
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 25 June 2019
Sec. Food Microbiology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.01419
This article is part of the Research TopicResearch Efforts, Challenges and Opportunities in Mitigating Aflatoxins in Food and Agricultural Crops and its Global Health ImpactsView all 18 articles
A correction has been applied to this article in:
Corrigendum: Identification of volatile organic compounds in extremophilic bacteria and their effective use in biocontrol of postharvest fungal phytopathogens
 An-Dong Gong1,2*
An-Dong Gong1,2* Nan-Nan Wu1
Nan-Nan Wu1 Xian-Wei Kong1
Xian-Wei Kong1 Yi-Mei Zhang1
Yi-Mei Zhang1 Meng-Jun Hu3
Meng-Jun Hu3 Shuang-Jun Gong4
Shuang-Jun Gong4 Fei-Yan Dong1
Fei-Yan Dong1 Jian-Hua Wang5
Jian-Hua Wang5 Zhi-Yong Zhao5
Zhi-Yong Zhao5 Yu-Cai Liao2
Yu-Cai Liao2Controlling aflatoxigenic Aspergillus flavus and aflatoxins (AFs) in grains and food during storage is a great challenge to humans worldwide. Alcaligenes faecalis N1-4 isolated from tea rhizosphere soil can produce abundant antifungal volatiles, and greatly inhibited the growth of A. flavus in un-contacted face-to-face dual culture testing. Gas chromatography tandem mass spectrometry revealed that dimethyl disulfide (DMDS) and methyl isovalerate (MI) were two abundant compounds in the volatile profiles of N1-4. DMDS was found to have the highest relative abundance (69.90%, to the total peak area) in N1-4, which prevented the conidia germination and mycelial growth of A. flavus at 50 and 100 μL/L, respectively. The effective concentration for MI against A. flavus is 200 μL/L. Additionally, Real-time quantitative PCR analysis proved that the expression of 12 important genes in aflatoxin biosynthesis pathway was reduced by these volatiles, and eight genes were down regulated by 4.39 to 32.25-folds compared to control treatment with significant differences. And the A. flavus infection and AFs contamination in groundnut, maize, rice and soybean of high water activity were completely inhibited by volatiles from N1-4 in storage. Scanning electron microscope further proved that A. flavus conidia inoculated on peanuts surface were severely damaged by volatiles from N1-4. Furthermore, strain N1-4 showed broad and antifungal activity to other six important plant pathogens including Fusarium graminearum, F. equiseti, Alternaria alternata, Botrytis cinerea, Aspergillus niger, and Colletotrichum graminicola. Thus, A. faecalis N1-4 and volatile DMDS and MI may have potential to be used as biocontrol agents to control A. flavus and AFs during storage.
Aspergillus flavus is an important agricultural fungus which can infect many grain and oil crops in pre- and post-harvest such as groundnuts, maize, rice and cottonseed et al. (Yu et al., 2004). Moreover, some aflatoxigenic A. flavus can produce large amount of toxic aflatoxins (AFs) in crops and food, and seriously affect human and livestock health (Waliyar et al., 2015). Currently, 18 types of AFs produced by Aspergillus spp. have been identified. Among them, aflatoxin B1 (AFB1) was considered as the most dangerous mycotoxins to mammals due to its high toxicity and carcinogenicity (Asters et al., 2014; Adhikari et al., 2016). International Agency for Research on Cancer (IARC) classified AFB1 as Group I carcinogen to humans (Robens and Cardwell, 2003; Zhuang et al., 2016).
Aspergillus flavus and AFs pose great risk of human health worldwide. Williams et al. (2004) estimated that more than 5 billion people are suffering from the chronic exposure to AFs. Vardon calculated that the annual economic losses caused by AFs was about $500 million in the United States (Wu, 2015; Outlaw et al., 2016), and the additional cost of disease management is more than $20–$50 million (Robens and Cardwell, 2003). In less developed countries, because of the poor income and scanty food supplication, AFs contamination in food is even more severe, resulting in serious harms to local people. In Burundi and Congo, the percentage of AFs contamination was 100% in 244 samples collected from local marker including crops, milk, and other products. In these samples, more than 50% crops were contaminated with large amount of AFs which were higher than the maximum limit set by the European Union (Udomkun et al., 2018). Azziz-Baumgartner et al. (2005) reported over 125 human deaths in Kenya due to the consumption of AFs contaminated foods during 2004–2005. More seriously, in hepatitis B/C virus endemic countries (such as China, India, Africa, etc.), AFs led to severe hazards to humans. Taking China as an example, under the dual pressure of hepatitis B/C virus and AFs, more than 3.7 million people died of liver cancer every year. This accounts for 50% of the world’s liver cancer deaths (Chen et al., 2013).
Because of the great damage and serious risks to humans, many countries have legislatively defined the maximum levels of AFs intended for human and animal consumption. For example, in groundnut and their products, United States Food and Drug Administration permits the maximum AFs at 20 ppb (Torres et al., 2014). In European Union, maximum level is 4 ppb for AFs, and 2 ppb for AFB1 (European Commission [EC], 2010). Upper limit of AFs is 10 and 15 ppb in Kenya (Wagacha et al., 2013) and Malaysia (Leong et al., 2010), respectively. In China, the maximum level for AFB1 is 20 ppb, and total AFs has not yet regulated. No regulation for total AFs and AFB1 in some developing counties (Kamika and Takoy, 2011).
Although legislations aimed to reduce the potential damage of AFs to humans is necessary, farmers in poor areas can not afford to monitor AFs (Probst et al., 2010). Thus, the effective strategies to eliminate AFs is controlling the initial infection of A. flavus in crops and grains (Abdel-Kareem et al., 2019). The A. flavus control in storage currently relies on physical drying, infected kernel sorting and chemical agents (Nurtjahtja, 2019). While the first two methods are safe and easy strategies and have been used for a long period (Bediako et al., 2018), the synthetic chemical agents may cause adverse effects on human health and environment due to long degradation period, toxic residues and potential undesirable biological effects (Prakash et al., 2015; Li et al., 2016). Bio-active compounds from organisms have been gradually applied in controlling A. flavus and AFs during storage (Kai et al., 2009; Passone and Etcheverry, 2014). Some essential oils extracted from plants have been widely used as promising agents in controlling A. flavus and AFs during storage, such as Curcuma longa (Hu et al., 2017), Litsea cubeba (Li et al., 2016), Zanthoxylum molle (Tian et al., 2014), Cinnamomum jensenianum (Tian et al., 2012), Peumus boldus, Lippia turbinata (Passone and Etcheverry, 2014), Oxalis corniculata (Rehman et al., 2015) and Cuminum cyminum (Kedia et al., 2014). Some compounds have been produced into commercial agents (DMC base Natural, owned by DOMCA S.A.), and successfully applied in disease control.
Despite the potential of plant extracts (i.e., bio-compounds and oils), plant growth requires a long period, dedicated maintenance and large cultivated areas. Moreover, extraction of bio-active compounds needs a large amount of plant sources which are not always available. Compared to plant materials, microbes are simple to obtain and culture, which are easier to obtain large amount. Till now, several microbes have been obtained and used in controlling A. flavus and AFs such as Bacillus megaterium (Chen et al., 2019), B. pumilus (Cho et al., 2009) and non-aflatoxigenic A. flavus strains (Ehrlich, 2013; Zanon et al., 2016). However, the biocontrol activity of these microbes mainly relies on antagonism (macromolecular metabolite) and competition, which always required direct contact with grains. Such contact may lead to secondary pollution to grains in storage. Compared to these contact phase, volatile phase can easily and uniformly distribute in the whole space of storage, and generated lower residual after volatilization. Recently, some evidences proved that the inhibitory effect of volatile phase on A. flavus in storage was consistently more effective than contact phase (Hu et al., 2017; Ma et al., 2017). To date, seldom microbe volatile was identified and used to control A. flavus and AFs during storage. Thus, the objectives of this study were to: (1) screening effective biocontrol microbes against A. flavus through the production of volatiles; (2) evaluating biocontrol efficacy of microbes against A. flavus and AFs in grain and food during storage; (3) identifying the primary antifungal bio-active volatiles; and (4) analyzing their antagonistic effect on cell ultra-structure of A. flavus and AFs biosynthesis.
Alcaligenes faecalis N1-4 was isolated from rhizosphere soil of tea plant in Cheyun mountain, Xinyang city, Henan, China (Fang et al., 2014). The bacteria was suspended in 25% (v/v) glycerol and stored at -80°C for long-term use.
Regular-sized maize (cultivar Zhengdan 958), peanut (Silihong) and soybean (W82) were purchased from local markets. Rice (Huanghuazhan) samples were supported by Huazhong Agricultural University. These samples were divided into two parts, and adjusted water activity (aw) to 0.8 and 0.9, respectively. Then, they were inoculated with A. flavus to detect the disease development and AFs contamination in storage.
Seven important phyto-pathogens were used in our test. These fungal strains included A. flavus, Fusarium graminearum, F. equiseti, Alternaria alternata, Botrytis cinerea, Aspergillus niger, and Colletotrichum graminicola. The pathogens were isolated from infected plants and stored in our lab for broad antifungal tests (Gong et al., 2014). A. flavus conidia was inoculated into PDB medium and cultured at 28°C for 48 h to produce mycelium coils.
The soil samples were collected from tea gardens containing large amounts of active humus. The soil was suspended in sterilized water and diluted to 10-5. The suspension (100 μL) was spread on the surface of NA medium and cultured at 28 for 48 h. The obtained microbe clones were streaked on NA medium to produce pure culture. Bacteria bodies on NA surface were washed off and co-cultured with A. flavus in Face-to-face (FTF) cultural method to screen antagonistic bacteria.
In FTF tests, A. flavus mycelia coil was placed to the center of PDA medium bacteria bodies (OD600 = 1.8, 100 μL) were spread on NA medium. The two petri dishes were placed FTF with A. flavus above and bacterial plate at the bottom. PDA plate inoculated with A. flavus mycelia challenged with NA plate was used as control. All sets were cultured at darkness and 28°C for 3 days. The mycelium diameter in each group was recorded, and the inhibitory rate was calculated following the forma below.
Inhibitory rate (%) = [(the diameter of control – the diameter of antagonist treatment)/the diameter of control] × 100.
Inhibitory effect of bacteria to A. flavus conidia germination was measured. Ten micro liter of A. flavus conidia (105 cfu/mL) were injected on a round filter paper (5 mm in diameter), and placed to the center of PDA plate. Isolated bacteria were spread on NA plate. The two petri dishes were located FTF with PDA above. PDA medium inoculated with A. flavus conidia was used as control. All sets were cultured at darkness and 28°C for 3 days. The diameter of conidia in each test was recorded, and the inhibition rate was calculated as before.
Single clone of N1-4 was inoculated into NB medium and cultured at 200 rpm and 28°C for 48 h. The cultured broth was centrifuged at 12,000 rpm for 10 min, and the collected cell bodies were used for DNA extraction (Gong et al., 2014). The 16S rRNA sequences of strain N1-4 were amplified using two universal primers (27 f and 1541 r). Obtained 16S rRNA were sequenced in TIANYI HUIYUAN company, and blasted in GenBank database. The strains in GenBank database homologous to N1-4 were selected and used to construct the phylogenetic tree through MEGA program and neighbor-joining method (Ding et al., 2015; Chen et al., 2017).
Biochemical activity of N1-4 was conducted through BIOLOG MicroStationTM System (Biolog Inc., United States). Exactly, strain N1-4 was streaked on NA medium. Fresh single clone was transferred into IF-A GEN III Inoculating Fluid. Then the suspension was transferred into GEN III MicroPlate with 120 μL in each cell. The plate was cultured at 37°C in darkness for 12 h, and the optical density of each cell in MicroPlate was recorded in BIOLOG MicroStationTM. All recorded data were aligned in bacterial database through BIOLOG MicroStationTM System (Banik et al., 2016).
Volatiles from N1-4 were enriched with solid-phase micro-extraction (SPME) fiber (divinylbenzene/carboxen/ polydimethlsiloxane), and identified through GC-MS/MS system (5975B-7890N, Agilent Technologies Inc.). N1-4 cell were spread on the surface of NA medium in a 150 mL flask. The flask containing NA medium without N1-4 bodies was used as control. Each flask was sealed with two layer of membrane, and placed at 37°C, and darkness for 24 h to produce volatiles. Volatiles in airspace of each flask were enriched with SPME system for 30 min, then analyzed with GC-MS/MS system equipped with an Agilent HP-5MS fused-C18 capillary column (length 30 m, internal diameter 0.25 mm, 0.25 μm thickness film). The samples were analyzed with splitless injection mode. The inlet temperature was 250°C, carrier gas was helium. The compounds were analyzed using the method as follows: initial temperature at 40°C for 3 min, following a linear heating rate of 3°C ⋅ min-1 to 160°C, and contained for 2 min, 8°C ⋅ min-1 to 220°C, and contained for 3 min. Each sample was conducted for two times. The compounds in N1-4 samples that were not present in the control samples were considered to be the final analyzes.
Identified compounds in N1-4 volatiles were selected for MIC analysis against A. flavus in FTF test (Gong et al., 2015). Ten micro liter fresh A. flavus conidia (5 × 105 cfu/mL) were inoculated to a round paper disk placed in the center of PDA plate. Another plate was inoculated with identified compound, respectively. Ethanol was used as control. All compounds was adjusted to the final concentration of 5, 10, 100 and 200 μg/L (compound weight to airspace volume), respectively. These two dishes were placed FTF and incubated at 28°C and darkness for 4 days. The diameter of A. flavus mycelium was calculated 4 dpi, and the inhibitory rate was calculated according to the following formula: Inhibitory rate (%) = [(the mycelia diameter of control – the diameter of N1-4 treatment)/the diameter of control] × 100.
Peanuts, maize, rice and soybean kernels were inoculated with A. flavus conidia, and challenged with N1-4 in sealed FTF culture test to detect the inhibitory effect of volatiles. In the tests, eight flasks was prepared, and two flasks as a group for one kind of kernel. 100 g of grains was added into each flask and autoclaved at 121°C and 1.01 MPa for 20 min, and cooled down to room temperature. 100 μL A. flavus conidia (at 1 × 105 cfu/mL) was added to each flask and mixed well. The two flasks in each group was inoculated with 10 mL (5 mL) of sterilized water, mixed well, and adjusted aw to 0.9 (0.8) with Aqualab Series 3 model TE (Decagon Devices, Pullman, WA, United States), respectively.
Peanuts, maize, rice and soybean kernels in each flask were equally divided into two parts, and placed into two petri dishes, respectively. One petri dish was challenged FTF with strain N1-4 coated on NA medium. The other petri dish challenged with NA medium was used as control. All dishes were cultured at 28°C and darkness for 7 days. The disease incidence of each treatment was recorded. The grains were collected, dried at 60°C for 4 days, and milled well for AFs extraction.
Milled samples of each grain were used for AFs extraction and quantitative analysis. One gram of milled sample was re-suspended in 5 ml acetonitrile/water (84/16, v/v), and vortex for 5 min. The suspension was centrifuged at 12,000 rpm for 10 min. The supernatant was transferred into a new tube, and same volume of hexane was added into the tube, mixed well. The upper layer was obtained and used for AFs analysis. The quantitative analysis of AFs were conducted through LC-ESI-MS system containing Thermo Surveyor plus HPLC system coupled to a TSQ Quantum Ultra mass spectrometer (Thermo Scientific, CA, United States) (Warth et al., 2012). AFs (AFB1, AFB2, AFG1, and AFG2) purchased from Sigma (Sigma-Aldrich, St. Louis, MO, United States) were used as standards for quantitative analysis.
Ultra-structural of A. flavus cell on peanuts coat of aw 0.92 were analyzed through JSM-4800 scanning electron microscope (SEM, Hitachi, Tokyo, Japan). Peanuts in control and N1-4 treatments were fixed with 0.1% (v/v) osmic acid for 1 h. A piece (3∼5 mm × 3∼5 mm) of peanut coat was ripped down, and affixed to SEM stubs, respectively. Then, all samples were sprayed with gold and examined in SEM (Boukaew and Prasertsan, 2014).
Broad antifungal tests of N1-4 against other 6 fungal pathogens were conducted with FTF method as mentioned above. Fresh hyphae block (5 cm in diameter) was inoculated to the center of PDA medium, respectively. The plate was challenged with strain N1-4 on NA plate with FTF method, respectively. All tests were cultured at 28°C and darkness for 5 days. The inhibition rate was calculated according the methods above.
Fresh A. flavus conidia (100 μl, 106 cfu/mL) were spread on PDA medium in one petri dish. Another dish containing NA medium was spread with N1-4 cell bodies (108 cfu/mL, 100 μl). Then, the two dishes were challenged FTF with A. flavus on the top. A. flavus conidia on PDA plate challenged with NA medium was used as control. All treatments were cultured at 28°C and darkness for 48 h. The mycelia on PDA surface were collect and used for RNA extraction.
Total RNA in A. flavus mycelia was extracted by Trizol (Invitrogen) method (Li et al., 2017). The RNA was treated with DNase at a concentration of 1.5 unit/μg. cDNA was synthesized using a PrimeScriptTM RT Reagent Kit (Takara). The cDNA products were diluted 20-fold with nuclease free deionized water. Reverse transcription quantitative PCR (RT-qPCR) was performed using a Bio-Rad iQ2 PCR system (Bio-Rad, United States). The PCR conditions were as follows: 95°C for 1 min; 40 cycles of 95°C for 20 s, 60°C for 20 s, and 72°C for 30 s. Twelve important genes in AFs biosynthesis pathway were used in our tests including aflR, AccC, aflCa, aflA, aflS, aflO, aflD, aflF, aflP, aflQ, aflX, and aflC (Cleveland et al., 2009; Hua et al., 2014). β-tubulin was used as the endogenous control due to its relatively stable expression level. All primers used for RT-qPCR were shown in Table 1. The relative expression of genes in N1-4 compared to control was calculated by using the 2∧-ΔΔCt method (Chen et al., 2017; Chai et al., 2018).
All statistical analyses were performed by one-way analysis of variance (ANOVA) with SPSS 17.0 statistics software for windows (SPSS Inc., Chicago, IL, United States). Experiments were arranged in a completely randomized design with at least two replications. Mean comparisons were performed with Student’s t test at P < 0.05.
In screening tests, 693 bacteria were isolated from tea soil, and six bacteria showed great antifungal activity against A. flavus in FTF tests. Among these strains, N1-4 could completely inhibit the growth of A. flavus mycelium, as well as conidia germination in vitro. N1-4 was selected for further biocontrol analysis in the tests.
In control treatment, mycelia in PDA medium could grow quickly, and extended to 5.2 cm in diameter 5 days post inoculation (dpi). Conidia on PDA surface germinated to hyphae, and extended quickly. When challenged with bacteria, N1-4 in NA medium without contact to A. flavus cell, can greatly inhibited the conidia germination, as well as mycelia growth of A. flavus (Figure 1). Seldom fresh mycelia were formed in N1-4 treatment 5 dpi. Hence, the inhibition rate of N1-4 to mycelia growth and conidia germination was up to 100% compared to control (Figure 1).
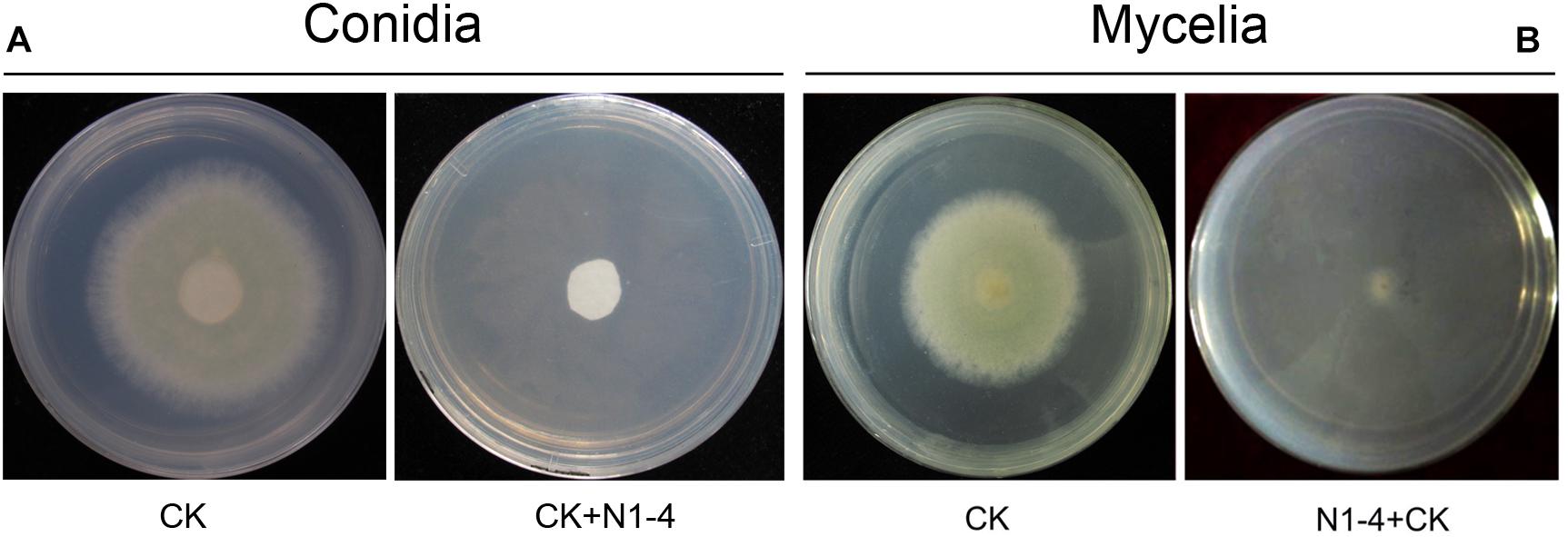
Figure 1. Antifungal activity of strain N1-4 against the growth of A. flavus mycelia and conidia. Mycelia pellet and conidia of A. flavus was inoculated to the center of PDA plate was used as control (CK), respectively. A. flavus challenged with bacteria N1-4 (CK+N1-4) with FTF method was used to test the antifungal activity, respectively. (A) The inhibitory effect of N1-4 against conidia germination of A. flavus. (B) The inhibitory effect of N1-4 against mycelia growth of A. flavus.
When active charcoal added into the tests, the diameter of A. flavus mycelia is relative equal to control treatment without the presence of N1-4. It means that active charcoal showed no inhibitory effect to A. flavus. When N1-4 added into the reaction, the diameter of A. flavus (CK+C+N1-4) is shorter than charcoal treatment (CK+C), but longer than N1-4 (CK+N1-4) (Figure 2). Thus, we could deduce that strain N1-4 can produce abundant volatiles, spread in the whole cultural space, and eventually inhibited the growth of A. flavus.
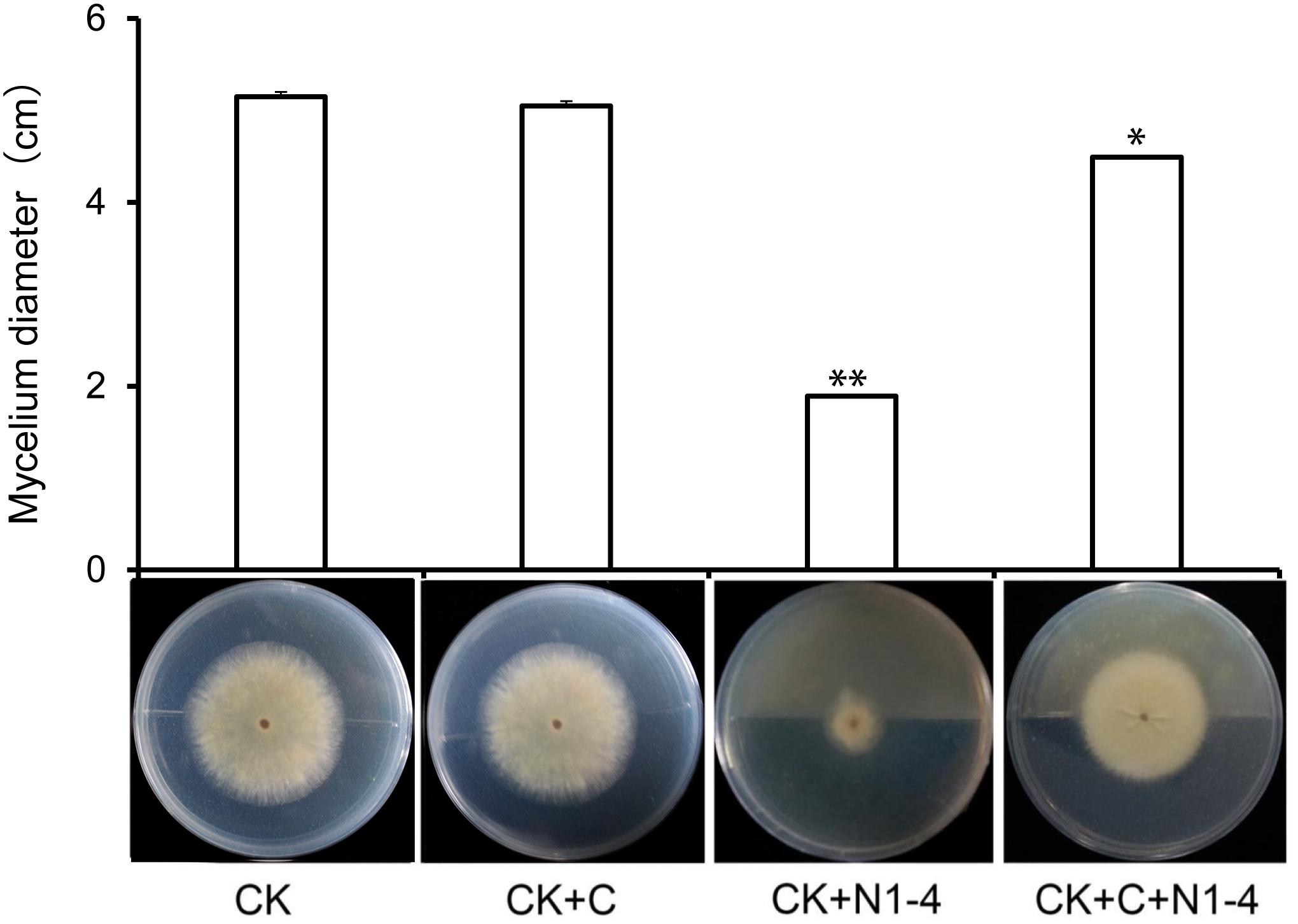
Figure 2. Antifungal activity of volatiles from strain N1-4 against A. flavus. Mycelia pellet of A. flavus was inoculated to the center of PDA plate was used as control (CK). A. flavus challenged with active charcoal (CK+C), N1-4 (CK+N1-4), and both of them (CK+C+N1-4), respectively. ∗ and ∗∗ mean significant difference at p < 0.05 and p < 0.01 leveles compared to CK treatment, respectively.
The 16S rRNA sequence of strain N1-4 was sequenced and blasted in GenBank database. The results indicated that sequences of N1-4 showed great similarity to the species of Alcaligenes faecalis, A. aquatilis and A. endophyticus. The physiological tree was constructed based on 16S rRNA from N1-4 and other homologous strains (Figure 3). Strain N1-4 was classified into a sub-cluster with A. faecalis, and showed highly homologous and close genetic distance to A. faecalis cb-4 (FJ588233.1) and A. faecalis 47N3 (KX302626.1). Thus, we deduced that strain N1-4 was initially identified to be A. faecalis based on 16S rRNA sequences.
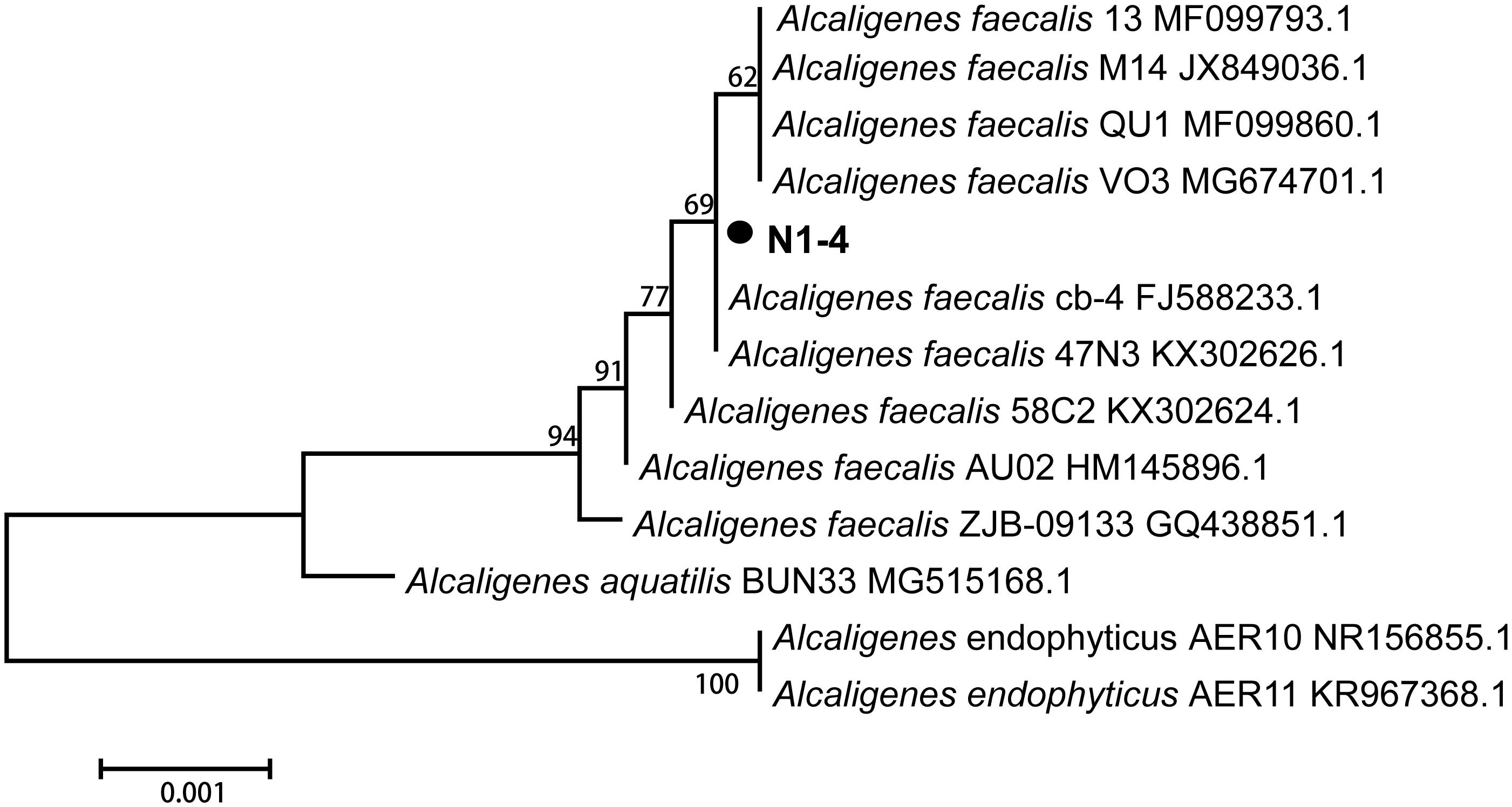
Figure 3. Phylogenetic tree analysis of N1-4 and other homologous strains based on 16S rRNA sequences. The tree was constructed with neighbor-joining methods and the scale bar represents the number of substitutions per base position.
Biochemical analysis further proved that strain N1-4 exhibited great similarity to A. faecalis strain in BIOLOG MicroStationTM System. Both of them can utilize more than 18 kinds of nutrients including methyl pyruvate, L-alanine, L-lactic acid and L-aspartic acid so on. They could grow at the presence of 1∼4% NaCl, and tolerant to 14 kinds of antibiotic such as troleandomycin, lincomycin, vancomycin, and rifamycin SV so on. But, bacteria N1-4 showed different activity to A. faecalis on the application of Tween 40 and D-Arabitol, as well as tolerance to 8% NaCl (Table 2).
More than 25 compounds were detected in the volatiles of N1-4 through SPME coupled with GC-MS/MS system. But, 23 fractions were also detected in control treatment. Only two compounds, including disulfide dimethyl (DMDS) and methyl isovalerate (MI) were proved exclusive in N1-4 volatiles. In the chromatogram, the retention time of DMDS and MI is between 3.04 and 4.34 min. And, both of them contain low molecular weight between 94 and 130 Dalton. DMDS showed the greatest relative abundance (compared to the total peak area) at 69.90%, which was higher than MI (3.38%) (Figure 4). Additionally, we purchased these two standards for further antagonistic effect analysis.
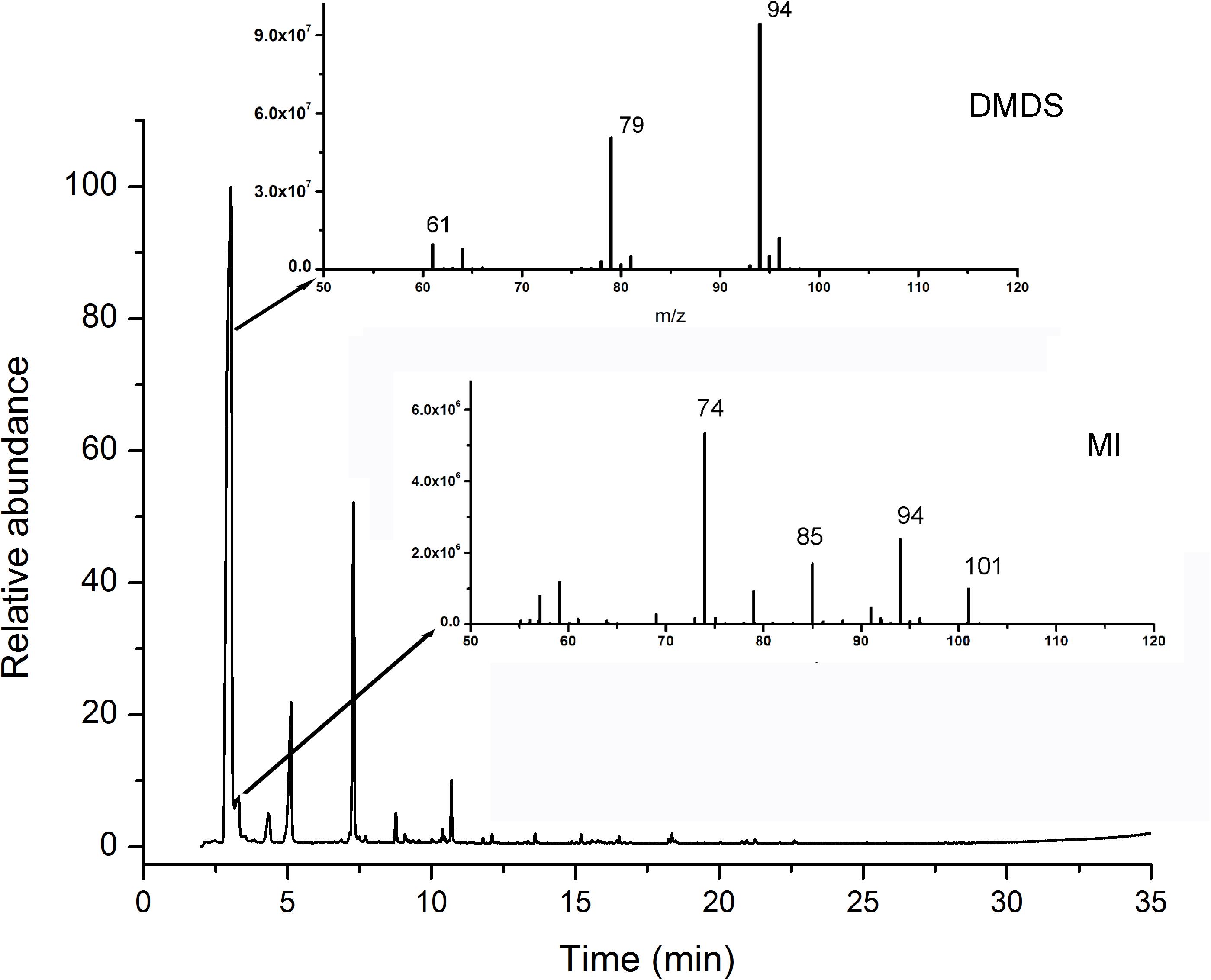
Figure 4. Identification of volatile compounds from strain N1-4 through GC-MS/MS. Volatiles were enriched with SPME system, and identified through GC-MS/MS system. Two compounds, DMDS and MI, in N1-4 profiles and without presence in NA profiles were considered as authentic compounds.
Two compounds, DMDS and MI, were serially diluted to 10, 50, 100, and 200 μL/L, and tested their inhibitory effect against A. flavus in FTF dual culture tests. In control treatment, A. flavus conidia germinated to hyphae, and mycelia grew quickly without the presence of volatiles. When DMDS or MI added into the treatment, the growth of A. flavus was greatly inhibited. Obviously, at 50 μL/L or higher concentration, DMDS can eventually prevent conidia germination of A. flavus. And at 100 μL/L or higher concentration, DMDS eventually prevented the growth of mycelia. Thus, the MIC of DMDS against conidia germination and mycelia growth of A. flavus was 50 and 100 μL/L, respectively. The MIC of MI against mycelia growth of A. flavus was 200 μL/L, but can not eventually prevent the germination of conidia (Figure 5). Thus, we can conclude that DMDS with the highest relative abundance (69.90%) and best antifungal activity is the main inhibitory factor in N1-4 volatiles.
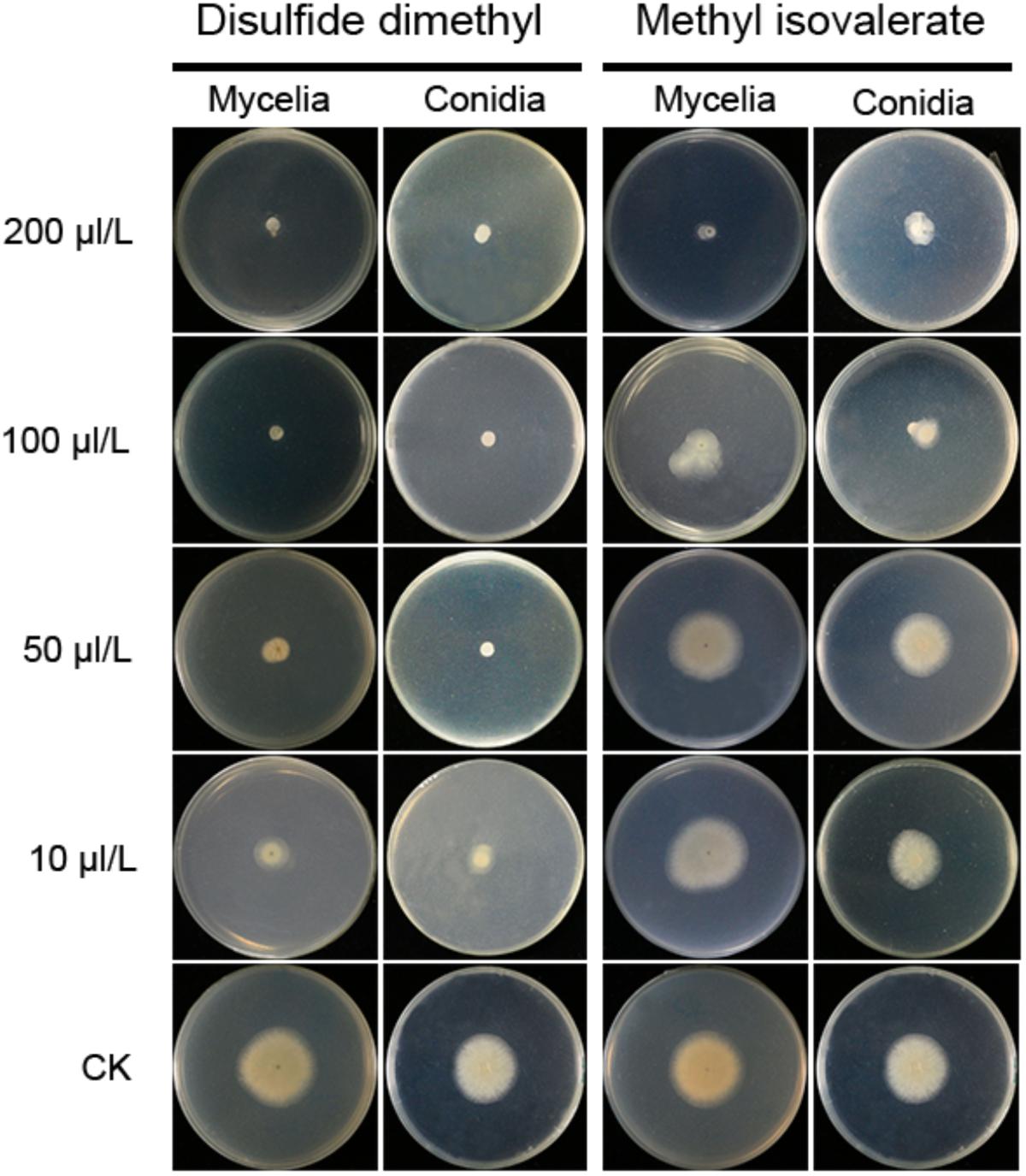
Figure 5. Minimal inhibitory concentration analysis of identified volatiles against mycelia growth and conidia germination of A. flavus. Two individual compounds DMDS and MI were diluted to gradient concentrations, and co-cultured with A. flavus conidia and mycelia with FTF methods.
Volatiles from N1-4 can eventually control A. flavus infection in maize, peanut, soybean and rice grains during storage. In control treatment, the A. flavus conidia germinated to hyphae, and quickly infected these grains at high aw. And the disease severity in peanuts was more serious than that in maize, soybean and rice samples. Additionally, in each grain, the disease severity of A. flavus at aw 0.9 was more severe than that at aw 0.8. Take peanuts as an example, the disease incidence in peanuts of aw 0.9 is up to 100% (Figure 6). And a mass of fresh conidia were formed and covered the grain surface. But, in N1-4 added treatment, A. flavus conidia did not germinate and infect these grains, and no disease symptom was shown in each grains of two aw. Hence, we could deduce that volatiles from N1-4 can greatly inhibit infection development in different grains during storage.
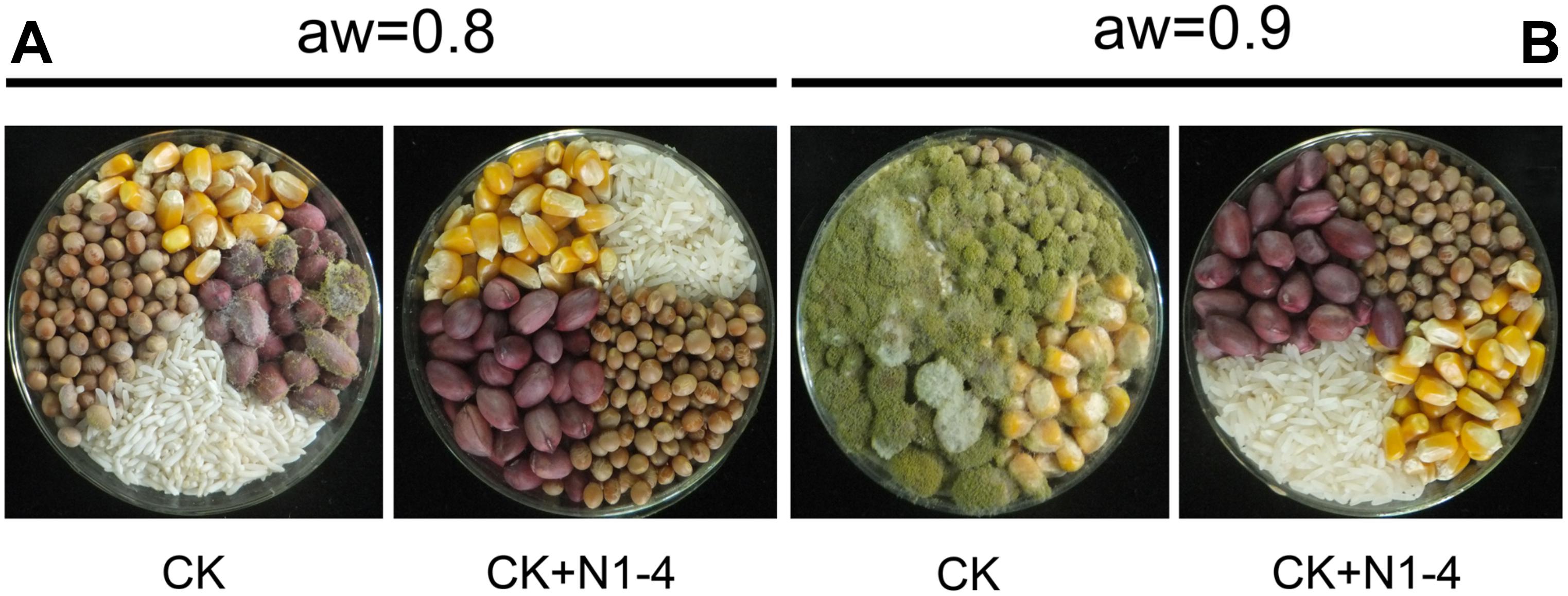
Figure 6. Biocontrol efficacy of volatiles from N1-4 against A. flavus and AFs on four different grains of aw 0.8 to 0.9. (A) Disease symptom of A. flavus inoculated grains with (N1-4+CK) or without (CK) the presence of strain N1-4 at 28°C for 7 days. (B) AFB1 and AFB2 in peanut samples were detected using LC-ESI-MS system.
Aflatoxins concentration in four grains showed similar tendency to infected symptom of A. flavus. In control treatment of aw 0.9, the total AFs in peanuts is 45.51 μg/g (AFB1 is 42.63 μg/g), the concentration is higher than that other grains including maize 37.54 μg/g (AFB1 is 34.80 μg/g), rice 22.05 μg/g (AFB1 is 20.86 μg/g) and soybean 2.13 μg/g (AFB1 is 2.01 μg/g). In control of aw 0.8, less AFs was detected in peanuts (0.37 μg/g), rice (0.01 μg/g) and soybean (0.01 μg/g), respectively. More importantly, no AFs was detected in each grain of 0.8 and aw 0.9 with the presence of N1-4. These results indicate that volatiles from N1-4 not only inhibit the infection of A. flavus, it also eventually prevented the contamination of AFs in storage (Table 3).
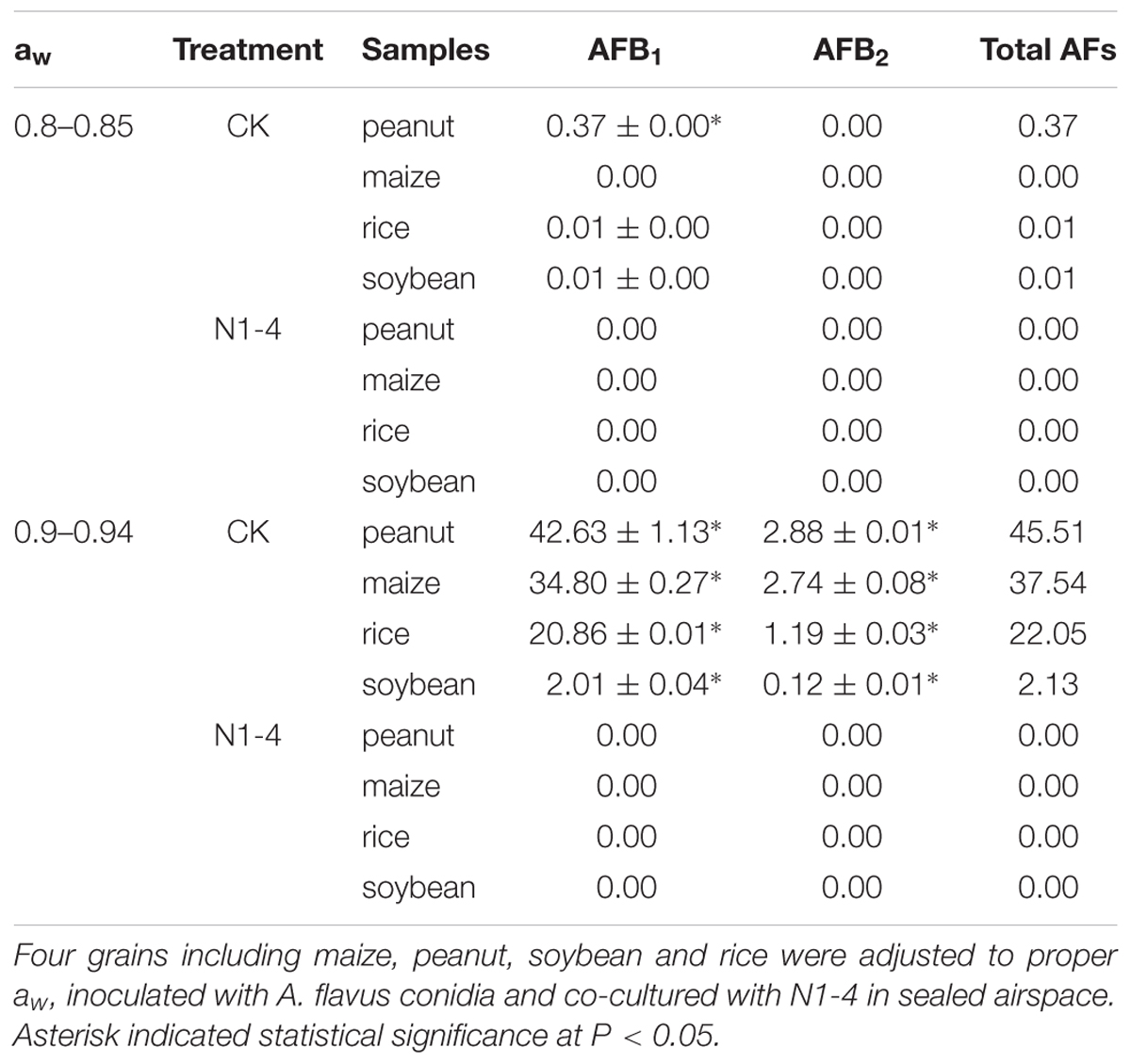
Table 3. Quantitative analysis of aflatoxins (AFs) in infected grains with or without the challenge of N1-4.
In scanning electron microscopy (SEM) analysis, A. flavus conidia germinated to hyphae, formed typical conidiophores, and produce abundant fresh conidia in control treatment (Figure 7). The hyphae and produced conidia covered the whole surface of inoculated peanut coat. But, the conidia in N1-4 added treatment, few conidia were found on peanut coat surface. These conidia were originated from the initial inoculation test. The germination of these conidia were completely inhibited by N1-4, they could not infect grains, produce conidiophore and fresh conidiospore, as well as induce secondary infection on grains. Additionally, these conidia appeared atypical structure with irregular surface and curving bodies. The regular ball around conidia surface were sunk and severely deformed. Finally, we deduce that strain N1-4 with effective antifungal volatiles production endowed valid functions in control A. flavus and AFs in grains during storage.
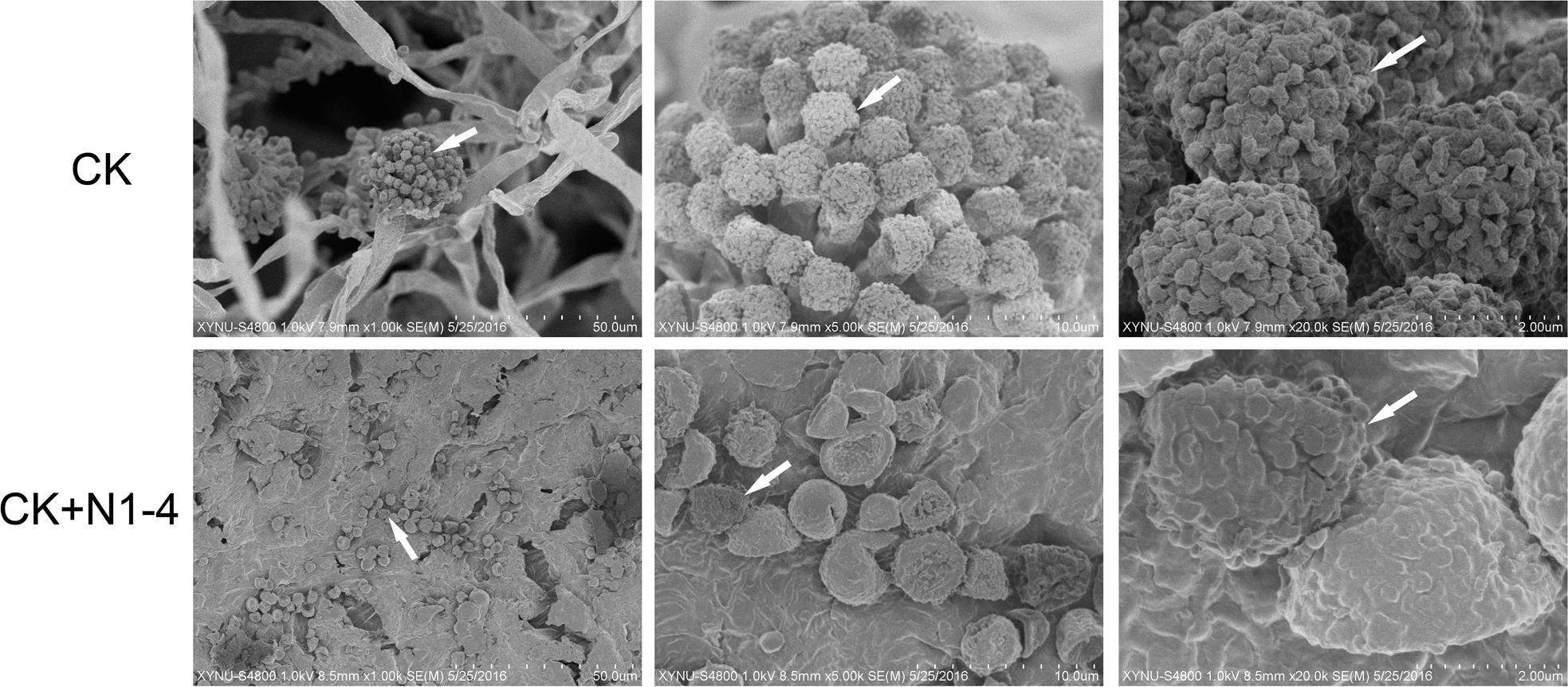
Figure 7. Scanning electron microscope analysis of A. flavus cell structure on peanut surface. Peanuts of aw 0.92 inoculated with A. flavus conidia was challenged with (N1-4+CK) or without (CK) the presence of strain N1-4 in FTF tests 7 dpi. Co, conidia; cp, conidiophore; my, mycelia; sg, sterigma.
Strain N1-4 was co-cultured with other six fungal pathogens by FTF methods to detect the broad antifungal activity. These fungi caused great damage to plant in pre- or post-harvest including Fusarium graminearum, F. equiseti, Alternaria alternata, Botrytis cinerea, Aspergillus niger, and Colletotrichum graminicola. The inhibitory rate was calculated 5 dpi. As shown in Figure 8, the mycelia in control grew quickly, and extended to 7 cm in length 5 dpi. And some pathogens including F. graminearum, A. alternata and C. graminicola produced different color of pigments in PDA medium, respectively. When N1-4 was added, the growth of all selected fungi was greatly inhibited by the produced volatiles. The inhibitory rate was ranged between 64.1 and 97.8%, respectively. These results proved that volatiles emitted from strain N1-4 exhibited a wide spectrum of antifungal activity against fungi from different genera.
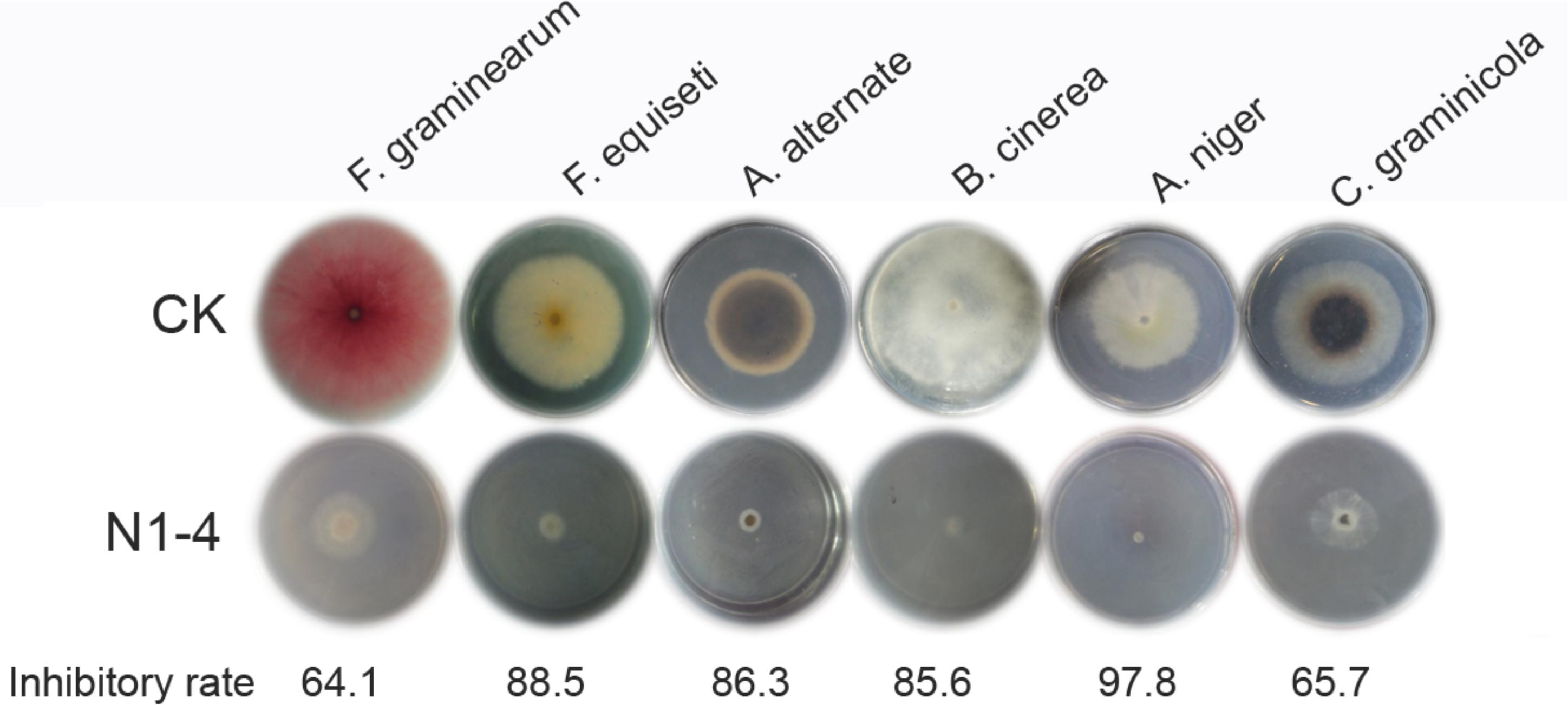
Figure 8. Broad spectrum antifungal activity of strain N1-4 against six important fungal pathogens. Fresh fungal block were inoculated to the center of PDA plate and challenged with strain N1-4 in FTF test, respectively. The diameter of each fungus on PDA plate was measured to calculate the inhibition rate.
In RT-qPCR analysis, the expression of 12 genes in N1-4 treatment was all reduced compared to control treatment. Eight genes were significantly down-regulated by 4.39 to 32.25-fold. The results further prove that volatiles from N1-4 greatly repress genes expression in AFs biosynthesis (Figure 9), and finally reduce AFs production.
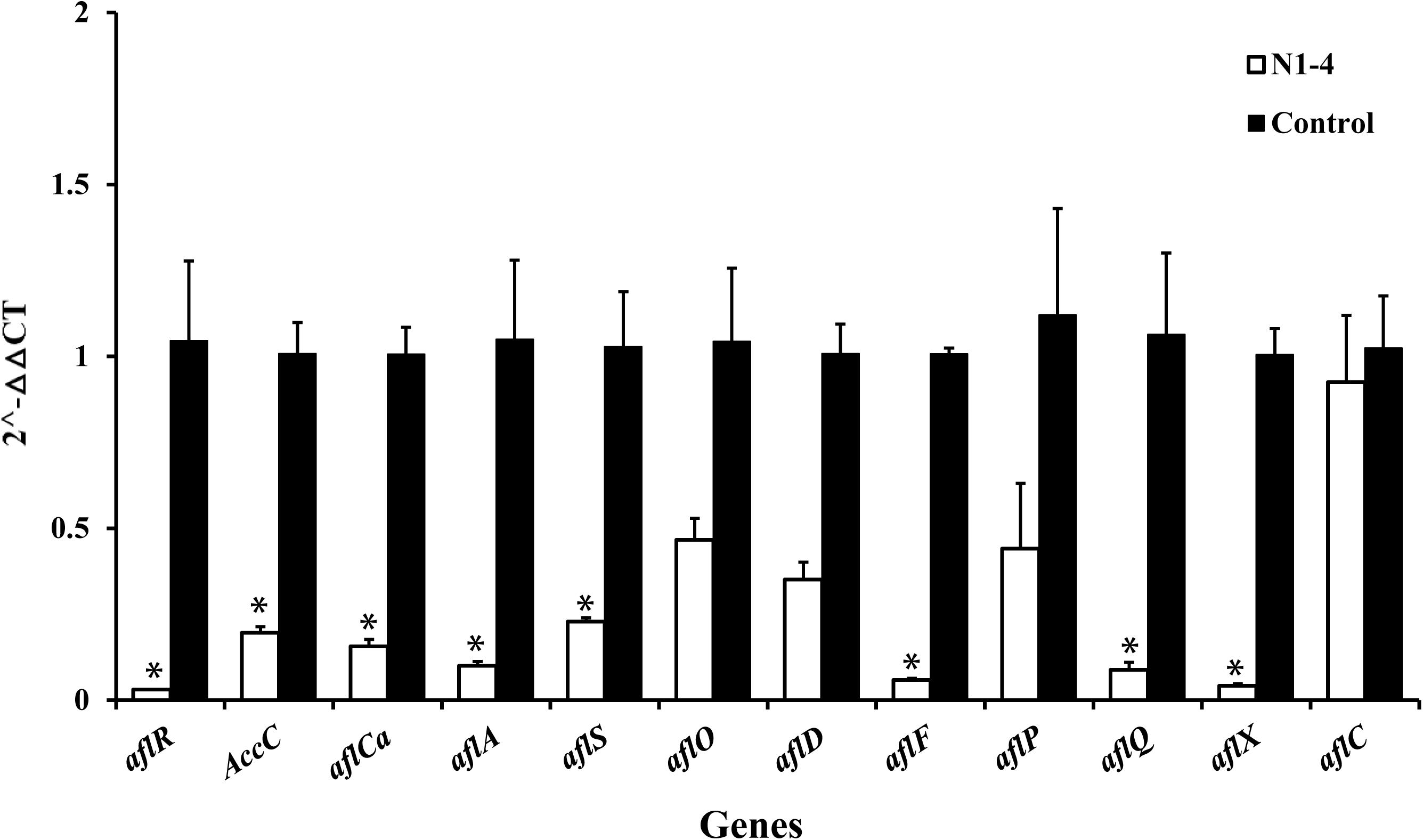
Figure 9. Expression of genes (RT-qPCR) involved in aflatoxin biosynthesis pathway of A. flavus effected by volatiles from N1-4. The fold change of genes in N1-4 treatment was calculated with 2∧-ΔΔct method. And ΔΔct = ΔCt in N1-4 treatment -ΔCt in control treatment. ∗means significant difference at p < 0.05 compared to CK treatment.
Aspergillus flavus can infect many grain, food and feedstuff in storage, and produce toxic and carcinogenic AFs which causes great hazardous to humans and livestock. The control of A. flavus and AFs at storage is an urgent and crucial problem to scientists worldwide. However, except for the traditional physical and chemical methods, few effective and safe agents are applied in control A. flavus and AFs during storage recently. In our current work, we innovatively prove that A. faecalis N1-4 isolated from tea rhizosphere could produce two antifungal volatiles including DMDS and MI, significantly inhibit the mycelia growth and gene expression in aflatoxin biosynthesis, eventually inhibit A. flavus infection and AFs production in four grains of high aw, and severely damage A. flavus cell structure. Thus, we deduce that strain N1-4 and the produced volatiles provide novel and alternative strategies for control A. flavus and AFs during storage, and open new views for screening effective biocontrol agents.
Alcaligenes faecalis is a gram-negative bacterium, commonly existing in soil and water. These bacteria have been broadly applied in several fields including organic acid production (Yamamoto et al., 1991; He et al., 2009), plant growth promoting (Sayyed and Chincholkar, 2010; Sayyed et al., 2010), NH4+-N removal in wastewater (Joo et al., 2005), biodegradation of phenol contamination (Jiang et al., 2007) and biocontrol activity (Shan et al., 2019). For example, Yokoyama et al. (2013) report that A. faecalis AD15 produce large amount of hydroxylamine (33.3 mg/L) in synthetic medium, and greatly inhibited the growth of two pathogens including Pantoea agglomerans and Colletotrichum gloeosporioides. Kakar et al. (2018) report that A. faecalis strains Bk1 and P1 greatly suppress the incidence of sheath blight diseases more than 70%. And both of them with phosphate solubilizing activity can produce indoleacetic acid, ammonia, siderophores, enrich the content of mineral nutrients in seedlings and improved plant growth. However, to our knowledge, the application of A. faecalis in preventing plant disease at storage has not been reported till now.
As we all know that A. flavus as an air-borne fungus produce abundant conidia during infection which spread in a long distance to infect grain and food. Hence, the control of A. flavus and AFs in storage is more difficult than other pathogens without conidia. Recently, Ma et al. (2017) report that the biocontrol activity of volatile phase on A. flavus is more effective than contact phase. Hence, screening antagonistic volatiles in control post-harvest A. flavus and AFs is urgent nowadays. So far, approximately 1,300 microbial volatiles have been obtained from microorganisms and registered in the volatile database1 (Lemfack et al., 2014). However, less than 10 volatiles are proved useful in control A. flavus or AFs till now. In our current work, two novel antifungal volatiles, DMDS and MI, are identified, and show effective in control A. flavus in vitro. They both are first proved effective in control A. flavus in grains during storage. Especially for DMDS, it shows the highest relative abundance at 69.90% in N1-4 volatiles, and best antifungal activity to A. flavus with MIC at 50 μL/L. MI shows less inhibitory activity compared to DMDS, The MIC for MI is 200 μL/L. These results prove that DMDS with effective antifungal activity will provide more valid and alternative bio-active agents in control A. flavus and other fungal pathogens in storage.
Dimethyl disulfide (molecular weight 94 Dalton) is easily spread in storage condition. Some research reported that it is naturally existed in fresh Allium porrum and Romanesco cauliflower plants (Valette et al., 2003; Sébastien et al., 2004). It is always considered as common and safe compounds, and be used as spice additive in food. Some microbe can also produce DMDS such as Bacillus cereus (Huang et al., 2012), pseudomonas aeruginosa (Briard et al., 2016), Serratia odorifera (Kai and Piechulla, 2010). DMDS with broad resource has been applied in plant disease control field. For example, DMDS shows effective activity in control root knot nematodes and cyst nematodes (Curto et al., 2014; Fritsch et al., 2014; Sasanelli et al., 2014), Recently, Papazlatani et al. (2016) proves that DMDS at low dose (56.4 g m-2) could drastically reduce the population of tomato soil borne pathogens F. oxysporum and Rhizoctonia solani. It also shows great activity to other pathogen including Sclerotium rolfsii, Verticillium dahliae and R. solani (Fritsch, 2004). Moreover, DMDS can induce systemic resistance in plant, and promote the growth of plant in field (Huang et al., 2012; Meldau et al., 2013; Piechulla et al., 2017). DMDS as novel bio-active compound with antagonistic activity in disease and pest control, plant growth promoting and ISR can be considered as potential biocontrol agent in field and storage.
Additionally, the volatiles produced by strain N1-4 also show broad antifungal activity against other 6 important fungal pathogens including Fusarium graminearum, F. equiseti, Alternaria alternata, Botrytis cinerea, Aspergillus niger and Colletotrichum graminicola. These fungi belong to different genera of two phylum including ascomycota, deuteromycotina. Each of them can cause great damage to crops, fruits and vegetables in field or storage. Our current work elucidates that volatiles from N1-4 can greatly prevent the growth of these different fungi in vitro. It may also inhibit the infection of these fungi in field or storage. Hence, we deduce that strain N1-4 and the produced volatiles may provide novel agents in controlling fungal pathogens and associated mycotoxins in practice.
GenBank accession numbers for our nucleotide sequences: N1-4: MK972333.
A-DG and Y-CL conceived and designed the experiments. A-DG, N-NW, X-WK, and F-YD performed the experiments. A-DG analyzed the data. J-HW, Z-ZY, S-JG, and Y-MZ contributed reagents, materials, and analysis tools. A-DG and M-JH wrote the manuscript.
This work was supported by the National Natural Science Foundation of China (31701740), Scientific and Technological Project of Henan Province (182102110018), Scientific Research Key Project Fund of Ministry of Education in Henan Province (16A180036), Opening Subject of Key Laboratory of Integrated Pest Management in Crops in Central China, Ministry of Agriculture (2018ZTSJJ2/2018-620-003-001), and Nanhu Scholars Program for Young Scholars of XYNU.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abdel-Kareem, M. M., Rasmey, A. M., and Zohri, A. A. (2019). The action mechanism and biocontrol potentiality of novel isolates of Saccharomyces cerevisiae against the aflatoxigenic Aspergillus flavus. Lett. Appl. Microbiol. 68, 104–111. doi: 10.1111/lam.13105
Adhikari, B. N., Bandyopadhyay, R., and Cotty, P. J. (2016). Degeneration of aflatoxin gene clusters in Aspergillus flavus from Africa and North America. AMB Express 6:62. doi: 10.1168/s13568-016-0228-6
Asters, M. C., Williams, W. P., Perkins, A. D., Mylroie, J. E., Windham, G. L., and Shan, X. (2014). Relating significance and relations of differentially expressed genes in response to Aspergillus flavus infection in maize. Sci. Rep. 4:4815. doi: 10.1038/srep04815
Azziz-Baumgartner, E., Lindblade, K., Gieseker, K., Rogers, H. S., Kieszak, S., Njapau, H., et al. (2005). Case–control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 113, 1779–1783. doi: 10.1289/ehp.8384
Banik, A., Mukhopadhaya, S. K., and Dangar, T. K. (2016). Characterization of N 2-fixing plant growth promoting endophytic and epiphytic bacterial community of Indian cultivated and wild rice (Oryza spp.) Genotypes. Planta 243, 799–812. doi: 10.1007/s00425-015-2444-8
Bediako, K. A., Ofori, K., Offei, S. K., Dzidzienyo, D., Asibuo, J. Y., and Amoah, R. A. (2018). Aflatoxin contamination of groundnut (Arachis hypogaea L.): predisposing factors and management interventions. Food Control 98, 61–67. doi: 10.1016/j.foodcont.2018.11.020
Boukaew, S., and Prasertsan, P. (2014). Suppression of rice sheath blight disease using a heat stable culture filtrate from Streptomyces philanthi RM-1-138. Crop Prot. 61, 1–10. doi: 10.1016/j.cripro.2014.02.012
Briard, B., Heddergott, C., and Latge, J. P. (2016). Volatile compounds emitted by Pseudomonas aeruginosa stimulate growth of the fungal pathogen Aspergillus fumigatus. mBio 7:e0219-16. doi: 10.1128/mBio.00219-16
Chai, L. Q., Li, W. W., and Wang, X. W. (2017). Identification and characterization of two arasin-like peptides in red swamp crayfish Procambarus clarkii. Fish Shellfish Immunol. 70, 673–681. doi: 10.1016/j.indcrop.2015.11.008
Chai, L. Q., Meng, J. H., Gao, J., Xu, Y. H., and Wang, X. W. (2018). Identification of a crustacean β-1,3-glucanase related protein as a pattern recognition protein in antibacterial response. Fish Shellfish Immunol. 80, 155–164. doi: 10.1016/j.fsi.2018.06.004
Chen, J. G., Egner, P. A., Ng, D., Jacobson, L. P., Munoz, A., Zhu, Y. R., et al. (2013). Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev. Res. 6, 1038–1045. doi: 10.1158/1940-6207
Chen, M. Y., Li, K., Li, H. P., Song, C. P., and Miao, Y. C. (2017). The glutathione peroxidase gene family in Gossypium hirsutum: genome-wide identification, classification, gene expression and functional analysis. Sci. Rep. 7:15. doi: 10.1038/srep44743
Chen, Y. J., Kong, Q., and Liang, Y. (2019). Three newly identified peptides from Bacillus megaterium strongly inhibit the growth and aflatoxin B1 production of Aspergillus flavus. Food Control 95, 41–49. doi: 10.1016/j.foodcont.2018.07.040
Cho, K. M., Math, R. K., Hong, S. Y., Asraful Islam, S. M., Mandanna, D. K., Cho, J. J., et al. (2009). Iturin produced by Bacillus pumilus HY1 from Korean soybean sauce (kanjang) inhibits growth of aflatoxin producing fungi. Food Control 20, 402–406. doi: 10.1016/j.foodcont.2008.07.010
Cleveland, T. E., Yu, J., Fedorova, N., Bhatnagar, D., Payne, G. A., Nierman, W. C., et al. (2009). Potential of Aspergillus flavus genomics for applications in biotechnology. Trends Biotechnol. 27, 151–157. doi: 10.1016/j.tibtech.2008.11.008
Curto, G., Dongiovanni, C., Sasanelli, N., Santori, A., and Myrta, A. (2014). Efficacy of dimethyl disulfide (DMDS) in the control of the root-knot nematode Meloidogyne incognita and the cyst nematode Heterodera carotae on carrot in field condition in Italy. Acta Hortic. 1044, 405–410. doi: 10.17660/actahortic.2014.1044.55
Ding, W., Si, M., Zhang, W., Zhang, Y., Chen, C., Zhang, L., et al. (2015). Functional characterization of a vanillin dehydrogenase in Corynebacterium glutamicum. Sci. Rep. 5:8044. doi: 10.1038/srep08044
Ehrlich, K. C. (2013). Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: advantages and limitations. Front. Microbiol. 5:50. doi: 10.3389/fmicb.2014.00050
European Commission [EC] (2010). Commission regulation (EC) No. 165/2010 of 26 February 2010, amending Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxin. Official J. Eur. Union L50, 8–12.
Fang, X., Zhang, M., Tang, Q., Wang, Y., and Zhang, X. (2014). Inhibitory effect of Xenorhabdus nematophila TB on plant pathogens Phytophthora capsici and Botrytis cinerea in vitro and in planta. Sci. Rep. 4:4300. doi: 10.1038/srep04300
Fritsch, J. (2004). Dimethyl disulfide as a new chemical potential alternative to methyl bromide in soil disinfestation in France. Acta Hortic. 698, 71–76. doi: 10.17660/actahortic.2005.698.8
Fritsch, J., Fouillet, T., Charles, P., Fargier-Puech, P., Ramponi-Bur, C., Descamps, S., et al. (2014). French experiences with dimethyl disulfide (DMDS) as a nematicide in vegetable crops. Acta Hortic. 1044, 427–433. doi: 10.17660/actahortic.2014.1044.59
Gong, A. D., Li, H. P., Shen, L., Zhang, J. B., Wu, A. B., He, W. J., et al. (2015). The Shewanella algae strain YM8 produces volatiles with strong inhibition activity against Aspergillus pathogens and aflatoxins. Front. Microbiol. 6:1091. doi: 10.3389/fmicb.2015.01091
Gong, A. D., Li, H. P., Yuan, Q. S., Song, X. S., Yao, W., He, W. J., et al. (2014). Antagonistic mechanism of iturin a and plipastatin a from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS One 10:e0116871. doi: 10.1371/journal.pone.0116871
He, Y. C., Xu, J. H., Su, J. H., and Zhou, L. (2009). Bioproduction of glycolic acid from glycolonitrile with a new bacterial isolate of alcaligenes sp. ecu 0401. Appl. Biochem. Biotechnol. 160, 1428–1440. doi: 10.1007/s12010-009-8607-y
Hu, Y. C., Zhang, J. M., Kong, W. J., Zhao, G., and Yang, M. H. (2017). Mechanisms of antifungal and antiaflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 220, 1–8. doi: 10.1016/j.foodchem.2016.09.179
Hua, S. S., Beck, J. J., Sarreal, S. B., and Gee, W. (2014). The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 30, 71–78. doi: 10.1007/s12550-014-0189-z
Huang, C. J., Tsay, J. F., Chang, S. Y., Yang, H. P., Wu, W. S., and Chen, C. Y. (2012). Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag. Sci. 68, 1306–1310. doi: 10.1002/ps.3301
Jiang, Y., Wen, J. P., Bai, J., Jia, X. Q., and Hu, Z. D. (2007). Biodegradation of phenol at high initial concentration by Alcaligenes faecalis. J. Hazard. Mater. 147, 672–676. doi: 10.1016/j.jhazmat.2007.05.031
Joo, H. S., Hirai, M., and Shoda, M. (2005). Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by alcaligenes faecalis no. 4. J. Biosci. Bioeng. 100, 184–191. doi: 10.1263/jbb.100.184
Kai, M., Haustein, M., Molina, F., Petri, A., Scholz, B., and Piechulla, B. (2009). Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 81, 1001–1012. doi: 10.1007/s00253-008-1760-3
Kai, M., and Piechulla, B. (2010). Impact of volatiles of the rhizobacteria serratia odorifera on the moss Physcomitrella patens. Plant Signal. Behav. 5, 444–446. doi: 10.4161/psb.5.4.11340
Kakar, K. U., Nawaz, Z., Cui, Z., Almoneafy, A. A., Ullah, R., and Shu, Q. Y. (2018). Rhizosphere-associated Alcaligenes and Bacillus strains that induce resistance against blast and sheath blight diseases, enhance plant growth and improve mineral contentin rice. J. Appl. Microbiol. 124, 779–796. doi: 10.1111/jam.13678
Kamika, I., and Takoy, L. L. (2011). Natural occurrence of Aflatoxin B1 in peanut collected from Kinshasa, Democratic Republic of Congo. Food Control 22, 1760–1764. doi: 10.1016/j.foodcont.2011.04.010
Kedia, A., Prakash, B., Mishra, P. K., and Dubey, N. K. (2014). Antifungal and antiaflatoxigenic properties of Cuminum cyminum (L.) Seed essential oil and its efficacy as a preservative in stored commodities. Int. J. Food Microbiol. 168-169, 1–7. doi: 10.1016/j.ifoodmicro.2013.10.008
Lemfack, M. C., Nickel, J., Dunkel, M., Preissner, R., and Piechulla, B. (2014). mVOC 2.0: a database of microbial volatiles. Nucleic Acids Res. 46, D1261–D1265. doi: 10.1093/nar/gkx1016
Leong, Y., Ismail, N., Latif, A. A., and Ahmad, R. (2010). Aflatoxin occurrence in nuts and commercial nutty products in Malaysia. Food Control 21, 334–338. doi: 10.1016/j.foodcont.2009.06.002
Li, Y. J., Kong, W. J., Li, M. H., Liu, H. M., Zhao, X., Yang, S. H., et al. (2016). Litsea cubeba essential oil as the potential natural fumigant: inhibition of Aspergillus flavus and AFB1 production in licorice. Ind. Crops Prod. 80, 186–193. doi: 10.1016/j.indcrop.2015.11.008
Li, Z. Q., Ding, B., Zhou, X. P., and Wang, G. L. (2017). The rice dynamin-related protein OsDRP1E negatively regulates programmed cell death by controlling the release of cytochrome c from Mitochondria. PLoS Pathog. 13:e1006157. doi: 10.1371/journal.ppat.1006157
Ma, W. B., Zhao, L. L., and Xie, Y. L. (2017). Inhibitory effect of (E)-2-hexenal as a potential natural fumigant on Aspergillus flavus in stored peanut seeds. Ind. Crop Prod. 107, 206–210. doi: 10.1016/j.indcrop.2017.05.051
Meldau, D. G., Meldau, S., Hoang, L. H., Underberg, S., Wünsche, H., and Baldwin, I. T. (2013). Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 25, 2731–2747. doi: 10.1105/tpc.113.114744
Nurtjahtja, K. (2019). Fungal infection and aflatoxin contaminationon dried-stored spices. Int. J. Ecophysiol. 1, 19–25. doi: 10.2174/1871530316666160823145817
Outlaw, J., Waller, M., Richardson, J., Richburg, N., Russell, L., Welch, M., et al. (2016). “Economics of Aflatoxin Risk Management in the Selected Southern States,” in Agricultural & Applied Economics Association Annual Meeting, Boston, MA.
Papazlatani, C., Rousidou, C., Katsoula, A., Kolyvas, M., Genitsaris, S., Papadopoulou, K. K., et al. (2016). Assessment of the impact of the fumigant dimethyl disulfide on the dynamics of major fungal plant pathogens in greenhouse soils. Eur. J. Plant Pathol. 146, 391–400. doi: 10.1007/s10658-016-0926-6
Passone, M. A., and Etcheverry, M. (2014). Antifungal impact of volatile fractions of Peumus boldus and Lippia turbinata on Aspergillus section Flavi and residual levels of these oils in irradiated peanut. Int. J. Food Microbiol. 168-169, 17–23. doi: 10.1016/j.ijfoodmicro.2013.10.009
Piechulla, B., Lemfack, M. C., and Kai, M. (2017). Effects of discrete bioactive microbial volatiles on plants and fungi. Plant Cell Environ. 40, 2042–2067. doi: 10.1111/pce.13011
Prakash, B., Kedia, A., Mishra, P. K., and Dubey, N. K. (2015). Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities – potentials and challenges. Food Control 47, 381–391. doi: 10.1016/jfoodcont.2014.07.023
Probst, C., Schulthess, F., and Cotty, P. J. (2010). Impact of aspergillus section flavi community structure on the development of lethal levels of aflatoxins in Kenyan maize (Zea mays). J. Appl. Microbiol. 108, 600–610. doi: 10.1111/j.1365-2672.2009.04458.x
Rehman, A., Rehman, A., and Ahmad, I. (2015). Antibacterial, antifungal, and insecticidal potentials of Oxalis corniculata and its isolated compounds. Int. J. Anal. Chem. 2015:842468. doi: 10.1155/2015/842468
Robens, J., and Cardwell, K. (2003). The costs of mycotoxin management to the USA: management of aflatoxins in the United States. J. Toxicol. Toxin Rev. 22, 139–152. doi: 10.1081/txr-120024089
Sasanelli, N., Dongiovanni, C., Santori, A., and Myrta, A. (2014). Control of the root-knot nematode Meloidogyne incognita by dimethyl disulfide (DMDS) applied in drip irrigation on melon and tomato in Apulia and Basilicata (Italy). Acta Hortic. 1044, 401–404. doi: 10.17600/actahortic.2014.1044.54
Sayyed, R. Z., and Chincholkar, S. B. (2010). Growth and siderophores production inalcaligenes faecalisis regulated by metal ions. Ind. J. Microbiol. 50, 179–182. doi: 10.1007/s12088-010-0021-1
Sayyed, R. Z., Gangurde, N. S., Patel, P. R., Joshi, S. A., and Chincholkar, S. B. (2010). Siderophore production by Alcaligenes faecalis and its application for growth promotion in arachis hypogaea. Ind. J. Biotechnol. 9, 302–307. doi: 10.1089/hum.2009.157
Sébastien, D., Eric, T., Ahmed, A. G., and Jacques, H. (2004). How a specialist and a non-specialist insect cope with dimethyl disulfide produced by Allium porrum. Entomol. Exp. Appl. 113, 173–179. doi: 10.1111/j.0013-8703.2004.00216.x
Shan, S. J., Wang, W. W., Song, C. X., Wang, M. G., Sun, B. J., and Li, Y. (2019). The symbiotic bacteria Alcaligenes faecalis of the entomopathogenic nematodes Oscheius spp. Exhibit potential biocontrol of plant-and entomopathogenic fungi. Microb. Biotechnol. 12, 459–471. doi: 10.1111/1751-7915.13365
Tian, J., Huang, B., Luo, X. L., Zeng, H., Ban, X. Q., He, J. S., et al. (2012). The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 130, 520–527. doi: 10.1016/j.foodchem.2011.07.061
Tian, J., Zeng, X. B., Feng, Z. Z., Miao, X. M., Peng, X., and Wang, Y. W. (2014). Zanthoxylum molle Rehd. Essential oil as a potential natural preservative in management of Aspergillus flavus. Ind. Crops Prod. 60, 151–159. doi: 10.1016/j.indcrop.2014.05.045
Torres, A. M., Barros, G. G., Palacios, S. A., Chulze, S. N., and Battilani, P. (2014). Review on pre- and post-harvest management of peanuts to minimize aflatoxin contamination. Food Res. Int. 62, 11–19. doi: 10.1016/j.foodres.2014.02.023
Udomkun, P., Mutegi, C., Wossen, T., Atehnkeng, J., Nabahungu, N. L., Njukwe, E., et al. (2018). Occurrence of aflatoxin in agricultural produce from local markets in burundi and eastern democratic Republic of Congo. Food Sci. Nutr. 6, 2227–2238. doi: 10.1002/fsn3.787
Valette, L., Fernandez, X., Poulain, S., Loiseau, A. M., Lizzani-Cuvelier, L., Levieil, R., et al. (2003). Volatile constituents from romanesco cauliflower. Food Chem. 80, 353–358. doi: 10.1016/s0308-8146(02)00272-8
Wagacha, J. M., Mutegi, C., Karanja, L., Kimani, J., and Christie, M. E. (2013). Fungal species isolated from peanuts in major kenyan markets: emphasis on aspergillus section flavi. Crop Prot. 52, 1–9. doi: 10.1016/j.cropro.2013.05.004
Waliyar, F., Umeh, V. C., Traore, A., Osiru, M., Ntare, B. R., Diarra, B., et al. (2015). Prevalence and distribution of aflatoxin contamination in groundnut (Arachishypogaea L.) in Mali, West Africa. Crop Prot. 70, 1–7. doi: 10.1016/j.cropro.2014.12.007
Warth, B., Sulyok, M., Fruhmann, P., Mikula, H., Berthiller, F., Schuhmacher, R., et al. (2012). Development and validation of a rapid multi-biomarker liquid chromatography/ tandem mass spectrometry method to assess human exposure to mycotoxins. Rapid Commun. Mass Spectrom. 26, 1533–1540. doi: 10.1002/rcm.6255
Williams, J. H., Phillips, T. D., Jolly, P. E., Stiles, J. K., Jolly, C. M., and Aggarwal, D. (2004). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 80, 1106–1122. doi: 10.1093/ajcn/80.5.1106
Wu, F. (2015). Global impacts of aflatoxin in maize: trade and human health. World Mycotoxin J. 8, 137–142. doi: 10.3920/wmj2014.1737
Yamamoto, K., Oishi, K., Fujimatsu, I., and Komatsu, K. (1991). Production of R-(-)-mandelic acid from mandelonitrile by Alcaligenes faecalis ATCC 8750. Appl. Environ. Microbiol. 57, 3028–3032.
Yokoyama, S. I., Adachi, Y., Asakura, S., and Kohyama, E. (2013). Characterization of Alcaligenes faecalis strain AD15 indicating biocontrol activity against plant pathogens. J. Gen. Appl. Microbiol. 59, 89–95. doi: 10.2323/jgam.59.089
Yu, J., Whitelaw, C. A., Nierman, W. C., Bhatnagar, D., and Cleveland, T. E. (2004). Aspergillus flavus expressed sequence tags for identification of genes with putative roles in aflatoxin contamination of crops. FEMS Microbiol. Lett. 237, 333–340. doi: 10.1111/j.1574-6968.2004.tb09715.x
Zanon, M. S. A., Barros, G. G., and Chulze, S. N. (2016). Non-aflatoxigenic Aspergillus flavus as potential biocontrol agents to reduce aflatoxin contamination in peanuts harvested in Northern Argentina. Int. J. Food Microbiol. 231, 63–68. doi: 10.1016/j.ijfoodmicro.2016.05.016
Keywords: Aspergillus flavus, aflatoxins, Alcaligenes faecalis, volatile, dimethyl disulfide, methyl isovalerate
Citation: Gong A-D, Wu N-N, Kong X-W, Zhang Y-M, Hu M-J, Gong S-J, Dong F-Y, Wang J-H, Zhao Z-Y and Liao Y-C (2019) Inhibitory Effect of Volatiles Emitted From Alcaligenes faecalis N1-4 on Aspergillus flavus and Aflatoxins in Storage. Front. Microbiol. 10:1419. doi: 10.3389/fmicb.2019.01419
Received: 01 April 2019; Accepted: 05 June 2019;
Published: 25 June 2019.
Edited by:
Masoomeh Shams-Ghahfarokhi, Tarbiat Modares University, IranReviewed by:
Fuguo Xing, Chinese Academy of Agricultural Sciences, ChinaCopyright © 2019 Gong, Wu, Kong, Zhang, Hu, Gong, Dong, Wang, Zhao and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: An-Dong Gong, Z29uZ2FkQHh5bnUuZWR1LmNu; Z2FkLTEyMy4xMjNAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.