- 1Innovative Institute for Plant Health, Zhongkai University of Agriculture and Engineering, Guangzhou, China
- 2Research Center of Microbial Diversity and Sustainable Utilization, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 3Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 4Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, Thailand
- 5Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Chiang Mai, Thailand
- 6Academy of Science, The Royal Society of Thailand, Bangkok, Thailand
In a cursory survey of fungi on Asteraceae in Yunnan Province, China, we report fungal species belonging to the family Leptosphaeriaceae (Pleosporales, Dothideomycetes). Two novel species have remarkable ascospores that are unusual for sexual ascomycetes. Multilocus phylogeny of large subunit, small subunit, and internal transcribed spacer sequence data showed one to be a novel genus, while the other is a new species. Praeclarispora artemisiae gen. et sp. nov. is introduced and is typical of Leptosphaeriaceae, but has unusual fusiform, versicolor ascospores with a brown median cell. Sphaerellopsis artemisiae sp. nov. has scolecosporous ascospores with deeply constricted septa that split into two parts, which resembles S. isthmospora but differs by ascospore dimension and molecular data. In addition, Plenodomus artemisiae is reported as a new collection from dead stems of Artemisia argyi in Qujing City. Plenodomus sinensis is reported as a new host record from Ageratina adenophora. All taxa are illustrated and described based on evidence of taxonomy and phylogeny.
Introduction
The plant family Asteraceae (= Compositae) is the major and widespread family of Angiosperms (flowering plants). The family comprises over 1,900 genera with over 32,000 accepted species (The Plant List, 2013). Most members of Asteraceae are herbaceous plants, but a significant number are shrubs, climbers, and trees. The family has a cosmopolitan distribution ranging from subpolar to tropical regions. The largest proportion of species occurs in arid and semiarid regions of subtropical and lower to middle temperate latitudes (Barkley et al., 2006). Several members of Asteraceae are economically important plants as food crops, including globe artichoke (Cynara cardunculus var. scolymus), lettuce (Lactuca spp.), safflower (Carthamus tinctorius), and sunflower (Helianthus spp.). Many genera are important in horticulture such as pot marigold (Calendula officinalis) and coneflowers (Echinacea spp.), and others are of herbal medicinal importance, including gumweed (Grindelia spp.), yarrow (Achillea millefolium), and silvery wormwood (Artemisia argyi). Many species in Asteraceae are also considered as invasive weeds including sticky snakeroot (Ageratina adenophora) and Siam weed (Chromolaena odorata).
Studying the fungi on Asteraceae will provide important information toward establishing the numbers of fungi (Hyde et al., 2020b), due to the fact that many species are herbaceous in arid and semi-arid areas, where we know little about fungal diversity. Several novel fungal species have been described from the plant family Asteraceae. For example, Hermatomyces chromolaenae from stems of Chromolaena odorata, Torula chromolaenae from a dead branch of C. odorata, and Dendryphion hydei from branch litter of Bidens pilosa were introduced by Li et al. (2017, 2020) and Tibpromma et al. (2017), respectively. A novel genus, Neocochlearomyces isolated from leaves of C. odorata, was described by Crous et al. (2018). Phookamsak et al. (2019) introduced novel fungal species from Cirsium arvense and Artemisia sp. Mapook et al. (2020) introduced 60 new taxa from Siam weed, including one new family Neomassarinaceae, 12 new genera, and 47 new species. Herein, fungal species belonging to the family Leptosphaeriaceae are reported from Ageratina adenophora and Artemisia argyi (Asteraceae).
Leptosphaeriaceae (Pleosporales, Dothideomycetes) was established by Barr (1987) and typified by Leptosphaeria. Leptosphaeriaceae is characterized by immersed, erumpent to superficial ascomata, scleroplectenchymatous peridium, cylindrical asci and hyaline to brown, transversely septate ascospores with coelomycetous or hyphomycetous asexual morphs (Alves et al., 2013; de Gruyter et al., 2013; Hyde et al., 2013; Ariyawansa et al., 2015). Historic reviews of Leptosphaeriaceae were detailedly provided by Hyde et al. (2013) and Ariyawansa et al. (2015). Species of Leptosphaeriaceae are widely distributed on various hosts and different regions (Dayarathne et al., 2015; Tennakoon et al., 2017; Phookamsak et al., 2019). They can be saprobic, hemibiotropic, pathogenic, or parasitic occurring on stems and leaves of herbaceous or woody plants in terrestrial and aquatic habitats (Alves et al., 2013; Hyde et al., 2013; Jones et al., 2015; Wanasinghe et al., 2016; Doilom et al., 2018). The early classification of taxa in Leptosphaeriaceae lacked DNA sequence data from ex-type strains. In addition, most strains in GenBank are named without a link to voucher specimens, which is not practical to verify their morphological characteristics to ensure accurate naming (Ariyawansa et al., 2015). Thus, phylogenetic analyses of taxa in Leptosphaeriaceae formed a paraphyletic clade (Dong et al., 1998; Zhang et al., 2012). Ariyawansa et al. (2015) provided a well-resolved backbone tree for Leptosphaeriaceae to resolve species and genera based on multilocus phylogeny with detailed morphology, and the results supported the monophyletic nature of 10 genera in Leptosphaeriaceae among the other families in Pleosporales. Currently, 14 genera are accepted in the family, viz., Alloleptosphaeria, Alternariaster, Chaetoplea, Heterosporicola, Leptosphaeria, Neoleptosphaeria, Ochraceocephala, Paraleptosphaeria, Plenodomus, Pseudoleptosphaeria, Querciphoma, Sclerenchymomyces, Sphaerellopsis, and Subplenodomus (Hongsanan et al., 2020a; Wijayawardene et al., 2020).
In this study, we introduce a new genus and two new species from Artemisia argyi that have remarkable ascospores. In addition, Plenodomus sinensis is reported as a new host record from Ageratina adenophora. Combined analyses of large subunit (LSU), small subunit (SSU), and internal transcribed spacer (ITS) sequence data with morphology supported the placement of our taxa in Leptosphaeriaceae.
Materials and Methods
Sample Collection, Specimen Examination, and Fungal Isolation
The specimens of Ageratina adenophora and Artemisia argyi belonging in Asteraceae were collected from Yunnan Province, China. Specimens were placed in zip-lock plastic bags and returned to the laboratory for fungal observation and isolation. Fungal structures on the host substrates were observed using the Motic SMZ 161 stereomicroscope and their ascomata on substrates were captured with a digital camera fitted on to the stereomicroscope. Micro-morphological characteristics were observed and photographed with a Nikon ECLIPSE Ni compound microscope fitted with a Canon EOS 600D digital camera. Indian Ink was used to observe mucilaginous sheaths surrounding the ascospores. Micro-morphological characteristics were measured by the Tarosoft (R) Image Frame Work program. Images used for figures were edited with Adobe Photoshop CS6 software (Adobe Systems, United States).
Fungal isolation was made from single spore as detailed in Chomnunti et al. (2014). Germinating ascospores were observed using the Motic SMZ 161 stereomicroscope and single ascospore was transferred using sterile needle and grown on potato dextrose agar (PDA) at room temperature (25–30°C). Pure cultures were kept for further studies.
Fungal Preservation and Fungal Registration Numbers
The herbaria were deposited at the herbarium of Cryptogams, Kunming Institute of Botany Academia Sinica (HKAS), Kunming, Yunnan Province, China and Key Laboratory of Industrial Microbiology and Fermentation Technology of Yunnan (YMF), Kunming, Yunnan Province, China. Living cultures were deposited at the Kunming Institute of Botany Culture Collection (KMUCC), Kunming, Yunnan Province, China. Facesoffungi (FoF) numbers and Index Fungorum (IF) numbers were registered as described by Jayasiri et al. (2015) and Index Fungorum (2021), respectively.
DNA Extraction, PCR Amplification, and Sequencing
Fungi were grown on PDA for 1 week at room temperature (25–30°C). Fungal mycelia were then scraped off and transferred to 1.5 ml sterilized micro-centrifuge tubes. Biospin Fungus Genomic DNA Extraction Kit–BSC14S1 (BioFlux®, China) was used to extract genomic DNA following the manufacturer’s protocol. The LSU 28S rRNA, the SSU 18S rRNA, the ITS, partial translation elongation factor 1-alpha (tef1-α) and partial RNA polymerase II second largest subunit (rpb2) were amplified and sequenced using primers LR0R/LR5 (Vilgalys and Hester, 1990; Rehner and Samuels, 1994), NS1/NS4, ITS5/ITS4 (White et al., 1990), EF1-983F/EF1-2218R (Rehner and Buckley, 2005), and fRPB2-5f/fRPB2-7cR (Liu et al., 1999), respectively.
The PCR amplification was performed in a total volume of 25 μl. PCR mixtures contained 12.5 μl of Easy Taq PCR Super Mix, 1 μl of dNTPs, 1 μl of each primer, and 9.5 μl of ddH2O. The PCR thermal cycle program for LSU, SSU, and ITS amplification was provided as initially 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 50 s, elongation at 72°C for 90 s, and a final extension at 72°C for 10 min. The annealing was adjusted to 52°C and 55°C for rpb2 and tef1-α, respectively. PCR products were purified and sequenced at Shanghai Sangon Biological Engineering Technology & Services Co., (Shanghai, China). GenBank accession numbers of tef1-α of our strains are provided in “Additional GenBank numbers.”
Phylogenetic Analysis
Consensus sequences were generated using BioEdit v.7.2.5 (Hall, 1999). Sequences of each strain were blasted using the MegaBLAST search of GenBank’s nucleotide database1 to examine their closest taxa. A total 89 sequences were used in phylogenetic analyses (Table 1). Didymella exigua (CBS 183.55) was used as the outgroup taxon. Individual dataset of the LSU, SSU, and ITS was aligned online with MAFFT version v.7.471 (Katoh et al., 2019)2 and manually edited where necessary using BioEdit v.7.2.5. Phylogenetic trees were inferred with maximum parsimony (MP), maximum likelihood (ML), and Bayesian inference (BI).
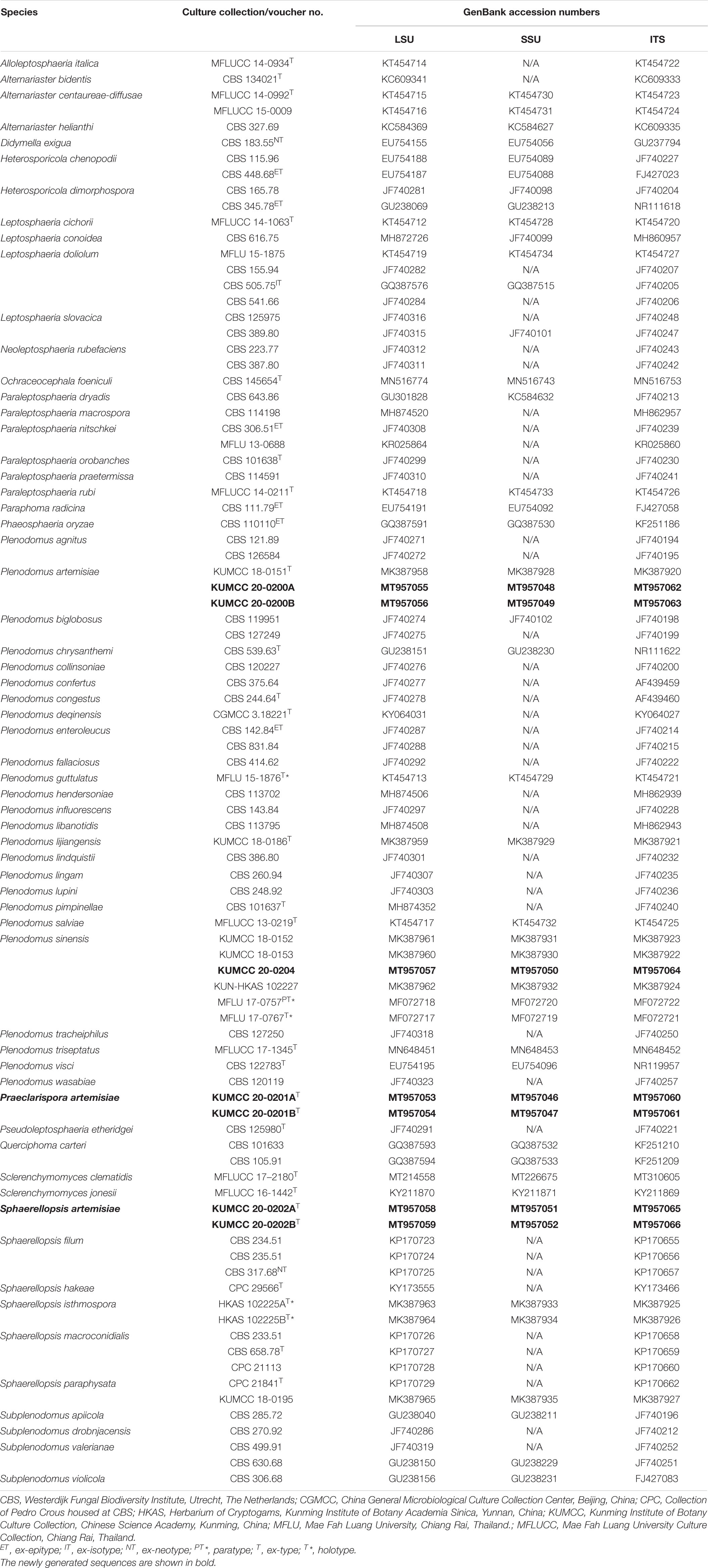
Table 1. GenBank accession numbers and culture collection numbers of species included in the present phylogenetic study.
Maximum parsimony analysis was performed with PAUP v. 4.0b10, with the parameter setting as the method described in Wanasinghe et al. (2018). Descriptive tree statistics for parsimony [Tree Length (TL), Consistency Index (CI), Retention Index (RI), Relative Consistency Index (RC), and Homoplasy Index (HI)] were calculated for trees generated under different optimality criteria. ML analysis was calculated as the method described in Doilom et al. (2017). All free model parameters will be estimated by RAxML and ML estimate of 25 per site rate categories. The model selected for ML was GTRGAMMA. BI analysis was conducted using the Markov Chain Monte Carlo (MCMC) method with MrBayes v. 3.2.7 (Huelsenbeck and Ronquist, 2001). By using MrModeltest 2.2 (Nylander, 2004), the GTR + I + G was selected as the best-fit nucleotide substitution models under the Akaike information criterion (AIC) for LSU, SSU, and ITS sequence data. Six chains were run for the individual and combined datasets. The MCMC algorithm was started from a random tree topology. Five million generations were selected with a sampling frequency every 100 generations. The Tracer v.1.6 program (Rambaut et al., 2013) was used to check the effective sampling sizes (ESS) that should be above 200, the stable likelihood plateaus, and burn-in value. The results suggest that the first 5,000 generations should be excluded as burn-in. Phylogenetic trees were visualized using FigTree v.1.4.0 (Rambaut, 2009) and formatted using PowerPoint 2010 (Microsoft Corporation, WA, United States).
Results
Phylogenetic Analysis
The alignment comprised 90 strains including the outgroup taxon, which consisted of 3,286 characters including alignment gaps (1–1331 bp for LSU, 1332–2680 bp for SSU, and 2681–3286 bp for ITS). The MP analysis for the combined dataset had 325 parsimony informative, 2,840 constant, and 121 parsimony uninformative characters and yielded 18 most parsimonious trees (TL = 2158, CI = 0.342, RI = 0.729, HI = 0.658, and RC = 0.249). The RAxML analysis resulted in a best scoring likelihood tree selected with a final combined dataset = −15205.152646. The matrix had 620 distinct alignment patterns, with 36.47% of undetermined characters or gaps.
Phylogenetic analysis of combined LSU, SSU, and ITS sequence data (Figure 1) showed that Praeclarispora artemisiae (KUMCC 20-0201A and KUMCC 20-0201B) clustered with Ochraceocephala (MP/ML/BI = 61%/96%/1.00) in the family Leptosphaeriaceae. Two strains, KUMCC 20-0200A and KUMCC 20-0200B, grouped with the ex-type strain of Plenodomus artemisiae (KUMCC 18-0151) with high bootstrap support (MP/ML/BI = 96%/95%/1.00). The collection KUMCC 20-0204 clustered with MFLU 17-0757 (paratype) and other strains of Plenodomus sinensis (MP/ML/BI = 70%/85%/1.00). Sphaerellopsis artemisiae (KUMCC 20-0202A and KUMCC 20-0202B) grouped separately from its closest relative Sphaerellopsis isthmospora with strong bootstrap support (MP/ML/BI = 100%/100%/1.00) (Figure 1).
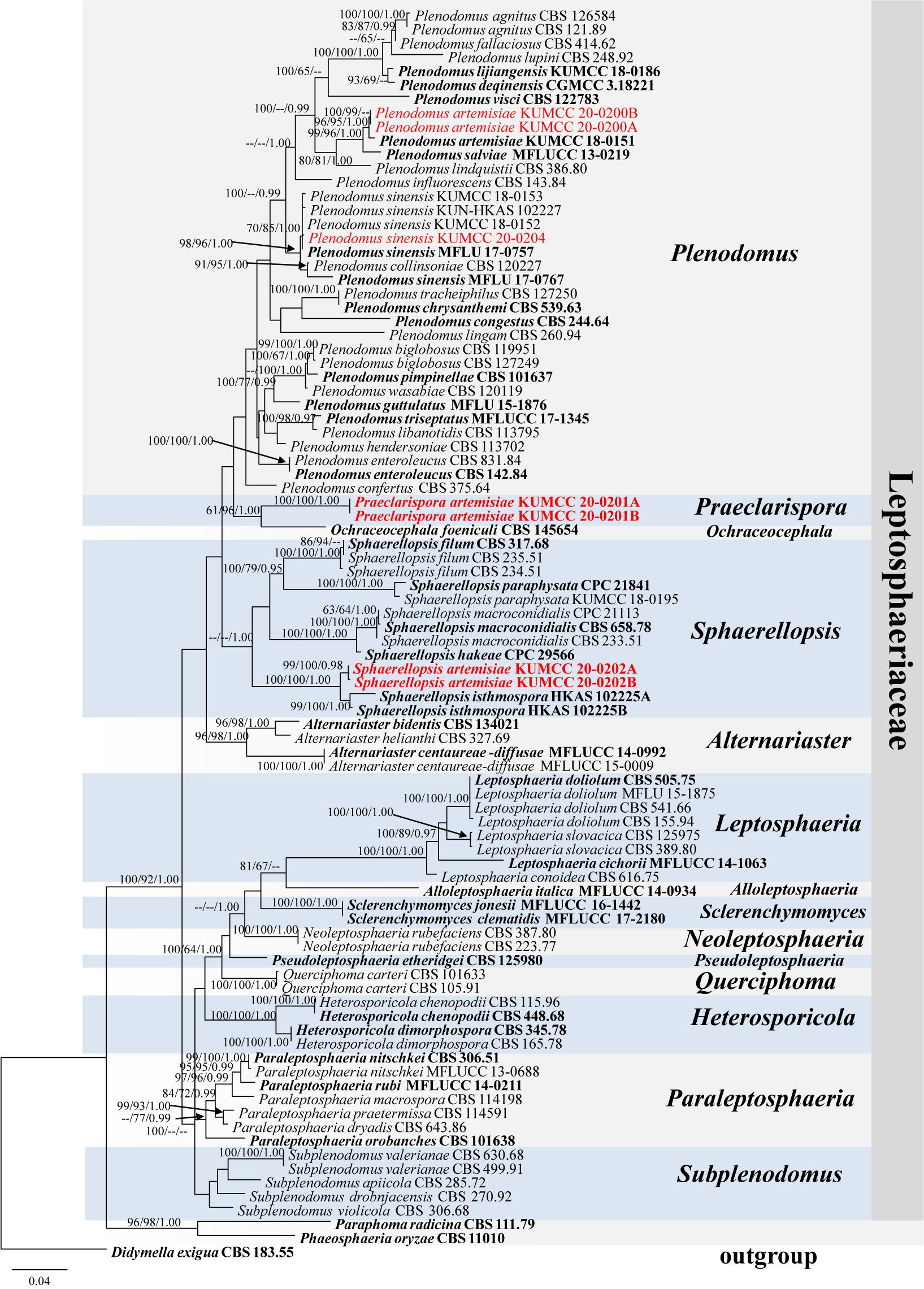
Figure 1. Phylogenetic tree generated from maximum likelihood analysis (RAxML) based on a combined LSU, SSU, and ITS sequence data. The tree is rooted to Didymella exigua (CBS 183.55). Maximum parsimony and maximum likelihood bootstrap values ≥60% and Bayesian posterior probabilities ≥0.95 (MPBS/MLBS/BYPP) are indicated at the nodes. Ex-epitype, ex-isotype, ex-neotype, ex-type, holotype, and paratype are bolded black, and the new isolates are in red.
Taxonomy
Praeclarispora Doilom, W. Dong, K. D. Hyde & C. F. Liao, gen. nov.
Index Fungorum number: IF558142; FoF number: FoF 09225
Etymology: The generic epithet “Praeclarispora” refers to remarkable-spored.
Saprobic on dead twigs of Artemisia argyi. Sexual morph: Ascomata black, scattered to gregarious, breaking the epidermis in linear fissures, semi-immersed, becoming erumpent to superficial, subglobose, uni- to multi-loculate, coriaceous, with ostiolate papilla. Peridium unevenly relatively thick, composed of several layers of thick-walled cells of textura angularis, outer layer black, inner layer brown. Hamathecium comprising numerous, filiform, septate, of cellular pseudoparaphyses embedded in a gelatinous matrix. Asci eight-spored, bitunicate, fissitunicate, narrowly obovoid, short pedicellate, apically rounded, with ocular chamber. Ascospores tri- to tetra-seriate, fusiform, curved, tapered toward the acute ends, versicolor, 0–1-septate when immature, becoming brown in median cell and hyaline to pale brown in other cells, middle cell larger than other cells, septate when mature, slightly constricted at the septa, thin- and smooth-walled. Asexual morph: Undetermined.
Type species: P. artemisiae Doilom, W. Dong, K. D. Hyde and C. F. Liao
Notes: Based on a blastn search of NCBIs GenBank, the closest hits using LSU sequence of Praeclarispora artemisiae matches with several genera in Leptosphaeriaceae and has highest similarity to Sphaerellopsis filum (CBS 234.51, identities = 99.18%) and S. paraphysata (CPC 21841, identities = 99.05%), followed by Ochraceocephala foeniculi (CBS 145654, identities = 98.83%). The closest hits using SSU sequence are Plenodomus lingam (CBS 260.94, identities = 99.81%), Pl. artemisiae (KUMCC 20-0200A, identities = 99.81%), Pl. biglobosus (CBS 119951, identities = 99.81%), and O. foeniculi (CBS 145654, identities = 99.71%). ITS sequence matches with published species Pl. hendersoniae (CBS 113702, identities = 92.14%), Pl. biglobosus (CBS 119951, identities = 89.66%), and O. foeniculi (CBS 145654, identities = 87.93%). However, in our multilocus analysis (Figure 1), P. artemisiae forms a sister branch with O. foeniculi with 96% ML and 1.00 BYPP but low MP bootstrap support, and separates from Leptosphaeria, Plenodomus, and Sphaerellopsis. The ITS phylogeny has similar results with the multilocus phylogeny (Supplementary Figure 1); LSU phylogenetic analysis clearly shows Praeclarispora separates from O. foeniculi as a distinct genus (Supplementary Figure 2). A single gene comparison between P. artemisiae and O. foeniculi shows that there are 1.17% (10/854), 0.29% (3/1029), and 13.81% (76/550) nucleotide difference in LSU, SSU, and ITS sequence data, respectively.
Ochraceocephala foeniculi is only known from its hyphomycetous asexual morph, which is characterized by hyaline, loosely or densely branched conidiophores, phialidic conidiogenous cells, and hyaline to yellowish, globose to subglobose conidia, and isolated as plant pathogen from living Foeniculum vulgare (Aiello et al., 2020). Praeclarispora artemisiae is reported herein from only its ascomycetous sexual morph, characterized by black ascomata, narrowly obovoid asci, and fusiform ascospores with a larger, brown, median cell, and isolated as saprobe from decaying twigs of Artemisia argyi. Unfortunately, we could not obtain the asexual morph from the culture for further morphological assessments. Even though we observed them under different conditions as described in Phookamsak et al. (2015) and Senanayake et al. (2020), neither conidia nor conidiomatal structures were produced. Therefore, we believe that it is wise to keep Ochraceocephala and Praeclarispora as separate genera in Leptosphaeriaceae for now. A different scenario may occur with the discovery of similar fungi from both of their asexual and sexual morphs with more fresh sampling.
Praeclarispora artemisiae Doilom, W. Dong, K. D. Hyde & C. F. Liao, sp. nov., Figure 2.
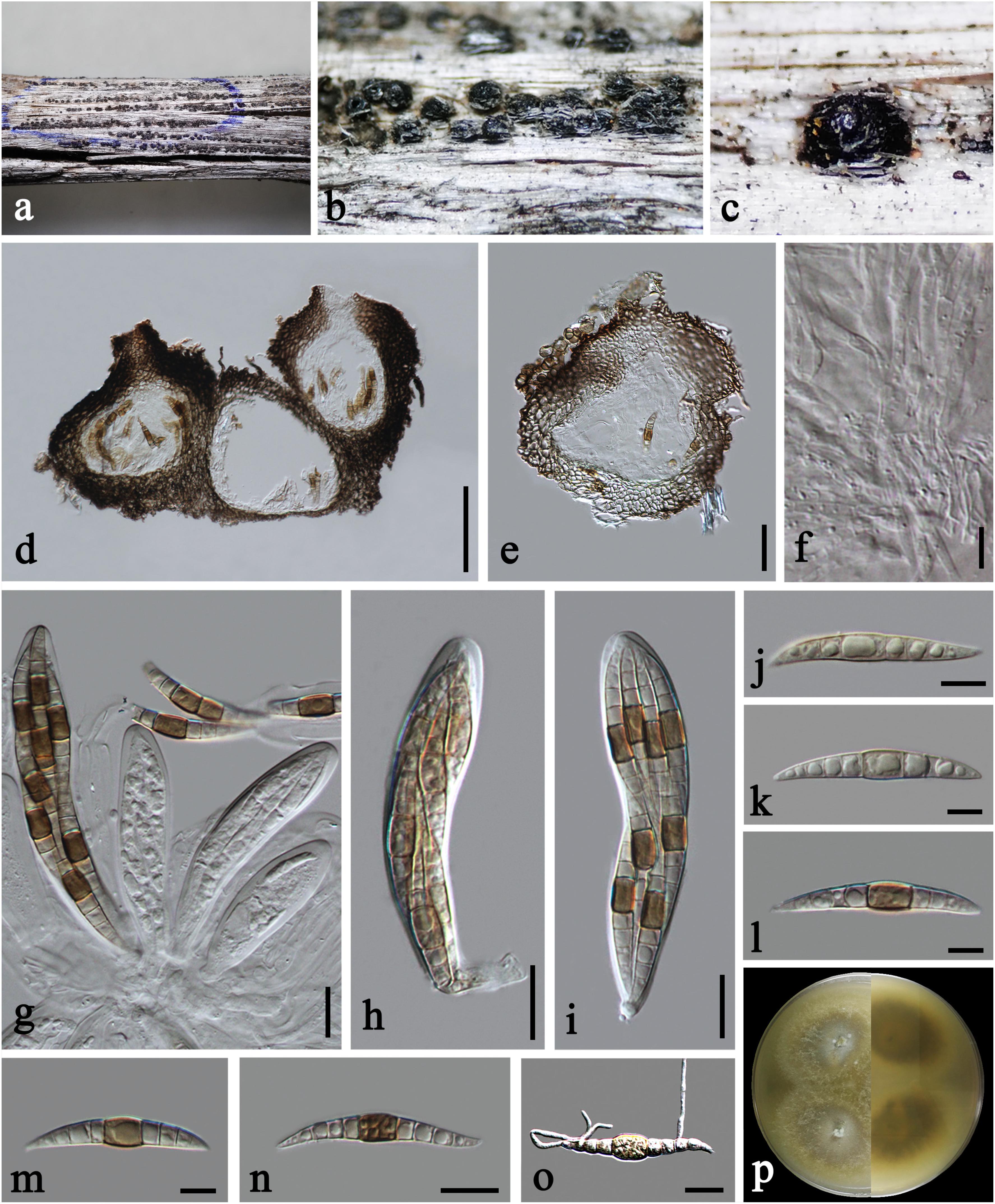
Figure 2. Praeclarispora artemisiae (HKAS 112654, holotype). (a–c) Appearance of ascomata on host substrate. (d,e) Vertical sections through ascomata. (f) Pseudoparaphyses. (g–i) Asci. (j–n) Ascospores. (o) Germinated ascospore. (p) Colony on PDA after 15 days (above and below views). Scale bars: (c) = 250 μm, (d) = 50 μm, (e) = 30 μm, (f,k–m) = 10 μm, (g–j,n,o) = 20 μm.
Index Fungorum number: IF558143; FoF number: FoF 09226
Etymology: The specific epithet “artemisiae” refers to the host genus Artemisia.
Holotype: HKAS 112654
Saprobic on dead twigs of Artemisia argyi. Sexual morph: Ascomata 170–245 μm high, 185–285 μm diam., black, scattered to gregarious, breaking the epidermis in linear fissures, semi-immersed, becoming erumpent to superficial, subglobose, uni- to multi-loculate, coriaceous, with ostiolate papilla. Ostioles 50–70 μm diam., central, brown, ostiolar canal filled with periphyses. Peridium 30–60 μm wide at the sides, unevenly thick, composed of scleroplectenchymatous cells, arrange in textura angularis, outer layer black, inner layer brown. Hamathecium 2–4.5 μm diam., numerous, filiform, septate, of cellular pseudoparaphyses embedded in a gelatinous matrix. Asci 100–140 × 19–27 μm ( = 120 × 23 μm, n = 15), eight-spored, bitunicate, fissitunicate, narrowly obovoid, short pedicellate, apically rounded, with ocular chamber. Ascospores 55–70 × 6–11 μm ( = 61 × 8.5 μm, n = 20), overlapping tri- to tetra-seriate, fusiform, curved, tapered to acute ends, versicolor, 0–1-septate when immature, becoming brown in median cell or occasionally two median cells, and hyaline to pale brown in other cells (Figure 2g), middle cell longer and slightly wider than the other cells, 8–10(–12)-euseptate when mature, slightly constricted at the septa, guttulate, thin- and smooth-walled. Asexual morph: Undetermined.
Culture characteristics: On PDA, colony circular, reaching 45 mm diam. in 15 days at room temperature (25–30°C), surface rough, with sparse mycelia on the surface, dry, umbonate from the side view, edge entire; from above, yellowish to cream at the margin, gray at the middle, white at the center; from below, yellowish at the margin, gray at the middle, yellowish brown at the center; producing yellowish pigmentation in culture.
Material examined: CHINA, Yunnan Province, Qujing City, dead twigs of Artemisia argyi (Asteraceae), October 1, 2019, C. F. Liao, (HKAS 112654, holotype); ex-type living culture KUMCC 20-0201; ibid., YMF 107390, isotype.
Additional GenBank numbers: tef1-α = MW396658 (KUMCC 20-0201A); MW396659 (KUMCC 20-0201B).
Plenodomus artemisiae A. Karunarathna, Phookamsak and K. D. Hyde, in Phookamsak et al., Fungal Diversity 95: 23 (2019), Figures 3, 4a
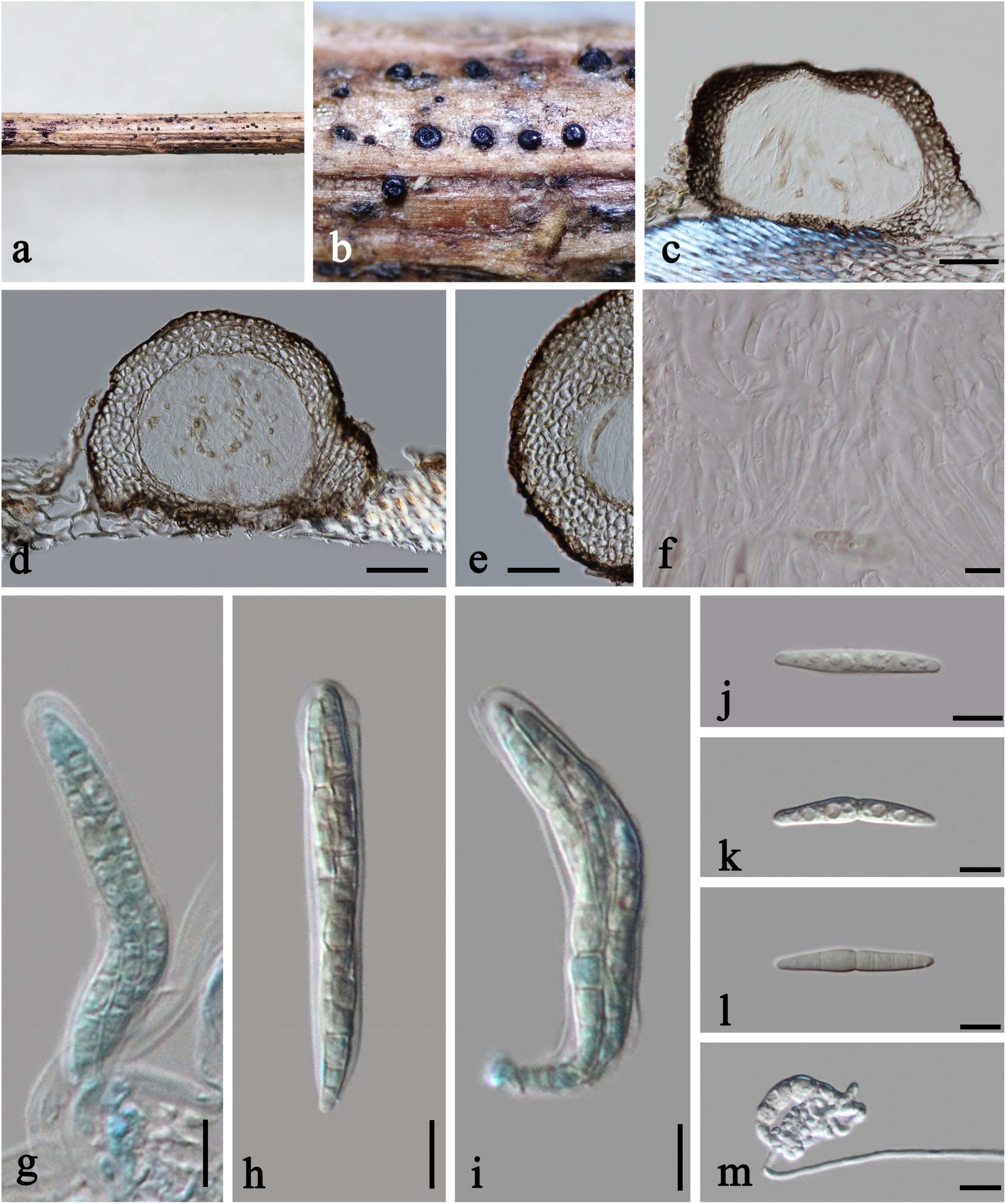
Figure 3. Plenodomus artemisiae (HKAS 112653, new collection). (a,b) Ascomata on host substrate. (c,d) Vertical sections of ascomata. (e) Structure of peridium. (f) Pseudoparaphyses. (g–i) Asci. (j–l) Ascospores (l showing the old ascospore occasionally with 5–7 septa). (m) Germinated ascospore. Scale bars: (c,d) = 50 μm, (e) = 30 μm, (f–m) = 10 μm.
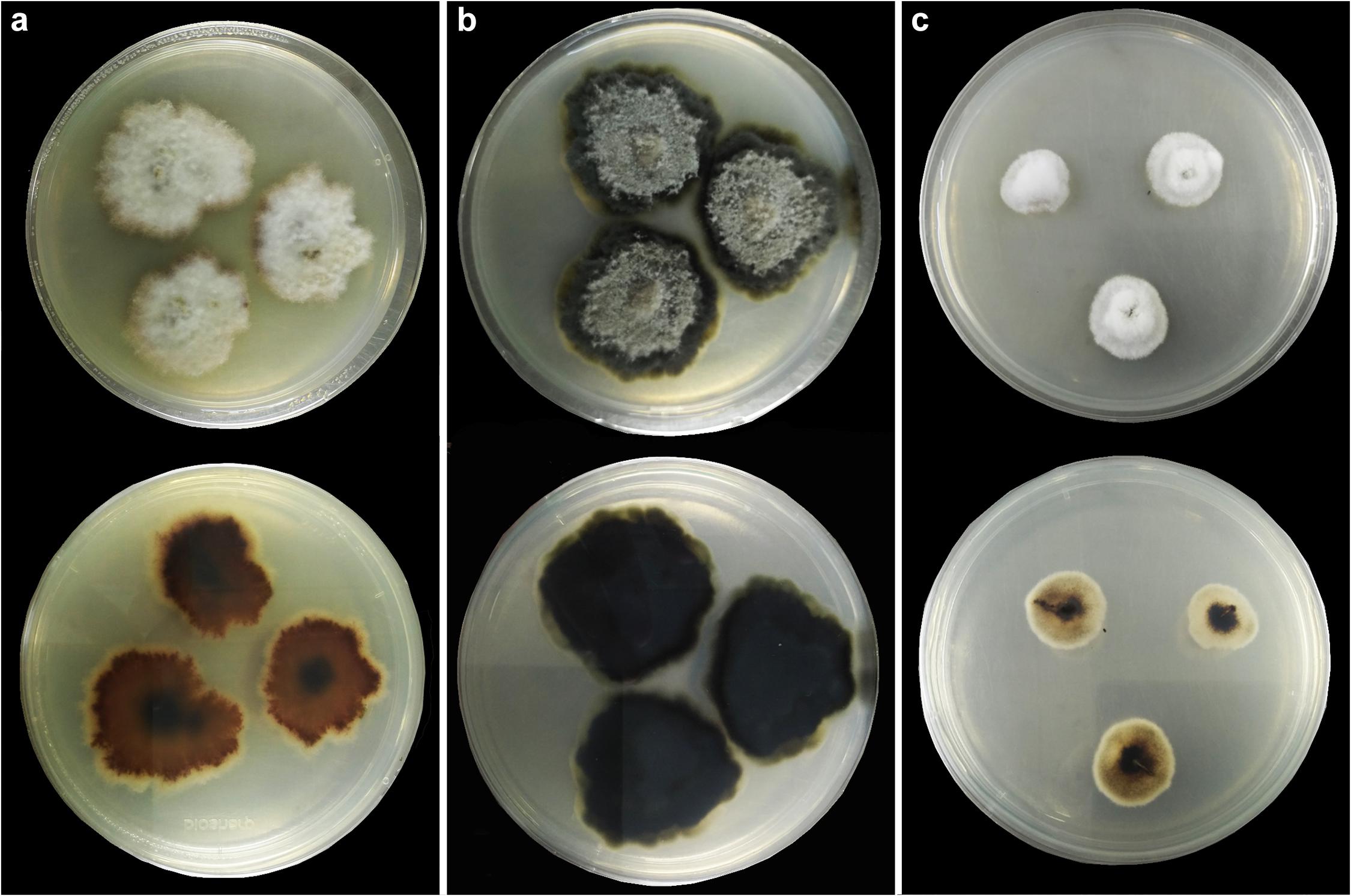
Figure 4. Colonies on PDA (up-front, down-reverse). (a) Plenodomus artemisiae (HKAS 112653). (b) Plenodomus sinensis (HKAS 112657). (c) Sphaerellopsis artemisiae (HKAS 112655).
Saprobic on dead stems of Artemisia argyi. Sexual morph: Ascomata 140–230 μm high, 210–260 μm diam., black, scattered, superficial with base seated in the substrate, compressed globose, uniloculate, glabrous, coriaceous, ostiolate, obscurely papillate. Peridium unevenly thick, 20–60 μm wide at the sides, with a poorly developed base, 10–15 μm wide, thinner toward the papilla, 12–15 μm wide, composed of several layers of thick-walled cells of textura angularis, outer layer black, inner layer brown. Hamathecium 2–2.5 μm diam., numerous, filiform, hyaline, septate, of cellular pseudoparaphyses embedded in a gelatinous matrix. Asci 60–80 × 9.5–11 μm ( = 68 × 10 μm, n = 15), eight-spored, bitunicate, fissitunicate, cylindric-clavate, short pedicellate, apically rounded. Ascospores 29–36 × 4.5–5.5 μm ( = 33 × 4.8 μm, n = 15), overlapping 2–3-seriate, narrowly fusiform, with tapering and rounded ends, hyaline, 1-septate, occasionally 5–7-septate when old, constricted at the median septum, guttulate, thin- and smooth-walled, without sheath or appendages. Asexual morph: Undetermined.
Culture characteristics: On PDA, colony irregular, reaching 40 mm diam. in 14 days at room temperature (25–30°C), surface rough, with dense mycelia, velvety and fluffy, dry, raised from the side view, edge undulate; from above, yellowish at the margin, cream to white at the center; from below, yellowish at the margin, orange brown at the middle, black at the center; producing yellowish pigmentation in culture.
Material examined: CHINA, Yunnan Province, Qujing City, dead stems of Artemisia argyi (Asteraceae), October 1, 2019, C. F. Liao, (HKAS 112653, new collection); living culture KUMCC 20-0200; ibid., YMF 112653, new collection.
Additional GenBank numbers: tef1-α = MW396660 (KUMCC 20-0200A); MW396661 (KUMCC 20-0200B).
Notes: Our collections KUMCC 20-0200A and KUMCC 20-0200B cluster with Plenodomus artemisiae (KUMCC 18-0151) with strong bootstrap support (MP/ML/BI = 99%/96%/1.00) (Figure 1). They have very similar morphological characteristics, except our collections have more septa (5–7-septate vs. only 5-septate) than the holotype (KUN-HKAS 102226) of Pl. artemisiae (Phookamsak et al., 2019). Our two collections have 99.65%, 99.90, 99.44, and 99.34% similarities with the ex-type strain (KUMCC 18-0151) of Pl. artemisiae in LSU, SSU, ITS, and tef1-α sequence data, respectively, which indicate them to be conspecific. We therefore identify our collections as Pl. artemisiae based on morphological and molecular evidences. Plenodomus artemisiae was initially reported from dead branches and stems of an unidentified Artemisia sp. in Yunnan Province, China (Phookamsak et al., 2019). Our new collection was collected from the same region, and we identify its host as Artemisia argyi.
Plenodomus sinensis Tennakoon, Phook. and K. D. Hyde, in Tennakoon et al., Phytotaxa 324(1): 76 (2017), Figures 4b, 5
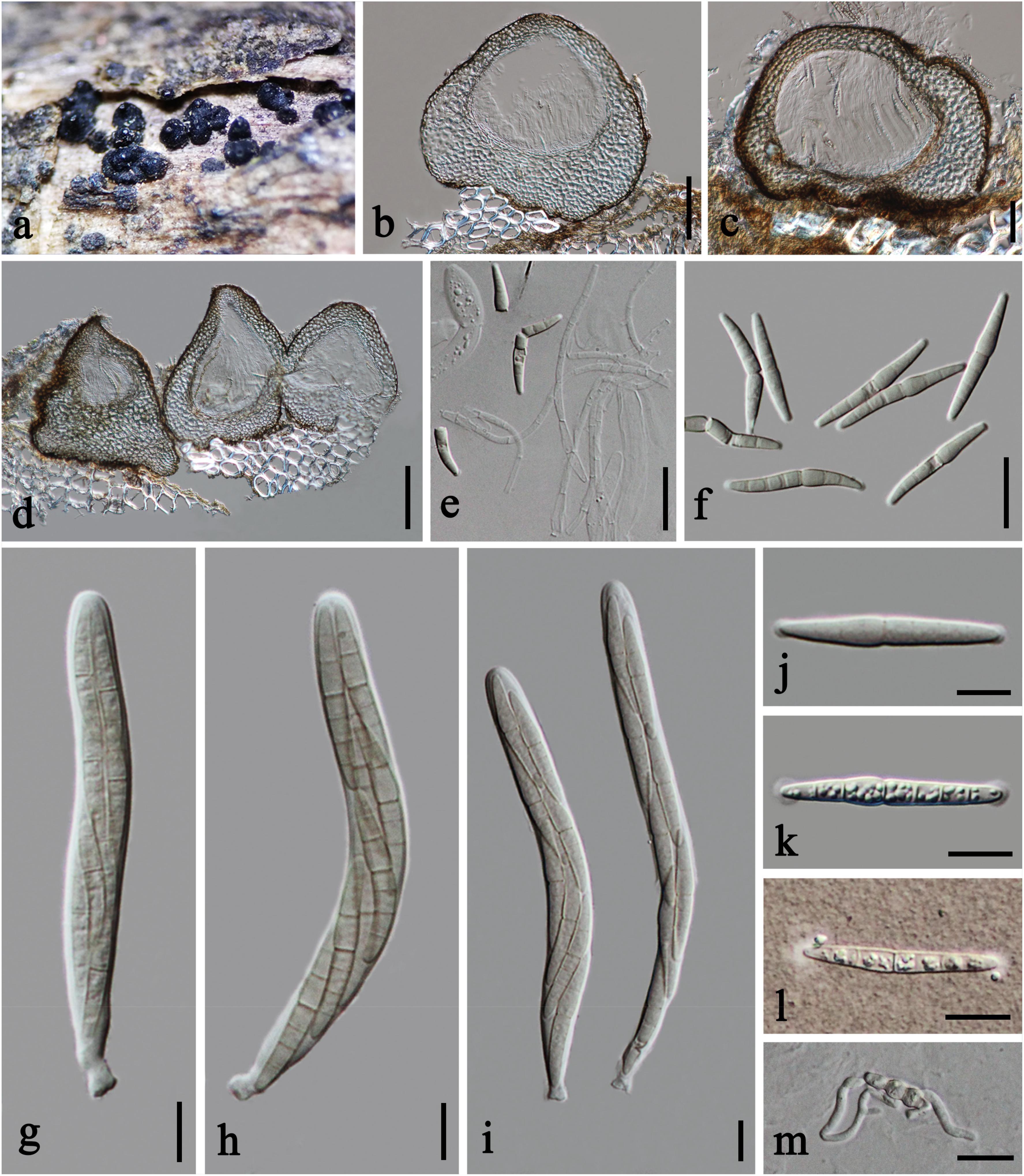
Figure 5. Plenodomus sinensis (HKAS 112657, new host record). (a) Ascomata on host substrate. (b–d) Vertical section of ascomata. (e) Structure of peridium. (g–i) Asci. (f,j–l) Ascospores (l Ascospore in Indian Ink). (m) Germinated ascospore. Scale bars: (b–d) = 50 μm, (e) = 30 μm, (f,m) = 20 μm, (g–l) = 10 μm.
Saprobic on Ageratina adenophora. Sexual morph: Ascomata 220–325 μm high, 205–345 μm diam., black, scattered to gregarious, raised, superficial, subglobose to conical, with flattened and thickened base, uniloculate, glabrous, coriaceous, with minutely ostiolate papilla, easily removed from the host substrate. Ostioles 55–95 μm diam., central, brown, ostiolar canal filled with some periphyses. Peridium unevenly thick, 25–115 μm wide at the base, 30–70 μm wide at the sides, mostly thickened at the base and thinner at the sides, three-layered, outer layer composed of dark brown, thick-walled cells of textura angularis, middle layer composed of pale brown to subhyaline, thick-walled, large cells of textura globulosa or textura angularis, inner layer composed of light brown, thin-walled, compressed cells of textura angularis. Hamathecium 2–4.5 μm wide, septate, branched, of cellular pseudoparaphyses embedded in a gelatinous matrix, slightly constricted at the septa. Asci 80–115 × 10–13 μm ( = 98 × 11 μm, n = 20), eight-spored, bitunicate, fissitunicate, cylindrical, with short furcate pedicel, apically rounded, with a distinct ocular chamber. Ascospores 29–39 × 4–5.5 μm ( = 33 × 4.9 μm, n = 30), overlapping 2–3-seriate, hyaline, 0–1-septate when immature, becoming olivaceous to yellowish, fusiform, with obtuse ends, 6–7-septate, constricted at the middle septum, not or slightly constricted at each septum, cell above central septum slightly wider, guttulate, thick- and smooth-walled, with mucilaginous globoid-shaped appendages at both ends. Asexual morph: Undetermined.
Culture characteristics: On PDA, colony irregular, reaching 40 mm diam. in 30 days at room temperature (25–30°C), surface rough and dull, with dense mycelia mostly immersed in culture, dry, umbonate from the side view, edge undulate; from above, dark gray at the margin, gray at the center; from below, greenish at the margin, black at the center; not producing pigmentation in culture.
Material examined: CHINA, Yunnan Province, Chuxiong City, Daguokou Township, Biji Village, dead branches of Ageratina adenophora (Asteraceae), September 14, 2019, C. F. Liao, (HKAS 112657, new host record); living culture KUMCC 20-0204; ibid., YMF 107633, new host record.
Notes: Our specimen HKAS 112657 and the holotype of Plenodomus sinensis (MFLU 17-0767) have 6–7-septate ascospores with mucilaginous globoid-shaped appendages at both ends, but they are slightly different in ascomatal base. Tennakoon et al. (2017) described flattened ascomatal base in the holotype, in addition, we observed the thickened one in our collection. Multilocus phylogeny shows that our collection KUMCC 20-0204 clusters with four collections of Pl. sinensis, including paratype MFLU 17-0757, but separates from the holotype MFLU 17-0767. Although MFLU 17-0767 clusters with Pl. collinsoniae (Figure 1), it differs in having larger asci, longer ascospores with olivaceous to yellowish pigmentation as discussed in Tennakoon et al. (2017). Our collection must be Pl. sinensis as its morphological characteristics are more similar to Pl. sinensis. Plenodomus sinensis appears to have a wide host range, occurring on Cirsium sp., Plukenetia volubilis, Tamarindus indica, and ferns in China (Tennakoon et al., 2017; Phookamsak et al., 2019). This is the first report of Pl. sinensis on Ageratina adenophora in China.
Sphaerellopsis artemisiae Doilom, W. Dong, K. D. Hyde and C. F. Liao, sp. nov., Figures 4c, 6

Figure 6. Sphaerellopsis artemisiae (HKAS 112655, holotype). (a, b) Ascomata on host substrate. (c,d) Vertical sections of ascomata. (e) Structure of peridium. (f) Splitted ascospores. (g–i) Asci. (j–l) Ascospores. (m) Germinated ascospore. Scale bars: (c,d) = 50 μm, (e) = 30 μm, (f–m) = 20 μm.
Index Fungorum number: IF557892; FoF number: FoF 09227
Etymology: The specific epithet “artemisiae” refers to the host genus Artemisia.
Holotype: HKAS 112655
Saprobic on dead stems of Artemisia argyi. Sexual morph: Ascomata 320–400 μm high, 230–300 μm diam., black, scattered or gregarious in small groups, superficial, subglobose, uniloculate, glabrous, coriaceous, with minutely ostiolate papilla. Ostioles 110–130 μm diam., central, dark brown to black, ostiolar canal filled with some periphyses. Peridium 30–55 μm at the sides, unevenly thick, thicker at the base, up to 70 μm wide, thinner at the ostiole, 13–18 μm wide, composed of several layers of brown to dark brown, thin-walled, large cells of textura angularis, inwardly compressed. Hamathecium 2–5.5 μm wide, sparse, hyaline, filamentous, septate, cellular pseudoparaphyses, constricted at the septa. Asci 105–170 × 17–25.5 μm ( = 122 × 20.5 μm, n = 15), eight-spored, bitunicate, fissitunicate, narrowly clavate, short pedicellate, apically rounded with a well-developed ocular chamber. Ascospores 80–117 × 5–7.5 μm ( = 92.5 × 6.5 μm, n = 25), overlapping 4–6-seriate, hyaline to yellowish, scolecosporous, bent at the fourth to fifth septum from the base, 10–13-septate, constricted at the septa, split into two part-spores at the bending point when old; upper part 45–75 μm long, cylindrical, 6–8-septate, with rounded apex and truncate base; lower part 30–42 μm long, subcylindric-clavate, 3–4-septate, with truncate apex and tapering or conical base, guttulate, without sheath or appendages. Asexual morph: Undetermined.
Culture characteristics: On PDA, colony circular, reaching 15 mm diam. in 7 days at room temperature (25–30°C), surface rough, with dense mycelia, velvety to fluffy, dry, raised from the side view, edge entire; from above, white to cream; from below, white at the margin, pale brown at the middle, black at the center; not producing pigmentation in culture.
Material examined: CHINA, Yunnan Province, Kunming City, dead stems of Artemisia argyi (Asteraceae), October 27, 2019, C. F. Liao, (HKAS 112655, holotype), ex-type living culture KUMCC 20-0202; ibid., YMF 107391, isotype.
Additional GenBank numbers: tef1-α = MW396662 (KUMCC 20-0202A); = MW396663 (KUMCC 20-0202B).
Notes: In our multilocus analysis, our collections Sphaerellopsis artemisiae (KUMCC 20-0202A and KUMCC 20-0202B) cluster with Sphaerellopsis isthmospora and separate from other Sphaerellopsis species with high bootstrap support (Figure 1). Sphaerellopsis artemisiae resembles S. isthmospora in having scolecosporous ascospores with deeply constricted septa that split into two parts at the fourth to fifth septum from the base (Phookamsak et al., 2019), but it differs in having longer and wider ascospores (92.5 × 6.5 μm vs. 87.1 × 5.9 μm). Phylogenetic analysis of combined LSU, SSU, and ITS sequence data also supports the idea that they are different species (Figure 1). In addition, a comparison of tef1-α sequence data shows that S. artemisiae has 4.04% differences with S. isthmospora. Based on morphological difference and molecular data, we therefore introduce S. artemisiae as a novel species.
Discussion
The members of the plant family Asteraceae are distributed throughout the world. Many novel fungal species have been reported from several genera in this family (Li et al., 2017; Tibpromma et al., 2017; Crous et al., 2018; Phookamsak et al., 2019; Mapook et al., 2020). Thus, Asteraceae is a promising cache of novel fungal species that warrant further study for basic science, use in biocontrol and biotechnology (Hyde et al., 2019). Our study reveals one new genus (Praeclarispora), two new species (Praeclarispora artemisiae and Sphaerellopsis artemisiae), one new collection of the sexual morph report (Plenodomus artemisiae), and one new host record (Pl. sinensis) on Ageratina adenophora in Yunnan Province, China. The two new species have remarkable ascospores that are unusual for sexual ascomycetes when compared with other genera (Doilom et al., 2018; Pem et al., 2019; Dong et al., 2020; Hongsanan et al., 2020a,b; Hyde et al., 2020c).
Praeclarispora has fusiform ascospores, with a larger median cell and tapering end cells which is slightly similar to Heptameria. However, Praeclarispora and Heptameria are different genera based on the distinct characteristics of ascomata, asci and ascospores. Heptameria has pseudothecial ascomata with rather thick pseudothecial wall (100–160 μm thick in H. obesa) (Lucas and Sutton, 1971), whereas Praeclarispora has euthecial ascomata with relatively thin peridium (30–60 μm thick in P. artemisiae). In addition, Heptameria often forms in several roundish groups on the substrate (Lucas and Sutton, 1971), while Praeclarispora mostly forms in linear fissures (never form in roundish groups). Heptameria has club-like asci (Lucas and Sutton, 1971), while they are narrowly obovoid in Praeclarispora. The ascospores of Heptameria are bi- or tri-seriate in the upper portion of the asci and uniseriate below (Lucas and Sutton, 1971), contrasting the tri- to tetra-seriate ascospores in Praeclarispora. Heptameria has distoseptate, rather thick-walled ascospores with a median, brown, rather large and muriform cell comprising of several transverse, longitudinal, and occasionally oblique septa (Lucas and Sutton, 1971), while Praeclarispora has euseptate, thin-walled ascospores and lacking the muriform median cell. Unfortunately, Heptameria cannot be incorporated in the phylogenetic tree as lacking sequence data and is referred to Dothideomycetes genera incertae sedis based on morphology (Lumbsch and Huhndorf, 2007, 2010; Zhang et al., 2012; Hyde et al., 2013; Wijayawardene et al., 2020). On the other hand, the available sequence data support Praeclarispora as a distinct genus within Leptosphaeriaceae (Figure 1).
Heptameria was introduced by Thümen with H. elegans as the type species (Thümen, 1879). However, H. elegans was considered as a synonym of an earlier proposed species H. obesa (≡ Sphaeria obesa) based on the examination of the holotype of H. elegans and H. obesa (Lucas and Sutton, 1971). Therefore, H. obesa is used as the type species. Although the current name of H. obesa is recorded as Leptosphaeria obesa in Index Fungorum (2021), Heptameria is not synonymized as Leptosphaeria and treated as a distinct genus by its cucurbitaria-like pseudothecia and characteristic ascospores (Petrak, 1951), which is also accepted by recent outline of fungi (Wijayawardene et al., 2020). However, sequence data derived from the type species H. obesa are indeed needed to confirm whether Heptameria is a valid genus, as most species of Heptameria have been transferred to other genera. Currently, only two species, i.e., H. obesa and H. uncinata, are accepted in the genus (Lucas and Sutton, 1971). It is very likely that the type species H. obesa will be extinct as it has been missing for nearly 150 years, especially in the increasingly serve climate change. Considering this circumstance and avoiding future confusion of Heptameria, Praeclarispora gen. nov. is introduced based on its distinct morphology.
One of the findings here is that Plenodomus sinensis has both flattened and thickened ascomatal bases, while the type that was studied by Tennakoon et al. (2017) has a flattened ascomatal base. The information of new collections and new records can be used to update fungal classification and improved identification of species (Hyde et al., 2020a). Our collection amends the morphology of P. sinensis, which is useful for fungal identification.
Additional protein-coding markers such as rpb2 and tef1-α are necessary to improve the phylogenetic resolution of genera and families in Pleosporales (Jaklitsch et al., 2018). However, most species of Leptosphaeriaceae lack tef1-α sequence data and other protein-coding markers, and some known species were sequenced using different tef1-α primer pairs. Thus, the phylogenetic analysis was constructed based on combined LSU, SSU, and ITS sequence data as provided in Dayarathne et al. (2015); Wanasinghe et al. (2016), Tennakoon et al. (2017), and this study. Nevertheless, we provide tef1-α sequence data for P. artemisiae, P. artemisiae, and S. artemisiae to facilitate the future identification of species. The rpb2 sequence data were unsuccessfully obtained even after several attempts.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
MD and WD designed the study. MD, WD, and KH wrote the manuscript. MD, WD, C-FL, and NS conducted the experiments, analyzed the data, and revised the manuscript. MD, NS, and SL contributed to research funds. All authors revised the manuscript.
Funding
This research work is partially supported by the Chiang Mai University. MD sincerely thanks the 5th batch of Postdoctoral Orientation Training Personnel in Yunnan Province (grant no. Y934283261) and the 64th batch of China Post-doctoral Science Foundation (grant no. Y913082271) for financial research support. KH thanks Chiang Mai University for the award of Visiting Professor, and the Foreign Experts Bureau of Yunnan Province, Foreign Talents Program (2018; grant no. YNZ2018002), and Thailand Research grant entitled Impact of climate change on fungal diversity and biogeography in the Greater Mekong Subregion (grant no. RDG6130001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully thank Shaun Pennycook (Landcare Research, New Zealand) for assistance in new epithets.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.660261/full#supplementary-material
Supplementary Figure 1 | Phylogenetic tree generated from maximum likelihood analysis (RAxML) based on ITS sequence data.
Supplementary Figure 2 | Phylogenetic tree generated from maximum likelihood analysis (RAxML) based on LSU sequence data.
Footnotes
References
Aiello, D., Vitale, A., Polizzi, G., and Voglmayr, H. (2020). Ochraceocephala foeniculi gen. et sp. nov., a new pathogen causing crown rot of fennel in Italy. MycoKeys 66, 1–22. doi: 10.3897/mycokeys.66.48389
Alves, J. L., Woudenberg, J. H. C., Duarte, L. L., Crous, P. W., and Barreto, R. W. (2013). Reappraisal of the genus Alternariaster (Dothideomycetes). Persoonia 31, 77–85. doi: 10.3767/003158513x669030
Ariyawansa, H. A., Phukhamsakda, C., Thambugala, K. M., Bulgakov, T. S., Wanasinghe, D. N., Perera, R. H., et al. (2015). Revision and phylogeny of Leptosphaeriaceae. Fungal Divers. 74, 19–51. doi: 10.1007/s13225-015-0349-2
Barkley, T. M., Brouillet, L., Strother, J. L., and Flora of North America Editorial Committee (eds) (2006). Asteraceae Martinov Tribe Cardueae Cassini. Flora of North America North of Mexico, Vol. 19: Magnoliophyta: Asteridae (In Part): Asteraceae, Part 1, 3–12. Available online at: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=10074 (accessed December 22, 2020)
Barr, M. E. (1987). New taxa and combinations in the Loculoascomycetes. Mycotaxon 29, 501–505. doi: 10.1127/0029-5035/2010/0091-0501
Chomnunti, P., Hongsanan, S., Aguirre-Hudson, B., Tian, Q., Peršoh, D., Dhami, M. K., et al. (2014). The sooty moulds. Fungal Divers. 66, 1–36. doi: 10.1007/s13225-014-0278-5
Crous, P. W., Luangsa-ard, J. J., Wingfield, M. J., Carnegie, A. J., Hernández-Restrepo, M., Lombard, L., et al. (2018). Fungal planet description sheets: 785– 867. Persoonia 41, 238–417. doi: 10.3767/persoonia.2018.41.12
Dayarathne, M. C., Phookamsak, R., Ariyawansa, H. A., Jones, E. B. G., Camporesi, E., and Hyde, K. D. (2015). Phylogenetic and morphological appraisal of Leptosphaeria italica sp. nov. Leptosphaeriaceae, Pleosporales from Italy. Mycosphere 6, 634–642. doi: 10.5943/mycosphere/6/5/13
de Gruyter, J., Woudenberg, J. H. C., Aveskamp, M. M., Verkley, G. J. M., Groenewald, J. Z., and Crous, P. W. (2013). Redisposition of phoma-like anamorphs in Pleosporales. Stud. Mycol. 75, 1–36. doi: 10.3114/sim0004
Doilom, M., Dissanayake, A. J., Wanasinghe, D. N., Boonmee, S., Liu, J. K., Bhat, D. J., et al. (2017). Microfungi on Tectona grandis (teak) in Northern Thailand. Fungal Divers. 82, 107–182. doi: 10.1007/s13225-016-0368-7
Doilom, M., Hyde, K. D., Phookamsak, R., Dai, D. Q., Tang, L. Z., Hongsanan, S., et al. (2018). Mycosphere notes 225–274: types and other specimens of some genera of Ascomycota. Mycosphere 9, 647–754. doi: 10.5943/mycosphere/9/4/3
Dong, J., Chen, W., and Crane, J. L. (1998). Phylogenetic studies of the Leptosphaeriaceae, Pleosporaceae and some other Loculoascomycetes based on nuclear ribosomal DNA sequences. Mycol. Res. 102, 151–156. doi: 10.1017/s0953756297004826
Dong, W., Wang, B., Hyde, K. D., McKenzie, E. H. C., Raja, H. A., Tanaka, K., et al. (2020). Freshwater Dothideomycetes. Fungal Divers. 105, 319–575. doi: 10.1007/s13225-020-00463-5
Hall, T. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Hongsanan, S., Hyde, K. D., Phookamsak, R., Wanasinghe, D. N., McKenzie, E. H. C., Sarma, V. V., et al. (2020a). Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 11, 1553–2107. doi: 10.5943/mycosphere/11/1/13
Hongsanan, S., Hyde, K. D., Phookamsak, R., Wanasinghe, D. N., McKenzie, E. H. C., Sarma, V. V., et al. (2020b). Refined families of Dothideomycetes: orders and families incertae sedis in Dothideomycetes. Fungal Divers. 105, 17–318. doi: 10.1007/s13225-020-00462-6
Huelsenbeck, J. P., and Ronquist, F. (2001). MRBAYES: bayesian inference of phylogeny. Bioinformatics 17, 754–755. doi: 10.1093/bioinformatics/17.8.754
Hyde, K. D., de Silva, N. I., Jeewon, R., Bhat, D. J., Phookamsak, R., Doilom, M., et al. (2020a). AJOM new records and collections of fungi: 1–100. Asian J. Mycol. 3, 22–294. doi: 10.5943/ajom/3/1/3
Hyde, K. D., Jeewon, R., Chen, Y. J., Bhunjun, C. S., Calabon, M. S., Jiang, H. B., et al. (2020b). The numbers of fungi: is the descriptive curve flattening? Fungal Divers. 103, 219–271. doi: 10.1007/s13225-020-00458-2
Hyde, K. D., Jones, E. B. G., Liu, J. K., Ariyawansa, H., Boehm, E., Boonmee, S., et al. (2013). Families of Dothideomycetes. Fungal Divers. 63, 1–313. doi: 10.1007/s13225-013-0263-4
Hyde, K. D., Norphanphoun, C., Maharachchikumbura, S. S. N., Bhat, D. J., Jones, E. B. G., Bundhun, D., et al. (2020c). Refined families of Sordariomycetes. Mycosphere 11, 305–1059. doi: 10.5943/mycosphere/11/1/7
Hyde, K. D., Xu, J. C., Rapior, S., Jeewon, R., Lumyong, S., Niego, A. G. T., et al. (2019). The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 97, 1–136. doi: 10.1007/s13225-019-00430-9
Index Fungorum (2021). Index Fungorum. Available online at: http://www.indexfungorum.org/names/names.asp (accessed January, 2021)
Jaklitsch, W. M., Checa, J., Blanco, M. N., Olariaga, I., Tello, S., and Voglmayr, H. (2018). A preliminary account of the Cucurbitariaceae. Stud. Mycol. 90, 71–118. doi: 10.1016/j.simyco.2017.11.002
Jayasiri, S. C., Hyde, K. D., Ariyawansa, H. A., Bhat, D. J., Buyck, B., Cai, L., et al. (2015). The faces of fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 74, 3–18. doi: 10.1007/s13225-015-0351-8
Jones, E. B. G., Suetrong, S., Sakayaroj, J., Bahkali, A. H., Abdel-Wahab, M. A., Boekhout, T., et al. (2015). Classification of marine ascomycota, basidiomycota, blastocladiomycota and chytridiomycota. Fungal Divers. 73, 1–72. doi: 10.1007/s13225-015-0339-4
Katoh, K., Rozewicki, J., and Yamada, K. D. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166. doi: 10.1093/bib/bbx108
Li, J. F., Jeewon, R., Mortimer, P. E., Doilom, M., Phookamsak, R., and Promputtha, I. (2020). Multigene phylogeny and taxonomy of Dendryphion hydei and Torula hydei spp. nov. from herbaceous litter in northern Thailand. PLoS One 15:e0228067. doi: 10.1371/journal.pone.0228067
Li, J. F., Phookamsak, R., Jeewon, R., Bhat, D. J., Mapook, A., Camporesi, E., et al. (2017). Molecular taxonomy and morphological characterization reveal new species and new host records of Torula species (Torulaceae, Pleosporales). Mycol. Prog. 16, 447–461. doi: 10.1007/s11557-017-1292-2
Liu, Y. J., Whelen, S., and Hall, B. D. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 16, 1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092
Lucas, M., and Sutton, B. (1971). Heptameria Rehm & Thüm. Trans. Br. Mycol. Soc. 57, 283–288. doi: 10.1016/S0007-1536(71)80010-4
Lumbsch, H. T., and Huhndorf, S. M. (2010). Outline of ascomycota–2009. Fieldiana Life Earth Sci. 1, 1–60.
Mapook, A., Hyde, K. D., McKenzie, E. H. C., Jones, E. B. G., Bhat, D. J., Jeewon, R., et al. (2020). Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (Siam weed). Fungal Divers. 101, 1–175. doi: 10.1007/s13225-020-00444-8
Nylander, J. A. A. (2004). MrModeltest v2.0. Program Distributed by the Author. Evolutionary Biology Centre, Uppsala University. Available online at: https://github.com/nylander/MrModeltest2/releases. (accessed March 15, 2020).
Pem, D., Jeewon, R., Bhat, D. J., Doilom, M., Boonmee, S., Hongsanan, S., et al. (2019). Mycosphere notes 275–324: a morphotaxonomic revision and typification of obscure Dothideomycetes genera (incertae sedis). Mycosphere 10, 1115–1246. doi: 10.5943/mycosphere/10/1/22
Phookamsak, R., Hyde, K. D., Jeewon, R., Bhat, D. J., Jones, E. B. G., Maharachchikumbura, S. S. N., et al. (2019). Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 95, 1–273. doi: 10.1007/s13225-019-00421-w
Phookamsak, R., Norphanphoun, C., Tanaka, K., Dai, D. Q., Luo, Z. L., Liu, J. K., et al. (2015). Towards a natural classification of Astrosphaeriella-like species; introducing Astrosphaeriellaceae and Pseudoastrosphaeriellaceae fam. nov. and Astrosphaeriellopsis, gen. nov. Fungal Divers. 74, 143–197. doi: 10.1007/s13225-015-0352-7
Rambaut, A. (2009). FigTree v1.3.1 Released. Institute of Evolutionary Biology. Ashworth Laboratories, University of Edinburgh, Scotland. Available online at: http://tree.bio.ed.ac.uk/software/figtree (accessed September 15, 2020)
Rambaut, A., Drummond, A. J., and Suchard, M. (2013). Tracer [Computer Program]. Available online at: http://tree.bio.ed.ac.uk/software/tracer/ (accessed September 20, 2020)
Rehner, S. A., and Buckley, E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97, 84–98. doi: 10.1080/15572536.2006.11832842
Rehner, S. A., and Samuels, G. J. (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 98, 625–634. doi: 10.1016/S0953-7562(09)80409-7
Senanayake, I. C., Rathnayake, A. R., Marasinghe, D. S., Calabon, M. S., Gentekaki, E., Lee, H. B., et al. (2020). Morphological approaches in studying fungi: collection, examination, isolation, sporulation and preservation. Mycosphere 11, 2678–2754. doi: 10.5943/mycosphere/11/1/20
Tennakoon, D. S., Phookamsak, R., Wanasinghe, D. N., Yang, J. B., Lumyong, S., and Hyde, K. D. (2017). Morphological and phylogenetic insights resolve Plenodomus sinensis (Leptosphaeriaceae) as a new species. Phytotaxa 324, 73–82. doi: 10.11646/phytotaxa.324.1.5
The Plant List (2013). Compositae. Available online at: http://www.theplantlist.org/ (accessed January 14, 2021)
Thümen, F. V. (1879). Contributiones ad floram mycologicam lusitanicam. Ser. II. Inst. Coimbra 27, 251–259.
Tibpromma, S., Hyde, K. D., Jeewon, R., Maharachchikumbura, S. S. N., Liu, J. K., Bhat, D. J., et al. (2017). Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 83, 1–261. doi: 10.1007/s13225-017-0378-0
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wanasinghe, D. N., Camporesi, E., and Hu, D. M. (2016). Neoleptosphaeria jonesii sp. nov., a novel saprobic sexual species, in Leptosphaeriaceae. Mycosphere 7, 1368–1377. doi: 10.5943/mycosphere/7/9/10
Wanasinghe, D. N., Phukhamsakda, C., Hyde, K. D., Jeewon, R., Lee, H. B., Jones, E. B. G., et al. (2018). Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 89, 1–236. doi: 10.1007/s13225-018-0395-7
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR Protocols: A Guide to Methods and Applications, eds M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (New York: Academic Press), 315–322. doi: 10.1016/b978-0-12-372180-8.50042-1
Wijayawardene, N. N., Hyde, K. D., Al-Ani, L. K. T., Tedersoo, L., Haelewaters, D., Rajeshkumar, K. C., et al. (2020). Outline of fungi and fungus-like taxa. Mycosphere 11, 1060–1456. doi: 10.5943/mycosphere/11/1/8
Keywords: Ageratina adenophora, Artemisia argyi, China, new record, phylogeny, Sphaerellopsis artemisiae, taxonomy
Citation: Doilom M, Hyde KD, Dong W, Liao C-F, Suwannarach N and Lumyong S (2021) The Plant Family Asteraceae Is a Cache for Novel Fungal Diversity: Novel Species and Genera With Remarkable Ascospores in Leptosphaeriaceae. Front. Microbiol. 12:660261. doi: 10.3389/fmicb.2021.660261
Received: 29 January 2021; Accepted: 19 March 2021;
Published: 13 May 2021.
Edited by:
Jadson Diogo Pereira Bezerra, Universidade Federal de Goiás, BrazilReviewed by:
Ning Jiang, Beijing Forestry University, ChinaXinlei Fan, Beijing Forestry University, China
Copyright © 2021 Doilom, Hyde, Dong, Liao, Suwannarach and Lumyong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saisamorn Lumyong, c2Nib2kwMDlAZ21haWwuY29t
 Mingkwan Doilom
Mingkwan Doilom Kevin D. Hyde
Kevin D. Hyde Wei Dong
Wei Dong Chun-Fang Liao
Chun-Fang Liao Nakarin Suwannarach
Nakarin Suwannarach Saisamorn Lumyong
Saisamorn Lumyong