
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 19 October 2022
Sec. Terrestrial Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1002198
This article is part of the Research Topic Ecological Distribution, Functional Diversity, and the Biogeochemical Cycle of Microorganisms in Karst View all 14 articles
The karst tiankeng is a special and grand negative terrain on the surface, that maintains a unique ecosystem. However, knowledge about bacterial and fungal communities in karst tiankengs is still limited. Therefore, soil samples from five karst tiankengs were collected and subjected to high-throughput sequencing of 16S rRNA and ITS genes, and multivariate statistical analysis. The results showed abundant and diversified bacterial and fungal communities in karst tiankeng. The bacterial communities were dominated by Proteobacteria and Acidobacteria, and the fungal communities were dominated by Ascomycota and Basidiomycota. Statistical analysis revealed significant differences in bacterial and fungal communities among the five karst tiankengs, which may indicate that the distribution of bacterial and fungal communities was driven by separate karst tiankengs. The co-occurrence network structure was characterized by highly modularized assembly patterns and more positive interactions. The keystone taxa were mainly involved in nutrient cycling and energy metabolism. The null model analysis results showed that the stochastic process, especially dispersal limitation, tended to be more important in controlling the development of bacterial and fungal communities in karst tiankeng. The bacterial community structure was significantly associated with soil properties (SWC, TN, AN, and BD), while the fungal community structure was significantly associated with soil properties (SWC and TP) and plant diversity. These results can expand our knowledge of the karst tiankeng microbiome.
Carbonate rocks are the material basis for the development of karst landforms, with a total distribution area of 3.44 million km2 in China, accounting for approximately 1/3 of the country’s land area (Yuan, 2005). China has the largest karst region in the world (Zhu and Chen, 2005). The karst tiankeng is a typical karst landscape that develops in specific karst geological, geomorphological, climatic, and hydrological environments (Zhu and Waltham, 2005; Shui et al., 2015). The latest research defines karst tiankengs as karst closed pits with a width and depth of more than 100 m, a small ratio of diameter to depth, a continuous circumference, and vertical or subvertical walls (Gunn, 2019). It can be seen that karst tiankeng is a large-scale negative topographic geological wonder on the surface. China is the karst tiankeng kingdom, accounting for more than 70% of the total number of karst tiankengs (Pu et al., 2017). Karst tiankeng tends to appear in groups and form complex cave-hydrogeological systems with underground rivers and caves (Xu et al., 2009; Shui et al., 2015).
Because karst tiankengs are characterized deep into the surface and cliffs, the internal habitat of karst tiankengs is relatively independent of the land surface environment, with unique hydrothermal conditions and primitive microclimates (Zhu and Chen, 2005; Pu et al., 2019). The unique habitat of karst tiankengs serves as an environment for an abundant and unique resource of animals, plants, and microorganisms (Batori et al., 2017; Su et al., 2017; Jian et al., 2018; Jiang et al., 2022a). Karst ecosystems are well known to be characterized by soil erosion, poor soil nutrients, and biodiversity loss (Clements et al., 2006). The karst tiankeng are similar to “oases” in degraded landscapes and play an important role in karst ecosystems. Existing studies have demonstrated that karst tiankengs are important repositories for plant diversity conservation (Su et al., 2017; Chen et al., 2018b; Shui et al., 2022). As the engine of biogeochemical cycles, the role of soil microorganisms in karst tiankengs cannot be ignored (Balser and Firestone, 2005; Fierer, 2017). Meta-analyses have shown that microbial communities are significantly affected by the habitat environment (Delmont et al., 2011; Wang et al., 2013). Due to their nutrient-rich conditions, microbial communities thrive in karst tiankengs, with higher alpha diversity of microbial communities than land surface habitats (Pu et al., 2019; Jiang et al., 2021). The DSE (dark septate endophyte) resources in Dashiwei karst tiankeng were abundant and some of them possess positive effects on plant growth (Lan et al., 2017). In addition, karst tiankengs resemble a natural open top chamber (OTC) and are ideal for studying the response of terrestrial ecosystems, especially fragile karst ecosystems, to climate warming (Yang et al., 2019). Climate warming will alter soil microbial community structure and activities, which is critical to ecosystem functioning and stability (Yuan et al., 2021). The study of microbial communities in karst tiankeng ecosystems can deepen our understanding of global microbial diversity.
The preliminary study of microorganisms in karst tiankeng focused on the macrofungal species and plant fungi (Deng and Wu, 2014; Lan et al., 2017). Jiang et al. (2014) isolated strains with high keratinase yield in karst tiankeng soil through traditional culture techniques, which had the best effect on feather protein degradation. With the advent of high-throughput sequencing technology, the soil microbial community structure and functionality in karst tiankengs have been gradually discovered (Pu et al., 2019; Jiang et al., 2022a,b). The soil microbial communities of karst tiankeng exhibit significant habitat heterogeneity. However, these studies tend to be limited to characterizing soil microbial communities in karst tiankeng, ignoring the interactions of karst tiankeng microbes. In isolated karst tiankeng ecosystems, microbial community survival activity and interactions are critical to ecosystem stability. However, our understanding of the characterization of the microbiome and interactions of karst tiankeng remains poorly understood. Molecular ecological network (MEN) models based on stochastic matrix theory (RMT) can better simulate the interaction between different species in the community (Deng et al., 2012; Yuan et al., 2021). Microbial interactions constrain their ecological functions via competition, syntrophism, or symbiosis (He et al., 2017; de Vries et al., 2018). The abundant soil nutrients, plant cover, and unique microclimates may distinguish the microbial trophic structures of karst tiankeng ecosystems from those of general terrestrial ecosystems. Understanding how microbial co-occurrence patterns occur in separate karst tiankeng ecosystems is urgently needed.
In this study, soil samples were collected from five karst tiankengs in the Zhanyi karst tiankeng group and subjected to high-throughput sequencing of bacterial 16S rRNA and fungal ITS1 genes and multivariate statistical analysis. The main purposes of this study were: (i) to determine the taxonomic composition and structure of microbial communities in karst tiankeng, (ii) to evaluate the microbial co-occurrence patterns and assembly processes of bacterial and fungal communities in karst tiankeng, and (iii) to determine the key factors driving bacterial and fungal communities in karst tiankeng.
We carried out this study in 2021 in Zhanyi District, Qujing City, Yunnan Province, China; the study was performed at Haifeng Natural Reserve (25°35′-25°57′N, 103°29′-103°39′E). The Zhanyi karst tiankeng group includes dozens of karst tiankengs of varying sizes. The annual precipitation ranges from 1073.5 to 1089.7 mm and is affected by the subtropical highland monsoon climate. The annual temperatures ranges from 13.8 to 14.0°C. The soil type was Yunnan red soil.
Among the Zhanyi karst tiankeng group, we selected five karst tiankengs, including Bajiaxiantang (BJXT), Shaojiaxiantang (SJXT), Shenxiantang (SXT), Wangjiaxiantang (WJXT), and Xiaotiankeng (XTK; Supplementary Figure 1). All five karst tiankengs are scattered in inaccessible areas, and only local residents occasionally enter these karst tiankengs, so they are largely kept in pristine conditions. The morphological characteristics of these five karst tiankengs are listed in Supplementary Table 1. Since the XTK slope is a vertical cliff, the seven sampling sites are all located at the bottom of the karst tiankeng. Of the other four karst tiankengs, the eight sampling sites include two at the bottom of the tiankeng and six at the slope of the tiankeng. Each sampling (10 × 10 m2) included three randomly established quadrats (1 × 1 m2). The plant species richness (R) and Shannon-Wiener (H′) index were calculated (Pan et al., 2014). The soil samples (0–15 cm soil layer) were collected and mixed as a composite soil sample. The soil samples were sieved (2 mm), divided into two parts, and transferred at 4°C. One part was for DNA extraction, and the other part was for soil physicochemical analyses. The ring sampler weighing method was used to measure the soil bulk density (BD). The soil water content (SWC) was determined by the gravimetric method. Soil pH was determined using a glass electrode meter (InsMark™ IS126, Shanghai, China) in a 1:2.5 soil:water (w/v) mixture. The soil organic carbon (SOC) and soil total nitrogen (TN) were determined by potassium dichromate oxidation and the Kjeldahl method, respectively. The available nitrogen (AN) was measured by the alkali-diffusion method. Soil determination of total phosphorus (TP) and available phosphorus (AP) was performed by alkali fusion-Mo-Sb anti-spectrophotometric and sodium hydrogen carbonate solution-Mo-Sb anti-spectrophotometric methods, respectively. The total potassium (TK) was determined by alkali fusion-atomic absorption spectrophotometry methods. The available potassium (AK) was measured by the acid fusion-atomic absorption spectrophotometry method.
Total DNA was extracted using the CTAB method in accordance with the instructions. The concentration and purity of DNA were examined with 1% agarose gel. The primer set of 515F (5’-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5’-GGACTACHVGGGTWTCTAAT-3′) targeting the bacterial 16S rRNA V4 region and ITS3F (5’-GCATCGATGAAGAACGCAGC) and ITS4R (5’-TCCTCCGCTTATTGATATGC) targeting the fungal IST2 region were used for bacterial and fungal genes sequencing, respectively (Caporaso et al., 2012; Jamil et al., 2020). The bacterial and fungal genes were amplified on Phusion® High-Fidelity PCR Master Mix (New England Biolabs). All the raw sequence reads were conducted using an Illumina NovaSeq 6,000 PE250 platform (Illumina, San Diego, CA, United States). All sequence data are deposited on the NCBI and accessible via BioProject IDs of PRJNA851199 for 16S sequences and PRJNA861802 for ITS sequences.
The QIIME2 system was used for raw data FASTQ files filtered and analyzed (Vazquez-Baeza et al., 2013; Bokulich et al., 2018). The demultiplexed sequences were quality filtered, trimmed, denoised, and merged, and then the chimeric sequences were obtained. After identification and removal by the QIIME2 dada2 plugin, the bacterial ASVs were obtained. Vsearch (2.15.1) software was used to identify the optimized sequences with 95% similarity into fungal OTUs. The representative bacterial sequences were taxonomically classified by alignment against the GREENGENES database. The fungal sequences were taxonomically classified by alignment against the UNITE database (Nilsson et al., 2019).
A Venn diagram was drawn using the R (v 4.1.2) package “plotrix.” The Sankey diagram was generated using the JShare online platform.1 The microbial diversity index (Shannon-Wiener and Chao1) was calculated by a core-diversity plugin of QIIME2. The analysis of similarities (ANOSIM) and nonmetric multidimensional scaling (NMDS) were performed using the R (v 4.1.2) package “vegan” (Dixon, 2003). The construction of the molecular ecological networks (MEN) of bacterial and fungal communities was based on the principle of the Molecular Ecological Network Analyses Pipeline (Zhou et al., 2010; Deng et al., 2012). Random matrix theory (RMT) was used to determine the appropriate similarity threshold (St) of molecular ecological networks (Deng et al., 2012). The calculation order follows the rules of decreasing the cutoff from the top, and scan speed refers to the method of regress Poisson distribution only. Cytoscape software (v 3.9.0) was used to visualize the co-occurrence microbial network. The within-module connectivity (Zi) and among-module connectivity (Pi) represent the ecological attributes of the network nodes (Rottjers and Faust, 2018). The network node topologies were classified as peripherals (Zi < 2.5 and Pi <0.62), connectors (Zi < 2.5 and Pi ≥0.62), module hubs (Zi ≥ 2.5 and Pi ≤0.62), and network hubs (Zi ≥ 2.5 and Pi ≥0.62). The connectors, module hubs, and network hubs acted as keystone taxa in the co-occurrence microbial network (He et al., 2017). To determine the ecological processes, phylogenetic and null model analyses were performed (Stegen et al., 2012, 2013). The phylogenic turnover across soil samples was measured by the weighted β-nearest taxon index (βNTI) using the R (v 4.1.2) package “picante” (Kembel et al., 2010). Ecological processes were divided into deterministic processes with |βNTI| values above 2 and stochastic processes with |βNTI| values below 2. If βNTI >2, variable selection plays crucial roles in shaping microbial communities. If βNTI < −2, homogeneous selection is the key assembly process in the microbial community. To further discern the stochastic processes, the Raup–Crick matrix (RCbray) was analyzed via the “vegan” package. The conditions of RCbray >0.95, |RCbray| < 0.95, and RCbray < −0.95 indicated drift, undominated processes, and homogenizing dispersal, respectively. Redundancy analysis (RDA) of bacterial or fungal communities and soil and plant properties was conducted in the R (v 4.1.2) package “vegan.” The selection principle of the RDA or CCA model was based on the results of DCA analysis; if the length was >4.0, the CCA model was chosen. Otherwise, the RDA model should be selected. In this study, the value of bacterial sample data was <4.0; thus, RDA was selected. A fungal sample date >4.0 was chosen; thus, CCA was chosen. The Mantel test was used to discern correlations among the bacterial and fungal communities and soil and plant properties based on the Spearman correlation coefficient, and was performed using the R (v 4.1.2) package “vegan.” The data were assessed for normality and homogeneity of variances, and ANOVAs were performed at the 95% confidence level. The ANOVAs were carried out using SPSS (v10.0).
Except for AK and AP, the physicochemical parameters of soils varied significantly between different tiankengs (Table 1). The SWC, TN, and AN were significantly higher in XTK. The SOC content was significantly higher in SJXT. The soil TK content ranged from 3.50 to 5.54. All tiankeng soils were slightly acidic, with pH values ranging from 6.19 to 6.67. The plant diversity of karst tiankengs was indicated by Shannon-Wiener (H) and richness (R) indices and differed among the five karst tiankengs (Supplementary Table 2). The plant species diversity was significantly higher in WJXT (p < 0.05).
After quality control, a total of 2,123,723 bacterial clean sequences and 2,508,449 fungal clean sequences were obtained. These sequences were grouped into 19,466 bacterial ASVs and 14,414 fungal OTUs (97% similarity threshold). Only 1,137 bacterial ASVs (5.8% of the total bacterial ASVs) and 195 fungal OTUs (1.4% of the total fungal OTUs) were shared by all karst tiankeng soils (Supplementary Figure 2). At the phylum of bacteria, Proteobacteria (39.3%–45.0.97%), Acidobacteria (17.2–24.8%), and Actinobacteria (14.9–22.1%) were predominant in all karst tiankeng soils (Figure 1A). Proteobacteria were represented by the classes Alphaproteobacteria (18.0–23.5%), Betaproteobacteria (8.7–11.3%), and Deltaproteobacteria (4.8–7.2%). Actinobacteria were represented by the classes Thermoleophilia (6.5–10.4%) and Actinobacteria (5.2–8.3%). In addition, the archaeal phyla Crenarchaeota and Euryarchaeota were also detected in the karst tiankeng soils. At the phyla of fungal, Basidiomycota (23.8–50.5%) and Ascomycota (36.2–63.6%) were predominant in all karst tiankeng soils. Ascomycota was mainly represented by the classes Eurotiomycetes (8.5–20.9%), Sordariomycetes (7.3–24.1%), and Dothideomycetes (3.5–16.2%; Figure 1B).
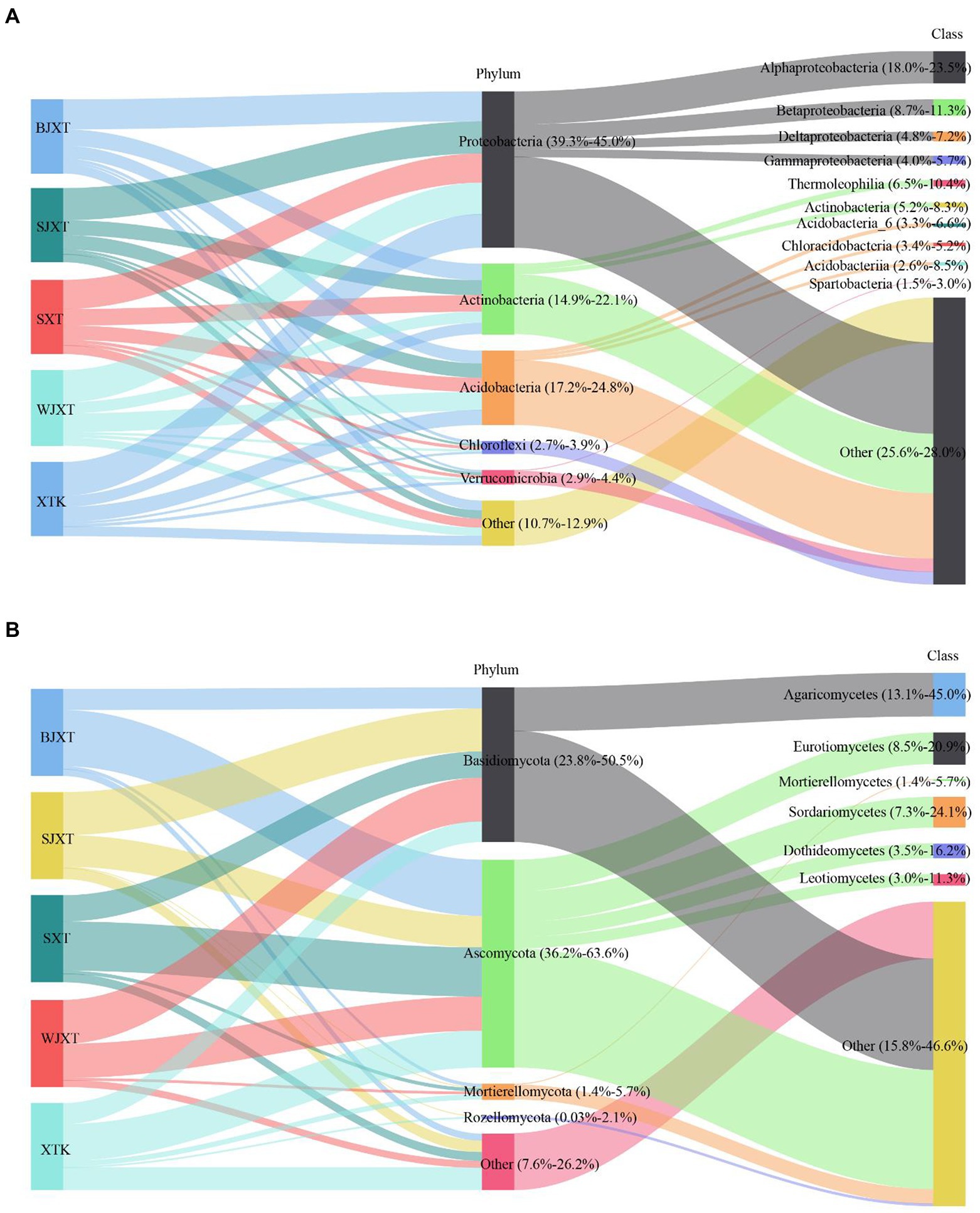
Figure 1. The Sankey diagram of the composition of bacterial (A) and fungal (B) communities at phylum and class level in five karst tiankengs. BJXT: Bajiaxiantang, SJXT: Shaojiaxiantang, SXT: Shenxiantang, WJXT: Wangjiaxiantang, XTK: Xiaotiankeng.
The alpha diversity of karst tiankeng microbial communities was indicated by the Shannon and Chao1 indices. There was no significant difference in the bacterial community Chao1 index among the five karst tiankengs (p = 0.25), whereas a significant difference was observed in the bacterial community Shannon index among the five karst tiankengs (p < 0.05). A significant difference was observed in fungal community alpha diversity among the five karst tiankengs (p < 0.05; Supplementary Figure 3). The analysis of similarities (ANOSIM; bacterial: R = 0.804, p = 0.001; fungal: R = 0.489, p = 0.001) revealed that bacterial and fungal communities from the different karst tiankengs significantly differed from each other (Supplementary Figure 4). The nonmetric multidimensional scaling (NMDS) plot showed that the bacterial communities in BJXT and SXT were closely clustered, while the fungal communities in BJXT, SJXT, SXT, and WJXT were closely clustered and separated from those in XTK (Figure 2).
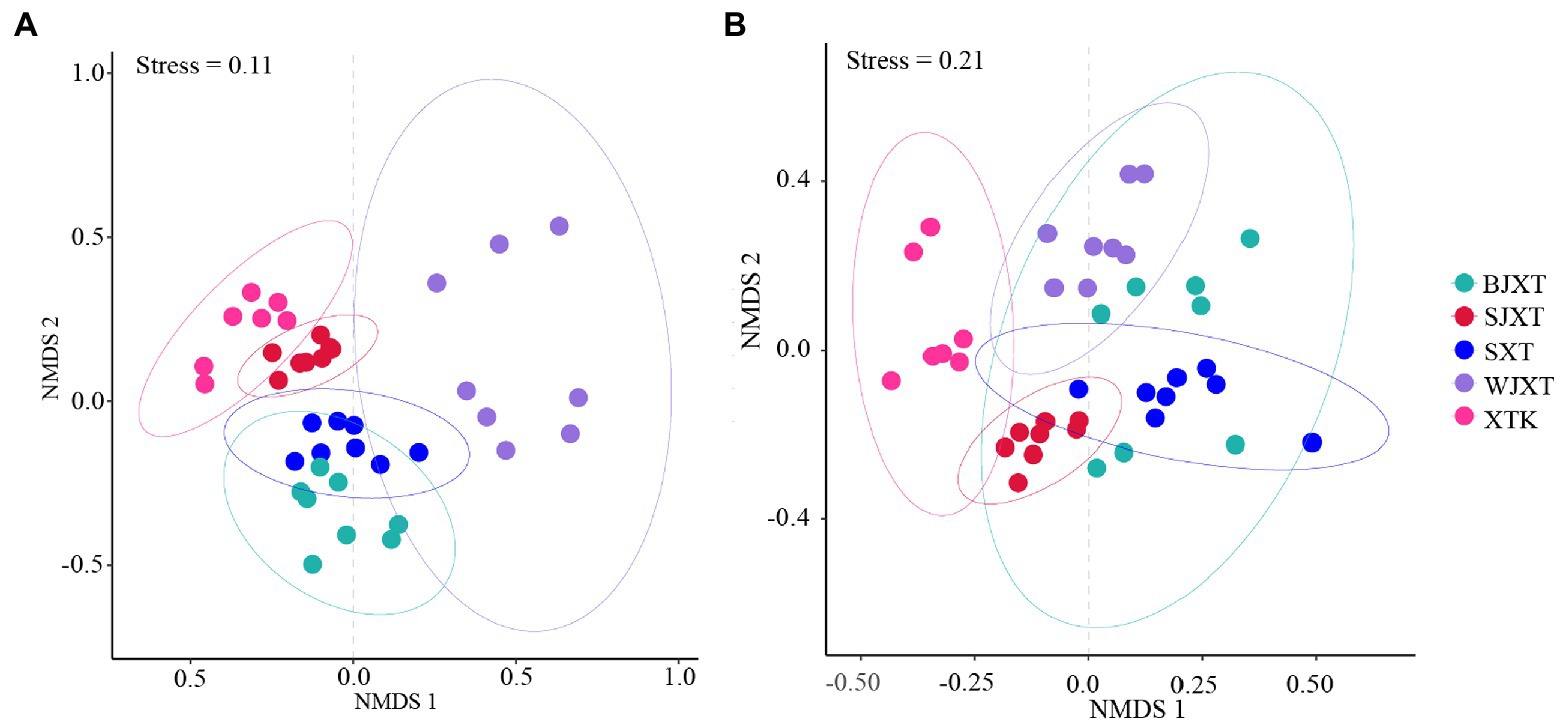
Figure 2. The non-metric multidimensional scaling (NMDS) analysis of bacterial (A) and fungal (B) communities in five karst tiankengs. BJXT: Bajiaxiantang, SJXT: Shaojiaxiantang, SXT: Shenxiantang, WJXT: Wangjiaxiantang, XTK: Xiaotiankeng.
The microbial random molecular ecological network (MENs) identified the microbe-microbe interactions in karst tiankeng. The karst tiankeng microbial network was a scale-free network (R2 = 0.923) and exhibited a good modular structure (modularity >0.4; Table 2). The karst tiankeng microbial network consisted of 185 nodes and 238 edges. More positive interaction edges (52.52%) were observed in the karst tiankeng microbial network. In the network of karst tiankeng soils, most nodes were grouped into six major modules (Figure 3A). Bacteria and fungi accounted for 90.58 and 9.42% of the total nodes, respectively (Figure 3B). The largest modules contain 17.84% of the total nodes. Proteobacteria (bacteria), Acidobacteria (bacteria), and Ascomycota (fungi) dominated in the major modules. Bacteria dominated in all the major modules, and fungi were mainly located in modules 0 and 1 (Figure 3C).
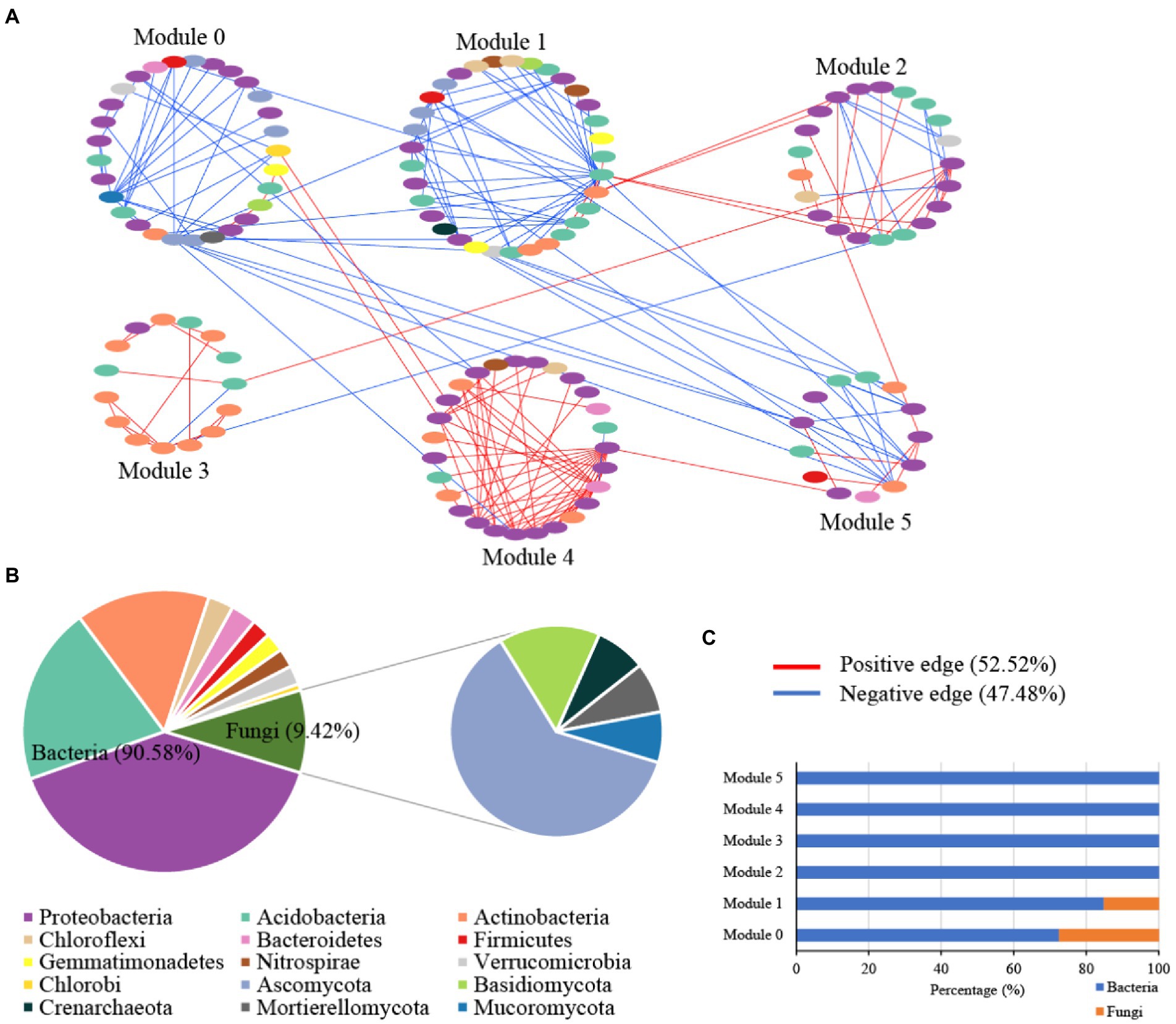
Figure 3. The microbial networks of bacterial and fungal communities of Zhanyi Karst Tiankeng Group by modules (A); The proportion of microbial composition (phylum level) in main module (B); The proportion of bacterial ASVs and fungal OTUs in main module (C).
Among all nodes, 96.22% belonged to peripherals in karst tiankengs (Figure 4). Seven keystone taxa (five bacteria keystone taxa and two fungi keystone taxa) were identified in the karst tiankeng network, including six module hubs (3.24%) and one connector (0.54%). The dominant keystone taxa were Proteobacteria (bacteria) with an abundance of 42.86% of all keystone taxa. Bacteria of Bradyrhizobium (genus), Steroidobacter (genus), Micrococcales (order), Ellin6513 (order), and fungi of Umbelopsis (genus) and Fusarium (genus) were the keystone taxa in the karst tiankeng network (Supplementary Table 3). All fungal keystone taxa of the karst tiankeng network were located in module 0.
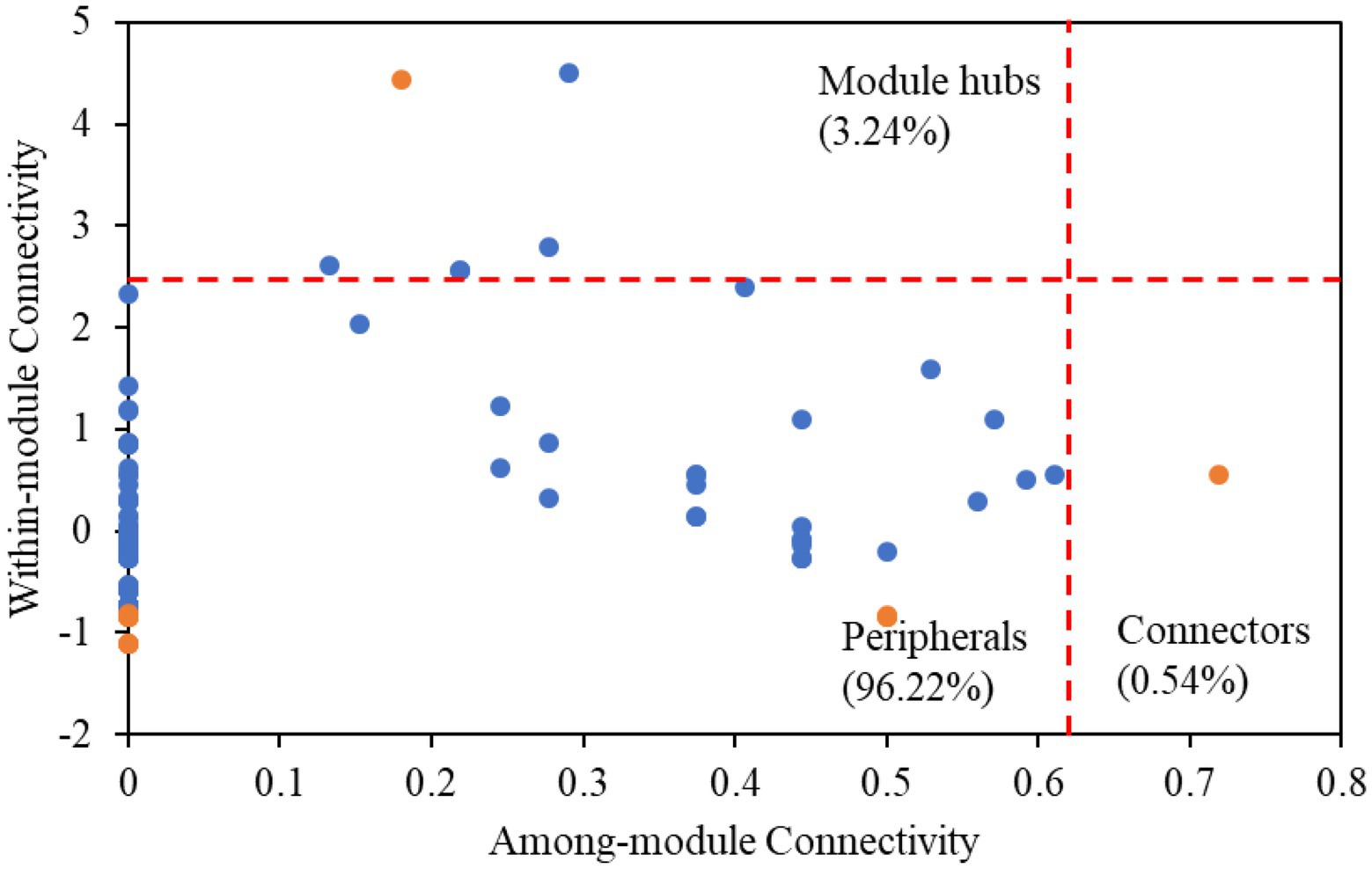
Figure 4. The ZP-plots shows distribution of ASVs/OTUs according to their module-based topological roles in the networks of five karst tiankengs. Each blue or orange dot represents a bacterial ASVs or fungal OTUs, respectively.
The median phylogenetic turnover was between −2 and 2, which indicated that stochastic processes controlled the bacterial community assembly. Dispersal limitation was the key process in bacterial community assembly, with a contribution of 92.5%. Similarly, stochastic processes are crucial assembly processes in fungal community composition. Dispersal limitation and undominated processes contributed 89.5 and 9.8%, respectively, to the fungal community assembly (Figure 5).

Figure 5. Phylogenetic and null model analyses revealing assembly processes of bacterial (A) and fungal (B) communities in five karst tiankeng tested by βNTI values, and percentage of each ecological process contributed to the assembly of bacterial and fungal communities in five karst tiankengs.
RDA was conducted to investigate the impact of soil properties and plant properties (richness and Shannon-Wiener) on bacterial and fungal communities. Among all the variables investigated, TN, AN, SWC, and BD had significant relationships with the bacterial communities (Figure 6A). SWC, R, TP, and TN were significantly associated with fungal communities (Figure 6B). The Mantel test results showed that BD, SWC, TN, TP, AP, and AN had significant relationships with the bacterial communities, while SWC, TN, AN, and H′ had significant relationships with the fungal communities (p < 0.05, Supplementary Table 4). Significant correlations were also observed between the main microbial phylum and soil or plant variables (Supplementary Table 5).
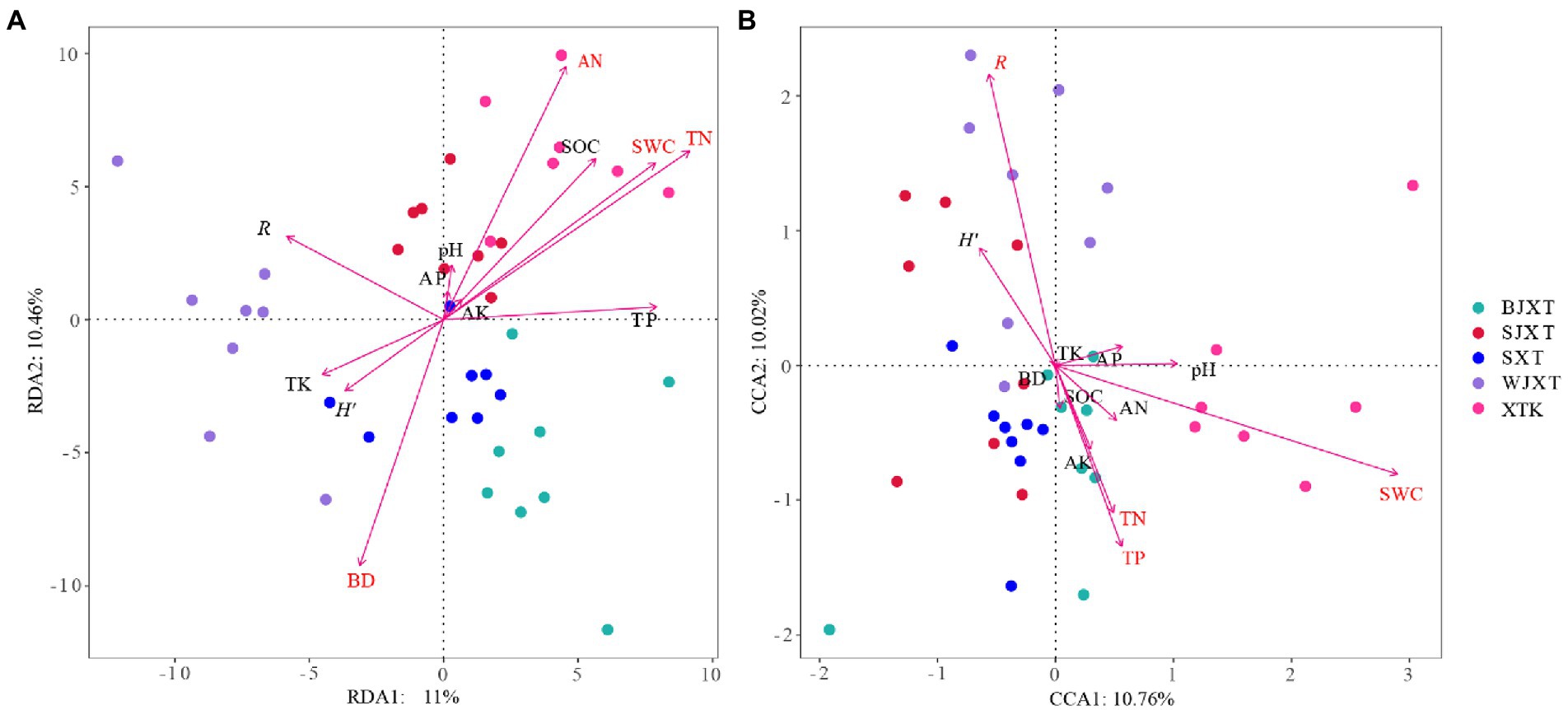
Figure 6. The redundancy analysis (RDA) of the bacterial (A) and fungal (B) communities with soil and plant properties in five karst tiankengs. Mark the significant factors in red font.
The constant physicochemical and microclimate conditions in the subsurface support a stable ecosystem in karst tiankeng. The bacterial communities in karst tiankeng were dominated by Proteobacteria and Acidobacteria. Similar results were reported for Shenmu karst tiankeng located in Guangxi, southwestern China (Pu et al., 2019). Proteobacteria are considered to play an important role in phylogenetic and ecological values and have highly diverse metabolic capabilities (Kang et al., 2019; von Borzyskowski et al., 2019). Acidobacteria species are considered to play a key role in nutrient cycles and perform the function of organic matter decomposition (Eichorst et al., 2018). In addition, the other abundant phyla included Actinobacteria, Chloroflexi, and Verrucomicrobia. Actinobacteria are considered the most important source of bioactive compounds, especially commercially available antibiotics (Barka et al., 2016; Rangseekaew and Pathom-aree, 2019). Actinobacteria (14.9–22.1%) ranked the third most abundant phyla in the karst tiankeng, which may indicate that Actinobacteria in the unique habitat of karst tiankeng are expected to be a good source of bioactive compound discovery. Chloroflexi and Verrucomicrobia are regarded as having a strong ability to survive in poor nutrient conditions (Pan et al., 2014; Yang et al., 2021), which may help karst tiankeng microbial communities resist external environmental disturbances. The fungal communities in karst tiankeng were dominated by Ascomycota and Basidiomycota. Previous research has shown that Ascomycota and Basidiomycota have a strong ability to decompose cellulose, and improve rock weathering in karst habitats (Baldrian et al., 2012; Xiao et al., 2022). Some of the fungal classes observed in our study were also detected in karst caves, such as Sordariomycetes and Dothideomycetes (Zhang et al., 2017; Man et al., 2018). The underground drainage system of karst landscapes is characterized by karst tiankengs and caves (Legatzki et al., 2011). The intricate hydrological system of the karst system links two unique habitats.
However, the shared bacterial ASVs and fungal OTUs among the five karst tiankengs accounted for less than 10% (Supplementary Table 1), indicating significant differences among bacterial and fungal communities among the five karst tiankengs. Different niches between karst tiankengs drive the evolution of microbial communities and maintain unique microbial populations. The results of the NMSD analysis also confirmed significant differences in bacterial and fungal communities in different karst tiankengs (Figure 2). Karst tiankengs, as isolated habitats, have a distinct island-like effect (Itescu, 2019). The isolation effect of the vertical cliff may vary between different karst tiankengs (Shui et al., 2018), leading to differences in microbial community evolution, composition, and genetic diversity. Affected by geological conditions, the external environment, and isolation effects (Wang et al., 2020), the different habitats between karst tiankengs may maintain diverse microbial communities.
Our study constructed an integrated microbial network by using different samples from five karst tiankengs, which suggests that the karst tiankeng microbial communities have more interactions within the niche (Figure 3). In the unique habitat of karst tiankengs, microbes may adopt different survival strategies (Jiang et al., 2022a). The inner microbial interactions play an important role in community stability and are determined by the basic dynamics of species to promote their survival (Zhou et al., 2011; Banerjee et al., 2018). A higher proportion of positive interaction edges of microbial networks (52.52%) suggested that bacterial and fungal communities form a tight organization through cooperation, thereby enhancing the complexity of the community structure and the stability of the karst tiankeng ecosystem (Wang Y. et al., 2022). Additionally, the collaboration between autotrophic and heterotrophic microorganisms contributes to their growth and metabolism and probably enhances community functions (Nadell et al., 2016). The microbial network showed good modularity, indicating nonrandom patterns of microbial interactions in karst tiankeng (Olesen et al., 2007).
The dominant phyla in the microbial network were Proteobacteria (bacteria) and Ascomycota (fungi), which are also the main soil microbial groups in various habitats (Sun et al., 2017; Cui et al., 2019). Based on the main functions of these microbial groups, it can be inferred that microbes involved in nutrient cycles and energy metabolism can survive well in karst tiankeng. Keystones are considered to play an important role in maintaining the stability of the microbial community (Mondav et al., 2017). A total of five bacterial keystones and two fungal keystones were identified in the karst tiankeng network (Supplementary Table 2). Previous studies demonstrated that Bradyrhizobium bacteria closely interact with plant roots and is abundantly present at high levels of plant diversity (Berg and Smalla, 2009; Xiao et al., 2021). Bradyrhizobium serves as a keystone in the network, which may be related to the abundant vegetation of karst tiankeng. As a member of Proteobacteria, Steroidobacter can access C and nutrients in oligotrophic conditions (Jeewani et al., 2020). The keystones of Bradyrhizobium and Steroidobacter play a key role in organic matter decomposition and nutrient cycling by connecting other microbial members in the network. In addition, fungal keystones of Fusarium are ubiquitous in karst ecosystems (Wang et al., 2016). Fusarium can secrete cellulase to decompose carbon, and participate in the dissolution of soil insoluble phosphorus, effectively improving the acquisition of phosphorus by plants (Yang et al., 2018). In general, keystones play a key role in maintaining the ecological function of the karst tiankeng ecosystem.
The assembly of bacterial and fungal communities is mainly a stochastic process, with the dominance of dispersal limitation in five karst tiankengs, indicating the spatial heterogeneity of karst tiankengs. The occurrence of dispersal limitation in the karst tiankeng indicates a weakening selection, which may result from the barrier of the vertical cliff. Most previous studies suggested that deterministic processes have a greater impact on the formation of bacterial communities (Cheng et al., 2021; Han et al., 2022; Wang H. et al., 2022). Our results may be explained by the complex environment in the karst tiankeng. The abundant vegetation cover of karst tiankeng leads to a large accumulation of vegetation litter, which enriches soil nutrients and ultimately promotes the abundance of bacterial communities (Liu et al., 2021). The nutrient-rich soil environment allows bacterial communities can thrive in the unique habitat of karst tiankengs. Additionally, bacterial communities can be transferred through the karst hydrological system (Mulec et al., 2019). These ecological processes were mixed to increase the stochastic process of bacterial community assembly in karst tiankeng. Deterministic processes were usually dominant in habitats with steep environmental gradients (Huang et al., 2022). Although the environments of these five karst tiankengs exhibit differences, it does not meet the characteristics of a steep environmental gradient. On the contrary, the interior of karst sinkholes maintains a relatively stable environment due to the effect of pit wall isolation. Thus, stochastic processes play a leading role in the construction of fungal communities in karst tiankengs. The dispersal limitation of fungal communities was consistent with previous studies (Li et al., 2020). The spread of fungal spores is usually limited to short distances, and the unique topography of karst tiankengs significantly reduces the spread of fungal spores. It is important to note that stochastic processes tend to occur before stable microbial communities form; our results may reflect that bacterial and fungal communities in karst tiankengs are still in an unstable state.
The soil and plant properties of the five karst tiankengs showed high habitat heterogeneity. Environmental variables are closely related to microbial diversity and community composition (Gao et al., 2017; Song et al., 2018). The RDA and mental test results showed that soil and plant properties were significantly associated with bacterial and fungal communities of the five karst tiankengs (Figure 6; Supplementary Table 1). SWC has been widely demonstrated to construct soil bacterial and fungal communities (Kaisermann et al., 2015; Long et al., 2021; Lin et al., 2022). Soil moisture content can directly affect the survival activity of microbes, and affect microbial growth by indirectly regulating the distribution of soil nutrients (Clark et al., 2009; Manzoni et al., 2014). For fungal communities, the soil water may help the expansion of soil hyphal networks (Hawkes et al., 2011). Our previous studies also indicated that the abundant soil water content in the karst tiankeng may make it easy for microorganisms to colonize (Jiang et al., 2022a). High SOC contents in karst regions, and soil nitrogen contents are more critical for bacterial communities (e.g., Proteobacteria; Li et al., 2022). Previous studies have indicated that soil structure with different bulk densities affects the spread and activity of bacteria in soil (Juyal et al., 2021). The high heterogeneity of karst habitats may affect bacterial communities by altering soil bulk density. Karst areas are generally limited by phosphorus (Chen et al., 2018a) and thus affect microbial communities. Liu et al. (2012) studies also reported that soil TP content is the limiting factor of microbial growth. Yang et al. (2017) study found that soil phosphorus has a more important effect on soil fungal communities than soil carbon-nitrogen ratios, which is consistent with our results. Fungal communities have a close relationship with plant diversity (He et al., 2008). Fungal communities are a key player in breaking down plant litter (Voriskova and Baldrian, 2013). The abundant plants in the karst tiankeng underground forest provide a high-quality environment for fungal communities.
This study provides a comprehensive assessment of the bacterial and fungal communities in karst tiankeng. The dominant phyla in the five karst tiankengs were mainly included Proteobacteria, Acidobacteria (bacterial), Ascomycota, and Basidiomycota (fungal). The diversity and composition of bacterial and fungal communities significantly differed among the five karst tiankengs. The co-occurrence network indicated that microorganisms preferred to survive in karst tiankengs in a modular manner, and abundant taxa relied more on partnerships to adapt to the environment. The keystones might play a critical role in nutrient cycles and energy metabolism. The bacterial communities were mainly related to SWC, TN, AN, and BD, while the fungal communities were mainly related to SWC, TP, and plant diversity. These observations provide improved knowledge about the structure, composition, community assembly processes, and co-occurrence patterns of bacterial and fungal communities in karst tiankeng ecosystems. This study contributes to further studies of the diversity and interaction patterns of bacterial and fungal communities in karst tiankeng worldwide and explores the value of the biodiversity conservation pool of karst tiankeng.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
CJ and WS designed the study. CJ, XS, YL, KW, and HL carried out field sampling and plot surveys. CJ performed the experiments, analyzed the data, and wrote the manuscript. SZ provided technical support to this study. WS provided critical comments and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (41871198).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1002198/full#supplementary-material
Baldrian, P., Kolarik, M., Stursova, M., Kopecky, J., Valaskova, V., Vetrovsky, T., et al. (2012). Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 6, 248–258. doi: 10.1038/ismej.2011.95
Balser, T. C., and Firestone, M. K. (2005). Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73, 395–415. doi: 10.1007/s10533-004-0372-y
Banerjee, S., Schlaeppi, K., and van der Heijden, M. G. A. (2018). Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 16, 567–576. doi: 10.1038/s41579-018-0024-1
Barka, E. A., Vatsa, P., Sanchez, L., Gaveau-Vaillant, N., Jacquard, C., Meier-Kolthoff, J. P., et al. (2016). Taxonomy, physiology, and natural products of Actinobacteria (vol 80, pg 1, 2016). Microbiol. Mol. Biol. Rev. 80:III. doi: 10.1128/mmbr.00044-16
Batori, Z., Vojtko, A., Farkas, T., Szabo, A., Havadtoi, K., Vojtko, A. E., et al. (2017). Large- and small-scale environmental factors drive distributions of cool-adapted plants in karstic microrefugia. Ann. Bot. 119, 301–309. doi: 10.1093/aob/mcw233
Berg, G., and Smalla, K. (2009). Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 68, 1–13. doi: 10.1111/j.1574-6941.2009.00654.x
Bokulich, N. A., Kaehler, B. D., Rideout, J. R., Dillon, M., Bolyen, E., Knight, R., et al. (2018). Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2 ' s q2-feature-classifier plugin. Microbiome 6:90. doi: 10.1186/s40168-018-0470-z
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Huntley, J., Fierer, N., et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624. doi: 10.1038/ismej.2012.8
Chen, Y. P., Jiang, C., Jian, X. M., Shui, W., Hu, Y., Ma, T., et al. (2018b). Spatial distribution characteristics of grassland plant communities in a moderately degraded tiankeng in Zhanyi. Yunnan. Acta Ecol Sin 38, 134–147.
Chen, H., Li, D. J., Xiao, K. C., and Wang, K. L. (2018a). Soil microbial processes and resource limitation in karst and non-karst forests. Funct. Ecol. 32, 1400–1409. doi: 10.1111/1365-2435.13069
Cheng, X., Yun, Y., Wang, H., Ma, L., Tian, W., Man, B., et al. (2021). Contrasting bacterial communities and their assembly processes in karst soils under different land use. Sci. Total Environ. 751:142263. doi: 10.1016/j.scitotenv.2020.142263
Clark, J. S., Campbell, J. H., Grizzle, H., Acosta-Martinez, V., and Zak, J. C. (2009). Soil microbial community response to drought and precipitation variability in the Chihuahuan Desert. Microb. Ecol. 57, 248–260. doi: 10.1007/s00248-008-9475-7
Clements, R., Sodhi, N. S., Schilthuizen, M., and Ng, P. K. L. (2006). Limestone karsts of Southeast Asia: imperiled arks of biodiversity. Bioscience 56, 733–742. doi: 10.1641/0006-3568(2006)56[733:Lkosai]2.0.Co;2
Cui, Y., Fang, L., Guo, X., Wang, X., Wang, Y., Zhang, Y., et al. (2019). Responses of soil bacterial communities, enzyme activities, and nutrients to agricultural-to-natural ecosystem conversion in the loess plateau. China. J. Soils Sediments 19, 1427–1440. doi: 10.1007/s11368-018-2110-4
de Vries, F. T., Griffiths, R. I., Bailey, M., Craig, H., Girlanda, M., Gweon, H. S., et al. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9:3033. doi: 10.1038/s41467-018-05516-7
Delmont, T. O., Malandain, C., Prestat, E., Larose, C., Monier, J.-M., Simonet, P., et al. (2011). Metagenomic mining for microbiologists. ISME J. 5, 1837–1843. doi: 10.1038/ismej.2011.61
Deng, Y., Jiang, Y.-H., Yang, Y., He, Z., Luo, F., and Zhou, J. (2012). Molecular ecological network analyses. Bmc Bioinformatics 13:113. doi: 10.1186/1471-2105-13-113
Deng, C., and Wu, X. (2014). The component and assessment of macro-fungi in Leye County, Guangxi autonomous region. China. Guizhou Sci 32, 1–18.
Dixon, P. (2003). VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x
Eichorst, S. A., Trojan, D., Roux, S., Herbold, C., Rattei, T., and Woebken, D. (2018). Genomic insights into the Acidobacteria reveal strategies for their success in terrestrial environments. Environ. Microbiol. 20, 1041–1063. doi: 10.1111/1462-2920.14043
Fierer, N. (2017). Embracing the unknown: disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 15, 579–590. doi: 10.1038/nrmicro.2017.87
Gao, C., Shi, N.-N., Chen, L., Ji, N.-N., Wu, B.-W., Wang, Y.-L., et al. (2017). Relationships between soil fungal and woody plant assemblages differ between ridge and valley habitats in a subtropical mountain forest. New Phytol. 213, 1874–1885. doi: 10.1111/nph.14287
Gunn, J. (2019). Tiankeng (giant doline) definitions with particular reference to the Hanzhong depressions, Shaanxi, China. Cave Karst Sci 46, 51–60.
Han, S., Tan, S., Wang, A., Chen, W., and Huang, Q. (2022). Bacterial rather than fungal diversity and community assembly drive soil multifunctionality in a subtropical forest ecosystem. Environ. Microbiol. Rep. 14, 85–95. doi: 10.1111/1758-2229.13033
Hawkes, C. V., Kivlin, S. N., Rocca, J. D., Huguet, V., Thomsen, M. A., and Suttle, K. B. (2011). Fungal community responses to precipitation. Glob. Chang. Biol. 17, 1637–1645. doi: 10.1111/j.1365-2486.2010.02327.x
He, D., Shen, W., Eberwein, J., Zhao, Q., Ren, L., and Wu, Q. L. (2017). Diversity and co-occurrence network of soil fungi are more responsive than those of bacteria to shifts in precipitation seasonality in a subtropical forest. Soil Biol. Biochem. 115, 499–510. doi: 10.1016/j.soilbio.2017.09.023
He, X.-Y., Wang, K.-L., Zhang, W., Chen, Z.-H., Zhu, Y.-G., and Chen, H.-S. (2008). Positive correlation between soil bacterial metabolic and plant species diversity and bacterial and fungal diversity in a vegetation succession on karst. Plant Soil 307, 123–134. doi: 10.1007/s11104-008-9590-8
Huang, L., Bai, J., Wang, J., Zhang, G., Wang, W., Wang, X., et al. (2022). Different stochastic processes regulate bacterial and fungal community assembly in estuarine wetland soils. Soil Biol. Biochem. 167:108586. doi: 10.1016/j.soilbio.2022.108586
Itescu, Y. (2019). Are island-like systems biologically similar to islands? A review of the evidence. Ecography 42, 1298–1314. doi: 10.1111/ecog.03951
Jamil, A., Yang, J.-Y., Su, D.-F., Tong, J.-Y., Chen, S.-Y., Luo, Z.-W., et al. (2020). Rhizospheric soil fungal community patterns of Duchesnea indica in response to altitude gradient in Yunnan, Southwest China. Can. J. Microbiol. 66, 359–367. doi: 10.1139/cjm-2019-0589
Jeewani, P. H., Gunina, A., Tao, L., Zhu, Z., Kuzyakov, Y., Van Zwieten, L., et al. (2020). Rusty sink of rhizodeposits and associated keystone microbiomes. Soil Biol. Biochem. 147:107840. doi: 10.1016/j.soilbio.2020.107840
Jian, X. M., Shui, W., Wang, Y. N., Wang, Q. F., Chen, Y. P., Jiang, C., et al. (2018). Species diversity and stability of grassland plant community in heavily-degraded karst tiankeng: a case study of Zhanyi tiankeng in Yunnan, China. Acta Ecologica Sinica 38, 4704–4714. doi: 10.5846/stxb201706281163
Jiang, C., Feng, J., Zhu, S. F., and Shui, W. (2021). Characteristics of the soil microbial communities in different slope positions along an inverted stone slope in a degraded karst Tiankeng. Biology-Basel 10:474. doi: 10.3390/biology10060474
Jiang, Z., Lu, B., Zhan, B., and Lu, Q. (2014). Screening, identification and enzyme-producing conditions of strains with high keratinase yield. Guizhou Agric Sci 42, 139–141.
Jiang, C., Sun, X.-R., Feng, J., Zhu, S.-F., and Shui, W. (2022a). Metagenomic analysis reveals the different characteristics of microbial communities inside and outside the karst tiankeng. BMC Microbiol. 22:115. doi: 10.1186/s12866-022-02513-1
Jiang, C., Zhu, S., Feng, J., and Shui, W. (2022b). Slope aspect affects the soil microbial communities in karst tiankeng negative landforms. BMC ecology and evolution 22:54. doi: 10.1186/s12862-022-01986-y
Juyal, A., Otten, W., Baveye, P. C., and Eickhorst, T. (2021). Influence of soil structure on the spread of Pseudomonas fluorescens in soil at microscale. Eur. J. Soil Sci. 72, 141–153. doi: 10.1111/ejss.12975
Kaisermann, A., Maron, P. A., Beaumelle, L., and Lata, J. C. (2015). Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Appl. Soil Ecol. 86, 158–164. doi: 10.1016/j.apsoil.2014.10.009
Kang, C. S., Dunfield, P. F., and Semrau, J. D. (2019). The origin of aerobic methanotrophy within the Proteobacteria. FEMS Microbiol. Lett. 366:096. doi: 10.1093/femsle/fnz096
Kembel, S. W., Cowan, P. D., Helmus, M. R., Cornwell, W. K., Morlon, H., Ackerly, D. D., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Lan, T., Chen, Y., Huang, C., Zhang, W., Xie, L., Shi, G., et al. (2017). Community constituent of dark septate endophytic fungi in Dashiwei Doline group and their effects on Pioneer Plants'Drought resistance capability. J. Mirobiol. 37, 26–34.
Legatzki, A., Ortiz, M., Neilson, J., Dominguez, S., Andersen, G. L., Toomey, R. S., et al. (2011). Bacterial and archaeal community structure of two adjacent calcite speleothems in Kartchner caverns, Arizona, USA. Geomicrobiol J. 28, 99–117. doi: 10.1080/01490451003738465
Li, Y., Gong, J., Liu, J., Hou, W., Moroenyane, I., Liu, Y., et al. (2022). Effects of different land use types and soil depth on soil nutrients and soil bacterial communities in a karst area, Southwest China. Soil Systems 6:20. doi: 10.3390/soilsystems6010020
Li, S.-P., Wang, P., Chen, Y., Wilson, M. C., Yang, X., Ma, C., et al. (2020). Island biogeography of soil bacteria and fungi: similar patterns, but different mechanisms. ISME J. 14, 1886–1896. doi: 10.1038/s41396-020-0657-8
Lin, Y., Kong, J., Yang, L., He, Q., Su, Y., Li, J., et al. (2022). Soil bacterial and fungal community responses to Throughfall reduction in a eucalyptus plantation in southern China. Forests 13:37. doi: 10.3390/f13010037
Liu, L., Gundersen, P., Zhang, T., and Mo, J. (2012). Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 44, 31–38. doi: 10.1016/j.soilbio.2011.08.017
Liu, M., Li, X., Zhu, R., Chen, N., Ding, L., and Chen, C. (2021). Vegetation richness, species identity and soil nutrients drive the shifts in soil bacterial communities during restoration process. Environ. Microbiol. Rep. 13, 411–424. doi: 10.1111/1758-2229.12913
Long, H., Wu, X., Wang, Y., Yan, J., Guo, X., An, X., et al. (2021). Effects of revegetation on the composition and diversity of bacterial and fungal communities of sandification land soil, in southern China. Environ. Monit. Assess. 193:706. doi: 10.1007/s10661-021-09508-x
Man, B., Wang, H., Yun, Y., Xiang, X., Wang, R., Duan, Y., et al. (2018). Diversity of fungal communities in Heshang cave of Central China revealed by Mycobiome-sequencing. Front. Microbiol. 9:1400. doi: 10.3389/fmicb.2018.01400
Manzoni, S., Schaeffer, S. M., Katul, G., Porporato, A., and Schimel, J. P. (2014). A theoretical analysis of microbial eco-physiological and diffusion limitations to carbon cycling in drying soils. Soil Biol. Biochem. 73, 69–83. doi: 10.1016/j.soilbio.2014.02.008
Mondav, R., McCalley, C. K., Hodgkins, S. B., Frolking, S., Saleska, S. R., Rich, V. I., et al. (2017). Microbial network, phylogenetic diversity and community membership in the active layer across a permafrost thaw gradient. Environ. Microbiol. 19, 3201–3218. doi: 10.1111/1462-2920.13809
Mulec, J., Petric, M., Kozelj, A., Brun, C., Batagelj, E., Hladnik, A., et al. (2019). A MULTIPARAMETER analysis of environmental gradients related to hydrological conditions in a binary karst system (underground course of the PIVKA river, SLOVENIA). Acta Carsologica 48, 313–327. doi: 10.3986/ac.v48i3.7145
Nadell, C. D., Drescher, K., and Foster, K. R. (2016). Spatial structure, cooperation and competition in biofilms. Nat. Rev. Microbiol. 14, 589–600. doi: 10.1038/nrmicro.2016.84
Nilsson, R. H., Larsson, K.-H., Taylor, A. F. S., Bengtsson-Palme, J., Jeppesen, T. S., Schigel, D., et al. (2019). The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264. doi: 10.1093/nar/gky1022
Olesen, J. M., Bascompte, J., Dupont, Y. L., and Jordano, P. (2007). The modularity of pollination networks. Proc. Natl. Acad. Sci. U. S. A. 104, 19891–19896. doi: 10.1073/pnas.0706375104
Pan, Y., Cassman, N., de Hollander, M., Mendes, L. W., Korevaar, H., Geerts, R. H. E. M., et al. (2014). Impact of long-term N, P, K, and NPK fertilization on the composition and potential functions of the bacterial community in grassland soil. FEMS Microbiol. Ecol. 90, 195–205. doi: 10.1111/1574-6941.12384
Pu, G. Z., Lv, Y. N., Dong, L. N., Zhou, L. W., Huang, K. C., Zeng, D. J., et al. (2019). Profiling the bacterial diversity in a typical karst Tiankeng of China. Biomol. Ther. 9:187. doi: 10.3390/biom9050187
Pu, G. Z., Lv, Y. N., Xu, G. P., Zeng, D. J., and Huang, Y. Q. (2017). Research Progress on karst Tiankeng ecosystems. Bot. Rev. 83, 5–37. doi: 10.1007/s12229-017-9179-0
Rangseekaew, P., and Pathom-aree, W. (2019). Cave Actinobacteria as producers of bioactive metabolites. Front. Microbiol. 10:387. doi: 10.3389/fmicb.2019.00387
Rottjers, L., and Faust, K. (2018). From hairballs to hypotheses biological insights from microbial networks. Fems Microbiol. Rev. 10. doi: 10.3389/fmicb.2019.00387
Shui, W., Chen, Y., Jian, X., Jiang, C., Wang, Q., and Guo, P. (2018). Spatial pattern of plant community in original karst tiankeng: a case study of Zhanyi tiankeng in Yunnan, China. J. applied ecology 29, 1725–1735. doi: 10.13287/j.1001-9332.201806.010
Shui, W., Chen, Y., Jian, X., Jiang, C., Wang, Q., Zeng, Y., et al. (2022). Original karst tiankeng with underground virgin forest as an inaccessible refugia originated from a degraded surface flora in Yunnan, China. Sci. Rep. 12:9408. doi: 10.1038/s41598-022-13678-0
Shui, W., Chen, Y. P., Wang, Y. W., Su, Z. A., and Zhang, S. (2015). Origination, study progress and prospect of karst tiankeng research in China. Acta Geograph. Sin. 70, 431–446. doi: 10.11821/dlxb201503007
Song, M., Peng, W., Zeng, F., Du, H., Peng, Q., Xu, Q., et al. (2018). Spatial patterns and drivers of microbial taxa in a karst broadleaf Forest. Front. Microbiol. 9:1691. doi: 10.3389/fmicb.2018.01691
Stegen, J. C., Lin, X., Fredrickson, J. K., Chen, X., Kennedy, D. W., Murray, C. J., et al. (2013). Quantifying community assembly processes and identifying features that impose them. ISME J. 7, 2069–2079. doi: 10.1038/ismej.2013.93
Stegen, J. C., Lin, X., Konopka, A. E., and Fredrickson, J. K. (2012). Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 6, 1653–1664. doi: 10.1038/ismej.2012.22
Su, Y. Q., Tang, Q. M., Mo, F. Y., and Xue, Y. G. (2017). Karst tiankengs as refugia for indigenous tree flora amidst a degraded landscape in southwestern China. Sci. Rep. 7:4249. doi: 10.1038/s41598-017-04592-x
Sun, S., Li, S., Avera, B. N., Strahm, B. D., and Badgley, B. D. (2017). Soil bacterial and fungal communities show distinct recovery patterns during Forest ecosystem restoration. Appl. Environ. Microbiol. 83:17. doi: 10.1128/aem.00966-17
Vazquez-Baeza, Y., Pirrung, M., Gonzalez, A., and Knight, R. (2013). EMPeror: a tool for visualizing high-throughput microbial community data. Gigascience 2:16. doi: 10.1186/2047-217x-2-16
von Borzyskowski, L. S., Severi, F., Krueger, K., Hermann, L., Gilardet, A., Sippel, F., et al. (2019). Marine Proteobacteria metabolize glycolate via the beta-hydroxyaspartate cycle. Nature 575:500. doi: 10.1038/s41586-019-1748-4
Voriskova, J., and Baldrian, P. (2013). Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 7, 477–486. doi: 10.1038/ismej.2012.116
Wang, Y., Cheng, X., Wang, H., Zhou, J., Liu, X., and Tuovinen, O. H. (2022). The characterization of microbiome and interactions on weathered rocks in a subsurface karst cave, Central China. Front. Microbiol. 13:909494. doi: 10.3389/fmicb.2022.909494
Wang, P., Li, S.-P., Yang, X., Zhou, J., Shu, W., and Jiang, L. (2020). Mechanisms of soil bacterial and fungal community assembly differ among and within islands. Environ. Microbiol. 22, 1559–1571. doi: 10.1111/1462-2920.14864
Wang, P. C., Mo, B. T., Chen, Y., Zeng, Q. F., and Wang, L. B. (2016). Effect of karst rocky desertification on soil fungal communities in Southwest China. Genet. Mol. Res. 15:460. doi: 10.4238/gmr.15038460
Wang, H., Qi, Z., Zheng, P., Jiang, C., and Diao, X. (2022). Abundant and rare microbiota assembly and driving factors between mangrove and intertidal mudflats. Appl. Soil Ecol. 174:104438. doi: 10.1016/j.apsoil.2022.104438
Wang, J., Shen, J., Wu, Y., Tu, C., Soininen, J., Stegen, J. C., et al. (2013). Phylogenetic beta diversity in bacterial assemblages across ecosystems: deterministic versus stochastic processes. ISME J. 7, 1310–1321. doi: 10.1038/ismej.2013.30
Xiao, D., Chen, Y., He, X., Xu, Z., Bai, S. H., Zhang, W., et al. (2021). Temperature and precipitation significantly influence the interactions between arbuscular mycorrhizal fungi and diazotrophs in karst ecosystems. For. Ecol. Manag. 497:119464. doi: 10.1016/j.foreco.2021.119464
Xiao, D., He, X., Zhang, W., Hu, P., Sun, M., and Wang, K. (2022). Comparison of bacterial and fungal diversity and network connectivity in karst and non-karst forests in Southwest China. Sci. Total Environ. 822:153179. doi: 10.1016/j.scitotenv.2022.153179
Xu, S., Zhang, Y., Huang, B., and Chen, W. (2009). Comparative analysis on value of typical geological trace landscapes of Guangxi Fengshan karst National Geopark. J. Mountain Res. 27, 373–380.
Yang, T., Adams, J.M., and Shi, Y., He, J.-s., Jing, X., Chen, L., et al. (2017). Soil fungal diversity in natural grasslands of the Tibetan plateau: associations with plant diversity and productivity. New Phytol. 215, 756–765. doi: 10.1111/nph.14606
Yang, L., Barnard, R., Kuzyakov, Y., and Tian, J. (2021). Bacterial communities drive the resistance of soil multifunctionality to land-use change in karst soils. Eur. J. Soil Biol. 104:103313. doi: 10.1016/j.ejsobi.2021.103313
Yang, G., Peng, C. H., Liu, Y. Z., and Dong, F. Q. (2019). Tiankeng: an ideal place for climate warming research on forest ecosystems. Environ. Earth Sci. 78. doi: 10.1007/s12665-018-8033-y
Yang, S., Yang, T., Lin, B., Liu, X., and Xiang, M. (2018). Isolation and evaluation of two phosphate-dissolving fungi. Acta Microbiol Sin. 58, 264–273.
Yuan, D. (2005). The development of Morden karstology in China. Sci. Foundation in China 19, 139–141.
Yuan, M. M., Guo, X., Wu, L., Zhang, Y., Xiao, N., Ning, D., et al. (2021). Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 11, 343–348. doi: 10.1038/s41558-021-00989-9
Zhang, Z. F., Liu, F., Zhou, X., Liu, X. Z., Liu, S. J., and Cai, L. (2017). Culturable mycobiota from karst caves in China, with descriptions of 20 new species. Persoonia 39, 1–31. doi: 10.3767/persoonia.2017.39.01
Zhou, J., Deng, Y., Luo, F., He, Z., Tu, Q., and Zhi, X. (2010). Functional molecular ecological networks. MBio 1:10. doi: 10.1128/mBio.00169-10
Zhou, J., Deng, Y., Luo, F., He, Z., and Yang, Y. (2011). Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. MBio 2:11. doi: 10.1128/mBio.00122-11
Keywords: unique habitat, co-occurrence network, amplicon sequencing, karst tiankeng, microbiome
Citation: Jiang C, Liu Y, Li H, Zhu S, Sun X, Wu K and Shui W (2022) The characterization of microbial communities and associations in karst tiankeng. Front. Microbiol. 13:1002198. doi: 10.3389/fmicb.2022.1002198
Received: 24 July 2022; Accepted: 01 September 2022;
Published: 19 October 2022.
Edited by:
Xiangyu Guan, China University of Geosciences, ChinaReviewed by:
J. D. Lewis, Fordham University, United StatesCopyright © 2022 Jiang, Liu, Li, Zhu, Sun, Wu and Shui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Shui, c2h1aXdlaUBmenUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.