
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 09 December 2022
Sec. Virology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1013439
 Xia Yu1†
Xia Yu1† Hai Li2,3†
Hai Li2,3† Wenting Tan4†
Wenting Tan4† Xianbo Wang5†
Xianbo Wang5† Xin Zheng6†
Xin Zheng6† Yan Huang7†
Yan Huang7† Beiling Li8†
Beiling Li8† Zhongji Meng9
Zhongji Meng9 Yanhang Gao10
Yanhang Gao10 Zhiping Qian11
Zhiping Qian11 Feng Liu12,13
Feng Liu12,13 Xiaobo Lu14
Xiaobo Lu14 Jia Shang15
Jia Shang15 Huadong Yan16
Huadong Yan16 Yubao Zheng17
Yubao Zheng17 Weituo Zhang18
Weituo Zhang18 Shan Yin2,3
Shan Yin2,3 Wenyi Gu2,3
Wenyi Gu2,3 Guohong Deng4
Guohong Deng4 Xiaomei Xiang4
Xiaomei Xiang4 Yi Zhou4
Yi Zhou4 Yixin Hou5
Yixin Hou5 Qun Zhang5
Qun Zhang5 Shue Xiong6
Shue Xiong6 Jing Liu6
Jing Liu6 Ruochan Chen7
Ruochan Chen7 Liyuan Long7
Liyuan Long7 Jinjun Chen8
Jinjun Chen8 Xiuhua Jiang8
Xiuhua Jiang8 Sen Luo9
Sen Luo9 Yuanyuan Chen9
Yuanyuan Chen9 Chang Jiang10
Chang Jiang10 Jinming Zhao10
Jinming Zhao10 Liujuan Ji11
Liujuan Ji11 Xue Mei11
Xue Mei11 Jing Li13
Jing Li13 Tao Li13
Tao Li13 Rongjiong Zheng14
Rongjiong Zheng14 Xinyi Zhou14
Xinyi Zhou14 Haotang Ren1
Haotang Ren1 Jifang Sheng1*
Jifang Sheng1* Yu Shi1*
Yu Shi1*Background: The accurate prediction of the outcome of hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) is impeded by population heterogeneity. The study aimed to assess the impact of underlying cirrhosis on the performance of clinical prediction models (CPMs).
Methods: Using data from two multicenter, prospective cohorts of patients with HBV-ACLF, the discrimination, calibration, and clinical benefit were assessed for CPMs predicting 28-day and 90-day outcomes in patients with cirrhosis and those without, respectively.
Results: A total of 919 patients with HBV-ACLF were identified by Chinese Group on the Study of Severe Hepatitis B (COSSH) criteria, including 675 with cirrhosis and 244 without. COSSH-ACLF IIs, COSSH-ACLFs, Chronic Liver Failure-Consortium Acute-on-Chronic Liver Failure score (CLIF-C ACLFs), Tongji Prognostic Predictor Model score (TPPMs), Model for End-Stage Liver Disease score (MELDs), and MELD-Sodium score (MELD-Nas) were all strong predictors of short-term mortality in patients with HBV-ACLF. In contrast to a high model discriminative capacity in ACLF without cirrhosis, each prognostic model represents a marked decline of C-index, net reclassification index (NRI), and integrated discrimination improvement (IDI) in predicting either 28-day or 90-day prognosis of patients with cirrhosis. The hazard analysis identified largely overlapping risk factors of poor outcomes in both subgroups, while serum bilirubin was specifically associated with short-term mortality in patients with cirrhosis and blood urea nitrogen in patients without cirrhosis. A subgroup analysis in patients with cirrhosis showed a decline of discrimination of CPMS in those with ascites or infections compared to that in those without.
Conclusion: Predicting the short-term outcome of HBV-ACLF by CPMs is optimal in patients without cirrhosis but limited in those with cirrhosis, at least partially due to the complicated ascites or infections.
Acute-on-chronic liver failure (ACLF) is a critical illness caused by the acute exacerbation of a chronic liver disease, which leads to organ failure(s) and high short-term mortality (Bernal et al., 2015; Hernaez et al., 2017). The etiologies of ACLF differ in countries and regions across the world. In the Asian Pacific region, especially in China, HBV-related ACLF remains to be the major subtype of ACLF and one of the main causes of death in individuals with chronic HBV infection (Shi et al., 2015b).
Due to the shortage of donor organs and high mortality on the waiting list, it is of paramount importance at hospital admission to differentiate patients who would die in a short-term period despite standard care from those who would recover. Many efforts have been put to utilize clinical prediction models (CPMs) in the prognostication of HBV-ACLF, including non-specific scoring models [the model for end-stage liver disease (MELD) (Malinchoc et al., 2000) and Child–Turcotte–Pugh (CTP) score (Pugh et al., 1973)] and those specifically developed for HBV-ACLF (Yan et al., 2015). Among them, the MELD score was the most widely assessed (Wong and Cai, 2005). A wide variation in MELD performance was reported among different studies which represents significant heterogeneity of study populations (Yu et al., 2021). It is speculated that the presence of liver cirrhosis would be an important variable affecting the predictive accuracy of one specific prognostic model, as indicated by the fact that non-cirrhotic ACLF displays distinct patterns of organ failures from those with cirrhosis (Arroyo et al., 2016). While current reported studies have focused on prognostic factors of various types and forms of ACLF (Yu et al., 2021), how the presence of cirrhosis affects the performance of a clinical predictive model for patients with HBV-ACLF remained unknown. To confirm the hypothesis, we aimed to assess the accuracy of selected scoring models [that were MELD (Malinchoc et al., 2000) and MELD-Sodium score (MELD-Nas) (Biggins et al., 2006) representative of current donor organ allocation systems, Chronic Liver Failure-Consortium Acute-on-Chronic Liver Failure Score (CLIF-C ACLFs) (Jalan et al., 2014b) representative of score systems for general ACLF population, Tongji Prognostic Predictor Model score (TPPMs) (Wang et al., 2014), Chinese Group on the Study of Severe Hepatitis B (COSSH-ACLF), (Wu et al., 2018) and COSSH-ACLF IIs (Li et al., 2021) representative of score systems specific for HBV-ACLF] in predicting the short-term mortality of HBV-ACLF with or without cirrhosis, respectively, using data from a large multicenter, prospective cohort [the Chinese Acute-on-Chronic Liver Failure (CATCH-LIFE)] study (Gu et al., 2018; Qiao et al., 2021).
We retrospectively used data from two prospective multicenter cohorts of the CATCH-LIFE study (NCT02457637 and NCT03641872) from January 2015 to December 2016 and September 2018 to January 2019. The CATCH-LIFE study was designed to investigate the natural history of patients with chronic liver disease and acute exacerbation. The multicenter study is held by the Chinese Chronic Liver Failure (CLIF) Consortium, which is composed of 15 tertiary hospitals in China [Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai; Southwest Hospital, Third Military Medical University, Chongqing; Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Hubei; Nanfang Hospital, Southern Medical University, Guangzhou; Beijing Ditan Hospital, Capital Medical University, Beijing; Xiangya Hospital, Central South University, Hunan; First Hospital of Jilin University (JU), Jilin; Taihe Hospital, Hubei University of Medicine, Hubei; Shanghai Public Health Clinical Centre (SPHCC), Fudan University, Shanghai; Second Hospital of Shandong University (SDU), Shandong; First Affiliated Hospital of Xinjiang Medical University (XMU), Xinjiang; Henan Provincial People’s Hospital, Henan; Tianjin, Affiliated Hospital of Logistics University of People’s Armed Police Force, Tianjin; Fuzhou General Hospital of Nanjing Military Command, Fujian; and The First Affiliated Hospital of Zhejiang University in Zhejiang province, Zhejiang].
The patient inclusion criteria of the CATCH-LIFE study were: (1) inpatients (length of stay >1 day), including patients in the emergency observation ward; (2) patients with chronic liver disease including patients with non-alcoholic fatty liver disease, patients with chronic hepatitis without cirrhosis, and patients with compensated/decompensated cirrhosis; and (3) patients with acute liver injury (ALI) (ALT or AST > 3 × Upper normal limit or total bilirubin > 2 × Upper normal limit (within 1 week before enrollment) or acute decompensation (AD) event(s) (ascites, hepatic encephalopathy, bacterial infection, or gastrointestinal bleeding within 1 month before enrollment). The exclusion criteria were: (1) age ≤15 or ≥80; (2) pregnant women; (3) hepatocellular carcinoma or other liver malignancies were detected before or during the first admission; (4) malignancies in other organs; and (5) severe chronic extrahepatic disease. With these criteria, we further identified patients with HBV-ACLF in the CATCH-LIFE cohort by applying the Chinese Group on the Study of Severe Hepatitis B (COSSH) diagnostic criteria (Wu et al., 2018). In addition, to exclude the heterogeneity of the included patients with HBV-ACLF by different diagnostic criteria, we further adapted the APASL consensus diagnostic criteria (Sarin et al., 2009, 2014, 2019) to include patients with HBV-ACLF to verify the conclusion. Cirrhosis was diagnosed by endoscopic signs of portal hypertension or radiological evidence of liver nodularity in patients with chronic liver diseases as previously described (Tsochatzis et al., 2014). Chronic HBV infection is defined as a prolonged serum HBsAg positivity for 6 months.
After enrollment, demographic, laboratory, radiological, and other clinical information were collected for each patient. After discharge, patients were followed up through outpatient records, telephone, or WeChat. Death or liver transplantation was recorded during follow-up. The primary endpoint of the study was death within 28 days and 90 days post-enrollment.
The study adheres to the Declaration of Helsinki and was approved by the Renji Hospital Ethics Committee of Shanghai Jiao Tong University School of Medicine, and written consent was obtained from all the study patients or their legal representatives.
We selected COSSH-ACLF IIs and COSSH-ACLFs representative of CPMs specific for HBV-ACLF, CLIF-C ACLFs for ACLF, MELD, and MELD-Nas for end-stage liver diseases. The COSSH-ACLF IIs calculation formula is as follows: 1.649 × ln (international normalized ratio) + 0.457 × hepatic encephalopathy score + 0.425 × ln (neutrophil) + 0.396 × ln (total bilirubin) + 0.576 × ln (serum urea) + 0.033 × age (Li et al., 2021). The COSSH-ACLFs calculation formula is as follows: 0.741 × INR + 0.523 × HBV − sequential organ failure assessment score (SOFAs) + 0.026 × age + 0.003 × TB (μmol/L) (Wu et al., 2018). The CLIF-C ACLFs calculation formula is as follows: 10 × {0.33 × CLIF − organ failure (OFs) score + 0.04 × age + 0.63 × ln [white blood cell (WBC)) − 2} (Jalan et al., 2014b). The TPPMs calculation formula is as follows: P = 1 / (1 + e–logit(P)), logit(P) = 0.003 × [TBil (μmol/L)] + 0.951 × INR + 2.258 × [constant for complications: 0 if without or with one complication; 1 with 2 or more complications] + 0.114 × [lg HBV DNA (copies/ml)] − 5.012 (Wang et al., 2014). The MELDs calculation formula is as follows: 3.78 × ln [TB (mg/dl)] + 11.2 × ln (INR) + 9.57 × ln [serum creatinine (mg/dl)] + 6.43 (Malinchoc et al., 2000). The MELD-Nas calculation formula is as follows: MELD + 1.59 (135–serum sodium) (Biggins et al., 2006).
Continuous variables were expressed as mean ± standard deviation (SD) or median (range), and categorical variables were expressed as counts (percentage). Student’s t-test or the Mann–Whitney U-test was used for the comparison of continuous variables, and χ2-test was used for the comparison of categorical variables. The 28-day and 90-day survival of HBV-ACLF patients with or without cirrhosis were shown by the cumulative correlation function (CIF) following a Fine–Gray competing risk model, in which the liver transplantation (LT) was regarded as a competing event with death (Fine and Gray, 1999). The hazard ratio (HR) of each scoring model associated with death was estimated by a Cox proportional hazards regression model.
The performance of scoring models in overall patients and cirrhosis/non-cirrhosis subgroups was compared in aspects of discrimination and calibration. The discrimination of models was measured by the C-index, integrated discrimination improvement (IDI), and the net reclassification improvement (NRI) metric. A C-index of >0.80 indicates a good discriminative performance of a prognostic model. An NRI or IDI of >0 indicates the improvement of discrimination in the new model over the reference model. The calibration of models was assessed by the Hosmer–Lemeshow (H–L) test, a calibration plot, Nagelkerke’s R2, and the Brier score. For the H–L test, the smaller the χ2, the greater the correlation p-value and the better the goodness-of-fit. Suitable calibration is indicated by an H–L p-value of ≥0.05. A higher R2 and a lower Brier score indicate better calibration. In addition, the clinical benefit of scoring models was evaluated by decision curve analysis (DCA). All the statistical analyses were undertaken with R software (version 4.0.5; The R Foundation for Statistical Computing1), and differences were considered significant at a p-value of <0.05.
As shown in Figure 1 and Table 1, a total of 919 patients with HBV-ACLF were included after excluding patients who were not eligible, with 675 with cirrhosis and 244 without. HBV-ACLF patients with cirrhosis were elder than those without cirrhosis (48 ± 11 vs. 43 ± 12, p < 0.001). They were more likely to develop gastrointestinal bleeding [39 (5.8%) vs. 0 (0%), p < 0.001], ascites [456 (69.0%) vs. 87 (35.7%), p < 0.001], and infection [258 (38.2%) vs. 62 (25.4%), p < 0.001). Patients with cirrhosis were more likely to develop renal failure [58 (8.6%) vs. 7 (2.9%), p = 0.003] and coagulation failure [256 (37.9%) vs. 75 (30.7%), p = 0.045] than patients without cirrhosis. As to the laboratory tests, patients with cirrhosis had a lower level of albumin (g/L) [30.6 (6.8) vs. 32.6 (6.2), p < 0.001], alanine aminotransferase (ALT) (U/L) [173.7 (436.0) vs. 602.8 (973.0), p < 0.001], aspartate aminotransferase (AST) (U/L) [185.2 (315.0) vs. 367.0 (626.2), p < 0.001], glutamyl transferase (GGT) (U/L) [72.0 (74.2) vs. 87.2 (75.0), p = 0.004], sodium (mmol/L) [136.0 (7.0) vs. 137.0 (5.2), p < 0.001], hemoglobin [120.0 (29.0) vs. 133.0 (26.0), p < 0.001], and platelets (109/L) [83.0 (61.0) vs. 120.5 (65.0), p < 0.001] than without cirrhosis. In contrast, patients with cirrhosis had a higher level of creatinine (μmol/L) [74.0 (38.8) vs. 68.0 (26.7), p < 0.001], urea nitrogen (mmol/L) [4.8 (3.9) vs. 3.7 (1.8), p < 0.001], higher neutrophil-to-lymphocyte ratio (NLR) [4.0 (4.3) vs. 3.3 (3.2), p = 0.030], and higher international normalized ratio (INR) [2.1 (1.0) vs. 2.0 (0.8), p = 0.025]. In addition, patients with cirrhosis had higher disease risk scores including COSSH-ACLF IIs [7.2 (1.2) vs. 6.7 (1.2), p < 0.001], COSSH-ACLFs [7.2 (1.8) vs. 6.3 (1.5), p < 0.001], CLIF-C ACLFs [40.5 (8.6) vs. 37.6 (10.3), p < 0.001], TPPMs [0.4 (0.6) vs. 0.2 (0.3), p < 0.001], MELDs [27.1 (7.0) vs. 25.4 (5.5), p < 0.001], and MELD-Nas [28.9 (10.6) vs. 26.3 (6.3), p < 0.001].
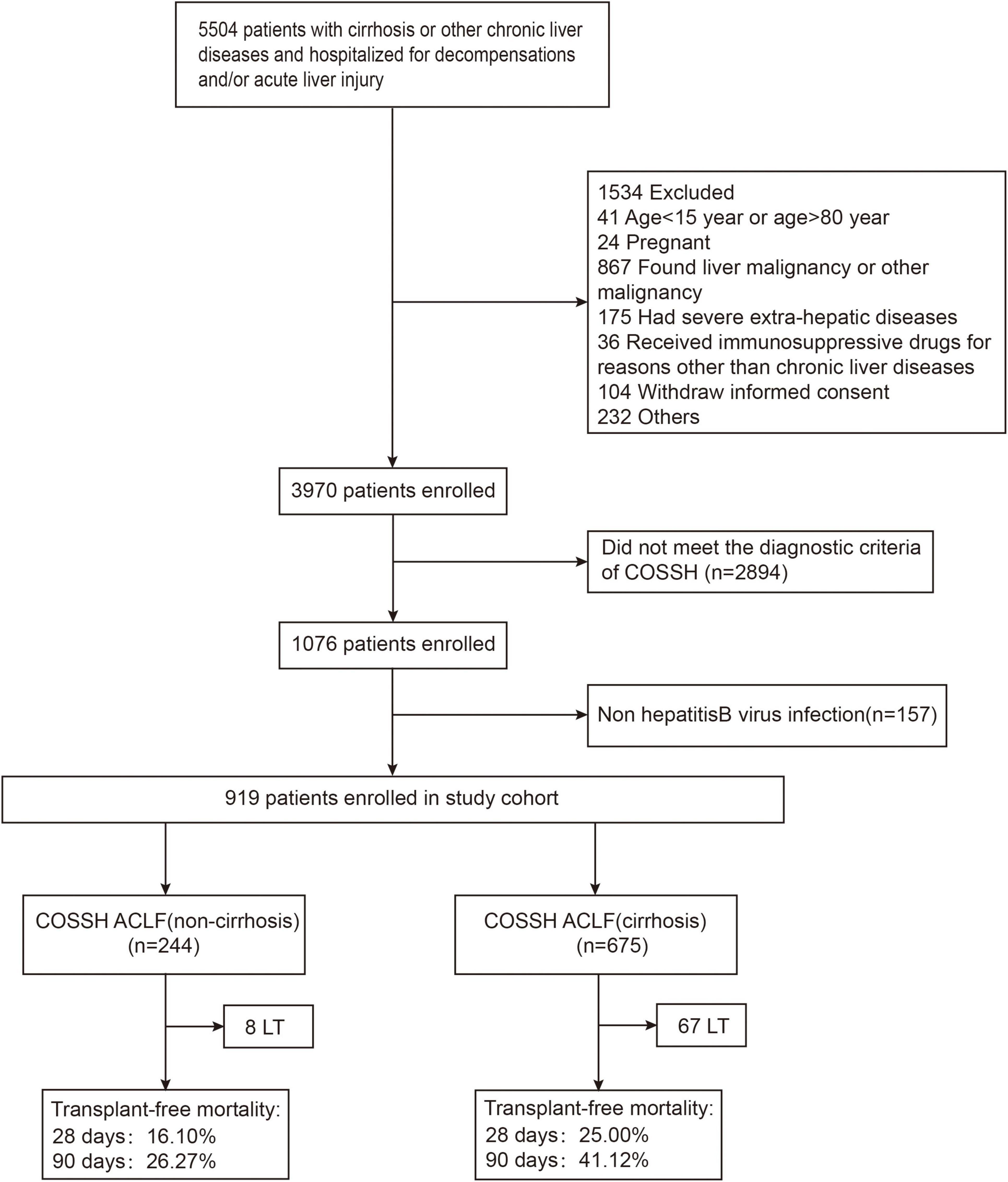
Figure 1. The flowchart of study selection. LT, liver transplantation; COSSH, Chinese Group on the Study of Severe Hepatitis B.
The median follow-up time of the study was 331 days (data not shown). A total of 312 deaths and 75 LT were recorded during follow-up. As shown in Table 1, 28-day and 90-day mortality in all patients with HBV-ACLF were 22.5 and 37.0%, respectively. Patients with cirrhosis had significantly higher both 28-day and 90-day mortality than those without (24.4/41.1 vs. 15.9/26.3%; 28-day/90-day). Liver transplantation is a common competing event with death in HBV-ACLF. As shown in Figure 2, by a Fine–Gray competing risk model, after controlling the competing event, there was a still significant difference in 28-day and 90-day cumulative survival between the cirrhotic and non-cirrhotic groups (28-day: p = 0.032; 90-day: p = 0.002). It was also noted that the incidence of LT was more frequent in HBV-ACLF with cirrhosis, concurring with the higher mortality in this subgroup.
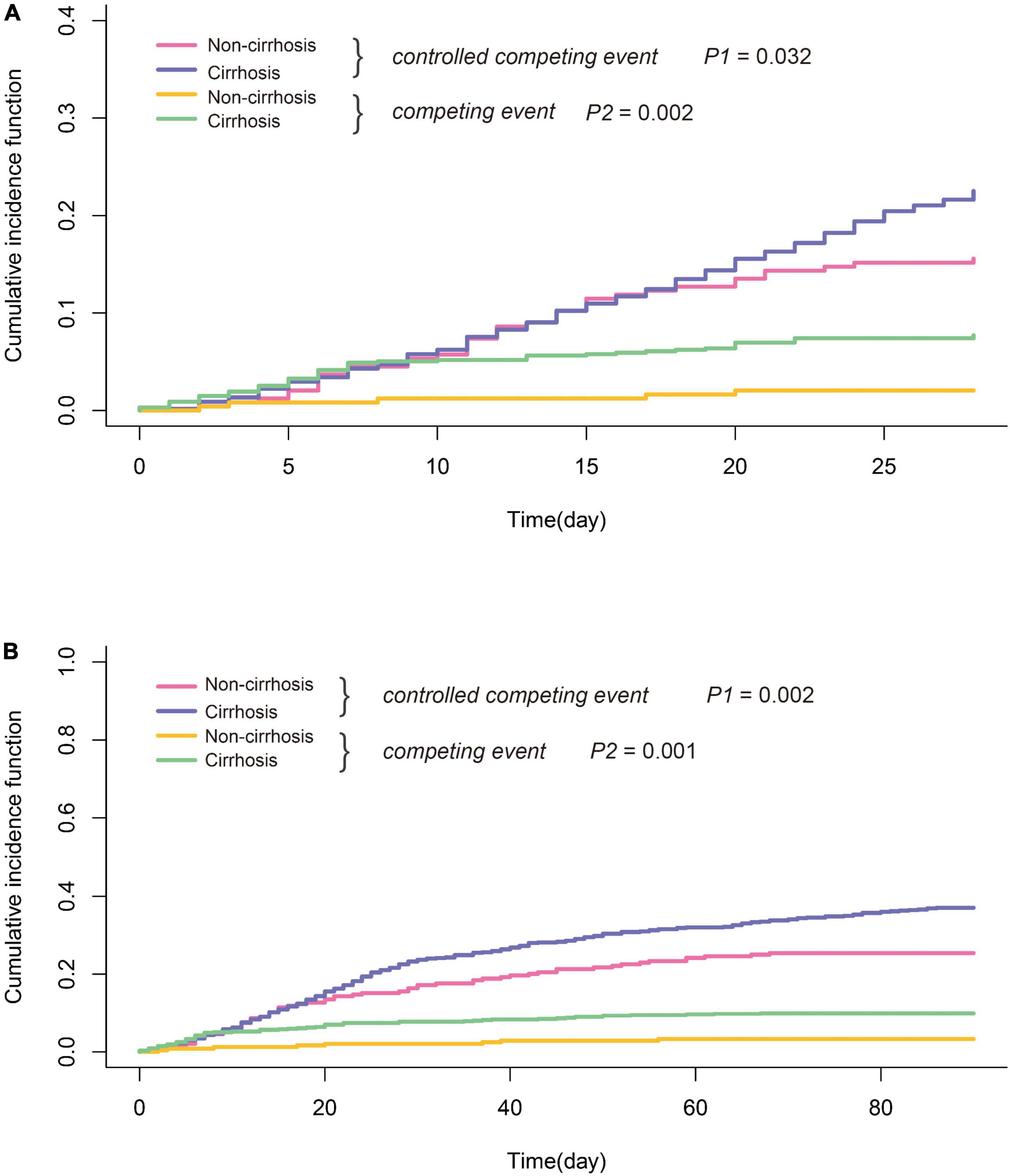
Figure 2. Comparison of short-term survival of HBV-ACLF with or without cirrhosis (A) 28-day and (B) 90-day; liver transplantation was considered as competing events. Statistical analysis was performed by cumulative incidence function. P1: HBV-ACLF with cirrhosis vs. HBV-ACLF without cirrhosis after controlling for competing risk. P2: HBV-ACLF with cirrhosis vs. HBV-ACLF without cirrhosis without controlling for competing risk.
As expected, in a Cox proportional model, each prognostic score was a strong predictor of short-term mortality of HBV-ACLF (Figure 3). The magnitude of risk estimates, as indicated by HR, was greater in patients without cirrhosis for each score, demonstrating a sharper increase in the risk of death with an increment of prognostic scores.
As shown in Table 2, in overall patients, COSSH-ACLF IIs exhibited the highest C-index both in predicting 28-day (0.773) and 90-day outcomes (0.739) in comparison to other scoring models including COSSH-ACLFs, CLIF-C ACLFs, TPPMs, MELDs, and MELD-Nas. COSSH-ACLF IIs remained to have the highest C-index in predicting the prognosis of either patients with cirrhosis or without, except in 90-day outcomes of patients without cirrhosis. By comparison, COSSH-ACLF IIs had better discrimination than other scoring models. The superiority was less significant over COSSH-ACLFs, particularly in the non-cirrhotic group. It was noted that the C-index of each model was shown to be better in patients without cirrhosis than those with. The conclusions remained to be unchanged regardless of whether LT was censored, excluded, or combined with death (Supplementary Tables 1, 2).
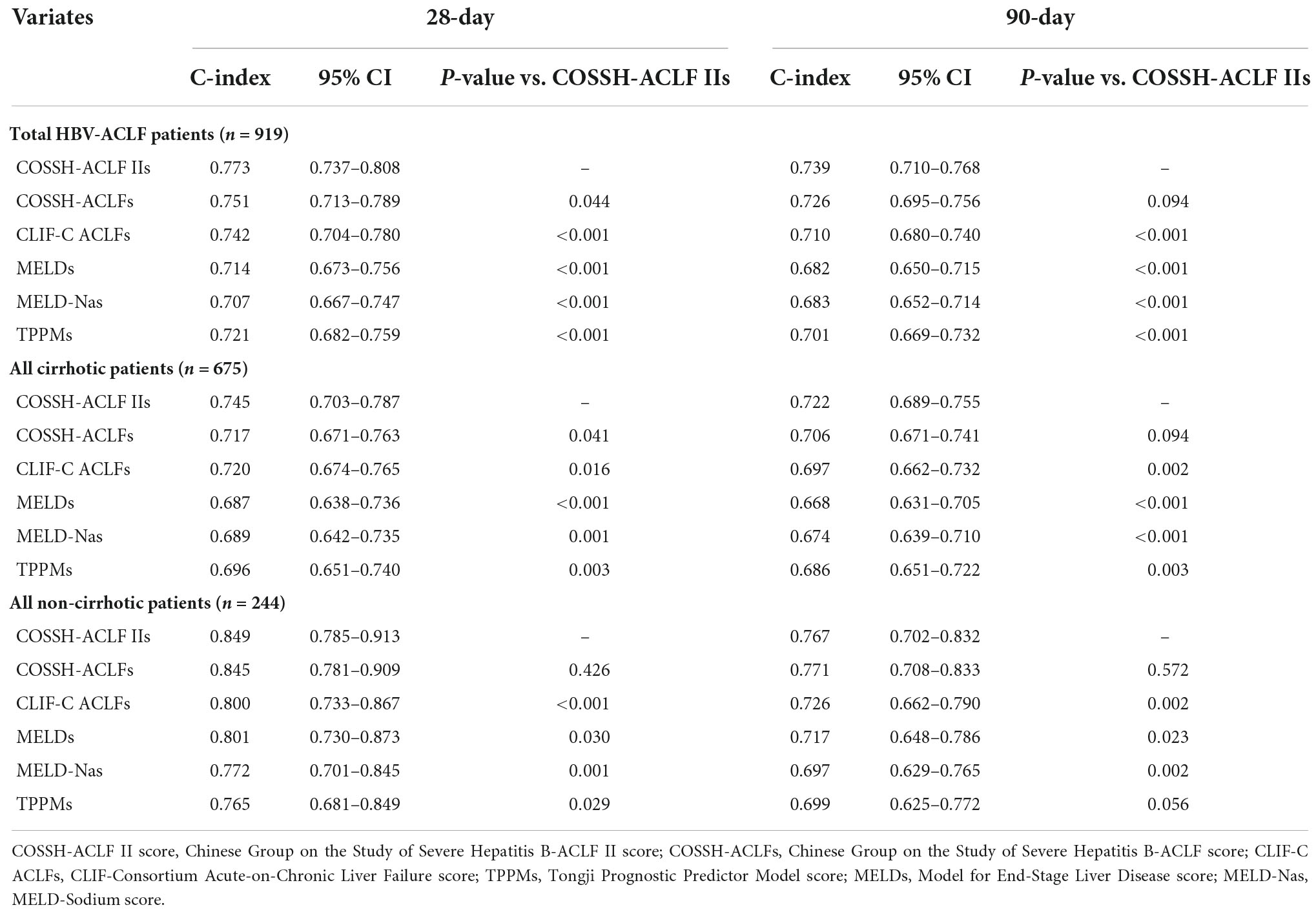
Table 2. Comparisons of C-index of prognostic models in predicting short-term mortality in patients with HBV-ACLF (liver transplantation regarded as censored).
In the study, a total of 919 patients with HBV-ACLF were included under COSSH criteria, of which 434 (47.2%), 550 (59.8%), and 48 (5.2%) patients were ACLF as per EASL-CLIF, APASL, and NASCELD criteria, respectively. As EASL-CLIF and NACSELD criteria only include ACLF with cirrhosis, we compared the predictive performance of CPMs between cirrhotic and non-cirrhotic patients with HBV-ACLF defined by APASL criteria. The baseline characteristics of patients within APASL-ACLF or without are shown in Supplementary Table 3. As shown in Supplementary Table 4, COSSH-ACLF IIs remained to have better discriminative performance than CLIF-C ACLFs, TPPMs, MELDs, and MELD-Nas in predicting both 28-day and 90-day mortality, in either whole population or cirrhosis/non-cirrhosis subgroups. COSSH-ACLF IIs was only better in predicting 28-day outcome in overall patients and patients with cirrhosis when compared to COSSH-ACLFs. Likewise, the C-index of each model in the prediction of 28-day mortality was shown to be better in patients without cirrhosis than those with. However, the trend was not observed in predicting the 90-day outcome.
Then, we compared the NRI and IDI of COSSH-ACLFs, CLIF-C ACLFs, TPPMs, MELDs, and MELD-Nas with COSSH-ACLF IIs, respectively. As shown in Supplementary Table 5, there was no significant difference between CLIF-C ACLFs and COSSH-ACLF IIs. Except for CLIF-C ACLFs, the NRI of COSSH-ACLF IIs significantly improved compared with other models (p < 0.05). Moreover, the NRI of patients without cirrhosis is greater than that of cirrhosis, indicating that the prediction efficiency of non-cirrhosis is better. Similarly, as shown in Supplementary Table 6, in the comparison of IDI, there was no significant difference between CLIF-C ACLFs and COSSH-ACLF IIs. Except for CLIF-C ACLFs, COSSH-ACLF IIs had significant improvement in IDI compared with other models (p < 0.05). Moreover, the IDI of patients without cirrhosis was greater than that of cirrhosis, also indicating that the prediction efficiency of non-cirrhosis is better.
We further assessed the calibration of scoring models. From an intuitive perspective, COSSH-ACLF IIs and CLIF-C ACLFs represented good calibration, irrespective of 28-day or 90-day outcome and the presence of cirrhosis or not (Supplementary Figure 2). The goodness-of-fit test demonstrated no significant deviation from observed risk in all models except MELDs and TPPMs (Table 3). A quantitative evaluation of model calibration was further by Nagelkerke’s R2 and the Brier score (Table 4). COSSH-ACLF IIs had the largest Nagelkerke’s R2 and the least Brier score in predicting 28-day or 90-day outcome in patients with or without cirrhosis, indicating a better calibration. Notably, a larger Nagelkerke’s R2 and a less Brier score of each model were seen in predicting prognosis in patients without cirrhosis than those with.
Finally, to evaluate the performance of clinical prognostic models, we used the DCA (Vickers and Elkin, 2006; Vickers, 2008). Of course, this is a theoretical model, which may overestimate or even underestimate the usefulness of the model. As shown in Figure 4, we can find that the six models (COSSH-ACLF IIs, COSSH-ACLFs, CLIF-C ACLFs, TPPMs, MELDs, and MELD-Nas) were all useful between the threshold probability of 30–50%, and the threshold probability range is large in the 90-day prognosis evaluation. In addition, it can be seen from the figure that the clinical net benefits of COSSH-ACLF IIs and COSSH-ACLFs are better than the other three models, especially in patients without cirrhosis.
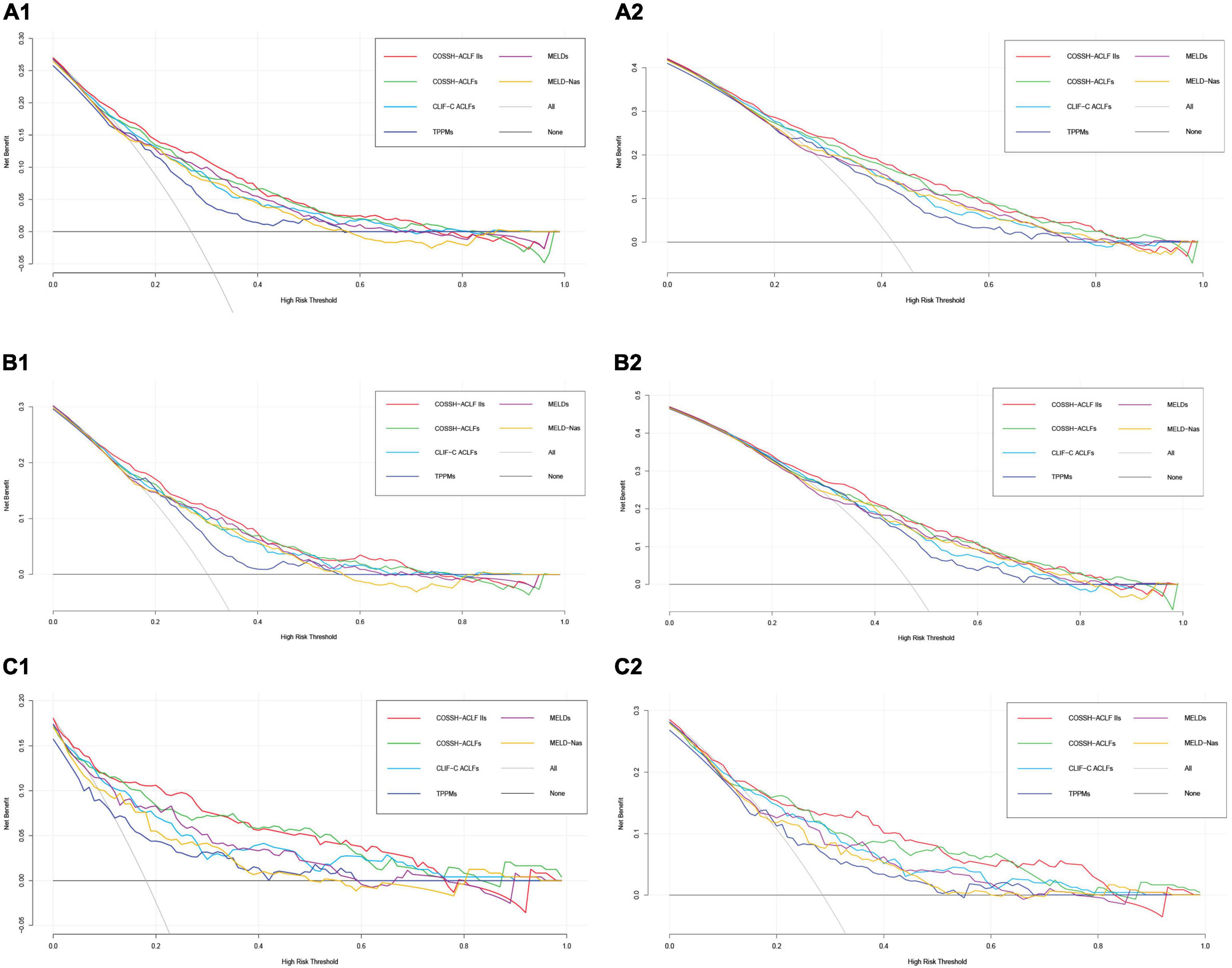
Figure 4. Decision analysis curve in predicting 28/90-day prognosis of overall patients with HBV-ACLF (A1,A2), HBV-ACLF patients with cirrhosis (B1,B2), and HBV-ACLF patients without cirrhosis (C1,C2).
We first performed a hazard analysis of risk factors associated with poor short-term outcomes in patients with or without cirrhosis, and the findings showed elder age, high INR and neutrophil count, and the presence of HE were independent predictors of 28-day or 90-day LT-free mortality in both HBV-ACLF patients with cirrhosis or without cirrhosis (see Supplementary Table 7). Notably, serum bilirubin was specifically associated with short-term mortality in patients with cirrhosis [28-day and 90-day outcome: HR = 1.002 (1.001–1.002), p < 0.001] and blood urea nitrogen in patients without cirrhosis [28-day outcome: HR = 1.111 (1.026–1.204), p = 0.010; 90-day outcome: HR = 1.086 (1.017–1.158), p = 0.013].
We further performed a subgroup analysis in patients with cirrhosis based on combined complications, such as ascites, hepatic encephalopathy, infections, and gastrointestinal hemorrhage. As shown in Supplementary Tables 8, 9, patients with ascites or infections had a lower discriminative performance of CPMs in predicting 28-day or 90-day LT-free mortality than those without, while the presence of hepatic encephalopathy and gastrointestinal hemorrhage had little impact.
A prior study that developed COSSH-ACLF IIs has shown a high discriminative accuracy of this model in predicting 28-day and 90-day outcomes of overall patients with HBV-ACLF, each C-index exceeding 0.80 (Wu et al., 2018). However, 0.773 (28-day) and 0.739 (90-day) C-indexes were significantly lower in our study. The discrepancy can be attributed to the difference in the constitution of patients with cirrhosis and non-cirrhosis between the two studies. Nevertheless, a relative discriminative superiority of COSSH-ACLF IIs over other scoring systems has been confirmed in both subgroups. In particular, COSSH-ACLF IIs achieved optimal accuracy in predicting 28-day outcomes of patients without cirrhosis, with a C-index close to 0.85. It should be noted that blood urea nitrogen is adopted instead of creatinine in the formula of COSSH-ACLF IIs. Although blood urea nitrogen is a less specific surrogate marker for kidney function than creatinine, creatinine is affected by increased tubular secretion, sarcopenia, and hyperbilirubinemia, and thereby commonly leads to underestimates of kidney function decline in patients with cirrhosis (Velez et al., 2020). In addition to acute kidney injury, other circumstances associated with the elevated level of blood urea nitrogen include gastrointestinal hemorrhage and high levels of catabolism (Zaccherini et al., 2021), which had a negative influence on the outcome of ACLF.
Second, our data clearly demonstrated that each prognostic model had better performance in HBV-ACLF patients without cirrhosis than those with cirrhosis in the study which is consistent with the study of Dong et al. (2020). The limited performance of prognostic scores in ACLF patients with cirrhosis reflects the intrinsic heterogeneity of this subtype, which results from both the wide spectrum of cirrhosis and the diversity of acute precipitating events (Shi et al., 2015a). There is a wide continuum of disease stages during the natural history of cirrhosis. According to the absence or presence of esophageal varices and/or ascites, four clinical stages or statuses of cirrhosis can be identified, each with distinct clinical features and a markedly different prognosis. To be more complicated, progression may be accelerated by the development of other complications such as (re)bleeding, renal impairment (refractory ascites and hepatorenal syndrome), hepatopulmonary syndrome, and sepsis (spontaneous bacterial peritonitis) (Wu et al., 2018; Tang et al., 2021). Thereby, ACLF patients with cirrhosis have varying stages of cirrhosis, which may impact the prognosis. To support it, our prior study has demonstrated that ACLF in patients with cirrhosis with the previous decompensation had higher mortality after 28 days. Our data suggested that the accuracy of CPMs declined with the presence of ascites or infections in patients with cirrhosis. In contrast, the clinical manifestations of ACLF patients with cirrhosis were complicated by the differential effect of hepatic (hepatitis B flare, superimposed infection of HAV or HEV, etc.) and extrahepatic (bacterial infection, upper gastrointestinal bleeding, etc.) precipitating events (Shi et al., 2015a). As shown by our prior study, hepatic-ACLF was classically characterized by liver and coagulation failures, whereas patients with extrahepatic-ACLF displayed diverse phenotypes of organ failures. In addition, 29–44% of ACLF in cirrhosis that occur without an evident precipitating event represent another unique population. Taken together, it is likely that the interaction between precipitating events and underlying cirrhosis leads to complex clinical phenotypes in ACLF patients with cirrhosis. In contrast, ACLF develops in compensatory non-cirrhotic chronic liver diseases that are precipitated by hepatic insults representing a more homogeneous population.
Third, there may be a lack of some key parameters in these available CPMs that reflects the pathophysiology mechanisms of ACLF in cirrhosis. For example, portal hypertension is the initial and main consequence of cirrhosis and is responsible for the majority of its complications. In the compensated phase, portal pressure may be normal or below the threshold level of clinically significant portal hypertension defined by a hepatic venous pressure gradient (HVPG) of at least 10 mmHg. During the transition to decompensated stage, an increase in portal pressure results in the development of complications such as ascites, portal hypertensive gastrointestinal (GI) bleeding, and encephalopathy. Portal hypertension was also observed in ACLF, as shown by increased intrahepatic resistance and HVPG, and associated with mortality (Mehta et al., 2015). Furthermore, relapse of acute variceal bleeding is higher in patients with ACLF and increased with ACLF grades, and in contrast, pre-emptive TIPS placement improved survival (Trebicka et al., 2020; Kumar et al., 2021). Collectively, these findings implied portal hypertension as an essential element in the pathophysiological alterations of ACLF in cirrhosis; however, there is no surrogate marker for portal hypertension in these available CPMs. Second, it has been recognized that systemic inflammation is a major driver of ACLF progression, and WBC or PMN count is usually adopted as the surrogate marker. However, a limitation of WBC or PMN count is that patients with advanced cirrhosis frequently manifest a reduced baseline WBC or PMN count due to hypersplenism, and thereby the systemic inflammation in these patients can be underestimated (Cazzaniga et al., 2009).
In addition, the frequent complications occurring during the natural history of ACLF in cirrhosis render it to be highly dynamic. For example, a prospective–retrospective cohort study enrolling 985 patients from the APASL-ACLF Research Consortium (AARC) database and the Chinese Study Group showed that the cirrhotic group exhibited higher rates of complication, including ascites, infection, and upper gastrointestinal bleeding than the non-cirrhotic group (Chen et al., 2019). The development of complications was demonstrated to be a major risk factor for mortality in HBV-ACLF patients with cirrhosis, with an increase of 1.9–4.7-fold, and 2.6–8.3-fold of death risk in presence of two and three complications, respectively. In line with it, several reports have shown that a high incidence of bacterial or fungal infections occurred secondary to ACLF in patients with cirrhosis and was associated with poor clinical course and high mortality (Jalan et al., 2014a; Fernández et al., 2018; Wong et al., 2021). Also, it has been shown that variceal bleeding was less likely to be controlled by initial endoscopy in patients with ACLF than in patients with non-ACLF cirrhosis, thereby leading to high rebleeding (Trebicka et al., 2020; Kumar et al., 2021).
In conclusion, our data revealed a differential performance of common scoring models in predicting short-term mortality between HBV-ACLF patients with and without cirrhosis. Common CPMs reached optimal performance in patients without cirrhosis. Therefore, the present study identified a subgroup of HBV-ACLF in which CPMs can be translated into clinical practice, for instance, liver donor allocation and tailoring indications for liver transplantation. However, it remains to be a challenge to predict the short-term prognosis of HBV-ACLF patients with cirrhosis at a precise level, especially those with ascites or infections. More effort should be put on to refine the current prognostication systems for HBV-ACLF with cirrhosis in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Renji Hospital Ethics Committee of Shanghai Jiao Tong University School of Medicine (NCT02457637 and NCT03641872). The patients/participants provided their written informed consent to participate in this study.
XY, SY, WG, WT, YZo, YHo, QZ, SX, JLiu, RC, LL, BL, XJ, SL, YC, CJ, JZ, LJ, XM, JLi, TL, RZ, XZho, and HR collected and analyzed the data. JSe, YS, HL, GD, XX, XW, XZhe, YHu, JC, ZM, YG, ZQ, FL, XL, JSa, HY, YZe, and WZ designed the research study. XY wrote the manuscript. YS and JSe critically reviewed the manuscript. All authors approved the final version of the manuscript.
This work was supported by the National Key Research and Development Program of China (2021YFC2301800), Shanghai Hospital Development Commission (SHDC2020CR1037B), the National Key Research and Development Program of China (2017YFC0908100 and 2017YFC0908103), the National Science and Technology Major Project (2018ZX10723203 and 2017ZX10202202), the National Natural Science Foundation of China (81930061, 81900579, 81473641, 81271884, 81461130019, 81700561, 81470038, 82070650, 81660333, and 81870425), the Chongqing Natural Science Foundation (CSTC2019jcyj-zdxmX0004), the Department of Science and Technology of Guangdong Province (2015B020226004), the Foundation for Innovative Research Groups of Natural Science Foundation of Hubei Province of China (2018CFA031), the Shandong Province Natural Science Foundation (ZR2019PH052), the 12-5 State S&T Projects of China (2018ZX10302-206), the Fundamental Research Funds for the Central Universities, and a project supported by Scientific Research Fund of Zhejiang University (XY2021030).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1013439/full#supplementary-material
ACLF, acute-on-chronic liver failure; HBV, hepatitis B virus; HBV-ACLF, hepatitis B virus-related acute-on-chronic liver failure; CPM, clinical prediction model; MELD, model for end-stage liver disease; MELD-Na, MELD-sodium; CLIF-C ACLFs, chronic liver failure-consortium acute-on-chronic liver failure score; COSSH-ACLF II score, Chinese Group on the Study of Severe Hepatitis {B-ACLF} II score; COSSH-ACLFs, Chinese Group on the Study of Severe Hepatitis {B-ACLF} score; TPPMs, Tongji Prognostic Predictor Model score; MAP, mean arterial pressure; GIH, gastrointestinal hemorrhage; HE, hepatic encephalopathy; SBP, spontaneous bacterial peritonitis; Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AKP, alkaline phosphatase; TB, total bilirubin; GGT, glutamyl transferase; Cr,~creatinine; K, serum potassium; Na, serum sodium; WBC, white blood cell count; NLR, neutrophil-to-lymphocyte ratio; PLT, platelet count; INR, international normalized ratio; NRI, net reclassification index; IDI, integrated discrimination improvement; AD, acute decompensation; ALI, acute liver injury; APASL, Asian Pacific Association for the Study of the Liver.
Arroyo, V., Moreau, R., Kamath, P. S., Jalan, R., Ginès, P., Nevens, F., et al. (2016). Acute-on-chronic liver failure in cirrhosis. Nat. Rev. Dis. Primers 2:16041. doi: 10.1038/nrdp.2016.41
Bernal, W., Jalan, R., Quaglia, A., Simpson, K., Wendon, J., and Burroughs, A. (2015). Acute-on-chronic liver failure. Lancet 386:157687. doi: 10.1016/S0140-6736(15)00309-8
Biggins, S. W., Kim, W. R., Terrault, N. A., Saab, S., Balan, V., Schiano, T., et al. (2006). Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 130, 1652–1660. doi: 10.1053/j.gastro.2006.02.010
Cazzaniga, M., Dionigi, E., Gobbo, G., Fioretti, A., Monti, V., and Salerno, F. (2009). The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J. Hepatol. 51, 475–482. doi: 10.1016/j.jhep.2009.04.017
Chen, T., Yang, Z., Choudhury, A. K., Al Mahtab, M., Li, J., Chen, Y., et al. (2019). Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: a multi-national study from the Asia-Pacific region. Hepatol. Int. 13, 695–705. doi: 10.1007/s12072-019-09992-x
Dong, X., He, J., Chen, W., Su, R., Xu, Y., Sheng, X., et al. (2020). Characteristics and outcomes of acute-on-chronic liver failure patients with or without cirrhosis using two criteria. Sci. Rep. 10:8577. doi: 10.1038/s41598-020-65529-5
Fernández, J., Acevedo, J., Wiest, R., Gustot, T., Amoros, A., Deulofeu, C., et al. (2018). Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 67, 1870–1880. doi: 10.1136/gutjnl-2017-314240
Fine, J. P., and Gray, R. J. (1999). A proportional hazards model for the subdistribution of a competing risk. J. Am. Statist. Assoc. 94, 496–509. doi: 10.1080/01621459.1999.10474144
Gu, W. Y., Xu, B. Y., Zheng, X., Chen, J., Wang, X. B., Huang, Y., et al. (2018). Acute-on-Chronic liver failure in china: rationale for developing a patient registry and baseline characteristics. Am. J. Epidemiol. 187, 1829–1839. doi: 10.1093/aje/kwy083
Hernaez, R., Solà, E., Moreau, R., and Ginès, P. (2017). Acute-on-chronic liver failure: an update. Gut 66, 541–553. doi: 10.1136/gutjnl-2016-312670
Jalan, R., Fernandez, J., Wiest, R., Schnabl, B., Moreau, R., Angeli, P., et al. (2014a). Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J. Hepatol. 60, 1310–1324. doi: 10.1016/j.jhep.2014.01.024
Jalan, R., Saliba, F., Pavesi, M., Amoros, A., Moreau, R., Ginès, P., et al. (2014b). Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 61, 1038–1047. doi: 10.1016/j.jhep.2014.06.012
Kumar, R., Kerbert, A. J. C., Sheikh, M. F., Roth, N., Calvao, J. A. F., Mesquita, M. D., et al. (2021). Determinants of mortality in patients with cirrhosis and uncontrolled variceal bleeding. J. Hepatol. 74, 66–79. doi: 10.1016/j.jhep.2020.06.010
Li, J., Liang, X., You, S., Feng, T., Zhou, X., Zhu, B., et al. (2021). Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J. Hepatol. 75, 1104–1115. doi: 10.1016/j.jhep.2021.05.026
Malinchoc, M., Kamath, P. S., Gordon, F. D., Peine, C. J., Rank, J., and ter Borg, P. C. (2000). A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 31, 864–871. doi: 10.1053/he.2000.5852
Mehta, G., Mookerjee, R. P., Sharma, V., and Jalan, R. (2015). Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 35, 724–734. doi: 10.1111/liv.12559
Pugh, R. N., Murray-Lyon, I. M., Dawson, J. L., Pietroni, M. C., and Williams, R. (1973). Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 60, 646–649. doi: 10.1002/bjs.1800600817
Qiao, L., Wang, X., Deng, G., Huang, Y., Chen, J., Meng, Z., et al. (2021). Cohort profile: a multicentre prospective validation cohort of the Chinese Acute-on-Chronic Liver Failure (CATCH-LIFE) study. BMJ Open 11:e037793. doi: 10.1136/bmjopen-2020-037793
Sarin, S. K., Choudhury, A., Sharma, M. K., Maiwall, R., Al Mahtab, M., Rahman, S., et al. (2019). Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol. Int. 13, 353–390. doi: 10.1007/s12072-019-09946-3
Sarin, S. K., Kedarisetty, C. K., Abbas, Z., Amarapurkar, D., Bihari, C., Chan, A. C., et al. (2014). Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol. Int. 8, 453–471. doi: 10.1007/s12072-014-9580-2
Sarin, S. K., Kumar, A., Almeida, J. A., Chawla, Y. K., Fan, S. T., Garg, H., et al. (2009). Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol. Int. 3, 269–282. doi: 10.1007/s12072-008-9106-x
Shi, Y., Yang, Y., Hu, Y., Wu, W., Yang, Q., Zheng, M., et al. (2015a). Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology 62, 232–242. doi: 10.1002/hep.27795
Shi, Y., Zheng, M. H., Yang, Y., Wei, W., Yang, Q., Hu, A., et al. (2015b). Increased delayed mortality in patients with acute-on-chronic liver failure who have prior decompensation. J. Gastroenterol. Hepatol. 30, 712–718. doi: 10.1111/jgh.12787
Tang, X., Qi, T., Li, B., Li, H., Huang, Z., Zhu, Z., et al. (2021). Tri-typing of hepatitis B-related acute-on-chronic liver failure defined by the world gastroenterology organization. J. Gastroenterol. Hepatol. 36, 208–216. doi: 10.1111/jgh.15113
Trebicka, J., Gu, W., Ibáñez-Samaniego, L., Hernández-Gea, V., Pitarch, C., Garcia, E., et al. (2020). Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J. Hepatol. 73, 1082–1091. doi: 10.1016/j.jhep.2020.04.024
Tsochatzis, E. A., Bosch, J., and Burroughs, A. K. (2014). Liver cirrhosis. Lancet 383, 1749–1761. doi: 10.1016/S0140-6736(14)60121-5
Velez, J. C. Q., Therapondos, G., and Juncos, L. A. (2020). Reappraising the spectrum of AKI and hepatorenal syndrome in patients with cirrhosis. Nat. Rev. Nephrol. 16, 137–155. doi: 10.1038/s41581-019-0218-4
Vickers, A. J. (2008). Decision analysis for the evaluation of diagnostic tests, prediction models and molecular markers. Am. Stat. 62, 314–320. doi: 10.1198/000313008X370302
Vickers, A. J., and Elkin, E. B. (2006). Decision curve analysis: a novel method for evaluating prediction models. Med. Decis. Making 26, 565–574. doi: 10.1177/0272989X06295361
Wang, J., Ma, K., Han, M., Guo, W., Huang, J., Yang, D., et al. (2014). Nucleoside analogs prevent disease progression in HBV-related acute-on-chronic liver failure: validation of the TPPM model. Hepatol. Int. 8, 64–71. doi: 10.1007/s12072-013-9485-5
Wong, F., Piano, S., Singh, V., Bartoletti, M., Maiwall, R., Alessandria, C., et al. (2021). Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure. J. Hepatol. 74, 330–339. doi: 10.1016/j.jhep.2020.07.046
Wong, Z. H., and Cai, S. Q. (2005). [Analysis of prognosis on patients with severe viral hepatitis using the model for end-stage liver disease]. Zhonghua Gan Zang Bing Za Zhi 13, 249–251. doi: 10.3748/wjg.v11.i6.899
Wu, T., Li, J., Shao, L., Xin, J., Jiang, L., Zhou, Q., et al. (2018). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 67, 2181–2191. doi: 10.1136/gutjnl-2017-314641
Yan, H., Wu, W., Yang, Y., Wu, Y., Yang, Q., and Shi, Y. (2015). A novel integrated model for end-stage liver disease model predicts short-term prognosis of hepatitis B virus-related acute-on-chronic liver failure patients. Hepatol. Res. 45, 405–414. doi: 10.1111/hepr.12362
Yu, X., Lu, Y., Sun, S., Tu, H., Xu, X., Gong, K., et al. (2021). Clinical prediction models for hepatitis B virus-related acute-on-chronic liver failure: a technical report. J. Clin. Trans. Hepatol. 9, 838–849. doi: 10.14218/JCTH.2021.00005
Keywords: hepatitis B virus, acute-on-chronic liver failure, clinical prediction models, cirrhosis, performance
Citation: Yu X, Li H, Tan W, Wang X, Zheng X, Huang Y, Li B, Meng Z, Gao Y, Qian Z, Liu F, Lu X, Shang J, Yan H, Zheng Y, Zhang W, Yin S, Gu W, Deng G, Xiang X, Zhou Y, Hou Y, Zhang Q, Xiong S, Liu J, Chen R, Long L, Chen J, Jiang X, Luo S, Chen Y, Jiang C, Zhao J, Ji L, Mei X, Li J, Li T, Zheng R, Zhou X, Ren H, Sheng J and Shi Y (2022) Prognosis prediction performs better in patients with non-cirrhosis hepatitis B virus-related acute-on-chronic liver failure than those with cirrhosis. Front. Microbiol. 13:1013439. doi: 10.3389/fmicb.2022.1013439
Received: 07 August 2022; Accepted: 18 November 2022;
Published: 09 December 2022.
Edited by:
Baibaswata Nayak, All India Institute of Medical Sciences, IndiaReviewed by:
Shalimar, All India Institute of Medical Sciences, IndiaCopyright © 2022 Yu, Li, Tan, Wang, Zheng, Huang, Li, Meng, Gao, Qian, Liu, Lu, Shang, Yan, Zheng, Zhang, Yin, Gu, Deng, Xiang, Zhou, Hou, Zhang, Xiong, Liu, Chen, Long, Chen, Jiang, Luo, Chen, Jiang, Zhao, Ji, Mei, Li, Li, Zheng, Zhou, Ren, Sheng and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jifang Sheng, amlmYW5nX3NoZW5nQHpqdS5lZHUuY24=; Yu Shi, emp1c2hpeXVAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.