
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 01 December 2022
Sec. Infectious Agents and Disease
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1063578
This article is part of the Research Topic Animal Emerging and Reemerging Diseases View all 24 articles
In the poultry industry worldwide, Clostridium perfringens has been causing major economic loss as it can cause necrotic enteritis (NE). The coccidial infection has been considered as the most important predisposing factor of NE caused by C. perfringens. In this study, we aimed to advance our knowledge on ileal microbiota of yellow feather broilers under C. perfringens and/or Eimeria challenge. Total of 80 healthy day old yellow feather broilers were randomly assigned to four groups including: Control, C. perfringens challenge group (C. Per), Eimeria challenge group (Cocc), and C. perfringens plus Eimeria challenge group (Comb). On day 14, the Cocc and Comb group broilers were orally gavaged 1 ml PBS solution containing 25,000 oocysts of Eimeria brunetti and 25,000 oocysts of Eimeria maxima. Starting on day 17, the C. Per and Comb group broilers were orally gavaged 10 mL of C. perfringens per bird (4 × 107 CFU/mL, ATCC® 13124™ Strain) every day for 6 days. 16S rRNA gene sequencing was performed on extracted DNA of ileal digesta samples. The results showed that C. perfringens alone did not affect the alpha diversity of ileal microbiome in yellow feather broilers but co-infection with Eimeria significantly decreased the diversity of ileal microbiota. C. perfringens and Eimeria challenge also decreased the relative abundance of beneficial bacteria including Bacteroidetes at the phylum level and Faecalibacterium at the genus level. At the species level, the relative abundance of Candidatus Arthromitus was significantly decreased in the Eimeria challenged groups. This microbial shift information of ileal microbiota under C. Perfringens and Eimeria challenge provide important reference data for the development of therapeutic approaches to necrotic enteritis in yellow-feather broiler chickens.
Clostridium perfringens (C. perfringens) is a constituent of normal flora in the digestive tract of animals and humans (Miller et al., 2010). In the poultry industry worldwide, C. perfringens has been causing major economic loss as it can cause necrotic enteritis (NE). Clinical or subclinical NE usually occurs in broiler chickens between the ages of 2–6 weeks (Skinner et al., 2010). In healthy chickens, C. perfringens almost always exist at levels less than 105 CFU/g intestinal content (Caly et al., 2015). Predisposing factors including high levels of dietary non-starch polysaccharide grains or fish meal proteins, physiological stress, Fusarium mycotoxins in feed as well as coccidial infection normally exist to induce the outbreak of NE (Timbermont et al., 2011; Moore, 2016; Zaytsoff et al., 2020).
The coccidial infection has been considered the most important predisposing factor of NE caused by C. perfringens. The species of obligate intracellular parasites of the genus Eimeria can cause potentially severe enteritis (Attree et al., 2021), resulting significantly economic loss to the poultry industry. Four species of Eimeria, including E. acervulina, E. maxima, E. necatrix, and E. tenella, are considered most important due to their pathogenicity, global prevalence, and overall economic impact (Attree et al., 2021). When co-infected with Eimeria, the NE incidence and the mortality rate of chickens are higher (Baba et al., 1992). Eimeria can cause damage to the epithelium or induce mucogenesis, thus providing a niche for C. perfringens colonization and proliferation (Collier et al., 2008; Van Immerseel et al., 2009). Infectious dose, age, and immune status of the host could all affect the response of the chicken to coccidial infection, ranging from few clinical signs or reduction in weight gain, feed conversion, or egg production to severe enteritis and death (Attree et al., 2021). Coccidiosis prevention would be one way to reduce the incidence of NE caused by C. perfringens.
Due to the significant economic loss and compromised animal welfare caused by C. perfringens and Eimeria, effective control of NE necessitates explorations of alternative strategies due to chemoprophylaxis resistance and limited cost-effective vaccines (Giannenas et al., 2012; Ritzi et al., 2014). In 2020, the ban of antibiotic growth promoters in China has resulted in re-emergence of NE in poultry industry. Gut microbial shift plays a role in the progress of disease development. It was reported that NE development in chickens is associated with microbial shift within the GI tract (Kim et al., 2015). It has not been clear if microbial shift is a predisposing factor or more of a consequence of NE. To prevent C. perfringens and Eimeria infection and improve the gut health of broilers, the gut microbial information of infected chickens is important to know. In this study, we aimed to advance our knowledge on ileal microbiome of yellow feather broilers under C. perfringens and/or Eimeria challenge and provide reference data for future therapeutic strategies for disease control.
This experimental protocol was approved by the Ethical Committee and conducted under the supervision of the Institutional Animal Care and Use Committee of Foshan University (Foshan, China).
Total of 80 healthy day old yellow feather broilers were randomly assigned to four groups including: Control, C. perfringens challenge group (C. Per), Eimeria challenge group (Cocc), and C. perfringens plus Eimeria challenge group (Comb). Birds under different treatments were raised in different pens. On day 14, the Cocc and Comb groups broilers were orally gavaged 1 mL mixed-species Eimeria oocysts solution. The mixed-species Eimeria spp. PBS-based solution contained 25,000 oocysts of Eimeria brunetti and 25,000 oocysts E. maxima. Starting on day 17, the C. Per and Comb groups broilers were orally gavaged 10 mL of C. perfringens/bird (4 × 107 CFU/mL; Clostridium perfringens ATCC® 13124™ Strain; cultured in Reinforced Clostridium Medium) everyday for 6 days. Fot the control groups birds, 1 ml of sterile PBS solution was orally gavaged. On day 24, six broilers randomly selected from each group were sacrificed by cervical dislocation and exsanguinated. The ileal digesta was collected from each broiler and immediately placed into a 2 mL Eppendorf tube. The digesta samples were stored at −80°C for later analysis.
The facility was thoroughly cleaned and disinfected before the bird placement. Temperature was maintained at 33–34°C initially and gradually decreased until 22–24°C by the third week. During the study, all the birds had free access to the same feed and clean water.
The DNA extraction and high-throughput sequencing analysis for this study were the same as our previous study (Feng et al., 2020). Briefly, total genome DNA from ileal digesta was extracted using the Cetyltrimethyl Ammonium Bromide method. Extracted DNA was monitored on 1% agarose gels before being diluted to 1 ng/μL to prepare amplicons for high-throughput sequencing. Conventional PCR was used to amplify the V3-V4 regions of the 16S rRNA genes using primers 515F (5′- GTGYCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). The PCR reaction mix consisted of 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs, MA, USA), 0.2 μM of forward and reverse primers, and about 10 ng template DNA. Sequencing libraries were generated using TruSeq® DNA PCR-Free sample preparation kit (Illumina, San Diego, CA, USA). The library quality was assessed on a Qubit @ 2.0 Fluorometer (Thermo Fisher Scientific, MA, USA) and Agilent Bioanalyzer 2100 system (Agilent Technologies, Inc., Santa Clara, CA, USA). The bar-coded amplicons were sequenced on an Illumina NovaSeq system and 250 bp paired-end reads were generated.
Paired-end reads were merged using Fast Length Adjustment of Short reads software (FLASH; V1.2.11) to obtain raw tags. Then quality control was conducted using fastp software to obtain high-quality clean tags. Finally, the Vsearch software was used to compare the clean tags with the database to detect and remove chimeras to obtain the effective tags (Haas et al., 2011). The obtained effective tags were denoised using the DADA2 module in QIIME2. Sequences with an abundance less than five were filtered out to obtain the final amplicon sequence variants (ASVs). Subsequently, the obtained ASVs were compared with the database (Silva138.1) using the classify-sklearn module in QIIME2 to obtain the species information of each ASV. Alpha diversity analysis (shannon, simpson, chao1, goods coverage, dominance, and pielou) and Beta diversity analysis were conducted in QIIME2. Principal component analysis (PCA) plot was generated using the “ade4” and “ggplot2” packages of the R software (v. 3.5.3).1 Differentially abundant genera among groups were identified using linear discriminant analysis (LDA) effect size (LEfSe) analysis (Segata et al., 2011). Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) was performed to make inferences about the metabolic functions of the microbial community and metagenome metabolic functions were assessed using the KEGG orthology database (Douglas et al., 2020).
Alpha diversity index and taxonomic data were analyzed using the PROC GLIMMIX procedure of SAS (SAS Institute, Inc., Cary, NC, USA) including treatment as fixed effect in the model. The significance was declared at P < 0.05 and trends at P < 0.1. Tukey multiple comparison method was used to detect difference between two groups when treatment effect was significant.
To evaluate how infection of C. perfringens and Eimeria shift the microbial composition of chickens, 16S rRNA gene sequencing was performed on extracted DNA of ileal digesta samples. Total number of Amplicon Sequence Variants (ASVs) in all four groups was 1,185 and 58 ASVs were shared by all groups (Figure 1A). Compared to the control group, all the other three groups had lower number of group specific ASVs (control 373 vs. C. Per 183, Cocc 148, and Comb 111) indicating lower diversity of bacterial species. Rarefaction curve of observed otus revealed that there was sufficient sequence coverage to describe the bacterial composition of each group (Figure 1B). Principal component analysis revealed that the first and second principal components explained 11.48 and 10.55% of the variation among samples, respectively (Figure 2). Samples from different groups could not be clearly separated from each other. However, it can be seen from the PCA plot that the three groups under challenge clustered together. Effects of C. Perfringens and Eimeria challenge on alpha diversity of ileal microbiota in yellow feather broilers are present in Table 1. Compared to the control group, challenge with C. perfringens alone did not affect alpha indices including Shannon, and Chao1. But Eimeria challenge alone or combined infection with C. perfringens all significantly decreased the values of these indices (P < 0.05). The indices of Simpson, Pielou, and Dominance were not different among four groups (P > 0.05).
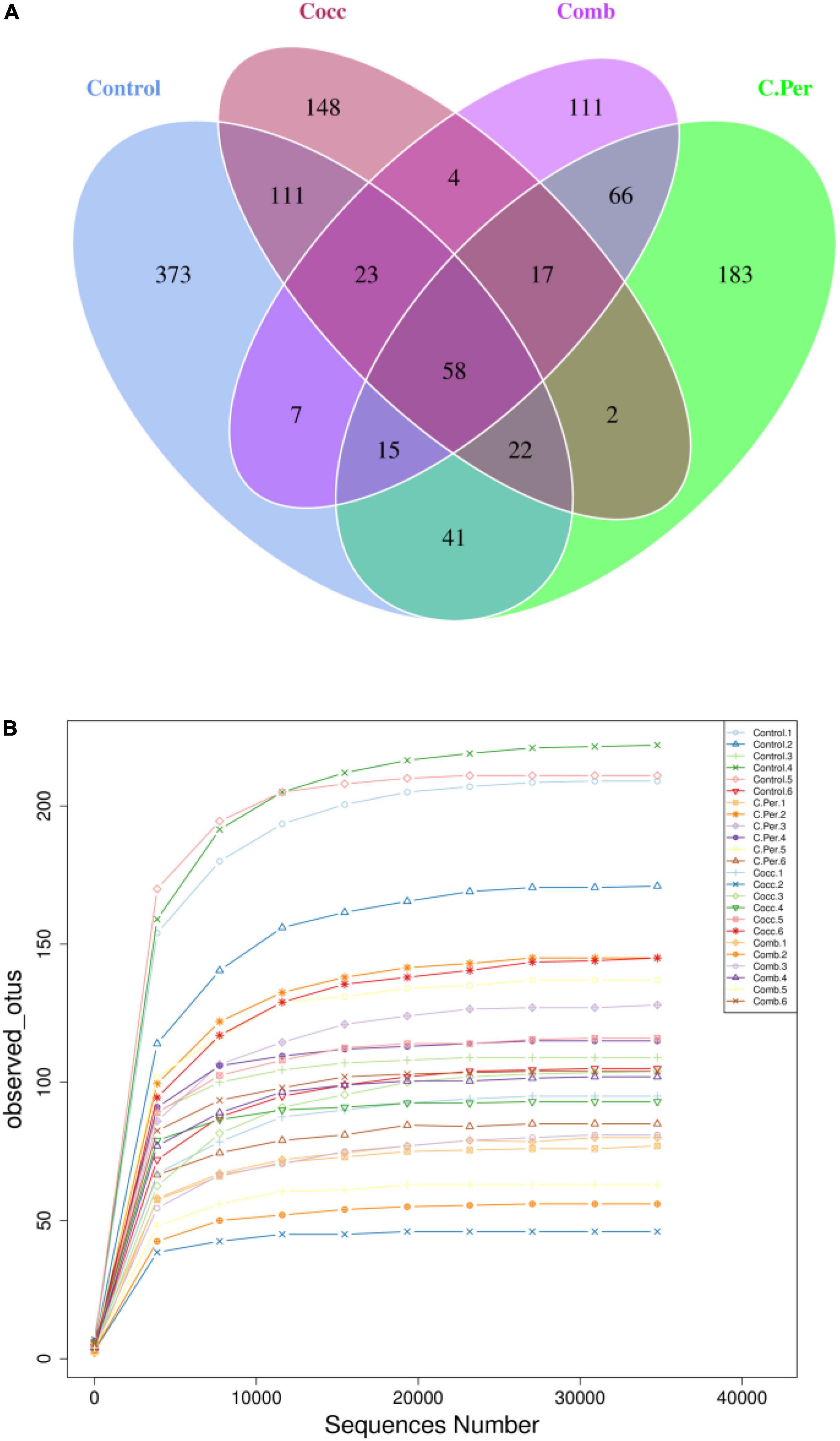
Figure 1. Number of Amplicon Sequence Variants (ASVs) in each group. (A) Venn diagram of shared and specific ASVs in the four groups. (B) Rarefaction curve of observed otus in all samples. C. Per, C. perfringens challenge; Cocc, Eimeria challenge; Comb, C. perfringens and Eimeria challenge.
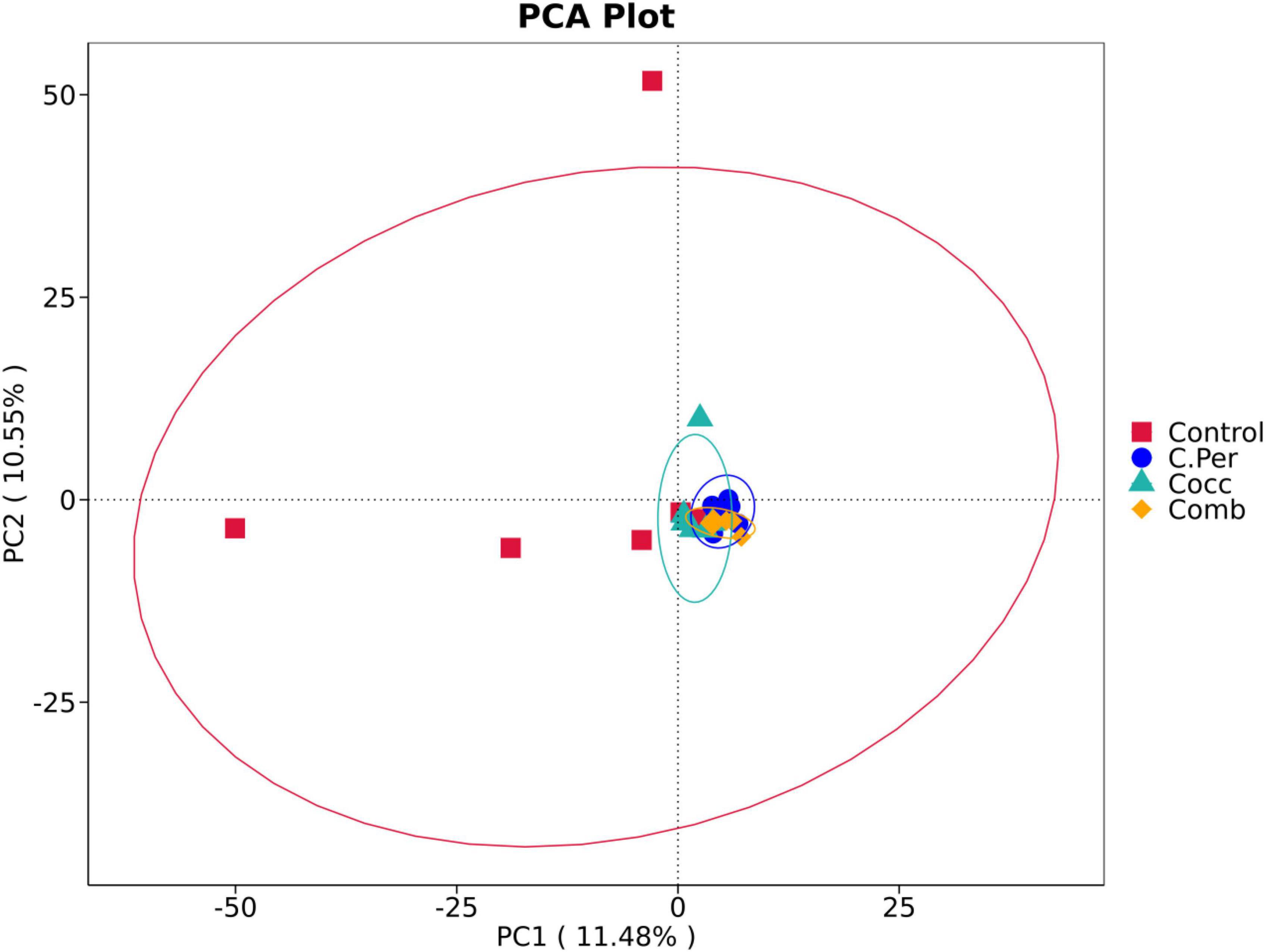
Figure 2. Principle component analysis of the ileal microbiota in different groups. C. Per, C. perfringens challenge; Cocc, Eimeria challenge; Comb, C. perfringens and Eimeria challenge.
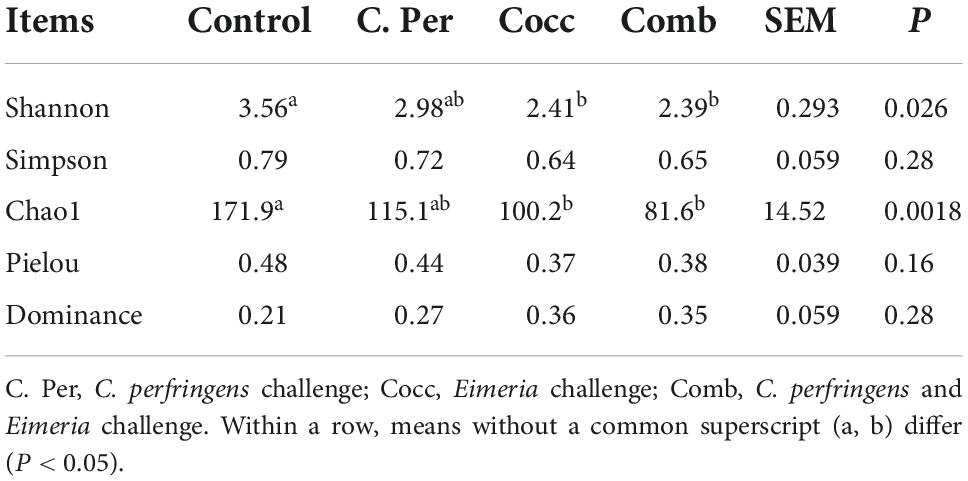
Table 1. Effects of Clostridium perfringens and/or Eimeria challenge on alpha diversity indices of ileal microbiota in yellow-feather broilers.
The main phyla present in the ileal digesta microbiota of the birds are shown in Table 2 and Figure 3. It was observed that Firmicutes and Bacteroidetes were the most frequent phyla, regardless of the treatment. Among the top 10 phyla, only the relative abundance of Bacteroidetes and Desulfobacterota were different among four groups. The C. perfringens and/or Eimeria challenge significantly decreased the relative abundance of Bacteroidetes compared to the control group (P < 0.05). In the ileal microbiota of yellow feather broilers, Bacteroidetes has the third highest abundance. At the genus level, among the top 15 genera analyzed, the relative abundance of Faecalibacterium was lower in the C. Per and Comb group in comparison with the control group. No difference between the control group and Cocc group was observed regarding the relative abundance of Faecalibacterium (Table 3). The relative abundance of the top 15 species in the ileal digesta microbiota of the broilers is shown in Table 4. The Lactobacillus aviarius was the most abundant species and its relative abundance was not different among the four groups (P = 0.90). Compared to the control group, the C. Per group had higher relative abundance of Motilimonas eburnea (P = 0.04). Its relative abundance in Cocc and Comb groups were not different from the control group. The control and C. Per groups had significantly higher abundance of Candidatus Arthromitus than the Cocc and Comb groups (P = 0.018).
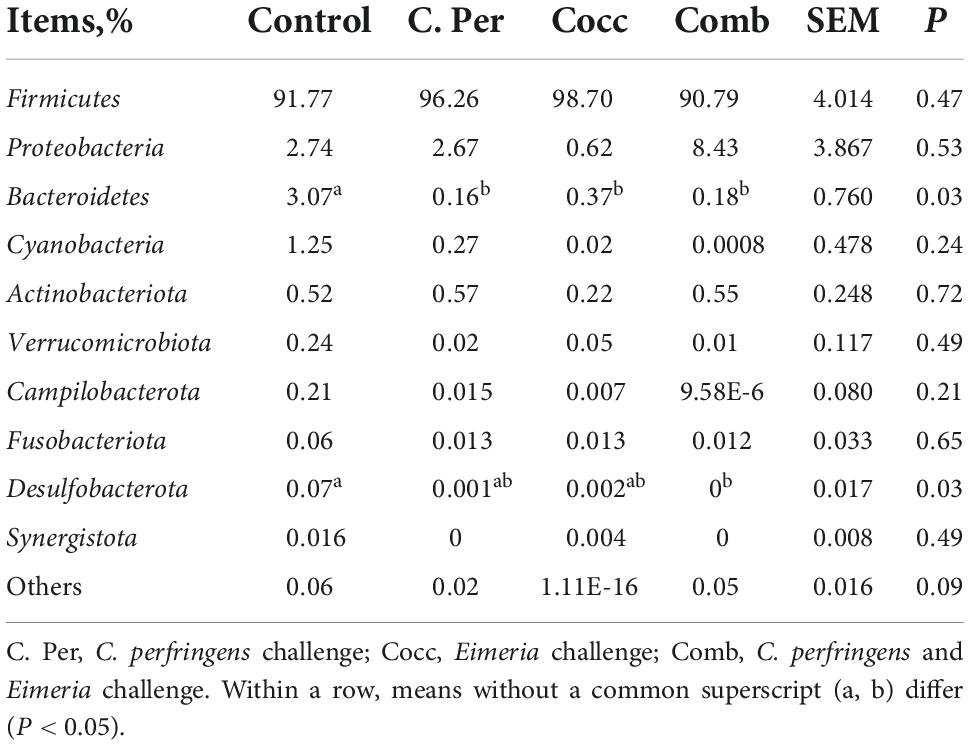
Table 2. Effects of Clostridium perfringens and/or Eimeria challenge on taxonomic composition of ileal microbiota at phylum level in yellow-feather broilers.
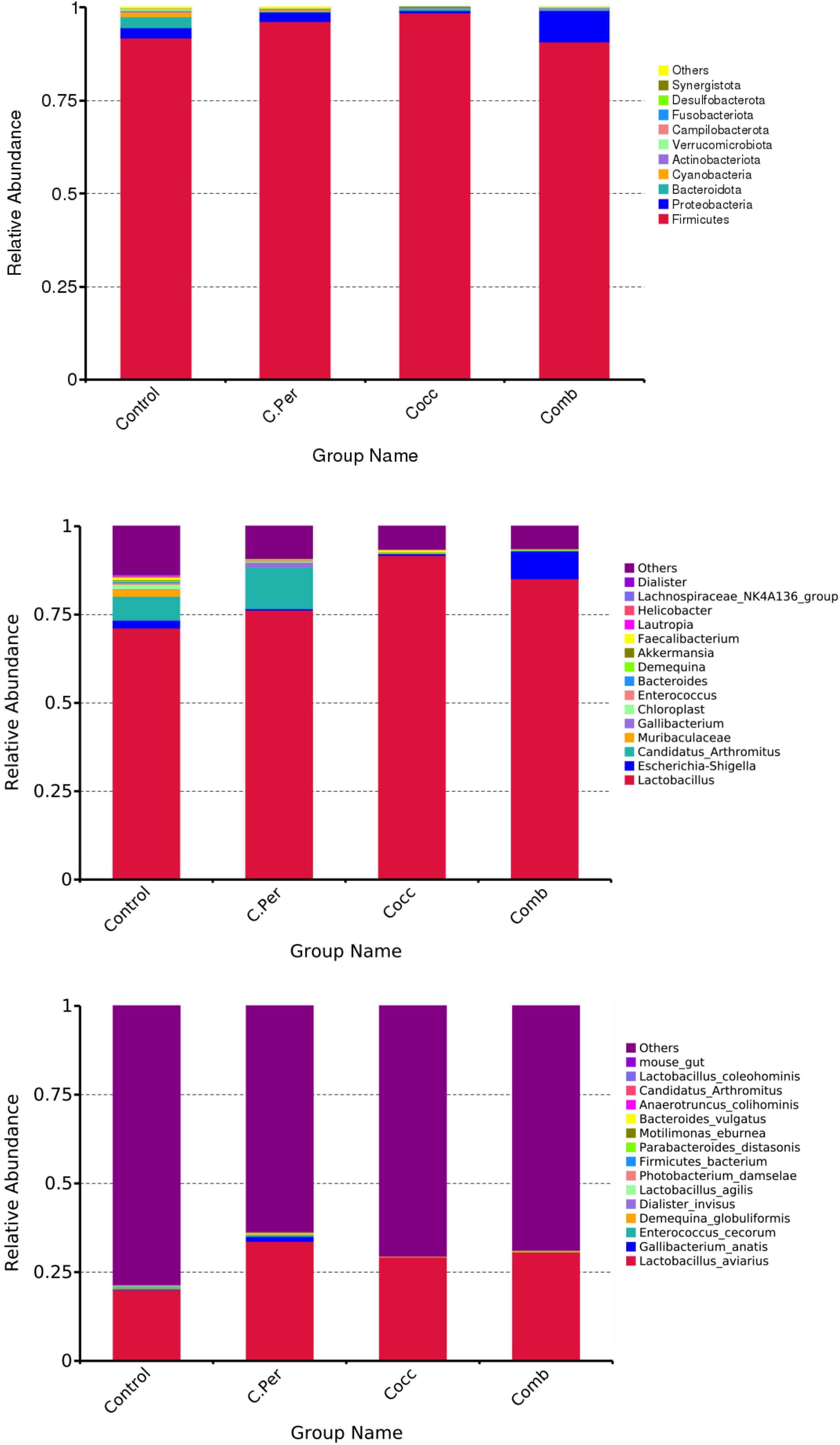
Figure 3. Phylum-level, genus-level, and species level taxonomic composition of the ileal bacterial communities in four groups. C. Per, C. perfringens challenge; Cocc, Eimeria challenge; Comb, C. perfringens and Eimeria challenge.
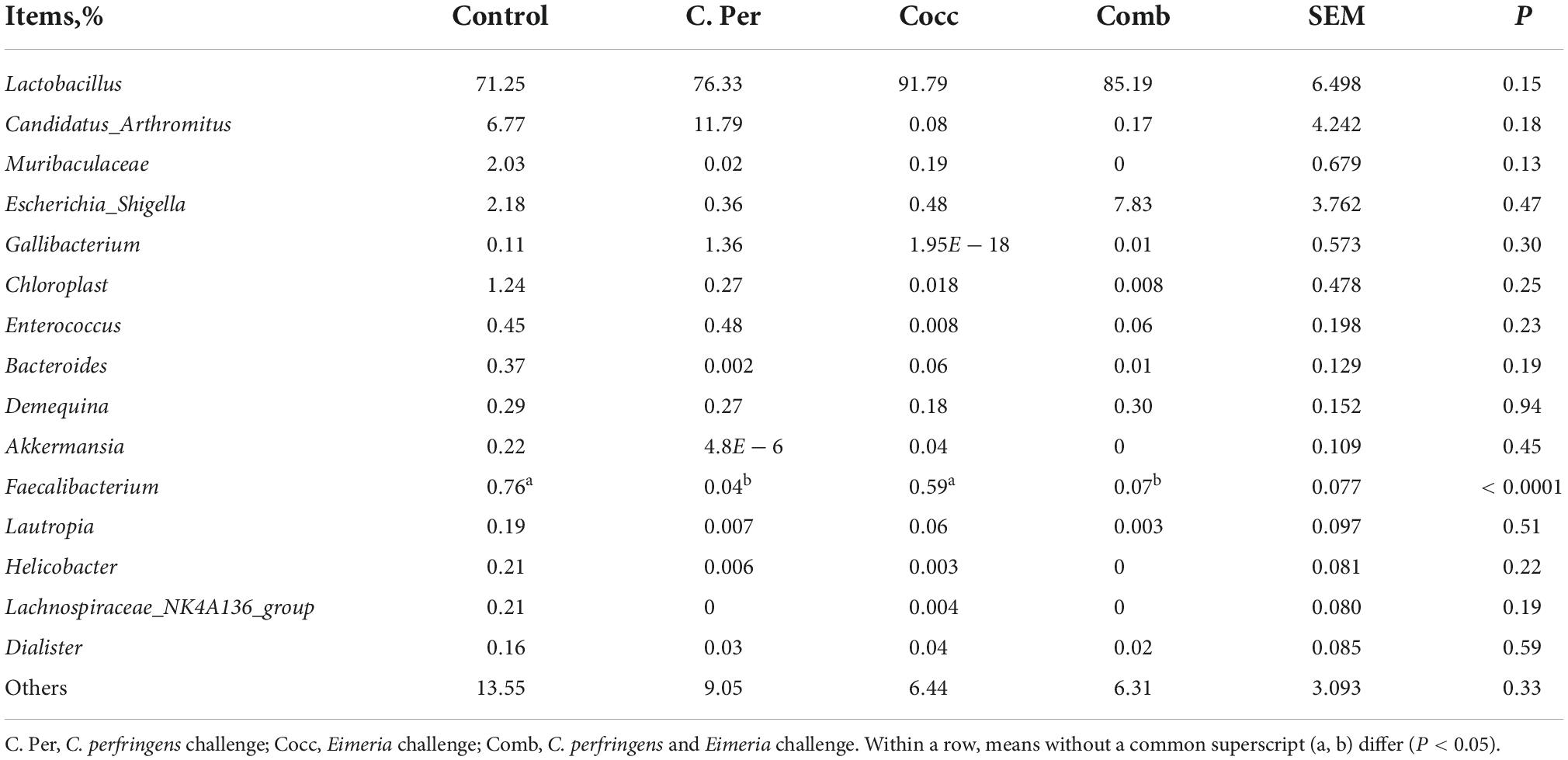
Table 3. Effects of Clostridium perfringens and/or Eimeria challenge on taxonomic composition of ileal microbiota at genus level in yellow-feather broilers.
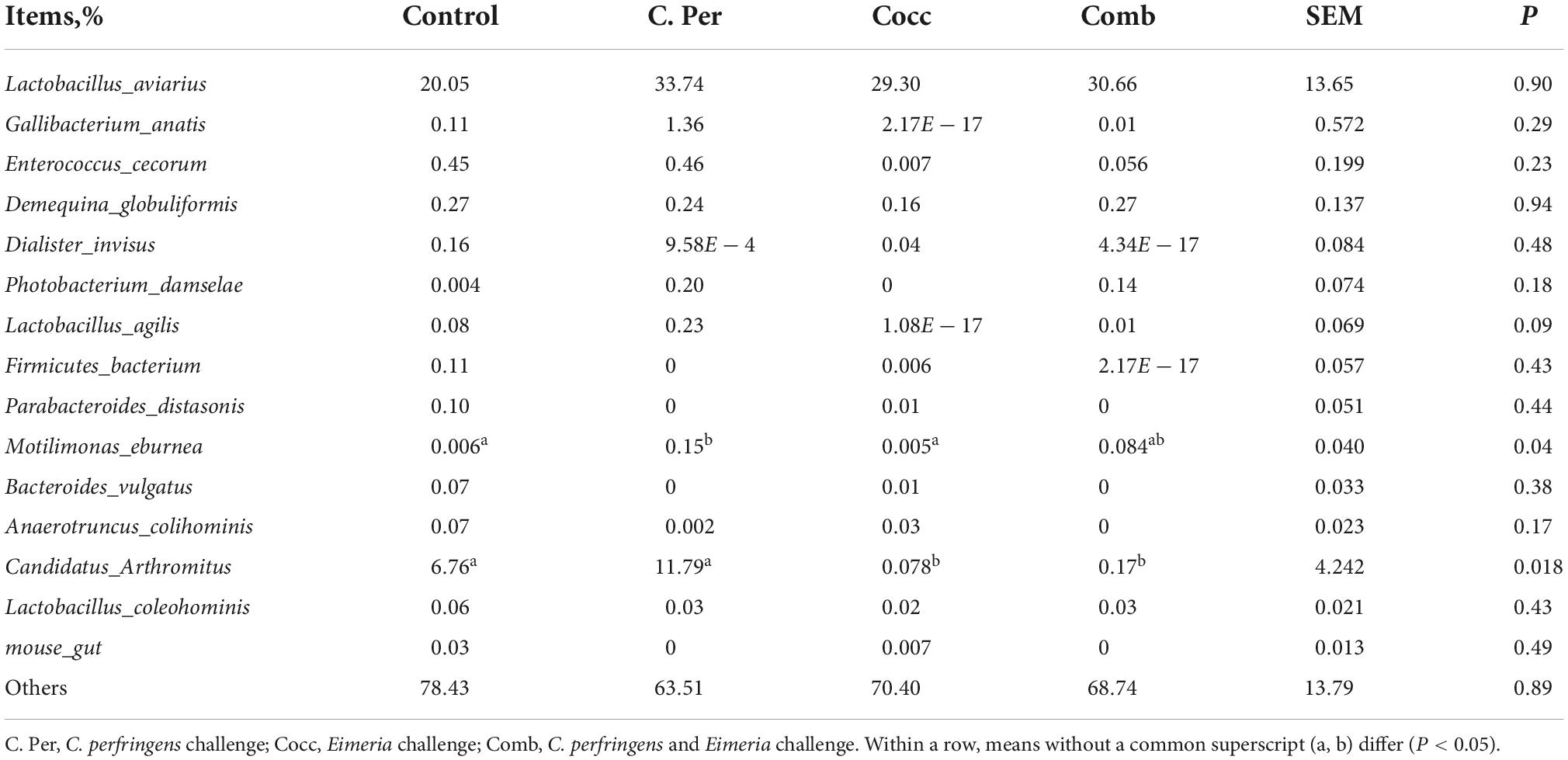
Table 4. Effects of Clostridium perfringens and/or Eimeria challenge on taxonomic composition of ileal microbiota at species level in yellow-feather broilers.
Linear discriminant analysis (LDA) effect size analysis (LDA > 3.5) was performed to discriminate the differences on the community composition between groups. A total of 25 biomarkers bacterial genera were identified in the four groups (Figure 4). Bacteroidales, Muribaculaceae (both at the family and genus level), Oscillospirales, Cyanobacteria, Cyanobacteria, Chloroplast (at the order, family, and genus levels), Lachnospirales, Lachnospiraceae, Ruminococcaceae were enriched in the control group. The C. Per group was enriched with Clostridiales, Clostridiaceae, Candidatus Arthromitus, Clostridia, Gallibacterium, Gallibacterium anatis, Pasteurellales, and Pasteurellaceae. In addition, Bacilli was enriched in Cocc group and Weissella and Leuconostocaceae were enriched in the Comb group. Consistent with results from Table 2, the control group had the highest abundance of Cyanobacteria, but not statistically different compared with other groups. This also applies to Candidatus Arthromitus, Muribaculaceae, Gallibacterium, and Chloroplast meaning that the groups in which they were enriched had the highest abundance but not significantly different from other groups. Based on the LEfSe analysis, Faecalibacterium was enriched in the control group. The control group did have significantly higher abundance of Faecalibacterium compared to the C. Per and Comb group (P < 0.05), but not different from the Cocc group.
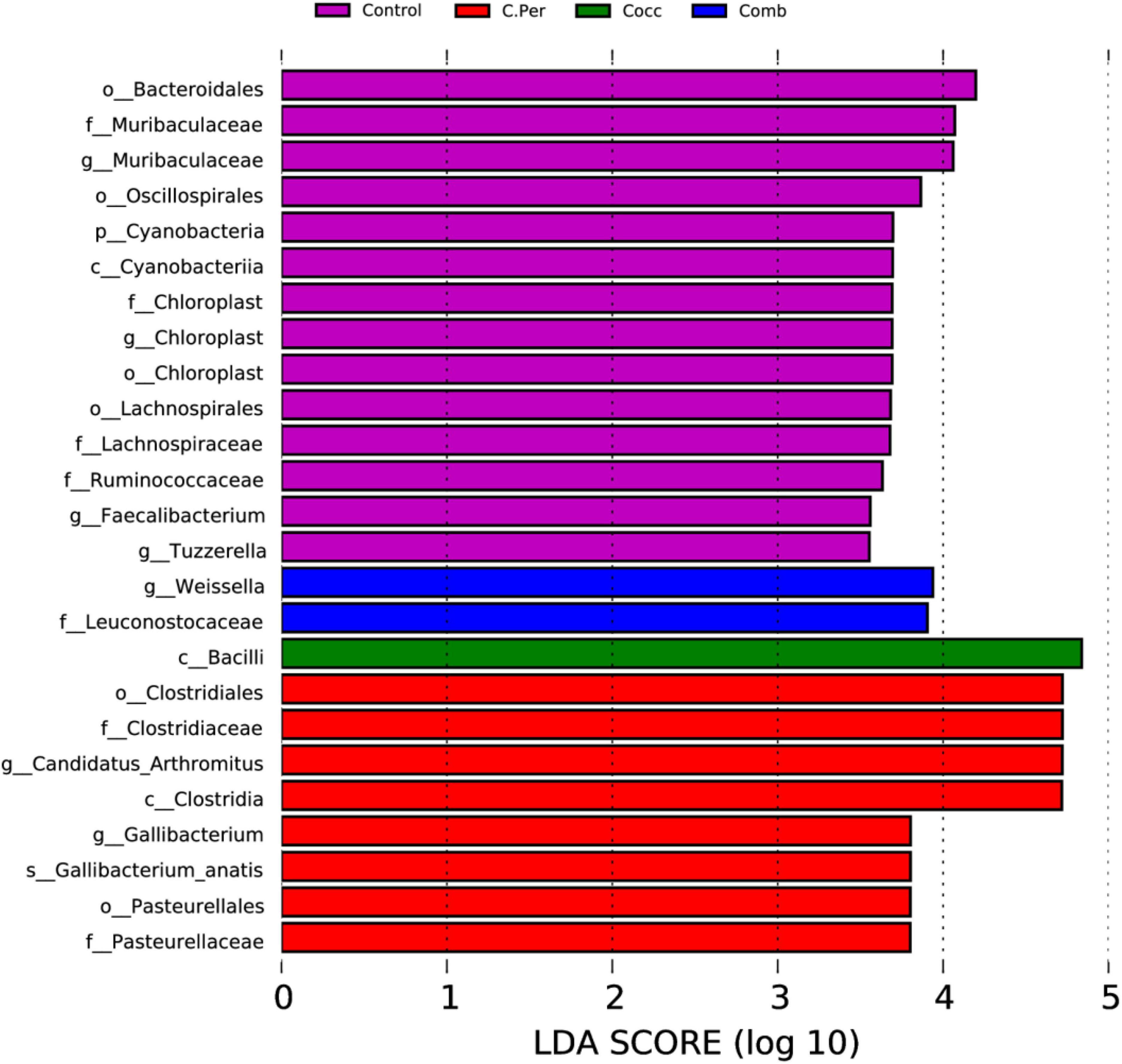
Figure 4. linear discriminant analysis (LDA) effect size (LEfSe) Analysis of differential species among all groups shown in the LDA value distribution histogram. The presented species are biomarkers with statistical differences between groups (LDA > 3.5). The length of the histogram (LDA score) represents the impact of the different species.
The predicted functions against the KEGG orthology database are presented in Figure 5. Total of 6,378 KOs were predicted among four groups and 5,215 were shared by all groups (Figure 5A). Compared to the control group, the other three groups had lower number of group specific microbial functions (330 for control vs. 61 for C. Per, 20 for Cocc, and 68 for Comb). The relative abundance of the top 10 microbial functions (Figure 5B) are most involved in the metabolism (K07024, K02761, K15634, and K01223), Cellular Processes (K02035 and K15508); genetic information processing (K02529 and K07496), and Environmental Information Processing (K01990 and K02004). All these functions were not statistically different among the four groups. The principal component analysis plot showed clusters of samples based on their microbial function similarity. It can be seen that samples from four groups could not be separated completely. The first and second components explained 36.37 and 22.67% of the variation, respectively (Figure 5C).
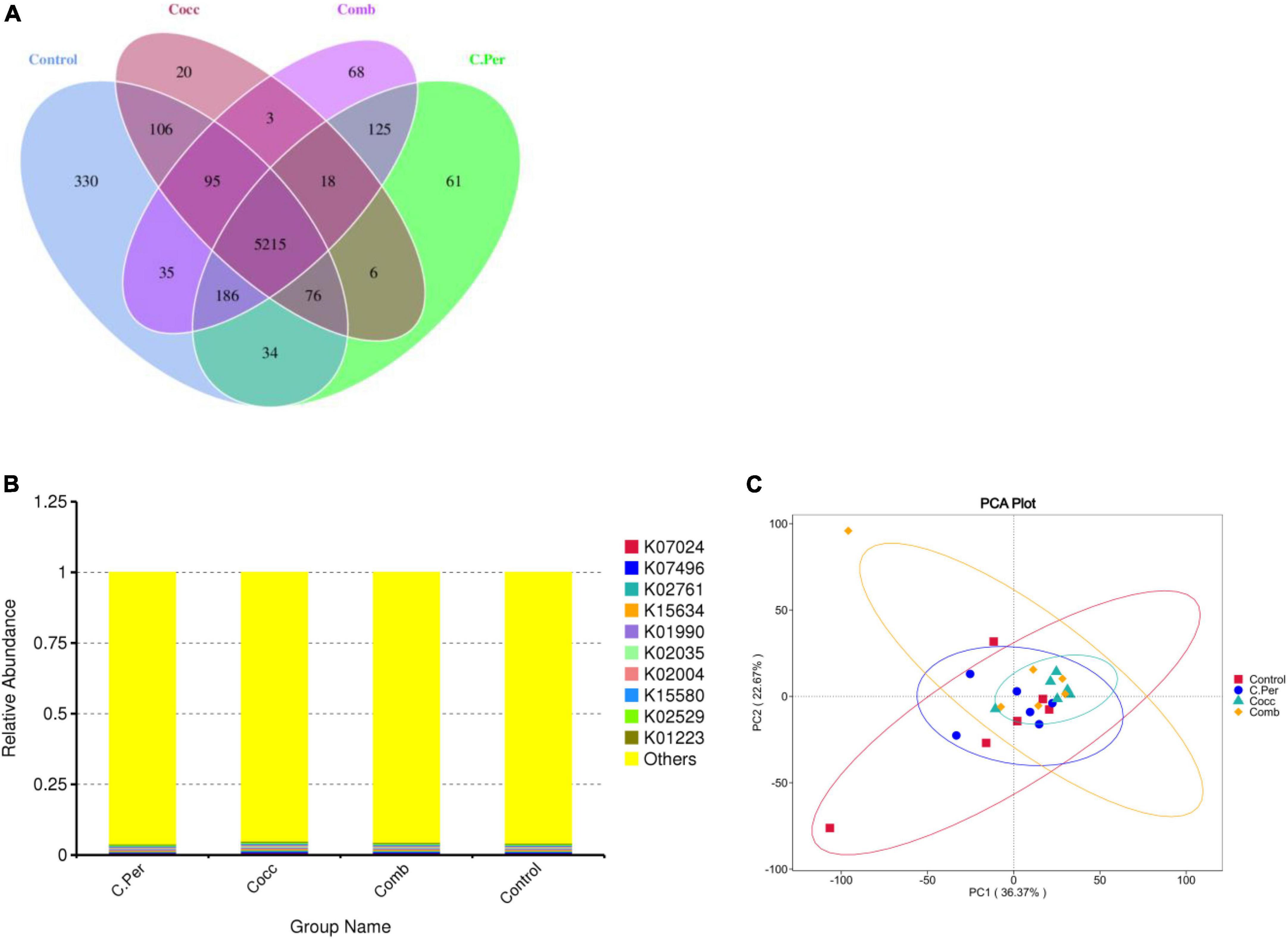
Figure 5. Function predictions using phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt2). (A) Venn diagram of shared and specific functions predicted in the four groups. (B) Barplot of the top functions predicted against KEGG orthology (KO) database. (C) Principle component analysis (PCA) of the functions predicted in different groups; C. Per, C. perfringens challenge; Cocc, Eimeria challenge; Comb, C. perfringens and Eimeria challenge.
Necrotic enteritis (NE) is a disease of the small intestine of chickens caused by C. perfringens (Zaytsoff et al., 2020). When C. Perfringens colonizes and proliferates in the small intestine, extracellular toxins produced can damage the intestinal wall thus cause necrotic enteritis. Modulation of C. perfringens on intestinal tight junctions and host immune response was reported earlier (Mitchell and Koval, 2010; Daneshmand et al., 2022). Predisposing factors such as high dietary protein or Eimeria infection have been utilized in the experimental reproduction of NE to investigate its pathogenesis or prevention (Shojadoost et al., 2012). Physiological stress as predisposing factor to NE has also been shown to increase densities of C. perfringens in the small intestine and weight gain impairment in chickens (Zaytsoff et al., 2020).
We used coccidia to facilitate an optimal NE reproduction in yellow feather broilers. During coccidiosis, Eimeria colonize the intestine and destroy intestinal epithelium cells which create an optimal environment for C. perfringens proliferation (Williams, 2005). In our study, lesions were not observed in the small intestine of the broilers inoculated with C. perfringens and/or Eimeria. Similarly, Wu et al. (2014) reported no observed lesions in the jejunum and ileum of Eimeria challenged birds. Daneshmand et al. (2022) observed that birds challenged with both Eimeria and C. Perfringens either on control diet or wheat-based diet had reduced performance coupled with enteric gross lesions and epithelial damage. The inconsistent findings might be related to the dose amount and Eimeria species used.
It has been shown that gut microbial community can alter the susceptibility of poultry to necrotic enteritis as they can affect gene expression in the ileum of the host (Tang et al., 2020). Six indices were often used to measure alpha diversity including Shannon, Simpson, Chao1, Good’s coverage, dominance, and Pielou. The greater the Chao1 and dominance indices, the higher the expected species richness of the microbiome. The greater the Shannon or the smaller the Simpson indices, the higher the diversity of the microbiome (Forbes et al., 2018). Pielou’s evenness index measures diversity along with species richness. A calculated value of Pielou’s evenness ranges from 0 (no evenness) to 1 (complete evenness). Compared to the control group, C. perfringens alone did not affect the richness or diversity of ileal microbiota. Eimeria challenge with or without C. perfringens all significantly decreased the Shannon and Chao1 indices indicating lower richness and diversity of intestinal microbiota in yellow feather broilers. Inconsistent to our results, increased chao1 index was observed in challenged birds (Bortoluzzi et al., 2019). Eimeria infection can significantly reduce the gut microbial diversity, most through reducing low abundance of operational taxonomic units (OTUs) and increasing dominance in the community (Perez et al., 2011). This was also observed in our study as groups challenged with Eimeria all had lower microbial diversity compared to the control group. Yang et al. (2021) investigated the link between ileal microbiota and disease severity in a chicken model of clinical NE. The authors reported that NE can significantly reduce the richness and Shannon index of ileal microbiota. Yang et al. (2019) reported that co-infection of C. perfringens and Eimeria significantly reduced species diversity in jejunal microbiota of broiler chicks but not cecal microbiota. Gastrointestinal tract is highly complex with numerous bacterial species, which would all contribute the development of NE. As shown in our results, changes in gut microbiota diversity induced by Eimeria may play an important role in predisposing the C. Perfringens infected birds to NE.
Healthy intestinal microbiota can enhance the host immune system and protection against intestinal pathogens (Ritzi et al., 2014). The dominance of beneficial microorganisms is essential to maintain gut homeostasis. Reduction of beneficial probiotic bacteria can predispose chickens to the onset of NE (Wu et al., 2014). LEfSe (Linear discriminant analysis Effect Size) determines the features most likely to explain differences between groups (Segata et al., 2011). Based on the LEfSe analysis, Faecalibacterium was enriched in the control group. Statistical analysis showed that the control group had significantly higher relative abundance of Faecalibacterium compared to the C. Per and Comb group. Surprisingly, no difference between the control group and Cocc group was observed. The sole known species of Faecalibacterium, Faecalibacterium prausnitzii, is a probiotic and its decline is associated with the development of chronic inflammation (Maioli et al., 2021). The decreased relative abundance of Faecalibacterium in the C. Per and Comb group indicated possible intestinal inflammation after challenge with C. perfringens. In chickens with severe NE, Firmicutes was significantly decreased whereas Proteobacteria was increased (Yang et al., 2021). The increase in the relative abundance of pathogenic group indicates that the birds are carriers of pathogenic bacteria, which might lead to future health issues (Akerele et al., 2022). As stated previously, in our study no lesions are observed in our study and the relative abundance of Firmicutes and Proteobacteria were not affected either. Bortoluzzi et al. (2019) observed no difference on the relative abundance of the most abundant phyla (Firmicutes, Bacteroidetes, and Proteobacteria) between the challenged and unchallenged birds. But in our study, the challenge groups had lower abundance of Bacteroidetes which was inconsistent to Bortoluzzi et al. (2019). The Bacteroidetes phylum has the ability to degrade a wide range of complex carbohydrates, making its dominance in many diverse environments (McKee et al., 2021). Decreased abundance of Bacteroidetes indicates lower ability of the ileal microbes on degradation of complex carbohydrates. Coccidial infection can dramatically decrease the resident microbiota and increase conditionally pathogenic bacteria such as Clostridiales (Ramanan et al., 2016; Lu et al., 2021). It can lead to more severe clinical manifestations by providing an environment which is conducive for pathogenic bacteria, such as Campylobacter jejuni, C. perfringens, Salmonella, and other bacteria (Kogut et al., 1994; Ficko-Blean et al., 2012; Macdonald et al., 2019). Major perturbations in lactic acid-producing and butyrate-producing families of bacteria in chickens with NE were observed (Antonissen et al., 2016). However, the results have been inconsistent among studies due to the varying severity degree of NE. Lactobacillus is known as beneficial bacteria by competition with pathogens and produce lactic acid which can inhibit pathogenic bacteria (Belenguer et al., 2007). Lactobacillus was more abundant in the upper GI tract compared with the lower tract. C. Perfringens challenge could decrease the Lactobacillus population in ileum (Antonissen et al., 2016; Akerele et al., 2022). But this was not observed in our study. Lactobacillus reuteri, L. johnsonii, L. acidophilus, L. crispatus, L. salivarius, and L. aviarius were the predominant Lactobacillus species and present throughout the GI tract of chickens (Wang et al., 2014). Our results showed that at the species level, L. aviarius had the highest relative abundance which is similar to Gong et al. (2007)’s findings. Lactobacillus aviarius was first isolated from the intestine of chickens in Fujisawa et al. (1984) and is very useful enhancing immunity systems of the organisms (Al-Shaer et al., 2019). It has been reported that probiotics supplementation shifted bacterial community in broiler digestive tract, with Lactobacillus salivarius and Lactobacillus aviarius as dominant species in the treatment group. Some researchers reported suppressed Lactobacillus at the early stage of C. perfringens challenge (Fasina et al., 2016) whereas opposite findings were also reported by others (Liu et al., 2010). C. perfringens infection could suppress L. aviarius in the ileum of broilers (Du et al., 2015). In our study, the relative abundance of Lactobacillus aviaries was not different among all four groups. However, it should be noted that the variation of Lactobacillus aviaries abundance within groups was fairly high. Lactobacillaceae shift in broilers with NE induced by dual E. maxima and C. perfringens are dependent on breed whereas relative abundance of Lactobacillus was increasing in the ileal content of Cobb 500 broilers but decreasing in Ross 308 (Kim et al., 2015). In addition, the time of challenge may also play a role. In Yang et al. (2021)’s study, the chickens were inoculated with E. maxima on day 10 and 4 × 108 CFU of C. perfringens on day 14. In our Comb group, we did this on day 14 and day 17. Rearing conditions could also affect the gut microbial compositions for NE challenged birds. Bortoluzzi et al. (2019) found that the cecal microbiota composition and function was more affected than the ileal microbiota for NE challenged bird raised in floor pens with reused litter compared to birds raised in cages. Motilimonas eburnea in the order Alteromonadales, was first isolated from coastal sediment and this novel species was named by the authors (Ling et al., 2017). The reason that the C. Per and Comb groups had higher abundance is not clear as limited research was reported on this species. Candidatus Arthromitus, also known as Candidatus Savagella, may provide a protective role in preventing the onset of the enteric condition in Turkeys (Hedblom et al., 2018). It has the potential to serve as an immune-stimulatory probiotic making it an organism of great interest to poultry researchers. Stanley et al. (2012) reported decreased abundance of Candidatus Arthromitus in the cecal digesta of broiler chickens challenged with oocysts of E. acervulina, E. maxima, and E. brunette. This was similar to our study as that the Cocc and Comb groups with Eimeria challenge had significantly lower abundance than the control and the C. Per groups.
Studying the composition of the gut microbiota not only can reflect the relationship between microorganisms and the host but also provide information of the microbial functions on the gut (Bortoluzzi et al., 2019). To further understand the functions performed by the gut microbes, we used PICRUSt2 to make inferences about the metabolic functions of the gut microbes against the KEGG orthology database. We found that the top 10 microbial functions are most involved in the metabolism (starch and sucrose metabolism), Cellular Processes (Quorum sensing), genetic information processing (transcription factors), and Environmental Information Processing (ABC transporters). All these predicted functions were not affected by treatments. Bortoluzzi et al. (2019) reported that NE mainly affected microbial functions of cecal microorganisms not ileal microorganism. They observed NE challenge enriched pathways related to DNA replication, proteins, amino acids related enzymes and metabolism, and transcription factors (Bortoluzzi et al., 2019). In that study, the challenged birds groups had enriched “ion channel” function compared to the control groups. We did not observe any difference regarding specific functions among the four groups and this might be because we analyzed the ileal digesta samples. Enriched pathways related to amino acid metabolism was observed in the challenged birds (Zhang et al., 2018; Yang et al., 2021). The possible reasons could be due to pathogens such as Helicobacter pylori and Salmonella Typhimurium which use amino acids as energy source (Zhang et al., 2018) or activated amino acid synthetic pathways in the remaining bacterial population to compensate for an inability of C. perfringens to synthesize amino acids (Yang et al., 2021). Yang et al. (2021) also suggested that metabolism of cofactors and vitamins may be enhanced in severe NE cases. In our study, the microbial functions were all predicted based on 16S rRNA sequencing data and follow-up metagenomics of the microbiota warrants further investigation.
Yellow-feather broiler, also known as three-yellow chicken (yellow feather, yellow skin, and yellow shank.) are high-quality chicken with excellent meat quality and flavor. They are mainly raised in south China. Researches on the inter relationship of NE and gut microbiota of yellow feather broilers were limited. An better understanding of the importance of gut microbiota in disease onset, progression, and treatment is utmost. The disease model would lay foundation for microbial manipulation of NE in yellow feather broilers. Further studies are needed to determine and improve the robustness and reproducibility of NE disease model.
The present study used Eimeria for NE reproduction to evaluate the effects of C. perfringens on ileal microbiota of yellow feather broilers. In summary, C. perfringens alone did not affect the alpha diversity of ileal microbiota in yellow feather broilers but co-infection with Eimeria significantly decreased the Shannon and Chao1 indices. Eimeria or C. perfringens challenge also decreased the relative abundance of beneficial bacteria including Bacteroidetes at the phylum level, Faecalibacterium at the genus level and Candidatus Arthromitus at the species level.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Bioproject accession number: PRJNA892261.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Foshan University.
XF, TL, and HZ conducted the experiment and wrote the manuscript together. LL and SB analyzed the samples. XC analyzed the data. HHZ provided critical feedback and helped shape the research. All authors contributed to the article and approved the submitted version.
This work received financial support from the Discipline Construction Program of Foshan University (CGZ0400162), the Scientific Research Foundation in the Higher Education Institutions of Educational Commission of Guangdong Province (2017GCZX006), the Guangdong Basic and Applied Basic Research Foundation (2019A1515110780), the Guangdong Province Modern Agriculture Poultry Industry Technology System Innovation Team Construction Project (2022KJ128), Special Projects in Key Fields of Guangdong Provincial Department of Education (2019KZDZX2006), the Guangdong Science and Technology Innovation Strategy Special Fund (DZX20192520309), the Research Start-Up Fund for Postdoctoral Fellows from Foshan City (BKS209059), and the Scientific Research Start-Up Fund for High-Level Talents of Foshan University (Gg07145).
Author LL was employed by Foshan Zhengdian Biology Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akerele, G., Al Hakeem, W. G., Lourenco, J., and Selvaraj, R. K. (2022). The effect of necrotic enteritis challenge on production performance, cecal microbiome, and cecal tonsil transcriptome in broilers. Pathogens 11:839. doi: 10.3390/pathogens11080839
Al-Shaer, B. M., Al-Batshan, H. A., and Al-Atiyat, R. M. (2019). Effect of probiotics on diversity of bacterial community in the gastrointestinal tract of chickens. Singapore J. Sci. Res. 9, 86–94.
Antonissen, G., Eeckhaut, V., Van Driessche, K., Onrust, L., Haesebrouck, F., Ducatelle, R., et al. (2016). Microbial shifts associated with necrotic enteritis. Avian Pathol. 45, 308–312. doi: 10.1080/03079457.2016.1152625
Attree, E., Sanchez-Arsuaga, G., Jones, M., Xia, D., Marugan-Hernandez, V., Blake, D., et al. (2021). Controlling the causative agents of coccidiosis in domestic chickens; an eye on the past and considerations for the future. CABI Agric. Biosci. 2:37. doi: 10.1186/s43170-021-00056-5
Baba, E., Wakeshima, H., Fukui, K., Fukata, T., and Arakawa, A. (1992). Adhesion of bacteria to the cecal mucosal surface of conventional and germ-free chickens infected with Eimeria tenella. Am. J. Vet. Res. 53, 194–197.
Belenguer, A., Duncan, S. H., Holtrop, G., Anderson, S. E., Lobley, G. E., and Flint, H. J. (2007). Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl. Environ. Microbiol. 73, 6526–6533. doi: 10.1128/AEM.00508-07
Bortoluzzi, C., Vieira, B. S., Hofacre, C., and Applegate, T. J. (2019). Effect of different challenge models to induce necrotic enteritis on the growth performance and intestinal microbiota of broiler chickens. Poult. Sci. 98, 2800–2812. doi: 10.3382/ps/pez084
Caly, D. L., D’Inca, R., Auclair, E., and Drider, D. (2015). Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: A microbiologist’s perspective. Front. Microbiol. 6:1336. doi: 10.3389/fmicb.2015.01336
Collier, C. T., Hofacre, C. L., Payne, A. M., Anderson, D. B., Kaiser, P., Mackie, R. I., et al. (2008). Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 122, 104–115. doi: 10.1016/j.vetimm.2007.10.014
Daneshmand, A., Kermanshahi, H., Mohammed, J., Sekhavati, M. H., Javadmanesh, A., Ahmadian, M., et al. (2022). Intestinal changes and immune responses during Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult. Sci. 101:101652. doi: 10.1016/j.psj.2021.101652
Douglas, G. M., Maffei, V. J., Zaneveld, J. R., Yurgel, S. N., Brown, J. R., Taylor, C. M., et al. (2020). PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 38, 685–688. doi: 10.1038/s41587-020-0548-6
Du, E., Gan, L., Li, Z., Wang, W., Liu, D., and Guo, Y. (2015). In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 6:58. doi: 10.1186/s40104-015-0055-7
Fasina, Y. O., Newman, M. M., Stough, J. M., and Liles, M. R. (2016). Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 95, 247–260. doi: 10.3382/ps/pev329
Feng, X., Zhu, H., Chen, B., Zhu, C., Gong, L., Hu, Z., et al. (2020). Effects of phytosterols supplementation on growth performance and intestinal microflora of yellow-feather broilers. Poult. Sci. 99, 6022–6030. doi: 10.1016/j.psj.2020.07.036
Ficko-Blean, E., Stuart, C. P., Suits, M. D., Cid, M., Tessier, M., Woods, R. J., et al. (2012). Carbohydrate recognition by an architecturally complex alpha-N-acetylglucosaminidase from Clostridium perfringens. PLoS One 7:e33524. doi: 10.1371/journal.pone.0033524
Forbes, J. D., Chen, C. Y., Knox, N. C., Marrie, R. A., El-Gabalawy, H., de Kievit, T., et al. (2018). A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome 6:221. doi: 10.1186/s40168-018-0603-4
Fujisawa, T., Shirasaka, S., Watabe, J., and Mitsuoka, T. (1984). Lactobacillus aviarius sp. nov.: A new species isolated from the intestine of chickens. Syst. Appl. Microbiol. 5, 414–420. doi: 10.1016/S0723-2020(84)80042-9
Giannenas, I., Papadopoulos, E., Tsalie, E., Triantafillou, E., Henikl, S., Teichmann, K., et al. (2012). Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet. Parasitol. 188, 31–40. doi: 10.1016/j.vetpar.2012.02.017
Gong, J., Si, W., Forster, R. J., Huang, R., Yu, H., Yin, Y., et al. (2007). 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: From crops to ceca. FEMS Microbiol. Ecol. 59, 147–157. doi: 10.1111/j.1574-6941.2006.00193.x
Haas, B. J., Gevers, D., Earl, A. M., Feldgarden, M., Ward, D. V., Giannoukos, G., et al. (2011). Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21, 494–504. doi: 10.1101/gr.112730.110
Hedblom, G. A., Reiland, H. A., Sylte, M. J., Johnson, T. J., and Baumler, D. J. (2018). Segmented filamentous bacteria - metabolism meets immunity. Front. Microbiol. 9:1991. doi: 10.3389/fmicb.2018.01991
Kim, J. E., Lillehoj, H. S., Hong, Y. H., Kim, G. B., Lee, S. H., Lillehoj, E. P., et al. (2015). Dietary Capsicum and Curcuma longa oleoresins increase intestinal microbiome and necrotic enteritis in three commercial broiler breeds. Res. Vet. Sci. 102, 150–158. doi: 10.1016/j.rvsc.2015.07.022
Kogut, M. H., Fukata, T., Tellez, G., Hargis, B. M., Corrier, D. E., and DeLoach, J. R. (1994). Effect of Eimeria tenella infection on resistance to Salmonella typhimurium colonization in broiler chicks inoculated with anaerobic cecal flora and fed dietary lactose. Avian Dis. 38, 59–64. doi: 10.2307/1591837
Ling, S. K., Guo, L. Y., Chen, G. J., and Du, Z. J. (2017). Motilimonas eburnea gen. nov., sp. nov., isolated from coastal sediment. Int. J. Syst. Evol. Microbiol. 67, 306–310. doi: 10.1099/ijsem.0.001621
Liu, D., Guo, Y., Wang, Z., and Yuan, J. (2010). Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. 39, 17–24. doi: 10.1080/03079450903447404
Lu, C., Yan, Y., Jian, F., and Ning, C. (2021). Coccidia-microbiota interactions and their effects on the host. Front. Cell. Infect. Microbiol. 11:751481. doi: 10.3389/fcimb.2021.751481
Macdonald, S. E., van Diemen, P. M., Martineau, H., Stevens, M. P., Tomley, F. M., Stabler, R. A., et al. (2019). Impact of Eimeria tenella Coinfection on Campylobacter jejuni Colonization of the Chicken. Infect. Immun. 87:e00772-18. doi: 10.1128/IAI.00772-18
Maioli, T. U., Borras-Nogues, E., Torres, L., Barbosa, S. C., Martins, V. D., Langella, P., et al. (2021). Possible benefits of Faecalibacterium prausnitzii for obesity-associated gut disorders. Front. Pharmacol. 12:740636. doi: 10.3389/fphar.2021.740636
McKee, L. S., La Rosa, S. L., Westereng, B., Eijsink, V. G., Pope, P. B., and Larsbrink, J. (2021). Polysaccharide degradation by the Bacteroidetes: Mechanisms and nomenclature. Environ. Microbiol. Rep. 13, 559–581. doi: 10.1111/1758-2229.12980
Miller, R. W., Skinner, E. J., Sulakvelidze, A., Mathis, G. F., and Hofacre, C. L. (2010). Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 54, 33–40. doi: 10.1637/8953-060509-Reg.1
Mitchell, L. A., and Koval, M. (2010). Specificity of interaction between Clostridium perfringens Enterotoxin and Claudin-Family tight junction proteins. Toxins 2, 1595–1611. doi: 10.3390/toxins2071595
Moore, R. J. (2016). Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 45, 275–281. doi: 10.1080/03079457.2016.1150587
Perez, V. G., Jacobs, C. M., Barnes, J., Jenkins, M. C., Kuhlenschmidt, M. S., Fahey, G. C. Jr., et al. (2011). Effect of corn distillers dried grains with solubles and Eimeria acervulina infection on growth performance and the intestinal microbiota of young chicks. Poult. Sci. 90, 958–964. doi: 10.3382/ps.2010-01066
Ramanan, D., Bowcutt, R., Lee, S. C., Tang, M. S., Kurtz, Z. D., Ding, Y., et al. (2016). Helminth infection promotes colonization resistance via type 2 immunity. Science 352, 608–612. doi: 10.1126/science.aaf3229
Ritzi, M. M., Abdelrahman, W., Mohnl, M., and Dalloul, R. A. (2014). Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 93, 2772–2778. doi: 10.3382/ps.2014-04207
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12:R60. doi: 10.1186/gb-2011-12-6-r60
Shojadoost, B., Vince, A. R., and Prescott, J. F. (2012). The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: A critical review. Vet. Res. 43:74. doi: 10.1186/1297-9716-43-74
Skinner, J. T., Bauer, S., Young, V., Pauling, G., and Wilson, J. (2010). An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 54, 1237–1240. doi: 10.1637/9399-052110-Reg.1
Stanley, D., Keyburn, A. L., Denman, S. E., and Moore, R. J. (2012). Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 159, 155–162. doi: 10.1016/j.vetmic.2012.03.032
Tang, D., Li, Z., Mahmood, T., Liu, D., Hu, Y., and Guo, Y. (2020). The association between microbial community and ileal gene expression on intestinal wall thickness alterations in chickens. Poult. Sci. 99, 1847–1861. doi: 10.1016/j.psj.2019.10.029
Timbermont, L., Haesebrouck, F., Ducatelle, R., and Van Immerseel, F. (2011). Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 40, 341–347. doi: 10.1080/03079457.2011.590967
Van Immerseel, F., Rood, J. I., Moore, R. J., and Titball, R. W. (2009). Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 17, 32–36. doi: 10.1016/j.tim.2008.09.005
Wang, L., Fang, M., Hu, Y., Yang, Y., Yang, M., and Chen, Y. (2014). Characterization of the most abundant Lactobacillus species in chicken gastrointestinal tract and potential use as probiotics for genetic engineering. Acta Biochim. Biophys. Sin. (Shanghai) 42, 612–619. doi: 10.1093/abbs/gmu037
Williams, R. B. (2005). Intercurrent coccidiosis and necrotic enteritis of chickens: Rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 34, 159–180. doi: 10.1080/03079450500112195
Wu, S. B., Stanley, D., Rodgers, N., Swick, R. A., and Moore, R. J. (2014). Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 169, 188–197. doi: 10.1016/j.vetmic.2014.01.007
Yang, Q., Liu, J., Wang, X., Robinson, K., Whitmore, M. A., Stewart, S. N., et al. (2021). Identification of an intestinal microbiota signature associated with the severity of necrotic enteritis. Front. Microbiol. 12:703693. doi: 10.3389/fmicb.2021.703693
Yang, W.-Y., Lee, Y., Lu, H., Chou, C. H., and Wang, C. (2019). Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS One 14:e0205784. doi: 10.1371/journal.pone.0205784
Zaytsoff, S. J. M., Lyons, S. M., Garner, A. M., Uwiera, R. R. E., Zandberg, W. F., Abbott, D. W., et al. (2020). Host responses to Clostridium perfringens challenge in a chicken model of chronic stress. Gut Pathog. 12:24. doi: 10.1186/s13099-020-00362-9
Keywords: challenge, C. perfringens, Eimeria, microbiota, yellow feather broiler
Citation: Feng X, Li T, Zhu H, Liu L, Bi S, Chen X and Zhang H (2022) Effects of challenge with Clostridium perfringens, Eimeria and both on ileal microbiota of yellow feather broilers. Front. Microbiol. 13:1063578. doi: 10.3389/fmicb.2022.1063578
Received: 07 October 2022; Accepted: 17 November 2022;
Published: 01 December 2022.
Edited by:
Qing Pan, Qingdao Agricultural University, ChinaReviewed by:
Mangesh Vasant Suryavanshi, Lerner Research Institute - Cleveland Clinic, United StatesCopyright © 2022 Feng, Li, Zhu, Liu, Bi, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huihua Zhang, aGh6aGFuZzJAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.