
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 03 November 2021
Sec. Microbe and Virus Interactions with Plants
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.737541
This article is part of the Research Topic Systematics, Genomics, and Pathogenesis of Forest Pathogens View all 8 articles
 Ying Zhang1
Ying Zhang1 Yupei Zhou1
Yupei Zhou1 Wei Sun1
Wei Sun1 Lili Zhao1
Lili Zhao1 D. Pavlic-Zupanc2
D. Pavlic-Zupanc2 Pedro W. Crous3
Pedro W. Crous3 Bernard Slippers4
Bernard Slippers4 Yucheng Dai1*
Yucheng Dai1*The genus Botryosphaeria includes more than 200 epithets, but only the type species, Botryosphaeria dothidea and a dozen or more other species have been identified based on DNA sequence data. The taxonomic status of the other species remains unconfirmed because they lack either morphological information or DNA sequence data. In this study, types or authentic specimens of 16 “Botryosphaeria” species are reassessed to clarify their identity and phylogenetic position. nuDNA sequences of four regions, ITS, LSU, tef1-α and tub2, are analyzed and considered in combination with morphological characteristics. Based on the multigene phylogeny and morphological characters, Botryosphaeria cruenta and Botryosphaeria hamamelidis are transferred to Neofusicoccum. The generic status of Botryosphaeria aterrima and Botryosphaeria mirabile is confirmed in Botryosphaeria. Botryosphaeria berengeriana var. weigeliae and B. berengeriana var. acerina are treated synonyms of B. dothidea. Botryosphaeria mucosa is transferred to Neodeightonia as Neodeightonia mucosa, and Botryosphaeria ferruginea to Nothophoma as Nothophoma ferruginea. Botryosphaeria foliicola is reduced to synonymy with Phyllachorella micheliae. Botryosphaeria abuensis, Botryosphaeria aesculi, Botryosphaeria dasylirii, and Botryosphaeria wisteriae are tentatively kept in Botryosphaeria sensu stricto until further phylogenetic analysis is carried out on verified specimens. The ordinal status of Botryosphaeria apocyni, Botryosphaeria gaubae, and Botryosphaeria smilacinina cannot be determined, and tentatively accommodate these species in Dothideomycetes incertae sedis. The study demonstrates the significance of a polyphasic approach in characterizing type specimens, including the importance of using of DNA sequence data.
Botryosphaeria Ces. and De Not. was formally established by Cesati and De Notaris (1863) based on 12 species, but the generic type was not designated, neither were detailed descriptions provided for these species. Furthermore, Botryosphaeria was discussed as being heterogeneous, and considered to probably represent three genera, i.e., Botryosphaeria, Gibberella Sacc. and Lisea Sacc. (Cesati and De Notaris, 1863). Subsequently, De Notaris (1863) described four additional species in Botryosphaeria, viz., B. berengeriana De Not., Botryosphaeria dispersa De Not., Botryosphaeria juglandina De Not., Botryosphaeria moricola Ces. and De Not. Saccardo (1877) emended the generic description of Cesati and De Notaris (1863) to exclude hypocreaceous species, and formally introduced Gibberella and Lisea to accommodate them. von Höhnel (1909) designated B. berengeriana as the generic type. Based on Saccardo’s (1877) amendment, Theissen and Sydow (1915) suggested Botryosphaeria quercuum (Schwein.) Sacc. as the generic type, which was supported by von Arx and Müller (1954). However, neither B. berengeriana nor B. quercuum had been included in the original description of Botryosphaeria. By 1954, more than 100 species had been described in Botryosphaeria. von Arx and Müller (1954) reduced 108 taxa to synonymy with B. quercuum, and 24 taxa with Botryosphaeria dothidea (Moug.) Ces. and De Not. Only 11 species remained accepted in Botryosphaeria. However, the sexual morph on which these synonymies were known to be morphologically conserved, and the treatment was therefore not widely accepted (Shoemaker, 1964; Sivanesan, 1984; Slippers et al., 2004). Barr (1972) proposed B. dothidea as the lectotype of Botryosphaeria, since it conformed to Saccardo’s (1877) amendment of the genus, and was one of the original species described in Botryosphaeria by Cesati and De Notaris (1863). This typification has subsequently been widely accepted (Smith et al., 2001; Slippers et al., 2004; Phillips et al., 2013).
Early researchers described Botryosphaeria species mostly based on their sexual morphs and host associations, which led to the addition of numerous species (Cesati and De Notaris, 1863; De Notaris, 1863; Saccardo, 1877, 1882; Grossenbacher and Duggar, 1911; Putterill, 1919; Trotter, 1928). Currently, more than 200 epithets are included in Botryosphaeria (November 2020)1, and the genus is considered as being heterogeneous (Slippers et al., 2004; Crous et al., 2006; Phillips et al., 2008, 2013).
Slippers et al. (2004) designated an epitype for B. dothidea, with a modified description, ex-type culture and DNA sequence data, which shed light on the circumscription of Botryosphaeria sensu stricto. Based on a LSU phylogeny, Crous et al. (2006) identified 10 phylogenetic lineages in Botryosphaeria sensu lato, which corresponded to different asexual genera. Using a phylogeny based on the analyses of sequence data for five loci (SSU, LSU, ITS, tub2, and tef1-α), Phillips et al. (2008) clarified the morphology of several genera in the Botryosphaeriaceae, and introduced two new genera, i.e., Barriopsis A.J.L. Phillips, A. Alves and Crous and Spencermartinsia A.J.L. Phillips, A. Alves and Crous. Furthermore, Phillips et al. (2013) recognized seven species in Botryosphaeria sensu stricto, i.e., Botryosphaeria agaves (Henn.) E.J. Butler, Botryosphaeria corticis (Demaree and Wilcox) Arx and E. Müll., B. dothidea, Botryosphaeria fabicerciana (S.F. Chen, Pavlic, M.J. Wingf. and X.D. Zhou) A.J.L. Phillips and A. Alves, Botryosphaeria fusispora Boonmee, Jian K. Liu and K.D. Hyde, Botryosphaeria ramosa (Pavlic, T.I. Burgess and M.J. Wingf.) A.J.L. Phillips and A. Alves and Botryosphaeria scharifii Abdollahz., Zare and A.J.L. Phillips. Subsequently, a few more species of Botryosphaeria, e.g., Botryosphaeria auasmontanum F.J.J. Van der Walt Slippers and G.J. Marais, Botryosphaeria minutispermatia Ariyawansa, K.D. Hyde and Z.Y. Liu, Botryosphaeria qingyuanensis G.Q. Li and S.F. Chen, Botryosphaeria sinensia Y.P. Zhou and Y. Zhang ter. and Botryosphaeria rosaceae Y.P. Zhou and Y. Zhang ter. were described (Slippers et al., 2014; Ariyawansa et al., 2016; Dissanayake et al., 2016; Zhou et al., 2016, 2017; Li et al., 2017). Zhang et al. (2021) reviewed the species within Botryosphaeriales, and accepted eight species within Botryosphaeria sensu stricto, viz., B. agaves, B. corticis, B. dothidea, B. fabicerciana, Botryosphaeria kuwatsukai, B. qingyuanensis, B. ramosa, and B. scharifii. To date, however, the taxonomic status of most taxa accommodated in Botryosphaeria sensu lato remains uncertain.
As a fundamental element in the current Code of Nomenclature for algae, fungi, and plants, type studies play a critical role in epitypification, as well as in defining species or genera of Ascomycetes (Zhang et al., 2009). Specifically for taxa in the Botryosphaeriales, there are few studies based on DNA sequence data. Almost all the older names linked to Botryosphaeria lack cultures or DNA sequence data, and they can consequently not be classified to genus or even family rank with confidence. Thus, these names are unusable unless they are either epitypified or supplemented with DNA sequence data (Slippers et al., 2014). The aims of the present study were thus to verify the identity of 17 selected type or authentic specimens (representing 16 species) currently placed in Botryosphaeria, using morphological characteristics and nuDNA sequence data.
Type specimens of 16 putative Botryosphaeria species were obtained on loan from the Conservatoire et Jardin botaniques de la Ville de Genève (G), Naturhistorisches Museum Wien (W), Field Museum of Natural History (F), Royal Botanic Gardens (K), University of Michigan (MICH) and New York State Museum (NYS) (Table 1). The type specimens were described and illustrated following the protocol by Zhang et al. (2012). Sections made from specimens were studied at × 1,000 magnification using a Nikon E600 compound microscope. Ascomata were examined under a Leica M125 dissecting microscope. Sections of ascomata, hamathecia, asci, and ascospores were mounted in water or 10–100% lactic acid. Micrographs were made from tissues mounted in water with 10–100% lactic acid or cotton blue. Question marks (?) indicate possible type specimens.
After getting the DNA extraction permission, nuDNA was extracted from ascomata or conidiomata using a Forensic DNA kit (OMEGA Bio-tek). The internal transcribed spacer of regions (1 and 2) of the nuDNA (ITS) was amplified and sequenced with primers ITS-4 and ITS-5 (White et al., 1990). The 28S large subunit nuDNA (LSU) was amplified and sequenced with primers LROR and LR5 (Vilgalys and Hester, 1990). Sections of the translation elongation factor-1α (tef1-α) with primers EF1-688F and EF1-1251R (Alves et al., 2008) and the β-tubulin gene (tub2) with primers Bt2a and Bt2b (Glass and Donaldson, 1995). PCR amplification and sequencing was conducted following the protocol by Zhang et al. (2009). Some of the resulting sequences had ambiguous base calls, possibly due to the contamination of the other fungi occurring on the specimens. All PCR products exhibiting this phenomenon were cloned using the pGEM-T Vector System I cloning kit (Promega).
For the sequences obtained, a search was conducted using BLAST (Basic Local Alignment Search Tool) in GenBank sequences2 to confirm the generic status of the related specimens. Sequence data for each individual gene region, ITS, LSU, tef1-α and tub2, as well as the combined datasets were used to infer the phylogenetic relationships among all confirmed Botryosphaeria, Neofusicoccum, Nothophoma species for which sequence data were available from GenBank (see text footnote 2), together with the sequences generated in this study. Alignments were made in MEGA v. 6 (Tamura et al., 2013) and phylogenetic analyses performed in PAUP v. 4.0b10 (Swofford, 2002) and MrBayes v. 3.1.2 (Ronquist and Huelsenbeck, 2003). Prior to phylogenetic analyses, ambiguous sequences at the start and the end of sequences were deleted and gaps manually adjusted to optimize the alignments. Maximum Parsimony (MP) was used to conduct heuristic searches as implemented in PAUP with the default options method (Zhang et al., 2008). Analyses were made under different parameters of maximum parsimony criteria as outlined in Zhang et al. (2008). Clade stability was assessed in a bootstrap analysis with 1,000 replicates, random sequence additions with MaxTrees set to 1,000 and other default parameters as implemented in PAUP. Maximum likelihood (ML) was also conducted using heuristic searches with the default options method as implemented in PAUP. For the ML analysis, best-fit model of nucleotide evolution was selected by hierarchical likelihood ratio test (hLRT) in MrModeltest 2.3. A bootstrap analysis with 1,000 replicates was used to test the statistical support of the branches. For the MrBayes analyses, the best-fit model of nucleotide evolution was selected by Akaike information criterion (AIC; Posada and Buckley, 2004) in MrModeltest v. 2.3. The metropolis-coupled Markov Chain Monte Carlo (MCMCMC) approach was used to calculate posterior probabilities (Huelsenbeck and Ronquist, 2005). Trees were viewed in TREEVIEW. Phylograms obtained based on combined loci or for a single locus were all deposited in TreeBASE. The nucleotide sequences reported in this study were deposited in GenBank (Supplementary Table 1).
Based on the results of BLAST in GenBank, Botryosphaeria aterrima (Fuckel) Sacc., B. berengeriana var. weigelae Rehm and Botryosphaeria mirabile (Fuckel) Cooke belong to Botryosphaeria, B. cruenta and B. hamamelidis to Neofusicoccum, Botryosphaeria ferruginea to Nothophoma Qian Chen and L. Cai. The phylogenetic analysis of the Botryosphaeria dataset included 14 ingroup taxa and two outgroup taxa (Supplementary Figure 4). The combined ITS, LSU, tef1-a, and tub2 matrix contained 2,268 characters, of which 1,940 were constant and 39 were variable and parsimony-uninformative. Maximum parsimony analysis of the remaining 289 parsimony-informative characters resulted in 2,275 equally most parsimonious trees (Supplementary Figure 4). The phylogenetic tree resulting from the Bayesian analysis using the general time reversible model of nuDNA evolution (Rodríguez et al., 1990), including estimation of non-variable sites and assuming a discrete gamma distribution with six rate categories (GTR+Γ+G), had a topology identical to the MP tree presented. In both analyses (MP and Bayesian), the clade of Botryosphaeria had a high bootstrap support (100% for MP) and high posterior probabilities (1.00 for MrBayes). The phylogenetic status of three species B. agaves (Henn.) E.J. Butler, B. ramosa and B. scharifii was resolved in a well-supported clade with B. agaves basal to all other species of Botryosphaeria. The phylogenetic relationships among B. aterrima, B. auasmontanum, B. berengeriana var. weigeliae Rehm, B. corticis, B. dothidea, B. fabicerciana, B. fusispora, B. minutispermatia, B. mirabile, B. rosaceae, and B. sinensia could not be resolved (Supplementary Figure 4, TreeBASE number S21054).
The analysis for Neofusicoccum involved 38 taxa including two outgroup species, i.e., B. corticis and B. dothidea. The combined ITS, tef1-α and tub2 nuDNA sequence matrix included 920 characters, 136 were constant and 39 were variable and parsimony-uninformative. Maximum parsimony analysis for the remaining 181 parsimony-informative characters resulted in 5,000 equally most parsimonious trees (Supplementary Figure 5, TreeBASE number S21059). The phylogenetic tree resulting from the Bayesian analysis using the general time reversible model of nuDNA evolution (Rodríguez et al., 1990), including estimation of invariable sites and assuming a discrete gamma distribution with six rate categories (GTR+Γ+G), had a topology identical to the MP tree presented. In both analyses (MP and Bayesian) the clade accommodating Neofusicoccum had a high level of support (100% for MP and 1.00 PP for MrBayes). Isolates of N. hamamelidis formed sub-clade representing an individual species of Neofusicoccum (Supplementary Figure 5).
The analysis for Neofusicoccum LSU sequences included 18 taxa with two outgroup species, B. corticis and B. dothidea. The LSU nuDNA sequence dataset contained 847 characters, of which 779 were constant and 42 were variable and parsimony-uninformative. Maximum parsimony analysis of the remaining 26 parsimony-informative characters resulted in 22 equally most parsimonious trees (Supplementary Figure 6, TreeBASE number S21050). Neofusicoccum cruenta and N. hamamelidis formed a sub-clade representing an individual species, respectively, while lacked of support (Supplementary Figure 6).
The analysis for Nothophoma spp. involved 22 taxa including one outgroup species, i.e., Didymella calidophila. The combined ITS and LSU sequence matrix included 1,821 characters, of which 1,751 were constant and 38 were variable and parsimony-uninformative. Maximum parsimony analysis for the remaining 32 parsimony-informative characters resulted in 1,000 equally most parsimonious trees. For the Bayesian analysis, TNe+I was selected as the best-fit model for the ITS and LSU dataset, had a topology identical to the MP tree and ML tree presented. Phylogenetically, species of Nothophoma formed a robust clade. Isolates of No. ferruginea formed sub-clade representing an individual species of Nothophoma spp. Only the Bayesian tree is presented herein with MP, PP, and ML values plotted on the branches (Supplementary Figure 7).
Botryosphaeria aterrima (Fuckel) Sacc. Syll. fung. (Abellini) 1: 458 (1882). Figure 1
≡ Melanops aterrima Fuckel, Jb. nassau. Ver. Naturk. 23–24: 225 (1870) [1869–1870]
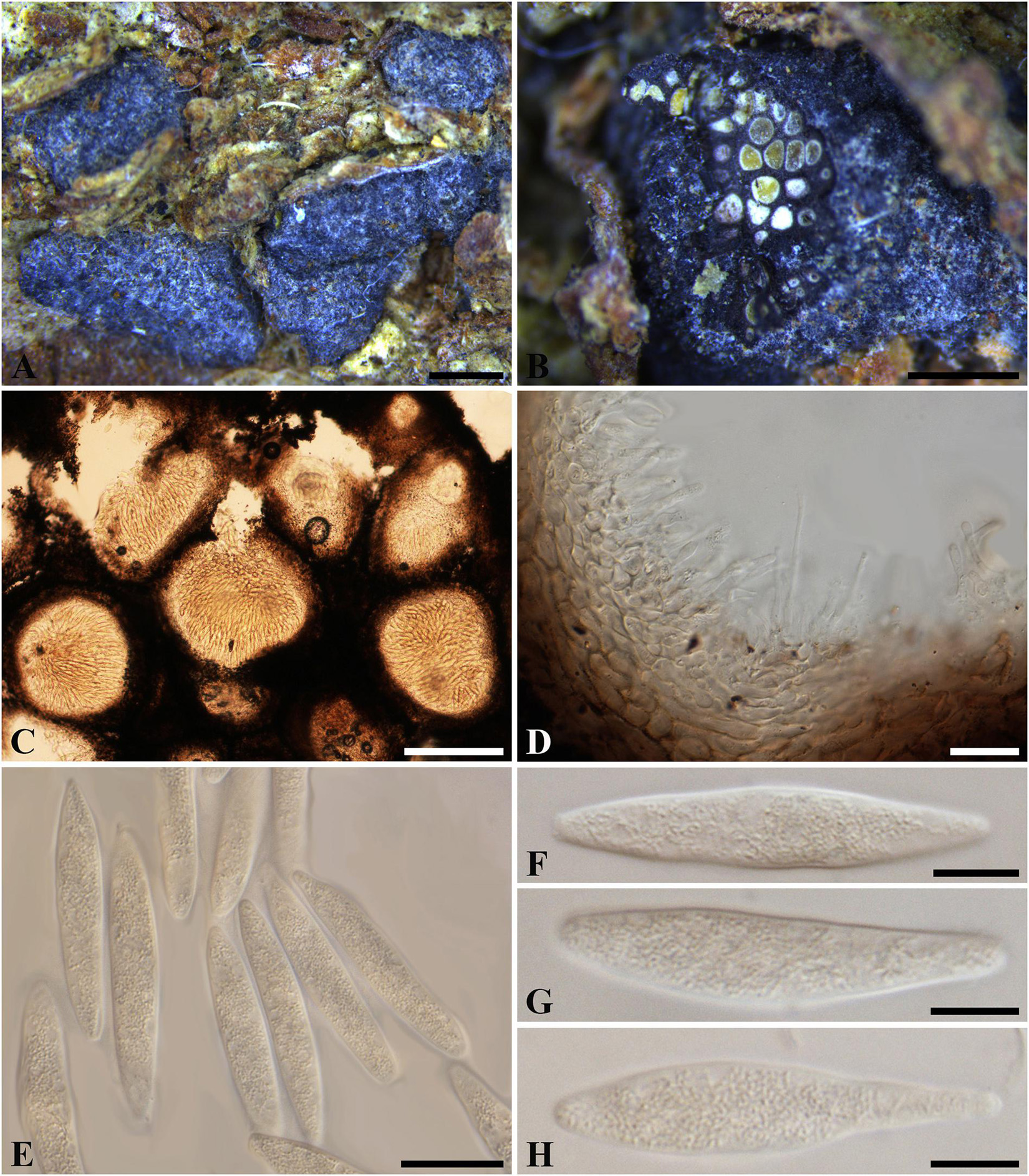
Figure 1. Botryosphaeria aterrima (G 00266252, holotype). (A) Dense botryose cluster of conidiomata erumpent through the bark. (B) Transverse section of the conidiomata. (C) Longitudinal section of conidiomata. (D) Conidiogenous cells and paraphyses. (E–H) Conidia. Scale bars: (A) = 5 mm, (B) = 1 mm, (C) = 200 μm, (D,E) = 20 μm, (F–H) = 10 μm.
Ascostromata not observed. Conidiostromata forming dense botryose aggregate, 2–7 mm diam., pseudothecial, aggregated into botryose clusters, 220–420 μm diam., spherical to globose with a central ostiole, ½ to ¾ emergent, rarely embedded, black. Peridium comprising 7–15 layers of textura angularis, outer region of dark brown cells, inner region of 3–7 layers of pale brown cells lining the locule. Paraphyses when present hyaline, septate, up to 70 μm long, 2–4 μm broad at the base, tapering to acutely rounded apices, 1.5–2 μm broad at the tip. Conidiogenous cells holoblastic, hyaline, sub-cylindrical, 8–20 × 3–5 μm. Conidia hyaline, narrowly fusiform, or irregularly fusiform, base subtruncate to bluntly rounded, (40–)42–60(–62) × (7–)9–11 μm (−x = 51.3 × 9.9 μm, n = 20), L/W = 5.2.
Specimen examined – GERMANY, Hessen, Ulmus sp. (Ulmaceae), Fuckel, K.W.G. (G 00266252, holotype).
Notes – Although phylogenetic analysis based on combined loci of ITS, tef1-a and LSU confirmed Botryosphaeria aterrima within Botryosphaeria, it cannot be distinguished from B. auasmontanum, B. berengeriana var. weigeliae, B. dothidea, B. minutispermatia, and B. mirabile (Supplementary Figure 4). The two isolates of B. dothidea used in this study are ex-epitype (CBS 115476) and a verified isolate (CBS 110302), respectively (Slippers et al., 2004; Phillips et al., 2013). Botryosphaeria dothidea is known as a cosmopolitan species associated with woody plants in numerous families (Slippers et al., 2004; Marsberg et al., 2017). The type of B. aterrima reassessed here was collected from Ulmus sp. in Germany, and only the asexual morph was observed. Conidia of B. aterrima examined in this study were much larger [(40–)42–60(–62) × (7–)9–11 μm] than those of B. dothidea [(17–)18–20(–22) × 4–5 μm, as reported by Slippers et al. (2004), Table 2]. A remarkable feature of B. aterrima was its multiloculate conidiomata, which was comparable with members of Aplosporellaceae and Melanopsaceae. The hyaline, fusiform conidia lacking mucous sheath, however, differed from these two families. Thus, we treat B. aterrima as a separate species within Botryosphaeria sensu stricto.
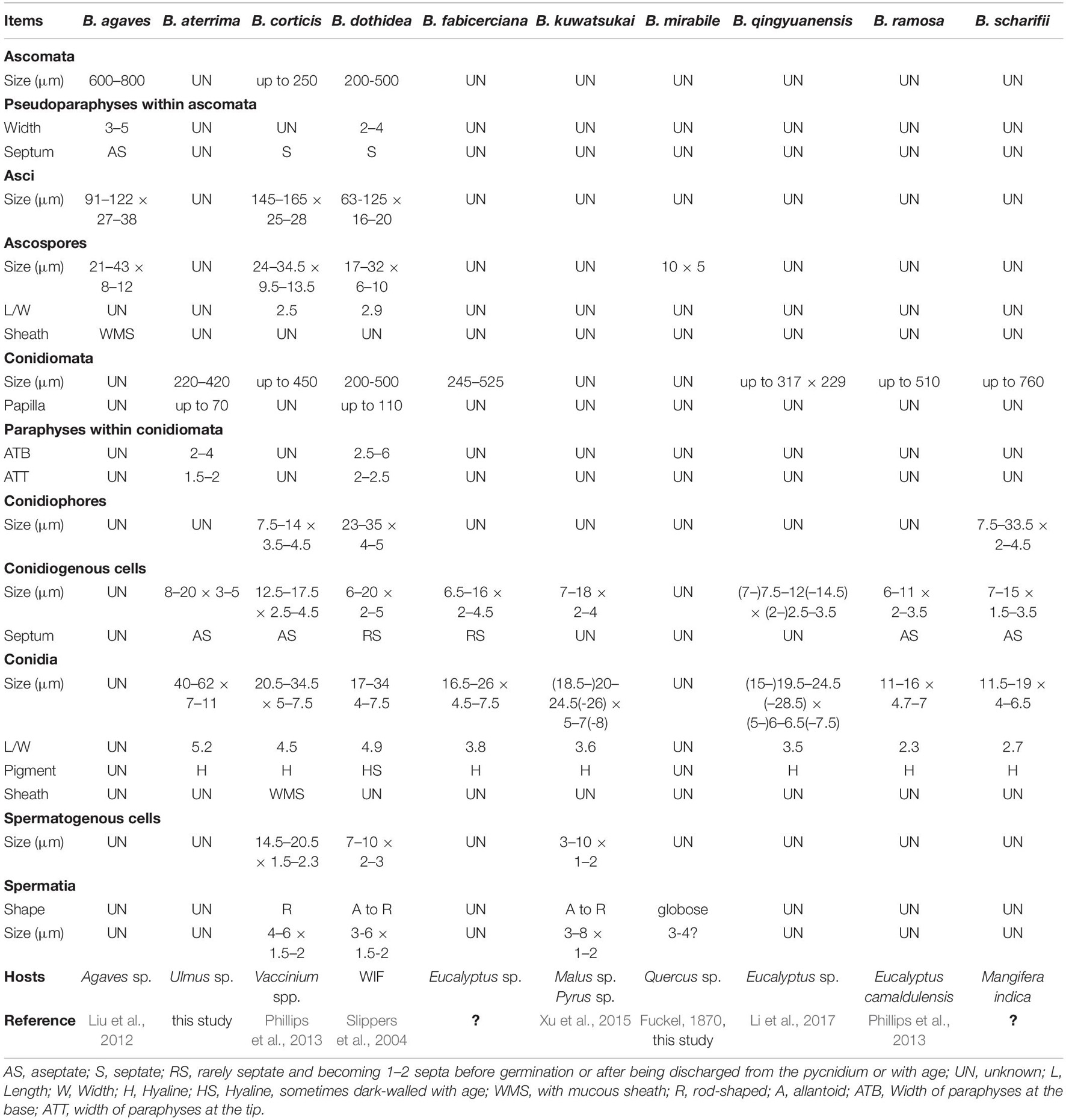
Table 2. Morphological characteristics of Botryosphaeria spp. surely assigned within Botryosphaeria so far.
Botryosphaeria dothidea (Moug. : Fr.) Ces. and De Not., Comment. Soc. Crittog. Ital. 1:212. 1863. Figure 2
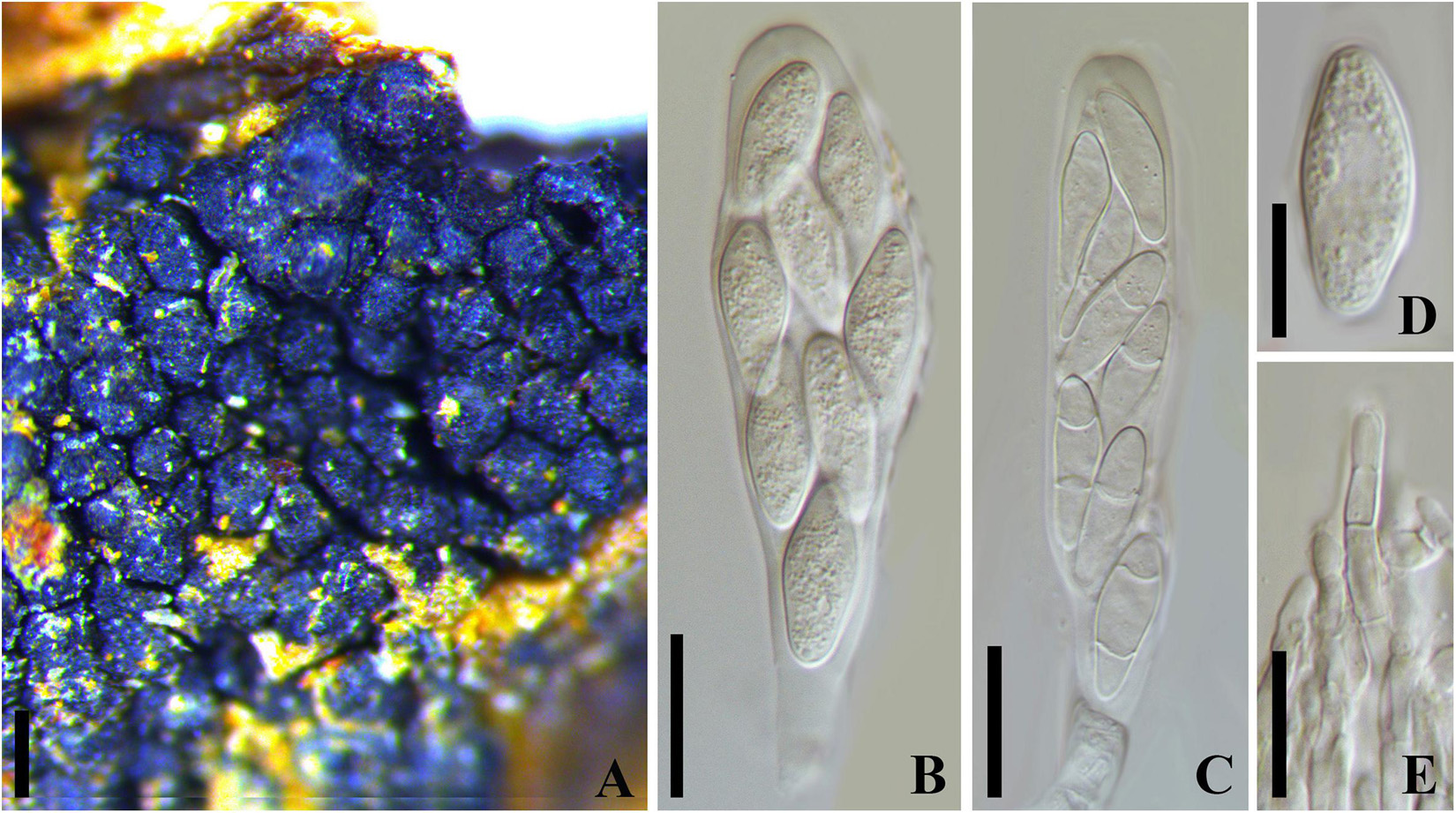
Figure 2. Botryosphaeria dothidea (MICH 13862, isotype of B. berengeriana var. weigeliae). (A) Botryose clusters of ascomata erumpent through twig epidermis. (B,C) Asci with hyaline aseptate or septate ascospores. (D) Hyaline ascospore. (E) Hyaline, septate, and cellulous pseudoparaphyses. Scale bars: (A) = 200 μm, (B,C) = 20 μm, (D,E) = 10 μm.
= Sphaeria dothidea Moug.: Fr. in Fries, Syst. Mycol. 2:423. 1823
= Botryosphaeria berengeriana De Not., Sfer. Ital. 82.1863[1864]
= Botryosphaeria berengeriana var. acerina Rehm, Annls mycol. 7(6): 533 (1909)
= Botryosphaeria berengeriana var. weigeliae Rehm, Annls mycol. 12(2): 168 (1914)
Ascostroma erumpent to nearly superficial, 1.5-4.5 mm diam. Ascomata 110-240 μm diam., pseudothecial, forming botryose clusters of up to 50 locules, globose with a central ostiole, papillate or not, black. Peridium comprising 6–15 layers of textura angularis, outer region of dark brown cells, inner region of 2–3 layers of hyaline cells lining the locule (Slippers et al., 2004). Pseudoparaphyses filiform, cellular, septate, 3–4 μm wide. Asci 8-spored, bitunicate, cylindric-clavate to clavate with a short pedicel, 72-130 × 17–27 μm, forming among pseudoparaphyses. Ascospores hyaline, broadly fusoid to ellipsoidal, smooth or with granular contents, sometimes become 1–2 septa with age, biseriate in the ascus, (18–)20–26(–28) × 7–10 μm (−x = 23.3 × 8.8 μm, n = 20), L/W = 2.6. Spermatia not observed.
Specimen examined – RUSSIA, Batum (i), Caucasus, on cortex Weigela sp. (Caprifoliaceae), Newodowski (MICH 13862, isotype of B. berengeriana var. weigeliae). United States, Washington, on bark of Acer macrophyllum (Aceraceae), June 1906, S. A. Harper (F C0003484F, holotype of Botryosphaeria berengeriana var. acerina).
Notes – The phylogeny based on ITS and tub2 nuDNA sequence analysis indicated that Botryosphaeria berengeriana var. weigeliae and B. dothidea cluster together with ITS [only one base-pair differences (of 202 base-pairs) and tub2 totally identical (of 344 base-pairs), Supplementary Figure 4]. All the morphological characteristics of B. berengeriana var. weigeliae agree with B. dothidea but the 1–2-septate mature ascospores (Slippers et al., 2004; Figure 2), which is insufficient to separate a species. Thus, we treated B. berengeriana var. weigeliae a synonym of B. dothidea. In addition, B. berengeriana var. acerina was reduced to synonymy with B. dothidea because of their morphological similarities.
Botryosphaeria mirabile (Fuckel) Cooke, Grevillea 13 (no. 68): 108 (1885). Figure 3
≡ Melanops mirabilis Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 225 (1870)
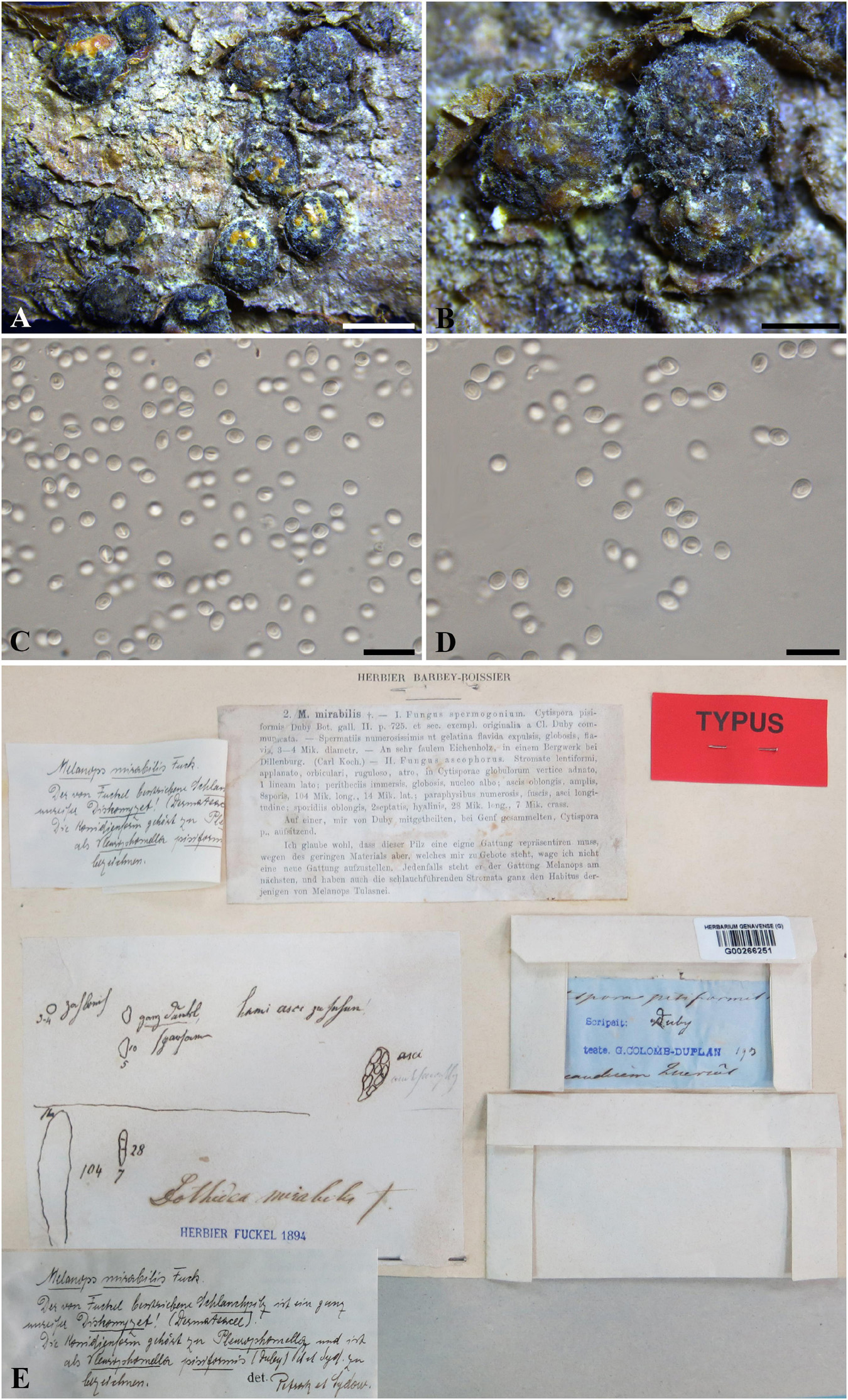
Figure 3. Botryosphaeria mirabile (G 00266251, holotype). (A,B) Fruiting bodies erumpent through the epidermis of twigs. (C,D) Spermatia produced in the fruiting bodies. (E) Herbarium label. Scale bars: (A) = 2 mm, (B) = 1 mm, (C,D) = 10 μm.
Ascostromata erumpent, clustered, black, with yellow globules on the top, 1–2 mm diam., globose, gregarious. Asci 8-spored, bitunicate, broadly clavate. Pseudoparaphyses not observed. Ascospores fusiform, irregularly biseriate to triseriate in asci, up to 10 × 5 μm. Spermatia numerous, ellipsoid to subglobose, hyaline, 3–4 μm (data of sexual stage are obtained from the label of the type specimen).
Specimen examined – SWITZERLAND, Genève, on trunk of Quercus sp. (Fagaceae), J.E. Duby (G 00266251, holotype).
Notes – Melanops mirabilis was introduced by Fuckel (1870), and was assigned to Botryosphaeria as B. mirabilis by Cooke (1885). Some large spermatogonia (up to 5 mm diam.) were found on the type specimen examined in this study, which contained numerous subglobose, pale brown spermatial-like cells (ca. 3–5 μm diam.) (Figure 3), from which the nuDNA was extracted in this study. Some immersed ascomata were also sectioned, but they were in ordinately old to enable a morphological study. Both spermatia and the sexual morph were considered in the original description (Fuckel, 1870). The sexual morph was described as having immersed pseudothecia, numerous, dark brown pseudoparaphyses, narrowly clavate asci, and oblong, hyaline, 2-septate ascospores which disagree with the concept of Botryosphaeria. Two distinct sexual morphs were illustrated on the envelope of the type specimen (Figure 3). Besides the one mentioned above, the other was illustrated as having broadly clavate asci, hyaline, aseptate ascospores (10 × 5 μm), which fits the concept of Botryosphaeria (Figure 3). Thus, the second illustrated sexual morph corresponds with B. mirabile (Figure 3). nuDNA sequence comparisons based on ITS and LSU indicated that Melanops mirabilis resides in Botryosphaeria (Supplementary Figure 4), although it cannot be distinguishable from B. aterrima, B. auasmontanum, B. berengeriana var. weigelae, B. dothidea, and B. minutispermatia. In addition, B. mirabile differs from B. dothidea by its nuDNA loci, e.g., 6 bp differences in ITS (1%) and 5 bp in LSU (1.2%). Based on its subglobose pale brown spermatia, small-sized ascospores as well as its unique nuDNA loci, we retain B. mirabile as a separate species in Botryosphaeria sensu stricto.
Neodeightonia mucosa (S.J. Kaur) Y. Zhang ter and Y.P. Zhou, comb. nov.
MycoBank number: 840943; Facesoffungi number: FoF 03579; Figure 4
≡ Botryosphaeria mucosa S.J. Kaur, Indian J. Mycol. Plant Path. 25(3): 333 (1996) [1995]
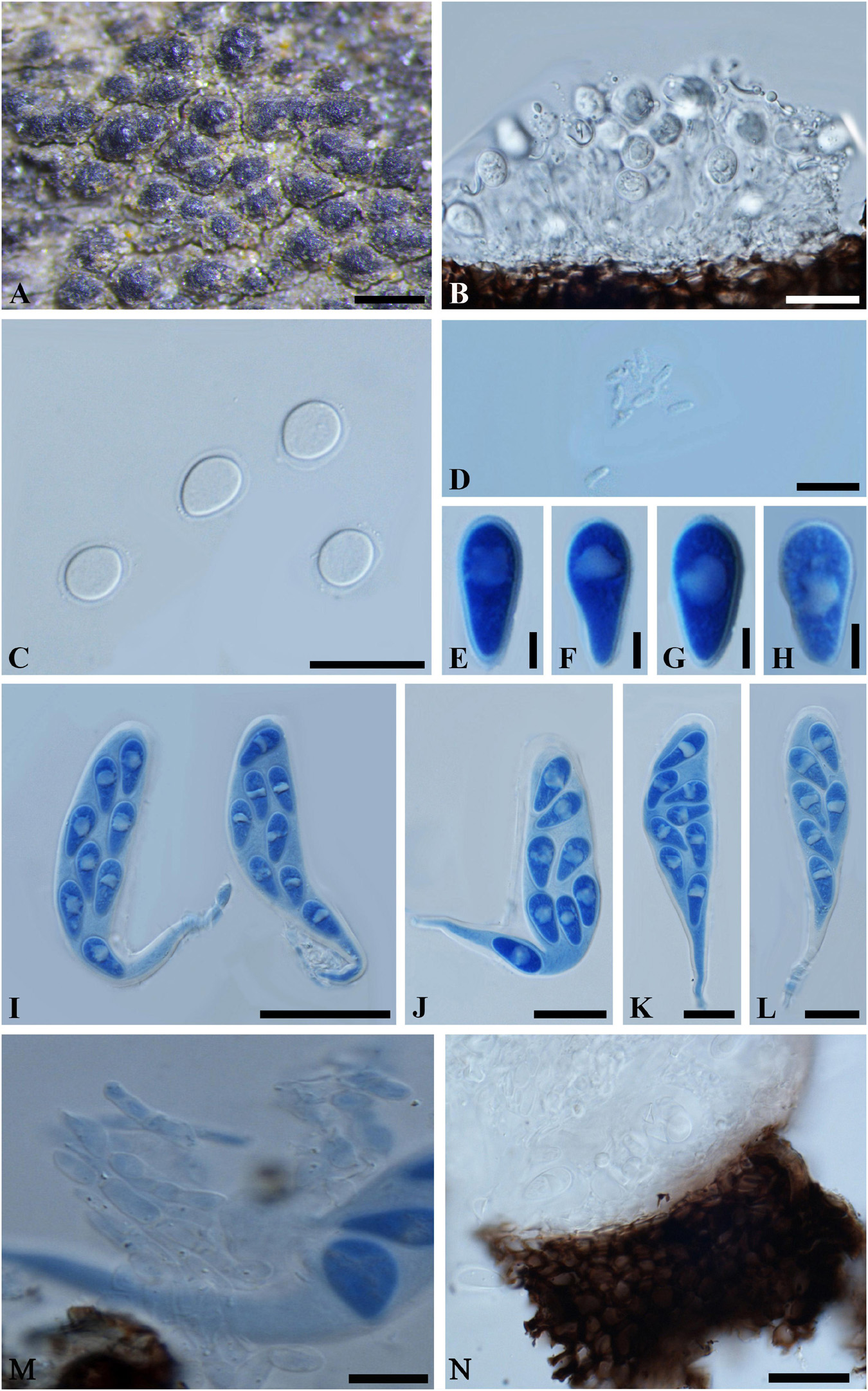
Figure 4. Neodeightonia mucosa (IMI 204341, type). (A) Aggregated ascomata erumpent through the twig. (B) Longitudinal section of a conidioma. (C) Conidia surrounded by a thin gelatinous sheath. (D) Spermatia. (E-H) Ascospores in cotton blue. Note the distinct oil drop in the ascospores. (I–L) Broadly clavate asci with a long, narrowed, and twisted pedicel in cotton blue. (M) Fragments of cellular pseudoparaphyses. (N) Part of peridium. Scale bars: (A) = 500 μm, (B,C,J-L) = 20 μm, (D,M,N) = 10 μm, (E-H) = 5 μm, (I) = 50 μm.
Ascomata 170-320 μm diam., erumpent, pseudothecial, scattered, solitary or aggregated, globose with a central ostiole, ¼ to ½ emergent, almost embedded, papillate or not, black. Peridium comprising 5–15 layers of textura angularis, outer region of dark brown cells, inner region of 2–4 layers of hyaline cells lining the locule. Asci bitunicate, broadly clavate, pedicellate, 100-135 × 20–35 μm, 8-spored, with a long, narrowed and twisted pedicel, forming between pseudoparaphyses. Pseudoparaphyses filiform, cellular, septate, 3–5 μm wide. Ascospores hyaline, aseptate, thin-walled, obovoid to obpyriform, with a distinct oil drop, biseriate in the ascus, 17–20 × 8–10 μm (−x = 18.8 × 8.9 μm, n = 20), L/W = 2.1. Conidiomata stromatic, pycnidial, solitary or aggregated, morphologically indistinguishable from the ascomata, walls composed of dark brown, thick-walled textura angularis, becoming thin-walled and hyaline toward the inner layer. Ostioles single, central, papillate or not. Paraphyses, hyaline, septate, 2–3 μm wide. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, 8–9(–11) × (1–)2–3 μm. Conidia hyaline, aseptate, moderately thick-walled, ovoid or broadly ellipsoidal, surrounded by mucilaginous sheath, 1 μm thick, (7–)9–14 × 6–10(–12) μm (−x = 11.5 × 8.6 μm, n = 20), L/W = 1.3. Spermatia unicellular, hyaline, allantoid to rod-shaped, 4-6 × 1-2 μm.
Specimen examined – INDIA, Rajasthan, on dead bamboo wood, 25 May 1976 (K 204341, holotype).
Notes – The bambusicolous lifestyle, aggregated ascostroma, broadly clavate asci with long pedicel, hyaline, aseptate, thin-walled, obovoid to obpyriform ascospores and its hyaline, aseptate, moderately thick-walled, ovoid or broadly ellipsoidal conidia surrounded by mucilaginous sheath suggest Neodeightonia being an appropriate genus for this species. Neodeightonia was introduced by Booth (Punithalingam, 1969), and species are typically associated with monocotyledonous plants and especially bamboo (Punithalingam, 1969; Phillips et al., 2008, 2013; Liu et al., 2012; Adamčík et al., 2015; Dai et al., 2017). Morphologically, Neodeightonia mucosa is most similar to Neodeightonia microspora, while the smaller ascospores (10–12 × 4.5–6 μm) of N. microspora, can be readily distinguished from those of N. mucosa (Dai et al., 2017).
So far, six species have been assigned in Neodeightonia, namely Neodeightonia licuriensis, N. microspora, N. mucosa, Neodeightonia palmicola, Neodeightonia phoenicum, and Neodeightonia subglobosa. Of these, N. palmicola and N. phoenicum have been reported on palms (Phillips et al., 2008, 2013; Liu et al., 2012), N. subglobosa, N. microspora, and N. mucosa are reported as bambusicolous (Dai et al., 2017; this study), and N. licuriensis has been reported on Syagrus coronata (Adamčík et al., 2015). Neodeightonia subglobosa has also been reported causing keratomycosis in a human eyes (Phillips et al., 2008).
Neofusicoccum cruenta (Petr.) Y.P. Zhou, Y. Zhang ter., comb. nov.
MycoBank number: 840944; Facesoffungi number: FoF 03578; Figure 5
≡ Melanops cruenta Petr., Annls mycol. 25(3/4): 226 (1927)
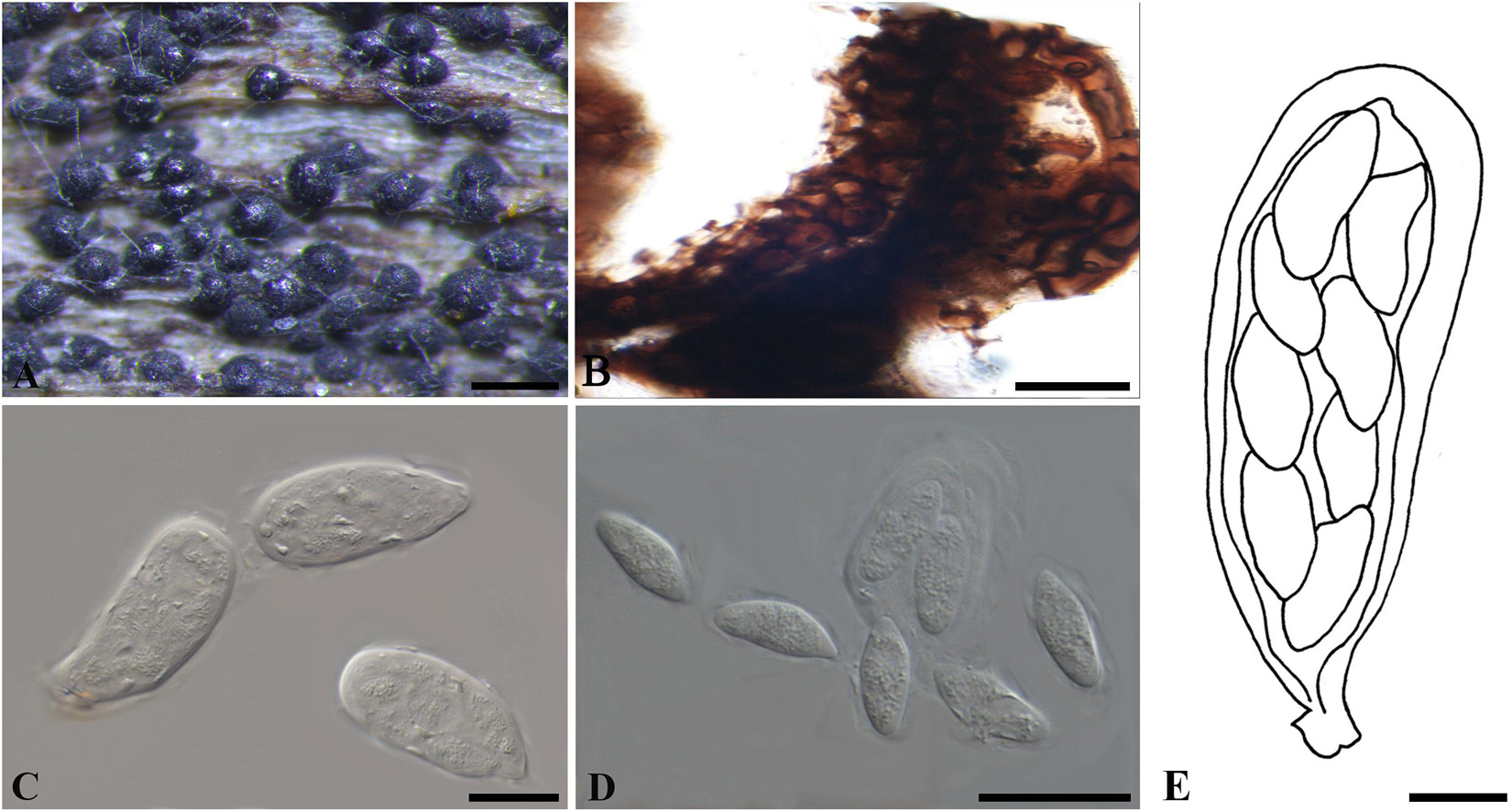
Figure 5. Neofusicoccum cruenta (W 10992, holotype). (A) Ascomata erumpent through a leaf surface. (B) Part of the peridium. (C,E) Asci. (D) Ascospores. Scale bars: (A) = 200 μm, (B,E) = 10 μm, (C,D) = 20 μm.
Ascomata erumpent, 70–140 μm diam., pseudothecial, solitary or gregarious, globose with a central ostiole, ¼ to ½ emergent, rarely embedded, black. Peridium composed of 6–10 layers of textura angularis, outer region of dark brown cells, inner region of 1–3 layers of hyaline cells lining the locule. Asci bitunicate, clavate, 38–65 × 17–22 μm. Pseudoparaphyses not observed. Ascospores hyaline, fusoid to ellipsoid, sometimes with tapered ends, bi- to triseriate, 13–20 × 5–9 μm (−x = 16.9 × 7 μm, n = 20), L/W = 2.4.
Specimen examined – CZECHIA, Prerov, on leaves of Polygonatum officinale (Liliaceae), April 1926, F. Petrak (W 1978-0010992/24018, holotype).
Notes – Only the sexual morph was observed on the type material, the morphology of which is consistent with members of Botryosphaeriaceae in having gregarious ascomata, broadly clavate asci and hyaline, aseptate ascospores as well as lacking pseudoparaphyses. Only LSU sequence was obtained for the type material of Melanops cruenta in this study, and a few other species of Neofusicoccum have LSU sequences available from GenBank as well. The phylogenetic analysis based on these LSU sequences suggested that M. cruenta resides in Neofusicoccum, being sibling to other species in the genus (Supplementary Figure 6). Thus, we have assigned M. cruenta to Neofusicoccum as a new combination, N. cruenta.
Neofusicoccum hamamelidis (Rehm) Y.P. Zhou, Y. Zhang ter., comb. nov.
MycoBank number: 840945; Facesoffungi number: FoF 03577; Figure 6
≡ Botryosphaeria hamamelidis Rehm, Annls mycol. 11(2): 168 (1913)
= Physalospora laricina Sawada, Bull. Gov. Forest Exp. Stn 46: 126 (1950)
= Guignardia laricina (Sawada) W. Yamam. and Kaz. Itô, Sci. Rep. Hyogo Univ. Agric. 5(1): 9 (1961)
= Botryosphaeria laricina (Sawada) Y.Z. Shang, Acta Mycol. Sin. 6(4): 249 (1987)

Figure 6. Neofusicoccum hamamelidis (W 29850, type?). (A) Ascomata erumpent through a twig epidermis. (B) Longitudinal section of a peridium showing the cells of textura angularis. (C-F) Broadly clavate, 8-spored asci with ascospores inside. (G–J). Hyaline, aseptate ascospores. Scale bars: (A) = 1 mm, (B-F) = 20 μm, (G-J) = 10 μm.
Ascomata erumpent, 170–400 μm diam., pseudothecial, scattered, solitary or aggregated, globose with a central ostiole, ¼ to ½ emergent, almost embedded, black. Peridium comprising 5–15 layers of textura angularis, outer region of dark brown cells, inner region of 4–6 layers of hyaline cells lining the locule. Asci bitunicate, clavate, 110–150 × 30–45 μm, 8-spored, forming among pseudoparaphyses. Pseudoparaphyses filiform, septate, constricted at the septa, rarely branched, 2–5 μm broad. Ascospores hyaline, thin-walled, ellipsoidal to ovoid, usually broadest in the middle, smooth, contents granular, biseriate in the ascus, 33–39(–48) × 13–16 μm (−x = 36.5 × 14.9 μm, n = 20), L/W = 2.4 (some data referred to Kobayashi, 1962).
Specimen examined – CANADA, near London, Ontario, on dead twigs of Hamamelidis virginiana (Hamamelidaceae), 18 May 1912, J. Dearness (W 07238/29850, type?). CHINA, Heilongjiang Province, Tieling, Langxiang, on twigs of Larix gmelinii (Rupr.) Kuzen. (Pinaceae), 10 July 2015, W. He (HMAS 246968, HMAS 246969).
Notes – The two isolates of Neofusicoccum hamamelidis (CGMCC3.18002/CGMCC3.18003) included in the phylogram (Supplementary Figure 5) were obtained from twigs of larch (Larix gmelinii) with shoot blight in Heilongjiang Province in China, which had been named as Physalospora laricina (on Larix kaempferi, Japan, Sawada, 1950), and subsequently combined to Guignardia as G. laricina (Sawada) W. Yamam. & Kaz. Itô and Botryosphaeria and B. laricina (Sawada) Y.Z. Shang (Yamamoto, 1961; Shang, 1987). Morphologically, “Botryosphaeria hamamelidis” and P. laricina were almost indistinguishable, but their ascospore broadness (9–12 vs. 13–16 μm, data from HMAS 246968 and HMAS 246969), which was insufficient to split them into two species. The phylogeny based on ITS, tef1-a, tub2 and LSU also supported the conspecific status of B. hamamelidis and P. laricina (Supplementary Figures 5, 6). Botryosphaeria hamamelidis, as an earlier epithet, had priority over Physalospora laricina. Thus, Physalospora laricina was reduced to a synonym of B. hamamelidis, which was assigned to Neofusicoccum as N. hamamelidis herein.
Botryosphaeria abuensis S.J. Kaur, 1996, Indian J. Mycol. Plant Path. 25(3): 334 (1996) [1995]. Figure 7
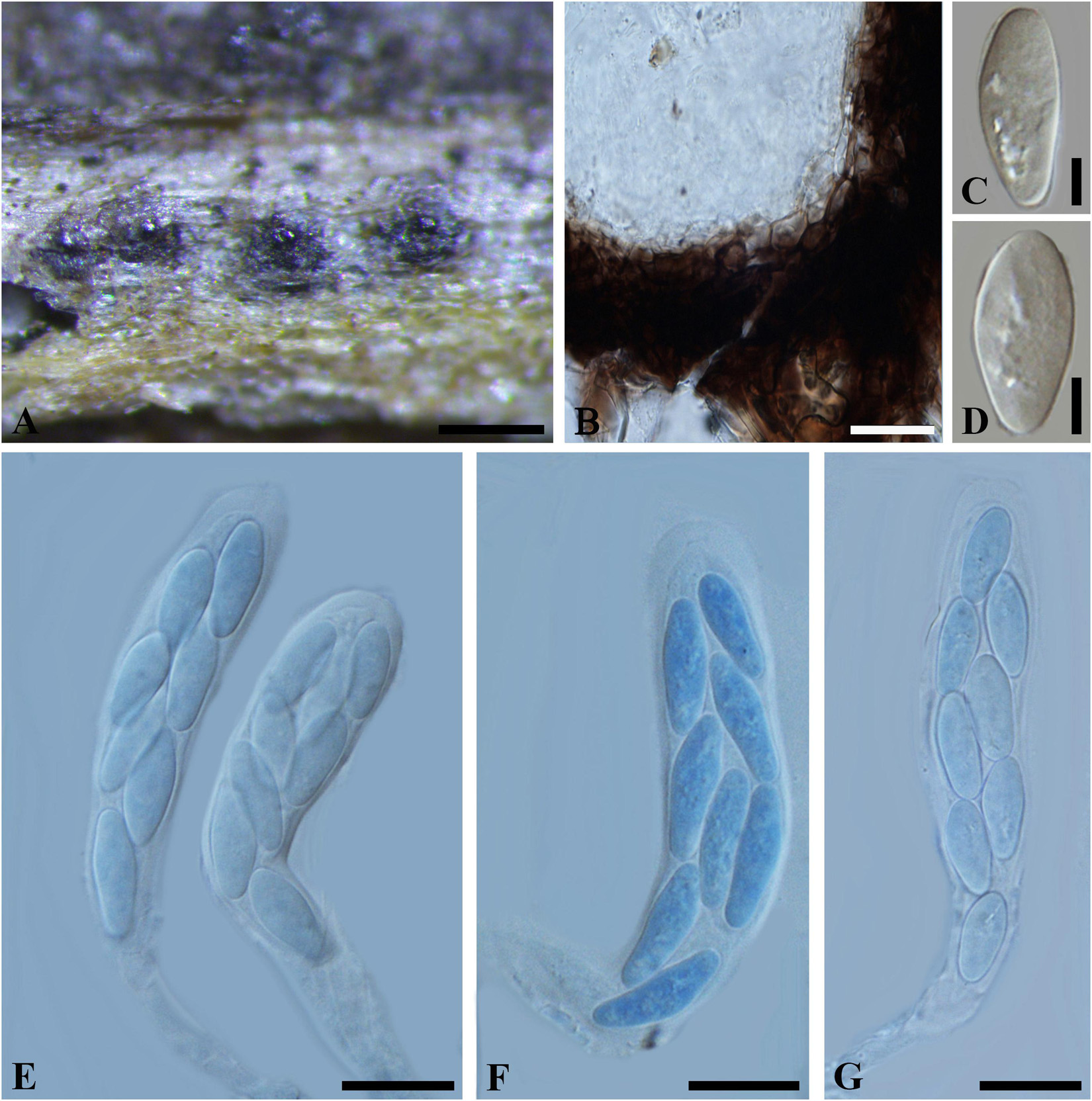
Figure 7. Botryosphaeria abuensis (IMI 192142, type). (A) Ascostroma erumpent through a twig. (B) Part of the peridium. (C,D) Released, hyaline ascospores. (E-G) Broadly clavate asci in cotton blue. Scale bars: (A) = 200 μm, (E-G) = 20 μm, (B-D) = 5 μm.
Ascomata 120-300 μm diam., pseudothecial, erumpent, scattered, solitary, globose with a central ostiole, ¼ to ½ emergent, rarely embedded, papillate or not, black. Peridium comprising 5–15 layers of textura angularis, outer region of dark brown cells, inner region of 2–4 layers of hyaline cells lining the locule. Asci bitunicate, clavate, pedicellate, up to 20 μm, 8-spored, 80-135 × 18–30 μm, forming among pseudoparaphyses. Pseudoparaphyses filiform, cellular, septate, 2–5 μm broad. Ascospores oblong to narrowly fusoid, broadest in the upper third, biseriate to triseriate in the ascus, hyaline, smooth, sometimes granular to guttulate, aseptate, (17–)20–30(–32) × 6–11 μm (−x = 23.4 × 8.4 μm, n = 30), L/W = 2.8.
Specimen examined – INDIA, Rajasthan, on dead twigs of Lantana camara (Verbenaceae), 4 March 1975 (K 192142, type).
Notes – The solitary or scattered ascomata, clavate asci, hyaline, aseptate, oblong to narrowly fusiform ascospores fit Botryosphaeriales. Taxonomic status of this species, however, could not be determined due to a lack of morphological features of the asexual morph and DNA sequence data. Thus B. abuensis is tentatively kept in Botryosphaeria sensu stricto herein.
Botryosphaeria aesculi (Peck) M.E. Barr, Contr. Univ. Mich. Herb. 2: 561 (1972). Figure 8
≡ Laestadia aesculi Peck, Rep. (Annual) Trustees State Mus. Nat. Hist., New York 39: 51 (1887) [1886]
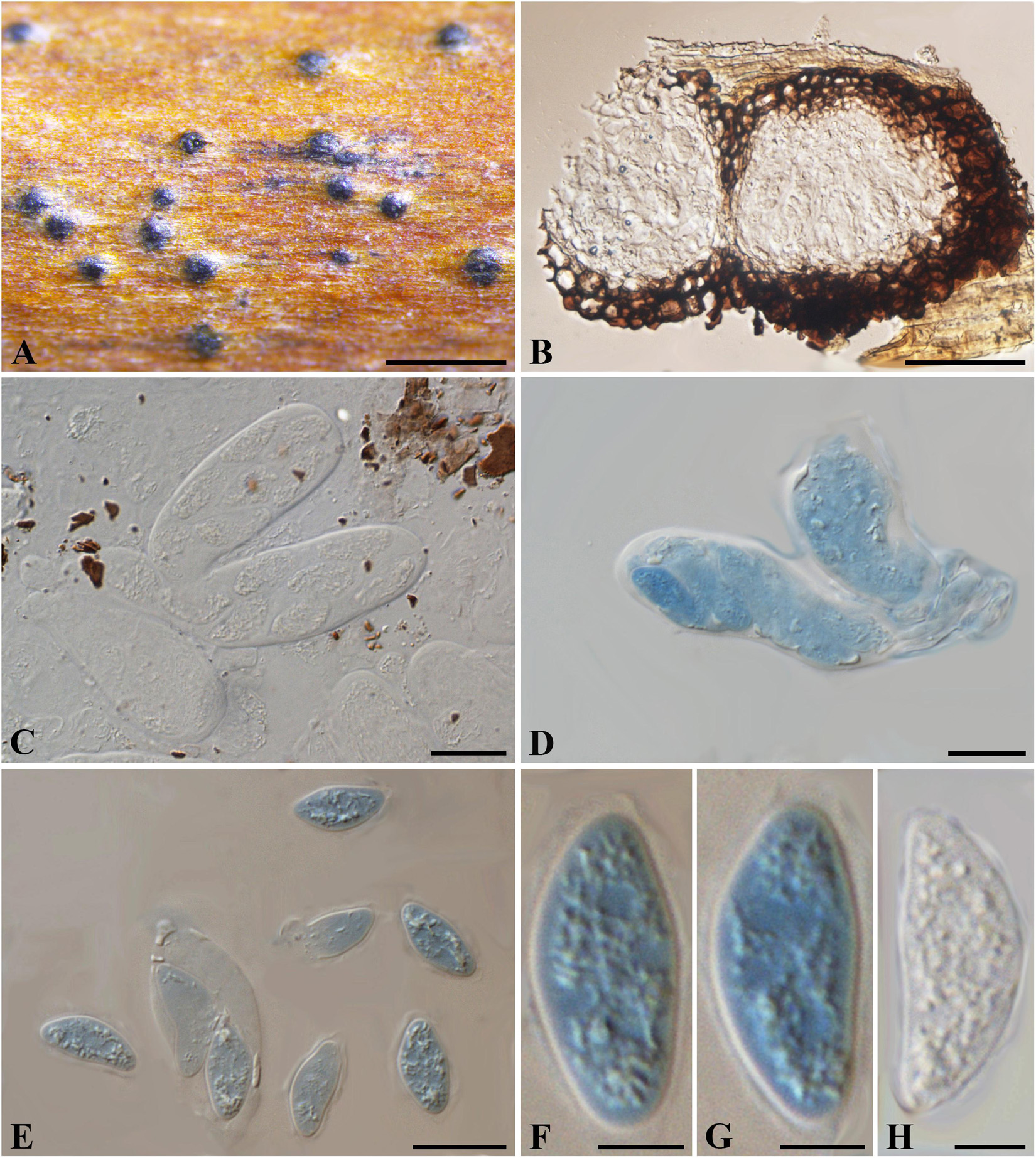
Figure 8. Botryosphaeria aesculi (NYS f93, holotype). (A) Ascomata erumpent through the bark of host twig. (B) Longitudinal section of ascomata. (C,D) Squash showing asci (C in water, D in cotton blue). (E-H) Released ascospores (E-G in cotton blue, H in water). Scale bars: (A) = 500 μm, (B) = 50 μm, (C-E) = 20 μm, (F-H) = 5 μm.
Ascomata erumpent, 80-200 μm diam., black, immersed to semi-immersed in the host, becoming erumpent, scattered, sometimes in small groups of 2 locules, globose with a central ostiole, papillate or not. Peridium comprising 4–8 layers of textura angularis, outer region of dark brown cells, inner region of 1–2 layers of hyaline cells lining the locule. Pseudoparaphyses not observed. Asci bitunicate, subclavate to clavate, 8-spored, 46-66 × 12–22 μm. Ascospores hyaline, ellipsoidal-fusiform or fusiform, broadest in the upper third, irregularly biseriate in the ascus, 16–20(–23) × 6–11 μm (−x = 19 × 8.9 μm, n = 20), L/W = 2.1.
Specimen examined – United States, Albany, on petioles of Aesculus hippocastanum (Sapindaceae), May 1885, G. W. Clinton (NYS f93, holotype).
Notes – Botryosphaeria aesculi was introduced as Laestadia aesculi, which was subsequently assigned to Botryosphaeria as B. aesculi by Barr (1972). Morphologically, the scattered and erumpent ascomata, small-sized and ellipsoidal asci differ from the species of Botryosphaeria sensu stricto, while the hyaline, aseptate and ellipsoidal to fusiform ascospores are consistent with taxa in the Botryosphaeriales. Herein we tentatively retain it within Botryosphaeria with its taxonomical status remaining to be undetermined until further phylogenetic analysis is carried out on verified specimens.
Botryosphaeria dasylirii (Peck) Theiss. and Syd., Annls mycol. 13(5/6): 663 (1915). Figure 9
≡ Dothidea dasylirii Peck, Bot. Gaz. 7(5): 57 (1882)
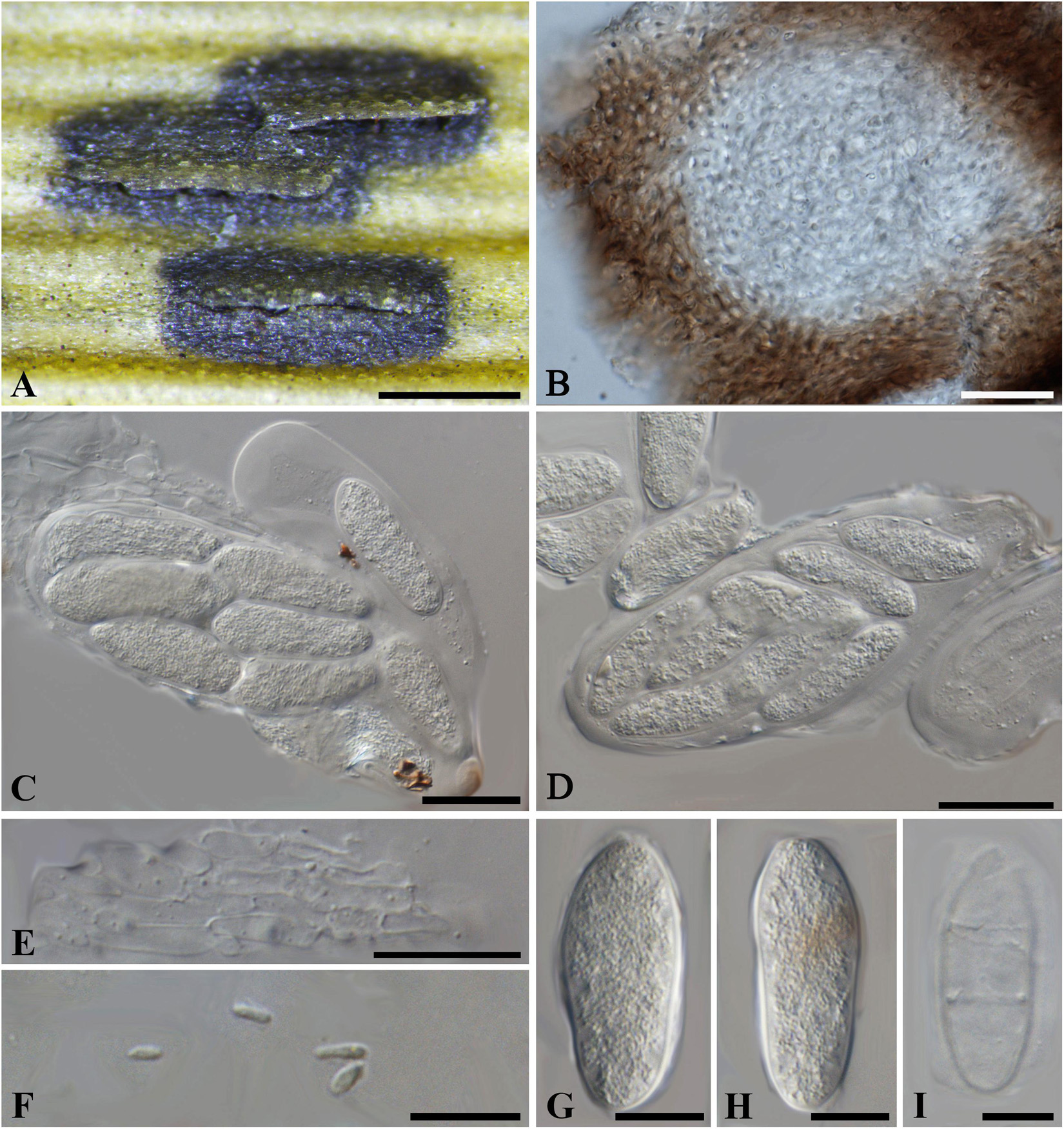
Figure 9. Botryosphaeria dasylirii (NYS f950, holotype). (A) Ascostroma erumpent through the leaf surface. (B) Section of the ascoma showing the peridium. (C) Ellipsoidal to narrowly fusiform asci. (D) Breaking asci with released ascospores. (E) Septate pseudoparaphyses. (F) Spermatia. (G,H) Hyaline aseptate ascospores with granular content. (I) Aged, 2-septate, hyaline ascospores. Scale bars: (A) = 500 μm, (C-E) = 20 μm, (B,F-I) = 10 μm.
Ascostroma erumpent through the leave surface, 0.3-1.8 mm diam. Ascomata 90-150 μm diam., pseudothecial, usually forming botryose clusters of 3-4 locules, globose with a central ostiole, covered with epidermal leaf tissue, black. Peridium comprising 9–12 layers of textura angularis, outer region of dark brown cells, inner region of 2–3 layers of hyaline cells lining the locule. Asci 8-spored, bitunicate, ellipsoidal to subclavate, 100-115 × 23–40 μm, forming between pseudoparaphyses. Pseudoparaphyses filiform, cellular, septate, 3–4 μm wide. Ascospores hyaline, ovoid to narrowly ellipsoid with granular content, aseptate, biseriate to triseriate, (24–)26–37(–40) × 10–15 μm (−x = 33 × 14 μm, n = 20), L/W = 2.3, sometimes becoming 0–2 septa with aging. Spermatia unicellular, hyaline, allantoid to rod-shaped, 4.5-6 × 1.5-2 μm.
Specimen examined – United States, Arizona, on leaves of Dasylirion sp. (Asparagaceae), May 1881, C. G. Pringle (NYS f950, holotype).
Notes – The erumpent botryose ascostroma, cellular pseudoparaphyses, hyaline, aseptate, large-sized ascospores suggest an affiliation in the Botryosphaeriaceae, while the ovoid to narrowly ellipsoid ascospores fit both Botryosphaeria and Neofusicoccum. Since there are no obvious morphological differences between Neofusicoccum and Botryosphaeria, the generic status of B. dasylirii remains uncertain until DNA sequence data can be obtained for it. Thus B. dasylirii is tentatively kept in Botryosphaeria sensu stricto herein until further phylogenetic analysis is carried out on verified specimens.
Botryosphaeria wisteriae (Rehm) Sacc., Syll. fung. (Abellini) 1: 459 (1882) Figure 10
≡ Thuemenia wisteriae Rehm, Mycoth. Univ., cent. 10: no. 971 (in sched.) (1878)

Figure 10. Botryosphaeria wisteriae (MICH 15081, isotype). (A) Botryose clustered ascomata erumpent through a twig epidermis. (B) Broken ascus showing hyaline, narrowly fusiform, ascospores within it. (C-F) Clavate ascus with aseptate or separated ascospores. Scale bars: (A) = 500 μm, (B-F) = 10 μm.
Ascostroma erumpent from bark of host, 0.5–1.5 mm diam. Ascomata 140-220 μm diam., pseudothecial, botryose clustered, globose with a central ostiole, black. Peridium comprising 7–13 layers of textura angularis, outer region of dark brown cells, inner region of 2–3 layers of hyaline cells lining the locule. Asci 8-spored, bitunicate, clavate with a short pedicel, 70-118 × 17–27 μm, forming between pseudoparaphyses. Pseudoparaphyses filiform, cellular, septate, 3–5 μm broad. Ascospores hyaline, ellipsoid to fusiform, granular content not sure, partially overlapping to biseriate in ascus, sometimes become 1-septate with aging, (17–)20–26(–30) × 7–10.5 μm (−x = 23.6 × 9.1 μm, n = 20), L/W = 2.6. Spermatia not observed.
Specimen examined – United States, South Carolina, Aiken, on dead twig of Wisteria chinensis (Fabaceae), Thuemen (MICH 15081, isotype).
Notes – The botryose ascomata, cellular pseudoparaphyses, and aseptate, hyaline ascospores are consistent with members of the Botryosphaeria sensu stricto. Thus B. wisteriae is tentatively kept in Botryosphaeria sensu stricto herein until further phylogenetic analysis is carried out on verified specimens.
Nothophoma ferruginea (Fuckel) Y.P. Zhou and Y. Zhang ter., comb. nov.
MycoBank number: 840946; Facesoffungi number: FoF 03576; Figure 11
≡ Melanops ferruginea Fuckel, Jb. nassau. Ver. Naturk. 25-26: 96 (1873)
≡ Botryosphaeria ferruginea (Fuckel) Sacc., Syll. fung. (Abellini) 1: 465 (1882)
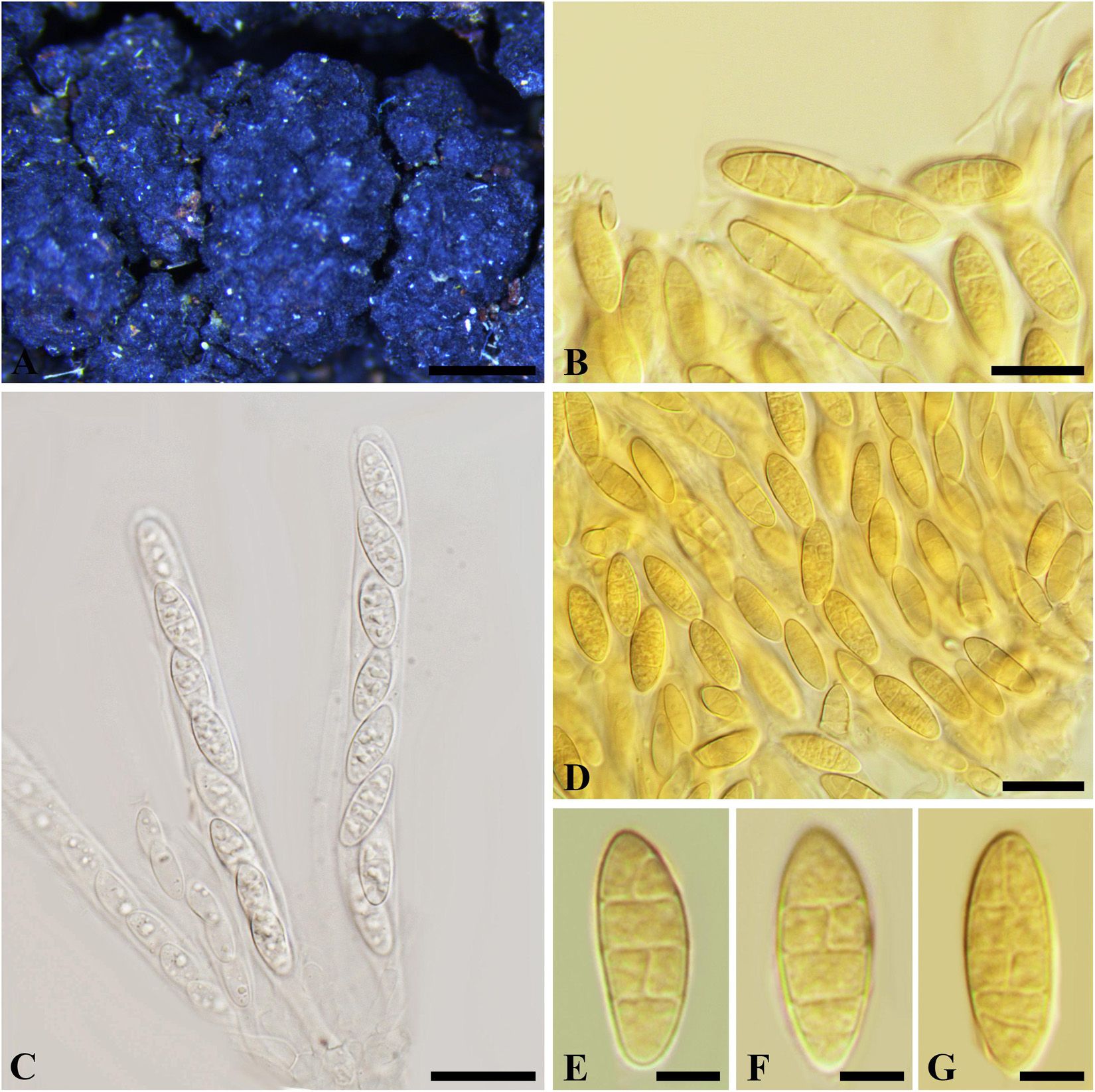
Figure 11. Nothophoma ferruginea (G 00127285, holotype). (A) Densely gregarious ascomata. (B,D-G) Ascospores in Melzer’s reagent. (C) Ascus with ascospores in water. Scale bars: (A) = 1 mm, (B) = 10 μm, (C,D) = 20 μm, (E-G) = 5 μm.
Ascostroma immersed to erumpent, 2–8 mm diam. Ascomata 220-550 μm diam., densely gregarious, with elongate, rounded, obtuse or acute papilla, black. Peridium comprising 12–15 layers of textura angularis, outer region of dark brown cells, inner region of 3–5 layers of hyaline cells lining the locule. Asci 8-spored, bitunicate, cylindrical with a short, narrowed, twisted, furcate pedicel, 115-145 × 10–13 μm, forming between pseudoparaphyses. Pseudoparaphyses filiform, cellular, septate, 3–4 μm wide. Ascospores uniseriate to partially overlapping, ellipsoidal to ovoid, muriform, with 3–4 transversal septa and 1–2 longitudinal septa in first, second or third cell(s), hyaline, (14–)17–20 × 6–9 μm (−x = 15.6 × 7.5 μm, n = 20), L/W = 2.5.
Specimen examined – SWITZERLAND, Neuchâtel, on the trunks of corruption Alnus glutinosa (Betulaceae), February 1872, Morthier (G 00127285, holotype).
Notes –The bitunicate asci, cellular pseudoparaphyses, the hyaline, broadly ellipsoid ascospores with 3–4 transversal septa and 1–2 longitudinal septa in central cells of Melanops ferruginea differ from members of Botryosphaeriales. Phylogeny based on ITS and LSU nuDNA sequences indicated that M. ferruginea closely related to Nothophoma Qian Chen and L. Cai (Didymellaceae, Pleosporales). Therefore, we assign it to Nothophoma as N. ferruginea.
Phyllachorella micheliae Syd., Annls mycol. 12(5): 489 (1914). Figure 12
= Botryosphaeria foliicola (Sivanesan and Nair, 1988)
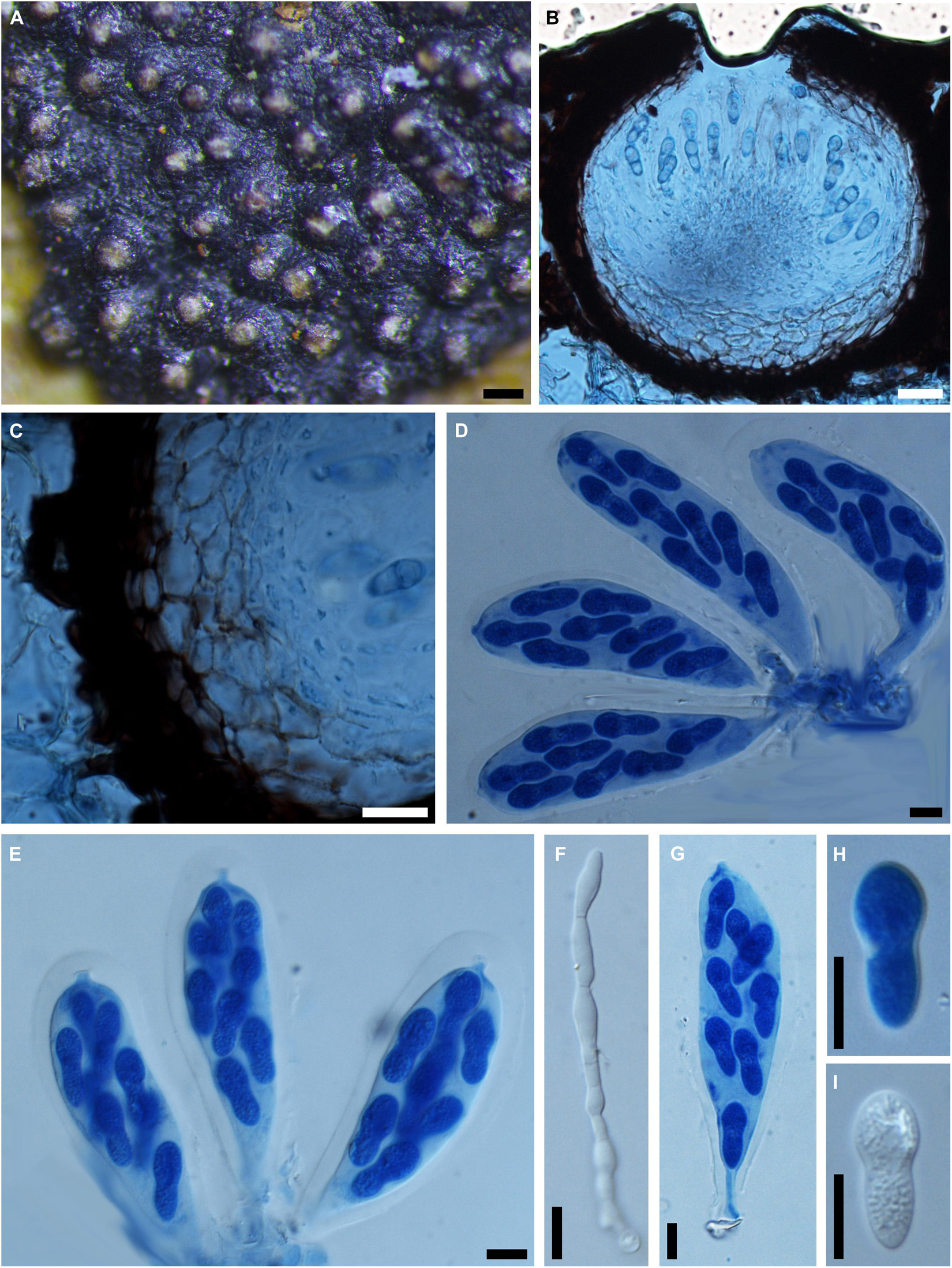
Figure 12. Phyllachorella micheliae (IMI 316002, holotype). (A) Botryose clusters of ascomata erumpent through the lower side of the leaf. (B) Longitudinal section through an ascoma in cotton blue. (C) Section of the peridium comprising cells of textura angularis in cotton blue. (D,E,G) Squash mounts showing broadly clavate asci with wide ocular chamber near the apex and short pedicels at the base in cotton blue. (F) Septate pseudoparaphyses in water. (H,I) Hyaline, aseptate ascospores in cotton blue (H) or in water (I). Scale bars: (A) = 100 μm, (B) = 20 μm, (C-I) = 20 μm.
Ascomata 140-250 μm diam., pseudothecial, aggregated forming a large botryose, irregularly rounded, 2–8 mm diam., lower side of the leaves, globose with a central white ostiole, ostiole 20–40 μm broad, ½ to ¾ emergent, rarely embedded, black. Peridium thick-walled, comprising 5–10 layers of textura angularis, outer region of dark brown cells, inner region of 4–6 layers of hyaline cells lining the locule. Asci 8-spored, bitunicate, broadly clavate with a long, narrow pedicel, which is up to 80 μm, with obvious apical chamber, 90-140 × 20–30 μm, forming between pseudoparaphyses. Pseudoparaphyses filiform, hyaline, cellular, septate, obviously constricted at the septa, rarely branched, 4–5 μm wide. Ascospores hyaline, aseptate, thin-walled, unequally gourd-shaped with upper part broader than the lower part, biseriate to triseriate in the ascus, (16–)18–21(–22) × 8–9(–11) μm (−x = 19.4 × 8.8 μm, n = 20), L/W = 2.2.
Specimen examined – INDIA, Kodaikanal, Tamil Nadu, on leaves of Michelia nilgarica (Magnoliaceae), 10 January 1987, L. N. Nair (K 316002, holotype).
Notes – Botryosphaeria foliicola was introduced by Sivanesan and Nair (1988) from the leaves of Michelia nilgarica in India, which distinguishes it from other species of Botryosphaeria by its obovoid and characteristically constricted ascospores. Phyllachorella micheliae, the generic type of Phyllachorella, was reported from the leaves of the same host in India. The strong morphological similarity of Botryosphaeria foliicola and Phyllachorella micheliae warranted their conspecific status (Liu et al., 2012). Based on priority, the later name Botryosphaeria foliicola is reduced to synonymy with Phyllachorella micheliae, which is retained in Botryosphaeriales genera incertae sedis (Wijayawardene et al., 2020).
Botryosphaeria gaubae Petr., Sydowia 21: 235 (1968). Supplementary Figure 1
Ascomata erumpent, 200-360 μm diam. Ascomata scattered, solitary, externally black, globose with a central ostiole, ¼ to ½ emergent, embedded in hairy seta on lower side of the leaves. Peridium comprising 5–15 layers of textura angularis, outer region of dark brown cells, inner region of 3–5 layers of hyaline cells lining the locule. Asci bitunicate, cylindrical or broadly cylindrical, pedicellate or not, pedicles up to 20 μm, 8-spored, 140-180 × 25–35 μm, forming among pseudoparaphyses. Pseudoparaphyses filiform, narrowly cellular, rarely branched, 2–3 μm broad. Ascospores hyaline, aseptate, fusiform to ellipsoid, with tapered ends and appearing spindle-shaped, sometimes slightly narrower at the middle, irregularly biseriate in the ascus, (23–)27–36(–46) × (6–)10–15(–17) μm (−x = 32.2 × 12.6 μm, n = 20), L/W = 2.6.
Specimen examined – AUSTRALIA, on leaves of Grevillea victoriae (Proteaceae), Mt Franklin, ca. 4500 ft., 27 January 1953, leg. E. Gauba (W 1992-05937, holotype).
Notes – Botryosphaeria gaubae was introduced and assigned in Botryosphaeria sensu lato, which included Botryosphaeria, Gibberella, and Lisea or even Melanops (Petrak, 1967). While the foliicolous habitation, scattered ascomata, cylindrical asci, fusiform to ellipsoid, aseptate ascospores with tapered ends and the presence of filiform pseudoparaphyses differ from these genera. We consequently treat it as a species in the Dothideomycetes incertae sedis.
Laestadia apocyni Ellis and Everh., Proc. Acad. nat. Sci. Philad. 42: 230 (1890). Supplementary Figure 2
= Botryosphaeria apocyni (Ellis and Everh.) M.E. Barr, Contr. Univ. Mich. Herb. 9(8): 560 (1972)
Ascomata 100-215 μm diam., pseudothecial, scattered or clustered, globose with a central ostiole, papillate or not, black. Peridium thin, comprising 2–3 layers of textura angularis. Asci 8-spored, bitunicate, slightly obclavate, lack of pedicel, 47 × 27 μm (only a single complete mature ascus observed). Pseudoparaphyses not observed. Ascospores hyaline, fusiform with rounded ends, 1-septate, biseriate in the ascus, 14–20 × 4–8 μm (−x = 17.2 × 6.1 μm, n = 10), L/W = 2.8.
Specimen examined – CANADA, Ontario, Middlesex: London, on dead stems of Apocynum sp. (Apocynaceae), J. Dearness (MICH 14281, isotype).
Notes – The small-sized ascomata, bitunicate, slightly obclavate asci, lack of a pedicel, hyaline, 1-septate ascospores disagree with Botryosphaeria sensu stricto. Laestadia apocyni had been assigned to Guignardia as G. apocyni, while its 1-septate ascospores disagree with the non-septate ascospores of Guignardia (Vasyagina et al., 1987; Barr, 1993). Thus, its taxonomic status cannot be determined yet, and tentatively assigned in Dothideomycetes incertae sedis.
Sphaeria smilacinina Peck, Ann. Rep. N.Y. St. Mus. nat. Hist. 29: 62 (1878) [1876] Supplementary Figure 3
= Botryosphaeria smilacinina (Peck) M.E. Barr, Contr. Univ. Mich. Herb. 9(8): 560 (1972)
= Discochora smilacinina (Peck) Bissett, Can. J. Bot. 64(8): 1721 (1986)
Ascomata 150-280 μm diam., pseudothecial, solitary or scattered, globose with a central ostiole, immersed or ¼ to ½ emergent, black. Peridium comprising 6–12 layers of textura angularis, outer region of dark brown cells, inner region of 2–4 layers of hyaline cells lining the locule. Asci bitunicate, broadly clavate to broadly cylindrical, 100-150 × 23–36 μm, forming among pseudoparaphyses. Pseudoparaphyses filiform, cellular, septate, 3–5 μm broad. Ascospores fusiform to narrowly fusiform, 1-septate, obviously constricted in the middle, biseriate in the ascus, 26–32 × 8–11 μm (−x = 29.6 × 9.4 μm, n = 10), L/W = 3.2.
Specimen examined – United States, Albany, on dead stems of Smilacina stellate (Liliaceae), Charles H. Peck (NYS f2818, holotype).
Notes – Sphaeria smilacinina has been assigned to Discochora as D. smilacinina (Phyllostictaceae, Botryosphaeriales) (Bissett, 1986). The 1-septate ascospore of S. smilacinina, however, disagree with the non-septate ascospores of Discochora. The immersed and scattered, ostiolate pseudothecia, clavate to broadly cylindrical bitunicate asci, 1-septate, constricted, hyaline ascospores suggest that this taxon probably resides in the Didymellaceae (Pleosporales), while its taxonomic status cannot be determined until further phylogenetic analysis is carried out on verified specimens (Zhang et al., 2012; Chen et al., 2015). Thus, we tentatively keep this species in Dothideomycetes incertae sedis.
Botryosphaeria sensu lato was described mainly on the basis of morphological characters of its sexual morph and host associations, which led to 286 epithets being assigned to the genus (Index Fungorum, 22/08/2021)3. Botryosphaeria sensu lato was characterized based on its pseudothecia, ostiolate, often multiloculate ascostroma, cellular pseudoparaphyses, bitunicate, uni- to biseriate, 8-spored asci with or without pedicels, aseptate, ovoid to fusoid to ellipsoid ascospores which may become brown and 1–2-septate with age. The genus has been connected with numerous asexual genera including Diplodia, Dothiorella, Lasiodiplodia, Macrophoma, Sphaeropsis, and Fusicoccum (Sivanesan, 1984; Barr, 1987; Denman et al., 2000). Based on the phylogenetic analysis of 28S rDNA sequences, Crous et al. (2006) recognized ten clades in the Botryosphaeriaceae, and noted that the morphology of the conidial morphs was more informative in generic circumscription. Thus far, Botryosphaeria sensu lato was reported being highly polyphyletic with only eight species being treated in Botryosphaeria sensu stricto (Crous et al., 2006; Phillips et al., 2008, 2013; Slippers et al., 2014; Ariyawansa et al., 2016; Zhou et al., 2016, 2017; Hattori et al., 2021; Zhang et al., 2021).
Four of the 17 taxa of Botryosphaeria sensu lato considered in the present study have been confirmed as members of Botryosphaeria sensu stricto, which include B. berengeriana var. acerina, B. aterrima, B. berengeriana var. weigelae, and B. mirabile with both B. berengeriana var. acerina and B. berengeriana var. weigelae reduced to synonyms of B. dothidea. Two other species of Botryosphaeria sensu lato were assigned in Neofusicoccum, viz., N. cruenta and N. hamamelidis. Neofusicoccum was separated from Botryosphaeria and introduced as a new genus based on combined multigene phylogenetic analysis and subtle morphological differences, i.e., pycnidial paraphyses only exist in Botryosphaeria (Fusicoccum), which have never been reported in any Neofusicoccum species (Crous et al., 2006; Phillips et al., 2013). Because of their morphological similarities, it is possible or even probable that some other species of Botryosphaeria sensu lato may actually more appropriately reside in Neofusicoccum.
Based on the phylogenetic analyses of combined ITS, LSU, tef1-a and tub2 loci, B. laricina and N. hamamelidis (= B. hamamelidis) form a conspecific clade. The morphological characteristics of their sexual morphs also support their conspecific status (see comments above). Neofusicoccum hamamelidis was originally reported from the dead twigs of Hamamelidis virginiana in Canada, while B. laricina causes shoot blight of larch, which is one of the most important quarantine diseases in China (Liu et al., 2009). The conspecific status of N. hamamelidis and B. laricina supports its broad host range and wide distribution, and these will help in making practical quarantine rules, as a comprehensive knowledge as well as accurate identification of pathogens are extremely important when formulating quarantine regulations (Kumar et al., 2008).
The sexual morph of Botryosphaeria sensu lato is morphologically conserved (for example, in the size of the asci and ascospores), while the morphology of asexual morph and host association varies considerably more, which contributes to a natural classification of this group of fungi (Shoemaker, 1964; Pennycook and Samuels, 1985; Phillips et al., 2013; Slippers et al., 2014). For example, Botryosphaeria mucosa was assigned to Neodeightonia (as N. mucosa, Botryosphaeriaceae) based on its bambusicolous host association, aggregated ascostroma, shape of asci, ascospore shape and septation, as well as conidial morphology. Based on the morphological characteristics or DNA sequences comparisons, many specimens considered in this study should be excluded from Botryosphaeriales. For example, B. ferruginea in Nothophoma as N. ferruginea (Pleosporales), Botryosphaeria apocyni (Basionym: Laestadia apocyni), B. gaubae and B. smilacinina (Basionym: Sphaeria smilacinina) in Dothideomycetes incertae sedis. Thus, a polyphasic taxonomic approach should be applied in type studies of Botryosphaeria sensu lato, including the use of host association, morphological characteristics of both sexual and asexual morphs, geographical distribution, DNA sequences as well as epitypification where possible.
Based on both morphological characters and results of nuDNA sequence analysis, Botryosphaeria sensu stricto now includes ten species, namely B. agaves, B. aterrima, B. corticis, B. dothidea, B. fabicerciana, B. kuwatsukai, B. mirabile, B. qingyuanensis, B. ramosa and B. scharifii, of which B. fabicerciana and B. qingyuanensis have previously been reported from China. The current study shows that further studies are necessary on other type specimens of Botryosphaeria sensu lato in order to clarify their taxonomic status. Fresh collections are also needed to facilitate their epitypification.
In summary, Botryosphaeria sensu lato is highly polyphyletic, and species belong to various genera or families of Botryosphaeriales or even other orders within Dothideomycetes. Studying the type material of Botryosphaeria sensu lato helps to understand the circumscription of genera or families within Botryosphaeriales. Redescribing and obtaining DNA sequences of the type specimens makes it possible to epitypify those species and clarify their taxonomic status (Zhang et al., 2008). Of the 286 epithets within Botryosphaeria sensu lato, less than 20% have DNA sequences available from the type materials (Denman et al., 1999; Smith et al., 2001; Slippers et al., 2004; Ariyawansa et al., 2016; Zhang et al., 2021). Thus, further study is required to obtain a more natural classification for species presently accommodated in Botryosphaeria sensu lato.
The data presented in the study are deposited in the TreeBASE and GenBank repository, accession numbers are S21054 for Botryosphaeria, S21059 and S21050 for Neofusicoccum, GenBank accession are listed in Supplementary Table 1.
YZ designed the experiments. YZ and YPZ prepared the samples, conducted the molecular experiments, and analyzed the data. WS, LZ, DP-Z, PC, BS, and YD revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (General Programs, 31971658, 31770015, and 31370063) and NSFC Projects of International Cooperation and Exchanges (3155461143028).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The curators of the herbarium G, W, CUP, F, K, MICH, and NYS are thanked for providing herbarium specimens on loan and for their assistance in locating specimens. YZ and YD thank the Beijing Forestry University for research support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.737541/full#supplementary-material
Supplementary Figure 1 | Botryosphaeria gaubae (W 1992-05937, holotype). (A,B) Ascomata erumpent through the lower side of the leaf. (C) Squash showing cylindrical or broadly cylindrical asci in cotton blue. (D) Part of the peridium. (E) Septate pseudoparaphyses in cotton blue. (F-H) Aseptate, fusiform to ellipsoid ascospores in cotton blue. Scale bars: (A) = 1 mm, (B) = 200 μm, (C) = 50 μm, (E) = 20 μm, (D,F-H) = 10 μm.
Supplementary Figure 2 | Laestadia apocyni (MICH 14281, isotype). (A) Ascomata erumpent through a piece of twig epidermis. (B) Released, hyaline, 1-septate ascospores. (C) Ascus in water. (D) Line drawing of ascus in water. Scale bars: (A) = 200 μm, (B-D) = 20 μm.
Supplementary Figure 3 | Sphaeria smilacinina (NYS f2818, holotype). (A) Ascomata erumpent through the twig epidermis. (B,C) Immature asci. (D) Released ascospores. (E) Line drawing of broadly clavate ascus. Scale bars: (A) = 500 μm, (B-D) = 20 μm, (E) = 40 μm.
Supplementary Figure 4 | One of the most parsimonious trees obtained from combined ITS, LSU, tub2, and tef1-a sequence data of Botryosphaeria spp. Outgroup taxa are Neofusicoccum luteum and Neofusicoccum parvum. Maximum parsimony (MP) support values above 70% and Bayesian posterior probabilities (PP) support above 80% are shown with MP bootstrap followed by Bayesian PP (MP/PP) values at the nodes. The species characterized in this study are in boldface.
Supplementary Figure 5 | One of the most parsimonious trees obtained based on combined ITS, tef1-α, and tub2 sequence data of Neofusicoccum spp. Outgroup taxon are Botryosphaeria dothidea and B. corticis. Maximum parsimony (MP) support values above 60% and Bayesian posterior probabilities (PP) support above 80% are shown with MP/PP, values at the nodes. The species characterized in this study are in boldface.
Supplementary Figure 6 | One of the most parsimonious trees obtained from LSU sequence dataset of Neofusicoccum spp. Outgroup taxa are Botryosphaeria corticis and B. dothidea. Maximum parsimony (MP) support values above 70% and Bayesian posterior probabilities (PP) support above 80% are shown with MP bootstrap followed by Bayesian PP (MP/PP) values at the nodes. The species characterized in this study are in boldface.
Supplementary Figure 7 | One of the most parsimonious trees obtained from ITS and LSU sequence dataset of Nothophoma spp. Outgroup taxa is Didymella calidophila. Maximum likelihood (ML) support values above 50%, Maximum parsimony (MP) support values above 50%, and Bayesian posterior probabilities (PP) support above 95% are shown with ML and MP bootstrap followed by Bayesian PP (MP/PP/ML) values at the nodes. The species characterized in this study are in boldface.
Supplementary Table 1 | Species, specimens and GenBank accession numbers of sequences used in this study (newly generated sequences are indicated in bold).
Abdollahzadeh, J., Zare, R., and Phillips, A. J. L. (2013). Phylogeny and taxonomy of Botryosphaeria and Neofusicoccum species in Iran, with description of Botryosphaeria scharifii sp. nov. Mycologia 105, 210–220. doi: 10.3852/12-107
Adamčík, S., Cai, L., Chakraborty, D., Chen, X. H., Cotter, H. V. T., Dai, D. Q., et al. (2015). Fungal biodiversity profiles 1-10. Crypt. Mycol. 36, 121–166. doi: 10.7872/crym/v36.iss2.2015.121
Alves, A., Crous, P. W., Correia, A., and Phillips, A. J. L. (2008). Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 28, 1–13.
Ariyawansa, H. A., Hyde, K. D., Liu, J. K., Wu, S. P., and Liu, Z. Y. (2016). Additions to Karst Fungi 1: Botryosphaeria minutispermatia sp. nov., from Guizhou Province, China. Phytotaxa 275, 35–44. doi: 10.11646/phytotaxa.275.1.4
Barr, M. E. (1972). Preliminary studies on the Dothideales in temperate North America. Contr. Univ. Michigan Herb. 9, 523–638.
Bissett, J. (1986). Discochora yuccae sp. nov. with Phyllosticta and Leptodothiorella synanamorphs. Can. J. Bot. 64, 1720–1726. doi: 10.1139/b86-230
Cesati, V., and De Notaris, G. (1863). Schema di classificazione degli sferiacei italici aschigeri piu’ o meno appartenenti al genere Sphaeria nell’antico significato attribuitoglide Persoon. Comment. Soc. Crittog. Ital. 14, 177–240.
Chen, Q., Jiang, J. R., Zhang, G. Z., Cai, L., and Crous, P. W. (2015). Resolving the Phoma enigma. Stud. Mycol. 82, 137–217. doi: 10.1016/j.simyco.2015.10.003
Chen, S. F., Pavlic, D., Roux, J., Slippers, B., Xie, Y. J., Wingfield, M. J., et al. (2011). Characterization of Botryosphaeriaceae from plantation-grown Eucalyptus species in South China. Plant Path. 60, 739–751. doi: 10.1111/j.1365-3059.2011.02431.x
Cooke, M. C. (1885). Synopsis pyrenomycetum. Grevillea 13, 100–109. doi: 10.1175/1520-0493(1885)13[100b:TOW]2.0.CO;2
Crous, P. W., Slippers, B., Wingfield, M. J., Rheeder, J., Marasas, W. F. O., Philips, A. J. L., et al. (2006). Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 55, 235–253. doi: 10.3114/sim.55.1.235
Dai, D. Q., Phookamsak, R., Wijayawardene, N. N., Li, W. J., Bhat, D. J., Xu, J. C., et al. (2017). Bambusicolous fungi. Fungal Divers. 8, 1–105. doi: 10.1007/s13225-016-0367-8
Denman, S., Crous, P. W., and Wingfield, M. J. (1999). A taxonomic reassessment of Phyllachora proteae, a leaf pathogen of Proteaceae. Mycologia 91, 510–516. doi: 10.2307/3761351
Denman, S., Crous, P. W., Taylor, J. E., Kang, J. C., Pascoe, I., and Wingfield, M. J. (2000). An overview of the taxonomic history of Botryosphaeria and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Stud. Mycol. 45, 129–140. doi: 10.1007/s005720050286
Dissanayake, A. J., Phillips, A. J. L., Li, X. H., and Hyde, K. D. (2016). Botryosphaeriaceae: Current status of genera and species. Mycosphere 7, 1001–1073. doi: 10.5943/mycosphere/si/1b/13
Fuckel, L. (1870). Symbolae mycologicae. Beiträge zur Kenntniss der Rheinischen Pilze. Jahrbücher Nassauischen Vereins Naturkunde 23-24, 1–459. doi: 10.5962/bhl.title.47117
Glass, N. L., and Donaldson, G. C. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl. Environ. Microbiol. 61, 1323–1330. doi: 10.1002/bit.260460112
Grossenbacher, J. G., and Duggar, B. M. (1911). A contribution to the life-history, parasitism, and biology of Botryosphaeria ribis. N. Y. Agric. Exp. Station Geneva Tech. Bull. 18, 113–190.
Hattori, Y., Ando, Y., Sasaki, A., Uechi, N., and Nakashima, C. (2021). Taxonomical study of noteworthy species of Botryosphaeria in Japan. Mycoscience 49, 122–132. doi: 10.1080/12298093.2021.1895486
Huelsenbeck, J. P., and Ronquist, F. (2005). “Bayesian analysis of molecular evolution using MrBayes,” in Statistical Methods in Molecular Evolution, ed. R. Nielsen (New York, NY: Springer), 183–232. doi: 10.1007/0-387-27733-1_7
Kobayashi, T. (1962). A blight disease of larch caused by Guignardia cryptomeriae Sawada in comparison with the shoot blight caused by Physalospora laricina Sawada (in Japanese). J. Jpn. For. Soc. 44, 282–286.
Kumar, A., Singh, U. S., Kumar, J., and Garg, G. K. (2008). Application of molecular and immuno-diagnostic tools for detection, surveillance and quarantine regulation of Karnal bunt (Tilletia indica) of wheat. Food. Agric. Immunol. 19, 293–311. doi: 10.1080/09540100802478194
Li, G. Q., Liu, F. F., Li, J. Q., Liu, Q. L., and Chen, S. F. (2017). Botryosphaeriaceae from Eucalyptus plantations and adjacent plants in China. Persoonia 40, 63–95. doi: 10.3767/persoonia.2018.40.03
Liu, J. K., Phookamsak, R., Doilom, M., Wikee, S., Li, Y. M., Ariyawansha, H., et al. (2012). Towards a natural classification of Botryosphaeriales. Fungal Divers. 57, 149–210. doi: 10.1007/s13225-012-0207-4
Liu, X. W., Wang, F., and Liu, X. F. (2009). The molecular diagnosis of the larch shoot bligh. Plant Quar. 23, 1–4.
Marsberg, A., Kemler, M., Jami, F., Nagel, J. H., Postma-Smidt, A., Naidoo, S., et al. (2017). Botryosphaeria dothidea: a latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 18, 477–488. doi: 10.1111/mpp.12495
Pennycook, S. R., and Samuels, G. J. (1985). Botryosphaeria and Fusicoccum species associated with ripe fruit rot of Actinidia deliciosa (kiwifruit) in New Zealand. Mycotaxon 24, 445–458.
Phillips, A. J. L., Alves, A., Abdollahzaden, J., Slippers, B., Wingfield, M. J., Groenewald, J., et al. (2013). The Botryosphaeriaceae: genera and species known from culture. Stud. Mycol. 76, 51–167. doi: 10.3114/sim0021
Phillips, A. J. L., Alves, A., Pennycook, S. R., Johnston, P. R., Ramaley, A., Akulov, A., et al. (2008). Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21, 29–55. doi: 10.3767/003158508X340742
Posada, D., and Buckley, T. R. (2004). Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst. Biol. 53, 793–808. doi: 10.1080/10635150490522304
Rodríguez, F., Oliver, J. L., Marín, A., and Medina, J. R. (1990). The general stochastic model of nucleotide substitution. J. Theor. Biol. 142, 485–501. doi: 10.1016/S0022-5193(05)80104-3
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Saccardo, P. A. (1882). Sylloge Fungorum Omnium Hucusque Cognitorum, Vol. 1. Patavii: Sumptibus Auctoris, 456–466. doi: 10.5962/bhl.title.80010
Sawada, K. (1950). Fungi inhabiting conifers in the Tohoku district. II. Fungi on various conifers except ‘Suji’. B. Gov. For. Exp. Station Meguro 46, 111–150.
Shang, Y. Z. (1987). Taxonomic study on the pathogen fungus of shoot blight of larch. Acta Mycol. Sin. 6, 248–249.
Shoemaker, R. A. (1964). Conidia states of some Botryosphaeria species on Vitis and Quercus. Can. J. Bot. 42, 1297–1301. doi: 10.1139/b64-122
Sivanesan, A., and Nair, L. N. (1988). Four interesting members of the Dothideales. T. Brit. Mycol. Soc. 91, 323–329. doi: 10.1016/S0007-1536(88)80221-3
Slippers, B., Crous, P. W., Denman, S., Coutinho, T. A., Wingfield, B. D., and Wingfield, M. J. (2004). Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96, 83–101. doi: 10.1080/15572536.2005.11833000
Slippers, B., Roux, J., Wingfield, M. J., van der Walt, F. J. J., Jami, F., Mehl, J. W. M., et al. (2014). Confronting the constraints of morphological taxonomy in the Botryosphaeriales. Persoonia 33, 155–168. doi: 10.3767/003158514X684780
Smith, H. E., Crous, P. W., Wingfield, M. J., Coutinho, T. A., and Wingfield, B. D. (2001). Botryosphaeria eucalyptorum sp. nov., a new species in the B. dothidea-complex on Eucalyptus in South Africa. Mycologia 93, 277–285. doi: 10.2307/3761649
Swofford, D. L. (2002). PAUP∗. Phylogenetic Analysis Using Parsimony (∗and Other Methods). Version 4. Sunderland, MA: Sinauer Associates.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Theissen, F., and Sydow, H. (1915). Die Dothideales. Kritisch-systematische Originalunter-suchungen (Continuatio). Ann. Mycol. 13, 431–746.
Trotter, A. (1928). Sylloge Fungorum Omnium Hucusque Cognitorum, Vol. 24 (London, MA: Forgotten Books), 810–815.
Vasyagina, M. P., Byzova, Z. M., and Tartenova, M. A. (1987). Lokuloaskomitsety. Flora Sporovykh Rastenii Kazachstana 12, 1–294.
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
von Arx, J. A., and Müller, E. (1954). Die Gattungen der amerosporen Pyrenomyceten. Beiträge Kryptogamenflora Schweiz Vol. 11, 1–434. Bern: Buächler
von Höhnel, F. (1909). Fragmente zur Mykologie (VII. Mitteilung, Nr. 289 bis 353). Sitzungsberichten Kaiserliche Akad. Wiss. Wien Math. Naturwissenschaftliche Klasse Abt. 1118, 813–904.
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR Protocols: A Guide to Methods and Applications, eds M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (New York, NY: Academic Press), 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wijayawardene, N. N., Hyde, K. D., Al-Ani, L. K. T., Tedersoo, L., Haelewaters, D., Rajeshkumar, K. C., et al. (2020). Outline of Fungi and fungus-like taxa. Mycosphere 11, 1060–1456. doi: 10.5943/mycosphere/11/1/8
Xu, C., Wang, C. S., Ju, L. L., Zhang, R., Biggs, A. R., Tanaka, E., et al. (2015). Multiple locus genealogies and phenotypic characters reappraise the causal agents of apple ring rot in China. Fungal Divers. 71, 215–231. doi: 10.1007/s13225-014-0306-5
Yamamoto, W. (1961). Species of the genera of Glomerella and Guignardia with special reference to their imperfect stages. Sci. Rep. Hyogo Univ. Agric. 5, 1–12.
Zhang, W., Groenewald, J. Z., Lombard, L., Schumacher, R. K., Phillips, A. J. L., and Crous, P. W. (2021). Evaluating species in Botryosphaeriales. Persoonia 46, 63–115. doi: 10.3767/persoonia.2021.46.03
Zhang, Y., Crous, P. W., Schoch, C. L., and Hyde, K. D. (2012). Pleosporales. Fungal Divers. 52, 1–221. doi: 10.1007/s13225-011-0117-x
Zhang, Y., Fournier, J., Jeewon, R., and Hyde, K. D. (2008). Multi-gene phylogeny and morpho-taxonomy of Amniculicola lignicola: a novel freshwater fungus from France and its relationships to the Pleosporales. Mycol. Res. 112, 1186–1194. doi: 10.1016/j.mycres.2008.04.004
Zhang, Y., Wang, H. K., Fournier, J., Crous, P. W., Jeewon, R., Pointing, S. B., et al. (2009). Towards a phylogenetic clarification of Lophiostoma / Massarina and morphologically similar genera in the Pleosporales. Fungal Divers. 38, 225–251. doi: 10.1002/yea.1704
Zhou, Y. P., Dou, Z. P., He, W., Zhang, X. D., and Zhang, Y. (2016). Botryosphaeria sinensia sp. nov., a new species from China. Phytotaxa 245, 43–50. doi: 10.11646/phytotaxa.245.1.4
Keywords: Botryosphaeriales, phylogeny, sexual stage, taxonomy, type specimens
Citation: Zhang Y, Zhou Y, Sun W, Zhao L, Pavlic-Zupanc D, Crous PW, Slippers B and Dai Y (2021) Toward a Natural Classification of Botryosphaeriaceae: A Study of the Type Specimens of Botryosphaeria sensu lato. Front. Microbiol. 12:737541. doi: 10.3389/fmicb.2021.737541
Received: 07 July 2021; Accepted: 13 September 2021;
Published: 03 November 2021.
Edited by:
Jun-Jun Liu, Canadian Forest Service, Natural Resources Canada, CanadaReviewed by:
Artur Alves, University of Aveiro, PortugalCopyright © 2021 Zhang, Zhou, Sun, Zhao, Pavlic-Zupanc, Crous, Slippers and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yucheng Dai, eXVjaGVuZ2RAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.