- 1Janssen Research & Development, Janssen Pharmaceutical Companies, Beerse, Belgium
- 2Early Discovery Biology, Charles River Laboratories, Beerse, Belgium
- 3Janssen Research & Development, Janssen Pharmaceutical Companies, Springhouse, PA, United States
Despite the availability of a prophylactic vaccine, chronic hepatitis B (CHB) caused by the hepatitis B virus (HBV) is a major health problem affecting an estimated 292 million people globally. Current therapeutic goals are to achieve functional cure characterized by HBsAg seroclearance and the absence of HBV-DNA after treatment cessation. However, at present, functional cure is thought to be complicated due to the presence of covalently closed circular DNA (cccDNA) and integrated HBV-DNA. Even if the episomal cccDNA is silenced or eliminated, it remains unclear how important the high level of HBsAg that is expressed from integrated HBV DNA is for the pathology. To identify therapies that could bring about high rates of functional cure, in-depth knowledge of the virus’ biology is imperative to pinpoint mechanisms for novel therapeutic targets. The viral proteins and the episomal cccDNA are considered integral for the control and maintenance of the HBV life cycle and through direct interaction with the host proteome they help create the most optimal environment for the virus whilst avoiding immune detection. New HBV-host protein interactions are continuously being identified. Unfortunately, a compendium of the most recent information is lacking and an interactome is unavailable. This article provides a comprehensive review of the virus-host relationship from viral entry to release, as well as an interactome of cccDNA, HBc, and HBx.
Introduction
Hepatitis B virus (HBV) is a member of the Hepadnaviridae family which is transmitted via bodily fluids as well as by vertical transmission (Davis et al., 1989; Schweitzer et al., 2015). The outcome of HBV infection is determined by multiple host and viral factors, and determines whether the infection will be acute, chronic, or occult (Fanning et al., 2019). Despite the availability of a prophylactic vaccine and potent antiviral treatments, chronic hepatitis B (CHB) infection affects 292 million individuals worldwide (Lazarus et al., 2018). The current standard of care is treatment with nucleos(t)ide analogs (NUCs) (i.e., lamivudine, adefovir, entecavir, telbivudine, and tenofovir), that inhibit the HBV polymerase reverse transcription (Liang et al., 2015). These therapies lead to suppression of viral replication, visible by a decrease in viral load, the normalization of serum alanine transaminase and improvement of liver histology (Bitton Alaluf and Shlomai, 2016). However, even prolonged treatment with NUCs rarely results (<10%) in functional cure of CHB and most often leads to virological relapse after treatment cessation (Liang et al., 2015; Kim, 2018).
Also pegylated interferon alpha (peg-IFNα) is approved for use in CHB patients although it is not the preferred therapy due to the occurrence of side effects. Furthermore, it is counter indicated for some patients such as those with liver cirrhosis (Saracco et al., 1994).
Untreated or off-treatment chronic patients are at risk to develop life threatening conditions such as fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma (HCC). In 2015, 887,000 people died from HBV-related cirrhosis and liver cancer alone (WHO, 2017). The ultimate therapeutic goal in CHB is preventing these life-limiting outcomes and to achieve a functional cure characterized by the loss of surface antigen (HBsAg) and HBV-DNA in the blood off-treatment.
Hepatitis B virus functional cure will be achieved when the high viral load, the antigen burden and inadequate host immune responses are overcome and thus may need a broader therapeutic approach involving multiple targets, both viral and host. With regard to the latter, in-depth knowledge of the HBV life cycle is indispensable for identifying mechanisms, that are targetable with new therapeutics.
Part of the therapeutic approach may be to target the interface between viral proteins and cellular targets. The HBV viral proteins have pluripotent functions and our understanding of how they interact with host proteins is continuously evolving. The interactions of these viral factors with the host cell proteome are complex and helps to shape the cellular environment for the virus to replicate. In addition, cccDNA, the template of all viral mRNAs, behaves as a minichromosome and attracts a multitude of protein partners. However, all these reported interactions are scattered in literature, and currently there is no overview bringing together the interactome of HBV. This review aims to provide such an overview, from entry to viral release, it summarizes the known interactions between viral proteins and host proteins. Because cccDNA, HBc, and HBx have been described in many interactions, we focused the construction of an interactome network around these three entities.
Interactions During the Early Phases of HBV Infection
The HBV particle consists of an incomplete 3.2 kb double-stranded (ds)DNA genome [relaxed circular DNA (RC-DNA)] packaged together with the viral polymerase in an icosahedral capsid assembled by HBV core (HBc) proteins (Summers et al., 1975). This nucleocapsid is enveloped by a lipid membrane studded with three forms of HBV surface antigen protein (collectively referred to as HBsAg) to compose the virus or Dane particle [reviewed by Bruss (2004)].
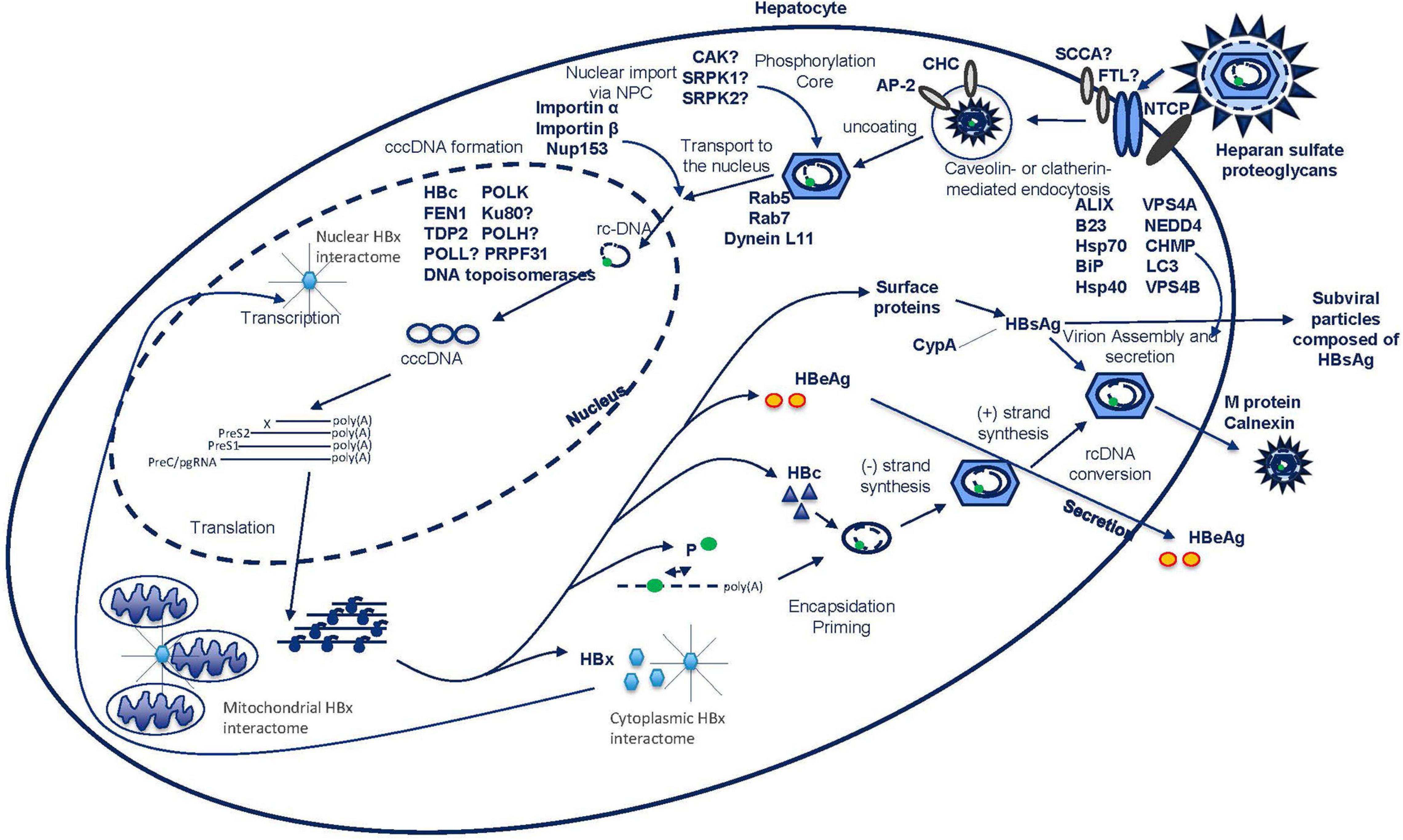
The life cycle of HBV begins upon its interaction with heparan sulfate proteoglycans (HSPGs) and subsequent binding to the sodium taurocholate co-transporting polypeptide (NTCP) receptor on the surface of the hepatocyte (Watashi et al., 2014; Yan et al., 2014; Figure 1). The interaction between virus and cell induces conformational changes of the membrane embedded myristoylated N-terminal preS1-domain of the viral large surface protein (L-HBsAg) leading to exposure of the receptor binding site for the NTCP receptor, which enables binding of the virus and entrance into the cell (Schulze et al., 2007, 2010; Yan et al., 2012, 2013, 2014; Nkongolo et al., 2014; Watashi et al., 2014). Recently, a crucial role in mediating HBV-NTCP internalization of epidermal growth factor receptor (EGFR) was published (Iwamoto et al., 2019). Besides the NTCP receptor, squamous cell carcinoma antigen 1 (SCCA1) and ferritin light chain (FTL) have also been identified as HBV co-receptors (Figure 1). Triple complexes of preS1, FTL, and SCCA1 were observed and overexpression assays with these proteins showed increased infection rates both in vitro and in vivo (Hao et al., 2012). The prevention of entry has been of interest as an antiviral target to circumvent viral spread by blocking de novo infection. In recent years molecules such as Myrcludex B (also known as bulevirtide), ezetimibe, cyclosporin derivates (CsA), and monoclonal antibodies against HBsAg epitopes were identified to interfere with this process (Gripon et al., 2005; Lucifora et al., 2013; Shimura et al., 2017).
The virus enters the cell by inducing endocytosis via caveolin-mediated endocytosis or via clathrin-mediated pathways (Macovei et al., 2010; Umetsu et al., 2018; Figure 1). In differentiated HepaRG cells, HBV infection has shown to be dependent on caveolin-mediated endocytosis. However, in Umetsu et al. (2018), the formation of a complex between the L-HBsAg, the clathrin heavy chain (CHC) and the clathrin adaptor protein-2 (AP-2) was described, suggesting an alternative endocytosis pathway (Figure 1). Indeed, inhibition of the clathrin-mediated pathway by silibinin and chlorpromazine has been reported to impair HBV uptake (Huang et al., 2012). Further work will be needed to understand the relative importance of these two pathways. After endocytosis, subsequent movement of the virus through the endocytic pathway is regulated by Rab proteins. These are guanosine triphosphatases (GTPases) that occupy specific endocytic compartments and direct endocytic vesicles to different cellular compartments. Silencing of Rab5 or Rab7, in contrast with Rab9 and Rab11, resulted in the inhibition of the early stages of HBV infection implying that the transport of virus to late endosomes is important for a successful infection (Macovei et al., 2013; Figure 1).
The precise location and timing of nucleocapsid release from the envelope remains unclear, but this process is required prior to nuclear entry. Transport of the nucleocapsid to the nucleus is facilitated by the microtubule network and the dynein L11 motor proteins through a direct interaction with the capsid (Osseman et al., 2018; Figure 1). In the nucleocapsid “uncoating” process, phosphorylation of the C-terminus of HBc destabilizes the capsid and allows the binding of importins α and β (Kann et al., 1999; Barrasa et al., 2001; Nguyen et al., 2008). Although a direct interaction has not been established, a number of kinases including core associated kinase (CAK), SR protein-specific kinase 1 (SRPK1) and SR protein-specific kinase 2 (SRPK2), have been reported to be involved in this phosphorylation process (Kau and Ting, 1998; Daub et al., 2002; Figure 1).
Once the nucleocapsid arrives at the nuclear pore complex (NPC), it can pass the complex as an intact particle (Pante and Kann, 2002; Fay and Pante, 2015). Interestingly, HBV seems to utilize a unique way of triaging immature from mature capsids at the level of the NPC as only mature capsids disassemble. In this process, importin β and Nup153 play a role via direct interaction with the capsid (Schmitz et al., 2010; Figure 1). Once through the NPC, the capsid is deposited in the nuclear basket where only mature capsids can pass. In the nucleus, the final uncoating, where capsid structures and viral DNA separate, takes place in an importin α and β-dependent manner (Gallucci and Kann, 2017).
The cccDNA Minichromosome
Once inside the nucleus, the RC-DNA is converted into cccDNA (Summers et al., 1975; Tuttleman et al., 1986; Wu et al., 1990; Lieberman, 2016). Early research using duck hepatitis B virus (DHBV) showed that the cccDNA was in fact organized as a minichromosome similar to host chromatin and SV40 (Newbold et al., 1995). Further DHBV studies showed that in vitro between 1 and 56 copies cccDNA reside in the nuclei of infected cells (Kock et al., 2010). These copy numbers were slightly lower (1–17 copies/cell) in in vivo studies in ducks. Further it was determined that the half-life of DHBV cccDNA is between 35 and 57 days (Addison et al., 2002; Zhang et al., 2003) although shorter half-lives have described (Tuttleman et al., 1986; Wu et al., 1990; Newbold et al., 1995). In vitro kinetic studies were also done using HBV, cccDNA formation is an early life cycle event (Tuttleman et al., 1986) and it was shown that the cccDNA pool grows over the course of 3 days after which a stable pool is reached (5–12 copies/cell) with a half-life of about 40 days (Ko et al., 2014). Similar findings were done using woodchuck HBV (Dandri et al., 2000). Patient samples of HBV infected individuals showed that cccDNA copy numbers were much lower in vivo ranging from 0.01 to 9 copies/cell but at the same time had a much longer half-life of months to a year (Werle-Lapostolle et al., 2004; Bourne et al., 2007; Boyd et al., 2016; Huang et al., 2021). Interestingly, the size and half-life of the cccDNA pool in patients has been suggested to depend on the antigen status (Lythgoe et al., 2021) as much more cccDNA has been shown in HBeAg positive patients while only 0.002 copies/cell were observed in patients that showed HBsAg seroclearance (Werle-Lapostolle et al., 2004).
The cccDNA genome is transcribed to different viral RNAs coding for HBx (0.7-kb RNA), three forms of HBsAg (2.4-kb RNA encoding the large and 2.1-kb RNA encoding the middle and small HBsAg), pre-core protein or HBeAg (3.5-kb RNA) and the core and polymerase protein (pre-genomic RNA or pgRNA, 3.5-kb). This pgRNA also becomes incorporated in the nucleocapsid thereby providing the template for the viral polymerase to produce RC-DNA.
Host Factors Involved in cccDNA Formation
Little is known about the host factors involved in the formation of the cccDNA. The L-HBsAg is not directly involved in cccDNA formation, but is part of a negative feedback mechanism in which high levels of surface protein shut down nuclear shuttling of mature nucleocapsids and direct the cell to produce virions instead (Summers et al., 1990). HBc is suggested to be present during the cccDNA formation process (Kock et al., 2010; Schreiner and Nassal, 2017) which is further evidenced by the fact that capsid modifiers inhibit cccDNA formation (Berke et al., 2017).
Several host factors have been reported to interact with HBV cccDNA during its formation and have quite diverse roles. The Flap endonuclease 1 (FEN1), an endonuclease that plays a role in DNA replication and repair, was shown to interact with RC-DNA in the nucleus and additionally could promote cccDNA formation in vitro (Kitamura et al., 2018; Figure 1). The discovery of a protein partner involved in DNA damage repair is coherent with the previous finding that this machinery is exploited by viruses to their own benefit (Schreiner and Nassal, 2017). Ku80, a component of non-homologous end joining DNA repair pathway, was essential for synthesis of cccDNA from dsDNA, but not from RC-DNA (Guo et al., 2012; Figure 1). In these processes, HBx could be an adaptor to link cccDNA formation with DNA damage response pathways, under the assumption that HBx is already present in the cell when cccDNA is being formed (Hodgson et al., 2012; Guo et al., 2014; Murphy et al., 2016; Niu C. et al., 2017). The link with the host DNA damage and repair machinery does not end with this interaction, the tyrosyl-DNA-phosphodiesterase (TDP2) also plays a partial role in cccDNA formation by releasing the viral transcriptase from the RC-DNA (Koniger et al., 2014; Cui et al., 2015; Figure 1). The host DNA polymerases K (POLK), H (POLH), and L (POLL) have all been reported to have a positive impact on cccDNA formation, however, the exact mechanism(s) is (are) not yet clear (Qi et al., 2016; Figure 1). In addition to DNA polymerases, knockout experiments showed the importance of cellular DNA ligase 1 and 2 in cccDNA formation (Long et al., 2017). Recently, it was shown that the plus-strand and the minus-strand require different cellular proteins. The plus-strand repair required proliferating cell nuclear antigen (PCNA), replication factor C (RFC) complex, DNA polymerase delta (POLδ), flap endonuclease 1 (FEN1), and DNA ligase 1 (LIG1) while the repair of the minus-strand only required FEN1 and LIG1 (Wei and Ploss, 2020). Also cellular DNA topoisomerases are required for cccDNA formation and amplification (Sheraz et al., 2019). Finally, pre-mRNA processing factor 31 (PRPF31) was identified as a cccDNA-associating factor involved in cccDNA formation (Kinoshita et al., 2017; Figure 1).
The Interactome of the cccDNA
Similar to a cellular chromosome, the cccDNA is bound to histones to form a minichromosome. These host-derived histones (H2A, H2B, H3, and H4) provide, together with the viral HBc, the stable scaffold for the cccDNA to be supercoiled (Newbold et al., 1995; Chong et al., 2017). That being said, the role of HBc in both cccDNA formation and maintenance is still under investigation. For example, despite their involvement in several processes regarding cccDNA formation, maintenance and transcription, capsid modifying compounds do not eliminate the cccDNA pool (Berke et al., 2017) nor is HBc essential for transcription (Zhang et al., 2014).
On the cccDNA of Duck hepatitis B virus (DHBV), nucleosomes are non-randomly positioned, suggesting that, like host cellular chromatin, positioning of the nucleosomes and histone modifications of the cccDNA may regulate cccDNA transcription (Bock et al., 1994; Pollicino et al., 2006). Methylation, acetylation, phosphorylation or other posttranslational modifications (PTMs) of these cccDNA-bound histone tails can fine tune the gene expression by altering the chromatin structure (Tropberger et al., 2015). This change in structure can wind the chromatin more tightly to prevent access of transcription factors and repress gene transcription. On the other hand, histone modifications can also result in increased DNA accessibility, transcription factor binding and therefore promoting gene activation (Li et al., 2007; Voss and Hager, 2014). In addition, the minichromosome attracts several other partners, many of which are transcription factors that further determine whether the cccDNA is transcriptionally active or inactive (Table 1).
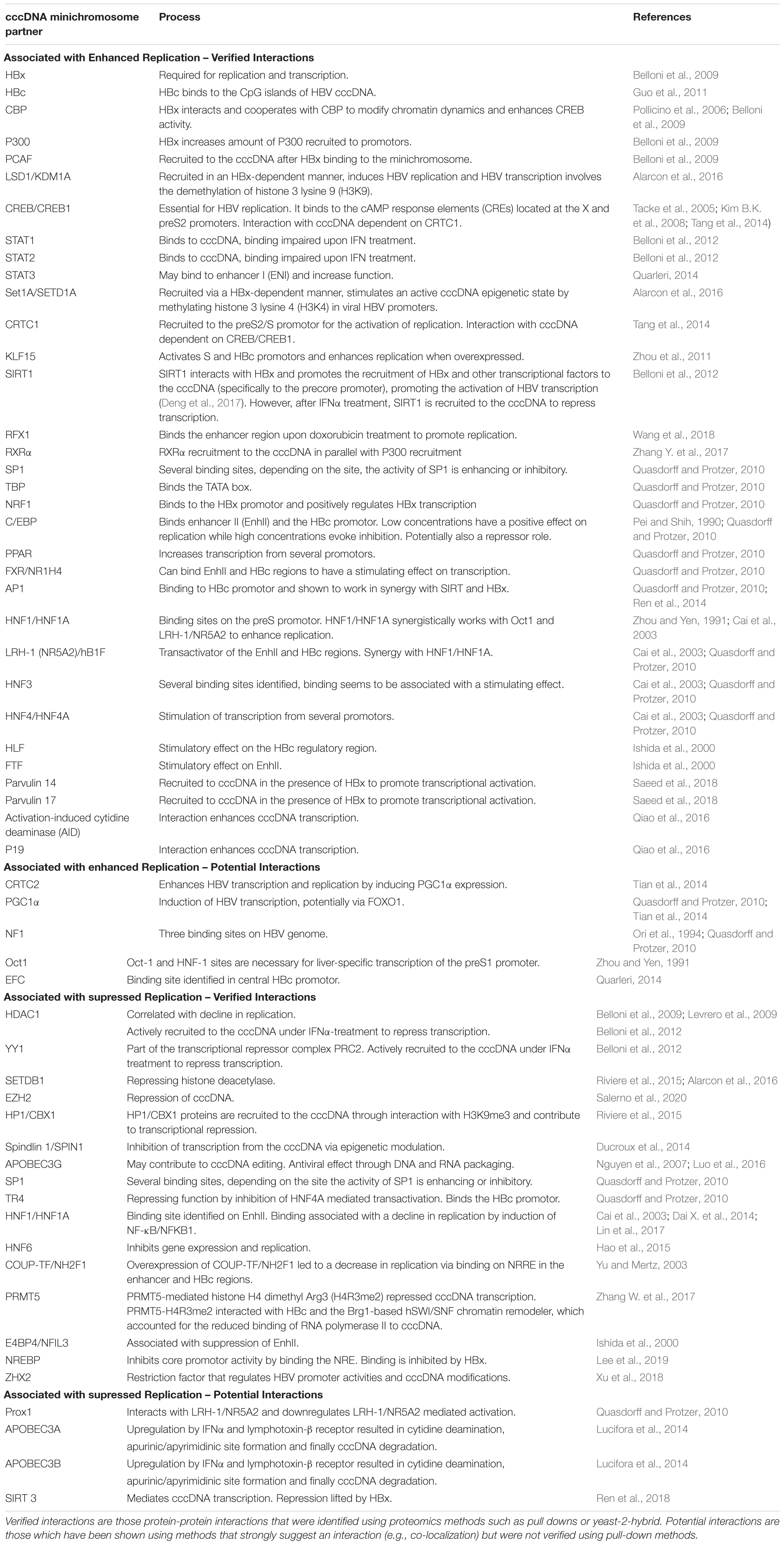
Table 1. List of known protein-cccDNA interactions associated with increased or decreased transcriptional regulation.
As previously mentioned, HBx and HBc proteins are bound to cccDNA. HBc has been described to modulate transcription from the cccDNA. Zlotnick et al. showed that the presence of HBc on a CpG island in the cccDNA can be linked to increased cccDNA activity, while methylation of the CpG island correlated with decreased cccDNA activity (Zlotnick et al., 2015). In addition, the presence of HBc appears to have a role in the maintenance of the structure of the cccDNA (Bock et al., 2001). Together these data suggest that HBc contributes to the epigenetic regulation of the cccDNA, which in turn contributes to its longevity.
Modalities Acting on cccDNA
A role in viral rebound made cccDNA a target for new antiviral drug development. Success of such tactics relies on complete inhibition of cccDNA throughout the lifespan of the hepatocyte. A first approach is to target the formation of cccDNA, although it can be questioned how much benefit CHB patients will have of such a therapy in the event the cccDNA does not become reduced. Several molecules reported to act through this mechanism have been described in literature. However, to date, these molecules have either been stopped at pre-clinical stage or did not progress far in clinical trials (Cai et al., 2012; Liu et al., 2016). The only assets which encompass this capacity and are still under clinical investigation are the entry inhibitor bulevirtide and capsid assembly modulators. The latter are small molecules that accelerate capsid formation but turned out to have a dual mode of action in preventing cccDNA formation when added in vitro at early stages of infection (Berke et al., 2017; Vandenbossche et al., 2019). Secondly, a number of molecules have been described that silence the cccDNA, either by inhibiting cccDNA transcription [e.g., Tamibarotene (Nkongolo et al., 2019)] or by diminishing HBV RNA levels post-transcription (e.g., RNA destabilizers such as RG7834 (Mueller et al., 2019); RNA interference). Tamibarotene never made it to clinical trials for HBV, while RG7834 was stopped in Phase I. Transcriptional control of cccDNA expression may also be achieved by interfering with the function of HBx, HBc or an interaction partner. An example is the interference between HBx and DNA damage-binding protein 1 (DDB1). HBx was found to hijack DDB1 which in turn recruits the ubiquitylation machinery to send Structural Maintenance of Chromosomes protein 5/6(SMC5/6), a transcriptional repressor of cccDNA, to the proteasome for degradation. Two molecules, pevonedistat, a NEDD8-activating enzyme inhibitor, and nitazoxanide, a thiazolide anti-infective agent, have been shown to restore SMC5/6 levels and suppress viral transcription (Decorsiere et al., 2016; Sekiba et al., 2019a,b). Recently, epigenetic modifiers that specifically target viral factors involved in the regulation of cccDNA expression have been described and are currently being evaluated. Several selective inhibitors (e.g., C646) for histone acetyltransferase like CBP and P300 have been used to study the inhibitory effect on HBV transcription (Tropberger et al., 2015). The prodrug GS-5801 has also been shown to inhibit transcription from cccDNA by blocking the activity of lysine demethylase 5 (KDM5) (Gilmore et al., 2017). Although these observations show that silencing of HBV transcription is possible, the main throwback of most of these targets is the lack of desired selectivity for cccDNA and their potential to impact cellular processes.
Complete elimination of cccDNA by compromising the stability or the half-life of the molecule is often dubbed the “Holy Grail” of HBV research. Many molecules have been described that phenotypically reduce the quantity or transcription level of cccDNA. Recently, a small molecule, ccc_R08, with an unknown mode of action was shown to decrease the pool of cccDNA together with a decrease in viral transcripts and viral antigens in primary human hepatocytes (PHH) and in an HBV minicircle mouse model (Wang et al., 2019). In most instances, information on the exact mechanism of such molecules is lacking implying a need to conduct target deconvolution studies to identify the respective interaction partner or process. We created a cccDNA network map, not only to visualize the currently known cccDNA interacting proteins but also to be put alongside such exercises (Figure 2).
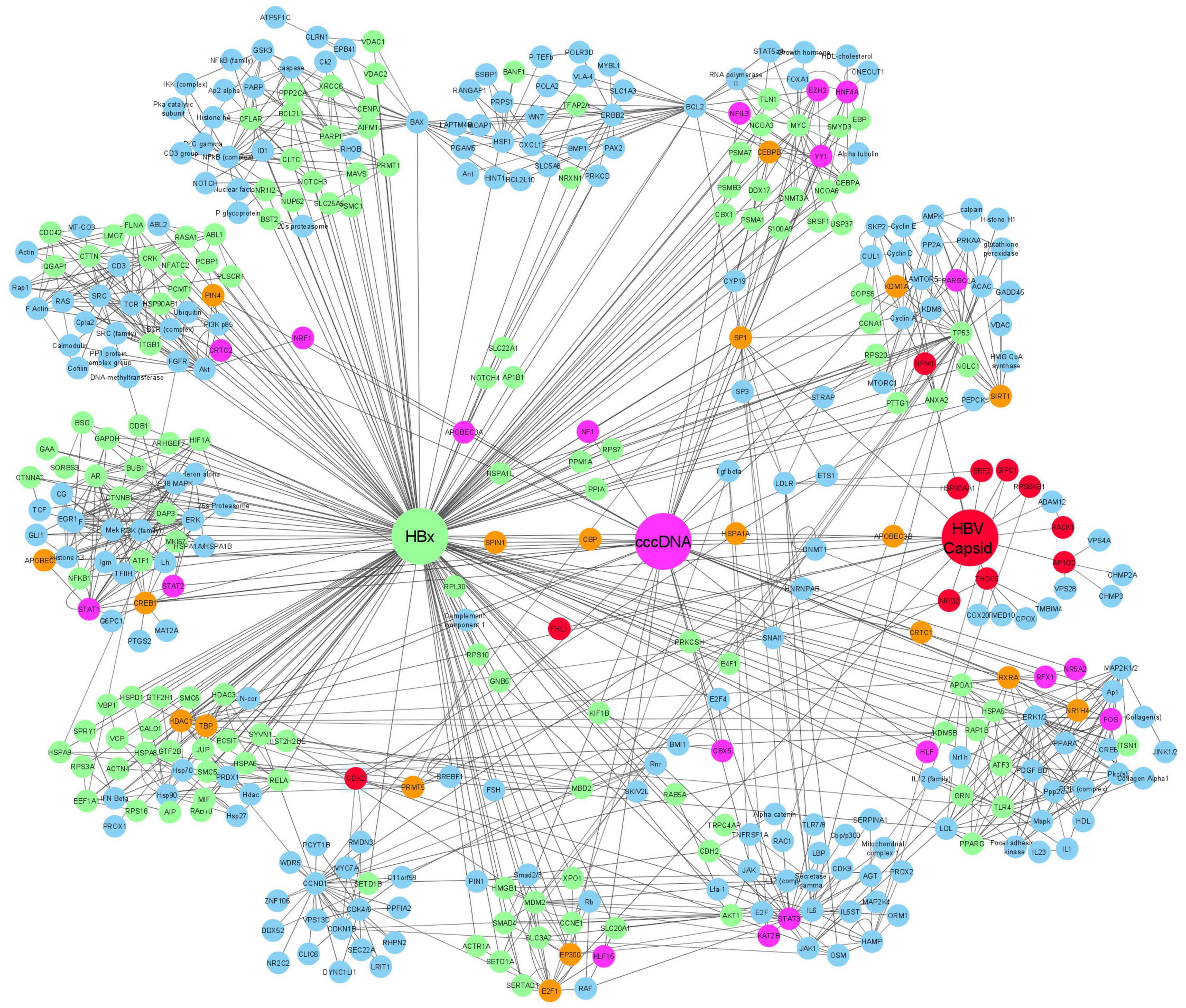
Figure 2. Gene association network showing the relationship between HBx, HBc, and HBV cccDNA interacting proteins. In the network, proteins which only interact with HBx are indicated in green, proteins which only interact with cccDNA are shown in pink, and proteins that only interact with HBc are shown in red. Proteins that were shown to interact with more than two of the founding nodes (cccDNA, HBc, and HBx) are depicted in orange. proteins that were extrapolated to connect to one or more interacting proteins are shown in blue.
The HBx Interactome
The interactome of HBx extends beyond its interaction with the cccDNA and associated proteins. Besides nuclear interaction partners, HBx also interacts with various proteins in the cytoplasm, the endoplasmic reticulum (ER) and the mitochondria (Henkler et al., 2001; Huh and Siddiqui, 2002; Belloni et al., 2009; Li et al., 2017; Figure 1). This may explain why this small viral protein (17-kDa) is not only involved in HBV replication, but is also shown to contribute to the development of HCC and interfere with cell cycle regulation, glucose metabolism, oxidative stress, calcium signaling, apoptosis and DNA repair (Luber et al., 1993; Waris et al., 2001; Bouchard et al., 2006; Benhenda et al., 2009; Table 2). The pivotal nature of HBx is demonstrated by Table 2 in which more than 250 HBx interaction partners are summarized. However, it does need to be mentioned that some of these interactions may be very weak or very brief and their relevance may be limited.
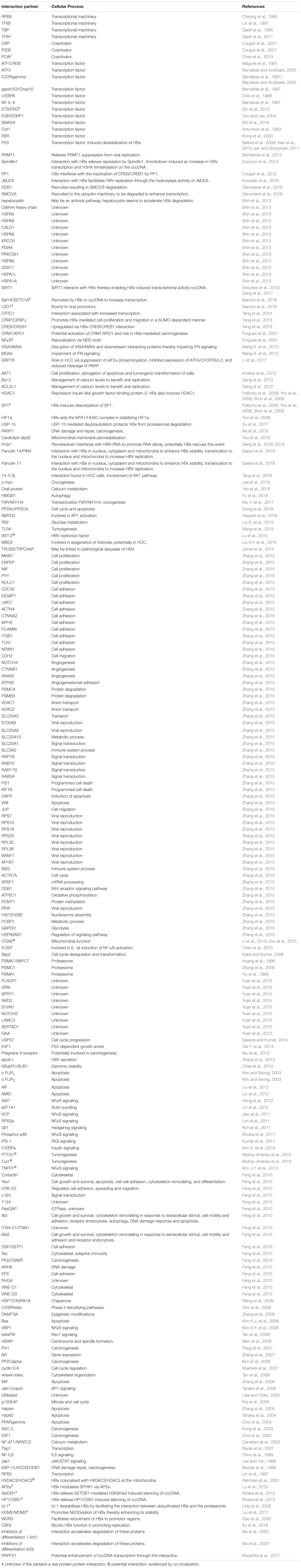
Table 2. HBx interacting proteins listed together with the cellular processes or pathways in which they are involved.
Besides the transcriptional modulation of cccDNA, HBx has also been described to modulate gene expression of multiple proteins involved in signaling pathways such as the AKT serine/threonine kinase 1 (AKT1), Ras-Raf-mitogen-activated protein (MAP) kinase, MAPK8/pSMAD3L, (TβRI)/pSMAD3C, nuclear factor-kappa B (NF-kB) pathways and potential restriction factors such as STIM1, zinc finger E-box binding homeobox 2 (ZEB2), and proteasome activator subunit 4 (PSME4) (Benn et al., 1996; Klein and Schneider, 1997; Waris et al., 2001; Yoo et al., 2008; Zhang et al., 2012; Liu et al., 2014; Lu et al., 2015; Rawat and Bouchard, 2015; Wu et al., 2016; Yu et al., 2016; Cheng et al., 2018; Zheng et al., 2019; Minor et al., 2020; Table 2). Interestingly, HBx expression itself is also influenced by cellular proteins, for example, NRF1 has shown to bind the HBx promotor to activate it in contrast to ATF2, which showed the opposite effect (Choi et al., 1997; Tokusumi et al., 2004; Quasdorff and Protzer, 2010).
The HBc Interactome
HBc is mostly known as the building block of the HBV capsid (Summers et al., 1975) but in recent years it has been shown that its function is not limited to this and also plays a role in cccDNA stability, transcription and epigenetic regulation (Newbold et al., 1995; Bock et al., 2001; Zlotnick et al., 2015; Chong et al., 2017), evasion of antiviral mechanisms (Lucifora et al., 2014), reverse transcription (Tan et al., 2015), cellular trafficking (Schmitz et al., 2010; Yang et al., 2014), genomic replication (Lott et al., 2000), and viral egress (Bardens et al., 2011). The field is also discovering more and more that HBc expression is extensively regulated by core promotor regulation, core mRNA modulation and post-translational modifications which highlights its importance in the life cycle (Buckwold et al., 1997; Sohn et al., 2006; Kohno et al., 2014; Qian et al., 2015; He et al., 2016; Bartusch et al., 2017; Lubyova et al., 2017; Heger-Stevic et al., 2018; Makokha et al., 2019).
Initially, the impact on the capsid made HBc an appealing drug-target (Berke et al., 2017). However, given that there is also an interplay with cccDNA and HBx these molecules may have more far-reaching consequences. As more protein interactions between HBc and the host are elucidated, we also compiled the interactome of the core protein and linked it to the HBx and cccDNA interactomes (Figure 2).
A cccDNA and HBx Gene Association Network: Expanding the Potential cccDNA and HBx Interactome
Tables 1–3 summarize what is currently known in the literature (manual curation) about cccDNA, HBx protein, and HBc protein-DNA and protein-protein interactions, respectively. However, to utilize this information to predict and potentially identify new protein interactions, network pathway analysis was performed (Ingenuity Pathway Analysis, IPA, Qiagen). IPA enables gene network generation from the Ingenuity Knowledge Base, a data repository of biological interactions and functional annotations.
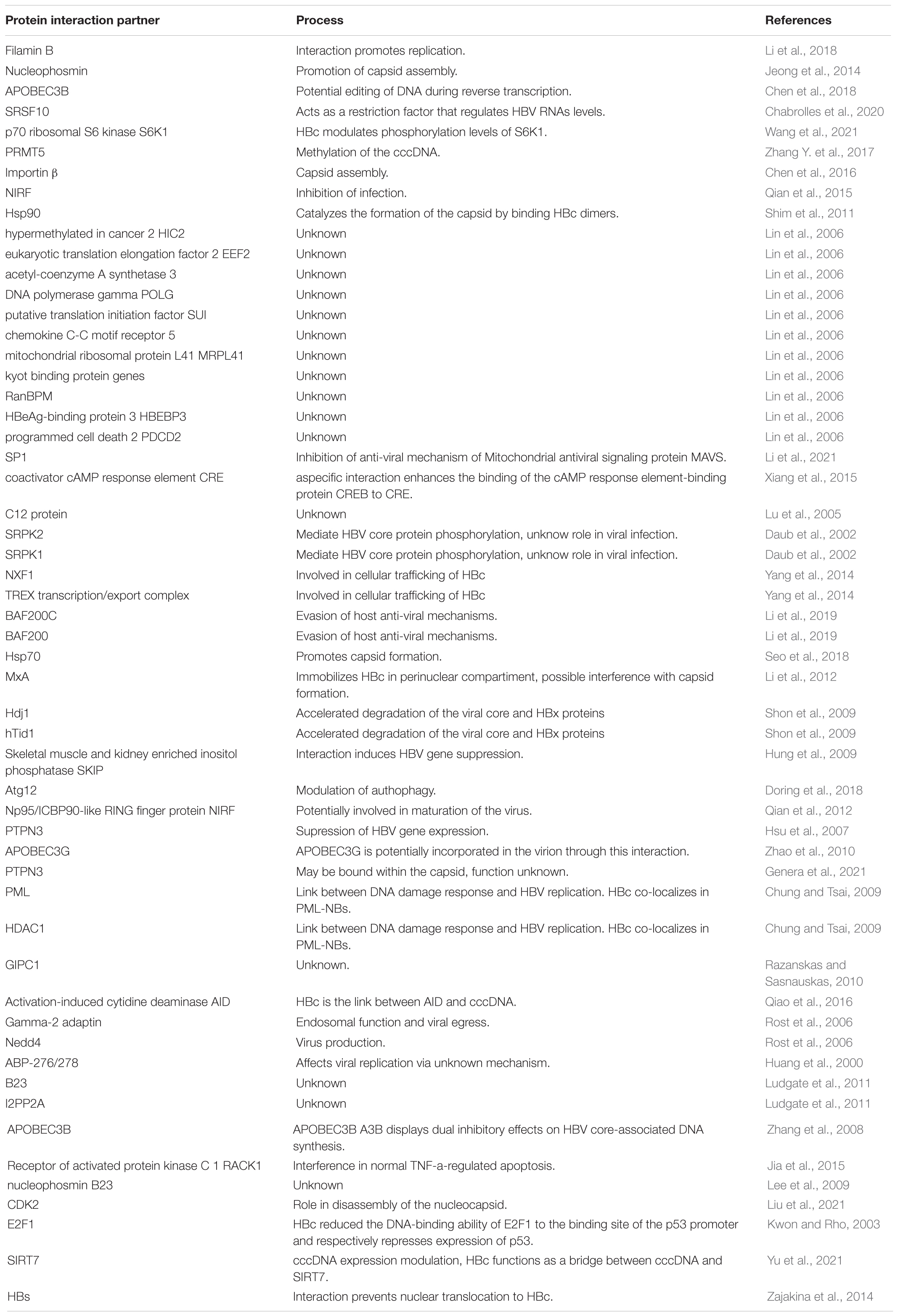
Table 3. HBc interacting proteins listed together with the cellular processes or pathways in which they are involved.
To generate gene association networks, the HBx, HBc, and HBV cccDNA interacting proteins were individually analyzed to create three separate network schemes. The database was filtered and core analysis performed to only query the following: (1) Species = Human, (2) Molecules per networks = 35, Networks per analysis = 10, (3) Node Types = All, (4) Data Source = All, (5) Confidence = Experimentally Observed, (6) Species = Human, (7) Tissues and Cell Types = Liver, Hepatocytes, Hepatoma Cell Lines not otherwise specified, HuH7 cell line, Hep3B cell line, HepG2 cell line and “Other” Hepatoma cell lines, and (8) Mutation = All.
If proteins selected as network “seeds” were not apparently connected or networks had less than 35 gene products, IPA added proteins from the IPA Knowledge data base to maximize the connectivity of the “seed” molecules within the filter limits. We also filtered out those proteins that were only interacting with either cccDNA, HBx, or HBc and had no extrapolated nodes. This kept the networks to a manageable size and reduced redundancy while deriving as much as possible biological context from the analysis. When adding molecules from the knowledge database, IPA uses a connectivity metric (edge-weighted spring layout) that prioritizes molecules that have the greatest overlap with the existing network. This means that the organization of the network in clusters is not based on proteins sharing similar pathways but is based on the number of described interactions in between those proteins. Upon completion of the IPA network generating algorithm, two networks were produced showing both “direct” and “indirect” relationships for either HBx, HBc, or HBV cccDNA interacting proteins. These were then merged and exported to Cytoscape 3.7.1 using an edge weighted spring layout to create the final network illustration showing the relationship between HBx, HBc, and HBV cccDNA interactingproteins (Figure 2).
In the network, proteins that interact with HBc are depicted in red, those interacting with cccDNA in pink and those interacting with HBx in green. We also highlighted those proteins that were described to interact with 2 or more of our founding (HBc, HBx, and cccDNA) nodes (Figure 2). Nineteen proteins are identified (P300, TBP, PIN4, CBP, SPIN1, CEBPB, SP1, CRTC1, RXRA, NR1H4, KDM1A, HSPA1A, APOBEC3G, APOBEC 3B, CREB1, PRMT5, HDAC1, E2F1, and SIRT1) as interacting proteins of HBx, HBc, and cccDNA. These are interesting because these components may be a driving force in cccDNA transcription and maintenance. Moreover, these may be interesting proteins for further functional research as they seem to play a connecting role in the viral life cycle (Figure 2). Most of these proteins, 12 out of 19, are regulators of transcription, for example, TBP and CRTC1 are both involved in transcription initiation; CREB1 and E2F1 are enhancers of transcription; P300, CBP, SPIN1, SP1, PRMT5, HDAC1, and SETD1B are all epigenetic modifiers that can influence the chromatin to a specific transcriptionally accessible, active state. Finally, PIN4 was described as a chromatin remodeler. Any of these proteins could be a potentially interesting target to influence transcriptional status of cccDNA. Notable is that all these transcription-related proteins have interactions with HBc, hereby confirming a role for HBc beyond capsid assembly. Also interesting is the occurrence of two APOBEC proteins as partners for HBx, HBc and cccDNA. APOBEC proteins play a role in anti-viral immunity (Stavrou and Ross, 2015) and is a means of the cell to counteract the effect of infection (Lucifora et al., 2014).
Through the IPA approach, the network was expanded from just those proteins with known interactions with HBx, HBc, or cccDNA to an additional 210 proteins (indicated in light blue) which may play a role in protein-protein or protein-cccDNA interactions. While the interactions itself are verified in literature, their involvement in the HBV pathology and viral replication cycle is not confirmed yet. Hence, these proteins provide an interesting starting point for further research. Analysis of the network shows that via these interacting proteins, HBV also taps into host pathways such as cell cycle, cell signaling, DNA repair, transcription regulation and apoptosis. This is not surprising as many of these processes have been described in relation to HBV already. However, this is the first time description of proteins which may be involved in these processes and how they relate to the cccDNA minichromosome, HBc or HBx. An additional interesting observation is that many heat shock proteins (HSP) (Hsp70, Hsp90, Hsp27, HSPD1, HSPA1A, HSPA1B, HSPA8, HSPA9, HSPA1L, HSP90AB1, HSPA5, and HSPA6) were observed as interacting proteins in this network. Literature has already described that viruses rely on host HSPs for viral protein folding and induce overexpression of HSPs in the infected cells (Bolhassani and Agi, 2019). Moreover, several HSPs were associated with some viral particles (Fust et al., 2005; Bolhassani and Agi, 2019). In HBV, downregulation of Hsp70 and Hsp90 by small interfering RNA significantly inhibited HBV production. Furthermore, also a significant reduction of HBV secretion could be observed in HepG2.2.15 cells treated with an Hsp90 inhibitor (Liu et al., 2009; Bolhassani and Agi, 2019). Further research will be required to confirm the additional protein partners identified in this network analysis.
Interactions During the Late Phases of HBV Infection
In HBV infection, besides the budding of virions, there is also the shedding of an excess amount of subviral particles (Figure 1). These particles are non-infectious 22 nm spheres or filaments of variable length consisting solely of the HBsAg envelope protein, which may be expressed from either cccDNA or HBV DNA that is integrated into the human genome (Heermann et al., 1984; Figure 1). Budding of infectious virus and shedding of subviral particles happen via distinct pathways (Selzer and Zlotnick, 2015).
Although redundant in viral assembly, the M-protein and its interaction with calnexin has been shown to be involved in the secretion of subviral particles (Werr and Prange, 1998). In the cytoplasm, HBsAg interacts with cyclophilin A (CypA) and stimulates the extracellular secretion of CypA (Tian et al., 2010; Figure 1). Interestingly, it seems that the presence of CypA reciprocally stimulates HBsAg secretion, as inhibitors against CypA reduce the amount of secreted HBsAg (Phillips et al., 2015).
To construct new virions, the pgRNA is packaged together with the viral polymerase in the nucleocapsid, which is formed in the cytoplasm by assembly of 120 HBc dimers (Lambert et al., 2007). Although not well understood, the interaction between HBc dimers and cellular protein nucleophosmin (B23) was shown to promote this assembly (Jeong et al., 2014; Figure 1). This nucleocapsid is surrounded by a cellular lipid layer embedded with three viral S glycoproteins, which originate from the endoplasmic reticulum (Bruss, 2007). Virion assembly depends solely on the L-protein, whereas the S-protein is required but not sufficient, and the M-protein is redundant (Bruss, 2004). To aid in building this unusual composition, Hsp70, and mammalian BiP were described as interaction partners of the L-protein in vitro and in vivo (Loffler-Mary et al., 1997; Lambert and Prange, 2003; Wang Y.P. et al., 2010; Figure 1). In the assembly of the mature virion, the S-protein needs to interact with the nucleocapsid (Loffler-Mary et al., 2000).
Once the mature virion is formed, it is ready to bud on the surface of the cells. The whole orchestration of this process is not clear at all, let alone accurately described in terms of interacting proteins. HBV makes use of the ESCRT, a machinery essential for the sorting of cellular cargo proteins in multivesicular bodies (Bardens et al., 2011). In this process, aryl hydrocarbon receptor interacting protein (AIP1)/ALIX and vacuolar protein sorting 4 homolog B (VPS4B) were found to colocalize with HBV particles (Kian Chua et al., 2006; Watanabe et al., 2007; Figure 1). Also, expression of dominant negative mutants of ESCRT-III complex-forming charged multivesicular body protein (CHMP) proteins (CHMP3, 4B, and 4C), as well as vacuolar protein sorting 4 homolog A (VPS4A) or VPS4B mutants, and knockout of γ2-adaptin blocked HBV assembly and egress (Hartmann-Stuhler and Prange, 2001; Rost et al., 2006; Lambert et al., 2007; Figure 1). However, the manipulation of these proteins did not alter the secretion of subviral particles. Also involved in viral egress is Neural precursor cell Expressed, Developmentally Down-regulated 4 (NEDD) E3 ubiquitin protein ligase, which appears to control virus production by binding to the late assembly domain-like PPAY motif of HBV capsids (Rost et al., 2006; Garcia et al., 2013). It is also known that at some point, autophagy is involved in HBV production as the S-protein was shown to interact with the autophagy factor LC3 and manipulations to the pathway result in changes in HBV secretion (Li et al., 2011).
Concluding Remarks
The interactome we build of the cccDNA, HBc and HBx protein in this review emphasizes the vast amount of knowledge there is about the interactions between HBV proteins and in particular HBx, HBc and the cccDNA. To our knowledge, this is the first time the information has been brought together in a comprehensive overview. Bringing this information together, it shows that there are still clear gaps in knowledge. For example, the network shows that several proteins were only described in a single publication as an interacting protein of cccDNA, HBc, or HBx. Further characterization of this kind of interactions and potentially understanding the reason behind these interactions will greatly benefit the understanding of HBV-related processes. In addition, through analysis of the known interacting proteins, we predicted 210 proteins which potentially interact with either cccDNA, HBx, HBc, or with multiple key modalities of HBV.
Experimental verification of these proteins can lead to the discovery of novel mechanisms and expansion of known protein interaction networks.
Being able to position cccDNA, HBc and HBx in the greater whole of the cellular environment is paramount to better understand how HBV hijacks the cellular environment.
Author Contributions
EVD, JV, LV, and FP conceived, designed, and wrote the manuscript. BS performed the network pathway analysis, designed the gene association network, and contributed to scientific discussions about the generated network. All authors have read and edited the manuscript.
Conflict of Interest
EVD, FP, LV, and BS are employees of Janssen Research and Development and may be Johnson & Johnson stockholders. JV was employed at Janssen Research and Development at the time of the work and drafting of the manuscript and may be Johnson & Johnson stockholder.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
JV did most of the work under the first affiliation at Johnson & Johnson and moved then to Charles River laboratories (present address) during the writing and finalizing of the manuscript.
References
Addison, W. R., Walters, K. A., Wong, W. W., Wilson, J. S., Madej, D., Jewell, L. D., et al. (2002). Half-life of the duck hepatitis B virus covalently closed circular DNA pool in vivo following inhibition of viral replication. J. Virol. 76, 6356–6363. doi: 10.1128/jvi.76.12.6356-6363.2002
Alarcon, V., Hernandez, S., Rubio, L., Alvarez, F., Flores, Y., Varas-Godoy, M., et al. (2016). The enzymes LSD1 and Set1A cooperate with the viral protein HBx to establish an active hepatitis B viral chromatin state. Sci. Rep. 6:25901. doi: 10.1038/srep25901
Antunovic, J., Lemieux, N., and Cromlish, J. A. (1993). The 17 kDa HBx protein encoded by hepatitis B virus interacts with the activation domains of Oct-1, and functions as a coactivator in the activation and repression of a human U6 promoter. Cell Mol. Biol. Res. 39, 463–482.
Barak, O., Aronheim, A., and Shaul, Y. (2001). HBV X protein targets HIV Tat-binding protein 1. Virology 283, 110–120. doi: 10.1006/viro.2001.0883
Bardens, A., Doring, T., Stieler, J., and Prange, R. (2011). Alix regulates egress of hepatitis B virus naked capsid particles in an ESCRT-independent manner. Cell Microbiol. 13, 602–619. doi: 10.1111/j.1462-5822.2010.01557.x
Barnabas, S., and Andrisani, O. M. (2000). Different regions of hepatitis B virus X protein are required for enhancement of bZip-mediated transactivation versus transrepression. J. Virol. 74, 83–90. doi: 10.1128/jvi.74.1.83-90.2000
Barnabas, S., Hai, T., and Andrisani, O. M. (1997). The hepatitis B virus X protein enhances the DNA binding potential and transcription efficacy of bZip transcription factors. J. Biol. Chem. 272, 20684–20690. doi: 10.1074/jbc.272.33.20684
Barrasa, M. I., Guo, J. T., Saputelli, J., Mason, W. S., and Seeger, C. (2001). Does a cdc2 kinase-like recognition motif on the core protein of hepadnaviruses regulate assembly and disintegration of capsids? J. Virol. 75, 2024–2028. doi: 10.1128/JVI.75.4.2024-2028.2001
Bartusch, C., Doring, T., and Prange, R. (2017). Rab33B Controls Hepatitis B Virus Assembly by Regulating Core Membrane Association and Nucleocapsid Processing. Viruses 9:9060157. doi: 10.3390/v9060157
Becker, S. A., Lee, T. H., Butel, J. S., and Slagle, B. L. (1998). Hepatitis B virus X protein interferes with cellular DNA repair. J. Virol. 72, 266–272.
Belloni, L., Allweiss, L., Guerrieri, F., Pediconi, N., Volz, T., Pollicino, T., et al. (2012). IFN-alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J. Clin. Invest. 122, 529–537. doi: 10.1172/JCI58847
Belloni, L., Pollicino, T., De Nicola, F., Guerrieri, F., Raffa, G., Fanciulli, M., et al. (2009). Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. Proc. Natl. Acad. Sci. U S A 106, 19975–19979. doi: 10.1073/pnas.0908365106
Benhenda, S., Cougot, D., Buendia, M. A., and Neuveut, C. (2009). Hepatitis B virus X protein molecular functions and its role in virus life cycle and pathogenesis. Adv. Cancer Res. 103, 75–109. doi: 10.1016/S0065-230X(09)03004-8
Benhenda, S., Ducroux, A., Riviere, L., Sobhian, B., Ward, M. D., Dion, S., et al. (2013). Methyltransferase PRMT1 is a binding partner of HBx and a negative regulator of hepatitis B virus transcription. J. Virol. 87, 4360–4371. doi: 10.1128/JVI.02574-12
Benn, J., Su, F., Doria, M., and Schneider, R. J. (1996). Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J. Virol. 70, 4978–4985.
Berke, J. M., Dehertogh, P., Vergauwen, K., Van Damme, E., Mostmans, W., Vandyck, K., et al. (2017). Capsid Assembly Modulators Have a Dual Mechanism of Action in Primary Human Hepatocytes Infected with Hepatitis B Virus. Antimicrob. Agents Chemother. 61:517. doi: 10.1128/AAC.00560-17
Bitton Alaluf, M., and Shlomai, A. (2016). New therapies for chronic hepatitis B. Liver Int. 36, 775–782. doi: 10.1111/liv.13086
Bock, C. T., Schranz, P., Schroder, C. H., and Zentgraf, H. (1994). Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes 8, 215–229.
Bock, C. T., Schwinn, S., Locarnini, S., Fyfe, J., Manns, M. P., Trautwein, C., et al. (2001). Structural organization of the hepatitis B virus minichromosome. J. Mol. Biol. 307, 183–196. doi: 10.1006/jmbi.2000.4481
Bolhassani, A., and Agi, E. (2019). Heat shock proteins in infection. Clin. Chim. Acta 498, 90–100. doi: 10.1016/j.cca.2019.08.015
Bouchard, M. J., Wang, L., and Schneider, R. J. (2006). Activation of focal adhesion kinase by hepatitis B virus HBx protein: multiple functions in viral replication. J. Virol. 80, 4406–4414. doi: 10.1128/JVI.80.9.4406-4414.2006
Bourne, E. J., Dienstag, J. L., Lopez, V. A., Sander, T. J., Longlet, J. M., Hall, J. G., et al. (2007). Quantitative analysis of HBV cccDNA from clinical specimens: correlation with clinical and virological response during antiviral therapy. J. Viral. Hepat. 14, 55–63. doi: 10.1111/j.1365-2893.2006.00775.x
Boyd, A., Lacombe, K., Lavocat, F., Maylin, S., Miailhes, P., Lascoux-Combe, C., et al. (2016). Decay of ccc-DNA marks persistence of intrahepatic viral DNA synthesis under tenofovir in HIV-HBV co-infected patients. J. Hepatol. 65, 683–691. doi: 10.1016/j.jhep.2016.05.014
Bruss, V. (2004). Envelopment of the hepatitis B virus nucleocapsid. Virus Res. 106, 199–209. doi: 10.1016/j.virusres.2004.08.016
Buckwold, V. E., Chen, M., and Ou, J. H. (1997). Interaction of transcription factors RFX1 and MIBP1 with the gamma motif of the negative regulatory element of the hepatitis B virus core promoter. Virology 227, 515–518. doi: 10.1006/viro.1996.8360
Cai, D., Mills, C., Yu, W., Yan, R., Aldrich, C. E., Saputelli, J. R., et al. (2012). Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob. Agents Chemother. 56, 4277–4288. doi: 10.1128/AAC.00473-12
Cai, Y. N., Zhou, Q., Kong, Y. Y., Li, M., Viollet, B., Xie, Y. H., et al. (2003). LRH-1/hB1F and HNF1 synergistically up-regulate hepatitis B virus gene transcription and DNA replication. Cell Res. 13, 451–458. doi: 10.1038/sj.cr.7290187
Carretero, M., Gomez-Gonzalo, M., Lara-Pezzi, E., Benedicto, I., Aramburu, J., Martinez-Martinez, S., et al. (2002). The hepatitis B virus X protein binds to and activates the NH(2)-terminal trans-activation domain of nuclear factor of activated T cells-1. Virology 299, 288–300.
Chabrolles, H., Auclair, H., Vegna, S., Lahlali, T., Pons, C., Michelet, M., et al. (2020). Hepatitis B virus Core protein nuclear interactome identifies SRSF10 as a host RNA-binding protein restricting HBV RNA production. PLoS Pathog. 16:e1008593. doi: 10.1371/journal.ppat.1008593
Chae, S., Ji, J. H., Kwon, S. H., Lee, H. S., Lim, J. M., Kang, D., et al. (2013). HBxAPalpha/Rsf-1-mediated HBx-hBubR1 interactions regulate the mitotic spindle checkpoint and chromosome instability. Carcinogenesis 34, 1680–1688. doi: 10.1093/carcin/bgt105
Chan, C., Wang, Y., Chow, P. K., Chung, A. Y., Ooi, L. L., and Lee, C. G. (2013). Altered binding site selection of p53 transcription cassettes by hepatitis B virus X protein. Mol. Cell Biol. 33, 485–497. doi: 10.1128/MCB.01189-12
Chen, C., Wang, J. C., Pierson, E. E., Keifer, D. Z., Delaleau, M., Gallucci, L., et al. (2016). Importin beta Can Bind Hepatitis B Virus Core Protein and Empty Core-Like Particles and Induce Structural Changes. PLoS Pathog. 12:e1005802. doi: 10.1371/journal.ppat.1005802
Chen, W. N., Liu, L. L., Jiao, B. Y., Lin, W. S., Lin, X. J., and Lin, X. (2015). Hepatitis B virus X protein increases the IL-1beta-induced NF-kappaB activation via interaction with evolutionarily conserved signaling intermediate in Toll pathways (ECSIT). Virus Res. 195, 236–245. doi: 10.1016/j.virusres.2014.10.025
Chen, Y., Hu, J., Cai, X., Huang, Y., Zhou, X., Tu, Z., et al. (2018). APOBEC3B edits HBV DNA and inhibits HBV replication during reverse transcription. Antiviral. Res. 149, 16–25. doi: 10.1016/j.antiviral.2017.11.006
Cheng, S. T., Ren, J. H., Cai, X. F., Jiang, H., and Chen, J. (2018). HBx-elevated SIRT2 promotes HBV replication and hepatocarcinogenesis. Biochem. Biophys. Res. Commun. 496, 904–910. doi: 10.1016/j.bbrc.2018.01.127
Cheong, J. H., Yi, M., Lin, Y., and Murakami, S. (1995). Human RPB5, a subunit shared by eukaryotic nuclear RNA polymerases, binds human hepatitis B virus X protein and may play a role in X transactivation. EMBO J. 14, 143–150.
Cho, I. J., Ki, S. H., Brooks, C. III, and Kim, S. G. (2009). Role of hepatitis B virus X repression of C/EBPbeta activity in the down-regulation of glutathione S-transferase A2 gene: implications in other phase II detoxifying enzyme expression. Xenobiotica 39, 182–192. doi: 10.1080/00498250802549808
Choi, B. H., Park, G. T., and Rho, H. M. (1999). Interaction of hepatitis B viral X protein and CCAAT/enhancer-binding protein alpha synergistically activates the hepatitis B viral enhancer II/pregenomic promoter. J. Biol. Chem. 274, 2858–2865. doi: 10.1074/jbc.274.5.2858
Choi, C. Y., Choi, B. H., Park, G. T., and Rho, H. M. (1997). Activating transcription factor 2 (ATF2) down-regulates hepatitis B virus X promoter activity by the competition for the activating protein 1 binding site and the formation of the ATF2-Jun heterodimer. J. Biol. Chem. 272, 16934–16939.
Choi, M., Lee, H., and Rho, H. M. (2002). E2F1 activates the human p53 promoter and overcomes the repressive effect of hepatitis B viral X protein (Hbx) on the p53 promoter. IUBMB Life 53, 309–317. doi: 10.1080/15216540213466
Choi, Y. H., Kim, H. I., Seong, J. K., Yu, D. Y., Cho, H., Lee, M. O., et al. (2004). Hepatitis B virus X protein modulates peroxisome proliferator-activated receptor gamma through protein-protein interaction. FEBS Lett. 557, 73–80.
Chong, C. K., Cheng, C. Y. S., Tsoi, S. Y. J., Huang, F. Y., Liu, F., Seto, W. K., et al. (2017). Role of hepatitis B core protein in HBV transcription and recruitment of histone acetyltransferases to cccDNA minichromosome. Antiviral. Res. 144, 1–7. doi: 10.1016/j.antiviral.2017.05.003
Chung, Y. L., and Tsai, T. Y. (2009). Promyelocytic leukemia nuclear bodies link the DNA damage repair pathway with hepatitis B virus replication: implications for hepatitis B virus exacerbation during chemotherapy and radiotherapy. Mol. Cancer Res. 7, 1672–1685. doi: 10.1158/1541-7786.MCR-09-0112
Cougot, D., Allemand, E., Riviere, L., Benhenda, S., Duroure, K., Levillayer, F., et al. (2012). Inhibition of PP1 phosphatase activity by HBx: a mechanism for the activation of hepatitis B virus transcription. Sci. Signal 5:ra1. doi: 10.1126/scisignal.2001906
Cougot, D., Wu, Y., Cairo, S., Caramel, J., Renard, C. A., Levy, L., et al. (2007). The hepatitis B virus X protein functionally interacts with CREB-binding protein/p300 in the regulation of CREB-mediated transcription. J. Biol. Chem. 282, 4277–4287. doi: 10.1074/jbc.M606774200
Cui, X., Mcallister, R., Boregowda, R., Sohn, J. A., Cortes Ledesma, F., Caldecott, K. W., et al. (2015). Does Tyrosyl DNA Phosphodiesterase-2 Play a Role in Hepatitis B Virus Genome Repair? PLoS One 10:e0128401. doi: 10.1371/journal.pone.0128401
Dai, X., Zhang, W., Zhang, H., Sun, S., Yu, H., Guo, Y., et al. (2014). Modulation of HBV replication by microRNA-15b through targeting hepatocyte nuclear factor 1alpha. Nucleic Acids Res. 42, 6578–6590. doi: 10.1093/nar/gku260
Dai, Y., Cros, M. P., Pontoizeau, C., Elena-Hermann, B., Bonn, G. K., and Hainaut, P. (2014). Downregulation of transcription factor E4F1 in hepatocarcinoma cells: HBV-dependent effects on autophagy, proliferation and metabolism. Carcinogenesis 35, 635–650. doi: 10.1093/carcin/bgt353
Dandri, M., Burda, M. R., Will, H., and Petersen, J. (2000). Increased hepatocyte turnover and inhibition of woodchuck hepatitis B virus replication by adefovir in vitro do not lead to reduction of the closed circular DNA. Hepatology 32, 139–146. doi: 10.1053/jhep.2000.8701
Daub, H., Blencke, S., Habenberger, P., Kurtenbach, A., Dennenmoser, J., Wissing, J., et al. (2002). Identification of SRPK1 and SRPK2 as the major cellular protein kinases phosphorylating hepatitis B virus core protein. J. Virol. 76, 8124–8137.
Davis, L. G., Weber, D. J., and Lemon, S. M. (1989). Horizontal transmission of hepatitis B virus. Lancet 1, 889–893.
Decorsiere, A., Mueller, H., Van Breugel, P. C., Abdul, F., Gerossier, L., Beran, R. K., et al. (2016). Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature 531, 386–389. doi: 10.1038/nature17170
Deng, J. J., Kong, K. E., Gao, W. W., Tang, H. V., Chaudhary, V., Cheng, Y., et al. (2017). Interplay between SIRT1 and hepatitis B virus X protein in the activation of viral transcription. Biochim. Biophys. Acta 1860, 491–501. doi: 10.1016/j.bbagrm.2017.02.007
Deng, L., Gan, X., Ito, M., Chen, M., Aly, H. H., Matsui, C., et al. (2018). Peroxiredoxin 1, a novel HBx-interacting protein, interacts with Exosc5 and negatively regulates HBV propagation through degradation of HBV RNA. J. Virol. 2018:2218. doi: 10.1128/JVI.02203-18
Deng, L., Gan, X., Ito, M., Chen, M., Aly, H. H., Matsui, C., et al. (2019). Peroxiredoxin 1, a Novel HBx-Interacting Protein, Interacts with Exosome Component 5 and Negatively Regulates Hepatitis B Virus (HBV) Propagation through Degradation of HBV RNA. J. Virol. 93:2218.
Doring, T., Zeyen, L., Bartusch, C., and Prange, R. (2018). Hepatitis B Virus Subverts the Autophagy Elongation Complex Atg5-12/16L1 and Does Not Require Atg8/LC3 Lipidation for Viral Maturation. J. Virol. 92:1517. doi: 10.1128/JVI.01513-17
Ducroux, A., Benhenda, S., Riviere, L., Semmes, O. J., Benkirane, M., and Neuveut, C. (2014). The Tudor domain protein Spindlin1 is involved in intrinsic antiviral defense against incoming hepatitis B Virus and herpes simplex virus type 1. PLoS Pathog. 10:e1004343. doi: 10.1371/journal.ppat.1004343
Fanning, G. C., Zoulim, F., Hou, J., and Bertoletti, A. (2019). Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat. Rev. Drug Discov. 18, 827–844. doi: 10.1038/s41573-019-0037-0
Fay, N., and Pante, N. (2015). Nuclear entry of DNA viruses. Front. Microbiol. 6:467. doi: 10.3389/fmicb.2015.00467
Feng, H., Tan, T. L., Niu, D., and Chen, W. N. (2010). HBV X protein interacts with cytoskeletal signaling proteins through SH3 binding. Front. Biosci. 2, 143–150.
Forgues, M., Marrogi, A. J., Spillare, E. A., Wu, C. G., Yang, Q., Yoshida, M., et al. (2001). Interaction of the hepatitis B virus X protein with the Crm1-dependent nuclear export pathway. J. Biol. Chem. 276, 22797–22803. doi: 10.1074/jbc.M101259200
Fu, S., Wang, J., Hu, X., Zhou, R. R., Fu, Y., Tang, D., et al. (2018). Crosstalk between hepatitis B virus X and high-mobility group box 1 facilitates autophagy in hepatocytes. Mol. Oncol. 12, 322–338. doi: 10.1002/1878-0261.12165
Fust, G., Beck, Z., Banhegyi, D., Kocsis, J., Biro, A., and Prohaszka, Z. (2005). Antibodies against heat shock proteins and cholesterol in HIV infection. Mol. Immunol. 42, 79–85. doi: 10.1016/j.molimm.2004.07.003
Gallucci, L., and Kann, M. (2017). Nuclear Import of Hepatitis B Virus Capsids and Genome. Viruses 9:9010021. doi: 10.3390/v9010021
Gao, W., Jia, Z., Tian, Y., Yang, P., Sun, H., Wang, C., et al. (2020). HBx Protein Contributes to Liver Carcinogenesis by H3K4me3 Modification Through Stabilizing WD Repeat Domain 5 Protein. Hepatology 71, 1678–1695. doi: 10.1002/hep.30947
Garcia, M. L., Reynolds, T. D., Mothes, W., and Robek, M. D. (2013). Functional characterization of the putative hepatitis B virus core protein late domain using retrovirus chimeras. PLoS One 8:e72845. doi: 10.1371/journal.pone.0072845
Genera, M., Quioc-Salomon, B., Nourisson, A., Colcombet-Cazenave, B., Haouz, A., Mechaly, A., et al. (2021). Molecular basis of the interaction of the human tyrosine phosphatase PTPN3 with the hepatitis B virus core protein. Sci. Rep. 11:944. doi: 10.1038/s41598-020-79580-9
Geng, X., Huang, C., Qin, Y., Mccombs, J. E., Yuan, Q., Harry, B. L., et al. (2012). Hepatitis B virus X protein targets Bcl-2 proteins to increase intracellular calcium, required for virus replication and cell death induction. Proc. Natl. Acad. Sci. U S A 109, 18471–18476. doi: 10.1073/pnas.1204668109
Gilmore, D. T., Dick, R., Appleby, T., Birkus, G., Willkom, M., Delaney, W. E., et al. (2017). Antiviral activity of GS-5801, a liver-targeted prodrug of a lysine demethylase 5 inhibitor, in a hepatitis B virus primary human hepatocyte infection model. J. Hepatol. 66, S690–S691. doi: 10.1016/S0168-8278(17)31855-X
Gong, S. J., Feng, X. J., Song, W. H., Chen, J. M., Wang, S. M., Xing, D. J., et al. (2016). Upregulation of PP2Ac predicts poor prognosis and contributes to aggressiveness in hepatocellular carcinoma. Cancer Biol. Ther. 17, 151–162. doi: 10.1080/15384047.2015.1121345
Gripon, P., Cannie, I., and Urban, S. (2005). Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J. Virol. 79, 1613–1622. doi: 10.1128/JVI.79.3.1613-1622.2005
Guo, H., Xu, C., Zhou, T., Block, T. M., and Guo, J. T. (2012). Characterization of the host factors required for hepadnavirus covalently closed circular (ccc) DNA formation. PLoS One 7:e43270. doi: 10.1371/journal.pone.0043270
Guo, L., Wang, X., Ren, L., Zeng, M., Wang, S., Weng, Y., et al. (2014). HBx affects CUL4-DDB1 function in both positive and negative manners. Biochem. Biophys. Res. Commun. 450, 1492–1497. doi: 10.1016/j.bbrc.2014.07.019:10.1016/j.bbrc.2014.07.019
Guo, Y. H., Li, Y. N., Zhao, J. R., Zhang, J., and Yan, Z. (2011). HBc binds to the CpG islands of HBV cccDNA and promotes an epigenetic permissive state. Epigenetics 6, 720–726.
Hao, R., He, J., Liu, X., Gao, G., Liu, D., Cui, L., et al. (2015). Inhibition of hepatitis B virus gene expression and replication by hepatocyte nuclear factor 6. J. Virol. 89, 4345–4355. doi: 10.1128/JVI.03094-14
Hao, Z., Zheng, L., Kluwe, L., and Huang, W. (2012). Ferritin light chain and squamous cell carcinoma antigen 1 are coreceptors for cellular attachment and entry of hepatitis B virus. Int. J. Nanomedicine 7, 827–834. doi: 10.2147/IJN.S27803:10.2147/IJN.S27803
Hartmann-Stuhler, C., and Prange, R. (2001). Hepatitis B virus large envelope protein interacts with gamma2-adaptin, a clathrin adaptor-related protein. J. Virol. 75, 5343–5351. doi: 10.1128/JVI.75.11.5343-5351.2001
Hayashi, M., Deng, L., Chen, M., Gan, X., Shinozaki, K., Shoji, I., et al. (2016). Interaction of the hepatitis B virus X protein with the lysine methyltransferase SET and MYND domain-containing 3 induces activator protein 1 activation. Microbiol. Immunol. 60, 17–25. doi: 10.1111/1348-0421.12345
He, Q., Li, W., Ren, J., Huang, Y., Huang, Y., Hu, Q., et al. (2016). ZEB2 inhibits HBV transcription and replication by targeting its core promoter. Oncotarget 7, 16003–16011. doi: 10.18632/oncotarget.7435
Heermann, K. H., Goldmann, U., Schwartz, W., Seyffarth, T., Baumgarten, H., and Gerlich, W. H. (1984). Large surface proteins of hepatitis B virus containing the pre-s sequence. J. Virol. 52, 396–402.
Heger-Stevic, J., Zimmermann, P., Lecoq, L., Bottcher, B., and Nassal, M. (2018). Hepatitis B virus core protein phosphorylation: Identification of the SRPK1 target sites and impact of their occupancy on RNA binding and capsid structure. PLoS Pathog. 14:e1007488. doi: 10.1371/journal.ppat.1007488
Henkler, F., Hoare, J., Waseem, N., Goldin, R. D., Mcgarvey, M. J., Koshy, R., et al. (2001). Intracellular localization of the hepatitis B virus HBx protein. J. Gen. Virol. 82, 871–882. doi: 10.1099/0022-1317-82-4-871
Hodgson, A. J., Hyser, J. M., Keasler, V. V., Cang, Y., and Slagle, B. L. (2012). Hepatitis B virus regulatory HBx protein binding to DDB1 is required but is not sufficient for maximal HBV replication. Virology 426, 73–82. doi: 10.1016/j.virol.2012.01.021
Hong, A., Han, D. D., Wright, C. J., Burch, T., Piper, J., Osiowy, C., et al. (2012). The interaction between hepatitis B virus X protein and AIB1 oncogene is required for the activation of NFkappaB signal transduction. Biochem. Biophys. Res. Commun. 423, 6–12. doi: 10.1016/j.bbrc.2012.05.021:10.1016/j.bbrc.2012.05.021
Hsu, E. C., Lin, Y. C., Hung, C. S., Huang, C. J., Lee, M. Y., Yang, S. C., et al. (2007). Suppression of hepatitis B viral gene expression by protein-tyrosine phosphatase PTPN3. J. Biomed. Sci. 14, 731–744. doi: 10.1007/s11373-007-9187-x
Hu, Z., Zhang, Z., Doo, E., Coux, O., Goldberg, A. L., and Liang, T. J. (1999). Hepatitis B virus X protein is both a substrate and a potential inhibitor of the proteasome complex. J. Virol. 73, 7231–7240.
Huang, C. J., Chen, Y. H., and Ting, L. P. (2000). Hepatitis B virus core protein interacts with the C-terminal region of actin-binding protein. J. Biomed. Sci. 7, 160–168. doi: 10.1007/BF02256623
Huang, H. C., Chen, C. C., Chang, W. C., Tao, M. H., and Huang, C. (2012). Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J. Virol. 86, 9443–9453. doi: 10.1128/JVI.00873-12
Huang, J., Kwong, J., Sun, E. C., and Liang, T. J. (1996). Proteasome complex as a potential cellular target of hepatitis B virus X protein. J. Virol. 70, 5582–5591.
Huang, Q., Zhou, B., Cai, D., Zong, Y., Wu, Y., Liu, S., et al. (2021). Rapid Turnover of Hepatitis B Virus Covalently Closed Circular DNA Indicated by Monitoring Emergence and Reversion of Signature-Mutation in Treated Chronic Hepatitis B Patients. Hepatology 73, 41–52. doi: 10.1002/hep.31240
Huh, K. W., and Siddiqui, A. (2002). Characterization of the mitochondrial association of hepatitis B virus X protein, HBx. Mitochondrion 1, 349–359.
Hung, C. S., Lin, Y. L., Wu, C. I., Huang, C. J., and Ting, L. P. (2009). Suppression of hepatitis B viral gene expression by phosphoinositide 5-phosphatase SKIP. Cell Microbiol. 11, 37–50. doi: 10.1111/j.1462-5822.2008.01235.x
Ishida, H., Ueda, K., Ohkawa, K., Kanazawa, Y., Hosui, A., Nakanishi, F., et al. (2000). Identification of multiple transcription factors, HLF, FTF, and E4BP4, controlling hepatitis B virus enhancer II. J. Virol. 74, 1241–1251.
Iwamoto, M., Saso, W., Sugiyama, R., Ishii, K., Ohki, M., Nagamori, S., et al. (2019). Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc. Natl. Acad. Sci. U S A 116, 8487–8492. doi: 10.1073/pnas.1811064116
Iyer, S., and Groopman, J. D. (2011). Interaction of mutant hepatitis B X protein with p53 tumor suppressor protein affects both transcription and cell survival. Mol. Carcinog. 50, 972–980. doi: 10.1002/mc.20767:10.1002/mc.20767
Jamal, A., Swarnalatha, M., Sultana, S., Joshi, P., Panda, S. K., and Kumar, V. (2015). The G1 phase E3 ubiquitin ligase TRUSS that gets deregulated in human cancers is a novel substrate of the S-phase E3 ubiquitin ligase Skp2. Cell Cycle 14, 2688–2700. doi: 10.1080/15384101.2015.1056946
Jeong, H., Cho, M. H., Park, S. G., and Jung, G. (2014). Interaction between nucleophosmin and HBV core protein increases HBV capsid assembly. FEBS Lett. 588, 851–858. doi: 10.1016/j.febslet.2014.01.020
Jia, B., Guo, M., Li, G., Yu, D., Zhang, X., Lan, K., et al. (2015). Hepatitis B virus core protein sensitizes hepatocytes to tumor necrosis factor-induced apoptosis by suppression of the phosphorylation of mitogen-activated protein kinase kinase 7. J. Virol. 89, 2041–2051. doi: 10.1128/JVI.03106-14
Jiao, B. Y., Lin, W. S., She, F. F., Chen, W. N., and Lin, X. (2011). Hepatitis B virus X protein enhances activation of nuclear factor kappaB through interaction with valosin-containing protein. Arch. Virol. 156, 2015–2021. doi: 10.1007/s00705-011-1099-4
Kalra, N., and Kumar, V. (2006). The X protein of hepatitis B virus binds to the F box protein Skp2 and inhibits the ubiquitination and proteasomal degradation of c-Myc. FEBS Lett. 580, 431–436. doi: 10.1016/j.febslet.2005.12.034
Kann, M., Sodeik, B., Vlachou, A., Gerlich, W. H., and Helenius, A. (1999). Phosphorylation-dependent binding of hepatitis B virus core particles to the nuclear pore complex. J. Cell Biol. 145, 45–55.
Kau, J. H., and Ting, L. P. (1998). Phosphorylation of the core protein of hepatitis B virus by a 46-kilodalton serine kinase. J. Virol. 72, 3796–3803.
Khattar, E., Mukherji, A., and Kumar, V. (2012). Akt augments the oncogenic potential of the HBx protein of hepatitis B virus by phosphorylation. FEBS J. 279, 1220–1230. doi: 10.1111/j.1742-4658.2012.08514.x
Kian Chua, P., Lin, M. H., and Shih, C. (2006). Potent inhibition of human Hepatitis B virus replication by a host factor Vps4. Virology 354, 1–6. doi: 10.1016/j.virol.2006.07.018
Kim, B. K., Lim, S. O., and Park, Y. G. (2008). Requirement of the cyclic adenosisne monophosphate response element-binding protein for hepatitis B virus replication. Hepatology 48, 361–373. doi: 10.1002/hep.22359
Kim, H. J., Kim, S. Y., Kim, J., Lee, H., Choi, M., Kim, J. K., et al. (2008). Hepatitis B virus X protein induces apoptosis by enhancing translocation of Bax to mitochondria. IUBMB Life 60, 473–480. doi: 10.1002/iub.68
Kim, H. Y., Cho, H. K., Hong, S. P., and Cheong, J. (2011). Hepatitis B virus X protein stimulates the Hedgehog-Gli activation through protein stabilization and nuclear localization of Gli1 in liver cancer cells. Cancer Lett. 309, 176–184. doi: 10.1016/j.canlet.2011.05.033
Kim, J. S., Rho, B., Lee, T. H., Lee, J. M., Kim, S. J., and Park, J. H. (2006). The interaction of hepatitis B virus X protein and protein phosphatase type 2 Calpha and its effect on IL-6. Biochem. Biophys. Res. Commun. 351, 253–258. doi: 10.1016/j.bbrc.2006.10.028
Kim, J. Y., Song, E. H., Lee, H. J., Oh, Y. K., Choi, K. H., Yu, D. Y., et al. (2010). HBx-induced hepatic steatosis and apoptosis are regulated by TNFR1- and NF-kappaB-dependent pathways. J. Mol. Biol. 397, 917–931. doi: 10.1016/j.jmb.2010.02.016
Kim, K. H., and Seong, B. L. (2003). Pro-apoptotic function of HBV X protein is mediated by interaction with c-FLIP and enhancement of death-inducing signal. EMBO J. 22, 2104–2116. doi: 10.1093/emboj/cdg210
Kim, K., Kim, K. H., and Cheong, J. (2010). Hepatitis B virus X protein impairs hepatic insulin signaling through degradation of IRS1 and induction of SOCS3. PLoS One 5:e8649. doi: 10.1371/journal.pone.0008649
Kim, S. Y., Kim, J. C., Kim, J. K., Kim, H. J., Lee, H. M., Choi, M. S., et al. (2008). Hepatitis B virus X protein enhances NFkappaB activity through cooperating with VBP1. BMB Rep. 41, 158–163.
Kim, W. R. (2018). Emerging Therapies Toward a Functional Cure for Hepatitis B Virus Infection. Gastroenterol. Hepatol. 14, 439–442.
Kinoshita, W., Ogura, N., Watashi, K., and Wakita, T. (2017). Host factor PRPF31 is involved in cccDNA production in HBV-replicating cells. Biochem. Biophys. Res. Commun. 482, 638–644. doi: 10.1016/j.bbrc.2016.11.085
Kitamura, K., Que, L., Shimadu, M., Koura, M., Ishihara, Y., Wakae, K., et al. (2018). Flap endonuclease 1 is involved in cccDNA formation in the hepatitis B virus. PLoS Pathog. 14:e1007124. doi: 10.1371/journal.ppat.1007124
Klein, N. P., and Schneider, R. J. (1997). Activation of Src family kinases by hepatitis B virus HBx protein and coupled signaling to Ras. Mol. Cell Biol. 17, 6427–6436.
Ko, C., Lee, S., Windisch, M. P., and Ryu, W. S. (2014). DDX3 DEAD-box RNA helicase is a host factor that restricts hepatitis B virus replication at the transcriptional level. J. Virol. 88, 13689–13698. doi: 10.1128/JVI.02035-14
Kock, J., Rosler, C., Zhang, J. J., Blum, H. E., Nassal, M., and Thoma, C. (2010). Generation of covalently closed circular DNA of hepatitis B viruses via intracellular recycling is regulated in a virus specific manner. PLoS Pathog. 6:e1001082. doi: 10.1371/journal.ppat.1001082
Kohno, T., Tsuge, M., Murakami, E., Hiraga, N., Abe, H., Miki, D., et al. (2014). Human microRNA hsa-miR-1231 suppresses hepatitis B virus replication by targeting core mRNA. J. Viral. Hepat. 21, e89–e97. doi: 10.1111/jvh.12240
Kong, H. J., Hong, S. H., Lee, M. Y., Kim, H. D., Lee, J. W., and Cheong, J. (2000). Direct binding of hepatitis B virus X protein and retinoid X receptor contributes to phosphoenolpyruvate carboxykinase gene transactivation. FEBS Lett. 483, 114–118. doi: 10.1016/s0014-5793(00)02091-3
Kong, H. J., Park, M. J., Hong, S., Yu, H. J., Lee, Y. C., Choi, Y. H., et al. (2003). Hepatitis B virus X protein regulates transactivation activity and protein stability of the cancer-amplified transcription coactivator ASC-2. Hepatology 38, 1258–1266. doi: 10.1053/jhep.2003.50451
Koniger, C., Wingert, I., Marsmann, M., Rosler, C., Beck, J., and Nassal, M. (2014). Involvement of the host DNA-repair enzyme TDP2 in formation of the covalently closed circular DNA persistence reservoir of hepatitis B viruses. Proc. Natl. Acad. Sci. U S A 111, E4244–E4253. doi: 10.1073/pnas.1409986111
Kouwaki, T., Okamoto, T., Ito, A., Sugiyama, Y., Yamashita, K., Suzuki, T., et al. (2016). Hepatocyte Factor JMJD5 Regulates Hepatitis B Virus Replication through Interaction with HBx. J. Virol. 90, 3530–3542. doi: 10.1128/JVI.02776-15
Kumar, M., Jung, S. Y., Hodgson, A. J., Madden, C. R., Qin, J., and Slagle, B. L. (2011). Hepatitis B virus regulatory HBx protein binds to adaptor protein IPS-1 and inhibits the activation of beta interferon. J. Virol. 85, 987–995. doi: 10.1128/JVI.01825-10
Kwon, J. A., and Rho, H. M. (2003). Transcriptional repression of the human p53 gene by hepatitis B viral core protein (HBc) in human liver cells. Biol. Chem. 384, 203–212. doi: 10.1515/BC.2003.022
Lambert, C., and Prange, R. (2003). Chaperone action in the posttranslational topological reorientation of the hepatitis B virus large envelope protein: Implications for translocational regulation. Proc. Natl. Acad. Sci. U S A 100, 5199–5204. doi: 10.1073/pnas.0930813100
Lambert, C., Doring, T., and Prange, R. (2007). Hepatitis B virus maturation is sensitive to functional inhibition of ESCRT-III, Vps4, and gamma 2-adaptin. J. Virol. 81, 9050–9060. doi: 10.1128/JVI.00479-07
Lazarus, J. V., Block, T., Brechot, C., Kramvis, A., Miller, V., Ninburg, M., et al. (2018). The hepatitis B epidemic and the urgent need for cure preparedness. Nat. Rev. Gastroenterol. Hepatol. 15, 517–518. doi: 10.1038/s41575-018-0041-6
Lee, H., Jeong, H., Lee, S. Y., Kim, S. S., and Jang, K. L. (2019). Hepatitis B Virus X Protein Stimulates Virus Replication Via DNA Methylation of the C-1619 in Covalently Closed Circular DNA. Mol. Cells 42, 67–78. doi: 10.14348/molcells.2018.0255
Lee, S. J., Shim, H. Y., Hsieh, A., Min, J. Y., and Jung, G. (2009). Hepatitis B virus core interacts with the host cell nucleolar protein, nucleophosmin 1. J. Microbiol. 47, 746–752. doi: 10.1007/s12275-009-2720-z
Lee, S., Kim, W., Ko, C., and Ryu, W. S. (2016). Hepatitis B virus X protein enhances Myc stability by inhibiting SCF(Skp2) ubiquitin E3 ligase-mediated Myc ubiquitination and contributes to oncogenesis. Oncogene 35, 1857–1867. doi: 10.1038/onc.2015.251
Lee, Y. H., and Yun, Y. (1998). HBx protein of hepatitis B virus activates Jak1-STAT signaling. J. Biol. Chem. 273, 25510–25515.
Levrero, M., Pollicino, T., Petersen, J., Belloni, L., Raimondo, G., and Dandri, M. (2009). Control of cccDNA function in hepatitis B virus infection. J. Hepatol. 51, 581–592. doi: 10.1016/j.jhep.2009.05.022
Li, B., Carey, M., and Workman, J. L. (2007). The role of chromatin during transcription. Cell 128, 707–719. doi: 10.1016/j.cell.2007.01.015
Li, D., Ding, J., Chen, Z., Chen, Y., Lin, N., Chen, F., et al. (2015). Accurately mapping the location of the binding site for the interaction between hepatitis B virus X protein and cytochrome c oxidase III. Int. J. Mol. Med. 35, 319–324. doi: 10.3892/ijmm.2014.2018
Li, J., He, J., Fu, Y., Hu, X., Sun, L. Q., Huang, Y., et al. (2017). Hepatitis B virus X protein inhibits apoptosis by modulating endoplasmic reticulum stress response. Oncotarget 8, 96027–96034. doi: 10.18632/oncotarget.21630
Li, J., Liu, Y., Wang, Z., Liu, K., Wang, Y., Liu, J., et al. (2011). Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J. Virol. 85, 6319–6333. doi: 10.1128/JVI.02627-10
Li, N., Zhang, L., Chen, L., Feng, W., Xu, Y., Chen, F., et al. (2012). MxA inhibits hepatitis B virus replication by interaction with hepatitis B core antigen. Hepatology 56, 803–811. doi: 10.1002/hep.25608
Li, T., Ke, Z., Liu, W., Xiong, Y., Zhu, Y., and Liu, Y. (2019). Human Hepatitis B Virus Core Protein Inhibits IFNalpha-Induced IFITM1 Expression by Interacting with BAF200. Viruses 11:11050427. doi: 10.3390/v11050427
Li, T., Yang, X., Li, W., Song, J., Li, Z., Zhu, X., et al. (2021). ADAR1 Stimulation by IFN-alpha Downregulates the Expression of MAVS via RNA Editing to Regulate the Anti-HBV Response. Mol. Ther. 29, 1335–1348. doi: 10.1016/j.ymthe.2020.11.031
Li, Y., Sun, Y., Sun, F., Hua, R., Li, C., Chen, L., et al. (2018). Mechanisms and Effects on HBV Replication of the Interaction between HBV Core Protein and Cellular Filamin B. Virol. Sin. 33, 162–172. doi: 10.1007/s12250-018-0023-4
Liang, T. J., Block, T. M., Mcmahon, B. J., Ghany, M. G., Urban, S., Guo, J. T., et al. (2015). Present and future therapies of hepatitis B: From discovery to cure. Hepatology 62, 1893–1908. doi: 10.1002/hep.28025
Lieberman, P. M. (2016). Epigenetics and Genetics of Viral Latency. Cell Host Microbe 19, 619–628. doi: 10.1016/j.chom.2016.04.008
Lim, K. H., Kim, K. H., Choi, S. I., Park, E. S., Park, S. H., Ryu, K., et al. (2011). RPS3a over-expressed in HBV-associated hepatocellular carcinoma enhances the HBx-induced NF-kappaB signaling via its novel chaperoning function. PLoS One 6:e22258. doi: 10.1371/journal.pone.0022258
Lin, J., Gu, C., Shen, Z., Liu, Y., Wang, W., Tao, S., et al. (2017). Hepatocyte nuclear factor 1alpha downregulates HBV gene expression and replication by activating the NF-kappaB signaling pathway. PLoS One 12:e0174017. doi: 10.1371/journal.pone.0174017
Lin, S. M., Cheng, J., Lu, Y. Y., Zhang, S. L., Yang, Q., Chen, T. Y., et al. (2006). Screening and identification of interacting proteins with hepatitis B virus core protein in leukocytes and cloning of new gene C1. World J. Gastroenterol. 12, 1043–1048. doi: 10.3748/wjg.v12.i7.1043
Lin, W. S., Jiao, B. Y., Wu, Y. L., Chen, W. N., and Lin, X. (2012). Hepatitis B virus X protein blocks filamentous actin bundles by interaction with eukaryotic translation elongat ion factor 1 alpha 1. J. Med. Virol. 84, 871–877. doi: 10.1002/jmv.23283
Lin, Y., Nomura, T., Cheong, J., Dorjsuren, D., Iida, K., and Murakami, S. (1997). Hepatitis B virus X protein is a transcriptional modulator that communicates with transcription factor IIB and the RNA polymerase II subunit 5. J. Biol. Chem. 272, 7132–7139.
Ling, M. T., Chiu, Y. T., Lee, T. K., Leung, S. C., Fung, M. K., Wang, X., et al. (2008). Id-1 induces proteasome-dependent degradation of the HBX protein. J. Mol. Biol. 382, 34–43. doi: 10.1016/j.jmb.2007.06.020
Liu, B., Fang, M., He, Z., Cui, D., Jia, S., Lin, X., et al. (2015). Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf2 activation. Cell Death Dis. 6:e1980. doi: 10.1038/cddis.2015.322
Liu, C., Cai, D., Zhang, L., Tang, W., Yan, R., Guo, H., et al. (2016). Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antiviral. Res. 134, 97–107. doi: 10.1016/j.antiviral.2016.08.026
Liu, H., Lou, G., Li, C., Wang, X., Cederbaum, A. I., Gan, L., et al. (2014). HBx inhibits CYP2E1 gene expression via downregulating HNF4alpha in human hepatoma cells. PLoS One 9:e107913. doi: 10.1371/journal.pone.0107913
Liu, H., Xi, J., and Hu, J. (2021). Regulation of Hepatitis B Virus Replication by Cyclin Docking Motifs in Core Protein. J. Virol. 95:21. doi: 10.1128/JVI.00230-21
Liu, H., Yuan, Y., Guo, H., Mitchelson, K., Zhang, K., Xie, L., et al. (2012). Hepatitis B virus encoded X protein suppresses apoptosis by inhibition of the caspase-independent pathway. J. Proteome Res. 11, 4803–4813. doi: 10.1021/pr2012297
Liu, K., Qian, L., Wang, J., Li, W., Deng, X., Chen, X., et al. (2009). Two-dimensional blue native/SDS-PAGE analysis reveals heat shock protein chaperone machinery involved in hepatitis B virus production in HepG2.2.15 cells. Mol. Cell Proteomics 8, 495–505. doi: 10.1074/mcp.M800250-MCP200
Liu, N., Zhang, J., Yang, X., Jiao, T., Zhao, X., Li, W., et al. (2017). HDM2 Promotes NEDDylation of Hepatitis B Virus HBx To Enhance Its Stability and Function. J. Virol. 91, 340–317. doi: 10.1128/JVI.00340-17
Liu, X. Y., Tang, S. H., Wu, S. L., Luo, Y. H., Cao, M. R., Zhou, H. K., et al. (2015). Epigenetic modulation of insulin-like growth factor-II overexpression by hepatitis B virus X protein in hepatocellular carcinoma. Am. J. Cancer Res. 5, 956–978.
Loffler-Mary, H., Dumortier, J., Klentsch-Zimmer, C., and Prange, R. (2000). Hepatitis B virus assembly is sensitive to changes in the cytosolic S loop of the envelope proteins. Virology 270, 358–367. doi: 10.1006/viro.2000.0268
Loffler-Mary, H., Werr, M., and Prange, R. (1997). Sequence-specific repression of cotranslational translocation of the hepatitis B virus envelope proteins coincides with binding of heat shock protein Hsc70. Virology 235, 144–152. doi: 10.1006/viro.1997.8689
Long, Q., Yan, R., Hu, J., Cai, D., Mitra, B., Kim, E. S., et al. (2017). The role of host DNA ligases in hepadnavirus covalently closed circular DNA formation. PLoS Pathog. 13:e1006784. doi: 10.1371/journal.ppat.1006784
Lott, L., Beames, B., Notvall, L., and Lanford, R. E. (2000). Interaction between hepatitis B virus core protein and reverse transcriptase. J. Virol. 74, 11479–11489. doi: 10.1128/jvi.74.24.11479-11489.2000
Lu, Y. Y., Cheng, J., Yang, Y. P., Liu, Y., Wang, L., Li, K., et al. (2005). Cloning and characterization of a novel hepatitis B virus core binding protein C12. World J. Gastroenterol. 11, 5666–5671. doi: 10.3748/wjg.v11.i36.5666
Lu, Z. P., Xiao, Z. L., Yang, Z., Li, J., Feng, G. X., Chen, F. Q., et al. (2015). Hepatitis B virus X protein promotes human hepatoma cell growth via upregulation of transcription factor AP2alpha and sphingosine kinase 1. Acta Pharmacol. Sin. 36, 1228–1236. doi: 10.1038/aps.2015.38
Luber, B., Lauer, U., Weiss, L., Hohne, M., Hofschneider, P. H., and Kekule, A. S. (1993). The hepatitis B virus transactivator HBx causes elevation of diacylglycerol and activation of protein kinase C. Res. Virol. 144, 311–321.
Lubyova, B., Hodek, J., Zabransky, A., Prouzova, H., Hubalek, M., Hirsch, I., et al. (2017). PRMT5: A novel regulator of Hepatitis B virus replication and an arginine methylase of HBV core. PLoS One 12:e0186982. doi: 10.1371/journal.pone.0186982
Lucifora, J., Esser, K., and Protzer, U. (2013). Ezetimibe blocks hepatitis B virus infection after virus uptake into hepatocytes. Antiviral. Res. 97, 195–197. doi: 10.1016/j.antiviral.2012.12.008
Lucifora, J., Xia, Y., Reisinger, F., Zhang, K., Stadler, D., Cheng, X., et al. (2014). Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 343, 1221–1228. doi: 10.1126/science.1243462
Ludgate, L., Adams, C., and Hu, J. (2011). Phosphorylation state-dependent interactions of hepadnavirus core protein with host factors. PLoS One 6:e29566. doi: 10.1371/journal.pone.0029566
Luo, X., Huang, Y., Chen, Y., Tu, Z., Hu, J., Tavis, J. E., et al. (2016). Association of Hepatitis B Virus Covalently Closed Circular DNA and Human APOBEC3B in Hepatitis B Virus-Related Hepatocellular Carcinoma. PLoS One 11:e0157708. doi: 10.1371/journal.pone.0157708
Lv, M., Zhang, B., Shi, Y., Han, Z., Zhang, Y., Zhou, Y., et al. (2015). Identification of BST-2/tetherin-induced hepatitis B virus restriction and hepatocyte-specific BST-2 inactivation. Sci. Rep. 5:11736. doi: 10.1038/srep11736
Lwa, S. H., and Chen, W. N. (2005). Hepatitis B virus X protein interacts with beta5 subunit of heterotrimeric guanine nucleotide binding protein. Virol. J. 2:76. doi: 10.1186/1743-422X-2-76
Lythgoe, K. A., Lumley, S. F., Pellis, L., Mckeating, J. A., and Matthews, P. C. (2021). Estimating hepatitis B virus cccDNA persistence in chronic infection. Virus Evol. 7:veaa063. doi: 10.1093/ve/veaa063
Macovei, A., Petrareanu, C., Lazar, C., Florian, P., and Branza-Nichita, N. (2013). Regulation of hepatitis B virus infection by Rab5, Rab7, and the endolysosomal compartment. J. Virol. 87, 6415–6427. doi: 10.1128/JVI.00393-13
Macovei, A., Radulescu, C., Lazar, C., Petrescu, S., Durantel, D., Dwek, R. A., et al. (2010). Hepatitis B virus requires intact caveolin-1 function for productive infection in HepaRG cells. J. Virol. 84, 243–253. doi: 10.1128/JVI.01207-09
Maguire, H. F., Hoeffler, J. P., and Siddiqui, A. (1991). HBV X protein alters the DNA binding specificity of CREB and ATF-2 by protein-protein interactions. Science 252, 842–844. doi: 10.1126/science.1827531
Makokha, G. N., Abe-Chayama, H., Chowdhury, S., Hayes, C. N., Tsuge, M., Yoshima, T., et al. (2019). Regulation of the Hepatitis B virus replication and gene expression by the multi-functional protein TARDBP. Sci. Rep. 9:8462. doi: 10.1038/s41598-019-44934-5
Minor, M. M., Hollinger, F. B., Mcnees, A. L., Jung, S. Y., Jain, A., Hyser, J. M., et al. (2020). Hepatitis B Virus HBx Protein Mediates the Degradation of Host Restriction Factors through the Cullin 4 DDB1 E3 Ubiquitin Ligase Complex. Cells 9:9040834. doi: 10.3390/cells9040834
Molina-Jimenez, F., Benedicto, I., Murata, M., Martin-Vilchez, S., Seki, T., Antonio Pintor-Toro, J., et al. (2010). Expression of pituitary tumor-transforming gene 1 (PTTG1)/securin in hepatitis B virus (HBV)-associated liver diseases: evidence for an HBV X protein-mediated inhibition of PTTG1 ubiquitination and degradation. Hepatology 51, 777–787. doi: 10.1002/hep.23468
Mueller, H., Lopez, A., Tropberger, P., Wildum, S., Schmaler, J., Pedersen, L., et al. (2019). PAPD5/7 Are Host Factors That Are Required for Hepatitis B Virus RNA Stabilization. Hepatology 69, 1398–1411. doi: 10.1002/hep.30329
Mukherji, A., Janbandhu, V. C., and Kumar, V. (2007). HBx-dependent cell cycle deregulation involves interaction with cyclin E/A-cdk2 complex and destabilization of p27Kip1. Biochem. J. 401, 247–256. doi: 10.1042/BJ20061091
Murphy, C. M., Xu, Y., Li, F., Nio, K., Reszka-Blanco, N., Li, X., et al. (2016). Hepatitis B Virus X Protein Promotes Degradation of SMC5/6 to Enhance HBV Replication. Cell Rep. 16, 2846–2854. doi: 10.1016/j.celrep.2016.08.026
Na, T. Y., Ka, N. L., Rhee, H., Kyeong, D., Kim, M. H., Seong, J. K., et al. (2016). Interaction of hepatitis B virus X protein with PARP1 results in inhibition of DNA repair in hepatocellular carcinoma. Oncogene 35, 5435–5445. doi: 10.1038/onc.2016.82
Newbold, J. E., Xin, H., Tencza, M., Sherman, G., Dean, J., Bowden, S., et al. (1995). The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J. Virol. 69, 3350–3357.
Nguyen, D. H., Gummuluru, S., and Hu, J. (2007). Deamination-independent inhibition of hepatitis B virus reverse transcription by APOBEC3G. J. Virol. 81, 4465–4472. doi: 10.1128/JVI.02510-06
Nguyen, D. H., Ludgate, L., and Hu, J. (2008). Hepatitis B virus-cell interactions and pathogenesis. J. Cell Physiol. 216, 289–294. doi: 10.1002/jcp.21416
Niu, C., Livingston, C. M., Li, L., Beran, R. K., Daffis, S., Ramakrishnan, D., et al. (2017). The Smc5/6 Complex Restricts HBV when Localized to ND10 without Inducing an Innate Immune Response and Is Counteracted by the HBV X Protein Shortly after Infection. PLoS One 12:e0169648. doi: 10.1371/journal.pone.0169648
Niu, Y., Wu, Z., Shen, Q., Song, J., Luo, Q., You, H., et al. (2013). Hepatitis B virus X protein co-activates pregnane X receptor to induce the cytochrome P450 3A4 enzyme, a potential implication in hepatocarcinogenesis. Dig. Liver Dis. 45, 1041–1048. doi: 10.1016/j.dld.2013.06.004
Niu, Y., Xu, M., Slagle, B. L., Huang, H., Li, S., Guo, G. L., et al. (2017). Farnesoid X receptor ablation sensitizes mice to hepatitis b virus X protein-induced hepatocarcinogenesis. Hepatology 65, 893–906. doi: 10.1002/hep.28924
Nkongolo, S., Ni, Y., Lempp, F. A., Kaufman, C., Lindner, T., Esser-Nobis, K., et al. (2014). Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J. Hepatol. 60, 723–731. doi: 10.1016/j.jhep.2013.11.022
Nkongolo, S., Nussbaum, L., Lempp, F. A., Wodrich, H., Urban, S., and Ni, Y. (2019). The retinoic acid receptor (RAR) alpha-specific agonist Am80 (tamibarotene) and other RAR agonists potently inhibit hepatitis B virus transcription from cccDNA. Antiviral. Res. 168, 146–155. doi: 10.1016/j.antiviral.2019.04.009
Ohno, H., Kaneko, S., Lin, Y., Kobayashi, K., and Murakami, S. (1999). Human hepatitis B virus X protein augments the DNA binding of nuclear factor for IL-6 through its basic-leucine zipper domain. J. Med. Virol. 58, 11–18.
Ori, A., Atzmony, D., Haviv, I., and Shaul, Y. (1994). An NF1 motif plays a central role in hepatitis B virus enhancer. Virology 204, 600–608. doi: 10.1006/viro.1994.1574
Osseman, Q., Gallucci, L., Au, S., Cazenave, C., Berdance, E., Blondot, M. L., et al. (2018). The chaperone dynein LL1 mediates cytoplasmic transport of empty and mature hepatitis B virus capsids. J. Hepatol. 68, 441–448. doi: 10.1016/j.jhep.2017.10.032
Pang, R., Lee, T. K., Poon, R. T., Fan, S. T., Wong, K. B., Kwong, Y. L., et al. (2007). Pin1 interacts with a specific serine-proline motif of hepatitis B virus X-protein to enhance hepatocarcinogenesis. Gastroenterology 132, 1088–1103. doi: 10.1053/j.gastro.2006.12.030
Pante, N., and Kann, M. (2002). Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol. Biol. Cell 13, 425–434. doi: 10.1091/mbc.01-06-0308
Pei, D. Q., and Shih, C. H. (1990). Transcriptional activation and repression by cellular DNA-binding protein C/EBP. J. Virol. 64, 1517–1522.
Phillips, S., Chokshi, S., Chatterji, U., Riva, A., Bobardt, M., Williams, R., et al. (2015). Alisporivir inhibition of hepatocyte cyclophilins reduces HBV replication and hepatitis B surface antigen production. Gastroenterology 148, 403–414e407. doi: 10.1053/j.gastro.2014.10.004
Pollicino, T., Belloni, L., Raffa, G., Pediconi, N., Squadrito, G., Raimondo, G., et al. (2006). Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology 130, 823–837. doi: 10.1053/j.gastro.2006.01.001
Qadri, I., Fatima, K., and Abde, L. H. H. (2011). Hepatitis B virus X protein impedes the DNA repair via its association with transcription factor, TFIIH. BMC Microbiol. 11:48. doi: 10.1186/1471-2180-11-48
Qadri, I., Maguire, H. F., and Siddiqui, A. (1995). Hepatitis B virus transactivator protein X interacts with the TATA-binding protein. Proc. Natl. Acad. Sci. U S A 92, 1003–1007. doi: 10.1073/pnas.92.4.1003
Qi, Y., Gao, Z., Xu, G., Peng, B., Liu, C., Yan, H., et al. (2016). DNA Polymerase kappa Is a Key Cellular Factor for the Formation of Covalently Closed Circular DNA of Hepatitis B Virus. PLoS Pathog. 12:e1005893. doi: 10.1371/journal.ppat.1005893
Qian, G., Hu, B., Zhou, D., Xuan, Y., Bai, L., and Duan, C. (2015). NIRF, a Novel Ubiquitin Ligase, Inhibits Hepatitis B Virus Replication Through Effect on HBV Core Protein and H3 Histones. DNA Cell Biol. 34, 327–332. doi: 10.1089/dna.2014.2714
Qian, G., Jin, F., Chang, L., Yang, Y., Peng, H., and Duan, C. (2012). NIRF, a novel ubiquitin ligase, interacts with hepatitis B virus core protein and promotes its degradation. Biotechnol. Lett. 34, 29–36. doi: 10.1007/s10529-011-0751-0
Qiao, Y., Han, X., Guan, G., Wu, N., Sun, J., Pak, V., et al. (2016). TGF-beta triggers HBV cccDNA degradation through AID-dependent deamination. FEBS Lett. 590, 419–427. doi: 10.1002/1873-3468.12058
Qin, D., Li, K., Qu, J., Wang, S., Zou, C., Sheng, Y., et al. (2013). HBx and HBs regulate RhoC expression by upregulating transcription factor Ets-1. Arch. Virol. 158, 1773–1781. doi: 10.1007/s00705-013-1655-1
Quarleri, J. (2014). Core promoter: a critical region where the hepatitis B virus makes decisions. World J. Gastroenterol. 20, 425–435. doi: 10.3748/wjg.v20.i2.425
Quasdorff, M., and Protzer, U. (2010). Control of hepatitis B virus at the level of transcription. J. Viral. Hepat. 17, 527–536. doi: 10.1111/j.1365-2893.2010.01315.x
Rahmani, Z., Huh, K. W., Lasher, R., and Siddiqui, A. (2000). Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J. Virol. 74, 2840–2846.
Rawat, S., and Bouchard, M. J. (2015). The hepatitis B virus (HBV) HBx protein activates AKT to simultaneously regulate HBV replication and hepatocyte survival. J. Virol. 89, 999–1012. doi: 10.1128/JVI.02440-14
Razanskas, R., and Sasnauskas, K. (2010). Interaction of hepatitis B virus core protein with human GIPC1. Arch. Virol. 155, 247–250. doi: 10.1007/s00705-009-0561-z
Ren, J. H., Hu, J. L., Cheng, S. T., Yu, H. B., Wong, V. K. W., Law, B. Y. K., et al. (2018). SIRT3 restricts hepatitis B virus transcription and replication through epigenetic regulation of covalently closed circular DNA involving suppressor of variegation 3-9 homolog 1 and SET domain containing 1A histone methyltransferases. Hepatology 68, 1260–1276. doi: 10.1002/hep.29912
Ren, J. H., Tao, Y., Zhang, Z. Z., Chen, W. X., Cai, X. F., Chen, K., et al. (2014). Sirtuin 1 regulates hepatitis B virus transcription and replication by targeting transcription factor AP-1. J. Virol. 88, 2442–2451. doi: 10.1128/JVI.02861-13
Riviere, L., Gerossier, L., Ducroux, A., Dion, S., Deng, Q., Michel, M. L., et al. (2015). HBx relieves chromatin-mediated transcriptional repression of hepatitis B viral cccDNA involving SETDB1 histone methyltransferase. J. Hepatol. 63, 1093–1102. doi: 10.1016/j.jhep.2015.06.023
Rost, M., Mann, S., Lambert, C., Doring, T., Thome, N., and Prange, R. (2006). Gamma-adaptin, a novel ubiquitin-interacting adaptor, and Nedd4 ubiquitin ligase control hepatitis B virus maturation. J. Biol. Chem. 281, 29297–29308. doi: 10.1074/jbc.M603517200
Rui, E., Moura, P. R., Goncalves, K. A., Rooney, R. J., and Kobarg, J. (2006). Interaction of the hepatitis B virus protein HBx with the human transcription regulatory protein p120E4F in vitro. Virus Res. 115, 31–42. doi: 10.1016/j.virusres.2005.07.003
Saeed, U., Kim, J., Piracha, Z. Z., Kwon, H., Jung, J., Chwae, Y. J., et al. (2018). Parvulin 14 and parvulin 17 bind to HBx and cccDNA and upregulate HBV replication from cccDNA to virion in a HBx-dependent manner. J. Virol. 2018:18. doi: 10.1128/JVI.01840-18
Salerno, D., Chiodo, L., Alfano, V., Floriot, O., Cottone, G., Paturel, A., et al. (2020). Hepatitis B protein HBx binds the DLEU2 lncRNA to sustain cccDNA and host cancer-related gene transcription. Gut 69, 2016–2024. doi: 10.1136/gutjnl-2019-319637
Saracco, G., Rizzetto, M., and Verme, G. (1994). Interferon in chronic hepatitis B. Antiviral. Res. 24, 137–143. doi: 10.1016/0166-3542(94)90062-0
Saxena, N., and Kumar, V. (2014). The HBx oncoprotein of hepatitis B virus deregulates the cell cycle by promoting the intracellular accumulation and re-compartmentalization of the cellular deubiquitinase USP37. PLoS One 9:e111256. doi: 10.1371/journal.pone.0111256
Schmitz, A., Schwarz, A., Foss, M., Zhou, L., Rabe, B., Hoellenriegel, J., et al. (2010). Nucleoporin 153 arrests the nuclear import of hepatitis B virus capsids in the nuclear basket. PLoS Pathog. 6:e1000741. doi: 10.1371/journal.ppat.1000741
Schreiner, S., and Nassal, M. (2017). A Role for the Host DNA Damage Response in Hepatitis B Virus cccDNA Formation-and Beyond? Viruses 9:9050125. doi: 10.3390/v9050125
Schulze, A., Gripon, P., and Urban, S. (2007). Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 46, 1759–1768. doi: 10.1002/hep.21896
Schulze, A., Schieck, A., Ni, Y., Mier, W., and Urban, S. (2010). Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J. Virol. 84, 1989–2000. doi: 10.1128/JVI.01902-09
Schweitzer, A., Horn, J., Mikolajczyk, R. T., Krause, G., and Ott, J. J. (2015). Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 386, 1546–1555. doi: 10.1016/S0140-6736(15)61412-X
Sekiba, K., Otsuka, M., Ohno, M., Yamagami, M., Kishikawa, T., Seimiya, T., et al. (2019b). Pevonedistat, a Neuronal Precursor Cell-Expressed Developmentally Down-Regulated Protein 8-Activating Enzyme Inhibitor, Is a Potent Inhibitor of Hepatitis B Virus. Hepatology 69, 1903–1915. doi: 10.1002/hep.30491
Sekiba, K., Otsuka, M., Ohno, M., Yamagami, M., Kishikawa, T., Suzuki, T., et al. (2019a). Inhibition of HBV Transcription From cccDNA With Nitazoxanide by Targeting the HBx-DDB1 Interaction. Cell Mol. Gastroenterol. Hepatol. 7, 297–312. doi: 10.1016/j.jcmgh.2018.10.010
Selzer, L., and Zlotnick, A. (2015). Assembly and Release of Hepatitis B Virus. Cold Spring Harb. Perspect. Med. 5:21394. doi: 10.1101/cshperspect.a021394
Seo, H. W., Seo, J. P., and Jung, G. (2018). Heat shock protein 70 and heat shock protein 90 synergistically increase hepatitis B viral capsid assembly. Biochem. Biophys. Res. Commun. 503, 2892–2898. doi: 10.1016/j.bbrc.2018.08.065
Sheraz, M., Cheng, J., Tang, L., Chang, J., and Guo, J. T. (2019). Cellular DNA Topoisomerases Are Required for the Synthesis of Hepatitis B Virus Covalently Closed Circular DNA. J. Virol. 93:2218. doi: 10.1128/JVI.02230-18
Shi, Y., Zhang, H., Han, Z., Mi, X., Zhang, W., and Lv, M. (2016). HBx interacted with Smad4 to deprive activin a growth inhibition function in hepatocyte HL7702 on CRM1 manner. Tumour Biol. 37, 3405–3415. doi: 10.1007/s13277-015-4076-9
Shim, H. Y., Quan, X., Yi, Y. S., and Jung, G. (2011). Heat shock protein 90 facilitates formation of the HBV capsid via interacting with the HBV core protein dimers. Virology 410, 161–169. doi: 10.1016/j.virol.2010.11.005
Shimura, S., Watashi, K., Fukano, K., Peel, M., Sluder, A., Kawai, F., et al. (2017). Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity. J. Hepatol. 66, 685–692. doi: 10.1016/j.jhep.2016.11.009
Shin, G. C., Ahn, S. H., Choi, H. S., Lim, K. H., Choi, D. Y., Kim, K. P., et al. (2013). Hepatocystin/80K-H inhibits replication of hepatitis B virus through interaction with HBx protein in hepatoma cell. Biochim. Biophys. Acta 1832, 1569–1581. doi: 10.1016/j.bbadis.2013.04.026
Shon, J. K., Shon, B. H., Park, I. Y., Lee, S. U., Fa, L., Chang, K. Y., et al. (2009). Hepatitis B virus-X protein recruits histone deacetylase 1 to repress insulin-like growth factor binding protein 3 transcription. Virus Res. 139, 14–21. doi: 10.1016/j.virusres.2008.09.006
Shukla, R., Yue, J., Siouda, M., Gheit, T., Hantz, O., Merle, P., et al. (2011). Proinflammatory cytokine TNF-alpha increases the stability of hepatitis B virus X protein through NF-kappaB signaling. Carcinogenesis 32, 978–985. doi: 10.1093/carcin/bgr057
Sohn, S. Y., Kim, S. B., Kim, J., and Ahn, B. Y. (2006). Negative regulation of hepatitis B virus replication by cellular Hsp40/DnaJ proteins through destabilization of viral core and X proteins. J. Gen. Virol. 87, 1883–1891. doi: 10.1099/vir.0.81684-0
Srisuttee, R., Koh, S. S., Kim, S. J., Malilas, W., Boonying, W., Cho, I. R., et al. (2012). Hepatitis B virus X (HBX) protein upregulates beta-catenin in a human hepatic cell line by sequestering SIRT1 deacetylase. Oncol. Rep. 28, 276–282. doi: 10.3892/or.2012.1798
Stavrou, S., and Ross, S. R. (2015). APOBEC3 Proteins in Viral Immunity. J. Immunol. 195, 4565–4570. doi: 10.4049/jimmunol.1501504
Su, Z. J., Cao, J. S., Wu, Y. F., Chen, W. N., Lin, X., Wu, Y. L., et al. (2017). Deubiquitylation of hepatitis B virus X protein (HBx) by ubiquitin-specific peptidase 15 (USP15) increases HBx stability and its transactivation activity. Sci. Rep. 7:40246. doi: 10.1038/srep40246
Summers, J., O’connell, A., and Millman, I. (1975). Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc. Natl. Acad. Sci. U S A 72, 4597–4601.
Summers, J., Smith, P. M., and Horwich, A. L. (1990). Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J. Virol. 64, 2819–2824.
Tacke, F., Liedtke, C., Bocklage, S., Manns, M. P., and Trautwein, C. (2005). CREB/PKA sensitive signalling pathways activate and maintain expression levels of the hepatitis B virus pre-S2/S promoter. Gut 54, 1309–1317. doi: 10.1136/gut.2005.065086
Tan, T. L., Fang, N., Neo, T. L., Singh, P., Zhang, J., Zhou, R., et al. (2008). Rac1 GTPase is activated by hepatitis B virus replication–involvement of HBX. Biochim. Biophys. Acta 1783, 360–374. doi: 10.1016/j.bbamcr.2007.10.024
Tan, T. L., Feng, Z., Lu, Y. W., Chan, V., and Chen, W. N. (2006). Adhesion contact kinetics of HepG2 cells during Hepatitis B virus replication: Involvement of SH3-binding motif in HBX. Biochim. Biophys. Acta 1762, 755–766. doi: 10.1016/j.bbadis.2006.06.016
Tan, Z., Pionek, K., Unchwaniwala, N., Maguire, M. L., Loeb, D. D., and Zlotnick, A. (2015). The interface between hepatitis B virus capsid proteins affects self-assembly, pregenomic RNA packaging, and reverse transcription. J. Virol 89, 3275–3284. doi: 10.1128/JVI.03545-14
Tanaka, Y., Kanai, F., Ichimura, T., Tateishi, K., Asaoka, Y., Guleng, B., et al. (2006). The hepatitis B virus X protein enhances AP-1 activation through interaction with Jab1. Oncogene 25, 633–642. doi: 10.1038/sj.onc.1209093
Tanaka, Y., Kanai, F., Kawakami, T., Tateishi, K., Ijichi, H., Kawabe, T., et al. (2004). Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis. Biochem. Biophys. Res. Commun. 318, 461–469. doi: 10.1016/j.bbrc.2004.04.046
Tang, H. M., Gao, W. W., Chan, C. P., Cheng, Y., Chaudhary, V., Deng, J. J., et al. (2014). Requirement of CRTC1 coactivator for hepatitis B virus transcription. Nucleic Acids Res. 42, 12455–12468. doi: 10.1093/nar/gku925
Tang, Y., Zhang, Y., Wang, C., Sun, Z., Li, L., Dong, J., et al. (2018). 14-3-3zeta binds to hepatitis B virus protein X and maintains its protein stability in hepatocellular carcinoma cells. Cancer Med. 7, 5543–5553. doi: 10.1002/cam4.1512
Tian, X., Zhao, C., Zhu, H., She, W., Zhang, J., Liu, J., et al. (2010). Hepatitis B virus (HBV) surface antigen interacts with and promotes cyclophilin a secretion: possible link to pathogenesis of HBV infection. J. Virol. 84, 3373–3381. doi: 10.1128/JVI.02555-09
Tian, X., Zhao, F., Sun, W., Zhi, X., Cheng, Z., Zhou, M., et al. (2014). CRTC2 enhances HBV transcription and replication by inducing PGC1alpha expression. Virol. J. 11:30. doi: 10.1186/1743-422X-11-30:10.1186/1743-422X-11-30
Tokusumi, Y., Zhou, S., and Takada, S. (2004). Nuclear respiratory factor 1 plays an essential role in transcriptional initiation from the hepatitis B virus x gene promoter. J. Virol. 78, 10856–10864. doi: 10.1128/JVI.78.20.10856-10864.2004
Tropberger, P., Mercier, A., Robinson, M., Zhong, W., Ganem, D. E., and Holdorf, M. (2015). Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proc. Natl. Acad. Sci. U S A 112, E5715–E5724. doi: 10.1073/pnas.1518090112
Tuttleman, J. S., Pourcel, C., and Summers, J. (1986). Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell 47, 451–460.
Umetsu, T., Inoue, J., Kogure, T., Kakazu, E., Ninomiya, M., Iwata, T., et al. (2018). Inhibitory effect of silibinin on hepatitis B virus entry. Biochem. Biophys. Rep. 14, 20–25. doi: 10.1016/j.bbrep.2018.03.003
Vandenbossche, J., Jessner, W., Van Den Boer, M., Biewenga, J., Berke, J. M., Talloen, W., et al. (2019). Pharmacokinetics, Safety and Tolerability of JNJ-56136379, a Novel Hepatitis B Virus Capsid Assembly Modulator, in Healthy Subjects. Adv. Ther. 36, 2450–2462. doi: 10.1007/s12325-019-01017-1
Voss, T. C., and Hager, G. L. (2014). Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat. Rev. Genet. 15, 69–81. doi: 10.1038/nrg3623
Wang, J., Jia, J., Chen, R., Ding, S., Xu, Q., Zhang, T., et al. (2018). RFX1 participates in doxorubicin-induced hepatitis B virus reactivation. Cancer Med. 7, 2021–2033. doi: 10.1002/cam4.1468
Wang, L. Z., Qihui, Z., Jing, Y., Zhipeng, F., Andrew, Y., and John, G. (2019). PS-074-A first-in-class orally available HBV cccDNA destabilizer ccc_R08 achieved sustainable HBsAg and HBV DNA suppression in the HBV circle mouse model through elimination of cccDNA-like molecules in the mouse liver. J. Hepatol. 70:e48. doi: 10.1016/S0618-8278(19)30086-6
Wang, X., Li, Y., Mao, A., Li, C., Li, Y., and Tien, P. (2010). Hepatitis B virus X protein suppresses virus-triggered IRF3 activation and IFN-beta induction by disrupting the VISA-associated complex. Cell Mol. Immunol. 7, 341–348. doi: 10.1038/cmi.2010.36
Wang, X., Zhou, Y., Sun, L., Chen, W., Li, X., Wang, Q., et al. (2008). Complex formation between heat shock protein 72 and hepatitis B virus X protein in hepatocellular carcinoma tissues. J. Proteome Res. 7, 5133–5137. doi: 10.1021/pr800435g
Wang, Y. P., Liu, F., He, H. W., Han, Y. X., Peng, Z. G., Li, B. W., et al. (2010). Heat stress cognate 70 host protein as a potential drug target against drug resistance in hepatitis B virus. Antimicrob. Agents Chemother. 54, 2070–2077. doi: 10.1128/AAC.01764-09
Wang, Y., Cai, J., Zeng, X., Chen, Y., Yan, W., Ouyang, Y., et al. (2015). Downregulation of toll-like receptor 4 induces suppressive effects on hepatitis B virus-related hepatocellular carcinoma via ERK1/2 signaling. BMC Cancer 15:821. doi: 10.1186/s12885-015-1866-9
Wang, Y., Han, M., Liu, S., Yuan, X., Zhao, J., Lu, H., et al. (2021). S6K1 inhibits HBV replication through inhibiting AMPK-ULK1 pathway and disrupting acetylation modification of H3K27. Life Sci. 265:118848. doi: 10.1016/j.lfs.2020.118848
Waris, G., Huh, K. W., and Siddiqui, A. (2001). Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol. Cell Biol. 21, 7721–7730. doi: 10.1128/MCB.21.22.7721-7730.2001
Watanabe, T., Sorensen, E. M., Naito, A., Schott, M., Kim, S., and Ahlquist, P. (2007). Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc. Natl. Acad. Sci. U S A 104, 10205–10210. doi: 10.1073/pnas.0704000104
Watashi, K., Urban, S., Li, W., and Wakita, T. (2014). NTCP and beyond: opening the door to unveil hepatitis B virus entry. Int. J. Mol. Sci. 15, 2892–2905. doi: 10.3390/ijms15022892
Wei, L., and Ploss, A. (2020). Core components of DNA lagging strand synthesis machinery are essential for hepatitis B virus cccDNA formation. Nat. Microbiol. 5, 715–726. doi: 10.1038/s41564-020-0678-0
Wen, Y., Golubkov, V. S., Strongin, A. Y., Jiang, W., and Reed, J. C. (2008). Interaction of hepatitis B viral oncoprotein with cellular target HBXIP dysregulates centrosome dynamics and mitotic spindle formation. J. Biol. Chem. 283, 2793–2803. doi: 10.1074/jbc.M708419200
Werle-Lapostolle, B., Bowden, S., Locarnini, S., Wursthorn, K., Petersen, J., Lau, G., et al. (2004). Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126, 1750–1758. doi: 10.1053/j.gastro.2004.03.018
Werr, M., and Prange, R. (1998). Role for calnexin and N-linked glycosylation in the assembly and secretion of hepatitis B virus middle envelope protein particles. J. Virol. 72, 778–782.
Wu, T. T., Coates, L., Aldrich, C. E., Summers, J., and Mason, W. S. (1990). In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology 175, 255–261.
Wu, Y. H., Ai, X., Liu, F. Y., Liang, H. F., Zhang, B. X., and Chen, X. P. (2016). c-Jun N-terminal kinase inhibitor favors transforming growth factor-beta to antagonize hepatitis B virus X protein-induced cell growth promotion in hepatocellular carcinoma. Mol. Med. Rep. 13, 1345–1352. doi: 10.3892/mmr.2015.4644
Xia, L., Wang, S., Zhang, H., Yang, Y., Wei, J., Shi, Y., et al. (2020). The HBx and HBc of hepatitis B virus can influence Id1 and Id3 by reducing their transcription and stability. Virus Res. 284:197973. doi: 10.1016/j.virusres.2020.197973
Xian, L., Zhao, J., Wang, J., Fang, Z., Peng, B., Wang, W., et al. (2010). p53 Promotes proteasome-dependent degradation of oncogenic protein HBx by transcription of MDM2. Mol. Biol. Rep. 37, 2935–2940. doi: 10.1007/s11033-009-9855-1
Xiang, A., Ren, F., Lei, X., Zhang, J., Guo, R., Lu, Z., et al. (2015). The hepatitis B virus (HBV) core protein enhances the transcription activation of CRE via the CRE/CREB/CBP pathway. Antiviral. Res. 120, 7–15. doi: 10.1016/j.antiviral.2015.04.013
Xu, F., Song, H., Xiao, Q., Li, N., Zhang, H., Cheng, G., et al. (2019). Type III interferon-induced CBFbeta inhibits HBV replication by hijacking HBx. Cell Mol. Immunol. 16, 357–366. doi: 10.1038/s41423-018-0006-2
Xu, L., Wu, Z., Tan, S., Wang, Z., Lin, Q., Li, X., et al. (2018). Tumor suppressor ZHX2 restricts hepatitis B virus replication via epigenetic and non-epigenetic manners. Antiviral. Res. 153, 114–123. doi: 10.1016/j.antiviral.2018.03.008
Yan, H., Peng, B., He, W., Zhong, G., Qi, Y., Ren, B., et al. (2013). Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J. Virol. 87, 7977–7991. doi: 10.1128/JVI.03540-12
Yan, H., Zhong, G., Xu, G., He, W., Jing, Z., Gao, Z., et al. (2012). Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1:e00049. doi: 10.7554/eLife.00049
Yan, H., Zhong, G., Xu, G., He, W., Jing, Z., Gao, Z., et al. (2014). Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 3:49.
Yang, C. C., Huang, E. Y., Li, H. C., Su, P. Y., and Shih, C. (2014). Nuclear export of human hepatitis B virus core protein and pregenomic RNA depends on the cellular NXF1-p15 machinery. PLoS One 9:e106683. doi: 10.1371/journal.pone.0106683
Yang, S. T., Yen, C. J., Lai, C. H., Lin, Y. J., Chang, K. C., Lee, J. C., et al. (2013). SUMOylated CPAP is required for IKK-mediated NF-kappaB activation and enhances HBx-induced NF-kappaB signaling in HCC. J. Hepatol. 58, 1157–1164. doi: 10.1016/j.jhep.2013.01.025
Yao, J. H., Liu, Z. J., Yi, J. H., Wang, J., and Liu, Y. N. (2018). Hepatitis B Virus X Protein Upregulates Intracellular Calcium Signaling by Binding C-terminal of Orail Protein. Curr. Med. Sci. 38, 26–34. doi: 10.1007/s11596-018-1843-z
Yoo, Y. G., and Lee, M. O. (2004). Hepatitis B virus X protein induces expression of Fas ligand gene through enhancing transcriptional activity of early growth response factor. J. Biol. Chem. 279, 36242–36249. doi: 10.1074/jbc.M401290200
Yoo, Y. G., Na, T. Y., Seo, H. W., Seong, J. K., Park, C. K., Shin, Y. K., et al. (2008). Hepatitis B virus X protein induces the expression of MTA1 and HDAC1, which enhances hypoxia signaling in hepatocellular carcinoma cells. Oncogene 27, 3405–3413. doi: 10.1038/sj.onc.1211000
You, D. G., Cho, Y. Y., Lee, H. R., Lee, J. H., Yu, S. J., Yoon, J. H., et al. (2019). Hepatitis B virus X protein induces size-selective membrane permeabilization through interaction with cardiolipin. Biochim. Biophys. Acta Biomembr. 1861, 729–737. doi: 10.1016/j.bbamem.2019.01.006
Yu, H. B., Cheng, S. T., Ren, F., Chen, Y., Shi, X. F., Wong, V. K. W., et al. (2021). SIRT7 restricts HBV transcription and replication through catalyzing desuccinylation of histone H3 associated with cccDNA minichromosome. Clin. Sci. 135, 1505–1522. doi: 10.1042/CS20210392
Yu, X., and Mertz, J. E. (2003). Distinct modes of regulation of transcription of hepatitis B virus by the nuclear receptors HNF4alpha and COUP-TF1. J. Virol. 77, 2489–2499.
Yu, Y., He, Z., Cao, Y., Tang, H., and Huang, F. (2016). TAGLN2, a novel regulator involved in Hepatitis B virus transcription and replication. Biochem. Biophys. Res. Commun. 477, 1051–1058. doi: 10.1016/j.bbrc.2016.07.034
Yuan, Y., Tian, C., Gong, Q., Shang, L., Zhang, Y., Jin, C., et al. (2015). Interactome map reveals phospholipid scramblase 1 as a novel regulator of hepatitis B virus x protein. J. Proteome Res. 14, 154–163. doi: 10.1021/pr500943x
Zajakina, A., Bruvere, R., and Kozlovska, T. (2014). A Semliki Forest virus expression system as a model for investigating the nuclear import and export of hepatitis B virus nucleocapsid protein. Acta Virol. 58, 173–179. doi: 10.4149/av_2014_02_173
Zhang, J. L., Zhao, W. G., Wu, K. L., Wang, K., Zhang, X., Gu, C. F., et al. (2005). Human hepatitis B virus X protein promotes cell proliferation and inhibits cell apoptosis through interacting with a serine protease Hepsin. Arch. Virol. 150, 721–741. doi: 10.1007/s00705-004-0446-0
Zhang, S., Lin, R., Zhou, Z., Wen, S., Lin, L., Chen, S., et al. (2006). Macrophage migration inhibitory factor interacts with HBx and inhibits its apoptotic activity. Biochem. Biophys. Res. Commun. 342, 671–679. doi: 10.1016/j.bbrc.2006.01.180
Zhang, T., Xie, N., He, W., Liu, R., Lei, Y., Chen, Y., et al. (2013). An integrated proteomics and bioinformatics analyses of hepatitis B virus X interacting proteins and identification of a novel interactor apoA-I. J. Proteomics 84, 92–105. doi: 10.1016/j.jprot.2013.03.028
Zhang, T., Zhang, J., You, X., Liu, Q., Du, Y., Gao, Y., et al. (2012). Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology 56, 2051–2059. doi: 10.1002/hep.25899
Zhang, W., Chen, J., Wu, M., Zhang, X., Zhang, M., Yue, L., et al. (2017). PRMT5 restricts hepatitis B virus replication through epigenetic repression of covalently closed circular DNA transcription and interference with pregenomic RNA encapsidation. Hepatology 66, 398–415. doi: 10.1002/hep.29133
Zhang, W., Zhang, X., Tian, C., Wang, T., Sarkis, P. T., Fang, Y., et al. (2008). Cytidine deaminase APOBEC3B interacts with heterogeneous nuclear ribonucleoprotein K and suppresses hepatitis B virus expression. Cell Microbiol. 10, 112–121. doi: 10.1111/j.1462-5822.2007.01020.x
Zhang, Y. Y., Zhang, B. H., Theele, D., Litwin, S., Toll, E., and Summers, J. (2003). Single-cell analysis of covalently closed circular DNA copy numbers in a hepadnavirus-infected liver. Proc. Natl. Acad. Sci. U S A 100, 12372–12377. doi: 10.1073/pnas.2033898100
Zhang, Y., He, S., Guo, J. J., Peng, H., Fan, J. H., and Li, Q. L. (2017). Retinoid X Receptor alpha-Dependent HBV Minichromosome Remodeling and Viral Replication. Ann. Hepatol. 16, 501–509. doi: 10.5604/01.3001.0010.0275
Zhang, Y., Liu, J., Liu, H., He, Y., Yi, R., Niu, Y., et al. (2015). Comparative study of the different activities of hepatitis B virus whole-X protein and HBx in hepatocarcinogenesis by proteomics and bioinformatics analysis. Arch Virol 160, 1645–1656. doi: 10.1007/s00705-015-2421-3
Zhang, Y., Mao, R., Yan, R., Cai, D., Zhang, Y., Zhu, H., et al. (2014). Transcription of hepatitis B virus covalently closed circular DNA is regulated by CpG methylation during chronic infection. PLoS One 9:e110442. doi: 10.1371/journal.pone.0110442
Zhang, Z., Torii, N., Furusaka, A., Malayaman, N., Hu, Z., and Liang, T. J. (2000). Structural and functional characterization of interaction between hepatitis B virus X protein and the proteasome complex. J. Biol. Chem. 275, 15157–15165. doi: 10.1074/jbc.M910378199
Zhao, D., Wang, X., Lou, G., Peng, G., Li, J., Zhu, H., et al. (2010). APOBEC3G directly binds Hepatitis B virus core protein in cell and cell free systems. Virus Res. 151, 213–219. doi: 10.1016/j.virusres.2010.05.009
Zheng, D. L., Zhang, L., Cheng, N., Xu, X., Deng, Q., Teng, X. M., et al. (2009). Epigenetic modification induced by hepatitis B virus X protein via interaction with de novo DNA methyltransferase DNMT3A. J. Hepatol. 50, 377–387. doi: 10.1016/j.jhep.2008.10.019
Zheng, Y., Chen, W. L., Ma, W. L., Chang, C., and Ou, J. H. (2007). Enhancement of gene transactivation activity of androgen receptor by hepatitis B virus X protein. Virology 363, 454–461. doi: 10.1016/j.virol.2007.01.040:10.1016/j.virol.2007.01.040
Zheng, Y., Ming, P., Zhu, C., Si, Y., Xu, S., Chen, A., et al. (2019). Hepatitis B virus X protein-induced SH2 domain-containing 5(SH2D5) expression promotes hepatoma cell growth via an SH2D5-transketolase interaction. J. Biol. Chem. 2019:5739. doi: 10.1074/jbc.RA118.005739
Zhou, D. X., and Yen, T. S. (1991). The ubiquitous transcription factor Oct-1 and the liver-specific factor HNF-1 are both required to activate transcription of a hepatitis B virus promoter. Mol. Cell Biol. 11, 1353–1359.
Zhou, J., Tan, T., Tian, Y., Zheng, B., Ou, J. H., Huang, E. J., et al. (2011). Kruppel-like factor 15 activates hepatitis B virus gene expression and replication. Hepatology 54, 109–121. doi: 10.1002/hep.24362
Zlotnick, A., Venkatakrishnan, B., Tan, Z., Lewellyn, E., Turner, W., and Francis, S. (2015). Core protein: A pleiotropic keystone in the HBV lifecycle. Antiviral. Res. 121, 82–93. doi: 10.1016/j.antiviral.2015.06.020
Keywords: hepatitis B virus, interactome, viral-host life cycle, cccDNA, HBx, HBc
Citation: Van Damme E, Vanhove J, Severyn B, Verschueren L and Pauwels F (2021) The Hepatitis B Virus Interactome: A Comprehensive Overview. Front. Microbiol. 12:724877. doi: 10.3389/fmicb.2021.724877
Received: 14 June 2021; Accepted: 17 August 2021;
Published: 16 September 2021.
Edited by:
Julie Lucifora, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Yuchen Xia, Wuhan University, ChinaBarbara Testoni, Institut National de la Santé et de la Recherche Médicale (INSERM), France
Gregor Ebert, Technical University of Munich, Germany
Copyright © 2021 Van Damme, Vanhove, Severyn, Verschueren and Pauwels. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ellen Van Damme, RVZBTkRBTU1AaXRzLmpuai5jb20=
†These authors have contributed equally to this work and share first authorship
 Ellen Van Damme
Ellen Van Damme Jolien Vanhove1,2†
Jolien Vanhove1,2† Bryan Severyn
Bryan Severyn