- 1Center of Infectious Disease and Pathogen Biology, Department of Infectious Diseases, The First Hospital of Jilin University, Changchun, China
- 2Department of Tuberculosis, Changchun Infectious Disease Hospital, Changchun, China
- 3Intensive Care Unit, The First Hospital of Jilin University, Changchun, China
- 4Department of Infectious Diseases, Changchun Infectious Disease Hospital, Changchun, China
Background: Under the wave of the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) variant Omicron epidemic, the number of infectious cases has increased dramatically in Jilin Province, China since March 2022.The clinical features and severity of SARS-CoV-2 Omicron variant infection in tuberculosis (TB) patients are not yet clear.
Methods: Data were obtained from 153 patients with the Omicron variant and TB coinfection and 153 non-TB COVID-19 patients who had been hospitalized at Changchun Infectious Disease Hospital from March to June 2022.
Results: Among these coinfection patients, 17 patients showed COVID-19-related pneumonia on chest imaging and 11 were diagnosed with severe COVID-19. The median duration of SARS-CoV-2 clearance was 13 days. The negative conversion time was associated with age, COVID-19-related pneumonia and antibody IgG. A higher white blood cell count, a lower lymphocyte percentage, a higher CRP level, and a higher D-dimer level were found in the severe group. Age and increased PCT were individual risk factors for the severity of COVID-19. Compared with the non-TB patients, the coinfection patients had higher severity of COVID-19 and the elder coinfection patients had a longer negative conversion time.
Conclusion: This study found an association between age, pneumonia, antibody IgG and RNA negative conversion time in COVID-19 and TB coinfection patients, and age and increased PCT were risk factors for the severity of COVID-19.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has quickly spread around the world (Lai et al., 2020).To adapt to human hosts, SARS-CoV-2 has been prone to genetic evolution with the development of mutations over time, resulting in mutant variants that may have different characteristics than its ancestral strains (Aleem et al., 2022). Several variants of SARS-CoV-2 have been described by the World Health Organization (WHO), especially given their impact on global public health. Alpha, Beta, Gamma, Delta, and Omicron have been identified as the five SARS-CoV-2 variants of concern (VOCs) since the beginning of the pandemic (Hirabara et al., 2021). The SARS-CoV-2 Omicron variant was first reported from Botswana on November 11, 2021, and it has now replaced the other variants as the main epidemic strain, and this strain is characterized by multiple mutations, stronger infectivity, immune escape characteristics, and a lower risk of severe disease (Karim and Karim, 2021).Under the wave of the Omicron epidemic, the number of infectious cases has increased dramatically in Jilin Province, China from March 2022 (Fan et al., 2022; Li et al., 2022a,b).
The WHO estimated that 9.9 million people fell ill with tuberculosis (TB) worldwide in 2020, and TB is always one of the most important public health concerns in the world. China has the second-highest number of TB cases (8.5%) in the world and is still one of the countries with the highest TB burden (Long et al., 2021). In the past 2 years, the COVID-19 pandemic has caused enormous health, social and economic impacts, including the provision of and access to essential TB services, the number of people diagnosed with TB and the notifications of TB cases through the national disease surveillance systems and TB disease burden (Umubyeyi Nyaruhirira et al., 2022). While the experience of COVID-19 infection in TB patients remains limited, it is anticipated that people ill with both TB and COVID-19 may have poorer COVID-19 treatment outcomes, and TB should be considered a risk factor for severe COVID-19 (TB/COVID-19 Global Study Group, 2022). In a previous study, there were high cases of COVID-19 infections in countries that had less BCG vaccination as compared to countries that immensely use BCG vaccine. It indicated that those vaccinated against TB have been found to have lower susceptibility to COVID-19 (Mariita and Musila, 2020). To report the available evidence on the interaction between COVID-19 and TB, especially for the Omicron variant coinfection, we retrospectively reviewed the epidemiological and clinical manifestations of 153 hospitalized patients with COVID-19 and TB coinfection at Changchun infectious disease hospital (Changchun, China) during March 2022–June 2022.
Materials and methods
Ethics statement
Ethical approval for this study was obtained from the Ethics Committee of Changchun Infectious Disease Hospital and the Ethics Committee of the 1stHospital of Jilin University, China. Before data analysis and reporting, all personal identifiers of the patients were removed. All patients provided written informed consent.
Study population and data collection
In this study, hospital COVID-19 and TB coinfection admissions at Changchun Infectious Disease Hospital (Changchun, China) from March 2022 to June 2022 during the outbreak were involved. To compare the time of SARS-CoV-2 RNA negative conversion, a group of COVID-19 patients without TB who were hospitalized in the same hospital during the same period was chosen as a control, who were chosen with a 1:1 ratio (n = 153) and matched for age and gender. The inclusion criteria for COVID-19 and TB coinfection patients included the following: (1) age ≥ 14 years; (2) diagnosis of COVID-19 by confirmed SARS-CoV-2 PCR results in accordance with Chinese Guidelines for Prevention and Control of Novel Coronavirus (Edition 9; CDC, 2022); (3) TB patients who were diagnosed in accordance with the Chinese national guidelines for the prevention and control of TB. Several tests are used to diagnose TB, including acid-fast bacilli smear, GeneXpert, culture, a tuberculin skin test (TST), interferon-gamma release assays and computer tomography (CT); and (4) patients who complied with the study procedures and agreed to participate in the study. All the patients’ records were reviewed for admission, clinical severity, medical history, vaccination, and prognosis. All patients were included in the sample.
Statistical analysis
Descriptive statistics are presented as the mean ± standard deviation (SD), median (interquartile range), or number (percentage) for continuous variables and frequencies for categorical variables. Comparisons between the groups were analyzed with a 2-tailed Student’s t test for parametric analysis and a Mann–Whitney U test for nonparametric data analysis. Categorical variables are presented as counts and percentages, and differences between the groups were analyzed with a Pearson χ2 test or Fisher’s exact test to assess categorical variables. Univariate logistic regression analysis with a p < 0.1. Then, multivariate logistic regression analysis was performed on the indicators with p < 0.05 in the univariate logistic regression analysis. Statistical analyses were performed using SPSS ver. 22.0 (SPSS, Inc., Chicago, IL, United States). A p < 0.05 (two-sided) was considered statistically significant.
Results
Demographic, epidemiological, and clinical characteristics
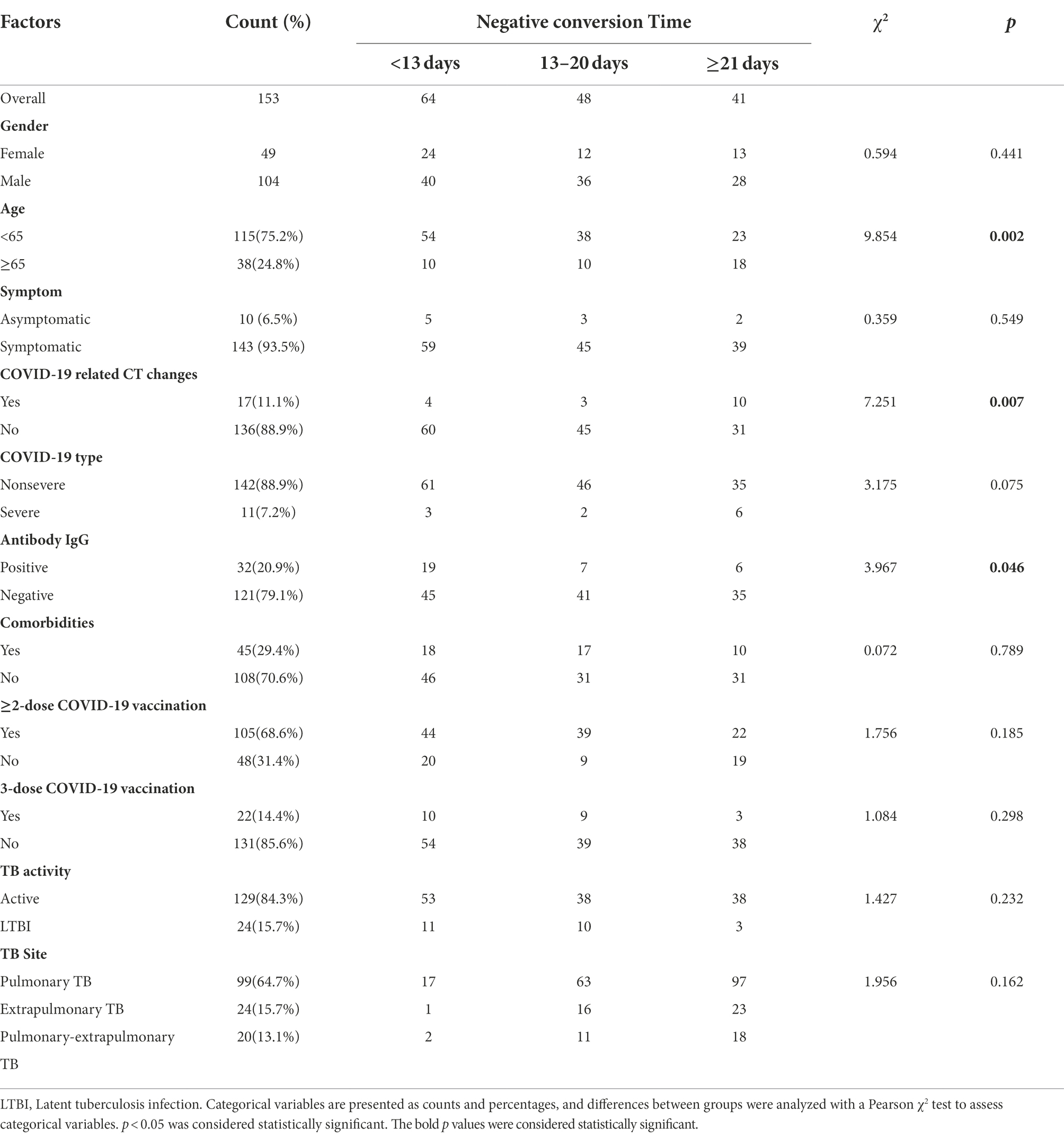
A total of 153 patients (104 males and 49 females) were enrolled in this study. The age of these patients (median [interquartile range]) was 53 (41–64). The youngest patient was 15 years old, and the oldest patient was 89 years old. Patients aged 65 and older counted for 24.8% (Figure 1A). Forty-five patients had chronic diseases, including diabetes, coronary heart disease, hypertension, chronic lung disease, tumors, cerebral infarction, and HIV infection. The most common comorbidity was diabetes (n, %; 24, 15.7%), followed by coronary heart disease (16, 10.5%), and hypertension (15, 10.5%; Figure 1B).
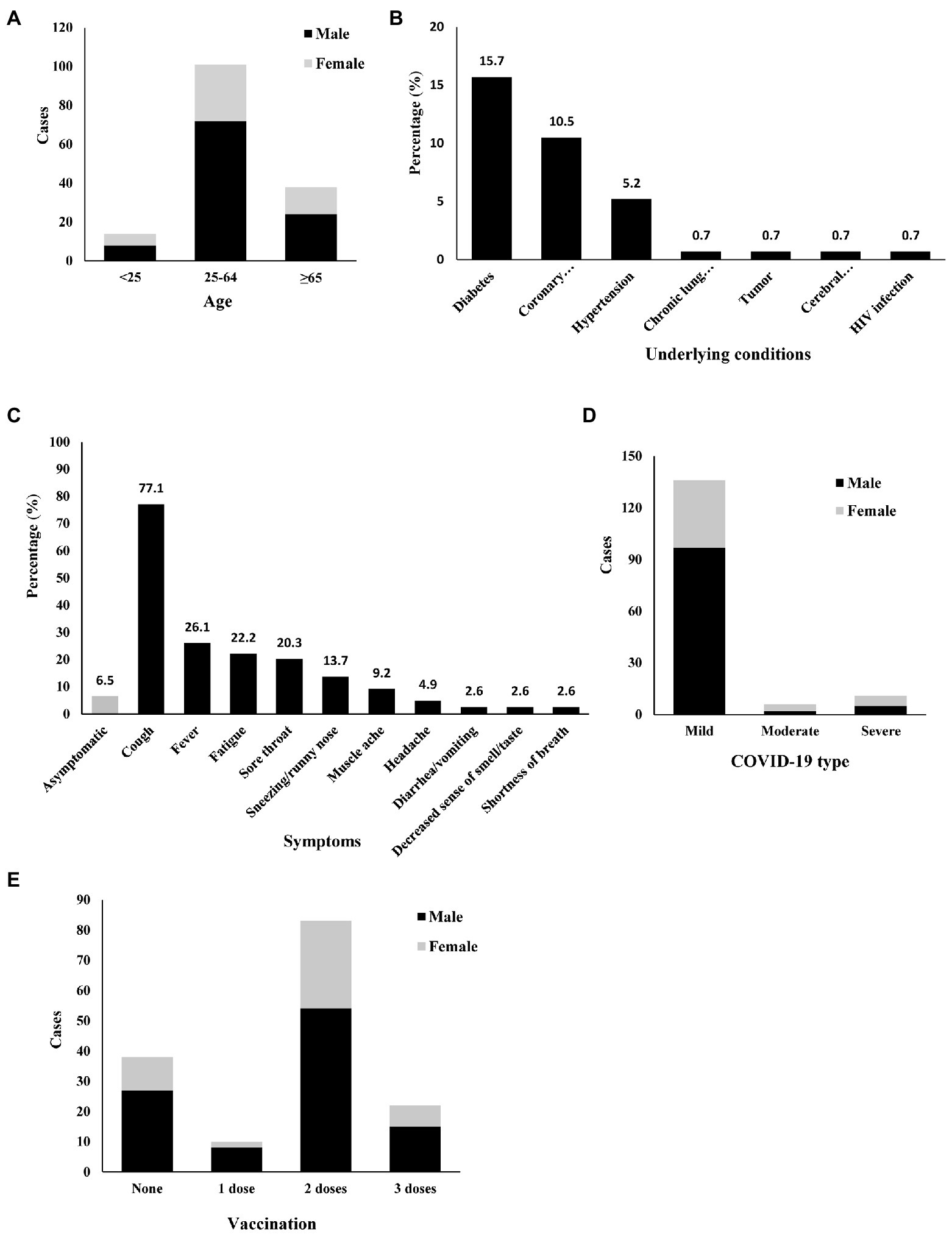
Figure 1. Demographic, epidemiological, and clinical characteristics of 153 COVID-19 and TB coinfection patients. Diabetes, Coronary heart disease, Hypertension, Chronic lung disease, Tumor, Cerebral infarction, HIV infection. (A) Age, (B) Underlying conditions, (C) Symptoms, (D) COVID-19 type, (E) Vaccination.
One hundred and twenty-nine patients had active TB, and 124 were under TB treatment. Five active TB patients were not under TB treatment. Among them, one did not start TB treatment because of his abnormal liver function. The other four refused TB treatment because of the side effects and long course of anti-TB drugs. Among the 124 patients undergoing anti-TB treatment, the diagnosis of COVID-19 was made during months 1–2 for 70 (56.5%) patients, during months 3–6 for eight (6.5%) patients, during months 7–12 for seven (5.6%) patients, and after 12 months for 39 (31.5%) patients. The longest TB history of the active TB patients was 30 years. One hundred and eight patients had pulmonary TB only, and 21 had both pulmonary TB and extrapulmonary TB. Twenty-four patients only had extrapulmonary TB without lung involvement. Among the extrapulmonary TB cases, pleural TB and bone TB were the most common. Among the 129 active TB patients, 12 patients (7.8%) had drug-resistant TB. In China, the BCG vaccine is part of the legal immunization program. China began to enforce BCG vaccination in 1949. After 1978, the planned immunization policy was well implemented. In the past 20 years, the BCG vaccination rate of newborns in Jilin Province was higher than 90%. Through history taking, most patients did not remember whether they had been vaccinated with BCG. So the percentage of BCG immunized history was not involved here.
Only 10 patients had no symptoms related to COVID-19. In these symptomatic coinfection patients, the major symptoms related to COVID-19 included cough, fever, fatigue, sore throat, sneezing/runny nose, muscle ache, headache, diarrhea/vomiting, decreased sense of smell/taste, and shortness of breath. Cough (77.1%) and fever (26.1%) were the most common symptoms (Figure 1C).
Radiological information was available for all patients (100%). Only 17 patients (11.1%) had chest high-resolution computed tomography (CT) findings highly suggestive of COVID-19-related pneumonia (bilateral ground-glass opacities). Among these 17 patients, 11 were diagnosed with severe COVID-19 (oxygen saturation ≤ 93% in the resting state or arterial partial pressure of oxygen/inhaled oxygen concentration ≤ 300 mmHg), while the other 6 patients with typical COVID-19-related changes were diagnosed with moderate COVID-19. All patients without typical COVID-19-related changes (136, 88.9%) were diagnosed with mild COVID-19, and their oxygen saturation was more than 95% (Figure 1D).
Most patients (68.6%) had more than 2 doses of a vaccine (inactivated COVID-19 vaccine), and 22 (14.4%) received three doses. However, 10 (6.5%) received only a single dose of vaccine, and 38 (24.8%) were still unvaccinated (Figure 1E). We compared the symptoms between fully more than fully vaccinated and less than 2-dose vaccinated patients. Full vaccination patients had lower incidence of cough (Supplementary Table S1). Because of the Chinese government’s prevention and control policy for COVID-19, all the patients were hospitalized. Nirmatrelvir plus ritonavir (Paxlovid) is available in China (CDC, 2022). However, Paxlovid was not used in these COVID-19 and TB coinfection patients since it could not be used in combination with rifampin. The patients with severe COVID-19 were given oxygen therapy. Two of them needed ICU admission. No specific anti-COVID-19 treatment was used for any of these patients. Chinese traditional medicine was given to most patients who prefer to be accepted. No patient died by the end of the study.
Laboratory characteristics
SARS-CoV-2 antibody tests were performed for all patients on admission. The IgM antibody result of seven patients (4.6%) was positive, and the immunoglobulin G (IgG) antibody result of 32 patients (20.9%) was positive. All 153 patients were tested by SARS-CoV-2 PCR every day from admission for confirmation of SARS-CoV-2 infection. The median duration of viral clearance was 13 days (10, 21). The shortest time was 2 days, and the longest time was 61 days. We investigated factors associated with the negative conversion time of viral RNA (Table 1). The negative conversion time in the old age group (≥ 65) was significantly longer than that in the young age and middle age groups [<65; 19.5 (12, 31) vs. 13 (9, 15), p = 0.002]. We also compared the data between the patients without COVID-19-related CT changes (the mild type of COVID-19) and the patients with those typical changes (the moderate and severe types of COVID-19). The RNA negative conversion time in the patients with COVID-19-related CT changes was significantly longer than that in those without CT typical changes [25 (12, 36.5) vs. 13 (10, 19), p = 0.007]. The patients with positive antibody IgG had an obviously shorter RNA negative conversion time than the patients with negative antibody IgG [10 (5, 15) vs. 14 (11, 21), p = 0.046]. Sex, comorbidities, symptoms, COVID-19 vaccination and the site of TB did not appear to affect the time of SARS-CoV-2 RNA negative conversion. However, when the mean duration of viral clearance was counted, the time in the 3-dose vaccination group was shorter than that in the less than 3-dose shot group (13.55 ± 7.63 days vs. 17.48 ± 11.62 days), and the time in the single-dose vaccination and nonvaccination groups was even longer (19.66 ± 14.04 days).
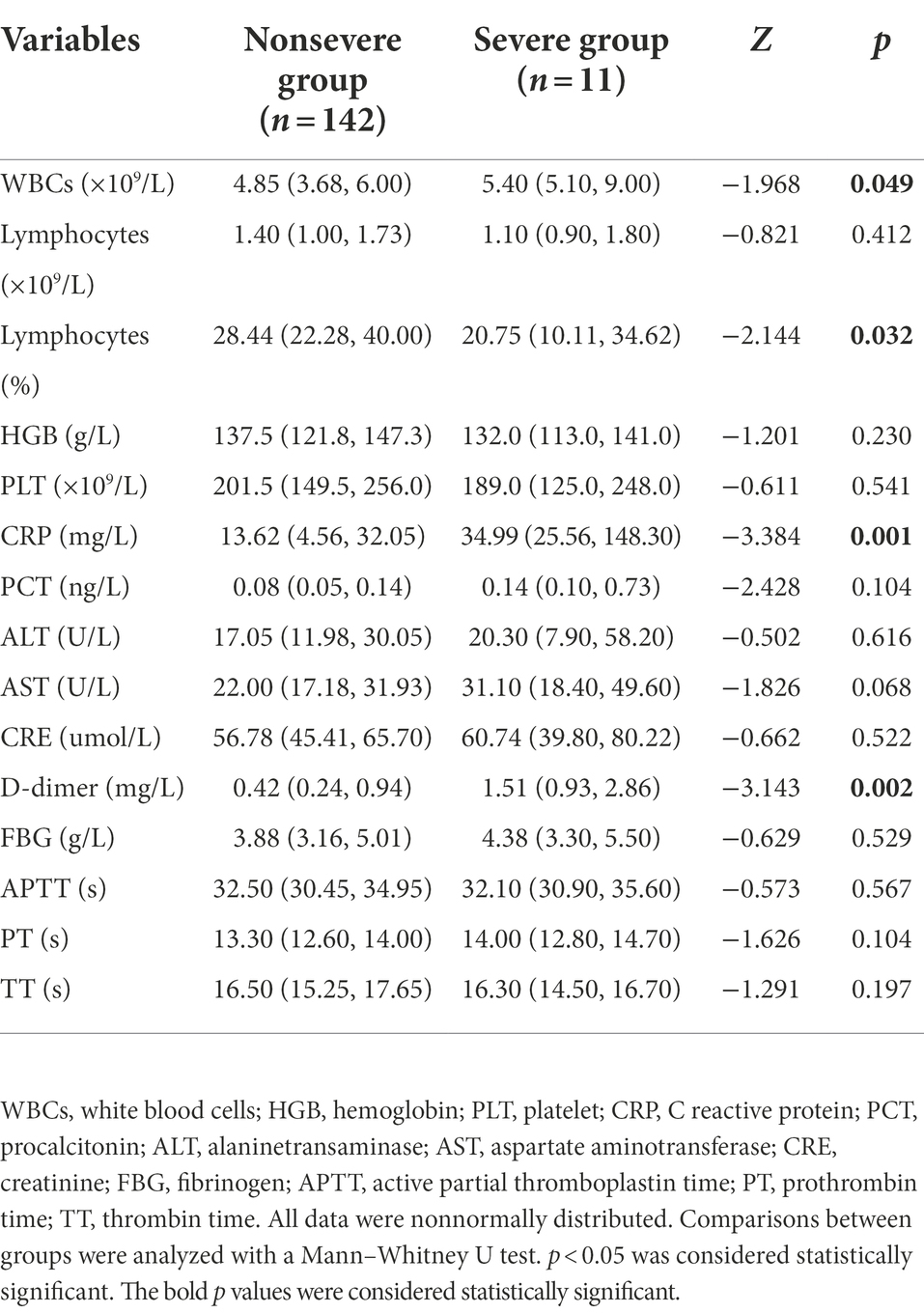
Routine blood tests, liver function tests, and kidney function tests were performed for all patients on admission (Table 2). We compared the results between the nonsevere COVID-19 and severe COVID-19 groups. As demonstrated in Table 3, a higher white blood cell count (p = 0.049), a lower lymphocyte percentage (p = 0.032), and a higher C reactive protein (CRP; p = 0.001) was observed in the severe group than in the nonsevere group. The procalcitonin (PCT) level in the severe group also seemed higher, but the differences were not significant (p = 0.104). The D-dimer level in the severe group was significantly higher than that in the nonsevere group (p = 0.002).
Univariate and multivariate analysis of risk factors for the severity of COVID-19
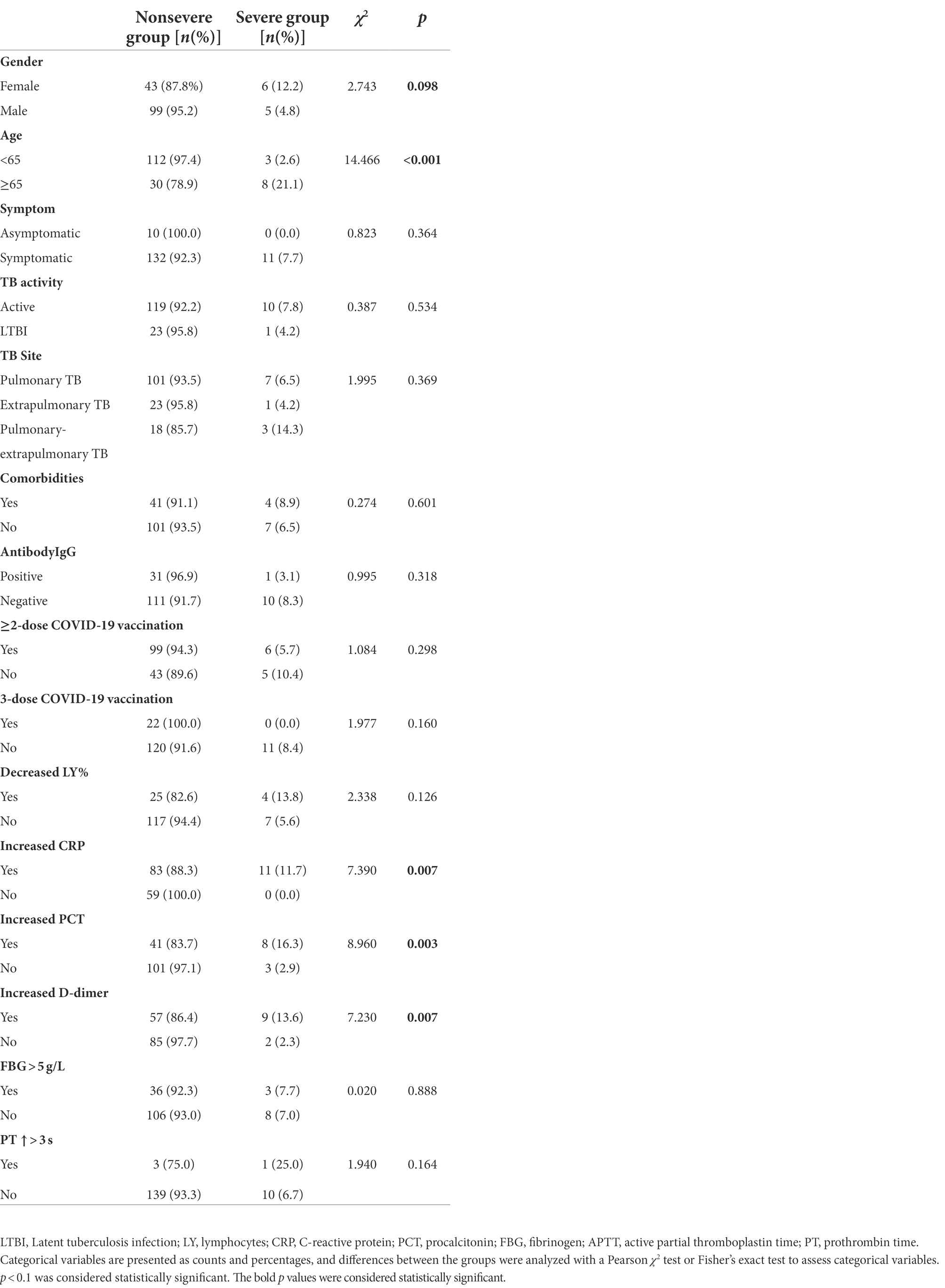
The univariate logistic regression analysis showed that older age, female sex, and increased CRP, PCT and D-dimer were associated with the severity of COVID-19 (Table 3). Some previous reports regarded an increased D-dimer level as a risk factor for the severity of COVID-19. However, different cutoff values for D-dimer were used (Simadibrata and Lubis, 2020). The most common cutoff values of D-dimer were 0.5 and 1 μg/ml. We used these two cutoff values in the univariate logistic regression analysis and found that both were associated with the severity of COVID-19. In Table 3, a cutoff value of 0.5 μg/ml was used in the data for D-dimer. All variables with significant differences in the univariate logistic regression analysis were used to construct a multivariable logistic regression model. In multivariable analysis, age ≥ 65 years (OR 9.550; 95% CI 2.283–39.949; p = 0.002) and increased PCT (OR 6.253; 95% CI 1.483–26.367; p = 0.013) were individual risk factors for the severity of COVID-19.
Differences between COVID-19 and TB coinfection cases and non-TB COVID-19 cases
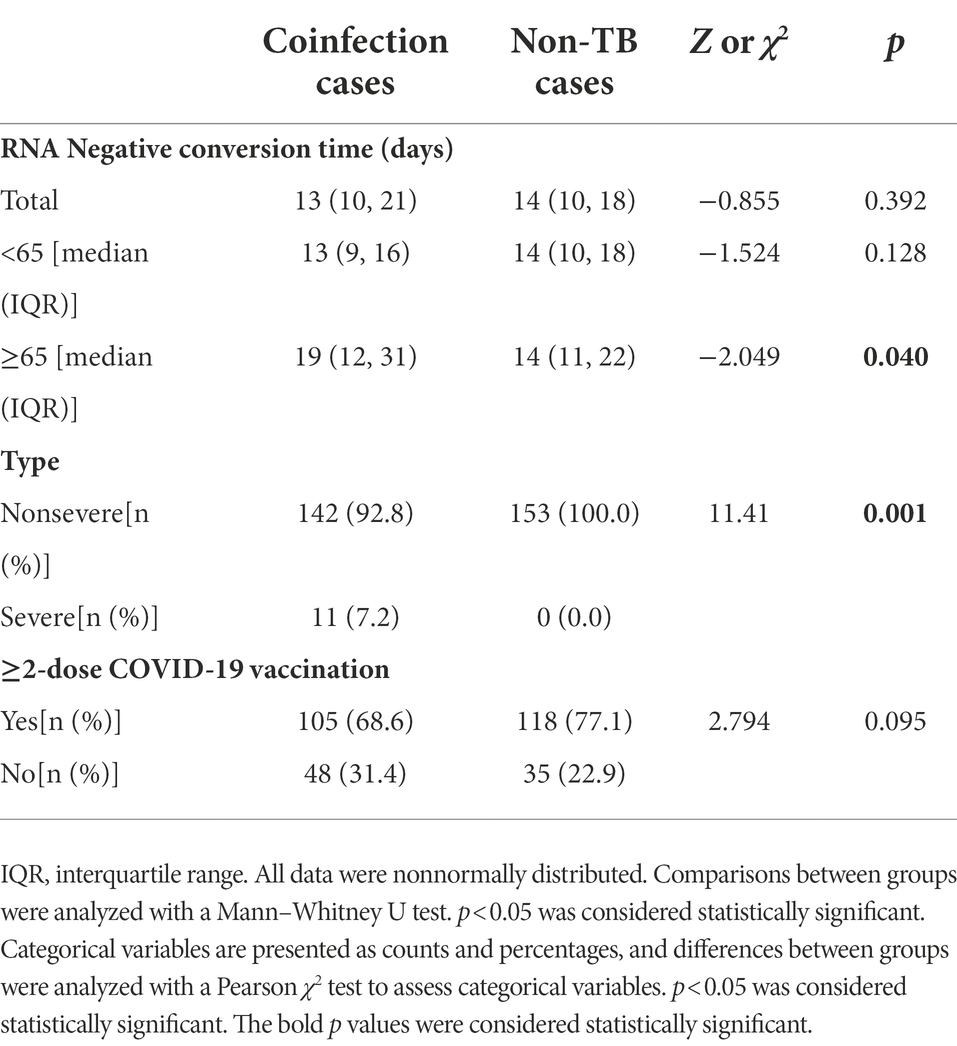
We chose a group of COVID-19 patients without TB to investigate the influence of TB infection on RNA negative conversion time and the severity of COVID-19. Control cases were chosen with 1:1 ratio and matched for age and gender. No specific treatment for COVID-19 was used. We compared the negative conversion time and COVID-19 vaccination status between coinfection cases and non-TB cases (Table 4). There’s no significant difference (p = 0.392). However, the negative conversion time of elder cases in coinfection group was much longer than that in non-TB group (p = 0.040). Among the 153 non-TB cases, 11 with typical COVID-19-related changes were diagnosed with moderate COVID-19, and no case was diagnosed with severe COVID-19. The severity rate of coinfecion group was much higher than that of control group (p = 0.001). There was no significant difference in full doses of COVID-19 vaccination between the coinfection group and the control group (p = 0.095). But the ratio of more than 2-dose vaccination of the coinfection group was lower than the control group (68.6% vs. 77.1%).
Discussion
Since being declared a global pandemic by the WHO, SARS-CoV-2, the virus responsible for COVID-19, has spread to 223 countries with more than 513 million cases, and more than 6.2 million deaths have been reported globally. The Omicron variant was first documented in the City of Tshwane, Gauteng Province, South Africa, on 9 November 2021 and led to exponential increases in the number of cases and a sharp rise in hospital admissions. Because the Omicron variant spreads easily and quickly, the number of new cases increased all over the world after December 2021. Omicron has three lineages or subvariants, BA.1 (B.1.1.529.1), BA.2 (B.1.1.529.2), and BA.3 (B.1.1.529.3). Earlier reports showed that the BA.2 Preliminary data from Denmark and the United Kingdom indicate that the BA.2 variant may be more transmissible than the BA.1 variant (Björk et al., 2022). The subvariant of SARS-CoV-2 is identified as Omicron BA.2 in Changchun (Fan et al., 2022; Li et al., 2022a,b). Compared with Omicron BA.1, Omicron BA.2 has a greater superspreading potential (Guo et al., 2022). Infections with the Omicron variant are relatively milder, and the proportion of asymptomatic patients with Omicron variant infection is much higher than with infections with the former variants. It was reported that the proportion of asymptomatic patients was 36.5–90.7% (Houhamdi et al., 2022; Kim et al., 2022; Zhang et al., 2022). In the latest report from another research team of our hospital, 35% patients with an Omicron variant BA.2 infection were asymptomatic (Li et al., 2022b). The most common symptoms were reported as fatigue and sore throat. Fever and cough are also major symptoms, but only 20% of patients will have fever or cough (Meo et al., 2021; Kim et al., 2022). Given the high transmissibility of the Omicron variant and the large TB infection population, the problem and burden of COVID-19 and TB coinfection patients might it’s more prominent (Dheda et al., 2022; Pai et al., 2022). Among TB patients, we found that the proportion of asymptomatic patients (6.5%) was much lower. The most common symptoms observed in our study were cough (77.1%), followed by fever (26.1%), fatigue (22.2%), and sore throat (20.3%). Although infections with the Omicron variant are considered much milder than infections with the former variant, patients who have coinfections with COVID-19 and TB do not have the mild disease that the general population has.
According to WHO website data, the global vaccine doses administered per 100 population are 148.34. While the Chinese vaccine doses that were administered per 100 population are 228.66 and the proportion of persons fully vaccinated with the last dose of the primary series have reached 85%. Several studies have shown that the Omicron variant can dodge some of the immune protection conferred by previous infection and vaccination, especially in patients who had the inactivated COVID-19 vaccine. However, it has been confirmed by several reports that full-scale vaccination (both mRNA COVID-19 vaccine or inactivated COVID-19 vaccine) could decrease the hospitalization rate, severity rate, and fatality rate of COVID-19 patients (Fan et al., 2021). Sinovac COVID-19 Vaccine and Sinopharm BIBI COVID-19 Vaccine were used in mainland of China and both of them are inactivated COVID-19 vaccine and shared similar technology. Most patients were randomized to inject any of them. Some patients got 2 or 3 doses of the vaccine, but the vaccine was not from the same brand. In our study, 68.6% of COVID-19 and TB coinfection patients received more than two doses of inactivated COVID-19 vaccine. In addition, only 14.4% of the patients received 3 doses of vaccination. More patients (77.1%) received more than two doses of inactivated COVID-19 vaccine in the non-TB COVID-19 group. The vaccination rate in TB patients was obviously lower than that in the general population.
The WHO’s current estimate of the global case fatality rate for the Omicron variant is 0.02–0.31% (Duong et al., 2022). In our study, both the ICU admission rate (1.3%) and fatality rate (0%) of COVID-19 and TB coinfection patients were lower than those reported in Omicron variant-infected patients (Abdullah et al., 2022; Modes et al., 2022). But the severity of COVID-19 in the coinfection group was obviously higher than the non-TB group. The severity and case fatality rate of COVID-19 are affected by many factors. Age, D-dimer, C-reactive protein, sequential organ failure assessment score and body temperature, decreasing albumin, and a history of diabetes were regarded as the major risk factors for COVID-19 severity (Rod et al., 2020). Although the Omicron variant BA.2 has evolvedtoward being less virulent, a higher rate of severe outcomes and mortality have been observed in unvaccinated people, especially older adults (Cheung et al., 2022). Comorbidities are still the major risk factor for hospitalization and death (Zhang et al., 2022). However, in our study, in the patients with COVID-19 and TB coinfection, the patients with underlying medical did not show an increased risk of developing severe COVID-19 infection. The sample size was limited in our study, and more studies with larger samples might show clearer and more definitive conclusions. In our study, older age and increased PCT were individual risk factors associated with the severity of COVID-19.In the elder group, 19 patients (50%) received more than 2 doses of vaccination, and only 3 (7.9%) had 3 shots of the vaccination. Sixteen patients (42.1%) did not receive even a single dose of the vaccination in this group. We also note that among these coinfected patients, all 3-dose vaccinated patients belonged to the nonsevere group, although COVID-19 vaccination did not appear to affect the severity of COVID-19 statistically. Three doses of inactivated COVID-19 vaccine seemed to effectively reduce the severity of COVID-19 in TB patients. In mainland China, the mRNA COVID-19 vaccine is not yet available; therefore, 3-dose inactivated COVID-19 vaccination is recommended in TB patients according to the findings of this study.
Because of the Chinese government’s prevention and control policy for COVID-19, patients could not be discharged from the hospital until the tests for SARS-CoV-2 RNA turned negative. The SARS-CoV-2 PCR test was performed for all patients every day. Therefore, we have a more in-depth understanding of the duration of viral clearance in these patients. In our study, the median duration of viral clearance was 13 days. The shortest time of RNA negative conversion was 2 days, while the longest time was 61 days. Age, COVID-19-related CT changes, and IgG antibodies against SARS-CoV-2 are associated with the duration of viral RNA clearance in TB patients. And the negative conversion time of elder cases in the coinfection group was much longer than that in the non-TB group.
Paxlovid is an antiviral therapeutic being developed by Pfizer for the treatment and postexposure prophylaxis of COVID-19. It has been reported to efficiently reduce the viral load, hospitalization and fatality rate (Lamb, 2022). Given the reported efficacy of Paxlovid against COVID-19, it is recommended to mild or moderate COVID-19 patients within 5 days onset with risk factors of disease progression. Paxlovid is a strong cytochrome P450 (CYP) 3A4 inhibitor and is contraindicated in patients receiving rifampicin, which is a strong CYP3A4 inducer. Because rifampin is one of the first-line agents for treating drug-sensitive TB patients, Paxlovid was not considered for treating TB and COVID-19 coinfection patients. In fact, among the 6 moderate and 11 severe COVID-19 patients, 3 were latent tuberculosis infection patients, and another had rifampin-resistant TB. These four patients did not take rifampin on admission. Among the mild COVID-19 patients, 21 patients were inactive TB patients, 11 patients were rifampin-resistant and 5 active TB patients were not under TB treatment. The application of Paxlovid in tuberculosis patients still needs further individualized evaluation, especially for those patients with risk factors.
Finally, we did not find a higher severity rate or fatality rate in the patients who were infected with both the Omicron variant of COVID-19 and TB in this wave. The variant is milder, and wide vaccination in the population might also play important roles. It is an important task to promote COVID-19 vaccination in TB patients, especially among elderly patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval for this study was obtained from the Ethics Committee of Changchun Infectious Disease Hospital and the Ethics Committee of the 1stHospital of Jilin University, China. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
JZ and KZ: conceptualization and project administration. YW and YC: validation and writing—original draft preparation. YW, LL, and KZ: writing—review and editing. LG: visualization. KZ: supervision. All authors agree to be accountable for the content of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81801972) to YW, Science and Technology Research Project of Jilin Provincial Department of Education (JKH20211179KJ) to YW, the Jilin Provincial Nature Science Foundation of Jilin Provincial Department of Science and Technology (20210101341JC and 20200201616JC) to YW and KZ, and Jilin Province Health Science and Technology Capacity Improvement Project (2021JC001) to KZ.
Acknowledgments
The authors are grateful to Junqi Niu and Songling Zhang for helpful discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1061879/full#supplementary-material
References
Abdullah, F., Myers, J., Basu, D., Tintinger, G., Ueckermann, V., Mathebula, M., et al. (2022). Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, South Africa. Int. J. Infect. Dis. 116, 38–42.
Aleem, A., Akbar Samad, A. B., and Slenker, A. K. (2022) in Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). ed. T. Island (FL: StatPearls Publishing)
Björk, J., Bonander, C., Moghaddassi, M., Rasmussen, M., Malmqvist, U., Inghammar, M., et al. (2022). COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 omicron BA.1 and BA.2 subvariants - surveillance results from southern Sweden, December 2021 to March 2022. Euro Surveill. 27:2200322. doi: 10.2807/1560-7917.ES.2022.27.18.2200322
CDC (2022). Guidelines for prevention and control of novel coronavirus (edition 9). Available at: http://www.nhc.gov.cn/jkj/s3577/202206/de224e7784fe4007b7189c1f1c9d5e85/files/235082ee2add42bd9a9eac93adea3b63.pdf (Accessed June 27, 2022).
Cheung, P. H., Chan, C. P., and Jin, D. Y. (2022). Lessons learned from the fifth wave of COVID-19 in Hong Kong in early 2022. Emerg. Microbes Infect. 11, 1072–1078. doi: 10.1080/22221751.2022.2060137
Dheda, K., Perumal, T., Moultrie, H., Perumal, R., Esmail, A., Scott, A. J., et al. (2022). The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 10, 603–622. doi: 10.1016/S2213-2600(22)00092-3
Duong, B. V., Larpruenrudee, P., Fang, T., Hossain, S. I., Saha, S. C., Gu, Y., et al. (2022). Is the SARS CoV-2 omicron variant deadlier and more transmissible than Delta variant? Int. J. Environ. Res. Public Health 19:4586. doi: 10.3390/ijerph19084586
Fan, Y. J., Chan, K. H., and Hung, I. F. (2021). Safety and efficacy of COVID-19 vaccines: a systematic review and meta-analysis of different vaccines at phase 3. Vaccines 9:989. doi: 10.3390/vaccines9090989
Fan, X., Lu, S., Bai, L., Liu, H., Fu, J., Jin, X., et al. (2022). Preliminary study of the protectiveness of vaccination against the COVID-19 in the outbreak of VOC omicron BA.2 - Jilin City, Jilin Province, China, March 3-April 12, 2022. Chin. CDC Wkly 4, 377–380. doi: 10.46234/ccdcw2022.081
Guo, Z., Zhao, S., Lee, S. S., Mok, C. K. P., Wong, N. S., Wang, J., et al. (2022). Superspreading potential of COVID-19 outbreak seeded by Omicron variants of SARS-CoV-2 in Hong Kong. J. Travel Med. 29:taac049. doi: 10.1093/jtm/taac049
Hirabara, S. M., Serdan, T. D. A., Gorjao, R., Masi, L. N., Pithon-Curi, T. C., Covas, D. T., et al. (2021). SARS-COV-2 variants: differences and potential of immune evasion. Front. Cell. Infect. Microbiol. 11:781429. doi: 10.3389/fcimb.2021.781429
Houhamdi, L., Gautret, P., Hoang, V. T., Fournier, P. E., Colson, P., and Raoult, D. (2022). Characteristics of the first 1119 SARS-CoV-2 Omicron variant cases, in Marseille, France, November-December 2021. J. Med. Virol. 94, 2290–2295. doi: 10.1002/jmv.27613
Karim, S. S. A., and Karim, Q. A. (2021). Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 398, 2126–2128. doi: 10.1016/S0140-6736(21)02758-6
Kim, M. K., Lee, B., Choi, Y. Y., Um, J., Lee, K. S., Sung, H. K., et al. (2022). Clinical characteristics of 40 patients infected with the SARS-CoV-2 omicron variant in Korea. J. Korean Med. Sci. 37:e31. doi: 10.3346/jkms.2022.37.e31
Lai, C. C., Shih, T. P., Ko, W. C., Tang, H. J., and Hsueh, P. R. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents 55:105924. doi: 10.1016/j.ijantimicag.2020.105924
Lamb, Y. N. (2022). Nirmatrelvir plus ritonavir: first approval. Drugs 82, 585–591. doi: 10.1007/s40265-022-01692-5
Li, H., Gao, M., You, H., Zhang, P., Pan, Y., Li, N., Qin, L., Wang, H., Li, D., Li, Y., Qiao, H., Gu, L., Xu, S., Guo, W., Wang, N., Liu, C., Gao, P., Niu, J., Cao, J., and Zheng, Y. (2022a). Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS-CoV-2 RT-PCR negative conversion rates among high-risk patients with COVID-19. Clin. Infect. Dis. doi: 10.1093/cid/ciac600 [Epub ahead of print].
Li, H., Zhu, M., Zhang, P., Yan, X., Niu, J., Wang, Z., et al. (2022b). Milder symptoms and shorter course in patients with re-positive COVID-19: a cohort of 180 patients from Northeast China. Front. Microbiol. 13:989879. doi: 10.3389/fmicb.2022.989879
Long, Q., Guo, L., Jiang, W., Huan, S., and Tang, S. (2021). Ending tuberculosis in China: health system challenges. Lancet Public Health 6, e948–e953. doi: 10.1016/S2468-2667(21)00203-6
Mariita, R. M., and Musila, J. M. (2020). A study on the relationship between bacillus Calmette– Guérin (BCG) vaccination and Covid-19 prevalence: do other confounders warrant investigation? J. Public Health Epidemiol. 12, 142–150. doi: 10.5897/JPHE2020.1230
Meo, S. A., Meo, A. S., Al-Jassir, F. F., and Klonoff, D. C. (2021). Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur. Rev. Med. Pharmacol. Sci. 25, 8012–8018. doi: 10.26355/eurrev_202112_27652
Modes, M. E., Directo, M. P., Melgar, M., Johnson, L. R., Yang, H., Chaudhary, P., et al. (2022). Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (omicron) variant predominance - one hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb. Mortal. Wkly Rep. 71, 217–223. doi: 10.15585/mmwr.mm7106e2
Pai, M., Kasaeva, T., and Swaminathan, S. (2022). Covid-19's devastating effect on tuberculosis care - a path to recovery. N. Engl. J. Med. 386, 1490–1493. doi: 10.1056/NEJMp2118145
Rod, J. E., Oviedo-Trespalacios, O., and Cortes-Ramirez, J. (2020). A brief-review of the risk factors for covid-19 severity. Rev. Saude Publica 54:60. doi: 10.11606/s1518-8787.2020054002481
Simadibrata, D. M., and Lubis, A. M. (2020). D-dimer levels on admission and all-cause mortality risk in COVID-19 patients: a meta-analysis. Epidemiol. Infect. 148:e202. doi: 10.1017/S0950268820002022
TB/COVID-19 Global Study Group (2022). Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur. Respir. J. 59:2102538. doi: 10.1183/13993003.02538-2021
Umubyeyi Nyaruhirira, A., Scholten, J. N., Gidado, M., and Suarez, P. G. (2022). Coronavirus disease 2019 diagnosis in low- and middle-income countries: the big new bully disrupting TB and HIV diagnostic services. J. Mol. Diagn. 24, 289–293. doi: 10.1016/j.jmoldx.2021.12.008
Keywords: COVID-19, tuberculosis, coinfection, epidemiological, clinical characteristics, RNA negative conversion time
Citation: Wang Y, Chen Y, Gu L, Lou L, Zhang J and Zhang K (2022) The clinical characteristics and risk factors for severe COVID-19 in patients with COVID-19 and tuberculosis coinfection. Front. Microbiol. 13:1061879. doi: 10.3389/fmicb.2022.1061879
Edited by:
Maria Alejandra Mussi, Consejo Nacional de Investigaciones Científicas y Técnicas, ArgentinaReviewed by:
Zuoren Yu, Tongji University, ChinaRichard M. Mariita, Crystal IS Inc., United States
Radha Gopalaswamy, National Institute of Research in Tuberculosis (ICMR), India
Copyright © 2022 Wang, Chen, Gu, Lou, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaiyu Zhang, a2FpeXVAamx1LmVkdS5jbg==; Jian Zhang, amlhbnpoYW5nMjAwODA4MDhAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
 Yang Wang
Yang Wang Yanping Chen2†
Yanping Chen2† Kaiyu Zhang
Kaiyu Zhang