microbiol. 10:1506. doi: 10.3389/fmicb.2019.01506
Han, Q. Q., Lü, X. P., Bai, J. P., Qiao, Y., Paré, P. W., Wang, S. M., et al. (2014). Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 5:525. doi: 10.3389/fpls.2014.00525
Hoffer, S. M., Schoondermark, P., van Veen, H. W., and Tommassen, J. (2001). Activation by gene amplification of pitB, encoding a third phosphate transporter of Escherichia coli K-12. J. Bacteriol. 183, 4659–4663. doi: 10.1128/JB.183.15.4659-4663.2001
Holguin, G., and Glick, B. R. (2001). Expression of the ACC deaminase gene from Enterobacter cloacae UW4 in Azospirillum brasilense. Microb. Ecol. 41, 281–288. doi: 10.1007/s002480000040
Honma, M., and Shimomura, T. (1978). Metabolism of 1-Aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 42, 1825–1831. doi: 10.1080/00021369.1978.10863261
Ibarra-Galeana, J. A., Castro-Martínez, C., Fierro-Coronado, R. A., Armenta-Bojórquez, A. D., and Maldonado-Mendoza, I. E. (2017). Characterization of phosphate-solubilizing bacteria exhibiting the potential for growth promotion and phosphorus nutrition improvement in maize (Zea mays L.) in calcareous soils of Sinaloa. Mexico. Ann. Microbiol. 67, 801–811. doi: 10.1007/s13213-017-1308-9
James, E. K., and Baldani, J. I. (2012). The role of biological nitrogen fixation by non-legumes in the sustainable production of food and biofuels. Plant Soil 356, 1–3. doi: 10.1007/s11104-012-1317-1
Jha, C. K., Annapurna, K., and Saraf, M. (2012). Isolation of Rhizobacteria from Jatropha curcas and characterization of produced ACC deaminase. J. Basic Microbiol. 52, 285–295. doi: 10.1002/jobm.201100113
Katiyar, V., and Goel, R. (2004). Siderophore mediated plant growth promotion at low temperature by mutant of fluorescent pseudomonad. Plant Growth Regul. 42, 239–244. doi: 10.1023/B:GROW.0000026477.10681.d2
Khan, A. R., Park, G. S., Asaf, S., Hong, S. J., Jung, B. K., and Shin, J. H. (2017). Complete genome analysis of Serratia marcescens RSC-14: a plant growth-promoting bacterium that alleviates cadmium stress in host plants. PLoS One 12:e0171534. doi: 10.1371/journal.pone.0171534
Li, J., Ovakim, D. H., Charles, T. C., and Glick, B. R. (2000). An ACC deaminase minus mutant of Enterobacter cloacae UW4 no longer promotes root elongation. Curr. Microbiol. 41, 101–105. doi: 10.1007/s002840010101
Liu, S., Mi, X., Zhang, R., An, Y., Zhou, Q., Yang, T., et al. (2019). Integrated analysis of miRNAs and their targets reveals that miR319c/TCP2 regulates apical bud burst in tea plant (Camellia sinensis). Planta 250, 1111–1129. doi: 10.1007/s00425-019-03207-1
Lucy, M., Reed, E., and Glick, B. R. (2004). Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86, 1–25. doi: 10.1023/B:ANTO.0000024903.10757.6e
Mayer, E., de Quadros, P. D., and Fulthorpe, R. (2019). Plantibacter flavus, Curtobacterium herbarum, Paenibacillus taichungensis, and Rhizobium selenitireducens endophytes provide host-specific growth promotion of Arabidopsis thaliana, Basil, Lettuce, and Bok Choy Plants. Appl. Environ. Microbiol. 85:e383-19. doi: 10.1128/AEM.00383-19
Mohamed, E., Farag, A., and Youssef, S. A. (2018). Phosphate solubilization by Bacillus subtilis and Serratia marcescens Isolated from tomato plant Rhizosphere. J. Environ. Prot. 9, 266–277. doi: 10.4236/jep.2018.93018
Moriya, Y., Itoh, M., Okuda, S., Yoshizawa, A. C., and Kanehisa, M. (2007). KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 35, W182–W185. doi: 10.1093/nar/gkm321
Mukhtar, S., Shahid, I., Mehnaz, S., and Malik, K. A. (2017). Assessment of two carrier materials for phosphate solubilizing biofertilizers and their effect on growth of wheat (Triticum aestivum L.). Microbiol. Res. 205, 107–117. doi: 10.1016/j.micres.2017.08.011
Nascimento, F. X., Tavares, M. J., Franck, J., Ali, S., Glick, B. R., and Rossi, M. J. (2019). ACC deaminase plays a major role in Pseudomonas fluorescens YsS6 ability to promote the nodulation of Alpha- and Betaproteobacteria rhizobial strains. Arch. Microbiol. 201, 817–822. doi: 10.1007/s00203-019-01649-5
Numan, M., Bashir, S., Khan, Y., Mumtaz, R., Shinwari, Z. K., Khan, A. L., et al. (2018). Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol. Res. 209, 21–32. doi: 10.1016/j.micres.2018.02.003
Overbeek, R., Olson, R., Pusch, G. D., Olsen, G. J., Davis, J. J., Disz, T., et al. (2014). The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res. 42, D206–D214. doi: 10.1093/nar/gkt1226
Penrose, D. M., and Glick, B. R. (2003). Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant 118, 10–15. doi: 10.1034/j.1399-3054.2003.00086.x
Pérez-Flores, P., Valencia-Cantero, E., Altamirano-Hernández, J., Pelagio-Flores, R., López-Bucio, J., García-Juárez, P., et al. (2017). Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma 254, 2201–2213. doi: 10.1007/s00709-017-1109-9
Ponmurugan, P., and Baby, U. I. (2007). Morphological, physiological and biochemical changes in resistant and susceptible cultivars of tea in relation to phomopsis disease. Plant Pathol. J. 6, 91–94. doi: 10.3923/ppj.2007.91.94
Rahman, A., Sitepu, I. R., Tang, S. Y., and Hashidoko, Y. (2010). Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci. Biotechnol. Biochem. 74, 2202–2208. doi: 10.1271/bbb.100360
Ramakrishna, W., Yadav, R., and Li, K. (2019). Plant growth promoting bacteria in agriculture: two sides of a coin. Appl. Soil Ecol. 138, 10–18. doi: 10.1016/j.apsoil.2019.02.019
Shen, P., Liu, W., Yan, S., and Chen, S. (2008). Culture conditions and character of extracellular enzyme ACC deaminase excreted by bacterium strain XG32. J. Nanjing Normal Univers. 31, 104–108.
Tatusov, R. L., Galperin, M. Y., Natale, D. A., and Koonin, E. V. (2000). The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 28, 33–36. doi: 10.1093/nar/28.1.33
Tiwari, G., Duraivadivel, P., and Sharma, S. H. (2018). 1-Aminocyclopropane-1-carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. by mitigating drought and salt stress. Sci. Rep. 8:17513. doi: 10.1038/s41598-018-35565-3
Todorovic, B., and Glick, B. R. (2008). The interconversion of ACC deaminase and D-cysteine desulfhydrase by directed mutagenesis. Planta 229, 193–205. doi: 10.1007/s00425-008-0820-3
Tripura, C., Sashidhar, B., and Podile, A. R. (2007). Ethyl methanesulfonate mutagenesis-enhanced mineral phosphate solubilization by groundnut-associated Serratia marcescens GPS-5. Curr. Microbiol. 54, 79–84. doi: 10.1007/s00284-005-0334-1
Upadhyaya, H., and Panda, S. K. (2013). Abiotic stress responses in tea [Camellia sinensis L (O) Kuntze]: an overview. Rev. Agric. Sci. 1, 1–10. doi: 10.7831/ras.1.1
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255, 571–586. doi: 10.1023/A:1026037216893
Wang, H., and Han, L. Z. (2019). Identification of four plant growth-promoting rhizobacteria isolated from tea rhizosphere. Microbiol. Chin. 46, 548–562.
Wang, W., Xin, H., Wang, M., Ma, Q., Wang, L., Kaleri, N. A., et al. (2016). Transcriptomic analysis reveals the molecular mechanisms of drought-stress-induced decreases in Camellia sinensis leaf quality. Front. Plant Sci. 7:385. doi: 10.3389/fpls.2016.00385
Watanabe, F. S., and Olsen, S. R. (1965). Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. J. 29, 677–678. doi: 10.2136/sssaj1965.03615995002900060025x
Xie, X., and Wu, X. (2021). Antagonism against forest pathogens and stability of fermentation broth of Burkholderia multivorans WS-FJ9. Microbiol. Chin. 48, 1985–1996.
Yehuda, Z., Shenker, M., Romheld, V., Marschner, H., Hadar, Y., and Chen, Y. (1996). The role of ligand exchange in the uptake of iron from microbial siderophores by gramineous plants. Plant Physiol. 112, 1273–1280. doi: 10.1104/pp.112.3.1273
Keywords: plant growth promoting rhizobacteria, tea plant, ACC deaminase, genome sequencing, Serratia marcescens
Citation: Liu H, Chen G-H, Sun J-J, Chen S, Fang Y and Ren J-H (2022) Isolation, Characterization, and Tea Growth-Promoting Analysis of JW-CZ2, a Bacterium With 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Activity Isolated From the Rhizosphere Soils of Tea Plants. Front. Microbiol. 13:792876. doi: 10.3389/fmicb.2022.792876
Received: 11 October 2021; Accepted: 24 January 2022;
Published: 28 February 2022.
Edited by:
Wanting Ling, Nanjing Agricultural University, ChinaReviewed by:
Anastasia Venieraki, Agricultural University of Athens, GreeceCopyright © 2022 Liu, Chen, Sun, Chen, Fang and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Liu, bGl1aHVpaGs4MkAxNjMuY29t; Jia-Hong Ren,cmVuamlhaG9uZ0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.