
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 18 February 2021
Sec. Food Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.634555
This article is part of the Research Topic Emerging Frontiers in the Formation of Viable but Non-Culturable Microorganisms and Biofilms During Food Processing View all 25 articles
Salmonella enterica is a typical foodborne pathogen with multiple toxic effects, including invasiveness, endotoxins, and enterotoxins. Viable but nonculturable (VBNC) is a type of dormant form preserving the vitality of microorganisms, but it cannot be cultured by traditional laboratory techniques. The aim of this study is to develop a propidium monoazide-crossing priming amplification (PMA-CPA) method that can successfully detect S. enterica rapidly with high sensitivity and can identify VBNC cells in food samples. Five primers (4s, 5a, 2a/1s, 2a, and 3a) were specially designed for recognizing the specific invA gene. The specificity of the CPA assay was tested by 20 different bacterial strains, including 2 standard S. enterica and 18 non-S. enterica bacteria strains covering Gram-negative and Gram-positive isolates. Except for the two standard S. enterica ATCC14028 and ATCC29629, all strains showed negative results. Moreover, PMA-CPA can detect the VBNC cells both in pure culture and three types of food samples with significant color change. In conclusion, the PMA-CPA assay was successfully applied on detecting S. enterica in VBNC state from food samples.
During food processing, food is frequently contaminated by foodborne bacteria, including Staphylococcus aureus, Salmonella enterica, and Escherichia coli O157 (Kirk et al., 2015; Miao et al., 2017a; Sharma et al., 2019). S. enterica is a typical foodborne pathogen with multiple toxic effects, including invasiveness, endotoxins, and enterotoxins (Eng et al., 2015). Various serotypes of Salmonella are implicated in foodborne infections and contaminate food products, including eggs, milk, poultry, meat, and vegetables. It is the main cause of human gastrointestinal and other related diseases (Bao et al., 2017a, b; Wen et al., 2020). Recently, studies confirmed that S. enterica is capable of entering into the viable but non-culturable state (VBNC) state under an adverse environment, which could include low-temperature, salt stress, and nutrient starvation (Roszak et al., 1984; Chmielewski and Frank, 1995; Gupte et al., 2003; Zeng et al., 2013; Morishige et al., 2017; Highmore et al., 2018). VBNC cells cannot be detected by traditional culture-based methods (Xu et al., 2011a; Lin et al., 2017; Miao et al., 2017a; Xie et al., 2017a). Therefore, it is urgent to develop a rapid and sensitive assay to detect S. enterica, especially in the VBNC state.
The molecular biological method, classified into polymerase chain reaction (PCR) and isothermal amplification, is a new strategy to detect pathogens (Xu et al., 2012; Xu et al., 2012a; Liu et al., 2019). PCR and PCR-based assays have been well developed in the last few decades (You et al., 2012; Zhong et al., 2013; Xu et al., 2016c). However, these assays require complicated procedures that may increase the uncertainty in result determination (Tada et al., 1992; Zhong et al., 2013; Xu et al., 2017a; Xie et al., 2017b; Jia et al., 2018). Quantitative real-time PCR can achieve the result interpretation by digital curve without electrophoresis but with lower detection limits (Xu et al., 2007; Miao et al., 2017b, c). Isothermal amplification assays include loop-mediated isothermal amplification (LAMP), rolling circle amplification (RCA), and strand displacement amplification (SDA) (Fire and Xu, 1995; Zhao et al., 2009; Zhao et al., 2010a, b; Bao et al., 2017c; Xu et al., 2017b). Reverse transcription LAMP (RT-LAMP) or other isothermal amplification assays have been utilized to replace the PCR assay (Parida et al., 2005).
Cross Priming Amplification (CPA) is a novel isothermal method relying on five primers (2a/1s, 2a, 3a, 4s, and 5a) to amply the target nucleotide sequences (Xu et al., 2012). It does not require any special instrumentation and presents high rapidity, specificity, and sensitivity. Recently, CPA assays have been used for the detection of E. coli O157:H7, Listeria monocytogenes, Enterobacter sakazakii, Yersinia enterocolitica, and other pathogens (Wang et al., 2014; Zhang et al., 2015; Wang et al., 2018; Xu et al., 2020). Therefore, CPA is a potentially valuable tool for the rapid detection of foodborne pathogens, and the combination of propidium monoazide (PMA) may achieve the detection of VBNC state (Xu et al., 2010; Wang et al., 2011; Xu et al., 2011c).
To standardize and evaluate the reaction system of CPA assay, non-S. enterica bacteria strains, including various species of Gram-negative and Gram-positive non-target strains, were used in this study (Table 1). Two standard S. enterica ATCC14028 and ATCC29629 were used as positive controls. All strains used in this study had been preliminarily identified in the Lab of Clinical Microbiology, Zhongshan Supervision Testing Institute of Quality and Metrology.
All bacteria were prepared for genomic DNA isolation after incubation in trypticase soy broth (TSB, Huankai Microbial, China) at 37°C at 200 rpm overnight. The genomic DNA was isolated by Bacterial DNA extraction Kit (Dongsheng Biotech, Guangzhou, China) according to the manufacturer’s protocol. The concentration and quality of the DNA were measured using Nano Drop 2000 (Thermo Fisher Scientific Inc., Waltham, MA, United States) at 260 and 280 nm. The isolated DNA was stored at −20°C for further use.
As a species specific gene in Salmonella, invA has been selected, and its specificity in Salmonella has been previously confirmed. The CPA primers were specifically designed for invA gene in S. enterica using Primer Premier 5, including five primers recognizing five distinct regions in the gene open reading frame sequence (Table 2). All primers were assessed for specificity by BLAST against the sequences in Genebank.
The PCR reaction was conducted to serve as a control in a total 25 μL volume with 12.5 μL 2× Taq PCR Master Mix (Dongsheng Biotech, Guangzhou, China), 3 μM each of forward and reverse primers, 2 μL of DNA template and the total volume was added up to 25 μL with nuclease-free water. The amplification procedure included a 5-min denaturation at 95°C, 32 cycles of amplification at 95°C for 30 s, 52°C 30 s, 72°C for 35 s, and final amplification at 72°C for 5 min. The PCR products were detected by electrophoresis on 1.5% agarose gels.
The CPA reaction system was performed using thermostatic equipment or a water bath in a 26 μL system, containing 20 mM Tris–HCl, 10 mM (NH4)2SO4, 10 mM KCl, 8.0 mM MgSO4, 0.1% Tween 20, 0.7 M betain (sigma), 1.4 mM dNTP (each), 8 U Bst DNA polymerase (NEB, United States), 1.0 μM primer of 2a/1s, 0.5 μM (each) primer of 2a and 3a, 0.6 μM (each) primer of 4s and 5a, 1 μL mixture chromogenic agent (mixture with calcein and Mn2+), 1 μL template DNA, and a volume of up to 26 μL of nuclease-free water. The mixed chromogenic agent consists of 0.13 mM calcein and 15.6 mM MnCl2⋅4H2O. And mixed reaction solution was incubated at 65°C for 60 min and heated at 80°C for 2 min to terminate the reaction. Nuclease-free water substituted target DNA was used as a negative control. Subsequently, the amplified products were analyzed by electrophoresis on 1.5% agarose gels and observed the color change by naked eyes.
The specificity of CPA was evaluated by amplifying the genomic DNA extracted from 2 standard S. enterica strains and 18 non-S. enterica strains.
To determine the sensitivity of the CPA assay, serial 10-fold dilutions of the genomic DNA of S. enterica ATCC14280 were prepared and used in the reaction. The sensitivity of the CPA method was compared with the PCR method. All the tests were performed in triplicate.
The application of CPA assay in the detection of S. enterica was conducted in three rice products (Cantonese rice cake, steamed bread, and rice noodle purchased from Guangzhou Restaurant, Guangzhou, China). Different concentrations (from 108 CFU/mL to 10 CFU/mL) of S. enterica ATCC14028 were applied to contaminate food samples. Subsequently, genomic DNA was extracted from the contaminated food samples and subjected to CPA and PCR methods in triplicate (Xu et al., 2020).
The VBNC state of S. enterica was induced by oligotrophic medium (sterile saline) at a low temperature. The bacterial overnight culture (∼108 CFU/mL) was washed three times and resuspended by sterile saline and then stored at −20°C. The culturable cell number was measured by plate counting method, and viable cells were determined by LIVE/DEAD® BacLight kitTM (ThermoFisher scientific, United States) with a fluorescence microscope after the cells were no longer culturable. The culturable and viable cell enumerations were performed every three days.
The PMA-CPA were developed to detect the VBNC cells of S. enterica with the observation of color change. The PMA-CPA was further applied in the detection of VBNC cells in contaminated rice food products (Cantonese rice cake, steamed bread, and rice noodle from Guangzhou Restaurant, Guangzhou, China).
The CPA assays for the detection of invA gene were set up using S. enterica ATCC14028. The products were analyzed by 1.5% agarose gel electrophoresis, and the bands were observed under UV light (Figure 1A). The results of electrophoresis revealed that the amplicons of CPA are of various sizes, showing as a ladder pattern on agarose gel instead of a single band. The fluorescent dye (MgCl2 and calcein) changes the reaction system from orange to green in the reaction system (Figure 1B).
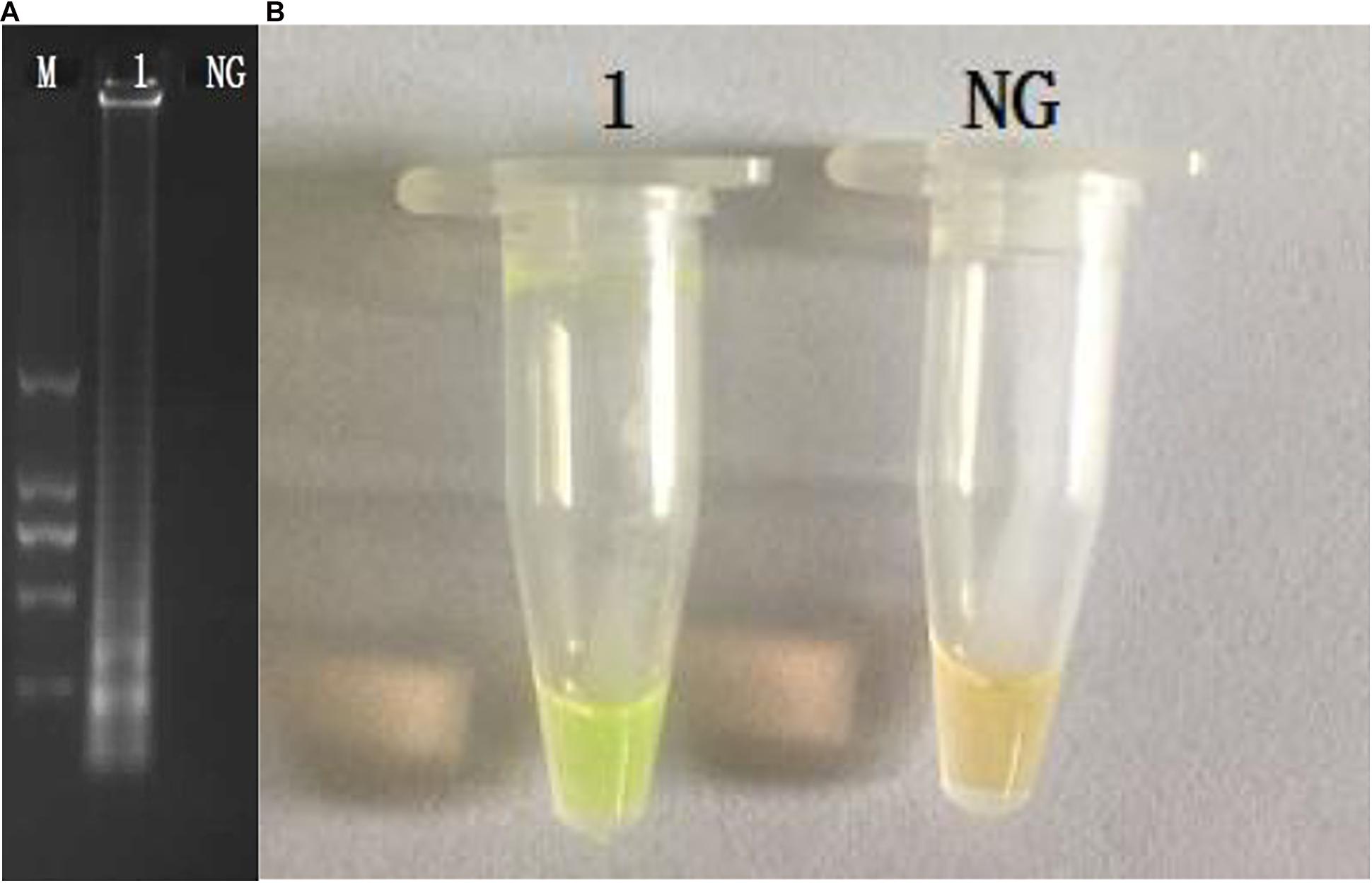
Figure 1. Amplification products were visually detected both by 1.5% agarose gel electrophoresis under UV light (A) and observation at the color change by naked eye (B). M, DNA marker; lane 1, positive control; lane NC, negative products (A); tube 1, positive products; tube NC, negative control.
The evaluation of the specificity of CPA assay was performed in 2 S. enterica strains and 18 non-S. enterica reference strains. Results were recorded by 1.5% agarose gel electrophoresis and color change. Ladder pattern bands and orange to yellow color changes were only observed in the 2 S. enterica strains (Figure 2). It indicated that only the target S. enterica strains were detected with positive results, showing the high specificity of the CPA assay.
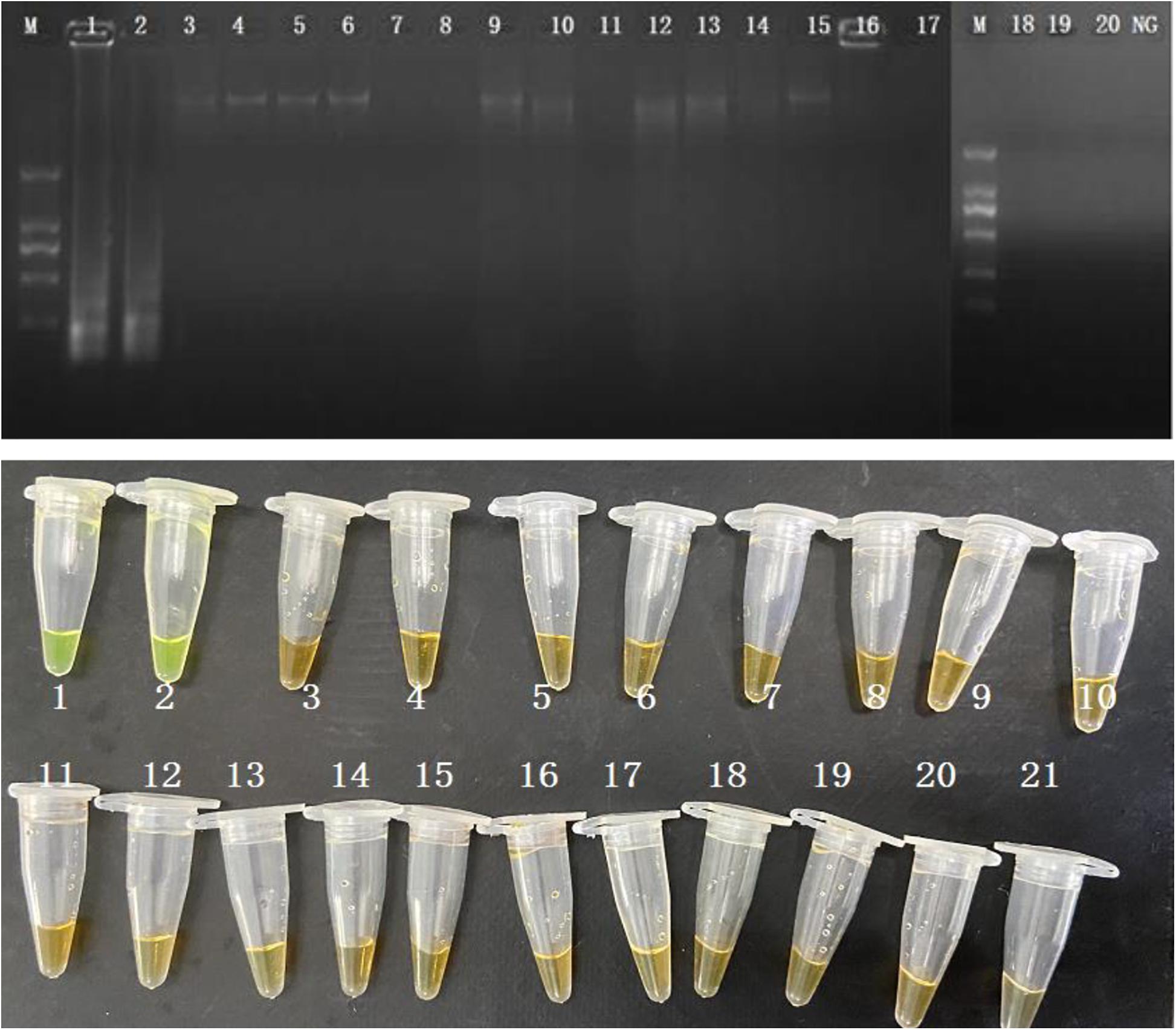
Figure 2. Specificity of CPA assay for detection S. enterica strains with invA genes by 1.5% agarose gel electrophoresis and observation at the color change by naked eye; M-DNA marker; lane 1–2, S. enterica ATCC 29629 and ATCC 14028; lane 2–20, non-S. enterica strains. NG/21, negative control.
The CPA assays were applied in the detection of S. enterica in three food samples (Cantonese rice cake, steamed bread, and rice noodle). The homogenized rice products (9 mL) were inoculated with 1 mL 10-fold serial dilution of pure S. enterica culture. The concentration of artificial contamination food samples ranging from 108 CFU/mL to 10 CFU/mL with 10-fold series dilutions was applied. All three food samples were included for the application. As expected, the LOD shows an insignificant difference among food samples, and the results are identical. Only the samples with a concentration higher than 103 CFU/mL were able to be detected (Figure 3). Thus, the detection limit of the CPA assay was 103 CFU/mL.

Figure 3. Sensitivity of the CPA assay in food samples of S. enterica with invA genes by 1.5% agarose gel electrophoresis (A) and agent mixture (B); M-DNA marker; lane 1–8, 107 CFU/mL, 106 CFU/mL, 105 CFU/mL, 104 CFU/mL, 103 CFU/mL, 102 CFU/mL, 10 CFU/mL negative control.
The PMA-CPA assay was established using VBNC cells of S. enterica. The PMA dye was added to either pure culture or flour samples with a final concentration of 5 μg/mL. After incubation at room temperature for 10 min in dark, the samples were exposed to a 650 W halogen lamp with a distance of 15 cm for 5 min, which inactivates unbinding PMA molecules rather than PMA-DNA molecules. All the dying process was performed in an ice bath to prevent DNA damage. Results showed that PMA-CPA can detect the VBNC cells both in pure culture and food samples with significant color change from orange to yellow (Figure 4). The samples with dead cells remain orange in both pure culture and food samples.
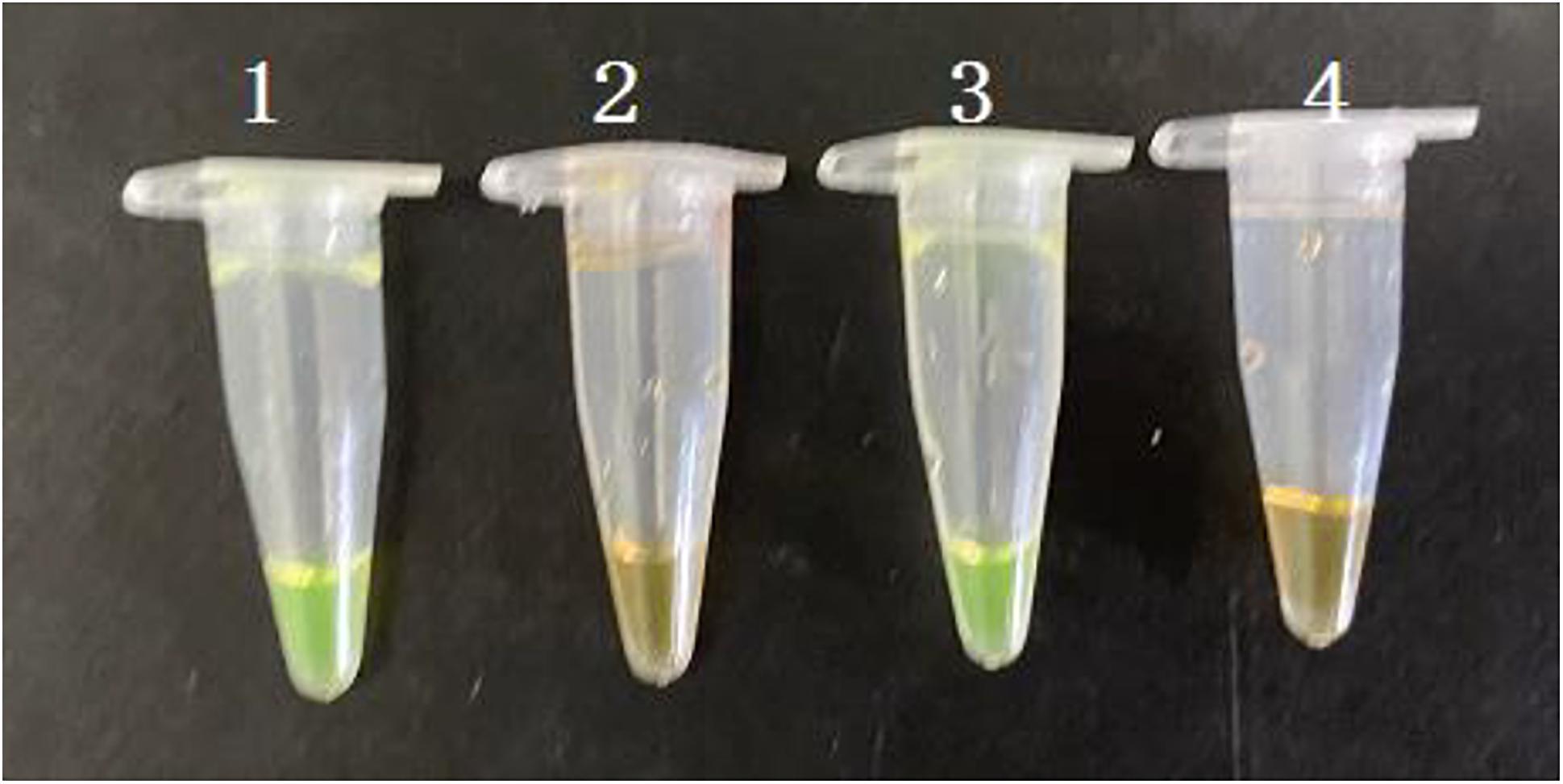
Figure 4. Detection of VBNC state of S. enterica in pure cultures and food samples by PMA-CPA assay with observation at the color change by naked eye. 1 – VBNC cells in pure cultures; 2 – dead cells in pure cultures; 3 – VBNC cells in food samples; 4 – dead cells in food samples.
Salmonella enterica is a common foodborne pathogen that may cause serve illness. The generation of S. enterica VBNC state occurs during the chlorination of wastewater or food (Oliver et al., 2005; Zeng et al., 2013). Non-ionic detergents and sanitizers can also induce S. enterica into VBNC state (Morishige et al., 2013; Purevdorj-Gage et al., 2018; Robben et al., 2018). Furthermore, a multi-stress environment in complex components of food and storage conditions may induce the VBNC state formation of foodborne pathogens (Lin et al., 2016; Miao et al., 2016; Xu et al., 2016a,b).
Various molecular techniques have been developed to identify microbes. PCR is a mature method to detect foodborne microbes, but it has low sensitivity and complex variable temperature programs (Kim et al., 1999). RT-PCR has been used to detect microbes compared with the method in ISO 21872-1:2007. RT-PCR achieved higher sensitivity but with long pre-enrichment and 24 h of complicate procedure. Although RT-PCR is able to show results via a digital curve, the sensitivity of this method is limited and the procedure is complicated (Xu et al., 2008a; Miao et al., 2018; Xu et al., 2018; Zhao et al., 2018a,b). RT-LAMP or other isothermal amplification has been used reported to replace RT-PCR to increase the detection limit (Xu et al., 2008b; Xu et al., 2009; Liu et al., 2018a,b; Lv et al., 2020). However, sophisticated equipment with these technologies has brought certain difficulties to rapid on-site testing (Xu et al., 2011b; Xu et al., 2012b). CPA technique is a new strategy to achieve rapid detection and can be performed under constant temperature using a simple water bath. Furthermore, with the improvement of fluorescence dye, results can be identified by the color change in the reaction tube by the naked eye. For VBNC and dead cells, they are both nonculturable. However, VBNC cells differ from dead cells in their intact cell membrane and thus could be differentiated via PMA, which is capable of differentiating viable and dead cells.
Therefore, the developed CPA method can successfully detect the S. enterica with high rapidity and sensitivity and can identify the VBNC cells in food samples when combining with PMA.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JL conceived of the study and participated in its design and coordination. AO and KW performed the experimental work and collected the data. YY and XG organized the database. LC and LQ performed the statistical analysis. AO wrote the manuscripts. All authors contributed to manuscript revision, read, and approved the submitted manuscript.
This work was supported by the National Key Research and Development Program of China (2016YFD04012021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bao, X. R., Jia, X. Y., Chen, L. Q., Peters, B. M., Lin, C. W., Chen, D. Q., et al. (2017c). Effect of Polymyxin Resistance (pmr) on Biofilm Formation of Cronobacter sakazakii. Microb. Pathog. 106, 16–19.
Bao, X. R., Yang, L., Chen, L. Q., Li, B., Li, L., Li, Y. Y., et al. (2017b). Analysis on pathogenic and virulent characteristics of the Cronobacter sakazakii strain BAA-894 by whole genome sequencing and its demonstration in basic biology science. Microb. Pathog. 109, 280–286.
Bao, X. R., Yang, L., Chen, L. Q., Li, B., Li, L., Li, Y. Y., et al. (2017a). Virulent and pathogenic features on the Cronobacter sakazakii polymyxin resistant pmr mutant strain s-3. Microb. Pathog. 110, 359–364.
Chmielewski, R. A., and Frank, J. F. (1995). Formation of viable but nonculturable Salmonella during starvation in chemically defined solutions. Lett. Appl. Microbiol. 20, 380–384.
Eng, S.-K., Pusparajah, P., Ab Mutalib, N.-S., Ser, H.-L., Chan, K.-G., and Lee, L.-H. (2015). Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 8:284–293. doi: 10.1080/21553769.2015.1051243
Fire, A., and Xu, S. Q. (1995). Rolling replication of short DNA circles. Proc. Natl. Acad. Sci. U.S.A. 92, 4641–4645.
Gupte, A. R., de Rezende, C. L. E., and Joseph, S. W. (2003). Induction and resuscitation of viable but nonculturable Salmonella enterica serovar typhimurium DT104. Appl. Environ. Microbiol. 69, 6669–6675.
Highmore, C. J., Warner, J. C., Rothwell, S. D., Wilks, S. A., and Keevil, C. W. (2018). Viable-but-nonculturable Listeria monocytogenes and Salmonella enterica serovar thompson induced by chlorine stress remain infectious. mBio 9:e540–18.
Jia, X. Y., Hua, J. J., Liu, L., Xu, Z. B., and Li, Y. Y. (2018). Phenotypic characterization of pathogenic Cronobacter spp. strains. Microb. Pathog. 121, 232–237.
Kim, Y. B., Okuda, J., Matsumoto, C., Takahashi, N., Hashimoto, S., and Nishibuchi, M. (1999). Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J. Clin. Microbiol. 37:1173.
Kirk, M. D., Pires, S. M., Black, R. E., Caipo, M., Crump, J. A., Devleesschauwer, B., et al. (2015). World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 12:e1001921. doi: 10.1371/journal.pmed.1001921
Kusumoto, A., Asakura, H., and Kawamoto, K. (2012). General stress sigma factor RpoS influences time required to enter the viable but non-culturable state in Salmonella enterica. Microbiol. Immunol. 56, 228–237.
Lin, S. Q., Li, L., Li, B., Zhang, X. H., Lin, C. W., Deng, Y., et al. (2016). Development and evaluation of quantitative detection of N-epsilon-carboxymethyl-lysine in Staphylococcus aureus biofilm by LC-MS method. Basic Clin. Pharmacol. Toxicol. 118:33.
Lin, S. Q., Yang, L., Chen, G., Li, B., Chen, D. Q., Li, L., et al. (2017). Pathogenic features and characteristics of food borne pathogens biofilm: Biomass, viability and matrix. Microb. Pathog. 111, 285–291.
Liu, L., Ye, C., Soteyome, T., Zhao, X., and Harro, J. M. (2019). Inhibitory effects of two types of food additives on biofilm formation by foodborne pathogens. MicrobiologyOpen 8:e00853.
Liu, L. Y., Lu, Z. R., Li, L., Li, B., Zhang, X., Zhang, X. M., et al. (2018a). Physical relation and mechanism of ultrasonic bactericidal activity on pathogenic E. coli with WPI. Microb. Pathog. 117, 73–79.
Liu, L. Y., Xu, R. R., Li, L., Li, B., Zhang, X., Zhang, X. M., et al. (2018b). Correlation and in vitro mechanism of bactericidal activity on E. coli with whey protein isolate during ultrasonic treatment. Microb. Pathog. 115, 154–158.
Lv, X., Wang, L., Zhang, J., Zeng, H., Chen, X., Shi, L., et al. (2020). Rapid and sensitive detection of VBNC Escherichia coli O157: H7 in beef by PMAxx and real-time LAMP. Food Control 115:107292.
Miao, J., Chen, L. Q., Wang, J. W., Wang, W. X., Chen, D. Q., Li, L., et al. (2017a). Evaluation and application of molecular genotyping on nosocomial pathogen-methicillin-resistant Staphylococcus aureus isolates in Guangzhou representative of Southern China. Microb. Pathog. 107, 397–403.
Miao, J., Chen, L. Q., Wang, J. W., Wang, W. X., Chen, D. Q., Li, L., et al. (2017c). Current methodologies on genotyping for nosocomial pathogen methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathog. 107, 17–28.
Miao, J., Liang, Y., Chen, L., Wang, W., Wang, J., Li, B., et al. (2017a). Formation and development of Staphylococcus biofilm: with focus on food safety. J. Food Safety 37:e12358.
Miao, J., Liang, Y. R., Chen, L. Q., Wang, W. X., Wang, J. W., Li, B., et al. (2017b). Formation and development of Staphylococcus biofilm: With focus on food safety. J. Food Safety 7:e12358.
Miao, J., Peters, B. M., Li, L., Li, B., Zhao, X. H., Xu, Z. B., et al. (2016). Evaluation of ERIC-PCR for fingerprinting methicillin-resistant staphylococcus aureus strains. Basic Clin. Pharmacol. Toxicol. 118:33.
Miao, J., Wang, W. X., Xu, W. Y., Su, J. Y., Li, L., Li, B., et al. (2018). The fingerprint mapping and genotyping systems application on methicillin-resistant Staphylococcus aureus. Microb. Pathog. 125, 246–251.
Morishige, Y., Fujimori, K., and Amano, F. (2013). Differential resuscitative effect of pyruvate and its analogues on VBNC (viable but non-culturable) Salmonella. Microb. Environ. 28, 180–186.
Morishige, Y., Koike, A., Tamura-Ueyama, A., and Amano, F. (2017). Induction of viable but nonculturable Salmonella in exponentially grown cells by exposure to a low-humidity environment and their resuscitation by catalase. J. Food Prot. 80, 288–294.
Oliver, J. D., Dagher, M., and Linden, K. (2005). Induction of Escherichia coli and Salmonella typhimurium into the viable but nonculturable state following chlorination of wastewater. J. Water Health 3, 249–257.
Parida, M., Horioke, K., Ishida, H., Dash, P. K., Saxena, P., Jana, A. M., et al. (2005). Rapid detection and differentiation of dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. J. Clin. Microbiol. 43:2895.
Purevdorj-Gage, L., Nixon, B., Bodine, K., Xu, Q., and Doerrler, W. T. (2018). Differential effect of food sanitizers on formation of viable but nonculturable Salmonella enterica in poultry. J. Food Protect. 81, 386–393.
Robben, C., Fister, S., Witte, A. K., Schoder, D., Rossmanith, P., and Mester, P. (2018). Induction of the viable but non-culturable state in bacterial pathogens by household cleaners and inorganic salts. Sci. Rep. 8:15132.
Roszak, D. B., Grimes, D. J., and Colwell, R. R. (1984). Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can. J Microbiol. 30, 334–338.
Sharma, J., Kumar, D., Hussain, S., Pathak, A., Shukla, M., Prasanna Kumar, V., et al. (2019). Prevalence, antimicrobial resistance and virulence genes characterization of nontyphoidal Salmonella isolated from retail chicken meat shops in Northern India. Food Control 102, 104–111.
Tada, J., Ohashi, T., Nishimura, N., Shirasaki, Y., Ozaki, H., Fukushima, S., et al. (1992). Detection of the thermostable direct hemolysin gene (tdh) and the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus by polymerase chain reaction. Mol. Cell. Probe. 6, 477–487.
Wang, L., Zhao, X. H., Chu, J., Li, Y., Li, Y. Y., Li, C. H., et al. (2011). Application of an improved loop-mediated isothermal amplification detection of Vibrio parahaemolyticus from various seafood samples. African J. Microbiol. Res. 5, 5765–5771.
Wang, Y., Wang, Y., Ma, A., Li, D., and Ye, C. (2014). Rapid and sensitive detection of Listeria monocytogenes by cross-priming amplification of lmo0733 gene. FEMS Microbiol. Lett. 361, 43–51.
Wang, Y. X., Zhang, A. Y., Yang, Y. Q., Lei, C. W., Cheng, G. Y., Zou, W. C., et al. (2018). Sensitive and rapid detection of Salmonella enterica serovar Indiana by cross-priming amplification. J. Microbiol. Methods 153, 24–30.
Wen, S. X., Feng, D. H., Chen, D. Q., Yang, L., and Xu, Z. B. (2020). Molecular epidemiology and evolution of Haemophilus influenzae. Infect. Genet. Evol. 80:104205.
Xie, J., Yang, L., Peters, B. M., Chen, L., Chen, D., Li, B., et al. (2017a). A 16-year retrospective surveillance report on the pathogenic features and antimicrobial susceptibility of Pseudomonas aeruginosa isolated from Guangzhou representative of Southern China. Microb. Pathog. 110, 37–41.
Xie, J. H., Peters, B. M., Li, B., Li, L., Yu, G. C., Xu, Z. B., et al. (2017b). Clinical features and antimicrobial resistance profiles of important Enterobacteriaceae pathogens in Guangzhou representative of Southern China, 2001-2015. Microb. Pathog. 107, 206–211.
Xu, G., Hu, L., Zhong, H., Wang, H., Yusa, S.-I., Weiss, T. C., et al. (2012). Cross priming amplification: mechanism and optimization for isothermal DNA amplification. Sci. Rep. 2:246.
Xu, Z., Luo, Y., Soteyome, T., Lin, C.-W., Xu, X., Mao, Y., et al. (2020). Rapid detection of food-borne Escherichia coli O157:H7 with visual inspection by crossing priming amplification (CPA). Food Anal. Methods 13, 474–481.
Xu, Z. B., Gui, Z. Y., Li, L., Li, B., Su, J. Y., Zhao, X. H., et al. (2012b). Expression and purification of gp41-gp36 Fusion protein and application in serological screening assay of HIV-1 and HIV-2. African J. Microbiol. Res. 6, 6295–6299.
Xu, Z. B., Hou, Y. C., Peters, B. M., Chen, D. Q., Li, B., and Li, L. (2016b). Chromogenic media for MRSA diagnostics. Mol. Biol. Rep. 43, 1205–1212.
Xu, Z. B., Hou, Y. C., Qin, D., Liu, X. C., Li, B., Li, L., et al. (2016a). Evaluation of current methodologies for rapid identification of methicillin-resistant staphylococcus aureus strains. Basic Clin. Pharmacol. Toxicol. 118:33.
Xu, Z. B., Li, L., Alam, M. J., Zhang, L. Y., Yamasaki, S., and Shi, L. (2008b). First confirmation of integron-bearing methicillin-resistant Staphylococcus aureus. Curr. Microbiol. 57, 264–268.
Xu, Z. B., Li, L., Chu, J., Peters, B. M., Harris, M. L., Li, B., et al. (2012a). Development and application of loop-mediated isothermal amplification assays on rapid detection of various types of staphylococci strains. Food Res. Int. 47, 166–173.
Xu, Z. B., Li, L., Shi, L., and Shirliff, M. E. (2011c). Class 1 integron in staphylococci. Mol. Biol. Rep. 38, 5261–5279.
Xu, Z. B., Li, L., Shirliff, M. E., Peters, B. M., Li, B., Peng, Y., et al. (2011b). Resistance class 1 integron in clinical methicillin-resistant Staphylococcus aureus strains in southern China, 2001-2006. Clin. Microbiol. Infect. 17, 714–718.
Xu, Z. B., Li, L., Shirtliff, M. E., Alam, M. J., Yamasaki, S., and Shi, L. (2009). Occurrence and characteristics of class 1 and 2 integrons in Pseudomonas aeruginosa Isolates from patients in Southern China. J. Clin. Microbiol. 47, 230–234.
Xu, Z. B., Li, L., Shirtliff, M. E., Peters, B. M., Peng, Y., Alam, M. J., et al. (2010). First report of class 2 integron in clinical Enterococcus faecalis and class 1 integron in Enterococcus faecium in South China. Diagn. Microbiol. Infect. Dis. 68, 315–317.
Xu, Z. B., Li, L., Zhao, X. H., Chu, J., Li, B., Shi, L., et al. (2011a). Development and application of a novel multiplex polymerase chain reaction (PCR) assay for rapid detection of various types of staphylococci strains. African J. Microbiol. Res. 5, 1869–1873.
Xu, Z. B., Liang, Y. R., Lin, S. Q., Chen, D. Q., Li, B., Li, L., et al. (2016c). Crystal Violet and XTT assays on staphylococcus aureus biofilm quantification. Curr. Microbiol. 73, 474–482.
Xu, Z. B., Shi, L., Alam, M. J., Li, L., and Yamasaki, S. (2008a). Integron-bearing methicillin-resistant coagulase-negative staphylococci in South China, 2001-2004. FEMS Microbiol. Lett. 278, 223–230.
Xu, Z. B., Shi, L., Zhang, C., Zhang, L. Y., Li, X. H., Cao, Y. C., et al. (2007). Nosocomial infection caused by class 1 integron-carrying Staphylococcus aureus in a hospital in South China. Clin. Microbiol. Infect. 13, 980–984.
Xu, Z. B., Xie, J. H., Peters, B. M., Li, B., Li, L., Yu, G. C., et al. (2017b). Longitudinal surveillance on antibiogram of important gram-positive pathogens in Southern China, 2001 to 2015. Microb. Pathog. 103, 80–86.
Xu, Z. B., Xie, J. H., Yang, L., Chen, D. Q., Peters, B. M., and Shirtliff, M. E. (2018). Complete sequence of pCY-CTX, a plasmid carrying a phage-like region and ISEcp1-mediated Tn2 element from Enterobacter cloacae. Microb. Drug Resist. 24, 307–313.
Xu, Z. B., Xu, X. Y., Yang, L., Li, B., Li, L., Li, X. X., et al. (2017a). Effect of aminoglycosides on the pathogenic characteristics of microbiology. Microb. Pathog. 113, 357–364.
You, R., Gui, Z. Y., Xu, Z. B., Shirtliff, M. E., Yu, G. C., Zhao, X. H., et al. (2012). Methicillin-resistance staphylococcus aureus detection by an improved rapid PCR assay. African J. Microbiol. Res. 6, 7131–7133.
Zeng, B., Zhao, G., Cao, X., Yang, Z., Wang, C., and Hou, L. (2013). Formation and resuscitation of viable but nonculturable Salmonella typhi. BioMed. Res. Int. 2013:907170.
Zhang, H., Feng, S., Zhao, Y., Wang, S., and Lu, X. (2015). Detection of Yersinia enterocolitica in milk powders by cross-priming amplification combined with immunoblotting analysis. Int. J. Food Microbiol. 214, 77–82.
Zhao, X., Li, Y., Wang, L., You, L., Xu*, Z., and Li, L., et al. (2010a). Development and application of a loop-mediated isothermal amplification method on rapid detection Escherichia coli O157 strains from food samples. Mol. Biol. Rep. 37, 2183–2188. doi: 10.1007/s11033-009-9700-6
Zhao, X., Li, Y., Chu, J., Wang, L., and Shirtliff, M. E., He, X., et al., (2010b). Rapid detection of Vibrio parahaemolyticus strains and virulent factors by loop-mediated isothermal amplification assays. Food Sci. Biotechnol. 19, 1191–1197. doi: 10.1007/s10068-010-0170-3
Zhao, X., Wang, L., Chu, J., Li, Y., Li, L., Shirtliff, M. E., He, X., et al. (2009). Development and application of a rapid and simple loop-mediated isothermal amplification method for food-borne Salmonella detection. Food Sci. Biotechnol. 19, 1655–1659. doi: 10.1007/s10068-010-0234-4
Zhao, X. H., Li, M., and Xu, Z. B. (2018b). Detection of foodborne pathogens by surface enhanced raman spectroscopy. Front. Microbiol. 9:1236.
Zhao, X. H., Yu, Z. X., and Xu, Z. B. (2018a). Study the features of 57 confirmed CRISPR Loci in 38 strains of staphylococcus aureus. Front. Microbiol. 9:1591. doi: 10.3389/fmicb.2018.01591
Keywords: Salmonella enterica, viable but non-culturable (VBNC), crossing priming amplification (CPA), propidium monoazide (PMA), rapid detection
Citation: Ou A, Wang K, Ye Y, Chen L, Gong X, Qian L and Liu J (2021) Direct Detection of Viable but Non-culturable (VBNC) Salmonella in Real Food System by a Rapid and Accurate PMA-CPA Technique. Front. Microbiol. 12:634555. doi: 10.3389/fmicb.2021.634555
Received: 28 November 2020; Accepted: 19 January 2021;
Published: 18 February 2021.
Edited by:
Yang Deng, College of Food Science and Engineering, Qingdao Agricultural University, ChinaReviewed by:
Wensen Jiang, Cedars Sinai Medical Center, United StatesCopyright © 2021 Ou, Wang, Ye, Chen, Gong, Qian and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyan Liu, amxpdTgxQHV0aHNjLmVkdQ==; Lu Qian, cWlhbmx1QHNjdXQuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.