
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 21 June 2021
Sec. Microbial Physiology and Metabolism
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.689527
This article is part of the Research Topic Fungal Biotechnology View all 13 articles
Plant diseases caused by phytopathogenic fungi can lead to huge losses in the agricultural fields and therefore remain a continuous threat to the global food security. Chemical-based fungicides contributed significantly in securing crop production. However, indiscriminate application of fungicides has led to increased chemical resistance and potential risks to human health and environment. Thus, there is an urgent need for searching for new bioactive natural products and developing them into new biopesticides. Fungal endophytes, microorganisms that reside in the fresh tissues of living plants, are regarded as untapped sources of novel natural products for exploitation in agriculture and/or medicine. Chemical examination of endophytic fungi has yielded enormous antifungal natural products with potential use in the development of biopesticides. This review summarizes a total of 132 antifungal metabolites isolated from fungal endophytes in the past two decades. The emphasis is on the unique chemical diversity of these metabolic products, together with their relevant antifungal properties. Moreover, some “star molecules,” such as griseofulvin and trichothecene, as well as their synthetic derivatives that possess high potential as candidates of new natural fungicides, are also presented herein.
Plant diseases caused by phytopathogenic fungi are continuing to be a huge threat in the agricultural fields. It is estimated that the global loss caused by plant diseases is more than 20% of the crop yield in the major food and cash crops worldwide (Kim et al., 2004). Fungal pathogens were responsible for the considerable postharvest losses of grain crops, fruits, and vegetables, which, in addition to causing decay, can produce mycotoxins that are harmful to humans and animals (Bai et al., 2013; Shi et al., 2017). Therefore, effective and sustained control of these fungal pathogens has become an urgent task. Chemical control has been widely adopted in crop production. During the past years, a great variety of chemical fungicides (agrochemicals), such as thiophanate-methyl, carbendazim, and imazalil, are designed and applied to control these diseases. It is indisputable that these chemicals have resulted in substantial increases in productivity and contributed significantly in agricultural industry. However, these most-applied chemical fungicides have been limited by many serious problems. Indiscriminate use of these fungicides has led to the appearance of pathogens with multiple fungicide resistances, which further complicated the management of the diseases (Talibi et al., 2014). Furthermore, repeated and exclusive use of fungicides is increasingly restricted owing to their undesirable effects on non-target organisms (carcinogenicity, high and acute residual toxicity) and potential risks to environmental pollution (long degradation period) (Bai et al., 2013; Talibi et al., 2014). Thus, these synthetic chemical fungicides are subject to registration and permission for use, and are even no longer authorized in various countries.
Based on above questions, the challenge is to develop new and eco-friendly alternatives for the safe control of these pathogens, which pose low risk to human health and environment. Nowadays, many promising biological approaches were considered to be potential alternatives to synthetic fungicides, including (i) use of biocontrol microorganisms, (ii) application of naturally sourced metabolites, and (iii) induction of natural resistance (Talibi et al., 2014). Among them, naturally sourced secondary metabolites from microbes have attracted great attention. Microorganisms are well known for their ability to synthesize bioactive secondary metabolites, which have provided abundant chemical entities for pharmaceuticals and agrochemicals. Many natural antifungal fungicides, such as kasugamycin, polyoxins, validamycin, and blasticidin-S, have been obtained from microbial resources (Copping and Duke, 2007; Wang et al., 2016).
Fungal endophytes, microorganisms that asymptomatically reside in the internal tissues of plants, have been widely distributed in almost every plant and are rich in diversity (Jia et al., 2016). Endophytic fungi have a close and complex interaction with their hosts, which involve mutualism, antagonism and rarely parasitism (Gouda et al., 2016). These species are known to promote host growth and gain essential nutrition. They also provide tolerance to plants against various types of abiotic and/or biotic stresses. Most importantly, they possess the ability to produce plenty of structurally diverse and biologically active secondary metabolites to protect their hosts from pathogenic microorganisms and pests. In this sense, endophytic fungi are a treasure source of searching for novel secondary metabolites with immense potential agricultural applications. It has been reported that a large number of metabolites with different chemical skeletons have been deciphered from endophytic fungi, such as alkaloids, terpenoids, steroids, peptides, benzopyranones, quinones, and isocoumarins (Gouda et al., 2016). Chemically speaking, the discovery of these metabolites provided impressive chemical basis in the development of agrochemicals. Moreover, most of them exhibited promising bioactivities, such as antifungal, antibacterial, herbicidal, nematocidal, insecticidal, and other agricultural activities.
Therefore, the topical subject of antifungal secondary metabolites produced by fungal endophytes has been searched and analyzed. The literature search was conducted using the combined keywords “antifungal,” “secondary metabolites,” and “endophytic fungus” in the databases such as Web of Science, Google Scholar, and SciFinder Scholar, with a previously reported search method (Zhang et al., 2020). As a result, a total of 132 metabolites with anti-phytopathogenic activities from fungal endophytes in the past two decades (covering from 2000 to 2020) were included in this review. The present compounds possess diverse chemical structures, which were classified into alkaloids (including cytochalasins, indoles, diketopiperazines, and other nitrogen-containing compounds), terpenoids, polyketides (quinones, macrolides, benzopyrones and unsaturated lactones), and other miscellaneous compounds within a biogenetic context. Moreover, we also describe the sources, the producing strains, target plant pathogenic fungi, and their potential use as lead compounds in the development of biopesticides.
Prior to the beginning of this review, there are three issues that need to be addressed. (i) The level of antifungal potency should be standardized. As we can see from literatures, the literatures are replete with uncalibrated potency descriptors, including ‘mild, moderate, strong, pronounced, significant, remarkable, and potent.’ Apparently these descriptions confused the readers that which level means strong and which should be defined as weak. The unified standardization has not yet formed since the definition of the level of bioactivity just represents the individual judgment of the authors. However, in this review, the antifungal potency was distinguished as ‘potent,’ ‘moderate,’ and ‘weak’ based on the comparison with positive controls. We discretionarily define ‘potent’ as the antifungal activity higher than that of the positive controls, ‘moderate’ as the bioactivity equal to that of the positive controls, and ‘weak’ as that of lower than positive controls (or inactive) throughout this review. (ii) Quantitative comparisons of the results of the bioactivity tests between different studies are problematic. As the fungal pathogens used in bioassays may have different provenance and viability/sensitivity with different assay protocols, the comparisons should be qualified by consideration of positive/negative controls, and even then should be best limited within individual studies, rather than between different studies. (iii) The selection of different positive controls may lead to radically different results. Commercial fungicides were generally chosen as positive controls. However, since they belong to different chemical classes, they may present distinct potency and mechanisms of action.
The cytochalasin alkaloids are a class of structurally related fungal metabolic products. To date, more than 300 cytochalasin analogs have been isolated from many genera of ascomycetes and basidiomycetes, including Aspergillus, Chaetomium, Penicillium, Phomopsis, Phoma, Spicaria, Xylaria, and so on (Wei et al., 2017). Structurally, cytochalasins are characterized by a highly substituted perhydroisoindol-1-one moiety which is fused with a 9- to 15-membered macrocyclic ring. Many of cytochalasins exhibit a wide range of biological activities, such as cytotoxic, antimicrobial, and phytotoxic properties (Wei et al., 2017; Xu et al., 2017). 14 cytochalasins (Figure 1) isolated from fungal endophytes were reported to possess moderate to potent antifungal activity. Cytochalasin D (1) was produced by various endophytic fungi Xylaria sp., which were isolated from leaves of guarana plant (Elias et al., 2018). 1 showed fungistatic activity against the phytopathogen Colletotrichum gloeosporioides, which causes the anthracnose disease, with an MIC of 2.46 mM. Commercial fungicides captan and difenoconazole were applied as positive controls (MICs 16.63 and 0.02 mM, respectively) (Elias et al., 2018). Bioassay-guided separation of Xylaria sp. XC-16, an endophyte from Toona sinensis led to the discovery of five agriculturally active cytochalasin alkaloids, including a new compound cytochalasin Z28 (2) (Zhang Q. et al., 2014). Compound 2 showed potent fungicidal effect (MIC = 12.5 μM) against the phytopathogen Gibberella saubinetti, which was better than that of the positive control hymexazol (MIC = 25 μM) (Zhang Q. et al., 2014). Chemical investigation of the biocontrol potential endophytic fungus Aspergillus capensis CanS-34A in Brassica napus has resulted in the isolation and identification the antifungal metabolite rosellichalasin (3) (Qin et al., 2019). 3 inhibited the plant pathogenic fungi Botrytis cinerea, Monilinia fructicola, Sclerotinia sclerotiorum, and S. trifoliorum with the EC50 values of 36.8, 87.1, 5.3, and 41.1 μM, respectively. Thus, S. sclerotiorum was the most sensitive target fungus (Qin et al., 2019). A new chaetoglobosin, penochalasin K (4) possessing a rare six-cyclic 6/5/6/5/6/13 fused ring system, was isolated from the solid culture of the mangrove endophytic fungus Penicillium chrysogenum V11 (Zhu et al., 2017b). Compound 4 displayed potent selective activities against C. gloeosporioides and Rhizoctonia solani, with MIC values of 6.13 and 12.26 μM, respectively, which were about ten-fold and two-fold better than that of the positive control carbendazim (Zhu et al., 2017b). Five metabolites, chaetoglobosins A (5), B (6), E (7), F (8), and penochalasin G (9), were obtained from endophytic Chaetomium globosum, isolating from the seeds of Panax notoginseng (Li et al., 2016). Some of them exhibited remarkable inhibition against phytopathogenic fungi causing root rot disease. For example, chaetoglobosin E (7) and penochalasin G (9) indicated potent inhibition against Epicoccum nigrum with the MICs < 2 μM (Li et al., 2016). A new chaetoglobosin named penochalasin J (10), as well as two known chaetoglobosins, chaetoglobosin C (11) and armochaetoglobosin I (12), were isolated from the mangrove endophytic fungus P. chrysogenum V11 (Huang et al., 2016). Compound 10 showed more potent antifungal activity against plant pathogen C. gloeosporioides with an MIC value of 25.08 μM, than the positive control carbendazim (MIC = 65.38 μM). Simultaneously, compounds 11 and 12 remarkably inhibited R. solani, with MIC values of 23.66 and 12.11 μM, respectively (Huang et al., 2016). Chaetoglobosins V (13) and G (14) were isolated from the culture of the endophytic fungus C. globosum, associated with the leaves of Ginkgo biloba tree (Xue et al., 2012). Compounds 13 and 14 exhibited potent antifungal activity against Alternaria solani, with MICs of 47.3 μM, while 14 also possessed potent activity against A. alternate, with an MIC of 47.3 μM (Xue et al., 2012). In total, the above results indicated that cytochalasin alkaloids could be used as fungicides or as leads of new fungicides to the related phytopathogenic fungi.
Except for the cytochalasins, alkaloids also include indoles, diketopiperazines, cyclopeptides, amides, and other N-containing compounds (Figure 2). Two prenylated tryptophan analogs, a previously reported 14-hydroxyterezine D (15) and a new terezine E (16), were isolated from an endophytic Mucor sp. from the medicinal plant Centaurea stoebe (Abdou et al., 2018). Both 15 and 16 exerted weak antifungal efficacy against Aspergillus terreus, with MICs of 127.8 and 111.2 μM, respectively (Abdou et al., 2018). Chemical investigation of the endophytic fungus C. gloeosporioides from the leaves of Michelia champaca resulted in the isolation of one new indole alkaloid, 2-phenylethyl 1H-indol-3-yl-acetate (17) (Chapla et al., 2014). 17 exhibited moderate activity against Cladosporium cladosporioides and C. sphaerospermum at 5 μg, which was comparable to that observed for the positive control nystatin (Chapla et al., 2014). Three prenylated indole diketopiperazine alkaloids, 12β-hydroxy-13α-methoxyverruculogen TR-2 (18), fumitremorgin B (19), and verruculogen (20), were isolated from Aspergillus fumigatus LN-4, an endophytic fungus isolated from the stem bark of Melia azedarach (Li et al., 2012). Compounds 18-20 showed broad-spectrum anti-phytopathogenic activities against eight fungi (B. cinerea, A. solani, A. alternata, C. gloeosporioides, Fusarium solani, F. oxysporum f. sp. niveum, F. oxysporum f. sp. vasinfectum, and G. saubinettii), with MIC values of 13.7-100 μM, which were comparable to the positive controls carbendazim and hymexazol (Li et al., 2012). Two quinolinones 3-O-methylviridicatin (21) and viridicatol (22), together with a new isoquinolone alkaloid named 5-hydroxy-8-methoxy-4-pheny lisoquinolin-1(2H)-one (23) were isolated from the fermentation of an endophytic fungus Penicillium sp. R22 in Nerium indicum (Ma et al., 2018). Compounds 21-23 exhibited weak to moderate antifungal activities against Alternaria brassicae, A. alternate, B. cinerea, and Valsa mali with MIC values of 124.3, 123.3, and 116.8 μM, respectively (Ma et al., 2018). A new fusaric acid derivative, atransfusarin (24), and (3R,6R)-3-benzyl-6-isopropyl-4-methylmorpholine-2,5-dione (25) were isolated from the culture of an endophyte Alternaria atrans MP-7, associated with the medicinal plant Psidium guajava (Yang Z. et al., 2019). Compound 25 exhibited potent antifungal activities against A. solani, C. gloeosporioides, and Phyricularia grisea with MICs of 6.25 μM, better than that of a broad-spectrum fungicide carbendazim. In contrast, 24 only exerted weak activities against B. cinerea and A. solani (MIC = 50 μM) (Yang Z. et al., 2019). A new pyrrolidinone derivative, named nigrosporamide A (26), was obtained from an endophytic fungus Nigrospora sphaerica ZMT05, which was isolated from Oxya chinensis Thunberg (Zhu et al., 2017a). 26 exhibited higher antifungal activity against C. gloeosporioides with an MIC value of 25.14 μM, than the positive control triadimefon (MIC = 272.39 μM) (Zhu et al., 2017a). It should be pointed out that the MIC of the positive control triadimefon (272.39 μM) seems to be outside the error measurements, probably because triadimefon is insensitive to C. gloeosporioides. As we discussed above, other appropriate positive controls should be rechose. From the culture extracts of the endophytic fungus Phoma sp. isolated from the plant Salsola oppositifolia, a new pyridione epoxide derivative, (+)-flavipucine (27), was isolated and characterized (Loesgen et al., 2011). This metabolite showed strong antifungal inhibition down to 7.81 ppm (Inhibitory concentration, 90% of growth inhibition) against Phytophthora infestans and down to 31.3 ppm against Septoria tritici (Loesgen et al., 2011). Two cyclic pentapeptides 28 and 29 were isolated from Cryptosporiopsis sp., an endophytic fungus from Zanthoxylum leprieurii (Rutaceae) (Talontsi et al., 2012). Compounds 28 and 29 exhibited motility inhibitory and lytic activities against Plasmopara viticola zoospores, a grapevine downy mildew pathogen, at 17.7-44.3 μM. Moreover, both of them also displayed potent inhibitory effects against mycelial growth of Pythium ultimum, Aphanomyces cochlioides, and R. solani (Talontsi et al., 2012). A metabolite cercosporamide (30) was isolated from the endophytic fungus Cadophora orchidicola from Kalimeris indica (Wang et al., 2019). Antifungal assay revealed that 30 had potent growth inhibition against five plant pathogens, Pestalotia diospyri, B. cinerea, Fusarium oxysporum, Sclerotium rolfsii, and Penicillum digitatum, with EC50 values of 16.0 × 10–3, 1.8, 2.8, 2.89, and 20.2 μM, respectively (Wang et al., 2019). Two new solanapyrone analogs, solanapyrones N (31) and O (32), and the known solanapyrone C (33), were isolated from Nigrospora sp. YB-141, an endophytic fungus obtained from Azadirachta indica (Wu et al., 2009). Compound 32 was regarded as an inseparable mixture of (E)-32 and (Z)-32. Compound 31 showed moderate activity against Penicillium islandicum at equivalent concentration of 98.6 μM compared with the positive control nystatin (Wu et al., 2009). Bipolamide B (34), a new triene fatty acid amide, was discovered from the endophytic fungus Bipolaris sp. MU34 of Thai medicinal plants Gynura hispida (Siriwach et al., 2014). Compound 34 showed weak to meoderate broad-spectrum antifungal activities against C. cladosporioides, C. cucumerinum, Saccharomyces cerevisiae, Aspergillus niger, and Rhizopus oryzae, with MICs of 82.9, 165.8, 165.8, 331.6, and 331.6 μM, respectively (Siriwach et al., 2014). An endophytic Xylaria sp. with broad antimicrobial activity was isolated from Ginkgo biloba L. Bioactivity-guided fractionation led to the identification of 7-amino-4-methylcoumarin (35) (Liu et al., 2008). Antimicrobial assay showed that 35 inhibited the growth of the tested 13 pathogens including Penicillium expansum (MIC, 228.6 μM) and A. niger (142.8 μM) (Liu et al., 2008).
Terpenoids produced by fungi are one of the most numerous and structurally diverse secondary metabolites with a wide array of pharmacological properties (Figure 3). The coculture of the phytopathogenic Nigrospora oryzae and endophytic Irpex lacteus from the same host Dendrobium officinale afforded a new tremulane sesquiterpene 5-demethyl conocenol C (36), conocenol B (37), and a new squalene irpenigirin B (38) (Wu et al., 2019). The new compounds 36 and 38 were active against C. gloeosporioides, with MICs of 31.7 and 13.4 μM, while 36 showed antifungal activity against Didymella glomerata with an MIC of 3.9 μM (Wu et al., 2019). It was also found that the mutually antagonistic relationship between the phytopathogens and endophytes can lead to the production of antibiotics, which inhibit the growth of phytopathogens and hinder certain phytotoxins (Wu et al., 2019). A new natural sesquiterpene 5-(hydroxymethyl)-2-(2′,6′,6′-trimethyltetrahydro-2H-pyran-2-yl)phenol (39) was characterized from endophytes belonging to the Lophodermium sp., which were isolated from the needles of superior Pinus strobus (eastern white pine) trees (Sumarah et al., 2011). 39 was antifungal against the rust Microbotryum violaceum with an MIC of 2 μM (Sumarah et al., 2011). Trichothecium roseum LZ93, an endophyte from medicinal plant Maytenus hookeri, was found to antagonize various phytopathogens in vitro. Chemical investigation of this fungal strain afforded a trichothecene, trichothecin (40), with weak to moderate inhibition on phytopathogenic fungi Typhula incarnate (MIC, 150.6 μM), Gaeumannomyces graminis (MIC, 90.4 μM), Phytophthora infestans (MIC, 90.4 μM), A. solani (MIC, 15.1 μM), and Phyricularia oryzae (MIC, 60.2 μM) (Zhang et al., 2010). A monosesquiterpene rhinomilisin B (41), a new dimeric sesquiterpene divirensol H (42), and an unprecedented trimeric sesquiterpene trivirensol A (43), were purified from T. virens FY06, an endophyte derived from Litchi chinensis Sonn (Hu et al., 2019). Compounds 41-43 exhibited moderate to potent activities on Penicillium italicum, F. oxysporum, F. graminearum, Colletotrichum musae, and C. gloeosporioides. 41 showed potent inhibitory activity against C. musae, with an MIC value of 37.4 μM, which was superior to that of the positive control triadimefon (273.0 μM). Moreover, 42 was more active toward F. oxysporum, C. gloeosporioides, C. musae, P. italicm, and F. graminearum, which were 8, 8, 3.2, 8, and 24 times as high as those of triadimefon (Hu et al., 2019). Interestingly, as metabolites of the endophytic fungus from L. chinensis, all the isolated sesquiterpenes presented potent antifungal activities against C. gloeosporioides. The phytopathogenic fungus C. gloeosporioides can cause anthracnose in L. chinensis, indicating that the metabolites produced by endophytes from its host may play a defensive role by inhibiting invasive phytopathogens. Two tetranorlabdane diterpenoids, botryosphaerin H (44) and 13,14,15,16-tetranorlabd-7-en-19,6β:12,17-diolide (45), were isolated from the endophytic fungus Botryosphaeria sp. P483 of the Chinese herbal medicine Huperzia serrata (Chen et al., 2015). Compounds 44 and 45 showed strong antifungal activities against G. graminis, F. moniliforme, F. solani, F. oxysporum and Pyricularia oryzae at 100 μg/disk (Chen et al., 2015). Three diterpenes, conidiogenones C (46), D (47), and G (48), were isolated from an endophytic fungus Leptosphaeria sp. XL026 derived from the leaves of Panax notoginseng (Chen et al., 2019). Compounds 46 and 48 showed moderate antifungal activity against Rhizoctonia cerealis, as well as 47 against Verticillium dahlia, with an MIC value of 41.4 μM (Chen et al., 2019). A nordammarane triterpenoid helvolic acid (49) was obtained from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach (Li et al., 2012). 49 exhibited broad-spectrum and potent antifungal activities against B. cinerea, A. solani, A. alternata, C. gloeosporioides, F. solani, F. oxysporum, F. oxysporum, and G. saubinettii, with MIC values of 11.0-88.1 μM (Li et al., 2012).
Polyketides are a large class of structurally diverse natural products that exhibit a wide range of bioactivities. These metabolites are generally biosynthesized by polyketide synthases, and simultaneously, some of them possess hybrid chemotypes derived from different biosynthetic pathways. In this review, chromones, quinines, macrolides, benzopyrones, unsaturated lactones, and phenols are all classified into polyketides (Figures 4–6). The culture of the fungus Botryosphaeria dothidea KJ-1, isolated from the stems of white cedar (Melia azedarach L.), yielded a perylenequinone derivative stemphyperylenol (50) (Xiao et al., 2014b). 50 displayed potent antifungal activity against A. solani with an MIC of 1.57 μM, compared to the commercially available fungicide carbendazim (Xiao et al., 2014b). Cultivation of the newly discovered fungal strain Edenia gomezpompae, an endophyte obtained from the leaves of Callicarpa acuminata (Verbenaceae), afforded three new naphthoquinone spiroketals, preussomerins EG1 (51), EG2 (52), and EG3 (53) (Macías-Rubalcava et al., 2008). Spiroketals 51-53 displayed significant growth inhibition against four economically important phytopathogens (Phythophtora capsici, P. parasitica, F. oxysporum, and A. solani), with IC50 values ranging from 57.5 to 447.4 μM (Macías-Rubalcava et al., 2008). Fonsecinone A (54), a dimeric naphtha-γ-pyrone, was characterized from the culture of Aspergillus sp. KJ-9, an endophytic fungus residing in the stem bark of Melia azedarach (Xiao et al., 2014a). Compound 54 had marked inhibition of G. saubinetti, Magnaporthe grisea, B. cinerea, and A. solani, with MICs in the range of 6.25-50 μM, which were better than or similar to that of hymexazol (MIC 50 μM) (Xiao et al., 2014a). An anthraquinone, macrosporin (55), was isolated from the mangrove endophytic fungus Phoma sp. L28 (Huang et al., 2017). 55 possessed broad-spectrum antifungal activities against F. oxysporum, F. graminearum, C. musae, P. italic, R. solani, and C. gloeosporioides, with MIC values ranging from 13.2 to 252.1 μM. It should be pointed out that the inhibitory activity of 55 against F. oxysporum (MIC = 13.2 μM) was higher than that of the positive control carbendazim (MIC = 32.7 μM) (Huang et al., 2017). Then, from the same host plant (semi-mangrove plant Myoporum bontioides), an endophytic fungus Alternaria sp. R6 was isolated. A racemic new cyclopentenone derivative, (±)-(4S∗,5S∗)-2,4,5-trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten-1-one (56), and a new xanthone 4-chloro-1,5-dihydroxy-3-hydroxymethyl-6-methoxycarbonyl-xanthen-9-one (57), were characterized from this fungal strain (Wang et al., 2015). In comparison to the positive control triadimefon (MIC 510.64 μM), compounds 56 and 57 exhibited inhibitory activities against F. graminearum with MICs of 215.52 and 107.14 μM, respectively, while 57 also showed potent antifungal activity against C. musae with an MIC of 214.29 μM (Wang et al., 2015). Cryptosporiopsis sp., an endophytic fungus from the medicinal plant Zanthoxylum leprieurii, was the source of new polyketides, cryptosporiopsin A (58), ponchonin D (59), hydroxypropan-2′,3′-diol orsellinate (60), and (-)-phyllostine (61) (Talontsi et al., 2012). Compounds 59-61 exhibited motility inhibitory and zoosporicidal activities against P. viticola zoospores at 28.6-71.4 μM. Meanwhile, they also displayed mycelial growth of P. ultimum, A. cochlioides, and R. solani with MICs of 20-40 μg/disk (Talontsi et al., 2012). An endophytic fungus, Epicoccum sp. CAFTBO, obtained from the cocoa tree Theobroma cacao was found to produce three new unprecedented polyoxygenated polyketides, epicolactone (62), epicoccolides A (63) and B (64) (Talontsi et al., 2013). Compounds 62-64 significantly inhibited the growth of three notorious crop-devastating phytopathogens P. ultimum, A. cochlioides, and R. solani with MICs of 20-80 μg/disk (Talontsi et al., 2013). Two chlorine-substituted azaphilones, chaetomugilins A (65) and D (66), were isolated from endophytic C. globosum of Panax notoginseng (Li et al., 2016). Compounds 65 and 66 had moderate activity against E. nigrum with the MIC values of 17.8 and 36.9 μM, respectively (Li et al., 2016). Viburspiran (67), a structurally novel maleic anhydride natural products with an additional ethylene bridge, was isolated the fungal endophyte Cryptosporiopsis sp. from Viburnum tinus (Saleem et al., 2011). 67 was active against Microbotryum violaceum and B. cinerea, with inhibition radius of 6 and 10 mm at 50 μg substance/filter disk (Saleem et al., 2011). A new macrocyclic metabolite, chaetoglobosin X (68), was isolated from an endophytic fungus C. globosum obtained from the medicinal plant Curcuma wenyujin (Wang Y. et al., 2012). 68 possessed reasonably potent fungistatic activities on Exserohilum turcicum, F. oxysporum, and Curvularia lunata with an MIC of 7.5 μM and showed moderate activity against F. graminearum and F. moniliforme with an MIC of 15.1 μM (Wang Y. et al., 2012). Two benzopyran derivatives, 2-methyl-5-methoxy-benzopyran-4-one (69) and (2′S)-2-(propan-2′-ol)-5-hydroxy-benzopyran-4-one (70), were isolated from the isolate of Curvularia sp., which was obtained from the leaves of a native plant Ocotea corymbosa (Teles et al., 2005). Compounds 69 and 70 exhibited moderate antifungal activity against C. sphaerospermum and C. cladosporioides with a detection limit of 10 μg (Teles et al., 2005). Two new chromones, phomochromones A and B (71 and 72), and one new natural cyclopentenone derivative, phomotenone (73), was obtained from Phomopsis sp., an endophyte isolated from Cistus monspeliensis (Ahmed et al., 2011). Compounds 71-73 showed moderate antifungal properties toward Microbotryum violaceum, with the radius of the inhibition zone of 8, 5, and 8 mm, respectively, at concentration of 50 μL of 1 mg/mL (Ahmed et al., 2011).
5-carboxy-6-hydroxy-3-methyl-3,4-dihydroisocoumarin (74) was produced from the endophyte Xylaria sp., associated with leaves of Casearia sylvestris (Chapla et al., 2018). 74 exhibited potent antifungal activities against two phytopathogenic fungi C. cladosporioides and C. sphaerospermum at 10 μg (Chapla et al., 2018). Bioassay-guided fractionation of the endophytic fungus Phoma sp. ZJWCF006 in the Chinese medicinal plant Arisaema erubescens afforded a new α-tetralone derivative, (3S)-3,6,7-trihydroxy-α-tetralone (75) (Wang L. W. et al., 2012). 75 showed selective growth inhibition against F. oxysporium and R. solani with EC50 values of 2.1 and 0.3 mM, respectively, whereas no obvious activity was observed against C. gloeosporioides and Magnaporthe oryzae (Wang L. W. et al., 2012). Three known isobenzofuranones including diaporthelactone (76), 7-hydroxy-4,6-dimethy-3H-isobenzofuran-1-one (77), and 7-methoxy-4,6-dimethyl-3H-isobenzofuran-1-one (78) were obtained from the mangrove endophytic fungus Phomopsis sp. A123 from the foliage of Kandelia candel (Zhang W. et al., 2014). Compounds 76 and 77 displayed antifungal activity against A. niger with MICs of 243 and 485 μM, respectively, while 78 inhibited the growth of A. alternaria with an MIC of 500 μM (Zhang W. et al., 2014). Chemical and biological study on an unidentified Ascomycete, an endophyte isolated from Meliotus dentatus, led to the isolation of 5-methoxy-7-hydroxyphthalide (79) and (3R,4R)-cis-4-hydroxymellein (80) (Hussain et al., 2009). Compounds 79 and 80 showed antifungal activity against Microbotryum violaceum with the radius of zone of inhibition of 7 and 8 mm at a concentration of 50 μL of 1 mg/mL (Hussain et al., 2009). An antifungal strain Verticillium sp. from the roots of Rehmannia glutinosa yielded 2,6-dihydroxy-2-methyl-7-(prop-1E-enyl)-1-benzofuran-3(2H)-one (81) (You et al., 2009). 81 clearly inhibited biomass accumulation at a low concentration of 4.4 μM on the pathogens Septoria sp. and Fusarium sp. The growth of the producing strain Verticillium sp. itself was also inhibited to some degree by 81 (You et al., 2009). Pestafolide A (82), a novel reduced spiro azaphilone derivative, and pestaphthalides A (83) and B (84), two new isobenzofuranones, were isolated from Pestalotiopsis foedan, an endophyte from an unidentified tree (Ding et al., 2008). 82 displayed antifungal activity against A. fumigatus, affording a zone inhibition of 10 mm at 100 μg/disk, whereas 83 and 84 showed activity against C. albicans and G. candidum, with the zone inhibition of 13 and 11 mm, respectively (Ding et al., 2008). Griseofulvin (85) was produced by Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam (Zhao et al., 2012). In vitro antifungal assay indicated that 85 displayed potent inhibition of the test eight plant pathogenic fungi; of particular note was the antifungal activity against B. cinerea and Colletotrichum orbiculare with the EC50 of 0.6 and 1.4 μM, respectively (Zhao et al., 2012). Four new metabolites, pestalotheols E-H (86-89), containing a reduced tetrahydro-2H-furo[3,2-g]chromene unit, were isolated from a fungal endophyte, an unidentified Ascomycete from the tree Arbutus unedo (Qin et al., 2011). Compounds 86-89 showed antifungal activity against Microbotryum violaceum, with the radius of the inhibition zone of 6, 8, 6, and 7 mm, respectively, at 50 μg of test substance/test filter disk (Qin et al., 2011). Phytochemical studies of the active constituents of the endophytic fungus Phomopsis sp. led to the isolation of two new pyrenocine derivatives pyrenocine K (90) and M (91) (Hussain et al., 2012a). Both compounds also showed antifungal activity against M. violaceum in an agar diffusion assay, with inhibition zone of 5 mm at a concentration of 0.05 mg (Hussain et al., 2012a). A new fungal polyketide, koningiopisin C (92), was characterized from the culture broth of the fungus Trichoderma koningiopsis YIM PH 30002 from the medicinal plant Panax notoginseng (Liu et al., 2016). 92 exhibited antifungal activity against Plectosphaerella cucumerina with an MIC of 57.1 μM (Liu et al., 2016). The endophyte Drechslera sp. strain 678 was isolated from the roots of an Australian native grass Neurachne alopecuroidea, which demonstrated efficacy against several plant pathogens (d’Errico et al., 2020). Metabolomic analysis revealed the presence of two major bioactive metabolites, an alkynyl substituted epoxycyclohexenone derivative (5-hydroxy-1-(3-oxo-but-1-ynyl)-7-oxa-bicyclo[4.1.0]hept-3-en-2-one) (93) and monocerin (94). 93 and 94 were active against B. cinerea and S. sclerotiorum at 10 and 100 μg (d’Errico et al., 2020). A new epoxydon derivate epoxydine B (95), along with two related metabolites, epoxydon (96) and (4R,5R,6S)-6-acetoxy-4,5-dihydroxy-2-(hydroxymethyl)cyclohex-2-en-1-one (97), were obtained from an endophytic fungus, Phoma sp., isolated from the plant Salsola oppostifolia (Qin et al., 2010). Compounds 95-97 were biologically active exhibiting antifungal activity against M. violaceum at a concentration of 0.05 mg (Qin et al., 2010). A new α-pyrone (2H-pyran-2-one), ficipyrone A (98), was isolated from solid cultures of the plant endophytic fungus Pestalotiopsis fici from the tea plant Camellia sinensis (Liu et al., 2013). 98 displayed weak antifungal activity against Gibberella zeae, with an IC50 value of 15.9 μM, compared with the positive control ketoconazole (IC50 6.02 μM) (Liu et al., 2013). Structural elucidation of the metabolites produced by the endophytic Phomopsis sp. revealed four new α-pyrone derivatives, phomopsinones A-D (99-102) (Hussain et al., 2012b). 99 showed potent antifungal activity against B. cinerea (inhibition zone of 17 mm), P. oryzae (25 mm), and Septoria tritici (20 mm), while 102 was active against B. cinerea (10 mm) and S. tritici (10 mm) (Hussain et al., 2012b). The fermentation of the endophytic fungus Nigrospora sp. YB-141 isolated from A. indica yielded two lactones, nigrosporalactone (103) and phomalactone (104) (Wu et al., 2009). Compounds 103 and 104 were active against B. cinerea with MIC values of 200.3 and 405.8 μM (Wu et al., 2009). Preliminary screening for antimicrobial activity of the endophytic fungi from Cinnamomum mollisimum yielded a polyketide, 5-hydroxyramulosin (105), which was identified as the major constituent of the bioactive fungal extracts (Santiago et al., 2012). 105 inhibited the fungal pathogen A. niger with the IC50 value of 7.9 μM (Santiago et al., 2012).
Five new polyoxygenated cyclohexenoids, phomopoxides B (106), C (107), D (108), F (109), and G (110), were isolated from an endophytic fungal strain Phomopsis sp. YE3250 from the medicinal plant Paeonia delavayi (Huang et al., 2018). Compounds 106-110 were active against five pathogenic fungi (C. albicans, A. niger, P. oryzae, F. avenaceum, and Hormodendrum compactum) with MICs of 46.5-372.1 μM (Huang et al., 2018). Investigatation of the metabolites produced by the endophytic Fusidium sp., isolated from the leaves of Mentha arvensis, found two new bicyclic fusidilactones D (111) and E (112) (Qin et al., 2009). Both compounds had only moderate activity toward Microbotryum violaceum, with the radius of the inhibition zone of 7 and 10 mm, respectively (Qin et al., 2009). Through screening antifungal activity of endophytic fungi and subsequent bioassay-guided fractionation, sporothriolide (113) was isolated from the selected endophyte Nodulisporium sp. A21 in Ginkgo biloba (Cao et al., 2016). 113 was validated to be potently antifungal against the mycelia growth of R. solani, S. sclerotiorum and inhibit conidium germination of M. oryzae in vitro and in vivo (Cao et al., 2016). The screening of fungal extracts of Lophodermium sp. isolated from P. strobus resulted in the discovery of a new aliphatic polyketide, (2Z,4E)-6(acetyloxy)-5-formyl-7-oxoocta-2,4-dienoate (114) (Sumarah et al., 2011). 114 was antifungal against M. violaceum with MIC of 2 μM (Sumarah et al., 2011). Terrein (115) was isolated from the endophytic fungus Aspergillus terreus JAS-2 associated with medicinal plant Achyranthus aspera (Goutam et al., 2017). In antifungal assay, 10 μg/μL concentration of 115 showed inhibition of Bipolaris Sorokiniana (57.14%), A. flavus (52.5%), and A. alternata (91.25%) as compared to control (Goutam et al., 2017). Six new halogenated cyclopentenones, including four chlorinated, bicolorins A (116), B (117), D (118), and E (119), and two brominated bicolorins G (120) and H (121), were isolated from the endophytic fungus Saccharicola bicolor of Bergenia purpurascens (Zhao et al., 2020). Compounds 116-121 possessed weak to moderate activity against five pathogenic fungi-Uromyces viciae-fabae, Pythium dissimile, G. zeae, A. niger, and S. sclerotiorum, with MICs of 26.8-380.9 μM. 117 and 118, in particular, exhibited moderate activity against P. dissimile with the MICs of 33.0 and 44.7 μM, respectively, compared with the positive control cycloheximide (MIC 30.6 μM). Additionally, 118 was proven to be potently antifungal against S. sclerotiorum in vivo, indicating its potential as a candidate of new natural fungicides (Zhao et al., 2020). A new dibenzo-α-pyrone rhizopycnin D (122) and a known congener TMC-264 (123) were isolated from the endophytic fungus Rhizopycnis vagum Nitaf22 obtained from Nicotiana tabacum (Lai et al., 2016). Both compounds inhibited the spore germination of M. oryzae with IC50 values of 33.9 and 34.1 μM, respectively (Lai et al., 2016).
A new furan derivative named 3-(5-oxo-2,5-dihydrofuran-3-yl) propanoic acid (124) was isolated from an endophytic Aspergillus tubingensis of Decaisnea insignis (Figure 7; Yang X. F. et al., 2019). 124 exhibited potent antifungal activity against F. graminearum with MIC value of 102.6 μM (Yang X. F. et al., 2019). Piliformic acid (125), derived from octanoate that originates from a fatty acid synthase, was obtained from endophytic fungi Xylaria sp., which were isolated from leaves of guarana plant (Elias et al., 2018). 125 had antifungal activity against C. gloeosporioides with an MIC of 2.92 μM (Elias et al., 2018). The endophytic fungus Aspergillus sp. from Moringa oleifera produced one phenolic acid, ferulic acid (126), which showed a weak antifungal activity at 500 μg/mL against A. niger with an inhibition zone diameter of 2 mm (Abonyi et al., 2018). Cordycepsidone A (127), a depsidone metabolite, was isolated from Cordyceps dipterigena, an endophytic fungus from Desmotes incomparabilis antagonistic to the phytopathogen Gibberella fujikuroi (Varughese et al., 2012). 127 showed a moderate to potent growth inhibitory activity against G. fujikuroi (MIC, 23.3 μM) and Pythium ultimum (MIC, 3.4 μM) (Varughese et al., 2012). An endophyte Botryosphaeria rhodina was isolated from the stems of the medicinal plant Bidens pilosa and was chosen for further chemical study due to its potent antifungal effects (Abdou et al., 2010). Bioactivity-guided fractionation of this strain yielded two new depsidones, botryorhodine A (128) and B (129), which were significantly active against A. terreus with MICs of 26.03 and 49.70 μM (Abdou et al., 2010). A new tridepside, colletotric acid (130), was characterized from C. gloeosporioides, an endophytic fungus colonized inside the stem of Artemisia mongolica (Zou et al., 2000). 130 inhibited the growth of the crop pathogenic fungus Helminthosporium sativum with an MIC of 95.4 μM (Zou et al., 2000). From an isolate of Aspergillus from a healthy plant of oilseed rape (Brassica napus), two chlorinated diphenyl ethers, penicillither (131) and methyl dichloroasterrate (132), were characterized (Qin et al., 2019). Both of them inhibited four plant pathogenic fungi (B. cinerea, M. fructicola, S. sclerotiorum, and S. trifoliorum) with the EC50s of 21.7-151.2 μM (Qin et al., 2019).
As mentioned above, biologically active and structurally diverse fungal metabolites constitute a rich resource for drugs and pesticides discovery. These recently discovered metabolites 1-132, which possessed extensive chemical skeletons, exhibited moderate to potent anti-phytopathogenic activities. Therefore, some of them might have the potential use in the development of new biopesticides. Especially, based on these potential compounds, a series of novel derivatives with agricultural and pharmaceutical importance were designed and synthesized.
Griseofulvin (85), a secondary metabolite possessing spirocyclic benzofuran-3-one skeleton, was initially isolated from the fungus Penicillium griseofulvum in 1939 by Oxford et al. (Petersen et al., 2014). Then, this polyketide has also been found to be produced by several Ascomycetes including Penicillium sp., Aspergillus sp., and Xylaria sp. (Zhao et al., 2012). Griseofulvin has a rich chemical diversity, and until now, more than 400 griseofulvin analogs have been isolated and synthesized (Figure 8; Petersen et al., 2014). Griseofulvin was one of the first antifungal metabolic products in filamentous fungi, offered in vitro fungistatic effect against dermatomycoses. Recently, it has gained renewed attention due to many reports of antifungal properties against plant pathogenic fungi. Zhao et al. reported that griseofulvin, produced by an endophyte Nigrospora sp., displayed clear growth inhibition of the test eight plant pathogenic fungi (B. cinerea, Colletotrichum orbiculare, F. oxysporum f.sp. cucumerinum, F. oxysporum f.sp. melonis, Pestalotia diospyri, Pythium ultimum, R. solani, and S. sclerotiorum) (Zhao et al., 2012). Among them, it exhibited potent activity against B. cinerea and C. orbiculare with the EC50 values of 0.6 and 1.4 μM, respectively (Zhao et al., 2012). It should be pointed out that, its dechlorinated derivative, dechlorogriseofulvin, only showed weak activity, indicating that the chlorine played a decisive role in the antifungal activity. Tang et al. reported that griseofulvin isolated from Penicillium brasilianum displayed strong inhibitory effect on the growth of A. solani with an MIC of 3.13 μM (Tang et al., 2015). These impressive activities make this compound suitable candidate for biopesticide discovery and trigger the following synthesis studies, including semisynthesis from griseofulvin and de novo synthesis.
Bai et al. designed and synthesized 22 griseofulvin derivatives from commercially available griseofulvin (Bai et al., 2019). In vitro antifungal assay indicated that griseofulvin and its derivatives possessed remarkable activities against five phytopathogenic fungi (Cytospora sp., C. gloeosporioides, B. cinerea, A. solani, and F. solani) (Bai et al., 2019). Of significance was that, compounds numbered 6a-6f were found to have significant potential, which were superior to commercial fungicides hymexazol and thiophanate-methyl. The three-dimensional quantitative structure-activity relationship analysis revealed that the modification of the 4’ position, for example, the suitable bulky and electronegative acyl-substituted groups at the 4’ position, can significantly improve the antifungal activity, even up to 10-fold higher than inhibitory effect of the parent compound griseofulvin (Bai et al., 2019). Kartsev et al. carried out the synthetic studies of griseofulvin derivatives (Kartsev et al., 2019). As a result, a total of 42 new griseofulvin derivatives were designed and synthesized. These newly synthesized griseofulvin derivatives exhibited potent antifungal activity against A. niger, A. ochraceus, A. fumigatus, A. versicolor, Penicillium funiculosum, P. ochrochloron, P. verucosum var. cyclopium, Trichoderma viride. All compounds showed higher activity than the commercial antifungal drugs ketoconazole (7-42 times) and bifonazole (3-16 fold) (Kartsev et al., 2019). Interestingly, the synthesized compounds were more active than the parent compound griseofulvin (up to 4 times). Therefore, in conclusion, griseofulvin especially its derivatives can be further used for the development of new agricultural fungicides.
Trichothecenes, a group of sesquiterpene-based fungal metabolites with a common tricyclic 12,13-epoxytrichothec-9-ene core, are found to be produced by various microorganisms such as Fusarium sp., Myrothecium sp., Spicellum sp., Stachybotrys sp., Cephalosporium sp., Trichoderma sp., and Trichothecium sp. Based on the substitution pattern, trichothecenes are classified into different families of nivalenols, neosolaniols, isotrichodermins, calonectrins, trichothecins, and trichobreols (Figure 9; Takahashi-Ando et al., 2020). Trichothecenes are well-known as mycotoxins, causing significant negative effects on agriculture and human health. Initial study of trichothecenes was focused on the phytotoxicity and mammalian intoxications, and later emphasis was on exploring their complementary bioactivities. Some related compounds exhibited potent antitumor effect and have been used for clinical trials (Li et al., 2017). Meanwhile, trichothecenes showed varied role in the field of agriculture and may act as bio-control agents, strengthen the defense system of the plants against pathogens (Kumari et al., 2016). Previous structure–activity relationships study revealed that a slight modification of trichothecenes could dramatically decrease the toxicity but still retain its bioactivity (Li et al., 2017). In this case, there seems to be a special significance to search for new trichothecenes with agricultural applications.
Yamazaki et al. reported three new antifungal trichothecenes, trichobreols A-C, from the NaI-containing fermentation of the marine-derived Trichoderma cf. brevicompactum (Yamazaki et al., 2020a). Then, from the same fungus, another two new trichothecenes, trichobreols D and E, were obtained (Yamazaki et al., 2020b). Trichobreols showed good antifungal activity, especially toward yeast-like pathogenic fungi. Moreover, five semisynthetic derivatives were prepared from trichobreol A to evaluate the structure–activity relationship of antifungal trichothecenes. The results indicated that the substituents at C-3 and C-4 positions were responsible for the potency of antifungal activity (Yamazaki et al., 2020b). Li et al. reported three new macrocyclic trichothecenes possessing rare 6’-ketal moieties, roridoxins A-C, from the insect-associated fungus Myrothecium roridum (Li et al., 2019). Roridoxins A and C were found to possess potent antifungal activity against Alternaria tenuissima, A. niger, Pyricularia grisea, and F. oxysporum (Li et al., 2019). These findings suggested that trichothecenes are also promising leads which are applicable for the development of new agrochemicals.
Fungal endophytes, which are ubiquitous in plants and symbiotic with their hosts, are well-known for producing a variety of antimicrobial metabolites and enhancing plant resistance to pathogens and pests. These bioactive metabolites play a defensive role in protecting the host plants against pathogenic attacks. Therefore, antibiotic metabolites from the endophytes have the potential to be applied as agrochemicals to control pathogens. This review summarizes the structural/biogenetic types of 132 antifungal metabolites isolated from fungal endophytes in the past two decades. These present metabolites possess diverse chemical structures. Based on their putative biogenetic origin, they were classified into alkaloids (including 1-14 for cytochalasins and 15-35 for other alkaloids), terpenoids (36-49), polyketides (50-123), and other miscellaneous compounds (124-132). It is worth mentioning that the structural classifications based on biogenetic categories are somewhat arbitrary, as many compounds are derived from mixed biosynthetic pathways. Taking compounds 86-89 as an example, they are new members of the chromenone-type of metabolites biogenetically derived from isoprenoids and a polyketide. As shown in Figure 10, among the 132 active metabolites, approximately 56% were polyketides. Molecules grouped as polyketides are significant in natural products research due to their biosynthetic complexity and high value in pharmaceutical and/or agrochemical industries. This finding revealed that polyketides are the most potential classes for the discovery of novel antifungal lead compounds. It should be pointed out that we divided cytochalasins into an individual group, since this kind of compounds constitutes 10.6% of the metabolites reported, nearly as many as terpenoids (10.6%) and other alkaloids (15.9%). Cytochalasins are a diverse group of fungal polyketide synthase-non-ribosomal peptide synthetase (PKS-NRPS) hybrid metabolites. This class of compounds are worthy of particular attention and may be applied in the field of bio-pesticides.
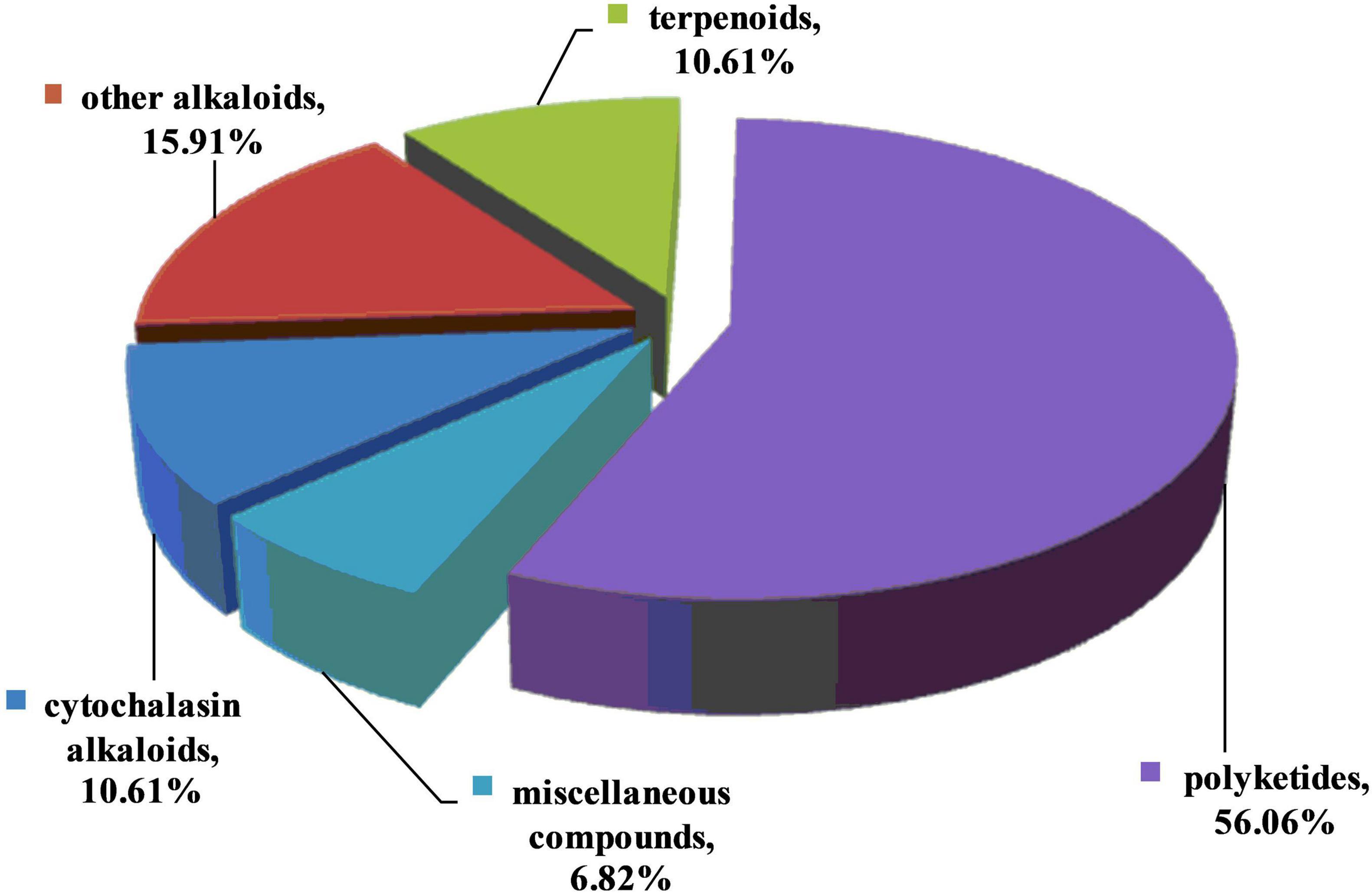
Figure 10. Percentage distributions of antifungal metabolites based on their putative biogenetic origin.
It is well-known that the endophytic fungi from terrestrial plants are a treasure house of bioactive secondary metabolites. Moreover, marine-derived endophytic fungi have also been considered as a non-negligible resource to search for antifungal lead compounds. In this review, compounds 4, 10-12, 55-57, and 76-78 were isolated from marine mangrove-associated endophytic fungi. The marine environment is quite different from the terrestrial environment, which indicates that marine-derived endophytic fungi may possess unique metabolic pathways to produce interesting antifungal compounds with novel structures. As for the producing strains, the fungal genera Phomopsis, Aspergillus, Chaetomium, and Nigrospora are predominant genera as producers of these antifungal metabolites, with 17, 11, 10, and 10 compounds described, respectively (Figure 11). As shown in Figure 11, these metabolites are scattered across a variety of fungi belonging to 29 various genera, including some rare species. Among them, C. globosum is a creative species known for making a large number of exclusive and structurally significant bioactive chaetoglobosins. The antifungal activity of these metabolites against the phytopathogenic fungi indicated that the endophytes could protect their host plants by producing bioactive molecules, which may be toxic or even lethal to phytopathogens and highlighted the potential of endophytic fungi in producing valuable metabolites. Moreover, a chemical interaction between the endophytes and the host plants, which produces metabolites as chemical defense compounds, needs further investigation.
Most importantly, among the 132 antifungal metabolites presented in this review, some of them not only possessed intriguing chemical structures but also showed novel antifungal activities comparable to those of widely used chemical pesticides. These include cytochalasin alkaloids cytochalasin Z28 (2), penochalasin K (4), and penochalasin J (10), indole diketopiperazines 12β-hydroxy-13α-methoxyverruculogen TR-2 (18), fumitremorgin B (19), and verruculogen (20), N-containing compounds (3R,6R)-3-benzyl-6-isopropyl-4-methylmorpholine-2,5-dione (25) and nigrosporamide A (26), sesquiterpenes rhinomilisin B (41) and divirensol H (42), quinones stemphyperylenol (50), fonsecinone A (54), and macrosporin (55), cyclopentenones (4S∗,5S∗)-2,4,5-trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten-1-one (56), bicolorin B (117), and D (118) (Figure 12). The above mentioned compounds showed potent (or significant) antifungal activities compared to those of positive controls (usually chemical pesticides such as difenoconazole, hymexazol, carbendazim, and triadimefon), which indicates that they could be used as potential alternatives to traditional pesticides.
Overall, endophytes are considered to be a treasure house of antifungal metabolites. This review summarizes 132 metabolites with moderate to potent antifungal activities isolated from fungal endophytes. We also list some “star molecules” such as griseofulvin and its derivatives that possess high potential as candidates of new natural fungicides herein. It is believed that in the near future, research on antifungal metabolites of endophytic fungi will become more prolific and be beneficial for the development of new agrochemicals.
KX and X-QL performed the literature material’s collection and reorganization. PZ and D-LZ wrote the manuscript. All authors reviewed the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research work was financially supported by the National Natural Science Foundation of China (32070391) and the Agricultural Science and Technology Innovation Program (ASTIP-TRIC05).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.689527/full#supplementary-material
Supplementary Table 1 | The producing strain, environment source, and antifungal activities of compounds 1–132.
Abdou, R., Scherlach, K., Dahse, H. M., Sattler, I., and Hertweck, C. (2010). Botryorhodines A-D, antifungal and cytotoxic depsidones from Botryosphaeria rhodina, an endophyte of the medicinal plant Bidens pilosa. Phytochemistry 71, 110–116. doi: 10.1016/j.phytochem.2009.09.024
Abdou, R., Shabana, S., and Rateb, M. E. (2018). Terezine E, bioactive prenylated tryptophan analogue from an endophyte of Centaurea stoebe. Nat. Prod. Res. 34, 503–510. doi: 10.1080/14786419.2018.1489393
Abonyi, D. O., Eze, P. M., Abba, C. C., Ujam, N. T., Proksch, P., Okoye, F. B. C., et al. (2018). Biologically active phenolic acids produced by Aspergillus sp., an endophyte of Moringa oleifera. Eur. J. Biol. Res. 8, 158–168. doi: 10.5281/zenodo.1404981
Ahmed, I., Hussain, H., Schulz, B., Draeger, S., Padula, D., Pescitelli, G., et al. (2011). Three new antimicrobial metabolites from the endophytic fungus Phomopsis sp. Eur. J. Org. Chem. 2011, 2867–2873.
Bai, Y. B., Gao, Y. Q., Nie, X. D., Tuong, T. M., Li, D., and Gao, J. M. (2019). Antifungal activity of griseofulvin derivatives against phytopathogenic fungi in vitro and in vivo and three-dimensional quantitative structure-activity relationship analysis. J. Agric. Food Chem. 67, 6125–6132. doi: 10.1021/acs.jafc.9b00606
Bai, Y. B., Zhang, A. L., Tang, J. J., and Gao, J. M. (2013). Synthesis and antifungal activity of 2-chloromethyl-1H-benzimidazole derivatives against phytopathogenic fungi in vitro. J. Agric. Food Chem. 61, 2789–2795. doi: 10.1021/jf3053934
Cao, L. L., Zhang, Y. Y., Liu, Y. J., Yang, T. T., Zhang, J. L., Zhang, Z. G., et al. (2016). Anti-phytopathogenic activity of sporothriolide, a metabolite from endophyte Nodulisporium sp. A21 in Ginkgo biloba. Pestic. Biochem. Physiol. 129, 7–13. doi: 10.1016/j.pestbp.2015.10.002
Chapla, V. M., Zeraik, M. L., Cafeu, M. C., Silva, G. H., Cavalheiro, A. J., Bolzani, V. S., et al. (2018). Griseofulvin, diketopiperazines and cytochalasins from endophytic fungi colletotrichum crassipes and Xylaria sp., and their antifungal, antioxidant and anticholinesterase activities. J. Braz Chem. Soc. 29, 1707–1713. doi: 10.21577/0103-5053.20180045
Chapla, V. M., Zeraik, M. L., Leptokarydis, I. H., Silva, G. H., Bolzani, V. S., Young, M. C., et al. (2014). Antifungal compounds produced by Colletotrichum gloeosporioides, an endophytic fungus from Michelia champaca. Molecules 19, 19243–19252. doi: 10.3390/molecules191119243
Chen, H. Y., Liu, T. K., Shi, Q., and Yang, X. L. (2019). Sesquiterpenoids and diterpenes with antimicrobial activity from Leptosphaeria sp. XL026, an endophytic fungus in Panax notoginseng. Fitoterapia 137:104243. doi: 10.1016/j.fitote.2019.104243
Chen, Y. M., Yang, Y. H., Li, X. N., Zou, C., and Zhao, P. J. (2015). Diterpenoids from the endophytic fungus Botryosphaeria sp. P483 of the Chinese herbal medicine Huperzia serrata. Molecules 20, 16924–16932. doi: 10.3390/molecules200916924
Copping, L. G., and Duke, S. O. (2007). Natural products that have been used commercially as crop protection agents. Pest. Manag. Sci. 63, 524–554. doi: 10.1002/ps.1378
d’Errico, G., Aloj, V., Flematti, G. R., Sivasithamparam, K., Worth, C. M., Lombardi, N., et al. (2020). Metabolites of a Drechslera sp. endophyte with potential as biocontrol and bioremediation agent. Nat. Prod. Res. Online ahead of print. doi: 10.1080/14786419.2020.1737058
Ding, G., Liu, S., Guo, L., Zhou, Y., and Che, Y. (2008). Antifungal metabolites from the plant endophytic fungus Pestalotiopsis foedan. J. Nat. Prod. 71, 615–618. doi: 10.1021/np070590f
Elias, L. M., Fortkamp, D., Sartori, S. B., Ferreira, M. C., Gomes, L. H., Azevedo, J. L., et al. (2018). The potential of compounds isolated from Xylaria spp. as antifungal agents against anthracnose. Braz J. Microbiol. 49, 840–847. doi: 10.1016/j.bjm.2018.03.003
Gouda, S., Das, G., Sen, S. K., Shin, H. S., and Patra, J. K. (2016). Endophytes: a treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 7:1538. doi: 10.3389/fmicb.2016.01538
Goutam, J., Sharma, G., Tiwari, V. K., Mishra, A., Kharwar, R. N., Ramaraj, V., et al. (2017). Isolation and characterization of “Terrein” an antimicrobial and antitumor compound from endophytic fungus Aspergillus terreus (JAS-2) associated from Achyranthus aspera Varanasi, India. Front. Microbiol. 8:1334. doi: 10.3389/fmicb.2017.01334
Hu, Z., Tao, Y., Tao, X., Su, Q., Cai, J., Qin, C., et al. (2019). Sesquiterpenes with phytopathogenic fungi inhibitory activities from fungus Trichoderma virens from Litchi chinensis Sonn. J. Agric. Food Chem. 67, 10646–10652. doi: 10.1021/acs.jafc.9b04053
Huang, R., Jiang, B. G., Li, X. N., Wang, Y. T., Liu, S. S., Zheng, K. X., et al. (2018). Polyoxygenated cyclohexenoids with promising α-glycosidase inhibitory activity produced by Phomopsis sp. YE3250, an endophytic fungus derived from Paeonia delavayi. J. Agric. Food Chem. 66, 1140–1146. doi: 10.1021/acs.jafc.7b04998
Huang, S., Chen, H., Li, W., Zhu, X., Ding, W., and Li, C. (2016). Bioactive chaetoglobosins from the mangrove endophytic fungus Penicillium chrysogenum. Mar. Drugs 14:172. doi: 10.3390/md14100172
Huang, S., Xu, J., Li, F., Zhou, D., Xu, L., and Li, C. (2017). Identification and antifungal activity of metabolites from the mangrove fungus Phoma sp. L28. Chem. Nat. Compd. 53, 237–240. doi: 10.1007/s10600-017-1961-z
Hussain, H., Ahmed, I., Schulz, B., Draeger, S., and Krohn, K. (2012a). Pyrenocines J-M: four new pyrenocines from the endophytic fungus, Phomopsis sp. Fitoterapia 83, 523–526. doi: 10.1016/j.fitote.2011.12.017
Hussain, H., Krohn, K., Ahmed, I., Draeger, S., Schulz, B., Di Pietro, S., et al. (2012b). Phomopsinones A-D: four new pyrenocines from endophytic fungus Phomopsis sp. Eur. J. Org. Chem. 2012, 1783–1789.
Hussain, H., Krohn, K., Draeger, S., Meier, K., and Schulz, B. (2009). Bioactive chemical constituents of a sterile endophytic fungus from Meliotus dentatus. Rec. Nat. Prod. 3, 114–117. doi: 10.1007/978-1-4020-8804-9_25
Jia, M., Chen, L., Xin, H. L., Zheng, C. J., Rahman, K., Han, T., et al. (2016). A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front. Microbiol. 7:906. doi: 10.3389/fmicb.2016.00906
Kartsev, V., Geronikaki, A., Petrou, A., Lichitsky, B., Kostic, M., Smiljkovic, M., et al. (2019). Griseofulvin derivatives: synthesis, molecular docking and biological evaluation. Curr. Top. Med. Chem. 19, 1145–1161. doi: 10.2174/1568026619666190523080136
Kim, Y. M., Lee, C. H., Kim, H. G., and Lee, H. S. (2004). Anthraquinones isolated from Cassiatora (Leguminosae) seed show an antifungal property against phytopathogenic fungi. J. Agric. Food Chem. 52, 6096–6100. doi: 10.1021/jf049379p
Kumari, I., Ahmed, M., and Akhter, Y. (2016). Multifaceted impact of trichothecene metabolites on plant-microbe interactions and human health. Appl. Microbiol. Biotechnol. 100, 5759–5771. doi: 10.1007/s00253-016-7599-0
Lai, D., Wang, A., Cao, Y., Zhou, K., Mao, Z., Dong, X., et al. (2016). Bioactive dibenzo-α-pyrone derivatives from the endophytic fungus Rhizopycnis vagum Nitaf22. J. Nat. Prod. 79, 2022–2031. doi: 10.1021/acs.jnatprod.6b00327
Li, T. X., Xiong, Y. M., Chen, X., Yang, Y. N., Wang, Y., Jia, X. W., et al. (2019). Antifungal macrocyclic trichothecenes from the insect-associated fungus Myrothecium roridum. J. Agric. Food Chem. 67, 13033–13039. doi: 10.1021/acs.jafc.9b04507
Li, W., Yang, X., Yang, Y., Duang, R., Chen, G., Li, X., et al. (2016). Anti-phytopathogen, multi-target acetylcholinesterase inhibitory and antioxidant activities of metabolites from endophytic Chaetomium globosum. Nat. Prod. Res. 30, 2616–2619. doi: 10.1080/14786419.2015.1129328
Li, X. J., Zhang, Q., Zhang, A. L., and Gao, J. M. (2012). Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 60, 3424–3431. doi: 10.1021/jf300146n
Li, Y., Liu, D., Cheng, Z., Proksch, P., and Lin, W. (2017). Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 7:7259. doi: 10.1039/C6RA26956G
Liu, K., Yang, Y., Miao, C. P., Zheng, Y. K., Chen, J. L., Chen, Y. W., et al. (2016). Koningiopisins A-H, polyketides with synergistic antifungal activities from the endophytic fungus Trichoderma koningiopsis. Planta Med. 82, 371–376. doi: 10.1055/s-0035-1558228
Liu, S., Liu, X., Guo, L., Che, Y., and Liu, L. (2013). 2H-Pyran-2-one and 2H-furan-2-one derivatives from the plant endophytic fungus Pestalotiopsis fici. Chem. Biodivers. 10, 2007–2013. doi: 10.1002/cbdv.201200361
Liu, X., Dong, M., Chen, X., Jiang, M., Lv, X., and Zhou, J. (2008). Antimicrobial activity of an endophytic Xylaria sp. YX-28 and identification of its antimicrobial compound 7-amino-4-methylcoumarin. Appl. Microbiol. Biotechnol. 78, 241–247. doi: 10.1007/s00253-007-1305-1
Loesgen, S., Bruhn, T., Meindl, K., Dix, I., Schulz, B., Zeeck, A., et al. (2011). (+)-Flavipucine, the missing member of the pyridione epoxide family of fungal antibiotics. Eur. J. Org. Chem. 2011, 5156–5162.
Ma, Y. M., Qiao, K., Kong, Y., Li, M. Y., Guo, L. X., Miao, Z., et al. (2018). A new isoquinolone alkaloid from an endophytic fungus R22 of Nerium indicum. Nat. Prod. Res. 32, 2375–2381. doi: 10.1080/14786419.2016.1258556
Macías-Rubalcava, M. L., Hernández-Bautista, B. E., Jiménez-Estrada, M., González, M. C., Glenn, A. E., Hanlin, R. T., et al. (2008). Naphthoquinone spiroketal with allelochemical activity from the newly discovered endophytic fungus Edenia gomezpompae. Phytochemistry 69, 1185–1196. doi: 10.1016/j.phytochem.2007.12.006
Petersen, A. B., Rønnest, M. H., Larsen, T. O., and Clausen, M. H. (2014). The chemistry of griseofulvin. Chem. Rev. 114, 12088–12107. doi: 10.1021/cr400368e
Qin, J., Lyu, A., Zhang, Q. H., Yang, L., Zhang, J., Wu, M. D., et al. (2019). Strain identification and metabolites isolation of Aspergillus capensis CanS-34A from Brassica napus. Mol. Biol. Rep. 46, 3451–3460. doi: 10.1007/s11033-019-04808-5
Qin, S., Hussain, H., Schulz, B., Draeger, S., and Krohn, K. (2010). Two new metabolites, epoxydine A and B, from Phoma sp. Helv. Chim. Acta 93, 169–174. doi: 10.1002/hlca.200900199
Qin, S., Krohn, K., Flörke, U., Schulz, B., Draeger, S., Pescitelli, G., et al. (2009). Two new fusidilactones from the fungal endophyte Fusidium sp. Eur. J. Org. Chem. 2009, 3279–3284.
Qin, S., Krohn, K., Hussain, H., Schulz, B., and Draeger, S. (2011). Pestalotheols E-H: antimicrobial metabolites from an endophytic fungus isolated from the tree Arbutus unedo. Eur. J. Org. Chem. 2011, 5163–5166.
Saleem, M., Hussain, H., Ahmed, I., Draeger, S., Schulz, B., Meier, K., et al. (2011). Viburspiran, an antifungal member of the octadride class of maleic anhydride natural products. Eur. J. Org. Chem. 2011, 808–812.
Santiago, C., Fitchett, C., Munro, M. H. G., Jalil, J., and Santhanam, J. (2012). Cytotoxic and antifungal activities of 5-hydroxyramulosin, a compound produced by an endophytic fungus isolated from Cinnamomum mollisimum. Evid. Based Complement. Alternat. Med. 2012:689310. doi: 10.1155/2012/689310
Shi, D., An, R., Zhang, W., Zhang, G., and Yu, Z. (2017). Stilbene derivatives from Photorhabdus temperata SN259 and their antifungal activities against phytopathogenic fungi. J. Agric. Food Chem. 65, 60–65. doi: 10.1021/acs.jafc.6b04303
Siriwach, R., Kinoshita, H., Kitani, S., Igarashi, Y., Pansuksan, K., Panbangred, W., et al. (2014). Bipolamides A and B, triene amides isolated from the endophytic fungus Bipolaris sp. MU34. J. Antibiot. 67, 167–170. doi: 10.1038/ja.2013.103
Sumarah, M. W., Kesting, J. R., Sørensen, D., and Miller, J. D. (2011). Antifungal metabolites from fungal endophytes of Pinus strobus. Phytochemistry 72, 1833–1837. doi: 10.1016/j.phytochem.2011.05.003
Takahashi-Ando, N., Matsui, K., Suzuki, T., Sadamatsu, K., Azuhata, H., Okada, A., et al. (2020). Trichothecene biosynthesis in different fungal genera: resistance mechanisms, pathway enzymes, and their product applications. JSM Mycotoxins 70, 67–74. doi: 10.2520/myco.70-2-3
Talibi, I., Boubaker, H., Boudyach, E. H., and Ait Ben, and Aoumar, A. (2014). Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 117, 1–17. doi: 10.1111/jam.12495
Talontsi, F. M., Dittrich, B., Schüffler, A., Sun, H., and Laatsch, H. (2013). Epicoccolides: antimicrobial and antifungal polyketides from an endophytic fungus Epicoccum sp. associated with Theobroma cacao. Eur. J. Org. Chem. 2013, 3174–3180. doi: 10.1002/ejoc.201300146
Talontsi, F. M., Facey, P., Tatong, M. D., Tofazzal Islam, M., Frauendorf, H., Draeger, S., et al. (2012). Zoosporicidal metabolites from an endophytic fungus Cryptosporiopsis sp. of Zanthoxylum leprieurii. Phytochemistry 83, 87–94. doi: 10.1016/j.phytochem.2012.06.006
Tang, H. Y., Zhang, Q., Li, H., and Gao, J. M. (2015). Antimicrobial and allelopathic metabolites produced by Penicillium brasilianum. Nat. Prod. Res. 29, 345–348. doi: 10.1080/14786419.2014.940347
Teles, H. L., Silva, G. H., Castro-Gamboa, I., Bolzani Vda, S., Pereira, J. O., Costa-Neto, C. M., et al. (2005). Benzopyrans from Curvularia sp., an endophytic fungus associated with Ocotea corymbosa (Lauraceae). Phytochemistry 66, 2363–2367. doi: 10.1016/j.phytochem.2005.04.043
Varughese, T., Riosa, N., Higginbotham, S., Arnold, A. E., Coley, P. D., Kursar, T. A., et al. (2012). Antifungal depsidone metabolites from Cordyceps dipterigena, an endophytic fungus antagonistic to the phytopathogen Gibberella fujikuroi. Tetrahedron Lett. 53, 1624–1626. doi: 10.1016/j.tetlet.2012.01.076
Wang, J., Ding, W., Wang, R., Du, Y., Liu, H., Kong, X., et al. (2015). Identification and bioactivity of compounds from the mangrove endophytic fungus Alternaria sp. Mar. Drugs 13, 4492–4504. doi: 10.3390/md13074492
Wang, J., He, W., Huang, X., Tian, X., Liao, S., Yang, B., et al. (2016). Antifungal new oxepine-containing alkaloids and xanthones from the deep-sea-derived fungus Aspergillus versicolor SCSIO 05879. J. Agric. Food Chem. 64, 2910–2916. doi: 10.1021/acs.jafc.6b00527
Wang, L. W., Xu, B. G., Wang, J. Y., Su, Z. Z., Lin, F. C., Zhang, C. L., et al. (2012). Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl. Microbiol. Biotechnol. 93, 1231–1239. doi: 10.1007/s00253-011-3472-3
Wang, L., Shen, J., Xu, L., Gao, J., Zhang, C., Wang, Y., et al. (2019). A metabolite of endophytic fungus Cadophora orchidicola from Kalimeris indica serves as a potential fungicide and TLR4 agonist. J. Appl. Microbiol. 126, 1383–1390. doi: 10.1111/jam.14239
Wang, Y., Xu, L., Ren, W., Zhao, D., Zhu, Y., and Wu, X. (2012). Bioactive metabolites from Chaetomium globosum L18, an endophytic fungus in the medicinal plant Curcuma wenyujin. Phytomedicine 19, 364–368. doi: 10.1016/j.phymed.2011.10.011
Wei, G., Tan, D., Chen, C., Tong, Q., Li, X. N., Huang, J., et al. (2017). Flavichalasines A–M, cytochalasan alkaloids from Aspergillus flavipes. Sci. Rep. 7:42434. doi: 10.1038/srep42434
Wu, S. H., Chen, Y. W., Shao, S. C., Wang, L. D., Yu, Y., Li, Z. Y., et al. (2009). Two new solanapyrone analogues from the endophytic fungus Nigrospora sp. YB-141 of Azadirachta indica. Chem. Biodivers. 6, 79–85. doi: 10.1002/cbdv.200700421
Wu, Y. M., Zhou, Q. Y., Yang, X. Q., Luo, Y. J., Qian, J. J., Liu, S. X., et al. (2019). Induction of antiphytopathogenic metabolite and squalene production and phytotoxin elimination by adjustment of the mode of fermentation in cocultures of phytopathogenic Nigrospora oryzae and Irpex lacteus. J. Agric. Food Chem. 67, 11877–11882. doi: 10.1021/acs.jafc.9b04209
Xiao, J., Zhang, Q., Gao, Y. Q., Shi, X. W., and Gao, J. M. (2014a). Antifungal and antibacterial metabolites from an endophytic Aspergillus sp. associated with Melia azedarach. Nat. Prod. Res. 28, 1388–1392. doi: 10.1080/14786419.2014.904308
Xiao, J., Zhang, Q., Gao, Y. Q., Tang, J. J., Zhang, A. L., and Gao, J. M. (2014b). Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 62, 3584–3590. doi: 10.1021/jf500054f
Xu, D., Luo, M., Liu, F., Wang, D., Pang, X., Zhao, T., et al. (2017). Cytochalasan and tyrosine-derived alkaloids from the marine sediment-derived fungus Westerdykella dispersa and their bioactivities. Sci. Rep. 7:11956. doi: 10.1038/s41598-017-12327-1
Xue, M., Zhang, Q., Gao, J. M., Li, H., Tian, J. M., and Pescitelli, G. (2012). Chaetoglobosin Vb from endophytic Chaetomium globosum: absolute configuration of chaetoglobosins. Chirality 24, 668–674. doi: 10.1002/chir.22068
Yamazaki, H., Takahashi, O., Kirikoshi, R., Yagi, A., Ogasawara, T., Bunya, Y., et al. (2020a). Epipolythiodiketopiperazine and trichothecene derivatives from the NaI-containing fermentation of marine-derived Trichoderma cf. brevicompactum. J. Antibiot. 73, 559–567.
Yamazaki, H., Yagi, A., Takahashi, O., Yamaguchi, Y., Saito, A., Namikoshi, M., et al. (2020b). Antifungal trichothecene sesquiterpenes obtained from the culture broth of marine-derived Trichoderma cf. brevicompactum and their structure-activity relationship. Bioorg. Med. Chem. Lett. 30:127375. doi: 10.1016/j.bmcl.2020.127375
Yang, X. F., Wang, N. N., Kang, Y. F., and Ma, Y. M. (2019). A new furan derivative from an endophytic Aspergillus tubingensis of Decaisnea insignis (Griff.) Hook.f. & Thomson. Nat. Prod. Res. 33, 2777–2783. doi: 10.1080/14786419.2018.1501687
Yang, Z., Dan, W. J., Li, Y. X., Peng, G. R., Zhang, A. L., and Gao, J. M. (2019). Antifungal metabolites from Alternaria atrans: an endophytic fungus in Psidium guajava. Nat. Prod. Commun. 14:1934578X1984411. doi: 10.1177/1934578X19844116
You, F., Han, T., Wu, J. Z., Huang, B. K., and Qin, L. P. (2009). Antifungal secondary metabolites from endophytic Verticillium sp. Biochem. Syst. Ecol. 37, 162–165. doi: 10.1016/j.bse.2009.03.008
Zhang, P., Wei, Q., Yuan, X., and Xu, K. (2020). Newly reported alkaloids produced by marine-derived Penicillium species (covering 2014–2018). Bioorg. Chem. 99:103840. doi: 10.1016/j.bioorg.2020.103840
Zhang, Q., Xiao, J., Sun, Q. Q., Qin, J. C., Pescitelli, G., and Gao, J. M. (2014). Characterization of cytochalasins from the endophytic Xylaria sp. and their biological functions. J. Agric. Food Chem. 62, 10962–10969. doi: 10.1021/jf503846z
Zhang, W., Xu, L., Yang, L., Huang, Y., Li, S., and Shen, Y. (2014). Phomopsidone A, a novel depsidone metabolite from the mangrove endophytic fungus Phomopsis sp. A123. Fitoterapia 96, 146–151. doi: 10.1016/j.fitote.2014.05.001
Zhang, X., Li, G., Ma, J., Zeng, Y., Ma, W., and Zhao, P. (2010). Endophytic fungus Trichothecium roseum LZ93 antagonizing pathogenic fungi in vitro and its secondary metabolites. J. Microbiol. 48, 784–790. doi: 10.1007/s12275-010-0173-z
Zhao, J. H., Zhang, Y. L., Wang, W., Wang, J. Y., and Zhang, C. L. (2012). Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 28, 2107–2112. doi: 10.1007/s11274-012-1015-4
Zhao, M., Guo, D. L., Liu, G. H., Fu, X., Gu, Y. C., Ding, L. S., et al. (2020). Antifungal halogenated cyclopentenones from the endophytic fungus Saccharicola bicolor of Bergenia purpurascens by the one strain-many compounds strategy. J. Agric. Food Chem. 68, 185–192. doi: 10.1021/acs.jafc.9b06594
Zhu, X., Chen, J., Zhu, S., He, Y., Ding, W., and Li, C. (2017a). Two new compounds from Nigrospora sphaerica ZMT05, a fungus derivated from Oxya chinensis Thunber. Nat Prod Res. 31, 951–958. doi: 10.1080/14786419.2017.1413566
Zhu, X., Zhou, D., Liang, F., Wu, Z., Shi, Z., and Li, C. (2017b). Penochalasin K, a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semisynthesis. Fitoterapia 123, 23–28. doi: 10.1016/j.fitote.2017.09.016
Keywords: fungal endophytes, secondary metabolites, chemical diversity, phytopathogenic fungi, antifungal activities, biopesticides
Citation: Xu K, Li X-Q, Zhao D-L and Zhang P (2021) Antifungal Secondary Metabolites Produced by the Fungal Endophytes: Chemical Diversity and Potential Use in the Development of Biopesticides. Front. Microbiol. 12:689527. doi: 10.3389/fmicb.2021.689527
Received: 01 April 2021; Accepted: 10 May 2021;
Published: 21 June 2021.
Edited by:
Peng Fu, Ocean University of China, ChinaReviewed by:
Fengyu Du, Qingdao Agricultural University, ChinaCopyright © 2021 Xu, Li, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Lin Zhao, emhhb2RvbmdsaW5AY2Fhcy5jbg==; Peng Zhang, emhhbmdwZW5nQGNhYXMuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.