
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 09 April 2019
Sec. Microbial Immunology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.00734
 Bin Zheng1,2
Bin Zheng1,2 Jianzu Ding1,2
Jianzu Ding1,2 Di Lou1,2
Di Lou1,2 Qunbo Tong1,2
Qunbo Tong1,2 Xunhui Zhuo1,2
Xunhui Zhuo1,2 Haojie Ding1,2
Haojie Ding1,2 Qingming Kong1,2
Qingming Kong1,2 Shaohong Lu1,2*
Shaohong Lu1,2*Toxoplasma gondii causes serious public health problems, but there is no effective treatment strategy against it currently. DNA vaccines have shown promising findings in this regard. MYR1 is a new virulence factor identified in T. gondii that may have potential as a DNA vaccine candidate. We constructed a recombinant eukaryotic plasmid, pVAX1-MYR1, as a DNA vaccine, injected it intramuscularly into BALB/c mice, and evaluated its immunoprotective effects. pVAX1-MYR1 immunization induced a sequential Th1 and Th2 T-cell response, as indicated by high levels of Th1 and mixed Th1/Th2 cytokines at 2 and 6 weeks after immunization, respectively. These findings were corroborated by the antibody assays too. In addition, increased levels of antigen-specific lymphocyte proliferation, CD4+ and CD8+ T lymphocytes, cytotoxic T lymphocyte activity and cytokine (IFN-γ, IL-12, and IL-10) production were also observed in the immunized mice. These findings showed that pVAX1-MYR1 stimulated humoral and cellular immune responses in the immunized mice. The increased production of IFN-γ and IL-12 was correlated with increased expression of the T-bet and p65 genes of the NF-κB pathway. However, no significant increase was observed in the level of IL-4. The survival of mice immunized with pVAX1-MYR1 was also significantly prolonged compared with the control group mice. Based on all the above findings, the current study proposes that pVAX1-MYR1 can induce a T. gondii-specific immune response and should therefore be considered as a promising vaccine candidate against toxoplasmosis. To the best of our knowledge, this is the first report to evaluate the immunoprotective value of an MYR1-based DNA vaccine against T. gondii.
Toxoplasma gondii is an intracellular protozoa that belongs to the phylum Apicomplexa, which has a global distribution and can cause toxoplasmosis in humans as well as animals (Dubey, 2008). More than one-third of the world’s population has chronic T. gondii infection (Pappas et al., 2009). Cats are the only final host of T. gondii, and most warm-blooded mammals, including humans, rodents, and birds, are intermediate hosts (Blader et al., 2015). This protozoan can be transmitted through close contact with cats or a pet, eating raw meat, and exposure to soil (Belluco et al., 2016). In humans, toxoplasmosis is usually asymptomatic in individuals with normal immune function. However, in immunocompromised people, such as those with malignant tumors, human immunodeficiency virus-positive individuals and organ transplant recipients, toxoplasmosis may cause serious and progressive complications with poor prognosis or may even lead to death (White et al., 2014). In addition, pregnant women are also at risk of T. gondii infection (Hunter and Sibley, 2012). During pregnancy, maternal T. gondii infection may have serious consequences such as fetal abortion (Torgerson and Mastroiacovo, 2013).
In general, T. gondii tachyzoites actively invade all nucleated cells of the intermediate host, but their replication is ultimately limited by a protective immune response (Sullivan and Jeffers, 2012). A common primary control measure for toxoplasmosis in humans and animals is chemotherapy. Chemotherapy is administered through a combination of pyrimethamine and sulfadiazine, which have a variety of side effects and may cause toxic allergic reactions in and have teratogenic effects on the fetus; further, these two drugs do not prevent the entry of bradyzoites into the tissue cyst (Antczak et al., 2016). As an another treatment modality, a commercial attenuated vaccine (ToxoVax®, Intervet B.V.) has been used in the veterinary industry in some areas, but the side effects as well as it high cost have limited the use of this vaccine. Thus, at present, there is no effective control strategy to limit toxoplasmosis in humans and many warm-blooded animals around the world (Li and Zhou, 2018).
Effective and safe anti-T. gondii vaccines may be the answer to preventing T. gondii infections. In recent years, a large number of studies have been carried out on T. gondii vaccines, including attenuated vaccines (Wang J.L. et al., 2017, 2018; Xia et al., 2018), subunit vaccines (Zheng et al., 2013; Ching et al., 2016; Sonaimuthu et al., 2016; Wang S. et al., 2017), exosome vaccines (Beauvillain et al., 2009; Li et al., 2018), DNA vaccines (Zhang et al., 2015; Li and Zhou, 2018) and other types of vaccines (Lee et al., 2018; Zhang N.Z. et al., 2018). Many studies have shown that using antigen-encoding DNA as experimental immunogens can effectively induce humoral and cellular immunity against T. gondii. In one of these studies, Zhou et al. reported that the SAG4 DNA vaccine had significant immune responses and improved protection against T. gondii (Zhou and Wang, 2017). Further, Zhang Z. et al. (2018) demonstrated that intense cell-mediated and humoral immunity was triggered and defense against T. gondii was partially induced after administration of the TgROP21 DNA vaccine. In yet another study, Zheng et al. (2017) found that immunization of mice with pVAX1-TgSPATR can produce humoral and cellular immune responses against T. gondii and significantly prolong the survival of mice. Thus, the future of DNA vaccines for the prevention of T. gondii infection looks promising.
In recent years, great progress has been made in identifying candidate vaccines for T. gondii infection that can induce a protective immune response. Of these potential vaccine antigens, T. gondii Myc regulation 1 (MYR1) seems to be particularly promising. MYR1 is a new virulence factor identified in T. gondii. It is a secreted protein that is ultimately localized within the parasitophorous vacuole and at the parasitophorous vacuole membrane, and it plays a key role in infected host cells. During T. gondii infection, MYR1 can upregulate the expression of c-Myc in host cells, mediating the interaction between the host and host cells, for example, by affecting the host cell cycle. In addition, the MYR1 protein is required for T. gondii tachyzoites to regulate several other important signaling pathways in the host, including those mediated by the dense particle effectors GRA16 and GRA24. MYR1 is also important for the transfer of T. gondii effector molecules from parasite vacuoles to the host cytoplasm or nucleus. In a mouse infection model, the virulence of MYR1-knockout T. gondii was found to be severely attenuated, and it did not result in death of the mice (Franco et al., 2016). Moreover, we have used some bioinformatics software to predict that MYR1 show good B-cell and T-cell epitopes (Zhou et al., 2016). These findings indicate that MYR1 may be a great potential vaccine candidate, but no studies have explored this possibility.
The aim of this study was to evaluate the potential of MYR1 as a candidate vaccine against T. gondii infection in mice. We constructed an MYR1 eukaryotic plasmid and intramuscularly administered it to BALB/c mice to evaluate the immunoprotective effect of this DNA vaccine on infection of the BALB/c mouse model with the highly virulent T. gondii RH strain.
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals according to the Animal Ethics Procedures and Guidelines of the People’s Republic of China. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Zhejiang Academy of Medical Sciences.
Six-week-old BALB/c mice were purchased from the Experimental Animal Center of Zhejiang Academy of Medical Sciences, China. All the mice were maintained under specific-pathogen-free standard conditions and the experimental procedures were in accordance with Chinese legislation on the use and care of laboratory animals. T. gondii tachyzoites of the virulent wild-type RH strain (type I) were maintained in our laboratory through serial passage in human foreskin fibroblasts grown in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA, United States) supplemented with 5% fetal bovine serum (Gibco, Carlsbad, CA, United States) in a humidified chamber containing 5% CO2 at 37°C. Soluble tachyzoite antigen was prepared from T. gondii tachyzoites as described previously (Wang et al., 2013).
Total RNA from the tachyzoites of the T. gondii RH strain was extracted by Trizol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s protocol, and the cDNA was constructed. The entire coding sequence of the T. gondii MYR1 (TGGT1_254470) gene was amplified by PCR with synthetic primers for MYR1: forward primer, 5′-GCCGATATCATGCCGCCACAGAATCGTAACG-3′; reverse primer, 5′-GCCGCGGCCGCTCACGAATTATGTGACTGAC-3′ (the introduced EcoRV and NotI recognition sites are underlined). The PCR product of the MYR1 gene was subcloned into the pMD19-T vector (Takara, Dalian, China). MYR1-positive clones were selected for sequencing.
The MYR1 gene was digested with the restriction enzymes corresponding to the EcoRV and NotI sites, and purified from agarose gels. The MYR1 gene fragment was inserted into the eukaryocyte vector pVAX1, which was then named pVAX1-MYR1. The recombinant plasmids were then transformed into Escherichia coli DH5a, and the transformation was confirmed by sequencing. pVAX1-MYR1 was purified using an Endofree plasmid giga kit (Qiagen, Chatsworth, CA, United States). The DNA concentration was determined by absorbance at 260 nm using NanoDrop 2000. Finally, the recombinant plasmid was diluted to a concentration of 1 mg/ml with sterile endotoxin-free phosphate-buffered saline (PBS) and stored at −20°C until use. pVAX1-MYR1 expression was analyzed by transfection of HEK293 cells with the Lipo2000 reagent (Invitrogen, Carlsbad, CA, United States), according to the manufacturer’s instructions. After 48 h, cell monolayers and supernatants were collected and stored at −20°C. Expression of MYR1 in the transfected cells was then analyzed by real-time PCR (RT-PCR), western blotting and indirect immunofluorescence assay.
The pET28a-MYR1 plasmid was constructed using the same method used to construct the pVAX1-MYR1 plasmid. This pET28a-MYR1 plasmid was transformed into E. coli BL21(DE3) and induced to express the rMYR1 protein in vitro using Isopropyl β-D-Thiogalactoside. The rMYR1 protein was purified by affinity chromatography using Ni2+-NTA agarose columns (Qiagen, Hilden, Germany). The purity of the eluted proteins was analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining. rMYR1 was dialyzed against PBS (pH 7.2), filtered throughout a 0.22-μm pore membrane and stored at −70°C until use. To generate polyclonal antibodies against rMYR1, New Zealand experimental rabbits were immunized subcutaneously with 200 μg of the rMYR1 protein emulsified with an equal volume of Freund’s complete adjuvant (Sigma-Aldrich, United Kingdom). Two weeks later, booster immunization was performed after emulsification with 200 μg rMYR1 protein and an equal volume of incomplete Freund’s adjuvant (Sigma-Aldrich, United Kingdom). A second booster immunization was performed after 2 weeks. Two weeks after the second booster immunization, the rabbits were sacrificed and serum was extracted.
Four groups of female BALB/c mice (25 mice per group) were inoculated three times at 2-week intervals through a single subcutaneous injection: Group I received no treatment and served as a healthy control group; Group II was administered 100 μL of sterile PBS and served as the negative control group; Group III was administered 100 μL of PBS containing 100 μg of the empty pVAX1 (vector control group); and Group IV was administered 100 μL of sterile PBS containing 100 μg pVAX1-MYR1. Blood samples from each group of mice were collected before immunization and on days 13, 27, and 41 after first immunization, and serum was stored at −20°C.
Two weeks after the final inoculation, ten mice each from the experimental group and the control group were sacrificed, and their spleens were collected for lymphocyte proliferation assay, cytokine measurement, analysis of T cell subsets and cytotoxic T lymphocyte (CTL) activity assay. Further, 100 tachyzoites were obtained from the highly virulent T. gondii RH strains and were used to intraperitoneally challenge ten immunized mice per group 3 weeks after the final inoculation. Survival was monitored daily.
Indirect ELISA was used to determine mouse serum IgG, IgG1, and IgG2a antibody levels. Briefly, 96-well microtiter plates were coated overnight at 4°C with rMYR1 (1 μg/well). These plates were then washed three times with PBS containing 0.05% Tween-20. Non-specific binding sites were blocked with PBS containing 10% bovine serum albumin and incubated with mouse sera diluted 1:100 with PBS for 1 h at 37°C. HRP-conjugated goat anti-mouse IgG, IgG1, and IgG2a (Abcam, Cambridge, United Kingdom) diluted to 1:10,000 were used to detect the bound antibodies. Immune complexes were revealed with 3,3′,5,5′-Tetramethylbenzidine dihydrochloride as the substrate. An automatic ELISA reader (BioTek, United States) was utilized to measure the absorbance values at 450 nm, and all the tests were run in triplicate.
Two weeks after the final booster injection, spleens were harvested from five mice in each group. Splenocyte suspensions were prepared and cultured in 96-well plates in triplicate at a density of 2 × 105 cells/well in Dulbecco’s modified Eagle’s medium supplemented with fetal bovine serum. Thereafter, the cultures were stimulated with either 10 μg/ml rMYR1 or 5 μg/ml concanavalin A (ConA) (Sigma, St. Louis, MO, United States). According to the manual, the plates were incubated in 5% CO2 at 37°C for 68 h, after which 50 μl of CCK-8 solution (Dojindo, Japan) was added to each well and incubated for 4 h. When the incubation was completed, 200 μl of dimethyl sulfoxide was added to each well before all the contents were discarded, and absorbance was measured at 450 nm with an ELISA reader. The results were expressed as the stimulation index (SI) and were calculated as follows: (OD570 rMYR1/OD570 Control)/(OD570 ConA/OD570 Control).
As described for the lymphocyte proliferation assay, splenocytes harvested from each group were cultured with rMYR1 or medium alone (negative control) in 96-well microtiter plates. According to the manufacturer’s instructions and published methods (Wang J.L. et al., 2017), cell-free supernatant was collected and assessed for the level of IL-4 at 24 h, IL-10 at 72 h, and IFN-γ and IL-12 at 96 h, using commercial ELISA kits (eBioscience, United States). The analysis was performed through three independent experiments.
The expression of the transcription factors p65 and T-bet of the NF-κB pathway was detected by RT-PCR and western blotting, in order to determine their roles in mediating the increased production of T-cell cytokines (e.g., IFN-γ and IL-12). The SYBR Green qPCR Mix was purchased from Bio-Rad (United States). RT-PCR was performed on CFX96 Touch (Bio-Rad). The amplification reactions were performed under the following conditions: 50°C 2 min, 95°C 2 min, 40 cycles of 95°C for 15 s, and 60°C for 30 s. Melting curve analysis was carried out under the following conditions: 1 min at 95°C, 65°C for 2 min, and progressive increase from 65 to 95°C to ensure that a single product was amplified in each reaction. All measurements were run in triplicate. The primers for amplification of p65 and T-bet genes are listed in Table 1.
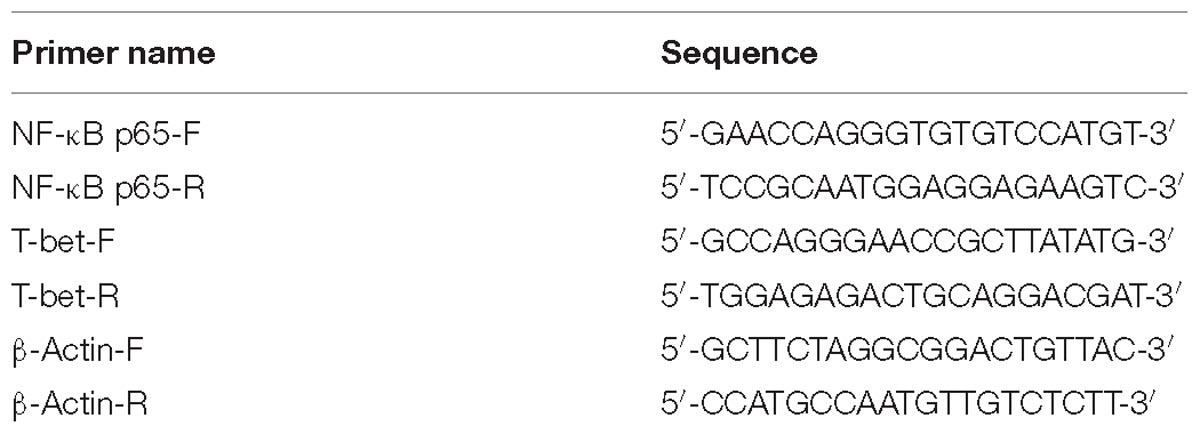
Table 1. RT-PCR primers used to amplify the NF-κB p65, T-bet, and β-actin genes designed by DNASTAR software.
Flow cytometry was used to analyze the percentage of T-cell subsets CD4+ and CD8+ in the splenocytes of mice from the four groups (pVAX1-rMYR1, pVAX1, PBS, and healthy control). Splenocyte suspensions (5 × 105 cells/ml) were dually stained with anti-mouse CD3e-FITC + anti-mouse CD8-PE and anti-mouse CD3e-FITC + anti-mouse CD4-PE (eBioscience, United States) for 30 min at 4°C in the dark. Cell population analysis was conducted with the FACScan flow cytometer using the CellQuest software (BD Biosciences, Franklin Lakes, NJ, United States).
Mouse spleen lymphocytes were obtained and prepared as described in the lymphocyte proliferation experiment. CTL activity was measured using CytoTox96® Non-Radioactive Cytotoxicity Assay Kits (Promega, United States). Briefly, spleen cell cultures were co-cultured with 100 U/ml recombinant murine IL-12 (eBioscience, United States) and used as effector cells. Five days later, mouse Sp2/0 cells were transfected with pVAX1-MYR1 using LipofectamineTM 2000 reagent (Invitrogen, United States) according to the manufacturer’s instructions and used as target cells. The effector cells were mixed with the target cells at a ratio of 10:1, 20:1, 40:1, 80:1, and incubated for 6 h in a humidified chamber at 37°C, 5% CO2. The percentage of specific lysis is calculated as follows: (Experimental – Effector Spontaneous – Target Spontaneous)/(Target Maximum – Target Spontaneous) × 100.
Survival time was analyzed using the Kaplan–Meier method. Differences in the levels of antibodies, lymphocyte proliferation and cytokines between groups were evaluated using independent-sample t-test, with the SSPS statistical analysis software (SPSS Inc., Chicago, IL, United States). p-values that were <0.05 were considered to indicate statistical significance.
The coding sequence of MYR1 was 2199 bp in size and encoded a 733-amino acid protein with a predicted molecular weight of 80.57 kDa (Figure 1A). Most of the recombinant MYR1 was expressed in E. coli as a soluble fusion protein with His-tag when bacterial growth occurred at 37°C. Soluble rMYR1 was purified by Ni affinity chromatography. rMYR1 had a molecular weight of approximately 80 kDa, as determined by SDS-PAGE (Figure 1B). The rabbit polyclonal antibody prepared using rMYR1 specifically recognized native MYR1 in the soluble antigen of T. gondii (Figure 1D).
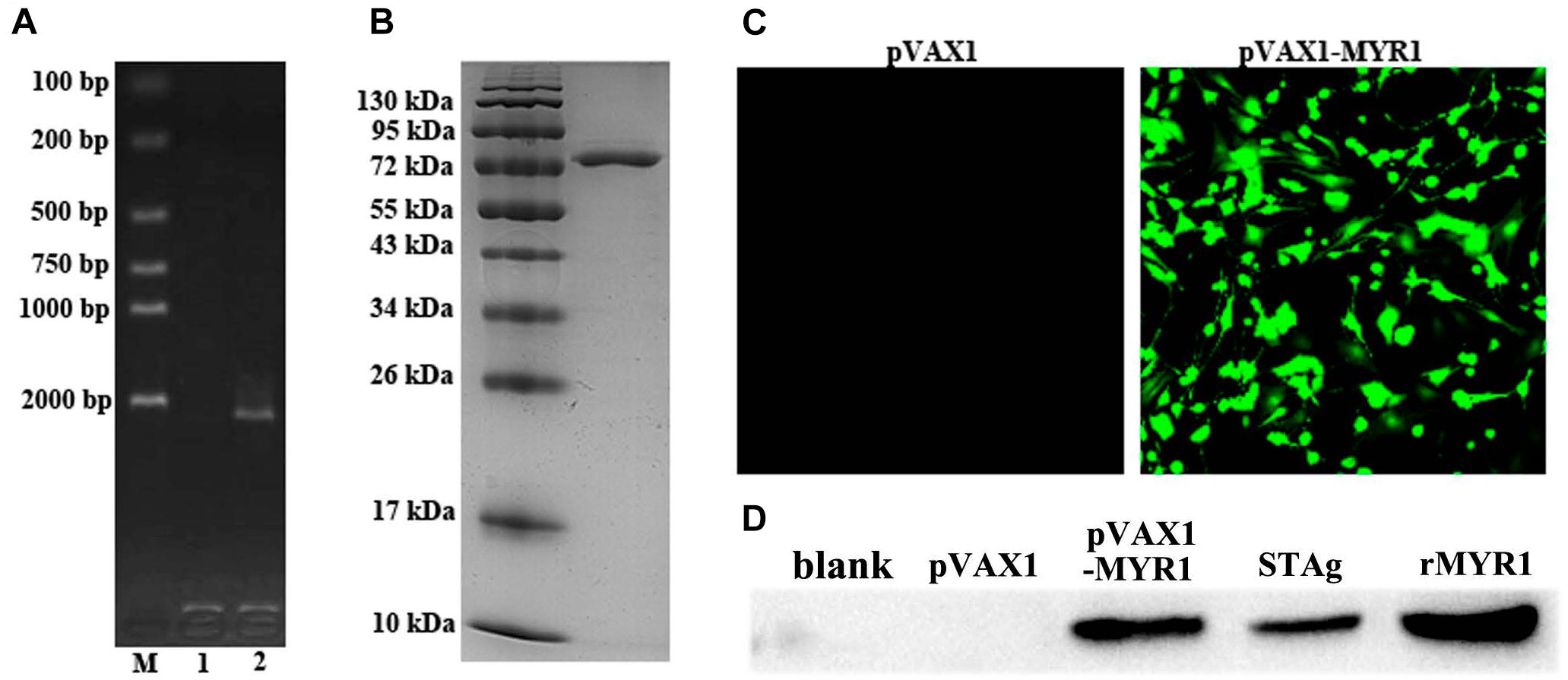
Figure 1. Expression of pET-28a-MYR1 in E. coli BL21 (DE3) and pVAX1-MYR1 in HEK293 cells. (A) Agarose gel analysis of the PCR-amplified myr1 gene. M: Marker, 1: Blank control, 2: T. gondii CDS. (B) SDS-PAGE of recombinant MYR1 protein after purification by Ni-NTA. (C) Immunofluorescence analysis of the expression of pVAX1-MYR1 in HEK293 cells. (D) Western blotting was used to detect the expression of MYR1 in HEK293 cells and STAg. Blank, non-transfected cells; pVAX1, empty pVAX1 transfected cells; pVAX1-MYR1, pVAX1-MYR1-transfected cells; STAg, soluble antigen of T. gondii; rMYR1, positive control.
We evaluated in vitro expression of pVAX1-MYR1 by immunofluorescence assay at 48 h after transfection of HEK293 cells. Cells transfected with pVAX1-MYR1 showed specific green fluorescence, whereas cells transfected with empty pVAX1 did not display cellular immunofluorescence (Figure 1C). The western blotting results revealed a single band (∼80 kDa) of the expected molecular size, indicating that pVAX1-MYR1 was expressed in HEK293 cells (Figure 1D). No protein band was detected in cells transfected with the empty pVAX1 vector and non-transfected cells. This finding indicates that the MYR1 protein was expressed by pVAX1-MYR1 in HEK293 cells.
In order to detect the levels of anti-T. gondii MYR1 antibodies, we tested sera samples from all the groups with ELISA. As shown in Figure 2A, anti-MYR1 antibody was detectable as early as 2 weeks after first inoculation in the pVAX1-MYR1 group: the OD450 value of total IgG antibody was 0.82 ± 0.03 (mean ± SD) at 2 weeks after the last immunization. IgG levels were significantly higher in the serum of mice immunized with pVAX1-MYR1 than in the serum of the control group mice (p < 0.05), and the antibody levels increased with continuous immunization. As expected, no increase in antibody levels was detected in any of three control groups (Figure 2A).
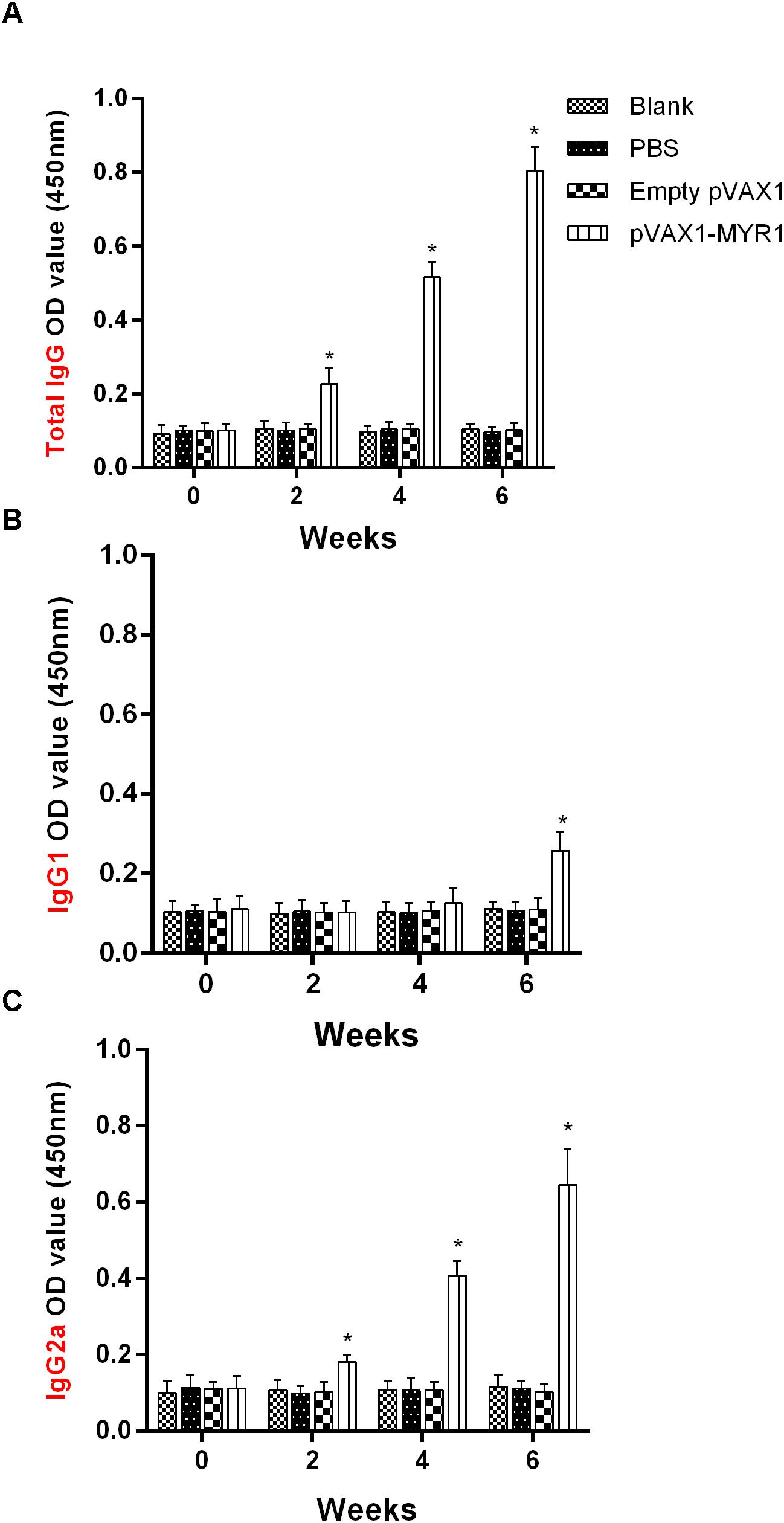
Figure 2. Humoral response in BALB/c mice induced by pVAX1-MYR1 immunization. (A) Total IgG, (B) IgG1, and (C) IgG2a. The total IgG and IgG subclass (IgG1 and IgG2a) titers were determined at 0, 2, 4, and 6 weeks after first vaccination. Data are expressed as the mean ± SD (n = 9) values from three independent experiments. ∗p < 0.05, indicates a significant difference in comparison with the control groups.
The distribution of the IgG subclasses IgG1 and IgG2a was analyzed in the serum of mice immunized with pVAX1-MYR1 using rMYR1 as the coating antigen. Compared to non-vaccinated mice, the level of IgG2a was significantly higher in vaccinated mice at 2, 4, and 6 weeks after first inoculation (p < 0.05) (Figure 2B). In contrast, the level of IgG1 only increased in the vaccinated mice at 6 weeks after vaccination (p < 0.05), as compared with the non-vaccinated mice (Figure 2C). These results indicate that vaccination with pVAX1-MYR1 in mice elicits a Th1-type immune response at 2 weeks after vaccination and a mixed Th1/Th2 immune response at 6 weeks after vaccination.
The lymphocyte proliferation assay was carried out to analyze the proliferation of splenocytes stimulated with rMYR1 or ConA at 2 weeks after the last immunization. A significantly higher SI value was obtained in the pVAX1-MYR1-immunized groups compared with the control groups (p < 0.05) (Table 2). There was no statistically significant difference between the three control groups (p > 0.05). These results showed that the lymphocytes of the vaccinated mice were successfully stimulated.
In order to determine whether pVAX1-MYR1 immunization enhances the Th1 or Th2 cytokine response, IFN-γ, IL-12, IL-4, and IL-10 levels in the supernatants of antigen-stimulated splenic cells were determined (Table 2). Compared to the control groups, significantly higher levels of IFN-γ and IL-12 were produced in the supernatants of restimulated splenocyte cultures from mice immunized with pVAX1-MYR1 (p < 0.05). IFN-γ and IL-12 are Th1-associated cytokines that are critical for protection against T. gondii. The concentration of IFN-γ and IL-12 in the pVAX1-MYR1-immunized BALB/c mice was 839.87 ± 60.39 pg/ml and 405.29 ± 67.81 pg/ml, respectively. In addition, mice immunized with pVAX1-MYR1 also showed significantly higher levels of IL-10 (p < 0.05). However, in the pVAX1-MYR1-immunized group, the concentration of IL-4 did not increase compared to the control groups (p > 0.05). These findings confirm the results for the anti-T. gondii IgG isotype experiment, which showed that immunization with pVAX1-MYR1 induces a mixed Th1/Th2 response.
We further characterized the T-cell subsets by flow cytometry to detect the number of CD3+CD4+CD8− and CD3+CD8+CD4− T cells in the spleens of mice from each group (Table 3). In the mice immunized with pVAX1-MYR1, the levels of CD3+CD4+CD8− T cells was 25.64 ± 3.19% and the levels of CD3+CD8+CD4− T cells was 11.23 ± 1.15%; these amounts were significantly higher than those in the control groups (p < 0.05). As expected, the proportion of these two T-cell subtypes was not significantly different between the three control groups (p > 0.05). These findings indicate that the frequency of CD4+ and CD8+ T cells was augmented after pVAX1-MYR1 immunization.
Expression of the transcription factors T-bet and p65 was detected using RT-PCR and western blotting. We examined the difference in the mRNA level and nuclear protein expression of these two genes between control mice and pVAX1-MYR1-immunized mice. We found that the expression of both T-bet and p65 was significantly higher in pVAX1-MYR1-immunized mice than in the control group (p < 0.05) (Figure 3). These results indicate that T-bet and p65 are induced by pVAX1-MYR1 immunization and are likely to increase the production of IFN-γ and IL-12.
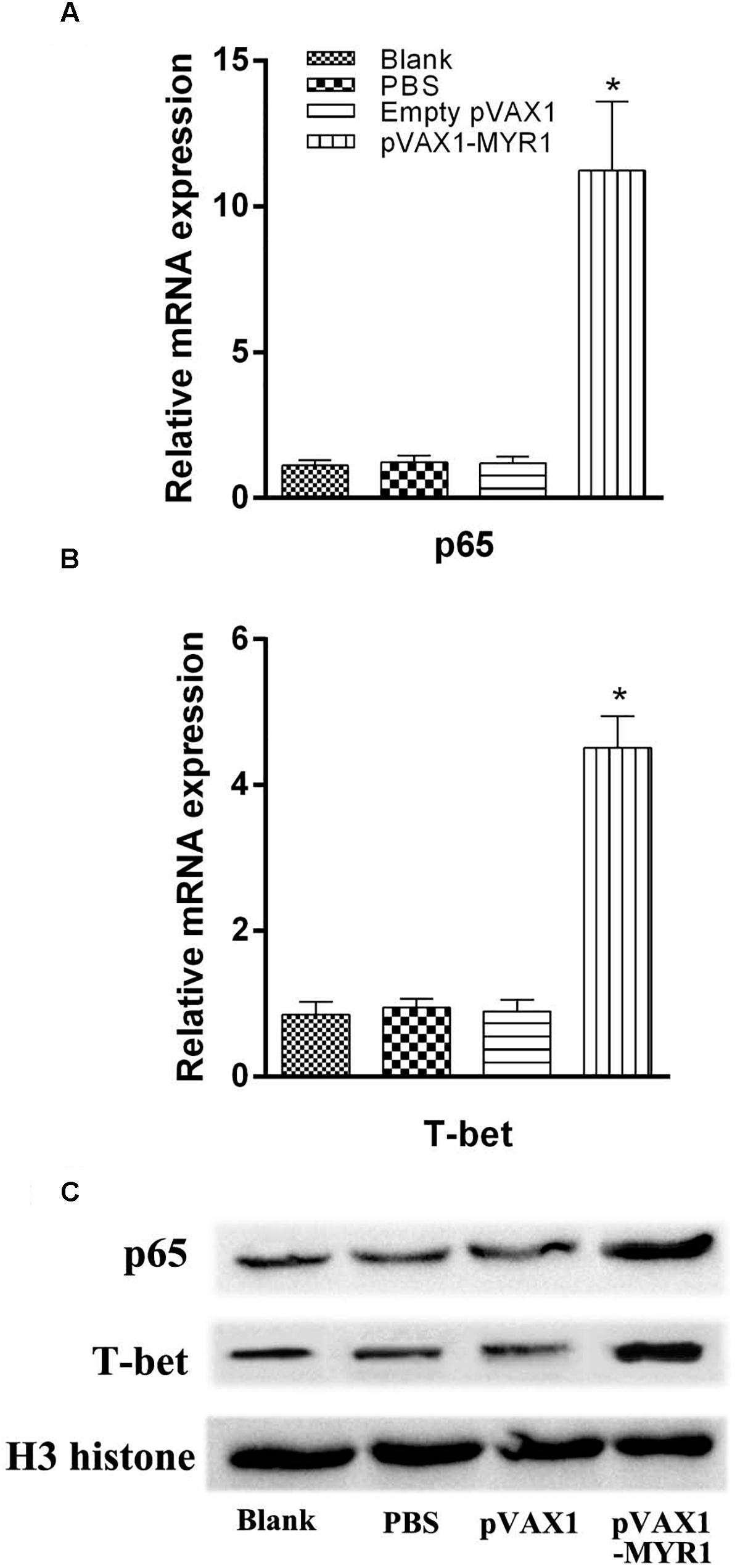
Figure 3. Expression of the transcription factors T-bet and p65. The mRNA and protein expression levels of p65 and T-bet. (A,B) The mRNA levels of p65 and T-bet; (C) The protein levels of p65 and T-bet. Data are expressed as the mean ± SD values (n = 5) from three independent experiments. ∗p < 0.05, indicates a significant difference in comparison with the control groups.
We found that as the ratio of effector cells to target cells gradually increased, CTL activity was gradually enhanced. The highest cytotoxic activity was observed at an effector cell/target cell ratio of 80:1 (Figure 4), and it was significantly higher than that of the control groups (p < 0.05). In addition, CTL activities were not significantly different between the three control groups (p > 0.05).
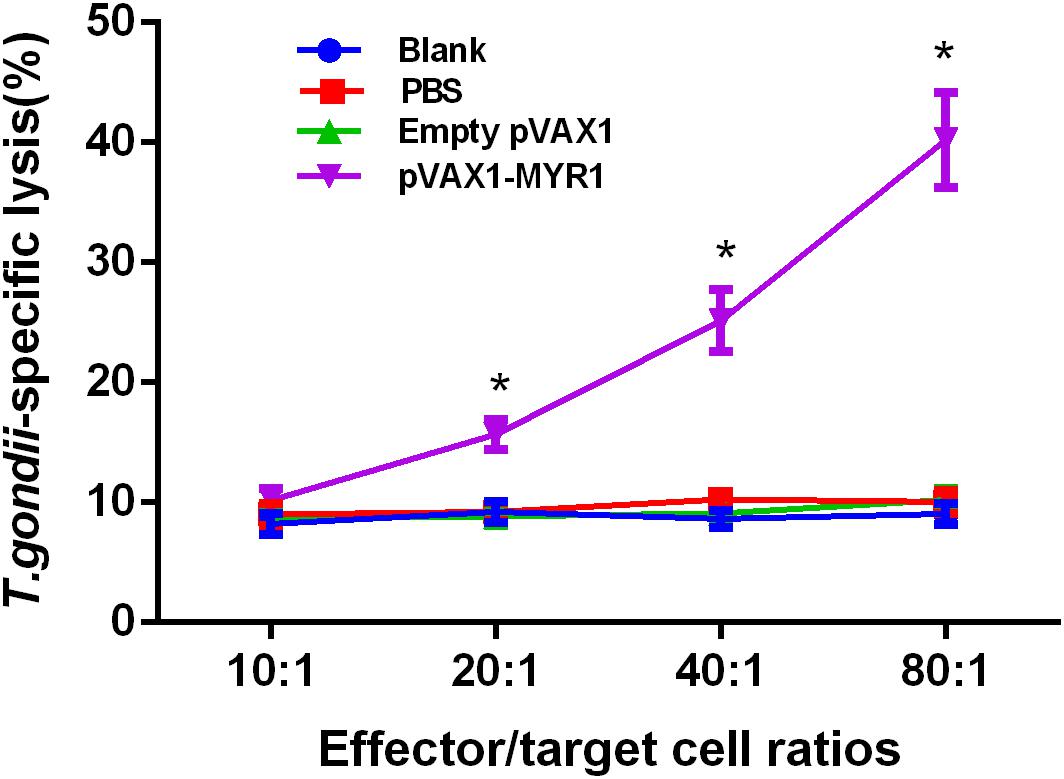
Figure 4. CTL activities of spleen lymphocytes in pVAX1-MYR1-immunized mice. The effector-to-target cell ratios are indicated along the horizontal axis. The vertical axis shows the percentage of T. gondii-specific lysis. Data are expressed as the mean ± SD values (n = 5) from three independent experiments. ∗p < 0.05, indicates a significant difference in comparison with the control groups.
We challenged mice from all the groups with tachyzoites of the highly virulent T. gondii strain RH 3 weeks after inoculation and observed the survival time (Figure 5). No differences were observed between the three control groups with regard to the mean survival time at 6 days after challenge (p > 0.05). A significant increase in survival time was observed in the group immunized with pVAX1-MYR1 compared with the control groups. The average survival time of the pVAX1-MYR1 immunization group was 27.3 ± 6.3 days, while the control group mice died by the 7th day after infection.
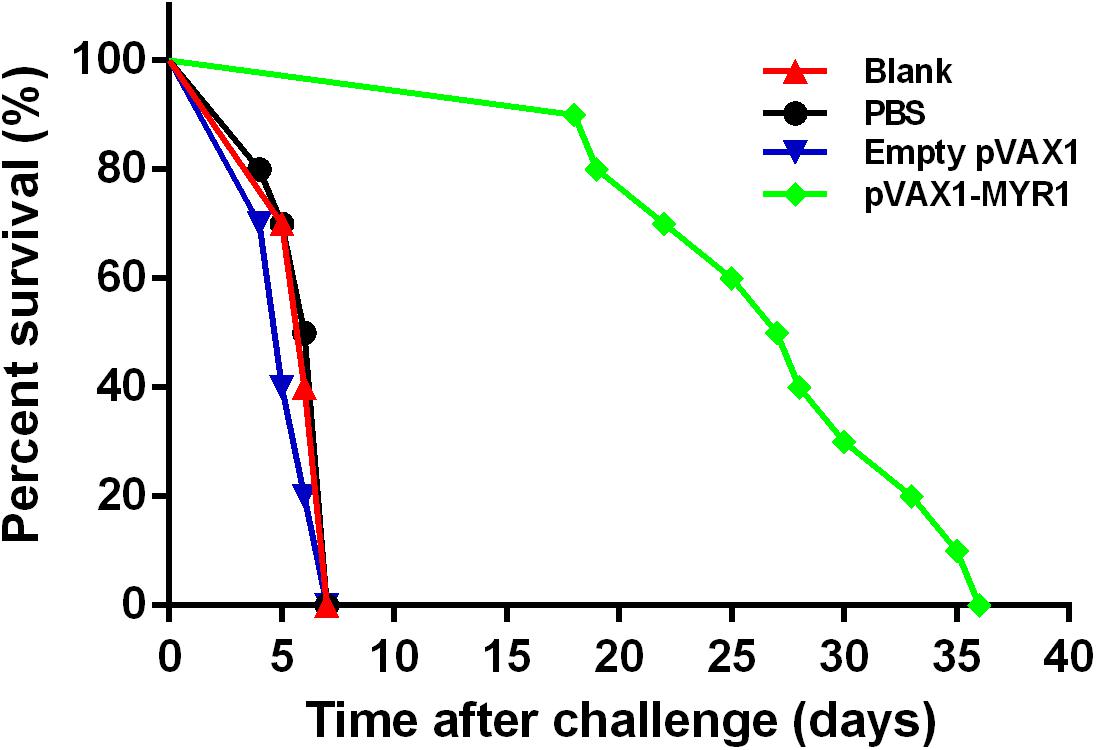
Figure 5. Survival curves of BALB/c mice after challenge. Each group comprised 10 mice. Survival was significantly higher in the pVAX1-MYR1-immunized mice than in the control mice. ∗p < 0.05, indicates a significant difference in comparison with the control groups.
In the present study, we investigated the possibility of using the novel virulence-related T. gondii protein MYR1 as a candidate DNA vaccine to prevent animal toxoplasmosis. We used pVAX1 as a DNA vaccine vector which is consistent with the Food and Drug Administration (FDA) document. The results showed that immunization of mice with pVAX1-MYR stimulated efficiently prolonged protective immunity against the highly virulent RH strain of T. gondii. The humoral response is thought to be critical for the immunity of T. gondii by inhibition of the adsorption on cells and promoting macrophage killing of intracellular parasites (Yoshida et al., 2017). In the present study, mice immunized with pVAX1-MYR1 gradually produced high levels of T. gondii-specific IgG at 2, 4, and 6 weeks after immunization. This antibody plays an important role in preventing subsequent T. gondii infection and controlling cyst reactivation during chronic infection (Lakhrif et al., 2018). Many studies have demonstrated that a Th1-biased response is required for effective protection against naturally occurring T. gondii infections (Yarovinsky, 2014). We therefore determined the antibody types triggered by pVAX1-MYR1 immunization by assaying the antibody subclasses IgG1 and IgG2a. pVAX1-MYR1-immunized mice displayed IgG2a as the primary antibody class during early infection; later, upregulation of IgG1 was observed in the chronic phase, possibly as a result of the conversion of Th1 to Th2. Two weeks after immunization, we observed high IgG2a antibody titers, which indicate induction of a Th1 immune response. However, the IgG1 antibody levels only increased 6 weeks after immunization, indicating induction of a mixed Th1/2 immune response. These results demonstrate that the anti-T. gondii IgG2a and IgG1 antibodies are upregulated in sequence. These results are confirmed by the results of the cytokine assay conducted on spleen cell culture supernatants, in which the levels of Th1 cytokines (IFN-γ and IL-12) and Th2 cytokines (IL-10) in mice immunized with pVAX1-MYR1 were significantly higher than those in the control mice; however, IL-4 production was not induced. Altogether, our findings indicate that IgG2a is triggered by IFN-γ in mice, and that a bias in Th1 response is associated with early IFN-γ production. These results are similar to those of previous research (Wang J.L. et al., 2017, 2018). Our results thus indicate that immunization with pVAX1-MYR1 promotes both cell (Th1)- and humoral (Th2)-mediated immune responses in BABL/c mice.
Increased production of Th1 cytokines is a key mechanism for an effective immune response against T. gondii infection (Pifer and Yarovinsky, 2011). During natural T. gondii invasion, the level of IFN-γ determines the fate of the infection. IFN-γ can inhibit the replication of T. gondii in infected cells through various mechanisms, including induction of the inhibitory protein guanamine 2,3-dioxygenase (IDO), inducible nitric oxide synthase (iNOS) and the effector proteins immunity-related GTPases (IRGs) and guanylate-binding proteins (GBPs). IDO depletes tryptophan, which is necessary for the growth of T. gondii. iNOS is capable of producing the highly toxic metabolite nitric oxide and consuming arginine, which is also necessary for the growth of T. gondii, to restrict parasite replication. IRGs and GBPs can destroy the parasitophorous vacuole, which once damaged leads to the elimination of T. gondii from the cytoplasm of the infected cells (Sturge and Yarovinsky, 2014; Yarovinsky, 2014). In the present study, in vitro re-stimulation of spleen lymphocytes with rMYR1 resulted in a significant increase in IFN-γ and IL-12 production in pVAX1-MYR1-immunized mice and also improved survival. These results indicate that the pVAX1-MYR1 vaccine can trigger increased IFN-γ and IL-12 production via the Th1-type immune response and help to effectively prevent acute T. gondii infection. In our study, an increase in IL-10 levels was also observed in pVAX1-MYR1-immunized mice. IL-10 functions as a regulatory cytokine that is very important for the prevention of immunopathological damage caused by high levels of IFN-γ as part of the Th1 response. The Th1/Th2 cytokine balance in the pVAX1-MYR1-immunized mice was adequate to control the dissemination of tachyzoites, but not excessive enough to elicit significant inflammation. Thus, the high IL-10 levels contributed to longer survival time in the pVAX1-MYR1-immunized mice (Wang J.L. et al., 2017, 2018). However, the levels of IL-4 in pVAX1-MYR1-immunized mice was similar to those in the controls; this means that immunization with this DNA vaccines did not promote sufficient B cell proliferation and mast cell responses (Zhou and Wang, 2017). This finding also explains why the pVAX1-MYR1 vaccine did not provide complete protection from acute T. gondii infection. In the present study, we observed increased IL-12 levels in the immunized mice. IL-12 is important for IFN-γ production, which in turn induces the differentiation of Th1 T lymphocytes and possibly CD8+ and NK cells to control T. gondii infection. It was reported that inhibition of IL-12 expression resulted in 100% mortality in mice infected with T. gondii (Morgado et al., 2014). The NF-κB pathway plays a role in inducing IL-12 secretion in the response to toxoplasmosis (Jensen et al., 2011). In the present study, a significant increase in the expression level of the transcription factor p65 of the NF-κB pathway was observed in pVAX1-MYR1-immunized mice compared to mice in the control group. This indicates activation of the NF-κB pathway as an additional mechanism for increased IFN-γ production in immunized mice. In addition, the expression level of the transcription factor T-bet was also significantly increased in the pVAX1-MYR1-immunized mice. This indicates that increased T-bet-mediated activation of CD4+ T cells and natural killer cells may also play a role in increased IFN-γ production (Zhu et al., 2012).
Given the intracellular localization of T. gondii, specific T lymphocyte activation (CD4+ T cells and CD8+ T cells) may play an important role in controlling the spread and development of T. gondii infection. CD8+ T cell-mediated CTL activity is particularly important in this regard (Kemball et al., 2007; Dupont et al., 2012). In the present study, we found that a significantly higher level of splenocyte proliferation was induced by pVAX1-MYR1 immunization; this indicates that an activated cellular immune response was induced in the immunized mice. In addition, the levels of both CD4+ and CD8+ T cells were also significantly increased in the immunized mice. Thus, all these immune cells may play a synergistic role in the response against T. gondii, and may also be responsible for the increased secretion of IFN-γ. Moreover, T. gondii-specific CD8+ CTL activity plays an important role in controlling T. gondii replication and clearance. Therefore, T. gondii-specific CTL induction is an important strategy for the development of effective T. gondii vaccines. However, in previous studies on T. gondii vaccines, detection of CTL activity was rarely found. In the present study, we found that mouse spleen cells immunized with pVAX1-MYR1 and stimulated again with rMYR1 were able to induce high levels of CTL activity. Taken together, the results show that the pVAX1-MYR1 DNA vaccine can induce strong humoral and cellular immune responses against T. gondii.
The survival rate of vaccinated mice against T. gondii challenge is considered as the most direct indicator of the effectiveness of a candidate vaccine. Therefore, 3 weeks after the last immunization with pVAX1-MYR1, we conducted a survival assessment by infecting the vaccinated mice intraperitoneally with tachyzoites from the highly virulent T. gondii RH strain. Although the mice did not survive after the infection, survival time was significantly and efficiently increased in the mice immunized with pVAX1-MYR1. Thus, the pVAX1-MYR1 vaccine may have potential for preventing T. gondii infection. In future experiments, it would be helpful to evaluate the efficacy of pVAX1-MYR1 immunization by comparing the brain tissue cyst burden in vaccinated and control groups by using low virulence strains of T. gondii and analyzing the potential immune response mechanisms involved.
We have analyzed the efficacy of a new T. gondii DNA vaccine, pVAX1-MYR1, which expresses a novel virulence-related T. gondii protein, MYR1. Our results indicate that intramuscular immunization of BABL/c mice with pVAX1-MYR1 can generate an immunoprotective response against T. gondii infection and also increase survival time. Thus, pVAX1-MYR1 may have potential as a promising candidate vaccine against T. gondii infection.
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals according to the Animal Ethics Procedures and Guidelines of the People’s Republic of China. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Zhejiang Academy of Medical Sciences.
BZ, SL, XZ, HD, DL, and QK developed the study protocol. BZ, JD, QT, and DL did the experiments. BZ analyzed the data and wrote the manuscript. SL revised the report. All authors read and approved the final manuscript.
This research was supported by National Natural Science Foundation of China (Grant Nos. 81702028 and 81871684), Natural Science Foundation of Zhejiang Province of China (LQ17H190007), and Zhejiang Medical and Health Science and Technology Plan (2018KY347).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the native English speaking scientists of Elixigen Company (Huntington Beach, CA, United States) for editing our manuscript.
Antczak, M., Dzitko, K., and Dlugonska, H. (2016). Human toxoplasmosis-searching for novel chemotherapeutics. Biomed. Pharmacother. 82, 677–684. doi: 10.1016/j.biopha.2016.05.041
Beauvillain, C., Juste, M. O., Dion, S., Pierre, J., and Dimier-Poisson, I. (2009). Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine 27, 1750–1757. doi: 10.1016/j.vaccine.2009.01.022
Belluco, S., Mancin, M., Conficoni, D., Simonato, G., Pietrobelli, M., and Ricci, A. (2016). Investigating the determinants of Toxoplasma gondii prevalence in meat: a systematic review and meta-regression. PLoS One 11:e0153856. doi: 10.1371/journal.pone.0153856
Blader, I. J., Coleman, B. I., Chen, C. T., and Gubbels, M. J. (2015). Lytic cycle of Toxoplasma gondii: 15 years later. Annu. Rev. Microbiol. 69, 463–485. doi: 10.1146/annurev-micro-091014-104100
Ching, X. T., Fong, M. Y., and Lau, Y. L. (2016). Evaluation of immunoprotection conferred by the subunit vaccines of GRA2 and GRA5 against acute toxoplasmosis in BALB/c mice. Front. Microbiol. 7:609. doi: 10.3389/fmicb.2016.00609
Dubey, J. P. (2008). The history of Toxoplasma gondii–the first 100 years. J. Eukaryot. Microbiol. 55, 467–475. doi: 10.1111/j.1550-7408.2008.00345.x
Dupont, C. D., Christian, D. A., and Hunter, C. A. (2012). Immune response and immunopathology during toxoplasmosis. Semin. Immunopathol. 34, 793–813. doi: 10.1007/s00281-012-0339-3
Franco, M., Panas, M. W., Marino, N. D., Lee, M. C., Buchholz, K. R., Kelly, F. D., et al. (2016). A novel secreted protein, MYR1, is central to toxoplasma’s manipulation of host cells. mBio 7:e02231-15. doi: 10.1128/mBio.02231-15
Hunter, C. A., and Sibley, L. D. (2012). Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10, 766–778. doi: 10.1038/nrmicro2858
Jensen, K. D., Wang, Y., Wojno, E. D., Shastri, A. J., Hu, K., Cornel, L., et al. (2011). Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host. Microbe 9, 472–483. doi: 10.1016/j.chom.2011.04.015
Kemball, C. C., Pack, C. D., Guay, H. M., Li, Z. N., Steinhauer, D. A., Szomolanyi-Tsuda, E., et al. (2007). The antiviral CD8+ T cell response is differentially dependent on CD4+ T cell help over the course of persistent infection. J. Immunol. 179, 1113–1121. doi: 10.4049/jimmunol.179.2.1113
Lakhrif, Z., Moreau, A., Herault, B., Di-Tommaso, A., Juste, M., Moire, N., et al. (2018). Targeted delivery of Toxoplasma gondii antigens to dendritic cells promote immunogenicity and protective efficiency against toxoplasmosis. Front. Immunol. 9:317. doi: 10.3389/fimmu.2018.00317
Lee, S. H., Kang, H. J., Lee, D. H., Kang, S. M., and Quan, F. S. (2018). Virus-like particle vaccines expressing Toxoplasma gondii rhoptry protein 18 and microneme protein 8 provide enhanced protection. Vaccine 36, 5692–5700. doi: 10.1016/j.vaccine.2018.08.016
Li, Y., Liu, Y., Xiu, F., Wang, J., Cong, H., He, S., et al. (2018). Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses. Int. J. Nanomed. 13, 467–477. doi: 10.2147/IJN.S151110
Li, Y., and Zhou, H. (2018). Moving towards improved vaccines for Toxoplasma gondii. Expert Opin. Biol. Ther. 18, 273–280. doi: 10.1080/14712598.2018.1413086
Morgado, P., Sudarshana, D. M., Gov, L., Harker, K. S., Lam, T., Casali, P., et al. (2014). Type II Toxoplasma gondii induction of CD40 on infected macrophages enhances interleukin-12 responses. Infect. Immun. 82, 4047–4055. doi: 10.1128/IAI.01615-14
Pappas, G., Roussos, N., and Falagas, M. E. (2009). Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 39, 1385–1394. doi: 10.1016/j.ijpara.2009.04.003
Pifer, R., and Yarovinsky, F. (2011). Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol. 27, 388–393. doi: 10.1016/j.pt.2011.03.009
Sonaimuthu, P., Ching, X. T., Fong, M. Y., Kalyanasundaram, R., and Lau, Y. L. (2016). Induction of protective immunity against toxoplasmosis in BALB/c mice vaccinated with Toxoplasma gondii rhoptry-1. Front. Microbiol. 7:808. doi: 10.3389/fmicb.2016.00808
Sturge, C. R., and Yarovinsky, F. (2014). Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection. Infect. Immun. 82, 3090–3097. doi: 10.1128/IAI.01722-14
Sullivan, W. J. Jr., and Jeffers, V. (2012). Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol. Rev. 36, 717–733. doi: 10.1111/j.1574-6976.2011.00305.x
Torgerson, P. R., and Mastroiacovo, P. (2013). The global burden of congenital toxoplasmosis: a systematic review. Bull. World Health Organ. 91, 501–508. doi: 10.2471/BLT.12.111732
Wang, J. L., Elsheikha, H. M., Zhu, W. N., Chen, K., Li, T. T., Yue, D. M., et al. (2017). Immunization with Toxoplasma gondii GRA17 deletion mutant induces partial protection and survival in challenged mice. Front. Immunol. 8:730. doi: 10.3389/fimmu.2017.00730
Wang, S., Zhang, Z., Wang, Y., Gadahi, J. A., Xu, L., Yan, R., et al. (2017). Toxoplasma gondii elongation factor 1-alpha (TgEF-1alpha) is a novel vaccine candidate antigen against toxoplasmosis. Front. Microbiol. 8:168. doi: 10.3389/fmicb.2017.00168
Wang, J. L., Li, T. T., Elsheikha, H. M., Chen, K., Cong, W., Yang, W. B., et al. (2018). Live attenuated Pru: deltacdpk2 strain of Toxoplasma gondii protects against acute, chronic, and congenital toxoplasmosis. J. Infect. Dis. 218, 768–777. doi: 10.1093/infdis/jiy211
Wang, S., Fang, Z., Huang, X., Luo, X., Fang, Z., Gong, N., et al. (2013). The soluble tachyzoite antigen of Toxoplasma gondii has a protective effect on mouse allografts. Trans. Proc. 45, 677–683. doi: 10.1016/j.transproceed.2012.02.036
White, M. W., Radke, J. R., and Radke, J. B. (2014). Toxoplasma development - turn the switch on or off? Cell Microbiol. 16, 466–472. doi: 10.1111/cmi.12267
Xia, N., Zhou, T., Liang, X., Ye, S., Zhao, P., Yang, J., et al. (2018). A lactate fermentation mutant of toxoplasma stimulates protective immunity against acute and chronic toxoplasmosis. Front. Immunol. 9:1814. doi: 10.3389/fimmu.2018.01814
Yarovinsky, F. (2014). Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 14, 109–121. doi: 10.1038/nri3598
Yoshida, N., Frickel, E. M., and Mostowy, S. (2017). Macrophage-microbe interactions: lessons from the zebrafish model. Front. Immunol. 8:1703. doi: 10.3389/fimmu.2017.01703
Zhang, N. Z., Gao, Q., Wang, M., Elsheikha, H. M., Wang, B., Wang, J. L., et al. (2018). Immunization with a DNA vaccine cocktail encoding TgPF, TgROP16, TgROP18, TgMIC6, and TgCDPK3 genes protects mice against chronic toxoplasmosis. Front. Immunol. 9:1505. doi: 10.3389/fimmu.2018.01505
Zhang, Z., Li, Y., Wang, M., Xie, Q., Li, P., Zuo, S., et al. (2018). Immune protection of rhoptry protein 21 (ROP21) of Toxoplasma gondii as a DNA vaccine against toxoplasmosis. Front. Microbiol. 9:909. doi: 10.3389/fmicb.2018.00909
Zhang, N. Z., Xu, Y., Wang, M., Petersen, E., Chen, J., Huang, S. Y., et al. (2015). Protective efficacy of two novel DNA vaccines expressing Toxoplasma gondii rhomboid 4 and rhomboid 5 proteins against acute and chronic toxoplasmosis in mice. Expert Rev. Vaccines 14, 1289–1297. doi: 10.1586/14760584.2015.1061938
Zheng, B., Ding, J., Chen, X., Yu, H., Lou, D., Tong, Q., et al. (2017). Immuno-efficacy of a T. gondii secreted protein with an altered thrombospondin repeat (TgSPATR) as a novel DNA vaccine candidate against acute toxoplasmosis in BALB/c mice. Front. Microbiol. 8:216. doi: 10.3389/fmicb.2017.00216
Zheng, B., Lu, S., Tong, Q., Kong, Q., and Lou, D. (2013). The virulence-related rhoptry protein 5 (ROP5) of Toxoplasma Gondii is a novel vaccine candidate against toxoplasmosis in mice. Vaccine 31, 4578–4584. doi: 10.1016/j.vaccine.2013.07.058
Zhou, J., Lu, G., and He, S. (2016). Analysis of structures and epitopes of a novel secreted protein MYR1 in Toxoplasma gondii. Folia Parasitol. 63:2016.028. doi: 10.14411/fp.2016.028
Zhou, J., and Wang, L. (2017). SAG4 DNA and peptide vaccination provides partial protection against T. gondii infection in BALB/c mice. Front. Microbiol. 8:1733. doi: 10.3389/fmicb.2017.01733
Keywords: Toxoplasma gondii, immunization, DNA vaccine, MYR1, Th1/Th2 cytokines
Citation: Zheng B, Ding J, Lou D, Tong Q, Zhuo X, Ding H, Kong Q and Lu S (2019) The Virulence-Related MYR1 Protein of Toxoplasma gondii as a Novel DNA Vaccine Against Toxoplasmosis in Mice. Front. Microbiol. 10:734. doi: 10.3389/fmicb.2019.00734
Received: 23 January 2019; Accepted: 25 March 2019;
Published: 09 April 2019.
Edited by:
Juarez Antonio Simões Quaresma, Instituto Evandro Chagas, BrazilReviewed by:
Parthasarathy Sonaimuthu, Children’s National Health System, United StatesCopyright © 2019 Zheng, Ding, Lou, Tong, Zhuo, Ding, Kong and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohong Lu, bHNoQHpqYW1zLmNvbS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.