
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 04 March 2021
Sec. Aquatic Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.640066
Phagotrophic protists are key players in aquatic food webs. Although sequencing-based studies have revealed their enormous diversity, ecological information on in situ abundance, feeding modes, grazing preferences, and growth rates of specific lineages can be reliably obtained only using microscopy-based molecular methods, such as Catalyzed Reporter Deposition-Fluorescence in situ Hybridization (CARD-FISH). CARD-FISH is commonly applied to study prokaryotes, but less so to microbial eukaryotes. Application of this technique revealed that Paraphysomonas or Spumella-like chrysophytes, considered to be among the most prominent members of protistan communities in pelagic environments, are omnipresent but actually less abundant than expected, in contrast to little known groups such as heterotrophic cryptophyte lineages (e.g., CRY1), cercozoans, katablepharids, or the MAST lineages. Combination of CARD-FISH with tracer techniques and application of double CARD-FISH allow visualization of food vacuole contents of specific flagellate groups, thus considerably challenging our current, simplistic view that they are predominantly bacterivores. Experimental manipulations with natural communities revealed that larger flagellates are actually omnivores ingesting both prokaryotes and other protists. These new findings justify our proposition of an updated model of microbial food webs in pelagic environments, reflecting more authentically the complex trophic interactions and specific roles of flagellated protists, with inclusion of at least two additional trophic levels in the nanoplankton size fraction. Moreover, we provide a detailed CARD-FISH protocol for protists, exemplified on mixo- and heterotrophic nanoplanktonic flagellates, together with tips on probe design, a troubleshooting guide addressing most frequent obstacles, and an exhaustive list of published probes targeting protists.
Protists (unicellular eukaryotes) play a central role in carbon and energy flow and nutrient recycling in aquatic food webs (Azam et al., 1983; Sherr and Sherr, 1988). Small heterotrophic and mixotrophic flagellated (HF and MF, respectively) protists with cell size < 8 μm are the key predators of pelagic prokaryotes (Porter et al., 1985; Šimek and Straškrabová, 1992; Rychert, 2006; Zubkov and Tarran, 2008). They control size distribution and composition of prokaryotic communities by selectively grazing on specific morpho- and phylotypes (Šimek and Chrzanowski, 1992; Šimek et al., 1997b; Jürgens and Matz, 2002; Jezbera et al., 2005, 2006). The trophic role of larger protists (8–20 μm) is far less understood (Arndt and Mathes, 1991; Arndt et al., 2000; Šimek et al., 2020). These apparently omnivorous predators appear ahead of the ciliates in the energy transfer and are the main consumers of bacterivorous protists and pico-sized algae (Piwosz and Pernthaler, 2011; Mukherjee et al., 2017, 2019; Grujčić et al., 2018). These considerably understudied but ecologically highly relevant trophic interactions significantly modulate carbon flow efficiency, nutrient regeneration, and overall trophic cascading within the grazer food chain (Goldman and Caron, 1985; Goldman et al., 1985; Andersen et al., 1986; Arndt et al., 2000; Šimek et al., 2019, 2020). Our review focuses on phagotrophic nanoplanktonic HF and MF (with cell size 3–20 μm; Jürgens and Matz, 2002; Massana, 2011), whose grazing impacts modulate their prey communities, and vice versa, shifts in prokaryotic prey communities rapidly cascade to the predator communities (Šimek et al., 2013, 2020; Grujčić et al., 2018), while our knowledge on these key predator-prey interactions at an individual level is quite limited.
Here, we review how the application of the CARD-FISH technique helped to advance our understanding of microbial trophic interactions and energy fluxes through microbial food webs, with the focus on aquatic nanoplanktonic flagellates (NF). First, we shortly discuss the currently used CARD-FISH protocols for protists and their combinations with other fluorescence-labeling techniques that allow determination of feeding modes and preferred prey at a single cell level in natural environments without any sample manipulation. Subsequently, we demonstrate how these approaches revealed hidden ecological and ecophysiological traits of so far unknown and morphologically almost indistinguishable protistan lineages in marine and freshwater habitats. Based on this novel knowledge that considerably modifies the current views on the carbon fluxes in pelagic environments, we propose an updated model of microbial food webs that more realistically reflects the complex trophic interactions and specific roles of NF. Our overarching aim is to attract more attention of microbial ecologists to the intriguing aspects of protistan ecology that these single-cell resolution approaches enabled to discover, thus considerably advancing our understanding of microbial food web structure and functioning.
The existence of protists was discovered in the 17th century by Antonie van Leeuwenhoek, who was the inventor of a light microscope, the main tool used in ecological studies of these captivating organisms until the late 20th century. This technique provided basic information on the morphology and gave preliminary hints on feeding modes of the most commonly observed and cultured microplanktonic protists (cell size > 20 μm) that possess diverse cell structures and shapes allowing for tentative discrimination of species. Thus, ciliates or dinoflagellates can be microscopically classified, and a large number of planktonic taxa have been described (Foissner and Berger, 1996; Steidinger and Jangen, 1997; Foissner et al., 1999; Hoppenrath et al., 2009, 2014; Madoni, 2011). Consequently, in ecological studies of ciliates, feeding modes of the major bacterivorous and omnivorous taxa can be identified to the level of genus, species or of a genus-like morphotype in environmental samples (e.g., Montagnes and Lynn, 1987; Šimek et al., 2000, 2019; Posch et al., 2015; Šimek and Sirova, 2019).
In contrast, it is far harder to differentiate NF to a species, genus, or even phylum level. The research on their community composition and ecological traits has been largely hindered by virtual lack of distinguishable morphological features of these mostly uncultivated protists (Arndt et al., 2000; Bass and Cavalier-Smith, 2004; Adl et al., 2019). Their enormous phylogenetic diversity is largely hidden behind simple, oval cells usually containing a single nucleus and 1–2 flagella. Only a few NF groups, e.g., cryptophytes, pedinellids, haptophytes, kinetoplastids and choanoflagellates, can be identified by experts via phase contrast, fluorescence microscopy, electron microscopy, or live sample observations (Swale, 1969; Patterson and Larsen, 1991; Boenigk and Arndt, 2002; Sekiguchi et al., 2003; Wasmund et al., 2011; Majaneva et al., 2012; Jeuck and Arndt, 2013; Weber et al., 2017). Consequently, in classical grazing research on heterotrophic prokaryotes, bacterivorous NF were treated as one functional unit, which reacts more or less uniformly to certain environmental factors (Berninger et al., 1991; Sanders et al., 1992; Gasol and Vagué, 1993). Although such microscopy-based studies reported, for example, similar looking small HF with two unequally long flagella as “Spumella-like,” “(Pseudo)Bodo ssp.,” or “Paraphysomonas spp.” functional guilds of key bacterivores in marine and freshwaters, they failed to uncover their phylogenetic affiliation and diversity (Fenchel, 1982; Andersen and Fenchel, 1985; Jürgens and Güde, 1994; Hansen, 1996; Šimek et al., 1997a; Matz et al., 2002). Nevertheless, epifluorescence microscopy, combined with the use of fluorescent food tracers or radiolabeled prey, allowed to recognize smaller flagellated protists (ca. 2–8 μm in size) as the most prominent pelagic bacterivorous protistan groups, omnipresent in both marine and freshwater habitats (Sherr and Sherr, 1988; Chrzanowski and Šimek, 1990; González et al., 1990b; Nygaard and Hessen, 1990; Berninger et al., 1991; Šimek et al., 1997b; Zubkov et al., 1998; Jezbera et al., 2005; Weisse et al., 2016). Moreover, even this simplifying “black box approach,” ranking HF and prokaryotes only as large functional guilds, has brought many indications that bacterioplankton responds to strong HF grazing pressure by multitude of adaptive mechanisms. For instance, selective grazing of HF predators shape morphological and compositional structure of prokaryotes (Chrzanowski and Šimek, 1990; González et al., 1990a; Šimek and Chrzanowski, 1992; del Giorgio et al., 1996; Corno and Jürgens, 2006; Zubkov and Tarran, 2008), resulting in a broad variety of grazing-resistant strategies of prokaryotes (see e.g., reviews by Hahn and Höfle, 2001; Jürgens and Matz, 2002; Pernthaler, 2005) that lead to complete avoidance or a considerable decrease of their grazing-induced mortality rates (Jürgens and Güde, 1994; Šimek et al., 1999, 2001; Langenheder and Jürgens, 2001). However, only the application of FISH techniques to target specific lineages of prokaryotes brought detailed insights to diverse impacts of protistan grazing on prokaryotic morphology and community composition (Šimek et al., 1997b, 2001, 2007; Langenheder and Jürgens, 2001; Jürgens and Matz, 2002; Pernthaler, 2005).
Cultivation approaches and live sample observations (e.g., Arndt et al., 2000; Boenigk and Arndt, 2002; Jeuck and Arndt, 2013; Weber et al., 2017) provided additional details for protists determination and thus also facilitated progress in the field, but have been limited to a restricted number of easily cultivable flagellates and ciliates. Unfortunately, the vast majority of free-living protists cannot be easily isolated and cultivated under close to in situ conditions (Weber et al., 2017), and thus, in fact this bottleneck effect recalls the well-known bacterial story of “great plate count anomaly” (Staley and Konopka, 1985; Lim et al., 1999).
The vast diversity of protists that encompass all the branches of eukaryotic phylogenetic tree (Figure 1A) has been recognized only since the last two decades (Pawlowski et al., 2012; Adl et al., 2019; Burki et al., 2020). The progress in describing the diversity of nanoplanktonic protists has been possible thanks to the development of molecular techniques and their increased application in microbial ecology. The increasing amount of information about their phylogenetic diversity, discoveries of cryptic species and of novel lineages made it evident that the knowledge on their diversity had been superficial (Šlapeta et al., 2006; Caron et al., 2012). These unexpected results have changed not only our perception of protistan diversity, but also of their role in ecosystem functioning and evolution of eukaryotes (Worden et al., 2015; Adl et al., 2019; Burki et al., 2020).
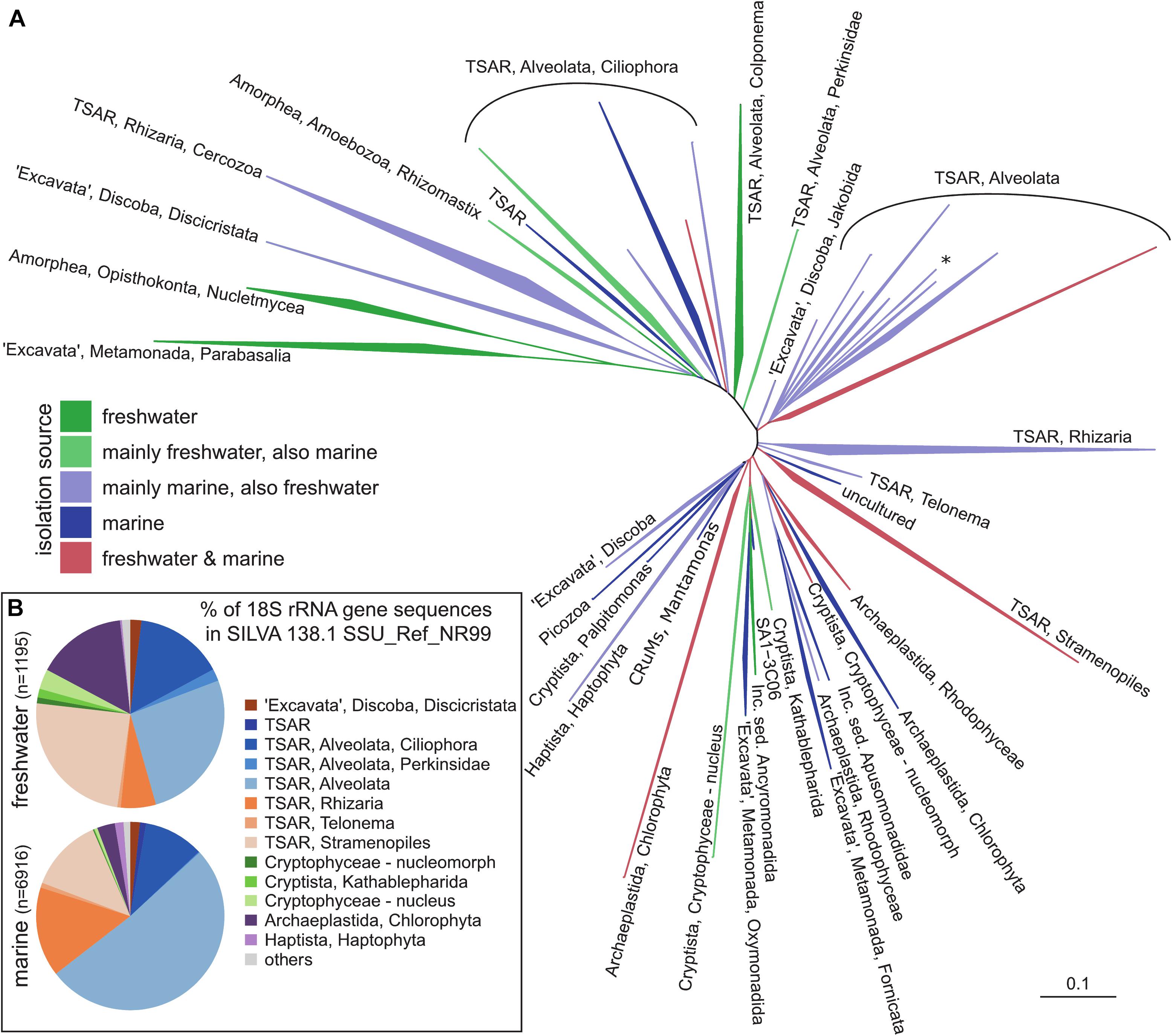
Figure 1. A general phylogenetic tree of protistan groups known to be important in pelagic environments. (A) 18S rRNA phylogenetic guide tree provided by SILVA release 138.1 SSU_Ref_NR99. Only branches containing sequences recovered either from freshwater and marine habitats were included by parsing the isolation_source field for respective entries, higher organisms (plants, animals) have been removed. (B) Taxonomic classification of eukaryotic 18S rRNA gene sequences recovered either from freshwater or marine habitats. The names of the groups follow notation by Adl et al. (2019) and Burki et al. (2020). TSAR – telonemids, stramenopiles, alveolates, and Rhizaria; CRuMs – collodictyonids (syn. diphylleids), Rigifilida and Mantamonas. The asterisk in TSAR, Alveolata indicates a lineage of non-TSAR protists (Archaeplastida, Rhodophyceae, Galdieria).
As in the case of prokaryotes, a gene encoding the small subunit (SSU) ribosomal RNA (18S rRNA) became widely used as a marker gene in community analyses of protists (Figure 1A). Due to its patchy nature of conserved and non-conserved regions, it enables both discrimination between closely related organisms and primer design for specific groups at various taxonomical levels. The first studies by Sanger sequencing of 18S rRNA clone libraries revealed an unexpected diversity and presence of novel lineages of protists from marine ecosystems (Lopez-Garcia et al., 2001; Moon-van der Staay et al., 2001). A wide range of studies followed in various ecosystems such as marine, freshwaters, sediments, soils, and insect guts, to name a few (Edgcomb et al., 2002; López-García et al., 2003; Romari and Vaulot, 2004; Lovejoy et al., 2006; Moon-van der Staay et al., 2006; Stoeck et al., 2006; Ohkuma and Brune, 2010; Piwosz and Pernthaler, 2010; Sauvadet et al., 2010; Scheckenbach et al., 2010; Stock et al., 2012; Medlin et al., 2017; Mukherjee et al., 2017). With the advent of sequencing technologies, Sanger sequencing of clone libraries has been replaced by high-throughput sequencing (HTS, also known as NGS – Next generation sequencing) techniques, such as already obsolete Ion torrent and 454 pyrosequencing (Behnke et al., 2011; Bachy et al., 2013; Egge et al., 2013, 2015; Georges et al., 2014; Balzano et al., 2015; Piwosz et al., 2018), currently the most often used Illumina MiSeq/HiSeq platforms (Logares et al., 2014; de Vargas et al., 2015; Hu et al., 2016), and the most recent PacBio and Oxford Nanopore MinION platforms (Orr et al., 2018; Davidov et al., 2020; Hatfield et al., 2020). HTS enables to process large number of samples at once at low cost, providing qualitative information on composition of protistan communities even up to the species level (Amaral-Zettler et al., 2009; Stoeck et al., 2010; Logares et al., 2014). The Malaspina (Duarte, 2015) and TARA Oceans (Bork et al., 2015) oceanographic circumnavigation campaigns made it evident that the majority of marine protistan lineages remain morphologically unknown, even if they belong to the recognized groups, such as Stramenopiles, Alveolata, Rhizaria, and “Excavata” (Figure 1B, de Vargas et al., 2015; Pernice et al., 2016). Similar pictures also emerged from localized sampling campaigns (Parris et al., 2014; Massana et al., 2015). Highly diverse communities of Dinophyta, Cercozoa, Stramenopiles, and Kinetoplastida were reported from extreme environments like abyssal depths and hydrothermal vents (Sauvadet et al., 2010; Scheckenbach et al., 2010). A novel picture on protistan diversity also emerged from the studies of freshwater and brackish habitats (Figure 1B), which discovered an unexpected diversity of the well-known groups: Cryptophyta, Stramenopiles, and Choanozoa (del Campo and Massana, 2011; Simon et al., 2015a, b; Grossmann et al., 2016; Hu et al., 2016; Grujčić et al., 2018; Piwosz et al., 2018). One of the biggest surprises from these studies was the discovery of highly diverse marine lineages of diplonemids (Flegontova et al., 2016), with a recently discovered lineage also in freshwaters (Mukherjee et al., 2020). Similarly, another member of the excavate protists, kinetoplastids, were reported to be highly diverse and abundant in the deep waters of both oceans and freshwater lakes (von der Heyden and Cavalier-Smith, 2005; Scheckenbach et al., 2010; Salani et al., 2011; Flegontova et al., 2018; Mukherjee et al., 2019). Novel lineages were discovered within well-known groups, such as the freshwater CRY1 lineage of cryptophytes (Shalchian-Tabrizi et al., 2008). Finally, Perkinsozoa (Mangot et al., 2012; Taib et al., 2013) and Telonemia were also found to be widely distributed in both marine and freshwaters (Shalchian-Tabrizi et al., 2007; Brate et al., 2010; Triado-Margarit and Casamayor, 2012; Simon et al., 2015b). However, the ecological role of most of these novel groups is not yet well understood.
To circumvent this lack of ecophysiological information, HTS sequencing studies have been combined with advanced statistical analyses, e.g., multivariate or co-occurrence networks (Steele et al., 2011; Cram et al., 2013; Guidi et al., 2016). Such correlative approaches provide only indirect hints about the biotic and abiotic interactions that may be crucial for structuring microbial communities over time and space (Posch et al., 2015; Qu et al., 2021), but may enable informed decision on the microbial groups to be targeted by CARD-FISH. However, the relative ease of obtaining sequencing data from large amounts of samples at once compared to analyzing individual samples by laborious microscopic methods resulted in a disproportional boom in diversity research. Unfortunately, the rapid progress in sequence-based investigation has not been accompanied by an increase in studies on other aspects of protistan ecology, such as morphology, abundances, feeding modes, or trophic roles of new lineages (Stern et al., 2018). Most strikingly, for most recovered sequences, neither the organisms themselves have been visualized yet nor their abundance and distribution have been quantified. In addition, much more efforts have been put on prokaryotes than on protists, although the latter play key roles as primary producers, predators or parasites. This evident imbalance in the research progress represents likely one of the largest knowledge gaps in the field, which justifies also the timing of this review.
Moreover, the quantitative accuracy of HTS data is compromised by the uneven effectiveness of DNA extraction protocols (Martin-Laurent et al., 2001), biased efficiency of PCR amplification (Hansen et al., 1998), incomplete coverage of primers across phylogenetic groups (Klindworth et al., 2013; Mukherjee et al., 2015), and a highly variable number of 18S rDNA gene copies in protists ranging from 1 in picoplanktonic Nannochloropsis salina (Eustigmatophyceae) to 315,000 in the peritrich ciliate Vorticella sp. (Zhu et al., 2005; Gong et al., 2013). Moreover, high intra-genomic diversity of 18S rRNA gene sequences (i.e., the same cell can harbor multiple variants of 18S rRNA genes beyond the species level) can artificially inflate the diversity of some protistan groups (Caron and Hu, 2019; Mukherjee et al., 2020). The relative abundances of specific protistan phylotypes obtained by HTS poorly correspond to their relative abundances in the original samples (Figure 2), and provide only a limited possibility to conduct hypothesis-driven research on the ecology of particular protistan taxa (Pitsch et al., 2019; Piwosz et al., 2020). In results, little is known about the ecology of majority of the newly discovered protistan lineages. Even the latest single cell sequencing or metagenomic techniques cannot yet provide ecological data on uncultured protistan lineages due to technological obstacles and the knowledge gaps in eukaryotic genomics (Seeleuthner et al., 2018).
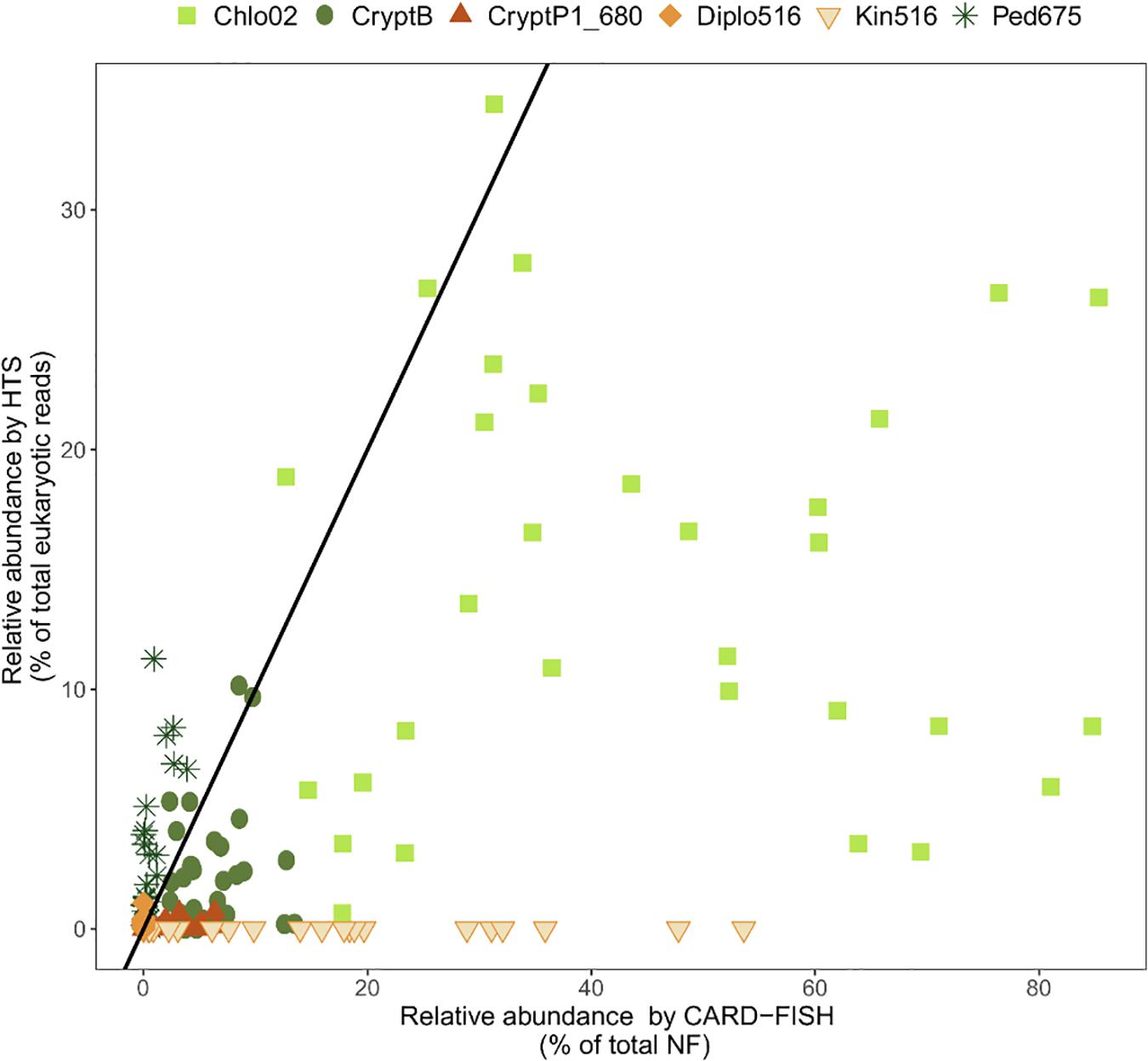
Figure 2. Relationships between relative abundance of selected protistan lineages from high throughput sequencing (HTS) and CARD-FISH. Black line indicates a perfect 1:1 correspondence between the two methods. Points above this line indicate overrepresentation, and below the line underrepresentation of a phylotype by HTS. Chlo2, chlorophytes hybridizable with Chl02 probe; CryptB, cryptophytes hybridizable with CryptB; CryptP1_680, CRY1 cryptophytes hybridizable with CryptP1_680 probe; Diplo516, diplonemids hybridizable with Diplo516 probe; Kin516, kinetoplastids hybridizable with Kin516 probe; Ped675, pedinellids hybridizable with Ped675 probe. Detail on the probes can be found in Supplementary Table 1. Data on Chlo02, CryptB, CryptP1_680 and Ped675 are from Piwosz et al. (2020), on Diplo516 from Mukherjee et al. (2020), and on Kin516 from Mukherjee et al. (2019).
The hindrances caused due to the sole use of sequencing techniques to address ecological questions can be solved by complementary use of microscopy-based molecular methods, such as fluorescence in situ hybridization (FISH) (Amann et al., 1995). FISH and its improved version with enzymatic signal amplification (catalyzed reporter deposition “CARD-FISH,” known also as tyramide signal amplification “TSA-FISH”), provide estimates of relative abundance (a percent contribution to total eukaryotic numbers) of individual microbial lineages defined by their rRNA gene phylogeny (Not et al., 2002; Pernthaler et al., 2004). The key steps in the CARD-FISH protocol are depicted in Figure 3. CARD-FISH provides up to 200-fold brighter signals than FISH with monolabeled probes, enabling detection of almost inactive cells with a low number of ribosomes (Lim et al., 1993). It has become a verified quantitative tool in numerous studies on prokaryotic communities, providing hints on their ecological niche, functions, and interactions (Amann and Fuchs, 2008). However, its use for eukaryotes has been less common (e.g., Not et al., 2005, 2008; Massana et al., 2006a; Piwosz and Pernthaler, 2010; Unrein et al., 2014; Mukherjee et al., 2015). The main advantage of CARD-FISH over the HTS methods is that the relative abundance of a particular lineage can be evaluated independently from all other taxa in the samples. Moreover, the CARD-FISH procedure can be separately optimized for each target group (probe), which is not possible for PCR with primers that target many different templates. It can be also combined with results from direct enumeration methods, such as microscopy or flow cytometry, providing absolute abundance estimates of microbial lineages in the samples. Finally, simultaneous use of CARD-FISH and double CARD-FISH with fluorescent tracers and food vacuole content observations provides information on grazing rates, prey-selectivity, taxonomic information on bacterial, archaeal and eukaryotic prey, and ultimately trophic roles of specific protistan lineages (Jezbera et al., 2005; Chambouvet et al., 2008; Piwosz and Pernthaler, 2010, 2011; Ballen-Segura et al., 2017; Grujčić et al., 2018; Šimek et al., 2020). Double CARD-FISH is a powerful tool for examining bacterivory and omnivory by combining two probes at different trophic levels, targeting protistan predators as well as their prey in food vacuoles (both prokaryotes or other protists). This combination gives new insights into predator–prey interactions, directly demonstrating which bacteria or small protists are preferentially consumed and which groups of flagellates are their grazers in aquatic ecosystems (Piwosz and Pernthaler, 2010, 2011; Grujčić et al., 2018). Further, this method can be applied to identify symbiotic relationships between pro- and eukaryotic microbes (Chambouvet et al., 2008; Dirren et al., 2014; Lepère et al., 2016).
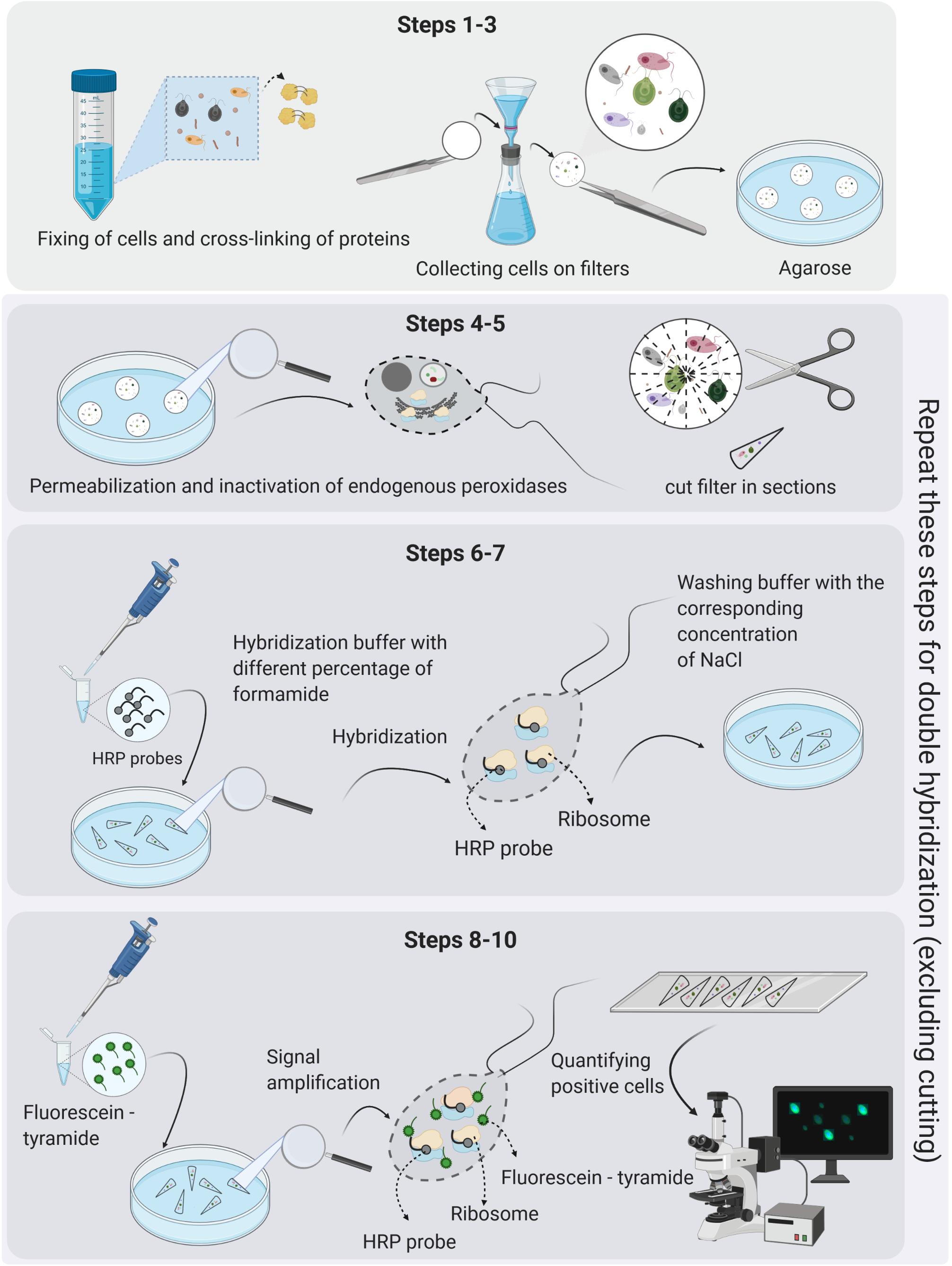
Figure 3. The key steps in the CARD-FISH and double CARD-FISH procedures. The detailed protocol is given in Supplementary File 2, and a single page printable version in Supplementary File 3. Figure created with BioRender.
The main strength of the FISH technique is that it is based on SSU rRNA gene phylogeny, and thus, it allows for the direct detection of phylotypes, the presence of which was identified in the samples by sequencing. Probes for novel lineages discovered with the HTS methods can be designed using full-length sequences of the 18S rRNA genes that are identical or have high similarities to short reads obtained via HTS (Piwosz, 2019), obtaining full-length sequence with amplicon-based specific and general eukaryotic primers combined with Sanger sequencing (Gimmler and Stoeck, 2015), or with Nanopore or PacBio long-read sequencing of amplicons (Orr et al., 2018; Davidov et al., 2020; Hatfield et al., 2020). Regions of 18S rRNA unique to the selected, monophyletic group of interest can be then identified in an alignment and a robust phylogeny, at best a phylogenetic inference using the maximum likelihood (ML) criterion (Felsenstein, 1981). Such approach makes it possible to visualize the morphology of novel phylogenetic lineages (Figures 4–6), estimate their abundance and biomass, determine their role in food webs by inspecting food vacuole contents (Figures 4–7), and also reveal symbiotic interaction between different protist species (Figures 4I–L,O), and protists and prokaryotes (Figures 5A–H,P–T, 6A–F,M–Q) (Chambouvet et al., 2008; Dirren et al., 2014; Schulz et al., 2014; Lepère et al., 2016; Piwosz, 2019; Šimek et al., 2020). This powerful approach has completely changed our views on abundance, functions, and dynamics of microbial populations in different environments. We strongly encourage researchers interested in protistan ecology to explore CARD-FISH in combination with other ecological approaches (see examples in Figures 4–7), as they provide taxonomic resolution of trophic interactions between different microbes and address the question “who eats whom” in microbial food webs at a single cell level. Below, we provide a brief discussion of CARD-FISH protocols and an overview of new insights into protistan ecology facilitated by this technique, a detailed description of all steps can be found in Supplementary Files 1–5.
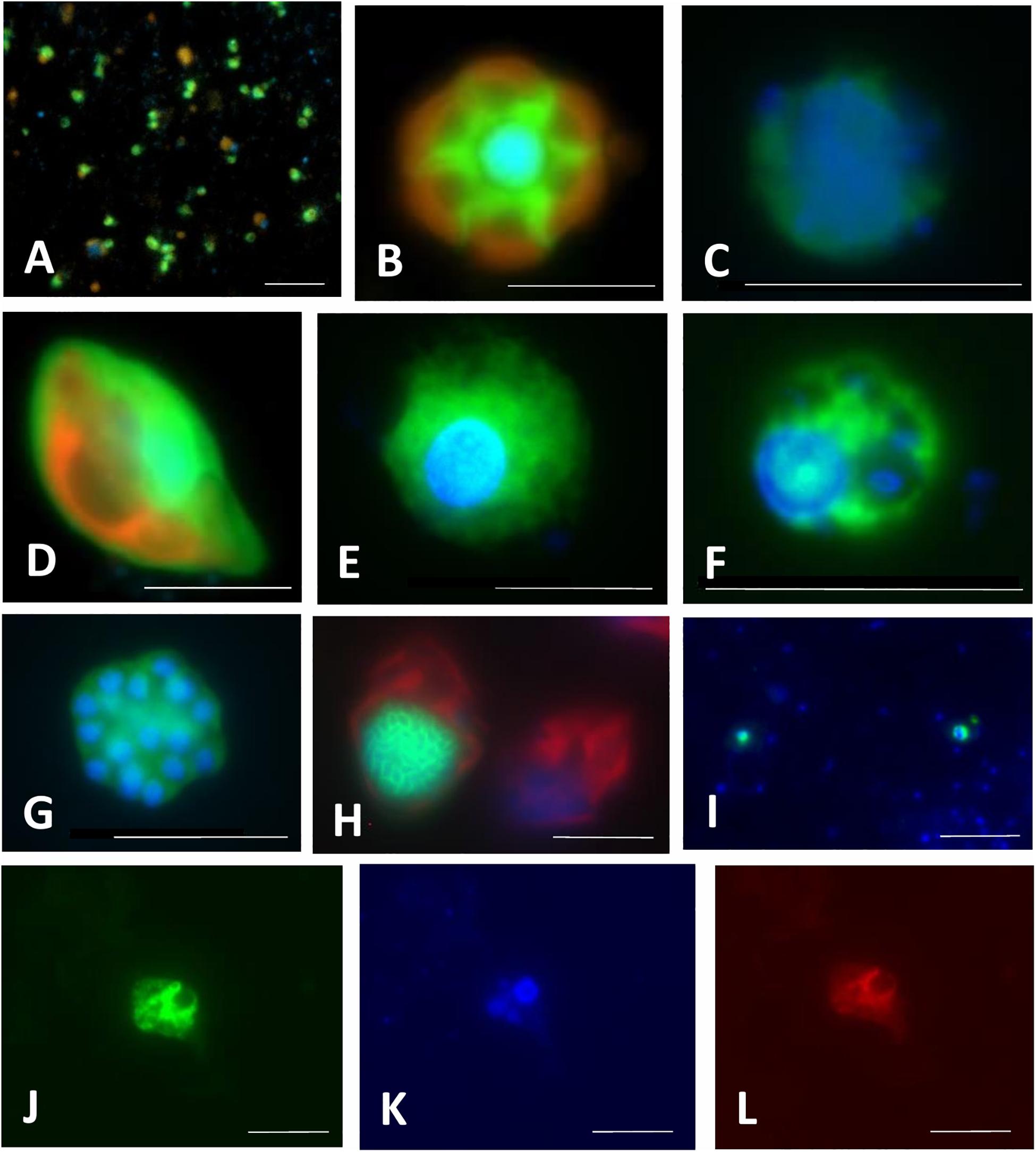
Figure 4. Microphotographs showing typical flagellate size and morphology of cells targeted by CARD-FISH probes from brackish waters of the Baltic Sea (A–H) and freshwater lakes (I–L). Shown are overlay images of DAPI-stained flagellate nuclei (blue), FITC or Alexa488 stained flagellates targeted by probes (green) and chlorophyll autofluorescence (orange/red), except for (J–L), where individual pictures of different channels are shown. (A) Picoplanktonic chlorophytes (probe Chlo02) and other unhybridized algae; (B) plastidic pedinellid (probe Ped675); (C) aplastidic pedinellid (probe Ped675); (D) plastidic cryptophyte (probe Crypto B); (E,F) large and small morphotypes of MAST-6 stramenopiles, respectively (probe MAST-6); (G) parasitic Syndiniales (probe Alv_Bal02): a trophont outside a host, (H) parasitic Syndiniales (probe Alv_Bal02): a trophont inside a host cell (the dinoflagellate Heterocapsa triquetra); (I) tiny perkinsozoans (probe PERKIN_01); (J–L) a diplonemid cell hybridized with probe DiploR1792 (green) (J); corresponding DAPI staining (blue) (K) and double CARD-FISH with general eukaryotic probe [Euk1209, Alexa546 (red), L]. Scale bar = 10 μm.
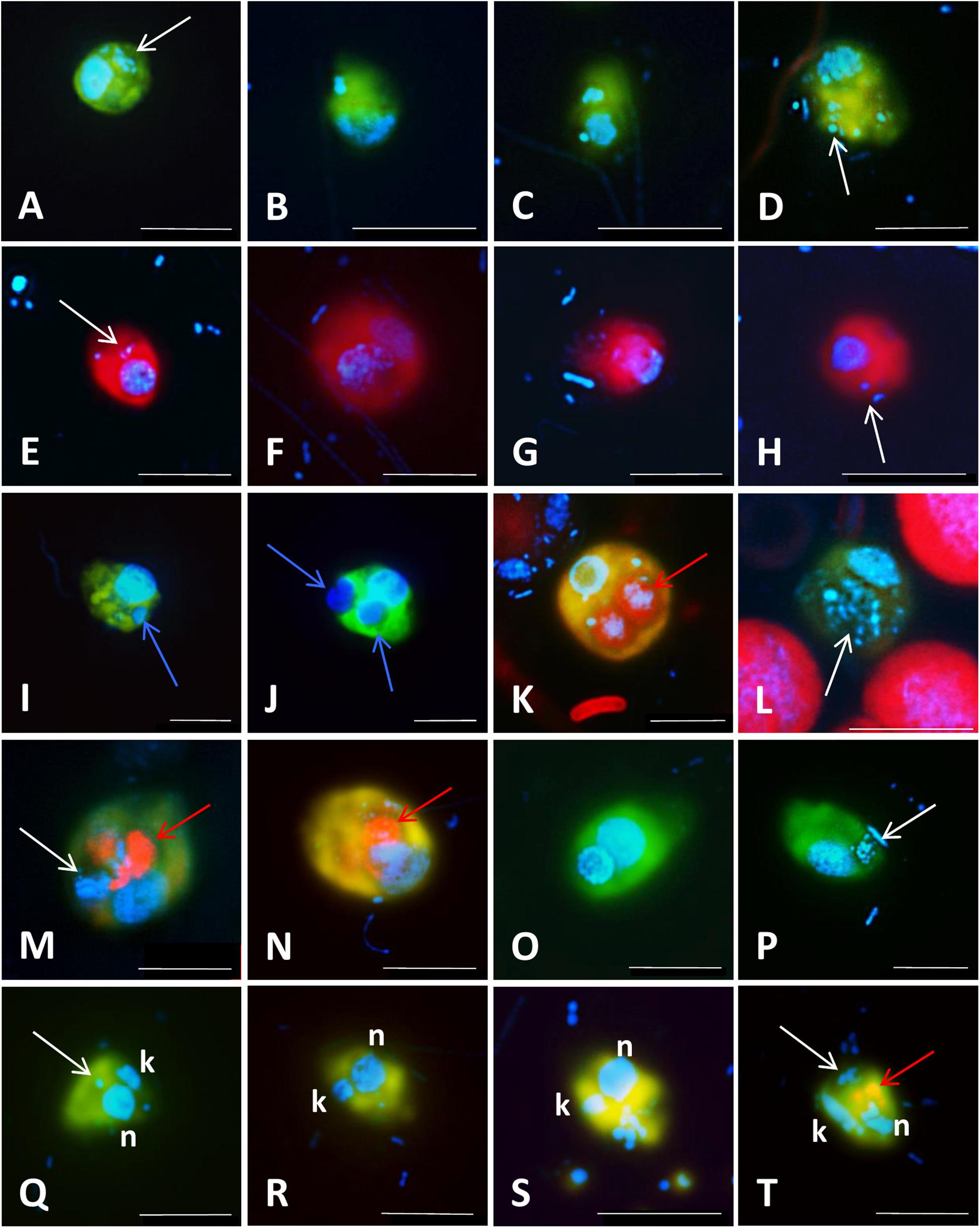
Figure 5. Microphotographs showing food preferences, i.e., bacterivory (A–H,M,P–T), predatory (I,J), or omnivory (M,N,P) of freshwater flagellates from different eutrophic ponds (A–D,I–T) and from mesotrophic Římov reservoir (E–H) in Czech Republic. Shown are overlay Z-stack images (following the methods described in Šimek et al., 2019) of flagellates targeted by probes [A–D,I–T: FITC-stained flagellates (yellow); E–H: Alexa546-stained flagellates (red)], DAPI-stained bacteria and flagellate nuclei (blue) and autofluorescence of algae and cyanobacteria (red). (A–D) Heterotrophic lineage of Cryptophyta (probe Cry1-652) with ingested bacteria; (E–H) other Cryptophyta (general probe Crypto B) with ingested bacteria; (I,J) Cercozoans of Novel Clade 7 (probe Cerc-193) with ingested flagellate prey or their cell remains; (K,L) other Cercozoa (general probe Cerc-02) with ingested cyanobacterial cells of Microcystis (K) or bacteria (L); (M–P) Katablepharidacea (probe Kat-1452) with ingested algal (M,N) or bacterial prey (P); (Q–T) kinetoplastids (probe Kin516) with visible DAPI-stained nucleus (n), kinetoplast (k), and ingested bacteria. White, blue, and red arrows highlight examples of ingested bacteria (A–H,L,M,P,Q,T), flagellate prey (I,J) and algae and cyanobacteria (K,M,N,T), respectively, visible in the grazer food vacuoles. Two arrows (images M,N,T) point to parallel appearance of algae and bacteria in food vacuoles of the same flagellate cells, thus indicating omnivory of the grazer. The scale bar shows length of 5 μm in all images except for (F,I,J,M), with the scale bar showing 10 μm.
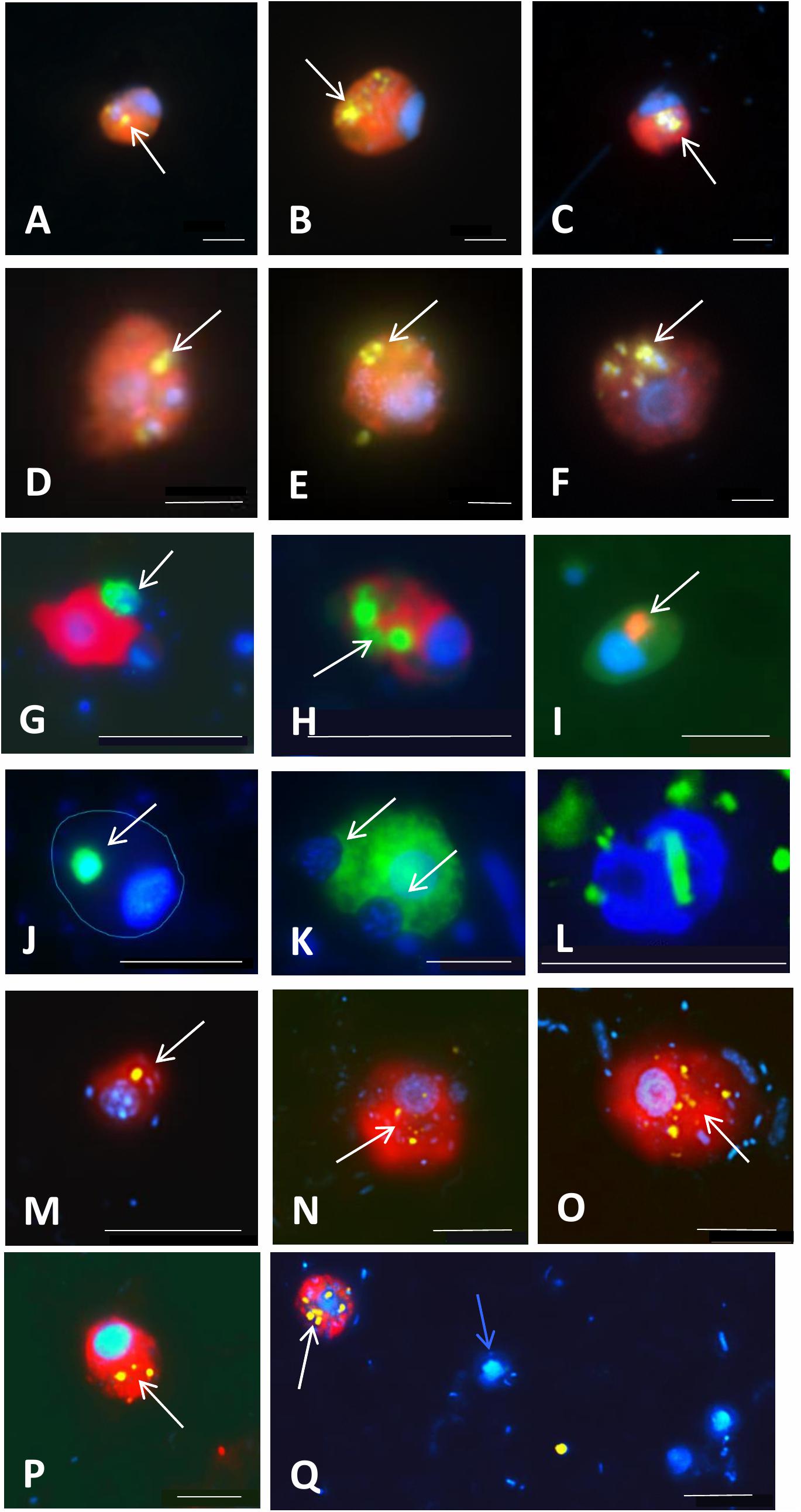
Figure 6. Examples of application of double CARD-FISH (A–H), food vacuole content observations (I–K), or a combination of CARD-FISH and uptake of fluorescently labeled bacteria (FLB; L–Q) to examine food preferences or bacterial uptake rates in freshwater and brackish environments. Double CARD-FISH of (A–C) aplastidic CRY1 lineage of Cryptophyta [probe Cry1-652, (red)]; and (D–F) aplastidic Cryptophyta [probe Crypto B, (red)] feeding on various Betaproteobacteria (yellow) (Grujčić et al., 2018); (G) Cercozoa [probe Bal_02, (red)] feeding on other Cercozoa [probe Bal_01, (green)]; (H) Cercozoa [probe Bal_02, (red)] feeding on prymnesiophytes [probe Prym02, (green)]; (I) Cercozoa [probe Bal_02, (red)] grazed by an unidentified protist; (J) MAST-6 stramenopile [probe MAST-6, (green)] grazed by an unidentified protist (cell shape indicated with blue line); (K) MAST-6 stramenopile [probe MAST-6, (green)] engulfing algae. Each image is an overlay of three pictures of the same flagellate cell observed under ultraviolet excitation (showing the blue nucleus after DAPI-staining), green light excitation (red color corresponding to different flagellate groups labeled with Alexa546 using CARD-FISH) and blue light excitation (yellow-green color corresponding to ingested Betaproteobacteria labeled with FITC using CARD-FISH and green color corresponding to ingested protists labeled with Alexa488 using CARD-FISH). Grazing on FLB [yellow] by (L) Cercozoa [probe Bal_01, labeled with Alexa350 (blue)]; and aplastidic cryptophytes [probe Crypto B, (red)] (N–Q) and CRY1 lineage of Cryptophyta [probe Cry1-652, (red)] (M)]. White arrows indicate ingested prokaryotes and eukaryotes targeted by CARD-FISH probes or FLB in food vacuoles of grazer cells. A blue arrow indicates a non-target flagellate cell close to a Cry1-positive cell with ingested FLB (Q). Scale bar = 2 μm (A–F), 10 μm (G–L), and 5 μm (M–Q).
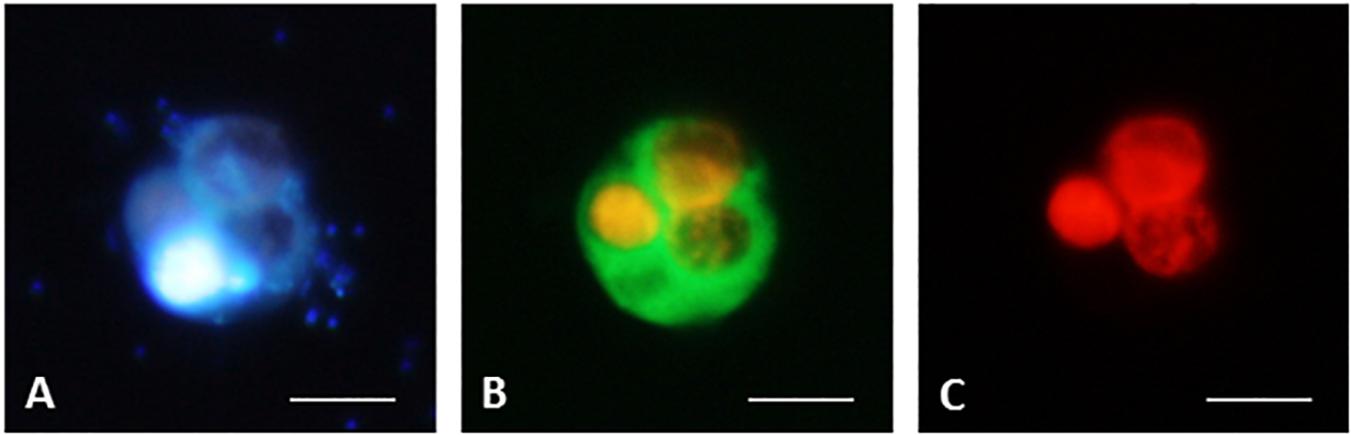
Figure 7. CARD-FISH preparations of ciliate B. planctonicum with the probe Bal-651 (Bühler, 2020). Pictures taken with an epifluorescence microscope using appropriate filter setting. (A) Cells stained with DAPI; (B) Cells at blue light excitation to visualize the green hybridization signal. Orange color comes from autofluorescence of ingested Cryptomonas sp.; (C) Cells excited with green light to show autofluorescence of ingested Cryptomonas sp. Scale bars = 10 μm.
In general, fluorescence in situ hybridization employs oligonucleotide probes that target short regions (usually 15–25 nucleotides in length) of rRNA genes and bind to a specific sequence of rRNA molecules in intact ribosomes. The first step of the CARD-FISH approach is selecting an already published probe or designing a novel probe for the lineage of interest based on a phylogenetic tree. Although most probes have been so far designed for bacteria and archaea, protists can be targeted as well, requiring only slight modifications in the general probe-design process described in great detail by Hugenholtz et al. (2001) and in Supplementary File 1. Table 1 provides basic information about general probes targeting main protistan lineages, while Supplementary Table 1 gives a comprehensive list of published probes targeting diverse protistan lineages and an evaluation of their specificity and coverage. The probes are labeled with a fluorescent dye, such as CY3 and FITC, or with horseradish peroxidase (HRP) that amplifies the signal from fluorescently labeled tyramides via catalyzed reporter deposition (CARD). The signal from the hybridized probes can be subsequently visualized via epifluorescence microscopy or less frequently by flow cytometry (Amann and Fuchs, 2008). The CARD-FISH protocol itself consists of 10 steps (Figure 3), discussed in detail in Supplementary Files 2–5.
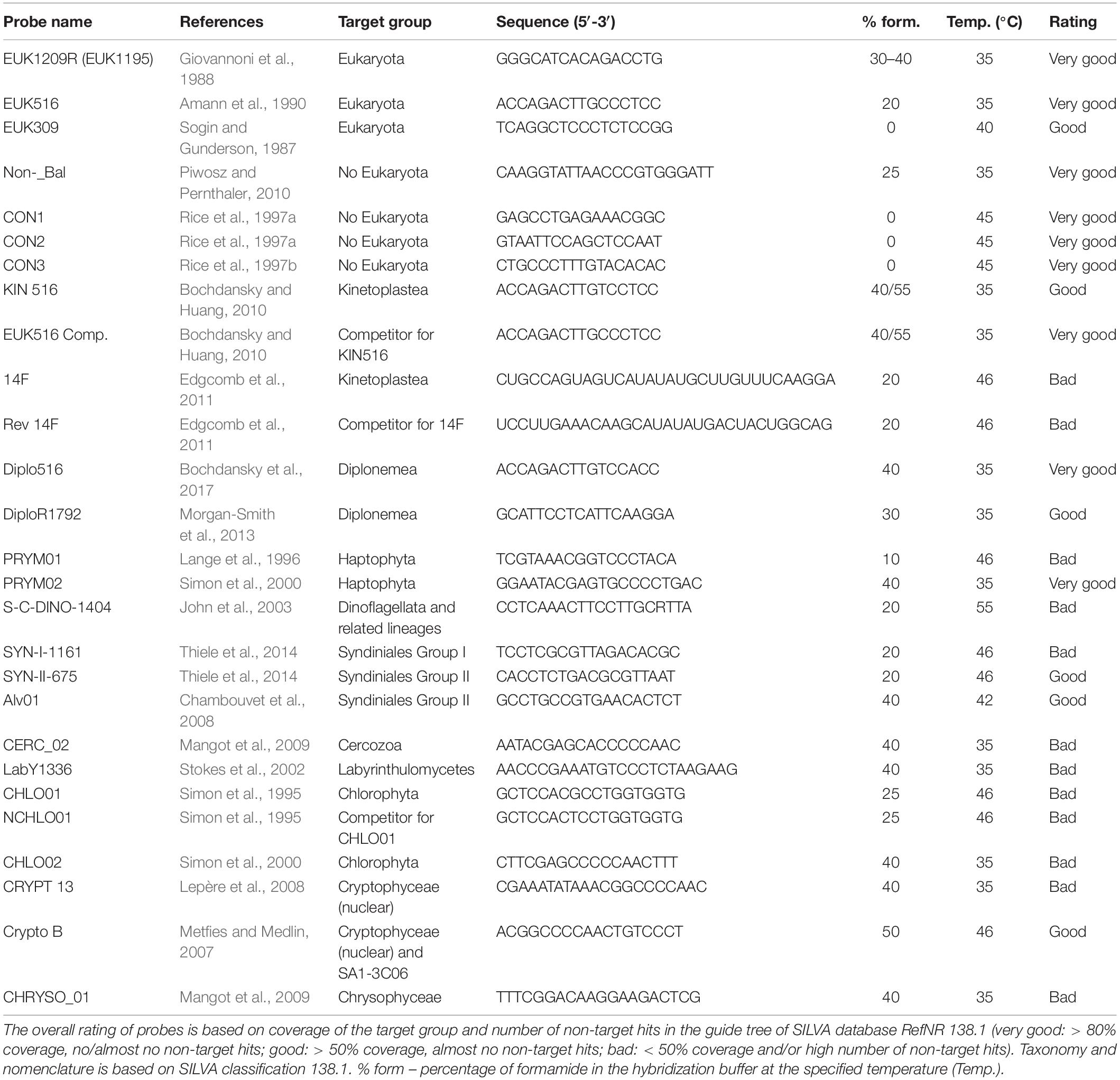
Table 1. List of oligonucleotide probes developed for protistan lineages, hybridization conditions, and overall recommendation for usage based in their current coverage and specificity (see Supplementary Table 1 for details and additional almost 100 probes for more specific protistan lineages).
Sample fixation is the key step for the preservation of protistan cells for the CARD-FISH procedure (Figure 3, step 1). It increases cell permeability, which can be a critical factor for protists with thick and/or complex cellular structures (Ku and Sebé-Pedrós, 2019). Insufficiently fixed cells will degrade and rupture during filtration, while harsh and highly concentrated fixatives can shrink and deform cells (Fried et al., 2002), eventually resulting in the ejection of particles ingested in their food vacuoles (Sherr et al., 1989). More information on different fixatives is available in Supplementary File 2. However, we recommend fixing samples with the neutral Lugol’s solution (final concentration 0.5%) for max. 1 min, followed by an immediate addition of particle-free formalin or PFA (final concentration 1–2%), and complete decolorization with a few drops of 3% sodium thiosulphate solution. Samples preserved this way must not be stored longer than 1 h at room temperature or 24 h at 4°C before filtration.
Samples for CARD-FISH should be filtered with low vacuum underpressure onto white polycarbonate filters that retain microbial cells on their surface (Figure 3, step 2). The filtered sample volume needs to be carefully adjusted to avoid multiple layers of cells, which would hamper microscopic analysis and may cause detachment of cells during embedding in agarose (Figure 3, step 3). The latter step minimizes cell loss during the follow up sample handling steps and incubations in buffers required for the CARD-FISH procedure. So prepared filters can be piled up in a Petri dish and stored at –20°C or at –80°C at least for a few months to years.
In contrast to prokaryotic cells (Pernthaler et al., 2002), enzymatic permeabilization is not required for hybridization of some protists (Bochdansky and Huang, 2010), and it is enough to simply dip the filters into 0.01 M HCl solution, which at the same time inactivates endogenous peroxidase (Figure 3, steps 4–5). We obtained strong signals without additional enzymatic pretreatment for protists with thick cellulose cell walls like chlorophytes (Figure 4A), scales-bearing flagellates such as pedinellids (Figures 4B,C) or those having periplasmic scales such as cryptophytes (Figure 4D). However, obtaining good signals from both the predatory NF and prey (prokaryotes or other protists) in their food vacuoles using double CARD-FISH method depends largely on the permeabilization of the cells so that probes can enter, and this can sometimes be quite tricky (Keeling, 2019; Ku and Sebé-Pedrós, 2019).
Stringent hybridization and washing conditions are of crucial importance for obtaining the specific binding of probes. They need to be experimentally optimized for each probe. Stringency of the hybridization at a certain temperature is determined by the concentration of formamide in the hybridization buffer and an appropriate concentration of NaCl in the washing buffer (Pernthaler et al., 2004). The standard duration of the hybridization step (Figure 3, step 6) is 3 h (Not et al., 2002; Piwosz and Pernthaler, 2010), but its prolongation (even up to 4 days) may improve the signal intensity from less active cells (Lim et al., 1993; Amann and Fuchs, 2008; Morgan-Smith et al., 2013; Mukherjee et al., 2015). The washing step (Figure 3, step 7) is done at temperature 2°C higher than the hybridization. It removes probes weakly hybridized to non-target cells and also non-hybridized probes, so they do not produce false positive signals during the CARD step.
Catalyzed reporter deposition or tyramide signal amplification (TSA; Figure 3, step 8) enhances the fluorescent signals by deposition of a large number of fluorescently labeled tyramines (Bobrow et al., 1991). There is a wide array of available dyes, but those emitting green light (emission maximum at 520 nm), such as Fluorescein isothiocyanate (FITC) or Alexa488, are the most commonly used (see Figures 4–6). In double CARD-FISH, two probes targeting two different microbial populations are used subsequently in the same filter section on the same microscopic slide. The choice of fluorescent colors is quite critical for visualization and differentiation of targeted cells with this approach. We advise, based on our experience, to use brighter green or yellow colors for detection of prey in the food vacuoles, and darker blue, orange, or red colors for HF or blue or far red for MF that show chlorophyll autofluorescence in orange and red. The available dyes are discussed in Supplementary File 2. Examples of the well distinguishable color combinations are shown in Figures 6A–H.
Hybridized and dried filter pieces need to be counterstained with a DNA dye, such as commonly used DAPI stain (Coleman, 1980; Porter and Feig, 1980), which can be added to a glycerol containing anti-fading mixture like Vectashield (Vector laboratories), used to mount the samples on microscopic slides and to reduce the signal fading (Figure 3, step 9). Hybridized protistan cells are counted as percent of all protists detected with a general nucleic acid stain. Independent total NF counts are required to estimate absolute numbers of the targeted HF and MF lineages. However, the absolute number of protists should be assessed from counts obtained with general eukaryotic probes, because nuclei of small protists can be mistaken for prokaryote cells (Beardsley et al., 2005; Pernice et al., 2014). Moreover, the contribution of specific lineages to higher taxonomic levels can be estimated as well. Counting precision (expressed as 95% confidence limits) depends on the number of counted hybridized cells and the number of counted fields of view. It is ±20% for 100 counted cells, 10% for 400 cells, and 6% for thousand cells (Edler and Elbrächter, 2010). However, such numbers may be impossible to reach for rare taxa, and thus, we recommend counting 500–1000 cells counterstained with a DNA dye. Alternatively, percent proportion of counted cells hybridized with a specific probe can be plotted against the number of counted DNA-stained cells until the plateau is reached, indicating the sufficient counting effort. Flow cytometry or automatic image acquisition microscopy may allow for statistically robust abundance estimates of rare lineages (Simon et al., 1995; Biegala et al., 2003; Mangot et al., 2018).
Unsuccessful CARD-FISH procedure can be identified by weak or lack of signals, high background fluorescence, and unspecific probe binding that can be recognized by diverse shape and size variability of hybridized cells, especially in case of very specific probes. For detailed troubleshooting guide, please refer to the Supplementary File 5.
As we show below, CARD-FISH is a powerful tool to unveil ecological traits of poorly studied and uncultured protists in their natural environments at a single cell level. However, as every method, it has its limitations. First, sample fixation might results in a certain cell loss, and fixatives do not have uniform efficiency for all protists (Jeuck et al., 2017). Moreover, CARD-FISH accuracy may be compromised by imperfect probe coverage and specificity, differences in the amount and activity of endogenous peroxidases between phylogenetic groups and environmental samples, and poor detection of low abundance or inactive community members, and difficulties in counting aggregated cells (Pernthaler et al., 2004; Amann and Fuchs, 2008). Moreover, tyramides sometimes bind unspecifically to cell walls of some protists, such as dinoflagellates or diatoms, making microscopic evaluation more challenging. Autofluorescence of chloroplasts might also interfere with probe signals depending on used fluorochromes and filter sets of the epifluorescence microscope used for quantification (Figure 7, Bühler, 2020). However, appropriate fluorochrome combinations considerably improve distinction of such objects as exemplified in Figures 4B,D, 5K,L. Other parameters may reduce the fluorescence of target cells, in particular a low rRNA content linked to low growth rate (Lim et al., 1993; Amann et al., 1995; Simon et al., 1995; Head et al., 1998). Nevertheless, most of these issues can be addressed as described in Supplementary Files 1, 5.
Understanding the in situ distribution and seasonal dynamics of individual protistan groups is important for obtaining a clear picture of the microbial processes in any ecosystem (Sherr et al., 2007; Kim et al., 2014). Although analyses of samples by CARD-FISH and double CARD-FISH is laborious, these single-cell approaches have revealed exciting discoveries on the importance of various flagellate taxa in marine and freshwater pelagic food webs. They have provided completely new insights into the life strategies of so far unknown or morphologically indistinguishable protists and will help to elucidate yet unknown trophic interactions of uncultured protists (Figures 4–6) that form highly complex microbial food webs. Below, we shortly summarize some key discoveries enabled by these techniques.
One of the first surprises upon applying FISH to marine samples was that the described species of bacterivorous HF were not abundant in natural environments. For instance, the genus Paraphysomonas was found to contribute below 1% to the total abundance of HF (Lim et al., 1999). In contrast, novel lineages of marine Stramenopiles (MAST), especially nanoplanktonic MAST-1 (cell size: 4–7 μm) and picoplanktonic MAST-4 (cell size: 2–3 μm), turned out to be ubiquitous in open oceans, contributing together to about 20% of all HF (Massana et al., 2006b; Mangot et al., 2018), and exhibiting growth rates between 0.4 and 0.8 per day (Massana et al., 2006a). Two morphotypes of the MAST-6 lineage were observed in the brackish Baltic Sea: larger (approximately 15 μm, Figure 4E) that formed a conspicuous peak when the salinity dropped to 6.2, and smaller (around 6 μm, Figure 6F) that dominated at salinities > 7 (Piwosz and Pernthaler, 2010). The MAST-2 lineage (cell size 4–5 μm) was found to be more abundant in first-year sea-ice (2–10%, Piwosz et al., 2013) than in pelagial (0.3%, Massana et al., 2006b). In contrast, cercozoans of the genus Cryothecomonas (cell size 2.5–29 μm) were also found in the sea-ice, but in rather low abundances (Thaler and Lovejoy, 2012). Another lineages of Cercozoa from Novel Clade 2 (Bass and Cavalier-Smith, 2004) turned out to be rare in the brackish Baltic Sea (Figures 6G–L), but they exhibited rapid growth rates of >1 per day in experimental incubations (Piwosz and Pernthaler, 2011).
Another unexpected discovery was that parasitic HF may appear in high concentrations in pelagic environments. Members of the order Syndiniales have been shown to control blooms of toxic dinoflagellates that form red-tides (Chambouvet et al., 2008, 2011). Free-living dinospores or multicellular trophonts were also found in the Baltic Sea (Piwosz and Pernthaler, 2010), where they may infect a bloom forming dinoflagellate Heterocapsa triquetra (Figures 4G,H), and in the first-year sea-ice (Piwosz et al., 2013). Syndiniales are more abundant in coastal waters, where they can contribute up to 40% of all eukaryotes and infect up to 25% of the target dinoflagellate species (Siano et al., 2011). In contrast, parasitic fungi were shown to be important nanophytoplankton parasites in the open ocean, where they can contribute up to 14% of abundance of all eukaryotes and infect up to 12% of haptophyte and 6% of chrysophyte algae (Lepère et al., 2016).
Completely different eukaryotic communities were found using CARD-FISH in the deep sea below 1000 m depth, where seven groups contributed to 50–70% of all eukaryotes: kinetoplastids (7–20%), labyrinthulomycetes (2–25%), fungi (2–25%), diplonemids (1–3%), Syndiniales group II (2–8%), MAST-4 lineage (1–6%), and an unidentified HF with a peculiar nuclear morphology (Edgcomb et al., 2011; Morgan-Smith et al., 2011, 2013). Fungi and labyrinthulomycetes seem to be more abundant on marine snow particles (Bochdansky et al., 2017) and in oxygen minimum zones (Morgan-Smith et al., 2013). In contrast to HF from the sun lit ocean, deep sea HF, especially those living on marine snow, might be saprotrophic rather than bacterivorous (Bochdansky et al., 2017).
The signal amplification was of crucial importance for detection of algae and MF showing strong autofluorescence from chlorophylls that may mask weak signals of monolabeled probes (Medlin and Strieben, 2010) (Figures 4A,B,D). Chlorophytes (Figure 4A), predominantly genus Micromonas, turned out to dominate the abundance of picophytoplankton in coastal oceans and seas, with contributions exceeding 80% (Not et al., 2005, 2008; Thiele et al., 2014; Unrein et al., 2014; Piwosz et al., 2015; Cabello et al., 2016; Piwosz, 2019). Their contribution was below 40% in other marine habitats, such as surface waters or deep chlorophyll maxima in the open oceans, Arctic fjords or the first-year sea-ice (Not et al., 2008; Piwosz et al., 2013, 2015). Larger haptophytes (cell sizes between 2–6 μm, Cabello et al., 2016; Piwosz, 2019) are the second most abundant group in the marine waters, with contributions to nanophytoplankton abundance of about 15% in the open sea, up to 30% in the brackish Baltic Sea, and above 35% in the polar regions (Not et al., 2005, 2008; Piwosz, 2019). Genus Pheocystis and family Pavlovales substantially contribute to haptophyte numbers in the open ocean (Thiele et al., 2014; Piwosz et al., 2015) and sea ice (Piwosz et al., 2013), while genera of Haptolina and Chrysochromulina were abundant in the brackish Baltic Sea (Piwosz, 2019). Pelagophyceae were found to be typically cells < 3 μm and contributing more to the deep chlorophyll maximum (about 24%) than in surface waters (below 10%, Cabello et al., 2016), but were of little importance in the brackish waters (Piwosz, 2019). In contrast, cryptophytes (Figure 4D) seem to be more important in coastal brackish waters (Piwosz et al., 2016; Piwosz, 2019) or in sea-ice (Piwosz et al., 2013) than in open ocean (Piwosz et al., 2015). A set of probes targeting photosynthetic cryptophyte clades has been developed and optimized for CARD-FISH (Table 1 and Supplementary Table 1, Metfies and Medlin, 2007; Medlin and Schmidt, 2010). However, the most abundant cryptophytes are members of the heterotrophic CRY1 lineage (Figures 5A–D), at least in the coastal waters of the Baltic Sea and freshwater habitats (Piwosz et al., 2016; Shiratori and Ishida, 2016; Grujčić et al., 2018; Piwosz, 2019; Šimek et al., 2020). Finally, bolidophytes and pedinellids seem to be rare (<1%) in truly marine waters (Guillou et al., 1999; Beardsley et al., 2005; Not et al., 2005; Piwosz et al., 2015), but the latter group sometimes form blooms in the Baltic Sea (Figures 4B,C, Piwosz and Pernthaler, 2010; Piwosz, 2019).
Freshwater lakes are much more accessible than open oceans, thus they are ideal ecosystems to understand the seasonal dynamics of small protists (Simon et al., 2015a; Mukherjee et al., 2017). However, in situ distributions, including vertical water column abundance patterns, and seasonal dynamics of protists remain still poorly studied in freshwater lakes, and only a handful of lineages have been quantified using CARD-FISH probes (Mangot et al., 2009; Mukherjee et al., 2017; Grujčić et al., 2018; Mukherjee et al., 2019; Šimek et al., 2020). Nevertheless, similar to marine environments, even a small number of such studies have brought unexpected results. One of the biggest surprises was the finding that the most abundant and omnipresent HF in various freshwater and even brackish habitats are not so called “Spumella-like” chrysomonads (Šimek et al., 1997a; Boenigk and Arndt, 2002; Matz et al., 2002; Grossmann et al., 2016), but tiny aplastidic cryptophytes and especially the cryptophyte CRY1 lineage therein (Piwosz et al., 2016; Shiratori and Ishida, 2016; Grujčić et al., 2018; Šimek et al., 2020). Concerning their typical cell size between 3 and 6 μm (Figures 5A–D), CRY1 were initially suggested to be bacterivores (Piwosz et al., 2016), which was later confirmed with observations of visibly ingested bacteria (Figures 5A–D, 6A–C) in their food vacuoles (Grujčić et al., 2018; Šimek et al., 2020). In different seasonal aspects of a freshwater reservoir, the aplastidic cryptophytes and cryptophyte CRY1 lineage accounted for, on average, ca. 50% and 20–25% of total HF numbers, respectively (Grujčić et al., 2018; Šimek et al., 2020). Moreover, the application of FLBs in combination with CARD-FISH clearly demonstrated their high bacterial consumption rates (Figures 6M–Q). In contrast, plastidic cryptophytes (Figure 4D) are important members of spring phytoplankton blooms, where they can account for up to 15% of all eukaryotes (Mangot et al., 2009), but they mostly do not show any uptake of prokaryotes.
Kinetoplastids are another surprisingly common and abundant lineage of HF in freshwaters. These flagellates are known as poor swimmers, being associated with detritus (Caron et al., 1982; Zubkov and Sleigh, 2000), where they glide around the particles to feed on surface-associated bacteria (Boenigk and Arndt, 2000). They were known to be ubiquitous in aquatic ecosystems (von der Heyden and Cavalier-Smith, 2005; Simpson et al., 2006), and they were even detected using light microscopy (Brandt and Sleigh, 2000; Weitere and Arndt, 2003). However, only upon application of CARD-FISH and kinetoplastid-specific probes (Table 1 and Supplementary Table 1), these flagellates were found to be widely distributed in freshwater lakes and, similarly to their deep ocean counterparts, to dominate in deep oxygenated hypolimnion waters, especially in summer when their contribution to total abundance of protists can reach up to 54% (Mukherjee et al., 2015, 2019). The timing of kinetoplastids maxima in the hypolimnetic waters was linked to the termination of phytoplankton blooms and high numbers of detritus particles sinking from the surface waters. The appearance of kinetoplastids in the surface waters may also correspond to the end of phytoplankton blooms, but it seems to be low in all the seasons (Mukherjee et al., 2015). However, their seasonal contributions to total HF in hypertrophic ponds, rich in small algae and detrital particles, were rather high, with examples of their typical bacterivorous morphotypes with well visible nuclei, kinetoplasts, and food vacuole contents shown in Figures 5Q–T.
Interestingly, a sister group of kinetoplastids, diplonemids, have been considered an exclusively marine group until a recent discovery of a distinct freshwater lineage widespread in various freshwater lakes around the world (Mukherjee et al., 2019, 2020) (Figures 4J–L). Their diversity seems to be low compared to the marine lineages and due to their low abundance, a clear seasonal pattern could not be deduced using CARD-FISH probes (Mukherjee et al., 2020). However, all the probe-positive cells clearly showed several bacteria inside their cytoplasm indicating their bacterial uptake (Mukherjee et al., 2020).
Cercozoans are smaller heterotrophic protists (size range 2–20 μm) belonging to Rhizaria (Figure 1A). Large Rhizaria are among the most dominant group of protists in the world oceans (Biard et al., 2016), but the abundance and distribution patterns of small cercozoans are less understood (Bass and Cavalier-Smith, 2004). Application of CARD-FISH revealed that cercozoans are consistently present in freshwater lakes, contributing around 11–12% of total protists (Mangot et al., 2009; Lepère et al., 2010), and they can ingest even cyanobacterial Microcystis cells (Figure 5K). Cercozoans of Novel Clade 7 (Bass and Cavalier-Smith, 2004) can account for up to 28% of the total community in food-web manipulation experiments amended with bacterial prey, which induced rapid growth of small HF and in turn also the growth of the predatory cercozoans with well visible small prey protists in their food vacuoles (Šimek et al., 2020) (Figures 5I,J).
Chlorophytes, cryptophytes, and haptophytes have also been reported to be consistently present throughout the year in freshwater lakes, mainly in the epilimnion waters, where their contribution to total eukaryotes can be > 50% (Mangot et al., 2009; Lepère et al., 2010). Autotrophic chlorophytes and haptophytes are considered as typical components of summer phytoplankton blooms in various lakes (Sommer et al., 1986, 2012). Chrysophytes are another dominant group of freshwater protists (del Campo and Massana, 2011) and are consistently present year round in freshwater lakes contributing up to 35% of total CARD-FISH positive eukaryotes (Mangot et al., 2009).
Parasitic perkinsozoans are regularly reported from freshwater lakes (Lepère et al., 2008, 2010; Mangot et al., 2011; Mukherjee et al., 2017). Their abundance peaks during summer, where they can account for up to 31% of the total eukaryotic community (Mangot et al., 2009). Perkinsea have been recently shown to infect green algae of the genus Sphaerocystis, and cells attached to filamentous cyanobacteria were also observed (Jobard et al., 2019). These parasites made up to 24% of all protists in the hypolimnion of a reservoir, where they were found attached to unidentified protistan cells and lake snow (Figure 4I).
Newly designed probes, targeting in situ morphologically indistinguishable HF and MF, have brought invaluable new information on absolute numbers of particular protistan lineages with the possibility to inspect also their food vacuole contents, thus unveiling their feeding modes (see examples in Figures 4–7) and examining grazing and growth rates of specific bacterivorous HF and MF. The method to estimate bacterivory based on FLB uptake rate during short-term incubations (Sherr et al., 1987) can be combined with identification of the grazers via CARD-FISH using the protocol described in Supplementary Files 2–4. This approach has unveiled that heterotrophic members of Cryptophyceae and its CRY1 lineage are important freshwater pelagic bacterivores (Grujčić et al., 2018; Šimek et al., 2020) (Figures 5A–H, 6A–F,M–Q). In marine waters, FISH combined with FLBs enabled detecting that HF of the MAST-1C lineage have higher grazing rates (∼4 bacteria HF–1 h–1) than members of the MAST-4 lineage (<1.5 bacteria HF–1 h–1), with an overall average cell-specific rate slightly above 2 bacteria HF–1 h–1 (Massana et al., 2009). Marine haptophytes were also found to be important bacterivores, with ingestion rates > 2.8 bacteria MF–1 h–1 and contributing even more than 25% to total protistan bacterivory (Unrein et al., 2014).
Furthermore, CARD-FISH enabled to reveal how bacterial food characteristics modulate growth and community dynamics of major bacterivores and consequently carbon flow rates to higher trophic levels. Monitoring abundance of specific HF lineages using CARD-FISH has evidenced that their growth response differs depending on a bacterioplankton species available as a main food source (Grujčić et al., 2018; Šimek et al., 2018, 2020). For instance, compositional shifts in bacterivorous HF communities tracked by CARD-FISH (Grujčić et al., 2018) cascaded through the food chain even to the level of predatory and omnivorous HF and small ciliates (Šimek et al., 2020).
The double CARD-FISH technique provides even higher resolution by allowing simultaneous phylogenetic identification of both predator and prey (Massana et al., 2009; Piwosz and Pernthaler, 2011; Grujčić et al., 2018; Šimek et al., 2020), thus ultimately opening the “black box” of microbial food webs and addressing the intriguing questions “who eats whom” (see examples in Figure 6). Ingested bacteria can be identified directly in food vacuoles (Figures 5, 6), providing information on positive or negative selections for particular bacterial and archaeal phylotypes (Jezbera et al., 2005, 2006; Massana et al., 2009; Anderson et al., 2012; Gerea et al., 2013; Šimek et al., 2014, 2020; Ballen-Segura et al., 2017).
Finally, newly designed CARD-FISH probes also shed light on the feeding modes of medium sized (5–20 μm) HF. Trophic relationships between bacterivorous cercozoans of the Novel Clade 2 (Bass and Cavalier-Smith, 2004) targeted by probe Cerc_Bal01 (Figure 6L), larger, omnivorous predator also of the Novel Clade 2 targeted by probe Cerc_Bal02 (Figure 6G), and heterotrophic dinoflagellates (Figure 6I) were deduced from experimental manipulations of a natural plankton community from coastal waters of the brackish Baltic Sea (Piwosz and Pernthaler, 2011). Similarly, experiments with a freshwater plankton community led to enrichments of 7–12 μm large HF affiliated with cercozoan Novel Clade 7 (Figures 5I,J) (Bass and Cavalier-Smith, 2004) and uncultured kathablepharids (Figures 5M–P) that grazed on small aplastidic bacterivorous cryptophytes or small algae rather than bacteria (Šimek et al., 2020). Time-course data of different HF lineages also allow for calculating lineage-specific growth rates, typically ranging from 1 to 1.8 d–1 for bacterivorous cryptophytes (Figures 5E–H), their CRY1 lineage (Figures 5A–D), and kinetoplastids (Figures 5Q–T), but also for larger omnivorous and predatory kathablepharids (Figures 5M–P) and Cercozoa (Figures 5I,J) (Šimek et al., 2020). Similarly, consumption rates on bacteria or probe-targeted lineages of prey protists can be estimated (Šimek et al., 2020). For instance, a comparison of cell biovolumes of prey cells from the CRY1 lineage (Figures 5A–D) with their cercozoan predator from the Novel Clade 7 (Figures 5I,J), and also taking into account the growth rate of the predatory cercozoans (doubling time of 10–20 h) indicated that these predators had to ingest ∼25–45 small CRY1 flagellates to meet their carbon requirements per one doubling (Šimek et al., 2020). Moreover, the rapid growth of both bacterivorous heterotrophic cryptophytes and omnivorous cercozoans and kathablepharids (in hours to days) tightly corresponded to typical doubling times reported for fast growing bacterioplankton groups (Eckert et al., 2012; Šimek et al., 2014; Neuenschwander et al., 2015). These findings allowed proposing a conceptual model explaining the tight linkages between rapid bacterial community shifts and succeeding HF community shifts in freshwaters (Šimek et al., 2018), now detectable using specific FISH probes (Figures 4–6).
The current view of aquatic microbial food webs assumes a relatively simple transfer of energy from prokaryotes via HF and MF to ciliates, and finally to zooplankton (Figure 8A). HF and MF are typically considered to prey predominantly on prokaryotes, even though it has been repeatedly shown that major bacterivores are actually those smaller than 5–8 μm (e.g., Chrzanowski and Šimek, 1990; Šimek et al., 1997b; Jürgens and Matz, 2002). The discovery of an enormous diversity in taxonomy, morphology, feeding modes, and life strategies of nanoflagellates provides compelling evidence that microbial food webs are far more complex than initially proposed, and the classical model requires a considerable revision in the light of recent findings (Arndt et al., 2000; Massana et al., 2009; Piwosz and Pernthaler, 2011; Grujčić et al., 2018; Šimek et al., 2020). The major obstacles in assigning the appropriate trophic roles to middle-sized flagellates in planktonic habitats are: (i) limited morphological features, which hamper rapid and reliable taxonomic identification by microscopy (Jeuck and Arndt, 2013), and (ii) a limited possibility to visualize and characterize ingested prey in food vacuoles of the protistan cells in natural communities that would indicate their food preference (Jezbera et al., 2005; Šimek et al., 2020). CARD-FISH based approaches are a perfect tool to solve these hindrances at a single cell level, as we showed above and exemplified in numerous microphotographs (Figures 4–7). Middle-sized NF (8–15 μm) are either omnivores feeding simultaneously on bacteria, algae, and bacterivorous HF and MF, or specific predators feeding primarily on motile algae and bacterivorous HF and MF (Piwosz and Pernthaler, 2010, 2011; Šimek et al., 2020). In general, Cercozoa and Katablepharida seem to be among the key consumers of bacterivorous HF and MF, at least in freshwaters and coastal brackish waters (Piwosz and Pernthaler, 2011; Grujčić et al., 2015; Mukherjee et al., 2017, 2019; Šimek et al., 2020). A single predatory cercozoan cell from Novel Clade 7 with an estimated doubling time of ∼12–24 h consumes daily 35–50 bacterivorous HF (Šimek et al., 2020). Similar doubling times were observed for predatory cercozoans from Novel Clade 2 (Piwosz and Pernthaler, 2011). These growth rates point to the dynamics and crucial role of the proposed trophic links to the overall energy and carbon transfer rates in microbial food webs. Similarly, small ciliates (12–20 μm), currently assumed to be primarily omnivorous, actually belong to the key planktonic bacterivores in most eutrophic and hypertrophic freshwater habitats (Šimek et al., 2000, 2019; Posch et al., 2015). These trophic interactions significantly modulate carbon flow efficiency, but their contribution to energy transfer in aquatic ecosystems is neither understood nor well quantified (Diehl and Feiáe, 2000).
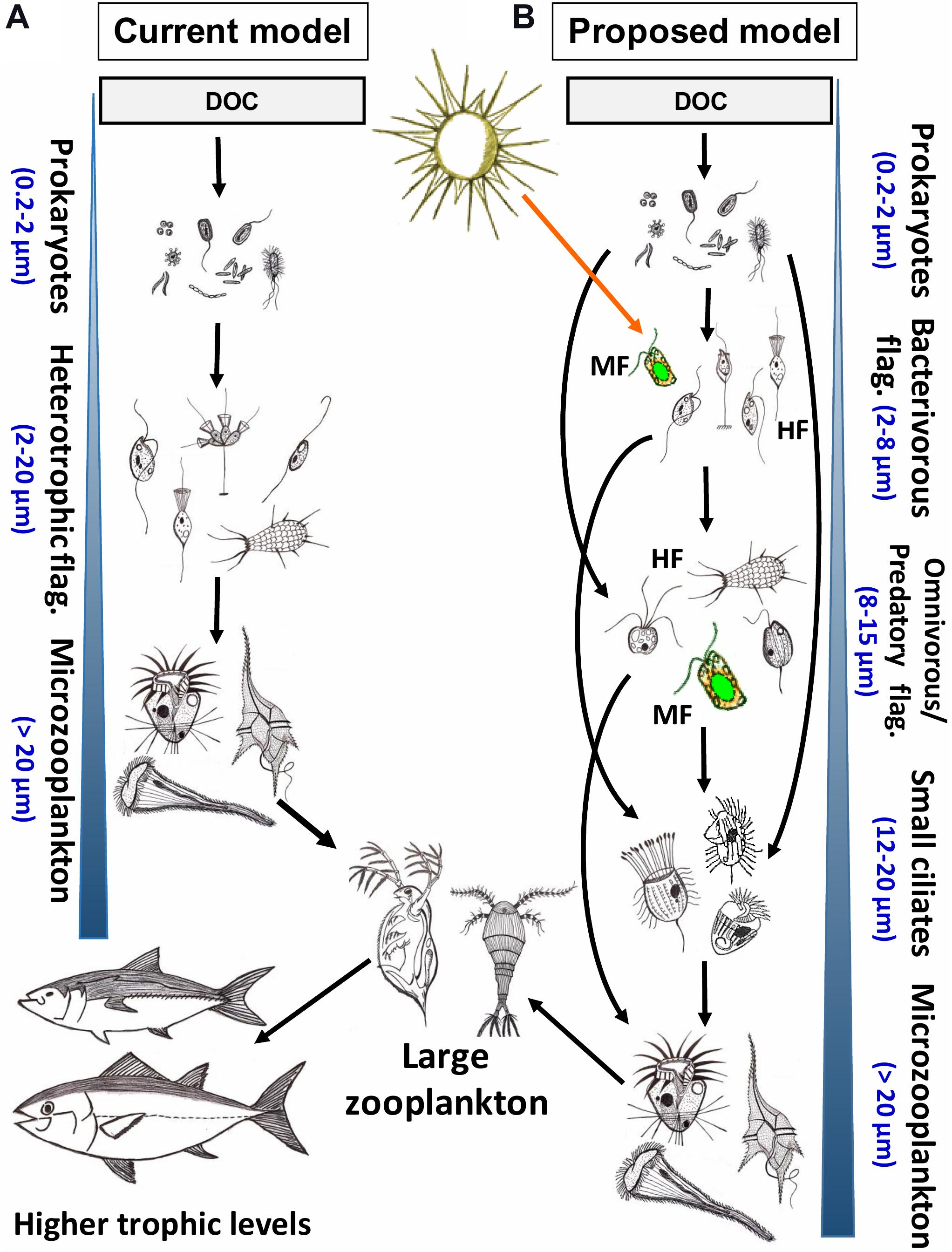
Figure 8. Conceptual models of aquatic microbial food webs aimed at describing major feeding and predatory relationships in pelagic systems. For simplicity of depiction, viruses, parasites and primary producers (phytoplankton) are not included, their roles have been reviewed recently elsewhere (Suttle, 2007; Winter et al., 2010; Sommer et al., 2012; Grossart et al., 2019; Zimmerman et al., 2020). (A) The original, most commonly applied model of major trophic interactions in microbial communities assumes generally linear carbon fluxes between neighboring trophic levels in the trophic food chain (Azam and Malfatti, 2007). All nanoplanktonic flagellates (NF) are considered to be primarily heterotrophic bacterivores that are grazed by microplanktonic protists, mainly ciliates and dinoflagellates. (B) Our proposed updated model of microbial communities includes recently recognized importance of novel trophic relationships within the nanoplankton size fraction (2–20 μm) described and also extensively documented in this review (see Figures 4–6). In this model, prokaryotes are grazed generally by small (2–8 μm) HF and MF. Larger NF (8–15 μm) are either omnivores (grazing on both bacteria and small NF) or predators (grazing mostly on small NF). However, these omnivorous and predatory NF prey also on each other (indicated by a blue circular arrow), and are themselves grazed by small filter-feeding and predatory ciliates (12–20 μm), which might also consume bacteria and bacterivorous NF. The larger omnivorous and predatory NF and small ciliates are controlled mainly by microplankton size fraction dominated by ciliates and dinoflagellates. Microzooplankton is consumed by mesozooplankton, thus channeling the carbon flow to higher trophic levels. Blue arrowhead indicated increasing cell- or body-size of planktonic organisms within a microbial community at different trophic levels. DOC, dissolved organic carbon. HF and MF, heterotrophic and mixotrophic flagellates, respectively.
To provoke a further debate on this topic and to advance our understanding of these interactions, we propose a new, considerably more complex but also more realistic model of microbial food webs (Figure 8B). The major refinement is related to the fact that the seemingly single level link (generally attributed to bacterivorous MF and HF) between prokaryotes and microzooplankton (mainly ciliates and dinoflagellates) actually consists of multiple linear and parallel trophic levels of omnivorous and predatory protists in the size range of ca. 8–15 μm. Recognition of these additional trophic levels and their complexity is the first step to quantify the contribution of phylogenetically and functionally diverse HF and MF in pelagic food webs. Consequences of additional trophic levels generally imply that more carbon is dissipated as CO2 within the microbial food web, and thus, a less efficient carbon transfer to higher trophic levels is anticipated. On the other hand, the energetic consequences of omnivory follow the opposite direction, as it reduces the number of trophic steps, resulting in more direct carbon flow from microbes to higher trophic levels (Goldman and Caron, 1985; Šimek et al., 2020). Omnivory has been also suggested to stabilize dynamics of food webs and communities (Fagan, 1997; McCann and Hastings, 1997; Holyoak and Sachdev, 1998).
CARD-FISH opens the door to a new era for studying small MF and HF by revealing their identity and feeding modes, thus providing novel insights into the ecology of otherwise poorly distinguishable groups. Moreover, it may also advance our understanding of the ecology of groups with generally larger cell sizes, such as ciliates or dinoflagellates. These groups are morphologically highly diverse, and a battery of methods has been developed to visualize characteristic features using light microscopy and numerous taxonomic keys, but reliable identification of smaller species requires substantial experience and it is generally time-consuming (Montagnes and Lynn, 1987; Foissner and Berger, 1996; Foissner et al., 1999; Madoni, 2011). So far, FISH-based identification has been proposed for economically important species and genera, such as the toxic dinoflagellates Alexandrium and Azadinium (John et al., 2003; Touzet et al., 2010; Toebe et al., 2012), or the pathogenic ciliate Pseudocohnilembus persalinus (Zhan et al., 2014), but it has not been routinely applied in ecological studies yet. FISH with monolabeled probes was successfully combined with Protargol staining and silver nitrate impregnation methods (Fried et al., 2002). Recently, a promising FISH and CARD-FISH protocol for freshwater planktonic ciliates has been developed (Bühler, 2020). This protocol was reliable for cultured ciliate strains and in multispecies mock assemblages (Figure 7, courtesy of D. Bühler and T. Posch). However, the FISH signal to noise ratio was insufficient for reliable in situ quantification. Even so, CARD-FISH proved to be a promising tool for the in situ detection and enumeration of tiny, barely distinguishable planktonic ciliates (Bühler, 2020).
CARD-FISH can be also used to study ecology of cryptic species. Multiple ribosomal lineages within one morpho-species have been discovered in many groups, such as dinoflagellates, diatoms, ciliates, and diplonemids (Zhu et al., 2005; Potvin and Lovejoy, 2009; Santoferrara et al., 2014; Stoeck et al., 2014; Caron and Hu, 2019; Mukherjee et al., 2020). Cryptic species differ in their eco-physiological characteristics, for instance, toxin production, as observed for dinoflagellate Alexandrium tamarense in the North Sea (Töbe et al., 2013), or habitat preference of Micromonas pusilla (Šlapeta et al., 2006). Such studies would enhance not only our understanding of protistan ecology and functional role in an ecosystem but also of their evolution and adaptation to different environments.
Fluorescence in situ hybridization-based methods are constantly being modified and developed. Recently, simultaneous analysis of even hundreds of lineages has been made possible with multi-color FISH approaches combined with high resolution confocal microscopy (Valm et al., 2011; Lukumbuzya et al., 2019; Shi et al., 2020). These novel techniques could be used, for example, to analyze food vacuole content for the presence of different prey items in multiple protistan lineages simultaneously, allowing for more direct determination of food preferences, and likely also accelerating sample processing. Moreover, FISH methods such as GeneFISH that combines the detection of specific genes and ribosomal RNA (rRNA) at the single cell level (Moraru et al., 2010) and mRNA FISH that detects synthesis and stability of mRNAs (Wendeberg et al., 2012; Xie et al., 2018) have great potential to be applied on protists. For example, with mRNA FISH, it may be possible to assess how localization patterns of mRNAs change during different stages of protist feeding on bacteria.
CARD-FISH can be also applied to samples gained from soils and sediments, where elevated organic matter content may cause high background, as shown for prokaryotes (Ferrari et al., 2006; Eickhorst and Tippkötter, 2008). As in the case of nanoplanktonic MF and HF, CARD-FISH protocols likely can be optimized for soil protists. Due to a lack of experience, we cannot recommend specific solutions, but considering the recent discovery of the impact of protistan grazing on decomposition rate (Geisen et al., 2020) or putatively high abundances of parasitic apicomplexans in soils (Mahé et al., 2017), the application of CARD-FISH might open a new universe of protistan ecology in soils and sediments, too.
Finally, we also suggest using various experimental manipulations with natural microbial communities (in order to considerably enrich the target protistan populations) in combination with novel probe design for both eukaryotic grazers and prokaryotic prey as highly relevant and useful approaches (Massana et al., 2009; Piwosz and Pernthaler, 2011; Šimek et al., 2013, 2020; Grujčić et al., 2018). This methods’ combination provides completely new insights into the life strategies of so far unknown or morphologically indistinguishable protists and to elucidate yet unknown trophic interactions and feeding modes (see examples in Figures 4–6) of uncultured nano-sized protists that form highly complex microbial food webs (Figure 8B). The current level of understanding of microbial interactions would undoubtedly profit from more frequent applications of FISH and other single cell approaches to considerably deepen our so far only mosaic knowledge on the ecology of most aquatic protists.
KP and KŠ conceptualized the study. All authors contributed to drafting the manuscript, preparing the figures, making critical revisions, and approved the final version of the manuscript.
This work was supported by several research grants of the Czech Science Foundation: KP was supported by the grant 18-14095Y, IM was supported by the grant 20-12496X, MMS was supported by the grant 19-23469S, and KŠ was supported by the grant 13-00243S. KP was also supported by a statutory project of National Marine Fisheries Research Institute no. DOT21/CERCOZOA. Additional support for KŠ and IM was provided by the European Union within ESIF in frame of Operational Programme Research, Development and Education (project no. CZ.02.1.01/0.0/0.0/16 025/0007417 administrated by the Ministry of Education, Youth and Sports of the Czech Republic) and by the Swiss National Science Foundation (SNF project 310030_185108 awarded to MS). VG was supported by the Swedish Research Council (FORMAS) grant 2017-00694. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Jiri Nedoma for assistance in taking photomicrographs and Thomas Posch and Dominique Bühler for sharing data on CARD-FISH for ciliates.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.640066/full#supplementary-material
Supplementary Figure 1 | Ready-to-print examples of circles for cutting filters into sections.
Supplementary File 1 | Guide for oligonucleotide probe design for protists.
Supplementary File 2 | Detailed description of the recommended CARD-FISH protocol.
Supplementary File 3 | Single page versions of the recommended CARD-FISH and double CARD-FISH protocols.
Supplementary File 4 | Recipes for all buffer, solutions and reagents used during CARD-FISH procedure.
Supplementary File 5 | Troubleshooting guide.
Supplementary File 6 | Reference list for Supplementary Table 1.
Supplementary Table 1 | List of FISH and CARD-FISH probes developed for protistan lineages, hybridization conditions, their current coverage and specificity, and overall recommendation for usage. The overall rating of probes is based on coverage of the target group and number of non-target hits in the guide tree of SILVA database RefNR 138.1 (very good: >80 coverage, no/almost no non-target hits; good: >50% coverage, almost no non-target hits; bad: <50 coverage and/or high number of non-target hits). Taxonomy is based on SILVA classification 138.1. List of references is available as Supplementary File 6. If two references to one probe are given, the first corresponds to the work that developed the probe, and the second to the work that tested it for FISH/CARD-FISH application.
Supplementary Table 2 | Volumes of formamide (form.) and water for 20 ml of hybridization buffer (HB), volumes of 5 M NaCl in 50 ml of washing buffer at 37°C (for hybridization at 35°C) and 48°C (for hybridization at 46°C). The recipes for HB and WB can be found in Supplementary File 4.
Supplementary Table 3 | Composition of buffers according to two most commonly used protocols: Not et al. (2002) and Piwosz and Pernthaler (2010). Concentrations of formamide in the hybridization buffer need to be optimized for each probe. The detailed instructions on how to prepare the buffers are given in Supplementary File 4.
Adl, S. M., Bass, D., Lane, C. E., Lukeš, J., Schoch, C. L., Smirnov, A., et al. (2019). Revisions to the classification, nomenclature, and diversity of Eukaryotes. J. Eukaryot. Microbiol. 66, 4–119.
Amann, R., and Fuchs, B. M. (2008). Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat. Rev. Microbiol. 6, 339–348. doi: 10.1038/nrmicro1888
Amann, R. I., Binder, B. J., Olson, R. J., Chisholm, S. W., Devereux, R., and Stahl, D. A. (1990). Combination of 16S ribosomal-RNA-targeted oligonucleotide probes with flow-cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56, 1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990
Amann, R. I., Ludwig, W., and Schleifer, K. H. (1995). Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59, 143–169. doi: 10.1128/mmbr.59.1.143-169.1995
Amaral-Zettler, L., Mccliment, E. A., Ducklow, H. W., and Huse, S. M. (2009). A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of Small-Subunit Ribosomal RNA Genes. PLoS One 4:e6372. doi: 10.1371/journal.pone.0006372
Andersen, O. K., Goldman, J. C., Caron, D. A., and Dennett, M. R. (1986). Nutrient cycling in a microflagellate food chain: III. Phosphorus dynamics. Mar. Ecol. Prog. Ser. 31, 47–55. doi: 10.3354/meps031047
Andersen, P., and Fenchel, T. (1985). Bacterivory by microheterotrophic flagellates in seawater samples. Limnol. Oceanogr. 30, 198–202. doi: 10.4319/lo.1985.30.1.0198
Anderson, R., Winter, C., and Jürgens, K. (2012). Protist grazing and viral lysis as prokaryotic mortality factors at Baltic Sea oxic-anoxic interfaces. Mar. Ecol. Prog. Ser. 467, 1–14. doi: 10.3354/meps10001
Arndt, H., Dietrich, D., Auer, B., Cleven, E.-J., Gräfenhan, T., Weitere, M., et al. (2000). “Functional diversity of heterotrophic flagellates in aquatic ecosystems,” in The Flagellates, eds B. S. C. Leadbeater and J. C. Green (London: Taylor & Francis), 240–268.
Arndt, H., and Mathes, J. (1991). Large heterotrophic flagellates form a significant part of protozooplankton biomass in lakes and rivers. Ophelia 33, 225–234. doi: 10.1080/00785326.1991.10429713
Azam, F., Fenchel, T., Field, J. G., Gray, J. S., Meyer-Reil, L., and Thingsted, F. (1983). The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257–263. doi: 10.3354/meps010257
Azam, F., and Malfatti, F. (2007). Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 5, 782–791. doi: 10.1038/nrmicro1747
Bachy, C., Dolan, J. R., Lopez-Garcia, P., Deschamps, P., and Moreira, D. (2013). Accuracy of protist diversity assessments: morphology compared with cloning and direct pyrosequencing of 18S rRNA genes and ITS regions using the conspicuous tintinnid ciliates as a case study. ISME J. 7, 244–255. doi: 10.1038/ismej.2012.106
Ballen-Segura, M., Felip, M., and Catalan, J. (2017). Some mixotrophic flagellate species selectively Graze on Archaea. Appl. Environ. Microbiol. 83:e2317-16.
Balzano, S., Abs, E., and Leterme, S. C. (2015). Protist diversity along a salinity gradient in a coastal lagoon. Aquat. Microb. Ecol. 74, 263–277. doi: 10.3354/ame01740
Bass, D., and Cavalier-Smith, T. (2004). Phylum-specific environmental DNA analysis reveals remarkably high global biodiversity of Cercozoa (Protozoa). Int. J. Syst. Evol. Microbiol. 54, 2393–2404. doi: 10.1099/ijs.0.63229-0
Beardsley, C., Knittel, K., Amann, R., and Pernthaler, J. (2005). Quantification and distinction of aplastidic and plastidic marine nanoplankton by fluorescence in situ hybridization. Aquat. Microb. Ecol. 41, 163–169. doi: 10.3354/ame041163
Behnke, A., Engel, M., Christen, R., Nebel, M., Klein, R. R., and Stoeck, T. (2011). Depicting more accurate pictures of protistan community complexity using pyrosequencing of hypervariable SSU rRNA gene regions. Environ. Microbiol. 13, 340–349. doi: 10.1111/j.1462-2920.2010.02332.x
Berninger, U.-G., Finlay, B. J., and Kuuppo-Leinikki, P. (1991). Protozoan control of bacterial abundances in freshwater. Limnol. Oceanogr. 36, 139–147. doi: 10.4319/lo.1991.36.1.0139
Biard, T., Stemmann, L., Picheral, M., Mayot, N., Vandromme, P., Hauss, H., et al. (2016). In situ imaging reveals the biomass of giant protists in the global ocean. Nature 532, 504–507. doi: 10.1038/nature17652
Biegala, I. C., Not, F., Vaulot, D., and Simon, N. (2003). Quantitative assessment of picoeukaryotes in the natural environment by using taxon-specific oligonucleotide probes in association with tyramide signal amplification-fluorescence in situ hybridization and flow cytometry. Appl. Environ. Microbiol. 69, 5519–5529. doi: 10.1128/aem.69.9.5519-5529.2003
Bobrow, M. N., Shaughnessy, K. J., and Litt, G. J. (1991). Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. J. Immunol. Methods 137, 103–112. doi: 10.1016/0022-1759(91)90399-z
Bochdansky, A., Clouse, M., and Herndl, G. (2017). Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 11, 362–373. doi: 10.1038/ismej.2016.113
Bochdansky, A. B., and Huang, L. (2010). Re-evaluation of the EUK516 probe for the domain Eukarya results in a suitable probe for the detection of kinetoplastids, an important group of parasitic and free-living flagellates. J. Eukaryot. Microbiol. 57, 229–235.
Boenigk, J., and Arndt, H. (2000). Comparative studies on the feeding behavior of two heterotrophic nanoflagellates: the filter-feeding choanoflagellate Monosiga ovata and the raptorial-feeding kinetoplastid Rhynchomonas nasuta. Aquat. Microb. Ecol. 22, 243–249. doi: 10.3354/ame022243
Boenigk, J., and Arndt, H. (2002). Bacterivory by heterotrophic flagellates: community structure and feeding strategies. Antonie Van Leeuwenhoek 81, 465–480.
Bork, P., Bowler, C., De Vargas, C., Gorsky, G., Karsenti, E., and Wincker, P. (2015). Tara Oceans studies plankton at planetary scale. Science 348, 873–873. doi: 10.1126/science.aac5605
Brandt, S. M., and Sleigh, M. A. (2000). The quantitative occurrence of different taxa of heterotrophic flagellates in Southampton water, U.K. Estuar. Coast. Shelf Sci. 51, 91–102. doi: 10.1006/ecss.2000.0607
Brate, J., Klaveness, D., Rygh, T., Jakobsen, K. S., and Shalchian-Tabrizi, K. (2010). Telonemia-specific environmental 18S rDNA PCR reveals unknown diversity and multiple marine-freshwater colonizations. BMC Microbiol. 10:168. doi: 10.1186/1471-2180-10-168
Bühler, D. (2020). ’FISHing for Ciliates’ Fluorescence in situ Hybridization for the Detection of Planktonic Freshwater Ciliates. Master’s thesis, University of Zurich, Zürich.
Burki, F., Roger, A. J., Brown, M. W., and Simpson, A. G. B. (2020). The new tree of Eukaryotes. Trends Ecol. Evol. 35, 43–55. doi: 10.1016/j.tree.2019.08.008
Cabello, A. M., Latasa, M., Forn, I., Morán, X. A., and Massana, R. (2016). Vertical distribution of major photosynthetic picoeukaryotic groups in stratified marine waters. Environ. Microbiol. 18, 1578–1590. doi: 10.1111/1462-2920.13285
Caron, D. A., Countway, P. D., Jones, A. C., Kim, D. Y., and Schnetzer, A. (2012). Marine protistan diversity. Annu. Rev. Mar. Sci. 4, 467–493. doi: 10.1146/annurev-marine-120709-142802
Caron, D. A., Davis, P. G., Madin, L. P., and Sieburth, J. M. (1982). Heterotrophic bacteria and bacterivorous protozoa in oceanic macroaggregates. Science 218, 795–797. doi: 10.1126/science.218.4574.795
Caron, D. A., and Hu, S. (2019). Are we overestimating protistan diversity in nature? Trends Microbiol. 27, 197–205. doi: 10.1016/j.tim.2018.10.009
Chambouvet, A., Alves-De-Souza, C., Cueff, V., Marie, D., Karpov, S., and Guillou, L. (2011). Interplay between the parasite Amoebophrya sp (Alveolata) and the cyst formation of the red tide dinoflagellate Scrippsiella trochoidea. Protist 162, 637–649. doi: 10.1016/j.protis.2010.12.001
Chambouvet, A., Morin, P., Marie, D., and Guillou, L. (2008). Control of toxic marine dinoflagellates blooms by serial parasitic killers. Science 322, 1254–1257. doi: 10.1126/science.1164387
Chrzanowski, T. H., and Šimek, K. (1990). Prey-size selection by freshwater flagellated protozoa. Limnol. Oceanogr. 35, 1429–1436. doi: 10.4319/lo.1990.35.7.1429
Coleman, A. W. (1980). Enhanced detection of bacteria in natural environments by fluorochrone staining of DNA. Limnol. Oceanogr. 25, 948–951. doi: 10.4319/lo.1980.25.5.0948
Corno, G., and Jürgens, K. (2006). Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity. Appl. Environ. Microbiol. 72, 78–86. doi: 10.1128/aem.72.1.78-86.2006
Cram, J., Sun, F., and Fuhrman, J. A. (2013). “Marine bacterial, Archaeal, and Protistan Association Networks,” in Encyclopedia of Metagenomics, ed. E. K. Nelson (New York, NY: Springer), 1–10. doi: 10.1007/978-1-4614-6418-1_721-3
Davidov, K., Iankelevich-Kounio, E., Yakovenko, I., Koucherov, Y., Rubin-Blum, M., and Oren, M. (2020). Identification of plastic-associated species in the Mediterranean Sea using DNA metabarcoding with Nanopore MinION. Sci. Rep. 10:17533.
de Vargas, C., Audic, S., Henry, N., Decelle, J., Mahe, F., Logares, R., et al. (2015). Eukaryotic plankton diversity in the sunlit ocean. Science 348:1261605.
del Campo, J., and Massana, R. (2011). Emerging diversity within chrysophytes, choanoflagellates and bicosoecids based on molecular surveys. Protist 162, 435–448. doi: 10.1016/j.protis.2010.10.003
del Giorgio, P. A., Gasol, J. M., Vaque, D., Mura, P., Agusti, S., and Duarte, C. M. (1996). Bacterioplankton community structure: protists control net production and the proportion of active bacteria in a coastal marine community. Limnol. Oceanogr. 41, 1169–1179. doi: 10.4319/lo.1996.41.6.1169
Diehl, S., and Feiáe, M. (2000). Effects of enrichment on three-level food chains with omnivory. Am. Nat. 155, 200–218. doi: 10.2307/3078943
Dirren, S., Salcher, M. M., Blom, J. F., Schweikert, M., and Posch, T. (2014). Menage-a-trois: the amoeba Nuclearia sp from Lake Zurich with its ecto- and endosymbiotic bacteria. Protist 165, 745–758. doi: 10.1016/j.protis.2014.08.004
Duarte, C. M. (2015). Seafaring in the 21St century: the Malaspina 2010 circumnavigation expedition. Limnol. Oceanogr. Bull. 24, 11–14. doi: 10.1002/lob.10008
Eckert, E. M., Salcher, M. M., Posch, T., Eugster, B., and Pernthaler, J. (2012). Rapid successions affect microbial N-acetyl-glucosamine uptake patterns during a lacustrine spring phytoplankton bloom. Environ. Microbiol. 14, 794–806. doi: 10.1111/j.1462-2920.2011.02639.x
Edgcomb, V. P., Kysela, D. T., Teske, A., De Vera Gomez, A., and Sogin, M. L. (2002). Benthic eukaryotic diversity in the Guaymas Basin hydrothermal vent environment. Proc. Natl. Acad. Sci. U.S.A. 99, 7658–7662. doi: 10.1073/pnas.062186399
Edgcomb, V. P., Orsi, W., Breiner, H. W., Stock, A., Filker, S., Yakimov, M. M., et al. (2011). Novel active kinetoplastids associated with hypersaline anoxic basins in the Eastern Mediterranean deep-sea. Deep Sea Res. 1 Oceanogr. Res. Pap. 58, 1040–1048. doi: 10.1016/j.dsr.2011.07.003
Edler, L., and Elbrächter, M. (2010). “The Utermöhl method for quantitative phytoplankton analysis,” in IOC Manuals and Guides, eds B. Karlson, C. Cusack, and E. Bresnan (Paris: Intergovernmental Oceanographic Commission of © UNESCO), 13–20.
Egge, E., Bittner, L., Andersen, T., Audic, S., De Vargas, C., and Edvardsen, B. (2013). 454 pyrosequencing to describe microbial eukaryotic community composition, diversity and relative abundance: a test for marine haptophytes. PLoS One 8:e74371. doi: 10.1371/journal.pone.0074371
Egge, E. S., Eikrem, W., and Edvardsen, B. (2015). Deep-branching novel lineages and high diversity of haptophytes in the Skagerrak (Norway) uncovered by 454 pyrosequencing. J. Eukaryot. Microbiol. 62, 121–140. doi: 10.1111/jeu.12157
Eickhorst, T., and Tippkötter, R. (2008). Improved detection of soil microorganisms using fluorescence in situ hybridization (FISH) and catalyzed reporter deposition (CARD-FISH). Soil Biol. Biochem. 40, 1883–1891. doi: 10.1016/j.soilbio.2008.03.024
Fagan, W. F. (1997). Omnivory as a stabilizing feature of natural communities. Am. Nat. 150, 554–567. doi: 10.1086/286081
Felsenstein, J. (1981). Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17, 368–376. doi: 10.1007/bf01734359
Fenchel, T. (1982). Ecology of heterotrophic microflagellates. IV. Quantitative occurrence and importance as bacterial consumers. Mar. Ecol. Prog. Ser. 9, 35–42. doi: 10.3354/meps009035
Ferrari, B. C., Tujula, N., Stoner, K., and Kjelleberg, S. (2006). Catalyzed reporter deposition-fluorescence in situ hybridization allows for enrichment-independent detection of microcolony-forming soil bacteria. Appl. Environ. Microbiol. 72, 918–922. doi: 10.1128/aem.72.1.918-922.2006
Flegontova, O., Flegontov, P., Malviya, S., Audic, S., Wincker, P., De Vargas, C., et al. (2016). Extreme diversity of diplonemid eukaryotes in the ocean. Curr. Biol. 26, 3060–3065. doi: 10.1016/j.cub.2016.09.031
Flegontova, O., Flegontov, P., Malviya, S., Poulain, J., De Vargas, C., Bowler, C., et al. (2018). Neobodonids are dominant kinetoplastids in the global ocean. Environ. Microbiol. 20, 878–889. doi: 10.1111/1462-2920.14034
Foissner, W., and Berger, H. (1996). A user-friendly guide to the ciliates (Protozoa, Ciliophora) commonly used by hydrobiologists as bioindicators in rivers, lakes, and waste waters, with notes on their ecology. Freshw. Biol. 35, 375. doi: 10.1111/j.1365-2427.1996.tb01775.x
Foissner, W., Berger, H., and Schaumburg, J. (1999). Identification and Ecology of Limnetic Plankton Ciliates. Munich: Bayer.
Fried, J., Ludwig, W., Psenner, R., and Schleifer, K. H. (2002). Improvement of ciliate identification and quantification: a new protocol for fluorescence in situ hybridization (FISH) in combination with silver stain techniques. Syst. Appl. Microbiol. 25, 555–571. doi: 10.1078/07232020260517706
Gasol, J. M., and Vagué, D. (1993). Lack of coupling between heterotrophic nanoflagellates and bacteria: A general phenomenon across aquatic systems? Limnol. Oceanogr. 38, 657–665. doi: 10.4319/lo.1993.38.3.0657
Geisen, S., Hu, S., Dela Cruz, T. E. E., and Veen, G. F. (2020). Protists as catalyzers of microbial litter breakdown and carbon cycling at different temperature regimes. ISME J. 15, 618–621. doi: 10.1038/s41396-020-00792-y
Georges, C., Monchy, S., Genitsaris, S., and Christaki, U. (2014). Protist community composition during early phytoplankton blooms in the naturally iron-fertilized Kerguelen area (Southern Ocean). Biogeosciences 11, 5847–5863. doi: 10.5194/bg-11-5847-2014
Gerea, M., Queimaliños, C., Schiaffino, M. R., Izaguirre, I., Forn, I., Massana, R., et al. (2013). In situ prey selection of mixotrophic and heterotrophic flagellates in Antarctic oligotrophic lakes: an analysis of the digestive vacuole content. J. Plankton Res. 35, 201–212. doi: 10.1093/plankt/fbs085
Gimmler, A., and Stoeck, T. (2015). Mining environmental high-throughput sequence data sets to identify divergent amplicon clusters for phylogenetic reconstruction and morphotype visualization. Environ. Microbiol. Rep. 7, 679–686. doi: 10.1111/1758-2229.12307
Giovannoni, S. J., Delong, E. F., Olsen, G. J., and Pace, N. R. (1988). Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J. Bacteriol. 170, 720–726. doi: 10.1128/jb.170.2.720-726.1988
Goldman, J. C., and Caron, D. A. (1985). Experimental studies on an omnivorous microflagellate: implications for grazing and nutrient regeneration in the marine microbial food chain. Deep Sea Res. A Oceanogr. Res. Pap. 32, 899–915. doi: 10.1016/0198-0149(85)90035-4
Goldman, J. C., Caron, D. A., Andersen, O. K., and Dennett, M. R. (1985). Nutrient cycling in a microflagellate food chain: I. Nitrogen dynamics. Mar. Ecol. Prog. Ser. 24, 231–242. doi: 10.3354/meps024231
Gong, J., Dong, J., Liu, X., and Massana, R. (2013). Extremely high copy numbers and polymorphisms of the rDNA operon estimated from single cell analysis of oligotrich and peritrich ciliates. Protist 164, 369–379. doi: 10.1016/j.protis.2012.11.006
González, J. M., Iriberri, J., Egea, L., and Barcina, I. (1990a). Differential rates of digestion of bacteria by freshwater and marine phagotrophic protozoa. Appl. Environ. Microbiol. 56, 1851–1857. doi: 10.1128/aem.56.6.1851-1857.1990
González, J. M., Sherr, E. B., and Sherr, B. F. (1990b). Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl. Environ. Microbiol. 56, 583–589. doi: 10.1128/aem.56.3.583-589.1990
Grossart, H.-P., Van Den Wyngaert, S., Kagami, M., Wurzbacher, C., Cunliffe, M., and Rojas-Jimenez, K. (2019). Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 17, 339–354.
Grossmann, L., Bock, C., Schweikert, M., and Boenigk, J. (2016). Small but manifold – hidden diversity in ‘Spumella-like flagellates’. J. Eukaryot. Microbiol. 63, 419–439. doi: 10.1111/jeu.12287
Grujčić, V., Kasalický, V., and Šimek, K. (2015). Prey-specific growth responses of freshwater flagellate communities induced by morphologically distinct bacteria from the genus Limnohabitans. Appl. Environ. Microbiol. 81, 4993–5002. doi: 10.1128/aem.00396-15
Grujčić, V., Nuy, J. K., Salcher, M. M., Shabarova, T., Kasalický, V., Boenigk, J., et al. (2018). Cryptophyta as major bacterivores in freshwater summer plankton. ISME J. 12, 1668–1681. doi: 10.1038/s41396-018-0057-5
Guidi, L., Chaffron, S., Bittner, L., Eveillard, D., Larhlimi, A., Roux, S., et al. (2016). Plankton networks driving carbon export in the oligotrophic ocean. Nature 532, 465–470. doi: 10.1038/nature16942
Guillou, L., Chretiennot-Dinet, M.-J., Medlin, L. K., Claustre, H., Loiseaux-Goer, S., and Vaulot, D. (1999). Bolidomonas: a new genus with two species belonging to a new algal class, the Bolidophyceae (Heterkonta). J. Phycol. 35, 368–381. doi: 10.1046/j.1529-8817.1999.3520368.x
Hahn, M. W., and Höfle, M. G. (2001). Grazing of protozoa and its effects on populations of aquatic bacteria. FEMS Microbiol. Ecol. 35, 113–121. doi: 10.1111/j.1574-6941.2001.tb00794.x
Hansen, M. C., Tolker-Nielsen, T., Givskov, M., and Molin, S. (1998). Biased 16S rDNA PCR amplification caused by interference from DNA flanking the template region. FEMS Microbiol. Ecol. 26, 141–149. doi: 10.1111/j.1574-6941.1998.tb00500.x
Hansen, P. (1996). Silica-scaled Chrysophyceae and Synurophyceae from Madagascar. Arch. Protistenkunde 147, 145–172. doi: 10.1016/s0003-9365(96)80030-3
Hatfield, R. G., Batista, F. M., Bean, T. P., Fonseca, V. G., Santos, A., Turner, A. D., et al. (2020). The application of Nanopore sequencing technology to the study of dinoflagellates: a proof of concept study for rapid sequence-based discrimination of potentially harmful algae. Front. Microbiol. 11:844. doi: 10.3389/fmicb.2020.00844
Head, I., Saunders, J., and Pickup, R. (1998). Microbial evolution, diversity, and ecology: a decade of ribosomal RNA analysis of uncultivated microorganisms. Microb. Ecol. 35, 1–21. doi: 10.1007/s002489900056
Holyoak, M., and Sachdev, S. (1998). Omnivory and the stability of simple food webs. Oecologia 117, 413–419. doi: 10.1007/s004420050675
Hoppenrath, M., Elbrächter, M., and Debres, G. (2009). Marine Phytoplankton. Stuttgart: Schweizerbart Science Publishers.
Hoppenrath, M., Murray, S. A., Chomérat, N., and Horiguchi, T. (2014). Marine Benthic Dinoflagellates - Unveiling their Worldwide Biodiversity. Stuttgart: Schweizerbart Science Publishers.
Hu, Y. O. O., Karlson, B., Charvet, S., and Andersson, A. F. (2016). Diversity of pico- to mesoplankton along the 2000 km salinity gradient of the Baltic Sea. Front. Microbiol. 7:679. doi: 10.3389/fmicb.2016.00679
Hugenholtz, P., Tyson, G., and Blackall, L. (2001). Design and evaluation of 16S rRNA-targeted oligonucleotide probes for fluorescence in situ hybridization. Methods Mol. Biol. 179, 29–42.
Jeuck, A., and Arndt, H. (2013). A short guide to common heterotrophic flagellates of freshwater habitats based on the morphology of living organisms. Protist 164, 842–860. doi: 10.1016/j.protis.2013.08.003
Jeuck, A., Nitsche, F., Wylezich, C., Wirth, O., Bergfeld, T., Brutscher, F., et al. (2017). A comparison of methods to analyze aquatic heterotrophic flagellates of different taxonomic groups. Protist 168, 375–391. doi: 10.1016/j.protis.2017.04.003
Jezbera, J., Horňák, K., and Šimek, K. (2005). Food selection by bacterivorous protists: insight from the analysis of the food vacuole content by means of fluorescence in situ hybridization. FEMS Microbiol. Ecol. 52, 351–363. doi: 10.1016/j.femsec.2004.12.001
Jezbera, J., Hornak, K., and Šimek, K. (2006). Prey selectivity of bacterivorous protists in different size fractions of reservoir water amended with nutrients. Environ. Microbiol. 8, 1330–1339. doi: 10.1111/j.1462-2920.2006.01026.x
Jobard, M., Wawrzyniak, I., Bronner, G., Marie, D., Vellet, A., Sime-Ngando, T., et al. (2019). Freshwater Perkinsea: diversity, ecology and genomic information. J. Plankton Res. 42, 3–17. doi: 10.1093/plankt/fbz068
John, U., Cembella, A., Hummert, C., Elbrächter, M., Groben, R., and Medlin, L. (2003). Discrimination of the toxigenic dinoflagellates Alexandrium tamarense and A. ostenfeldii in co-occurring natural populations from Scottish coastal waters. Eur. J. Phycol. 38, 25–40.
Jürgens, K., and Güde, H. (1994). The potential importance of grazing-resistant bacteria in planktonic systems. Mar. Ecol. Prog. Ser. 112, 169–188.
Jürgens, K., and Matz, C. (2002). Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Van Leeuwenhoek 81, 413–434.
Keeling, P. J. (2019). Combining morphology, behaviour and genomics to understand the evolution and ecology of microbial eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20190085.
Kim, D. Y., Countway, P. D., Jones, A. C., Schnetzer, A., Yamashita, W., Tung, C., et al. (2014). Monthly to interannual variability of microbial eukaryote assemblages at four depths in the eastern North Pacific. ISME J. 8, 515–530.
Klindworth, A., Pruesse, E., Schweer, T., Peplies, J., Quast, C., Horn, M., et al. (2013). Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41:e1.
Ku, C., and Sebé-Pedrós, A. (2019). Using single-cell transcriptomics to understand functional states and interactions in microbial eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20190098.
Lange, M., Guillou, L., Vaulot, D., Simon, N., Amann, R. I., Ludwig, W., et al. (1996). Identification of the class prymnesiophyceae and the genus Phaeocystis with ribosomal RNA-targeted nucleic acid probes detected by flow cytometry. J. Phycol. 32, 858–868. doi: 10.1111/j.0022-3646.1996.00858.x
Langenheder, S., and Jürgens, K. (2001). Regulation of bacterial biomass and community structure by metazoan and protozoan predation. Limnol. Oceanogr. 46, 121–134. doi: 10.4319/lo.2001.46.1.0121
Lepère, C., Domaizon, I., and Debroas, D. (2008). Unexpected importance of potential parasites in the composition of the freshwater small-eukaryote community. Appl. Environ. Microbiol. 74, 2940–2949. doi: 10.1128/AEM.01156-07
Lepère, C., Masquelier, S., Mangot, J.-F., Debroas, D., and Domaizon, I. (2010). Vertical structure of small eukaryotes in three lakes that differ by their trophic status: a quantitative approach. ISME J. 4, 1509–1519. doi: 10.1038/ismej.2010.83
Lepère, C., Ostrowski, M., Hartmann, M., Zubkov, M. V., and Scanlan, D. J. (2016). In situ associations between marine photosynthetic picoeukaryotes and potential parasites - a role for fungi? Environ. Microbiol. Rep. 8, 445–451. doi: 10.1111/1758-2229.12339
Lim, E. L., Amaral, L. A., Caron, D. A., and Delong, E. F. (1993). Application of rRNA-based probes for observing marine nanoplanktonic protists. Appl. Environ. Microbiol. 59, 1647–1655. doi: 10.1128/AEM.59.5.1647-1655.1993
Lim, E. L., Dennett, M. R., and Caron, D. A. (1999). The ecology of Paraphysomonas imperforata based on studies employing oligonucleotide probe identification in coastal water samples and enrichment cultures. Limnol. Oceanogr. 44, 37–51. doi: 10.4319/lo.1999.44.1.0037
Logares, R., Audic, S., Bass, D., Bittner, L., Boutte, C., Christen, R., et al. (2014). Patterns of rare and abundant marine microbial eukaryotes. Curr. Biol. 24, 813–821. doi: 10.1016/j.cub.2014.02.050
López-García, P., Philippe, H., Gail, F., and Moreira, D. (2003). Autochthonous eukaryotic diversity in hydrothermal sediment and experimental microcolonizers at the Mid-Atlantic Ridge. Proc. Natl. Acad. Sci. U.S.A. 100, 697–702. doi: 10.1073/pnas.0235779100
Lopez-Garcia, P., Rodriguez-Valera, F., Pedros-Alio, C., and Moreira, D. (2001). Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature 409, 603–607. doi: 10.1038/35054537
Lovejoy, C., Massana, R., and Pedros-Alio, C. (2006). Diversity and ditribution of marine microbial eukaryotes in the Arctic ocean and adjacent seas. Appl. Environ. Microbiol. 72, 3085–3095. doi: 10.1128/AEM.72.5.3085-3095.2006
Lukumbuzya, M., Schmid, M., Pjevac, P., and Daims, H. (2019). A multicolor fluorescence in situ hybridization approach using an extended set of fluorophores to visualize microorganisms. Front. Microbiol. 10:1383. doi: 10.3389/fmicb.2019.01383
Madoni, P. (2011). Protozoa in wastewater treatment processes: a minireview. Ital. J. Zool. 78, 3–11. doi: 10.1080/11250000903373797
Mahé, F., De Vargas, C., Bass, D., Czech, L., Stamatakis, A., Lara, E., et al. (2017). Parasites dominate hyperdiverse soil protist communities in Neotropical rainforests. Nat. Ecol. Evol. 1:91. doi: 10.1038/s41559-017-0091
Majaneva, M., Rintala, J.-M., Hajdu, S., Hallfors, S., Hallfors, G., Skjevik, A.-T., et al. (2012). The extensive bloom of alternate-stage Prymnesium polylepis (Haptophyta) in the Baltic Sea during autumn-spring 2007-2008. Eur. J. Phycol. 47, 310–320. doi: 10.1080/09670262.2012.713997
Mangot, J.-F., Debroas, D., and Domaizon, I. (2011). Perkinsozoa, a well-known marine protozoan flagellate parasite group, newly identified in lacustrine systems: a review. Hydrobiologia 659, 37–48. doi: 10.1007/s10750-010-0268-x
Mangot, J.-F., Domaizon, I., Taib, N., Marouni, N., Duffaud, E., Bronner, G., et al. (2012). Short-term dynamics of diversity patterns: evidence of continual reassembly within lacustrine small eukaryotes. Environ. Microbiol. 15, 1745–1758. doi: 10.1111/1462-2920.12065
Mangot, J.-F., Forn, I., Obiol, A., and Massana, R. (2018). Constant abundances of ubiquitous uncultured protists in the open sea assessed by automated microscopy. Environ. Microbiol. 20, 3876–3889. doi: 10.1111/1462-2920.14408
Mangot, J. F., Lepere, C., Bouvier, C., Debroas, D., and Domaizon, I. (2009). Community structure and dynamics of small eukaryotes targeted by new oligonucleotide probes: new insight into the lacustrine microbial food web. Appl. Environ. Microbiol. 75, 6373–6381. doi: 10.1128/AEM.00607-09
Martin-Laurent, F., Philippot, L., Hallet, S., Chaussod, R., Germon, J. C., Soulas, G., et al. (2001). DNA extraction from soils: old bias for new microbial diversity analysis methods. Appl. Environ. Microbiol. 67, 2354–2359. doi: 10.1128/AEM.67.5.2354-2359.2001
Massana, R. (2011). Eukaryotic picoplankton in surface oceans. Annu. Rev. Microbiol. 65, 91–110. doi: 10.1146/annurev-micro-090110-102903
Massana, R., Gobet, A., Audic, S., Bass, D., Bittner, L., Boutte, C., et al. (2015). Marine protist diversity in European coastal waters and sediments as revealed by high-throughput sequencing. Environ. Microbiol. 17, 4035–4049. doi: 10.1111/1462-2920.12955
Massana, R., Guillou, L., Terrado, R., Forn, I., and Pedros-Alio, C. (2006a). Growth of uncultured heterotrophic flagellates in unamended seawater incubations. Aquat. Microb. Ecol. 45, 171–180. doi: 10.3354/ame045171
Massana, R., Terrado, R., Forn, I., Lovejoy, C., and Pedros-Alio, C. (2006b). Distribution and abundance of uncultured heterotrophic flagellates in the world oceans. Environ. Microbiol. 8, 1515–1522. doi: 10.1111/j.1462-2920.2006.01042.x
Massana, R., Unrein, F., Rodríguez-Martínez, R., Forn, I., Lefort, T., Pinhassi, J., et al. (2009). Grazing rates and functional diversity of uncultured heterotrophic flagellates. ISME J. 3, 588–596. doi: 10.1038/ismej.2008.130
Matz, C., Boenigk, J., Arndt, H., and Jürgens, K. (2002). Role of bacterial phenotypic traits in selective feeding of the heterotrophic nanoflagellate Spumella sp. Aquat. Microb. Ecol. 27, 137–148. doi: 10.3354/ame027137
McCann, K., and Hastings, A. (1997). Re-evaluating the omnivory - stability relationship in food webs. Proc. Biol. Sci. 264, 1249–1254. doi: 10.1098/rspb.1997.0172
Medlin, L., and Schmidt, C. (2010). Molecular probes improve the taxonomic resolution of cryptophyte abundance in Arcachon Bayy, France. Vie Milieu 60, 9–15.
Medlin, L. K., Piwosz, K., and Metfies, K. (2017). Uncovering hidden biodiversity in the cryptophyta: clone library studies at the Helgoland time series site in the southern German Bight identifies the cryptophycean clade potentially responsible for the majority of its genetic diversity during the spring bloom. Vie Milieu 67, 27–32.
Medlin, L. K., and Strieben, S. (2010). Refining cryptophyte identification: matching cell fixation methods to FISH hybridisation of cryptomonads. J. Appl. Phycol. 22, 725–731. doi: 10.1007/s10811-010-9512-z
Metfies, K., and Medlin, L. (2007). Refining cryptophyte identification with DNA-microarrays. J. Plankton Res. 12, 1071–1075. doi: 10.1093/plankt/fbm080
Montagnes, D., and Lynn, D. (1987). A quantitative protargol stain (QPS) for ciliates: method description and test of its quantitative nature. Mar. Microb. Food Webs 2, 83–93.
Moon-van der Staay, S. Y., De Wachter, R., and Vaulot, D. (2001). Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity. Nature 409, 607–610. doi: 10.1038/35054541
Moon-van der Staay, S. Y., Tzeneva, V. A., Van Der Staay, G. W., De Vos, W. M., Smidt, H., and Hackstein, J. H. (2006). Eukaryotic diversity in historical soil samples. FEMS Microbiol. Ecol. 57, 420–428. doi: 10.1111/j.1574-6941.2006.00130.x
Moraru, C., Lam, P., Fuchs, B. M., Kuypers, M. M. M., and Amann, R. (2010). GeneFISH – an in situ technique for linking gene presence and cell identity in environmental microorganisms. Environ. Microbiol. 12, 3057–3073. doi: 10.1111/j.1462-2920.2010.02281.x
Morgan-Smith, D., Clouse, M. A., Herndl, G. J., and Bochdansky, A. B. (2013). Diversity and distribution of microbial eukaryotes in the deep tropical and subtropical North Atlantic Ocean. Deep Sea Res. I Oceanogr. Res. Pap. 78, 58–69. doi: 10.1016/j.dsr.2013.04.010
Morgan-Smith, D., Herndl, G. J., Van Aken, H. M., and Bochdansky, A. B. (2011). Abundance of eukaryotic microbes in the deep subtropical North Atlantic. Aquat. Microb. Ecol. 65, 103–115. doi: 10.3354/ame01536
Mukherjee, I., Hodoki, Y., and Nakano, S.-I. (2015). Kinetoplastid flagellates overlooked by universal primers dominate in the oxygenated hypolimnion of Lake Biwa, Japan. FEMS Microbiol. Ecol. 91:fiv083. doi: 10.1093/femsec/fiv083
Mukherjee, I., Hodoki, Y., and Nakano, S.-I. (2017). Seasonal dynamics of heterotrophic and plastidic protists in the water column of Lake Biwa, Japan. Aquat. Microb. Ecol. 80, 123–137. doi: 10.3354/ame01843
Mukherjee, I., Hodoki, Y., Okazaki, Y., Fujinaga, S., Ohbayashi, K., and Nakano, S.-I. (2019). Widespread dominance of kinetoplastids and unexpected presence of diplonemids in deep freshwater lakes. Front. Microbiol. 10:2375. doi: 10.3389/fmicb.2019.02375
Mukherjee, I., Salcher, M. M., Andrei, A.-Ş., Kavagutti, V. S., Shabarova, T., Grujčić, V., et al. (2020). A freshwater radiation of diplonemids. Environ. Microbiol. 22, 4658–4668. doi: 10.1111/1462-2920.15209
Neuenschwander, S. M., Pernthaler, J., Posch, T., and Salcher, M. M. (2015). Seasonal growth potential of rare lake water bacteria suggest their disproportional contribution to carbon fluxes. Environ. Microbiol. 17, 781–795. doi: 10.1111/1462-2920.12520
Not, F., Latasa, M., Scharek, R., Viprey, M., Karleskind, P., Balagué, V., et al. (2008). Protistan assemblages across the Indian Ocean, with a specific emphasis on the picoeukaryotes. Deep Sea Res. I Oceanogr. Res. Pap. 55, 1456–1473. doi: 10.1016/j.dsr.2008.06.007
Not, F., Massana, R., Latasa, M., Marie, D., Colson, C., Eikrem, W., et al. (2005). Late summer community composition and abundance of photosynthetic picoeukaryotes in Norwegian and Barents Seas. Limnol. Oceanogr. 50, 1677–1686. doi: 10.4319/lo.2005.50.5.1677
Not, F., Simon, N., Biegala, I. C., and Vaulot, D. (2002). Application of fluorescent in situ hybridization coupled with tyramide signal amplification (FISH-TSA) to assess eukaryotic picoplankton composition. Aquat. Microb. Ecol. 28, 157–166. doi: 10.3354/ame028157
Nygaard, K., and Hessen, D. (1990). Use of 14C-protein-labelled bacteria for estimating clearance rates by heterotrophic and mixotrophic flagellates. Mar. Ecol. Prog. Ser. 68, 7–14. doi: 10.3354/meps068007
Ohkuma, M., and Brune, A. (2010). “Diversity, structure, and evolution of the termite gut microbial community,” in Biology of Termites: A Modern Synthesis, eds D. Bignell, Y. Roisin, and N. Lo (Dordrecht: Springer), 413–438. doi: 10.1007/978-90-481-3977-4_15
Orr, R. J. S., Zhao, S., Klaveness, D., Yabuki, A., Ikeda, K., Watanabe, M. M., et al. (2018). Enigmatic Diphyllatea eukaryotes: culturing and targeted PacBio RS amplicon sequencing reveals a higher order taxonomic diversity and global distribution. BMC Evol. Biol. 18:115. doi: 10.1186/s12862-018-1224-z
Parris, D. J., Ganesh, S., Edgcomb, V. P., Delong, E. F., and Stewart, F. J. (2014). Microbial eukaryote diversity in the marine oxygen minimum zone off northern Chile. Front. Microbiol. 5:543. doi: 10.3389/fmicb.2014.00543
Patterson, D., and Larsen, J. (1991). The Biology of Free-Living Heterotrophic Flagellates. Oxford: Clarendon Press.
Pawlowski, J., Audic, S., Adl, S., Bass, D., Belbahri, L., Berney, C., et al. (2012). CBOL Protist Working Group: barcoding eukaryotic richness beyond the animal, plant, and fungal kingdoms. PLoS Biol. 10:e1001419. doi: 10.1371/journal.pbio.1001419
Pernice, M. C., Forn, I., Gomes, A., Lara, E., Alonso-Sáez, L., Arrieta, J. M., et al. (2014). Global abundance of planktonic heterotrophic protists in the deep ocean. ISME J. 9, 782–792. doi: 10.1038/ismej.2014.168
Pernice, M. C., Giner, C. R., Logares, R., Perera-Bel, J., Acinas, S., Duarte, C., et al. (2016). Large variability of bathypelagic microbial eukaryotic communities across the world’s oceans. ISME J. 10, 945–958. doi: 10.1038/ismej.2015.170
Pernthaler, A., Pernthaler, J., and Amann, R. (2002). Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 68, 3094–3101. doi: 10.1128/AEM.68.6.3094-3101.2002
Pernthaler, A., Pernthaler, J., and Amann, R. (2004). “Sensitive multi-color fluorescence in situ hybridization for the identification of environmental microorganisms,” in Molecular Microbial Ecology Manual, ed. G. E. A. Kowalchuk (Dordrecht: Kluwer Academic Press), 711–726.
Pernthaler, J. (2005). Predation on prokaryotes in the water column and its ecological implications. Nat. Rev. Microbiol. 3, 537–546. doi: 10.1038/nrmicro1180
Pitsch, G., Bruni, E. P., Forster, D., Qu, Z., Sonntag, B., Stoeck, T., et al. (2019). Seasonality of planktonic freshwater ciliates: Are analyses based on V9 regions of the 18S rRNA gene correlated with morphospecies counts? Front. Microbiol. 10:248. doi: 10.3389/fmicb.2019.00248
Piwosz, K. (2019). Weekly dynamics of abundance and size structure of specific nanophytoplankton lineages in coastal waters (Baltic Sea). Limnol. Oceanogr. 64, 2172–2186. doi: 10.1002/lno.11177
Piwosz, K., Całkiewicz, J., Gołębiewski, M., and Creer, S. (2018). Diversity and community composition of pico- and nanoplanktonic protists in the Vistula River estuary (Gulf of Gdañsk, Baltic Sea). Estuar. Coast. Shelf Sci. 207, 242–249. doi: 10.1016/j.ecss.2018.04.013
Piwosz, K., Kownacka, J., Ameryk, A., Zalewski, M., and Pernthaler, J. (2016). Phenology of cryptomonads and the CRY1 lineage in a coastal brackish lagoon (Vistula Lagoon, Baltic Sea). J. Phycol. 52, 626–637. doi: 10.1111/jpy.12424
Piwosz, K., and Pernthaler, J. (2010). Seasonal population dynamics and trophic role of planktonic nanoflagellates in coastal surface waters of the Southern Baltic Sea. Environ. Microbiol. 12, 364–377. doi: 10.1111/j.1462-2920.2009.02074.x
Piwosz, K., and Pernthaler, J. (2011). Enrichment of omnivorous cercozoan nanoflagellates from coastal Baltic Sea waters. PLoS One 6:e24415. doi: 10.1371/journal.pone.0024415
Piwosz, K., Shabarova, T., Pernthaler, J., Posch, T., Šimek, K., Porcal, P., et al. (2020). Bacterial and eukaryotic small-subunit amplicon data do not provide a quantitative picture of microbial communities, but they are reliable in the context of ecological interpretations. mSphere 5:e00052-20. doi: 10.1128/mSphere.00052-20
Piwosz, K., Spich, K., Całkiewicz, J., Weydmann, A., Kubiszyn, A. M., and Wiktor, J. M. (2015). Distribution of small phytoflagellates along an Arctic fjord transect. Environ. Microbiol. 17, 2393–2406. doi: 10.1111/1462-2920.12705
Piwosz, K., Wiktor, J. M., Niemi, A., Tatarek, A., and Michel, C. (2013). Mesoscale distribution and functional diversity of picoeukaryotes in the first-year sea ice of the Canadian Arctic. ISME J. 7, 1461–1471. doi: 10.1038/ismej.2013.39
Porter, K. G., and Feig, Y. S. (1980). The use of DAPI for identifying and counting aquatic bacteria. Limnol. Oceanogr. 25, 943–948. doi: 10.4319/lo.1980.25.5.0943
Porter, K. G., Sherr, E. B., Sherr, B. F., Pace, M. L., and Sanders, R. W. (1985). Protozoa in planktonic food webs. J. Protozool. 32, 409–415. doi: 10.1111/j.1550-7408.1985.tb04036.x
Posch, T., Eugster, B., Pomati, F., Pernthaler, J., Pitsch, G., and Eckert, E. M. (2015). Network of interactions between ciliates and phytoplankton during spring. Front. Microbiol. 6:1289. doi: 10.3389/fmicb.2015.01289
Potvin, M., and Lovejoy, C. (2009). PCR-based diversity estimates of artificial and environmental 18S rRNA gene libraries. J. Eukaryot. Microbiol. 56, 174–181. doi: 10.1111/j.1550-7408.2008.00386.x
Qu, Z., Forster, D., Bruni, E. P., Frantal, D., Kammerlander, B., Nachbaur, L., et al. (2021). Aquatic food webs in deep temperate lakes: key species establish through their autecological versatility. Mol. Ecol. doi: 10.1111/mec.15776 [Epub ahead of print].
Rice, J., Oconnor, C. D., Sleigh, M. A., Burkill, P. H., Giles, I. G., and Zubkov, M. V. (1997a). Fluorescent oligonucleotide rDNA probes that specifically bind to a common nanoflagellate, Paraphysomonas vestita. Microbiology 143, 1717–1727. doi: 10.1099/00221287-143-5-1717
Rice, J., Sleigh, M. A., Burkill, P. H., Tarran, G. A., O’connor, C. D., and Zubkov, M. V. (1997b). Flow cytometric analysis of characteristics of hybridization of species-specific fluorescent oligonucleotide probes to rRNA of marine nanoflagellates. Appl. Environ. Microbiol. 63, 938–944. doi: 10.1128/AEM.63.3.938-944.1997
Romari, K., and Vaulot, D. (2004). Composition and temporal variability of picoeukaryote communities at a coastal site of the English Channel from 18S rDNA sequences. Limnol. Oceanogr. 49, 784–798. doi: 10.4319/lo.2004.49.3.0784
Rychert, K. (2006). Nanoflagellates in the Gdañsk Basin: coexistence between form belonging to different trophic types. Oceanologia 48, 323–330.
Salani, F., Arndt, H., Hausmann, K., Nitsche, F., and Scheckenbach, F. (2011). Analysis of the community structure of abyssal kinetoplastids revealed similar communities at larger spatial scales. ISME J. 6, 713–723. doi: 10.1038/ismej.2011.138
Sanders, R., Caron, D. A., and Berninger, U. G. (1992). Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters: an inter-ecosystem comparison. Mar. Ecol. Prog. Ser. 86, 1–14. doi: 10.3354/meps086001
Santoferrara, L. F., Grattepanche, J.-D., Katz, L. A., and Mcmanus, G. B. (2014). Pyrosequencing for assessing diversity of eukaryotic microbes: analysis of data on marine planktonic ciliates and comparison with traditional methods. Environ. Microbiol. 16, 2752–2763. doi: 10.1111/1462-2920.12380
Sauvadet, A. L., Gobet, A., and Guillou, L. (2010). Comparative analysis between protist communities from the deep-sea pelagic ecosystem and specific deep hydrothermal habitats. Environ. Microbiol. 12, 2946–2964.
Scheckenbach, F., Hausmann, K., Wylezich, C., Weitere, M., and Arndt, H. (2010). Large-scale patterns in biodiversity of microbial eukaryotes from the abyssal sea floor. Proc. Natl. Acad. Sci. U.S.A. 107, 115–120. doi: 10.1111/j.1462-2920.2010.02272.x
Schulz, F., Lagkouvardos, I., Wascher, F., Aistleitner, K., Kostanjšek, R., and Horn, M. (2014). Life in an unusual intracellular niche: a bacterial symbiont infecting the nucleus of amoebae. ISME J. 8, 1634–1644.
Seeleuthner, Y., Mondy, S., Lombard, V., Carradec, Q., Pelletier, E., Wessner, M., et al. (2018). Single-cell genomics of multiple uncultured stramenopiles reveals underestimated functional diversity across oceans. Nat. Commun. 9:310.
Sekiguchi, H., Kawachi, M., Nakayama, T., and Inouye, I. (2003). A taxonomic re-evaluation of the Pedinellales (Dictyochophyceae), based on morphological, behavioural and molecular data. Phycologia 42, 165–182.
Shalchian-Tabrizi, K., Brate, J., Logares, R., Klaveness, D., Berney, C., and Jakobsen, K. S. (2008). Diversification of unicellular eukaryotes: cryptomonad colonization of marine and fresh waters inferred from revised 18S rRNA phylogeny. Environ. Microbiol. 10, 2635–2644.
Shalchian-Tabrizi, K., Kauserud, H. A. E., Massana, R., Klaveness, D., and Jakobsen, K. (2007). Analysis of environmental 18S ribosomal RNA sequences reveals unknown diversity of the cosmopolitan phylum Telonemia. Protist 158, 173–180.
Sherr, B. F., Sherr, E. B., and Fallon, R. D. (1987). Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl. Environ. Microbiol. 53, 958–965.
Sherr, B. F., Sherr, E. B., and Pedros-Alio, C. (1989). Simultaneous measurement of bacterio-plankton production and protozoan bacterivory in estuarine water. Mar. Ecol. Prog. Ser. 54, 209–219.
Sherr, E., and Sherr, B. (1988). Role of microbes in pelagic food webs: A revised concept. Limnol. Oceanogr. 33, 1225–1227.
Sherr, E. B., Sherr, B. F., Caron, D. A., Vaulot, D., and Worden, A. Z. (2007). Oceanic Protists. Oceanography 20, 130–134.
Shi, H., Shi, Q., Grodner, B., Lenz, J. S., Zipfel, W. R., Brito, I. L., et al. (2020). Highly multiplexed spatial mapping of microbial communities. Nature 588, 676–681.
Shiratori, T., and Ishida, K.-I. (2016). A new heterotrophic Cryptomonad: Hemiarma marina n. g., n. sp. J. Eukaryot. Microbiol. 63, 804–812.
Siano, R., Alves-De-Souza, C., Foulon, E., Bendif, E. M., Simon, N., Guillou, L., et al. (2011). Distribution and host diversity of Amoebophryidae parasites across oligotrophic waters of the Mediterranean Sea. Biogeosciences 8, 267–278.
Šimek, K., and Chrzanowski, T. H. (1992). Direct and indirect evidence of size-selective grazing on pelagic bacteria by freshwater nanoflagellates. Appl. Environ. Microbiol. 58, 3715–3720.
Šimek, K., Grujčić, V., Hahn, M. W., Horňák, K., Jezberová, J., Kasalický, V., et al. (2018). Bacterial prey food characteristics modulate community growth response of freshwater bacterivorous flagellates. Limnol. Oceanogr. 63, 484–502.
Šimek, K., Grujčić, V., Mukherjee, I., Kasalický, V., Nedoma, J., Posch, T., et al. (2020). Cascading effects in freshwater microbial food webs by predatory Cercozoa, Katablepharidacea and ciliates feeding on aplastidic bacterivorous cryptophytes. FEMS Microbiol. Ecol. 96:fiaa121.
Šimek, K., Grujčić, V., Nedoma, J., Jezberová, J., Šorf, M., Matoušů, A., et al. (2019). Microbial food webs in hypertrophic fishponds: omnivorous ciliate taxa are major protistan bacterivores. Limnol. Oceanogr. 64, 2295–2309.
Šimek, K., Hartman, P., Nedoma, J., Pernthaler, J., Vrba, J., Springmann, D., et al. (1997a). Community structure, picoplankton grazing and zooplankton control of heterotrophic nanoflagellates in a eutrophic reservoir during the summer phytoplankton maximum. Aquat. Microb. Ecol. 12, 49–63.
Šimek, K., Jurgens, K., Nedoma, J., Comerma, M., and Armengol, J. (2000). Ecological role and bacterial grazing of Halteria spp.: small freshwater oligotrichs as dominant pelagic ciliate bacterivores. Aquat. Microb. Ecol. 22, 43–56.
Šimek, K., Kasalický, V., Jezbera, J., Hornak, K., Nedoma, J., Hahn, M. W., et al. (2013). Differential freshwater flagellate community response to bacterial food quality with a focus on Limnohabitans bacteria. ISME J. 7, 1519–1530.
Šimek, K., Kojecká, P., Nedoma, J., Hartman, P., Vrba, J., and Dolan, J. (1999). Shifts in bacterial community composition associated with different microzooplankton size fractions in a eutrophic reservoir. Limnol. Oceanogr. 44, 1634–1644.
Šimek, K., Nedoma, J., Znachor, P., Kasalický, V., Jezbera, J., Horňák, K., et al. (2014). A finely tuned symphony of factors modulates the microbial food web of a freshwater reservoir in spring. Limnol. Oceanogr. 59, 1477–1492.
Šimek, K., Pernthaler, J., Weinbauer, M. G., Horňák, K., Dolan, J. R., Nedoma, J., et al. (2001). Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl. Environ. Microbiol. 67, 2723–2733.
Šimek, K., and Sirova, D. (2019). Fluorescently labeled bacteria as a tracer to reveal novel pathways of organic carbon flow in aquatic ecosystems. J. Vis. Exp. 151:e59903.
Šimek, K., and Straškrabová, V. (1992). Bacterioplankton production and protozoan bacterivory in a mesotrophic Reservoir. J. Plankton Res. 14, 773–787.
Šimek, K., Vrba, J., Pernthaler, J., Posch, T., Hartman, P., and Nedoma, J. (1997b). Morphological and compositional shifts in an experimental bacterial community influenced by protists with contrasting feeding modes. Appl. Environ. Microbiol. 63, 587–595.
Šimek, K., Weinbauer, M. G., Horňák, K., Jezbera, J., Nedoma, J., and Dolan, J. R. (2007). Grazer and virus-induced mortality of bacterioplankton accelerates development of Flectobacillus populations in a freshwater community. Environ. Microbiol. 9, 789–800.
Simon, M., Jardillier, L., Deschamps, P., Moreira, D., Restoux, G., Bertolino, P., et al. (2015a). Complex communities of small protists and unexpected occurrence of typical marine lineages in shallow freshwater systems. Environ. Microbiol. 17, 3610–3627.
Simon, M., Lopez-Garcia, P., Deschamps, P., Moreira, D., Restoux, G., Bertolino, P., et al. (2015b). Marked seasonality and high spatial variability of protist communities in shallow freshwater systems. ISME J. 9, 1941–1965.
Simon, N., Campbell, L., Ornolfsdottir, E., Groben, R., Guillou, L., Lange, M., et al. (2000). Oligonucleotide probes for the identification of three algal groups by dot blot and fluorescent whole-cell hybridization. J. Eukaryot. Microbiol. 47, 76–84.
Simon, N., Lebot, N., Marie, D., Partensky, F., and Vaulot, D. (1995). Fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes to identify small phytoplankton by flow cytometry. Appl. Environ. Microbiol. 61, 2506–2513.
Simpson, A. G. B., Stevens, J. R., and Lukeš, J. (2006). The evolution and diversity of kinetoplastid flagellates. Trends Parasitol. 22, 168–174.
Sogin, M. L., and Gunderson, J. H. (1987). Structural diversity of eukaryotic small subunit ribosomal RNAs. Evolutionary implications. Ann. N. Y. Acad. Sci. 503, 125–139.
Sommer, U., Adrian, R., De Senerpont Domis, L., Elser, J. J., Gaedke, U., Ibelings, B., et al. (2012). Beyond the Plankton Ecology Group (PEG) model: mechanisms driving plankton succession. Annu. Rev. Ecol. Evol. Syst. 43, 429–448.
Sommer, U., Gliwicz, M., Lampert, W., and Duncan, A. (1986). The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 106, 433–471.
Staley, J. T., and Konopka, A. (1985). Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu. Rev. Microbiol. 39, 321–346.
Steele, J. A., Countway, P. D., Xia, L., Vigil, P. D., Beman, J. M., Kim, D. Y., et al. (2011). Marine bacterial, archaeal and protistan association networks reveal ecological linkages. ISME J. 5, 1414–1425.
Steidinger, K. A., and Jangen, K. (1997). “Dinoflagellates,” in Identifying Marine Phytoplankton, ed. C. R. Tomas (San Diego, CA: Academic Press), 387–584.
Stern, R., Kraberg, A., Bresnan, E., Kooistra, W. H. C. F., Lovejoy, C., Montresor, M., et al. (2018). Molecular analyses of protists in long-term observation programmes—current status and future perspectives. J. Plankton Res. 40, 519–536.
Stock, A., Breiner, H.-W., Pachiadaki, M., Edgcomb, V., Filker, S., Cono, V., et al. (2012). Microbial eukaryote life in the new hypersaline deep-sea basin Thetis. Extremophiles 16, 21–34.
Stoeck, T., Bass, D., Nebel, M., Christen, R., Jones, M. D. M., Breiner, H.-W., et al. (2010). Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 19, 21–31.
Stoeck, T., Breiner, H.-W., Filker, S., Ostermaier, V., Kammerlander, B., and Sonntag, B. (2014). A morphogenetic survey on ciliate plankton from a mountain lake pinpoints the necessity of lineage-specific barcode markers in microbial ecology. Environ. Microbiol. 16, 430–444.
Stoeck, T., Hayward, B., Taylor, G. T., Varela, R., and Epstein, S. S. (2006). A multiple PCR-primer approach to access the microeukaryotic diversity in environmental samples. Protist 157, 31–43.
Stokes, N. A., Calvo, L. M. R., Reece, K. S., and Burreson, E. M. (2002). Molecular diagnostics, field validation, and phylogenetic analysis of Quahog Parasite Unknown (QPX), a pathogen of the hard clam Mercenaria mercenaria. Dis. Aquat. Organ. 52, 233–247.
Suttle, C. A. (2007). Marine viruses - major players in the global ecosystem. Nat. Rev. Microbiol. 5, 801–812.
Swale, E. M. F. (1969). A study of nanoplankton flagellate Pedinella hexacostata Vysotskii by light and electron microscopy. Eur. J. Phycol. 4, 65–86. doi: 10.1080/00071616900650051
Šlapeta, J., Lopez-Garcia, P., and Moreira, D. (2006). Global dispersal and ancient cryptic species in the smallest marine eukaryotes. Mol. Biol. Evol 23, 23–29. doi: 10.1093/molbev/msj001
Taib, N., Mangot, J.-F., Domaizon, I., Bronner, G., and Debroas, D. (2013). Phylogenetic affiliation of SSU rRNA genes generated by massively parallel sequencing: new insights into the freshwater protist diversity. PLoS One 8:e58950. doi: 10.1371/journal.pone.0058950
Thaler, M., and Lovejoy, C. (2012). Distribution and diversity of a protist predator Cryothecomonas (Cercozoa) in Arctic marine waters. J. Eukaryot. Microbiol. 59, 291–299. doi: 10.1111/j.1550-7408.2012.00631.x
Thiele, S., Wolf, C., Schulz, I. K., Assmy, P., Metfies, K., and Fuchs, B. M. (2014). Stable composition of the nano- and picoplankton community during the ocean iron fertilization experiment LOHAFEX. PLoS One 9:e113244. doi: 10.1371/journal.pone.0113244
Töbe, K., Alpermann, T., Tillmann, U., Krock, B., Cembella, A., and John, U. (2013). Molecular discrimination of toxic and non-toxic Alexandrium species (Dinophyta) in natural phytoplankton assemblages from the Scottish coast of the North Sea. Eur. J. Phycol. 48, 12–26. doi: 10.1080/09670262.2012.752870
Toebe, K., Joshi, A. R., Messtorff, P., Tillmann, U., Cembella, A., and John, U. (2012). Molecular discrimination of taxa within the dinoflagellate genus Azadinium, the source of azaspiracid toxins. J. Plankton Res. 35, 225–230. doi: 10.1093/plankt/fbs077
Touzet, N., Davidson, K., Pete, R., Flanagan, K., Mccoy, G., Amzil, Z., et al. (2010). Co-Occurrence of the West European (Gr.III) and North American (Gr.I) Ribotypes of Alexandrium tamarense (Dinophyceae) in Shetland, Scotland. Protist 161, 370–384. doi: 10.1016/j.protis.2009.12.001
Triado-Margarit, X., and Casamayor, E. O. (2012). Genetic diversity of planktonic eukaryotes in high mountain lakes (Central Pyrenees, Spain). Environ. Microbiol. 14, 2445–2456. doi: 10.1111/j.1462-2920.2012.02797.x
Unrein, F., Gasol, J. M., Not, F., Forn, I., and Massana, R. (2014). Mixotrophic haptophytes are key bacterial grazers in oligotrophic coastal waters. ISME J. 8, 164–176. doi: 10.1038/ismej.2013.132
Valm, A. M., Welch, J. L. M., Rieken, C. W., Hasegawa, Y., Sogin, M. L., Oldenbourg, R., et al. (2011). Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc. Natl. Acad. Sci. U.S.A. 108, 4152–4157. doi: 10.1073/pnas.1101134108
von der Heyden, S., and Cavalier-Smith, T. (2005). Culturing and environmental DNA sequencing uncover hidden kinetoplastid biodiversity and a major marine clade within ancestrally freshwater Neobodo designis. Int. J. Syst. Evol. Microbiol. 55, 2605–2621. doi: 10.1099/ijs.0.63606-0
Wasmund, N., Tuimala, J., Suikkanen, S., Vandepitte, L., and Kraberg, A. (2011). Long-term trends in phytoplankton composition in the western and central Baltic Sea. J. Mar. Syst. 87, 145–159. doi: 10.1016/j.jmarsys.2011.03.010
Weber, F., Mylnikov, A., Jürgens, K., and Wylezich, C. (2017). Culturing heterotrophic protists from the Baltic Sea: mostly the ‘usual suspects’ but a few novelties as well. J. Eukaryot. Microbiol. 64, 153–163. doi: 10.1111/jeu.12347
Weisse, T., Anderson, R., Arndt, H., Calbet, A., Hansen, P., and Djs, M. (2016). Functional ecology of aquatic phagotrophic protists - Concepts, limitations, and perspectives. Eur. J. Protistol. 55, 50–74. doi: 10.1016/j.ejop.2016.03.003
Weitere, M., and Arndt, H. (2003). Structure of the heterotrophic flagellate community in the water column of the River Rhine (Germany). Eur. J. Protistol. 39, 287–300. doi: 10.1078/0932-4739-00913
Wendeberg, A., Zielinski, F. U., Borowski, C., and Dubilier, N. (2012). Expression patterns of mRNAs for methanotrophy and thiotrophy in symbionts of the hydrothermal vent mussel Bathymodiolus puteoserpentis. ISME J. 6, 104–112. doi: 10.1038/ismej.2011.81
Winter, C., Bouvier, T., Weinbauer, M. G., and Thingstad, T. F. (2010). Trade-offs between competition and defense specialists among unicellular planktonic organisms: the ‘Killing the Winner’ hypothesis revisited. Microbiol. Mol. Biol. Rev. 74, 42–57. doi: 10.1128/MMBR.00034-09
Worden, A. Z., Follows, M. J., Giovannoni, S. J., Wilken, S., Zimmerman, A. E., and Keeling, P. J. (2015). Rethinking the marine carbon cycle: factoring in the multifarious lifestyles of microbes. Science 347:1257594. doi: 10.1126/science.1257594
Xie, F., Timme, K. A., and Wood, J. R. (2018). Using Single Molecule mRNA Fluorescent in Situ Hybridization (RNA-FISH) to Quantify mRNAs in Individual Murine Oocytes and Embryos. Sci. Rep. 8:7930. doi: 10.1038/s41598-018-26345-0
Zhan, Z., Stoeck, T., Dunthorn, M., and Xu, K. (2014). Identification of the pathogenic ciliate Pseudocohnilembus persalinus (Oligohymenophorea: Scuticociliatia) by fluorescence in situ hybridization. Eur. J. Protistol. 50, 16–24. doi: 10.1016/j.ejop.2013.09.004
Zhu, F., Massana, R., Not, F., Marie, D., and Vaulot, D. (2005). Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol. Ecol. 52, 79–92. doi: 10.1016/j.femsec.2004.10.006
Zimmerman, A. E., Howard-Varona, C., Needham, D. M., John, S. G., Worden, A. Z., Sullivan, M. B., et al. (2020). Metabolic and biogeochemical consequences of viral infection in aquatic ecosystems. Nat. Rev. Microbiol. 18, 21–34. doi: 10.1038/s41579-019-0270-x
Zubkov, M. V., and Sleigh, M. A. (2000). Comparison of growth efficiencies of protozoa growing on bacteria deposited on surfaces and in suspension. J. Eukaryot. Microbiol. 47, 62–69. doi: 10.1111/j.1550-7408.2000.tb00012.x
Zubkov, M. V., Sleigh, M. A., and Burkill, P. H. (1998). Measurement of bacterivory by protists in open ocean waters. FEMS Microbiol. Ecol. 27, 85–102. doi: 10.1111/j.1574-6941.1998.tb00527.x
Keywords: aquatic microbial food webs, CARD-FISH, grazing by protists, protists, unicellular eukaryotes, bacterivorous, omnivorous and predatory flagellates, heterotrophic and mixotrophic flagellates
Citation: Piwosz K, Mukherjee I, Salcher MM, Grujčić V and Šimek K (2021) CARD-FISH in the Sequencing Era: Opening a New Universe of Protistan Ecology. Front. Microbiol. 12:640066. doi: 10.3389/fmicb.2021.640066
Received: 10 December 2020; Accepted: 09 February 2021;
Published: 04 March 2021.
Edited by:
Télesphore Sime-Ngando, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Klaus Jürgens, Leibniz Institute for Baltic Sea Research (LG), GermanyCopyright © 2021 Piwosz, Mukherjee, Salcher, Grujčić and Šimek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kasia Piwosz, a3Bpd29zekBtaXIuZ2R5bmlhLnBs; Karel Šimek, a3NpbWVrQGhidS5jYXMuY3o=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.