
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 15 November 2022
Sec. Virology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1036955
This article is part of the Research TopicHTLV-1: Addressing Unmet Research Needs, Vol IIView all 6 articles
 Nahoko Komatsu1
Nahoko Komatsu1 Masako Iwanaga2
Masako Iwanaga2 Yuri Hasegawa1
Yuri Hasegawa1 Shoko Miura1
Shoko Miura1 Naoki Fuchi1
Naoki Fuchi1 Hiroyuki Moriuchi3
Hiroyuki Moriuchi3 Katsunori Yanagihara4
Katsunori Yanagihara4 Kiyonori Miura1*
Kiyonori Miura1*Background: Human T-cell leukemia virus type-1 (HTLV-1) is transmitted vertically from an infected mother to her child via breastfeeding during infancy or horizontally via sexual contact. However, little information is available on the HTLV-1 seroconversion rate in pregnant mothers and the impact of new HTLV-1 infection on mothers and babies during the perinatal period.
Methods: From the database of a prefecture-wide antenatal adult T-cell leukemia prevention program in Nagasaki, Japan, we extracted data on 57,323 pregnant women who were screened for anti-HTLV-1 antibody during 2011–2018. Data on the 16,863 subjects whose HTLV-1 proviral load (PVL) was measured more than twice were included in our analyses.
Results: In total, 133 (0.79%) pregnant women were HTLV-1-positive during their first pregnancy and nine (0.05%) seroconverted before or during subsequent pregnancies (between pregnancies). The median PVL (per 100 peripheral blood mononuclear cells) was significantly lower in the seroconverted mothers (0.10%) than in the initially seropositive mothers (0.15%). A repeated measures correlation analysis for the individual PVLs of the HTLV-1-positive pregnant women showed that PVL increased with parity number (rrm = 0.25) with no perinatal problems.
Conclusion: The HTLV-1 seroconversion rate between pregnancies was 0.05%, and their HTLV-1 PVL increased annually but no perinatal problems were noted.
Human T-cell leukemia virus-1 (HTLV-1) is a retrovirus that causes adult T-cell leukemia (ATL), HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), and a variety of HTLV-1-associated inflammatory disorders (Watanabe, 1997). Globally, HTLV-1 infects at least 5–10 million people (Gessain and Cassar, 2012), particularly in southwest Japan, Brazil, the Caribbean islands, Central and South America, sub-Saharan Africa, and central Australia (Gessain and Cassar, 2012; World Health Organization, 2020). HTLV-1 infects mainly CD4-positive T-cells and is known to transmit from person-to-person via cell-to-cell contact between HTLV-1-infected cells and uninfected cells through three modes (Bangham, 2003; Matsuoka and Jeang, 2007; Pique and Jones, 2012): vertical mother-to-child transmission from HTLV-1-infected mothers via breastfeeding (Hino et al., 1985), horizontal transmission from HTLV-1-infected partners via sexual intercourse (Stuver et al., 1993; Kaplan et al., 1996; Roucoux et al., 2005; Satake et al., 2016; Paiva et al., 2017), and iatrogenic transmission via HTLV-1-infected donated blood or organs. However, the risk of iatrogenic transmission through transfusion can be reduced by performing leukoreduction prior to transfusion (Okochi et al., 1984; Armstrong et al., 2012).
The first scientific evidence of vertical transmission from a HTLV-1-infected mother to their infant via prolonged breastfeeding by the infected mother was presented in 1985 by researchers from Nagasaki University, located in southwest Japan (Hino et al., 1985; Figure 1). They presented the following findings: (1) the first nationwide survey of HTLV-1 infection in Japan identified Nagasaki Prefecture as one of the regions with the highest prevalence of HTLV-1-infected individuals (Tajima et al., 1982); (2) HTLV-1-infected cells are present in breastmilk from HTLV-1-carrier mothers, and infection through breastfeeding is associated with a higher probability of developing ATL (Kinoshita et al., 1984); and (3) experimentally, HTLV-1 can be transmitted via intraoral inoculation among common marmosets (Kinoshita et al., 1985; Yamanouchi et al., 1985). Soon after, a prefecture-wide, multidisciplinary, antenatal intervention program, known as the “ATL Prevention Program (APP),” was established in 1987 in Nagasaki (APP Nagasaki; Hino et al., 1987) through collaboration with virologists, obstetric gynecologists, hematologists, pediatricians, and clinical laboratory scientists at Nagasaki University, obstetric gynecologists throughout Nagasaki Prefecture, and public health officers of the Maternal and Child Health Division (MCHD) of the Nagasaki Prefectural Government. The purpose of APP Nagasaki was to reduce the vertical transmission of HTLV-1 via breastfeeding and to reduce the future development of HTLV-1-associated diseases through systematic screening for anti-HTLV-1 antibodies in pregnant women and the avoidance of breastfeeding in identified HTLV-1-infected mothers, in Nagasaki Prefecture.
Figure 1. Location of the study area in Nagasaki Prefecture, Japan. The present study was carried out in Nagasaki Prefecture, which is located in the most western part of Japan. Only the part of Nagasaki Prefecture colored pink is included in this study. (Tsushima islands are not included).
As previously reported, APP Nagasaki led to a marked reduction in mother-to-child HTLV-1 transmission rates from 20.5% before the APP began to 2.4% after its implementation (Hino et al., 1997; Katamine, 1999; Hino, 2011; Moriuchi et al., 2013; Miura and Masuzaki, 2017). Other intervention programs similar to APP Nagasaki have been implemented in several areas endemic for HTLV-1 in Japan (Takahashi et al., 1991; Kashiwagi et al., 2004). However, HTLV-1 infection is currently widespread across geographical regions (Watanabe, 2011; Satake et al., 2012), and the number of HTLV-1 carriers in the metropolitan areas in Japan has increased. This situation probably reflects the migration of individuals from HTLV-1-endemic areas (Watanabe, 2011). A nationwide antenatal screening program for HTLV-1 was introduced in Japan in 2011 (Itabashi et al., 2020). Currently, it is estimated that each year ~1,600 HTLV-1-infected pregnant mothers in Japan (almost 0.16% of all pregnant mothers in Japan; Suzuki et al., 2014) are recommended to refrain from breastfeeding to avoid mother-to-child HTLV-1 transmission by methods similar to that of APP Nagasaki.
Recently, researchers from APP Nagasaki reported that the HTLV-1 proviral load (PVL), induced regulatory T-cell population, and soluble interleukin-2 receptor (sIL-2R) levels of HTLV-1-infected pregnant mothers remained stable during pregnancy but became elevated after delivery (Fuchi et al., 2018). However, many questions regarding the impact of HTLV-1 infection on pregnant mothers remain unanswered. For example, the number of pregnant mothers who seroconvert from HTLV-1 negative to positive between pregnancies, how the HTLV-1 PVL changes in HTLV-1-infected pregnant mothers through delivery (between pregnancies), and the specific risks to pregnancy outcomes for HTLV-1-infected and HTLV-1-seroconverted mothers are not currently known.
The present study therefore aimed to assess the HTLV-1 seroconversion status in multiparous mothers who participated in APP Nagasaki, the individual changes of the HTLV-1 PVL between pregnancies in HTLV-1-positive mothers, and the impact of HTLV-1 positivity on pregnancy outcomes.
From its initiation in July 1987 to April 2018, the APP Nagasaki database included information on ~330,000 pregnant mothers, from almost all areas of Nagasaki Prefecture, who were tested for HTLV-1 infection (Figure 1). The database is managed by the Department of Obstetrics–Gynecology at Nagasaki University Hospital (Miura and Masuzaki, 2017).
Figure 2 shows the protocol employed for APP Nagasaki (Miura and Masuzaki, 2017). Briefly, any pregnant mothers who were ~30 weeks of gestation and living in most areas of Nagasaki Prefecture were asked to participate in APP Nagasaki when visiting their obstetric gynecologists. After providing consent to participate in the project, each pregnant mother was required to provide a blood sample for an anti-HTLV-1 antibody test. From 2011 onwards, such blood samples were sent to one of five commercial clinical laboratory companies (see Acknowledgment) where a screening test for anti-HTLV-1 antibody was performed using either a particle agglutination (PA) assay, a chemiluminescent immunoassay (CLIA), or a chemiluminescent enzyme immunoassay (CLEIA). The definition of “HTLV-1-positive” as a result of the screening test is a titer of 8 or higher in the PA assay or a cutoff index (COI) of ≥1.0 in the CLEIA or CLIA, in accordance with the manufacturer’s criteria (Fujirebio, Japan).
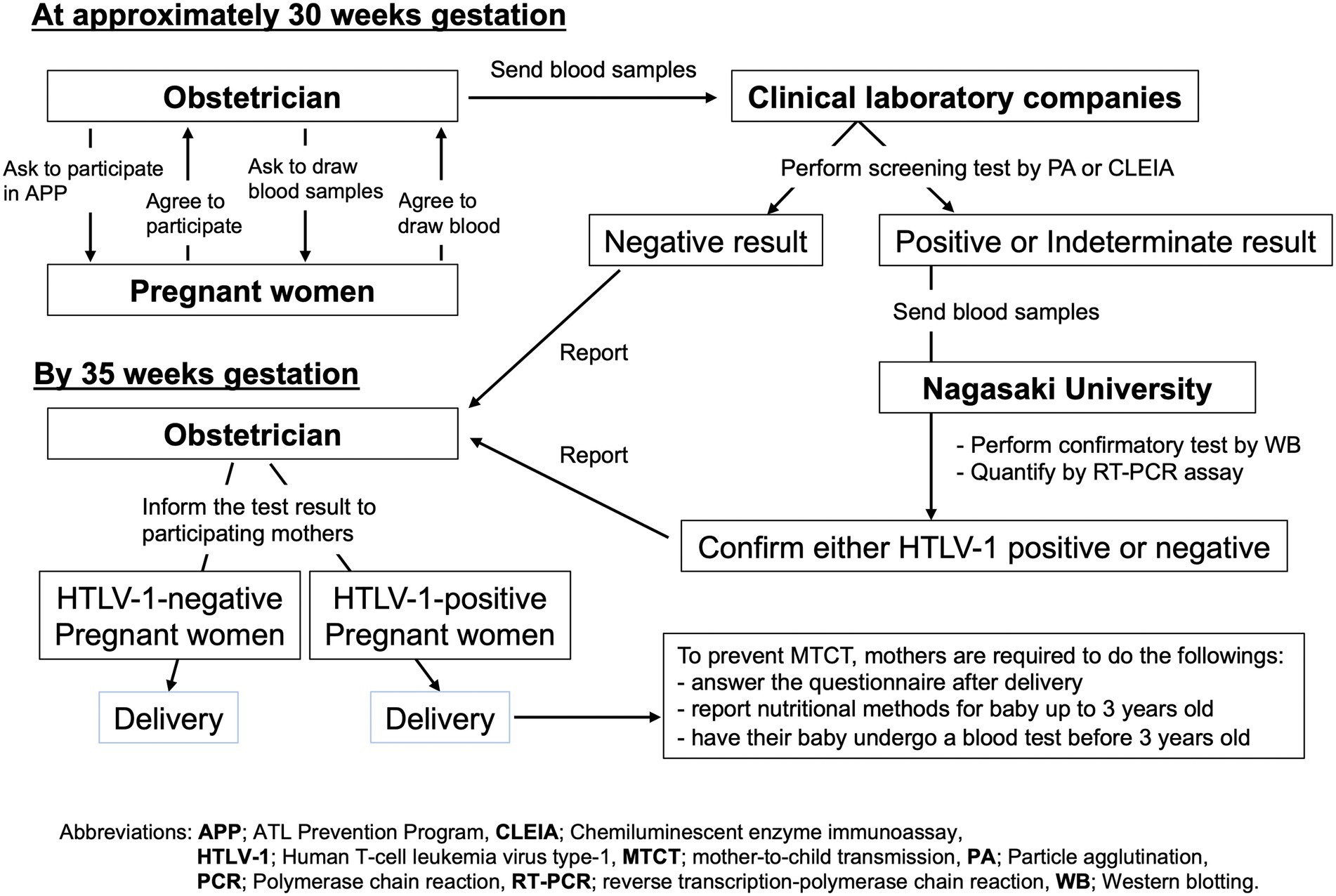
Figure 2. Study protocol of the APP Nagasaki project. By 30 weeks of gestation, blood samples were obtained from the pregnant mothers who participated in APP Nagasaki to screen for anti-HTLV-1 antibody. Negative test results were reported directly to the respective obstetrician. For positive or indeterminate test results, confirmatory tests were performed. All of the test results were scheduled to be returned to the respective obstetricians by 35 weeks of gestation.
All of the negative screening test results from the clinical laboratory companies were sent back to the respective obstetric gynecologists, for them to inform the respective pregnant mothers. From 2011 onward, in the case of a positive or suspected positive screening test result, the residual blood sample was forwarded to the APP Nagasaki office at Nagasaki University Hospital where a confirmatory test was performed by western blotting and the HTLV-1 PVL was quantified by a real-time polymerase chain reaction (RT-PCR) assay in the Department of Laboratory Medicine. The final test results were reported to the respective obstetric gynecologists, who then informed the respective mothers by 35 weeks of gestation (Figure 2). The obstetric gynecologists also asked their public health nurses to explain to HTLV-1-positive pregnant mothers how to prevent HTLV-1 mother-to-child transmission, preferentially through exclusive bottle-feeding or alternatively by freeze–thawing breast milk or only short-term breastfeeding for no more than 3 months. According to the APP Nagasaki report, the majority of HTLV-1-positive pregnant mothers chose the first choice option of exclusive bottle-feeding to prevent mother-to-child transmission (Miura and Masuzaki, 2017).
In APP Nagasaki, a quantitative RT-PCR assay was routinely performed from 2011 onward to quantify the HTLV-1 PVL in peripheral blood mononuclear cells (PBMCs) from anti-HTLV-1 antibody-positive pregnant mothers and those with indeterminate western blotting results. The details of the method used to quantify the HTLV-1 PVL have been previously published (Kamihira et al., 2003; Sasaki et al., 2010), and the resulting value was expressed as the copy number per 100 PBMCs. All measurements were performed by the Department of Laboratory Medicine at Nagasaki University Hospital. Coefficient variation (CV) of HTLV-1 quantitative PCR test in Nagasaki university hospital was 13.3% (Kuramitsu et al., 2015).
Within 1 week of delivery, each of the HTLV-1-positive mothers who participated in APP Nagasaki was required to answer questions about their birthplace, pregnancy history, the feeding method chosen by their own mother (i.e., the grandmother) during their infancy, and the feeding method that they chose for their own baby. Their obstetric gynecologist was also required to answer questions regarding pregnancy/delivery complications, the health condition of the baby, and the medical history of the pregnant mother (Supplementary material S1).
The dataset used for analysis in this study was extracted from the APP Nagasaki database after obtaining approval from the Ethics Committee of the Institutional Review Board of Nagasaki University Hospital, Nagasaki, Japan (approval number: 20051810, September 2019). The APP Nagasaki was initiated in 1987, and modern techniques to measure the HTLV-1 PVL using an RT-PCR assay have been applied to participating mothers only since 2011 (Kamihira et al., 2003; Sasaki et al., 2010). Therefore, for the present analysis, we limited our data extraction to the years 2011–2018.
We analyzed the available data on pregnant mothers who were tested for HTLV-1 infection at least twice during pregnancy. The demographic data were summarized with standard descriptive statistics, such as frequencies (percentages) for categorical variables, and median (min–max) or median (interquartile range, IQR) values for continuous variables. The pregnant mother age at enrollment in the APP was treated as a continuous variable or categorized into four groups (<25, 26–30, 31–35, and >36 years old). The method of feeding that the pregnant mothers themselves received from their own mothers was categorized into three groups: long-term breastfeeding (6 months or longer), short-term breastfeeding (shorter than 6 months), and formula feeding alone. The mothers were also categorized into subgroups based on their history of gravidity (the number of pregnancies), parity (the number of live births), and divorce. Continuous variables were compared using Wilcoxon rank-sum tests. Categorical variables were compared using the Chi-squared test or Fisher’s exact test, as appropriate. All of the basic statistical analyses were performed using OpenEpi Statistical Software version 3.01 (http://www.openepi.com, Atlanta, GA, United States; Sullivan and Dean, 2009) and R package version 3.4.2 (R-Project for Statistical Computing, https://CRAN.R-project.org). To analyze changes over time in the HTLV-1 PVL by age and by parity of the HTLV-1-positive mothers, we conducted repeated measures correlation (rmcorr) analyses using the R package version 0.3.0 (R-Project for Statistical Computing, https://CRAN.R-project.org; Bakdash and Marusich, 2017) and calculated the correlation coefficient for repeated measures (rrm). All statistical tests were performed at the two-tailed 5% level of significance.
Figure 3 shows the flowchart for the present analysis. In the years 2011–2018, a total of 57,323 pregnant mothers were screened for anti-HTLV-1 antibody, and 471 (seropositive rate of ~0.82%) were identified as being HTLV-1-positive. Among the 57,323 screened women, 40,460 (70.6%) were examined only once, and the remaining 16,863 (29.4%) were examined more than twice. Of those examined only once, 338 (0.84%) were HTLV-1-positive; of those examined more than twice, 133 (0.79%) were HTLV-1-positive. Among the 133 HTLV-1-positive pregnant mothers screened during their first pregnancy, 61 (45.9%) had already been diagnosed as asymptomatic HTLV-1 carriers in a hospital before their enrollment with APP Nagasaki, but the remaining 72 had no prior HTLV-1 diagnosis information. To date, there have been no reports about ATL, HAM/TSP, or other HTLV-1-associated diseases from their obstetric gynecologists.
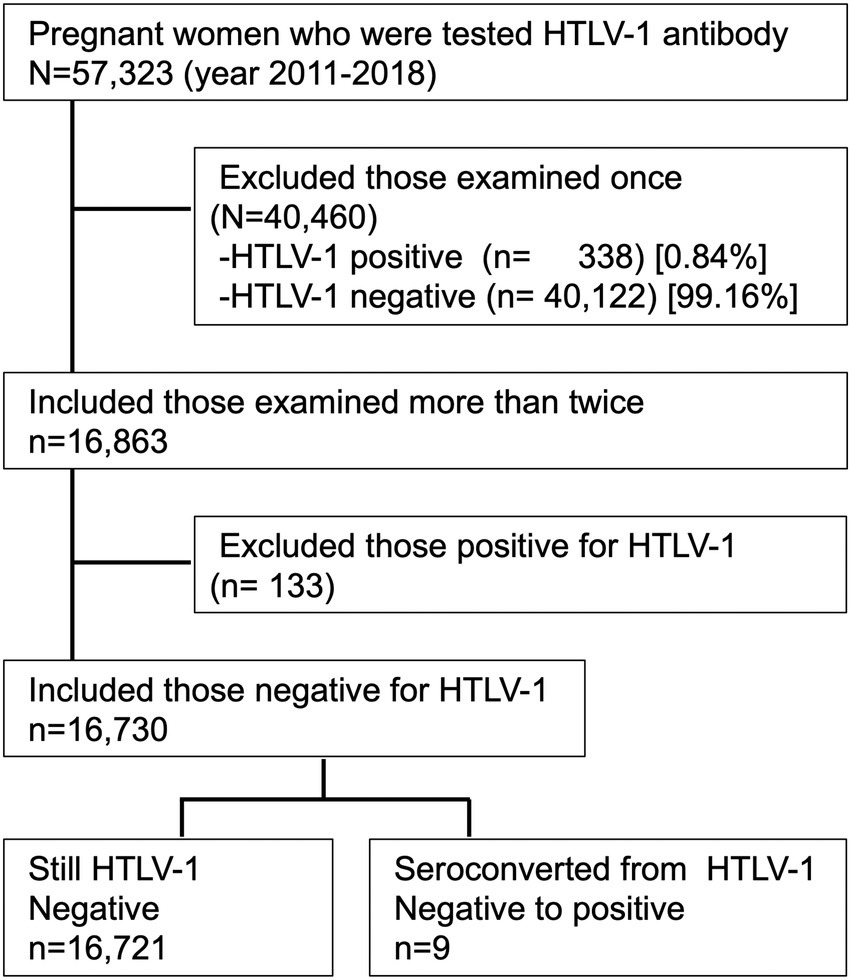
Figure 3. Study flowchart for the present analysis. Of the 57,323 pregnant mothers who were screened for anti-HTLV-1 antibody during 2011–2018, the 16,863 mothers who were examined more than twice were included in the study. Of those included, the 16,730 mothers who tested negative for anti-HTLV-1 antibody were included in the analysis. Nine of these women later seroconverted to HTLV-1-positive.
During the period between January 2011 and December 2018, nine (0.054%) of the 16,730 pregnant mothers who were found to be negative for HTLV-1 during the initial screening subsequently seroconverted to HTLV-1 positive (Figure 3). Thus, the incidence rate of HTLV-1 seroconversion among HTLV-1-negative pregnant mothers was 17.5 (95% confidence interval: 11.4–26.0) per 100,000 person-years at follow-up.
Table 1 summarizes the characteristics of pregnant mothers who were already known to be HTLV-1 seropositive before their entry into the study (n = 133) and those of mothers who were HTLV-1 seronegative at study entry but later seroconverted to anti-HTLV-1 antibody-positive (n = 9). The median age at first entry to APP Nagasaki was 30 years old (range, 17–47 years old) in mothers of known HTLV-1-seropositive status and 31 years old (range, 23–38 years old) in HTLV-1-seroconverted mothers. Although there was no statistically significant difference in the age distribution between the two groups, approximately half of those already known to be HTLV-1-seropositive were in their late 20 s at their first pregnancy, whereas approximately half of the HTLV-1-seroconverted mothers were in their 30 s at their first pregnancy. There was also no significant difference in the proportions of mothers with a family history of ATL, a divorce history, or particular screening test values or in the proportion of western blot-positive or -indeterminate individuals between the two groups.
In contrast, there were significant differences between the two groups regarding the feeding method that the participating mothers themselves received from their mothers (i.e., the grandmothers), gravidity history, parity history, and HTLV-1 PVL. In brief, compared with the group of those already known to be HTLV-1-positive before pregnancy, the group of mothers that underwent HTLV-1 seroconversion between pregnancies had lower HTLV-1 PVL (0.15% vs. 0.10% per 100 PBMCs; p < 0.024). Regarding whether the mothers had known about the HTLV-1 infection status of their husband or sexual partner, only two pregnant mothers reported HTLV-1 infection in their partner; the rest of the study participants did not know their partner’s HTLV-1 infection status.
An analysis of the HTLV-1-positive mothers revealed a statistically significant positive correlation between an increase in parity number and an increase in HTLV-1 PVL (rrm = +0.25, p = 0.012; Figure 4).
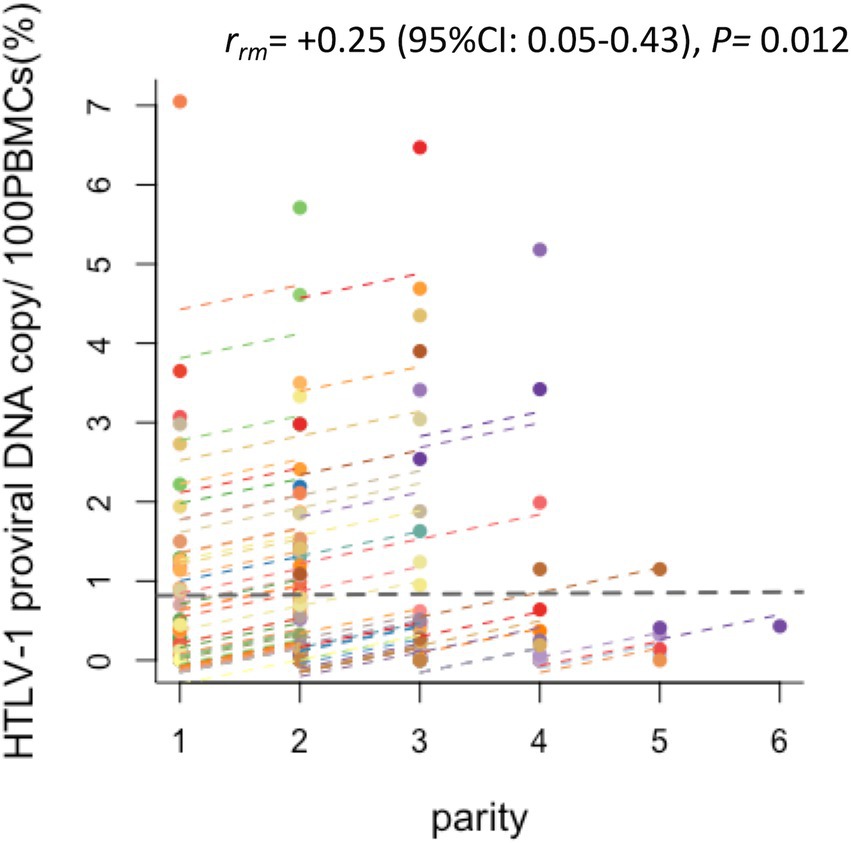
Figure 4. Changes in the HTLV-1 PVL of HTLV-1-positive pregnant women by parity. Plots of the correlation between the HTLV-1 PVL and parity of HTLV-1-positive pregnant women. Of the study participants, the 87 who were HTLV-1 seropositive and had their PVL measured more than twice were analyzed by repeated measures correlation (rmcorr). Each colored plot line shows the chronological change in HTLV-1 PVL of an individual HTLV-1-carrier pregnant woman. Positive correlations were found between increased HTLV-1 PVL and increased parity (r = 0.25, p = 0.012, 95% CI 0.05, 0.43).
Among the nine HTLV-1-seroconverted pregnant mothers, there was only one mother (No. 2 in the Supplementary material S2) for whom the HTLV-1 PVL had been measured more than twice after HTLV-1 seroconversion. Her HTLV-1 PVL increased from 1.09 at her second delivery to 3.90 at her third delivery, which suggests that, even in HTLV-1-seroconverted pregnant mothers, the HTLV-1 PVL increased over time.
Table 2 summarizes the pregnancy outcomes of mothers of known HTLV-1-positive status and mothers that seroconverted to HTLV-1 between pregnancies. There was no statistically significant difference between the two groups in the frequency of any complications that occurred during pregnancy, the length of delivery time, or the sex distribution or birth weight of the babies.
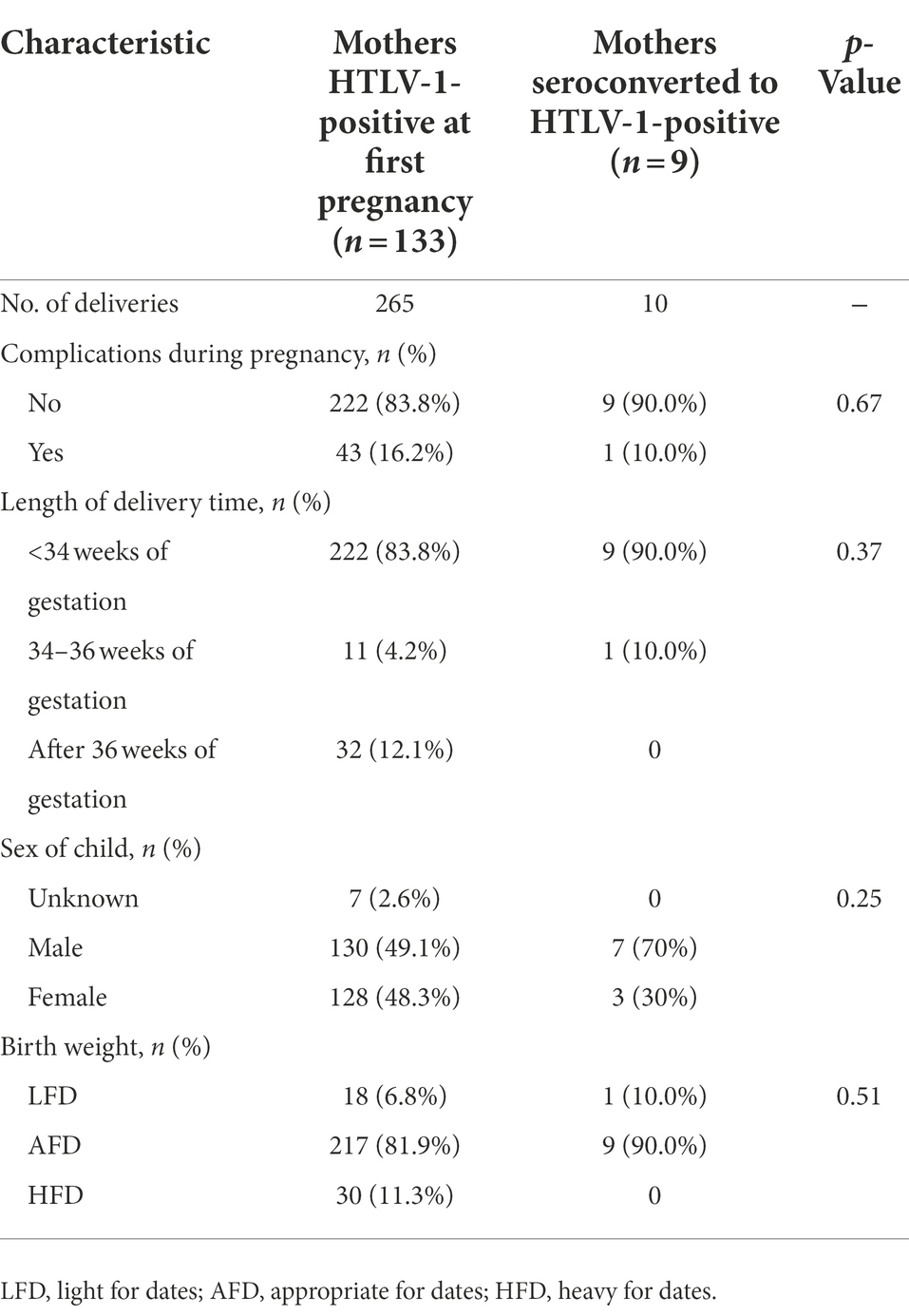
Table 2. Pregnancy outcomes in mothers with known HTLV-1-positive status and mothers with HTLV-1 seroconversion during pregnancy.
This is the first report on the HTLV-1-positive and -seroconversion status of pregnant mothers that participated in APP Nagasaki, and the first to report HTLV-1 PVL chronological data. Our main findings were that: (1) the proportion of those who underwent seroconversion from anti-HTLV-1 antibody negative to positive among pregnant mothers was 0.054%, with a seroconversion incidence rate of 17.5 per 100,000 person-years during the period 2011–2018, (2) HTLV-1 infection had no adverse effect on pregnancy outcomes in either the HTLV-1-infected or HTLV-1-seroconverted mothers; however, (3) the HTLV-1 PVL increased with increased parity number, and this showed a statistically significant positive correlation in both the HTLV-1-infected and the HTLV-1-seroconverted pregnant mothers.
The estimated incidence rate (17.5 per 100,000 person-years) of HTLV-1 seroconversion among pregnant mothers aged 17–41 years in this analysis was higher than that for female blood donors aged 16–49 years (2.03–8.54 per 100,000 person-years) in Kyushu-Okinawa, where HTLV-1 is endemic (Satake et al., 2016), despite the similar age range of these groups. A possible explanation for this difference might be the eligibility criteria for blood donation, because pregnant women or recently postpartum mothers are not eligible to donate blood in Japan. Instead, the estimated crude incidence rate in this analysis was close to the HTLV-1 seroconversion rate in female repeated blood donors aged over 50 years in Kyushu-Okinawa (11.8–17.0 per 100,000 person-years; Satake et al., 2016). One possible explanation for the HTLV-1 seroconversion rate similarity in these two groups might be related to unprotected sexual contact with HTLV-1-infected partners—the majority of pregnant mothers in our study did not know the HTLV-1 infection status of their partner.
Regarding the PVL in HTLV-1-positive pregnant mothers, we previously reported that the PVL was the highest during the postpartum period (i.e., the period just after delivery) as compared with that during pregnancy terms (i.e., the first, second, and third trimesters; Fuchi et al., 2018). The present study supported the previous report by performing time-sequential analyses of the individual PVL of both HTLV-1-positive and HTLV-1-seroconverted pregnant mothers. In other words, we hypothesize that the positive correlation between the number of deliveries and HTLV-1 PVL found in this study indicates that the elevated HTLV-1 PVL that occurs during the first postpartum period is maintained and then the HTLV-1 PVL increases further during the second postpartum period. Thus, the individual HTLV-1 PVL increased with increased parity number among multiparous women (Figure 4). Alternatively, this finding could suggest that these women are continuously exposed to HTLV-1 from their HTLV-1-positive partners (Stuver et al., 1993; Sullivan et al., 1993; Kaplan et al., 1996). Therefore, they require regular medical check-ups and monitoring of the HTLV-1 PVL for the early detection of HTLV-1-associated diseases, not only by obstetric gynecologists, but also by hematologists. This is primarily because it has been well-established that the higher the PVL in HTLV-1-infected asymptomatic carriers, the higher their risk of developing HTLV-1-associated diseases (Nagai et al., 1998; Iwanaga et al., 2010), but also because a number of case reports have indicated the development of ATL in HTLV-1-positive pregnant mothers with a high PVL (Ohba et al., 1988; Utsumi et al., 1996; Safdar et al., 2002; Amor et al., 2013; Motedayen Aval et al., 2020; Ramassamy et al., 2020). In APP Nagasaki, one asymptomatic HTLV-1-positive pregnant woman developed ATL (Fuchi et al., 2016).
We also report here, for the first time, that even in a HTLV-1-seroconverted mother, the PVL increased between her second and third pregnancies with her increase in age (the second case in Supplementary material S2). This finding supports the idea that the HTLV-1 PVL increases with parity (or age) even in HTLV-1-seroconverted pregnant mothers. Therefore, we recommend that all HTLV-1-positive women of reproductive age be checked regularly for their HTLV-1 PVL and HTLV-1-associated diseases.
Regarding pregnancy complications in HTLV-1-positive pregnant mothers, previous studies reported no difference in the degree of pregnancy complications between those who were positive and those who were negative for anti-HTLV-1 antibody (Kusuhara et al., 1987; Rosadas et al., 2019). In the present study, among the 10 deliveries from nine HTLV-1-seroconverted pregnant mothers, only one baby (10% of all deliveries) was born prematurely at 29 weeks of gestation. Although only one premature case occurred, and therefore the sample size was small, the proportion (10%) was greater than the national average for premature cases (5.6%–5.7%) in Japan during 2015–2018 (Vital Statistics of Japan, 2015–2018). Further large-scale analyses are required to determine whether the occurrence of preterm/premature birth/delivery/labor is higher in HTLV-1-infected and/or HTLV-1-seroconverted pregnant mothers than in uninfected individuals.
Regarding the babies born from HTLV-1-positive mothers, Kusuhara et al. reported that such babies may acquire their own anti-HTLV-1 antibodies by the age of 3 years and continue to acquire such antibodies until the age of 18 (Kusuhara et al., 1987). Therefore, the obstetric gynecologists participating in APP Nagasaki advised each of the HTLV-1-positive mothers to have their children tested for HTLV-1 antibodies when they reach 3 years of age. In APP Nagasaki, 10 children were born from HTLV-1-seroconverted mothers; of these, one baby girl was given artificial nutrition and tested HTLV-1-negative, while no information was available on the other nine babies.
The major limitation of this study is that the findings were based on a single area of Nagasaki Prefecture; therefore, the results may not apply to other regions of Japan. Another limitation is that our analyses were based only on healthy pregnant women; as such, information on pregnant mothers with possible HTLV-1-associated diseases was lacking. Furthermore, our method was not capable of identifying cases in which a pregnant mother is HTLV-1-negative at the screening date but seroconverts to positive after the screening date. Therefore, our results might be an underestimation. Furthermore, we have little information on the HTLV-1-infection status of the partners of the pregnant mothers. Taking these limitations into account, the actual number of pregnant mothers horizontally infected with HTLV-1 might be higher than what we observed in this study. Also, since our APP project is a kind of a public-health project to avoid mother-to-child HTLV-1-transmission rather than a research-oriented project, the accuracy of data collection may not be perfect and only peripheral blood test and HTLV-1 PVL are regularly measured. Therefore, even if we are willing to perform a multivariate analysis, it is possible to include only factors such as age and the number of deliveries. However, since aging and the number of deliveries are almost proportional, therefore it is difficult to perform a multivariate analysis as another limitation of our study.
In conclusion, this is the first report on the individual change in HTLV-1 PVL of HTLV-1-positive and HTLV-1-seroconverted pregnant mothers and the infection status of their babies in APP Nagasaki. Although there were no serious adverse effects in the HTLV-1-infected pregnant mothers and their babies, we found that the HTLV-1 PVL of the mothers increased with parity (or age), which suggests that HTLV-1-infected women of reproductive age are continuously exposed to HTLV-1 horizontally from their HTLV-1-infected partners. Therefore, further careful follow-up is needed for both HTLV-1-positive pregnant mothers and their babies.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Institutional Review Board of Nagasaki University Hospital, Nagasaki, Japan (approval number: 20051810, September 2019). The patients/participants provided their written informed consent to participate in this study.
NK and NF analysis the data of HTLV-1 test among pregnant women. MI and KM organized the study protocol. NK, MI, and KM wrote the manuscript. YH, SM, NF, and KM collected the data of HTLV-1 tests among pregnant women. HM collected the data regarding Mother to Child transmission of HTLV-1. KY and KM performed the HTLV-1 screening test of pregnant women in Nagasaki. All authors contributed to the article and approved the submitted version.
This work was partially supported by the Research Program (20fk0108124j0001, 21fk0108124j0002, 22fk0108124j0003) on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development (AMED) awarded to KM. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of AMED.
We thank all of the participating mothers in the projects, the 69 obstetric gynecologists across Nagasaki Prefecture, the Nagasaki Association of Obstetrics and Gynecology, the commercial clinical laboratory companies [SRL Inc. (Tokyo, Japan), BML Inc. (Tokyo, Japan), Shikoku Chuken Inc. (Kagawa, Japan), and CRC, LaboTech (Tokyo, Japan)], all of the experts at Nagasaki University (virologists, hematologists, pediatricians, clinical laboratory physicians, and obstetric gynecologists), all of the public health staff in Nagasaki prefectural local authorities, as well as those involved in the APP Nagasaki. We also thank Mami Machida, Maasa Miyamoto, Yuko Sakakida, Ayako Ueyama, and Yasuko Noguchi for their technical assistance, and Kate Fox and Katie Oakley from Edanz (https://jp.edanz.com/ac) for editing drafts of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1036955/full#supplementary-material
Amor, M. M., Olaso, A. S., Atienza, R., Stueben, B., Cohen, S., Kossev, P., et al. (2013). Adult T-cell leukemia-lymphoma during pregnancy. Case Rep. Oncol. Med. 2013:631825. doi: 10.1155/2013/631825
Armstrong, M. J., Corbett, C., Rowe, I. A., Taylor, G. P., and Neuberger, J. M. (2012). HTLV-1 in solid-organ transplantation: current challenges and future management strategies. Transplantation 94, 1075–1084. doi: 10.1097/TP.0b013e318263ad7a
Bakdash, J. Z., and Marusich, L. R. (2017). Repeated measures correlation. Front. Psychol. 8:456. doi: 10.3389/fpsyg.2017.00456
Bangham, C. R. M. (2003). The immune control and cell-to-cell spread of human T-lymphotropic virus type 1. J. Gen. Virol. 84, 3177–3189. doi: 10.1099/vir.0.19334-0
Fuchi, N., Miura, K., Imaizumi, Y., Hasegawa, H., Yanagihara, K., Miyazaki, Y., et al. (2016). Adult T-cell leukemia-lymphoma in a pregnant woman diagnosed as a human T-cell lymphotropic virus type 1 carrier. J. Obstet. Gynaecol. Res. 42, 336–340. doi: 10.1111/jog.12904
Fuchi, N., Miura, K., Tsukiyama, T., Sasaki, D., Ishihara, K., Tsuruda, K., et al. (2018). Natural course of human T-cell leukemia virus type 1 proviral DNA levels in carriers during pregnancy. J. Infect. Dis. 217, 1383–1389. doi: 10.1093/infdis/jiy017
Gessain, A., and Cassar, O. (2012). Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 3:388. doi: 10.3389/fmicb.2012.00388
Hino, S. (2011). Establishment of the milk-borne transmission as a key factor for the peculiar endemicity of human T-lymphotropic virus type 1 (HTLV-1): the ATL prevention program Nagasaki. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 87, 152–166. doi: 10.2183/pjab.87.152
Hino, S., Katamine, S., Miyata, H., Tsuji, Y., Yamabe, T., Miyamoto, T., et al. (1997). Primary prevention of HTLV-1 in Japan. Leukemia S3, 57–59.
Hino, S., Sugiyama, H., Doi, H., Ishimaru, T., Yamabe, T., Tsuji, Y., et al. (1987). Breaking the cycle of HTLV-I transmission via carrier mothers' milk. Lancet 2, 158–159.
Hino, S., Yamaguchi, K., Katamine, S., Sugiyama, H., Amagasaki, T., Kinoshita, K., et al. (1985). Mother-to-child transmission of human T-cell leukemia virus type-I. Jpn. J. Cancer Res. 76, 474–480.
Itabashi, K., Miyazawa, T., Sekizawa, A., Tokita, A., Saito, S., Moriuchi, H., et al. (2020). A nationwide antenatal human T-cell leukemia virus type-1 antibody screening in Japan. Front. Microbiol. 11:595. doi: 10.3389/fmicb.2020.00595
Iwanaga, M., Watanabe, T., Utsunomiya, A., Okayama, A., Uchimaru, K., Koh, K. R., et al. (2010). Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood 116, 1211–1219. doi: 10.1182/blood-2009-12-257410
Kamihira, S., Dateki, N., Sugahara, K., Hayashi, T., Harasawa, H., Minami, S., et al. (2003). Significance of HTLV-1 proviral load quantification by real-time PCR as a surrogate marker for HTLV-1-infected cell count. Clin. Lab. Haematol. 25, 111–117. doi: 10.1046/j.1365-2257.2003.00503.x
Kaplan, J. E., Khabbaz, R. F., Murphy, E. L., Hermansen, S., Roberts, C., Lal, R., et al. (1996). Male-to-female transmission of human T-cell lymphotropic virus types I and II: association with viral load. The retrovirus epidemiology donor study group. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12, 193–201. doi: 10.1097/00042560-199606010-00014
Kashiwagi, K., Furusyo, N., Nakashima, H., Kubo, N., Kinukawa, N., Kashiwagi, S., et al. (2004). A decrease in mother-to-child transmission of human T lymphotropic virus type I (HTLV-I) in Okinawa, Japan. Am. J. Trop. Med. Hyg. 70, 158–163. doi: 10.4269/ajtmh.2004.70.158
Katamine, S. (1999). Milk-bone transmission of HTLV-1 and its intervention in Nagasaki. Acta Med. Nagasaki 44, 1–6.
Kinoshita, K., Hino, S., Amagasaki, T., Ikeda, S., Yamada, Y., Suzuyama, J., et al. (1984). Demonstration of adult T-cell leukemia virus antigen in milk from three sero-positive mothers. Gan 75, 103–105.
Kinoshita, K., Yamanouchi, K., Ikeda, S., Momita, S., Amagasaki, T., Soda, H., et al. (1985). Oral infection of a common marmoset with human T-cell leukemia virus type-I (HTLV-1) by inoculating fresh human milk of HTLV-1 carrier mothers. Jpn. J. Cancer Res. 76, 1147–1153.
Kuramitsu, M., Okuma, K., Yamochi, T., Sato, T., Sasaki, D., Hasegawa, H., et al. (2015). Standardization of quantitative PCR for human T-cell leukemia virus type 1 in Japan: a collaborative study. J. Clin. Microbiol. 53, 3485–3491. doi: 10.1128/JCM.01628-15
Kusuhara, K., Sonoda, S., Kazuo, T., Tokugawa, K., Fukushige, J., Ueda, K., et al. (1987). Mother-to-child transmission of human T-cell leukemia virus type 1 (HTLV-1): a fifteen-year follow-up study in Okinawa, Japan. Int. J. Cancer 40, 755–757. doi: 10.1002/ijc.2910400607
Matsuoka, M., and Jeang, K. T. (2007). Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7, 270–280. doi: 10.1038/nrc2111
Miura, K., and Masuzaki, H. (2017). “Prevention of human T-cell leukemia virus type 1 (HTLV-1) mother-to-child transmission” in Adult T-cell Leukemia/Lymphoma. Jpn. J. Obstet. Gynecol. Neonatal Hematol. (Japan: Springer), 157–169.
Moriuchi, H., Masuzaki, H., Doi, H., and Katamine, S. (2013). Mother-to-child transmission of human T-cell lymphotropic virus type 1. Pediatr. Infect. Dis. J. 32, 175–177. doi: 10.1097/INF.0b013e31827efc39
Motedayen Aval, L., Boullier, M., Lyall, H. P., Collins, G., Ayto, R. F., Kelly, D., et al. (2020). Adult T cell leukaemia/lymphoma (ATL) in pregnancy: a UK case series. eJHaem 2, 134–138. doi: 10.1002/jha2.142
Nagai, M., Usuku, K., Matsumoto, W., Kodama, D., Takenouchi, N., Moritoyo, T., et al. (1998). Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 4, 586–593. doi: 10.3109/13550289809114225
Ohba, T., Matsuo, I., Katabuchi, H., Nishimura, H., Fujisaki, S., Okamura, H., et al. (1988). Adult T-cell leukemia/lymphoma in pregnancy. Obstet. Gynecol. 72, 445–447.
Okochi, K., Sato, H., and Hinuma, Y. (1984). A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 46, 245–253. doi: 10.1111/j.1423-0410.1984.tb00083.x
Paiva, A., Smid, J., Haziot, M. E. J., Assone, T., Pinheiro, S., Fonseca, L. A. M., et al. (2017). High risk of heterosexual transmission of human T-cell lymphotropic virus type 1 infection in Brazil. J. Med. Virol. 89, 1287–1294. doi: 10.1002/jmv.24745
Pique, C., and Jones, K. S. (2012). Pathways of cell-cell transmission of HTLV-1. Front. Microbiol. 3, 1–14. doi: 10.3389/fmicb.2012.00378
Ramassamy, J. L., Tortevoye, P., Ntab, B., Seve, B., Carles, G., Gaquière, D., et al. (2020). Adult T-cell leukemia/lymphoma incidence rate in French Guiana: a prospective cohort of women infected with HTLV-1. Blood Adv. 4, 2044–2048. doi: 10.1182/bloodadvances.2020001628
Rosadas, C., Tosswill, J. H., Tedder, R., and Taylor, G. P. (2019). Pregnancy does not adversely impact diagnostic tests for HTLV-1/2 infection. PLoS Negl. Trop. Dis. 13:e0007736. doi: 10.1371/journal.pntd.0007736
Roucoux, D. F., Wang, B., Smith, D., Nass, C. C., Smith, J., Hutching, S. T., et al. (2005). HTLV outcomes study investigators. A prospective study of sexual transmission of human T lymphotropic virus (HTLV)-I and HTLV-II. J. Infect. Dis. 191, 1490–1497. doi: 10.1086/429410
Safdar, A., Johnson, N., Gonzalez, F., and Busowski, J. D. (2002). Adult T-cell leukemia-lymphoma during pregnancy. N. Engl. J. Med. 346, 2014–2015. doi: 10.1056/NEJM200206203462519
Sasaki, D., Doi, Y., Hasegawa, H., Yanagihara, K., Tsukasaki, K., Iwanaga, M., et al. (2010). High human T cell leukemia virus type-1 (HTLV-1) provirus load in patients with HTLV-1 carriers complicated with HTLV-1-unrelated disorders. Virol. J. 7:81. doi: 10.1186/1743-422X-7-81
Satake, M., Iwanaga, M., Sagara, Y., Watanabe, T., Okuma, K., and Hamaguchi, I. (2016). Incidence of human T-lymphotropic virus 1 infection in adolescent and adult blood donors in Japan: a nationwide retrospective cohort analysis. Lancet Infect. Dis. 16, 1246–1254. doi: 10.1016/S1473-3099(16)30252-3
Satake, M., Yamaguchi, K., and Tadokoro, K. (2012). Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. J. Med. Virol. 84, 327–335. doi: 10.1002/jmv.23181
Stuver, S. O., Tachibana, N., Okayama, A., Shioiri, S., Tsunetoshi, Y., Tsuda, K., et al. (1993). Heterosexual transmission of human T cell leukemia/lymphoma virus type I among married couples in southwestern Japan: an initial report from the Miyazaki cohort study. J. Infect. Dis. 167, 57–65. doi: 10.1093/infdis/167.1.57
Sullivan, K. M., and Dean, A. (2009). Open epi: a web-based epidemiologic and statistical calculator for public health. Public Health Rep. 124, 471–474. doi: 10.1177/003335490912400320
Sullivan, M. T., Williams, A. E., Fang, C. T., Notari, E. P., Poiesz, B. J., and Ehrlich, G. D. (1993). Human T-lymphotropic virus (HTLV) types I and II infection in sexual contacts and family members of blood donors who are seropositive for HTLV type I or II. American red Cross HTLV-I/II collaborative study group. Transfusion 33, 585–590. doi: 10.1046/j.1537-2995.1993.33793325055.x
Suzuki, S., Tanaka, M., Matsuda, H., Tsukahara, Y., Kuribayashi, Y., Gomibuchi, H., et al. (2014). Current status of HTLV-1 carrier in Japanese pregnant women. J. Matern. Fetal Neonatal Med. 27, 312–313. doi: 10.3109/14767058.2013.814631
Tajima, K., Tominaga, S., Suchi, T., Kawagoe, T., Komoda, H., Hinuma, Y., et al. (1982). Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan 73, 893–901.
Takahashi, K., Takezaki, T., Oki, T., Kawakami, K., Yashiki, S., Fujiyoshi, T., et al. (1991). Inhibitory effect of maternal antibody on mother-to-child transmission of human T-lymphotropic virus type I. the mother-to-child transmission study group. Int. J. Cancer 49, 673–677. doi: 10.1002/ijc.2910490508
Utsumi, T., Tsuda, A., Hirano, H., Goto, K., Tsubaki, H., and Tanaka, T. (1996). A case of pregnancy with adult T-cell leukemia. J. Obstet. Gynaecol. Res. 22, 599–601. doi: 10.1111/j.1447-0756.1996.tb01077.x
Vital Statistics of Japan (2015–2018). Ministry of internal affairs and communications Japan. e-Stat, Vital Statistics of Japan. Available at: https://www.e-stat.go.jp
Watanabe, T. (2011). Current status of HTLV-1 infection. Int. J. Hematol. 94, 430–434. doi: 10.1007/s12185-011-0934-4
World Health Organization (2020). Human T-lymphotropic virus type 1: technical report. Available at: https://apps.who.int/iris/handle/10665/339773 (Accessed March 3, 2021).
Keywords: HTLV-1, pregnant woman, carrier, screening test, seroconversion, horizontal transmission, proviral load, adult T-cell leukemia-lymphoma
Citation: Komatsu N, Iwanaga M, Hasegawa Y, Miura S, Fuchi N, Moriuchi H, Yanagihara K and Miura K (2022) Frequency of HTLV-1 seroconversion between pregnancies in Nagasaki, Japan, 2011–2018. Front. Microbiol. 13:1036955. doi: 10.3389/fmicb.2022.1036955
Received: 05 September 2022; Accepted: 20 October 2022;
Published: 15 November 2022.
Edited by:
Wei Zhang, University of Minnesota Twin Cities, United StatesReviewed by:
Yorifumi Satou, Kumamoto University, JapanCopyright © 2022 Komatsu, Iwanaga, Hasegawa, Miura, Fuchi, Moriuchi, Yanagihara and Miura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kiyonori Miura, a2l5b25vcmlAbmFnYXNha2ktdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.