- Key Laboratory of Molecular Microbiology and Technology, Ministry of Education, College of Life Sciences, Nankai University, Tianjin, China
Transient receptor potential (TRP) channel Yvc1 was related with hyphal growth, oxidative stress response, and pathogenicity. Calcineurin subunit Cnb1 was activated immediately in yeasts when exposed to severe stimulation. However, the relationship between Yvc1 and Cnb1-governed calcium ions and endoplasmic reticulum (ER) stress response remains unrevealed. In this study, we found that the mutant cnb1Δ/Δ was sensitive to TN, which was related with the overexpression of membrane calcium ion channels that could increase the cytosol calcium concentration. However, the growth of the cnb1Δ/Δyvc1Δ/Δ mutant was recovered and its cell vitality was better than the cnb1Δ/Δ strain. Meanwhile, the cellular calcium concentration was decreased and its fluctuation was weakened under ER stress in the cnb1Δ/Δyvc1Δ/Δ strain. To verify the regulation role of Yvc1 in the calcium concentration, we found that the addition of CaCl2 led to the worse viability, while the growth state was relieved under the treatment of EGTA in the cnb1Δ/Δ strain. In conclusion, the deletion of YVC1 could reduce the cellular calcium and relieve the ER stress sensitivity of the cnb1Δ/Δ strain. Thereby, our findings shed a novel light on the relationship between the Yvc1-governed cellular calcium concentration and ER stress response in C. albicans.
Introduction
Candida albicans, as the most well-known pathogenic fungi, may cause lethal systemic infection of humans (Dantas Ada et al., 2015). Calcium ion is an important signal messenger, which serves various activities and structural functions in all eukaryote cells. Normally, the cytosolic calcium ions are maintained at low concentrations in C. albicans, in which some calcium pumps and calcium exchangers play an important role (Cagnac et al., 2010; Ghanegolmohammadi et al., 2017). However, in response to specific stress, the extracellular calcium ions enter into the cytoplasm through the high-affinity calcium uptake system (HACS) (Cyert and Philpott, 2013). HACS consists of Cch1, Mid1, and Ecm7, which interact with each other (Iida et al., 2017). Besides, the organelle vacuole is the natural calcium ion pool in yeast cells, containing over 90% of the total cellular calcium ions (Bianchi et al., 2019). Under environmental stress, the calcium ions stored in vacuolar cavity are released through transient receptor potential (TRP) channel Yvc1 into the cytosol (Palmer et al., 2001).
In C. albicans, calcineurin is the conserved Ca2+/calmodulin-dependent phosphatase, composed of catalytic subunit Cna1 and regulatory subunit Cnb1 (Connolly et al., 2018). By dephosphorylation of transcription factor Crz1, calcineurin triggers downstream signaling events and regulates the cytosol ion concentration (Chow et al., 2017; Xu et al., 2020). In Saccharomyces cerevisiae, calcineurin is activated when exposed to severe stimulation, such as high pH, cell membrane damage, or antifungal drugs (LaFayette et al., 2010; Li Y. et al., 2018). The most important is that calcineurin regulated CCH1 negatively, which inhibits the expression of Cch1 once the cellular calcium ions are overloaded (Karababa et al., 2006; Teng et al., 2013; Xu et al., 2019). Yvc1 is the unique TRP-type calcium ion channel in yeast cells, a homolog with the TRP family of mammalian cells (de Castro et al., 2014). CaYvc1, similar with ScYvc1, located on the vacuolar membrane, is important for the calcium transport under environmental stress. In the previous study, we found that the deletion of YVC1 caused hypersensitivity to oxidative stress (Yu et al., 2014b). Meanwhile, Yvc1 is important in the process of hyphal growth, and its specific localization on the vacuolar membrane is necessary for the normal function of V-ATPase in C. albicans (Peng et al., 2019, 2020).
Generally, the proteins are synthesized, folded, and secreted in the endoplasmic reticulum (ER) (Zhang et al., 2019). Tunicamycin (TN) inhibits N-glycosylation and blocks the formation of glycoprotein, thereby leading to ER stress (Cherepanova et al., 2019). The unfolded protein response (UPR) is the typical strategy in yeast cells for relieving the stress (Bravo et al., 2013). bZIP transcription factor Hac1, a homolog with Xbp1 in mammalian cells, is spliced at the C terminus and transported from cytosol into nucleus, triggering the immediate expression of PMT4 or PRB1 to alleviate the ER stress (Cheon et al., 2011; Cherry et al., 2019). In mammalian cells, the nuclear factor E2-related factor 2 (Nrf2) was activated under oxidative stress, thereby modulating ER calcium levels by the regulation of glutathione peroxidase (Granatiero et al., 2019). Besides, since ER stress contributes to intracellular calcium and stress response, overloaded calcium induced mitochondrial dysfunction in cardiac, especially complex I (Mohsin et al., 2020).
In this study, we found that cnb1Δ/Δ was sensitive to ER stress, which was related with the overexpression of cytoplasm membrane channel CCH1 and irrelevant to the oxidative stress reaction. Besides, we found that the growth of the cnb1Δ/Δyvc1Δ/Δ strain was faster than that of the cnb1Δ/Δ strain, and the cell death rate, vacuolar membrane permeability, and mitochondrial activity were relieved in the double mutant. Interestingly, the classical UPR pathway was activated normally in all of the strains, indicating that the mechanism of relieving the growth in cnb1Δ/Δyvc1Δ/Δ was unrelated with the UPR pathway. However, the calcium flux was enhanced and its concentration was increased in the cnb1Δ/Δ strain under ER stress, and their level decreased obviously in the cnb1Δ/Δyvc1Δ/Δ strain. CaCl2 or its chelating reagent EGTA was added to verify the regulatory role of Yvc1 in the calcium ion concentration, and we found that the addition of CaCl2 led to poor viability and weakened the functions of vacuole and mitochondria under ER stress of the cnb1Δ/Δ strain. However, the growth state or organelle activity was relieved under the treatment of EGTA, indicating that Yvc1 alters the cellular calcium concentration in response to ER stress to improve the cnb1Δ/Δ growth. Overall, our work shed a novel light on the interaction between Yvc1-mediated calcium homeostasis and ER stress response in C. albicans.
Materials and Methods
Strains and Culture Conditions
The strains and primers used in our study are listed in Tables 1, 2. Wild-type (WT) strain BWP17 was used as the background strain to construct the cnb1Δ/Δ, yvc1Δ/Δ, and cnb1Δ/Δyvc1Δ/Δ mutant strains by the PCR-mediated homologous recombination method. The ARG4 cassettes were amplified and transformed into the WT. The heterozygous mutants (cnb1:ARG4/CNB1) were identified by PCR. After that, the URA3 fragment was transformed into the heterozygous mutant above to construct the cnb1Δ/Δ mutant strains (cnb1:ARG4/cnb1:URA3). The yvc1Δ/Δ and double mutant cnb1Δ/Δyvc1Δ/Δ were constructed with a similar method.
Besides, the pPCCH1-GFP plasmid was digested with StuI for 1 h and transferred into the WT and other mutant strains to measure the CCH1 expression level. In general, the strains were cultured in YPD (10 g/l yeast extract, 20 g/l peptone, 20 g/l glucose) medium. The SC (2% glucose, 0.67% yeast nitrogen base, 0.2% amino acid mixture) medium without uracil was used to separate and select the URA3-tagged strains.
Spot Assay
The YPD plates containing different concentrations of TN, β-mercaptoethanol, dithiothreitol (DTT), or calcofluor white (CFW) were used to measure the sensitivity to ER stress or cell wall stress (Su et al., 2021). Besides, 5 mM reductive agent ascorbic acid (VC) was added into these stress-related plates to investigate the relationship between stress susceptibility and oxidative stress response (OSR).
Cellular Calcium Levels
The content of cellular calcium and the calcium flux were measured with a Fluo-4 (C51H50F2N2O23, Beyotime, Shanghai, China, dissolved in DMSO) probe (Bartoli et al., 2019). Log-phase cells were treated with 2 μg/ml TN for 2 h. The cells were collected, washed with phosphate-buffered saline (PBS, 8 g/l NaCl, 0.2 g/l KCl, 1.42 g/l Na2HPO4, 0.27 g/l KH2PO4, pH 7.4), and resuspended with PBS buffer. The 2-mM Fluo-4 probe was added and incubated with 70 r/min at 30°C for 1 h. The fluorescence intensity (excitation wavelength at 488 nm, emission wavelength at 525 nm) was detected using a fluorescent microplate reader (Bode et al., 2020). The scanning time was sustained for 7 min with a scan gap for 1 s.
Vacuolar Membrane Permeability Assay
5-(6)-Carboxy-2′,7′-dichlorofluorescein diacetate (C-DCFDA) or 7-amino-4 chloromethyl coumarin (CMAC) was used to measure the vacuolar membrane permeability (VMP) (Andrei-Selmer et al., 2001). In the normal cells, C-DCFDA with green fluorescence or CMAC with blue fluorescence was concentrated in the vacuolar cavity, while it was spread to the whole cell in VMP-positive cells. The TN-treated strains were resuspended in PBS buffer and added with 1 mg/ml C-DCFDA (C25H14Cl2O9, Heowns, Tianjin, China, dissolved in DMSO) or 1 mg/ml CMAC (C10H8ClNO2, Beyotime, China, dissolved in DMSO). The cells were incubated for 10 min and observed by fluorescence microscopy. At least 1,000 cells for each group were photographed to count the percent of VMP-positive cells.
Mitochondrial Membrane Potential (ΔΨm) Assays
The log-phase cells treated with 2 μg/ml TN were collected, washed, and resuspended in PBS buffer. Two micrograms per milliliter of JC-1 (2 mg/ml, dissolved in DMSO, Sigma) was added into the suspension. The cells were incubated for 1 h, and the mitochondrial membrane potential (MMP) was recorded with the flow cytometer (FACSCalibur, BD, San Jose, CA, United States). The red fluorescence (excitation wavelength at 525 nm, emission wavelength at 590 nm) with J-aggregates could be detected in the cells with normal MMP. The green fluorescence (excitation wavelength at 490 nm, emission wavelength at 530 nm) with J-monomer was detected in the cells with decreased MMP (Yu et al., 2021).
HAC1 Splicing Assay
The strains treated with TN were cultured to log-phage. Total RNA was extracted with EastepTM Total RNA Extraction Kit (Promega, Madison, WI, United States) and transcribed reversely to cDNA. The HAC1 splicing assay was detected by the PCR method with primers HAC1-5RT and HAC1-3RT. The PCR product was separated in agarose gel electrophoresis for 3 h (Li J. et al., 2018).
MTT Assay
The cellular viability was detected by MTT reagent [3-(4,5)-dimethylthiazo(-z-y1)-3,5-di-phenytetrazoliumromide, 4 mg/ml, Beyotime, China] (Rong et al., 2020). The log-phage cells were collected and resuspended in PBS buffer. The 0.5-mg/ml MTT reagent was added and incubated with 70 r/min at 37°C for 1 h. The cells were centrifuged, and the supernatant was removed. Dimethyl sulfoxide (DMSO, Beyotime, China) was added into the precipitated cells, and the cells were centrifuged. The dissolved supernatant was collected to measure the absorption wavelength at 570 nm.
Propidium Iodide Assay
Propidium iodide (PI) was used to measure the cell death rate for the PI-positive cells which were stained with red fluorescence completely (Priante et al., 2018). The strains treated with TN were cultured to log-phase. Five micrograms per milliliter of PI (1 mg/ml, Sigma) was added into cells and incubated for 5 min. Mortality rate was represented by the PI-positive cells with a flow cytometer.
Real-Time PCR Assay
Real-time PCR assay was used to measure the expression level of the calcium channel-related gene CCH1 and UPR response-related genes PMT4 and PRB1. Cells were collected, and the total RNA was extracted with EastepTM Total RNA Extraction Kit (Promega, United States) and transcribed reversely to cDNA (Meng et al., 2018). The RealMasterMix (SYBR Green) kit (TransGen, China) was used for real-time PCR analysis (Gomes-Neto et al., 2017). The following primers used are listed in Table 2: ACT1-5RT, ACT1-3RT, CCH1-5RT, CCH1-3RT, PRB1-5RT, PRB1-3RT, PMT4-5RT, and PMT4-3RT. The 2–ΔΔ CT method was used to calculate the expression level of different genes, and ACT1 was used as the internal control.
Statistical Analysis
Each experiment mentioned above was repeated at least three times under the tested conditions. The standard deviations and means were calculated by the separate three replicates. The one-tailed Student’s t test was used to calculate p values. The p values less than 0.05 were considered as statistically significant difference.
Results
The Deletion of CNB1 Caused Sensitivity to Endoplasmic Reticulum Stress and Led to the CCH1 Overexpression
Firstly, we constructed the cnb1Δ/Δ mutant and measured its sensitivity to ER stress reagent TN. The results showed that on the YPD plate, cnb1Δ/Δ grew normally as a wild-type (WT) strain, while cnb1Δ/Δ could hardly grow on the 2-μg/ml TN plate (Figure 1A). Besides, the growth of liquid medium indicated that the WT strain grew rapidly during the 24-h culture period, whose value increased from 0.8 to 24. However, the OD600 of cnb1Δ/Δ was always maintained at 0.8 and the maximum value at 24 h was just 3 (Figure 1B). Since calcineurin regulated the cellular calcium concentration through inhibition of plasma membrane channel CCH1 (Xu et al., 2019), we assumed that the deletion of CNB1 might have an impact on the CCH1 expression. It was interesting that under TN treatment, the expression level of the CCH1 promoter in the cnb1Δ/Δ strain was near as 1.5 times as WT (Figure 1C). Moreover, the qPCR analysis indicated that CCH1 was upregulated in the transcription level of cnb1Δ/Δ in response to ER stress (Figure 1D). In general, the deletion of CNB1 leads to the sensitivity to ER stress and overexpression of CCH1.
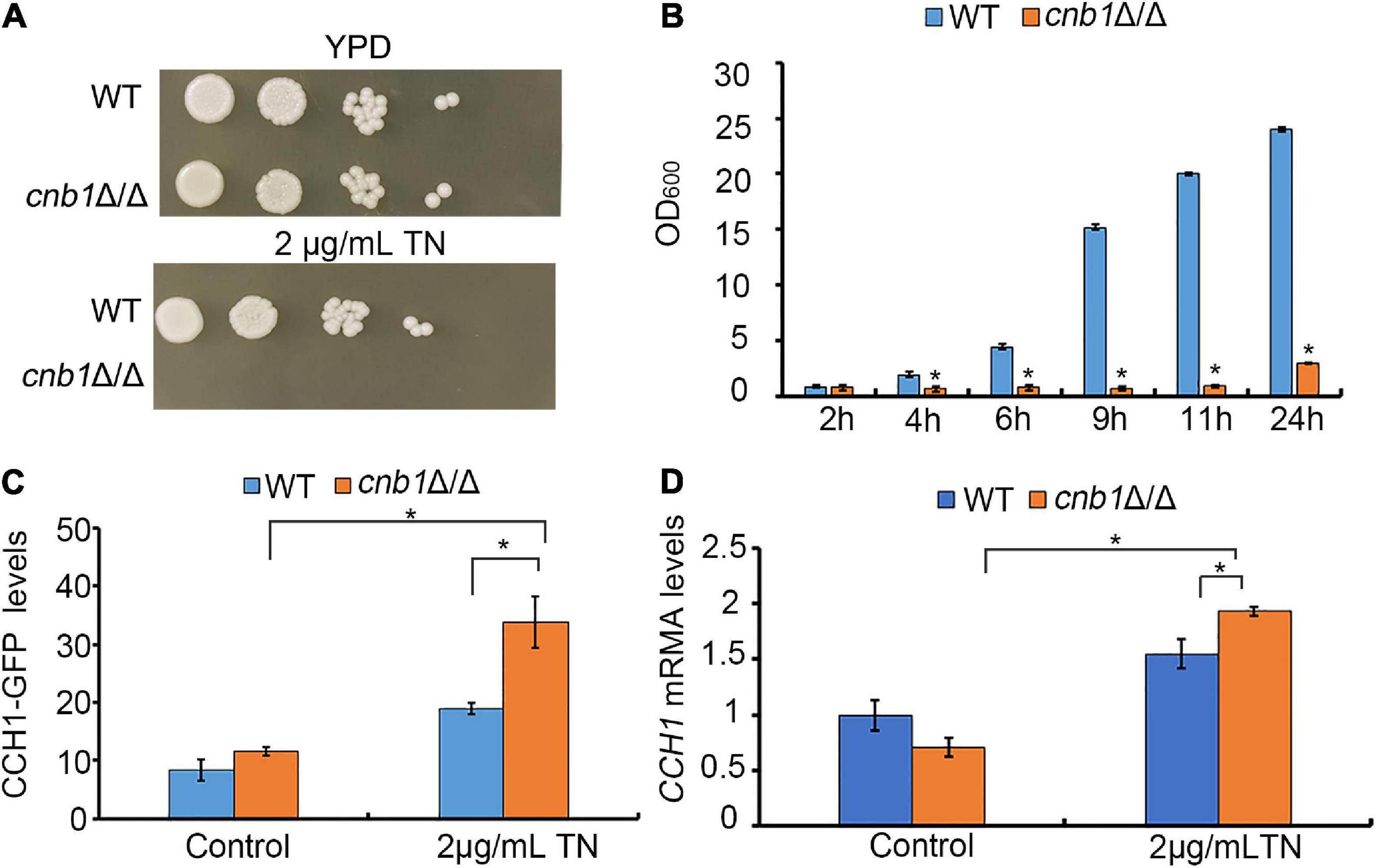
Figure 1. Effect of the CNB1 deletion on sensitivity to ER stress. (A) Cells were spotted on the YPD plates containing TN and photographed after being cultured for 2–3 days. (B) The liquid growth assay of cnb1Δ/Δ. (C) The CCH1-GFP fluorescence levels under ER stress. Strains tagged with the CCH1-GFP fragment were cultured to log phase, and the fluorescence intensity was detected with fluorescent microplate reader. (D) The CCH1 expression level. Total RNA was extracted and transcribed reversely to cDNA. CCH1-5RT CCH1-3RT were used for real-time quantitative PCR analysis. * means significant difference between the mutant and WT strains (p < 0.05). The experiments above were repeated three times independently.
The Inhibition of the Plasma Membrane Calcium Channel Could Recover the Growth Defect of cnb1Δ/Δ Under Endoplasmic Reticulum Stress
Now that the sensitivity to ER stress of the cnb1Δ/Δ strain was related with the overexpression of CCH1, we speculated that the inhibition of the plasma membrane calcium channel might improve its growth status. Verapamil or nifedipine, as the universal calcium channel blocker, was usually applied to cure the hypertension or angina. A different concentration of verapamil or nifedipine was added into the YPD plates containing 2 μg/ml TN. The spot assay result showed that the colony of cnb1Δ/Δ could grow in both the plates treated with verapamil and nifedipine (Figure 2A). Meanwhile, the liquid growth measurement indicated that during the 24-h culture, 40 or 80 μg/ml verapamil could improve the growth of cnb1Δ/Δ under ER stress (Figure 2B). These results implied that the inhibition of the plasma membrane calcium channel to decrease the cytosolic calcium concentration could recover the cnb1Δ/Δ growth under ER stress.
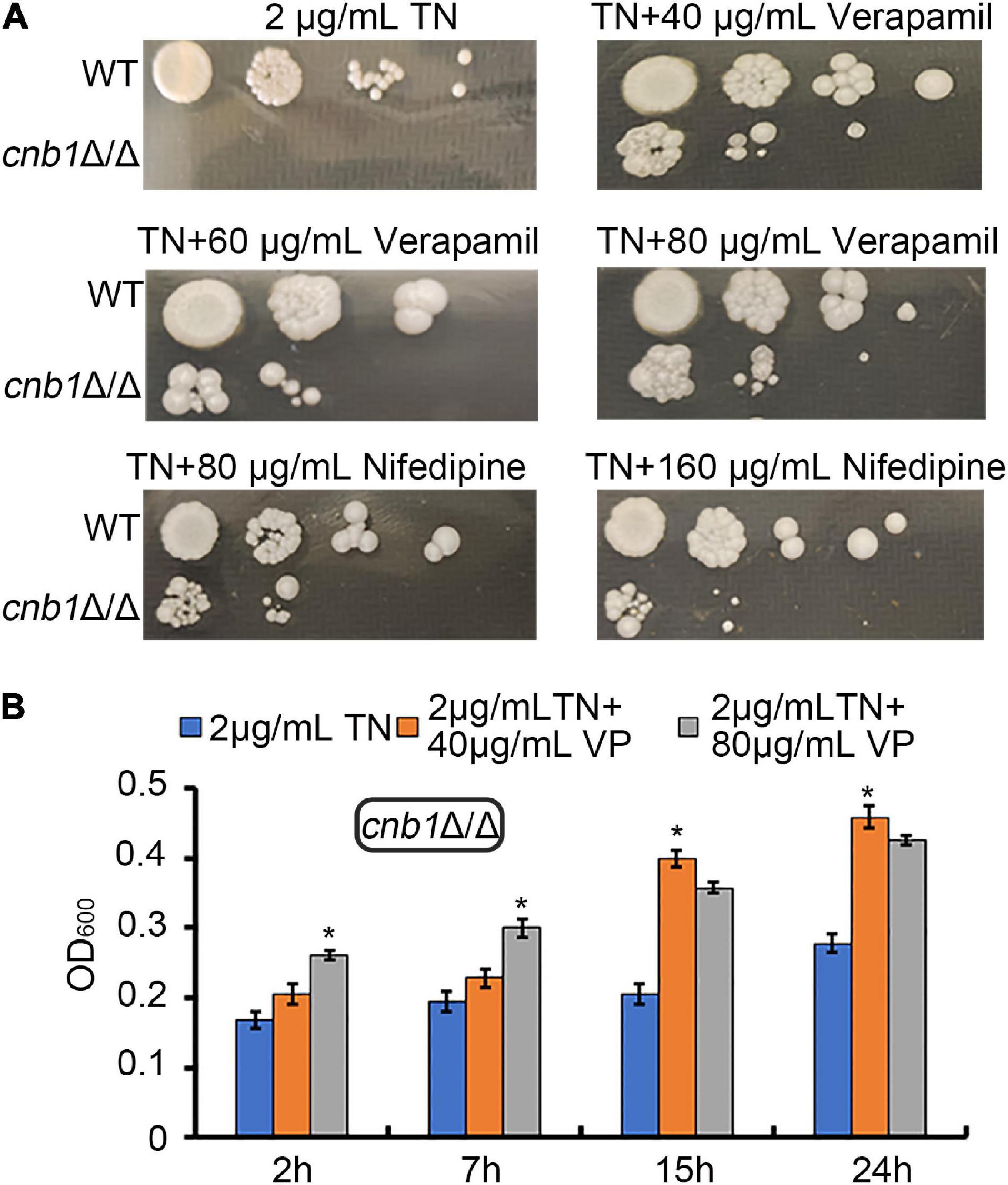
Figure 2. The supplement of verapamil and nifedipine recovered the growth defect of cnb1Δ/Δ under ER stress. (A) The different concentrations of verapamil and nifedipine were added into TN-containing YPD plates. WT and cnb1Δ/Δ strains were cultured and spotted on these plates. (B) Forty micrograms per milliliter of verapamil or 80 μg/ml verapamil was added to the TN-treated YPD medium. Strains were cultured, and OD600 was measured at 2, 7, 15, and 24 h. * means significant difference between TN treated alone and TN with verapamil-treated strains (<0.05). The experiments above were repeated three times independently.
Deletion of YVC1 Decreased the Cell Death Rate of cnb1Δ/Δ Under Endoplasmic Reticulum Stress
Since the inhibition of the cytosolic calcium concentration recovered the growth of cnb1Δ/Δ and Yvc1 was the unique vacuolar membrane calcium channel in yeast, we doubted whether the deletion of YVC1 could improve the growth status of cnb1Δ/Δ under ER stress. We constructed yvc1Δ/Δ and cnb1Δ/Δyvc1Δ/Δ strains and measured their susceptibility to environmental stress. CFW and caspofungin could cause the cell wall stress. Besides, both β-mercaptoethanol and DTT could interfere the formation of disulfide bonds, and TN could inhibit glycosylation. These three reagents could lead to ER stress. We found that similar with the WT strain, cnb1Δ/Δ, yvc1Δ/Δ, and cnb1Δ/Δyvc1Δ/Δ strains were resistant to CFW, β-mercaptoethanol, and DTT (Figure 3A, panels 2–4). However, the cnb1Δ/Δ and cnb1Δ/Δyvc1Δ/Δ strains were sensitive to caspofungin and TN. Since Yvc1 regulated the OSR in yeast (Yu et al., 2014b), the antioxidant vitamin C (VC) was added into the plates to verify whether the sensitivity was related with abnormal OSR. It was interesting that the addition of VC recovered the strains on the caspofungin-treated plate, indicating that the sensitivity to cell wall stress was related with impaired OSR reaction (Figure 3A, panels 5–6). Nevertheless, the addition of VC could not change the strains’ susceptibility to TN (Figure 3A, panels 7–8), which revealed the novel interaction between Yvc1 and Cnb1 with ER stress independently of OSR.

Figure 3. Deletion of CNB1 and YVC1 recovered the growth defect of the cnb1Δ/Δ strain. (A) ER stress or cell wall stress-related reagents were added into YPD plates. WT, cnb1Δ/Δ, yvc1Δ/Δ, or cnb1Δ/Δyvc1Δ/Δ strains were cultured and spotted on the plates. (B) The growth curve of each strain was drawn. The initial OD600 value was rectified at 0.2 and strains were cultured in liquid YPD medium, and 2 μg/ml TN was added into these medium and cultured for 25 h. The OD600 value in each strain was measured at specific times. (C) The death rate of each strain under ER stress. Log-phase strains were collected and washed twice with PBS buffer. Five micrograms per milliliter of PI was added into the strains and incubated for 5 min. The cell death rate was measured by flow cytometry. The cells without TN treatment were as the control group. (D) The statistical analysis of PI death rate. * means significant difference between the cnb1Δ/Δ and cnb1Δ/Δyvc1Δ/Δ strains under TN-treated conditions (p < 0.05). The experiments were repeated three times separately.
Under TN treatment, we measured the strain growth in the liquid medium during the 24-h cultivation. WT and yvc1Δ/Δ strains grew rapidly to the log phase and eventually maintained with the maximum quantity. Although cnb1Δ/Δ and cnb1Δ/Δyvc1Δ/Δ mutants grew slowly, cnb1Δ/Δyvc1Δ/Δ grew marginally faster than the cnb1Δ/Δ strain did (Figure 3B). Besides, the cell death rates under ER stress of these strains were calculated by flow cytometry with PI dye. All of the strains grew well in the control group, and their dead rates were low (Figures 3C,D, control). Under the TN treatment, WT and yvc1Δ/Δ strains were still with the small dead cells, and the death rate was 26.8% in the cnb1Δ/Δyvc1Δ/Δ strains, which was increased to 39.72% in the cnb1Δ/Δ strain (Figures 3C,D). These results indicated that even if cnb1Δ/Δyvc1Δ/Δ showed sensitivity to TN, the cell death rate was lower than that of the cnb1Δ/Δ strain, indicating that the deletion of YVC1 recovered the cell vitality of cnb1Δ/Δ.
The Deletion of YVC1 Recovered Vacuolar Membrane Permeability and Mitochondrial Membrane Potential of cnb1Δ/Δ Under Endoplasmic Reticulum Stress
Normally, the vacuole cavity could be dyed by CMAC, while the damaged cells were stained in the whole cell or failed to be stained (Andrei-Selmer et al., 2001). The vacuolar membrane permeability of the mutants showed that deletion of CNB1 caused the damaged vacuolar membrane under TN treatment, with the whole cells stained by CMAC or failing to be stained (Figure 4A). Besides, the calculation of the VMP-positive rate showed that cultured in the TN-treated medium, the WT and yvc1Δ/Δ strains maintained the integrity of vacuoles, with a low percentage of the VMP-positive rate. However, the positive rate was up to 70% in the vacuolar severely impaired cnb1Δ/Δ strain, although for the cnb1Δ/Δyvc1Δ/Δ strain with impaired vacuolar membrane, the VMP-positive rate was lower than cnb1Δ/Δ, just about 45% (Figure 4B).
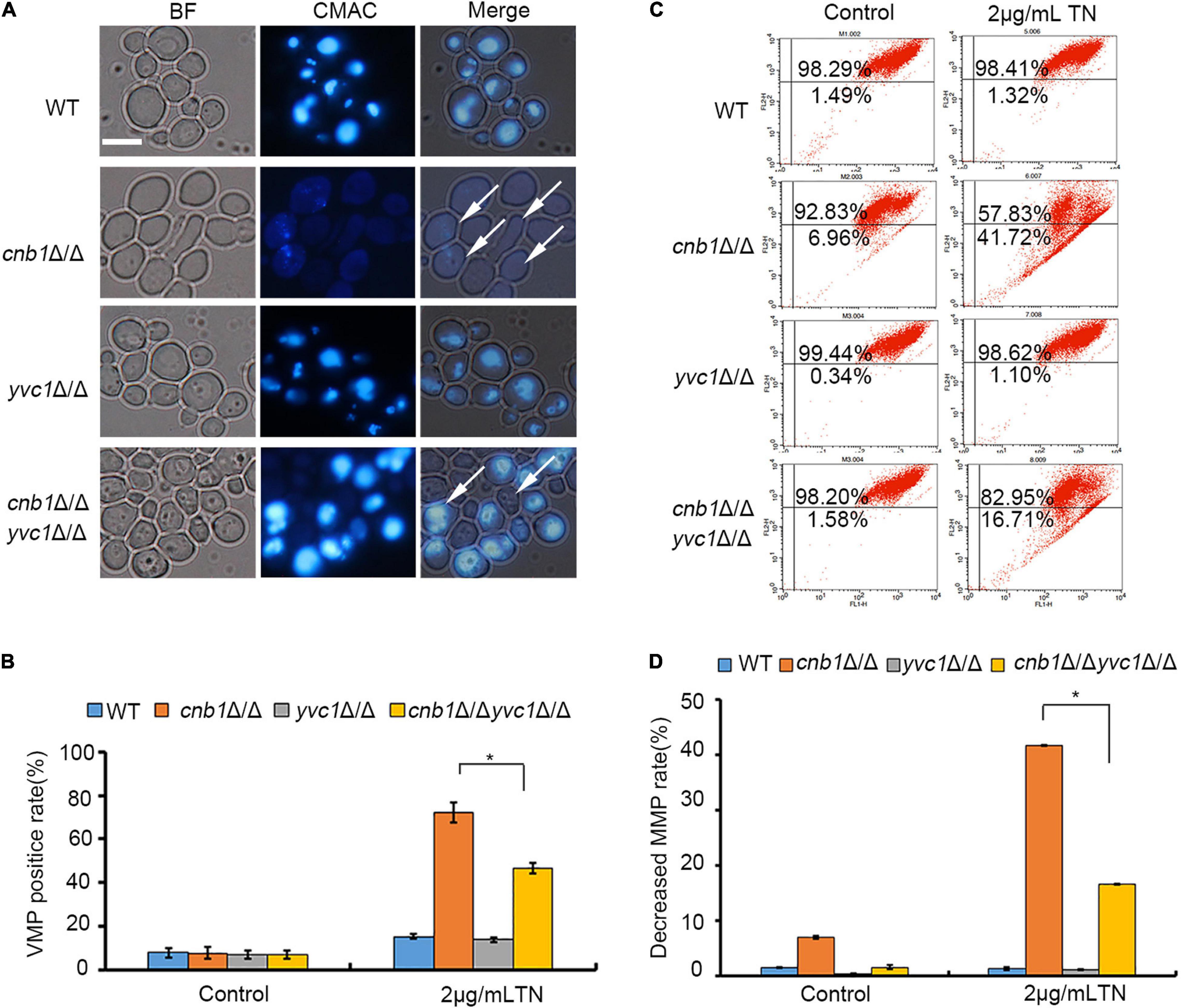
Figure 4. The deletion of YVC1 recovered the vacuolar membrane permeability (VMP) and mitochondrial membrane potential (MMP) under ER stress. (A) The detection reagent CMAC was added into the TN-treated strains. The cells were incubated with a gentle shaker at 30°C for 30 min and observed by fluorescence microscopy for each group. White arrows indicated the VMP-positive cells. Bar = 10 μm. (B) The calculation of VMP-positive cells. At least 1,000 cells in each strain were photographed and counted. (C) The log-phase cells were collected and resuspended in PBS buffer. JC-1 was added into the cells and incubated. The mitochondrial membrane potential (MMP) was detected by flow cytometry. (D) The calculation of MMP-decreased cells. The experiments were repeated three times separately. * means significant difference between the cnb1Δ/Δ and cnb1Δ/Δyvc1Δ/Δ strains under TN-treated conditions (p < 0.05).
The mitochondrial activities under ER stress were measured as well. In the control group, the cnb1Δ/Δyvc1Δ/Δ, cnb1Δ/Δ, and yvc1Δ/Δ strain, as the WT strain, maintained the normal mitochondrial function, with the low percentage of damaged rate (Figures 4C,D, control). However, compared with the other strains, the mitochondrial function was interfered by TN in the cnb1Δ/Δ strain, and the rate of decreased MMP was up to 41.72%. Nevertheless, the rate of impaired mitochondria of cnb1Δ/Δyvc1Δ/Δ was just 16.71% (Figures 4C,D). In conclusion, the deletion of YVC1 recovered the function of vacuole and mitochondria of the cnb1Δ/Δ strain.
The Unfolded Protein Response Pathway Was Activated Effectively in the cnb1Δ/Δ and cnb1Δ/Δyvc1Δ/Δ Strains Under Endoplasmic Reticulum Stress
The UPR pathway is the classical response process in the ER stress of yeast strains (Zhang et al., 2019). Since the deletion of YVC1 recovered the cell vitality of cnb1Δ/Δ under the treatment of TN, we speculated whether the UPR pathway was overactivated in this mutant. To verify the possibility, the total RNA of these three mutants and WT strains was extracted and transcribed reversely to cDNA (Gomes-Neto et al., 2017). The Hac1 splicing level and the expression level of PRB1 and PMT4 were measured. However, much unexpectedly, similar with the WT or yvc1Δ/Δ mutants, the UPR pathway was activated in cnb1Δ/Δ and cnb1Δ/Δyvc1Δ/Δ effectively. The unspliced HAC1 was 581 bp in the WT and mutant strains of the control group (Figure 5A). Moreover, under TN treatment for 2 h, HAC1 was spliced normally in the cnb1Δ/Δ and cnb1Δ/Δyvc1Δ/Δ strains and other strains with the size at 562 bp (Figure 5A).
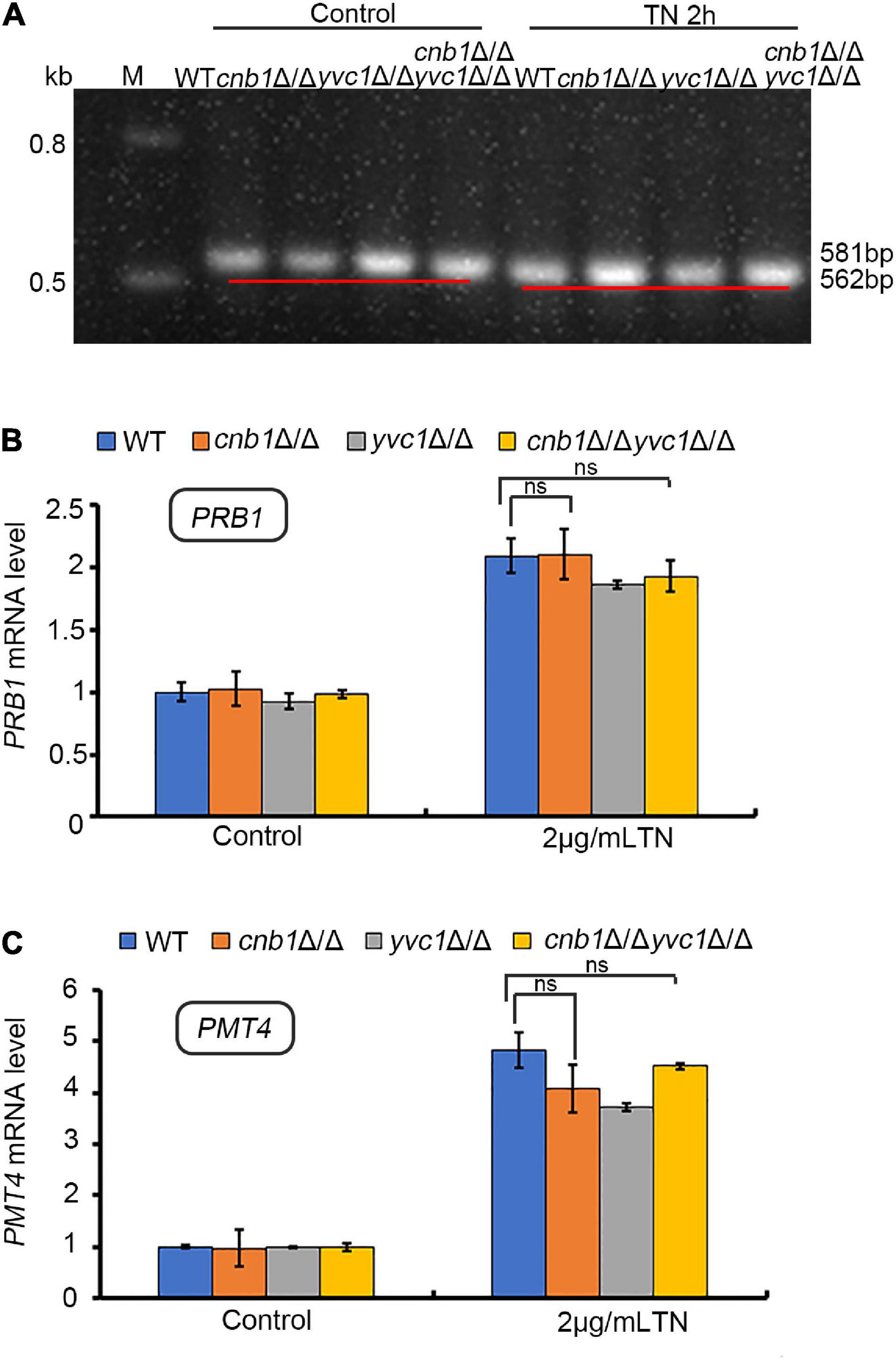
Figure 5. UPR pathway was activated effectively in both WT and other mutant strains under ER stress. (A) HAC1 splicing assay. The primers HAC1-5RT and HAC1-3RT were used to amplify the HAC1 fragment. (B) UPR gene PRB1 expression levels. The primers PRB1-5RT and PRB1-3RT were used to amplify the cDNA fragment of PRB1. (C) Real-time quantitative PCR to detect the expression level of PMT4. “ns” means no significant difference between the WT and other mutant strains under TN-treated conditions (p < 0.05). The experiments above were repeated three times independently.
Besides, qPCR analysis indicated that the expression levels of PMT4 and PRB1 in the cnb1Δ/Δ and cnb1Δ/Δyvc1Δ/Δ strains were similar with WT, which were all upregulated in the transcriptional level under ER stress (Figures 5B,C). In summary, these figures showed that the URP pathway in all of the mutants did not fail to evoke, implying a novel regulation mechanism within susceptibility.
The Deletion of YVC1 Decreased the Calcium Fluctuation and Cellular Calcium Concentration of cnb1Δ/Δ Under Endoplasmic Reticulum Stress
Yvc1 and Cnb1 were related with the cellular calcium regulation (Cyert and Philpott, 2013), and the inhibition of CCH1 to decrease the cytosolic calcium content could improve the growth status in the cnb1Δ/Δ strain. Therefore, we speculated whether the disruption of YVC1 reduces the vacuolar calcium release to improve the cell vitality. Moreover, the results showed that calcium fluctuation was enhanced in the cnb1Δ/Δ strain under ER stress. In the detected period, the calcium fluctuation was increased within 3 min, and the maximum concentration was higher than other strains. Nevertheless, the calcium flux of the cnb1Δ/Δyvc1Δ/Δ strain was steady with a low peak value like the WT strain (Figure 6A).
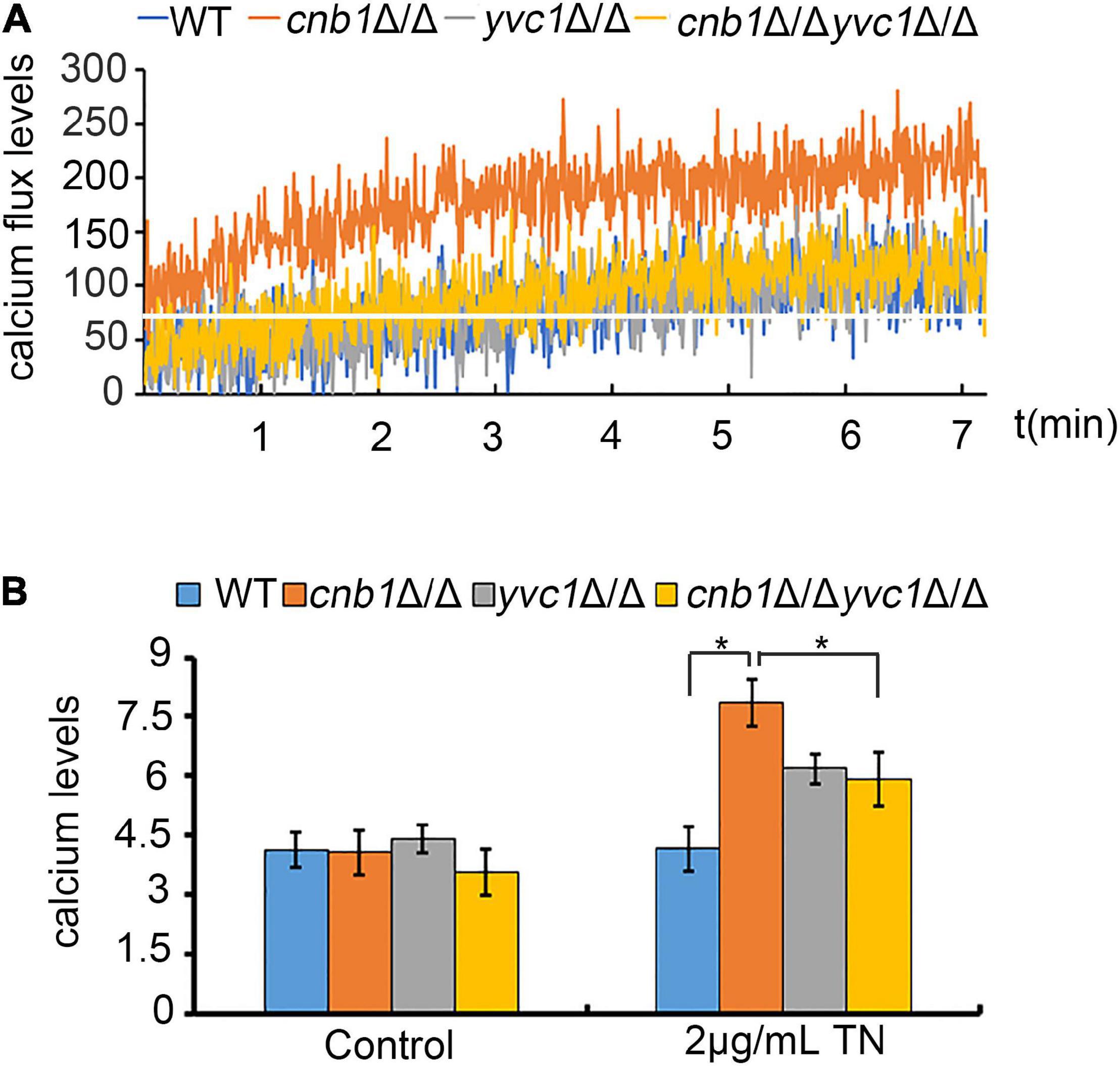
Figure 6. Deletion of CNB1 and YVC1 caused decreased calcium flux and calcium concentration. (A) The calcium flux assay. The experiment was detected for 7 min with a 1-s gap by a fluorescent microplate reader. The white straight line indicated the initial calcium concentration of cnb1Δ/Δ. (B) The cellular calcium concentration was detected with the fluorescent value in which the excitation wavelength was 488 nm and the emission wavelength was 525 nm. * means significant difference between the WT and other mutant strains under TN-treated conditions (p < 0.05).
Next, we measured the specific concentration of cellular calcium, which showed a similar tendency with the calcium flux. We found that the calcium concentration was increased significantly with TN treatment for 2 h of cnb1Δ/Δ, in which the concentration was much higher than those of other strains. The calcium content of the cnb1Δ/Δyvc1Δ/Δ strain was close to that of yvc1Δ/Δ, lower than that of cnb1Δ/Δ (Figure 6B). In conclusion, the calcium concentration was decreased significantly and its fluctuation was reduced obviously under ER stress in the cnb1Δ/Δyvc1Δ/Δ strain, indicating that the disruption of YVC1 could reduce the cellular calcium concentration, thereby improving the vitality.
The Supplement of EGTA Could Recover the Growth of cnb1Δ/Δ Under Endoplasmic Reticulum Stress
To test and verify our hypothesis that the deletion of YVC1 could decrease the cytosolic calcium concentration and recover the cell growth, CaCl2 or its chelating agent EGTA was added into the culture medium in the presence of TN. Although TN treatment in cnb1Δ/Δ caused a decreased MTT level, the supplement of EGTA recovered the cellular vitality, in which the MTT level was increased. Moreover, the supplement of CaCl2 led to significantly decreased vitality level in cnb1Δ/Δ (Figure 7A). Moreover, the PI death rate in the EGTA group was 4.08%, which increased to 12.28% in the CaCl2 group. It indicated that the addition of EGTA improved the cell growth as well (Figure 7B). The VMP assay showed that the permeability was improved in the addition of EGTA, while CaCl2 caused severely damaged VMP in the cnb1Δ/Δ strain (Figure 7C). Furthermore, the VMP-positive rate calculation displayed that under TN treatment, the addition of CaCl2 led to a 60% positive rate of the mutant, while under EGTA treatment, the positive rate was down to 48% (Figure 7D). In conclusion, we determine that it is the overloaded calcium ions that cause the susceptibility to ER stress in the cnb1Δ/Δ strain, and the disruption of YVC1 reduces the cytosolic calcium and improves the cell vitality (Figure 8).
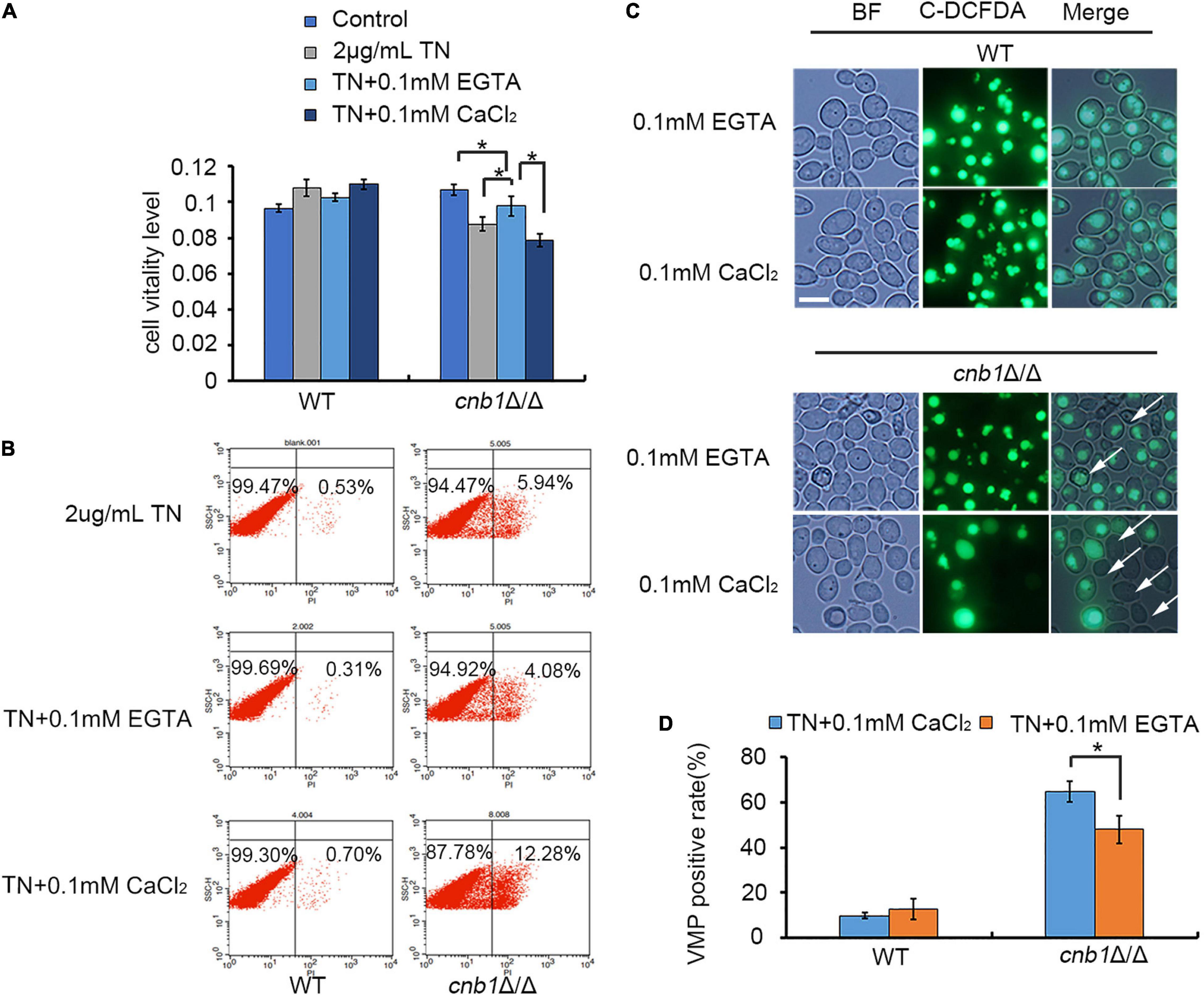
Figure 7. The supplement of EGTA could recover the cell viability of the cnb1Δ/Δ strain under ER stress. (A) WT and cnb1Δ/Δ were cultured in the medium containing TN supplied with EGTA or CaCl2. The MTT reagent was added, and the absorption wavelength of 570 nm was detected. (B) After the supplement of EGTA or CaCl2 into the TN-treated cnb1Δ/Δ, the PI death rate was measured by flow cytometry. (C) The observation of vacuolar membrane permeability under TN treatment supplied with EGTA or CaCl2. The method was similar with that in Figure 4A. Bar = 10 μm. (D) The count of VMP-positive cells treated by TN supplied with CaCl2 or EGTA. The method was similar with that in Figure 4B. * means significant difference among the different treated strains (p < 0.05). The experiments were repeated three times separately.
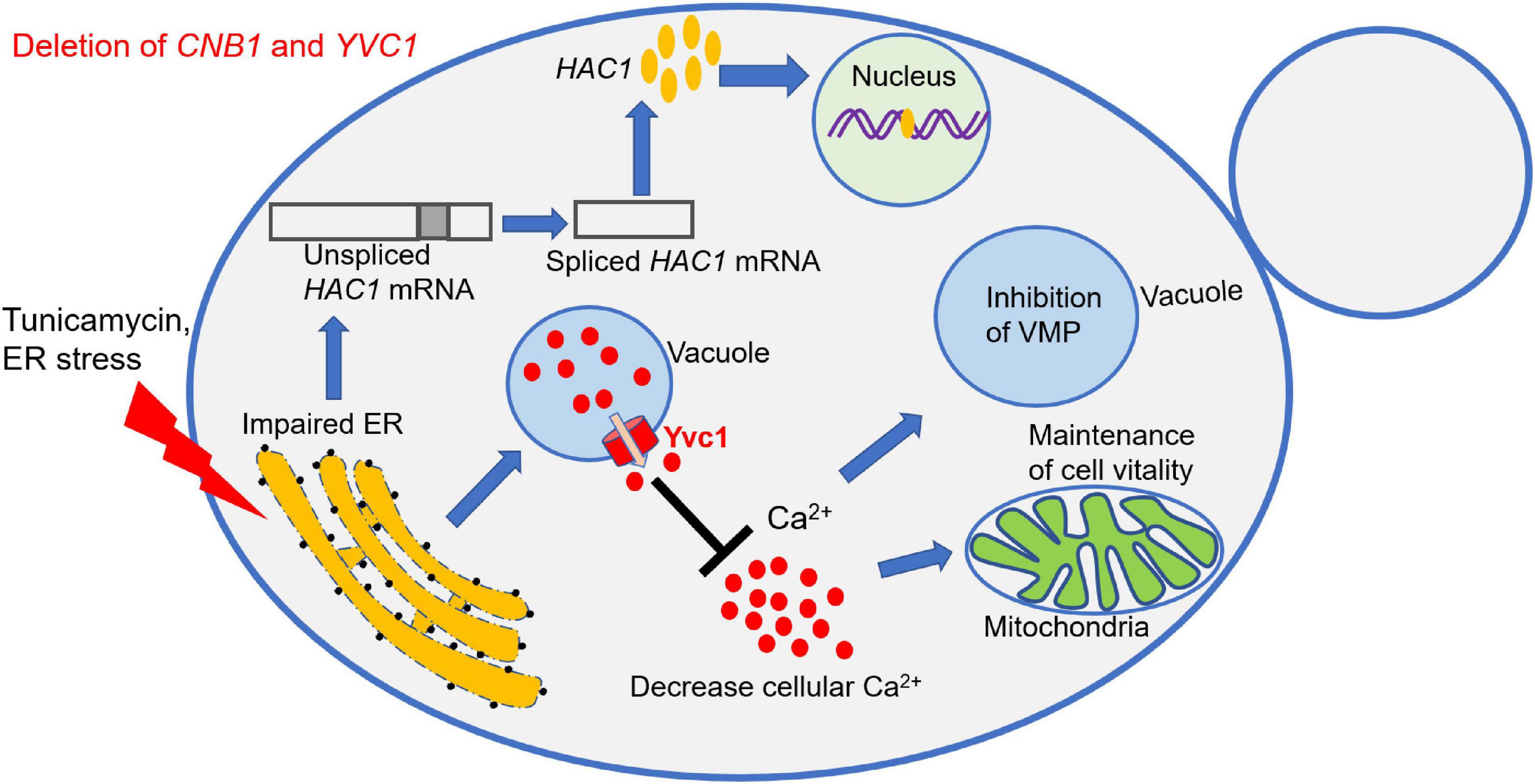
Figure 8. Schematic model of Yvc1 regulation cellular calcium concentration to improve growth in the cnb1Δ/Δyvc1Δ/Δ strain under ER stress. The calcineurin mutant strain cnb1Δ/Δ was sensitive to ER stress while the double mutant cnb1Δ/Δyvc1Δ/Δ recovered the growth. The URP pathway was effectively activated in these mutants. However, the deletion of TRP channel Yvc1 could inhibit the release of Ca2+ from vacuole and reduce the cellular calcium concentration in response to ER stress. Thereby, the vacuolar membrane permeability (VMP) was inhibited and the cell vitality was maintained, leading to the improvement of cell growth.
Discussion
In this study, we found that the deletion of YVC1 recovered the growth defect of cnb1Δ/Δ in the regulation of calcium ions in response to ER stress (Figure 8). cnb1Δ/Δ was hypersensitive to TN, which was relevant with the overexpression of the cytoplasm membrane channel CCH1 (Figures 1, 2). This revealed the interaction between the sensitivity to ER stress and the regulation of cytosolic calcium content of the cnb1Δ/Δ strain. Although cnb1Δ/Δyvc1Δ/Δ was sensitive to TN, the growth was recovered and the death rate was obviously decreased (Figure 3). In the spot assay, the strains were sensitive to TN instead of DTT, the former inhibited the N-glycosylation, and the latter influenced the disulfide bond. The results indicated that the disruption strain might have no impact on the disulfide bond, which could be verified subsequently. Moreover, the vacuolar membrane permeability and mitochondrial activity were improved in the double mutant (Figure 4).
The UPR pathway was a classical regulation method in response to ER stress; much unexpectedly, the UPR pathway was evoked in all of the tested strains, indicating their normal activation (Figure 5). However, we measured the calcium flux and the cellular calcium content. The calcium fluctuation was enhanced with the highest ion concentration in the cnb1Δ/Δ strain. Also, the calcium flux was steady with a lower content in the cnb1Δ/Δyvc1Δ/Δ strain, indicating the effect of the deletion of YVC1 on the decrease in cellular calcium (Figure 6). The further results show that addition of EGTA recovered the cell growth, cell vitality, and vacuolar membrane permeability, which corresponded with our conjectures (Figure 7).
Endoplasmic reticulum stress leads to the activation of the IRE1/Xbp1 signal pathway in mammalian cells. Regulated by Hac1, Pmt4 is expressed in the nucleus to alleviate the misfolded or unfolded proteins. In S. cerevisiae, ER stress upregulated the expression of Ptp2 tyrosine phosphatase and Cmp2 calcineurin phosphatase; the former was mediated by Mpk1 MAP kinase, and the latter could downregulate the activity of Hog1 MAP kinase (Mizuno et al., 2018). The Cmp2 homolog with Cna1 in C. albicans is the catalytic subunit which maintains the principal role for the stress response. Besides, calcineurin is essential for cells in many biological processes and for the long-term survival of cells undergoing ER stress (Liu et al., 2015). Connected with our findings, we doubt whether the Hog1-MAPK pathway is related with the ER stress response in the mutants.
It was reported that for humans, the nephrotic syndrome was associated with the activation of the ER calcium release channel, which led to podocyte injury (Park et al., 2019). Moreover, the connection of ER and mitochondria was complex. Through shuttling of calcium ions, the connection was involved not only in ion homeostasis but also in many structural and apoptotic proteins (Kumar and Maity, 2021). A stimulator of IFN genes, called STING, regulated not only calcium homeostasis but also ER stress and T cell survival, which were associated with lung disease (Wu et al., 2019). In yeast, cadmium exposure led to the interrupted calcium homeostasis, induced the lipid dysregulation, and finally caused ER stress (Rajakumar et al., 2016).
Verapamil and nifedipine are the cytoplasm membrane channel blockers in mammalian cells, used as the clinical drugs in curing heart diseases like angina pectoris and supraventricular arrhythmias (Xing et al., 2020). In our previous work, verapamil inhibited the Hwp1 expression, indicating its regulatory role in both morphogenesis-associated proteins and the secretory pathway (Yu et al., 2014a). Moreover, the combined use of verapamil and antifungal drug fluconazole had a synergetic inhibitory effect on hyphal development (unpublished data). Thereby, the cumulative effect of verapamil and TN could be measured in-depth. Besides, the effect of CNB1 or YVC1 deletion was interesting since the sensitivity to TN was irrelevant with the UPR pathway; indeed, it was the ROS-independent and UPR-independent calcium overloading. Connected with our previous cognition, it was subversive and enlightening.
Moreover, the relationship between the calcium signaling pathway members and the environmental stress response needs further investigation, for instance, the relationship between Cch1 and Yvc1 for their regulation role in calcium ion content. Since Cnb1 regulates the ER stress response, does the transcription factor Crz1 or the other subunit of calcineurin Cna1 regulate CCH1 in C. albicans. Besides, the specific regulation site of CCH1, the mechanism by which deletion of Cnb1 and Yvc1 caused cellular calcium ions to be increased under TN treatment, and the sensitivity to other environmental stimulus of the mutant are still unknown. Furthermore, we will work in-depth to figure out more mechanisms about the calcium signal pathway regulation under environmental stress.
In summary, our study revealed a novel interaction between the Yvc1-regulated cellular calcium ions and ER stress response, which was independent on the antioxidative reaction or UPR pathway (Figure 8). This work will extend our knowledge of the cellular sensor role of the TRP channel and calcineurin under environmental stress and uncover the new targets against fungal infections.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LP and ML conceived and designed the experiments and wrote the manuscript. LP, JD, and RZ performed the experiments. HZ, NZ, and QZ analyzed the data. ML and QY did supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (81873961, 31870139, and 32070145) and Natural Science Foundation of Tianjin (19JCZDJC33800).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andrei-Selmer, C., Knuppel, A., Satyanarayana, C., Heese, C., and Schu, P. V. (2001). A new class of mutants deficient in dodecamerization of aminopeptidase 1 and vacuolar transport. J. Biol. Chem. 276, 11606–11614. doi: 10.1074/jbc.M003846200
Bartoli, F., Moradi Bachiller, S., Antigny, F., Bedouet, K., Gerbaud, P., Sabourin, J., et al. (2019). Specific upregulation of TRPC1 and TRPC5 channels by mineralocorticoid pathway in adult rat ventricular cardiomyocytes. Cells 9:47. doi: 10.3390/cells9010047
Bianchi, F., Van’t Klooster, J. S., Ruiz, S. J., and Poolman, B. (2019). Regulation of amino acid transport in Saccharomyces Cerevisiae. Microbiol. Mol. Biol. Rev. 83, e00024–e00119. doi: 10.1128/mmbr.00024-19
Bode, D., Wen, Y., Hegemann, N., Primessnig, U., Parwani, A., Boldt, L. H., et al. (2020). Oxidative stress and inflammatory modulation of Ca(2+) handling in metabolic HFpEF-related left atrial cardiomyopathy. Antioxidants (Basel) 9:860. doi: 10.3390/antiox9090860
Bravo, R., Parra, V., Gatica, D., Rodriguez, A. E., Torrealba, N., Paredes, F., et al. (2013). Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 301, 215–290. doi: 10.1016/b978-0-12-407704-1.00005-1
Cagnac, O., Aranda-Sicilia, M. N., Leterrier, M., Rodriguez-Rosales, M. P., and Venema, K. (2010). Vacuolar cation/H+ antiporters of Saccharomyces cerevisiae. J. Biol. Chem. 285, 33914–33922. doi: 10.1074/jbc.M110.116590
Cheon, S. A., Jung, K. W., Chen, Y. L., Heitman, J., Bahn, Y. S., and Kang, H. A. (2011). Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans. PLoS Pathog. 7:e1002177. doi: 10.1371/journal.ppat.1002177
Cherepanova, N. A., Venev, S. V., Leszyk, J. D., Shaffer, S. A., and Gilmore, R. (2019). Quantitative glycoproteomics reveals new classes of STT3A-and STT3B-dependent N-glycosylation sites. J. Cell Biol. 218, 2782–2796. doi: 10.1083/jcb.201904004
Cherry, P. D., Peach, S. E., and Hesselberth, J. R. (2019). Multiple decay events target HAC1 mRNA during splicing to regulate the unfolded protein response. Elife 8:e42262 doi: 10.7554/eLife.42262
Chow, E. W., Clancey, S. A., Billmyre, R. B., Averette, A. F., Granek, J. A., Mieczkowski, P., et al. (2017). Elucidation of the calcineurin-Crz1 stress response transcriptional network in the human fungal pathogen Cryptococcus neoformans. PLoS Genet. 13:e1006667. doi: 10.1371/journal.pgen.1006667
Connolly, S., Quasi-Woode, D., Waldron, L., Eberly, C., Waters, K., Muller, E. M., et al. (2018). Calcineurin regulatory subunit calcium-binding domains differentially contribute to calcineurin signaling in Saccharomyces cerevisiae. Genetics 209, 801–813. doi: 10.1534/genetics.118.300911
Cyert, M. S., and Philpott, C. C. (2013). Regulation of cation balance in Saccharomyces cerevisiae. Genetics 193, 677–713. doi: 10.1534/genetics.112.147207
Dantas Ada, S., Day, A., Ikeh, M., Kos, I., Achan, B., and Quinn, J. (2015). Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 5, 142–165. doi: 10.3390/biom5010142
de Castro, P. A., Chiaratto, J., Winkelströter, L. K., Bom, V. L., Ramalho, L. N., Goldman, M. H., et al. (2014). The involvement of the Mid1/Cch1/Yvc1 calcium channels in Aspergillus fumigatus virulence. PLoS One 9:e103957. doi: 10.1371/journal.pone.0103957
Ghanegolmohammadi, F., Yoshida, M., Ohnuki, S., Sukegawa, Y., Okada, H., Obara, K., et al. (2017). Systematic analysis of Ca(2+) homeostasis in Saccharomyces cerevisiae based on chemical-genetic interaction profiles. Mol. Biol. Cell 28, 3415–3427. doi: 10.1091/mbc.E17-04-0216
Gomes-Neto, J. C., Mantz, S., Held, K., Sinha, R., Segura Munoz, R. R., Schmaltz, R., et al. (2017). A real-time PCR assay for accurate quantification of the individual members of the altered schaedler flora microbiota in gnotobiotic mice. J. Microbiol. Methods 135, 52–62. doi: 10.1016/j.mimet.2017.02.003
Granatiero, V., Konrad, C., Bredvik, K., Manfredi, G., and Kawamata, H. (2019). Nrf2 signaling links ER oxidative protein folding and calcium homeostasis in health and disease. Life Sci. Alliance 2, doi: 10.26508/lsa.201900563
Iida, K., Teng, J., Cho, T., Yoshikawa-Kimura, S., and Iida, H. (2017). Post-translational processing and membrane translocation of the yeast regulatory Mid1 subunit of the Cch1/VGCC/NALCN cation channel family. J. Biol. Chem. 292, 20570–20582. doi: 10.1074/jbc.M117.810283
Karababa, M., Valentino, E., Pardini, G., Coste, A. T., Bille, J., and Sanglard, D. (2006). CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 59, 1429–1451. doi: 10.1111/j.1365-2958.2005.05037.x
Kumar, V., and Maity, S. (2021). ER stress-sensor proteins and ER-mitochondrial crosstalk-signaling beyond (ER) stress response. Biomolecules 11:173. doi: 10.3390/biom11020173
LaFayette, S. L., Collins, C., Zaas, A. K., Schell, W. A., Betancourt-Quiroz, M., Gunatilaka, A. A., et al. (2010). PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 6:e1001069. doi: 10.1371/journal.ppat.1001069
Li, J., Yu, Q., Zhang, B., Xiao, C., Ma, T., Yi, X., et al. (2018). Stress-associated endoplasmic reticulum protein 1 (SERP1) and Atg8 synergistically regulate unfolded protein response (UPR) that is independent on autophagy in Candida albicans. Int. J. Med. Microbiol. 308, 378–386. doi: 10.1016/j.ijmm.2018.03.004
Li, Y., Sun, L., Lu, C., Gong, Y., Li, M., and Sun, S. (2018). Promising antifungal targets against Candida albicans based on ion homeostasis. Front. Cell. Infect. Microbiol. 8:286. doi: 10.3389/fcimb.2018.00286
Liu, S., Hou, Y., Liu, W., Lu, C., Wang, W., and Sun, S. (2015). Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot Cell 14, 324–334. doi: 10.1128/ec.00271-14
Meng, F., Yan, J., Ma, Q., Jiao, Y., Han, L., Xu, J., et al. (2018). Expression status and clinical significance of lncRNA APPAT in the progression of atherosclerosis. PeerJ 6:e4246. doi: 10.7717/peerj.4246
Mizuno, T., Nakamura, M., and Irie, K. (2018). Induction of Ptp2 and Cmp2 protein phosphatases is crucial for the adaptive response to ER stress in Saccharomyces cerevisiae. Sci. Rep. 8:13078. doi: 10.1038/s41598-018-31413-6
Mohsin, A. A., Thompson, J., Hu, Y., Hollander, J., Lesnefsky, E. J., and Chen, Q. (2020). Endoplasmic reticulum stress-induced complex I defect: central role of calcium overload. Arch. Biochem. Biophys. 683:108299. doi: 10.1016/j.abb.2020.108299
Palmer, C. P., Zhou, X. L., Lin, J., Loukin, S. H., Kung, C., and Saimi, Y. (2001). A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. U.S.A. 98, 7801–7805. doi: 10.1073/pnas.141036198
Park, S. J., Kim, Y., Yang, S. M., Henderson, M. J., Yang, W., Lindahl, M., et al. (2019). Discovery of endoplasmic reticulum calcium stabilizers to rescue ER-stressed podocytes in nephrotic syndrome. Proc. Natl. Acad. Sci. U.S.A. 116, 14154–14163. doi: 10.1073/pnas.1813580116
Peng, L., Yu, Q., Wei, H., Zhu, N., Ren, T., Liang, C., et al. (2019). The TRP Ca(2+) channel Yvc1 regulates hyphal reactive oxygen species gradient for maintenance of polarized growth in Candida albicans. Fungal Genet. Biol. 133:103282. doi: 10.1016/j.fgb.2019.103282
Peng, L., Yu, Q., Zhu, H., Zhu, N., Zhang, B., Wei, H., et al. (2020). The V-ATPase regulates localization of the TRP Ca(2+) channel Yvc1 in response to oxidative stress in Candida albicans. Int. J. Med. Microbiol. 310:151466. doi: 10.1016/j.ijmm.2020.151466
Priante, G., Quaggio, F., Gianesello, L., Ceol, M., Cristofaro, R., Terrin, L., et al. (2018). Caspase-independent programmed cell death triggers Ca(2)PO(4) deposition in an in vitro model of nephrocalcinosis. Biosci. Rep. 38:BSR20171228. doi: 10.1042/bsr20171228
Rajakumar, S., Bhanupriya, N., Ravi, C., and Nachiappan, V. (2016). Endoplasmic reticulum stress and calcium imbalance are involved in cadmium-induced lipid aberrancy in Saccharomyces cerevisiae. Cell Stress Chaperones 21, 895–906. doi: 10.1007/s12192-016-0714-4
Rong, F., Liu, L., Zou, C., Zeng, J., and Xu, Y. (2020). MALAT1 promotes cell tumorigenicity through regulating miR-515-5p/EEF2 axis in non-small cell lung cancer. Cancer Manag. Res. 12, 7691–7701. doi: 10.2147/cmar.S242425
Su, X., Yan, X., Chen, X., Guo, M., Xia, Y., and Cao, Y. (2021). Calcofluor white hypersensitive proteins contribute to stress tolerance and pathogenicity in entomopathogenic fungus, Metarhizium acridum. Pest Manag. Sci. 77, 1915–1924. doi: 10.1002/ps.6218
Teng, J., Iida, K., Imai, A., Nakano, M., Tada, T., and Iida, H. (2013). Hyperactive and hypoactive mutations in Cch1, a yeast homologue of the voltage-gated calcium-channel pore-forming subunit. Microbiology (Reading) 159(Pt 5), 970–979. doi: 10.1099/mic.0.064030-0
Wu, J., Chen, Y. J., Dobbs, N., Sakai, T., Liou, J., Miner, J. J., et al. (2019). STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J. Exp. Med. 216, 867–883. doi: 10.1084/jem.20182192
Xing, H., Luo, X., Li, Y., Fan, C., Liu, N., Cui, C., et al. (2020). Effect of verapamil on the pharmacokinetics of hydroxycamptothecin and its potential mechanism. Pharm. Biol. 58, 152–156. doi: 10.1080/13880209.2020.1717550
Xu, H., Fang, T., Omran, R. P., Whiteway, M., and Jiang, L. (2020). RNA sequencing reveals an additional Crz1-binding motif in promoters of its target genes in the human fungal pathogen Candida albicans. Cell Commun. Signal. 18:1. doi: 10.1186/s12964-019-0473-9
Xu, H., Fang, T., Yan, H., and Jiang, L. (2019). The protein kinase Cmk2 negatively regulates the calcium/calcineurin signalling pathway and expression of calcium pump genes PMR1 and PMC1 in budding yeast. Cell Commun. Signal. 17:7. doi: 10.1186/s12964-019-0320-z
Yu, Q., Ding, X., Zhang, B., Xu, N., Jia, C., Mao, J., et al. (2014a). Inhibitory effect of verapamil on Candida albicans hyphal development, adhesion and gastrointestinal colonization. FEMS Yeast Res. 14, 633–641. doi: 10.1111/1567-1364.12150
Yu, Q., Zhang, B., Yang, B., Chen, J., Wang, H., Jia, C., et al. (2014b). Interaction among the vacuole, the mitochondria, and the oxidative stress response is governed by the transient receptor potential channel in Candida albicans. Free Radic. Biol. Med. 77, 152–167. doi: 10.1016/j.freeradbiomed.2014.09.011
Yu, Q., Zhang, B., Li, J., Zhang, B., Wang, H., and Li, M. (2021). Corrigendum to “Endoplasmic reticulum-derived reactive oxygen species (ROS) is involved in toxicity of cell wall stress to Candida albicans” [Free Radic. Biol. Med. 99 (2016) 572-583]. Free Radic. Biol. Med. 162, 176–178. doi: 10.1016/j.freeradbiomed.2020.07.001
Keywords: calcineurin, TRP channel, calcium transport, endoplasmic reticulum stress, Candida albicans
Citation: Peng L, Du J, Zhang R, Zhu N, Zhao H, Zhao Q, Yu Q and Li M (2021) The Transient Receptor Potential Channel Yvc1 Deletion Recovers the Growth Defect of Calcineurin Mutant Under Endoplasmic Reticulum Stress in Candida albicans. Front. Microbiol. 12:752670. doi: 10.3389/fmicb.2021.752670
Received: 03 August 2021; Accepted: 07 October 2021;
Published: 30 November 2021.
Edited by:
Matteo Barberis, University of Surrey, United KingdomReviewed by:
Marco Vanoni, University of Milano-Bicocca, ItalyAshutosh Singh, University of Lucknow, India
Copyright © 2021 Peng, Du, Zhang, Zhu, Zhao, Zhao, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qilin Yu, eXVxaWxpbkBtYWlsLm5hbmthaS5lZHUuY24=; Mingchun Li, bmtsaW1pbmdjaHVuQDE2My5jb20=
 Liping Peng
Liping Peng Mingchun Li
Mingchun Li