- Birbal Sahni Institute of Palaeosciences, Lucknow, India
Isolated elongate spore-like cells present in the >1600 Ma-old Salkhan Limestone of the Semri Group, Vindhayan Supergroup, India are considered akinetes of the heterocystous cyanobacteria. Small to large size, and young (single walled) to mature (double walled) akinetes – namely, Archaeoellipsoides bactroformis, A. conjuctivus, A. dolichos, A. elongatus, A. grandis, A. major and A. minor – found in the stromatolitic and bedded cherts have been reported in the present paper. Their role in understanding extreme environmental conditions is a subject matter of this paper. Additionally, the occurrence of doubly-terminated quartz crystals and fan-fabrics in the Salkhan Limestone indicates adverse conditions for the survival of life forms. The depositional environment of the Salkhan Limestone, Vindhyan Supergroup is suggested to be shallow marine intertidal with pulses of the intermittent hypersaline regime during which akinetes, closely resembling those of extant Nostocaceans, were formed by cyanobacteria for survival in the extreme conditions.
Introduction
The origin of varied life forms during the Proterozoic Eon (2500-541 Ma) and their biology, ecology, and preservation in the different depositional environments are a subject of great interest to Precambrian paleobiologists. Some of these issues and associated processes are being investigated to understand life forms in extreme conditions as proxies for and signatures of life on other planetary bodies. Heterocystous fossil cyanobacteria and their various degradational states are potentially useful for inferring the prevailing extreme environmental conditions in the geological past. Documentation of silicified microfossils in the Precambrian Gunflint chert of Canada is considered to be the beginning of the Precambrian paleobiology (Tyler and Barghoorn, 1954). Initial studies conducted on Precambrian successions across the globe demonstrated that silicified microfossils entombed in the chert are conspicuously dominated by filamentous and coccoid microfossils, most of which are comparable to modern cyanobacteria (Barghoorn and Tyler, 1965; Schopf, 1968; Schopf and Blacic, 1971; Knoll, 1982, 1984, see Sergeev et al., 2012 for synthesis of such studies). These studies are, however, primarily based on a taxonomic approach; therefore, the evolutionary inferences drawn from these studies are few. Subsequently, paleoenvironmental interpretations based on microfossil assemblages were also recorded (Golubic, 1973, 1976; Golubic and Hofmann, 1976; Hofmann, 1976; Golubic and Barghoorn, 1977; Golubic and Campbell, 1979; Horodyski and Donaldson, 1980, 1983; Knoll, 1985a; Green et al., 1988, 1989; Knoll and Golubic, 1992; Golubic and Seong-Joo, 1999; Sharma and Sergeev, 2004; Sharma, 2006a,b).
The Precambrian period is generally known as the most crucial time in geological history for understanding the growth and evolution of surviving life forms, especially those in extreme environmental conditions (Young, 2013). Studies conducted on the Salkhan Limestone of the Semri Group in India demonstrated the diversity of life forms thriving during the deposition of the Salkhan Limestone and suggested how environmental conditions prevailed during the late Paleoproterozoic period (Kumar, 1978a,b; McMenamin et al., 1983; Venkatachala et al., 1990; Kumar and Srivastava, 1992a,b, 1995; Sharma and Sergeev, 2004; Prasad et al., 2005; Srivastava, 2005; Sharma, 2006a,b; Sergeev et al., 2008; Srivastava and Tewari, 2011). However, the relation of these life forms to extreme environmental conditions is largely unknown and requires a thorough explanation.
Well-preserved cyanobacterial remains in the form of microfossils in the chert samples of the Salkhan Limestone, Vindhyan Supergroup have been recorded (Kumar and Srivastava, 1995). In extant aquatic environments, both marine and freshwater, some cyanobacteria develop two specialized types of cells: (i) heterocysts, which help fix atmospheric nitrogen and make cyanobacteria important for N2-fixation, and (ii) akinetes, which act as a survival strategy and become dormant cells that form under adverse conditions, re-germinating to form new cyanobacterial cells upon the onset of more favorable conditions (Adams and Duggan, 1999; Pinto et al., 2016). As such, akinetes are huge, resting-state cyanobacterial cells that are surrounded by a thick cell wall and a multilayered extracellular envelope (Nichols and Adams, 1982; Herdman, 1987, 1988). Therefore, the occurrence of akinetes in any depositional environment can potentially be used to interpret adverse/extreme environmental conditions in the geological past (Tomitani et al., 2006). Studies conducted on the microfossil assemblage documented from the chert samples of the Salkhan Limestone of the Newari locality were characterized by the presence of different types of akinetes (Srivastava, 2005; Sharma, 2006b); however, the occurrences of these akinetes and their relation to extreme environmental conditions during the late Paleoproterozoic period is yet to be explained thoroughly. We present here the data on akinetes recorded in the thin sections of the chert samples of the Salkhan Limestone, Vindhyan Supergroup, India. Additional data on the depositional environment, associated minerals, and petrological studies on the Salkhan Limestone helped to understand prevailing extreme environmental conditions and the strategies adopted by the akinetes for their survival. The conspicuous occurrence of authigenic doubly-terminated quartz crystals in the surroundings of akinete-bearing cherts is intriguing. The authigenic doubly-terminated quartz crystals are widely reported from different geological settings worldwide and considered to form under evaporitic conditions (Tarr and Lonsdale, 1929; Folk, 1952; Grimm, 1962; Wilson, 1966; Zenger, 1976; Ulmer-Scholle et al., 1993; Chafetz and Zhang, 1998; Albright and Lueth, 2003).
Study Area
The Vindhyan Supergroup is situated in central India and is characterizes by a 4500–5000-m-thick sedimentary succession belonging to the Proterozoic Era. This mostly undeformed and unmetamorphosed thick succession represents a sequence of sandstone, shale, limestone, and dolomite with minor conglomerate as well as volcanoclastic rocks (Auden, 1933). The Vindhyan Supergroup is exposed over a large area from Bihar (Sasaram) in the east to Chittorgarh, Rajasthan in the west, and Dholpur, Rajasthan in the north to Hoshangabad (Madhya Pradesh) in the southwest (Figure 1). The outcrops of the Vindhyan Supergroup are present between the Archaean Aravalli-Bundelkhand province (to the north and east) and the Cretaceous Deccan Traps (to the south and bounded by the Great Boundary Fault to the west) (Mazumder et al., 2000). Lithostratigraphically, the Vindhyan Supergroup represents four groups, namely the Semri Group, the Kaimur Group, the Rewa Group, and the Bhander Group. The Semri Group is considered as the Lower Vindhyans, whereas the Kaimur, Rewa, and Bhander Groups together constitute the Upper Vindhyans. The Kheinjua Subgroup, part of the Semri Group, is lithostratigraphically divided into the three formations, namely the Olive Shale, the Salkhan Limestone, and the Glauconitic Sandstone (Figure 2). In a broader context, the Vindhyan Basin is further divided into two parts which are the eastern part and the western part. The eastern part is known as the Son Valley Section, whereas the western part is the Chambal Valley Section. The best exposure of the Semri Group is in the Son Valley area, from east to west, Rohtas district, Bihar, Sonbhadra district, Uttar Pradesh (Figure 2), and Satna district, Madhya Pradesh. The Salkhan Limestone is a ∼90-m-thick unit of fawn and dark grayish color, mainly constituted of dolomitic, siliceous limestone, and chert bands. The Vindhyan basin is believed to be deposited under the shallow marine environment (Chakraborty et al., 2012 and references therein). More specifically, the depositional environment of the Salkhan Limestone of the Semri Group has been proposed to be intertidal to a supratidal environment based on the microbial assemblages (Sharma, 2006a).
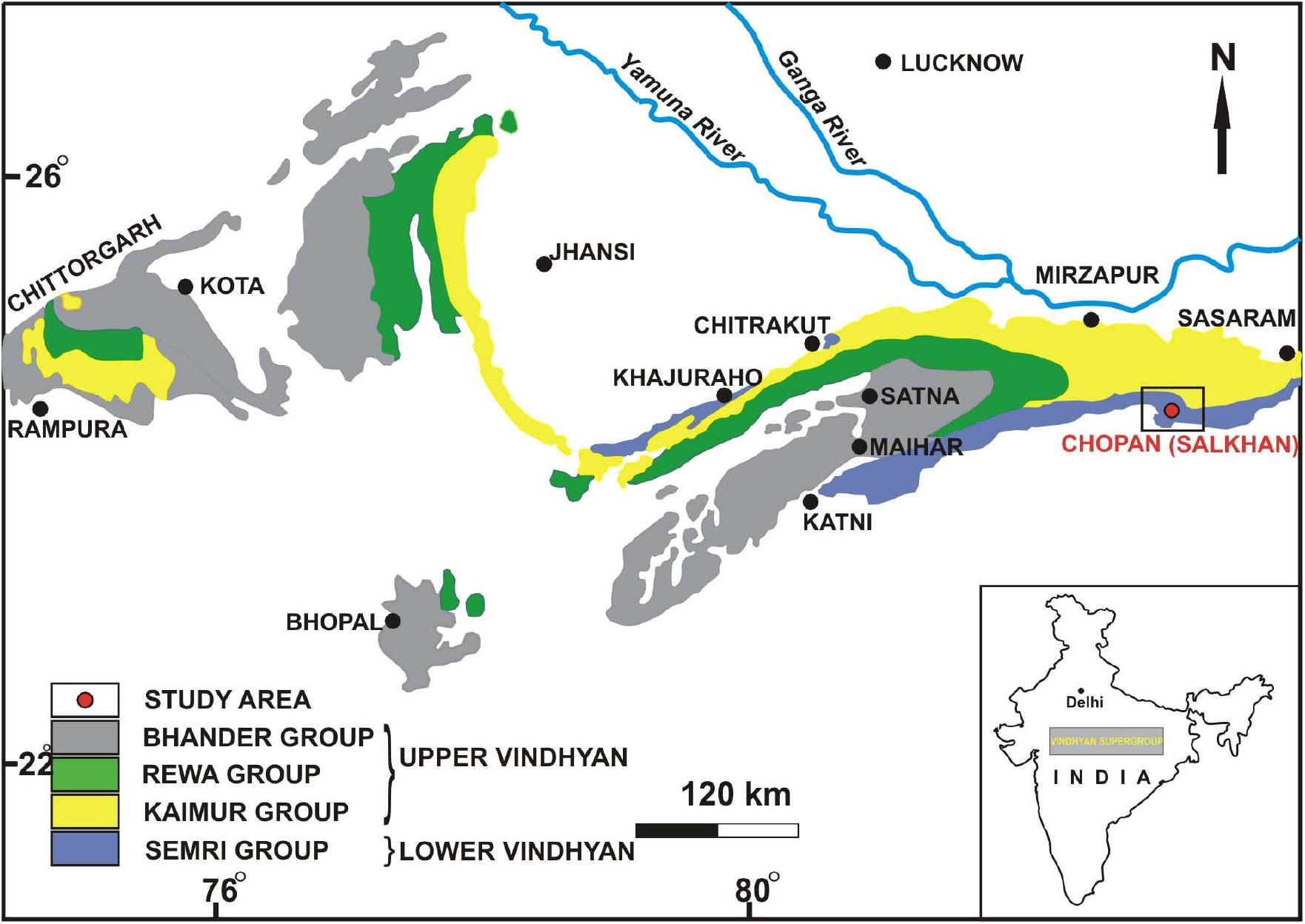
Figure 1. Generalized geological map of Vindhyan Basin, inset map of India showing position of the Vindhyan Supergroup (after Krishnan and Swaminath, 1959).
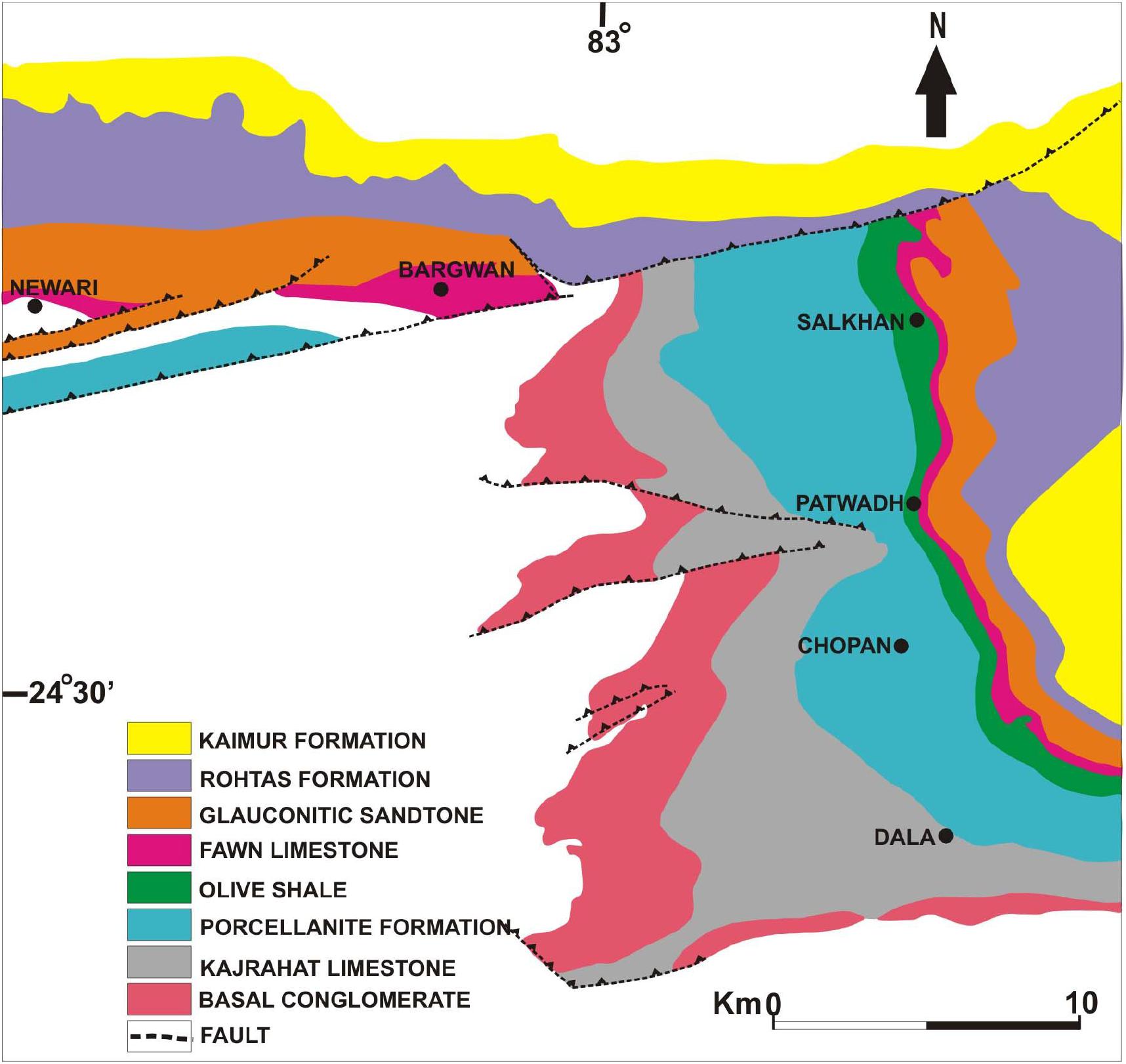
Figure 2. Geological map showing lithostratigraphic units of the Son Valley Section (after Auden, 1933).
Materials and Methods
Akinete-yielding chert samples were collected from the various localities of the Salkhan Limestone of the Semri Group (exposed in the Sonbhadra and Rohtas districts of India) whereas doubly-terminated quartz crystals were collected from the Newari area (Figure 3). The thin sections of the chert samples were studied for the diversity of microbial life, including the akinetes (Archaeoellipsoides). Akinetes and fan-fabrics were examined and photographed under transmitted light using a Nikon Eclipse 80i Microscope. All the photographs included in different plates were taken using the NIS Element F 3.0 program of the microscope. It should be noted that this photo-imaging technique sometimes shows duplicating images of the content; therefore, all the focused zones were sutured using the COREL Draw 12 program to make completely focused specimens. The size measurements of akinetes were carried out using an eyepiece micrometer. Two main techniques, namely maceration and thin sections, were used to recover and study the microfossil assemblages in chert samples. Slide No. (S.No. BSIP-10907, 14993, 14994, 14997-14999, 15107, 15723-15741) and England Finder co-ordinates (EF) are also provided for all the documented akinetes and listed in Supplementary Data. All the slides are deposited in the repository of the Birbal Sahni Institute of Palaeosciences, Lucknow, India under statement nos. 825, 1386, and 1431. The doubly-terminated quartz crystals were observed visually and photographed.
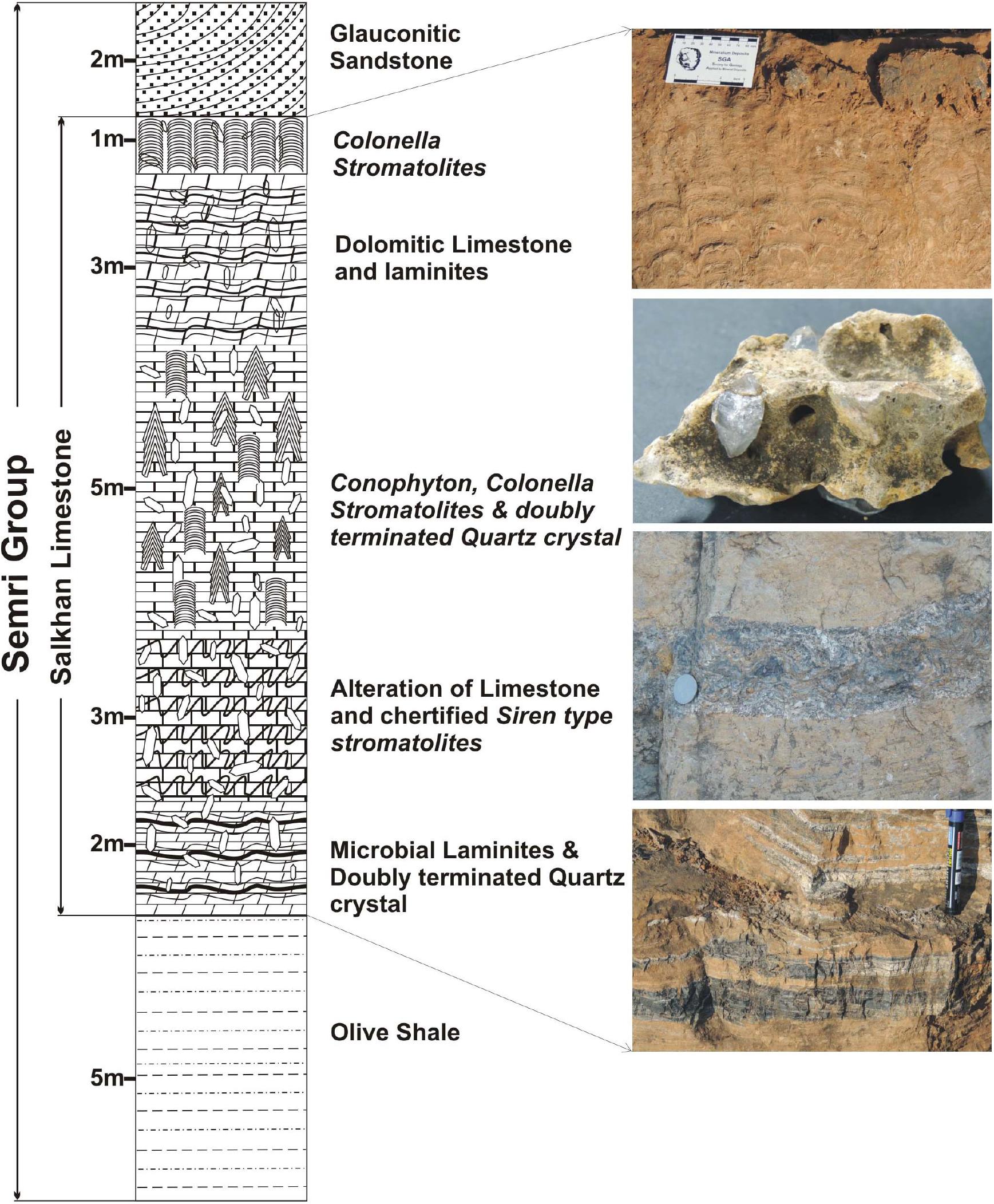
Figure 3. Litho-column showing the Salkhan Limestone section exposed in the Newari in Sonbhadra district, Uttar Pradesh, India.
Results
Akinetes
We present here the results of observations on the akinetes obtained from the chert samples of the Vindhyan Supergroup, India. The size measurements of akinetes revealed a wide range of size with length ranging from 2 to 110 μm and width ranging from 2 to 35 μm (Figure 4). A detailed account of Archaeoellipsoides reported from the Salkhan chert samples is tabulated in Table 1. The taxonomic characterization of the akinetes is as follows:
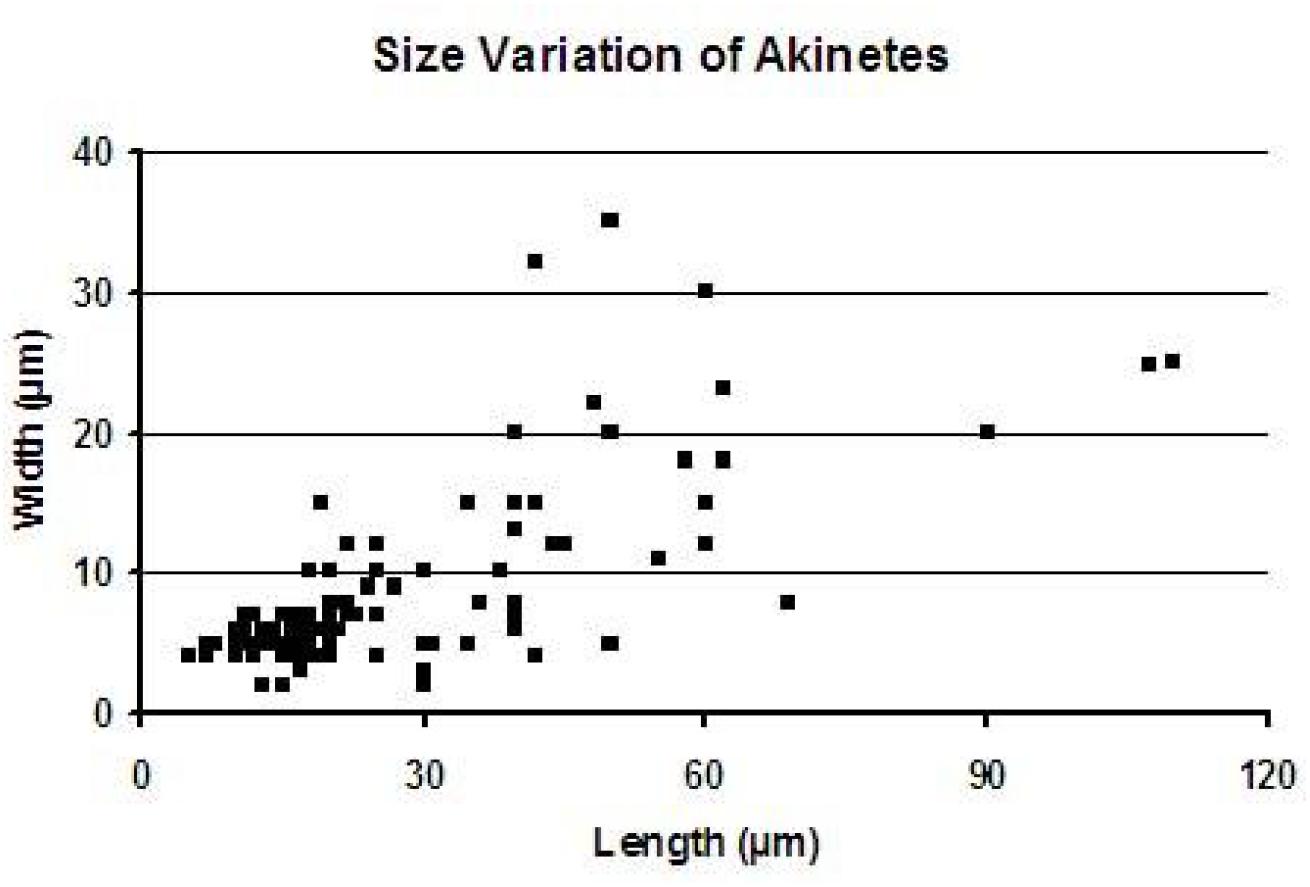
Figure 4. Size variation diagram of akinetes occurring in the thin section of cherts from the Salkhan Limestone.
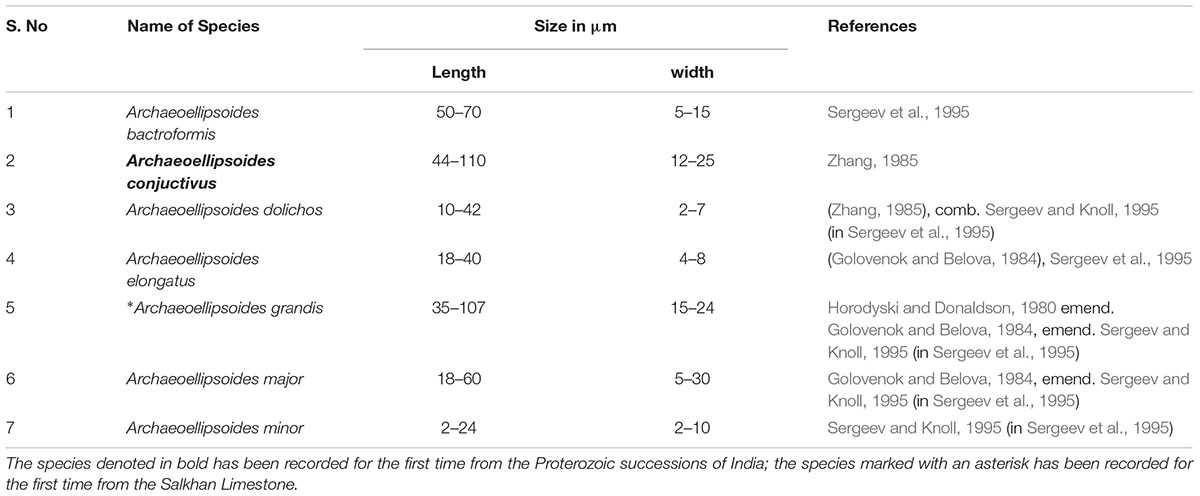
Table 1. A list of recorded akinetes in the present study showing size range from the Salkhan Limestone.
Taxonomy
Kingdom : Eubacteria Woese and Fox, 1977
Phylum : Cyanobacteria Stainer et al., 1978
Class : Coccogoneae Thuret, 1875
Order : Nostocales Geitler, 1925
Family : Nostocaceae Kützing, 1843
Genus : Archaeoellipsoides Horodyski and Donaldson, 1980 emend. Sergeev et al., 1995.
Type Species : Archaeoellipsoides bactroformis Sergeev et al., 1995 (Figures 5c,e,l, 6d,f,h–j,l).
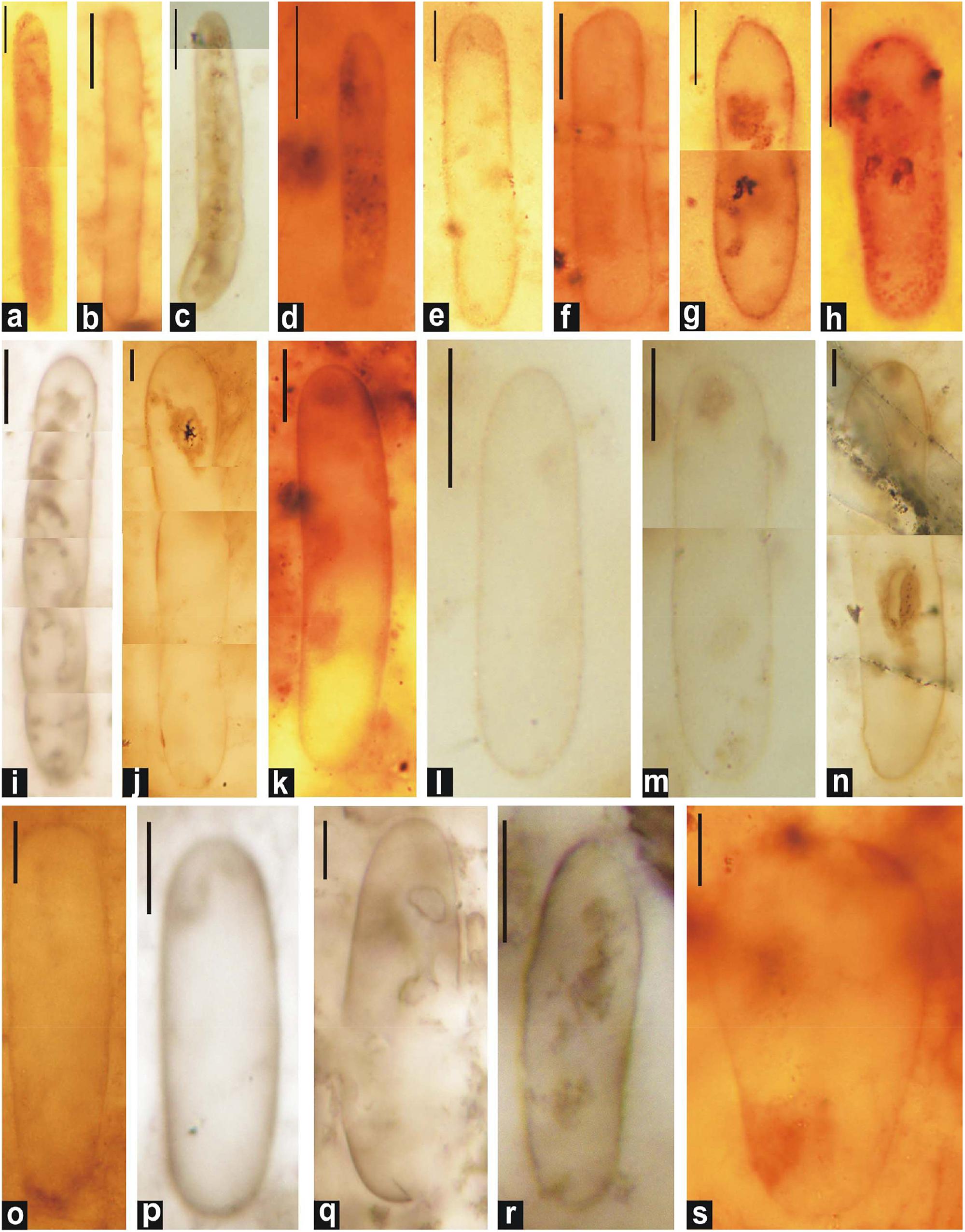
Figure 5. Photomicrographs of single-walled akinetes in petrographic thin sections of chert. (c, e, and l) Archaeoellipsoides bactroformis; (i,o) A. conjuctivus; (b,d) A. dolichos; (a,f) A. elongatus; (j,k,n,q, and s) A. grandis; (g,m,p, and r) A. major; (h) A. minor. Scale bar = 10 μm.
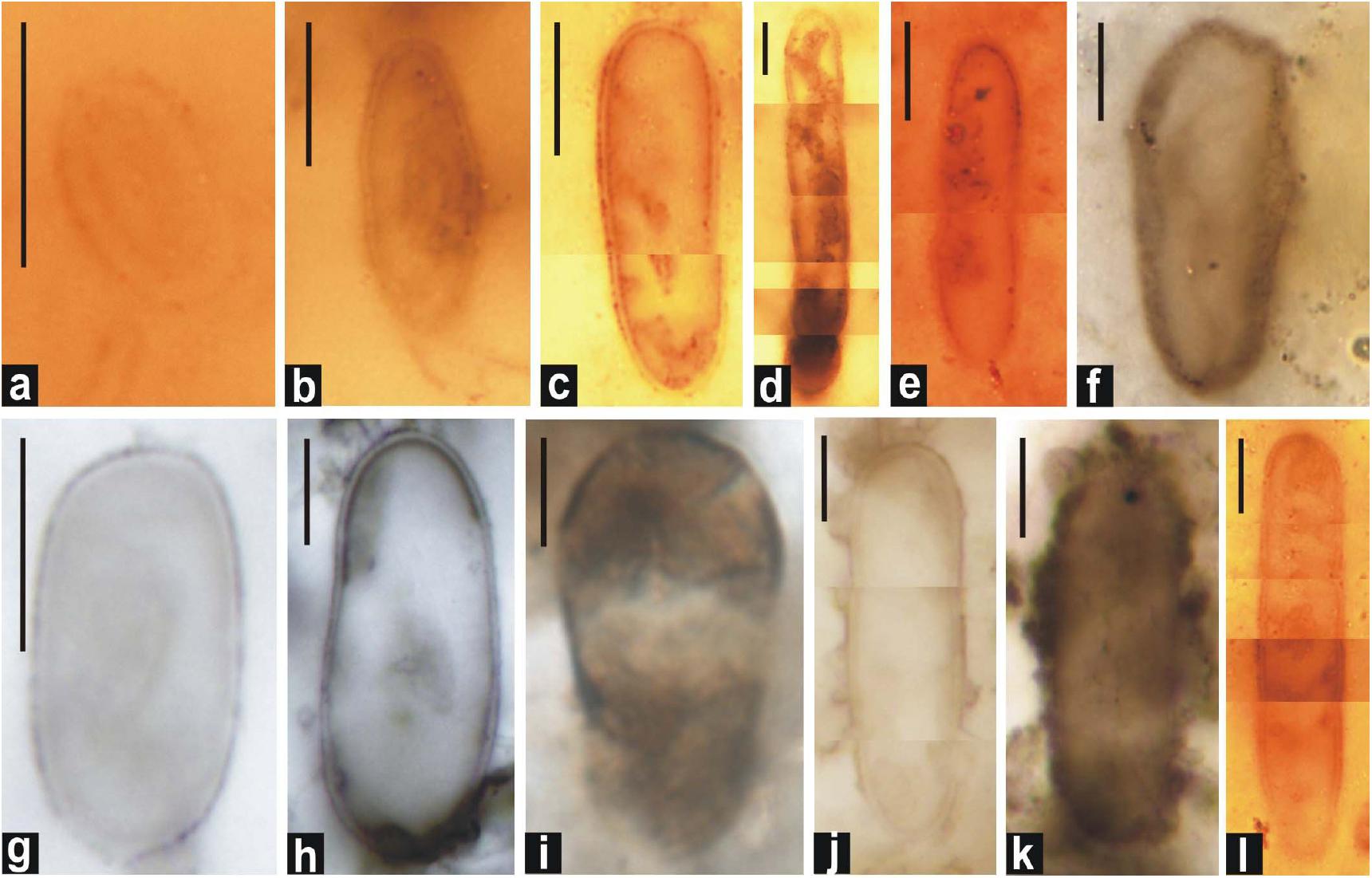
Figure 6. Photomicrographs of double-walled akinetes formed in petrographic thin sections of black chert. (d,f,h–j, and l): A. bactroformis; (c,k) A. major; (a,b,e, and g): A. minor. Scale bar = 10 μm.
Description: Solitary, single-, and double-layered ellipsoids with rounded ends, cell walls of ellipsoids medium to coarse-grained; empty or containing amorphous organic matter, elongated dark bodies. Length of ellipsoids 50–70 μm, width 5–15 μm, length width (L/W) ratio 4–10.
Remarks: The Salkhan ellipsoidal microfossils are small in cross-section and comparable with the Billyakh and Shorikha microfossils. There is no evidence of binary cell divisions. The close similarity in L/W ratio of these specimens with A. bactroformis and the wide variation in length allow these to be considered as A. aff. bactroformis.
Age and Locality: Paleo- to Mesoproterozoic; Nauhatta (Bihar), Newari and Jata Shankar Pahari (Sonbhadra district, Uttar Pradesh) localities of the Salkhan Limestone, India.
Archaeoellipsoides conjuctivus Zhang (1985) (Figures 5i,o, 7h,i).
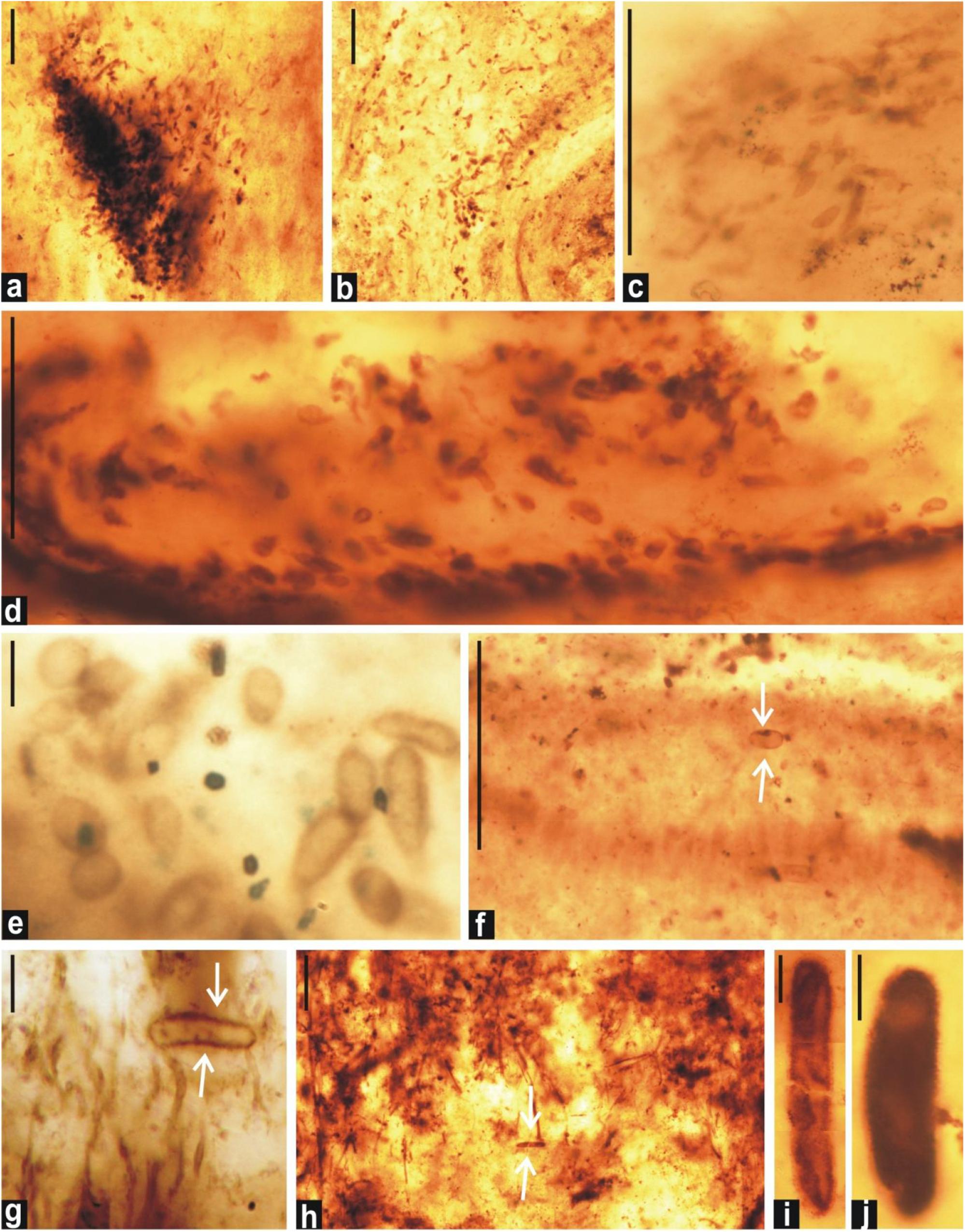
Figure 7. Distribution pattern of small-sized akinetes observed in petrographic thin sections of the black chert of the Salkhan Limestone. Assemblage showing the cluster and random distribution of Archaeoellipsoides (a–e). Note the occurrence of solitary individual of Archaeoellipsoides (marked by arrow) entangled in the radial fan-fabric (f). Individual Archaeoellipsoides (marked by arrow) are shown along with the filamentous cyanobacteria (g,h). Archaeoellipsoides shown in (a–g) represent A. minor, (h,i) A. conjuctivus, and (j) A. elongatus. Specimens a–d, f, and h are photographed at low magnification and scale bars represent 100 μm whereas scale bars in e,g,i, and j represent 10 μm.
Description: Ellipsoids solitary, single-layered, and slightly curved with flattened ends; basically empty but sometimes containing blebs of irregular micron-sized granules attached to the inner side of the ellipsoid; walls of ellipsoids medium- or fine-grained about 0.5 μm thick. The range of the length of ellipsoids is 44–110 μm, width 12–25 μm, and the length-width ratio is 1.4 to 4.5.
Remarks: Zhang (1985) diagnosed A. conjunctivus as chains of ellipsoidal bodies. That distinction is retained here but is purely formal; most Kotuikan and Yusmastakh akinetes were possibly arranged in chains originally. On the other hand, the modern cyanobacterial species Aulosira forms a chain of akinetes which is surrounded by a common sheath (Elenkin, 1938, p. 872) similar to A. conjunctivus.
Age and Locality: Paleo- to Mesoproterozoic; Nauhatta (Bihar), Newari, and Jata Shankar Pahari (Sonbhadra district, Uttar Pradesh) localities of the Salkhan Limestone, India.
Archaeoellipsoides dolichos (Zhang, 1985), comb. Sergeev and Knoll, 1995 (in Sergeev et al., 1995) (Figures 5b,d).
Description: Single-layered solitary, straight or gently curved rod-like ellipsoids with rounded ends; vesicles empty and 10–42 μm long but only 2–7 μm wide, length-width ratio 2 to 15.
Remarks: A. dolichos differs from other species of Archaeoellipsoides by its small cross-sectional diameter and high length-width ratio.
Age and Locality: Paleo to Mesoproterozoic; Newari (Sonbhadra district, Uttar Pradesh) locality of the Salkhan Limestone, India.
Archaeoellipsoides elongatus (Golovenok and Belova, 1984), comb. Sergeev and Knoll, 1995 (in Sergeev et al., 1995) (Figures 5a,f, 7j).
Description: Solitary, single-layered vesicles with rounded ends; vesicles generally empty, but may contain sparse blebs of amorphous organic matter. Ellipsoidal vesicles 18–40 μm long and 4–8 μm wide, length-width ratio 2.5 to 6.6.
Remarks: These elongated ellipsoidal microfossils were described by Golovenok and Belova (1984) as a distinct species of Eosynechococcus. Subsequently, Zhang (1985) described similar fossils as Bactrophycus oblongus from the Wumishan Formation. Some elongated bodies from the Dismal Lakes Group referred to by Horodyski and Donaldson as Oscillatoriopsis curta are probably remnants of akinetes comparable to Archaeoellipsoides elongatus (Horodyski and Donaldson, 1980, Figures 13A,B,G and Horodyski and Donaldson, 1983, Figure 5AA).
Age and Locality: Paleo to Mesoproterozoic; Newari (Sonbhadra district, Uttar Pradesh) locality of the Salkhan Limestone, India.
Archaeoellipsoides grandis Horodyski and Donaldson, 1980 emend. Golovenok and Belova, 1984, emend. Sergeev and Knoll, 1995 (in Sergeev et al., 1995) (Figures 5j,k,n,q,s).
Description: Solitary, single- and double-layered ellipsoids with rounded ends; ellipsoids usually empty and slightly curved; while blebs of amorphous organic matter found inside ellipsoids. Vesicle wall medium to coarse-grained, ellipsoids 35–107 μm long and 15–24 μm wide; length-width ratio of 2 to 4.3.
Remarks: Sergeev (1992) described ellipsoidal bodies as A. longus from the Chichkan Formation of South Kazakhstan, which was in accordance with the formal classification of genus Archaeoellipsoides (Sergeev et al., 1995), and should be transferred to A. grandis.
Age and Locality: Paleo- to Mesoproterozoic; Nauhatta (Bihar) and Jata Shankar Pahari (Sonbhadra district, Uttar Pradesh) localities of the Salkhan Limestone, India.
Archaeoellipsoides major Golovenok and Belova, 1984, comb. Sergeev and Knoll, 1995 (in Sergeev et al., 1995) (Figures 5g,m,p,r, 6c,k).
Description: Solitary, double-layered ellipsoids with rounded ends; ellipsoids basically empty, length 18–60 μm and width 5–30 μm, length-width ratio of 1.2 to 4.8, defined by translucent medium-grained walls, 1.0 to 1.5 μm thick. Vesicles tend to be aggregated in loose clusters or in short unconnected chain-like groups.
Remarks: Like the other species of Archaeoellipsoides, A. major is also distinguished by its characteristic range of diameters.
Age and Locality: Paleo- to Mesoproterozoic; Nauhatta (Bihar), Newari, and Jata Shankar Pahari (Sonbhadra district, Uttar Pradesh) localities of the Salkhan Limestone, India.
Archaeoellipsoides minor Sergeev and Knoll, 1995 (in Sergeev et al., 1995) (Figures 5h, 6a,b,e,g, 7a–g).
Description: Mostly found in the cluster, sometimes solitary, single and double layered ellipsoids with rounded and tapering ends; mainly empty vesicles sometimes containing amorphous organic matter. Vesicle wall medium to coarse-grained, ellipsoids are 5–24 μm long and 2–10 μm wide. Length-width ratio is 1.2 to 7.5.
Remarks: Sergeev et al. (1995) reassigned the ellipsoids described by Golovenok and Belova (1984) as Eosynechococcus grandis to Archaeoellipsoides minor and also the specimens described by Horodyski and Donaldson (1980) as a plausible chain of A. minor. They compared the specimens to akinetes of living Anabaena flos-aquae that commonly occur as clumps. The Salkhan population of A. minor is smaller in size.
Age and Locality: Paleo- to Mesoproterozoic; Nauhatta (Bihar), Newari, and Bargwan (Sonbhadra district, Uttar Pradesh) localities of the Salkhan Limestone, India.
Authigenic Doubly-Terminated Quartz Crystals and Fan-Fabrics
Doubly-terminated quartz crystals and fan-fabrics have been recorded in the Salkhan Limestone of the Vindhyan Supergroup. More than a hundred such crystals have been collected from the field, and a few hand specimens of massive limestone that contain quartz crystals are available for study. All the quartz crystals are euhedral. Their sizes range from a few millimeters to 15 centimeters. These quartz crystals are associated with the dolostone bed of the Salkhan Limestone and are found embedded in the stromatolitic chert unit of the Salkhan Limestone. These are prominently found near the Newari and Bargawan localities in Sonbhadra district. Petrographic thin sections show the presence of early diagenetic chert replacing the micritic part of the limestone. Some of the petrological thin sections of the chert also show the presence of micron-sized quartz crystals. No preferred orientation of these crystals was noted, however, some of them were found cut across the stromatolitic laminae. However, most of these crystals were regular in shape a few were found to be incomplete at one end. Intergrown crystals and twinning were rare. Prismatic faces were smooth in most of the crystals, with a few pits on the rhombohedral faces. On the color parameter, small quartz crystals were mostly clear and colorless. A few were smoky in color, and the larger crystals had some black spots (Figure 8). Doubly-terminated complete crystals were considered as authigenic to the Salkhan Limestone.

Figure 8. Variety of doubly-terminated quartz crystals from the Salkhan Limestone recorded at Newari locality. Hand specimens of colorless, prismatic, white, and smoky quartz crystals are shown (a–d) and scale bars for the same are 1 cm. Field photograph shows the relationship of quartz crystal with associated stromatolite, length of pen is 13.2 cm (e). Doubly-terminated quartz crystal in petrographic thin sections under the plane and cross polarized light indicated by white arrow (f,g).
The observed radial fan-fabrics in the thin sections, in which numerous microfossils were found embedded, are very peculiar. The size of fan-fabrics ranged from 2 to 10 μm (Figure 9).
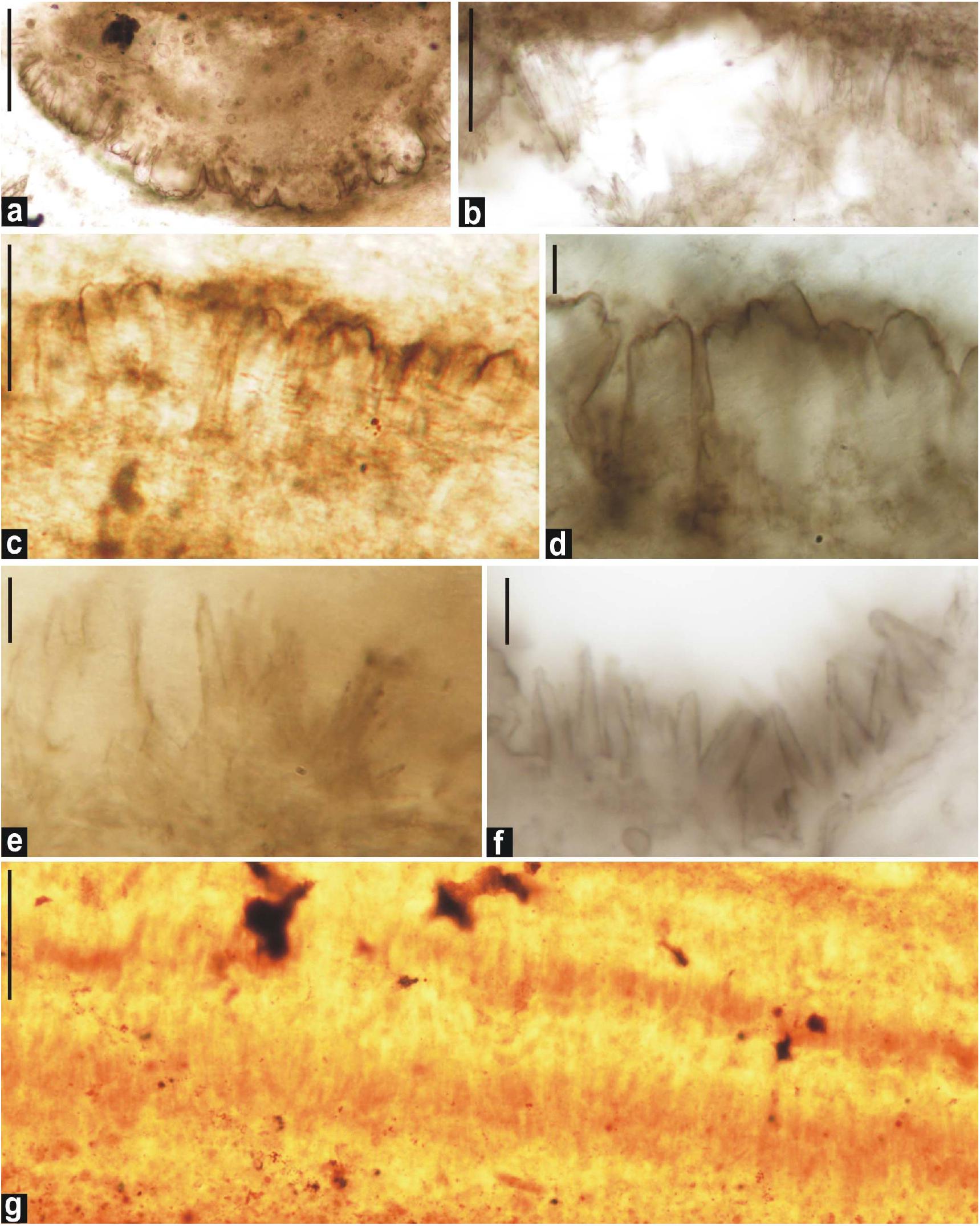
Figure 9. Fan-fabrics from the thin sections of chert from the Salkhan Limestone. (a): Distribution of coccoid cyanobacterial microfossils over fan-shaped blades of aragonite crystals pseudomorphs; (b–f): gradual enlargements of the blade shaped crystal pseudomoprhs of aragonite; (g): akinetes entrapped in crystal pseudomorphs. Scale bars = a,b,g = 100 μm and c–f = 10 μm.
Discussion
We reconciled our data on the akinetes (size variations and taxonomic diversity) vis-à-vis authigenic doubly-terminated quartz crystals and demonstrated that the depositional environment of the Salkhan Limestone experienced hypersaline conditions intermittently during the late Paleoproterozoic to early Mesoproterozoic (∼1600 Ma), which forced life forms to adopt survival strategies for the extreme environmental conditions of heat and dryness.
Formation of Akinetes in the Modern Environment
The heterocystous cyanobacteria usually form akinetes during unfavorable conditions, for which various factors are noted in the modern environment such as light, temperature, nutrient availability, and salinity. Akinetes can remain dormant for years and germinate to develop as new filaments when conditions become favorable (Nichols and Adams, 1982; Herdman, 1987). Both light limitation (Fay, 1969; Sutherland et al., 1979; Nichols et al., 1980; Fay et al., 1984) and increased light intensity (Rother and Fay, 1977; Moore et al., 2005) are important factors that influence the formation of akinetes in some cyanobacterial species. Likewise, nutrient availability (Fisher and Wolk, 1976; Sinclair and Whitton, 1977; Sutherland et al., 1979; Nichols and Adams, 1982; Fay et al., 1984; Rao et al., 1987; Sarma and Khattar, 1993; van Dok and Hart, 1996; Sukenik et al., 2007) and temperature (Li et al., 1997; Moore et al., 2005) also play an important role for the formation of akinetes in certain species, but akinetes do not survive in long exposures to high temperature (Oren, 2014). Salinity is also an important factor for the formation of akinetes, both in freshwater and marine species of cyanobacteria, and akinetes are readily formed when salinity levels are low or high compared to the required growth level (Pandey and Talpasayi, 1981; Huber, 1985; Baker and Bellifemine, 2000). The occurrence of akinetes in shales may indicate such conditions (noted above) in planktonic cyanobacteria in water bodies of normal chemistry. In such conditions, the akinetes form at the end of the optimal vegetation period, sink and accumulate in the sediment, where they survive over winter. They germinate in the next spring and return to the plankton stage during the isothermic turnovers.
Fossil Records of Akinetes
The recorded forms of akinetes from the Salkhan Limestone belong to the genus Archaeoellipsoides Horodyski and Donaldson, 1980. The occurrence of these isolated rod-shaped and large ellipsoidal vesicles (up to 100 μm) was recorded from the Dismal Lakes Group in Canada (Horodyski and Donaldson, 1980). A similar type of assemblage in the chert samples of the Mesoproterozoic Billyakh Group of the Western Anabar region, Northern Siberia was, however, assigned as Synechococcus-like cells (Golovenok and Belova, 1981, 1984). The ellipsoidal akinetes preserved in shales were retrieved either through the maceration technique and assigned as Brevitrichoides species (Jankauskas et al., 1989) or those found in the thin sections were regarded as Archaeoellipsoides. However, some akinetes from the chert were also assigned as Brevitrichoides, for instance from the Paleoproterozoic Epworth Group, Canada (Hofmann and Grotzinger, 1985). Heterocysts and akinetes are reported from Paleoproterozoic rocks of Franceville Group, Canada (Amard and Bertrand-Sarfati, 1997), Odjick and Rocknest Formations, and Epworth Group, northwestern Canada (Hofmann and Grotzinger, 1985).
Akinete populations have also been reported from many peritidal carbonates of Mesoproterozoic age, including the Gaoyuzhang and Wumishan Formations, China (Zhang, 1981, 1985; Zhang, 1982; Zhang and Li, 1985; Cao, 1992; Seong-Joo and Golubic, 1999), the Uluksan Group of Baffin Island, Canada (Hofmann and Jackson, 1991), the Kheinjua Formation, India (McMenamin et al., 1983; Kumar and Srivastava, 1995; Srivastava, 2005; Sharma, 2006b), the Deoban Limestone Formation, Garhwal Lesser Himalaya, India (Srivastava and Kumar, 2003), the Sukhaya Tunguska Formation, Turukhansk Uplift, Siberia (Sergeev et al., 1997; Sergeev, 1997, 1999), the Kotuikan and Yusmastakh Formations, Anabar Uplift, north-eastern Siberia (Golubic et al., 1995; Sergeev et al., 1995), and the Debengda Formation, northern Siberia (Sergeev et al., 1994). The akinetes of genera Archaeoellipsoides declined after the Mesoproterozoic; the two Neoproterozoic occurrences described to date come from the Chickhan Formation of southern Kazakhstan (Sergeev, 1989, 1992) and the Shorikha Formation, Turukhansk Uplift, Siberia (Sergeev, 2001).
Expanding investigations for the search of microbial remains revealed the presence of a large population of akinetes from the Salkhan Limestone. Sharma and Sergeev (2004) recorded varied precipitate patterns and entrapped Archaeoellipsoides in the cherts of the Salkhan Limestone from the Nauhatta area, Rohtas district, Bihar. Srivastava (2005) and Sharma (2006b) recorded Archaeoellipsoides in the thin sections of the chert from the Salkhan Limestone. They suggested that these fossil akinetes are similar to those of Anabaena species. Besides akinetes, there are also few reports of heterocystous cyanobacteria from Proterozoic sedimentary rocks. Licari and Cloud (1968); Cloud (1976), and Awramik and Barghoorn (1977) interpreted Gunflint fossil Gunflintia as heterocysts or akinetes but Knoll (1986) considered them as diagenetic embolisms or differential shrinkage within the filamentous sheaths. Schopf (1968) described heterocystous trichomes from the 800 Ma-old Bitter Springs Formation of Australia. Anabaenidium johnsonii has alternatively been interpreted as a preservational artifact (Golubic and Barghoorn, 1977; Gerasimenks and Krylov, 1983). Nagy (1978) reported heterocyst-forming cyanobacteria from the Paleoproterozoic Malmani Dolomite Formation, which later proved to be modern endoliths that penetrated older rocks (Mendelson and Schopf, 1982). Sastry et al. (1972) reported heterocysts from the subsurface Ganga Basin sediments of India. The unequivocal occurrence of Proterozoic heterocysts, demonstrating their unique functional morphology, remains undemonstrated (Wilmotte and Golubic, 1991). Golubic et al. (1995) suggested that the Proterozoic heterocyst-forming cyanobacteria existed, lived in terrestrial and coastal marine environments, and were preserved but remained unrecognized. In spite of these limitations in our understanding of akinetes, based on morphometric and sedimentary behavior comparison with the akinetes of modern bloom-forming Anabaena, Golubic et al. (1995) suggested that Archaeoellipsoides are fossilized akinetes. Butterfield (2015) also considered that the fossil Archaeoellipsoides possibly represents akinetes of Nostocalean cyanobacteria. Nonetheless, at present, the supporting evidence and arguments are less than compelling. To date, the most convincing Stigonematalean heterocysts and associated akinetes have been recorded in terrestrial hot springs known from the early Devonian Rhynie chert (Croft and George, 1959).
Implications for Paleoenvironmental Settings
The occurrence of authigenic doubly-terminated quartz crystals is usually indicative of a hypersaline environment (Flügel, 2004). The profused development of authigenic doubly-terminated quartz crystals in the Salkhan Limestone suggests the input of silica solution in the depositional milieu in the colloidal form and its subsequent gradual growth as euhedral quartz crystals. Such developments have been noted in various geological settings and ages and are common in evaporitic and Sabkha conditions (Tarr and Lonsdale, 1929; Grimm, 1962; Albright and Lueth, 2003). The source of colloidal silica which played a crucial role in the formation of quartz crystals is not yet fully clear in the context of the Salkhan Limestone; however, precipitation of chert/silica in peritidal carbonate sequences is well established (Knoll, 1985a). The occurrence of radial fan-fabrics in the same chert unit also suggests early diagenetic replacement of aragonitic and gypsum crystals (Figure 9) (Sharma and Sergeev, 2004). Tarr and Lonsdale (1929) suggested the critical role of divalent calcium in gypsum which induces coagulation and precipitation of silica as quartz near the sediment/water interface. Aragonite and gypsum precipitation is common in Sabkha environments, intertidal zones, and lagoonal environments where hypersaline conditions frequently develop over time. The occurrence of different varieties of stromatolites and microfossil assemblages in the Salkhan Limestone was used to interpret their depositional environments; intertidal to supratidal (Kumar, 1978a,b; Sharma, 2006b); intertidal to subtidal (McMenamin et al., 1983); shallow intertidal (Venkatachala et al., 1990; Kumar and Srivastava, 1995); and lagoonal intertidal to shallow marine subtidal environment (Gupta et al., 2003).
Extant Entophysalis usually found in the warm shallow hypersaline water bodies of the intertidal zone (Fritsch, 1945; Golubic and Hofmann, 1976) and Eoentophysalis dominated the microfossil assemblage recorded at Newari as well as other localities of the Salkhan Limestone, suggesting an intertidal depositional environment (Kumar and Srivastava, 1995; Sharma, 2006a). Radial fan-fabric development after aragonite crystals (Sharma and Sergeev, 2004), silica replacement features characteristics of shallow marine saline water (Sharma, 2006a), and profused occurrence of Eoentophysalis (Sharma, 2006a; Shukla and Sharma unpublished data) in the Salkhan Limestone indicate the evaporitic hypersaline depositional environment for the stromatolitic fine laminated chert and doubly-terminated quartz crystal-bearing unit in the Newari and Bargawan localities of the Sonbhadra district. The prevalence of apparently excessive mucilage-encompassed Eoentophysalis suggests harsh environmental conditions during the deposition of this unit. The occurrence of akinetes further supports the notion that the depositional conditions were not conducive for normal cyanobacterial growth. Similar depositional conditions have been noted at several places throughout the Proterozoic and are consistent with the Salkhan Limestone shallow hypersaline depositional environment. These places include the Upper Mt. Isa Group, Queensland, Australia (Neudert and Russell, 1981); the Dismal Lakes Group, Canada (Horodyski and Donaldson, 1983); the Sibley Group, Ontario, Canada (Bartley et al., 2015); and Sabkha deposits reviewed by Knoll (1985a,b).
Conclusion
Study of the stromatolitic chert unit of the Salkhan Limestone (>1600 Ma) exposed at Newari and Bargwan localities reveals the presence of akinetes belonging to the morphogenus Archaeoellipsoides and its seven different species, authigenic doubly-terminated quartz crystals, and radial fan-fabrics after the formation of aragonitic crystals have recorded in the petrographic thin sections. The extensive occurrence of mucilage-encompassed Eoentophysalis (although not part of the present study) and Archeaoellipsoides suggests extreme stress in the depositional environment which triggered mucilage production in certain coccoidal benthic cyanobacteria to overcome extreme conditions such as desiccation and high temperature, leading to akinete production in filamentous cyanobacteria. The occurrence of akinetes can also be used as a proxy to interpret the extremely harsh conditions for the thriving entities in the depositional environment. To this end, the Salkhan Limestone has provided important indicators which can also be used in various geological stages.
Author Contributions
MS conceived and designed the project, undertook multiple field visits in different localities for collection of samples, placed them in the proper stratigraphic order, prepared and completed the initial scanning of the slides from productive samples for microfossils, and discussed the implications of the data with BS and finalized the manuscript. BS jointly conceived the project, undertook field visit in one of the productive localities for collection of samples, prepared a large set of slides and scanned them for microfossils, generated statistical data, and jointly discussed and prepared the manuscript with MS.
Funding
The fieldwork of MS and BS was supported by the Birbal Sahni Institute of Palaeosciences.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to the Director, Birbal Sahni Institute of Palaeosciences (BSIP) for providing necessary laboratory and library facilities, and permission to publish this study (BSIP/RDCC/07/2016-17). BS sincerely thanks to Birbal Sahni Research Scholarship (BSRS) awarded by BSIP. We are thankful to Mónica Sánchez-Roman for the insightful comments on the earlier draft of the manuscript that helped us to improve the contents of the paper.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00397/full#supplementary-material
SUPPLEMENTARY DATA | Localities and coordinates for all the species of Archaeoellipsoides recorded from the Salkhan Limestone.
References
Adams, D. G., and Duggan, P. S. (1999). Heterocyst and akinete differentiation in cyanobacteria. New Phytol. 144, 3–33.
Albright, J. L., and Lueth, V. W. (2003). Pecos diamonds – Quartz and dolomite crystals from the Seven Rivers Formation outcrops of southeastern New Mexico. New Mexico Geol. 25, 63–74.
Amard, B., and Bertrand-Sarfati, J. (1997). Microfossils in 2000 ma old cherty stromatolites of the Franceville group, Gabon. Precambr. Res. 81,197–221.
Auden, J. B. (1933). Vindhyan sedimentation in the Son Valley, Mirzapur district. Mem. Geol. Surv. India 62, 141–250.
Baker, P. D., and Bellifemine, D. (2000). Environmental influences on akinete germination of Anabaena circinalis and implications for management of cyanobacterial blooms. Hydrobiologia 427, 65–73.
Barghoorn, B. S., and Tyler, S. A. (1965). Microorganisms from the gunflint chert. Science 147, 563–577.
Bartley, J. K., Firmin, S., Reiners, L., Hilgren, B., Berger, J., and Eischen, T. (2015). “A hypersaline environment for stromatolite growth in the Mesoproterozoic Sibley Group (Ontario, Canada),” in Sixth International Limnogeology Congress, Reno, 24–25.
Butterfield, N. J. (2015). Proterozoic photosynthesis- A critical review. Palaeontology 58, 953–972.
Cao, F. (1992). Algal microfossils of the middle proterozoic gaoyuzhuang formation in Pinggu County, Beijing. Geol. Rev. 38,382–387.
Chafetz, H. S., and Zhang, J. (1998). Authigenic euhedral megaquartz crystals in a quaternary dolomite. J. Sediment Res. 68,994–1000.
Chakraborty, P. P., Sarkar, S., and Patranabis-Deb, S. (2012). Tectonics and sedimentation of proterozoic basin of peninsular India. Proc. Indian Nat. Sci. Acad. 78, 393–400.
Cloud, P. (1976). Beginnings of biospheric evolution and their biogeochemical consequences. Paleobiology 2, 351–357.
Croft, W. N., and George, E. A. (1959). Blue-green algae from the middle devonian of rhynie, aberdeenshire. Geology 3, 341–353.
Elenkin, A. A. (1938). Monographie algarum Cyanophycearum Aquidulcium et Terrestrium Infinibus URSS Inventarum. Moscow: Izdetelstvo Akademii Nauk SSSR, 1, 984.
Fay, P. (1969). Metabolic activities of isolated spores of Anabaena cylindrica. J. Exp. Bot. 20, 100–109.
Fay, P., Lynn, J. A., and Majer, S. C. (1984). Akinete development in the planktonic blue-green alga Anabaena circinalis. Br. Phycol. J. 19,163–174.
Fisher, R. W., and Wolk, C. P. (1976). Substance stimulating the differentiation of spores of the blue-green alga Cylindrospermum licheniforme. Nature 259, 394–395.
Flügel, E. (2004). Microfacies of Carbonate Rocks: Analysis, Interpretation and Application. Berlin: Springer-Verlag, 984.
Folk, R. L. (1952). Petrography and Petrology of the Lower Ordovician Beekmantown Carbonate Rocks in the Vicinity of State College, Ph.D. dissertation, Pennsylvania State University, University Park, PA, 366.
Geitler, L. (1925). “Cyanophyceae,” in Die Susswasserflora Deutschlands, Osterreichs and der Schweiz, Vol. 12, ed. A. Pascher’s (Jena: Gustav Fischer), 450.
Gerasimenks, L. M., and Krylov, I. N. (1983). Post-mortem changes of cyanobacteria in algal-bacterial mats of thermal springs of Kamchatka. Doklady Akademii Nauk SSSR. 272, 201–203 (in Russian).
Golovenok, V. K., and Belova, M. Y. (1981). Precambrian microfossils in cherts from the Anabar Uplift. Doklady Akademii Nauk SSSR, 261, 713–715 (English version).
Golovenok, V. K., and Belova, M. Y. (1984). Riphean microbiotas in cherts of the Billyakh Group on the Anabar Uplift. Paleontologicheskyi Zhurnal. 4, 20–30 (English version).
Golubic, S. (1973). “The relationship between blue-green algae and carbonate deposits,” in The Biology of Blue-Green Algae, eds N. Y. Carr and B. A. Whitton (Oxford: Blackwell Scientific Publications), 434–472.
Golubic, S. (1976). “Organisms that build stromatolites,” in Stromatolites, ed. M. R. Walter (Amsterdam: Elsevier), 113–126.
Golubic, S., and Barghoorn, E. S. (1977). “Interpretation of microbial fossils with special reference to the Precambrian,” in Fossil Algae, ed. E. Flügel (Berlin: Springer), 1–14.
Golubic, S., and Campbell, S. E. (1979). Analogous microbial forms in recent subaerial habitats and in Precambrian cherts: Gloeothece coerulea geitler and Eosynechococcus moorei Hofmann. Precambr. Res. 8, 201–217.
Golubic, S., and Hofmann, H. J. (1976). Comparison of holocene and mid precambrian entophysalidaceae (Cyanophyta) in stromatolitic algal mats: cell division and degradation. J. Paleontol. 50, 1074–1082.
Golubic, S., and Seong-Joo, L. (1999). Early cyanobacterial fossil record: preservation, palaeoenvironments and interpretation. Eur. J. Phycol. 34,339–348.
Golubic, S., Sergeev, V. N., and Knoll, A. H. (1995). Mesoproterozoic Archaeoellipsoides: akinetes of heterocystous cyanobacteria. Lethaia 28,285–298.
Green, J. W., Knoll, A. H., and Swett, K. (1988). Microfossils from oolites and pisolites of the Upper Proterozoic Eleonora Bay Group, Central East Greenland. J. Paleontol. 62, 835–852.
Green, J. W., Knoll, A. H., and Swett, K. (1989). Microfossils from silicified stromatolithic carbonates of the Upper Proterozoic Limestones Dolomite ‘Series’, Central East Greenland. Geol. Mag. 119, 567–585.
Grimm, W. D. (1962). Idiomorphe quarze als leeitmineralien fur salinare fazies. Erdöl Kohle 15, 880–887.
Gupta, S., Jain, K. C., Srivastava, V. C., and Mehrotra, R. D. (2003). Depositional environment and tectonism during the sedimentation of the Semri and Kaimur groups of rocks, Vindhyan Basin. J. Palaeonl. Soc. Ind. 48, 181–190.
Herdman, M. (1987). “Akinetes: structure and function,” in The Cyanobacteria, eds P. Fay and C. van Baalen (Amsterdam: Elsevier), 227–250.
Hofmann, H. J. (1976). Precambrian microflora, Belcher Island, Canada: significance and systematics. J. Paleontol. 50, 1040–1073.
Hofmann, H. J., and Grotzinger, J. P. (1985). Shelf facies microbiotas from the odjick and rocknest formations (Epworth Group; 1.89 Ga), north-western Canada. Can. J. Earth Sci. 22, 1781–1792.
Hofmann, H. J., and Jackson, G. D. (1991). Shelf facies microfossilsfrom the Uluksan Group (Late Proterozoic) Bylot Supergroup, Baffin Island, Canada. J. Palaeontol. 65, 361–382.
Horodyski, R. J., and Donaldson, J. A. (1980). Microfossils from the Middle Proterozoic Dismal Lakes Group, Arctic Canada. Precambr. Res. 11, 125–159.
Horodyski, R. J., and Donaldson, J. A. (1983). Distribution and significance of microfossils in cherts of the Middle Proterozoic Dismal Lakes Group, District of Mackenzie, Northwest Territories, Canada. J. Paleontol. 57, 271–278.
Huber, A. L. (1985). Factors affecting the germination of akinetes of Nodularia spumigena (cyanobacteriaceae). Appl. Environ. Microbiol. 49, 73–78.
Jankauskas, T. V., Mikhailova, N. S., and Hermann, T. N. (eds) (1989). Precambrian Microfossils of the USSR. Leningrad: Nauka, 190.
Knoll, A. H. (1982). Microfossils from the late precambrian draken conglomerate, ny friesland, svalbard. J. Paleontol. 56, 577–790.
Knoll, A. H. (1984). Microbiotas of the late precambrian hunnberg formation, nordaustlandet, svalbard. J. Paleontol. 58, 131–162.
Knoll, A. H. (1985a). “A paleobiological perspective on sabkhas,” in Ecological Studies: Hypersaline Ecosystems, Vol. 53, eds G. M. Friedman and W. E. Krumbein (Berlin: Springer), 407–425. doi: 10.1007/978-3-642-70290-7_24
Knoll, A. H. (1985b). The distribution and evolution of microbial life in the late Proterozoic era. Ann. Rev. Microbiol. 39, 391–417. doi: 10.1146/annurev.mi.39.100185.002135
Knoll, A. H., and Golubic, S. (1992). “Living and Proterozoic cyanobacteria,” in Early Organic Evolution: Implication for Mineral and Energy Resources, eds M. Schidlowski, S. Golubic, and M. M. Kimberley (Berlin: Springer-Verlag), 450–462. doi: 10.1007/978-3-642-76884-2_35
Krishnan, M. S., and Swaminath, J. (1959). The great vindhyan basin of northern India. J. Geol. Soc. India 1, 10–30.
Kumar, S. (1978a). Discovery of microorganisms from the black cherts of the fawn limestone, late precambrian, semri group, son valley, Mirzapur Distt.U.P. Curr. Sci. 47:461.
Kumar, S. (1978b). On the kheinjua formation of semri group (Lower Vindhyan), Newari Area, Mirzapur district, U.P. Proc. Indian Nat. Sci. Acad. 44, 144–154.
Kumar, S., and Srivastava, P. (1992a). Discovery of microfossils from the nonstromatolitic middle Proterozoic Vindhyan chert, Chitrakut area, U.P. J. Geol. Soc. India 38, 511–515.
Kumar, S., and Srivastava, P. (1992b). Middle to late proterozoic microbiota from the deoban limestone, Garhwal Himalaya, India. Precambr. Res. 56, 291–318. doi: 10.1016/0301-9268(92)90106-X
Kumar, S., and Srivastava, P. (1995). Microfossils from the kheinjua formation, mesoproterozoic semri group, Newari area, Central India. Precambr. Res. 74, 91–117. doi: 10.1016/0301-9268(94)00098-C
Kützing, T. F. (1843). Phycologia Generalis, Oder Anatomie, Physiologie, und Systematik der Tange. Leipzig: F. A. Brockhaus, 458.
Li, R., Watanabe, M., and Watanabe, M. M. (1997). Akinete formation in planktonic Anabaena spp. (Cyanobacteria) by treatment with low temperature. J. Phycol. 33, 576–584. doi: 10.1111/j.0022-3646.1997.00576.x
Licari, G. R., and Cloud, P. (1968). Reproductive structures and taxonomic affinities of some nannofossils from the Aphebian Gunflint Iron Formation. Proc. Natl. Acad. Sci. U.S.A. 59, 1053–1960. doi: 10.1073/pnas.59.4.1053
Mazumder, R., Bose, P. K., and Sarkar, S. (2000). A commentary on the tectonic-sedimentary record of the Pre-2. 0 Ga evolution of. (Indian )craton vis-à-vis pre-gondwana afro-indian supercontinent. J. Afr. Earth Sci. 30, 201–217. doi: 10.1016/S0899-5362(00)00016-6
McMenamin, D. S., Kumar, S., and Awramik, S. M. (1983). Microbiota fossils from the kheinjua formation. Middle Proterozoic, Semri Group (Lower Vindhyan), Son Valley area, Central India. Precambr. Res. 21, 247–271. doi: 10.1016/0301-9268(83)90043-8
Mendelson, C. V., and Schopf, J. W. (1982). Proterozoic microfossils from the Sukhaya Tunguska, Shorikha and Yudoma Formations of the Siberian Platform, USSR. J. Paleontol. 56, 42–83.
Moore, D., O’Donohue, M., Garnett, C., Critchley, C., and Shaw, G. (2005). Factors affecting akinete differentiation in Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria). Freshw. Biol. 50, 345–352. doi: 10.1111/j.1365-2427.2004.01324.x
Nagy, I. A. (1978). New filamentous and cystous microfossils, 2300 M. Y. old from the Transvaal sequence. J. Paleontol. 52, 141–154.
Neudert, M. K., and Russell, R. E. (1981). Shallow-water and hypersaline features from the Proterozoic Mt Isa sequence. Nature 293, 284–286. doi: 10.1038/293284a0
Nichols, J. M., and Adams, D. G. (1982). “Akinetes,” in The Biology of Cyanobacteria, eds N. G. Carr and B. A. Whitton (Oxford: Blackwell), 387–412.
Nichols, J. M., Adams, D. G., and Carr, N. G. (1980). Effect of canavanine and other amino acid analogues on akinete formation in the cyanobacterium Anabaena cylindrica. Arch. Microbiol. 127, 67–75. doi: 10.1007/BF00414357
Oren, A. (2014). “Cyanobacteria: biology, ecology and evolution,” in Stress Biology of Cyanobacteria: Molecular Mechanisms to Cellular Responses, eds N. K. Sharma, A. K. Rai, and L. Stal (New York, NY: Wiley), 3–20.
Pandey, R. K., and Talpasayi, E. R. S. (1981). Factors affecting germination of spores in a blue-green alga Nodularia spumigena. Acta Bot. Ind. 9, 35–42.
Pinto, P. T., Kust, A., Devercelli, M., and Kozlíková-Zapomìlová, E. (2016). Morphological traits in nitrogen fixing heterocytous cyanobacteria: possible links between morphology and eco-physiology. Hidrobiologia 764, 271–281. doi: 10.1007/s10750-015-2516-6
Prasad, B., Uniyal, S. N., and Asher, R. (2005). Organic walled microfossils from the proterozoic, vindhyan supergroup of son valley, Madhya Pradesh, India. Palaeobotanist 54, 13–60.
Rao, V. V., Ghosh, R., and Singh, H. N. (1987). Diazotrophic regulation of akinete development in the cyanobacterium Anabaena doliolum. New Phytol. 106, 161–168. doi: 10.1111/j.1469-8137.1987.tb04800.x
Rother, J. A., and Fay, P. (1977). Sporulation and the development of planktonic blue-green algae in two salopian meres. Proc. R. Soc. Lond. B 196, 317–332. doi: 10.1098/rspb.1977.0043
Sarma, T. A., and Khattar, J. I. S. (1993). Akinete differentiation in phototrophic, photoheterotrophic and chemoheterotrophic conditions in Anabaena torulosa. Folia Microbiol. 38, 335–340. doi: 10.1007/BF02898604
Sastry, V. V., Venkatachala, B. S., and Desikachary, T. V. (1972). “A fossil nostocaceae from India,” in Proceedings of the Symposium on Taxonomy and Biology of Blue Green algae (Madras: Madras University),159–161.
Schopf, J. W. (1968). Microflora of the bitter springs formation, late precambrian, Central Australia. J. Paleontol. 42, 651–688.
Schopf, J. W., and Blacic, J. M. (1971). New microorganisms from the bitter springs formation (late precambrian) of the North-Central Amadeus basin, Australia. J. Paleontol. 45, 925–960.
Seong-Joo, L., and Golubic, S. (1999). Microfossils population in the context of synsedimentary micritic deposition and acicular carbonate precipitation: mesoproterozoic Gaoyuzhuang Formation, China. Precambr. Res. 96, 183–208. doi: 10.1016/S0301-9268(99)00004-2
Sergeev, V. N. (1989). Microfossils from transitional Precambrian Phanerozoic strata of Central Asia. Himal. Geol. 13, 269–278.
Sergeev, V. N. (1992). Silicified Microfossils from the Precambrian and Cambrian Deposits of the Southern Ural Mountains and Middle Asia. Moscow:Nauka, 136.
Sergeev, V. N. (1997). “Mesoproterozoic microbiotas of the northern hemisphere and the meso-neoproterozoic transition,” in Proceedings of the 30th International Geological Congress, Beijing 1, 177–185.
Sergeev, V. N. (1999). Silicified microfossils from transitional Meso- Neoproterozoic deposits of the Turukhansk Uplift, Siberia. Bolletinodella Soc. Paleontol. Ital. 38, 287–295.
Sergeev, V. N. (2001). Palaeobiology of neoproterozoic (Upper Riphean), shorikha and burovaya silicified microbiotas, turukhansk uplift, siberia. J. Paleontol. 75, 427–448. doi: 10.1017/S0022336000018229
Sergeev, V. N., and Knoll, A. H. (1995). Taphonomic and evolutionary changes across the Mesoproterozoic-Neoproterozoic boundary. Neues. Jahrb. Geol. Palaontol. Abh. 195, 289–302.
Sergeev, V. N., Knoll, A. H., and Grotzinger, J. P. (1995). Paleobiology of the mesoproterozoic billyakh group, Anabar Uplift, Northeastern Siberia. Paleontol. Soc. Memoir. 39:37.
Sergeev, V. N., Knoll, A. H., Kolosova, S. P., and Kolosov, P. N. (1994). Microfossils in cherts from the mesoproterozoic debengda formation, olenek Uplift, Northeastern Siberia. Stratigr. Geol. Correl. 2, 23–38.
Sergeev, V. N., Knoll, A. H., and Petrov, P. Y. (1997). Paleobiology of the mesoproterozoic-neoproterozoic transition: the sukhaya tunguska formation, Turukhansk Uplift, Siberia. Precambr. Res. 85, 201–239. doi: 10.1016/S0301-9268(97)00035-1
Sergeev, V. N., Sharma, M., and Shukla, Y. (2008). Mesoproterozoic silicified microbiotas of Russia and India characteristics and contrasts. Palaeobotanist 57, 323–358.
Sergeev, V. N., Sharma, M., and Shukla, Y. (2012). Proterozoic fossil cyanobacteria. Palaeobotanist 61, 189–358.
Sharma, M. (2006a). Palaeobiology of mesoproterozoic salkhan limestone, semri group, Rohtas, India; Systematic and significance. J. Earth Syst. Sci. 115, 67–98. doi: 10.1007/BF02703027
Sharma, M. (2006b). Small-sized akinetes from the mesoproterozoic salkhan limestone, Semri Group, Bihar, India. J. Palaeontol. Soc. India 51, 109–118.
Sharma, M., and Sergeev, V. N. (2004). Genesis of carbonate precipitates patterns and associated microfossils in Mesoproterozoic formations of India and Russia- a comparative study. Precambr. Res. 134, 317–347. doi: 10.1016/j.precamres.2004.07.001
Sinclair, C., and Whitton, B. A. (1977). Influence of nitrogen source on morphology of Rivulariaceae (cyanophyta). J. Phycol. 13, 335–340.
Srivastava, P. (2005). Vindhyan akinetes: an indicator of mesoproterozoic biospheric evolution. Orig. Life Evol. Biosphs. 35, 175–185. doi: 10.1007/s11084-005-8765-z
Srivastava, P., and Kumar, S. (2003). New microfossils from the Meso- Neoproterozoic Deoban Limestone, Garhwal Lesser Himalaya India. Palaeobotanist 52, 13–47.
Srivastava, P., and Tewari, V. C. (2011). “Morphological changes in mikroscopic-megascopic life and stromatolites, recorded during Late Palaeoproterozoic-Neoproterozoic transition: the Vindhyan Supergroup,” in Stromatolites: Interaction of Microbes with Sediments. Cellular Origin, Life in Extreme Habitats and Astrobiology, eds V. C. Tewari and J. Seckback (Dordrecht: Springer), 18, 87–114.
Stainer, R. Y., Sistrom, W. R., Hansen, T. A., Castenholz, R. W., Pfennig, N., Gorlenko, V. N., et al. (1978). Proposal to place nomenclature of the cyanobacteria (blue green algae) under the rules of International Code of Nomenclature of bacteria. Int. J. Syst. Bacteriol. 28, 335–336. doi: 10.1099/00207713-28-2-335
Sukenik, A., Beardall, J., and Hadas, O. (2007). Photosynthetic characterization of developing and mature akinetes of Aphanizomenon ovalisporum (Cyanoprokaryota). J. Phycol. 43, 780–788. doi: 10.1111/j.1529-8817.2007.00374.x
Sutherland, J. M., Herdman, M., and Stewart, W. D. P. (1979). Akinetes of the cyanobacterium Nostoc PCC7524: macromolecular composition, structure and control of differentiation. J. Gen. Microbiol. 115, 273–287. doi: 10.1099/00221287-115-2-273
Tarr, W. A., and Lonsdale, J. T. (1929). Pseudocubic quartz crystals from Artesia, New Mexico. Am. Mineral. 14, 50–53.
Thuret, G. (1875). Essai de Classification des Nostocines. Paris (Botanique): Annales des Sciences Naturelles, 6, 372–382.
Tomitani, A., Knoll, A. H., Cavanaugh, C. M., and Ohno, T. (2006). The evolutionary diversification of cyanobacteria: molecular-phylogenetic and paleontological perspectives. Proc. Natl. Acad. Sci. U.S.A. 103, 5442–5447. doi: 10.1073/pnas.0600999103
Tyler, S. A., and Barghoorn, E. S. (1954). Occurrence of structurally preserved plants in Precambrian rocks of Canadian Shield. Science 119, 606–608. doi: 10.1126/science.119.3096.606
Ulmer-Scholle, D. S., Scholle, P. A., and Brady, P. V. (1993). Silicification of evaporites in Permian (Guadalupian) back-reef carbonates of the Delaware Basin, west Texas and New Mexico. J. Sediment Petrol. 63, 955–965.
van Dok, W., and Hart, B. T. (1996). Akinete differentiation in Anabaena circinalis (Cyanophyta). J. Phycol. 32, 557–565. doi: 10.1111/j.0022-3646.1996.00557.x
Venkatachala, B. S., Yadav, V. K., and Shukla, M. (1990). “Middle proterozoic microbiota from nauhatta limestone (Vindhyan Supergroup) Rohatasgarh India. Development in Precambrian Geology,” in Precambrian Continental Crust and Economic Resources, ed. S. M. Naqvi (Amsterdam: Elsevier), 471–478. doi: 10.1016/S0166-2635(08)70180-4
Wilmotte, A., and Golubic, S. (1991). Morphological and genetic criteria in the taxonomy of Cyanophyta/Cyanobacteria. Algol. Stud. 64, 1–24.
Wilson, R. C. L. (1966). Silica diagenesis in Upper Jurassic limestones of southern England. J. Sediment Petrol. 36, 1036–1049.
Woese, C., and Fox, G. (1977). Phylogenetic structure of prokaryotic domain. Proc. Natl. Acad. Sci. U.S.A. 74, 5088–5090. doi: 10.1073/pnas.74.11.5088
Young, G. M. (2013). Precambrian supercontinents, glaciations, atmospheric oxygenation, metazoan evolution and an impact that may have changed the second half of Earth history. Geosci. Front. 4, 247–261. doi: 10.1016/j.gsf.2012.07.003
Zenger, D. H. (1976). Definition of type little falls dolostone (Late Cambrian), east-central New York. Am. Assoc. Pet. Geol. Bull. 60,xyb 1570–1575.
Zhang, P. (1982). Microfossils from the Wumishan Formation of Jixian County. Acta Geol. Sin. 53, 87–90.
Zhang, Y. (1981). Proterozoic stromatolite microfloras of the gaoyuzhuang formation (Early Sinian: Riphean), Hebei, China. J. Paleontol. 55, 485–506.
Zhang, Y. (1985). Stromatolitic microbiota from the Middle Proterozoic Wumishan Formation (Jixian Group) of the Ming Tombs, Beijing, China. Precambr. Res. 30, 277–302. doi: 10.1016/0301-9268(85)90024-5
Keywords: akinetes, authigenic doubly-terminated quartz crystal, chert, extreme environment, fan-fabrics, Salkhan Limestone, India
Citation: Sharma M and Shukla B (2019) Akinetes From Late Paleoproterozoic Salkhan Limestone (>1600 Ma) of India: A Proxy for Understanding Life in Extreme Conditions. Front. Microbiol. 10:397. doi: 10.3389/fmicb.2019.00397
Received: 15 May 2018; Accepted: 14 February 2019;
Published: 12 March 2019.
Edited by:
David Emerson, Bigelow Laboratory for Ocean Sciences, United StatesReviewed by:
Stjepko Golubić, Boston University, United StatesHenderson James Cleaves, Earth-Life Science Institute, Tokyo Institute of Technology, Japan
Copyright © 2019 Sharma and Shukla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mukund Sharma, bXVrdW5kX3NoYXJtYUBic2lwLnJlcy5pbg==
 Mukund Sharma
Mukund Sharma Bandana Shukla
Bandana Shukla