
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 14 October 2022
Sec. Food Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1020628
This article is part of the Research TopicInteraction Between Food Homologous Plants and Intestinal MicrobiotaView all 22 articles
A correction has been applied to this article in:
Frontiers Corrigendum Template for Authors
 Yexun Zhou1,2†
Yexun Zhou1,2† Liang Chen1†
Liang Chen1† Hui Han1,2
Hui Han1,2 Bohui Xiong1
Bohui Xiong1 Ruqing Zhong1
Ruqing Zhong1 Yue Jiang1
Yue Jiang1 Lei Liu1
Lei Liu1 Haiqing Sun3
Haiqing Sun3 Jiajian Tan3
Jiajian Tan3 Xiaowei Cheng4
Xiaowei Cheng4 Martine Schroyen2
Martine Schroyen2 Yang Gao5*
Yang Gao5* Yong Zhao1*
Yong Zhao1* Hongfu Zhang1*
Hongfu Zhang1*Taxifolin (TAX), as a natural flavonoid, has been widely focused on due to its strong anti-oxidation, anti-inflammation, anti-virus, and even anti-tumor activity. However, the effect of TAX on semen quality was unknown. The purpose of this study was to analyze the beneficial influences of adding feed additive TAX to boar semen in terms of its quality and potential mechanisms. We discovered that TAX increased sperm motility significantly in Duroc boars by the elevation of the protein levels such as ZAG, PKA, CatSper, and p-ERK for sperm quality. TAX increased the blood concentration of testosterone derivatives, antioxidants such as melatonin and betaine, unsaturated fatty acids such as DHA, and beneficial amino acids such as proline. Conversely, TAX decreased 10 different kinds of bile acids in the plasma. Moreover, TAX increased “beneficial” microbes such as Intestinimonas, Coprococcus, Butyrivibrio, and Clostridium_XlVa at the Genus level. However, TAX reduced the “harmful” intestinal bacteria such as Prevotella, Howardella, Mogibacterium, and Enterococcus. There was a very close correlation between fecal microbes, plasma metabolites, and semen parameters by the spearman correlation analysis. Therefore, the data suggest that TAX increases the semen quality of Duroc boars by benefiting the gut microbes and blood metabolites. It is supposed that TAX could be used as a kind of feed additive to increase the semen quality of boars to enhance production performance.
The decreasing quality of semen is a serious issue that has contributed to a worldwide increase in infertility rates (10–15%) during the past few decades (Zhou et al., 2016; Wang et al., 2018). It is reported that semen quality (including sperm concentration and sperm motility) was reduced by about 50% worldwide between 1973 and 2011 (Centola et al., 2016; Levine et al., 2017). Environmental toxins, high fat diets, cancer treatments, and many other factors have been reported as involved in the rapid decline of semen quality (Vakalopoulos et al., 2015; Checa Vizcaíno et al., 2016; Skakkebaek et al., 2016; Virtanen et al., 2017; Han et al., 2019; Zhang et al., 2019; Ding et al., 2020). Many investigations have attempted to improve semen quality, and nutritional factors (protein, fatty acids, vitamins, and others) play crucial roles in semen quality. Lower protein or excessive protein can decrease sperm quality (Louis et al., 1994; Dong et al., 2016). Omega-3 (n-3) polyunsaturated fatty acids (PUFA), linolenic acid, eicosahexaenoic acid, and docosahexaenoic acid (DHA) can improve semen quality (Singh et al., 2021). Vitamins could also benefit spermatogenesis (Sanjo et al., 2020). Many dietary additives have been reported to regulate spermatogenesis and benefit semen quality. It has been reported that olive leaf extract, Korean red ginseng, and Genistein can improve spermatogenesis (Chi et al., 2013; Jung et al., 2015; Ganjalikhan Hakemi et al., 2019). Furthermore, we found that alginate oligosaccharides, beta-carotene, and chestnut polysaccharides improved spermatogenesis at various levels (Yu et al., 2020; Zhao et al., 2020; Ma et al., 2021).
Taxifolin (TAX) is a flavonoid present in a variety of plants, such as Douglas fir and fruits (grapes and oranges; Fukui, 1960; Topal et al., 2016). Due to its biological functions of anti-oxidation, anti-inflammation, anti-virus, anti-cardiovascular disease, and even anti-tumor activity, TAX has been used in food additives (in milk, cheese, and other foods), healthy products, and medicines (Galochkina et al., 2016a; Jomová et al., 2019; Turck et al., 2020; Zhang X. et al., 2020). TAX is a strong antioxidant that is mainly manifested in scavenging active oxygen and preventing the production of active oxygen (Jomová et al., 2019). It can alleviate LPS-induced acute lung injury, which triggers inflammation and apoptosis (Chen et al., 2017). TAX acts as an anti-fibrotic substance to effectively inhibit the fibrosis of the heart, kidney, liver, and lungs (Guo et al., 2015; Impellizzeri et al., 2015) via TGF-β/Smads and PI3K/AKT/mTOR pathways (Liu et al., 2021a). Moreover, TAX is an anti-viral molecule that inhibits Coxsackievirus B4 (Galochkina et al., 2016a, 2016b), and it can modulate the colorectal cancer cell cycle and apoptosis by regulating the Wnt/β-catenin signaling pathway (Razak et al., 2018). TAX has been found to have beneficial advantages for the reproductive systems. TAX could recover ovarian damage and reproductive dysfunctions through its antioxidant characteristics (Ince et al., 2020) and quite a few investigations have reported that it has beneficial effects on semen quality. The current research aimed to study the potentially positive effects of TAX on boar semen quality and the potential mechanisms involved to provide a basis for improving boar fertility. A few important proteins for sperm quality have been determined in the current study. The cation channel of sperm (CatSper), which is a kind of sperm calcium ion channel protein, plays a vital role in fertility via modification of the calcium entry and sperm hyperactivated motility (Lishko et al., 2012). Protein kinase A (PKA; the cyclic adenosine monophosphate (cAMP) dependent protein kinase) and ERK signaling have been reported to play important roles in sperm maturation, capacitation, and motility (Baro Graf et al., 2020; Li Q. et al., 2020). Zn-alpha2-glycoprotein (ZAG), via the cAMP/PKA signaling pathway, regulates sperm motility (Qu et al., 2007). Pigs have been used as an animal model to explore human nutrition because their physiology is to humans (O'Shea et al., 2015; Sun et al., 2021). In the current study, we discovered that TAX increased semen quality via the improvement of gut microbiota and systemic metabolome.
The animal experiments were followed by the Animal Care and Use Committee of the Institute of Animal Sciences of the Chinese Academy of Agricultural Sciences (IAS2021-67). Twenty Duroc boars of similar age (2-year-old), health status, and weight (300 kg) were chosen along with a Tian Ti mountain boar stud from Yangxiang Joint Stock Company (Guigang, China; Guo et al., 2020). The boar feeding conditions we used have been previously reported by Wu et al. (2019). We divided these 20 Duroc boars into 2 groups randomly, each group included 10 boars in a control group (CON) and the Taxifolin group (TAX). The control group (CON) was fed a basal diet (Supplementary Table S1), and boars in the TAX group (TAX) were fed a basal diet with 15 mg/kg body weight TAX (Hou et al., 2021). TAX was provided by Yinuo Biopharmaceutical Co., Ltd., Harbin, China. The boars lived in individual pens and the whole feeding period was 63 days (Figure 1A).
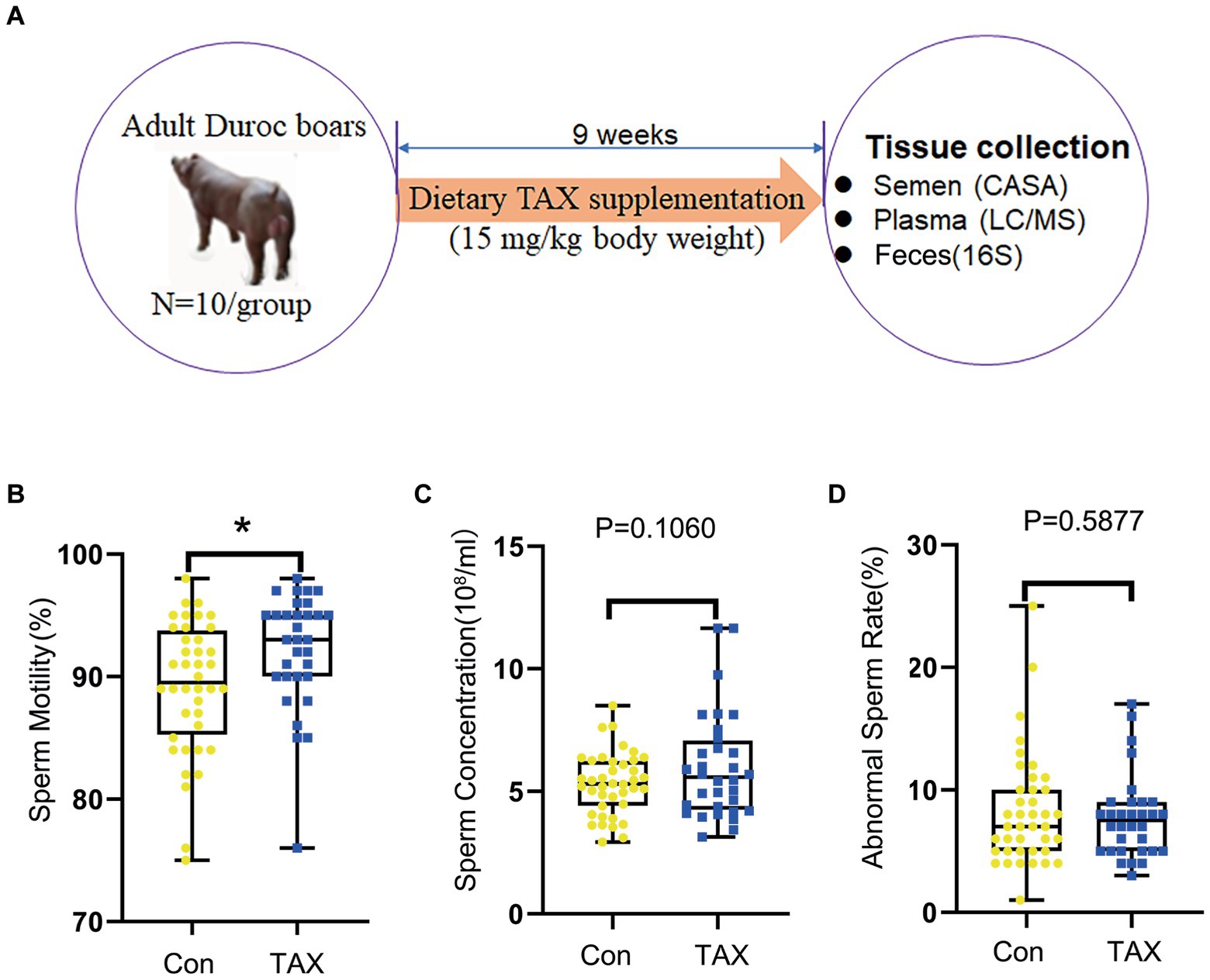
Figure 1. Effects of TAX on semen quality. (A) Study design. (B) Sperm motility. The y-axis represents the percentage of total cells. The x-axis represents the treatment (n = 10/group). *p < 0.05. (C) Sperm concentration. The y-axis represents the concentration. The x-axis represents the treatment (n = 10/group). (D) Abnormal sperm rate. The y-axis represents the percentage of total cells. The x-axis represents the treatment. Data were expressed as the mean ± SEM.
The semen samples of Duroc boars were collected by a breeder who used gloved-hand technology. After that, sperm concentration, sperm motility, and abnormal sperm rate were assessed by CASA software according to the reported methods (Wu et al., 2019; Guo et al., 2020). Blood samples were taken from boar hind leg veins when they were working. We used anticoagulant tubes containing EDTA-2Na. Then, blood samples were centrifuged at 3000× g for 10 min to separate blood plasma, then transferred to a −80°C refrigerator until the experiments. Fecal samples were taken from the boar rectum, then placed in liquid nitrogen, and finally stored in a −80°C freezer for 16S analysis (Guo et al., 2020).
The boar semen quality, including sperm concentration, sperm motility, and abnormal sperm rate were analyzed by a computer-assisted sperm analysis (CASA) system (Shanghai Kasu Biotechnology Co., Ltd., Shanghai, China; n = 10 per group; WHO, 2010; Zhao et al., 2016; Zhang et al., 2018, 2019). The evaluated criteria of sperm motility were as follows: grade A fast forward movement >22 μ m s−1; grade B forward movement <22 μ m s−1; grade C curve movement <5 μ m s−1; grade D none movement.
The protocol for the analysis of fecal microbiota was reported in our previous study (n = 10 per group; Zhang P. et al., 2020).
We used an E.Z.N.A.® Stool DNA Kit (Omega Bio-tek Inc., United States) to separate the total genomic DNA that came from the feces of the boars, according to the manufacturer’s instructions. A NanoDrop 2000 (Thermo Scientific, United States) and 1% agarose gel were used to detect the DNA quantity and quality, respectively. Primer pairs at 338F (5′- ACTCCTACGGGAGGCAGCAG-3′) and 806R (5’-GGACTACHVGGGTWTCTAAT-3′) amplified the V3–V4 region of the microbial 16S rRNA genes. The conditions of the PCR system and amplification were undertaken by following the technique used in our previous study (Wan et al., 2021). PCR amplification products can be extracted by 2% agarose gel and AxyPrep DNA Gel Extraction Kit (AXYGEN, New York, United States), which followed the instructions to purify them. After that, the sequences were assigned to the same operational taxonomic units ((OTUs) > 97% similarity).
The plasma metabolites were detected as reported (n = 10 per group). Boar plasma was stored at −80°C. Firstly, the protein was removed from the samples and then analyzed by LC/MS using our previous research method (Zhang P. et al., 2020). Next, An ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 × 100 mm) was employed in both positive and negative modes. Solvent A is an aqueous solution containing 0.1% formic acid. Solvent B is an aqueous solution containing 0.1% acetonitrile. The following program was: 5–20% B over 0–2 min; 20–60% B over 2–4 min; 60–100% B over 4–11 min. The composition was held at 100% B for 2 min, then 13–13.5 min, 100 to 5% B, and 13.5–14.5 min holding at 5% B. The flow rate was set at 0.4 ml/min and the column temperature was 45°C. The plasma samples were all kept at 4°C and the volume of the injection was 5 μl. ESI was used in the mass spectrometry program.
The methods for IHF of boar sperm have been reported in our previous articles (n = 10 per group; Zhao et al., 2016; Zhang et al., 2019). Primarily, we fixed the boar sperm in 4% paraformaldehyde for 1 h, then air-dried the sperm, which was spread on slides covered with poly-L-lysine. After being performed 3 times (each time for 5 min) and then being washed with PBS, the sperm were incubated with 2% Triton X-100 in PBS for 1 h at room temperature. Next, they were washed 3 times (each time for 5 min) again with PBS, the sperm were blocked with PBS, which contained 1% BSA and 1% goat serum for 30 min at 17°C, and then incubated with diluted primary antibody (1:100; Supplementary Table S2) overnight at 4°C. The next morning, after being washed three times with Tween 20, the slides were combined with Alexa Fluor 546 goat anti-rabbit IgG (1,200) in the dark for 30 min at RT. The negative controls were only incubated with the secondary antibody. After washing the slides 3 times with the Tween-20, we then used DAPI (4.6-diamidino-2-phenylindole hydrochloride, 100 ng/ml) as a nuclear stain and incubated them for 5 min. After washing with ddH2O, we used a fade-resistant mounting medium (Vector, Burlingame, United States) to cover the slides. Therefore, the fluorescence images were obtained by the Microscope (LEICA TCS SP5 II, Germany).
The procedure of Western blotting experiments, which are related to some beneficial sperm proteins, followed our previous publications (n = 6 per group; Zhao et al., 2016; Zhang et al., 2019). Sperm cells have to first be lysed in RIPA buffer that contains the protease inhibitor cocktail purchased from Sangong Biotech, Ltd. (Shanghai, China). Second, we detected the protein concentration followed by the instruction of BCA kits (Beyotime Institute of Biotechnology, Shanghai, China). In this study, Actin was used as a reference. The primary antibodies (Abs) are shown in Supplementary Table S2. The secondary donkey anti-goat and goat anti-rabbit was purchased from Beyotime Institute of Biotechnology (Shanghai, P.R. China) and Novex® by Life Technologies (United States) respectively. Next, we loaded 50 ug of total protein in each sample to 10% SDS polyacrylamide electrophoresis gels, which were transferred to a polyvinylidene fluoride (PVDF) membrane at the electric current of 300 mA for 2.5 h at 4°C. Then, we used 5% BSA to block the membranes for 1 h at 17°C, after washing them 3 times with 0.1% Tween-20 in TBS, the membranes were incubated with primary antibody, which was diluted at 1:500 in TBST with 1% BSA overnight at 4°C. The next day, Using TBST to wash three times, the blots were incubated with the secondary goat anti-rabbit or donkey anti-goat, respectively, for 1 h at 17°C. After being washed three times, the blots were imaged by a camera (Kodak, Beijing, China). Finally, we used ImageJ to analyze the bands.
Data are expressed as the mean ± SEM. p < 0.05 was considered a significant difference. The student’s t-test (SPSS 21 software) was used to perform the statistical analyses. Spearman’s correlation analysis was completed by RStudio (version 4.0.3) platform. Plots were performed by using GraphPad Prism 8.0.2.
As shown in Figure 1A (Study scheme), the adult Duroc boars were fed TAX at 15 mg/kg body weight for 9 weeks. Dietary supplementation of TAX significantly increased sperm motility compared to the control (CON) group (Figure 1B; p < 0.05). Meanwhile, TAX tended to increase sperm concentration (Figure 1C; p = 0.106). However, there were no differences in the abnormal sperm rate between the TAX and CON groups (Figure 1D). The data suggested that TAX improved semen quality by increasing sperm motility and raising the tendency of sperm concentration.
To understand how TAX improved boar semen quality, the protein levels (p-ERK, PKA, ZAG, and CatSper; Liu et al., 2012; Li et al., 2016; Xu et al., 2018) of important genes for sperm quality were quantified. TAX increased the protein levels of p-ERK, PKA and ZAG significantly compared to the CON group by IHF staining (Figure 2A,B; p < 0.05). Then, we used Western Blotting experiments to further confirm the results above (Figures 2C,D; p < 0.05). The results indicated that TAX could improve sperm quality by increasing the proteins related to spermatogenesis.
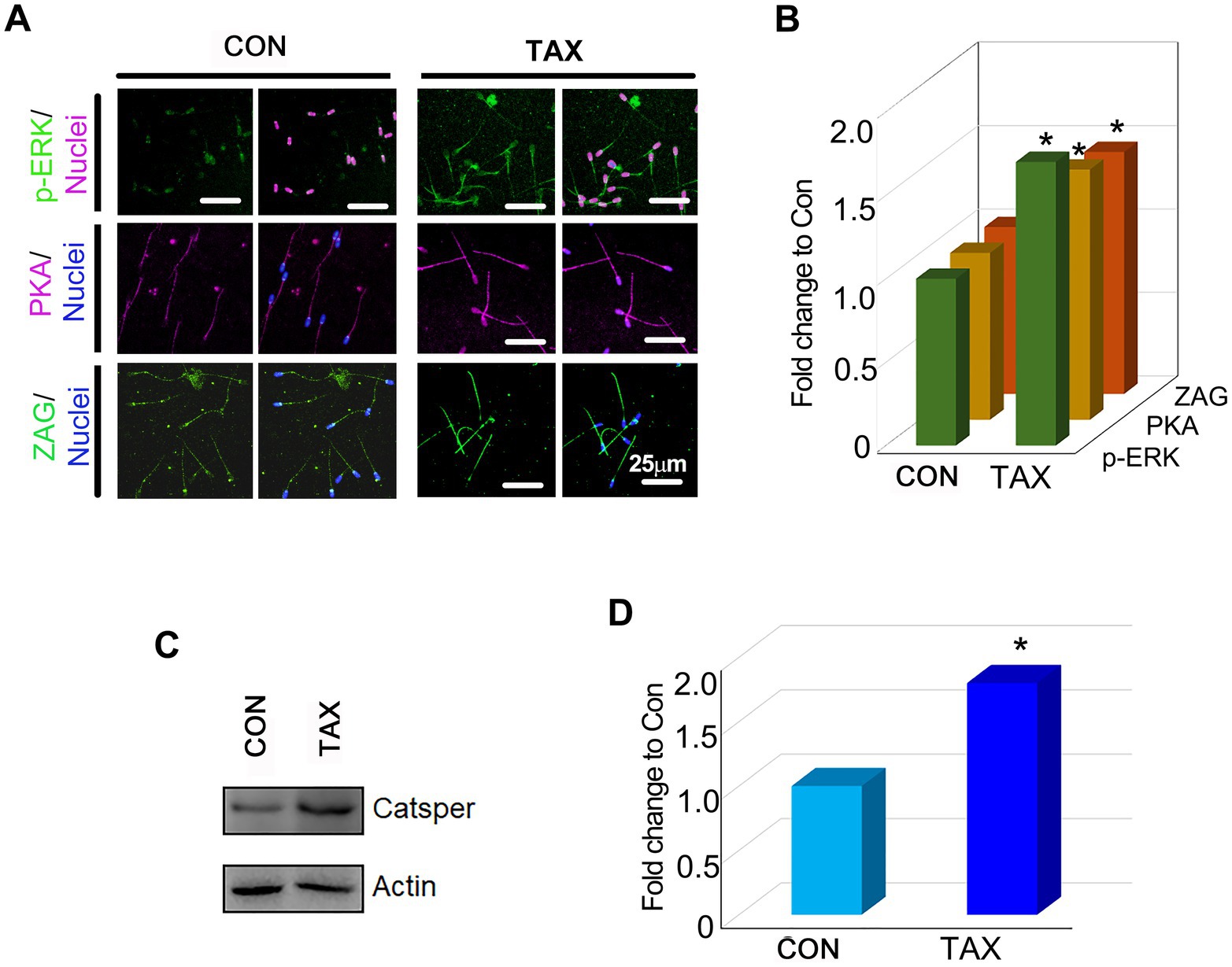
Figure 2. Effects of TAX on the protein expression of important genes related to sperm quality. (A) Immunofluorescence staining (IHF) of p-ERK, PKA, and ZAG. (B) Quantitative data for IHF staining of p-ERK, PKA, and ZAG (Fold change to CON). (C) Western blotting (WB) of Catsper. (D) Quantitative data for Catsper staining (Fold change to CON). *p < 0.05.
TAX altered the blood metabolites, which were determined by LC/MS analysis (Data File 1). Firstly, TAX increased the level of blood steroid hormone testosterone glucuronide (Figure 3A; *p < 0.05). TAX elevated a batch of blood antioxidants such as Betaine (*p < 0.05), Melatonin (p = 0.46), and 3-Oxooctanoyl-CoA (*p < 0.05) compared to the CON group (Figures 3B–D). Meanwhile, we also found that a few fatty acids, including Oleic acid (p = 0.082), Ricinoleic acid (p = 0.2464), DHA (p = 0.243), Inosine cyclic phosphate (**p < 0.01), Nonadecanoic acid (***p < 0.001), and Methyl hexadecanoic acids (***p < 0.001) were increased in TAX group than CON group (Figures 3E–J). Moreover, TAX significantly increased 5 amino acids and derivatives such as N-Acetylglutamine (*p < 0.05), 4-Hydroxyproline (*p < 0.05), Serylproline (*p < 0.05), Glycyl-Threonine (*p < 0.05), and 2-Furoylglycine (*p < 0.05) compared to the CON group (Figures 3K–P). It was very interesting to notice that TAX reduced ten different kinds of bile acids and derivatives compared to the CON group (Figures 4A–J).
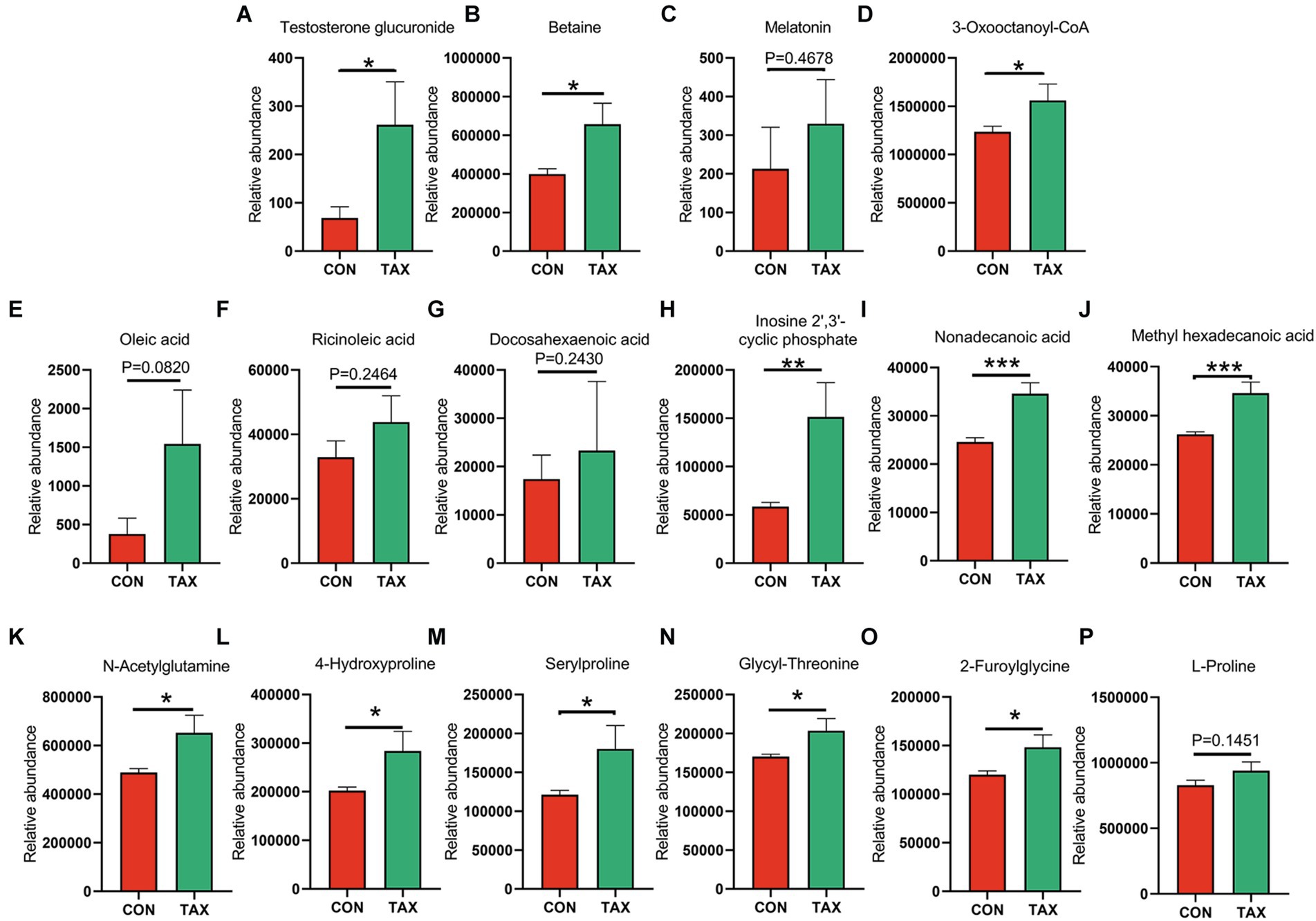
Figure 3. TAX increased blood metabolites. (A) Blood testosterone glucuronide level. (B) Blood betaine level. (C) Blood melatonin level. (D) Blood 3-Oxooctanoyl-CoA level. (E) Blood Oleic acid level. (F) Blood Ricinoleic acid level. (G) Blood DHA level. (H) Blood Inosine 2′,3′-cyclic phosphatel level. (I) Blood Nonadecanoic acid level. (J) Blood Methyl hexadecanoic acid level. (K) Blood N-Acetylglutamine level. (L) Blood 4-Hydroxyproline level. (M) Blood Serylproline level. (N) Blood Glycyl-Threonine level. (O) Blood 2-Furoylglycine level. (P) Blood L-Proline level. Data were expressed as the mean ± SEM. The y-axis represents the relative amount. The x-axis represents the treatments. *p < 0.05. **p < 0.01. ***p < 0.001.
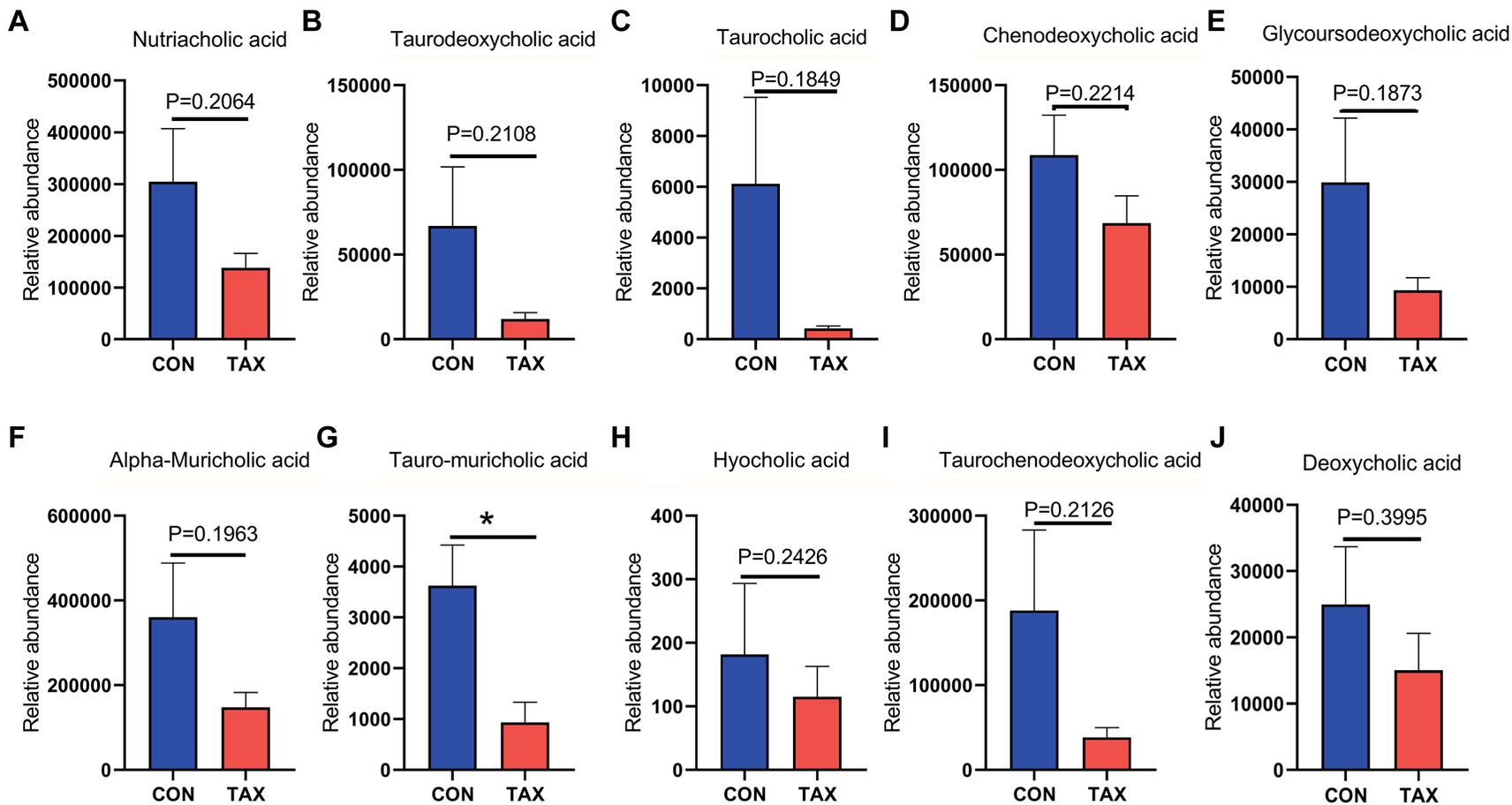
Figure 4. TAX decreased blood bile acids. (A) Blood nutriacholic acid level. (B) Blood Taurodeoxycholic acid level. (C) Blood taurocholic acid level. (D) Blood Chenodeoxycholic acid level. (E) Blood Glycoursodeoxycholic acid level. (F) Blood Alpha-Muricholic acid level. (G) Blood Tauro-muricholic acid level. (H) Blood Hyocholic acid level. (I) Blood Taurochenodeoxycholic acid level. (J) Blood Deoxycholic acid level. Data were expressed as the mean ± SEM. The y-axis represents the relative amount. The x-axis represents the treatment. *p < 0.05.
To search for the beneficial advantages of TAX on gut microbes, fecal microbes were determined. The microbes were different between the TAX and CON groups by PLS-DA analysis (Figure 5A), however, the total OUT and α-diversity were not changed much (Figures 5B,C). TAX increased the abundance of beneficial microbiota such as Intestinimonas (p = 0.1496), Coprococcus (*p < 0.05), Butyrivibrio (p = 0.2951), and Clostridium_XlVa (p = 0.6702) at the Genus level (Figures 5E–H). However, the harmful microbes were decreased by TAX such as Prevotella (p = 0.1867), Howardella (**p < 0.01), Mogibacterium (*p < 0.05), and Enterococcus (*p < 0.05; Figures 5I–L).
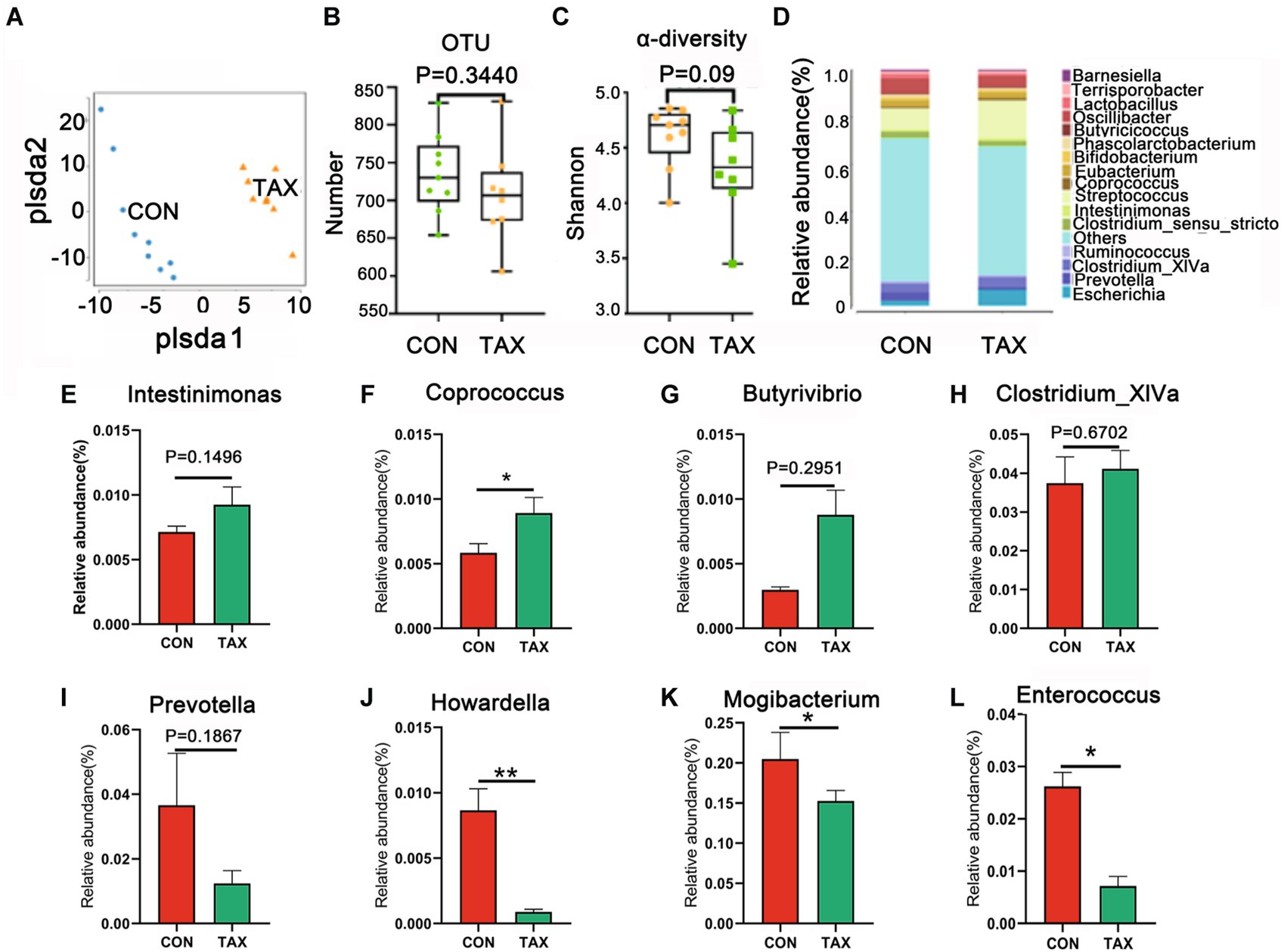
Figure 5. Effects of TAX on the fecal microbial composition. (A) The PLS-DA analysis of OUT of fecal microbes. (B) The levels of OUTs. (C) α-diversity with Shannon. (D) The relative amount of microbiota in feces at the genus level. The relative amount of individual microbiota in feces at the genus level (E–L). Data were expressed as the mean ± SEM. *p < 0.05. **p < 0.01.
Spearman correlation analysis (Figure 6) indicated that the fecal microbes, plasma metabolites, and semen parameters were well correlated. Firstly, the blood metabolites were well correlated with each other. Secondly, there was also a good correlation between blood metabolites and gut microbes. In the TAX group, the elevated beneficial bacteria were positively correlated with amino acids and unsaturated fatty acids, and negatively correlated with bile acids. Conversely, decreased harmful bacteria were negatively correlated with amino acids and unsaturated fatty acids and positively correlated with bile acids. Among them. The beneficial bacteria Butyrivibrio was significantly positively correlated with amino acids. The harmful bacteria Prevotella was significantly positively correlated with bile acids. In terms of semen quality, unsaturated fatty acids Ricinoleic acid and DHA had a strong positive correlation with sperm motility, while harmful bacteria Prevotella were significantly positively correlated with abnormal sperm rate. There was also a trend of positive correlation between bile acids and abnormal sperm.
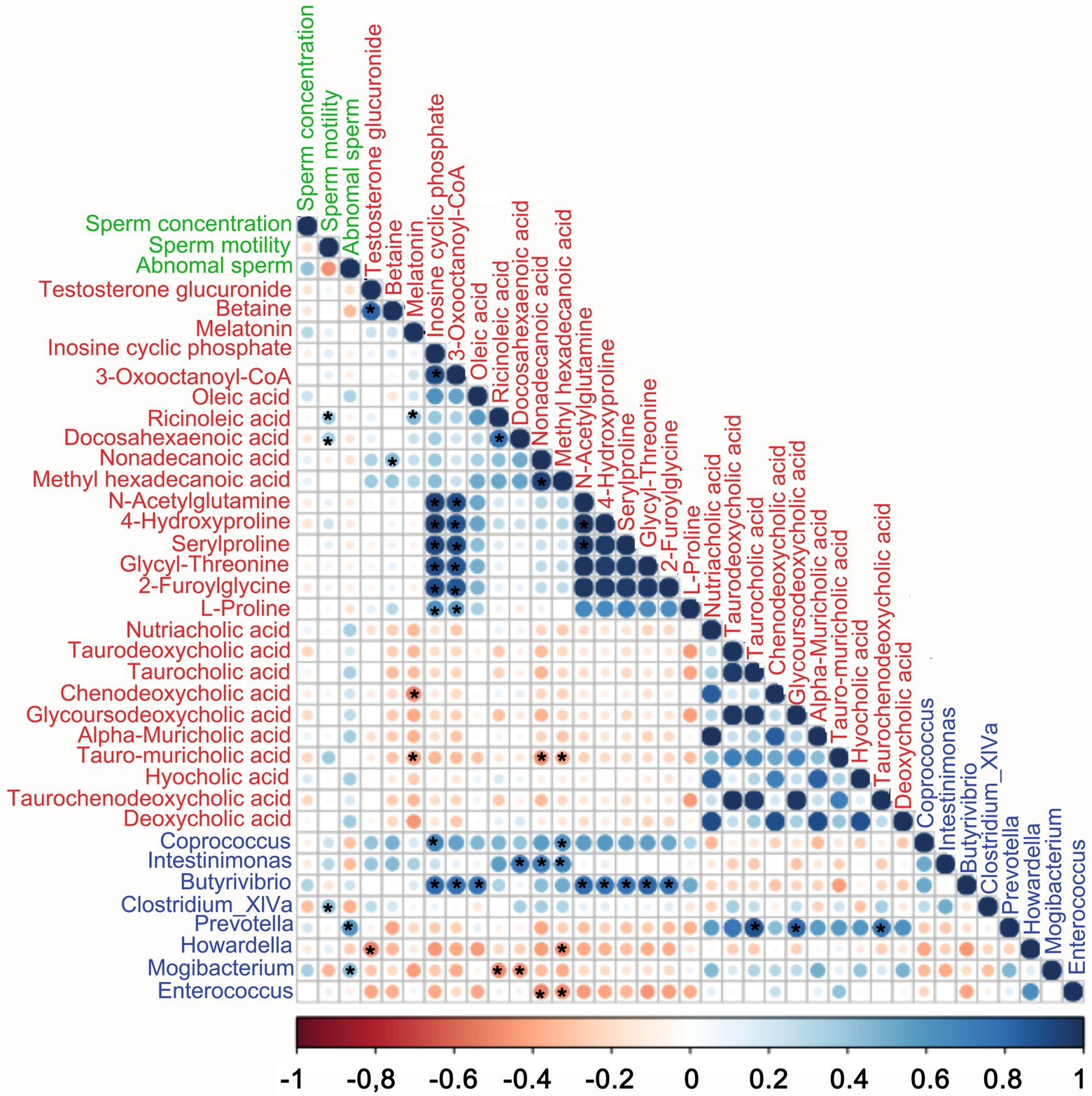
Figure 6. Correlations among fecal microbes, blood metabolites, and semen quality parameters. The color of the circle represents a positive or negative correlation, and the size of the circle represents the strength of the correlation. (large circle = stronger correlation, color green represents semen quality parameters, the color red represents blood metabolites, the color blue represents fecal microbes). *p < 0.05.
As a natural product, TAX has multiple biological functions. In recent years, it has been used in many fields, such as anti-oxidation to scavenge free radicals (Li et al., 2017; Gustiene et al., 2019; Lektemur Alpan et al., 2020), anti-obesity (Su et al., 2022), anti-inflammation (Wu et al., 2019), and other areas. In the current research, we found that adding TAX to the basal diet could improve boar semen quality by increasing sperm motility and sperm concentration. It has been reported that adding TAX to the cryopreservation extender can improve the ram sperm quality (Bucak et al., 2020). We also found that TAX increased the levels of some important proteins related to spermatogeneses such as ZAG, PKA, CatSper, and p-ERK. ZAG has been found to increase sperm motility (Qu et al., 2007). Catsper regulates sperm tail calcium entry and sperm hyperactivated motility (Lishko et al., 2012). PKA is related to sperm capacitation in mammalian (Baro Graf et al., 2020). p-ERK was related to sperm concentration and sperm activity (Li et al., 2016). In another study, TAX could rescue di-n-butyl phthalate disrupted testicular development in prenatal rats (Li Z. et al., 2020). Therefore, TAX improves the semen quality of Duroc boars by increasing some protein levels that benefit spermatogenesis.
Intestinal microbes not only regulate host health but also play an important role as a bridge between diet and host. The beneficial effects of TAX on biological systems may be through changing gut microbial composition. It has been reported that TAX improved dysbiosis caused by a high fat diet and regulated the gut microbiota diversity, also decreasing the ratio of Firmicutes/Bacteroidetes, which inhibit Proteobacteria from blooming (Su et al., 2022). Dietary TAX prevented dextran sulfate sodium (DSS)-induced colitis by reducing the abundance of Bacteroides, Clostridium ramosum, Clostridium saccharogumia, Sphingobacterium multivorum. Meanwhile, there was an increase in the abundance of Desulfovibrio and Gemmiger formicilis at the genus level (Hou et al., 2021). TAX ameliorated the aging process by modifying the gut microbes: Enterorhabdus, Clostridium, Bifidobacterium, and Parvibacter (Liu et al., 2021b). In our previous study, we found that alginate oligosaccharides (AOS; a natural antioxidant) could increase the “beneficial” bacteria such as Bacteroidales, Lactobacillaceae, and Campylobacterales to improve spermatogenesis and semen quality (Zhao et al., 2020; Zhang P. et al., 2021; Zhang C. et al., 2021). In this study, we found that dietary addition TAX increased the level of Coprococcus (butyrate producing; Keshavarzian et al., 2015), Intestinimonas (butyrate producing; Afouda et al., 2019), Butyrivibrio which is fermented glucose to produce butyric acid, then synthesizes short-chain fatty acids to protect the function of the intestinal epithelium, and some short-chain fatty acids can also be used in spermatogenesis (Moon et al., 2008; Olia Bagheri et al., 2021). On the other hand, TAX decreased the levels of “harmful” bacteria such as Enterococcus, Prevotella, Howardella, and Mogibacterium. A study has shown that Enterococcus can induce Bacteriospermia in rabbit semen (Duracka et al., 2019). Prevotella appeared to exert a negative effect on sperm quality (Farahani et al., 2021). In our investigation, we also found that Prevotella was significantly positively correlated with abnormal sperm rate. Therefore, our results were consistent with previous studies. It was interesting to notice that Howardella was associated with obesity (Zhu et al., 2021), which was also an important factor affecting semen quality and male infertility (Leisegang et al., 2021). TAX could decrease the abundance of Mogibacterium which promoted inflammation and is associated with obesity (Wu et al., 2018; Li Q. et al., 2020). Therefore, dietary supplementation of TAX can improve the semen quality of boars by increasing “beneficial” bacteria and decreasing “harmful” bacteria.
Metabolic regulation plays a crucial role in spermatogenesis (Rato et al., 2012; Al-Asmakh et al., 2014; Dai et al., 2015), and gut microbiota can produce metabolites to modulate systemic metabolome (Hou et al., 2021; Su et al., 2022). It has been reported that TAX could improve the blood metabolites of pigs to prevent oxidative stress (Nekrasov et al., 2021). In current experiments, TAX increased blood testosterone and the derivatives which were essential for maintaining spermatogenesis and boar fertility (Smith and Walker, 2014). Moreover, TAX increased blood antioxidant molecules melatonin (increased trend) and betaine. Melatonin has been reported to improve spermatogenesis via the alleviation of oxidative stress and DNA damage (Pang et al., 2017; Xu et al., 2020). Betaine could improve sperm quality and ameliorate oxidative damage in testis (Elsheikh et al., 2020). Furthermore, TAX increased (n-3) polyunsaturated fatty acids in the blood such as docosahexaenoic acid (DHA) which was very crucial for spermatogenesis and sperm quality (Hale et al., 2019; Bunay et al., 2021). In addition, TAX elevated some essential amino acids such as proline, which is important for spermatogenesis and semen quality (Dawra et al., 2015; Dong et al., 2016). However, TAX reduced blood bile acid derivatives, which have been reported to induce oxidative stress (Bomzon et al., 1997). Various studies have reported that bile acids can result in oxidative stress by promoting the production of oxygen free radicals from mitochondria (Bomzon et al., 1997). Moreover, bile acids contributed to infertility by activating farnesoid X receptor and G-protein-coupled bile acid receptor expressed in sperm, which then affected glucose and lipid metabolism and led to abnormal sperm (Baptissart et al., 2014; Malivindi et al., 2018). Our data were consistent with the previous studies described above. Therefore, dietary supplementation of TAX can improve the blood metabolites of boars to keep them healthy. However, there are some limitations to this study, meaning that the underlying mechanism of TAX improved sperm motility or concentration was not fully revealed. In our previrous research, we found that Hydroxytyrosol which is a kind of antioxidant benefits the semen quality of Duroc boar through improving gut microbes and blood metabolites (Han et al., 2021). In our current research, we also found that the blood metabolites and gut microbes have a good correlation with each other, so the improvement of boar semen quality by TAX was mainly mediated by both blood metabolites and gut microbes.
The present study indicated that TAX improves the semen quality of boars by ameliorating gut microbiota and blood metabolome. Our study confirms that TAX may be a good feed additive for improving the semen quality of boars, increasing the conception rate and litter size of sows to meet demands for pork consumption.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
The animal study was reviewed and approved by Animal Care and Use Committee of the Institute of Animal Sciences of Chinese Academy of Agricultural Sciences (IAS2021-67).
YZ, HZ, and YG designed the experiment. YZ, LC, HH, RZ, BX, LL, HS, JT, XC, and YG conducted the experiment and analyzed the data. MS, HZ, and YZ wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by funding from China Academy of Agriculture Sciences, the Agricultural Science and Technology Innovation Program (CAAS-ZDRW202006-02, ASTIPIAS07), and the State Key Laboratory of Animal Nutrition (2004DA125184G2102).
We thank the Beijing Genomics Institute (BGI) and Shanghai LUMING Biotechnology CO., LCD for technical support.
XC was employed by the company Yinuo Biopharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1020628/full#supplementary-material
Afouda, P., Durand, G. A., Lagier, J. C., Labas, N., Cadoret, F., Armstrong, N., et al. (2019). Noncontiguous finished genome sequence and description of Intestinimonas massiliensis sp. nov strain GD2T, the second Intestinimonas species cultured from the human gut. Microbiology 8:e00621. doi: 10.1002/mbo3.621
Al-Asmakh, M., Stukenborg, J. B., Reda, A., Anuar, F., Strand, M. L., Hedin, L., et al. (2014). The gut microbiota and developmental programming of the testis in mice. PLoS One 9:103809. doi: 10.1371/journal.pone.0103809
Baptissart, M., Vega, A., Martinot, E., Pommier, A. J., Houten, S. M., Marceau, G., et al. (2014). Bile acids alter male fertility through G-protein-coupled bile acid receptor 1 signaling pathways in mice. Hepatology 60, 1054–1065. doi: 10.1002/hep.27204
Baro Graf, C., Ritagliati, C., Stival, C., Luque, G. M., Gentile, I., Buffone, M. G., et al. (2020). Everything you ever wanted to know about PKA regulation and its involvement in mammalian sperm capacitation. Mol. Cell. Endocrinol. 518:110992. doi: 10.1016/j.mce.2020.110992
Bomzon, A., Holt, S., and Moore, K. (1997). Bile acids, oxidative stress, and renal function in biliary obstruction. Semin. Nephrol. 17, 549–562.
Bucak, M. N., Keskin, N., Ili, P., Bodu, M., Akalın, P. P., Öztürk, A. E., et al. (2020). Decreasing glycerol content by co-supplementation of trehalose and taxifolin hydrate in ram semen extender: microscopic, oxidative stress, and gene expression analyses. Cryobiology 96, 19–29. doi: 10.1016/j.cryobiol.2020.09.001
Bunay, J., Gallardo, L. M., Torres-Fuentes, J. L., Aguirre-Arias, M. V., Orellana, R., Sepúlveda, N., et al. (2021). A decrease of docosahexaenoic acid in testes of mice fed a high-fat diet is associated with impaired sperm acrosome reaction and fertility. Asian J. Androl. 23, 306–313. doi: 10.4103/aja.aja_76_20
Centola, G. M., Blanchard, A., Demick, J., Li, S., and Eisenberg, M. L. (2016). Decline in sperm count and motility in young adult men from 2003 to 2013: observations from a U.S sperm bank. Andrology 4, 270–276. doi: 10.1111/andr.12149
Checa Vizcaíno, M. A., González-Comadran, M., and Jacquemin, B. (2016). Outdoor air pollution and human infertility: a systematic review. Fertil. Steril. 106, 897–904.e1. doi: 10.1016/j.fertnstert.2016.07.1110
Chen, X., Huang, J., Hu, Z., Zhang, Q., Li, X., and Huang, D. (2017). Protective effects of dihydroquercetin on an APAP-induced acute liver injury mouse model. Int. J. Clin. Exp. Pathol. 10, 10223–10232.
Chi, H., Chun, K., Son, H., Kim, J., Kim, G., and Roh, S. (2013). Effect of genistein administration on the recovery of spermatogenesis in the busulfan-treated rat testis. Clin. Exp. Reprod. Med. 40, 60–66. doi: 10.5653/cerm.2013.40.2.60
Dai, Z., Wu, Z., Hang, S., Zhu, W., and Wu, G. (2015). Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 21, 389–409. doi: 10.1093/molehr/gav003
Dawra, V., Yadav, B., and Yadav, S. (2015). Effect of glutamine supplementation and replacement of tris-egg yolk based extender with defatted cow milk on spermatozoa quality after equilibration and thawing. Vet. World. 8, 1027–1031. doi: 10.14202/vetworld.2015.1027-1031
Ding, N., Zhang, X., Zhang, X. D., Jing, J., Liu, S. S., Mu, Y. P., et al. (2020). Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 69, 2259–2260. doi: 10.1136/gutjnl-2020-321220
Dong, H., Wu, D., Xu, S., Li, Q., Fang, Z., Che, L., et al. (2016). Effect of dietary supplementation with amino acids on boar sperm quality and fertility. Anim. Reprod. Sci. 172, 182–189. doi: 10.1016/j.anireprosci.2016.08.003
Duracka, M., Lukac, N., Kacaniova, M., Kantor, A., Hleba, L., Ondruska, L., et al. (2019). Antibiotics versus natural biomolecules: the case of in vitro induced bacteriospermia by enterococcus Faecalis in rabbit semen. Molecules 24:4329. doi: 10.3390/molecules24234329
Elsheikh, N. A. H., Omer, N. A., Yi-Ru, W., Mei-Qian, K., Ilyas, A., Abdurahim, Y., et al. (2020). Protective effect of betaine against lead-induced testicular toxicity in male mice. Andrologia 52:13600. doi: 10.1111/and.13600
Farahani, L., Tharakan, T., Yap, T., Ramsay, J. W., Jayasena, C. N., and Minhas, S. (2021). The semen microbiome and its impact on sperm function and male fertility. Andrology 9, 115–144. doi: 10.1111/andr.12886
Fukui, Y. (1960). Studies on the chemical constituents of the plants of coniferae and allied orders. XL. Yakugaku zasshi 80, 752–756. doi: 10.1248/yakushi1947.80.6_752
Galochkina, A. V., Anikin, V. B., Babkin, V. A., Ostrouhova, L. A., and Zarubaev, V. V. (2016b). Virus-inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against coxsackievirus B4 in a model of viral pancreatitis. Arch. Virol. 164, 929–938. doi: 10.1007/s00705-016-2749-3
Galochkina, A. V., Zarubaev, V., Kiselev, O., Babkin, V., and Ostroukhova, L. (2016a). Antiviral activity of the dihydroquercetin during the Coxsackievirus b4 replication in vitro. Vopr. Virusol. 61, 27–31. doi: 10.18821/0507-4088-2016-61-1-27-31
Ganjalikhan Hakemi, S., Sharififar, F., Haghpanah, T., Babaee, A., and Eftekhar-Vaghefi, S. H. (2019). The effects of olive leaf extract on the testis, sperm quality and testicular germ cell apoptosis in male rats exposed to busulfan. Int. J. Fertil. Steril. 13, 57–65. doi: 10.22074/ijfs.2019.5520
Guo, L., Wu, Y., Wang, C., Wei, H., Tan, J., Sun, H., et al. (2020). Gut microbiological disorders reduce semen utilization rate in duroc boars. Front. Microbiol. 11:581926. doi: 10.3389/fmicb.2020.581926
Guo, H., Zhang, X., Cui, Y., Zhou, H., Xu, D., Shan, T., et al. (2015). Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Toxicol. Appl. Pharmacol. 287, 168–177. doi: 10.1016/j.taap.2015.06.002
Gustiene, S., Zaborskiene, G., Rokaityte, A., and Riešute, R. (2019). Effect of biofermentation with taxifolin on physicochemical and microbiological parameters of cold-smoked pork sausages. Food Technol. Biotechnol. 57, 481–489. doi: 10.17113/ftb.57.04.19.6250
Hale, B. J., Fernandez, R. F., Kim, S. Q., Diaz, V. D., Jackson, S. N., Liu, L., et al. (2019). Acyl-CoA synthetase 6 enriches seminiferous tubules with the ω-3 fatty acid docosahexaenoic acid and is required for male fertility in the mouse. J. Biol. Chem. 294, 14394–14405. doi: 10.1074/jbc.RA119.009972
Han, X., Zhang, P., Shen, W., Zhao, Y., and Zhang, H. (2019). Estrogen receptor-related DNA and histone methylation may be involved in the transgenerational disruption in spermatogenesis by selective toxic chemicals. Front. Pharmacol. 10:1012. doi: 10.3389/fphar.2019.01012
Han, H., Zhong, R., Zhou, Y., Xiong, B., Chen, L., Jiang, Y., et al. (2021). Hydroxytyrosol benefits boar semen quality via improving gut microbiota and blood Metabolome. Front. Nutr. 8:815922. doi: 10.3389/fnut.2021.815922
Hou, J., Hu, M., Zhang, L., Gao, Y., Ma, L., and Xu, Q. (2021). Dietary Taxifolin protects against dextran sulfate sodium-induced colitis via NF-κB signaling, enhancing intestinal barrier and modulating gut microbiota. Front. Immunol. 11:631809. doi: 10.3389/fimmu.2020.631809
Impellizzeri, D., Talero, E., Siracusa, R., Alcaide, A., Cordaro, M., Maria Zubelia, J., et al. (2015). Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice. Br. J. Nutr. 114, 853–865. doi: 10.1017/S0007114515002597
Ince, S., Ozer, M., Kadioglu, B. G., Kuzucu, M., Ozkaraca, M., Gezer, A., et al. (2020). The effect of taxifolin on oxidative ovarian damage and reproductive dysfunctions induced by antipsychotic drugs in female rats. J. Obstet. Gynaecol. Res. 47, 2140–2148. doi: 10.1111/jog.14769
Jomová, K., Hudecova, L., Lauro, P., Simunkova, M., Alwasel, S. H., Alhazza, I. M., et al. (2019). A switch between antioxidant and prooxidant properties of the phenolic compounds yyricetin, morin, 3′,4′-dihydroxyflavone, taxifolin and 4-hydroxy-coumarin in the presence of copper (II) ions: a spectroscopic, absorption titration and DNA damage study. Molecules 24:4335. doi: 10.3390/molecules24234335
Jung, S., Kim, H., Lee, B., Choi, S., Kim, H., Choi, Y., et al. (2015). Effects of Korean red ginseng extract on busulfan-induced dysfunction of the male reproductive system. J. Ginseng Res. 39, 243–249. doi: 10.1016/j.jgr.2015.01.002
Keshavarzian, A., Green, S. J., Engen, P. A., Voigt, R. M., Naqib, A., Forsyth, C. B., et al. (2015). Colonic bacterial composition in Parkinson's disease. Mov. Disord. 30, 1351–1360. doi: 10.1002/mds.26307
Leisegang, K., Sengupta, P., Agarwal, A., and Henkel, R. (2021). Obesity and male infertility: mechanisms and management. Andrologia 53:e13617. doi: 10.1111/and.13617
Lektemur Alpan, A., Kızıldağ, A., Özdede, M., Karakan, N. C., and Özmen, Ö. (2020). The effects of taxifolin on alveolar bone in experimental periodontitis in rats. Arch. Oral Biol. 117:104823. doi: 10.1016/j.archoralbio.2020.104823
Levine, H., Jørgensen, N., Martino-Andrade, A., Mendiola, J., Weksler-Derri, D., Mindlis, I., et al. (2017). Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum. Reprod. Update 23, 646–659. doi: 10.1093/humupd/dmx022
Li, J., Mao, R., Zhou, Q., Ding, L., Tao, J., Ran, M. M., et al. (2016). Exposure to bisphenol a (BPA) in Wistar rats reduces sperm quality with disruption of ERK signal pathway. Toxicol. Mech. Methods 26, 180–188. doi: 10.3109/15376516.2016.1139024
Li, Q., Pu, Y., Lu, H., Zhao, N., Wang, Y., Guo, Y., et al. (2020). Porphyromonas, Treponema, and Mogibacterium promote IL8/IFNγ/TNFα-based pro-inflammation in patients with medication-related osteonecrosis of the jaw. J. Oral Microbiol. 13:1851112. doi: 10.1080/20002297.2020.1851112
Li, X., Xie, H., Jiang, Q., Wei, G., Lin, L., Li, C., et al. (2017). The mechanism of (+) taxifolin's protective antioxidant effect for OH-treated bone marrow-derived mesenchymal stem cells. Cell. Mol. Biol. Lett. 22:31. doi: 10.1186/s11658-017-0066-9
Li, Z., Yu, Y., Li, Y., Ma, F., Fang, Y., Ni, C., et al. (2020). Taxifolin attenuates the developmental testicular toxicity induced by di-n-butyl phthalate in fetal male rats. Food Chem. Toxicol. 142:111482. doi: 10.1016/j.fct.2020.111482
Lishko, P. V., Kirichok, Y., Ren, D., Navarro, B., Chung, J. J., and Clapham, D. E. (2012). The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol. 74, 453–475. doi: 10.1146/annurev-physiol-020911-153258
Liu, X., Liu, W., Ding, C., Zhao, Y., Chen, X., Ling, D., et al. (2021a). Taxifolin, extracted from waste larix olgensis roots, attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR and TGF-β1/Smads signaling pathways. Drug Des. Devel. Ther. 15, 871–887. doi: 10.2147/DDDT.S281369
Liu, Y., Qu, F., Cao, X., Chen, G., Guo, Q., Ying, X., et al. (2012). Con A-binding protein Zn-2-glycoprotein on human sperm membrane is related to acrosome reaction and sperm fertility. Int. J. Androl. 35, 145–157. doi: 10.1111/j.1365-2605.2011.01195.x
Liu, X., Zhao, Y., Zhu, H., Wu, M., Zheng, Y., Yang, M., et al. (2021b). Taxifolin retards the D-galactose-induced aging process through inhibiting Nrf2-mediated oxidative stress and regulating the gut microbiota in mice. Food Funct. 10:1039. doi: 10.1039/d1fo01349a
Louis, G. F., Lewis, A. J., Weldon, W. C., Miller, P. S., Kittok, R. J., and Stroup, W. W. (1994). The effect of protein intake on boar libido, semen characteristics, and plasma hormone concentrations. J. Anim. Sci. 72, 2038–2050. doi: 10.2527/1994.7282038x
Ma, D., Han, P., Song, M., Zhang, H., Shen, W., Huang, G., et al. (2021). β-carotene rescues busulfan disrupted spermatogenesis through elevation in testicular antioxidant capability. Front. Pharmacol. 12:593953. doi: 10.3389/fphar.2021.593953
Malivindi, R., Santoro, M., De Rose, D., Panza, S., Gervasi, S., Rago, V., et al. (2018). Activated-farnesoid X receptor (FXR) expressed in human sperm alters its fertilising ability. Reproduction 156, 249–259. doi: 10.1530/REP-18-0203
Moon, C. D., Pacheco, D. M., Kelly, W. J., Leahy, S. C., Li, D., Kopecny, J., et al. (2008). Reclassification of clostridium proteoclasticum as Butyrivibrio proteoclasticus comb. nov., a butyrate-producing ruminal bacterium. Int. J. Syst. Evol. Microbiol. 58, 2041–2045. doi: 10.1099/ijs.0.65845-0
Nekrasov, R. V., Bogolyubova, N. V., Semenova, A. A., Nasonova, V. V., and Polishchuk, E. K. (2021). Dihydroquercetin influence on clinical and biochemical blood parameters of pigs under conditions of stress load. Vopr. Pitan. 90, 74–84. doi: 10.33029/0042-8833-2021-90-1-74-84
Olia Bagheri, F., Alizadeh, A., Sadighi Gilani, M. A., and Shahhoseini, M. (2021). Role of peroxisome proliferator-activated receptor gamma (PPARγ) in the regulation of fatty acid metabolism related gene expressions in testis of men with impaired spermatogenesis. Reprod. Biol. 21:100543. doi: 10.1016/j.repbio.2021.100543
O'Shea, C. J., Doyle, D. N., Heim, G., and O'Doherty, J. (2015). Effect of maternal dietary supplementation of laminarin and fucoidan, independently or in combination, on pig growth performance and aspects of intestinal health. J. Ani. Feed Sci. 204, 28–41. doi: 10.1016/j.anifeedsci.2015.02.007
Pang, J., Zhou, Q., Sun, X., Li, L., Zhou, B., Zeng, F., et al. (2017). Effect of low-dose zearalenone exposure on reproductive capacity of male mice. Toxicol. Appl. Pharmacol. 333, 60–67. doi: 10.1016/j.taap.2017.08.011
Qu, F., Ying, X., Guo, W., Guo, Q., Chen, G., Liu, Y., et al. (2007). The role of Zn-alpha2 glycoprotein in sperm motility is mediated by changes in cyclic AMP. Reproduction 134, 569–576. doi: 10.1530/REP-07-0145
Rato, L., Alves, M. G., Socorro, S., Duarte, A. I., Cavaco, J. E., and Oliveira, P. F. (2012). Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 9, 330–338. doi: 10.1038/nrurol.2012.77
Razak, S., Afsar, T., Ullah, A., Almajwal, A., Alkholief, M., Alshamsan, A., et al. (2018). Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/β-catenin signaling pathway. BMC Cancer 18:1043. doi: 10.1186/s12885-018-4959-4
Sanjo, H., Yao, T., Katagiri, K., Sato, T., Matsumura, T., Komeya, M., et al. (2020). Antioxidant vitamins and lysophospholipids are critical for inducing mouse spermatogenesis under organ culture conditions. FASEB J. 37, 9480–9487. doi: 10.1096/fj.202000245R
Singh, M., Talimoa Mollier, R., Sharma, P. R., Kadirvel, G., Doley, S., Sanjukta, R. K., et al. (2021). Dietary flaxseed oil improve boar semen quality, antioxidant status and in-vivo fertility in humid sub-tropical region of North East India. Theriogenology 159, 123–131. doi: 10.1016/j.theriogenology.2020.10.023
Skakkebaek, N. E., Rajpert-De Meyts, E., Louis, G. M. B., Toppari, J., Andersson, A. M., Eisenberg, M. L., et al. (2016). Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol. Rev. 96, 55–97. doi: 10.1152/physrev.00017.2015
Smith, L. B., and Walker, W. H. (2014). The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 30, 2–13. doi: 10.1016/j.semcdb.2014.02.012
Su, H., Wang, W. J., Zheng, G. D., Yin, Z. P., Li, J. E., Chen, L. L., et al. (2022). The anti-obesity and gut microbiota modulating effects of taxifolin in C57BL/6J mice fed with a high-fat diet. J. Sci. Food Agric. 102, 1598–1608. doi: 10.1002/jsfa.11496
Sun, S., Meng, Q., Bai, Y., Cao, C., Li, J., Cheng, B., et al. (2021). Lycopene improves maternal reproductive performance by modulating milk composition and placental antioxidative and immune status. Food Funct. 10:1039. doi: 10.1039/d1fo01595h
Topal, F., Nar, M., Gocer, H., Kalin, P., Kocyigit, U. M., Gülçin, İ., et al. (2016). Antioxidant activity of taxifolin: an activity-structure relationship. J. Enzyme Inhib. Med. Chem. 31, 674–683. doi: 10.3109/14756366.2015.1057723
Turck, D., Castenmiller, J., De Henauw, S., Hirsch-Ernst, K. I., Kearney, J., Maciuk, A., et al. (2020). Safety of hot water extract of fruits and peduncles of Hovenia dulcis as a novel food pursuant to Regulation 1(EU) 2015/2283. EFSA J. 18:6196. doi: 10.2903/j.efsa.2020.6196
Vakalopoulos, I., Dimou, P., Anagnostou, I., and Zeginiadou, T. (2015). Impact of cancer and cancer treatment on male fertility. Hormones (Athens) 14, 579–589. doi: 10.14310/horm.2002.1620
Virtanen, H. E., Jørgensen, N., and Toppari, J. (2017). Semen quality in the 21st century. Nat. Rev. Urol. 14, 120–130. doi: 10.1038/nrurol.2016.261
Wan, F., Zhong, R., Wang, M., Zhou, Y., Chen, Y., Yi, B., et al. (2021). Caffeic acid supplement alleviates colonic inflammation and oxidative stress potentially through improved gut microbiota community in mice. Front. Microbiol. 12:784211. doi: 10.3389/fmicb.2021.784211
Wang, M., Liu, X., Chang, G., Chen, Y., An, G., Yan, L., et al. (2018). Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell 23, 599–614.e4. doi: 10.1016/j.stem.2018.08.007
WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th Edn. Cambridge: Cambridge University Press (2010).
Wu, Y., Chi, X., Zhang, Q., Chen, F., and Deng, X. (2018). Characterization of the salivary microbiome in people with obesity. PeerJ 6:e4458. doi: 10.7717/peerj.4458
Wu, Y., Lai, W., Liu, Z., Wei, H., Zhou, Y., Tan, J., et al. (2019). Serum and seminal plasma element concentrations in relation to semen quality in Duroc boars. Biol. Trace Elem. Res. 189, 85–94. doi: 10.1007/s12011-018-1459-y
Xu, Y., Fan, Y., Fan, W., Jing, J., Xue, K., Zhang, X., et al. (2018). RNASET2 impairs the sperm motility via PKA/PI3K/calcium signal pathways. Reproduction 155, 383–392. doi: 10.1530/REP-17-0746
Xu, D., Liu, L., Zhao, Y., Yang, L., Cheng, J., Hua, R., et al. (2020). Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J. Pineal Res. 69:12690. doi: 10.1111/jpi.12690
Yu, S., Zhao, Y., Zhang, F. L., Li, Y. Q., Shen, W., and Sun, Z. Y. (2020). Chestnut polysaccharides benefit spermatogenesis through improvement in the expression of important genes. Aging (Albany NY) 12, 11431–11445. doi: 10.18632/aging.103205
Zhang, P., Feng, Y., Li, L., Ge, W., Yu, S., Hao, Y., et al. (2021). Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut 70, 222–225. doi: 10.1136/gutjnl-2020-320992
Zhang, X., Li, D., Dong, C., Shi, J., Sun, Y., Ye, B., et al. (2020). Molybdenum sulfide-based electrochemical platform for high sensitive detection of taxifolin in Chinese medicine. Anal. Chim. Acta 1099, 85–93. doi: 10.1016/j.aca.2019.11.057
Zhang, P., Liu, J., Xiong, B., Zhang, C., Kang, B., Gao, Y., et al. (2020). Microbiota from alginate oligosaccharide dosed mice successfully mitigated small intestinal mucositis. Microbiome 8:112. doi: 10.1186/s40168-020-00886-x
Zhang, C., Xiong, B., Chen, L., Ge, W., Yin, S., Feng, Y., et al. (2021). Rescue of male fertility following faecal microbiota transplantation from alginate oligosaccharide-dosed mice. Gut 70, 2213–2215. doi: 10.1136/gutjnl-2020-323593
Zhang, W., Zhao, Y., Zhang, P., Hao, Y., Yu, S., Min, L., et al. (2018). Decrease in male mouse fertility by hydrogen sulfide and/or ammonia can be inheritable. Chemosphere 194, 147–157. doi: 10.1016/j.chemosphere.2017.11.164
Zhang, P., Zhao, Y., Zhang, H., Liu, J., Feng, Y., Yin, S., et al. (2019). Low dose chlorothalonil impairs mouse spermatogenesis through the intertwining of estrogen receptor pathways with histone and DNA methylation. Chemosphere 230, 384–395. doi: 10.1016/j.chemosphere.2019.05.029
Zhao, Y., Zhang, P., Ge, W., Feng, Y., Li, L., Sun, Z., et al. (2020). Alginate oligosaccharides improve germ cell development and testicular microenvironment to rescue busulfan disrupted spermatogenesis. Theranostics 10, 3308–3324. doi: 10.7150/thno.43189
Zhao, Y., Zhang, W. D., Liu, X. Q., Zhang, P. F., Hao, Y. N., Li, L., et al. (2016). Hydrogen sulfide and/or ammonia reduces spermatozoa motility through AMPK/AKT related pathways. Sci. Rep. 6:37884. doi: 10.1038/srep37884
Zhou, Q., Wang, M., Yuan, Y., Wang, X., Fu, R., Wan, H., et al. (2016). Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell 18, 330–340. doi: 10.1016/j.stem.2016.01.017
Keywords: Taxifolin, semen quality, blood metabolite, gut microbiota, boar
Citation: Zhou Y, Chen L, Han H, Xiong B, Zhong R, Jiang Y, Liu L, Sun H, Tan J, Cheng X, Schroyen M, Gao Y, Zhao Y and Zhang H (2022) Taxifolin increased semen quality of Duroc boars by improving gut microbes and blood metabolites. Front. Microbiol. 13:1020628. doi: 10.3389/fmicb.2022.1020628
Received: 16 August 2022; Accepted: 09 September 2022;
Published: 14 October 2022.
Edited by:
Kun Li, Nanjing Agricultural University, ChinaReviewed by:
Kong Qinghui, College of Agricultural and Animal Husbandry, Tibet University, ChinaCopyright © 2022 Zhou, Chen, Han, Xiong, Zhong, Jiang, Liu, Sun, Tan, Cheng, Schroyen, Gao, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongfu Zhang, emhhbmdob25nZnVAY2Fhcy5jbg==; Yong Zhao, eXpoYW84MThAaG90bWFpbC5jb20=; WW9uZy5aaGFvQG11cmRvY2guZWR1LmF1; Yang Gao, MTc5NjkyMDU4QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.