- 1National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Chinese Center for Tropical Diseases Research), NHC Key Laboratory of Parasite and Vector Biology, WHO Collaborating Center for Tropical Diseases, National Center for International Research on Tropical Diseases, Shanghai, China
- 2School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu, China
- 3Ganzr Tibetan Autonomous Prefecture Center for Disease Control and Prevention, Kangding, Sichuan, China
- 4National Health Commission Key Laboratory of Echinococcosis Prevention and Control, Tibet Autonomous Region Center for Disease Control and Prevention, Lhasa, Tibet, China
- 5Department of Microbiology and Microbial Engineering, School of Life Sciences, Fudan University, Shanghai, China
Echinococcus multilocularis, the causative agent of alveolar echinococcosis (AE), severely threats human health and livestock farming. The first line of chemotherapeutic drug for AE is albendazole, which limits rapid extension of E. multilocularis metacestodes, but is rarely curative for AE, with severe side effects in long-term use, thus development of new anti-echinococcal drugs is mandated. Pseudolaric acid B (PAB) has long been used to treat fungal-infected dermatosis, and exerted anti-tumor, -fertility, -angiogenesis, -tubulin and antiparasitic activity. However, the effect of PAB against Echinococcus spp. remains unclear. The present study is to understand the effect of PAB against E. multilocularis in vitro and in vivo, and identify potential anti-echinococcal mechanism, as well as its toxicity. After exposure to PAB at 20 μg/ml, significant reduction of the survival rate and substantial ultrastructural destructions in E. multilocularis protoscoleces were observed in vitro. Furthermore, the wet weight of E. multilocularis cysts in the infected mice was significantly decreased after treatment with PAB (40, 20 or 10 mg/kg) for 12 weeks. Meanwhile, significant increase of both protein and mRNA expression of transforming growth factor beta 1 (TGF-β1) was detected in the serum and liver of the infected mice, whereas PAB administration lowered its expression significantly. The toxicity tests demonstrated that PAB displayed lower cytotoxicity to human liver and kidney cells (HL-7702 and HK-2 cell) with IC50 = 25.29 and 42.94 μg/ml than albendazole with IC50 = 3.71 and 21.22 μg/ml in vitro, and caused lower hepatoxicity and nephrotoxicity in mice than ABZ. Our findings indicated that PAB possesses potent anti-echinococcal effect, with lower toxicity than albendazole, implying a potential chemotherapeutic agent for AE. Additionally, the present study demonstrated that the suppressive effect of PAB on the parasite may involve down-regulation of TGF-β1 signaling.
Introduction
Alveolar echinococcosis (AE) is a neglected zoonotic parasitosis caused by the larval stage of Echinococcus multilocularis. It is a cosmopolitan public health issue posing severe harm to human and livestock health, mainly spreading in Central Asia and Tibetan plateau of Western China, Europe and North America (Deplazes et al., 2017). It was reported that more than 91% of annual new AE cases worldwide occurred in rural communities on the Qinghai-Tibet Plateau of Western China, which causes more than 90% of the global disease burden (Kern et al., 2017). E. multilocularis metacestodes often reside in the liver of human and rodents as the intermediate host, and show tumor-like infiltrative growth, leading to liver fibrosis and organ-failure, which is often termed “parasitic cancer” (Wang et al., 2016; Gao et al., 2021). If untreated or insufficiently treated, AE patients prognose a mortality rate of > 90% within 10–15 years after diagnosed (Cheng et al., 2020; Wang et al., 2020). Currently, clinical therapeutic strategies of AE include radical resection, liver transplantation and chemotherapy, in which, drug treatment is indispensable for AE patients before and after the surgical treatment (Stamatakos et al., 2009; Kern et al., 2017). Albendazole (ABZ), a derivative of benzimidazole, is the first choice for treatment of AE, while it disrupts the microtubule polymerization and interferes with its energy metabolism (Horton, 2000; Hemphill et al., 2014). However, even if ABZ limits the rapid extension of E. multilocularis metacestodes, the curative effect is poor, and its long-term use produces severe adverse reactions (Hemphill and Muller, 2009). Therefore, development of new and effective treatment drugs for AE is urged.
Pseudolaric acid B (PAB), a diterpene acid extracted from the root of Pseudolarix kaempferi Gordon, is a traditional Chinese medicine that has been used for treatment of fungal-infected dermatosis for many years (Zhang et al., 2014; Liu et al., 2017). Increasing evidences indicated that PAB have a wide range of antitumor effects. For example, in a nude mouse experiment, PAB inhibits gastric cancer cell lung metastasis, inducing cell apoptosis by suppressing phosphatidylinositol-4,5-bisphosphate 3-kinases and protein kinase B (PI3K/Akt), ERK1/2 and mitochondrial signaling pathways (Wang D. et al., 2017). In addition, our recent study has demonstrated that PAB blocks hepatocellular carcinoma (HCC) cells proliferation and invasion through inhibiting Notch1/Akt signaling pathway (Gao et al., 2022). Moreover, PAB inhibited immunomodulatory functions in T regulatory cells by reducing the expression of protein kinase B (PKB or Akt) and mitogen activated protein kinases (MAPK; Li et al., 2014; Liu et al., 2017), and a derivant of PAB was also evidenced to boost the expression of TGF-β1 in regulatory T cells (Li et al., 2014). Overall, PAB may be a novel effective candidate agent in treatment of cancer, immune disorders, inflammatory diseases, and immunosuppression, even though the mechanism responsible for PAB exerting the biological functions is still poorly understood. For instance, it was noted that PAB has a strong insecticidal effect described in ancient book-Compendium of Materia Medica (Yao et al., 2014), and later studies found it has a strong anti-parasitic effect on flatworm Dactylogyrus in fish (Ji, 2013). More importantly, the excessive disorder of transforming growth factor beta 1 (TGF-β1) and many other signaling pathways, such as Notch/Akt, PI3K/Akt, and MAPKs signaling, were found to cross-drive the initiation and progression of many liver diseases, such as liver fibrosis induced by Echinococcus infection (Brehm and Koziol, 2017; Wang Y. et al., 2017; Chen et al., 2022). However, the information of PAB against E. multilocularis metacestodes and the underlying anti-echinococcal mechanism remain unclear as yet.
The present study is to explore the anti-echinococcal activity of PAB on E. multilocularis in vitro and in vivo, and the target signaling regulated by PAB during this process. In addition, the cytotoxicity of PAB in normal liver and kidney cell line in vitro, and its sub-acute hepatotoxicity and nephrotoxicity in the mice will be assessed.
Materials and methods
Biochemical reagents
PAB, ABZ and ABZ sulfoxide (ABZ-SO, an anthelmintic active form of ABZ) were purchased from Aladdin Industrial Corporation (Shanghai, China) and Sigma-Aldrich (St. Louis, MO, United States); cell culture media were obtained from Gibco (Wisent, Canada); human liver cancer cell line (HepG2 cell) and normal human renal epithelial cell line (HK-2 cell) were purchased from Cell bank of Chinese Academy of Sciences (Shanghai, China), and human normal liver cell line (HL-7702 cell) was warmly presented by Dengfeng Yang from Gangxi Academy of Sciences. Mongolian gerbils were purchased from Zhejiang Academy of Medical Sciences (Hangzhou, China).
Isolation and culture of Echinococcus multilocularis protoscoleces
E. multilocularis protoscoleces (PSC) were separated from the metacestodes in E. multilocularis-infected Mongolian gerbils, and rinsed with dulbecco’s modified eagle’s medium (DMEM) containing 1% of penicillin–streptomycin (P-S) until the viability of > 97%, and then seeded into 24-well culture plates (120 PSC per well) to be incubated under the condition of 37°C and 5% CO2, as described previously (Gao et al., 2021).
Drug treatment of Echinococcus multilocularis protoscoleces in vitro
The experimental group was assigned as follows: (i) the vehicle group treated with 0.1% dimethyl sulfoxide (DMSO) (n = 3); (ii) the ABZ-SO group treated with ABZ-SO of 40, 20 and 10 μg/ml resolved in 0.1% DMSO (n = 3), respectively; (iii) the PAB group treated with PAB of 80, 40, 20, 10, 5, 2, 1 and 0.5 μg/ml resolved in 0.1% DMSO (n = 3), respectively. After treatment with different doses of drugs for 48 h, the PSC were stained with 0.4% trypan blue and examined morphologically under an inverted light microscope (BX43, Olympus, Japan). The survival rate of PSC were calculated as follows: PSC survival rate (%) = (live PSC count/total PSC count) × 100, with three independent biological replicates, as described previously (Gao et al., 2021), and half maximal inhibitory concentration (IC50) values were calculated from the graph plotted inhibition percentage against the concentration of sample solution using the Graphpad/Prism program version 8.0 (San Diego, CA). Further, the PSC exposed to different compounds were fixed with 4% glutaraldehyde to observe the ultrastructural alterations under scanning electron microscope (SEM, JSM-5600LV, JEOL, Japan) and transmission electron microscope (TEM, HT7800, Hitachi Consumer Marketing, Japan), as described previously (Gao et al., 2021).
Culture and treatment of Echinococcus multilocularis microcyst in vitro
After PSC co-cultured with the nurse cell (HepG2 cell) for 4 weeks in complete culture media containing 89% DMEM, 1% P-S and 10% fetal bovine serum (FBS) at 37°C, 5% CO2, the microcyst developed from PSC were seeded into 24-well culture plate with 3 microcysts per well. The test groups were assigned as: (i) the vehicle group treated with 0.1% DMSO, (ii) the PAB group treated with PAB of 40 and 20 μg/ml resolved in 0.1% DMSO, respectively. The vitality and morphology of E. multilocularis microcysts after treatment with PAB for 1, 2, and 4 weeks were inspected, respectively. On wk. 1, alkaline phosphatase (ALP) activity in the culture supernatant of the microcysts was examined using chemiluminescence method under a multifunctional microplate reader (BioTek, US), according to the manufacturer’s reagent specification (Solarbio Science & Technology Co., Ltd., Beijing, China). On week 4 after treatment with PAB, these microcysts were stained by 0.4% trypan blue to observe the morphological alterations by inverted microscopy.
Echinococcus multilocularis infection and drug treatment in vivo
Kunming mice (n = 30) were infected with E. multilocularis PSC (2000 PSC per mouse) by in situ surgical intrahepatic implantation in Specific Pathogen Free (SPF) laboratory for 3 months, and meanwhile, other healthy mice (n = 5) were processed with a sham procedure as a negative control group. After 3 months post-infection, the infected mice were divided into: (i) the untreated group (n = 5), daily treated with only honey/PBS (1:1 v/v), (ii) the ABZ group (n = 5), treated with ABZ daily of 40 mg/kg in honey/PBS (1:1 v/v), (iii) the PAB group (n = 5), daily treated with PAB (40, 20, 10 and 5 mg/kg) in honey/PBS (1:1 v/v), respectively, and at the same time, the uninfected mice were only administrated with honey/PBS (1:1 v/v). After oral administration of different drugs for 12 weeks, the wet weight of E. multilocularis cysts was weighed, the serum and the liver of the mice were collected to examine the expression of TGF-β1.
Examination of TGF-β1 protein expression in Echinococcus multilocularis PSC by immunofluorescence assay
To examine the expression of TGF-β1 by immunofluorescence (IF) assay, E. multilocularis PSC in the vehicle (0.1% DMSO) group and PAB-treated (20 μg/ml) group were rinsed with phosphate buffer saline (PBS), and then fixed with 4% paraformaldehyde, as described previously (Wang et al., 2022). The paraffin-embedded PSC sections were blocked with 20% normal goat serum and incubated with rabbit anti-TGF-β1 antibody (1:100) overnight at 4°C. After reaction with Cy3 conjugated goat anti-rabbit IgG (H + L) (1:200; Servicebio technology Co., LTD., Wuhan, China) and DAPI (1:100; abcam, Cambridge, UK), these slides were imaged under a fluorescence microscope (Olympus, Japan). Mean fluorescence intensity indicating TGF-β1 protein expresssion in the images were calculated using ImageJ software (ImageJ, RRID:SCR_003070), as described previously (Jansen et al., 2016).
Analysis of TGF-β1 protein expression by ELISA, immunohistochemistry-paraffin, and Western blot assays
TGF-β1 level in the serum of the mice was measured by enzyme-linked immunosorbent assay (ELISA), following the manufacturer’s instructions (CUSABIO, Wuhan, China). The content of TGF-β1 was presented as ng/ml after the subtraction of the appropriate control. Further, to observe the expression of TGF-β1 in Echinococcus metacestodes and the mice after treatment with PAB, E. multilocularis cysts and the liver tissues were fixed with 4% paraformaldehyde for immunohistochemistry-paraffin (IHC-P) examination, as described previously (Liu et al., 2021). Brifely, the slides coated with E. multilocularis cysts and the liver tissues were reacted with rabbit anti-TGF-β1 polyclonal antibody (Bioss Co., Beijing, China) and goat anti-rabbit IgG (ZSGB-BIO, Beijing, China) at the dilution of 1:200 and 1:800, respectively, and then these slides were imaged under a light microscope (Olympus, Japan) (10 fields/group), and the semi-quantitative analysis of TGF-β1 protein expression in each field was performed by ImageJ software (National Institutes of Health, Bethesda, MD, USA), as described previously (Antarianto et al., 2022).
Western blot (WB) was used to examine the TGF-β1 expression, briefly, E. multilocularis cysts and the liver tissues of the mice were lysed by tissue homogenizer (Tiangen biotech Co., LTD, Beijing, China), the total protein was extracted using Nuclear and Cytoplasmic Protein Extraction Kit (Yeasen Biotechnology (Shanghai) Co., Ltd.), and the protein concentration was detected using BCA Assay Kit (Servicebio, Wuhan, China), as described previously (Wang et al., 2022). Every sample of 60 μg per lane was separated by 12% SDS-PAGE, and then transferred to a polyvinylidene difluoride membrane, and blocked with 5% non-fat milk for 2 h at room temperature. The membrane was incubated with 1:500 diluted rabbit anti-TGF-β1 antibodies overnight at 4°C, and then with 1:2000 diluted goat anti-rabbit IgG for 2 h at room temperature. In addition, the rabbit anti-β-actin antibody was used as an internal standard at the dilution of 1:300 (Bioss, Beijing, China). Semiquantitative analysis was performed by ImageJ software, as described previously (Jansen et al., 2016).
Determination of TGF-β1 mRNA expression by real-time quantitative PCR
Total RNA in mice livers and E. multilocularis cysts was extracted using TRIzol reagent (Invitrogen, San Diego, USA), and reverse-transcribed into cDNA, referring to the reagent instruction (No. RR036A). The RT-qPCR was performed according to the protocols as described (Liu et al., 2021), and the used primers were as follows: mouse TGF-β1 (forward, 5′-CTTCAATACGTCAG ACATTCGGG-3′; reverse, 5′-GTAACGCCAGGAATTGTTGCTA-3′), mouse β-actin (forward, 5′-TTGTTACCAACTGGGACG-3′; reverse, 5′-GGCATAGAGGTCTTTACGG-3′). The semi-quantitative analysis of cDNA was performed by the SYBR Green PCR Master Mix (TaKaRa, Tokyo, Japan), following the manufacturer’s protocols. The relative expression level of TGF-β1 mRNA was normalized to that of β-actin, and the comparison of cycle threshold (CT) values of each group was measured using the 2–ΔΔCT method (Xiao et al., 2022).
Assessment of cytotoxicity of PAB on human normal hepatocytes and kidney cell in vitro
To assess the cytotoxicity of PAB, human normal hepatocyte (HL-7702 cell line) and renal cell line (HK-2 cell) were seeded into 96-well culture plates (5 × 103 cells per well) and 24-well culture plates (2 × 104 cells per well) with complete culture media, and followed by incubation at 37°C and 5% CO2 for 6 h. PAB and ABZ-SO at a final concentration of 80, 40, 20, 10, 5, 2, 1, 0.5, 0.2 and 0 μg/ml were added into the cell culture plates, respectively (n = 6). After treatment with PAB and ABZ-SO for 48 h, cell counting kit-8 (CCK-8) solution (10 μl per well) was added into 96-well culture plates to continuously incubate for 2 h, and the optical density was read under a multifunctional enzyme marking instrument (BioTek, US), and then cell viability was calculated using the following formula: cell viability = [(ODtreatment − ODblank)/(ODcontrol − ODblank)] × 100%, and IC50 values were calculated using the Graphpad/Prism software. In addition, cell morphologic alterations in 24-well culture plates were observed after staining with 1% crystal violet (Solarbio Co., Ltd., Beijing, China) under the indicated inverted microscope. These experimental processes were conducted with three independent biological replicates following the manufacturer’s specifications (Beyotime Biotechnology Co., Ltd., Shanghai, China).
Evaluation of sub-acute hepatotoxicity and nephrotoxicity of PAB in mice
To evaluate the sub-acute hepatotoxicity and nephrotoxicity of PAB in vivo, 15 healthy Kunming mice were assigned as: (i) the control group (n = 5), orally administrated with honey/PBS daily (1:1 v/v), (ii) the PAB group (n = 5), orally administrated with PAB at 40 mg/kg in honey/PBS daily (1:1 v/v), and (iii) the ABZ group (n = 5), orally administrated with ABZ at 100 mg/kg (the dosage of 136.3 mg/kg for mice calculated by the recommended dosage for human with 10 mg/kg; Kern et al., 2017) in honey/PBS daily (1:1 v/v). After treatment with PAB and ABZ for 6 weeks, those mice sera were collected to detect 11 biochemical indexes that were related to the liver and kidney function by chemiluminescent immunoassay (CLIA). At the same time, the liver and kidney tissues of those mice were fixed with 4% paraformaldehyde to observe the pathological alterations by hematoxylin–eosin (HE) staining.
Statistics
The experimental data were described as the mean ± standard deviation (mean ± SD). The different reading in fluorescence intensity between two groups was analyzed by student’s t-test, and the differences in cysts weight, TGF-β1 level, and biochemical indexes among three or more groups were assessed by one-way ANOVA with multiple comparisons. Statistical analysis was performed using GraphPad Prism version 8.0 (San Diego, CA) and SPSS version (IBM, Chicago, IL). p < 0.05 indicates significant difference.
Results
Effect of PAB on the activity of Echinococcus multilocularis protoscoleces in vitro
To investigate insecticidal effect of PAB on E. multilocularis in vitro, the survival rate and morphological alterations in PSC were measured after treatment with PAB at different concentrations. The survival rate of PSC showed a time- and dose-dependent decreasing after treatment with 0.5–80 μg/ml PAB within 7 days. All PSC examined were killed by 1–80 μg/ml PAB in 1 week. Among them, all PSC were killed after treatment with PAB at 10, 20 and 40 μg/ml for 3, 2 and 1 day, respectively (Figure 1A). Treatment with PAB (IC50 = 12.63 μg/ml) at 40, 20 and 10 μg/ml for 3 days significantly reduced the PSC survival rate in comparison with ABZ-SO treatment at the same concentrations, respectively (all p < 0.0001) (Figure 1B).
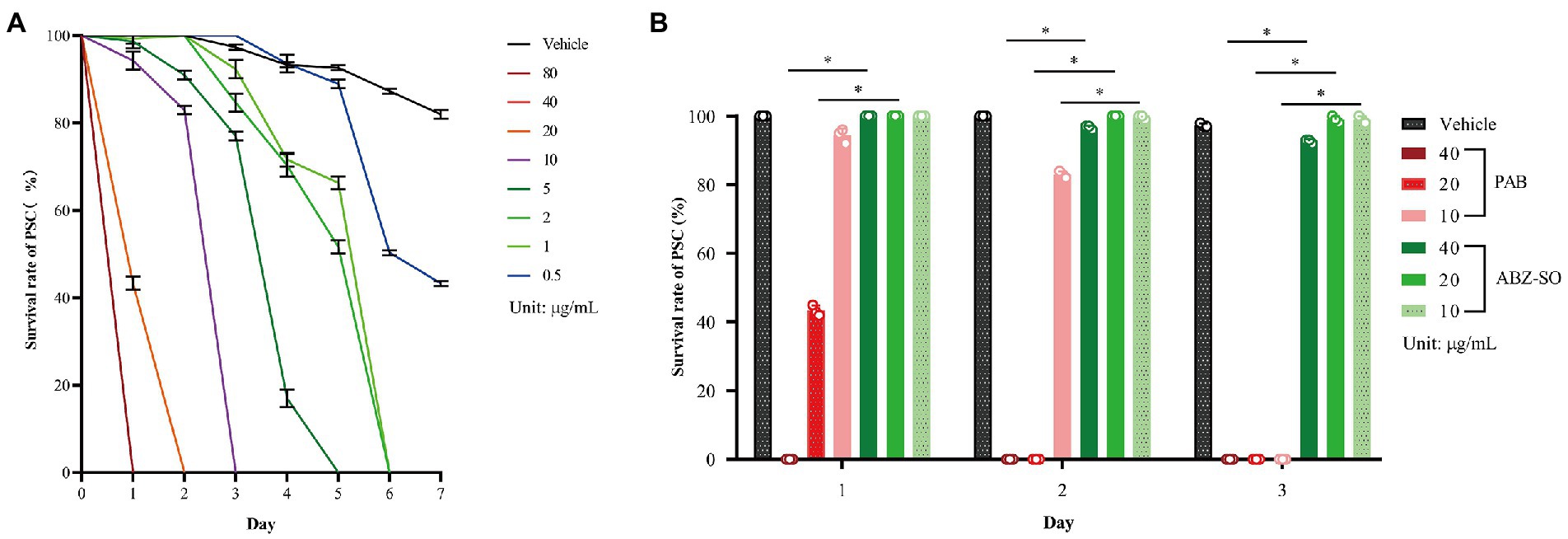
Figure 1. Changes in survival rate of E. multilocularis PSC before and after PAB treatment in vitro. (A) Survival rate of E. multilocularis PSC of the vehicle group exposed to 0.5–80 μg/ml PAB for 7 days, and (B) 10, 20 and 40 μg/ml PAB and ABZ-SO for 3 days, as measured by morphological alterations under a light microscope. Data are expressed as mean ± SD, n = 3. The independent experiments were processed in triplicate.
Light microscopy indicated that structural damage was observed in the PSC after treatment with 20 μg/ml PAB, showing disappearance of calcareous corpuscles, breakage of tegument and suckers, but not seen in the vehicle group with typan blue staining (Figures 2D–F) or not (Figures 2A–C). Furthermore, SEM assay revealed that the ultrastructure of PSC treated with 20 μg/ml PAB was different from that treated with 20 μg/ml ABZ-SO or DMSO. The PSC exposed to 20 μg/ml PAB exhibited ultrastructural destructions, including shedding of tegument, breakage of rostellum and suckers (Figures 2G–I). At the same time, TEM assay indicated that in comparison with the microstructure of PSC treated with ABZ-SO and DMSO, the PSC exposed to PAB showed disappearance of periodic acid-Schiff stain (PAS) positive materials, microvillus and syncytial layer (SL), contraction of parenchymal cells, and presentation of abundant cytoplasm vacuolization, condensed chromatin and apoptotic bodies (Figures 2J–L).
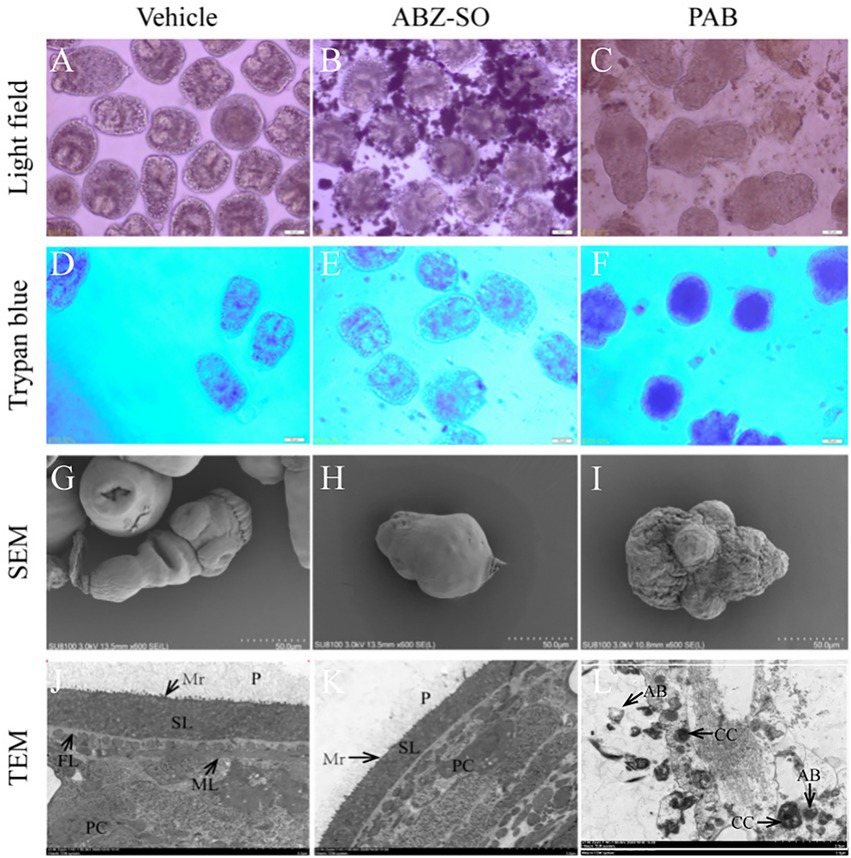
Figure 2. Structural morphology changes of E. multilocularis PSC after treatment with PAB in vitro. The PSC treated with PAB (20 μg/ml) and ABZ-SO (20 μg/ml) for 2 days were observed using trypan blue staining (D–L) or unstained (A–C) under light microscope (A–F), 200 × magnification, SEM (G–I), scale-bars: 50 μm and TEM (J–L), scale-bars: 5 μm. Abbreviations: P, periodic acid-schiff stain positive material; Mv, microvillus; SL, syncytial layer, FL, fibrous layer, ML, muscular layer, AB, apoptotic bodies, CC, condensed chromatin.
Effect of PAB on the development of Echinococcus multilocularis protoscoleces in vitro
To further investigate anti-echinococcal effect of PAB in vitro, the development of E. multilocularis PSC after treatment with PAB was measured. Microscopic observation at week 1 of in vitro test found that, E. multilocularis microcysts upon exposure to PAB of 20 and 40 μg/ml showed morphological alterations, including collapse of the microcyst, shrinking of the germinal layer from the laminated layer, but not in the vehicle group. At weeks 2 and 4, the solid protrusions (SP) and new PSC in the microcysts were observed in the vehicle group, but the microcysts treated with PAB exhibited breakage. At week 4, E. multilocularis microcysts after treatment with PAB were found stained by trypan blue and no new PSC was found inside (Figure 3A). In addition, after treatment with 40 and 20 μg/ml PAB for 1 week, the activity of ALP in the culture supernatant of the microcysts exhibited significant increase from (0.42 ± 0.04) U/L in the vehicle group to (0.76 ± 0.08) U/L and (0.89 ± 0.04) in 20 and 40 μg/ml PAB-treated groups, respectively (p = 0.0002 and p = 0.0085; Figure 3B).
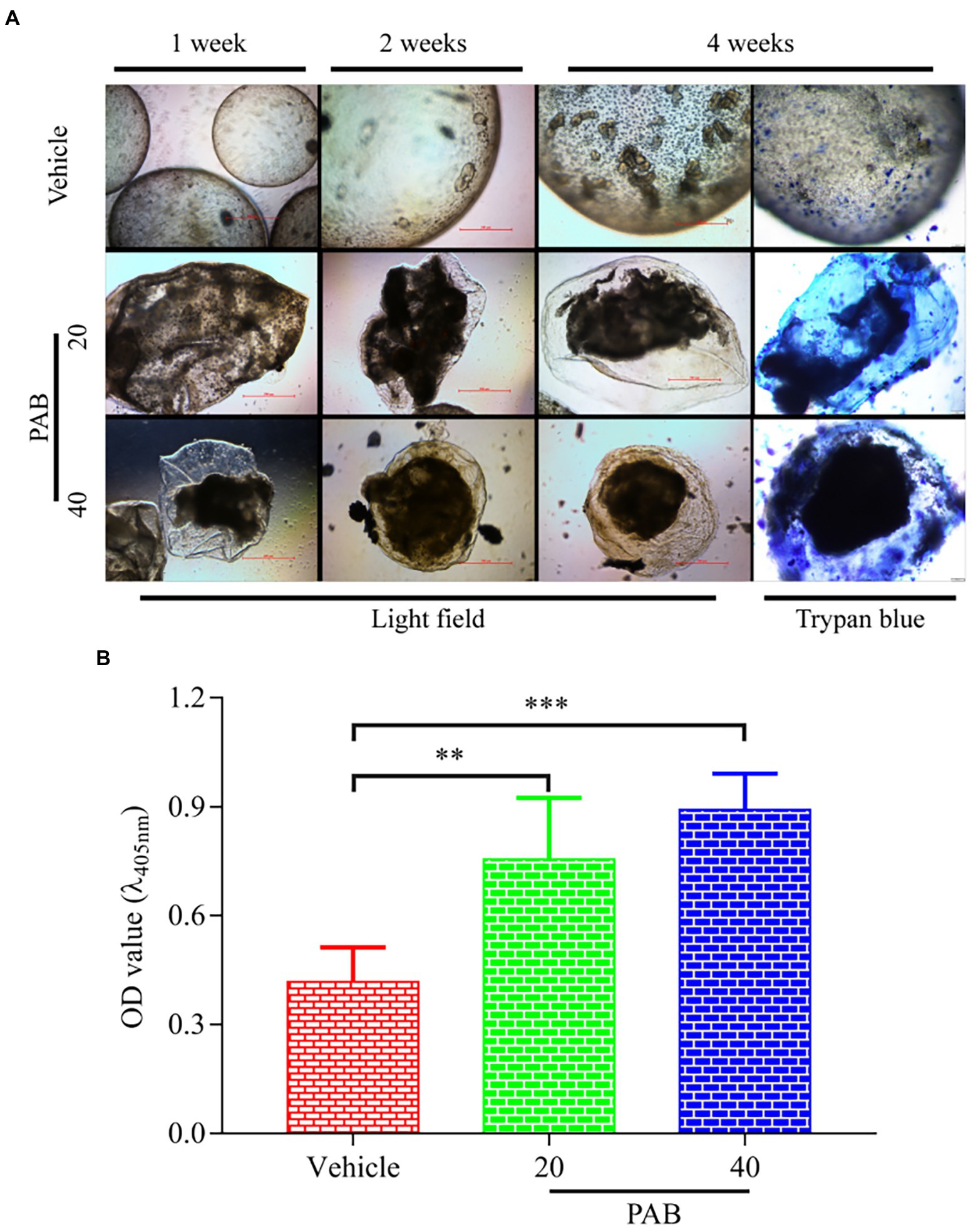
Figure 3. Viability changes of E. multilocularis microcysts after treated with PAB in vitro. (A) Microcysts exposed to 20 and 40 μg/ml PAB for 4 weeks showed ultrastructural damage, such as detachment of the germinal layer from the laminated layer, however the typical germinal layer (GL) (week 1) producing solid protrusions (SP) (week 2), present of daughter PSC (week 4) in the vehicle group. Images were measured by light microscopy at weeks 1, 2 and 4 without any staining; and at week 4 with trypan blue staining. (B) ALP levels in the culture supernatants of E. multilocularis microcysts after treatment with 20 and 40 μg/ml PAB for 1 week, as measured by chemiluminescence method. All data were assessed using One-way ANOVA with multiple comparisons. The independent experiments were processed in triplicate.
Effect of PAB against Echinococcus multilocularis metacestodes in vivo
Furthermore, in vivo anti-echinococcal effect of PAB was evaluated in a mouse model with liver echinococcosis. The wet weight of E. multilocularis cysts isolated from the group treated with 40, 20 and 10 mg/kg PAB was (1.94 ± 0.30) g, (2.38 ± 0.22) g and (2.64 ± 0.48) g, respectively, which were significantly lower than those from the infected group [(3.82 ± 0.44) g] (p < 0.0001, p < 0.0001 and p = 0.0002), respectively, but not seen in 5 mg/kg PAB treatment group [(3.16 ± 0.37) g] (p = 0.0597). In addition, the wet weight of the cysts from 40 mg/kg ABZ treatment group [(2.29 ± 0.15) g] was significantly lower than that in the infected group [(3.82 ± 0.44) g] (p = 0.011), but no significant difference was seen when compared with the 40 mg/kg PAB treatment group (p = 0.6091; Table 1).
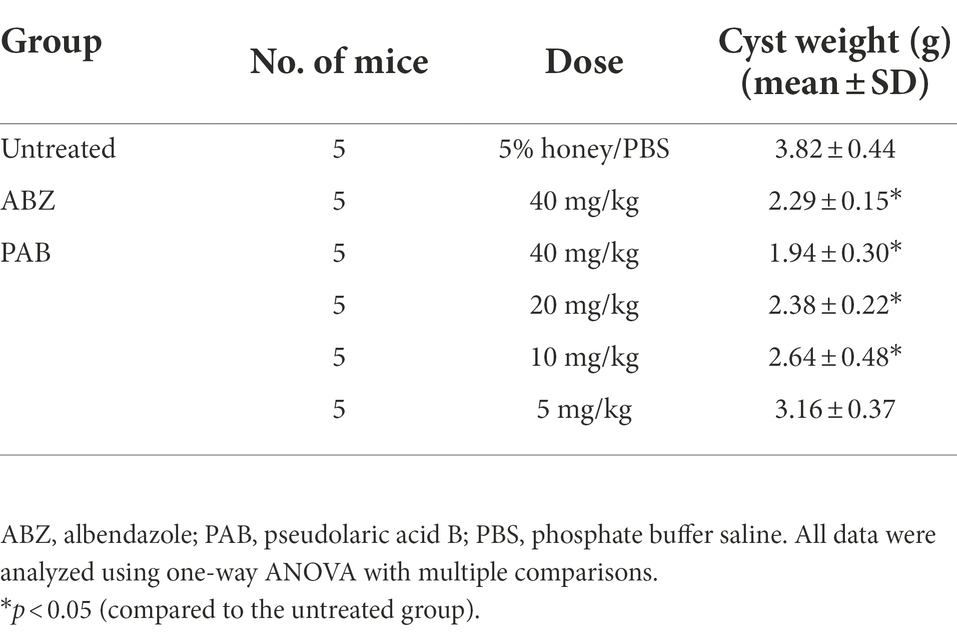
Table 1. Changes in the wet weight of E. multilocularis metacestodes in mice orally treated with PAB for 12 weeks after 3 months post-infection.
PAB down-regualting TGF-β1 protein and mRNA expression in Echinococcus multilocularis metacestodes
Possible anti-echinococcal mechanism of PAB was investigated by observing the expression of TGF-β1 protein and mRNA in E. multilocularis. By immunofluorescence assay, red fluorescence-stained TGF-β1 was found widely distributed in the natural parenchymatous tissue of the PSC, but the distribution was diminished after treatment with 20 μg/ml PAB in vitro (Figure 4A), and the semiquantitative analysis indicated that compared with the vehicle group, PSC in the PAB-treated group showed a significant decrease of fluorescence-labeled TGF-β1 (p < 0.0001; Figure 4B).
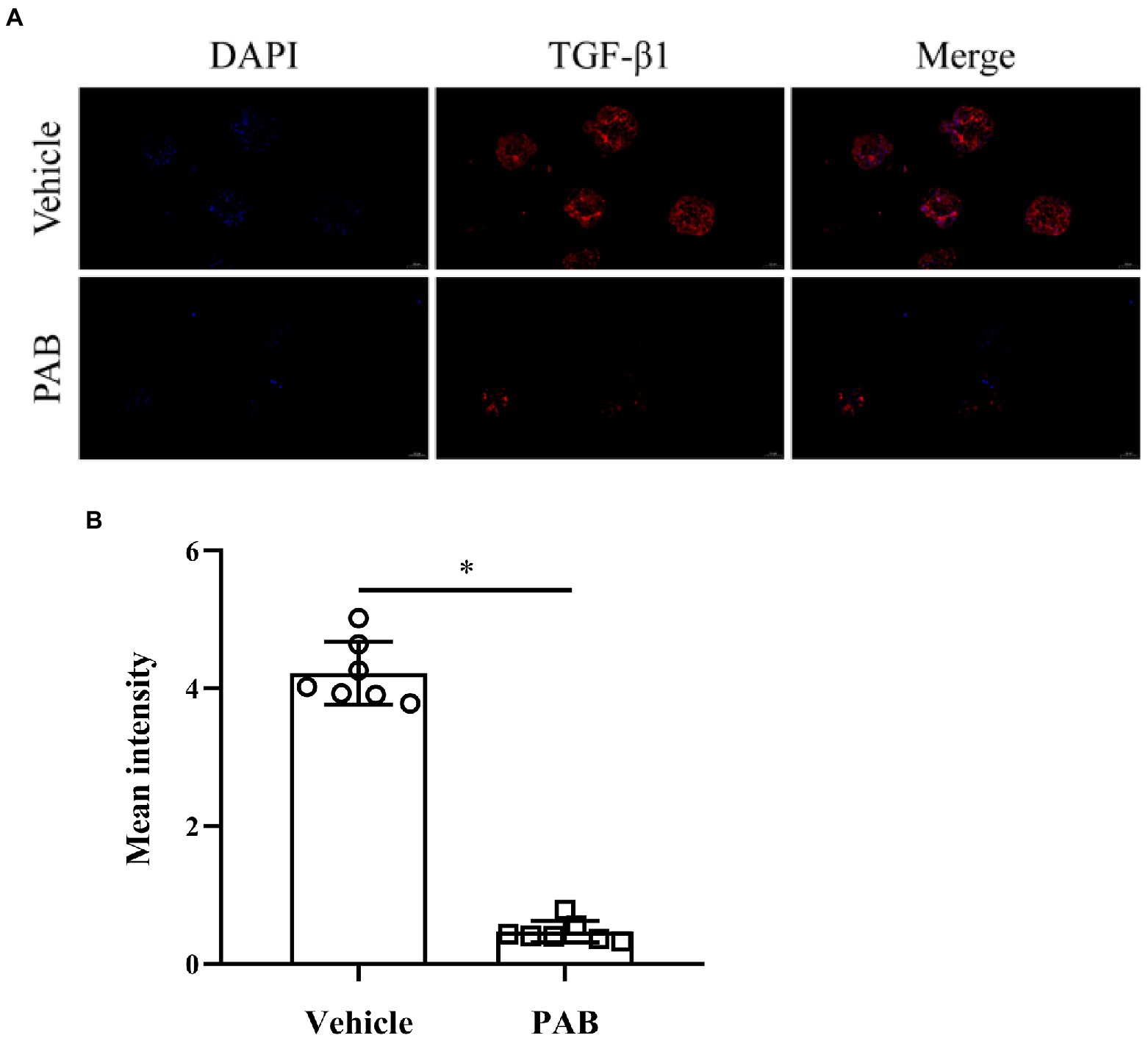
Figure 4. Expression of TGF-β1 in E. multilocularis PSC after treatment with 20 μg/ml PAB for 2 days by immunofluorescence assay. (A) Location of TGF-β1 protein (red) and DAPT-indicated DNA (blue) in the PSC in immunofluorescence staining images. Scale-bars: 50 μm. (B) Analysis of mean fluorescence intensity indicated TGF-β1 protein expression, as measured by ImageJ software. The data was assessed using t-test.
IHC-P assay revealed that the expression of TGF-β1 in E. multilocularis cysts [(6.29 ± 1.37)% and (6.29 ± 1.37)%] exhibited a significant decrease after treatment with 40 and 20 mg/kg PAB (p = 0.0211 and p = 0.0499), respectively, but 10 and 5 mg/kg of PAB caused no change [(7.30 ± 1.21)% and (7.58 ± 1.40)%] (p = 0.6247 and p = 0.9168) (Figures 5Aa–f), in comparison with that in the infected mice [(7.95 ± 1.10)%].
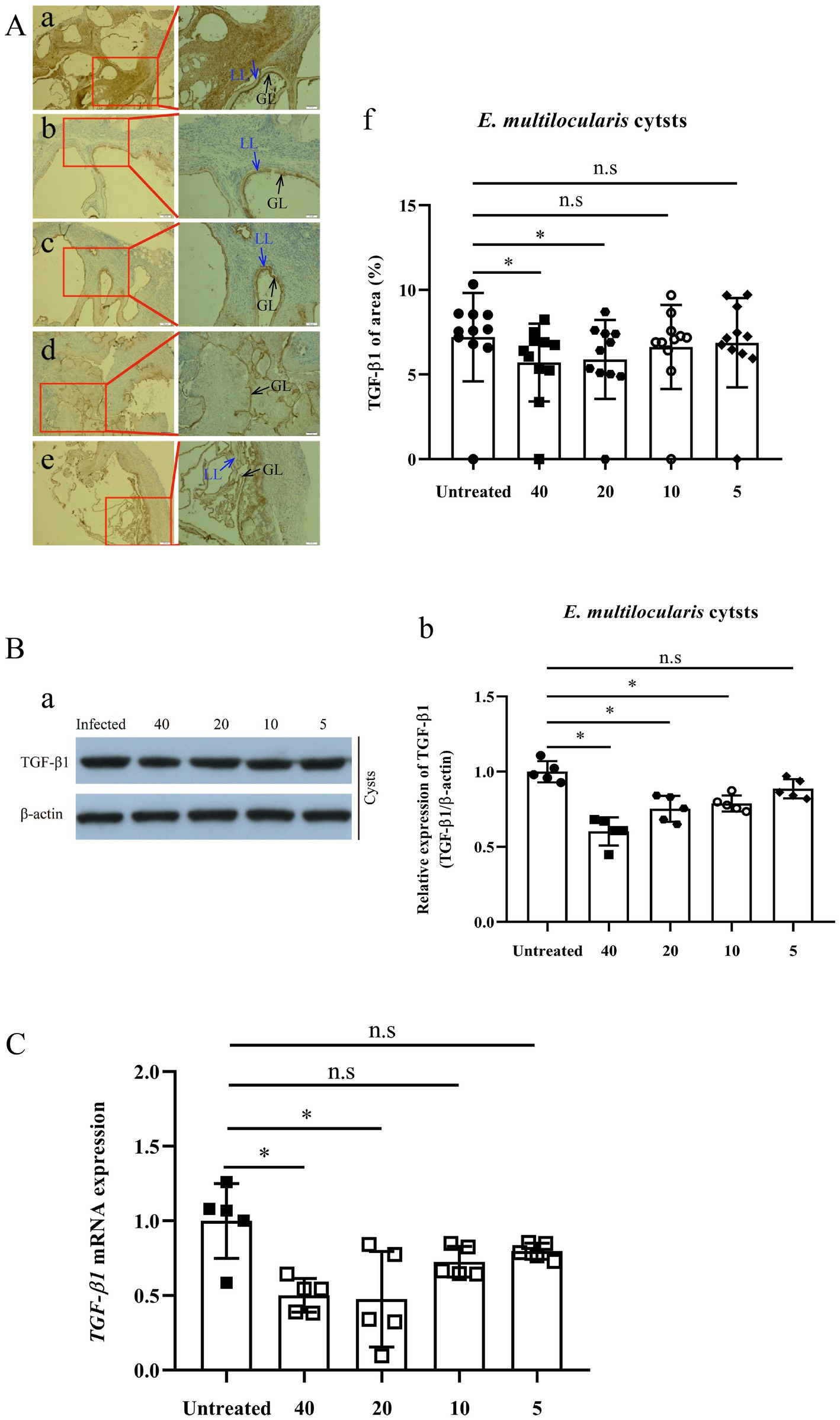
Figure 5. Expression of TGF-β1 protein and mRNA in Echinococcus multilocularis cysts after treatment with PAB for 12 weeks, as measured by immunohistochemistry-paraffin (IHC-P), Western blot (WB) and real-time quantitative PCR (RT-qPCR) assay. (A) Images of TGF-β1 protein expression in E. multilocularis cysts from the mice (a) untreated, and treated with PAB (b) 40, (c) 20, (d) 10, and (e) 5 mg/kg, as measured by IHC-P assay; and (f) semi-quantitative analysis of TGF-β1 protein, as measured by ImageJ software. Brown area indicates expression of TGF-β1 protein, Laminated layer (LL, blue arrow) and germinal layer (GL, black arrow) in E. multilocularis cysts. Magnification, × 100 and × 200. (B) WB analysis of (a) TGF-β1 and β-actin expression in E. multilocularis cysts in the untreated, and treated with PAB of 40, 20, 10, and 5 mg/kg PAB mice, and (b) the semi-quantitative analysis of TGF-β1 protein, as measured by ImageJ software. (C) Analysis of TGF-β1 mRNA expression in E. multilocularis cysts by RT-qPCR. All data were assessed using One-way ANOVA with multiple comparisons. LL, laminated layer; GL, germinal layer.
Furthermore, WB assay showed that the expression of TGF-β1 in E. multilocularis cysts [(0.60 ± 0.04), (0.75 ± 0.04) and (0.79 ± 0.02)], were significantly lower after treatment with 40, 20 and 10 mg/kg of PAB (all p < 0.0001), respectively, than those in the PAB-untreated mice (1.00 ± 0.03), but no significant reduction was observed at 5 mg/kg of PAB (0.87 ± 0.06) (p = 0.0649; Figures 5Ba–b).
Detected by RT-qPCR, expression of TGF-β1 mRNA in the cysts of infected mice with 40 and 20 mg/kg PAB were reduced to 54.49% and 32.40% (p = 0.0024 and p = 0.0015), respectively, comparing with the non-treated infected group, but the expression in the group treated with 10 and 5 mg/kg of PAB only showed mild reduction at 64.80% and 84.91% (p = 0.1196 and p = 0.3189; Figure 5C).
PAB down-regualting TGF-β1 protein and mRNA expression in the liver of Echinococcus multilocularis-infected mice
Furthermore, the expression of TGF-β1 protein and mRNA in Echinococcus-infected mouse was also assessed after treatment with PAB. Detected by ELISA, the level of TGF-β1 protein in the serum of the mice was elevated 1.7-fold after infection of E. multilocularis (p < 0.0001). However, compared with that of the uninfected group, TGF-β1 level in the serum of the infected mice after treatment with 40, 20 and 10 mg/kg of PAB were reduced by 1.9-fold, 1.6-fold and 1.3-fold (p < 0.0001, p < 0.0001 and p = 0.0035), respectively, but 5 mg/kg of PAB treatment group only showed 1.1-fold decrease (p = 0.2239; Table 2).
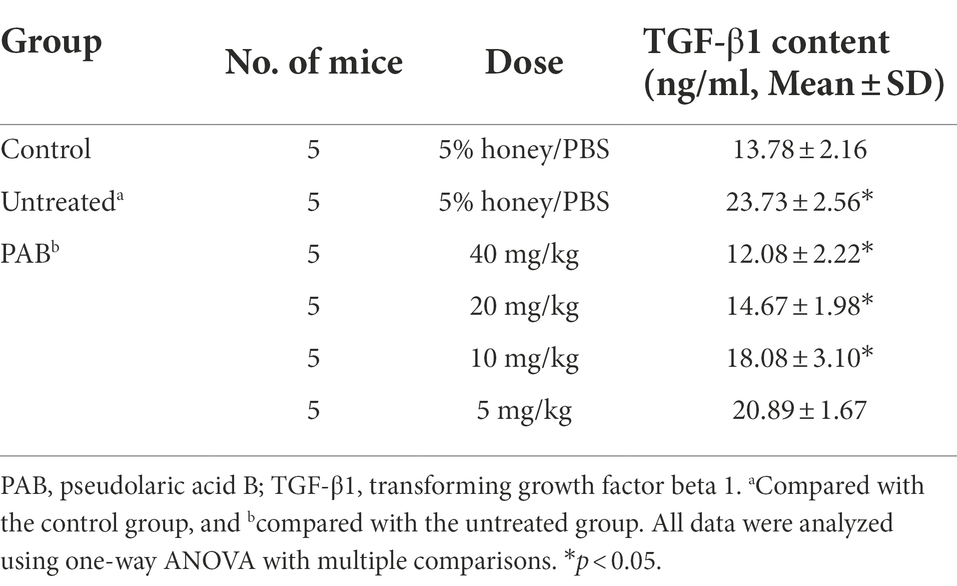
Table 2. Changes of TGF-β1 content in the serum of E. multilocularis-infected mouse orally treated with PAB for 12 weeks after 3 months post-infection.
Furthermore, IHC-P assay indicated that the expression of TGF-β1 in the mice liver was boosted fivefold after the infection of E. multilocularis (p < 0.0001). However, the expression of TGF-β1 in the infected mice treated with 40 and 20 mg/kg PAB was decreased significantly (p < 0.0001 and p = 0.0167), whilst no significant reduction was found in 10 and 5 mg/kg PAB groups (p = 0.999 and p = 0.9979; Figures 6Aa–g).
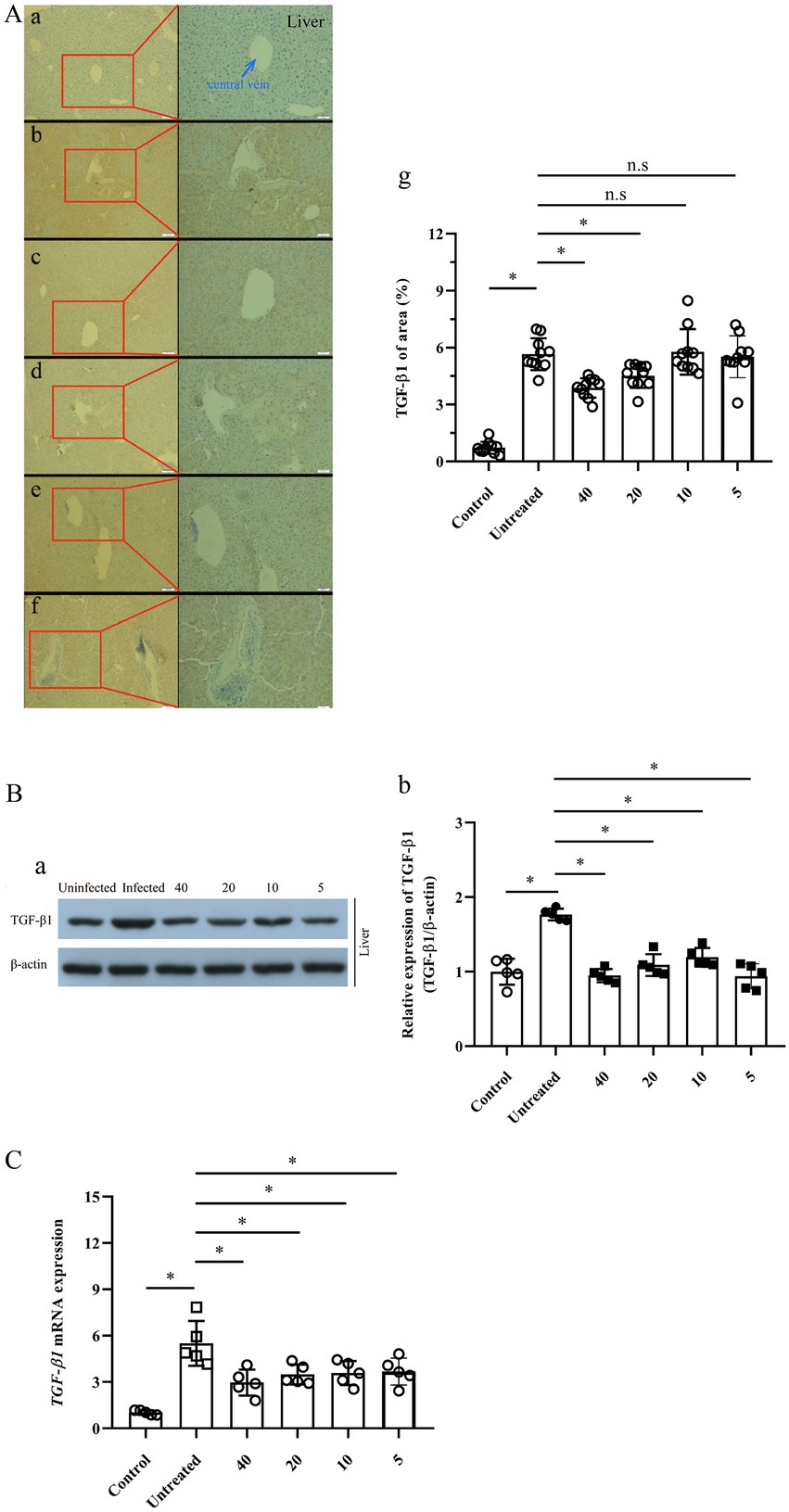
Figure 6. Expression of TGF-β1 protein and mRNA in the liver of Echinococcus-infected mouse after treatment with PAB for 12 weeks, as measured by IHC-P, WB and RT-qPCR assay. (A) Images of TGF-β1 protein expression in the liver of the mice, (a) uninfected, (b) untreated, and treated with PAB of (c) 40, (d) 20, (e) 10 and (f) 5 mg/kg, as measured by IHC-P assay; and (g) the semi-quantitative analysis of TGF-β1 protein, as measured by ImageJ software. Brown area indicates expression of TGF-β1 protein, central vein (blue arrow) in the liver of E. multilocularis-infected mice. Magnification, × 100 and × 200. (B) WB analysis of (a) TGF-β1 and β-actin expression in the mice liver, and (b) the semi-quantitative analysis of TGF-β1 protein, as measured by ImageJ software. (C) Analysis of TGF-β1 mRNA expression in the liver of the mice by RT-qPCR. All data were assessed using One-way ANOVA with multiple comparisons.
Western blot revealed that the TGF-β1 expression in the liver of E. multilocularis infected mouse was significantly increased (p < 0.0001), but significantly decreased after treatment with PAB of 40, 20, 10 and 5 mg/kg (all p < 0.0001), respectively (Figures 6Ba–b).
In addition, RT–qPCR indicated that the expression of TGF-β1 mRNA in the liver of the mice was elevated 5.5-fold after infection of E. multilocularis (p < 0.0001). However, compared with that in Echinococcus-infected mice, RT-qPCR indicated that the expression of TGF-β1 mRNA in the liver of the infected mice treated with 40, 20, 10 and 5 mg/kg of PAB was decreased to be 2.9-, 3.5-, 3.6- and 3.7-folds (p = 0.0006, p = 0.0059, p = 0.012 and p = 0.0136), respectively (Figure 6C).
Hepatorenal cytotoxicity of PAB in vitro
To assess the toxicity of PAB, in vitro the proliferation and morphology of the normal liver and kidney cell were first measured using cell counting kit (CCK-8) assay and crystal violet staining. CCK-8 indicated the survival rates of HL-7702 cell showed a dose-dependent decrease after treatment with different concentrations of PAB and ABZ-SO at 0.2, 0.5, 1, 2, 5, 10, 20, 40 and 80 μg/ml (Figure 7Aa). Among them, treated with 10 μg/ml PAB (IC50 = 25.29 μg/ml), the cell survival rate showed a significant increase [(50.79 ± 1.80)%] in comparison with that [(26.9 ± 0.81)%] using the same concentration of ABZ-SO (IC50 = 3.71 μg/ml) (p = 0.0001). When the concentrations of PAB and ABZ-SO reached 40 μg/ml, the cell survival rate in PAB-treated group [(46.07 ± 1.77)%] was also significantly higher than that in ABZ-SO-treated group [(24.65 ± 0.93)%] (p = 0.0001). In addition, crystal violet staining assay showed reduction in the number of viable HL-7702 cells after treated with 10 and 40 μg/ml PAB or ABZ-SO. Among them, ABZ-SO presented a stronger inhibitory effect than the same concentrations of PAB (Figure 7Ab).
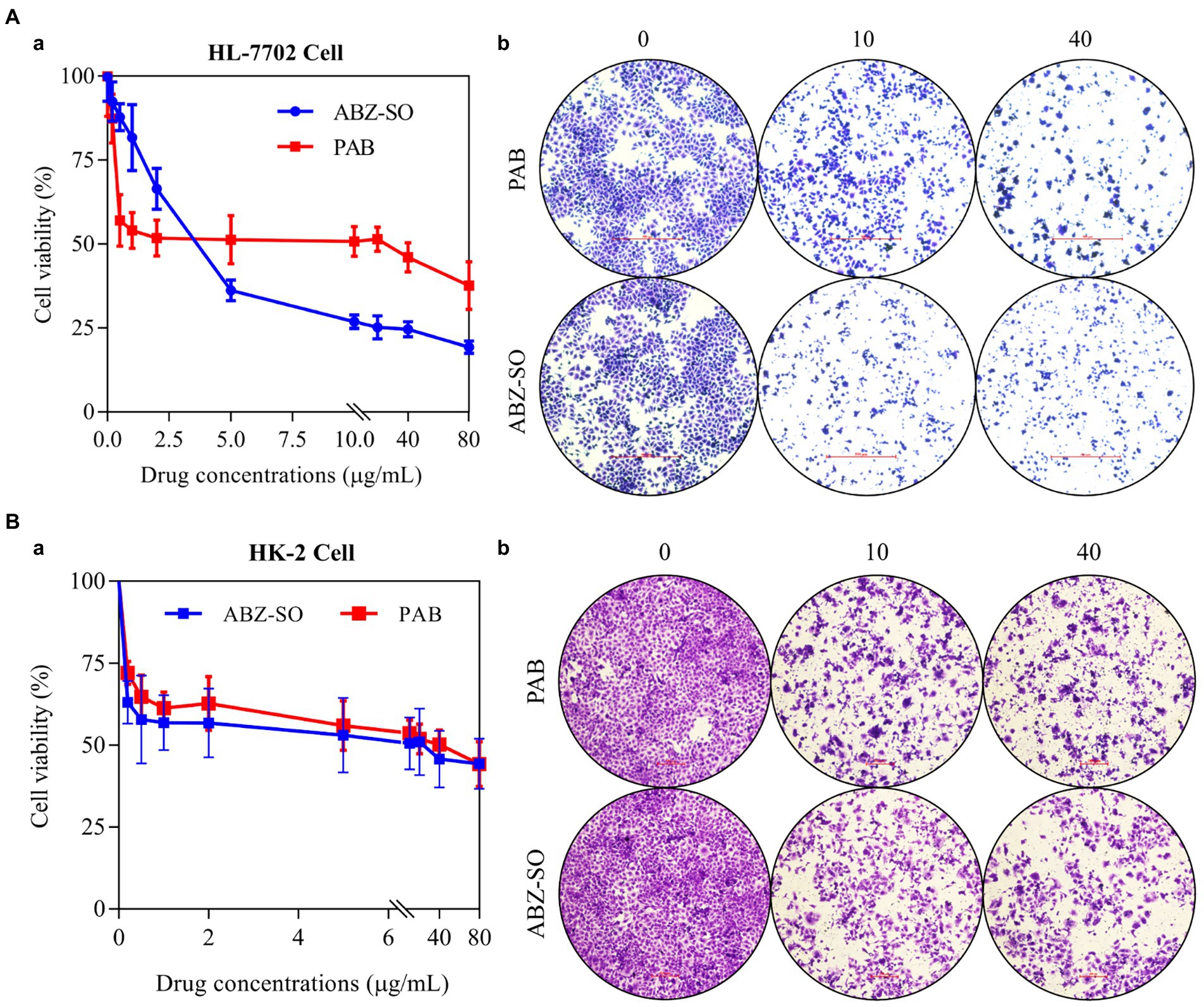
Figure 7. Cytotoxicity of PAB on human hepatocytes and renal cells in vitro. The cell livability (a) and number (b) of HL-7702 cell (A) and HK-2 cell (B) after treatment with different concentrations of PAB and ABZ-SO (0–80 μg/ml) for 48 h by CCK-8 assay (A) and crystal violet staining (B). The independent experiments were processed in triplicate.
Furthermore, CCK-8 assay indicated dose-dependent inhibitory effect of both PAB and ABZ-SO at 0.2, 0.5, 1, 2, 5, 10, 20, 40 and 80 μg/ml on HK-2 cells, respectively. Among them, the survival rate of HK-2 cells exposed to 40 μg/ml PAB (IC50 = 42.94 μg/ml) was (50.16 ± 1.82)%, whereas, showing no significant difference in comparison with that [(45.73 ± 3.52)%] upon the same concentration of ABZ-SO (IC50 = 21.22 μg/ml) (p = 0.2907) (Figure 7Ba). Similar to the response of HL-7702 cells, the number of HK-2 cells also decreased by crystal violet staining after treatment with 10 and 40 μg/ml PAB and ABZ-SO, respectively (Figure 7Bb).
Hepatotoxicity and nephrotoxicity of PAB in mice
To assess the sub-acute hepatotoxicity and nephrotoxicity of PAB in vivo, Kunming mice were orally administrated with PAB for 6 weeks. Compared with the control group, the PAB group showed significant difference in serum direct bilirubin (DBIL) content with 79.68% decrease (p = 0.0015), total protein (TP) content with 1.1-fold increase (p = 0.0012), aspartate aminotransferase (AST) content with 1.1-fold increase (p = 0.0038), but the ABZ group showed significant difference in serum DBIL content with 79.69% decrease (p = 0.0015), indirect bilirubin (IBIL) content with 1.2-fold increase (p = 0.0063), TP content with 1.1-fold increase (p = 0.0027), alanine aminotransferase (ALT) content with 1.1-fold increase (p < 0.0001), AST content with 1.1-fold increase (p = 0.0373), alkaline phosphatase (ALP) with 1.2-fold increase (p = 0.0007). When compared with the PAB group, the ABZ group showed significance increase in serum IBIL content (p = 0.0408), ALT level (p = 0.0002), ALP content (p = 0.0005). Additionally, compared with the control group, the PAB group showed significant difference in serum creatinine (CRE) content with 1.2-fold increase (p = 0.0013) and blood urea nitrogen (BUN) content with 1.1-fold increase (p = 0.0329), and the ABZ group showed significant difference in serum CRE content with 1.2-fold increase (p = 0.0032) and BUN content with 1.1-fold increase (p < 0.0001). When compared with the PAB group, the ABZ group showed significance increase in serum BUN content (p = 0.0005; Table 3). In addition, no death and adverse reactions were observed in mice during treatment with PAB and ABZ.
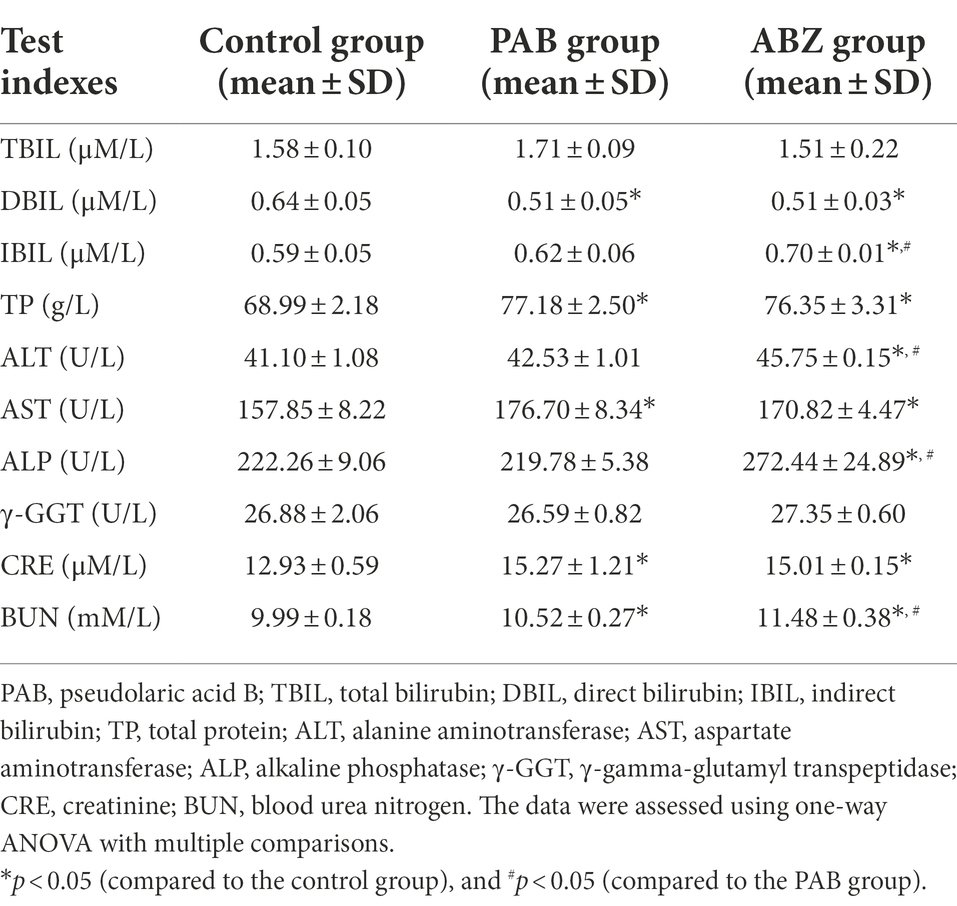
Table 3. Changes of biochemical indexes in the serum of E. multilocularis-infected mice after treatment with PAB for 6 weeks (n = 5).
It was demonstrated that pathological changes of the liver and kidney tissues in the mice after treatment with PAB were examined by HE staining. In comparison with that in the liver of untreated mice, the liver in the mice treated with PAB or ABZ for 6 weeks did not show obvious pathological damage, such as infiltration of inflammatory cells in portal area, disorganization of hepatic lobule structure and ballooning or fatty degeneration of hepatocytes (Figure 8A). As well, compared with the liver of untreated mice, the kidney in PAB- or ABZ-treated mice did not show obvious pathological damage, such as renal tubular lesions, interstitial edema and accumulation of inflammatory cells (Figure 8B).
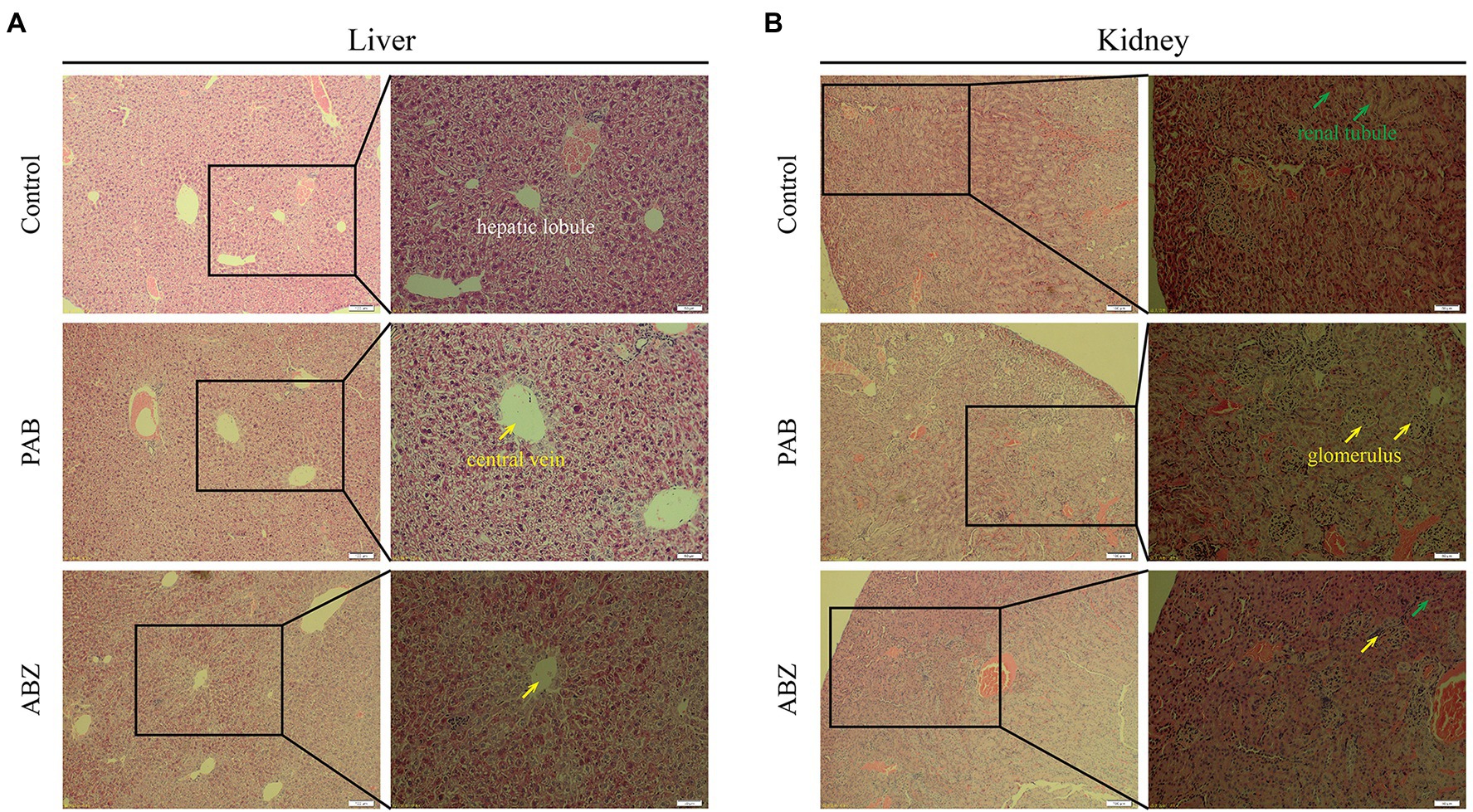
Figure 8. Hepatotoxicity and nephrotoxicity of PAB in mice. (A) Liver and (B) kidney histopathological images from Kunming mice treated with 40 mg/kg PAB and 100 mg/kg ABZ for 6 weeks (100 × and 200 × magnification), as detected by HE staining assay.
Discussion
E. multilocularis metacestodes often reside in the liver of humans and plateau pikas, and is also termed “parasitic cancer” due to its tumor-like invasive growth pattern, causing a great concern in public health (Li et al., 2018; Gao et al., 2021). Currently, the drug of choice for AE is ABZ, which can limit rapid extension of E. multilocularis metacestodes, but it is difficult to meet curative goal for AE, and its long-term use produces strong adverse reactions (Liu et al., 2020b). Hence, development of new and effective anti-echinococcal drugs is imperative.
In the present study, the survival rate of E. multilocularis PSC exhibited a time- and dose-dependent decrease in vitro after treatment with PAB. Observed by SEM and TEM, we found that PSC upon exposure to PAB exhibited ultrastructural destructions, such as disappearance of syncytial layer, PAS stained materials and microvillus, collapse of parenchymae cells, and presentation of cytoplasm vacuolization, condensed chromatin and apoptotic bodies, which is similar to the pro-apoptotic activity of PAB in HCC cells (Gao et al., 2022). It is documented that E. multilocularis PSC has a duality: developing into an adult worm with sexual reproduction pattern in the definitive host, and initiating new cycles of asexual multiplication in the intermediate host, including development into microcyst, mature microcyst (production of cellular protrusions and brood capsules) and multiple cysts (production of abundant new PSC and multiple cysts) (Wang et al., 2016; Li et al., 2018). In the in vitro experiment, we observed that PSC grew into microcyst and completed a new cycle of asexual multiplication under the support of HepG2 cells, but this asexual cycle of PSC was blocked by PAB, showing collapse of microcyst, shrinking of germinal layer from the laminated layer after treatment with PAB. In addition, we found that after treatment with PAB, ALP activity in the culture supernatants of PSC in vitro was elevated significantly, which indicated a serious damage to E. mutilocularis metacestodes by PAB (Xin et al., 2020; Gao et al., 2021). At the same time, in the in vivo experiment, we also observed that the growth of E. multilocularis metacestodes was inhibited by PAB with a dose-dependent manner, evidenced by significant lower cyst weight after treatment with PAB. In short, PAB exhibits a strong parasiticidal effect on E. multilocularis larvae both in vitro and in vivo.
Previous study demonstrated that PAB exerts antitumor effects by activated apoptosis, autophagy and cell cycle arrest in certain types of cancer cells (Yu et al., 2008, 2016, Liu et al., 2020a). It was recognized that TGF-β1 signaling could trigger a variety of cellular responses, including inhibition of cell growth, migration, differentiation and apoptosis (Sanchez-Capelo, 2005). Later research exhibited that overexpressed TGF-β1 causes epithelial-mesenchymal transition (EMT), extracellular matrix (ECM) deposition and cancer-associated fibroblast (CAF) formation, which lead to fibrotic disease, and cancer (Peng et al., 2022). Thus, use of TGF-β1 chemical inhibitors as appears to be a new line of defenses against fibrotic disorders or cancer (Caja et al., 2018). Genome study indicated that E. multilocularis possesses TGF-β1, MAPK and Akt signaling pathways (Tsai et al., 2013; Zheng et al., 2013), beyond that, strong evidence suggest that during echinococccosis, the impaired host immune response is paralleled by an increased expression of TGF-β1 signaling components in periparasitic host cells and tissues (Nono et al., 2020). A later study on AE transplant treatment showed that through modulating the activity level of the TGF-β/Smad7 signaling pathway, liver fibrosis induced by E. multilocularis infection can be alleviated (Yang et al., 2022). In the present study, we observed apoptotic bodies in E. multilocularis PSC after treatment with PAB in vitro, referring apoptosis might be triggered. It was also demonstrated in our in vitro test that the TGF-β1 expression in the PSC was significantly reduced after treatment with PAB. On the other hand, we noted that the infection of E. multilocularis could induce over-expression of TGF-β1 protein and/or mRNA in the serum and liver of the mice, which was similar to the previous descriptions (Dissous et al., 2006; Brehm and Koziol, 2017). However, the over-expression of TGF-β1 in infected mice was significantly decreased after treatment with PAB, and meanwhile, the growth and proliferation of cysts was significantly inhibited in vivo. We thus deemed that the anti-parasite effect of PAB on E. multilocularis metacestodes was associated with the down-regulation of TGF-β1 signaling. Nevertheless, future study needs to elucidate the mechanism on how PAB regulate TGF-β1 signaling pathway to exert suppressive effect on the growth and proliferation of E. multilocularis. Current studies suggest that PAB is a promising immunosuppressive and anti-inflammatory agent candidate. It was evidenced that the derivative of PAB can promotes the production of Tregs and this inductive effect on Tregs production might be TGF-β1 dependent (Li et al., 2014). Some experiments demonstrated that TGF-β1 is secreted into the granuloma area around E. multilocularis, and the signaling may contribute to parasitic evasion of the host’s immunity (Zhang et al., 2008; Anthony et al., 2010). It was indicated that Treg/Th17 imbalance was observed at the middle and even late stage of E. multilocularis infection and it may be regulated by the TGF-β/Smad signaling pathway. TGF-β1 alone supports Treg cell expansion, TGF-β1 together with IL-6 promotes Th17 expansion (Gottstein et al., 2017; Wang et al., 2018). We expect future study on the action mechanism of PAB may include specific target effectors in TGF-β1 signaling possibly regulated by PAB, and other cytokine signaling pathway contributing to PAB’s suppressive effect on the growth and development of Echinococcus parasite.
PAB possesses potent cytotoxic effects to target cells; however, its cytotoxicity to the host is a research concern for future clinical application. To this end, a PAB derivative, hexahydropseudolaric acid B (HPAB) was synthesized and was demonstrated to be able to substantially reduce the cytotoxicity, while its inhibitory efficacy on T cell proliferation remains high (Li et al., 2014). Another experimental study indicated that PAB displays potent anti-chronic myeloid leukaemia (CML) cells activity and inhibits the growth of the tumors, but without being toxic to mice, suggesting PAB could be used as a potential treatment for CML patients (Liu et al., 2017; Choi et al., 2019; Jiang et al., 2020). In the present study, in vitro PAB showed inhibitory effect in human normal liver and kidney cells with IC50 = 25.29 and 42.94 μg/ml, which was in agree with the previous findings of the cytostatic effect of PAB on mouse liver and renal cells (Mei, 2011). Interestingly, the cytotoxicity of PAB to the two cells exhibited a weak effect superior to ABZ with IC50 = 3.71 and 21.22 μg/ml. Furthermore, we assessed the sub-acute hepatotoxicity and nephrotoxicity of PAB in the healthy mice, and found significant increase of serum IBIL, ALT, ALP and BUN level in mice treated with ABZ compared to PAB, which further indicates that PAB exerts a weaker hepatotoxicity and nephrotoxicity than ABZ. However, further studies are needed to elucidate whether chronic and genetic toxicity of PAB is also weaker than ABZ.
In conclusion, PAB exhibited evident suppressive effect on E. multilocularis metacestodes in our in vitro and in vivo tests. Meanwhile, we found that PAB reduces the expression of TGF-β1 protein and mRNA in E. multilocularis metacestodes in vitro and in infected mice, indicative of the anti-echinococcal effect may involve the reduction of the expression of TGF-β1. In addition, PAB exhibits lower cytotoxicity than ABZ-SO in the normal liver and kidney cell lines in vitro, and presents no sub-acute hepatic and renal toxicity in the healthy mice. Our study suggests the potential of PAB to be developed as a therapeutic agent. However, the chronic toxicity of PAB needs clarified. Moreover, future study is expected to further elucidate the mechanism of PAB exerting suppressive effect on the growth and proliferation of E. multilocularis metacestodes to gain insight into the passway of TGF-β1 signaling involved.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by The Experimental Animal Ethics Committee of School of Basic Medical Sciences, Lanzhou University, and the Ethics Committee of the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Chinese Center for Tropical Diseases Research).
Author contributions
TZ, HG, TJ, and WH designed the study. HG, XMo, BJ, YL, and BX carried out the experiments. HG, LH, and XMo analyzed data. TZ, WH, XMa, and JL provided experimental material. HG, TZ, and LH wrote the manuscript. HG, TZ, and ZF revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (grant no. 2021YFC2300800 and 2021YFC2300802), NHC Key Laboratory of Parasite and Vector Biology (National Institute of Parasitic Diseases, Chinese Center for Diseases Control and Prevention; grant no. NHCKFKT2022-02), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (grant no. 2019PT320004), NHC Key Laboratory of Echinococcosis Prevention and Control (grant no. 2021WZK1003) and the foundation of Shanghai Municipal Health Commission (grant no. 201940302). The funders had no role in the study design, data collection, data analysis, data interpretation, or the writing of this report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Antarianto, R. D., Pragiwaksana, A., Septiana, W. L., Mazfufah, N. F., and Mahmood, A. (2022). Hepatocyte differentiation from iPSCs or MSCs in decellularized liver scaffold: cell-ECM adhesion, spatial distribution, and hepatocyte maturation profile. Organogenesis 18:2061263. doi: 10.1080/15476278.2022.2061263
Anthony, B., Allen, J. T., Li, Y. S., and McManus, D. P. (2010). Hepatic stellate cells and parasite-induced liver fibrosis. Parasit Vectors 3:60. doi: 10.1186/1756-3305-3-60
Brehm, K., and Koziol, U. (2017). Echinococcus-host interactions at cellular and molecular levels. Adv. Parasitol. 95, 147–212. doi: 10.1016/bs.apar.2016.09.001
Caja, L., Dituri, F., Mancarella, S., Caballero-Diaz, D., Moustakas, A., Giannelli, G., et al. (2018). TGF-β and the tissue microenvironment: relevance in fibrosis and cancer. Int. J. Mol. Sci. 19:E1294. doi: 10.3390/ijms19051294
Chen, Z., Lin, Z., Yu, J., Zhong, H., Zhuo, X., Jia, C., et al. (2022). Mitofusin-2 restrains hepatic stellate cells’ proliferation via PI3K/Akt signaling pathway and inhibits liver fibrosis in rats. J. Healthc. Eng. 2022:6731335. doi: 10.1155/2022/6731335
Cheng, Z., Xu, Z., Tian, H., Liu, F., Li, X., Luo, D., et al. (2020). In vitro and in vivo efficacies of the EGFR/MEK/ERKh signaling inhibitors in the treatment of alveolar echinococcosis. Antimicrob. Agents Chemother. 64:e00341–20. doi: 10.1128/AAC.00341-20
Choi, S.-J., Ahn, C.-H., Yang, I.-H., Jin, B., Lee, W. W., Kim, J.-H., et al. (2019). Pseudolaric acid B induces growth inhibition and caspase-dependent apoptosis on head and neck cancer cell lines through death receptor 5. Molecules 24, 24:3715. doi: 10.3390/molecules24203715
Deplazes, P., Rinaldi, L., Alvarez Rojas, C. A., Torgerson, P. R., Harandi, M. F., Romig, T., et al. (2017). Global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 95, 315–493. doi: 10.1016/bs.apar.2016.11.001
Dissous, C., Khayath, N., Vicogne, J., and Capron, M. (2006). Growth factor receptors in helminth parasites: signalling and host-parasite relationships. FEBS Lett. 580, 2968–2975. doi: 10.1016/j.febslet.2006.03.046
Gao, H., Sun, X., Luo, Y., Pang, H., Ma, X., Zhang, T., et al. (2021). Anti-echinococcal effect of verapamil involving the regulation of the calcium/calmodulin-dependent protein kinase II response in vitro and in a murine infection model. Parasit Vectors 14:108:108. doi: 10.1186/s13071-021-04618-4
Gao, H., Zhang, Y., Mo, X., Huo, L., Luo, Y., Zhang, T., et al. (2022). Antitumor effect of pseudolaric acid B involving regulation of notch1/Akt signaling response in human hepatoma cell in vitro. Evid. Based Complement. Altern. Med. 2022:5353686, –5353611. doi: 10.1155/2022/5353686
Gottstein, B., Soboslay, P., Ortona, E., Wang, J., Siracusano, A., and Vuitton, D. A. (2017). Immunology of alveolar and cystic echinococcosis (AE and CE). Adv. Parasitol. 96, 1–54. doi: 10.1016/bs.apar
Hemphill, A., and Muller, J. (2009). Alveolar and cystic echinococcosis: towards novel chemotherapeutical treatment options. J. Helminthol. 83, 99–111. doi: 10.1017/S0022149X0928936X
Hemphill, A., Stadelmann, B., Rufener, R., Spiliotis, M., Boubaker, G., Muller, J., et al. (2014). Treatment of echinococcosis: albendazole and mebendazole--what else? Parasite 21:70. doi: 10.1051/parasite/2014073
Horton, J. (2000). Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology 121, S113–S132. doi: 10.1017/s0031182000007290
Jansen, S. R., Poppinga, W. J., Jager, W., Lezoualc’h, F., Cheng, X., Wieland, T., et al. (2016). Epac1 links prostaglandin E2 to β-catenin-dependent transcription during epithelial-to-mesenchymal transition. Oncotarget 7, 46354–46370. doi: 10.18632/oncotarget.10128
Ji, J. (2013). Study on active constituents from Pseudolarix kaempferi against Dactylogyrus infecting fish. North West agriculture and forestry university. (In Chinese).
Jiang, L., Wen, C., He, Q., Sun, Y., Wang, J., Lan, X., et al. (2020). Pseudolaric acid B induces mitotic arrest and apoptosis in both imatinib-sensitive and -resistant chronic myeloid leukaemia cells. Eur. J. Pharmacol. 876:173064. doi: 10.1016/j.ejphar.2020.173064
Kern, P., Menezes da Silva, A., Akhan, O., Mullhaupt, B., Vizcaychipi, K. A., Budke, C., et al. (2017). The echinococcoses: diagnosis, clinical management and burden of disease. Adv. Parasitol. 96, 259–369. doi: 10.1016/bs.apar.2016.09.006
Li, L., Chen, B., Yan, H., Zhao, Y., Lou, Z., Li, J., et al. (2018). Three-dimensional hepatocyte culture system for the study of Echinococcus multilocularis larval development. PLoS Negl. Trop. Dis. 12:e0006309. doi: 10.1371/journal.pntd.0006309
Li, T., Chen, H., Yang, Z., Wang, W., Wang, Y. T., Zhang, L. M., et al. (2014). A novel pseudolaric acid B derivative, Hexahydropseudolaric acid B, exterts an immunomodulatory effect in vitro/in vivo evaluation. Eur. J. Pharmacol. 745, 10–18. doi: 10.1016/j.ejphar.2014.10.009
Liu, F., Sun, C., Chen, Y., Du, F., Yang, Y., and Wu, G. (2021). Indole-3-propionic acid-aggravated CCl4-induced liver fibrosis via the TGF-beta1/Smads signaling pathway. J. Clin. Transl. Hepatol. 9, 917–930. doi: 10.14218/JCTH.2021.00032
Liu, M. L., Sun, D., Li, T., and Chen, H. (2017). A systematic review of the immune-regulating and anticancer activities of pseudolaric acid B. Front. Pharmacol. 8:394. doi: 10.3389/fphar.2017.00394
Liu, C., Wang, F., Wang, B., Wu, T., Wang, Y., Huo, W., et al. (2020a). Pseudolaric acid B induces apoptosis in human rhabdomyosarcoma RD cells. Oncol. Lett. 20:1. doi: 10.3892/ol.2020.12222
Liu, C., Yin, J., Hu, W., and Zhang, H. (2020b). Glycogen phosphorylase: a drug target of amino alcohols in Echinococcus granulosus, predicted by a computer-aided method. Front. Microbiol. 11:557039. doi: 10.3389/fmicb.2020.557039
Mei, X. (2011). Research on treatment effect of pseudolaric acid B on the murine allergic contact dermatitis and its mechanism. Hebei Medical University. In Chinese
Nono, J. K., Lutz, M. B., and Brehm, K. (2020). Expansion of host regulatory T cells by secreted products of the tapeworm Echinococcus multilocularis. Front. Immunol. 11:798. doi: 10.3389/fimmu.2020.00798
Peng, D., Fu, M., Wang, M., Wei, Y., and Wei, X. (2022). Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 21:104. doi: 10.1186/s12943-022-01569-x
Sanchez-Capelo, A. (2005). Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev. 16, 15–34. doi: 10.1016/j.cytogfr.2004.11.002
Stamatakos, M., Sargedi, C., Stefanaki, C., Safioleas, C., Matthaiopoulou, I., and Safioleas, M. (2009). Anthelminthic treatment: an adjuvant therapeutic strategy against Echinococcus granulosus. Parasitol. Int. 58, 115–120. doi: 10.1016/j.parint.2009.01.002
Tsai, I. J., Zarowiecki, M., Holroyd, N., Garciarrubio, A., Sanchez-Flores, A., Brooks, K. L., et al. (2013). The genomes of four tapeworm species reveal adaptations to parasitism. Nature 496, 57–63. doi: 10.1038/nature12031
Wang, J., Cardoso, R., Marreros, N., Müller, N., Lundström-Stadelmannet, B., Siffert, M., et al. (2018). Foxp3+ T regulatory cells as a potential target for immunotherapy against primary infection with Echinococcus multilocularis eggs. Infect. Immun. 86, e00542–e00518. doi: 10.1128/iai.00542-18
Wang, H., Li, J., Guo, B., Zhao, L., Zhang, Z., McManus, D. P., et al. (2016). In vitro culture of Echinococcus multilocularis producing protoscoleces and mouse infection with the cultured vesicles. Parasit Vectors 9:411. doi: 10.1186/s13071-016-1687-y
Wang, Y., Shen, R. W., Han, B., Li, Z., Xiong, L., Zhang, F. Y., et al. (2017). Notch signaling mediated by TGF-beta/Smad pathway in concanavalin A-induced liver fibrosis in rats. World J. Gastroenterol. 23, 2330–2336. doi: 10.3748/wjg.v23.i13.2330
Wang, D., Xin, Y., Tian, Y., Li, W., Sun, D., and Yang, Y. (2017). Pseudolaric acid B inhibits gastric cancer cell metastasis in vitro and in haematogenous dissemination model through PI3K/AKT, ERK1/2 and mitochondria-mediated apoptosis pathways. Exp. Cell Res. 352, 34–44. doi: 10.1016/j.yexcr.2017.01.012
Wang, X. J., Xue, Y. Q., Zhang, H. L., Yu, Y., and Liu, P. (2022). PINK1 deficiency aggravates the β-amyloid-attenuated Mitophagy-lysosomal degradation in PC12 cells. J. Explor. Res. Pharmacol. 7, 30–36. doi: 10.14218/jerp.2021.00053
Wang, H., Zhang, C. S., Fang, B. B., Hou, J., Li, W. D., Li, Z. D., et al. (2020). Dual role of hepatic macrophages in the establishment of the Echinococcus multilocularis metacestode in mice. Front. Immunol. 11:600635. doi: 10.3389/fimmu.2020.600635
Xiao, Y., Wang, Y. J., Xie, J. G., and Wang, X. C. (2022). Transcriptomic identification of key genes in human peripheral blood mononuclear cells for the prognosis of sepsis. J. Explor. Res Pharmacol. doi: 10.14218/JERP.2022.00023
Xin, Q., Yuan, M., Li, H., Song, X., Lu, J., and Jing, T. (2020). In vitro effects of lonidamine and 6-aminonicotinamide against Echinococcus granulosussensu stricto and Echinococcus multilocularis. Vet. Res. 51:29. doi: 10.1186/s13567-020-00744-6
Yang, N., Ma, W., Ke, Y., Liu, H., Chu, J., Sun, L., et al. (2022). Transplantation of adipose-derived stem cells ameliorates Echinococcus multilocularis-induced liver fibrosis in mice. PLoS Negl. Trop. Dis. 16:e0010175. doi: 10.1371/journal.pntd.0010175
Yao, G., Qi, M., Ji, X., Fan, S., Xu, L., Hayashi, T., et al. (2014). ATM-p53 pathway causes G2/M arrest, but represses apoptosis in pseudolaric acid B-treated HeLa cells. Arch. Biochem. Biophys. 558, 51–60. doi: 10.1016/j.abb.2014.05.029
Yu, J., Chen, C., Xu, T., Yan, M., Xue, B., Wang, Y., et al. (2016). Pseudolaric acid B activates autophagy in MCF-7 human breast cancer cells to prevent cell death. Oncol. Lett. 11, 1731–1737. doi: 10.3892/ol.2016.4103
Yu, J. H., Wang, H. J., Li, X. R., Tashiro, S. I., Onodera, S., and Ikejima, T. (2008). Protein tyrosine kinase, JNK, and ERK involvement in pseudolaric acid B-induced apoptosis of human breast cancer MCF-7 cells. Acta Pharmacol. Sin. 29, 1069–1076. doi: 10.1111/j.1745-7254.2008.00835.x
Zhang, S., Hüe, S., Sène, D., Penfornis, A., Bresson-Hadni, S., Kantelip, B., et al. (2008). Expression of major histocompatibility complex class I chain-related molecule a, NKG2D, and transforming growth factor-beta in the liver of humans with alveolar echinococcosis: new actors in the tolerance to parasites? J. Infect. Dis. 197, 1341–1349. doi: 10.1086/586709
Zhang, J., Yan, L. T., Yuan, E. L., Ding, H. X., Ye, H. C., Zhang, Z. K., et al. (2014). Antifungal activity of compounds extracted from cortex pseudolaricis against Colletotrichum gloeosporioides. J. Agric. Food Chem. 62, 4905–4910. doi: 10.1021/jf500968b
Keywords: alveolar echinococcosis, pseudolaric acid B, Echinococcus multilocularis, protoscoleces, TGF-β1
Citation: Gao H, Huo L, Mo X, Jiang B, Luo Y, Xu B, Li J, Ma X, Jing T, Feng Z, Zhang T and Hu W (2022) Suppressive effect of pseudolaric acid B on Echinococcus multilocularis involving regulation of TGF-β1 signaling in vitro and in vivo. Front. Microbiol. 13:1008274. doi: 10.3389/fmicb.2022.1008274
Edited by:
Quan Liu, Foshan University, ChinaReviewed by:
Yong Fu, Washington University in St. Louis, United StatesNamrata Anand, University of Kentucky, United States
Copyright © 2022 Gao, Huo, Mo, Jiang, Luo, Xu, Li, Ma, Jing, Feng, Zhang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Zhang, emhhbmd0aW5nQG5pcGQuY2hpbmFjZGMuY24=; Wei Hu, aHV3QGZ1ZGFuLmVkdS5jbg==
†These authors have contributed equally to this work
 Haijun Gao
Haijun Gao Lele Huo1†
Lele Huo1† Bin Xu
Bin Xu Xingming Ma
Xingming Ma Zheng Feng
Zheng Feng Ting Zhang
Ting Zhang Wei Hu
Wei Hu