
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 16 September 2020
Sec. Virology
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.588427
This article is part of the Research Topic Next-Generation Sequencing and CRISPR-Cas Editing in Plant Virology View all 17 articles
The use of high-throughput sequencing (HTS) for virus diagnostics, as well as the importance of this technology as a valuable tool for discovery of novel viruses has been extensively investigated. In this review, we consider the application of HTS approaches to uncover novel plant viruses with a focus on the negative-sense, single-stranded RNA virosphere. Plant viruses with negative-sense and ambisense RNA (NSR) genomes belong to several taxonomic families, including Rhabdoviridae, Aspiviridae, Fimoviridae, Tospoviridae, and Phenuiviridae. They include both emergent pathogens that infect a wide range of plant species, and potential endophytes which appear not to induce any visible symptoms. As a consequence of biased sampling based on a narrow focus on crops with disease symptoms, the number of NSR plant viruses identified so far represents only a fraction of this type of viruses present in the virosphere. Detection and molecular characterization of NSR viruses has often been challenging, but the widespread implementation of HTS has facilitated not only the identification but also the characterization of the genomic sequences of at least 70 NSR plant viruses in the last 7 years. Moreover, continuing advances in HTS technologies and bioinformatic pipelines, concomitant with a significant cost reduction has led to its use as a routine method of choice, supporting the foundations of a diverse array of novel applications such as quarantine analysis of traded plant materials and genetic resources, virus detection in insect vectors, analysis of virus communities in individual plants, and assessment of virus evolution through ecogenomics, among others. The insights from these advancements are shedding new light on the extensive diversity of NSR plant viruses and their complex evolution, and provide an essential framework for improved taxonomic classification of plant NSR viruses as part of the realm Riboviria. Thus, HTS-based methods for virus discovery, our ‘new eyes,’ are unraveling in real time the richness and magnitude of the plant RNA virosphere.
Viruses are the most numerous biological entities on Earth, but the number of reported and formally described virus species, the known virosphere, is exiguous. This underestimated global virus landscape has led to a distorted view of virus diversity and function. Some aspects of virus biology including their small size, rapid rate of evolution, or lack of universally conserved viral genetic markers are likely the major cause for the lack of knowledge of most of the viruses present on our planet (Koonin et al., 2015; Zhang et al., 2019). Thus, as we explore the virosphere, it becomes evident that only a tiny proportion (about 1% according to some estimates) has been characterized, with a major bias against the identification of the most divergent genomes (Zhang et al., 2018).
High-throughput sequencing (HTS) technology, also referred to as next generation sequencing, has revolutionized the nucleic acid characterization process since it allows the parallel sequencing of millions of nucleotides in a short period of time at a very high redundancy (depth of sequencing) without any a priori knowledge. Thus, when combined with specific bioinformatics tools, HTS provides a powerful, efficient and economical alternative that has enabled not only the untargeted detection of both known and unknown viruses that inhabit a particular organism or environment but also a rapid characterization of their genomes (Massart et al., 2017; Villamor et al., 2019). Thus, the steady increase in the adoption of HTS in the past decade has accelerated virus discovery of both wild and cultivated plant species, which led to the advancement of our knowledge about the diversity of viruses in nature.
High-throughput sequencing is also widely used to advance the molecular characterization not only of those viruses which have a poorly characterized genome, but also of preserved historic virus isolates which had been subjected to studies of their biological properties some decades ago. For instance, sterile stunt disease of maize, characterized by severe stunting and top necrosis of susceptible maize genotypes, was first reported in 1977 in Australia, but the genome of its etiological agent, maize sterile stunt virus was only characterized by HTS and recently reported (Dietzgen and Higgins, 2019). Similarly, sowthistle yellow vein virus (SYVV) was recently rediscovered and its genome characterized after a hiatus of over 30 years following pioneering virus and vector biology research (Stenger et al., 2020).
In spite of the many advantages of HTS, there are some limitations and constraints that should be considered when using this platform for virus discovery. Given the high sensitivity of HTS, this technology may detect contaminant viral sequences or viruses that may not be actually replicating in the sampled plant tissue where they were found (Blawid et al., 2017). In this context it is worth emphasizing the importance of traditional wet bench virology experiments to complement viral HTS data where possible, such as virus isolation and transmission experiments, in situ virus particle detection by electron microscopy or immunoassays, to name a few.
Most of the plant viruses described so far, whose nucleotide sequences are available in public databases, were discovered in exemplars of cultivated plant species that showed conspicuous disease symptoms, limiting our view of viral diversity (Wren et al., 2006). However, the steady increase in the last few years of metagenomic studies to characterize the viromes of wild plant species, many of which did not show any visible symptoms, led to the identification of many new viruses. Moreover, several recent reports provide evidence of viruses which appear to be essentially cryptic, identified from cultivated plants (Bernardo et al., 2018; Schoelz and Stewart, 2018; Susi et al., 2019; Ma et al., 2020). Nevertheless, for most of the newly discovered viruses, subsequent studies to characterize their biological properties, such as symptoms in different hosts, and potential vectors, among others, in both cultivated and wild plant species are scarce, and likely will be neglected due to lack of economic significance or unavailability of preserved samples. The huge gap between virus discovery and biological characterization of new viruses, is due to the latter requiring time-consuming research efforts. Therefore, we are advancing into a scenario where the classification of most novel viruses will be based only on their genomic sequences which constitutes a paradigm shift in their taxonomical classification (Simmonds et al., 2017; Kuhn et al., 2019), thus representing a major challenge for virologists and the International Committee on Taxonomy of Viruses (ICTV).
Although many pipelines are available for plant virus discovery through HTS, all share a common backbone (Villamor et al., 2019). Various HTS technologies are available commercially, but Illumina short read shotgun sequencing platforms are the most popular choice and most widely used for HTS of viruses because of their high throughput, low error rate and high cost effectiveness among currently available HTS platforms (Villamor et al., 2019). Four main classes of nucleic acids have been targeted as templates for HTS, (i) total plant RNA extracts, usually with a ribosomal depletion step (ii) virion-associated nucleic acids (VANA) extracted from purified viral particles, (iii) double-stranded RNA (dsRNA), enriched through cellulose chromatography or monoclonal antibody pull-down, and (iv) small interfering RNAs (siRNAs) (Roossinck et al., 2015; Wu et al., 2015; Blouin et al., 2016). These different strategies are characterized by diverse caveats. For instance, VANA is inefficient in detecting viruses that lack virions, or siRNA-based protocols are not as reliable for complete genome assembly of novel viruses. Sequencing of siRNAs and total RNA are the two approaches most generically applicable to viruses with different genome types and replication strategies, and can be relatively easily integrated into workflows of diagnostic laboratories (Pecman et al., 2017).
Recent technological advances have led to the appreciation of exciting novel aspects of negative-sense and ambisense RNA (NSR) viruses, as recently reviewed by German et al. (2020). Plant NSR viruses are considered emerging viral pathogens (German et al., 2020). In light of the growing interest in these viruses, in this review we delve into the application of HTS approaches to uncover the abundance and diversity of the negative-sense, single-stranded RNA virosphere associated with plants.
Hundreds of plant viruses have been characterized by HTS in the last few years (Blawid et al., 2017; Villamor et al., 2019), and most of them have positive-sense RNA genomes. On the other hand, RNA viruses with negative-sense and ambisense genomes discovered through HTS represent only a small fraction of the total number of viruses discovered, thus representing a tiny fraction of the plant virosphere. For example, the characterization of the virome of different Solanum species resulted in the identification of viruses belonging to 20 different families, but only one was a NSR virus (Ma et al., 2020). In addition, the characterization of a tomato virome resulted in the identification of 22 viruses belonging to 12 genera, but only three genomes corresponded to NSR viruses (Xu et al., 2017). Moreover, the characterization of a papaya virome resulted in the identification of 52 viruses, but only one was a NSR virus (Alcalá-Briseño et al., 2020). It is worth mentioning that typically, virome studies based on HTS generate a significant amount of sequence contigs that lack detectable homology to both the sampled host and any microorganism. It has been suggested that a fraction of these sequences may correspond to viral ‘dark’ matter, which may imply that many deeply divergent viruses, or viruses lacking common ancestry or similarity with known virus families, remain to be discovered; this may not happen until better frameworks are implemented to identify viral sequences regardless of their sequence similarity to known viruses (Obbard et al., 2020). Current virome analyses usually rely on sequence similarity searches to identify virus-like sequences through inferred homology. This approach limits the identification of new viruses that can be discovered through traditional empirical search algorithms such as BLAST using identity thresholds of target sequences to genomes, genes, proteins or protein motifs of known viruses (Obbard et al., 2020). As a consequence, in contrast to the more straightforward hypothesis that plant NSR viruses are relatively rare, it is plausible that NSR virus sequences may have been overlooked due to extreme divergence from known viruses, thus providing an alternative reason why so few NSR viruses have been identified when the viromes of different plant hosts are characterized. Nevertheless, as novel bioinformatics tools are developed to increase the sensitivity of similarity search algorithms and more refined ab initio probabilistic methods are implemented, such as hidden Markov models that incorporate position-specific information into the alignment process of a group of highly divergent, evolutionarily related sequences and use these profiles to identify virus sequences (Skewes-Cox et al., 2014) or methods that rely on support vector machines (Liao and Noble, 2003), the identification of novel, more divergent viruses will likely become possible. This may result in an increased number of identified NSR viruses, which will allow us to deepen our understanding about the evolution and diversity of NSR viruses. This is clearly illustrated by the discovery of two NSR viruses associated with apple rubbery wood disease. The initial attempt to determine by NGS if any virus was associated with the disease was unsuccessful (Jakovljevic et al., 2016). However, the NGS data was later reanalyzed in depth using a bioinformatics approach focused on viral conserved protein motifs that resulted in the identification and genome assembly of two novel NSR viruses (Rott et al., 2018).
The use of HTS technologies has not only allowed the identification and characterization of novel NSR viruses in several plant hosts (Table 1), but has also enabled the completion of genome sequences of NSR viruses for which biological properties and only partial genome fragments were known (Table 2). The two nucleic acid classes mainly targeted when using HTS to sequence NSR viruses are total RNA and siRNAs (Tables 1, 2). When Pecman et al. (2017) compared these approaches they found that both can be used to detect and identify a wide array of known plant viruses in the tested samples including orthotospoviruses. However, on this occasion a putative novel cytorhabdovirus genome could only be assembled de novo from the sequencing data generated from total RNA and not from the small RNA dataset, due to the low number of short reads in the latter (Pecman et al., 2017), thus the choice of nucleic acid types used in HTS may have an effect on the range of viruses that can be identified. However, a few novel plant rhabdoviruses including cytorhabdoviruses have been identified using small RNA as sequencing template (Table 1).

Table 2. NSR viruses with partial genome sequence already know, whose genomes were characterized using HTS.
Most plant NSR viruses are transmitted by arthropods in a persistent-circulative and propagative manner, thus they are adapted to infect both arthropod and plant cells. In fact, it has been suggested that plant NSR viruses may have originated from arthropod viruses that evolved to also infect plants (Whitfield et al., 2018; Dolja et al., 2020). Therefore, another potential source to discover novel plant-associated NSR viruses is the characterization of arthropod vector viromes. For instance, Li et al. (2015) performed deep transcriptome sequencing of 70 arthropod species that resulted in the identification of three novel cytorhabdoviruses (Wuhan insect viruses 4–6) associated with the mealy plum aphid (Hyalopterus pruni). Wuhan insect viruses 4–6 may be plant viruses based on their clear phylogenetic relationship with plant rhabdoviruses in the genus Cytorhabdovirus and that their genomes encode a P3 protein that most resembles plant rhabdovirus cell-to-cell movement proteins.
Since most NSR viruses replicate in both the plant host and the arthropod vector, the true virus host origins cannot be unambiguously discerned from the HTS data. Some of the newly discovered NSR viruses are highly divergent which makes it even more difficult to unequivocally determine if they are plant or plant-associated viruses, and it will require a significant amount of research to confirm their status, which is rarely carried out. This is exemplified by the recently discovered coguviruses and rubodviruses, where the host assignment based on their phylogenetic relationships is only preliminary. Thus, further biological studies will be required to determine if they are plant, plant-associated, or insect viruses (Navarro et al., 2018b; Diaz-Lara et al., 2019; Chiapello et al., 2020).
Negative-sense and ambisense RNA viruses belong to the phylum Negarnaviricota, which includes members characterized by (i) negative-sense or ambisense single-stranded unsegmented or segmented genomes, (ii) the presence or absence of a lipid membrane enveloping the capsid, and (iii) a diverse host range (Diaz-Lara et al., 2019; Koonin et al., 2020; Kuhn et al., 2020). This phylum contains major groups of pathogenic viruses and our current knowledge of these viruses is strongly biased toward agents with special importance for human and animal health, such as influenza virus (Orthomyxoviridae), Zaire ebolavirus (Filoviridae) or Crimean-Congo hemorrhagic fever virus (Nairovirus). Most of the genera contain NSR viruses that infect vertebrates and only about 10% contain phytoviruses (Käfer et al., 2019) (Figure 1).
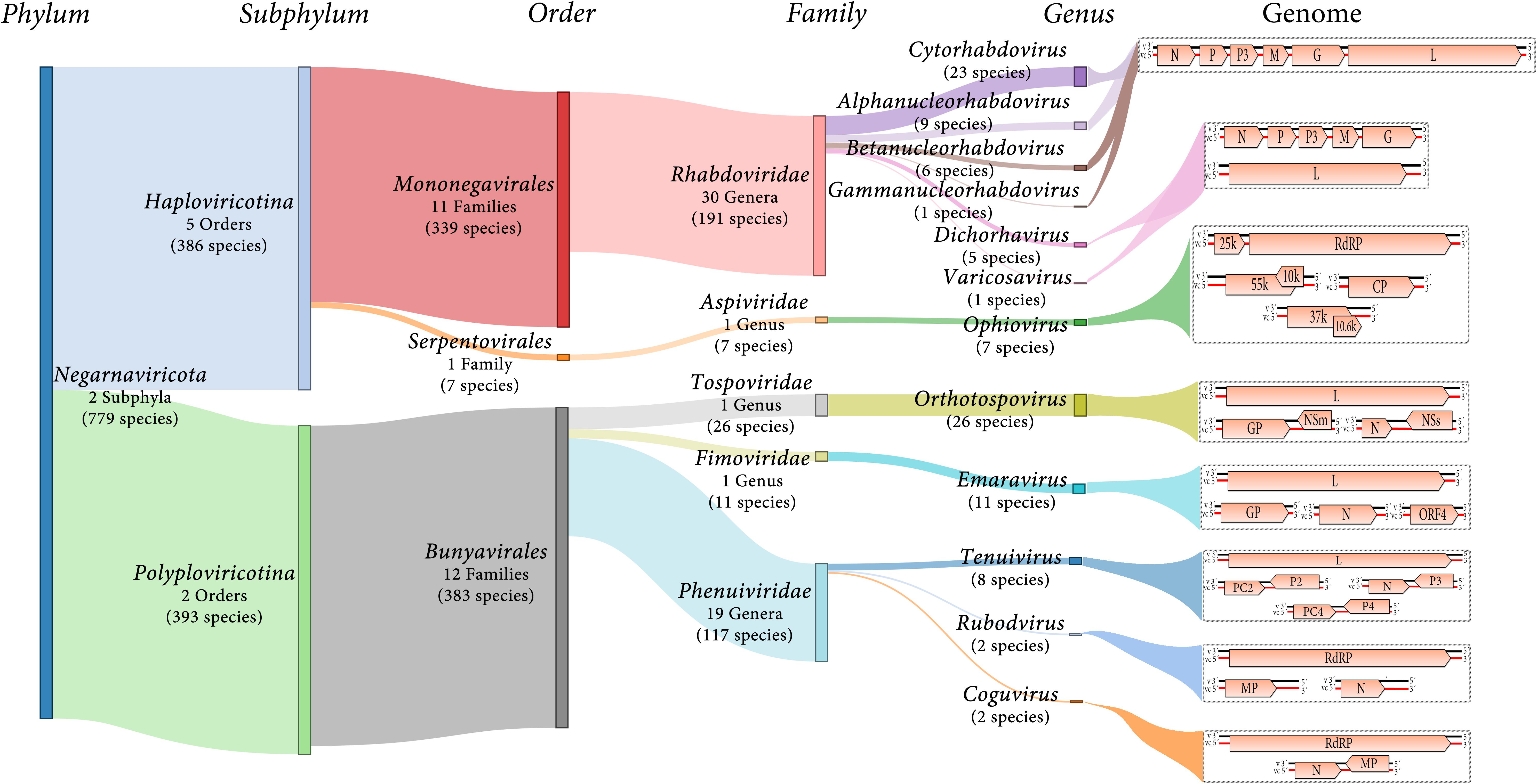
Figure 1. Diagram showing an overview of plant NSR virus taxonomy and schematic genome graphs depicting organization and gene products of representative members of each taxon. The predicted coding sequences are shown in orange arrowed boxes, virion-sense RNA (v) is depicted in black, while virion-complementary sense RNA (vc) is depicted in red. The number of taxonomic categories included in each taxon are indicated. Species number indicate 2019 ICTV-accepted virus species corresponding to each taxonomic rank.
Most of the NSR viruses are divided into two large lineages based on whether their RNA genomes are unsegmented or segmented (Koonin et al., 2020) (Figure 1). The unsegmented and some bi-segmented viruses with negative-sense RNA genomes, belong to the order Mononegavirales (Koonin et al., 2020; Kuhn et al., 2020). In contrast, most of the segmented viruses with both negative-sense and ambisense RNA genomes belong to the order Bunyavirales (Koonin et al., 2020; Kuhn et al., 2020) (Figure 1). Other orders such as Serpentovirales (which is the only one of these including NSR phytoviruses), Muvirales, Articulavirales, among others, have been created to accommodate diverse viruses which have been placed in the major phylogenetic gap between the two large groups of NSR viruses (Wolf et al., 2018).
Twelve genera (Alphanucleorhabdovirus, Betanucleor- habdovirus, Coguvirus, Cytorhabdovirus, Dichorhavirus, Emara- virus, Gammanucleorhabdovirus, Ophiovirus, Orthotospovirus, Rubodvirus, Tenuivirus, and Varicosavirus) belonging to five different families (Rhabdoviridae, Aspiviridae, Fimoviridae, Tospoviridae, and Phenuiviridae) include species of phytoviruses (Figure 1). Two of the genera (Coguvirus and Rubodvirus) were recently created to accommodate novel species of NSR viruses related to members of the Phenuiviridae family (Navarro et al., 2018b; Diaz-Lara et al., 2019). Furthermore, the ongoing discovery of many novel nucleorhabdoviruses and dichorhaviruses by HTS in the last few years resulted in a split of the genus Nucleorhabdovirus into three new genera (Freitas-Astúa et al., 2019). Therefore, as the pace of discovery of new NSR plant viruses using HTS is speedily increasing, the creation of new genera and families to accommodate some of these newly discovered viruses will be a common classification task in future years.
The family Rhabdoviridae currently comprises 30 genera for 191 species for viruses infectin vertebrates, invertebrates and plants. Six of these genera include 45 species of phytoviruses: Cytorhabdovirus, Alphanucleorhabdovirus, Betanu- cleorhabdovirus and Gammanucleorhabdovirus (unsegmented genomes), and Dichorhavirus and Varicosavirus (bi-segmented genomes) (Walker et al., 2018; Kuhn et al., 2020) (Figure 1). The virions of these phytorhabdoviruses have bacilliform or rod-shaped morphology, and those with unsegmented genomes are enveloped. Plant-infecting rhabdovirus genomes are 10–16 kb in size and are composed of 6 to 10 genes (Walker et al., 2018) (Figure 1).
The increased application of HTS has seen a significant rise in the number of novel plant rhabdoviruses (Table 1), as well as the completion of genomic sequences of those viruses with poorly characterized genomes (Table 2).
High-throughput sequencing was used successfully to complete the genome sequences of previously reported plant rhabdoviruses where only partial sequence fragments were available, such as Iranian citrus ringspot-associated virus (Sadeghi et al., 2016), ivy vein banding virus (Petrzik, 2012), and soybean blotchy mosaic virus (Lamprecht et al., 2010). Furthermore, HTS could be a key tool to characterize the genomes of some cyto- and nucleorhabdoviruses which have only been characterized biologically, such as broccoli necrotic yellows virus (Lin and Campbell, 1972) and festuca leaf streak virus (Lundsgaard and Albrechtsen, 1976).
Moreover, partial genome fragments of three putative cytorhabdoviruses and one unassigned rhabdovirus were reported, when the viromes of water lily, common bean, Lamprocephalus sp. and kalanchoe were analyzed by HTS (Kreuze, 2014; Verdin et al., 2017; Bernardo et al., 2018; Mwaipopo et al., 2018). However, the obtained sequences are not available in any public database, so it is not possible to know if these viruses are novel or may correspond to already known viruses. Thus, it would be useful to apply HTS to complete the genome characterization of these viruses to increase our understanding of plant rhabdovirus diversity. Furthermore, since a tentative nucleorhabdovirus associated with papaya was recently discovered in a papaya virome (Alcalá-Briseño et al., 2020), it would be useful to employ HTS to characterize the molecular properties of papaya apical necrosis virus, a putative nucleorhabdovirus associated with papaya, which was only characterized biologically almost four decades ago (Lastra and Quintero, 1981), to determine if both viruses are related.
The application of HTS has facilitated the discovery of at least 17 novel cytorhabdoviruses during the last 7 years (Table 1). Furthermore, the use of HTS has allowed characterization of the genomes of five cytorhabdoviruses, for which only a fragment or only the biological properties were known (Table 2). The template mostly used as a source for HTS of these viruses was total RNA, sequenced usually on Illumina platforms (Tables 1, 2).
The application of HTS has facilitated the discovery of ten novel nucleorhabdoviruses during the last 4 years, four alphanucleorhabdoviruses and six betanucleorhabdoviruses (Table 1). Furthermore, the use of HTS has allowed re-sequencing the genomes of two alphanucleorhabdoviruses which had previously been determined using Sanger dideoxy sequencing (maize mosaic virus and maize Iranian mosaic virus). HTS also allowed sequencing of the genome of a betanucleorhabdovirus for which only a genome fragment and the biological properties were known (datura yellow vein virus), and one other for which only the biological properties had been investigated 30 years earlier (sowthistle yellow vein virus) (Table 2). The template mostly used as a source for the HTS was total RNA, sequenced usually with Illumina instruments (Tables 1, 2).
The application of HTS has facilitated the discovery of three novel dichorhaviruses during the last 3 years (Table 1). Furthermore, the use of HTS has allowed characterization of the genome of a dichorhavirus for which only a fragment and the biological properties were known (coffee ringspot virus) (Table 2). The template used as a source for the HTS was total RNA, sequenced on Illumina platforms (Tables 1, 2).
The complete genome of only one varicosavirus, lettuce big-vein associated virus, was previously characterized using Sanger dideoxy sequencing (Sasaya et al., 2002, 2004); however, the application of HTS has facilitated the discovery of two novel varicosaviruses during the last 3 years (Table 1). The template used as a source for the HTS was total RNA and dsRNA, and Illumina and Roche 454 were the technologies employed in the HTS projects (Table 1).
This family is composed of only one genus, Emaravirus, for viruses that are distantly related to orthotospoviruses, and exclusively comprise members that have plants as their hosts. Emaraviruses have enveloped, spherical virions, with a diameter of 80–100 nm and a segmented, linear, single-stranded genome with generally four to eight RNA segments (Elbeaino et al., 2018), but a recently described novel emaravirus, perilla mosaic virus, has 10 RNA segments (Kubota et al., 2020).
Most of the members of the genus Emaravirus have been recently discovered (Table 1) or characterized in depth by HTS techniques (Table 2). The application of HTS resulted in the discovery of 13 emaraviruses during the last 5 years (Table 1). Different templates, such as total RNA, small RNAs and dsRNA were used as sources for the HTS, and Illumina platforms have been mostly used in the HTS projects (Table 1).
The use of HTS has revealed the genome sequence of two emaraviruses for which only a genomic fragment and some biological properties were known (high plains wheat mosaic virus and pigeonpea sterility mosaic virus 1) (Table 2). Furthermore, the application of HTS has resulted in the detailed characterization of the complete genomes of three emaraviruses. A clearer picture of the genome organization of emaraviruses was obtained by the identification of additional RNA segments. HTS- based research resulted in the identification of three novel genome segments of raspberry leaf blotch virus and rose rosette virus (Di Bello et al., 2015; Lu et al., 2015), and two novel segments for European mountain ash ringspot-associated virus genome (von Bargen et al., 2019). Different templates, including total nucleic acids, total RNA, dsRNA and RNA from partially purified virions were used as sources for the HTS. Illumina platforms were mostly used in these HTS projects (Table 2).
Finally, partial fragments of RNAs1, 2 and 4 of a putative novel emaravirus infecting alfalfa in Australia, named alfalfa ringspot-associated virus were recently identified using HTS (Samarfard et al., 2020). PCR amplification of the conserved termini of the genome segments (Babu et al., 2016) and HTS could be used to characterize the complete genome sequence of this tentative novel emaravirus.
This family, formerly named Ophioviridae, contains only one genus, Ophiovirus, which is exclusively composed of members that have plants as their hosts (Figure 1). Ophioviruses have non-enveloped, naked filamentous virions and a segmented, linear open circle, serpentine, single-stranded RNA genome, consisting of three to four segments (García et al., 2017).
One novel ophiovirus, blueberry mosaic associated ophiovirus, has been discovered using HTS during the last 6 years (Table 1). Partial genome fragments of three ophioviruses, freesia sneak virus, ranunculus white mottle virus, and tulip mild mottle mosaic virus have been known for some years but their complete genomes remain elusive (García et al., 2017). HTS could be a crucial tool to obtain the complete genomes of these viruses and to expand our understanding of the genomic cues and evolutionary diversity of ophioviruses.
This family contains the single genus Orthotospovirus, which is exclusively composed of species for viruses that have plants as their hosts (Figure 1). Orthotospoviruses have enveloped, spherical virions of 80–120 nm diameter and are transmitted by thrips insects in which they also replicate. The genome of orthotospoviruses is segmented with three linear single-stranded RNA segments named large (L), middle (M), and small (S). The L RNA contains one open reading frame in negative-sense polarity, whereas the other two segments, M and S RNAs, are ambisense and have two open reading frames encoding proteins in opposite orientation (Oliver and Whitfield, 2016).
The application of HTS led to the discovery of two novel orthotospoviruses in 2019 and 2020 (Table 1). Total RNA was used as the template and Illumina platforms as the sequencing technology.
The use of HTS has also facilitated completion of the sequence of eight orthotospoviruses for which only one genome segment was available (mostly the S RNA) and their biological properties were known (Table 2). Different templates, including total RNA, small RNA and virus-enriched RNA from purified ribonucleocapsids were used as RNA source, and Illumina platforms were mostly used as sequencing technology in the HTS projects (Table 2).
At the time of this review, only partial genome sequence of four other reported orthotospoviruses are available: groundnut yellow spot virus (S RNA), lisianthus necrotic ringspot virus (S RNA), pepper necrotic spot virus (S RNA) and tomato necrotic ringspot virus (S and M RNAs) (Satyanarayana et al., 1998; Seepiban et al., 2011; Torres et al., 2012; Shimomoto et al., 2014); HTS could enable completion of the genome of these agronomically important viruses.
Most phenuiviruses have enveloped particles with helical morphology, except tenuiviruses that have non-enveloped filamentous particles, and their genomes are comprised of two to four single-stranded, linear RNA segments. As of 2019, the family Phenuiviridae includes 19 ICTV-recognized genera (Figure 1). Only the established genus Tenuivirus and likely two recently accepted new genera Coguvirus and Rubodvirus (2019.026M.A.v1.Phenuiviridae_4gen79sp.xlsx) contain species of plant-infecting viruses (Navarro et al., 2018b; Diaz-Lara et al., 2019). Phylogenetic relationships with other phenuiviruses support their classification within this family (Diaz-Lara et al., 2019).
This genus is composed of two previously unknown viruses that have bi-segmented genomes, which have been discovered using HTS of citrus (Navarro et al., 2018a,b), while two tentative coguviruses that have tri-segmented genomes, have been discovered using HTS in watermelon (Xin et al., 2017) (Table 1). Total RNA and small RNAs were used as template sources for HTS on Illumina platforms (Table 1). Recently, the complete tri-segmented genomes of four cogu-like viruses were determined when the virome of grapevine was characterized (Bertazzon et al., 2020; Chiapello et al., 2020) (Table 1).
Moreover, partial segments of a cogu-like virus were discovered when the virome of ornamental flowers was analyzed by HTS (Wylie et al., 2019). However, it will be necessary to complete the genome characterization and perform further analyses of these viruses to confirm if they are in fact plant-infecting viruses or arthropod-infecting viruses whose RNA was co-purified along with the plant RNA. This additional information would enable further characterization of these putative coguviruses, thus increasing our understanding of the plant-infecting phenuivirus diversity and evolutionary history.
This genus is composed of species for two previously unknown viruses that have tri-segmented genomes which have been discovered using HTS in apple (Rott et al., 2018; Wright et al., 2018), while two tentative rubodviruses were discovered using HTS in grapevine (Diaz-Lara et al., 2019) (Table 1). DsRNA and total RNA were the templates used as sources for HTS on Illumina platforms (Table 1).
Tenuiviruses have a segmented genome of four to eight single-stranded RNA segments with negative-sense or ambisense polarity (Gaafar et al., 2019a). The application of HTS led to the discovery of two new tenuiviruses, one infecting melon and black medic (Gaafar et al., 2019a; Lecoq et al., 2019) and the other from sugarcane associated with Ramu stunt disease (Mollov et al., 2016) (Table 1). In addition, a recent HTS study resulted in the molecular characterization of a wheat tenuivirus, the biological properties of which had been previously characterized in the 1960s (Sõmera et al., 2020) (Table 1). Total RNA and small RNAs were used as sources for HTS on Illumina platforms (Table 1). Moreover, the use of HTS facilitated sequencing of a tenuivirus from rice, for which only the sequence of RNA4 and its biological properties were known previously (Table 2). At the time of writing this review, only partial genomic sequence of four other tenuiviruses are known: maize stripe virus (complete sequences of RNAs 2, 3, 4 and 5), maize yellow stripe virus (partial sequences of RNAs 1, 2, 3, 4 and complete sequence of RNA 5), Iranian wheat stripe virus (complete sequences of RNAs 2, 3 and 4), wheat yellow head virus (complete sequence of the nucleoprotein gene) (Huiet et al., 1991; Estabrook et al., 1996; Seifers et al., 2005; Heydarnejad et al., 2006; Mahmoud et al., 2007). HTS would be an essential tool to obtain the complete genomes of these viruses and expand our knowledge of genomic architecture of tenuiviruses.
The number of NSR plant viruses identified so far represents a negligible fraction of the potential number of NSR viruses present in the virosphere. The discovery and in-depth molecular characterization of these viruses has been challenging given their extensive divergence, their outstanding diversity in terms of genomic architecture, and mostly, the negligible share of plant species studied for virus discovery. However, the widespread implementation of HTS, a cost-effective and efficient technology platform, allowed the discovery and molecular characterization of the genome sequences of at least 70 NSR plant viruses in the last few years. Thus, we predict that the increasing use of HTS, not only for plant samples but also in arthropod vectors, will allow the identification of many novel NSR phytoviruses which will be crucial to unravel the evolutionary landscapes of many NSR virus clades that are poorly characterized today. Nevertheless, as many more novel NSR viruses are being discovered, careful analysis will be essential to confirm their correct ecological context, to determine whether the new viral agents are plant-infecting viruses, fungi- or arthropod-infecting viruses whose RNA was co-purified along with the plant RNA, or just contamination-inadvertently sampled environmental viruses.
As soon as the virome characterization of multiple samples of cultivated and wild plants becomes more routine, more viruses with unique features and novel phylogenetic relationships will likely be discovered. As we assess the viral ‘dark matter,’ we will start to grasp the evolutionary history of plant viruses, eventually leading us to untangle the diversity of the NSR virosphere and gain increased knowledge about its complex evolutionary and phylogenetic relationships. A rich picture of the plant virome landscape will also provide an essential framework for improved taxonomic classification of plant NSR viruses as part of the realm Riboviria. In conclusion, HTS-based methods for virus discovery, our ‘new eyes,’ are unraveling in real time the richness and magnitude of the plant RNA virosphere.
All authors wrote the manuscript and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adegbola, R., Kemerait, R., Adkins, S., and Naidu, R. (2019). Complete genome segment sequences of tomato chlorotic spot virus from peanut in Haiti. Microbiol. Resour. Announc. 8:e00306-19.
Alcalá-Briseño, R. I., Casarrubias-Castillo, K., López-Ley, D., Garrett, K. A., and Silva-Rosales, L. (2020). Network analysis of the papaya orchard virome from two agroecological regions of Chiapas, Mexico. mSystems 5:e00423-19.
Alves-Freitas, D. M. T., Pinheiro-Lima, B., Faria, J. C., Lacorte, C., Ribeiro, S. G., and Melo, F. L. (2019). Double-stranded RNA high-throughput sequencing reveals a new cytorhabdovirus in a bean golden mosaic virus-resistant common bean transgenic line. Viruses 11:90. doi: 10.3390/v11010090
Babu, B., Washburn, B. K., Poduch, K., Knox, G. W., and Paret, M. L. (2016). Identification and characterization of two novel genomic RNA segments RNA5 and RNA6 in rose rosette virus infecting roses. Acta Virol. 60, 156–165. doi: 10.4149/av_2016_02_156
Baek, D., Lim, S., Ju, H., Kim, H., Lee, S., and Moon, J. (2019). The complete sequence of a novel nucleorhabdovirus (apple rootstock virus A) identified in apple rootstock. Arch. Virol. 164, 2641–2644. doi: 10.1007/s00705-019-04348-0
Bejerman, N., Acevedo, R., de Breuil, S., Ruiz, O., and Sansberro, P. (2020). Molecular characterization of a novel cytorhabdovirus with a unique genomic organization infecting yerba mate (Ilex paraguariensis) in Argentina. Arch. Virol. 165, 1475–1479. doi: 10.1007/s00705-020-04609-3
Bejerman, N., de Breuil, S., Debat, H., Miretti, M., Badaracco, A., and Nome, C. (2017). Molecular characterization of yerba mate chlorosis-associated virus, a putative cytorhabdovirus infecting yerba mate (Ilex paraguariensis). Arch. Virol. 162, 2481–2484. doi: 10.1007/s00705-017-3363-8
Bejerman, N., Giolitti, F., de Breuil, S., Trucco, V., Nome, C., Lenardon, S., et al. (2015). Complete genome sequence and integrated protein localization and interaction map for alfalfa dwarf virus, which combines properties of both cytoplasmic and nuclear plant rhabdoviruses. Virology 483, 275–283. doi: 10.1016/j.virol.2015.05.001
Bernardo, P., Charles-Dominique, T., Barakat, M., Ortet, P., Fernandez, E., Filloux, D., et al. (2018). Geometagenomics illuminates the impact of agriculture on the distribution and prevalence of plant viruses at the ecosystem scale. ISME J. 12, 173–184. doi: 10.1038/ismej.2017.155
Bertazzon, N., Chitarra, W., Angelini, E., and Nerva, L. (2020). Two new putative plant viruses from wood metagenomics analysis of an esca diseased vineyard. Plants 9:835. doi: 10.3390/plants9070835
Bhat, A. I., Pamitha, N. S., Naveen, K. P., and Biju, C. N. (2020). Identification and characterization of cardamom vein clearing virus, a novel aphid-transmitted nucleorhabdovirus. Eur. J. Plant Pathol. 156, 1053–1062. doi: 10.1007/s10658-020-01958-2
Blawid, R., Silva, J. M. F., and Nagata, T. (2017). Discovering and sequencing new plant viral genomes by next-generation sequencing: description of a practical pipeline. Ann. Appl. Biol. 170, 301–314. doi: 10.1111/aab.12345
Blouin, A. G., Ross, H. A., Hobson-Peters, J., O’Brien, C., Warren, B., and MacDiarmid, R. (2016). A new virus discovered by immunocapture of double-stranded RNA, a rapid method for virus enrichment in metagenomic studies. Mol. Ecol. Resour. 16, 1255–1263. doi: 10.1111/1755-0998.12525
Buzkan, N., Chiumenti, M., Massart, S., Sarpkaya, K., Karadað, S., and Minafra, A. (2019). A new emaravirus discovered in Pistacia from Turkey. Virus Res. 26, 159–163. doi: 10.1016/j.virusres.2019.01.012
Cao, M., Zhang, S., Li, M., Liu, Y., Dong, P., Li, S., et al. (2019). Discovery of four novel viruses associated with flower yellowing disease of green Sichuan pepper (Zanthoxylum armatum) by virome analysis. Viruses 11:696. doi: 10.3390/v11080696
Chabi-Jesus, C., Ramos-Gonzalez, P., Tassi, A. D., Guerra-Peraza, O., Kitajima, E. W., Harakava, R., et al. (2018). Identification and characterization of citrus chlorotic spot virus, a new dichorhavirus associated with citrus leprosis-like symptoms. Plant Dis. 102, 1509–1519.
Chiapello, M., Rodríguez-Romero, J., Nerva, L., Forgia, M., Chitarra, W., Ayllón, M. A., et al. (2020). Putative new plant viruses associated with Plasmopara viticola-infected grapevine samples. Ann. Appl. Biol. 176, 180–191. doi: 10.1111/aab.12563
Chou, W. C., Lin, S. S., Yeh, S. D., Li, S. L., Peng, Y. C., Fan, Y. H., et al. (2017). Characterization of the genome of a phylogenetically distinct tospovirus and its interactions with the local lesion-induced host Chenopodium quinoa by whole-transcriptome analyses. PLoS One 12:e0182425. doi: 10.1371/journal.pone.0182425
Ciuffo, M., Nerva, L., and Turina, M. (2017). Full-length genome sequence of the tospovirus melon severe mosaic virus. Arch. Virol. 162, 1419–1422. doi: 10.1007/s00705-017-3237-0
Debat, H. J., and Bejerman, N. (2019). Novel bird’s-foot trefoil RNA viruses provide insights into a clade of legume-associated enamoviruses and rhabdoviruses. Arch. Virol. 164, 1419–1426. doi: 10.1007/s00705-019-04193-1
Di Bello, P. L., Ho, T., and Tzanetakis, I. E. (2015). The evolution of emaraviruses is becoming more complex: seven segments identified in the causal agent of Rose rosette disease. Virus Res. 210, 241–244. doi: 10.1016/j.virusres.2015.08.009
Di Bello, P. L., Laney, A. G., Druciarek, T., Ho, T., and Gergerich, R. C. (2016). A novel emaravirus is associated with redbud yellow ringspot disease. Virus Res. 222, 41–47. doi: 10.1016/j.virusres.2016.05.027
Diaz-Lara, A., Navarro, B., Di Serio, F., Stevens, K., Hwang, M. S., Kohl, J., et al. (2019). Two novel negative-sense RNA viruses infecting grapevine are members of a newly proposed genus within the family Phenuiviridae. Viruses 11:685. doi: 10.3390/v11080685
Dietzgen, R. G., and Higgins, C. (2019). Complete genome sequence of maize sterile stunt virus. Arch. Virol. 164, 1221–1223. doi: 10.1007/s00705-019-04164-6
Dietzgen, R. G., Innes, D. J., and Bejerman, N. (2015). Complete genome sequence and intracellular protein localization of Datura yellow vein nucleorhabdovirus. Virus Res. 205, 7–11. doi: 10.1016/j.virusres.2015.05.001
Ding, X., Chen, D., Du, Z., Zhang, J., and Wu, Z. (2019). The complete genome sequence of a novel cytorhabdovirus identified in strawberry (Fragaria ananassa Duch.). Arch. Virol. 164, 3127–3131. doi: 10.1007/s00705-019-04390-y
Dolja, V. V., Krupovic, M., and Koonin, E. V. (2020). Deep roots and splendid boughs of the global plant virome. Annu. Rev. Phytopathol. 58, 23–53. doi: 10.1146/annurev-phyto-030320-041346
Dullemans, A. M., Verhoeven, J. T., Kormelink, R., and van der Vlugt, R. A. (2015). The complete nucleotide sequence of chrysanthemum stem necrosis virus. Arch. Virol. 160, 605–608. doi: 10.1007/s00705-014-2282-1
Elbeaino, T., Digiaro, M., Mielke-Ehret, N., Mühlbach, H.-P., and Martelli, G. P. (2018). ICTV virus taxonomy profile: fimoviridae. J. Gen. Virol. 99, 1478–1479. doi: 10.1099/jgv.0.001143
Elbeaino, T., Digiaro, M., Uppala, M., and Sudini, H. (2014). Deep sequencing of pigeonpea sterility mosaic virus discloses five RNA segments related to emaraviruses. Virus Res. 188, 27–31. doi: 10.1016/j.virusres.2014.03.022
Elbeaino, T., Digiaro, M., Uppala, M., and Sudini, H. (2015). Deep sequencing of dsRNAs recovered from mosaic-diseased pigeonpea reveals the presence of a novel emaravirus: pigeonpea sterility mosaic virus 2. Arch. Virol. 160, 2019–2029. doi: 10.1007/s00705-015-2479-y
Estabrook, E. M., Suyenaga, K., Tsai, J. H., and Falk, B. W. (1996). Maize stripe tenuivirus RNA2 transcripts in plant and insect analysis of pvc2, a protein similar to the Phlebovirus virion glycoproteins. Virus Genes 12, 239–247. doi: 10.1111/j.1365-3059.1995.tb02774.x
Fagundes Silva, J., Silvas de Oliveira, A., Severo de Almeida, M. M., Kormelink, R., Nagata, T., and Resende, R. O. (2019). Tomato chlorotic spot virus (TCSV) putatively incorporated a genomic segment of Groundnut ringspot virus (GRSV) upon a reassortment event. Viruses 11, 187–194. doi: 10.3390/v11020187
Franova, J., Pøibylova, J., and Koloniuk, I. (2019a). Molecular and biological characterization of a new strawberry cytorhabdovirus. Viruses 11:982. doi: 10.3390/v11110982
Franova, J., Sarkisová, T., Jakešová, H., and Koloniuk, I. (2019b). Molecular and biological properties of two putative new cytorhabdoviruses infecting Trifolium pratense. Plant Pathol. 11, 1276–1286. doi: 10.1111/ppa.13065
Freitas-Astúa, J., Dietzgen, R. G., Walker, P. J., Blasdell, K. R., and Breyta, S. (2019). Split the Genus Nucleorhabdovirus, Creating Three New Genera (Alphanucleorhabdovirus, Betanucleorhabdovirus and Gammanucleorhabdovirus) Comprising Sixteen Species, Including 23 Six New Species, in the Family Rhabdoviridae. London: International Committee on Taxonomy of Viruses.
Gaafar, Y., Abdelgalil, M., Knierim, D., Richert-Pöggeler, K., and Menzel, W. (2018). First report of physostegia chlorotic mottle virus on tomato (Solanum lycopersicum) in Germany. Plant Dis. 102:255. doi: 10.1094/pdis-05-17-0737-pdn
Gaafar, Y., Richert-Poggeler, K., Sieg-Muller, A., Luddecke, P., and Herz, K. (2019a). A divergent strain of melon chlorotic spot virus isolated from black medic (Medicago lupulina) in Austria. Virol. J. 16:89.
Gaafar, Y., Richert-Pöggeler, K. R., Maaß, C., Vetten, H. J., and Ziebell, H. (2019b). Characterization of a novel nucleorhabdovirus infecting alfalfa (Medicago sativa). Virol. J. 16:55. doi: 10.1007/978-1-4020-6754-9_468
Gallo, Y., Toro, L. F., Jaramillo, H., Gutiérrez, P., and Marín, M. (2018). Identificación y caracterización molecular del genoma completo de tres virus en cultivos de lulo (Solanum quitoense) de Antioquia (Colombia). Rev. Colomb. Cienc. Hortíc. 1, 281–292. doi: 10.17584/rcch.2018v12i2.7692
García, M. L., Dal Bó, E., da Graça, J. V., Gago-Zachert, S., and Hammond, J. (2017). ICTV virus taxonomy profile: Ophioviridae. J. Gen. Virol. 98, 1161–1162. doi: 10.1099/jgv.0.000836
German, T. L., Lorenzen, M. D., Grubbs, N., and Whitefield, A. E. (2020). New technologies for studying negative-strand RNA viruses in plant and arthropod hosts. Mol. Plant Microbe Interact. 33, 382–393. doi: 10.1094/mpmi-10-19-0281-fi
Ghorbani, A., Izadpanah, K., and Dietzgen, R. G. (2018). Completed sequence and corrected annotation of the genome of maize Iranian mosaic virus. Arch. Virol. 163, 767–770. doi: 10.1007/s00705-017-3646-0
Goh, C., Park, D., and Hahn, Y. (2020). Identification of Trichosanthes associated rhabdovirus 1, a novel member of the genus cytorhabdovirus of the family Rhabdoviridae, in the Trichosanthes kirilowii transcriptome. Acta Virol. 64, 36–43. doi: 10.4149/av_2020_105
Hassan, M., Di Bello, P. L., Keller, K. E., Martin, R. R., Sabanadzovic, S., and Tzanetakis, I. E. (2017). A new, widespread emaravirus discovered in blackberry. Virus Res. 235, 1–5. doi: 10.1016/j.virusres.2017.04.006
Hassani-Mehraban, A., Dullemans, A. M., Verhoeven, J. T. J., Roenhorst, J. W., and Peters, D. (2019). Alstroemeria yellow spot virus (AYSV): a new orthotospovirus species within a growing Eurasian clade. Arch. Virol. 164, 117–126. doi: 10.1007/s00705-018-4027-z
Heydarnejad, J., Barclay, W. S., Hunter, F. R., Izadpanah, K., and Gooding, M. J. (2006). Molecular characterization of Iranian wheat stripe virus shows its taxonomic position as a distinct species in the genus Tenuivirus. Arch. Virol. 151, 217–227. doi: 10.1007/s00705-005-0652-4
Higgins, C. M., Bejerman, N., Li, M., James, A. P., and Dietzgen, R. G. (2016). Complete genome sequence of Colocasia bobone disease-associated virus, a putative cytorhabdovirus infecting taro. Arch. Virol. 161, 745–748. doi: 10.1007/s00705-015-2713-7
Huiet, L., Klaassen, V., Tsai, J. H., and Falk, B. W. (1991). Nucleotide sequence and RNA hybridization analyses reveal an ambisense coding strategy for maize stripe virus RNA-3. Virology 182, 47–53. doi: 10.1016/0042-6822(91)90646-s
Ilyas, M., Avelar, A. S., Schuch, U., and Brown, J. K. (2018). First report of an emaravirus associated with witches broom disease and eriophyid mite infestations of the blue palo verde tree in Arizona. Plant Dis. 102:1863. doi: 10.1094/pdis-01-18-0124-pdn
Ito, T., Suzaki, K., and Nakano, M. (2013). Genetic characterization of novel putative rhabdovirus and dsRNA virus from Japanese persimmon. J. Gen. Virol. 94, 1917–1921. doi: 10.1099/vir.0.054445-0
Jakovljevic, V., Otten, P., Berwarth, C., and Jelkmann, W. (2016). Analysis of the apple rubbery wood disease by next generation sequencing of total RNA. Eur. J. Plant Pathol. 148, 637–646. doi: 10.1007/s10658-016-1119-z
Jimenez, J., Carvajal-Yepes, M., Leiva, A. M., Cruz, M., Romero, L. E., Bolaños, C. A., et al. (2018). Complete genome sequence of Rice hoja blanca tenuivirus isolated from a susceptible rice cultivar in Colombia. Genome Announc. 6:e01490-17.
Jones, S., McGavin, W., and MacFarlane, S. (2019). The complete sequences of two divergent variants of the rhabdovirus raspberry vein chlorosis virus and the design of improved primers for virus detection. Virus Res. 265, 162–165. doi: 10.1016/j.virusres.2019.03.004
Käfer, S., Paraskevopoulou, S., Zirkel, F., Wieseke, N., Donath, A., Peterson, M., et al. (2019). Re-assessing the diversity of negative strand RNA viruses in insects. PLoS Pathog. 15:e1008224. doi: 10.1371/journal.ppat.1008224
Koloniuk, I., Fránová, J., Sarkisova, T., Pøibylová, J., and Lenz, O. (2018a). Identification and molecular characterization of a novel varicosa-like virus from red clover. Arch. Virol. 163, 2213–2218. doi: 10.1007/s00705-018-3838-2
Koloniuk, I., Fránová, J., Sarkisova, T., and Pøibylová, J. (2018b). Complete genome sequences of two divergent isolates of strawberry crinkle virus coinfecting a single strawberry plant. Arch. Virol. 163, 2539–2542. doi: 10.1007/s00705-018-3860-4
Koonin, E. V., Dolja, V. V., and Krupovic, M. (2015). Origins and evolution of viruses of eukaryotes: the ultimate modularity. Virology 479–480, 2–25. doi: 10.1016/j.virol.2015.02.039
Koonin, E. V., Dolja, V. V., Krupovic, M., Varsani, A., Wolf, Y. I., Yutin, N., et al. (2020). Global organization and proposed megataxonomy of the virus world. Microbiol. Mol. Biol. Rev. 84, 1–33.
Kreuze, J. (2014). “siRNA deep sequencing and assembly: piecing together viral infections,” in Detection and Diagnostics of Plant Pathogens, Plant Pathology in the 21st Century 5, eds M. L. Gullino and P. J. M. Bonants (Dordrecht: Springer Science+Business Media), 21–38. doi: 10.1007/978-94-017-9020-8_2
Kubota, K., Usugi, T., Tomitaka, Y., Shimomoto, Y., and Takeuchi, S. (2020). Perilla mosaic virus is a highly divergent emaravirus transmitted by Shevtchenkella Sp. (Acari: Eriophyidae). Phytopatology 110, 1352–1361. doi: 10.1094/PHYTO-01-20-0013-R
Kuhn, J. H., Adkins, S., and Alioto, D. (2020). 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. (in press). doi: 10.1007/s00705-020-04731-2
Kuhn, J. H., Wolf, Y. I., Krupovic, M., Zhang, Y.-Z., Maes, P., Dolja, V. V., et al. (2019). Classify viruses—the gain is worth the pain. Nature 566, 318–320. doi: 10.1038/d41586-019-00599-8
Kumar, S., Subbarao, B., and Hallan, V. (2017). Molecular characterization of emaraviruses associated with Pigeonpea sterility mosaic disease. Sci. Rep. 7:11831.
Lamprecht, R. L., Kasdorf, G. G. F., Stiller, M., Staples, S. M., Nel, L. H., and Pietersen, G. (2010). Soybean blotchy mosaic virus, a new Cytorhabdovirus found in South Africa. Plant Dis. 94, 1348–1354. doi: 10.1094/pdis-09-09-0598
Lastra, R., and Quintero, E. (1981). Papaya apical necrosis, a new diseases associated with a rhabdovirus. Plant Dis. 65, 439–440. doi: 10.1094/pd-65-439
Lecoq, H., Wipf-Scheibel, C., Verdin, E., and Desbiez, C. (2019). Characterization of the first tenuivirus naturally infecting dicotyledonous plants. Arch. Virol. 164, 297–301. doi: 10.1007/s00705-018-4057-6
Li, C. X., Shi, M., Tian, J. H., Lin, X. D., Kang, Y. J., Chen, L. J., et al. (2015). Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. eLife 4:e05378.
Liao, L., and Noble, W. S. (2003). Combining pairwise sequence similarity and support vector machines for detecting remote protein evolutionary and structural relationships. J. Comput. Biol. 10, 857–868.
Lima, R. N., De Oliveira, A. S., Leastro, M. O., Blawid, R., Nagata, T., Resende, R. O., et al. (2016). The complete genome of the tospovirus Zucchini lethal chlorosis virus. Virol. J. 13:123.
Lin, M. T., and Campbell, R. N. (1972). Characterization of broccoli necrotic yellows virus. Virology 48, 30–40. doi: 10.1016/0042-6822(72)90111-0
Liu, Y., Du, Z., Wang, H., Zhang, S., Cao, M., and Wang, X. (2018). Identification and characterization of wheat yellow striate virus, a novel leafhopper-transmitted nucleorhabdovirus infecting wheat. Front. Microbiol. 9:468. doi: 10.3389/fmicb.2018.00468
Lu, Y., McGavin, W., Cock, P. J., Schnettler, E., Yan, F., Chen, J., et al. (2015). Newly identified RNAs of raspberry leaf blotch virus encoding a related group of proteins. J. Gen. Virol. 96, 3432–3439. doi: 10.1099/jgv.0.000277
Lundsgaard, T., and Albrechtsen, S. E. (1976). Electron microscopy of rhabdovirus-like particles in Festuca gigantea with leaf streak mosaic. J. Phytopathol. 87, 12–16. doi: 10.1111/j.1439-0434.1976.tb01715.x
Ma, Y., Marais, A., Lefebvre, M., Faure, C. H., and Candresse, T. (2020). Metagenomic analysis of virome cross-talk between cultivated Solanum lycopersicum and wild Solanum nigrum. Virology 540, 38–44. doi: 10.1016/j.virol.2019.11.009
Mahmoud, A., Royer, M., Granier, M., Ammar, E.-D., Thouvenel, J. C., and Peterschmitt, M. (2007). Evidence for a segmented genome and partial nucleotide sequences of Maize yellow stripe virus, a proposed new Tenuivirus. Arch. Virol. 152, 1757–1762. doi: 10.1007/s00705-007-0980-7
Margaria, P., Miozzi, L., Ciuffo, M., Pappu, H., and Turina, M. (2014). The complete genome sequence of polygonum ringspot virus. Arch. Virol. 159, 3149–3152. doi: 10.1007/s00705-014-2166-4
Martin, K., and Whitfield, A. (2019). Complete genome sequence of Maize Mosaic Nucleorhabdovirus. Microbiol. Resour. Announc. 8:e00637-19.
Martínez, R. T., de Almeida, M. M. S., Rodriguez, R., de Oliveira, A. S., Melo, F. L., and Resende, R. O. (2018). Identification and genome analysis of tomato chlorotic spot virus and dsRNA viruses from coinfected vegetables in the Dominican Republic by high-throughput sequencing. Virol. J. 15:24.
Massart, S., Candresse, T., Gil, J., Lacomme, C., Predajna, L., Ravnikar, M., et al. (2017). A framework for the evaluation of biosecurity, commercial, regulatory, and scientific impacts of plant viruses and viroids identified by NGS technologies. Front. Microbiol. 8:45. doi: 10.3389/fmicb.2017.00045
Maurino, F., Dumón, A. D., Llauger, G., Alemandri, V., and de Haro, L. (2018). Complete genome sequence of maize yellow striate virus, a new cytorhabdovirus infecting maize and wheat crops in Argentina. Arch. Virol. 163, 291–295. doi: 10.1007/s00705-017-3579-7
Medina-Salguero, A. X., Cornejo-Franco, J. F., Grinstead, S., Mollov, D., Mowery, J. D., Flores, F., et al. (2019). Sequencing, genome analysis and prevalence of a cytorhabdovirus discovered in Carica papaya. PLoS One 14:e0215798. doi: 10.1371/journal.pone.0215798
Meng, J., Liu, P., Zhu, L., Zou, C., Li, J., and Chen, B. (2015). Complete genome sequence of mulberry vein banding associated virus, a new tospovirus infecting mulberry. PLoS One 10:e0136196. doi: 10.1371/journal.pone.0136196
Menzel, W., Knierim, D., Richert-Pöggeler, K., and Winter, S. (2016). Charakterisierung eines nucleorhabdovirus aus physostegia. Julius Kühn Arch. 454, 283–284.
Mollov, D., Maroon-Lango, C., and Kuniata, L. (2016). Detection by next generation sequencing of a multi-segmented viral genome from sugarcane associated with Ramu stunt disease. Virus Genes 52, 152–155. doi: 10.1007/s11262-015-1279-5
Mwaipopo, B., Msolla, N. S., Njau, P., Mark, D., and Mbanzibwa, D. R. (2018). Comprehensive surveys of Bean common mosaic virus and Bean common mosaic necrosis virus and molecular evidence for occurrence of other Phaseolus vulgaris viruses in Tanzania. Plant Dis. 102, 2361–2370. doi: 10.1094/pdis-01-18-0198-re
Navarro, B., Minutolo, M., Stradis, A. D., Palmisano, F., Alioto, D., and Di Serio, F. (2018a). The first phlebo-like virus infecting plants: a case study on the adaptation of negative-stranded RNA viruses to new hosts. Mol. Plant Pathol. 19, 1075–1089. doi: 10.1111/mpp.12587
Navarro, B., Zicca, S., Minutolo, M., Saponari, M., Alioto, D., and Di Serio, F. (2018b). A negative-stranded RNA virus infecting citrus trees: the second member of a new genus within the order Bunyavirales. Front. Microbiol. 9:2340. doi: 10.3389/fmicb.2018.02340
Obbard, D. J., Shi, M., Roberts, K. E., Longdon, B., and Dennis, A. B. (2020). A new lineage of segmented RNA viruses infecting animals. Virus Evol. 6:vez061.
Oliver, J., and Whitfield, A. (2016). The genus Tospovirus: emerging bunyaviruses that threaten food security. Annu. Rev. Virol. 3, 101–124. doi: 10.1146/annurev-virology-100114-055036
Olmedo-Velarde, A., Park, A. C., Sugano, J., Uchida, J. Y., and Kawate, M. (2019). Characterization of Ti ringspot-associated virus, a novel Emaravirus associated with an emerging ringspot disease of Cordyline fruticosa. Plant Dis. 103, 2345–2352. doi: 10.1094/pdis-09-18-1513-re
Orfanidou, C. G., Beta, C., Reynard, J., Tsiolakis, G., Katis, N. I., and Maglioka, V. I. (2020). Identification, molecular characterization and prevalence of a novel cytorhabdovirus infecting zucchini crops in Greece. Virus Res. 287:198095. doi: 10.1016/j.virusres.2020.198095
Pecman, A., Kutnjak, D., Gutiérrez-Aguirre, I., Adams, I., Fox, A., Boonham, N., et al. (2017). Next generation sequencing for detection and discovery of plant viruses and viroids: comparison of two approaches. Front. Microbiol. 8:1998. doi: 10.3389/fmicb.2017.01998
Peracchio, C., Fogia, M., Chiapello, M., Vallino, M., Turina, M., and Ciuffo, M. (2020). A complex virome including two distinct emaraviruses associated with virus-like symptoms in Camellia japonica. Virus Res. 286:197964. doi: 10.1016/j.virusres.2020.197964
Petrzik, K. (2012). Bioinformatic analysis of the L polymerase gene leads to discrimination of new rhabdoviruses. J. Phytopathol. 160, 377–381. doi: 10.1111/j.1439-0434.2012.01919.x
Ramalho, T. O., Figueira, A. R., Sotero, A. J., Wang, R., Geraldino Duarte, P. S., Farman, M., et al. (2014). Characterization of coffee ringspot virus-Lavras: a model for an emerging threat to coffee production and quality. Virology 464–465, 385–396. doi: 10.1016/j.virol.2014.07.031
Ramos-González, P. L., Chabi-Jesus, C., Banguela-Castillo, A., Tassi, A. D., and Rodrigues, M. D. C. (2018). Unveiling the complete genome sequence of clerodendrum chlorotic spot virus, a putative dichorhavirus infecting ornamental plants. Arch. Virol. 163, 2519–2524. doi: 10.1007/s00705-018-3857-z
Ramos-González, P. L., Chabi-Jesus, C., Guerra-Peraza, O., Tassi, A. D., and Kitajima, E. W. (2017). Citrus leprosis virus N: a new dichorhavirus causing citrus leprosis disease. Phytopathology 107, 963–976. doi: 10.1094/phyto-02-17-0042-r
Read, D. A., Featherston, J., Rees, D. J. G., Thompson, G. D., and Roberts, R. (2019). Molecular characterization of Morogoro maize-associated virus, a nucleorhabdovirus detected in maize (Zea mays) in Tanzania. Arch. Virol. 164, 1711–1715. doi: 10.1007/s00705-019-04212-1
Roossinck, M. J., Martin, D. P., and Roumagnac, P. (2015). Plant virus metagenomics: advances in virus discovery. Phytopathology 105, 716–727. doi: 10.1094/phyto-12-14-0356-rvw
Rott, M. E., Kesanakurti, P., Berwarth, C., Rast, H., Boyes, I., Phelan, J., et al. (2018). Discovery of negative-sense RNA viruses in trees infected with apple rubbery wood disease by next-generation sequencing. Plant Dis. 102, 1254–1263.
Sabbadin, F., Glover, R., Stafford, R., Rozado-Aguirre, Z., and Boonham, N. (2017). Transcriptome sequencing identifies novel persistent viruses in herbicide resistant wild-grasses. Sci. Rep. 7:41987.
Sadeghi, M. S., Afsharifar, A., Izadpanah, K., Loconsole, G., De Stradis, A., Martelli, G. P., et al. (2016). Isolation and partial characterization of a novel cytorhabdovirus from citrus trees showing foliar symptoms in Iran. Plant Dis. 100, 66–71.
Samarfard, S., McTaggart, A. R., Sharman, M., Bejerman, N. E., and Dietzgen, R. G. (2020). Viromes of ten alfalfa plants in Australia reveal diverse known viruses and a novel RNA virus. Pathogens 9:214. doi: 10.3390/pathogens9030214
Sasaya, T., Ishikawa, K., and Koganezawa, H. (2002). The nucleotide sequence of RNA1 of Lettuce big-vein virus, genus Varicosavirus, reveals its relation to nonsegmented negative-strand RNA viruses. Virology 297, 289–297.
Sasaya, T., Kusaba, S., Ishikawa, K., and Koganezawa, H. (2004). Nucleotide sequence of RNA2 of Lettuce big-vein virus and evidence for a possible transcription termination/initiation strategy similar to that of rhabdoviruses. J. Gen. Virol. 85, 2709–2717.
Satyanarayana, T., Gowda, S., Reddy, K. L., Mitchell, S. E., and Dawson, W. O. (1998). Peanut yellow spot virus is a member of a new serogroup of Tospovirus genus based on small (S) RNA sequence and organization. Arch. Virol. 143, 353–364.
Schoelz, J. E., and Stewart, L. R. (2018). The role of viruses in the phytobiome. Annu. Rev. Virol. 5, 93–111.
Seepiban, C., Gajanandana, O., Attathom, T., and Attathom, S. (2011). Tomato necrotic ringspot virus, a new tospovirus isolated in Thailand. Arch. Virol. 156, 263–274.
Seifers, D. L., Harvey, T. L., Martin, T. J., Haber, S., and She, Y.-M. (2005). Association of a virus with wheat displaying yellow head disease symptoms in the Great Plains. Plant Dis. 89, 888–895.
Shimomoto, Y., Kobayashi, K., and Okuda, M. (2014). Identification and characterization of Lisianthus necrotic ringspot virus, a novel distinct tospovirus species causing necrotic disease of lisianthus (Eustoma grandiflorum). J. Gen. Plant Pathol. 80, 169–175.
Simmonds, P., Adams, M. J., Benkõ, M., Breitbart, M., Brister, J. R., and Carstens, E. B. (2017). Consensus statement: virus taxonomy in the age of metagenomics. Nat. Rev. Microbiol. 15, 161–168.
Skewes-Cox, P., Sharpton, T. J., Pollard, K. S., and DeRisi, J. L. (2014). Profile hidden Markov models for the detection of viruses within metagenomic sequence data. PLoS One 9:e105067. doi: 10.1371/journal.pone.0105067
Sõmera, M., Kvarnheden, A., Desbiez, C., Blystad, D., and Sooväli, P. (2020). Sixty years after the first description: genome sequence and biological characterization of European wheat striate mosaic virus infecting cereal crops. Phytopathology 110, 68–79.
Stenger, D., Burbank, L., Wang, R., Stewart, A., Mathias, C., and Goodin, M. (2020). Lost and found: rediscovery and genomic characterization of sowthistle yellow vein virus after a 30+ year hiatus. Virus Res. 284:197987.
Susi, H., Filloux, D., Frilander, M. J., Roumagnac, P., and Laine, A.-L. (2019). Diverse and variable virus communities in wild plant populations revealed by metagenomic tools. PeerJ 7:e6140.
Tatineni, S., McMechan, A. J., Wosula, E. N., Wegulo, S. N., and Graybosch, R. A. (2014). An eriophyid mite-transmitted plant virus contains eight genomic RNA segments with unusual heterogeneity in the nucleocapsid protein. J. Virol. 88, 11834–11845.
Thekke-Veetil, T., Ho, T., Keller, K. E., Martin, R. R., and Tzanetakis, I. E. (2014). A new ophiovirus is associated with blueberry mosaic disease. Virus Res. 189, 92–96.
Torres, R., Larenas, J., Fribourg, C., and Romero, J. (2012). Pepper necrotic spot virus, a new tospovirus infecting solanaceous crops in Peru. Arch. Virol. 157, 609–615.
Verdin, E., Wipf-Scheibel, C., Gognalons, P., Aller, F., Jacquemond, M., and Tepfer, M. (2017). Sequencing viral siRNAs to identify previously undescribed viruses and viroids in a panel of ornamental plant samples structured as a matrix of pools. Virus Res. 241, 19–28.
Villamor, D. E. V., Ho, T., Al Rwahnih, M., Martin, R. R., and Tzanetakis, I. E. (2019). High throughput sequencing for plant virus detection and discovery. Phytopathology 109, 716–725.
von Bargen, S., Al Kubrusli, R., Gaskin, T., Fürl, S., and Hüttner, F. (2020). Characterisation of a novel emaravirus identified in mosaic-diseased Eurasian aspen (Populus tremula). Ann. Appl. Biol. 176, 210–222.
von Bargen, S., Dieckmann, H. L., Candresse, T., Mühlbach, H. P., Roßbach, J., and Büttner, C. (2019). Determination of the complete genome of European mountain ash ringspot-associated emaravirus from Sorbus intermedia reveals two additional genome segments. Arch. Virol. 164, 1937–1941.
Walker, P. J., Blasdell, K. R., Calisher, C. H., Dietzgen, R. G., and Kondo, H. (2018). ICTV virus taxonomy profile: Rhabdoviridae. J. Gen. Virol. 99, 447–448.
Wang, Y.-Q., Song, Y., Cao, M., Cheng, Q., Wu, J., and Hu, T. (2020). Identification of a novel emaravirus infecting lilac through next generation sequencing. J. Integr. Agric. 19, 2064–2071.
Whitfield, A. E., Huot, O. B., Martin, K. M., Kondo, H., and Dietzgen, R. G. (2018). Plant rhabdoviruses: their origins and vector interactions. Curr. Opin. Virol. 33, 198–207.
Willie, K., and Stewart, L. R. (2017). Complete genome sequence of a new maize-associated cytorhabdovirus. Genome Announc. 5:e00591-17.
Wolf, Y. I., Kazlauskas, D., Iranzo, J., Lucia-Sanz, A., Kuhn, J. H., Krupovic, M., et al. (2018). Origins and evolution of the global RNA virome. mBio 9:e02329-18.
Wren, J. D., Roossinck, M. J., Nelson, R. S., Sheets, K., Palmer, M. W., and Melcher, U. (2006). Plant virus biodiversity and ecology. PLoS Biol. 4:e80. doi: 10.1371/journal.pbio.0040080
Wright, A. A., Szostek, S. A., Beaver-Kanuya, E., and Harper, S. J. (2018). Diversity of three bunya-like viruses infecting apple. Arch. Virol. 163, 3339–3343.
Wu, L. P., Yang, T., Liu, H. W., Postman, J., and Li, R. (2018). Molecular characterization of a novel rhabdovirus infecting blackcurrant identified by high-throughput sequencing. Arch. Virol. 162, 2493–2494.
Wu, Q., Ding, S. W., Zhang, Y., and Zhu, S. (2015). Identification of viruses and viroids by next-generation sequencing and homology- dependent and homology- independent algorithms. Annu. Rev. Phytopathol. 53, 425–444.
Wylie, S. J., Tran, T. T., Nguyen, D. Q., Koh, S.-H., and Chakraborty, A. (2019). A virome from ornamental flowers in an Australian rural town cites. Arch. Virol. 164, 2255–2263.
Xin, M., Cao, M., Liu, W., Ren, Y., Zhou, X., and Wang, X. (2017). Two negative-strand RNA viruses identified in watermelon represent a novel clade in the order Bunyavirales. Front. Microbiol. 8:1514. doi: 10.3389/fmicb.2017.01514
Xu, C., Sun, X., Taylor, A., Jiao, C., Xu, Y., Cai, X., et al. (2017). Diversity, distribution, and evolution of tomato viruses in china uncovered by small RNA sequencing. J. Virol. 91:e00173-17.
Yan, T., Zhu, J. R., Di, D., Gao, Q., and Zhang, Y. (2015). Characterization of the complete genome of barley yellow striate mosaic virus reveals a nested gene encoding a small hydrophobic protein. Virology 478, 112–122.
Yang, C., Zhang, S., Han, T., Fu, J., Di Serio, F., and Cao, M. (2019). Identification and characterization of a novel emaravirus associated with jujube (Ziziphus jujuba Mill.) yellow mottle disease. Front. Microbiol. 25:1417. doi: 10.3389/fmicb.2019.01417
Yang, X., Huang, J. L., Liu, C. H., Chen, B., Zhang, T., and Zhou, G. H. (2017). Rice stripe mosaic virus, a novel cytorhabdovirus infecting rice via leafhopper transmission. Front. Microbiol. 7:2140. doi: 10.3389/fmicb.2016.02140
Zhang, S., Huang, A., Zhou, X., Li, Z., and Dietzgen, R. G. (2020). Natural defect of a plant rhabdovirus glycoprotein gene: a case study of virus-plant co-evolution. Phytopathology doi: 10.1094/PHYTO-05-20-0191-FI [Epub ahead of print].
Zhang, Y.-Z., Chen, Y.-M., Wang, W., Qin, X.-C., and Holmes, E. C. (2019). Expanding the RNA virosphere by unbiased metagenomics. Annu. Rev. Virol. 6, 119–139.
Zhang, Y. Z., Shi, M., and Holmes, E. C. (2018). Using metagenomics to characterize an expanding virosphere. Cell 172, 1168–1172.
Zheng, K., Chen, T.-H., Wu, K., Kang, Y.-C., and Yeh, S.-D. (2020). Characterization of a new orthotospovirus from chilli pepper in Yunnan Province, China. Plant Dis. 104, 1175–1182.
Zheng, Y., Navarro, B., Wang, G., Wang, Y., and Yang, Z. (2017). Actinidia chlorotic ringspot-associated virus: a novel emaravirus infecting kiwifruit plants. Mol. Plant Pathol. 18, 569–581.
Keywords: HTS, virus discovery, plant NSR viruses, virosphere, bioinformactics
Citation: Bejerman N, Debat H and Dietzgen RG (2020) The Plant Negative-Sense RNA Virosphere: Virus Discovery Through New Eyes. Front. Microbiol. 11:588427. doi: 10.3389/fmicb.2020.588427
Received: 28 July 2020; Accepted: 27 August 2020;
Published: 16 September 2020.
Edited by:
John Wesley Randles, University of Adelaide, AustraliaReviewed by:
Elvira Fiallo-Olivé, Institute of Subtropical and Mediterranean Horticulture La Mayora, SpainCopyright © 2020 Bejerman, Debat and Dietzgen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolás Bejerman, bmljb2JlamVybWFuQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.