
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 14 January 2020
Sec. Food Microbiology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.03128
Recent multistate outbreaks and recalls of fresh apples due to Listeria monocytogenes contamination have increased consumer concerns regarding fresh and processed apple safety. This study aimed to evaluate the antimicrobial efficacy of two sanitizers, mineral oxychloride (JC9450) and neutral electrolyzed water (NEW), for inactivation of L. monocytogenes on fresh apples. A 2-min treatment of 0.125% (v/v) JC9450 with 100 ppm free available chlorine (FAC) or NEW with 110 ppm FAC caused 0.9–1.2 log10 CFU/apple reduction of L. monocytogenes on both Granny Smith and Fuji apples 24 h post-inoculation. Increasing JC9450 concentration to 0.25 and 0.50% significantly improved its bactericidal effect and reduced L. monocytogenes on Granny Smith apples by ~2.0 and 3.8 log10 CFU/apple, respectively, after a contact time of 2 min. At a shorter contact time of 30 sec, the inactivation efficacy of chlorine and 0.25–0.50% JC9450 against L. monocytogenes on apples was significantly reduced compared with the respective 2-min wash. Furthermore, no L. monocytogenes was recovered in deionized water prepared antimicrobial wash solution or on non-inoculated apples post-NEW with 110 ppm FAC or 0.125–0.5% JC9450 washes, indicating their ability to prevent cross-contamination. In addition, a 2-min exposure to NEW with 110 ppm FAC and 0.50% JC9450 reduced apple native microbiota including total plate count by 0.14 and 0.65 log10 CFU/apple, respectively, and yeast and mold counts by 0.55 and 1.63 log10 CFU/apple, respectively. In summary, L. monocytogenes attached on apples was difficult to eliminate. JC9450 and NEW demonstrated a dose-dependent reduction in L. monocytogenes on apples and successfully prevented cross-contamination, indicating their application potential in post-harvest washes of apples.
Listeria monocytogenes is a major foodborne pathogen, which causes more than 1,500 illnesses annually in the United States with a high mortality rate of ~16% (Scallan et al., 2011). Multistate outbreaks attributed to contamination of L. monocytogenes on caramel apples (CDC, 2015) and recalls of fresh apples (FDA, 2016, 2017b) and sliced apples (CFIA, 2015) due to potential L. monocytogenes contamination highlight the importance of preventive control programs directed at L. monocytogenes on fresh apples. L. monocytogenes population remained stable on fresh apple surface during 3 months of cold storage (Sheng et al., 2017).
Antimicrobial sanitizer interventions have been widely used for the processing and packing of fresh produce to minimize the risk of foodborne pathogen and prevent the cross-contamination. Chlorine, typically in the form of sodium/calcium hypochlorite, is the most commonly used antimicrobial in the fresh produce industry (Banach et al., 2015). The major antimicrobial components in chlorine solutions are hypochlorous acid (HOCl) and hypochlorite ion (OCl−), namely free available chlorine (FAC), where HOCl has a stronger oxidation capacity and antimicrobial efficacy than OCl− (Fukuzaki, 2006; Rahman et al., 2016). The efficacy of chlorine is related to the FAC level, which varies depending on the washing conditions such as pH and organic matter (Francis et al., 2012). Chlorine at commonly used concentration, 50–200 ppm (Suslow, 2005), has a limited efficacy against L. monocytogenes on fresh produce (Prado-Silva et al., 2015). A 1-min spray of sodium hypochlorite solution with 200 ppm FAC reduced L. monocytogenes inoculated on whole Red Delicious apples at ~1.7 log10 CFU/cm2 by ~0.9 log10 CFU/cm2 (Beuchat et al., 1998). Dipping in 100 ppm chlorine for 2 min reduced L. monocytogenes inoculated on Granny Smith apples at ~6.4 log10 CFU/apple by 0.7–0.9 log10 CFU/apple (Shen et al., 2019). There is also safety concern about the production of carcinogenic halogenated by-products resulted from chlorinated organic compounds (Brown et al., 2011; Gil et al., 2016). As a result, chemical and fresh produce industries have been seeking for alternative antimicrobial sanitizers with an improved efficacy and convenience.
JC9450 is a novel sanitizer certified by the National Sanitation Foundation (NSF) and approved for use in potable water systems as a disinfectant (NSF, 2012). The active antimicrobial ingredient of JC9450 is mineral oxychloride or metal oxychloride (MOxCly), which is a chemical compound where oxygen (O) and chlorine (Cl) atoms are bonded to a metal (M) (Yu et al., 2017). Unlike the well-known fungicide copper oxychloride [Cu4(OH)6Cl2], inhibitory effect of which relies on the antifungal efficacy of copper (Cioffi et al., 2005; Peter, 2016), the metals in mineral oxychloride act as catalysts to generate reactive oxygen species (ROS) especially hydroxyl radicals through interaction with water (Yang et al., 2013; Hayyan et al., 2016; Li and Zhang, 2016; Jenfitch, 2018). JC9450 is reported to have a superior antimicrobial efficacy than chlorine; 0.0002% JC9450 and chlorine reduced Salmonella enterica ATCC 10708 in liquid broth by ~6.7 and 0.1 log10 CFU/ml, respectively (DisinfectWater, 2017). The concentration of mineral oxychloride that inhibited 99% of germination (EC99) of conidia of Penicillium digitatum was 0.003%, while the EC99 of chlorine was 0.01% (Smilanick et al., 2014). However, there is no information available regarding its efficacy against L. monocytogenes on fresh apples or in water.
Neutral electrolyzed water (NEW) is produced from the electrochemical reaction of water and salt, and on-site generation creates a sodium-free solution of hypochlorous acid (HOCl) with a high oxidation-reduction potential (Rahman et al., 2016). Over the years, NEW has been evaluated against foodborne pathogens and spoilage microorganisms on various fresh and fresh-cut produce. A 5-min wash with NEW containing 100 ppm FAC (pH ~8.4) reduced L. innocua on apple slices by ~0.9 log10 CFU/g (Graca et al., 2011). Using NEW wash with 89 ppm FAC (pH ~8.0), there was more than 4.0 log10 CFU/cm2 reductions of L. monocytogenes, Salmonella, and E. coli O157:H7 inoculated on tomato surface within 1 min (Deza et al., 2003). NEW with 50 ppm FAC (pH ~7.5) reduced Alicyclobacillus acidoterrestris spores on apples by ~2.0 log10 CFU/apple after 1-min exposure (Torlak, 2014). However, its efficacy against L. monocytogenes on fresh apples or in washing solution has not been evaluated. Therefore, the objectives of this study were to evaluate the efficacy of JC9450 and NEW to control L. monocytogenes on fresh apples and to prevent cross-contamination.
L. monocytogenes NRRL B-57618 (1/2a, human clinical isolate), NRRL B-33053 (4b, Coleslaw outbreak isolate), and NRRL-33466 (1/2b, environmental isolate) were obtained from the USDA-ARS culture collection [National Center for Agricultural Utilization Research (NRRL), Peoria, IL, USA] and maintained at −80°C in trypticase soy broth [Becton, Dickinson and Company (BD), Sparks, MD, USA] supplemented with 0.6% yeast extract (TSBYE; Fisher Scientific, Fair Lawn, NJ, USA) and 20% glycerol. Each frozen stock culture was reconstituted in TSBYE at 37°C for 24 h. For test cultures, these were sub-cultured into fresh TSBYE and incubated as before. All the experiments were conducted in a biosafety level 2 lab.
Following incubation, the cultures were centrifuged at 8,000 × g for 5 min at 4°C, and the resulting pellets were washed once and re-suspended in phosphate buffered saline (PBS, pH 7.4). Equal population of each washed L. monocytogenes strain (~5 × 108 CFU/ml per strain) was combined to make a three-strain cocktail and diluted to achieve ~106 CFU/ml for apple inoculation. The high contamination level was chosen to enable accurate detection of bacterial count reductions.
Different lots of unwaxed Granny Smith apples (GSA) and Fuji apples of commercial maturity, the optimum maturity for either the fresh market or the storage (Girschik et al., 2017), were donated by Allan Brothers Inc. (Naches, WA, USA) and Stemilt Growers LLC (Wenatchee, WA, USA) and stored at 4°C. Apples of uniform size (~200 g/apple) with stems left fully intact and devoid of cuts, bruises, or scars were selected for the experiment. The whole apples were rinsed with cold tap water, dried and then dip-inoculated in the Listeria cocktail (~106 CFU/ml) at room temperature (RT, ~22.5°C) as previously described (Sheng et al., 2017), and dried at RT and ambient environmental relative humidity (RH) for 24 or 48 h. Twelve apples were randomly sampled immediately following inoculation (0 h) and at 24 and 48 h post-inoculation for enumeration following the procedure described in section “Listeria monocytogenes Enumeration” to confirm the persistent population density of inoculated L. monocytogenes.
JC9450 was kindly provided by Jenfitch LLC (Walnut Creek, CA, USA). JC9450 consists of 6.7–9.5% mineral oxychloride (CAS#1332-17-8), 0.02–0.67% sodium hydroxide, and water. JC9450 was tested at 0.125, 0.25, and 0.50% (v/v). NEW (Disinfectant 275) was donated by AquaOx (Loxahatchee, FL, USA) and tested at 1:15, 1:7, and 1:3 dilutions corresponding to 22.5, 55, 110 ppm FAC, respectively. Chlorine, prepared from Accu-Tab (Calcium hypochlorite, Pace International, Wapato, WA, USA) with ~100 ppm FAC, was used as control. All solutions were prepared with deionized water and applied right after preparation. The pH and oxidation/reduction potential (ORP) of wash solutions were measured at RT with Orion 8302BNUMD ROSS Ultra pH/ATC Triode (Thermo Scientific, Waltham, WA, USA) and Orion 9678BNWP electrode (Thermo Scientific), respectively, connected to an Orion Versa Star Pro advanced electrochemistry meter (Thermo Scientific). FAC was measured with a Taylor K-2006 complete test kit (Taylor Technologies, Sparks, MD, USA), and there was no difference in FAC in tested solutions before and after wash. The pH, ORP, and FAC content for all wash solutions are listed in Table 1.
For each sanitizer and contact time treatment combination, a set of 12 apples was submerged in 3 L of respective antimicrobial solution at RT and agitated manually for 2 min or 30 sec. The deionized water wash was used as a negative control. After treatment, the apples were removed immediately from the sanitizing solution and analyzed to estimate viable L. monocytogenes populations. Each sanitizer and time treatment combination was repeated independently three times.
To evaluate the efficacy of the aforementioned antimicrobial sanitizers in preventing cross-contamination of L. monocytogenes during apple washing, inoculated apples were introduced along with non-inoculated apples at either 1:10 or 6:6 ratio to respective sanitizer solution (Pao et al., 2007; Nou and Luo, 2010). They were treated as described in section “Antimicrobial Intervention on Apple Surfaces”. Each treatment was repeated independently three times.
To enumerate survival of L. monocytogenes on inoculated apples, each apple was placed into a stomacher bag with 10 ml sterile PBS and hand-rubbed for 1.5 min to detach adherent cells from apple surfaces (Sheng et al., 2018). Rub solutions were 10-fold serially diluted with sterile PBS, and 0.1 or 1 ml (333 μl/plate, 3 plates) from appropriate dilutions was plated on duplicate TSAYE (TSBYE with 1.5% agar) plates and then overlaid with modified Oxford agar (MOX, BD) to differentiate L. monocytogenes from indigenous apple microbiota (Kang and Fung, 1999).
Residual populations of L. monocytogenes in the wash water were enumerated by serially diluting and then plating following the above quantitative method. Residual L. monocytogenes in the spent antimicrobial solutions was enumerated by filtration method. Briefly, 100 ml wash solution was filtrated through a disposable 0.45 μm analytical test filter funnel (Thermo Scientific), rinsed twice with sterile PBS, and placed on TSAYE and CHROMagar™ Listeria (DRG International Inc. Springfield, NJ, USA) plates followed by incubation at 37°C for 48 h. Presumptive colonies were further confirmed by PCR targeting invasion-associated secreted endopeptidase (iap) gene (FDA, 2017a).
To examine the potential cross-contamination of L. monocytogenes, non-inoculated apples were hand rubbed in the same manner as inoculated apples, and 1 ml of rub solution was transferred into 9 ml buffered Listeria enrichment broth (BLEB, BD), incubated at 30°C for 48 h, and streaked onto CHROMagar™ Listeria plates (FDA, 2017a). The plates were incubated at 37°C for 48 h to determine the presence or absence of L. monocytogenes. Presumptive colonies were confirmed by PCR as mentioned above.
Non-inoculated apples post-sanitizer treatments were rubbed for 1.5 min to detach the resident microbiota on apple surface. The rub solutions were serially diluted and then plated onto TSAYE for total plate count (TPC) and potato dextrose agar (PDA, BD) plates for yeast and mold (Y/M) counts, respectively. TSAYE plates were incubated at 35 ± 1°C for 48 h, while PDA plates were incubated at RT for 3~5 days.
Data were analyzed by GLM from Statistical Analysis Systems (SAS, Cary, NC, USA). Mean values were compared by least significant difference (LSD) multiple-comparison test. p values of less than 0.05 were considered statistically significant. Each experiment was repeated three times independently. Results were reported as mean ± standard error mean (SEM). Log reduction of L. monocytogenes or background microbiota from apples was averaged from three independent experiments with 12 apples/treatment in each independent study, n = 36. Three spent water samples were collected in each independent cross-contamination study, hence a total of nine samples per treatment.
Antimicrobial efficacy of JC9450 and NEW was first assessed and compared against L. monocytogenes on GSA, the variety associated with a recent listeriosis caramel apple outbreak (CDC, 2015), and Fuji apple, which is a commercially important variety and has a different surface structure than GSA (Hall, 1966). The initial L. monocytogenes inoculation level on GSA and Fuji apples was 6.24 ± 0.06 and 6.18 ± 0.03 log10 CFU/apple, respectively. After 24 h at RT, the populations of L. monocytogenes on GSA and Fuji apples were 6.48 ± 0.07 and 6.36 ± 0.05 log10 CFU/apple, respectively (Figure 1). NEW with 110 ppm FAC and 0.125% JC9450 reduced L. monocytogenes on GSA by ~1.0 log10 CFU/apple (Figure 1A). Increasing JC9450 concentration improved its efficacy; 0.25 and 0.50% of JC9450 caused ~2.1 and 3.8 log10 CFU/apple reductions of L. monocytogenes on GSA, respectively (Figure 1A). Reducing NEW concentrations to 55 and 22.5 ppm FAC significantly decrease its efficacy against L. monocytogenes on fresh apples. L. monocytogenes on Fuji apples exhibited the same degree of resistance (p < 0.05) in response to respective antimicrobial treatment as GSA (Figure 1B). Therefore, only GSA was used in subsequent experiments.
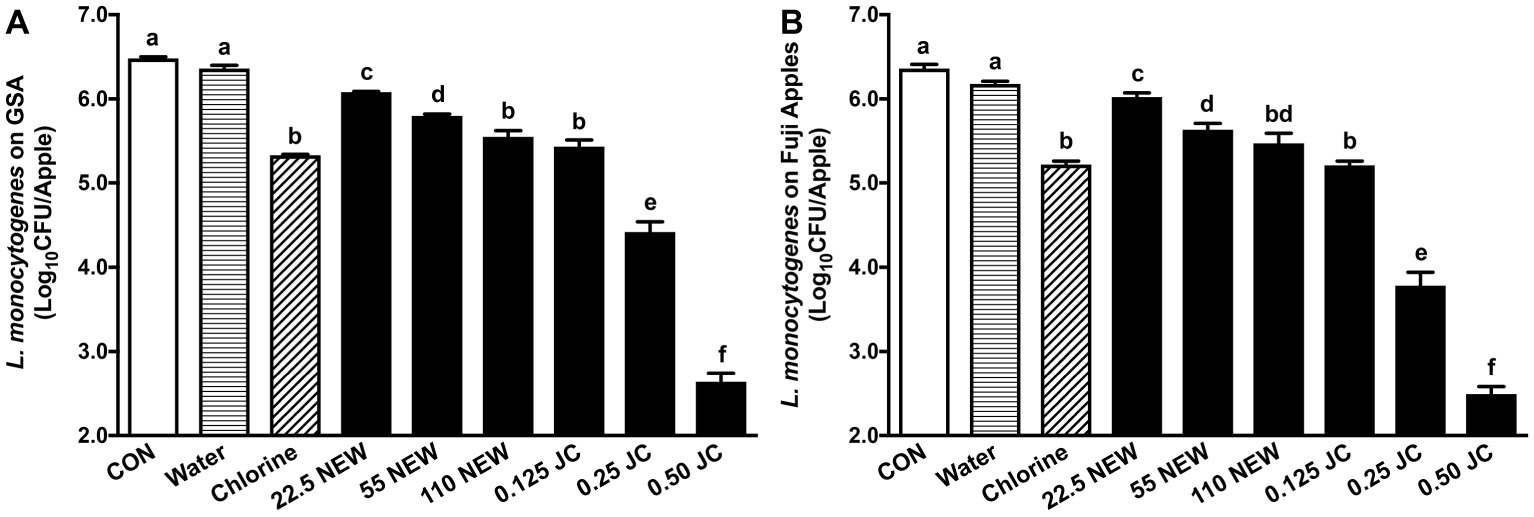
Figure 1. Efficacy of JC9450 and neutral electrolyzed water against Listeria monocytogenes on fresh apples 24 h post-inoculation with a contact time of 2 min. (A) L. monocytogenes count on Granny Smith apples (GSA) post-sanitizer treatments; (B) L. monocytogenes count on Fuji apples post-sanitizer treatments. Mean ± SEM, averaged from three independent studies with 12 apples/treatment in each independent study (n = 36). CON, untreated control; JC, JC9450, mineral oxychloride, %; NEW, neutral electrolyzed water, tested at 22.5, 55, and 110 ppm free available chlorine. Chlorine at ~110 ppm free available chlorine was used as a positive control. Histogram bars without common letter differ significantly (p < 0.05).
Bacterial attachment time before sanitizer intervention is reported to impact antimicrobial efficacy of sanitizers (Lang et al., 2004). Therefore, the antimicrobial efficacy of selected sanitizers was tested against L. monocytogenes on apples at 24 and 48 h post-inoculation. The counts of L. monocytogenes on apples 24 and 48 h post-inoculation were 6.48 ± 0.02 and 6.45 ± 0.02 log10 CFU/apple, respectively. At 24 h post-inoculation, reducing contact time from 2 min to 30 sec significantly decreased the antimicrobial efficacy of chlorine and 0.25–0.50% JC9450 (Table 2). At 48 h post-inoculation, shortening contact time to 30 sec had no influence on the inhibitory effect of all the tested sanitizers except 0.50% JC9450 (Table 2).
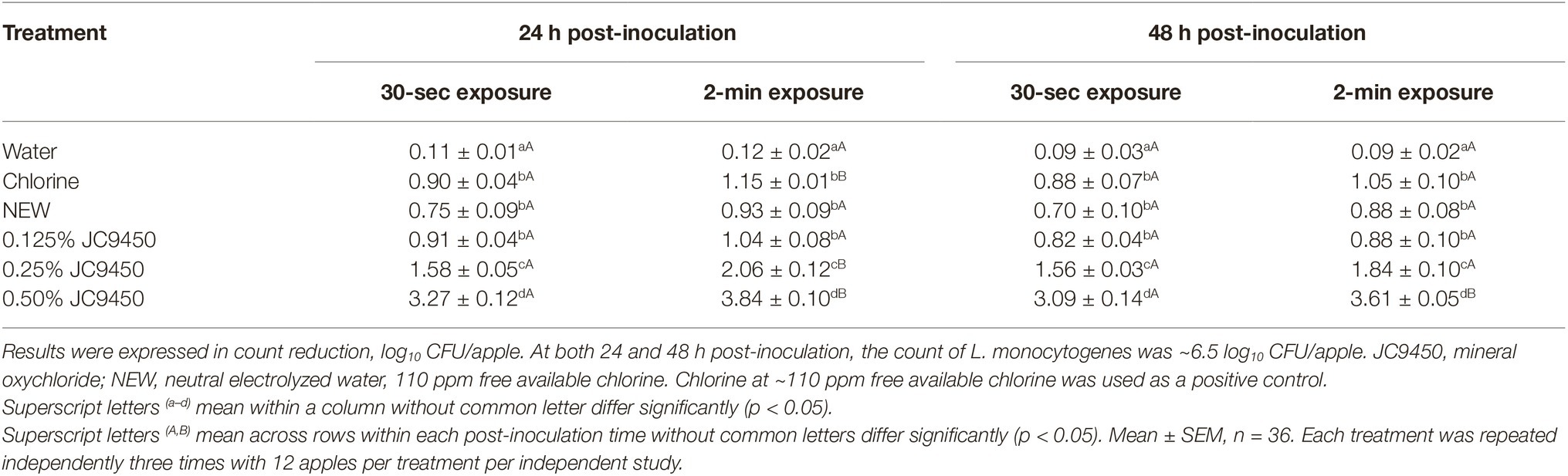
Table 2. Influence of Listeria monocytogenes attachment time and sanitizer contact time on the antimicrobial efficacy of neutral electrolyzed water and JC9450 against Listeria monocytogenes on fresh apples.
A 2-min wash with water alone transferred ~4.6 log10 CFU/ml L. monocytogenes from inoculated apples to wash solution, while residual L. monocytogenes in wash solutions of chlorine, NEW with 110 ppm FAC, and 0.125–0.50% JC9450 were reduced to under the detectable level (1 CFU/100 ml) at contact times of 30 sec–2 min (data not shown). No L. monocytogenes was detected on non-inoculated apples following 2-min NEW (110 ppm FAC) or JC9450 (0.125–0.50%) washes regardless of the contamination level (Table 3). However, the 2-min water wash without any antimicrobial transferred 3.8–4.2 log10 CFU/apple of L. monocytogenes to non-inoculated apples (Table 3). These data, collectively, indicated that JC9450 and NEW have the potential to prevent cross-contamination from both fruit-to-water and fruit-to-fruit.
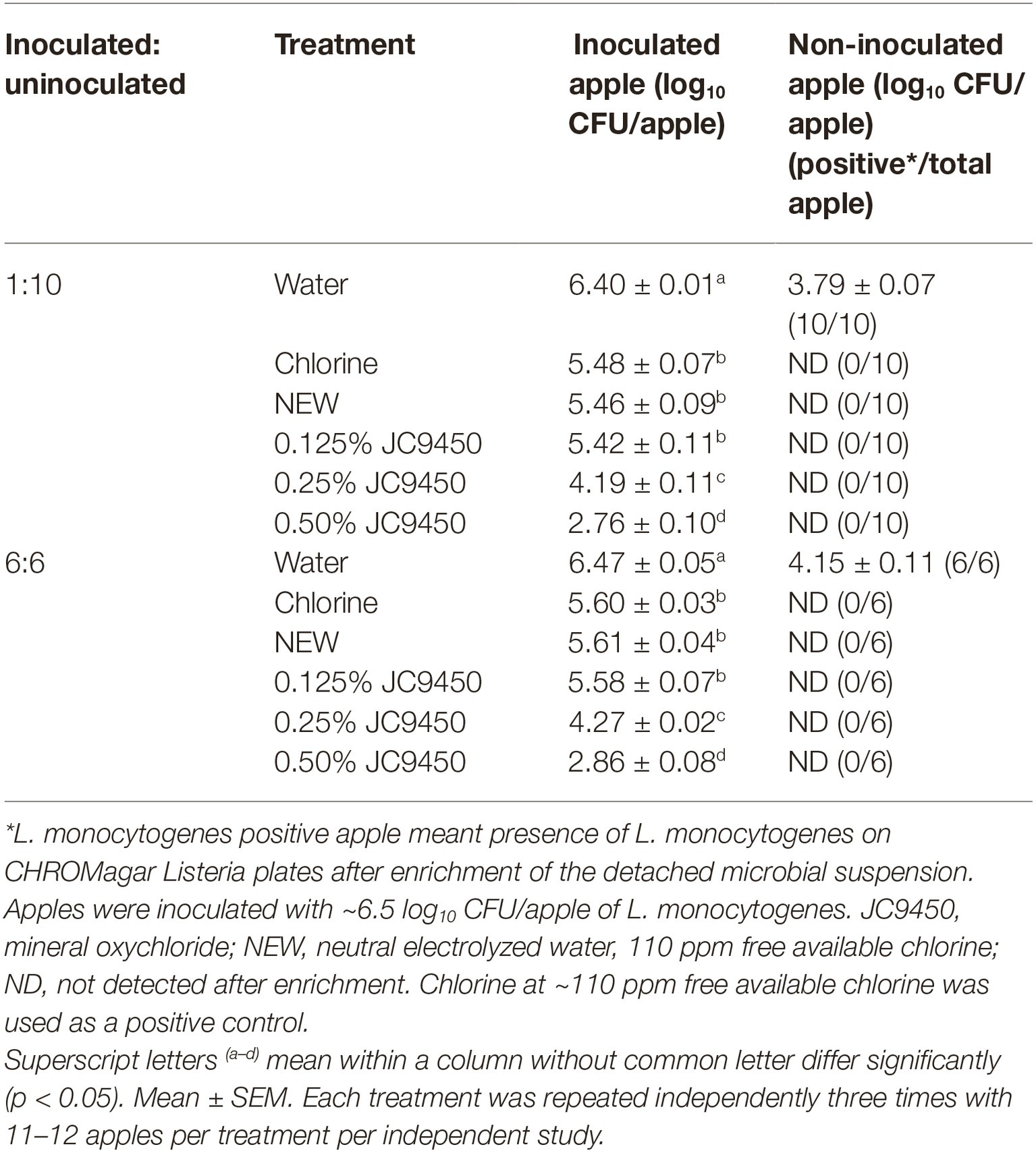
Table 3. Efficacy of neutral electrolyzed water and JC9450 for the prevention of cross-contamination of Listeria monocytogenes among apples.
Both NEW at 110 ppm FAC and 0.125% JC9450 along with chlorine have a similar but limited efficacy against naturally presented background microbiota; they caused 0.14–0.15 and 0.50–0.60 log10 CFU/apple reduction of TPC and Y/M, respectively (Figure 2). Increasing JC9450 concentrations improved its efficacy; a 2-min exposure to 0.50% JC9450 reduced TPC and Y/M counts by 0.65 and 1.63 log10 CFU/apple, respectively (Figure 2).
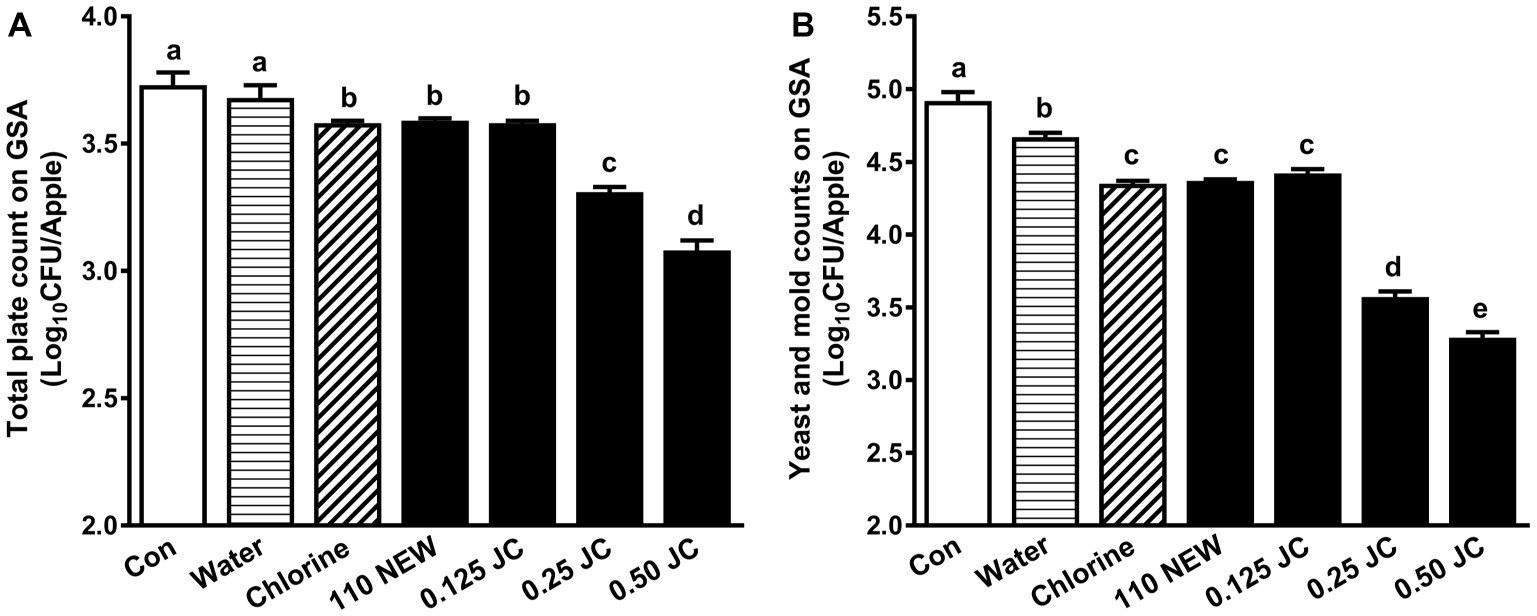
Figure 2. Effects of JC9450 and neutral electrolyzed water against background microbiota on Granny Smith apples (GSA). (A) Total plate counts (TPC) of resident bacteria on GSA post-sanitizer treatments; (B) yeast and mold (Y/M) counts on GSA post-sanitizer treatments; mean ± SEM, averaged from three independent studies with 12 apples/treatment in each independent study (n = 36). CON, untreated control; JC, JC9450, mineral oxychloride, %; NEW, neutral electrolyzed water, 110 ppm free available chlorine. Chlorine at ~110 ppm free available chlorine was used as a positive control. Histogram bars without common letter differ significantly (p < 0.05).
NEW at 110 ppm FAC and 0.125% JC9450 showed a comparable but limited antimicrobial efficacy against L. monocytogenes on apples as did conventional chlorine treatment. Similarly, spray application of sodium hypochlorite solution with 200 ppm FAC reduced L. monocytogenes inoculated on whole Red Delicious apples by ~0.9 log10 CFU/cm2 after a contact time of 1 min (Beuchat et al., 1998). A 1-min exposure to NEW at 89 ppm FAC reduced L. innocua, a non-pathogenic species closely related to L. monocytogenes, in lettuce by ~1.2 log10 CFU/g (Abadias et al., 2008). Increasing JC9450 concentration to 0.50% enhanced its antimicrobial efficacy, possibly due to the increased production of bactericidal ROS.
The morphology of surface wax, where pathogens attach on intact apple surface (Burnett et al., 2000), varies among different apple varieties (Hall, 1966). These surface morphologies might influence the interaction between bacteria and apple surface and subsequent inactivation. However, our results suggested that the JC9450 and NEW had similar efficacies against L. monocytogenes on GSA and Fuji apples. In support of our findings, a 30-sec exposure to 200 ppm chlorine reduced Penicillium expansum by 1.3–1.6 log10 spores/g on Red Delicious, Gala, and Fuji apples (Salomao et al., 2008). The survival of L. monocytogenes on GSA and Fuji apples was similar during a 3-month cold storage (Sheng et al., 2017).
Reducing contact times significantly reduced the efficacy of chlorine and 0.25–0.50% JC9450 on freshly contaminated apples (24 h post-inoculation). Similarly, a 25 ppm HOCl wash for 15 sec and 2 min reduced Salmonella 2 h post-inoculated on tomatoes by ~2.4 and 6.0 log10 CFU/tomato, respectively (Gereffi et al., 2015). By contrast, a 200 ppm chlorine wash for both 3 and 10 min reduced L. monocytogenes on cantaloupe surface 2 h post-inoculation by ~1.0 log10 CFU/cm2 (Upadhyay et al., 2014). Chlorine wash with 100 ppm FAC for 1 and 3 min reduced E. coli O157:H7 2 h post-inoculated onto Red Delicious apples by ~4.6 log10 CFU/apple (Baskaran et al., 2013).
The impact of contact time was lost for L. monocytogenes on apples after prolonged drying, indicating an increased antimicrobial tolerance of L. monocytogenes on fresh apples to JC9450 and NEW. Similarly, antimicrobial efficacy of 100 ppm chlorine against L. monocytogenes on lettuce 24 h post-inoculation was reduced compared to that at 6 h post-inoculation, which resulted in ~2.2 and 3.5 log10 CFU/g reduction after a 2-min exposure, respectively (Olmez and Temur, 2010). Chlorine at 200 ppm with an exposure time of 5 min caused ~3.8 and 1.8 log10 CFU/tomato reductions in E. coli O157:H7 post-1 h and -24 h inoculation, respectively (Lang et al., 2004). A 2-min exposure to 80 ppm PAA reduced L. monocytogenes on GSA by ~2.2 and 1.7 log10 CFU/apple after 24 and 48 h of attachment, respectively (Shen et al., 2019). The increased resistance with prolonged attachment time might be due to the increased binding strength of bacteria to produce surface and/or maturation of cell aggregates and protective extracellular materials (Elhariry, 2011). Lengthened attachment time might also induce bacterial desiccation stress response, which may cross-protect the bacterial cells against sanitizers (Lou and Yousef, 1997). Biofilm formation and development of cell aggregates during extended adhesion could further contribute to the increased antimicrobial resistance (Olmez and Temur, 2010). However, Salmonella attached on whole cantaloupe rind for 24 and 72 h demonstrated a similar resistance to 200 ppm chlorine, as its levels were reduced by ~2.5 log10 CFU/cm2 after a contact time of 2 min (Ukuku and Fett, 2006).
During washing, pathogens in contaminated fresh produce can be directly transferred to wash water, which further transfers pathogens to contaminant-free produce (Allende et al., 2008). In this study, a single apple harboring L. monocytogenes was able to contaminate a whole batch of 10 clean apples with ~3.8 log10 CFU/apple in the absence of sanitizer. Similarly, a 2-min water wash transferred ~5.0 log10 CFU/tomato of Salmonella from inoculated tomato (8.3 log10 CFU/tomato) to non-inoculated tomatoes (Gereffi et al., 2015). These data indicated that during post-harvest processing, a single contaminated fresh produce has the potential to compromise the whole batch or entire lot of fresh produce. It is of great necessity that the antimicrobial used in fresh produce wash can prevent cross-contamination. NEW and JC9450 at ~100 ppm FAC eliminated residual L. monocytogenes in the spent wash solutions and prevented cross-contamination to clean apples at both low and high contamination levels. In support of our data, NEW with 89 ppm FAC wash for 30 sec or 1 min eliminated L. monocytogenes in the spent solution post-L. monocytogenes-inoculated tomato washing (Deza et al., 2003).
Washes with acidic electrolyzed water (pH ~2.5) at 50 ppm FAC have been reported to reduce TPC and Y/M on cabbage and shredded carrots by ~2.0 log10 CFU/g after a 3-min exposure (Rahman et al., 2010, 2011). NEW at 50 ppm FAC and 3-min exposure reduced TPC on fresh-cut carrot slices by 1.8 log10 CFU/g (Izumi, 1999). In this study, however, JC9450 or NEW at 100 FAC had a limited efficacy in removing background bacteria and Y/M. A similar phenomenon was observed where a 30 sec treatment with 2% sodium orthophenylphenate formulated detergent did not decrease background bacteria of citrus fruit (Pao and Brown, 1998). The different efficacy against background microbiota might be due to the different produce surface composition and structure as well as indigenous bacterial species and ecology.
JC9450 and NEW at ~100 ppm FAC successfully prevented L. monocytogenes cross-contamination from both fruit-to-water and fruit-to-fruit but showed similar yet limited efficacy against L. monocytogenes on fresh apples. Future research is needed to evaluate the performance of both sanitizers in water with organic matter to mimic the commercial conditions. Meanwhile, the apple industry should continue to implement good manufacturing practices and preventive controls to minimize the contamination of products with L. monocytogenes or other pathogens, through a system-based framework and approach.
All datasets generated for this study are included in the article.
LS performed the experiment and wrote the manuscript. XS and OU assisted the sample analyses. M-JZ and LS designed the experiment. M-JZ, IH, and TS revised the manuscript.
This activity was funded by the Center for Produce Safety (2017CPS10) and Washington Tree Fruit Research Commission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Allan Brothers Inc. and Stemilt Growers LLC for their generous donation of fresh apples. We also thank Pace International, Jenfitch, and AquaOx for their kind donation of Accu-Tab, JC9450, and NEW, respectively. We acknowledge Tonia Green, Xia Song, Yuan Su, and Zi Hua for their assistance in sample preparation and processing.
Abadias, M., Usall, J., Oliveira, M., Alegre, I., and Vinas, I. (2008). Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally-processed vegetables. Int. J. Food Microbiol. 123, 151–158. doi: 10.1016/j.ijfoodmicro.2007.12.008
Allende, A., Selma, M. V., Lopez-Galvez, F., Villaescusa, R., and Gil, M. I. (2008). Impact of wash water quality on sensory and microbial quality, including Escherichia coli cross-contamination, of fresh-cut escarole. J. Food Prot. 71, 2514–2518. doi: 10.4315/0362-028X-71.12.2514
Banach, J. L., Sampers, I., Van Haute, S., and van der Fels-Klerx, H. J. (2015). Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int. J. Environ. Res. Public Health 12, 8658–8677. doi: 10.3390/ijerph120808658
Baskaran, S. A., Upadhyay, A., Kollanoor-Johny, A., Upadhyaya, I., Mooyottu, S., Amalaradjou, M. A. R., et al. (2013). Efficacy of plant-derived antimicrobials as antimicrobial wash treatments for reducing enterohemorrhagic Escherichia coli O157:H7 on apples. J. Food Sci. 78, M1399–M1404. doi: 10.1111/1750-3841.12174
Beuchat, L. R., Nail, B. V., Adler, B. B., and Clavero, M. R. S. (1998). Efficacy of spray application of chlorinated water in killing pathogenic bacteria on raw apples, tomatoes, and lettuce. J. Food Prot. 61, 1305–1311. doi: 10.4315/0362-028X-61.10.1305
Brown, D., Bridgeman, J., and West, J. R. (2011). Predicting chlorine decay and THM formation in water supply systems. Rev. Environ. Sci. Biotechnol. 10, 79–99. doi: 10.1007/s11157-011-9229-8
Burnett, S. L., Chen, J., and Beuchat, L. R. (2000). Attachment of Escherichia coli O157:H7 to the surfaces and internal structures of apples as detected by confocal scanning laser microscopy. Appl. Environ. Microbiol. 66, 4679–4687. doi: 10.1128/AEM.66.11.4679-4687.2000
CDC (2015). Multistate outbreak of listeriosis linked to commercially produced, prepackaged caramel apples made from Bidart Bros. apples (final update). Available at: https://www.cdc.gov/listeria/outbreaks/caramel-apples-12-14/index.html (Accessed October 17, 2019).
CFIA (2015). Sliced apples and products containing sliced apples recalled due to Listeria monocytogenes. Available at: http://www.inspection.gc.ca/about-the-cfia/newsroom/food-recall-warnings/complete-listing/2015-04-30/eng/1430431517170/1430431518530 (Accessed October 17, 2019).
Cioffi, N., Torsi, L., Ditaranto, N., Tantillo, G., Ghibelli, L., Sabbatini, L., et al. (2005). Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 17, 5255–5262. doi: 10.1021/cm0505244
Deza, M. A., Araujo, M., and Garrido, M. J. (2003). Inactivation of Escherichia coli O157:H7, Salmonella enteritidis and Listeria monocytogenes on the surface of tomatoes by neutral electrolyzed water. Lett. Appl. Microbiol. 37, 482–487. doi: 10.1046/j.1472-765X.2003.01433.x
DisinfectWater (2017). JC9400 SERIES Does it fit with EPA’s green chemistry program? Available at: http://disinfectwater.com/wp-content/uploads/2017/08/JC-9450-GREEN-CHEMISTRY-REVIEW.pdf (Accessed October 17, 2019).
Elhariry, H. M. (2011). Attachment strength and biofilm forming ability of Bacillus cereus on green-leafy vegetables: cabbage and lettuce. Food Microbiol. 28, 1266–1274. doi: 10.1016/j.fm.2011.05.004
FDA (2016). Fresh from Texas recalls apple product beacuse of possible health risk. Available at: https://www.fda.gov/Food/NewsEvents/ucm494345.htm (Accessed October 17, 2019).
FDA (2017a). BAM: detection and enumeration of Listeria monocytogenes. Available at: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071400.htm (Accessed October 17, 2019).
FDA (2017b). Jack Brown Produce, Inc. recalls Gala, Fuji, Honeycrisp and Golden Delicious apples due to possible health risk. Available at: https://www.fda.gov/Safety/Recalls/ucm589722.htm (Accessed October 17, 2019).
Francis, G. A., Gallone, A., Nychas, G. J., Sofos, J. N., Colelli, G., Amodio, M. L., et al. (2012). Factors affecting quality and safety of fresh-cut produce. Crit. Rev. Food Sci. Nutr. 52, 595–610. doi: 10.1080/10408398.2010.503685
Fukuzaki, S. (2006). Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 11, 147–157. doi: 10.4265/bio.11.147
Gereffi, S., Sreedharan, A., and Schneider, K. R. (2015). Control of Salmonella cross-contamination between green round tomatoes in a model flume system. J. Food Prot. 78, 1280–1287. doi: 10.4315/0362-028X.JFP-14-524
Gil, M. I., Marin, A., Andujar, S., and Allende, A. (2016). Should chlorate residues be of concern in fresh-cut salads? Food Control 60, 416–421. doi: 10.1016/j.foodcont.2015.08.023
Girschik, L., Jones, J. E., Kerslake, F. L., Robertson, M., Dambergs, R. G., and Swarts, N. D. (2017). Apple variety and maturity profiling of base ciders using UV spectroscopy. Food Chem. 228, 323–329. doi: 10.1016/j.foodchem.2017.02.012
Graca, A., Abadias, M., Salazar, M., and Nunes, C. (2011). The use of electrolyzed water as a disinfectant for minimally processed apples. Postharvest Biol. Technol. 61, 172–177. doi: 10.1016/j.postharvbio.2011.04.001
Hall, D. (1966). A study of the surface wax deposits on apple fruit. Aust. J. Biol. Sci. 19, 1017–1026. doi: 10.1071/BI9661017
Hayyan, M., Hashim, M. A., and AlNashef, I. M. (2016). Superoxide ion: generation and chemical implications. Chem. Rev. 116, 3029–3085. doi: 10.1021/acs.chemrev.5b00407
Izumi, H. (1999). Electrolyzed water as a disinfectant for fresh-cut vegetables. J. Food Sci. 64, 536–539. doi: 10.1111/j.1365-2621.1999.tb15079.x
Jenfitch (2018). Advanced oxidant using mineral oxychloride technology. Available at: https://jenfitch.com/advanced-oxidant-using-mineral-oxychloride-technology-to-help-improve-the-removal-of-pathogens-organic-contaminants-and-inorganic-contaminants/ (Accessed October 17, 2019).
Kang, D. H., and Fung, D. Y. C. (1999). Thin agar layer method for recovery of heat-injured Listeria monocytogenes. J. Food Prot. 62, 1346–1349. doi: 10.4315/0362-028X-62.11.1346
Lang, M. M., Harris, L. J., and Beuchat, L. R. (2004). Evaluation of inoculation method and inoculum drying time for their effects on survival and efficiency of recovery of Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes inoculated on the surface of tomatoes. J. Food Prot. 67, 732–741. doi: 10.4315/0362-028X-67.4.732
Li, J., and Zhang, L. Z. (2016). Synthesis and facet-dependent properties of layered BiOCl photocatalysts. Cham: Springer.
Lou, Y., and Yousef, A. E. (1997). Adaptation to sublethal environmental stresses protects Listeria monocytogenes against lethal preservation factors. Appl. Environ. Microbiol. 63, 1252–1255.
Nou, X., and Luo, Y. (2010). Whole-leaf wash improves chlorine efficacy for microbial reduction and prevents pathogen cross-contamination during fresh-cut lettuce processing. J. Food Sci. 75, M283–M290. doi: 10.1111/j.1750-3841.2010.01630.x
NSF (2012). Drinking water treatment chemicals-health effects. Available at: http://info.nsf.org/Certified/PwsChemicals/Listings.asp?Company=C0165759& (Accessed March 04, 2018).
Olmez, H., and Temur, S. D. (2010). Effects of different sanitizing treatments on biofilms and attachment of Escherichia coli and Listeria monocytogenes on green leaf lettuce. LWT-Food Sci. Technol. 43, 964–970. doi: 10.1016/j.lwt.2010.02.005
Pao, S., and Brown, G. E. (1998). Reduction of microorganisms on citrus fruit surfaces during packinghouse processing. J. Food Prot. 61, 903–906. doi: 10.4315/0362-028X-61.7.903
Pao, S., Kelsey, D. F., Khalid, M. F., and Ettinger, M. R. (2007). Using aqueous chlorine dioxide to prevent contamination of tomatoes with Salmonella enterica and Erwinia carotovora during fruit washing. J. Food Prot. 70, 629–634. doi: 10.4315/0362-028X-70.3.629
Peter, K. A. (2016). Demystifying copper for disease management. Available at: https://agresearch.umd.edu/sites/agresearch.umd.edu/files/_docs/locations/wye/2016%20Winter%20meeting_Copper.pdf (Accessed October 17, 2019).
Prado-Silva, L., Cadavez, V., Gonzales-Barron, U., Rezende, A. C. B., and Sant'Ana, A. S. (2015). Meta-analysis of the effects of sanitizing treatments on Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes inactivation in fresh produce. Appl. Environ. Microbiol. 81, 8008–8021. doi: 10.1128/AEM.02216-15
Rahman, S. M. E., Jin, Y. G., and Oh, D. H. (2010). Combined effects of alkaline electrolyzed water and citric acid with mild heat to control microorganisms on cabbage. J. Food Sci. 75, M111–M115. doi: 10.1111/j.1750-3841.2009.01507.x
Rahman, S. M. E., Jin, Y. G., and Oh, D. H. (2011). Combination treatment of alkaline electrolyzed water and citric acid with mild heat to ensure microbial safety, shelf-life and sensory quality of shredded carrots. Food Microbiol. 28, 484–491. doi: 10.1016/j.fm.2010.10.006
Rahman, S. M. E., Khan, I., and Oh, D. H. (2016). Electrolyzed water as a novel sanitizer in the food industry: current trends and future perspectives. Compr. Rev. Food Sci. Food Saf. 15, 471–490. doi: 10.1111/1541-4337.12200
Salomao, B. C. M., Aragao, G. M. F., Churey, J. J., and Worobo, R. W. (2008). Efficacy of sanitizing treatments against Penicillium expansum inoculated on six varieties of apples. J. Food Prot. 71, 643–647. doi: 10.4315/0362-028X-71.3.643
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 17, 7–15. doi: 10.3201/eid1701.P11101
Shen, X., Sheng, L., Gao, H., Hanrahan, I., Suslow, T., and Zhu, M. J. (2019). Enhanced efficacy of peroxyacetic acid against Listeria monocytogenes on fresh apples at elevated temperature. Front. Microbiol. 10:1196. doi: 10.3389/fmicb.2019.01196
Sheng, L., Edwards, K., Tsai, H. C., Hanrahan, I., and Zhu, M. J. (2017). Fate of Listeria monocytogenes on fresh apples under different storage temperatures. Front. Microbiol. 8:1396. doi: 10.3389/fmicb.2017.01396
Sheng, L., Hanrahan, I., Sun, X., Taylor, M. H., Mendoza, M., and Zhu, M.-J. (2018). Survival of Listeria innocua on Fuji apples under commercial cold storage with or without low dose continuous ozone gaseous. Food Microbiol. 76, 21–28. doi: 10.1016/j.fm.2018.04.006
Smilanick, J. L., Mansour, M., and Sorenson, D. (2014). Performance of fogged disinfectants to inactivate conidia of Penicillium digitatum within citrus degreening rooms. Postharvest Biol. Technol. 91, 134–140. doi: 10.1016/j.postharvbio.2013.12.020
Suslow, T. (2005). Chlorination in the production and postharvest handling of fresh fruits and vegetables. Available at: https://www.siphidaho.org/env/pdf/Chlorination_of_fruits_and_veggies.PDF (Accessed August 10, 2019).
Torlak, E. (2014). Inactivation of Alicyclobacillus acidoterrestris spores in aqueous suspension and on apples by neutral electrolyzed water. Int. J. Food Microbiol. 185, 69–72. doi: 10.1016/j.ijfoodmicro.2014.05.022
Ukuku, D. O., and Fett, W. F. (2006). Effects of cell surface charge and hydrophobicity on attachment of 16 Salmonella serovars to cantaloupe rind and decontamination with sanitizers. J. Food Prot. 69, 1835–1843. doi: 10.4315/0362-028X-69.8.1835
Upadhyay, A., Upadhyaya, I., Mooyottu, S., Kollanoor-Johny, A., and Venkitanarayanan, K. (2014). Efficacy of plant-derived compounds combined with hydrogen peroxide as antimicrobial wash and coating treatment for reducing Listeria monocytogenes on cantaloupes. Food Microbiol. 44, 47–53. doi: 10.1016/j.fm.2014.05.005
Yang, X. J., Xu, X. M., Xu, J., and Han, Y. F. (2013). Iron oxychloride (FeOCl): an efficient Fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants. J. Am. Chem. Soc. 135, 16058–16061. doi: 10.1021/ja409130c
Keywords: fresh apple, Listeria monocytogenes, neutral electrolyzed water, JC9450, antimicrobial, cross-contamination
Citation: Sheng L, Shen X, Ulloa O, Suslow TV, Hanrahan I and Zhu M-J (2020) Evaluation of JC9450 and Neutral Electrolyzed Water in Controlling Listeria monocytogenes on Fresh Apples and Preventing Cross-Contamination. Front. Microbiol. 10:3128. doi: 10.3389/fmicb.2019.03128
Received: 18 July 2019; Accepted: 24 December 2019;
Published: 14 January 2020.
Edited by:
Gianluigi Mauriello, Università degli Studi di Napoli Federico II, ItalyReviewed by:
Keith Warriner, University of Guelph, CanadaCopyright © 2020 Sheng, Shen, Ulloa, Suslow, Hanrahan and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei-Jun Zhu, bWVpanVuLnpodUB3c3UuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.