- 1Department of Veterinary Clinical Science, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 2Laboratory of Anatomy of Domestic Animals, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 3Key Lab of Animal Epidemiology and Zoonosis of Ministry of Agriculture, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 4Modern Animal Research Center, Nanjing University, Nanjing, China
- 5Research and Development Department, Artron BioResearch Inc., Vancouver, BC, Canada
The incidence of zoonotic Staphylococcus pseudintermedius and Microsporum canis infections is rapidly growing worldwide in the context of an increasing frequency of close contact between animals and humans, presenting challenges in both human and veterinary medicine. Moreover, the development of microbial resistance and emergence of recalcitrant biofilms, accompanied by the insufficiency of new antimicrobial agents, have become major obstacles in treating superficial skin infections caused by various microbes including S. pseudintermedius and M. canis. Over recent years, the prospects of antimicrobial peptides as emerging antimicrobials to combat microbial infections have been demonstrated. In our study, two novel short-chain peptides, namely, allomyrinasin and andricin B, produced by Allomyrina dichotoma and Andrias davidianus, were revealed to exhibit potent antimicrobial efficacy against clinical isolates of S. pseudintermedius and M. canis with remarkable and rapid fungicidal and bactericidal effects, while allomyrinasin exhibited inhibition of biofilm formation and eradication of mature biofilm. These peptides displayed synergistic activity when combined with amoxicillin and terbinafine against S. pseudintermedius and M. canis. Cytoplasmic leakage via cytomembrane permeabilization serves as a mechanism of action. Extremely low hemolytic activity and serum stability in vitro, as well as superior anti-infective efficacy in reducing bacterial counts and relieving the inflammatory response in vivo, were detected. The potent antibacterial, antifungal, and anti-inflammatory activities of allomyrinasin and andricin B might indicate promising anti-infective alternatives for the treatment of S. pseudintermedius and M. canis infections in the context of human and veterinary medicine.
Introduction
In human history, the most challenging epidemics with pandemic potential, such as pestis, HIV, tuberculosis, SARS-2003, brucellosis, and COVID-19, are attributed to microbes that develop from their counterparts that naturally inhabit animals (Jones et al., 2008; Zhou et al., 2020). Animals have been reported as reservoirs for more than 60% of human infectious diseases, which is also the case for approximately 75% of emerging diseases. It is evident that the majority of human infectious diseases derive from animals (Taylor et al., 2001). Among them, zoonotic skin infection has attracted increasing interest in the fields of human and veterinary medicine, as well as animal husbandry.
Staphylococci have been associated with a plethora of human medical issues, including localized skin infections, surgical site infections (SSIs), and medical device-related infections (Yong et al., 2019). Notably, Staphylococcus pseudintermedius has appeared over the last decade as a critically significant bacterial pathogen responsible for skin, soft tissue, wound, and SSIs and possesses increasing zoonotic potential for cutaneous infections in humans (Murayama et al., 2013). S. pseudintermedius infections have been widely reported in recent years because of their potential for widespread transmission in human populations and significant implications for public health. This pathogen was blamed for apparent zoonotic infections in humans via close contact with affected animals, such as animals on farms or in household (Stegmann et al., 2010; Weese and van Duijkeren, 2010). Staphylococcal infections can result in local and systemic infections, extended inflammation, and delayed wound healing (Wolcott et al., 2010). Worryingly, recent studies have reported that the emergence of drug resistance of S. pseudintermedius against demonstrating the emergence of drug resistance to antimicrobial agents applied in Europe and North America, indicating the urgency of novel therapeutic alternatives (Perreten et al., 2010; Ong et al., 2014).
Apart from staphylococci, Microsporum canis, a worldwide distributed zoophilic dermatophyte, is another significant pathogen frequently related to integumentary and hair infections in humans and animals (Pasquetti et al., 2017). Animals can act as reservoirs and the major route for M. canis infection spreading in humans (Cafarchia et al., 2006). However, M. canis infections usually have a high recurrence rate and therefore require a long-cycle treatment. Medical therapy with conventional antifungal antimicrobials like triazole agents is prone to induce hepatotoxicity and nephrotoxicity (Kyriakidis et al., 2017). In light of the increasing frequency of S. pseudintermedius and complexity of treatment for M. canis, there is a compelling demand for new antimicrobial agents with novel mechanisms of action to reduce the incidence of bacterial resistance and drug toxicity.
Antimicrobial peptides (AMPs) have arisen as novel therapeutic alternatives against microbial infections in recent years. These peptides possess rapid and broad-spectrum antimicrobial activity against bacterial and fungal pathogens via membrane disruption or endocellular constituent binding, leading to a low potential for the development of resistance (Brunetti et al., 2017). In addition, they display an effective ability to prevent biofilm formation and to eradicate sessile communities of microbes. With the ability to modulate the host inflammatory response and prevent cellular toxicity, AMPs have shown significant potential for treating skin infections and accelerating the wound healing (Woodburn et al., 2019). However, several limitations of AMPs, including proteinase degradation, serum binding, and high cost of production (Sharma et al., 2018), have substantially stunted their pharmaceutical utilization. Therefore, exploration of short-chain AMPs with antibacterial and antifungal potency, synergism with conventional therapeutics, and suitability for animal application will be a direction for future development.
Several studies have reported the efficacy of certain types of AMPs in killing S. pseudintermedius and M. canis in vitro (Santoro and Maddox, 2014; Simonetti et al., 2014); however, there are little data about the application of newly discovered AMPs to in vitro antimicrobial assays for clinical isolates of S. pseudintermedius and M. canis from China and their activity in a S. pseudintermedius-induced mouse skin infection model. Two short synthetic peptides, namely, allomyrinasin and andricin B, identified from Allomyrina dichotoma and Andrias davidianus, and three other original short-chain peptides have been previously described as effective against staphylococcal and fungal strains, which may serve as good candidates for further research (Pei et al., 2018; Bao et al., 2019; Bessa et al., 2019; Chaparro-Aguirre et al., 2019; Lee et al., 2019). In our present study, we confirmed that allomyrinasin and andricin B exhibited potent antimicrobial activities by the disruption of microbial cell membrane. In addition, we evaluated the antibiofilm activities of the peptides and investigated synergistic activities with conventional antimicrobials. The toxicity of these peptides to mammalian erythrocytes and their antimicrobial stability in serum were assessed to identify their potential for animal application. Moreover, the mouse model of S. pseudintermedius skin infection was established; and bacterial counts, histopathological techniques, and Western blotting were performed to explore the antibacterial and anti-inflammatory efficacy of allomyrinasin and andricin B in vivo.
Materials and Methods
Peptides
The characteristics of five AMPs, allomyrinasin (AAVTRR ILCWFA-NH2) (Figure 1A), andricin B (GLTRLFSVIK) (Figure 1B), pinipesin (VAEARQGSFSY), nigrocin-HLM (GLLS GILGAGKKIVF), Hs02 (KWAVRIIRKFIKGFIS), and nisin (ITSISLCTPGCKTGALMGCNMKTATCHCSIHVSK), were described previously (Rogers, 1928; Pei et al., 2018; Bao et al., 2019; Bessa et al., 2019; Chaparro-Aguirre et al., 2019; Lee et al., 2019). Allomyrinasin and andricin B are cationic AMPs identified from A. dichotoma and A. davidianus blood, respectively. These short-chain peptides possess net positive charge of +3 and +2, as depicted in the schematic in Figure 1. Pinipesin, nigrocin-HLM, and Hs02 are another three novel AMPs and exhibit potent antimicrobial effects against a wide spectrum of microorganisms, simultaneously with low hemolytic and cytotoxic activities. All peptides in this study were obtained by chemical synthesis using a standard solid-phase 9-fluorenylmethoxycarbonyl (Fmoc) and refined by reverse-phase high-performance liquid chromatography (RP-HPLC) (GL Biochem, Shanghai, China). A purity greater than 99.6% was identified by liquid chromatography–mass spectrometry (LC-MS) (Oddo et al., 2015).
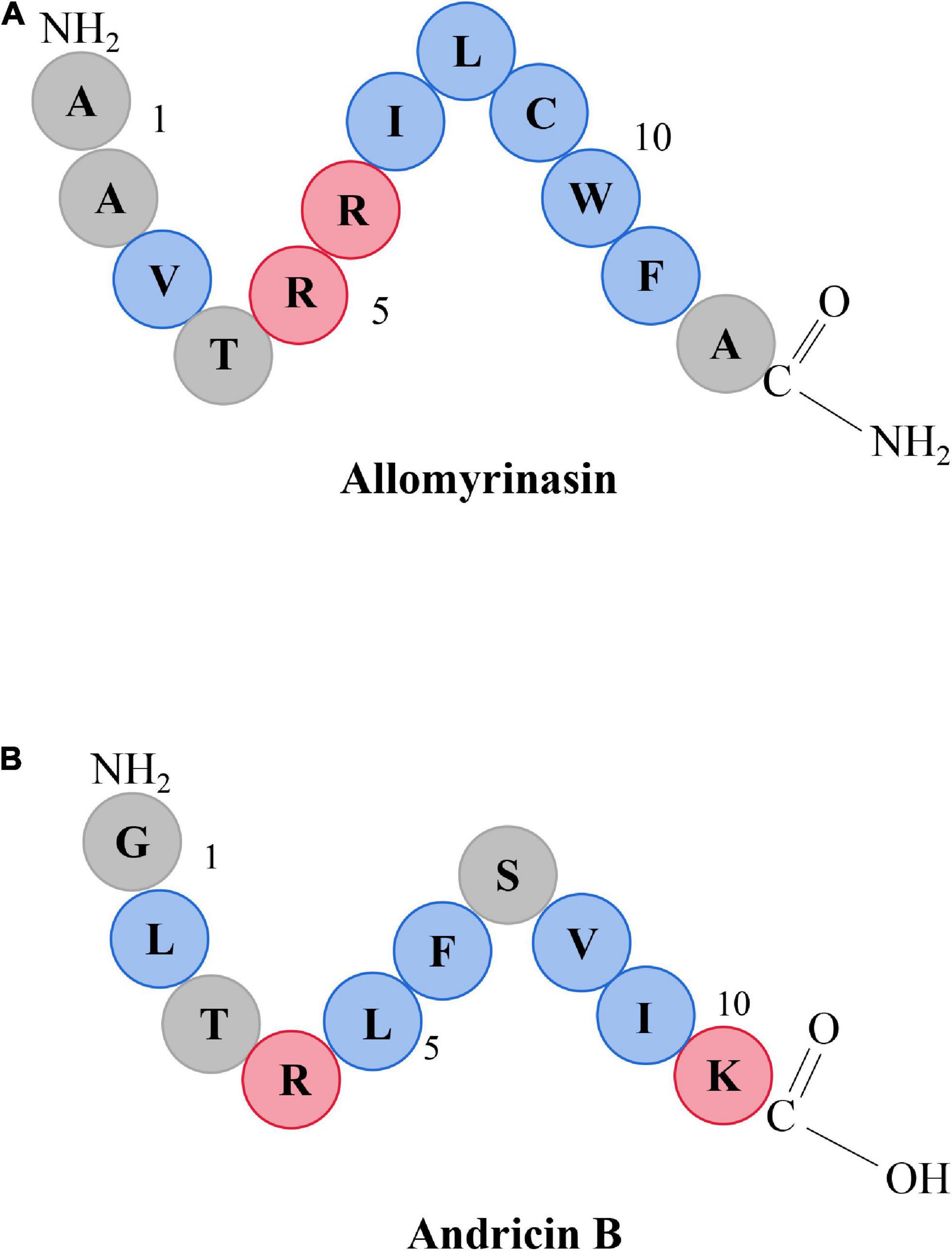
Figure 1. Schematic representation of allomyrinasin (A) and andricin B (B). Hydrophobic residues are in blue, while those residues with a net positive charge are in red.
Pathogens
Bacterial strains included in the study were isolated from clinical specimens obtained from the skin of dogs with pyoderma at the Small Animal Protection Center (Hainan, China), and fungal strains were acquired from the skin of dogs admitted to the Veterinary Teaching Hospital of China Agricultural University (Table 1). Bacterial and fungal strains were examined at the Diagnostic Laboratory of China Agricultural University by 16S rRNA gene amplification and rDNA ITS sequence analysis by polymerase chain reaction (PCR), respectively. The amplified PCR products were then electrophoresed and sequenced by the Sanger method at Majorbio Sanger Bio-pharm Technology (Beijing, China). The data of sequencing analysis were submitted to the GenBank database of National Center for Biotechnology Information (NCBI, Bethesda, MD, United States).
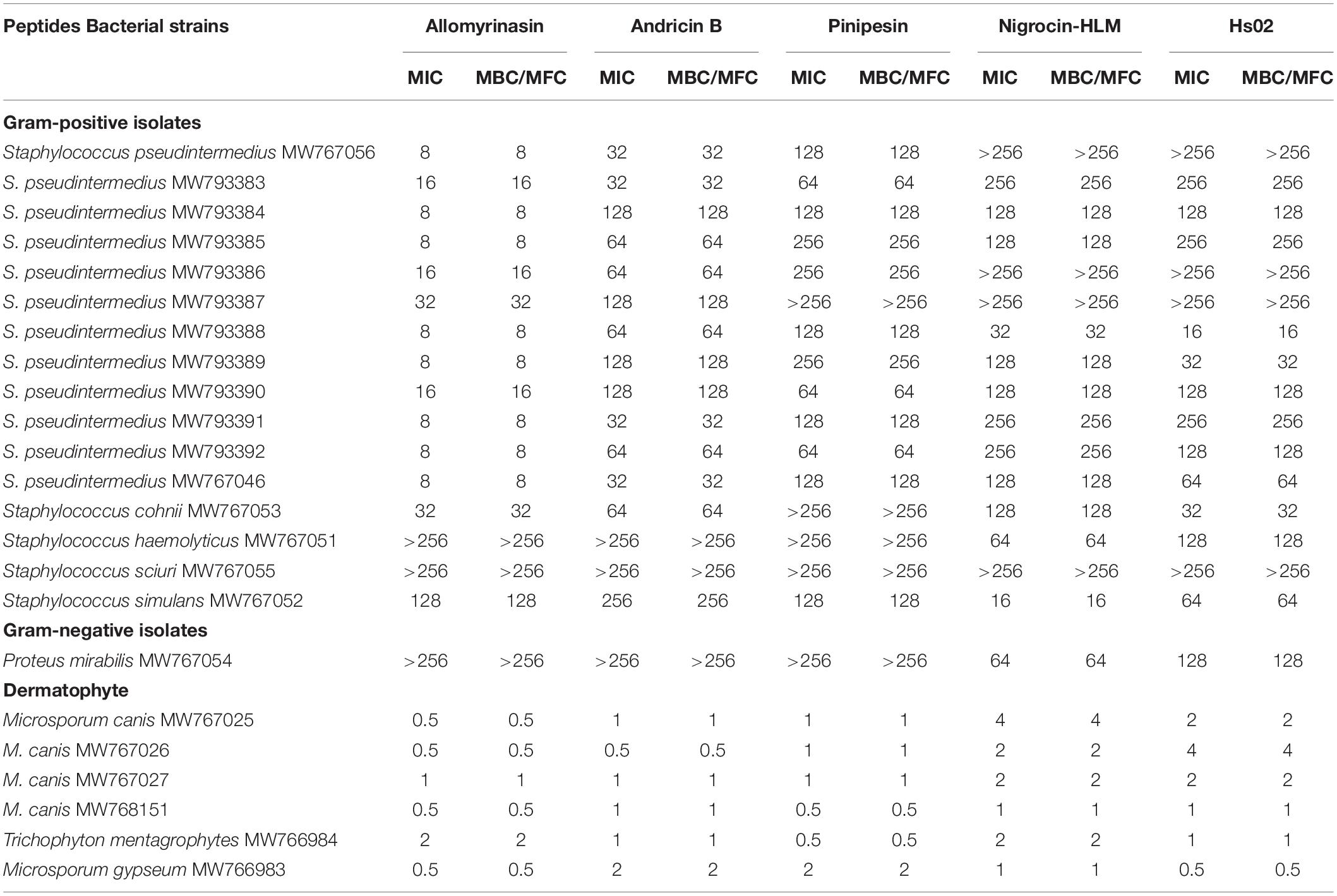
Table 1. Minimum inhibitory concentrations and minimum bactericidal/fungicidal concentrations (MICs and MBCs/MFCs, μg/ml) of selected peptides against clinical isolates of planktonic bacteria and dermatophytes.
Antimicrobial Assays
The broth microdilution technique was applied to measure the minimal inhibitory concentrations (MICs) of the tested peptides (Wiegand et al., 2008). In brief, the concentrations of bacterial strains (MW767051–MW793392) were diluted to 1 × 105 CFU/ml in Mueller–Hinton (MH) broth (MHB) and added to a twofold serial dilution of peptides ranging from 1 to 512 μg/ml in 96-well microplates. The MIC values were recorded as the lowest concentration preventing visible bacterial growth after 24 h of incubation at 37°C. Next, 10 μl of overnight suspension cultivated on a MH plate was performed with colony counting for determination of minimum bactericidal concentrations (MBCs). The lowest concentrations with no evidence of colonies were considered MBCs. Similarly, a twofold serial dilution of peptides varying from 0.25 to 16 μg/ml was added to the fungal suspension (MW766983–MW767027) at concentrations of 1 × 103 CFU/ml in RPMI-1640 (Gibco, Grand Island, NY, United States). The culture was observed daily, and the MICs were determined at 7 days after incubation as the lowest concentration with no microbial growth at 30°C. Next, 100 μl of the fungal culture was smeared on Sabouraud dextrose agar (SDA) plates to determine the minimal fungicidal concentration (MFC), considered the lowest concentration of drug inhibiting 100% growth. Experiments were run in triplicate.
Time-Kill Assays
The microbial killing activity of allomyrinasin and andricin B against S. pseudintermedius, Staphylococcus cohnii and M. canis strains (MW767056, MW767053, and MW768151) was determined based on prior antimicrobial susceptibility assays. Briefly, bacteria and dermatophytes were incubated aerobically overnight at 37°C in MHB and RPMI-1640 media and then diluted to 1 × 105 CFU/ml. Peptides (at 0.5×, 1×, 2×, and 4× MIC) and equivalent saline were applied to inocula with rotation speed at 250 r.p.m. After 0, 15, 30, 60, 120, 180, and 300 min, 100 μl of aliquots was removed for 10-fold serial dilution and then plated on brain heart infusion (BHI) and SDA plates for viable counts in triplicate (Blondeau et al., 2012; Simonetti et al., 2014).
Anti-biofilm Assays
Minimal biofilm inhibitory concentration (MBIC) and minimal biofilm eradication concentration (MBEC) assays were performed in accordance with assays utilizing prototype tetrazolium salt, 2,3,5-triphenyl-tetrazolium chloride (TTC) or 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) (Delattin et al., 2014; Sabaeifard et al., 2014). S. pseudintermedius MW767056, S. cohnii MW767053, and M. canis MW768151 strains were applied in these assays. Briefly, a suspension of overnight inoculum, rinsed with phosphate-buffered saline (PBS), was adjusted to 1 × 106 CFU/ml with culture media and then incubated with peptides of the same concentration range applied in the MIC assay in a round-bottomed 96-well microplate for MBIC determination. Finally, biofilms were washed and quantified after 24 h of static incubation at 37°C.
Microbial biofilm eradication was determined using a previously described protocol. One hundred microliters of aliquots of overnight culture diluted to 106 CFU/ml was added to a round-bottomed 96-well microplate and then incubated at 37°C for 3 h for adhesion, followed by the removal of planktonic cells with PBS and the addition of 100 μl of fresh media. After 48 h of static incubation at 37°C for biofilm maturation, mature biofilms were washed and incubated with a twofold serial dilution series of peptides at 37°C for 24 h. Following sufficient growth, inocula were rinsed with PBS and quantified with TTC and XTT. Evaluations were done in triplicate.
Combination Therapy Analysis
Allomyrinasin and andricin B were investigated for their interaction with antibiotics and antifungals such as amoxicillin (a major drug for Staphylococcus-induced skin infection) (Summers et al., 2014) and terbinafine hydrochloride (a main drug for M. canis-induced dermatophytosis) (Aneke et al., 2021). S. pseudintermedius MW767056, S. cohnii MW767053, and M. canis MW768151 were applied as tested strains in this assay. Overnight cultures were diluted to a final concentration of 1 × 105 CFU/ml in a total of 200 μl. The effect of combined medication was determined by testing a twofold serial dilution series of antimicrobials in combination with a constant concentration (1/4 × MIC) of peptides, which was unable to inhibit microbial growth independently. This assay was carried out in triplicate. A fractional inhibitory concentration (FIC) index was calculated to indicate the underlying interaction between two antimicrobials, and interpretation of the interaction type was performed as follows.
A FIC was derived for each well containing the lowest inhibitory combination of antimicrobials from the following calculation:
A FIC index=FIC of antimicrobial A + FIC of antimicrobial B. Synergism and antagonism presented as FIC indices less than 0.5 and more than 4, respectively. All values ranging from 0.5 to 4.0 suggested an indifferent interaction, except for an additive effect, classified as a FIC index equivalent to 1 (Yan and Hancock, 2001).
Bacteriolysis Analysis
Cytolysis, indicated by a reduction in the absorbance at 600 nm, was identified as described previously (Oliva et al., 2003). Briefly, overnight culture of S. pseudintermedius MW767056 incubated in MHB at 37°C with an OD600 of ≈0.6 was diluted to 1 × 107 CFU/ml. Aliquots (100 ml) were placed in 96-well microplates, followed by the addition of allomyrinasin and andricin B at 4× MIC. Nisin at 4× MIC and 0.9% NaCl (w/v), normal saline, were used as the positive and negative controls, respectively. Turbidity was recorded every 2 h until 10 h by a microplate reader at 600-nm absorbance. The assay was carried out in triplicate.
Calcein Leakage Analysis
The cell membrane permeabilization induced by peptides was measured by variations in preloaded calcein leakage from lysed cells as described previously (Xiong et al., 2005). In brief, the inocula of S. pseudintermedius MW767056 were cultivated in MHB at 37°C overnight, collected by centrifugation, rinsed with PBS, and then diluted to 108 CFU/ml. After incubation with 5 μM of calcein for 2 h at 37°C, cells, gathered by rinses and centrifugation, were diluted to a suspension of 105 CFU/ml. Then, 100 μl of aliquots was placed in black 96-well microplates, followed by the addition of allomyrinasin and andricin B at a series of concentrations of 0.5, 1, 5, and 10× MIC. Bacterial cultures treated with 0.9% NaCl (w/v) and nisin at 10× MIC were defined as the negative and positive controls, respectively. Leakage was monitored every 10 min for up to 1 h using a fluorescence plate reader. Membrane permeabilization (%) was determined as the absolute percent calcein leakage in peptide-treated samples in contrast to peptide-untreated samples. Experiments were done in triplicate and repeated independently twice.
Hemolytic Activity
The hemolysis assay was carried out by inoculating mammalian erythrocytes obtained from fresh defibrinated sheep and mouse blood (Thermo Fisher Scientific, Waltham, MA, United States) with the peptides. Briefly, peptides were incubated at 37°C with a 4% erythrocyte suspension with a twofold serial dilution series of peptides ranging from 512 to 1 μg/ml for 2 h. PBS and 1% nonionic detergent Triton X-100TM (Sigma-Aldrich, St. Louis, MO, United States) diluted in PBS served as negative and positive controls, respectively. Cytolysis was evaluated by the measurement of absorption using a microplate reader at 550 nm. Experiments were done in triplicate. The hemolysis is derived from the following calculation:
A represents the absorbance of samples treated with peptide, while APBS and ATriton indicate the absorbance of the negative and positive controls, respectively.
Antimicrobial Activities in Serum
Peptide stability was identified as the variation in antimicrobial activity after inoculation with serum. In this assay, S. pseudintermedius MW767056, S. cohnii MW767053, and M. canis MW768151 were applied as tested strains. Twenty-five percent fetal bovine serum (FBS) diluted in RPMI-1640 medium was stored at 37°C for 15 min for pre-equilibration and then inoculated with peptides with a twofold serial dilution of concentrations from 512 to 1 μg/ml. After incubation at 37°C for 4 h, peptides were collected by centrifugation, and 50 μl of aliquots was added to the same amount of culture at a concentration of 1 × 105 CFU/ml in 96-well microplates. The MICs were defined as the lowest concentration of peptides preventing any discernible growth after incubation with serum. All the peptide concentrations and controls had three replicates in a single 96-well plate, and two independent experiments were performed.
In vivo Mouse Skin Infection Model
All experiments on animals were employed in accordance with the Chinese Regulations for Laboratory Animals—The Guidelines for the Care of Laboratory Animals and Laboratory Animal Requirements of Environment and Housing Facilities (GB 14925-2010, National Laboratory Animal Standardization Technical Committee). The animal studies and research protocols were authorized by the Animal Care and Use Committee of China Agricultural University. The murine skin abrasion model was established using overnight culture of S. pseudintermedius (Abe et al., 1992). Forty specific pathogen-free (SPF), 6- to 8-week-old female BALB/c mice weighing 15–21 g were obtained from Charles River Laboratories (Beijing, China). All mice were raised singly in cages under a 12-h light/dark cycle and allowed ad libitum access to feed and water. Skin abrasion wounds of 3 cm2 were performed using sand paper on the shaved dorsal skin after anesthetization with 2.5% isoflurane. Damage was confined to the epidermis, and bleeding was carefully avoided. Fifty microliters of S. pseudintermedius MW767056 suspension at concentrations of 108 CFU/ml was spread on scratched skin areas loaded with a total inoculum of 5 × 106 CFU. Four hours after inoculation, 40 mice were randomly divided into four groups (three antimicrobial treatment groups and one infected control group treated with 50 μl 0.9% NaCl w/v). Treatment groups were topically treated daily for 3 days with 50 μl of allomyrinasin, andricin B, or amoxicillin at 6× MIC with direct application onto the infected skin, followed by the placement of a transparent film dressing of 3 cm2 (TegadermTM, 3M, St. Paul, MN, United States). The dosage of AMPs was determined by in vitro hemolysis study, which indicated the minimum hemolytic concentration (MHC), required to cause 10% hemolysis, was between 128 and 256 μg/ml and around 512 μg/ml for allomyrinasin and andricin B, respectively. In order to minimize hemolytic effect induced by peptides on mice and to simultaneously not negatively influence the therapeutic effects, 6× MIC of allomyrinasin and andricin B was chosen as the dosage for application. Body weight, clinical observations, and skin wound areas were also recorded daily. On the first and third days of administration, five mice were randomly removed from each group for euthanasia after anesthetization with 2.5% isoflurane. The infected skin and liver tissues were harvested separately for further analysis with a portion homogenized for bacterial culture and Western blotting analysis and another portion fixed in 4% formalin for histopathological assessment. Treatment efficacy was determined according to wound healing, inflammatory response, and bacterial load in skin. Diluted samples for bacterial culture were added and spread in BHI plates incubated at 37°C for 24 h following viable bacterial counting. Evaluation of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) protein expression in infected skin was performed by Western blotting as described previously (Tang et al., 2017).
Histopathological Examination
The treatment segments of dorsal skin tissues were cut out, rinsed with sterile physiologic saline, and then fixed in 4% paraformaldehyde. The formalin-fixed tissues, embedded in paraffin, were sectioned at 5-μm thickness and then stained with hematoxylin and eosin (Qi et al., 2016). Three sections of each tissue were evaluated.
Western Blotting Analysis
To examine the expression of IL-6 and TNF-α at the protein level, immunoblotting was performed as reported previously (Tang et al., 2017). Proteins from dorsal skin tissues were extracted for Western blotting assays. Primary antibodies included anti-IL-6 (1:1,000, Abcam, Waltham, MA, United States) and anti-TNF-α (1:1,000, Abcam, United States). To verify equal sample loading, the membrane was incubated with rabbit polyclonal anti-β-actin antibody (1:1,000, Abcam, United States) as an internal control. Horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000, Abcam, United States) was used as the secondary antibody. The membranes were exposed under a chemiluminescent imaging analysis system (GE Healthcare Life Science, Danderyd, Sweden). Samples not treated with primary antibodies were considered negative controls. Relative levels of each protein were normalized to those of β-actin in each sample.
Statistical Analysis
Statistical analysis of data was performed with SPSS 22 (Systat Software, Erkrath, Germany) by one-way ANOVA followed by Tukey’s multiple comparisons test and presented as the means ± SD. Log transformations were performed as needed to maintain homogeneity of variance and normality. p-Values lower than 0.05 were considered indicative of statistical significance.
Results
Strain Identification
The identification of bacterial and fungal isolates using Sanger method confirmed that the strains studied in this work were S. pseudintermedius, S. cohnii, Staphylococcus haemolyticus, Staphylococcus simulans, Proteus mirabilis and Staphylococcus sciuri, M. canis, Microsporum gypseum, and Trichophyton mentagrophytes. Among all isolates, 12 were identified as S. pseudintermedius and four were M. canis. Upon submission of the bacterial 16S rRNA and fungal ITS sequences to the NCBI GenBank database, the following accession numbers have been assigned: MW766983–MW793392 (Supplementary Table 1).
Antimicrobial Assays
The peptides exerted microbicidal effects on the tested bacteria and fungi but to different degrees (Table 1). Allomyrinasin and andricin B possessed comparatively strong bioactivity against S. pseudintermedius (MIC and MBC of 8 and 32 μg/ml, respectively). In contrast, pinipesin, nigrocin-HLM, and Hs02 showed relatively weak bioactivity against S. pseudintermedius, and their MIC and MBC values were 128 μg/ml, > 256μg/ml, and > 256μg/ml, respectively. In addition to pinipesin and nigrocin-HLM, allomyrinasin, andricin B, and HS02 were also potent against S. cohnii (MIC and MBC of 32, 64, and 32 μg/ml, respectively). Allomyrinasin, andricin B, and pinipesin were moderately inhibitory against S. simulans, but no obvious inhibitory effect was observed against S. haemolyticus and P. mirabilis. Nigrocin-HLM and Hs02 exhibited strong potency against S. simulans and moderate inhibitory activity against S. haemolyticus and P. mirabilis with MICs and MBCs of 128 μg/ml. No antibacterial activity of peptides against S. sciuri was detected. Interestingly, all of the tested peptides had strong antifungal activities against M. canis, M. gypseum, and T. mentagrophytes, with MIC values varying from 0.5 to 2 μg/ml. Considering the potent antimicrobial effect of allomyrinasin and andricin B on S. pseudintermedius, S. cohnii, and M. canis, we selected them as the tested targets to perform the following antimicrobial assays.
Microbial Killing Kinetics
The excellent antimicrobial activity of allomyrinasin and andricin B against clinical isolates of S. pseudintermedius, S. cohnii, and M. canis was identified; and next, the killing kinetics of these two AMPs were examined. Both peptides exhibited complete eradication of these strains in a time- and concentration-dependent manner (Figure 2). Allomyrinasin displayed rapid bactericidal efficacy and has the capability of totally clearing an inoculum of S. pseudintermedius (1 × 105 CFU/ml) within 100 and 180 min at 4× and 2× MIC, respectively, but more moderate bactericidal effects were observed, presented as the requirement of more time for complete elimination of S. cohnii. At a low concentration of 0.5× MIC, allomyrinasin could only inhibit the growth of S. pseudintermedius and S. cohnii, and the bacteria began to grow in large quantities after 3 h.
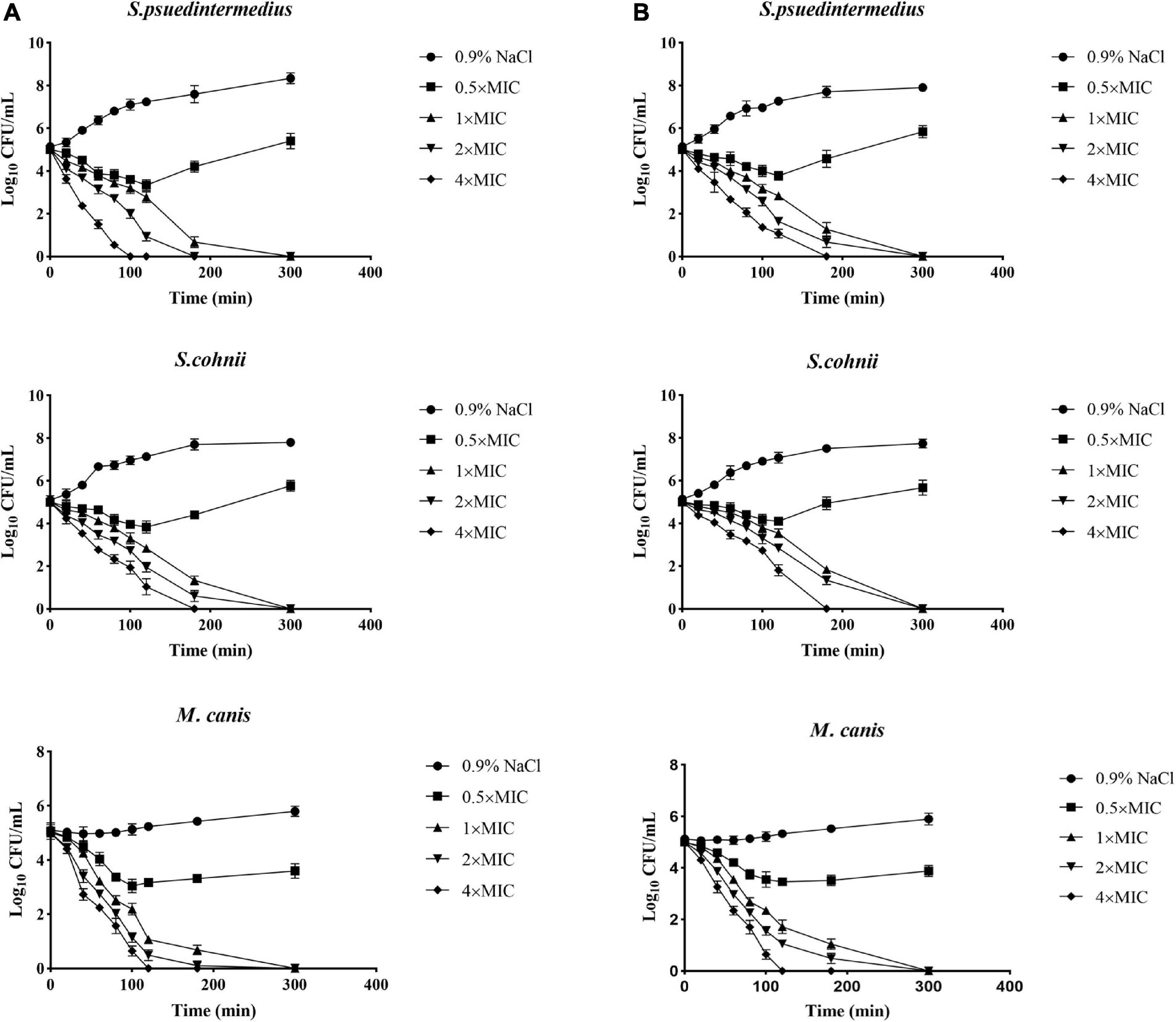
Figure 2. Microbial killing kinetics of allomyrinasin (A) and andricin B (B) against Staphylococcus pseudintermedius MW767056, Staphylococcus cohnii MW767053, and Microsporum canis MW768151 at four different concentrations. Inocula treated with physiological saline (0.9% NaCl) were used as a control. The error bars represent the standard deviation (SD) for three repeats.
In contrast to allomyrinasin, andricin B demonstrated relatively strong killing kinetics with the complete elimination of a starting inoculum of S. cohnii (1 × 105 CFU/ml) within a range from 180 to 300 min at 4×, 2×, and 1× MIC. For the S. cohnii strain, andricin B showed slower bactericidal activity against S. pseudintermedius. At a low concentration of 0.5× MIC, obvious inhibitory efficacy against S. pseudintermedius and S. cohnii was not detected.
Interestingly, allomyrinasin and andricin B displayed potent fungicidal activity at >0.5× MIC within 2 h. These peptides completely killed the initial inoculum at different concentrations within 5 h. The results show that allomyrinasin and andricin B exerted rapid and strong fungicidal effects on M. canis.
Biofilm Disruption
Microbial biofilms have posed serious challenges on account of their role in recurrent infections and drug resistance. In our study, the tested peptides were able to eradicate mature biofilms and prevent their formation (Table 2). Allomyrinasin maintained potent elimination of established biofilms and inhibition of the sessile cells of S. pseudintermedius and M. canis (MBIC of 32 and 2 μg/ml, respectively) with remarkable increases in MBEC values (128 and 8 μg/ml, respectively) but showed a moderate inhibitory effect on biofilm formation by S. cohnii. Compared with andricin B and pinipesin, allomyrinasin at concentrations higher than or equal to 8 and 0.25 μg/ml began to exhibit an obvious inhibitory effect on bacterial biofilm growth by S. pseudintermedius and M. canis (Figures 3A,C). In contrast, andricin B showed a weak effect on the formation of biofilms by S. pseudintermedius and S. cohnii with relatively high MBIC and MBEC values (MBIC of 128 and 256 μg/ml; MBEC of 256 and >256 μg/ml, respectively), but similar to allomyrinasin, andricin B completely inhibited biofilm formation and eradicated mature biofilms of M. canis exhibiting MBIC and MBEC of 4 and 16 μg/ml, respectively. A concentration ≥16 μg/ml had a more obvious inhibitory effect than allomyrinasin and pinipesin on bacterial biofilm growth by S. cohnii (Figure 3B). No obvious inhibition of pinipesin on the biofilm of S. pseudintermedius and S. cohnii was found; however, pinipesin exerted a strong effect on the biofilm formation and mature biofilm of M. canis with MBIC and MBEC of 4 and 16 μg/ml, respectively.
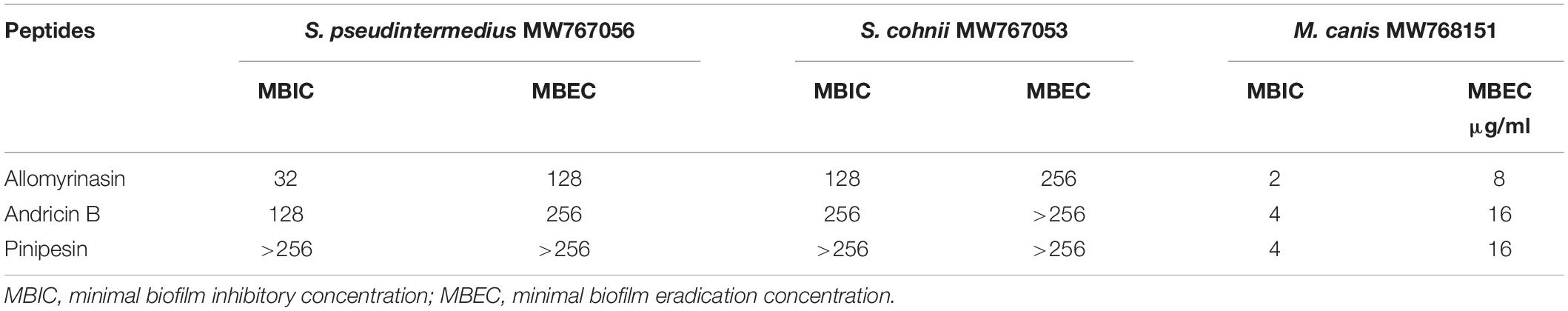
Table 2. The effects of allomyrinasin, andricin B, and pinipesin on mature biofilms and their formation by Staphylococcus pseudintermedius MW767056, Staphylococcus cohnii MW767053, and Microsporum canis MW768151.
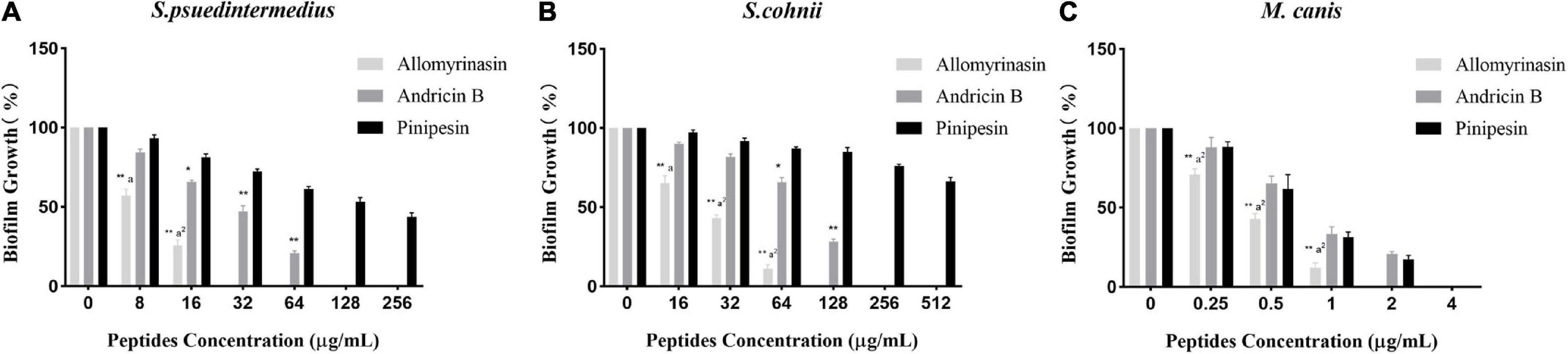
Figure 3. Effect of antimicrobial peptides on biofilm formation rate of Staphylococcus pseudintermedius MW767056 (A), Staphylococcus cohnii MW767053 (B), and Microsporum canis MW768151 (C). All data are shown as the mean ± SD from the experiment performed in triplicate. *p < 0.05, **p < 0.01 significant difference between pinipesin and the other two peptides; ap < 0.05, a2p < 0.01 significant difference between allomyrinasin and andricin B.
Synergistic Activity Analysis
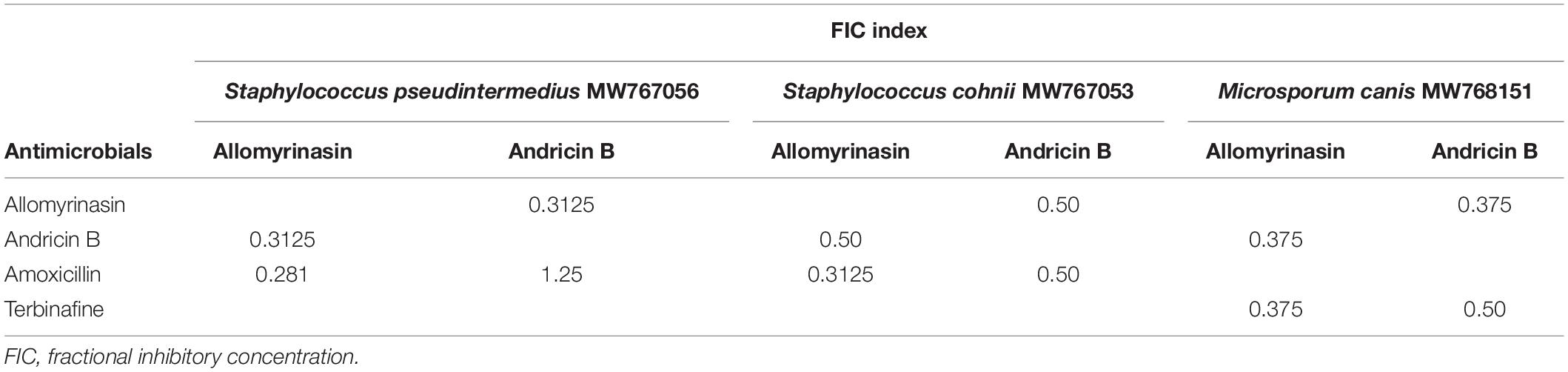
The in vitro antimicrobial assays indicated that the tested peptides had the potential to be applied independently. Thus, there is a necessity to assess synergism between peptides and conventional therapeutics widely used in skin infections to optimize dosage regimens. The synergistic interaction of allomyrinasin and andricin B with conventional antibiotics and antifungals against S. pseudintermedius, S. cohnii, and M. canis isolates was evaluated. Allomyrinasin and andricin B exhibited synergistic activity in combination against S. pseudintermedius, S. cohnii, and M. canis, with FIC indices ranging from 0.3125 to 1.25. Andricin B exhibited synergistic activity with amoxicillin and terbinafine hydrochloride against S. cohnii and M. canis, with FIC indices ranging from 0.375 to 1.25. Interestingly, allomyrinasin was shown to be superior to andricin B, as potent synergism with amoxicillin and terbinafine hydrochloride was observed, with FIC indices varying from 0.3125 to 0.50. Most notably, only one indifferent interaction between andricin B and amoxicillin against S. pseudintermedius was detected (Table 3).
Membrane Disruption Activity
Rapid cytolysis is an indication of damage to the membrane by AMPs (Boman et al., 1993; Park et al., 1998). The membrane-destroying activities of peptides were evaluated by culture turbidity measurement. The turbidity of inocula treated with 4× MIC peptides was monitored by a microplate reader at OD600 over the course of 10 h. The results revealed that both allomyrinasin and andricin B led to a rapid reduction in the total bacterial count in inocula with a high concentration of 108 CFU/ml. Allomyrinasin resulted in >30% and 75% decreases in absorbance after 2.5 and 6 h, respectively, while the data produced by andricin B were similar to those by allomyrinasin but to a lower degree. Nisin (4× MIC), a well-known membrane-perturbing peptide (Ruhr and Sahl, 1985), induced cell lysis at a higher rate than allomyrinasin, resulting in a >60% reduction in turbidity after 2.5 h. In contrast, the variations in turbidity of samples treated with 4× MIC amoxicillin, a cell wall synthesis-inhibiting antibiotic, were not recorded (Figure 4).
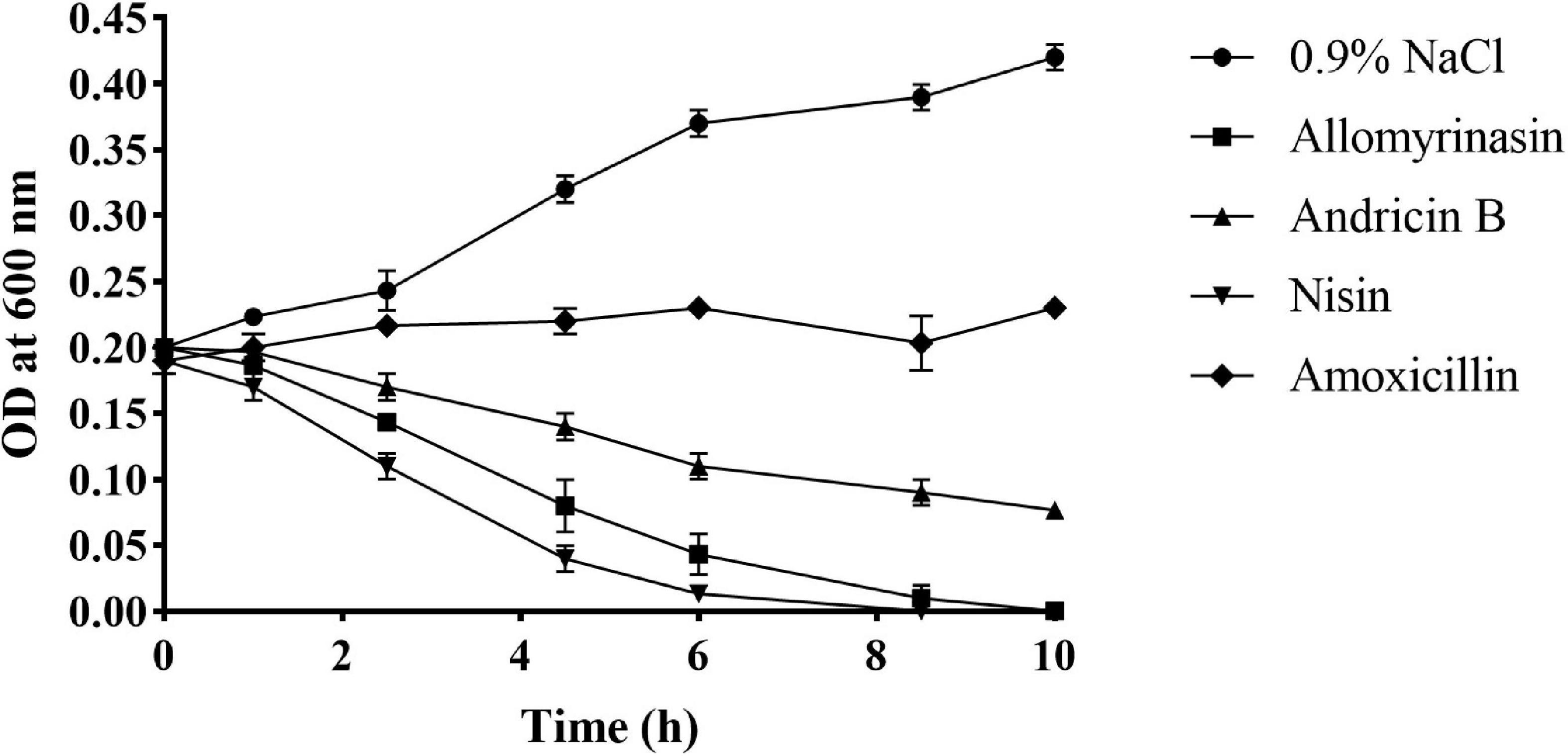
Figure 4. Bacterial killing kinetics of Staphylococcus pseudintermedius MW767056 culture treated with 4× minimal inhibitory concentration (MIC) allomyrinasin, andricin B, and amoxicillin by measuring absorbance at 600 nm over time. Nisin and saline served as positive and negative controls, respectively. All data represent the mean ± SD from the experiments performed in triplicate.
Membrane Permeabilization
The calcein leakage assay was applied to assess the membrane permeabilization of the tested peptides as described previously (Xiong et al., 2005). Calcein leakage from cells indicated membrane integrity damage, represented as a decrease in fluorescence intensity. The tested peptides damaged the bacterial cell membrane, resulting in intracellular calcein leakage in a time- and concentration-dependent manner. The greater efficiency and potency of allomyrinasin than andricin B in membrane perturbation were recorded. At a concentration of 0.5× MIC, peptides resulted in calcein leakage lower than 10%, while peptides with a concentration of 1× MIC caused more than 20% leakage. Peptides brought about at least 70% and 50% leakage, respectively, within 1 h at a concentration of 5× MIC. When concentrations increased to 10× MIC, an obvious membrane damage reaction was detected for both peptides. More than 95% and 80% decreases in fluorescence intensity were measured for the two tested peptides. Nisin (10× MIC) led to at least 95% calcein leakage from cells, while amoxicillin exerted no obvious disruptive reaction on membrane integrity at a high concentration of 10× MIC, as expected (Figure 5).
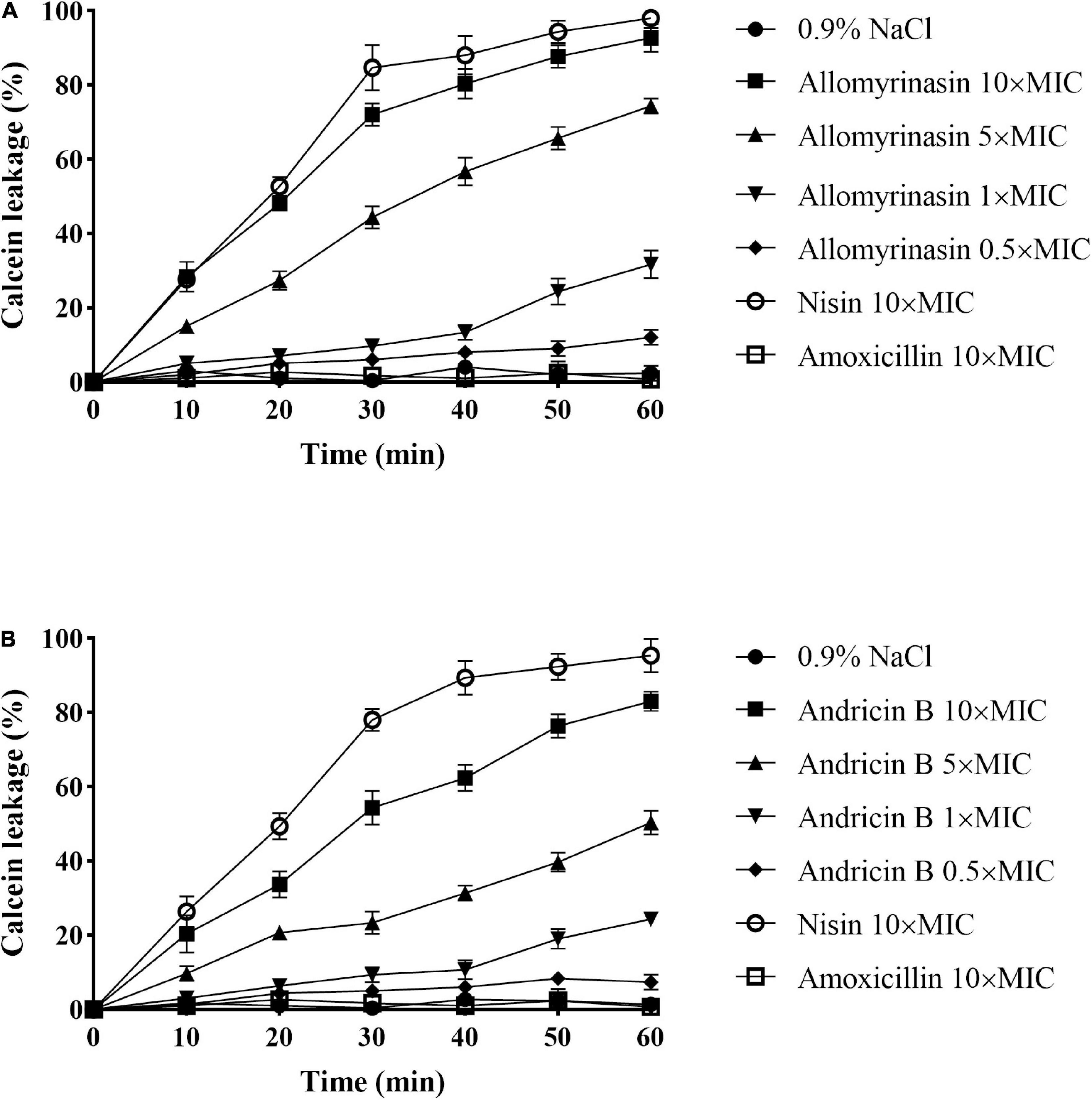
Figure 5. Cytoplasmic membrane permeabilization of Staphylococcus pseudintermedius MW767056 as a consequence of actions of allomyrinasin (A) and andricin B (B) at 0.5, 1, 5, and 10× minimal inhibitory concentration (MIC) μg/ml, suggested by calcein leakage over 1 h of exposure. All data are shown as the mean ± SD from two independent experiments performed in triplicate.
Hemolytic Activity
With the increase in the concentration of the tested peptides, the relative hemolysis rate also rose (Figure 6). Allomyrinasin, andricin B, and pinipesin showed slight hemolytic activity at high concentrations (>64 μg/ml) but displayed little or no obvious hemolytic activity at lower concentrations ranging from 1 to 64 μg/ml, which is closely associated with their MICs against microbes. Compared with those of allomyrinasin and andricin B, the relative hemolysis rates of pinipesin were still lower at high concentrations (>64 μg/ml), and no obvious hemolysis was observed at higher concentrations, which may be ascribed to their moderate antimicrobial bioactivities.
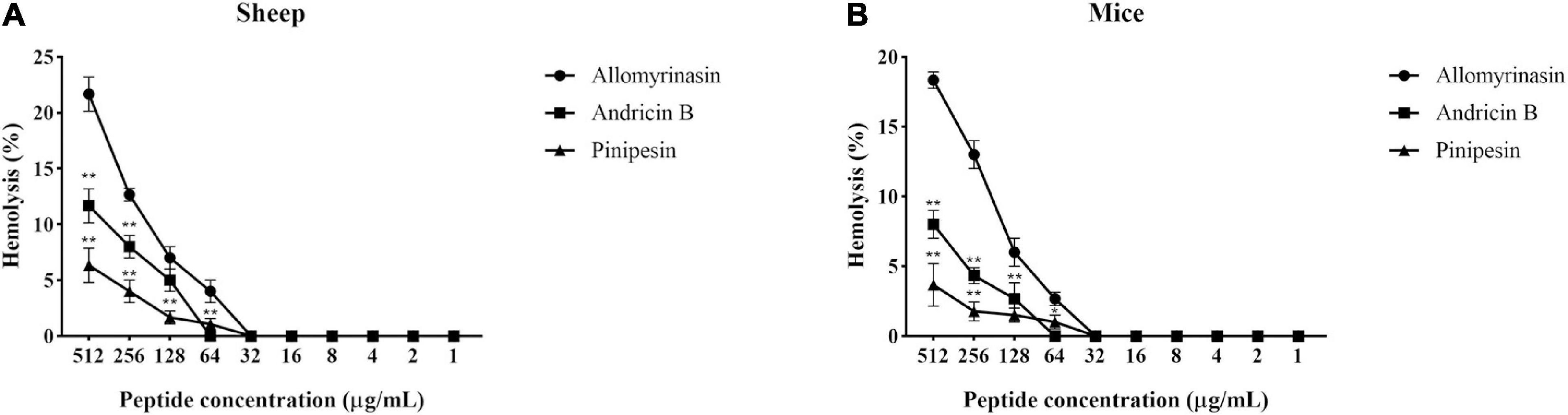
Figure 6. The hemolytic reaction was observed in 4% suspensions of sheep (A) and mouse (B) erythrocytes treated with an increasing peptide concentration. All data recorded after incubation for 2 h are shown as the mean ± SD from the experiment performed in triplicate. Triton X-100 (2%) and phosphate-buffered saline (PBS) served as positive and negative controls, respectively. Statistically significance was performed in comparison with allomyrinasin (*p < 0.05; **p < 0.01), using one-way ANOVA followed by Tukey’s analysis.
Antimicrobial Activities in Serum
Both allomyrinasin and andricin B exhibited stable antimicrobial effects on S. pseudintermedius, S. cohnii, and M. canis in the presence of serum (Figure 7), with no obvious functional changes noticed. After treatment with FBS for 4 h, allomyrinasin maintained stable antimicrobial activity against S. pseudintermedius, S. cohnii, and M. canis. Similar to the MIC, MBIC, and MBEC results, the MIC of serum-incubated andricin B was also higher than that of allomyrinasin, but its bioactivity decreased to a certain extent. The MIC values of andricin B serum against S. pseudintermedius, S. cohnii, and M. canis increased from 32 to 64, 64 to 256, and 0.5 to 8 μg/ml, respectively. The biological activity of andricin B slightly decreased after 4 h of inoculation with serum.
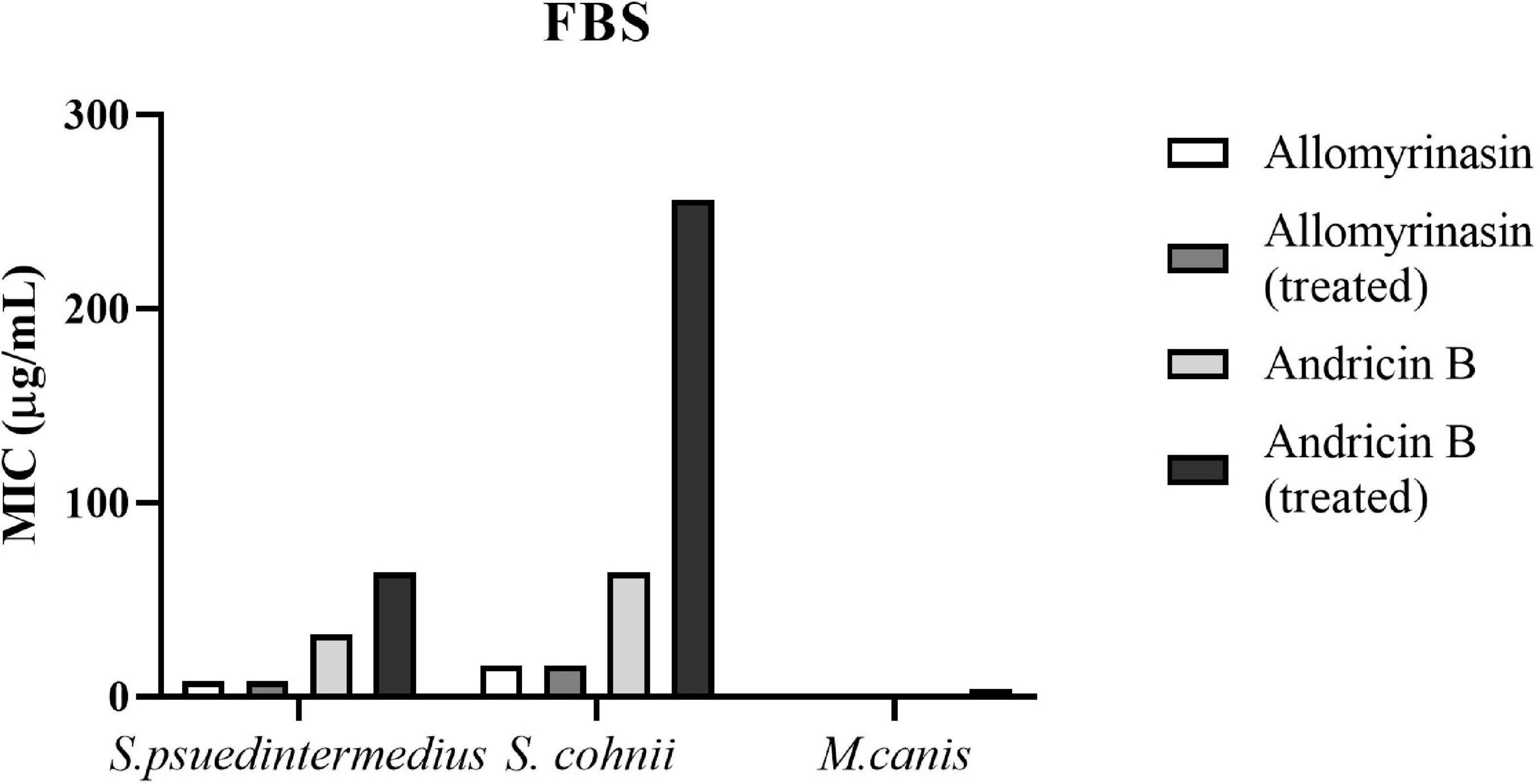
Figure 7. Minimal inhibitory concentrations (MICs) of allomyrinasin and andricin B with or without serum treatment against Staphylococcus pseudintermedius MW767056, Staphylococcus cohnii MW767053, and Microsporum canis MW768151. The experiments are carried out in triplicate from two independent experiments.
Efficacy of Peptides in the Mouse Skin Infection Model
A mouse model of S. pseudintermedius skin infection was established. During the observation period, there was no obvious abnormality in food intake, drinking water, or mental state changes of mice treated with low-dose 6× MIC AMPs. There was no significant difference in body weight among the groups (Figure 8).
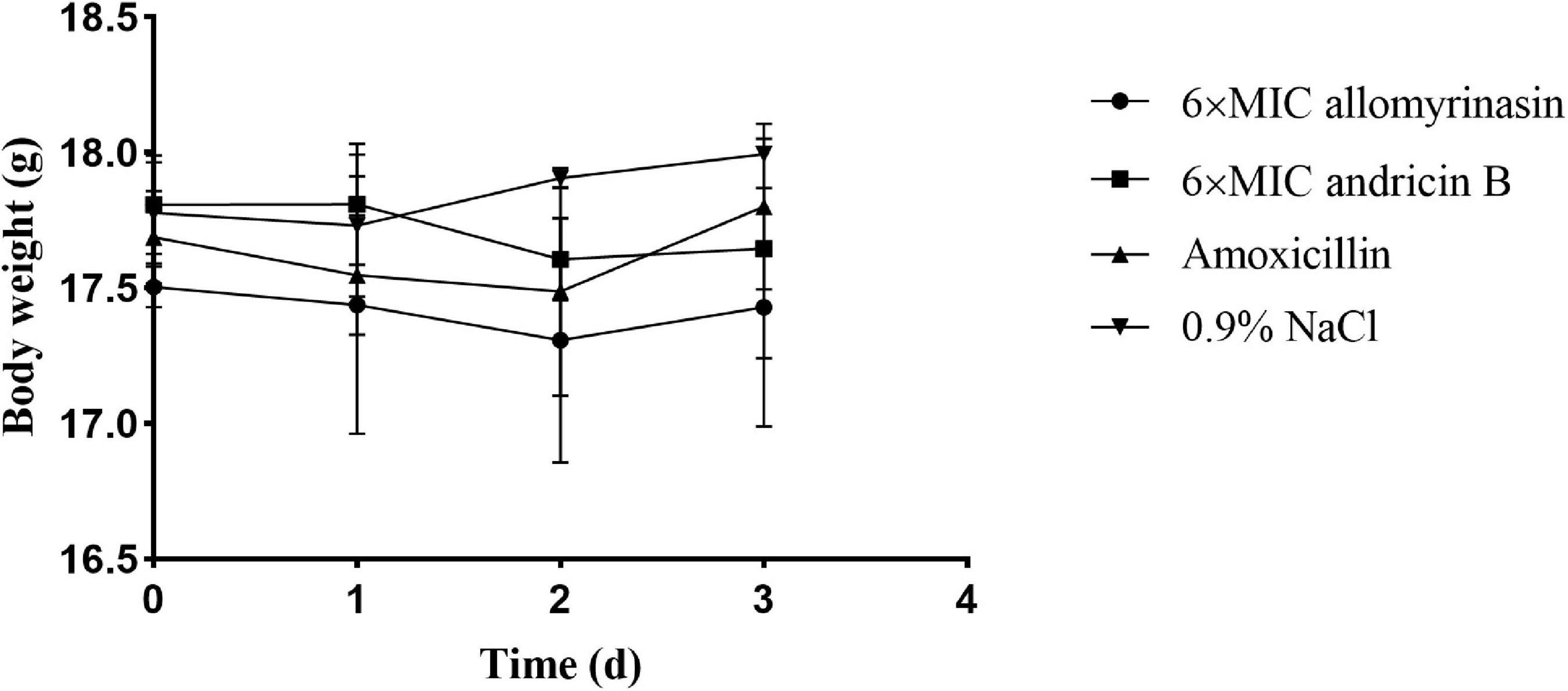
Figure 8. Body weights of mice in different groups treated with allomyrinasin, andricin B, amoxicillin, and saline at 6× minimal inhibitory concentration (MIC) in a mouse skin infection model induced by Staphylococcus pseudintermedius MW767056. Data are presented as means ± SD (n = 5).
Significant differences in skin and hepatic infection prevention were observed between groups treated with antimicrobials and saline. On day 1, all administrations significantly decreased the bacterial load of S. pseudintermedius in infected tissues compared with saline (p < 0.01, Figure 9A). Specifically, the bacterial counts of S. pseudintermedius-infected skin tissue treated with allomyrinasin, andricin B, and amoxicillin were 5.68, 6.2, and 5.94 log10 CFU/g, respectively, which were significantly lower than the 7.52 log10 CFU/g in the saline-treated group (Figure 9A). No bacterial load was detected in the liver of each AMP and amoxicillin-treated group 1 day after skin infection. However, bacteria were found in the liver homogenate of two mice in the saline-treated group (Figure 9C).
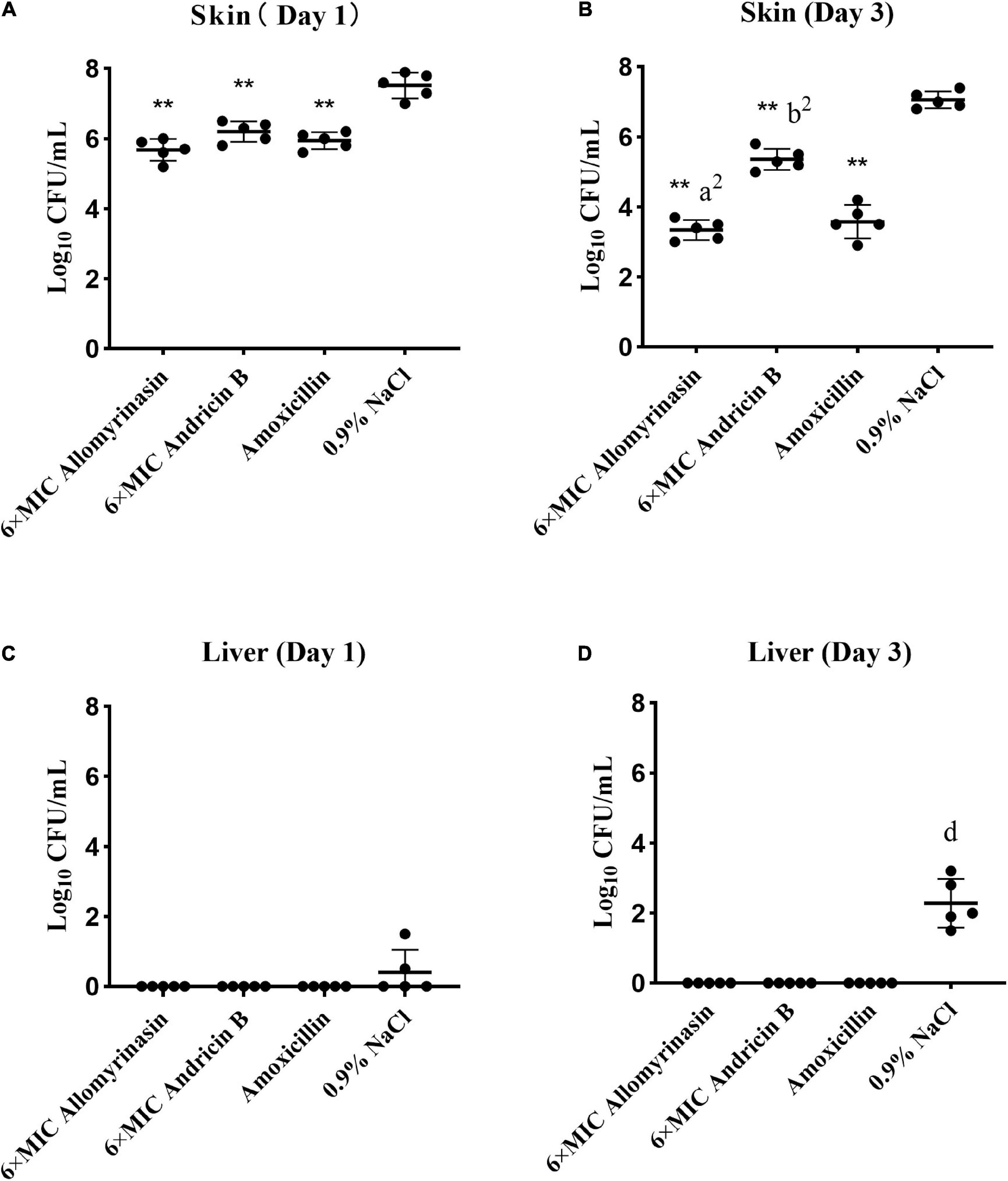
Figure 9. (A–D) Efficacy of allomyrinasin and andricin B at 6× minimal inhibitory concentration (MIC) on skin and hepatic infections in a mouse model of Staphylococcus pseudintermedius (MW767056) skin infection. The skin was treated daily with allomyrinasin, andricin B, amoxicillin, or 0.9% NaCl after inoculation of 5 × 106 CFU of S. pseudintermedius MW767056. The mean bacterial counts (log10 CFU/g) were measured on the first and third days after inoculation with S. pseudintermedius. Data are presented as means ± SD (n = 5). *p < 0.05, **p < 0.01, compared with saline group; ap < 0.05, a2p < 0.01, compared with group treated with andricin B; bp < 0.05, b2p < 0.01, compared with group treated with amoxicillin.
On day 3, further reductions in bacterial counts up to 3.34, 5.36, and 3.58 log10 CFU/g in infected skin tissues could be achieved by treatment with allomyrinasin, andricin B, and amoxicillin, respectively, while treatment with saline rose to 7.06 log10 CFU/g (p < 0.01, Figure 9B). Furthermore, allomyrinasin and amoxicillin were more effective in killing bacteria in infected skin than andricin B, but there was no significant difference in antimicrobial efficacy between allomyrinasin and amoxicillin (allomyrinasin vs. andricin B, p < 0.01; andricin B vs. amoxicillin, p < 0.01, Figure 9B). Interestingly, treatment with allomyrinasin, andricin B, and amoxicillin completely prevented S. pseudintermedius infection in the liver, but in the group treated with saline, liver infections were recorded (Figure 9D). These results indicate that low concentrations of allomyrinasin, andricin B, and amoxicillin can effectively reduce the number of S. pseudintermedius in the epidermis of infected mice.
Pathology and Histological Analysis
Treatment with AMPs and amoxicillin alleviated inflammation in the infected skin among the treatment groups. An inflammatory infiltrate was mainly observed in the dermis and scattered in the epidermis in all mouse groups on day 1 (Figure 10A). On day 3, the skin of treated mice exhibited increased epidermal thickness but with significantly diminished dermal inflammation, whereas the saline-treated mice displayed a large number of inflammatory cells infiltrating the dermis (Figure 10B).
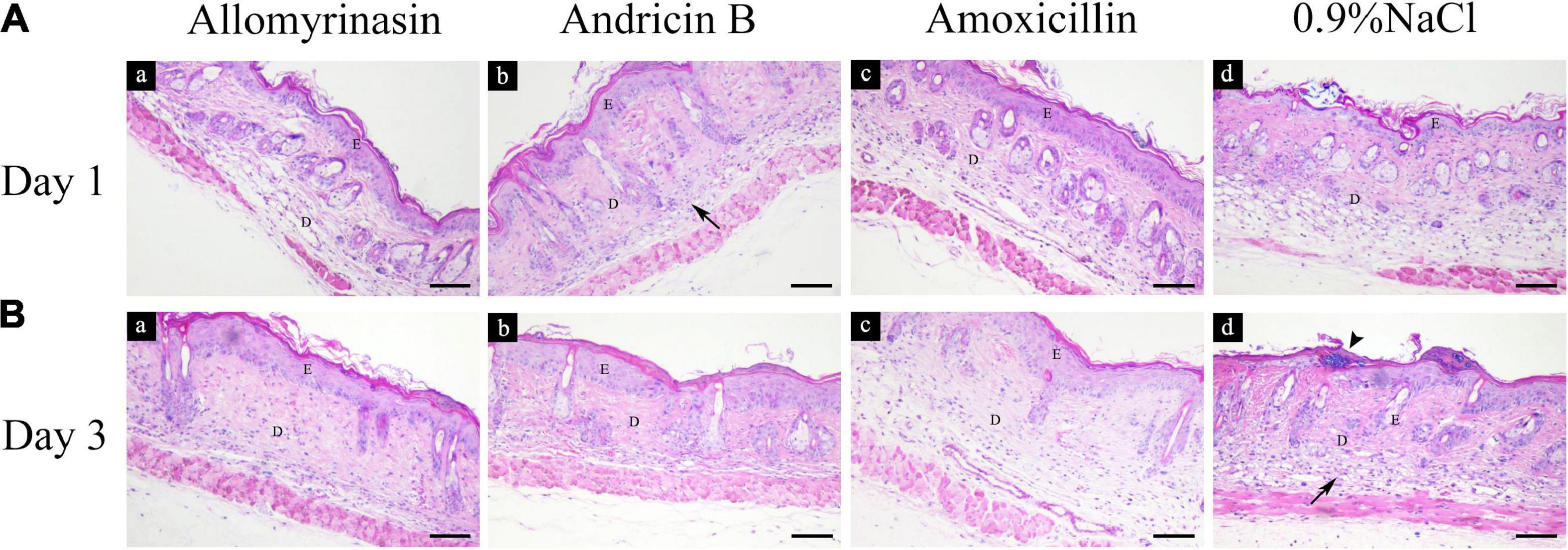
Figure 10. Histopathological analysis of mouse skin tissues infected by Staphylococcus pseudintermedius MW767056, evaluated by hematoxylin and eosin staining. (A) Inflammatory infiltrate (arrow) was mainly detected in the dermis and scattered in the epidermis on day 1. (B) Increased epidermal thickness and an alleviated inflammatory response were observed in all treatment groups, accompanied by staphylococci colonizing the stratum corneum (arrowhead) in the saline-treated group on day 3. The bar represents 100 μm. E, epidermis; D, dermis.
Allomyrinasin Inhibited the Proinflammatory Cytokines IL-6 and TNF-α Induced by Staphylococcus pseudintermedius
The antimicrobial and anti-inflammatory properties of the tested peptides were investigated via quantification of the expression levels of the proinflammatory cytokines TNF-α and IL-6 by Western blotting (Figures 11A, 12A).
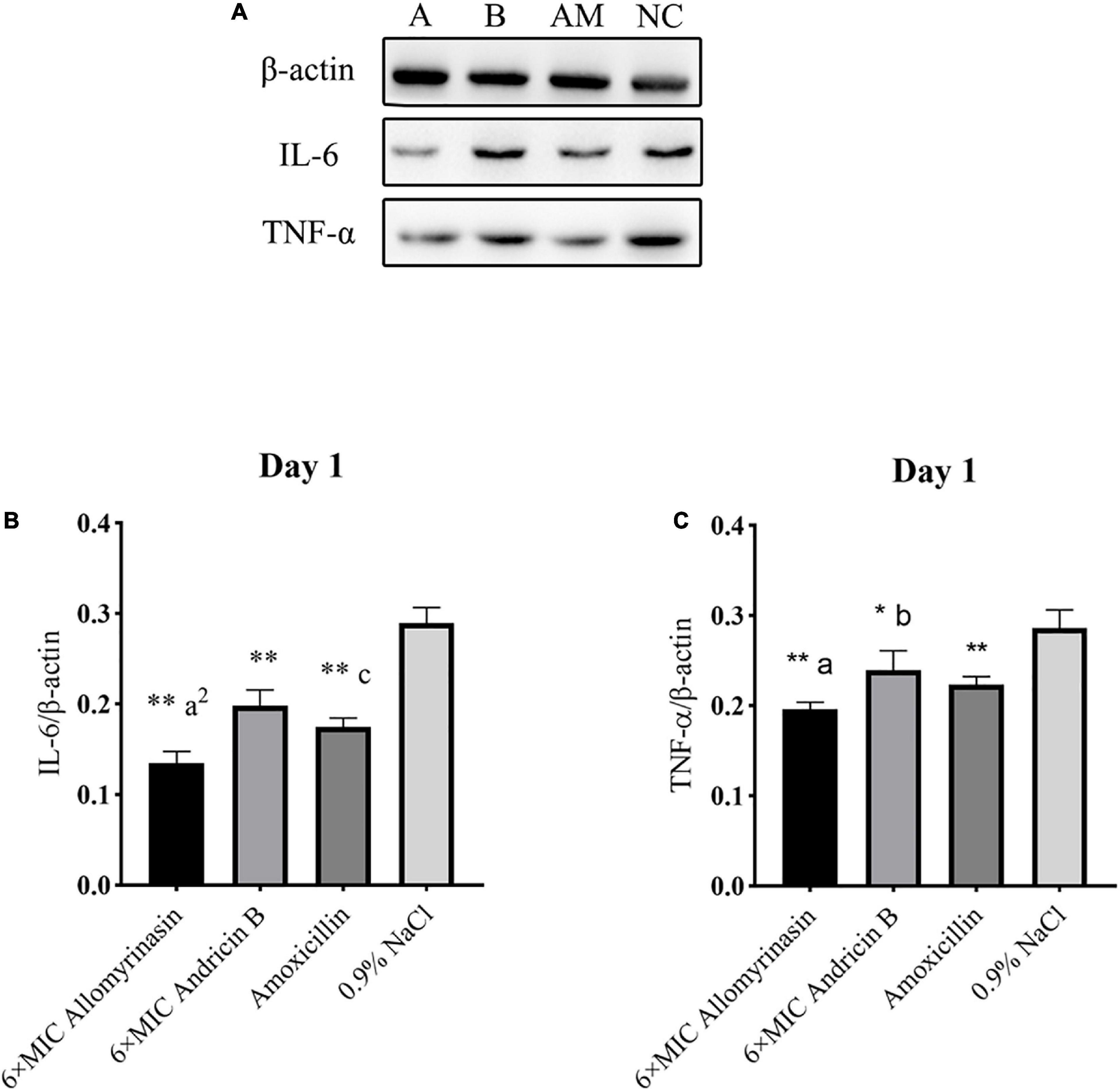
Figure 11. Allomyrinasin inhibited the proinflammatory cytokines IL-6 and TNF-α on day 1 after skin infections induced by Staphylococcus pseudintermedius MW767056. (A) Western blotting analysis of IL-6 and TNF-α expression in the skin tissues of mice. (B,C) Expression of IL-6 and TNF-α, with β-actin as the internal control, in the infected skin is shown as bar graphs. Data with error bars are presented as the mean ± SD (n = 3). (A) 6× minimal inhibitory concentration (MIC) allomyrinasin. (B) 6× MIC andricin B. AM, amoxicillin; NC, 0.9% NaCl. *p < 0.05, **p < 0.01, compared with saline group; ap < 0.05, a2p < 0.01, compared with group treated with andricin B; bp < 0.05, b2p < 0.01, compared with group treated with amoxicillin.
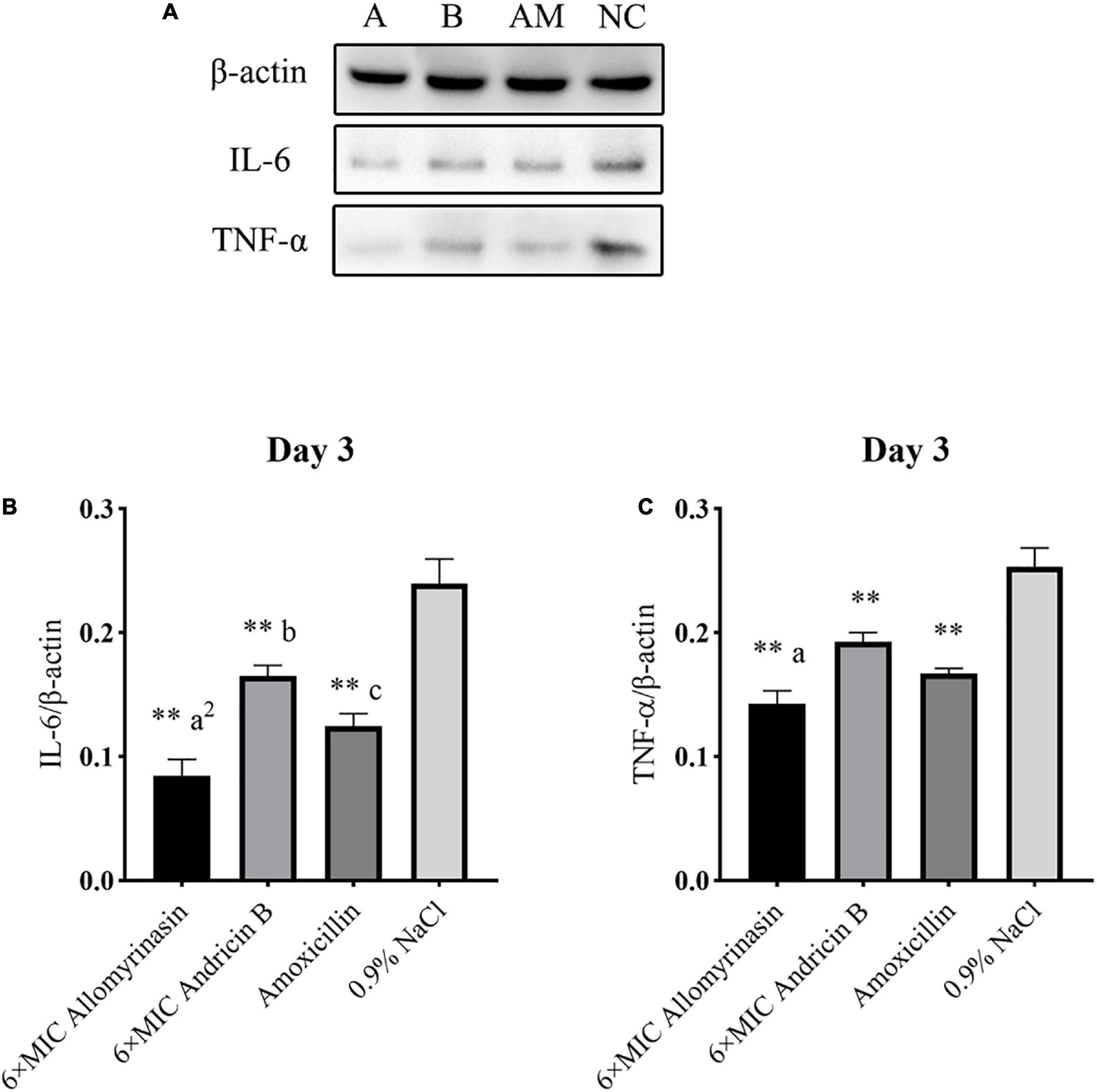
Figure 12. Allomyrinasin inhibited the proinflammatory cytokines IL-6 and TNF-α on day 3 after skin infections induced by Staphylococcus pseudintermedius MW767056. (A) Western blotting analysis of IL-6 and TNF-α expression in the skin tissues of mice. (B,C) Expression of IL-6 and TNF-α, with β-actin as the internal control, in the infected skin is presented in bar graphs. Data with error bars are presented as the mean ± SD (n = 3). (A) 6× minimal inhibitory concentration (MIC) allomyrinasin. (B) 6× MIC andricin B. AM, amoxicillin; NC, 0.9% NaCl. *p < 0.05, **p < 0.01, compared with saline group; ap < 0.05, a2p < 0.01, compared with group treated with andricin B; bp < 0.05, b2p < 0.01, compared with group treated with amoxicillin.
On day 1, the expression level of TNF-α protein in each treated group decreased, and there was a significant difference between the treated groups and the saline-treated group (allomyrinasin vs. 0.9% NaCl, p < 0.01; andricin B vs. 0.9% NaCl, p < 0.05; amoxicillin vs. 0.9% NaCl, p < 0.01, Figure 11C). These results indicate that the tested peptides at low concentrations can effectively reduce the expression of TNF-α in infected mice. The expression of TNF-α protein in the group treated with allomyrinasin and amoxicillin was significantly lower than that in the group treated with andricin B (allomyrinasin vs. andricin B, p < 0.05; amoxicillin vs. andricin B, p < 0.05, Figure 11C). Similarly, the protein expression level of IL-6 in each treated group decreased, and there was a significant difference between the treated groups and the saline-treated group (p < 0.01, Figure 11B). Furthermore, the protein expression of IL-6 in the allomyrinasin-treated group was significantly lower than that in the groups treated with andricin B and amoxicillin (allomyrinasin vs. andricin B, p < 0.01; allomyrinasin vs. amoxicillin, p < 0.05, Figure 11B).
On day 3, the protein expression of TNF-α in each group was further reduced, with a significant difference between the treated groups and the saline-treated group. Additionally, the expression of TNF-α protein in the allomyrinasin group was significantly lower than that in the andricin B group (p < 0.05, Figure 12C). Similarly, the protein expression level of IL-6 in the group treated with peptides and antibiotics significantly decreased compared with that in the saline-treated group (p < 0.01, Figure 12B). Meanwhile, the expression of TNF-α in the group treated with allomyrinasin was significantly lower than that in the groups treated with andricin B and amoxicillin (allomyrinasin vs. andricin B, p < 0.01; allomyrinasin vs. amoxicillin, p < 0.05), and the expression of IL-6 protein in the group treated with andricin B was significantly higher than that in the group treated with amoxicillin (p < 0.05, Figure 12B).
Discussion
In the present study, the antimicrobial activities of the selected short synthetic peptides and their potential for animal application were evaluated.
Allomyrinasin is a cationic peptide that exhibits a broad spectrum of antibacterial and antifungal activities (Lee et al., 2019). In the present study, we observed that allomyrinasin displayed potent antibacterial activities against the clinical isolates of S. pseudintermedius and S. cohnii, while andricin B showed moderate activity. It should be emphasized that all tested peptides demonstrated powerful antifungal activities against the zoonotic fungi, M. canis, T. mentagrophytes, and M. gypseum, the most common dermatophytes with the high potential for zoonotic infections by contact with animals (Cafarchia et al., 2006).
It is universally known that the structure–function relationship of AMPs is largely determined by numerous physicochemical properties of peptides, including charge, hydrophobicity, sequence, and size (Yeaman and Yount, 2003). Among them, the biological activity and specificity of AMPs are closely associated with cationicity. Cationic AMPs are initially bound to the bacterial cytomembrane by the mean of electrostatic force between cationic amino acid residues within AMPs and negatively charged phospholipids of the bacterial cytomembrane. The cationic charge of AMPs strongly correlates with their activity as the capability of binding to the membrane interface began to improve with an increase in net positive charge (Bonucci et al., 2014; Fillion et al., 2015). Allomyrinasin and andricin B are both cationic AMPs with net positive charge of +3 and +2, respectively. The stronger effect of allomyrinasin on bacterial strains and erythrocytes than andricin B may be attributed to its higher net positive charge. Theoretically, AMPs with more cationic residues should exhibit more robust antimicrobial activity; however, there is an optimum charge beyond which higher or lower cationicity can considerably decrease their biological activity. The addition of cationic residues of magainin 2 resulted in increased hemolysis and the decreased selectivity for microbial cells (Dathe et al., 2001). Similarly, removal of the cationic C-terminal residues of melittin also led to a significant drop in hemolytic activity and membrane binding ability (Hall et al., 2011; Wang et al., 2015). In addition, hydrophobicity has been considered as another crucial parameter to regulate the antimicrobial potency and hemolytic capacity of AMPs. Hydrophobicity plays a significant role in interaction with cell membranes by impacting the extent to which AMPs are capable of penetrating into the membrane layer. Similar to charge, modulating hydrophobicity to an optimal percentage can boost antimicrobial activity against microbial cytomembranes, but once beyond this optimum point, impaired antimicrobial activity and increased mammalian cytotoxicity can appear (Pasupuleti et al., 2012). In our study, although allomyrinasin possessed the same amount of hydrophobic residues with andricin B, it may possess an optimum balance between cationicity and hydrophobicity, as indicated by the more potent antimicrobial efficacy and biofilm inhibition activity with acceptable mammalian hemolysis (Wimley, 2010).
Rapid antibiosis at the epidermis is pivotal for the recovery of skin barrier function and is capable of inhibiting the emergence of drug resistance and preventing infection spreading (Finberg et al., 2004). In vitro time-kill kinetics revealed complete and rapid eradication of S. pseudintermedius, S. cohnii, and M. canis within hours in a time- and concentration-dependent manner. This rapid antimicrobial elimination by AMPs may display great suitability for animal applications and may result in better treatment outcomes than conventional antimicrobials.
Allomyrinasin and andricin B exhibited antibacterial patterns similar to membrane-lytic peptides, such as the antibiotic nisin, rather than nonlytic peptides (Park et al., 1998), identified by bacteriolysis analysis of S. pseudintermedius exposed to peptides. These figures indicated that disrupting bacterial membrane integrity is the underlying mechanism of action. Furthermore, we investigated the action of peptides on S. pseudintermedius membranes using calcein leakage assays for validation. Interestingly, both allomyrinasin and andricin B exhibited cytomembrane permeabilization in a dose- and time-dependent manner, which is similar to the data arising from the well-studied membrane-dissolving peptide nisin. These findings clearly illustrated that the accomplishment of bacterial lysis by peptides is attributable to pore formation, membrane permeabilization, and cytoplasmic leakage.
Microbial biofilms, constituting a thorny issue facing current antimicrobial agents, are pivotal in the pathogenic mechanism of skin and wound infections. Clinical treatment failure usually results from impediment by biofilms to penetration of antimicrobials to microbes (Batoni et al., 2021). Actively metabolizing cells are the main target of traditional therapeutics rather than quiescent cells due to inhibition of essential biomacromolecule synthesis in these growing cells (Hurdle et al., 2011). However, the capability of some AMPs to directly disrupt the cellular membranes of microbes, a cellular structure in both proliferative and quiescent cells, is anticipated to be more efficacious in biofilm eradication (Nuti et al., 2017). In our study, allomyrinasin was shown to inhibit S. pseudintermedius and S. cohnii biofilm formation and disrupt mature biofilms efficiently, while andricin B and pinipesin exhibited moderate activity with relatively high values of MBIC and MBEC. Furthermore, biofilm formation also plays a significant role in the pathogenesis of dermatophytosis (Sherry et al., 2017). Notably, allomyrinasin was highly efficient in impeding M. canis biofilm formation and in clearing mature biofilms, while andricin B and pinipesin were relatively ineffective.
Since the ability of peptides to hinder microbial growth alone was evaluated, we then investigated their synergistic ability with other traditional antimicrobials. Amoxicillin is a widely applied antibiotic that is capable of blocking the synthesis of glycopeptides, a significant component of staphylococcal cell walls, by specifically inactivating transpeptidase in bacteria (de Marco et al., 2017). Terbinafine is a allylamine antifungal agent frequently used in the treatment of the superficial fungal infections. It exclusively inhibits squalene oxygenase in the process of ergosterol biosynthesis, leading to excessive accumulation of squalene in fungal cells and cell death. It has been reported that amoxicillin and terbinafine are widely applied in the treatment of bacterial and fungal infections; however, these pathogens have evolved resistance by different mechanisms (Ghannoum, 2016; Monod and Méhul, 2019; de Jong et al., 2020; Wegener et al., 2020). In the present study, we observed a synergistic relationship between tested peptides and conventional antimicrobials, as indicated by complete clearance of S. pseudintermedius, S. cohnii, and M. canis within extremely low concentrations. We speculated that the synergistic effect between peptides and antibiotics may be ascribed to the inhibition of the bacterial cell wall synthesis by amoxicillin, allowing more peptides to access the bacterial membrane.
One of the primary limitations of the application in the treatment of skin and wound infections is inactivation of AMPs by serum either through protease cleavage or protein binding (Yeung et al., 2011). In our study, allomyrinasin and andricin B are capable of maintaining their antimicrobial activity at different ratios in the presence of 25% FBS. Their capacity to withstand degradation in serum provides a potential for administration of peptides in physiological solutions. Hemolysis is another noticeable obstacle in antimicrobial development, especially when the cell membrane acts as the drug target. The discovery of clinically feasible AMPs has been hindered by unexpected cytotoxicity to eukaryotic cells, particularly erythrocytes at therapeutic dosages (Edwards et al., 2017). The selectivity of AMPs can be indicated by the therapeutic index, defined as the ratio between MHC and MIC (MHC/MIC). AMPs with the higher therapeutic index often exhibit more effective activity as an antibiotic (Maturana et al., 2017). In our study, allomyrinasin with a therapeutic index in the range from 16 to 32 can be considered as a more effective antimicrobial than andricin B with a therapeutic index of 16.
The results from in vitro antimicrobial assays laid a solid groundwork for further research on animals. In this study, the anti-infective and anti-inflammatory effects of the peptides were assessed using a mouse skin infection model. Microbial clearance is the ultimate objective of anti-infectious treatments, with bacterial burden being the major determinant of therapeutic effects (Ball et al., 2002). Daily topical treatment with allomyrinasin and andricin B at a low concentration (6× MIC) efficiently decreased the skin bacterial load in a time-dependent fashion and achieved complete prevention of hepatic infection, whereas liver colonization appeared in 20% and 100% of mice after 1 and 3 days of treatment with saline, respectively. Compared with amoxicillin, andricin B was less effective (p < 0.01) in clearing the bacterial load in infected skin, while allomyrinasin showed a similar efficacy (p > 0.05). Based on the outstanding synergism between allomyrinasin and amoxicillin exhibited by in vitro assays, the combination therapy may provide an ideal option for treatment of skin infections in the context of animal husbandry and medicine. However, complete removal of S. pseudintermedius from skin was not realized by the applied antimicrobials, indicating a requirement for prolonging treatment duration.
The skin infections induced by S. pseudintermedius can be aggravated by excess expression of host proinflammatory factors more than by bacterial load. An exacerbated inflammatory response not only largely defers wound healing but also is likely to induce cicatrization. AMPs with integrated microbicidal and immunomodulatory abilities are capable of promoting epithelialization and wound healing and should occupy a dominant position in the treatment of S. pseudintermedius skin infections (Mangoni et al., 2016). Inflammatory reactions in infected skin were observed in the entire pathological process as anticipated due to manual scratch and S. pseudintermedius infection. However, with the increase of the medication duration, the protein expression of inflammatory factors (TNF-α and IL-6) in the antimicrobial-treated groups significantly decreased compared with those in the saline-treated group (p < 0.01), indicating the alleviation of the inflammatory response. In particular, allomyrinasin seemed to be superior in anti-inflammatory efficacy when compared with two other antibacterial agents, which can be attributed to its potent antibacterial ability in vitro and high stability in physiological solutions, such as serum (Figure 6).
Although AMPs have exhibited the unique anti-infective actions and there is an emergent need for new antimicrobials, they have previously been disregarded due to high production costs (Mulani et al., 2019). However, the remarkable reductions in the excessive manufacturing cost of AMPs have been achieved due to technological progress and wide application of solid-phase peptide synthesis. These newly synthesized peptides exhibited microbicidal potency with a unique mechanistic action, robust stability, biofilm formation prevention, and mature biofilm-eradicating effectiveness, as well as synergism with conventional antimicrobials. Topical application significantly reduced the wound bacterial load, prevented hepatic dissemination, and alleviated the skin inflammatory response. In conclusion, the clinical application of allomyrinasin may provide an ideal option for treating animal skin infections, particularly in the context of medicine and animal husbandry, despite additional investigation required to optimize pharmaceutical formulation and delivery.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www. ncbi.nlm.nih.gov/nuccore/?term=MW767025:MW767027[accn], MW767025-MW767027; https://www.ncbi.nlm.nih.gov/nuccore/MW766984, MW766984; https://www.ncbi.nlm.nih.gov/nuccore/MW768151, MW768151; https://www.ncbi.nlm.nih.gov/nuccore/MW766983, MW766983; https://www.ncbi.nlm.nih.gov/nuccore/?term=MW793383:MW793392[accn], MW793383-MW793392; https://www.ncbi.nlm.nih.gov/nuccore/MW767046, MW767046; and https://www.ncbi.nlm.nih.gov/nuccore/?term=MW767051: MW767056[accn], MW767051-MW767056.
Ethics Statement
The animal study was reviewed and approved by Animal Care and Use Committee of China Agricultural University.
Author Contributions
QT and DL contributed conception and design of the study. QT, YZ, and XW organized the database, performed the statistical analysis, and wrote the first draft of the manuscript. QT, CY, and WL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The authors would like to express their gratitude to the National Natural Science Foundation of China (Grant No. 31372489) for the financial support.
Conflict of Interest
ZM was employed by the company Artron BioResearch Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.684650/full#supplementary-material
References
Abe, Y., Akiyama, H., and Arata, J. (1992). Production of experimental staphylococcal impetigo in mice. J. Dermatol. Sci. 4, 42–48. doi: 10.1016/0923-1811(92)90055-g
Aneke, C. I., Rhimi, W., Hubka, V., Otranto, D., and Cafarchia, C. (2021). Virulence and antifungal susceptibility of Microsporum canis strains from animals and humans. Antibiotics (Basel) 10:296. doi: 10.3390/antibiotics10030296
Ball, P., Baquero, F., Cars, O., File, T., Garau, J., Klugman, K., et al. (2002). Antibiotic therapy of community respiratory tract infections: strategies for optimal outcomes and minimized resistance emergence. J. Antimicrob. Chemother. 49, 31–40. doi: 10.1093/jac/49.1.31
Bao, K., Yuan, W., Ma, C., Yu, X., Wang, L., Hong, M., et al. (2019). Modification targeting the “rana box” motif of a novel nigrocin peptide from Hylarana latouchii enhances and broadens its potency against multiple bacteria. Front. Microbiol. 9:2846. doi: 10.3389/fmicb.2018.02846
Batoni, G., Maisetta, G., and Esin, S. (2021). Therapeutic potential of antimicrobial peptides in polymicrobial biofilm-associated infections. Int. J. Mol. Sci. 22:482. doi: 10.3390/ijms22020482
Bessa, L. J., Manickchand, J. R., Eaton, P., Leite, J. R. S. A., Brand, G. D., and Gameiro, P. (2019). Intragenic antimicrobial peptide Hs02 hampers the proliferation of single- and dual-species biofilms of P. aeruginosa and S. aureus: a promising agent for mitigation of biofilm-associated infections. Int. J. Mol. Sci. 20:3604. doi: 10.3390/ijms20143604
Blondeau, J. M., Borsos, S., Blondeau, L. D., and Blondeau, B. J. (2012). In vitro killing of Escherichia coli, Staphylococcus pseudintermedius and Pseudomonas aeruginosa by enrofoxacin in combination with its active metabolite ciprofoxacin using clinically relevant drug concentrations in the dog and cat. Vet. Microbiol. 155, 284–290. doi: 10.1016/j.vetmic.2011.08.015
Boman, H. G., Agerberth, B., and Boman, A. (1993). Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun. 61, 2978–2984. doi: 10.1128/iai.61.7.2978-2984.1993
Bonucci, A., Balducci, E., Martinelli, M., and Pogni, R. (2014). Human neutrophil peptide 1 variants bearing arginine modified cationic side chains: effects on membrane partitioning. Biophys. Chem. 190-191, 32–40. doi: 10.1016/j.bpc.2014.04.003
Brunetti, J., Falciani, C., Bracci, L., and Pini, A. (2017). Models of in vivo bacterial infections for the development of antimicrobial peptide-based drugs. Curr. Top. Med. Chem. 17, 613–619. doi: 10.2174/1568026616666160713143017
Cafarchia, C., Romito, D., Capelli, G., Guillot, J., and Otranto, D. (2006). Isolation of Microsporum canis from the hair coat of pet dogs and cats belonging to owners diagnosed with M. canis tinea corporis. Vet. Dermatol. 17, 327–331. doi: 10.1111/j.1365-3164.2006.00533.x
Chaparro-Aguirre, E., Segura-Ramírez, P. J., Alves, F. L., Riske, K. A., Miranda, A., and Silva Júnior, P. I. (2019). Antimicrobial activity and mechanism of action of a novel peptide present in the ecdysis process of centipede Scolopendra subspinipes subspinipes. Sci. Rep. 9:13631. doi: 10.1038/s41598-019-50061-y
Dathe, M., Nikolenko, H., Meyer, J., Beyermann, M., and Bienert, M. (2001). Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Lett. 501, 146–150. doi: 10.1016/s0014-5793(01)02648-5
de Jong, A., Youala, M., El Garch, F., Simjee, S., Rose, M., Morrissey, I., et al. (2020). Antimicrobial susceptibility monitoring of canine and feline skin and ear pathogens isolated from European veterinary clinics: results of the ComPath Surveillance programme. Vet. Dermatol. 31, 431–e114. doi: 10.1111/vde.12886
de Marco, B. A., Natori, J., Fanelli, S., Tótoli, E. G., and Salgado, H. (2017). Characteristics, properties and analytical methods of amoxicillin: a review with green approach. Crit. Rev. Anal. Chem. 47, 267–277. doi: 10.1080/10408347.2017.1281097
Delattin, N., De Brucker, K., Vandamme, K., Meert, E., Marchand, A., Chaltin, P., et al. (2014). Repurposing as a means to increase the activity of amphotericin B and caspofungin against Candida albicans biofilms. J. Antimicrob. Chemother. 69, 1035–1044. doi: 10.1093/jac/dkt449
Edwards, I. A., Elliott, A. G., Kavanagh, A. M., Blaskovich, M. A. T., and Cooper, M. A. (2017). Structure-activity and -toxicity relationships of the antimicrobial peptide Tachyplesin-1. ACS Infect. Dis. 3, 917–926. doi: 10.1021/acsinfecdis.7b00123
Fillion, M., Valois-Paillard, G., Lorin, A., Noel, M., Voyer, N., and Auger, M. (2015). Membrane interactions of synthetic peptides with antimicrobial potential: effect of electrostatic interactions and amphiphilicity. Probiotics Antimicrob. Proteins 7, 66–74. doi: 10.1007/s12602-014-9177-z
Finberg, R. W., Moellering, R. C., Tally, F. P., Craig, W. A., Pankey, G. A., Dellinger, E. P., et al. (2004). The importance of bactericidal drugs: future directions in infectious disease. Clin. Infect. Dis. 39, 1314–1320. doi: 10.1086/425009
Ghannoum, M. (2016). Azole resistance in dermatophytes: prevalence and mechanism of action. J. Am. Podiatr. Med. Assoc. 106, 79–86. doi: 10.7547/14-109
Hall, K., Lee, T. H., and Aguilar, M. I. (2011). The role of electrostatic interactions in the membrane binding of melittin. J. Mol. Recog. 24, 108–118. doi: 10.1002/jmr.1032
Hurdle, J. G., O’Neill, A. J., Chopra, I., and Lee, R. E. (2011). Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 9, 62–75. doi: 10.1038/nrmicro2474
Jones, K. E., Patel, N. G., Levy, M. A., Storeygard, A., Balk, D., Gittleman, J. L., et al. (2008). Global trends in emerging infectious diseases. Nature 451, 990–993.
Kyriakidis, I., Tragiannidis, A., Munchen, S., and Groll, A. H. (2017). Clinical hepatotoxicity associated with antifungal agents. Expert Opin. Drug Saf. 16, 149–165.
Lee, J. H., Seo, M., Lee, H. J., Baek, M., Kim, I. W., Kim, S. Y., et al. (2019). Anti-inflammatory activity of antimicrobial peptide allomyrinasin derived from the dynastid beetle, Allomyrina dichotoma. J. Microbiol. Biotechnol. 29, 687–695. doi: 10.4014/jmb.1809.09031
Mangoni, M. L., McDermott, A. M., and Zasloff, M. (2016). Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp. Dermatol. 25, 167–173. doi: 10.1111/exd.12929
Maturana, P., Martinez, M., Noguera, M. E., Santos, N. C., Disalvo, E. A., Semorile, L., et al. (2017). Lipid selectivity in novel antimicrobial peptides: implication on antimicrobial and hemolytic activity. Colloids Surf. B Biointerfaces 153, 152–159. doi: 10.1016/j.colsurfb.2017.02.003
Monod, M., and Méhul, B. (2019). Recent findings in onychomycosis and their application for appropriate treatment. J. Fungi (Basel) 5:20. doi: 10.3390/jof5010020
Mulani, M. S., Kamble, E. E., Kumkar, S. N., Tawre, M. S., and Pardesi, K. R. (2019). Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front. Microbiol. 10:539. doi: 10.3389/fmicb.2019.00539
Murayama, N., Nagata, M., Terada, Y., Okuaki, M., Takemura, N., Nakaminami, H., et al. (2013). In vitro antiseptic susceptibilities for Staphylococcus pseudintermedius isolated from canine superficial pyoderma in Japan. Vet. Dermatol. 24, 126–129. doi: 10.1111/j.1365-3164.2012.01103.x
Nuti, R., Goud, N. S., Saraswati, A. P., Alvala, R., and Alvala, M. (2017). Antimicrobial peptides: a promising therapeutic strategy in tackling antimicrobial resistance. Curr. Med. Chem. 24, 4303–4314.
Oddo, A., Thomsen, T. T., Kjelstrup, S., Gorey, C., Franzyk, H., Frimodt-Møller, N., et al. (2015). An amphipathic undecapeptide with all d-amino acids shows promising activity against colistin-resistant strains of Acinetobacter baumannii and a dual mode of action. Antimicrob. Agents Chemother. 60, 592–599. doi: 10.1128/aac.01966-15
Oliva, B., Miller, K., Caggiano, N., O’Neill, A. J., Cuny, G. D., Hoemann, M. Z., et al. (2003). Biological properties of novel antistaphylococcal quinoline-indole agents. Antimicrob. Agents Chemother. 47, 458–466. doi: 10.1128/aac.47.2.458-466.2003
Ong, Z. Y., Cheng, J., Huang, Y., Xu, K., Ji, Z., Fan, W., et al. (2014). Effect of stereochemistry, chain length and sequence pattern on antimicrobial properties of short synthetic β-sheet forming peptide amphiphiles. Biomaterials 35, 1315–1325. doi: 10.1016/j.biomaterials.2013.10.053
Park, C. B., Kim, H. S., and Kim, S. C. (1998). Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 244, 253–257. doi: 10.1006/bbrc.1998.8159
Pasquetti, M., Min, A. R. M., Scacchetti, S., Dogliero, A., and Peano, A. (2017). Infection by microsporum canis in paediatric patients: a veterinary perspective. Vet. Sci. 4:46. doi: 10.3390/vetsci4030046
Pasupuleti, M., Schmidtchen, A., and Malmsten, M. (2012). Antimicrobial peptides: key components of the innate immune system. Crit. Rev. Biotechnol. 32, 143–171. doi: 10.3109/07388551.2011.594423
Pei, J., Feng, Z., Ren, T., Sun, H., Han, H., Jin, W., et al. (2018). Purification, characterization and application of a novel antimicrobial peptide from Andrias davidianus blood. Lett. Appl. Microbiol. 66, 38–43. doi: 10.1111/lam.12823
Perreten, V., Kadlec, K., Schwarz, S., Grönlund Andersson, U., Finn, M., Greko, C., et al. (2010). Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: an international multicentre study. J. Antimicrob. Chemother. 65, 1145–1154. doi: 10.1093/jac/dkq078
Qi, X., Lam, S. S., Liu, D., Kim, D. Y., Ma, L., Alleruzzo, L., et al. (2016). Development of inCVAX, in situ cancer vaccine, and its immune response in mice with hepatocellular cancer. J. Clin. Cell. Immunol. 7:438. doi: 10.4172/2155-9899.1000438
Rogers, L. A. (1928). The inhibiting effect of Streptococcus Lactis on Lactobacillus bulgaricus. J. Bacteriol. 16, 321–325. doi: 10.1128/jb.16.5.321-325.1928
Ruhr, E., and Sahl, H. G. (1985). Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob. Agents Chemother. 27, 841–845. doi: 10.1128/aac.27.5.841
Sabaeifard, P., Abdi-Ali, A., Soudi, M. R., and Dinarvand, R. (2014). Optimization of tetrazolium salt assay for Pseudomonas aeruginosa biofilm using microtiter plate method. J. Microbiol. Methods 105, 134–140. doi: 10.1016/j.mimet.2014.07.024
Santoro, D., and Maddox, C. W. (2014). Canine antimicrobial peptides are effective against resistant bacteria and yeasts. Vet. Dermatol. 25, 35–e12. doi: 10.1111/vde.12091
Sharma, K., Aaghaz, S., Shenmar, K., and Jain, R. (2018). Short antimicrobial peptides. Recent Pat. Antiinfect. Drug Discov. 13, 12–52.
Sherry, L., Kean, R., McKloud, E., O’Donnell, L. E., Metcalfe, R., and Jones, B. L. (2017). Biofilms formed by isolates from recurrent vulvovaginal candidiasis patients are heterogeneous and insensitive to fluconazole. Antimicrob. Agents Chemother. 24:e01065-17. doi: 10.1128/AAC.01065-17
Simonetti, O., Silvestri, C., Arzeni, D., Cirioni, O., Kamysz, W., Conte, I., et al. (2014). In vitro activity of the protegrin IB-367 alone and in combination compared with conventional antifungal agents against dermatophytes. Mycoses 57, 233–239. doi: 10.1111/myc.12148
Stegmann, R., Burnens, A., Maranta, C. A., and Perreten, V. (2010). Human infection associated with methicillinresistant Staphylococcus pseudintermedius ST71. J. Antimicrob. Chemother. 65, 2047–2048. doi: 10.1093/jac/dkq241
Summers, J. F., Hendricks, A., and Brodbelt, D. C. (2014). Prescribing practices of primary-care veterinary practitioners in dogs diagnosed with bacterial pyoderma. BMC Vet. Res. 10:240. doi: 10.1186/s12917-014-0240-5
Tang, Q. Y., Li, W. T., Dai, N., Gao, Y. M., Han, Y., Cheng, G. F., et al. (2017). The role of necroptosis, apoptosis, and inflammation in fowl cholera-associated liver injury in a chicken model. Avian Dis. 61, 491–502. doi: 10.1637/11732-073017-reg.1
Taylor, L. H., Latham, S. M., and Woolhouse, M. E. J. (2001). Risk factors for human disease emergence. Philos. Trans. R. Soc. B Biol. Sci. 356, 983–989.
Wang, G., Mishra, B., Lau, K., Lushnikova, T., Golla, R., and Wang, X. (2015). Antimicrobial peptides in 2014. Pharmaceuticals (Basel) 8, 123–150. doi: 10.1007/978-3-0348-0541-4_5
Weese, J. S., and van Duijkeren, E. (2010). Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet. Microbiol. 140, 418–429. doi: 10.1016/j.vetmic.2009.01.039
Wegener, A., Damborg, P., Guardabassi, L., Moodley, A., Mughini-Gras, L., Duim, B., et al. (2020). Specific staphylococcal cassette chromosome mec (SCCmec) types and clonal complexes are associated with low-level amoxicillin/clavulanic acid and cefalotin resistance in methicillin-resistant Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 75, 508–511. doi: 10.1093/jac/dkz509
Wiegand, I., Hilpert, K., and Hancock, R. E. W. (2008). Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175. doi: 10.1038/nprot.2007.521
Wimley, W. C. (2010). Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 5, 905–917. doi: 10.1021/cb1001558
Wolcott, R. D., Rhoads, D. D., Bennett, M. E., Wolcott, B. M., Gogokhia, L., Costerton, J. W., et al. (2010). Chronic wounds and the medical biofilm paradigm. J. Wound Care 19, 45–53. doi: 10.12968/jowc.2010.19.2.46966
Woodburn, K. W., Jaynes, J. M., and Clemens, L. E. (2019). Evaluation of the antimicrobial peptide, RP557, for the broad-spectrum treatment of wound pathogens and biofilm. Front. Microbiol. 10:1688. doi: 10.3389/fmicb.2019.01688
Xiong, Y. Q., Mukhopadhyay, K., Yeaman, M. R., Adler-Moore, J., and Bayer, A. S. (2005). Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrob. Agents Chemother. 49, 3114–3121. doi: 10.1128/aac.49.8.3114-3121.2005
Yan, H., and Hancock, R. E. (2001). Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 45, 1558–1560. doi: 10.1128/aac.45.5.1558-1560.2001
Yeaman, M. R., and Yount, N. Y. (2003). Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55, 27–55. doi: 10.1124/pr.55.1.2
Yeung, A. T., Gellatly, S. L., and Hancock, R. E. (2011). Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 68, 2161–2176. doi: 10.1007/s00018-011-0710-x
Yong, Y. Y., Dykes, G. A., and Choo, W. S. (2019). Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit. Rev. Microbiol. 45, 201–222. doi: 10.1080/1040841x.2019.1573802
Keywords: antimicrobial peptide, Microsporum canis, Staphylococcus pseudintermedius, anti-inflammation, anti-biofilm, mouse skin infection model, synergistic efficacy
Citation: Tang Q, Yang C, Li W, Zhang Y, Wang X, Wang W, Ma Z, Zhang D, Jin Y and Lin D (2021) Evaluation of Short-Chain Antimicrobial Peptides With Combined Antimicrobial and Anti-inflammatory Bioactivities for the Treatment of Zoonotic Skin Pathogens From Canines. Front. Microbiol. 12:684650. doi: 10.3389/fmicb.2021.684650
Received: 23 March 2021; Accepted: 16 July 2021;
Published: 11 August 2021.
Edited by:
Alexey S. Vasilchenko, Tyumen State University, RussiaReviewed by:
Song Lin Chua, Hong Kong Polytechnic University, Hong KongJyawei Cheng, National Tsing Hua University, Taiwan
Copyright © 2021 Tang, Yang, Li, Zhang, Wang, Wang, Ma, Zhang, Jin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Degui Lin, Y3NhbWFAc2luYS5jb20=
 Qiyu Tang
Qiyu Tang Chunyi Yang1
Chunyi Yang1 Xinying Wang
Xinying Wang