
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 04 December 2018
Sec. Food Microbiology
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.02745
This article is part of the Research Topic Advances in Postharvest Pathology of Fruits and Vegetables View all 17 articles
Chitosan is a natural biopolymer from crab shells that is known for its biocompatibility, biodegradability, and bioactivity. In human medicine, chitosan is used as a stabilizer for active ingredients in tablets, and is popular in slimming diets. Due to its low toxicity, it was the first basic substance approved by the European Union for plant protection (Reg. EU 2014/563), for both organic agriculture and integrated pest management. When applied to plants, chitosan shows triple activity: (i) elicitation of host defenses; (ii) antimicrobial activity; and (iii) film formation on the treated surface. The eliciting activity of chitosan has been studied since the 1990’s, which started with monitoring of enzyme activities linked to defense mechanisms (e.g., chitinase, β-1,3 glucanase, phenylalanine ammonia-lyase) in different fruit (e.g., strawberry, other berries, citrus fruit, table grapes). This continued with investigations with qRT-PCR (Quantitative Real-Time Polymerase Chain Reaction), and more recently, with RNA-Seq. The antimicrobial activity of chitosan against a wide range of plant pathogens has been confirmed through many in-vitro and in-vivo studies. Once applied to a plant surface (e.g., dipping, spraying), chitosan forms an edible coating, the properties of which (e.g., thickness, viscosity, gas and water permeability) depend on the acid in which it is dissolved. Based on data in literature, we propose that overall, the eliciting represents 30 to 40% of the chitosan activity, its antimicrobial activity 35 to 45%, and its film-forming activity 20 to 30%, in terms of its effectiveness in the control of postharvest decay of fresh fruit. As well as being used alone, chitosan can be applied together with many other alternatives to synthetic fungicides, to boost its eliciting, antimicrobial and film-forming properties, with additive, and at times synergistic, interactions. Several commercial chitosan formulations are available as biopesticides, with their effectiveness due to the integrated combination of these three mechanisms of action of chitosan.
Chitosan is the linear polysaccharide of glucosamine and N-acetylglucosamine units joined by β-1,4-glycosidic links and it is obtained by deacetilation of chitin through exposure to NaOH solutions or to the enzyme chitinase. Chitosan and chitin are naturally occurring polymers. For their biocompatibility and biosafety, their applications are widespread in many industries, such as cosmetology, food, biotechnology, pharmacology, medicine, and agriculture (Ding et al., 2013; Lei et al., 2014). In particular, chitosan has increasing interest in plant protection as a natural fungicide and plant defense booster, and meets the interest of many researchers, that used it to prolong the storage of an array of fruit and vegetables worldwide. Chitosan was the first compound in the list of basic substances approved in the European Union for plant protection purposes (Reg. EU 66 2014/563), for both organic agriculture and integrated pest management. A comprehensive review on the available data on the effectiveness of chitosan was published recently, for its preservation of fruit and vegetables, both alone and in combination with other treatments, and its mechanisms of action (Romanazzi et al., 2017). However, the increasing knowledge of this biopolymer (Figure 1) and the fast advances in basic and applied research in this field require a more focused and schematic update based on the last 5 years of investigations (2013–2018). The reader can then focus on specific aspects from the long list of other reviews that have appeared on the subject, among which some have focused on the applications of chitosan to fruit and vegetables (Bautista-Baňos et al., 2006; Bautista-Baòos et al., 2016; Zhang et al., 2011). When applied to plants, chitosan shows triple activity: (i) elicitation of host defenses; (ii) antimicrobial activity; and (iii) film formation on the treated surface. We will cover the recent information on these issues in the following sections, which is also listed comprehensively in the Tables, with examples of these applications.
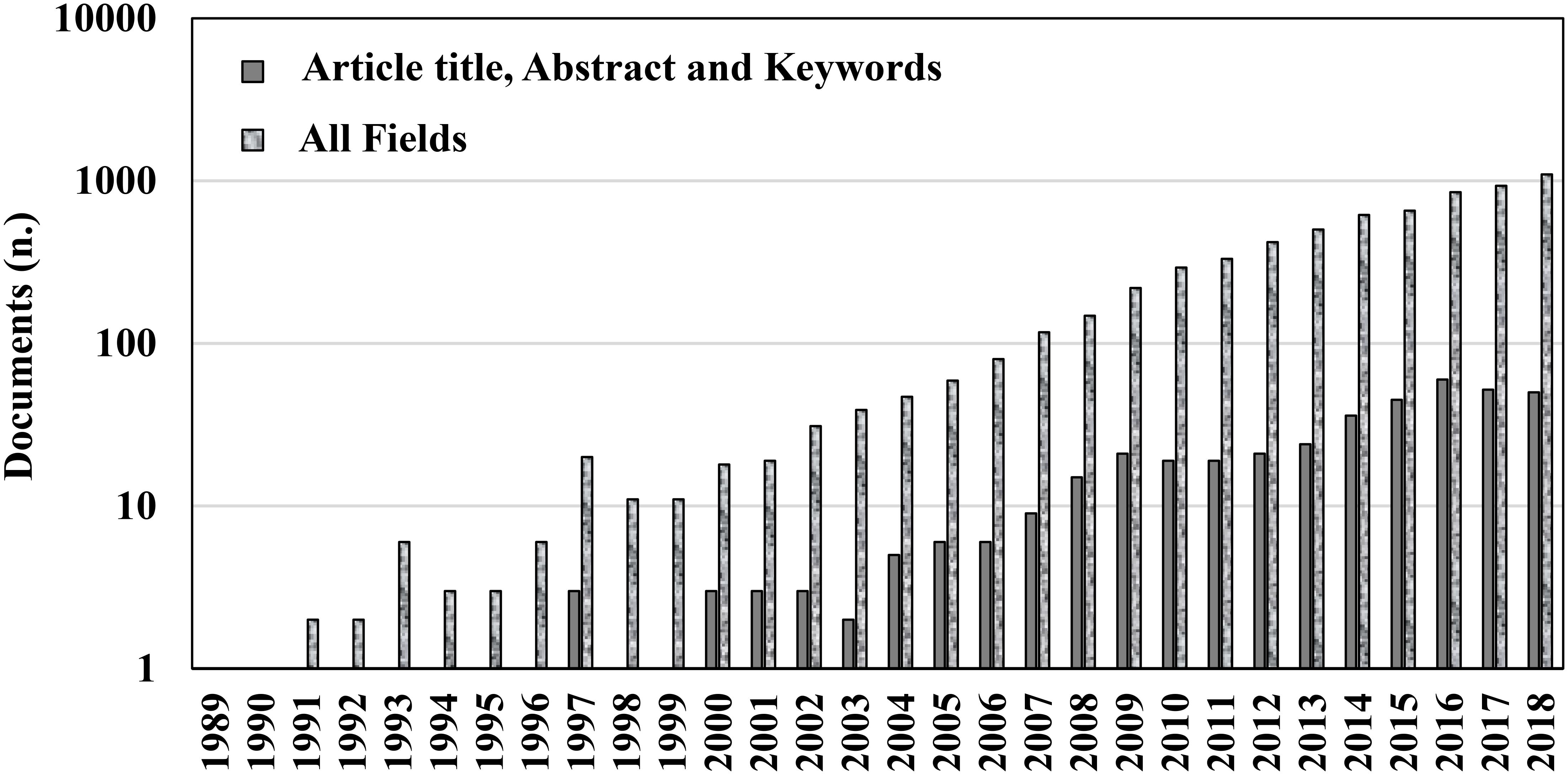
FIGURE 1. Number of documents available on Scopus through searches with keywords ‘chitosan’ and ‘postharvest’ in ‘Article title, Abstract, and Keywords’ or in ‘All fields’ published over the last 30 years (accessed on 6 November 2018).
The potential effectiveness of chitosan as a coating for fresh fruit was first proposed by Muzzarelli (1986). The first in-vivo application of chitosan on fruit was in the Josep Arul Laboratory, by Ahmed El Ghaouth, who produced a list of papers through the last decade of the last century. These included El Ghaouth et al. (1992), where they applied chitosan to strawberries and other fruit, both alone and in combinations with other potential biocontrol agents, which then contributed to the develop of some commercial formulations. Following these promising investigations, and with the growing need for alternatives to the use of synthetic fungicides, chitosan use became popular, and it was proposed to be part of a new class of plant protectants (Bautista-Baňos et al., 2006). Chitosan coatings have now been applied to numerous temperate and subtropical fruit, both alone and in combination with other treatments (Tables 1–3), with generally additive, and in some cases synergistic, effectiveness (Romanazzi et al., 2012).
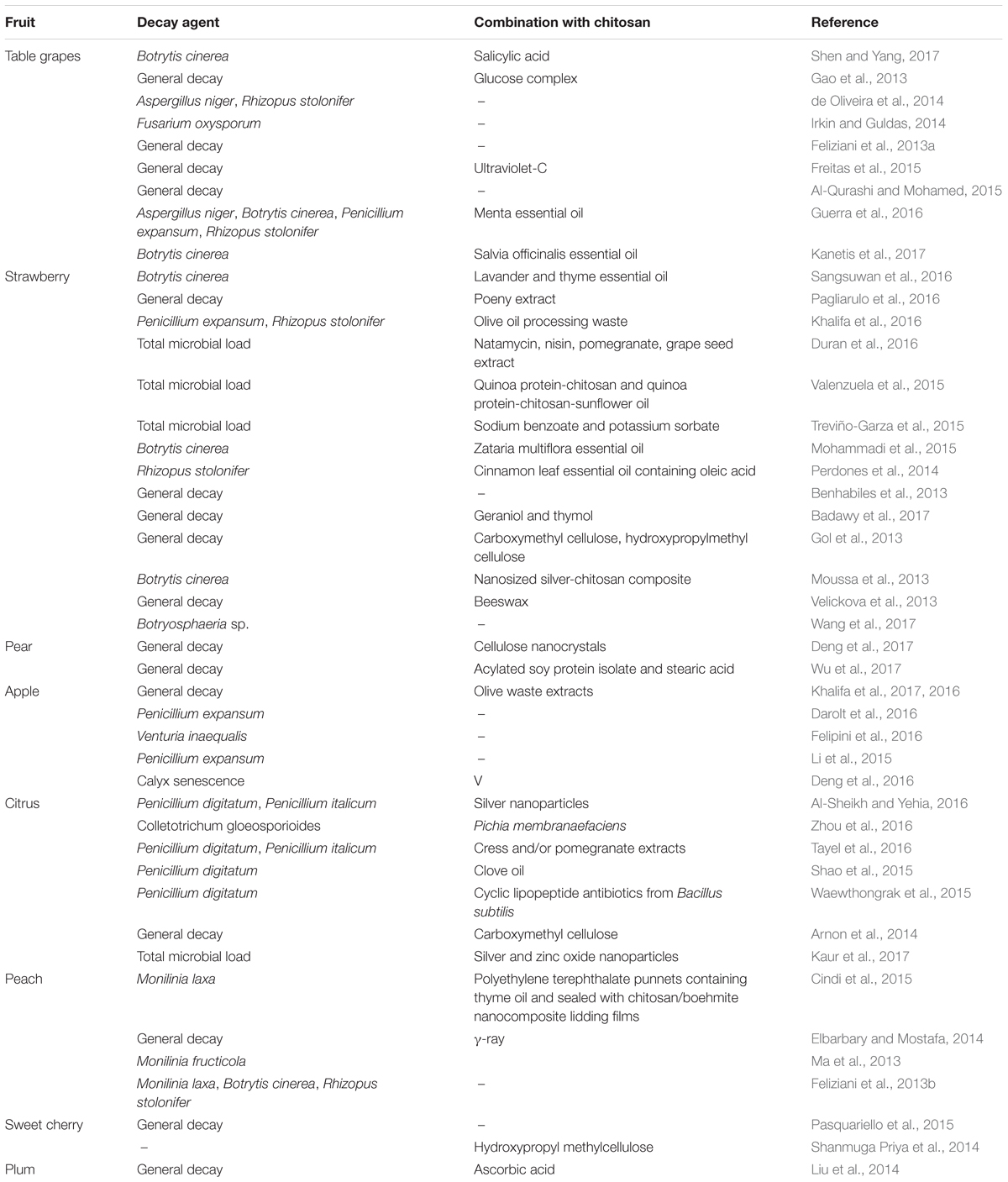
TABLE 1. Postharvest chitosan treatments with other applications for storage decay of temperate fruit.
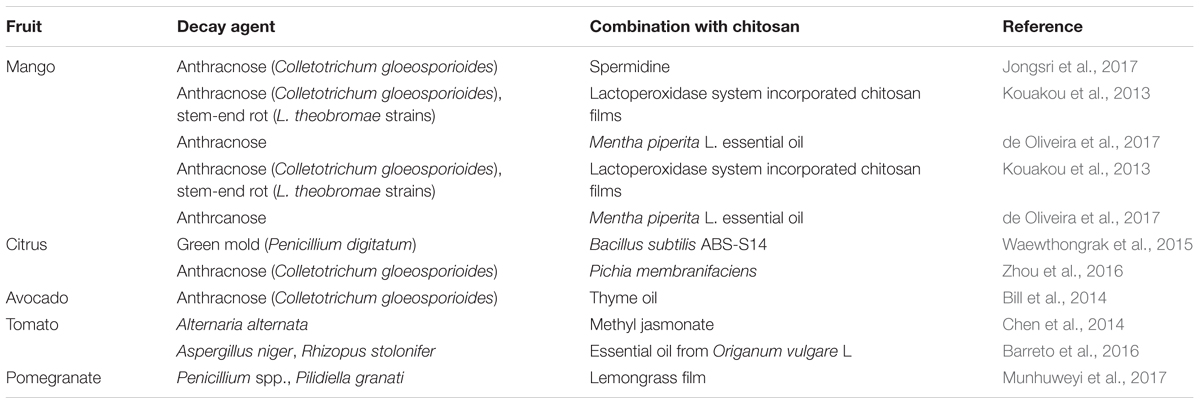
TABLE 2. Postharvest chitosan treatments with other applications for storage decay of subtropical fruit.
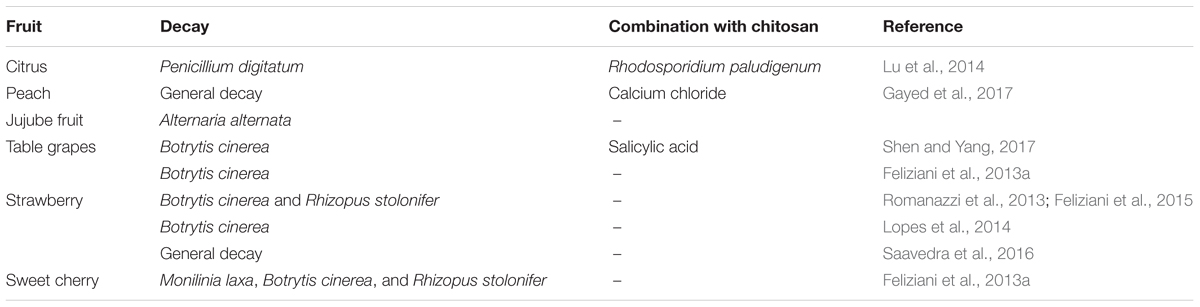
TABLE 3. Preharvest chitosan treatments with other applications for storage decay of temperate fruit.
Chitosan is known to elicit plant defences against several classes of pathogens, including fungi, viruses, bacteria and phytoplasma (El Hadrami et al., 2010). Moreover, in some studies, its eliciting activity was reported to be effective on pests (Badawy and Rabea, 2016). Based on our experience, the eliciting activity of chitosan accounts for 30 to 40% of its effectiveness in the control of postharvest decay of fresh fruit (Figure 2). The extent of this eliciting activity depends on the reactivity of the fruit tissues, and it is well known that fruit responses to stress decline with ripening (Romanazzi et al., 2016). This eliciting activity of chitosan has been studied since the 1990’s, which started with monitoring of the activities of enzymes linked to the defense mechanisms (e.g., chitinase) in different fruit (e.g., strawberries) (El Ghaouth et al., 1992). This was followed by investigations on other berries, citrus fruit and table grapes, among others. More recently, tools such as qRT-PCR and in recent years RNA-Seq (RNA-Sequencing) have allowed important information to be gained, first at the level of single gene expression, and then later at the level of global gene expression (Xoca-Orozco et al., 2017). This has provided good understanding of the multiple actions of chitosan applications and how they affect a number of physiological changes in fruit. As an example, the application of chitosan to strawberries at different times before harvest can affect the expression of a thousand or more genes (Landi et al., 2017). Some examples that have become available in the literature over the last 5 years are listed in Table 4, which deal with the physiological changes that can occur in chitosan-treated fruit, both when the biopolymer is applied alone, and when it is combined with other treatments. The eliciting activity of chitosan is particularly effective toward latent infections, as a more reactive fruit can stop the infection process, through a balance that resembles quorum sensing, which is well known for bacterial infections (Papenfort and Bassler, 2016).

TABLE 4. Physiological changes that can occur in fresh fruit after chitosan treatment, alone or in combination with other applications.
Numerous studies on chitosan inhibitory activities toward numerous microrganisms have been carried out since the first report of almost half a century ago (Allan and Hadwiger, 1979). The antimicrobial activities of chitosan against a wide range of plant pathogens have been confirmed by any of in-vitro and in-vivo studies. The antimicrobial activity of chitosan is one of its main properties, and this depends on the concentration at which it is applied. In the control of postharvest decay of fresh fruit, the antimicrobial activity can account for 35–45% of its effectiveness, as an antifungal barrier on a fruit inhibits the germination of fungal spores and slows down the rate of decay-causing fungi of already infected fruit, both latently and actively (Figure 2). A standard application rate of chitosan to provide a significant control of postharvest decay of fruit and vegetables can be considered 1%, except for the control of Penicillia, where higher concentrations may be needed to provide a good effectiveness. The degree of deacetylation and the molecular weight of chitosan characterize its properties, such as the number of positively charges of amino groups and therefore, its electrostatic interactions with different substrate and organisms at different pH. Chitosan with a higher degree of deacetylation, which has greater numbers of positive charges, would also be expected to have stronger antibacterial activities. On the other hand, numerous studies have generated different results relating to correlations between the chitosan bactericidal activities and its molecular weight (Romanazzi et al., 2017). In addition, there are many differences between the chitosan antifungal and antibacterial activities and several mechanisms relating to these remain still unclear and further researches are needed (Romanazzi et al., 2017).
Once applied to a plant surface by dipping or spraying, chitosan can form an edible coating, the properties of which (e.g., thickness, viscosity, gas, and water permeability) greatly depend on the acid in which the biopolymer is dissolved. The film-forming properties of chitosan account for 20–30% of the chitosan effectiveness in the control of postharvest decay of fruit and vegetables (Figure 2). Coating produces a barrier for gas exchanges and reduced respiration, and slows down fruit ripening. Of note, a less ripe fruit is less sensitive to postharvest decay.
When first used in experimental trials, chitosan needed to be dissolved in an acid (e.g., hydrochloric acid, acetic acid, which were among the most effective ones; see Romanazzi et al., 2009), and then taken to the optimal pH (∼5.6) This approach can even take 1–2 days, and it is impractical for use by growers. More recently, several commercial chitosan formulations that can be dissolved in water have become available on the market to be used as a biopesticides (Table 5). Some of these are formulated as powders, and then the cost of shipping is lower (although still higher compared to most of the commercially available synthetic fungicides), although the chitosan needs to be dissolved in water, in some cases a few hours before its application. This makes chitosan more difficult to use, as the grower wants to use an alternative to synthetic fungicides in the same way as a commercial compound, such that it should have the same effectiveness. This objective can be achieved with liquid formulations, which have concentrations of 2–15%. In this case, the cost of shipping is higher, as the volumes are larger due to the amounts of water that travel with the chitosan. In tests of three different commercial products, even when used at the same concentration, differential effectiveness was seen (Feliziani et al., 2013a). The higher cost of chitosan treatment compared to standard applications might also induce companies toward the use of low doses (e.g., even well below 0.1%), Based on data in literature, the optimal dose is around 1%, while decreasing the concentration, the effectiveness declines. Furthermore, when the concentration of chitosan is decreased, its effectiveness also declines. However, applications to the plant canopy also need to take in account possible phytotoxic effects, mainly if repeated applications occur. This has been shown for grapevines (Romanazzi et al., 2016a), such that for these purposes a good concentration might be 0.5%. However, under some particular conditions, even low concentrations of chitosan (e.g., 0.02%) in a commercial formulation can be beneficial, such as for the improved storage of litchi (Jiang et al., 2018).
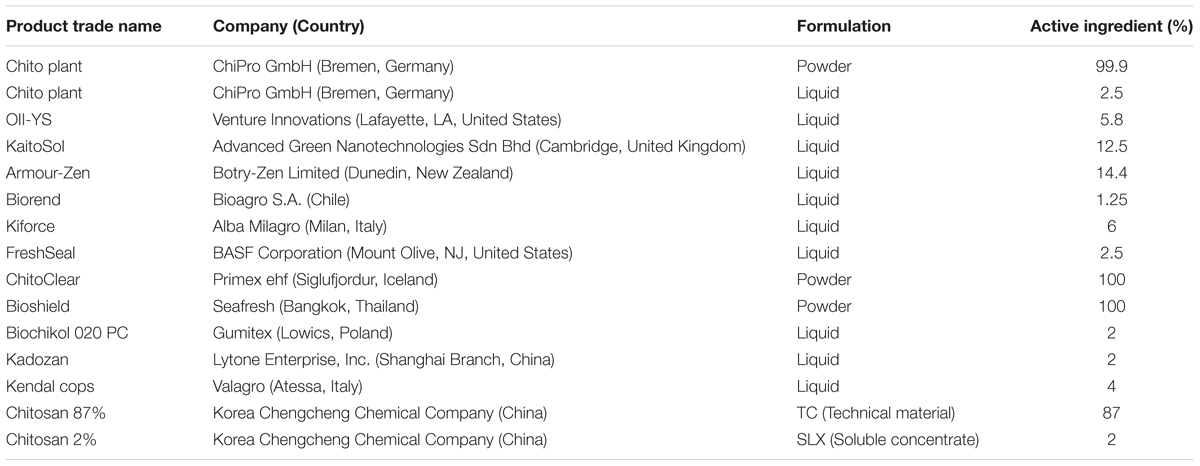
TABLE 5. Some chitosan-based commercial products that are available for control of postharvest diseases of fruit and vegetables.
The effectiveness of chitosan application arises from the integrated combination of its three mechanisms of action. There are increasing consumer requests for fruit and vegetables to be free from residues of synthetic pesticides, such that the rules defined by the public administration have become more limiting in terms of the active ingredients allowed and the maximum residue limits. Also, large stores compete with each other to further reduce these limits, compared to the legal thresholds (Romanazzi et al., 2016b). These trends make the concept of the application of alternatives to synthetic fungicides more popular, and among these the main one that is already used in human medicine is chitosan, which is particularly welcomed by public opinion. These aspects have promoted further studies based on the multiple actions of chitosan on fruit and vegetables. Therefore, further increases in our knowledge are expected following the widespread practical application of chitosan due to the regulation of its use in agriculture and the interest of companies to promote chitosan-based products, with potential benefits for the growers, the consumers and the environment.
GR proposed the review, collected data on chitosan popularity over time and on commercial products, coordinated the authors, and wrote the article. EF collected papers on effectiveness of chitosan on temperate fruit and on the mechanisms of action in the tables, and helped with the writing. DS collected papers on effectiveness of chitosan on tropical fruit and on the mechanisms of action in the tables, and helped with the writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Allan, C. R., and Hadwiger, L. A. (1979). The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 3, 285–287. doi: 10.1016/S0147-5975(79)80054-7
Al-Qurashi, A. D., and Mohamed, S. A. (2015). Postharvest chitosan treatment affects quality, antioxidant capacity, antioxidant compounds and enzymes activities of ‘El-Bayadi’ table grapes after storage. Sci. Hortic. 197, 393–398. doi: 10.1016/j.scienta.2015.09.065
Al-Sheikh, H., and Yehia, R. S. S. (2016). In vitro antifungal efficacy of Aspergillus niger ATCC 9642 chitosan-AgNPs composite against post-harvest disease of citrus fruits. Appl. Biochem. Microbiol. 52, 413–420. doi: 10.1134/S0003683816040177
Arnon, H., Zaitsev, Y., Porat, R., and Poverenov, E. (2014). Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharv. Biol. Technol. 87, 21–26. doi: 10.1016/j.postharvbio.2013.08.007
Badawy, M., and Rabea, I. (2016). “Chitosan and its derivatives as active ingredients against plant pests and diseases,” in Chitosan in the Preservation of Agricultural Commodities, eds S. Bautista-Baòos, G. Romanazzi, and A. Jiménez-Aparicio (Amsterdam: Elsevier), 179–219. doi: 10.1016/B978-0-12-802735-6.00007-0
Badawy, M. E. I., Rabea, E. I., El-Nouby, M. A. M., Ismail, R. I. A., and Taktak, N. E. M. (2017). Strawberry shelf life, composition, and enzymes activity in response to edible chitosan coatings. Int. J. Fruit Sci. 17, 117–136. doi: 10.1080/15538362.2016.1219290
Barreto, T., Andrade, S. C., Maciel, J. F., Arcanjo, N. M. O., Madruga, M. S., Meireles, B., et al. (2016). A Chitosan coating containing essential oil from Origanum vulgare L. to control postharvest mold infections and keep the quality of cherry tomato fruit. Front. Microbiol. 7:1724. doi: 10.3389/fmicb.2016.01724
Bautista-Baòos, S., Hernandez-Lauzardo, A. N., Velazquez-del Valle, M. G., Hernandez-Lopez, M., Ait Barka, E., Bosquez-Molina, E., et al. (2006). Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 25, 108–118. doi: 10.1016/j.cropro.2005.03.010
Bautista-Baòos, S., Romanazzi, G., and Jiménez-Aparicio, A. (eds) (2016). Chitosan in the Preservation of Agricultural Commodities. Amsterdam: Elsevier.
Benhabiles, M. S., Drouiche, N., Lounici, H., Pauss, A., and Mameri, N. (2013). Effect of shrimp chitosan coatings as affected by chitosan extraction processes on postharvest quality of strawberry. J. Food Meas. Charact. 7, 215–221. doi: 10.1007/s11694-013-9159-y
Bill, M., Sivakumar, D., Korsten, L., and Thompson, A. K. (2014). The efficacy of combined application of edible coating and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 64, 159–167. doi: 10.1016/j.cropro.2014.06.015
Chen, J., Zou, X., Liu, Q., Wang, F., Feng, W., and Wan, N. (2014). Combination effect of chitosan and methyl jasmonate on controlling Alternaria alternata and enhancing activity of cherry tomato fruit defence mechanisms. Crop Prot. 56, 31–36. doi: 10.1016/j.cropro.2013.10.007
Cindi, M. D., Shittu, T., Sivakumar, D., and Bautista-Baños, S. (2015). Chitosan boehmite-alumina nanocomposite films and thyme oil vapour control brown rot in peaches (Prunus persica L.) during postharvest storage. Crop Prot. 72, 127–131. doi: 10.1016/j.cropro.2015.03.011
Coqueiro, D. S. O., de Souza, A. A., Takita, M. A., Rodrigues, C. M., Kishi, L. T., and Machado, M. A. (2015). Transcriptional profile of sweet orange in response to chitosan and salicylic acid. BMC Genomics 16:288. doi: 10.1186/s12864-015-1440-5
Darolt, J. C., Rocha Neto, A. C., and Di Piero, R. M. (2016). Effects of the protective, curative, and eradicative applications of chitosan against Penicillium expansum in apples. Braz. J. Microbiol. 47, 1014–1019. doi: 10.1016/j.bjm.2016.07.007
de Oliveira, C. E. V., Magnani, M., de Sales, C. V., de Souza Pontes, A. L., Campos-Takaki, G. M., Stamford, T. C. M., et al. (2014). Effects of post-harvest treatment using chitosan from Mucor circinelloides on fungal pathogenicity and quality of table grapes during storage. Food Microbiol. 44, 211–219. doi: 10.1016/j.fm.2014.06.007
de Oliveira, K. ÁR., Berger, L. R. R., de Araújo, S. A., Camara, M. P. S., and de Souza, E. L. (2017). Synergistic mixtures of chitosan and Menta piperita L. essential oil to inhibit Colletorichum species and anthracnose development in mango cultivar Tommy Atkins. Food Microbiol. 66, 96–103. doi: 10.1016/j.fm.2017.04.012
Deng, L., Yin, B., Yao, S., Wang, W., and Zeng, K. (2016). Postharvest application of oligochitosan and chitosan reduces calyx alterations of citrus fruit induced by ethephon degreening treatment. J. Agric. Food Chem. 64, 7394–7403. doi: 10.1021/acs.jafc.6b02534
Deng, Z., Jung, J., Simonsen, J., Wang, Y., and Zhao, Y. (2017). Cellulose nanocrystal reinforced chitosan coatings for improving the storability of postharvest pears under both ambient and cold storages. J. Food Sci. 82, 453–462. doi: 10.1111/1750-3841.13601
Ding, F., Nie, Z., Deng, H., Xiao, L., Du, Y., and Shi, X. (2013). Antibacterial hydrogel coating by electrophoretic co-deposition of chitosan/alkynyl chitosan. Carbohydr. Polym. 98, 1547–1552. doi: 10.1016/j.carbpol.2013.07.042
Duran, M., Aday, M. S., Zorba, N. N. D., Temizkan, R., Büyükcan, M. B., and Caner, C. (2016). Potential of antimicrobial active packaging “containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating” to extend shelf life of fresh strawberry. Food Bioprod. Process. 98, 354–363. doi: 10.1016/j.fbp.2016.01.007
El Ghaouth, A., Arul, J., Grenier, J., and Asselin, A. (1992). Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopatology 82, 398–402. doi: 10.1094/Phyto-82-398
El Hadrami, A., Adam, L. R., El Hadrami, I., and Daayf, F. (2010). Chitosan in plant protection. Mar. Drugs 8, 968–987. doi: 10.3390/md8040968
Elbarbary, A. M., and Mostafa, T. B. (2014). Effect of γ-rays on carboxymethyl chitosan for use as antioxidant and preservative coating for peach fruit. Carbohydr. Polym. 104, 109–117. doi: 10.1016/j.carbpol.2014.01.021
Felipini, R. B., Boneti, J. I., Katsurayama, Y., Neto, A. C. R., Veleirinho, B., Maraschin, M., et al. (2016). Apple scab control and activation of plant defence responses using potassium phosphite and chitosan. Eur. J. Plant Pathol. 145, 929–939. doi: 10.1007/s10658-016-0881-2
Feliziani, E., Landi, L., and Romanazzi, G. (2015). Preharvest treatments with chitosan and other alternatives to conventional fungicides to control postharvest decay of strawberry. Carbohydr. Polym. 132, 111–117. doi: 10.1016/j.carbpol.2015.05.078
Feliziani, E., Romanazzi, G., Margosan, D. A., Mansour, M. F., Smilanick, J. L., Gu, S., et al. (2013a). Preharvest fungicide, potassium sorbate, or chitosan use on quality and storage decay of table grapes. Plant Dis. 97, 307–314. doi: 10.1094/pdis-12-11-1043-re
Feliziani, E., Santini, M., Landi, L., and Romanazzi, G. (2013b). Pre- and postharvest treatment with alternatives to synthetic fungicides to control postharvest decay of sweet cherry. Postharv. Biol. Technol. 78, 133–138. doi: 10.1016/j.postharvbio.2012.12.004
Freitas, P. M., López-Gálvez, F., Tudela, J. A., Gil, M. I., and Allende, A. (2015). Postharvest treatment of table grapes with ultraviolet-C and chitosan coating preserves quality and increases stilbene content. Postharv. Biol. Technol. 105, 51–57. doi: 10.1016/j.postharvbio.2015.03.011
Gao, P., Zhu, Z., and Zhang, P. (2013). Effects of chitosan-glucose complex coating on postharvest quality and shelf life of table grapes. Carbohydr. Polym. 95, 371–378. doi: 10.1016/j.carbpol.2013.03.029
Gayed, A. A. N. A., Shaarawi, S. A. M. A., Elkhishen, M. A., and Elsherbini, N. R. M. (2017). Pre-harvest application of calcium chloride and chitosan on fruit quality and storability of “Early Swelling” peach during cold storage. Ciência Agrot. 41, 220–231. doi: 10.1590/1413-70542017412005917
Gol, N. B., Patel, P. R., and Rao, T. V. R. (2013). Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharv. Biol. Technol. 85, 185–195. doi: 10.1016/j.postharvbio.2013.06.008
Guerra, I. C. D., De Oliveira, P. D. L., Santos, M. M. F., Lúcio, A. S. S. C., Tavares, J. F., Barbosa-Filho, J. M., et al. (2016). The effects of composite coatings containing chitosan and Mentha (piperita L. or x villosa Huds) essential oil on postharvest mold occurrence and quality of table grape cv. Isabella. Innov. Food Sci. Emerg. Technol. 34, 112–121. doi: 10.1016/j.ifset.2016.01.008
Guo, H., Xing, Z., Yu, Q., Zhao, Y., and Zhu, E. (2017). Effectiveness of preharvest application of submicron chitosan dispersions for controlling Alternaria rot in postharvest jujube fruit. J. Phytopathol. 165, 425–431. doi: 10.1111/jph.12576
Gutierrez-Martinez, P., Bautista-Baños, S., Berúmen-Varela, S., Ramos-Guerrero, A., and Hernández-Ibaéz, A. M. (2017). In vitro response of Colletotrichum to chitosan. Effect on incidence and quality on tropical fruit. Enzymatic expression in mango. Acta Agron. 62, 282–289. doi: 10.15446/acag.v66n2.53770
Irkin, R., and Guldas, M. (2014). Chitosan coating of red table grapes and fresh-cut honey melons to inhibit Fusarium oxysporum growth. J. Food Process. Preserv. 38, 1948–1956. doi: 10.1111/jfpp.12170
Jiang, X., Lin, H., Shi, J., Neethirajan, S., Lin, Y., Chen, Y., et al. (2018). Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chem. 252, 134–141. doi: 10.1016/j.foodchem.2018.01.095
Jongsri, P., Rojsitthisak, P., Wangsomboondee, T., and Seraypheap, K. (2017). Influence of chitosan coating combined with spermidine on anthracnose disease and qualities of ‘Nam Dok Mai’ mango after harvest. Sci. Hortic. 224, 180–187. doi: 10.1016/j.scienta.2017.06.011
Kanetis, L., Exarchou, V., Charalambous, Z., and Goulas, V. (2017). Edible coating composed of chitosan and Salvia fruticosa Mill. extract for the control of grey mould of table grapes. J. Sci. Food Agric. 97, 452–460. doi: 10.1002/jsfa.7745
Kaur, M., Kalia, A., and Thakur, A. (2017). Effect of biodegradable chitosan–rice-starch nanocomposite films on post-harvest quality of stored peach fruit. Starch 69:1600208. doi: 10.1002/star.201600208
Khalifa, I., Barakat, H., El-Mansy, H. A., and Soliman, S. A. (2016). Improving the shelf-life stability of apple and strawberry fruits applying chitosan-incorporated olive oil processing residues coating. Food Packag. Shelf Life 9, 10–19. doi: 10.1016/j.fpsl.2016.05.006
Khalifa, I., Barakat, H., El-Mansy, H. A., and Soliman, S. A. (2017). Preserving apple (Malus domestica var. Anna) fruit bioactive substances using olive wastes extract-chitosan film coating. Inform. Process. Agric. 4, 90–99. doi: 10.1016/j.inpa.2016.11.001
Kou, X. H., Guo, W. L., Guo, R. Z., Li, X. Y., and Xue, Z. H. (2014a). Effects of chitosan, calcium chloride, and pullulan coating treatments on antioxidant activity in Pear cv. “Huang guan” during storage. Food Bioprocess. Technol. 7, 671–681. doi: 10.1007/s11947-013-1085-9
Kou, X. H., Wang, S., Zhang, Y., Guo, R. Z., Wu, M. S., Chen, Q., et al. (2014b). Effects of chitosan and calcium chloride treatments on malic acid-metabolizing enzymes and the related gene expression in post-harvest pear cv. “Huang guan.” Sci. Hortic. 165, 252–259. doi: 10.1016/j.scienta.2013.10.034
Kouakou, I. M., Clementine, M., Didier, M., Gérard, L., and Ducamp-Collin, M. N. (2013). Antimicrobial and physical properties of edible chitosan films enhanced by lactoperoxidase system. Food Hydrocoll. 30, 576–580. doi: 10.1016/j.foodhyd.2012.07.018
Landi, L., De Miccolis Angelini, R. M., Pollastro, S., Feliziani, E., Faretra, F., and Romanazzi, G. (2017). Global transcriptome analysis and identification of differentially expressed genes in strawberry after preharvest application of benzothiadiazole and chitosan. Front. Plant Sci. 8:235. doi: 10.3389/fpls.2017.00235
Landi, L., Feliziani, E., and Romanazzi, G. (2014). Expression of defense genes in strawberry fruits treated with different resistance inducers. J. Agric. Food Chem. 62, 3047–3056. doi: 10.1021/jf404423x
Lei, J., Yang, L., Zhan, Y., Wang, Y., Ye, T., Deng, H., et al. (2014). Plasma treated polyethylene terephthalate/polypropylene films assembled with chitosan and various preservatives for antimicrobial food packaging. Coll. Surf. Biointerfaces 114, 60–66. doi: 10.1016/j.colsurfb.2013.09.052
Li, H., Wang, Y., Liu, F., Yang, Y., Wu, Z., Cai, H., et al. (2015). Effects of chitosan on control of postharvest blue mold decay of apple fruit and the possible mechanisms involved. Sci. Hortic. 186, 77–83. doi: 10.1016/j.scienta.2015.02.014
Liu, K., Yuan, C., Chen, Y., Li, H., and Liu, J. (2014). Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 176, 45–53. doi: 10.1016/j.scienta.2014.06.027
Lopes, U. P., Zambolim, L., Costa, H., Pereira, O. L., and Finger, F. L. (2014). Potassium silicate and chitosan application for gray mold management in strawberry during storage. Crop Prot. 63, 103–106. doi: 10.1016/j.cropro.2014.05.013
Lu, L., Liu, Y., Yang, J., Azat, R., Yu, T., and Zheng, X. (2014). Quaternary chitosan oligomers enhance resistance and biocontrol efficacy of Rhodosporidium paludigenum to green mold in satsuma orange. Carbohydr. Polym. 113,174–181. doi: 10.1016/j.carbpol.2014.06.077
Ma, Z., Yang, L., Yan, H., Kennedy, J. F., and Meng, X. (2013). Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 94, 272–277. doi: 10.1016/j.carbpol.2013.01.012
Mohammadi, A., Hashemi, M., and Hosseini, S. M. (2015). Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol. 28, 73–80. doi: 10.1016/j.ifset.2014.12.011
Moussa, S. H., Tayel, A. A., Alsohim, A. S., and Abdallah, R. R. (2013). Botryticidal activity of nanosized silver-chitosan composite and its application for the control of gray mold in strawberry. J. Food Sci. 78, 1589–1594. doi: 10.1111/1750-3841.12247
Munhuweyi, K., Oluwafemi, J. C., Lennox, C. L., van Reenen, A. J., and Opara, L. U. (2017). In vitro and in vivo antifungal activity of chitosan-essential oils against pomegranate fruit pathogens. Postharv. Biol. Technol. 129, 9–22. doi: 10.1016/j.postharvbio.2017.03.002
Muzzarelli, R. A. A. (1986). “Filmogenic properties of chitin/chitosan,” in Chitin in Nature and Technology, eds R. A. A. Muzzarelli, C. Jeuniaux, and G. W. Gooday (New York, NY: Plenum Press), 389–396. doi: 10.1007/978-1-4613-2167-5_48
Pagliarulo, C., Sansone, F., Moccia, S., Russo, G. L., Aquino, R. P., Salvatore, P., et al. (2016). Preservation of strawberries with an antifungal edible coating using peony extracts in chitosan. Food Bioprocess. Technol. 9, 1951–1960. doi: 10.1007/s11947-016-1779-x
Papenfort, K., and Bassler, B. L. (2016). Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 14, 576–588. doi: 10.1038/nrmicro.2016.89
Pasquariello, M. S., Di Patre, D., Mastrobuoni, F., Zampella, L., Scortichini, M., and Petriccione, M. (2015). Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharv. Biol. Technol. 109, 45–56. doi: 10.1016/j.postharvbio.2015.06.007
Perdones, Á, Vargas, M., Atarés, L., and Chiralt, A. (2014). Physical, antioxidant and antimicrobial properties of chitosan-cinnamon leaf oil films as affected by oleic acid. Food Hydrocoll. 36, 256–264. doi: 10.1016/j.foodhyd.2013.10.003
Petriccione, M., Mastrobuoni, F., Zampella, L., Nobis, E., Capriolo, G., and Scortichini, M. (2017). Effect of chitosan treatment on strawberry allergen-related gene expression during ripening stages. J. Food Sci. Technol. 54, 1340–1345. doi: 10.1007/s13197-017-2554-3
Romanazzi, G., Feliziani, E., Bautista-Baòos, S., and Sivakumar, D. (2017). Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 57, 579–601. doi: 10.1080/10408398.2014.900474
Romanazzi, G., Feliziani, E., Santini, M., and Landi, L. (2013). Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharv. Biol. Technol. 75, 24–27. doi: 10.1016/j.postharvbio.2012.07.007
Romanazzi, G., Lichter, A., Mlikota Gabler, F., and Smilanick, J. (2012). Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharv. Biol. Technol. 63, 141–147. doi: 10.1016/j.postharvbio.2011.06.013
Romanazzi, G., Mlikota Gabler, F., Margosan, D. A., Mackey, B. E., and Smilanick, J. L. (2009). Effect of chitosan dissolved in different acids on its ability to control postharvest gray mold of table grape. Phytopathology 99, 1028–1036. doi: 10.1094/PHYTO-99-9-1028
Romanazzi, G., Sanzani, S. M., Bi, Y., Tian, S., Gutierrez-Martinez, P., and Alkan, N. (2016). Induced resistance to control postharvest decay of fruit and vegetables. Postharv. Biol. Technol. 122, 82–94. doi: 10.1016/j.postharvbio.2016.08.003
Romanazzi, G., Mancini, V., Feliziani, E., Servili, A., Endeshaw, S., and Neri, D. (2016a). Impact of alternative fungicides on grape downy mildew control and vine growth and development. Plant Dis. 100, 739–748. doi: 10.1094/PDIS-05-15-0564-RE
Romanazzi, G., Smilanick, J. L., Feliziani, E., and Droby, S. (2016b). Integrated management of postharvest gray mold on fruit crops. Postharv. Biol. Technol. 113, 69–76. doi: 10.1016/j.postharvbio.2015.11.003
Saavedra, G. M., Figueroa, N. E., Poblete, L. A., Cherian, S., and Figueroa, C. R. (2016). Effects of preharvest applications of methyl jasmonate and chitosan on postharvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem. 190, 448–453. doi: 10.1016/j.foodchem.2015.05.107
Sangsuwan, J., Pongsapakworawat, T., Bangmo, P., and Sutthasupa, S. (2016). Effect of chitosan beads incorporated with lavender or red thyme essential oils in inhibiting Botrytis cinerea and their application in strawberry packaging system. Food Sci. Technol. 74, 14–20. doi: 10.1016/j.lwt.2016.07.021
Shanmuga Priya, D., Suriyaprabha, R., Yuvakkumar, R., and Rajendran, V. (2014). Chitosan-incorporated different nanocomposite HPMC films for food preservation. J. Nanopart. Res. 16:2248. doi: 10.1007/s11051-014-2248-y
Shao, X., Cao, B., Xu, F., Xie, S., Yu, D., and Wang, H. (2015). Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharv. Biol. Technol. 99, 37–43. doi: 10.1016/j.postharvbio.2014.07.014
Shen, Y., and Yang, H. (2017). Effect of preharvest chitosan-g-salicylic acid treatment on postharvest table grape quality, shelf life, and resistance to Botrytis cinerea-induced spoilage. Sci. Hortic. 224, 367–373. doi: 10.1016/j.scienta.2017.06.046
Tayel, A. A., Moussa, S. H., Salem, M. F., Mazrou, K. E., and El-Tras, W. F. (2016). Control of citrus molds using bioactive coatings incorporated with fungal chitosan/plant extracts composite. J. Sci. Food Agric. 96, 1306–1312. doi: 10.1002/jsfa.7223
Treviño-Garza, M. Z., García, S., Flores-González, M., del, S., and Arévalo-Niño, K. (2015). Edible active coatings based on pectin, pullulan, and chitosan increase quality and shelf life of strawberries (Fragaria × ananassa). J. Food Sci. 80, M1823–M1830. doi: 10.1111/1750-3841.12938
Valenzuela, C., Tapia, C., López, L., Bunger, A., Escalona, V., and Abugoch, L. (2015). Effect of edible quinoa protein-chitosan based films on refrigerated strawberry (Fragaria × ananassa) quality. Electron. J. Biotechnol. 18, 406–411. doi: 10.1016/j.ejbt.2015.09.001
Velickova, E., Winkelhausen, E., Kuzmanova, S., Alves, V. D., and Moldão-Martins, M. (2013). Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria × ananassa cv Camarosa) under commercial storage conditions. Food Sci. Technol. 52, 80–92. doi: 10.1016/j.lwt.2013.02.004
Waewthongrak, W., Pisuchpen, S., and Leelasuphakul, W. (2015). Effect of Bacillus subtilis and chitosan applications on green mould (Penicilium digitatum Sacc.) decay in citrus fruit. Postharv. Biol. Technol. 99, 44–49. doi: 10.1016/j.postharvestbio.2014.07.016
Wang, Y., Li, B., Zhang, X., Peng, N., Mei, Y., and Liang, Y. (2017). Low molecular weight chitosan is an effective antifungal agent against Botryosphaeria sp. and preservative agent for pear (Pyrus) fruits. Int. J. Biol. Macromol. 95, 1135–1143. doi: 10.1016/j.ijbiomac.2016.10.105
Wu, T., Dai, S., Cong, X., Liu, R., and Zhang, M. (2017). Succinylated soy protein film coating extended the shelf life of apple fruit. J. Food Process. Preserv. 41, 13024–13034. doi: 10.1111/jfpp.13024
Xoca-Orozco, L. A., Cuellar-Torres, E. A., González-Morales, S., Gutiérrez-Martinez, P., López-Garcia, U., Herrera-Estrella, L., et al. (2017). Transcriptomic analysis of avocado Hass (Persea americana Mill) in the interaction system fruit-chitosan- Colletotrichum. Front. Plant Sci. 8:956. doi: 10.3389/fpls.2017.00956
Zhang, H., Li, R., and Liu, W. (2011). Effects of chitin and its derivative chitosan on postharvest decay of fruits: a review. Int. J. Mol. Sci. 12, 917–934. doi: 10.3390/ijms12020917
Zheng, W., Li, L., Pan, S., Liu, M., Zhang, W., Liu, H., et al. (2017). Controls postharvest decay and elicits defense response in kiwifruit. Food Bioprocess. Technol. 11, 1937–1945. doi: 10.1007/s11947-017-1957-5
Keywords: antimicrobial activity, biopolymer, coating, induced resistance, natural fungicide
Citation: Romanazzi G, Feliziani E and Sivakumar D (2018) Chitosan, a Biopolymer With Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Front. Microbiol. 9:2745. doi: 10.3389/fmicb.2018.02745
Received: 04 July 2018; Accepted: 26 October 2018;
Published: 04 December 2018.
Edited by:
Boqiang Li, Institute of Botany (CAS), ChinaReviewed by:
Xianghong Meng, Ocean University of China, ChinaCopyright © 2018 Romanazzi, Feliziani and Sivakumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gianfranco Romanazzi, Zy5yb21hbmF6emlAdW5pdnBtLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.