- 1Department of Psychiatry, The Second Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center on Mental Disorders, Changsha, China
- 3National Technology Institute on Mental Disorders, Changsha, China
- 4Hunan Medical Center for Mental Health, Changsha, China
- 5Hunan Key Laboratory of Psychiatry and Mental Health, Changsha, China
- 6Mental Health Institute of Central South University, Changsha, China
- 7Department of Laboratory Medicine, The Third Xiangya Hospital, Central South University, Changsha, China
Substance addiction is a complex worldwide public health problem. It endangers both personal life and social stability, causing great loss on economy. Substance-related disorder is considered to be a complicated chronic brain disorder. It resulted from interactions among pharmacological properties of addictive substances, individual susceptibility, and social–environmental factors. Unfortunately, there is still no ideal treatment for this disorder. Recent lines of evidence suggest that gut microbiome may play an important role in the pathogenesis of neuropsychiatric disorders, including substance-related disorders. This review summarizes the research on the relationship between gut microbiome and substance-related disorders, including different types of substance, different individual susceptibility, and the occurrence and development of substance-induced mental disorders. We also discuss the potentiation of gut microbiome in the treatment of substance-related disorders, especially in the treatment of substance-induced mental disorders and manipulation on individuals’ responsiveness to addictive substances.
Introduction
Substance addiction is a complex worldwide public health problem. According to the 2020 World Drug Report, under the COVID-19 pandemic, a global health menace caused by substance abuse becomes more and more complicated and brings tremendous threats to social security and economy (WHO, 2020), such as the global opioid crisis, the rapidly spreading synthetic substance market, the legalization of non-medical marijuana, and the supply chains on the dark web. In addition to illegal drugs, addiction of tobacco and alcohol also had huge impact on human health. Substance-related disorder in DSM-5 refers to a cluster of cognitive, behavioral, and physiology symptoms related to the continuous use of substances, encompassing substance use disorder, intoxication, withdrawal, other substance-induced disorders, and unspecified substance-related disorders (Saunders, 2017). The reward circuit, ventral tegmental area–nucleus accumbens (VTA–NAc) circuit (Kim et al., 2016), is considered to be the structural basis of substance-related disorders (Koob and Volkow, 2016). Long-term drug abuse leads to abnormal activation of dopaminergic neurons in reward circuits (Koob and Le Moal, 2001). However, the mechanism of substance-related disorders is unclear, and more importantly, treatments with comparable efficacy and tolerable side effects for these disorders are still under development.
In recent decades, the impact of gut microbiome on the nervous system has aroused extensive interest. Studies show that there is a bidirectional communication system between gut microbiome and brain, which is known as the microbiome–gut–brain axis (MGBA) (Dinan and Cryan, 2017). Increasing evidence revealed the role of intestinal microbial community in neuropsychiatric diseases (Burokas et al., 2015), including reward process and substance use disorders (Garcia-Cabrerizo et al., 2021). It has been found that the composition and diversity of gut microbiome in substance-addicted individuals changed remarkably (Meckel and Kiraly, 2019). A study showed the specific impacts of sex in the correlation of addictive related behaviors and gut microbiome according to comprehensive behavioral characterization and gut microbiome analysis in rats (Peterson et al., 2020). It was also becoming clear the impact of the microbiome–gut–brain axis on hypothalamic–pituitary–adrenal (HPA) axis stress response and depression, which were all risk factors for substance use disorders. The underlying mechanisms were explored not only in the overall modulation of immune responses and intestinal barrier integrity, but also in the neurotransmitter synthesis and metabolism, such as serotonin and dopamine (Leclercq et al., 2014a; Skosnik and Cortes-Briones, 2016). Recently, “psychobiotic” treatment strategies had great promise as novel treatment for drug addiction (Skosnik and Cortes-Briones, 2016). Here, we reviewed the preclinical and clinical studies of correlation between intestinal microbial community and different addictive substances (alcohol, opioid, cocaine, amphetamine, etc.) and then focused on the role of gut microbiome in individual susceptibility to addictive substances and substance-related mental disorders. We also discussed the therapeutic potential of gut microbiome in the treatment of substance-related disorders, especially in the treatment of substance-induced mental disorders and manipulation on individuals’ responsiveness to addictive substances. However, further systematic investigations were required to expand towards casual and mechanistic relationships between gut microbiome and drug addiction (Temko et al., 2017). More basic researches and clinical trials were needed to examine the efficacy of probiotic or combination treatments.
The Role of the Microbiome–Gut–Brain Axis in Alcohol Use Disorders (AUD)
Alcohol directly affects intestinal micro-ecology. Both animal and clinical studies had shown that alcohol can cause changes in the diversity and composition of the gut microbiome (Bajaj, 2019; Wang S. C. et al., 2020), which induced intestinal mucosal damage and increases intestinal permeability in some patients (Leclercq et al., 2014b; Peterson et al., 2017). As intestinal permeability increases, intestinal bacteria and metabolites including neurotransmitters produced by gut microbiome may enter the peripheral blood circulation and affect the host’s health (Leclercq et al., 2014b; Mayer et al., 2014; Szabo, 2015).
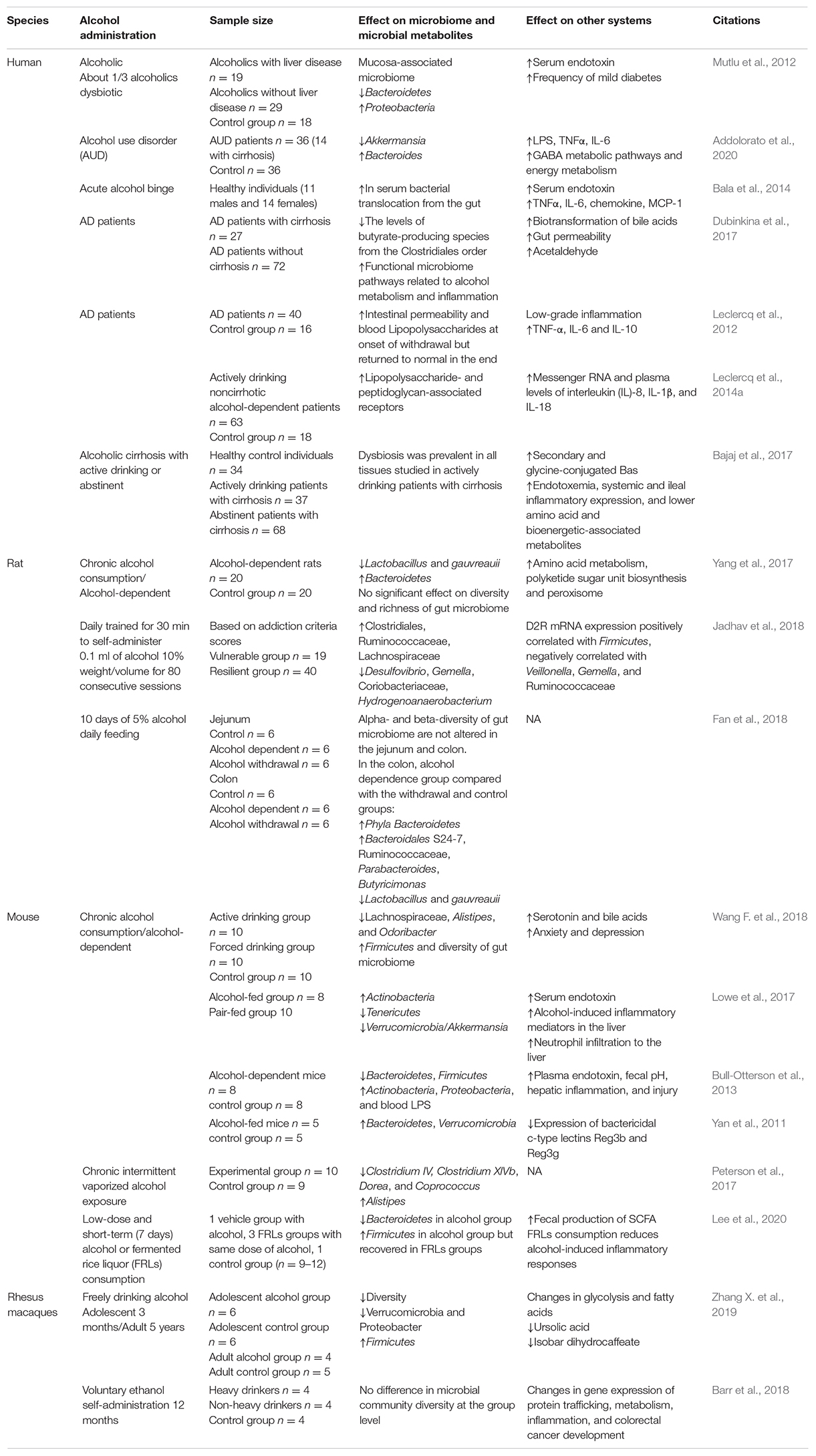
Although the impact of alcohol on gut microbiome was clear, as we summarized in Table 1, the effects of alcohol on gut microbiome and its metabolites were diverse in different studies. In preclinical studies, firstly, different species, mice, rats, and rhesus macaques, showed different gut microbiome responses to alcohol (Yan et al., 2011; Bull-Otterson et al., 2013; Barr et al., 2018; Fan et al., 2018; Wang G. H. et al., 2018). Even though the same mouse models were employed, alcohol administration was different, namely, 5% ethanol Lieber-DeCarli pair-fed diet daily feeding (Lowe et al., 2017), chronic intermittent vaporized ethanol for 4 weeks (Peterson et al., 2017), and fermented rice liquor consumption (Lee et al., 2020), which showed diverse alteration in gut microbiome. One study of rhesus macaques showed that the voluntary alcohol self-administration for 12 months did not alter the microbial community diversity (Barr et al., 2018), while another study showed that microbiome change induced by 3-month alcohol consumption can be reversed by 5-day withdrawal (Zhang X. et al., 2019). In one clinical study that compared with 24 healthy controls, the number of Bifidobacteria, Lactobacilli, and Enterococci were significantly reduced in 66 alcoholic patients (Kirpich et al., 2008). Patients with alcohol use disorder presented a unique pattern of gut microbiome: Akkermansia decreased and Bacteroides increased, with an accuracy of 93.4% (Addolorato et al., 2020). In alcoholics with dysbiosis, the median abundance of Proteobacteria was lower, which correlated with high levels of serum endotoxin in some subjects, and they had a higher frequency of mild diabetes (Mutlu et al., 2012). Patients diagnosed with alcohol dependence had higher intestinal permeability and their gut microbiome profile was altered, with more microbiome from Lachnospiraceae and Incertae Sedis XIV and less from Ruminococcaceae and Incertae Sedis XIII at the family level. The family Erysipelotrichaceae and the genus Holdemania decreased significantly after 3 weeks of detoxification, compared to the beginning of withdrawal (Leclercq et al., 2014b). In summary, although preclinical and clinical studies were complicated by many factors, including different species, alcohol concentration, types of ferment, stages of alcohol use disorders (AUD), mode and duration of alcohol administration, and the duration of withdrawal, there was still evidence to support the safety and benefit of microbial manipulation in short-term alcohol craving and consumption. However, further trials with larger number of patients are needed.
The Role of the Microbiome–Gut–Brain Axis in Alcohol-Induced Liver Damage (ALD)
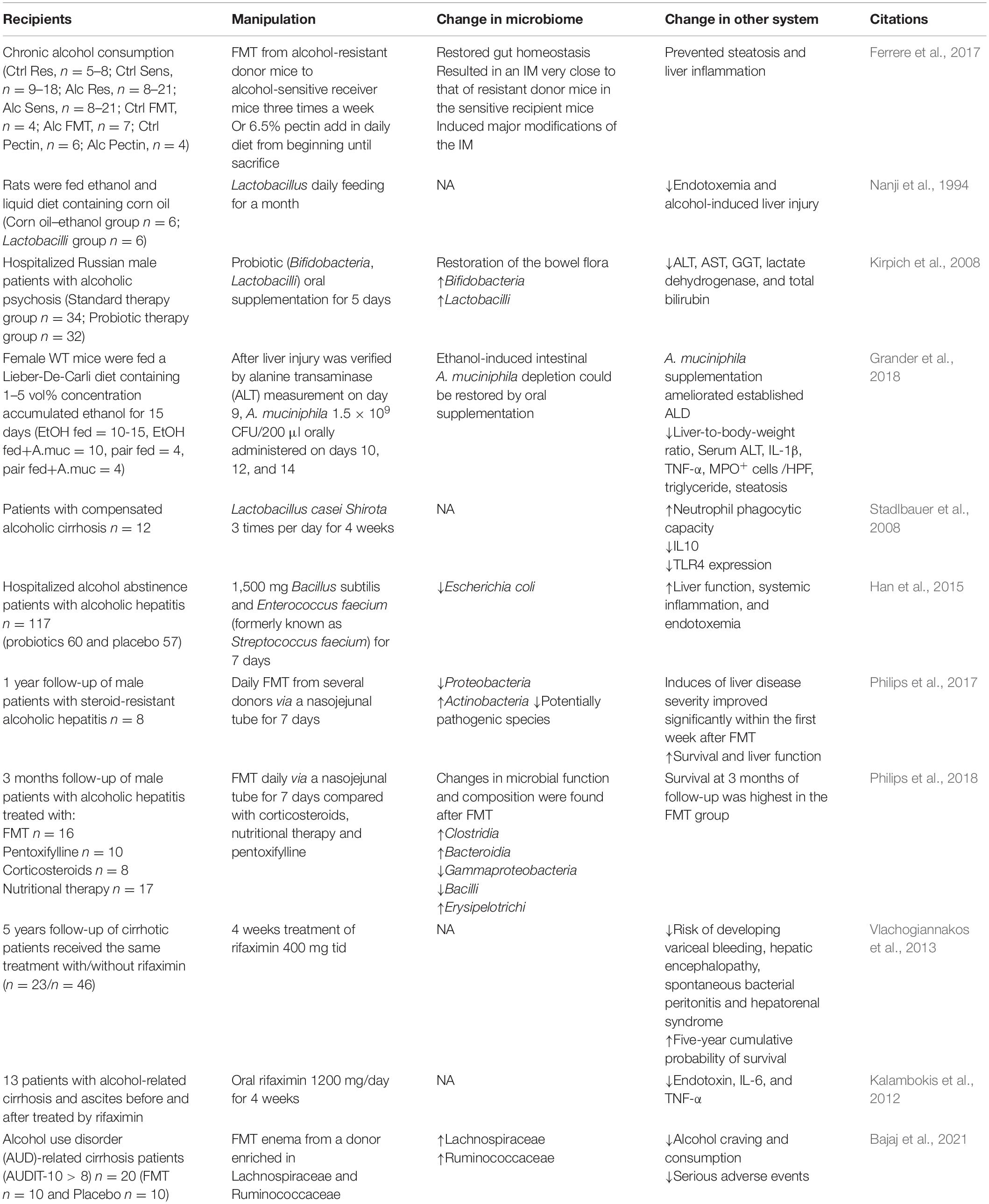
Alcohol mainly metabolized in liver. So far, a large number of studies have confirmed the important role of gut–liver axis in ALD (Bajaj, 2019). Fecal microbiome transplantation (FMT) from alcohol-resistant mice into alcohol-sensitive mice or pectin prebiotics can reshape intestinal micro-ecology homeostasis and prevent alcohol-induced fatty liver and inflammation (Ferrere et al., 2017). Specifically, a decrease in Lactobacillus and Bifidobacterium was seen in patients with alcohol dependence, and both clinical and animal studies suggested that restoration of Lactobacillus and Bifidobacterium could reduce endotoxemia and alcohol-induced liver injury (Nanji et al., 1994; Kirpich et al., 2008). Furthermore, the loss of Akkermansia was also considered as an early marker of alcohol-induced gut dysbiosis (Lowe et al., 2017). Patients with alcohol use disorder presented decreased Akkermansia and increased Bacteroides (Addolorato et al., 2020). Consistently, a study found that the decrease of relative abundance of Akkermansia was accompanied by more severe alcoholic liver disease, and the relative abundance of Akkermansia was the lowest in patients with severe alcoholic hepatitis. Oral supplementation of Akkermansia restored the ethanol-induced Akkermansia depletion and alleviated the liver function damage in acute and chronic alcohol-exposed mice (Grander et al., 2018). After FMT enema from a donor rich in Lachnospiraceae and Ruminococcaceae, patients with alcohol use disorder-related cirrhosis showed decreased alcohol craving and consumption, reduced serious adverse events, and improved cognitive function (Bajaj et al., 2021). Table 2 summarized preclinical studies and clinical trials of gut microbiome-based therapies for ALD. These results suggested that intervention in the gut microbiome, such as Akkermansia, Lactobacillus, and Bifidobacterium, is likely to be effective targets to prevent and treat ALD. Interestingly, some bacteria such as Clostridium genus, had been recognized as ethanol-producing bacteria (Wiegel et al., 2006), Clostridium -sensu stricto, -XI, -XVIII, Lactococcus, Turicibacter, and Akkermansia carried the genes encoding alcohol dehydrogenase and aldehyde dehydrogenase, indicating the direct functional ability of gut microbiome in alcohol production and/or metabolism (Konkit et al., 2016; Leclercq et al., 2020). Ruminococcus was identified as a potential acetaldehyde accumulator that is responsible for the pathogenesis of ethanol-related colorectal cancer (Tsuruya et al., 2016). These lines of evidence also revealed promising therapeutic strategies for ALD by metabolizing alcohol and aldehyde.
The Role of the Microbiome–Gut–Brain Axis in Alcohol-Induced Anxiety and Depression
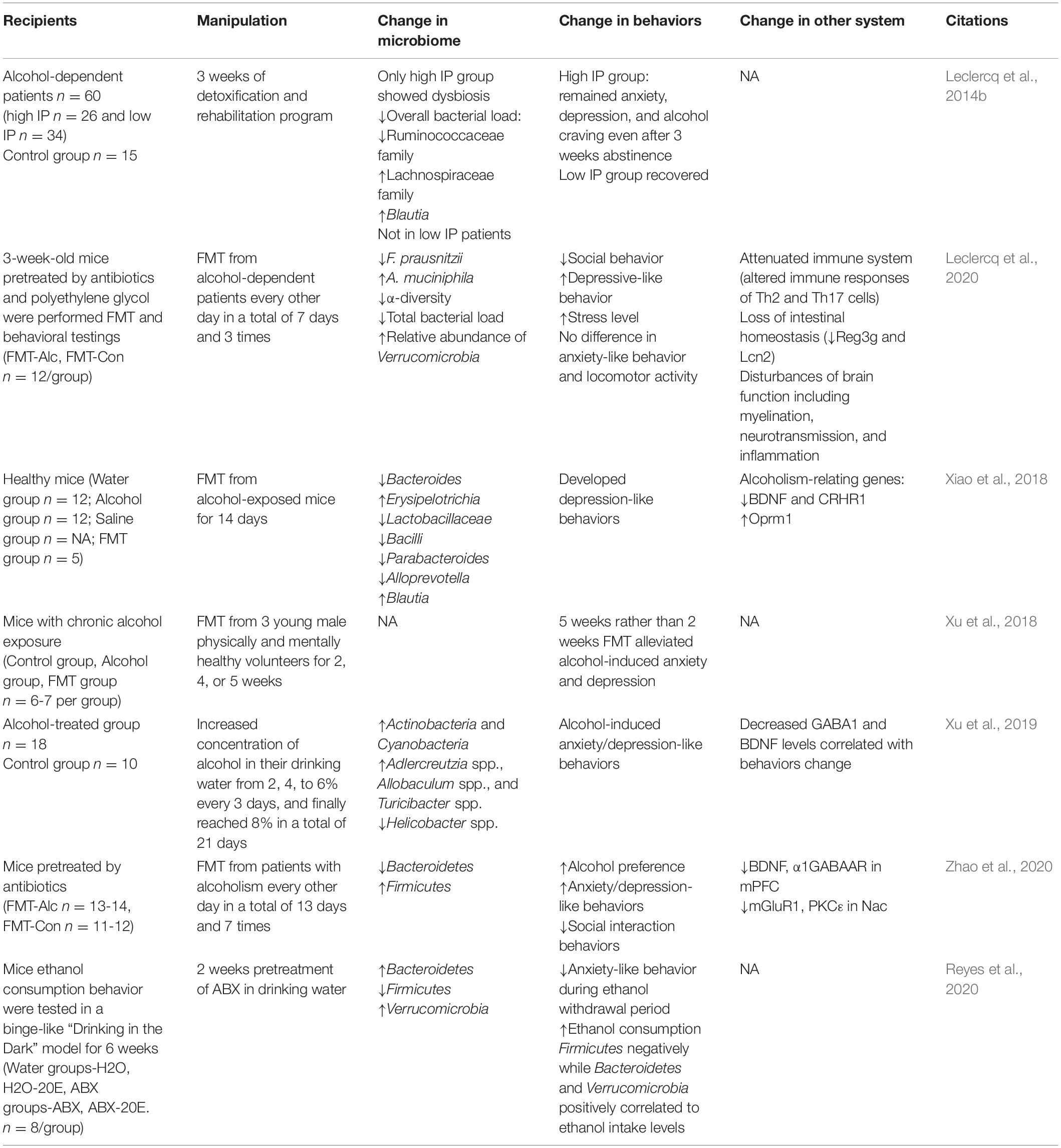
Psychiatric disorders, such as depression, bipolar disorders, and anxiety disorders, preceded the onset of alcohol abuse, and chronic alcohol exposure induced anxiety and depression symptoms, further aggregating AUD. Alcohol-induced anxiety and depression can bidirectionally interact with gut microbiome (Carbia et al., 2021). As summarized in Table 3, a series of studies from the same research group showed that only part of the alcohol-dependent patients showed dysbiosis in gut microbiome, while the dysbiosis-induced gut leakiness was highly correlated with alcohol craving, alcohol-induced depression, anxiety, and possibility of relapse. Low-grade inflammation in alcohol-dependent patients was also found to be correlated to depression and alcohol craving. In addition, the levels of Bifidobacterium spp. and Lactobacillus spp. increased significantly in alcohol-dependent subjects with high intestinal permeability during withdrawal period and returned to the level of control group. Besides, the intestinal permeability of alcohol-dependent patients completely recovered after a 3-week withdrawal. Blood lipopolysaccharides produced from gut microbiome increased in alcohol-dependent patients, which can stimulate specific inflammatory pathways correlated to alcohol craving, and gut leakiness favored the translocation of lipopolysaccharides from gut into the circulation system (Leclercq et al., 2012, 2014a,b, 2020). Direct evidence was from preclinical studies. One study established a chronic alcoholism withdrawal mouse model and transplanted gut microbiome of alcoholism and withdrawal mice into normal mice. The results came out that the normal mice developed anxiety behaviors caused by alcohol withdrawal (Xiao et al., 2018). Another study established an animal model of chronic alcohol exposure and innovatively designed three FMT schemes to explore the potential effects of three healthy donors’ fecal microbiome on alcohol-induced neuropsychological behaviors (Xu et al., 2018). Alcohol-induced anxiety and depression in mice can be relieved by transplantation of gut microbiome from healthy human donors, and conversely, healthy mice developed behavioral signs of alcohol withdrawal-induced anxiety by FMT from alcohol-exposed mice (Xiao et al., 2018; Xu et al., 2018). In 2019, Xu’s team used the same mouse model and control group to investigate the gut microbiome and anxiety/depression-like behavior testing, and found increases in Actinobacteria and Cyanobacteria at the phylum level and Adlercreutzia spp., Allobaculum spp., and Turicibacter spp. at the genus level, but Helicobacter spp. decreased. Meanwhile, the expression of BDNF/Gabra1 decreased in the prefrontal cortex (Xu et al., 2019). This team also found that the abundance of Firmicutes increased while Bacteroidetes decreased in alcoholism, and mice receiving FMT from these patients showed anxiety/depression-like behaviors (Zhao et al., 2020). Another research found that after 2 weeks of antibiotic pre-treatment, the level of Firmicutes decreased importantly and the levels of Bacteroidetes and Verrucomicrobia increased, accompanied by increased alcohol intake and relief of anxiety symptoms during alcohol withdrawal (Reyes et al., 2020). An interesting study collected fecal samples from human donors of alcohol use disorders who showed higher depression scores and alcohol craving with lower F. prausnitzii and A. muciniphila. After 45 days, AUD FMT mice showed decreased social behavior, more depression-like behavior, and higher cortisol level indicating higher stress, surprisingly with significantly higher A. muciniphila, indicating that there might be a comprehensive interaction among gut microbiome, alcohol use disorders, depression, and anxiety behaviors, or an interaction among different species in gut microbiomes (Leclercq et al., 2020). These studies presented a complex interaction among gut microbiome, alcohol exposure, and alcohol-induced anxiety/depression. It might be a challenge as well as a chance, for it was difficult to distinguish the impact of alcohol craving and alcohol-induced anxiety/depression on gut microbiome. Meanwhile, the treatment that relieved alcohol-induced anxiety/depression could also reduce alcohol craving.
In sum, the change of gut microbiome is closely related to alcohol-related disorders. However, currently the relationship between alcohol-related disorders and changes in gut microbiome is not consistent. They might be confounded by lots of factors, including different species, alcohol concentration and types of ferment, mode and duration of alcohol administration, stages of AUD, and the duration of withdrawal. Additionally, alcohol caused complicated symptoms accompanied by liver damage, depression, and anxiety, which, in turn, affect gut microbiome, making this field more mysterious. The interactions among gut microbiome–AUD–ALD–alcohol-related/induced depression and anxiety are complicated, and lots of confounding factors could not be easily controlled in research; the alteration in gut microbiome was diverse and inconsistent with each other. However, gut microbiome was irreversible, and after cessation of alcohol exposure or intervention such as probiotics, antibiotics, and FMT, alcohol-induced gut microbiome imbalance can be partially reconstructed and restored. Meanwhile, it can also improve alcohol-induced endotoxemia, liver function impairment, intestinal permeability, alcohol craving, and psychiatric symptoms such as anxiety and depression. Furthermore, some bacteria were considered to directly contribute to alcohol production and metabolism. Due to the persistence and adjustability of gut microbiome, therapy focused on it may be a promising target to treat alcohol-related disorders.
The Role of the Microbiome–Gut–Brain Axis in Opioid Use Disorders
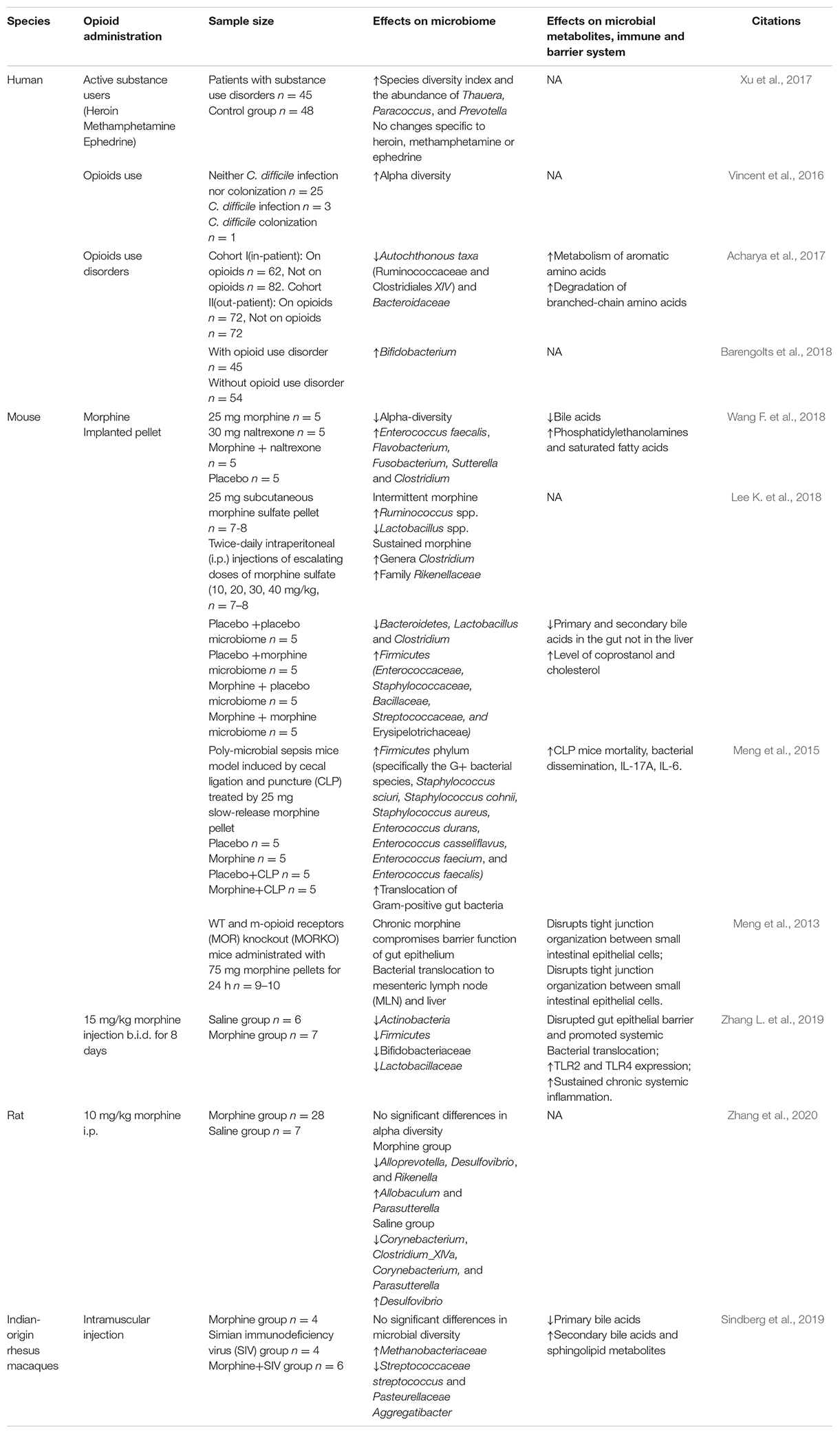
Studies on the relationship between opioid use disorders and gut microbiome are accumulating, although much fewer than those of alcohol (shown in Table 4). Preclinically, five studies employing mice implanted morphine pellets (Meng et al., 2013, 2015; Banerjee et al., 2016; Wang F. et al., 2018, 2020; Wang G. H. et al., 2018); one study of mice (Zhang L. et al., 2019), one study of rats (Zhang et al., 2020), and one study of rhesus macaques (Sindberg et al., 2019) with injected morphine showed the significantly altered composition of microbiome, but they showed inconsistent results. Intermittent morphine injection and sustained morphine-implanted pellet showed a different impact on gut microbiome in the same way (Lee K. et al., 2018). The discrepancy could be attributed to the limited sample size per group and confounded by the type of administration, dose and duration of morphine exposure, etc. Clinical studies also showed diverse results. A study recruited 48 healthy controls and 45 patients with substance use disorders. All of these patients with daily alcohol and tobacco were poly-drug abusers, including heroin or methamphetamine (MA) and/or ephedrine. The study found that the diversity indexes of patients were higher than controls, with increases in Prevotella, Paracoccus, and Thauera and a decrease in Bacteroides (Xu et al., 2017). Vincent et al. found that in 90 patients with neither C. difficile infection nor colonization, 25 patients who used opioid showed increased diversity of gut microbiome (Vincent et al., 2016). Acharya et al. in 2017 recruited two cohorts with/without opioid use from inpatients and outpatients, respectively, and found a significant dysbiosis in opioid use patients, especially those with hepatic encephalopathy (Acharya et al., 2017). Barengolts et al. conducted a cross-sectional study among African American men with obesity and type 2 diabetes comorbid psychiatric and opioid use disorders. Microbiome analysis showed that Bifidobacterium and Prevotella could be significantly affected by opioids and metformin interaction (Barengolts et al., 2018). These studies indicated the complex nature of gut microbiome especially in the studies of substance use disorders; more solid and direct evidence are required to figure out the relationship between opioid use disorders and gut microbiome.
The Role of the Microbiome–Gut–Brain Axis in Tolerance of Morphine Analgesia
Several animal studies focused on the role of microbiome in an individual’s response to morphine analgesia. Opioid analgesics represented by morphine have been widely used as medication all over the world. However, the long-term medical use of opioid as pain management is limited by its analgesic tolerance. It would be promising if microbiome could be a modulator for an individual’s response to morphine analgesia. Firstly, the study of Kang et al. showed that antibiotics can increase the tolerance to morphine, thus inhibiting the analgesic effect of morphine, comparing the control group with the antibiotic group and the antibiotic–morphine chronic exposure group (Kang et al., 2017). Later, Lee et al. transplanted fecal microbiome from the opioid-dependent group to antibiotics-pretreated mice, significantly increasing the response to morphine analgesia of these mice and restoring their cocaine location preference behavior (Lee K. et al., 2018). In 2018 and 2019, two studies from the same group also reached a consistent conclusion. The germ-free mice or antibiotic pretreatment presented prolonged efficacy of morphine in the treatment of mice and reduced the tolerance of morphine analgesic effect (Wang F. et al., 2018; Zhang L. et al., 2019). The evidence above supported the idea that gut microbiome played an important role in the tolerance to morphine analgesia, and manipulations of gut microbiome by antibiotic treatment may be a potential treatment to improve the response to morphine analgesia. Furthermore, Wang et al. further found significantly increased Enterococcus faecalis induced by morphine, and E. faecalis colonized mice showed significant increase in morphine tolerance (Wang F. et al., 2018). A further study by Zhang et al. reported that FMT from normal mice did not significantly prevent the occurrence of tolerance to morphine analgesia after chronic morphine exposure, but after 21 days of intragastric pretreatment with probiotic VSL # 3, composed of eight different strains of bacteria mainly belonging to Bifidobacterium and Lactobacillus, the tolerance significantly decreased. This study further explored the underlying mechanism and found that morphine tolerance was mediated by probiotics through activating TLR2/4 and pro-inflammatory cytokine cascades (Zhang L. et al., 2019). In sum, all evidence supported the idea that gut microbiome, especially Bifidobacteria and Lactobacillus, played an important role in tolerance to morphine analgesia. In addition to germ-free or antibiotic pretreatment, Bifidobacteria and Lactobacillus transplantation might be a safer, more effective, and more economical treatment to improve the individual’s response to morphine analgesics. All of these studies are summarized in Table 5.
Compared with alcohol-related disorders, studies on the correlation between morphine-related disorders and gut microbiome were much fewer. Similarly, the changes of gut microbiome in morphine-related disorders were also affected by many factors such as different animal models, different routes of administration, duration of exposure, and different stages of the disorders. Most of the studies focused on the role and mechanism of gut microbiome in morphine analgesia. Probiotics mainly containing Bifidobacteria and Lactobacillus might become a promising therapeutic drug to prolong the analgesic effect of morphine.
The Role of the Microbiome–Gut–Brain Axis in Psychostimulant Use Disorders
The abuse of psychostimulants, including cocaine and amphetamines, especially novel synthetic drugs, has posed serious impact all over the world. However, studies on the microbiome–gut–brain axis and addiction to psychostimulants were much fewer and mainly based on animal models.
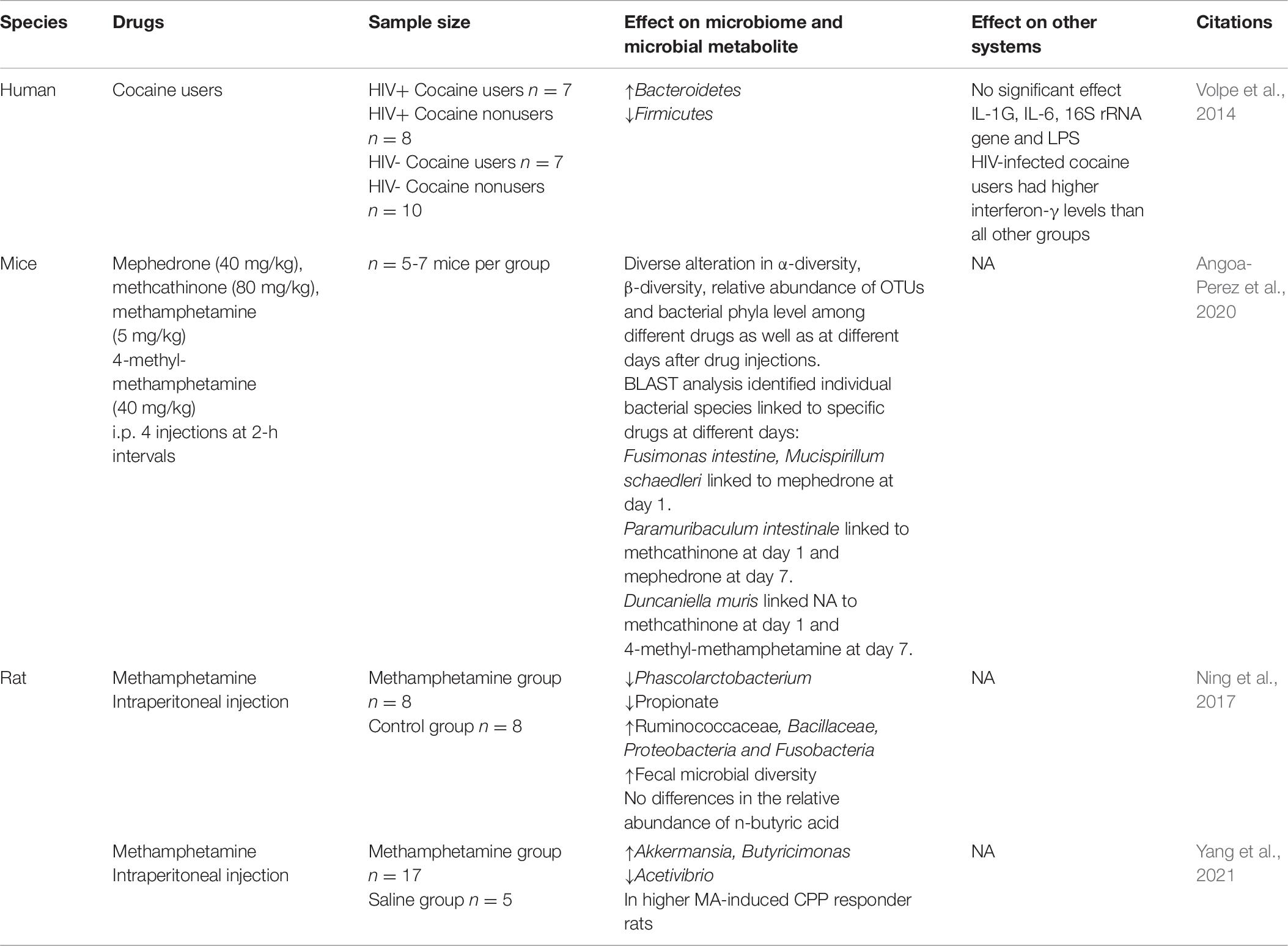
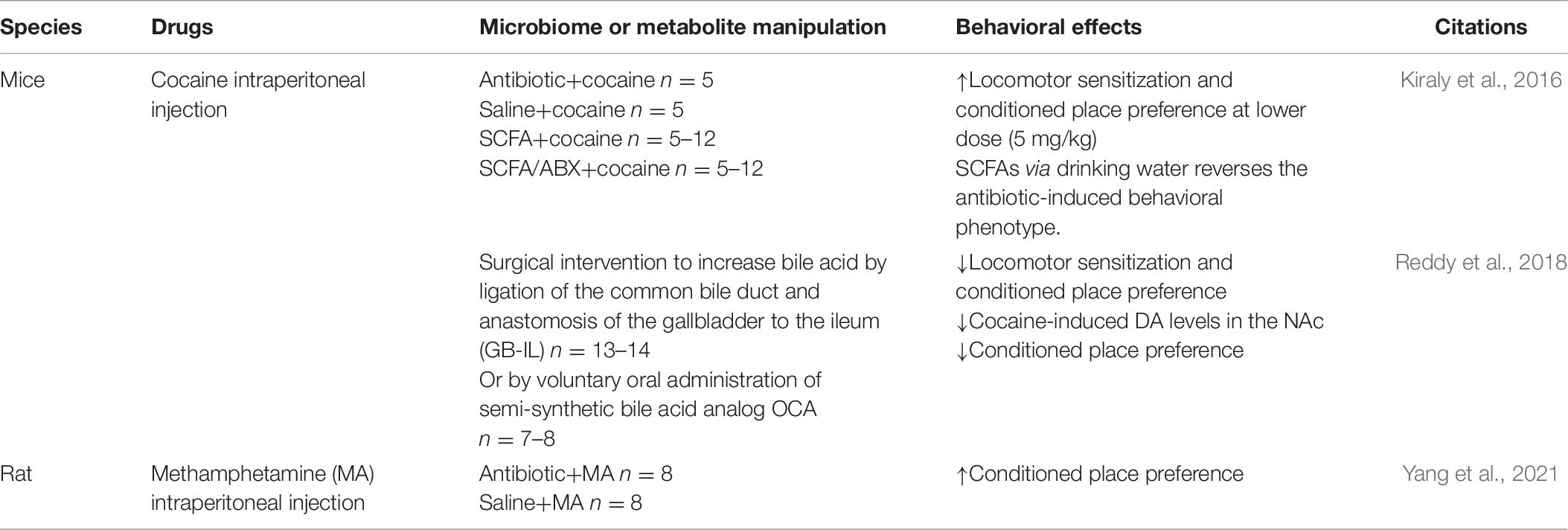
As shown in Table 6, Ning et al. focused on the relationship between MA and gut microbiome, in which a dysregulation of gut microbiome was detected after a 14-day induction of conditioned place preference (CPP) using MA, including a significant decrease of the relative abundance of Phascolarctobacterium and a decrease of propionate. What is more, unlike other addictive drugs, the diversity of microbial community in the MA group was slightly higher than that in the control group (Ning et al., 2017). This result has been further verified by our team in 2020, and our study further found the association between gut microbiome and an individual’s response to MA reward property (Yang et al., 2021). Similarly, Kiraly et al. (2016) also found that the sensitivity of mice to cocaine reward and their behavioral response to chronic cocaine use were also enhanced after antibiotic treatment before CPP training. A recent research showed that compared with amphetamine stimulants such as MA and 4-methylmethamphetamine, the effect on gut microbiome in mice treated with selected synthetic psychoactive cathinones (including methoxyephedrine and methylcathinone) was significantly different (Angoa-Perez et al., 2020), which indicates that different substances, even different kinds of psychostimulants, have different effects on gut microbiome, and this may be caused by different pharmacological mechanisms of drugs themselves; nevertheless, many other confounding factors may exist. So far, there was only one clinical study by Volpe et al. that examined the gut microbiome of patients with cocaine addiction and healthy controls, and the results showed that the Bacteroides in patients significantly increased (Volpe et al., 2014). An elegant study showed that a surgery ligating the common bile duct and anastomosis of the gallbladder to the ileum (GB-IL) or voluntary oral administration of semi-synthetic bile acid analog OCA blocked the increased DA levels in the NAc and reduced cocaine locomotor sensitization as well as cocaine CPP, and further demonstrated the mechanism of bile acid signaling in regulating the behavioral response to cocaine via Takeda G protein-coupled bile acid receptor (TGR5), utilizing a TGR5 knockout mouse model and viral restoration of NAc, which is sufficient to restore cocaine reward. This study opens up novel ways of gut-to-brain communication (Reddy et al., 2018). As summarized in Table 7, antibiotic-treated mice showed increased cocaine CPP response while antibiotic-pretreated rats showed elevated MA-induced CPP (Yang et al., 2021). In sum, although there is a lack of clinical studies about the role of gut microbiome in psychostimulant use disorders, preclinical studies showed discrepancy in gut microbiome induced by the exposure of psychostimulants, compared to other substances or among different kinds of psychostimulants. Manipulations of gut microbiome by antibiotic treatment consistently showed the increased sensitivity to drug reward and behavioral response to psychostimulant use; additionally, bile acid signaling regulated the behavioral response to cocaine via TGR5. These data indicated the potential role of gut microbiome in individual susceptibility of psychostimulants addiction. However, compared to alcohol and opioid, the roles of gut microbiome in psychostimulant use disorders still need to be clarified.
The Role of the Microbiome–Gut–Brain Axis in Other Substance Use Disorders
Other addictive substances, such as nicotine (Chi et al., 2017), marijuana (Panee et al., 2018), and ketamine (Yang et al., 2017), also have an impact on the gut microbiome. A population-based study found significant differences in gut microbiome between current smokers and non-smokers (Lee S. H. et al., 2018; Nolan-Kenney et al., 2020). Research has proved that nicotine, as the main active content in tobacco, has effects on gut microbiome community composition, functional bacterial genes, and fecal metabolites (Chi et al., 2017). Similarly, preliminary results demonstrated a close correlation between the use of cannabis and the change of gut microbiome, such as a significantly increased Prevotella:Bacteroides ratio, which may further lead to cognitive impairment (Panee et al., 2018). Chronic Δ9-tetrahydrocannabinol (THC)-administrated mice showed altered gut microbiome composition (Cluny et al., 2015). Furthermore, studies showed that cannabinoid cannabidiol (CBD) or THC showed preclinical promising in alcohol use disorders and diet-induced obesity collectively through the microbiome–gut–brain axis (Cluny et al., 2015; Cani et al., 2016; Karoly et al., 2020). Ketamine, an inhibitor of the central nervous system, also has specific effects on the levels of Mollicutes and Butyricimonas, and may exert its antidepressant effect through the microbiome–gut–brain axis (Yang et al., 2017). These lines of evidence suggest that gut microbiome may contribute to substance use disorders through the microbiome–gut–brain axis, providing a new perspective for the study of addiction mechanism and therapeutic target investigation.
The Role of the Microbiome–Gut–Brain Axis in Individual Susceptibility of Addiction
Through studies about the correlation between addiction and gut microbiome, it turns out that gut microbiome plays a role in individual’s susceptibility of addiction. The susceptibility of addiction appears that, after an initial exposure to the same dose of the same addictive substance, some individuals remain in the stage of occasional use for a long time, while some gradually develop into regular use, and finally compulsive drug-seeking and addiction (Hao et al., 2016). One study found that uncontrollable drug-seeking behavior only occurred in 15% of the mice with alcohol self-administration. According to the behavioral indicators related to alcohol addiction, these rats were divided into alcohol-susceptible groups and alcohol-resistant groups. Comparing the gut microbiome between the two groups, significant differences were found, which was related to the response of rats to alcohol. The relative abundances of Clostridiales, Ruminococcaceae, and Lachnospiraceae were positively correlated with alcohol-use behavior, while the relative abundances of Desulfovibrio, Gemella, Coriobacteriaceae, and Hydrogenoanaerobacterium were negatively correlated with total alcohol consumption (Jadhav et al., 2018). In 2020, a study from Reyes et al. found that after 2 weeks of antibiotic pretreatment, the spontaneous alcohol intake of mice in the drinking in the dark model increased significantly, and the intestinal Bacteroides increased while Firmicutes decreased (Reyes et al., 2020). These findings suggest that gut microbiome is significantly associated with individual susceptibility to alcohol, and changing gut microbiome can change an individual’s susceptibility to alcohol. Furthermore, Bareli’s study on rats showed that cocaine self-administration altered gut microbiome, while dehydroepiandrosterone treatment, an endogenous neurosteroid and a food supplement, attenuated drug-seeking behavior by shifting the gut microbial diversity towards the control group and raised a certain bacterium, then reduced the drug-seeking behavior of rats (Bareli et al., 2019). Kiraly et al. (2016) found that the sensitivity of mice to cocaine reward and their behavioral response to chronic cocaine use were also enhanced after antibiotic treatment of mice before CPP training. In addition, Lee et al. found that after intermittent morphine administration, antibiotic therapy can reduce opioid analgesic efficacy and damage brain cocaine reward circuit by reproducing neuroinflammation, and reduce the CPP value of cocaine in mice. To further verify the experimental results, they colonized microbiome of untreated normal controls in mice treated with sustained antibiotics, and found that it can help restore the activation state of microglia and the CPP behavior to cocaine in mice (Lee K. et al., 2018). Our team found that only part of the rats exposed to the same dose of MA showed a significant preference for drug testing in CPP training. Furthermore, comparing the changes of gut microbiome in rats grouped with high MA preference and low MA preference, we found that Verrucomicrobia and Butyricimonas increased significantly in the high-preference group, and the relative abundance was positively correlated with the increased preference, while Acetivibrio increased significantly in the low-preference group, and was correlated with the decreased preference. Furthermore, rats pretreated with broad-spectrum antibiotics for 1 week showed a significantly increased MA preference (Yang et al., 2021) (see Table 7). Following a similar strategy, we found that the altered bacterial phyla level also correlated with morphine-induced preference (Zhang et al., 2020). These lines of evidence suggested the important role of gut microbiome in individual susceptibility of addiction or behavior response to alcohol, opioid, cocaine, and MA. Manipulation of gut microbiome might be an efficient modulator for individual sensitivity to drug award, morphine analgesia, and risk of development of addiction. However, this field is still under development, and more direct and strong evidence from both clinical and preclinical studies are required.
Tridirectional Interaction Among Gut Microbiome, Substance Addiction, and Substance-Induced/Related Mental Disorders
Substance use disorder is a comprehensive disorder with lots of symptoms overlapping with other psychiatric disorders. First of all, various psychotic symptoms induced by substance use usually appear during or immediately after substance use (within 48 h); onset excluding delirium and withdrawal symptoms or after 2 weeks of substance use can be diagnosed as mental disorders caused by substance use in DSM-5, including psychotic disorders, bipolar and related disorders, depressive disorders, anxiety disorders, sleep disorders, sexual dysfunction, delirium, and neurocognitive disorders. Secondly, the comorbidity in substance use disorder and mental disorders is also very common. According to the National Comorbidity Study, 50.9% of Americans who have suffered from lifelong mental disorders have also suffered from substance abuse. Meanwhile, among Americans who have suffered from lifelong drug abuse, the lifetime prevalence of mental disorders is 51.4%. Mood disorders, anxiety disorders, and personality disorders are more common (Hawkins, 2009; Green and Drake, 2011). As mentioned above, alcohol-induced anxiety/depression could be produced by FMT from alcohol-exposed donors and relived by probiotic treatment (Reyes et al., 2020; Zhao et al., 2020). In a study by Reyes et al. (2020), 2 weeks of antibiotic pretreatment relieved anxiety symptoms during alcohol withdrawal, and the level of Firmicutes negatively correlated while Bacteroidetes and Verrucomicrobia positively correlated to alcohol intake. Plenty of other addictive substances, such as MA or ketamine, are frequently reported as various psychotic symptoms (McKetin et al., 2017), while tons of clinical and basic studies have proved the interaction between these mental disorders and gut microbiome (Sharon et al., 2016; Barbosa and Vieira-Coelho, 2020; Chinna Meyyappan et al., 2020; Zagorska et al., 2020). How could we rule out the confounding effects of substance-induced/related mental disorders when we focus on the correlation between gut microbiome and substance addiction? Is there any commonality or difference about the role of gut microbiome in mental disorders and substance-induced/related mental disorders? Could the microbiome manipulation that provides relief for alcohol-induced anxiety/depression be a treatment for anxiety/depression disorders? Further investigations are required to answer these questions.
Challenge and Prospect
Research on gut microbiome and substance-related disorders is promising but still under development. As showed in Figure 1, present evidence reveals that gut microbiome plays an important role in substance use disorders via the microbiome–gut–brain axis, and preclinical studies suggest the therapeutic potential of gut microbiome manipulation in substance-related disorders, which has a very promising future. However, more evidence especially from clinical studies is needed and there are lots of challenges to further demonstrate the causal relationship between gut microbiome and substance-related disorders, to investigate the underlying mechanism and explore therapeutic targets.
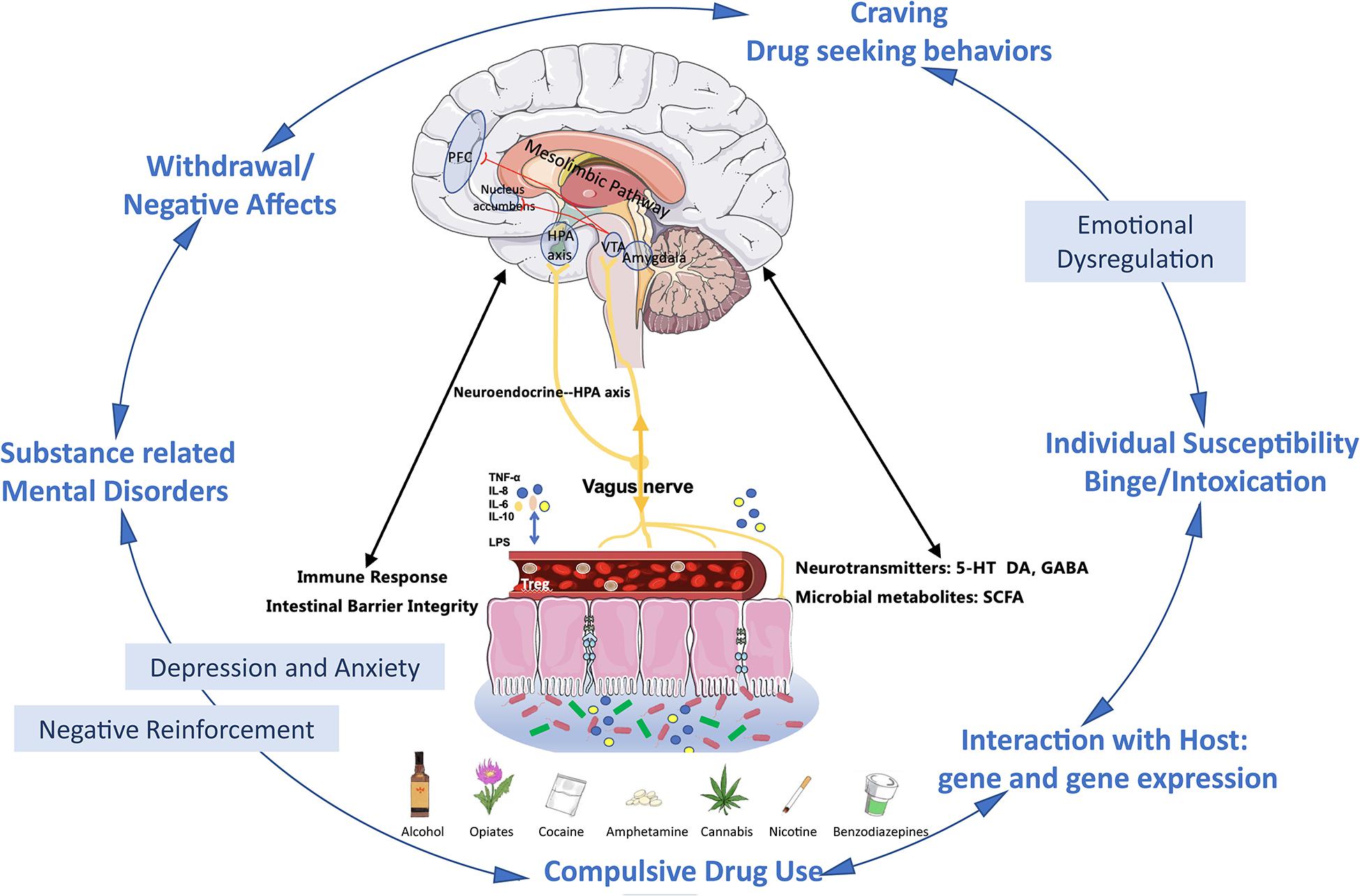
Figure 1. Microbiome–gut–brain in substance use disorders and substance-related mental disorders. First, via the microbiome–gut–brain axis, substance exposure-induced dysbiosis leads to the damage of the gut epithelial barrier. In turn, it further causes the increased permeability (“leaky gut”) of bacterial products such as LPS lipopolysaccharide (LPS), immune cytokines (TNF-α, IL-6, IL-8, IL-10, etc.), hormones, neurotransmitters, and microbial metabolites produced by the gut microbiome. These products can be released to the systemic circulation such as blood stream and then transported to the brain, and these gut derived components also target the HPA axis and vagus nerve. These all together further impact on brain barrier integrity and immunoactivities in brains and alter the reward system (Carbia et al., 2021; Garcia-Cabrerizo et al., 2021). Second, the gut microbiome plays an important role in the cycle of development and maintenance of addiction. At the binge/intoxication stage, substance use is mainly initiated by individual susceptibility of the drug, which might be determined by the interaction between the microbiome and the host genetic background. In this stage, the same emotional problems might also precede the use of the drug. Repeated binge induces the progressively microbiome imbalances and contributes to early emotional dysregulation, and further motivates the craving and drug seeking behaviors. At the withdrawal/negative effect stage, exaggerated emotional disturbances and dysbiosis combine with the negative effects related to withdrawal driven negative reinforcement and finally accelerate the transition to compulsive alcohol use (Wise and Koob, 2014; Carbia et al., 2021; Garcia-Cabrerizo et al., 2021). Additionally, the substance-related mental disorders, especially depression and anxiety, could further exacerbate dysbiosis (Reyes et al., 2020; Carbia et al., 2021). Abbreviations: PFC, prefrontal cortex; VTA, ventral tegmental area; NAc, nucleus accumbens; AMYG, amygdala; NTS, nucleus tractus solitarii; IL, interleukin; TNF, tumor necrosis factor; LPS, lipopolysaccharide; SCFA, short-chain fatty acids; 5-HT, 5-hydroxytryptamine; DA, dopamine; GABA, γ-aminobutyric acid; HPA, hypothalamic–pituitary–adrenal.
Confounding Factors Complicate the Problem
Substance use disorders are comprehensive disorders. There might be lots of confounding factors in the study of gut microbiome and substance use disorders. On the one hand, firstly, gut microbiome itself could be affected by various factors including age, gender, genetic background, diet, infection and diseases, medications, and stressors. Secondly, specifically, there are several substance use-related confounding factors, such as different addictive substances, different dosages and duration of the same substance, at different stages of addiction (acute use, chronic use, withdrawal and relapse, etc.), different combinations and dosages of antibiotics/probiotic treatment, liver function damage, endotoxemia, and other systemic diseases such as HIV or bacterial infection accompanied or induced by the use of substances. Additionally, other physical diseases, psychotic symptoms, or psychiatric disorders such as schizophrenia, mood dysfunction, anxiety neurosis, or personality disorders associated with substance use also interacted with gut microbiome. All of the above factors complicated this problem. On the other hand, there is a very comprehensive mechanism in substance-related disorders, and what we can see is based on a one-sided viewpoint. Firstly, it is likely that there are multiple gut microbiomes rather than a single bacterium that plays an essential role in substance-related disorders. Gut microbiome, host genetic background, gene expression difference, and other factors combined together affect the onset and development of substance-related disorders. Therefore, to further study the role of gut microbiome in substance-related disorders, well-designed experiments, adequate sample size, and proper analysis are needed.
Possibility of Shared Biomarkers in Gut Microbiome for Different Substance Addiction
It is worth mentioning that different substance-related disorders have common behavioral characteristics and neurobiological mechanisms, including irrepressible and uncontrollable drug craving and repeated compulsive drug-seeking behavior related to drug addiction (Hao et al., 2016). The common neural structural basis of addiction is the VTA–NAc circuit of reward circuit located in the limbic system (Kim et al., 2016; Koob and Volkow, 2016). Long-term abuse of addictive substance can lead to abnormal activation of dopaminergic neurons in reward circuits (Koob and Le Moal, 2001). There will also likely be a common specific pattern about correlated changes of gut microbiome in different addictive substance-related disorders when the variables are strictly controlled. It will not only identify novel common biomarkers of gut microbiome for substance addiction but also become a collective therapeutic target for substance use disorders.
Summary and Conclusion
Current studies illustrate a complex relationship between gut microbiome and substance-related disorders, involving multiple confounding factors. Also, gut microbiome may interact with the genetic background and gene expression of host cells to influence the occurrence and development of substance-related disorders. However, plenty of preclinical animal studies have found that different gut microbiome of different individuals or the change of gut microbiome will in turn affect the host, including the response to addictive substances and the onset and development of substance-related/induced mental diseases. These lines of evidence suggest that gut microbiome plays an important role in substance-related disorders and has a potential to be a new therapeutic target. However, it is still lacks strong and direct evidence especially from clinical trials to make a final conclusion. In a word, the research on the relationship between substance use disorder and gut microbiome is still in its infancy with a bright future ahead, but there is a long way to go.
Author Contributions
XZ designed the overall idea. XZ, XF, TC, and JC performed the review of the literature and wrote the manuscript. BL and YZ participated in reviewing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Hunan Province Natural Science Foundation Project (2021JJ40894), the Scientific Research Project of Hunan Provincial Health Commission (202103090528), the Changsha Natural Science Foundation Project (kq2014239) for XZ, and the Central South University Undergraduate Research and Innovation (URI) Program (XCX20190547) for XF.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acharya, C., Betrapally, N. S., Gillevet, P. M., Sterling, R. K., Akbarali, H., White, M. B., et al. (2017). Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment. Pharmacol. Ther. 45, 319–331. doi: 10.1111/apt.13858
Addolorato, G., Ponziani, F. R., Dionisi, T., Mosoni, C., Vassallo, G. A., Sestito, L., et al. (2020). Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol-associated liver disease. Liver Int. 40, 878–888. doi: 10.1111/liv.14383
Angoa-Perez, M., Zagorac, B., Winters, A. D., Greenberg, J. M., Ahmad, M., Theis, K. R., et al. (2020). Differential effects of synthetic psychoactive cathinones and amphetamine stimulants on the gut microbiome in mice. PLoS One 15:e0227774. doi: 10.1371/journal.pone.0227774
Bajaj, J. S. (2019). Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 235–246. doi: 10.1038/s41575-018-0099-1
Bajaj, J. S., Gavis, E. A., Fagan, A., Wade, J. B., Thacker, L. R., Fuchs, M., et al. (2021). A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology 73, 1688–1700. doi: 10.1002/hep.31496
Bajaj, J. S., Kakiyama, G., Zhao, D., Takei, H., Fagan, A., Hylemon, P., et al. (2017). Continued alcohol misuse in human cirrhosis is associated with an impaired gut–liver axis. Alcohol. Clin. Exp. Res. 41, 1857–1865. doi: 10.1111/acer.13498
Bala, S., Marcos, M., Gattu, A., Catalano, D., and Szabo, G. (2014). Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One 9:e96864. doi: 10.1371/journal.pone.0096864
Banerjee, S., Sindberg, G., Wang, F., Meng, J., Sharma, U., Zhang, L., et al. (2016). Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. 9, 1418–1428. doi: 10.1038/mi.2016.9
Barbosa, R. S. D., and Vieira-Coelho, M. A. (2020). Probiotics and prebiotics: focus on psychiatric disorders - a systematic review. Nutr. Rev. 78, 437–450. doi: 10.1093/nutrit/nuz080
Bareli, T., Haput, R., Levi-Ahdoot, H., Nissan, H., Koren, O., and Yadid, G. (2019). Cocaine alters the gut microbiome in a self-administration rat model: reversal by dehydroepiandrosterone treatment. Eur. Neuropsychopharmacol. 29, S495–S496. doi: 10.1016/j.euroneuro.2019.09.773
Barengolts, E., Green, S. J., Eisenberg, Y., Akbar, A., Reddivari, B., Layden, B. T., et al. (2018). Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS One 13:e0194171. doi: 10.1371/journal.pone.0194171
Barr, T., Sureshchandra, S., Ruegger, P., Zhang, J., Ma, W., Borneman, J., et al. (2018). Concurrent gut transcriptome and microbiota profiling following chronic ethanol consumption in nonhuman primates. Gut Microbes 9, 338–356. doi: 10.1080/19490976.2018.1441663
Bull-Otterson, L., Feng, W., Kirpich, I., Wang, Y., Qin, X., Liu, Y., et al. (2013). Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 8:e53028. doi: 10.1371/journal.pone.0053028
Burokas, A., Moloney, R. D., Dinan, T. G., and Cryan, J. F. (2015). Microbiota regulation of the Mammalian gut-brain axis. Adv. Appl. Microbiol. 91, 1–62. doi: 10.1016/bs.aambs.2015.02.001
Cani, P. D., Plovier, H., Van Hul, M., Geurts, L., Delzenne, N. M., Druart, C., et al. (2016). Endocannabinoids–at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 12, 133–143. doi: 10.1038/nrendo.2015.211
Carbia, C., Lannoy, S., Maurage, P., Lopez-Caneda, E., O’riordan, K. J., Dinan, T. G., et al. (2021). A biological framework for emotional dysregulation in alcohol misuse: from gut to brain. Mol. Psychiatry 26, 1098–1118. doi: 10.1038/s41380-020-00970-6
Chi, L., Mahbub, R., Gao, B., Bian, X., Tu, P., Ru, H., et al. (2017). Nicotine alters the gut microbiome and metabolites of gut-brain interactions in a sex-specific manner. Chem. Res. Toxicol. 30, 2110–2119. doi: 10.1021/acs.chemrestox.7b00162
Chinna Meyyappan, A., Forth, E., Wallace, C. J. K., and Milev, R. (2020). Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry 20:299. doi: 10.1186/s12888-020-02654-5
Cluny, N. L., Keenan, C. M., Reimer, R. A., Le Foll, B., and Sharkey, K. A. (2015). Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with delta9-tetrahydrocannabinol. PLoS One 10:e0144270. doi: 10.1371/journal.pone.0144270
Dinan, T. G., and Cryan, J. F. (2017). The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am. 46, 77–89. doi: 10.1016/j.gtc.2016.09.007
Dubinkina, V. B., Tyakht, A. V., Odintsova, V. Y., Yarygin, K. S., Kovarsky, B. A., Pavlenko, A. V., et al. (2017). Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 5:141. doi: 10.1186/s40168-017-0359-2
Fan, Y., Ya, E. Z., Ji-Dong, W., Yu-Fan, L., Ying, Z., Ya-Lun, S., et al. (2018). Comparison of microbial diversity and composition in jejunum and colon of the alcohol-dependent rats. J. Microbiol. Biotechnol. 28, 1883–1895. doi: 10.4014/jmb.1806.06050
Ferrere, G., Wrzosek, L., Cailleux, F., Turpin, W., Puchois, V., Spatz, M., et al. (2017). Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J. Hepatol. 66, 806–815. doi: 10.1016/j.jhep.2016.11.008
Garcia-Cabrerizo, R., Carbia, C., Kj, O. R., Schellekens, H., and Cryan, J. F. (2021). Microbiota-gut-brain axis as a regulator of reward processes. J. Neurochem. 157, 1495–1524. doi: 10.1111/jnc.15284
Grander, C., Adolph, T. E., Wieser, V., Lowe, P., Wrzosek, L., Gyongyosi, B., et al. (2018). Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67, 891–901. doi: 10.1136/gutjnl-2016-313432
Green, A. I., and Drake, R. E. (2011). Current epidemiology and emerging interventions for people with co-occurring mental illness and substance use disorders. J. Dual Diagn. 7, 1–3. doi: 10.1080/15504263.2011.571624
Han, S. H., Suk, K. T., Kim, D. J., Kim, M. Y., Baik, S. K., Kim, Y. D., et al. (2015). Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur. J. Gastroenterol. Hepatol. 27, 1300–1306. doi: 10.1097/MEG.0000000000000458
Hao, W., Zhao, M., and Li, J. (2016). Addiction Medicine: Theory and Practice. Beijing: People’s Medical Publishing House.
Hawkins, E. H. (2009). A tale of two systems: co-occurring mental health and substance abuse disorders treatment for adolescents. Annu. Rev. Psychol. 60, 197–227. doi: 10.1146/annurev.psych.60.110707.163456
Jadhav, K. S., Peterson, V. L., Halfon, O., Ahern, G., Fouhy, F., Stanton, C., et al. (2018). Gut microbiome correlates with altered striatal dopamine receptor expression in a model of compulsive alcohol seeking. Neuropharmacology 141, 249–259. doi: 10.1016/j.neuropharm.2018.08.026
Kalambokis, G. N., Mouzaki, A., Rodi, M., Pappas, K., Fotopoulos, A., Xourgia, X., et al. (2012). Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin. Gastroenterol. Hepatol. 10, 815–818. doi: 10.1016/j.cgh.2012.02.025
Kang, M., Mischel, R. A., Bhave, S., Komla, E., Cho, A., Huang, C., et al. (2017). The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci. Rep. 7:42658. doi: 10.1038/srep42658
Karoly, H. C., Mueller, R. L., Bidwell, L. C., and Hutchison, K. E. (2020). Cannabinoids and the microbiota-gut-brain axis: emerging effects of cannabidiol and potential applications to alcohol use disorders. Alcohol. Clin. Exp. Res. 44, 340–353. doi: 10.1111/acer.14256
Kim, J., Ham, S., Hong, H., Moon, C., and Im, H. I. (2016). Brain reward circuits in morphine addiction. Mol. Cells 39, 645–653. doi: 10.14348/molcells.2016.0137
Kiraly, D. D., Walker, D. M., Calipari, E. S., Labonte, B., Issler, O., Pena, C. J., et al. (2016). Alterations of the host microbiome affect behavioral responses to cocaine. Sci. Rep. 6:35455. doi: 10.1038/srep35455
Kirpich, I. A., Solovieva, N. V., Leikhter, S. N., Shidakova, N. A., Lebedeva, O. V., Sidorov, P. I., et al. (2008). Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 42, 675–682. doi: 10.1016/j.alcohol.2008.08.006
Konkit, M., Choi, W. J., and Kim, W. (2016). Aldehyde dehydrogenase activity in Lactococcus chungangensis: application in cream cheese to reduce aldehyde in alcohol metabolism. J. Dairy Sci. 99, 1755–1761. doi: 10.3168/jds.2015-10549
Koob, G. F., and Le Moal, M. (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24, 97–129. doi: 10.1016/S0893-133X(00)00195-0
Koob, G. F., and Volkow, N. D. (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. doi: 10.1016/S2215-0366(16)00104-8
Leclercq, S., Cani, P. D., Neyrinck, A. M., Stärkel, P., Jamar, F. O., Mikolajczak, M. R., et al. (2012). Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav. Immun. 26, 911–918. doi: 10.1016/j.bbi.2012.04.001
Leclercq, S., De Saeger, C., Delzenne, N., De Timary, P., and Starkel, P. (2014a). Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol. Psychiatry 76, 725–733. doi: 10.1016/j.biopsych.2014.02.003
Leclercq, S., Le Roy, T., Furgiuele, S., Coste, V., Bindels, L. B., Leyrolle, Q., et al. (2020). Gut microbiota-induced changes in beta-hydroxybutyrate metabolism are linked to altered sociability and depression in alcohol use disorder. Cell Rep. 33:108238. doi: 10.1016/j.celrep.2020.108238
Leclercq, S., Matamoros, S., Cani, P. D., Neyrinck, A. M., Jamar, F., Starkel, P., et al. (2014b). Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. U.S.A. 111, E4485–E4493. doi: 10.1073/pnas.1415174111
Lee, J. E., Ha, J. S., Park, H. Y., and Lee, E. (2020). Alteration of gut microbiota composition by short-term low-dose alcohol intake is restored by fermented rice liquor in mice. Food Res. Int. 128:108800.
Lee, K., Vuong, H. E., Nusbaum, D. J., Hsiao, E. Y., Evans, C. J., and Taylor, A. M. W. (2018). The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43, 2606–2614. doi: 10.1016/j.foodres.2019.108800
Lee, S. H., Yun, Y., Kim, S. J., Lee, E. J., Chang, Y., Ryu, S., et al. (2018). Association between cigarette smoking status and composition of gut microbiota. J. Clin. Med. 7:282. doi: 10.3390/jcm7090282
Lowe, P. P., Gyongyosi, B., Satishchandran, A., Iracheta-Vellve, A., Ambade, A., Kodys, K., et al. (2017). Alcohol-related changes in the intestinal microbiome influence neutrophil infiltration, inflammation and steatosis in early alcoholic hepatitis in mice. PLoS One 12:e0174544. doi: 10.1371/journal.pone.0174544
Mayer, E. A., Knight, R., Mazmanian, S. K., Cryan, J. F., and Tillisch, K. (2014). Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 34, 15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014
McKetin, R., Baker, A. L., Dawe, S., Voce, A., and Lubman, D. I. (2017). Differences in the symptom profile of methamphetamine-related psychosis and primary psychotic disorders. Psychiatry Res. 251, 349–354. doi: 10.1016/j.psychres.2017.02.028
Meckel, K. R., and Kiraly, D. D. (2019). A potential role for the gut microbiome in substance use disorders. Psychopharmacology 236, 1513–1530. doi: 10.1007/s00213-019-05232-0
Meng, J., Banerjee, S., Li, D., Sindberg, G. M., Wang, F., Ma, J., et al. (2015). Opioid exacerbation of gram-positive sepsis, induced by gut microbial modulation, is rescued by IL-17A neutralization. Sci. Rep. 5:10918. doi: 10.1038/srep10918
Meng, J., Yu, H., Ma, J., Wang, J., Banerjee, S., Charboneau, R., et al. (2013). Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 8:e54040. doi: 10.1371/journal.pone.0054040
Mutlu, E. A., Gillevet, P. M., Rangwala, H., Sikaroodi, M., Naqvi, A., Engen, P. A., et al. (2012). Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 302, 966–978. doi: 10.1152/ajpgi.00380.2011
Nanji, A. A., Khettry, U., and Sadrzadeh, S. M. (1994). Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc. Soc. Exp. Biol. Med. 205, 243–247. doi: 10.3181/00379727-205-43703
Ning, T., Gong, X., Xie, L., and Ma, B. (2017). Gut microbiota analysis in rats with methamphetamine-induced conditioned place preference. Front. Microbiol. 8:1620. doi: 10.3389/fmicb.2017.01620
Nolan-Kenney, R., Wu, F., Hu, J., Yang, L., Kelly, D., Li, H., et al. (2020). The association between smoking and gut microbiome in Bangladesh. Nicotine Tob. Res. 22, 1339–1346. doi: 10.1093/ntr/ntz220
Panee, J., Gerschenson, M., and Chang, L. (2018). Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J. Neuroimmune Pharmacol. 13, 113–122. doi: 10.1007/s11481-017-9767-0
Peterson, V. L., Jury, N. J., Cabrera-Rubio, R., Draper, L. A., Crispie, F., Cotter, P. D., et al. (2017). Drunk bugs: chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav. Brain Res. 323, 172–176. doi: 10.1016/j.bbr.2017.01.049
Peterson, V. L., Richards, J. B., Meyer, P. J., Cabrera-Rubio, R., Tripi, J. A., King, C. P., et al. (2020). Sex-dependent associations between addiction-related behaviors and the microbiome in outbred rats. EBioMedicine 55:102769. doi: 10.1016/j.ebiom.2020.102769
Philips, C. A., Pande, A., Shasthry, S. M., Jamwal, K. D., Khillan, V., Chandel, S. S., et al. (2017). Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin. Gastroenterol. Hepatol. 15, 600–602. doi: 10.1016/j.cgh.2016.10.029
Philips, C. A., Phadke, N., Ganesan, K., Ranade, S., and Augustine, P. (2018). Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J. Gastroenterol. 37, 215–225. doi: 10.1007/s12664-018-0859-4
Reddy, I. A., Smith, N. K., Erreger, K., Ghose, D., Saunders, C., Foster, D. J., et al. (2018). Bile diversion, a bariatric surgery, and bile acid signaling reduce central cocaine reward. PLoS Biol. 16:e2006682. doi: 10.1371/journal.pbio.2006682
Reyes, R. E. N., Al Omran, A. J., Davies, D. L., and Asatryan, L. (2020). Antibiotic-induced disruption of commensal microbiome linked to increases in binge-like ethanol consumption behavior. Brain Res. 1747:147067. doi: 10.1016/j.brainres.2020.147067
Saunders, J. B. (2017). Substance use and addictive disorders in DSM-5 and ICD 10 and the draft ICD 11. Curr. Opin. Psychiatry 30, 227–237. doi: 10.1097/YCO.0000000000000332
Sharon, G., Sampson, T. R., Geschwind, D. H., and Mazmanian, S. K. (2016). The central nervous system and the gut microbiome. Cell 167, 915–932. doi: 10.1016/j.cell.2016.10.027
Sindberg, G. M., Callen, S. E., Banerjee, S., Meng, J., Hale, V. L., Hegde, R., et al. (2019). Morphine potentiates dysbiotic microbial and metabolic shifts in acute SIV infection. J. Neuroimmune Pharmacol. 14, 200–214. doi: 10.1007/s11481-018-9805-6
Skosnik, P. D., and Cortes-Briones, J. A. (2016). Targeting the ecology within: the role of the gut-brain axis and human microbiota in drug addiction. Med. Hypotheses 93, 77–80. doi: 10.1016/j.mehy.2016.05.021
Stadlbauer, V., Mookerjee, R. P., Hodges, S., Wright, G. A., Davies, N. A., and Jalan, R. (2008). Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J. Hepatol. 48, 945–951. doi: 10.1016/j.jhep.2008.02.015
Szabo, G. (2015). Gut-liver axis in alcoholic liver disease. Gastroenterology 148, 30–36. doi: 10.1053/j.gastro.2014.10.042
Temko, J. E., Bouhlal, S., Farokhnia, M., Lee, M. R., Cryan, J. F., and Leggio, L. (2017). The microbiota, the gut and the brain in eating and alcohol use disorders: a ‘menage a trois’? Alcohol Alcohol. 52, 403–413. doi: 10.1093/alcalc/agx024
Tsuruya, A., Kuwahara, A., Saito, Y., Yamaguchi, H., Tenma, N., Inai, M., et al. (2016). Major anaerobic bacteria responsible for the production of carcinogenic acetaldehyde from ethanol in the colon and rectum. Alcohol Alcohol. 51, 395–401. doi: 10.1093/alcalc/agv135
Vincent, C., Miller, M. A., Edens, T. J., Mehrotra, S., Dewar, K., and Manges, A. R. (2016). Bloom and bust: intestinal microbiota dynamics in response to hospital exposures and Clostridium difficile colonization or infection. Microbiome 4:12. doi: 10.1186/s40168-016-0156-3
Vlachogiannakos, J., Viazis, N., Vasianopoulou, P., Vafiadis, I., Karamanolis, D. G., and Ladas, S. D. (2013). Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J. Gastroenterol. Hepatol. 28, 450–455. doi: 10.1111/jgh.12070
Volpe, G. E., Ward, H., Mwamburi, M., Dinh, D., Bhalchandra, S., Wanke, C., et al. (2014). Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J. Stud. Alcohol Drugs 75, 347–357. doi: 10.15288/jsad.2014.75.347
Wang, F., Meng, J., Zhang, L., and Roy, S. (2020). Opioid use potentiates the virulence of hospital-acquired infection, increases systemic bacterial dissemination and exacerbates gut dysbiosis in a murine model of Citrobacter rodentium infection. Gut Microbes 11, 172–190. doi: 10.1080/19490976.2019.1629237
Wang, F., Meng, J., Zhang, L., Johnson, T., Chen, C., and Roy, S. (2018). Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 8:3596. doi: 10.1038/s41598-018-21915-8
Wang, G. H., Liu, Q., Guo, L., Zeng, H. J., Ding, C. C., Zhang, W. T., et al. (2018). Gut microbiota and relevant metabolites analysis in alcohol dependent mice. Front. Microbiol. 9:14. doi: 10.3389/fmicb.2018.01874
Wang, S. C., Chen, Y. C., Chen, S. J., Lee, C. H., and Cheng, C. M. (2020). Alcohol addiction, gut microbiota, and alcoholism treatment: a review. Int. J. Mol. Sci. 21:6413. doi: 10.3390/ijms21176413
WHO (2020). World Drug Report 2020. Available online at: https://wdr.unodc.org/wdr2020/en/exsum.html (accessed December 25, 2020).
Wiegel, J., Tanner, R., and Rainey, F. A. (2006). “An introduction to the family clostridiaceae,” in The Prokaryotes: Volume 4: Bacteria: Firmicutes, Cyanobacteria, eds M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (New York, NY: Springer), 654–678.
Wise, R. A., and Koob, G. F. (2014). The development and maintenance of drug addiction. Neuropsychopharmacology 39, 254–262. doi: 10.1038/npp.2013.261
Xiao, H. W., Ge, C., Feng, G. X., Li, Y., Luo, D., Dong, J. L., et al. (2018). Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol. Lett. 287, 23–30. doi: 10.1016/j.toxlet.2018.01.021
Xu, Y., Xie, Z., Wang, H., Shen, Z., Guo, Y., Gao, Y., et al. (2017). Bacterial diversity of intestinal microbiota in patients with substance use disorders revealed by 16S rRNA gene deep sequencing. Sci. Rep. 7:3628. doi: 10.1038/s41598-017-03706-9
Xu, Z., Liu, Z., Dong, X., Hu, T., Wang, L., Li, J., et al. (2018). Fecal microbiota transplantation from healthy donors reduced alcohol-induced anxiety and depression in an animal model of chronic alcohol exposure. Chin. J. Physiol. 61, 360–371.
Xu, Z., Wang, C., Dong, X., Hu, T., Wang, L., Zhao, W., et al. (2019). Chronic alcohol exposure induced gut microbiota dysbiosis and its correlations with neuropsychic behaviors and brain BDNF/Gabra1 changes in mice. Biofactors 45, 187–199. doi: 10.1002/biof.1469
Yan, A. W., Fouts, D. E., Brandl, J., Starkel, P., Torralba, M., Schott, E., et al. (2011). Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 53, 96–105. doi: 10.1002/hep.24018
Yang, C., Fu, X., Hao, W., Xiang, X., Liu, T., Yang, B. Z., et al. (2021). Gut dysbiosis associated with the rats’ responses in methamphetamine-induced conditioned place preference. Addict. Biol. 26:e12975. doi: 10.1111/adb.12975
Yang, C., Qu, Y., Fujita, Y., Ren, Q., Ma, M., Dong, C., et al. (2017). Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl. Psychiatry 7:1294. doi: 10.1038/s41398-017-0031-4
Zagorska, A., Marcinkowska, M., Jamrozik, M., Wisniowska, B., and Pasko, P. (2020). From probiotics to psychobiotics - the gut-brain axis in psychiatric disorders. Benef. Microbes 11, 717–732. doi: 10.3920/BM2020.0063
Zhang, J., Yang, J., Yang, C., Chen, T., Wang, Z., Li, J., et al. (2020). Sensitivity to morphine reward associates with gut dysbiosis in rats with morphine-induced conditioned place preference. Front. Psychiatry 11:631. doi: 10.3389/fpsyt.2020.00631
Zhang, L., Meng, J., Ban, Y., Jalodia, R., Chupikova, I., Fernandez, I. A., et al. (2019). Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc. Natl. Acad. Sci. U.S.A. 116, 13523–13532. doi: 10.1073/pnas.1901182116
Zhang, X., Yasuda, K., Gilmore, R. A., Westmoreland, S. V., Platt, D. M., Miller, G. M., et al. (2019). Alcohol-induced changes in the gut microbiome and metabolome of rhesus macaques. Psychopharmacology (Berl) 236, 1531–1544. doi: 10.1007/s00213-019-05217-z
Keywords: microbiome-gut-brain axis (MGBA), microbiota manipulation, substance-related disorders, gut microbiota, fecal microbiome transplantation
Citation: Fu X, Chen T, Cai J, Liu B, Zeng Y and Zhang X (2021) The Microbiome–Gut–Brain Axis, a Potential Therapeutic Target for Substance-Related Disorders. Front. Microbiol. 12:738401. doi: 10.3389/fmicb.2021.738401
Received: 08 July 2021; Accepted: 31 August 2021;
Published: 06 October 2021.
Edited by:
Miguel Gueimonde, Institute of Dairy Products of Asturias (IPLA), SpainReviewed by:
Aurelijus Burokas, Vilnius University, LithuaniaDiling Chen, Guangdong Institute of Microbiology, Guangdong Academy of Science, China
Copyright © 2021 Fu, Chen, Cai, Liu, Zeng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojie Zhang, eGlhb2ppZXpoYW5nMjAxNEBjc3UuZWR1LmNu
†These authors have contributed equally to this work
 Xuan Fu
Xuan Fu Ti Chen7†
Ti Chen7† Jingda Cai
Jingda Cai Xiaojie Zhang
Xiaojie Zhang