- 1Crop Protection Division, ICAR-National Rice Research Institute, Cuttack, India
- 2Department of Botany and Biotechnology, Ravenshaw University, Cuttack, India
- 3Crop Improvement Division, ICAR-National Rice Research Institute, Cuttack, India
- 4Division of Crop Production, ICAR-National Rice Research Institute, Cuttack, India
- 5Nuclear Agriculture and Biotechnology Division, Bhabha Atomic Research Centre, Trombay, India
- 6International Rice Research Institute, New Delhi, India
This study is a unique report of the utilization of Trichoderma strains collected from even tree barks for rice plant growth, its health management, and paddy straw degradation. Seven different spp. of Trichoderma were characterized according to morphological and molecular tools. Two of the isolated strains, namely Trichoderma hebeiensis and Trichoderma erinaceum, outperformed the other strains. Both of the strains controlled four important rice pathogens, i.e., Rhizoctonia solani (100%), Sclerotium oryzae (84.17%), Sclerotium rolfsii (66.67%), and Sclerotium delphinii (76.25%). Seed bio-priming with respective Trichoderma strains reduced the mean germination time, enhanced the seedling vigor and total chlorophyll content which could be related to the higher yield observed in two rice varieties; Annapurna and Satabdi. All the seven strains accelerated the decomposition of rice straw by producing higher straw degrading enzymes like total cellulase (0.97–2.59 IU/mL), endoglucanase (0.53–0.75 IU/mL), xylanase (145.35–201.35 nkat/mL), and laccase (2.48–12.60 IU/mL). They also produced higher quantities of indole acetic acid (19.19–46.28 μg/mL), soluble phosphate (297.49–435.42 μg/mL), and prussic acid (0.01–0.37 μg/mL) which are responsible for plant growth promotion and the inhibition of rice pathogen populations. Higher expression of defense enzymes like catalase (≥250% both in shoot and root), peroxidase (≥150% in root and ≥100% in shoot), superoxide dismutase (≥ 150% in root and ≥100% in shoot), polyphenol oxidase (≥160% in shoot and ≥120% in shoot), and total phenolics (≥200% in root and ≥250% in shoot) as compared to the control indicates stress tolerance ability to rice crop. The expression of the aforementioned enzymes were confirmed by the expression of corresponding defense genes like PAL (>3-fold), DEFENSIN (>1-fold), POX (>1.5-fold), LOX (>1-fold), and PR-3 (>2-fold) as compared to the non-treated control plants. This investigation demonstrates that Trichoderma strains obtained from tree bark could be considered to be utilized for the sustainable health management of rice crop.
Introduction
The population of the world is increasing rapidly and it is expected that the world population will be around 9.6 billion in 2050. To attain food security for all, the production of food must be increased to 70% by 2050. Crops should be protected from biotic stresses in order to achieve this goal. This should be done in a more eco-friendly and sustainable way, potentially by using certain biocontrol agents (BCA).
Different BCA like bacteria, fungi, and viruses are being used frequently for the management of diseases in different crops (Abraham et al., 2013). Fungal biocontrol agents are popular as they may be reproduced easily in an artificial nutrient media and are suitable for commercial multiplication (Singh et al., 2013). Genus Trichoderma is compelling as a biocontrol operator against various pathogens (Parmar et al., 2015). The primary natural habitat of Trichoderma is traditionally seen as soil or plant rhizosphere, even though maximum diversity of these species happens over-the-ground (Druzhinina et al., 2011). With the expanding dangers to nature and to our food security, determination of Trichoderma spp. as a BCA has been expecting centrality in giving security for plant protection and development (Swain and Mukherjee, 2020). Trichoderma spp. also induces plant growth by the creation of various phytohormones and activates plant supplements for better boost. Trichoderma spp. is not only marketed as a biopesticide, biofertilizer, and growth promoter, but also used as a nutrient solubilizer and organic matter decomposer (Woo et al., 2014). There are just a couple of reports on the assessment of Trichoderma as a biocontrol specialist obtained from above the ground territories (Jahagirdar et al., 2019). As per Whipps and Lumsden (2001), almost 60% of fungal BCA market is shared by Trichoderma spp. and there are significant challenges to investigate. The activity or mode of action of Trichoderma spp. is as per the following:
1. Generation of trichodermin, trichothecenes, trichorzianins, or gliotoxins (Mukherjee et al., 2013).
2. Seeking sustenance and space (Celar, 2003).
3. Antibiosis (Swain and Mukherjee, 2020).
4. Mycoparasitic capacities—a relationship in which one living fungus goes about as a supplement hotspot for another (Punja and Utkhede, 2003).
Trichoderma spp. produces auxins that are chargeable for plant bloom and root improvement in each symbiotic and pathogenic communication with plants (Swain et al., 2018). An amazing effect on plant improvement has been demonstrated for several Trichoderma secondary metabolites. Koninginins, 6-pentyl-α-pyrone, trichocaranes A–D, harzianopyridone, cyclonerodiol, harzianolide, and harzianic acid are instances of exacerbates that affect plant development in a considerably subordinate way (Swain and Mukherjee, 2020). To understand the plant growth promotion activity, rice seeds were bioprimed with the Trichoderma strains. Biopriming will help in increase in colonization, proliferation, and establishment of BCA on the seed surface. Consequently, it will boost seedling vigor and will be able to induce systemic resistance to biotic and abiotic stresses (Singh D. P. et al., 2020). Plant-microbe interactions setup by distant, distinct, and assorted microbial associations tend to instigate various common beneficial systemic changes in the expression level of plant genes that encode for proteins to detoxify reactive oxygen species (ROS). The beneficial microorganisms because of their presence in the plant rhizosphere help plants in easing biotic and abiotic stresses (Singh P. et al., 2020).
Faster decomposition of rice straw can be achieved by inoculating microorganisms, like ligno-cellulolytic microbes. Trichoderma produces high levels of several biomass degrading enzymes like cellulase and xylanase (Juturu and Wu, 2014). These enzymes degrade cellulose and hemicellulose, respectively. Lignin was degraded by ligninolytic enzymes into simpler phenyl rings (Sancez, 2009). In this investigation, we analyzed seven distinctive spp. of Trichoderma segregated from the bark of various trees in the Odisha province of India to consider biocontrol properties and rice straw decomposition capacity alongside their growth promotion activity in rice by the production of various enzymes and the expression of genes related to this.
Materials and Methods
Isolation, Characterization, Growth Condition and Biocontrol Potential of Trichoderma spp.
Seven Trichoderma strains were collected from the bark of various trees. The isolation and purification of all the strains were made according to standard techniques described by Mukherjee et al. (2014). Trichoderma species were identified by using the typical conidiophores structure (Gams and Bissett, 1998) and according to ISTH guidelines. Molecular characterization, identification, and construction of the phylogenetic tree of all the fungal isolates were done according to Swain et al. (2018). Total genomic DNA from the young mycelia was secluded by utilizing standard SDS (sodium dodecyl sulfate) technique (Mukherjee et al., 2014). The molecular characterization of all the fungal segregates were based on the sequences of Internal Transcribed Spacer (ITS) regions, Translation Elongation Factor 1 (TEF1) regions and RNA Polymerase B-larger subunit-II (RPB-II) regions according to standard strategies1. The species were recognized by BLASTN search on the NCBI site and the character was affirmed by contrasting the sequences and with authentic sequences from GenBank, and a phylogenetic tree constructed on http://www.phylogeny.fr. Biocontrol potential of isolated Trichoderma strains was evaluated against above Rhizoctonia solani CRRI-RS-8 (MTCC-12232) causing sheath blight of rice, Sclerotium oryzae CRRI-S.O (MTCC-12230) causing seedling blight of rice, Sclerotium rolfsii causing foot rot of rice and Sclerotium delphinii (MTCC11568) causing seedling rot of rice.
The confrontation assay was carried out by concurrent inoculation of both Trichoderma and the pathogen close to the edge of the plate, put opposite to one another. Plates inoculated with pathogens only were utilized as control. The percentage of mycelial growth inhibition was determined by Swain et al. (2018)
where, R1, radial growth of the pathogen in control plate; R2, radial growth of the pathogen in test plate.
Quantification of Production of Indole Acetic Acid, Prussic Acid, and Solubilization of Inorganic Phosphate by Trichoderma spp.
The indole acetic acid (IAA) produced by different strains of Trichoderma was quantified as per Sureshrao et al. (2016). For the quantitative estimation of IAA, agar plugs (5 mm) from the edge of actively growing colonies of Trichoderma were inoculated to 20 ml DF (Dworkin and Foster) salts minimal media and incubated for 3 days at 28°C. The medium was supplemented with L-tryptophan at a concentration of 1.02 g l–1. After incubation for 72 h, the mycelia were removed from the culture medium by centrifugation at 5,000 rpm for 5 min. One ml aliquot of the supernatant was mixed vigorously with 4 ml of Salkowski’s reagent and allowed to stand at room temperature for 20 min. The absorbance at 535 nm was measured. The concentration of IAA in each culture supernatant was determined by using an IAA (Himedia) as standard curve.
For prussic acid production, Trichoderma spp. was grown on Tryptic Soya Agar (TSA) supplemented with 4.4 g L–1 of glycine for 2 days. White filter paper discs were cut in the same size and soaked in picric acid solution. The sheets of filter papers were placed on the upper lid of each plate. The plates were sealed with Parafilm and incubated for 7 days at 28°C. After incubation, prussic acid production was observed by the color changes of the filter paper from yellow to light brown or reddish brown (Meera and Balabaskar, 2012). The colored filter paper was then eluted by placing the filter paper in a clean test tube containing 10 mL distilled water and the absorbance was measured at 625 nm by using a spectrophotometer (Manwar et al., 2011).
Quantitative estimation of phosphate solubilization was performed in Pikovskaya broth (Himedia) containing tricalcium phosphate as a phosphate source. Freshly grown Trichoderma isolates were inoculated to 50 ml of Pikovskaya’s broth and incubated at 28°C and allowed to shake at 100 rpm. After 5 days the broth culture was centrifuged at 10,000 rpm for 10 min. To the 0.5 ml of the culture supernatant, 5 ml of chloromolybdic acid was added and mixed thoroughly. Volume was made up to 10 ml with distilled water and 125 μl chlorostannous acid was added to it. Immediately, the final volume was made-up to 25 ml with distilled water and mixed thoroughly. The absorbance was measured at 610 nm by using a spectrophotometer. The corresponding amount of soluble phosphorous was calculated from a standard curve of potassium dihydrogen phosphate (KH2PO4). Phosphate solubilizing activity was expressed in terms of tricalcium phosphate solubilization which in turn was measured by μg ml–1 of available orthophosphate as calibrated from the standard curve of KH2PO4 (Jackson, 1973).
Qualitative and Quantitative Screening of Straw Degrading Enzymes Produced by Trichoderma spp.
Seven Trichoderma strains were screened for cellulase activity on carboxy-methyl cellulose (CMC) (Analytical Reagent grade, Himedia, India) and xylanase activity on xylan agar medium containing 1% beech wood xylan (Molecular biology grade, Himedia, India) as the substrate (Teather and Wood, 1982). The Trichoderma isolates were cultured on petriplates (90 mm × 15 mm, Himedia, India) containing 1% CMC agar media or 1% beech wood xylan media at 26°C. After 5 days’ incubation, plates were stained with 1% solution of Congo red and followed by destained with 1N NaOH solution on a Gyrotory Shaker (Model G2, New Brunswick Scientific Co., Inc., Edison, NJ, United States) at 50 rpm for 15 min to detect clear distinct zone (Saczi et al., 1986). The Enzymatic Index (EI) was calculated for the above two enzymes by measuring the clearance zone as per Florencio et al. (2012).
The qualitative screening for laccase activity of the Trichoderma strains were examined by culturing the respective isolates on PDA media supplemented with 0.04% guaiacol (Extra Pure, Himedia, India) and 0.01% (w/v) chloramphenicol with pH 5.5. They were examined for the development of a mixture of red-brown colored zone around the fungal colonies after incubation at 28°C for 72 h (Kalra et al., 2013). Here; three independent experiments were performed with three imitates per isolate. For each isolate the average EI of the three analyses was determined alongside the standard deviation.
The quantitative enzymatic screening of seven Trichoderma strains was carried out. Assay of four major enzymes, i.e., total cellulase, endoglucanase, xylanase, and laccase were carried out. The extraction of the crude enzyme was done using rice straw (Variety, Swarna sub-1, Indica type) as the base material as per Pathak et al. (2014) with minor modifications. Trichoderma grown on PDA plates (2 days’ culture) were sub-cultured and grown on rice bran (4%) agar media for a period of 5 days at 27°C. After completion of the incubated period, 10 mL of sterile double distilled water having 0.1% polyoxyethylene (20) sorbitan monooleate was added to the plates to collect the fungal spores. Fungal spores (2 × 106 CFU/mL) were inoculated in 250 mL flask containing grinded sterilized rice straw of 5 g and 15 mL of Mandel and Reese nutrient salt solution (NSS). After 5 days of incubation, crude enzyme extraction was done by using citrate buffer (pH 4.8) in NSS: extraction buffer (1:2: V/V). The fermented matter was shaken for 15 min at room temperature. Multilayered cheese cloth was used for the filtration of the fermented product and the centrifugation of the filtrate was done at 10,000 rpm for 15 min at 4°C (Hermel, Labortechnik GmbH, Type-Z36HK, Nr-580901000). The clear supernatant was used as a crude enzyme sample.
The total cellulase activity and endo-β-1,4-glucanase of the above Trichoderma strains was determined as units per milliliter (IU/mL) by Dinitro Salicylic Acid (DNS) method using Whatman No.1 filter paper and 2% CMC as substrates, respectively (Ghosh, 1987). The activity of xylanase and laccase enzyme produced by the Trichoderma isolates were measured by using 1% xylan (Bailey et al., 1992) and guaiacol (Monssef et al., 2016) as substrates, respectively. The activity of xylanase and laccase was expressed in nkat/mL and IU/mL, respectively.
In vitro Preparation of Rice Straw Compost by Trichoderma spp.
The in vitro preparation of rice straw compost was carried out by all the strains of Trichoderma. The moisture content in rice straw was measured. Inoculums of seven different isolates (107 cfu mL–1) were poured into different conical flasks (250 mL) containing 25 gm of straw (Variety, Swarna sub-1, Indica type) and kept at ambient temperature. The weight of the flask was measured at regular intervals along with control until 60 days after inoculation. The C/N ration of the decomposed rice straw was determined. Total carbon and nitrogen content was measured according to the methods given by Walkley and Black (1934) and Keeney and Bremner (1965), respectively.
Measurement of Plant Growth Promoting Parameters, Vigor Index, and Chlorophyll Content Under Greenhouse Conditions
Biocontrol potential and growth promotion property of Trichoderma strains were confirmed under controlled conditions. Rice seeds (Annapurna, Indica type and Satabdi, Indica type) were bio-primed with respective Trichoderma formulations (1 × 107 cfu g–1) and set in soils (double autoclaved) filled in 30 cm × 30 cm pots. Soapstone (a mix of Talcum powder, hydrous magnesium silicate, and Talc) was used to prepare the respective Trichoderma formulation as per Indian Patent File no. 1240/KOL/2015. Seeds not primed with Trichoderma formulation were treated as control. Germination capacity and vigor index of the seeds sown in the pot were examined as per Abdul-Baki and Anderson (1973) and Swain et al. (2018). For the assessment of vigor index germination percentage, seedling length and dry weight of seedling was taken into consideration. The effect of Trichoderma on the plant chlorophyll content of the leaf was evaluated as per Porra (2002).
Measurement of Plant Growth Promoting Parameters Under Field Conditions
For the assessment of growth promotion under in vivo conditions two direct seeded rice varieties (“Annapurna” and “Satabdi”) were used. Seeds dressed with respective Trichoderma formulations were treated as treatments. The experiment was conducted in completely randomized design with four replications. The agronomical parameters (root length, shoot length, dry root weight, dry shoot weight, no of tiller/hill, and yield/hill) were recorded. Expression of plant stress responsive enzymes like peroxidase (PER), catalase (CAT), superoxide dismutase (SOD), polyphenol oxidase (PPO), and total phenolics (TP) conveyed by the plants treated with Trichoderma were investigated at active tillering stage (Arnon, 1949; Sadasivam and Manickum, 2011; Swain et al., 2018).
Extraction of RNA, cDNA Synthesis and Examination of Gene Articulation by Real-Time-PCR
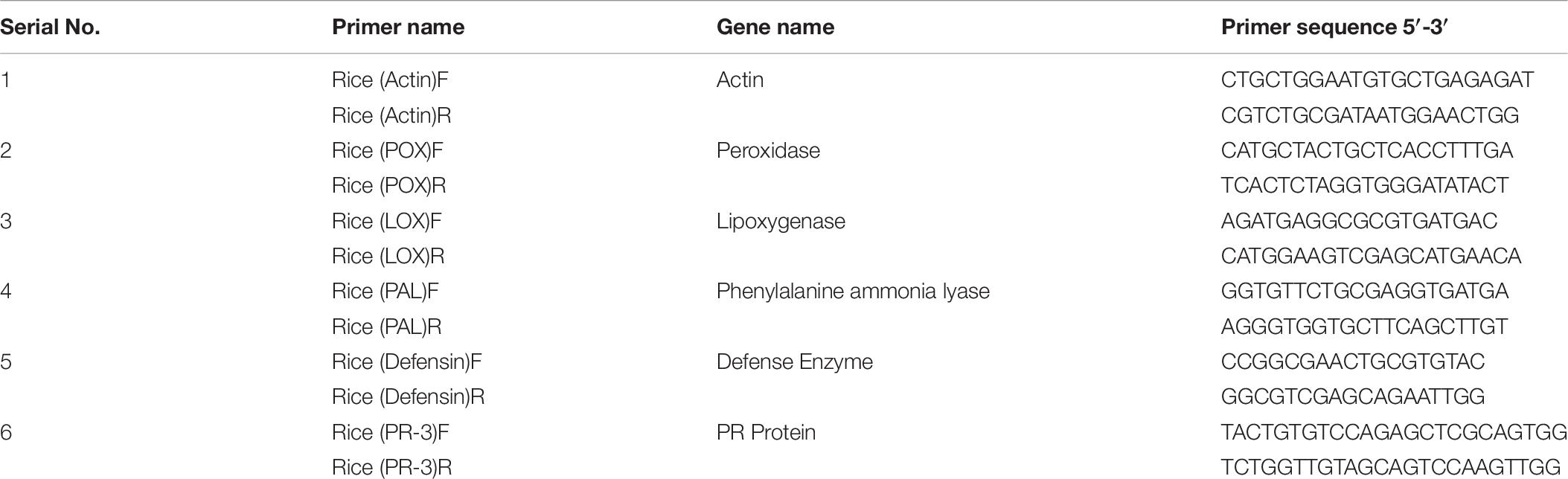
Total RNA was extracted from the fresh plant leaves by utilizing RNeasy Plant Mini Kit (Qiagen, Germany). RNA concentration was quantified by using Nano Drop 2000 (Thermo Fisher Scientific). cDNA synthesis was carried out by using Maxima H minus first strand cDNA synthesis Kit with dsDNase (Thermo Fisher Scientific) as per the manufacturer’s guidelines. Quantitative real-time (RT-PCR) reaction was performed in the Insta-Q96 RT-PCR system (Himedia Laboratories, Mumbai, India) using Maxima SYBRGreen/ROX qPCR master mix (Thermo Fisher Scientific) for five growth promotion and antioxidant genes, i.e., POX (peroxidase), LOX (lipoxigenase), PAL (phenylalanine ammonia lyase), DEFENSIN, PR-3 (PR protein). Act (actin) was utilized as housekeeping genes for normalization of relative gene articulation level. All primers were designed by IDT programming. All the treatments were in sets of three for each primer pair in a similar plate. The PCR reactions were set by convention formulated by Singh P. et al. (2020) with minor adjustments.
Statistical Analysis
Statistical analysis was performed by utilizing the Statistical Analysis Software (SAS) of ICAR-IASRI, New Delhi2 was used for statistical analysis. All the investigations were replicated three times. Seed germination rate was ARCSINE transformed. The other seed quality boundaries were examined with no change. All the information was exposed to single way characterized analysis of variance (ANOVA) and means of treatments were compared based on Tukey’s honestly significant difference test (HSD) at 0.05 probability level using SAS.
Results and Discussion
Identification of Trichoderma Strains
Based on morphological characteristics and molecular identification, the isolates were identified as Trichoderma harzianum (CRRI-T1), Trichoderma erinaceum (CRRI-T2), Trichoderma atroviride (CRRI-T3), Trichoderma hebeiensis (CRRI-T15), Trichoderma parareesei (CRRI-T16), Trichoderma longibrachiatum (CRRI-T22), and Trichoderma reesei (CRRI-T27) (Figure 1 and Supplementary Figure 1). The ITS sequence data from CRRI-T1, CRRI-T2, CRRI-T3, CRRI-T22, CRRI-T27, and TEF sequence data from CRRI-T15, CRRI-T16 have been deposited with NCBI (Table 1). Out of these 7 isolates 4 are rarely found in India (i.e., T. erinaceum, T. hebeiensis, T. parareesei, and T. reesei). This provides the evidence for the maximum diversity of this genus occurring above ground. This may be the first time in India, we are reporting T. hebeiensis, T. parareesei, and T. reesei from the above ground. T. reesei reported earlier were isolated from soil and obtained from the mutation of other species of Trichoderma (Kar et al., 2006; Saravanan et al., 2008).
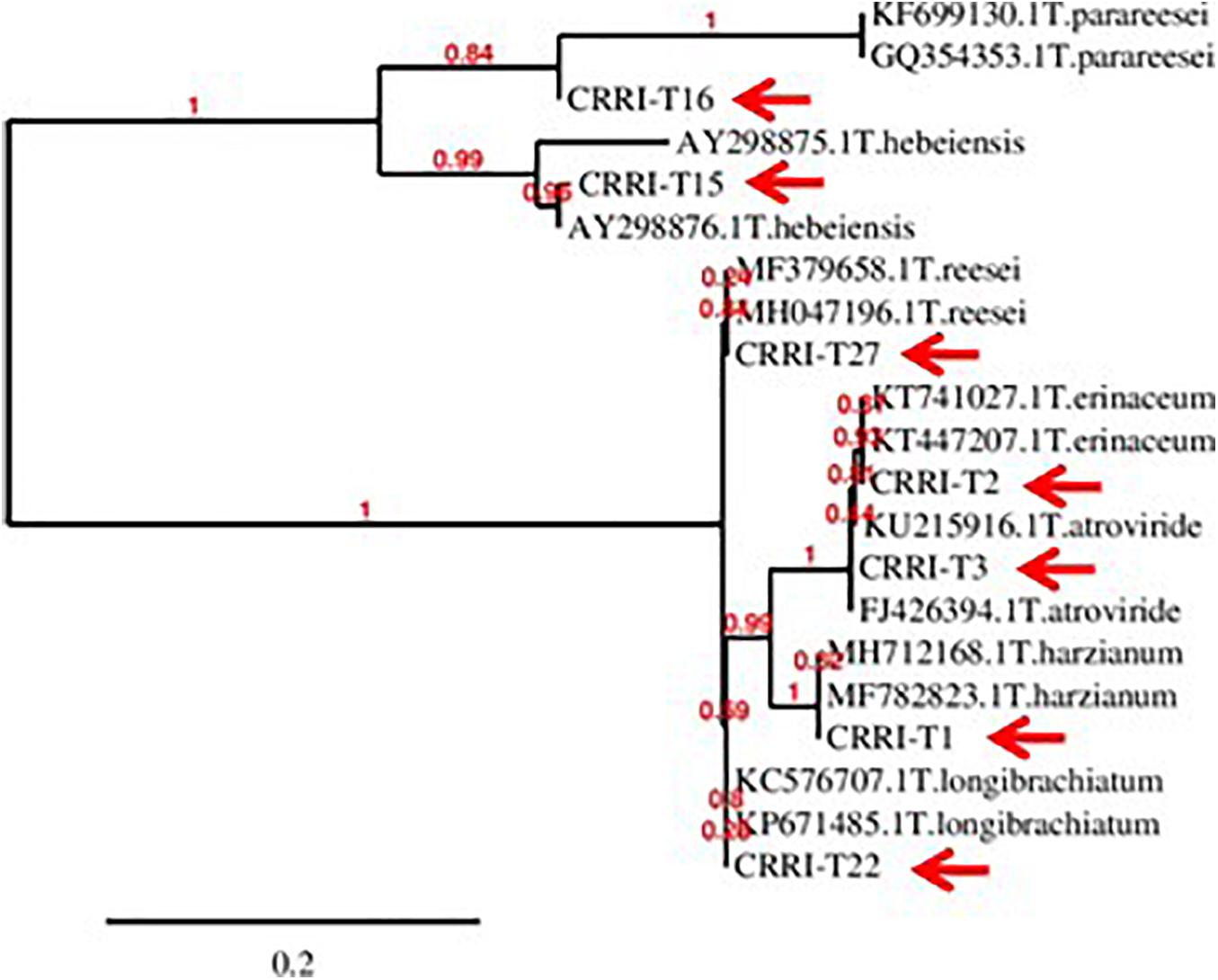
Figure 1. Phylogeny of the isolated Trichoderma spp. (CRRI-T1 to CRRI-T27) used for the recent study.
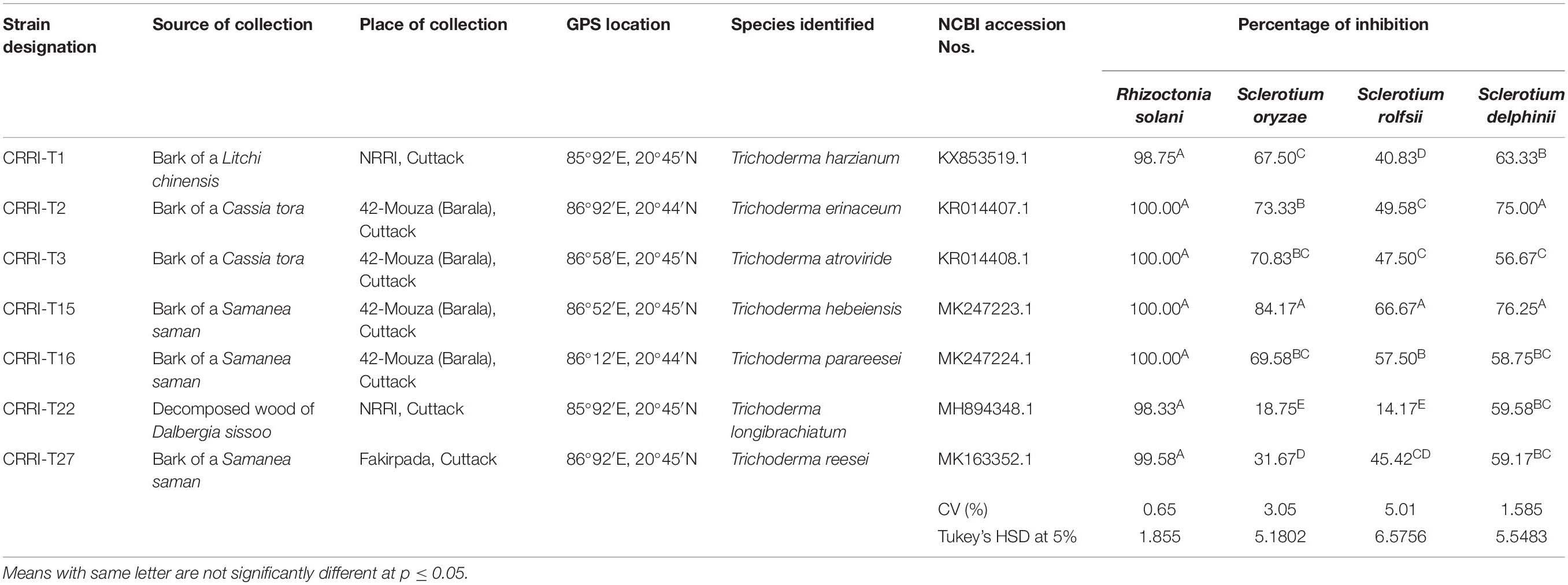
Table 1. Details of Trichoderma isolates and confrontation assay showing the inhibition of pathogen growth by different Trichoderma isolates on PDA medium.
Biocontrol Capability of Trichoderma Strains and Its Mechanism
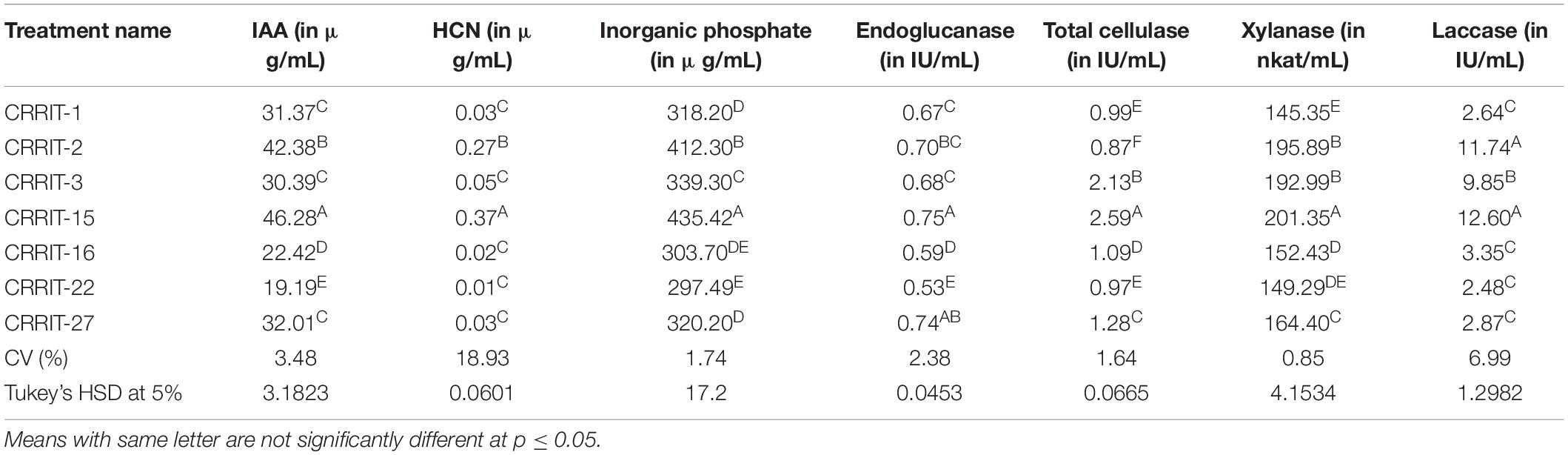
Mycelial growth of R. solani, S. oryzae, S. rolfsii, and S. delphinii were inhibited by 98.33–100.00, 18.75–84.17, 14.17–66.67, and 56.67–76.25%, respectively. CRRIT-2, CRRIT-3, CRRIT-15, CRRIT-16 overgrew R. solani within 3 days. Similarly, these isolates grew quicker in dual culture against S. oryzae and covered minimum of 70% of the medium surface within 3 days (Table 1). In case of S. delphinii, CRRIT-15, CRRIT-2 exhibited more than 70% inhibitory effect whereas all other strains were able to colonize in between 58 and 63% of the medium surface. Among the seven strains, T. hebeiensis (CRRIT-15) and T. erinaceum (CRRIT-2) was superior antagonist against four rice pathogens (Supplementary Figure 2). Biocontrol potential of these isolates can be correlated with prussic acid (HCN) production. The highest quantity of HCN was produced by CRRI-T15 (0.37 μg/mL) which was significantly higher as compared to other isolates. Other isolates such as CRRI-T1, CRRI-T2, CRRI-T3, CRRI-T16, and CRRI-T27 produced 0.03, 0.02, 0.05, 0.02, and 0.03 μg/mL HCN (Table 2).
Many species of Trichoderma were accounted for as biocontrol specialists for a wide range of plant pathogens (Abdollahi et al., 2012; Kumar et al., 2012; Swain et al., 2018). Mycoparasitism is clearly one of the mechanisms for biocontrol action of Trichoderma (Mukherjee et al., 2013). Besides mycoparasitism, release of prussic acid has been proposed as a significant antifungal component. Cyanide produced by microbes may act as an inhibitor to soil borne pathogens without any harm to the host plant (Noori and Saud, 2012). Hence, for the management of soil borne pathogens, the prussic acid produced by Trichoderma spp. played a vital role. Biocontrol potential of Trichoderma spp. mainly depends on the host plant, agro climatic conditions and nutrient availability (Mukherjee et al., 2013). To the best of our knowledge, T. hebeiensis and T. parareesei from the above ground part have been explored as potential biocontrol for the first time.
Trichoderma as a Decomposer
Cellulase activity of fungal isolates with EI values of more than 1.5 were considered to be potential cellulase producers (Florencio et al., 2012; Saroj et al., 2018). All the seven strains were potential cellulase producers as the EI value was more than 1.5. Earlier Saroj et al. (2018) reported thermophilic fungi isolated from soil, i.e., Aspergillus fumigatus JCM 10253 and Aspergillus terreus with highest EI values of 1.50 and 1.24 for cellulase activity, respectively. Florencio et al. (2012) reported T. harzianum CEN 139, T. sp. 104 NH and T. harzianum CEN 155 exhibited 1.74, 1.72, and 1.61 EI value for cellulase activity, respectively. Similarly, EI values of xylanase activity of Trichoderma isolates ranged from 0.50 to 1.33. CRRIT-15 exhibited the highest xylanase activity, i.e., 1.68 EI value followed by CRRIT-27 and CRRIT-2. The strains reported by Saroj et al. (2018) exhibited range 1.01–1.13 of EI value for xylanase activity. However, CRRIT-26, CRRIT-2, and CRRIT-27 had better cellulase and xylanase activities in comparison to others as reported earlier. All the strains exhibited positive reddish-brown zones around the fungal colonies indicating laccase activity. Monssef et al. (2016) examined 24 fungal isolates for the production laccase enzyme and found only T. harzianum could produce the enzyme. These results are in line with Gochev and Krastanov (2007) who found that many of Trichoderma spp. with cellulase activity could also be a good source of laccase (Figure 2).
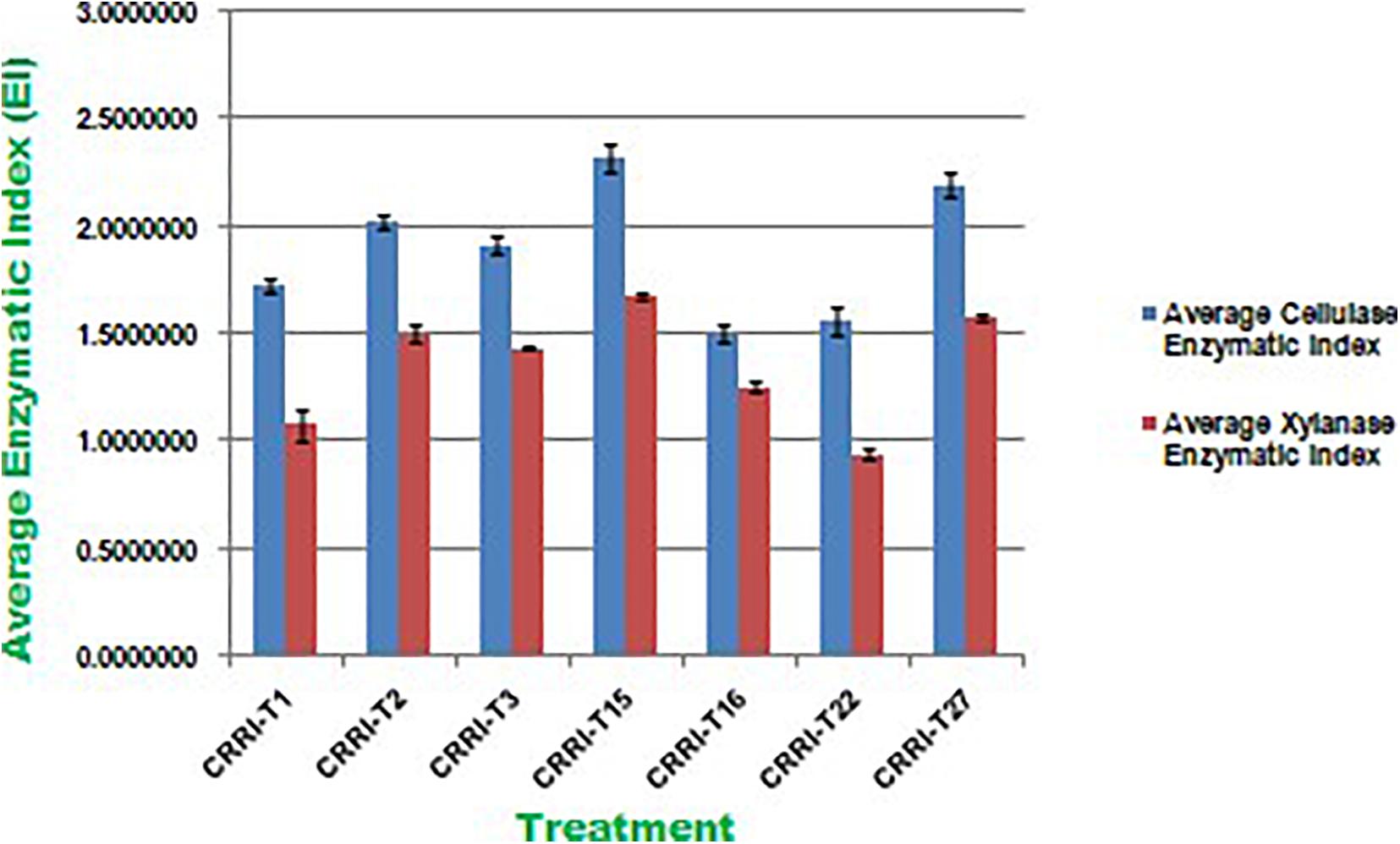
Figure 2. Enzymatic Index of cellulase and xylanase activity of Trichoderma isolates used in the present study.
All the isolates were examined for endoglucanase, total cellulase, xylanase, and laccase activity. CRRIT-15 showed maximum endoglucanase activities, i.e., 0.75 IU/mL whereas CRRIT-22 isolate showed lowest endoglucanase activities (i.e., 0.53 IU/mL). Similarly, CRRIT-15 and CRRIT-3 showed maximum total cellulase activities, i.e., 2.59 and 2.13 IU/mL, respectively. Similarly, CRRIT-15 released maximum xylanase activity of 201.35 nkat/mL followed by CRRIT-2 195.89 nkat/mL. Among four isolates, CRRIT-15 showed 12.60 IU/mL of laccase activity followed by CRRIT-3 9.85 IU/mL of activity (Table 2).
Earlier Druzhinina et al. (2011) reported that T. reesei is a major source of hydrolytic enzymes like cellulase and hemicellulase. T. harzianum CEN 139, T. sp. 104 NH, and T. harzianum CEN 155 exhibited 1.74, 1.72, and 1.61 EI value and 0.27, 0.23, and 0.22 IU/mL of endoglucanase activity, respectively (Florencio et al., 2012). Lee et al. (2011) reported T. harzianum isolated from post-harvest rice straw possesses 0.095 IU/mL of endoglucanase activity and 0.222 IU/mL of total cellulase activity. Similarly, Pathak et al. (2014) reported T. harzianum isolated from soil, rotting wood, and manure from different locales of northern India possesses 1.28 IU/mL endoglucanase and 0.37 IU/mL of total cellulase activity. So, both the isolates NRRIT-26 and NRRIT-27 seem to be a very good potential cellulase and xylanase producer and they may be used as a better option for the preparation of rice straw compost as compared to the previous reports. According to Pathak et al. (2014) 100.2 IU/mL of xylanase activity was observed in the case of T. harzianum collected from various location of northern India. NRRIT-26, i.e., T. reesei showed highest xylanase activity as compared to the other three isolates that can be considered as the best candidate for rice straw compost. The other three strains, i.e., NRRIT-27, CRRIT-13, and CRRIT-5 also showed higher xylanase activity in comparison to isolates reported by Lee et al. (2011) and Pathak et al. (2014).
Moreover, T. harzianum and T. longibrachiatum are the wellsprings of laccase production as portrayed by Holker et al. (2002) and Velazquez-Cedeno et al. (2004), respectively. We reported here the creation of laccase enzyme in all the Trichoderma isolates isolated from tree bark. Trichoderma are adapted well to rice straw and they could be utilized to degrade straw as reported by previous researchers (Kang et al., 2004). Overall, the isolate, CRRIT-15 released the highest number of enzymes as compared to other isolates. Hence, it may be used as a candidate for the preparation of rice straw compost in an economically way.
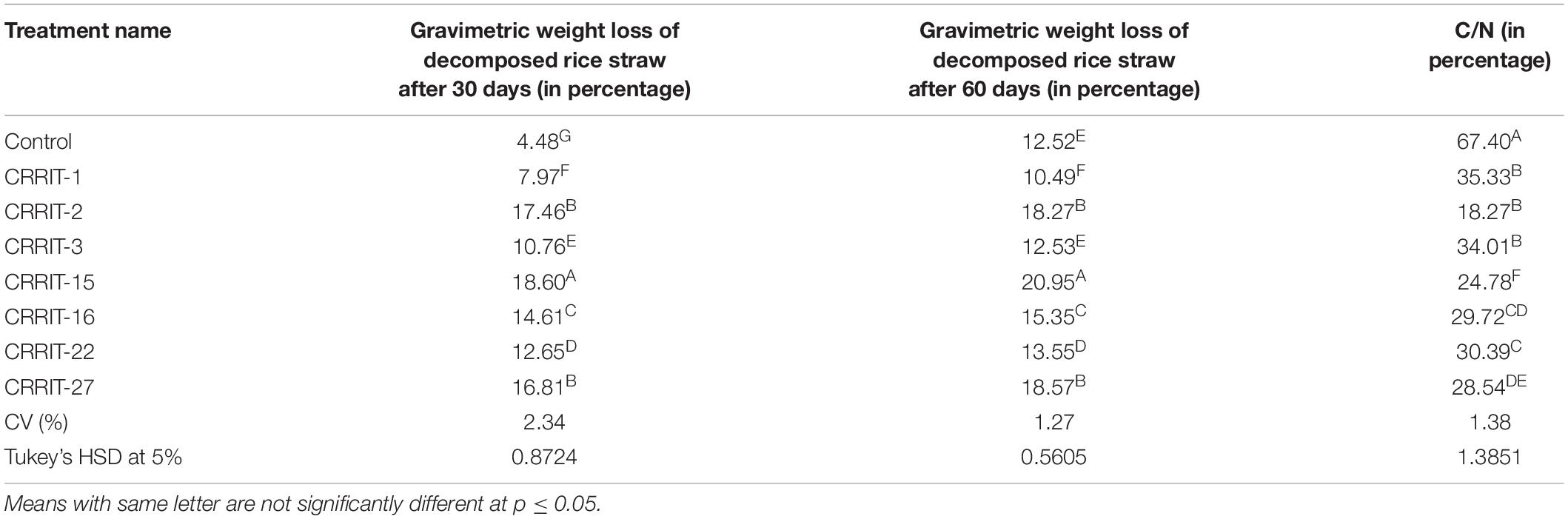
The Trichoderma inoculated straw was decomposed at a faster rate as compared to the non-inoculated one. Among the seven Trichoderma strains, NRRIT-15, CRRIT-2, and NRRIT-27 could be able to produce compost from rice straw at a faster rate. There was 18.60, 17.46, and 16.81% of weight loss of the rice straw with NRRIT-15, CRRIT-2, and NRRIT-27 treatments, respectively, after 30 days (Table 3). The loss of weight of the rice straw was 20.95, 18.27, and 18.57%, respectively, after 60 days when treated with NRRIT-15, CRRIT-2, and NRRIT-27. The above mentioned three isolates had also secreted higher quantities of ligno-cellulolytic enzymes as stated above. These enzymes decomposed the rice straw at a faster rate. There were insignificant changes in weight loss after 60 days of incubation as compared to 30 days of incubation. Similarly, the C/N ratio did not vary between 30 and 60 days. So based on these data, we can conclude the compost is generally stable after 30 days of incubation. As Trichoderma mediated rice straw compost has a low C/N ratio in in vitro condition, the technique can be extended to field conditions which will improve organic matter along with fertility of the soil.
Trichoderma as a Plant Growth Promoter
Auxins play a critical role for both the plant growth and root development. The quantity of IAA synthesized by various Trichoderma strains in the broth was ranged from 19.19 to 46.28 μg/mL (Table 4). The highest IAA was produced by CRRI-T15 (46.28 μg/mL) which was significantly higher followed by CRRI-T2 (42.38 μg/mL).
Quantitative assessment of soluble phosphate concentrations in Pikovskaya’s broth was varied from 297.49 to 435.42 μg/mL (Table 2). CRRI-T15 may be treated as the best inducer of phosphate mobilization as it exhibited higher phosphate solubilization capacity in Pikovaskaya’s broth. The amount of inorganic phosphate solubilized was 435.42 μg/mL. Other Trichoderma strains, i.e., CRRI-T1, CRRI-T2, CRRI-T3, CRRI-T16, CRRI-T22, and CRRI-T27 were also good phosphate solubilizers.
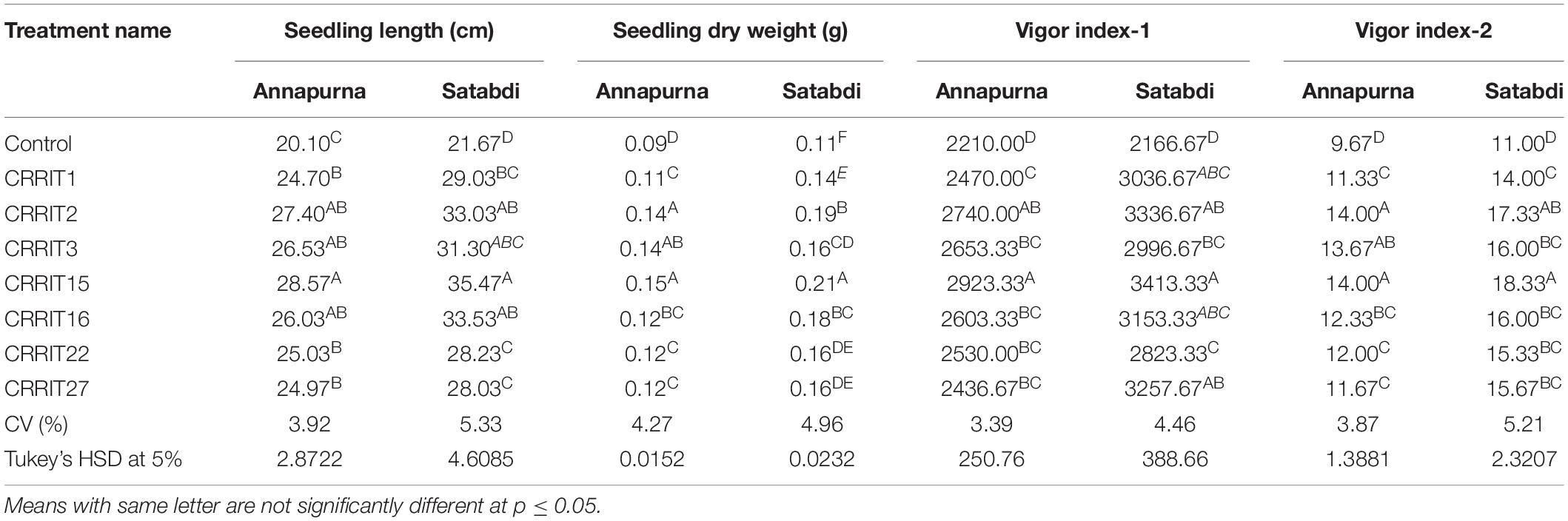
Parameters related to seed vigor and seed germination of different Trichoderma dressed seeds varied from 2.13–4.13 days (Mean germination time), 2166.67–3413.33 (Vigor index-I) and 11.00–18.33 (Vigor index-II), respectively in variety Satabdi (Figure 3). A similar trend was also found in the case of the Annapurna rice variety. Physiological parameters among the treatments were significantly varied in both the varieties. Absolute chlorophyll content went from 4.61 to 18.78 mg/g in the Annapurna rice variety. Seeds inoculated with T. hebeiensis (CRRIT-15), T. parareesei (CRRIT-16), T. erinaceum (CRRIT-2), and T. longibrachiatum (CRRIT-22) had significantly higher chlorophyll parameters when contrasted with other isolates depicted in the current examination (Table 5). Seed biopriming with beneficial microbes have been reported by several workers for their ability to mitigate biotic stress in an efficient way (Singh P. et al., 2020). During biopriming, antagonistic PGPR increases on the seed surface, thereby defending the plant from pathogen attack and enabling it to be sustained under various stress conditions (Rajput et al., 2019).
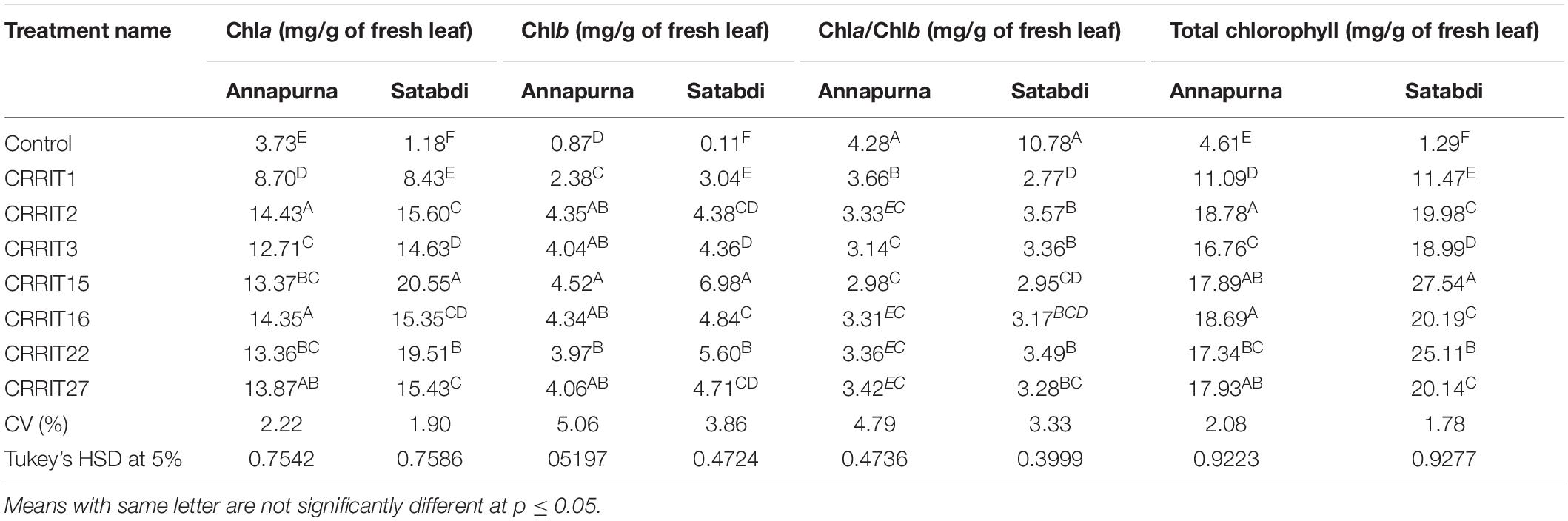
Table 5. Chlorophyll content in different rice varieties due to the application of different Trichoderma isolates.
In the field study, all the strains of Trichoderma controlled the plant growth along with various agronomical parameters. The highest yield (31.14 g/hill) was recorded from CRRIT-15 followed by CRRIT-16 and CRRIT-13. Similar trends were observed in the case of the Satabdi rice variety (Table 6). As an overall study, all the isolates performed better than the control one (Supplementary Figures 3, 4). Previously Swain et al. (2018) reported higher total chlorophyll content, plant vigor in direct seeded rice treated with Trichoderma. Previously Mukherjee et al. (2013) and Mukherjee et al. (2018) explained the role of Trichoderma as a plant growth promoter. The enhancement of seed vigor parameters may be due to the production of phenolic compounds and secondary metabolite namely harzianolide by Trichoderma spp. (Cai et al., 2013). This result was also found in case of chickpea and wheat (Zhang et al., 2019). All these positive impacts of vigor property helped the plant in uptake and mobilization of nutrients for a longer time, which leads to a better yield.
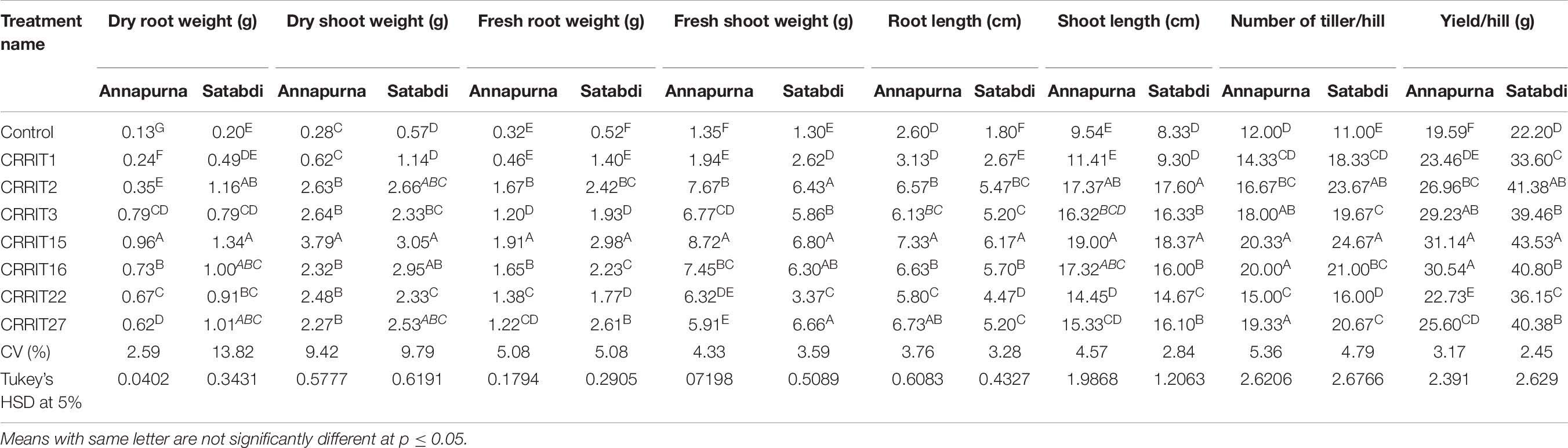
Table 6. Growth promotion in different rice varieties due to Trichoderma application as indicated by various agronomical parameters.
Among the secondary metabolites, IAA (auxin) helps in plant growth and potentially increases the root length as well. Laboratory studies have emphasized the role of plant growth promoting fungi as auxin producers and biocontrol operators (Hossain et al., 2007; Contreras-Cornejo et al., 2009) in plant development. This key hormone was synthesized by the fungus Trichoderma in symbiotic as well as in pathogenic interactions (Gravel et al., 2007). Similarly, plants can only uptake and mobilize essential micronutrients if they are solubilized by microbes (Rudresh et al., 2005). As indicated by Dunaitsev et al. (2008) Trichoderma spp. can deliver phosphate from mineral crude materials as plant accessible structures. It was seen that Trichoderma isolates a demonstrated higher capacity to solubilize the phosphate as they additionally displayed great reactions to plant growth promotion action after direct seed treatments. Trichoderma as plant symbionts for updated supplement take-up, extended root and shoot advancement, improved plant influence and biotic/abiotic stress flexibility have been widely discussed (Harman, 2011).
Improvement of Plant Resistance by the Articulation of Stress Responsive Enzymes
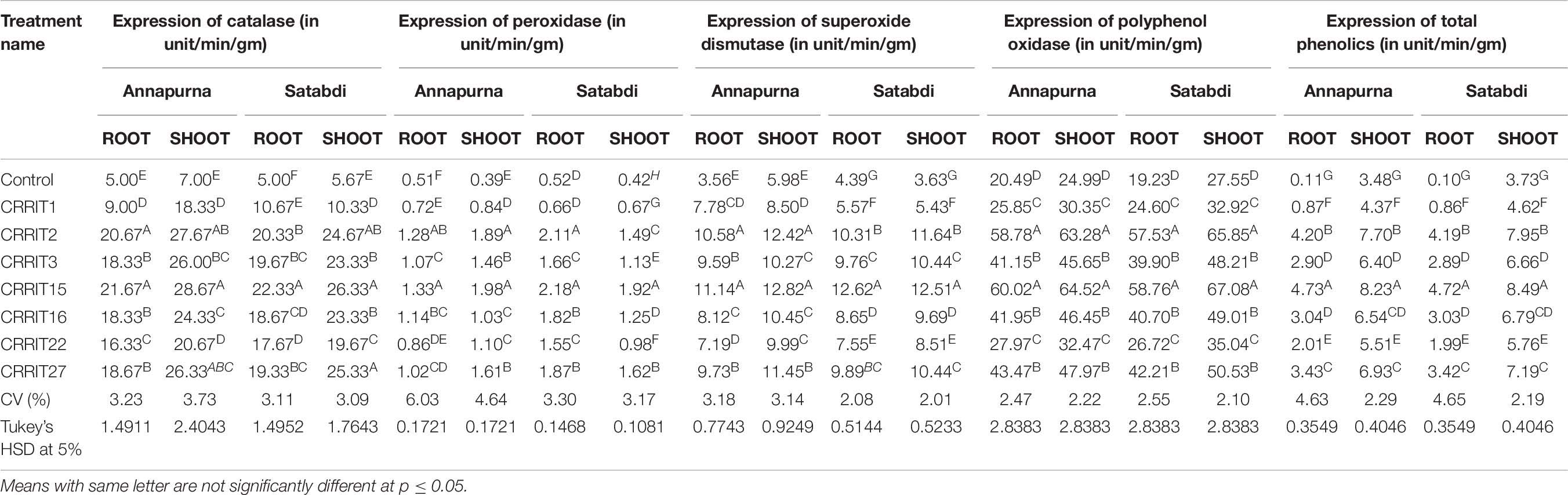
Inside and out higher verbalization of the enzymes related to stress was seen in Trichoderma seed treated plants when appeared differently in relation to untreated plants against both the rice assortments. Correspondingly, CRRIT-15, and CRRIT-2 treated root and shoots of rice assortment Annapurna and Satabdi had exceptionally higher PER, SOD, PPO, and TP activity contrasted with other treatment. Also, catalase action was higher in CRRIT-15 and CRRIT-2 treatment in both root and shoot. Comparative examples were found in peroxidase action in root and shoot of both the rice assortments (Table 7).
In blend with their immediate impact on the pathogen structure and action, Trichoderma spp. has additionally been found to invigorate plant resistance mechanisms (Yedidia et al., 2001). Successful Trichoderma strains can instigate a more grounded reaction in the plant contrasted with pathogen-triggered immunity by creating an assortment of microorganisms related molecular patterns (MAMPs), for example, hydrophobins, expansin-like proteins, metabolites, and catalysts like catalase, peroxidase, and superoxide dismutage, having a direct antimicrobial movement (Vargas et al., 2008). Trichoderma can likewise improve ETI by causing a quicker reaction (preparing), or initiate it by discharging exacerbates that, similarly as with some pathogen molecules, are explicitly perceived by plant cell receptors (Bailey et al., 1993). In the present exploration, the stress enzymes expressed in a higher amount in Trichoderma treatments rather than the control group. Mohapatra and Mittra (2017) and Swain et al. (2018) documented that Trichoderma spp. triggered the fabrication of antioxidant enzymes in wheat and rice seedlings, respectively. Trichoderma isolates were accounted to help fundamental defense reactions through different catalysts (Małolepsza et al., 2017). PPO is engaged with the plant guard system against pathogens by catalyzing the oxidation of phenols to quinines in an oxygen-subordinate way (Daw et al., 2008). An increase in defense enzymes activity (Mandal et al., 2013) and TP content (Mandal et al., 2013) in plants with a reaction to pathogen assault has also been previously reported.
Trichoderma Seed Biopriming Induces Antioxidant Gene Expression in Rice Plants
Plants have created solid cell antioxidant molecular systems because of the utilization of biocontrol specialists like Trichoderma (Rejeb et al., 2014; Swain et al., 2018), however the initiation and articulation of these genes change under plant organism communication conditions (Singh D. P. et al., 2020). Albeit, both organism-treated and non-treated plants were developed under typical development conditions. We investigated the plant molecular reactions as far as the outflow of prominent defense (PAL and DEFENSIN) and antioxidant (POX, LOX, and PR-3) genes following microbial inoculation (Table 8). We observe multifold over expression of genes in both the strains. However, Trichoderma seed biopriming caused >2-fold up regulation of every gene in both the rice varieties. Besides, CRRIT-15 exhibited the highest level of fold expression (i.e., >3) of all the genes as compared to control one. CRRIT-16 and CRRIT-2 treatment performed the second highest level of fold expression (i.e., >2.5) by following CRRIT-15 (Figures 4, 5).
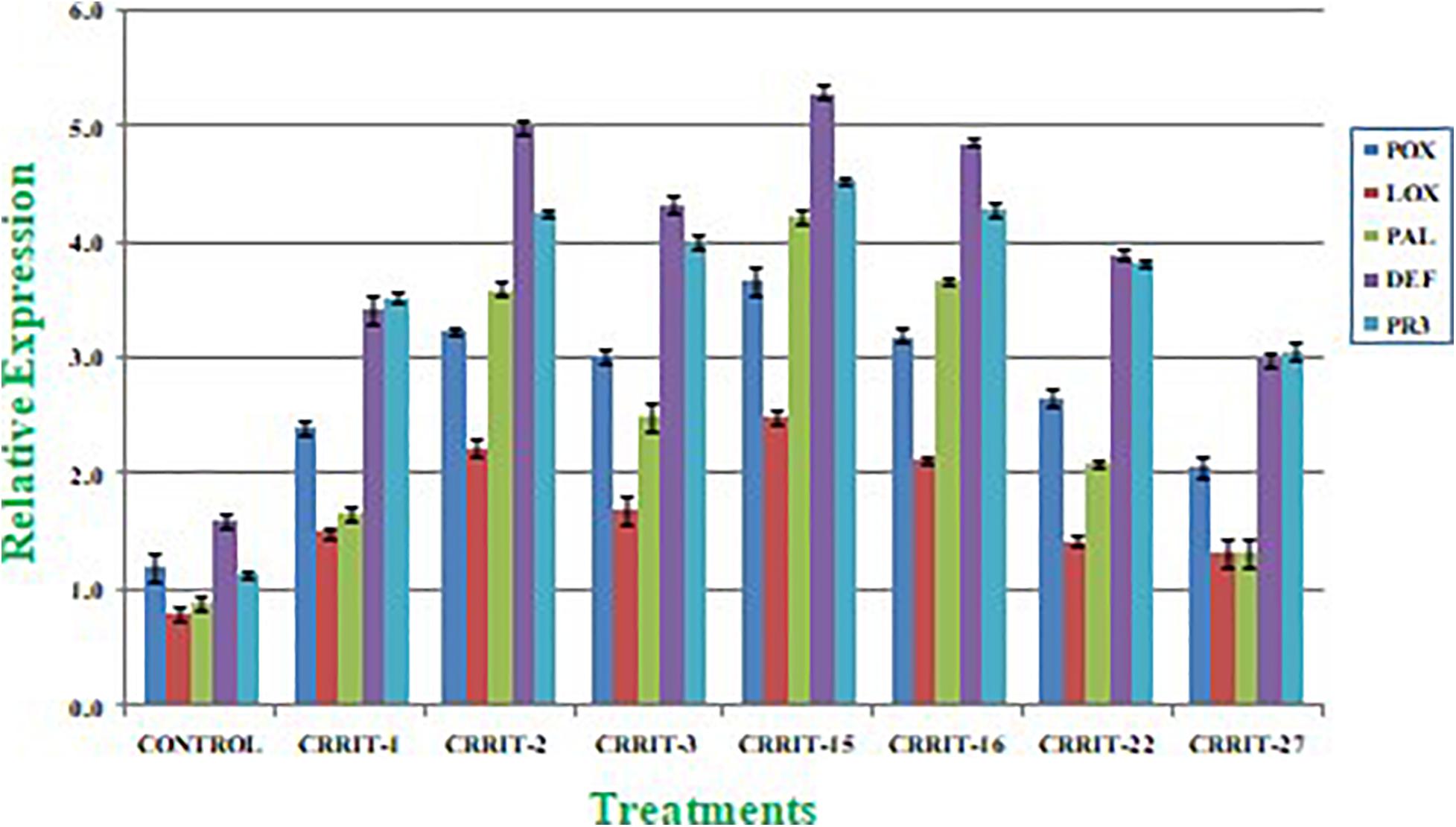
Figure 4. Expression of antioxidant and defense related genes in rice variety (Annapurna). Results are expressed as means of three replicates and vertical bars indicate the standard deviation of the means.
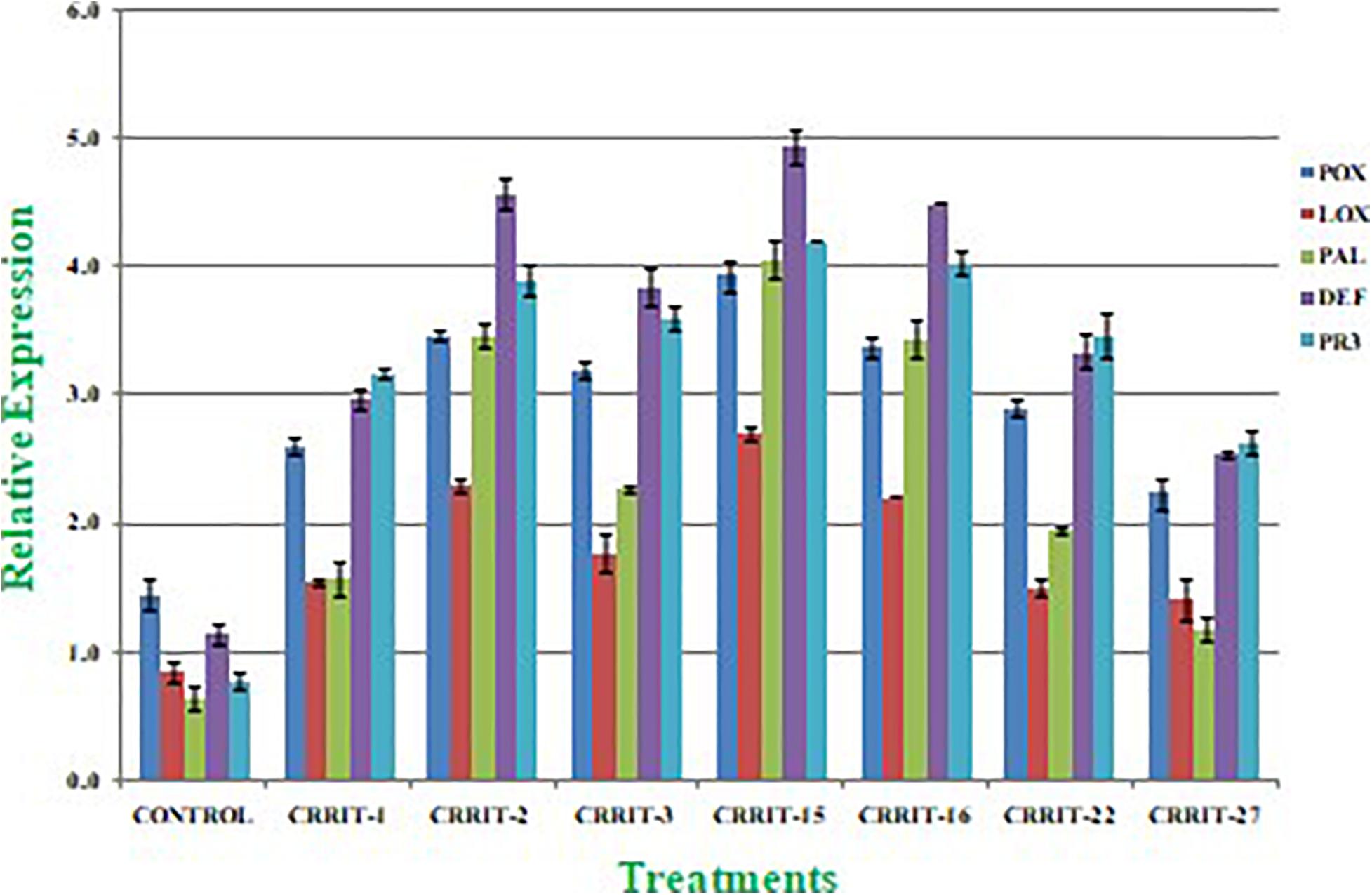
Figure 5. Expression of antioxidant and defense related genes in rice variety (Satabdi). Results are expressed as means of three replicates and vertical bars indicate the standard deviation of the means.
Seed biopriming with beneficial microbes have been accounted for their capacity to relieve biotic stress in an effective way. During the process of seed biopriming, antagonistic plant growth promotive activity increases on the seed surface, hence not only defending the plant from pathogen attack but also promoting the plant growth (Swain et al., 2018; Rajput et al., 2019). Co-vaccination of Paenibacillus polymyxa and Rhizobium tropici mitigated drought in common bean (Phaseolus vulgaris L.) (Figueiredo et al., 2008). In the present study it was found that Trichoderma treated plants exhibited an increase in total phenol content catalase content, peroxidase content superoxide dismutase content and expression of defense gene (POX, LOX, PAL, DFENSIN, and PR-3) as compared to control one. This further authenticates the induction of growth responses and defense response in rice up on application of Trichoderma in rice seeds. Plant phenolics are normally framed in light of both biotic and abiotic stress through enactment of phenyl propanoid pathway and include in cellulase, lignin, xylanase, and biosynthesis. Thus, PPO catalyzes phenolics, exacerbating the production of quinines through a secondary reaction, which further prompts the arrangement of an earthy colored complex polymer, melanin; a physical hindrance to microbe ingression (Taranto et al., 2017). In this study all the Trichoderma strains exhibited higher total phenol content, PPO activity as compared to the control one. Besides, CRRIT-15, CRIT-16, and CRRIT-2 out-perform the others. This suggests a synergistic effect of Trichoderma in induction of plant growth and defense in rice varieties. Notwithstanding these, acceptance of different development promotive genes and antioxidant agent responsive genes mitigates oxidative stress in plant cells. Our outcomes are validated with Singh et al. (2013) in which they revealed improved movement of PAL, PPO, PO, and SOD content in chickpea treated with Pseudomonas, Trichoderma, and Rhizobium. Contrasting these discoveries and our outcomes prompts the presumption that stronghold of rice with microbial inoculants characteristically balanced molecular components to give resilience against ROS scavenging in a manner to making plants fortified against stress difficulties. Rice seed biopriming of Trichoderma could, accordingly, become a proficient methodology for raising yield for better profitability and resistance against stress conditions.
Conclusion
In India alone, an excess of 250 commercial formulations are available, however, a large portion of them are from a solitary strain, i.e., Trichoderma viride (presently renamed as Trichoderma asperelloides). Most of the Trichoderma strains defined in literature were isolated from the soil or rhizosphere, but very few are isolated from the above ground aerial parts. In the present study we evaluated both the biocontrol and growth promotion activity of seven different Trichoderma strains isolated from above ground parts. The rice seed treatment with Trichoderma strains not only promoted germination, seedling vigor, and growth of the plant, but also increase the level of gene expression related to plant defense. Apart from growth promotion these strains imparted intrinsic stress tolerance to rice by producing a higher amount of defense enzymes like catalase, peroxidase, superoxide dismutase, polyphenol oxidase, and total phenolics content as evidenced by the expression of their respective genes. Our recent investigation tries to fill the gap by isolating and identifying above ground Trichoderma strains for rice health management. Two strains, namely T. hebeiensis and T. erinaceum may be promoted in sustainable crop management for their beneficial role.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
HS: investigation, statistical analysis, and writing – original draft. TA: conceptualization and writing – review and editing. AM: conceptualization, writing – review and editing, project administration, resources, and supervision. PS, SS, AK, and RJ: investigation. PB: methodology. SN: writing – review and editing, and project administration. SM: project administration. MB: statistical analysis. SKM and NZ: conducted the field trials and recorded the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors are immensely thankful to Department of Science and Technology (DST), Govt. of India, for providing DST-INSPIRE Fellowship to Harekrushna Swain having Fellowship No. IF140749 (NRRI EAP-195). Authors duly acknowledge the Board of Research in Nuclear Science (BRNS), Department of Atomic Energy, and Govt. of India SANCTION No. 35/14/35/2016-BRNS/35159, dated on December 1, 2016 (NRRI EAP-233) and International Rice Research Institute, New Delhi, India (NRRI EAP-186) for providing funds. Authors are also thankful to Director, ICAR-NRRI, Cuttack-753006 and HOD, Department of Botany and Biotechnology, Ravenshaw University, Cuttack-753003 for providing necessary technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.633881/full#supplementary-material
Footnotes
References
Abdollahi, M., Rahnama, K., Marabadi, M., Ommati, F., and Zaker, M. (2012). The in vitro efficacy of Trichoderma isolates against Pythium aphanidermatum, the causal agent of sugar beet root rot. J. Res. Agric. Sci. 8, 79–87.
Abdul-Baki, A., and Anderson, J. D. (1973). Vigor determination in Soybean seed by multiple criteria. Crop Sci. 13, 630–633. doi: 10.2135/cropsci1973.0011183x001300060013x
Abraham, A., Philip, S., Jacob, C. K., and Jayachandran, K. (2013). Novel bacterial endophytes from Hevea brasiliensis as biocontrol agent against Phytophthora leaf fall disease. Biocontrol 58, 675–684. doi: 10.1007/s10526-013-9516-0
Arnon, D. I. (1949). Copper enzymes in isolated chloroplast: polyphenol oxidase in Beta vulgaris. Plant Physiol. 24, 1–15. doi: 10.1104/pp.24.1.1
Bailey, B. A., Korcak, R. F., and Anderson, J. D. (1993). Sensitivity to an ethylene biosynthesis-inducing endoxylanase in Nicotiana tabacum-L cv xanthi is controlled by a single dominant gene. Plant Physiol. 101, 1081–1088. doi: 10.1104/pp.101.3.1081
Bailey, M. J., Biely, P., and Poutanen, K. (1992). Inter-laboratory testing of methods for assay of xylanase activity. J. Biotechnol. 23, 257–270. doi: 10.1016/0168-1656(92)90074-j
Cai, F., Yu, G., Wang, P., Wei, Z., Fu, L., Shen, Q., et al. (2013). Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Biochem. 73, 106–113. doi: 10.1016/j.plaphy.2013.08.011
Celar, F. (2003). Competition for ammonium and nitrate forms of nitrogen between some phytopathogenic and antagonistic soil fungi. Biol. Control 28, 19–24. doi: 10.1016/s1049-9644(03)00049-5
Contreras-Cornejo, H. A., Macias-Rodríguez, L., Cortés-Penagos, C., and López-Bucio, J. (2009). Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 149, 1579–1592. doi: 10.1104/pp.108.130369
Daw, B. D., Zhang, L. H., and Wang, Z. Z. (2008). Salicylic acid enhances antifungal resistance to Magnaporthe grisea in rice plants. Austral. Plant Pathol. 37, 637–644. doi: 10.1071/ap08054
Druzhinina, I. S., Seidl-Seiboth, V., Herrera-Estrella, A., Horwitz, B. A., Kenerley, C. M., Monte, E., et al. (2011). Trichoderma: the genomics of opportunistic success. Nat. Rev. Microbiol. 9, 749–759. doi: 10.1038/nrmicro2637
Dunaitsev, I. A., Kolombet, L. V., Zhigletsova, S. K., Bystrova, E. V., Besaeva, S. G., Klykova, M. V., et al. (2008). Phosphate releasing microorganisms with antagonistic activity against phytopathogenic microorganisms. Mikol. Fitopatol. 42, 264–269.
Figueiredo, M. V. B., Buritya, H. A., Martínez, C. R., and Chanway, C. P. (2008). Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 40, 182–188. doi: 10.1016/j.apsoil.2008.04.005
Florencio, C., Couri, S., and Farinas, C. S. (2012). Correlation between agar plate screening and solid state fermentation for the prediction of cellulase production by Trichoderma strains. Enzyme Res. 2012:793708. doi: 10.1155/2012/793708
Gams, W., and Bissett, J. (1998). “Morphology and identification of Trichoderma,” in Trichoderma and Gliocladium, Vol. 1, eds C. P. Kubicek and G. E. Harman (London: Taylor and Francis), 1–34.
Ghosh, T. K. (1987). Measurement of cellulase activities. Pure Appl. Chem. 59, 257–268. doi: 10.1351/pac198759020257
Gochev, V. K., and Krastanov, A. I. (2007). Isolation of laccase producing trichoderma species. Bulgarian J. Agric. Sci. 13, 171–176.
Gravel, V., Antoun, H., and Tweddell, R. J. (2007). Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol. Biochem. 39, 1968–1977. doi: 10.1016/j.soilbio.2007.02.015
Harman, G. E. (2011). Multifunctional fungal plant symbionts: new tools to enhance plant growth and productivity. New Phytol. 189, 647–649. doi: 10.1111/j.1469-8137.2010.03614.x
Holker, U., Dohse, J., and Hofer, M. (2002). Extracellular Laccase in ascomycetes Trichoderma atroviridae and Trichoderma harzianum. Folia Microbiol. 47, 423–427.
Hossain, M., Sultana, F., Kubota, M., Koyama, H., and Hyakumachi, M. (2007). The plant growth-promoting fungus Penicillium simplicissimum GP17-2 induces resistance in Arabidopsis thaliana by activation of multiple defense signals. Plant Cell Physiol. 48, 1724–1736. doi: 10.1093/pcp/pcm144
Jahagirdar, S., Kambrekar, D. N., Navi, S. S., and Kunta, M. (2019). “Plant growth-promoting fungi: diversity and classification,” in Bioactive Molecules in Plant Defense, eds S. Jogaiah and M. Abdelrahman (Cham: Springer).
Juturu, V., and Wu, J. C. (2014). Microbial cellulases: engineering production and applications. Renew. Sustain. Energy Rev. 33, 188–203. doi: 10.1016/j.rser.2014.01.077
Kalra, K., Chauhan, R., Shavez, M., and And Sachdeva, S. (2013). Isolation of laccase producing Trichoderma species and effect of pH and temperature on its activity. Int. J. ChemTech Res. 5, 2229–2235.
Kang, S. W., Park, Y. S., Lee, J. S., Hong, S. I., and Kim, S. W. (2004). Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour. Technol. 91, 153–156. doi: 10.1016/s0960-8524(03)00172-x
Kar, S., Mandal, A., Das Mohapatra, P. K., Mondal, K. C., and Pati, B. R. (2006). Production of cellulase free xylanase by Trichoderma reesei SAF3. Braz. J. Microbiol. 37, 462–464. doi: 10.1590/s1517-83822006000400011
Keeney, D. R., and Bremner, J. M. (1965). Steam distillation methods for determining of ammonium, nitrate and nitrite. Anal. Chim. Acta 32, 485–497. doi: 10.1016/s0003-2670(00)88973-4
Kumar, K., Amaresan, N., Bhagat, S., Madhuri, K., and Srivastava, R. C. (2012). Isolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian J. Microbiol. 52, 137–144. doi: 10.1007/s12088-011-0205-3
Lee, S., Jang, Y., Lee, Y. M., Lee, J., Lee, H., Kim, G. H., et al. (2011). Rice straw-decomposing fungi and their cellulolytic and Xylanolytic enzymes. J. Microbiol. Biotechnol. 21, 1322–1329. doi: 10.4014/jmb.1107.07022
Małolepsza, U., Nawrocka, J., and Szczech, M. (2017). Trichoderma virens 106 inoculation stimulates defense enzyme activities and enhances phenolics levels in tomato plants leading to lowered Rhizoctonia solani infection. Biocontrol Sci. Technol. 27, 180–199. doi: 10.1080/09583157.2016.1264570
Mandal, S., Kar, I., Mukherjee, A. K., and Acharya, P. (2013). Elicitor-induced defense responses in Solanum lycopersicum against Ralstonia solanacearum. Sci. World J. 2013:561056. doi: 10.1155/2013/561056
Manwar, A. V., Rakh, R. R., Raut, L. S., and Dalvi, S. M. (2011). Biological control of Sclerotium rolfsii, causing stem rot of groundnut by Pseudomonas cf. monteilii. Recent Res. Sci. Technol. 3, 26–34.
Meera, T., and Balabaskar, P. (2012). Isolation and characterization of Pseudomonas fluorescens from rice fields. Int. J. Food Agric. Vet. Sci. 2, 113–120.
Mohapatra, S., and Mittra, B. (2017). Alleviation of Fusarium oxysporum induced oxidative stress in wheat by Trichoderma viride. Arch. Phytopathol. Plant Protect. 50, 84–96. doi: 10.1080/03235408.2016.1263052
Monssef, R., Abd Hassan, A., Enas, A., and Ramadan, M. E. (2016). Production of Laccase enzyme for their potential application to decolorize fungal pigments on aging and paper parchment. Ann. Agric. Sci. 61, 145–154. doi: 10.1016/j.aoas.2015.11.007
Mukherjee, A. K., Sampath, Kumar, A., Kranthi, S., and Mukherjee, P. K. (2014). Biocontrol potential of three novel Trichoderma strains: isolation, evaluation and formulation. 3Biotech 4, 275–281. doi: 10.1007/s13205-013-0150-4
Mukherjee, A. K., Swain, H., Adak, T., and Chattopadhyaya, K. (2018). Evaluation of Trichoderma based product ‘RiceVit’ in farmers field of Chandol, Kendrapada, Odisha. NRRI Newslett. 39, 20–21.
Mukherjee, P. K., Horwitz, B. A., Singh, U. Shankar, Mukherjee, M., and Schmoll, M. (2013). Trichoderma: Biology and Applications. London: CAB International.
Noori, M. S. S., and Saud, H. M. (2012). Potential plant growth-promoting activity of Pseudomonas spp. isolated from paddy soil in Malaysia as biocontrol agent. J. Plant Pathol. Microbiol. 3:2. doi: 10.4172/2157-7471.1000120
Parmar, H. J., Bodar, N. P., Lakhani, H. N., Patel, S. V., Umrania, V. V., and Hassan, M. M. (2015). Production of lytic enzymes by Trichoderma strains during in vitro antagonism with Sclerotium rolfsii, the causal agent of stem rot of groundnut. Afr. J. Microbiol. Res. 9, 365–372. doi: 10.5897/ajmr2014.7330
Pathak, P., Bharadwaj, N., and Singh, A. K. (2014). Production of crude cellulase and xylanase from Trichoderma harzianum PPDDN10NFCCI-2925 and its application in photocopier waste paper recycling. Appl. Biochem. Biotechnol. 172, 3776–3797. doi: 10.1007/s12010-014-0758-9
Porra, R. J. (2002). The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 73, 149–156. doi: 10.4324/9781351187596-10
Punja, Z. K., and Utkhede, R. S. (2003). Using fungi and yeasts to manage vegetable crop diseases. Trends Biotechnol. 21, 400–407. doi: 10.1016/s0167-7799(03)00193-8
Rajput, R. S., Singh, P., Singh, J., Vaishnav, A., Ray, S., and Singh, H. B. (2019). Trichoderma mediated seed biopriming augments antioxidant and phenylpropanoid activities in tomato plant against Sclerotium rolfsii. J. Pharmacogn. Phytochem. 8, 2641–2647.
Rejeb, I. B., Pastor, V., and Mauch-Mani, B. (2014). Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants 3, 458–475. doi: 10.3390/plants3040458
Rudresh, D. L., Shivaprakash, M. K., and Prasad, R. D. (2005). Tricalcium phosphate solubilizing abilities of Trichoderma spp. in relation to P uptake and growth and yield parameters of chickpea (Cicer arietinum L.). Can. J. Microbiol. 51, 217–222. doi: 10.1139/w04-127
Saczi, A., Radford, A., and Erenler, K. (1986). Detection of cellulolytic fungi by using congo red as an indicator: a comparative study with the dinitrosalicylic acid reagent method. J. Appl. Microbiol. 61, 559–562. doi: 10.1111/j.1365-2672.1986.tb01729.x
Sadasivam, S., and Manickum, A. (2011). Biochemical Methods, third Edn. New Delhi: New Age International (P) Limited Publishers, 203–204.
Sancez, C. (2009). Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol. Adv. 27, 185–194. doi: 10.1016/j.biotechadv.2008.11.001
Saravanan, D., Dinesh, C., Kartikeyan, S., Vivekanandan, A., Nalankilli, G., and Ramachandran, T. (2008). Biopolishing of cotton fabrics with total cellulase of Trichoderma reesei and optimization using Taguchi methods. J. Appl. Polym. Sci. 112, 3402–3409. doi: 10.1002/app.29826
Saroj, P., Manasa, P., and Narasimhulu, K. (2018). Characterization of thermophilic fungi producing extracellular lignocellulolytic enzymes for lignocellulosic hydrolysis under solid state fermentation. Bioresour. Bioprocess 5:31. doi: 10.1186/s40643-018-0216-6
Singh, A., Sarma, B. K., Upadhyay, R. S., and Singh, H. B. (2013). Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiol. Res. 168, 33–40. doi: 10.1016/j.micres.2012.07.001
Singh, D. P., Singh, V., Shukla, R., Sahu, P., Prabha, R., Gupta, A., et al. (2020). Stage-dependent concomitant microbial fortification improves soil nutrient status, plant growth, antioxidative defense system and gene expression in rice. Microbiol. Res. 239:126538. doi: 10.1016/j.micres.2020.126538
Singh, P., Singh, J., Ray, S., Rajput, R. S., Vaishnav, A., Singh, R. K., et al. (2020). Seed biopriming with antagonistic microbes and ascorbic acid induce resistance in tomato against Fusarium wilt. Microbiol. Res. 237:126482. doi: 10.1016/j.micres.2020.126482
Sureshrao, K. S., Pradeeprao, K. V., Dnyanobarao, G. S., Agrawal, T., and Kotasthane, A. S. (2016). Efficiency of different trichoderma isolates on plant growth promoting activity in rice (Oryza sativa L.). Int. J. Bio Resour. Stress Manag. 7, 489–500.
Swain, H., Adak, T., Mukherjee, A. K., Mukherjee, P. K., Bhattacharyya, P., Behera, S., et al. (2018). Novel Trichoderma strains. isolated from tree barks as potential biocontrol agents and biofertilizers for direct seeded rice. Microbiol. Res. 214, 83–90. doi: 10.1016/j.micres.2018.05.015
Swain, H., and Mukherjee, A. K. (2020). “Host–pathogen–trichoderma interaction,” in Trichoderma, eds A. Sharma and P. Sharma (Singapore: Springer), 149–165. doi: 10.1007/978-981-15-3321-1_8
Taranto, F., Pasqualone, A., Mangini, G., Tripodi, P., Miazzi, M., Pavan, S., et al. (2017). Polyphenol oxidases in crops: biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 18:377. doi: 10.3390/ijms18020377
Teather, R. M., and Wood, P. J. (1982). Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 43, 777–780. doi: 10.1128/aem.43.4.777-780.1982
Vargas, W. A., Djonovic, S., Sukno, S. A., and Kenerley, C. M. (2008). Dimerization controls the activity of fungal elicitors that trigger systemic resistance in plants. J. Biochem. Chem. 283, 19804–19815. doi: 10.1074/jbc.m802724200
Velazquez-Cedeno, M. A., Farnet, A. M., Ferre, E., and Savoie, J. M. (2004). Variations of lignocellulosic activities in dual cultures of Pleurotuso streatus and Trichoderma longibrachiatum on unsterilized wheat straw. Mycologia 96, 712–719. doi: 10.2307/3762105
Walkley, A., and Black, I. A. (1934). An examination of the Degtareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Whipps, J. M., and Lumsden, R. D. (2001). “Commercial use of fungi as plant disease biological control agents: status and prospects,” in Fungi as Biocontrol Agents: Progress, Problems and Potential, eds T. M. Butt, C. Jackson, and N. Magan (Wallingford: CABI Publishing), 9–22. doi: 10.1079/9780851993560.0009
Woo, S. L., Ruoco, M. F., and Vinale, F. (2014). Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 8(Suppl. 1, M4), 71–126. doi: 10.2174/1874437001408010071
Yedidia, I., Srivastva, A. K., Kapulnik, Y., and Chet, I. (2001). Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 235, 235–242.
Keywords: Trichoderma hebeiensis, indole acetic acid, prussic acid, straw degrading enzyme, vigor index, stress responsive enzyme, antioxidant genes, biofertilizer
Citation: Swain H, Adak T, Mukherjee AK, Sarangi S, Samal P, Khandual A, Jena R, Bhattacharyya P, Naik SK, Mehetre ST, Baite MS, Kumar M S and Zaidi NW (2021) Seed Biopriming With Trichoderma Strains Isolated From Tree Bark Improves Plant Growth, Antioxidative Defense System in Rice and Enhance Straw Degradation Capacity. Front. Microbiol. 12:633881. doi: 10.3389/fmicb.2021.633881
Received: 26 November 2020; Accepted: 25 January 2021;
Published: 26 February 2021.
Edited by:
Giuseppe Colla, University of Tuscia, ItalyReviewed by:
Birinchi Kumar Sarma, Banaras Hindu University, IndiaOrlando Borras-Hidalgo, Qilu University of Technology, China
Copyright © 2021 Swain, Adak, Mukherjee, Sarangi, Samal, Khandual, Jena, Bhattacharyya, Naik, Mehetre, Baite, Kumar M and Zaidi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arup K. Mukherjee, dGl0aXJ0dWFAZ21haWwuY29t; YXJ1cG11a2hlcmplZUB5YWhvby5jb20=
 Harekrushna Swain
Harekrushna Swain Totan Adak
Totan Adak Arup K. Mukherjee
Arup K. Mukherjee Sarmistha Sarangi
Sarmistha Sarangi Pankajini Samal
Pankajini Samal Ansuman Khandual
Ansuman Khandual Rupalin Jena
Rupalin Jena Pratap Bhattacharyya
Pratap Bhattacharyya Soumendra K. Naik
Soumendra K. Naik Sayaji T. Mehetre
Sayaji T. Mehetre Mathew S. Baite
Mathew S. Baite Sunil Kumar M
Sunil Kumar M Najam Waris Zaidi6
Najam Waris Zaidi6