
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 25 January 2023
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1060050
The emergence of antimicrobial resistance among microorganisms is a serious public health concern, and extended-spectrum β-lactamases (ESBL)-producing Enterobacterales is one of the major concerns among antibiotic-resistant bacteria. Although the prevalence of ESBL in Enterobacterales has been increasing with time, the prevalence of ESBL could differ according to the species, hospital allocation, sources of infections, nosocomial or community acquisitions, and geographic regions. Therefore, we conducted a comprehensive review of the epidemiology of ESBL-producing Enterobacterales in Taiwan. Overall, the rates of ESBL producers are higher in northern regions than in other parts of Taiwan. In addition, the genotypes of ESBL vary according to different Enterobacterales. SHV-type ESBLs (SHV-5 and SHV-12) were the major types of Enterobacter cloacae complex, but Serratia marcescens, Proteus mirabilis, Escherichia coli, and Klebsiella pneumoniae were more likely to possess CTX-M-type ESBLs (CTX-M-3 and CTX-M-14). Moreover, a clonal sequence type of O25b-ST131 has been emerging among urinary or bloodstream E. coli isolates in the community in Taiwan, and this clone was potentially associated with virulence, ESBL (CTX-M-15) production, ciprofloxacin resistance, and mortality. Finally, the evolution of the genetic traits of the ESBL-producing Enterobacterales isolates helps us confirm the interhospital and intrahospital clonal dissemination in several regions of Taiwan. In conclusion, continuous surveillance in the investigation of ESBL production among Enterobacterales is needed to establish its long-term epidemiology.
The rapid development of antimicrobial resistance among microorganisms is a serious public health concern, and there were an estimated 4.95 million deaths associated with antimicrobial resistance. Based on the estimation of the global burden of antibiotic resistance by Antimicrobial Resistance Collaborators, Escherichia coli and Klebsiella pneumoniae were the first and third leading pathogens associated with resistance. In addition, third-generation cephalosporin-resistant E. coli and K. pneumoniae would cause 50,000–100,000 deaths. Many antibiotic-resistance mechanisms have been identified among Enterobacterales and extended-spectrum β-lactamases (ESBLs), which remained one of the most common mechanisms (Lai et al., 2014; Colmenarejo et al., 2020; Vink et al., 2020; Estaleva et al., 2021; Karlowsky et al., 2022; Sader et al., 2022; Selmi et al., 2022). ESBLs that mediate resistance to newer β-lactam antibiotics, including extended-spectrum cephalosporins and monobactams, are plasmid-mediated class A enzymes commonly found in the family Enterobacterales and frequently detected among E. coli and K. pneumoniae. For adult patients with community-onset bacteremia, ESBL-producing E. coli, Klebsiella species, and Proteus mirabilis pathogens were linked to inappropriate empirical antibiotic therapy and the 4-week mortality (Lee et al., 2017). Therefore, it should be concerned that the ESBL-producing Enterobacterales are associated with a high mortality rate and increased medical care costs. Based on the finding of the Regional Resistance Surveillance program that monitored susceptibility rates and developing resistance by geographic region, including 12 Asia-Pacific countries (Mendes et al., 2013), the resistance rates among nations within the area can be diverse, being generally lower in Japan and Australia/New Zealand and higher in eastern Asia countries. In detail, ESBL phenotype rates in E. coli and Klebsiella spp. were 48 and 47%, respectively, ranging from 11%/10% in New Zealand to 91%/75% in Taiwan (Mendes et al., 2013). Another study focusing on Enterobacterales isolates that caused intra-abdominal infections in hospitalized patients in the Asia-Pacific region revealed a higher resistance burden in Vietnam and the Philippines than in other Asia-Pacific countries (Jean and Hsueh, 2017). However, the high prevalence of ESBL producers in Taiwan might be due to selection bias. Therefore, this study aimed to conduct a comprehensive review of the epidemiology of ESBL-producing Enterobacterales in Taiwan.
The prevalence of ESBL in Enterobacterales could be variable according to the species, hospital allocation, sites of infection, as well as nosocomial or community acquisition, however, the prevalence of ESBL was increasing over time in Taiwan (Yu et al., 2006c; Table 1). In intensive care units (ICUs), a surveillance investigation of 574 Enterobacterales isolates in 10 major teaching hospitals in Taiwan in 2005 showed the prevalence of ESBL production was 26, 16, 14, and 13% in K. pneumoniae, Serratia marcescens, E. coli and Proteus mirabilis, respectively (Jean et al., 2009). Another surveillance of 336 Enterobacterales isolates from patients with intra-abdominal infections in a north medical center between 2000 and 2006 showed the overall prevalence of ESBL production was 26, 23, and 19% among E. coli, Klebsiella spp., and Enterobacter spp. (Chen et al., 2009). During the seven-year study period, the highest rate of ESBL production was found in 2005 for E. coli (38%) and in 2003 for Klebsiella spp. (38%) and Enterobacter spp. (40%). The incidence of ESBL-producing isolates declined in 2005 and 2006 (Chen et al., 2009). Shu et al. reported that the prevalence of ESBL increased from 4.8 to 10.0% during seven-year surveillance in a large teaching hospital in north Taiwan (Shu et al., 2010). Another surveillance of pathogens isolated from patients with complicated intra-abdominal infections at five medical centers from 2006 to 2010 found that the rate of ESBL-producing species was three-fold higher among patients with nosocomial IAIs than among patients with community-acquired IAIs (Lee et al., 2012). Moreover, the rate of ESBL producers was highest in northern Taiwan and lowest in central Taiwan (Lee et al., 2012). One of the reasons for the differences in prevalence of ESBL producers in different regions in Taiwan could be associated with the prescription patterns by physicians. For example, the highest rate ceftazidime resistance among Pseudomonas aeruginosa isolates and the highest rates of ESBL producers in northern Taiwan could be attributed to the highest ceftazidime utilization in this region. Likewise, the highest rates of carbapenem resistance among P. aeruginosa isolates and the lowest rates of ESBL producers in central Taiwan implying the highest carbapenem utilization in the area (Lee et al., 2012). For bacteremia, a retrospective study in a medical center in southern Taiwan between 2008 and 2013 including 1,141 adult patients with community-onset bacteremia due to E. coli, K. pneumoniae, and P. mirabilis found that only 65 (5.7%) isolates were ESBL producers and were associated with poor prognosis (Lee et al., 2018). Regarding community-onset urinary tract infections (UTIs), a medical center in northern Taiwan reported a prospective study of 393 isolates from urine cultures, including 253 E. coli and 42 K. pneumoniae isolates. Fifty-three (13.5%) isolates were phenotypically positive for ESBL production (Kung et al., 2015). All these findings suggest that regular monitoring and surveillance investigation of ESBL production among Enterobacterales is needed to establish its associated epidemiology.
In addition to the clinical settings, we cannot neglect the presence of multi-drug resistant (MDR) organisms in wastewater, which could be a major source of antibiotic-resistant bacteria released into the environment (Gomi et al., 2018). The occurrence of ESBL-producing Enterobacterales recovered from hospital wastewater is increasing, and these MDR organisms can be eventually discharged into the environment and might contaminate the food chain (Fadare and Okoh, 2021). In addition, Tao et al. also reported that wastes from animal husbandry could be a potential environmental source of antibiotic-resistant pathogens (Tao et al., 2014). Moreover, one study sampling river water from 40 stations in southern Taiwan found that ESBL-producing E. coli strains were commonly isolates, accounting for 30% of the 621 E. coli strains (Chen et al., 2016). Further analysis showed that the most commonly resistant mechanism revealed CTX-M group 9 and the proportion of ESBL-producing E. coli was significantly higher in regions with a large number of chickens being raised (Chen et al., 2016).
Two studies evaluated the risk factors of healthy carriers with ESBL-producing Enterobacterales in Taiwan (Huang Y. S. et al., 2020; Cheng et al., 2022). One prospective cohort study including healthy adults who attended a health examination in the local community reported a high carrier rate of third-generation cephalosporin-resistant E. coli or K. pneumoniae (27.4% ESBL-producing strains), especially in those being an employee of a technology company [adjusted odds ratio (OR), 4.127; 95% confidence interval (CI), 1.824–9.336], and traveling to Southeast Asia in the past year (adjusted OR, 6.545; 95% CI, 1.071–40.001; Huang Y. S. et al., 2020). However, in another cohort of asymptomatic adults in Taiwan, travel to Asian countries and food habits were not associated with fecal carriage of ESBL-producing E. coli isolates (Wu et al., 2019). Another prospective study collected stool samples from children aged 0–18 years within 3 days of hospitalization and found several anthropogenic risk factors including drinking water process, pork consumption, pets, and household density might be associated with ESBL-producing E. coli (Cheng et al., 2022).
In addition to healthy carriers, many factors have been found to be associated with the acquisition of ESBL-producing Enterobacterales infections in Taiwan. A case–control study in neonatal ICU found that previous usage of 3rd generation cephalosporin (OR, 4.72; 95% CI, 2.03–10.97) and underlying renal disease (OR, 4.07; 95% CI, 1.10–15.08) were identified as independent risk factors for ESBL acquisition (Tsai et al., 2016). Among children, Kuo et al. reported that recent antibiotic exposure (within 30 days before the episode) was the most important predisposing factor associated with infection of ESBL-producing K. pneumoniae (Kuo et al., 2007). Additionally, other possible risk factors included recent surgery, the application of mechanical ventilation, nasogastric tubes, and central venous catheter insertion (Kuo et al., 2007). For elderly patients, multiple underlying comorbidities (OR, 2.88; p < 0.05) or receiving more than two antimicrobial agents (OR, 3.71; p < 0.05) were associated with increased risks for acquiring the ESBL-producing microorganisms (Lin et al., 2013). Moreover, tracheostomy (OR, 5.13; 95% CI, 1.24–21.1) and ceftazidime use (OR, 13.40; 95% CI, 1.21–148.85) were independently associated with ESBL-producing K. pneumoniae (Lin et al., 2003). The acquisition of ESBL-producing microorganism infections has been reported to be associated with recent hospitalization, hospital-acquired infection, and urinary catheter placement (Hsieh et al., 2010; Wu et al., 2010, 2014a; Lin et al., 2011; Tsui et al., 2012). Finally, nasogastric tube placement and hospitalization within the previous 3 months were significantly associated with the acquisition of ESBL-producing pathogens in community-onset UTIs (Kung et al., 2015).
Increased antimicrobial consumption and inappropriate empirical use have been presumed associated factors with ESBL production in Enterobacterales in Taiwan. A 13-year study in a university hospital demonstrated a significant correlation between antimicrobial resistance and increased antimicrobial consumption of some antibiotics in Taiwan (Hsueh et al., 2005). For example, a significant positive association was found between cefotaxime-resistant E. coli and annual consumption of cefotaxime (r = 0.764 and p = 0.002). Another study showed that adult patients with ESBL-producing E. coli bacteremia had more antimicrobial agents used in the previous 3 months (50.0 vs. 11.1%, p = 0.017) than did those with non-ESBL-producing E. coli bacteremia (Tsai et al., 2018). The diverse antibiotic usage by physicians’ prescription might also have contributed to different rates of ESBL producers among different regions in Taiwan (Lee et al., 2012). This study also showed that more adult patients with ESBL-producing E. coli bacteremia received inappropriate initial antimicrobial treatment after hospitalization than those with non-ESBL-producing E. coli bacteremia (96.3 vs. 35.7%, p < 0.001; Tsai et al., 2018).
The genotypes of ESBL are also different in various studies in Taiwan (Table 2). In 1999, Siu et al. reported that SHV-5 was the main genotype of ESBL and the presence of 11 in 16 ESBL-producing gram-negative bacteria caused nosocomial bacteremia in a pediatric oncology ward in a north Taiwan hospital (Siu et al., 1999). In 2004, Yu et al. first reported CTX-M-15 from 2 K. pneumoniae isolates in Taiwan (Yu et al., 2004a). In 2005, Chia et al. demonstrate that SHV-12 (n = 80) was the most prevalent genotype of 199 ESBL-producers, followed in order of frequency by CTX-M-3 (n = 65) and CTX-M-14 (n = 36; Chia et al., 2005). In addition, 17 (9%) clinical isolates harbored both SHV- and CTX-M-type ESBLs. SHV-type ESBL was the major type of Enterobacter cloacae complex, but E. coli and K. pneumoniae were more likely to possess CTX-M-type ESBLs (Chia et al., 2005). In 2006, Yu et al. reported that the most prevalent types of ESBLs were SHV-5, SHV-12, CTX-M-3, and CTX-M-14 in isolates of K. pneumoniae and E. coli, however, the prevalence differed according to the institutions (Yu et al., 2006c). Furthermore, SHV-12 and CTX-M-3 have been reported as the most common ESBLs in isolates of E. cloacae complex and S. marcescens, respectively (Yu et al., 2006c). In 2010, surveillance in a regional hospital in central Taiwan found that CTX-M-14 type (53.6%) was the most prevalent ESBL among 69 E. coli isolates, while SHV type (57.6%) was the most dominant among 33 K. pneumoniae isolates (Lin C. F. et al., 2010). Moreover, the co-existence of two or more kinds of ESBL in a single isolate was observed in 40.6 and 72.7% of E. coli and K. pneumoniae isolates, respectively (Lin C. F. et al., 2010). In 2017, Jean et al. using the surveillance investigation of Enterobacterales isolates from 2008 to 2014 reported that CTX-M-14, CTX-M-55, CTX-M-15, CTX-M-27, and SHV-12 had been the dominant ESBL-producing Enterobacterales in Taiwan (Jean and Hsueh, 2017).
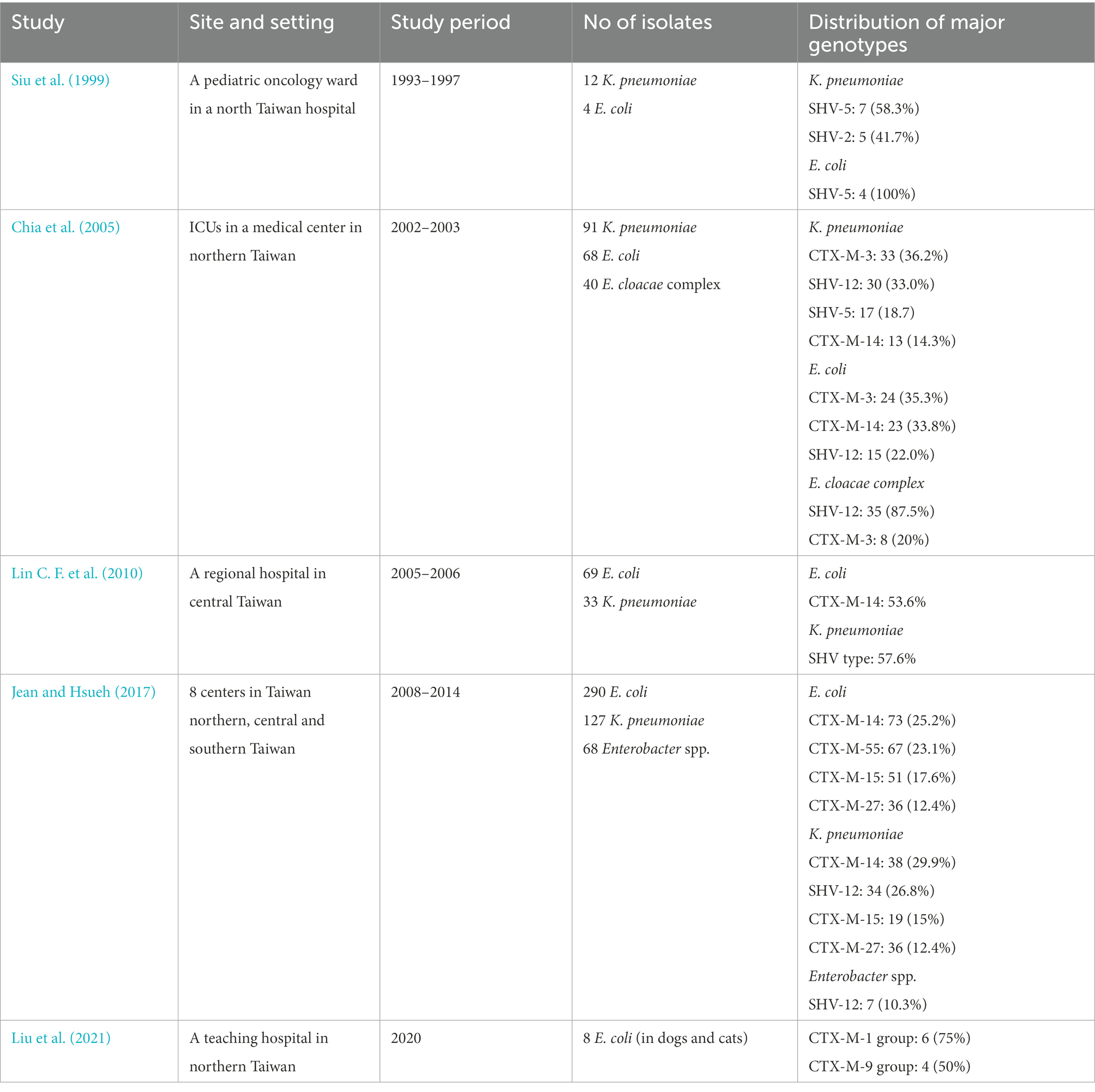
Table 2. Genotypic distribution of extended-spectrum β-lactamases (ESBLs) among Enterobacterales in Taiwan.
Generally, SHV-types (SHV-5 and SHV-12) and CTX-M-types (CTX-M-3 and CTX-M-14) were equally prevalent among K. pneumoniae isolates, whereas CTX-M-types (CTX-M-3 and CTX-M-14) were highly prevalent in E. coli isolates in Taiwan. Currently, CTX-M-55, −17 and −27 were increasingly emerging in Taiwan. Globally, TEM- and SHV-type ESBLs were the predominant families of ESBLs in the past. For example, TEM-3 was very common in France, but TEM-10 was the most prevalent TEM-type ESBL in the USA (Soilleux et al., 1996; Wiener et al., 1999). SHV-type ESBLs were most often found in clinical isolates of K. pneumoniae worldwide. At present, CTX-M-type enzymes are the most commonly found ESBL type with the CTX-M-15 variant dominating worldwide, followed in prevalence by CTX-M-14, and CTX-M-27 emerging in many parts of the world, including Japan, Europe, and the US (Matsumura et al., 2016; Merino et al., 2018; Cameron et al., 2021). TEM-type ESBLs have been infrequent in Taiwan and less detected in ESBL-producing E. coli and Klebsiella pneumoniae in European isolates as the CTX-M-type enzymes become the most prevalent ESBL worldwide (Castanheira et al., 2021).
In 2021, an animal study involving 50 samples of E. coli from dogs and cats detected eight ESBL producers with ESBL genes including the CTX-M-1 and CTX-M-9 groups in Taipei in northern Taiwan (Liu et al., 2021). According to the phylogenic study of CTX-M enzymes (Bonnet, 2004), the CTX-M group 1 from human isolates is mainly composed of CTX-M-1, CTX-M-3, CTX-M-10, CTX-M-12, and CTX-M-15. While the CTX-M-9 group mainly includes nine enzymes (CTX-M-9, CTXM-M-13, CYX-M-14, CTX-M-16, CTX-M-17, CTX-M-19, CTX-M-21, CTX-M-27, and Toho-2). CTX-M-55 is a variant of CTX-M-15 with only one amino acid substitution (Ala-80-Val). Both CTX-M-15 and CTX-M-55 belong to the CTX-M-1 group, however, the prevalence of CTX-M-55 has gradually increased probably due to multiple spreading mechanisms in China (Zeng et al., 2021) and is even higher than CTX-M-15 in E. coli with community-onset infections, especially in South China (Zhang et al., 2014). Therefore, it is a warning for the emerging CTX-M-55 as potential wide spreading in Taiwan.
The following sections review and discuss specific pathogens commonly isolated in Taiwan, including E. coli, K. pneumoniae, E. cloacae complex, S. marcescens, and P. mirabilis.
In Taiwan, the prevalence of ESBL production has been reported in many studies, however, the prevalence can vary according to different settings, infection sources, and patient groups (Biedenbach et al., 1999).
Table 3 summarized the prevalence of ESBL-producing E. coli according to specific allocation. In ICU, Hsueh et al. conducted an analysis of clinical specimens from patients in five major teaching hospitals in 2020 and found that ESBL was found in 11.9% of E. coli (Hsueh et al., 2001). Further study by Shu et al. showed the prevalence of ESBL increased from 4.8 to 10.0% during seven-year surveillance in a large teaching hospital in north Taiwan (Shu et al., 2010). Further analysis of the distribution of ESBL-producing isolates in different ICUs showed that the most significant increase occurred in medical ICUs, with a peak prevalence rate of 35.9% in 2006 for E. coli (Shu et al., 2010). In a respiratory care center, one retrospective analysis in a tertiary care center from January 2001 to December 2002 reported that the ESBL phenotype was found in 31.4% of E. coli (Lee C. M. et al., 2009). In the respiratory care ward involving patients who required prolonged or long-term mechanical ventilation, Lin et al. reported that the prevalence of ESBL-producing isolates of E. coli was 39.5% (Lin et al., 2013). For nursing home residents, a retrospective study conducted in medical wards of a district hospital in southern Taiwan between July 2009 and June 2011 reported that 52.7–69.5% of E. coli isolates had an ESBL (Liu et al., 2016). Due to chronic exposure to multiple antibiotics, E. coli isolates from a nursing home, respiratory care center, or respiratory care ward seemed to have higher ESBL rates than those from an acute ICU setting.
Among the 404 clinical specimens from patients with community-onset E. coli bacteremia in a medical center in southern Taiwan, Hsieh et al. identified only 19 (4.7%) isolates were ESBL producers (Hsieh et al., 2010). For premature babies with E. coli bacteremia, a small study including 27 cases showed five (18.5%) isolates were ESBL producers (Chen I. L. et al., 2017). Additionally, this study found that the level of serum alanine aminotransferase was significantly lower in the ESBL-producing E. coli group than that in the non-ESBL-producing E. coli group (Chen I. L. et al., 2017).
Regarding the genotype of E. coli causing bacteremia, one study in northern Taiwan including 60 patients with ESBL-producing E. coli bacteremia detected 41 (68.3%) isolates with CTX-M β-lactamases. CTX-M-14 accounted for 31 (75.6%) and CTX-M-3 for 9 (22.0%) of the 41 CTX-M isolates, which were associated with chronic renal failure and ICU stay (Wu et al., 2011). In contrast, the study in southern Taiwan found CTX-M group 9 was the most common genotype causing E. coli bacteremia, especially in pediatric patients (85.7%), and there were more E. coli ST131 in ESBL isolates than in non-ESBL isolates (Tsai et al., 2018). Finally, one retrospective study enrolled 121 adults from southern Taiwan with ESBL-producing E. coli bloodstream infections to investigate their sequence type and virulence factors and showed that positivity for the virulence genes iha, hlyD, sat, iutA, fyuA, malX, ompT, and traT was associated with ST131 positivity (all p < 0.05; Hung et al., 2019). Moreover, iroN positivity was associated with 30-day mortality among bacteremia patients without UTIs (Hung et al., 2019).
E. coli was the most common pathogen causing UTIs. For young patients (0–18 years) with UTI, a 10-year study reported that the ESBL rate had increased from 2% in 2003 to 11% in 2012 (Chen et al., 2014). Another study involving young children aged from 1 day to 36 months reported that the ESBL rate was 3.3% (n = 13) among 421 E. coli isolates and it accounted for 93.3% of ESBL-producing Enterobacterales (Wu et al., 2016). In contrast, another study reported that the prevalence of UTIs due to ESBL-producing E. coli in hospitalized children with community-onset UTIs only increased slightly from 0.59% in 2002 to 0.96% in 2006, however, children with ESBL-producing E. coli UTIs had a longer hospital stay (p = 0.031) than those without (Fan et al., 2014). But a retrospective of 159 infant patients with UTIs found that most of them had no prior history of illness, and no significant risk factors for acquiring ESBL-producing E. coli, such as prior antimicrobial use, hospitalization, UTI, and underlying renal diseases (Cheng et al., 2016). For adult patients, a prospective study included 136 patients with gram-negative bacilli causing community-onset UTIs between August 2009 and January 2012 showed that nine (8.0%) of 111 E. coli isolates had ESBL production (Wu et al., 2014a).
Intra-abdominal infection was another common type of E. coli infection. Surveillance of the pathogens isolated from patients with complicated intra-abdominal infections at five tertiary-care hospitals in Taiwan during the period 2006 to 2010 showed that ESBL production was detected in 7.6% (71/935) of E. coli isolates, and the rate of ESBL production in E. coli was steady (Lee et al., 2012). Moreover, the rate of ESBL production among E. coli isolates collected from patients with nosocomial IAI was higher than those from community-acquired IAI (14.6 vs. 4.7%).
ESBL of ST131 has emerged in many parts of the world since the 2000s, including the US, UK, France, Japan, Canada, India, Kuwait, France, Switzerland, Portugal, and Spain (Coque et al., 2008; Komatsu et al., 2018; Day et al., 2019; Broussier et al., 2020; Duffy et al., 2022). There is no exception for Taiwan. The estimated overall ST131 prevalence in this community UTI cohort increased from 11.2% (in 2002–2004) to 17.4% in 2014–2016 (p < 0.01). Especially ST131 with ESBL phenotype (cefotaxime-resistant group) increased from 33.3% in 2002–2004 to 72.1% in 2014–2016 (p < 0.01; Wang et al., 2021).
In some countries, including the US and Korea, the multidrug-resistant ST131 clone producing CTX-M-15 has emerged as a major clone in both the community and hospital (Peirano et al., 2010; Park et al., 2012; Cho et al., 2015). However, the study about the association between ST131 and CTX-M-15 in Taiwan is scarce. A study of 122 patients with ESBL-producing E. coli bacteremia during a 6-year period from 2005 to 2010 in southern Taiwan reported that the most common ST was ST131 (29.5%, n = 36). CTX-M-producing isolates were identified in 72.2% (26/36) ST131 isolates, including CTX-M-14 (n = 13), CTX-M-15 (n = 9) and CTX-M-3 (n = 4; Chung et al., 2012). In addition, CTX-M has been the most common ESBL in E. coli from Taiwan and in UTI causing ST131 E. coli (Wang et al., 2021). CTX-M-type ESBL gene was detected in 83.5% (66/79) of the ST131 isolates. Among them, ST131 isolates carrying group 1 CTX-M ESBL have emerged in 2014–2016, especially in 2016 (Wang et al., 2021). Since CTX-M-15 belongs to group 1 CTX-M, it is possible that CTX-M-15 might be associated with Taiwanese ST131 E. coli clinical isolates, albeit with no further precise identification in the report (Wang et al., 2021).
One study using the database of healthy inhabitants attending health examinations at a medical center in southern Taiwan in 2017 reported that the prevalence rate of asymptomatic ESBL-producing E. coli fecal carriage in adults was 1.9% (14/724; Wu et al., 2019). In this study, ST131 was found in 22 (3.0%) adults, and underlying cancer and stroke were associated with ST131 E. coli fecal carriage (Wu et al., 2019). Although the ST131 E. coli fecal carriage rate was low among asymptomatic adults in this study (Wu et al., 2019), the prevalence of ST131 among ESBL-producing E. coli was much higher in the following clinical entities. Wu et al. conducted a retrospective analysis of 371 consecutive community-onset non-ESBL producing E. coli bloodstream infections in a 1,200-bed hospital in southern Taiwan in 2010 and found that 60 (16.2%) patients belonged to the ST131 group and clonal group (O25b-ST131) accounted for 5.9% of total isolates (Wu et al., 2014b). In the same institution, Chung et al. showed that ST131 remained the major sequence type, which accounted for 29.5% (36/122) among adult patients with ESBL-producing E. coli bacteremia (Chung et al., 2012). In this retrospective study from 2005 to 2010, clone ST131 were more likely to have secondary bacteremia (OR, 5.05; 95% CI, 1.08 to 23.56) and non-use of the urinary catheter (OR, 3.77; 95% CI, 1.17 to 12.18; Chung et al., 2012). Additionally, another study involving 843 adults presenting with community-onset monomicrobial E. coli bacteremia at a medical center between 2008 and 2013 reported that more elderly (76.5 vs. 64.0%; p = 0.01) or nursing-home residents (12.7 vs. 3.8%; p < 0.001) were found in 102 adults infected by the ST131 clone in comparison to 741 adults by non-ST131 isolates (Wang et al., 2018). Although Chung et al. reported that the ST131 clone was not associated with higher mortality compared with the non-ST131 clone (Chung et al., 2012), Wang et al. found that the ST131 clone was associated with higher 28-day mortality, particularly in those infected by ESBL producers (Wang et al., 2018).
Among patients with UTI, ST131 E. coli still plays an important role in this clinical entity. A national surveillance analyzed the temporal trend of the ST131 clone among urinary E. coli isolates in the community from 2002 to 2016, in which 2,997 outpatient urine E. coli isolates were included and 542 were selected for detection of ST131 based on ciprofloxacin and/or cefotaxime resistance (Wang et al., 2021). Overall, the estimated ST131 prevalence gradually increased from 11.2% (in 2002–2004), 12.2% (in 2006–2008), 13.6% (in 2010–2012), to 17.4% in 2014–2016 (p < 0.01). In the ciprofloxacin-resistant/cefotaxime-resistant group, ST131 increased from 33.3% in 2002–2004 to 72.1% in 2014–2016 (p < 0.01). Moreover, age (≥65 years) and ciprofloxacin resistance were independently associated with ST131 (Wang et al., 2021).
For children aged 0–18 years, a prospective study included 157 isolates from stool specimens collected within 3 days of hospitalization between 2013 and 2014 and showed that among 157 E. coli isolates, 26 (16.6%) and 13 (8.3%) were O25b and ST131 positive, respectively (Huang et al., 2018). Among 13 ESBL-producing E. coli isolates, five (38.5%) belonged to CTX-M group 9, among which 4 (80%) were CTXM-14 and O25b-ST131 positive (Huang et al., 2018). For infants <1 year, a study included infant patients hospitalized for ESBL-producing E. coli-associated UTI between 2009 and 2012 and found that O25b-ST131 accounted for 65% of the 111 isolates (Cheng et al., 2015). Although E. coli O25b-ST131 isolates were more susceptible to trimethoprim/sulfamethoxazole, there were more resistant to ciprofloxacin (Cheng et al., 2015).
Several anthropogenic factors, including the drinking water process, pork consumption, pets, and household density might be associated with ST131 E. coli (Cheng et al., 2022). Compared with families who live in less crowded houses, participants with pets had a similar trend of higher risks of ESBL-producing E. coli, ST131 E. coli, and extraintestinal pathogenic E. coli fecal carriage among those living in houses accommodating relatively more people (Cheng et al., 2022). In addition to the above clinical settings, Chen et al. investigated the epidemiology of ESBL-producing E. coli from multiple rivers in southern Taiwan (Chen et al., 2016). They found that ESBL-producing E. coli mostly belonged to clonal complexes ST10 and ST58, which were geographically related to chicken farms, however, ESBL-producing E. coli ST131 was not detected among the isolates from river water (Chen et al., 2016).
The ST131/O25b strain is a global zoonotic clone of public health concern, however, the prevalence of ST131 E. coli in animal studies was various. One study including 275 E. coli isolated from piglets with diarrhea in swine farms showed that the occurrence rate of ESBL-producing E. coli was 19.7% (n = 54) in southern Taiwan in 2015 (Lee and Yeh, 2017). The ST10 clonal complexes comprised most of the ESBL-producing E. coli strains and the most detected β-lactamase genes were the CTX-M-15 gene (16 of 54; 29.6%) and CTX-M-55 gene (34 of 54; 63.0%), which belong to the CTX-M-1 group (Lee and Yeh, 2017). Another surveillance screened for 283 E. coli isolates in dogs and cats from 2014 to 2017 and found 65 (23%) E. coli (54 from dogs and 11 from cats) with the ESBL phenotype (Huang Y. H. et al., 2020). Additionally, the CTX-M-1 group and CTX-M-2 group were the most identified ESBL gene groups. CTX-M-55 gene was the main ESBL gene within the CTX-M-1 group, whereas the CTX-M-2 group contained only CTX-M-124. Further multilocus sequence typing indicated that ST457, ST131, and ST648 were the most common sequence types, and eight ST131/O25b isolates were identified (Huang Y. H. et al., 2020). Another study investigated the rectal swab specimens from 299 non-infectious dogs and found that the prevalence of ESBL-producing E. coli was 9.4%, and seven isolates were positive for ST131, in which the most predominant subtypes were FimH41 and FimH22 (Chen et al., 2020). Overall, it is worth noting that the potentially wide-spreading CTX-M-55 has been emerging in E. coli isolates from both humans and animals in Taiwan (Jean and Hsueh, 2017).
In Taiwan, K. pneumoniae was the prevalent pathogen in many types of infections, such as liver abscess, community-acquired pneumonia, lung abscess, empyema thoracic, urinary tract infections, spontaneous peritonitis, and peritoneal dialysis-related peritonitis (Chu et al., 1992; Wang et al., 2005; Lee et al., 2006; Chan et al., 2007; Lin et al., 2010b, 2014, 2015). However, the emergence of antibiotic-resistant K. pneumoniae, including ESBL-producing K. pneumoniae has largely limited the therapeutic options and posed a great threat to public health. Table 4 summarized the prevalence of ESBL-producing K. pneumoniae over time in Taiwan.
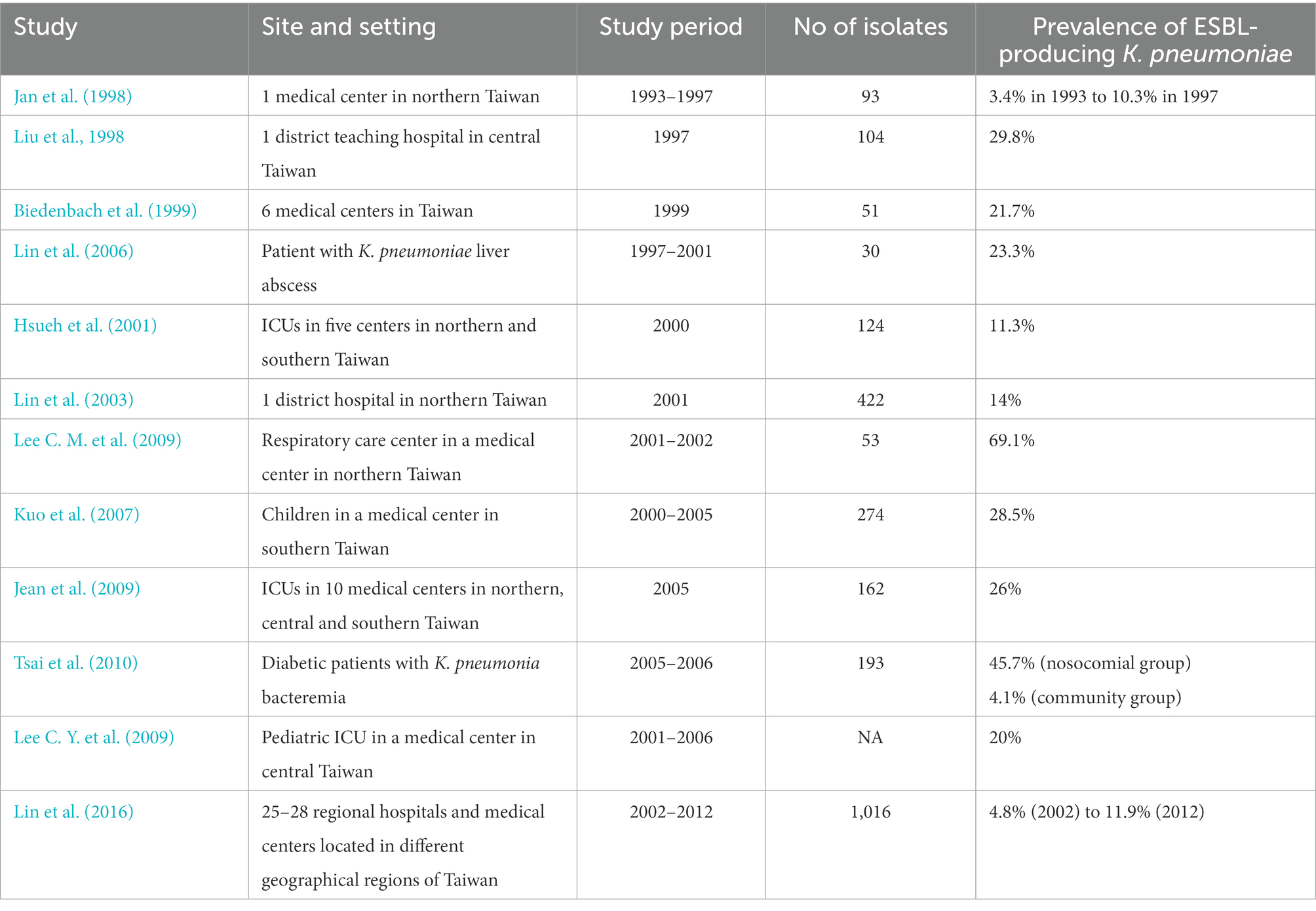
Table 4. Prevalence of extended-spectrum β-lactamases (ESBLs) among Klebsiella pneumoniae in Taiwan.
The prevalence of ESBL-producing K. pneumoniae has been higher in hospitals than in the community (Table 4). Although less reported for ESBL production in E. coli, the prevalence of ESBL-producing E. coli strains has been high in ICUs, respiratory care centers, and nursing homes in Taiwan (Table 3).
Three studies before 2000 had reported the prevalence of ESBL-producing K. pneumoniae isolates. In 1998, Jan et al. reported that the frequency of ESBL-producing K. pneumoniae isolates (according to the disk-diffusion method) had increased markedly in the years from 3.4% in 1993 to 10.3% in 1997 in a single center in northern Taiwan (Jan et al., 1998). At the same time, Liu et al. showed that 31 (29.8%) of 104 clinical isolates of K. pneumoniae collected over a period of 8 months were found to be ESBL producers in a district teaching hospital in central Taiwan in 1997 (Liu et al., 1998). In 1999, a multicenter study showed that ESBL production in Klebsiella spp. was found to be 21.7% from isolates in six medical centers in Taiwan (Biedenbach et al., 1999).
Between 2000 and 2010, many studies in different parts of Taiwan or settings showed the various prevalence of ESBL-producing K. pneumoniae. A study in a district hospital in northern Taiwan found that 59 (14%) of 422 isolates of K. pneumoniae collected in 2001 were ESBL-producing strains (Lin et al., 2003). In addition, they demonstrated that tracheostomy and ceftazidime use were risk factors in the acquisition of K. pneumoniae with ESBLs (Lin et al., 2003). Moreover, a significantly rising prevalence of ESBL production among K. pneumoniae in national surveillance for 3 months in 2005 was noted when compared with a previous Taiwanese survey in 2000 (p = 0.002; Jean et al., 2009). Additionally, clonal dissemination (both interhospital and intrahospital dissemination) of ESBL-producing isolates of K. pneumoniae occurred in several regions of Taiwan, mainly in the hospitals located in the northern and central regions of Taiwan (Yu et al., 2004b).
For critically ill patients, a multicenter study in 2000 showed that the percentage of ESBL-producing K. pneumoniae was 11.3% of isolates from various clinical specimens from patients in ICUs (Hsueh et al., 2001). For pediatrics requiring ICU admission, a single medical center in central Taiwan showed that ESBL-producing K. pneumoniae accounted for 20% of K. pneumoniae isolates since 2005 (Lee C. Y. et al., 2009). In a respiratory care center, a retrospective analysis from January 2001 to December 2002 revealed the prevalence of ESBL phenotype was as high as 69.1% of 53 K. pneumoniae isolates (Lee C. M. et al., 2009). For patients with community-acquired liver abscess, a small study showed that seven of 30 K. pneumoniae strains associated with primary liver abscess were found to be ESBL-producers, in which SHV-5a was found in 5, whereas SHV-5 and CTX-M-9 group were detected in 1 strain (Lin et al., 2006). For diabetic patients, a retrospective analysis involving 193 adult diabetic patients with K. pneumoniae bacteremia hospitalized between January 2005 and December 2006 showed that the rate of ESBL infections in the nosocomial group was 11 times higher than that in the community group (45.7 vs. 4.1%, p < 0.001; Tsai et al., 2010). Moreover, ESBL infection accounted for 53% of mortality in the nosocomial group (Tsai et al., 2010).
One study including 57 fistula or graft- or catheter-related ESBL-producing K. pneumoniae bacteremia in patients on maintenance hemodialysis (HD) found most of the patients were elderly, malnourished, and with a history of severe illnesses, broad-spectrum antibiotic use before the onset of bacteremia, and severe septicemia (Yang et al., 2012). Further multivariate analyses revealed that flomoxef use (OR, 3.52; 95% CI, 1.19–58.17), Pitt bacteremia score (OR, 2.92; 95% CI, 1.36–6.26), and catheter-dependent HD >30 days (OR, 5.73; 95% CI, 1.21–63.2) were independently associated with increased mortality (Yang et al., 2012). A longitudinal study involving 1,016 K. pneumoniae isolates from the community- outpatients or those visiting emergency rooms collected during 2002–2012 from the Taiwan Surveillance of Antimicrobial Resistance program showed that the prevalence of ESBL-producers significantly increased from 4.8% in 2002 to 11.9% in 2012 (p = 0.012; Lin et al., 2016).
In 1998, Liu et al. first reported the molecular epidemiology of ESBL-producing K. pneumoniae clinical isolates in Taiwan and found that 22 (71%) of 31 isolates were found to produce SHV-5 (Liu et al., 1998). Additional analytical isoelectric focusing also found seven isolates that produced two unknown ESBLs (with pIs of 7.9 and 7.75), and the pI of 7.9 was later presumed to be CTXM-14 (Liu et al., 1998). In 2000, Yan et al. detected 20 (8.5%) ESBL producers among 234 nonrepetitive clinical isolates of K. pneumoniae, in which a predominance of SHV-12 in 10 strains followed by SHV-5 in 4 strains (Yan et al., 2000). In 2001, Chang et al. repdqorted the stepwise mutations initiated from SHV-1 or SHV-11 into SHV-2, SHV-5, and SHV-12 comprise the evolutionary change responsible for ESBL production based on the analysis of a total of 113 blood culture isolates of K. pneumoniae from 10 hospitals in northern Taiwan (Chang et al., 2001).
In 2002, a multicenter study first identified another rapidly evolving group of CTX-M family enzymes (CTX-M-3 and CTX-M-14) with inter-and intrahospital clonal dissemination in Taiwan (Yu et al., 2002). In 2004, CTX-M-15, an Asp-240-Gly variant of CTX-M-3 with increased catalytic efficiency against ceftazidime, was first described in two of 211 ESBL-producing K. pneumoniae isolates (Yu et al., 2004a). In 2006, Yu et al. found that the insertion sequence IS26 and IS5 were found downstream from the SHV-5 gene in a transferable plasmid pKP53 (Yu et al., 2006a). In 2009, nationwide surveillance from seven medical centers and 13 regional hospitals showed that 102 (43.4%) of 235 ESBL-producing K. pneumoniae isolates were resistant to amikacin (Ma et al., 2009). Moreover, 92 of these 102 (90.2%) isolates were carrying CTX-M-type β-lactamases individually or concomitantly with SHV-type or CMY-2 β-lactamases and CTX-M-type β-lactamase genes belonging to either group 1 (CTX-M-3 and CTX-M-15) or group 9 (CTX-M-14) were found in all amikacin-resistant ESBL-producing K. pneumoniae isolates (Ma et al., 2009). Further molecular typing revealed that the amikacin-resistant ESBL-producing K. pneumoniae isolates were epidemiologically unrelated, and suggested that the acquisition of resistance was not through the spread of a resistant clone (Ma et al., 2009). In 2017, Chang et al. included 51 E. coli transconjugant strains with plasmids from ESBL-producing K. pneumoniae from the Taiwan Surveillance of Antimicrobial Resistance III Program and found that all the 51 plasmids carried a CTX-M gene, the majority of which were CTX-M-3 gene [28/51 (54.9%); Chang et al., 2017]. They found that the most common replicon type of plasmids was incompatibility group (Inc)A/C (60.8%), in which all carried CTX-M-3, CTX-M-14, and CTX-M-15 genes, and some also carried SHV-5 and SHV-12 genes (Chang et al., 2017). In this surveillance, greater than 50% of plasmids fell into clusters, and >60% of cluster-classified plasmids were present in clonally unrelated isolates, which should suggest that horizontal transfer of plasmids in the spread of ESBL genes (Chang et al., 2017).
Enterobacter cloacae complex is an important nosocomial pathogen. One study in central Taiwan demonstrated that ESBL-producing strains could be associated with higher mortality than non-ESBL strains in patients with bloodstream infections (Chen and Huang, 2013). Furthermore, they found that the risk factors for ESBL among E. cloacae complex isolates included diseases severity (p = 0.03), category of healthcare-associated infection (p = 0.04), prior use of antibiotics (p = 0.023), and prior use of a ventilator (p = 0.037; Chen and Huang, 2013). One study tested a total of 116 clinical isolates of E. cloacae complex in northern Taiwan and found that the overall prevalence of ESBL-producing E. cloacae complex was 21.6% (Yang et al., 2009). Similar findings were shown in another study in a medical center in southern Taiwan, in which ESBL producers were identified in 20 (27.0%) of 74 clinical isolates by polymerase chain reaction-based methods (Su et al., 2010). Additionally, one more study in southern Taiwan showed that 17 of 110 E. cloacae complex isolates were ESBL producers, and further gene analysis reveal the presence of the SHV-12 gene in all ESBL producers (Yu et al., 2006b). In addition, one and two isolates carried the CTX-M-3 gene and CTX-M-9 gene, respectively. However, no major epidemic clone of ESBL producers was identified by pulsed-field gel electrophoresis (Yu et al., 2006b). In ICU, a study conducted at four ICUs of a tertiary hospital showed that SHV-12 (59%), CTX-M- 3 (36%), and CTX-M-14 (14%) were the three most frequent ESBLs among a total of 125 nonrepetitive ESBL-producing isolates of E. cloacae complex, E. coli, and K. pneumoniae (Wu T. L. et al., 2006). Furthermore, SHV-12 was predominant among the E. cloacae complex in the burn unit (Wu T. L. et al., 2006). A large study of 610 E. cloacae complex bacteremic isolates in a medical center during an 8-year period showed that 138 (22.6%) carried ESBL genes (Lee et al., 2010). In addition, 133 (96.3%) carried the SHV-12 gene, 3 (2.1%) had CTX-M-3, and 2 (1.4%) had both the SHV-12 and CTX-M-3 genes (Lee et al., 2010). Mover, they observed that the in-hospital sepsis-related mortality rate of patients definitively treated with a carbapenem was lower than that of those treated by non-carbapenem β-lactams (5/53, or 9.4%, vs. 13/44, or 29.5%; p = 0.01), and suggested that the survival benefit by carbapenem therapy for ESBL-producing E. cloacae complex bacteremia may provide therapeutic benefits (Lee et al., 2010).
The study investigating the ESBL-producing Serratia marcescens is limited. One single-center study reported that ESBL producers could be identified in 11 (15.9%) of 69 S. marcescens isolates collected from patients hospitalized at a medical center in southern Taiwan from 1999 to 2002 by polymerase chain reaction-based methods (Su et al., 2010). In ICU, a national surveillance reported that ESBL production was found in 16% of S. marcescens isolates (Jean et al., 2009). The clinical spectrum of ESBL-producing S. marcescens-related infections included urinary tract infection; pneumonia, spontaneous bacterial peritonitis, secondary bacteremia, primary bacteremia, and colonization of the central catheter tip (Cheng et al., 2006). The 30-day mortality rate of ESBL-producing S. marcescens-related infections was 33.3% (5/15) in one study, in which their outcome varied according to the severity of the underlying disorder and the site of infection (Cheng et al., 2006). Among 123 nonrepetitive S. marcescens clinical isolates, 15 (12%) were ESBL-producers with exclusively revealing CTX-M-3 (Cheng et al., 2006). A similar finding was shown in the survey in a medical center in middle Taiwan, which found that CTX-M-3 was the most common ESBL gene in S. marcescens isolates (Wu et al., 2004).
ESBL-producing P. mirabilis is another common Enterobacterales in Taiwan. In central Taiwan, a two-center study reported that 34 (30.6%) of 111 clinical isolates of P. mirabilis from patients with respiratory or urinary tract infection had ESBLs, in which CTX-M-14 in 33 strains and CTX-M-3 in 6 strains (5 strains harboring both CTX-M-14 and CTX-M-3 enzymes; Wu L. T. et al., 2006). This study was also the first one to demonstrate the clonal spreading (both intra- and interhospital spread) of CTX-M-type P. mirabilis (Wu L. T. et al., 2006). These findings were consistent with another study in central Taiwan including 314 P. mirabilis isolates from urine, in which 79 (25%) clinical isolates were ESBL-producing and most ESBL-producing P. mirabilis isolates were positive for CTX-M (Chen C. M. et al., 2017). Additionally, they found that class 1 integrons were more frequently found in ESBL-positive (55/79, 70%) than ESBL-negative (21/235, 8.9%) P. mirabilis isolates (Chen C. M. et al., 2017). However, the study in southern Taiwan found a lower prevalence of ESBL among P. mirabilis, which was 2.8% (44/1574) but it increased from 0.7% in 1999 to approximately 6% after 2002 (Wu et al., 2008). Nonetheless, they showed a similar finding that all ESBL-producing P. mirabilis were positive for CTX-M, including 22 CTX-M-14, 18 CTX-M-3, two CTX-M-24, and two CTX-M-66 producers (Wu et al., 2008). In an additional study focusing on nursing home residents in southern Taiwan, the prevalence of ESBL in 102 P. mirabilis isolated was 4.9% (n = 5; Liu et al., 2016). Two nationwide surveillance showed similar findings that the prevalence of ESBL was 8.2% in 25 to 28 hospitals between 2002 and 2012 (Wang et al., 2014), and 13% in P. mirabilis isolates at the ICUs of 10 major teaching hospitals in 2015 (Jean et al., 2009). As previously described (Wu L. T. et al., 2006; Wu C. M. et al., 2008; Chen et al., 2017), the CTX-M type remained the predominant ESBL gene in the nationwide study (Wang et al., 2014).
The prevalence of ESBL in Enterobacterales has been increasing over time in Taiwan, however, the prevalence of ESBL could be various. The rates of ESBL producers have been highest in northern Taiwan. The genotypes of ESBL are also different in various studies in Taiwan. SHV-type ESBLs (SHV-5 and SHV-12) were the major type of E. cloacae complex, but S. marcescens, E. coli, and K. pneumoniae were more likely to possess CTX-M-type ESBLs (CTX-M-3 and CTX-M-14). A clonal sequence type of O25b-ST131 among urinary or bloodstream E. coli isolates, potentially associated with virulence, ESBL production, ciprofloxacin resistance, and mortality has been emerging in the community in Taiwan. The evolution of the genetic traits of the ESBL-producing Enterobacterales isolates has established clonal dissemination (both interhospital and intrahospital dissemination) in several regions of Taiwan. The cause of different prevalence and genotypes of ESBL in Enterobacterales in different regions might be due to different hospital allocation, clinical specimens, sources of infections, antibiotic prescription by physicians, and nosocomial or community acquisitions. Continuous monitoring and surveillance in the investigation of ESBL production among Enterobacterales are needed to establish its long-term epidemiology.
C-MC, C-CL, and W-LY contributed to conception and design of the study. C-MC and C-CL wrote the first draft of the manuscript. W-LY critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Biedenbach, D. J., Johnson, D. M., and Jones, R. N. (1999). In vitro evaluation of cefepime and other broad-spectrum beta-lactams in Taiwan medical centers. The Taiwan antimicrobial resistance study group. Diagn. Microbiol. Infect. Dis. 35, 299–305. doi: 10.1016/S0732-8893(99)00106-6
Bonnet, R. (2004). Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48, 1–14. doi: 10.1128/AAC.48.1.1-14.2004
Broussier, M., Gbaguidi-Haoré, H., Rachidi-Berjamy, F., Bertrand, X., and Slekovec, C. (2020). Prevalence, genetic diversity of and factors associated with ESBL-producing Enterobacterales carriage in residents of French nursing homes. J. Hosp. Infect. 104, 469–475. doi: 10.1016/j.jhin.2019.12.008
Cameron, A., Mangat, R., Mostafa, H. H., Taffner, S., Wang, J., Dumyati, G., et al. (2021). Detection of CTX-M-27 β-lactamase genes on two distinct plasmid types in ST38 Escherichia coli from three U.S. States. Antimicrob. Agents Chemother. 65:e0082521. doi: 10.1128/AAC.00825-21
Castanheira, M., Simner, P. J., and Bradford, P. A. (2021). Extended-spectrum β-lactamases: an update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 3:dlab092. doi: 10.1093/jacamr/dlab092
Chan, K. S., Yu, W. L., Tsai, C. L., Cheng, K. C., Hou, C. C., Lee, M. C., et al. (2007). Pyogenic liver abscess caused by Klebsiella pneumoniae: analysis of the clinical characteristics and outcomes of 84 patients. Chin. Med. J. 120, 136–139. doi: 10.1097/00029330-200701020-00012
Chang, C. Y., Lin, H. J., Chang, L. L., Ma, L., Siu, L. K., Tung, Y. C., et al. (2017). Characterization of extended-Spectrum β-lactamase-carrying plasmids in clinical isolates of Klebsiella pneumoniae from Taiwan. Microb. Drug Resist. 23, 98–106. doi: 10.1089/mdr.2015.0212
Chang, F. Y., Siu, L. K., Fung, C. P., Huang, M. H., and Ho, M. (2001). Diversity of SHV and TEM beta-lactamases in Klebsiella pneumoniae: gene evolution in northern Taiwan and two novel beta-lactamases, SHV-25 and SHV-26. Antimicrob. Agents Chemother. 45, 2407–2413. doi: 10.1128/AAC.45.9.2407-2413.2001
Chen, P. C., Chang, L. Y., Lu, C. Y., Shao, P. L., Tsai, I. J., Tsau, Y. K., et al. (2014). Drug susceptibility and treatment response of common urinary tract infection pathogens in children. J. Microbiol. Immunol. Infect. 47, 478–483. doi: 10.1016/j.jmii.2013.07.011
Chen, C. H., and Huang, C. C. (2013). Risk factor analysis for extended-spectrum β-lactamase-producing Enterobacter cloacae bloodstream infections in Central Taiwan. BMC Infect. Dis. 13:417. doi: 10.1186/1471-2334-13-417
Chen, J. W., Huang, H. H., Chang, S. M., Scaria, J., Chiu, Y. L., Chen, C. M., et al. (2020). Antibiotic-resistant Escherichia coli and sequence type 131 in fecal colonization in dogs in Taiwan. Microorganisms 8:1439. doi: 10.3390/microorganisms8091439
Chen, I. L., Huang, H. C., Wu, C. T., Ou-Yang, M. C., Chung, M. Y., Chen, C. C., et al. (2017). Analysis of early-onset bloodstream infection due to Escherichia coli infection in premature babies. Medicine (Baltimore) 96:e7748. doi: 10.1097/MD.0000000000007748
Chen, P. A., Hung, C. H., Huang, P. C., Chen, J. R., Huang, I. F., Chen, W. L., et al. (2016). Characteristics of CTX-M extended-spectrum β-lactamase-producing Escherichia coli strains isolated from multiple rivers in southern Taiwan. Appl. Environ. Microbiol. 82, 1889–1897. doi: 10.1128/AEM.03222-15
Chen, W. Y., Jang, T. N., Huang, C. H., and Hsueh, P. R. (2009). In vitro susceptibilities of aerobic and facultative anaerobic gram-negative bacilli isolated from patients with intra-abdominal infections at a medical center in Taiwan: results of the study for monitoring antimicrobial resistance trends (SMART) 2002-2006. J. Microbiol. Immunol. Infect. 42, 317–323.
Chen, C. M., Lai, C. H., Wu, H. J., and Wu, L. T. (2017). Genetic characteristic of class 1 integrons in proteus mirabilis isolates from urine samples. Biomedicine 7:9. doi: 10.1051/bmdcn/2017070202
Cheng, M. F., Chen, W. L., Huang, I. F., Chen, J. R., Chiou, Y. H., Chen, Y. S., et al. (2016). Urinary tract infection in infants caused by extended-spectrum beta-lactamase-producing Escherichia coli: comparison between urban and rural hospitals. Pediatr. Nephrol. 31, 1305–1312. doi: 10.1007/s00467-016-3338-0
Cheng, M. F., Chen, W. L., Hung, W. Y., Huang, I. F., Chiou, Y. H., Chen, Y. S., et al. (2015). Emergence of extended spectrum-β-lactamase-producing Escherichia coli O25b-ST131: a major community-acquired uropathogen in infants. Pediatr. Infect. Dis. J. 34, 469–475. doi: 10.1097/INF.0000000000000623
Cheng, K. C., Chuang, Y. C., Wu, L. T., Huang, G. C., and Yu, W. L. (2006). Clinical experiences of the infections caused by extended-spectrum beta-lactamase-producing Serratia marcescens at a medical center in Taiwan. Jpn. J. Infect. Dis. 59, 147–152.
Cheng, M. F., Ho, P. Y., Wang, J. L., Tseng, F. C., Chang, J. T., Huang, I. F., et al. (2022). Prevalence and household risk factors for fecal carriage of ESBL-producing, sequence type 131, and extraintestinal pathogenic Escherichia coli among children in southern Taiwan. J. Microbiol. Immunol. Infect. 55, 695–707. doi: 10.1016/j.jmii.2022.04.001
Chia, J. H., Chu, C., Su, L. H., Chiu, C. H., Kuo, A. J., Sun, C. F., et al. (2005). Development of a multiplex PCR and SHV melting-curve mutation detection system for detection of some SHV and CTX-M beta-lactamases of Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae in Taiwan. J. Clin. Microbiol. 43, 4486–4491. doi: 10.1128/JCM.43.9.4486-4491.2005
Cho, S. Y., Kang, C. I., Cha, M. K., Wi, Y. M., Ha, Y. E., Chung, D. R., et al. (2015). Clinical features and treatment outcomes of bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli sequence type 131. Microb. Drug Resist. 21, 463–469. doi: 10.1089/mdr.2014.0261
Chu, C. M., Chiu, K. W., and Liaw, Y. F. (1992). The prevalence and prognostic significance of spontaneous bacterial peritonitis in severe acute hepatitis with ascites. Hepatology 15, 799–803. doi: 10.1002/hep.1840150509
Chung, H. C., Lai, C. H., Lin, J. N., Huang, C. K., Liang, S. H., Chen, W. F., et al. (2012). Bacteremia caused by extended-spectrum-β-lactamase-producing Escherichia coli sequence type ST131 and non-ST131 clones: comparison of demographic data, clinical features, and mortality. Antimicrob. Agents Chemother. 56, 618–622. doi: 10.1128/AAC.05753-11
Colmenarejo, C., Hernández-García, M., Muñoz-Rodríguez, J. R., Huertas, N., Navarro, F. J., Mateo, A. B., et al. (2020). Prevalence and risks factors associated with ESBL-producing faecal carriage in a single long-term-care facility in Spain: emergence of CTX-M-24- and CTX-M-27-producing Escherichia coli ST131-H30R. J. Antimicrob. Chemother. 75, 2480–2484. doi: 10.1093/jac/dkaa219
Coque, T. M., Novais, A., Carattoli, A., Poirel, L., Pitout, J., Peixe, L., et al. (2008). Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg. Infect. Dis. 14, 195–200. doi: 10.3201/eid1402.070350
Day, M. J., Hopkins, K. L., Wareham, D. W., Toleman, M. A., Elviss, N., Randall, L., et al. (2019). Extended-spectrum β-lactamase-producing Escherichia coli in human-derived and foodchain-derived samples from England, Wales, and Scotland: an epidemiological surveillance and typing study. Lancet Infect. Dis. 19, 1325–1335. doi: 10.1016/S1473-3099(19)30273-7
Duffy, N., Karlsson, M., Reses, H. E., Campbell, D., Daniels, J., Stanton, R. A., et al. (2022). Epidemiology of extended-spectrum β-lactamase-producing Enterobacterales in five US sites participating in the emerging infections program, 2017. Infect. Control Hosp. Epidemiol. 43, 1586–1594. doi: 10.1017/ice.2021.496
Estaleva, C. E. L., Zimba, T. F., Sekyere, J. O., Govinden, U., Chenia, H. Y., Simonsen, G. S., et al. (2021). High prevalence of multidrug resistant ESBL- and plasmid mediated AmpC-producing clinical isolates of Escherichia coli at Maputo Central Hospital, Mozambique. BMC Infect. Dis. 21:16. doi: 10.1186/s12879-020-05696-y
Fadare, F. T., and Okoh, A. I. (2021). Distribution and molecular characterization of ESBL, pAmpC β-lactamases, and non-β-lactam encoding genes in Enterobacteriaceae isolated from hospital wastewater in Eastern Cape Province, South Africa. PLoS One 16:e0254753. doi: 10.1371/journal.pone.0254753
Fan, N. C., Chen, H. H., Chen, C. L., Ou, L. S., Lin, T. Y., Tsai, M. H., et al. (2014). Rise of community-onset urinary tract infection caused by extended-spectrum β-lactamase-producing Escherichia coli in children. J. Microbiol. Immunol. Infect. 47, 399–405. doi: 10.1016/j.jmii.2013.05.006
Gomi, R., Matsuda, T., Yamamoto, M., Chou, P. H., Tanaka, M., Ichiyama, S., et al. (2018). Characteristics of carbapenemase-producing Enterobacteriaceae in wastewater revealed by genomic analysis. Antimicrob. Agents Chemother. 62:62. doi: 10.1128/AAC.02501-17
Hsieh, C. J., Shen, Y. H., and Hwang, K. P. (2010). Clinical implications, risk factors and mortality following community-onset bacteremia caused by extended-spectrum β-lactamase (ESBL) and non-ESBL producing Escherichia coli. J. Microbiol. Immunol. Infect. 43, 240–248. doi: 10.1016/S1684-1182(10)60038-2
Hsueh, P. R., Liu, Y. C., Yang, D., Yan, J. J., Wu, T. L., Huang, W. K., et al. (2001). Multicenter surveillance of antimicrobial resistance of major bacterial pathogens in intensive care units in 2000 in Taiwan. Microb. Drug Resist. 7, 373–382. doi: 10.1089/10766290152773383
Huang, Y. H., Kuan, N. L., and Yeh, K. S. (2020). Characteristics of extended-spectrum β-lactamase-producing Escherichia coli from dogs and cats admitted to a veterinary teaching Hospital in Taipei, Taiwan from 2014 to 2017. Front. Vet. Sci. 7:395. doi: 10.3389/fvets.2020.00395
Huang, Y. S., Lai, L. C., Chen, Y. A., Lin, K. Y., Chou, Y. H., Chen, H. C., et al. (2020). Colonization with multidrug-resistant organisms among healthy adults in the community setting: prevalence, risk factors, and composition of gut microbiome. Front. Microbiol. 11:1402. doi: 10.3389/fmicb.2020.01402
Huang, I. F., Lee, W. Y., Wang, J. L., Hung, C. H., Hu, H. H., Hung, W. Y., et al. (2018). Fecal carriage of multidrug-resistant Escherichia coli by community children in southern Taiwan. BMC Gastroenterol. 18:86. doi: 10.1186/s12876-018-0807-x
Hung, W. T., Cheng, M. F., Tseng, F. C., Chen, Y. S., Shin-Jung Lee, S., Chang, T. H., et al. (2019). Bloodstream infection with extended-spectrum beta-lactamase-producing Escherichia coli: the role of virulence genes. J. Microbiol. Immunol. Infect. 52, 947–955. doi: 10.1016/j.jmii.2019.03.005
Hsueh, P. R., Chen, W. H., and Luh, K. T. (2005). Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991-2003 at a university hospital in Taiwan. Int. J. Antimicrob. Agents. 26, 463–472. doi: 10.1016/j.ijantimicag.2005.08.016
Jan, I. S., Hsueh, P. R., Teng, L. J., Ho, S. W., and Luh, K. T. (1998). Antimicrobial susceptibility testing for Klebsiella pneumoniae isolates resistant to extended-spectrum beta-lactam antibiotics. J. Formos. Med. Assoc. 97, 661–666.
Jean, S. S., and Hsueh, P. R. (2017). Distribution of ESBLs, AmpC β-lactamases and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal and urinary tract infections in the Asia-Pacific region during 2008-14: results from the study for monitoring antimicrobial resistance trends (SMART). J. Antimicrob. Chemother. 72, 166–171. doi: 10.1093/jac/dkw398
Jean, S. S., Hsueh, P. R., Lee, W. S., Chang, H. T., Chou, M. Y., Chen, I. S., et al. (2009). Nationwide surveillance of antimicrobial resistance among Enterobacteriaceae in intensive care units in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 28, 215–220. doi: 10.1007/s10096-008-0610-7
Karlowsky, J. A., Bouchillon, S. K., Benaouda, A., Soraa, N., Zerouali, K., Mohamed, N., et al. (2022). Antimicrobial susceptibility testing of clinical isolates of gram-negative bacilli collected in Morocco by the ATLAS global surveillance program from 2018 to 2020. J. Glob. Antimicrob. Resist. 30, 23–30. doi: 10.1016/j.jgar.2022.04.011
Komatsu, Y., Kasahara, K., Inoue, T., Lee, S. T., Muratani, T., Yano, H., et al. (2018). Molecular epidemiology and clinical features of extended-spectrum beta-lactamase- or carbapenemase-producing Escherichia coli bacteremia in Japan. PLoS One 13:e0202276. doi: 10.1371/journal.pone.0202276
Kung, C. H., Ku, W. W., Lee, C. H., Fung, C. P., Kuo, S. C., Chen, T. L., et al. (2015). Epidemiology and risk factors of community-onset urinary tract infection caused by extended-spectrum β-lactamase-producing Enterobacteriaceae in a medical center in Taiwan: a prospective cohort study. J. Microbiol. Immunol. Infect. 48, 168–174. doi: 10.1016/j.jmii.2013.08.006
Kuo, K. C., Shen, Y. H., and Hwang, K. P. (2007). Clinical implications and risk factors of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae infection in children: a case-control retrospective study in a medical center in southern Taiwan. J. Microbiol. Immunol. Infect. 40, 248–254.
Lai, C. C., Lee, K., Xiao, Y., Ahmad, N., Veeraraghavan, B., Thamlikitkul, V., et al. (2014). High burden of antimicrobial drug resistance in Asia. J. Glob. Antimicrob. Resist. 2, 141–147. doi: 10.1016/j.jgar.2014.02.007
Lee, C. Y., Chen, P. Y., Huang, F. L., and Lin, C. F. (2009). Microbiologic spectrum and susceptibility pattern of clinical isolates from the pediatric intensive care unit in a single medical center - 6 years' experience. J. Microbiol. Immunol. Infect. 42, 160–165.
Lee, Y. L., Chen, Y. S., Toh, H. S., Huang, C. C., Liu, Y. M., Ho, C. M., et al. (2012). Antimicrobial susceptibility of pathogens isolated from patients with complicated intra-abdominal infections at five medical centers in Taiwan that continuously participated in the study for monitoring antimicrobial resistance trends (SMART) from 2006 to 2010. Int. J. Antimicrob. Agents 40, S29–S36. doi: 10.1016/S0924-8579(12)70007-9
Lee, H. C., Chuang, Y. C., Yu, W. L., Lee, N. Y., Chang, C. M., Ko, N. Y., et al. (2006). Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J. Intern. Med. 259, 606–614. doi: 10.1111/j.1365-2796.2006.01641.x
Lee, C. C., Lee, C. H., Hong, M. Y., Hsieh, C. C., Tang, H. J., and Ko, W. C. (2018). Propensity-matched analysis of the impact of extended-spectrum β-lactamase production on adults with community-onset Escherichia coli, Klebsiella species, and Proteus mirabilis bacteremia. J. Microbiol. Immunol. Infect. 51, 519–526. doi: 10.1016/j.jmii.2017.05.006
Lee, C. C., Lee, N. Y., Yan, J. J., Lee, H. C., Chen, P. L., Chang, C. M., et al. (2010). Bacteremia due to extended-spectrum-beta-lactamase-producing Enterobacter cloacae: role of carbapenem therapy. Antimicrob. Agents Chemother. 54, 3551–3556. doi: 10.1128/AAC.00055-10
Lee, C. C., Wang, J. L., Lee, C. H., Hung, Y. P., Hong, M. Y., Chang, C. M., et al. (2017). Age-related trends in adults with community-onset bacteremia. Antimicrob. Agents Chemother. 61:61. doi: 10.1128/AAC.01050-17
Lee, W. C., and Yeh, K. S. (2017). Characteristics of extended-spectrum β-lactamase-producing Escherichia coli isolated from fecal samples of piglets with diarrhea in central and southern Taiwan in 2015. BMC Vet. Res. 13:66. doi: 10.1186/s12917-017-0986-7
Lee, C. M., Yeh, S. C., Lim, H. K., Liu, C. P., and Tseng, H. K. (2009). High prevalence rate of multidrug resistance among nosocomial pathogens in the respiratory care center of a tertiary hospital. J. Microbiol. Immunol. Infect. 42, 401–404.
Lin, J. N., Chen, Y. H., Chang, L. L., Lai, C. H., Lin, H. L., and Lin, H. H. (2011). Clinical characteristics and outcomes of patients with extended-spectrum β-lactamase-producing bacteremias in the emergency department. Intern. Emerg. Med. 6, 547–555. doi: 10.1007/s11739-011-0707-3
Lin, Y. T., Chen, T. L., Siu, L. K., Hsu, S. F., and Fung, C. P. (2010b). Clinical and microbiological characteristics of community-acquired thoracic empyema or complicated parapneumonic effusion caused by Klebsiella pneumoniae in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 29, 1003–1010. doi: 10.1007/s10096-010-0961-8
Lin, C. F., Hsu, S. K., Chen, C. H., Huang, J. R., and Lo, H. H. (2010). Genotypic detection and molecular epidemiology of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a regional hospital in Central Taiwan. J. Med. Microbiol. 59, 665–671. doi: 10.1099/jmm.0.015818-0
Lin, M. F., Huang, M. L., and Lai, S. H. (2003). Risk factors in the acquisition of extended-spectrum beta-lactamase Klebsiella pneumoniae: a case-control study in a district teaching hospital in Taiwan. J. Hosp. Infect. 53, 39–45. doi: 10.1053/jhin.2002.1331
Lin, W. H., Kao, C. Y., Yang, D. C., Tseng, C. C., Wu, A. B., Teng, C. H., et al. (2014). Clinical and microbiological characteristics of Klebsiella pneumoniae from community-acquired recurrent urinary tract infections. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1533–1539. doi: 10.1007/s10096-014-2100-4
Lin, H. C., Lai, L. A., Wu, J. Y., Su, Y. M., Chang, S. P., and Hsueh, Y. M. (2013). Risk factors for acquiring extended-spectrum β-lactamase-producing Enterobacteriaceae in geriatric patients with multiple comorbidities in respiratory care wards. Geriatr Gerontol Int 13, 663–671. doi: 10.1111/j.1447-0594.2012.00961.x
Lin, T. L., Tang, S. I., Fang, C. T., Hsueh, P. R., Chang, S. C., and Wang, J. T. (2006). Extended-spectrum beta-lactamase genes of Klebsiella pneumoniae strains in Taiwan: recharacterization of shv-27, shv-41, and tem-116. Microb. Drug Resist. 12, 12–15. doi: 10.1089/mdr.2006.12.12
Lin, W. H., Tseng, C. C., Wu, A. B., Yang, D. C., Cheng, S. W., Wang, M. C., et al. (2015). Clinical and microbiological characteristics of peritoneal dialysis-related peritonitis caused by Klebsiella pneumoniae in southern Taiwan. J. Microbiol. Immunol. Infect. 48, 276–283. doi: 10.1016/j.jmii.2013.10.002
Lin, W. P., Wang, J. T., Chang, S. C., Chang, F. Y., Fung, C. P., Chuang, Y. C., et al. (2016). The antimicrobial susceptibility of Klebsiella pneumoniae from community settings in Taiwan, a trend analysis. Sci. Rep. 6:36280. doi: 10.1038/srep36280
Liu, H. C., Hung, Y. P., Lin, H. J., Liu, H. C., Lee, J. C., Wu, Y. H., et al. (2016). Antimicrobial susceptibility of clinical Enterobacteriaceae isolates at the emergency department in a regional hospital: a threat of extended spectrum beta-lactamase-producers among nursing home residents. J. Microbiol. Immunol. Infect. 49, 584–590. doi: 10.1016/j.jmii.2015.10.001
Liu, F. L., Kuan, N. L., and Yeh, K. S. (2021). Presence of the extended-spectrum-β-lactamase and plasmid-mediated AmpC-encoding genes in Escherichia coli from companion animals-a study from a university-based veterinary Hospital in Taipei, Taiwan. Antibiotics 10:10. doi: 10.3390/antibiotics10121536
Liu, P. Y., Tung, J. C., Ke, S. C., and Chen, S. L. (1998). Molecular epidemiology of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a district hospital in Taiwan. J. Clin. Microbiol. 36, 2759–2762. doi: 10.1128/JCM.36.9.2759-2762.1998
Ma, L., Lin, C. J., Chen, J. H., Fung, C. P., Chang, F. Y., Lai, Y. K., et al. (2009). Widespread dissemination of aminoglycoside resistance genes armA and rmtB in Klebsiella pneumoniae isolates in Taiwan producing CTX-M-type extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 53, 104–111. doi: 10.1128/AAC.00852-08
Matsumura, Y., Pitout, J. D., Gomi, R., Matsuda, T., Noguchi, T., Yamamoto, M., et al. (2016). Global Escherichia coli sequence type 131 clade with Bla (CTX-M-27) gene. Emerg. Infect. Dis. 22, 1900–1907. doi: 10.3201/eid2211.160519
Mendes, R. E., Mendoza, M., Banga Singh, K. K., Castanheira, M., Bell, J. M., Turnidge, J. D., et al. (2013). Regional resistance surveillance program results for 12 Asia-Pacific nations (2011). Antimicrob. Agents Chemother. 57, 5721–5726. doi: 10.1128/AAC.01121-13
Merino, I., Hernández-García, M., Turrientes, M. C., Pérez-Viso, B., López-Fresneña, N., Diaz-Agero, C., et al. (2018). Emergence of ESBL-producing Escherichia coli ST131-C1-M27 clade colonizing patients in Europe. J. Antimicrob. Chemother. 73, 2973–2980. doi: 10.1093/jac/dky296
Park, S. H., Byun, J. H., Choi, S. M., Lee, D. G., Kim, S. H., Kwon, J. C., et al. (2012). Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in the community and hospital in Korea: emergence of ST131 producing CTX-M-15. BMC Infect. Dis. 12:149. doi: 10.1186/1471-2334-12-149
Peirano, G., Costello, M., and Pitout, J. D. (2010). Molecular characteristics of extended-spectrum beta-lactamase-producing Escherichia coli from the Chicago area: high prevalence of ST131 producing CTX-M-15 in community hospitals. Int. J. Antimicrob. Agents 36, 19–23. doi: 10.1016/j.ijantimicag.2010.02.016
Sader, H. S., Mendes, R. E., Streit, J. M., Carvalhaes, C. G., and Castanheira, M. (2022). Antimicrobial susceptibility of Gram-negative bacteria from intensive care unit and non-intensive care unit patients from United States hospitals (2018-2020). Diagn. Microbiol. Infect. Dis. 102:115557. doi: 10.1016/j.diagmicrobio.2021.115557
Selmi, R., Tayh, G., Srairi, S., Mamlouk, A., Ben Chehida, F., Lahmar, S., et al. (2022). Prevalence, risk factors and emergence of extended-spectrum β-lactamase producing-, carbapenem- and colistin-resistant Enterobacterales isolated from wild boar (Sus scrofa) in Tunisia. Microb. Pathog. 163:105385. doi: 10.1016/j.micpath.2021.105385
Shu, J. C., Chia, J. H., Kuo, A. J., Su, L. H., and Wu, T. L. (2010). A 7-year surveillance for ESBL-producing Escherichia coli and Klebsiella pneumoniae at a university hospital in Taiwan: the increase of CTX-M-15 in the ICU. Epidemiol. Infect. 138, 253–263. doi: 10.1017/S0950268809990409
Siu, L. K., Lu, P. L., Hsueh, P. R., Lin, F. M., Chang, S. C., Luh, K. T., et al. (1999). Bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric oncology ward: clinical features and identification of different plasmids carrying both SHV-5 and TEM-1 genes. J. Clin. Microbiol. 37, 4020–4027. doi: 10.1128/JCM.37.12.4020-4027.1999
Soilleux, M. J., Morand, A. M., Arlet, G. J., Scavizzi, M. R., and Labia, R. (1996). Survey of Klebsiella pneumoniae producing extended-spectrum beta-lactamases: prevalence of TEM-3 and first identification of TEM-26 in France. Antimicrob. Agents Chemother. 40, 1027–1029. doi: 10.1128/AAC.40.4.1027
Su, P. A., Wu, L. T., Cheng, K. C., Ko, W. C., Chuang, Y. C., and Yu, W. L. (2010). Screening extended-spectrum beta-lactamase production in Enterobacter cloacae and Serratia marcescens using antibiogram-based methods. J. Microbiol. Immunol. Infect. 43, 26–34. doi: 10.1016/S1684-1182(10)60004-7
Tao, C. W., Hsu, B. M., Ji, W. T., Hsu, T. K., Kao, P. M., Hsu, C. P., et al. (2014). Evaluation of five antibiotic resistance genes in wastewater treatment systems of swine farms by real-time PCR. Sci. Total Environ. 496, 116–121. doi: 10.1016/j.scitotenv.2014.07.024
Tsai, S. S., Huang, J. C., Chen, S. T., Sun, J. H., Wang, C. C., Lin, S. F., et al. (2010). Characteristics of Klebsiella pneumoniae bacteremia in community-acquired and nosocomial infections in diabetic patients. Chang Gung Med. J. 33, 532–539.
Tsai, W. L., Hung, C. H., Chen, H. A., Wang, J. L., Huang, I. F., Chiou, Y. H., et al. (2018). Extended-spectrum β-lactamase-producing Escherichia coli bacteremia: comparison of pediatric and adult populations. J. Microbiol. Immunol. Infect. 51, 723–731. doi: 10.1016/j.jmii.2017.08.005
Tsai, M. H., Lee, I. T., Chu, S. M., Lien, R., Huang, H. R., Chiang, M. C., et al. (2016). Clinical and molecular characteristics of neonatal extended-spectrum β-lactamase-producing gram-negative bacteremia: a 12-year case-control-control study of a referral Center in Taiwan. PLoS One 11:e0159744. doi: 10.1371/journal.pone.0159744
Tsui, K., Wong, S. S., Lin, L. C., Tsai, C. R., Chen, L. C., and Huang, C. H. (2012). Laboratory identification, risk factors, and clinical outcomes of patients with bacteremia due to Escherichia coli and Klebsiella pneumoniae producing extended-spectrum and AmpC type β-lactamases. J. Microbiol. Immunol. Infect. 45, 193–199. doi: 10.1016/j.jmii.2011.11.003
Vink, J., Edgeworth, J., and Bailey, S. L. (2020). Acquisition of MDR-GNB in hospital settings: a systematic review and meta-analysis focusing on ESBL-E. J. Hosp. Infect. 106, 419–428. doi: 10.1016/j.jhin.2020.09.006
Wang, J. T., Chen, P. C., Chang, S. C., Shiau, Y. R., Wang, H. Y., Lai, J. F., et al. (2014). Antimicrobial susceptibilities of Proteus mirabilis: a longitudinal nationwide study from the Taiwan surveillance of antimicrobial resistance (TSAR) program. BMC Infect. Dis. 14:486. doi: 10.1186/1471-2334-14-486
Wang, J. L., Chen, K. Y., Fang, C. T., Hsueh, P. R., Yang, P. C., and Chang, S. C. (2005). Changing bacteriology of adult community-acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin. Infect. Dis. 40, 915–922. doi: 10.1086/428574
Wang, J. L., Ko, W. C., Hung, C. H., Cheng, M. F., Wang, H. Y., Shiau, Y. R., et al. (2021). Temporal trend of ST131 clone among urinary Escherichia coli isolates in the community: a Taiwan National Surveillance from 2002 to 2016. Microorganisms 9:963. doi: 10.3390/microorganisms9050963
Wang, J. L., Lee, C. C., Lee, C. H., Lee, N. Y., Hsieh, C. C., Hung, Y. P., et al. (2018). Clinical impact of sequence type 131 in adults with community-onset monomicrobial Escherichia Coli bacteremia. J. Clin. Med. 7:7. doi: 10.3390/jcm7120508
Wiener, J., Quinn, J. P., Bradford, P. A., Goering, R. V., Nathan, C., Bush, K., et al. (1999). Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA 281, 517–523. doi: 10.1001/jama.281.6.517
Wu, Y. H., Chen, P. L., Hung, Y. P., and Ko, W. C. (2014a). Risk factors and clinical impact of levofloxacin or cefazolin nonsusceptibility or ESBL production among uropathogens in adults with community-onset urinary tract infections. J. Microbiol. Immunol. Infect. 47, 197–203. doi: 10.1016/j.jmii.2012.09.001
Wu, J. J., Chen, H. M., Ko, W. C., Wu, H. M., Tsai, S. H., and Yan, J. J. (2008). Prevalence of extended-spectrum beta-lactamases in Proteus mirabilis in a Taiwanese university hospital, 1999 to 2005: identification of a novel CTX-M enzyme (CTX-M-66). Diagn. Microbiol. Infect. Dis. 60, 169–175. doi: 10.1016/j.diagmicrobio.2007.08.004
Wu, Y. H., Cheng, M. F., Lai, C. H., Lin, H. H., Hung, C. H., and Wang, J. L. (2014b). The role of sequence type (ST) 131 in adult community-onset non-ESBL-producing Escherichia coli bacteraemia. BMC Infect. Dis. 14:579. doi: 10.1186/s12879-014-0579-z
Wu, T. L., Chia, J. H., Su, L. H., Chu, C., Kuo, A. J., and Chiu, C. H. (2006). Dissemination of extended-spectrum beta-lactamase-producing Enterobacteriaceae in intensive care units of a medical center in Taiwan. Microb. Drug Resist. 12, 203–209. doi: 10.1089/mdr.2006.12.203
Wu, C. T., Lee, H. Y., Chen, C. L., Tuan, P. L., and Chiu, C. H. (2016). High prevalence and antimicrobial resistance of urinary tract infection isolates in febrile young children without localizing signs in Taiwan. J. Microbiol. Immunol. Infect. 49, 243–248. doi: 10.1016/j.jmii.2015.05.016
Wu, L. T., Tsou, M. F., Wu, H. J., Chen, H. E., Chuang, Y. C., and Yu, W. L. (2004). Survey of CTX-M-3 extended-spectrum beta-lactamase (ESBL) among cefotaxime-resistant Serratia marcescens at a medical center in middle Taiwan. Diagn. Microbiol. Infect. Dis. 49, 125–129. doi: 10.1016/j.diagmicrobio.2004.02.004
Wu, U. I., Wang, J. L., Chen, W. C., Chang, S. C., and Chen, Y. C. (2011). Risk factors and outcomes of Escherichia coli bacteremia caused by strains that produce CTX-M or non-CTX-M extended-spectrum-beta-lactamases. Eur. J. Clin. Microbiol. Infect. Dis. 30, 33–39. doi: 10.1007/s10096-010-1048-2
Wu, P. C., Wang, J. L., Hsueh, P. R., Lin, P. H., Cheng, M. F., Huang, I. F., et al. (2019). Prevalence and risk factors for colonization by extended-spectrum β-lactamase-producing or ST 131 Escherichia coli among asymptomatic adults in community settings in southern Taiwan. Infect. Drug Resist. 12, 1063–1071. doi: 10.2147/IDR.S201086
Wu, L. T., Wu, H. J., Chung, J. G., Chuang, Y. C., Cheng, K. C., and Yu, W. L. (2006). Dissemination of Proteus mirabilis isolates harboring CTX-M-14 and CTX-M-3 beta-lactamases at 2 hospitals in Taiwan. Diagn. Microbiol. Infect. Dis. 54, 89–94. doi: 10.1016/j.diagmicrobio.2005.09.005
Wu, U. I., Yang, C. S., Chen, W. C., Chen, Y. C., and Chang, S. C. (2010). Risk factors for bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli. J. Microbiol. Immunol. Infect. 43, 310–316. doi: 10.1016/S1684-1182(10)60048-5
Yan, J. J., Wu, S. M., Tsai, S. H., Wu, J. J., and Su, I. J. (2000). Prevalence of SHV-12 among clinical isolates of Klebsiella pneumoniae producing extended-spectrum beta-lactamases and identification of a novel AmpC enzyme (CMY-8) in southern Taiwan. Antimicrob. Agents Chemother. 44, 1438–1442. doi: 10.1128/AAC.44.6.1438-1442.2000
Yang, C. C., Li, S. H., Chuang, F. R., Chen, C. H., Lee, C. H., Chen, J. B., et al. (2012). Discrepancy between effects of carbapenems and flomoxef in treating nosocomial hemodialysis access-related bacteremia secondary to extended spectrum beta-lactamase producing Klebsiella pneumoniae in patients on maintenance hemodialysis. BMC Infect. Dis. 12:206. doi: 10.1186/1471-2334-12-206
Yang, J. L., Wang, J. T., Lauderdale, T. L., and Chang, S. C. (2009). Prevalence of extended-spectrum beta-lactamases in Enterobacter cloacae in Taiwan and comparison of 3 phenotypic confirmatory methods for detecting extended-spectrum beta-lactamase production. J. Microbiol. Immunol. Infect. 42, 310–316.
Yu, W. L., Chen, S. C., Hung, S. W., Chuang, Y. C., Chung, J. G., Chen, I. C., et al. (2006a). Genetic association of blaSHV-5 with transposable elements IS26 and IS5 in Klebsiella pneumoniae from Taiwan. Clin. Microbiol. Infect. 12, 806–809. doi: 10.1111/j.1469-0691.2006.01488.x
Yu, W. L., Cheng, K. C., Chi, C. J., Chen, H. E., Chuang, Y. C., and Wu, L. T. (2006b). Characterisation and molecular epidemiology of extended-spectrum beta-lactamase-producing Enterobacter cloacae isolated from a district teaching hospital in Taiwan. Clin. Microbiol. Infect. 12, 579–582. doi: 10.1111/j.1469-0691.2006.01384.x
Yu, W. L., Cheng, K. C., Wu, L. T., Pfaller, M. A., Winokur, P. L., and Jones, R. N. (2004a). Emergence of two Klebsiella pneumoniae isolates harboring plasmid-mediated CTX-M-15 beta-lactamase in Taiwan. Antimicrob. Agents Chemother. 48, 362–363. doi: 10.1128/AAC.48.1.362-364.2004
Yu, W. L., Chuang, Y. C., and Walther-Rasmussen, J. (2006c). Extended-spectrum beta-lactamases in Taiwan: epidemiology, detection, treatment and infection control. J. Microbiol. Immunol. Infect. 39, 264–277.
Yu, W. L., Winokur, P. L., Jones, R. N., and Sader, H. S. (2004b). Surveillance in Taiwan using molecular epidemiology for extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect. Control Hosp. Epidemiol. 25, 812–818. doi: 10.1086/502301
Yu, W. L., Winokur, P. L., Von Stein, D. L., Pfaller, M. A., Wang, J. H., and Jones, R. N. (2002). First description of Klebsiella pneumoniae harboring CTX-M beta-lactamases (CTX-M-14 and CTX-M-3) in Taiwan. Antimicrob. Agents Chemother. 46, 1098–1100. doi: 10.1128/AAC.46.4.1098-1100.2002
Zeng, S., Luo, J., Li, X., Zhuo, C., Wu, A., Chen, X., et al. (2021). Molecular epidemiology and characteristics of CTX-M-55 extended-spectrum β-lactamase-producing Escherichia coli from Guangzhou, China. Front. Microbiol. 12:730012. doi: 10.3389/fmicb.2021.730012
Keywords: Enterobacterales, ESBL, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis
Citation: Chao C-M, Lai C-C and Yu W-L (2023) Epidemiology of extended-spectrum β-lactamases in Enterobacterales in Taiwan for over two decades. Front. Microbiol. 13:1060050. doi: 10.3389/fmicb.2022.1060050
Received: 02 October 2022; Accepted: 22 December 2022;
Published: 25 January 2023.
Edited by:
Alberto Antonelli, University of Florence, ItalyReviewed by:
Haiquan Kang, Affiliated Hospital of Xuzhou Medical University, ChinaCopyright © 2023 Chao, Lai and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Liang Yu, ✉ eXUyMjMxQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.