- Cytokine Research Laboratory, Department of Experimental Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
by Tyagi, A. K., and Prasad, S. (2015). Front. Microbiol. 6:433. doi: 10.3389/fmicb.2015.00433
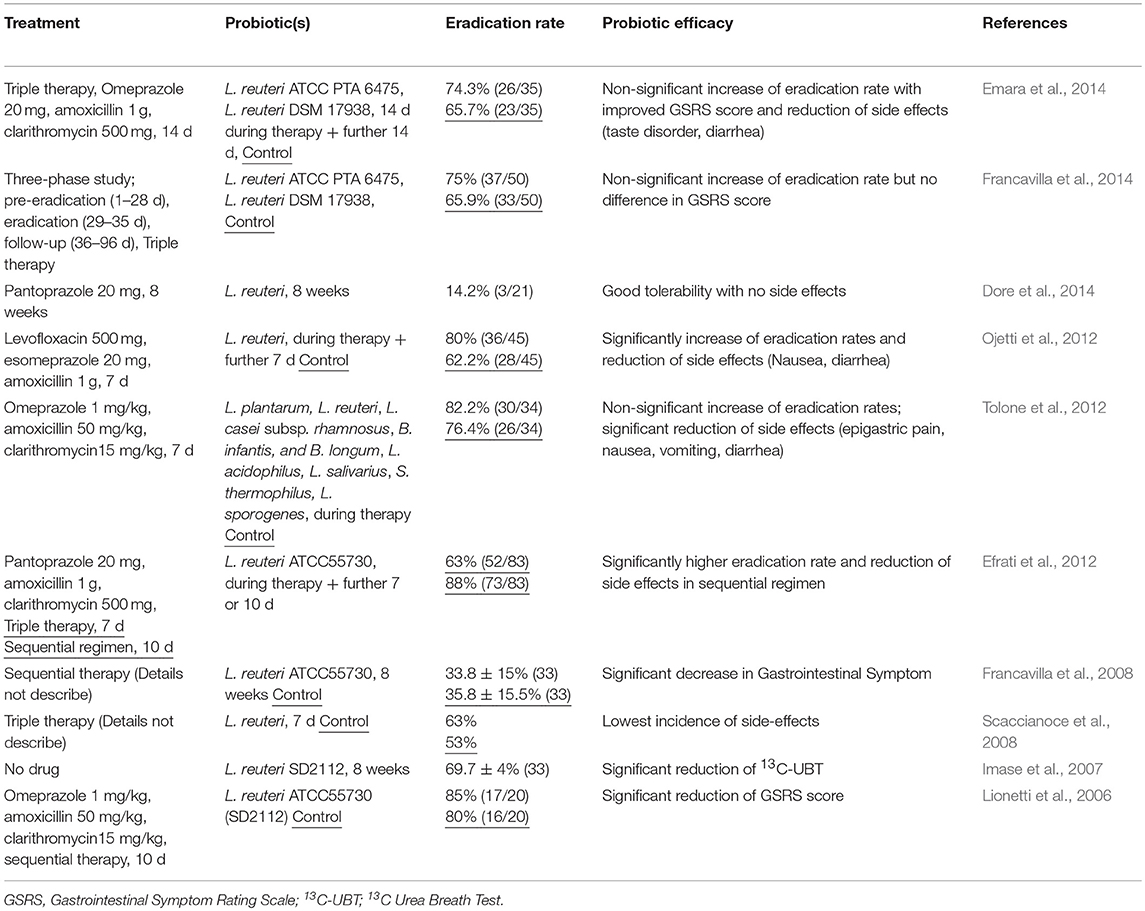
In the original article, there was error in Table 1 as published. The probiotic efficacy for Emara 2014 and Francavilla 2014 reported a significant increase instead of a non-significant increase.
Additionally, the reference for “Francavilla et al., 2008” in Table 1 was incorrectly written as “Francavilla, R., Lionetti, E., and Cavallo, L. (2008). Sequential treatment for Helicobacter pylori eradication in children. Gut 57:1178.” It should be “Francavilla, R., Lionetti, E., Castellaneta, S. P., Magistà, A. M., Maurogiovanni, G., Bucci, N., et al. (2008). Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter 13, 127–134. doi: 10.1111/j.1523-5378.2008.00593.x”.
The corrected Table 1 and Reference appear below.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
References
Dore, M. P., Cuccu, M., Pes, G. M., Manca, A., and Graham, D. Y. (2014). Lactobacillus reuteri in the treatment of Helicobacter pylori infection. Intern. Emerg. Med. 9, 649–654. doi: 10.1007/s11739-013-1013-z
Efrati, C., Nicolini, G., Cannaviello, C., O'sed, N. P., and Valabrega, S. (2012). Helicobacter pylori eradication: sequential therapy and Lactobacillus reuteri supplementation. World J. Gastroenterol. 18, 6250–6254. doi: 10.3748/wjg.v18.i43.6250
Emara, M. H., Mohamed, S. Y., and Abdel-Aziz, H. R. (2014). Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial. Therap. Adv. Gastroenterol. 7, 4–13. doi: 10.1177/1756283X13503514
Francavilla, R., Lionetti, E., Castellaneta, S. P., Magistà, A. M., Maurogiovanni, G., Bucci, N., et al. (2008). Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter 13, 127–134. doi: 10.1111/j.1523-5378.2008.00593.x
Francavilla, R., Polimeno, L., Demichina, A., Maurogiovanni, G., Principi, B., Scaccianoce, G., et al. (2014). Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J. Clin. Gastroenterol. 48, 407–413. doi: 10.1097/MCG.0000000000000007
Imase, K., Tanaka, A., Tokunaga, K., Sugano, H., Ishida, H., and Takahashi, S. (2007). Lactobacillus reuteri tablets suppress Helicobacter pylori infection–a double-blind randomised placebo-controlled cross-over clinical study. Kansenshogaku Zasshi 81, 387–393. doi: 10.11150/kansenshogakuzasshi1970.81.387
Lionetti, E., Miniello, V. L., Castellaneta, S. P., Magistá, A. M., de Canio, A., Maurogiovanni, G., et al. (2006). Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment. Pharmacol. Ther. 24, 1461–1468. doi: 10.1111/j.1365-2036.2006.03145.x
Ojetti, V., Bruno, G., Ainora, M. E., Gigante, G., Rizzo, G., Roccarina, D., et al. (2012). Impact of Lactobacillus reuteri supplementation on anti-Helicobacter pylori levofloxacin-based second-line therapy. Gastroenterol. Res. Pract. 2012:740381. doi: 10.1155/2012/740381
Scaccianoce, G., Zullo, A., Hassan, C., Gentili, F., Cristofari, F., Cardinale, V., et al. (2008). Triple therapies plus different probiotics for Helicobacter pylori eradication. Eur. Rev. Med. Pharmacol. Sci. 12, 251–256.
Keywords: Lactobacillus reuteri, Helicobacter pylori, probiotics, inflammation, triple therapy
Citation: Tyagi AK and Prasad S (2019) Corrigendum: Commentary: Probiotic and technological properties of Lactobacillus spp. strains from the human stomach in the search for potential candidates against gastric microbial dysbiosis. Front. Microbiol. 10:1628. doi: 10.3389/fmicb.2019.01628
Received: 02 April 2019; Accepted: 02 July 2019;
Published: 18 July 2019.
Edited and reviewed by: Fabio Minervini, University of Bari Aldo Moro, Italy
Copyright © 2019 Tyagi and Prasad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amit K. Tyagi, YWt1bWFyNkBtZGFuZGVyc29uLm9yZw==; YW1pdHR5YWdpaWl0ZEBnbWFpbC5jb20=
 Amit K. Tyagi
Amit K. Tyagi Sahdeo Prasad
Sahdeo Prasad