
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 01 October 2019
Sec. Infectious Agents and Disease
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.02199
This article is part of the Research Topic Role Of Small Colony Variants (SCVs) And Persisters In Staphylococcus aureus Pathogenesis And Development Of Therapeutic Approaches View all 9 articles
Staphylococcus aureus is an opportunistic pathogen that causes acute and chronic infections. Due to S. aureus’s highly resistant and persistent nature, it is paramount to identify better drug targets in order to eradicate S. aureus infections. Despite the efforts in understanding bacterial cell death, the genes, and pathways of S. aureus cell death remain elusive. Here, we performed a genome-wide screen using a transposon mutant library to study the genetic mechanisms involved in S. aureus cell death. Using a precisely controlled heat-ramp and acetic acid exposure assays, mutations in 27 core genes (hsdR1, hslO, nsaS, sspA, folD, mfd, vraF, kdpB, USA300HOU_2684, 0868, 0369, 0420, 1154, 0142, 0930, 2590, 0997, 2559, 0044, 2004, 1209, 0152, 2455, 0154, 2386, 0232, 0350 involved in transporters, transcription, metabolism, peptidases, kinases, transferases, SOS response, nucleic acid, and protein synthesis) caused the bacteria to be more death-resistant. In addition, we identified mutations in 10 core genes (capA, gltT, mnhG1, USA300HOU_1780, 2496, 0200, 2029, 0336, 0329, 2386, involved in transporters, metabolism, transcription, and cell wall synthesis) from heat-ramp and acetic acid that caused the bacteria to be more death-sensitive or with defect in persistence. Interestingly, death-resistant mutants were more virulent than the parental strain USA300 and caused increased mortality in a Caenorhabditis elegans infection model. Conversely, death-sensitive mutants were less persistent and formed fewer persister cells upon exposure to different classes of antibiotics. These findings provide new insights into the mechanisms of S. aureus cell death and offer new therapeutic targets for developing more effective treatments for infections caused by S. aureus.
Staphylococcus aureus is a major bacterial pathogen that colonizes on the skin of over one-third of the human population and can cause acute infections such as bacteremia, pneumonia, meningitis and persistent infections such as osteomyelitis, endocarditis, and biofilm infections such as on prosthetic implants (Tong et al., 2015). Due to emerging resistance and high risk of nosocomial infections and community-acquired infections, S. aureus infections are a major public health concern. To develop better treatments for S. aureus and other bacterial infections, understanding how bacterial cells die is crucial.
By definition, bacteria treated with bactericidal antibiotics, such as the β-lactams, quinolones, and aminoglycosides, are killed. Much is known about how the drug makes contact with its target in the bacterial cell. β-lactam antibiotics bind to penicillin binding protein (PBP) disrupting proper cell wall synthesis; quinolones bind to topoisomerase or gyrase blocking DNA replication, and aminoglycosides bind to ribosomal proteins resulting in mistranslated proteins (Davis, 1987; Arthur and Courvalin, 1993; Drlica et al., 2008). Despite having different drug-target interactions, the downstream effects of drug lethality of cidal antibiotics are similar. It has been proposed that bacteria treated with cidal antibiotics have pathways such as SOS response, TCA cycle, ROS formation damaging Fe-S cluster regardless of the drug target (Kohanski et al., 2007, 2010), however, the ROS theory of cidal antibiotic lethality has been challenged as killing of bacteria can still occur in apparently anaerobic conditions that do not produce ROS (Malik et al., 2007; Keren et al., 2013; Liu and Imlay, 2013).
Mechanisms pertaining to bacterial cell death were mainly characterized in toxin-antitoxin systems. One of the better characterized Toxin-Antitoxin modules in the context of bacterial cell death is the MazEF module found in E. coli and other species such as Listeria, Enterococcus, Neisseria, Streptococcus, and Mycobacterium (Engelberg-Kulka and Glaser, 1999; Engelberg-Kulka et al., 2006; Zhu et al., 2006; Amitai et al., 2009). Upon exposure to stresses such as nutrient depletion, DNA damage, temperature, antibiotics, and oxidative radicals, the MazF antitoxin is degraded and hence, the MazE toxin can degrade cellular mRNA causing cellular shutdown (Engelberg-Kulka and Glaser, 1999; Amitai et al., 2009). In particular to S. aureus, the CidA and LrgA proteins, which are holin-like molecules with analogous functions to apoptotic regulators of the BCL-2 family in eukaryotes, were proposed to play a role in death and lysis of S. aureus (Groicher et al., 2000; Rice et al., 2003). However, the specific process as to how CidA and LrgA regulate cell death is poorly defined.
Although cell death is an important process of a living organism, little is known about the mechanisms. High-throughput screens have been developed to study the cell death mechanism of unicellular eukaryotic organisms such as S. cerevisiae upon stress signals from high temperature and acetic acid (Ludovico et al., 2001; Teng and Hardwick, 2009, 2013; Sousa et al., 2013), both of which also induce death in bacteria. Here, we performed a high-throughput genetic screen using a transposon mutant library of USA300 to identify genes involved in cell death in S. aureus (Fey et al., 2013). Under multiple death stimuli, we identified 27 genes whose mutations caused the bacteria to be more death-resistant, while mutations in 10 genes caused the bacteria to be more death-sensitive.
Meropenem, moxifloxacin, rifampicin, gentamicin, and erythromycin were obtained from Sigma-Aldrich Co. (St. Louis, MO, United States). Stock solutions were prepared in the laboratory, filter-sterilized and used at indicated concentrations. Bacterial strains used in this study included USA300 and the Nebraska-Transposon Mutant Library (NTML) (Fey et al., 2013). S. aureus strains were cultured in tryptic soy broth (TSB) and tryptic soy agar (TSA) with the appropriate antibiotics and growth conditions. Transposon-insertion mutants were grown in the presence of erythromycin (50 μg/ml), the antibiotic selective marker.
For the heat-ramp, we performed the assay as described (Teng and Hardwick, 2013). Briefly, we normalized the concentration of the bacteria to OD600 = 0.5 using PBS as the diluent. Then, we placed the samples in the thermocycler with a protocol following: 30°C for 1 min, ramp from 30°C to 62°C with a step-interval of 0.5°C per 30 s. For acid stress, acetic acid (6 mM) were added into stationary phase cultures (when the pH changed slightly from 5.0 to 4.7) and incubated overnight. To enumerate for cell counts, the mutant library was replica transferred to TSA plates to score for mutants that failed to grow after stress. For viability staining, SYBR Green I/PI staining was performed as described (Feng et al., 2014, 2018). SYBR Green I (10,000 × stock, Invitrogen) was mixed with PI (20 mM, Sigma) in distilled H2O with a ratio of 1:3 (SYBR Green I to PI) in 100 ul distilled H2O and stained for 30 min in room temperature. Prior to the heat-ramp, the SYBR Green I/PI dye was added to the bacteria as the heat-ramp impaired the uptake of the dye. For the acetic acid stress, the dyes were added at the end of the exposure (Teng and Hardwick, 2013). The green and red fluorescence intensity was detected using a Synergy H1 microplate reader by BioTek Instruments (VT, United States) at excitation wavelength of 485, 538, and 612 nm for green and red emission, respectively. The live/dead ratio was calculated by dividing the green/red fluorescence.
Selected drugs were added to overnight stationary phase cultures for 6 days as described previously (Yee et al., 2015). Briefly, at the selected time points, bacterial cultures were washed with 1 × PBS to remove stress, serially diluted, and plated onto TSA with no drugs for cell enumeration (Yee et al., 2015). Drugs were tested at 10X MIC (minimal inhibitory concentration): gentamicin (60 μg/ml), meropenem (20 μg/ml), moxifloxacin (40 μg/ml), and rifampin (2 μg/ml).
Caenorhabditis elegans N2 Bristol worms (Caenorhabditis Genetics Center) were synchronized to the same growth stage by treatment with alkaline hypochlorite solution as described (Porta-de-la-Riva et al., 2012). Worms of adult stage were washed and suspended in bleaching solution with 5% hypochlorite for 9 min to lyse all the adult stages but keeping the eggs intact. Bleach was removed by centrifugation at 1,500 rpm for 1 min and washed three times with M9 buffer. The pellet was incubated in M9 buffer at 20°C with gentle agitation and proper aeration. L4 stage adult worms were obtained after 48 h at 20°C. For each assay, 20–30 worms were added to liquid M9 buffer supplemented with 5- Fluoro-2′-deoxyuridine (10 μM) to inhibit progeny formation. S. aureus (106 CFU) (Yee et al., 2019) that were grown overnight at 30°C in TSB containing the appropriate antibiotics as needed were added into the buffer containing the worms. The samples were scored for live and dead worms every 24 h.
To better understand the mechanisms of cell death, we performed a genetic screen using the Nebraska Transposon Mutant Library (NTML) which contains mutations in all the non-essential genes of S. aureus USA300, the most common circulating community-acquired MRSA strain in the United States (Fey et al., 2013). To design our assay, we utilized the heat-ramp assay that has been used to study cell death programs in yeast (Teng and Hardwick, 2013). To determine viability, we employed both the traditional agar replica plating for visualization of viable growth on solid media but also stained cells with SYBR Green I/PI, a viability stain that can detect both live and dead cells (Feng et al., 2014, 2018; Supplementary Figure S1). Using a cidA mutant (Rice et al., 2003) which has been shown to be death-resistant as a control and the parental strain of USA300 as a death sensitive control, we optimized the condition of our heat-ramp experiment to show the biggest difference between both the death-resistant and death-sensitive phenotypes based on agar plating and the live/dead ratio from viability staining with SYBR Green I/PI.
For identification of death-resistant mutants, we searched for clones that survive heat-ramp and grow on agar plating (as opposed to USA300 which no longer show colony growth from replica plating) and a live/dead ratio that is higher than our death-resistant mutant, cidA control. After the heat-ramp exposure, we identified 74 mutants that were death-resistant. While we cannot pinpoint a specific gene to be the ultimate regulator of cell death, we generated a list of potential regulators of cell death. In order to identify core genes and pathways involved in cell death, we exposed the transposon mutant library to acetic acid stress.
Acetic acid has been shown to induce cell death in S. aureus and in yeast (Ludovico et al., 2001; Rice et al., 2003; Teng and Hardwick, 2009; Windham et al., 2016). Upon treatment with acetic acid (6 mM) overnight, a condition in which USA300 was death-sensitive and the cidA mutant was death-resistant, 27 (hsdR1, hslO, nsaS, sspA, folD, mfd, vraF, kdpB, USA300HOU_2684, 0868, 0369, 0420, 1154, 0142, 0930, 2590, 0997, 2559, 0044, 2004, 1209, 0152, 2455, 0154, 2386, 0232, and 0350) out of the 74 heat-ramp resistant mutants were also acetic acid resistant (Figure 1). A majority of the genes identified encode transporters (n = 9), involved in transcription (n = 4), metabolism (n = 3), peptidases (n = 2), and phosphatases and kinases (n = 2). For genes encoding transferases and proteins involved in stress response, nucleic acid synthesis and protein synthesis, one candidate was found in each category (Table 1).
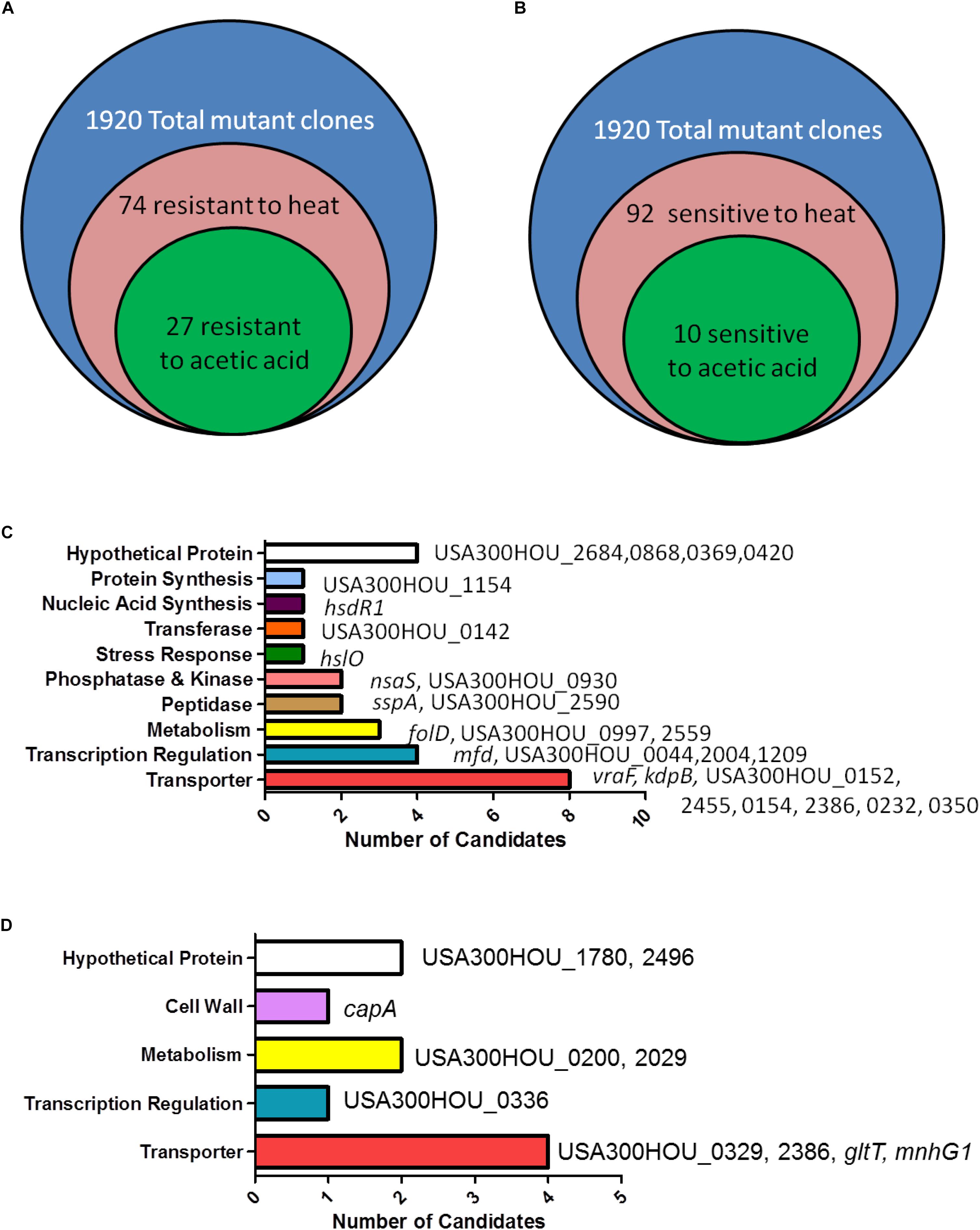
Figure 1. Identification of genes involved in causing bacterial cell death-resistance and cell death-sensitivity. (A) Summary of the number of candidates identified from the Nebraska Transposon Mutant Library (NTML) as being more death-resistant (A) and death-sensitive (B) in heat-ramp and acetic acid stress. The breakdown of the respective categories of genes whose mutations caused death-resistance (C) and death-sensitivity (D) to both stresses.
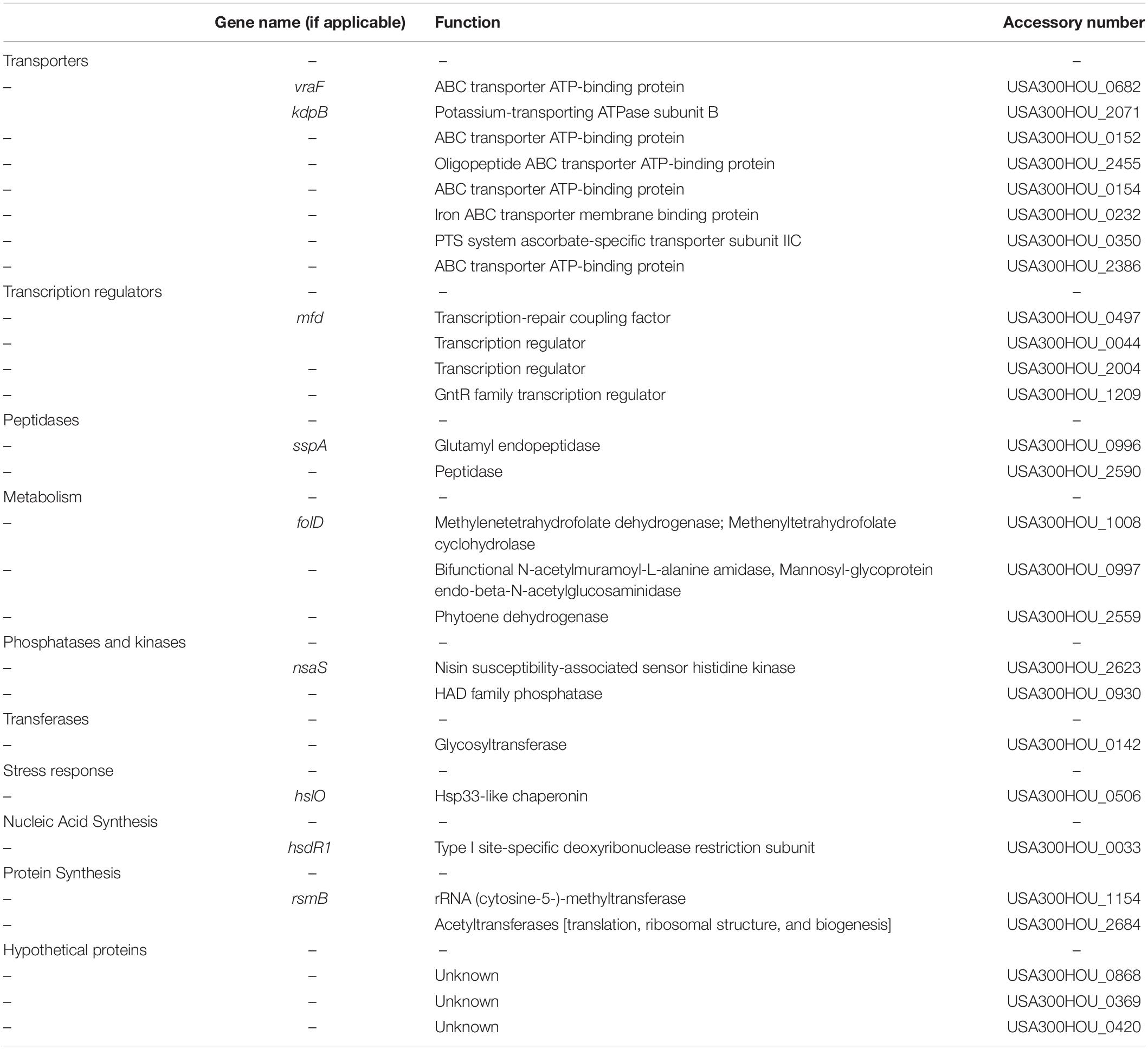
Table 1. Genes whose mutations resulted in cell death resistance to both heat-ramp stress and acetic acid stress.
Upon infection inside a host, in addition to drug stress, the bacteria are exposed to various types of stresses such as oxidative stress especially in the phagosomes of immune cells. We then tested if our death-resistant mutants were more virulent in causing an infection inside the host. After infection of C. elegans with the top 4 death-resistant mutants (folD, USA300HOU_0997, sspA, USA300HOU_0232) with the highest viability (e.g., live/dead ratio) as determined by SYBR Green I/PI staining and parental strain USA300, we observed that all four mutants significantly decreased the survival of the C. elegans and killed the worms faster than USA300 (Figure 2). By 2 days post-infection, the survival of worms infected with our death-resistant mutants had a survival rate of 36% or lower while worms infected with USA300 had a survival of 50%. The most virulent strain was Tn:USA300HOU_0232, a mutation in an iron transporter, as it caused the greatest mortality in the worms, resulting with only 22% survival of the worms by day 2. Our data suggest that bacteria that are more death-resistant could potentially cause more serious infections.
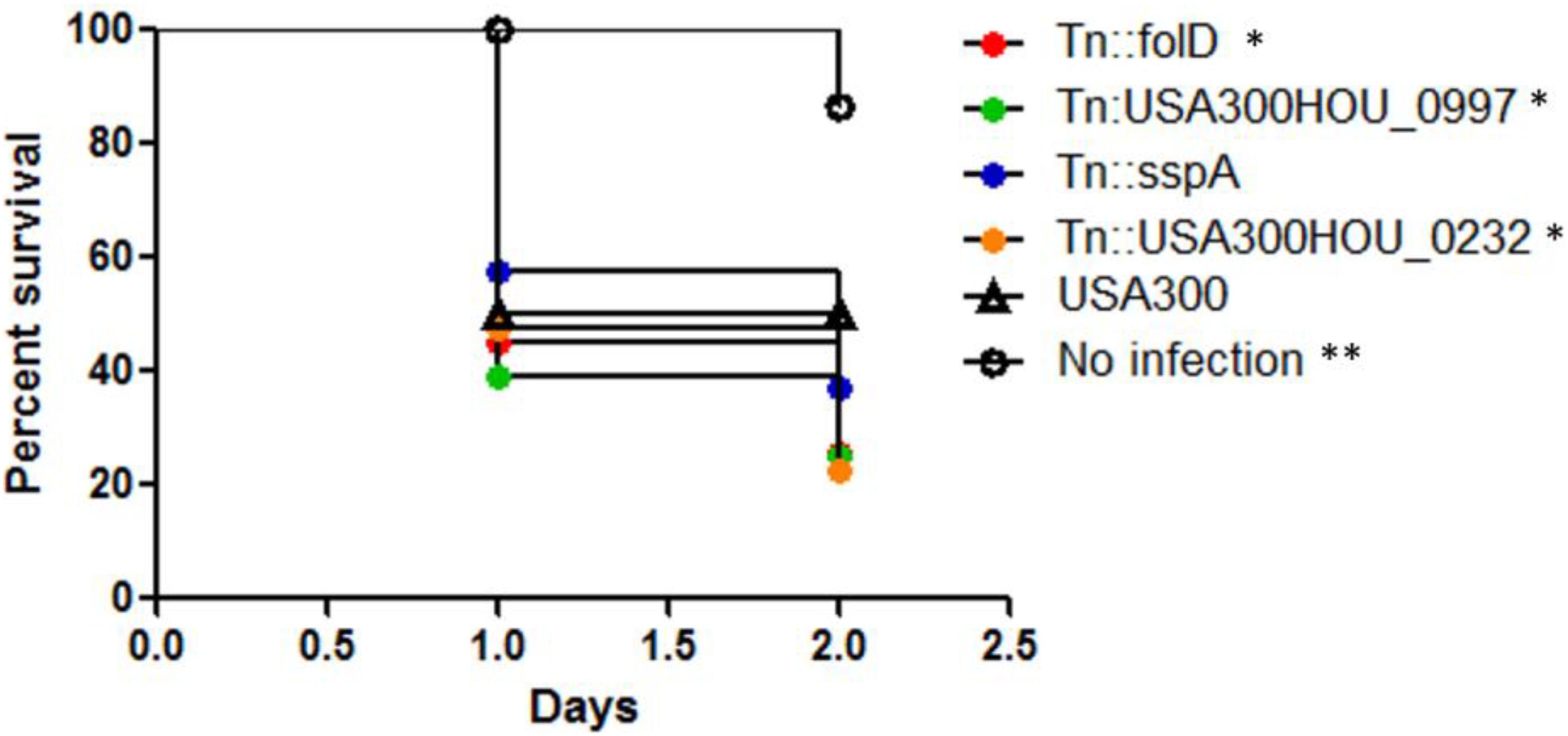
Figure 2. Strains that were more death-resistant caused more virulent infections in Caenorhabditis elegans. C. elegans (n = 20–30) infected (106 CFU) with mutants showing resistance to cell death showed increased mortality than C. elegans infected with S. aureus parental strain USA300 (Log–rank test, ∗p < 0.05 and ∗∗p < 0.005).
In order to fully understand the regulatory networks of cell death pathways, it is crucial to examine the genes whose mutations cause the cells to be more sensitive to stress as well. Using the data from our two screens (heat-ramp and acetic acid stress), we adjusted the parameters of data analysis to distinguish mutants that were cell death sensitive. Unlike the cut-offs mentioned previously, cell death sensitive mutants were identified as having no viable growth on agar media and a live/dead ratio that was lower than USA300.
Our screen revealed that 92 mutants were hypersensitive to the heat-ramp, of which 10 (capA, gltT, mnhG1, USA300HOU_1780, 2496, 0200, 2029, 0336, 0329, and 2386) were also sensitive to acetic acid stress (Figure 1 and Table 2). Transporters were the more abundant (n = 4) followed by genes involved in metabolism (n = 2), lastly with genes involved in transcription (n = 1), and cell wall synthesis (n = 1). Two of the candidates were hypothetical proteins. Interestingly, two (USA300HOU_2029, gltT) of the top four death-sensitive mutants (USA300HOU_0329, USA300HOU_2029, USA300HOU_2386, and gltT) harbored mutations pertaining to glutamate metabolism.
One of the reasons why S. aureus can cause persistent and recalcitrant infections is due to its ability to form persister cells. Persisters are dormant cells that are formed during stressed conditions and upon stress removal, the bacteria can revert back to a growing state and consequently, cause a relapse in infection (Zhang, 2014). Bacterial persistence can also be viewed as cells with a strong anti-death program. Given now that we have identified genes whose mutation renders the bacteria death-sensitive, we then wanted to know if these mutations also lead to defect in persistence, forming lower amounts of persister cells. Such mutations could then potentially be drug targets for clearing persistent infections.
Persisters can be obtained by treating stationary phase bacteria with high concentrations of bactericidal antibiotics (usually at least 10 × MIC). Cells are then washed to rid of stress and plated on solid medium with no drug for CFU enumeration (Yee et al., 2015). We exposed the top 4 death-sensitive mutants to bactericidal antibiotics with different mechanisms of action: gentamicin, meropenem, rifampin, and moxifloxacin. Upon 6-day exposure of antibiotics, all 4 mutants showed a defect in persistence to different classes of antibiotics (Figure 3). The amount of persisters is dependent on the type of stress which can be seen here since the absolute amount of persisters changes among the drugs tested (Zhang, 2014). However, the overall amount of persister cells formed by the death-sensitive mutants was significantly lower than the parent strain USA300. The defect in persistence was the most prominent for gentamicin stress. Under gentamicin stress, mutations in gltT (proton/sodium-glutamate symport protein) and USA300HOU_2029 (amidohydrolase), both involved in glutamate metabolism, were completely killed by 4 day-post exposure while USA300 still had over 107 CFU/ml. In non-stressed conditions, no growth defects and decrease in viability of cells were observed.
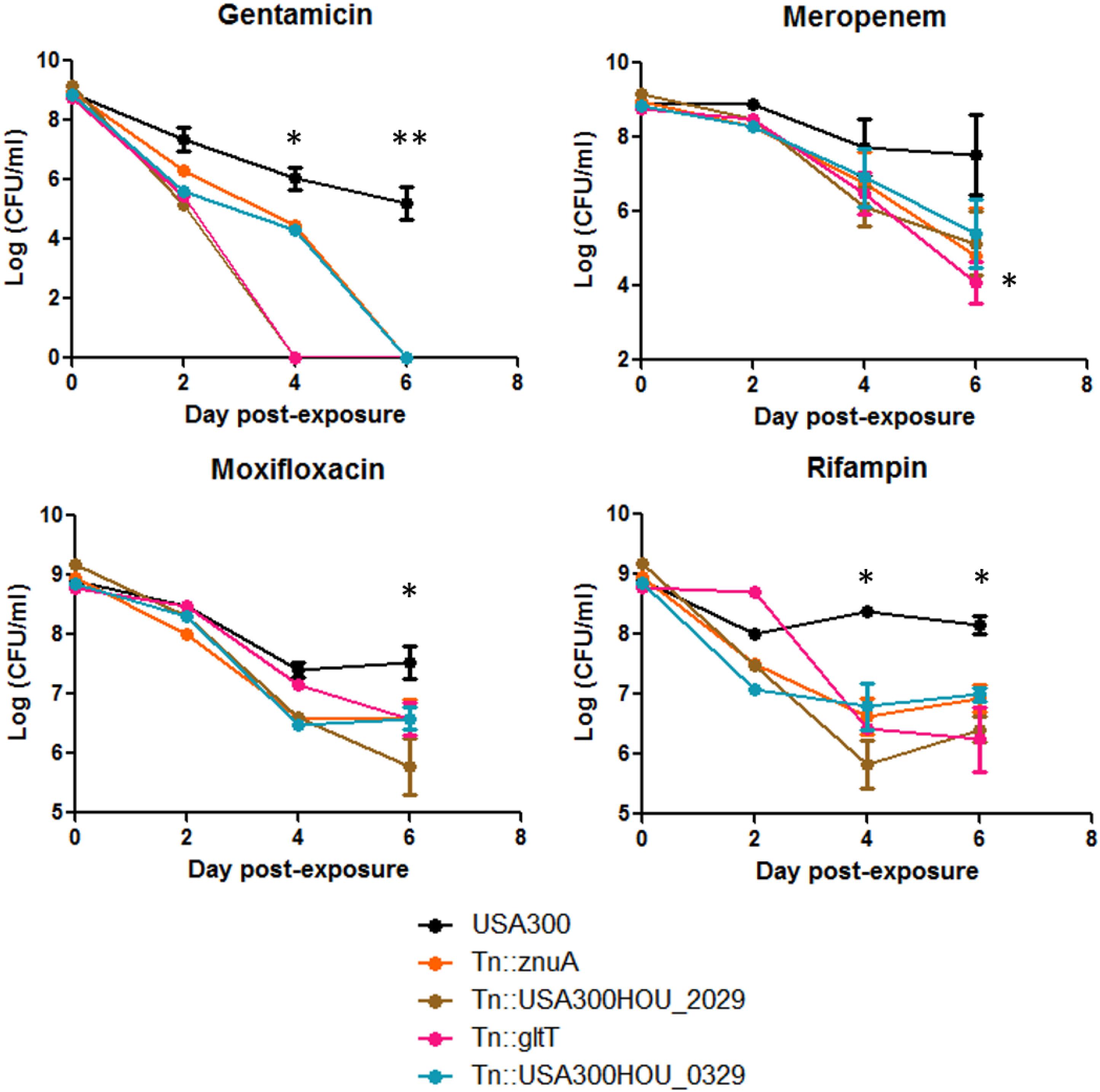
Figure 3. Strains that were more death-sensitive also show decreased persistence to bactericidal antibiotics. Mutants that were more cell-death sensitive showed defective persistence to gentamicin (60 μg/ml), meropenem (20 μg/ml), moxifloxacin (40 μg/ml), and rifampin (2 μg/ml) upon prolonged drug exposure up to 6 days (Student’s t-test, ∗p < 0.05 and ∗∗p < 0.005).
To our knowledge, this is the first comprehensive study to identify genes and pathways that play a role in anti-death and pro-death programs in S. aureus. In particular, we found 27 core genes (folD, mfd, kdpB, vraF, sspA, hsdR1, hslO, nsaS, USA300HOU_2684, 0868, 0369, 0420, 1154, 0142, 0930, 2590, 0997, 2559, 0044, 2004, 1209, 0152, 2455, 0154, 2386, 0232, and 0350) involved in transporters, transcription, metabolism, peptidases, kinases, transferases, SOS response, nucleic acid, and protein synthesis) that caused the bacteria to be more death-resistant. The findings that glutamate metabolism and glutamate transport are important for transformation of a bacterial cell into both a more death-resistant and death-sensitive phenotype under stressed conditions are of particular interest. We show that mutations in genes involved in intracellular glutamate level regulation such as gltT and USA300HOU_2029 can decrease cell viability and persistence under antibiotic stresses, and environmental stresses such as temperature changes (heat-ramp) and low pH (acetic acid).
While other studies have searched for cell death genes in S. aureus (Groicher et al., 2000; Rice and Bayles, 2003; Rice et al., 2003; Sadykov et al., 2013), the specific pathway of glutamate metabolism has never been identified as core cell death proteins. Previous studies suggest that TCA cycle activity, inhibition of ROS production, and activation of stress response pathways can promote cell survival during stress conditions. Sadykov et al. (2013) identified that inactivation of the phosphotransacetylase-acetate kinase (Pta-AckA) pathway which normally generates acetate from acetyl-CoA, a byproduct of the TCA cycle, leads to cell death in S. aureus under glucose, and oxygen excess. In bacteria, glutamate fermentation can occur via 3-methylaspartate which produces pyruvate followed by acetyl-CoA. Considering that acetate can be produced from acetyl-CoA, our findings may help explain the events that occur upstream of Pta-AckA activation (Herrmann et al., 2008). Additionally, the lethality induced by cidal antibiotics has been shown to be due to ROS generation and radical generation from the Fenton reaction suggesting that death mechanisms result in oxidative responses within the cell (Kohanski et al., 2007). In Francisella, glutamate transporter GadC has been shown to neutralize reactive oxygen species (Ramond et al., 2014). A study performed to evaluate the bactericidal effect of CO-releasing molecules (CO-RMs) showed that CO-RMs stimulated the production of intercellular ROS in the bacteria which was abolished when glutamate was supplemented to the culture (Tavares et al., 2011). Thus, increased glutamate levels in the stressed cells may protect the cell from ROS-mediated cell death under stress.
Findings from our previous screens performed to identify genes involved in persistence to rifampicin (Yee et al., 2015) showed that genes gltS, gltD, gltA, all of which are involved in glutamate synthesis, were important. Intriguingly, the protein ArgJ was shown to be a potential core regulator for S. aureus persistence in various stresses (different classes of drugs, heat, and low pH) (Yee et al., 2017). Glutamate is the substrate for ArgJ (Figure 4) and since mutants with impaired glutamate biosynthesis and transport showed both death-sensitive and defective persistence phenotypes, it can be speculated that glutamate can be the main driver of anti-death or equivalent to elevated persistence programs where arginine synthesis is a potential downstream effector pathway. Further biochemical studies and metabolomic studies are needed to understand how glutamate and arginine are involved in cell death. Based on our findings, the current knowledge about TCA cycle, ROS, and glutamate, we propose a model that links these events together in the context of cell death as shown in Figure 4, which can offer new insights into how intracellular glutamate levels affect the cell death program of S. aureus.
Our screen also identified mutants whose phenotypes are cell death resistant and when the top four death-resistant mutants were tested in an in vivo C. elegans model, we found that they are indeed more virulent than the parental strain USA300 (Figure 2). The mutant that was the most virulent harbored a mutation in USA300HOU_0232 which encodes an iron transporter. Similarly, the nsaS mutant was also identified to be death-resistant from our screens and previous studies in S. aureus have shown that NsaS is part of the cell-envelope two-component sensing system (Blake et al., 2011; Kolar et al., 2011). In a mutant of nsaS in S. aureus, there is decreased association with metal ions on the cell surface and as a result, the intracellular concentration of metal ions were reduced (Kolar et al., 2011). Considering how ROS may play a role in cell death, a mutated iron transporter may cause the cells to be death-resistant by decreasing the amount of ROS inside the cell (Birben et al., 2012) as the transporter limits the amount of iron needed for the Fenton reaction. USA300HOU_0997 encodes an autolysin family protein that plays a role in peptidoglycan turnover (Kajimura et al., 2005). Gene expression of autolysins has been implicated in increasing cell wall stability under stressed conditions (Utaida et al., 2006). In vancomycin resistant strains, autolysin genes were downregulated which led to changes in membrane charges and thickness of the cell wall enabling the cell to survive treatment of cell wall inhibitors (Cafiso et al., 2012). It can be speculated here that decreased cell division and peptidoglycan turnover in our USA300HOU_0997 mutant may lead to death resistance. folD is a bifunctional protein that allows the production of continuous tetrahydrofolate which is a key metabolite for amino acid and nucleic acid biosynthesis (Umbarger, 1978). Folate metabolism is important for bacteria in providing folate cofactors for the synthesis of purines, thymidylate, glycine, methionine, and tRNAMet. Mutation in folD may lead to lower metabolism and thus allow the bacteria to enter dormant state for continued survival under stressed conditions. Lastly, the SspA is a serine protease a member of the glutamyl endopeptidase family of enzymes that cleaves proteins rich in glutamate such as fibronectin binding proteins. Serine proteases such as SspA are under the agr quorum-sensing control operon (Thoendel et al., 2011) which coordinates a series of gene expression cascades to withstand stresses such as oxidative stress and the human immune response and facilitate bacterial cell growth and pathogenesis (Dunny and Leonard, 1997; Sun et al., 2012). It has been shown that agr negative cells do not undergo cell death as rapidly and were more resistant to cell lysis due to the increased expression of RNAIII (Fujimoto and Bayles, 1998; Paulander et al., 2018). The increased mortality of C. elegans infected with the SspA mutant suggests that downregulation of agr gene could potentially be involved in causing cell death resistance because agr-negative S. aureus strains can be found in hosts with chronic infections and cause infections with increased mortality compared to those infected with agr-positive strains (Schweizer et al., 2011).
Interestingly, our screen revealed that mutants of hslO and mfd were more death-resistant which may appear contradictory given that expression of HslO and Mfd are protective for the cell during stress (Utaida et al., 2003; Darrigo et al., 2016). HslO (or Hsp33) has been shown to be upregulated especially during oxidative stress and that HslO levels were decreased when S. aureus cells were transitioning to slow/non-growing status (Michalik et al., 2017). It is well known that Mfd is involved in preventing DNA damage from oxidative stress, immune response, and drugs (Wang and Maier, 2015; Darrigo et al., 2016). Decreased expression of Mfd in S. aureus led to decreased biofilm formation (Tu Quoc et al., 2007) and a mfd mutant of C. difficle has increased toxin production (Willing et al., 2015). For B. subtilis, Mfd-deficient cells formed less endospores (Ramirez-Guadiana et al., 2013) and resulted in a 35-fold overexpression of OhrR, a transcription factor involved in peroxide stress response (Martin et al., 2019). Given that Mfd has many non-conical roles, we speculate that under stressed conditions decrease of expression in our mfd mutant may allow expression of another transcription factor (such as the OhrR) to activate stress response pathways. It is also possible that the contradictory phenotypes of the hslO and mfd mutants are due to compensatory mutations in the hslO and mfd mutants, which are known to occur in loss of function mutants in yeast (Teng et al., 2013). Additionally, our speculation as to why a hsdR mutant is more death-resistant could also be associated to the genetic background of the mutant. Inactivation of HsdR, an endonuclease of the type 1 restriction system in S. aureus, allowed S. aureus to become readily transformable due to decreased cleavage of foreign DNA (Waldron and Lindsay, 2006; Corvaglia et al., 2010). While there is a possibility of secondary mutations developing in the mutant libraries, we performed the screen twice followed by individual confirmation of the cell death phenotype. Sequencing of all our mutants did not reveal any mutations.
Our work adapted assays that are used in the yeast community to identify genes involved in yeast cell death (Ludovico et al., 2001; Teng and Hardwick, 2009, 2013; Sousa et al., 2013). In the high-throughput screen consisting of yeast mutants and even in yeast strains of different backgrounds, categories of genes that were redundant among the studies include carbohydrate metabolism, transcription factors, and ion transport (Kawahata et al., 2006; Mira et al., 2010; Sousa et al., 2013). Interestingly, amino acid transport was the most significantly enriched term for genes involved in positive regulation of acetic acid-induced death; two of the identified transporters were involved in metabolism of glutamate (GDH1 and GDH2) (Sousa et al., 2013). A similar approach of a “heat-ramp” stress was used in one study on B. subtilis using a water bath (Kort et al., 2008). Their study revealed that heat shock proteins, sporulation, competence, and carbon metabolism were important. While we did not identify similar genes, carbon metabolism was identified in both our screens. Heat shock proteins were heavily enriched in their study but not ours which can be attributed to the candidates in our library which only contain mutants of non-essential genes and only 2 candidates out of 1,952 were heat shock proteins (Bose et al., 2013).
Despite the significant findings of this study, there are some limitations. First, while we generated a list of the mutants that are death-sensitive and death-resistant to both heat ramp and acetic acid stress, the screens performed here are only a snapshot of what is happening in the cell. The phenotypes seen here were determined by the condition of our assays and can be affected by the level of stress (e.g., concentration of the acetic acid, ramping time, and temperature for the heat ramp) (Teng and Hardwick, 2013). Although we included both a wild type USA300 strain and a CidA mutant, a known death-resistant mutant (Rice et al., 2003; Ranjit et al., 2011), to optimize our conditions, it is important to recognize that our screen may not be comprehensive in finding all the mediators of cell death. While our goal was more conservative and was to search for core regulators, the significant role of other genes and pathways involved in cell death that were not classified as core regulators should not be ignored. Our screen only explored cell death at the DNA level and further protein studies would provide more comprehensive insight into the effects of transposon insertions on regulating cell death. Even though this mutant library contained all the non-essential genes in USA300, we cannot overrule the fact that cell death is an important program for any living organism and cell death regulators may be essential genes which are not included in our library. In addition, complementation of the mutants with the wild type genes would be needed to confirm the role of the key genes in the future. Additionally, we did not observe any small colony variants in our screen but that could possibly be due to our agar plating method which does not allow for visualization of a single colony.
Given that we know the killing activity of antibiotics extend beyond the drug-target interactions (Kohanski et al., 2007), understanding the effector proteins and downstream events of bacterial cell death can help provide novel drug treatment approaches for bacterial infections. For example, cell death can be artificially induced by disruption of the negative regulator of cell death in a similar fashion to how apoptotic pathways are exploited to treat cancer cells. Recently, it was shown that extracellular death peptides from both E. coli and P. aeruginosa can induce toxic endoribonucleolytic activities of MazEF in M. tuberculosis suggesting promising therapeutic outcomes upon manipulation of the cell death mediators in bacteria (Nigam et al., 2018). Similarly, since ROS formation serves an important role in the lethality of cidal antibiotics, a drug combination with metabolic perturbations may enhance killing of currently used antibiotics. For example, defects in peroxide-detoxifying enzymes have been shown to increase antimicrobial lethality (Wang and Zhao, 2009). On the other hand, it is of concern that more than half of the population in the US consumes nutritional supplements such as vitamins which are antioxidants (Radimer et al., 2004) and the potential antagonistic effects of an antioxidant diet in a patient taking antibiotics will require further investigation.
In conclusion, we report the molecular basis of cell death in S. aureus. To our knowledge, this is the first report on identification of S. aureus cell death genes from a whole genome perspective. Our extensive screen also offers insights to common core mechanisms that are relevant to not only cell death but bacterial persistence, a phenomenon that’s at the core of recalcitrant infections and biofilm formations. Our studies provide insights to possible new druggable targets, biomarkers for recalcitrant infections for diagnostic purposes and novel vaccine targets for prevention of bacterial infections. The similarity in functional groups found between our study and other yeast studies suggest that our work also sheds light into cell death pathways of eukaryotic systems such as pathogenic fungi and cancer stem cells.
All datasets generated for this study are included in the manuscript/Supplementary Files.
RY and YZ contributed to the design of the study and wrote the manuscript. RY, JF, JW, JC, and YZ helped with the acquisition, analysis, and interpretation of the data.
RY was partially supported by the NIH training grant T32 AI007417.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We gratefully acknowledge the BEI Resources for provision of the NTML mutant library. We thank Zachary Stolp and Marie Hardwick for helpful discussions regarding the heat-ramp experiment.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02199/full#supplementary-material
FIGURE S1 | Comparison of agar plating and viability staining of SYBR Green I/PI.
Amitai, S., Kolodkin-Gal, I., Hananya-Meltabashi, M., Sacher, A., and Engelberg-Kulka, H. (2009). Escherichia coli MazF leads to the simultaneous selective synthesis of both “death proteins” and “survival proteins”. PLoS Genet. 5:e1000390. doi: 10.1371/journal.pgen.1000390
Arthur, M., and Courvalin, P. (1993). Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 37, 1563–1571. doi: 10.1128/aac.37.8.1563
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., and Kalayci, O. (2012). Oxidative stress and antioxidant defense. World Allergy Organ J. 5, 9–19. doi: 10.1097/WOX.0b013e3182439613
Blake, K. L., Randall, C. P., and O’Neill, A. J. (2011). In vitro studies indicate a high resistance potential for the lantibiotic nisin in Staphylococcus aureus and define a genetic basis for nisin resistance. Antimicrob. Agents Chemother. 55, 2362–2368. doi: 10.1128/aac.01077-10
Bose, J. L., Fey, P. D., and Bayles, K. W. (2013). Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl. Environ. Microbiol. 79, 2218–2224. doi: 10.1128/aem.00136-13
Cafiso, V., Bertuccio, T., Spina, D., Purrello, S., Campanile, F., Di Pietro, C., et al. (2012). Modulating activity of vancomycin and daptomycin on the expression of autolysis cell-wall turnover and membrane charge genes in hVISA and VISA strains. PLoS One 7:e29573. doi: 10.1371/journal.pone.0029573
Corvaglia, A. R., Francois, P., Hernandez, D., Perron, K., Linder, P., and Schrenzel, J. (2010). A type III-like restriction endonuclease functions as a major barrier to horizontal gene transfer in clinical Staphylococcus aureus strains. Proc. Natl. Acad. Sci. U.S.A. 107, 11954–11958. doi: 10.1073/pnas.1000489107
Darrigo, C., Guillemet, E., Dervyn, R., and Ramarao, N. (2016). The bacterial Mfd protein prevents DNA damage induced by the host nitrogen immune response in a NER-independent but RecBC-dependent pathway. PLoS One 11:e0163321. doi: 10.1371/journal.pone.0163321
Davis, B. D. (1987). Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 51, 341–350.
Drlica, K., Malik, M., Kerns, R. J., and Zhao, X. (2008). Quinolone-mediated bacterial death. Antimicrob. Agents Chemother. 52, 385–392. doi: 10.1128/aac.01617-6
Dunny, G. M., and Leonard, B. A. (1997). Cell-cell communication in gram-positive bacteria. Annu. Rev. Microbiol. 51, 527–564. doi: 10.1146/annurev.micro.51.1.527
Engelberg-Kulka, H., Amitai, S., Kolodkin-Gal, I., and Hazan, R. (2006). Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2:e135. doi: 10.1371/journal.pgen.0020135
Engelberg-Kulka, H., and Glaser, G. (1999). Addiction modules and programmed cell death and antideath in bacterial cultures. Annu. Rev. Microbiol. 53, 43–70. doi: 10.1146/annurev.micro.53.1.43
Feng, J., Wang, T., Zhang, S., Shi, W., and Zhang, Y. (2014). An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS One 9:e111809. doi: 10.1371/journal.pone.0111809
Feng, J., Yee, R., Zhang, S., Tian, L., Shi, W., Zhang, W. H., et al. (2018). A rapid growth-independent antibiotic resistance detection test by SYBR green/propidium iodide viability assay. Front. Med. 5:127. doi: 10.3389/fmed.2018.00127
Fey, P. D., Endres, J. L., Yajjala, V. K., Widhelm, T. J., Boissy, R. J., Bose, J. L., et al. (2013). A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12
Fujimoto, D. F., and Bayles, K. W. (1998). Opposing roles of the Staphylococcus aureus virulence regulators, Agr and Sar, in Triton X-100- and penicillin-induced autolysis. J. Bacteriol. 180, 3724–3726.
Groicher, K. H., Firek, B. A., Fujimoto, D. F., and Bayles, K. W. (2000). The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J. Bacteriol. 182, 1794–1801. doi: 10.1128/jb.182.7.1794-1801.2000
Herrmann, G., Jayamani, E., Mai, G., and Buckel, W. (2008). Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J. Bacteriol. 190, 784–791. doi: 10.1128/jb.01422-7
Kajimura, J., Fujiwara, T., Yamada, S., Suzawa, Y., Nishida, T., Oyamada, Y., et al. (2005). Identification and molecular characterization of an N-acetylmuramyl-L-alanine amidase Sle1 involved in cell separation of Staphylococcus aureus. Mol. Microbiol. 58, 1087–1101. doi: 10.1111/j.1365-2958.2005.04881.x
Kawahata, M., Masaki, K., Fujii, T., and Iefuji, H. (2006). Yeast genes involved in response to lactic acid and acetic acid: acidic conditions caused by the organic acids in Saccharomyces cerevisiae cultures induce expression of intracellular metal metabolism genes regulated by Aft1p. FEMS Yeast Res. 6, 924–936. doi: 10.1111/j.1567-1364.2006.00089.x
Keren, I., Wu, Y., Inocencio, J., Mulcahy, L. R., and Lewis, K. (2013). Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 339, 1213–1216. doi: 10.1126/science.1232688
Kohanski, M. A., Dwyer, D. J., and Collins, J. J. (2010). How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 8, 423–435. doi: 10.1038/nrmicro2333
Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A., and Collins, J. J. (2007). A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130, 797–810. doi: 10.1016/j.cell.2007.06.049
Kolar, S. L., Nagarajan, V., Oszmiana, A., Rivera, F. E., Miller, H. K., Davenport, J. E., et al. (2011). NsaRS is a cell-envelope-stress-sensing two-component system of Staphylococcus aureus. Microbiology 157(Pt 8), 2206–2219. doi: 10.1099/mic.0.049692-0
Kort, R., Keijser, B. J., Caspers, M. P., Schuren, F. H., and Montijn, R. (2008). Transcriptional activity around bacterial cell death reveals molecular biomarkers for cell viability. BMC Genomics 9:590. doi: 10.1186/1471-2164-9-590
Liu, Y., and Imlay, J. A. (2013). Cell death from antibiotics without the involvement of reactive oxygen species. Science 339, 1210–1213. doi: 10.1126/science.1232751
Ludovico, P., Sousa, M. J., Silva, M. T., Leao, C., and Corte-Real, M. (2001). Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology 147(Pt 9), 2409–2415. doi: 10.1099/00221287-147-9-2409
Malik, M., Hussain, S., and Drlica, K. (2007). Effect of anaerobic growth on quinolone lethality with Escherichia coli. Antimicrob. Agents Chemother. 51, 28–34. doi: 10.1128/aac.00739-6
Martin, H. A., Porter, K. E., Vallin, C., Ermi, T., Contreras, N., Pedraza-Reyes, M., et al. (2019). Mfd protects against oxidative stress in Bacillus subtilis independently of its canonical function in DNA repair. BMC Microbiol. 19:26. doi: 10.1186/s12866-019-1394-x
Michalik, S., Depke, M., Murr, A., Gesell Salazar, M., Kusebauch, U., Sun, Z., et al. (2017). A global Staphylococcus aureus proteome resource applied to the in vivo characterization of host-pathogen interactions. Sci. Rep. 7:9718. doi: 10.1038/s41598-017-10059-w
Mira, N. P., Palma, M., Guerreiro, J. F., and Sa-Correia, I. (2010). Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb. Cell Fact. 9:79. doi: 10.1186/1475-2859-9-79
Nigam, A., Kumar, S., and Engelberg-Kulka, H. (2018). Quorum sensing extracellular death peptides enhance the endoribonucleolytic activities of Mycobacterium tuberculosis MazF toxins. mBio 9:e00685-18. doi: 10.1128/mBio.00685-18
Paulander, W., Varming, A. N., Bojer, M. S., Friberg, C., Baek, K., and Ingmer, H. (2018). The agr quorum sensing system in Staphylococcus aureus cells mediates death of sub-population. BMC Res. Notes 11:503. doi: 10.1186/s13104-018-3600-6
Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A., and Ceron, J. (2012). Basic Caenorhabditis elegans methods: synchronization and observation. J. Vis. Exp. 64, e4019. doi: 10.3791/4019
Radimer, K., Bindewald, B., Hughes, J., Ervin, B., Swanson, C., and Picciano, M. F. (2004). Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am. J. Epidemiol. 160, 339–349. doi: 10.1093/aje/kwh207
Ramirez-Guadiana, F. H., Del Carmen Barajas-Ornelas, R., Ayala-Garcia, V. M., Yasbin, R. E., Robleto, E., and Pedraza-Reyes, M. (2013). Transcriptional coupling of DNA repair in sporulating Bacillus subtilis cells. Mol. Microbiol. 90, 1088–1099. doi: 10.1111/mmi.12417
Ramond, E., Gesbert, G., Rigard, M., Dairou, J., Dupuis, M., Dubail, I., et al. (2014). Glutamate utilization couples oxidative stress defense and the tricarboxylic acid cycle in Francisella phagosomal escape. PLoS Pathog. 10:e1003893. doi: 10.1371/journal.ppat.1003893
Ranjit, D. K., Endres, J. L., and Bayles, K. W. (2011). Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J. Bacteriol. 193, 2468–2476. doi: 10.1128/jb.01545-10
Rice, K. C., and Bayles, K. W. (2003). Death’s toolbox: examining the molecular components of bacterial programmed cell death. Mol. Microbiol. 50, 729–738. doi: 10.1046/j.1365-2958.2003.t01-1-03720.x
Rice, K. C., Firek, B. A., Nelson, J. B., Yang, S. J., Patton, T. G., and Bayles, K. W. (2003). The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J. Bacteriol. 185, 2635–2643. doi: 10.1128/jb.185.8.2635-2643.2003
Sadykov, M. R., Thomas, V. C., Marshall, D. D., Wenstrom, C. J., Moormeier, D. E., Widhelm, T. J., et al. (2013). Inactivation of the Pta-AckA pathway causes cell death in Staphylococcus aureus. J. Bacteriol. 195, 3035–3044. doi: 10.1128/jb.00042-13
Schweizer, M. L., Furuno, J. P., Sakoulas, G., Johnson, J. K., Harris, A. D., Shardell, M. D., et al. (2011). Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob. Agents Chemother. 55, 1082–1087. doi: 10.1128/aac.00918-0
Sousa, M., Duarte, A. M., Fernandes, T. R., Chaves, S. R., Pacheco, A., Leao, C., et al. (2013). Genome-wide identification of genes involved in the positive and negative regulation of acetic acid-induced programmed cell death in Saccharomyces cerevisiae. BMC Genomics 14:838. doi: 10.1186/1471-2164-14-838
Sun, F., Liang, H., Kong, X., Xie, S., Cho, H., Deng, X., et al. (2012). Quorum-sensing agr mediates bacterial oxidation response via an intramolecular disulfide redox switch in the response regulator AgrA. Proc. Natl. Acad. Sci. U.S.A. 109, 9095–9100. doi: 10.1073/pnas.1200603109
Tavares, A. F., Teixeira, M., Romao, C. C., Seixas, J. D., Nobre, L. S., and Saraiva, L. M. (2011). Reactive oxygen species mediate bactericidal killing elicited by carbon monoxide-releasing molecules. J. Biol. Chem. 286, 26708–26717. doi: 10.1074/jbc.M111.255752
Teng, X., Dayhoff-Brannigan, M., Cheng, W. C., Gilbert, C. E., Sing, C. N., Diny, N. L., et al. (2013). Genome-wide consequences of deleting any single gene. Mol. Cell 52, 485–494. doi: 10.1016/j.molcel.2013.09.026
Teng, X., and Hardwick, J. M. (2009). Reliable method for detection of programmed cell death in yeast. Methods Mol. Biol. 559, 335–342. doi: 10.1007/978-1-60327-017-5_23
Teng, X., and Hardwick, J. M. (2013). Quantification of genetically controlled cell death in budding yeast. Methods Mol. Biol. 1004, 161–170. doi: 10.1007/978-1-62703-383-1_12
Thoendel, M., Kavanaugh, J. S., Flack, C. E., and Horswill, A. R. (2011). Peptide signaling in the staphylococci. Chem. Rev. 111, 117–151. doi: 10.1021/cr100370n
Tong, S. Y., Davis, J. S., Eichenberger, E., Holland, T. L., and Fowler, V. G. Jr. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661. doi: 10.1128/cmr.00134-14
Tu Quoc, P. H., Genevaux, P., Pajunen, M., Savilahti, H., Georgopoulos, C., Schrenzel, J., et al. (2007). Isolation and characterization of biofilm formation-defective mutants of Staphylococcus aureus. Infect. Immun. 75, 1079–1088. doi: 10.1128/iai.01143-6
Umbarger, H. E. (1978). Amino acid biosynthesis and its regulation. Annu. Rev. Biochem. 47, 532–606. doi: 10.1146/annurev.bi.47.070178.002533
Utaida, S., Dunman, P. M., Macapagal, D., Murphy, E., Projan, S. J., Singh, V. K., et al. (2003). Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology 149(Pt 10), 2719–2732. doi: 10.1099/mic.0.26426-0
Utaida, S., Pfeltz, R. F., Jayaswal, R. K., and Wilkinson, B. J. (2006). Autolytic properties of glycopeptide-intermediate Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 50, 1541–1545. doi: 10.1128/aac.50.4.1541-1545.2006
Waldron, D. E., and Lindsay, J. A. (2006). Sau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J. Bacteriol. 188, 5578–5585. doi: 10.1128/jb.00418-6
Wang, G., and Maier, R. J. (2015). A novel DNA-binding protein plays an important role in Helicobacter pylori stress tolerance and survival in the host. J. Bacteriol. 197, 973–982. doi: 10.1128/jb.02489-14
Wang, X., and Zhao, X. (2009). Contribution of oxidative damage to antimicrobial lethality. Antimicrob. Agents Chemother. 53, 1395–1402. doi: 10.1128/aac.01087-8
Willing, S. E., Richards, E. J., Sempere, L., Dale, A. G., Cutting, S. M., and Fairweather, N. F. (2015). Increased toxin expression in a Clostridium difficile mfd mutant. BMC Microbiol. 15:280. doi: 10.1186/s12866-015-0611-5
Windham, I. H., Chaudhari, S. S., Bose, J. L., Thomas, V. C., and Bayles, K. W. (2016). SrrAB modulates Staphylococcus aureus cell death through regulation of cidABC transcription. J. Bacteriol. 198, 1114–1122. doi: 10.1128/jb.00954-15
Yee, R., Cui, P., Shi, W., Feng, J., and Zhang, Y. (2015). Genetic screen reveals the role of purine metabolism in Staphylococcus aureus persistence to Rifampicin. Antibiotics 4, 627–642. doi: 10.3390/antibiotics4040627
Yee, R., Cui, P., Xu, T., Shi, W., Feng, J., Zhang, W., et al. (2017). Identification of a novel gene argJ involved in arginine biosynthesis critical for persister formation in Staphylococcus aureus. bioRxiv. doi: 10.1101/114827
Yee, R., Yuan, Y., Shi, W., Brayton, C., Tarff, A., Feng, J., et al. (2019). Infection with persister forms of Staphylococcus aureus causes a persistent skin infection with more severe lesions in mice: failture to clear the infection by the current standard of care treatment. Discov. Med. 28, 7–16.
Zhang, Y. (2014). Persisters, persistent infections and the Yin-Yang model. Emerg. Microbes Infect. 3:e3. doi: 10.1038/emi.2014.3
Keywords: Staphylococcus aureus, cell death, genetic screen, mutants, genes
Citation: Yee R, Feng J, Wang J, Chen J and Zhang Y (2019) Identification of Genes Regulating Cell Death in Staphylococcus aureus. Front. Microbiol. 10:2199. doi: 10.3389/fmicb.2019.02199
Received: 25 June 2019; Accepted: 09 September 2019;
Published: 01 October 2019.
Edited by:
Mattias Collin, Lund University, SwedenReviewed by:
Nayeli Alva-Murillo, University of Guanajuato, MexicoCopyright © 2019 Yee, Feng, Wang, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhang, eXpoYW5nQGpoc3BoLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.