
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 09 August 2021
Sec. Microbiotechnology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.727670
This article is part of the Research Topic Bioactive Compounds with Potential Medicinal Properties Derived from Fungi: Recent and Future Developments in Microbial Biotechnology View all 15 articles
 Yan Ge1,2,3†
Yan Ge1,2,3† Wen-Li Tang2†
Wen-Li Tang2† Qing-Rong Huang1,4
Qing-Rong Huang1,4 Mao-Lian Wei2
Mao-Lian Wei2 You-Zhi Li2
You-Zhi Li2 Lin-Lin Jiang1,2,3
Lin-Lin Jiang1,2,3 Cheng-Lin Li5
Cheng-Lin Li5 Xin Yu1,2,4
Xin Yu1,2,4 Hong-Wei Zhu1,2,3
Hong-Wei Zhu1,2,3 Guo-Zhong Chen1,3,4
Guo-Zhong Chen1,3,4 Jian-Long Zhang1,2,3*
Jian-Long Zhang1,2,3* Xing-Xiao Zhang1,3,4*
Xing-Xiao Zhang1,3,4*Marine-derived fungi are a treasure house for the discovery of structurally novel secondary metabolites with potential pharmaceutical value. In this study, a pair of new nor-bisabolane derivative enantiomers (±)−1 and two new phthalides (4 and 5), as well as four known metabolites, were isolated from the culture filtrate of the marine algal-derived endophytic fungus Penicillium chrysogenum LD-201810. Their structures were established by detailed interpretation of spectroscopic data (1D/2D NMR and ESI-MS). The optical resolution of compound (±)−1 by chiral HPLC successfully afforded individual enantiomers (+)−1 and (−)−1, and their absolute configurations were determined by TDDFT-ECD calculations. Compound (±)−1 represents the first example of bisabolane analogs with a methylsulfinyl substituent group, which is rare in natural products. All of the isolated compounds 1–7 were evaluated for their cytotoxic activity against A549, BT-549, HeLa, HepG2, MCF-7, and THP-1 cell lines, as well as for antifungal activity against four plant pathogenetic fungi (Alternaria solani, Botrytis cinerea, Fusarium oxysporum, and Valsa mali). Compound 2, a bisabolane-type sesquiterpenoid, was shown to possess excellent activity for control of B. cinerea with half-maximal inhibitory concentration (IC50) of 13.6 μg/mL, whereas the remaining investigated compounds showed either weak or no cytotoxic/antifungal activity in this study.
Marine-derived fungi that inhabit the marine environment possess the unique metabolic pathways to produce a great diversity of bioactive secondary metabolites, which play an important role in agrochemical and pharmaceutical industries (Xu et al., 2020; Zhang et al., 2020). It is well-known that a large number of new marine natural products have been discovered and reported every year (Carroll et al., 2021). Mining natural products with novel structures and remarkable bioactivities from marine-derived fungi is still a research hotspot.
Filamentous fungi belonging to the genus Penicillum are important and untapped producers of structurally diverse metabolites (Bai et al., 2019). Penicillum from marine environment have gained particular attention, not only due to their unusual chemical skeletons but also their significant bioactivities with pharmaceutical potential (Zhang et al., 2020). In our continuing study on bioactive metabolites of marine-derived fungi, we investigated Penicillium chrysogenum LD-201810, a marine alga-associated fungus isolated from the marine red alga Grateloupia turuturu (Jiang et al., 2020). Previous solid cultivation of this fungus on rice medium led to the isolation of a new pentaketide derivative, two new hydroxyphenylacetic acid derivatives, as well as the known bisabolane-type sesquiterpenoids and meroterpenoids (Jiang et al., 2020). Motivated by OSMAC (one strain-many compounds) strategy (Zhao et al., 2020), the fungal strain was cultivated on liquid PDB medium. A follow-up examination of this cultivation yielded a pair of new nor-bisabolane derivative enantiomers (±)−1, two previously reported bisabolenes (2 and 3), and two new phthalides (4 and 5) (Figure 1). The aromatic bisabolenes are a rarely found family of sesquiterpenes. Mulholland et al. firstly reported a new trisnor-bisabolane sesquiterpene boivinianin A (Mulholland et al., 2006). Then Li et al. (2015) reported the second occurrence of a new nor-bisabolane derivative, 1-hydroxyboivinianin A. Herein we reported the first example of nor-bisabolane analogs with a methylsulfinyl substituent group, which is rare in natural products. Moreover, the structure elucidation of the new phthalides (4 and 5), as well as the cytotoxicity and antifungal activity of the isolated compounds, are also described.
The UV and optical rotations data were obtained on a Shimadzu UV-2700 spectrometer (Shimadzu Co., Ltd., Kyoto, Japan) and Jasco P-1020 automatic polarimeter (JASCO, Tokyo, Japan), respectively. 1H (500 MHz), 13C (125 MHz), and 2D NMR spectra were measured on an Agilent DD2 spectrometer (Agilent Technologies, Waldbronn, Germany). The mass spectra (ESI-MS) were measured under the positive and negative ion modes by a Waters Xevo G2-XS QTof mass spectrometer (Waters, Milford, MA, United States). Column chromatography was performed on silica gel (100–200 and 200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China), Lobar LiChroprep RP-18 (40–60 μm, Merck, Darmstadt, Germany), and on Sephadex LH-20 (Merck). Preparative TLC plates precoated with silica gel GF254 were purchased from Qingdao Marine Chemical Industry Company.
The producing fungal strain P. chrysogenum LD-201810 was isolated from Grateloupia turuturu, a marine red alga which collected in Qingdao coastal zone. The gene sequencing in the ITS region of the rDNA (GenBank no. MT075873) was applied to identify the fungus (Jiang et al., 2020). To identify the phylogenetic location of this fungus, phylogenetic trees were constructed based on the ITS region sequences using maximum likelihood (ML) method, and the bootstrap support was calculated using 1,000 replicates. The fungus has been deposited in School of Life Sciences, Ludong University, Yantai, China.
The selected fungal strain P. chrysogenum LD-201810 was cultured on potato dextrose agar (PDA) medium (Solarbio Life Sciences, Beijing) at 28°C. After 5 days, the agar blocks were cut into small pieces (0.5 × 0.5 cm), and then inoculated to 100 erlenmeyer flasks containing the liquid potato dextrose broth (PDB) medium (Solarbio Life Sciences) under static conditions at room temperature for 30 days. The culture filtrate was collected, combined, and extracted with equivoluminal EtOAc (30 L) for three times. The organic phase was subsequently dried under reduced pressure to afford 12.6 g of crude extract. Then the detailed separation process was as follows: (i) The crude extract was subjected to open silica gel column chromatography (CC) (100−200 mesh), eluted with a mixed petroleum ether (PE)−EtOAc gradient system (30:1, 10:1, 5:1, 2:1, 1:1, and 0:1) to yield six fractions (Fr. 1 to Fr. 6); (ii) Fr. 4 (2.5 g, eluted with PE−EtOAc 2:1, v/v) was re-fractionated by reversed-phase CC over Lobar LiChroprep RP-18 with a MeOH−H2O gradient system (from 10% MeOH−H2O to 100% MeOH, v/v) to give nine subfractions (Fr. 4.1 to Fr. 4.9); (iii) Fr. 4.3 (0.5 g, eluted with 30% MeOH−H2O, v/v) was separated by preparative thin layer chromatography [prep.-TLC, 20 × 20 cm; developing solvents: dichloromethane (DCM)−methanol (MeOH), 20:1, v/v] to furnish the new compounds 4 (4.5 mg) and 5 (8.0 mg); (iv) Fr. 4.4 (0.8 g, eluted with 40% MeOH−H2O, v/v) was also separated by prep.-TLC (developing solvents: DCM−MeOH, 20:1, v/v) to obtain compound 6 (12.0 mg); (v) Fr. 4.6 (0.6 g, eluted with 60% MeOH−H2O, v/v) was repeatedly subjected to Sephadex LH-20 (MeOH) to give compound 7 (20.5 mg); (vi) Fr. 5 (1.9 g, eluted with PE−EtOAc 1:1, v/v) was subjected to Sephadex LH-20 (MeOH) to yield five subfractions (Fr. 5.1 to Fr. 5.5); (vii) Fr. 5.2 (0.8 g) was applied to silica gel CC (200–300 mesh; DCM−MeOH 20:1, v/v) to yield compounds 2 (9.6 mg) and 3 (31.8 mg); (viii) Fr. 5.3 (0.4 g) was purified by prep.-TLC (developing solvents: DCM−MeOH, 10:1, v/v) to give the racemic compound 1 (7.0 mg). Compound 1 was well resolved into the pure enantiomers (+)−1 (3.0 mg, tR = 16.3 min) and (−)−1 (2.8 mg, tR = 18.3 min) by HPLC using a (R,R) Whelk-O1 chiral column (10 μm; 4.6 × 250 mm; n-hexane-ethanol eluent 7:3, v/v; 1.0 mL/min).
Methylsulfinyl-1-hydroxyboivinianin A (±1): white amorphous powder; UV (MeOH) λmax (log ε) 200 (2.65), 282 (1.52) nm; 1H and 13C NMR data were assigned and listed in Table 1; (−)-HR-ESI-MS m/z 267.0680 [M − H]– (calcd for C13H15O4S, 267.0691).
(+)−1: [α]25D + 23.8° (c 0.05, MeOH); ECD (0.125 mg/mL, MeOH) λmax (Δε) 220 (+ 1.54) nm.
(−)−1: [α]25D −22.2° (c 0.04, MeOH); ECD (0.125 mg/mL, MeOH) λmax (Δε) 218 (−1.52) nm.
Chrysoalide A (4): white amorphous powder; [α]25D + 31.5° (c 0.03, MeOH); UV (MeOH) λmax (log ε) 216 (2.58), 239 (2.03), 330 (1.96) nm; 1H and 13C NMR data were assigned and listed in Table 1; (−)-HRESIMS m/z 209.0431 [M − H]– (calcd for C10H9O5, 209.0450).
Chrysoalide B (5): white amorphous powder; [α]25D + 15.8° (c 0.03, MeOH); UV (MeOH) λmax (log ε) 215 (2.13), 237 (1.63), 329 (1.50) nm; 1H and 13C NMR data were assigned and listed in Table 1; (−)-HRESIMS m/z 223.0584 [M − H]– (calcd for C11H11O5, 223.0606).
The conformational search was performed by the molecular mechanics with MM + method in HyperChem 8.0 software. Next, the geometries were optimized at B3LYP/6-31G(d) level with Gaussian 09 software to afford the energy-minimized conformers (Frisch et al., 2013). The optimized conformers were subjected to TD-DFT ECD calculations at PBE0/TZVP, CAM-B3LYP/TZVP, and BH&HLYP/TZVP level. The solvent effects (MeCN) were evaluated at the same DFT level with the SCRF/PCM method.
Cytotoxicity of compounds 1−7 toward A549, BT-549, HeLa, HepG2, MCF-7, and THP-1 cell lines was tested by the Cell Counting Kit-8 (CCK-8) method (Yuan et al., 2020). All of the cell lines were purchased from the Chinese Academy of Sciences Committee on Type Culture Collection Cell Bank (Shanghai, China). The six cell lines (3.0 × 104 cells per well) were initially inoculated into 96-well plates for 24 h. Subsequently, the cells were exposed to various concentrations of tested compounds (0, 5, 10, 20, 40, 80, and 100 μg/mL). With the treatment of 24, 48, and 72 h, 10 μL of 5 g/L CCK-8 solution (CCK-8 Cell Proliferation and Cytotoxicity Assay Kit, #CA1210, Solarbio, Beijing, China) was applied to each well and the cells were cultured for 1.5 h at 37°C. Absorbance data were obtained with a microplate spectrophotometer reader (Multiskan GO, Thermo Fisher Scientific, Waltham, MA, United States) at 490 nm.
The antifungal activities against four phytopathogenic fungi (Alternaria solani, Botrytis cinerea, Fusarium oxysporum, and Valsa mali) were evaluated in 96-well microtiter plates using a modified broth microdilution method (Shi et al., 2017). Carbendazim was used as a positive control. The tested compounds were added to autoclaved PDA medium to a final concentration of 3.12, 6.25, 12.5, 25, and 50 μg/mL, while 95% ethanol was treated as black control. The blocks (about 5 mm diameter) from four phytopathogenic fungi were cultured in the center of plates at 20°C. Colony diameters were measured with the cross method. The mycelial growth inhibition rate was calculated as follows, while IC50 values were obtained by the logarithm method.
The mycelial growth inhibition rate = (control colony diameter – treatment colony diameter)/(control colony diameter – 5) × 100%
To clarify the evolutionary position of the producing strain LD-201810, we performed phylogenetic analysis based on its ITS sequence, together with those from other Penicillium species. Results indicated that the strain LD-201810 located at the basal position of the whole tree with high confidence (100%, Figure 2). The result demonstrated that Penicillium chrysogenum LD-201810 belongs to the Penicillium genus.
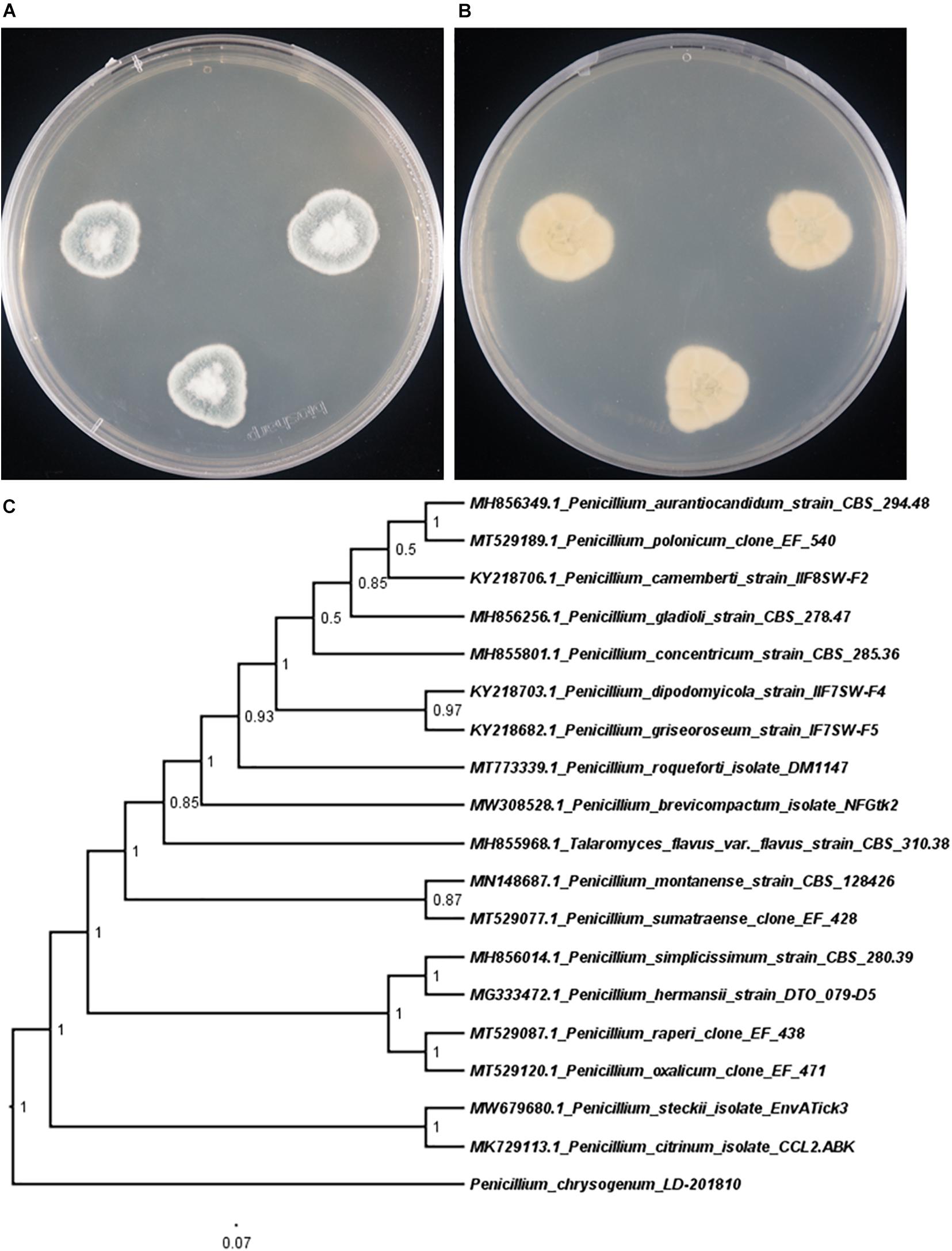
Figure 2. Morphology of P. chrysogenum LD-201810 on PDA medium (A, front view; B reverse view). (C) Neighbor-joining tree based on ITS nucleotide sequences.
Compound (±)−1 was a white amorphous powder (MeOH), and its molecular formula was determined to be C13H16O4S by negative-mode HR-ESI-MS (m/z 267.0680 [M − H]–, calcd 267.0691). The 1H NMR data for 1 (Table 1) clearly revealed signals of two methyl singlets at δH 1.76 (s, H3-11) and 2.58 (s, H3-13), two sets of methylene multiplets at δH 2.67 (m, H-8α), 2.65 (m, H-9α), 2.49 (m, H-8β), and 2.45 (m, H-9β), a pair of methylene doublets at δH 4.07 (d, J = 13.0 Hz, H-12α) and 3.94 (d, J = 13.0 Hz, H-12β), and three aromatic protons at δH 7.33 (d, J = 8.4 Hz, H-3), 6.83 (d, J = 8.4 Hz, H-4), and 6.82 (s, H-6). The 13C NMR data identified 13 carbon signals that were highly resolved, categorized as two methyls, three methylenes, three sp2 methines, and five quaternary carbons including three sp2, one oxygenated sp3, and one carbonyl carbon at δC 179.6 (C-10). Detailed analysis of the 1D and 2D NMR (Figure 3) spectra of 1 indicated that they were similar to those of 1-hydroxyboivinianin A, a trisnor-bisabolane derivative identified from the culture of a deep-sea sediment-derived fungus Penicillium aculeatum SD-321 (Li et al., 2015). By comparison of the NMR data of 1-hydroxyboivinianin A with those of 1, the main differences in 1 were the presence of an additional methylene group at δC 59.7 (C-12) and a distinctive methyl at δC 37.4 (C-13). The downfield chemical shifts of 13-CH3 (δH/C 2.58/37.4) and 12-CH2 (δC 59.7) were ascribed to that bearing a heteroatom between them. Initially, the common-observed heteroatoms, such as oxygen (1a), nitrogen (1b), and sulfur (1c) atoms, were assumed between C-13 and C-12. However, the predicted 13C NMR shifts in ChemBioDraw didn’t match well with that for measured data (Figure 3). Furthermore, combined with positive-mode ESI-MS (m/z 269.0843 [M + H]+ and 537.1672 [2M + H]+) and negative-mode ESI-MS (m/z 267.0644 [M − H]– and 535.1417 [2M − H]–), the molecular weight of 1 was determined as 268. In view of its molecular weight, a remaining S and O atom could be accounted for by inserting the S = O group between C-13 and C-12 to form a methylsulfinyl substituent. The predicted data for 1d were in good agreement with the authentic data. Moreover, Fu et alreported a series of synthetic compounds with a methylsulfinyl group (Fu et al., 2020). The chemical shifts of C-12 and C-13 in 1 were accordant with those of known compounds, which further confirmed the presence of such a rare substituent in natural products. On the basis of the above discussion, the structure of compound 1 was determined as methylsulfinyl-1-hydroxyboivinianin A.
Compound 1 had only one chiral center at C-7. The zero specific rotation value and baseline ECD curve indicated its racemic nature (Meng et al., 2016). Subsequent chiral HPLC analysis of (±)−1 successfully led to the separation of the two individual enantiomers (+)−1 and (−)−1 with a ratio of approximately 1:1, which exhibited opposite optical rotations (Figure 4A). To determine the absolute configurations of (+)−1 and (−)−1, their ECD spectra were measured in MeOH and simulated by the time-dependent density function theory (TD-DFT) method. The experimental ECD spectrum of (+)−1 showed a positive (+220 nm) Cotton effect, whereas the experimental (−)−1 showed an almost mirror image ECD curve (Figure 4B). The calculated ECD curves of 7R and 7S matched the experimental ECD curves of (+)−1 and (−)−1, thus the absolute configurations of (+)−1 and (−)−1 were proposed as 7R and 7S, respectively.
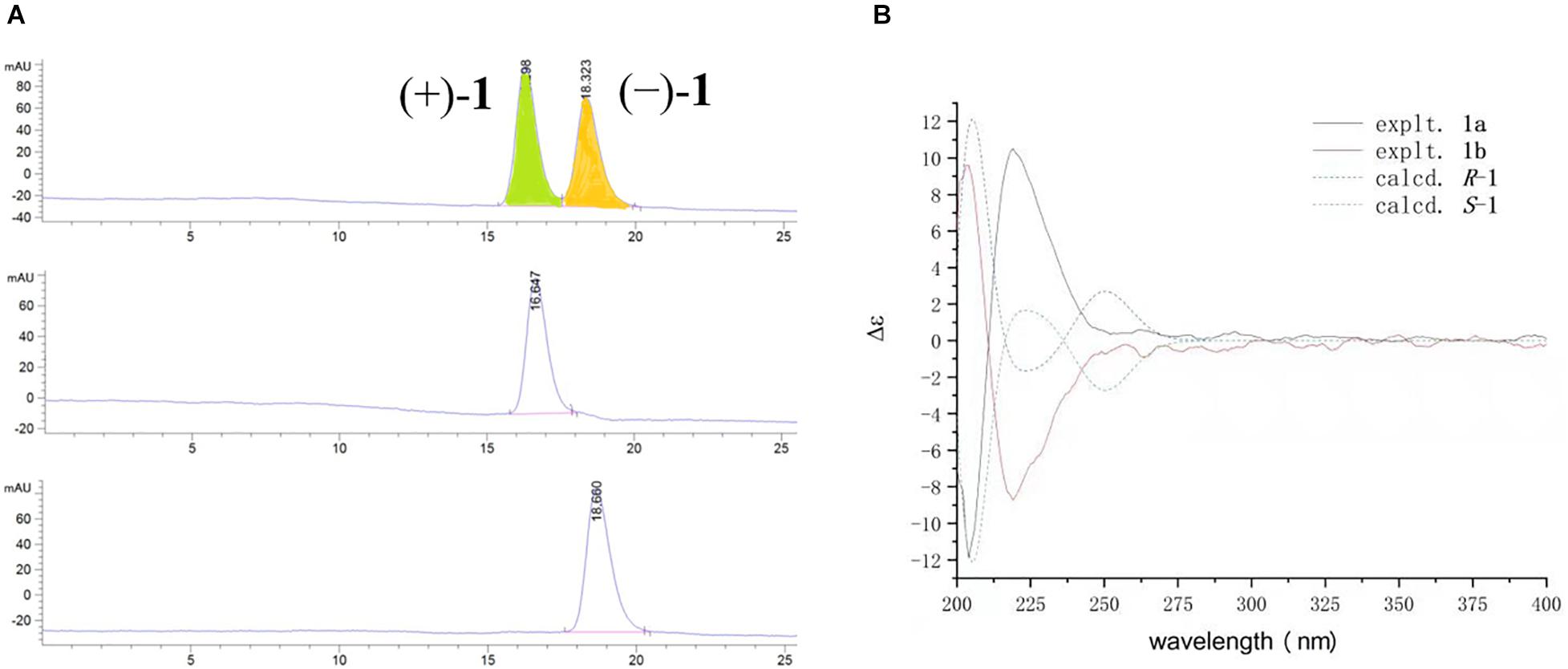
Figure 4. (A) Chromatogram of the chiral HPLC analysis of 1. (B) Experimental and calculated ECD spectra of (±)−1 in MeOH.
Chrysoalide A (4), a white amorphous powder, was shown to possess a molecular formula of C10H10O5 by its HR-ESI-MS (m/z 209.0431 [M − H]–, calcd 209.0450). Its UV spectrum showed absorption peaks at 216, 239, and 330 nm, indicating the presence of a conjugated carbonyl chromophore (Phainuphong et al., 2018; Saetang et al., 2021). The 1H NMR data for 4 (Table 1) showed signals of one methyl singlet at δH 1.73 (s, H3-8), one methoxy singlet at δH 2.93 (s, H3-9), and a pair of intercoupling aromatic protons at δH 7.01 (d, J = 8.7 Hz, H-5) and 6.84 (d, J = 8.7 Hz, H-6), which can be easily deduced the presence of a 1,2,3,4-tetrasubstituted benzene group. The 13C NMR spectrum (Table 1) displayed signals for two methyls at δC 24.2 (C-8) and 51.0 (C-9), two sp2 methines at δC 124.3 (C-5) and 119.7 (C-6), five quaternary carbons, and one ester carbonyl at δC 166.2 (C-1). Detailed analysis of 2D NMR data established the planar structure of 4 (Figure 5A). Moreover, the experimental ECD spectrum of 4 displayed a similar shape of curves and Cotton effects to those of the calculated ECD spectrum of the R-configuration (Figure 5B), which established the absolute configuration of C-3 to be R.
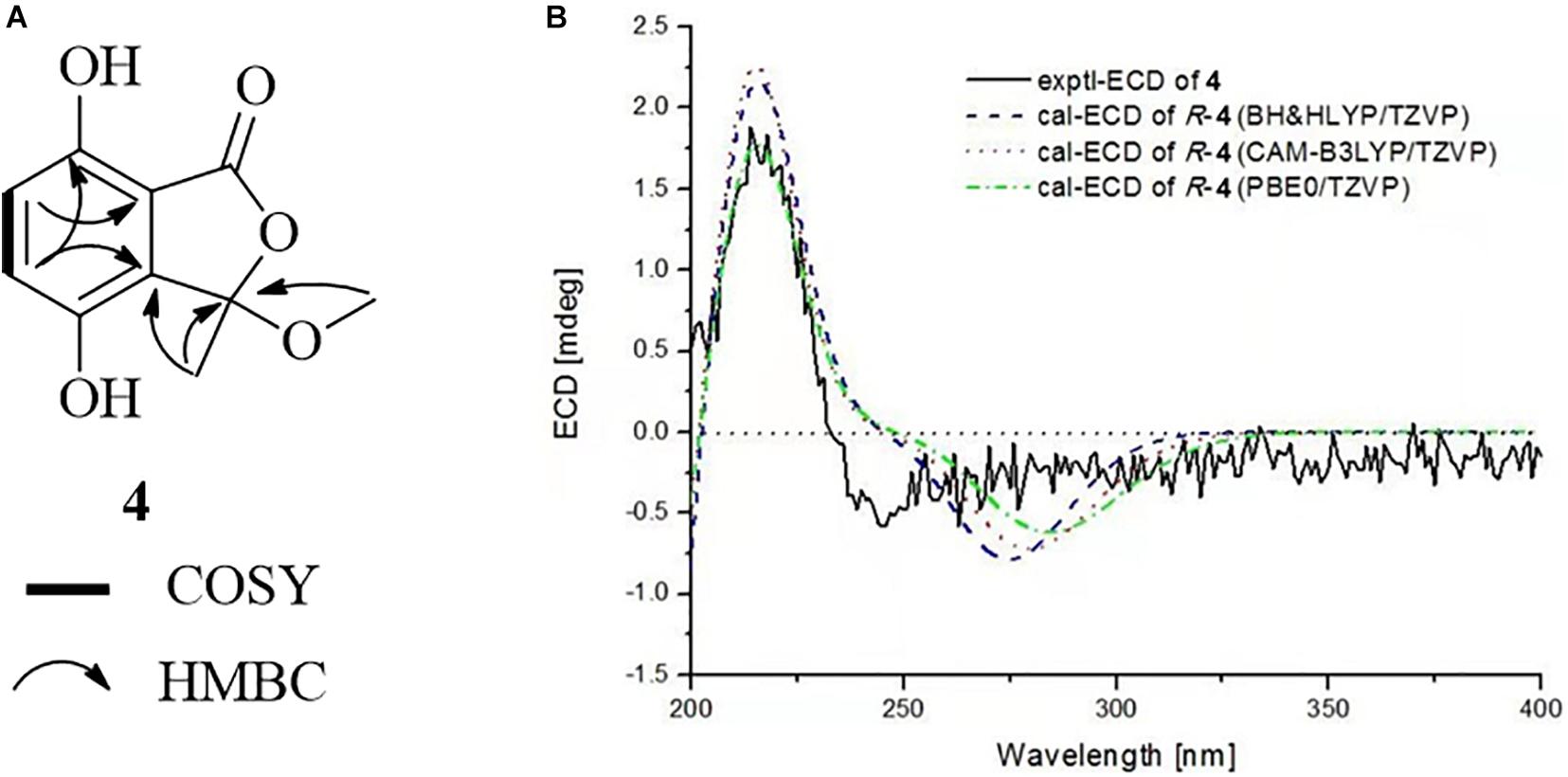
Figure 5. (A) COSY and HMBC correlations of 4. (B) Experimental and calculated ECD spectra of 4 in MeOH.
Chrysoalide B (5) was also obtained as a white amorphous powder with a molecular formula of C11H12O5 as determined by HR-ESI-MS. The molecular weight of 5 was more than that of 4 by 14 units (CH2). The 1D and 2D NMR spectra of 5 (Table 1 and Figure 6A) suggested that it resembled 4 structurally, but possessed an additional methoxy group at δH/C 3.80/56.3. The extra methoxy group was shown to be linked to C-7, as evidenced from the HMBC correlation from 10-CH3 to C-7. Compound 5 was elucidated as a methoxy derivative of 4. The absolute configuration of 5 was considered to be identical with that of 4 by its similar ECD curve, which gave a positive Cotton effect at 220 nm. Comparison of the experimental ECD data with those of calculated spectra further proved the above assignment (Figure 6B).
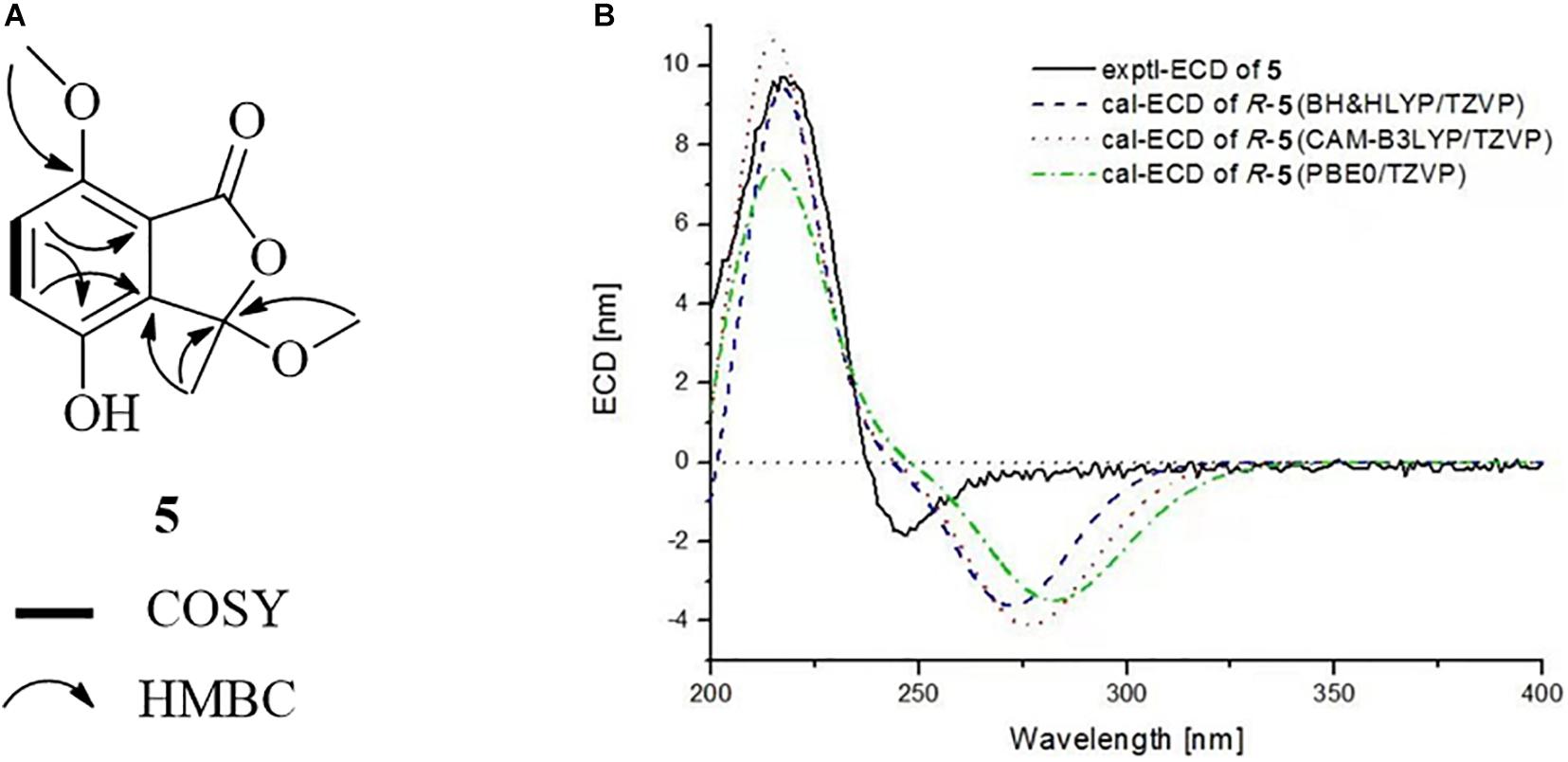
Figure 6. (A) COSY and HMBC correlations of 5. (B) Experimental and calculated ECD spectra of 5 in MeOH.
In addition to the new compounds, two previously reported bisabolane sesquiterpenes 2 and 3, a phthalide derivative 6, and a chromone 7, were also isolated from this fungal strain. Based on detailed spectroscopic analysis as well as by comparisons with literature data, their structures were identified as hydroxysydonic acid (2) (Hamasaki et al., 1978), sydowic acid (3) (Hamasaki et al., 1975), rubralide C (6) (Kimura et al., 2007), and 2,5-dimethyl-7-hydroxychromone (7) (Kashiwada et al., 1984).
All of the isolated compounds were evaluated for their cytotoxicity against six different types of cancer cell lines, A549 (a human lung adenocarcinoma epithelial cell line), BT-549 (a human breast cancer cell line), HeLa (a human cervix carcinoma cell line), HepG2 (a human liver carcinoma cell line), MCF-7 (a human breast adenocarcinoma cell line), and THP-1 (a human monocytic cell line). However, none of them exhibited obvious inhibitory activity at 20 μg/mL (the highest concentration tested, data were shown in Supplementary Table 1).
Previous studies indicated that bisabolane sesquiterpenoids and phthalides possessed promising antimicrobial activity (Li et al., 2015; Saetang et al., 2021). Marine natural products considered to be new sources of lead molecules with agrochemical significance (Oppong-Danquah et al., 2020). To discover new marine fungal agrochemicals, the isolated compounds were evaluated for antifungal activity against several plant pathogenetic fungi (A. solani, B. cinerea, F. oxysporum, and V. mali). The bisabolane-type sesquiterpenoid 2 was shown to possess excellent activity for control of B. cinerea with an IC50 value of 13.6 μg/mL (Figure 7) (compared with the positive control carbendazim, with an IC50 value of 19.2 μg/mL), whereas other compounds showed either weak or no activity (Data were shown in Supplementary Table 2). It should be pointed out that limited amounts of these metabolites were obtained, which prevented us to perform more biological experiments. Further study should be particularly focused on more agricultural activities, such as antifeedant and phytotoxic activities, to fully evaluate their agricultural potentials.
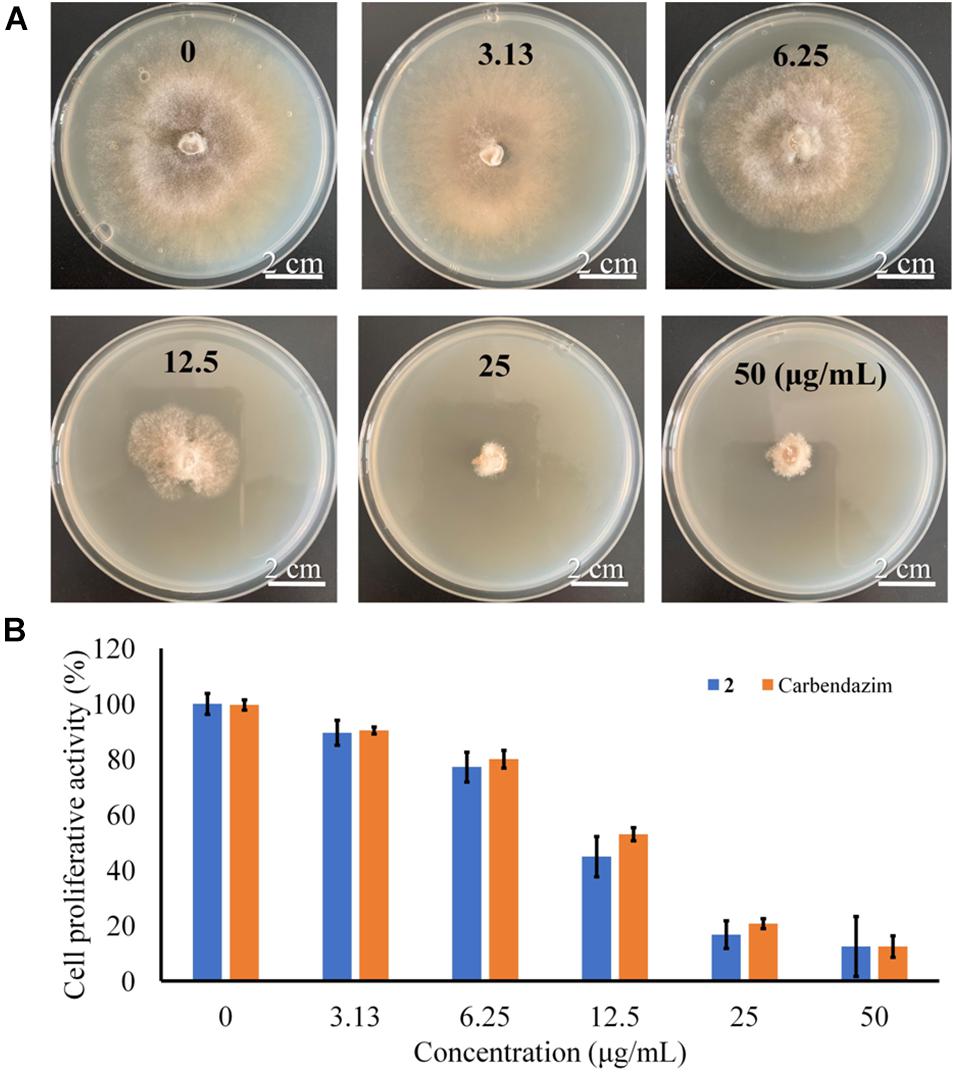
Figure 7. (A) Antifungal assay against B. cinerea with different concentrations. (B) Inhibition of proliferative activity against B. cinerea induced by 2.
Marine-derived fungi have been proven to be prolific producers of secondary metabolites with potent bioactivities. In this study, chemical investigation of a marine algal-derived endophytic fungus P. chrysogenum LD-201810 led to the isolation and identification of a pair of new nor-bisabolane derivative enantiomers (±)−1 and two new phthalides (4 and 5), as well as four known metabolites (2, 3, 6, and 7). Compound (±)−1 represents the first example of bisabolane analogs with a methylsulfinyl substituent group, which is rare in natural products. The aromatic bisabolanes are a rarely found family of sesquiterpenes, and the discovery of (±)−1 added greatly to the diversity of this kind of molecules. The cytotoxic and antifungal activities were evaluated. Hydroxysydonic acid (2), a bisabolane-type sesquiterpenoid, showed strong inhibition against B. cinerea, compared with that of the positive control carbendazim. The results indicated that some marine natural products may be regarded as candidate agents of antifungal agrochemicals.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
YG and W-LT: writing-original draft preparation. Q-RH: methodology. M-LW and Y-ZL: investigation. L-LJ and C-LL: formal analysis. XY, H-WZ, and G-ZC: data curation. J-LZ and X-XZ: writing-review and editing. J-LZ: supervision. X-XZ: funding acquisition. All authors have read and agreed to the published version of the manuscript.
This work was funded by the National Key Research and Development Program of China (Grant Nos. 2016YFD0501010, 2017YFD0500806, and 2018YFD0501402), the Major Agricultural Applied Technological Innovation Projects of Shandong Province (to Xingxiao Zhang), the Key Research and Development Plan of Yantai (Nos. 2020XDRH101, 2021YT06000060, 2021YT06000636, and 2018XSCC045), the Natural Science Foundation of Shandong Province (Nos. ZR201911120018 and ZR2014HL061), Development of Medical and Health Science and Technology in Shandong Province (No. 2016WS0224), and the Innovation Team Project for Modern Agricultural Industrious Technology System of Shandong Province (SDAIT-11-10).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.727670/full#supplementary-material
Bai, M., Zheng, C. J., Huang, G. L., Mei, R. Q., Wang, B., Luo, Y. P., et al. (2019). Bioactive meroterpenoids and isocoumarins from the mangrove-derived fungus Penicillium sp. TGM112. J. Nat. Prod. 82, 1155–1164.
Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R. (2021). Marine natural products. Nat. Prod. Rep. 38, 362–413.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., and Cheeseman, J. R. (2013). Gaussian 09, Revision D.01. Wallingford: Gaussian, Inc.
Fu, D., Dong, J., Du, H., and Xu, J. (2020). Methanesulfinylation of benzyl halides with dimethyl sulfoxide. J. Org. Chem. 85, 2752–2758. doi: 10.1021/acs.joc.9b03041
Hamasaki, T., Nagayama, K., and Hatsuda, Y. (1978). Two new metabolites, sydonic acid and hydroxysydonic acid from Aspergillus sydowi. Agric. Biol. Chem. 42, 37–40. doi: 10.1271/bbb1961.42.37
Hamasaki, T., Sato, Y., Hatsuda, Y., Tanabe, M., and Cary, L. W. (1975). Sydowic acid, a new metabolite from Aspergillus sydowi. Tetrahedron Lett. 16, 659–660. doi: 10.1016/s0040-4039(00)71947-2
Jiang, L. L., Tang, J. X., Bo, Y. H., Li, Y. Z., Feng, T., Zhu, H. M., et al. (2020). Cytotoxic secondary metabolites isolated from the marine alga-associated fungus Penicillium chrysogenum LD-201810. Mar. Drugs 18:276. doi: 10.3390/md18050276
Kashiwada, Y., Nonaka, G. I., and Nishioka, I. (1984). Studies on rhubarb (Rhei Rhizoma). V. isolation and characterization of chromone and chromanone derivatives. Chem. Pharm. Bull. 32, 3493–3500. doi: 10.1248/cpb.32.3493
Kimura, Y., Yoshinari, T., Koshino, H., Fujioka, S., Okada, K., and Shimada, A. (2007). Rubralactone, rubralides A, B and C, and rubramin produced by Penicillium rubrum. Biosci. Biotechnol. Biochem. 71, 1896–1901. doi: 10.1271/bbb.70112
Li, X. D., Li, X. M., Xu, G. M., Zhang, P., and Wang, B. G. (2015). Antimicrobial phenolic bisabolanes and related derivatives from Penicillium aculeatum SD-321, a deep sea sediment-derived fungus. J. Nat. Prod. 78, 844–849. doi: 10.1021/acs.jnatprod.5b00004
Meng, L. H., Mándi, A., Li, X. M., Liu, Y., Kurtán, T., and Wang, B. G. (2016). Isolation, stereochemical study, and antioxidant activity of benzofuranone derivatives from a mangrove-derived fungus Eurotium rubrum MA-150. Chirality 28, 581–584. doi: 10.1002/chir.22613
Mulholland, D. A., McFarland, K., and Randrianarivelojosia, M. (2006). Sesquiterpenoid derivatives from Cipadessa boiviniana (Meliaceae). Biochem. Syst. Ecol. 34, 365–369. doi: 10.1016/j.bse.2005.11.005
Oppong-Danquah, E., Budnicka, P., Blümel, M., and Tasdemir, D. (2020). Design of fungal co-cultivation based on comparative metabolomics and bioactivity for discovery of marine fungal agrochemicals. Mar. Drugs. 18:73. doi: 10.3390/md18020073
Phainuphong, P., Rukachaisirikul, V., Phongpaichit, S., Sakayaroj, J., Kanjanasirirat, P., Borwornpinyo, S., et al. (2018). Depsides and depsidones from the soil-derived fungus Aspergillus unguis PSU-RSPG204. Tetrahedron 74, 5691–5699. doi: 10.1016/j.tet.2018.07.059
Saetang, P., Rukachaisirikul, V., Phongpaichit, S., Preedanon, S., Sakayaroj, J., Hadsadee, S., et al. (2021). Antibacterial and antifungal polyketides from the fungus Aspergillus unguis PSU-MF16. J. Nat. Prod. 84, 1498–1506. doi: 10.1021/acs.jnatprod.0c01308
Shi, D., An, R., Zhang, W., Zhang, G., and Yu, Z. (2017). Stilbene derivatives from Photorhabdus temperata SN259 and their antifungal activities against phytopathogenic fungi. J. Agric. Food Chem. 65, 60–65.
Xu, K., Yuan, X. L., Li, C., and Li, X. D. (2020). Recent discovery of heterocyclic alkaloids from marine-derived Aspergillus species. Mar. Drugs 18:54. doi: 10.3390/md18010054
Yuan, X. L., Li, X. Q., Xu, K., Hou, X. D., Zhang, Z. F., Xue, L., et al. (2020). Transcriptome profiling and cytological assessments for identifying regulatory pathways associated with diorcinol N-induced autophagy in A3 cells. Front. Pharmacol. 11:570450. doi: 10.3389/fphar.2020.570450
Zhang, P., Wei, Q., Yuan, X. L., and Xu, K. (2020). Newly reported alkaloids produced by marine-derived Penicillium species (covering 2014–2018). Bioorg. Chem. 99:103840. doi: 10.1016/j.bioorg.2020.103840
Keywords: marine fungus, Penicillium chrysogenum, secondary metabolites, bisabolane derivatives, phthalides, antifungal activity
Citation: Ge Y, Tang W-L, Huang Q-R, Wei M-L, Li Y-Z, Jiang L-L, Li C-L, Yu X, Zhu H-W, Chen G-Z, Zhang J-L and Zhang X-X (2021) New Enantiomers of a Nor-Bisabolane Derivative and Two New Phthalides Produced by the Marine-Derived Fungus Penicillium chrysogenum LD-201810. Front. Microbiol. 12:727670. doi: 10.3389/fmicb.2021.727670
Received: 19 June 2021; Accepted: 21 July 2021;
Published: 09 August 2021.
Edited by:
Paola Angelini, University of Perugia, ItalyReviewed by:
Hemraj Chhipa, Agriculture University, Kota, IndiaCopyright © 2021 Ge, Tang, Huang, Wei, Li, Jiang, Li, Yu, Zhu, Chen, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Long Zhang, emhhbmdqaWFubG9uZ0BsZHUuZWR1LmNu; Xing-Xiao Zhang, emhhbmd4aW5neGlhb0BsZHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.