
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 06 September 2021
Sec. Food Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.720485
This article is part of the Research Topic Rapid and Cost-effective Technologies to Detect the Pathogens in Food and Environment View all 14 articles
 Tuhong Wang1†
Tuhong Wang1† Haojun Ji1,2†
Haojun Ji1,2† Yongting Yu1†‡
Yongting Yu1†‡ Xiaojie Wang2
Xiaojie Wang2 Yi Cheng1
Yi Cheng1 Zhimin Li1
Zhimin Li1 Jia Chen1
Jia Chen1 Litao Guo1
Litao Guo1 Jianping Xu1,3*
Jianping Xu1,3* Chunsheng Gao1*
Chunsheng Gao1*Brown root rot caused by Phytopythium vexans is a new destructive root disease on many plants such as Gingko, Citrus, kiwifruit, and ramie. The establishment of loop-mediated isothermal amplification (LAMP) technology for detecting P. vexans can help monitor and control brown root rot quickly, efficiently, and accurately. LAMP technology is known for its simplicity, sensitivity, and speed; and it does not require any specialized equipment – a water bath or a thermoblock is sufficient for isothermal amplifications. LAMP products can be visualized by using hydroxy naphthol blue (HNB) dye or agarose gel electrophoresis. In this study, by searching and comparing the internal transcribed spacer (ITS) sequences of P. vexans and the related species in oomycete genera Pythium, Phytopythium, and Phytophthora, we designed specific primers targeting the ITS gene region of P. vexans. Using HNB dye, we established a LAMP technique for rapid detection of P. vexans by visible color change. In addition, we optimized the protocol to enhance both sensitivity and specificity for P. vexans detection. Under the optimized condition, our protocol based on LAMP technology could detect as low as 24 copies of the P. vexans genomic DNA, which is ∼100 times more sensitive than conventional PCR. This method can successfully detect P. vexans using cell suspensions from P. vexans – infected ramie root tissues.
Oomycetes belong to the Kingdom Stramenopila (Baldauf et al., 2000; Yoon et al., 2002), which includes diverse microorganisms living in marine, freshwater, and terrestrial environments (Sparrow, 1960; Karling, 1981). Phytopythium is a genus in the family Pythiaceae of oomycete. At present, there are over 20 species in this genus, with most of them showing strong association with the freshwater environment (Tkaczyk, 2020) and being saprophytes. However, a few Phytopythium species have been observed to cause diseases in plants, such as Phytopythium litorale infecting the Old-World sycamore Platanus orientalis (Derviş et al., 2020); Phytopythium helicoides causing citrus fruit and root rot (Chen X. R. et al., 2016); and Phytopythium vexans (de Bary) (Lévesque et al., 2008; Bala et al., 2010) causing root rot in many economically important plants (Adhikari et al., 2013; de Cock et al., 2015).
Phytopythium vexans, formerly known as Pythium vexans, is found in many parts of the world. In recent years, diseases caused by P. vexans have been frequently reported. In Africa, the species was identified to infect citrus trees and grapevine (Spies et al., 2009; Benfradj et al., 2017; Langenhoven et al., 2018). In the United States, P. vexans was reported to cause root and crown rot of woody ornamentals (flowering cherry, Ginkgo, and red maple) (Panth et al., 2020; Baysal-Gurel et al., 2021). In China, P. vexans has been reported to cause diseases on ramie, tobacco, rubber trees, and dendrobium (Zeng et al., 2005; Tao et al., 2011; Bian et al., 2015; Chen B. et al., 2016; Yu et al., 2016). In addition, P. vexans can cause patch canker, damping-off, and crown rot, stem rot, and root rot in many economically important fruit trees, such as durian, kiwifruit, apple, and avocado in Vietnam, Turkey, Morocco, and Mexico, respectively (Polat et al., 2017; Hernández et al., 2019; Jabiri et al., 2020; Thao et al., 2020). The increasing reports of diseases caused by P. vexans suggest that this pathogen has the potential to cause large-scale disease outbreaks across multiple plants in the future.
Pathogen detection is a fundamental part of disease control. At present, the identification method of P. vexans mainly relies on visible disease symptoms and microbial cultures. However, due to (i) difficulties in isolation and cultivation of Phytopythium spp. and Pythium spp., (ii) similarities in hyphal and spore morphologies among related Phytopythium species (Schroeder et al., 2006), and (iii) similarities in disease symptoms caused by P. vexans and many other disease agents belonging to genera Phytopythium and Pythium, and even plant fungal pathogens, it’s been difficult to identify P. vexans based on disease symptom and culture characteristics as the causal agent of diseases (Li et al., 2018). Indeed, traditional identification methods require a wealth of knowledge and experience, is time-consuming and laborious, and lacks accuracy, all of which contribute to making them difficult to apply for the analysis and identification of large samples (Wang et al., 2020). In recent years, with the rapid development of molecular biological technology, a variety of detection technologies based on polymerase chain reaction (PCR) and real-time fluorescent quantitative PCR have been developed. These methods have become the main means for rapid, accurate and specific identification of pathogens, avoiding the shortcomings of uncertainty in traditional morphology-based identifications (Schroeder et al., 2013). However, most current molecular detection technologies require professional instruments, reagents, and strict environmental conditions in a lab setting. The instruments and reagents are often expensive, the operation requirements are high, the process is complex, and the detection time is still long. These shortcomings make most of these methods not suitable in the field or in resource-limited laboratories.
Loop-mediated isothermal amplification (LAMP) is a molecular detection technology with limited technical and instrumental requirements. It uses four to six specific primers to amplify the target DNA in a short time under constant temperature by using Bst DNA polymerase (Notomi et al., 2000). The reaction products can be detected using multiple methods, of which gel electrophoresis is the most accurate and generates typical ladder-like banding patterns. The amplified products can also be detected visually by adding different dyes, including SYBR green, hydroxynaphthol blue (HNB), or calcein, which can be seen with the naked eye (Mori et al., 2001; Goto et al., 2009). The advantages of LAMP include being simple, having easy detection of products, and low cost, as well as being suitable for rapid detection of pathogens in both lab and field conditions (Parida et al., 2010). So far, LAMP technology has been widely used in the rapid detection of oomycete, fungi, bacteria, viruses, nematodes, including many plant and animal pathogens (Liu et al., 2010; Fukuta et al., 2013a, b; Feng L. et al., 2015; Wei et al., 2016; Zeng et al., 2018). However, a LAMP rapid detection method for P. vexans has not been reported.
The objective of this study is to develop a LAMP assay for the detection of P. vexans. Here, we selected the internal transcribed spacer (ITS) regions of the nuclear ribosomal RNA gene cluster as the detection target sequence. We designed and evaluated the specificity of the primers, optimized the LAMP protocol, and determined the sensitivity of this method. Using this protocol, we successfully identified P. vexans in laboratory-infected ramie samples. Our method should facilitate the rapid diagnosis of P. vexans in agricultural and forestry environments.
Although Phytopythium is a relatively newly defined genus different from the Pythium and Phytophthora genera, organisms in all three genera have similar micromorphology and growth patterns. Therefore, in this study, 20 representative isolates of Phytopythium, Pythium, and Phytophthora, as well as of selected Fusarium species that are also common causes of brown root rot in plants (Table 1) were selected for this study. The 20 isolates representing 19 brown root rot species were collected from different provinces in China and were maintained at the Institute of Bast Fiber Crops, Chinese Academy of Agricultural Sciences (Changsha, China). All isolates were grown on potato dextrose agar (PDA) medium at 25°C for 3–5 days before extracting DNA. Mycelia were harvested, collected, and stored at −20°C. Mycelial DNA was extracted by a Rapid Extraction Kit for Fungi Genomic DNA (Aidlab, Beijing, China) following the user’s manual. Conventional PCR amplification was carried out with the specific primers of P. vexans (PvF1/PvR1) (Spies et al., 2011b). The ITS region (ITS1, 5.8S, and ITS2) of all 20 isolates was amplified and sequenced using primers V9G (5′-TTACGTCCCTGCCCTTTGTA-3′) and LS266 (5′-GCATTCCCAAACAACTCGACTC-3′) (van den Ende and de Hoog, 1999).
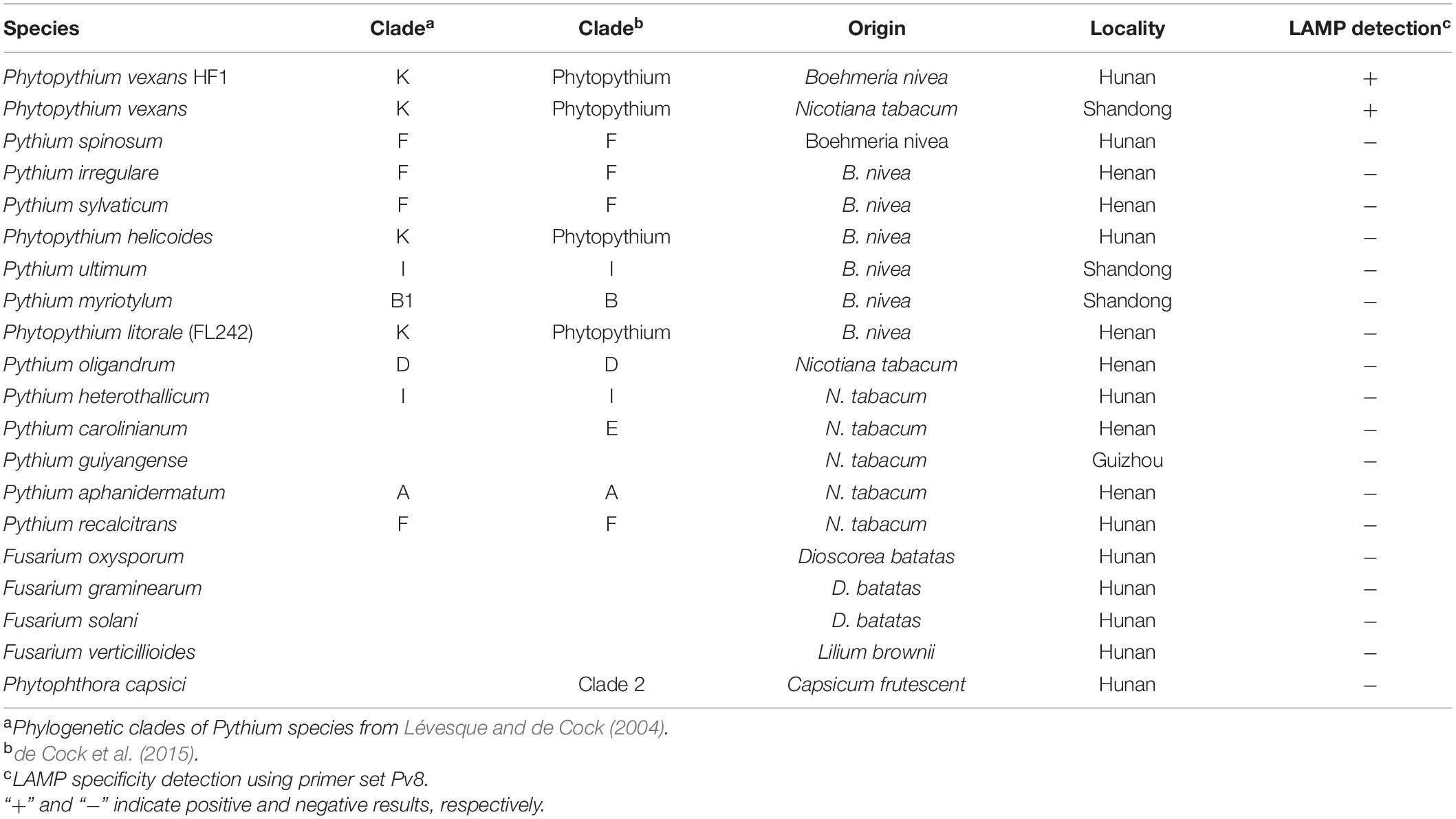
Table 1. Isolates used to test the specificity of the loop-mediated isothermal amplification (LAMP) method and the assay results.
In this study, the ITS sequence was selected as the target gene for LAMP assay. To identify the portions of ITS sequence that are unique to P. vexans, we performed multiple sequence alignment including sequences of tested isolates as well as sequences of type strains of P. vexans (GenBank accession number: HQ643400.2), P. irregulare (AY598702.2), Pythium ultimum (AY598657.2), Pythium spinosum (AY598701.2), Pythium aphanidermatum (AY598622.2), Pythium myriotylum (AY598678.2), P. litorale (NNIBRFG9359), Pythium sylvaticum (AY598645), P. helicoides (AB108026), Pythium recalcitrans (KJ716861), Pythium oligandrum (AY598618), Pythium heterothallicum (AY598654), Pythium carolinianum (HQ643484.1), Pythium guiyangense (AY987037), and Phytophthora capsici (FN257939) that were retrieved from the NCBI databases. The program ClustalW21 (Larkin et al., 2007) was used to perform multiple sequence alignment to find the regions within ITS which were specific for P. vexans. Based on the aligned sequences, a set of primers was designed. The LAMP primers were designed using PrimerExplore V5,2 all the parameters were set by default, and primers were synthesized by TSINGKE Biotechnology Co. Ltd. Nine primers sets were tested (Supplementary Table 1), however, the best primers (Pv8 primers set) were screened by specificity and sensitivity test. In addition, a phylogenetic tree was constructed with the maximum likelihood method implemented in MEGA 7.0 (Kumar et al., 2016). A Poisson correction was used for multiple substitution models and a pairwise deletion was used for handling missing data. Statistical support for individual branches of the phylogenetic tree showing taxa relationships was assessed with 1,000 bootstrap replicates.
The LAMP reaction was carried out in a total volume of 25 μL containing 1.6 μM of each FIP and BIP primer, 0.2 μM of each F3 and B3 primer, 20 mM Tris–HCL (pH 8.8), 10 mM KCl, 0.1% Tween20, 0.8 M betaine (Shanghai Yuanye Bio-Technology, Shanghai, China), 8 mM MgSO4, 10 mM (NH4)2SO4, 1.4 mM dNTPs, and 8U Bst DNA polymerase large fragment (New England Biolabs Japan, Tokyo, Japan), 180 μM HNB (Macklin, Shanghai, China), and 2 μL template DNA (Feng W. et al., 2015). The mixture was incubated at 64°C for 60 min to perform the LAMP reaction. The genomic DNA template of P. vexans was diluted in a 10-fold series after concentration determination, so that the DNA concentration gradient was 100 ng/μL, 10 ng/μL, 1 ng/μL, 100 pg/μL, 10 pg/μL, 1 pg/μL, 100 fg/μL, 10 fg/μL, and 1 fg/μL. Among them, 1 pg DNA contained 23.4 copies of the P. vexans genome.
Based on the above LAMP reaction system, the reaction time and temperature, the concentration of MgSO4, betaine, dNTPs were optimized using a gradient of values. Specifically, the reaction temperatures were set at 60, 62, 64, 66, and 68°C; the reaction times were set at 10, 20, 30, 40, 50, 60, 70, and 80 min; the concentrations of betaine were 0, 0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 M; of MgSO4 were 0,2, 4, 6, 8, and 10 mM; and of dNTP were 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, and 1.6 mM. In all optimizations, a negative control was set up with sterilized distilled water instead of DNA template. The LAMP products were visualized by adding HNB before amplification. Samples that turned blue were considered positive, while samples that remained violet were negative. In addition, the LAMP products were detected through electrophoresis in 1.5% agarose gel by staining with GelRed (TSINGKE Biological Technology, Beijing, China), and positive reactions resulted in a ladder-like banding pattern. The reaction was performed with three biological repeats.
Using the selected primers, the genomic DNA of 20 strains were used as templates, and sterilized distilled water instead of DNA template was used as negative control. The optimized LAMP reaction system was used for amplification. After the reaction, a color change in the products was assessed with the naked eyes of at least two independent investigators.
To evaluate the application of the developed LAMP protocol as a tool for the diagnosis of P. vexans in infected plants, ramie cultivar “Zhongzhu No. 1” roots were inoculated as described below. Two-week-old seedlings (approximately 20–30 cm tall) were prepared via the cutting propagation method (Yu et al., 2015). To inoculate ramie plants, mycelial disks (8 mm in diameter) obtained from 3-day-old culture of each of the 20 isolates of Phytopythium spp., Pythium spp., and Phytophthora spp. on PDA were placed upside-down on the roots. Plants inoculated with agar disks without mycelia were used as controls. Both the inoculated and control plants were grown in the greenhouse at 26 ± 1°C with a 12 h photoperiod. After 10 days, tissues surrounding the infected region was harvested for DNA extraction by a Rapid Extraction Kit for Fungi Genomic DNA (Aidlab, Beijing, China). These DNA samples were evaluated using the developed LAMP technique.
In total, two P. vexans isolates, two isolates of two other Phytopythium species, 11 isolates of 11 Pythium species, one P. capsici isolate, and four isolates of four Fusarium species obtained from different provinces in China (Table 1) were used to test the specificity of the LAMP primers and reaction conditions. All tested strains were first analyzed with the above-mentioned P. vexans diagnostic primers PvF1 and PvR1, a reported specific primer of P. vexans. The results showed that lanes 1 and 2 are strains of P. vexans, and the other tested strains were not P. vexans (Figure 1). For all 20 isolates, their species identities were confirmed based on their ITS sequences. Based on the ITS sequences of type strains of these species, we constructed a phylogeny among the tested species (Supplementary Figure 1). The phylogenetic relationships among these strains and species are overall consistent with what has been described previously for these organisms. Specifically, the fungal genus Fusarium is distinct from the oomycetes. Among the three oomycete genera, the genus Phytophthora was diverged first, followed by Phytopythium and Pythium (Supplementary Figure 1).
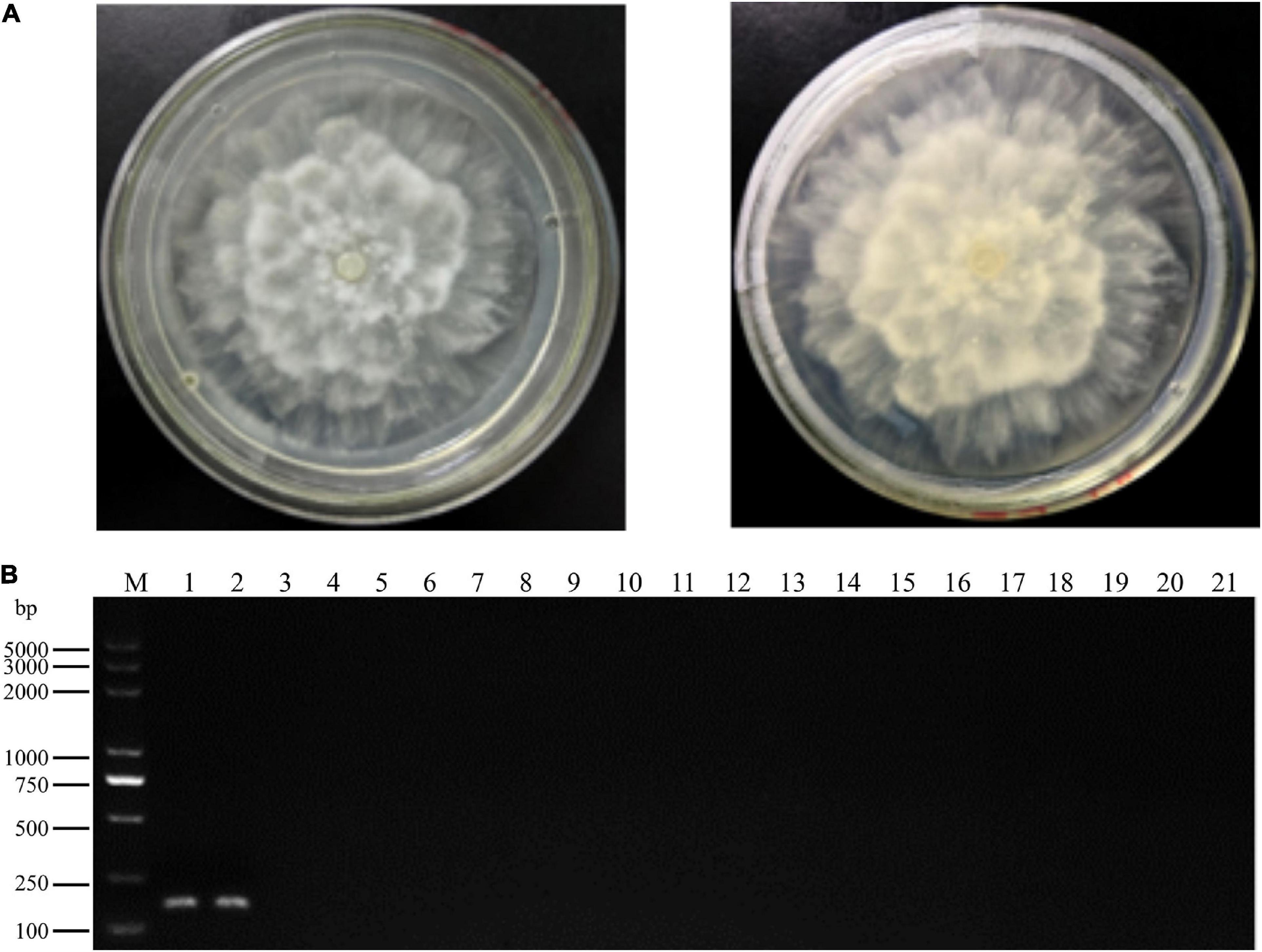
Figure 1. Colony morphology and specific polymerase chain reaction (PCR) primer detection of Phytopythium vexans. (A) Colony morphology of P. vexans. (B) Confirmation of primer specificity for P. vexans, lanes 1 and 2 are strains of P. vexans; lanes 3–20 correspond to the following species Pythium spinosum, Pythium irregulare, Pythium sylvaticum, Phytopythium helicoides, Pythium ultimum, Pythium myriotylum, Phytopythium litorale, Pythium oligandrum, Pythium heterothallicum, Pythium carolinianum, Pythium guiyangense, Pythium aphanidermatum, Pythium recalcitrans, Fusarium oxysporum, Fusarium graminearum, Fusarium solani, Fusarium verticillioides, and Phytophthora capsici, respectively; lane 21 is a negative control.
To identify P. vexans -specific LAMP primers at the ITS region, multiple sequence alignment of ITS sequences between P. vexans and other 18 isolates was carried out. The variable regions of ITS between P. vexans and other isolates were found (Figure 2). Based on the sequence variability patterns, nine primer sets were designed using the online tool PrimerExplore V5 (Supplementary Table 1). Then, according to the LAMP amplification system and procedure, reactions were set up for all the 20 isolates. The specificity and sensitivity of various primer combinations were compared. Finally, among these nine sets of primer combinations, the Pv8 set consists of four core primers Pv8F3 (5′-CGTGTAGTCGTCGGTTGTT-3′), Pv8B3 (5′-CGCAAATCGAGCAATCCACT-3′), Pv8FIP (5′-CGCGTCCGACTTTAAAGGGACTTGCAGATGTGAGGTTG TCTC-3′), and Pv8BIP (5′-GTTTTGTGCTTGATGGGGTG CGGCCATCGCCAAAGGTCAC-3′) performed the best and was the most consistent for detecting P. vexans (Figure 2). Using the Pv8 primer combinations, positive amplifications were obtained only with the two P. vexans isolates in Table 1 while no amplification product (Figure 3A) nor color change (Figure 3B) was observed for the remaining 18 isolates.
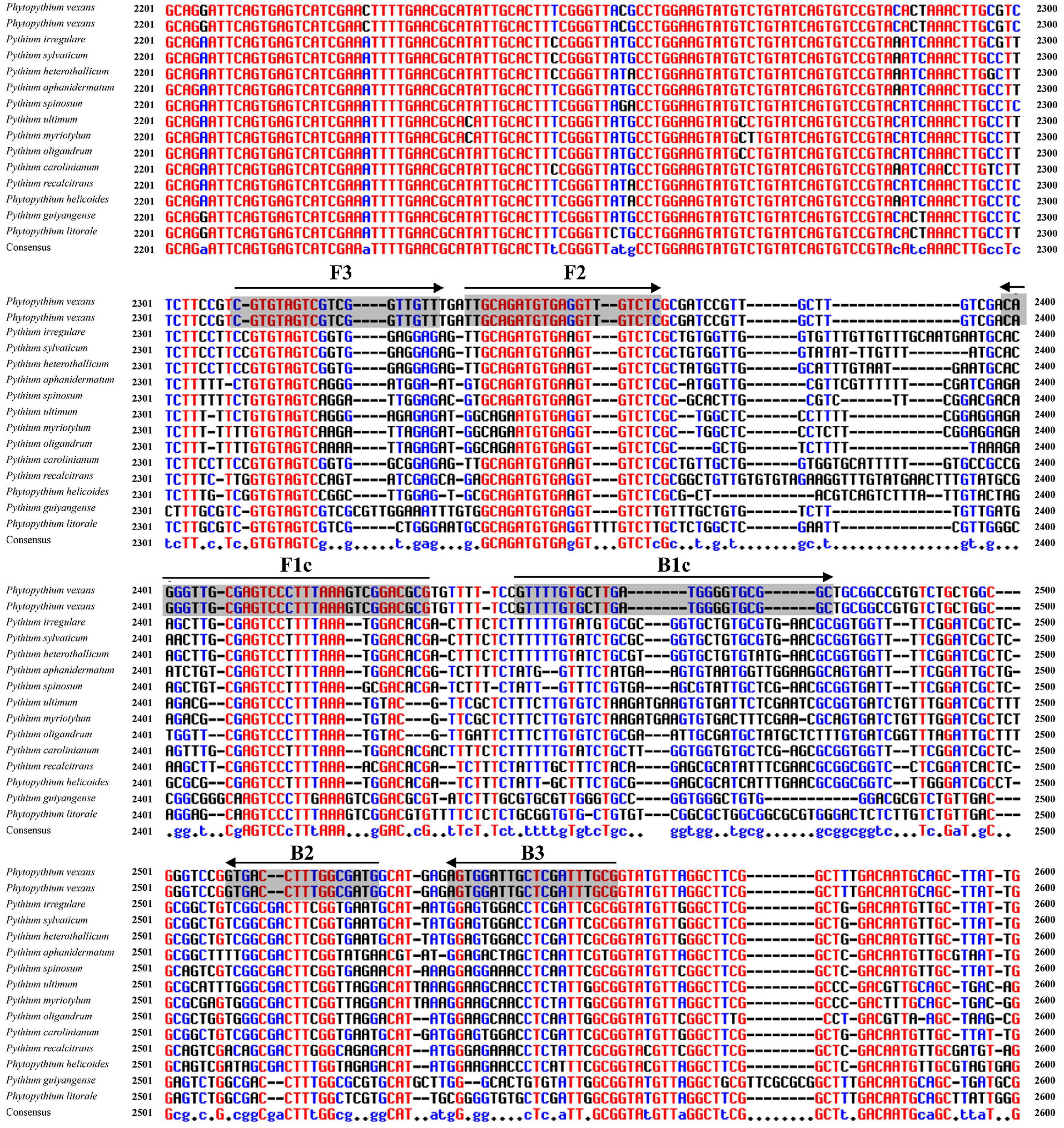
Figure 2. Nucleotide sequence alignment of internal transcribed spacer (ITS) sequences from P. vexans and closely related isolates. Partial sequences of ITS and the location of the Pv8 set consists of four loop-mediated isothermal amplification (LAMP) core primers [Pv8F3, Pv8B3, Pv8FIP (F1c-F2), and Pv8BIP (B1c-B2)] are shown. Arrows indicate the 5′->3′ direction of primer extension during amplification.
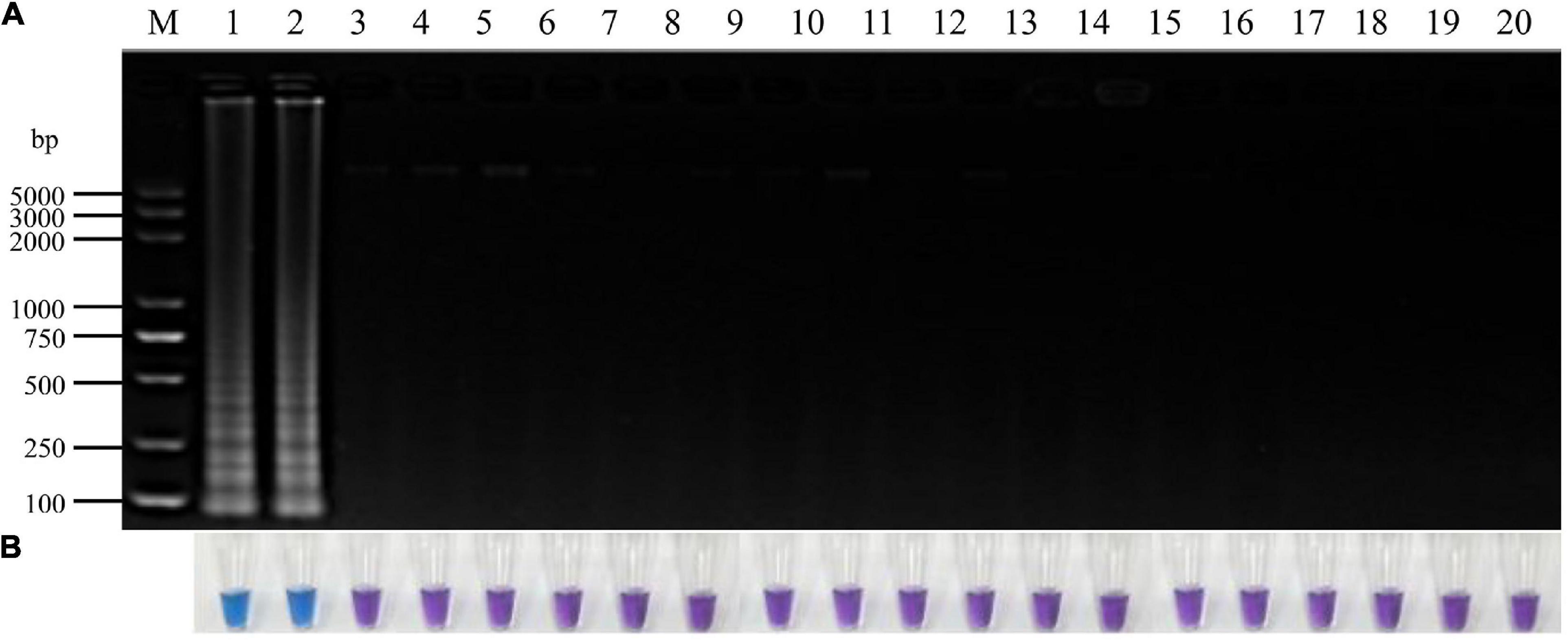
Figure 3. Specificity of LAMP detection of P. vexans. (A) Detection of LAMP products by 2% agarose gel electrophoresis. (B) Detection of LAMP products detected by HNB, 1 and 2, P. vexans; 3–20 indicate P. spinosum, P. irregulare, P. sylvaticum, P. helicoides, P. ultimum, P. myriotylum, P. litorale, P. oligandrum, P. heterothallicum, P. carolinianum, P. guiyangense, P. aphanidermatum, P. recalcitrans, F. oxysporum, F. graminearum, F. solani, F. verticillioides, and P. capsici, respectively.
A 25 μL LAMP reaction system was established with P. vexans DNA as template for optimizing the reaction temperature and time, and the concentrations of dNTPs, MgSO4 and betaine in the system. The evaluated reaction temperatures were set at 60, 62, 64, 66, and 68°C; the reaction times were set at 10, 20, 30, 40, 50, 60, 70, and 80 min; the concentrations of betaine were 0, 0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 M; of MgSO4 were 0, 2, 4, 6, 8, and 10 mM; and of dNTP were 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, and 1.6 mM. In each series of amplifications, we included a negative control where pure sterilized distilled water without any DNA template was used. The results showed that under the recommended LAMP condition, the amplification products could be detected between reaction time 20–80 min. However, the brightest amplification products and the amplification efficiency was reached at a reaction time of 60 min (Figure 4A). Thus, we chose 60 min as the reaction time to observe the LAMP amplified products, and with coloration intensity from HNB staining and/or DNA band brightness through agarose gel electrophoresis as indicators of amplification efficiency in each reaction. Among the tested temperature range 60–68°C, we found little difference among the five temperature treatments in both color change and in the amounts of amplified products as shown on the electrophoretic gel. The temperature experiments indicated that the LAMP reaction condition was robust in the 60–68°C temperature range; therefore, for the following experiments, we chose the optimum temperature of Bst DNA polymerase, i.e., at 64°C (Figure 4B). Among the concentrations of dNTP from 0.2 to 1.6 mM, the amplification products could all be detected, but the highest amplification product was observed at the dNTP concentration of 1.4 mM (Figure 4C). Similar to the limited effect of dNTP concentration change, the change of concentration had relatively little effect on amplification efficiency, but the highest amplification efficiency was observed at the concentration of 6.0 mM MgSO4 (Figure 4D). In contrast to MgSO4, where no MgSO4 resulted in no amplification product, betaine was found to be not essential in our LAMP protocol. However, because betaine has the effect of stabilizing enzyme activity and promoting melting (Rees et al., 1993; Notomi et al., 2000), to improve the stability of the reaction system, especially for field applications, 0.8 M betaine where the color change was the most obvious was added to the system (Figure 4E). In conclusion, the optimal LAMP reaction system was determined as follows: 1.6 μM FIP/BIP, 0.2 μM F3/B3, and 8U Bst DNA polymerase, 1× isothermal amplification buffer [20 mM Tris–HCL (pH 8.8), 10 mM (NH4)2SO4, 50 mM KCl, 0.1% Tween20], 6 mM MgSO4, 1.4 mM dNTPs, 0.8 M betaine, 180 μM HNB, 2 μL DNA template in a total volume of 25 μL with the final volume adjusted with sterilized distilled water, with the reaction temperature of 64°C for 60 min.
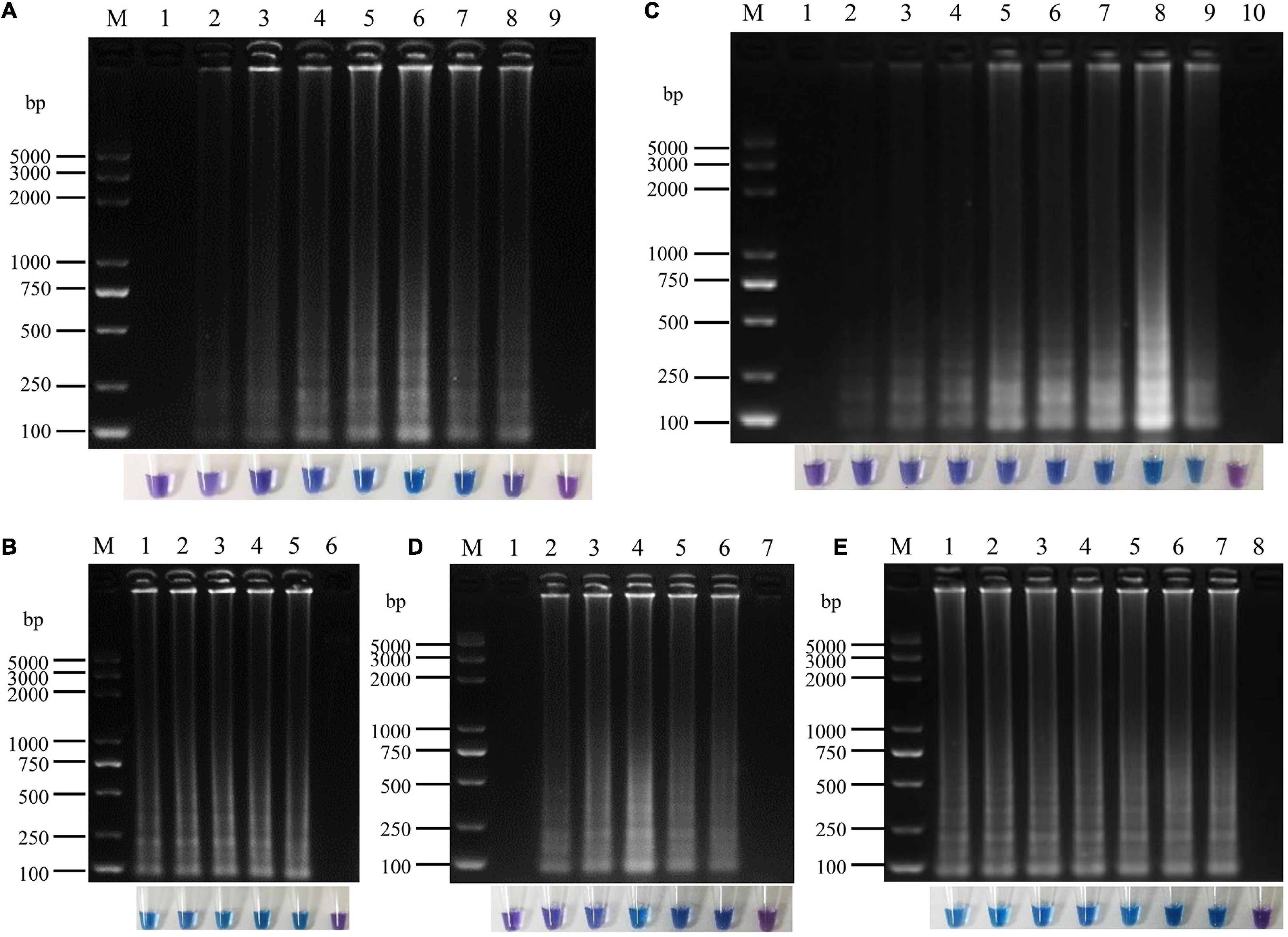
Figure 4. Optimization of loop-mediated isothermal amplification (LAMP) reaction system for P. vexans. (A) The reaction time of lanes 1–8 were 10, 20, 30, 40, 50, 60, 70, and 80 min, lane 9 was negative control. (B) The reaction temperature of lanes 1–5 were 60, 62, 64, 66, and 68°, lane 6 was negative control. (C) Lanes 1–9 corresponded to 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, and 1.6 mM dNTPs, respectively, and lane 10 was negative control. (D) Lanes 1–6 indicated that the concentrations of MgSO4 were 0, 2, 4, 6, 8, and 10 mM, respectively, lane 7 was negative control. (E) Lanes 1–7 indicated that the concentrations of betaine were 0, 0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 M, lane 8 was negative control. In all optimizations, a negative control was set up that contained pure sterilized distilled water without any template DNA.
To determine the sensitivity of primer set Pv8, ten-fold serial dilutions of P. vexans genomic DNA were tested. The DNA template concentrations ranged from 10 ng/μL to 1 fg/μL. The detection limit of the conventional PCR assay using the specific primers Pv8F3 and Pv8R3 was 100 pg/μL DNA (∼2,350 copy of the P. vexans genome) (Figure 5A). In comparison, as indicated by a color change and DNA amplification of the reaction products, the lowest limit of P. vexans detection was 1 pg/μL DNA (∼24 copy of the P. vexans genome) for the LAMP assay (Figures 5B,C). This indicates that the LAMP assay has a wider dynamic range, with a sensitivity at least 100 times greater than that of conventional PCR for the detection of P. vexans.
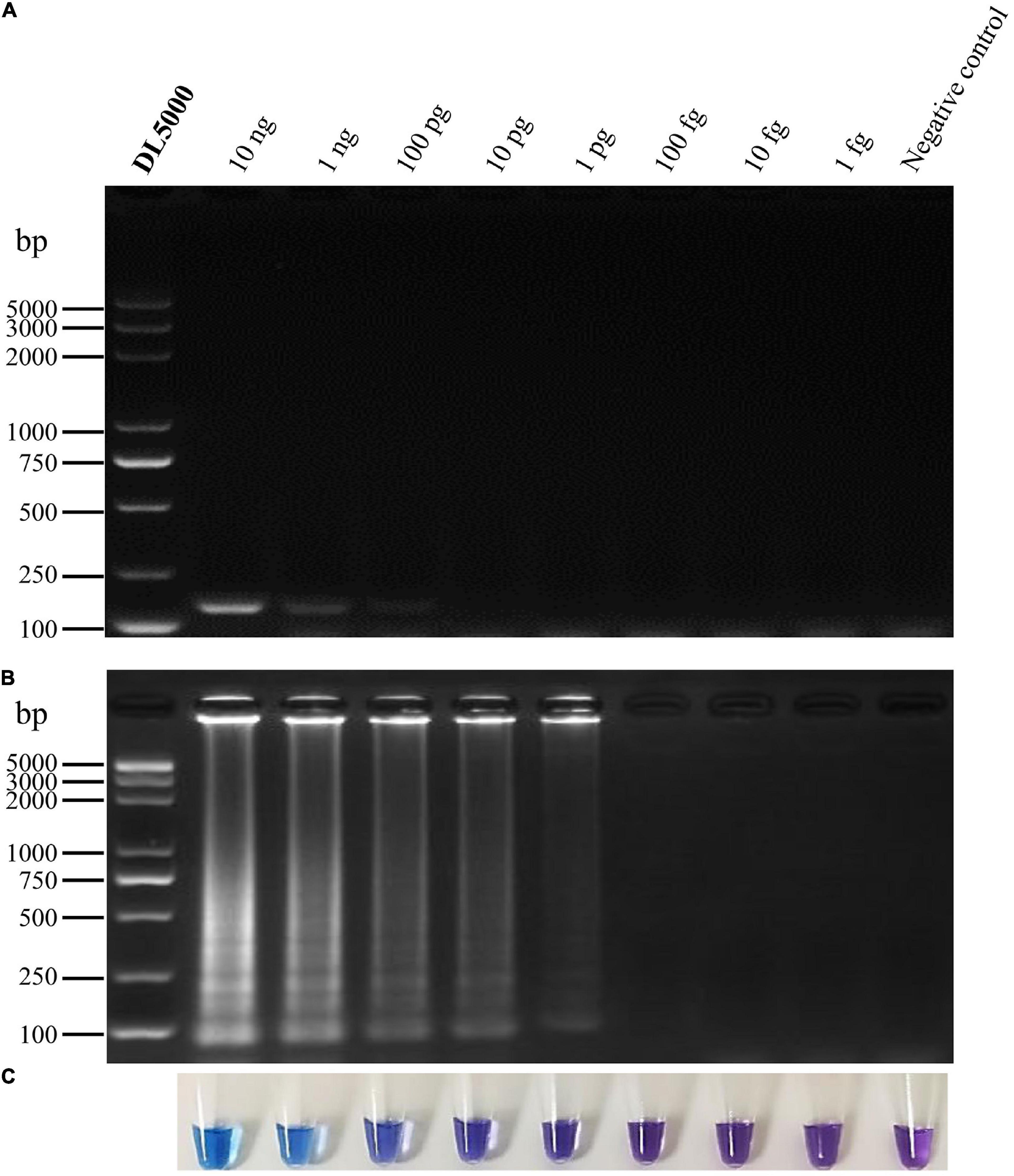
Figure 5. Sensitivity of conventional PCR and the LAMP assays for detecting P. vexans. (A) Detection of conventional PCR products by agarose gel electrophoresis. (B) Detection of LAMP products by agarose gel electrophoresis. (C) Detection of LAMP products using HNB as a visual indicator.
All 15 Phytopythium and Pythium isolates were inoculated onto the “Zhongzhu No. 1” cultivar of ramie plants. After 10 days at 25°C, the fibrous roots of all inoculated plants turned red-brown surrounding the inoculation site. However, no symptom was observed on the roots of negative control plants (Supplementary Figure 2). Genomic DNA was extracted from the infected region and subjected to the LAMP reaction. The results of electrophoresis showed that, except for P. vexans, no DNA bands were produced in the DNA system of ramie roots infected by other 13 tested pathogens (Figure 6A). The results were confirmed based on color change as shown in Figure 6B. Both assay systems indicate that LAMP amplification reaction using primer set Pv8 can accurately detect P. vexans in ramie roots.
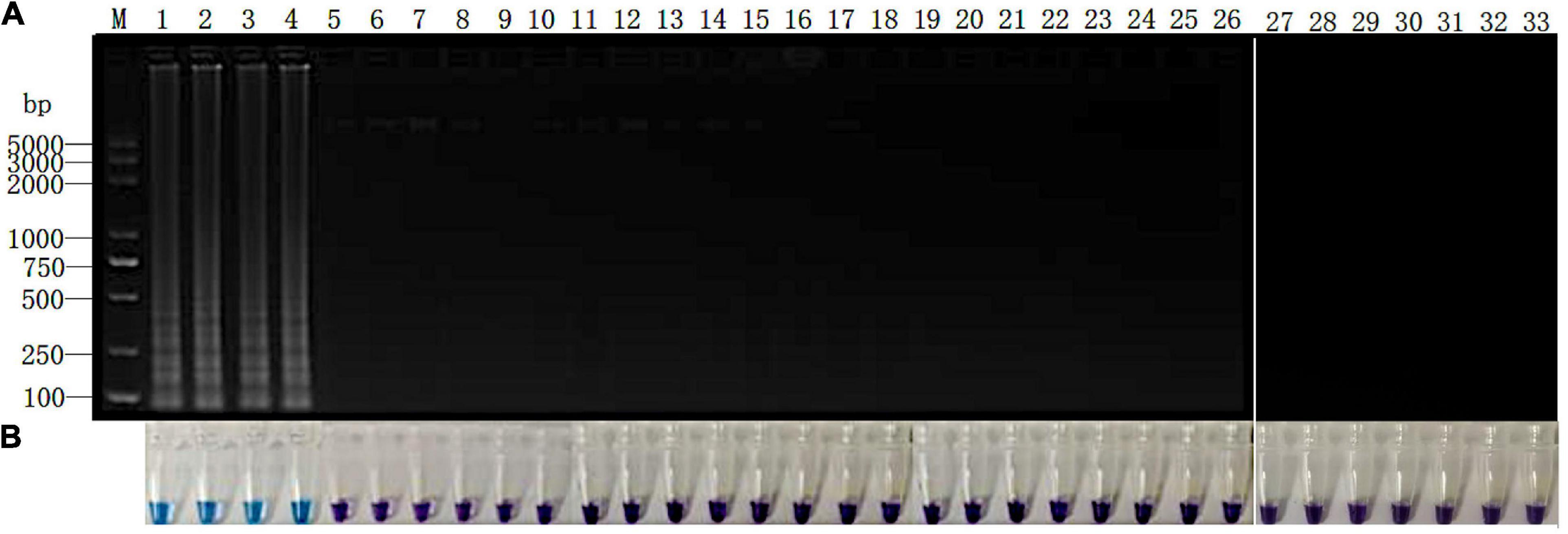
Figure 6. Detection of P. vexans in infected ramie roots. (A) Detection of LAMP products by 2% agarose gel electrophoresis. (B) Detection of LAMP products detected by HNB. Lanes 1–4, P. vexans; lanes 5 and 6, P. spinosum; lanes 7 and 8, P. irregulare; lanes 9 and 10, P. sylvaticum; lanes 11 and 12, P. helicoides; lanes 13 and 14, P. ultimum; lanes 15 and 16, P. myriotylum; lanes 17 and 18, P. litorale; lanes 19 and 20, P. oligandrum; lanes 21 and 22, P. heterothallicum; lanes 23 and 24, P. carolinianum; lanes 25 and 26, P. guiyangense; lanes 27 and 28, P. aphanidermatum; lanes 29 and 30, P. recalcitrans; lanes 31 and 32, CK. Lane 33, negative control.
Phytopythium vexans is the most well-known plant-pathogenic species of the genus Phytopythium. This pathogen can attack roots, stems, and crown of a wide range of plants worldwide (Spies et al., 2011a; Panth et al., 2020). For example, brown root rot of ramie caused by P. vexans has become an increasingly important disease endangering ramie production in China, resulting in >40% yield loss in some ramie plantations (Zhu et al., 2014; Yu et al., 2016). Therefore, a rapid and accurate detection of P. vexans is especially important for its identification and disease management. In this study, a rapid, sensitive, and accurate LAMP detection method for P. vexans based on color observation was established by using ITS gene sequence as the detection target. This technique was successfully used to detect P. vexans in infected ramie root tissues. It should be directly used in field conditions for the rapid diagnosis of ramie brown root rot caused by P. vexans.
A key factor influencing the LAMP method is target selection. Due to the divergence of ITS sequences among many closely-related species and its high copy number in each genome, ITS is often used as the detection target gene in oomycete. For example, ITS has been used as target gene for sequence-based identification of P. aphanidermatum (Fukuta et al., 2013), P. helicoides (=P. helicoides) (Takahashi et al., 2014), P. myriotylum (Fukuta et al., 2014), Pythium irregulare (Feng W. et al., 2015), and Phytophthora infestans (Ristaino et al., 2019). In our study, ITS sequence was successfully used in LAMP detection of P. vexans. However, in other pathogen groups such as P. ultimum and related Pythium species (Shen et al., 2017), Phytophthora alni and Phytophthora cambivora (Brasier et al., 2004), the ITS sequences of closely related sister species are often too similar to allow effective discrimination. In the Phytopythium genus, Phytopythium chamaehyphon, and P. helicoides also have highly similar ITS sequences that can’t be used to design LAMP primers for detection (de Cock et al., 2015). In the case of similar ITS sequences among closely related species, researchers have recently developed other genes for LAMP-based species-specific detection. For example, a target gene encoding a spore wall protein was developed to detect P. ultimum (Shen et al., 2017); while a target gene encoding trypsin protease was used to detect P. aphanidermatum (Li et al., 2018).
Another key factor of molecular detection technology is sensitivity. The higher the sensitivity is, the more favorable it is to detect target pathogens from samples. It is generally believed that compared with conventional and real-time PCR assays, the LAMP assay is simple, rapid, specific, and sensitive. However, previous studies also suggest that the LAMP assay has the same sensitivity than the PCR assay (Fukuta et al., 2013; Ishiguro et al., 2013; Feng W. et al., 2015). Due to differences in target gene copy number among species and strains in their genomes and to differences in their genome sizes, the detection limit among species and strains could be different. For example, previous studies have shown the detection limits of LAMP ranging from 100 fg/μL to 1 pg/μL, while the detection limits of conventional PCR ranging from 100 fg/μL to 100 pg/μL (Takahashi et al., 2014; Shen et al., 2017; Ristaino et al., 2019). In this study, the LAMP assay was about 100 times more sensitive than conventional PCR, even though the primers of LAMP assay and conventional PCR primers were all designed according to ITS sequence. Specifically, the sensitivity evaluation showed that the detection limit of LAMP was 1 pg/μL (∼24 copies of P. vexans genome), while that of conventional PCR was 100 pg/μL (∼2,350 copies of P. vexans genome).
Our analyses showed that our LAMP protocol could become a new reliable method for the diagnosis of P. vexans infection. However, the method has only been tested on pure DNA and laboratory-infected plant tissues. Direct field applicability has not been conducted and additional optimization may be needed in agricultural and forestry field application. In addition, our analyses used fresh cultures and recently infected plant tissues, the applicability of our LAMP technology to old samples and shipped materials from distant locations needs further testing. Regardless, the technology described here represents a promising approach for rapid, specific, and sensitive identification of P. vexans using DNA from pure cultures and from plant tissues infected with this pathogen.
In conclusion, we developed a visual LAMP assay for the diagnosis of P. vexans. This LAMP method has high specificity, sensitivity, and accuracy, and can identify P. vexans in infected plants. This method should facilitate the early and accurate diagnosis of P. vexans in the field so that timely plant protection measures can be initiated.
The datasets presented in this study can be found in online repositories. Representative isolates can be obtained from the corresponding author CG upon request.
YY and CG designed and supervised the research. TW and HJ performed the research, analyzed the data, and prepared the draft manuscript. XW, YC, ZL, JC, LG, CG, and JX contributed to the literature search, reviewing, and finalizing the manuscript. All authors have read and approved the final manuscript.
This work was financially supported by the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2015-IBFC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely thank all the researchers who provided the test strains.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.720485/full#supplementary-material
Supplementary Figure 1 | Maximum likelihood tree showing phylogenetic relationships among the 19 tested species. The tree was constructed based on ITS sequence of oomycetes listed in Table 1. Statistical support for the branches was assessed by bootstrap with 1,000 replicates. Bootstrap values above 50 are shown near the branch node.
Supplementary Figure 2 | Symptoms of ramie roots after infected with Phytopythium and Pythium spp.
Adhikari, B. N., Hamilton, J. P., Zerillo, M. M., Tisserat, N., Lévesque, C. A., and Buell, C. R. (2013). Comparative genomics reveals insight into virulence strategies of plant pathogenic oomycetes. PLoS One 8:e75072. doi: 10.1371/journal.pone.0075072
Bala, K., Robideau, G. P., Lévesque, C. A., De Cock, A. W. A. M., Abad, Z. G., Lodhi, A. M., et al. (2010). Phytopythium gen. nov. and Phytopythium sindhum sp. nov. Persoonia 24, 36–137.
Baldauf, S. L., Roger, A. J., Wenk-Siefert, I., and Doolittle, W. F. (2000). A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 290, 972–977. doi: 10.1126/science.290.5493.972
Baysal-Gurel, F., Liyanapathiranage, P., Panth, M., Avin, F. A., and Simmons, T. (2021). First report of Phytopythium vexans causing root and crown rot on flowering cherry in Tennessee. Plant Dis. 105:232. doi: 10.1094/PDIS-06-20-1166-PDN
Benfradj, N., Migliorini, D., Luchi, N., Santini, A., and Boughalleb-M’Hamdi, N. (2017). Occurrence of Pythium and Phytopythium species isolated from citrus trees infected with gummosis disease in Tunisia. Arch. Phytopathol. Plant Protect. 50, 286–302. doi: 10.1080/03235408.2017.1305479
Bian, C., Wang, W., Zhao, S., and Kang, Y. (2015). Molecular identification and pathogenicity of Pythium vexans isolated from tobacco (In Chinese). J. Agric. 5, 32–36.
Brasier, C. M., Kirk, S. A., Delcan, J., Cooke, D. E. L., Jung, T., and Veld, W. A. M. I. (2004). Phytophthora alni sp. nov. and its variants: designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycol. Res. 108, 1172–1184. doi: 10.1017/S0953756204001005
Chen, B., Chen, J., Mu, B., Zeng, M., Yu, J., Zhao, J., et al. (2016). Advances in medicinal health protection studies of Boehmeria Jacq. Spp. (In Chinese). Plant Fiber Sci. China 38, 237–241. doi: 10.3969/j.issn.1671-3532.2016.05.008
Chen, X. R., Liu, B. B., Xing, Y. P., Cheng, B. P., Liu, M. L., Tong, Y. H., et al. (2016). Identification and characterization of Phytopythium helicoides causing stem rot of shatangju mandarin seedlings in china. Eur. J. Plant Pathol. 146, 715–727. doi: 10.1007/s10658-016-0952-4
de Cock, A. W., Lodhi, A. M., Rintoul, T. L., Bala, K., Robideau, G. P., Abad, Z. G., et al. (2015). Phytopythium: molecular phylogeny and systematics. Persoonia 34, 25–39. doi: 10.3767/003158515X685382
Derviş, S., Türkölmez, Ş, Çiftçi, O., Özer, G., and Ulubaş Serçe, Ç, and Dikilitas, M. (2020). Phytopythium litorale: A novel killer pathogen of plane (Platanus orientalis) causing canker stain and root and collar rot. Plant Dis. 104, 2642–2648. doi: 10.1094/PDIS-01-20-0141-RE
Feng, L., Ni, X., Wu, X., Lun, C., Wu, C., and Luan, J. (2015). Loop-mediated isothermal amplification (LAMP) for detection of Pantoea stewartii subsp. stewartii (In Chinese). J. Plant Protect. 42, 347–352. doi: 10.13802/j.cnki.zwbhxb.2015.03.010
Feng, W., Ishiguro, Y., Hotta, K., Watanabe, H., Suga, H., and Kageyama, K. (2015). Simple detection of Pythium irregulare using loop-mediated isothermal amplification assay. FEMS Microbiol. Lett. 362:fnv174. doi: 10.1093/femsle/fnv174
Fukuta, S., Takahashi, R., Kuroyanagi, S., Ishiguro, Y., Miyake, N., Nagai, H., et al. (2014). Development of loop-mediated isothermal amplification assay for the detection of Pythium myriotylum. Lett. Appl. Microbiol. 59, 49–57. doi: 10.1111/lam.12244
Fukuta, S., Takahashi, R., Kuroyanagi, S., Miyake, N., Nagai, H., Suzuki, H., et al. (2013a). Detection of Pythium aphanidermatum in tomato using loop-mediated isothermal amplification (LAMP) with species-specific primers. Eur. J. Plant Pathol. 136, 689–701. doi: 10.1007/s10658-013-0198-3
Fukuta, S., Tamura, M., Maejima, H., Takahashi, R., Kuwayama, S., Tsuji, T., et al. (2013b). Differential detection of wheat yellow Mosaic virus, Japanese soil-borne wheat mosaic virus and Chinese wheat mosaic virus by reverse transcription loot-mediated isothermal amplification reaction. J. Virol. Methods 1089, 348–354. doi: 10.1016/j.jviromet.2013.03.005
Goto, M., Honda, E., Ogura, A., Nomoto, A., and Hanaki, K. (2009). Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 46, 167–172. doi: 10.2144/000113072
Hernández, P. A., Chávez, E. C., Ortiz, J. D., Beache, M. B., Vargas, L. T., and Fuentes, Y. O. (2019). First report of Phytopythium vexans causing the “Avocado sadness” in Michoacan, Mexico. Phyton Int. J. Exp. Bot. 88, 11–13. doi: 10.32604/phyton.2019.04608
Ishiguro, Y., Asano, T., Otsubo, K., Suga, H., and Kageyama, K. (2013). Simultaneous detection by multiplex PCR of the high-temperature-growing Pythium species: P. aphanidermatum. P. helicoides and P. myriotylum. J. Gen. Plant Pathol. 79, 350–358. doi: 10.1007/s10327-013-0466-2
Jabiri, S., Lahlali, R., Bahra, C., Amraoui, M. B., Tahiri, A., and Amiri, S. (2020). First report of Phytopythium vexans associated with dieback disease of apple trees in Morocco. J. Plant Pathol. 102, 1319–1319. doi: 10.1007/s42161-020-00606-2
Karling, J. S. (1981). Predominantly Holocarpic and Eucarpic Simple Biflagellate Phycomycetes, 2nd Edn. Germany: J. Cramer.
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Langenhoven, S., Halleen, F., Spies, C., Stempien, E., and Mostert, L. (2018). Detection and quantification of black foot and crown and root rot pathogens in grapevine nursery soils in the Western Cape of South Africa. Phytopathol. Mediterr. 57, 519–537. doi: 10.14601/Phytopathol_Mediterr-23921
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. doi: 10.1093/bioinformatics/btm404
Lévesque, C. A., and de Cock, A. W. (2004). Molecular phylogeny and taxonomy of the genus Pythium. Mycol Res. 108, 1363–1383. doi: 10.1017/s0953756204001431
Lévesque, C. A., Robideau, G. P., Desaulniers, N., Bala, K., Chen, W., and Tambong, J. T. (2008). “Molecular phylogeny, barcoding and ecology of Pythium species. (Abstr.),” in 3rd International Workshop on Phytophthora/Pythium and related genera, Turin.
Li, Q., Shen, D., Yu, J., Zhao, Y., Zhu, Y., and Dou, D. (2018). Rapid detection of Pythium aphanidermatum by loop-mediated isothermal amplification (In Chinese). J. Nanjing Agric. Univ. 41, 79–87. doi: 10.7685/jnau.201703025
Liu, J., Huang, C., Wu, Z., Zhang, X., and Wang, Y. (2010). Detection of Tomato aspermy virus infecting chrysanthemums by LAMP (In Chinese). Sci. Agric. Sin 43, 1288–1294. doi: 10.4028/www.scientific.net/AMM.37-38.1549
Mori, Y., Nagamine, K., Tomita, N., and Notomi, T. (2001). Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 289, 150–154. doi: 10.1006/bbrc.2001.5921
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., et al. (2000). Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:e63. doi: 10.1097/RLU.0b013e3181f49ac7
Panth, M., Baysal-Gurel, F., Avin, F. A., and Simmons, T. (2020). Identification and chemical and biological management of Phytopythium vexans, the causal agent of Phytopythium root and crown rot of woody ornamentals. Plant Dis. 105, 1091–1100. doi: 10.1094/PDIS-05-20-0987-RE
Parida, M., Sannarangaiah, S., Dash, P. K., Rao, P. V. L., and Morita, K. (2010). Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 18, 407–421. doi: 10.1002/rmv.593
Polat, Z., Awan, Q. N., Hussain, M., and Akgül, D. S. (2017). First report of Phytopythium vexans causing root and collar rot of kiwifruit in Turkey. Plant Dis. 101:1058. doi: 10.1094/PDIS-11-16-1554-PDN
Rees, W. A., Yager, T. D., Korte, J., and von Hippel, P. H. (1993). Betaine can eliminate the base pair composition dependence of DNA melting. Biochemistry 32, 137–144. doi: 10.1021/bi00052a019
Ristaino, J. B., Saville, A. C., Paul, R., Cooper, D. C., and Wei, Q. (2019). Detection of Phytophthora infestans by LAMP, real-time LAMP and droplet digital PCR. Plant Dis. 104, 1–9.
Schroeder, K. L., Martin, F. N., de Cock, A. W. A. M., Lévesque, C. A., and Paulitz, T. C. (2013). Molecular detection and quantification of Pythium species: evolving taxonomy, new tools, and challenges. Plant Dis. 97, 4–20. doi: 10.1094/PDIS-03-12-0243-FE
Schroeder, K. L., Okubara, P. A., Tambong, J. T., Lévesque, C. A., and Paulitz, T. C. (2006). Identification and quantification of pathogenic Pythium spp. from soils in eastern Washington using real-time polymerase chain reaction. Phytopathology 96, 637–647. doi: 10.1094/PHYTO-96-0637
Shen, D., Li, Q., Yu, J., Zhao, Y., Zhu, Y., Xu, H., et al. (2017). Development of a loop-mediated isothermal amplification method for the rapid detection of Pythium ultimum. Australas. Plant Pathol. 46, 571–576. doi: 10.1007/s13313-017-0517-9
Spies, C. F. J., Mazzola, M., Botha, W. J., Koopman, T. A., Meitz, J., Paarwater, H., et al. (2009). “Characterisation of the Pythium vexans species complex from fruit crops in South Africa,” in In 46th SASPP Congress. Gordon’s Bay: SASPP council.
Spies, C. F. J., Mazzola, M., Botha, W. J., Van der Rijst, M., Mostert, L., and McLeod, A. (2011a). Oogonial biometry and phylogenetic analyses of the Pythium vexans species group from woody agricultural hosts in South Africa reveal distinct groups within this taxon. Fungal Biol. 115, 157–168. doi: 10.1016/j.funbio.2010.11.005
Spies, C. F. J., Mazzola, M., and McLeod, A. (2011b). Characterisation and detection of Pythium and Phytophthora species associated with grapevines in South Africa. Eur. J. Plant Pathol. 131, 103–119. doi: 10.1007/s10658-011-9791-5
Takahashi, R., Fukuta, S., Kuroyanagi, S., Miyake, N., Nagai, H., Kageyama, K., et al. (2014). Development and application of a loop-mediated isothermal amplification assay for rapid detection of Pythium helicoides. FEMS Microbiol. Lett. 355, 28–35. doi: 10.1111/1574-6968.12453
Tao, Y., Zeng, F., Ho, H., Wei, J., Wu, Y., Yang, L., et al. (2011). Pythium vexans causing stem rot of Dendrobium in Yunnan Province, China. J. Phytopathol. 159, 255–259. doi: 10.1111/j.1439-0434.2010.01756.x
Thao, L., Hien, L., Liem, N., Thanh, H., Khanh, T., Binh, V., et al. (2020). First report of Phytopythium vexans causing root rot disease on durian in Vietnam. New Dis. Rep. 41:2. doi: 10.5197/j.2044-0588.2020.041.002
Tkaczyk, M. (2020). Phytopythium: origin, differences and meaning in modern plant pathology. Folia For. Pol. Ser. A For. 62, 227–232. doi: 10.2478/ffp-2020-0022
van den Ende, A. H. G., and de Hoog, G. S. (1999). Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Stud. Mycol. 43, 151–162.
Wang, T., Gao, C., Cheng, Y., Li, Z., Chen, J., Guo, L., et al. (2020). Molecular diagnostics and detection of oomycetes on fiber crops. Plants (Basel) 2020:769. doi: 10.3390/plants9060769
Wei, H., Wang, X., Li, H., Sun, W., and Gu, J. (2016). Loop-mediated isothermal amplification assay for rapid diagnosis of Meloidogyne mali (In Chinese). J. Plant Protect. 43, 260–266. doi: 10.13802/j.cnki.zwbhxb.2016.02.012
Yoon, H. S., Hackett, J. D., and Bhattacharya, D. (2002). A single origin of the peridinin-and fucoxanthin-containing plastids in dinoflagellates through tertiary endosymbiosis. Proc. Natl. Acad. Sci. U. S. A. 99, 11724–11729. doi: 10.1073/pnas.172234799
Yu, Y., Chen, J., Gao, C. S., Zeng, L. B., Li, Z. M., Zhu, T. T., et al. (2016). First report of brown root rot caused by Pythium vexans on ramie in Hunan, China. Can. J. Plant Pathol. 38, 405–410. doi: 10.1080/07060661.2016.1230150
Yu, Y., Zeng, L., Yan, Z., Liu, T., Sun, K., Zhu, T., et al. (2015). Identification of ramie genes in response to Pratylenchus coffeae infection challenge by digital gene expression analysis. Int. J. Mol. Sci. 16, 21989–22007. doi: 10.3390/ijms160921989
Zeng, D., Rong, Z., Yuan, Y., Wang, X., and Zheng, X. (2018). Rapid diagnosis of rice bakanae disease caused by Fusarium verticillioides using a loop-mediated isothermal amplification assay (In Chinese). J. Nanjing Agric. Univ. 41, 286–292. doi: 10.7685/jnau.201705023
Zeng, H. C., Ho, H. H., and Zheng, F. C. (2005). Pythium vexans causing patch canker of rubber trees on Hainan Island, China. Mycopathologia 159, 601–606. doi: 10.1007/s11046-005-5258-6
Keywords: Phytopythium vexans, loop mediated isothermal amplification, internal transcribed spacer (ITS) sequence, hydroxynaphthol blue (HNB), diagnosis
Citation: Wang T, Ji H, Yu Y, Wang X, Cheng Y, Li Z, Chen J, Guo L, Xu J and Gao C (2021) Development of a Loop-Mediated Isothermal Amplification Method for the Rapid Detection of Phytopythium vexans. Front. Microbiol. 12:720485. doi: 10.3389/fmicb.2021.720485
Received: 04 June 2021; Accepted: 06 August 2021;
Published: 06 September 2021.
Edited by:
Xiyang Wu, Jinan University, ChinaReviewed by:
Antonella Amicucci, University of Urbino Carlo Bo, ItalyCopyright © 2021 Wang, Ji, Yu, Wang, Cheng, Li, Chen, Guo, Xu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunsheng Gao, Z2FvY2h1bnNoZW5nQGNhYXMuY24=; Jianping Xu, anB4dUBtY21hc3Rlci5jYQ==
†These authors share first authorship
‡Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.