- 1School of Food Science and Engineering, Guangdong Province Key Laboratory for Green Processing of Natural Products and Product Safety, Engineering Research Center of Starch and Vegetable Protein Processing Ministry of Education, South China University of Technology, Guangzhou, China
- 2Research Institute for Food Nutrition and Human Health, Guangzhou, China
- 3Department of Diagnostics, Second Affiliated Hospital of Shantou University Medical College, Shantou, China
- 4State Key Laboratory of Respiratory Diseases, National Clinical Research Center for Respiratory Diseases, National Center for Respiratory Medicine, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Pseudomonas aeruginosa (P. aeruginosa) is a notorious gram-negative pathogenic microorganism, because of several virulence factors, biofilm forming capability, as well as antimicrobial resistance. In addition, the appearance of antibiotic-resistant strains resulting from the misuse and overuse of antibiotics increases morbidity and mortality in immunocompromised patients. However, it has been underestimated as a foodborne pathogen in various food groups for instance water, milk, meat, fruits, and vegetables. Chemical preservatives that are commonly used to suppress the growth of food source microorganisms can cause problems with food safety. For these reasons, finding effective, healthy safer, and natural alternative antimicrobial agents used in food processing is extremely important. In this review, our ultimate goal is to cover recent advances in food safety related to P. aeruginosa including antimicrobial resistance, major virulence factors, and prevention measures. It is worth noting that food spoilage caused by P. aeruginosa should arouse wide concerns of consumers and food supervision department.
1. Introduction of Pseudomonas aeruginosa
Pseudomonas aeruginosa is an aerobic, motile, slightly curved Gram-negative bacterium that is ubiquitous in diverse environments, including air, water, soil, and plant and animal tissues. As a well-known opportunistic human pathogen, P. aeruginosa possesses the ability to cause a whole host of severe acute and chronic life-threatening infections such as meningitis, otitis media, urinary tract infections, and pneumonia, especially in cystic fibrosis individuals. It is in the top three causes of opportunistic infections in humans and annually affects over 2 million patients and kills about 90 thousand each year (Cross et al., 1983). Because of the extracellular enzyme secretion capacity, P. aeruginosa is also a common spoilage bacterium, particularly in higher water content and nutrient-rich foods.
2. Pseudomonas aeruginosa is a leading human pathogen and super-bug
Pseudomonas aeruginosa, a ubiquitous opportunistic pathogen, could cause serious acute and chronic infections in immunodeficient patients, commonly in the bloodstream, urinary tract, respiratory tract, soft tissue, or wound. The pathogenicity of P. aeruginosa stems from diverse virulence factors and remarkable genetic flexibility which allows it to adapt to various habitats and escape host immune defenses (Klockgether and Tümmler, 2017). The emergence of carbapenem-resistant strains of P. aeruginosa is a sanitary health threat (Wang L. et al., 2021; Wang L. J. et al., 2021; Liu et al., 2022c) and a source of extreme concern to the World Health Organization (WHO). In 2007, the number of infections arising from P. aeruginosa, which is multidrug- or carbapenem-resistant, was approximately 23,575 and the number of deaths was approximately 1,573, according to reports from the EU region. And then in 2015, the number of infections increased to over 68,278, and the number of deaths increased to about 4,564 in these areas (Cassini et al., 2019). Therefore, the WHO considers study and exploitation of novel treatments for carbapenem-resistant P. aeruginosa to be of great urgency (Tacconelli and Magrini, 2021).
Bacterial virulence factors include lipopolysaccharide (LPS), adhesins, lectins, flagella, and so on, which contribute to the P. aeruginosa pathogenesis and disrupt signal transduction pathways in host cells. As a physical barrier, LPS causes tissue damage and antibiotic resistance due to its endotoxic activity (Chadha et al., 2022). The polar flagellum responsible for motility in liquid or on solid surfaces is also an important virulence factor. The attachment ability of the flagellum aids initial binding to the cystic fibrosis epithelia and initiates biofilm establishment (Jurado-Martin et al., 2021). The type IV pili play an essential role for P. aeruginosa in adhesion to several cell types, initiation of biofilm formation, and attachment to specific tissues (Barken et al., 2008). Pili is able to control twitching motility which is used for colonization on different cell surfaces. Among the five secretory systems secreting various hydrolytic enzymes and toxins to attack the host, the most important secretion system is type III, which directly injects the virulence factors into host cells and can also destruct the host’s immune system. Four well-known anti-host factors, ExoS (exoenzyme S), ExoT, ExoU, and ExoY are injected via the type III secretion system. ExoS mainly inhibits the function of neutrophils and type I pneumocytes in the early and later infection phase, respectively. 92%–100% of clinical isolates produce ExoT which impedes wound healing and inhibit cell division. ExoU, a phospholipase, can rapidly destroy the membrane of host cells and consequently leads to severe lung injury, proinflammatory response, sepsis, and mortality. ExoY irreversibly causes proapoptotic processes and actin microtubule disassembly (Jurado-Martin et al., 2021). Exotoxin A (ETA), an ADP-ribosyl transferase is the most toxic virulence factor of P. aeruginosa causing necrotizing at the site of colonization and inhibiting host cell protein synthesis. The various extracellular proteolytic enzymes and lipolytic enzymes all secreted by the type II secretion system are also weapons for P. aeruginosa invasion. Elastase A (LasA), a serine protease secreted by P. aeruginosa is proven to be relevant to antibiotic resistance in clinical isolates. The most abundant protease elastase B (LasB) is the major virulence factor. Due to its protein cleavage activity, LasB could interfere with bacterial clearance, disrupt epithelial junctions, and affect biofilm formation (Behzadi et al., 2021). To adapt to the diverse environmental conditions, lipases synergy with other virulence factors is expressed. P. aeruginosa has also been considered to be a “Super-bug,” as a number of antimicrobial resistance determinants and mechanisms have been commonly reported, including formation of biofilm, carriage of plasmids and integrons (Xu et al., 2021c).
3. Pseudomonas aeruginosa is a typical biofilm former
Pseudomonas aeruginosa is a critical biofilm-forming species and is also a paradigm bacterium for the investigation of biofilms. It was reported that 65%–80% of nosocomial infections were related to biofilms. Biofilm is a structure built primarily of autogenous extracellular polymeric substances (EPS) that serves as a scaffold to wrap bacteria on a surface and shield them from external pressures and hinder phagocytosis (Ma et al., 2022). Bacteria within biofilms demonstrate distinct properties from those of planktonic growth. Particularly, bacteria within biofilms are much less susceptible to antibiotics, disinfectants, and host defenses (Xu et al., 2021a). The EPS of P. aeruginosa biofilms matrix attached to a variety of surfaces or tissues consists of proteins, exopolysaccharides (Psl, Pel, and alginate), and extracellular DNA (eDNA) which can be hydrolyzed by DNase I (Swartjes et al., 2013). The process of biofilm formation by P. aeruginosa progresses begins with attachment to the surfaces suitable for growth including medical instruments and food, followed by the formation of microcolonies, and finally, maturation involving the expression of matrix polymers (Sauer et al., 2002). In addition, P. aeruginosa has been often found in polymicrobial interaction, including S. aureus in bacteria and Candida albicans in fungi (Liu J. et al., 2021).
4. Pseudomonas aeruginosa often exists in various food types
Despite being widely known, P. aeruginosa is a leading human opportunistic pathogen, in food safety areas it is an underrecognized microorganism. Due to its high metabolic versatility, rapid reproducible ability, high adaptive capacity, and growth abilities at low temperatures, P. aeruginosa is common worldwide and consequently is a prevalent causative agent of food infection (Gao et al., 2023).
4.1. Water
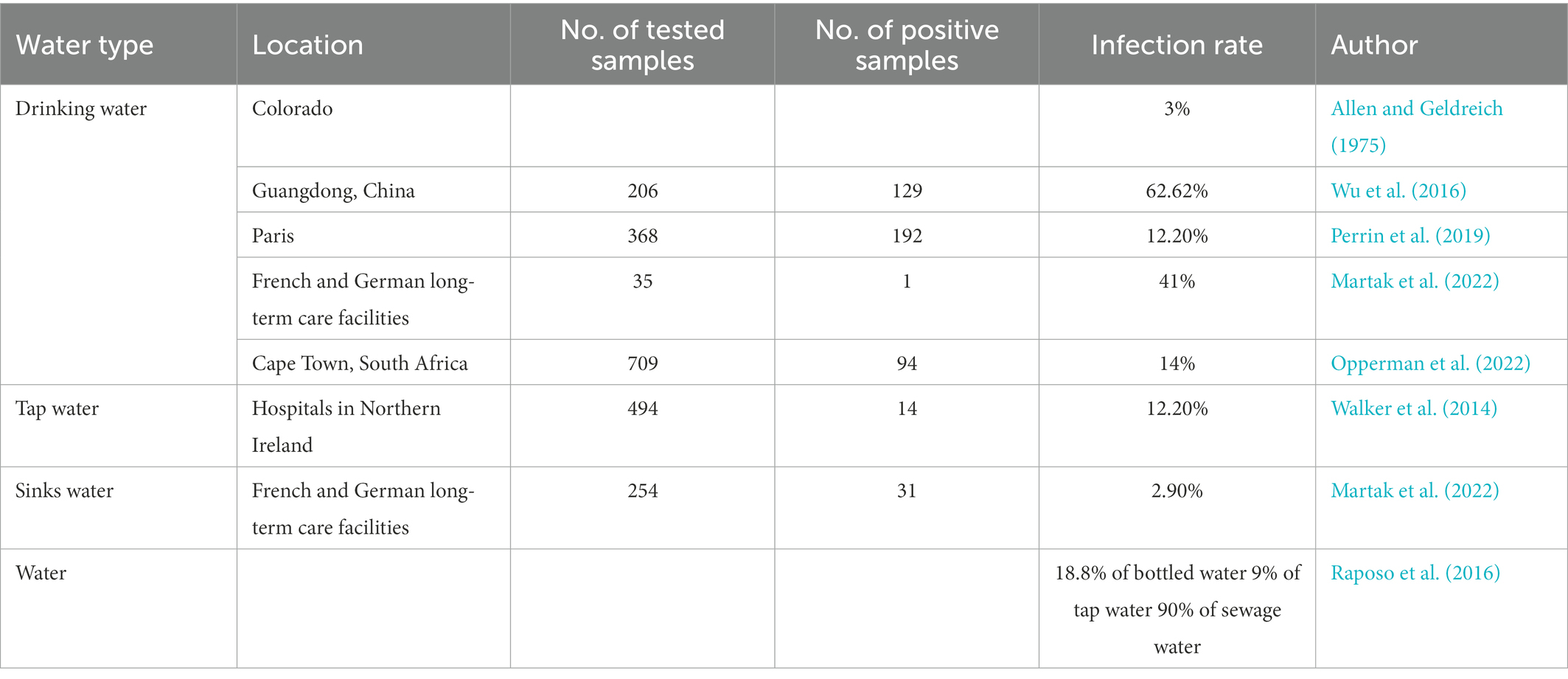
Pseudomonas aeruginosa usually exists in humid environments and is an important source of pollution in drinking water. In China, the presence of P. aeruginosa in a 250 mL sample of potable water is explicitly prohibited. At the same time, the United States, Europe, Japan, Canada, Brazil and the World Health Organization limit the amount of P. aeruginosa in potable water to the maximum possible number is less than 3 CFU/L or no P. aeruginosa can be detected in 250 mL water samples. The pollution of barreled potable water by P. aeruginosa is frequently reported and is gradually becoming the main indicator of unqualified barreled drinking water quality. Due to incomplete disinfection or cross-contamination of source water, water injection tanks, pipelines, filtration in the water production process, reverse osmosis facilities, etc., the recovery of empty barrels, the inadequate cleaning of the empty barrels, and the poor sealing of the lid are all possible causes P. aeruginosa to pollute the bottled water. The pollution of potable water by P. aeruginosa occurs from time to time, and symptoms such as vomiting and diarrhea may occur when drinking water contaminated by P. aeruginosa. Pseudomonas aeruginosa was identified in 3% of drinking water (Allen and Geldreich, 1975), 18.8% of bottled water, 9% of tap water, and 90% of sewage water samples (Raposo et al., 2016). In addition, 206 water samples collected from Guangzhou, China were tested for P. aeruginosa and the results showed 10 for finished water, 52 positive for source water, and 67 for carbonated water (Wu et al., 2016). In Paris, by testing water samples in 4 monomer distribution systems, P. aeruginosa was positive in 52.17% of the samples. Transformation, P. aeruginosa changes, and the isolation rate is relatively high from March to May (Perrin et al., 2019). In Venezuela, the isolation rate of P. aeruginosa is as high as 92.5% in drinking water (Table 1).
4.2. Milk
The abundant nutrients such as protein, fat, carbohydrates, and vitamins make milk susceptible to contamination by a number of microorganisms. Due to the biofilm production ability facilitated it attaches to the wall of milk cooling tanks, milk cans, and pipes for milk delivery, P. aeruginosa is a bacterium that is often separated from milk. Consequently, milk is an efficient route for transmitting pathogens (Chen et al., 2011). Psychrophilic bacterium P. aeruginosa is able to continuously develop its population at the temperature of 4–6°C. The short generation time of less than 4 h at temperature of the 4°C of P. aeruginosa has enabled its number to increase to over 106 CFU/mL in milk after 8 days, although contamination with only a single P. aeruginosa (Meesilp and Mesil, 2019). In Ethiopia, 54 different bacterial species were identified from 107 raw milk and pasteurized milk samples. Specifically, P. aeruginosa (18.5%), Escherichia coli (29.6%), and Klebsiella pneumoniae (16.7%) were detected in milk samples (Garedew et al., 2012). In the Czech Republic, 18 (8.87%) of P. aeruginosa were identified out of 203 samples of fresh milk and two strains (4%) were identified from 50 samples of pasteurized milk during a year. Exposed to pasteurization temperatures (72°C) for 20 s, P. aeruginosa would be devitalized. At refrigerator temperatures, strain cells multiplied by an average of two orders of magnitude (Mickova et al., 1989). Pseudomonas aeruginosa not only exists in cow’s milk but can also be detected in goat milk and camel milk. In pasteurized milk, the isolation rate of P. aeruginosa may also be relatively high, sometimes as high as 40% (Mickova et al., 1989).
4.3. Meat
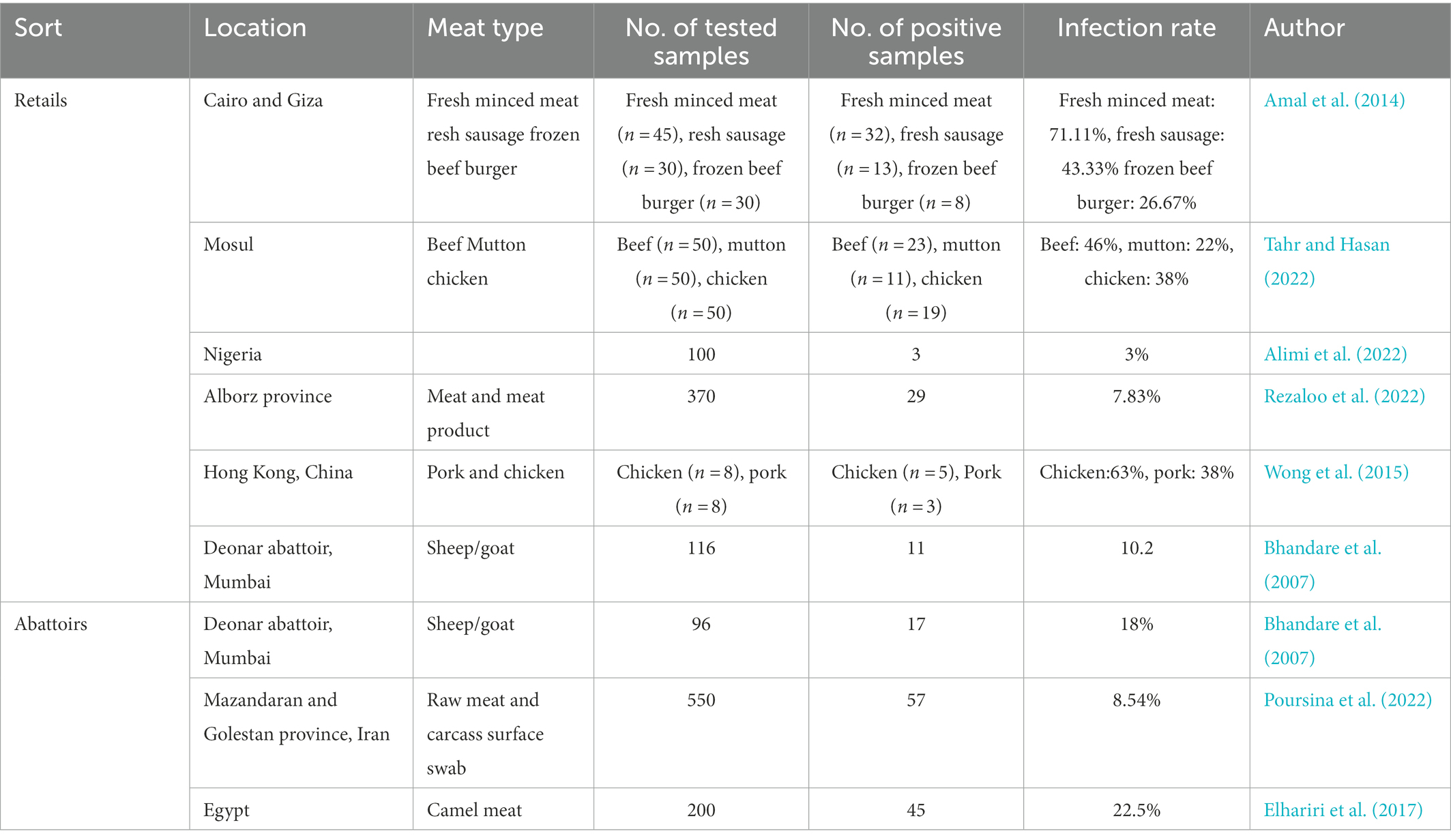
Due to the abilities of proteolytic, lipolytic, and saccharolytic processes, P. aeruginosa dominates and spoils the meat. Fresh meat sold in the market is more likely to be contaminated by P. aeruginosa, which may be related to exposure, handling processes, and cleaning processes using contaminated water (Alimi et al., 2022). One hundred and five retail meat product samples, including fresh minced meat (n = 45), fresh sausage (n = 30), and frozen beef burger samples (n = 30) were collected from Cairo and Giza markets to isolate and identify P. aeruginosa. The examined meat product samples showed that Pseudomonas species were detected in 32 (71.11%), 13 (43.33%) and, 8 (26.67%) with an average count/g of 3.2 × 104, 1.07 × 103, and 2.2 × 103 of the investigated samples, respectively, (Amal et al., 2014). From November to February, all 150 retail meat samples including beef (n = 50), mutton (n = 50), and chicken meat (n = 50) were collected in Mosul city to isolate Pseudomonas species. And the result showed that 53 Pseudomonas strains were isolates from all these meat samples examined (35.33%), including 46% (23) from beef, 22% (11) from lamb, and 38% (19) from chicken (Dong et al., 2022), with an average count/g of 1.47 × 104, 1.92 × 104, 2.13 × 105 cfu/g, respectively (Tahr and Hasan, 2022). The occurrence frequency of P. aeruginosa is 3% from meat and meat product samples (n = 100) in north-central Nigeria (Alimi et al., 2022). In addition, camel meat may be contaminated by P. aeruginosa. In camel meat, the totality of Pseudomonas spp. was isolated at a rate of 10/100, 8/10 for P. aeruginosa, and 2/10 for Pseudomonas fluorescens, respectively. The isolation of P. aeruginosa in meat was not caused by camel infection with P. aeruginosa, because P. aeruginosa tested positive in 45 out of 200 healthy camel meat samples (22.5%; Elhariri et al., 2017; Table 2).
4.4. Fruits and vegetables
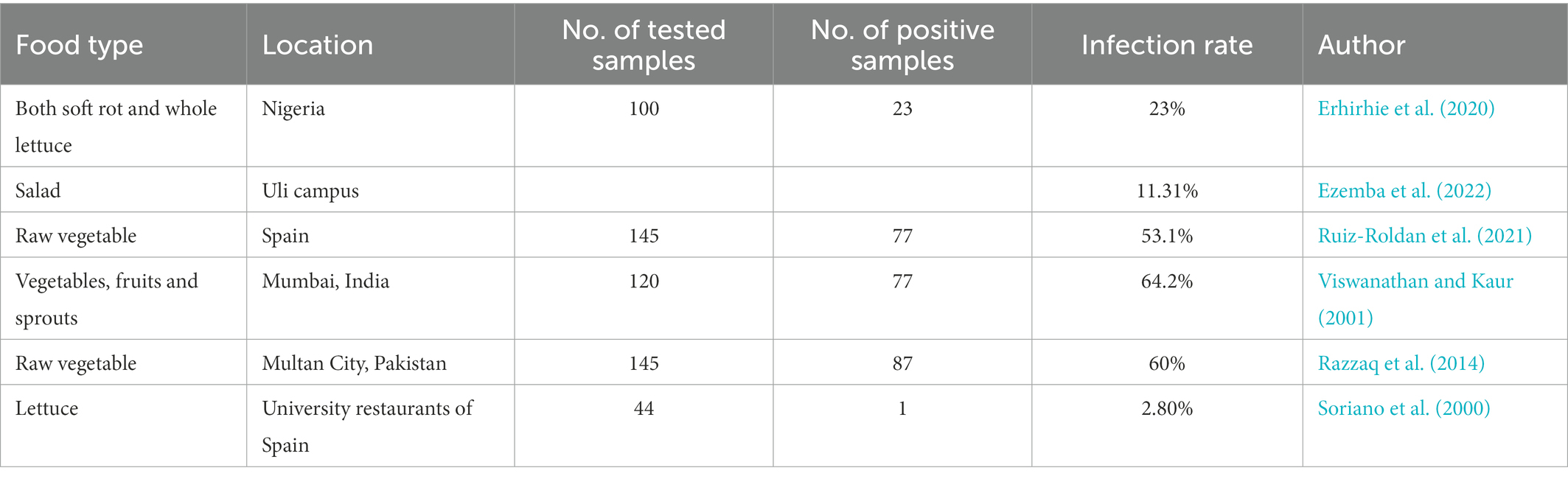
Complex microbial communities also exist in a range of vegetables and fruits besides the foods described above (Li et al., 2020a,b,c,d), and endophytic and exterior parasitic P. aeruginosa has been normally isolated in fresh, raw vegetables and fruits, for example, cucumber, minitomatoes, tomatoes, onion, carrots, lettuces, spinaches, celeries, and different types of salads (Remington and Schimpff, 1981; Xu et al., 2019). Pseudomonas spp. (23%) was the dominant microbial agent found in both soft rot and whole lettuce samples (n = 100) in Nigeria (Erhirhie et al., 2020). Ezemba, C. C found that in salad and four other ready-to-eat samples on Uli campus, the probability of identifying E.coli, Klebsiella pneumoniae, and P. aeruginosa was 68.45%, 20.24%, and, 11.31%, respectively, (Ezemba et al., 2022). Raw and fresh salad vegetables act as a vector that transmits opportunistic P. aeruginosa to humans including immunocompetent people. Immunocompetent people, like cystic fibrosis patients, are particularly vulnerable to acute or chronic lung infections arising from P. aeruginosa. Therefore, it has been suggested that salads should not be consumed by high-risk patients (Remington and Schimpff, 1981; Table 3).
5. The outbreak of Pseudomonas aeruginosa infection
Pseudomonas aeruginosa is increasingly known for its potential to cause outbreaks of diseases associated with public places such as hospitals and its resistance to a wide range of drugs. The results of the study showed that at least 106 P. aeruginosa are required to establish a flora in the intestinal tract of a healthy adult. In patients with reduced colonization resistance due to antimicrobials, 1 gram of salad containing 103 Gram-negative rods may be sufficient to cause sustained colonization of the intestine (Remington and Schimpff, 1981). Pseudomonas aeruginosa was examined in salads, cold meat, other cold food, and hot food, in public spaces including eight hospitals, two schools, and 11 canteens (Shooter et al., 1971). Most hospitals and canteens contain enough organisms to cause the swallowed organisms to build up in the bowel. Due to the contaminated hospital waste-water system, multidrug-resistant P. aeruginosa outbreaks, involving 89 patients, occurred in two English hospitals (File et al., 1995). Shooter et al. (1971) reported P. aeruginosa was detected in medicines and food, the organism is detected in the feces of patients after a period of time following the ingestion of food contaminated with the organism.
6. Antimicrobial resistant Pseudomonas aeruginosa has posed another concern for food safety
Continued overuse and misuse of antibiotics in food animals in order to treat disease or promote growth have triggered the emergence of drug-resistant superbugs and the increased mortality rates caused by bacterial infections pose a significant threat to human health (Xu et al., 2009; Kumar et al., 2020). Unlike another species of ESKAPE, Staphylococcus aureus, for which resistance primarily is mediated by an element in chromosome- SCCmec (Liu et al., 2016), resistance mechanisms in P aeruginosa has been commonly found to be associated with mobile elements, such as plasmids and integrons (Yu et al., 2016). Class 1 integrons have been commonly reported in P. aeruginosa since 1990s, then we had firstly reported class 2 integrons in P. aeruginosa in 2000s (Xu et al., 2009). Class 2 integrons were occasionally reported as we had also firstly reported class 1 integrons in S. aureus and 4 species of MRCNS (Xu et al., 2007, 2008, 2011a,b; Deng et al., 2015), class 1 and 2 integrons in Enterococcus (Xu et al., 2010), during similar period in the same medical setting. Since 2010s, we had observed more novel resistance genes on mega plasmids in P. aeruginosa (Liu et al., 2018a,b; Chen et al., 2019). Fluoroquinolone resistance is rapidly emerging in Gram-negative bacteria Pseudomonas spp., and the use of quinolones in food animal husbandry was found to be one of the main causes of the prevalence of fluoroquinolone-resistant P. aeruginosa (Gasink et al., 2006). In bovine meat (n = 230), fresh fish (n = 130), and smoked fish (n = 140), the prevalence of P. aeruginosa multidrug-resistance was 47.8%, 33.1%, and 20.0%, respectively (Vega-Mercado et al., 1995; Quintieri et al., 2019). Pseudomonas aeruginosa has a high level of drug resistance (Xie et al., 2017; Liu et al., 2018b), with the largest proportion of resistance to aztreonam being 36.4%, the following being ceftazidime, cefepime, and tobramycin. About one fifth of P. aeruginosa was resistant to beta-lactamases and about one tenth of P. aeruginosa was resistant to metallo-beta-lactamases and carbapenemases (Correa Rivas et al., 2015). The growing prevalence of resistance to carbapenem is a severe worldwide threat to public health, posing a risk to human and animal health. From minced meat in Egypt, one P. aeruginosa was isolated, which resists all antibiotics except colistin (Sadek et al., 2021). In Dhaka city, 100% of P. aeruginosa isolated from frozen meat and chicken nuggets showed resistance to ampicillin, penicillin, cefixime, and cefpodoxime, and three-tenths of isolates were not susceptible to cefotaxime (Bhuiya et al., 2018). The 19 P. aeruginosa isolates in milk samples (n = 125) obtained from dairy farms in Tirupati showed high resistance to ampicillin, penicillin, and oxacillin (100%; Swetha et al., 2017). In Jamaica, vegetable samples collected from supermarkets (55.6%) and groceries (72.3%) were extensively polluted with P. aeruginosa, which were resistant to ampicillin (100%), chloramphenicol (84%), trimethoprim (83%), and aztreonam (41%), with 35% of these isolates being insensitive to all four antibacterial compounds (Allydice-Francis and Brown, 2012).
7. Prevention and safety control of Pseudomonas aeruginosa in food
Preventing food spoilage and inhibiting the growth of pathogenic microorganisms is often achieved through the use of synthetic preservatives. However, the use of chemicals can be harmful to human health and can lead to the acquisition of resistance by microorganisms (Liu Z. et al., 2021). As a result of these concerns, there is an urgent need for scientists to find healthier, natural, effective, and environmentally friendly alternative antimicrobial agents. Various plant extracts belonging to plant secondary metabolites (PSMs) have therapeutic potential and are useful for the reduction of pathogens (Singh et al., 2003). Ethanolic extracts of Punica granatum peels and Syzygium aromaticum flowers exhibited inhibitory and germicidal activity and the MIC values for some of the more sensitive food borne P. aeruginosa range from 2.0 to 5.0 mg/mL (Mostafa et al., 2018). The essential oils extracted from basil, marjoram, oregano, rosemary, sage, grapefruit, citrus, lemon, Thymus vulgaris, Mentha piperita, Eucalyptus globulus, and Lonicera japonica could be used to inhibit P. aeruginosa virulence (Stojanović-Radić et al., 2016). Juglone from walnut husk, is highly effective, natural, non-hazardous, low residue and has the potential to inhibit P. aeruginosa. The test findings indicated that juglone at a concentration of 35 μg/mL was effective in inhibiting the colony formation of P. aeruginosa even at a concentration of around 107 CFU/mL (Han et al., 2021).
The main reason why these PSMs control P. aeruginosa virulence is that the small molecules from a number of common plants and foods were obtained and selected for their QSI activity to try to combat the widespread opportunistic pathogen P. aeruginosa. Some bacteria modulate their phenotypes associated with pathogenicity via chemical signaling molecules, also termed quorum sensing (QS; Xu et al., 2021b; Liu et al., 2022a,b). The expression of genes related to QS in P. aeruginosa can be specifically inhibited by Iberin from Horseradish manufactured by a wide range of members of the Brassicaceae family (Jakobsen et al., 2012). In addition, biofilm formation inhibition and cell structure change can also effectively impede the development of P. aeruginosa. Because of the increased resistance of biofilm to disinfectants and bactericides, higher disinfectant concentrations and more contact time are needed to effectively inhibit bacterial activity. Carvacrol, available in the majority of antimicrobial essential oils, maybe a potential agent for QS inhibition because of its capacity to bind to proteins which are combined on cell membranes or involved in biofilm formation (Tapia-Rodriguez et al., 2017). Thymol is thought to be effective in inhibiting planktonic P. aeruginosa by disrupting cell integrity and increasing the permeability of the membrane, allowing the cell contents to escape (Liu T. et al., 2021).
Some other substances could also be utilized as healthy alternative preventive tools to control food spoilage due to microorganisms and to reduce the health risks posed by P. aeruginosa. Honey shows excellent antibacterial activity toward clinical bacterial samples of P. aeruginosa and Escherichia coli (Mandal et al., 2010). The growth and biofilm form of P. aeruginosa can also be inhibited by the papain hydrolysis product from the camel milk whey (Abdel-Hamid et al., 2020). Cell membrane disruption caused by Arg-Ser-Ser (RSS) handling results in leakage of intracellular contents from P. aeruginosa cells. Thus, RSS exhibits potential inhibition of P. aeruginosa (Liu et al., 2020).
8. Conclusion
As one of the most common foodborne pathogens, P. aeruginosa can cause severe acute and chronic life-threatening infections. Factors contributing to bacterial virulence include LPS, adhesins, agglutinins, and flagellates. P. aeruginosa often exists in various types of food, such as water, milk, meat, vegetable and fruit. Unfortunately, in the face of outbreaks of P. aeruginosa infections, overuse and misuse of antibiotic have made them resistant to antibiotics. The prevention of foodborne poisoning from P. aeruginosa is based on hygienic measures to avoid or reduce contamination of food by P. aeruginosa. Chemical preservatives can be used as appropriate to suppress the growth of P. aeruginosa. But as a typical biofilm former, P. aeruginosa attaches to a variety of surfaces or tissues, making it difficult to remove P. aeruginosa from food and medical instruments. Therefore, more research on this issue is urgently needed to be able to reduce the public health burden.
Author contributions
XL writing—original draft and data curation. NG resources and conceptualization. TH methodology and supervision. FZ writing—review and editing. GP supervision and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by grants from National Natural Science Foundation of China (82170056 and 81570045), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01S155), Natural Science Foundation of Guangdong Province (2021A1515011024, 2017A030313683, and 2014A030313486), Science and Technology Program of Guangzhou (202201020404 and 201510010226), National Key Research and Development Program of China (2018YFC1311600, 2018YFC1311604, 2016YFC1304100, and 2016YFC1304104).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Hamid, M., Romeih, E., Saporito, P., Osman, A., Mateiu, R. V., Mojsoska, B., et al. (2020). Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control 111:107056. doi: 10.1016/j.foodcont.2019.107056
Alimi, B. A., Lawal, R., and Odetunde, O. N. (2022). Food safety and microbiological hazards associated with retail meat at butchery outlets in north-Central Nigeria. Food Control 139:109061. doi: 10.1016/j.foodcont.2022.109061
Allen, M. J., and Geldreich, E. E. (1975). Bacteriological criteria for ground-water quality. Groundwater 13, 45–52. doi: 10.1111/j.1745-6584.1975.tb03064.x
Allydice-Francis, K., and Brown, P. D. (2012). Diversity of antimicrobial resistance and virulence determinants in Pseudomonas aeruginosa associated with fresh vegetables. International journal of microbiology. 2012, 1–7. doi: 10.1155/2012/426241
Amal, A., Seham, A. I., and Marouf, H. A. (2014). Prevalence of Pseudomonas aeruginosa and its toxins in some meat products. Glob. J. Agric Food Saf. Sci. 139-50, 39–50. doi: 10.33314/jnhrc.1877
Barken, K. B., Pamp, S. J., Yang, L., Gjermansen, M., Bertrand, J. J., Klausen, M., et al. (2008). Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 10, 2331–2343. doi: 10.1111/j.1462-2920.2008.01658.x
Behzadi, P., Barath, Z., and Gajdacs, M. (2021). It’s not easy being green: a narrative review on the microbiology, virulence and therapeutic prospects of multidrug-resistant Pseudomonas aeruginosa. Antibiotics-Basel 10:42. doi: 10.3390/antibiotics10010042
Bhandare, S. G., Sherikar, A. T., Paturkar, A. M., Waskar, V. S., and Zende, R. J. (2007). A comparison of microbial contamination on sheep/goat carcasses in a modern Indian abattoir and traditional meat shops. Food Control 18, 854–858. doi: 10.1016/j.foodcont.2006.04.012
Bhuiya, M., Sarkar, M. K., Sohag, M. H., Ali, H., Roy, C. K., Akther, L., et al. (2018). Enumerating antibiotic susceptibility patterns of Pseudomonas aeruginosa isolated from different sources in Dhaka City. Open. Microbiol. J. 12, 172–180. doi: 10.2174/1874285801812010172
Cassini, A., Högberg, L. D., Plachouras, D., Quattrocchi, A., Hoxha, A., Simonsen, G. S., et al. (2019). Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 19, 56–66. doi: 10.1016/S1473-3099(18)30605-4
Chadha, J., Harjai, K., and Chhibber, S. (2022). Revisiting the virulence hallmarks of Pseudomonas aeruginosa: a chronicle through the perspective of quorum sensing. Environ. Microbiol. 24, 2630–2656. doi: 10.1111/1462-2920.15784
Chen, T. R., Wei, Q. K., and Chen, Y. J. (2011). Pseudomonas spp. and Hafnia alvei growth in UHT milk at cold storage. Food Control 22, 697–701. doi: 10.1016/j.foodcont.2010.10.004
Chen, D. Q., Yang, L., Peters, B. M., Liu, J. Y., Li, L., Li, B., et al. (2019). Complete sequence of a novel multidrug-resistant Pseudomonas putida strain carrying two copies of qnrVC6. Microb. Drug Resist. 25, 1–7. doi: 10.1089/mdr.2018.0104
Correa Rivas, K. A., Bravo Torrealba, M. V., Silva Alvarado, R. A., and Montiel, M. (2015). Antibiotic susceptibility of Pseudomonas aeruginosa isolated from drinking water from Santa Rosa de Agua community, Maracaibo Zulia state. Rev. Soc. Venez. Microbiol. 35, 83–88.
Cross, A., Allen, J. R., Burke, J., Ducel, G., Harris, A., John, J., et al. (1983). Nosocomial infections due to Pseudomonas aeruginosa: review of recent trends. Rev. Infect. Dis. 5, S837–S845. doi: 10.1093/clinids/5.supplement_5.s837
Deng, Y., Liu, J., Peters, B. M., Chen, L., Miao, J., Li, B., et al. (2015). Antimicrobial resistance investigation on staphylococcus strains in a local Hospital in Guangzhou, China, 2001–2010. Microb. Drug Resist. 21, 102–104. doi: 10.1089/mdr.2014.0117
Dong, Q. L., Sun, L. J., Fang, T. S., Wang, Y., Li, Z. S., Wang, X., et al. (2022). Biofilm formation of listeria monocytogenes and Pseudomonas aeruginosa in a simulated chicken processing environment. Foods 11:1917. doi: 10.3390/foods11131917
Elhariri, M., Hamza, D., Elhelw, R., and Dorgham, S. M. (2017). Extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa in camel in Egypt: potential human hazard. Ann. Clin. Microbiol. Antimicrob. 16, 21–26. doi: 10.1186/s12941-017-0197-x
Erhirhie, E. O., Omoirri, M. A., Chikodiri, S. C., Ujam, T. N., Emmanuel, K. E., and Oseyomon, J. O. (2020). Microbial quality of fruits and vegetables in Nigeria. Int. J. Nutr. Sci. 5, 2–11. doi: 10.30476/IJNS.2020.86034.1065
Ezemba, C. C., Mmaduekwe, C. J., Udeze, C. P., Ogumuo, F. C., Ozoekwe, N., Duru, B. N., et al. (2022). Isolation identification and antibiogram of Pseudomonas aeruginosa, Klebsiella pneumonia and Escherichia coli isolates from ready to eat food samples in Uli campus. Asian J. Food Res. Nutr. 1, 016–021. doi: 10.56355/ijfrms.2022.1.1.0027
File, T. M., Tan, J. S., Thomson, R. B., Stephens, C., and Thompson, P. (1995). An outbreak of Pseudomonas aeruginosa ventilator-associated respiratory infections due to contaminated food coloring dye-further evidence of the significance of gastric colonization preceding nosocomial pneumonia. Infect. Control Hosp. Epidemiol. 16, 417–418. doi: 10.2307/30141899
Gao, X., Li, C., He, R., Zhang, Y., Wang, B., Zhang, Z., et al. (2023). Research advances on biogenic amines in traditional fermented foods: emphasis on formation mechanism, detection and control methods. Food Chem. 405:134911. doi: 10.1016/j.foodchem.2022.134911
Garedew, L., Berhanu, A., Mengesha, D., and Tsegay, G. (2012). Identification of gram-negative bacteria from critical control points of raw and pasteurized cow milk consumed at Gondar town and its suburbs, Ethiopia. BMC Public Health 12, 1–7. doi: 10.1186/1471-2458-12-950
Gasink, L. B., Fishman, N. O., Weiner, M. G., Nachamkin, I., Bilker, W. B., and Lautenbach, E. (2006). Fluoroquinolone-resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact. Am. J. Med. 119, 526–e19. doi: 10.1016/j.amjmed.2005.11.029
Han, Q., Yan, X., Zhang, R., Wang, G., and Zhang, Y. (2021). Juglone inactivates Pseudomonas aeruginosa through cell membrane damage, biofilm blockage, and inhibition of gene expression. Molecules 26:5854. doi: 10.3390/molecules26195854
Jakobsen, T. H., Bragason, S. K., Phipps, R. K., Christensen, L. D., van Gennip, M., Alhede, M., et al. (2012). Food as a source for quorum sensing inhibitors: iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 78, 2410–2421. doi: 10.1128/AEM.05992-11
Jurado-Martin, I., Sainz-Mejias, M., and McClean, S. (2021). Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Int. J. Mol. Sci. 22:3128. doi: 10.3390/ijms22063128
Klockgether, J., and Tümmler, B. (2017). Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Research. 6:1261. doi: 10.12688/f1000research.10506.1
Kumar, S. B., Arnipalli, S. R., and Ziouzenkova, O. (2020). Antibiotics in food chain: the consequences for antibiotic resistance. Antibiotics. 9:688. doi: 10.3390/antibiotics9100688
Li, Y., Huang, T., Bai, C., Fu, J., Chen, L., Liang, Y., et al. (2020b). Reduction, prevention, and control of salmonella enterica viable but non-culturable cells in flour food. Front. Microbiol. 11:1859. doi: 10.3389/fmicb.2020.01859
Li, Y., Huang, T., Mao, Y., Chen, Y., Shi, F., Peng, R., et al. (2020c). Study on the viable but non-culturable (VBNC) state formation of Staphylococcus aureus and its control in food system. Front. Microbiol. 11:599739. doi: 10.3389/fmicb.2020.599739
Li, Y., Huang, T., Mao, Y., Chen, Y., Shi, F., Peng, R., et al. (2020d). Effect of environmental conditions on the formation of the viable but nonculturable state of Pediococcus acidilactici BM-PA17927 and its control and detection in food system. Front. Microbiol. 11:586777. doi: 10.3389/fmicb.2020.586777
Li, Y., Huang, T. Y., Ye, C., Chen, L., Liang, Y., Wang, K., et al. (2020a). Formation and control of the viable but non-culturable state of foodborne pathogen Escherichia coli O157:H7. Front. Microbiol. 11:1202. doi: 10.3389/fmicb.2020.01202
Liu, J., Chen, D., Peters, B. M., Li, L., Li, B., Xu, Z., et al. (2016). Staphylococcal chromosomal cassettes mec (SCCmec): a mobile genetic element in methicillin-resistant Staphylococcus aureus. Microb. Pathog. 101, 56–67. doi: 10.1016/j.micpath.2016.10.028
Liu, Z., Hu, S., Soteyome, T., Bai, C., Liu, J., Wang, Z., et al. (2021). Intense pulsed light for inactivation of foodborne gram-positive bacteria in planktonic cultures and bacterial biofilms. LWT 152:112374. doi: 10.1016/j.lwt.2021.112374
Liu, J., Huang, T., Hong, W., Peng, F., Lu, Z., Peng, G., et al. (2022a). A comprehensive study on ultrasonic deactivation of opportunistic pathogen Saccharomyces cerevisiae in food processing: from transcriptome to phenotype. LWT 170:114069. doi: 10.1016/j.lwt.2022.114069
Liu, J., Huang, T. Y., Liu, G., Ye, Y., Soteyome, T., Seneviratne, G., et al. (2022b). Microbial interaction between Lactiplantibacillus plantarum and Saccharomyces cerevisiae: transcriptome level mechanism of cell-cell antagonism. Microbiol. Spectr. 10:e0143322. doi: 10.1128/spectrum.01433-22
Liu, T., Kang, J., and Liu, L. (2021). Thymol as a critical component of Thymus vulgaris L. essential oil combats Pseudomonas aeruginosa by intercalating DNA and inactivating biofilm. LWT 136:110354. doi: 10.1016/j.lwt.2020.110354
Liu, H., Li, S., Brennan, C. S., and Wang, Q. (2020). Antimicrobial activity of Arg–Ser–Ser against the food-borne pathogen Pseudomonas aeruginosa. Int. J. Food Sci. Technol. 55, 379–388. doi: 10.1016/j.lwt.2020.110354
Liu, J., Lin, X., Bai, C., Soteyome, T., Bai, X., Wang, J., et al. (2022c). Verification and application of a modified carbapenem inactivation method (mCIM) on Pseudomonas aeruginosa: a potential screening methodology on carbapenemases phenotype in Bacillus cereus. Bioengineered 13, 12088–12098. doi: 10.1080/21655979.2022.2072601
Liu, J., Willems, H., Sansevere, E. A., Allert, S., Barker, K. S., Lowes, D. J., et al. (2021). A variant ECE1 allele contributes to reduced pathogenicity of Candida albicans during vulvovaginal candidiasis. PLoS Pathog. 17:e1009884. doi: 10.1371/journal.ppat.1009884
Liu, J., Yang, L., Chen, D., Peters, B. M., Li, L., Li, B., et al. (2018b). Complete sequence of pBM413, a novel multidrug resistance megaplasmid carrying qnrVC6 and Bla IMP-45 from Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 51, 145–150. doi: 10.1016/j.ijantimicag.2017.09.008
Liu, J., Yang, L., Li, L., Li, B., Chen, D., and Xu, Z. (2018a). Comparative genomic analyses of two novel qnrVC6 carrying multidrug-resistant Pseudomonas spp. strains. Microb. Pathog. 123, 269–274. doi: 10.1016/j.micpath.2018.07.026
Ma, L. Z., Wang, D., Liu, Y., Zhang, Z., and Wozniak, D. J. (2022). Regulation of biofilm exopolysaccharide biosynthesis and degradation in Pseudomonas aeruginosa. Annu. Rev. Microbiol. 76, 413–433. doi: 10.1146/annurev-micro-041320-111355
Mandal, S., DebMandal, M., Pal, N. K., and Saha, K. (2010). Antibacterial activity of honey against clinical isolates of Escherichia coli, Pseudomonas aeruginosa and Salmonella enterica serovar Typhi. Asian Pac. J. Trop. Med. 3, 961–964. doi: 10.1016/S1995-7645(11)60009-6
Martak, D., Gbaguidi-Haore, H., Meunier, A., Valot, B., Conzelmann, N., Eib, M., et al. (2022). High prevalence of Pseudomonas aeruginosa carriage in residents of French and German long-term care facilities. Clin. Microbiol. Infect. 28, 1353–1358. doi: 10.1016/j.cmi.2022.05.004
Meesilp, N., and Mesil, N. (2019). Effect of microbial sanitizers for reducing biofilm formation of Staphylococcus aureus and Pseudomonas aeruginosa on stainless steel by cultivation with UHT milk. Food Sci. Biotechnol. 28, 289–296. doi: 10.1007/s10068-018-0448-4
Mickova, V., Lukasova, J., and Konecny, S. (1989). Pseudomonas aeruginosa in raw and pasteurized milk. Vet. Med. (Praha). 34, 411–419. doi: 10.1016/0093-691X(89)90572-4
Mostafa, A. A., Al-Askar, A. A., Almaary, K. S., Dawoud, T. M., Sholkamy, E. N., and Bakri, M. M. (2018). Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi journal of biological sciences. 25, 361–366. doi: 10.1016/j.sjbs.2017.02.004
Opperman, C. J., Moodley, C., Lennard, K., Smith, M., Ncayiyana, J., Vulindlu, M., et al. (2022). A citywide, clonal outbreak of Pseudomonas aeruginosaa. Int. J. Infect. Dis. 117, 74–86. doi: 10.1016/j.ijid.2022.01.039
Perrin, Y., Bouchon, D., Héchard, Y., and Moulin, L. (2019). Spatio-temporal survey of opportunistic premise plumbing pathogens in the Paris drinking water distribution system. Int. J. Hyg. Environ. Health 222, 687–694. doi: 10.1016/j.ijheh.2019.04.010
Poursina, S., Ahmadi, M., Fazeli, F., and Ariaii, P. (2022). Assessment of virulence factors and antimicrobial resistance among the Pseudomonas aeruginosa strains isolated from animal meat and carcass samples. Vet. Med. Sci. doi: 10.1002/vms3.1007
Quintieri, L., Zuhlke, D., Fanelli, F., Caputo, L., Liuzzi, V. C., Logrieco, A. F., et al. (2019). Proteomic analysis of the food spoiler Pseudomonas fluorescens ITEM 17298 reveals the antibiofilm activity of the pepsin-digested bovine lactoferrin. Food Microbiol. 82, 177–193. doi: 10.1016/j.fm.2019.02.003
Raposo, A., Pérez, E., de Faria, C. T., Ferrús, M. A., and Carrascosa, C. (2016). Food spoilage by Pseudomonas spp.—an overview. Foodborne pathogens and antibiotic resistance., 41–71. doi: 10.1002/9781119139188.ch3
Razzaq, R., Farzana, K., Mahmood, S., and Murtaza, G. (2014). Microbiological analysis of street vended vegetables in Multan City, Pakistan: a public health concern. Pak. J. Zool. 46, 1133–1138.
Remington, J. S., and Schimpff, S. C. (1981). Occasional notes. Please don’t eat the salads. N. Engl. J. Med. 304, 433–435. doi: 10.1056/NEJM198102123040730
Rezaloo, M., Motalebi, A., Mashak, Z., and Anvar, A. (2022). Prevalence, antimicrobial resistance, and molecular description of Pseudomonas aeruginosa isolated from meat and meat products. J. Food Qual. 2022, 1–11. doi: 10.1155/2022/9899338
Ruiz-Roldan, L., Rojo-Bezares, B., Lozano, C., Lopez, M., Chichon, G., Torres, C., et al. (2021). Occurrence of pseudomonas spp. in raw vegetables: molecular and phenotypical analysis of their antimicrobial resistance and virulence-related traits. Int. J. Mol. Sci. 22:12626. doi: 10.3390/ijms222312626
Sadek, M., Soliman, A. M., Nariya, H., Shimamoto, T., and Shimamoto, T. (2021). Genetic characterization of carbapenemase-producing Enterobacter cloacae complex and Pseudomonas aeruginosa of food of animal origin from Egypt. Microb. Drug Resist. 27, 196–203. doi: 10.1089/mdr.2019.0405
Sauer, K., Camper, A. K., Ehrlich, G. D., Costerton, J. W., and Davies, D. G. (2002). Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. Am. Soc. Microbiol. 184, 1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002
Shooter, R. A., Cooke, E. M., Faiers, M. C., Breaden, A. L., and O’Farrell, S. M. (1971). Isolation of Escherichia coli, Pseudomonas aeruginosa, and Klebsiella from food in hospitals, canteens, and schools. Lancet (London, England). 2, 390–392. doi: 10.1016/s0140-6736(71)90111-5
Singh, B., Bhat, T. K., and Singh, B. (2003). Potential therapeutic applications of some antinutritional plant secondary metabolites. J. Agric. Food Chem. 51, 5579–5597. doi: 10.1021/jf021150r
Soriano, J. M., Rico, H., Molto, J. C., and Manes, J. (2000). Assessment of the microbiological quality and wash treatments of lettuce served in university restaurants. Int. J. Food Microbiol. 58, 123–128. doi: 10.1016/S0168-1605(00)00288-9
Stojanović-Radić, Z., Stojanović, N., Sharifi-Rad, J., and Stanković, N. (2016). Potential of Ocimum basilicum L. and Salvia officinalis L. essential oils against biofilms of P. aeruginosa clinical isolates. Cell. Mol. Biol. 62, 27–33.
Swartjes, J. J., Das, T., Sharifi, S., Subbiahdoss, G., Sharma, P. K., Krom, B. P., et al. (2013). A functional DNase I coating to prevent adhesion of bacteria and the formation of biofilm. Adv. Funct. Mater. 23, 2843–2849. doi: 10.1002/adfm.201202927
Swetha, C. S., Babu, A. J., Rao, K. V., Bharathy, S., Supriya, R. A., and Rao, T. M. (2017). A study on the antimicrobial resistant patterns of Pseudomonas aeruginosa isolated from raw milk samples in and around Tirupati, Andhra Pradesh. Asian J. Dairy Food Res. 36, 100–105. doi: 10.18805/ajdfr.v36i02.7951
Tacconelli, E., and Magrini, N. (2021). Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Organ. Mund La Salud. 2017:76. doi: 10.4103/jms.jms_25_17
Tahr, I. M., and Hasan, M. G. (2022). Molecular identification of Pseudomonas aeruginosa in meat at Mosul city retails using PCR technique. Iraqi J. Vet. Sci. 36, 1083–1087. doi: 10.33899/ijvs.2022.133086.2173
Tapia-Rodriguez, M. R., Hernandez-Mendoza, A., Gonzalez-Aguilar, G. A., Martinez-Tellez, M. A., Martins, C. M., and Ayala-Zavala, J. F. (2017). Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 75, 255–261. doi: 10.1016/j.foodcont.2016.12.014
Vega-Mercado, H., Powers, J. R., Barbosa-Canovas, G. V., Swanson, B. G., and Luedecke, L. (1995). Inactivation of a protease from Pseudomonas fluorescens M3/6 using high voltage pulsed electric fields. In Proceedings IFT Annual Meeting. 267.
Viswanathan, P., and Kaur, R. (2001). Prevalence and growth of pathogens on salad vegetables, fruits and sprouts. Int. J. Hyg. Environ. Health 203, 205–213. doi: 10.1078/S1438-4639(04)70030-9
Walker, J. T., Jhutty, A., Parks, S., Willis, C., Copley, V., Turton, J. F., et al. (2014). Investigation of healthcare-acquired infections associated with Pseudomonas aeruginosa biofilms in taps in neonatal units in Northern Ireland. J. Hosp. Infect. 86, 16–23. doi: 10.1016/j.jhin.2013.10.003
Wang, L. J., Chen, E. Z., Yang, L., Feng, D. H., Xu, Z., and Chen, D. Q. (2021). Emergence of clinical Pseudomonas aeruginosa isolate Guangzhou-PaeC79 carrying crpP, Bla(GES-5), and Bla(KPC-2) in Guangzhou of China. Microb. Drug Resist. 27, 965–970. doi: 10.1089/mdr.2020.0420
Wang, J., Ye, C., Fan, X., Liu, J., Huang, Y., Lin, X., et al. (2021). Letter to the Editor: Four Novel Types of Gene Cassettes from Carbapenem-Resistant Pseudomonas Aeruginosa in Southern China-First Report of qnrVC7. Microb. Drug Resist. 27, 1011–1012. doi: 10.1089/mdr.2020.0453
Wong, M. H., Chan, E. W. C., and Chen, S. (2015). Isolation of carbapenem-resistant Pseudomonas spp. from food. J. Glob. Antimicrob. Resist. 3, 109–114. doi: 10.1016/j.jgar.2015.03.006
Wu, Q., Ye, Y., Li, F., Zhang, J., and Guo, W. (2016). Prevalence and genetic characterization of Pseudomonas aeruginosa in drinking water in Guangdong Province of China. LWT-Food Sci. Technol. 26, 57–64. doi: 10.1016/j.cofs.2019.03.006
Xie, J., Yang, L., Peters, B. M., Chen, L., Chen, D., Li, B., et al. (2017). A 16-year retrospective surveillance report on the pathogenic features and antimicrobial susceptibility of Pseudomonas aeruginosa isolates from FAHJU in Guangzhou representative of southern China. Microb. Pathog. 110, 37–41. doi: 10.1016/j.micpath.2017.06.018
Xu, Z., Li, L., Shi, L., and Shirtliff, M. E. (2011b). Class 1 integron in staphylococci. Mol. Biol. Rep. 38, 5261–5279. doi: 10.1007/s11033-011-0676-7
Xu, Z., Li, L., Shirtliff, M. E., Alam, M. J., Yamasaki, S., and Shi, L. (2009). Occurrence and characteristics of class 1 and 2 integrons in Pseudomonas aeruginosa isolates from patients in southern China. J. Clin. Microbiol. 47, 230–234. doi: 10.1128/JCM.02027-08
Xu, Z., Li, L., Shirtliff, M. E., Peters, B. M., Li, B., Peng, Y., et al. (2011a). Resistance class 1 integron in clinical methicillin-resistant Staphylococcus aureus strains in southern China, 2001–2006. Clin. Microbiol. Infect. 17, 714–718. doi: 10.1111/j.1469-0691.2010.03379.x
Xu, Z., Li, L., Shirtliff, M. E., Peters, B. M., Peng, Y., Alam, M. J., et al. (2010). First report of class 2 integron in clinical enterococcus faecalis and class 1 integron in enterococcus faecium in South China. Diagn. Microbiol. Infect. Dis. 68, 315–317. doi: 10.1016/j.diagmicrobio.2010.05.014
Xu, Z., Lin, X., Soteyome, T., Ye, Y., Chen, D., Yang, L., et al. (2021c). Significant downtrend of antimicrobial resistance rate and rare β-lactamase genes and plasmid replicons carriage in clinical Pseudomonas aeruginosa in southern China. Microb. Pathogensis 159:105124. doi: 10.1016/j.micpath.2021.105124
Xu, Z., Liu, Z., Soteyome, T., Hua, J., Zhang, L., Yuan, L., et al. (2021a). Impact of pmrA on Cronobacter sakazakii planktonic and biofilm cells: a comprehensive transcriptomic study. Food Microbiol. 98:103785. doi: 10.1016/j.fm.2021.103785
Xu, Z., Lu, Z., Soteyome, T., Ye, Y., Huang, T., Liu, J., et al. (2021b). Polymicrobial interaction between lactobacillus and Saccharomyces cerevisiae: coexistence-relevant mechanisms. Crit. Rev. Microbiol. 47, 386–396. doi: 10.1080/1040841X.2021.1893265
Xu, Z., Shi, L., Alam, M. J., Li, L., and Yamasaki, S. (2008). Integron-bearing methicillin-resistant coagulase-negative staphylococci in South China, 2001–2004. FEMS Microbiol. Lett. 278, 223–230. doi: 10.1111/j.1574-6968.2007.00994.x
Xu, Z., Shi, L., Zhang, C., Zhang, L., Li, X., Cao, Y., et al. (2007). Nosocomial infection caused by class 1 integron-carrying Staphylococcus aureus in a hospital in South China. Clin. Microbiol. Infect. 13, 980–984. doi: 10.1111/j.1469-0691.2007.01782.x
Xu, Z., Xie, J., Soteyome, T., Peters, B. M., Shirtliff, M. E., Liu, J., et al. (2019). Polymicrobial interaction and biofilms between Staphylococcus aureus and Pseudomonas aeruginosa: an underestimated concern in food safety. Curr. Opin. Food Sci. 26, 57–64. doi: 10.1016/j.cofs.2019.03.006
Yu, G., Wen, W., Peters, B. M., Liu, J., Ye, C., Che, Y., et al. (2016). First report of novel genetic array aacA4-Bla(IMP-25)-oxa30-catB3 and identification of novel metallo-beta-lactamase gene Bla(IMP25): a retrospective study of antibiotic resistance surveillance on Psuedomonas aeruginosa in Guangzhou of South China, 2003-2007. Microb. Pathog. 95, 62–67. doi: 10.1016/j.micpath.2016.02.021
Keywords: Pseudomonas aeruginosa, foodborne, pathogen, biofilm, antimicrobial resistance
Citation: Li X, Gu N, Huang TY, Zhong F and Peng G (2023) Pseudomonas aeruginosa: A typical biofilm forming pathogen and an emerging but underestimated pathogen in food processing. Front. Microbiol. 13:1114199. doi: 10.3389/fmicb.2022.1114199
Edited by:
Yang Deng, Qingdao Agricultural University, ChinaReviewed by:
Xihong Zhao, Wuhan Institute of Technology, ChinaWensen Jiang, Cedars Sinai Medical Center, United States
Copyright © 2023 Li, Gu, Huang, Zhong and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gongyong Peng, ✉ Z29uZ3lvbmcxOTc2MUAxNjMuY29t
†These authors have contributed equally to this work
 Xuejie Li
Xuejie Li Nixuan Gu1†
Nixuan Gu1† Gongyong Peng
Gongyong Peng