- 1Laboratory Medicine, Guangdong Provincial People's Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, Guangdong, China
- 2Centre for Precision Health, School of Medical and Health Sciences, Edith Cowan University, Perth, WA, Australia
- 3Beijing Key Laboratory for Animal Models of Emerging and Reemerging Infectious Diseases, Beijing, China
- 4Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences, Comparative Medicine Center, Peking Union Medical College, Beijing, China
- 5Department of Computer Science and Software Engineering, The University of Western Australia, Perth, WA, Australia
- 6The Marshall Centre for Infectious Diseases Research and Training, The University of Western Australia, Perth, WA, Australia
Editorial on the Research Topic
What does not kill you makes you stronger: Interactions between environmental stresses and microbial virulence
The intriguing sit-and-wait hypothesis predicts that “virulence should be positively correlated with durability in the external environment because high durability reduces the dependence of transmission on host mobility” (Walther and Ewald, 2004) (Figure 1). Since the hypothesis was first proposed in the late 20th century (Ewald, 1983), both theoretical and computational studies have been reporting the relationships between environmental durability and microbial virulence (Sundberg et al., 2014; Wang et al., 2017, 2018, 2019a,b, 2020; Rafaluk-Mohr, 2019; Li et al., 2021). For example, Sundberg et al. (2014) investigated how long-term starvation influences the virulence of the fish pathogen Flavobacterium columnare, which revealed that abiotic selection did not solely facilitate high virulence but diversified virulence of the environmentally transmitting bacterial pathogen. In contrast, Wang et al. (2017) applied computational methods to investigate the relationships between bacterial durability and virulence, which showed that “non-vector-borne pathogens with sit-and-wait potentials have higher number of virulence and durability genes compared with other bacterial groups”, providing theoretically genetic basis for the hypothesis. However, debates have been revolving around the plausibility of the hypothesis due to the lack of direct molecular and genetic evidence (Turner et al., 2021; Pandey et al., 2022). Under this Research Topic, we sought to highlight an exciting set of groundbreaking efforts proposed by frontline investigators, which mainly focused on implementing studies to get an in-depth understanding of the relationships between environmental stress and bacterial virulence. Articles can be fitted into either of the five categories: (1) measures of virulence vs. abiotic stress tolerance; (2) application of sit-and-wait hypothesis to viral, fungal, archaeal or bacterial pathogens; (3) cross kingdom analyses; (4) patterns of abiotic stress tolerance; and (5) computational analysis of the links between abiotic stress tolerance and virulence.
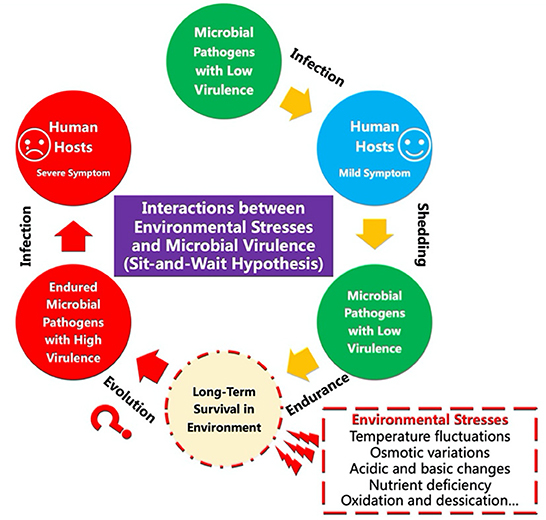
Figure 1. Schematic illustration of the sit-and-wait hypothesis in terms of the potential interactions between environmental stresses and the evolution of microbial virulence.
In particular, a total of four original research articles are enclosed in this Research Topic, which are either about bacterial responses to environmental stresses or about bacterial virulence factors and phenotypes. For example, Liu et al. studied the microevolution of the laboratory strains of Pseudomonas aeruginosa PAO1 attributed to selective media culture or prolonged passage in the laboratory by focusing on two genes, mexT and lasR, which are the two key components of the quorum sensing (QS) circuit. Through introducing these mutations into P. aeruginosa, it was found that they were related with QS, virulence, motility, and biofilm formation, which indicated that stress-induced microevolution could be correlated with bacterial virulence phenotypes, providing genetic basis for the sit-and-wait hypothesis. In the study of the impacts of proton irradiation on gut microbiota and its metabolome characteristics, Li et al. explored the changes of intestinal flora before and after proton irradiation stress by comparing the feces of Balb/c and C57BL/6J mice, which revealed that intestinal microbiome and some metabolites were related to the repair of intestinal injury. Therefore, the influences of the proton irradiation stress on complex systems like gut microbiota are more complex and difficult to establish the relationships between environmental stress and microbial virulence, which requires further investigations. In another P. aeruginosa study by Dela Ahator et al., virulence factor regulator (Vfr) controlling CRISPR-Cas system in response to abiotic stresses e.g., calcium deficiency was identified, which not only facilitated the design of effective phage therapies against P. aeruginosa but also revealed the presence of a regulatory network between virulence and abiotic stress in the bacterial pathogen P. aeruginosa. Lastly, Kuhl et al. sequenced and analyzed a plant-associated bacterium Rhodococcus qingshengii RL1, which revealed its stress tolerance features through the identification of a repertoire of genes enabling it to survive under different abiotic stress conditions. Although the study only revealed the benefits of these stress tolerance during plant-microbe interactions, it established methods for studying microbial stress tolerance and its related genes (Kuhl et al.), which could provide methodological references for studies focusing on the interactions between environmental stress and microbial virulence.
Taken together, through the studies enclosed in this Research Topic, recent progresses in the field of interactions between environmental stress and microbial virulence have been discussed, which provides insights into their complex relationships and facilitates our understanding of the sit-and-wait hypothesis. However, the relationship between environmental stresses and microbial virulence are still under intensive investigations for specific molecular mechanisms due to their extreme complexity on evolutionary time-scales, which will provide better guidance toward management and prevention of infectious diseases in future.
Author contributions
LW drafted the manuscript. LW, L-JZ, and MW revised the draft. LW and MW made substantial contributions to the work through in-depth discussion. LW is a bioinformatician and microbiologist who leads the Theoretical and Experimental Microbiology (TEM) Group as a principal investigator at Laboratory Medicine, Guangdong Provincial People's Hospital (Guangdong Academy of Medical Science), Southern Medical University, Guangdong Province, China. LW is also an adjunct associate professor at Edith Cowan University, Perth, Western Australia, Australia. All authors proposed the Research Topic theme, made a direct and intellectual contribution to the work, and approved the final version of the editorial for publication.
Funding
This study was supported by Xuzhou Key R&D Plan Social Development Project (Grant No. KC22300) and Jiangsu Qinglan Project (2022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ewald, P. W. (1983). Host-parasite relations, vectors, and the evolution of disease severity. Annu. Rev. Ecol. Syst. 14, 465–485. doi: 10.1146/annurev.es.14.110183.002341
Li, F., Xiong, X.-S., Yang, Y.-Y., Wang, J.-J., Wang, M.-M., Tang, J.-W., et al. (2021). Effects of NaCl concentrations on growth patterns, phenotypes associated with virulence, and energy metabolism in Escherichia coli BW25113. Front. Microbiol. 12, 705326. doi: 10.3389/fmicb.2021.705326
Pandey, A., Mideo, N., and Platt, T. G. (2022). Virulence evolution of pathogens that can grow in reservoir environments. Am. Nat. 199, 141–158. doi: 10.1086/717177
Rafaluk-Mohr, C. (2019). The relationship between parasite virulence and environmental persistence: a meta-analysis. Parasitology 146, 897–902. doi: 10.1017/S0031182019000015
Sundberg, L.-R., Kunttu, H. M. T., and Valtonen, E. (2014). Starvation can diversify the population structure and virulence strategies of an environmentally transmitting fish pathogen. BMC Microbiol. 14, 1–6. doi: 10.1186/1471-2180-14-67
Turner, W. C., Kamath, P. L., Van Heerden, H., Huang, Y.-H., Barandongo, Z. R., Bruce, S. A., et al. (2021). The roles of environmental variation and parasite survival in virulence-transmission relationships. R Soc Open Sci. 8, 1–21. doi: 10.1098/rsos.210088
Walther, B. A., and Ewald, P. W. (2004). Pathogen survival in the external environment and the evolution of virulence. Biol. Rev. 79, 849–869. doi: 10.1017/S1464793104006475
Wang, L., Liu, Z., Dai, S., Yan, J., and Wise, M. J. (2017). The sit-and-wait hypothesis in bacterial pathogens: a theoretical study of durability and virulence. Front. Microbiol. 8, 2167. doi: 10.3389/fmicb.2017.02167
Wang, L., Yan, J., Wise, M. J., Liu, Q., Asenso, J., Huang, Y., et al. (2018). Distribution patterns of polyphosphate metabolism pathway and its relationships with bacterial durability and virulence. Front Microbiol. 9, 782. doi: 10.3389/fmicb.2018.00782
Wang, L., Yang, J., Huang, Y., Liu, Q., Xu, Y., Piao, X., et al. (2019a). Systematic analysis of metabolic pathway distributions of bacterial energy reserves. G3 Genes Genomes Genet. 9, 2489–2496. doi: 10.1534/g3.119.400123
Wang, L., Yang, J., Xu, Y., Piao, X., and Lv, J. (2019b). Domain-based comparative analysis of bacterial proteomes: uniqueness, interactions, and the dark matter. Curr. Genom. 20, 115–123. doi: 10.2174/1389202920666190320134438
Keywords: environmental stress, microbial virulence, sit-and-wait hypothesis, durability, transmission
Citation: Wang L, Zhan L-J and Wise MJ (2023) Editorial: What does not kill you makes you stronger: Interactions between environmental stresses and microbial virulence. Front. Microbiol. 13:1127058. doi: 10.3389/fmicb.2022.1127058
Received: 19 December 2022; Accepted: 28 December 2022;
Published: 09 January 2023.
Edited and reviewed by: John R. Battista, Louisiana State University, United States
Copyright © 2023 Wang, Zhan and Wise. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael J. Wise,  bWljaGFlbC53aXNlQHV3YS5lZHUuYXU=; Liang Wang,
bWljaGFlbC53aXNlQHV3YS5lZHUuYXU=; Liang Wang,  d2FuZ2xpYW5nQGdkcGgub3JnLmNu
d2FuZ2xpYW5nQGdkcGgub3JnLmNu
†These authors have contributed equally to this work
 Liang Wang
Liang Wang Ling-Jun Zhan
Ling-Jun Zhan Michael J. Wise
Michael J. Wise