
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 12 January 2023
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1039687
β-Lactams are a broad class of antimicrobial agents with a high safety profile, making them the most widely used class in clinical, agricultural, and veterinary setups. The widespread use of β-lactams has induced the extensive spread of β-lactamase hydrolyzing enzymes known as β-lactamases (BLs). To neutralize the effect of β-lactamases, newer generations of β-lactams have been developed, which ultimately led to the evolution of a highly diverse family of BLs. Based on sequence homology, BLs are categorized into four classes: A–D in Ambler’s classification system. Further, each class is subdivided into families. Class B is first divided into subclasses B1–B3, and then each subclass is divided into families. The class to which a BL belongs gives a lot of insight into its hydrolytic profile. Traditional methods of determining the hydrolytic profile of BLs and their classification are time-consuming and require resources. Hence we developed a machine-learning-based in silico method, named as β-LacFamPred, for the prediction and annotation of Ambler’s class, subclass, and 96 families of BLs. During leave-one-out cross-validation, except one all β-LacFamPred model HMMs showed 100% accuracy. Benchmarking with other BL family prediction methods showed β-LacFamPred to be the most accurate. Out of 60 penicillin-binding proteins (PBPs) and 57 glyoxalase II proteins, β-LacFamPred correctly predicted 56 PBPs and none of the glyoxalase II sequences as non-BLs. Proteome-wide annotation of BLs by β-LacFamPred showed a very less number of false-positive predictions in comparison to the recently developed BL class prediction tool DeepBL. β-LacFamPred is available both as a web-server and standalone tool at http://proteininformatics.org/mkumar/blacfampred and GitHub repository https://github.com/mkubiophysics/B-LacFamPred respectively.
The rapid emergence of antimicrobial resistance (AMR) in bacteria due to the overuse of antibiotics is compromising the efficacy of antibiotics globally (Gould and Bal, 2013; Sengupta et al., 2013; Golkar et al., 2014; Wright, 2014). Unfortunately, the drying up of the new antibiotic development pipelines and the rapid spread of antibiotic resistance has become a significant global health crisis (Piddock, 2012; Bartlett et al., 2013; Gould and Bal, 2013; Gross, 2013; Sengupta et al., 2013; The antibiotic alarm, 2013; Lushniak, 2014; Michael et al., 2014; Read and Woods, 2014; Viswanathan, 2014). Bacteria employ multiple ways to neutralize the lethal effect of antibiotics. The most common mechanisms are (a) altering the permeability of cell membrane to stop/reduce the entry of antibiotics inside the cell, (b) enzymatic breakdown of antibiotics, (c) pumping out of drug molecules from the cell, and (d) altering the target of antibiotics.
β-lactams are the most commonly prescribed drug for treatment of Gram-negative bacterial infection. The resistance against β-lactam antibiotics is due to development of a highly diverse group of enzymes, collectively called β-lactamases (BLs), that hydrolyze the amide bond of a β-Lactam ring to make it ineffective (Abraham and Chain, 1940/1988; Petrosino et al., 1998; McKeegan et al., 2002; Zervosen et al., 2012). BLs is a highly diverged super-family of enzymes both in terms of sequence and functional diversity (Singh et al., 2009). Over the years, several classification systems have been developed to classify BLs. However, the most popular schemes are (i) Ambler’s classification scheme, which was based on the amino acid sequence similarity, and (ii) Bush, Jacoby, and Medeiros classification scheme, which was based on substrate and inhibitor profiles (Ambler, 1980; Bush et al., 1995; Mack et al., 2020). Ambler’s classification scheme categorized BLs into four classes: A–D. Class A, C, and D are also known as serine BLs because they have an active-site serine to catalyze the hydrolysis. Class B BLs is known as Metallo β-lactamases (MBLs) since they use zinc ions (Zn2+) for their activity (Galleni et al., 2001). MBLs are distinct from the serine BL in sequence, structure fold, and catalytic mechanism (Bush, 1998) and they are further divided into three subclasses, B1–B3, based on their active site geometry and overall homology (Walsh et al., 2005).
The Bush, Jacoby, and Medeiros classification scheme attempted to correlate the phenotype of clinical isolates with substrate and inhibitor profiles. It classified BLs into three major groups: Group 1 BLs (class C BLs) is cephalosporinases that are not well inhibited by clavulanic acid; Group 2 (classes A and D) is the largest group of BLs. It includes penicillinases, cephalosporinases, and broad spectrum BLs generally inhibited by active site-directed BL inhibitors; Group 3 are MBLs that hydrolyze penicillins, cephalosporins, and carbapenems but are poorly inhibited by a majority of beta-lactam containing molecules (Petrosino et al., 1998; Zervosen et al., 2012). Based on the differences among the enzymes, each group is further divided into several subgroups and families (Petrosino et al., 1998). Diversity in the amino acid sequences of different BL families also affects the clinical outcome. The family of BL ultimately decides whether the prescribed β-lactam antibiotics would be able to kill the drug-resistant pathogen infection or not.
Several screening tests have been developed to identify the family of BLs at both gene and whole genome levels (Livermore et al., 2001; Sharma et al., 2004). However, these methods are resource and time-consuming. An alternative approach for rapid annotation of BLs family is to use computational methods (Moradigaravand et al., 2018), which can quickly identify BLs genes/proteins and classify them into the family. The most popular computational approach is using BLAST search against either general-purpose molecular biology databases such as NCBI NR/NT or UniProtKB/SwissProt or BL-specific databases such as BLDB (Naas et al., 2017), BLAD (Danishuddin et al., 2013), LacED (Thai et al., 2009), ARDB (Liu and Pop, 2009), CARD (McArthur et al., 2013; Jia et al., 2017), and CBMAR (Srivastava et al., 2014b). Other approaches to predict, classify, and annotate BLs and/or its families are by prediction of family-specific motifs or patterns using LactFP (Srivastava et al., 2014a) or by using machine learning-based algorithms such as Bayes (Nath and Karthikeyan, 2017), support vector machine (SVM) (Kumar et al., 2015; Srivastava et al., 2018), convolutional neural network (CNN) (White et al., 2017) VGGNet architecture and TensorFlow deep learning (Wang et al., 2021). Machine learning-based algorithms present an opportunity for increasing the sensitivity of classification over alignment and thus have been previously used with high-throughput sequence data to characterize the resistome. For example, AMRFinderPlus (Feldgarden et al., 2021) and Meta-MARC (Lakin et al., 2019) used Hidden Markov Models (HMMs) to classify AMR-related protein/gene sequences from high-throughput sequence data and sequence reads. The existing methods and databases, both specific for BLs and general purpose, have made significant contributions in annotation of different variants of BLs. However, most prediction methods except LactFP were restricted only to the prediction up to class level [e.g., βLact-Pred (Ashraf et al., 2021), CNN-BLPred (White et al., 2017), PredLactamase (Kumar et al., 2015)], or subclass [e.g., BlaPred (Srivastava et al., 2018)]. LactFP predicts the class, sub-class, and family of a BL protein on the basis of presence of a family-specific motif called fingerprint in the primary amino acid sequence. However, there are a few limitations of LactFP. The most critical limitation of LactFP was that it was developed using a dataset compiled in 2014. Over time information about new family members and many mutations in different families has been accumulated in the databases. Hence LactFP might not be capable of predicting all BL families correctly. This indicates that a tool capable of predicting more BL families is the need of the hour.
To address the above-mentioned limitations, we present β-LacFamPred, a machine learning based classifier that can annotate BLs up to the family level. β-LacFamPred can be used on both genomic and proteomic data. To develop β-LacFamPred, the data were initially extracted from two BL databases namely, CBMAR and BLDB. Afterward, new sequences were added to each family from UniProtKB and NCBI NR. Using these sequences we constructed 96 HMMs, each specific for one family of BL. The consistency of prediction of each HMM was evaluated using leave-one-out approach. We benchmarked the effectiveness of β-LacFamPred on an independent dataset vis-a-vis LactFP and observed very high performance (≥98% precision & recall and ≥ 99% accuracy). We also developed a user-friendly web-server of β-LacFamPred that is available at http://proteininformatics.org/mkumar/blacfampred. The working schema of β-LacFamPred is shown in Figure 1.
The family-wise sequences of BLs were obtained from our earlier developed database, Comprehensive β-Lactamase Molecular Annotation Resource (CBMAR) and BLDB. The number of protein sequences in each family was also augmented from CARD, UniProtKB, and NCBI NR databases. Sequences of each family were manually curated using literature and UniProtKB annotations. We also removed the fragmented sequences from each family. BL families with less than five sequences or single sequences were also removed from further studies. If multiple copies of identical sequences were present in a family, then all except one sequence were removed. Finally, we found 96 BL families consisting of full-length sequences only. The number of sequences at each stage of data compilation is shown in Table 1.
We used the reference bla gene sequences obtained from Lee et al. (2015) for benchmarking. These BL sequences were used to develop molecular probes for PCR-based methods to detect bla genes in various pathogenic isolates (Lee et al., 2015). The total number of bla gene sequences were 1,342, belonging to all four Ambler’s classes, A–D, and 29 families of BLs.
Sequences of each BL family were multiply aligned using the Muscle 3.8 program (Edgar, 2004) at default parameters. Using the hmmbuild function of the HMMER tool (version 3.1) (Finn et al., 2011), we build HMM of each BL family.
To test the efficiency of each HMM in discriminating between the family and non-family members, we used the leave-one-out cross-validation (LOOCV) approach. During LOOCV, HMM was built using all but one family sequence for each BL family. Hence the total number of HMMs built for a family during LOOCV was equal to the number of BL sequences in that family. The performance of each model was evaluated against a dataset containing (a) excluded sequence of a particular family for which HMM was being evaluated and (b) sequences using which remaining 95 HMMs were built. The search efficiency of each HMM was determined based on the best hit, i.e., search results having a minimum e-value. If the best search result was the left-out sequence of the same family, the search result was categorized as True Positive (TP). If the best search result belonged to a different BL family, the search result was categorized as False Positive (FP). The performance of methods was assessed using the standard evaluation metrics namely, precision, recall, accuracy, and F-measure. These performance metrics have also been frequently used in several prediction and classification studies (Pandey et al., 2020). For example, during prediction for a BL protein that belongs to a hypothetical β-lactamase family ‘X’, if it is predicted to belong to the same class ‘X’, the prediction was categorized as TP prediction; if it were predicted to class non-‘X’, it would be an FN prediction.
Similarly, if a non-‘X’ is predicted as non-‘X’ and ‘X’, it is an example of TN and FP predictions, respectively. TP, TN, FP, and FN represent true positives, true negatives, false positives, and false negatives, respectively. The expressions used to calculate the above-mentioned parameters were:
The precision of each HMM was determined as the ratio of the number of proteins whose family was correctly predicted to the total number of proteins, which were predicted as a member of that BL family (Eq. 1). A precision value of 1.0 of an HMM indicates the correct prediction of all family proteins. Recall of an HMM was the ratio of proteins whose family was correctly predicted to the number of proteins in that BL family (Eq. 2). The recall value of 1.0 indicates that all proteins of that family were correctly predicted. The overall percentage of correctly predicted examples is calculated through accuracy (Eq. 3). To balance the precision and recall values due to unequal composition of family (positive) and non-family (negative) sequences during evaluation, F-measure was used, which is the harmonic mean of precision and recall (Powers, 2020).
All 96 BL HMMs were annotated using (a) ARG databases, namely DeepARG – ARGminer (Arango-Argoty et al., 2018), CARD, ARDB, (b) UniProtKB, and (c) published research papers. The annotation details mentioned with each HMM are resistance mechanisms, class, and name of antibiotic against which the family confers the resistance, family, class, subclass, and phenotypic information as per Jacoby and Bush classification scheme. Each HMM was also tagged with the information of their action in terms of their spectrum, namely broad spectrum, extended spectrum, and narrow spectrum (Supplementary Table S1). β-LacFamPred webserver is freely available at http://proteininformatics.org/mkumar/blacfampred.
We compared the performance of β-LacFamPred with well-known ARG annotation methods: AMRFinderPlus, RGI-CARD, ResFinder, and Meta-MARC. We have also included LactFP as it assigns the family of a BL sequence based on the presence of a conserved motif. The comparative evaluation was done on the independent test dataset consisting of 1,342 BL protein/gene sequences that belong to 29 BL families.
The class-wise distribution of BL families in CBMAR was as follows: Class A = 64, Class B = 36, Class C = 14, and Class D = 2. In BLDB, the number of Class A–D families, which were not present in CBMAR, was 13, 80, 9, and 18, respectively (Table 1). The total number of BL families with which we started the work was as follows: Class A = 77, Class B = 116, Class C = 23, and Class D = 20. After removing families that had less than five sequences we were left with 96 families, of which 60, 15, 19, and 2 belonged to classes A–D, respectively. The subfamily-wise distribution of class B families were 10, 1 and 4 in subclass B1–B3, respectively. In the final dataset, the total number of BL families obtained from CBMAR and BLDB was 72 and 24, respectively, with at least five sequences. We did pairwise multiple sequence alignment of sequences of each BL family and converted the alignment HMM. The current version contained 96 BL HMMs.
The consistency in search efficiency of all 96 HMMs was evaluated using the leave-one-out cross-validation (LOOCV) approach. We calculated the true positive and false positive prediction rate of each BL HMM of β-LacFamPred models. A detailed description of the LOOCV method was described in the materials and methods section under the sub-heading cross-validation and performance metrics. During LOOCV, all 96 HMMs gave 100% accuracy. In summary, the cross-validation (LOOCV) showed that all 96 HMMs could predict the family of BL with very high consistency and efficiency. The complete set of 96 HMMs capable of predicting 96 families of BLs is called β-LacFamPred henceforth.
In class A BL, except for one protein of family TEM, all were correctly predicted to their actual family and achieved 100% accuracy and 100% precision, recall, and F-measure in all cases of class A (Table 2). The wrongly predicted class A bla gene originally belonged to TEM, but our method wrongly predicted it as a CTXM family member. In the case of TEM, we have found accuracy was 99 and 99.38% precision, recall, and F1 score. During performance evaluation on the independent dataset we got the hits of CTX-M family against KLUA, KLUY, KLUC, TOHO and CTX-M. To find the reason behind this we did a profile HMM-HMM comparison using (hhalign module of HMMER) using the KLUA, KLUY, KLUC, TOHO and CTX-M, which showed they are highly similar to each other. To further check the evolutionary relationship among them, we also constructed a phylogenetic tree using profile HMMs that showed that CTX-M and KLUC shared the same branch but KLUA and TOHO did not belong to the same branch. We also saw KLUY forming a separate branch, which was shown in Supplementary Figure S1. Due to this we added these sequences to a separate family. In addition to this, we have also noticed that several databases mention these families as separate. We have marked the ‘CTX-M like families’ in front of KLUY, KLUA, KLUC and TOHO families.
In the case of class B BLs, all sequences were correctly predicted to their respective family; hence the number of false predictions was zero, and accuracy was also found to be 100%. In the case of class C BLs in eight of nine families, no false prediction was observed. The accuracy found in all eight cases was 100%. However, in one family, namely CMY/MOX/FOX/AmpC, there were 39 sequences, out of which 36 were correctly predicted as members of CMY/MOX/FOX/AmpC.
In contrast, three sequences were wrongly predicted to belong to family AQU, and the accuracy we achieved, in that case, was 99% and 92.30 precision, recall, and F1 score. In the case of class D BLs, out of 210 sequences, 209 BL sequences were correctly predicted as OXA family members. At the same time, one was incorrectly predicted as a member of the IMP family, and in that case, also we found 99% accuracy and 99.52 precision, recall, and F1 score, respectively (Table 2).
To further assess the capability of β-LacFamPred for identifying BL class, subclass, and families, we performed an additional independent evaluation using a Penicillin-Binding Proteins (PBPs) dataset. PBPs are membrane-associated proteins involved in the biosynthesis of peptidoglycan components of bacterial cell walls. PBP and BLs belong to the superfamily of serine penicillin-recognizing enzymes and have similar conserved protein folds (Knox et al., 1996; Meroueh et al., 2003). PBP and BLs are homologous proteins, but PBP does not provide antibiotic resistance against BLs. Also, BLs is considered to have evolved from penicillin-binding proteins. PBPs were not part of the dataset on which β-LacFamPred prediction models were developed. Out of 60 PBP sequences, only four were wrongly predicted as BLs. Three sequences were predicted as a member of the family AmpC (class C) and one sequence to family ARL (class A).
To further confirm the discriminatory capability of β-lactamase, and non-β-lactamase, we created a second independent dataset consisting of glyoxalase II, which belongs to the metallo-beta-lactamase (MBL) superfamily of proteins. The sequences of the glyoxalase II were retrieved from the UniProtKB database. We found a total of 57 full-length sequences of glyoxalase II. At e-value 1e-15 none of the glyoxalase II sequences were predicted as BL. When e-value was increased to 1e-10, 1e-6 and 0.1 the number gradually increased to 17, 43 and 43, respectively. The result was consistent with previous work that had shown the requirement of more stringent e-value cutoff to reduce the number of false positive predictions (Zankari et al., 2012; McArthur et al., 2013; Gibson et al., 2015).
Recently Wang et al. have developed a deep learning based method, DeepBL, for predicting and classifying BLs on the basis of their protein sequences (Wang et al., 2021). To characterize the complete repertoire of BLs, they annotated all reviewed bacterial protein sequences (334,542 in total) from the UniProtKB database. DeepBL identified 2,876 Class-A, 665 Class-B, 335 Class-C, and 231 Class-D BL protein sequences in this dataset. To examine the capability of β-LacFamPred in predicting BLs in screening of a proteome-wide high-throughput data, we also annotated the 334,542 protein sequences, compiled by DeepBL, using β-LacFamPred. We found 86 Class-A, 246 Class-B, 67 Class-C, and 29 Class-D BL proteins. Further, 86 Class-A BLs were classified into 26 families. Out of 246 Class-B proteins, 21 were predicted as subclass B1, 02 were predicted as subclass B2, and 223 were predicted to belong to subclass B3, which were further classified into different families as follows: B1 = 5, B2 = 1 and B3 = 3. In the case of 67 and 29 proteins, which were predicted to belong to class C and D respectively, they were predicted to belong to 10 and 2 families, respectively (Table 3; Supplementary Table S2). Since there was a significant difference in the number of proteins predicted as BLs by DeepBL and β-LacFamPred, we compared the UniProtKB annotations with predictions of BLs by DeepBL. We observed that out of 4,107, and 428 proteins predicted as BL by DeepBL and β-LacFamPred, only 199 and 252 were annotated as BLs by the UniProtKB database (Table 3; Supplementary Table S3).
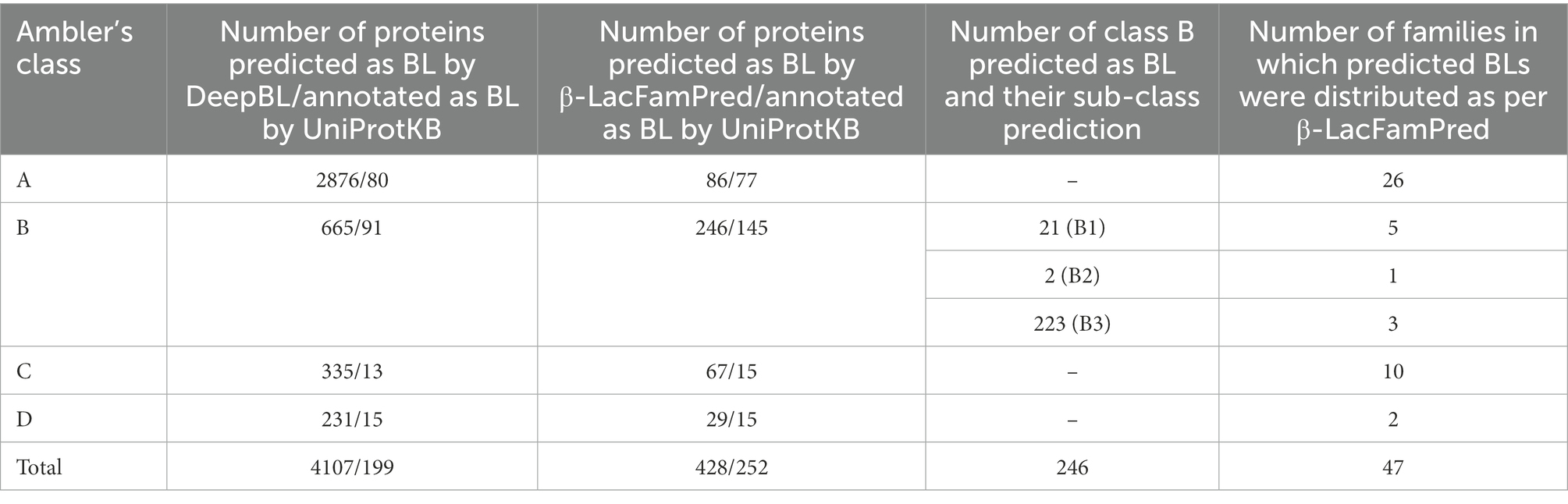
Table 3. Number of proteins predicted as BL by DeepBL and β-LacFamPred and annotation statistics of UniProtKB therein.
When we analyzed the prediction results of DeepBL and β-LacFamPred about the UniProtKB annotations, we found the four situations. A few examples are shown in Table 4 as an illustration. Four different situations we found were (a) both DeepBL and β-LacFamPred correctly predicted the nature and class of query protein (Sr. No 1–10 of Table 4), (b) DeepBL predicted non-BL but β-LacFamPred predicted the query sequence as BL and also predicted its corresponding family. The UniProtKB annotation also supported β-LacFamPred annotations (Sr. No 11–12 of Table 4), (c) DeepBL predicted the query sequence as BL, but β-LacFamPred predicted them as non-BL. UniProtKB also supported the β-LacFamPred prediction (Sr. No 13 of Table 4), and (d) both DeepBL and β-LacFamPred predicted the query sequence as non-BL and UniProtKB also supported the prediction (Sr. No 14–15 of Table 4). The results showed that the number of false positive predictions in β-LacFamPred was significantly lower than DeepBL and β-LacFamPred can be used to predict and annotate new BLs that are not known yet.
Table 4 also showed the advantage of β-LacFamPred over DeepBL. Whereas DeepBL can predict only up to Ambler’s class of query BL, β-LacFamPred can predict both the class and family of query BL and subclass in the case of class B BL.
Out of 1,342 BL sequences of independent dataset AMRFinderPlus, RGI-CARD, ResFinder, Meta-MARC, LactFP, and β-LacFamPred correctly predicted 1,026, 1,115, 1,242, 1,199, 742, and 1,320 BL sequences to their respective families Table 5. Family-wise performance of each method is shown in Supplementary Tables S4–S10. AMRFinderPlus obtained accuracy ranges from 1 to 0.95 in most BL families. Only in a few families, namely, SHV/LEN/OKP, GIM, and EC no correct prediction was done. In RGI-CARD, we obtained accuracy ranges from 1 to 0.92 in different BL families, except in the case of GIM and LAT, we found zero accuracy. Among all methods, LactFP showed the least performance. In 25 BL families, the accuracy range was 100 to 79%. In families GOB, PDC, ADC, and EC all proteins were correctly predicted (Supplementary Tables S7–S10). In Meta-MARC, accuracy ranges from 1 to 0.98 in different BL families, but in several cases, zero accuracy was also observed (SIM, DIM, TMB, GIM, and EC) (Supplementary Table S4). ResFinder predicted 28 families in a range of 98–100% accurately except for one family (EC) of class C, where it predicted the wrong family of all 87 sequences. Compared to other methods, our proposed method achieves a very high accuracy (ranges from 1 to 0.99) in all 29 families on an independent dataset of 1,342 BL gene datasets compared to other existing methods in Supplementary Table S6.
Moreover, we were unable to predict the BLs in the gene/protein independent dataset using the web servers of AMRFinderPlus, Meta-MARC, RGI-CARD, ResFinder, and LactFP, due to the limitations of their web services (The first two methods not available in the form of web servers which raises difficulty in handling for non-programmers, the third one allows a maximum of 20 Mb limit size of sequences per submission for prediction. ResFinder only allows genomic sequences as an input for predicting ARGs, not a proteome, and LactFP has a web server. However, it only deals with a single protein sequence for prediction, while β-LacFamPred provides a user-friendly web server and standalone tool for protein and gene sequence analysis. The web server of β-LacFamPred allows users to analyze 100 gene/protein sequences in one go). For proteome/genome/metagenome scanning. We also provided a standalone version of our tool for predicting families of BLs. The comparison clearly demonstrated better capability of BLs class, subclass, and family prediction of β-LacFamPred in comparison to other ARG annotation tools.
In the past, we developed a motif-based prediction method of BL classification named LactFP. Although LactFP and β-LacFamPred are based on BL family datasets, they were developed using different approaches. There are also several advantages of β-LacFamPred over LactFP. For example, (a) LactFP was developed using 71 families, 46 from class A, 15 from class B (10 from subclass B1, one from subclass B2, four from subclass B3), eight from class C, and two from class D. On the other hand, β-LacFamPred was developed using 96 families, 60 from class A, 15 from class B (10 from subclass B1, one from subclass B2, four from subclass B3), 19 from class C, two from class D. (b) In LactFP fingerprints were extracted by using only 605 protein sequences. In contrast, β-LacFamPred models were built using >8,000 protein sequences. (c) The total 605 protein sequence dataset of LactFP contained 325 sequences in class A, 58 sequences in subclass B1, 14 sequences in subclass B2, 58 sequences in subclass B3, 139 sequences in class C, and 11 sequences in class D, while the complete 8,060 protein sequences of β-LacFamPred contained 4,404 from class A, 682 from subclass B1, 67 from subclass B2, 132 from subclass B3, 2,438 from class C, 337 from class D. (d) The LactFP web server allows users to search the family-specific fingerprint in only protein sequences while the β-LacFamPred web server is capable of handling both protein/gene sequences or complete proteome/genome. Additionally, β-LacFamPred provides annotation along with the family information, which is not available in LactFP. (e) The family-specific patterns/motifs-based tool LactFP only identifies short conserved sequence patterns. In contrast, the domain-based HMM tool β-LacFamPred identifies more extended conserved regions in a protein or gene (Table 6).
To provide the β-LacFamPred prediction module to the scientific community, we also established a web server that can be used to query whether a gene/protein sequence is BL or not. If the query sequence is predicted as BL, then its probable class/subclass and families, along with other annotation details, will also be provided. The overall schema of the prediction methodology of the tool is explained in Figure 1. The β-LacFamPred web server can process a maximum of 100 sequences in one go. A snapshot of the search and prediction page of the ‘β-LacFamPred’ webserver is shown in Figure 2. The overall workflow depicting the methodology adopted for designing the β-LacFamPred tool has been illustrated in Figure 3. For high-throughput, whole genome, metagenomic and proteome/genome-scale annotation, a standalone version would be required, which is provided in the download section of the webserver at: http://proteininformatics.org/mkumar/blacfampred/download.html.
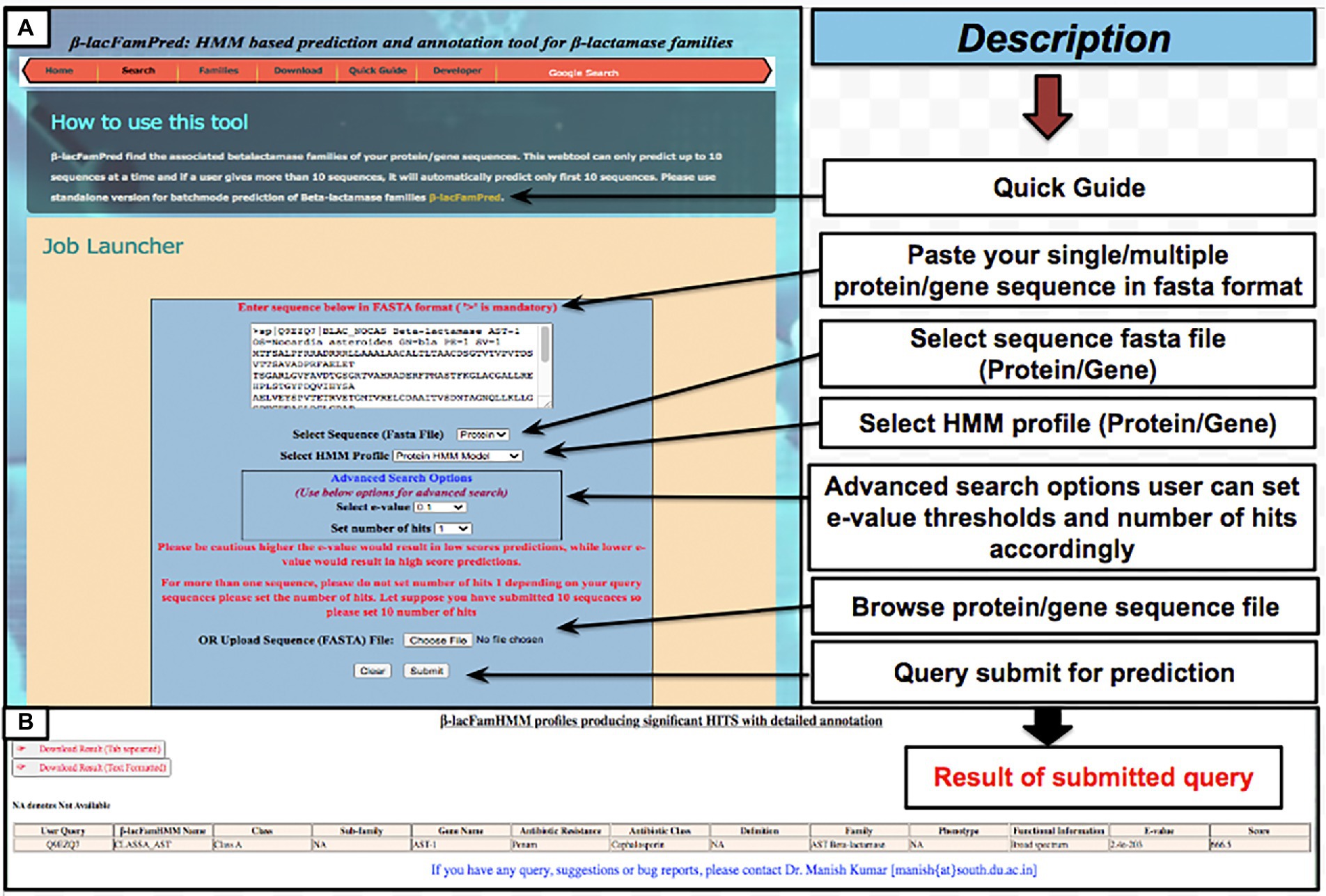
Figure 2. Screenshots of web pages of ‘β-LacFamPred’ tool (A) search page (B) prediction result page.
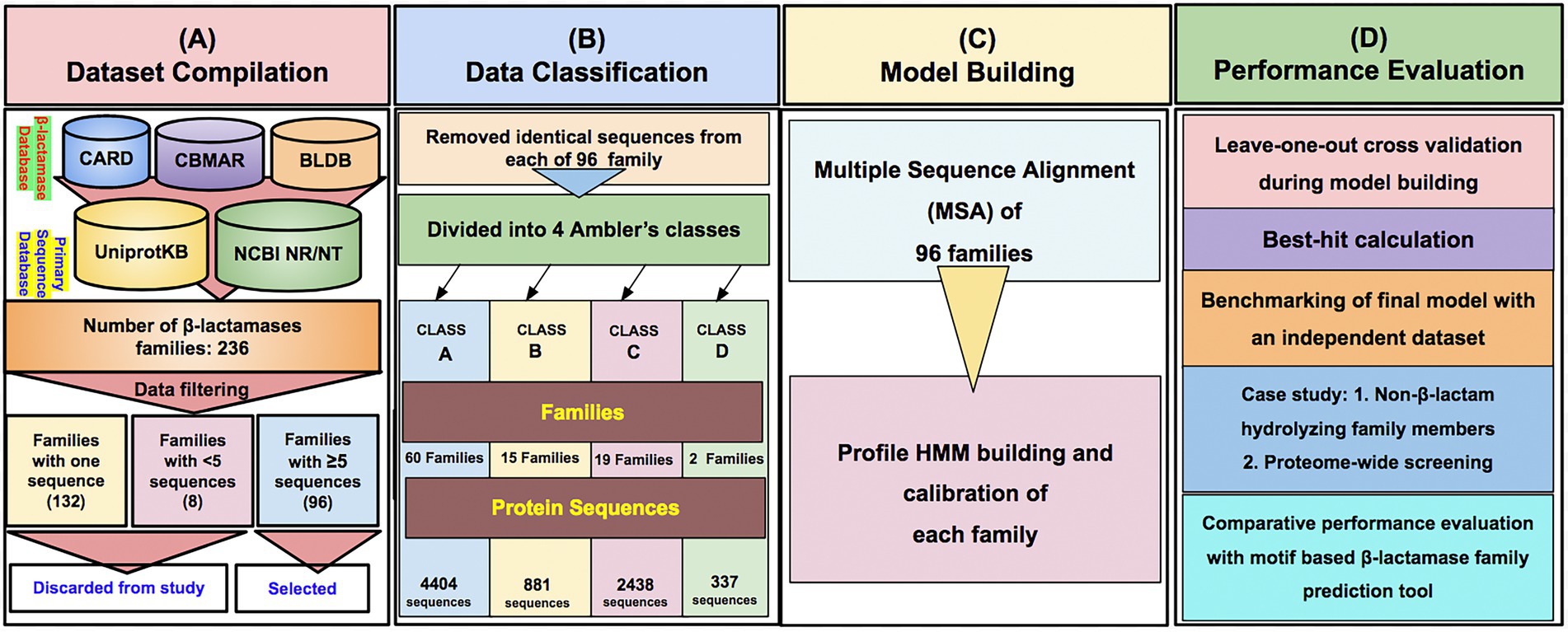
Figure 3. The overall workflow depicting the methodology used for designing the profile HMMs-based prediction tool β-LacFamPred. (A–D) represents the series of workflow.
Recent advancements in sequencing technologies have produced a large amount of data that might contain novel variants of BLs. As experimental characterization and annotation is an expensive and time-consuming exercise hence to facilitate rapid annotation of potential BLs, we developed an in silico tool named β-LacFamPred. It can predict BL and their families using only protein/gene sequences. β-LacFamPred can also be combined with traditional surveillance methods and thus can complement the traditional BL families’ annotation methods. The current version of β-LacFamPred can predict 96 BL families. In the near future, we strive to update the β-LacFamPred tool regularly to reflect the latest discoveries of BL families. We hope that β-LacFamPred would help in annotation of the novel BLs genes/proteins and help in the progress of studies related to BL-based antimicrobial resistance.
We reported an in silico tool, β-LacFamPred, for annotation and prediction of BLs in classes, subclasses, and families. Evaluation and comparison with other methods using independent datasets and proteome-wide screening showed β-LacFamPred to be a highly efficient tool.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
DP collected and organized the data and developed the web interface. DP and MK analyzed the results. DP, NS, and MK wrote the manuscript. MK conceived the idea and did overall supervision of the work. All authors contributed to the article and approved the submitted version.
DP was supported by the Department of Science and Technology (INSPIRE Program) (DST INSPIRE Fellowship/2016/IF160262) [Grant Number: DST/INSPIRE 03/2015/003022]. NS was supported by the Council of Scientific and Industrial Research under the Pool Scientist Scheme [Grant Number: 13(9089-A)/2019-POOL]. The work was carried out using the resources funded by the Indian Council of Medical Research [Grant Numbers: VIR (25)/2019/ECD-1 and ISRM/12(33)/2019].
We thank University of Delhi (South Campus), New Delhi (India) for providing facilities to pursue the research work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1039687/full#supplementary-material
Supplementary Figure S1 | Phylogenetic tree of Class A beta-lactamase families.
Abraham, E. P., and Chain, E. (1940/1988). An enzyme from bacteria able to destroy penicillin. Rev. Infect. Dis. 10, 677–678.
Ambler, R. P. (1980). The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 289, 321–331.
Arango-Argoty, G., Garner, E., Pruden, A., Heath, L. S., Vikesland, P., and Zhang, L. (2018). DeepARG: a deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome 6:23. doi: 10.1186/s40168-018-0401-z
Ashraf, M. A., Khan, Y. D., Shoaib, B., Khan, M. A., Khan, F., and Whangbo, T. (2021). βLact-Pred: a predictor developed for identification of beta-lactamases using statistical moments and PseAAC via 5-step rule. Comput. Intell. Neurosci. 2021:8974265. doi: 10.1155/2021/8974265
Bartlett, J. G., Gilbert, D. N., and Spellberg, B. (2013). Seven ways to preserve the miracle of antibiotics. Clin. Infect. Dis. 56, 1445–1450. doi: 10.1093/cid/cit070
Bush, K. (1998). Metallo-beta-lactamases: a class apart. Clin. Infect. Dis. 27, S48–S53. doi: 10.1086/514922
Bush, K., Jacoby, G. A., and Medeiros, A. A. (1995). A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39, 1211–1233. doi: 10.1128/AAC.39.6.1211
Danishuddin, M., Baig, M. H., Kaushal, L., and Khan, A. U. (2013). BLAD: a comprehensive database of widely circulated beta-lactamases. Bioinformatics 29, 2515–2516. doi: 10.1093/bioinformatics/btt417
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Feldgarden, M., Brover, V., Gonzalez-Escalona, N., Frye, J. G., Haendiges, J., Haft, D. H., et al. (2021). AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 11:12728. doi: 10.1038/s41598-021-91456-0
Finn, R. D., Clements, J., and Eddy, S. R. (2011). HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 39, W29–W37. doi: 10.1093/nar/gkr367
Galleni, M., Lamotte-Brasseur, J., Rossolini, G. M., Spencer, J., Dideberg, O., Frère, J. M., et al. (2001). Standard numbering scheme for Class B beta-lactamases. Antimicrob. Agents Chemother. 45, 660–663. doi: 10.1128/AAC.45.3.660-663.2001
Gibson, M. K., Forsberg, K. J., and Dantas, G. (2015). Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 9, 207–216. doi: 10.1038/ismej.2014.106
Golkar, Z., Bagasra, O., and Pace, D. G. (2014). Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries. 8, 129–136. doi: 10.3855/jidc.3573
Gould, I. M., and Bal, A. M. (2013). New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence 4, 185–191. doi: 10.4161/viru.22507
Gross, M. (2013). Antibiotics in crisis. Curr. Biol. 23, R1063–R1065. doi: 10.1016/j.cub.2013.11.057
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., et al. (2017). CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 45, D566–D573. doi: 10.1093/nar/gkw1004
Knox, J. R., Moews, P. C., and Frere, J. M. (1996). Molecular evolution of bacterial beta-lactam resistance. Chem. Biol. 3, 937–947. doi: 10.1016/S1074-5521(96)90182-9
Kumar, R., Srivastava, A., Kumari, B., and Kumar, M. (2015). Prediction of β-lactamase and its class by Chou’s pseudo-amino acid composition and support vector machine. J. Theor. Biol. 365, 96–103. doi: 10.1016/j.jtbi.2014.10.008
Lakin, S. M., Kuhnle, A., Alipanahi, B., Noyes, N. R., Dean, C., Muggli, M., et al. (2019). Hierarchical hidden Markov models enable accurate and diverse detection of antimicrobial resistance sequences. Commun. Biol. 2:294. doi: 10.1038/s42003-019-0545-9
Lee, J. J., Lee, J. H., Kwon, D. B., Jeon, J. H., Park, K. S., Lee, C.-R., et al. (2015). Fast and accurate large-scale detection of β-lactamase genes conferring antibiotic resistance. Antimicrob. Agents Chemother. 59, 5967–5975. doi: 10.1128/AAC.04634-14
Liu, B., and Pop, M. (2009). ARDB--antibiotic resistance genes database. Nucleic Acids Res. 37, D443–D447. doi: 10.1093/nar/gkn656
Livermore, D. M., Winstanley, T. G., and Shannon, K. P. (2001). Interpretative reading: recognizing the unusual and inferring resistance mechanisms from resistance phenotypes. J. Antimicrob. Chemother. 48, 87–102. doi: 10.1093/jac/48.suppl_1.87
Lushniak, B. D. (2014). Antibiotic resistance: a public health crisis. Public Health Rep. 129, 314–316. doi: 10.1177/003335491412900402
Mack, A. R., Barnes, M. D., Taracila, M. A., Hujer, A. M., Hujer, K. M., Cabot, G., et al. (2020). A standard numbering scheme for class C β-lactamases. Antimicrob. Agents Chemother. 64:3. doi: 10.1128/AAC.01841-19
McArthur, A. G., Waglechner, N., Nizam, F., Yan, A., Azad, M. A., Baylay, A. J., et al. (2013). The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 57, 3348–3357. doi: 10.1128/AAC.00419-13
McKeegan, K. S., Ines Borges-Walmsley, M., and Walmsley, A. R. (2002). Microbial and viral drug resistance mechanisms. Trends Microbiol. 10, S8–S14. doi: 10.1016/S0966-842X(02)02429-0
Meroueh, S. O., Minasov, G., Lee, W., Shoichet, B. K., and Mobashery, S. (2003). Structural aspects for evolution of beta-lactamases from penicillin-binding proteins. J. Am. Chem. Soc. 125, 9612–9618. doi: 10.1021/ja034861u
Michael, C. A., Dominey-Howes, D., and Labbate, M. (2014). The antimicrobial resistance crisis: causes, consequences, and management. Front. Public Health 2:145. doi: 10.3389/fpubh.2014.00145
Moradigaravand, D., Palm, M., Farewell, A., Mustonen, V., Warringer, J., and Parts, L. (2018). Prediction of antibiotic resistance in Escherichia coli from large-scale pan-genome data. PLoS Comput. Biol. 14:e1006258. doi: 10.1371/journal.pcbi.1006258
Naas, T., Oueslati, S., Bonnin, R. A., Dabos, M. L., Zavala, A., Dortet, L., et al. (2017). Beta-lactamase database (BLDB) – structure and function. J. Enzyme Inhib. Med. Chem. 32, 917–919. doi: 10.1080/14756366.2017.1344235
Nath, A., and Karthikeyan, S. (2017). Enhanced identification of β-lactamases and its classes using sequence, physicochemical and evolutionary information with sequence feature characterization of the classes. Comput. Biol. Chem. 68, 29–38. doi: 10.1016/j.compbiolchem.2017.02.006
Pandey, D., Kumari, B., Singhal, N., and Kumar, M. (2020). BacEffluxPred: a two-tier system to predict and categorize bacterial efflux mediated antibiotic resistance proteins. Sci. Rep. 10:9287. doi: 10.1038/s41598-020-65981-3
Petrosino, J., Cantu, C. 3rd, and Palzkill, T. (1998). Beta-lactamases: protein evolution in real time. Trends Microbiol. 6, 323–327. doi: 10.1016/S0966-842X(98)01317-1
Piddock, L. J. V. (2012). The crisis of no new antibiotics--what is the way forward? Lancet Infect. Dis. 12, 249–253. doi: 10.1016/S1473-3099(11)70316-4
Powers, D. M. W. (2020). Evaluation: from precision, recall and F-measure to ROC, informedness, markedness and correlation. arXiv preprint arXiv:2010.16061. doi:doi: 10.48550/arXiv.2010.16061
Read, A. F., and Woods, R. J. (2014). Antibiotic resistance management. Evol. Med. Public Health 2014:147. doi: 10.1093/emph/eou024
Sengupta, S., Chattopadhyay, M. K., and Grossart, H.-P. (2013). The multifaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 4:47. doi: 10.3389/fmicb.2013.00047
Sharma, S., Ramnani, P., and Virdi, J. S. (2004). Detection and assay of beta-lactamases in clinical and non-clinical strains of Yersinia enterocolitica biovar 1A. J. Antimicrob. Chemother. 54, 401–405. doi: 10.1093/jac/dkh365
Singh, R., Saxena, A., and Singh, H. (2009). Identification of group specific motifs in beta-lactamase family of proteins. J. Biomed. Sci. 16:109. doi: 10.1186/1423-0127-16-109
Srivastava, A., Kumar, R., and Kumar, M. (2018). BlaPred: predicting and classifying β-lactamase using a 3-tier prediction system via Chou’s general PseAAC. J. Theor. Biol. 457, 29–36. doi: 10.1016/j.jtbi.2018.08.030
Srivastava, A., Singhal, N., Goel, M., Virdi, J. S., and Kumar, M. (2014a). Identification of family specific fingerprints in β-lactamase families. Sci. World J. 2014:980572. doi: 10.1155/2014/980572
Srivastava, A., Singhal, N., Goel, M., Virdi, J. S., and Kumar, M. (2014b). CBMAR: a comprehensive β-lactamase molecular annotation resource. Database 2014:bau111. doi: 10.1093/database/bau111
Thai, Q. K., Bös, F., and Pleiss, J. (2009). The lactamase engineering database: a critical survey of TEM sequences in public databases. BMC Genomics 10:390. doi: 10.1186/1471-2164-10-390
Viswanathan, V. K. (2014). Off-label abuse of antibiotics by bacteria. Gut Microbes 5, 3–4. doi: 10.4161/gmic.28027
Walsh, T. R., Toleman, M. A., Poirel, L., and Nordmann, P. (2005). Metallo-Beta-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18, 306–325. doi: 10.1128/CMR.18.2.306-325.2005
Wang, Y., Li, F., Bharathwaj, M., Rosas, N. C., Leier, A., Akutsu, T., et al. (2021). DeepBL: a deep learning-based approach for in silico discovery of beta-lactamases. Brief. Bioinform. 22:bbaa301. doi: 10.1093/bib/bbaa301
White, C., Ismail, H. D., Saigo, H., and Dukka, B. K. (2017). CNN-BLPred: a convolutional neural network based predictor for β-lactamases (BL) and their classes. BMC Bioinformatics 18:577. doi: 10.1186/s12859-017-1972-6
Wright, G. D. (2014). Something old, something new: revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 60, 147–154. doi: 10.1139/cjm-2014-0063
Zankari, E., Hasman, H., Cosentino, S., Vestergaard, M., Rasmussen, S., Lund, O., et al. (2012). Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67, 2640–2644. doi: 10.1093/jac/dks261
Keywords: antibiotic resistance, β-lactamase, in silico prediction tool, classification, hidden Markov models, high-throughput, annotation
Citation: Pandey D, Singhal N and Kumar M (2023) β-LacFamPred: An online tool for prediction and classification of β-lactamase class, subclass, and family. Front. Microbiol. 13:1039687. doi: 10.3389/fmicb.2022.1039687
Received: 09 September 2022; Accepted: 19 December 2022;
Published: 12 January 2023.
Edited by:
Pablo Power, Universidad de Buenos Aires, ArgentinaReviewed by:
Jozsef Soki, University of Szeged, HungaryCopyright © 2023 Pandey, Singhal and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manish Kumar, ✉ bWFuaXNoQHNvdXRoLmR1LmFjLmlu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.