
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 22 July 2021
Sec. Food Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.690211
This article is part of the Research Topic From Traditional to Modern: Progress of Molds and Yeasts in Fermented-Food Production View all 45 articles
Filamentous fungi are a group of economically important fungi used in the production of fermented foods, industrial enzymes, and secondary metabolites. Glycosphingolipids (GSLs) as constituents of lipid rafts are involved in growth, differentiation, and response to environment stress in filamentous fungi. In addition to these key roles, GSLs are also important in the barrier function of skin to retain moisture as a moisturizing ingredient in cosmetics or health products for their strong biological activity as a functional component. GSLs found in filamentous fungi are divided in two major classes: neutral GSLs (glycosylceramides), glucosylceramides (GlcCers), and/or galactosylceramides (GalCers) and acidic GSLs, mannosylinositol phosphorylceramide (MIPC) and mannosyldiinositol phosphorylceramide [M(IP)2C]. Glycosylceramides are one of the abundant GSLs in Aspergillus and known to improve skin-barrier function and prevent intestinal impairment as a prebiotic. Some filamentous fungi of Aspergillus spp., synthesizing both GlcCer and GalCer, would be an amenable source to exploit glycosylceramides that wildly adding in cosmetics as moisturizing ingredients or health food as dietary supplements. In this minireview, the types, structures, and biosynthetic pathways of GSLs in filamentous fungi, and the relevance of GSLs in fungal growth, spore formation, and environmental stress response are explained. Furthermore, the advantage, potential development, and application of GlcCer and GalCer from filamentous fungi Aspergillus spp. are also investigate based on the use of plant GlcCer in health foods and cosmetics.
Filamentous fungi, particularly Aspergillus spp., Trichoderma reesei, and Neurospora crassa, are a group of economically important fungi used in the production of fermented foods, industrial enzymes, antibiotic substances, and organic acids (Brunt, 1986; Cherry and Fidantsef, 2003; Allgaier et al., 2009; Karaffa and Kubicek, 2019). For more than a century, filamentous fungi have been known to produce and secrete different types of enzymes in large quantities, which has resulted in an increased interest in studying them and using them in industrial applications. The production of more than 60% of total industrial enzymes is done by Aspergillus genus of filamentous fungi. In addition, filamentous fungi are well-known producers of secondary metabolites with various biological activities. Many of these compounds such as penicillin, cyclosporine or lovastatin are of great importance to human health (Nützmann et al., 2012). For example, two important antibiotics cephalosporin and penicillin are produced by Cephalosporium acremonium and Penicillium chrysogenum, respectively (Nash and Huber, 1971; Nielsen and Jorgensen, 1995). Furthermore, filamentous fungi are also widely used in the production of organic acids in industrial fermentation, such as citric acid, itaconic acid, fumaric acid, and malic acid (Papagianni, 2007; Knuf et al., 2013; Hu et al., 2014; Karaffa and Kubicek, 2019). Aspergillus niger and Aspergillus oryzae have a long history of use in the fermentation industry and are generally recognized as safe (GRAS) in accordance with the Food and Drug Administration (FDA). Amylase, a well-known enzyme is produced from A. niger and A. oryzae and applicated in diverse processes, ranging from food and beverage to medical (Wirsel et al., 2010; Silano et al., 2018). Soy sauce, a traditional fermented condiment widely consumed in China, Japan, Korea, and other Asian countries, is fermented from soybean by A. oryzae (Liang et al., 2009). Therefore, due to the economic importance of filamentous fungi, the secondary metabolite pathways, organic compound, and fermentation processes in these filamentous fungi have attracted attention of the scientists.
In the industrial application of filamentous fungi, the conditions of the fermentation play a vital role in the growth and metabolism of a microbial population. Filamentous fungi have the potential to grow or ferment under diverse environmental conditions by utilizing a wide variety of substrates as nutrients (Chen et al., 2011). The ability of microorganisms to adapt to different environmental factors has attracted considerable attention, with many studies investigating the molecular mechanisms of microorganism in response to environmental stress. Fungi adapt to environmental changes using a range of molecular mechanisms. One mechanism is via change in the composition of cellular lipids, such as phospholipids, neutral lipids, or unique glycosphingolipids (GSLs) (Suutari, 1995; Řezanka et al., 2016, 2018). Fungal GSLs, including neutral and acidic GSLs, are the main lipid components of microdomains in fungal membranes and are clustered along with sterols to form lipid rafts, which play crucial roles in cell polarization, hyphal growth, fungal fitness, and adaptation to most diverse environments as a signaling molecules (Sonnino et al., 2007; Guimarães et al., 2014; Řezanka et al., 2016, 2018). Although GSLs have been studied extensively, the details of their function are difficult to understand owing to complex and dynamic changing synthetic pathways of interconversion and utilization. Nonetheless, the role of yeast GSLs in response to heat stress has been investigated thoroughly (Chen et al., 2013; Řezanka et al., 2016, 2018). However, the GSL pathways and related genes that contribute to fungal growth, differentiation, morphogenesis, particularly those involved in response to environmental stress in filamentous fungi, are less appreciated.
In addition to fungal growth and response to environmental stress, neutral GSLs (also call glycosylceramides) are also known to have nutritive functions such as preventing intestinal impairment and enhancing the moisture content of skin (Ono et al., 2010; Duan et al., 2012; Hamajima et al., 2016). For example, neutral GSLs can be added to food as a “functional components” for their strong biological activity in health food products such as nutritional supplements, infant foods, and beverages that help in reduction of blood pressure, activation of immunity and inhibition of cancer cell proliferation. Previous studies reported that A. oryzae glycosylceramides functions as a prebiotic, can alter the intestinal microbial flora and increase Blautia coccoides (Hamajima et al., 2019). Since there are many reports of the effects of B. coccoides on health, an increase in intestinal B. coccoides by koji glycosylceramide might be the connection between intestinal microbial flora and healthy. Besides, neutral GSLs are also important due to their barrier function as a moisturizing ingredient in cosmetics that help of skin retain its moisture (Alessandrini et al., 2004; Tessema et al., 2017; Miyagawa et al., 2019). However, plant cell membrane contents such as neutral GSLs, are difficult to extract due to the thick cell wall and GSLs from neural tissues of animals are not acceptable for cosmetic or other human use due to the underlying risk of prion diseases. Therefore, filamentous fungi koji, which are safe for humans and contain abundant (0.5–2 mg/g dry weight koji) neutral GSLs, would be important resources for exploiting neutral GSLs in the future.
In this review, we discuss the relevance of GSLs in fungal growth, spore formation and environmental stress response, which are key to the production of fermented foods, commercial enzymes, and secondary metabolites in industrial fermentation, and the potential development and application of filamentous fungi GSLs in cosmetics and health foods.
Glycosphingolipids are ubiquitous membrane components and are involved in many biological processes crucial for filamentous fungi, such as growth, signal transduction, morphological transition, and pathogenesis (Heung et al., 2006; Huber et al., 2019). Our knowledge of GSL biosynthesis in filamentous fungi is mainly based on investigations of yeasts, the human pathogenic Candida albicans (Oura and Kajiwara, 2010), the model organisms N. crassa (Park et al., 2005) and Aspergillus nidulans (Levery et al., 2002; Fernandes et al., 2016), and the plant pathogen Fusarium graminearum (Duarte et al., 1998; Ramamoorthy et al., 2007, 2009; Zaüner et al., 2008). The basic structure of GSL consists of an 18-carbon-alcohol chain called long chain base (LCB) that is linked to a C16–26 fatty acid (FA) chain through an amide bond to form ceramide (Figure 1A), which in turn is linked to a polar head group (Figures 1B,C) (Barreto-Bergter et al., 2004, 2011; Del Poeta et al., 2014). GSLs have been isolated and identified from various filamentous fungi, such as C. albicans, F. graminearum, N. crassa, and Aspergillus spp., and divided in two major classes of neutral and acidic GSLs (Totani et al., 2001; Park et al., 2005; Zhang et al., 2007; Zaüner et al., 2008; Takahashi et al., 2009; Hirata et al., 2012; Guimarães et al., 2014; Tani et al., 2014; Fernandes et al., 2016).
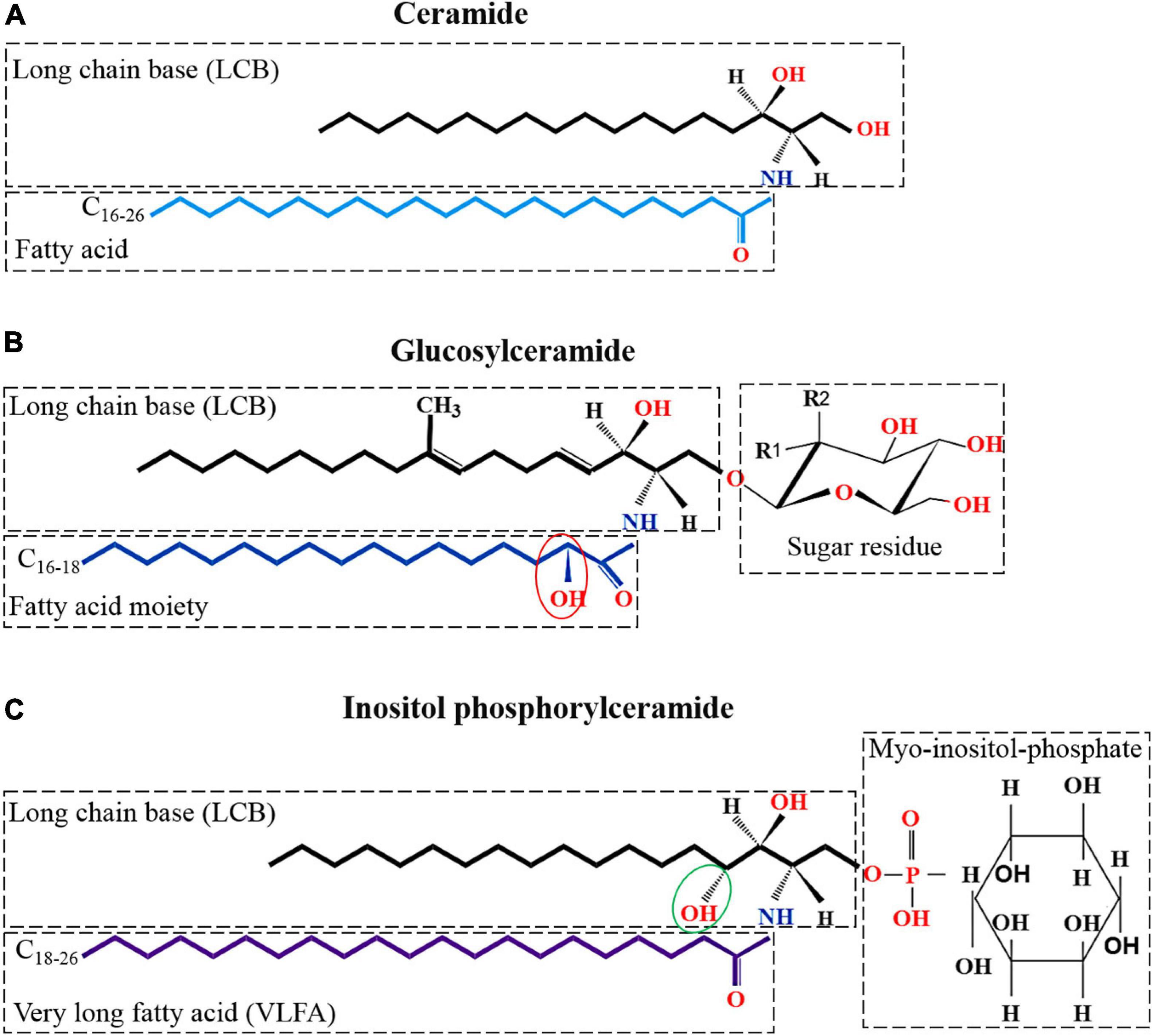
Figure 1. Kinds and structures of glycosphingolipid in filamentous fungi. (A) Structure of ceramide; the basic unit of neutral and acidic glycosphingolipids; made up of a LCBs and a FA moiety. (B) Structure of neutral glycosphingolipid which is linked to a sugar residue on the base of ceramide. Neutral glycosphingolipid contains an-OH moiety at C2 in the FA chain (showed with red ellipse); a double bond between C4–5 and C8–9; and a methyl C9 of the LCB. Monosaccharide may be either glucosyl (Glc) (R1 = OH, R2 = H) or galactosyl (Gal) residues (R1 = H, R2 = OH). (C) The basic structure of acidic glycosphingolipid, inositol phosphorylceramide, is shown. Inositol phosphorylceramide is different from neutral glycosphingolipids in that it contains an additional hydroxy at C4 of the LCB (showed with green ellipse) and lacks double bonds between C4–5 and C8–9, and the C9 methyl of the LCB. In addition, acidic glycosphingolipids are composed of a very long FA chain (C18–26) instead of the C16–18 chain that found in neutral glycosphingolipids.
In filamentous fungi, neutral GSLs (also call glycosylceramides) include glucosylceramide (GlcCer) and galactosylceramide (GalCer), while acidic GSLs consist of two forms of glycosyl inositol phosphoryl ceramides (GIPCs), mannosylinositol phosphorylceramide (MIPC) and mannosyldiinositol phosphorylceramide [M(IP)2C] (Barreto-Bergter et al., 2004, 2011; Buré et al., 2014). Neutral GSLs contain 9-methyl-Δ4,Δ8-sphingadienine as the LCB linked to a C16–18 N-2-hydroxy or unsaturated N-2-hydroxy-Δ3 FA chain and a glucose or galactose moiety to form GlcCer or GalCer. In contrast, the ceramide moiety of acidic GSLs is composed of a 4-hydroxysphinganine (phytosphingosine) as the LCB attached to a very long fatty acid (VLFA) chain (C18–26) and complex glycan groups via an inositol phosphate linker. All filamentous fungi investigated so far contain GlcCer or GalCer, with most mold species, such as Aspergillus fumigatus (Boas et al., 1994; Toledo et al., 1999; Fontaine, 2017), A. niger (Wagner and Fiegert, 1969; Levery et al., 2000), A. oryzae, Aspergillus sojae, and Aspergillus awamori (Tani et al., 2014), containing both GlcCer and GalCer (Table 1). Interestingly, Sporothrix schenckii mycelia synthesize only GlcCer, while the yeast forms are found to contain both GlcCer and GalCer (Table 1; Dickson and Lester, 1999; Toledo et al., 2000). In fungi, GlcCer and GalCer are considered the final step of the pathway, whereas in mammalian cells GlcCer and GalCer are then used to make hundreds of complex GSLs. In fungi and plants, acidic GSLs include inositol phosphorylceramides (IPCs), which are used as building blocks for more complex molecules, such as MIPC and M(IP)2C. In summary, GlcCer is the only GSLs found in all organisms studied, especially eukaryotic cell, such as fungi, plants as well as invertebrates and vertebrates (Table 2). In contrast, GIPCs occur only in fungi and plants, whereas GalCer is restricted to fungi, vertebrates, and invertebrates (Table 2).
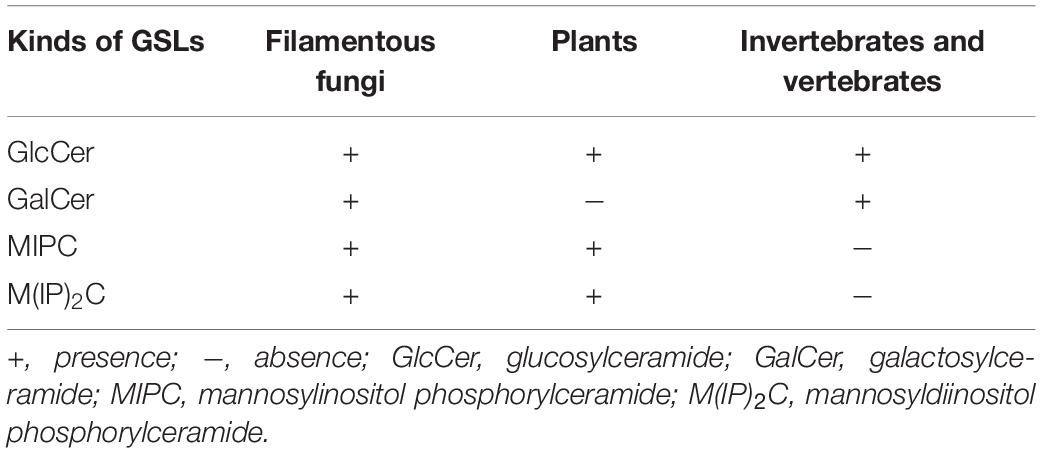
Table 2. Relative presence of glycosphingolipids (GSLs) in filamentous fungi, plants, invertebrates, and vertebrates.
Neutral and acidic GSLs have specific differences in the structures of their ceramide backbones. In filamentous fungi, the differences in hydroxylation, saturation levels and methylation levels result in different LCBs of both GSLs (Warnecke and Heinz, 2003; Gault et al., 2010). Neutral GSLs normally contain the monosaccharide, glucose or galactose, in glycosidic linkage with the 9-methyl-Δ4, Δ8-sphingoid (ceramide) (Takahashi et al., 2009). The structure of 9-methyl-Δ4, Δ8-sphingadienine, which contains two double bonds in C4–5 and C8–9 and a methylation at C9 (Fujino and Ohnishi, 1977; Barreto-Bergter et al., 2004, 2011; Takahashi et al., 2009; Del Poeta et al., 2014), is unique to neutral GSLs of filamentous fungi and distinguishes them from plant and mammalian sphingosines (Figure 1B). While Δ4-sphingenine with a double bond in C4–5 is predominantly found as LCB in mammals and is rare in fungi and plants, Δ4,Δ8-sphingadienine with two double bonds in C4–5 and C8–9 is found in plants (Sperling and Heinz, 2003; Barreto-Bergter et al., 2004; Takahashi et al., 2009). In contrast, acidic GSLs are different from neutral GSLs in that they contain an additional hydroxyl group at C4 of their LCB (4-hydroxyshinganine) (Figure 1C showed with green ellipse) and lack Δ4,Δ8-unsaturations and C9-methylation found in the LCBs of neutral GSLs in filamentous fungi (Figure 1C). In addition, the FA lengths of neutral and acidic GSLs is also different. In filamentous fungi, C16–18 FA chains are observed in neutral GSLs, and C24–26 chains are observed in acidic GSLs (Marques et al., 2018), while the mammalian FA length is predominantly a C16 or C18 FA chain. In plants, the lengths of FA chains range from C14 to C26 (Sperling and Heinz, 2003). Interestingly, some filamentous fungal GSLs contain a Δ3-desaturated in FA chain, which is a unique modification of fungal GSLs and has been reported in A. fumigatus (Boas et al., 1994; Toledo et al., 1999), A. niger (Wagner and Fiegert, 1969; Levery et al., 2000), and S. schenckii (Toledo et al., 2001; Table 1).
In summary, filamentous fungal GlcCer or GalCer mainly consists of a β-D-glucose or galactose attached to C1 of 9-methyl-Δ4,Δ8-sphingadienine which is N-acylated with a C16–18 N-2-hydroxy or N-2-hydroxy-Δ3 FA chain. In contrast, the ceramide moiety of acidic GSLs is usually formed by 4-hydroxysphinganine (phytosphingosine) as the LCB, bound to a VFFA chain (C18–26), linked to a polar head group. IPC is further modified upon addition of mannose and a second inositol phosphate group to generate MIPC (with a mannose sugar unit) and M(IP)2C (with two inositol groups).
Raw ceramide for the synthesis of GSLs is derived from three pathways, de novo GSL synthesis, sphingomyelin degradation and GSL recycling. Here we focus on the de novo synthesis pathway of GSLs that is conserved among filamentous fungi (Gault et al., 2010). The whole process of GSL synthesis pathway was divided into three modules for better understanding, the synthesis of dihydrosphingosine, synthesis of neutral GSLs, and synthesis of acidic GSLs (Figure 2). In this section, the productions of GSLs via the de novo biosynthetic pathway and the key genes involved are discussed.
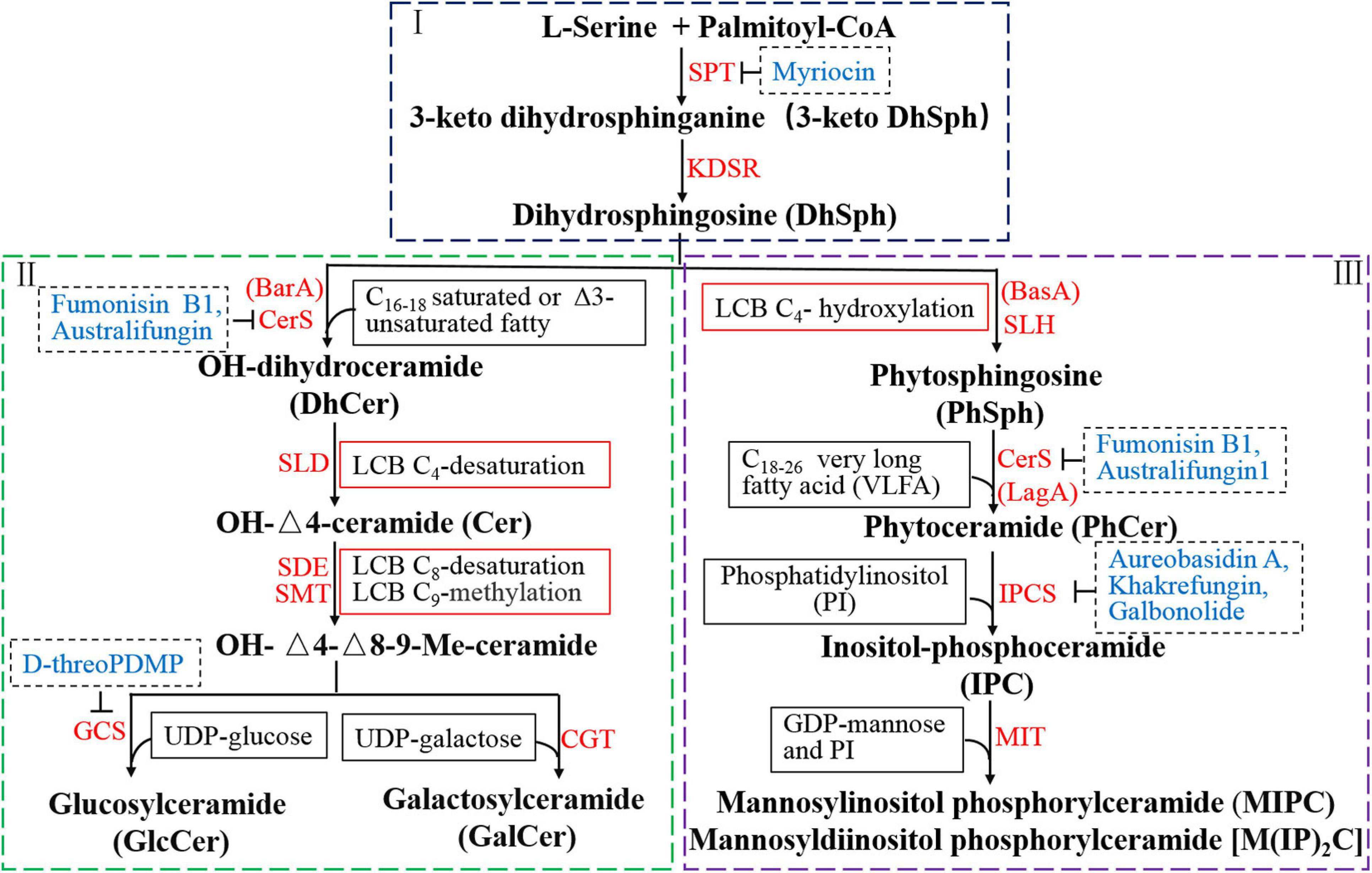
Figure 2. Reorganized biosynthetic pathway of glycosphingolipid in filamentous fungi. The first part (I) involves two key enzyme-catalyzed reactions and is the starting point and common to neutral and acidic GSL synthesis. The second part (II) represents the biosynthetic pathway of neutral GSLs (GlcCer and GalCer) from DhSph including four enzyme-catalyzed reactions. GlcCer and GalCer are the final products of neutral GSL pathway in filamentous fungi. The third part (III) is the biosynthetic process of acidic GSLs, including the production of IPC, MIPC, and M(IP)2C, which also starts from DhSph and includes three enzyme-catalyzed reactions. IPCs, used as building blocks for more complex molecules, are further modified upon addition of mannose and a second inositol phosphate group to generate MIPC and M(IP)2C. Red texts represent genes or relative enzymes involved in the GSL pathway. Blue texts indicate the inhibitors of the specific enzyme in the biosynthetic steps.
The biosynthesis of dihydrosphingosine (DhSph), involves two key enzyme-catalyzed reactions and is the starting point in the biosynthesis of all GSLs (Figure 2I). The first and rate-determining step is the condensation of palmitoyl coenzyme A (palmitoyl-CoA) and L-serine, catalyzed by serine palmitoyltransferase (SPT), to produce 3-keto dihydrosphingosine (3-keto DhSph), which is then reduced to DhSph by 3-keto DhSph reductase (KDSR) (Barreto-Bergter et al., 2004). The genes encoding SPT and KDSR are conserved among all organisms and have been identified from various species of fungi. Two genes, LCB1 and LCB2, which are necessary for SPT activity, have been identified in Saccharomyces cerevisiae (Buede et al., 1991; Nagiec et al., 1994). Deletion of either LCB1 or LCB2 was found to abolish GSL production in yeast (Mukherjee and Dekker, 1990; Zhao et al., 1994; Dickson, 2008). In A. nidulans, the gene encoding SPT was identified and named lcbA owing to its homology to the S. cerevisiae LCB1. The lcbA is essential for polarity and growth in A. nidulans and under the control of the alcA promoter, which is alcohol inducible and glucose repressible (Waring et al., 1989; Cheng et al., 2001). An important third subunit of SPT is Tsc3p, which coimmunoprecipitates with LCB1 or/and LCB2, and is required for optimal and high level SPT activity during maximal GSL biosynthesis in S. cerevisiae (Gable et al., 2000).
The role of 3-keto DhSph reductase remains poorly investigated in filamentous fungi. In A. fumigatus, KDSR is encoded by the ksrA gene, but the biological role of ksrA in Aspergillus remains to be elucidated (Fornarotto et al., 2006). Deletion of C. albicans ksr1 shows compromised filamentation, suggesting that the expression of ksr1 may be important for polarized growth (Fornarotto et al., 2006).
Although poorly studied, the production of DhSph is a critical and common pathway in the biosynthesis of neutral and acidic GSLs. This compound can generate two distinct ceramide pools of dihydroceramide and phytoceramide, which are utilized for the formation of neutral and acidic GSLs, respectively. Two genes, BarA and BasA, are involved in this step in filamentous fungi (Li et al., 2006; Rittenour et al., 2011; Cheon et al., 2012; Fontaine, 2017). The ceramide pool involved in the neutral GSL synthesis (dihydroceramide) is generated by BarA, while acidic GSL synthesis (phytoceramide) is catalyzed by BasA (also called Sur2 in S. cerevisiae). Therefore, filamentous fungi possess two distinct ceramide pools that make independent contributions to polarized hyphal growth, through the formation of specialized lipid microdomains that regulate the organization of the cytoskeleton (Li et al., 2006).
Synthesis of the neutral GlcCer and GalCer from DhSph includes four enzyme-catalyzed reactions (Figure 2II).
Dihydrosphingosine is first N-acylated with saturated or Δ3-unsaturated C16–18 FA chain catalyzed by ceramide synthase (CerS) to produce dihydroceramide (DhCer). A hydroxyl group is then inserted at C2 in the FA chain of DhCer generating OH-DhCer. The ceramide pool involved in the DhCer synthesis is unique to filamentous fungi and catalyzed by ceramide synthase encoded by BarA (or Cer1, Bar1) (Li et al., 2006; Rittenour et al., 2011). BarA is specifically necessary to produce neutral GSLs in fungi and contributes differentially to polarized hyphal growth. The ΔBarA or ΔCer1 or ΔBar1mutant can produce normal IPC but completely lacks GlcCer, fails to display the distinct sterol-rich domain at the hyphal tip and is incapable of producing perithecia (Rittenour et al., 2011; Cheon et al., 2012; Munshi et al., 2018).
The LCB of OH-DhCer is reduced by sphingolipid Δ4-desaturase (SLD) to generate OH-Δ4-ceramide in the cytosolic face of the endoplasmic reticulum (ER) (Michel and van Echten-Deckert, 1997; Ternes et al., 2002). Sphingolipid Δ4-desaturase, encoded by the DEGS1, is involved in the double bond formation at C4–5 of DhCer (Michel et al., 1997; Rodriguez-Cuenca et al., 2015).
Then, a double bond at C8–9 and a methyl group at C9 are introduced into the LCB by sphingolipid Δ8-desaturase (SDE) and sphingolipid C9-methyltransferase (SMT), respectively, resulting in the formation of OH-Δ4,Δ8-9-methyl-ceramide (Ternes et al., 2006; Rhome et al., 2007). Sphingolipid Δ8-desaturase is encoded by SdeA in A. nidulans. There are two genes encoding C9-methyltransferases in A. nidulans (smtA and smtB) and F. graminearum (FgMT1 and FgMT2), but only one candidate has been identified in N. crassa (Ternes et al., 2006). OH-Δ4,Δ8-9-methyl-ceramide is produced in the ER and transported to the Golgi apparatus for synthesis of GalCer and GlcCer.
The final step of the neutral GSL biosynthetic pathway is the transfer of a sugar residue from UDP-glucose or UDP-galactose to the OH-Δ4,Δ8-9-methyl-ceramide catalyzed by glucosylceramide synthase (GCS) or ceramide galactosyltransferase (CGT), respectively in the Golgi apparatus (Leipelt et al., 2001; Warnecke and Heinz, 2003; Ternes et al., 2011). GCS is encoded by GCSA or GCS1 that is essential for mycelial growth and filamentation in filamentous fungi (Ramamoorthy et al., 2007; Fernandes et al., 2016). Although most of the genes responsible for GlcCer biosynthesis have been identified and cloned, the gene encoding ceramide galactosyltransferase has been detected only in N. crassa and Magnaporthe grisea (Lester et al., 1974; Maciel et al., 2002). Limited information is available on the occurrence of GalCer in fungi and information on their function or on the galactosyltransferase responsible for their formation is lacking.
The synthesis of acidic GSLs, including the productions of IPCs, MIPCs, and M(IP)2Cs, also starts from DhSph and includes three enzyme-catalyzed reactions (Figure 2III).
The LCB at C4 of DhSph is hydroxylated by the sphingolipid C4-hydroxylase (SLH) that is present only in fungi and plants. This reaction generates phytosphingosine (PhSph) that is essential for filamentous fungal growth and viability (Li et al., 2006). In S. cerevisiae, Sur2 encoding sphingolipid C4-hydroxylase is essential for sphingolipid C4-hydroxylation activity but is not essential for normal growth (Cliften et al., 1996; Haak et al., 1997). In A. nidulans, deletion of basA leads to a reduced growth with an hyperbranching of hyphae, an aberrant cell wall thickening and a strong defect in conidiation (Li et al., 2006, 2007).
A C18–26 FA chain is linked to PhSph via an amide by ceramide synthase (CerS) to form phytoceramide (PhCer) that seems to be relevant for fungal viability and hyphal morphogenesis. In A. nidulans, ceramide synthase is encoded by the lagA and is required for the apical growth and morphology (Li et al., 2006). PhCer is transported from the ER to the outer leaflet of the Golgi membrane for the synthesis of IPC in the next step (Dickson et al., 2006).
The myo-inositol-1-phosphate group is transferred from phosphatidylinositol (PI) to the C1 hydroxyl of PhCer to produce IPC, catalyzed by the IPC synthase (Cheng et al., 2001; Dickson et al., 2006). IPC synthase is also a rate-limiting enzyme encoded by the IPC1 or AUR1, found in S. cerevisiae, Schizosaccharomyces pombe, Candida spp., and Aspergillus spp. (Hashida-Okado et al., 1996; Nagiec et al., 1997; Kuroda et al., 1999; Georgopapadakou, 2000; Cheng et al., 2001). The aurA is required for polarized cell growth in A. nidulans; repression of aurA in A. nidulans alcA::aurA causes a terminal phenotype of germinating spores and lacks polarized hyphal growth (Cheng et al., 2001). In filamentous fungi, IPC is further modified by addition of mannose and a second inositol phosphate group in a reaction catalyzed by MIPC transferase (MIT, encoded by mitA) to generate two products, MIPC that has a mannose sugar unit and M(IP)2C that has a mannose unit as well as two inositol groups (Leber et al., 1997). The mitA gene is essential for the addition of the first mannose residue to the inositol ring. Although A. fumigatus ΔmitA abolishes the production of MIPCs and MIPC-derived GSLs, leading to accumulation of the precursor IPC, the ΔmitA mutant shows normally growth and no defects in cell wall or membrane organization, suggesting that MIPC is not critical for fungal differentiation (Kotz et al., 2010). MIPC and M(IP)2C are two forms of GIPCs found in several fungi that are particularly regulated during morphogenesis (Takahashi et al., 2009; Guimarães et al., 2014; Buré et al., 2014).
Blocking GSL biosynthesis has become a target for developing antifungal therapies and understanding fungal biology. Thus, enzyme inhibitors of synthetic GSLs for a variety of biological processes have already been demonstrated. There are five key steps in which enzymes can be blocked by inhibitors in the GSL biosynthetic pathway (Figure 2 the blue fonts). As the first rate-limiting enzyme of the GSL biosynthetic pathway, SPT has been the subject of many studies to identify inhibitors. One of the earliest SPT inhibitors identified is the mechanism-based inhibitor L-cysteine (Zheng et al., 1994, 1998). The most widely used and studied natural product SPT inhibitors are myriocin and sphingofungin that have been shown to block biofilm formation and polarized growth of fungal hyphae in Candida and Aspergillus species (Cheng et al., 2001; Lattif et al., 2011; Perdoni et al., 2015; Harrison et al., 2018). In addition, β-chloroalanine, β-fluoroalanine, and halide can also be used to inhibit SPT (Lev and Milford, 1981; Medlock and Merrill, 1988; Smith and Merrill, 1995). Fumonisin B1 and australifungin are inhibitors of ceramide synthase that alter GSL metabolism by inhibiting ceramide synthesis, and act as antifungal agents in Cryptococcus, Candida, and Aspergillus species (Mandala et al., 1995; Merrill et al., 2001). Glucosylceramide synthase is involved in the final step of GlcCer synthesis and can be blocked by D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (D-threoPDMP) that cause a reduction in hyphal germination and colony growth in A. nidulans, A. fumigatus, and Aspergillus terreus (Levery et al., 2002).
Inositol phosphorylceramides synthases are unique structures in fungi involved in many cellular processes, including growth, differentiation, and morphogenesis, but are not found in mammals (Barr and Lester, 1984; Levery et al., 1998, 2002; Loureiro y Penha et al., 2001). Therefore, IPC synthases constitute potential targets for the development of new antifungal drugs, as inhibiting it will lead to the accumulation of PhSph and PhCer and, ultimately fungal death. Aureobasidin A, khakrefungin, and galbonolide inhibitors of IPC synthase can block transfer of myo-inositol-1-phosphate from PI to ceramide, resulting in accumulation of PhSph and PhCer, and complete inhibition of IPC production, along with cell cycle arrest in C. albicans, Cryptococcus neoformans, S. cerevisiae, and A. nidulans (Cheng et al., 2001; Cerantola et al., 2009; Tan and Tay, 2013). Therefore, IPC inhibitors, with low toxicity in mammals due to the lack of a mammalian IPC synthase, might be possible candidates as ideal antifungal drugs (Takesako et al., 1993; Mandala et al., 1997, 1998; Georgopapadakou, 2000; Rollin-Pinheiro et al., 2016).
Glycosphingolipids are the main lipid components of microdomains and are clustered along with sterols in specialized membrane microdomains to form lipid rafts in filamentous fungi (Sonnino et al., 2007). These regions play crucial roles in cell polarization and hyphal growth, mediated by the cytoskeleton and accumulation of lipid raft components at the growing tip of hyphal cells (Cheng et al., 2001; Barreto-Bergter et al., 2004; Pearson et al., 2004; Nimrichter and Rodrigues, 2011; Guimarães et al., 2014). Biological functions of GSLs have been studied through gene deletion and inhibitory approaches in filamentous fungi (Rittershaus et al., 2006; Oura and Kajiwara, 2008, 2010; Ramamoorthy et al., 2009; Zhu et al., 2014; Fernandes et al., 2016). Several studies have demonstrated that impairment of any steps of GlcCer biosynthetic pathway leads to compromised mycelium growth, hyphal elongation, and lipid raft mislocalization. For example, disruption of the initial step of GlcCer synthesis by deletion of barA or by inhibition in presence of HSAF strongly reduces polarized growth and leads to the formation of enlarged and hyperbranched hyphae, with altered cell wall (Li et al., 2006, Li et al., 2009). Similarly, disruption of the final step of GlcCer synthesis by deletion of GCS or by glucosylceramide synthase inhibitors also impairs hyphal extension in A. nidulans and inhibits morphological transitions such as budding and germ tube formation in A. fumigatus (Levery et al., 2002; Ramamoorthy et al., 2007; Fernandes et al., 2016). These results suggest that GlcCer synthase is essential for the establishment and maintenance of the polarity axis, fungal growth, and differentiation in filamentous fungi (Figure 3).
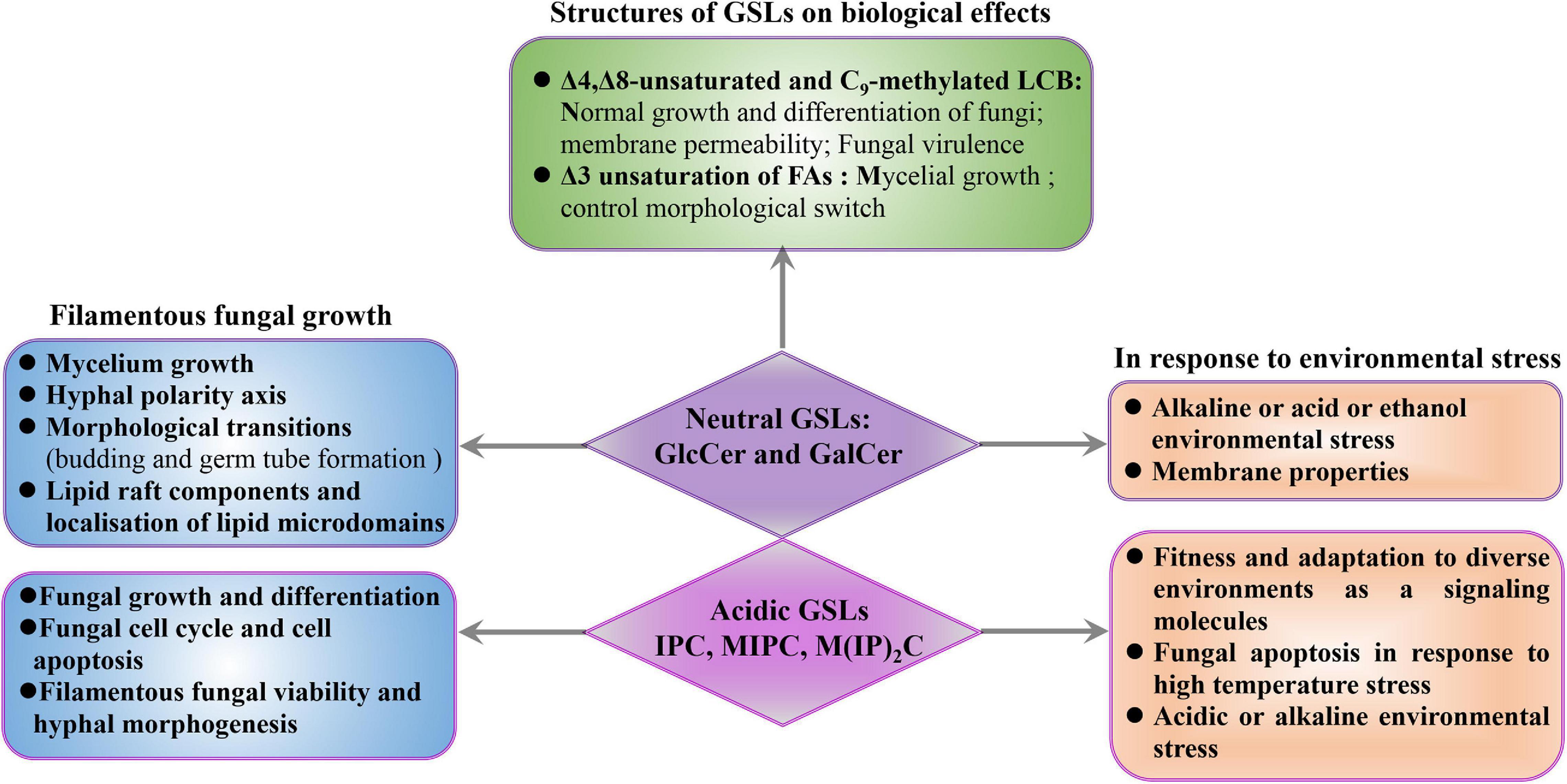
Figure 3. Biological roles of GSLs in filamentous fungi. The effects of GSLs on filamentous fungi were discussed from three aspects, mainly including fungal growth and environmental stress. Meanwhile, the functions of the Δ3-unsaturated FAs, and Δ4, Δ8-unsaturated, and C9-methylated structures of GlcCer and GalCer were also summarized.
In filamentous fungi, not only is growth and differentiation reduced upon impairment of neutral GSL synthesis, but growth also relies on the production of IPC (Figure 3). Inhibition of IPC synthase is followed by an accumulation of PhSph and PhCer intermediates that lead to fungal cell cycle arrest and apoptosis (Cheng et al., 2001). Integrity of the IPC synthesis pathway is relevant for filamentous fungal viability, not only due to the role of IPC in fungal differentiation but also due to the need for highly regulation of DhSph, PhSph, and PhCer levels, as DhSph and PhSph are highly toxic to fungal cells. Thus, changes in their levels may have uncontrolled effects on signaling events, resulting in fungal cell death which indicate that PhCer synthase and IPC synthase can be potential targets for development of new antifungal drugs, as inhibiting these enzymes will lead to the accumulation of PhSph and PhCer, and, ultimately, fungal death.
In addition to fungal polarization and hyphal growth, GSLs also play important role in filamentous fungal fitness and adaptation to diverse environments as a signaling molecules (Grimm et al., 2006; Lingwood and Simons, 2010; Figure 3). The fungal heat shock response is characterized by the production of heat shock proteins that function as chaperonins, and in the processes of protein degradation. GSLs possess the capacity to serve as bioactive signaling molecules in early heat stress response. This signaling function influences the regulation of the cell cycle and the synthesis of heat shock proteins, which have important secondary effects, especially if heat shock proteins are not available to serve as protectors of other proteins (Jenkins, 2003). For example, C. albicans treatment with inhibitor of SPT, leads to the decrease in lipid domain accumulation, resulting in less recruitment of heat-shock proteins (Insenser et al., 2006). In addition, environmental stresses directly affect the representation of fungal GSL classes (Řezanka et al., 2018). Thermophilic microorganisms can change the contents of IPC, MIPC, or M(IP)2C to mediate fungal apoptosis in response to high temperature stress (Řezanka et al., 2016, 2018). N. crassa synthesizes PhCer as signal molecules to mediate fungal apoptosis in response to heat stress (Plesofsky et al., 2008). In S. cerevisiae, the accumulation of IPC is detrimental to yeast under low pH conditions, and downregulation of IPC levels is one of the adaptation mechanisms for low pH conditions (Otsu et al., 2020). In contrast, reduction of the IPC level increases the sensitivity of C. neoformans to low pH and impairs the growth and the pathogenicity of C. neoformans (Luberto, 2001). Besides, blocking the pathway of GSL synthesis or adding exogenous GSLs can significantly affect fungal tolerance to acidic or alkaline environmental stress. For example, in C. neoformans, the Δcer1 mutant cannot survive in acid or alkaline environments due to its inability to synthesize GlcCer (Ishibashi et al., 2012; Munshi et al., 2018). Besides, the C. neoformansΔsld8 mutant that only synthesizes saturated GlcCer is more susceptible to membrane stressors and shows increased membrane permeability, resulting in decreased stress resistance (Raj et al., 2017). Similarly, S. cerevisiae, which is incapable of synthesizing GlcCer, can adapt to alkaline and ethanol stress after exposure to Aspergillus kawachii-derived GlcCer via altering the yeast membrane properties by exogenous GlcCer (Sawada et al., 2015). These results demonstrate that GSLs play important roles in response to environmental stress in fungi (Figure 3).
Impairment of polarized hyphal growth and mislocalization of lipid microdomains were observed when the biosynthetic steps of sphingolipid desaturase and sphingolipid C9-methyltransferase were interrupted, demonstrating that unsaturation and C9-methylation of the neutral GSLs are essential for growth and differentiation in filamentous fungi. The structures of Δ4,Δ8-unsaturated and C9-methylated LCB, distinguishing filamentous fungi from plant and mammalian sphingosines, are unique to fungal neutral GSLs (Barreto-Bergter et al., 2004, 2011; Del Poeta et al., 2014). Disruption of the A. nidulans sdeA leads to increase accumulation of saturated and unmethylated GlcCer and reduce mycelium growth (Fernandes et al., 2016). Previous studies in C. neoformans showed that Δsld8 mutant synthesizing only saturated GlcCer is more susceptible to membrane stressors follow by increasing membrane permeability, even though saturated GlcCer produced more lipid rafts than unsaturated GlcCer species (Raj et al., 2017). The C9-methylated found in LCB is required for normal growth and differentiation in filamentous fungi (Ramamoorthy et al., 2009; Fernandes et al., 2016). A. nidulans smtA deletion combined with conditional repression of smtB significantly increases unmethylated GlcCer levels and compromises filamentous growth (Fernandes et al., 2016). The ΔFgmt2 mutant produces 65–75% unmethylated GlcCer shows severe growth defects when compared to the wild-type strain (Ramamoorthy et al., 2009). In pathogenic yeasts, deletion of the gene encoding C9-methyltransferase results in a mutant with attenuated virulence (Noble et al., 2010; Singh et al., 2012). Interestingly, certain plant defensins require the C9-methylation for fungal GSL recognition (Oguro et al., 2014; Fernandes et al., 2016), which suggest that plant defensins may have a therapeutic potential for treatment of fungal infections for fungus specific C9-methylation (Figure 3).
The unsaturated FAs of neutral GSLs are also important for the mycelial growth in filamentous fungi. The Δ3-unsaturated FA is a unique modification of fungal GSLs, which has been reported in neutral GSLs of A. oryzae (Yasuhiko and Masao, 1997), A. fumigatus (Toledo et al., 1999), and S. schenckii (Toledo et al., 2001), and is involved in signaling pathways that control morphological switch (Figure 3). In fact, the ratio of saturated and Δ3-unsaturated 2-hydroxy FAs vary among the GSLs from different fungal morphotypes. For example, only 15% of GlcCer extracted from the Paracoccidioides brasiliensis yeasts is composed of Δ3-unsaturated FAs, while 50% of GlcCer contains the Δ3-unsaturation in P. brasiliensis mycelium (Toledo et al., 1999). Similarly, GlcCer from Histoplasma capsulatum yeast contains a higher proportion of saturated FAs, while the GlcCer from mycelium is almost exclusively constituted by the Δ3-unsaturated FAs (Toledo et al., 2001). The higher contents of Δ3-unsaturated GlcCer in mycelial forms of P. brasiliensis and H. capsulatum may be ascribed to the activation of desaturase activity during the yeast-to-hypha transition, suggesting that Δ3-unsaturation of GlcCer may be involved in signaling pathways that control morphological switch.
In contrast to GlcCer, most studies of GalCer have been performed in mammals and its biological function in filamentous fungi remains unknown. It has been previously shown that sphingolipids regulate the activity of protein kinases, such as protein kinase C and protein phosphatases, involved in signaling cascades that ultimately modulate cell growth, differentiation, and proliferation (Hannun and Obeid, 2008). S. schenckii mycelia synthesize only GlcCer, while yeast forms are found to contain both GlcCer and GalCer, suggesting that the ceramide galactosyltransferase may be activated during the S. schenckii mycelium-yeast switch or inhibited during the yeast-to-hypha transition (Dickson and Lester, 1999; Toledo et al., 2000). Structural analyses of neutral GSLs between A. fumigatus and S. schenckii show that GalCer and GlcCer possess identical ceramide backbones, except that GalCer contains a higher proportion of Δ3-unsaturated FA (Toledo et al., 1999, 2000). Similarly, the GalCer production and differential Δ3-unsaturation of FAs may constitute a molecular mechanism of GSL control over fungal morphogenesis through the activation/inactivation of signal transduction pathways (Fernandes et al., 2018).
Acidic GSLs in plants and fungi are more structurally diverse and difficult to analyze due to unavailability of commercial standards required by mass spectrometry. Therefore, neutral GSLs are mainly exploited and applicated in the cosmetics and health food products. Neutral GSLs are a common natural component in plants, animals, and fungi. Experiments show that neutral GSLs are not toxic, and no adverse reactions in humans. Therefore, neutral GSLs, mainly GlcCers obtained from plants, are now widely added in cosmetics as moisturizing ingredients and health foods as functional component (Alessandrini et al., 2004; Tessema et al., 2017).
Ceramide is thought to be a critical molecule in the maintenance and formation of the epidermal permeability barrier and an important component in water-retention of skin (Hamanaka et al., 2005). GlcCer can be hydrolyzed to ceramide by β-glucocerebrosidase in keratinocytes, and the resultant ceramide is stored in stratum corneum (Figure 4). Levels of GlcCer significantly increase during epidermal differentiation, and then GlcCer is enzymatically hydrolyzed to ceramides to regulate permeability barrier function. The level of epidermal ceramide is regulated by a balance between β-glucocerebrosidase, sphingomyelinase, and ceramidase (Holleran et al., 2006). A deficiency or inhibition of β-glucocerebrosidase in the epidermis can alter the distribution of ceramide and GlcCer, resulting in decreased the epidermal permeability barrier function (Alessandrini et al., 2004; Carneiro et al., 2011). Low levels of GluCer and ceramide in atherosclerosis-prone mice led to skin inflammation and hair discoloration and loss (Bedja et al., 2018). Prior studies demonstrated that all molecular species of stratum corneum ceramides are derived from GlcCer (Uchida et al., 2000; Hamanaka et al., 2002). In addition, GlcCer also prevents dehydration of the stratum corneum and repaired the barrier function of skin. Therefore, the GlcCers play critical role in enhancing the epidermal permeability barrier function.
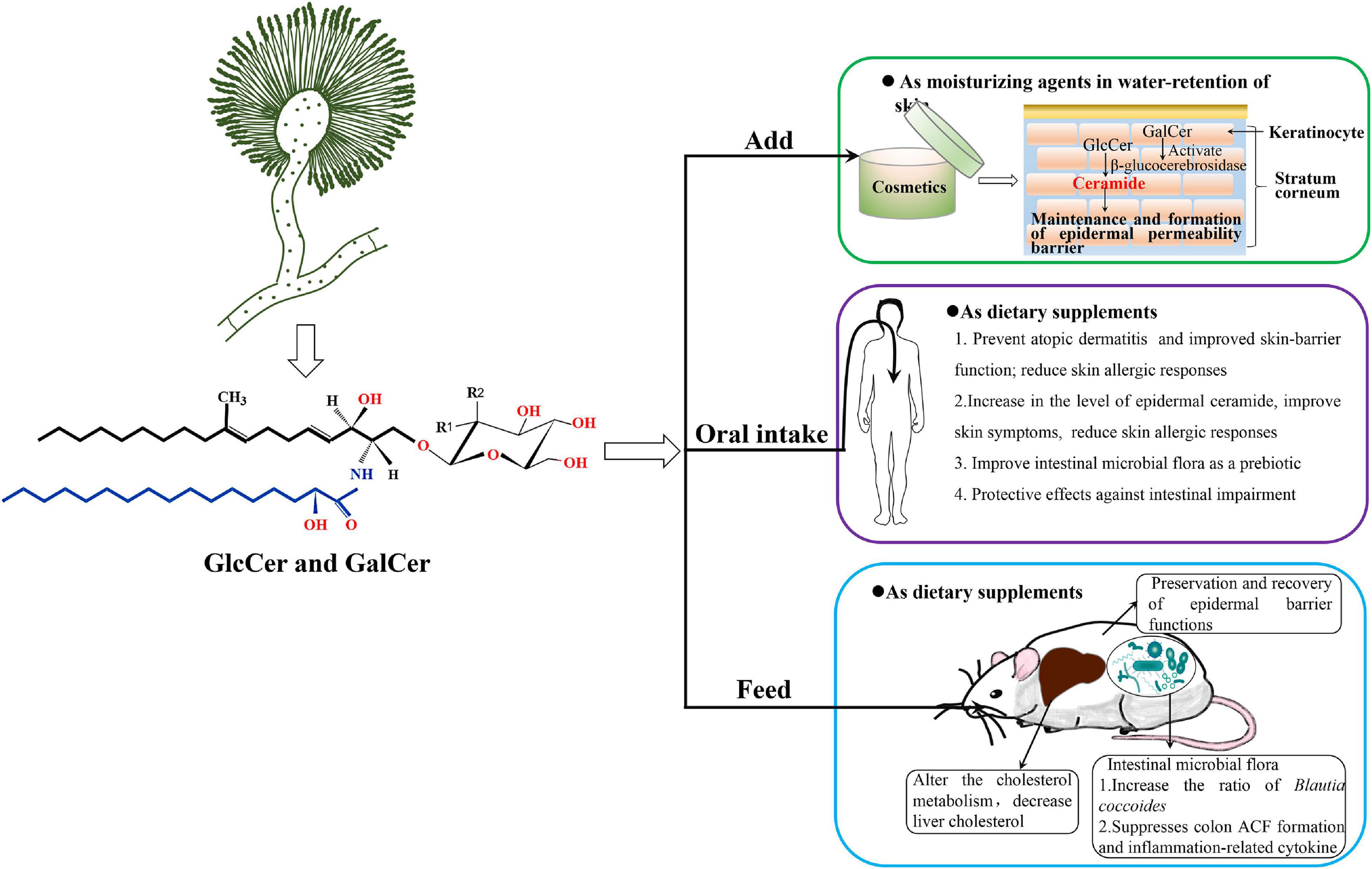
Figure 4. The potential application diagram of filamentous fungal glycosylceramides, taking A. oryzae as an example. A. oryzae produces GlcCers and GalCers of glycosylceramides which might be safe to add in cosmetics as moisturizing ingredient or health food products as nutritional supplements. A. oryzae glycosylceramides, which function as a prebiotic, have been confirmed to increase ratio of Blautia coccoides and significantly decrease liver cholesterol in obese mice fed with koji glycosylceramide. Monosaccharide may be either glucosyl (Glc) (R1 = OH, R2 = H) or galactosyl (Gal) residues (R1 = H, R2 = OH) in the chemical structure.
Furthermore, the beneficial effects of oral intake of plant GlcCer for skin hydration and skin barrier reinforcement have also been established in several studies involving animal models (Tsuji et al., 2006; Uchiyama et al., 2008; Yeom et al., 2012; Kawano and Umemura, 2013) as well as human subjects (Miyanishi et al., 2005; Kimata, 2006; Uchiyama et al., 2008; Guillou et al., 2011; Figure 4). The present studies suggest that the lipophilic fraction of GlcCer, present in plants has protective effects against intestinal impairment, but it requires extraction since digestion alone is not enough to elicit its complete protective action. Therefore, purified GlcCers are usually added in health food as a function complement. Some studies report that an intake of 0.6–1.8 mg/day of GlcCer from supplements is enough to improve human skin health (Uchiyama et al., 2008; Hirakawa et al., 2013). Shimoda et al. (2012) established the effects of GlcCer on the changes in epidermal ceramide and GlcCer in mice after oral intake of rice-derived GlcCer, as well as in a human epidermal equivalent. These findings demonstrated an increase in the level of epidermal ceramide follow by a decrease in the amounts of GlcCer (accompanied with enhanced β-glucocerebrosidase and GlcCer synthase expressions), suggesting epidermal GlcCer metabolism enhancing effect by oral intake of GlcCers. Oral administration of GluCer from maize significantly reduced UVA-induced wrinkle formation in the skin as well as epidermal hypertrophy of hairless mice (Shimada et al., 2011). Oral intake of konjac GlcCer has been reported to decrease the transepidermal water loss (TEWL) in atopic dermatitis (AD) patients (Miyanishi et al., 2005), and improve skin symptoms (including TEWL reduction) and reduce skin allergic responses in children with moderate AD (Kimata, 2006). These studies support the beneficial effects of oral intake of plant GlcCer and their potential complementary and alternative therapeutic applications in the restoration and maintenance of skin barrier function. Therefore, GlcCers exhibit a wide potential for development and application as an important biological resource in improving epidermal barrier function.
In addition to prevent AD and improved skin-barrier function, plant GlcCer also prevent intestinal impairment as dietary supplements (Figure 4). Recently, the incidence rate of intestinal impairments, such as colon cancer and inflammatory bowel disease (IBD), has increased in East Asian countries and Western countries (Molodecky et al., 2012). It is difficult to recover completely from IBD and these patients have an increased risk of developing colon cancer (Triantafillidis et al., 2009). There are many studies on the effect of GlcCer contained in food on intestinal impairment. In vitro experiments indicate the possibility that GlcCer protects the colon surface from the harmful effects of various drugs (Yamashita et al., 2017). In addition, GlcCer has been shown to have an apoptosis-inducing effect on colon cancer cells in vitro (Aida et al., 2004). Previous studies demonstrate that uptake of dietary GlcCer may improve the microenvironment of intestinal tract via modulating the intestinal microbiota (Schmelz et al., 2015). Yamashita et al. (2019) had confirmed that dietary GlcCer can significantly suppress aberrant crypt foci (ACF) formation and the production of inflammation-related cytokines in mice fed with rice-derived extracted GlcCer, which suggest GlcCer extracted from polished rice has protective effects against intestinal impairment. Collectively, dietary GlcCers can be digested by the intestinal microbial flora and display preventive and chemotherapeutic effects on colon cancer in animal models; however, clinical trials are urgently needed to investigate the response of colon carcinogenesis to dietary GlcCer in the future.
Although there are few studies on the applications of fungal glycosylceramides, we summarized several applications of them in cosmetics or foods. Interestingly, Takahashi confirmed that GlcCer produced by A. oryzae constituted the most abundant species (43% of the total GlcCer) in the sake lees, which is brewed with A. oryzae and S. cerevisiae, and has long been recognized for its moisture-holding activity in cosmetics in Japan (Takahashi et al., 2014). Torula yeast (Candida utilis)-derived GlcCer has been reported to increase dermal fibroblast proliferation and collagen production to maintain dermal elasticity (Fukunaga et al., 2019). Miyagawa et al. (2019) investigated the effects of glycosylceramides on gene expression in normal human epidermal keratinocytes, which reveal that koji and Aspergillus luchuensis and A. oryzae glycosylceramides increased the expression of occludin (OCLN, an epidermal tight junction protein) and ATP-binding cassette sub-family A member 12 (ABCA12, a cellular membrane transporter) to increase ceramide in the keratinocytes. These results indicated that glycosylceramides have an effect of increasing genes expression which involved in skin barrier function and the transport of lipids in the keratinocytes, and suggest that koji exerts its cosmetic effect by increasing ceramide and tight junctions via glycosylceramides. In addition to function in cosmetics, fungal neutral GSLs also play an important role in foods. For example, Ferdouse et al. (2018) have proved that addition of A. oryzae glycosylceramide can affect the flavor and metabolic profiles of sake yeast in the manufacture of sake. They also demonstrate that addition of A. oryzae, Glycine max, and Grifola frondosa GlcCers confer a similar effect on the flavor profiles of sake yeast (Ferdouse et al., 2018), which indicate that the effects of plant GlcCer and A. oryzae GlcCer on flavor profiles are similar. In addition, A. oryzae glycosylceramide, used as a prebiotic in obese mice, can be digested by the intestinal microbial flora and increased ratio of B. coccoides, which is a potentially beneficial species in the intestine (Hamajima et al., 2019). Besides, feeding of A. oryzae glycosylceramide to obese mice can alter the cholesterol metabolism that liver cholesterol is significantly decreased in obese mice fed with A. oryzae glycosylceramide (Figure 4). These results will be of value in the utilization of fungal GlcCer and GalCer for cosmetics and functional foods.
Although neutral GSLs are originally derived from soybean and bovine sources, currently almost all the GlcCers used in cosmetics or health foods are extracted from plants. GlcCers from wheat flour (Ohnishi et al., 1985), potatoes (Bartke et al., 2006), maize, rice (Sugawara et al., 2010), and konjac (Uchiyama et al., 2008; Usuki et al., 2015) are now widely added in cosmetics as moisturizing agents or in health foods as “functional components.” The most abundant classes of GSLs in plant tissue are mono-GlcCers. In contrast, the GalCer of glycosylceramides is rarely detected or reported in plants (Sullards et al., 2000; Spassieva and Hille, 2003). In cosmetics, GlcCer must be hydrolyzed to ceramide that is then deposited in stratum corneum to form the epidermal barrier. The more selective and efficient approach of transforming GlcCer to ceramide is enzymatic hydrolysis of β-glucocerebrosidase. Interestingly, GalCer, restricted to mainly in neural tissues in mammals, can activate β-glucocerebrosidase to hydrolyze GlcCer in keratinocytes and increase ceramide content to improve dry skin and AD (Alessandrini et al., 2004). However, GalCer is mainly extracted from neural tissues of animals such as cows and has been for the subject of several studies until the discovery of bovine spongiform encephalopathy. Although the animal glycosylceramides obtained from bovine brain and biotechnological sources have been investigated, the safety profile for cosmetic and food applications has not yet been established (Adam, 2001; Ono et al., 2010). Thus, GalCer from neural tissues of animals is not acceptable for cosmetic or other human use due to the underlying risk for prion diseases. As an alternative, glycosylceramides isolated from edible plants are highly safe and preferable for cosmetic and therapeutic applications. Unfortunately, plant glycosylceramides only contain GlcCer and are totally devoid of GalCer. Therefore, it is important to seek new biological resources that can synthesize both GlcCer and GalCer, along with attempts to improve their contents by modifying the biosynthetic pathway of neutral GSLs using genetic engineering technology in known resources.
Glycosylceramides, which have now been commercialized as moisturizing agents or dietary supplements for dry skin, are mainly sourced from edible plants that only contain GlcCer and are completely without GalCer. Surprisingly, there are many reports that filamentous fungi Aspergillus species, such as A. oryzae, A. awamori, and A. sojae that are used in various Japanese fermented foods and drinks, can produce both GlcCer and GalCer (Fujino and Ohnishi, 1977; Zhang et al., 2007; Hirata et al., 2012; Tani et al., 2014). Therefore, glycosylceramides from Aspergillus that include both GlcCer and GalCer can make up for the lack of GalCer in plants and would be more effective for their barrier function of skin in cosmetics as compared to plant GlcCer, due to the GalCer can activate β-glucocerebrosidase to promote the hydrolysis of GlcCer to ceramide (Figure 4; Alessandrini et al., 2004; Tani et al., 2014). Furthermore, previous studies have elucidated that koji (A. oryzae, A. awamori, and A. sojae) contains abundant glycosylceramides (0.5–2 mg/g dry weight koji), which are comprised of GalCer (30.3%) and GlcCer (69.7%), and are one of the highest amounts found in any cuisine (Hirata et al., 2012; Hamajima et al., 2016). Therefore, since filamentous fungi Aspergillus are economically important in the industrial production and can synthesize both GlcCer and GalCer, they would be new sources for exploiting neutral glycosylceramides in future.
Another advantage of filamentous fungi in the production of GlcCer and GalCer is that the cell growth cycle of fungi is much shorter than that of plants, which can provide a large amount of mycelium for extraction of GlcCer and GalCer. In addition, most filamentous fungi used in the production of fermented foods are safe for humans, especially A. oryzae, which is generally regarded as safe according to the FDA and has been used for making fermented foods such as saké, shoyu (soy sauce), and miso (soybean paste) for thousands of years. Therefore, the A. oryzae would be a suitable source for production of GlcCer and GalCer due to their food safety. Furthermore, the availability of genome sequences of A. oryzae and the availability of DNA arrays, GeneChips, and RNA sequence have provided an unprecedented resource for studying and modifying the biosynthetic pathways of GlcCer and GalCer. In summary, we believe that filamentous fungi would be important resources for exploiting neutral glycosylceramides in the future, especially GalCer.
There is no doubt that exogenous or endogenous GSLs as moisturizing ingredients or dietary supplements continue to surprise us today and there are exciting times for the field. GSLs are a chemically complex group of substances, widely existing in fungi, plant, and animals. In this review, we summarized the kinds and structures of filamentous fungi GSLs. The synthetic pathway and biological roles of GSLs in filamentous fungi were also discussed. Importantly, we particularly focused on the important role of neutral GSLs adding in cosmetics as moisturizing ingredients and dietary supplements as functional components. Meanwhile we also drawn attention to the limitation of GSLs from plants and animals and the advantage of GSLs from filamentous fungi. Collectively, neutral GSLs from filamentous fungi Aspergillus will play important roles in the barrier function of skin and intestinal impairment of human health, which greatly increase the demand for neutral GSL. Nowadays, the market demand of GSLs as a new raw ingredient in beauty products is increasing rapidly at an annual growth rate of about 15%.
Since neutral GSLs are benefit to human health, a broader assessment of the types of GSLs adding in cosmetics and health foods is needed because they may have beneficial effects on human health. Not only are endogenous GSLs involved in obesity-related pathologies, prevention or treatment of obesity, and reducing skin allergic responses, but exogenous GSLs may be beneficial in the barrier function of skin (Walls et al., 2013; Miyagawa et al., 2019; Le Barz et al., 2020). Nowadays neutral GSLs, mainly GlcCers obtained from plants, are now widely included in cosmetics or health food. Previous studies done on improving AD and skin moisture suggest that oral intake of GlcCer activates enteric canal immunity and ceramide metabolism in the skin, rather than the direct reutilization of dietary GlcCer (Ono et al., 2010; Duan et al., 2012), which conclude that the maintenance of intestinal homeostasis by dietary GlcCer might be indirectly related to these mechanisms. In addition, GlcCers mast be hydrolyzed by β-glucocerebrosidase to ceramide in keratinocytes or mucosal cells of the small intestine and colon; and then ceramide is taken up directly by intestinal cells in human intestinal epithelial cell models (Nicolas et al., 2005). GalCer was reported to activate β-glucocerebrosidase in keratinocytes and increase ceramide content to improve dry skin and prevent AD (Alessandrini et al., 2004; Carneiro et al., 2011). Unfortunately, neutral GSLs derive from plants without any GalCer; while GalCer extracted from neural tissue of animals such as bovine has been applied for several research until the finding of bovine spongiform encephalopathy. What is more, the production of GalCer from neural tissues of animals would not be acceptable for cosmetic or other human uses. Also, GalCer from pathogenic fungi is not suitable for oral intake. Since neutral GSLs from plants do not contain GalCer, and GalCer from animals is at risk of viral infection, those from filamentous fungi Aspergillus that include both GlcCer and GalCer would be highly effective for barrier function of skin and safe for health food as dietary supplements. Therefore, the filamentous fungi Aspergillus, synthesizing both GlcCer and GalCer, could be an amenable source to produce glycosylceramides, because they are food safe. We believe that the development of GlcCer and GalCer, especially GalCer, from Aspergillus would become a trend and major source in the future.
JG: conceptualization and writing—original draft preparation. CJ: writing—review and editing, conceptualization, and funding acquisition. BZ: supervision and funding acquisition. BH: project administration. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (NSFC), grant number 31900063, the Science and Technology Research Project of Jiangxi Provincial Department of Education, grant number GJJ180630, and Natural Science Foundation of Jiangxi Province, grant number 20192ACBL20012.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We Thank to those who made critical review on this manuscript.
Aida, K., Kinoshita, M., Sugawara, T., Ono, J., Miyazawa, T., and Ohnishi, M. (2004). Apoptosis inducement by plant and fungus sphingoid bases in human colon cancer cells. J. Oleo Sci. 53, 503–510. doi: 10.5650/jos.53.503
Alessandrini, F., Pfister, S., Kremmer, E., Gerber, J. K., Ring, J., and Behrendt, H. (2004). Alterations of glucosylceramide-beta-glucosidase levels in the skin of patients with psoriasis vulgaris. J. Invest. Dermatol. 123, 1030–1036. doi: 10.1111/j.0022-202x.2004.23469.x
Allgaier, S., Taylor, R. D., Brudnaya, Y., Jacobson, D. J., Cambareri, E., and Stuart, W. D. (2009). Vaccine production in Neurospora crassa. Biological 37, 128–132. doi: 10.1016/j.biologicals.2009.02.006
Barr, K., and Lester, R. L. (1984). Occurrence of novel antigenic phosphoinositol-containing sphingolipids in the pathogenic yeast Histoplasma capsulatum. Biochemistry 23, 5581–5588. doi: 10.1021/bi00318a031
Barreto-Bergter, E., Pinto, M. R., and Rodrigues, M. L. (2004). Structure and biological functions of fungal cerebrosides. An. Acad. Bras. Cienc. 76, 67–84. doi: 10.1590/s0001-37652004000100007
Barreto-Bergter, E., Sassaki, G. L., and De Souza, L. M. (2011). Structural analysis of fungal cerebrosides. Front. Microbiol. 2:239. doi: 10.3389/fmicb.2011.00239
Bartke, N., Fischbeck, A., and Humpf, H. U. (2006). Analysis of sphingolipids in potatoes (Solanum tuberosum L.) and sweet potatoes (Ipomoea batatas (L.) lam.) by reversed phase high-performance liquid chromatography electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS). Mol. Nutr. Food Res. 50, 1201–1211. doi: 10.1002/mnfr.200600140
Bedja, D., Yan, W., Lad, V., Iocco, D., Sivakumar, N., Bandaru, V. V. R., et al. (2018). Inhibition of glycosphingolipid synthesis reverses skin inflammation and hair loss in apoe-/- mice fed western diet. Sci. Rep. 8:11463.
Boas, M. H., Egge, H., Pohlentz, G., Hartmann, R., and Bergter, E. B. (1994). Structural determination of N-20-hydroxyoctadecenoyl-1O-b-D-glucopyranosyl-9-methyl-4,8-sphingadieninefrom species of Aspergillus. Chem. Phys. Lipids 70, 11–19.
Brunt, J. V. (1986). Fungi: the perfect hosts? Nat. Biotechnol. 4, 1057–1062. doi: 10.1038/nbt1286-1057
Buede, R., Rinker-schaffer, C., Pinto, W. J., Lester, R. L., and Dickson, R. C. (1991). Cloning and characterization of LCB1, a Saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids. J. Bacteriol. 173, 4325–4332. doi: 10.1128/jb.173.14.4325-4332.1991
Buré, C., Cacas, J. L., Mongrand, S., and Schmitter, J. M. (2014). Characterization of glycosyl inositol phosphoryl ceramides from plants and fungi by mass spectrometry. Anal. Bioanal. Chem. 406, 995–1010. doi: 10.1007/s00216-013-7130-8
Carneiro, R., Salgado, A., Raposo, S., Marto, J., Simoes, S., Urbano, M., et al. (2011). Topical emulsions containing ceramides: effects on the skin barrier function and anti-inflammatory properties. Eur. J. Lipid Sci. Tech. 113, 961–966. doi: 10.1002/ejlt.201000495
Cerantola, V., Guillas, I., Roubaty, C., Vionnet, C., Uldry, D., Knudsen, J., et al. (2009). Aureobasidin A arrests growth of yeast cells through both ceramide intoxication and deprivation of essential inositolphosphorylceramides. Mol. Microbiol. 71, 1523–1537. doi: 10.1111/j.1365-2958.2009.06628.x
Chen, P. W., Fonseca, L. L., Hannun, Y. A., and Voit, E. O. (2013). Coordination of rapid sphingolipid responses to heat stress in yeast. PLoS Comput. Biol. 9:e1003078. doi: 10.1371/journal.pcbi.1003078
Chen, T., Xiong, S., Jiang, S., Wang, M., Wu, Q., and Wei, H. (2011). Molecular identification of microbial community in Chinese douchi during post-fermentation process. Food Sci. Biotechnol. 20, 1633–1638. doi: 10.1007/s10068-011-0225-0
Cheng, J., Park, T. S., Fischl, A. S., and Ye, X. S. (2001). Cell cycle progression and cell polarity require sphingolipid biosynthesis in Aspergillus nidulans. Mol. Cell. Biol. 21, 6198–6209. doi: 10.1128/mcb.21.18.6198-6209.2001
Cheon, S. A., Bal, J., Song, Y., Hwang, H. M., Kim, A. R., Kang, W. K., et al. (2012). Distinct roles of two ceramide synthases, CaLag1p and CaLac1p, in the morphogenesis of Candida albicans. Mol. Microbiol. 83, 728–745. doi: 10.1111/j.1365-2958.2011.07961.x
Cherry, J. R., and Fidantsef, A. L. (2003). Directed evolution of industrial enzymes: an update. Curr. Opin. Biotechnol. 14, 438–443. doi: 10.1016/s0958-1669(03)00099-5
Cliften, P., Wang, Y., Mochizuki, D., Miyakawa, T., Wangspa, R., Hughes, J., et al. (1996). SYR2, a gene necessary for syringomycin growth inhibition of Saccharomyces cerevisiae. Microbiology 142, 477–484. doi: 10.1099/13500872-142-3-477
Dickson, R. C. (2008). Thematic review series: sphingolipids. New insights into sphingolipid metabolism and function in budding yeast. J. Lipid Res. 49, 909–921. doi: 10.1194/jlr.r800003-jlr200
Dickson, R. C., and Lester, R. L. (1999). Yeast sphingolipids. Biochim. Biophys. Acta 1426, 347–357.
Dickson, R. C., Sumanasekera, C., and Lester, R. L. (2006). Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog. Lipid Res. 45, 447–465. doi: 10.1016/j.plipres.2006.03.004
Duan, J., Sugawara, T., Hirose, M., Aida, K., Sakai, S., Fujii, A., et al. (2012). Dietary sphingolipids improve skin barrier functions via the upregulation of ceramide synthases in the epidermis. Exp. Dermatol. 21, 448–452. doi: 10.1111/j.1600-0625.2012.01501.x
Duarte, R. S., Polycarpo, C. R., Wait, R., Hartmann, R., and Bergter, E. B. (1998). Structural characterization of neutral glycosphingolipids from Fusarium species. Biochim. Biophys. Acta 1390, 186–196. doi: 10.1016/s0005-2760(97)00179-3
Ferdouse, J., Yamamoto, Y., Taguchi, S., Yoshizaki, Y., Takamine, K., and Kitagaki, H. (2018). Glycosylceramide modifies the flavor and metabolic characteristics of sake yeast. Peer J. 6:e4768. doi: 10.7717/peerj.4768
Fernandes, C. M., de Castro, P. A., Singh, A., Fonseca, F. L., Pereira, M. D., Vila, T. V., et al. (2016). Functional characterization of the Aspergillus nidulans glucosylceramide pathway reveals that LCB Δ8-desaturation and C9-methylation are relevant to filamentous growth, lipid raft localization and Psd1 defensin activity. Mol. Microbiol. 102, 488–505. doi: 10.1111/mmi.13474
Fernandes, C. M., Goldman, G. H., Poeta, M. D., and Garsin, D. A. (2018). Biological roles played by sphingolipids in dimorphic and filamentous fungi. Mbio 9:2472555217719372.
Fontaine, T. (2017). Sphingolipids from the human fungal pathogen Aspergillus fumigatus. Biochimie 141, 9–15. doi: 10.1016/j.biochi.2017.06.012
Fornarotto, M., Xiao, L., Hou, Y., Koch, K. A., Chang, E., O’Malley, R. M., et al. (2006). Sphingolipid biosynthesis in pathogenic fungi: identification and characterization of the 3-ketosphinganine reductase activity of Candida albicans and Aspergillus fumigatus. Biochim. Biophys. Acta 1761, 52–63. doi: 10.1016/j.bbalip.2005.11.013
Fujino, Y., and Ohnishi, M. (1977). Structure of cerebroside in Aspergillus oryzae. Biochim. Biophys. Acta 486, 161–171.
Fujino, Y., and Ohnishi, M. (2003). Structure of cerebroside in Aspergillus oryzae. Biochim. Biophys. Acta 486, 161–171.
Fukunaga, S., Wada, S., Yamashita, M., Morita, M., Aoi, W., Naito, Y., et al. (2019). Torula yeast (Candida utilis)-derived glucosylceramide contributes to dermal elasticity in vitro. J. Food Biochem. 43:e12847.
Gable, K., Slife, H., Bacikova, D., Monaghan, E., and Dunn, T. M. (2000). Tsc3p Is an 80-amino acid protein associated with serine Palmitoyltransferase and required for optimal Enzyme activity. J. Biol. Chem. 275, 7597–7603. doi: 10.1074/jbc.275.11.7597
Gault, C. R., Obeid, L. M., and Hannun, Y. A. (2010). An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 688, 1–23. doi: 10.1007/978-1-4419-6741-1_1
Georgopapadakou, N. H. (2000). Antifungals targeted to sphingolipid synthesis: Focus on inositol phosphorylceramide synthase. Expert Opin. Investig. Drugs 9, 1787–1796. doi: 10.1517/13543784.9.8.1787
Grimm, M. O. W., Tschpe, J. A., Grimm, H. S., Zinser, E. G., and Hartmann, T. (2006). Altered membrane fluidity and lipid raft composition in presenilin-deficient cells. Acta Neurol. Scandinavica 114, 27–32. doi: 10.1111/j.1600-0404.2006.00682.x
Guillou, S., Ghabri, S., Jannot, C., Gaillard, E., Lamour, I., and Boisnic, S. (2011). The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int. J. Cosmet. Sci. 33, 138–143. doi: 10.1111/j.1468-2494.2010.00600.x
Guimarães, L. L., Toledo, M. S., Ferreira, F. A., Straus, A. H., and Takahashi, H. K. (2014). Structural diversity and biological significance of glycosphingolipids in pathogenic and opportunistic fungi. Front. Cell Infect. Microbiol. 4:138. doi: 10.3389/fcimb.2014.00138
Haak, D., Gable, K., Beeler, T., and Dunn, T. (1997). Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 272, 29704–29710. doi: 10.1074/jbc.272.47.29704
Hamajima, H., Fujikawa, A., Yamashiro, M., Ogami, T., Kitamura, S., Tsubata, M., et al. (2016). Chemical analysis of the sugar moiety of monohexosylceramide contained in koji, Japanese traditional rice fermented with Aspergillus. Fermentation 2:2. doi: 10.3390/fermentation2010002
Hamajima, H., Tanaka, M., Miyagawa, M., Sakamoto, M., Nakamura, T., Yanagita, T., et al. (2019). Koji glycosylceramide commonly contained in Japanese traditional fermented foods alters cholesterol metabolism in obese mice. Biosci. Biotechnol. Biochem. 83, 1514–1522. doi: 10.1080/09168451.2018.1562877
Hamanaka, S., Hara, M., Nishio, H., Otsuka, F., and Uchida, Y. (2002). Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J. Invest. Dermatol. 119, 416–423. doi: 10.1046/j.1523-1747.2002.01836.x
Hamanaka, S., Nakazawa, S., Yamanaka, M., Uchida, Y., and Otsuka, F. (2005). Glucosylceramide accumulates preferentially in lamellar bodies in differentiated keratinocytes. Br. J. Dermatol. 152, 426–434. doi: 10.1111/j.1365-2133.2004.06333.x
Hannun, Y. A., and Obeid, L. M. (2008). Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell. Biol. 9, 139–150. doi: 10.1038/nrm2329
Harrison, P. J., Dunn, T. M., and Campopiano, D. J. (2018). Sphingolipid biosynthesis in man and microbes. Nat. Prod. Rep. 35, 921–954. doi: 10.1039/c8np00019k
Hashida-Okado, T., Ogawa, A., Endo, M., Yasumoto, R., Takesako, K., and Kato, I. (1996). AUR1, a novel gene conferring aureobasidin resistance on Saccharomyces cerevisiae: a study of defective morphologies in Aur1p-depleted cells. Mol. Gen. Genet. 251, 236–244. doi: 10.1007/bf02172923
Heung, L. J., Luberto, C., and Del Poeta, M. (2006). Role of sphingolipids in microbial pathogenesis. Infect. Immun. 74, 28–39. doi: 10.1128/iai.74.1.28-39.2006
Hirakawa, S., Sato, A., Hattori, Y., Matsumoto, T., Yokoyama, K., and Kanai, A. (2013). Dietary rice bran extract improves TEWL of whole body. Jpn. Pharmacol. Ther. 41, 1051–1059.
Hirata, M., Tsuge, K., Jayakody, L., Urano, Y., Sawada, K., Inaba, S., et al. (2012). Structural determination of glucosylceramides in the distillation remnants of shochu, the Japanese traditional liquor, and its production by Aspergillus kawachii. J. Agric. Food Chem. 60, 11473–11482. doi: 10.1021/jf303117e
Holleran, W. M., Takagi, Y., and Uchida, Y. (2006). Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 580, 5456–5466. doi: 10.1016/j.febslet.2006.08.039
Hu, W., Liu, J., Chen, J. H., Wang, S. Y., Lu, D., Wu, Q. H., et al. (2014). A mutation of Aspergillus niger for hyper-production of citric acid from corn meal hydrolysate in a bioreactor. J. Zhejiang Univ. Sci. B 15, 1006–1010. doi: 10.1631/jzus.b1400132
Huber, A., Oemer, G., Malanovic, N., Lohner, K., Kovács, L., Salvenmoser, W., et al. (2019). Membrane sphingolipids regulate the fitness and antifungal protein susceptibility of Neurospora crassa. Front. Microbiol. 10:605. doi: 10.3389/fmicb.2019.00605
Insenser, M., Nombela, C., Molero, G., and Gil, C. (2006). Proteomic analysis of detergentresistant membranes from Candida albicans. Proteomics 6, S74–S81.
Ishibashi, Y., Ikeda, K., Sakaguchi, K., Okino, N., Taguchi, R., and Ito, M. (2012). Quality control of fungus-specific glucosylceramide in Cryptococcus neoformans by endoglycoceramidase-related protein 1 (EGCrP1). J. Biol. Chem. 287, 368–381. doi: 10.1074/jbc.m111.311340
Jenkins, G. M. (2003). The emerging role for sphingolipids in the eukaryotic heat shock response. Cell Mol. Life Sci. 60, 701–710. doi: 10.1007/s00018-003-2239-0
Karaffa, L., and Kubicek, C. P. (2019). Citric acid and itaconic acid accumulation: variations of the same story? Appl. Microbiol. Biotechnol. 103, 2889–2902. doi: 10.1007/s00253-018-09607-9
Kawano, K., and Umemura, K. (2013). Oral intake of beet extract provides protection against skin barrier impairment in hairless mice. Phytother Res. 27, 775–783. doi: 10.1002/ptr.4792
Kimata, H. (2006). Improvement of atopic dermatitis and reduction of skin allergic responses by oral intake of konjac ceramide. Pediatr. Dermatol. 23, 386–389. doi: 10.1111/j.1525-1470.2006.00268.x
Knuf, C., Nookaew, I., Brown, S. H., McCulloch, M., Berry, A., and Nielsen, J. (2013). Investigation of malic acid production in Aspergillus oryzae under nitrogen starvation conditions. Appl. Environ. Microbiol. 79, 6050–6058. doi: 10.1128/aem.01445-13
Kotz, A., Wagener, J., Engel, J., Routier, F., Echtenacher, B., Pich, A., et al. (2010). The mitA gene of Aspergillus fumigatus is required for mannosylation of inositol-phosphorylceramide, but is dispensable for pathogenicity. Fungal Genet. Biol. 47, 169–178. doi: 10.1016/j.fgb.2009.10.001
Kuroda, M., Hashida-Okado, T., Yasumoto, R., Gomi, K., Kato, I., and Takesako, K. (1999). An aureobasidin a resistance gene isolated from Aspergillus is a homolog of yeast AUR1, a gene responsible for inositol phosphorylceramide (IPC) synthase activity. Mol. Gen. Genet. 261, 290–296. doi: 10.1007/s004380050969
Lattif, A. A., Mukherjee, P. K., Chandra, J., Roth, M. R., Welti, R., Rouabhia, M., et al. (2011). Lipidomics of Candida albicans biofilms reveals phase-dependent production of phospholipid molecular classes and role for lipid rafts in biofilm formation. Microbiology 157, 3232–3242. doi: 10.1099/mic.0.051086-0
Le Barz, M., Boulet, M. M., Calzada, C., Cheillan, D., and Michalski, M. C. (2020). Alterations of endogenous sphingolipid metabolism in cardiometabolic diseases: Towards novel therapeutic approaches. Biochimie 169, 133–143. doi: 10.1016/j.biochi.2019.10.003
Leber, A., Fischer, P., Schneiter, R., Kohlwein, S. D., and Daum, G. (1997). The yeast mic2 mutant is defective in the formation of mannosyldiinositolphosphorylceramide. FEBS Lett. 411, 211–214.
Leipelt, M., Warnecke, D., Zähringer, U., Ott, C., Müller, F., Hube, B., et al. (2001). Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. J. Biol. Chem. 276, 33621–33629. doi: 10.1074/jbc.m104952200
Lester, R. L., Smith, S. W., Wells, G. B., Rees, D. C., and Angus, W. W. (1974). The isolation and partial characterization of two novel sphingolipids from Neurospora crassa: di(inositolphosphoryl)ceramide and ((gal)3glu) ceramide. J. Biol. Chem. 249, 3388–3394. doi: 10.1016/s0021-9258(19)42584-2
Lev, M., and Milford, A. F. (1981). The 3-ketodihydrosphingosine synthetase of Bacteroides melaninogenicus: Partial purification and properties. Arch. Biochem. Biophys. 212, 424–431. doi: 10.1016/0003-9861(81)90384-2
Levery, S. B., Momany, M., Lindsey, R., Toledo, M. S., Shayman, J. A., Fuller, M., et al. (2002). Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett. 525, 59–64. doi: 10.1016/s0014-5793(02)03067-3
Levery, S. B., Toledo, M. S., Doong, R. L., Straus, A. H., and Takahashi, H. K. (2000). Comparative analysis of ceramide structural modification found in fungal cerebrosides by electrospray tandem mass spectrometry with low energy collision-induced dissociation of Li+ adduct ions. Rapid. Commun. Mass Spectrom 14, 551–563. doi: 10.1002/(sici)1097-0231(20000415)14:7<551::aid-rcm909>3.0.co;2-l
Levery, S. B., Toledo, M. S., Straus, A. H., and Takahashi, H. K. (1998). Structure elucidation of sphingolipids from the mycopathogen Paracoccidioides brasiliensis: an immunodominant beta-galactofuranose residue is carried by a novel glycosylinositol phosphorylceramide antigen. Biochemistry 37, 8764–8775. doi: 10.1021/bi9730083
Li, S., Bao, D., Yuen, G., Harris, S. D., and Calvo, A. M. (2007). basA regulates cell wall organization and asexual/sexual sporulation ratio in Aspergillus nidulans. Genetics 176, 243–253. doi: 10.1534/genetics.106.068239
Li, S., Du, L., Yuen, G., and Harris, S. D. (2006). Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 17, 1218–1227. doi: 10.1091/mbc.e05-06-0533
Li, S., Calvo, A. M., Yuen, G. Y., Du, L., and Harris, S. D. (2009). Induction of cell wall thickening by the antifungal compound dihydromaltophilin disrupts fungal growth and is mediated by sphingolipid biosynthesis. J. Eukaryot. Microbiol. 56, 182–187.
Liang, Y., Pan, L., and Lin, Y. (2009). Analysis of extracellular proteins of Aspergillus oryzae grown on Soy Sauce Koji. Biosci. Biotechnol. Biochem. 73, 192–195. doi: 10.1271/bbb.80500
Lingwood, D., and Simons, K. (2010). Lipid rafts as a membrane-organizing principle. Science 327, 46–50. doi: 10.1126/science.1174621
Loureiro y Penha, C. V., Todeschini, A. R., Lopes-Bezerra, L. M., Wait, R., Jones, C., Mattos, K. A., et al. (2001). Characterization of novel structures of mannosylinositolphosphorylceramides from the yeast forms of Sporothrix schenckii. Eur. J. Biochem. 268, 4243–4250. doi: 10.1046/j.1432-1327.2001.02339.x
Luberto, C. (2001). Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev. 15, 201–212. doi: 10.1101/gad.856001
Maciel, D. M., Rodrigues, M. L., Wait, R., Villas Boas, M. H., Tischer, C. A., and Barreto-Bergter, E. (2002). Glycosphingolipids from Magnaporthe grisea cells: expression of a ceramide dihexoside presenting phytosphingosine as the long-chain base. Arch. Biochem. Biophys. 405, 205–213. doi: 10.1016/s0003-9861(02)00365-x
Mandala, S. M., Thornton, R. A., Frommer, B. R., Curotto, J. E., Rozdilsky, W., Kurtz, M. B., et al. (1995). The discovery of australifungin, a novel inhibitor of sphinganine N-acyltransferase from Sporormiella australis. Producing organism, fermentation, isolation, and biological activity. J. Antibiot. 48, 349–356. doi: 10.7164/antibiotics.48.349
Mandala, S. M., Thornton, R. A., Milligan, J., Rosenbach, M., Garcia-Calvo, M., Bull, H. G., et al. (1998). Rustmicin, a potent antifungal agent, inhibits sphingolipid synthesis at the inositol phosphoceramide synthase. J. Biol. Chem. 273, 14942–14949. doi: 10.1074/jbc.273.24.14942
Mandala, S. M., Thornton, R. A., Rosenbach, M., Milligan, J., Garcia-Calvo, M., Bull, H. G., et al. (1997). Khafrefungin, a novel inhibitor of sphingolipid synthesis. J. Biol. Chem. 272, 32709–32714. doi: 10.1074/jbc.272.51.32709
Marques, J. T., Marinho, H. S., and De Almeida, R. F. M. (2018). Sphingolipid hydroxylation in mammals, yeast and plants-an integrated view. Prog. Lipid Res. 71, 18–42. doi: 10.1016/j.plipres.2018.05.001
Medlock, K. A., and Merrill, A. H. (1988). Inhibition of serine palmitoyltransferase in vitro and long-chain base biosynthesis in intact Chinese hamster ovary cells by β-chloroalanine. Biochemistry 27, 7079–7084. doi: 10.1021/bi00418a061
Merrill, A. H. J., Sullards, M. C., Wang, E., Voss, K. A., and Riley, R. T. (2001). Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins. Environ. Health Perspect. 109, 283–289. doi: 10.2307/3435020
Michel, C., and van Echten-Deckert, G. (1997). Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum. FEBS Lett. 416, 153–155. doi: 10.1016/s0014-5793(97)01187-3
Michel, C., van Echten-Deckert, G., Rother, J., Sandhoff, K., Wang, E., and Merrill, A. H. (1997). Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J. Biol. Chem. 272, 22432–22437.
Miyagawa, M., Fujikawa, A., Nagadome, M., Kohama, K., Ogami, T., Kitamura, S., et al. (2019). Glycosylceramides purified from the Japanese traditional Non-Pathogenic Fungus Aspergillus and Koji increase the expression of genes involved in tight junctions and ceramide delivery in normal human epidermal Keratinocytes. Fermentation 5:43. doi: 10.3390/fermentation5020043
Miyanishi, K., Shiono, N., Shirai, H., Dombo, M., and Kimata, H. (2005). Reduction of transepidermal water loss by oral intake of glucosylceramides in patients with atopic eczema. Allergy 60, 1454–1455. doi: 10.1111/j.1398-9995.2005.00915.x
Molodecky, N. A., Soon, I. S., Rabi, D. M., Ghali, W. A., Ferris, M., Chernoff, G., et al. (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54. doi: 10.1053/j.gastro.2011.10.001
Mukherjee, J. J., and Dekker, E. E. (1990). 2-Amino-3-ketobutyrate CoA ligase of Escherichia coli: stoichiometry of pyridoxal phosphate binding and location of the pyridoxyllysine peptide in the primary structure of the enzyme. Biochim. Biophys. Acta 1037, 24–29. doi: 10.1016/0167-4838(90)90097-y
Munshi, M. A., Gardin, J. M., Singh, A., Luberto, C., Rieger, R., Bouklas, T., et al. (2018). The role of ceramide synthases in the pathogenicity of Cryptococcus neoformans. Cell Rep. 22, 1392–1400. doi: 10.1016/j.celrep.2018.01.035
Nagiec, M. M., Baltisberger, J. A., Wells, G. B., Lester, R. L., and Dickson, R. C. (1994). The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 91, 7899–7902. doi: 10.1073/pnas.91.17.7899
Nagiec, M. M., Nagiec, E. E., Baltisberger, J. A., Wells, G. B., Lester, R. L., and Dickson, R. C. (1997). Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J. Biol. Chem. 272, 9809–9817.
Nash, C. H., and Huber, F. M. (1971). Antibiotic synthesis and morphological differentiation of cephalosporium acremonium. Appl. Microbiol. 22, 6–10. doi: 10.1128/aem.22.1.6-10.1971
Nicolas, G., Nadira, T. E., Nouara, Y., and Jacques, F. (2005). Apical uptake and transepithelial transport of sphingosine monomers through intact human intestinal epithelial cells: Physicochemical and molecular modeling studies. Arch. Biochem. Biophys. 440, 91–100. doi: 10.1016/j.abb.2005.06.001
Nielsen, J., and Jorgensen, H. S. (1995). Metabolic control analysis of the penicillin biosynthetic pathway in a high-yielding strain of Penicillium chrysogenum. Biotechnol. Prog. 11, 299–305. doi: 10.1021/bp00033a010
Nimrichter, L., and Rodrigues, M. L. (2011). Fungal glucosylceramides: from structural components to biologically active targets of new antimicrobials. Front. Microbiol. 2:212. doi: 10.3389/fmicb.2011.00212
Noble, S. M., French, S., Kohn, L. A., Chen, V., and Johnson, A. D. (2010). Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 42, 590–598. doi: 10.1038/ng.605
Nützmann, H. W., Schroeckh, V., and Brakhage, A. A. (2012). Regulatory cross talk and microbial induction of fungal secondary metabolite gene clusters. Methods Enzymol. 517, 325–341. doi: 10.1016/b978-0-12-404634-4.00016-4
Oguro, Y., Yamazaki, H., Takagi, M., and Takaku, H. (2014). Antifungal activity of plant defensin AFP1 in Brassica juncea involves the recognition of the methyl residue in glucosylceramide of target pathogen Candida albicans. Curr. Genet. 60, 89–97. doi: 10.1007/s00294-013-0416-8
Ohnishi, M., Ito, S., and Fujino, Y. (1985). Sphingolipid classes and their molecular species in wheat flour. Agric. Biol. Chem. Tokyo 49, 3609–3611. doi: 10.1271/bbb1961.49.3609
Ono, J., Kinoshita, M., Aida, K., Tamura, M., and Ohnishi, M. (2010). Effects of dietary glucosylceramide on dermatitis in atopic dermatitis model mice. Eur. J. Lipid Sci. Technol. 112, 708–711. doi: 10.1002/ejlt.200900268
Otsu, M., Toume, M., Yamaguchi, Y., and Tani, M. (2020). Proper regulation of inositolphosphorylceramide levels is required for acquirement of low pH resistance in budding yeast. Sci. Rep. 10:10792.
Oura, T., and Kajiwara, S. (2008). Disruption of the sphingolipid Delta8-desaturase gene causes a delay in morphological changes in Candida albicans. Microbiology 154, 3795–3803. doi: 10.1099/mic.0.2008/018788-0
Oura, T., and Kajiwara, S. (2010). Candida albicans sphingolipid C9-methyltransferase is involved in hyphal elongation. Microbiology 156, 1234–1243. doi: 10.1099/mic.0.033985-0
Papagianni, M. (2007). Advances in citric acid fermentation by Aspergillus niger: biochemical aspects, membrane transport and modeling. Biotechnol. Adv. 25, 244–263. doi: 10.1016/j.biotechadv.2007.01.002
Park, C., Bennion, B., Francois, I. E., Ferket, K. K., Cammue, B. P., Thevissen, K., et al. (2005). Neutral glycolipids of the filamentous fungus Neurospora crassa: altered expression in plant defensin-resistant mutants. J. Lipid Res. 46, 759–768. doi: 10.1194/jlr.m400457-jlr200
Pearson, C. L., Xu, K., Sharpless, K. E., and Harris, S. D. (2004). MesA, a novel fungal protein required for the stabilization of polarity axes in Aspergillus nidulans. Mol. Biol. Cell 15, 3658–3672. doi: 10.1091/mbc.e03-11-0803
Perdoni, F., Signorelli, P., Cirasola, D., Caretti, A., Galimberti, V., Biggiogera, M., et al. (2015). Antifungal activity of Myriocin on clinically relevant Aspergillus fumigatus strains producing biofilm. BMC Microbiol. 15:248. doi: 10.1186/s12866-015-0588-0
Plesofsky, N. S., Levery, S. B., Castle, S. A., and Brambl, R. (2008). Stress-induced cell death is mediated by ceramide synthesis in Neurospora crassa. Eukaryot Cell 7, 2147–2159. doi: 10.1128/ec.00147-08
Del Poeta, M, Nimrichter, L., Rodrigues, M. L., and Luberto, C. (2014). Synthesis and biological properties of fungal glucosylceramide. PLoS Pathog. 10:e1003832. doi: 10.1371/journal.ppat.1003832
Raj, S., Nazemidashtarjandi, S., Kim, J., Joffe, L., Zhang, X., Singh, A., et al. (2017). Changes in glucosylceramide structure affect virulence and membrane biophysical properties of Cryptococcus neoformans. Biochim. Biophys. Acta Biomembr. 1859, 2224–2233. doi: 10.1016/j.bbamem.2017.08.017
Ramamoorthy, V., Cahoon, E. B., Li, J., Thokala, M., Minto, R. E., and Shah, D. M. (2007). Glucosylceramide synthase is essential for alfalfa defensin-mediated growth inhibition but not for pathogenicity of Fusarium graminearum. Mol. Microbiol. 66, 771–786. doi: 10.1111/j.1365-2958.2007.05955.x
Ramamoorthy, V., Cahoon, E. B., Thokala, M., Kaur, J., Li, J., and Shah, D. M. (2009). Sphingolipid C-9 methyltransferases are important for growth and virulence but not for sensitivity to antifungal plant defensins in Fusarium graminearum. Eukaryot Cell 8, 217–229. doi: 10.1128/ec.00255-08
Řezanka, T., Kolouchová, I., Gharwalová, L., Doležalová, J., Nedbalová, L., and Sigle, K. (2018). Sphingolipidomics of thermotolerant yeasts. Lipids 53, 627–639. doi: 10.1002/lipd.12076
Řezanka, T., Kolouchová, I., and Sigle, K. (2016). Lipidomic analysis of psychrophilic yeasts cultivated at different temperatures. Biochim. Biophys. Acta 1861, 1634–1642. doi: 10.1016/j.bbalip.2016.07.005
Rhome, R., McQuiston, T., Kechichian, T., Bielawska, A., Hennig, M., Drago, M., et al. (2007). Biosynthesis and immunogenicity of glucosylceramide in Cryptococcus neoformans and other human pathogens. Eukaryot Cell 6, 1715–1726. doi: 10.1128/ec.00208-07
Rittenour, W. R., Chen, M., Cahoon, E. B., and Harris, S. D. (2011). Control of glucosylceramide production and morphogenesis by the Bar1 ceramide synthase in Fusarium graminearum. PLoS One 6:e19385. doi: 10.1371/journal.pone.0019385
Rittershaus, P. C., Kechichian, T. B., Allegood, J. C., Merrill, A. H. J., Hennig, M., Luberto, C., et al. (2006). Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Invest 116, 1651–1659. doi: 10.1172/jci27890
Rodriguez-Cuenca, S., Barbarroja, N., and Vidal-Puig, A. (2015). Dihydroceramide desaturase 1, the gatekeeper of ceramide induced lipotoxicity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1851, 40–50. doi: 10.1016/j.bbalip.2014.09.021
Rollin-Pinheiro, R., Singh, A., Barreto-Bergter, E., and Del Poeta, M. (2016). Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 8, 1469–1484. doi: 10.4155/fmc-2016-0053
Sawada, K., Sato, T., Hamajima, H., Jayakody, L. N., Hirata, M., Yamashiro, M., et al. (2015). Glucosylceramide contained in koji mold-cultured cereal confers membrane and flavor modification and stress tolerance to Saccharomyces cerevisiae during coculture fermentation. Appl. Environ. Microbiol. 81, 3688–3698. doi: 10.1128/aem.00454-15
Schmelz, E. M., Zhou, H., and Roberts, P. C. (2015). Dietary sphingolipids in colon cancer prevention. Cham: Springer International Publishing.
Shimada, E., Aida, K., Sugawara, T., and Hirata, T. (2011). Inhibitory effect of topical maize glucosylceramide on skin photoaging in UVA-irradiated hairless mice. J. Oleo Sci. 60, 321–325. doi: 10.5650/jos.60.321
Shimoda, H., Terazawa, S., Hitoe, S., Tanaka, J., Nakamura, S., Matsuda, H., et al. (2012). Changes in ceramides and glucosylceramides in mouse skin and human epidermal equivalents by rice-derived glucosylceramide. J. Med. Food 15, 1064–1072. doi: 10.1089/jmf.2011.2137
Silano, V., Bolognesi, C., Castle, L., and Chipman, K. (2018). Safety evaluation of the food enzyme α-amylase from a genetically modified Aspergillus niger (strain NZYM-MC). EFSA J. 16:e05451.
Singh, A., Wang, H., Silva, L. C., Na, C., Prieto, M., Futerman, A. H., et al. (2012). Methylation of glycosylated sphingolipid modulates membrane lipid topography and pathogenicity of Cryptococcus neoformans. Cell Microbiol. 14, 500–516. doi: 10.1111/j.1462-5822.2011.01735.x
Smith, E. R., and Merrill, A. H. J. (1995). Differential roles of de novo sphingolipid biosynthesis and turnover in the “burst” of free sphingosine and sphinganine, and their 1-phosphates and N-acyl-derivatives, that occurs upon changing the medium of cells in culture. J. Biol. Chem. 270, 18749–18758. doi: 10.1074/jbc.270.32.18749
Sonnino, S., Mauri, L., Chigorno, V., and Prinetti, A. (2007). Gangliosides as components of lipid membrane domains. Glycobiology 17, 1R–13R.
Spassieva, S., and Hille, J. (2003). Plant sphingolipids today-are they still enigmatic? Plant Biol. 5, 125–136. doi: 10.1055/s-2003-40726
Sperling, P., and Heinz, E. (2003). Plant sphingolipids: structural diversity, biosynthesis, first genes and functions. Biochim. Biophys. Acta 1632, 1–15. doi: 10.1016/s1388-1981(03)00033-7
Sugawara, T., Duan, J., Aida, K., Tsuduki, T., and Hirata, T. (2010). Identification of glucosylceramides containing sphingatrienine in maize and rice using ion trap mass spectrometry. Lipids 45, 451–455. doi: 10.1007/s11745-010-3417-0
Sullards, M. C., Lynch, D. V., Merrill, A. H. J., and Adams, J. (2000). Structure determination of soybean and wheat glucosylceramides by tandem mass spectrometry. J. Mass Spectr. 35, 347–353. doi: 10.1002/(sici)1096-9888(200003)35:3<347::aid-jms941>3.0.co;2-3
Suutari, M. (1995). Effect of growth temperature on lipid fatty acids of four fungi (Aspergillus niger, Neurospora crassa, Penicillium chrysogenum, andTrichoderma reesei). Arch. Microbiol. 164, 212–216. doi: 10.1007/bf02529973
Takahashi, H. K., Toledo, M. S., Suzuki, E., Tagliari, L., and Straus, A. H. (2009). Current relevance of fungal and trypanosomatid glycolipids and sphingolipids: studies defining structures conspicuously absent in mammals. An. Acad. Bras. Cienc. 81, 477–488. doi: 10.1590/s0001-37652009000300012
Takahashi, K., Izumi, K., Nakahata, E., Hirata, M., Sawada, K., Tsuge, K., et al. (2014). Quantitation and structural determination of glucosylceramides contained in sake lees. J. Oleo. Sci. 63, 15–23. doi: 10.5650/jos.ess13086
Takesako, K., Kuroda, H., Inoue, T., Haruna, F., Yoshikawa, Y., Kato, I., et al. (1993). Biological properties of Aureobasidin A, a cyclic depsipeptide antifungal antibiotic. J. Antibiot. 46, 1414–1420. doi: 10.7164/antibiotics.46.1414
Tan, H. W., and Tay, S. T. (2013). The inhibitory effects of aureobasidin A on Candida planktonic and biofilm cells. Mycoses 56, 150–156. doi: 10.1111/j.1439-0507.2012.02225.x
Tani, Y., Amaishi, Y., Funatsu, T., Ito, M., Itonori, S., Yamamoto, K., et al. (2014). Structural analysis of cerebrosides from Aspergillus fungi: the existence of galactosylceramide in A. oryzae. Biotechnol. Lett. 36, 2507–2513. doi: 10.1007/s10529-014-1631-1
Ternes, P., Franke, S., Zähringer, U., Sperling, P., and Heinz, E. (2002). Identification and characterization of a sphingolipid Δ4-desaturase family. J. Biol. Chem. 277, 25512–25518. doi: 10.1074/jbc.m202947200
Ternes, P., Sperling, P., Albrecht, S., Franke, S., Cregg, J. M., Warnecke, D., et al. (2006). Identification of fungal sphingolipid C9-methyltransferases by phylogenetic profiling. J. Biol. Chem. 281, 5582–5592. doi: 10.1074/jbc.m512864200
Ternes, P., Wobbe, T., Schwarz, M., Albrecht, S., Feussner, K., Riezman, I., et al. (2011). Two pathways of sphingolipid biosynthesis are separated in the yeast Pichia pastoris. J. Biol. Chem. 286, 11401–11414. doi: 10.1074/jbc.m110.193094
Tessema, E. N., Gebre-Mariam, T., Neubert, R. H. H., and Wohlrab, J. (2017). Potential applications of phyto-derived ceramides in improving epidermal barrier function. Skin Pharmacol. Physiol. 30, 115–138. doi: 10.1159/000464337
Toledo, M. S., Levery, S. B., Straus, A. H., Suzuki, E., Momany, M., Glushka, J., et al. (1999). Characterization of sphingolipids from mycopathogens: factors correlating with expression of 2-hydroxy fatty acyl (E)-Delta 3-unsaturation in cerebrosides of Paracoccidioides brasiliensis and Aspergillus fumigatus. Biochemistry 38, 7294–7306. doi: 10.1021/bi982898z
Toledo, M. S., Levery, S. B., Straus, A. H., and Takahashi, H. K. (2000). Dimorphic expression of cerebrosides in the mycopathogen Sporothrix schenckii. J. Lipid Res. 41, 797–806. doi: 10.1016/s0022-2275(20)32388-9
Toledo, M. S., Levery, S. B., Suzuki, E., Straus, A. H., and Takahashi, H. K. (2001). Characterization of cerebrosides from the thermally dimorphic mycopathogen Histoplasma capsulatum: expression of 2-hydroxy fatty N-acyl (E)-Δ3-unsaturation correlates with the yeast-mycelium phase transition. Glycobiology 11, 113–124. doi: 10.1093/glycob/11.2.113
Totani, K., Yasutake, N., Ohi, H., Murata, T., and Usui, T. (2001). Enzymatic synthesis of aliphatic beta-lactosides as mimic units of glycosphingolipids by use of Trichoderma reesei cellulase. Arch. Biochem. Biophys. 385, 70–77. doi: 10.1006/abbi.2000.2133
Triantafillidis, J. K., Nasioulas, G., and Kosmidis, P. A. (2009). Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 29, 2727–2737.
Tsuji, K., Mitsutake, S., Ishikawa, J., Takagi, Y., Akiyama, M., Shimizu, H., et al. (2006). Dietary glucosylceramide improves skin barrier function in hairless mice. J. Dermatol. Sci. 44, 101–107. doi: 10.1016/j.jdermsci.2006.08.005
Uchida, Y., Hara, M., Nishio, H., Sidransky, E., Inoue, S., Otsuka, F., et al. (2000). Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J. Lipid Res. 41, 2071–2082. doi: 10.1016/s0022-2275(20)32369-5
Uchiyama, T., Nakano, Y., Ueda, O., Mori, H., Nakashima, M., Noda, A., et al. (2008). Oral intake of glucosylceramide improves relatively higher level of transepidermal water loss in mice and healthy human subjects. J. Health Sci. 54, 559–566. doi: 10.1248/jhs.54.559
Usuki, S., Tamura, N., Sakai, S., Tamura, T., Mukai, K., and Igarashi, Y. (2015). Chemoenzymatically prepared konjac ceramide inhibits NGF-induced neurite outgrowth by a semaphorin 3A-like action. Biochem. Biophys. Rep. 5, 160–167. doi: 10.1016/j.bbrep.2015.11.016
Wagner, H., and Fiegert, E. (1969). Sphingolipids and glycolipids of fungi and higher plants. 3. Isolation of a cerebroside from Aspergillus niger (in German). Z Naturforsch B 24:359.
Walls, S. M., Attle, S. J., Brulte, G. B., Walls, M. L., Finley, K. D., Chatfield, D. A., et al. (2013). Identification of sphingolipid metabolites that induce obesity via mis-regulation of appetite, caloric intake and fat storage in drosophila. PLoS Genet. 9:e1003970. doi: 10.1371/journal.pgen.1003970
Waring, R. B., May, G. S., and Morris, N. R. (1989). Characterization of an inducible expression system in Aspergillus nidulans using alcA and tubulin coding genes. Gene 79, 119–130. doi: 10.1016/0378-1119(89)90097-8
Warnecke, D., and Heinz, E. (2003). Recently discovered functions of glucosyl ceramides in plants and fungi. Cell Mol. Life Sci. 60, 919–941. doi: 10.1007/s00018-003-2243-4
Wirsel, S., Lachmund, A., Wildhardt, G., and Ruttkowski, E. (2010). Three alpha-amylase genes of Aspergillus oryzae exhibit identical intron-exon organization. Mol. Microbiol. 3, 3–14. doi: 10.1111/j.1365-2958.1989.tb00097.x
Yamashita, S., Seino, T., Aida, K., and Kinoshita, M. (2017). Effects of plant sphingolipids on inflammatory stress in differentiated Caco-2 cells. J. Oleo. Sci. 66, 1337–1342. doi: 10.5650/jos.ess17171
Yamashita, S., Yamamoto, M., Hirakawa, K., Kikuchi, N., Kinoshita, M., and Miyazawa, T. (2019). Extraction of lipophilic fraction from polished rice improves its ameliorative effect on intestinal impairment. J. Oleo. Sci. 68, 463–470. doi: 10.5650/jos.ess19013
Yasuhiko, F., and Masao, O. (1997). Structure of cerebroside in Aspergillus oryzae. Biochim. Biophys. Acta 486, 161–171. doi: 10.1016/0005-2760(77)90080-7
Yeom, M., Kim, S. H., Lee, B., Han, J. J., Chung, G. H., Choi, H. D., et al. (2012). Oral administration of glucosylceramide ameliorates inflammatory dry-skin condition in chronic oxazolone-induced irritant contact dermatitis in the mouse ear. J. Dermatol. Sci. 67, 101–110. doi: 10.1016/j.jdermsci.2012.05.009
Zaüner, S., Zähringer, U., Lindner, B., Warnecke, D., and Sperling, P. (2008). Identification and functional characterization of the 2-hydroxy fatty N-acyl-Delta3(E)-desaturase from Fusarium graminearum. J. Biol. Chem. 283, 36734–36742. doi: 10.1074/jbc.m807264200
Zhang, Y., Wang, S., Li, X. M., Cui, C. M., Feng, C., and Wang, B. G. (2007). New sphingolipids with a previously unreported 9-methyl C20-sphingosine moiety from a marine algous endophytic fungus Aspergillus niger EN-13. Lipids 42, 759–764.
Zhao, C., Beeler, T., and Dunn, T. (1994). Suppressors of the Ca(2+)-sensitive yeast mutant (csg2) identify genes involved in sphingolipid biosynthesis. Cloning and characterization of SCS1, a gene required for serine palmitoyltransferase activity. Biol. Chem. 269, 21480–21488.
Zheng, L., Cash, V. L., Flint, D. H., and Dean, D. R. (1998). Assembly of iron-sulfur clusters-identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J. Biol. Chem. 273, 13264–13272.
Zheng, L., White, R. H., Cash, V. L., and Dean, D. R. (1994). Mechanism for the desulfurization of l-cysteine catalyzed by the nifS gene product. Biochemistry 33, 4714–4720.
Keywords: filamentous fungi, Aspergillus, glycosphingolipids, biosynthetic pathway, glucosylceramide and galactosylceramide, application of glycosylceramide
Citation: Jiang C, Ge J, He B and Zeng B (2021) Glycosphingolipids in Filamentous Fungi: Biological Roles and Potential Applications in Cosmetics and Health Foods. Front. Microbiol. 12:690211. doi: 10.3389/fmicb.2021.690211
Received: 02 April 2021; Accepted: 28 June 2021;
Published: 22 July 2021.
Edited by:
Wanping Chen, Georg-August-University Göettingen, GermanyReviewed by:
Leonardo Freire-de-Lima, Federal University of Rio de Janeiro, BrazilCopyright © 2021 Jiang, Ge, He and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin He, aGViaW4ubGlAZm94bWFpbC5jb20=; Bin Zeng, emVuZ3R4MDAxQGFsaXl1bi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.