- State Key Laboratory of Crop Stress Biology for Arid Areas, College of Plant Protection, Northwest A&F University, Yangling, China
Gramineous grasses are a large group of species, many of which act as accessory (secondary) host for a large number of rust fungi, including devastating rust pathogens of cereals. Among the rust fungi, some are known to be heteroecious and have a complete macrocyclic life cycle with five types of spores on distinct plant species, but for many others the complete life cycle is unknown. Puccinia achnatheri-sibirici, a rust fungus infecting grasses in the genus Achnatherum, has been known for only its uredinial and telial stages; however, the other (pycnial and aecial) stages have not been identified. In this study, we demonstrate that P. achnatheri-sibirici is a heteroecious, macrocyclic fungus with the sexual stage on barberry (Berberis spp.) through inoculation. Pycnia and aecia were successively produced on the inoculated barberry plants. Inoculation of Achnatherum extremiorientale leaves with aeciospores produced yellow-orange uredinia with high infection types, whereas inoculation of wheat variety Mingxian 169, highly susceptible to Puccinia striiformis f. sp. tritici which causes stripe rust on wheat, produced chlorotic flecks but no uredinia. The ITS sequence analysis of P. achnatheri-sibirici did not match with any sequence in the NCBI database and had the highest homology with 94% compared to Puccinia brachypodii and Puccinia aizazii. Observations of the uredinial, telial, and basidial stages on A. extremiorientale and pycnial and aecial stages on Berberis shensiana using light and scanning electron microscopes revealed its characteristics. Morphological characteristics of urediniospores and teliospores are most similar with those of P. achnatheri-sibirici described in the literature. This study proved (1) the life cycle of P. achnatheri-sibirici as heteroecious and macrocyclic and the alternate host as barberry; (2) the description of life stages in the sexual cycle, especially the morphologies of aecial, pycnial, and basidial stages; and (3) the expansion of knowledge on the rust flora on barberry.
Introduction
Gramineous plants around the globe harbor a large number of rust fungi with a wide range of morphologic and biologic differences (Anikster and Wahl, 1979). More than 380 species have been reported to belong to genera of Puccinia and Uromyces (Anikster and Wahl, 1979). Among the rust fungi on gramineous plants, some are important pathogens of cereal crops, such as Puccinia striiformis which causes stripe rust on wheat, barley, rye, triticale, and other cultivated or wild grasses; Puccinia triticina which causes leaf rust on wheat and grasses; P. hordei which causes leaf rust on barley; Puccinia graminis which causes stem rust on wheat, barley, rye, triticale, oats, and cultivated and non-cultivated grasses; and Puccinia coronata which causes crown rust on cereals and grasses.
Rust fungi belong to a group of obligate parasites that only depend on living plants. Many of them are heteroecious, requiring two unrelated plant species to complete their life cycle. Some heteroecious rust fungi with an asexual stage on gramineous grasses have a sexual stage on the alternate host barberry.
Barberry (Berberis spp.) plants are shrubs and can serve as alternate (aecial) hosts for several Puccinia species infecting cereals and gramineous grasses. So far, barberry spp. have been reported as an alternate host for P. graminis (de Bary, 1866; Roelfs, 1982), P. striiformis and P. pseudostriiformis (syn. P. striiformis f. sp. poae) (Jin et al., 2010), Puccinia brachypodii (Payak, 1965), Puccinia minshanensis (Zhuang and Wei, 1999), P. brachypodii var. poae-nemoralis (syn. Puccinia arrhenatheri, Puccinia poae-nemoralis) (Urban, 1967), Puccinia pygmaea, Puccinia montanesis, and Puccinia brachypodii-phoenicoidis (Cummins and Greene, 1966).
During an investigation on species and geographic distribution of barberry (Berberis spp.) plants and rusts on grasses in October 2013, abundant rust pustules in a mix of uredinia and telia were observed on leaves of a gramineous grass near Xinjie town, Baoji, Shaanxi, China (N34°41′30.09″, E106°52′16.23″; Elevation 1090 m). In November, 2015, uredinial and telial pustules on the same grass species were observed again in the same area. Because the grass plants were growing closely to barberry (Berberis shensiana) shrubs, and barberry plants have had heavy rust infection in the springs during our survey in this region since 2010 (Zhao et al., 2013; Wang et al., 2016), we postulated that the rust on the grass plants and rusts on the barberry plants might be related.
The grass plants were identified as Achnatherum extremiorientale (Hara) Keng ex P. C. Kuo and distributed in northeast, north, and northwest of China (Chu and Yang, 1990; Flora of China, e-version, website: http://www.iplant.cn/foc/). In the literature, the rust species Puccinia achnatheri-sibirici Wang was reported on several species of the genus Achnatherum as hosts, including A. extremiorientale, Achnatherum sibiricum (L.) Keng (Liu et al., 1991, 2015; Wang and Zhuang, 1998), and Achnatherum splendens (Trin.) Nevski (Wang et al., 2018). This rust has a narrow geographic distribution in the world, only in China and Japan (Wang and Wei, 1983, Wang et al., 2018). Teliospores of P. achnatheri-sibirici have a short pedicel or no pedicel, which is a distinguishable characteristics differentiating this species from any other rust fungi infecting Achnatherum and Stipa species (Wang and Zhuang, 1998). However, the rust pathogen was reported to have an incomplete life cycle with only uredinial and telial stages but other spore stages are unknown (Cummins, 1971; Wang and Zhuang, 1998).
The objectives of this study were to (1) determine if barberry is an alternate host for P. achnatheri-sibirici by artificial inoculation and (2) if so describe the morphological ontogeny of this rust fungus infecting A. extremiorientale and B. shensiana through light and scanning electron microscopy.
Materials and Methods
Plant and Pathogen
Plants and seeds of A. extremiorientale were collected at Xinjie town, Baoji, Shaanxi, China, in September 2014. Leaves of A. extremiorientale plants bearing uredinia and telia were sampled in the same region. Seeds of A. extremiorientale and wheat cv. Mingxian 169, which is highly susceptible to all races of P. striiformis f. sp. tritici (the wheat stripe rust pathogen) in China so far (Li and Zeng, 2002), were planted in plastic pots filled with soil mixture composed of turf, perlite, and vermiculite (Inner Mongolia Mengfei Biotech Co., Ltd., Hohhot, Inner Mongolia), and the plants were grown in a rust-free chamber of a greenhouse at 16–20°C and a diurnal 16-h light/8-h dark cycle.
Young plants of barberry (B. shensiana) collected from Taibai, Baoji, Shaanxi Province, were transplanted into pots filled with soil mixture and grown in the greenhouse at 16–20°C and a diurnal cycle of 16-h light/8-h dark.
To obtain isolates of the rust and increase urediniospores, a single uredinium of the grass leaf sample was transferred onto the surface of leaf of the grass A. extremiorientale. The inoculated grass were kept in a dew chamber at 10°C for 24 h in the dark and then moved to a growth chamber at 16–20°C with a diurnal cycle of 16-h light/8-h dark. Urediniospores of the single uredinium were used to increase spores by reinoculation.
Inoculation of Wheat and A. extremiorientale Using Urediniospores of the Grass Rust
Seedlings of wheat cv. Mingxian 169 that had been grown for 10 days after planting and those of A. extremiorientale that had been grown for 30 days after planting were used for inoculation. Approximately 2-mg urediniospores were diluted by adding 2–3 drops (approx. 50–100 μL) of deionized water to make urediniospore suspension. Wheat and grass plants were inoculated using urediniospore suspension of P. achnatheri-sibirici and then kept for 24 h at 10°C in a dew chamber in the dark. The inoculated wheat plants were moved to a growth chamber with temperature set at 13 ± 3°C and a photoperiod of 16 h light/8 h dark. Infection types (ITs) on wheat plants were observed at 15–20 days post inoculation (dpi) using a 0–4 scale (Stubbs, 1985).
Urediniospores of the pure isolate from A. extremiorientale samples that produced low ITs (0, 0; or 1) on wheat but high ITs (3 or 4) on A. extremiorientale were multiplied on A. extremiorientale to produce an adequate amount of urediniospores in a greenhouse set at the same temperature and light conditions as described above for late use. A parallel test was conducted using P. striiformis f. sp. tritici race CYR32 as a positive control. IT “0” indicates no visible symptom, “0;” obvious necrosis or chlorosis without uredinia, and “1” obvious chlorotic or necrotic patches with a trance amount of uredinial sporulation. IT “2” describes large necrotic patches with a moderate amount of sporulation, IT “3” abundant sporulation with some necrosis or chlorosis, and IT “4” abundant sporulation with little or no chlorosis.
To produce teliospores, urediniospores from A. extremiorientale-derived isolates were used to inoculate A. extremiorientale plants at the adult plant stage at the conditions described above. Approximately sixty days after inoculation, leaves bearing telia were harvested and kept at 4°C for late use.
Inoculation of B. shensiana With Basidiospores of the Grass Rust
Inoculation of barberry leaves with basidiospores was conducted following the procedure as previously described (Zhao et al., 2013). Leaves of A. extremiorientale bearing teliospores of P. achnatheri-sibirici within 10 days after harvest were wet–dry treated for 2–3 cycles at 16°C. In each cycle, the leaves bearing telia in a plastic box were soaked in water for 12 h and then dried under 16°C for 12 h. The leaves were cut into segments about 2 cm and placed on the surface of 2% (w/v) water agar medium in a Petri dish kept at 16°C. Teliospores were observed periodically for germination using a light microscope. Once teliospores began to germinate, the Petri dish was put over B. shensiana grown in a pot and kept for 3–4 days at 16°C in a dew chamber. After incubation, the inoculated B. shensiana plants were transferred to a growth chamber with the same conditions as mentioned above. Fertilization was performed by transferring nectar of one pycnium to another using a clean plastic inoculation loop.
The B. shensiana plant was covered for 1–2 days, and then the cover was removed. About 10–18 days after fertilization, aecial cups were excised with a sterile razor blade, and aeciospores were collected for inoculating A. extremiorientale and wheat plants.
Inoculation of A. extremiorientale and Wheat Seedlings With Aeciospores of the Grass Rust
Seedlings of 30-day-old A. extremiorientale and 10-day-old wheat Mingxian 169 were inoculated using a water suspension of aeciospores produced on leaves of B. shensiana using the methods described above for inoculation using urediniospores. ITs were observed 15–20 days post inoculation.
ITS Sequencing
Approximately 5 mg urediniospores produced on A. extremiorientale plants inoculated with aeciospores was placed into a 2-mL micorcentrifuge tube with two small steal balls ground to fine powder using a tissue grinding pestle (MGR 115, Sigma-Aldrich, MO, United States). Genomic DNA was extracted with Biospin Fungus Genomic DNA Extraction Kit (BioFlux, Tokyo, Japan). DNA concentration was measured by using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., DE, United States). Genomic DNA was also extracted from an isolate of race CYR32 of P. striiformis f. sp. tritici in the same way. PCR amplifications of the grass rust DNA, together with DNA of the race CYR32 used as positive control and sterile ion-free water as a negative control, were performed with universal primer pairs ITS1RustF10d/ITS4 (White et al., 1990; Barnes and Szabo, 2007). PCR amplification was conducted according to the same method as described by Barnes and Szabo (2007). PCR products were electrophoresed in 1% (w/v) agarose gel in 1 × TAE buffer (0.04 M Tris–HCl, 0.001 M EDTA pH 8.0) at 3–5 v/cm for 40–60 min. The agarose gel was placed in a 0.1-μg/mL ethidium bromide (EB) solution for staining for 20 min and visualized using a Gel Imaging System (Bio-Rad ChemiDoc XRS, United States). The target band was excised from the gel, and DNA amplicons were collected using a Gel DNA Extraction Kit (BioTeke Corp., Beijing, China). The collected DNA amplicons were cloned by transduction into the pMD19 vector using a T-vector pMD19 (simple) Kit [Takara Biotechnology (Dalian) Co., Ltd, Japan] and sequenced by Aoke Biotechnology Co., Ltd. (Beijing, China). We sequenced a single clone for each type spore and repeated it three times. For each type of spore, we choose one of the repeats for sequence analysis. All the sequences of P. achnatheri-sibirici were deposited in GenBank under accession numbers MN913585, MN913586, and MN913598, while the accession numbers deposited in GenBank of P. brachypodii (on Brachypodium distachyon) analyzed in this study were MN915123 and MN915138. The datasets analyzed for this study can be found in the NCBI GenBank1.
DNA sequences were blasted at the NCBI database (see footnote 1) for determination of the rust fungus. Sequence alignment was implemented using the BioEdit program2. Phylogenetic tree procedures of P. achnatheri-sibirici and other species in the genus Puccinia downloaded from the NCBI database (Table 1) were constructed based on ITS sequences using maximum parsimony analysis in software PAUP∗4.0b10 (Swofford, 2002).
Morphology of P. achnatheri-sibirici
To observe the morphological characteristics of P. achnatheri-sibirici spores, leaf samples of uredinia and telia were harvested from A. extremiorientale, together with leaf samples of pycnia and aecia from barberry. Approximately 3-cm A. extremiorientale leaf segments with uredinia and/or telia and 2 cm × 3 cm barberry leaf pieces with pycnia or aecia were detached. All leaf samples from both hosts were used for histological observations using a light and/or electron microscope. Leaf sections were fixed in phosphate buffer (pH 7.4) of a final concentration of 0.1 M with addition of 2.5% (v/v) glutaraldehyde at 4°C overnight. The samples were then dehydrated using a series of grade ethanol solution and substituted with isoamyl acetate. After CO2 critical-point drying, the samples were sputter coated with wolfram and observed using a scanning electron microscope (SEM; Hitachi S-4800, Tokyo, Japan) at 10 kV.
Urediniospores and teliospores of P. achnatheri-sibirici were scratched from leaf segments and put into a drop (10–20 μL) of ion-free water on a glass slide with a glass cover slip and observed morphologically using a light microscope (Olympus BX53, Tokyo, Japan). We measured the length, width, wall thickness, apex thickness, and pedicels of teliospores and the size of basidiospores by software cellSens Imaging coupled with a microscope. Urediniospores and teliospores were observed morphologically and identified according to recorded gramineous rust species (Wang and Wei, 1983).
Treatments of teliospore germination to produce basidiospores were conducted according to the method described by Zhao et al. (2013).
Results
Morphology of A. extremiorientale and Rust Infection
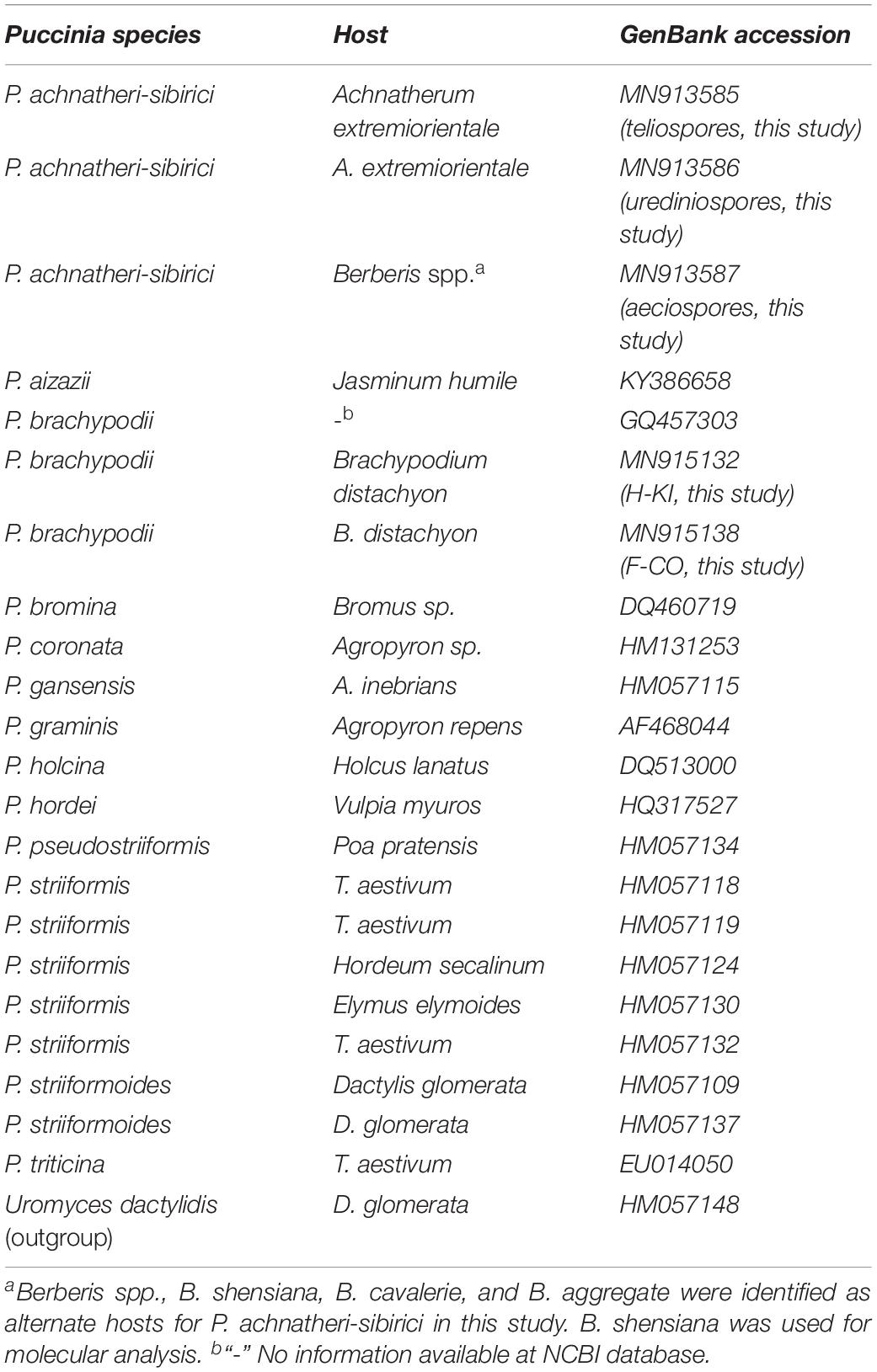
Achnatherum extremiorientale is a perennial gramineous grass, belonging to the tribe of Stipeae, the subfamily of Pooideae, and the family of Gramineae. This grass grows in a cluster (Figure 1A) and has a wide long leaf (up to 50 cm) often rolling upward at the margin, panicle with 3–6 branches and 20–40 cm in length, and fusiform caryopsis approximately 4 mm in length (Figures 1B,C).
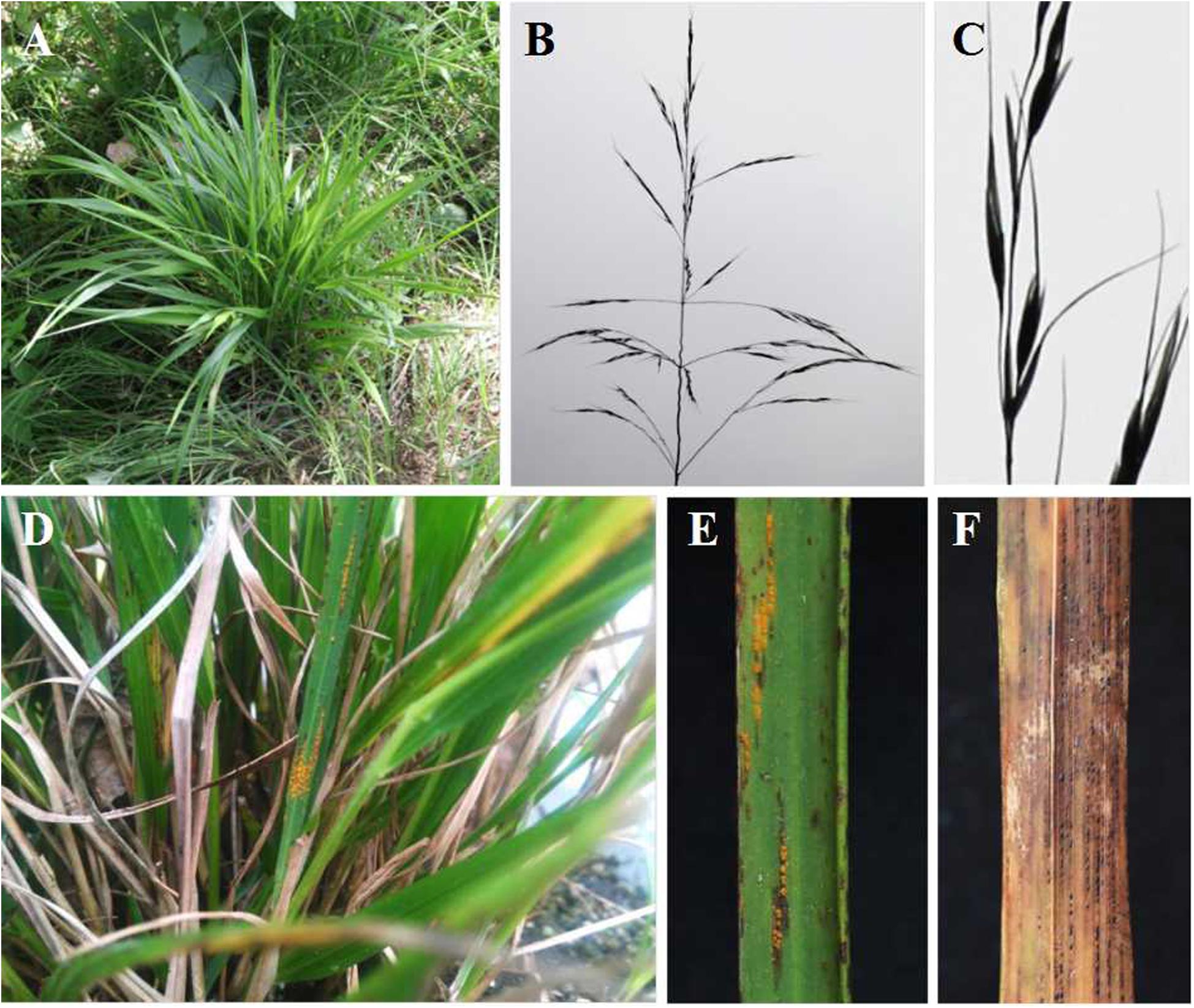
Figure 1. Morphology of gramineous grass, Achnatherum extremiorientale, and natural rust infection on the grass species by Puccinia achnatheri-sibirici, observed in Xinjie, Baoji, Shaanxi Province, in October 2013. (A–C) Plants, heads, and seeds of Achnatherum extremiorientale. (D–F) Uredinial and telial symptoms of natural infection of P. achnatheri-sibirici on grass A. extremiorientale, respectively.
Under natural conditions, A. extremiorientale plants infected with P. achnatheri-sibirici produced yellow- to brown-colored uredinia on both sides of a leaf we observed in fall (Figure 1D). The uredinia broke through the epidermis of the infected leaves presenting powdery pustules with obvious black necrosis around/on uredinia (Figure 1E). During the development of uredinia, black telia were produced and embedded underneath the epidermis (Figure 1F).
B. shensiana Serves as an Alternate Host for P. achnatheri-sibirici
After inoculation of B. shensiana with basidiospores that are produced from germinated teliospores of P. achnatheri-sibirici, pycnia appeared on the surface of the leaves at 7–9 dpi (Figure 2A). After fertilization, orange-colored aecial cups, variable in number, began to appear on the opposite side of the leaf from the pycnia at 11–16 dpi (Figure 2B).
Figure 2. Pycnial and aecial stages of Puccinia achnatheri-sibirici on barberry (Berberis shensiana) after infection by basidiospores and responses of Achnatherum extremiorientale and wheat cv. Mingxian 169 to aeciospores. (A) Pycnial stage of P. achnatheri-sibirici infecting the barberry (B. shensiana Ahrendt) after inoculation with basidiospores. (B) Aecial formation on the infected barberry. (C) The grass, Achnatherum extremiorientale, presenting orange uredinia on leaves after inoculation using P. achnatheri-sibirici aeciospores produced on the barberry in Figure 2B. (D) Black stripes formed and restricted between leaf veins of the grass. (E) Wheat cv. Mingxian 169 showed obvious chlorotic lesions after inoculation with aeciospores of P. achnatheri-sibirici. (F) Non-inoculated leaves of Mingxian 169 wheat used as control.
Inoculation of A. extremiorientale and wheat Mingxian 169 plants with aeciospores produced orange-colored uredinia accompanied with longitudinal black stripes around the pustules (Figures 2C,D). Symptoms produced by artificial inoculation with aeciospores were similar to that of rust infection on the grass under natural conditions (Figure 1E). However, inoculation of Mingxian 169 with aeciospores produced only chlorotic necrosis lesions with IT 0; (Figure 2E) in contrast to healthy leaves of wheat (Figure 2F).
Molecular Data
The ITS region of P. achnatheri-sibirici amplified with ITS1RustF10d/ITS4 primers produced a 541-bp amplicon. BLAST research of the NCBI database3 did not identify any sequences sharing above 95% homology. Top five Puccinia species sharing relatively high homologies included Puccinia aizazii (on Jasminum humile, 94%), P. brachypodii (92% or 94%), P. striiformis (on Dactylis glomerata, 92%), P. striiformoides (92%), and P. striiformis f. sp. tritici (on wheat, 91%). Phylogentic analysis of the ITS region indicated that teliospores on leaves of A. extremiorientale, aeciospores produced on B. shensiana, and urediniospores produced on the grass inoculated with the aeciospores were clustered into a clade with a bootstrap value of 92%, strongly distinguished from the other rust species used in the present study (Figure 3).
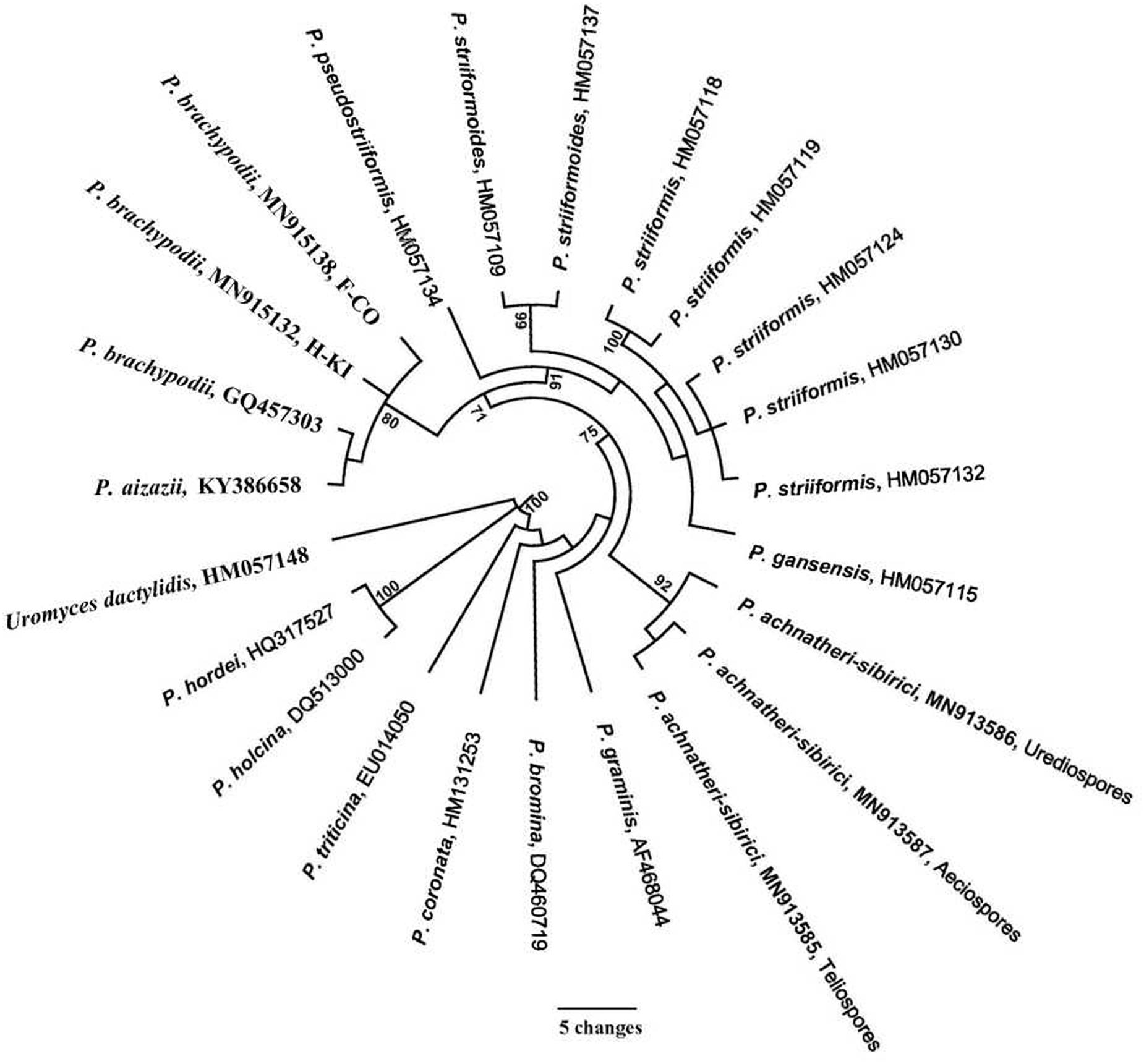
Figure 3. Phylogenetic tree of Puccinia achnatheri-sibirici and Puccinia species based on the sequence of nuclear ribosomal internal transcribed spacer (ITS) amplified with primer ITS1RustF10d/ITS4 (White et al., 1990; Barnes and Szabo, 2007). Clade robustness was assessed using a bootstrap analysis with 1,000 replicates. The numbers on the branches are bootstrap values from MP. Reference sequences used in this analysis are listed in Table 1.
Based on the tree in Figure 3, P. brachypodii is more closely related to the P. striiformis species complex. So, a cross-validation experiment was conducted. Two P. brachypodii isolates H-KI and F-CO which infect B. distachyon were used to inoculate grass A. extermiorientale, producing low infections with merely light necrosis. Similarly, B. distachyon represented a highly resistant reaction to P. achnatheri-sibirici after inoculation. This result indicated that the rust pathogen P. achnatheri-sibirici is not P. brachypodii, supporting the results of P. achnatheri-sibiri and P. brachypodii grouped into two subclades (Table 1 and Figure 3).
Spore Development and Morphology
After inoculation, B. shensiana leaves infected with P. achnatheri-sibirici basidiospores produced light yellow lesions 7–9 dpi, which continue to expand to about 3–5 mm in diameter after 18–20 dpi (Figure 2A). Simultaneously, pycniospores, paraphyses, and flexuous hyphae were produced in pycnia under the epidermis of barberry leaves, subsequently broke through the epidermis (Figure 4A), and congested around the ostioles of pycnia as the production of numerous pycniospores in succession (Figure 4B). The paraphyses and flexuous hyphae radiated to separate during the development of pycnia (Figures 4B,C). Mature pycniospores were oval and smooth-surfaced, 4.3 (3.5–5.0) μm-1.5 (1.3–1.7) μm in size (Figure 4D). Macroscopically, drops of hyaline nectars (honeydews) were formed on the ostioles.
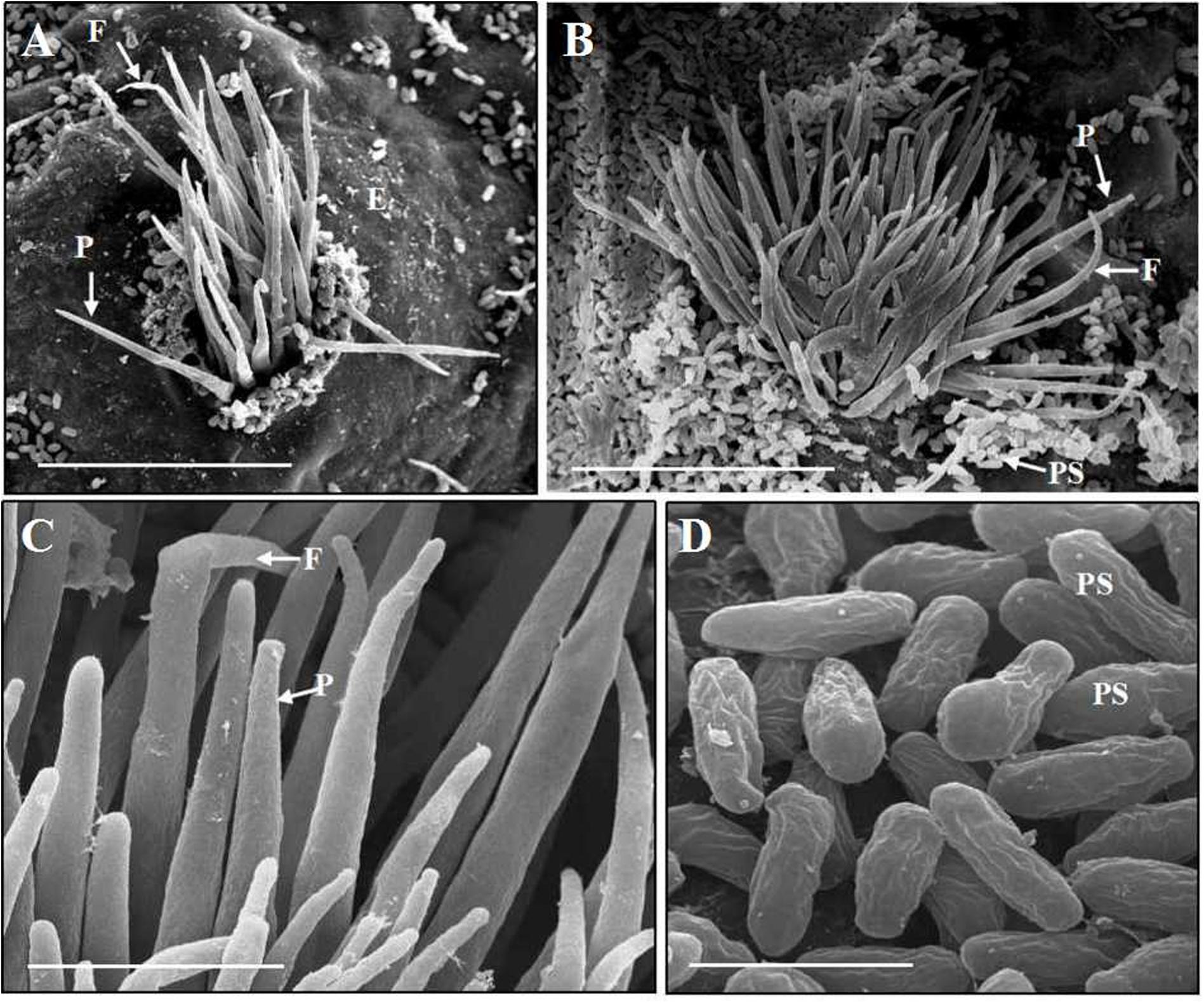
Figure 4. Scanning electron microscopy observations of Puccinia achnatheri-sibirici infecting Berberis shensiana Ahrendt at the pycnial stage. (A) A young pycnium at the early stage with eruption from epidermis (E) of the host, producing paraphyses (P) and flexuous hyphae (F). Bar = 50 μm. (B) Paraphyses (P) and flexuous hyphae (F) of a mature pycnium radiated on the ostiole of the pycnium with production of numerous pycniospores congesting around the opening. Bar = 50 μm. (C) Enlargement of the paraphyses (P) and flexuous hyphae (F). Bar = 5 μm. (D) Mature pycniospores (PS). Bar = 5 μm.
After fertilization, young aecia formed on the opposite surface, about 20 dpi (Figure 5A). Young aecia first appeared as dome-shaped and then developed into long finger-shaped horns (Figures 2B, 5B,C). As the aecium matured in the peridium, it split open and released aeciospores (Figure 5B). A ripe aeciospore was spherosome-shaped, having several germ pores and the ornaments (verrucae) consisting of a great number of short columns on the surface (Figures 5D–F).
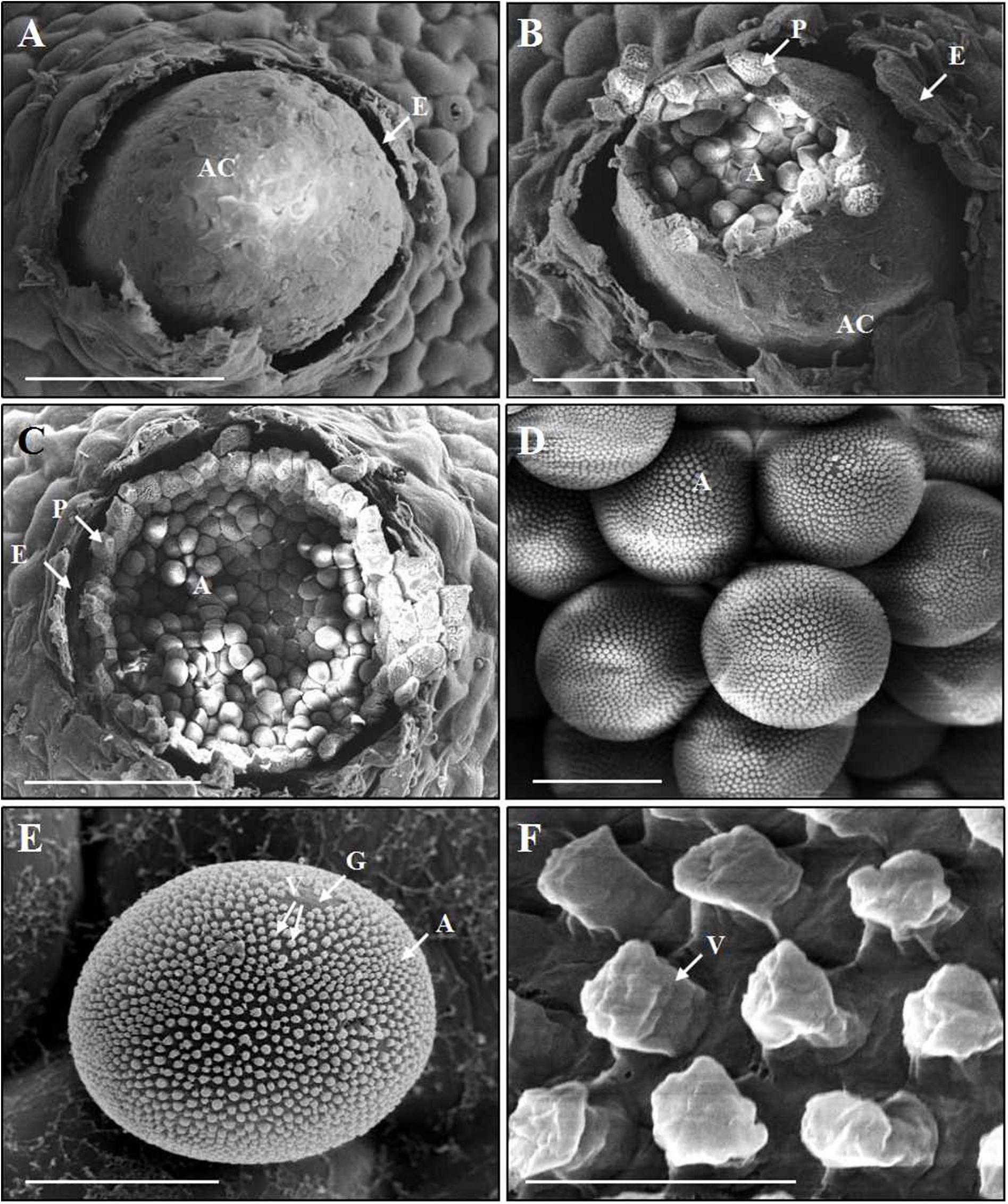
Figure 5. Scanning electron microscopy observations of the development of aecia and aeciospores of Puccinia achnatheri-sibirici infecting Berberis shensiana at the aecial stage. (A) An initial aecial cup (aecium, A) erupted from the epidermis (E) of the host. Bar = 100 μm. (B,C) A mature aecial cup (AC) broke and released aeciospores (A) embraced by a layer peridium (P) that was formed by peridial cells. Bar = 100 μm. (D,E) A mature aeciospore (A) was spherosome-shaped and has germ pores (G) and the numerous, column-shaped ornaments (verrucae, V) on the surface. Bar = 10 μm. (F) Close-up of verrucae (V). Bar = 1 μm.
After 18–25 dpi, aeciospores infected A. extremiorientale leaves to produce yellow uredinia (Figures 2C,D). Light and scanning electron microscopic observations showed that young urediniospores differentiated from the apex of long sporophores and surface-smoothed at the early stage (Figures 6A–C), becoming orange-colored, near sphere-shaped, mature urediniospores with numerous ornaments on the surface (Figures 6D–F). The ornament was broken through the surface of a urediniospore to produce a cone (thorn) in shape beyond the surface. The cone towered in the middle of the opening, looking like a crater of a volcano in shape (Figure 6G).
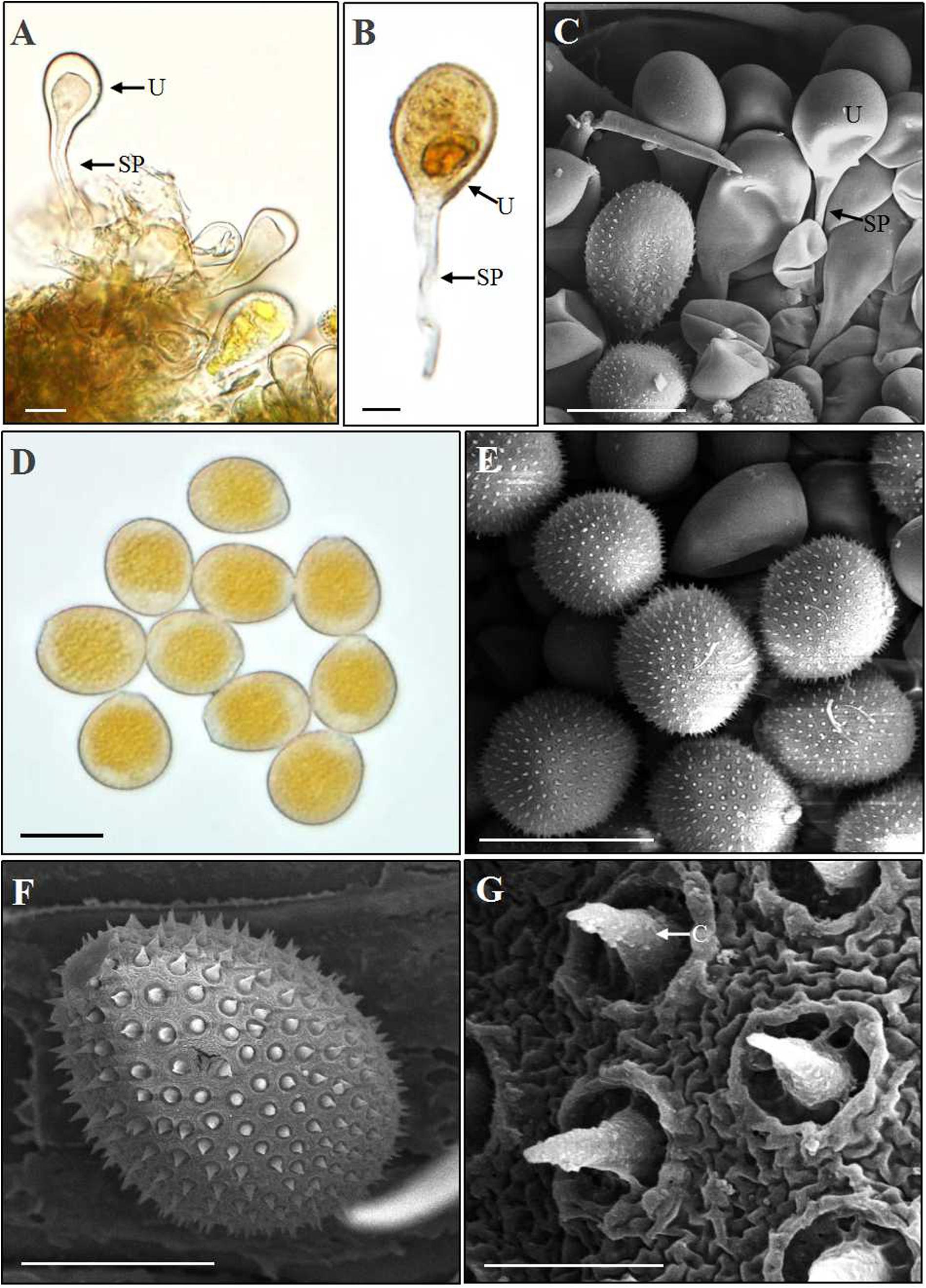
Figure 6. Light and scanning electron microscopy observations of urediniospores of Puccinia achnatheri-sibirici. (A–C). A young urediniospore (U) developing at the top of a long sporophore (SP) formed in a uredinium based on observations using light and scanning electron microscopy. Bar = 20 μm. (D) Light microscopy observation showing orange and near sphere-shaped urediniospores. Bar = 20 μm. (E) Scanning electron microscopy (SEM) observation presenting urediniospore production in a uredinium. Bar = 20 μm. (F) SEM observation showing a mature urediniospore with numerous cones on the surface. Bar = 10 μm. (G) Enlargement of the cones (C) on the surface of a urediniospore. Bar = 1 μm.
At the late stage of uredinial development, hyphae growing in a uredinium began to differentiate to form young dark-brown teliospores (Figures 7A,B). The young teliospores continuously developed to give rise to mature teliospores, which were two-celled and had or had no constriction at the connection (septum) of the two cells and are dark brown in color. The color of the upper cell was darker than that of the lower cell.
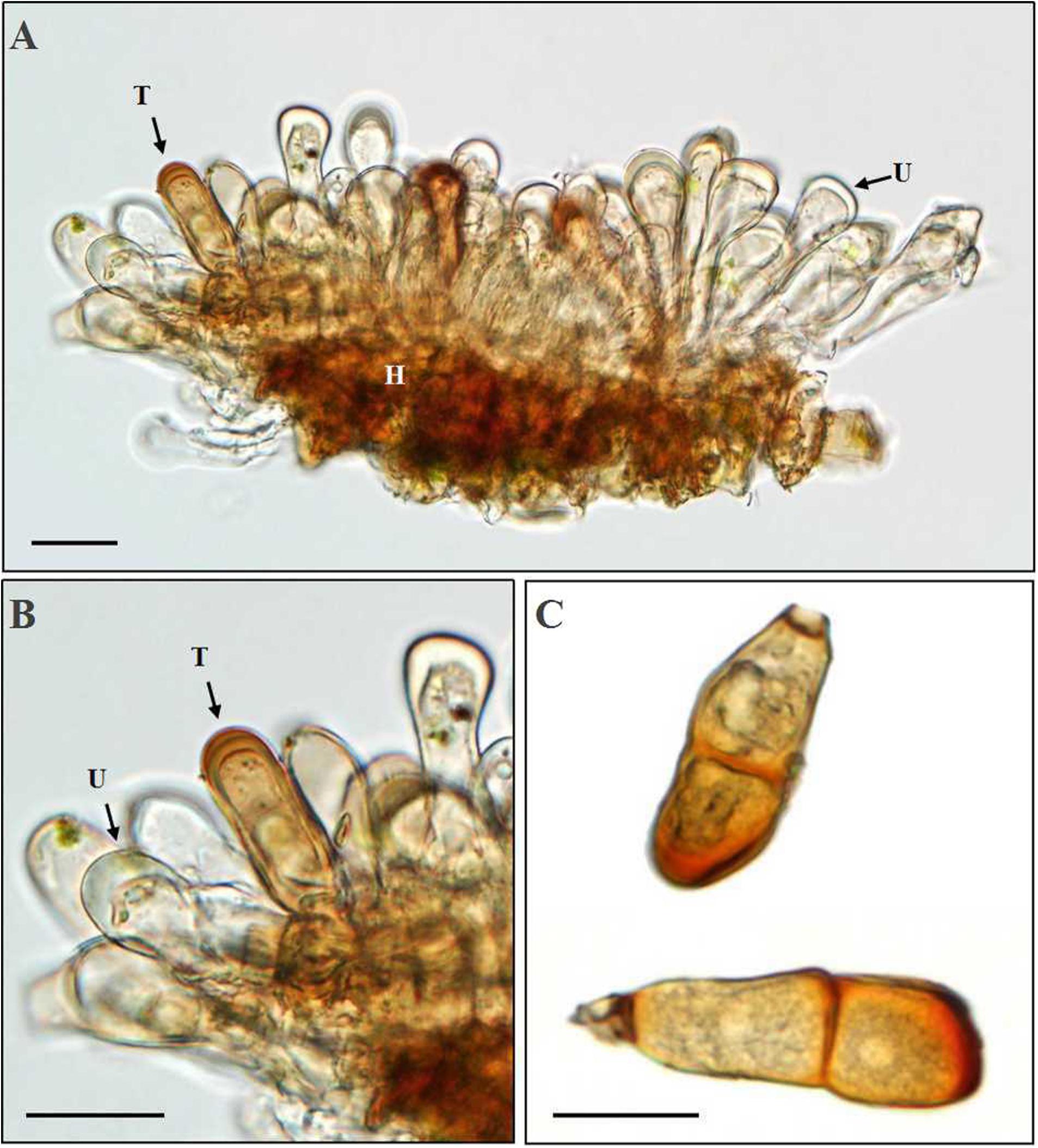
Figure 7. Morphologic observation of Puccinia achnatheri-sibirici teliospores by light microscopy. (A,B) Production of a teliospore (T) in a uredinium (U). Bar = 20 μm. (C) Mature teliospores with/without a short stalk cell. Bar = 20 μm.
Based on the data of measurement of 105 individual teliospores, teliospores generally were two-celled, with a short pedicle and brown-colored. The color of the upper cell of a teliospore was darker than that of the lower cell. The top of teliospores were nearly domed with a thick and dark-brown cell wall. The two-celled teliospores were 40.84 (27.64–57) μm long and 20.46 (7.83–29.11) μm wide. The thickness of the side cell wall were 1.73 (0.52–3.43) μm, and that of the apical cell wall was 4.07 (1.63–6.28) μm. Pedicels were short and averaged 4.85 (0.84–8.36) μm in length and light brownish in color. Sometimes, one-celled teliospores could be observed under microscopic observations (Figure 7C). Morphologic comparisons were made between with teliospores of the rust and species of the genus Puccinia-infecting gramineous plants described by Cummins (1971). The rust pathogen infecting the grass A. extremiorientale in morphological characteristics, similar to P. achnatheri-sibirici descried by Wang and Wei (1983) and Cummins (1971), was confirmed as P. achnatheri-sibirici. The differences among the rust fungi on teliospore morphology infecting Achnatherum and Stipa are shown in Table 2.
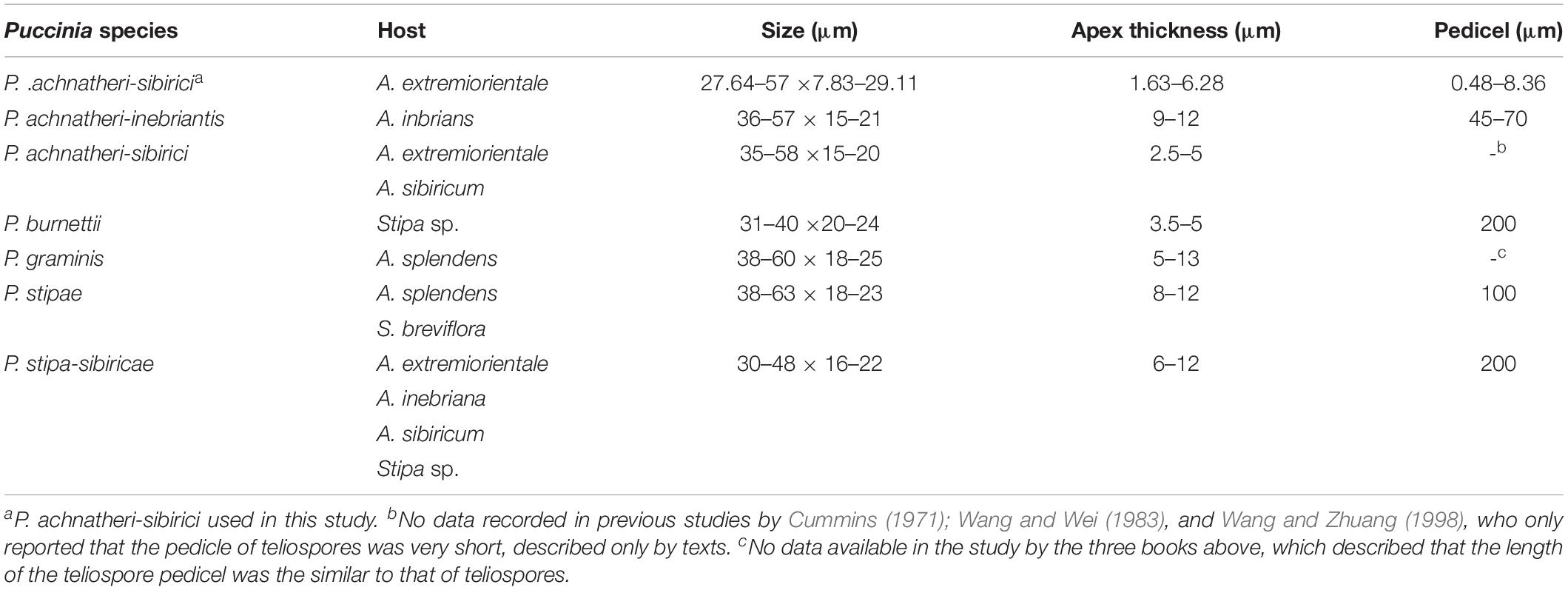
Table 2. The characteristics of teliospores of different rust fungi infecting Achnatherum and Stipa.
Teliospores started to germinate at 4 h after being transferred on the surface of the water agar, producing a tube-shaped metabasidium (promycelium) with migration of cellular contents and the nucleus from a cell of the P. achnatheri-sibirici two-celled teliospore into the top of the metabasidium (Figure 8A). With the extension of the metabasidium, a septum formed inside the metabasidium, separating from cellular contents and the nucleus from the basal cell (Figure 8B). Two daughter nuclei formed inside the top of the metabasidium after meiosis I and meiosis II continued to form a four-celled metabasidium separated by four speta inside (Figures 8C,D). A sterigma produced from each of four cells of a metabasidium and a basidiospore formed at the tip of the sterigma (Figure 8E). Basidiospores were tiny, ovoid in shape, and single-celled with the size of 13.4 (11.4–15.9) μm in length and 9.5 (7.4–11.5) μm in width based on measurement data of 102 basidiospores (Figure 8F). A metabasidium can be produced from each of two cells of a teliospore of P. achnatheri-sibirici (Figure 9A). Occasionally, formation of a secondary basidiospore at the tip of a primary basidiospore can be observed (Figure 9B).
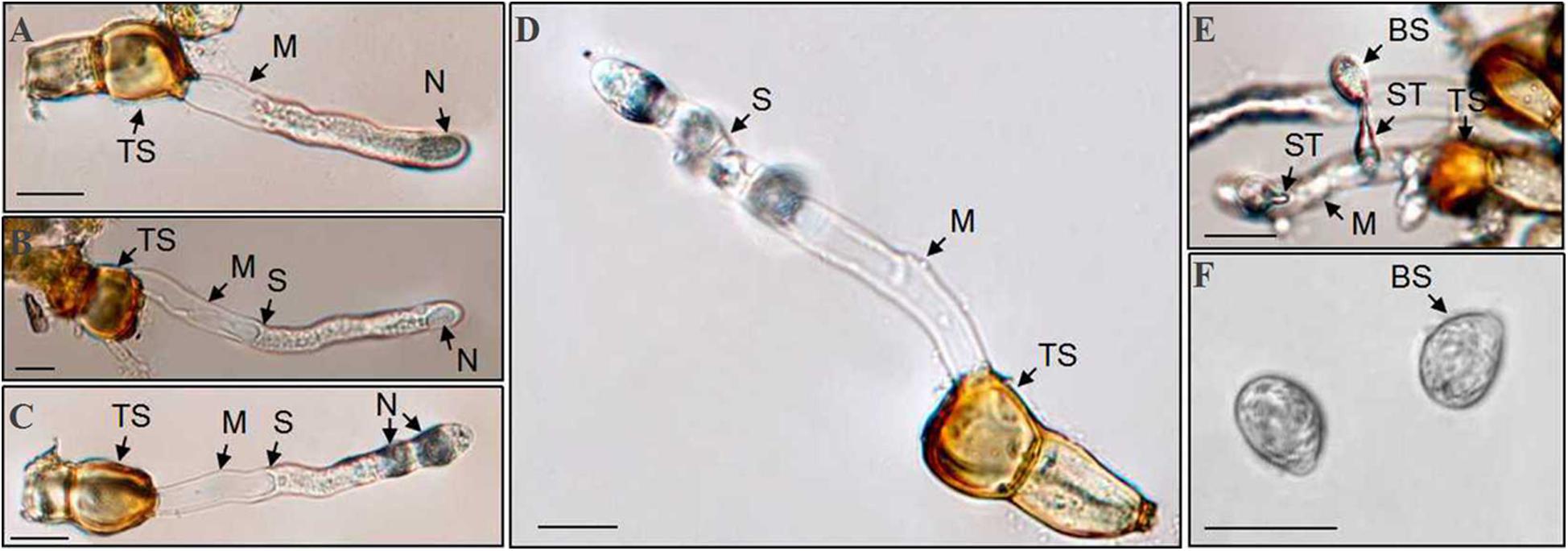
Figure 8. Puccinia achnatheri-sibirici teliospores (TS) germinated to produce basidiospores on the water agar medium. (A) Migration of nucleus (N) and cellular contents from the top cell of a two-celled teliospore (TS) into the top of a metabasidium (M) at the early stage of teliospore germination. (B) Formation of a septum (S) separated the top of a metabasidium (M) containing the nucleus (N) from the vacant basal cell of the metabasidium developed from a two-celled teliospore (TS). (C) Meiosis I showing formation of two daughter nuclei (N) at the top of a metabasidium (M) derived from a teliospore (TS). (D) Meiosis II showing formation of a four-celled metabasidium (M) separated by four speta (S). (E) A basidiospore (BS) growing at the tip of a sterigmata (ST) developed from a metabasidium (M). (F) Morphological single-celled and ovoid-shaped basidiospores (BS). Bars = 50 μm.
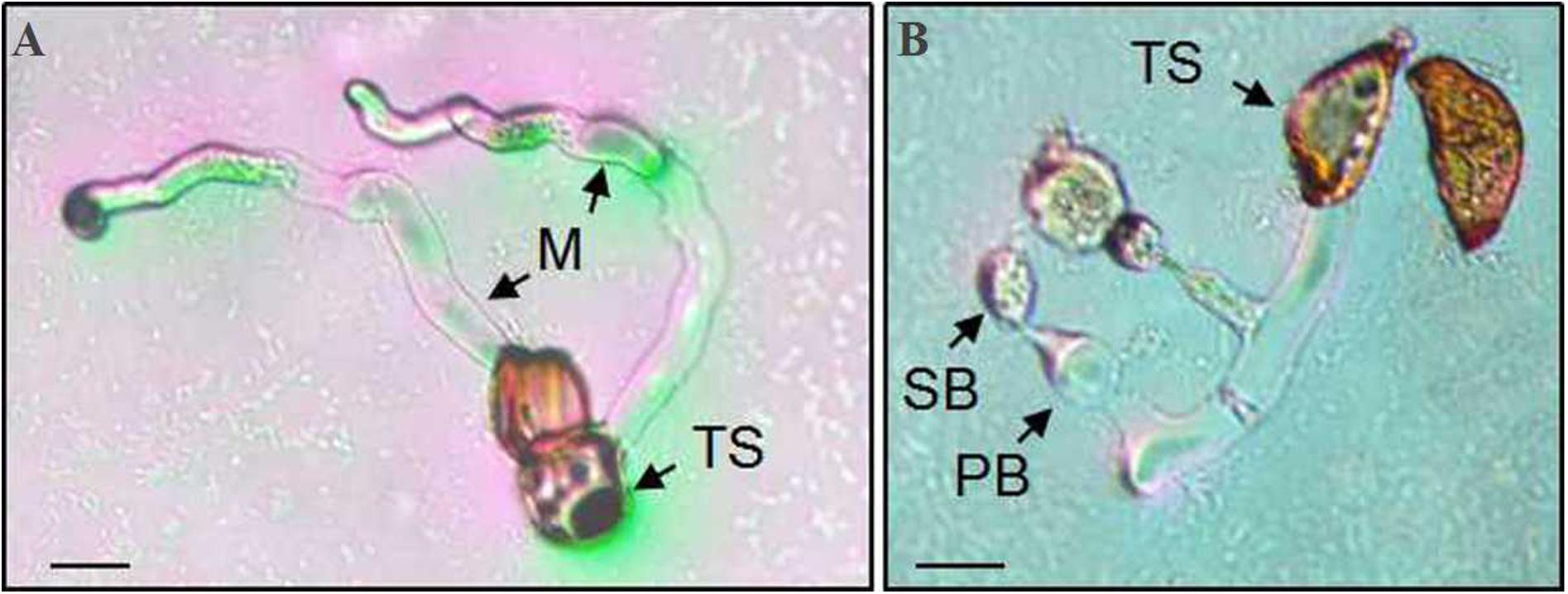
Figure 9. A Puccinia achnatheri-sibirici teliospore (TS) showing each of two cells could germinate to produce a metabasidium. Panel (A) showing that each cell of a two-celled teliospore (TS) of Puccinia achnatheri-sibirici can germinate to produce a metabasidium (M). Bars = 50 μm. (B) Formation of secondary basidiospores (SB) at the tips of primary basidiospore (PB). Bars = 50 μm.
Discussion
We demonstrated that P. achnatheri-sibirici is a heteroecious, macrocyclic rust fungus having five spore stages and B. shensiana can serve as an aecial host. This species, infecting gramineous grass A. extremiorientale, differs from any other rust species on Achnatherum spp. recorded (Cummins, 1971; Wang and Wei, 1983; Wang and Zhuang, 1998).
We also have tested other four Berberis species, including Berberis phanera, Berberis aggregata, Berberis soulieana, and Berberis cavaleriei, by inoculation using P. achnatheri-sibirici basidiospores developed from teliospores in the same conditions used in the present study. Based on three repeat tests, only two of them, B. cavalerie and B. aggregate, can be infected by P. achnatheri-sibirici and the rust fungus completed the whole sexual cycle on the two Berberis species. However, B. phanera and B. soulieana were not infected by P. achnatheri-sibirici, showing resistance to the rust fungus. In China, there are rich Berberis species resources and more than 250 Berberis species were recorded in the Chinese botanic monograph Flora of China (English E-version at website http://www.iplant.cn/foc/). This indicated that more Berberis species could serve as potential aecial hosts for the rust fungus.
Various Achnatherum species are infected by multiple rust fungi in the genus Puccinia, including P. achnatheri-sibirici. Some of them are morphologically similar to P. achnatheri-sibirici and have known aecial hosts. For example, P. achnatheri-inebriantis Z. Y. Zhao, a similar rust species to P. achnatheri-sibirici, was distinguished from the latter by its teliospores that have a long-stalk cell and a thick cell wall on the top of the upper cell. However, the aecial host of P. achnatheri-inebriantis is Aster altaicus Willd., which is not closely related to Berberis (Zhao, 1985).
Sexual reproduction is a vital stage of the life cycle of macrocyclic, heteroecious species of rust fungi and plays roles in surviving unfavorable climatic conditions, providing initial inoculum and/or generating diverse populations (Zhao, 2017). Barberry serves as an aecial host for several Puccinia species. Different formae speciales of P. graminis complete the sexual hybridization on barberry (de Bary, 1866; Johnson et al., 2006). Thus, barberry serves as a bio-platform for virulence recombination by nuclear heteromixis and reassembly at the sexual stage, which can greatly increase the virulence diversity of the progeny population after sexual hybridization. It is not clear whether P. achnatheri-sibirici could carry out sexual hybridization with other Puccinia species on susceptible Berberis species as their common aecial hosts. Recently, under laboratory conditions, we tried to make inter-species cross between P. achnatheri-sibirici and P. striiformis f. sp. tritici on barberry which successfully produced aeciospores. Currently, we are conducting studies to characterize the aecial and uredinial progenies.
In China, there are 1150 species belonging to 193 genera in Gramineae, which could be infected by 129 rust species (Wang and Wei, 1983). Sexual stages have been reported for many species in the genus Puccinia. However, sexual stages have not been found for most of the species (Mains, 1933; Wang and Wei, 1983; Wang and Zhuang, 1998). Therefore, we need to persevere in collecting Puccinia species infecting the grasses, belonging to hemicyclic rust fungi, to identify existence or absence of these rust parasites. This will be beneficial in understanding the diversity of the rust fungi in Puccinia and providing an insight into the evolution of Puccinia species.
Germination of basidiospores can be conducted in two ways. The basidiospore produces a germ tube directly or undergoes a process like sterigma, in which the sporidium (secondary basidiospore) is borne on the tip, called indirect germination (repetitive germination). It depends on environment conditions and the surface of hosts (Gold and Mendgen, 1991). Repetitive germination is common as the basidiospores survive in water or on the water agar (Mims and Richardson, 1990). Some scientists consider that it is an escape mechanism facing unseemly conditions (Alexopoulos et al., 1996).
Formation of secondary basidiospores in some of rust fungi is completed in different environmental conditions and temperatures. Biological phenomena on the production of secondary basidiospores derived from germinated teliospores have been reported in water in Gymnosporangium fuscum (Metzler, 1982), or in both water and air in Gymnosporangium, Coleosporium, Cronartium, Phragmidium, Puccinia, Melampsora, and Uromyces appendiculatus (Bauer, 1987; Freytag et al., 1988). This also was observed on the water agar in Melampsora larici-populina (Gong et al., 2015), Coleosporium phellodendri (Wei et al., 2013), and P. achnatheri-sibirici observed in this study. Secondary basidiospores can be produced at a range of temperatures and are reported in Melampsora larici-populina (Gong et al., 2015), C. phellodendri (Wei et al., 2013), and M. larici-tremulae (Shang and Pei, 1984). In addition, formation of tertiary spores has been reported in some rust fungi (Shang and Pei, 1984; Wei et al., 2013; Gong et al., 2015), but not in P. achnatheri-sibirici.
The pathogenicity of secondary basidiospores similar to or stronger than those of primary basidiospores has been reported in Cronartium quercuum f. sp. fusiforme (Roncadori, 1968) and Gymnosporangium haraeanum (Yu, 2009).
This study demonstrates that Berberis spp. are able to serve alternate hosts of P. achnatheri-sibirici on A. extremiorientale and showed that this rust species are heteroecious, macrocyclic rust fungi with five types of spores. The study also expands the knowledge on rust fungi that have Berberis species serving as alternate hosts.
Data Availability Statement
The datasets generated for this study can be found in the National Center for Biotechnology Information. MN913585, MN913586, MN913587, MN915132, and MN915138.
Author Contributions
JZ found the material of this study in the field and designed the study. ZK provided project support and platform for this study. XM, YL, XT, ZD, and QL performed the experiments. XM performed the statistical analysis. XM and JZ wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was finically supported by the National Key Technology R&D Program (2018YFD0200500), the Natural Science Basic Research Plan in Shaanxi Province of China (2020JZ-15 and 2017JM3006), the National Natural Science Foundation of China (31071641), the Fundamental Research Funds for the Central Universities (2452019046), and the Program for New Century Excellent Talents in University (NCET-13-0490).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Xianming Chen at USDA-ARS, Wheat Health, Genetics, and Quality Research Unit, and Department of Plant Pathology, Washington State University, Pullman 99164-6430, U.S.A., for critically reviewing this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01278/full#supplementary-material
Footnotes
- ^ http://www.ncbi.nlm.nih.gov/
- ^ http://www.mybiosoftware.com/bioedit-7-0-9-biological-sequence-alignment-editor.html
- ^ https://blast.ncbi.nlm.nih.gov/Blast.cgi
References
Alexopoulos, C. J., Blackwell, M., and Mims, C. W. (1996). “Basidiomycota,” in Introductory Mycology, ed. C. Donovan, (Hoboken, NJ: John Wiley & Sons Inc), 544.
Anikster, Y., and Wahl, I. (1979). Coevolution of the rust fungi on gramineae and liliaceae and their hosts. Ann. Rev. Phytopathol. 17, 367–403. doi: 10.1146/annurev.py.17.090179.002055
Barnes, C. W., and Szabo, L. J. (2007). Detection and identification of four common rust pathogens of cereals and grasses using real-time polymerase chain reaction. Phytopathology 97, 717–727. doi: 10.1094/phyto-97-6-0717
Bauer, R. (1987). Uredinales-germination of basidiospores and pycnospores. Stud. Mycologia 30, 111–125.
Chu, Q. G., and Yang, Y. C. (1990). Studies on the classification and morphological evolution of the genus Achnatherum in China. J. Laiyang Agri. Coll. 7, 282–290.
Cummins, G. B. (1971). The Rust Fungi of Cereals, Grasses and Bamboos. New York, NY: Springer, doi: 10.1007/978-3-642-88451-88451
Cummins, G. B., and Greene, H. C. (1966). A review of the grass rust fungi that have uredinial paraphyses and aecia on Berberis-Mahonia. Mycologia 58, 702–721. doi: 10.2307/3756846
de Bary, H. A. (1866). “Neue Untersuchungen über die Uredineen, Insbesondere,” in Die Entwicklung der Puccinia Graminis Und Den Zusammenhang Derselben Mit Aecidium Berberidis (New Researches into the Uredinales, in Particular The Development Of Puccinia Graminis And Its Connection With Aecidium Berberidis Monatsberichte der Koniglichen Preussichen). Berlin: Akademie der Wissenschaften.
Freytag, S., Bruscaglioni, L., Gold, R. E., and Mendgen, K. (1988). Basidiospores of rust fungi (Uromyces species) differentiate infection structures in vitro. Exper. Mycol. 12, 275–283. doi: 10.1016/0147-5975(88)90042-90044
Gold, R. E., and Mendgen, K. (1991). “Rust basidiospore germlings and disease initiation,” in Fungal Spore and Fisease Initiation in Plants and Animals, eds G. T. Cole and H. C. Hoch, (New York, NY: Plenum), 67–99. doi: 10.1007/978-1-4899-2635-7_4
Gong, W. C., Weng, Y. T., Wang, S. J., and Xue, Y. (2015). Germination pattern of Melampsora larici-populina basidiospores. J. Northeast Forest. Univ. 43, 121–123.
Jin, Y., Szabo, L. J., and Carson, M. (2010). Century-old mystery of Puccinia striiformis life history solved with the identification of Berberis as an alternate host. Phytopathology 100, 432–435. doi: 10.1094/PHYTO-100-5-0432
Johnson, T., Newton, M., and Brown, A. M. (2006). Hybridization of Puccinia graminis tritici with Puccinia graminis secalis and Puccinia graminis agrostidis. Can. J. Plant Pathol. 28, S78–S91. doi: 10.1080/07060660609507366
Liu, T. Z., Tian, H. M., and Hou, Z. S. (2015). Rust fungi in inner mongolia II. species of Puccinia. J. Inner. Mongol. Univ. 46, 277–291.
Liu, W. C., Song, Z. Q., and Bai, J. K. (1991). Taxonomic studies on the genus Puccinia in the Northeastern region of China. J. Shenyang Agri. Univ. 22, 305–311.
Mains, E. B. (1933). Studies concerning heteroecious rusts. Mycologia 25, 407–471. doi: 10.2307/3754016
Metzler, B. (1982). Untersuchungen an heterobasidiomyceten(23): basidiosporenkeimung und infectionsvorgang beim birnengitterrost. Phytopath. Z. 103, 126–138.
Mims, C. W., and Richardson, E. A. (1990). Ultrastructure of secondary spore formation in the rust Gymnosporangium Juniperi-Virginianae. Micrologia 82, 236–242. doi: 10.1080/00275514.1990.12025869
Payak, M. M. (1965). Berberis as the aecial host of Puccinia brachypodii in Simla Hills (India). J. Phytopathol. 52, 49–54. doi: 10.1111/j.1439-0434.1965.tb02165.x
Roelfs, A. P. (1982). Effects of barberry eradication on stem rust in the United States. Plant Dis. 66, 177–181. doi: 10.1094/PD-66-177
Roncadori, R. W. (1968). The pathogenicity of secondary and tertiary basidiospores of Cronartium fusiforme. Phytopathology 58, 712–713.
Shang, R. Z., and Pei, M. H. (1984). Study on the leaf rust of davids european aspen caused by melampsora larici-tremulae kleb. J. North East. Fores. Instit. 12, 47–55.
Stubbs, R. W. (1985). “Stripe rust,” in The Cereal Rust, Vol. II, eds A. P. Roelfs and W. R. Bushnell, (Orlando, FL: Academic Press), 61–101.
Swofford, D. L. (2002). PAUP Phylogenetic Analysis Using Parsimony and other methods, version 4.0b10. Sunderland, MA: Sinauer Associates.
Wang, L. L., Li, K. M., and Huang, Y. J. (2018). Four new records of the rust fungi in Xinjiang. J. Fungal Res. 16, 92–94.
Wang, Y. C., and Wei, S. X. (1983). Taxonomic Studies on Graminicolus Rust Fungi of China. Beijing: Science Press.
Wang, Y. Z., and Zhuang, J. Y. (1998). “Uredinales (I),” in Flora Fungorum Sinicorum, Consilio Florarum Cyptogamarum Sinicarum Academiae Sinicae Edita, (Beijing: Science Press), 32–33.
Wang, Z., Zhao, J., Chen, X., Peng, Y., Ji, J., Zhao, S., et al. (2016). Virulence variations of Puccinia striiformis f. sp. tritici isolates collected from Berberis spp. in China. Plant Dis. 100, 131–138. doi: 10.1094/PDIS-12-14-1296-RE
Wei, Y., Yan, J. M., Yang, W. F., and Ye, H. Z. (2013). Cytological studies of basidium development and basidiospore germination of Coleosporium phellodendri. Mycosystema 32, 978–985.
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR Protocols: A Guide to Methods and Applications (San Diego, CA: Academic Press), 315–322. doi: 10.1016/b978-0-12-372180-8.50042-1
Zhao, J., Wang, L., Wang, Z. Y., Chen, X. M., Zhang, H. C., Yao, J. N., et al. (2013). Identification of eighteen Berberis species as alternate hosts of Puccinia striiformis f. sp. tritici and virulence variation in the pathogen isolates form natural infection of barberry plants in China. Phytopathology 103, 927–934. doi: 10.1094/PHYTO-09-12-0249-R
Zhao, Y. Y. (2017). Study on Function of Rusted Barberry Adjacent to Wheat Fields in Occurrence of Wheat Stripe Rust. Ph.D. thesis, Northwest A&F University, Yangling.
Zhao, Z. Y. (1985). A new species of graminicolous Puccinia rust fungi in Xinjiang. Mycosystema 4, 35–37.
Keywords: Berberis spp., gramineous grass, Achnatherum, alternate host, Puccinia achnatheri-sibirici
Citation: Ma X, Liu Y, Li Q, Tian X, Du Z, Kang Z and Zhao J (2020) Identification of Berberis spp. as Alternate Hosts for Puccinia achnatheri-sibirici Under Controlled Conditions and Morphologic Observations of Sexual Stage Development of the Rust Fungus. Front. Microbiol. 11:1278. doi: 10.3389/fmicb.2020.01278
Received: 05 January 2020; Accepted: 19 May 2020;
Published: 25 June 2020.
Edited by:
Anindya Chanda, University of South Carolina, United StatesReviewed by:
Yue Jin, United States Department of Agriculture (USDA), United StatesLes J. Szabo, Cereal Disease Laboratory (USDA-ARS), United States
Copyright © 2020 Ma, Liu, Li, Tian, Du, Kang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Zhao, amllemhhb0Bud3N1YWYuZWR1LmNu; Zhensheng Kang, a2FuZ3pzQG53c3VhZi5lZHUuY24=
 Xinyao Ma
Xinyao Ma Yao Liu
Yao Liu Zhensheng Kang
Zhensheng Kang Jie Zhao
Jie Zhao