
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 08 November 2022
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1039297
 Meimei Zhang1†
Meimei Zhang1† Wenyu Han1,2†
Wenyu Han1,2† Jingmin Gu1,2†
Jingmin Gu1,2† Cao Qiu1
Cao Qiu1 Qiujie Jiang3
Qiujie Jiang3 Jianbao Dong4
Jianbao Dong4 Liancheng Lei1
Liancheng Lei1 Fengyang Li1*
Fengyang Li1*Biofilm formation is a fundamental part of life cycles of bacteria which affects various aspects of bacterial-host interactions including the development of drug resistance and chronic infections. In clinical settings, biofilm-related infections are becoming increasingly difficult to treat due to tolerance to antibiotics. Bacterial biofilm formation is regulated by different external and internal factors, among which quorum sensing (QS) signals and nucleotide-based second messengers play important roles. In recent years, different kinds of anti-biofilm agents have been discovered, among which are the Chinese herbal medicines (CHMs). CHMs or traditional Chinese medicines have long been utilized to combat various diseases around the world and many of them have the ability to inhibit, impair or decrease bacterial biofilm formation either through regulation of bacterial QS system or nucleotide-based second messengers. In this review, we describe the research progresses of different chemical classes of CHMs on the regulation of bacterial biofilm formation. Though the molecular mechanisms on the regulation of bacterial biofilm formation by CHMs have not been fully understood and there are still a lot of work that need to be performed, these studies contribute to the development of effective biofilm inhibitors and will provide a novel treatment strategy to control biofilm-related infections.
Biofilm is a self-protective state formed by bacteria to adapt to the poor living environment. It is a microbial community attached to biotic or abiotic surfaces and wrapped by self-produced extracellular polymeric matrix (EPS) that contains extracellular polysaccharides, nucleic acids (extracellular DNA and extracellular RNA), amyloid proteins, lipids, and many other biomolecules (Karygianni et al., 2020). All bacterial species can form biofilm under suitable conditions, and actually it is estimated that more than 90% of microorganisms exist in the form of biofilm (Costerton et al., 1999). Bacteria in biofilms are physiologically distinct from their planktonic cell state which makes them tolerant to harsh conditions and tolerance to antibacterial treatments such as antibiotics (Roy et al., 2018; Hawas et al., 2022). In clinical settings, biofilm formation of pathogens causes persist infections and biofilm-related infections are becoming increasingly difficult to treat due to tolerance to antibiotics which poses a great threat to human health. It is estimated that approximately 65%–80% of bacterial infections in humans are associated with biofilm formation (Chen et al., 2010; Bjarnsholt et al., 2018). Thus, it is urgent to develop effective and robust strategies to control biofilm formation of pathogens.
Strategies for combating bacterial biofilms have been classified into three main categories: (i) changing the properties of susceptible surfaces to prevent biofilm formation; (ii) regulating signaling pathways to inhibit biofilm formation; (iii) applying external forces to eradicate the biofilm (Yin et al., 2021; Figure 1). Besides the development of novel biofilm-resistant materials and application of physical forces to eradicate biofilms, most of the researchers focus on investigating the regulatory signaling pathways of biofilm formation including bacterial quorum sensing (QS) system and nucleotide-based second messengers cyclic dimeric guanosine monophosphate (c-di-GMP), cyclic dimeric adenosine monophosphate (c-di-AMP), cyclic guanosine monophosphate (cGMP), cyclic adenosine monophosphate (cAMP) and guanosine tetraphosphate ((p)ppGpp; Wu et al., 2015; Yin et al., 2021), and several kinds of anti-biofilm agents have been discovered so far, including Quorum Sensing Inhibitors (QSIs) such as quercetin which dampens QS signaling (Ouyang et al., 2016), and nitric oxide (NO)-generating agents such as sodium nitroprusside (SNP) that restricts c-di-GMP signaling (Barraud et al., 2009). Other anti-biofilm agents targeting bacterial adhesion and disruption of extracellular DNA have also been identified recently, such as Dispersin B which cleaves the major EPS polysaccharide poly-β 1,6-N-acetylglucosamine, and Deoxyribonuclease I which degrades extracellular DNA present in the EPS (Kaplan et al., 2003; Qin et al., 2007).
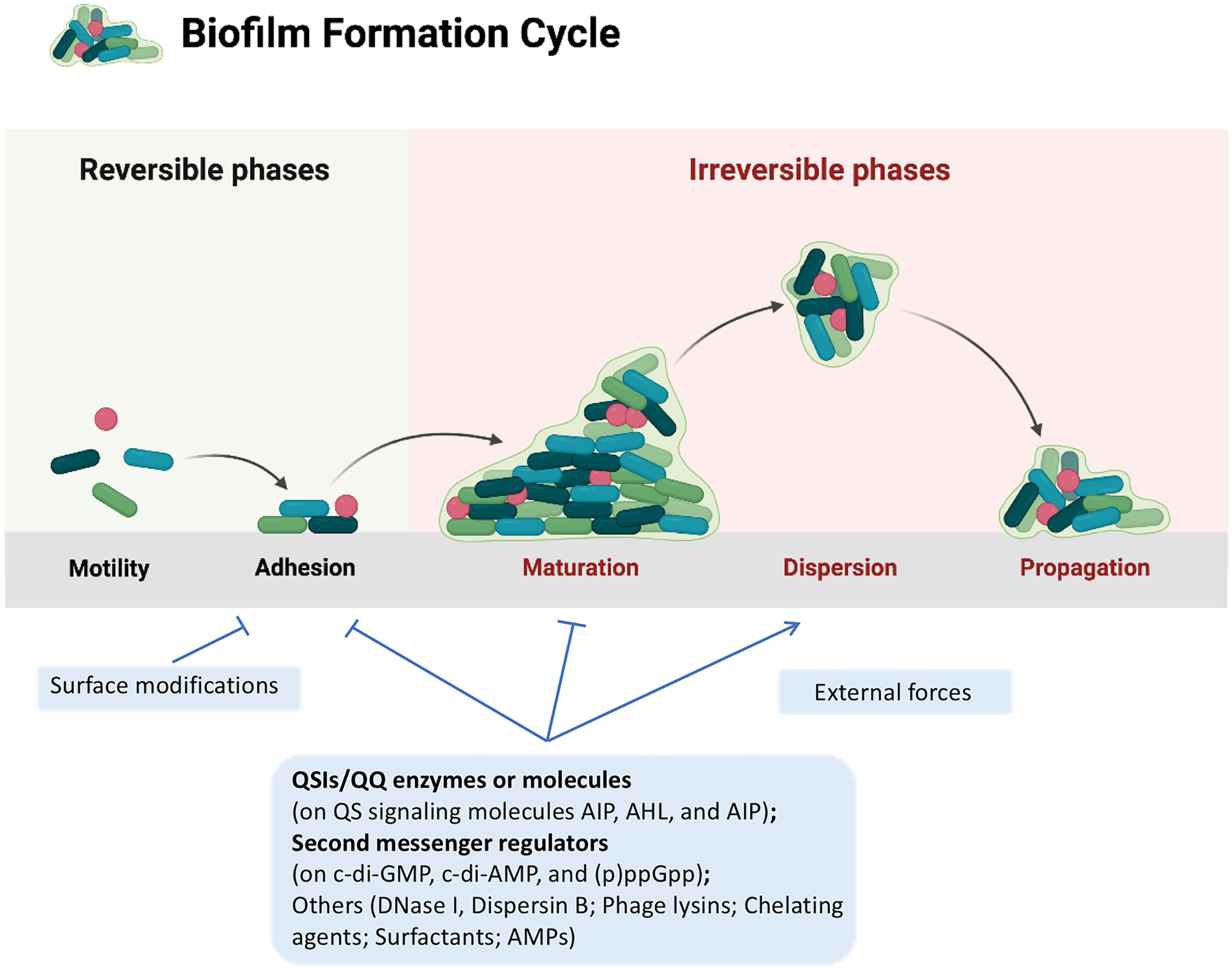
Figure 1. A typical biofilm cycle and the strategies to control biofilm formation. A typical biofilm formation consists of five stages: (i) reversible attachment to surface; (ii) irreversible attachment to surface; (iii) microcolony formation; (iv) maturation of biofilm; and (v) biofilm dispersal. Strategies for combating bacterial biofilms are classified into three main categories: (i) changing the properties of susceptible surfaces to prevent biofilm formation; (ii) regulating signaling pathways to inhibit biofilm formation; and (iii) applying external forces to eradicate the biofilm, which are displayed in light blue rectangles. Strategies discussed in this review are presented in bold. Lines with arrow head, positive regulation; Lines with stops, negative regulation. This figure was created with BioRender.com.
Traditional Chinese medicine (TCM) is one of the oldest healing systems which includes herbal medicine, acupuncture, moxibustion, massage, food therapy, and physical exercise, and have been used for a long history in China against various diseases (Tang et al., 2008). Many TCMs are derived from natural herbs and Chinese herbal medicines (CHMs) are important component of TCMs (Liu et al., 2021). CHMs are usually a mixture of herbal plants or extracts which comprise hundreds of different constituents with widely differing physiochemical properties (Tang et al., 2008). As such, roots, stems, leaves and/or fruits of diverse herbs species are commonly used in CHMs. The standardized formulae of CHMs are now commonly used as tablets, capsules, and even ampoules as well as the traditional decoctions of individualized prescriptions (Tang et al., 2008; Kong et al., 2009). As natural active drugs, CHMs have the advantages of abundant resources, higher safety, and lower toxicity compared with chemically synthesized drugs (Flower et al., 2015; Liao et al., 2022). However, due to the complex composition of CHMs, the large-scale application of TCMs is limited. Thus, more and more researchers have shifted their research focus to the identification and clarification of the antibacterial mechanisms of active components from CHMs, many of which exert anti-infection effect through inhibition of bacterial biofilm formation (Liu et al., 2011; Packiavathy et al., 2014). In exploring their antibacterial mechanisms, it was found that different chemical classes of CHMs metabolites, including flavonoids, terpenoids, phenols, organic acids, alkaloids and their derivatives, can inhibit bacterial biofilm formation by regulating bacterial QS system and nucleotide-based second messengers. In this review, we describe the research progresses of CHMs that act on bacterial QS system and second messengers in terms of bacterial biofilm formation, and to provide evidence of the potential of CHMs for the treatment and/or control of biofilms-associated infections and, in this way, encourage more and more advanced research on this area.
Quorum sensing (QS) is a bacterial communication system that plays a pivotal role in regulating bacterial biofilm formation (Irie and Parsek, 2008). QS is driven by signaling molecules in a density-dependent manner that contributes to a variety of biological functions, such as virulence factor secretion (Singh and Ray, 2014; Hernández-Ramírez et al., 2020), swimming/swarming motility (Daniels et al., 2004; Yang and Defoirdt, 2015), and bioluminescence (Nealson et al., 1970; Zhao et al., 2016). Various signaling molecules have been identified in bacteria so far, including N-acyl-homoserine lactone (AHL), autoinducing peptide (AIP), autoinducer-2 (AI-2), AI-3/epinephrine/norepinephrine signaling molecules, the diffusible signal factor (DSF), and 2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde (Irie and Parsek, 2008; Dickschat, 2010; Lee et al., 2013; Zhou et al., 2017). Among these molecules, AHL, AIP, and AI-2 are most widely studied. These different signaling molecules mediate different types of QS systems (Reading and Sperandio, 2006). While the QS system of most Gram-negative bacteria is the LuxI/LuxR type self-induction system that uses AHL as signaling molecule (Parsek and Greenberg, 2000), the QS system of Gram-positive bacteria is mediated by the small molecule peptide AIP (Kleerebezem et al., 1997). Moreover, there is a QS system that exists in both Gram-negative and Gram-positive bacteria, the LuxS/AI-2 type signaling system which uses AI-2 as the system’s signaling molecule for information exchange between bacterial species (Chen et al., 2002; Camilli and Bassler, 2006).
The regulatory mechanism of the bacterial QS system has been extensively studied. It has been found that the system can be targeted for the development of antibacterial inhibitors, and such inhibitors are called Quorum Sensing Inhibitors (QSIs; Chaieb et al., 2022). In addition to common antimicrobial peptides and antibiotics, many natural active substances extracted from TCMs and plants are also QSIs that can play an important role in the regulation of bacterial biofilm formation. The mechanisms of QSIs in blocking QS pathway are broadly classified into three types: (i) inhibition of signaling molecules synthesis; (ii) promotion of signaling molecules degradation; and (iii) competition with signaling molecules for receptor proteins binding (Zhou et al., 2020). Table 1 shows TCMs metabolites and their derivatives which displayed anti-bacterial biofilm formation via QS in the literatures, as well as their targets.
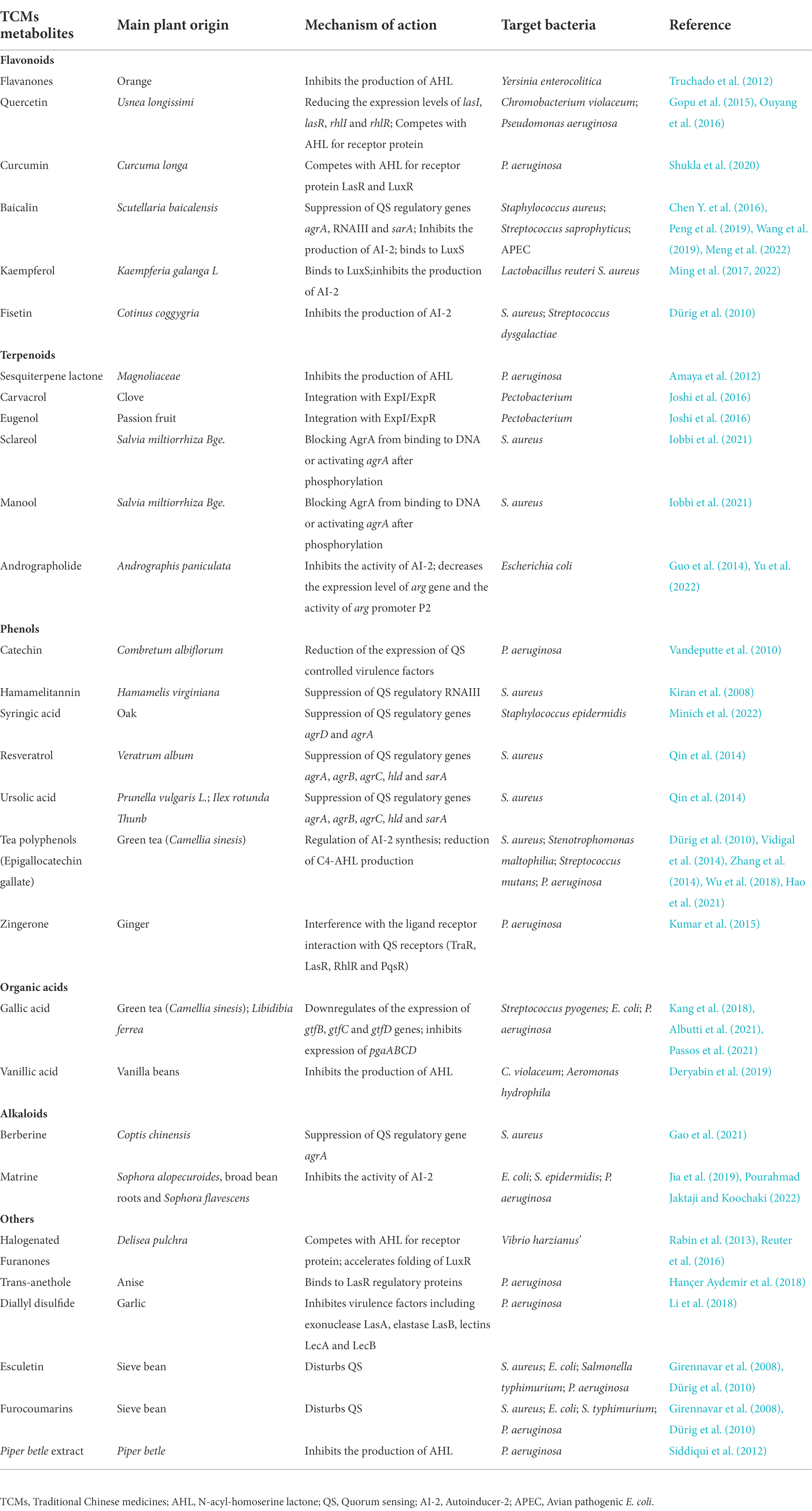
Table 1. Different classes of anti-biofilm TCMs metabolites and their mechanisms of action via bacterial QS system.
Flavonoids refer to a series of chemical compounds with two variable phenolic structure and many of them show various bioactive functions including antioxidant, antiviral, antibacterial, and anti-inflammation (Chu et al., 2015; Lee et al., 2018; Table 1; Figure 2). Plants are rich in flavonoids and many of which have been utilized as TCMs for a long period, such as quercetin leaves (Ouyang et al., 2016), Pericarpium Citri Reticulatae (Ma et al., 2021), and Scutellaria baicalensis (Chen Y. et al., 2016). Clinical studies have shown that flavonoids can protects gut microbiota from dysbiosis (Klinder et al., 2016), but whether this is through QS signaling is still unknown. Common flavonoids discovered so far including flavanone, quercetin, curcumin, baicalin, kaempferol, and fisetin, all of which exhibit different degrees of anti-biofilm activity via bacterial QS signaling (Table 1; Figure 1).
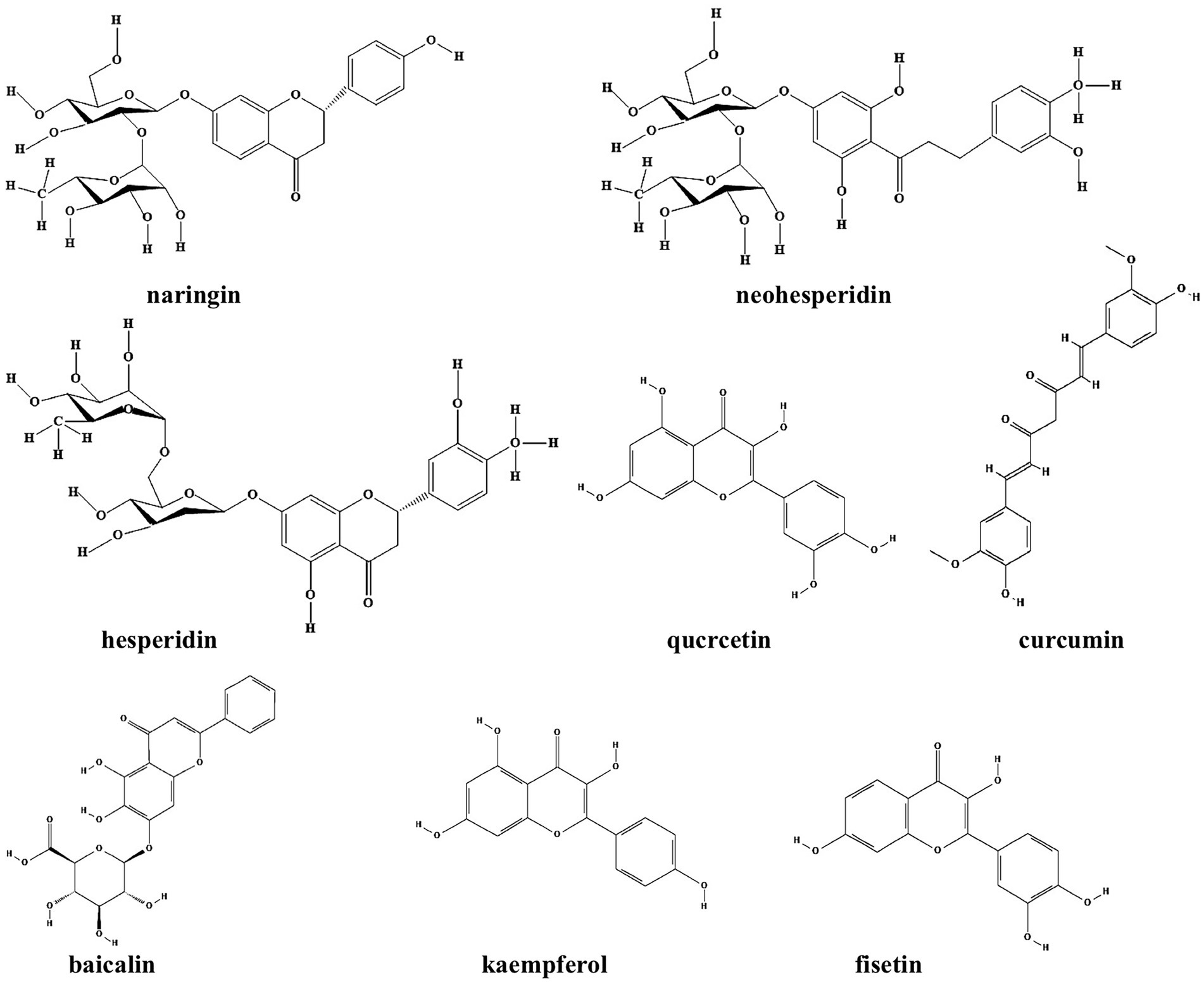
Figure 2. Chemical structures of the different flavonoids that inhibit biofilm formation via QS. ChemDraw software has been utilized to draw the chemical structures of the molecules.
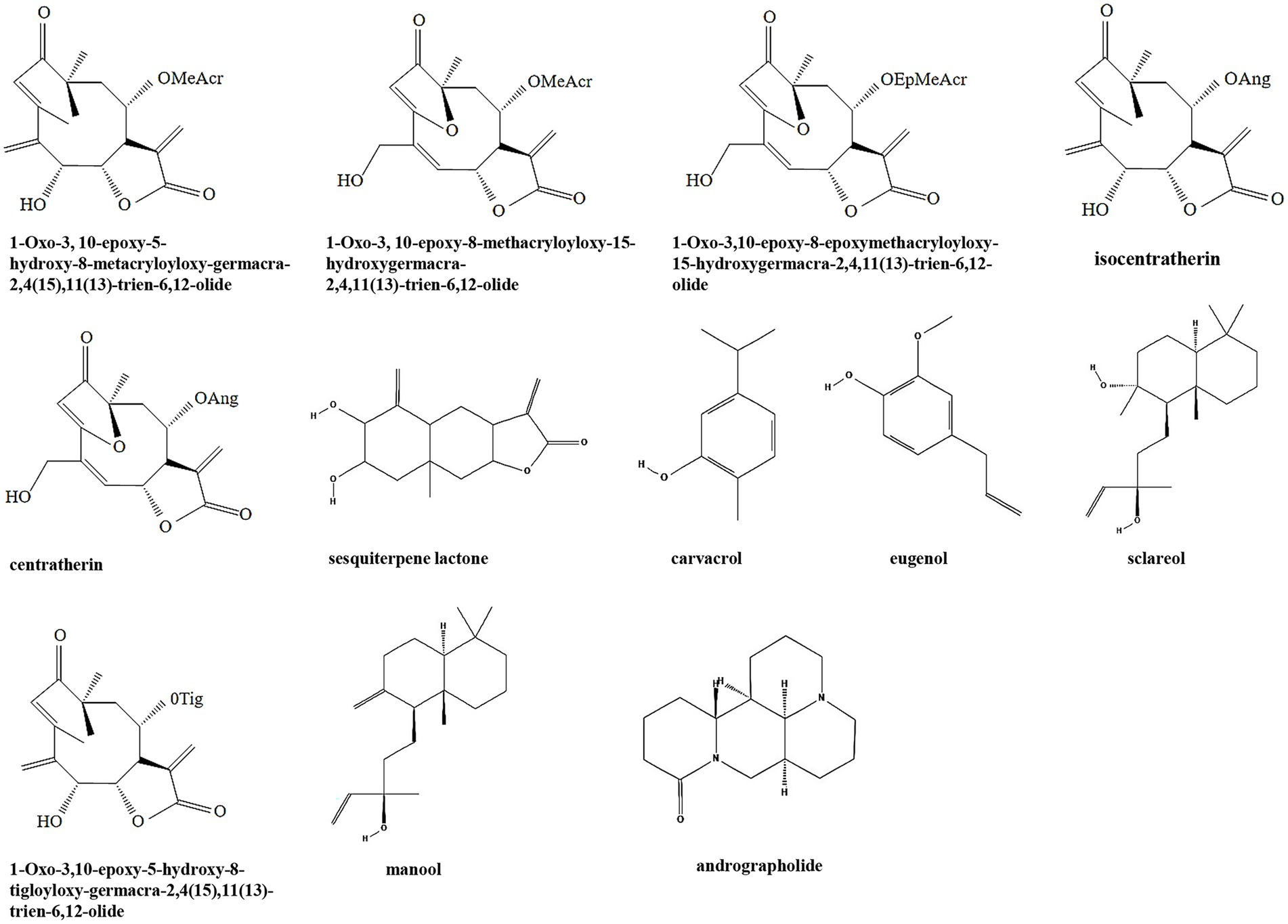
Figure 3. Chemical structures of the different terpenoids that inhibit biofilm formation via QS. ChemDraw software has been utilized to draw the chemical structures of the molecules.
The glycosylated flavanones naringin, neohesperidin, and hesperidin extracted from orange reduce the activity of Yersinia enterocolitica and inhibit its biofilm formation by interfering with the production of the signaling molecule AHL of QS system (Truchado et al., 2012). These flavanones downregulate the expression of genes involved in the synthesis of AHL (yenI and yenR) to impair QS signaling and biofilm formation. In vivo, naringin and hesperidin protects mice from endotoxin shock through inhibition of bacterial numbers and inflammatory cytokine release (Kawaguchi et al., 2004a,b).
Similarly, quercetin, another flavonoid from Usnea longissimi, inhibit the biofilm formation of diverse bacteria species including Pseudomonas aeruginosa (Ouyang et al., 2016), Chromobacterium violaceum (Skogman et al., 2016), and Klebsiella pneumoniae (Gopu et al., 2015) through QS signaling. Quercetin, although not affecting the growth of P. aeruginosa, significantly inhibit the production of biofilm and virulence factors by downregulation of the expression levels of lasI, lasR, rhlI, and rhlR (Ouyang et al., 2016). It further demonstrates that quercetin inhibit QS via binding with LuxI-type AHL synthases and/or LuxR-type AHL receptor proteins (Deryabin et al., 2019). In vivo, quercetin supplementation reduces the number of pathogenic species including Enterococcus, Neisseria and Pseudomonas and increases the number of non-pathogenic Streptococcus sp. and oral microbiome diversity (Mooney et al., 2021).
Moreover, curcumin from Curcuma longa also reduce the ability of P. aeruginosa to form biofilms and inhibit virulence factors expression. Curcumin binds to both LasR and LuxR that leads to the inactivation of these proteins and reduction in biofilm formation (Shukla et al., 2020). In a clinical study, curcumin treatment significantly diminishes the severity of dyspepsia and eradication of Helicobacter pylori in patients, indicating that curcumin can be used as a candidate drug for the treatment of functional dyspepsia (Panahi et al., 2021).
Baicalin, another flavonoid isolated from the root of Scutellaria baicalensis, downregulates the gene expression of Staphylococcus aureus QS regulators agrA, RNA III and sarA and ica to inhibit biofilm formation, leading to increased vancomycin permeability (Chen Y. et al., 2016). Wang et al. (2019) further demonstrated that the reduction of biofilm formation by baicalin was achieved by inhibiting the MsrA efflux pump and the Agr system in Streptococcus saprophyticus. Moreover, baicalin also inhibits QS signaling molecule AI-2 and the expression of virulence genes in avian pathogenic Escherichia coli (APEC; Peng et al., 2019). In vivo, baicalin significantly reduces APEC colonization and increases the abundance of short chain fatty acid (SCFA)-producing bacteria of gut microbiota to alleviate lung injury (Peng et al., 2021b).
Furthermore, in silico analyzation by molecular docking reveales the binding mode of four natural products, norathyriol, mangiferin, baicalein, kaempferol and baicalin, to LuxS. All of these products show good binding ability to LuxS and inhibit the production of AI-2 (Meng et al., 2022). In addition, kaempferol extracted from Kaempferia galanga L. could also reduce the biofilm formation of S. aureus by inhibit the activity sortase A and the expression of adhesion-related genes (Ming et al., 2017). This is also the case for fisetin, a compound extracted from Cotinus coggygria, which dramatically inhibit biofilm formation of both S. aureus and Streptococcus dysgalactiae via a similar mechanism (Dürig et al., 2010).
Terpenoids are a class of secondary metabolites that have the general formula of (C5H8) n. According to the number of isoprene or isopentane (C5H8), terpenoids and their derivatives are divided into several subclasses including monoterpenes, sesquiterpenes, diterpenes, triterpenes, tetriterpenes, and polyterpenes (Zhuang and Chappell, 2015). Terpenoids are widely distributed in nature and many of them play a wide range of pharmacological effects as TCMs, such as antiparasitic and antibacterial effects. Many terpenoids including sesquiterpene lactones, carvacrol, eugenol, sclareol, manool, and andrographolide have been discovered with anti-biofilm activity (Table 1; Figure 2). It is been shown that six sesquiterpene lactones, three of the goyazensolide-type and three of the isogoyazensolide-type extracted from Centratherum punctatum, inhibited biofilm formation of P. aeruginosa by downregulation of QS signaling molecule AHL and inhibit bacterial growth in a concentration dependent manner (Amaya et al., 2012), but the detailed molecular mechanisms still need to be investigated.
Carvacrol and eugenol, which are commonly isolated from clove and passion fruit, respectively, and utilized in essential oils, could also specifically interfere with the QS system of Pectobacterium. By constructing homology models for high serine lactone synthase (ExpI) or regulatory proteins (ExpR) and performing molecular docking simulation tests, carvacrol and eugenol have the ability to bind ExpI/ExpR, which in turn leads to decreased accumulation of the intracellular QS signaling molecule AHL and inhibit biofilm formation (Joshi et al., 2016; Deryabin et al., 2019). Moreover, eugenol inhibit the formation of Acinetobacter baumannii biofilms and disrupt biofilm structure by downregulation of the transcription of genes involved in biofilm formation (Karumathil et al., 2016). In vivo studies demonstrate that carvacrol inhibits the colonization of several pathogens, including Campylobacter jejuni (Mousavi et al., 2020), S. typhimurium (Kortman et al., 2014), and Listeria monocytogenes (Silva et al., 2015), to host cells and thus protest host from infections. Similarly, eugenol can also inhibit the colonization of S. typhimurium and restricts host inflammation (Zhao et al., 2022).
The labdane diterpenoids sclareol and manool from Salvia tingitana are considered potential QSIs against methicillin-resistant S. aureus (MRSA). They can inhibit MRSA biofilm formation and virulence factor expression by prevention of the activation of AgrA upon binding or phosphorylation of the helper gene regulator AgrA to DNA (Iobbi et al., 2021). Guo et al. investigated the effect of andrographolide, the main active ingredient of Andrographis paniculata, on the pathogenies of APEC O78. They found that andrographolide significantly decrease the lactate dehydrogenase release, F-actin cytoskeleton polymerization, and bacterial adhesion to chicken type II pneumocytes by inhibiting the expression of QS signaling molecule AI-2 and virulence factors (Guo et al., 2014). However, study also showed that andrographolide had no effect on the production of AI-2, but significantly decreased the expression level of arg gene and the activity of arg promoter P2, leading to inhibition of the biofilm formation and virulence of L. monocytogenes (Yu et al., 2022).
Plant phenols are found in the leaves, shells, pulp and seed coat of plants, and are second only to cellulose, hemicellulose and lignin in content. Plant phenols have a long history of medical applications and have been shown to have strong antioxidant activity, effective in preventing chronic diseases such as hyperglycemia (Westfall et al., 2018), hyperlipidemia (Yazdanparast et al., 2008), cardiovascular and cerebrovascular diseases (Wu et al., 2010), as well as reducing cancer risk (Cesmeli et al., 2021). Common plant phenols such as catechin, hamamelitannin, syringic acid, ursolic acid, zingerone, resveratrol, and tea polyphenols have been shown to inhibit the formation of biofilm by bacteria (Table 1; Figure 4).
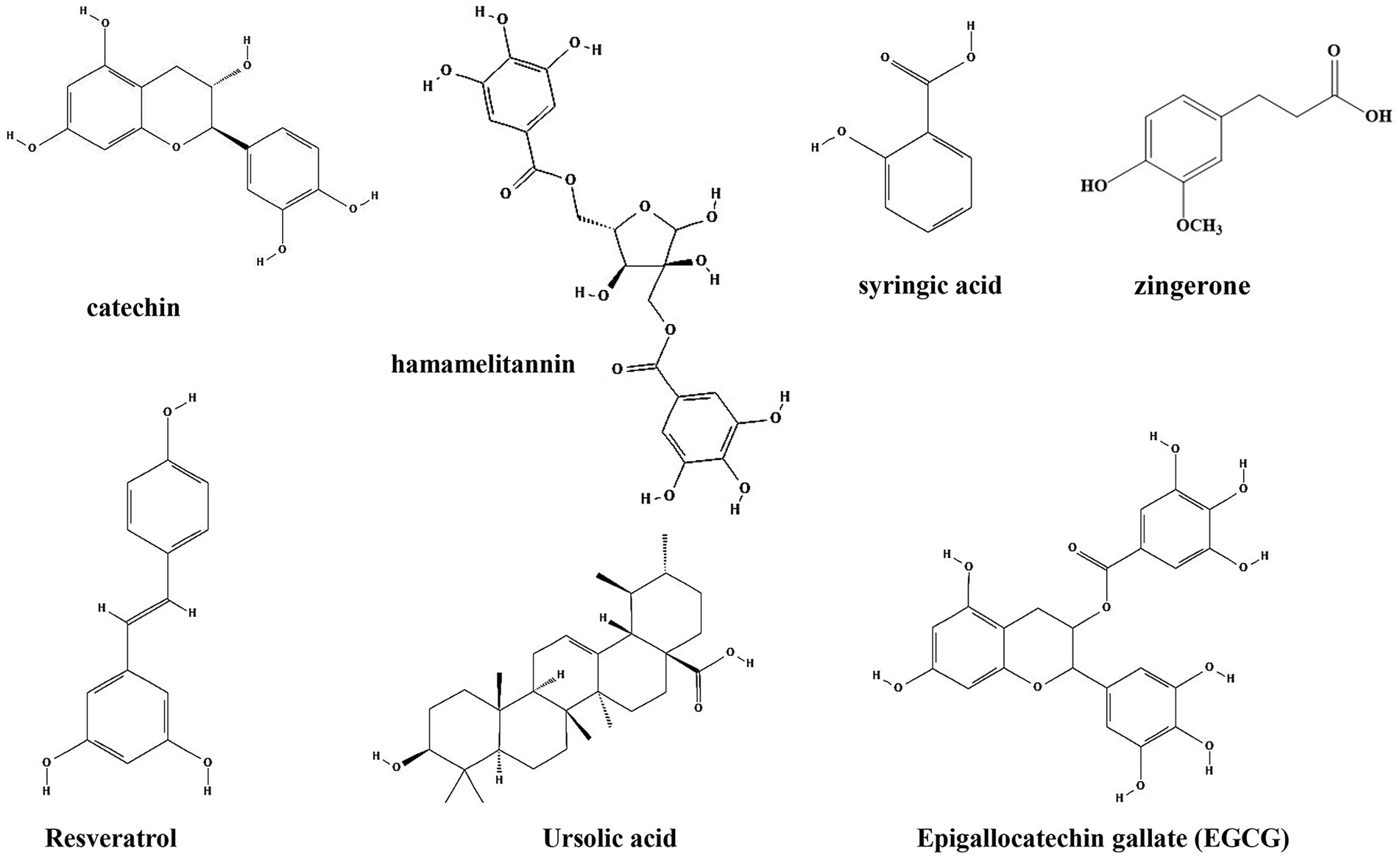
Figure 4. Chemical structures of the different phenols that inhibit biofilm formation via QS. ChemDraw software has been utilized to draw the chemical structures of the molecules.
Catechin, one of the phenols isolated from Combretum albiflorum leaves and bark extracts, inhibit the biofilm formation and pathogenesis by reduction of the expression of QS controlled virulence factors in P. aeruginosa (Vandeputte et al., 2010). The use of RhlR-and LasR-based biosensors indicated that catechin might interfere with the perception of the QS signal N-butanoyl-L-homoserine lactone by RhlR, thereby leading to a reduction of the production of QS factors. In vivo studies showed that catechin can promote the proliferation of beneficial intestinal bacteria and regulate the balance of intestinal flora to relieve the inflammatory bowel disease (Fan et al., 2017). Hamamelitannin, a polyphenolic natural product found in the bark of Hamamelis virginiana, has no effect on staphylococcal growth in vitro, but reduce biofilm formation by inhibiting the QS regulator RNA III (Kiran et al., 2008). Moreover, several synthetic hamamelitannin analogs have been identified as antibiotic potentiators for S. aureus treatment (Vermote et al., 2016). Hamamelitannin increases the susceptibility of S. aureus to antibiotic treatment in vivo Caenorhabditis elegans and mouse mammary gland infection models (Brackman et al., 2016). Syringic acid, which is also a phenolic compound isolated from oak bark lignin, reduce biofilm formation up to 80% and EPS up to 55% by downregulation of mRNA expression of two genes of the QS system, agrD and agrA in Staphylococcus epidermidis (Minich et al., 2022). Moreover, inhibition of biofilm formation by interfering with the QS system is also observed by treatment with resveratrol (extracted from Veratrum album, a plant of Liliaceae) and ursolic acid (found in the whole grass of Prunella vulgaris L., a labiatae plant, and the leaves of Ilex rotunda Thunb), upon which the expressions of genes related to the QS system (agrA, agrB, agrC, hld and sarA) are downregulated (Qin et al., 2014). Similar to catechin, resveratrol and ursolic acid have also shown protective effects on gut microbiota in vivo (Cai et al., 2020; Peng et al., 2021a).
Investigation of the molecular mechanism also identified several phenolic compounds that interacts with QS signaling molecules. Zingerone, which is mainly found in root of ginger (Zingiber officinale), reduces the ability of P. aeruginosa to form biofilms and inhibits virulence factors expression by competing with signaling molecules for receptor proteins (TraR, LasR, RhlR and PqsR), thereby blocked the QS signaling (Kumar et al., 2015). Of note, zingerone effectively reduced P. aeruginosa biofilm-associated murine acute pyelonephritis (Sharma et al., 2020), suggesting it is a potential effective therapeutic agent for clinical application. Zhang et al. investigated the effects of citral, cinnamaldehyde, and tea polyphenols on the formation of mixed biofilms of foodborne S. aureus and Salmonella enteritidis. The results showed that citral, cinnamaldehyde and tea polyphenols could significantly inhibit the formation of mixed biofilms. Interestingly, while citral could reduce the synthesis of AI-2, cinnamaldehyde and low concentrations of tea polyphenols increased AI-2 synthesis (Zhang et al., 2014). Similarly, Epigallocatechin gallate (EGCG, tea polyphenol), which is present in green tea, also showed anti-biofilm and anti-infection activities by Stenotrophomonas maltophilia and P. aeruginosa by reduction of C4-AHL production (Vidigal et al., 2014; Hao et al., 2021). In mice, these compounds protect mice from infections by different pathogens, including methicillin-resistant S. aureus (Long et al., 2019), H. pylori (Muhammad et al., 2015; Deng et al., 2022), and S. typhimurium (Wang et al., 2021; Zhao et al., 2021).
Natural organic acids are widely distributed in the leaves, roots and especially fruits of herbs such as umeboshi (pickled Japanese plum), schisandra (dry and mature fruit of Schisandra chinensis) and raspberry. Some natural organic acids have certain biological activities including antibacterial (Fontanay et al., 2008), anti-inflammatory (Wu et al., 2023), hypoglycemic (Pandey et al., 2022), antioxidant (Ma et al., 2018), and immune modulation (Wu et al., 2004; Fontanay et al., 2008; Ma et al., 2018). Common natural organic acids including gallic acid and vanillic acid have antibacterial biofilm effects (Table 1; Figure 5).
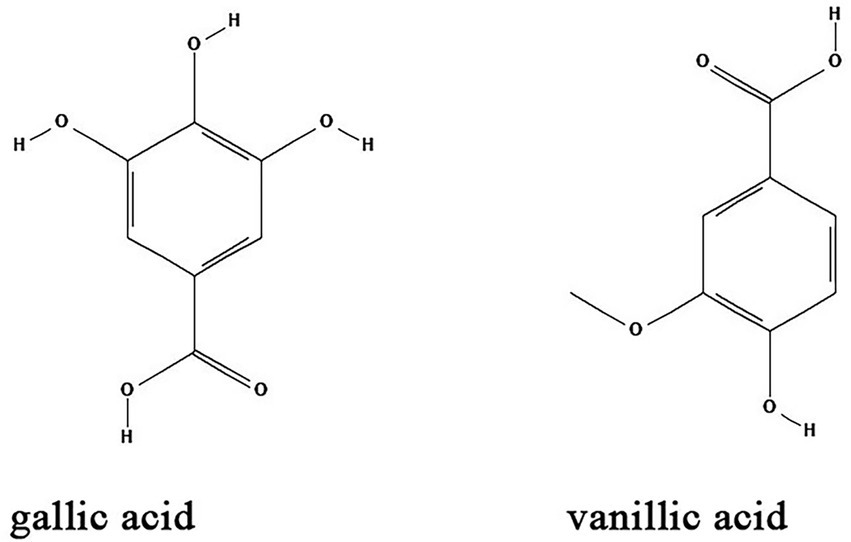
Figure 5. Chemical structures of the different organic acids that inhibit biofilm formation via QS. ChemDraw software has been utilized to draw the chemical structures of the molecules.
Gallic acid, also known as 3,4,5-trihydroxybenzoic acid, is a naturally occurring secondary metabolite. It is extracted from Green tea (Camellia sinesis) as a major component together with other anti-biofilm compounds such as EGCG, propyl gallate, and octyl gallate (Vidigal et al., 2014). The anti-biofilm activity of gallic acid has been investigated in diverse bacteria species. Gallic acid and ethyl gallate extracted from Libidibia ferrea (Mart. ex Tul.) inhibits Streptococcus pyogenes biofilms by downregulation of the expression of gtfB, gtfC and gtfD genes (Passos et al., 2021). Gallic acid at a concentration of 2 mg/ml significantly inhibits the expression of pgaABCD genes and effectively suppress the formation of E. coli biofilm in a dose-dependent manner (Kang et al., 2018). Moreover, high concentrations of gallic acid inhibited the biofilm formation and growth of Proteus spp., Pseudomonas spp., Salmonella spp., Streptococcus mutans, and S. aureus (Albutti et al., 2021). In vivo, gallic acid reduces inflammation and proliferation of Brucella abortus in spleens of mice (Reyes et al., 2018). Vanillic acid is a benzoic acid derivative that can be extracted from vanilla beans. Studies showed that vanillic acid inhibited the QS-dependent violacein biosynthesis in C. violaceum and biofilm formation in Aeromonas hydrophila by downregulation of AHL production (Deryabin et al., 2019). However, the detailed mechanisms of vanillic acid on biofilm formation needs to be further elucidated.
Alkaloids are nitrogen-containing heterocyclic compounds which are widely found in plants including Papaveraceae, Berberidaceae, and Fabaceae. Lots of alkaloids have been identified so far and many of them exert antibacterial effects with broad spectrum and fewer adverse effects (Table 1; Figure 6). Their main antibacterial mechanisms include (i) inhibition of bacterial cell wall synthesis; (ii) inhibition of bacterial biofilm formation; (iii) alteration of cell membrane permeability; (iv) inhibition of bacterial metabolism; and (v) inhibition of nucleic acid and protein synthesis (Larghi et al., 2015; Table 1; Figure 5).
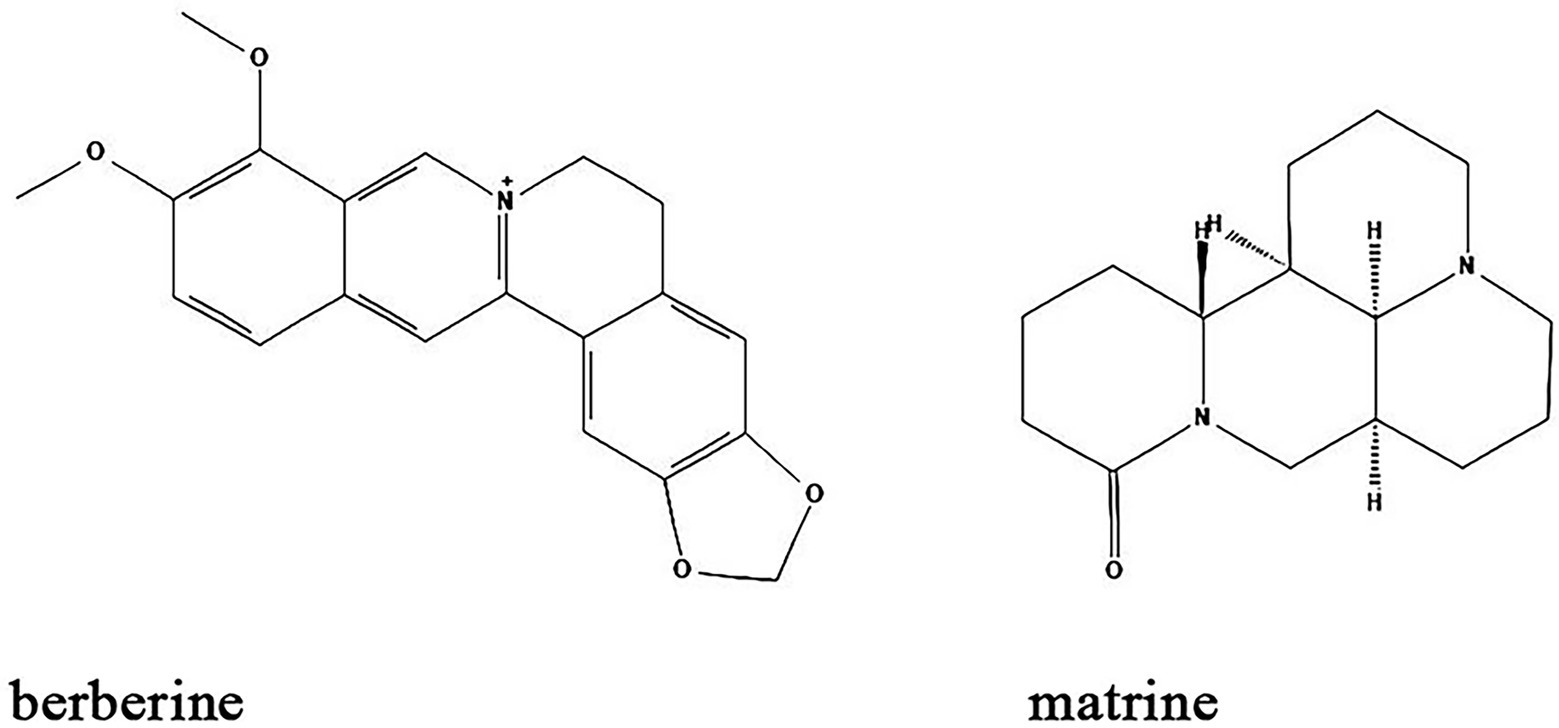
Figure 6. Chemical structures of the different alkaloids that inhibit biofilm formation via QS. ChemDraw software has been utilized to draw the chemical structures of the molecules.
Berberine is an alkaloid extracted from Coptis chinensis and also an isoquinoline derivative according to its chemical structure. Berberine has been reported to have antibacterial efficacy in eliminating bacterial and fungal biofilms. As such, berberine exerted synergistic effects on inhibiting Candida albicans/S. aureus dual strain biofilms in combination with amphotericin B, an efficient antibiotic that utilized for the treatment of fungal infections in clinic (Gao et al., 2021). A study by Ning et al. demonstrated that berberine inhibited biofilm formation via downregulation of the expression of the QS regulatory gene agrA in a concentration-dependent manner in S. aureus (Ning et al., 2022). Moreover, Ferrazzano et al. found that berberine exerted efficient antimicrobial efficacy against diverse endodontic pathogens including Fusobacterium nucleatum, Prevotella intermedia, and Enterococcus faecalis (Ferrazzano et al., 2011). Interestingly, berberine also regulates gut microbiota and microbial tryptophan catabolites to protect mice from inflammatory bowel diseases (Zhang et al., 2019; Jing et al., 2021).
Matrine is another alkaloid that is widely distributed in Sophora alopecuroides (a perennial leguminous herb distributed in northwestern and northern China), broad bean roots and Sophora flavescens. It has anti-inflammatory, antibacterial, antioxidant, immunomodulatory and anticancer effects (Sun et al., 2022). Similar to berberine, matrine is also found to inhibit the biofilm formation of different bacteria species. Matrine reduce the formation of antimicrobial-resistant E. coli (a strain that showed resistant to different antibiotics) biofilms by downregulation of QS-related genes luxS, pfS, sdiA, hflX, motA and fliA (Sun et al., 2019). In S. epidermidis, the biofilm formation is also inhibited by matrine through decreasing the QS signaling molecule AI-2 activity (Jia et al., 2019). In combination with antibiotics, matrine dramatically decreases the multidrug-resistant P. aeruginosa biofilms (Pourahmad Jaktaji and Koochaki, 2022). Moreover, in vivo studies found that matrine can modulate the composition and functions of gut microbiota to improve gut barrier integrity and reduce murine colitis (Yao et al., 2021).
Besides the major classes of anti-biofilm compounds mentioned above, many other compounds have been identified from natural sources or TCMs with anti-biofilm activity including but not limited to trans-anethole, diallyl disulfide, esculetin, and furocoumarins. (Table 1; Figure 7).
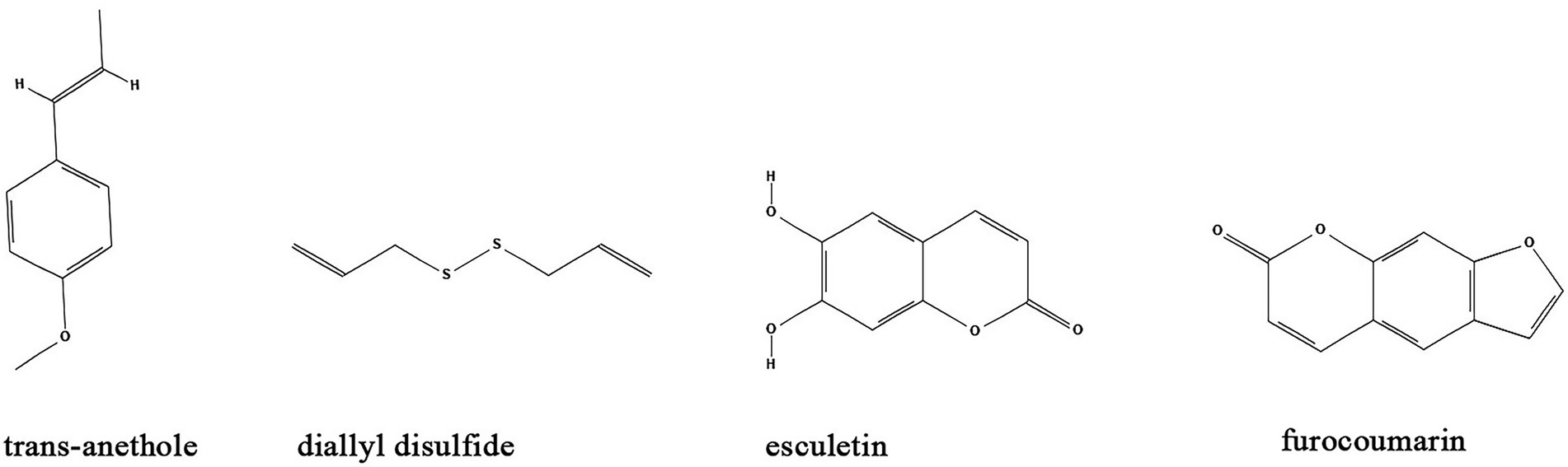
Figure 7. Chemical structures of other compounds that inhibit biofilm formation via QS. ChemDraw software has been utilized to draw the chemical structures of the molecules.
Trans-anethole, the main component of anise oil, exhibit inhibitory effect on biofilm formation and the expression of QS-regulated virulence factors in P. aeruginosa by binding to LasR regulatory protein (Hançer Aydemir et al., 2018). Similarly, the P. aeruginosa biofilms and virulence factors including exonuclease LasA, elastase LasB, lectins LecA and LecB can also be inhibited by diallyl disulfide, a compound utilized in garlic oil (Li et al., 2018). Moreover, diallyl disulfide had beneficial effects on establishment of microbiota biofilms and colonic mucus production that alleviate murine colitis (Motta et al., 2015). Coumarins are a class of organic compounds which are not only isolated from sieve bean, but also in many different plants, such as Tonka beans, verbena, wild vanilla and orchid (ElNaggar et al., 2022). Studies found that some coumarins including esculetin and furocoumarins have broad range anti-biofilm activity by disturbing QS in S. aureus, E. coli, S. typhimurium, and P. aeruginosa via reduction of AHL (Girennavar et al., 2008; Dürig et al., 2010). Further studies demonstrated that esculetin is structurally compatible with the TraR AHL-binding site and downregulates numerous genes associated with QS signaling (Zeng et al., 2008; Zhang et al., 2018).
Apart from these CHMs metabolites, the anti-biofilm activities of some plant’s crude extract have also been investigated. For example, halogenated furanone compounds extracted from red seaweed Delisea pulchra can inhibit colonization, swarming and biofilm formation of Gram-negative bacteria, attenuate bacterial virulence and prevent bacterial infections (Chang et al., 2019; Aburto-Rodríguez et al., 2021). The structure of halogenated furanones is similar to that of the signaling molecule AHL, which compete with AHL for the receptor protein and replace AHL molecules binding to the receptor (Rabin et al., 2013). In Vibrio fischeri and Vibrio harveyi, halogenated furanones are also found to accelerate the folding of luxR, which in turn diminishes the ability of LuxR to bind to DNA and the transcription initiation process (Reuter et al., 2016). Moreover, Siddiqui et al. demonstrate that Piper betle extract (PBE) inhibit P. aeruginosa biofilm formation by reduction of AHL and EPS (Siddiqui et al., 2012). Also, PBE can reduce the virulence of P. aeruginosa by affecting the QS system (Datta et al., 2016).
Nucleotide-based second messengers are small non-protein molecules produced intracellularly. Bacteria can respond to extracellular signals through changes in the concentration of second messenger molecules (increase or decrease) by binding to cell surface receptors, regulating the enzymatic activity of intracellular metabolic systems, amplifying the original signal and thus inducing intracellular expression of a series of specific genes, and ultimately affecting a variety of physiological and biochemical processes in bacteria (Römling et al., 2013; Opoku-Temeng et al., 2016). Second messenger molecules have been shown to be involved in regulating bacterial growth and metabolism and other physiological functions, such as virulence factor expression (Ahmad et al., 2011, 2013), fatty acid synthesis (Zhang et al., 2013; Gerhardt et al., 2020; Li et al., 2022), cell wall metabolic homeostasis (Witte et al., 2013; Commichau et al., 2018), extracellular polysaccharide synthesis and biofilm formation (da Aline Dias et al., 2020; Junkermeier and Hengge, 2021). Six major types of second messengers have been discovered in bacteria so far, including c-di-GMP (Römling et al., 2013), c-di-AMP (Peng et al., 2016), cGAMP (Davies et al., 2012; Li et al., 2019), cGMP (Linder, 2010), cAMP (Harman, 2001) and (p)ppGpp (van Delden et al., 2001). While c-di-GMP is recognized as an ubiquitous second messenger for the regulation of bacterial biofilm formation, biofilm formation regulated by the other second messengers is only found in certain bacteria species, including S. mutans (Lemos et al., 2004; Peng et al., 2016), Bacillus subtilis (Gundlach et al., 2016; Townsley et al., 2018), S. aureus (Gries et al., 2016), P. aeruginosa (Luo et al., 2015), K. pneumoniae (Ou et al., 2017), and E. coli (Hufnagel et al., 2016; Li et al., 2019). Therefore, the development of novel anti-biofilm agents in terms of nucleotide-based second messengers is mainly targeted on c-di-GMP signaling.
Given the important role of second messenger-regulated signaling pathways in bacterial biofilm formation, the development of antimicrobial compounds via second messenger-regulated signaling pathways to control infections has become a research priority. The mechanism of action for blocking second messenger signaling is broadly divided into three categories: (i) inhibition or activation of second messenger synthases; (ii) inhibition or activation of second messenger degradation enzymes; and (iii) competition for signaling pathway receptor proteins (Zhou et al., 2013; Sambanthamoorthy et al., 2014; Zheng et al., 2016). Although thousands of literatures have provided biological insights into second messenger signaling so far, the development of small-molecule inhibitors of second messengers on bacterial biofilm formation is significantly lagging behind, with even fewer studies on natural compounds such as TCMs metabolites as inhibitors (Opoku-Temeng et al., 2016). Compounds which inhibit bacterial second messengers signaling are listed in Table 2; Figure 8.
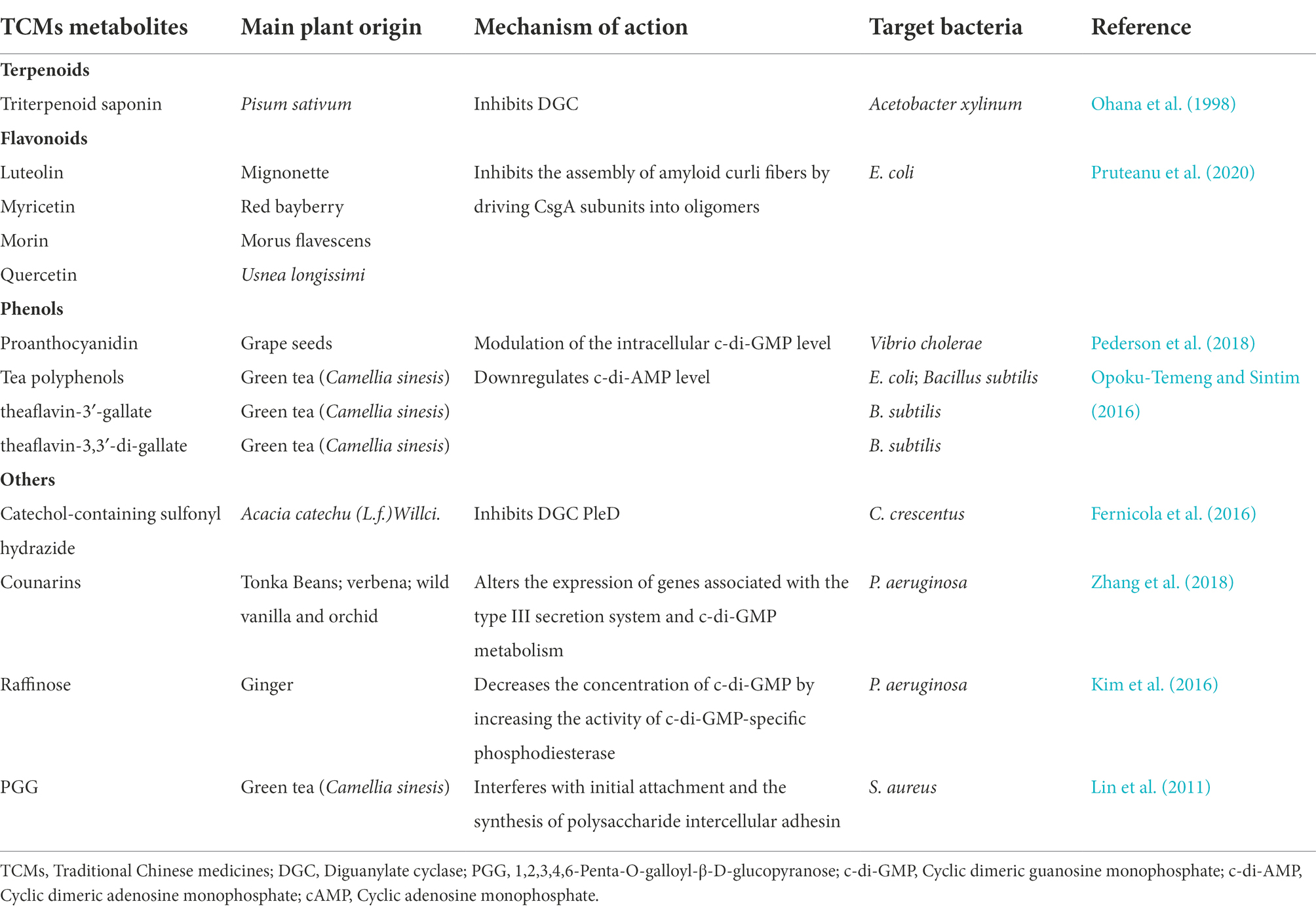
Table 2. Different classes of anti-biofilm TCMs metabolites and their mechanisms of action via bacterial second messengers (−related) signaling pathways.
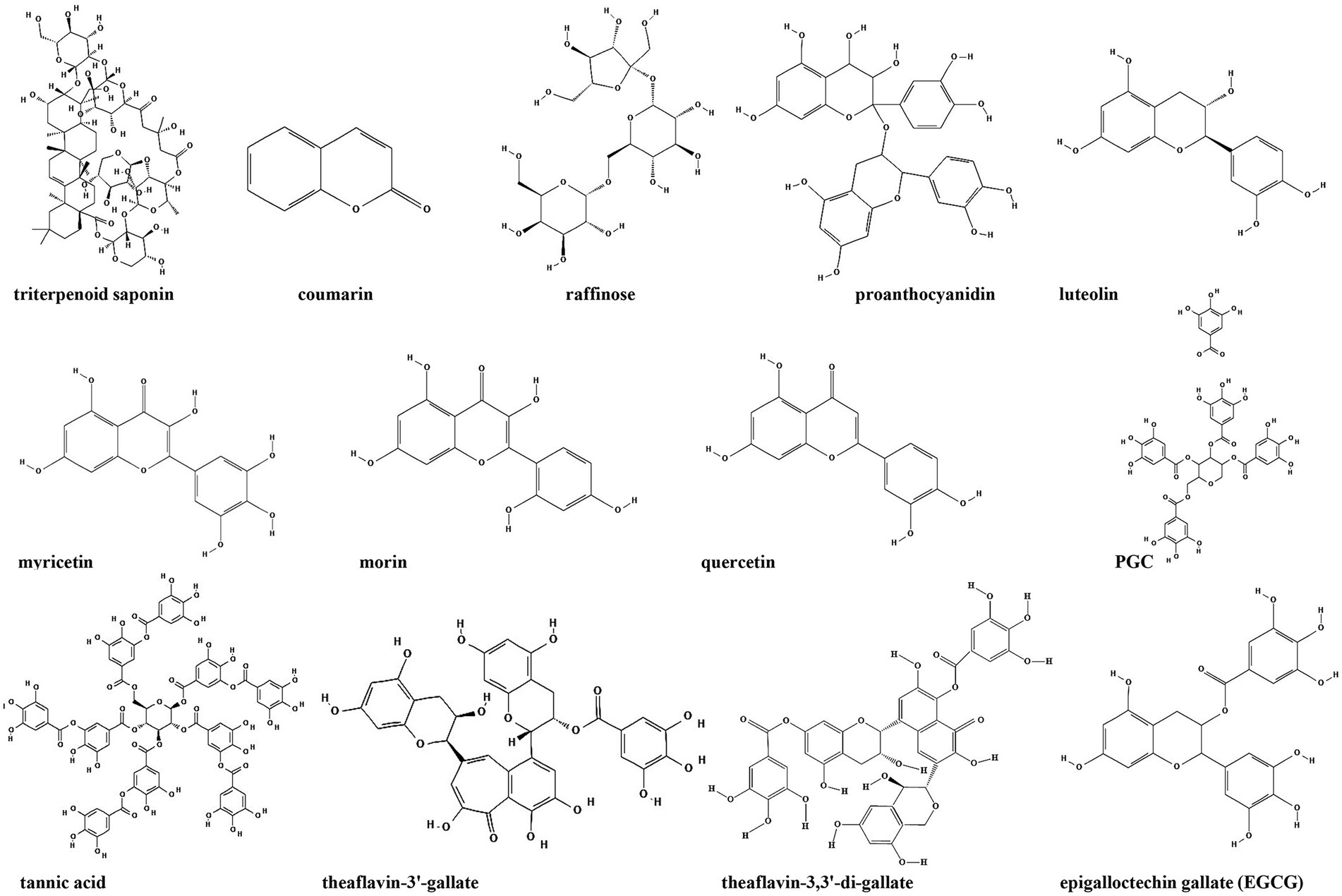
Figure 8. Chemical structures of the compounds that inhibit biofilm formation via second messengers (−related) signaling pathways. ChemDraw software has been utilized to draw the chemical structures of the molecules.
Cyclic di-GMP is recognized as an ubiquitous second messenger that regulates bacterial sessility/motility lifestyle transition (Simm et al., 2004), cell cycle (Xu et al., 2020), virulence (Ahmad et al., 2011), biofilm formation and dispersal (Ross et al., 1987; Miller et al., 2022). The intracellular concentrations of c-di-GMP depend on the rates of synthesis and degradation, which are regulated by diguanylate cyclase (DGC) and phosphodiesterase (PDE), respectively, that can sense different signals (Boyd and O’Toole, 2012; Römling et al., 2013). At present, c-di-GMP signaling inhibitors discovered in herbs are mostly c-di-GMP analogs or non-nucleotide small molecules that inhibit DGCs. Ohana et al. isolated a specific and efficient inhibitor of DGC, triterpenoid saponin, from extracts of Pisum sativum. Triterpenoid saponin inhibits DGC of Acetobacter xylinum, thereby reduces the intracellular concentration of c-di-GMP (Ohana et al., 1998). In vivo, triterpenoid saponin increase beneficial bacteria, while decreases sulfate-reducing bacteria, and alleviate intestinal inflammatory gut environment in mice (Chen L. et al., 2016). Moreover, using a virtual approach with a three-dimensional pharmacophore model, two catechol-containing sulfonyl hydrazide compounds are identified with the ability to competitively inhibit DGC PleD in Caulobacter crescentus and could serve as potential inhibitors of bacterial c-di-GMP signaling (Fernicola et al., 2016).
Coumarin is found in tonka beans, verbena, wild vanilla and orchid, and has the smell of fresh hay and fenugreek (ElNaggar et al., 2022). Coumarins have been shown to have antibacterial activity as a QSI in a broad spectrum of pathogens. Coumarin alters the expression of genes associated with the type III secretion system and c-di-GMP metabolism to inhibit biofilm formation. Coumarin significantly reduces the cellular c-di-GMP levels of P. aeruginosa PAO1 and clinical P. aeruginosa strains (Zhang et al., 2018). Raffinose, a plant galactose derived from ginger, can bind to a carbohydrate-binding protein LecA to effectively inhibit P. aeruginosa biofilm and alter bacterial phenotype without impairing bacterial growth (Kim et al., 2016). In addition, raffinose also decreases the concentration of c-di-GMP by increasing the activity of c-di-GMP-specific phosphodiesterase (Kim et al., 2016). Moreover, procyanidins are the general name of a large class of polyphenol compounds, which are abundant in grape seeds. Water-soluble extract from cranberry standardized to 4.0% proanthocyanidins could significantly inhibit Vibrio cholerae biofilm formation by reducing the biofilm matrix production and secretion via modulation of the intracellular c-di-GMP level (Pederson et al., 2018).
Besides the compounds mentioned above, it’s demonstrated that green tea polyphenol EGCG inhibits E. coli biofilms by elimination of the biofilm matrix via interfering with CsgD expression and the assembly of curli subunits into amyloid fibers (Serra et al., 2016). Study from the same group also identified several plant flavonoids including luteolin, myricetin, morin and quercetin as biofilm inhibitors. These flavonoids strongly reduce the extracellular matrix production by directly inhibiting the assembly of amyloid curli fibers through driving CsgA subunits into oligomers (Pruteanu et al., 2020). Additionally, 1,2,3,4,6-Penta-O-galloyl-β-D-glucopyranose (PGG), an active ingredient in plants, inhibits S. aureus biofilm formation by interfering with initial attachment and the synthesis of polysaccharide intercellular adhesin (Lin et al., 2011), but whether c-di-GMP is also involved in this process stills unknown.
Plant anti-biofilm compounds targeting other second messengers are quite few and still needs to be discovered. Opoku-Temeng et al. identified three tea polyphenols including tannic acid, theaflavin-3′-gallate and theaflavin-3,3′-di-gallate as c-di-AMP inhibitors in B. subtilis. They found that these polyphenols specifically inhibited DisA activity to downregulate c-di-AMP level (Opoku-Temeng and Sintim, 2016).
The majority of bacteria in nature live in a biofilm state, and infections due to biofilms pose a great threat to clinical treatment. The bacterial QS system and second messenger signaling pathways play an important role in the regulation of biofilm formation, but their complex regulatory mechanisms need to be further investigated. These works on bacterial biofilm formation have provided many potential therapeutic targets for the development of antibacterial drugs. Many TCMs from natural compounds are well-known for their safety and less toxicity to host (Flower et al., 2015; Liao et al., 2022). The different chemical classes of TCMs metabolites with antibacterial activity act in the QS system and second messenger signaling pathways mainly by reducing the production of signaling molecules or competing for receptor proteins, and no TCMs’ metabolites with enzymatic activity to degrade signaling molecules have been discovered. In addition, most TCMs’ metabolites work alone at high concentrations and take a long time to function without the ability to kill bacteria, but they work well in combination with antibiotics or as antibiotic potentiators. Strategies such as modification of chemical structures and precision delivery by nanomaterials to the target of action can be developed to enhance the antibacterial ability of TCMs’ metabolites. In conclusion, with the continuous development of life science, TCMs, as a valuable asset left to mankind by nature and our ancestors, must have a longer-term development prospect in the fight against bacterial infections.
FL and JG conceived and designed the manuscript. MZ wrote the draft of the manuscript. CQ, QJ, and JD prepared the figures and edited the tables. FL compiled and reviewed the draft of the manuscript. LL, WH, and JG co-administrated the project. All authors contributed to the article and approved the submitted version.
This work was financially supported through grants from the National Natural Science Foundation of China (grant no. U19A2038, 31872505, 32072824, and 32102670), the Natural Science Foundation of Jilin Province (grant nos. 20200201120JC, 20220101295JC), the Fundamental Research Funds for the Central Universities, Shandong Provincial Modern Agricultural Industry Technology System (SDAIT-27), and Key Technology Research and Development Program of Shandong (2021TZXD012).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aburto-Rodríguez, N. A., Muñoz-Cázares, N., Castro-Torres, V. A., González-Pedrajo, B., Díaz-Guerrero, M., García-Contreras, R., et al. (2021). Anti-pathogenic properties of the combination of a T3SS inhibitory halogenated Pyrrolidone with C-30 Furanone. Molecules 26:7635. doi: 10.3390/molecules26247635
Ahmad, I., Lamprokostopoulou, A., Le Guyon, S., Streck, E., Barthel, M., Peters, V., et al. (2011). Complex c-di-GMP signaling networks mediate transition between virulence properties and biofilm formation in salmonella enterica serovar Typhimurium. PLoS One 6:e28351. doi: 10.1371/journal.pone.0028351
Ahmad, I., Wigren, E., Le Guyon, S., Vekkeli, S., Blanka, A., El Mouali, Y., et al. (2013). The EAL-like protein STM1697 regulates virulence phenotypes, motility and biofilm formation in salmonella typhimurium. Mol. Microbiol. 90, 1216–1232. doi: 10.1111/mmi.12428
Albutti, A., Gul, M. S., Siddiqui, M. F., Maqbool, F., Adnan, F., Ullah, I., et al. (2021). Combating biofilm by targeting its formation and dispersal using Gallic acid against single and multispecies bacteria causing dental plaque. Pathogens 10:1486. doi: 10.3390/pathogens10111486
Amaya, S., Pereira, J. A., Borkosky, S. A., Valdez, J. C., Bardón, A., and Arena, M. E. (2012). Inhibition of quorum sensing in Pseudomonas aeruginosa by sesquiterpene lactones. Phytomedicine 19, 1173–1177. doi: 10.1016/j.phymed.2012.07.003
Barraud, N., Schleheck, D., Klebensberger, J., Webb, J. S., Hassett, D. J., Rice, S. A., et al. (2009). Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 191, 7333–7342. doi: 10.1128/jb.00975-09
Bjarnsholt, T., Buhlin, K., Dufrêne, Y. F., Gomelsky, M., Moroni, A., Ramstedt, M., et al. (2018). Biofilm formation—what we can learn from recent developments. J. Intern. Med. 284, 332–345. doi: 10.1111/joim.12782
Boyd, C. D., and O’Toole, G. A. (2012). Second messenger regulation of biofilm formation: breakthroughs in understanding c-di-GMP effector systems. Annu. Rev. Cell Dev. Biol. 28, 439–462. doi: 10.1146/annurev-cellbio-101011-155705
Brackman, G., Breyne, K., De Rycke, R., Vermote, A., Van Nieuwerburgh, F., Meyer, E., et al. (2016). The quorum sensing inhibitor Hamamelitannin increases antibiotic susceptibility of Staphylococcus aureus biofilms by affecting peptidoglycan biosynthesis and eDNA release. Sci. Rep. 6:20321. doi: 10.1038/srep20321
Cai, T. T., Ye, X. L., Li, R. R., Chen, H., Wang, Y. Y., Yong, H. J., et al. (2020). Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front. Pharmacol. 11:1249. doi: 10.3389/fphar.2020.01249
Camilli, A., and Bassler, B. L. (2006). Bacterial small-molecule signaling pathways. Science 311, 1113–1116. doi: 10.1126/science.1121357
Cesmeli, S., Goker Bagca, B., Caglar, H. O., Ozates, N. P., Gunduz, C., and Biray Avci, C. (2021). Combination of resveratrol and BIBR1532 inhibits proliferation of colon cancer cells by repressing expression of LncRNAs. Med. Oncol. 39:12. doi: 10.1007/s12032-021-01611-w
Chaieb, K., Kouidhi, B., Hosawi, S. B., Baothman, O. A. S., Zamzami, M. A., and Altayeb, H. N. (2022). Computational screening of natural compounds as putative quorum sensing inhibitors targeting drug resistance bacteria: molecular docking and molecular dynamics simulations. Comput. Biol. Med. 145:105517. doi: 10.1016/j.compbiomed.2022.105517
Chang, Y., Wang, P. C., Ma, H. M., Chen, S. Y., Fu, Y. H., Liu, Y. Y., et al. (2019). Design, synthesis and evaluation of halogenated furanone derivatives as quorum sensing inhibitors in Pseudomonas aeruginosa. Eur. J. Pharm. Sci. 140:105058. doi: 10.1016/j.ejps.2019.105058
Chen, L., Brar, M. S., Leung, F. C., and Hsiao, W. L. (2016). Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice. Oncotarget 7, 31226–31242. doi: 10.18632/oncotarget.8886
Chen, C., Liao, X., Jiang, H., Zhu, H., Yue, L., Li, S., et al. (2010). Characteristics of Escherichia coli biofilm production, genetic typing, drug resistance pattern and gene expression under aminoglycoside pressures. Environ. Toxicol. Pharmacol. 30, 5–10. doi: 10.1016/j.etap.2010.03.004
Chen, Y., Liu, T., Wang, K., Hou, C., Cai, S., Huang, Y., et al. (2016). Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS One 11:e0153468. doi: 10.1371/journal.pone.0153468
Chen, X., Schauder, S., Potier, N., Van Dorsselaer, A., Pelczer, I., Bassler, B. L., et al. (2002). Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415, 545–549. doi: 10.1038/415545a
Chu, M., Xu, L., Zhang, M. B., Chu, Z. Y., and Wang, Y. D. (2015). Role of Baicalin in anti-influenza virus a as a potent inducer of IFN-gamma. Biomed. Res. Int. 2015:263630. doi: 10.1155/2015/263630
Commichau, F. M., Gibhardt, J., Halbedel, S., Gundlach, J., and Stülke, J. (2018). A delicate connection: c-di-AMP affects cell integrity by controlling Osmolyte transport. Trends Microbiol. 26, 175–185. doi: 10.1016/j.tim.2017.09.003
Costerton, J. W., Stewart, P. S., and Greenberg, E. P. (1999). Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. doi: 10.1126/science.284.5418.1318
da Aline Dias, P., de Nathalia Marins, A., deGabriel Guarany, A., de Robson Francisco, S., and Cristiane Rodrigues, G. (2020). The world of cyclic Dinucleotides in bacterial behavior. Molecules 25:2462. doi: 10.3390/molecules25102462
Daniels, R., Vanderleyden, J., and Michiels, J. (2004). Quorum sensing and swarming migration in bacteria. FEMS Microbiol. Rev. 28, 261–289. doi: 10.1016/j.femsre.2003.09.004
Datta, S., Jana, D., Maity, T. R., Samanta, A., and Banerjee, R. (2016). Piper betle leaf extract affects the quorum sensing and hence virulence of Pseudomonas aeruginosa PAO1. 3 Biotech 6:18. doi: 10.1007/s13205-015-0348-8
Davies, B. W., Bogard, R. W., Young, T. S., and Mekalanos, J. J. (2012). Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cells 149, 358–370. doi: 10.1016/j.cell.2012.01.053
Deng, G., Wu, Y., Song, Z., Li, S., Du, M., Deng, J., et al. (2022). Tea polyphenol liposomes overcome gastric mucus to treat helicobacter pylori infection and enhance the intestinal microenvironment. ACS Appl. Mater. Interfaces 14, 13001–13012. doi: 10.1021/acsami.1c23342
Deryabin, D., Galadzhieva, A., Kosyan, D., and Duskaev, G. (2019). Plant-derived inhibitors of AHL-mediated quorum sensing in bacteria: modes of action. Int. J. Mol. Sci. 20:5588. doi: 10.3390/ijms20225588
Dickschat, J. S. (2010). Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 27, 343–369. doi: 10.1039/b804469b
Dürig, A., Kouskoumvekaki, I., Vejborg, R. M., and Klemm, P. (2010). Chemoinformatics-assisted development of new anti-biofilm compounds. Appl. Microbiol. Biotechnol. 87, 309–317. doi: 10.1007/s00253-010-2471-0
ElNaggar, M. H., Eldehna, W. M., Abourehab, M. A. S., and Abdel Bar, F. M. (2022). The old world salsola as a source of valuable secondary metabolites endowed with diverse pharmacological activities: a review. J. Enzyme Inhib. Med. Chem. 37, 2036–2062. doi: 10.1080/14756366.2022.2102005
Fan, F. Y., Sang, L. X., and Jiang, M. (2017). Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 22:484. doi: 10.3390/molecules22030484
Fernicola, S., Paiardini, A., Giardina, G., Rampioni, G., Leoni, L., Cutruzzolà, F., et al. (2016). In silico discovery and in vitro validation of catechol-containing sulfonohydrazide compounds as potent inhibitors of the diguanylate cyclase PleD. J. Bacteriol. 198, 147–156. doi: 10.1128/jb.00742-15
Ferrazzano, G. F., Roberto, L., Amato, I., Cantile, T., Sangianantoni, G., and Ingenito, A. (2011). Antimicrobial properties of green tea extract against cariogenic microflora: an in vivo study. J. Med. Food 14, 907–911. doi: 10.1089/jmf.2010.0196
Flower, A., Wang, L. Q., Lewith, G., Liu, J. P., and Li, Q. (2015). Chinese herbal medicine for treating recurrent urinary tract infections in women. Cochrane Database Syst. Rev. 2015:Cd010446. doi: 10.1002/14651858.CD010446.pub2
Fontanay, S., Grare, M., Mayer, J., Finance, C., and Duval, R. E. (2008). Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 120, 272–276. doi: 10.1016/j.jep.2008.09.001
Gao, S., Zhang, S., and Zhang, S. (2021). Enhanced in vitro antimicrobial activity of amphotericin B with berberine against dual-species biofilms of Candida albicans and Staphylococcus aureus. J. Appl. Microbiol. 130, 1154–1172. doi: 10.1111/jam.14872
Gerhardt, E. C. M., Parize, E., Gravina, F., Pontes, F. L. D., Santos, A. R. S., Araújo, G. A. T., et al. (2020). The protein-protein interaction network reveals a novel role of the signal transduction protein PII in the control of c-di-GMP homeostasis in Azospirillum brasilense. mSystems 5:e00817-20. doi: 10.1128/mSystems.00817-20
Girennavar, B., Cepeda, M. L., Soni, K. A., Vikram, A., Jesudhasan, P., Jayaprakasha, G. K., et al. (2008). Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 125, 204–208. doi: 10.1016/j.ijfoodmicro.2008.03.028
Gopu, V., Meena, C. K., and Shetty, P. H. (2015). Quercetin influences quorum sensing in food borne bacteria: in-vitro and in-Silico evidence. PLoS One 10:e0134684. doi: 10.1371/journal.pone.0134684
Gries, C. M., Bruger, E. L., Moormeier, D. E., Scherr, T. D., Waters, C. M., and Kielian, T. (2016). Cyclic di-AMP released from Staphylococcus aureus biofilm induces a macrophage type I interferon response. Infect. Immun. 84, 3564–3574. doi: 10.1128/iai.00447-16
Gundlach, J., Rath, H., Herzberg, C., Mäder, U., and Stülke, J. (2016). Second messenger signaling in Bacillus subtilis: accumulation of cyclic di-AMP inhibits biofilm formation. Front. Microbiol. 7:804. doi: 10.3389/fmicb.2016.00804
Guo, X., Zhang, L. Y., Wu, S. C., Xia, F., Fu, Y. X., Wu, Y. L., et al. (2014). Andrographolide interferes quorum sensing to reduce cell damage caused by avian pathogenic Escherichia coli. Vet. Microbiol. 174, 496–503. doi: 10.1016/j.vetmic.2014.09.021
Hançer Aydemir, D., Çifci, G., Aviyente, V., and Boşgelmez-Tinaz, G. (2018). Quorum-sensing inhibitor potential of trans-anethole aganist Pseudomonas aeruginosa. J. Appl. Microbiol. 125, 731–739. doi: 10.1111/jam.13892
Hao, S., Yang, D., Zhao, L., Shi, F., Ye, G., Fu, H., et al. (2021). EGCG-mediated potential inhibition of biofilm development and quorum sensing in Pseudomonas aeruginosa. Int. J. Mol. Sci. 22:4946. doi: 10.3390/ijms22094946
Harman, J. G. (2001). Allosteric regulation of the cAMP receptor protein. Biochim. Biophys. Acta 1547, 1–17. doi: 10.1016/s0167-4838(01)00187-x
Hawas, S., Verderosa, A. D., and Totsika, M. (2022). Combination therapies for biofilm inhibition and eradication: a comparative review of laboratory and preclinical studies. Front. Cell. Infect. Microbiol. 12:850030. doi: 10.3389/fcimb.2022.850030
Hernández-Ramírez, K. C., Valerio-Arellano, B., Valle-Maldonado, M. I., Ruíz-Herrera, L. F., Meza-Carmen, V., and Ramírez-Díaz, M. I. (2020). Virulence conferred by PumA toxin from the plasmid-encoded PumAB toxin-antitoxin system is regulated by quorum system. Curr. Microbiol. 77, 2535–2543. doi: 10.1007/s00284-020-02083-3
Hufnagel, D. A., Evans, M. L., Greene, S. E., Pinkner, J. S., Hultgren, S. J., and Chapman, M. R. (2016). The Catabolite repressor protein-cyclic AMP complex regulates csgD and biofilm formation in uropathogenic Escherichia coli. J. Bacteriol. 198, 3329–3334. doi: 10.1128/jb.00652-16
Iobbi, V., Brun, P., Bernabé, G., Dougué Kentsop, R. A., Donadio, G., Ruffoni, B., et al. (2021). Labdane Diterpenoids from Salvia tingitana Etl. Synergize with clindamycin against methicillin-resistant Staphylococcus aureus. Molecules 26:6681. doi: 10.3390/molecules26216681
Irie, Y., and Parsek, M. R. (2008). Quorum sensing and microbial biofilms. Curr. Top. Microbiol. Immunol. 322, 67–84. doi: 10.1007/978-3-540-75418-3_4
Jia, F., Zhou, Q., Li, X., and Zhou, X. (2019). Total alkaloids of Sophora alopecuroides and matrine inhibit auto-inducer 2 in the biofilms of Staphylococcus epidermidis. Microb. Pathog. 136:103698. doi: 10.1016/j.micpath.2019.103698
Jing, W., Dong, S., Luo, X., Liu, J., Wei, B., Du, W., et al. (2021). Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites. Pharmacol. Res. 164:105358. doi: 10.1016/j.phrs.2020.105358
Joshi, J. R., Khazanov, N., Senderowitz, H., Burdman, S., Lipsky, A., and Yedidia, I. (2016). Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci. Rep. 6:38126. doi: 10.1038/srep38126
Junkermeier, E. H., and Hengge, R. (2021). A novel locally c-di-GMP-controlled exopolysaccharide synthase required for bacteriophage N4 infection of Escherichia coli. MBio 12:e0324921. doi: 10.1128/mbio.03249-21
Kang, J., Li, Q., Liu, L., Jin, W., Wang, J., and Sun, Y. (2018). The specific effect of gallic acid on Escherichia coli biofilm formation by regulating pgaABCD genes expression. Appl. Microbiol. Biotechnol. 102, 1837–1846. doi: 10.1007/s00253-017-8709-3
Kaplan, J. B., Ragunath, C., Ramasubbu, N., and Fine, D. H. (2003). Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity. J. Bacteriol. 185, 4693–4698. doi: 10.1128/jb.185.16.4693-4698.2003
Karumathil, D. P., Surendran-Nair, M., and Venkitanarayanan, K. (2016). Efficacy of trans-cinnamaldehyde and Eugenol in reducing Acinetobacter baumannii adhesion to and invasion of human keratinocytes and controlling wound infection in vitro. Phytother. Res. 30, 2053–2059. doi: 10.1002/ptr.5713
Karygianni, L., Ren, Z., Koo, H., and Thurnheer, T. (2020). Biofilm Matrixome: extracellular components in structured microbial communities. Trends Microbiol. 28, 668–681. doi: 10.1016/j.tim.2020.03.016
Kawaguchi, K., Kikuchi, S., Hasunuma, R., Maruyama, H., Ryll, R., and Kumazawa, Y. (2004a). Suppression of infection-induced endotoxin shock in mice by a citrus flavanone naringin. Planta Med. 70, 17–22. doi: 10.1055/s-2004-815449
Kawaguchi, K., Kikuchi, S., Hasunuma, R., Maruyama, H., Yoshikawa, T., and Kumazawa, Y. (2004b). A citrus flavonoid hesperidin suppresses infection-induced endotoxin shock in mice. Biol. Pharm. Bull. 27, 679–683. doi: 10.1248/bpb.27.679
Kim, H. S., Cha, E., Kim, Y., Jeon, Y. H., Olson, B. H., Byun, Y., et al. (2016). Raffinose, a plant galactoside, inhibits Pseudomonas aeruginosa biofilm formation via binding to LecA and decreasing cellular cyclic diguanylate levels. Sci. Rep. 6:25318. doi: 10.1038/srep25318
Kiran, M. D., Adikesavan, N. V., Cirioni, O., Giacometti, A., Silvestri, C., Scalise, G., et al. (2008). Discovery of a quorum-sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol. Pharmacol. 73, 1578–1586. doi: 10.1124/mol.107.044164
Kleerebezem, M., Quadri, L. E., Kuipers, O. P., and de Vos, W. M. (1997). Quorum sensing by peptide pheromones and two-component signal-transduction systems in gram-positive bacteria. Mol. Microbiol. 24, 895–904. doi: 10.1046/j.1365-2958.1997.4251782.x
Klinder, A., Shen, Q., Heppel, S., Lovegrove, J. A., Rowland, I., and Tuohy, K. M. (2016). Impact of increasing fruit and vegetables and flavonoid intake on the human gut microbiota. Food Funct. 7, 1788–1796. doi: 10.1039/c5fo01096a
Kong, D. X., Li, X. J., and Zhang, H. Y. (2009). Where is the hope for drug discovery? Let history tell the future. Drug Discov. Today 14, 115–119. doi: 10.1016/j.drudis.2008.07.002
Kortman, G. A., Roelofs, R. W., Swinkels, D. W., de Jonge, M. I., Burt, S. A., and Tjalsma, H. (2014). Iron-induced virulence of salmonella enterica serovar typhimurium at the intestinal epithelial interface can be suppressed by carvacrol. Antimicrob. Agents Chemother. 58, 1664–1670. doi: 10.1128/aac.02060-13
Kumar, L., Chhibber, S., Kumar, R., Kumar, M., and Harjai, K. (2015). Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia 102, 84–95. doi: 10.1016/j.fitote.2015.02.002
Larghi, E. L., Bracca, A. B., Arroyo Aguilar, A. A., Heredia, D. A., Pergomet, J. L., Simonetti, S. O., et al. (2015). Neocryptolepine: a promising indoloisoquinoline alkaloid with interesting biological activity. Evaluation of the drug and its most relevant analogs. Curr. Top. Med. Chem. 15, 1683–1707. doi: 10.2174/1568026615666150427113937
Lee, Y. J., Beak, S. Y., Choi, I., and Sung, J. S. (2018). Quercetin and its metabolites protect hepatocytes against ethanol-induced oxidative stress by activation of Nrf2 and AP-1. Food Sci. Biotechnol. 27, 809–817. doi: 10.1007/s10068-017-0287-8
Lee, J., Wu, J., Deng, Y., Wang, J., Wang, C., Wang, J., et al. (2013). A cell-cell communication signal integrates quorum sensing and stress response. Nat. Chem. Biol. 9, 339–343. doi: 10.1038/nchembio.1225
Lemos, J. A., Brown, T. A. Jr., and Burne, R. A. (2004). Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infect. Immun. 72, 1431–1440. doi: 10.1128/iai.72.3.1431-1440.2004
Li, F., Cao, L., Bähre, H., Kim, S. K., Schroeder, K., Jonas, K., et al. (2022). Patatin-like phospholipase CapV in Escherichia coli—morphological and physiological effects of one amino acid substitution. NPJ Biofilms Microbiomes 8:39. doi: 10.1038/s41522-022-00294-z
Li, F., Cimdins, A., Rohde, M., Jänsch, L., Kaever, V., Nimtz, M., et al. (2019). DncV synthesizes cyclic GMP-AMP and regulates biofilm formation and motility in Escherichia coli ECOR31. mBio 10:e02492-18. doi: 10.1128/mBio.02492-18
Li, W. R., Ma, Y. K., Xie, X. B., Shi, Q. S., Wen, X., Sun, T. L., et al. (2018). Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa quorum sensing systems and corresponding virulence factors. Front. Microbiol. 9:3222. doi: 10.3389/fmicb.2018.03222
Liao, W., Huang, L., Han, S., Hu, D., Xu, Y., Liu, M., et al. (2022). Review of medicinal plants and active pharmaceutical ingredients against aquatic pathogenic viruses. Viruses 14, 1281. doi: 10.3390/v14061281.
Lin, M. H., Chang, F. R., Hua, M. Y., Wu, Y. C., and Liu, S. T. (2011). Inhibitory effects of 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose on biofilm formation by Staphylococcus aureus. Antimicrob. Agents Chemother. 55, 1021–1027. doi: 10.1128/aac.00843-10
Linder, J. U. (2010). cGMP production in bacteria. Mol. Cell. Biochem. 334, 215–219. doi: 10.1007/s11010-009-0321-0
Liu, G., Xiang, H., Tang, X., Zhang, K., Wu, X., Wang, X., et al. (2011). Transcriptional and functional analysis shows sodium houttuyfonate-mediated inhibition of autolysis in Staphylococcus aureus. Molecules 16, 8848–8865. doi: 10.3390/molecules16108848
Liu, S., Zhu, J. J., and Li, J. C. (2021). The interpretation of human body in traditional Chinese medicine and its influence on the characteristics of TCM theory. Anat Rec (Hoboken) 304, 2559–2565. doi: 10.1002/ar.24643
Long, N., Tang, H., Sun, F., Lin, L., and Dai, M. (2019). Effect and mechanism of citral against methicillin-resistant Staphylococcus aureus in vivo. J. Sci. Food Agric. 99, 4423–4429. doi: 10.1002/jsfa.9677
Luo, Y., Zhao, K., Baker, A. E., Kuchma, S. L., Coggan, K. A., Wolfgang, M. C., et al. (2015). A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. MBio 6:e02456-14. doi: 10.1128/mBio.02456-14
Ma, J., Li, M., Kalavagunta, P. K., Li, J., He, Q., Zhang, Y., et al. (2018). Protective effects of cichoric acid on H(2)O(2)-induced oxidative injury in hepatocytes and larval zebrafish models. Biomed. Pharmacother. 104, 679–685. doi: 10.1016/j.biopha.2018.05.081
Ma, Y., Shi, Q., He, Q., and Chen, G. (2021). Metabolomic insights into the inhibition mechanism of methyl N-methylanthranilate: a novel quorum sensing inhibitor and antibiofilm agent against Pseudomonas aeruginosa. Int. J. Food Microbiol. 358:109402. doi: 10.1016/j.ijfoodmicro.2021.109402
Meng, F., Zhang, F., Chen, Q., Yang, M., Yang, Y., Li, X., et al. (2022). Virtual screening and in vitro experimental verification of LuxS inhibitors from natural products for Lactobacillus reuteri. Biomed. Pharmacother. 147:112521. doi: 10.1016/j.biopha.2021.112521
Miller, A. L., Nicastro, L. K., Bessho, S., Grando, K., White, A. P., Zhang, Y., et al. (2022). Nitrate is an environmental Cue in the gut for salmonella enterica Serovar Typhimurium biofilm dispersal through Curli repression and flagellum activation via cyclic-di-GMP signaling. MBio 13:e0288621. doi: 10.1128/mbio.02886-21
Ming, D., Wang, D., Cao, F., Xiang, H., Mu, D., Cao, J., et al. (2017). Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 8:2263. doi: 10.3389/fmicb.2017.02263
Minich, A., Levarski, Z., Mikulášová, M., Straka, M., Liptáková, A., and Stuchlík, S. (2022). Complex analysis of vanillin and syringic acid as natural antimicrobial agents against Staphylococcus epidermidis biofilms. Int. J. Mol. Sci. 23:1816. doi: 10.3390/ijms23031816
Mooney, E. C., Holden, S. E., Xia, X. J., Li, Y., Jiang, M., Banson, C. N., et al. (2021). Quercetin preserves Oral cavity health by mitigating inflammation and microbial Dysbiosis. Front. Immunol. 12:774273. doi: 10.3389/fimmu.2021.774273
Motta, J. P., Flannigan, K. L., Agbor, T. A., Beatty, J. K., Blackler, R. W., Workentine, M. L., et al. (2015). Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm. Bowel Dis. 21, 1006–1017. doi: 10.1097/mib.0000000000000345
Mousavi, S., Schmidt, A. M., Escher, U., Kittler, S., Kehrenberg, C., Thunhorst, E., et al. (2020). Carvacrol ameliorates acute campylobacteriosis in a clinical murine infection model. Gut Pathog 12:2. doi: 10.1186/s13099-019-0343-4
Muhammad, J. S., Zaidi, S. F., Shaharyar, S., Refaat, A., Usmanghani, K., Saiki, I., et al. (2015). Anti-inflammatory effect of cinnamaldehyde in helicobacter pylori induced gastric inflammation. Biol. Pharm. Bull. 38, 109–115. doi: 10.1248/bpb.b14-00609
Nealson, K. H., Platt, T., and Hastings, J. W. (1970). Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 104, 313–322. doi: 10.1128/jb.104.1.313-322.1970
Ning, Y., Wang, X., Chen, P., Liu, S., Hu, J., Xiao, R., et al. (2022). Targeted inhibition of methicillin-resistant Staphylococcus aureus biofilm formation by a graphene oxide-loaded aptamer/berberine bifunctional complex. Drug Deliv. 29, 1675–1683. doi: 10.1080/10717544.2022.2079768
Ohana, P., Delmer, D. P., Carlson, R. W., Glushka, J., Azadi, P., Bacic, T., et al. (1998). Identification of a novel triterpenoid saponin from Pisum sativum as a specific inhibitor of the diguanylate cyclase of Acetobacter xylinum. Plant Cell Physiol. 39, 144–152. doi: 10.1093/oxfordjournals.pcp.a029351
Opoku-Temeng, C., and Sintim, H. O. (2016). Inhibition of cyclic diadenylate cyclase, DisA, by polyphenols. Sci. Rep. 6:25445. doi: 10.1038/srep25445
Opoku-Temeng, C., Zhou, J., Zheng, Y., Su, J., and Sintim, H. O. (2016). Cyclic dinucleotide (c-di-GMP, c-di-AMP, and cGAMP) signalings have come of age to be inhibited by small molecules. Chem. Commun. (Camb.) 52, 9327–9342. doi: 10.1039/c6cc03439j
Ou, Q., Fan, J., Duan, D., Xu, L., Wang, J., Zhou, D., et al. (2017). Involvement of cAMP receptor protein in biofilm formation, fimbria production, capsular polysaccharide biosynthesis and lethality in mouse of Klebsiella pneumoniae serotype K1 causing pyogenic liver abscess. J. Med. Microbiol. 66, 1–7. doi: 10.1099/jmm.0.000391
Ouyang, J., Sun, F., Feng, W., Sun, Y., Qiu, X., Xiong, L., et al. (2016). Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 120, 966–974. doi: 10.1111/jam.13073
Packiavathy, I. A., Priya, S., Pandian, S. K., and Ravi, A. V. (2014). Inhibition of biofilm development of uropathogens by curcumin - an anti-quorum sensing agent from Curcuma longa. Food Chem. 148, 453–460. doi: 10.1016/j.foodchem.2012.08.002
Panahi, Y., Karbasi, A., Valizadegan, G., Ostadzadeh, N., Soflaei, S. S., Jamialahmadi, T., et al. (2021). Effect of curcumin on severity of functional dyspepsia: a triple blinded clinical trial. Adv. Exp. Med. Biol. 1308, 119–126. doi: 10.1007/978-3-030-64872-5_10
Pandey, A. R., Ahmad, S., Singh, S. P., Mishra, A., Bisen, A. C., Sharma, G., et al. (2022). Furostanol saponins from Asparagus racemosus as potential hypoglycemic agents. Phytochemistry 201:113286. doi: 10.1016/j.phytochem.2022.113286
Parsek, M. R., and Greenberg, E. P. (2000). Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. U. S. A. 97, 8789–8793. doi: 10.1073/pnas.97.16.8789
Passos, M. R., Almeida, R. S., Lima, B. O., Rodrigues, J. Z. S., Macêdo Neres, N. S., Pita, L. S., et al. (2021). Anticariogenic activities of Libidibia ferrea, gallic acid and ethyl gallate against Streptococcus mutans in biofilm model. J. Ethnopharmacol. 274:114059. doi: 10.1016/j.jep.2021.114059
Pederson, D. B., Dong, Y., Blue, L. B., Smith, S. V., and Cao, M. (2018). Water-soluble cranberry extract inhibits Vibrio cholerae biofilm formation possibly through modulating the second messenger 3′, 5′ - cyclic diguanylate level. PLoS One 13:e0207056. doi: 10.1371/journal.pone.0207056
Peng, L. Y., Shi, H. T., Tan, Y. R., Shen, S. Y., Yi, P. F., Shen, H. Q., et al. (2021b). Baicalin inhibits APEC-induced lung injury by regulating gut microbiota and SCFA production. Food Funct. 12, 12621–12633. doi: 10.1039/d1fo02407h
Peng, L. Y., Yuan, M., Wu, Z. M., Song, K., Zhang, C. L., An, Q., et al. (2019). Anti-bacterial activity of baicalin against APEC through inhibition of quorum sensing and inflammatory responses. Sci. Rep. 9:4063. doi: 10.1038/s41598-019-40684-6
Peng, X., Zhang, Y., Bai, G., Zhou, X., and Wu, H. (2016). Cyclic di-AMP mediates biofilm formation. Mol. Microbiol. 99, 945–959. doi: 10.1111/mmi.13277
Peng, F., Zhang, H., He, X., and Song, Z. (2021a). Effects of ursolic acid on intestinal health and gut bacteria antibiotic resistance in mice. Front. Physiol. 12:650190. doi: 10.3389/fphys.2021.650190
Pourahmad Jaktaji, R., and Koochaki, S. (2022). In vitro activity of honey, total alkaloids of Sophora alopecuroides and matrine alone and in combination with antibiotics against multidrug-resistant Pseudomonas aeruginosa isolates. Lett. Appl. Microbiol. 75, 70–80. doi: 10.1111/lam.13705
Pruteanu, M., Hernández Lobato, J. I., Stach, T., and Hengge, R. (2020). Common plant flavonoids prevent the assembly of amyloid curli fibres and can interfere with bacterial biofilm formation. Environ. Microbiol. 22, 5280–5299. doi: 10.1111/1462-2920.15216
Qin, Z., Ou, Y., Yang, L., Zhu, Y., Tolker-Nielsen, T., Molin, S., et al. (2007). Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology (Reading) 153, 2083–2092. doi: 10.1099/mic.0.2007/006031-0
Qin, N., Tan, X., Jiao, Y., Liu, L., Zhao, W., Yang, S., et al. (2014). RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci. Rep. 4:5467. doi: 10.1038/srep05467
Rabin, N., Delago, A., Inbal, B., Krief, P., and Meijler, M. M. (2013). Tailor-made LasR agonists modulate quorum sensing in Pseudomonas aeruginosa. Org. Biomol. Chem. 11, 7155–7163. doi: 10.1039/c3ob41377b
Reading, N. C., and Sperandio, V. (2006). Quorum sensing: the many languages of bacteria. FEMS Microbiol. Lett. 254, 1–11. doi: 10.1111/j.1574-6968.2005.00001.x
Reuter, K., Steinbach, A., and Helms, V. (2016). Interfering with bacterial quorum sensing. Perspect Medicin Chem. 8, 1–15. doi: 10.4137/pmc.S13209
Reyes, A. W. B., Arayan, L. T., Hop, H. T., Ngoc Huy, T. X., Vu, S. H., Min, W., et al. (2018). Effects of gallic acid on signaling kinases in murine macrophages and immune modulation against Brucella abortus 544 infection in mice. Microb. Pathog. 119, 255–259. doi: 10.1016/j.micpath.2018.04.032
Römling, U., Galperin, M. Y., and Gomelsky, M. (2013). Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 77, 1–52. doi: 10.1128/mmbr.00043-12
Ross, P., Weinhouse, H., Aloni, Y., Michaeli, D., Weinberger-Ohana, P., Mayer, R., et al. (1987). Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325, 279–281. doi: 10.1038/325279a0
Roy, R., Tiwari, M., Donelli, G., and Tiwari, V. (2018). Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9, 522–554. doi: 10.1080/21505594.2017.1313372
Sambanthamoorthy, K., Luo, C., Pattabiraman, N., Feng, X., Koestler, B., Waters, C. M., et al. (2014). Identification of small molecules inhibiting diguanylate cyclases to control bacterial biofilm development. Biofouling 30, 17–28. doi: 10.1080/08927014.2013.832224
Serra, D. O., Mika, F., Richter, A. M., and Hengge, R. (2016). The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σ(E) -dependent sRNA RybB. Mol. Microbiol. 101, 136–151. doi: 10.1111/mmi.13379
Sharma, K., Bose, S. K., Chhibber, S., and Harjai, K. (2020). Exploring the therapeutic efficacy of Zingerone nanoparticles in treating biofilm-associated pyelonephritis caused by Pseudomonas aeruginosa in the murine model. Inflammation 43, 2344–2356. doi: 10.1007/s10753-020-01304-y
Shukla, A., Parmar, P., Rao, P., Goswami, D., and Saraf, M. (2020). Twin peaks: presenting the antagonistic molecular interplay of curcumin with LasR and LuxR quorum sensing pathways. Curr. Microbiol. 77, 1800–1810. doi: 10.1007/s00284-020-01997-2
Siddiqui, M. F., Sakinah, M., Ismail, A. F., Matsuura, T., and Zularisam, A. W. (2012). The anti-biofouling effect of Piper betle extract against Pseudomonas aeruginosa and bacterial consortium. Desalination 288, 24–30. doi: 10.1016/j.desal.2011.11.060
Silva, A., Genovés, S., Martorell, P., Zanini, S. F., Rodrigo, D., and Martinez, A. (2015). Sublethal injury and virulence changes in listeria monocytogenes and listeria innocua treated with antimicrobials carvacrol and citral. Food Microbiol. 50, 5–11. doi: 10.1016/j.fm.2015.02.016
Simm, R., Morr, M., Kader, A., Nimtz, M., and Römling, U. (2004). GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53, 1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x
Singh, R., and Ray, P. (2014). Quorum sensing-mediated regulation of staphylococcal virulence and antibiotic resistance. Future Microbiol. 9, 669–681. doi: 10.2217/fmb.14.31
Skogman, M. E., Kanerva, S., Manner, S., Vuorela, P. M., and Fallarero, A. (2016). Flavones as quorum sensing inhibitors identified by a newly optimized screening platform using Chromobacterium violaceum as reporter bacteria. Molecules 21:1211. doi: 10.3390/molecules21091211
Sun, X. Y., Jia, L. Y., Rong, Z., Zhou, X., Cao, L. Q., Li, A. H., et al. (2022). Research advances on Matrine. Front. Chem. 10:867318. doi: 10.3389/fchem.2022.867318
Sun, T., Li, X. D., Hong, J., Liu, C., Zhang, X. L., Zheng, J. P., et al. (2019). Inhibitory effect of two traditional Chinese medicine monomers, Berberine and Matrine, on the quorum sensing system of antimicrobial-resistant Escherichia coli. Front. Microbiol. 10:2584. doi: 10.3389/fmicb.2019.02584
Tang, J. L., Liu, B. Y., and Ma, K. W. (2008). Traditional Chinese medicine. Lancet 372, 1938–1940. doi: 10.1016/s0140-6736(08)61354-9
Townsley, L., Yannarell, S. M., Huynh, T. N., Woodward, J. J., and Shank, E. A. (2018). Cyclic di-AMP acts as an extracellular signal that impacts Bacillus subtilis biofilm formation and plant attachment. mBio 9:e00341-18. doi: 10.1128/mBio.00341-18
Truchado, P., Giménez-Bastida, J. A., Larrosa, M., Castro-Ibáñez, I., Espín, J. C., Tomás-Barberán, F. A., et al. (2012). Inhibition of quorum sensing (QS) in Yersinia enterocolitica by an orange extract rich in glycosylated flavanones. J. Agric. Food Chem. 60, 8885–8894. doi: 10.1021/jf301365a
van Delden, C., Comte, R., and Bally, A. M. (2001). Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183, 5376–5384. doi: 10.1128/jb.183.18.5376-5384.2001
Vandeputte, O. M., Kiendrebeogo, M., Rajaonson, S., Diallo, B., Mol, A., El Jaziri, M., et al. (2010). Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 76, 243–253. doi: 10.1128/aem.01059-09
Vermote, A., Brackman, G., Risseeuw, M. D., Vanhoutte, B., Cos, P., Van Hecke, K., et al. (2016). Hamamelitannin analogues that modulate quorum sensing as Potentiators of antibiotics against Staphylococcus aureus. Angew. Chem. Int. Ed. Engl. 55, 6551–6555. doi: 10.1002/anie.201601973
Vidigal, P. G., Müsken, M., Becker, K. A., Häussler, S., Wingender, J., Steinmann, E., et al. (2014). Effects of green tea compound epigallocatechin-3-gallate against Stenotrophomonas maltophilia infection and biofilm. PLoS One 9:e92876. doi: 10.1371/journal.pone.0092876
Wang, J., Jiao, H., Meng, J., Qiao, M., Du, H., He, M., et al. (2019). Baicalin inhibits biofilm formation and the quorum-sensing system by regulating the MsrA drug efflux pump in Staphylococcus saprophyticus. Front. Microbiol. 10:2800. doi: 10.3389/fmicb.2019.02800
Wang, R., Li, S., Jia, H., Si, X., Lei, Y., Lyu, J., et al. (2021). Protective effects of Cinnamaldehyde on the inflammatory response, oxidative stress, and apoptosis in liver of salmonella typhimurium-challenged mice. Molecules 26:2309. doi: 10.3390/molecules26082309
Westfall, S., Lomis, N., and Prakash, S. (2018). A polyphenol-rich prebiotic in combination with a novel probiotic formulation alleviates markers of obesity and diabetes in Drosophila. Journal of Functional Foods 48, 374–386. doi: 10.1016/j.jff.2018.07.012
Witte, C. E., Whiteley, A. T., Burke, T. P., Sauer, J. D., Portnoy, D. A., and Woodward, J. J. (2013). Cyclic di-AMP is critical for listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. mBio 4:e00282. doi: 10.1128/mBio.00282-13
Wu, K., Fu, M., Zhao, Y., Gerhard, E., Li, Y., Yang, J., et al. (2023). Anti-oxidant anti-inflammatory and antibacterial tannin-crosslinked citrate-based mussel-inspired bioadhesives facilitate scarless wound healing. Bioact Mater 20, 93–110. doi: 10.1016/j.bioactmat.2022.05.017
Wu, H. Z., Luo, J., Yin, Y. X., and Wei, Q. (2004). Effects of chlorogenic acid, an active compound activating calcineurin, purified from Flos Lonicerae on macrophage. Acta Pharmacol. Sin. 25, 1685–1689. doi: 10.1111/lam.13705
Wu, X., Kang, J., Xie, C., Burris, R., Ferguson, M. E., Badger, T. M., et al. (2010). Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J Nutr. 140, 1628–1632. doi: 10.3945/jn.110.123927
Wu, H., Moser, C., Wang, H. Z., Høiby, N., and Song, Z. J. (2015). Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 7, 1–7. doi: 10.1038/ijos.2014.65
Wu, C. Y., Su, T. Y., Wang, M. Y., Yang, S. F., Mar, K., and Hung, S. L. (2018). Inhibitory effects of tea catechin epigallocatechin-3-gallate against biofilms formed from Streptococcus mutans and a probiotic lactobacillus strain. Arch. Oral Biol. 94, 69–77. doi: 10.1016/j.archoralbio.2018.06.019
Xu, C., Weston, B. R., Tyson, J. J., and Cao, Y. (2020). Cell cycle control and environmental response by second messengers in Caulobacter crescentus. BMC Bioinformatics 21:408. doi: 10.1186/s12859-020-03687-z
Yang, Q., and Defoirdt, T. (2015). Quorum sensing positively regulates flagellar motility in pathogenic Vibrio harveyi. Environ. Microbiol. 17, 960–968. doi: 10.1111/1462-2920.12420
Yao, H., Shi, Y., Yuan, J., Sa, R., Chen, W., and Wan, X. (2021). Matrine protects against DSS-induced murine colitis by improving gut barrier integrity, inhibiting the PPAR-α signaling pathway, and modulating gut microbiota. Int. Immunopharmacol. 100:108091. doi: 10.1016/j.intimp.2021.108091
Yazdanparast, R., Bahramikia, S., and Ardestani, A. (2008). Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem. Biol. Interact 172, 176–184. doi: 10.1016/j.cbi.2008.01.006
Yin, W., Xu, S., Wang, Y., Zhang, Y., Chou, S. H., Galperin, M. Y., et al. (2021). Ways to control harmful biofilms: prevention, inhibition, and eradication. Crit. Rev. Microbiol. 47, 57–78. doi: 10.1080/1040841x.2020.1842325
Yu, T., Jiang, X., Xu, X., Jiang, C., Kang, R., and Jiang, X. (2022). Andrographolide inhibits biofilm and virulence in listeria monocytogenes as a quorum-sensing inhibitor. Molecules 27:3234. doi: 10.3390/molecules27103234
Zeng, Z., Qian, L., Cao, L., Tan, H., Huang, Y., Xue, X., et al. (2008). Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 79, 119–126. doi: 10.1007/s00253-008-1406-5
Zhang, L., Li, W., and He, Z. G. (2013). DarR, a TetR-like transcriptional factor, is a cyclic di-AMP-responsive repressor in mycobacterium smegmatis. J. Biol. Chem. 288, 3085–3096. doi: 10.1074/jbc.M112.428110
Zhang, Y., Sass, A., Van Acker, H., Wille, J., Verhasselt, B., Van Nieuwerburgh, F., et al. (2018). Coumarin reduces virulence and biofilm formation in Pseudomonas aeruginosa by affecting quorum sensing, type III secretion and C-di-GMP levels. Front. Microbiol. 9:1952. doi: 10.3389/fmicb.2018.01952
Zhang, W., Xu, J. H., Yu, T., and Chen, Q. K. (2019). Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 118:109131. doi: 10.1016/j.biopha.2019.109131
Zhang, H., Zhou, W., Zhang, W., Yang, A., Liu, Y., Jiang, Y., et al. (2014). Inhibitory effects of citral, cinnamaldehyde, and tea polyphenols on mixed biofilm formation by foodborne Staphylococcus aureus and Salmonella enteritidis. J. Food Prot. 77, 927–933. doi: 10.4315/0362-028x.Jfp-13-497
Zhao, Z., Feng, M., Wan, J., Zheng, X., Teng, C., Xie, X., et al. (2021). Research progress of epigallocatechin-3-gallate (EGCG) on anti-pathogenic microbes and immune regulation activities. Food Funct. 12, 9607–9619. doi: 10.1039/d1fo01352a
Zhao, W., Lorenz, N., Jung, K., and Sieber, S. A. (2016). Fimbrolide natural products disrupt bioluminescence of vibrio by targeting autoinducer biosynthesis and luciferase activity. Angew. Chem. Int. Ed. Engl. 55, 1187–1191. doi: 10.1002/anie.201508052
Zhao, X., Zheng, S., Wei, S., Tian, Q., Tao, Y., Bo, R., et al. (2022). The protective effect and potential mechanisms of eugenol against salmonella in vivo and in vitro. Poult. Sci. 101:101801. doi: 10.1016/j.psj.2022.101801
Zheng, Y., Tsuji, G., Opoku-Temeng, C., and Sintim, H. O. (2016). Inhibition of P. aeruginosa c-di-GMP phosphodiesterase RocR and swarming motility by a benzoisothiazolinone derivative. Chem. Sci. 7, 6238–6244. doi: 10.1039/c6sc02103d
Zhou, J., Watt, S., Wang, J., Nakayama, S., Sayre, D. A., Lam, Y. F., et al. (2013). Potent suppression of c-di-GMP synthesis via I-site allosteric inhibition of diguanylate cyclases with 2'-F-c-di-GMP. Bioorg. Med. Chem. 21, 4396–4404. doi: 10.1016/j.bmc.2013.04.050
Zhou, L., Zhang, L. H., Cámara, M., and He, Y. W. (2017). The DSF family of quorum sensing signals: diversity, biosynthesis, and turnover. Trends Microbiol. 25, 293–303. doi: 10.1016/j.tim.2016.11.013
Zhou, L., Zhang, Y., Ge, Y., Zhu, X., and Pan, J. (2020). Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front. Microbiol. 11:589640. doi: 10.3389/fmicb.2020.589640
Keywords: biofilm formation, traditional Chinese medicine, anti-biofilm agents, quorum sensing, second messenger
Citation: Zhang M, Han W, Gu J, Qiu C, Jiang Q, Dong J, Lei L and Li F (2022) Recent advances on the regulation of bacterial biofilm formation by herbal medicines. Front. Microbiol. 13:1039297. doi: 10.3389/fmicb.2022.1039297
Received: 08 September 2022; Accepted: 18 October 2022;
Published: 08 November 2022.
Edited by:
Abdelali Daddaoua, University of Granada, SpainReviewed by:
Mahmoud M. Tawfick, Al-Azhar University, EgyptCopyright © 2022 Zhang, Han, Gu, Qiu, Jiang, Dong, Lei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengyang Li, ZnlsZWUxOTg3QG91dGxvb2suY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.