- 1School of Public Health, Shantou University, Shantou, China
- 2College of Animal Science and Veterinary Medicine, Heilongjiang Bayi Agricultural University, Daqing, China
- 3Key Laboratory of Zoonosis Research, Ministry of Education, Institute of Zoonosis, College of Veterinary Medicine, Jilin University, Changchun, China
- 4State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology, Beijing, China
- 5Animal Health Laboratory, JRU BIPAR ANSES ENVA UPEC, Maisons-Alfort, France
Rickettsia raoultii is a tick-borne pathogen that infects humans; however, the vertebrate hosts of this pathogen have not been clearly defined. Our molecular examination of Rickettsia spp. infecting mammals and ticks in China, identified the gltA, ompA, and 17KD gene sequences of R. raoultii in horses and their ticks. This indicates a role of horses in R. raoultii epidemiology.
Introduction
Tick-borne rickettsioses are recognized as emerging vector-borne infections, infecting both human and animal hosts worldwide. Rickettsia raoultii was initially implicated as the causative agent of human infection in 2006 through the detection of DNA in the blood of a Spanish patient (Ibarra et al., 2006), and has since been reported in human infections in many countries of the world; in particular, China (Jia et al., 2014; Li et al., 2018; Dong et al., 2019). R. raoultii was first detected in Dermacentor nuttalli and Rhipicephalus pumilio ticks in 1999 (Rydkina et al., 1999). Although R. raoultii has been detected in bloodsucking insects and other tick species, the dominant vectors are generally considered to be Dermacentor spp. (Silaghi et al., 2011; Liu et al., 2016).
Rickettsia raoultii has been detected in a variety of ticks collected from dogs, cattle, and wildlife (Klitgaard et al., 2017; Chisu et al., 2018; Seo et al., 2020). All of these animals may serve as reservoir hosts for R. raoultii; however, experimental evidence is still lacking. Additionally, R. raoultii has been detected in squirrels, marbled polecats, red foxes, hedgehogs, yaks, camels and other wild mammals in China (Liu et al., 2018, 2021; Zhao et al., 2019; Li et al., 2020; Fang et al., 2021; Shao et al., 2021). Furthermore, there is a highly significant association of horse contact with tick-borne lymphadenopathy, the pathogens of which are R. slovaca and R. raoultii (Lakos et al., 2012). Horses also play a role in the epidemiology of Brazilian spotted fever, which is caused by R. rickettsii. Horses are considered the most suitable domestic agent for Brazilian spotted fever in some areas (Sangioni et al., 2005), which raises the question of whether horses may also play a role in the disease epidemiology of R. raoultii. The findings from this study provide evidence that Rickettsia species are in horses and sympatric ticks and that these hosts play a role in the circulation of the spotted fever group of rickettsiae.
The Study
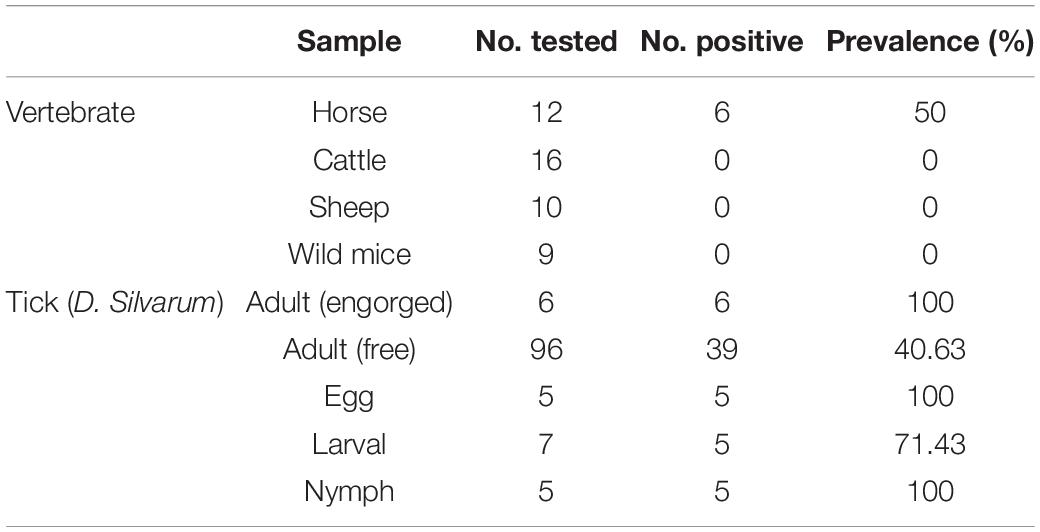
To identify putative vertebrate hosts of R. raoultii, we collected blood samples from horses (n = 12), cattle (n = 16), sheep (n = 10), and brown rats (Rattus norvegicus, n = 9). All the animals infested by ticks, and all were from the same rangeland in Daqing, northeastern China (46°58′N, 125°03′E), where spotted fever group rickettsiosis was previously reported (Jia et al., 2014). Free-living ticks (Dermacentor silvarum, n = 96) were collected by the flagging method and engorged adult ticks (D. silvarum, n = 6) were collected from horses in the same rangeland. Tick eggs (100 eggs, 5 pools) were laid in our laboratory by engorged wild-caught adult female D. silvarum from a PCR-positive horse. Larval and nymphal ticks of the same cohort that fed on mice were collected and pooled (35 larvae/10 nymphs; 12 pools in total). All the samples were tested for Rickettsia spp. This study was approved by the Research Ethics Committee of Heilongjiang Bayi Agricultural University, China.
Following the manufacturer’s instructions, DNA was extracted from the blood samples and ticks using a Tissue/Blood DNA Extraction Kit (Tiangen Biotech Inc., Beijing, China). PCR was performed to amplify the rickettsial citrate synthase gene (gltA), outer membrane protein A-encoding gene (ompA), and the 17-kDa antigen-encoding gene fragments; then, sequencing was performed (Li et al., 2018). The sequences obtained in this study were analyzed by an NCBI BLAST search. Rickettsia spp. were not detected in any of the samples from cattle, sheep, or brown rats, but sequences most closely related to R. raoultii were detected in 6/12 (50%) horses (Table 1). Additionally, R. raoultii was detected in 6/6 (100%) engorged ticks, 39/96 (41%) questing, 5/5 (100%) eggs, 5/7 (71%) larvae, and 5/5 (100%) nymphs (Table 1).
We deposited the sequences from 25 PCR-positive specimens into the GenBank database (accession nos. MH212168–92). For phylogenetic analysis, we used the MegAlign component to perform multiple sequence alignments with the ClustalX1.83 algorithm. A phylogenetic tree was constructed based on the gltA and ompA sequences identified in this study and other sequences from the GenBank database using the neighbor-joining method. This revealed that the test samples from a horse, questing tick and an engorged adult tick clustered with other R. raoultii strains and were most closely related to R. raoultii strain MDJ1, which had been isolated from a patient in Mudanjiang, China (Figure 1).
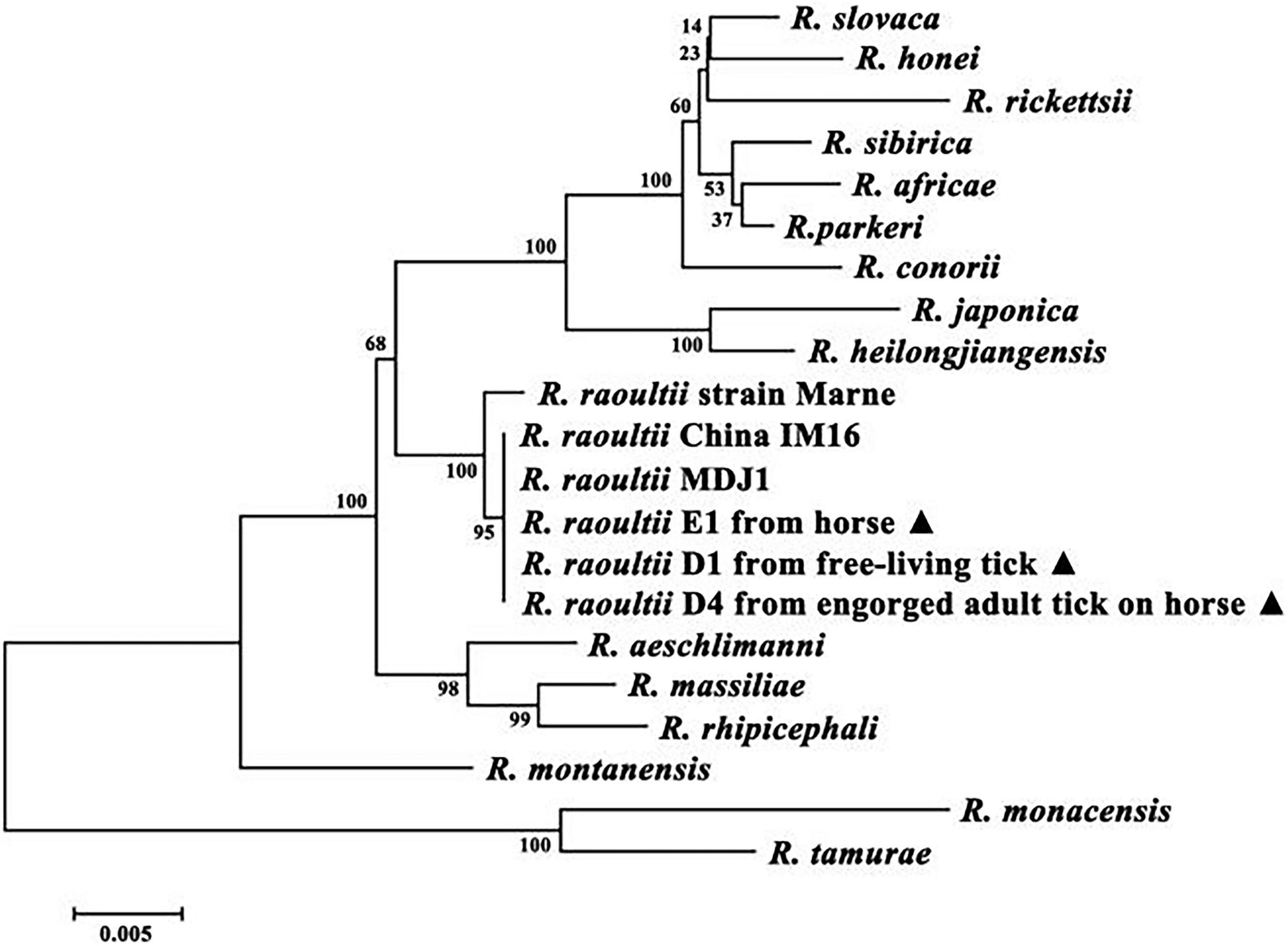
Figure 1. Molecular phylogenetic analysis of Rickettsia raoultii isolates from horse. Two genes were concatenated (gltA + ompA), a total of (1,047 + 498) positions were tested. GenBank accession numbers of the Sequences of the Rickettsia species used to make the concatenated analysis were as follows: R. rickettsii (U59729 + U43804), R. slovaca (U59725 + U43808), R. massiliae (U59719 + U43799), R. africae (U59733 + U43790), R. honei (U59726 + U43809), R. conorii (U59730 + U43806), R. japonica (U59724 + U43795), R. montanensis (U74756 + U43801), R. aeschlimanni (U59722 + U43800), R. heilongjiangensis (AF172943 + AF179364), R. parkeri (KY124257 + KY271186), R. rhipicephali (DQ865206 + DQ865208), R. monacensis (LN794217), R. sibirica (DQ097081 + DQ097082), R. tamurae (AF394896 + DQ103259), R. raoultii China IM16 (KY474576 + KY474577), R. raoultii strain Khabarovsk (DQ365804 + AH015610), R. raoultii strain Marne (DQ365803 + AH015609), R. raoultii D1 from free-living tick (MH212179 + MH212186), R. raoultii E1 from horse (MH212183 + MH212190) and R. raoultii D4 from engorged adult tick on horse (MH212181 + MH212188).
Discussion
Among our samples, R. raoultii was frequently detected in horses, but not in cattle, sheep, or brown rats, despite sharing pasture with the infected horses. A previous study in Xinjiang, China also reported the detection of R. raoultii in horses (Li et al., 2020). In this study, horses and other animals residing in the same environment were tested, but positive samples were only detected in horses. The results indicate that horses may be a vertebrate host of R. raoultii in the Daqing area. Horses are susceptible to a variety of rickettsial pathogens (Sangioni et al., 2005), and the prevalence rate is higher in some areas (Souza et al., 2016), indicating that horses may well be considered hosts. Therefore, horses may serve as a source of R. raoultii infection in ticks. Coincidentally, in a previous study, there were two human cases of R. raoultii infection in the same region (Jia et al., 2014).
In this study, the infection rate of R. raoultii in D. silvarum was 40.63%, which was higher than that (32.25%) in other studies (Wen et al., 2014). Furthermore, all engorged adult ticks and almost all egg/larval/nymphal tick pools were positive for R. raoultii. Our results support the idea that D. silvarum acts as a vector and a reservoir for R. raoultii. Schmuck et al. (2020) confirmed that transovarial transmission might be an efficient way of maintaining the infection cycle of R. raoultii, and both transstadial and transovarial transmission of R. raoultii have been demonstrated in D. nuttalli (Moore et al., 2018).
In conclusion, the main livestock grazing in the area were cattle, horses, and sheep, which were equally likely to be bitten by ticks, but R. raoultii was only detected in horses. Horses may therefore serve as a source of R. raoultii, contributing to the long-term preservation of R. raoultii in this area by promoting the infection of ticks and further increasing the chances of humans becoming infected with R. raoultii via tick bites.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, MH212168-92.
Author Contributions
J-FJ, A-QW, and Q-CC designed the project and experiments. YH, T-TW, and X-XM conducted the experiments. B-GJ and NJ analyzed the data. YH and Q-CC drafted the manuscript. All authors corrected, edited, and approved the manuscript.
Funding
This work was supported by the National Key Research Development Program of China (2019YFC1200501), the STU Scientific Research Foundation for Talents (NTF21043), and the National Natural Science Foundation of China (32072885).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Mallory Eckstut from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
References
Chisu, V., Foxi, C., and Masala, G. (2018). First molecular detection of the human pathogen Rickettsia raoultii and other spotted fever group Rickettsiae in ixodid ticks from wild and domestic mammals. Parasitol. Res. 117, 3421–3429. doi: 10.1007/s00436-018-6036-y
Dong, Z., Yang, Y., Wang, Q., Xie, S., Zhao, S., Tan, W., et al. (2019). A case with neurological abnormalities caused by Rickettsia raoultii in Northwestern China. BMC Infect. Dis. 19:796. doi: 10.1186/s12879-019-4414-4
Fang, L. Z., Lei, S. C., Yan, Z. J., Xiao, X., Liu, J. W., Gong, X. Q., et al. (2021). Detection of multiple intracellular bacterial pathogens in Haemaphysalis flava ticks collected from hedgehogs in central China. Pathogens 10:115. doi: 10.3390/pathogens10020115
Ibarra, V., Oteo, J. A., Portillo, A., Santibanez, S., Blanco, J. R., Metola, L., et al. (2006). Rickettsia slovaca infection: DEBONEL/TIBOLA. Ann. N. Y. Acad. Sci. 1078, 206–214. doi: 10.1196/annals.1374.040
Jia, N., Zheng, Y. C., Ma, L., Huo, Q. B., Ni, X. B., Jiang, B. G., et al. (2014). Human infections with Rickettsia raoultii, China. Emerg. Infect. Dis. 20, 866–868. doi: 10.3201/eid2005.130995
Klitgaard, K., Chriel, M., Isbrand, A., Jensen, T. K., and Bodker, R. (2017). Identification of Dermacentor Reticulatus ticks carrying Rickettsia raoultii on migrating jackal, Denmark. Emerg. Infect. Dis. 23, 2072–2074. doi: 10.3201/eid2312.170919
Lakos, A., Kőrösi, A., and Földvári, G. (2012). Contact with horses is a risk factor for tick-borne lymphadenopathy (TIBOLA): a case control study. Wien. Klin. Wochenschr. 124, 611–617. doi: 10.1007/s00508-012-0217-y
Li, H., Zhang, P. H., Huang, Y., Du, J., Cui, N., Yang, Z. D., et al. (2018). Isolation and Identification of Rickettsia raoultii in human cases: a surveillance study in 3 medical centers in China. Clin. Infect. Dis. 66, 1109–1115. doi: 10.1093/cid/cix917
Li, J., Li, Y., Moumouni, P. F. A., Lee, S. H., Galon, E. M., Tumwebaze, M. A., et al. (2020). First description of Coxiella burnetii and Rickettsia spp. infection and molecular detection of piroplasma co-infecting horses in Xinjiang Uygur autonomous region, China. Parasitol. Int. 76:102028. doi: 10.1016/j.parint.2019.102028
Liu, D., Wang, Y. Z., Zhang, H., Liu, Z. Q., Wureli, H. Z., Wang, S. W., et al. (2016). First report of Rickettsia raoultii and R. slovaca in Melophagus Ovinus, the sheep ked. Parasit. Vectors 9:600. doi: 10.1186/s13071-016-1885-7
Liu, G., Zhao, S., Tan, W., Hornok, S., Yuan, W., Mi, L., et al. (2021). Rickettsiae in Red Fox (Vulpes vulpes). Marbled Polecat (Vormela peregusna) and their ticks in Northwestern China. Parasit. Vectors 14:204. doi: 10.1186/s13071-021-04718-1
Liu, X., Yang, M., Liu, G., Zhao, S., Yuan, W., Xiao, R., et al. (2018). Molecular evidence of Rickettsia Raoultii, “Candidatus Rickettsia Barbariae” and a novel babesia genotype in marbled polecats (Vormela peregusna) at the China-Kazakhstan border. Parasit. Vectors 11:450. doi: 10.1186/s13071-018-3033-z
Moore, T. C., Pulscher, L. A., Caddell, L., von Fricken, M. E., Anderson, B. D., Gonchigoo, B., et al. (2018). Evidence for transovarial transmission of tick-borne Rickettsiae circulating in Northern Mongolia. PLoS Negl. Trop. Dis. 12:e0006696. doi: 10.1371/journal.pntd.0006696
Rydkina, E., Roux, V., Rudakov, N., Gafarova, M., Tarasevich, I., and Raoult, D. (1999). New Rickettsiae in ticks collected in territories of the former Soviet Union. Emerg Infect Dis. 5, 811–814. doi: 10.3201/eid0506.990612
Sangioni, L. A., Horta, M. C., Vianna, M. C., Gennari, S. M., Soares, R. M., Galvao, M. A., et al. (2005). Rickettsial Infection in animals and brazilian spotted fever endemicity. Emerg. Infect. Dis. 11, 265–270. doi: 10.3201/eid1102.040656
Schmuck, H. M., Chitimia-Dobler, L., Król, N., Kacza, J., and Pfeffer, M. (2020). Collection of Immature Dermacentor Reticulatus (Fabricius, 1794) ticks from vegetation and detection of Rickettsia Raoultii in them. Ticks Tick Borne Dis. 11:101543. doi: 10.1016/j.ttbdis.2020.101543
Seo, M. G., Kwon, O. D., and Kwak, D. (2020). High prevalence of Rickettsia raoultii and associated pathogens in canine ticks, South Korea. Emerg. Infect. Dis. 26, 2530–2532. doi: 10.3201/eid2610.191649
Shao, J. W., Yao, X. Y., Song, X. D., Li, W. J., Huang, H. L., Huang, S. J., et al. (2021). Molecular detection and genetic diversity of Rickettsia spp. in pet dogs and their infesting ticks in harbin, Northeastern China. BMC Vet. Res. 17:113. doi: 10.1186/s12917-021-02823-y
Silaghi, C., Hamel, D., Thiel, C., Pfister, K., and Pfeffer, M. (2011). Spotted fever group Rickettsiae in ticks, Germany. Emerg. Infect. Dis. 17, 890–892. doi: 10.3201/eid1705.101445
Souza, C. E., Camargo, L. B., Pinter, A., and Donalisio, M. R. (2016). High Seroprevalence for Rickettsia rickettsii in equines suggests risk of human infection in silent areas for the Brazilian spotted fever. PLoS One 11:e0153303. doi: 10.1371/journal.pone.0153303
Wen, J., Jiao, D., Wang, J., Yao, D., Liu, Z., Zhao, G., et al. (2014). Rickettsia raoultii, the predominant Rickettsia found in dermacentor silvarum ticks in China-Russia border areas. Exp. Appl. Acarol. 63, 579–585. doi: 10.1007/s10493-014-9792-0
Keywords: horse, infection, Rickettsia raoultii, tick-borne pathogens, China
Citation: Chang Q-C, Hu Y, Wu T-T, Ma X-X, Jiang B-G, Jia N, Wang A-Q and Jiang J-F (2022) The Role of Ranged Horses in Eco-Epidemiology of Rickettsia raoultii Infection in China. Front. Microbiol. 12:795500. doi: 10.3389/fmicb.2021.795500
Received: 15 October 2021; Accepted: 28 December 2021;
Published: 17 January 2022.
Edited by:
Axel Cloeckaert, Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement (INRAE), FranceReviewed by:
Pierre-Edouard Fournier, Aix-Marseille Université, FranceGabor Foldvari, Centre for Ecological Research, Hungary
Marina Eremeeva, Georgia Southern University, United States
Copyright © 2022 Chang, Hu, Wu, Ma, Jiang, Jia, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiao-Cheng Chang, Y2hhbmdxaWFvY2hlbmcyMDAxQDE2My5jb20=; An-Qi Wang, YW5naWV3YW5nOTFAeWFob28uY29t; Jia-Fu Jiang, amlhbmdqZjIwMDhAMTM5LmNvbQ==
 Qiao-Cheng Chang
Qiao-Cheng Chang Yang Hu
Yang Hu Ting-Ting Wu
Ting-Ting Wu Xiao-Xiao Ma
Xiao-Xiao Ma Bao-Gui Jiang
Bao-Gui Jiang Na Jia
Na Jia An-Qi Wang
An-Qi Wang Jia-Fu Jiang
Jia-Fu Jiang