
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 18 June 2021
Sec. Food Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.641034
Apples are naturally coated with a water-repelling hydrophobic wax layer, which may limit the antimicrobial efficacies of surface sanitizer solutions. Lauric arginate (LAE) is a cationic surfactant with antimicrobial efficacy against Listeria monocytogenes. In this study, we investigated the antimicrobial and the wettability effects of LAE in enhancing anti-L. monocytogenes efficacy of peracetic acid (PAA) and further verified the optimized treatment combinations in a pilot spray-bar brush bed system. Apples after 48 h of inoculation were treated with PAA surface sanitation in combination with different concentrations of LAE at 22 or 46°C. The effectiveness of PAA with LAE solutions in decontaminating L. monocytogenes significantly increased with the increased concentration of PAA (60–80 ppm) or LAE (0.01–0.05%) or the treatment temperature (from 22 to 46°C). A 30–120-sec wash by 80 ppm PAA with 0.01 and 0.05% LAE at 22°C reduced L. monocytogenes on apples by 2.10–2.25 and 2.48–2.58 log10 CFU/apple, respectively. Including LAE in the PAA solution decreased contact angles on apple surfaces. However, the increased wettability of the sanitizer solution may not be the main contributor to the enhanced antimicrobial efficacy of the PAA solution, given that the addition of Tween 80 or Tween 85 only slightly boosted the anti-L. monocytogenes efficacy of PAA solutions though both increased the wettability of the PAA solutions. The synergistic effects of PAA and LAE were further validated in a pilot spray-bar brush bed packing system, where a 30-sec spray wash with 80 ppm PAA and 0.05% LAE at 22 and 46°C caused 1.68 and 2.08 log reduction of Listeria on fresh apples, respectively. This study provides an improved PAA process/preventive strategy for ensuring microbial food safety of fresh apples that is applicable to commercial apple packing lines.
Listeria monocytogenes can potentially transfer to fresh produce including apples during the postharvest handling, which results in deadly outbreaks (McCollum et al., 2013; Angelo et al., 2017). Although listeriosis is rare, the mortality rate of listeriosis is very high (∼ 21%) (Silk et al., 2013). The recent foodborne outbreak of L. monocytogenes linked to caramel apples (Angelo et al., 2017) and multiple recall linked to fresh apples due to the potential contamination of L. monocytogenes (FDA, 2015, 2017b) highlight the importance of effective control strategies to minimize contamination risk of L. monocytogenes on fresh apples.
Antimicrobial wash interventions including chlorine-based sanitizers (Du et al., 2002; Rodgers et al., 2004; Sheng et al., 2020a), organic acids such as lactic acid and citric acid (Park et al., 2011), and peracetic acid (PAA) (Rodgers et al., 2004; Shen et al., 2019) were used to decontaminate L. monocytogenes on fresh apples. Of these antimicrobials, PAA is the most widely used sanitizer in apple packing lines during the spray-bar brush bed intervention (Zhu et al., 2020). PAA has broad-spectrum antimicrobial activity (Baert et al., 2009) and does not produce toxic by-products (Monarca et al., 2002). PAA applied at 80 ppm, a concentration approved by the Food and Drug Administration (FDA) to wash fresh produce without further rinsing requirement (FDA, 2017a), is more efficient in decontaminating L. monocytogenes on fresh apples compared with 100 ppm chlorine-based sanitizers (Shen et al., 2019; Sheng et al., 2020a). However, spray wash of 80 ppm PAA at the current industry practice (at ambient temperature for 30–120 sec contact time) only resulted in about one log reduction of L. monocytogenes (Shen et al., 2020), indicating the need to boost the anti-Listeria efficacy of PAA.
Fresh apples are naturally covered with a hydrophobic wax layer (Dong et al., 2012; Veraverbeke et al., 2001), which might limit the direct contact of sanitizer solutions on apple surfaces. Surfactants are amphipathic molecules that exist in hydrophobic/hydrophilic interfaces resulting in easy spreading of a liquid solution (Nakama, 2017). Lauric arginate (LAE) is a cationic surfactant derived from lauric acid, L-arginine, and ethanol (Infante et al., 1984), which was approved by the FDA as Generally Recognized as Safe (GRAS) when used at the concentration of 0.02% for direct addition to food products such as meat, cheese, and fruit juice (FDA, 2005). LAE has antimicrobial activity against Listeria when used alone or in combination with other sanitizers. Application of 0.01% LAE for 120 sec caused 3.1 log reduction of L. monocytogenes on lettuces at an initial bacterial population of 6.6 log10 CFU/g (Nübling et al., 2017). A 20-min treatment of 0.10% LAE reduced Listeria innocua by 2.3 log10 CFU/cm2 on lettuce surfaces (Huang and Nitin, 2017). A 3-min of 0.02% LAE exposure resulted in 1 log10 CFU/cm2 reduction of L. monocytogenes biofilm on lettuce surfaces (Sadekuzzaman et al., 2017). LAE was reported to enhance the anti-Listeria efficacy of potassium lactate and sodium diacetate on dairy and meat products (Stopforth et al., 2010; Soni et al., 2012). Existing data collectively suggest that LAE is a promising antimicrobial substance for the control of L. monocytogenes on food products. However, a recent laboratory scale study reported that the antimicrobial efficacy of 80 ppm PAA with 0.10% LAE against L. innocua on apple surfaces was not significantly different from that of 80 ppm PAA alone (Pietrysiak et al., 2019). Currently, no information is available about the strengthening effects of LAE in PAA solutions against L. monocytogenes on fresh apples.
The objectives of this study were to evaluate and optimize the effects of LAE in strengthening the anti-Listeria efficacy of PAA on fresh apples and further verify the optimized PAA and LAE treatments in a pilot spray-bar brush bed apple processing system. This study provides a practical and optimized PAA intervention strategy for the apple industry and other produce industries with similar postharvest handling and processing for their food safety programs.
Three L. monocytogenes strains, NRRL B-57618 (1/2a, 2011 cantaloupe outbreak isolate), NRRL-33466 (1/2b, processing plant isolate), and NRRL B-33053 (4b, 1983 coleslaw outbreak isolate) and L. innocua strains (NRRL B-33197, NRRL B-33314, and NRRL B-33554) were obtained from USDA-ARS culture collection [National Center for Agricultural Utilization Research (NRRL), Peoria, IL, United States). Stock cultures were stored at −80°C in trypticase soy broth [Becton, Dickinson and Company (BD), Sparks, MD, United States) supplemented with 6 g/L yeast extract (Fisher Scientific, Fair Lawn, NJ, United States) (TSBYE) and 20% (v/v) glycerol (J. T. Baker, Philipsburg, NJ, United States). Each frozen stock culture was subcultured in TSBYE at 37°C for 24 h and transferred into fresh TSBYE for a second 24-h subculture. Following incubation, the cultures were centrifuged at 8,000 × g for 5 min at 4°C and the resulting pellets were washed once and resuspended in sterile phosphate-buffered saline (PBS, pH 7.4) (EMD Millipore, Billerica, MA, United States). For apple inoculation, a three-strain L. monocytogenes or L. innocua cocktail was used, and each strain suspension was mixed in equal proportions and diluted to achieve ∼ 106 CFU/ml in sterile PBS solutions.
Cationic surfactant LAE (CytoGuardTM) was kindly provided by A&B Ingredients, Inc. (Field, NJ, United States). Nonionic surfactants of Tween 80 and Tween 85 were purchased from Sigma (St. Louis, MO, United States). Bioside HS (a stabilized mixture of 15% PAA and 22% hydrogen peroxide) (Pace International Inc., Wapato, WA, United States) was used to prepare the 60–80 ppm PAA solutions. The concentration of PAA in the respective solution was verified using a titration kit (Aquaphoenix Scientific, Hanover, PA, United States). LAE (0.001–0.10%, v/v), Tween 80 (0.10–0.20%, v/v) or Tween 85 (0.10–0.20%, v/v) were added to 80 ppm PAA solution to the specified concentration.
The above prepared PAA with LAE solutions were individually inoculated with three-strain L. monocytogenes cocktail at 5 × 108 CFU/ml, where 1 ml of three-strain L. monocytogenes cocktail suspension was added to 9 ml of respective PAA with LAE solution, treated for 30 sec, then the 1.0 ml of solution was sampled and immediately neutralized with 9.0 ml of D/E neutralizing broth (BD). The survivals were enumerated by 10-fold serial dilution with sterile PBS and plating on duplicate TSAYE (TSBYE with 1.5% agar) plates and incubated at 37°C for 24 h. The detection limit of L. monocytogenes is 10 CFU/ml.
Medium-sized (210–230 g) unwaxed Granny Smith apples (GSA) and Fuji apples devoid of cuts, bruising, or scars were used for this study. Before inoculation, 30 apples were randomly sampled for background microflora enumeration, which is about 3.3–4.0 log10 CFU/apples. To inoculate, apples were washed with cold tap water, equilibrated to room temperature (∼22°C, RT) overnight, then dip inoculated with the three-strain L. monocytogenes or L. innocua cocktail inoculum (∼ 6.8 Log10 CFU/ml) solution to have ∼ 6 log10 CFU/apple inoculation level (Sheng et al., 2017). The inoculated apples were held at RT for 48 h when apples were treated with different sanitizer solutions. For each batch of the inoculated apples, 10 inoculated apples were randomly sampled 0 and 48 h after inoculation to evaluate the initial L. monocytogenes or L. innocua population and the uniformity on apples.
The inoculated apples after 48 h inoculation were subjected to 80 ppm PAA solutions in the presence of 0.001–0.10% LAE at RT for a 2-min exposure unless specified. PAA with 0.10–0.20% Tween 80 or Tween 85 were included as a control.
We previously reported that PAA solution when applied at 46°C had enhanced antimicrobial activity against L. monocytogenes on apples compared with that at 22°C without compromising the quality of fruit (Shen et al., 2019). Therefore, the antimicrobial efficacies of PAA in combination with 0.01 or 0.05% LAE solutions were further evaluated at 46°C. Each experiment was independently repeated three times; 10 apples per treatment within an independent study. There were 30 apples in total for a selected treatment.
The spray wash of apples was conducted in a pilot spray-bar processing system equipped with a brush bed at Washington State University. L. innocua, a well-defined surrogate of L. monocytogenes (Sheng et al., 2020b; Zhu et al., 2020), was used in the pilot spray bar intervention. GSA inoculated with L. innocua were spray washed by 80 ppm PAA with or without LAE at 0.01 and 0.05% (v/v) at the specific temperature (22 or 46°C) for 30 or 120 sec. The flow rate of the spray bar was 0.977 L/min, and the brush bed is rotating at 47 revolutions per min. Each experiment was independently repeated three times with 10 inoculated apples per treatment within each independent study.
Immediately after sanitizer interventions, each apple was transferred into a stomacher bag with the addition of 10 ml of D/E neutralizing broth (BD). Each apple was hand-rubbed for 90 s as previously reported (Shen et al., 2019). Rub solutions were 10-fold serially diluted with sterile PBS, and the appropriate dilutions were plated in duplicate on TSAYE plates. The above TSAYE plates were first incubated at 35 ± 2°C for 4 h and then overlaid with a thin layer of modified Oxford agar (MOX, BD) to facilitate the recovery of the injured Listeria and to discern Listeria from apple background microflora (Kang and Fung, 1999; Shen et al., 2019).
The contact angles of each wash solution on apple surfaces were measured using the Theta Lite Tensiometer (Biolin Scientific, Stockholm, Sweden) at RT. Apple disks with a 2-cm diameter were cut from fresh apples using a sharp knife. To measure the contact angle, 10 μl of wash solution was deposited onto the above-prepared apple disk using a microliter syringe needle, then the static contact angle was recorded within 5-sec contact. To accurately measure the wettability, the contact angle of each solution was measured on 20 apple disks. Mean values of 20 replicates were reported. The larger water contact angle represents the lower wettability.
Mean differences were compared by one-way analysis of variance (ANOVA) and analyzed by Tukey’s multiple-comparison test (P < 0.05) using IBM SPSS 19.0 (Chicago, IL, United States). Microbiological data were reported as mean ± SEM (standard error mean) averaged from three independent experiments with 10 apples per treatment in each independent study, n = 30. Contact angles were presented as mean ± SEM, calculated from 20 apple disks, n = 20.
Adding LAE as low as 0.001% significantly (P < 0.05) enhanced anti-Listeria efficacy of 80 ppm PAA, which resulted in an additional ∼ 1.48 log10 CFU/ml reduction of L. monocytogenes in water compared with that treated with 80 ppm PAA alone (caused 3.12 ± 0.02 log10 CFU/ml) (Figure 1). The addition of 0.01% LAE in 80 ppm PAA solution caused more than a 5-log reduction of L. monocytogenes in water (Figure 1).
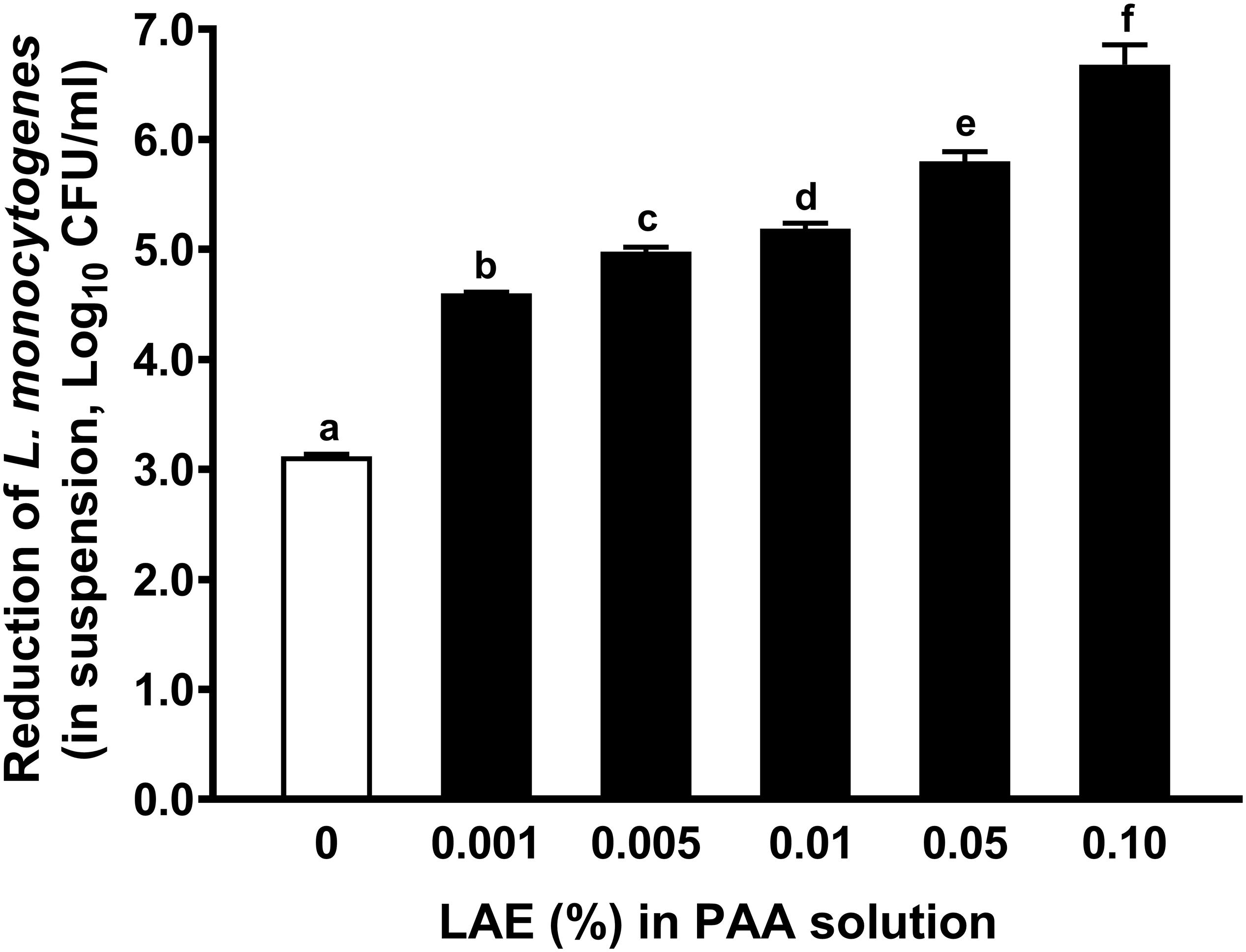
Figure 1. Antimicrobial efficacy of 80 ppm peracetic acid with different concentrations of lauric arginate against L. monocytogenes in water. The treatment time is 30 sec. The initial L. monocytogenes population was ∼ 7.06 log10 CFU/ml. In all treatments, peracetic acid (PAA) was used at 80 ppm. Data are reported as mean ± SEM from three independent studies; there are three replicates per treatment within an independent study. a–fMean among bars without common letters differ significantly (P < 0.05). LAE, lauric arginate.
The addition of LAE as low as 0.001% enhanced (P < 0.05) the effectiveness of PAA against L. monocytogenes on fresh apples (Figure 2A). A 2-min exposure of 80 ppm PAA in combination with 0.05 or 0.10% LAE reduced L. monocytogenes by 2.68 ± 0.02 or 2.81 ± 0.01 log10 CFU/apple (Figure 2A). However, including Tween 80 or Tween 85, up to 0.20% only slightly improved the efficacy of 80 ppm PAA solution against L. monocytogenes on apples, though they were statistically different (P < 0.05) (Figure 2B).
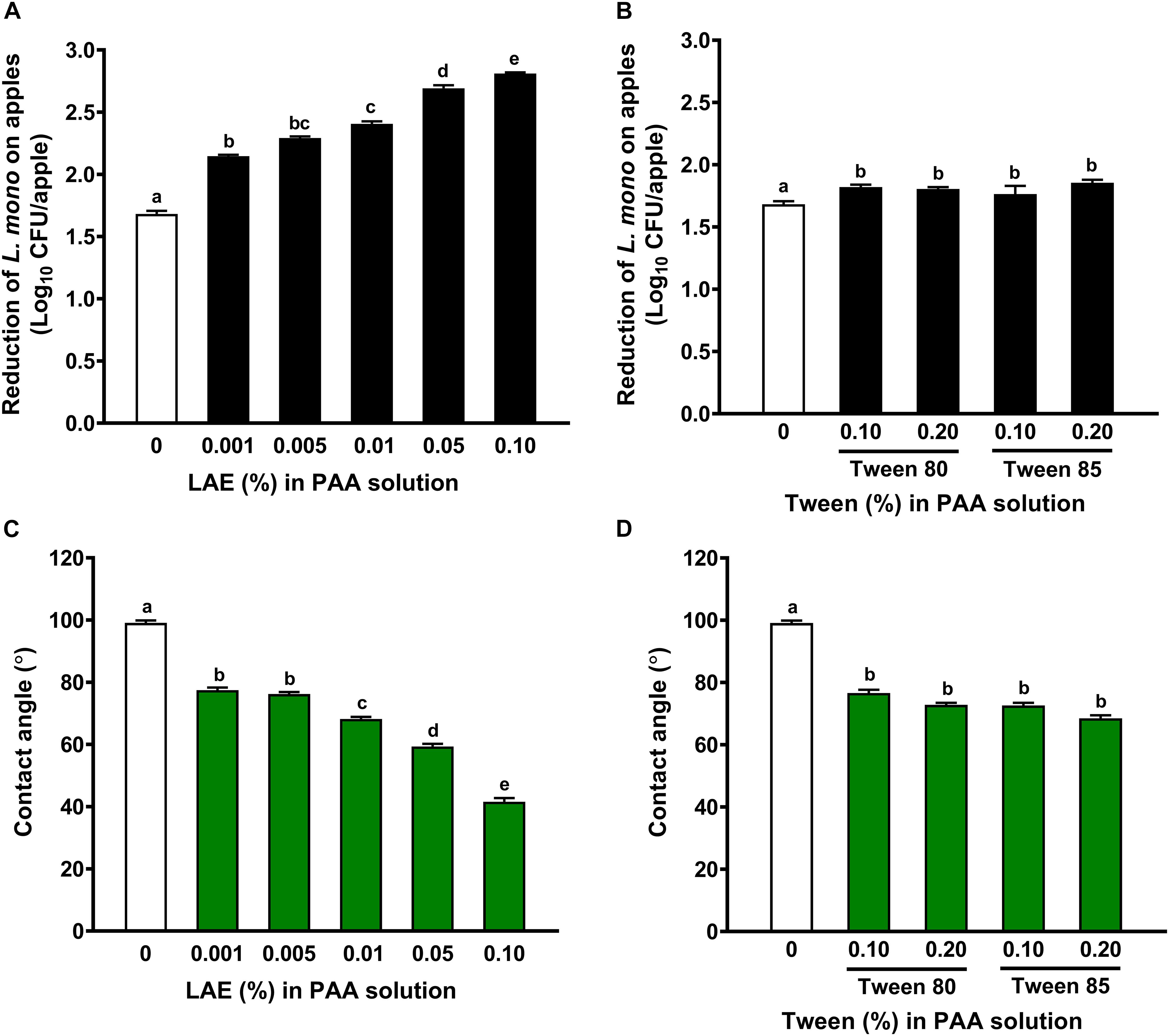
Figure 2. The effects of lauric arginate in enhancing the efficacies of peracetic acid against L. monocytogenes on Granny Smith apples may not be due to its wettability. Reduction of L. monocytogenes on apples treated with 80 ppm PAA in the presence of lauric arginate (LAE) (A) or Tween 80 or Tween 85 (B) for 2 min. The wettability of 80 ppm PAA solution with LAE (C) or Tween 80 or Tween 85 (D) as indicated by their contact angle on apple surfaces. Mean ± SEM, n = 30 for the reduction of L. monocytogenes and 20 for the contact angle. a–eMean among bars without common letters differ significantly (P < 0.05).
Including either LAE, Tween 80, or Tween 85 significantly increased (P < 0.05) the wettability of PAA solution on apple surfaces as indicated by decreased contact angles (Figures 2C,D). The PAA solutions with 0.001–0.005% and 0.01% LAE had a contact angle of 76–77° and 68.2°, respectively, which did not differ from those with 0.1 or 0.2% Tween 80/85 (Figures 2C,D). Data indicated that the enhanced anti-Listeria efficacy of LAE in PAA solutions might be mainly due to its antimicrobial activity instead of wettability.
Extending the contact time from 30 to 120 sec significantly enhanced (P < 0.05) anti-L. monocytogenes efficacies of PAA with or without LAE (Figure 3A). Regardless of contact time, the antimicrobial efficacies of PAA solutions against L. monocytogenes on GSA increased with elevated LAE concentrations (P < 0.05). Increasing LAE concentration to 0.05% caused 2.48–2.58 log10 CFU/apple reductions of L. monocytogenes on apples (Figure 3A). Decreasing the concentration of PAA from 80 to 60 or 70 ppm diminished (P < 0.05) the efficacy of PAA with LAE treatment against L. monocytogenes on GSA, regardless of LAE concentration (Figure 3B). The efficacies of PAA with 0.01 and 0.05% LAE against L. monocytogenes on Fuji apples were not significantly different from those on GSA; a 2-min exposure to PAA with 0.01–0.05% LAE reduced L. monocytogenes on Fuji apples by 2.40–2.62 log10 CFU/apple (Figure 3C).
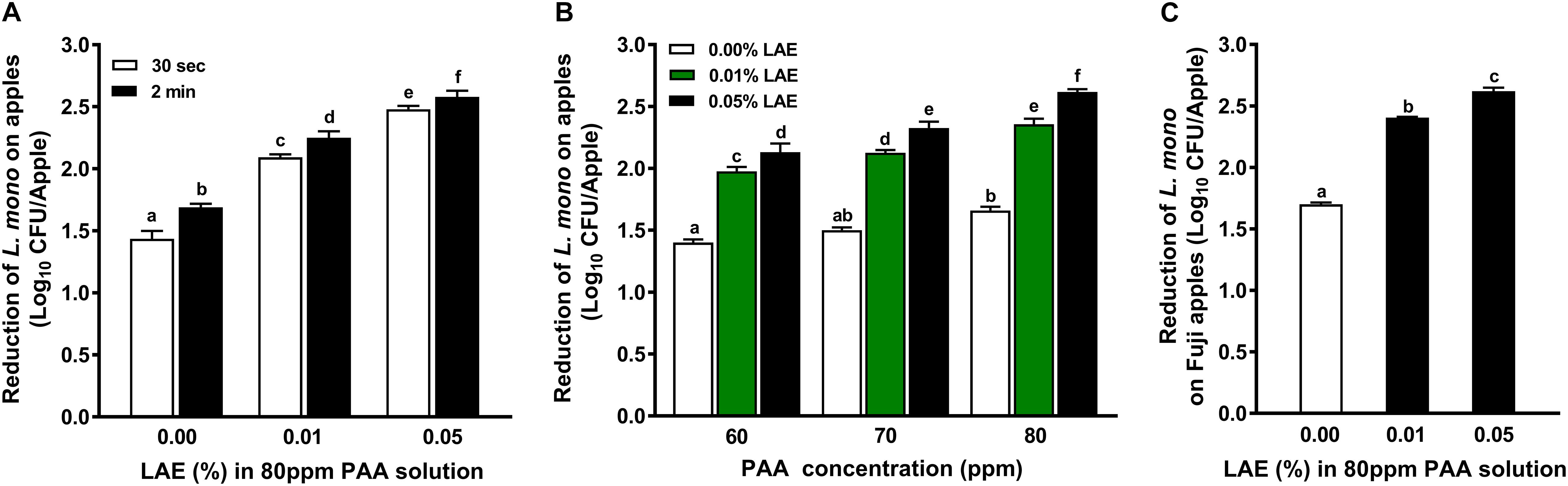
Figure 3. Efficacy of peracetic acid with lauric arginate in reducing L. monocytogenes on apples under different concentrations and contact times at 22°C. (A) Reduction of L. monocytogenes on Granny Smith apples subjected to 80 ppm peracetic acid (PAA) with or without lauric arginate (LAE) for a 30-sec or 2-min contact. (B) Reduction of L. monocytogenes on Granny Smith apples treated with different PAA and LAE combinations for 2 min. (C) Reduction of L. monocytogenes on Fuji apples after a 2-min exposure of 80 ppm PAA with different concentrations of LAE. The initial L. monocytogenes population on apple surfaces was ∼ 6.8 log10 CFU/apple. Mean ± SEM, averaged from three independent experiments; 10 apples per treatment within an independent study. a–fMean among bars without common letters differ significantly (P < 0.05).
Peracetic acid with LAE solutions at 46°C showed higher effectiveness against L. monocytogenes on GSA than that at 22°C (P < 0.05) (Figure 4). A 30–120-sec treatment of PAA at 80 ppm with 0.05% LAE at 46°C reduced L. monocytogenes by 2.90–2.95 log10 CFU/apple (Figure 4). The contact time has a diminished effect on enhancing effects of LAE in PAA solutions at 46°C compared with that at 22°C (Figures 3A, 4). The strengthening effects of 0.05% LAE on anti-Listeria efficacy of PAA, when applied at 46°C, provided an additional 0.54 log10 CFU/apple reduction compared with that at 22°C (Figures 3A, 4).
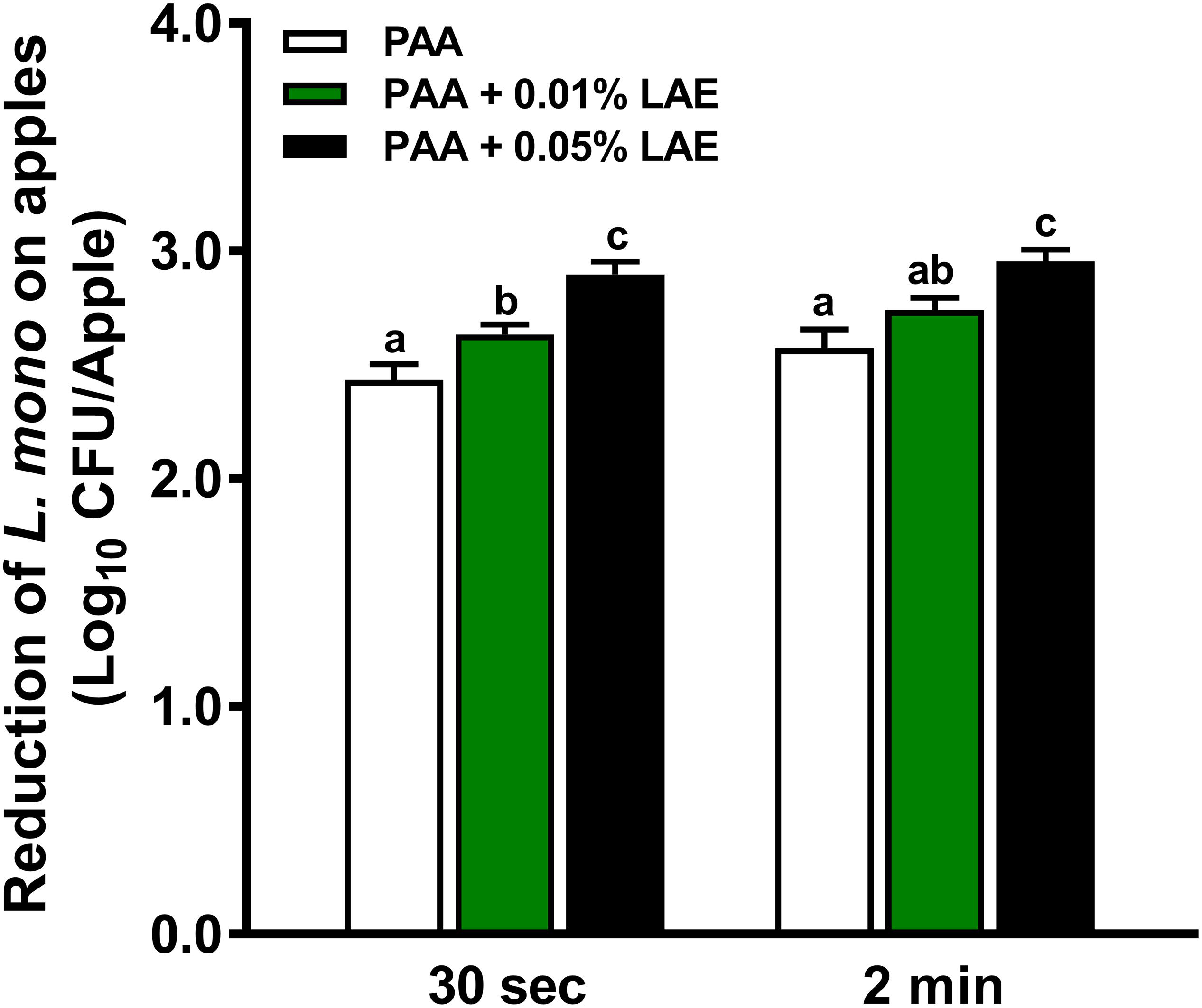
Figure 4. Efficacy of peracetic acid with lauric arginate against L. monocytogenes on apples at 46°C. Granny Smith apples subjected to 80 ppm peracetic acid (PAA) alone or in the presence of lauric arginate (LAE) for 30-sec or 2-min contact time. The initial L. monocytogenes level on apple surfaces was ∼ 6.5 log10 CFU/apple. Mean ± SEM, averaged from three independent experiments; each independent experiment has 10 apples per treatment. a–cMean among bars without common letters differ significantly (P < 0.05).
The antimicrobial efficacies of PAA in combination with LAE against Listeria on fresh apples were further verified in a pilot spray-bar brush bed system. Spray wash of PAA with 0.01 and 0.05% LAE at RT for 30–120 sec reduced Listeria by 1.47–1.64 and 1.68–1.76 log10 CFU/apple, respectively (Table 1). Consistently with the lab-scale testing, the addition of LAE significantly improved the antimicrobial efficacy of PAA solution (P < 0.05) (Table 1). Increasing the temperature of PAA with 0.01 and 0.05% LAE solution from 22 to 46°C further increased (P < 0.05) the efficacies and resulted in 1.83–1.94 and 2.08–2.14 log10 CFU/apple reductions of Listeria for 30–120 sec contact, respectively (Table 1).
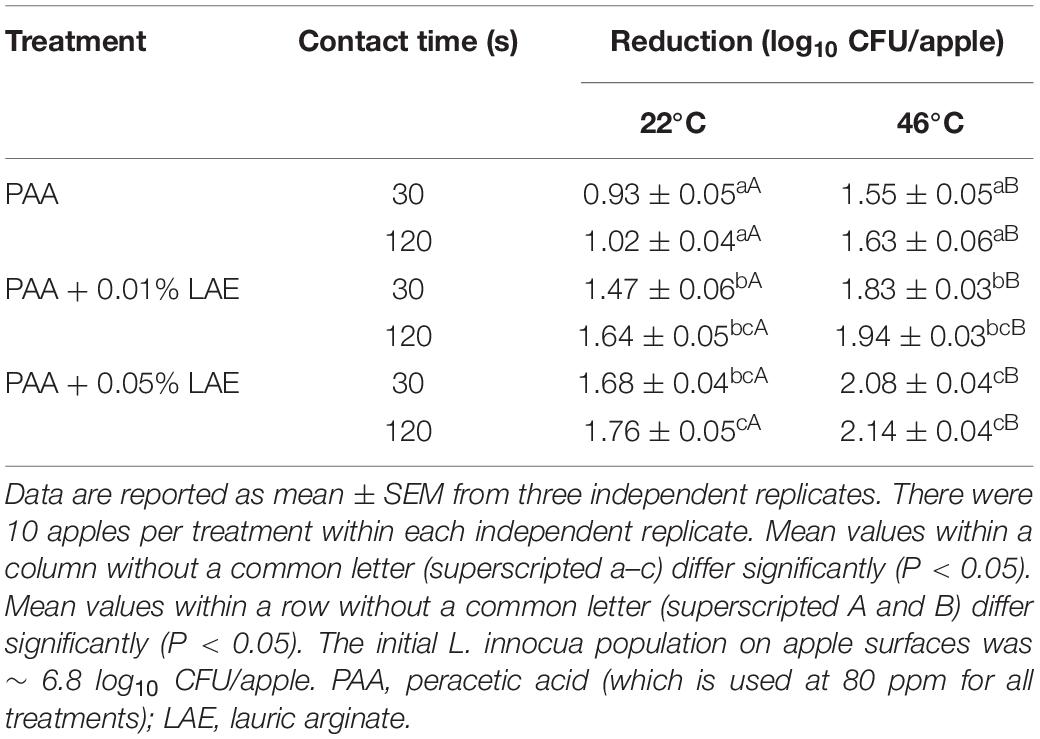
Table 1. Antimicrobial effectiveness of peracetic acid with lauric arginate solution against L. innocua on Granny Smith apples applied in a pilot spray-bar system.
Lauric arginate has received increasing attention in the fresh produce industry due to its antimicrobial activity against L. monocytogenes, used alone or in combination with other antimicrobials. However, the reported effectiveness of LAE against Listeria varied among different studies. For example, a 2-min exposure of 0.01% LAE resulted in 3.1 log10 CFU/g log reduction of L. monocytogenes spot-inoculated on lettuce surfaces with an initial bacterial population of 6.6 log10 CFU/g and treated 15-min after inoculation (Nübling et al., 2017). The application of 0.10% LAE for 20 min reduced L. innocua by 2.3 log10 CFU/cm2 on lettuce surfaces, where L. innocua was spot inoculated on the lettuce surfaces with an initial bacterial load of 6–8 log10 CFU/ml and exposure to LAE treatment 1 h after inoculation (Huang and Nitin, 2017). The antimicrobial strengthening effects of LAE when used with other antimicrobials have been mostly conducted on dairy and meat products (Stopforth et al., 2010; Soni et al., 2012; Ma et al., 2013). The addition of 0.075% LAE with essential oils (cinnamon, eugenol, and thymol oils) caused an additional 2–4 log10 CFU/ml reduction of L. monocytogenes in 2% reduced-fat milk compared with adding essential oils alone after 4-h treatment (Ma et al., 2013). The addition of 0.07% LAE in a mixture of 1.68% potassium lactate and 0.12% sodium diacetate caused an additional ∼ 1.0 log10 CFU/g reduction of L. monocytogenes on cooked cured ham after 24-h exposure at 4°C compared with no LAE control (Stopforth et al., 2010). Nonetheless, a recent study on fresh apples reported that the antimicrobial efficacy of 80 ppm PAA with 0.10% LAE against L. innocua on apple surfaces was not different from no LAE control (Pietrysiak et al., 2019), which was likely due to large standard deviations among replicates associated with that study. Consistent with studies on dairy and meat products, we herein showed that the addition of LAE significantly enhanced the antimicrobial efficacies of PAA solutions against L. monocytogenes in water or on apple surfaces in a concentration-dependent manner. This strengthening effect was further verified in a pilot apple spray-bar brush bed processing line.
The enhanced efficacy of LAE with PAA treatment could be due to their synergic antimicrobial activities. Both PAA and LAE disrupt the lipoprotein cytoplasmic membranes of bacteria and cause the leakage of intercellular components (Maris, 1995; Rodriguez et al., 2004; Becerril et al., 2013). PAA also oxidizes proteins, enzymes, lipids, and DNA by inducing the release of intracellular reactive oxygen species (Leaper, 1984; Maris, 1995; González-Aguilar et al., 2012). Additionally, LAE has an oxidizing property and has been shown to generate oxidative stress against Escherichia coli (Yang et al., 2019), which might be synergistic with PAA in releasing bactericidal reactive oxygen species to activate membrane lipid peroxidation and amplify oxidative damage of DNA, ultimately causing cell death. LAE also can interact with bacterial DNA and result in DNA secondary structure changes (Ma et al., 2016).
Lauric arginate, Tween 80, and Tween 85 are surface-active agents that lower the interfacial tension between a liquid and solid surface and increase the spreading property of a liquid solution (Yu et al., 2001; Huang and Nitin, 2017). Consistently, we showed that including LAE, Tween 80, or Tween 85 in PAA solution increased the spreading property on apple surfaces. Of note, the wettability of LAE is stronger than those of Tween 80 or Tween 85; the PAA solutions with 0.10% LAE and 0.10% Tween 80 or Tween 85 reduced contact angle from 99.1 to 41.6, 76.6, or 72.6°, respectively. PAA with 0.10% Tween 80 or Tween 85 solution had a comparable wettability as PAA with 0.005% LAE, but the antimicrobial strengthening effects of 0.005% LAE was much higher than those of PAA with 0.10% Tween 80 or Tween 85, indicating the enhanced anti-Listeria efficacies of LAE in PAA solutions should be mainly due to bactericidal reactive oxygen species instead of its wettability (Yang et al., 2019). In support of our findings, LAE is more effective against L. innocua on lettuce surface than that of Tween 20 (Huang and Nitin, 2017). Inclusion of 0.10% of Tween 80 in 2.0% citric acid solution failed to increase its antimicrobial efficacy against Salmonella on alfalfa seeds (Weissinger and Beuchat, 2000). In contrast, 80 ppm PAA solution with 0.10% Tween 20 and 0.10% LAE had a similar contact angle on Gala apple surface, but 80 ppm PAA with 0.10% Tween 20 exhibited a better antimicrobial efficacy against L. innocua on apples than that with 0.10% LAE (Pietrysiak et al., 2019).
The extended contact time from 30 sec to 2 min significantly increased the efficacy of PAA with LAE solution at RT, but the magnitude of increment was smaller than that of 80 ppm PAA only (Shen et al., 2019). Increased PAA concentration significantly increased the antimicrobial activities of PAA with LAE solutions against L. monocytogenes on fresh apples, and the effects of PAA concentration are more pronounced in PAA with LAE solutions compared with PAA alone (Shen et al., 2019). This is possibly due to the increased production of bactericidal reactive oxygen species in PAA with LAE solutions compared with PAA alone.
Our previous study showed that the efficacy of PAA against L. monocytogenes on fresh apples was enhanced when it was applied at 46°C, causing an additional 1.02 and 0.89 log10 CFU/apple reduction at a 30-sec or 2-min treatment, respectively, compared with those at RT (Shen et al., 2019). The addition of 0.05% LAE in 80 ppm PAA solution at RT obtained a comparable log reduction of L. monocytogenes as PAA only solution applied at 46°C (Shen et al., 2019). Similarly, the addition of LAE significantly enhanced PAA anti-Listeria efficacy at 46°C, but the strengthening effects were smaller than that applied at RT. The mild heat (Ebrahimi et al., 2018) and LAE (Becerril et al., 2013; Pattanayaiying et al., 2014) caused cellular membrane damages. Thus, the effects of mild heat on the bacterial cell membrane might diminish the strengthening effects of LAE. Spray wash of PAA with LAE in a pilot facility at either 22°C or 46°C was less effective than those applied via lab-scale immersion intervention, which is consistent with our previous finding on PAA intervention alone (Zhu et al., 2020). Similar to our findings, LAE combined with eugenol was less effective in reducing Salmonella on spinach when conducted by spray wash compared with immersion intervention (Ruengvisesh et al., 2015).
Low concentrations of LAE significantly enhanced the effectiveness of PAA against L. monocytogenes on fresh apples. The antimicrobial efficacies of PAA with LAE treatments increased with the increased concentration of PAA or LAE. The most efficacious treatment was a combination of 80 ppm PAA with 0.05% LAE conducted at 46°C. In this scenario, a 2.90 log10 CFU/apple reductions of L. monocytogenes on fresh apples was achieved. Data showed that LAE plus PAA is a practical and viable PAA spray-bar brush bed intervention strategy with enhanced anti-Listeria efficacy for the apple industry to prevent cross contamination of L. monocytogenes of fresh apples, facilitating their compliance with FSMA Preventive Controls requirements.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
XS conducted the experiment, analyzed the data, and wrote the manuscript. JC and JM helped with sample processing. M-JZ and IH revised the manuscript. M-JZ supervised the work, guided the experimental design, analyzed the data, and was in charge of the funding acquisition. All authors contributed to the article and approved the submitted version.
The research activity was partially supported by the Washington State Department of Agriculture (WSDA), the Center for Produce Safety 2017CPS10, and the Washington Tree Fruit Research Commission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to acknowledge Guardian Manufacturing and Pace International for donation and setting up the pilot-scale spray-bar brush bed system, A&B Ingredients, Inc. for providing CytoGuard LA 20, and Pace International Inc. for providing Bioside HS. We acknowledge To Chiu and Tonia Green for their assistance.
Angelo, K. M., Conrad, A. R., Saupe, A., Dragoo, H., West, N., Sorenson, A., et al. (2017). Multistate outbreak of Listeria monocytogenes infections linked to whole apples used in commercially produced, prepackaged caramel apples: United States, 2014-2015. Epidemiol. Infect. 145, 848–856. doi: 10.1017/s0950268816003083
Baert, L., Vandekinderen, I., Devlieghere, F., Van Coillie, E., Debevere, J., and Uyttendaele, M. (2009). Efficacy of sodium hypochlorite and peroxyacetic acid to reduce murine Norovirus 1, B40-8, Listeria monocytogenes, and Escherichia coli O157:H7 on shredded iceberg lettuce and in residual wash water. J. Food Prot. 72, 1047–1054. doi: 10.4315/0362-028x-72.5.1047
Becerril, R., Manso, S., Nerin, C., and Gomez-Lus, R. (2013). Antimicrobial activity of lauroyl arginate ethyl (LAE), against selected food-borne bacteria. Food Control 32, 404–408. doi: 10.1016/j.foodcont.2013.01.003
Dong, X. Q., Rao, J. P., Huber, D. J., Chang, X. X., and Xin, F. C. (2012). Wax composition of ‘Red Fuji’ apple fruit during development and during storage after 1-methylcyclopropene treatment. Hort. Environ. Biotechnol. 53, 288–297. doi: 10.1007/s13580-012-0036-0
Du, J. H., Han, Y., and Linton, R. H. (2002). Inactivation by chlorine dioxide gas (ClO2) of Listeria monocytogenes spotted onto different apple surfaces. Food Microbiol. 19, 481–490. doi: 10.1006/fmic.2002.0501
Ebrahimi, A., Csonka, L. N., and Alam, M. A. (2018). Analyzing thermal stability of cell membrane of Salmonella using time-multiplexed impedance sensing. Biophys. J. 114, 609–618. doi: 10.1016/j.bpj.2017.10.032
FDA (2005). Agency Response Letter GRAS Notice No. GRN 000164. Available online at: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=164. (accessed May 15, 2021)
FDA (2015). Northstar Produce Inc. recalls Granny Smith size 175 apples because of possible health risk. Available online at: https://fdarecall.wordpress.com/2015/10/30/northstar-produce-inc-recalls-granny-smith-size-175-apples-because-of-possible-health-risk/. (accessed May 15, 2021).
FDA (2017a). 21CFR173.315; Chemicals Used in Washing or to Assist in the Peeling of Fruits and Vegetables. Available online at: https://www.accessdata.fda.gov/SCRIPTs/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=173.315&SearchTerm=chemicals. (accessed May 15, 2021)
FDA (2017b). Jack Brown Produce, Inc. recalls Gala, Fuji, Honeycrisp and Golden Delicious Apples due to Possible Health Risk. Available online at: https://www.fda.gov/Safety/Recalls/ucm589722.html. (accessed May 15, 2021)
González-Aguilar, G., Ayala-Zavala, J. F., Chaidez-Quiroz, C., and Heredia, J. B. (2012). “Peroxyacetic acid,” in Decontamination of Fresh and Minimally Processed Produce, ed. V. M. Gómez-López (Hoboken, NJ: John Wiley & Sons), 215.
Huang, K., and Nitin, N. (2017). Enhanced removal of Escherichia coli O157:H7 and Listeria innocua from fresh lettuce leaves using surfactants during simulated washing. Food Control 79, 207–217. doi: 10.1016/j.foodcont.2017.03.032
Infante, R., Dominguez, J. G., Erra, P., Julia, R., and Prats, M. (1984). Surface active molecules: preparation and properties of long chain n-acyl-l-alpha-amino-omega-guanidine alkyl acid derivatives. Int. J. Cosmet. Sci. 6, 275–282. doi: 10.1111/j.1467-2494.1984.tb00385.x
Kang, D. H., and Fung, D. Y. C. (1999). Thin agar layer method for recovery of heat-injured Listeria monocytogenes. J. Food Prot. 62, 1346–1349. doi: 10.4315/0362-028x-62.11.1346
Leaper, S. (1984). Influence of temperature on the synergistic sporicidal effect of peracetic acid plus hydrogen peroxide on Bacillus subtilis SA22 (NCA 72-52). Food Microbiol. 1, 199–203. doi: 10.1016/0740-0020(84)90034-0
Ma, Q., Davidson, P. M., and Zhong, Q. (2013). Antimicrobial properties of lauric arginate alone or in combination with essential oils in tryptic soy broth and 2% reduced fat milk. Int. J. Food Microbiol. 166, 77–84. doi: 10.1016/j.ijfoodmicro.2013.06.017
Ma, Q. M., Davidson, P. M., Critzer, F., and Zhong, Q. X. (2016). Antimicrobial activities of lauric arginate and cinnamon oil combination against foodborne pathogens: improvement by ethylenediaminetetraacetate and possible mechanisms. LWT Food Sci. Technol. 72, 9–18. doi: 10.1016/j.lwt.2016.04.021
Maris, P. (1995). Modes of action of disinfectants. Rev. Sci. Tech. 14, 47–55. doi: 10.20506/rst.14.1.829
McCollum, J. T., Cronquist, A. B., Silk, B. J., Jackson, K. A., O’Connor, K. A., Cosgrove, S., et al. (2013). Multistate outbreak of listeriosis associated with cantaloupe. N. Engl. J. Med. 369, 944–953.
Monarca, S., Richardson, S. D., Feretti, D., Grottolo, M., Thruston, A. D., Zani, C., et al. (2002). Mutagenicity and disinfection by-products in surface drinking water disinfected with peracetic acid. Environ. Toxicol. Chem. 21, 309–318. doi: 10.1002/etc.5620210212
Nakama, Y. (2017). “Chapter 15–Surfactants,” in Cosmetic Science and Technology, eds K. Sakamoto, R. Y. Lochhead, H. I. Maibach, and Y. Yamashita (Amsterdam: Elsevier), 231–244.
Nübling, S., Wohlt, D., Saile, N., Weiss, A., and Schmidt, H. (2017). Antimicrobial effect of lauroyl arginate ethyl on Escherichia coli O157: H7 and Listeria monocytogenes on red oak leaf lettuce. Eur. Food Res. Technol. 243, 879–887. doi: 10.1007/s00217-016-2802-1
Park, S. H., Choi, M. R., Park, J. W., Park, K. H., Chung, M. S., Ryu, S., et al. (2011). Use of organic acids to inactivate Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh apples and lettuce. J. Food Sci. 76, M293–M298.
Pattanayaiying, R., H-Kittikun, A., and Cutter, C. N. (2014). Effect of lauric arginate, nisin Z, and a combination against several food-related bacteria. Int. J. Food Microbiol. 188, 135–146. doi: 10.1016/j.ijfoodmicro.2014.07.013
Pietrysiak, E., Kummer, J. M., Hanrahan, I., and Ganjyal, G. M. (2019). Efficacy of surfactant combined with peracetic acid in removing Listeria innocua from fresh apples. J. Food Prot. 82, 1965–1972. doi: 10.4315/0362-028x.jfp-19-064
Rodgers, S. L., Cash, J. N., Siddiq, M., and Ryser, E. T. (2004). A comparison of different chemical sanitizers for inactivating Escherichia coli O157:H7 and Listeria monocytogenes in solution and on apples, lettuce, strawberries, and cantaloupe. J. Food Prot. 67, 721–731. doi: 10.4315/0362-028x-67.4.721
Rodriguez, E., Seguer, J., Rocabayera, X., and Manresa, A. (2004). Cellular effects of monohydrochloride of L-arginine, N-lauroyl ethylester (LAE) on exposure to Salmonella typhimurium and Staphylococcus aureus. J. Appl. Microbiol. 96, 903–912. doi: 10.1111/j.1365-2672.2004.02207.x
Ruengvisesh, S., Loquercio, A., Castell-Perez, E., and Taylor, T. M. (2015). Inhibition of bacterial pathogens in medium and on spinach leaf surfaces using plant-derived antimicrobials loaded in surfactant micelles. J. Food Sci. 80, M2522–M2529.
Sadekuzzaman, M., Yang, S., Kim, H. S., Mizan, M. F. R., and Ha, S. D. (2017). Evaluation of a novel antimicrobial (lauric arginate ester) substance against biofilm of Escherichia coli O157: H7, Listeria monocytogenes, and Salmonella spp. Int. J. Food Sci. Tech. 52, 2058–2067. doi: 10.1111/ijfs.13484
Shen, X., Sheng, L., Gao, H., Hanrahan, I., Suslow, T., and Zhu, M.-J. (2019). Enhanced efficacy of peroxyacetic acid against Listeria monocytogenes on fresh apples at elevated temperature. Front. Microbiol. 10:1196. doi: 10.3389/fmicb.2019.01196
Shen, X., Su, Y., Hua, Z., Cong, J., Dhowlaghar, N., Sun, Q., et al. (2020). Validation of peroxyacetic acid treatment against L. monocytogenes on fresh apples using E. faecium NRRL B-2354 as a surrogate in commercial spray-bar operations. Food Microbiol. 92:103590. doi: 10.1016/j.fm.2020.103590
Sheng, L., Edwards, K., Tsai, H. C., Hanrahan, I., and Zhu, M. J. (2017). Fate of Listeria monocytogenes on fresh apples under different storage temperatures. Front. Microbiol. 8:1396. doi: 10.3389/fmicb.2017.01396
Sheng, L., Shen, X., Ulloa, O., Suslow, T. V., Hanrahan, I., and Zhu, M.-J. (2020a). Evaluation of JC9450 and neutral electrolyzed water in controlling Listeria monocytogenes on fresh apples and preventing cross-contamination. Front. Microbiol. 10:3128. doi: 10.3389/fmicb.2019.03128
Sheng, L., Shen, X., and Zhu, M.-J. (2020b). Screening of non-pathogenic surrogates of Listeria monocytogenes applicable for chemical antimicrobial interventions of fresh apples. Food Control 110:106977. doi: 10.1016/j.foodcont.2019.106977
Silk, B. J., Mahon, B. E., Griffin, P. M., Gould, L. H., Tauxe, R. V., Crim, S. M., et al. (2013). Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. Morb. Mortal. Wkly. Rep. 62:448.
Soni, K. A., Desai, M., Oladunjoye, A., Skrobot, F., and Nannapaneni, R. (2012). Reduction of Listeria monocytogenes in queso fresco cheese by a combination of listericidal and listeriostatic GRAS antimicrobials. Int. J. Food Microbiol. 155, 82–88. doi: 10.1016/j.ijfoodmicro.2012.01.010
Stopforth, J. D., Visser, D., Zumbrink, R., van Dijk, L., and Bontenbal, E. W. (2010). Control of Listeria monocytogenes on cooked cured ham by formulation with a lactate-diacetate blend and surface treatment with lauric arginate. J. Food Prot. 73, 552–555. doi: 10.4315/0362-028x-73.3.552
Veraverbeke, E. A., Lammertyn, J., Saevels, S., and Nicolai, B. M. (2001). Changes in chemical wax composition of three different apple (Malus domestica Borkh.) cultivars during storage. Postharvest Biol. Technol. 23, 197–208. doi: 10.1016/s0925-5214(01)00128-4
Weissinger, W. R., and Beuchat, L. R. (2000). Comparison of aqueous chemical treatments to eliminate Salmonella on alfalfa seeds. J. Food Prot. 63, 1475–1482. doi: 10.4315/0362-028x-63.11.1475
Yang, X., Rai, R., Huu, C. N., and Nitin, N. (2019). Synergistic antimicrobial activity by light or thermal treatment and lauric arginate: membrane damage and oxidative stress. Appl. Environ. Microbiol. 85: e01033-19..
Yu, K. H., Newman, M. C., Archbold, D. D., and Hamilton-Kemp, T. R. (2001). Survival of Escherichia coli O157 : H7 on strawberry fruit and reduction of the pathogen population by chemical agents. J. Food Prot. 64, 1334–1340. doi: 10.4315/0362-028x-64.9.1334
Keywords: Listeria monocytogenes, peracetic acid, lauric arginate, apples, spray application, temperature
Citation: Shen X, Cong J, Mugendi J, Hanrahan I and Zhu M-J (2021) Synergistic Effects of Lauric Arginate and Peracetic Acid in Reducing Listeria monocytogenes on Fresh Apples. Front. Microbiol. 12:641034. doi: 10.3389/fmicb.2021.641034
Received: 13 December 2020; Accepted: 20 May 2021;
Published: 18 June 2021.
Edited by:
Riadh Hammami, University of Ottawa, CanadaReviewed by:
Krzysztof Skowron, Nicolaus Copernicus University in Toruń, PolandCopyright © 2021 Shen, Cong, Mugendi, Hanrahan and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei-Jun Zhu, bWVpanVuLnpodUB3c3UuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.