
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 26 November 2020
Sec. Food Microbiology
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.599739
This article is part of the Research Topic Emerging Frontiers in the Formation of Viable but Non-Culturable Microorganisms and Biofilms During Food Processing View all 25 articles
 Yanmei Li1†
Yanmei Li1† Teng-Yi Huang2†
Teng-Yi Huang2† Yuzhu Mao3
Yuzhu Mao3 Yanni Chen3
Yanni Chen3 Fan Shi3
Fan Shi3 Ruixin Peng3
Ruixin Peng3 Jinxuan Chen3
Jinxuan Chen3 Lei Yuan4
Lei Yuan4 Caiying Bai5
Caiying Bai5 Ling Chen3,6
Ling Chen3,6 Kan Wang7*
Kan Wang7* Junyan Liu8*
Junyan Liu8*A Viable but non-culturable (VBNC) state is a bacterial survival strategy under reverse conditions. It poses a significant challenge for public health and food safety. In this study, the effect of external environmental conditions including acid, nutrition, and salt concentrations on the formation of S. aureus VBNC states at low temperatures were investigated. Different acidity and nutritional conditions were then applied to food products to control the VBNC state formation. Four different concentration levels of each factor (acid, nutrition, and salt) were selected in a total of 16 experimental groups. Nutrition showed the highest influence on the VBNC state formation S. aureus, followed by acid and salt. The addition of 1% acetic acid could directly kill S. aureus cells and inhibit the formation of the VBNC state with a nutrition concentration of 25, 50, and 100%. A propidium monoazide-polymerase chain reaction (PMA-PCR) assay was applied and considered as a rapid and sensitive method to detect S. aureus in VBNC state with the detection limit of 104 CFU/mL.
- The stress of nutrition, acid and salt could induce the VBNC state formation of S. aureus under low temperature.
- The effect of external environmental conditions on the state of VBNC formation of S. aureus was: nutrition > acid > salt concentrations.
- Addition of 1% acetic acid could directly kill the S. aureus and inhibit VBNC state formation with nutrition concentration of 25, 50, and 100%.
- The PMA-PCR assay is able to be applied on the detection of S. aureus VBNC cells with a detection limit at 104 CFU/mL.
Food-borne pathogens can cause diseases by contaminating food products and are the cause of serious concerns in public health and food safety. Staphylococcus aureus is widely distributed in the environment including air, water, and the surface of the skin, and has been found in raw meat, milk and dairy products, frozen products, and cooked foods (Xu et al., 2012a,b; Bao et al., 2017a,c). It is the source of major concern in the food industry due to its multi-drug resistance and virulence (Miao et al., 2017c; Jia et al., 2018). Foodborne outbreaks with vomiting cases caused by S. aureus have been frequently reported in recent years (Bennett et al., 2013). It can produce Staphylococcus enterotoxins (SEs) including SEA, SEB, SEC, SED, and SEE which can cause severe food poisoning incidents (Xu et al., 1982). Besides, Panton-Valentine leukocidin (PVL) can also cause food poisoning with a high mortality rate (Kraushaar and Fetsch, 2014; Liu et al., 2019).
The “Viable but non-culturable” (VBNC) state, first reported by Xu et al. in 1982, is considered to be a survival strategy of non-spore-forming bacteria in response to adverse conditions (Xu et al., 1982; Oliver, 2010; Liu et al., 2018, 2018a,b). Environmental stresses including low temperature, nutrient-limited conditions, high salt, low pH, and even UV-induced conditions have been reported to induce the formation of a VBNC state (Foster, 1999; Ramaiah et al., 2002; Xu et al., 2008a,b; Cunningham et al., 2009; Wang et al., 2011; Guo et al., 2019). At present, 85 species of bacteria have been confirmed as capable of entering into a VBNC state, including 18 non-pathogenic and 67 pathogenic species (Li et al., 2014; Bao et al., 2017b; Miao et al., 2017a,b , c). VBNC cells are alive with low metabolic activity, and capable of producing biological toxins. Shigella dysenteriae type1 retained Shiga toxin encoding gene (stx) and produced toxin in the VBNC state (Rahman et al., 1996; Lin et al., 2016). VBNC Escherichia coli O157 cells had a higher expression of rfbE and relatively lower expression of stx1 and stx2 genes compared to normal cells (Liu et al., 2017b; Xu X. et al., 2017; Xu J. et al., 2017; Zhao et al., 2018a,b). Furthermore, VBNC cells can resuscitate when in suitable conditions (Pinto et al., 2015; Xu et al., 2016a,b, c; Miao et al., 2018). Therefore, VBNC pathogens pose a serious threat to food safety and human health.
The traditional detection method for foodborne microbes is culturing-based. However, in the VBNC state, bacteria remain metabolic activity but below detection levels, indicating the ability to cause false negative detection by culturing-based method (Xu M. E. et al., 2011; Xu L. et al., 2011; Xu Z. et al., 2011; Pinto et al., 2015; Miao et al., 2016). Thus, food safety incidents may occur if contaminated by the foodborne pathogen in the VBNC state (Xu et al., 2012a,b; Liu et al., 2017b) and traditional culturing-based methods cannot be trusted to detect VBNC cells. Furthermore, this method cannot identify living and dead cells which is a major limitation in nucleic acid diagnosis (Xu et al., 2010; Zhong et al., 2016; Lin et al., 2017). However, some reagents including photoreactive DNA-binding dyes ethidium bromide monoazide (EMA) and propidium monoazide (PMA) can be used to amplify DNA in dead cells. Nucleic acid amplification methods have been combined with EMA/PMA and widely developed for the detection of pathogenic bacteria in a VBNC state, including PMA-PCR and PMA-LAMP (Li et al., 2017; Xie et al., 2017a,b; Liu et al., 2018; Zhong and Zhao, 2018).
This study aimed to investigate the effect of nutrition, acid, and salt concentrations on the viability and culturability of S. aureus at low temperature (4 and −20°C) to obtain a better understanding of the conditions of the formation of the VBNC state in food systems and to enable us to control it. A PMA-PCR assay was applied to detect the VBNC cells of S. aureus.
The stain used in this study was S. aureus ATCC25923, which was maintained as glycerol stock and stored at −80°C before use. The strain was streaked on tryptic soy agar (TSA) plate and incubated at 37°C for 24 h to recover. A single colony was then inoculated into 2 mL of TSB and incubated at 37°C with 150 rpm for 12 h prior to further experiments.
The bacterial culture was inoculated into TSB with 1:100 dilution and was incubated until it reached the exponential phase according to the growth curve (data not shown). The exponential phase culture was centrifuged at 5,000 × g for 10 min and the cells were washed with 1 × phosphate buffer solution (PBS). The washed culture was resuspended in induction groups (Table 1) to a final concentration of approximately 107 CFU/mL. To avoid the effects of continuous freeze-thawing, the induction system was separated into multiple 1.5 mL centrifuge tubes. Subsequently, the tubes were placed at 4 and −20°C, respectively, to induce the VBNC state.
To determine the culturability of S. aureus cells, the plate counting method was applied to identify the culturable cell number. The induction culture was serially diluted with 0.9% NaCl and inoculated on TSA followed by incubation at 37°C for 24 h. When culturable, the cell number was < 1 CFU/mL for 3 days, and the cells were considered to be non-culturable (Deng et al., 2015). In addition, the LIVE/DEAD® BacLightTM bacterial viability kit (Thermo Fisher Scientific, China) combined with fluorescence microscopy was used to determine whether the non-culturable cells were in the VBNC state following the manufacturer’s instructions.
According to VBNC state induction results, suitable concentrations of nutrition, salt, and acid were selected to inhibit the formation of the VBNC state. The bacterial culture was washed and resuspended at the concentration of 5 × 107 CFU/mL with a total volume of 30 mL and stored at 4 and −20°C, respectively. The culturable cell number was measured by plate counting after 3 days (Tables 2, 3).
Twenty-five grams of Cantonese rice cake (Guangzhou Restaurant, Guangzhou, China) was added to 225 mL of 0.9% NaCl and determined as a 100% food sample medium. Accordingly, 25 and 50% food sample medium were prepared with sterilization. 2 mL of S. aureus culture at exponential growth phase were centrifuged at 4°C and washed with 0.9% NaCl before resuspended with sterilized food sample medium to a final concentration of 5 × 107 CFU/mL as initial induction concentration. Simultaneously, the filtered acetic acid solution was added into the food induction group at a final concentration of 1% (v/v). Then, the final induction group was stored at 4°C for 3 days and the culturability and viability were identified by plate counting method and LIVE/DEAD® BacLightTM bacterial viability kit, respectively.
Twenty-five grams of Cantonese rice cake mixed with 225 mL saline was prepared as a diluting solution. The bacterial culture of S. aureus in the VBNC state was diluted to the final concentrations of 106, 105, 104, 103, 102, and 10 cells/mL using the diluting solution, respectively. The PMA reagent was used at the concentration of 5 μg/mL. Subsequently, the detection samples mixed with PMA were incubated in the dark at room temperature for 10 min before the tubes were placed horizontally on ice exposed to a halogen lamp (650 W) at a distance of 15 cm for 15 min to complete the combination of DNA and PMA (Chen et al., 2020). The mixed samples were centrifuged at 10,000 rpm for 5 min. DNA from the precipitated cells was isolated using a DNA extraction kit (Dongsheng Biotech, Guangzhou) following the manufacturer’s instruction.
The PCR assay was performed at a total volume of 25 μL. The reaction system consists of 12.5 μL 2 × Taq PCR MasterMix (Dongsheng Biotech, Guangzhou), 3 μM each of forward and reverse primers (femA-F: AGGTATAGACTTCGATG TTTCAAATCGCGGTCCAGTG; femA-R: TTGTAGCTTCAGATATGGAAACCAA TCATTAC CAGCA), 2 μL of DNA template and added up to 25 μL with nuclease-free water. A mixture with 2 μL of nuclease-free water was used (instead of DNA) as a negative control. The protocol of PCR assay was as followed: 5 min denaturation at 95°C, 32 cycles of amplification at 95°C for 30 s, 52°C 30 s, 72°C for 35 s and final inactivation at 72°C for 5 min. The PCR products were detected by electrophoresis on 1.5% agarose gels and observed under UV light.
The changes of culturable cell numbers during VBNC state induction are shown in Figure 1. In induction group 1, for which stored at −20°C, the culturable cell number decreased to 0 in 52 days, while at 4°C the culturable cell number dropped in the first 3 days and remained unchanged (Figure 1A). In induction group 2, the culturable number declined to 0 in 43 days (−20°C) and 46 days (4°C), respectively (Figure 1B), which was similar to the result of induction groups 3 and 4 but with the longer induction time (Figures 1C,D). As for induction group 7, the culturable cell number reduced to 0 after stored at 4 and −20°C for 43 days and 54 days, respectively (Figure 1F). And in induction groups 5, 9, 10, 13, and 14, the culturable cell numbers remain the same which indicated the cells were unable to enter into the VBNC state (Figures 1E,G–J). The cells in induction groups 6, 8, 11, 12, 15, and 16 were non-culturable in 3 days. The total times for S. aureus cells to become non-culturable are listed in Table 4.
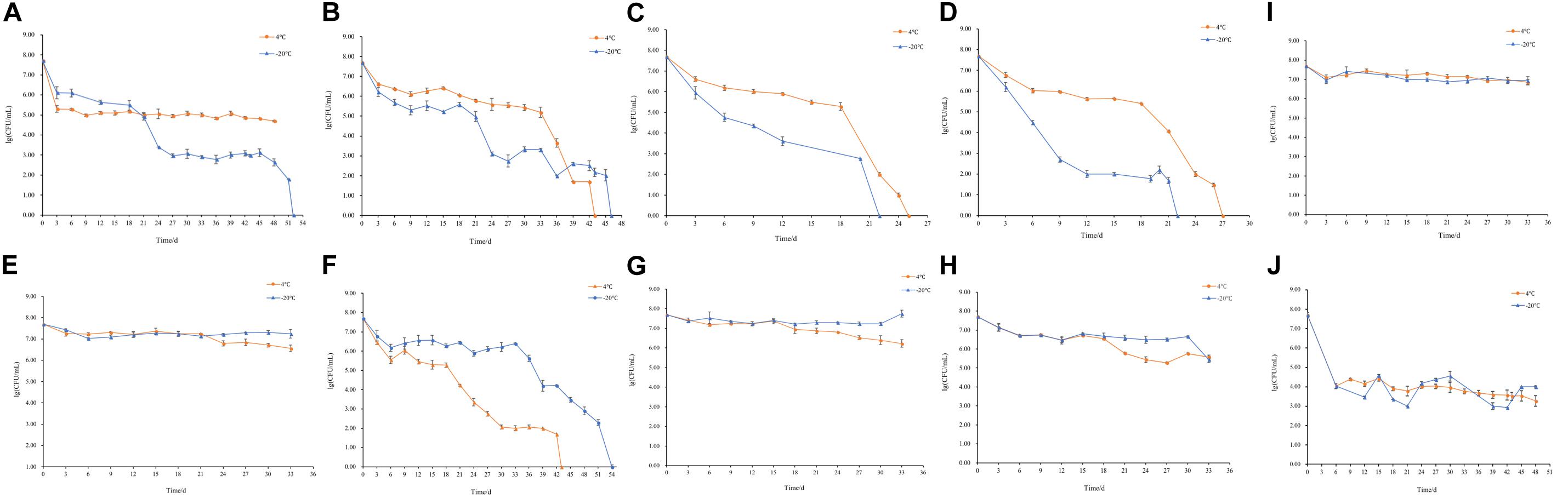
Figure 1. Culturable cell numbers of S. aureus under different conditions stored at 4 and –20°C (A–J, represent the cell culturability of S. aureus in induction groups 1, 2, 3, 4, 5, 7, 9, 10, 13, and 14, respectively).
The viability of S. aureus during the induction was observed by the fluorescence microscope after the treatment of the LIVE/DEAD® BacLightTM bacterial viability kit. In induction group 1 at −20°C, when the culturable cell number decreased to 0, viable cells still existed indicating that S. aureus can enter into VBNC state in saline at −20°C (Figure 2). The same results were obtained in induction group 2 at 4 and −20°C (Figure 2). As for induction groups 3, 4, and 7 at 4°C, a small percentage of cells entered into VBNC state (Figure 2). However, most cells were dead at −20°C (Figure 2).
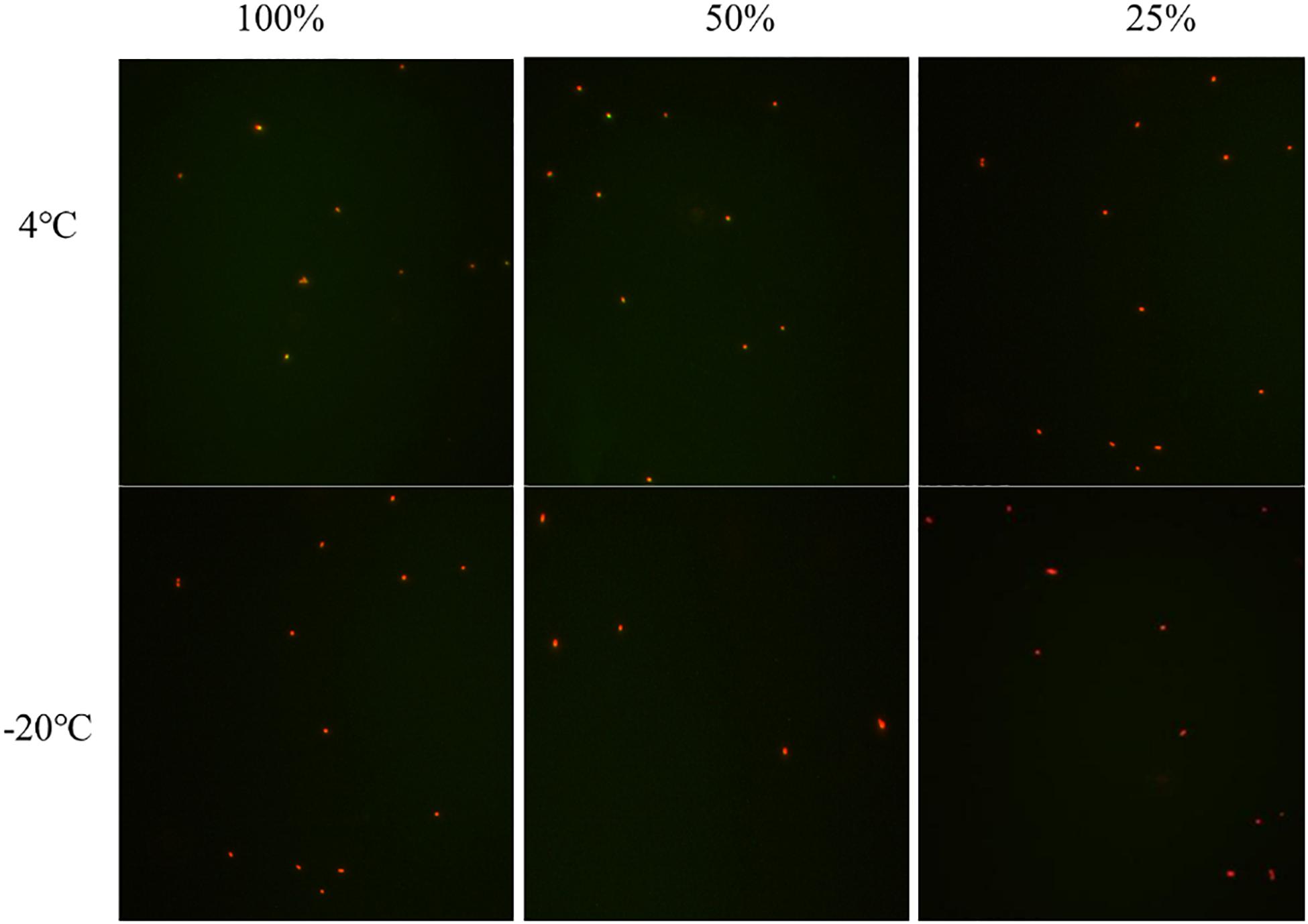
Figure 2. The viability of nonculturable S. aureus stored at different conditions with fluorescence microscope.
In summary, under low temperature (4°C) and strong acidity with sufficient nutrition (medium concentration ≥ 50%), S. aureus could enter into VBNC state within a short time. Similar results were obtained under low salt and weak acidic environment with insufficient nutrition but with longer induction time, as well as the in conditions that were oligotrophic and acid-free. Briefly, under reverse conditions, including insufficient nutrition with weak acid and sufficient nutrition with a strong acid, it was easier for S. aureus to enter into VBNC state at 4°C but more difficult to survive in freezing conditions (−20°C). These results showed that the key conditions for the VBNC state formation of S. aureus were adequate nutrition with strong acid at 4°C, insufficient nutrition with weak acid at 4°C, and oligotrophic system at −20°C.
S. aureus was able to enter into VBNC state under strong acid with adequate nutrition but not under strong acid that lacked nutrition, indicating with the treatment of strong acid, nutrition plays an important role during the formation of VBNC state. By comparing induction groups 1 and 2, under weak acid, it took cells in group 2 a shorter time to enter into VBNC state, demonstrating that weak acid may have an active contribution to the formation of VBNC state. Due to the salt-tolerant property of S. aureus, salt concentration had no significant effect. Under insufficient nutrition (medium concentration ≤ 50%) with strong acid [concentration of acetic acid (v/v) ≥ 0.7%] and high salt concentration (≥10%) without nutrition, the cells died within 3 days. Therefore, nutrition had the strongest effect on the formation of the VBNC state, followed by the concentration of acetic acid and salt.
S. aureus cells with higher ATP concentration would enter into the VBNC state instead of dying. Similar phenomenon has been found in the VBNC L. monocytogenes (Lindbäck et al., 2010; Bai et al., 2019). ATP synthase was also found upregulated in VBNC Vibrio parahaemolyticus cells (Lai et al., 2009). The upregulation of genes or proteins related to ATP accumulation offset ATP consumption in VBNC bacteria might be due to the survival mechanism of VBNC S. aureus under the reverse condition and need to be confirmed by further study on the expression of ATP-related genes or proteins in VBNC S. aureus cells (Bai et al., 2019). In the VBNC S. aureus cells, the mutational inactivation of catalase (KatA) or superoxide dismutase (SodA) encoded by katA and sodA gene was present. The changes on the expression of genes rendered cell hypersensitive to seawater with a high concentration of salt at 4°C (Masmoudi et al., 2010).
Under weak acid with insufficient nutrition (medium concentration ≤ 25%) conditions, S. aureus was capable of entering into VBNC state and acid concentration influenced the survival of S. aureus cells. Thus, a further experiment on the inhibitory effect of 0.3, 0.7, and 1.0% acetic acid on the control of VBNC state was performed.
In saline with 0.3, 0.7, and 1.0% acetic acid (induction groups 1, 2, and 3), S. aureus lost culturability and viability in 3 days, while low salt concentration with nutrition (medium concentration ≤ 25%) and 0.7%, 1.0% acetic acid (induction group 4 and 5), S. aureus remained culturability when stored at 4°C for 3 days. Among all, only the cells in induction group 5 stored at −20°C were non-culturable, indicating that eliminating VBNC state formation only by acetic acid treatment is not sufficient. S. aureus died at low nutrition, high salt, and strong acidity, indicating salt concentration can restrain the formation of the VBNC state However, given the low salt concentration in the food processing and storage of rice product, only acid treatment is less effective in eliminating S. aureus and its VBNC state (Table 5).
Since S. aureus could enter into VBNC state with strong acid treatment, the elimination of VBNC state by different nutrition conditions with strong acid were studied. Under low temperature (4 and −20°C), all cells were dead within 3 days in groups with no nutrition and strong acid (induction groups 1, 3, and 5). S. aureus may enter into VBNC under the treatment of some nutrition and low salt with strong acid. These results indicated that in low salt and strong acid environment, the VBNC state of S. aureus cannot be eliminated by only reducing nutrients. Thus, the control of VBNC state formation can be achieved by changing nutrition concentration in combination with other treatments. One way of eliminating the VBNC state of S. aureus, which could be applicable in food processing or used when cleaning equipment, would be to provide a condition of no nutrition with acid (Table 6).
Recently, several studies have reported on the formation of Staphylococcus biofilm on different surfaces during food processing, including polystyrene, polypropylene, stainless steel, and glass (Sattar et al., 2001; DeVita et al., 2007; Simon and Sanjeev, 2007). VBNC state induced in S. aureus biofilm under the antibiotic pressure has also been confirmed by RT-PCR (Pasquaroli et al., 2013, 2014). S. aureus, as well as its VBNC state formation, is emerging as a major concern of food product contamination and poses a threat to human health.
The culturable cell numbers under low temperature stress are shown in Figure 3. In food systems with 100, 50, and 25% nutrients and 1.0% acetic acid, the cells lost their culturability in 3 days. Observation under a fluorescence microscope (Figure 4) confirmed that all cells were dead, which is different from the results in the induction in TSB due to complex food matrix with unfavorable factors. Thus, 1% of acetic acid can be applied in the control of normal and VBNC state S. aureus cells in rice products.
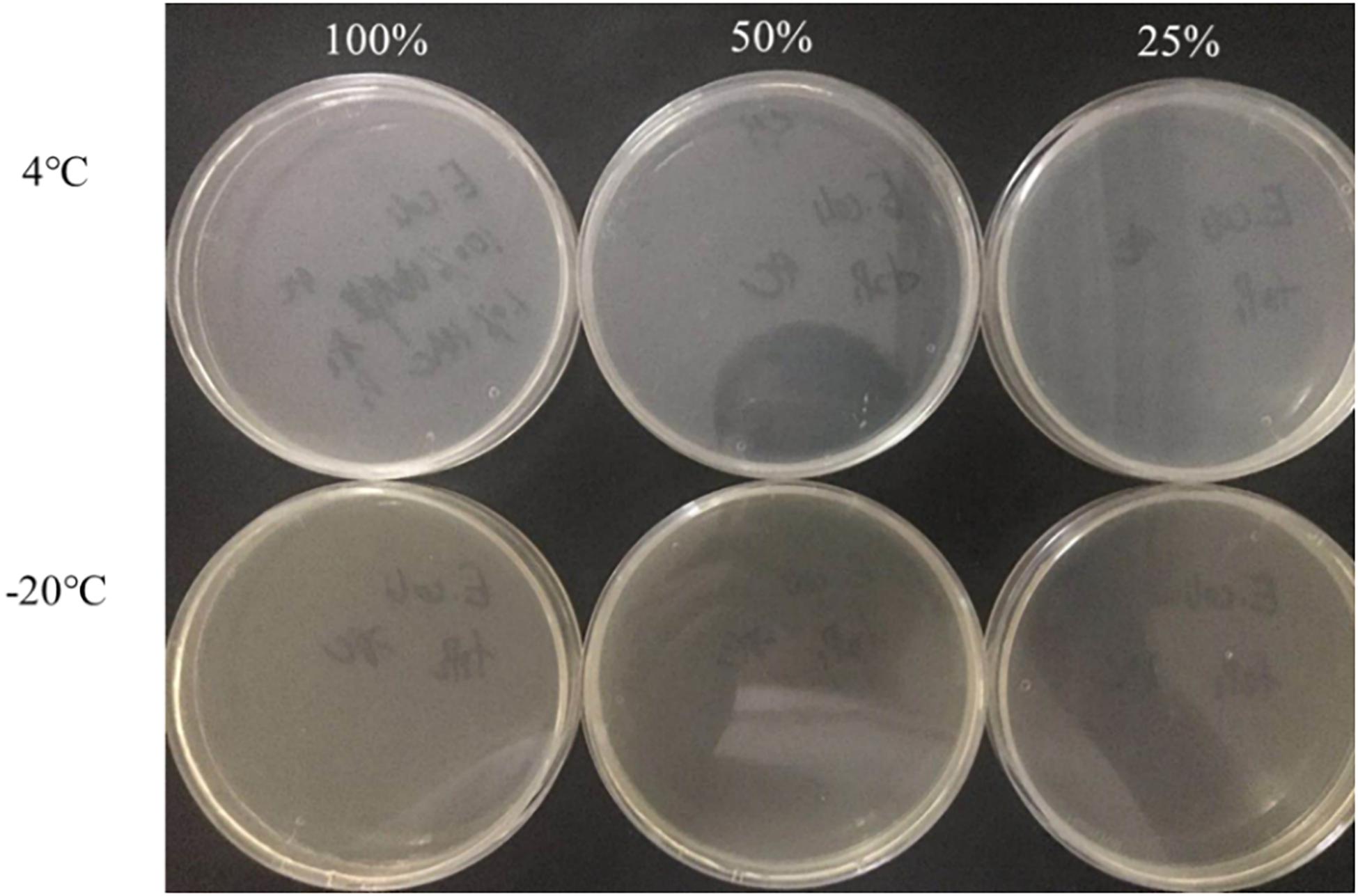
Figure 3. The culturable cell number of S. aureus inoculated in the 1.0% (v/v) acetic acid containing 100, 50, 25% nutrients at low temperature for 3 days.
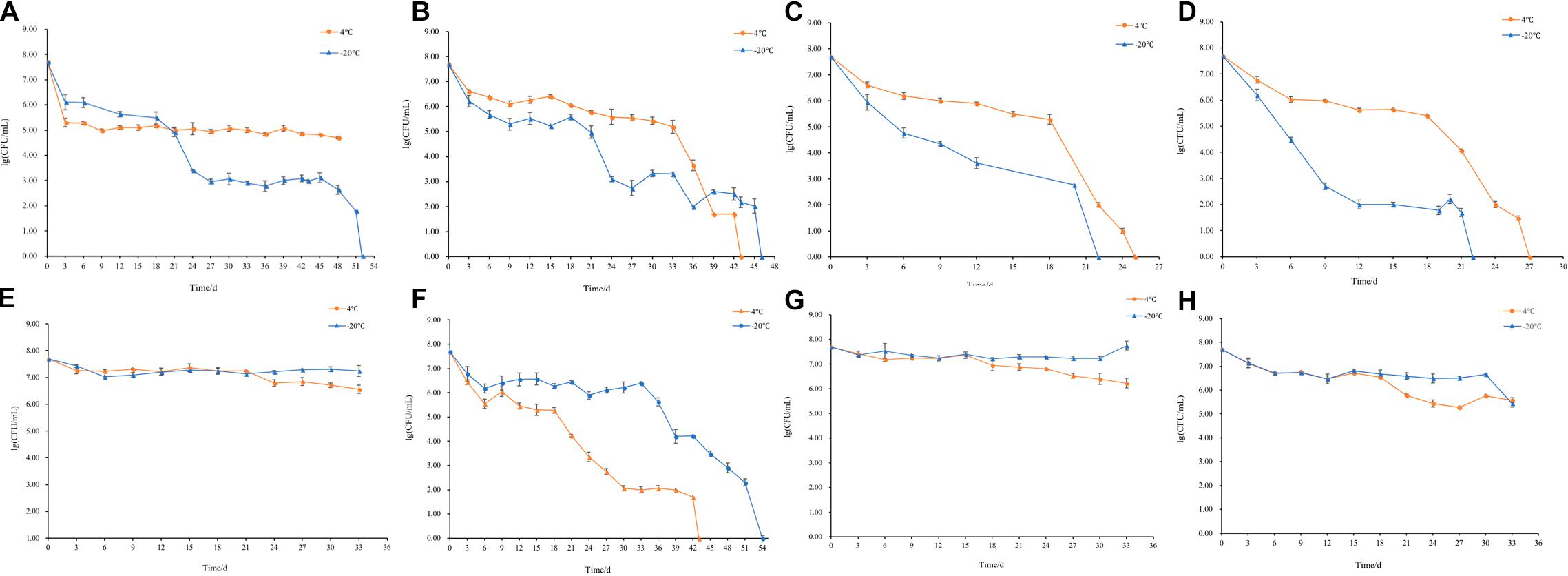
Figure 4. The viability of non-culturable S. aureus stored at different conditions (A) group 1 (–20°C); (B,C) group 2 (4°C, –20°C); (D,E) group 3 (4°C, –20°C); (F,G) group 4 (4°C, 20°C); (H) group 7 (4°C).
Foodborne pathogens and spoilage bacteria in the VBNC state can produce toxins and cause food spoilage. This is emerging as a leading concern for the food industry (Xu et al., 2009; Liu et al., 2017a). Over the past few decades, it has been confirmed that VBNC cells are capable of recovering, with restored metabolic activity. However, resuscitation conditions vary among species and strains. VBNC E. coli O157:H7 and Vibrio vulnificus cells can recover with the treatment of fresh TSB and a temperature upshift (Dinu and Bach, 2013; Zhao et al., 2013; Zhong et al., 2013; Rao et al., 2014; Xu et al., 2018). Moreover, the VBNC state pathogens recover or maintain virulence after resuscitation (Cappelier et al., 2007; You et al., 2012; Zeng et al., 2013). Several foodborne outbreaks were due to the resuscitation of VBNC cells (Xu et al., 2007; Zhao et al., 2017; Wen et al., 2020). Therefore, the control of the VBNC state formation in food products is of importance.
The detection limit of PMA-PCR for detection of the VBNC state S. aureus in rice products was 104 CFU/mL. Compared with the conventional culturing-based method, which uses the LIVE/DEAD® BacLightTM fluorescence staining method to detect VBNC cells, the PMA-PCR assay can detect specific concentrations of VBNC cells with high rapidity and sensitivity (Yoon et al., 2019).
This study investigated the impact of three elements including nutrition, acid and salt concentrations in food systems on the VBNC state formation of S. aureus. Nutrition showed the highest influence on the VBNC state formation S. aureus, followed by acid and salt. The addition of 1% acetic acid could directly kill S. aureus cells and inhibit the formation of VBNC states with a nutrition concentration of 25, 50, and 100%. Propidium monoazide-polymerase chain reaction (PMA-PCR) assay was applied and considered to be a rapid and sensitive method for detecting S. aureus in the VBNC state, with the detection limit of 104 CFU/mL.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
JL and KW conceived of the study and participated in its design and coordination. T-YH, YM, YC, FS, RP, and JC performed the experimental work. CB, LY, and LC analyzed the data. JL prepared and revised this manuscript. All authors reviewed and approved the final manuscript.
This study was funded by the National Key Research and Development Program of China (2016YFD04012021), the Science and Technology Planning Project of Guangdong Province (2017A050501007), the Fundamental Research Funds for the Central Universities (2017ZD092), and the 111 Project (B17018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bai, H., Zhao, F., Li, M., Qin, L., Yu, H., Lu, L., et al. (2019). Citric acid can force Staphylococcus aureus into viable but nonculturable state and its characteristics. Int. J. Food Microbiol. 305:108254. doi: 10.1016/j.ijfoodmicro.2019.108254
Bao, X., Jia, X., Chen, L., Peters, B. M., Lin, C., Chen, D., et al. (2017a). Effect of polymyxin resistance (pmr) on biofilm formation of Cronobacter sakazakii. Microb. Pathog 106, 16–19. doi: 10.1016/j.micpath.2016.12.012
Bao, X., Yang, L., Chen, L., Li, B., Li, L., Li, Y., et al. (2017b). Analysis on pathogenic and virulent characteristics of the Cronobacter sakazakii strain BAA-894 by whole genome sequencing and its demonstration in basic biology science. Microb. Pathog. 109, 280–286. doi: 10.1016/j.micpath.2017.05.030
Bao, X., Yang, L., Chen, L., Li, B., Li, L., Li, Y., et al. (2017c). Virulent and pathogenic features on the Cronobacter sakazakii polymyxin resistant pmr mutant strain s-3. Microb. Pathog. 110, 359–364. doi: 10.1016/j.micpath.2017.07.022
Bennett, S. D., Walsh, K. A., and Gould, L. H. (2013). Foodborne disease outbreaks caused by bacillus cereus. clostridium perfringens, and staphylococcus aureus–united states, 1998-2008. Clin. Infect. Dis. 57, 425–433. doi: 10.1093/cid/cit244
Cappelier, J. M., Besnard, V., Roche, S. M., Velge, P., and Federighi, M. (2007). Avirulent viable but non culturable cells of listeria monocytogenes need the presence of an embryo to be recovered in egg yolk and regain virulence after recovery. Vet. Res. 38, 573–583. doi: 10.1051/vetres:2007017
Chen, H., Zhong, C., Zhang, T., Shu, M., Lin, L., Luo, Q., et al. (2020). Rapid and sensitive detection of viable but non-culturable Salmonella induced by low temperature from chicken using EMA-Rti-LAMP combined with BCAC. Food Anal. Methods 13, 313–324. doi: 10.1007/s12161-019-01655-9
Cunningham, E., O’Byrne, C., and Oliver, J. D. (2009). Effect of weak acids on listeria monocytogenes survival: evidence for a viable but nonculturable state in response to low pH. Food Control 20, 1141–1144. doi: 10.1016/j.foodcont.2009.03.005
Deng, Y., Liu, J., Li, L., Fang, H., Tu, J., Li, B., et al. (2015). Reduction and restoration of culturability of beer-stressed and low-temperature-stressed Lactobacillus acetotolerans strain 2011-8. Int. J. Food Microbiol. 206, 96–101. doi: 10.1016/j.ijfoodmicro.2015.04.046
DeVita, M. D., Wadhera, R. K., Theis, M. L., and Ingham, S. C. (2007). Assessing the potential of Streptococcus pyogenes and Staphylococcus aureus transfer to foods and customers via a survey of hands, hand-contact surfaces and food-contact surfaces at foodservice facilities. J. Foodservice 18, 76–79. doi: 10.1111/j.1745-4506.2007.00049.x
Dinu, L.-D., and Bach, S. (2013). Detection of viable but non-culturable Escherichia coli O157:H7 from vegetable samples using quantitative PCR with propidium monoazide and immunological assays. Food Control 31, 268–273. doi: 10.1016/j.foodcont.2012.10.020
Foster, J. W. (1999). When protons attack: microbial strategies of acid adaptation. Curr. Opin. Microbiol. 2, 170–174. doi: 10.1016/s1369-5274(99)80030-7
Guo, L., Ye, C., Cui, L., Wan, K., Chen, S., Zhang, S., et al. (2019). Population and single cell metabolic activity of UV-induced VBNC bacteria determined by CTC-FCM and D(2)O-labeled raman spectroscopy. Environ. Int. 130:104883. doi: 10.1016/j.envint.2019.05.077
Jia, X., Hua, J., Liu, L., Xu, Z., and Li, Y. (2018). Phenotypic characterization of pathogenic Cronobacter spp. strains. Microb. Pathog. 121, 232–237. doi: 10.1016/j.micpath.2018.05.033
Kraushaar, B., and Fetsch, A. (2014). First description of PVL-positive methicillin-resistant staphylococcus aureus (MRSA) in wild boar meat. Int. J. Food Microbiol. 186, 68–73. doi: 10.1016/j.ijfoodmicro.2014.06.018
Lai, C. J., Chen, S. Y., Lin, I. H., Chang, C. H., and Wong, H. C. (2009). Change of protein profiles in the induction of the viable but nonculturable state of vibrio parahaemolyticus. Int. J. Food Microbiol. 135, 118–124. doi: 10.1016/j.ijfoodmicro.2009.08.023
Li, L., Mendis, N., Trigui, H., Oliver, J. D., and Faucher, S. P. (2014). The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 5:258. doi: 10.3389/fmicb.2014.00258
Li, Y., Yang, L., Fu, J., Yan, M., Chen, D., and Zhang, L. (2017). The novel loop-mediated isothermal amplification based confirmation methodology on the bacteria in viable but non-culturable (VBNC) state. Microb. Pathog. 111, 280–284. doi: 10.1016/j.micpath.2017.09.007
Lin, S., Li, L., Li, B., Zhao, X., Lin, C., Deng, Y., et al. (2016). Development and evaluation of quantitative detection of N-epsilon-carboxymethyl-lysine in Staphylococcus aureus biofilm by LC-MS method. Basic Clin. Pharmacol. Toxicol. 118:33.
Lin, S., Yang, L., Chen, G., Li, B., Chen, D., Li, L., et al. (2017). Pathogenic features and characteristics of food borne pathogens biofilm: biomass, viability and matrix. Microb. Pathog. 111, 285–291. doi: 10.1016/j.micpath.2017.08.005
Lindbäck, T., Rottenberg, M. E., Roche, S. M., and Rørvik, L. M. (2010). The ability to enter into an avirulent viable but non-culturable (VBNC) form is widespread among listeria monocytogenes isolates from salmon, patients and environment. Vet. Res. 41:8. doi: 10.1051/vetres/2009056
Liu, J., Li, L., Li, B., Peters, B. M., Deng, Y., Xu, Z., et al. (2017a). First study on the formation and resuscitation of viable but nonculturable state and beer spoilage capability of Lactobacillus lindneri. Microb. Pathog. 107, 219–224. doi: 10.1016/j.micpath.2017.03.043
Liu, J., Zhou, R., Li, L., Peters, B. M., Li, B., Lin, C. W., et al. (2017b). Viable but non-culturable state and toxin gene expression of enterohemorrhagic Escherichia coli O157 under cryopreservation. Res. Microbiol. 168, 188–193. doi: 10.1016/j.resmic.2016.11.002
Liu, L., Lu, Z., Li, L., Li, B., Zhang, X., Zhang, X., et al. (2018a). Physical relation and mechanism of ultrasonic bactericidal activity on pathogenic E. coli with WPI. Microb. Pathog. 117, 73–79. doi: 10.1016/j.micpath.2018.02.007
Liu, L., Xu, R., Li, L., Li, B., Zhang, X., Zhang, X., et al. (2018b). Correlation and in vitro mechanism of bactericidal activity on E. Coli with whey protein isolate during ultrasonic treatment. Microb. Pathog. 115, 154–158. doi: 10.1016/j.micpath.2017.12.062
Liu, L., Ye, C., Soteyome, T., Zhao, X., Xia, J., Xu, W., et al. (2019). Inhibitory effects of two types of food additives on biofilm formation by foodborne pathogens. Microbiol. Open 8:e853. doi: 10.1002/mbo3.853
Liu, Y., Zhong, Q., Wang, J., and Lei, S. (2018). Enumeration of vibrio parahaemolyticus in VBNC state by PMA-combined real-time quantitative PCR coupled with confirmation of respiratory activity. Food Control 91, 85–91. doi: 10.1016/j.foodcont.2018.03.037
Masmoudi, S., Denis, M., and Maalej, S. (2010). Inactivation of the gene katA or sodA affects the transient entry into the viable but non-culturable response of Staphylococcus aureus in natural seawater at low temperature. Mar. Pollut. Bull. 60, 2209–2214. doi: 10.1016/j.marpolbul.2010.08.017
Miao, J., Chen, L., Wang, J., Wang, W., Chen, D., Li, L., et al. (2017a). Current methodologies on genotyping for nosocomial pathogen methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathog. 107, 17–28. doi: 10.1016/j.micpath.2017.03.010
Miao, J., Chen, L., Wang, J., Wang, W., Chen, D., Li, L., et al. (2017b). Evaluation and application of molecular genotyping on nosocomial pathogen-methicillin-resistant Staphylococcus aureus isolates in guangzhou representative of southern china. Microb. Pathog. 107, 397–403. doi: 10.1016/j.micpath.2017.04.016
Miao, J., Liang, Y., Chen, L., Wang, W., Wang, J., Li, B., et al. (2017c). Formation and development of staphylococcus biofilm: with focus on food safety. J. Food Safety 37:e12358. doi: 10.1111/jfs.12358
Miao, J., Peters, B. M., Li, L., Li, B., Zhao, X., Xu, Z., et al. (2016). Evaluation of ERIC-PCR for fingerprinting methicillin-resistant Staphylococcus aureus Strains. Basic Clin. Pharmacol. Toxicol. 118:33.
Miao, J., Wang, W., Xu, W., Su, J., Li, L., Li, B., et al. (2018). The fingerprint mapping and genotyping systems application on methicillin-resistant Staphylococcus aureus. Microb. Pathog. 125, 246–251. doi: 10.1016/j.micpath.2018.09.031
Oliver, J. D. (2010). Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 34, 415–425. doi: 10.1111/j.1574-6976.2009.00200.x
Pasquaroli, S., Citterio, B., Cesare, A. D., Amiri, M., Manti, A., Vuotto, C., et al. (2014). Role of daptomycin in the induction and persistence of the viable but non-culturable state of Staphylococcus aureus biofilms. Pathogens 3, 759–768. doi: 10.3390/pathogens3030759
Pasquaroli, S., Zandri, G., Vignaroli, C., Vuotto, C., Donelli, G., and Biavasco, F. (2013). Antibiotic pressure can induce the viable but non-culturable state in staphylococcus aureus growing in biofilms. J. Antimicrob. Chemother. 68, 1812–1817. doi: 10.1093/jac/dkt086
Pinto, D., Santos, M. A., and Chambel, L. (2015). Thirty years of viable but nonculturable state research: unsolved molecular mechanisms. Crit. Rev. Microbiol. 41, 61–76. doi: 10.3109/1040841x.2013.794127
Rahman, I., Shahamat, M., Chowdhury, M. A., and Colwell, R. R. (1996). Potential virulence of viable but nonculturable Shigella dysenteriae type 1. Appl. Environ. Microbiol. 62, 115–120.
Ramaiah, N., Ravel, J., Straube, W. L., Hill, R. T., and Colwell, R. R. (2002). Entry of vibrio harveyi and vibrio fischeri into the viable but nonculturable state. J. Appl. Microbiol. 93, 108–116. doi: 10.1046/j.1365-2672.2002.01666.x
Rao, N. V., Shashidhar, R., and Bandekar, J. R. (2014). Induction, resuscitation and quantitative real-time polymerase chain reaction analyses of viable but nonculturable vibrio vulnificus in artificial sea water. World J. Microbiol. Biotechnol. 30, 2205–2212. doi: 10.1007/s11274-014-1640-1
Sattar, S. A., Springthorpe, S., Mani, S., Gallant, M., Nair, R. C., Scott, E., et al. (2001). Transfer of bacteria from fabrics to hands and other fabrics: development and application of a quantitative method using Staphylococcus aureus as a model. J. Appl. Microbiol. 90, 962–970. doi: 10.1046/j.1365-2672.2001.01347.x
Simon, S. S., and Sanjeev, S. (2007). Prevalence of enterotoxigenic staphylococcus aureus in fishery products and fish processing factory workers. Food Control 18, 1565–1568. doi: 10.1016/j.foodcont.2006.12.007
Wang, L., Zhao, X., Chu, J., Li, Y., Li, Y., Li, C., et al. (2011). Application of an improved loop-mediated isothermal amplification detection of Vibrio parahaemolyticus from various seafood samples. Afr. J. Microbiol. Res. 5, 5765–5771. doi: 10.5897/AJMR11.1237
Wen, S., Feng, D., Chen, D., Yang, L., and Xu, Z. (2020). Molecular epidemiology and evolution of Haemophilus influenzae. Infect. Genet. Evol. 80:104205. doi: 10.1016/j.meegid.2020.104205
Xie, J., Peters, B. M., Li, B., Li, L., Yu, G., Xu, Z., et al. (2017a). Clinical features and antimicrobial resistance profiles of important Enterobacteriaceae pathogens in guangzhou representative of southern china, 2001-2015. Microb. Pathog. 107, 206–211. doi: 10.1016/j.micpath.2017.03.038
Xie, J., Yang, L., Peters, B. M., Chen, L., Chen, D., Li, B., et al. (2017b). A 16-year retrospective surveillance report on the pathogenic features and antimicrobial susceptibility of Pseudomonas aeruginosa isolated from guangzhou representative of southern china. Microb. Pathog. 110, 37–41. doi: 10.1016/j.micpath.2017.06.018
Xu, H. S., Roberts, N., Singleton, F. L., Attwell, R. W., Grimes, D. J., and Colwell, R. R. (1982). Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8, 313–323. doi: 10.1007/bf02010671
Xu, Z., Hou, Y., Peters, B. M., Chen, D., Li, B., and Li, L. (2016a). Chromogenic media for MRSA diagnostics. Mol. Biol. Rep. 43, 1205–1212. doi: 10.1007/s11033-016-4062-3
Xu, Z., Hou, Y., Qin, D., Liu, X., Li, B., Li, L., et al. (2016b). Evaluation of current methodologies for rapid identification of methicillin-resistant staphylococcus aureus strains. Basic Clin. Pharmacol. Toxicol. 118:33.
Xu, Z., Liang, Y., Lin, S., Chen, D., Li, B., Li, L., et al. (2016c). Crystal violet and XTT assays on Staphylococcus aureus biofilm quantification. Curr. Microbiol. 73, 474–482. doi: 10.1007/s00284-016-1081-1
Xu, Z., Gui, Z., Li, B., Li, L., Su, J., Zhao, X., et al. (2012a). Expression and purification of gp41-gp36 Fusion protein and application in serological screening assay of HIV-1 and HIV-2. Afr. J. Microbiol. Res. 6, 6295–6299. doi: 10.5897/AJMR12.1075
Xu, Z., Li, L., Chu, J., Peters, B. M., Harris, M. L., Li, B., et al. (2012b). Development and application of loop-mediated isothermal amplification assays on rapid detection of various types of staphylococci strains. Food Res. Int. 47, 166–173.
Xu, Z., Li, L., Mark, E., Shirtliff, M. E., Peters, B. M., Peng, Y., et al. (2010). First report of class 2 integron in clinical Enterococcus faecalis and class 1 integron in Enterococcus faecium in south china. Diag. Microbiol. Infect. Dis. 68, 315–317. doi: 10.1016/j.diagmicrobio.2010.05.014
Xu, L., Li, Z., Shirliff, M. E., Peters, B. M., Li, B., Peng, Y., et al. (2011). Resistance class 1 integron in clinical methicillin-resistant Staphylococcus aureus strains in southern china, 2001-2006. Clin. Microbiol. Infect. 17, 714–718. doi: 10.1111/j.1469-0691.2010.03379.x
Xu, M. E., Li, L., Shi, L., and Shirliff, Z. (2011). Class 1 integron in staphylococci. Mol. Biol. Rep. 38, 5261–5279. doi: 10.1007/s11033-011-0676-7
Xu, Z., Li, L., Zhao, X., Chu, J., Li, B., Shi, L., et al. (2011). Development and application of a novel multiplex polymerase chain reaction (PCR) assay for rapid detection of various types of staphylococci strains. Afr. J. Microbiol. Res. 5, 1869–1873. doi: 10.1186/1471-2105-12-291
Xu, Z., Li, L., Shirtliff, M. E., Alam, M. J., Yamasaki, S., and Shi, L. (2009). Occurrence and characteristics of class 1 and 2 integrons in Pseudomonas aeruginosa isolates from patients in southern china. J. Clin. Microbiol. 47, 230–234. doi: 10.1128/JCM.02027-08
Xu, Z., Li, L., Li, L., Zhang, L., Yamasaki, S., and Shi, L. (2008a). First confirmation of integron-bearing methicillin-resistant Staphylococcus aureus. Curr. Microbiol 57, 264–268. doi: 10.1007/s00284-008-9187-8
Xu, Z., Shi, L., Alam, M. J., Li, L., and Yamasaki, S. (2008b). Integron-bearing methicillin-resistant coagulase-negative staphylococci in south china, 2001-2004. FEMS Microbiol. Lett. 278, 223–230. doi: 10.1111/j.1574-6968.2007.00994.x
Xu, Z., Shi, L., Zhang, C., Zhang, L., Li, X., Cao, Y., et al. (2007). Nosocomial infection caused by class 1 integron-carrying Staphylococcus aureus in a hospital in south china. Clin. Microbiol. Infect. 13, 980–984. doi: 10.1111/j.1469-0691.2007.01782.x
Xu, Z., Xie, J., Yang, L., Chen, D., Peters, B. M., and Shirtliff, M. E. (2018). Complete Sequence of pCY-CTX, a plasmid carrying a phage-like region and ISEcp1-Mediated Tn2 element from Enterobacter cloacae. Microb. Drug. Resist. 24, 307–313. doi: 10.1089/mdr.2017.0146
Xu, X., Xu, Z., Yang, L., Li, B., Li, L., Li, X., et al. (2017). Effect of aminoglycosides on the pathogenic characteristics of microbiology. Microb. Pathog. 113, 357–364. doi: 10.1016/j.micpath.2017.08.053
Xu, J., Xie, Z., Peters, B. M., Li, B., Li, L., Yu, G., et al. (2017). Longitudinal surveillance on antibiogram of important gram-positive pathogens in southern china, 2001 to 2015. Microb. Pathog 103, 80–86. doi: 10.1016/j.micpath.2016.11.013
Yoon, J. H., Moon, S. K., Choi, C., Ryu, B. Y., and Lee, S. Y. (2019). Detection of viable but nonculturable vibrio parahaemolyticus induced by prolonged cold-starvation using propidium monoazide real-time polymerase chain reaction. Lett. Appl. Microbiol. 68, 537–545. doi: 10.1111/lam.13157
You, R., Gui, Z., Xu, Z., Shirtliff, M. E., Yu, G., Zhao, X., et al. (2012). Methicillin-resistance Staphylococcus aureus detection by an improved rapid PCR assay. Afr. J. Microbiol. Res. 6, 7131–7133. doi: 10.5897/AJMR12.708
Zeng, B., Zhao, G., Cao, X., Yang, Z., Wang, C., and Hou, L. (2013). Formation and resuscitation of viable but nonculturable Salmonella typhi. Biomed. Res. Int. 2013:907170. doi: 10.1155/2013/907170
Zhao, F., Bi, X., Hao, Y., and Liao, X. (2013). Induction of viable but nonculturable Escherichia coli O157:H7 by high pressure CO2 and its characteristics. PLoS One 8:e62388. doi: 10.1371/journal.pone.0062388
Zhao, X., Li, M., and Xu, Z. (2018a). Detection of foodborne pathogens by surface enhanced raman spectroscopy. Front. Microbiol. 9:1236. doi: 10.3389/fmicb.2018.01236
Zhao, X., Yu, Z., and Xu, Z. (2018b). Study the features of 57 confirmed CRISPR loci in 38 strains of Staphylococcus aureus. Front. Microbiol. 9:1591. doi: 10.3389/fmicb.2018.01591
Zhao, X., Zhong, J., Wei, C., Lin, C. W., and Ding, T. (2017). Current perspectives on viable but non-culturable state in foodborne pathogens. Front. Microbiol. 8:580. doi: 10.3389/fmicb.2017.00580
Zhong, J., and Zhao, X. (2018). Detection of viable but non-culturable Escherichia coli O157:H7 by PCR in combination with propidium monoazide. 3 Biotech 8:28. doi: 10.1007/s13205-017-1052-7
Zhong, N., Gui, Z., Liu, X., Huang, J., Hu, K., Gao, X., et al. (2013). Solvent-free enzymatic synthesis of 1, 3-Diacylglycerols by direct esterification of glycerol with saturated fatty acids. Lipids Health Dis. 12, 2–7. doi: 10.1186/1476-511X-12-65
Keywords: Staphylococcus aureus, VBNC state, induction, control, formation
Citation: Li Y, Huang T-Y, Mao Y, Chen Y, Shi F, Peng R, Chen J, Yuan L, Bai C, Chen L, Wang K and Liu J (2020) Study on the Viable but Non-culturable (VBNC) State Formation of Staphylococcus aureus and Its Control in Food System. Front. Microbiol. 11:599739. doi: 10.3389/fmicb.2020.599739
Received: 28 August 2020; Accepted: 14 October 2020;
Published: 26 November 2020.
Edited by:
Xihong Zhao, Wuhan Institute of Technology, ChinaReviewed by:
Wei Wu, Qingdao Agricultural University, ChinaCopyright © 2020 Li, Huang, Mao, Chen, Shi, Peng, Chen, Yuan, Bai, Chen, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyan Liu, amxpdTgxQHV0aHNjLmVkdQ==; Kan Wang, MTIwMzg0NzQ2QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.