
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 04 October 2019
Sec. Infectious Agents and Disease
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.02198
Rationale: Although frequently retrieved in tracheal secretions of critically ill patients on mechanical ventilation, the existence of pneumonia caused by coagulase-negative staphylococci (CoNS) remains controversial.
Objective: To assess whether Staphylococcus haemolyticus (S. haemolyticus) inoculated in mice’s trachea can infect normal lung parenchyma, increasing concentrations of S. haemolyticus were intratracheally administered in 221 immunocompetent mice.
Methods: Each animal received intratracheally phosphate-buffered saline (PBS) (n = 43) or live (n = 141) or inactivated (n = 37) S. haemolyticus at increasing load: 1.0 × 106, 1.0 × 107, and 1.0 × 108 colony forming units (CFU). Forty-three animals were sacrificed at 12 h and 178 were sacrificed at 36 h; 64 served for post-mortem lung histology, 157 served for pre-mortem bronchoalveolar lavage (BAL) analysis, and 42 served for post-mortem quantitative bacteriology of lung tissue. The distribution of biofilm-associated genes was investigated in the S. haemolyticus strain used in our in vivo experiment as well as among 19 other clinical S. haemolyticus strains collected from hospitals or nursing houses.
Measurements and Main Results: Intratracheal inoculation of 1.0 × 108 CFU live S. haemolyticus caused macroscopic and histological confluent pneumonia with significant increase in BAL white cell count, tumor necrosis factor-α (TNF-α), and macrophage inflammatory protein (MIP)-2. At 12 h, high concentrations of S. haemolyticus were identified in BAL. At 36 h, lung injury and BAL inflammation were less severe than at 12 h and moderate concentrations of species belonging to the oropharyngeal flora were identified in lung tissue. The inoculation of 1.0 × 106 and 1.0 × 107 CFU live S. haemolyticus caused histologic interstitial pneumonia and moderate BAL inflammation. Similar results were observed after inoculation of inactivated S. haemolyticus. Moreover, biofilm formation was a common phenotype in S. haemolyticus isolates. The low prevalence of the ica operon in our clinical S. haemolyticus strain collection indicated icaA and icaD independent-biofilm formation.
Conclusion: In immunocompetent spontaneously breathing mice, inoculation of S. haemolyticus causes concentration-dependent lung infection that spontaneously recovers over time. icaA and icaD independent biofilm formation is a common phenotype in S. haemolyticus isolates.
Coagulase-negative staphylococci (CoNS), ubiquitous colonizers of healthy skin and mucosal surfaces, are among the most frequently isolated bacteria in the microbiology laboratory. Initially considered as non-pathogenic, CoNS has been recognized over the past three decades as nosocomial pathogens causing significant morbidity, mortality, and healthcare costs worldwide (Becker et al., 2014), in adults (Wisplinghoff et al., 2004; Rogers et al., 2009) and newborns (Cunha Mde et al., 2002; Venkatesh et al., 2006; Rogers et al., 2009). Because of their propensity to form biofilms on device materials (John and Harvin, 2007), they frequently infect catheters inserted in critically ill patients (Rogers et al., 2009; Becker et al., 2014; Grasselli et al., 2017) and prosthesis implemented during orthopedic surgery (Wisplinghoff et al., 2004; Becker et al., 2014). CoNS have also been increasingly reported as a cause of bacteremia in immunocompromised and hospitalized patients, especially with indwelling medical devices (Bearman and Wenzel, 2005; Cervera et al., 2009; Rogers et al., 2009; Becker et al., 2014). Although frequently retrieved in tracheal secretions (Agvald-Ohman et al., 2004; Qi et al., 2015), some controversy remains whether CoNS can infect lung parenchyma, particularly in critically ill patients requiring prolonged mechanical ventilation through endotracheal tube or tracheostomy.
The molecular basis of the virulence of CoNS remains largely unknown. It has been demonstrated that the formation of biofilm is responsible for the indolent, difficult-to-treat nature of CoNS infections, but the phenotype may vary among different species. Staphylococcus epidermidis (S. epidermidis) requires a functional ica locus (Loiez et al., 2007; Peer et al., 2011; Morfin-Otero et al., 2012) when producing a thick biofilm consisting mainly of polysaccharide intercellular adhesion (PIA) (von Eiff et al., 2005), thus creating an environment that results in antibiotic tolerance and evasion of host defense (Crass and Bergdoll, 1986; Lina et al., 1996). In contrast to S. epidermidis, proteins and extracellular DNA are of more functional relevance for biofilm accumulation in Staphylococcus haemolyticus (S. haemolyticus), whereas PIA plays only a minor role (Fredheim et al., 2009). Whether the phenotype of biofilm formation, especially ica locus, is critical for the virulence of CoNS remains unclear.
The aim of this study was to assess whether CoNS can infect normal lung parenchyma; we set up an experimental model in mice in which increasing concentrations from 106 to 108 colony forming units (CFU) of S. haemolyticus strains were inoculated in the trachea. In addition, the phenotype of biofilm formation in S. haemolyticus was investigated.
The clinical CoNS strain, obtained from the Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, was isolated from the blood culture specimen of a female hospitalized patient with Hodgkin’s lymphoma. The strain was identified as S. haemolyticus by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) (Biomerieux VITEK MSTM, France). The isolate was incubated overnight in Luria-Bertani (LB) broth, shaken at 37°C at the rate of 275 rpm. One hundred microliters of inoculum was pipetted into 5 ml of fresh LB broth, shaken at 37°C at the same rate, for approximately 2 h, with additional adjustment (either re-shaking or diluting) if needed, until optical density (OD) value (600 nm) reached 0.8. Number of CFU was previously calculated: OD600 nm = 0.8 corresponds to 5 × 108 CFU/ml (Figure 1B). The detailed antimicrobial resistance profile is shown in Figure 1A. To prepare the different bacterial concentrations, the adjusted inocula were centrifuged at 10,000 × g for 2 min and pellets were subsequently re-suspended in phosphate-buffered saline (PBS) with the final concentration of 2.5 × 107, 2.5 × 108, and 2.5 × 109 CFU/ml. In separate experiments, inactivated inocula were pretreated by incubating at 65°C for 30 min after centrifugation.
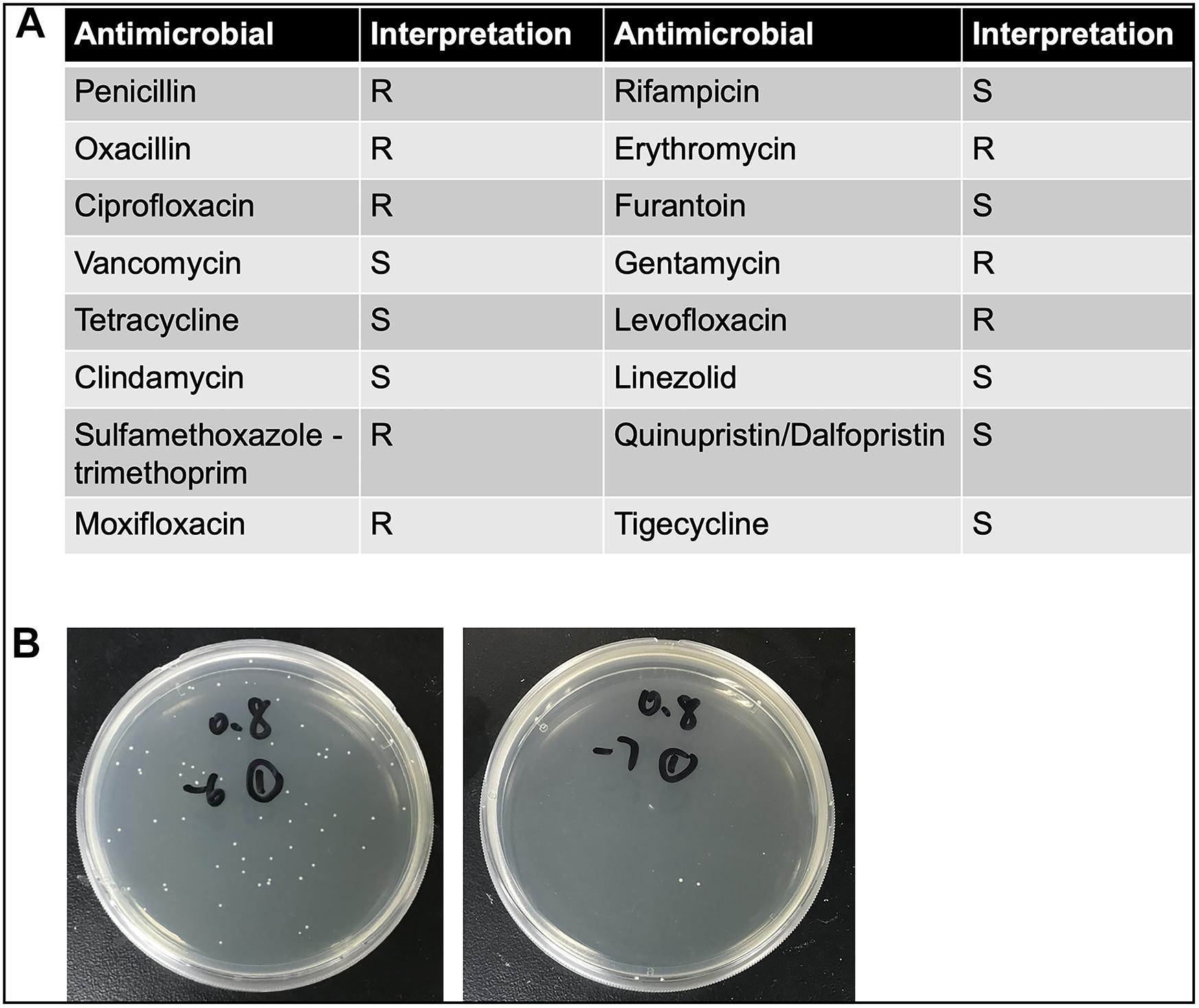
Figure 1. (A) The detailed antimicrobial resistance profile of S. haemolyticus isolated from the blood culture specimen of a female hospitalized patient with Hodgkin’s lymphoma. (B) The number of colony forming units (CFU) was calculated when optical density value was 0.8. The left panel showed 10–6 times diluted inoculum, and the right panel showed 10–7 times diluted inoculum.
C57BL/6 male mice (7–8 weeks) were obtained from Shanghai Laboratory Animal Co., Ltd., China. Animals were kept in a specific pathogen-free facility of the Research Center for Experimental Medicine of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, China. All animal procedures were approved by the Ruijin Hospital Animal Ethics Committee. Mice were anesthetized with pentobarbital sodium salt (40 mg/kg) intraperitoneally and received intratracheally: (1) PBS as carrier control; and (2) 40 μl of S. haemolyticus in concentrations of 2.5 × 107, 2.5 × 108, and 2.5 × 109 CFU/ml (equivalent to inocula of 1 × 106, 1 × 107, and 1 × 108 CFU, respectively), according to protocols previously reported (Su et al., 2004). In separate experiments, we inoculated with 40 μl of inactivated S. haemolyticus in concentrations of 2.5 × 107, 2.5 × 108, and 2.5 × 109 CFU/ml. After 12 or 36 h, both bronchoalveolar lavage (BAL) samples and lungs were collected following sacrifice.
Total cell count and differential in BAL were obtained using Sysmex pocH-100i (Sysmex Shanghai Ltd., China) according to the manufacturer’s protocols. BAL samples were centrifuged at 3000 rpm for 10 min, and the supernatants were collected and stored at −20°C. Macrophage inflammatory protein (MIP)-2, a mouse neutrophil chemokine, as well as tumor necrosis factor-α (TNF-α) were measured by ELISA (R&D Systems, Minneapolis, MN, United States) in the BAL supernatants. BAL protein concentration as a marker of lung endothelial and epithelial permeability was also measured using Bthe CA Protein Assay Kit1 (Beyotime, Shanghai, China).
Bacterial growth in the BAL and lung homogenate following CoNS-induced lung injury in vivo were quantitated by CFU. The samples were cultured on LB agar plate (TEKnova, Hollister, CA, United States) as well as blood plate (CP Adaltis Diagnostics, Shanghai, China) overnight at 37°C. Individuals colonies (CFU) were then counted and also identified as CoNS by MALDI-TOF-MS (Figures 2A–C).
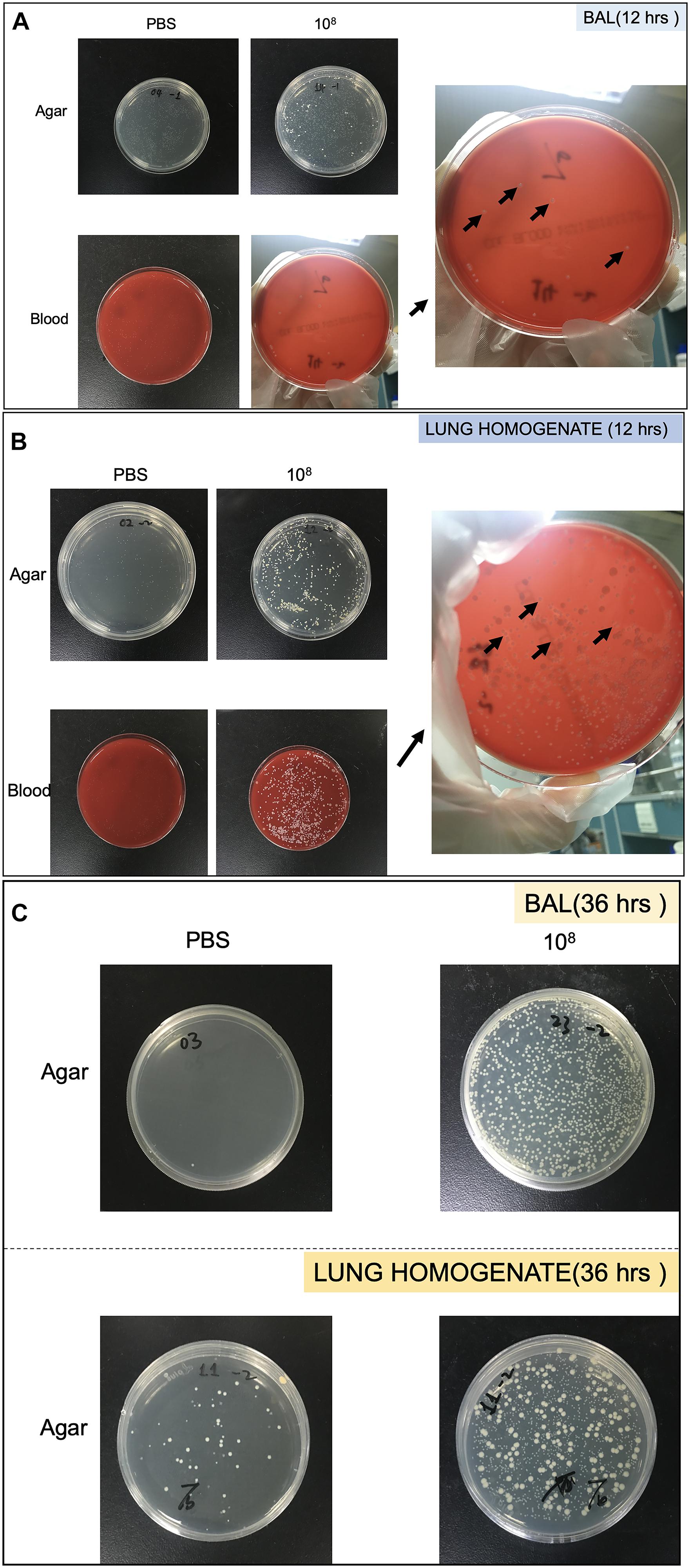
Figure 2. Pictures of Petri dish showing the heterogeneity of strains growth in bronchoalveolar lavage (BAL) and lung homogenate. (A) Strains collected from BAL at 12 h. (B) Strains collected from lung homogenate at 12 h. (C) Strains collected from BAL and lung homogenate at 36 h. The black arrows showed colonies identified as S. haemolyticus.
After excision, lungs were gently inflated with 0.5 ml of 4% formalin, followed by tracheal ligature, fixed in 4% formalin, and dehydrated through serially diluted graded ethyl alcohol baths. After fixation, lungs were embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin. An independent investigator, blinded to group assignments, assessed whether lung parenchyma was infected or not. Pneumonia was defined as the presence of polymorphonuclear neutrophils (PMNs) within septa and alveolar spaces (Mizgerd and Skerrett, 2008). Levels of lung injury were assessed using the lung injury score (LIS) (Matute-Bello et al., 2011), which varies from 0 to 1, where a higher score means more injury (Supplementary Table S1).
Genomic DNA isolation from the S. haemolyticus strain used in our experiment as well as 19 other clinical S. haemolyticus strains were carried out as previously described (Frebourg et al., 2000; Zhou et al., 2019). Standard strains of S. haemolyticus (ATCC29970) and S. epidermidis (ATCC12228) were used as controls. The presence of icaA and icaD genes (main component of PIA biofilm) along with a variety of significant biofilm-associated genes [encoding cell surface attachment proteins (ebpS, cbp, fbp, and srtA), quorum sensing (agrA, agrB, agrD, and luxS), and eDNA release (cidA, cidB, lrgA, lrgB, and atlE)] were detected in all the S. haemolyticus strains by polymerase chain reaction (PCR). Amplification conditions (20 μl volume) were as follows: denaturation for 3 min at 94°C, followed by 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, with final extension at 72°C for 5 min. The primer sequences of PCR used in this study are shown in Supplementary Table S2 (Panda and Singh, 2018). The amplified PCR products were analyzed on 1.5% agarose gel and were visualized using a UV trans-illuminator2 (FR980A, Furi Technology, China). In addition, the PCR products were sequenced to confirm the product identity.
The capacity of biofilm formed by S. haemolyticus was determined by a modified method (Fredheim et al., 2009; Panda and Singh, 2018). Bacterial suspension was prepared in lysogeny broth (LB) and adjusted to 0.5 McFarland (1 × 108 CFU/ml). This bacterial suspension was 20-fold (1/20) diluted to reach 5 × 106 CFU/ml. Then, 180 μl of LB and 20 μl of bacterial suspensions were inoculated into 96-well flat-bottomed sterile polystyrene microplate. Microplates were incubated at 37°C for 24 h and then were washed twice by PBS. Biofilms formed on the walls of microplate were fixed by adding 150 μl of methanol and then stained with 150 μl of crystal violet (Beyotime, Shanghai, China) for 15 min. Then crystal violet-stained microplates were washed twice with PBS to discharge crystal violet stain. The microplate was measured spectrophotometrically at 570 nm by a microplate reader. Isolates were considered biofilm positive if they had an absorbance ≥0.12. We performed the assay in a single run of four wells in biological triplicates.
Results are expressed as median and 25th–75th interquartile range (IQR). Comparisons between two groups were made by non-parametric Mann–Whitney U test. Comparisons between more than two groups were made by non-parametric Kruskal–Wallis analysis followed by pairwise comparisons tested using a post hoc analysis Dunn’s method. A p-value < 0.05 was considered statistically significant. All statistical analysis was performed using GraphPad Prism software (La Jolla, CA, United States).
As shown in Table 1, 221 mice were distributed in 33 groups. Each animal was anesthetized, inoculated, sent back to its cage, and sacrificed at 12 or 36 h.
As shown in the Supplementary Videos available on the online data supplement, mice behavior was deeply affected only in animals inoculated with 108 CFU live S. haemolyticus. Like in other models of inoculation pneumonia, mice cage physical activity and behavior were affected (reduced mobility and lack of food intake) only transitorily during the first 12 h. Subsequently, they progressively recovered, and all animals survived at 36 h.
As shown in Figures 3A,C, blood neutrophil counts significantly decreased in animals inoculated with 106 CFU as compared with PBS-treated mice (1.538 ± 1.661 for 106 CFU, 3.138 ± 1.645 for PBS, p = 0.02 for 106 CFU vs. PBS) or 107 CFU treated mice (1.538 ± 1.661 for 106 CFU, 2.783 ± 1.101 for 107 CFU, p = 0.03 for 106 CFU vs. 107 CFU), but did not change in animals inoculated with 108 CFU live or inactivated S. haemolyticus whatever the inoculum.
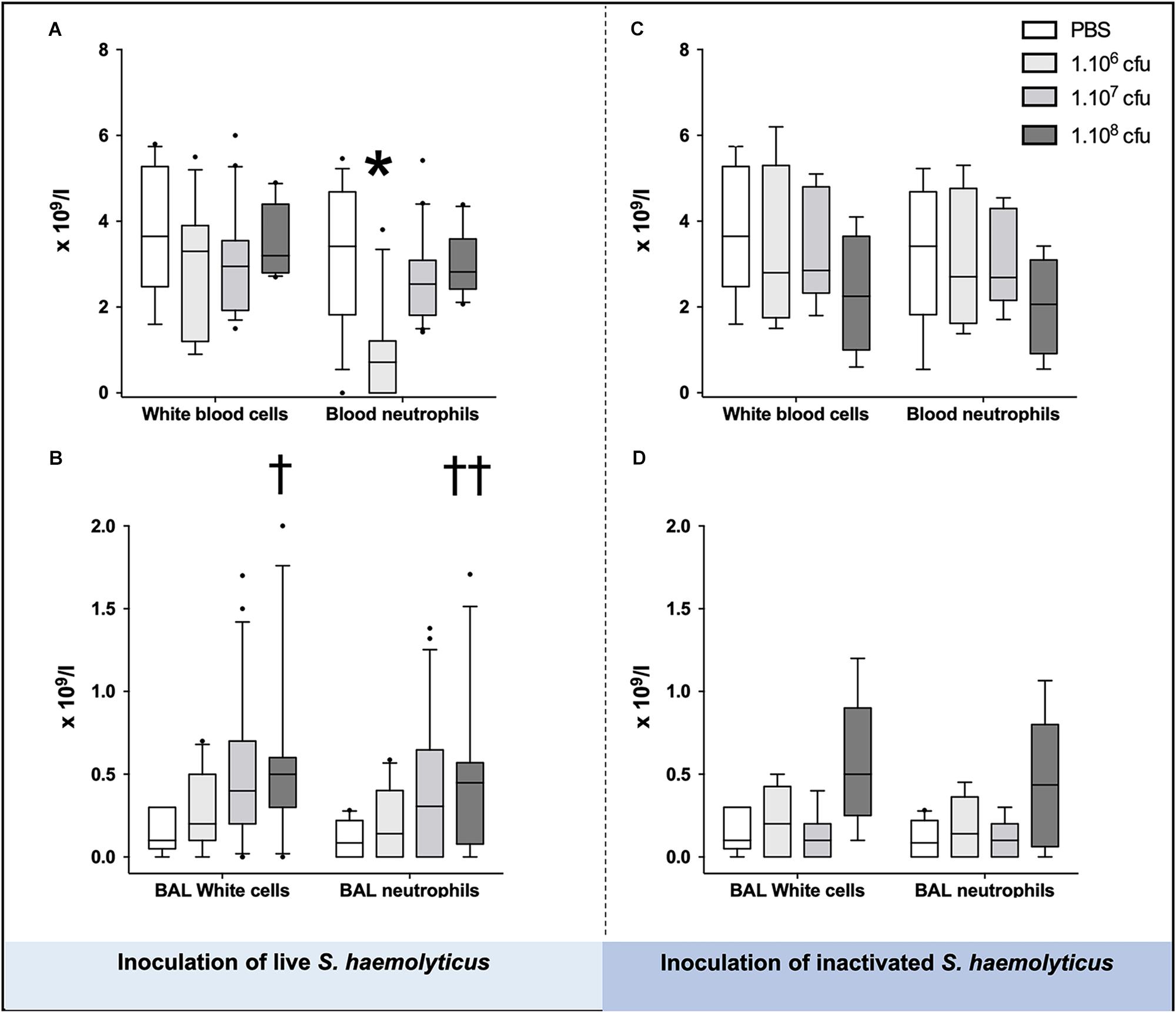
Figure 3. White cells and neutrophils in the blood (A,C) and BAL (B,D), 36 h following tracheal inoculation of phosphate-buffered saline (PBS), live and inactivated S. haemolyticus. Median values, interquartile 25th–75th range and extreme values (>2 SD) are represented. Blood neutrophils were significantly different between groups: Kruskal–Wallis p = 0.0095; ∗ indicates p < 0.05 for 106 CFU versus PBS and 107 CFU; BAL White cells were significantly different between groups: Kruskal–Wallis p = 0.0023; †indicates p = 0.0013 for 108 CFU versus PBS; BAL neutrophils were significantly different between groups: Kruskal–Wallis p = 0.01; ††indicates p = 0.0084 for 108 CFU versus PBS.
As shown in Figure 4, 12 and 36 h following inoculation of 108 CFU live S. haemolyticus, there was macroscopic evidence of pneumonia. Twelve hours after PBS inoculation, macroscopic injury was absent. Macroscopic injury was moderate 12 h after inoculation of 106 and 107 CFU live S. haemolyticus and severe 12 h after inoculation of 108 CFU live S. haemolyticus. Thirty-six hours later, macroscopic injury was less pronounced, corresponding to spontaneous improvement in behavior. Histologically, interstitial pneumonia was observed after inoculation of 106 and 107 CFU live S. haemolyticus and confluent pneumonia was observed after inoculation of 108 CFU live S. haemolyticus. At 12 h, LIS increased linearly with the tracheal inoculum (p < 0.001). At 36 h, LIS of animals inoculated with 106 CFU live S. haemolyticus returned to control values. LIS of animals inoculated with 107 CFU live S. haemolyticus remained elevated whereas LIS of animals inoculated with 108 CFU live S. haemolyticus also remained elevated, but significantly lower than after 12 h (0.780 [0.757–0.802] at 36 h versus 0.895 [0.860–0.947] at 12 h, p = 0.002).
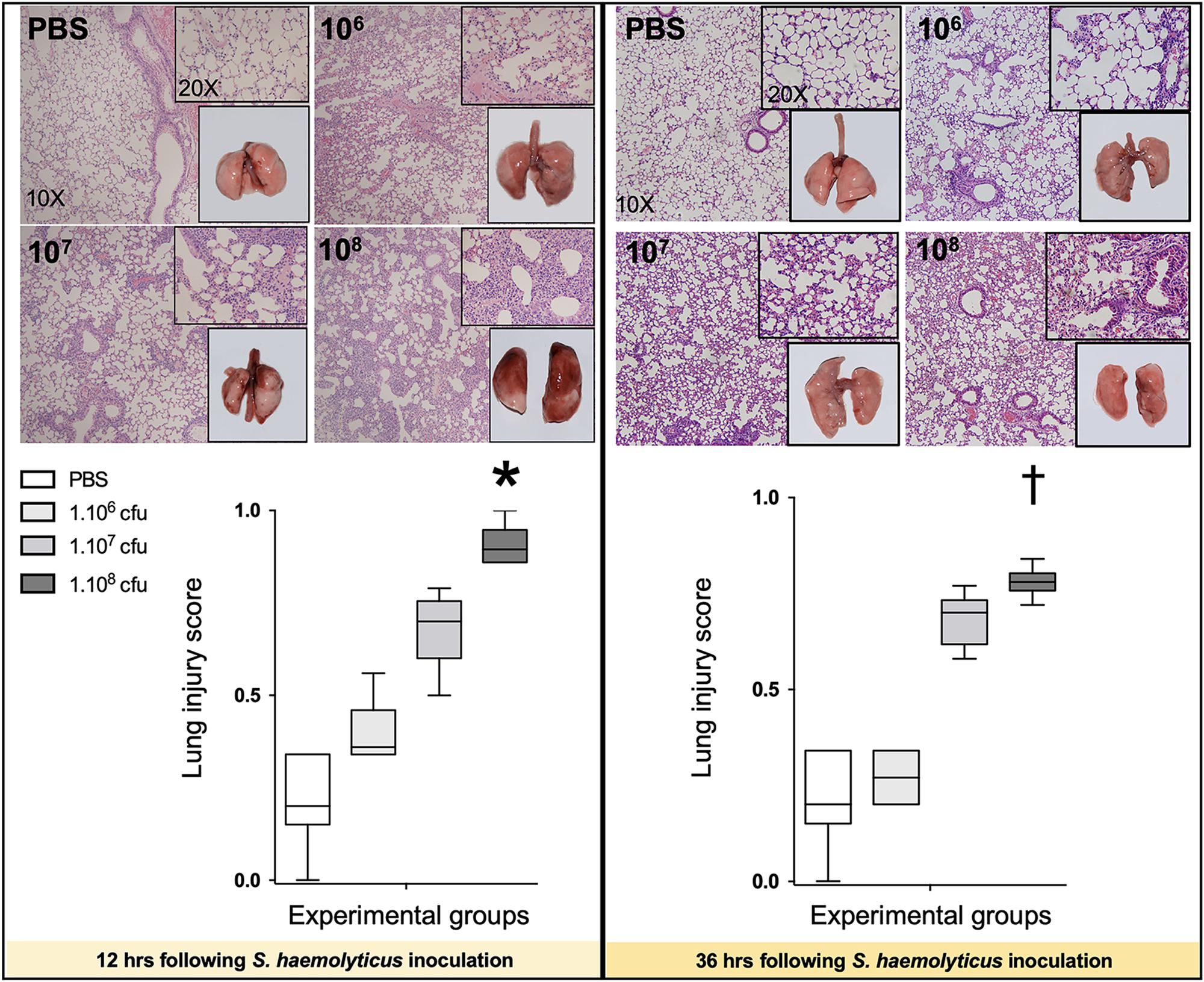
Figure 4. Macroscopic (square windows) and histological lung aspects 12 and 36 h following tracheal inoculation of PBS, and live S. haemolyticus at 106, 107, and 108 CFU. Lung injury score (LIS) is represented as median values and interquartile 25th–75th range and extreme values (>2 SD). Twelve and 36 h after inoculation, LIS was significantly different between groups: Kruskal–Wallis p = 0.0002; ∗indicates p < 0.05 for 108 CFU versus PBS and 106 CFU; †indicates p < 0.05 for 108 CFU versus PBS and 106 CFU. Twelve and 36 h after tracheal inoculation of 106 and 107 CFU, interstitial pneumonia is present, characterized by inflammatory cells infiltrating interalveolar septa and respecting alveolar space and lung architecture. Twelve and 36 h after tracheal inoculation of 108 CFU, confluent pneumonia is present, characterized by inflammatory cells invading alveolar space, hyaline membranes, lung consolidation, and loss of basal lung architecture.
As shown in Figure 5, tracheal inoculation of 108 CFU inactivated S. haemolyticus induced severe lung lesions at 36 h, whereas less histological injury was observed 36 h following the inoculation of 106 or 107 CFU inactivated S. haemolyticus.
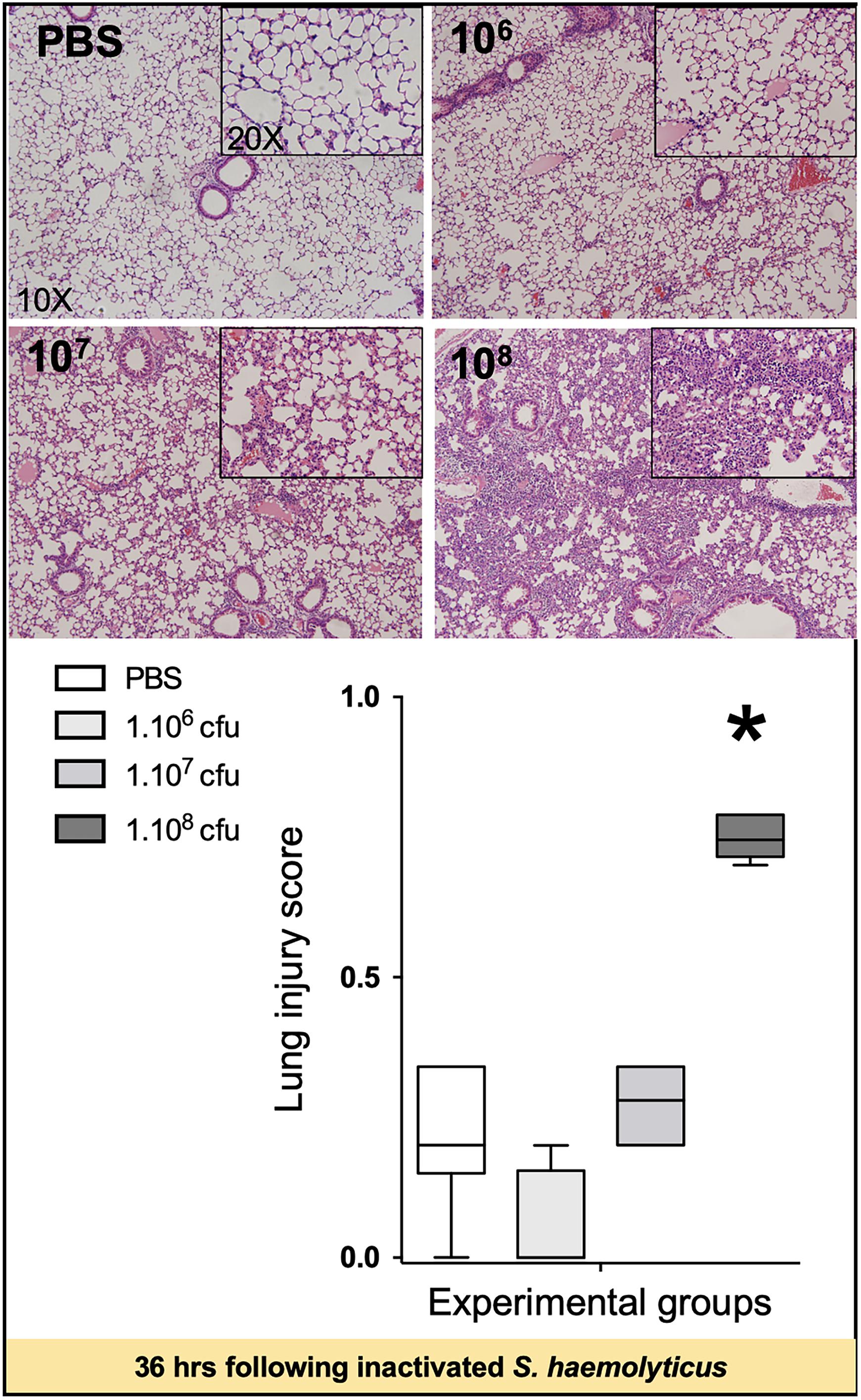
Figure 5. Lung histology 36 h following tracheal inoculation of PBS and inactivated S. haemolyticus at increasing load 106, 107, and 108 CFU. LIS is represented as median values and interquartile 25th–75th range and extreme values (>2 SD). LIS is significantly different between groups: Kruskal–Wallis p = 0.0004; ∗indicates p < 0.05 for 108 CFU versus PBS and 106 CFU. Thirty-six hours after tracheal inoculation of inactivated S. haemolyticus at 106 and 107 CFU, inflammatory cells infiltrate interalveolar septae, respecting alveolar space and lung architecture. The inoculation of 108 CFU of inactivated S. haemolyticus induced invasion of inflammatory cells within alveolar space, hyaline membrane formation, and lung consolidation.
As shown in Figure 3B, tracheal inoculation of 108 CFU live S. haemolyticus produced a significant increase in BAL influx of white cells, specifically neutrophils (p = 0.0013 for 108 CFU versus PBS for white cells and p = 0.0084 for 108 CFU versus PBS for neutrophils). As shown in Figure 3D, tracheal inoculation of increasing inocula of inactivated S. haemolyticus did not produce any significant increase of BAL white cells and neutrophils. As shown in Figure 6, high peaks of TNF-α and MIP-2 were observed in BAL 12 h following inoculation of live S. haemolyticus: TNF-α was (median, [25th–75th] percentile) 845.2 [717.8–1088] pg/ml in mice that received a 108 CFU inoculum and 0 [0–0] in mice that received PBS (p = 0.0021) and 0 [0–0] in mice that received a 106 inoculum (p = 0.0011); MIP-2 was 341.8 [232.9–388.2] pg/ml in mice that received a 108 CFU inoculum, 2.2 [0.1–18.5] in mice that received PBS (p = 0.0094), and 3.0 [0–22] in mice that received a 106 inoculum (p = 0.0084). Thirty-six hours following inoculation of live S. haemolyticus, TNF-α was (median, [25th–75th] percentile) 4.6 [0–22] pg/ml in mice that received a 108 CFU inoculum, 0 [0–0] in mice that received PBS (p = 0.047), and 0 [0–0] in mice that received a 106 inoculum (p = 0.004); TNF-α was significantly higher in mice that received a 107 inoculum compared to those that received a 106 inoculum: 0 [0–29.3] vs. 0 [0–0] (p = 0.03); MIP-2 was 45.7 [16.5–154] pg/ml in mice that received a 108 CFU inoculum, 0 [0–0] in mice that received PBS (p < 10–4), and 0 [0–1.1] in mice receiving 106 inoculum (p < 10–4); MIP-2 was significantly higher in mice that received a 107 inoculum compared to mice that received either PBS or a 106 inoculum: 13.2 [0–80.3] versus 0 [0–0], (p = 0.03), and 13.2 [0–80.3] versus 0 [0–1.1], (p = 0.04), respectively. Thirty-six hours following inoculation of inactivated S. haemolyticus, TNF-α was 7.3 [0–20.4] pg/ml in mice that received a 108 CFU inoculum, 0 [0–0] in mice that received PBS (p = 0.07), 0 [0–0] in mice that received a 106 inoculum (p = 0.02), and 0 [0–0] pg/ml in mice that received a 107 CFU inoculum (p = 0.01); MIP-2 was 54.7 [12.1–168.2] pg/ml in mice that received a 108 CFU inoculum, 0 [0–0] in mice that received PBS (p = 0.0064), 0 [0–0] in mice that received a 106 inoculum (p = 0.0031), and 0 [0–0] pg/ml in mice that received a 107 CFU inoculum (p = 0.0090).
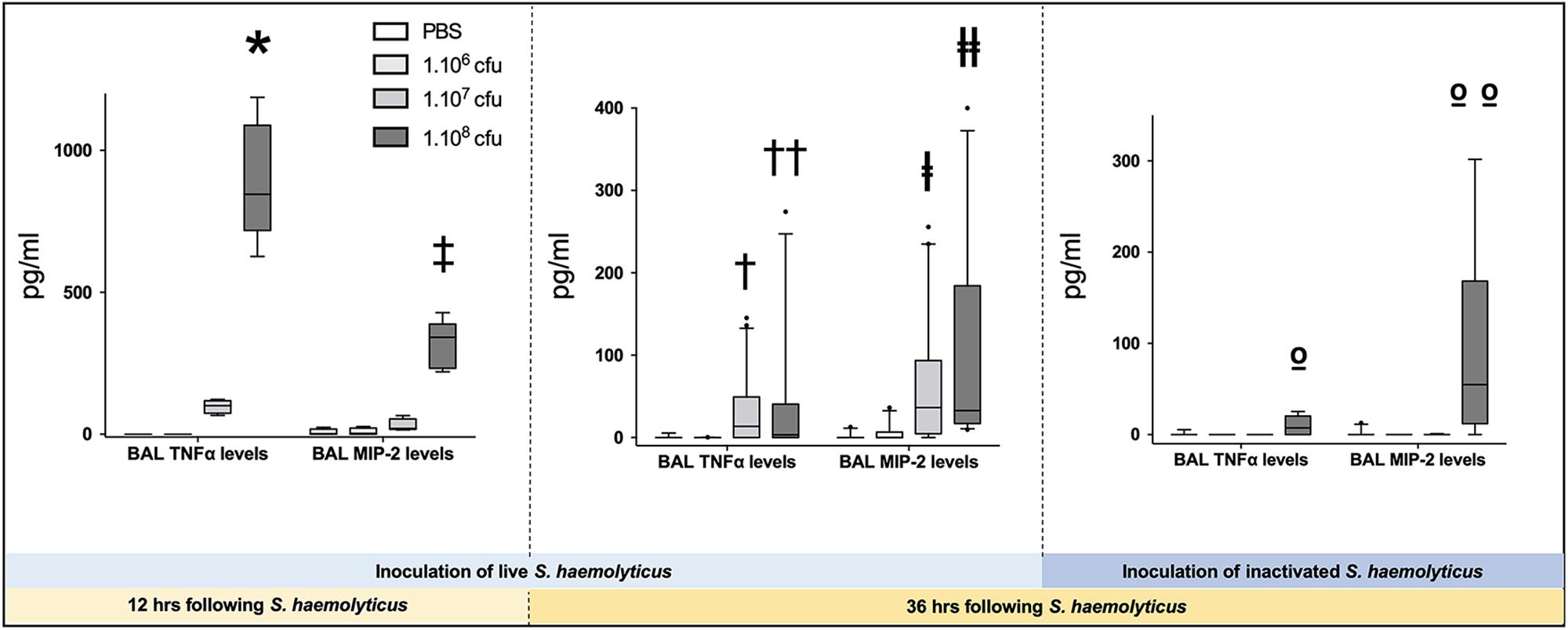
Figure 6. Tumor necrosis factor (TNF-α) and monocyte inflammatory protein-2 (MIP-2) in BAL following tracheal inoculation of PBS, and increasing inoculum (106, 107 and 108 CFU) of live and inactivated S. haemolyticus. Data are expressed as median values, interquartile 25th–75th range and extreme values (>2 SD). Twelve hours following tracheal inoculation of live S. haemolyticus, alveolar levels of TNF-α and MIP-2 significantly increased with the inoculum (Kruskal–Wallis p = 0.0002 for TNF-α and p = 0.004 for MIP-2; ∗ and ‡ indicate p < 0.05 for 108 CFU versus PBS and 106 CFU). Thirty-six hours following inoculation, alveolar levels of TNF-α and MIP-2 remained significantly elevated in mice that received the 108 CFU (Kruskal–Wallis p = 0.0012 for TNF-α and p < 10–4 for MIP-2; †† and ╪╪ indicate p < 0.05 for 108 CFU versus PBS and 106 CFU; †indicates p = 0.03 for 107 versus 106 CFU and ╪ indicates p < 0.05 for 107 versus PBS and 106 CFU). However, both cytokine levels were significantly lower than levels measured 12 h following inoculation of 108 CFU S. haemolyticus (p < 10–4 for TNF-α and MIP2 alveolar levels at 12 h versus 36 h). Thirty-six hours following tracheal inoculation of 108 CFU inactivated S. haemolyticus, BAL TNF-α and MIP-2 significantly increased with the inoculum (Kruskal–Wallis p = 0.0086 for TNF-α and p = 0.0013 for MIP-2; ο indicate p < 0.05 for 108 versus 107 and 106 CFU; οο indicate p < 0.05 for 108 versus 107 and 106 CFU, and PBS).
As shown in Figure 7, there were no significant differences in the total protein concentration in BAL following tracheal inoculation of PBS or 106, 107, and 108 CFU of live and inactivated S. haemolyticus.
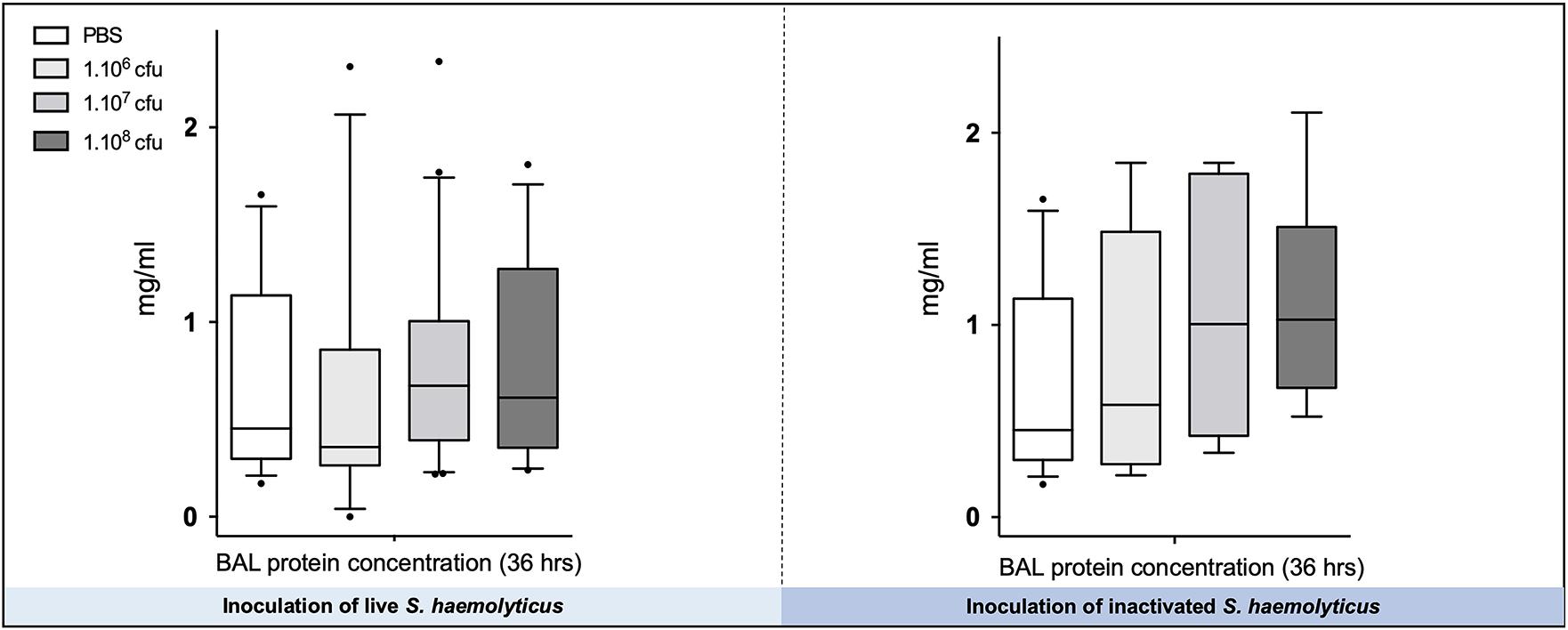
Figure 7. The total protein concentration in BAL following tracheal inoculation of PBS, and increasing inocula (106, 107, and 108 CFU) of live and inactivated S. haemolyticus remained unchanged. Data are expressed as median values, interquartile 25th–75th range, and extreme values (>2 SD).
As shown in Figure 8A, 12 h following the tracheal inoculation of 108 CFU S. haemolyticus, bacterial load in the BAL reached a median value of 390 CFU/ml [294 to 496], whereas BAL from the other groups remained sterile. At the 12-h time point, the overall identified strains in both BAL (Figure 2A) and lung homogenate (Figure 2B) samples were S. haemolyticus. As shown in Figure 8B, 36 h later, bacterial load remained elevated after inoculation of 108 CFU S. haemolyticus and appeared in BAL following PBS and lower inocula. As shown in Figure 8C, a significant bacterial load was observed in lung tissue homogenates 36 h following tracheal inoculation of 107 and 108 CFU S. haemolyticus (p = 0.003). However, colonies in both BAL and lung homogenate (Figure 2C) were identified as species originating from oropharyngeal flora, such as Achromobacter denitrificans and Bordetella avium. S. haemolyticus strains remained undetectable. As shown in Figure 8D, 36 h following tracheal inoculation of PBS and increasing inocula of inactivated S. haemolyticus, a weak and non-significant bacterial load was observed in BAL.
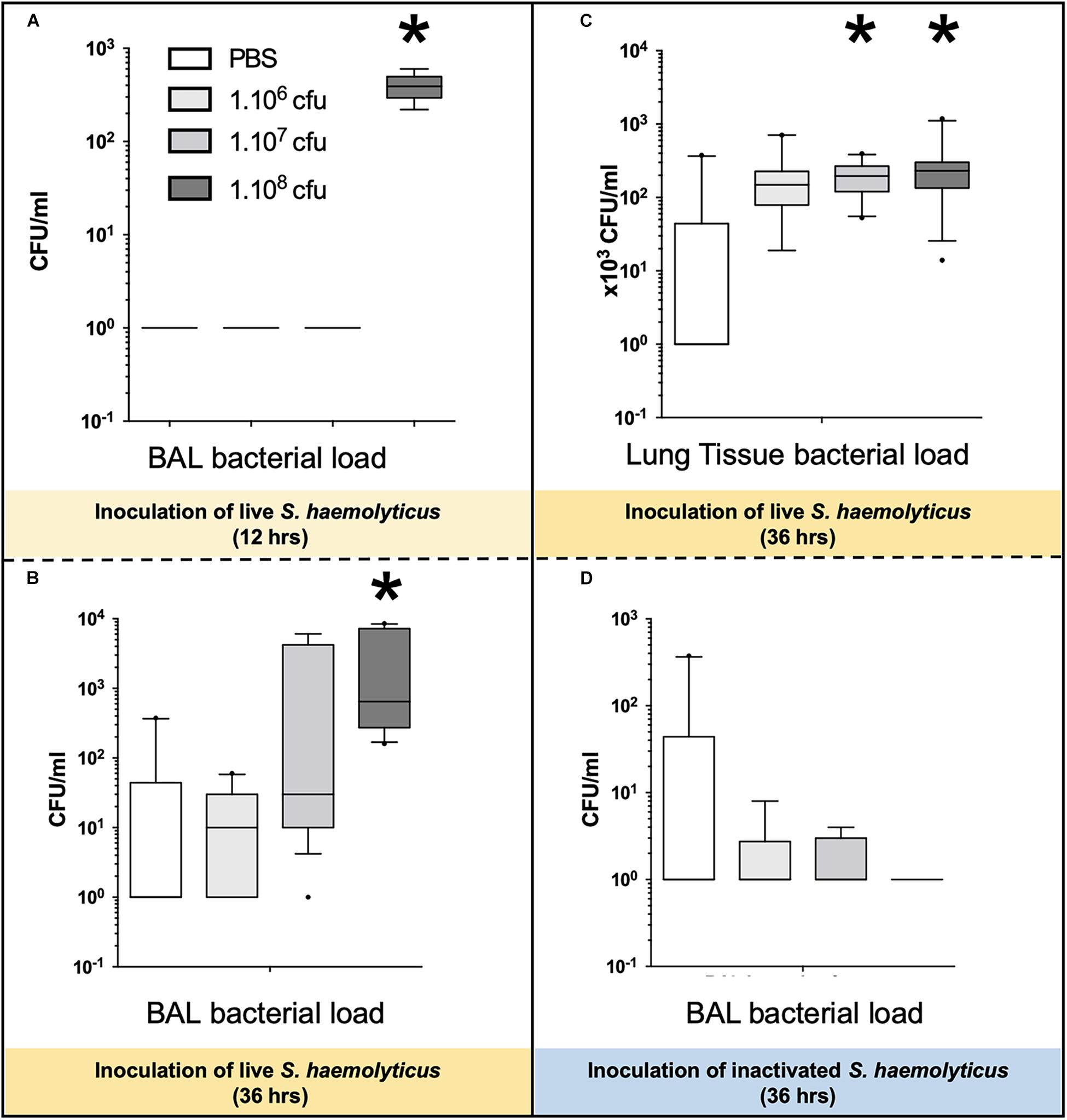
Figure 8. Bacterial load in BAL and lung tissue homogenate following tracheal inoculation of PBS, and increasing inocula (106, 107, and 108 CFU) of live and inactivated S. haemolyticus. Data are expressed as median values, interquartile 25th–75th range and extreme values (>2 SD). (A) Kruskal–Wallis p = 0.002; ∗indicates p < 0.05 for 108 CFU versus PBS and 106 CFU; (B) Kruskal–Wallis p = 0.04; ∗indicates p = 0.046 for 108 CFU versus 106 CFU; (C) Kruskal–Wallis p = 0.003; ∗indicates p < 0.05 for 108 CFU and 107 CFU versus PBS. (D) No significant differences of bacterial colonies in BALF among the inactivated S. haemolyticus groups.
We collected S. haemolyticus used in our vivo experiment as well as another 19 of clinical S. haemolyticus strains and screened for biofilm-associated genes. The details of origin and antimicrobial resistance profile are listed in Supplementary Tables S3, S4. All the S. haemolyticus isolates formed a biofilm on microplates (Figures 9A,B).
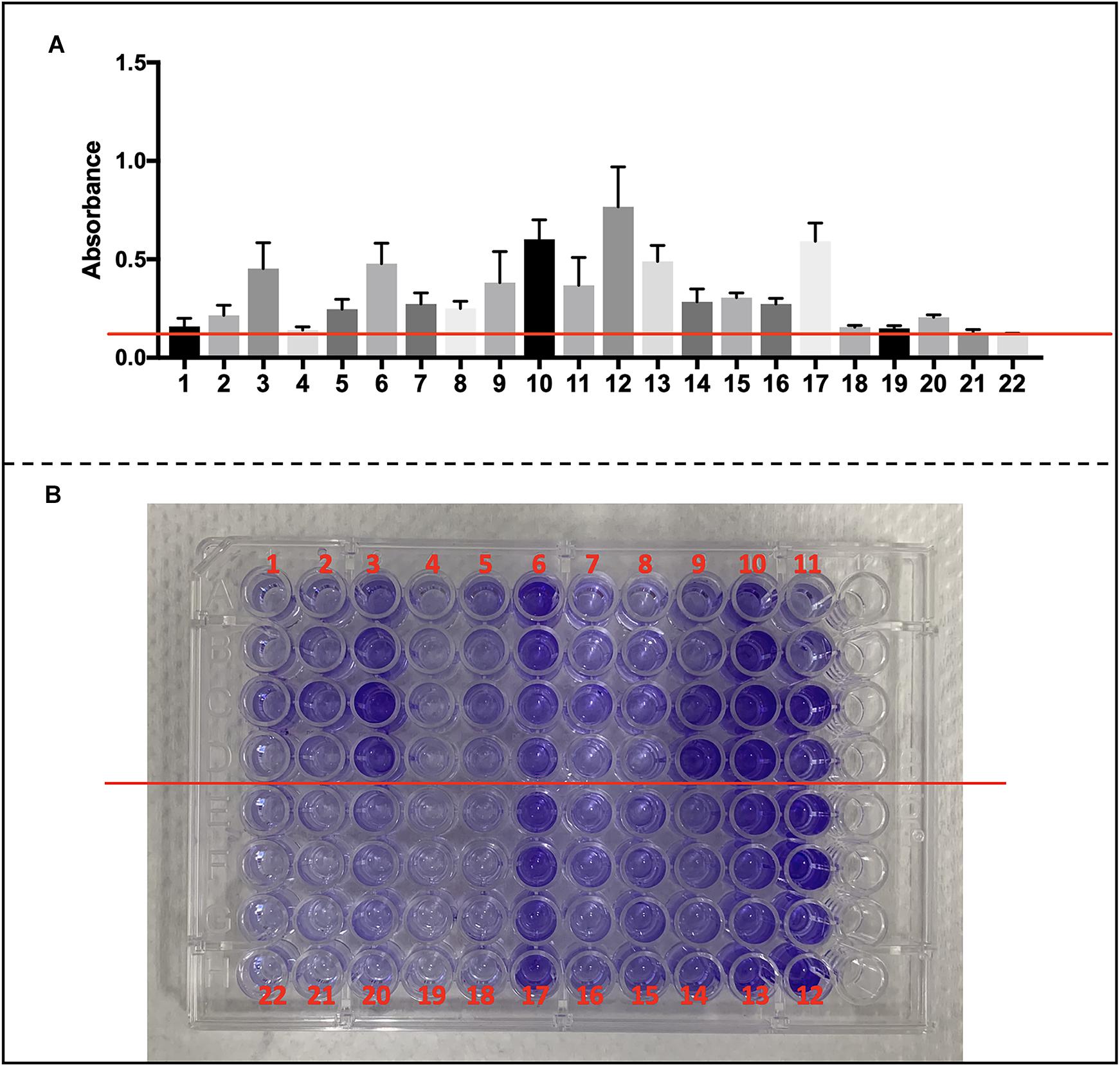
Figure 9. Biofilm formation of CoNS strains and detection of biofilm-associated genes. (A) Data are shown in mean ± SD. Mean level of absorbance of wells was measured spectrophotometrically at 570 nm by a microplate reader after staining by crystal violet (red cross line shows cutoff value = 0.12). (B) Images of wells represent each strain in crystal violet-stained microplates.
As shown in Supplementary Figure S1A, none of the S. haemolyticus carried icaA or icaD genes. As shown in Supplementary Figure S1B, 14 isolates (14/20, 70%) of clinical S. haemolyticus carried the cbp gene while 18 isolates (18/20, 90%) carried the argD gene. All the clinical S. haemolyticus isolates carried the genes encoding cell surface attachment proteins (ebpS, fbp, and srtA), quorum sensing (agrA, agrB, and luxS), and eDNA release (cidA, cidB, lrgA, lrgB, and atlE) (Supplementary Figures S1C–H).
The main findings of this work can be summarized as follows. (1) Twelve hours after the tracheal inoculation of 108 CFU live S. haemolyticus, macroscopic evidence of lung injury was observed, histologically characterized by confluent pneumonia and associated with marked increases in TNF-α and MIP-2 and the presence of significant concentrations of S. haemolyticus in the BAL and in the lung homogenate. (2) Thirty-six hours later, macroscopic and histologic evidence of confluent pneumonia and BAL inflammation persisted, but at a lesser degree. High concentrations of bacteria were found in lung tissue and BAL, but S. haemolyticus strains were replaced by various species belonging to the oropharyngeal flora. (3) Tracheal inoculation of lower concentrations of live S. haemolyticus produced less severe macroscopic lung injury, histological evidence of interstitial pneumonia, and moderate but significant inflammation in BAL. (4) Inoculation of increasing concentrations of inactivated S. haemolyticus produced similar histological lung injury with low-to-moderate alveolar inflammation, which were similar to those found 36 h following tracheal inoculation of 108 CFU live S. haemolyticus. (5) Biofilm formation was a common phenotype in S. haemolyticus isolates. The low prevalence of the ica operon in our clinical S. haemolyticus strain collection indicated icaA and icaD independent-biofilm formation.
CoNS are considered to be among the top common pathogens isolated in bloodstream infections (Moyo et al., 2010; Adam et al., 2011; Wilson et al., 2011). The S. haemolyticus isolate inoculated in our mice was obtained from a patient with nosocomial bacteremia and considered by clinicians as pathogenic, requiring antibiotic treatment. The presence of CoNS in distal bronchial specimens is generally believed to reflect lung colonization or contamination (van Saene et al., 1996). However, more and more physicians consider that CoNS can generate pulmonary infections in both immunocompetent and immunocompromised hosts (Rouby et al., 1992; Kloos and Bannerman, 1994; Kirtland et al., 1997; Rello et al., 1997; George et al., 1998; Hell et al., 1999). Staphylococci of the S. epidermidis group demonstrate a low to moderate virulence (Becker et al., 2014). They have a high ability to attach to foreign bodies’ surface and, sometimes, host cells. S. haemolyticus is able to induce biofilm on cultured cells (Shrestha et al., 2018) and Staphylococcus lugdunensis may adhere and invade cultured alveolar type II cells (Pereira et al., 2012). Formed on foreign bodies and endocardium cells, biofilm makes the cells less accessible to the defense system of the host, impairs antibiotic activity, and is the most important virulence factor for CoNS (Becker et al., 2014). However, the production of strongly cytotoxic phenol-soluble modulins by CoNS is low, whereas the release of pyrogenic toxin super-antigen like staphylococcal enterotoxins and toxic shock syndrome toxin1 is virtually absent. Cell wall components of S. epidermidis stimulate the release of TNF-α, IL-6, and IL-1β from activated monocytes (Mattsson et al., 1993, 1996). No data are available concerning the pathogenicity of S. haemolyticus on the lungs. Our study demonstrates for the first time that intratracheal inoculation of S. haemolyticus induces histological interstitial and confluent pneumonia in immunocompetent mice with influx of inflammatory cells and cytokines in BAL. Twelve hours after a bacterial inoculation of 108 CFU, low to moderate concentrations of S. haemolyticus were retrieved in the BAL. Twenty-four hours later, while we attempted to select as many colonies as possible with different morphologies (appearance, size, shape), we were unsuccessful in detecting S. haemolyticus, either massively diluted into colonizing strains, or cleared by the immune systems at those time points. Species such as A. denitrificans and B. avium, belonging to the oropharyngeal flora, were isolated at low concentrations in lung parenchyma. All these results tend to indicate that the S. haemolyticus colonies initially inoculated, and found at the 12-h time point, were eventually replaced by germs colonizing the airways between the 12- and 36-h time point. Supporting this hypothesis, BAL TNF-α and MIP-2 were quite elevated 12 h after the inoculation of 108 CFU S. haemolyticus and markedly decreased 24 h later, suggesting a decreased monocyte activation by wall components of the inoculated isolates. All these data confirmed the hypothesis of S. haemolyticus pathogenicity to cause pneumonia, from which immunocompetent mice are able to recover spontaneously.
We previously reported that pneumonia could be induced by tracheal inoculation of endotoxin (Zhu et al., 2014; Tang et al., 2017), 106 CFU Pseudomonas aeruginosa (Mao et al., 2015) and Escherichia coli (Monsel et al., 2015) in the same mice model. In the current study, 107 CFU inoculum was required to produce interstitial pneumonia and 108 CFU was required to produce confluent pneumonia, confirming indirectly that S. haemolyticus is less virulent than Gram-negative bacteria (van Saene et al., 1996; Lambotte et al., 2002; Becker et al., 2014). Interestingly, similar inflammatory cell influx, release of proinflammatory cytokines in the BAL, and lung parenchymal histological damage were also produced by high inoculum of inactivated S. haemolyticus, suggesting that wall debris may participate in lung injury. Another interesting finding is that inoculation pneumonia only transitorily impacted mice behavior. Animals inoculated by 108 CFU S. haemolyticus were initially prostrated with limited food access, but rapidly recovered, moving again within their cage and accessing food and water. Although histological pneumonia persisted, this behavior improvement was associated with a decreased lung inflammation and a partial eradication of S. haemolyticus from the lung.
It has been described that biofilm formation was a two-step process that was mediated by a number of factors like cell wall-anchored surface proteins (i.e., fbe and bhp) (Cucarella et al., 2001) and the cell wall lytic enzyme autolysin E (i.e., atlE) (Heilmann et al., 1997; Davis et al., 2001). S. haemolyticus biofilm formation in vitro has been reported, but the contribution of ica operon was not well elucidated (Cramton et al., 1999; Allignet et al., 2001; Foka et al., 2006; Frank and Patel, 2007). In our study, we have investigated the biofilm formation phenotypes and genotypes of a large collection of clinical S. haemolyticus isolates, and the genetic background of our vivo experiment strain for biofilm formation is mostly similar as what is commonly found in S. haemolyticus strains from other studies (Fredheim et al., 2009). We confirmed by sequencing that the icaA and icaD was absent in all the S. haemolyticus isolates, suggesting a low prevalence compared with S. epidermidis. In concurrence with previously reported sequences (Takeuchi et al., 2005; Watanabe et al., 2007), the instability of the ica operon may be explained by genomic rearrangements mediated by insertion elements that have been observed in S. haemolyticus. Interestingly, further studies are needed to define the role of cbp gene, which was more frequently present in clinically originated strains than nursing house-originated strains.
Several limitations associated with the present study warrant mention. First, only a single species, S. haemolyticus, was studied in our work, which may not be representative of the whole CoNS family. Second, some commensals were identified in lung homogenate, suggesting that they were introduced during the inoculation procedure consisting of transglottic tracheal administration. Interaction between CoNS and other microorganisms remains undocumented, and the possibility that they can play a role in lung parenchymal infection cannot be ruled out. Third, several differences exist between mice and humans, precluding the generalization of our findings. Tracheobronchial branching is less complex in mice than in humans. Respiratory bronchioles do not exist in mice, and airways terminate directly into alveolar ducts (Bal and Ghoshal, 1988). Therefore, foci of pneumonia are not centered on infected respiratory bronchioles like in humans and infection disseminates more easily in the lung parenchyma. In humans, bronchial submucosal glands secrete lysozyme, which keeps the lower respiratory tract sterile. Submucosal glands are absent from the conducting airways in mice (Skerrett, 2004), reducing local anti-infectious defenses. Circulating neutrophil counts are lower in mice than in humans and lack α-defensins (Eisenhauer and Lehrer, 1992), whereas natural killer cell activity is generally higher in murine than in human lungs (Matute-Bello et al., 2011). These species differences may facilitate or protect against S. haemolyticus virulence. Also, exploring other signaling pathways such as the anti-inflammatory side of the immune response may help better explain the underlying mechanisms of the pathophysiology of this S. haemolyticus-induced pneumonia.
This study provides evidence that tracheal inoculation of S. haemolyticus to immunocompetent mice produces interstitial and confluent pneumonia of moderate severity with a rapid and spontaneous recovery of inflammatory markers and clinical condition. Moreover, icaA and icaD independent biofilm formation is a common phenotype in S. haemolyticus isolates.
All datasets generated for this study are included in the manuscript/Supplementary Files.
The animal study was reviewed and approved by the Ruijin Hospital Ethics Committee, Shanghai Jiao Tong University School of Medicine.
M-MS and Y-PX contributed to the conception and design, the collection and/or assembly of data, the data analysis and the interpretation, and the writing of the manuscript. AM and J-JR contributed to the conception and design, the data analysis and the interpretation, and the writing of the manuscript. Y-GZ and J-MQ contributed to the conception and design, financial support, data analysis and interpretation, the writing of the manuscript, and final approval of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81570066 and 81630001), the Excellent youth talent project of the Shanghai Municipal Commission of Health and Family Planning (No. 2017YQ081), the Shanghai Key Discipline for Respiratory Diseases (No. 2017ZZ02014), and the National Innovative Research Team of High-level Local Universities in Shanghai.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02198/full#supplementary-material
Adam, H. J., DeCorby, M., Rennie, R., Karlowsky, J. A., Hoban, D. J., and Zhanel, G. G. (2011). Prevalence of antimicrobial resistant pathogens from blood cultures from Canadian hospitals: Results of the CANWARD 2007-2009 study. Diagn. Microbiol. Infect. Dis 69, 307–313. doi: 10.1016/j.diagmicrobio.2010.10.026
Agvald-Ohman, C., Lund, B., and Edlund, C. (2004). Multiresistant coagulase-negative staphylococci disseminate frequently between intubated patients in a multidisciplinary intensive care unit. Critical care (London, England) 8, R42–R47.
Allignet, J., Aubert, S., Dyke, K. G., and El Solh, N. (2001). Staphylococcus caprae strains carry determinants known to be involved in pathogenicity: A gene encoding an autolysin-binding fibronectin and the ica operon involved in biofilm formation. Infect. Immun. 69, 712–718. doi: 10.1128/iai.69.2.712-718.2001
Bal, H. S., and Ghoshal, N. G. (1988). Morphology of the terminal bronchiolar region of common laboratory mammals. Lab. Anim. 22, 76–82. doi: 10.1258/002367788780746539
Bearman, G. M., and Wenzel, R. P. (2005). Bacteremias: A leading cause of death. Arch. Med. Res. 36, 646–659. doi: 10.1016/j.arcmed.2005.02.005
Becker, K., Heilmann, C., and Peters, G. (2014). Coagulase-negative staphylococci. Clin. Microbiol. Rev 27, 870–926. doi: 10.1128/CMR.00109-13
Cervera, C., Almela, M., Martinez-Martinez, J. A., Moreno, A., and Miro, J. M. (2009). Risk factors and management of Gram-positive bacteraemia. Int. J. Antimicrob. Agents 34(Suppl. 4), S26–S30. doi: 10.1016/S0924-8579(09)70562-X
Cramton, S. E., Gerke, C., Schnell, N. F., Nichols, W. W., and Gotz, F. (1999). The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67, 5427–5433.
Crass, B. A., and Bergdoll, M. S. (1986). Involvement of coagulase-negative staphylococci in toxic shock syndrome. J. Clin. Microbiol. 23, 43–45.
Cucarella, C., Solano, C., Valle, J., Amorena, B., Lasa, I., and Penades, J. R. (2001). Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183, 2888–2896. doi: 10.1128/jb.183.9.2888-2896.2001
Cunha Mde, L., Lopes, C. A., Rugolo, L. M., and Chalita, L. V. (2002). Clinical significance of coagulase-negative staphylococci isolated from neonates. J. Pediatr. 78, 279–288.
Davis, S. L., Gurusiddappa, S., McCrea, K. W., Perkins, S., and Hook, M. (2001). SdrG, a fibrinogen-binding bacterial adhesin of the microbial surface components recognizing adhesive matrix molecules subfamily from Staphylococcus epidermidis, targets the thrombin cleavage site in the Bbeta chain. J. Biol. Chem. 276, 27799–27805. doi: 10.1074/jbc.m103873200
Eisenhauer, P. B., and Lehrer, R. I. (1992). Mouse neutrophils lack defensins. Infect. Immun. 60, 3446–3447.
Foka, A., Chini, V., Petinaki, E., Kolonitsiou, F., Anastassiou, E. D., Dimitracopoulos, G., et al. (2006). Clonality of slime-producing methicillin-resistant coagulase-negative staphylococci disseminated in the neonatal intensive care unit of a university hospital. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 12, 1230–1233. doi: 10.1111/j.1469-0691.2006.01565.x
Frank, K. L., and Patel, R. (2007). Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect. Immun. 75, 4728–4742. doi: 10.1128/iai.00640-07
Frebourg, N. B., Lefebvre, S., Baert, S., and Lemeland, J. F. (2000). PCR-Based assay for discrimination between invasive and contaminating Staphylococcus epidermidis strains. Journal of clinical microbiology 38, 877–880.
Fredheim, E. G., Klingenberg, C., Rohde, H., Frankenberger, S., Gaustad, P., Flaegstad, T., et al. (2009). Biofilm formation by Staphylococcus haemolyticus. J. Clin. Microbiol. 47, 1172–1180. doi: 10.1128/JCM.01891-08
George, D. L., Falk, P. S., Wunderink, R. G., Leeper, K. V. Jr., Meduri, G. U., Steere, E. L., et al. (1998). Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am. J. Respir. Crit. Care Med. 158, 1839–1847. doi: 10.1164/ajrccm.158.6.9610069
Grasselli, G., Scaravilli, V., Di Bella, S., Biffi, S., Bombino, M., Patroniti, N., et al. (2017). Nosocomial infections during extracorporeal membrane oxygenation: Incidence, etiology, and impact on patients’ outcome. Crit. Care Med 45, 1726–1733. doi: 10.1097/ccm.0000000000002652
Heilmann, C., Hussain, M., Peters, G., and Gotz, F. (1997). Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24, 1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x
Hell, W., Kern, T., and Klouche, M. (1999). Staphylococcus saprophyticus as an unusual agent of nosocomial pneumonia. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 29, 685–686. doi: 10.1086/598657
John, J. F., and Harvin, A. M. (2007). History and evolution of antibiotic resistance in coagulase-negative staphylococci: Susceptibility profiles of new anti-staphylococcal agents. Therapeutics and clinical risk management 3, 1143–1152.
Kirtland, S. H., Corley, D. E., Winterbauer, R. H., Springmeyer, S. C., Casey, K. R., Hampson, N. B., et al. (1997). The diagnosis of ventilator-associated pneumonia: A comparison of histologic, microbiologic, and clinical criteria. Chest 112, 445–457.
Kloos, W. E., and Bannerman, T. L. (1994). Update on clinical significance of coagulase-negative staphylococci. Clin. Microbiol. Rev 7, 117–140. doi: 10.1128/cmr.7.1.117
Lambotte, O., Timsit, J. F., Garrouste-Orgeas, M., Misset, B., Benali, A., and Carlet, J. (2002). The significance of distal bronchial samples with commensals in ventilator-associated pneumonia: Colonizer or pathogen? Chest 122, 1389–1399. doi: 10.1378/chest.122.4.1389
Lina, G., Fleer, A., Etienne, J., Greenland, T. B., and Vandenesch, F. (1996). Coagulase-negative staphylococci isolated from two cases of toxic shock syndrome lack superantigenic activity, but induce cytokine production. FEMS Immunol. Med. Microbiol. 13, 81–86. doi: 10.1016/0928-8244(95)00087-9
Loiez, C., Wallet, F., Pischedda, P., Renaux, E., Senneville, E., Mehdi, N., et al. (2007). First case of osteomyelitis caused by “Staphylococcus pettenkoferi”. Journal of clinical microbiology 45, 1069–1071. doi: 10.1128/jcm.02328-06
Mao, Y. X., Xu, J. F., Seeley, E. J., Tang, X. D., Xu, L. L., Zhu, Y. G., et al. (2015). Adipose tissue-derived mesenchymal stem cells attenuate pulmonary infection caused by Pseudomonas aeruginosa via inhibiting overproduction of prostaglandin E2. Stem cells (Dayton, Ohio) 33, 2331–2342. doi: 10.1002/stem.1996
Mattsson, E., Van Dijk, H., Verhoef, J., Norrby, R., and Rollof, J. (1996). Supernatants from Staphylococcus epidermidis grown in the presence of different antibiotics induce differential release of tumor necrosis factor alpha from human monocytes. Infect. Immun. 64, 4351–4355.
Mattsson, E., Verhage, L., Rollof, J., Fleer, A., Verhoef, J., and van Dijk, H. (1993). Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol. Med. Microbiol. 7, 281–287. doi: 10.1016/0928-8244(93)90023-w
Matute-Bello, G., Downey, G., Moore, B. B., Groshong, S. D., Matthay, M. A., Slutsky, A. S., et al. (2011). An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. American journal of respiratory cell and molecular biology 44, 725–738. doi: 10.1165/rcmb.2009-0210ST
Mizgerd, J. P., and Skerrett, S. J. (2008). Animal models of human pneumonia. American journal of physiology Lung cellular and molecular physiology 294, L387–L398.
Monsel, A., Zhu, Y. G., Gennai, S., Hao, Q., Hu, S., Rouby, J. J., et al. (2015). Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 192, 324–336. doi: 10.1164/rccm.201410-1765OC
Morfin-Otero, R., Martinez-Vazquez, M. A., Lopez, D., Rodriguez-Noriega, E., and Garza-Gonzalez, E. (2012). Isolation of rare coagulase-negative isolates in immunocompromised patients: Staphylococcus gallinarum, Staphylococcus pettenkoferi and Staphylococcus pasteuri. Ann. Clin. Lab. Sci. 42, 182–185.
Moyo, S., Aboud, S., Kasubi, M., and Maselle, S. Y. (2010). Bacteria isolated from bloodstream infections at a tertiary hospital in Dar es Salaam, Tanzania—Antimicrobial resistance of isolates. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde 100, 835–838.
Panda, S., and Singh, D. V. (2018). Biofilm Formation by ica-negative ocular isolates of Staphylococcus haemolyticus. Frontiers in microbiology 9:2687. doi: 10.3389/fmicb.2018.02687
Peer, M. A., Nasir, R. A., Kakru, D. K., Fomda, B. A., Bashir, G., and Sheikh, I. A. (2011). Sepsis due to linezolid resistant Staphylococcus cohnii and Staphylococcus kloosii: First reports of linezolid resistance in coagulase negative staphylococci from India. Indian journal of medical microbiology 29, 60–62. doi: 10.4103/0255-0857.76527
Pereira, E. M., Teixeira, C. A., Alvarenga, A. L., Schuenck, R. P., Giambiagi-Demarval, M., Holandino, C., et al. (2012). A Brazilian lineage of Staphylococcus lugdunensis presenting rough colony morphology may adhere to and invade lung epithelial cells. J. Med. Microbiol. 61, 463–469. doi: 10.1099/jmm.0.033001-0
Qi, F., Zhang, G. X., She, D. Y., Liang, Z. X., Wang, R. T., Yang, Z., et al. (2015). Healthcare-associated pneumonia: Clinical features and retrospective analysis over 10 years. Chin. Med. J. 128, 2707–2713. doi: 10.4103/0366-6999.167294
Rello, J., Gallego, M., Mariscal, D., Sonora, R., and Valles, J. (1997). The value of routine microbial investigation in ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 156, 196–200. doi: 10.1164/ajrccm.156.1.9607030
Rogers, K. L., Fey, P. D., and Rupp, M. E. (2009). Coagulase-negative staphylococcal infections. Infect. Dis. Clin. North Am. 23, 73–98. doi: 10.1016/j.idc.2008.10.001
Rouby, J. J., Martin De Lassale, E., Poete, P., Nicolas, M. H., Bodin, L., Jarlier, V., et al. (1992). Nosocomial bronchopneumonia in the critically ill. Histologic and bacteriologic aspects. The American review of respiratory disease 146, 1059–1066. doi: 10.1164/ajrccm/146.4.1059
Shrestha, L. B., Bhattarai, N. R., and Khanal, B. (2018). Comparative evaluation of methods for the detection of biofilm formation in coagulase-negative staphylococci and correlation with antibiogram. Infection and drug resistance 11, 607–613. doi: 10.2147/IDR.S159764
Skerrett, S. J. (2004). Lysozyme in pulmonary host defense: New tricks for an old dog. Am. J. Respir. Crit. Care Med. 169, 435–436. doi: 10.1164/rccm.2312018
Su, X., Looney, M., Robriquet, L., Fang, X., and Matthay, M. A. (2004). Direct visual instillation as a method for efficient delivery of fluid into the distal airspaces of anesthetized mice. Exp. Lung Res. 30, 479–493. doi: 10.1080/01902140490476382
Takeuchi, F., Watanabe, S., Baba, T., Yuzawa, H., Ito, T., Morimoto, Y., et al. (2005). Whole-genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J. Bacteriol. 187, 7292–7308. doi: 10.1128/jb.187.21.7292-7308.2005
Tang, X. D., Shi, L., Monsel, A., Li, X. Y., Zhu, H. L., Zhu, Y. G., et al. (2017). Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem cells (Dayton, Ohio) 35, 1849–1859. doi: 10.1002/stem.2619
van Saene, H. K., Damjanovic, V., Murray, A. E., and de la Cal, M. A. (1996). How to classify infections in intensive care units—The carrier state, a criterion whose time has come? The Journal of hospital infection 33, 1–12. doi: 10.1016/s0195-6701(96)90025-0
Venkatesh, M. P., Placencia, F., and Weisman, L. E. (2006). Coagulase-negative staphylococcal infections in the neonate and child: An update. Seminars in pediatric infectious diseases 17, 120–127. doi: 10.1053/j.spid.2006.06.005
von Eiff, C., Jansen, B., Kohnen, W., and Becker, K. (2005). Infections associated with medical devices: Pathogenesis, management and prophylaxis. Drugs 65, 179–214. doi: 10.2165/00003495-200565020-00003
Watanabe, S., Ito, T., Morimoto, Y., Takeuchi, F., and Hiramatsu, K. (2007). Precise excision and self-integration of a composite transposon as a model for spontaneous large-scale chromosome inversion/deletion of the Staphylococcus haemolyticus clinical strain JCSC1435. Journal of bacteriology 189, 2921–2925. doi: 10.1128/jb.01485-06
Wilson, J., Elgohari, S., Livermore, D. M., Cookson, B., Johnson, A., Lamagni, T., et al. (2011). Trends among pathogens reported as causing bacteraemia in England, 2004–2008. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 17, 451–458. doi: 10.1111/j.1469-0691.2010.03262.x
Wisplinghoff, H., Bischoff, T., Tallent, S. M., Seifert, H., Wenzel, R. P., and Edmond, M. B. (2004). Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 39, 309–317. doi: 10.1086/421946
Zhou, K., Tao, Y., Han, L., Ni, Y., and Sun, J. (2019). Piperacillin-Tazobactam (TZP) Resistance in Escherichia coli due to hyperproduction of TEM-1 beta-lactamase mediated by the promoter Pa/Pb. Frontiers in microbiology 10:833. doi: 10.3389/fmicb.2019.00833
Keywords: inoculation pneumonia, coagulase-negative staphylococci, Staphylococcus haemolyticus, tumor necrosis factor-α, macrophage inflammatory protein-2
Citation: Shi M-M, Monsel A, Rouby J-J, Xu Y-P, Zhu Y-G and Qu J-M (2019) Inoculation Pneumonia Caused by Coagulase Negative Staphylococcus. Front. Microbiol. 10:2198. doi: 10.3389/fmicb.2019.02198
Received: 21 June 2019; Accepted: 09 September 2019;
Published: 04 October 2019.
Edited by:
Miklos Fuzi, Semmelweis University, HungaryReviewed by:
Fufa Qu, Changsha University, ChinaCopyright © 2019 Shi, Monsel, Rouby, Xu, Zhu and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-gang Zhu, cm9iaW56eWdAZ21haWwuY29t; Jie-ming Qu, am1xdTA5MDZAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.