
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 24 December 2021
Sec. Food Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.812536
This article is part of the Research TopicProbiotic bacteria-derived effector molecules and their impact on the host in health and diseaseView all 16 articles
Probiotics for food or supplement use have been studied in numerous clinical trials, addressing a broad variety of diseases, and conditions. However, discrepancies were observed in the clinical outcomes stemming from the use of lactobacillaceae and bifidobacteria strains. These differences are often attributed to variations in the clinical trial protocol like trial design, included target population, probiotic dosage, or outcome parameters measured. However, a contribution of the methods used to produce the live bioactive ingredients should not be neglected as a possible additional factor in the observed clinical outcome variations. It is well established that manufacturing conditions play a role in determining the survival and viability of probiotics, but much less is known about their influence on the probiotic molecular composition and functionality. In this review, we briefly summarize the evidence obtained for Lacticaseibacillus rhamnosus GG and Lactiplantibacillus plantarum WCFS1, highlighting that expression and presence of probiotic niche factor (NF) and/or effector molecules (EM) may be altered during production of those two well-characterized lactobacillaceae probiotic strains. Subsequently, we summarize in more depth what is the present state of knowledge about bifidobacterial probiotic NF and EM; how their expression may be modified by manufacturing related environmental factors and how that may affect their biological activity in the host. This review highlights the importance of gathering knowledge on probiotic NF and EM, to validate them as surrogate markers of probiotic functionality. We further propose that monitoring of validated NF and/or EM during production and/or in the final preparation could complement viable count assessments that are currently applied in industry. Overall, we suggest that implementation of molecular level quality controls (i.e., based on validated NF and EM), could provide mode of action based in vitro tests contributing to better control the health-promoting reliability of probiotic products.
Initially formulated by the World Health Organization in 2002 (Joint Fao/Who Working Group Report on Drafting, 2002) and slightly corrected by experts in the field in 2014 (Hill et al., 2014), probiotics are today defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit to the host.” Overall, probiotic bacteria for food or supplement use (mainly lactobacillaceae and bifidobacteria) have been studied in a large number of clinical trials, targeting a wide array of diseases and conditions (Dronkers et al., 2020).
Two distinguishable classes of health benefits are attributed to probiotics: a “general” class of effects that groups beneficial effects exerted by various well-studied microbial species; and a “strain-specific” class of effects that are expected to be driven by specific probiotics strains. An expert panel convened in 2013 has acknowledged those two classes, concluding that “general” benefits such as “creating a more favorable gut environment” and “supporting a healthy digestive tract” (regrouping a diversity of clinical end points such as diarrhea, antibiotic-associated diarrhea (AAD), gut transit, abdominal pain, bloating, and necrotizing enterocolitis) are displayed by a large number of probiotic strains representing various commonly studied species. The mechanisms of action supporting those “general” probiotic beneficial effects (e.g., probiotic and/or microbiome mediated SCFA production, regulation of intestinal transit, competitive exclusion of pathogens) are similarly believed to be shared by a large number, if not all, of the probiotic strains (Hill et al., 2014). Furthermore, “general” benefits (e.g., AAD prevention) provided by the commonly used Lacticaseibacillus rhamnosus GG strain have be shown to be relatively consistent throughout different clinical trials in children (Szajewska and Hojsak, 2020).
In contrast, “strain-specific” benefits are defined as effects that are likely exerted by a limited number of strains, such as “prevention of allergic disease,” “downregulation of inflammation,” “enhancement of anti-infection activities,” or “support of specific organs health” (e.g., reproductive tract, lungs) (Hill et al., 2014). Those beneficial effects are believed to be driven by specific molecules present within or at the surface of the probiotic bacterial cells (Lebeer et al., 2008; Remus et al., 2012; Lee et al., 2013). In the last decade, it was shown that a range of molecules produced by probiotic contribute to their robustness and stress tolerance, supporting their survival and establishment when they transit through the gastro-intestinal tract (i.e., so-called niche factors; NF). In addition, various probiotic effector molecules (EM) have been identified to drive in situ host-microbe interactions, thus determining the specific health benefit of different probiotic strains (Lebeer et al., 2018). Disentangling the NF or EM role of specific probiotic molecules is not trivial, especially when adhesive-like phenotypes are affected. Adhesion to the host cells or to the intestinal mucus can be regarded as a factor promoting the bacterial colonization but could as well contribute to the exposure of different structures present on the bacterial cell envelope.
Variability in health effects is not uncommonly observed in clinical trials with probiotic for food or supplement use (Osborn and Sinn, 2007; Johnston et al., 2011; Guo et al., 2019). The inconsistency in results is usually attributed to variations in the design of the clinical studies, including differences in dosage of the probiotic, selection of different target population (i.e., inclusion and exclusion factors at enrollment), powering of the studies according to the primary and secondary objectives, duration of the studies, probiotic delivery format and schedule, data collection, and further analysis performed. Indeed, these factors have been suggested to explain part of the discrepancies observed in the reported clinical health-outcomes (Forssten et al., 2020). Furthermore, probiotics need to exert their effects in the complex microbiome. Inter-individual microbiota variability represent hence a challenge in ensuring probiotic effect consistency in different populations, and new stratification as well as personalized nutrition approaches have been recently proposed to improve the situation (Veiga et al., 2020). Moreover, the way the probiotic strains themselves are produced and formulated is often not well-described in clinical trial studies, while it could play an important role in the health-promoting efficacy that a product elicits. This is well illustrated by the discrepancies observed in randomized clinical trials using L. rhamnosus GG targeting the prevention of allergic disease, which are summarized by Segers and Lebeer (2014). Initially, Kalliomaki et al. (2001, 2007) showed in a landmark study that L. rhamnosus GG treatment significantly lowered the risk of eczema in young children belonging to families with a history of atopic disease. However, in a subsequent attempt to reproduce this Finnish study protocol, Kopp et al. (2008) failed to detect similar beneficial effects in a German cohort. Population (Finnish vs. Germans) and dosage differences (2E10 vs. 1E10 CFU daily) are potential confounding factor in those two studies, but it is important to note that the source (and possibly the manufacturing process) of L. rhamnosus GG in the studies by Kalliomaki et al. (2001, 2007) and Kopp et al. (2008) was different, which deserves attention because it may have as well contributed to the differences in clinical outcomes (Tripathi et al., 2012). At present, probiotic manufacturing procedures remain largely unexplored as a potential source of variation in probiotic clinical trials outcomes and hence deserves to be studied in more details (Sanders et al., 2014; Brussow, 2019), especially in the light of the increasing knowledge about specific NF and EM that play a role in the efficacy of intestinal delivery and health promotion following consumption of the product.
Production of dried probiotic supplements consists generally of (a) a series of fermentations of different scale where bacterial biomass is produced using specific media and growth conditions, (b) a centrifugation step to remove the culture supernatant and concentrate the biomass, (c) a mixing step where protectants are added, followed by (d) a drying step (Fenster et al., 2019). Throughout these manufacturing stages, probiotics encounter a range of different stress conditions, including variations in temperature, acid exposure, osmotic and oxidative stress, all of which can modulate their physiology and molecular composition. These modulations may impact their survival during manufacturing as well as their fitness during gastrointestinal tract transit (Corcoran et al., 2008; Gaucher et al., 2019). We suggest that not only the expression of NF (e.g., proteins that contribute to robustness and stress tolerance) can be affected by the production conditions, but also effector molecule expression levels may differ, leading to variable presence of molecules that have been shown to mediate the health-benefit elicited by the strain. The required presence of NF and/or EM is further supported by the fact that probiotic cells rendered metabolically inactive by the mean of heat-treatment can still elicit beneficial health effects (Pique et al., 2019), highlighting the potential limitation of using live cells enumeration alone to ascertain efficacy of probiotic preparations. Therefore, monitoring the expression of validated NF as well as EM during probiotic manufacturing may enable better control of product properties at a molecular level, which goes beyond the traditionally used colony forming units (CFU), and could contribute to an increased robustness of clinical outcomes (Figure 1).
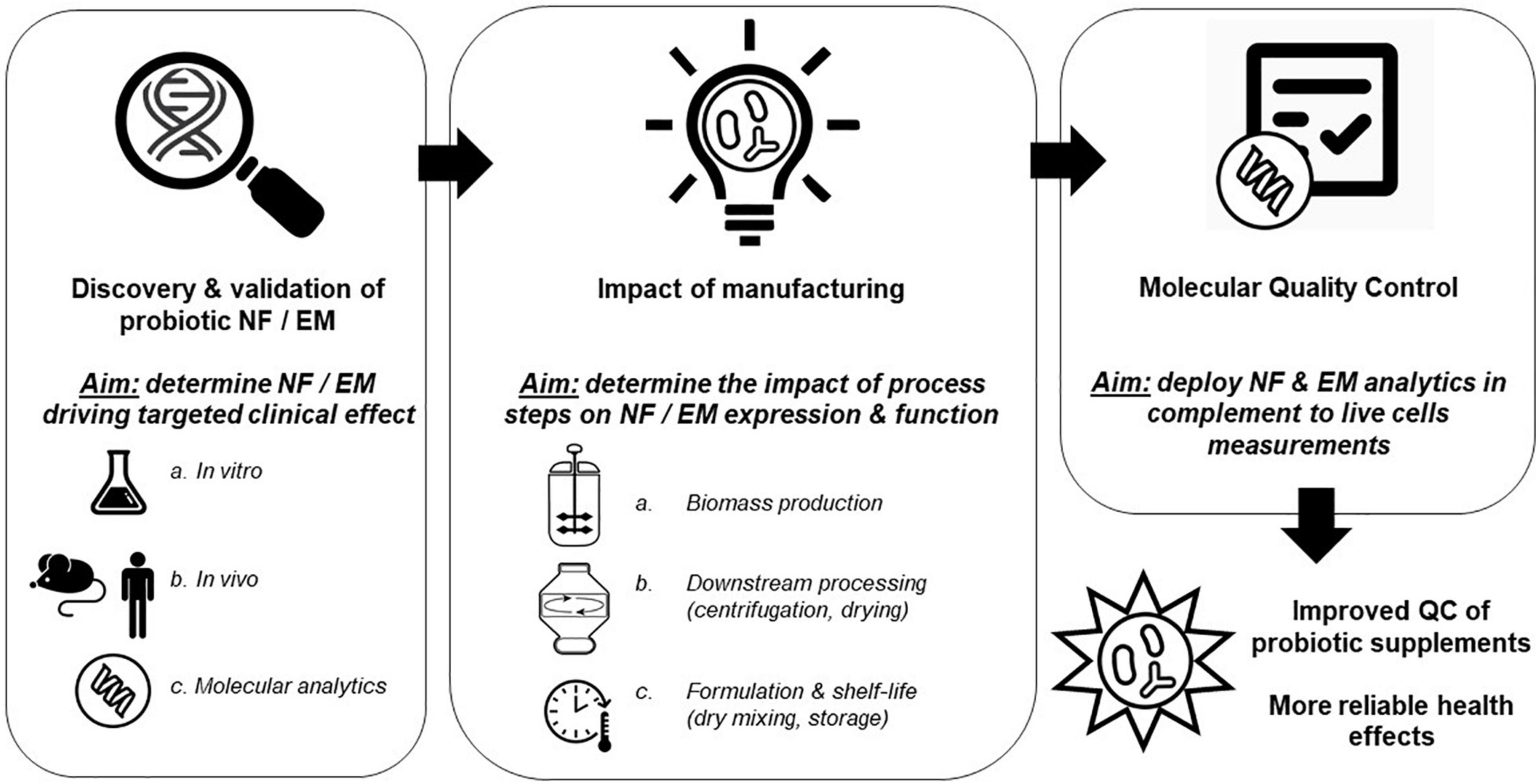
Figure 1. Stepwise approach leveraging probiotic niche factors (NF) and effector molecules (EM) knowledge to improve probiotic supplements consistency. As a first step, a robust link between the presence of probiotic NF or EM and the desired clinical outcome needs to be established. This step will also allow the development of sets of molecular analytics that can be used in the subsequent steps. Then, the different manufacturing steps need to be evaluated to understand their contribution to NF or EM expression and function. Finally, the NF/EM molecular analytics developed earlier can be used as quality control (QC), complementing traditional CFU/live cells measurements, which overall should enable production of probiotic supplements with increased consistency in their attributed health benefits.
In this review, we first briefly highlight that expression and presence of probiotic EM may be altered during production using two well-characterized examples among the lactobacillaceae probiotic, i.e., Lacticaseibacillus rhamnosus GG and Lactiplantibacillus plantarum WCFS1 (Figure 2). Subsequently, we focus with more depth on bifidobacteria and their probiotic NF and EM, summarizing what is the present state of knowledge about bifidobacterial NF and EM; how their expression may be modified by manufacturing related environmental factors and how that may affect their biological activity in the host (Table 1). Overall, this review highlights the importance of gathering knowledge on probiotic NF and EM, to validate them as surrogate markers of probiotic functionality. We further hypothesize that understanding the dynamics of these molecules during production could contribute to better control the health-promoting reliability of dried probiotic products. Hence, NF/EM based in vitro assays represent molecular-level quality controls that adds to the limited information coming from viable count assessments that are currently applied in industry and research.
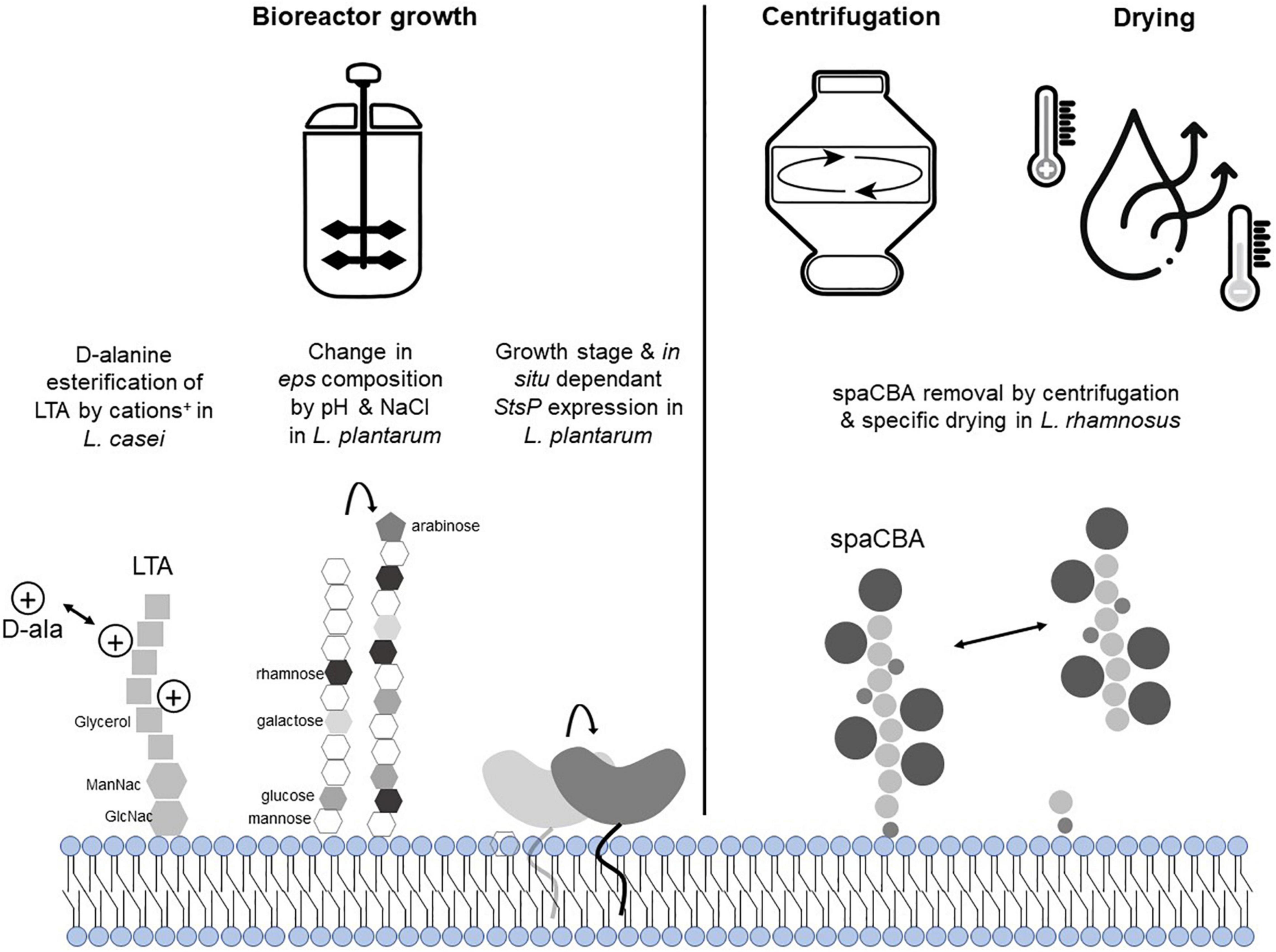
Figure 2. Overview of potential effects of manufacturing on lactobacillacae NF and EM. L. rhamnosus GG spaCBA pili has been shown to be at least partially removed by centrifugation (8,000 g, 30 min) or spray-drying. StsP has been shown to be predominantly produced in the stationary growth phase of L. plantarum WCFS1, and is regulated in response to intestinal conditions (Bron et al., 2004; Marco et al., 2010; Remus et al., 2012). In analogy to what has been shown in L. casei and L. plantarum, it can be proposed that lipoteichoic acid (LTA) and exopolysaccharides (EPS) structures of L. rhamnosus GG can be modified by cations and/or other positively charged compounds.
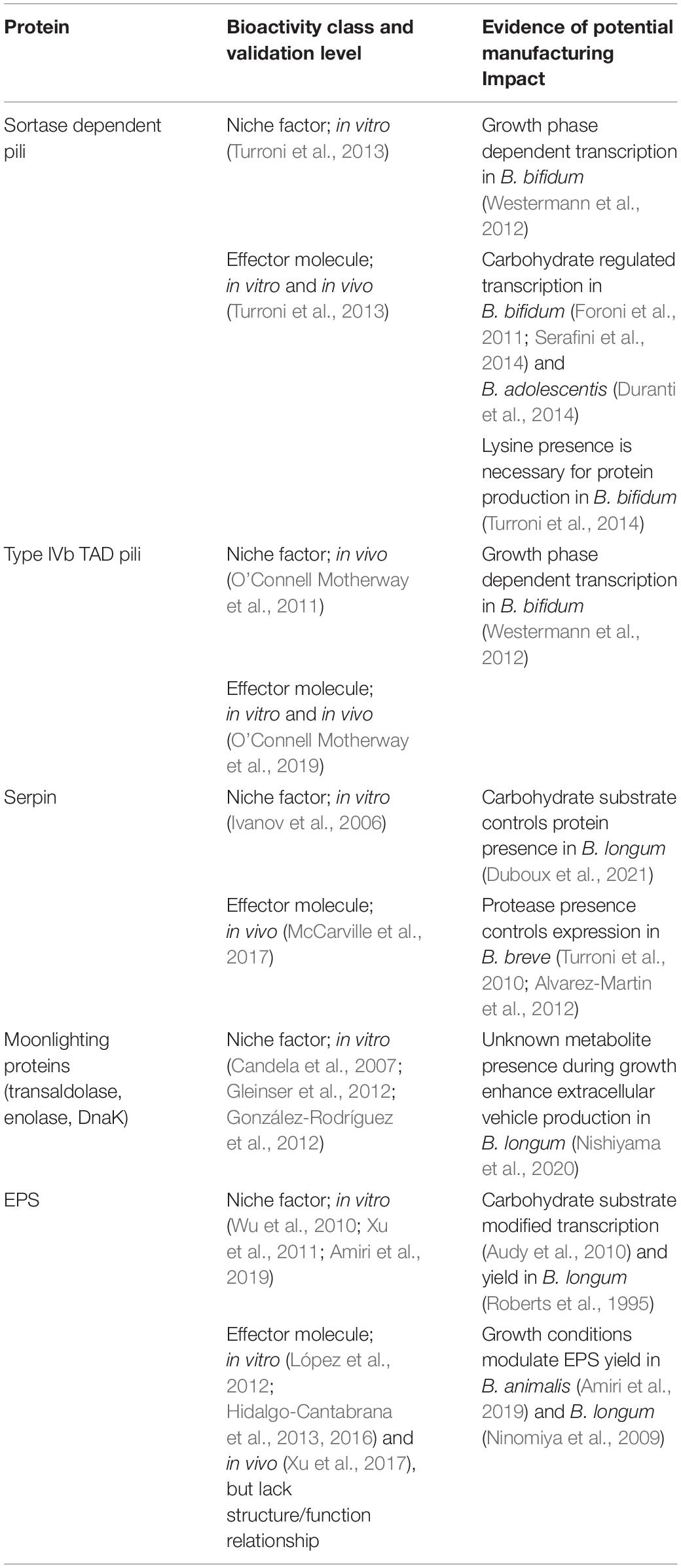
Table 1. Summary of known bifidobacterial NF and EM, their validation level and related evidence supporting an effect of manufacturing.
L. rhamnosus GG is among the probiotic strains with the best described set of EM, several of which play diverse roles in the probiotic activity of this strain as assessed mainly in preclinical models. Those include the major secreted proteins p40 and p75 that prevent cytokine-induced inflammatory damage, lipoteichoic acid (LTA) that negatively modulate colitis, CpG-rich DNA motifs that dampen allergen-specific IgE, and exopoly-saccharides (EPS) that reduce adipogenesis in high-fat-diet fed mice (Lebeer et al., 2018). L. rhamnosus GG spaCBA encoded sortase-dependent pilin anchored at the surface of the bacteria are important NF as they are involved in the mucus and intestinal epithelium adhesion capacity of the strain. However, they were also shown in vitro to act as EM as they contribute to the immunomodulatory capacities of the strain when incubated with monocytes and dentritic cells (Lebeer et al., 2012; Vargas Garcia et al., 2015), and stimulate cell proliferation that protects against radiologically induced intestinal epithelial damage (Ardita et al., 2014). Nevertheless, the regulation of expression and functional properties of the pili as well as the other EM during manufacturing of L. rhamnosus GG remains largely unexplored.
For example, the regulation of the genes encoding p40 and p75 remains unknown. Additionally, these bioactive molecules are derived from cell wall associated muramidases and are at least partially secreted in the culture supernatant (Yan et al., 2013), which is usually removed during dried probiotic manufacturing (Fenster et al., 2019), raising doubts about their functional availability in supplement products. Similarly, although the role of specific genes involved in LTA biosynthesis in lactobacilli (including L. rhamnosus GG) has been studied (Debabov et al., 1996, 2000), their regulation by environmental conditions remains largely unknown. For example, Dlt mediated LTA D-alanylation has been long recognized as an important modulator of the host-effects elicited by LTA (Claes et al., 2012), but we do not know whether dlt expression is regulated by environmental conditions in L. rhamnosus GG. Notably, it has been demonstrated that the dlt gene of Staphylococcus aureus, is regulated by cations levels in the medium [Na+, Mg(2+), Ca(2+)] (Koprivnjak et al., 2006), and in L. casei (a close relative of L. rhamnosus) is regulated by the presence of charged molecules like antimicrobial peptides (Revilla-Guarinos et al., 2013). These findings suggest that dlt regulation in lactobacilli may be coordinated similarly to what was observed in S. aureus, and that the concentrations of positively charged components in the growth medium may affect D-alanylation of LTA. A specific galactose-rich exopolysaccharide in L. rhamnosus GG has been previously identified to play a role in the adhesion capacity of the strain (Lebeer et al., 2009). Although regulation of the production of this EPS in L. rhamnosus GG has not been studied in detail, recent studies in L. plantarum VAL6 indicated that expression of eps genes eliciting structural changes of the polysaccharides produced in this species is regulated by pH and sodium chloride induced stress (Nguyen et al., 2021), conditions that may occur during industrial growth. Importantly, manufacturing was demonstrated to influence the presence of the SpaCBA pili at L. rhamnosus GG’s surface and could thus affect the presence and function of this important niche factor and effector molecule in preparations of this strain. It has been shown that centrifugation at 8,000 g for 30 min was sufficient to break and separate the pili from the surface of the bacteria (Tripathi et al., 2012), while a specific type of drying (spray-drying without addition of any protectants, which is not a common manufacturing practice) diminished the adherence capacity of L. rhamnosus GG correlated with the disappearance of the SpaCBA pili (Kiekens et al., 2019; Figure 2). It is not known today if the presence of this importance protein structure can be influenced by other types of drying. However, freeze-drying was shown to decrease the adherence capacity of L. rhamnosus GG, while it did not exert the same effect on L. casei Shirota (du Toit et al., 2013). Moreover, besides the physical presence or absence of the pili structure in preparations of this strain, the genetic region encoding the SpaCBA pili was shown to be relatively unstable (Sybesma et al., 2013), which may also contribute to variations in in vivo behavior of L. rhamnosus GG isolates originating from different products (Grzeskowiak et al., 2011). Altogether, these lines of evidence indicate that upstream (e.g., fermentation conditions) or downstream processing conditions (e.g., centrifugation, type of drying) can play a role in the presence and bioavailability of NF as well as EM of L. rhamnosus GG. In vivo demonstration of the impact of those processing induced modifications has not yet been pursued, but we hypothesize that they may have contributed to the different outcomes obtained in clinical trials like those reported by Kalliomaki et al. (2001, 2007) and Kopp et al. (2008).
To the best of our knowledge, the only substantiated example demonstrating that production parameters (i.e., growth phase harvesting) can influence the way a probiotic can interact with the human body has been obtained with the well-characterized L. plantarum WCFS1 strain. Freeze-dried preparations of heat-killed or live L. plantarum WCFS1 were administered to healthy adults. In addition, the live preparations consisted of cells harvested during mid-logarithmic or during the stationary phase of growth. Following consumption of these distinct preparations of the same strain, duodenal tissue biopsies were analyzed by array-based transcriptomics, revealing that both live and dead (heat treated) stationary phase harvested bacterial preparations were able to modulate Nfκ-B responses in human duodenum mucosal tissues, which were interpreted to play an important role in the establishment of immune tolerance. Conversely, the bacteria harvested mid-exponentially failed to induce such responses, but modified the expression of human genes involved in immune-suppressing activities such as BCL3, Iκ-B, and ADM, as well as several functions involved in cell-cycle and metabolic regulation (van Baarlen et al., 2009). As a follow-up, it was found that the lp_0800 gene, coding for a serine- and threonine-rich surface protein (StsP) that is anchored to the peptidoglycan by sortase was shown to be expressed predominantly during the stationary growth phase, albeit at low levels during growth under laboratory conditions. Notably, previous studies of L. plantarum had established that the expression of lp_0800 was in situ induced during the transit through the murine and human intestinal tract, supporting that specific environmental conditions can modulate its expression (Bron et al., 2004; Marco et al., 2007, 2010). Importantly, using isogenic L. plantarum WCFS1 lacking or overexpressing StsP, it was shown that StsP surface derived peptides obtained by whole-cell trypsin-shaving could strongly inhibit flagellin induced Nfκ-B activation in a CaCo-2-derived reporter cell line. Finally, gel-purified StsP protein derived tryptic peptides potently suppressed NFkB activation, unambiguously pinpointing this activity to peptides derived from this surface protein (Remus et al., 2012). These findings demonstrate that the growth phase as well as specific growth conditions (i.e., gut-like conditions) of L. plantarum WCFS1 can influence the expression level or bioavailability of the important immunomodulatory StsP, which was proposed to play a prominent role in the clinically observed duodenal transcriptional responses (Figure 2).
Of note, the host responses were determined in the duodenum of the participating volunteers. In fact, upon ingestion, the relatively short transit time to reach the duodenal mucosa likely allows a limited molecular adaptation of the probiotic bacteria. At this moment it is unclear whether similar transcriptome response differences would be observed in the colonic mucosa when applying these distinct L. plantarum WCFS1 preparations. One the one hand, the different molecular make-up of the preparations may change during gastrointestinal transit, and on the other hand it is known that stsP expression is induced in the intestinal tract. We hence hypothesize that ensuring NF and EM presence and function in probiotic products may be especially relevant when the probiotic is expected to elicit its health benefit in the proximal regions of the intestine.
Overall, the L. plantarum WCFS1 example strongly indicates that upstream processing (e.g., harvesting time) can impact probiotic bioactivities in vivo, and underlines the importance of harvesting probiotic cells at stationary phase, which is today a common practice in industry. Moreover, it supports that quantification of StsP in preparations of L. plantarum WCFS1 could serve as a molecular quality control parameter to complement the traditionally used CFU enumeration.
Similar to probiotics belonging to the Lactobacillaceae family, we propose that manufacturing procedures used for bifidobacteria should be investigated for their possible contribution to the discrepancies observed in clinical trial outcomes (Szajewska and Hojsak, 2020). To date, there are only few studies focusing on the effect of manufacturing on bifidobacteria bioactivities and most focused on the potential impact of downstream processing (i.e., drying). Moreover, the available studies did not include an assessment of specific effector molecule presence and bioavailability but were mostly driven by functional assays. For example, Laconelli et al. (2015) showed that different drying procedures (air-, freeze-, spray-drying) impacted on the anti-inflammatory properties of B. bifidum, as determined by cytokine production profiling in Peripheral Blood Mononuclear Cells (PBMC) following co-incubation with the differently processed bacterial preparations of the same strain. In addition, this study demonstrated that different down-stream process may affect the hydrophobicity of the strain, which is indicative of changes in cell-surface properties (Laconelli et al., 2015). Conversely, similar analyses showed that spray-drying did not alter the immunomodulatory potential of two B. animalis subsp. lactis strains (INL1 and BB12), nor did it modify their preventive effect on colitis in vivo (Burns et al., 2017). Similarly, although freeze-drying was proposed to enhance the adherence capacity of B. animalis subsp. lactis BB12, increasing its capacity to outcompete C. difficile in vitro (du Toit et al., 2013), these effects were not observed for other strains of B. animalis subsp. lactis (Charnchai et al., 2016). These studies illustrate the rather limited information concerning the potential impact of manufacturing on bifidobacteria bioactivity, and highlight the contradictory findings described to date on the potential role of downstream processing (e.g., drying) in influencing Bifidobacterium probiotic functionality. However, these studies mostly addressed the consequences of different downstream-processing conditions (i.e., drying procedures) on in vitro outcomes (O’Connell Motherway et al., 2011; Westermann et al., 2016), whereas the upstream processing (e.g., fermentation parameters) effects on the expression of NF and/or EM in these bacteria remain to be deciphered.
Two types of pili have been described in bifidobacteria to act as NF and EM, the sortase dependant pili and the Type IV TAD pili. Sortase dependent pili gene clusters consisting of major (fimA or fimP) and minor (fimB or fimQ) subunit structural proteins are widely distributed amongst Bifidobacterium species (Foroni et al., 2011). However, their genetic distribution among strains and species within this genus appears quite disperse. For example, B. adolescentis contains five distinct pili encoding gene clusters, while other bifidobacteria, like B. bifidum, contains “only” three of these clusters. Out of the three pili gene clusters found in B. bifidum PRL2010, only pil2 and pil3 were found to be functional, and pil1 being disrupted by a frameshift (Foroni et al., 2011; Turroni et al., 2013; Duranti et al., 2014). Importantly, and analogous to what was shown for L. rhamnosus GG, the sortase dependent pili of B. bifidum PRL2010 were demonstrated to play a role in both adhesive and anti-inflammatory properties of the strain using recombinant L. lactis harboring the pil2 or pil3 gene clusters. While Pil2 was shown to act as a NF and mediated binding to extracellular matrix, Pil3 was also able to modify both in vitro and in vivo inflammatory responses (Turroni et al., 2013).
Similarly to the sortase dependent pili, type IVb TAD pili are also conserved and widely distributed in both gram positive and gram negative bacteria (Pu et al., 2018), and has been identified in multiple B. breve and B. bifidum strains (O’Connell Motherway et al., 2011; Westermann et al., 2012). It was demonstrated that the Type IVb TAD pili of B. breve UCC2003 act as NF, as disruption of the ATPase encoding gene tadA2003, which is essential for its assembly, resulted in a decreased capacity of the strain to colonize the mouse intestine (O’Connell Motherway et al., 2011). Additionally, using a set of recombinant B. breve UCC 2003 strains it was shown that the same pilin structure (and particularly its TadE pilin subunit) could contribute to the maturation of the naïve gut, since it promoted epithelial proliferation both in vitro and in vivo (O’Connell Motherway et al., 2019), demonstrating its additional EM role.
Limited information is available about the regulation of production of the various pili that are encoded by bifidobacteria. In B. bifidum S17 it was demonstrated that sortase-dependent pili encoded by the pil2 and pil3 clusters were higher expressed during exponential growth as compared to the stationary phase of growth (Westermann et al., 2012). In another strain of the same species, B. bifidum PRL2010, the pil2 and pil3 clusters were transcribed both during growth in laboratory medium (MRS) as well as in the mouse cecum, whereas transcription of the pil1 cluster could not be detected under either of these conditions (Turroni et al., 2013). Culturing of this strain in bovine milk led to activation transcription of the pil1 cluster genes, indicating that growth (i.e., production) conditions can influence the repertoire of pilin produced by B. bifidum PRL2010. This observation was expanded by demonstrating that the other pil clusters in this strain were subject to substrate regulation, which is exemplified by the induction of transcription of the pil2 cluster during growth on fructo-oligosaccharides (FOS) and the induction of the pil3 cluster during both growth on bovine milk or polysaccharides derived from kefir (Foroni et al., 2011; Serafini et al., 2014). Analogously, the expression of pil gene clusters was also regulated by the carbon source used for growth in B. adolescentis 22L, where maltodextrin or cellobiose as substrates for growth resulted in an increase of gene expression (compared to growth on glucose) for pil3, pil4, and pil5, which coincided with increased adhesion of the strain to laminin, fibrinogen, and fibronectin, albeit that direct relatedness of these observations remains to be established (Duranti et al., 2014). Besides the carbon source for growth, also the available nitrogen source in the medium has been reported to control pilin expression. For example, the presence of lysine in the growth medium appeared to be essential for Pil2 and Pil3 production by B. bifidum PRL2010 (Turroni et al., 2014).
In both B. breve and B. bifidum, part of the genes encoding the Type IVb TAD pili were found to be expressed during standard growth conditions in the laboratory (O’Connell Motherway et al., 2011; Westermann et al., 2012). However, the transcriptional levels of the Type IVb TAD pilus encoding genes in B. breve were strongly induced (25–62-fold) when the bacteria were inhabiting the murine intestinal tract. This was further supported by immunogold staining demonstrating that the pili structures could only be observed when the strain was harvested from the murine gut (O’Connell Motherway et al., 2011). Even though the Type IVb TAD pili protein presence was not assessed in laboratory-grown (MRS) B. bifidum S17, the encoding genes (tadZ, tadA, and tadB) were expressed in a growth phase dependent manner, with higher transcriptional levels in the exponential phase compared to the stationary phase (Westermann et al., 2012).
Although, the specific environmental factors that regulate pili production of specific bifidobacterial pili are quite diverse (e.g., carbon source, nitrogen source, “intestinal conditions,” etc.) and/or remain unknown, it is clear from the observations presented above that pilin expression by Bifidobacterium probiotics may be modulated by the growth conditions (e.g., substrate) employed during production. In addition, more work deserves to be pursued to decipher the role of downstream processing, as analogous to what has been described for the pili of L. rhamnosus GG, we can hypothesis that the presence of the pili on the cell-surface of the bifidobacteria may be impacted by drying procedures.
The serine protease inhibitor (serpin) of pancreatic and neutrophilic elastases was has initially described in B. longum NCC 2705 (Ivanov et al., 2006). This protein was shown to be conserved in a broad range of bifidobacteria and has been proposed to protect them against host produced proteases, thus providing them with a survival and colonization advantage (Ivanov et al., 2006; Turroni et al., 2010). The serpin’s capacity to inhibit the Human Neutrophil Elastase (Ivanov et al., 2006) may also be involved in the immunomodulatory capacities of the strain (Riedel et al., 2006) as elastase is released by activated neutrophils at the sites of intestinal inflammation in the gastro-intestinal tract (Burg and Pillinger, 2001). In line with this role in dampening innate immunity, serpin was demonstrated to play a key role in the anti-inflammatory effect of B. longum NCC 2705 in a mouse model of gluten sensitivity (McCarville et al., 2017). In addition, it was recently reported that the serpin of the NCC 2705 strain prevented enteric nerve activation in vitro, which was proposed to potentially play a role in pain reduction in Irritable Bowel Syndrome (IBS) patients (Buhner et al., 2018). These findings indicate that analogous to the bifidobacterial pili, the role of the serpin is dualistic in the sense that it acts as both NF and EM.
Transcriptional regulation studies of the B. breve UCC2003 serpin-encoding gene showed that it involves a protease inducible two-component system encoded directly adjacent to the serpin encoding operon, which was shown to activate serpin production upon exposure of the strain to proteases (e.g., papain) (Alvarez-Martin et al., 2012). However, a similar two-component system appears to be absent in B. longum subsp. longum strains (including NCC 2705), and variable gene-syntenies encountered in the serpin encoded region in different bifidobacterial (sub-)species suggests that serpin regulation may involve (sub-)species specific mechanisms (Turroni et al., 2010). This notion is further confirmed by our recent study that demonstrated that in B. longum subsp. longum, serpin production is regulated by the carbohydrate-substrates used for growth, revealing galactose and fructose (or galacto- or fructo- di/oligo-saccharides) as inducing substrates, while the presence of glucose repressed serpin production almost completely (Duboux et al., 2021). These studies illustrate the diverse environmental factors and regulatory mechanisms involved in controlling serpin production in Bifidobacterium (sub-) species, indicating that growth conditions (e.g., substrate) could be tailored to ensure, or even enhance the production and function of this NF and/or EM in the final probiotic preparation.
Exopolysaccharides (EPS) are extracellular carbohydrates polymers synthesized by a vast variety of micro-organisms, including gram positive bacteria. In bifidobacteria, EPS can be covalently or non-covalently bound to the cell surface (sometimes referred to as capsular polysaccharides; CPS), or can be predominantly secreted. The EPS produced by bifidobacteria are heteropolysaccharides (HePS) that have been reported to vary in molecular weight (between 4.9 × 103 and 3 × 106 Da) (Leivers et al., 2011) and monosaccharide composition and linkage (Hidalgo-Cantabrana et al., 2012). The synthesis of HePS by Bifidobacterium strains involves gene clusters (eps clusters) and biosynthesis mechanisms that are similar to those described for other microbes, involving a membrane associated synthesis machinery that utilizes cytoplasmic sugar nucleotides as building blocks for the assembly of repeating units that are exported and polymerized to form the HePS (Altmann et al., 2016; Schiavi et al., 2016; Castro-Bravo et al., 2017). Most of the bifidobacterial HePS are predominantly composed of D-galactose and D-glucose, but can also contain other monosaccharides like L-rhamnose, D-mannose, L-arabinose, or D-fructose in ratios that can vary between species and likely also between strains of the same species (Xu et al., 2011; Amiri et al., 2019). This notion is supported by the fact that eps gene clusters are highly variable between different species and strains of bifidobacteria (Hidalgo-Cantabrana et al., 2014; Altmann et al., 2016; Schiavi et al., 2016; Castro-Bravo et al., 2017).
Purified EPS produced by B. longum BCRC14634 showed an anti-microbial effect on four pathogenic and three food spoiling bacteria (Wu et al., 2010), which could provide a competitive advantage to the strain in the complex gut ecosystem, supporting its role as NF. In addition, bifidobacterial EPS has also been proposed to affect host responses, suggesting that these molecules may also act as EM in bifidobacteria. Firstly, EPS produced by two B. animalis strains (B. animalis RH and B. animalis subsp. lactis BB12) was shown to possess anti-oxidant capacities in vitro (Xu et al., 2011; Amiri et al., 2019), which could be relevant in order to alleviate intestinal oxidative damages. Then, the EPS produced by different B. longum and B. animalis strains has been proposed to modulate the immune response of the host based on the role of these molecules in inducing immune cell proliferation and modulating cytokine production in peripheral blood mononuclear cells (PBMCs) (López et al., 2012; Xu et al., 2017). Notably, the immunomodulatory effect of EPS observed in this study was shown to be strain specific, which was exemplified by the finding that out of eight strains of B. longum and B. animalis tested, only the EPS produced by B. animalis A1 and B. longum NB667 elicited a significant increase of PBMC proliferation (López et al., 2012). The immunomodulatory capacities of specific bifidobacterial EPS molecules has been reported to be quite diverse, including reports on the induction of pro-inflammatory profiles in vitro (López et al., 2012; Hidalgo-Cantabrana et al., 2016) or in vivo (Xu et al., 2017) but also cases where anti-inflammatory responses were detected (Wu et al., 2010; Schiavi et al., 2016). However, these studies provided very limited information on the physical and chemical characteristics or the monosaccharide composition of the EPS molecules produced by these different bifidobacterial strains, leaving the relationships of EPS structure and its immunomodulatory function unaddressed. A study by Hidalgo-Cantabrana et al. (2013) partly elucidated how EPS characteristics might affect its biological activity. In this study they used B. animalis subsp. lactis A1 and two mutant derivatives (A1dO and A1dOxR) that produce EPS with distinct monosaccharide composition and molecular size characteristics compared to the wild-type strain. Strain A1dOxR harbored a mutation in the Balat_1410 tyrosine kinase encoding gene (Hidalgo-Cantabrana et al., 2015) and expressed the enzyme dTDP-glucose 4,6-dehydratase that catalyzes the production of dTDP-rhamnose at an elevated level, leading to the production of a high molecular weight, rhamnose-rich EPS. The changes in polymer length and monosaccharide composition in this mutant strain were associated with increased production of the anti-inflammatory cytokine IL-10 by PBMCs exposed to the A1dOxR strain relative to its parental strain (Hidalgo-Cantabrana et al., 2013). To the best of our knowledge this is one of the few studies where the EPS structure-function relationship is investigated in the context of immunomodulatory capacities, illustrating that there is a large gap in our mechanistic understanding of the postulated role of EPS as EM in bifidobacteria.
Several studies have demonstrated that growth conditions modulate both the level of production as well as the structural properties of EPS produced by bifidobacteria. As an example, the carbon substrate applied during growth influences the expression of eps related genes in B. longum CRC002 (Audy et al., 2010), which is potentially influencing the EPS produced by the strain. Indeed, the carbon source used for growth of B. longum BB79 affected the level of EPS produced, with lactose leading to the highest level of production when compared to glucose, fructose, or sucrose (Roberts et al., 1995). Besides the influence of the carbon source, differences in concentration of yeast extract, growth temperature and incubation time modulated the EPS production by B. animalis BB12 (Amiri et al., 2019), and the level of dissolved oxygen and CO2 concentrations affected EPS production in B. longum JBL05 (Ninomiya et al., 2009). Although these studies did not investigate the potential compositional changes in the EPS that was produced, they do highlight that various growth conditions can influence EPS production. In this context, it should be noted that in L. rhamnosus E/N, the carbon source used for growth did not only affect the quantity of EPS produced but also its monosaccharide composition (Berecka et al., 2013). Taken together, the existing information illustrates the limited and scattered knowledge of the regulatory mechanisms underlying the production of (different) EPS molecules in bifidobacteria. Especially when the role of EPS as a NF or EM is to be further substantiated, better understanding of the regulation of EPS production and composition will be required to reliably investigate the role played by these molecules in Bifidobacterium probiotics. Such knowledge would also be required to design manufacturing procedures that aim to improve the presence and abundance of bioactive EPS molecules in Bifidobacterium probiotic products, in order to enhance their health benefit reliability as we propose in this review.
Different cytoplasmic enzymes are also found on the surface of bacterial cells, such as transaldolase, enolase, and DnaK. These surface attached proteins were suggested to act as bifidobacterial NF, based on their in vitro demonstrated role in the adhesive properties of Bifidobacterium strains. The example proteins mentioned serve typical cytoplasmic functions in glycolysis (transaldolase and enolase) or stress response (DnaK is a chaperonin), but were suggested to be secreted via a yet unknown non-classical secretion mechanism (González-Rodríguez et al., 2012). This class of surface exposed cytoplasmic proteins is often referred to as moonlighting proteins (Candela et al., 2007; Gleinser et al., 2012) defined by the fact that they can perform two or more physiologically relevant biochemical or biophysical functions (Jeffery, 1999). It could be that these proteins become deposited on the cell surface upon lysis of surrounding bacteria that release their cytoplasmic content in the environment. Alternatively, it was recently proposed that in B. longum NCC 2705 those type of surface exposed cytoplasmic (moonlighting) proteins could be excreted through the formation of extracellular vesicles (Nishiyama et al., 2020), of which the formation was shown to occur through membrane bubbling upon peptidoglycan damage in Bacillus subtilis (Toyofuku et al., 2017).
Modulation of the expression of these cytoplasmic proteins could also affect their surface exposure levels. Although such relation has to the best of our knowledge not been investigated in detail, it has been reported that different environmental factor (pH, bile salts) or mild stress conditions (Sánchez et al., 2007; Candela et al., 2010) can increase expression levels of moonlighting proteins. Finally, extracellular vesicles are one of the purported export-mechanism for adhesive proteins and may be modulated during growth as the presence of yet unidentified gut microbiota derived metabolites (in in vitro fecal fermentations) increased their formation (Nishiyama et al., 2020). More work is required to understand how these proteins are ending up on the cell surface, and if different manufacturing steps (e.g., steps inducing lysis of cells or growth condition inducing extracellular vesicles formation) might influence this process.
Discrepancies in clinical trial outcomes stemming from the use of probiotics belonging to the Lactobacillaceae family or the Bifidobacterium genus has been previously reported. Variations in trial design (population, dose, outcome measurement, etc.) have been advocated to at least in part explain the differences in the outcomes. However, the information summarized in the present review indicates that manufacturing conditions can influence on the presence and/or function of probiotic molecules that play critical roles in the survival of the bacteria in the intestinal tract (NF) and/or their interaction with host cells (EM), which may in turn be an additional cause for the observed variations in clinical outcomes. This review argues that knowledge of probiotic NF and/or EM molecules can provide means to assess the impact of manufacturing conditions on the functionality of the studied strain. Ensuring the presence of validated NF and EM in the final probiotic product could ultimately contribute to improve the consistency of the probiotic’s clinical effect.
As mentioned above, ensuring the presence and function of NF and EM during manufacturing could be particularly relevant for upper-gastrointestinal tract mediated health-benefits. An interesting example supporting this hypothesis is the serpin produced by B. longum NCC 2705 that was shown to reduce gliadin-induced immune responses in a mouse model, which was proposed to compensate the duodenal serine protease inhibitor decrease observed in active celiac disease. Recently, it was shown that the level of serpin production by this strain can be strongly modulated by the carbon source applied for growth, which allows the production of serpin-rich and serpin-poor probiotic preparations that can subsequently be evaluated in vivo. Such approach could establish the relevance of EM presence and function in the probiotic product for health benefits elicited in the proximal region of the intestine. Conversely, the Type IVb TAD pili of B. breve UCC 2003 was demonstrated to act as EM in the promotion of colonic epithelial cell proliferation. In this case, it would be interesting to study if the presence or absence of those EM in the initial preparation influences their mediated effect in the distal regions of the intestine, as pili expression was shown to be induced during intestinal transit.
Overall, this review highlights that beyond an improvement of the current clinical trial designs, there is a need to better understand the impact of manufacturing on clinical efficacy of probiotic products. First, NF and EM molecules have to be established as surrogate markers for probiotic functionality, linking their presence or absence to functional readouts. Work around this topic is relatively advanced for Lactobacillaceae (e.g., with the cases of L. plantarum WCFS1 and L. rhamnosus GG). However, for Bifidobacterium probiotics, only a few molecules were identified to act as EM in preclinical animal studies, like the sortase dependant pili of B. bifidum PRL2010, the Type IVb TAD pili of B. breve UCC 2003 and the serpin of B. longum NCC 2705. Overall, additional work, including in vivo demonstrations, is needed to identify and validate the molecules driving the host-bifidobacteria interactions. Once NF or EM established as surrogate markers of functionality, implementation of molecular level quality controls (i.e., based on NF and EM), could nicely complement the traditional live cells (e.g., CFU) enumeration in the final probiotic preparation, hence providing more insight on the functional consistency of dried probiotics.
SD took the lead in writing the manuscript, under direct supervision of MK. MV provided initial literature survey for several sections, under supervision of SD. All authors provided critical feedback and helped shape the manuscript.
SD and MV performed the work as employees of Nestlé (Société des Produits Nestlé SA).
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank B. Bogicevic, A. Mercenier, J. A. Muller, E. Ananta, and E. van der Beek for their critical review of the manuscript.
Altmann, F., Kosma, P., O’Callaghan, A., Leahy, S., Bottacini, F., Molloy, E., et al. (2016). Genome analysis and characterisation of the exopolysaccharide produced by Bifidobacterium longum subsp. longum 35624™. PLoS One 11:e0162983. doi: 10.1371/journal.pone.0162983
Alvarez-Martin, P., O’Connell Motherway, M., Turroni, F., Foroni, E., Ventura, M., and van Sinderen, D. (2012). A two-component regulatory system controls autoregulated serpin expression in Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 78, 7032–7041. doi: 10.1128/AEM.01776-12
Amiri, S., Rezaei Mokarram, R., Sowti Khiabani, M., Rezazadeh Bari, M., and Alizadeh Khaledabad, M. (2019). Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: optimization of fermentation variables and characterization of structure and bioactivities. Int. J. Biol. Macromol. 123, 752–765. doi: 10.1016/j.ijbiomac.2018.11.084
Ardita, C. S., Mercante, J. W., Kwon, Y. M., Luo, L., Crawford, M. E., Powell, D. N., et al. (2014). Epithelial adhesion mediated by pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses. Appl. Environ. Microbiol. 80, 5068–5077. doi: 10.1128/AEM.01039-14
Audy, J., Labrie, S., Roy, D., and LaPointe, G. (2010). Sugar source modulates exopolysaccharide biosynthesis in Bifidobacterium longum subsp. longum CRC 002. Microbiology 156, 653–664. doi: 10.1099/mic.0.033720-0
Berecka, M. P., Waśko, A., Szwajgier, D., and Choma, A. (2013). Bifidogenic and antioxidant activity of exopolysaccharides produced by Lactobacillus rhamnosus E/N cultivated on different carbon sources. Pol. J. Microbiol. 62, 181–189.
Bron, P. A., Grangette, C., Mercenier, A., de Vos, W. M., and Kleerebezem, M. (2004). Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. J. Bacteriol. 186, 5721–5729. doi: 10.1128/JB.186.17.5721-5729.2004
Brussow, H. (2019). Probiotics and prebiotics in clinical tests: an update. F1000Res. 8, F1000FacultyRev–1157. doi: 10.12688/f1000research.19043.1
Buhner, S., Hahne, H., Hartwig, K., Li, Q., Vignali, S., Ostertag, D., et al. (2018). Protease signaling through protease activated receptor 1 mediate nerve activation by mucosal supernatants from irritable bowel syndrome but not from ulcerative colitis patients. PLoS One 13:e0193943. doi: 10.1371/journal.pone.0193943
Burg, N. D., and Pillinger, M. H. (2001). The neutrophil: function and regulation in innate and humoral immunity. Clin. Immunol. 99, 7–17. doi: 10.1006/clim.2001.5007
Burns, P., Alard, J., Hrdy, J., Boutillier, D., Paez, R., Reinheimer, J., et al. (2017). Spray-drying process preserves the protective capacity of a breast milk-derived Bifidobacterium lactis strain on acute and chronic colitis in mice. Sci. Rep. 7:43211. doi: 10.1038/srep43211
Candela, M., Bergmann, S., Vici, M., Vitali, B., Turroni, S., Eikmanns, B. J., et al. (2007). Binding of human plasminogen to Bifidobacterium. J. Bacteriol. 189, 5929–5936.
Candela, M., Centanni, M., Fiori, J., Biagi, E., Turroni, S., Orrico, C., et al. (2010). DnaK from Bifidobacterium animalis subsp. lactis is a surface-exposed human plasminogen receptor upregulated in response to bile salts. Microbiology 156, 1609–1618. doi: 10.1099/mic.0.038307-0
Castro-Bravo, N., Hidalgo-Cantabrana, C., Rodriguez-Carvajal, M. A., Ruas-Madiedo, P., and Margolles, A. (2017). Gene replacement and fluorescent labeling to study the functional role of exopolysaccharides in bifidobacterium animalis subsp. lactis. Front. Microbiol. 8:1405. doi: 10.3389/fmicb.2017.01405
Charnchai, P., Jantama, S. S., Prasitpuriprecha, C., Kanchanatawee, S., and Jantama, K. (2016). Effects of the food manufacturing chain on the viability and functionality of bifidobacterium animalis through simulated gastrointestinal conditions. PLoS One 11:e0157958. doi: 10.1371/journal.pone.0157958
Claes, I. J., Segers, M. E., Verhoeven, T. L., Dusselier, M., Sels, B. F., De Keersmaecker, S. C., et al. (2012). Lipoteichoic acid is an important microbe-associated molecular pattern of Lactobacillus rhamnosus GG. Microb. Cell Fact. 11:161. doi: 10.1186/1475-2859-11-161
Corcoran, B. M., Stanton, C., Fitzgerald, G., and Ross, R. P. (2008). Life under stress: the probiotic stress response and how it may be manipulated. Curr. Pharm. Des. 14, 1382–1399. doi: 10.2174/138161208784480225
Debabov, D. V., Heaton, M. P., Zhang, Q., Stewart, K. D., Lambalot, R. H., and Neuhaus, F. C. (1996). The D-Alanyl carrier protein in Lactobacillus casei: cloning, sequencing, and expression of dltC. J. Bacteriol. 178, 3869–3876. doi: 10.1128/jb.178.13.3869-3876.1996
Debabov, D. V., Kiriukhin, M. Y., and Neuhaus, F. C. (2000). Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in D-alanylation. J. Bacteriol. 182, 2855–2864. doi: 10.1128/JB.182.10.2855-2864.2000
Dronkers, T. M. G., Ouwehand, A. C., and Rijkers, G. T. (2020). Global analysis of clinical trials with probiotics. Heliyon 6:e04467.
du Toit, E., Vesterlund, S., Gueimonde, M., and Salminen, S. (2013). Assessment of the effect of stress-tolerance acquisition on some basic characteristics of specific probiotics. Int. J. Food Microbiol. 165, 51–56. doi: 10.1016/j.ijfoodmicro.2013.04.022
Duboux, S., Golliard, M., Muller, J. A., Bergonzelli, G., Bolten, C. J., Mercenier, A., et al. (2021). Carbohydrate-controlled serine protease inhibitor (serpin) production in Bifidobacterium longum subsp. longum. Sci. Rep. 11:7236. doi: 10.1038/s41598-021-86740-y
Duranti, S., Turroni, F., Lugli, G. A., Milani, C., Viappiani, A., Mangifesta, M., et al. (2014). Genomic characterization and transcriptional studies of the starch-utilizing strain bifidobacterium adolescentis 22L. Appl. Environ. Microbiol. 80, 6080–6090. doi: 10.1128/AEM.01993-14
Fenster, K., Freeburg, B., Hollard, C., Wong, C., Ronhave Laursen, R., and Ouwehand, A. C. (2019). The production and delivery of probiotics: a review of a practical approach. Microorganisms 7:83. doi: 10.3390/microorganisms7030083
Foroni, E., Serafini, F., Amidani, D., Turroni, F., He, F., Bottacini, F., et al. (eds) (2011). Genetic analysis and morphological identification of pilus-like structures in members of the genus Bifidobacterium. Microb. Cell Fact. 10:S16. doi: 10.1186/1475-2859-10-S1-S16
Forssten, S. D., Laitila, A., Maukonen, J., and Ouwehand, A. C. (2020). Probiotic triangle of success; strain production, clinical studies and product development. FEMS Microbiol. Lett. 367:fnaa167. doi: 10.1093/femsle/fnaa167
Gaucher, F., Bonnassie, S., Rabah, H., Marchand, P., Blanc, P., Jeantet, R., et al. (2019). Review: adaptation of beneficial propionibacteria, Lactobacilli, and bifidobacteria improves tolerance toward technological and digestive stresses. Front. Microbiol. 10:841. doi: 10.3389/fmicb.2019.00841
Gleinser, M., Grimm, V., Zhurina, D., Yuan, J., and Riedel, C. U. (2012). Improved adhesive properties of recombinant bifidobacteria expressing the Bifidobacterium bifidum-specific lipoprotein BopA. Microbial. Cell Fact. 11:80. doi: 10.1186/1475-2859-11-80
González-Rodríguez, I., Sánchez, B., Ruiz, L., Turroni, F., Ventura, M., Ruas-Madiedo, P., et al. (2012). Role of extracellular transaldolase from Bifidobacterium bifidum in mucin adhesion and aggregation. Appl. Environ. Microbiol. 78, 3992–3998. doi: 10.1128/AEM.08024-11
Grzeskowiak, L., Isolauri, E., Salminen, S., and Gueimonde, M. (2011). Manufacturing process influences properties of probiotic bacteria. Br. J. Nutr. 105, 887–894. doi: 10.1017/S0007114510004496
Guo, Q., Goldenberg, J. Z., Humphrey, C., El Dib, R., and Johnston, B. C. (2019). Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 4:CD004827.
Hidalgo-Cantabrana, C., Algieri, F., Rodriguez-Nogales, A., Vezza, T., Martínez-Camblor, P., Margolles, A., et al. (2016). Effect of a ropy exopolysaccharide-producing Bifidobacterium animalis subsp. lactis strain orally administered on DSS-induced colitis mice model. Front. Microbiol. 7:868. doi: 10.3389/fmicb.2016.00868
Hidalgo-Cantabrana, C., López, P., Gueimonde, M., Clara, G., Suárez, A., Margolles, A., et al. (2012). Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob. Proteins 4, 227–237. doi: 10.1007/s12602-012-9110-2
Hidalgo-Cantabrana, C., Sanchez, B., Alvarez-Martin, P., Lopez, P., Martinez-Alvarez, N., Delley, M., et al. (2015). A single mutation in the gene responsible for the mucoid phenotype of Bifidobacterium animalis subsp. lactis confers surface and functional characteristics. Appl. Environ. Microbiol. 81, 7960–7968. doi: 10.1128/AEM.02095-15
Hidalgo-Cantabrana, C., Sánchez, B., Milani, C., Ventura, M., Margolles, A., and Ruas-Madiedo, P. (2014). Genomic overview and biological functions of exopolysaccharide biosynthesis in Bifidobacterium spp. Appl. Environ. Microbiol. 80, 9–18. doi: 10.1128/AEM.02977-13
Hidalgo-Cantabrana, C., Sanchez, B., Moine, D., Berger, B., de Los Reyes-Gavilan, C. G., Gueimonde, M., et al. (2013). Insights into the ropy phenotype of the exopolysaccharide-producing strain Bifidobacterium animalis subsp. lactis A1dOxR. Appl. Environ. Microbiol. 79, 3870–3874. doi: 10.1128/AEM.00633-13
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document. the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Ivanov, D., Emonet, C., Foata, F., Affolter, M., Delley, M., Fisseha, M., et al. (2006). A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J. Biol. Chem. 281, 17246–17252. doi: 10.1074/jbc.M601678200
Johnston, B. C., Goldenberg, J. Z., Vandvik, P. O., Sun, X., and Guyatt, G. H. (2011). Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 4:CD004827.
Joint Fao/Who Working Group Report on Drafting (2002). Guidelines for the Evaluation of Probiotics in Food. London: Food and Agriculture Organization.
Kalliomaki, M., Salminen, S., Arvilommi, H., Kero, P., Koskinen, P., and Isolauri, E. (2001). Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357, 1076–1079. doi: 10.1016/S0140-6736(00)04259-8
Kalliomaki, M., Salminen, S., Poussa, T., and Isolauri, E. (2007). Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 1019–1021. doi: 10.1016/j.jaci.2006.12.608
Kiekens, S., Vandenheuvel, D., Broeckx, G., Claes, I., Allonsius, C., De Boeck, I., et al. (2019). Impact of spray-drying on the pili of Lactobacillus rhamnosus GG. Microb. Biotechnol. 12, 849–855. doi: 10.1111/1751-7915.13426
Kopp, M. V., Hennemuth, I., Heinzmann, A., and Urbanek, R. (2008). Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics 121, e850–e856. doi: 10.1542/peds.2007-1492
Koprivnjak, T., Mlakar, V., Swanson, L., Fournier, B., Peschel, A., and Weiss, J. P. (2006). Cation-induced transcriptional regulation of the dlt operon of Staphylococcus aureus. J. Bacteriol. 188, 3622–3630. doi: 10.1128/JB.188.10.3622-3630.2006
Laconelli, C., Lemetais, G., Kechaou, N., Chain, F., Bermudez-Humaran, L. G., Langella, P., et al. (2015). Drying process strongly affects probiotics viability and functionalities. J. Biotechnol. 214, 17–26. doi: 10.1016/j.jbiotec.2015.08.022
Lebeer, S., Bron, P. A., Marco, M. L., Van Pijkeren, J. P., O’Connell Motherway, M., Hill, C., et al. (2018). Identification of probiotic effector molecules: present state and future perspectives. Curr. Opin. Biotechnol. 49, 217–223. doi: 10.1016/j.copbio.2017.10.007
Lebeer, S., Claes, I., Tytgat, H. L., Verhoeven, T. L., Marien, E., von Ossowski, I., et al. (2012). Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 78, 185–193. doi: 10.1128/AEM.06192-11
Lebeer, S., Vanderleyden, J., and De Keersmaecker, S. C. (2008). Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 72, 728–764. doi: 10.1128/MMBR.00017-08
Lebeer, S., Verhoeven, T. L., Francius, G., Schoofs, G., Lambrichts, I., Dufrene, Y., et al. (2009). Identification of a gene cluster for the biosynthesis of a long, galactose-rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase. Appl. Environ. Microbiol. 75, 3554–3563. doi: 10.1128/AEM.02919-08
Lee, I. C., Tomita, S., Kleerebezem, M., and Bron, P. A. (2013). The quest for probiotic effector molecules–unraveling strain specificity at the molecular level. Pharmacol. Res. 69, 61–74. doi: 10.1016/j.phrs.2012.09.010
Leivers, S., Hidalgo-Cantabrana, C., Robinson, G., Margolles, A., Ruas-Madiedo, P., and Laws, A. P. (2011). Structure of the high molecular weight exopolysaccharide produced by Bifidobacterium animalis subsp. lactis IPLA-R1 and sequence analysis of its putative eps cluster. Carbohydr. Res. 346, 2710–2717. doi: 10.1016/j.carres.2011.09.010
López, P., Monteserín, D. C., Gueimonde, M., de los Reyes-Gavilán, C. G., Margolles, A., Suárez, A., et al. (2012). Exopolysaccharide-producing Bifidobacterium strains elicit different in vitro responses upon interaction with human cells. Food Res. Int. 46, 99–107.
Marco, M. L., Bongers, R. S., de Vos, W. M., and Kleerebezem, M. (2007). Spatial and temporal expression of Lactobacillus plantarum genes in the gastrointestinal tracts of mice. Appl. Environ. Microbiol. 73, 124–132. doi: 10.1128/AEM.01475-06
Marco, M. L., de Vries, M. C., Wels, M., Molenaar, D., Mangell, P., Ahrne, S., et al. (2010). Convergence in probiotic Lactobacillus gut-adaptive responses in humans and mice. ISME J. 4, 1481–1484. doi: 10.1038/ismej.2010.61
McCarville, J. L., Dong, J., Caminero, A., Bermudez-Brito, M., Jury, J., Murray, J. A., et al. (2017). A commensal Bifidobacterium longum strain improves gluten-related immunopathology in mice through expression of a serine protease inhibitor. Appl. Environ. Microbiol. 83, e1323–e1317. doi: 10.1128/AEM.01323-17
Nguyen, P. T., Nguyen, T. T., Vo, T. N., Nguyen, T. T., Hoang, Q. K., and Nguyen, H. T. (2021). Response of Lactobacillus plantarum VAL6 to challenges of pH and sodium chloride stresses. Sci. Rep. 11:1301. doi: 10.1038/s41598-020-80634-1
Ninomiya, K., Matsuda, K., Kawahata, T., Kanaya, T., Kohno, M., Katakura, Y., et al. (2009). Effect of CO2 concentration on the growth and exopolysaccharide production of Bifidobacterium longum cultivated under anaerobic conditions. J. Biosci. Bioeng. 107, 535–537. doi: 10.1016/j.jbiosc.2008.12.015
Nishiyama, K., Takaki, T., Sugiyama, M., Fukuda, I., Aiso, M., Mukai, T., et al. (2020). Extracellular vesicles produced by Bifidobacterium longum export mucin-binding proteins. Appl. Environ. Microbiol. 86, e1464–e1420. doi: 10.1128/AEM.01464-20
O’Connell Motherway, M., Houston, A., O’Callaghan, G., Reunanen, J., O’Brien, F., O’Driscoll, T., et al. (2019). A Bifidobacterial pilus-associated protein promotes colonic epithelial proliferation. Mol. Microbiol. 111, 287–301. doi: 10.1111/mmi.14155
O’Connell Motherway, M., Zomer, A., Leahy, S. C., Reunanen, J., Bottacini, F., Claesson, M. J., et al. (2011). Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc. Natl. Acad. Sci. U.S.A. 108, 11217–11222. doi: 10.1073/pnas.1105380108
Osborn, D. A., and Sinn, J. K. (2007). Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst. Rev. 4:CD006475. doi: 10.1002/14651858.CD006475.pub2
Pique, N., Berlanga, M., and Minana-Galbis, D. (2019). Health benefits of heat-killed (Tyndallized) probiotics: an overview. Int. J. Mol. Sci. 20:2534. doi: 10.3390/ijms20102534
Pu, M., Duriez, P., Arazi, M., and Rowe-Magnus, D. A. (2018). A conserved tad pilus promotes Vibrio vulnificus oyster colonization. Environ. Microbiol. 20, 828–841. doi: 10.1111/1462-2920.14025
Remus, D., Kleerebezem, M., and Bron, P. A. (2012). Molecular Analysis of Candidate Probiotic Effector Molecules of Lactobacillus plantarum. Netherlands: Wageningen University.
Revilla-Guarinos, A., Gebhard, S., Alcantara, C., Staron, A., Mascher, T., and Zuniga, M. (2013). Characterization of a regulatory network of peptide antibiotic detoxification modules in Lactobacillus casei BL23. Appl. Environ. Microbiol. 79, 3160–3170. doi: 10.1128/AEM.00178-13
Riedel, C. U., Foata, F., Philippe, D., Adolfsson, O., Eikmanns, B. J., and Blum, S. (2006). Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-kappaB activation. World J. Gastroenterol. 12, 3729–3735. doi: 10.3748/wjg.v12.i23.3729
Roberts, C. M., Fett, W. F., Osman, S. F., Wijey, C., O’Connor, J. V., and Hoover, D. G. (1995). Exopolysaccharide production by Bifidobacterium longum BB-79. J. Appl. Bacteriol. 78, 463–468.
Sánchez, B., Champomier-Vergès, M.-C., Collado, M. D. C., Anglade, P., Baraige, F., Sanz, Y., et al. (2007). Low-pH adaptation and the acid tolerance response of Bifidobacterium longum Biotype longum. Appl. Environ. Microbiol. 73, 6450–6459. doi: 10.1128/AEM.00886-07
Sanders, M. E., Klaenhammer, T. R., Ouwehand, A. C., Pot, B., Johansen, E., Heimbach, J. T., et al. (2014). Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Ann. N. Y. Acad. Sci. 1309, 1–18. doi: 10.1111/nyas.12363
Schiavi, E., Gleinser, M., Molloy, E., Groeger, D., Frei, R., Ferstl, R., et al. (2016). The surface-associated exopolysaccharide of Bifidobacterium longum 35624 plays an essential role in dampening host proinflammatory responses and repressing local TH17 responses. Appl. Environ. Microbiol. 82, 7185–7196. doi: 10.1128/AEM.02238-16
Segers, M. E., and Lebeer, S. (2014). Towards a better understanding of Lactobacillus rhamnosus GG–host interactions. Microb. Cell Fact. 13(Suppl. 1):S7. doi: 10.1186/1475-2859-13-S1-S7
Serafini, F., Turroni, F., Ruas-Madiedo, P., Lugli, G. A., Milani, C., Duranti, S., et al. (2014). Kefir fermented milk and kefiran promote growth of Bifidobacterium bifidum PRL2010 and modulate its gene expression. Int. J. Food Microbiol. 178, 50–59. doi: 10.1016/j.ijfoodmicro.2014.02.024
Sybesma, W., Molenaar, D., van, I. W., Venema, K., and Kort, R. (2013). Genome instability in Lactobacillus rhamnosus GG. Appl Environ Microbiol. 79, 2233–2239.
Szajewska, H., and Hojsak, I. (2020). Health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12 in children. Postgrad. Med. 132, 441–451. doi: 10.1080/00325481.2020.1731214
Toyofuku, M., Carcamo-Oyarce, G., Yamamoto, T., Eisenstein, F., Hsiao, C. C., Kurosawa, M., et al. (2017). Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat. Commun. 8:481. doi: 10.1038/s41467-017-00492-w
Tripathi, P., Dupres, V., Beaussart, A., Lebeer, S., Claes, I. J., Vanderleyden, J., et al. (2012). Deciphering the nanometer-scale organization and assembly of Lactobacillus rhamnosus GG pili using atomic force microscopy. Langmuir 28, 2211–2216. doi: 10.1021/la203834d
Turroni, F., Foroni, E., O’Connell Motherway, M., Bottacini, F., Giubellini, V., Zomer, A., et al. (2010). Characterization of the serpin-encoding gene of Bifidobacterium breve 210B. Appl. Environ. Microbiol. 76, 3206–3219. doi: 10.1128/AEM.02938-09
Turroni, F., Serafini, F., Foroni, E., Duranti, S., O’Connell Motherway, M., Taverniti, V., et al. (2013). Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium–host interactions. Proc. Natl. Acad. Sci. U.S.A. 110, 11151–11156. doi: 10.1073/pnas.1303897110
Turroni, F., Serafini, F., Mangifesta, M., Arioli, S., Mora, D., van Sinderen, D., et al. (2014). Expression of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in response to environmental gut conditions. FEMS Microbiol. Lett. 357, 23–33. doi: 10.1111/1574-6968.12509
van Baarlen, P., Troost, F. J., van Hemert, S., van der Meer, C., de Vos, W. M., de Groot, P. J., et al. (2009). Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc. Natl. Acad. Sci. U.S.A. 106, 2371–2376. doi: 10.1073/pnas.0809919106
Vargas Garcia, C. E., Petrova, M., Claes, I. J., De Boeck, I., Verhoeven, T. L., Dilissen, E., et al. (2015). Piliation of Lactobacillus rhamnosus GG promotes adhesion, phagocytosis, and cytokine modulation in macrophages. Appl. Environ. Microbiol. 81, 2050–2062. doi: 10.1128/AEM.03949-14
Veiga, P., Suez, J., Derrien, M., and Elinav, E. (2020). Moving from probiotics to precision probiotics. Nat. Microbiol. 5, 878–880. doi: 10.1038/s41564-020-0721-1
Westermann, C., Gleinser, M., Corr, S. C., and Riedel, C. U. (2016). A critical evaluation of bifidobacterial adhesion to the host tissue. Front. Microbiol. 7:1220. doi: 10.3389/fmicb.2016.01220
Westermann, C., Zhurina, D. S., Baur, A., Shang, W., Yuan, J., and Riedel, C. U. (2012). Exploring the genome sequence of Bifidobacterium bifidum S17 for potential players in host-microbe interactions. Symbiosis 58, 191–200.
Wu, M.-H., Pan, T.-M., Wu, Y.-J., Chang, S.-J., Chang, M.-S., and Hu, C.-Y. (2010). Exopolysaccharide activities from probiotic bifidobacterium: immunomodulatory effects (on J774A.1 macrophages) and antimicrobial properties. Int. J. Food Microbiol. 144, 104–110. doi: 10.1016/j.ijfoodmicro.2010.09.003
Xu, R., Shen, Q., Ding, X., Gao, W., and Li, P. (2011). Chemical characterization and antioxidant activity of an exopolysaccharide fraction isolated from Bifidobacterium animalis RH. Eur. Food Res. Technol. 232, 231–240.
Xu, R., Shen, Q., Wu, R., and Li, P. (2017). Structural analysis and mucosal immune regulation of exopolysaccharide fraction from Bifidobacterium animalis RH. Food Agric. Immunol. 28, 1226–1241.
Yan, F., Liu, L., Dempsey, P. J., Tsai, Y. H., Raines, E. W., Wilson, C. L., et al. (2013). A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J. Biol. Chem. 288, 30742–30751. doi: 10.1074/jbc.M113.492397
Keywords: probiotic, niche factors, effector molecules, lactobacillaceae, bifidobacteria, manufacturing
Citation: Duboux S, Van Wijchen M and Kleerebezem M (2021) The Possible Link Between Manufacturing and Probiotic Efficacy; a Molecular Point of View on Bifidobacterium. Front. Microbiol. 12:812536. doi: 10.3389/fmicb.2021.812536
Received: 10 November 2021; Accepted: 06 December 2021;
Published: 24 December 2021.
Edited by:
Corine Sandström, Swedish University of Agricultural Sciences, SwedenReviewed by:
Maria de los Angeles Serradell, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaCopyright © 2021 Duboux, Van Wijchen and Kleerebezem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stéphane Duboux, c3RlcGhhbmUuZHVib3V4QHJkbHMubmVzdGxlLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.