
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 28 February 2020
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.00180
This article is part of the Research Topic Antimicrobial Resistance As a Global Public Health Problem: How Can We Address It? View all 50 articles
 Jing-Wei Cheng1,2†
Jing-Wei Cheng1,2† Jian-Rong Su2†
Jian-Rong Su2† Meng Xiao1,3
Meng Xiao1,3 Shu-Ying Yu1,3,4
Shu-Ying Yu1,3,4 Ge Zhang1,3
Ge Zhang1,3 Jing-Jia Zhang1,3
Jing-Jia Zhang1,3 Yang Yang1,3
Yang Yang1,3 Si-Meng Duan1,3
Si-Meng Duan1,3 Timothy Kudinha5,6
Timothy Kudinha5,6 Qi-Wen Yang1,3*
Qi-Wen Yang1,3* Ying-Chun Xu1,3*
Ying-Chun Xu1,3*The objective of this study was to systematically evaluate the in vitro activity of cefoselis and other comparators against common bacterial pathogens collected from 18 hospitals across China. Minimum inhibitory concentrations (MICs) were determined by the broth microdilution method following Clinical and Laboratory Standards Institute (CLSI) guidelines. Cefoselis showed poor activity against extended-spectrum β-lactamase (ESBL)-producing Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis, with susceptibility rates of < 10% each, while the susceptibility rates of this antibiotic against non-ESBL-producing strains of these organisms were 100%, 94.3%, and 97.0%, respectively. Cefoselis exhibited susceptibility rates of 56.7–83.3% against other tested Enterobacteriaceae isolates. For Acinetobacter baumannii and Pseudomonas aeruginosa isolates, the susceptibility rates to cefoselis were 18.7% and 73.3%, respectively. All methicillin-resistant Staphylococcus aureus (MRSA) strains were resistant to cefoselis, while all methicillin-sensitive S. aureus (MSSA) strains were susceptible to this antibiotic. In conclusion, cefoselis showed good activity against non-ESBL-producing E. coli, K. pneumoniae, and P. mirabilis, MSSA, and was also potent against Enterobacteriaceae, P. aeruginosa, and Streptococcus.
Multidrug-resistant (MDR) pathogens, especially the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species), are the leading cause of nosocomial infections throughout the world, which is usually caused by excessive drug usage or prescription and inappropriate use of antimicrobials. Understanding the mechanisms, developing novel antimicrobial agents, and knowing the latest antimicrobial resistance patterns of bacterial pathogens are crucial to combat these public health challenges (Santajit and Indrawattana, 2016; Sheu et al., 2018; Gajdacs, 2019).
Cefoselis is a member of the fourth-generation cephalosporins which exhibit a wider antibacterial spectrum activity than the third-generation cephalosporins to both Gram-negative and Gram-positive bacteria (King et al., 1995). The wide antibacterial spectrum of cefoselis is attributed to the resistance to hydrolysis by the chromosomal β-lactamases and the rapid penetration through the bacterial cell wall (Giamarellos-Bourboulis et al., 2000). However, few reports have been published in China on the antimicrobial activity of cefoselis against common bacterial pathogens. The objective of this study was to better understand the in vitro activity of cefoselis against common Gram-positive and Gram-negative bacterial pathogens in China.
The protocol was approved by the Human Research Ethics Committee of Peking Union Medical College Hospital (no. S-K262). Peking Union Medical College Hospital did not require written informed consent from participants because this was an in vitro study on bacteria isolates without any private data of the human participants.
A total of 1188 bacterial isolates derived from 18 hospitals in China (January 2014–December 2016) were studied. The bacterial species distribution is shown in Table 1. The isolates from each of the participating hospitals were re-identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics GmbH, Bremen, Germany) at the Central Lab, Peking Union Medical College Hospital (Beijing), China.
Minimum inhibitory concentrations (MICs) were determined by the broth microdilution method following Clinical and Laboratory Standards Institute (CLSI) guidelines. Thirty-two antimicrobial agents were tested against the isolates, among which 17 agents were against Gram-negative organisms, 24 agents against Staphylococcus spp., and 19 agents against Streptococcus spp. Cefoselis was obtained from Hansoh Pharma, and the other agents were provided by AstraZeneca. Interpretation of the antimicrobial testing results was based on CLSI M100-S28 (CLSI, 2018). Escherichia coli ATCC 25922, P. aeruginosa ATCC 27853, K. pneumoniae ATCC 700603, S. aureus ATCC 29213, and Streptococcus pneumoniae ATCC 49619 were used as the quality control strains.
Phenotypic identification of extended-spectrum β-lactamase (ESBL) production in E. coli, K. pneumoniae, and Proteus mirabilis, was carried out using CLSI-recommended methods. If the cefotaxime or ceftazidime MICs were ≥ 2 μg/ml, the MICs of cefotaxime + clavulanic acid (4 μg/ml) or ceftazidime + clavulanic acid (4 μg/ml) were comparatively determined. ESBL production was defined as an eightfold or greater decrease in MICs for cefotaxime or ceftazidime tested in combination with clavulanic acid compared to their MICs without clavulanic acid.
Against ESBL-producing E. coli, K. pneumoniae, and P. mirabilis isolates, cefoselis, cefepime, cefotaxime, and ceftriaxone showed relatively low susceptibility rates, with drug resistance rates of > 87%. Against non-ESBL-producing strains, most antibiotics revealed good activity, of which cefoselis, cefepime, showed > 94% antimicrobial susceptibility rates. For Citrobacter freundii, Enterobacter aerogenes, Enterobacter cloacae, Serratia marcescens, and Proteus vulgaris isolates, the susceptibility rates for cefoselis ranged from 56.7% to 83.3%, which were slightly lower than that of cefepime, with susceptibility rates ranging from 70% to 100%. Meropenem and amikacin exhibited high activity against all the Enterobacteriaceae strains (Tables 2, 3).
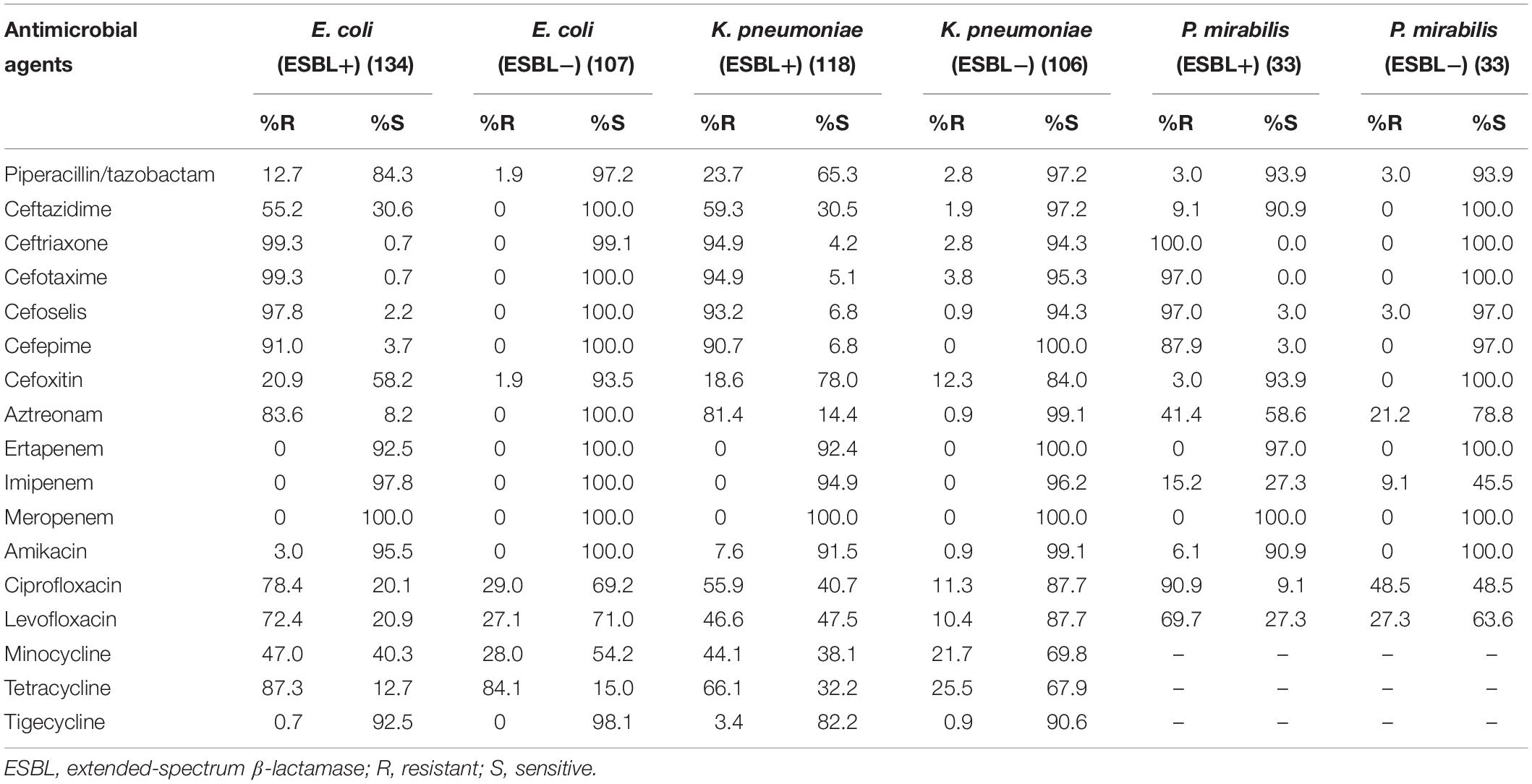
Table 2. In vitro activity of antimicrobial agents against ESBL-positive and ESBL-negative Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis strains.
The most active agents against A. baumannii were tigecycline and minocycline, with susceptibility rates of 58.6% and 45.5%, respectively. The other analyzed agents were less effective, with susceptibility rates of < 30%. Furthermore, against P. aeruginosa isolates, amikacin exhibited the highest in vitro activity, with a susceptibility rate of 93.3%. The susceptibility rates for cefoselis, cefepime, and ceftazidime were all 73.3% each for this organism (Table 4).
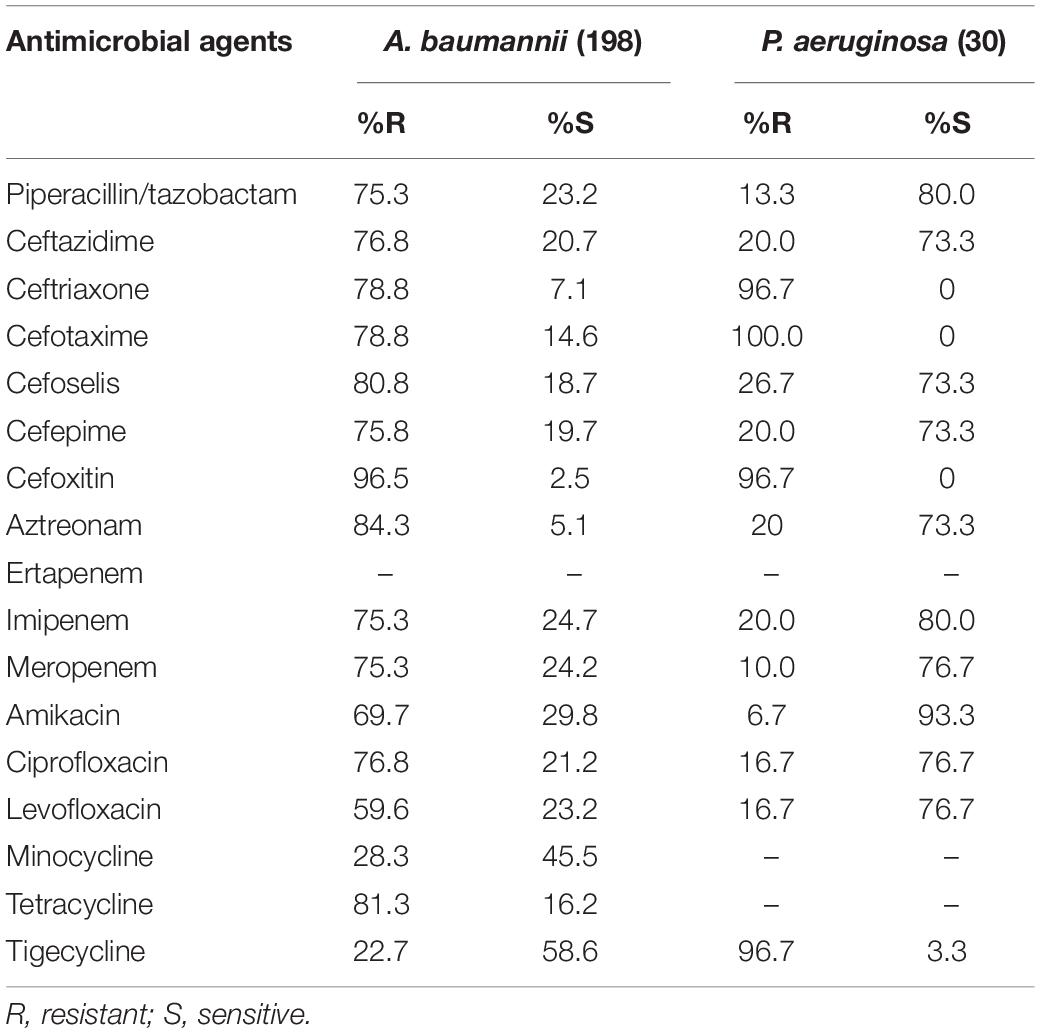
Table 4. In vitro activity of antimicrobial agents against Acinetobacter baumannii and Pseudomonas aeruginosa strains.
Against methicillin-resistant S. aureus (MRSA) strains, linezolid, vancomycin, and teicoplanin exhibited a susceptibility rate of 100% each, followed by tigecycline (97.9%), and trimethoprim–sulfamethoxazole (TMP-SMX) (94.8%). All strains were resistant to ceftazidime, ceftriaxone, cefoselis, and cefepime. Against 100 methicillin-sensitive S. aureus (MSSA) strains, most antibiotics showed good activity, except for ampicillin, tetracycline, and erythromycin. All strains were susceptible to ceftazidime, ceftriaxone, cefoselis, and cefepime (Table 5).
For the penicillin-susceptible S. pneumoniae (PSSP), beta-hemolytic Streptococcus strains, and viridans group Streptococcus strains, cefoselis and cefepime both showed very high antimicrobial activities, with susceptibility rates of > 90%. Against 15 penicillin-resistant S. pneumoniae (PRSP) strains, the susceptibility rate of cefoselis was higher than that of cefepime (60.0% vs. 40.0%). Linezolid, vancomycin, and tigecycline exhibited 100% antimicrobial activity against all the Streptococcus strains (Table 6).
Cefoselis exhibited a slightly lower antimicrobial activity than cefepime against Enterobacteriaceae and non-fermentative Gram-negative organisms, but a little higher activity than cefepime against MRSA, MSSA, PSSP, beta-hemolytic Streptococcus, and viridans group Streptococcus strains. The cumulative percentage MIC distributions of cefoselis and cefepime against common clinical pathogens are shown in Table 7.
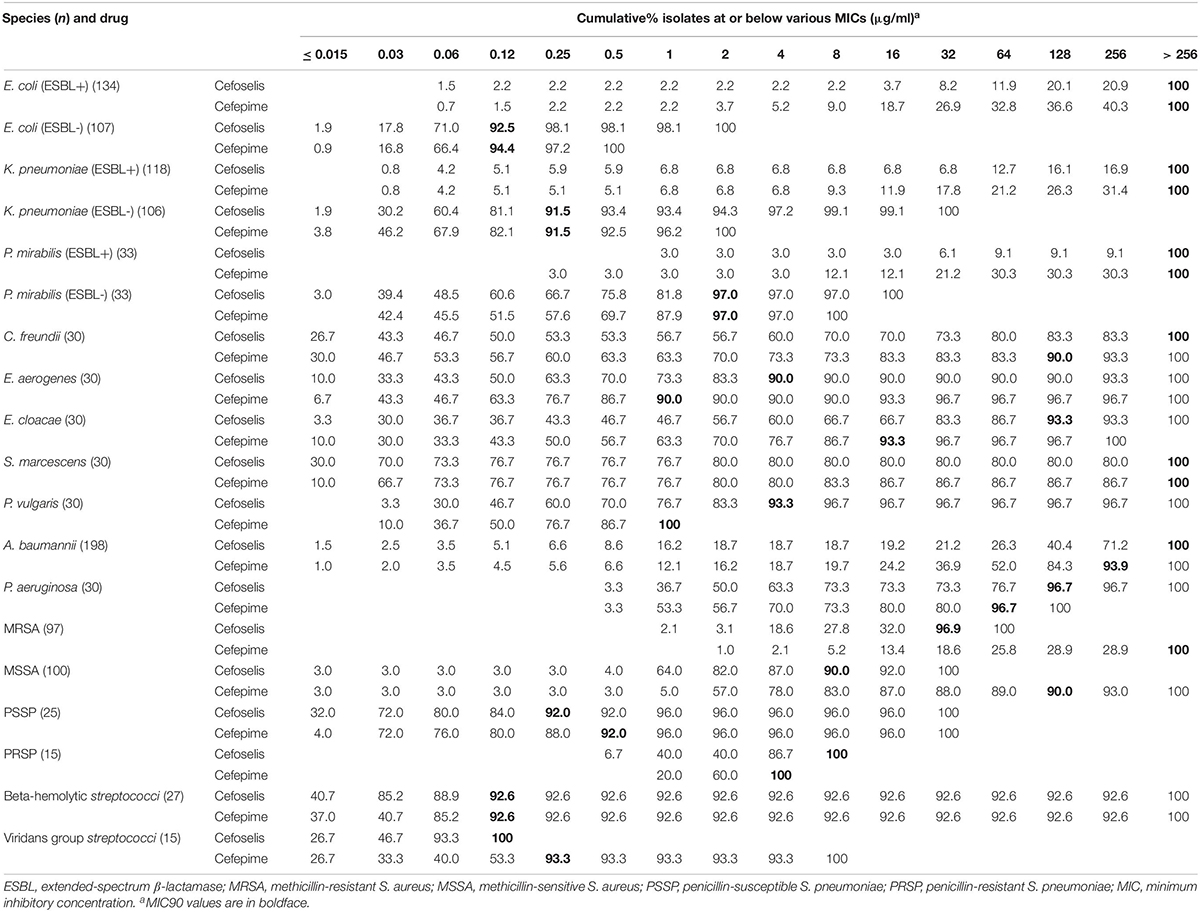
Table 7. Cumulative percentage MIC distributions of cefoselis and cefepime against common clinical pathogens collected in China.
The Enterobacteriaceae family is a major group of pathogens causing several community- and hospital-acquired infections, among which the ESBL rates in E. coli and K. pneumoniae in China have been reported as high as 60–70% and 30–40%, respectively (Yang et al., 2010, 2013). According to our previous study, the genotype distribution of ESBL-producing strains among bacterial species was diverse, and blaCTX-M was the major ESBL gene, with occurrences in 99.5% of E. coli, 91.1% of K. pneumoniae, and 97.5% of P. mirabilis strains (Yang et al., 2015). In the present study, cefoselis and cefepime both showed poor activities against ESBL-producing E. coli, K. pneumoniae, and P. mirabilis, which may be attributed to the specific ESBL genes present in China, albeit further studies are needed for confirmation.
Against other Enterobacteriaceae strains, cefoselis exhibited a slightly lower antimicrobial activity than cefepime, but a higher activity than third-generation cephalosporins. The relatively high activities of fourth-generation cephalosporins against Enterobacteriaceae may be attributed to the low affinity for chromosome-mediated AmpC β-lactamases (D’Angelo et al., 2016), which are the common β-lactamases in Enterobacteriaceae isolates from China. A multicenter, double-blind, randomized clinical trial in China revealed equal clinical efficacy and safety of intravenous cefoselis and cefepime injection for the treatment of acute, moderate, and severe bacterial infections (Liu et al., 2014).
Acinetobacter baumannii was one of the bacteria considered to be of maximum resistance and is classified as a priority category according to the bacterial groups classified by priority categories of need for new antibiotics (Tacconelli and Magrini, 2017). In this study, A. baumannii exhibited low susceptibility to most of the tested antibiotics, with susceptibility rates ranging from 5.1 to 58.6%. Against P. aeruginosa, cefoselis and cefepime showed equal antimicrobial activities for this organism, with susceptibility rates of 73.3% each, which are slightly higher than those in a previous study in China; thus, these two antibiotics could be used to treat infections caused by P. aeruginosa, in combination with other antibiotics (Zhang et al., 2016).
Although the prevalence of MRSA in China showed a markedly decreasing trend from 69.0% in 2005 to 35.3% in 2017, as per the China Antimicrobial Surveillance Network (CHINET) program, MRSA remains a major pathogen responsible for nosocomial infections (Hu et al., 2018). No isolates were found to be resistant to vancomycin, linezolid, and teicoplanin in this study. Tigecycline and TMP-SMX also showed good activities, which were similar to previous studies (Zhao et al., 2012; Zhang et al., 2015). Vancomycin, linezolid, and TMP-SMX were recommended by the Infectious Diseases Society of America (IDSA) to treat MRSA infections (Liu et al., 2011).
Teicoplanin can be an effective alternative to vancomycin for treating patients infected by MRSA as the two therapies are similar in both efficacy and safety (Peng et al., 2013). Tigecycline was often recommended as a second- or third-line agent for MRSA infections when alternative agents cannot be used (Rodvold and McConeghy, 2014). Ceftaroline fosamil was the first FDA-approved cephalosporin with any activity against MRSA, but the low susceptibility among MRSA isolates in China needs attention (Lodise and Low, 2012; Zhang et al., 2015).
The bacterial isolates were collected from 2014 to 2016, and the susceptibility has certainly changed in the last 5 years for most organisms. More recently collected strains should be involved in further studies. This is a limitation of the study. In conclusion, cefoselis exhibited good antimicrobial activity against non-ESBL[Frame1]-producing E. coli, K. pneumoniae, P. mirabilis, and MSSA and was also potent against other Enterobacteriaceae, P. aeruginosa, and Streptococcus.
The datasets generated for this study are available on request to the corresponding author.
J-WC, J-RS, and TK wrote the manuscript. S-YY, GZ, J-JZ, YY, and S-MD collaborated the strains and performed the antimicrobial susceptibility tests. MX, Q-WY, and Y-CX designed and supervised the study.
This work was supported by the National Key Research and Development Program of China (2018YFE0101800, 2018YFC1200100, and 2018YFC1200105), Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (Grant No. 2016-I2M-3–014), CAMS Innovation Fund for Medical Sciences (Grant No. 2016-I2M-1–014), Beijing Innovation Base Cultivation and Development Special Fund (Z171100002217068), Outstanding Talents Training Funding Project of Dongcheng District, Beijing (2017), Graduate Innovation Fund of Peking Union Medical College (Grant No. 2017-1002-1-21), and the National Natural Science Foundation of China (Grant No. 81101287). The funders had no role in the study design, data collection and analysis, decision to publish, or in the preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank all the 18 hospitals for providing the study strains.
CLSI (2018). Performance Standards for Antimicrobial Susceptibility Testing. 28th Ed. CLSI Supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute.
D’Angelo, R. G., Johnson, J. K., Bork, J. T., and Heil, E. L. (2016). Treatment options for extended-spectrum beta-lactamase (ESBL) and AmpC-producing bacteria. Expert Opin. Pharmacother. 17, 953–967. doi: 10.1517/14656566.2016.1154538
Gajdacs, M. (2019). The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics 8:E52. doi: 10.3390/antibiotics8020052
Giamarellos-Bourboulis, E. J., Grecka, P., Tsitsika, A., Tympanidou, C., and Giamarellou, H. (2000). In-vitro activity of FK 037 (Cefoselis), a novel 4(th) generation cephalosporin, compared to cefepime and cefpirome on nosocomial staphylococci and gram-negative isolates. Diagn. Microbiol. Infect. Dis. 36, 185–191. doi: 10.1016/s0732-8893(99)00131-5
Hu, F., Zhu, D., Wang, F., and Wang, M. (2018). Current status and trends of antibacterial resistance in China. Clin. Infect. Dis. 67, S128–S134. doi: 10.1093/cid/ciy657
King, A., Bethune, L., and Phillips, I. (1995). The comparative In Vitro activity of FK- 037 (Cefoselis), a new broad-spectrum cephalosporin. Clin. Microbiol. Infect. 1, 13–17. doi: 10.1111/j.1469-0691.1995.tb00018.x
Liu, C., Bayer, A., Cosgrove, S. E., Daum, R. S., Fridkin, S. K., Gorwitz, R. J., et al. (2011). Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52, e18–e55. doi: 10.1093/cid/ciq146
Liu, Y. B., Lv, X. J., Yu, R. J., Qiu, H. M., Bai, J. L., Jiang, N., et al. (2014). Multicenter, double- blind, randomized clinical trial of parenterally administered Cefoselis versus Cefepime for the treatment of acute bacterial infections. Eur. Rev. Med. Pharmacol. Sci. 18, 2006–2012.
Lodise, T. P., and Low, D. E. (2012). Ceftaroline fosamil in the treatment of community- acquired bacterial pneumonia and acute bacterial skin and skin structure infections. Drugs 72, 1473–1493. doi: 10.2165/11635660-000000000-00000
Peng, Y., Ye, X., Li, Y., Bu, T., Chen, X., Bi, J., et al. (2013). Teicoplanin as an effective alternative to vancomycin for treatment of MRSA infection in Chinese population: a meta-analysis of randomized controlled trials. PLoS One 8:e79782. doi: 10.1371/journal.pone.0079782
Rodvold, K. A., and McConeghy, K. W. (2014). Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin. Infect. Dis. 58(Suppl. 1), S20–S27. doi: 10.1093/cid/cit614
Santajit, S., and Indrawattana, N. (2016). Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016:2475067. doi: 10.1155/2016/2475067
Sheu, C. C., Lin, S. Y., Chang, Y. T., Lee, C. Y., Chen, Y. H., and Hsueh, P. R. (2018). Management of infections caused by extended-spectrum beta-lactamase- producing Enterobacteriaceae: current evidence and future prospects. Expert Rev. Anti Infect. Ther. 16, 205–218. doi: 10.1080/14787210.2018.1436966
Tacconelli, E., and Magrini, N. (2017). Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva: WHO.
Yang, Q., Wang, H., Chen, M., Ni, Y., Yu, Y., Hu, B., et al. (2010). Surveillance of antimicrobial susceptibility of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in China: the 2002-2009 study for monitoring antimicrobial resistance trends (SMART). Int. J. Antimicrob. Agents 36, 507–512. doi: 10.1016/j.ijantimicag.2010.09.001
Yang, Q., Zhang, H., Cheng, J., Xu, Z., Xu, Y., Cao, B., et al. (2015). In vitro activity of flomoxef and comparators against Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis producing extended-spectrum beta- Lactamases in China. Int. J. Antimicrob. Agents 45, 485–490. doi: 10.1016/j.ijantimicag.2014.11.012
Yang, Q., Zhang, H., Wang, Y., Xu, Y., Chen, M., Badal, R. E., et al. (2013). A 10 year surveillance for antimicrobial susceptibility of Escherichia coli and Klebsiella pneumoniae in community- and hospital-associated intra-abdominal infections in China. J. Med. Microbiol. 62, 1343–1349. doi: 10.1099/jmm.0.059816-0
Zhang, H., Xiao, M., Kong, F., O’sullivan, M. V., Mao, L. L., Zhao, H. R., et al. (2015). A multicentre study of meticillin-resistant Staphylococcus aureus in acute bacterial skin and skin-structure infections in China: susceptibility to ceftaroline and molecular epidemiology. Int. J. Antimicrob. Agents 45, 347–350. doi: 10.1016/j.ijantimicag.2014.12.014
Zhang, H., Yang, Q., Liao, K., Ni, Y., Yu, Y., Hu, B., et al. (2016). Antimicrobial susceptibilities of aerobic and facultative gram-negative Bacilli from intra- abdominal infections in patients from seven regions in China in 2012 and 2013. Antimicrob. Agents Chemother. 60, 245–251. doi: 10.1128/AAC.00956-15
Zhao, C. J., Sun, H. L., Wang, H., Liu, Y. D., Hu, B. J., Yu, Y. S., et al. (2012). Antimicrobial resistance trends among 5608 clinical Gram-positive isolates in China: results from the gram-positive CocciResistance surveillance program (2005-2010). Diagn. Microbiol. Infect. Dis. 73, 174–181. doi: 10.1016/j.diagmicrobio.2012.03.003
Keywords: cefoselis, cefepime, antimicrobial resistance, in vitro activity, China
Citation: Cheng J-W, Su J-R, Xiao M, Yu S-Y, Zhang G, Zhang J-J, Yang Y, Duan S-M, Kudinha T, Yang Q-W and Xu Y-C (2020) In vitro Activity of a New Fourth-Generation Cephalosporin, Cefoselis, Against Clinically Important Bacterial Pathogens in China. Front. Microbiol. 11:180. doi: 10.3389/fmicb.2020.00180
Received: 07 August 2019; Accepted: 24 January 2020;
Published: 28 February 2020.
Edited by:
Ilana L. B. C. Camargo, University of São Paulo, BrazilReviewed by:
Norma Margarita de la Fuente Salcido, Universidad Autónoma de Coahuila, MexicoCopyright © 2020 Cheng, Su, Xiao, Yu, Zhang, Zhang, Yang, Duan, Kudinha, Yang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi-Wen Yang, eWFuZ3Fpd2VuODFAdmlwLjE2My5jb20=; Ying-Chun Xu, eHljcHVtY2hAMTM5LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.