
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 02 July 2019
Sec. Virology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.01415
This article is part of the Research Topic Frontiers in Fungal Virus Research View all 16 articles
 Qianqian Wang1,2
Qianqian Wang1,2 Shufen Cheng1,2
Shufen Cheng1,2 Xueqiong Xiao2
Xueqiong Xiao2 Jiasen Cheng1,2
Jiasen Cheng1,2 Yanping Fu2
Yanping Fu2 Tao Chen2
Tao Chen2 Daohong Jiang1,2
Daohong Jiang1,2 Jiatao Xie1,2*
Jiatao Xie1,2*Sclerotinia sclerotiorum, an important phytopathogenic fungus, harbors rich diversity of mycoviruses. Lately, more mycoviruses can be successfully and accurately discovered by deep sequencing, especially those that could not be detected by traditional double-stranded RNA (dsRNA) extraction. Previously, we reported that the hypovirulent S. sclerotiorum strain SZ-150 is coinfected by Sclerotinia sclerotiorum hypovirus 1 (SsHV1) and its related satellite RNA. Here, aside from SsHV1, we detected two other mycoviruses, Sclerotinia sclerotiorum botybirnavirus 3 (SsBV3/SZ-150) and Sclerotinia sclerotiorum mycotymovirus 1 (SsMTV1/SZ-150), coinfecting strain SZ-150, by deep sequencing and assembly of mycovirus-derived small RNAs and determined their full-length genomes. The genome of SsBV3/SZ-150 was found to be composed of two linear dsRNA segments, 6,212, and 5,880 bp in size, respectively. Each dsRNA segment of SsBV3/SZ-150 contains a large open reading frame (ORF) encoding RNA-dependent RNA polymerase (RdRp) and a hypothetical protein. The whole genome of SsBV3/SZ-150 shares more than 95% sequence identity with Botrytis porri botybirnavirus 1 (BpBV1) at the nucleotide (nt) or amino acid level. Thus, SsBV3/SZ-150 was assumed to be a strain of BpBV1. The genome of SsMTV1/SZ-150 consists of 6,391 nt excluding the poly(A) tail. SsMTV1/SZ-150 was predicted to contain a large ORF that encodes a putative replication-associated polyprotein (RP) with three conserved domains of viral RNA methyltransferase, viral RNA helicase, and RdRp. Phylogenetic analyses suggest that SsMTV1/SZ-150 is related, albeit distantly, to members of the family Tymoviridae. Analysis of the small RNAs derived from SsBV3/SZ-150 and SsMTV1/SZ-150 revealed that small-RNA lengths mainly range from 20 to 24 nt, with a peak at 22 nt, and the most abundant 5′-terminal nucleotide is uridine, suggesting that the Dicer 2 and Argonaute 1, two key components in the RNA inference pathway, may play important roles in the resistance to mycoviral infection in S. sclerotiorum. Neither SsBV3/SZ-150 nor SsMTV1/SZ-150 is a causal agent of hypovirulence in strain SZ-150.
Mycoviruses (or fungal viruses) are viruses that infect fungi and Oomycete species and depend on their hosts for replication. Since the first mycovirus was reported in 1962 (Hollings, 1962), mycoviruses have been identified in all major taxonomic groups of fungi and have shown remarkable diversity (Ghabrial and Suzuki, 2009; Pearson et al., 2009; Ghabrial et al., 2015). For defense against mycovirus infection, fungi have evolved antiviral defense mechanisms of RNA silencing, which is similar to that of other eukaryote hosts including plants and animals (Torres-Martinez and Ruiz-Vazquez, 2017; Guo et al., 2019). RNA virus infection in fungi induces production of virus-derived small RNAs (vsRNAs) that have specific function in the RNA-silencing process (Wang et al., 2016; Yaegashi et al., 2016; Donaire and Ayllon, 2017; Yu et al., 2018). These vsRNAs share features with host endogenous small interfering RNAs (siRNAs) and can potentially mediate RNA silencing pathways or can regulate viral replication. Therefore, deep sequencing of small RNAs from virus-infected fungi is an efficient strategy to discover and identify mycoviruses, especially latent ones.
Sclerotinia sclerotiorum, a destructive Ascomycota fungus, is capable of attacking more than 400 species of plants, and cause great losses in a wide variety of crops throughout the world (Bolton et al., 2006). Since the first double-stranded RNA (dsRNA) element was found in a hypovirulent S. sclerotiorum strain 91 (Boland, 1992), this fungus is increasingly recognized to harbor a great variety of mycoviruses, including dsRNA viruses, single-stranded RNA (ssRNA) viruses, and one single-stranded circular DNA virus. Some of those mycoviruses confer hypovirulence to their hosts and show a great potential for virocontrol (Xie and Jiang, 2014). At present, with development of the high-throughput sequencing technology, novel mycoviruses that are difficult to find by conventional detection methods, such as dsRNA extraction, can be discovered in S. sclerotiorum (Marzano et al., 2016; Mu et al., 2017). These discovered S. sclerotiorum mycoviruses not only enrich known virus diversity but also supply new knowledge about viral evolution and its coevolution with the host (Jiang et al., 2013; Marzano et al., 2016).
The order Tymovirales was first established in 2004 and is currently composed of five families: Alphaflexiviridae, Betaflexiviridae, Gammaflexiviridae, Deltaflexiviridae, and Tymoviridae1. Members of this order possess an ssRNA genome from 5.9 to 9.0 kb in length, which is mostly polyadenylated. The largest protein, i.e., replication-associated polyprotein (RP), with molecular mass approximately 150–250 kDa, is encoded in all the members of the order Tymovirales, and the RP protein usually consists of a set of conserved functional domains (King et al., 2011). The members of Tymovirales show great diversity in virion morphology. The members of the families Alphaflexiviridae, Betaflexiviridae, and Gammaflexiviridae have virions in the form of flexuous filaments, whereas viruses in the family Tymoviridae have non-enveloped isometric particles (Adams et al., 2011b). The viruses belonging to Tymovirales also have diverse host ranges. Members of Betaflexiviridae and Tymoviridae are plant viruses (Adams et al., 2011b), a single member of Gammaflexiviridae is reported only from a filamentous fungus (Howitt et al., 2001; Adams et al., 2011b), whereas members of Alphaflexiviridae infect both plants and fungi (Adams et al., 2011a).
Botybirnaviruses are a group of bipartite viruses with dsRNA genomes of 5,800–6,500 bp for each segment. The first botybirnavirus, Botrytis porri botybirnavirus 1 (BpBV1), has been discovered in the phytopathogenic fungus B. porri and is responsible for hypovirulent traits (Wu et al., 2012). Two botybirnaviruses have been subsequently found in S. sclerotiorum (Liu et al., 2015; Ran et al., 2016). The botybirnavirus infection is associated with the hypovirulence of S. sclerotiorum as well. Recently, more candidate members of the genus of Botybirnavirus were detected in the fungi of Ascomycota, e.g., fungi of genera Alternaria and Bipolaris (Xiang et al., 2017; Wang et al., 2018). With the development of sequencing technology, more botybirnavirus-like sequences have also been identified during the metatranscriptomic analysis, e.g., the finding of botybirnavirus-like sequences in the metatranscriptomics survey of soybean phyllosphere phytobiomes (Marzano and Domier, 2016). Therefore, botybirnaviruses may have a wide distribution among different fungal groups.
Previously, we have reported three dsRNA segments, two similarly, sized at 9.5 kbp and a third one of approximately 3.6 kbp. One of the two large dsRNA segments, the replication form of Sclerotinia sclerotiorum hypovirus 1 (SsHV1/SZ-150), has been characterized (Xie et al., 2011). SsHV1/SZ-150 is closely related to CHV3/GH2 and CHV4/SR2 within Hypoviridae, whereas the 3.6-kbp dsRNA segment was recognized to be the satellite-like RNA (SatH) of SsHV1/SZ-150. The partial cDNA sequence of the other large dsRNA segment (the genome of the previously named Sclerotinia sclerotiorum RNA virus 1, SsRV1/SZ-150) was determined and putatively assigned to a plant virus (Xie et al., 2011). In the present study, we obtained sRNA from the hypovirulent strain SZ-150 of S. sclerotiorum by deep sequencing and discovered two other mycoviruses, Sclerotinia sclerotiorum botybirnavirus 3 (SsBV3/SZ-150, originally named SsRV1/SZ-150) and Sclerotinia sclerotiorum mycotymovirus 1 (SsMTV1/SZ-150) aside from SsHV1 and SatH. Combined with bioinformatics analysis and rapid amplification of cDNA end (RACE), full-length genomes of these two mycoviruses were determined via assembly of myco vsRNAs and were characterized. The assay of biological features on individual strains with different virus combination confirmed that neither SsBV3/SZ-150 nor SsMTV1/SZ-150 is a causal agent of hypovirulence of strain SZ-150.
Large-scale sequencing of sRNAs has been used for virus identification in insects, plants, and fungi (Kreuze et al., 2009; Wu et al., 2010; Vainio et al., 2015). To test whether the strain SZ-150 is infected by other new mycoviruses aside form SsHV1/SZ-150 and its SatH, the total RNA from the strain SZ-150 was prepared for deep sequencing of sRNAs. After removing of the adaptors and filtering out low-quality tags, the total number of reads produced by the Illumina sequencer was 12,999,900 [read length ranging from 18 to 30 nucleotides (nts)]. To obtain more information about mycovirus-related sequences, these sRNAs were first matched against the genome sequences of S. sclerotiorum, SsHV1/SZ-150 and SatH/SZ-150. The unmatched sRNAs were then assembled into longer contiguous sequences (contigs) before they were used for sequence similarity searches in reference databases. The newly assembled contigs could be subdivided into two groups based on BLASTX against NCBI GenBank database. One group of contigs contained sequences sharing a higher identity (95–100%) with BpBV1/GarlicBc-72, and those contigs were presumed to represent the genome of a putative mycovirus, namely, SsBV3/SZ-150 (accession numbers MK530703 and MK530704) to distinguish it from two botybirnaviruses previously found in S. sclerotiorum (Liu et al., 2015; Ran et al., 2016). The second group of sequences revealed that proteins encoded by two contigs shared limited sequence identity (27–39%) with members of the family Tymoviridae, suggesting that they represent the genome of a new virus. We tentatively named this virus SsMTV1/SZ-150 (accession number MK530705). Thus, SZ-150 is coinfected with three mycoviruses: two (+)ssRNA viruses (SsHV1 and SsMTV1) and one virus with dsRNA genome (SsBV3), along with a SatH of SsHV1/SZ-150.
As a result of a combination of the assembled vsRNA contigs and RACE experiments, the complete genomes of SsBV3/SZ-150 and SsMTV1/SZ-150 were determined. Genomes of both viruses were re-sequenced via RT-PCR with specific primers based on the assembled sequence to ensure maximum sequence accuracy (Supplementary Table S2).
The SsBV3/SZ-150 genome is composed of two linear dsRNA segments (L1-dsRNA and L2-dsRNA) of 6,212 and 5,880 bp in length, respectively (Figures 1A, 4C,E). SsBV3/SZ-150 was subjected to BLAST searches against NCBI GenBank, and results showed that SsBV3/SZ-150 shares 96% sequence identity with Botrytis porri botybirnavirus 1 (BpBV1/GarlicBc-72; Wu et al., 2012). Similar to BpBV1, each dsRNA segment of SsBV3 possesses a large open reading frame (ORF), designated as ORF I (on L1-dsRNA), and ORF II (on L2-dsRNA) (Figures 1A, 4C,E). The protein encoded by the 3′ proximal coding region of ORF I encompasses the RdRp_4 conserved domain sequence and shows sequence similarity (97.58%) to the corresponding region of BpBV1/GarlicBc-72. The hypothetical protein encoded by ORF II shares 97.30% identity with BpBV1/GarlicBc-72. In addition, the proteins encoded by the 5′-proximal region of ORF I and by the entire ORF II lack significant sequence similarity to the proteins of any other known virus groups aside from botybirnaviruses. The phylogenetic analysis confirmed that SsBV3/SZ-150 is most closely related to BpBV1/GarlicBc-72 but distant from two other botybirnaviruses reported from S. sclerotiorum (Figure 1B). All the above results suggested that SsBV3/SZ-150 and BpBV1/GarlicBc-72 belong to the same species.
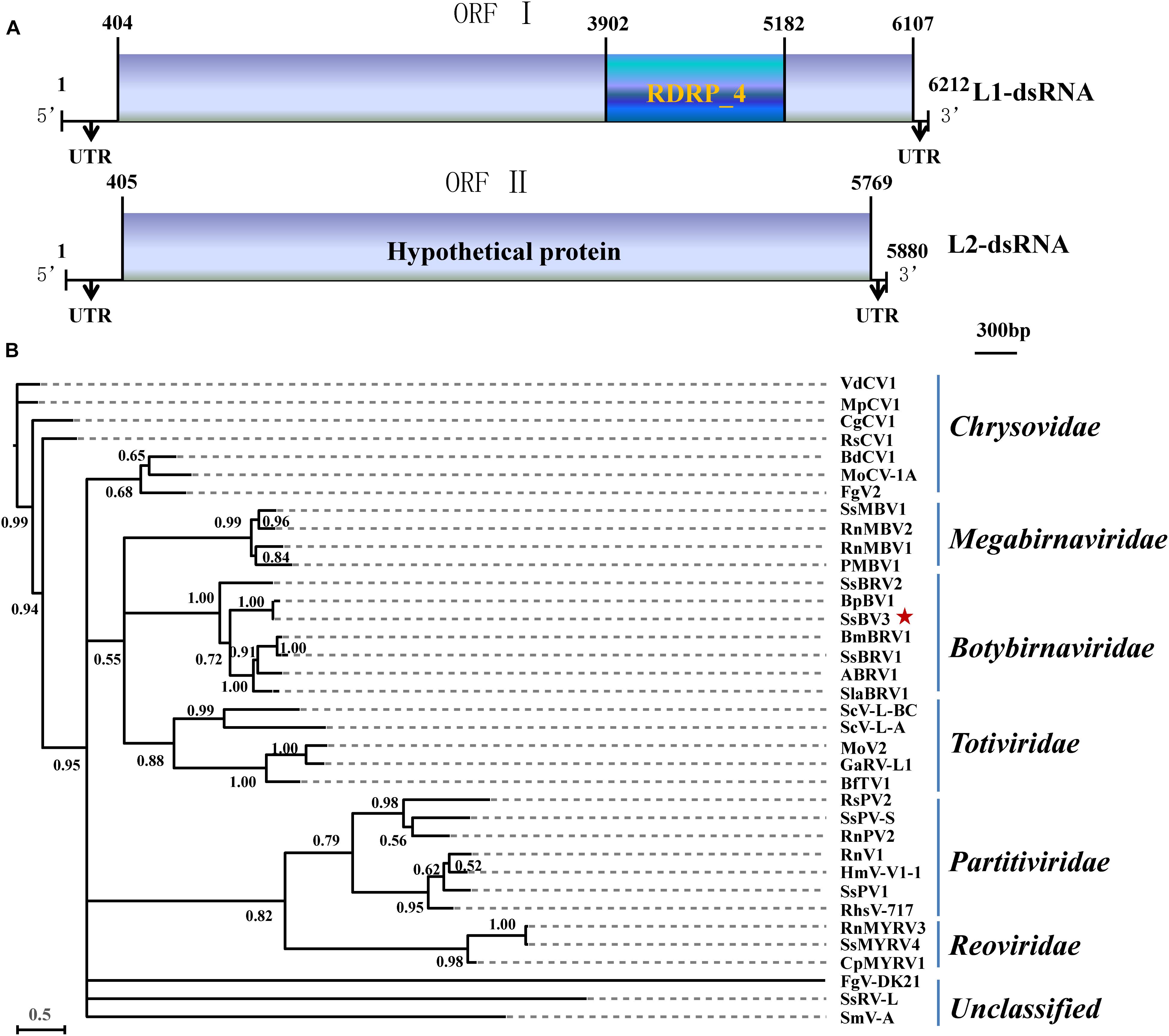
Figure 1. Schematic diagram and phylogenetic analysis of Sclerotinia sclerotiorum botybirnavirus 3 (SsBV3/SZ-150). (A) Genomic organization of SsBV3/SZ-150. The 5′- and 3′-UTRs (line) and the ORF (box) are displayed. The blue box indicates a conserved RdRp domain. The scale bar corresponds to a length of 300 bp. (B) A Bayesian phylogenetic tree illustrating the relationships of SsBV3/SZ-150 with selected viruses from Totiviridae, Chrysoviridae, Partitiviridae, Reoviridae, Megabirnaviridae, Botybirnavirius, and unassigned viruses, with the model of VT+G+F. The scale bars correspond to 0.5 amino acid substitutions per site, numbers at branch nodes show percentage posterior probabilities. The red star indicates the position of SsBV3/SZ-150. The detailed information on the selected viruses in the phylogenetic tree is shown in Supplementary Table S1.
The complete genomic sequence of SsMTV1/SZ-150 is 6391 nt in length excluding the 3′-terminal poly (A) tail (Figures 2A, 4A), with a G+C content of 53.3%. This mycovirus contains a 263- and 248-nt-long 5′- and 3′-UTRs, respectively. Sequence analyses revealed that the SsMTV1 genome has a putative single large ORF. This ORF is 5,880 nt long, beginning at AUG (nt positions 264–266) and terminating at UAG (nt positions 6,141–6,143). The ORF codes for a putative RP of 1,958 amino acids residues with a calculated molecular mass of 218.6 kDa. A conserved-motif search revealed that the RP contains three conserved domains (from the N terminus to the C terminus): viral methyltransferase (Mtr), viral RNA helicase (Hel), and RNA-dependent RNA polymerase (RdRP) (Figures 2A, 4A).
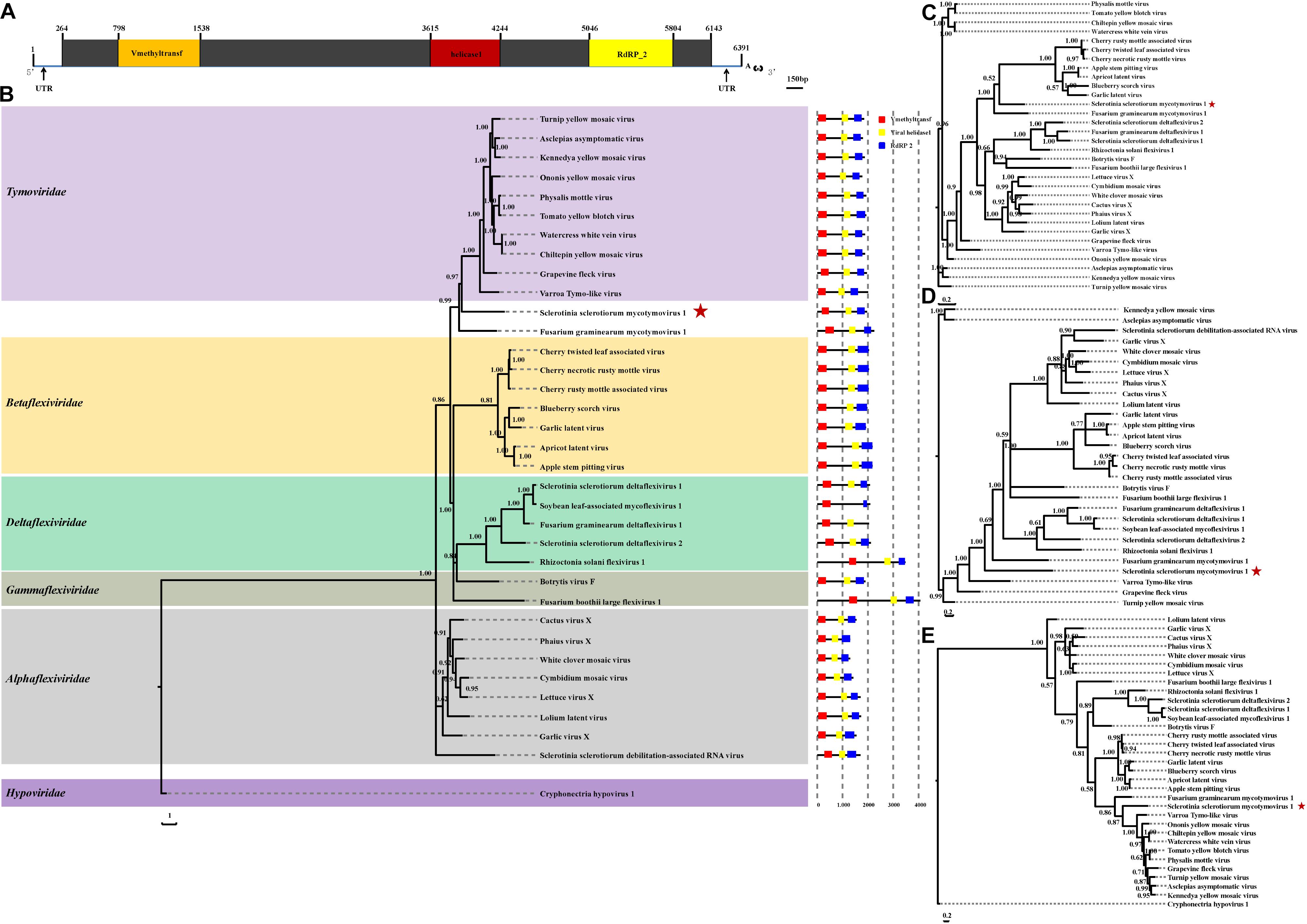
Figure 2. Analyses of identified Sclerotinia sclerotiorum mycotymovirus 1 (SsMTV1/SZ-150) genome. (A) Schematic diagrams of the genetic organization of SsMTV1/SZ-150. The ORF (nt positions 264–6,143) encodes a putative viral protein with 1,958 amino acid residues that contains three conserved domains represented by the box: Vmethyltransf (798–1,538 nt), helicase (3,615–4,244 nt), and RNA-dependent RNA polymerase (RdRP_2, 5,046–5,804 nt). 5′-UTR (nt positions 1–263 nt) and 3′-UTR (nt positions 6,144–6,391 nt) are indicated as lines. The scale bar corresponds to a length of 150 bp. Phylogenetic (Bayesian) trees were constructed from the sequences of three conserved domains of the entire RP domain (B), helicase (C), methyltransferase (D), RdRP (E), and the SsMTV1/SZ-150, with the models of VT+I+G+F, RtREV+I+G+F, LG+I+G+F, and LG+I+G+F, respectively. CHV1 (Cryphonectria hypovirus 1) served as an outgroup in the phylogenetic analysis. The scale bar indicates the number of amino acid substitutions per site; numbers at branch nodes show percentage posterior probabilities. The position of SsMTV1/SZ-150 is shown by red asterisks. The detailed information on the selected viruses in the phylogenetic tree is presented in Table 1.
Multiple-alignment analysis was conducted based on the full sequence of RP by the methods of pairwise comparisons and the percentage of amino acid sequence identities. The RP of SsMTV1/SZ-150 showed the highest identity (20.3%) with that of turnip yellow mosaic virus (NP_663297) compared to the RP of the representatives of the order Tymovirales (Table 1). Phylogenetic analyses of the viral RP of SsMTV1/SZ-150 and representatives of the order Tymovirales indicated that SsMTV1/SZ-150 is phylogenetically related to members of Tymoviridae but belongs to a distinct evolutionary lineage (Figure 2B).
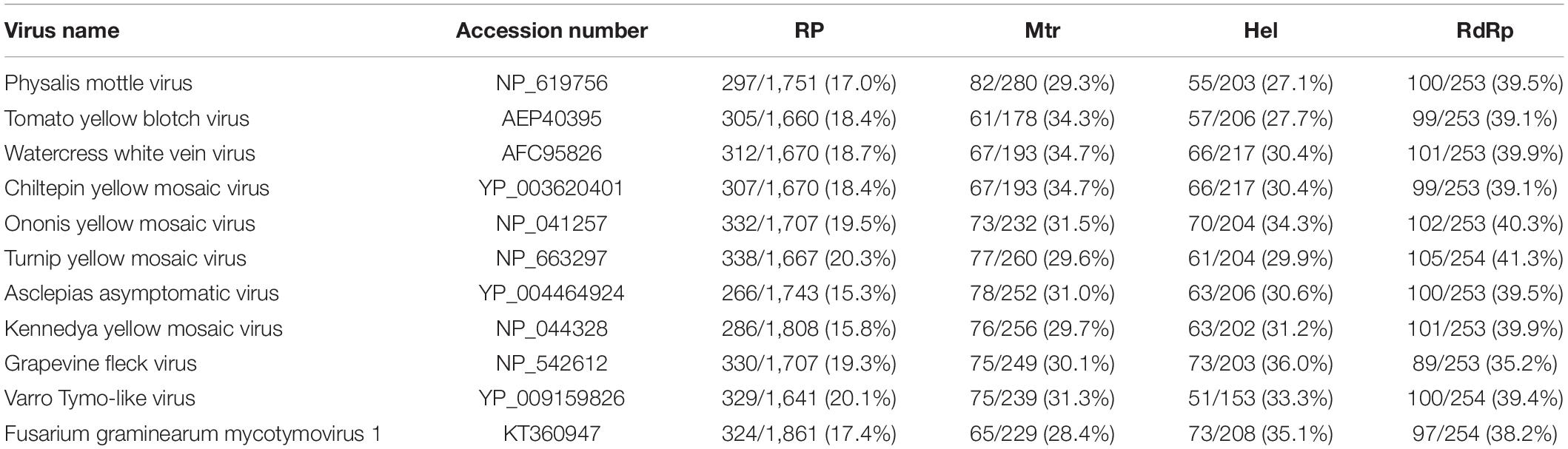
Table 1. Percent amino acid sequence identity between the RP polyprotein, methyltransferase (Mtr), helicase (Hel), and RdRp motifs of Sclerotinia sclerotiorum mycotymovirus 1 (SsMTV1/SZ-150) and those of selected viruses of the family Tymoviridae.
Multiple-alignment and phylogenetic analyses were next conducted based on three conserved domains (Mtr, Hel, and RdRp) of RP encoded by SsMTV1/SZ-150 (Figures 2C–E). The putative Mtr domain (nt positions 798–1,538) is located near the N-terminal region of the SsMTV1-encoded RP and contains three conserved motifs (I–III; Supplementary Figure S1). Multiple alignment suggested that this conserved domain shared 34.7% sequence identity with those of watercress white vein virus (AFC95826) and chiltepin yellow mosaic virus (YP_003620401; Table 1). The putative Hel domain (nt positions 3,615–4,244) with five conserved motifs (I–V; Supplementary Figure S1) shares 36.0 and 35.1% sequence identities with those of grapevine fleck virus (NP_542612) and Fusarium graminearum mycotymovirus 1 (KT360947), respectively (Table 1). An RdRP domain, containing six conserved motifs (I–VI; Supplementary Figure S1) was detected near the C-terminus of the RP. This domain of SsMTV1 shares highest level of sequence identity (41.3%) with that of turnip yellow mosaic virus (NP_663297; Table 1).
In total, 966,210 virus-derived sRNAs (vsRNAs) of 18–30 nt in length, accounting for 7.43% of all sRNA reads, matched the genome sequence of SsMTV1/SZ-150 (Table 2). In addition, 1,077,449 and 794,152 vsRNAs of the same length (accounting for 8.23 and 6.11% of all sRNA reads) matched the genome sequence of SsBV3-L1-dsRNA/SZ-150 and SsBV3-L2-dsRNA/SZ-150, respectively (Table 2). The length of these vsRNAs mainly ranges from 20 to 24 nt, with the 22-nt class being dominant in strain SZ-150. Indeed, the 22-nt class represented approximately 37.3% of the total SsMTV1-derived vsRNAs (Figure 3A and Table 2) and approximately 27.4 and 27.0% of the total SsBV3 vsRNAs (Figures 3C,E and Table 2). The second most abundant vsRNA species in SsMTV1/SZ-150 was the 23-nt class, accounting for 26.8% of the total vsRNAs (Figure 3A and Table 2), whereas the number of vsRNAs (21 and 23 species) was essentially equal in SsBV3/SZ-150 (Figures 3C,E and Table 2). A more specific analysis was performed on the distribution of the vsRNAs derived from the sense and antisense strands of SsMTV1. The results showed that there was an asymmetrical distribution, and the antisense vsRNAs were dominant (nearly 70%; Figure 3B and Table 2). In contrast to the vsRNAs from SsMTV1/SZ-150, the number of vsRNAs derived from the sense strand was almost equal to that from the antisense strain in SsBV3/SZ-150 (Figures 3D,F and Table 2).
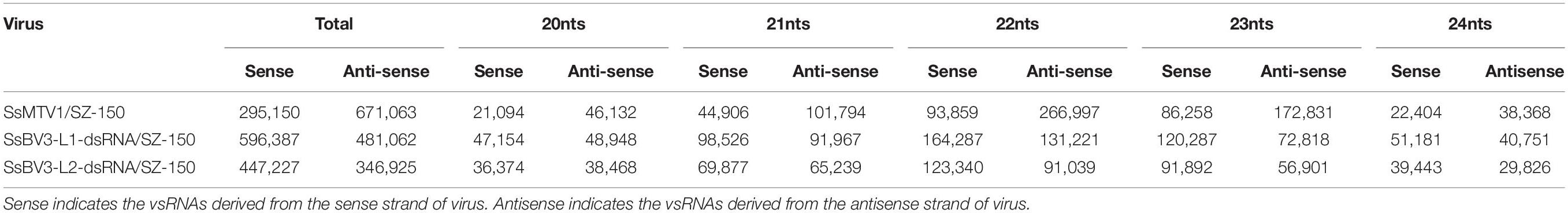
Table 2. The number of vsRNAs derived from Sclerotinia sclerotiorum mycotymovirus 1 (SsMTV1/SZ-150) and Sclerotinia sclerotiorum botybirnavirus 3 (SsBV3/SZ-150).
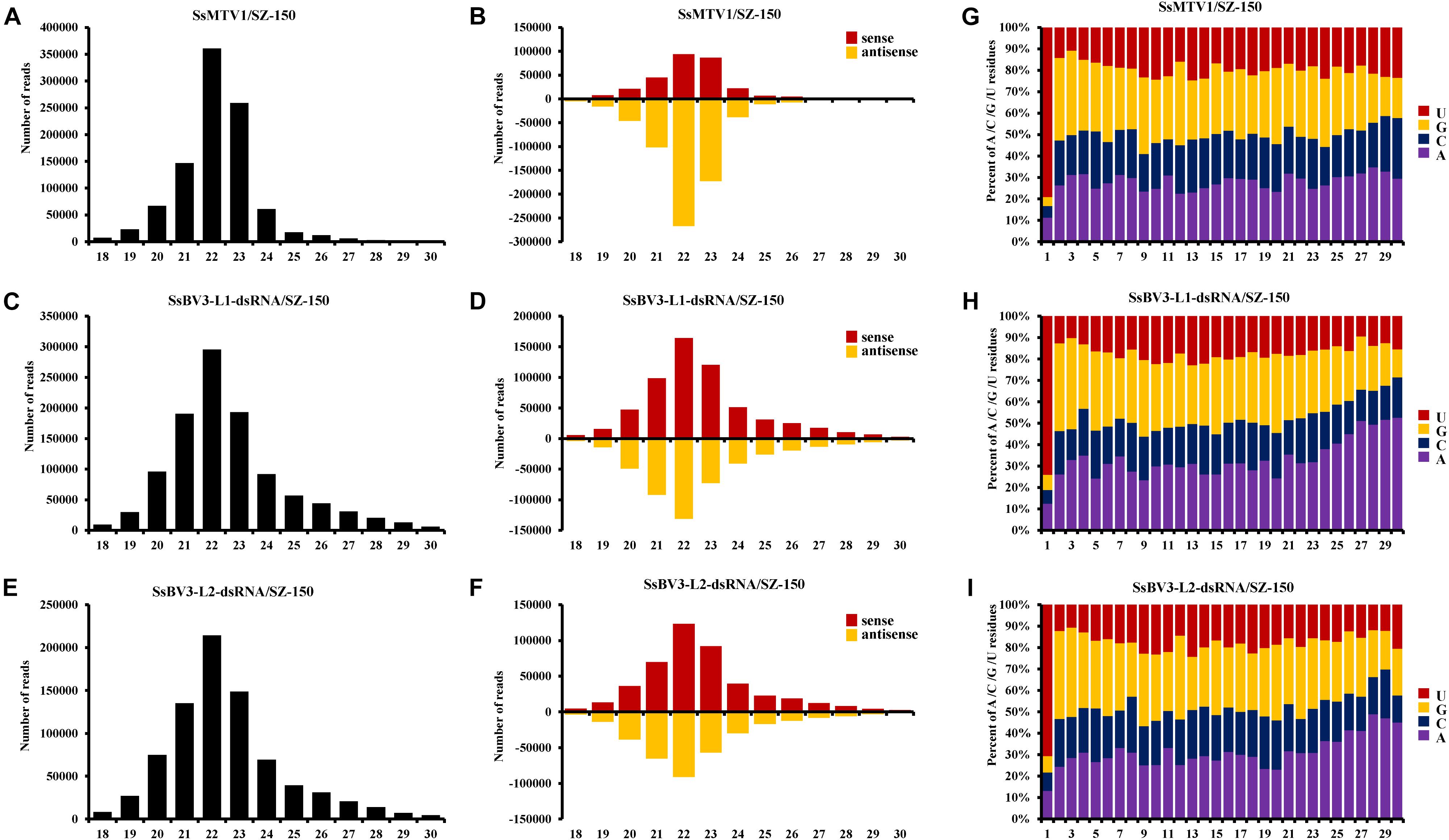
Figure 3. Characterization of vsRNAs from Sclerotinia sclerotiorum mycotymovirus 1 (SsMTV1/SZ-150) and Sclerotinia sclerotiorum botybirnavirus 3 (SsBV3/SZ-150) in mycovirus-infected strain SZ-150. The size distributions of vsRNAs derived from SsMTV1 (A), SsBV3-L1-dsRNA (C), and SsBV3-L2-dsRNA (E) in strain SZ-150. The histogram illustrating the number of vsRNA reads; the size distribution of clones 18 to 30 nt. Relative accumulation of vsRNAs from sense and antisense of SsMTV1 (B), SsBV3-L1-dsRNA (D), and SsBV3-L2-dsRNA (F) from strain SZ-150. Red and yellow bars indicate sense and antisense vsRNAs, respectively. The identities and the relative frequency of 5′-terminal nucleotide of SsMTV1-derived vsRNAs (G), SsBV3-L1-dsRNA-derived vsRNAs (H), and SsBV3-L2-dsRNA-derived vsRNAs (I). The y-axis shows the percentages of 5’-terminal nucleotides consisting of G, C, A, and U in the 18- to 30-nt vsRNAs class, and the x-axis represents the length distribution.
There was a clear preference for uridine (U) residues (79.2%) in the 5′ terminal nucleotide composition of SsMTV1-derived vsRNAs, while guanidine (G) was the least abundant (4.1%; Figure 3G). Similar results were obtained in the analysis of vsRNAs from SsBV3/SZ-150 (Figures 3H,I). To determine the distribution of the vsRNAs in genomes of SsMTV1/SZ-150 and SsBV3/SZ-150, vsRNAs reads were then aligned against the genomes of two mycoviruses. As presented in Figures 4B,D,F, vsRNAs were distributed along the whole viral genome, including the coding regions and 5′- or 3′-UTRs; the vsRNAs derived from SsMTV1/SZ-150 were distributed along the genome in a non-random pattern, with the majority derived from the negative strand, and the vsRNAs derived from SsBV3/SZ-150 were uniformly distributed along the positive and negative strands, as described previously. There was one hotspot region, which produced more vsRNAs; they were present on both the positive and negative strands in the 5′ region of genomes of SsMTV1/SZ-150 and SsBV3/SZ-150.
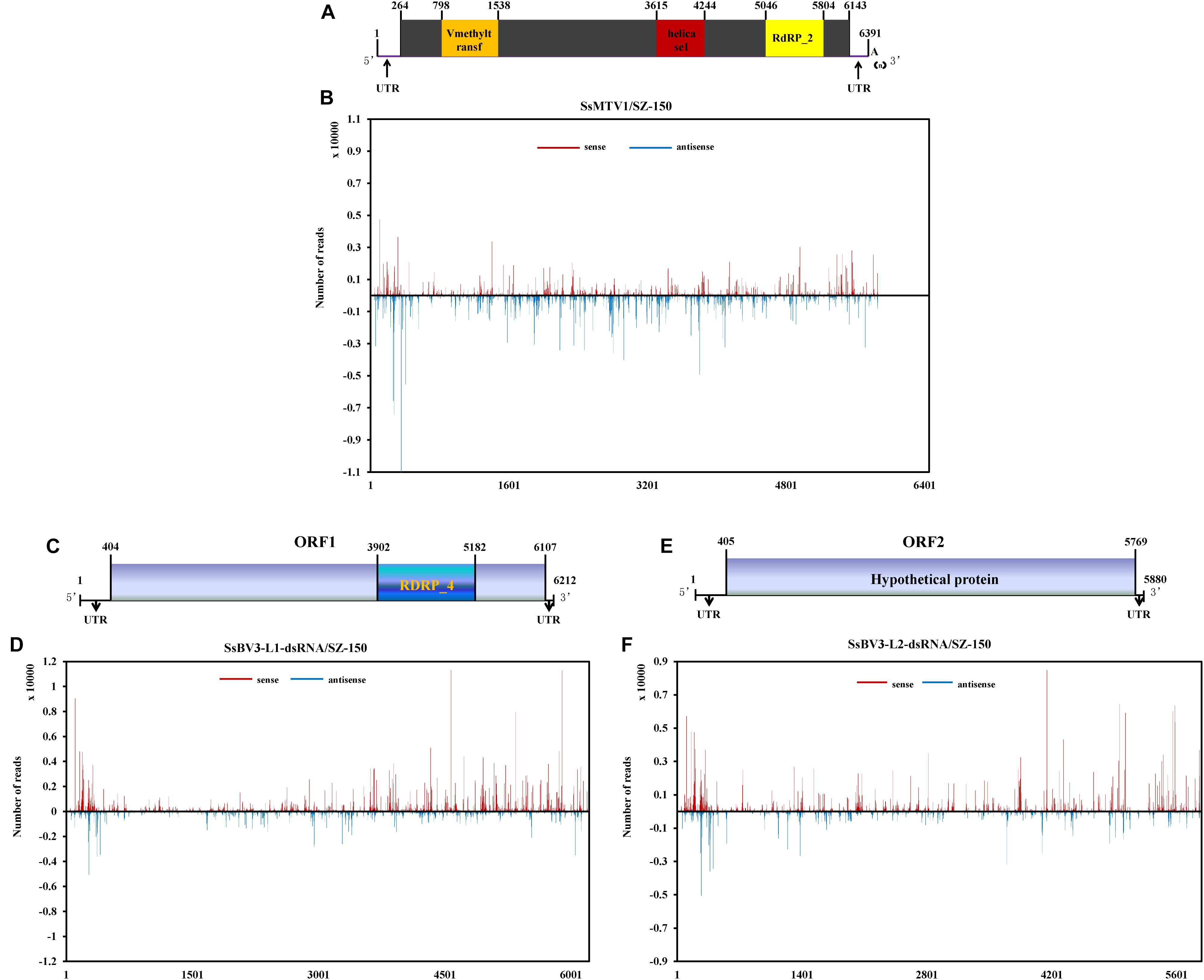
Figure 4. Profiles of vsRNAs along Sclerotinia sclerotiorum mycotymovirus 1 (SsMTV1/SZ-150) and Sclerotinia sclerotiorum botybirnavirus 3 (SsBV3/SZ-150) genomes. The identified vsRNAs were mapped onto the SsMTV1 (A), SsBV3-L1-dsRNA (C), and SsBV3-L2-dsRNA (E) genomes. (B,D,F) Distribution of the vsRNAs mapped on the corresponding viral genomes. The x-axis shows schematic representations of SsMTV1 and SsBV3 genomic organization. The y-axis indicates the numbers of vsRNAs matching the sense strand (red, above the x-axis) and antisense strand (blue, below the x-axis).
RT-PCR analysis was performed on total-RNA samples from mycelial extracts of the hypovirulent strain SZ-150, from an ascospore progeny derivative SZ-150/A6, and from two protoplast derivatives (SZ-150/R59 and SZ-150/R6) of strain SZ-150. Strain SZ-150/R59 was infected with SsBV3/SZ-150, while SZ-150/R6 was coinfected by three mycoviruses: SsMTV1/SZ-150, SsHV1/SZ-150, and SsBV3/SZ-150 (Figure 5A). Strain SZ-150/A6 was virus-free, while we failed to create a strain infected with SsMTV1 alone (Figure 5A). To determine the biological effects of SsBV3/SZ-150 and SsMTV1/SZ-150 on hypovirulence of S. sclerotiorum, biological features of the four individual strains were assayed. Strain SZ-150 had abnormal colony morphology characterized by a dark pigment, whereas its derivatives SZ-150/A6, SZ-150/R59, and SZ-150/R6 had normal colony morphology with no significant differences on the potato–dextrose–agar (PDA) medium (Figure 5B). The growth rate of SZ-150/R59 (15.97 ± 3.34 mm/day) was not significantly different from that of SZ-150/A6 (17.86 ± 2.33 mm/day), but was faster than that of SZ-150/R6 (8.40 ± 1.46 mm/day; Figure 5D). Virulence assays were conducted on the detached leaves of rapeseed plants that were kept at 20°C for 48 h post inoculation. Strain SZ-150 failed to infect rapeseed leaves, whereas SZ-150/A6, SZ-150/R59, and SZ-150/R6 induced typical lesions on the leaves (Figure 5C). The size of lesion induced by SZ-150/R59 (3.3 ± 0.17 cm) was not significantly different from that induced by strain SZ-150/A6 (3.48 ± 0.26 cm) but was larger than that of strain SZ-150/R6 (1.74 ± 0.21 cm; Figure 5E). These results suggest that SsBV3/SZ-150 does not induce phenotypic changes in the host, and SsMTV1/SZ-150 makes only a limited contribution to the hypovirulence.
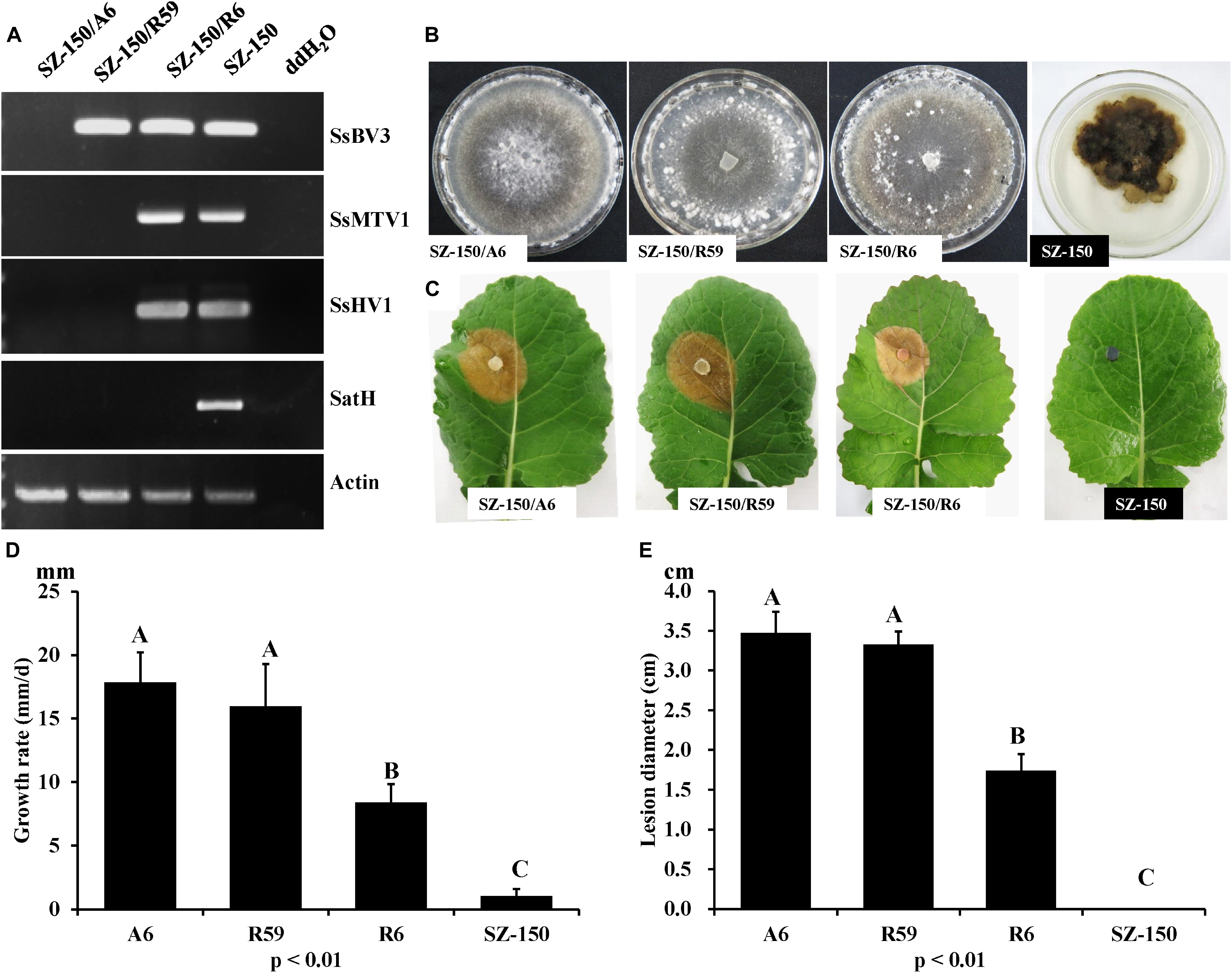
Figure 5. Biological properties of four individual strains: SZ-150/A6, SZ-150/R59, SZ-150/R6, and SZ-150. (A) Detection of mycoviruses Sclerotinia sclerotiorum hypovirus 1 (SsHV1/SZ-150) and its related satellite RNA (SatH), Sclerotinia sclerotiorum mycotymovirus 1 (SsMTV1/SZ-150), and Sclerotinia sclerotiorum botybirnavirus 3 (SsBV3/SZ-150) in four strains by RT-PCR. The actin gene served as an internal control. (B) Colony morphology and (D) growth rate of four individual strains. Four strains were grown on PDA for 15 days at 20∘C prior to photography. (C,E) Virulence assays of the four strains on the detached leaves of rapeseed plants. Statistical analysis of the growth rate and lesion diameter of the four individual strains was conducted at the P < 0.01 level. The error bars indicate the SD from three sample means.
Multiple infection by several mycoviruses is a common phenomenon in the fungi kingdom (Ghabrial and Suzuki, 2008). These viruses are usually detected by conventionally methods, such as dsRNA extraction. Nevertheless, hypovirulence-related mycoviruses receive more attention from researchers, while latent mycoviruses or mycoviruses with a lower copy number of dsRNA formed in a fungal host are often ignored or are difficult to detect by dsRNA extraction.
Previously, our study based on the dsRNA extraction and conventional cloning strategies revealed coinfection by two mycoviruses SsHV1/SZ-150 and SsBV3/SZ-150 (SsRV1/SZ-150 in Xie et al., 2011) in a hypovirulent strain SZ-150 of S. sclerotiorum. In the present study, the results of high-throughput sequencing of sRNAs revealed one additional mycovirus (SsMTV1/SZ-150) in the same strain and, combined with RACE, allowed for completion of genome sequences of two mycoviruses (SsMTV1/SZ-150 and SsBV3/SZ-150). High-throughput sequencing is a powerful tool for the discovery of many novel ssRNA mycoviruses, especially (−)ssRNA mycoviruses that have a lower copy number of dsRNA in fungal cells. Several studies have proved the ability of high-throughput sequencing to detect low-copy mycovirus infections, thereby discovering new mycoviruses and evaluating mycovirus diversity in phytopathogenic fungi including S. sclerotiorum, Rhizoctonia solani, Rosellinia necatrix, and Rhizophagus spp. (Bartholomaus et al., 2016; Marzano et al., 2016; Yaegashi et al., 2016; Mu et al., 2017).
The genome of SsBV3/SZ-150 shares 97% identity with that of BpBV1/GarlicBc-72 at the amino acid level, thus revealing that SsBV3/SZ-150 should be recognized as an isolate of species Botrytis porri botybirnavirus 1. BpBV1/GarlicBc-72 confers hypovirulence on its natural host B. porri (Wu et al., 2012), whereas SsBV3/SZ-150 is associated with a latent infection in S. sclerotiorum, suggesting that BpBV1 patterns of interaction with B. porri and S. sclerotiorum are different, even though these two fungi share 83% amino acid identity in the genome (Amselem et al., 2011). Actually, two mycoviruses of SsBV1 and SsBV2 belonging to the genus Botybirnavirus have already been characterized from S. sclerotiorum (Liu et al., 2015; Ran et al., 2016). SsBV2 infection induces hypovirulence in strains of S. sclerotiorum studied. Although infection by SsBV1 alone has no significant effects on culture morphology and virulence of S. sclerotiorum, infection by SsBV1 along with its SatH leads to slightly reduced virulence and a slower mycelial growth rate in its host. These combined results imply that the interaction between botybirnavirus and S. sclerotiorum is complex. Botybirnaviruses probably have a wide host range. Besides Botrytis spp. and S. sclerotiorum, botybirnaviruses have also been found in the fungi Bipolaris maydis and Alternaria spp. (Xiang et al., 2017; Wang et al., 2018). Although the genomes of these two botybirnaviruses have been fully sequenced, the influences on their fungal hosts are still unknown.
Notably, a botybirnavirus, BpBV1, was detected in both Botrytis squamosa and B. cinerea in addition to B. porri and S. sclerotiorum. These four phytopathogenic fungi have many similarities in the genome; more importantly, they have similar ecological niches and have opportunities to coinfect the same plants. Similar findings have been reported in other studies. Some viruses detected in R. necatrix have the closest relation to viruses infecting Fusarium spp., which are sympatric to R. necatrix (Arjona-Lopez et al., 2018). Recently, Schoebel et al. (2018) provided evidence that multiple interspecific virus transfers have occurred from Hymenoscyphus albidus to Hymenoscyphus fraxineus (Schoebel et al., 2018). These data suggest that interspecific transmission, even interfamily transmission, of BpBV1, and other mycoviruses may occur under field conditions, although the underlying mechanism is still unclear.
The complete genome sequence of SsMTV1/SZ-150 was determined in this study as well. Phylogenetic analyses of the putative polyprotein strongly suggest that SsMTV1/SZ-150 is related, albeit distantly, to members of the family Tymoviridae and forms an independent evolutionary clade. Therefore, SsMTV1/SZ-150 is a novel mycotymo-like virus related to members of Tymoviridae. Although most viruses in the order Tymovirales infect plants, a few members of this order infecting plant-pathogenic fungi, including B. cinerea, Fusarium boothii, Fusarium graminearum, R. solani, and S. sclerotiorum, have been characterized (Howitt et al., 2006; Li K. et al., 2016; Li P. et al., 2016; Bartholomaus et al., 2017; Mizutani et al., 2018). Nevertheless, those mycotymo-like viruses were placed into different evolutionary lineages, suggesting that mycotymo-like viruses are more diverse in fungi than previously thought, and have a complex evolutionary relationship with plant viruses within Tymovirales.
The genomic RNA of viruses belonging to the family Tymoviridae ranges from 6.0 to 7.5 kb in length and is mostly polyadenylated (King et al., 2011) and characterized by a high cytosine content (32–50%). Similar to other members of family Tymoviridae, the genomic sequence of SsMTV1/SZ-150 is 6,391 nt in size, excluding the 3′-terminal poly (A) tail. Nevertheless, the genomic sequence of SsMTV1/SZ-150 also contains a high content of cytosines with 30.1%. Consistently with the genomic RNA of Sclerotinia sclerotiorum debilitation-associated RNA virus (Xie et al., 2006), the genome of SsMTV1/SZ-150 has a single ORF encoding a polyprotein but lacks a gene encoding a coat protein (CP). All other recognized members of the order Tymovirales contain CP genes regardless of whether they are plant viruses, insect viruses, or two mycoviruses (BotVX and BotV-F).
In the present study, we analyzed the vsRNAs derived from SsMTV1/SZ-150 and SsBV3/SZ-150. vsRNAs from SsMTV1/SZ-150 and SsBV3/SZ-150 have a size from 18 to 30 nt with a peak at 22 nt. vsRNAs are uniformly distributed along the genomes of two viruses, with the majority derived from the negative strand in the case of SsMTV1/SZ-150. In the plant Arabidopsis thaliana, different Dicer proteins are responsible for the cleavage of sRNAs of different lengths. Dicer2 is responsible for the cleavage of 22-nt sRNAs (Ruiz-Ferrer and Voinnet, 2009), suggesting that the homolog of DCL2 in S. sclerotiorum might be the predominant Dicer ribonuclease involved in the biogenesis of vsRNAs. Some reports indicate that the loading of sRNA onto an AGO-containing effector complex is guided by the 5′-terminal nucleotide of the sRNA in Arabidopsis and rice (Mi et al., 2008; Wu et al., 2009). In Arabidopsis, AGO1 harbors microRNAs with 5′-terminal U residues (Mi et al., 2008). This finding suggests that most of the vsRNAs are preferentially recruited into AGO1 in S. sclerotiorum.
Thus, aside from SsHV1/SZ-150 and its SatH, in this study, we discovered and characterized two other mycoviruses, SsBV3/SZ-150 (belonging to genus Botybirnavirius), and SsMTV1/SZ-150 (related to viruses in the order Tymovirales), in the hypovirulent strain SZ-150 via high-throughput sequencing. The SsMTV1 and SsBV3-vsRNA analyses suggest that the DCL2 and AGO1 are key players in the defense against mycovirus infection in S. sclerotiorum.
Sclerotinia sclerotiorum strain SZ-150 was originally isolated from a sclerotium collected from a diseased rapeseed (Brassica napus) (Xie et al., 2011). SZ-150/R59 and SZ-150/R6 were derived from strain SZ-150 by protoplast isolation and regeneration. SZ-150/A6 was a single-ascospore isolation of strain SZ-150/R59. All fungal strains were grown at 18–22°C on the PDA medium and stored on PDA slants at 4–8°C.
To prepare total RNA for high-throughput sRNA sequencing, strain SZ-150 was cultured on cellophane membranes overloading PDA plate for 10 days. The harvested mycelium of SZ-150 was ground into a fine powder in liquid nitrogen with a mortar and pestle, and total RNA was extracted with TRIzol RNA extraction kit (Takara Bio, Inc., Japan). The total RNA was stored at −80°C until analysis. Deep sequencing of sRNA was performed on the Illumina HiSeq 2000 platform by the BGI Tech Company (Shenzhen, China). sRNA molecules (≤30 nt) were isolated and purified by polyacrylamide gel electrophoresis (PAGE). The purified sRNA was ligated with an adaptor, and then reverse-transcribed to cDNA, which was then amplified by polymerase chain reaction (PCR) and recovered by PAGE in a 6% gel for deep sequencing. To obtain clean sequences, the raw reads from deep sequencing were processed to remove the adaptor sequences and discard low-quality reads, and then were assembled into contigs in the Velvet software with a k-mer value of 17 excluding the sRNA generated from S. sclerotiorum. The assembled contigs were employed in searches in the GenBank database2 using BLASTN and BLASTX to find similar sequences.
Reads of vsRNAs were mapped to the virus genome using the Bowtie (1.0) software and only those having sequences identical or complementary to the viral genomic sequence (allowing for one mismatch with the reference genomes) were identified as vsRNAs. Then, the sorted and indexed BAM file was processed by viRome3.
To fill the gap between different contigs, virus-specific primers based on the sequences assembled from sRNAs were designed and used for RT-PCR (Supplementary Table S2). The amplicons were purified and then cloned into the pMD18-T vector (Takara, Dalian, China) for sequencing. Finally, we filled the gap between two different contigs. To obtain the complete cDNA sequences of mycovirus genomes, the terminal sequence was determined by RACE-PCR using the SMARTer RACE 5′/3′ Kit (Cat. No. 634858, Clontech) (Supplementary Table S2).
Sequence assembly and the basic features (e.g., lengths, G+C content, and ORF identification) of the full-length genome sequence were analyzed using the DNAMAN software. The conserved domains of mycovirus genomes were identified on a motif scan website4. The sequences of previously reported mycoviruses and other sequences referenced in this research were downloaded from the NCBI GenBank database5 and used for multiple-alignment and phylogenetic analyses. Multiple alignment was performed using the Clustal X program. On the basis of the aligned sequences, the best-fit evolution models of SsMTV1 and SsBV3 were obtained using Akaike’s information criterion (AIC), and searched using the ProtTest server. BI (Bayesian) trees were constructed with MrBayes-3.2.7, and then the tree files were viewed using FigTree-1.4.0.
To determine the contribution of SsMTV1 and SsBV3 to the hypovirulence in strain SZ-150, the presence of viruses in strains SZ-150, SZ-150/A6, SZ-150/R59, and SZ-150/R6 was analyzed via RT-PCR using specific primers for SsHV1, SsMTV1, SsBV3, and SatH (Supplementary Table S2). Four strains, SZ-150, SZ-150/A6, SZ-150/R59, and SZ-150/R6, were evaluated regarding colony morphology, growth rate, and virulence as previously described (Zhang et al., 2009; Xie et al., 2011). All assays were repeated three times. Experimental data were analyzed in SAS 8.0 software. Treatment means were compared by the least significant difference test at Pn SAS 8.
All datasets generated for this manuscript can be found in GenBank. Accession numbers are listed in the Supplementary Information.
QW, DJ, and JX designed the research and wrote the manuscript. QW, SC, and XX executed the experiments. QW, XX, JC, YF, TC, DJ, and JX performed the data and bioinformatics analyses. All authors read and approved the final manuscript.
This research was financially supported by the National Key Research and Development Program of China (2017YFD0201100), the National Natural Science Foundation of China (31571959 and 31722046), the Fund of Fok Ying Tung Education Foundation (151031), and the earmarked fund of China Agriculture Research System (CARS-13).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01415/full#supplementary-material
Adams, M. J., Candresse, T., Hammond, J., Kreuze, J. F., Martelli, G. P., Namba, S., et al. (2011a). “Alphaflexiviridae,” in Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses, eds A. M. Q. King, M. J. Adams, E. B. Carstens, and E. J. Lefkowitz (San Diego, CA: Elsevier), 904–919. doi: 10.1016/b978-0-12-384684-6.00077-x
Adams, M. J., Kreuze, J. F., and Martelli, G. P. (2011b). “Tymovirales,” in Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses, eds A. M. Q. King, M. J. Adams, E. B. Carstens, and E. J. Lefkowitz (San Diego, CA: Elsevier), 901–903. doi: 10.1016/b978-0-12-384684-6.00076-8
Amselem, J., Cuomo, C. A., van Kan, J. A., Viaud, M., Benito, E. P., Couloux, A., et al. (2011). Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 7:e1002230. doi: 10.1371/journal.pgen.1002230
Arjona-Lopez, J. M., Telengech, P., Jamal, A., Hisano, S., Kondo, H., Yelin, M. D., et al. (2018). Novel, diverse RNA viruses from mediterranean isolates of the phytopathogenic fungus, Rosellinia necatrix: insights into evolutionary biology of fungal viruses. Environ. Microbiol. 20, 1464–1483. doi: 10.1111/1462-2920.14065
Bartholomaus, A., Wibberg, D., Winkler, A., Puhler, A., Schluter, A., Varrelmann, M., et al. (2016). Deep sequencing analysis reveals the mycoviral diversity of the virome of an avirulent isolate of Rhizoctonia solani AG-2-2 IV. PLoS One 11:e0165965. doi: 10.1371/journal.pone.0165965
Bartholomaus, A., Wibberg, D., Winkler, A., Puhler, A., Schluter, A., Varrelmann, M., et al. (2017). Identification of a novel mycovirus isolated from Rhizoctonia solani (AG 2-2 IV) provides further information about genome plasticity within the order Tymovirales. Arch. Virol. 162, 555–559. doi: 10.1007/s00705-016-3085-3
Boland, G. J. (1992). Hypovirulence and double-stranded RNA in Sclerotinia sclerotiorum. Can. J. Plant Pathol. 14, 10–17. doi: 10.1080/07060669209500900
Bolton, M. D., Thomma, B. P., and Nelson, B. D. (2006). Sclerotinia sclerotiorum (Lib). de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 7, 1–16. doi: 10.1111/j.1364-3703.2005.00316.x
Donaire, L., and Ayllon, M. A. (2017). Deep sequencing of mycovirus-derived small RNAs from Botrytis species. Mol. Plant Pathol. 18, 1127–1137. doi: 10.1111/mpp.12466
Ghabrial, S. A., Caston, J. R., Jiang, D., Nibert, M. L., and Suzuki, N. (2015). 50-plus years of fungal viruses. Virology 479, 356–368. doi: 10.1016/j.virol.2015.02.034
Ghabrial, S. A., and Suzuki, N. (2008). “Encyclopedia of virology,” in Fungal viruses, 3rd Edn, Vol. 2, eds B. W. J. Mahy and M. H. V. Van Regenmortel (Elsevier: Oxford), 284–291.
Ghabrial, S. A., and Suzuki, N. (2009). Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 47, 353–384. doi: 10.1146/annurev-phyto-080508-081932
Guo, Z., Li, Y., and Ding, S. W. (2019). Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 19, 31–44. doi: 10.1038/s41577-018-0071-x
Hollings, M. (1962). Viruses associated with a die-back disease of cultivated mushroom. Nature 196, 962–965. doi: 10.1038/196962a0
Howitt, R. L., Beever, R. E., Pearson, M. N., and Forster, R. L. (2001). Genome characterization of botrytis virus F, aflexuous rod-shaped mycovirus resembling plant ‘potex-like’ viruses. J. Gen. Virol. 82, 67–78. doi: 10.1099/0022-1317-82-1-67
Howitt, R. L., Beever, R. E., Pearson, M. N., and Forster, R. L. (2006). Genome characterization of a flexuous rod-shaped mycovirus, botrytis virus X, reveals high amino acid identity to genes from plant ‘potex-like’ viruses. Arch. Virol. 151, 563–579. doi: 10.1007/s00705-005-0621-y
Jiang, D., Fu, Y., Guoqing, L., and Ghabrial, S. A. (2013). Viruses of the plant pathogenic fungus Sclerotinia sclerotiorum. Adv. Virus Res. 86, 215–248. doi: 10.1016/b978-0-12-394315-6.00008-8
King, A. M. Q., Adams, M. J., Carstens, E. B., and Lefkowitz, E. J. (2011). Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier: Amsterdam, 901–952.
Kreuze, J. F., Perez, A., Untiveros, M., Quispe, D., Fuentes, S., Barker, I., et al. (2009). Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: a generic method for diagnosis, discovery and sequencing of viruses. Virology 388, 1–7. doi: 10.1016/j.virol.2009.03.024
Li, K., Zheng, D., Cheng, J., Chen, T., Fu, Y., Jiang, D., et al. (2016). Characterization of a novel Sclerotinia sclerotiorum RNA virus as the prototype of a new proposed family within the order Tymovirales. Virus Res. 219, 92–99. doi: 10.1016/j.virusres.2015.11.019
Li, P., Lin, Y., Zhang, H., Wang, S., Qiu, D., and Guo, L. (2016). Molecular characterization of a novel mycovirus of the family tymoviridae isolated from the plant pathogenic fungus Fusarium graminearum. Virology 489, 86–94. doi: 10.1016/j.virol.2015.12.004
Liu, L., Wang, Q., Cheng, J., Fu, Y., Jiang, D., and Xie, J. (2015). Molecular characterization of a bipartite double-stranded RNA virus and its satellite-like RNA co-infecting the phytopathogenic fungus Sclerotinia sclerotiorum. Front. Microbiol. 6:406. doi: 10.3389/fmicb.2015.00406
Marzano, S. L., and Domier, L. L. (2016). Novel mycoviruses discovered from metatranscriptomics survey of soybean phyllosphere phytobiomes. Virus Res. 213, 332–342. doi: 10.1016/j.virusres.2015.11.002
Marzano, S. L., Nelson, B. D., Ajayi-Oyetunde, O., Bradley, C. A., Hughes, T. J., Hartman, G. L., et al. (2016). Identification of diverse mycoviruses through metatranscriptomics characterization of the viromes of five major fungal plant pathogens. J. Virol. 90, 6846–6863. doi: 10.1128/JVI.00357-16
Mi, S., Cai, T., Hu, Y., Chen, Y., Hodges, E., Ni, F., et al. (2008). Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133, 116–127. doi: 10.1016/j.cell.2008.02.034
Mizutani, Y., Abraham, A., Uesaka, K., Kondo, H., Suga, H., Suzuki, N., et al. (2018). Novel mitoviruses and a unique Tymo-like virus in hypovirulent and virulent strains of the Fusarium head blight fungus, Fusarium boothii. Viruses 10:E584. doi: 10.3390/v10110584
Mu, F., Xie, J., Cheng, S., You, M. P., Barbetti, M. J., Jia, J., et al. (2017). Virome characterization of a collection of S. sclerotiorum from Australia. Front. Microbiol. 8:2540. doi: 10.3389/fmicb.2017.02540
Pearson, M. N., Beever, R. E., Boine, B., and Arthur, K. (2009). Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 10, 115–128. doi: 10.1111/j.1364-3703.2008.00503.x
Ran, H., Liu, L., Li, B., Cheng, J., Fu, Y., Jiang, D., et al. (2016). Co-infection of a hypovirulent isolate of Sclerotinia sclerotiorum with a new botybirnavirus and a strain of a mitovirus. Virol. J. 13:92. doi: 10.1186/s12985-016-0550-2
Ruiz-Ferrer, V., and Voinnet, O. (2009). Roles of plant small RNAs in biotic stress responses. Annu. Rev. Plant Biol. 60, 485–510. doi: 10.1146/annurev.arplant.043008.092111
Schoebel, C. N., Prospero, S., Gross, A., and Rigling, D. (2018). Detection of a conspecific mycovirus in two closely related native and introduced fungal hosts and evidence for interspecific virus transmission. Viruses 10:628. doi: 10.3390/v10110628
Torres-Martinez, S., and Ruiz-Vazquez, R. M. (2017). The RNAi universe in fungi: a varied landscape of small RNAs and biological functions. Annu. Rev. Microbiol. 71, 371–391. doi: 10.1146/annurev-micro-090816-093352
Vainio, E. J., Jurvansuu, J., Streng, J., Rajamäki, M.-L., Hantula, J., Valkonen, J. P. T., et al. (2015). Diagnosis and discovery of fungal viruses using deep sequencing of small RNAs. J. Gen. Virol. 96, 714–725. doi: 10.1099/jgv.0.000003
Wang, H., Li, C., Cai, L., Fang, S., Zheng, L., Yan, F., et al. (2018). The complete genomic sequence of a novel botybirnavirus isolated from a phytopathogenic Bipolaris maydis. Virus Genes 54, 733–736. doi: 10.1007/s11262-018-1584-x
Wang, S., Li, P., Zhang, J., Qiu, D., and Guo, L. (2016). Generation of a high resolution map of sRNAs from Fusarium graminearum and analysis of responses to viral infection. Sci. Rep. 6:26151. doi: 10.1038/srep26151
Wu, L., Zhang, Q., Zhou, H., Ni, F., Wu, X., Qi, Y., et al. (2009). Rice microRNA effector complexes and targets. Plant Cell 21, 3421–3435. doi: 10.1105/tpc.109.070938
Wu, M., Jin, F., Zhang, J., Yang, L., Jiang, D., and Li, G. (2012). Characterization of a novel bipartite double-stranded RNA mycovirus conferring hypovirulence in the phytopathogenic fungus Botrytis porri. J. Virol. 86, 6605–6619. doi: 10.1128/JVI.00292-12
Wu, Q., Luo, Y., Lu, R., Lau, N., Lai, E. C., Li, W. X., et al. (2010). Virus discovery by deep sequencing and assembly of virus-derived small silencing RNAs. Proc. Natl. Acad. Sci. U.S.A. 107, 1606–1611. doi: 10.1073/pnas.0911353107
Xiang, J., Fu, M., Hong, N., Zhai, L., Xiao, F., and Wang, G. (2017). Characterization of a novel botybirnavirus isolated from a phytopathogenic Alternaria fungus. Arch. Virol. 162, 3907–3911. doi: 10.1007/s00705-017-3543-6
Xie, J., and Jiang, D. (2014). New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 52, 45–68. doi: 10.1146/annurev-phyto-102313-050222
Xie, J., Wei, D., Jiang, D., Fu, Y., Li, G., Ghabrial, S., et al. (2006). Characterization of debilitation-associated mycovirus infecting the plant-pathogenic fungus Sclerotinia sclerotiorum. J. Gen. Virol. 87(Pt 1), 241–249. doi: 10.1099/vir.0.81522-0
Xie, J., Xiao, X., Fu, Y., Liu, H., Cheng, J., Ghabrial, S. A., et al. (2011). A novel mycovirus closely related to hypoviruses that infects the plant pathogenic fungus Sclerotinia sclerotiorum. Virology 418, 49–56. doi: 10.1016/j.virol.2011.07.008
Yaegashi, H., Shimizu, T., Ito, T., and Kanematsu, S. (2016). Differential inductions of RNA silencing among encapsidated double-stranded RNA mycoviruses in the white root rot fungus Rosellinia necatrix. J. Virol. 90, 5677–5692. doi: 10.1128/JVI.02951-15
Yu, J., Lee, K. M., Cho, W. K., Park, J. Y., and Kim, K. H. (2018). Differential contribution of RNA interference components in response to distinct Fusarium graminearum virus infections. J. Virol. 92:e01756-17. doi: 10.1128/JVI.01756-17
Keywords: Sclerotinia sclerotiorum, mycovirus, botybirnavirus, Tymoviridae, high-throughput sequencing, small RNA
Citation: Wang Q, Cheng S, Xiao X, Cheng J, Fu Y, Chen T, Jiang D and Xie J (2019) Discovery of Two Mycoviruses by High-Throughput Sequencing and Assembly of Mycovirus-Derived Small Silencing RNAs From a Hypovirulent Strain of Sclerotinia sclerotiorum. Front. Microbiol. 10:1415. doi: 10.3389/fmicb.2019.01415
Received: 24 February 2019; Accepted: 05 June 2019;
Published: 02 July 2019.
Edited by:
Sead Sabanadzovic, Mississippi State University, United StatesReviewed by:
Carmen Hernandez, Polytechnic University of Valencia, SpainCopyright © 2019 Wang, Cheng, Xiao, Cheng, Fu, Chen, Jiang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiatao Xie, amlhdGFveGllQG1haWwuaHphdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.