
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 14 December 2020
Sec. Aquatic Microbiology
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.610974
This article is part of the Research TopicAdvancements in the Understanding of Anthropogenic Impacts on the Microbial Ecology and Function of Aquatic EnvironmentsView all 13 articles
 Xing Chen1,2
Xing Chen1,2 Huaxian Zhao1
Huaxian Zhao1 Gonglingxia Jiang1
Gonglingxia Jiang1 Jinli Tang1
Jinli Tang1 Qiangsheng Xu1
Qiangsheng Xu1 Lengjinghua Huang1
Lengjinghua Huang1 Si Chen2
Si Chen2 Shuqi Zou3
Shuqi Zou3 Ke Dong3
Ke Dong3 Nan Li1*
Nan Li1*Vibrio are widely distributed in aquatic environments and strongly associated with eutrophic environments and human health through the consumption of contaminated seafood. However, the response of the Vibrio community to seasonal variation in eutrophic environments is poorly understood. In this study, we used a Vibrio-specific 16S rRNA sequencing approach to reveal the seasonal distribution pattern and diversity of the Vibrio community in the Maowei Sea, Beibu Gulf of China. The Shannon diversity of the Vibrio community was highest in the summer, while β-diversity analysis showed that Vibrio community structures were significantly different between seasons. Distance-based redundancy analysis (dbRDA) and Mantel test analysis suggested that total dissolved nitrogen (TDN), total dissolved phosphorus (TDP), dissolved inorganic nitrogen (DIN), salinity, and temperature were the key environmental factors shaping the Vibrio community structure, indicating a strong filtering effect of trophic condition on Vibrio communities. Furthermore, through random forest analysis, V. fluvialis, V. alginolyticus, V. proteolyticus, V. splendidus, and the other eight Vibrio species were more sensitive to eutrophic changes. This study revealed seasonal changes in Vibrio communities and the influence of environmental variation on Vibrio community composition, contributing to a better understanding of their potential ecological roles in a subtropical inland bay.
The genus Vibrio consists of heterotrophic bacteria that are common in marine ecosystems, especially in coastal areas (Westrich, 2015; Shelford and Suttle, 2018; Zhang et al., 2019). Due to their remarkable capability for decomposition of a range of nutrient resources, including chitin and alginate, Vibrio may exert large impacts on biogeochemical cycling in coastal habitats (Kopprio et al., 2017; Zhang et al., 2018). Furthermore, the genus Vibrio encompasses many facultatively symbiotic and potential pathogenic strains, such as Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus, which are all capable of infecting humans through the consumption of contaminated seafood (Guin et al., 2019; hun Yoon and Waters, 2019; King et al., 2019). Since they drive major biogeochemical cycles and support food webs globally, Vibrio communities are a vital component of the marine ecosystem. Furthermore, the diversity and dynamics of the Vibrio community have attracted growing interest worldwide, including in the Northern Chinese marginal seas, coastal North Atlantic, and Gulf Coast of Mexico (López-Hernández et al., 2015; Vezzulli et al., 2016; Wang et al., 2019). Understanding when and where pathogenic Vibrio will break out is important for aquaculture and marine health. A range of studies (Hartwick et al., 2019; Lamon et al., 2019; Baddam et al., 2020) have indicated that the Vibrio community undergoes substantial temporal variation. However, the response of the Vibrio community to seasonal variation in eutrophic environments is still poorly understood.
Vibrio communities are highly diverse and complex and vary between habitats due to changing environmental conditions. This increases the necessity of studying the ecology of Vibrio. Previous studies have demonstrated that the dynamics of Vibrio populations are linked to multiple factors, including seasonal changes, biological parameters, and physicochemical properties such as temperature, salinity, pH, and dissolved oxygen (DO), as well as associations with nutrients (Urquhart et al., 2016; Greenfield et al., 2017; Froelich et al., 2019). For example, Siboni et al. (2016) revealed that shifts in the composition of the Vibrio community between seasons were primarily driven by changes in temperature, salinity, and NO2–. Takemura et al. (2014) found temperature-associated increases in the abundance of pathogenic Vibrio species observed in Australian coastal areas. Considering that the occurrence of specific vibrios has frequently been linked to the temperature, salinity, and nutrients, we hypothesized that seasonal variability in the coastal ecosystem could characterize the level of Vibrio community differentiation under ongoing environmental forcing.
Moreover, Vibrio disease events and community structure appear to be inextricably linked to anthropogenic disturbance of the aquatic environment. An influx of anthropogenically derived pollutants and nutrients into aquatic systems significantly disrupts the Vibrio community structure, contributing to changes in diversity and an increase in potentially pathogenic Vibrio species (Shore-Maggio et al., 2018). For example, the dense human population and low-lying geography of an estuarine environment were hypothesized to have allowed the enrichment of V. cholerae, which causes fatal damage to the human gut (Boucher et al., 2015). Besides, Vibrio are strongly associated with high nutrient environments, which are considered a potential indicator for environmental changes and eutrophication (Gregoracci et al., 2012; Takemura et al., 2014). Consequently, gaining deeper insight into the diversity of marine Vibrio and the role of environmental factors as filters of Vibrio community composition will help people evaluate their ecological role and public health effect in the marine coastal ecosystem.
To better understand the associations between the free-living Vibrio community and eutrophic perturbation, we collected surface water samples from the Maowei Sea, which is an estuarine-bay ecosystem that suffers from anthropogenic activities and receives large inputs of terrestrial nutrients from the QinJiang and Maoling rivers, existing serious eutrophication in the area (Zhu et al., 2019). Therefore, this research presented here focuses on the associations between Vibrio community composition and seasonal patterns under broadly eutrophic condition. Our aims were to (a) fill the gap in knowledge of the seasonal composition and variation in the Vibrio community in the Maowei Sea, (b) determine the gradients of the key controlling environmental factors driving the dynamics of the Vibrio community, (c) and identify the potential indicative Vibrio species for eutrophic conditions in the local ecosystem.
We collected a total of 140 surface seawater samples (0.5 m deep) seasonally from seven sites of the Maowei Sea from June 2017 through March 2018 (Figure 1). Two liters of seawater was collected from four corners and the middle of a 5 m × 5 m square area in each site by a five-point sampling method. Thereafter, all seawater samples were stored at 4°C on board and transferred to the laboratory before filtration. A vacuum pump was used to sequentially filter 1 L of seawater per sample through 3-μm filters (Port Washington, NY, United States) to remove debris and larger organisms, and the resulting samples were collected on 0.22-μm-pore-size polycarbonate membranes (Millipore Corporation, Billerica, MA, United States) for subsequent analysis. During sampling, seawater temperature, pH, and salinity in each sample were measured using a portable meter (556 MPS; YSI, United States). Other nutrients in each sample were measured by standard methods previously described by Yao et al. (2014). For example, dissolved inorganic nutrients (NO2–, NO3–, and NH4+) were measured using spectrophotometric and colorimetric methods (Han et al., 2012). Dissolved inorganic nitrogen (DIN) was composed of NO2–, NO3–, and NH4+, and dissolved inorganic phosphorus (DIP) represented the concentration of PO4-P (Lai et al., 2014). Chl a was measured using a fluorescence spectrophotometer (Holm-Hansen and Riemann, 1978). The total organic carbon (TOC) and the chemical oxygen demand (COD) were measured using the standard method (Visco et al., 2005). The total dissolved nitrogen (TDN) and the total dissolved phosphorus (TDP) were determined using the Cu–Cd column reduction and the spectrophotometric phosphomolybdate blue method, respectively (Sah, 1994; Cai and Guo, 2009). The eutrophication index (EI) was indicated as a Chinese eutrophication status index, which was calculated as the following formula: EI = DIN × DIP × COD × 106/4500 (Lai et al., 2014; Chen et al., 2016). According to the degree of eutrophication, we found that the samples could be classified into three eutrophication statues: low eutrophication (1 < EI < 3), medium eutrophication (3 < EI < 9), and high eutrophication (EI > 9) (Supplementary Table 1). Samples nearby the ocean area showed the lower eutrophication, whereas samples located next to the river mouth presented higher eutrophication.
Genomic DNA extraction was performed by a DNeasy PowerWater Kit (QIAGEN, United States) using 0.22-μm-pore-size polycarbonate membranes according to the manufacturer’s protocols. DNA yield and purity were evaluated using a Nanodrop-2000 Spectrophotometer (Thermo Scientific, United States). The DNA sample was preserved at −80°C. The extracted DNA was tested by PCR using the Vibrio-specific 16S rRNA primers 169F (5′-GGCGTAAAGCGCATGCAGGT-3′) and 680R (5′-GAAATTCTACCCCCCTCTACAG-3′) (Thompson et al., 2004). A total reaction volume of 20 μL PCR mixture containing 2 μL DNA template, 6 μL ddH2O, 10 μL 2 × Taq PCR Mastermix (TIANGEN, China), and 2 μL forward and reverse primers were conducted on a Bio-Rad thermocycler (Hercules, CA). The amplification process was as follows: an initial activation step at 94°C for 1 min, followed by 35 cycles of 30 s denaturation at 95°C, annealing at 56°C for 30 s, extension at 72°C for 30 s, and a final elongation step at 72°C for 10 min. Ultrapure water was used instead of a sample solution as a negative control, which ruled out the possibility of false-positive PCR results. The PCR products were verified by 2% agarose gel electrophoresis and visualized with a UV light and gel image system.
Sequencing was performed on the Illumina MiSeq platform of Lianchuan Bio-Technology Company (China) after preparing DNA libraries. Sequences with low-quality reads (quality scores <30) were removed using Trimmomatics (Bolger et al., 2014). Adapter and barcode sequences were eliminated by Cutadapt (Martin, 2011). The paired-end demultiplexed sequencing reads were processed in a QIIME2 environment (Bolyen et al., 2019). Using a VSEARCH plugin (Rognes et al., 2016), the paired-end reads were joined followed by dereplicating, chimeras filtering, and operational taxonomic units (OTUs) clustering (de novo) at a 97% sequence similarity level. Taxonomic classification of OTUs was assigned by a local Blastn (cutoff E-value 1e-10) against a Ribosomal Database Project (RDP) database (Release 11) (Cole et al., 2014).
Seasonal differences in environmental parameters were evaluated using a one-way analysis of variance (ANOVA) followed by Tukey’s HSD test in SPSS Statistics v24.0 (Subtype and Sloan, 2018). The α-diversity indices between different groups of samples, including Shannon, Simpson, Chao1, and observed number of OTUs were calculated using the vegan package in R (Dixon, 2003). The β-diversity was performed with Bray-Curtis dissimilarity, principal coordinate analysis (PCoA), ANOSIM, and PERMANOVA for comparison between the seasonal Vibrio communities. Random forest analysis was used to examine the important indicator taxa. Spearman correlation, Mantel test analysis, redundancy analysis (RDA), variation partitioning analysis (VPA), and linear regression were also run in R to reveal correlations between the Vibrio community and environmental factors. The R software packages used in this study are mainly the “vegan,” “random forest,” and “psych” packages (R Core Team, 2013). Analysis of the quantitative and evolutionary relationships of Vibrio species in different seasons was performed using TBtool (Chen C. et al., 2018).
The environmental parameters of seawater samples collected from the Maowei Sea in the Beibu Gulf were measured during the seasonal cruises (Supplementary Table 1). The environmental parameters of the seawater samples all varied significantly between seasons as per ANOVA (Tukey’s HSD test, p < 0.001) (Table 1). The temperature (24.58 ± 6.25°C), Chl a (2.23 ± 1.10 μg/L), TOC (1.20 ± 0.29 mg/L), and COD (2.82 ± 0.69 mg/L) were significantly higher in summer and autumn than in spring and winter. In contrast, the value for salinity (18.19 ± 5.13), DO (7.64 ± 1.37 mg/L), and TDP (0.06 ± 0.01 mg/L) showed higher in spring and winter. In TDN, there were differences between fall and winter, with the lowest value (0.57 ± 0.07) in winter and the highest value (0.79 ± 0.180) in fall. Moreover, NO2–, NO3–, and NH4+ concentrations all showed lower values in winter. According to EI indicators, the eutrophication level was significantly lowest in winter and highest in fall. Also, within each season, the eutrophication showed a decreasing spatial trend from the river mouth site M1 to the open sea site M7 (Supplementary Table 1). Overall, environmental parameters varied between all the sites, and the eutrophication conditions showed significant seasonal changes (p < 0.001; Table 1).
A total of 12,589 ± 5,735 sequences were generated from the samples after quality control and clustered into 85 ± 18 OTUs at a 97% similarity level (Supplementary Table 2). Good’s coverage values were greater than 99%, indicating that the current sequences reflected the actual situation of the majority of the Vibrio community. The α-diversity of the Vibrio community including Shannon, Simpson, Chao1, and the observed number of OTU indices varied and differed significantly across seasons (ANOVA, p < 0.001) (Figure 2). The summer group showed the highest α-diversity, including Shannon, Simpson, and Chao1 indices, indicating that evenness and richness were significantly higher than in other seasons (Figure 2). However, the Shannon and Simpson diversities of the Vibrio community were lowest in the spring samples and significantly different from summer and winter. According to Spearman’s correlation analysis, the α-diversity of the Vibrio community was negatively (p < 0.01) correlated to NO2–, NO3–, NH4+, TDN, DIN, DIP, and COD but only positively correlated to salinity (p < 0.001) (Supplementary Table 3). Since the EI is a synthetic index of the multiple nutrients of DIN (NO2–, NO3–, and NH4+), DIP, and COD, we determined that the occurrence of eutrophication, especially in summer and fall, was accompanied by a decrease in the α-diversity of the Vibrio community. In addition, TDN, DIN, NO2–, and salinity were the most influential (| r| > 0.28, p < 0.001) among these factors.
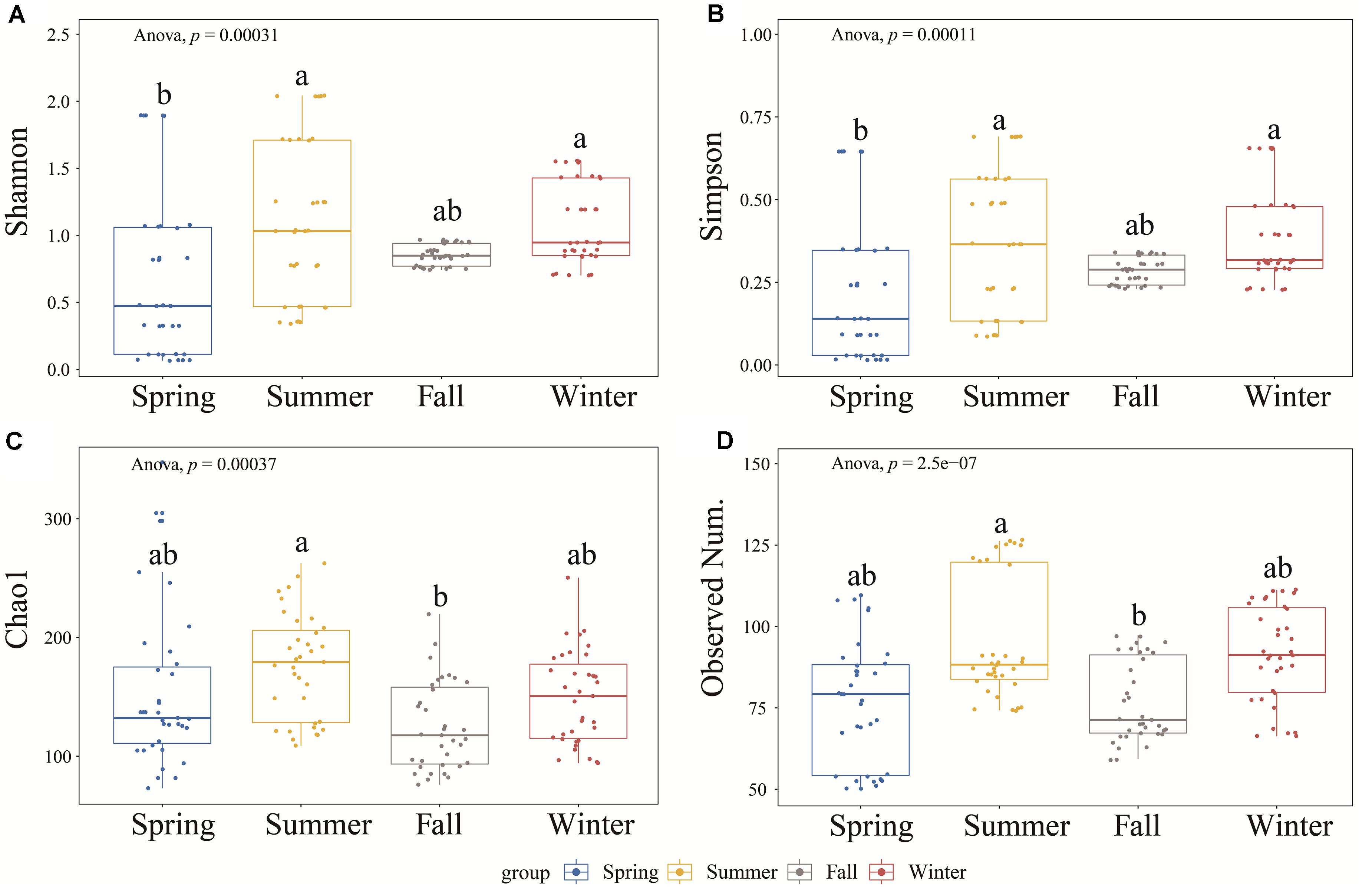
Figure 2. The α-diversity indices (A–D: Shannon, Simpson, Chao 1, and Observed Number of OTUs) among the four seasons by boxplot. The differences between any two groups were tested by Tukey’s HSD test, presented by lowercase letters on the plots.
The representative sequences of each OTU were analyzed to determine the taxonomic status and calculate the relative abundance (Supplementary Figure 1). The results showed that the total sequences were affiliated with 36 known Vibrio species. V. fluvialis, V. gigantis, V. natriegens, V. aestuarianus, V. plantisponsor, V. proteolyticus, and V. parahaemolyticus were predominant in all seasonal samples. V. fluvialis was the most abundant species occupying a large proportion (>50%) of each sample. The variation in community composition was largely attributable to the presence of V. fluvialis that dominated the Vibrio community. Meanwhile, a considerable proportion (∼10%) of the sequence was assigned to an unclassified group, which might represent novel Vibrio species. To better understand the seasonal differences of the Vibrio species, a heat map was generated to reflect the relative abundance among the four seasonal groups (Figure 3). A total of 21 Vibrio species were detected in all four seasonal groups, whereas 15 Vibrio species were found to be seasonally dependent. For example, V. azureus, V. brasiliensis, V. cidicii, and V. porteresiae existed only in summer, while V. anguillarum, V. renipiscarius, and V. splendidus were only found in winter and Vibrio nereis and V. mytili were only found in spring. In addition, V. cyclitrophicus and V. diazotrophicus existed widely in all groups except the summer group. The cluster cladogram analysis in the heat map illustrated that the fall and winter samples grouped together, while groups of spring and summer were clustered separately. Moreover, the main abundant Vibrio species were significantly correlated with the seasonal changes via ANOVA (Figure 3). The above results suggested that the Vibrio community composition was significantly different seasonally and followed a seasonal distribution pattern in the Beibu Gulf.
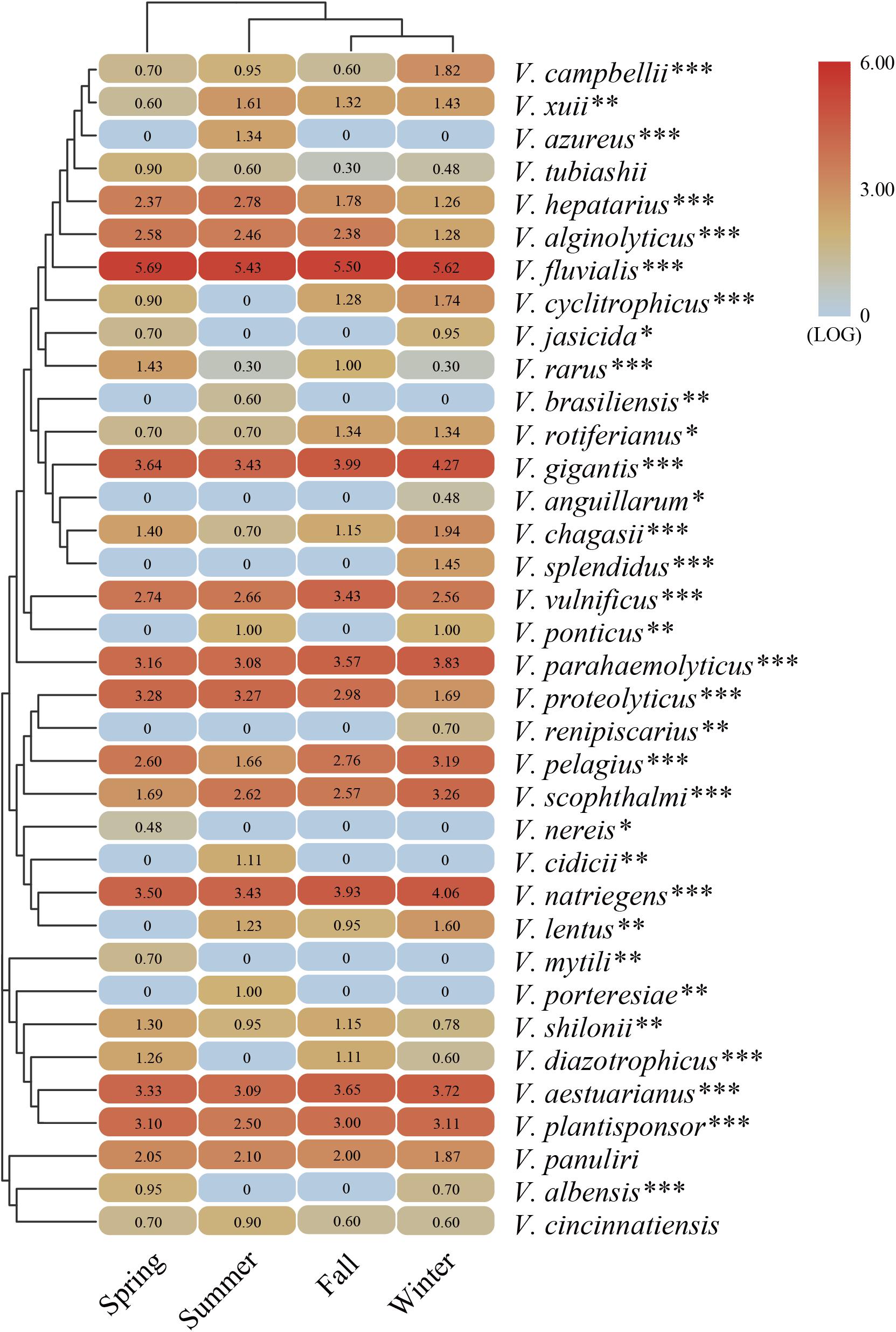
Figure 3. The heat map generated from the major abundant Vibrio species with representative sequences in four seasons. The color code represents the difference in relative abundance, ranging from blue to red. The top tree shows the cluster relationship of sampling groups. The significant differences between seasonal changes and distribution of Vibrio species are presented by one-way ANOVA test (*p < 0.05; **p < 0.01; ***p < 0.001).
Principal coordinate analysis (PCoA) based on the OTU level was performed to investigate the β-diversity of the Vibrio community in different seasonal groups (Figure 4A). The first two PCoA axes explained 22.76 and 11.61% of the total variation, respectively. The results demonstrated that summer samples were clearly separated from the others, indicating that the Vibrio community composition differed strongly between the summer and the other groups, whereas the Vibrio community of fall and winter groups was similar, which was consistent with the cluster cladogram (Figure 3). Both ANOSIM (r = 0.33, p = 0.001) and PERMANOVA (r2 = 0.29, p = 0.001) tests further confirmed that the differences in Vibrio community structures between the four seasons were significant. Furthermore, the Bray-Curtis dissimilarity showed that the dissimilarity of the Vibrio community structure among the different samples was highest in spring and lowest in fall (Supplementary Figure 2). The significant differences in the Bray-Curtis dissimilarity between seasons also further reflected the seasonal difference of the Vibrio community structure in the Maowei sea. To further determine the relationship between the Vibrio community dissimilarity and the eutrophic changes, linear regressions were performed based on Bray-Curtis dissimilarity and showed significantly increasing β-diversity correlated with the eutrophic variations in seasons (Figure 5).
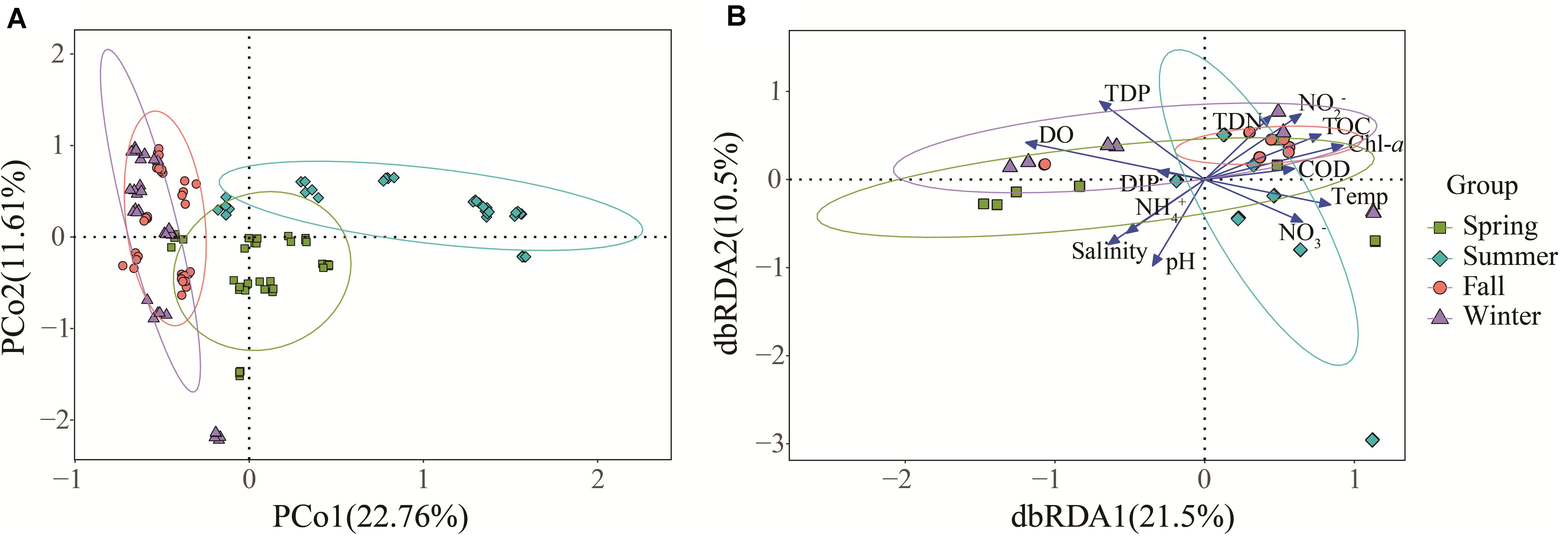
Figure 4. The β-diversity of the Vibrio community and its relationship with environmental factors in four seasons. (A) The PCoA of different samples based on Bray-Curtis dissimilarity of Vibrio communities. (B) The dbRDA plot shows the relationship between samples and environmental factors.
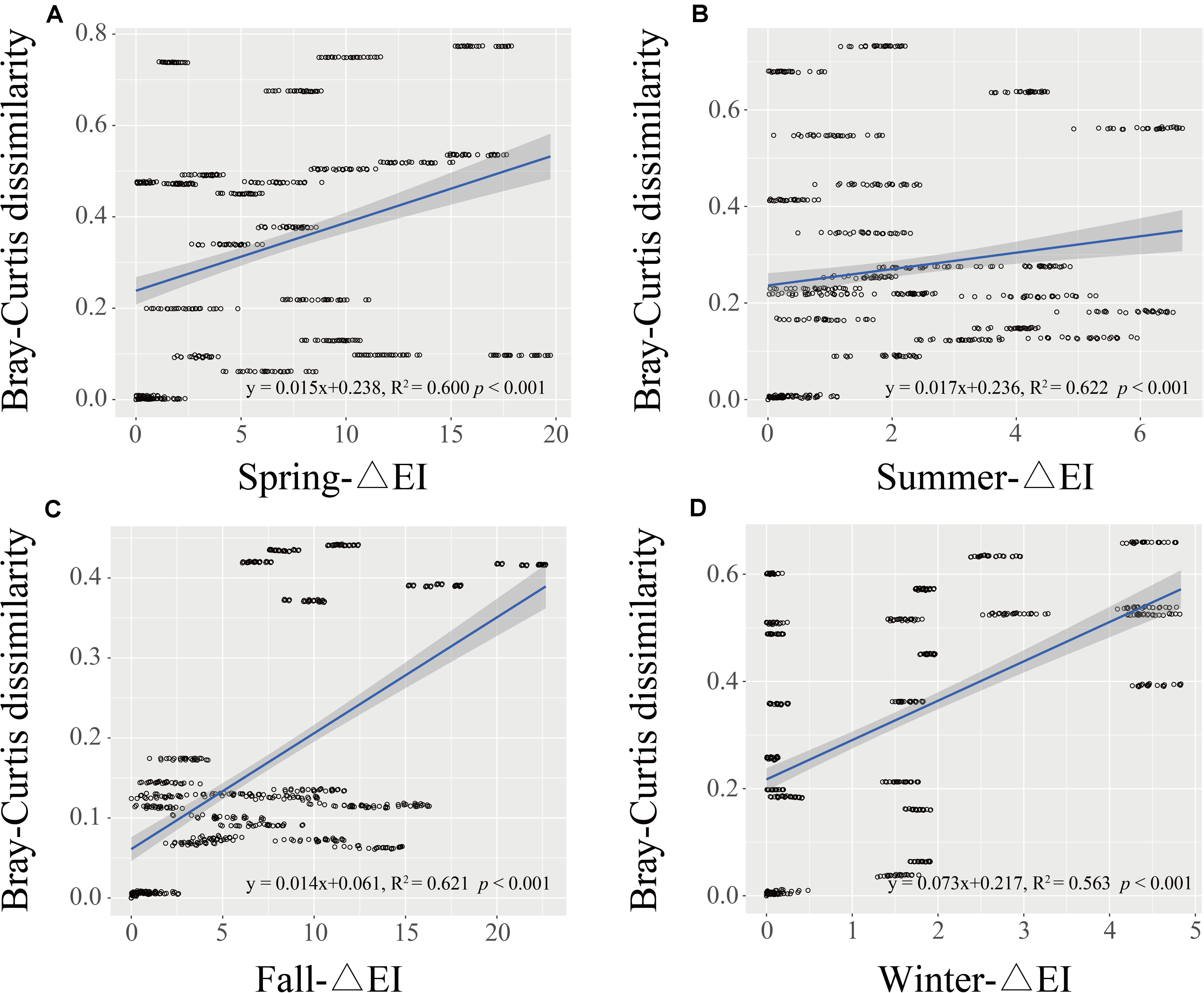
Figure 5. The regression between Bray-Curtis dissimilarity and variation of Delta EI in four seasons (A–D represents spring to winter). The black line represents the linear regression, and P-values were calculated to indicate significant differences.
Variation partitioning analysis (VPA) was used to analyze the effects of nutrient variables (NO2–, NO3–, NH4+, TDN, TDP, TOC, DIN, and DIP) and water properties (temperature, pH, salinity, DO, Chl a, and COD) on seasonal Vibrio community heterogeneity. The results showed that the pure effect of nutrient variables (20%) was higher than that of water properties (12%), and the combined effects explained 38% of the total community variation (Figure 6). In seasons, the pure effects of nutrient variables were almost all higher than those of water properties, except spring. For example, in winter samples, the pure effect of nutrient variables was 40%, and the combined effects explained of the total community variation was 91%. The dbRDA was performed to determine the specific environmental factors that governed the Vibrio community structure in different seasons. The dbRDA suggested that almost all environmental factors were strongly correlated with Vibrio community structures (Figure 4B), and shifts in the Vibrio community structure may be due to main environmental factors, such as TDN, TDP, DO, temperature, and salinity (Supplementary Table 4). Furthermore, the Mantel test calculated the correlation between environmental factors and the β-diversity of the Vibrio community (Table 2). The partial Mantel results showed that the Vibrio community composition along environmental gradients was significantly correlated with TDN, TDP, temperature, and DO, with shifts in community composition being better correlated with changes in TDP than TDN.
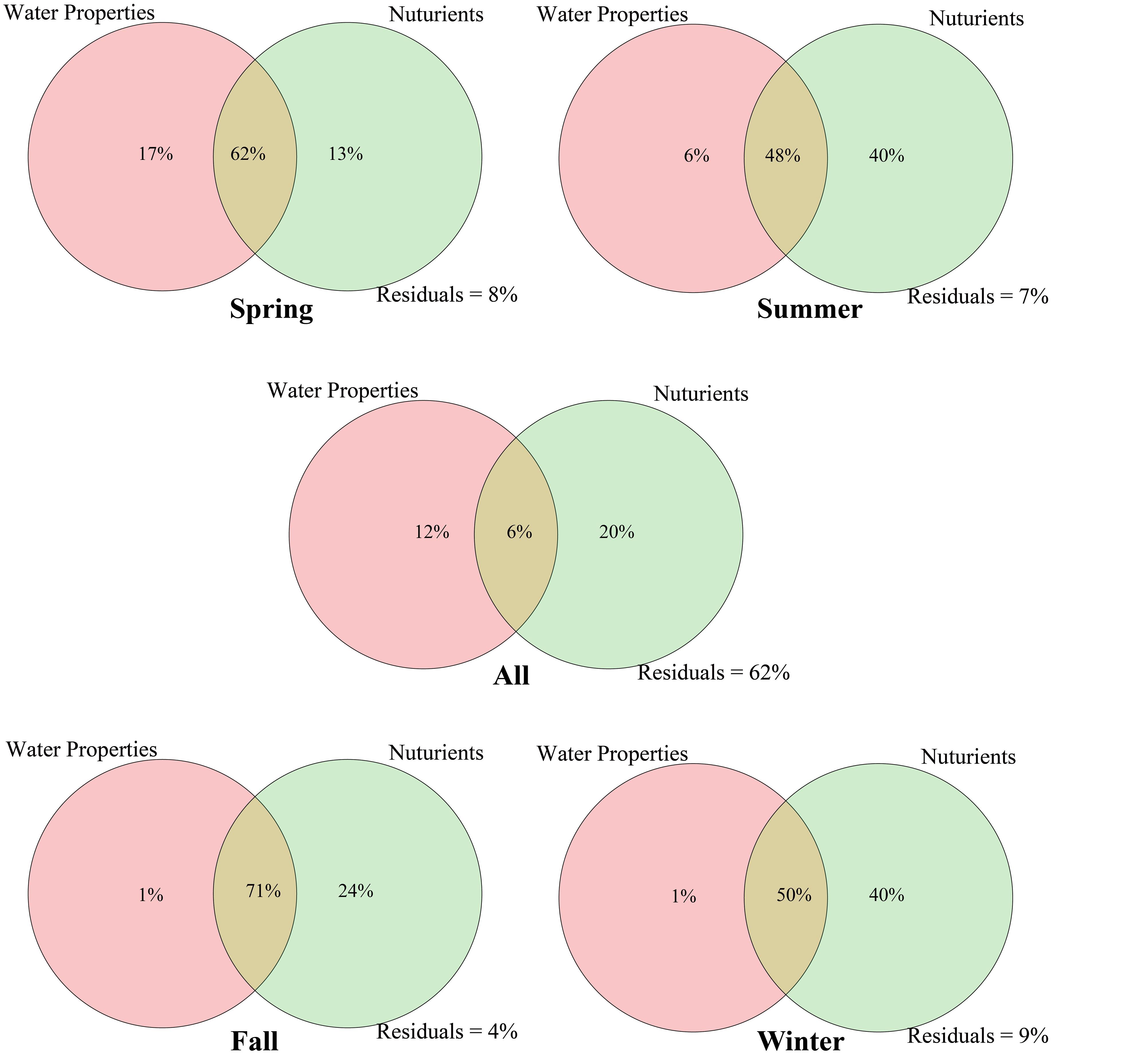
Figure 6. Variation partitioning analysis shows the effects of water properties (temperature, pH, salinity, DO, Chl a, and COD) and nutrients (NO2–, NO3–, NH4+, TDN, TDP, TOC, DIN, and DIP) on the Vibrio community.
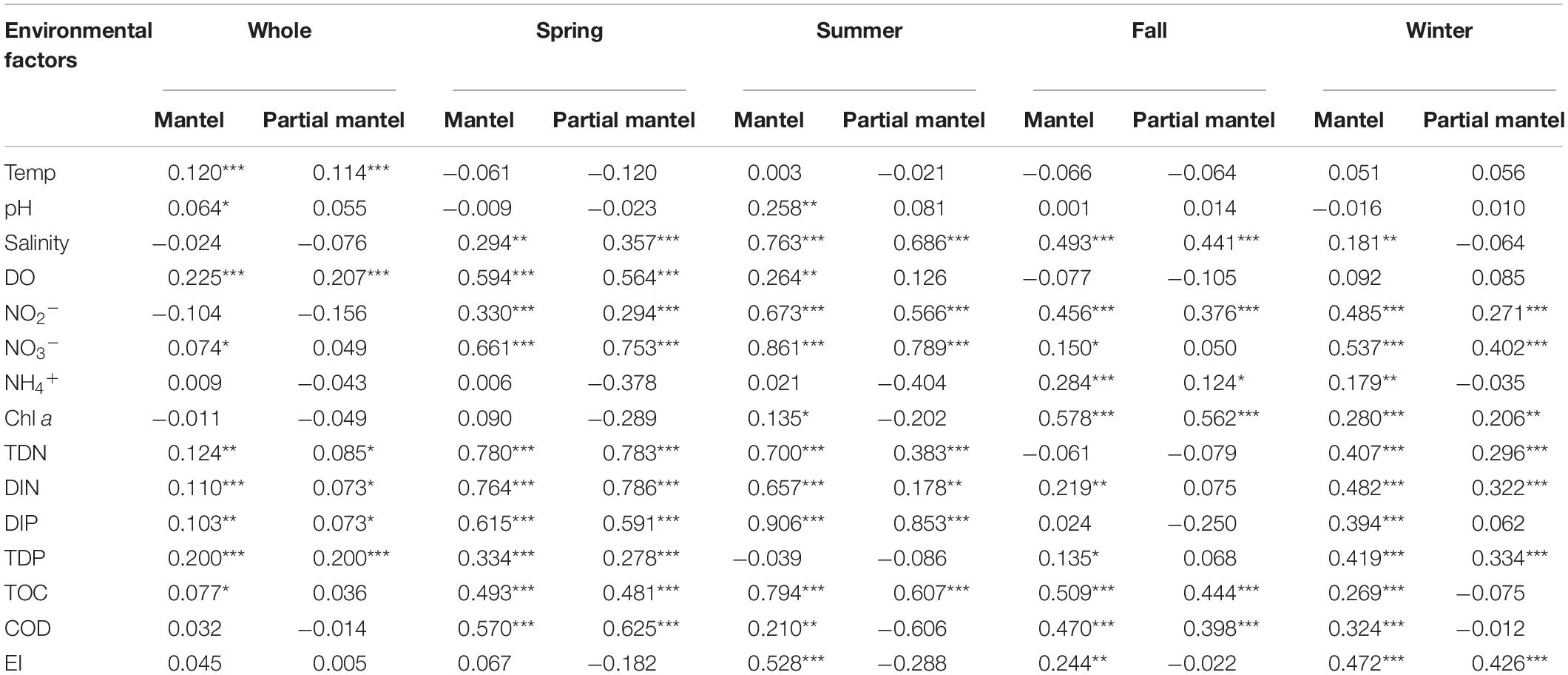
Table 2. The mantel and partial mantel tests (Pearson) show the correlations between Bray-Curtis dissimilarity of the Vibrio community and the environmental factors (*p < 0.05; **p < 0.01; ***p < 0.001).
The random forest method was utilized to analyze the most important Vibrio species for classifying the seasonal samples, indicating that V. scophthalmi, V. natriegens, V. proteolyticus, V. hepatarius, and V. vulnificus were the most important species with relatively high abundances (Figure 7). Furthermore, linear regression analysis was used to explore the trend of sensitive Vibrio species with EI (Supplementary Figure 3). The results revealed that 12 of the top 20 important Vibrio species were significantly correlated with the EI values. For example, V. splendidus was negatively correlated with EI changes (R2 = 0.535, p < 0.001), while V. fluvialis (R2 = 0.662, p < 0.001), V. alginolyticus (R2 = 0.576, p < 0.001), and V. proteolyticus (R2 = 0.386, p < 0.001) were positively correlated with the degree of eutrophication. These Vibrio species from the region with trophic gradients may be more sensitive to the eutrophication, which can assess whether nutrient contents had posed an important selective constraint on the communities.
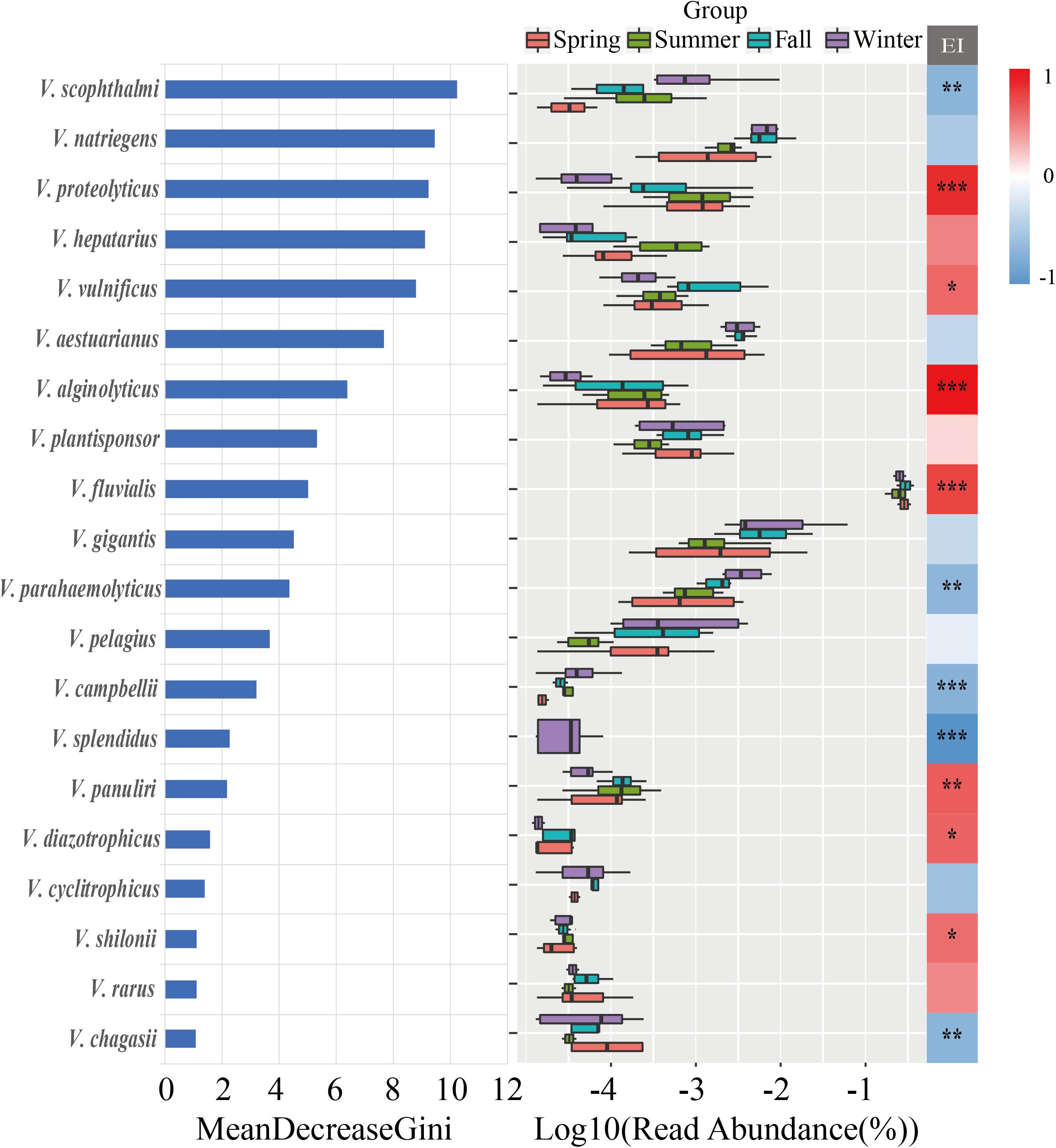
Figure 7. The random forest analysis of the top 20 important Vibrio species, ranked by Gini index. Middle: ranked by the richness of the top 20 Vibrio species with boxplot. Right: the correlations between the abundances of top 20 Vibrio species and EI variable by Spearman correlation analysis. *p < 0.05; **p < 0.01; ***p < 0.001.
This study investigated the seasonal variation in Vibrio diversity in a subtropical coastal sea system. Analysis of Vibrio-specific 16S rRNA sequences showed that V. fluvialis was present year-round and occupied the largest proportion of the Vibrio community, followed by V. gigantis, V. natriegens, and V. parahaemolyticus (Figure 3). V. fluvialis is known as an emerging foodborne pathogen, which was isolated from marine and estuarine environments and humans exhibiting severe diarrheal disease indicating its considerable adaptability (Ramamurthy et al., 2014). In Toulon harbor, France, detections of V. fluvialis have increased considerably (29.3%) in seawater during the process of filter feeding in marine mollusks (Martin and Bonnefont, 1990). Similarly, V. fluvialis was predominantly detected (>50%) in all samples, indicating the higher possibility and risk of causing gastroenteritis in humans year-round in the studied area. The second most abundant group was V. gigantis, which has been reported to originate from the hemolymph of diseased cultured oysters (Le Roux et al., 2005). The Maowei sea is a natural ecosystem rich in wild oyster populations, providing an opportunity for the bloom of pathogenic Vibrio species such as V. fluvialis, V. gigantis, V. parahaemolyticus, and V. vulnificus, which infect humans through oyster consumption (Chen X. et al., 2018). These may also lead to frequent outbreak of diseases in aquatic organisms and thereby impair aquaculture economy. Moreover, the relative abundance of the different Vibrio species varied between the different seasons, and several Vibrio species detected in this study showed season-specific prevalence, including V. brasiliensis and V. splendidus, which were detected only in summer and winter, respectively (Figure 3). These results indicated that Vibrio communities from the four seasons exhibited contrasting community compositions and were sensitive to seasonal environmental changes.
Regarding α-diversity, we elucidated the temporal changes in Vibrio diversity in the Beibu Gulf. The α-diversity of Vibrio was highest in summer, which is consistent with previous research in which the diversity of Vibrio was found to be greater in summer than in winter (Siboni et al., 2016). Meanwhile, the results of β-diversity revealed distinct seasonal changes in the Vibrio community composition of the Maowei Sea and the summer group showing a significant difference from the others on the PCoA (Figure 4A). From spring to summer, Shannon and Simpson diversities increased significantly, and the Vibrio community structure in summer was significantly different from those in other seasons. This could be partly due to the arrival of the wet season and an increase in runoff. Conversely, it could be that summer is the fastest growing season for shellfish, increasing their filter-feeding rate, and a large number of metabolites were excreted as the water temperature rose (Zheng et al., 2012). Moreover, one possible explanation for the seasonal fluctuation pattern of Bray-Curtis dissimilarity (high in spring and winter and low in summer and autumn) is that the river inflows and seawater temperature increase in summer can stimulate the rapid growth of the motile, chemotactic species of Vibrio making them the dominant species (Figure 5). This may be the cause of the higher similarity of the Vibrio community in summer and fall. When exposed to cold seawater in winter, Vibrio enter into a viable but non-culturable state, leading to the persistence of various Vibrio species (Oliver, 2016). In addition, previous studies have suggested that marine bacterial diversity increased with the input of human-derived pollution and nutrients, such as N, P, and C, which could contribute to the creation of favorable conditions for the proliferation of different microorganisms (Nogales et al., 2011; Numberger et al., 2019). Therefore, our results were consistent with these studies, indicating that nutrient variations may lead to the increase in Vibrio diversity in the Beibu Gulf.
We observed that the changes in the Vibrio community composition and diversity in relation to seasons were more likely to be a result of a direct influence of temperature, salinity, TDN, DIN, and TDP. Our findings confirmed that salinity was the most important environmental determinant of α-diversity in the Vibrio community as described in previous studies (Siboni et al., 2016; Wang et al., 2019), and observed a similar positive correlation (Spearman, p < 0.001). The Bray-Curtis dissimilarity of the Vibrio community was also significantly correlated with temperature in the Maowei sea (Table 2 Mantel test, p < 0.001) providing further evidence that warmer seawater increased the dissimilarity of the Vibrio community (Liang et al., 2019). Except for the temperature and salinity, RDA and Mantel test analysis indicated that the roles of TDN and TDP were significantly positively correlated with the seasonal variation in the Vibrio community (Figure 4 and Table 2). Moreover, the regression analysis showed that Vibrio community dissimilarity was significantly increased with the variation in main environmental factors (TDN, TDP, salinity, and DO) (Supplementary Figure 4). The positive correlation between DO and Vibrio community dissimilarity demonstrated that the relative accumulation of dissolved oxygen in seawater enhanced the heterogeneity of the Vibrio community, which was consistent with previous studies (Liang et al., 2019; Wang et al., 2019). With these environmental factors becoming the main factors constraining the community composition, the pool of Vibrio species that would have been capable of surviving these high trophic conditions and able to outcompete and replace less adapted ones may have decreased. In this study, the VPA result also revealed that the effects of nutrients were stronger than those of water properties, indicating that the variations of nitrogen and phosphorus availably shaped and filtered the observed variations in the Vibrio community.
High concentrations of TDN, TDP, and DIN were closely related to the occurrence of eutrophication (Ko et al., 2019). Recently, the eutrophication in the Maowei sea could be attributed to land-based runoff and pollution produced by aquaculture in the area due to a lack of water exchange and large quantities of oyster excreta (Yang et al., 2019). Particularly in summer, the discharge from the Qinjiang River and Maoling River into the Maowei sea led to a high concentration of nutrients (Lai et al., 2014). Consequently, the input of the nutrients led to the Vibrio community showing the highest diversity in summer and significant seasonal variation. Besides, previous studies found that the Vibrio diversity was increased with nutritional levels, suggesting that Vibrio species could be considered as indicators of trophic conditions (Gregoracci et al., 2012; Mansergh and Zehr, 2014). In this study, we identified 12 Vibrio species such as V. fluvialis, V. proteolyticus, V. alginolyticus, and V. splendidus that had significant positive or negative relationships with the eutrophication (Figure 7 and Supplementary Figure 3). For example, V. fluvialis has the capacity to utilize a wide range of organic and inorganic nutrients containing C, N, and P (such as L-arginine, L-arabinose, D-glucose, chitin, malonate, phosphate, and citrate) (Brenner et al., 1983). This strong capacity for nutrient utilization may cause increased population levels in these species with increased pollution levels. By contrast, V. splendidus is a pathogen associated with oysters (Vezzulli et al., 2015). The degradation and destruction of the host due to overfishing and eutrophication in the nearshore region may cause the loss of associated microbiomes. In summary, discovery of these indicative species may facilitate the assessment of a trophic shift and its subsequent ecological effects.
This study demonstrated that highly diverse Vibrio communities were present in the Maowei Sea with great temporal resolution. Additionally, our study observed that the variation in Vibrio diversity and community composition was mainly affected by changes of salinity, temperature, nitrogen, and phosphorus (TDN, TDP, and DIN). Several important Vibrio species, such as V. splendidus, V. proteolyticus, V. fluvialis, and V. alginolyticus were significantly correlated with trophic changes, which may be more sensitive to eutrophic status in coastal ecosystems. This investigation enhanced our understanding of the Vibrio seasonal distribution in response to environmental factors under eutrophication pressure and their ecological effects and public health in a subtropical inland bay.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA607420.
NL and HZ conceived and designed the experiments. XC performed the experiments and analyzed the data. XC, GJ, JT, QX, LH, SC, SZ, and KD wrote and helped increase the quality of this manuscript. All authors contributed to the article and approved the submitted version.
This study was financially supported by the following funding sources: Guangxi Natural Science Foundation (Nos. 2018GXNSFDA281006 and 2017GXNSFAA198081), the National Natural Science Foundation of China (No. 41966005), the National Research Foundation of Korea (No. 2018R1C1B6007755100), and the “One Hundred Talents” Project of Guangxi (No. 6020303891251).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.610974/full#supplementary-material
Baddam, R., Sarker, N., Ahmed, D., Mazumder, R., Abdullah, A., Morshed, R., et al. (2020). Genome dynamics of Vibrio cholerae isolates linked to seasonal outbreaks of cholera in Dhaka, Bangladesh. mBio 11:e3339-19. doi: 10.1128/mBio.03339-19
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Boucher, Y., Orata, F. D., and Alam, M. (2015). The out-of-the-delta hypothesis: dense human populations in low-lying river deltas served as agents for the evolution of a deadly pathogen. Front. Microbiol. 6:1120. doi: 10.3389/fmicb.2015.01120
Brenner, D. J., Hickmanbrenner, F. W., Lee, J. V., Steigerwalt, A. G., Fanning, G. R., Hollis, D. G., et al. (1983). Vibrio furnissii (formerly aerogenic biogroup of Vibrio fluvialis), a new species isolated from human feces and the environment. J. Clin. Microbiol. 18, 816–824. doi: 10.1128/jcm.18.4.816-824.1983
Cai, Y., and Guo, L. (2009). Abundance and variation of colloidal organic phosphorus in riverine, estuarine, and coastal waters in the northern Gulf of Mexico. Limnol. Oceanogr. 54, 1393–1402. doi: 10.4319/lo.2009.54.4.1393
Chen, C., Chen, H., He, Y., and Xia, R. (2018). TBtools, a Toolkit for Biologists integrating various biological data handling tools with a user-friendly interface. bioRxiv [Preprint] doi: 10.1101/289660
Chen, X., Lao, Y., Wang, J., Du, J., Liang, M., and Yang, B. (2018). Submarine groundwater-borne nutrients in a tropical bay (Maowei Sea, China) and their impacts on the oyster aquaculture. Geochem. Geophys. Geosyst. 19, 932–951. doi: 10.1002/2017GC007330
Chen, C. W., Ju, Y.-R., Chen, C.-F., and Dong, C.-D. (2016). Evaluation of organic pollution and eutrophication status of Kaohsiung Harbor, Taiwan. Int. Biodeterior. Biodegradation 113, 318–324. doi: 10.1016/j.ibiod.2016.03.024
Cole, J. R., Wang, Q., Fish, J. A., Chai, B., McGarrell, D. M., Sun, Y., et al. (2014). Ribosomal database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42, D633–D642. doi: 10.1093/nar/gkt1244
Dixon, P. (2003). VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x
Froelich, B., Gonzalez, R., Blackwood, D., Lauer, K., and Noble, R. (2019). Decadal monitoring reveals an increase in Vibrio spp. concentrations in the Neuse River Estuary, North Carolina, USA. PLoS One 14:e0215254. doi: 10.1371/journal.pone.0215254
Greenfield, D., Gooch Moore, J., Stewart, J., Hilborn, E., George, B., Li, Q., et al. (2017). Temporal and environmental factors driving Vibrio vulnificus and V. parahaemolyticus populations and their associations with harmful algal blooms in South Carolina detention ponds and receiving tidal creeks. GeoHealth 1, 306–317. doi: 10.1002/2017GH000094
Gregoracci, G. B., Nascimento, J. R., Cabral, A. S., Paranhos, R., Valentin, J. L., Thompson, C. C., et al. (2012). Structuring of bacterioplankton diversity in a large tropical bay. PLoS One 7:e31408. doi: 10.1371/journal.pone.0031408
Guin, S., Saravanan, M., Chowdhury, G., Pazhani, G. P., Ramamurthy, T., and Das, S. C. (2019). Pathogenic Vibrio parahaemolyticus indiarrhoeal patients, fish and aquatic environments and their potential for inter-source transmission. Heliyon 5:e01743. doi: 10.1016/j.heliyon.2019.e01743
Han, A., Dai, M., Kao, S.-J., Gan, J., Li, Q., Wang, L., et al. (2012). Nutrient dynamics and biological consumption in a large continental shelf system under the influence of both a river plume and coastal upwelling. Limnol. Oceanogr. 57, 486–502. doi: 10.4319/lo.2012.57.2.0486
Hartwick, M. A., Urquhart, E. A., Whistler, C. A., Cooper, V. S., Naumova, E. N., and Jones, S. H. (2019). Forecasting seasonal Vibrio parahaemolyticus concentrations in New England shellfish. Int. J. Env. Res. Public Health 16:4341. doi: 10.3390/ijerph16224341
Holm-Hansen, O., and Riemann, B. (1978). Chlorophyll a determination: improvements in methodology. Oikos 30, 438–447. doi: 10.2307/3543338
King, M., Rose, L., Fraimow, H., Nagori, M., Danish, M., and Doktor, K. (2019). Vibrio vulnificus infections from a previously Nonendemic Area. Ann. Intern. Med. 171, 520–521. doi: 10.7326/L19-0133
Ko, S.-R., Srivastava, A., Lee, N., Jin, L., Oh, H.-M., and Ahn, C.-Y. (2019). Bioremediation of eutrophic water and control of cyanobacterial bloom by attached periphyton. Int. J. Environ. Sci. Technol. 16, 4173–4180. doi: 10.1007/s13762-019-02320-8
Kopprio, G. A., Streitenberger, M. E., Okuno, K., Baldini, M., Biancalana, F., Fricke, A., et al. (2017). Biogeochemical and hydrological drivers of the dynamics of Vibrio species in two Patagonian estuaries. Sci. Total Environ. 579, 646–656.
Lai, J., Jiang, F., Ke, K., Xu, M., Lei, F., and Chen, B. (2014). Nutrients distribution and trophic status assessment in the northern Beibu Gulf, China. Chin. J. Oceanol. Limnol. 32, 1128–1144. doi: 10.1007/s00343-015-4289-1
Lamon, S., Consolati, S. G., Fois, F., Cambula, M. G., Pes, M., Porcheddu, G., et al. (2019). Occurrence, seasonal distribution, and molecular characterization of Vibrio vulnificus, Vibrio cholerae, and Vibrio parahaemolyticus in Shellfish (Mytilus galloprovincialis and ruditapes decussatus) collected in Sardinia (Italy). J. Food Prot. 82, 1851–1856. doi: 10.4315/0362-028x.jfp-19-021
Le Roux, F., Goubet, A., Thompson, F., Faury, N., Gay, M., Swings, J., et al. (2005). Vibrio gigantis sp. nov., isolated from the haemolymph of cultured oysters (Crassostrea gigas). Int. J. Syst. Evol. Microbiol. 55, 2251–2255. doi: 10.1099/ijs.0.63666-0
Liang, J., Liu, J., Wang, X., Lin, H., Liu, J., Zhou, S., et al. (2019). Spatiotemporal dynamics of free-living and particle-associated Vibrio communities in the northern Chinese marginal seas. Appl. Environ. Microbiol. 85, e00217–e00219.
López-Hernández, K. M., Pardío-Sedas, V. T., Lizárraga-Partida, L., Williams, J. D. J., Martínez-Herrera, D., Flores-Primo, A., et al. (2015). Environmental parameters influence on the dynamics of total and pathogenic Vibrio parahaemolyticus densities in Crassostrea virginica harvested from Mexico’s Gulf coast. Mar. Pollut. Bull. 91, 317–329.
Mansergh, S., and Zehr, J. (2014). Vibrio diversity and dynamics in the monterey bay upwelling region. Front. Microbiol. 5:48. doi: 10.3389/fmicb.2014.00048
Martin, M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12. doi: 10.14806/ej.17.1.200
Martin, Y., and Bonnefont, J. (1990). Annual variations and identification of Vibrios growing at 37 degrees C in urban sewage, in mussels and in seawater at Toulon harbour (Mediterranean, France). Can. J. Microbiol. 36, 47–52. doi: 10.1139/m90-008
Nogales, B., Lanfranconi, M. P., Piña-Villalonga, J. M., and Bosch, R. (2011). Anthropogenic perturbations in marine microbial communities. FEMS Microbiol. Rev. 35, 275–298. doi: 10.1111/j.1574-6976.2010.00248.x
Numberger, D., Ganzert, L., Zoccarato, L., Mühldorfer, K., Sauer, S., Grossart, H.-P., et al. (2019). Characterization of bacterial communities in wastewater with enhanced taxonomic resolution by full-length 16S rRNA sequencing. Sci. Rep. 9:9673. doi: 10.1038/s41598-019-46015-z
Oliver, J. D. (2016). The viable but nonculturable state for bacteria: status update. Microbe 11, 159–164. doi: 10.1128/microbe.11.159.1
Ramamurthy, T., Chowdhury, G., Pazhani, G. P., and Shinoda, S. (2014). Vibrio fluvialis: an emerging human pathogen. Front. Microbiol. 5:91. doi: 10.3389/fmicb.2014.00091
Rognes, T., Flouri, T., Nichols, B., Quince, C., and Mahé, F. (2016). VSEARCH: a versatile open source tool for metagenomics. PeerJ 4;e2584. doi: 10.7717/peerj.2584
Sah, R. (1994). Nitrate-nitrogen determination—a critical review. Commun. Soil Sci. Plant Anal. 25, 2841–2869. doi: 10.1080/00103629409369230
Shelford, E. J., and Suttle, C. A. (2018). Virus-mediated transfer of nitrogen from heterotrophic bacteria to phytoplankton. Biogeosciences 15, 809–819. doi: 10.5194/bg-15-809-2018
Shore-Maggio, A., Aeby, G., and Callahan, S. (2018). Influence of salinity and sedimentation on Vibrio infection of the Hawaiian coral Montipora capitata. Dis. Aquat. Org. 128, 63–71. doi: 10.3354/dao03213
Siboni, N., Balaraju, V., Carney, R., Labbate, M., and Seymour, J. R. (2016). Spatiotemporal dynamics of Vibrio spp. within the Sydney Harbour estuary. Front. Microbiol. 7:460. doi: 10.3389/fmicb.2016.00460
Subtype, H.-G. H., and Sloan, M. (2018). Lesions were Morphologically Different or had Differing Gene Mutation Status were Considered Separate Histologies (7). Data were Compared using the Pearson chi-Squared test with SPSS Statistics v24. 0. Endicott, NY: IBM Corporation.
Takemura, A. F., Chien, D. M., and Polz, M. F. (2014). Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front. Microbiol. 5:38. doi: 10.3389/fmicb.2014.00038
Thompson, J. R., Randa, M. A., Marcelino, L. A., Tomita-Mitchell, A., Lim, E., and Polz, M. F. (2004). Diversity and dynamics of a North Atlantic coastal Vibrio community. Appl. Environ. Microbiol. 70, 4103–4110. doi: 10.1128/AEM.70.7.4103-4110.2004
Vezzulli, L., Grande, C., Reid, P. C., Hélaouët, P., Edwards, M., Höfle, M. G., et al. (2016). Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. U.S.A. 113, E5062–E5071. doi: 10.1073/pnas.1609157113
Urquhart, E. A., Jones, S. H., Jong, W. Y., Schuster, B. M., Marcinkiewicz, A. L., Whistler, C. A., et al. (2016). Environmental conditions associated with elevated Vibrio parahaemolyticus concentrations in Great Bay Estuary, New Hampshire. PloS one 11:e0155018. doi: 10.1371/journal.pone.0155018
Vezzulli, L., Pezzati, E., Stauder, M., Stagnaro, L., Venier, P., and Pruzzo, C. (2015). Aquatic ecology of the oyster pathogens Vibrio splendidus and Vibrio aestuarianus. Environ. Microbiol. 17, 1065–1080. doi: 10.1111/1462-2920.12484
Visco, G., Campanella, L., and Nobili, V. (2005). Organic carbons and TOC in waters: an overview of the international norm for its measurements. Microchem. J. 79, 185–191. doi: 10.1016/j.microc.2004.10.018
Wang, X., Liu, J., Li, B., Liang, J., Sun, H., Zhou, S., et al. (2019). Spatial heterogeneity of Vibrio spp. in sediments of Chinese marginal seas. Appl. Environ. Microbiol. 85:e3064-18. doi: 10.1128/aem.03064-18
Yang, B., Zhou, J.-B., Lu, D.-L., Dan, S. F., Zhang, D., Lan, W.-L., et al. (2019). Phosphorus chemical speciation and seasonal variations in surface sediments of the Maowei Sea, northern Beibu Gulf. Mar. Pollut. Bull. 141, 61–69. doi: 10.1016/j.marpolbul.2019.02.023
Yao, P., Zhao, B., Bianchi, T. S., Guo, Z., Zhao, M., Li, D., et al. (2014). Remineralization of sedimentary organic carbon in mud deposits of the Changjiang Estuary and adjacent shelf: implications for carbon preservation and authigenic mineral formation. Cont. Shelf Res. 91, 1–11. doi: 10.1016/j.csr.2014.08.010
Zhang, R., Kelly, R. L., Kauffman, K. M., Reid, A. K., Lauderdale, J. M., Follows, M. J., et al. (2019). Growth of marine Vibrio in oligotrophic environments is not stimulated by the addition of inorganic iron. Earth Planet. Sci. Lett. 516, 148–155. doi: 10.1016/j.epsl.2019.04.002
Zhang, X., Lin, H., Wang, X., and Austin, B. (2018). Significance of Vibrio species in the marine organic carbon cycle—A review. Sci. China Earth Sci. 61, 1357–1368. doi: 10.1007/s11430-017-9229-x
Zheng, Q., Zhang, R., Wang, Y., Pan, X., Tang, J., and Zhang, G. (2012). Occurrence and distribution of antibiotics in the Beibu Gulf, China: impacts of river discharge and aquaculture activities. Mar. Environ. Res. 78, 26–33.
Keywords: 16S rRNA, Vibrio diversity, seasonal variation, eutrophication, Beibu Gulf
Citation: Chen X, Zhao H, Jiang G, Tang J, Xu Q, Huang L, Chen S, Zou S, Dong K and Li N (2020) Responses of Free-Living Vibrio Community to Seasonal Environmental Variation in a Subtropical Inland Bay. Front. Microbiol. 11:610974. doi: 10.3389/fmicb.2020.610974
Received: 28 September 2020; Accepted: 11 November 2020;
Published: 14 December 2020.
Edited by:
Sara Beier, Leibniz Institute for Baltic Sea Research (LG), GermanyReviewed by:
Peng Luo, South China Sea Institute of Oceanology (CAS), ChinaCopyright © 2020 Chen, Zhao, Jiang, Tang, Xu, Huang, Chen, Zou, Dong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Li, bmxpMDQxN0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.