- 1School of Life Sciences, Ludong University, Yantai, China
- 2Yantai Key Laboratory of Animal Pathogenetic Microbiology and Immunology, Yantai, China
- 3Shandong Provincial Key Laboratory of Quality Safety Monitoring and Risk Assessment for Animal Products, Jinan, China
- 4Shandong Breeding Environmental Control Engineering Laboratory, Yantai, China
Extracellular vesicles (EVs) are nanoscale membrane-enveloped vesicles secreted by prokaryotic and eukaryotic cells, which are commonly defined as membrane vesicles (MVs) and exosomes, respectively. They play critical roles in the bacteria–bacteria and bacteria–host interactions. In infectious diseases caused by bacteria, as the first line of defense against pathogens, the macrophage polarization mode commonly determines the success or failure of the host's response to pathogen aggression. M1-type macrophages secrete pro-inflammatory factors that support microbicidal activity, while alternative M2-type macrophages secrete anti-inflammatory factors that perform an antimicrobial immune response but partially allow pathogens to replicate and survive intracellularly. Membrane vesicles (MVs) released from bacteria as a distinctive secretion system can carry various components, including bacterial effectors, nucleic acids, or lipids to modulate macrophage polarization in host–pathogen interaction. Similar to MVs, bacteria-infected macrophages can secrete exosomes containing a variety of components to manipulate the phenotypic polarization of “bystander” macrophages nearby or long distance to differentiate into type M1 or M2 to regulate the course of inflammation. Exosomes can also repair tissue damage associated with the infection by upregulating the levels of anti-inflammatory factors, downregulating the pro-inflammatory factors, and regulating cellular biological behaviors. The study of the mechanisms by which EVs modulate macrophage polarization has opened new frontiers in delineating the molecular machinery involved in bacterial pathogenesis and challenges in providing new strategies for diagnosis and therapy.
Introduction
Macrophages are heterogeneous cells, distributed in various tissues and organs, and participate in innate immunity, antigen presentation, and anti-infectious immune regulation (Murray, 2017). During the inflammatory responses evoked by microorganisms, they can be activated and differentiated into two subtypes with distinct phenotypes and functions under the induction of microenvironment (Essandoh et al., 2016). M1 macrophages, known as an inflammatory subtype, have a strong bactericidal function by releasing pro-inflammatory cytokines, while excessive activation can also aggravate inflammatory responses, lead to tissue injury, and contribute to pathogenesis. M2 macrophages, also known as an anti-inflammatory subtype, can promote tissue repair and immune regulation by secreting anti-inflammatory or immunomodulatory cytokines. In the development of inflammation, these two subtypes are in a dynamic equilibrium and can convert into each other under certain conditions (Smith et al., 2016). There are many factors leading to different polarization of macrophages, including physiological and pathological environments, microbes and their products, cytokines, and activated lymphocytes (Lawrence and Natoli, 2011). In recent years, converging researchers have reported that the potential biological function of extracellular vesicles (EVs) released from cells in vivo and in vitro exerts essential roles in macrophage polarization to maintain physiological homeostasis (Schorey et al., 2015; Jurkoshek et al., 2016; Jones et al., 2018; Furuyama and Sircili, 2021).
Extracellular vesicles are small membranous vesicles secreted to the extracellular environment by various types of eukaryotic cells including immune and non-immune cells (Denzer et al., 2000). They can be used as carriers for information exchange and transmission between cells, modulate cellular activities, and reprogram the phenotype in recipient cells (Schorey et al., 2015). EVs contain abundant biomolecules, which can modulate the balance of M1/M2 macrophages polarization through different pathways in inflammatory diseases such as tuberculosis (TB), Crohn's disease (CD), sepsis, and pneumonia (Saadatpour et al., 2016). Bacteria can also secrete EVs, which are known as bacterial membrane vesicles (BMVs), containing distinct components including toxins, virulence factors, nucleic acids, and other molecules that promote survival in the host and modulate the host immune response positively or negatively (Jurkoshek et al., 2016; Owen et al., 2016; Jones et al., 2018). Some EVs stimulate innate and adaptive immune responses and promote major histocompatibility complex (MHC) class II antigen presentation of dendritic cells to eliminate bacterial infection (Jurkoshek et al., 2016; Cheng and Schorey, 2019; Lee et al., 2020). In contrast, other vesicles can inhibit the activation of naïve macrophages and suppress the expression of MHC-II in mouse bone marrow-derived macrophages (BMMs) or activate M2 macrophages, which play an anti-inflammatory role and contribute to bacteria evading from surveillance of the immune system (Singh et al., 2011; Ti et al., 2015; Furuyama and Sircili, 2021). Therefore, the regulation of macrophage polarization by EVs from varying sources is a double-edged sword. Revealing the composition of EVs in the course of bacterial infection and the mechanism of how these vesicles promote macrophage polarization can clearly help understand the development of inflammation progress and provide a new strategy for preventing microbe-induced inflammation aggravation. In this review, we systematically summarize the current state of knowledge about the roles of EVs secreted from both bacteria and bacteria-infected cells in modulating macrophage polarization in various inflammatory diseases caused by common bacteria and enumerate the possible mechanisms of how these EVs activated macrophages differentiation, which can be used as a diagnostic biomarker or target for the prevention and treatment of bacterial infection.
Mechanism of macrophage polarization
Macrophages are plastic and heterogeneous cells due to different mechanisms governing their differentiation. Tissue distribution with different microenvironments, such as intestines, alveolar space, or adipose tissue, may also constrain the functional properties of macrophages (Benoit et al., 2008). The presence or absence of microbial infection in vivo or in vitro is also necessary for macrophage polarization. Although the use of the terms M1 and M2 remains controversial due to the lack of a tightly defined criterion for scoring the increasing number of activated macrophage subtypes, efforts to define polarization are ongoing, and polarized macrophages have been a partial consensus to be classified into two groups: M1 and M2 macrophages (Murray et al., 2014; Murray, 2017). Macrophages typically exist between these two groups, as the polarization process is dynamic and cells often display characteristics of both states simultaneously. Summarizing the key experimental findings on macrophage polarization in bacterial infection will attempt to define some of the major questions in this field.
M1 activation in bacterial infection
Macrophage polarization can occur at any point in an inflammatory process and can also be typically evoked in vitro by treating cells with lipopolysaccharide (LPS) and/or interferon-γ (IFN-γ). Numerous studies suggest that macrophages exposed to pathogens respond with common transcriptional activation programs. In the early course of infection, as the first line of defense against pathogens, macrophages can recognize and respond rapidly to invading microbes by the expression of pattern recognition receptors (PRRs) such as toll-like receptor 2 (TLR2) or TLR4 and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), which show increased susceptibility to gram-negative and gram-positive bacterial infections, respectively (Elson et al., 2007). Then macrophages are activated to secrete inflammation-related cytokines such as interleukin-6 (IL-6), IL-1β, tumor necrosis factor-α (TNF-α), and chemokines such as C–C motif chemokine 2 (CCL2) and CCL5 to recruit more macrophages and neutrophils to eliminate invasive microorganisms (Murray, 2017). CD86 and CD80 are also expressed in M1-polarized macrophages as surface markers, and iNOS (also called NO synthase 2, NOS2) is upregulated to synthesize more NO, which is an important messenger and effector molecule in the defense system of macrophages (Jungi et al., 1997; Barley et al., 2022). These factors potently contribute to the establishment of a pro-inflammatory activation state, which is commonly referred to as classical M1 macrophage activation (Bhatnagar et al., 2007; Hui et al., 2018). The activation can be further induced by cytokines such as IFN-γ released from activated T helper 1 (Th1) cells. Nuclear factor-kappa B (NF-κB) and signal transducer and activator of transcription 1 (STAT1) impact on M1 polarization are involved in the infection progress (Murray, 2017). It is becoming clear that M1-polarized macrophages are associated with the control of acute infections such as active tuberculosis and gastroenteritis in the early phases of healing and high antigen presentation, while an excessive or prolonged M1 activation is deleterious for the host (Mège et al., 2011; Owen et al., 2016; Liu et al., 2021).
M2 activation in bacterial infection
The M2-polarized macrophages are known as an anti-inflammatory phenotype with a low phagocytic and bacterial killing ability, which are generally prominent in the later course of bacterial infections to prevent tissue damage (Atri et al., 2018). M2 macrophages are characterized by functional expression of anti-inflammatory cytokine mediators such as IL-10, transforming growth factor-beta (TGF-β), and Arginase-1 (Arg-1), as well as alternative activation markers CD206, CD163, mannose receptor, IL-4R, and chemokines that will extenuate inflammatory reactions and promote the wound healing process and tissue repair (Arnold et al., 2007; Paynich et al., 2017). STAT6, STAT3, peroxisome proliferator-activated receptor delta (PPARδ), or PPZRγ impact is involved in the progress of M2 polarization, respectively, which is similar in M2 program evoked by IL-4 and/or IL-13 treatment in vitro (Murray, 2017; Stapels et al., 2018; Panagi et al., 2020). The specific M2 phenotype of macrophages with low bactericidal ability is also thought to be beneficial to bacterial pathogens' immune escape for survival (Kyrova et al., 2012; Wang, Y et al., 2021). Though M2-polarized macrophages are prominent in the reparative phase of inflammation, they are also found in connection with parasitic and chronic bacterial infections such as sepsis and Buruli ulcer (Kiszewski et al., 2006; Wang, X et al., 2021). The balance of switching between the M1 and M2 polarization states is necessary to allow the beneficial processes of inflammation, resolution, and repair.
Biogenesis and composition of EVs
Membrane vesicles in bacterial infections
During the bacterial infection, nanosized EVs are released to extracellular space by both the host and bacteria (Pfeifhofer-Obermair et al., 2016; Escudé Martinez de Castilla et al., 2021; Tian et al., 2022). Bacterial vesicles are broadly defined as MVs with a size range from 20 to 300 nm (Palacios et al., 2021), which are enriched for LPS, phospholipids, peptidoglycan (PG), periplasmic and cytoplasmic proteins, and nucleic acids, respectively (Schwechheimer and Kuehn, 2015; Jan, 2017; Furuyama and Sircili, 2021). Proteomics analysis implied that MVs from different bacterial sources were diverse in composition (Williams et al., 2007; Choi et al., 2011; Lee et al., 2015), indicating that various bacterial infections caused different inflammatory responses. The selectivity of MV cargo seems a deliberate process, rather than a random event, though the biogenesis mechanism of cargo selection is still ambiguous (Liu et al., 2022). The biogenesis of MVs is supposed to be regulated by multiple elements, including genetic background and growth conditions such as temperature, stress factors, oxidation state, iron, vasculogenesis, and immune response regulators (VirRs) (Jan, 2017; Furuyama and Sircili, 2021; Palacios et al., 2021). A high-throughput screen of a whole-genome knockout library of Escherichia coli (E. coli) identified nearly 150 genes that affect vesicle biogenesis (Kulp et al., 2015). The vesicles of some bacteria have bacteriolytic enzymes capable of distinguishing between self and non-self microbes and killing other bacteria that surround them (Yaron et al., 2000; Caruana and Walper, 2020). Meanwhile, bacterial MVs during infection could be recognized by immune cells through cell surface TLRs (Jurkoshek et al., 2016). For instance, TLR2, TLR4, and TLR5 located at the host cell membrane are reported to recognize bacterial lipoproteins and flagellins, while TLR7, TLR8, and TLR9 located at the endosomal membranes can bind MV-associated nucleic acids, respectively (Jurkoshek et al., 2016; Liu et al., 2022). The pathways of MVs from different bacteria entry into host cells include endocytosis (e.g., clathrin-mediated, caveolin-mediated, and lipid raft-mediated endocytosis) or membrane fusion, respectively (O'Donoghue and Krachler, 2016). Several studies have shown that MVs have a multifaceted role both offensively and defensively due to their various components (MacDonald and Kuehn, 2012; Schorey et al., 2015; Guerrero-Mandujano et al., 2017). For instance, many of the vesicles stimulate the activation of the host immune response for the elimination of pathogens, and another mechanism for delivery of the autolysins and virulence factors or cytotoxins is for defending against the digestion and maintaining survival and replication in hosts (Jurkoshek et al., 2016; Li et al., 2018). Therefore, bacterial MVs containing different components bind to certain receptors on macrophages, which can affect the polarization of cells and thus affect the process of inflammation and infection.
Exosomes of the host in bacterial infections
Extracellular vesicles released by eukaryotes are generally divided into three main populations, including exosomes, microvesicles, and apoptotic bodies (Pfeifhofer-Obermair et al., 2016; Escudé Martinez de Castilla et al., 2021; Tian et al., 2022). In recent decades, exosomes have been found to be important in regulating cell function during bacterial infection. They can be secreted by hematopoietic origin, including macrophages, dendritic cells (DCs), B cells, mastocytes, platelets, and cells of non-hematopoietic origin, such as neurons and epithelial cells (Denzer et al., 2000; Tian et al., 2022; Wang et al., 2022; Zhang et al., 2022). In mammals, exosomes are present in all biofluids, including urine, blood, breast milk, saliva, cerebrospinal fluid, and ascites, and are observed among all kingdoms of life, from bacteria to mammals (Liu et al., 2022). Various databases including EVpedia, Vesiclepedia, and Exocarta have been shown to provide abundant resources for the study of exosomes (Kalra et al., 2012; Kim et al., 2013; Keerthikumar et al., 2016). The studies have demonstrated that exosomes carry proteins, lipids, nucleic acids, and even bacterial components for intercellular communication including the activation or inhibition of recipient cells. The cargoes vary depending on the cell type of origin and physiological/pathological state, which have a broad range of biological functions and participate in multiple physiological and pathological processes such as tumorigenesis, inflammation, immune response modulation, angiogenesis, and tissue repair (Giri et al., 2010; Cheng and Schorey, 2013; Schorey et al., 2015; Palacios et al., 2021). The best-described mechanism for the formation of exosomes is associated with the successive endocytosis of the endosomal system and the internal vesicles of multivesicular bodies (MVBs), which is driven in an endosomal sorting complex required for transport (ESCRT)-dependent or ESCRT-independent manner (Colombo et al., 2014). Certain proteins such as transport and fusion-related proteins, tetraspanins (CD9, CD63, and CD81), and heat shock proteins (HSPs) are contained in exosomes in various levels of expression (Denzer et al., 2000; Schorey et al., 2015), which are commonly used as markers to identify exosomes. Though exosome uptake by target cells depends on the type of recipient cells, phagocytosis and macropinocytosis seem to be involved in this process crucially. Moreover, exosomes can interact with target cells in a ligand-to-receptor manner, including transferrin receptors, TLRs, integrins, and CD94/56 with their individual ligands, respectively (Yáñez-Mó et al., 2015). Taken together, the content of the host-derived exosomes and bacteria-derived MVs can have significant effects on who has the advantage in the battle between the immune system and the pathogen during the infection. Further characterizing the composition and function of EVs will better reveal the biological relevance of these natural nanocarriers and provide new strategies for diagnosis and therapy (Zhang et al., 2021).
EVs in the regulation of macrophage polarization in microbial infections
Bacteria are major pathogens that develop resistance and cause distinct types of infectious diseases such as tuberculosis, legionnaires' disease, and acute fibrinopurulent pneumonia (Livermore, 2004; Kiszewski et al., 2006; Schwechheimer and Kuehn, 2015; Jung et al., 2016). Independent of the location, microbes have developed many tools to facilitate microbe–microbe, microbe–host, and microbe–environment interactions (Kaparakis et al., 2010; Furuyama and Sircili, 2021; Cui et al., 2022). One strategy is through the classical secretion system types (1~7), which have been widely studied and characterized (El Qaidi et al., 2017; Hui et al., 2018; Grigoryeva et al., 2021; Hardy et al., 2022). The other one depends on MVs, which are considered an alternative and independent secretion system that carries virulence effectors and toxins to regulate the function of recipient cells (Kaparakis et al., 2010; Guerrero-Mandujano et al., 2017; Furuyama and Sircili, 2021). Packaging virulence factors into or onto MV concentrates can increase their stability, allow toxins and virulence factors to be delivered intracellularly, target specific objects at different organelles in host cells to expand or alter their function, and allow them to be transported over long distances (Rüter et al., 2018; Rueter and Bielaszewska, 2020).
For mycobacterial infections
Mycobacterium tuberculosis (Mtb), the major causative agent of TB, is capable of surviving within the phagosomes of host alveolar macrophages and establishes latent infection for the lifetime of the host (O'Garra et al., 2013; Singhania et al., 2018). Previous reports have shown that SecA and Esx protein secretion systems are important for mycobacterial virulence effectors' delivery into the cytosol of the host (Feltcher and Braunstein, 2012; Gröschel et al., 2016). More recently, evolving evidence suggests that Mtb MVs transport various effectors including cell membranes, cell walls, and extracellular proteins into the recipient cells, particularly macrophages, to regulate the host immune response depending on but not limited to nutrient uptake, oxidative stress, envelope stress, and antimicrobial peptides (Jurkoshek et al., 2016; Palacios et al., 2021; Cui et al., 2022).
During the initial infection, Mtb vesicles are recognized and endocytosed by macrophages and then activate M1 polarization, which secretes large amounts of pro-inflammatory mediators, facilitates complement-mediated phagocytosis, and induces type I inflammation to eliminate infection (Anand et al., 2010; Singh et al., 2015; Chiplunkar et al., 2019; Palacios et al., 2021). Surprisingly, exosomes have also been shown to be carriers of some important soluble mediators like cytokines such as IL-1β, IL-6, TNFα, TGFβ, and CCL2/3/4/5 (Yáñez-Mó et al., 2015), which then regulate the function of naïve macrophages. These results indicate besides being released by the cell through the fusion of secretory lysosomes with the plasma membrane, the cytokines are also secreted in exosomes, which are then characterized as pro-inflammatory or anti-inflammatory exosomes, accelerate or suppress the progression of inflammation. It is speculated that cytokines encapsulated in exosomes could preserve more activity than in their soluble form (Schneider et al., 1998). Immune cells infected by Mtb can also release exosomes with or without MVs ingredients, which will influence macrophage polarization and mediate to generate both protective innate and adaptive immune responses against Mtb (Palacios et al., 2021). Mycobacterial antigens including lipoprotein, lipoarabinomannan (LAM), antigenic target protein-6 (ESAT-6), and HspX are reported to be detected in exosomes released from Mtb-infected macrophage J774 cells (Bhatnagar et al., 2007; Giri et al., 2010). Treatment with exosomes carrying these Mtb antigens in vitro could promote naïve macrophages to M1 phenotype with the production of pro-inflammatory cytokines (Bhatnagar and Schorey, 2007; Singh et al., 2012; Walters et al., 2013) and enhanced expression of membrane surface markers such as CD40, CD86, CD80, and HLA-DR (Wang et al., 2014, 2015), which indicate that developing immunity against exosomes will increase host resistance to Mtb infection. Taken together, during Mtb infection, in addition, to being released directly by Mtb in soluble form, virulence effectors can also occur in Mtb MVs or exosomes of infected host cells to affect the phenotype of uninfected macrophages.
Research has disclosed that exosomes secreted from other Mtb-infected immune cells such as dendritic cells and neutrophils, can also induce a significant pro-inflammatory response of macrophages, and endothelial cells can be also activated in this process (Marinho et al., 2013; Alvarez-Jiménez et al., 2018; Li et al., 2018). A significant upregulation of genes and proteins known to promote the recruitment and activation of leukocytes is involved in cell adhesion and the inflammatory process through several immune response-related pathways such as TLR2/NF-κB and the type I interferon pathways. This recruitment is supposed to activate a robust innate immune response and helps speed up the removal of pathogens, but sometimes can lead to tissue damage if it lasts too long. DCs, active naïve CD4+, and CD8+ T cells in vivo can be stimulated, indicating a possible application for these exosomes as a TB vaccine for immune defense. Mycobacterial antigen delivery by exosomes to bystander naïve cells to mediate host protection has been suggested to undergo through different mechanisms such as increased phagocytosis (Wang et al., 2015), high production of superoxide (Alvarez-Jiménez et al., 2018), or by phagosome maturation through a non-canonical LC3-associated phagosome pathway in macrophages (Cheng and Schorey, 2019). In summary, these results suggest that MVs are central mechanisms for intercellular communication between bacteria and host cells during infection, and exosome secretion-mediated signal transduction can be beneficial to the host and the pathogen.
Although studies suggest that EVs that promote persistent inflammation may be detrimental to the host, it is likely that MV-mediated Mtb–host interactions are more complex and multifactorial, relying on Mtb antigen availability as exosomal content at every step in the process (Wang et al., 2019; Mirzaei et al., 2021). Indeed, early secreted ESAT-6 from Mtb can also directly inhibit the activation of NF-κB and IFN regulatory factors downstream of TLR2 via Akt-dependent mechanisms, which suggested that acute mycobacterial infection interferes with M1 polarization (Pathak et al., 2007) and the signal divergence of the same molecule. High and sustained levels of type I interferons from the macrophage and other sources (e.g., T cells or DCs after viral infection) can also be detected at the later stage or latent period of Mtb infection and be harmful to induce the suppressive cytokine IL-10 secretion (McNab et al., 2014; Moreira-Teixeira et al., 2017; Singhania et al., 2018), which may be responsible for the tolerance of low Mtb loads in the host. A possible molecular mechanism is speculated due to the heterogeneous nature of the EVs released by Mtb-infected macrophages, with unknown bacterial molecules possibly present in a vesicle population distinct from the exosome, which then induces different functions on the recipient cells. A comprehensive proteomic analysis confirmed the inference, which identified two distinct, largely nonoverlapping vesicle subsets discovered from Mtb-infected macrophages (Athman et al., 2015; Lee et al., 2015). Besides, the common exosomes with host components, other entirely distinct vesicles are detected to contain a rich source of pathogen-associated molecular patterns (PAMPs) such as bacterial lipoproteins, glycolipids, and LAM. The vesicles harboring these molecules are predicted to suppress the function of macrophage and promote intracellular Mtb survival through activating cell surface and cytosol TLRs for the long term, respectively (Jurkoshek et al., 2016). Consequently, these antigens in MVs transferred to macrophages were released in exosomes which then inhibited IL-2 production and reduced T-cell proliferation to further suppress the adaptive immune response (Jurkoshek et al., 2016; Athman et al., 2017). Although TLR activation is typically important for promoting immunity, prolonged TLR2 signaling during Mtb infection contrary leads to the M2 program and inhibition of Th1 polarization of responding T cells for preventing tissue damage or intracellular survival of bacteria (Singh et al., 2011; Richardson et al., 2015). There is no consensus as to whether these PAMP molecules enter exosomes through MVs or the classical secretory system into MVBs and then are present in the host exosomes or both. MVs carrying metal ions and degradative enzymes could also contribute to nutrient acquisition for bacterial survival. Because metal ions are important for MVs transport during host invasion and transition of the bacterium, which can be confirmed by the presence of different metal ion binding proteins detected by proteomics analysis (Lee et al., 2007). Enzymes found in MVs can also degrade complex biomolecules in the culture medium to make nutrients available (Biller et al., 2014). In general, these results suggest that during acute Mtb infection, Mtb MVs carrying some antigens and virulence effectors can bind to cell surface TLRs and activate macrophages to express the M1 program and release inflammatory exosomes on naïve macrophages to activate them into a pro-inflammatory phenotype, and then participate in the process of eliminating the mycobacterium. On the other hand, prolonged activation by these antigens or other effectors in MVs can also restrain the M1 polarization for their replication and survival (Figure 1). In brief, the EV-modulated polarization of macrophages likely plays a critical role in charging the balance of immunity and immune evasion which is the characteristic of latent Mtb infection.

Figure 1. Potential mechanisms for the effect of EVs, including bacterial MVs and host-derived exosomes, on the polarization modulation of uninfected macrophages. Abbreviation: MVs, membrane vesicles.
At present, the mechanism of mycobacterial virulence effectors translocation into EVs and the specificity of this transport remain undefined. SecA2 and Esx-1 protein secretion systems are important for exporting a multitude of specific effectors out of the bacterial cytoplasm and into the cell envelope or extracellular space (Feltcher and Braunstein, 2012; Gröschel et al., 2016), and Esx-1 is required for mycobacterial DNA release into the cytosol of infected cells (Manzanillo et al., 2012). Moreover, Mtb RNA can also be delivered into MVs via a SecA2-dependent pathway, as well as through cytosolic and intracellular excesses of infected macrophage, which then induces a more pro-inflammatory response with an increased bacterial killing capacity of the macrophages (Biton et al., 2019; Cheng and Schorey, 2019). These results demonstrate that the SecA2-mediated secretion of bacterial nucleic acids packaged in MVs permits infected macrophages to efficiently detect the presence of viable and virulent Mtb in the cytosol via the immune sensory receptor RIG-I. The results not only reveal a novel cytosolic immune sensing strategy for Mtb infection but also suggest that such immune sensing is linked to the recognition of bacterial virulence because MVs transport cargo that serves as virulence factors and/or ligands for cytosolic immune sensory receptors. The mechanism of how these virulence effectors are transported from MVs to exosomes of infected hosts remains inexplicit. There are studies showing that mycobacterial MVs are delivered into the cytosol of macrophages via clathrin-mediated endocytosis (Chiplunkar et al., 2019). In addition, Smith et al. have shown that mycobacterial proteins either released by bacteria or endocytosed by macrophages required mono-ubiquitination for trafficking to the exosomes of infected hosts (Smith et al., 2015). Previous studies have shown that EsxB, an ESAT-6-like secreted protein, is encapsulated in Mtb MVs and then delivered into the host cytoplasm to modulate the immune response (Lee et al., 2015), but it remains to be shown whether the secreted protein ESAT-6 is involved in the MVs or only in a soluble state.
For Salmonella infection
Salmonella enterica serovar Typhimurium (S. typhimurium) is an important cause of gastroenteritis and can invade and survive within macrophages to cause enteric diseases (Gogoi et al., 2019). As a part of the innate immune response, macrophages sense the presence of Salmonella-derived PAMPs via TLRs. This subsequently leads to an antibacterial response that comprises reactive oxygen species (ROS), reactive nitrogen species (RNS), acidic environment, metal starvation, and antimicrobial peptides (AMPs), which are essential to activate Th1 responses against Salmonella to avoid chronic infection. Existing studies have shown that Salmonella invasion is mechanistically similar to Mtb infection. MVs released from Salmonella are heterogeneous and include mixtures of toxins, transporters, degradative enzymes, and transcriptional regulators which are responsible for the activation of the host immune system or survival of Salmonella inside macrophages (Li et al., 1998; Bai et al., 2014). As a defensive strategy, MVs serve as decoys that absorb antimicrobial peptides and neutralize host immune responses. On the other hand, invaded MVs also activate the host's innate immune response (Geddes et al., 2005; Furuyama and Sircili, 2021). It is speculated that macrophages are maintained in a dynamic equilibrium of pro-inflammatory M1 phenotype or anti-inflammatory M2 phenotype depending on the time and dose of bacterial infection and bacterial components, as well as the local environmental stimuli such as different cytokines and downstream signaling pathways (Murray, 2017; Wang, X et al., 2021). In some cases, recruitment, repair, and resolution are rapid (minutes to a few days) for minor damage. It is important to emphasize the time dependence of resolving inflammation, which requires more detailed future studies.
Various exosome subpopulations have been reported to be released from Salmonella-infected macrophages with distinct contents and functions. In the early phase of Salmonella infection, macrophages can be activated to an M1-like response by producing pro-inflammatory exosomes which then transfer cargo to naïve macrophages to induce their activation (Bhatnagar et al., 2007; Hui et al., 2018). The pro-inflammatory effects are partially attributed to virulence effectors of Salmonella such as LPS, Salmonella invasion protein A/C (SipA/C), flagellin, and T1SS-secreted agglutinin RTX, which are detected within exosomes. They partially trigger an increased production of pro-inflammatory cytokines in naïve macrophages. However, whether these virulence factors are encapsulated in MVs or delivered into host cytoplasm by T1SS directly as part of the host exosomes or both are undefined. In addition, the following studies suggest that some of the virulence factors encapsulated in MVs can cause violent IL-1β release through the cell pyroptosis pathway to prevent pathogen infection. MVs containing flagellin released from Salmonella or Pseudomonas aeruginosa (PA) are recognized by TLR5 and endocytosed by macrophages, which trigger inflammasome activation of BMMs through the NLR family CARD domain-containing protein 4 (NLRC4) and caspase-1 pathway and then enhance the production of IL-1β (Yang et al., 2020). However, flagellin-deficient Salmonella MVs induced NLRC4-independent non-canonical inflammasome activation and caused a weak interleukin-1β production in an NLR family, pyrin domain containing 3 (NLRP3)-dependent manner, which indicates that OMV-associated flagellin is crucial for Salmonella OMV-induced inflammasome response, and NLRC4 is a rapid sensor of bacterial OMV-bound flagellin as a host defense mechanism to promote the removal of bacterial pathogens against infection. Endocytosis partially depends on the transferrin receptor present on the surface of the macrophage, which binds to plasma ferric ion-bound transferring (Gogoi et al., 2019). All these changes in macrophage iron homeostasis are reported to be IFN-γ-mediated. Similar results are found in the treatment of Salmonella MVs on chicken macrophages (Cui et al., 2022). Therefore, MVs released from Salmonella, like those from Mtb, can carry virulence factors that induce macrophages to polarize toward type M1 and exert the ability to eliminate bacteria. In addition, infected macrophages can also secrete inflammatory exosomes with or without bacterial components to activate uninfected macrophages to type M1 and participate in the clearance of pathogens. The difference is that due to the presence of flagella, Salmonella can activate M1-like macrophages more strongly and the signaling pathways are more complex.
On the other hand, in order to survive and replicate in the host, bacterial pathogens are able to manipulate macrophage gene expression to induce the M2 program in order to escape the hostile environment present in M1-polarized macrophages (Owen et al., 2016; Saliba et al., 2016). Studies have shown that Salmonella can stimulate an M2 profile of macrophages via the production of a key anti-inflammatory cytokine IL-10 through its effector protein SteE (also known as Salmonella anti-inflammatory response activator, SarA) to trigger the activation of the host STAT3 and promote intracellular replication and increase virulence (Jaslow et al., 2018; Panagi et al., 2020). Deletion of Salmonella-secreted effector K1 (SseK1) can decrease the virulence of Salmonella in vivo and in vitro. SseK1 can downregulate the inflammation-related cytokines and prevent necroptotic cell death by inhibiting NF-κB signaling to maintain the M2 phenotype, which will be beneficial for Salmonella survival (Günster et al., 2017; Lu et al., 2021). Furthermore, iron supplementation is found to increase the intracellular survival of Salmonella. The nuclear peroxisome proliferator-activated receptors γ (PPARγ) and PPARδ, via their signal transduction, are pivotal in dictating the gene regulation patterns of M2 macrophages. Protein kinase C (PKC) isotypes such as PKCα, PKCβ, PKCδ, and PKCθ, have also been shown to have critical roles in antimicrobial immune responses of the macrophages (Gogoi et al., 2019; Mathieu et al., 2019). The mechanistic details of how SteE and SseK1 drive M2 polarization are lacking entirely, whether they are enclosed in Salmonella MVs or just delivered to host cytoplasm through the Salmonella SPI-1 and SPI-2 encoded type III secretion system (T3SS) is unknown. Since T3SS is a syringe-like apparatus exploited by some gram-negative bacteria to deliver virulence effectors into infected host cells (Kyrova et al., 2012; Lawrence et al., 2021; Wang, Y et al., 2021), it is possible that virulence factors may be released by the secretory system and then encapsulated in MVs other than in soluble form to maintain their activities.
For Escherichia coli infection
Acute infections with pathogenic E. coli cause gastroenteritis, urinary tract infections, acute lung injury (ALI), and sepsis (Mège et al., 2011; Nirujogi et al., 2022). However, E. coli strain Nissle 1917 (EcN) can be well colonized in the human intestinal tract and can modulate intestinal homeostasis and microflora balance, which has been developed as a microbial product or dietary supplement to treat intestinal inflammatory diseases such as inflammatory bowel disease (IBD) and infectious diarrhea (Lee et al., 2018; Ramos and Papadakis, 2019; Sanders et al., 2019). Compared to pathogenic E. coli, EcN is not pathogenic due to the lack of some defined virulence factor genes in its genome (Grozdanov et al., 2004). Therefore, the genome structures of pathogenic bacteria and probiotics determine the difference between their protective or harmful effects on the host immune system (Grozdanov et al., 2004; Kulp et al., 2015). Consequently, MVs from different strains of E. coli will likewise be wrapped with distinct components and perform entirely distinct regulatory roles on cells.
Studies have shown that MV-associated LPS from pathogenic E. coli leads to NLRP3-dependent M1-associated cytokine IL-1β secretion via LPS delivery into the host cytoplasm and triggers TLR4/TRIF signaling pathway to cause caspase-11-mediated non-canonical inflammasome activation (Santos et al., 2018). Similar results are found in that enterohemorrhagic E. coli (EHEC) MVs traffic LPS or heat-labile enterotoxin (LT) into the cytosol of host cells (Vanaja et al., 2016; Rueter and Bielaszewska, 2020). The mechanism further revealed that endotoxins were likely the ligands that mediated the binding of MVs to lipid rafts of host cells, thus leading to the uptake of MVs. Based on high sequence homology to SseK1, a unique T3SS effector of Salmonella, E. coli effector NleB1 can block TNF-mediated NF-κB pathway activation to inhibit antibacterial and inflammatory host responses (El Qaidi et al., 2017). Whether NleB1 is packaged in OMV remains undefined. Several articles have identified that bacterial MVs enter epithelial cells via NOD receptor-dependent NF-κB pathways or lipid rafts and caveolin-dependent endocytosis (Kaparakis et al., 2010; Cañas et al., 2018). For macrophages, the uptake of E. coli MVs may also be through random phagocytosis, classic endocytosis, or specific pathways, and the detailed mechanisms need further investigation.
As probiotics, EcN MVs can recapitulate the anti-inflammatory properties of EcN by modulating cytokine expression and production from various cells and tissues in different manners. In peripheral blood mononuclear cells (PBMCs) and intestinal epithelial cells, a mixed secretion of M1-associated pro-inflammatory cytokines IL-6, IL-8, and TNF-α with M2-associated anti-inflammatory cytokines IL-10 can be triggered (Fábrega et al., 2016; Cañas et al., 2018). In other studies performed in vivo and in vitro, EcN MVs have been shown to be effective in enhancing the antibacterial activity of macrophages, which can regulate the adaptive immune response to host defense (Hu et al., 2020). The reasonable causes are that the upregulation of pro-inflammatory cytokines activated by EcN MVs is probably due to LPS or other PRR ligands, while some other unidentified vesicular components may induce the activation of M2-associated cytokines (Kulp et al., 2015). These findings again emphasized that M1- and M2-polarized phenotypes could be provoked by bacterial MVs with different components at the same time and they will interact with each other in the presence of E. coli anti-inflammatory response.
Other than MVs, exosomal shuttles from E. coli-infected cells can transfer cargo from cell to cell and affect the function of recipient cells. The pathogenic adherent-invasive E. coli (AIEC), which abnormally colonizes the intestinal mucosa of patients with CD, is able to adhere to and invade intestinal epithelial cells (IECs), survive and replicate within macrophages (Lapaquette et al., 2012; Nguyen et al., 2014; Mitsuhashi et al., 2016; Ramos and Papadakis, 2019). Studies have reported that pro-inflammatory exosomes are enhanced in the patient's lumen after AIEC infection. The secretion of exosomes by human IECs and THP-1 macrophages in vitro is promoted as well, which are in turn entrapped by naïve THP-1, leading to increased pro-inflammatory response with the elevated secretion of M1-associated cytokines through pathways involving NF-κB, p38 MAPK, c-Jun N-terminal kinase, and impaired clearance of intracellular AIEC in exosome-receiving cells (Carrière et al., 2016; Larabi et al., 2020). Exosomes released by AIEC-infected IECs also inhibited autophagy-mediated clearance of uninfected AIEC due to increased levels of miRNA-30c and miRNA-130a packaged in exosomes through inhibiting ATG5 and ATG16L expression, thus favoring AIEC intracellular replication within IECs (Larabi et al., 2020). Whether such a similar signaling pathway also occurs in E. coli intracellular survival and replication of macrophages requires further verification.
Bacterial effectors can act on recipient cells by being encapsulated in MVs or transported through undefined mechanisms into the infected host exosomes. Exosomes released from Shiga toxin 2a (Stx2a)-treated human THP-1 macrophages contain Stx2a, modulate inflammatory responses, and induce cell death in human renal cortical epithelial cells expressing the toxin receptor globotriaosylceramide (Gb3) (Lee et al., 2020). The high expression of pro-inflammatory cytokines IL-6, TNFα, IL-1β, and IL-8 indicates an activated M1-polarized phenotype after infection. All pro-inflammatory cytokines are packaged randomly into diverse Stx2-associated exosomes, but only exo-mRNA levels of IL-1β and IL-8 are higher than those in uninfected THP-1 cells, which may be the reason for the exacerbated localized inflammation and death of recipient cells in Stx-mediated renal injury. Regardless of nucleotide and protein cargos packaged in exosomes, lipid mediators can also control the initiation and resolution of acute lung inflammation (Ott et al., 2011; Robb et al., 2016). A possible mechanism has been revealed recently, increased release of exosomes from alveolar macrophages which carried a diverse array of lipid mediators derived from ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) metabolite profile in part depend on the inflammatory status of the lung macrophages and their interaction with other lung cells in E.coli LPS-activated ALI (Nirujogi et al., 2022). However, the processes of lipid mediator synthesis and transportation are much more complicated, which will need to be further delineated in the future.
For other bacterial infections
In addition to the bacteria mentioned above, there are a number of other bacteria that affect the inflammatory process by regulating the proportion of M1/M2 macrophages by EVs after infection, which are shown in Table 1.
Besides virulence effectors, there are many other cofactors involved in MVs generation. TseF secreted by H3-T6SS of PA is incorporated into MVs by directly interacting with the iron-binding Pseudomonas quinolone signal (PQS), which suggests a possible approach of general secretion system effectors encapsulated into MVs and an important role of the quorum sensing (QS) system in OMV formation (Lin et al., 2017). In addition, sphingomyelin mutation and inhibition of Arg- and lys-gingipain in MVs of Porphyromonas gingivalis can significantly increase the secretion of inflammatory cytokines and chemokines by M1-type macrophages (Rocha et al., 2021; Castillo et al., 2022), possibly suggesting that natural MVs with normal sphingomyelin and Arg- and lys-gingipain activity may inhibit the M1-type polarization of macrophages during bacterial infection and prevent macrophages from playing a scavenging role. These results indicate that MVs secreted by different strains of bacteria or by different treatment methods contain distinct components that have greatly different effects on macrophage function. As an opportunistic nosocomial pathogen, Acinetobacter baumannii (A. baumannii) can activate pro-inflammatory macrophages reaction, evade neutrophil chemotaxis, and cause cell death through the cytotoxic Outer membrane protein A (OmpA) (Knapp et al., 2006; Bhuiyan et al., 2016; Skerniškyte et al., 2021; Tiku et al., 2021), which is reported to be translocated into the mitochondria and nucleus of target cells to induce fragmentation through being packaged in MVs (Choi et al., 2008; Bhuiyan et al., 2016; Tiku et al., 2021). The results indicate that these effectors are not only limited to inducing the production of inflammatory factors but also have toxic effects on the organelles of the host. Moreover, MVs isolated from two clinical A. baumannii strains exhibit different toxicity and proteome characteristics, which suggest that the multidrug-resistant strain containing more virulence factors might produce abundant MVs facilitating the worse outcome (Li et al., 2015). The abundance of proteins correlated with redox and iron metabolism in A. baumannii for infection and survival is identified, and others are enriched in the pathways such as platelet activation and signaling, high-density lipoprotein (HDL) remodeling, heme homeostasis, and apoptosis, which indicate the complicated pathogen–host interaction (Kho et al., 2022). Further projects should classify the virulence effectors contained in MVs of various bacteria and reveal the mechanisms of MVs transportation into the host.
The mechanisms of EVs on macrophage polarization are far more complicated. As important PRRs on macrophages, some TLRs reside at the host cell membrane and recognize bacterial LPS, lipoproteins, and flagellins in MVs or soluble form, while others locate at the endosomal membranes bind MV-associated nucleic acids, respectively (Lim and Staudt, 2013; Liu et al., 2022). The transducers of NF-κB and MAPK are conventional signaling pathways to induce pro-inflammatory cytokines released after EVs bind to individual receptors (Figure 2). For IL-1β, there is another secretion approach by caspase-1-dependent NLR4 activation pathway, which generally causes pyroptosis to prevent bacterial replication and diffusion (Lee et al., 2020; Rueter and Bielaszewska, 2020; Chen et al., 2022). These results provide a therapeutic basis for preventing the excessive release of inflammatory factors. Nevertheless, most of the published reports mainly focused on the mechanism of M1 polarization induced by EVs in macrophages. Future studies may focus on the mechanism of EVs on M2 polarization, to provide a reference for the prevention and treatment of chronic infection.
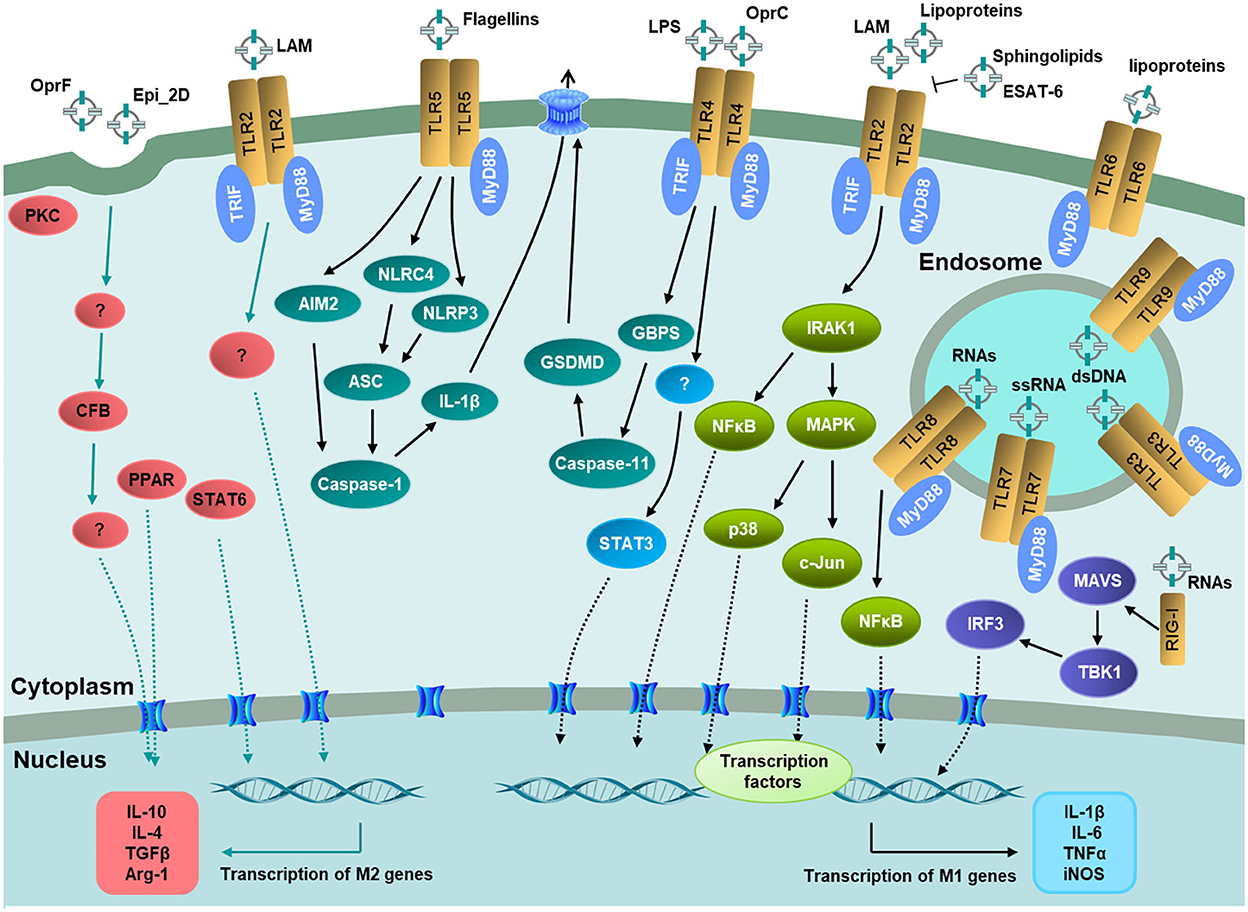
Figure 2. Recognition of EV-associated molecular patterns by host immune receptors and signaling pathways on macrophage polarization. TLR2, TLR4, TLR5, and TLR6 located at the host cell membrane. TLR3, TLR7, TLR88, and TLR9 located at the endosomal membranes. RIG-I located in the cytosol to recognize bacterial RNAs in EVs. The downstream signaling pathways lead to the activation of transcription factors including NF-κB, STAT3, PPAR, and STAT6, respectively, and then the transcription of pro-inflammatory or anti-inflammatory genes. Adaptor molecules: MyD88 and TRIF.
Conclusion
During a bacterial infection, the host immune system is exposed to intact bacterial and microbial components, and both are key compounds to control the infection program. Extensive studies have shown that bacterial invasion can regulate the polarization of macrophages by secreting virulence factors through the general secretion system, thus, affecting the development of inflammation. Various reviews have demonstrated the mechanism of macrophage polarization regulation at different stages of bacterial infection, but none focused on the effect of bacteria-derived MVs and host-derived exosomes on macrophage polarization in inflammation. Emerging studies have demonstrated that bacteria can secrete EVs like MVs containing virulence factors to modulate the polarization of macrophages. Host cells infected by bacteria can similarly secrete EVs such as exosomes bearing various proteins or nucleic acids to induce the polarization of uninfected and infected macrophages. Here, we outline the current state of the articles and summarize the effects of EVs from both bacteria and hosts on macrophage polarization in order to better understand the mechanisms underlying the development of disease caused by bacterial infection.
Extracellular vesicle-mediated polarization of macrophages may promote or inhibit the development of infection. Many bacterial productions are involved in MVs and then transported into recipient cells of the same species, other bacterial species, or eukaryotic cells to modulate the cellular processes. The bioactive component of MVs is different for each species and determines whether MV secretion promotes bacterial virulence, host immunity, or both. During the bacterial infection, immune cells like macrophages can uptake the bacterial MVs through different TLR pathways or endocytosis and then activate to release pro-inflammatory or anti-inflammatory cytokines in soluble form or in exosomes with or without bacterial effectors, which then affect the functions of other naïve recipient cells nearby or further apart. In the process of anti-infection, macrophages keep the balance of inflammatory response through the transformation of phenotype. The pro-inflammatory M1 phenotype is prominent during the initial stage of infection, while the anti-inflammatory M2 phenotype dominates during the late stage of infection to prevent excessive inflammatory reactions. There are so many intermediate stages that suggest dynamic equilibrium depending on the course of infection and the dose of bacteria. Some of the most important steps that must be taken in this area are a comprehensive comparison of various subtypes of EVs, such as MVs from bacteria and exosomes from host cells. Different EVs may be effectively distinguished based on their size, density, morphology, and specific surface marker proteins. This is crucial in determining which EVs should be targeted for any therapeutic approach. Moreover, in accordance with the specific biomarkers of different origins of exosomes isolated from bodily fluids such as blood, urine, and saliva, the infection-causing pathogens may be determined. The number of exosomes and their composition can also tell the infection progression, which could provide a targeted treatment program. However, the complex effect of EV secretion on disease pathogenesis must be assessed on a case-by-case basis for each pathogen. In addition, it remains to be further confirmed how these virulence factors enter the exosome of the host cell, whether they are encapsulated in the exosome after being released from the bacterial MVs or secreted into the extracellular cell through the entire vesicle. The exosomes secreted by macrophages after bacterial infection contain not only proteins and mRNA of the host but also miRNAs or lncRNAs (long non-coding RNAs), which should also be taken into account for regulating the gene expression of recipient cells. Defining and classifying the composition and effects of these EVs in the regulation of macrophage polarization, and how they disseminate during infection, is essential to our understanding of the pathogenesis of human diseases and how our immune system responds to the infection.
Author contributions
MQ wrote and edited the manuscript. HZ revised the manuscript. XZ reviewed and edited the manuscript. All authors read and approved the final version.
Funding
This study was supported by the Natural Science Foundation of Shandong Province (ZR2021QC167, ZR2020KC028, and ZR2020QC227), Innovation Team Project for Modern Agricultural Industrious Technology System of Shandong Province (SDAIT-11-10), and Cooperation Project of University and Local Enterprise in Yantai of Shandong Province (2020XDRHXMPT34, 2021XDRHXMXK23, and 2022-XUEYL).
Acknowledgments
The authors extend thanks to colleague Xiaoli Liu, for criticism that helped to improve the quality of content for a broader audience, and Gang Yuan, from the library of Ludong University, for guidance on graphics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, A. A. Q., Qi, F., Zheng, R., Xiao, L., Abdalla, A. M. E., Mao, L., et al. (2021). The impact of ExHp-CD (outer membrane vesicles) released from Helicobacter pylori SS1 on macrophage RAW 264.7 cells and their immunogenic potential. Life Sci. 279, 119644. doi: 10.1016/j.lfs.2021.119644
Alvarez, C. S., Giménez, R., Cañas, M. A., Vera, R., Díaz-Garrido, N., Badia, J., et al. (2019). Extracellular vesicles and soluble factors secreted by Escherichia coli Nissle 1917 and ECOR63 protect against enteropathogenic E. coli-induced intestinal epithelial barrier dysfunction. BMC Microbiol. 19, 166. doi: 10.1186/s12866-019-1534-3
Alvarez-Jiménez, V. D., Leyva-Paredes, K., García-Martínez, M., Vázquez-Flores, L., García-Paredes, V. G., Campillo-Navarro, M., et al. (2018). Extracellular vesicles released from mycobacterium tuberculosis-infected neutrophils promote macrophage autophagy and decrease intracellular mycobacterial survival. Front. Immunol. 9, 272. doi: 10.3389/fimmu.2018.00272
Anand, P. K., Anand, E., Bleck, C. K., Anes, E., and Griffiths, G. (2010). Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PloS ONE 5, e10136. doi: 10.1371/journal.pone.0010136
Armstrong, D. A., Lee, M. K., Hazlett, H. F., Dessaint, J. A., Mellinger, D. L., Aridgides, D. S., et al. (2020). Extracellular Vesicles from Pseudomonas aeruginosa Suppress MHC-Related Molecules in Human Lung Macrophages. ImmunoHorizons 4, 508–519. doi: 10.4049/immunohorizons.2000026
Arnold, L., Henry, A., Poron, F., Baba-Amer, Y., van Rooijen, N., Plonquet, A., et al. (2007). Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069. doi: 10.1084/jem.20070075
Athman, J. J., Sande, O. J., Groft, S. G., Reba, S. M., Nagy, N., Wearsch, P. A., et al. (2017). Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation. J. Immunol. (Baltimore, Md: 1950) 198, 2028–2037. doi: 10.4049/jimmunol.1601199
Athman, J. J., Wang, Y., McDonald, D. J., Boom, W. H., Harding, C. V., Wearsch, P. A., et al. (2015). Bacterial membrane vesicles mediate the release of mycobacterium tuberculosis lipoglycans and lipoproteins from infected macrophages. J. Immunol. 195, 1044–1053. doi: 10.4049/jimmunol.1402894
Atri, C., Guerfali, F. Z., and Laouini, D. (2018). Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 19. doi: 10.3390/ijms19061801
Bai, J., Kim, S. I., Ryu, S., and Yoon, H. (2014). Identification and characterization of outer membrane vesicle-associated proteins in Salmonella enterica serovar Typhimurium. Infrct. immunity 82, 4001–4010. doi: 10.1128/IAI.01416-13
Barley, T. J., Murphy, P. R., Wang, X., Bowman, B. A., Mormol, J. M., Mager, C. E., et al. (2022). Mitogen-Activated Protein Kinase Phosphatase-1 Controls PD-L1 Expression by Regulating Type I Interferon during Systemic Escherichia coli Infection. J. Biol. Chem. 101938. doi: 10.1016/j.jbc.2022.101938
Benoit, M., Desnues, B., and Mege, J. L. (2008). Macrophage polarization in bacterial infections. J. Immunol. 181, 3733–3739. doi: 10.4049/jimmunol.181.6.3733
Bhatnagar, S., and Schorey, J. S. (2007). Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J. Biol. Chem. 282, 25779–25789. doi: 10.1074/jbc.M702277200
Bhatnagar, S., Shinagawa, K., Castellino, F. J., and Schorey, J. S. (2007). Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110, 3234–3244. doi: 10.1182/blood-2007-03-079152
Bhuiyan, M. S., Ellett, F., Murray, G. L., Kostoulias, X., Cerqueira, G. M., Schulze, K. E., et al. (2016). Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc. Natl. Acad. Sci. U. S. A. 113, 9599–9604. doi: 10.1073/pnas.1523116113
Biller, S. J., Schubotz, F., Roggensack, S. E., Thompson, A. W., Summons, R. E., Chisholm, S. W., et al. (2014). Bacterial vesicles in marine ecosystems. Science (New York, NY) 343, 183–186. doi: 10.1126/science.1243457
Biton, M., Abou Karam, P., and Regev-Rudzki, N. (2019). Tuberculosis's cargoman: bacteria load RNA into host extracellular vesicles. EMBO Rep. 20. doi: 10.15252/embr.201947719
Burstein, D., Amaro, F., Zusman, T., Lifshitz, Z., Cohen, O., Gilbert, J. A., et al. (2016). Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires. Nat. Genet. 48, 167–175. doi: 10.1038/ng.3481
Cañas, M. A., Fábrega, M. J., Giménez, R., Badia, J., and Baldomà, L. (2018). Outer membrane vesicles from probiotic and commensal Escherichia coli activate NOD1-mediated immune responses in intestinal epithelial cells. Front. Microbiol. 9, 498. doi: 10.3389/fmicb.2018.00498
Carrière, J., Bretin, A., Darfeuille-Michaud, A., Barnich, N., and Nguyen, H. T. (2016). Exosomes released from cells infected with Crohn's disease-associated adherent-invasive Escherichia coli activate host innate immune responses and enhance bacterial intracellular replication. Inflamm. Bowel Dis. 22, 516–528. doi: 10.1097/MIB.0000000000000635
Caruana, J. C., and Walper, S. A. (2020). Bacterial membrane vesicles as mediators of microbe—microbe and microbe—host community interactions. Front. Microbiol. 11, 432. doi: 10.3389/fmicb.2020.00432
Castillo, Y., Castellanos, J. E., Lafaurie, G. I., and Castillo, D. M. (2022). Porphyromonas gingivalis outer membrane vesicles modulate cytokine and chemokine production by gingipain-dependent mechanisms in human macrophages. Arch. Oral Biol. 140, 105453. doi: 10.1016/j.archoralbio.2022.105453
Cecil, J. D., O'Brien-Simpson, N. M., Lenzo, J. C., Holden, J. A., Singleton, W., Perez-Gonzalez, A., et al. (2017). Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Front. Immunol. 8, 1017. doi: 10.3389/fimmu.2017.01017
Chen, G., Sun, Q., Cai, Q., and Zhou, H. (2022). Outer membrane vesicles from fusobacterium nucleatum switch M0-like macrophages toward the M1 phenotype to destroy periodontal tissues in mice. Front. Microbiol. 13, 815638. doi: 10.3389/fmicb.2022.815638
Cheng, Y., and Schorey, J. S. (2013). Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur. J. Immunol. 43, 3279–3290. doi: 10.1002/eji.201343727
Cheng, Y., and Schorey, J. S. (2019). Extracellular vesicles deliver Mycobacterium RNA to promote host immunity and bacterial killing. EMBO Rep. 20. doi: 10.15252/embr.201846613
Chiplunkar, S. S., Silva, C. A., Bermudez, L. E., and Danelishvili, L. (2019). Characterization of membrane vesicles released by Mycobacterium avium in response to environment mimicking the macrophage phagosome. Future Microbiol. 14, 293–313. doi: 10.2217/fmb-2018-0249
Chiu, C. H., Lee, Y. T., Lin, Y. C., Kuo, S. C., Yang, Y. S., Wang, Y. C., et al. (2020). Bacterial membrane vesicles from Acinetobacter baumannii induced by ceftazidime are more virulent than those induced by imipenem. Virulence 11, 145–158. doi: 10.1080/21505594.2020.1726593
Choi, C. H., Hyun, S. H., Lee, J. Y., Lee, J. S., Lee, Y. S., Kim, S. A., et al. (2008). Acinetobacter baumannii outer membrane protein A targets the nucleus and induces cytotoxicity. Cell. Microbiol. 10, 309–319.
Choi, D. S., Kim, D. K., Choi, S. J., Lee, J., Choi, J. P., Rho, S., et al. (2011). Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 11, 3424–3429. doi: 10.1002/pmic.201000212
Colombo, M., Raposo, G., and Théry, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Biol. 30, 255–289. doi: 10.1146/annurev-cellbio-101512-122326
Cui, H., Sun, Y., Lin, H., Zhao, Y., and Zhao, X. (2022). The outer membrane vesicles of salmonella enterica serovar typhimurium activate chicken immune cells through lipopolysaccharides and membrane proteins. Pathogens (Basel, Switzerland) 11, 339. doi: 10.3390/pathogens11030339
Denzer, K., Kleijmeer, M. J., Heijnen, H. F., Stoorvogel, W., and Geuze, H. J. (2000). Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113 Pt 19, 3365–3374. doi: 10.1242/jcs.113.19.3365
Deo, P., Chow, S. H., Hay, I. D., Kleifeld, O., Costin, A., Elgass, K. D., et al. (2018). Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLoS Pathogens 14, e1006945. doi: 10.1371/journal.ppat.1006945
El Qaidi, S., Chen, K., Halim, A., Siukstaite, L., Rueter, C., Hurtado-Guerrero, R., et al. (2017). NleB/SseK effectors from Citrobacter rodentium, Escherichia coli, and Salmonella enterica display distinct differences in host substrate specificity. J. Biol. Chem. 292, 11423–11430. doi: 10.1074/jbc.M117.790675
Elson, G., Dunn-Siegrist, I., Daubeuf, B., and Pugin, J. (2007). Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood 109, 1574–1583. doi: 10.1182/blood-2006-06-032961
Escudé Martinez de Castilla, P., Tong, L., Huang, C., Sofias, A.M., Pastorin, G., Chen, X., et al. (2021). Extracellular vesicles as a drug delivery system: A systematic review of preclinical studies. Adv. Drug Deliv. Rev. 175, 113801. doi: 10.1016/j.addr.2021.05.011
Essandoh, K., Li, Y., Huo, J., and Fan, G. C. (2016). MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock (Augusta, Ga) 46, 122–131. doi: 10.1097/SHK.0000000000000604
Fábrega, M. J., Aguilera, L., Giménez, R., Varela, E., Alexandra Cañas, M., Antolín, M., et al. (2016). Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic Escherichia coli Strains. Front. Microbiol. 7, 705. doi: 10.3389/fmicb.2016.00705
Feltcher, M. E., and Braunstein, M. (2012). Emerging themes in SecA2-mediated protein export. Nat. Rev. Microbiol. 10, 779–789. doi: 10.1038/nrmicro2874
Fleetwood, A. J., Lee, M. K. S., Singleton, W., Achuthan, A., Lee, M. C., O'Brien-Simpson, N. M., et al. (2017). Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by porphyromonas gingivalis and its outer membrane vesicles. Front. Cell. Infect. Microbiol. 7, 351. doi: 10.3389/fcimb.2017.00351
Furuyama, N., and Sircili, M. P. (2021). Outer Membrane Vesicles (OMVs) produced by gram-negative bacteria: structure, functions, biogenesis, and vaccine application. BioMed Res. Int. 2021, 1490732. doi: 10.1155/2021/1490732
Gao, P., Guo, K., Pu, Q., Wang, Z., Lin, P., Qin, S., et al. (2020). oprC impairs host defense by increasing the quorum-sensing-mediated virulence of Pseudomonas aeruginosa. Front. Immunol. 11, 1696. doi: 10.3389/fimmu.2020.01696
Geddes, K., Worley, M., Niemann, G., and Heffron, F. (2005). Identification of new secreted effectors in Salmonella enterica serovar Typhimurium. Infrct. immunity 73, 6260–6271. doi: 10.1128/IAI.73.10.6260-6271.2005
Giri, P. K., Kruh, N. A., Dobos, K. M., and Schorey, J. S. (2010). Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics 10, 3190–3202. doi: 10.1002/pmic.200900840
Gogoi, M., Shreenivas, M. M., and Chakravortty, D. (2019). Hoodwinking the big-eater to prosper: the salmonella-macrophage paradigm. J. Innate. Immun. 11, 289–299. doi: 10.1159/000490953
Grigoryeva, L. S., Rehman, S., White, R. C., Garnett, J. A., and Cianciotto, N. P. (2021). Assay for assessing mucin binding to bacteria and bacterial proteins. Bio-protocol 11, e3933. doi: 10.21769/BioProtoc.3933
Gröschel, M. I., Sayes, F., Simeone, R., Majlessi, L., and Brosch, R. (2016). ESX secretion systems: mycobacterial evolution to counter host immunity. Nat. Rev. Microbiol. 14, 677–691. doi: 10.1038/nrmicro.2016.131
Grozdanov, L., Raasch, C., Schulze, J., Sonnenborn, U., Gottschalk, G., Hacker, J., et al. (2004). Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol 186, 5432–5441. doi: 10.1128/JB.186.16.5432-5441.2004
Guerrero-Mandujano, A., Hernández-Cortez, C., Ibarra, J. A., and Castro-Escarpulli, G. (2017). The outer membrane vesicles: secretion system type zero. Traffic (Copenhagen, Denmark) 18, 425–432. doi: 10.1111/tra.12488
Günster, R. A., Matthews, S. A., Holden, D. W., and Thurston, T. L. M. (2017). SseK1 and SseK3 Type III secretion system effectors inhibit nf-κb signaling and necroptotic cell death in salmonella-infected macrophages. Infrct. Immunity 85, e00010–17. doi: 10.1128/IAI.00010-17
Han, E. C., Choi, S. Y., Lee, Y., Park, J. W., Hong, S. H., Lee, H. J., et al. (2019). Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood-brain barrier in mice. FASEB J. 33, 13412–13422. doi: 10.1096/fj.201901575R
Hardy, K. S., Tuckey, A. N., Housley, N. A., Andrews, J., Patel, M., Al-Mehdi, A. B., et al. (2022). The pseudomonas aeruginosa type iii secretion system exoenzyme effector exou induces mitochondrial damage in a murine bone marrow-derived macrophage infection model. Infrct. Immunity 90, e0047021. doi: 10.1128/iai.00470-21
Hu, R., Lin, H., Li, J., Zhao, Y., Wang, M., Sun, X., et al. (2020). Probiotic Escherichia coli Nissle 1917-derived outer membrane vesicles enhance immunomodulation and antimicrobial activity in RAW264.7 macrophages. BMC MicroBiol. 20, 268. doi: 10.1186/s12866-020-01953-x
Hui, W. W., Hercik, K., Belsare, S., Alugubelly, N., Clapp, B., Rinaldi, C., et al. (2018). Salmonella enterica serovar typhimurium alters the extracellular proteome of macrophages and leads to the production of proinflammatory exosomes. Infrct. immunity 86. doi: 10.1128/IAI.00386-17
Jan, A. T. (2017). Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front. Microbiol. 8, 1053. doi: 10.3389/fmicb.2017.01053
Jaslow, S. L., Gibbs, K. D., Fricke, W. F., Wang, L., Pittman, K. J., Mammel, M. K., et al. (2018). Salmonella activation of STAT3 signaling by SarA effector promotes intracellular replication and production of IL-10. Cell Rep. 23, 3525–3536. doi: 10.1016/j.celrep.2018.05.072
Jia, R., Cui, K., Li, Z., Gao, Y., Zhang, B., Wang, Z., et al. (2020). NK cell-derived exosomes improved lung injury in mouse model of Pseudomonas aeruginosa lung infection. J. Physiol Sci. 70, 50. doi: 10.1186/s12576-020-00776-9
Jones, L. B., Bell, C. R., Bibb, K. E., Gu, L., Coats, M. T., Matthews, Q. L., et al. (2018). Pathogens and their effect on exosome biogenesis and composition. Biomedicines 6, 79. doi: 10.3390/biomedicines6030079
Jung, A. L., Stoiber, C., Herkt, C. E., Schulz, C., Bertrams, W., Schmeck, B., et al. (2016). Legionella pneumophila-derived outer membrane vesicles promote bacterial replication in macrophages. PLoS Pathog 12, e1005592. doi: 10.1371/journal.ppat.1005592
Jungi, T. W., Brcic, M., Sager, H., Dobbelaere, D. A., Furger, A., Roditi, I., et al. (1997). Antagonistic effects of IL-4 and interferon-gamma (IFN-gamma) on inducible nitric oxide synthase expression in bovine macrophages exposed to gram-positive bacteria. Clin. Exp. Immunol. 109, 431–438. doi: 10.1046/j.1365-2249.1997.4891384.x
Jurkoshek, K. S., Wang, Y., Athman, J. J., Barton, M. R., and Wearsch, P. A. (2016). Interspecies communication between pathogens and immune cells via bacterial membrane vesicles. Front. Cell Dev. Biol. 4, 125. doi: 10.3389/fcell.2016.00125
Kalra, H., Simpson, R. J., Ji, H., Aikawa, E., Altevogt, P., Askenase, P., et al. (2012). Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 10, e1001450. doi: 10.1371/journal.pbio.1001450
Kaparakis, M., Turnbull, L., Carneiro, L., Firth, S., Coleman, H. A., Parkington, H. C., et al. (2010). Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell. Microbiol. 12, 372–385. doi: 10.1111/j.1462-5822.2009.01404.x
Keerthikumar, S., Chisanga, D., Ariyaratne, D., Al Saffar, H., Anand, S., Zhao, K., et al. (2016). ExoCarta: a web-based compendium of exosomal cargo. J. molecular Biol. 428, 688–692. doi: 10.1016/j.jmb.2015.09.019
Khan, J., Sharma, P. K., and Mukhopadhaya, A. (2015). Vibrio cholerae porin OmpU mediates M1-polarization of macrophages/monocytes via TLR1/TLR2 activation. ImmunoBiol. 220, 1199–1209. doi: 10.1016/j.imbio.2015.06.009
Kho, Z. Y., Azad, M. A. K., Han, M. L., Zhu, Y., Huang, C., Schittenhelm, R. B., et al. (2022). Correlative proteomics identify the key roles of stress tolerance strategies in Acinetobacter baumannii in response to polymyxin and human macrophages. PLoS Pathogens 18, e1010308. doi: 10.1371/journal.ppat.1010308
Kim, D. K., Kang, B., Kim, O. Y., Choi, D. S., Lee, J., Kim, S. R., et al. (2013). EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. ExtraCell. vesicles 2. doi: 10.3402/jev.v2i0.20384
Kiszewski, A. E., Becerril, E., Aguilar, L. D., Kader, I. T., Myers, W., Portaels, F., et al. (2006). The local immune response in ulcerative lesions of Buruli disease. Clin. Exp. Immunol. 143, 445–451. doi: 10.1111/j.1365-2249.2006.03020.x
Knapp, S., Wieland, C. W., Florquin, S., Pantophlet, R., Dijkshoorn, L., Tshimbalanga, N., et al. (2006). Differential roles of CD14 and toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am. J. Respir. Crit. Care Med. 173, 122–129. doi: 10.1164/rccm.200505-730OC
Kulp, A. J., Sun, B., Ai, T., Manning, A. J., Orench-Rivera, N., Schmid, A. K., et al. (2015). Genome-wide assessment of outer membrane vesicle production in Escherichia coli. PloS ONE 10, e0139200. doi: 10.1371/journal.pone.0139200
Kyrova, K., Stepanova, H., Rychlik, I., Faldyna, M., and Volf, J. (2012). SPI-1 encoded genes of Salmonella Typhimurium influence differential polarization of porcine alveolar macrophages in vitro. BMC Vet. Res. 8, 115. doi: 10.1186/1746-6148-8-115
Lapaquette, P., Bringer, M. A., and Darfeuille-Michaud, A. (2012). Defects in autophagy favour adherent-invasive Escherichia coli persistence within macrophages leading to increased pro-inflammatory response. Cell. Microbiol. 14, 791–807. doi: 10.1111/j.1462-5822.2012.01768.x
Larabi, A., Dalmasso, G., Delmas, J., Barnich, N., and Nguyen, H. T. T. (2020). Exosomes transfer miRNAs from cell-to-cell to inhibit autophagy during infection with Crohn's disease-associated adherent-invasive E. coli. Gut microbes 11, 1677–1694. doi: 10.1080/19490976.2020.1771985
Lawrence, A. E., Abuaita, B. H., Berger, R. P., Hill, D. R., Huang, S., Yadagiri, V. K., et al. (2021). Salmonella enterica serovar typhimurium SPI-1 and SPI-2 shape the global transcriptional landscape in a human intestinal organoid model system. mBio 12, e00399–21. doi: 10.1128/mBio.00399-21
Lawrence, T., and Natoli, G. (2011). Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761. doi: 10.1038/nri3088
Lee, E. S., Song, E. J., Nam, Y. D., and Lee, S. Y. (2018). Probiotics in human health and disease: from nutribiotics to pharmabiotics. J. Microbiol. (Seoul, Korea) 56, 773–782. doi: 10.1007/s12275-018-8293-y
Lee, E. Y., Bang, J. Y., Park, G. W., Choi, D. S., Kang, J. S., Kim, H. J., et al. (2007). Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 7, 3143–3153. doi: 10.1002/pmic.200700196
Lee, J., Kim, S. H., Choi, D. S., Lee, J. S., Kim, D. K., Go, G., et al. (2015). Proteomic analysis of extracellular vesicles derived from Mycobacterium tuberculosis. Proteomics 15, 3331–3337. doi: 10.1002/pmic.201500037
Lee, K. S., Lee, J., Lee, P., Kim, C. U., Kim, D. J., Jeong, Y. J., et al. (2020). Exosomes released from Shiga toxin 2a-treated human macrophages modulate inflammatory responses and induce cell death in toxin receptor expressing human cells. Cell MicroBiol. 22, e13249. doi: 10.1111/cmi.13249
Li, L., Cheng, Y., Emrich, S., and Schorey, J. (2018). Activation of endothelial cells by extracellular vesicles derived from Mycobacterium tuberculosis infected macrophages or mice. PloS ONE 13, e0198337. doi: 10.1371/journal.pone.0198337
Li, Z., Clarke, A. J., and Beveridge, T. J. (1998). Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 180, 5478–5483. doi: 10.1128/JB.180.20.5478-5483.1998
Li, Z. T., Zhang, R. L., Bi, X. G., Xu, L., Fan, M., Xie, D., et al. (2015). Outer membrane vesicles isolated from two clinical Acinetobacter baumannii strains exhibit different toxicity and proteome characteristics. Microbial pathogenesis 81, 46–52. doi: 10.1016/j.micpath.2015.03.009
Lim, K. H., and Staudt, L. M. (2013). Toll-like receptor signaling. Cold Spring Harb Perspect Biol. 5, a011247. doi: 10.1101/cshperspect.a011247
Lin, J., Zhang, W., Cheng, J., Yang, X., Zhu, K., Wang, Y., et al. (2017). A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat. Commun. 8, 14888. doi: 10.1038/ncomms14888
Liu, H., Zhang, Q., Wang, S., Weng, W., Jing, Y., Su, J., et al. (2022). Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioactive Mat. 14, 169–181. doi: 10.1016/j.bioactmat.2021.12.006
Liu, L., Liang, L., Yang, C., Zhou, Y., and Chen, Y. (2021). Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes 13, 1–20. doi: 10.1080/19490976.2021.1902718
Livermore, D. M. (2004). The need for new antibiotics. Clin. Microbiol. Infect. 10, 1–9. doi: 10.1111/j.1465-0691.2004.1004.x
Lu, X., Yu, C., Zhang, C., Zhang, H., Li, Y., Cheng, X., et al. (2021). Effects of Salmonella enterica serovar typhimurium sseK1 on macrophage inflammation-related cytokines and glycolysis. Cytokine 140, 155424. doi: 10.1016/j.cyto.2021.155424
MacDonald, I. A., and Kuehn, M. J. (2012). Offense and defense: microbial membrane vesicles play both ways. Res. Microbiol. 163, 607–618. doi: 10.1016/j.resmic.2012.10.020
Makepeace, B. L., Watt, P. J., Heckels, J. E., and Christodoulides, M. (2001). Interactions of Neisseria gonorrhoeae with mature human macrophage opacity proteins influence production of proinflammatory cytokines. Infect Immun 69, 1909–1913. doi: 10.1128/IAI.69.3.1909-1913.2001
Manzanillo, P. S., Shiloh, M. U., Portnoy, D. A., and Cox, J. S. (2012). Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11, 469–480. doi: 10.1016/j.chom.2012.03.007
Marinho, F. A., de Paula, R. R., Mendes, A. C., de Almeida, L. A., Gomes, M. T., Carvalho, M. V., et al. (2013). Toll-like receptor 6 senses Mycobacterium avium and is required for efficient control of mycobacterial infection. Eur. J. Immunol. 43, 2373–2385. doi: 10.1002/eji.201243208
Mathieu, M., Martin-Jaular, L., Lavieu, G., and Théry, C. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17. doi: 10.1038/s41556-018-0250-9
McCaig, W. D., Koller, A., and Thanassi, D. G. (2013). Production of outer membrane vesicles and outer membrane tubes by Francisella novicida. J. Bacteriol. 195, 1120–1132. doi: 10.1128/JB.02007-12
McNab, F. W., Ewbank, J., Howes, A., Moreira-Teixeira, L., Martirosyan, A., Ghilardi, N., et al. (2014). Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-γ for production of IL-12 and bacterial killing in Mycobacterium tuberculosis-infected macrophages. J. Immunol. (Baltimore, Md: 1950) 193, 3600–3612. doi: 10.4049/jimmunol.1401088
Mège, J. L., Mehraj, V., and Capo, C. (2011). Macrophage polarization and bacterial infections. Curr. Opin. Infect. Dis. 24, 230–234. doi: 10.1097/QCO.0b013e328344b73e
Mirzaei, R., Babakhani, S., Ajorloo, P., Ahmadi, R. H., Hosseini-Fard, S. R., Keyvani, H., et al. (2021). The emerging role of exosomal miRNAs as a diagnostic and therapeutic biomarker in Mycobacterium tuberculosis infection. Mol. Med. (Cambridge, Mass) 27, 34. doi: 10.1186/s10020-021-00296-1
Mitsuhashi, S., Feldbrügge, L., Csizmadia, E., Mitsuhashi, M., Robson, S. C., Moss, A. C., et al. (2016). Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm. Bowel Dis. 22, 1587–1595. doi: 10.1097/MIB.0000000000000840
Moreira-Teixeira, L., Redford, P. S., Stavropoulos, E., Ghilardi, N., Maynard, C. L., Weaver, C. T. Freitas do Rosário, A.P., et al. (2017). T cell-derived IL-10 impairs host resistance to mycobacterium tuberculosis infection. J. Immunol. (Baltimore, Md: 1950) 199, 613–623. doi: 10.4049/jimmunol.1601340
Moussouni, M., Berry, L., Sipka, T., Nguyen-Chi, M., and Blanc-Potard, A. B. (2021). Pseudomonas aeruginosa OprF plays a role in resistance to macrophage clearance during acute infection. Sci. Rep. 11, 359. doi: 10.1038/s41598-020-79678-0
Murray, P. J. (2017). Macrophage polarization. Annu. Rev. Physiol. 79, 541–566. doi: 10.1146/annurev-physiol-022516-034339
Murray, P. J., Allen, J. E., Biswas, S. K., Fisher, E. A., Gilroy, D. W., Goerdt, S., et al. (2014). Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. doi: 10.1016/j.immuni.2014.06.008
Nguyen, H. T., Dalmasso, G., Müller, S., Carrière, J., Seibold, F., Darfeuille-Michaud, A., et al. (2014). Crohn's disease-associated adherent invasive Escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology 146, 508–519. doi: 10.1053/j.gastro.2013.10.021
Nirujogi, T. S., Kotha, S. R., Chung, S., Reader, B. F., Yenigalla, A., Zhang, L., et al. (2022). Lipidomic profiling of bronchoalveolar lavage fluid extracellular vesicles indicates their involvement in lipopolysaccharide-induced acute lung injury. J. Innate Immun. 14, 555–568. doi: 10.1159/000522338
O'Donoghue, E. J., and Krachler, A. M. (2016). Mechanisms of outer membrane vesicle entry into host cells. Cell MicroBiol. 18, 1508–1517. doi: 10.1111/cmi.12655
O'Garra, A., Redford, P. S., McNab, F. W., Bloom, C. I., Wilkinson, R. J., Berry, M. P., et al. (2013). The immune response in tuberculosis. Ann. Rev. Immunol. 31, 475–527. doi: 10.1146/annurev-immunol-032712-095939
Ott, J., Hiesgen, C., and Mayer, K. (2011). Lipids in critical care medicine. Prostaglandins leukotrienes Essent. Fatty Acids 85, 267–273. doi: 10.1016/j.plefa.2011.04.011
Owen, K. A., Anderson, C. J., and Casanova, J. E. (2016). Salmonella suppresses the TRIF-dependent type I interferon response in macrophages. mBio 7, e02051–02015. doi: 10.1128/mBio.02051-15
Palacios, A., Gupta, S., Rodriguez, G. M., and Prados-Rosales, R. (2021). Extracellular vesicles in the context of Mycobacterium tuberculosis infection. Mol. Immunol. 133, 175–181. doi: 10.1016/j.molimm.2021.02.010
Panagi, I., Jennings, E., Zeng, J., Günster, R. A., Stones, C. D., Mak, H., et al. (2020). Salmonella effector SteE converts the mammalian serine/threonine kinase GSK3 into a tyrosine kinase to direct macrophage polarization. Cell Host Microbe 27, 41–53. e46. doi: 10.1016/j.chom.2019.11.002
Pathak, S. K., Basu, S., Basu, K. K., Banerjee, A., Pathak, S., Bhattacharyya, A., et al. (2007). Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat. Immunol. 8, 610–618. doi: 10.1038/ni1468
Paynich, M. L., Jones-Burrage, S. E., and Knight, K. L. (2017). Exopolysaccharide from Bacillus subtilis induces anti-inflammatory M2 macrophages that prevent T cell-mediated disease. J. Immunol. (Baltimore, Md: 1950) 198, 2689–2698. doi: 10.4049/jimmunol.1601641
Pfeifhofer-Obermair, C., Albrecht-Schgoer, K., Peer, S., Nairz, M., Siegmund, K., Klepsch, V., et al. (2016). Role of PKCtheta in macrophage-mediated immune response to Salmonella typhimurium infection in mice. Cell Commun. Signal. CCS 14, 14. doi: 10.1186/s12964-016-0137-y
Ramos, G. P., and Papadakis, K. A. (2019). Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 94, 155–165. doi: 10.1016/j.mayocp.2018.09.013
Richardson, E. T., Shukla, S., Sweet, D. R., Wearsch, P. A., Tsichlis, P. N., Boom, W. H., et al. (2015). Toll-like receptor 2-dependent extracellular signal-regulated kinase signaling in Mycobacterium tuberculosis-infected macrophages drives anti-inflammatory responses and inhibits Th1 polarization of responding T cells. Infrct. Immunity 83, 2242–2254. doi: 10.1128/IAI.00135-15
Robb, C. T., Regan, K. H., Dorward, D. A., and Rossi, A. G. (2016). Key mechanisms governing resolution of lung inflammation. Sem. Immunopathol. 38, 425–448. doi: 10.1007/s00281-016-0560-6
Rocha, F. G., Ottenberg, G., Eure, Z. G., Davey, M. E., and Gibson, F. C. 3rd (2021). Sphingolipid-containing outer membrane vesicles serve as a delivery vehicle to limit macrophage immune response to Porphyromonas gingivalis. Infrct. Immunity 89, e00614–20. doi: 10.1128/IAI.00614-20
Rueter, C., and Bielaszewska, M. (2020). Secretion and delivery of intestinal pathogenic escherichia coli virulence factors via outer membrane vesicles. Front. Cell. Infect. Microbiol. 10, 91. doi: 10.3389/fcimb.2020.00091
Rüter, C., Lubos, M. L., Norkowski, S., and Schmidt, M. A. (2018). All in-Multiple parallel strategies for intracellular delivery by bacterial pathogens. Int J. Med MicroBiol. 308, 872–881. doi: 10.1016/j.ijmm.2018.06.007
Saadatpour, L., Fadaee, E., Fadaei, S., Nassiri Mansour, R., Mohammadi, M., Mousavi, S. M., et al. (2016). Glioblastoma: exosome and microRNA as novel diagnosis biomarkers. Cancer Gene Ther. 23, 415–418. doi: 10.1038/cgt.2016.48
Saliba, A. E., Li, L., Westermann, A. J., Appenzeller, S., Stapels, D. A., Schulte, L. N., et al. (2016). Single-cell RNA-seq ties macrophage polarization to growth rate of intracellular Salmonella. Nat. Microbiol. 2, 16206. doi: 10.1038/nmicrobiol.2016.206
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R., and Rastall, R. A. (2019). Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 16, 605–616. doi: 10.1038/s41575-019-0173-3
Santos, J. C., Dick, M. S., Lagrange, B., Degrandi, D., Pfeffer, K., Yamamoto, M., et al. (2018). LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J. 37, e98089. doi: 10.15252/embj.201798089
Schneider, P., Holler, N., Bodmer, J. L., Hahne, M., Frei, K., Fontana, A., et al. (1998). Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp Med 187, 1205–1213. doi: 10.1084/jem.187.8.1205
Schorey, J. S., Cheng, Y., Singh, P. P., and Smith, V. L. (2015). Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 16, 24–43. doi: 10.15252/embr.201439363
Schwechheimer, C., and Kuehn, M. J. (2015). Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619. doi: 10.1038/nrmicro3525
Singh, P. P., LeMaire, C., Tan, J.C., Zeng, E., and Schorey, J.S. (2011). Exosomes released from M. tuberculosis infected cells can suppress IFN-γ mediated activation of naïve macrophages. PloS ONE 6, e18564. doi: 10.1371/journal.pone.0018564
Singh, P. P., Li, L., and Schorey, J. S. (2015). Exosomal RNA from Mycobacterium tuberculosis-infected cells is functional in recipient macrophages. Traffic (Copenhagen, Denmark) 16, 555–571. doi: 10.1111/tra.12278
Singh, P. P., Smith, V. L., Karakousis, P. C., and Schorey, J. S. (2012). Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J. Immunol. (Baltimore, Md: 1950) 189, 777–785. doi: 10.4049/jimmunol.1103638
Singhania, A., Wilkinson, R. J., Rodrigue, M., Haldar, P., and O'Garra, A. (2018). The value of transcriptomics in advancing knowledge of the immune response and diagnosis in tuberculosis. Nat. Immunol. 19, 1159–1168. doi: 10.1038/s41590-018-0225-9
Skerniškyte, J., Karazijaite, E., Lučiunaite, A., and SuŽiedeliene, E. (2021). OmpA protein-deficient Acinetobacter baumannii outer membrane vesicles trigger reduced inflammatory response. Pathogens (Basel, Switzerland) 10, 407. doi: 10.3390/pathogens10040407
Smith, T. D., Tse, M. J., Read, E. L., and Liu, W. F. (2016). Regulation of macrophage polarization and plasticity by complex activation signals. Integrative Biology: Quantitative Biosciences from Nano to Macro 8, 946–955. doi: 10.1039/c6ib00105j
Smith, V. L., Jackson, L., and Schorey, J. S. (2015). Ubiquitination as a mechanism to transport soluble mycobacterial and eukaryotic proteins to exosomes. J. Immunol. (Baltimore, Md: 1950) 195, 2722–2730. doi: 10.4049/jimmunol.1403186
Soudi, H., Falsafi, T., Gharavi, S., and Mahboubi, M. (2020). The role of helicobacter pylori proinflammatory outer membrane protein and propolis in immunomodulation on U937 macrophage cell model. Galen Med. J. 9, e1687. doi: 10.31661/gmj.v9i0.1687
Stapels, D. A. C., Hill, P. W. S., Westermann, A. J., Fisher, R. A., Thurston, T. L., Saliba, A. E., et al. (2018). Salmonella persisters undermine host immune defenses during antibiotic treatment. Science (New York, NY) 362, 1156–1160. doi: 10.1126/science.aat7148
Ti, D., Hao, H., Tong, C., Liu, J., Dong, L., Zheng, J., et al. (2015). LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 13, 308. doi: 10.1186/s12967-015-0642-6
Tian, Y., Cheng, C., Wei, Y., Yang, F., and Li, G. (2022). The role of exosomes in inflammatory diseases and tumor-related inflammation. Cells 11, 1005. doi: 10.3390/cells11061005
Tiku, V., Kofoed, E. M., Yan, D., Kang, J., Xu, M., Reichelt, M., et al. (2021). Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii. Sci. Rep 11, 618. doi: 10.1038/s41598-020-79966-9
Vanaja, S. K., Russo, A. J., Behl, B., Banerjee, I., Yankova, M., Deshmukh, S. D., et al. (2016). Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165, 1106–1119. doi: 10.1016/j.cell.2016.04.015
Volgers, C., Benedikter, B. J., Grauls, G. E., Savelkoul, P. H. M., and Stassen, F. R. M. (2017a). Immunomodulatory role for membrane vesicles released by THP-1 macrophages and respiratory pathogens during macrophage infection. BMC MicroBiol. 17, 216. doi: 10.1186/s12866-017-1122-3
Volgers, C., Grauls, G. E., Hellebrand, P. H. M., Savelkoul, P. H. M., and Stassen, F. R. M. (2017b). Budesonide, fluticasone propionate, and azithromycin do not modulate the membrane vesicle release by THP-1 macrophages and respiratory pathogens during macrophage infection. Inflammopharmacology 25, 643–651. doi: 10.1007/s10787-017-0359-7
Walters, S. B., Kieckbusch, J., Nagalingam, G., Swain, A., Latham, S. L., Grau, G. E., et al. (2013). Microparticles from mycobacteria-infected macrophages promote inflammation and cellular migration. J. Immunol. (Baltimore, Md: 1950) 190, 669–677. doi: 10.4049/jimmunol.1201856
Wang, J., Chen, C., Wang, L., Xie, M., Ge, X., Wu, S., et al. (2022). Patient-derived tumor organoids: new progress and opportunities to facilitate precision cancer immunotherapy. Front. Oncol. 12, 872531. doi: 10.3389/fonc.2022.872531
Wang, J., Yao, Y., Xiong, J., Wu, J., Tang, X., Li, G., et al. (2015). Evaluation of the inflammatory response in macrophages stimulated with exosomes secreted by Mycobacterium avium-infected macrophages. BioMed Res. Int. 2015, 658421. doi: 10.1155/2015/658421
Wang, J. J., Chen, C., Xie, P. F., Pan, Y., Tan, Y. H., Tang, L. J., et al. (2014). Proteomic analysis and immune properties of exosomes released by macrophages infected with Mycobacterium avium. Microbes Infecti. 16, 283–291. doi: 10.1016/j.micinf.2013.12.001
Wang, S., Gao, J., and Wang, Z. (2019). Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip. Rev. NanoMed. Nanobiotechnol. 11, e1523. doi: 10.1002/wnan.1523
Wang, X., Zhang, H., Guo, R., Li, X., Liu, H., Wang, Z., et al. (2021). MicroRNA-223 modulates the IL-4-medicated macrophage M2-type polarization to control the progress of sepsis. Int. Immunopharmacol. 96, 107783. doi: 10.1016/j.intimp.2021.107783
Wang, Y., Wu, C., Gao, J., Du, X., Chen, X., Zhang, M., et al. (2021). Host metabolic shift during systemic Salmonella infection revealed by comparative proteomics. Emerg. Microb. Infect. 10, 1849–1861. doi: 10.1080/22221751.2021.1974316
Williams, J. N., Skipp, P. J., Humphries, H. E., Christodoulides, M., O'Connor, C. D., Heckels, J. E., et al. (2007). Proteomic analysis of outer membranes and vesicles from wild-type serogroup B Neisseria meningitidis and a lipopolysaccharide-deficient mutant. Infrct. Immunity 75, 1364–1372. doi: 10.1128/IAI.01424-06
Yáñez-Mó, M., Siljander, P. R., Andreu, Z., Zavec, A. B., Borràs, F. E., Buzas, E. I., et al. (2015). Biological properties of extracellular vesicles and their physiological functions. J. ExtraCell. Vesicles 4, 27066. doi: 10.3402/jev.v4.27066
Yang, J., Hwang, I., Lee, E., Shin, S. J., Lee, E. J., Rhee, J. H., et al. (2020). Bacterial outer membrane vesicle-mediated cytosolic delivery of flagellin triggers host NLRC4 canonical inflammasome signaling. Front. Immunol. 11, 581165. doi: 10.3389/fimmu.2020.581165
Yaron, S., Kolling, G. L., Simon, L., and Matthews, K. R. (2000). Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 66, 4414–4420. doi: 10.1128/AEM.66.10.4414-4420.2000
Zhang, M., Xie, Y., Li, S., Ye, X., Jiang, Y., Tang, L., et al. (2021). Proteomics analysis of exosomes from patients with active tuberculosis reveals infection profiles and potential biomarkers. Front. Microbiol. 12, 800807. doi: 10.3389/fmicb.2021.800807
Keywords: extracellular vesicles, exosome, macrophage polarization, membrane vesicles, bacterial infectious diseases
Citation: Qu M, Zhu H and Zhang X (2022) Extracellular vesicle-mediated regulation of macrophage polarization in bacterial infections. Front. Microbiol. 13:1039040. doi: 10.3389/fmicb.2022.1039040
Received: 07 September 2022; Accepted: 25 November 2022;
Published: 22 December 2022.
Edited by:
Hridayesh Prakash, Maharishi Markandeshwar University, Mullana, IndiaReviewed by:
Supriya Shukla, National Institute of Allergy and Infectious Diseases (NIH), United StatesMonica Reis, University of Edinburgh, United Kingdom
Andreas Kupz, James Cook University, Australia
Copyright © 2022 Qu, Zhu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingxiao Zhang,  zhangxingxiao2017@163.com
zhangxingxiao2017@163.com
 Mingjuan Qu
Mingjuan Qu Hongwei Zhu
Hongwei Zhu Xingxiao Zhang
Xingxiao Zhang