- 1Novel Bacteria and Drug Discovery Research Group, Biomedicine Research Advancement Centre, School of Pharmacy, Monash University Malaysia, Bandar Sunway, Malaysia
- 2Biomedical Research Laboratory, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Bandar Sunway, Malaysia
- 3Center of Health Outcomes Research and Therapeutic Safety, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
- 4UKM Medical Molecular Biology Institute, UKM Medical Centre, National University of Malaysia, Bangi, Malaysia
- 5Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Shatin, Hong Kong
Vibrio parahaemolyticus, a Gram-negative halophilic bacterium is often associated with fish and fishery products, thus causing gastroenteritis in humans upon ingestion of contaminated food. V. parahaemolyticus has become a globally well-known pathogen with yearly reported cases in many countries. This study aimed to discover the antibiotic resistance patterns of V. parahaemolyticus as well as detect Carbapenem resistant isolates from marine and freshwater fish in Selangor. A total of 240 freshwater and marine fish samples collected from wet market and supermarket in Selangor were tested for the presence of V. parahaemolyticus. All the fish samples were determined positive for V. parahaemolyticus using conventional microbiological culture-based method. The toxR gene were detected via polymerase chain reaction (PCR) in 165/240 (69%) isolates. The two-virulence factor of V. parahaemolyticus, thermostable direct hemolysin (tdh) and TDH-related hemolysin (trh) was screened via PCR. As such, four isolates were trh+and none were tdh+. Majority of the isolates presented high resistance to ampicillin (88%), amikacin (64%), and kanamycin (50%). In addition, this study identified 19-imipenem resistant isolates isolated from freshwater and marine fish samples. Further analysis of these 19-imipenem resistant isolates revealed that the resistance toward imipenem was plasmid mediated after plasmid curing assay. The multiple antibiotics resistance index was >0.2 for 70% of the isolates. In summary, the results confirm the presence of V. parahaemolyticus in freshwater and marine fish samples in Selangor, Malaysia. To our best knowledge, this is the first report discovering the antibiotic resistant patterns and Carbapenem-resistant isolates of V. parahaemolyticus isolated from marine and freshwater fish samples in Selangor.
Introduction
Vibrionaceae family within the class of Gammaproteobacteria comprises of Gram-negative halophilic bacteria, straight or curved rods, ubiquitous and indigenous in aquatic environments (Tison and Kelly, 1984; Tantillo et al., 2004; Sawabe et al., 2013). The Vibrio genus consists of 142 species that are marine originated and its taxonomy is continuously been revised due to the discovery of new species (Sawabe et al., 2013). Vibrio parahaemolyticus is among the member of this genus that been regarded as important human pathogenic bacteria (Su and Liu, 2007; Iwamoto et al., 2010; Bier et al., 2015; Law et al., 2015). The species is widely distributed in marine and estuarine environments thus leading to gastrointestinal infections upon consumption of raw or undercooked seafood (Kubota et al., 2008; Letchumanan et al., 2014; Lee and Raghunath, 2018). Based on the published data by Centers for Disease Control and Prevention (CDC) in the United States during the year 2016, V. parahaemolyticus is considered as a major foodborne bacterium compared to other Vibrio species and accounted for nearly 34,664 foodborne cases annually in the United States (Scallan et al., 2011; Huang et al., 2016).
In terms of its pathogenicity, thermostable direct hemolysin (tdh) gene, TDH-related hemolysin (trh) gene, T3SS systems (T3SS1 and T3SS2) are among the virulence factors own by pathogenic V. parahaemolyticus in order to initiate an infection (Letchumanan et al., 2014, 2017). Usually, 99% of clinical V. parahaemolyticus isolates are known to be pathogenic because they carry tdh genes and/or trh genes, whereas majority of the environmental isolates are non-pathogenic (Sudha et al., 2012; Tsai et al., 2013). Nevertheless, around 0–6% of the environmental isolates are identified as pathogenic carrying tdh gene and/or trh gene (Letchumanan et al., 2014, 2015a).
The aquaculture industry in Malaysia is mainly associated with its economic gains from supplying domestic and foreign demands, and as well as generating a steady income for farmers (Witus and Vun, 2016). Fish is among the popular fishery products that been consumed in daily basis by consumers from Southeast Asian countries (Hajeb et al., 2009). Around 75% of the global fishery production is mainly for human consumptions (Teh, 2012). In Malaysia, the fish consumption has increased since 1970 and now its above 40 kg/capita/year (Teh, 2012). Professed has a healthy food, fish contains a high level of proteins, omega-3 fatty acids (n-3), essential vitamins and minerals that are required by an individual (Aremu and Ekunode, 2008; Hajeb et al., 2009). There are variety of fishes that been consumed by Malaysian in their daily life including the Indian mackerel, Spanish mackerel, black pomfret, silver pomfret, yellowstripe scad, catfish, fringe scale sardine, and tilapia (Osman et al., 2001; Hajeb et al., 2009; Taweel et al., 2013). The expanding and intense aquaculture industry has led to the suppression of immune systems and increases the susceptibility of fish to bacterial infections (Davies et al., 2001; Basti et al., 2006; Harikrishnan et al., 2011).
Intensified fish farming in order to meet consumers demand has prompted the use of antibiotics as treatment regime, prophylaxis and as growth promotion (Vaseeharan et al., 2005). Antibiotics are often been in-cooperated as feed additives or immersion bath in order to treat bacterial infections, promote fast growth of fish, and also prevent the growth of water plants (Abu Bakar et al., 2010). Oxytetracycline, tetracycline, quinolones, sulphonamides, trimethoprim, nalidixic acid, gentamicin, nitrofurazone, and trimethoprim-sulfamethoxazole are among the permitted antibiotics used in the Asian aquaculture industry (Harikrishnan et al., 2011; Manjusha and Sarita, 2011; Rico et al., 2012; Yano et al., 2014). Extensive use of antibiotics in aquaculture has resulted in the increase antibiotic resistance among bacteria including Vibrio species (Tendencia and de la Peña, 2001; Jerbi et al., 2011; Heng et al., 2017; Lee and Raghunath, 2018). Direct transmission of resistant bacteria through food to human, and transfer of resistance genes to other bacteria happens, thus causing a possible hazard to human wellbeing (Duran and Marshall, 2005; Guglielmetti et al., 2009; Kim et al., 2013).
Antimicrobial resistance (MDR) has been recognized as an important global threat issue to global public health and food safety (Food and Agriculture Organization [FAO], 2016). In hospitals, many clinical antibiotics are no longer effective to control bacterial infections (Tan et al., 2016). As a result of misuse of antibiotic to control infections during aquaculture production, V. parahaemolyticus has been reported to exhibit multidrug resistance, which raised the concern about public health and economic threat of this bacterium (Vaseeharan et al., 2005; Han et al., 2007; Lesley et al., 2011; Manjusha and Sarita, 2011; Noorlis et al., 2011). Carbapenems are always been regarded as the last treatment selection for Gram-positive and Gram-negative infections, and as well as infections caused by multidrug resistant bacteria (Nordmann et al., 2011; Martin et al., 2018). Nevertheless, their use has been compromised causing an increased incidence of carbapenem-resistant bacteria, and widely been discussed among medical practitioners, researchers, and public (Martin et al., 2018). A study by Nordmann and colleagues identified the novel New Delhi metallo-β-lactamase (NDM) encoded by the gene blaNDM-1 in members of the family Enterobacteriaceae. This gene was reported to be not only present largely in Enterobacteriaceae, but also in Vibrionaceae (Nordmann et al., 2011). Over the years, Carbapenem-resistant Vibrio sp. has been detected and isolated from environmental and seafood samples (Walsh et al., 2011; Mandal et al., 2012; Gu et al., 2014; Bier et al., 2015). Recently, in Kolkata, NDM-1 producing Vibrio fluvialis strains has been isolated from diarrheal fecal samples from patients (Chowdhury et al., 2016).
The increase in bacterial resistance toward many clinical antibiotics affects many countries healthcare sector and food production sectors (World Health Organization [WHO], 2014). In view of previous reports and the possible severity of infections, continuous investigation on antimicrobial resistance of V. parahaemolyticus is needed for epidemiological purpose and guidance in healthcare treatment. For this reason, our study aimed to assess antimicrobial susceptibility profiles of V. parahaemolyticus from marine and freshwater fish in Selangor, Malaysia. In addition, we also report the identification and antibiotic resistant characterization of Carbapenem-resistant isolates isolated from marine and freshwater fish samples. To our knowledge, this is the first study examining the antibiotic resistant profiles and Carbapenem-resistant isolates of V. parahaemolyticus from both marine and freshwater fish samples in Selangor, Malaysia.
Materials and Methods
Sampling
The study focused on two category fish – marine and freshwater fish. A total of 240 fish samples comprising of yellowstripe scad (Selaroides leptolepis) (n = 48), Indian mackerel (Rastrelliger kanagurta) (n = 48), black pomfret (Parastromateus niger) (n = 48), catfish (Clarias batrachus) (n = 48), and red tilapia (Oreochromis spp.) (n = 48) were collected from three wet markets and three supermarkets in Selangor (Table 1). From each sampling site, we collected eight fish samples and sampling was done weekly from January 2016 to May 2016. All the samples were kept in sterile sealed bags and transported to the laboratory in an ice box. Samples were analyzed immediately thereafter.
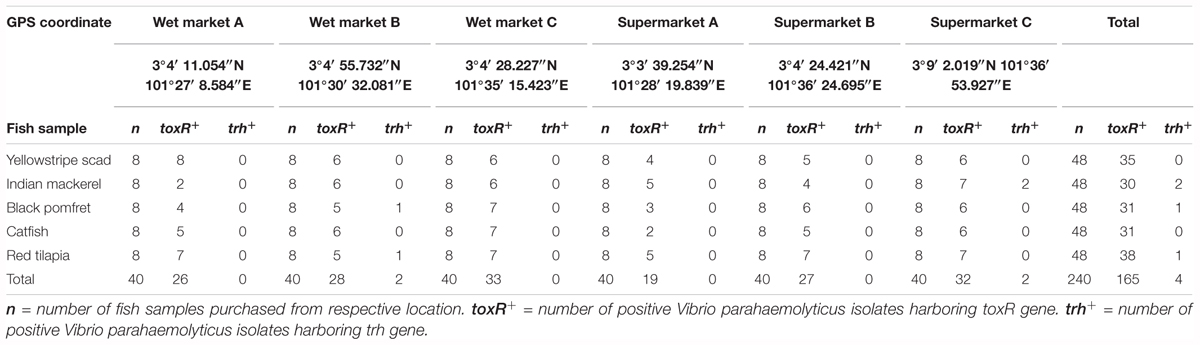
TABLE 1. The frequency of Vibrio parahaemolyticus detected in different fish samples from different sampling locations by using PCR assay.
Isolation of Vibrio sp. in Fish Samples
Isolation of Vibrio sp. was following Standard US Food and Drug Administration (FDA) protocol (Kaysner and DePaola, 2004) and FAO/WHO Risk Assessment of V. parahaemolyticus in Seafood (Food and Agriculture Organization/World Health Organization [FAO/WHO], 2011); this method was previously reported by Zarei et al. (2012) and Letchumanan et al. (2015a). 25 g of sample (gut and fish meat) was homogenized with 225 mL of alkaline peptone water (APW) with 2% w/v sodium chloride (NaCl), pH 8.5 for 60 s using a stomacher (BagMixer 400W, Interscience, Saint-Nom-la-Bretèche, France). The homogenate was enriched at 37°C for 18 h. After 18 h of incubation, a loopful of enriched mixture was streaked onto selective media, Thiosulfate Citrate Bile Salts Sucrose (TCBS) agar (HiMedia, India) and incubated at 37°C for 18 h. In each plate, one sucrose non-fermenting colony that has a green or bluish green color measuring about 3–5 mm suggestive of V. parahaemolyticus was selected from the TCBS plates. The isolate was purified by re-streaking onto Tryptic Soy Agar (TSA) (HiMedia, India) plates supplemented with 2% w/v sodium chloride (NaCl) (Vivantis, United States). The purified colony were inoculated into TSB semi-solid nutrient agar and stored until further identification.
DNA Extraction
Bacterial lysate was prepared following established protocol (Suzita et al., 2010; Vengadesh et al., 2012; Letchumanan et al., 2015a,c). The isolates were revived in tryptic soy broth (TSB) (HiMedia) supplemented with 2% w/v sodium chloride NaCl (Vivantis, United States). Overnight suspension was transferred into 1.5 mL of microcentrifuge tube and centrifuged. The supernatant was discarded and 1 mL of sterile ultrapure water was added and vortexed. The suspension was heated at 100°C for 7 min and then cooled on ice immediately into ice for 5 min. Cell debris from the cell lysate were pelleted by centrifugation at 13,000 rpm for 1 min. The supernatant was used as DNA templates for polymerase chain reaction (PCR) assays.
Identification of Vibrio parahaemolyticus Using toxR-PCR Assay
Specific primers targeting toxR gene with the expected amplicon size of 368 bp were used to identify V. parahaemolyticus (Kim et al., 1999; Letchumanan et al., 2015a). The PCR assay was performed in 20 μL reaction mixture containing 2 μL of DNA template, 10 μL of 2× Taq PLUS PCR Smart mix 1 (SolGentTM, South Korea), 6 μL of ultrapure water and 1 μL of each primer. toxR-based PCR amplification was performed using PCR thermocycler (Kyratec, Super Cycler Thermal Cycler, Australia) with the following cycling conditions: initial denaturation at 95°C for 4 min, 35 cycles of 94°C for 1 min, 68°C for 1 min and 72°C for 30 s, and a final elongation at 72°C for 5 min. PCR products was visualized by using 1.5% agarose gel and viewed under UV transilluminator using a Gel Documentation System (ChemiDocTM XRS, Bio-Rad, United States). The toxR-PCR results of a few presumptive V. parahaemolyticus isolates and type strain Vibrio parahaemolyticus NBRC 12711 were sequenced to confirm the identity of toxR gene (Supplementary Table S1). The Vibrio parahaemolyticus NBRC 12711 was used as the positive control and Vibrio vulnificus NBRC 15645 was used as the negative control.
Detection of Virulence Gene
Molecular identification of thermostable direct haemolysin (tdh) and thermostable-related direct haemolysin (trh) was performed using a duplex PCR assay (Bej et al., 1999; Letchumanan et al., 2015c). The PCR assay was done in 20 μL of reaction mixture containing 2 μL of DNA template, 10 μL of 2× Taq PLUS PCR Smart mix 1 (SolGentTM, South Korea), 4 μL of sterile distilled water and 1 μL of each primer. The PCR amplifications was performed using a Thermocycler (Kyratec, Super Cycler Thermal Cycler, Australia) with the following cycling conditions: initial denaturation at 94°C for 3 min, 30 cycles of 94°C for 1 min, 58°C for 1 min and 72°C for 1 min, and a final elongation at 72°C for 5 min. The PCR products was visualized by using 1.5% agarose gel and viewed under UV transilluminator using a Gel Documentation System (ChemiDocTM XRS, Bio-Rad, United States). The PCR results of a few presumptive V. parahaemolyticus isolates and type strain Vibrio parahaemolyticus NBRC 12711 were sequenced to confirm the identity of virulence gene (Supplementary Table S1). Vibrio parahaemolyticus NBRC 12711 was used as the positive control and Vibrio vulnificus NBRC 15645 was used as the negative control.
Antibiotic Susceptibility Test
The antibiotic susceptibility of V. parahaemolyticus isolates was determined using Kirby-Bauer disk diffusion method (Yano et al., 2014). Fourteen type of antibiotics disks (Oxoid, United Kingdom) was used: amplicon (10 μg), ampicillin/sulbactam (30 μg), amikacin (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), chloramphenicol (30 μg), gentamicin (30 μg), imipenem (10 μg), kanamycin (30 μg), levofloxacin (5 μg), nalidixic acid (30 μg), oxytetracycline (30 μg), sulfamethoxazole/trimethoprim (25 μg), and tetracycline (30 μg). E. coli ATCC 25922 with known sensitivity pattern was included as a positive control in each test.
V. parahaemolyticus isolates was grown in tryptic soy broth (TSB) (HiMedia, India) 2% w/v sodium chloride (NaCl) (Vivantis, United States) at 37°C for 18 h under constant agitation. The antibiotic disks were dispensed on Mueller Hilton agar (HiMedia, India) supplemented with 2% w/v NaCl (Vivantis, United States) plates with bacterial lawn. After incubation at 37°C for 18 h, the inhibition zone was measured and interpreted based on guidelines of the Clinical and Laboratory Standards Institute (CLSI) M45-A2 (Clinical and Laboratory Standards Institute [CLSI], 2010). The multiple antibiotic resistance (MAR) index was determined based on the ratio of antibiotic resistance exhibited by the isolate to the number of antibiotics to which the isolates were exposed (Krumperman, 1983).
Plasmid Curing
The 19-imipenem resistant V. parahaemolyticus isolate was subjected to plasmid curing assay to determine the antibiotic resistance mediation. The plasmid curing assay was performed using an intercalating agent, ethidium bromide (EB) (Lou et al., 2002; Molina-Aja et al., 2002; Letchumanan et al., 2015b). The isolates were revived in freshly prepared tryptic tryptic soy broth (TSB) supplemented with 0.2 mg/mL EB (Bio Basic, Canada), then incubated at 37°C for 18 h under constant agitation. After treatment with the curing agent, the antibiotic resistance profiles were re-examined and compared with the antibiotic resistance phenotype on non-treated group.
Genomic and Phylogenetic Analyses
Polymerase chain reaction amplification of the 16s rRNA gene for the 19-imipenem resistant V. parahaemolyticus was done according to the protocol described by Thomas et al. (2018) with slight modifications. The 16S rRNA gene sequence of each isolate was aligned with representative sequences of related type strains in the genus V. parahaemolyticus retrieved from the GenBank/EMBL/DDBJ databases using CLUSTAL-X software (Thompson et al., 1997). The alignment was first verified manually and adjusted, followed by construction of phylogenetic trees with neighbor-joining (Saitou and Nei, 1987; Figure 1) and maximum-likelihood algorithms (Felsenstein, 1981), utilizing the MEGA version 6.0 (Tamura et al., 2013). For neighbor-joining algorithm, the evolutionary distances were computed using the Kimura’s two-parameter model (Kimura, 1980). The calculations of level of sequence similarity were performed by GenBank server1. Bootstrap based on 1,000 resampling method of Felsenstein (1985) was used to analyze the stability of the resultant tree topologies.
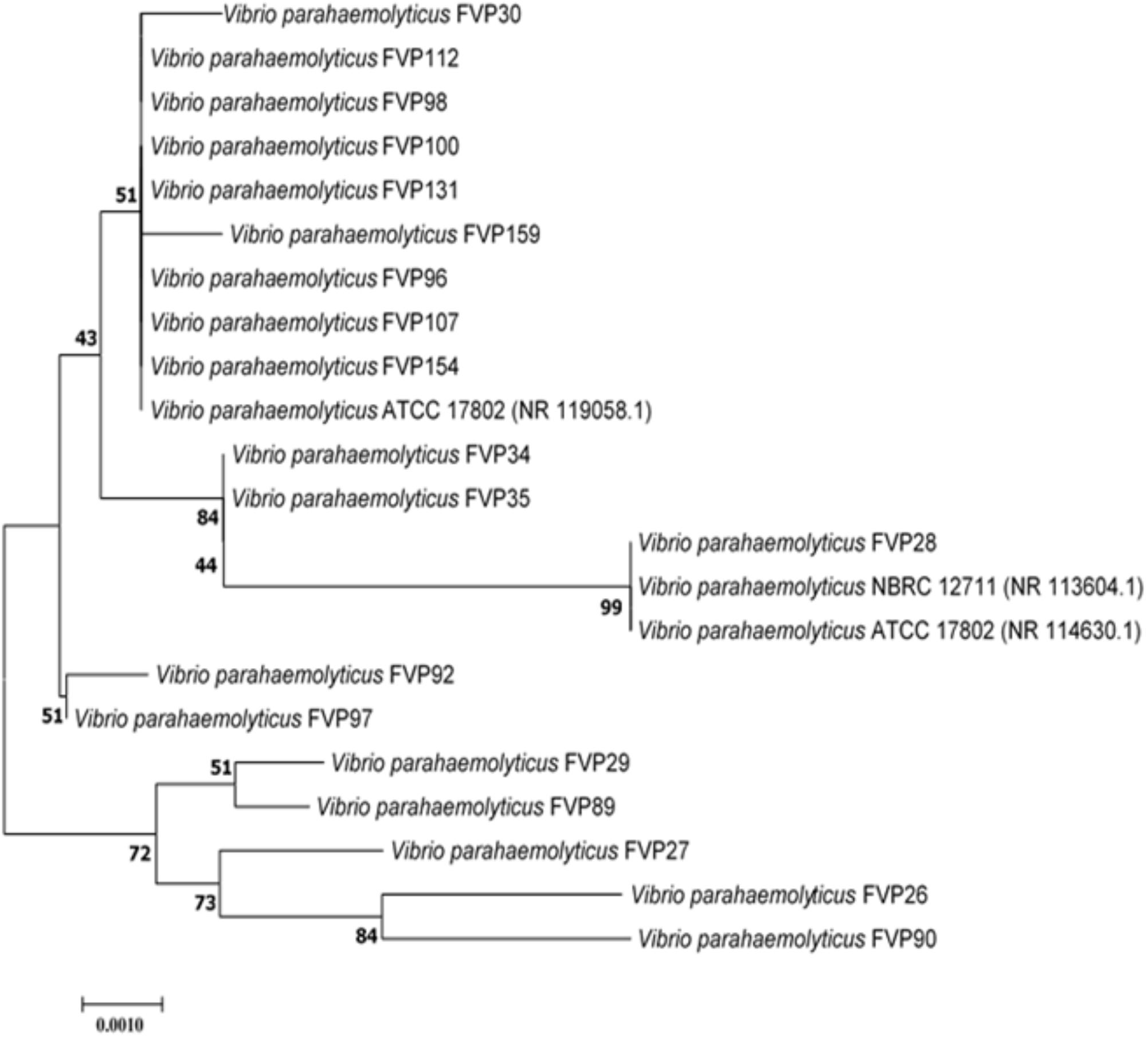
FIGURE 1. Neighbor-joining phylogenetic tree based on almost complete 16S rRNA sequences showing the relationship between 19-imipenem resistant isolate and representatives of some other related taxa. Numbers at nodes indicate percentages of 1,000 bootstrap re-samplings. Bar: 0.0010 substitutions per site.
Statistical Analysis
Data analysis was performed with SPSS statistical analysis software version 20. Statistical analysis was performed in order to determine whether there was any significant difference in between two types of fish (marine and freshwater fish) and the MAR index of resistant isolates using the independent t-test. The significance level was set at p ≤ 0.05. One-way analysis of variance (ANOVA) followed by appropriate post hoc text (Tukey) was performed to determine the significant differences between the type of fishes and MAR index of resistant isolates. A difference was considered statistically significant when p ≤ 0.05.
Results
Prevalence of Vibrio parahaemolyticus in Fish Samples
The present study isolated V. parahaemolyticus from freshwater and marine fish. A total of 240 fish samples comprising of yellowstripe scad (Selaroides leptolepis) (n = 48), Indian mackerel (Rastrelliger kanagurta) (n = 48), black pomfret (Parastromateus niger) (n = 48), catfish (Clarias batrachus) (n = 48), and red tilapia (Oreochromis spp.) (n = 48) were collected from three wet market and three supermarkets. Based on the colony morphology on TCBS agar, a total of 240 isolates was picked and purified on TSA agar. The toxR-PCR assay exhibited positive amplification of toxR gene with 368 bp amplicon band in 69% (165/240) of the presumptive V. parahaemolyticus isolates. Based on the sampling location site, 47% (78/165) of the isolates originated from the wet market and 53% (87/165) was from supermarket. A total of 96 (58%) of the isolates were isolated from marine fish samples and 69 (42%) of the isolates were isolated from freshwater fish samples.
Detection of Thermostable Direct Hemolysin (tdh) and tdh-Related Hemolysin (trh)
A duplex PCR assay was performed to detect the presences of tdh and trh gene in all isolates (Table 1). None of the 165 V. parahaemolyticus isolates yielded tdh-positive PCR amplification. Only 4 (2.4%) out of the total 165 V. parahaemolyticus showed positive PCR amplification of the trh gene. The trh-positive V. parahaemolyticus isolates was isolated from black pomfret (wet market B) (FVP81), red tilapia (wet market B) (FVP92), and two from Indian mackerel (supermarket C) (FVP47 and FVP49). The presence of trh-positive V. parahaemolyticus isolates in both types of fish samples indicates possible high risk of foodborne gastroenteritis transmission to humans upon ingestion of the fish.
Antimicrobial Susceptibilities of Vibrio parahaemolyticus Isolates
Most of the tested antibiotics in this study such as tetracycline, folate pathway inhibitors (trimethoprim-sulfamethoxazole), third-generation cephalosporins (cefotaxime and ceftazidime), aminoglycosides (gentamicin and amikacin) and fluoroquinolones (ciprofloxacin and levofloxacin), are among the recommended antibiotics by CDC for the treatment of Vibrio sp. infections (Daniels and Shafaie, 2000; Shaw et al., 2014). Table 2 summarizes the percentage of antibiotic resistant profiles of V. parahaemolyticus isolated from fish sample. Based on the results, the resistance rate of the 165 V. parahaemolyticus isolates in our study was 88% to ampicillin, 64% to amikacin, and 50% to kanamycin. A notable resistance pattern can be observed to the third generation cephalosporins (cefotaxime 52% and ceftazidime 28%). In contrast, high susceptibility rate was seen to chloramphenicol (93%), tetracycline (90%), imipenem (85%), levofloxacin (85%), gentamicin (84%), sulfamethoxazole/trimethoprim (80%), nalidixic acid (78%), oxytetracycline (72%), and ampicillin/sulbactam (70%).
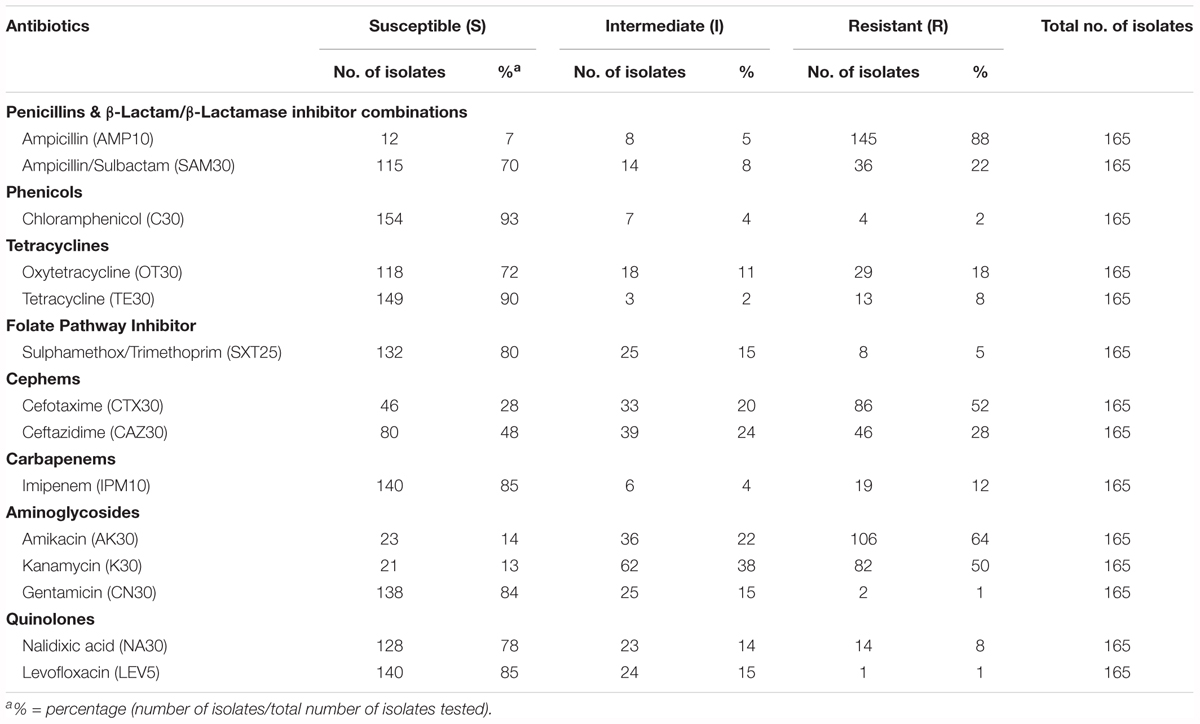
TABLE 2. Percentage of antibiotic susceptible, intermediate, and resistant of V. parahaemolyticus isolated from various fish samples.
Interestingly, 19 isolates (12%) from this study exhibited resistance to imipenem, an antibiotic in Carbapenem class. The detection of imipenem resistant isolates is of concern as Carbapenems are among the beta-lactams that is the last line antibiotic used for bacterial infection treatment (Meletis, 2016). These 19 isolates had an MAR index of 0.14 to 0.50, resistant to more than two different type of antibiotics tested. Majority of the imipenem resistant isolates were isolate from freshwater fish sample (15/19) and the remaining 4 isolates were isolated from marine fish samples.
In this study, the values of MAR index ranged from 0.00 to 0.57 (Table 3). Forty-two different resistance patterns had a significant MAR index more than 0.2. Two of the isolates (FVP24 – yellowstripe scad, marine fish and FVP67 – red tilapia, freshwater fish) has the highest MAR index of 0.57, resistant to 8/14 antibiotics tested. Further analysis was performed by comparing the MAR index between source of sample (marine and freshwater) and MAR index. The mean MAR index of marine fish sample was 0.26 where else, freshwater fish sample was 0.25. The results showed that there was no significant difference between source of fish sample and MAR index. According to the one-way ANOVA analysis, there was no significant difference between the fish types on the MAR index of V. parahaemolyticus isolates. The results suggest that isolates from the fish samples may have similar level of antibiotic exposure, regardless there are marine or freshwater originated. As shown in Figure 2, 8% of V. parahaemolyticus isolates (13 isolates) did not exhibited MAR as they were susceptible to all of the antibiotics tested.
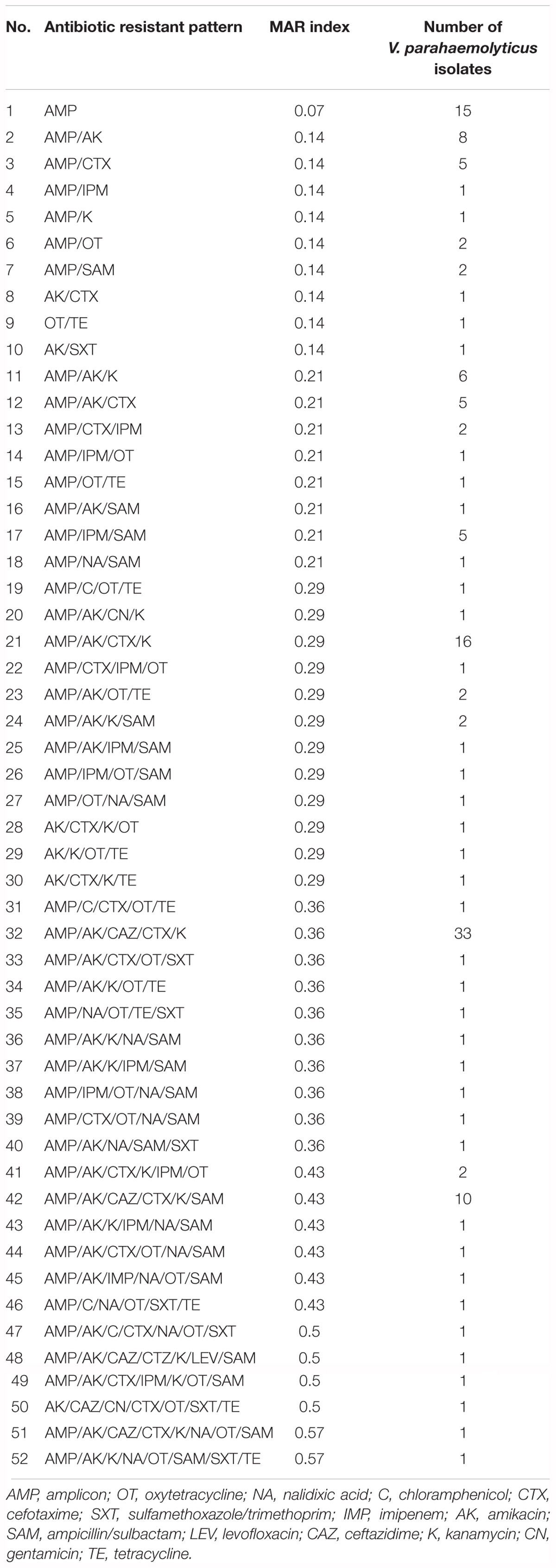
TABLE 3. Antibiogram and multiple antimicrobial resistance (MAR) indices of V. parahaemolyticus isolates.
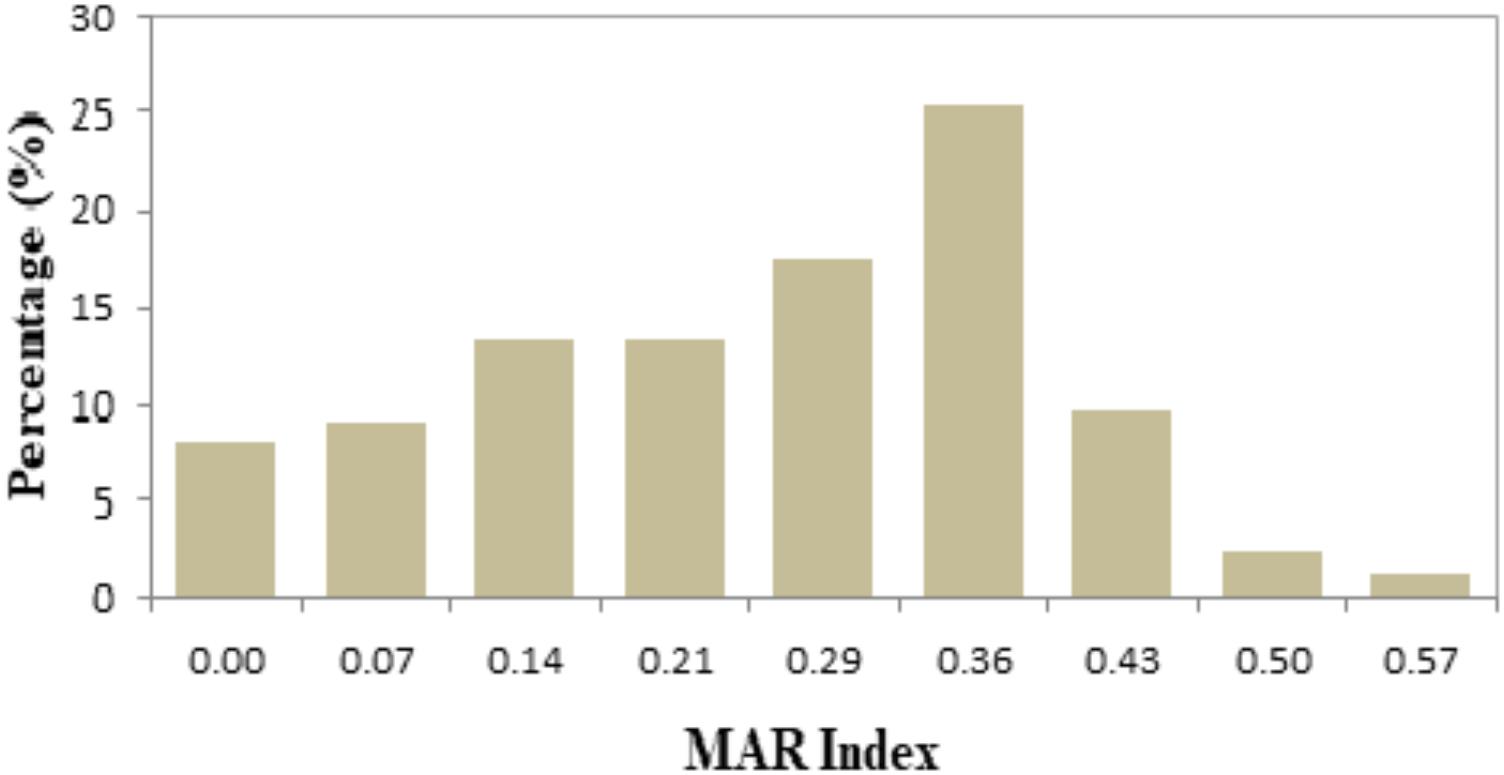
FIGURE 2. Percentage occurrence of MAR index of Vibrio parahaemolyticus isolates from all fish samples of different sampling locations. The isolates exhibited MAR index from 0.00 to 0.57, representing that the V. parahaemolyticus isolates are resistant to 0–8 types of antibiotics tested.
The antibiotic resistance patterns between freshwater and marine fish samples did not exhibit any significant profiles. Based on the analysis, both freshwater and marine fish samples were exposed to antibiotics and phenotypic assay showed a similar resistant profile to 0 to 8 types of antibiotics tested. For each fish sample, the mean MAR indices for V. parahaemolyticus isolates was 0.26 for yellowstripe scad, Indian mackerel was 0.24, black pomfret was 0.29, catfish was 0.25, and red tilapia was 0.24.
Plasmid Curing
Plasmid curing may server as an effective assay to determine the antibiotic resistance mediation of bacteria. This assay enables to eliminate desired bacterial plasmid and subsequently reassess the antibiotic resistance phenotype by antibiotic disk diffusion method. Table 4 summarizes the antibiotic resistance profile of the 19-imipenem resistant isolates before and after plasmid curing assay. All the phenotypically seen imipenem resistant isolates became susceptible to imipenem after curing assay, suggesting the resistance was plasmid mediated. All the isolates were still resistant to ampicillin and oxytetracycline, suggesting a possible chromosomal mediated resistance. Hence, the antibiotic resistance seen in 19-imipenem isolates are both plasmid and chromosomally mediated.
Genomic and Phylogenetic Analyses
The nearly complete 16S rRNA gene sequence was determined for all the 19-imipenem resistant V. parahaemolyticus isolates and manual alignment of these sequences was performed with the corresponding partial 16S rRNA gene sequences of the type strains of V. parahaemolyticus retrieved from GenBank/EMBL/DDBJ databases. Phylogenetic tree was constructed based on the 16S rRNA gene sequences to determine the phylogenetic position of the 19-imipenem resistant isolates (Figure 1). Phylogenetic analysis exhibited that closely related strains include Vibrio parahaemolyticus ATCC 17802 (NR 119058.1), Vibrio parahaemolyticus NBRC 12711 (NR 113604.1) and Vibrio parahaemolyticus ATCC 17802 (NR 114630.1), as the 19-imipenem resistant isolates form distinct five clades. The isolates within the same clade are closely related.
Discussion
The occurrence of pathogenic strains of V. parahaemolyticus in fish samples we studied does raise concern as this organism is known to cause foodborne gastroenteritis resulted from ingesting of uncooked or undercooked seafood (Ma et al., 2014; Romalde et al., 2014). However, while the microbiological culture-based method found all fish samples to be contaminated Vibrio sp., only 69% (165/240) of there were confirmed to be V. parahaemolyticus based on toxR PCR assay; and only 2.4% (4/165) of these were pathogenic strains (trh-positive) (Table 1). Our results came to an agreement with other researchers on the fact that the identity of V. parahaemolyticus could not be fully confirmed by conventional microbiological culture-based method (Kim et al., 1999; Zulkifli et al., 2009; Fabbro et al., 2010; Ottaviani et al., 2013). Affirming with previous research, we found that toxR PCR assay was specific and reliable technique for the identification of both pathogenic and non-pathogenic V. parahaemolyticus (Kim et al., 1999; Dileep et al., 2003; Zulkifli et al., 2009). This reliable and specific toxR-PCR assay has resulted in many promising V. parahaemolyticus identifications studies (Deepanjali et al., 2005; Das et al., 2009; Vimila et al., 2010; Elamparithi and Ramanathan, 2011; Noorlis et al., 2011; Paydar et al., 2013). The remaining 75 isolates had the morphology of V. parahaemolyticus in TCBS agar, however, the toxR gene was not present in these isolates. This result demonstrates the detection of V. parahaemolyticus thru toxR PCR assay is highly sensitive, specific and accurate compared to microbiological culture-based technique (Mandal et al., 2011).
The tdh and trh genes are considered major virulence factors in V. parahaemolyticus, so in many clinically isolated strains of V. parahaemolyticus have hemolytic activity that is produced by these two genes (Ceccarelli et al., 2013; Raghunath, 2015). Our study reported the isolation of trh-positive isolates of V. parahaemolyticus at a very low prevalence rate, and none of the isolates have tdh-position genes. Our results follow the trends of worldwide dispersed studies that have reported low number of virulent V. parahaemolyticus strains from environmental sources (Fuenzalida et al., 2006; Nordstrom et al., 2007; Thongjun et al., 2013). Many studies have reported low prevalence rate (less than 5%) of environmental and food source have pathogenic V. parahaemolyticus isolates carrying tdh and/or trh genes (Parveen et al., 2008; Zulkifli et al., 2009; Tsai et al., 2013). In addition, it is strongly suggested that putative pathogenic environmental V. parahaemolyticus isolates may be less virulent than the clinical V. parahaemolyticus isolates (Vongxay et al., 2008; Tsai et al., 2013). The presences of tdh+ and/or trh+ V. parahaemolyticus in the marine and freshwater fish samples in Selangor is of concern due to several factors. Firstly, the fact that these pathogenic isolates could potentially cause gastroenteritis (Jun et al., 2012). Secondly, pathogenic V. parahaemolyticus not only contaminate seafood and transmit pathogenesis, but it also causes huge economic loss in the aquaculture sector (Fuenzalida et al., 2006; Thongjun et al., 2013). Hence, the study results need the importance for continuous monitoring of seafood for pathogen contamination.
Our antibiotic susceptibility test placed ampicillin at the top of the V. parahaemolyticus resistance scope (88%). This finding signifying that ampicillin may longer be an effective antibiotic to treat Vibrio sp. infections. In fact, V. parahaemolyticus resistance to ampicillin is well reported in many literatures (Joseph et al., 1978; Lesmana et al., 2001; Zulkifli et al., 2009; Melo et al., 2011; Oh et al., 2011; Al-Othrubi et al., 2014). Interestingly, ampicillin resistance was reported 100% in study by Devi et al. (2009) and Ottaviani et al. (2013). The chromosomally encoded β-lactamase is the cause for V. parahaemolyticus resistance to ampicillin and other penicillin (Devi et al., 2009). In addition, more that 70% of the V. parahaemolyticus isolates in this study remained susceptible to tetracycline, levofloxacin, gentamicin, sulfamethoxazole/trimethoprim, chloramphenicol, imipenem, nalidixic acid, oxytetracycline, and ampicillin/sulbactam. Our findings are in line with previous studies that reported susceptibility of V. parahaemolyticus against chloramphenicol, tetracyclines, trimethoprim-sulfamethoxazole, nalidixic acid, and imipenem (Ottaviani et al., 2001; Devi et al., 2009; Melo et al., 2011; Al-Othrubi et al., 2014; Shaw et al., 2014). The MAR index values ranged from 0 to 0.57.
Forty-two different resistance patterns had a significant MAR value >0.2. Collectively, there were expressed by 70% of the V. parahaemolyticus isolates and resistant to 3 to 8 types of antibiotics tested. MAR index >0.2 are exposed to several antibiotics or isolated from contaminated sources as such dairy cattle, aquaculture, and agriculture farms. Where else, isolates with lesser than 0.2 MAR indices are lessen prone to antibiotic exposure (Noorlis et al., 2011; Subramani and Vignesh, 2012). In this study there was no significant difference been observed among the source of sample and MAR index. This result demonstrates that the isolates isolated from marine and freshwater samples are exposure of antibiotics. Our results came to an agreement with many studies that reported high percentage of V. parahaemolyticus isolated from seafood are resistant to more than one antibiotic tested (Zulkifli et al., 2009; Lesley et al., 2011; Manjusha and Sarita, 2011; Noorlis et al., 2011).
Imipenem, a member of Carbapenem class is an effective antibiotic used in the treatment of Gram-positive and Gram-negative infections (Papp-Wallace et al., 2011). Interestingly, in this study we detected 19-imipenem resistant V. parahaemolyticus strains isolated from marine and freshwater fish samples. Ever since the first detection case of carbapenemase producing Carbapenem-Resistant Enterobacteriaceae (Cp-Cre) in the United States, Cp-Cre have rapidly spread with more reported cases in another 50 states (Centers for Disease Control and Prevention [CDC], 2018). In fact, now carbapenem resistance is no longer associated with Enterobacteriaceae but also associated with other bacteria. As such, the resistance of Vibrio sp. to carbapenem has been reported by Bier et al. (2015) in Germany coastal line, Gu et al. (2014) in Southwest China, and Walsh et al. (2011) in India. Thus, our results agree with other findings and demonstrates the misuse of carbapenem that may cause a negative impact on the clinical treatment of Vibrio infections in future. Hence, a non-antibiotic approach is required in order to manage the occurrence of antibiotic resistance among Vibrio sp. in the environments (Tan et al., 2014; Letchumanan et al., 2016; Tan et al., 2016).
Further analysis on the 19-imipenem resistant isolates by plasmid curing assay exhibited interesting findings. The antibiotic resistance phenotype of these 19 isolates have been altered after plasmid curing. All the 19 isolate’s phenotypically seen resistance to imipenem has changed to susceptible, suggesting the resistance was plasmid mediated. All the isolates were still resistant to ampicillin, suggesting the resistance was chromosomal mediated. In addition, the isolate FVP92, FVP96, FVP98, FVP100, FVP107, FVP112, FVP131, FVP154, FVP159 (Table 3) remained resistant to oxytetracycline even after plasmid curing and its chromosomally mediated. It is usual to find oxytetracycline resistant isolates from aquaculture products because this antibiotic is among the permitted antimicrobial used in the seafood production. In summary, plasmids are transferable between different bacteria and the presence of antibiotic resistant genes in the bacterial plasmid have facilitated the fast spreading of antibiotic resistance among bacteria (Wilson and Salyers, 2003; Stepanauskas et al., 2006; Manjusha and Sarita, 2011). Hence, the acquisition of imipenem resistance by the 19 isolates are possibly due to horizontal gene transfer from other environmental bacteria.
The results from phylogenetic and genomic analyses indicated that the 19-imipenem resistant isolates are closely related forming five clades (Figure 1). The isolates are closely related to each another within the same clade. Isolate FVP28 was closely related to Vibrio parahaemolyticus NBRC 12711 and Vibrio parahaemolyticus ATCC 17802 at 99% bootstrap value, indicating the high confident level of the association. The isolate FVP28 and both type strains are isolated from food source. The FVP28 was isolated from freshwater red tilapia where else, Vibrio parahaemolyticus Nbrd 12711 and Vibrio parahaemolyticus ATCC 17802 was originally isolated from shirasu food poisoning case in Japan. This result exhibits a close relationship between these strains isolated from different types of seafood. In addition, there was another clade with nine isolates (FVP30, FVP112, FVP98, FVP100, FVP131, FVP159, FVP96, FVP107, and FVP154) that were closely related to Vibrio parahaemolyticus ATCC 17802 at 51% bootstrap value. 7/9 of the isolates (FVP30, FVP112, FVP98, FVP100, FVP159, FVP96, and FVP107) was isolated from freshwater fish sample. Majority of the isolates within this clade were resistant to oxytetracycline, an antibiotic that is permitted in Asian aquaculture industry (Yano et al., 2014). In summary, phylogenetic tree analysis revealed that there was no distinctive grouping based on the antibiogram of each isolate, however, the16S rRNA sequencing had a high discriminating power to group the isolates into different clades (Hoffmann et al., 2010).
The global increase of antibiotic resistant bacteria is of great public health concern and warrants a continuous monitoring (Xie et al., 2017). In the case of V. parahaemolyticus, the situation is aggravated due to excessive use of antimicrobial agents in aquaculture to protect infectious diseases and huge production loses (Xu et al., 2016). In addition, antimicrobial resistance is likely caused by exposure to antibiotics via agriculture runoff or wastewater treatment plants, and thru mobile genetic elements or horizontal gene transfers among bacteria (Stepanauskas et al., 2006; Kümmerer, 2009; Al-Othrubi et al., 2014; Xu et al., 2016). Recently, the Food and Agriculture Organization (FAO) have drawn action plans to increase awareness and promote prudent use of antimicrobials (Food and Agriculture Organization [FAO], 2015).
Conclusion
Our study confirms the presences of V. parahaemolyticus in freshwater and marine fish samples in Selangor by having use highly accurate detection and identification method (the combination of microbiological culture-based method and PCR). To our best knowledge, this study represents the first evidence of Carbapenem resistant isolate and as well as antibiotic resistance patterns of V. parahaemolyticus isolated from freshwater and marine fish samples. The detection of tdh and trh genes provides better understanding regarding the distribution of pathogenic V. parahaemolyticus strains in fish samples. Despite the fact that majority most of the environmental V. parahaemolyticus isolates are non-pathogenic, consumer should still be aware and ensure that fish is cooked properly before consumption. Adequate cooking of fish before consumption is the main safety measure to prevent foodborne disease caused by V. parahaemolyticus associated with fish (Zulkifli et al., 2009). Furthermore, several important measures including good hygiene practices while handling the fish and the cleanliness of the handlers and display area are very crucial in order to prevent cross-contamination in wet market and supermarket. In conclusion, the information presented serves as a baseline on future microbiological risk assessment of V. parahaemolyticus associated with fish consumption in Selangor, Malaysia.
Author Contributions
L-HL, VL, and JL conducted the experiments and data analysis, and wrote the manuscript. N-SAM and SW provided vital insight, technical support, guidance, and proofreading for the project. L-HL founded the project.
Funding
This work was supported by PVC Award Grant (Project No. PVC-ECR-2016) and External Industry Grant (Biotek Abadi – Vote Nos. GBA-808138 and GBA-808813) awarded to L-HL.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02513/full#supplementary-material
Footnotes
References
Abu Bakar, I., Ayub, M. K., Muhd Yatim, A., and Abdullah Sani, N. (2010). Pesticide and antibiotic residues in freshwater aquaculture fish: chemical risk assessment from farm to table. Asian J. Food Agro Ind. 3, 328–334.
Al-Othrubi, S. M. Y., Kqueen, C. Y., Mirhosseini, H., Hadi, Y. A., and Radu, S. (2014). Antibiotic resistance of Vibrio parahaemolyticus isolated from cockles and shrimp sea food marketed in Selangor, Malaysia. Clin. Microbiol. 3, 1–7.
Aremu, M. O., and Ekunode, O. E. (2008). Nutritional evaluation and functional properties of Clarias lazera (African Catfish) from River Tammah in Nasarawa States, Nigeria. Am. J. Food Tech. 3, 264–274. doi: 10.3923/ajft.2008.264.274
Basti, A. A., Misaghi, A., Salehi, T. Z., and Kamkar, A. (2006). Bacterial pathogens in fresh, smoked and salted Iranian fish. Food Control 17, 183–188. doi: 10.1016/j.foodcont.2004.10.001
Bej, A. K., Patterson, D. P., Brasher, C. W., Vickery, M. C. L., Jones, D. D., and Kaysner, C. A. (1999). Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. J. Microbiol. Methods 36, 215–225. doi: 10.1016/S0167-7012(99)00037-8
Bier, N., Schwartz, K., Guerra, B., and Strauch, E. (2015). Survey on antimicrobial resistance patterns in Vibrio vulnificus and Vibrio cholerae non-O1/non-O139 in Germany reveals carbapenemase-producing Vibrio cholerae in coastal waters. Front. Microbiol. 6:1179. doi: 10.3389/fmicb.2015.01179
Ceccarelli, D., Hasan, N, A., Hug, A., and Colwell, R, R. (2013). Distribution and dynamics of epidemic and pandemic Vibrio parahaemolyticus virulence factors. Front. Cell. Infect. Microbiol. 3:97. doi: 10.3389/fcimb.2013.00097
Centers for Disease Control and Prevention [CDC] (2018). Carbapenemase Producing Carbapenem-Resistant Enterobacteriaceae (CP-CRE). Atlanta, GA: Centers for Disease Control and Prevention. doi: 10.3201/eid2210.151612
Chowdhury, G., Pazhani, G. P., Sarkar, A., Rajendran, K., Mukhopadhyay, A. K., Bhattacharya, M. K., et al. (2016). Carbapenem resistance in clonally distinct clinical strains of Vibrio fluvialis isolated from diarrheal samples. Emerg. Infect. Dis. 22, 1754–1761. doi: 10.3201/eid2210.151612
Clinical and Laboratory Standards Institute [CLSI] (2010). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, Approved Guideline, CLSI Document M45-A2, 2nd Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
Daniels, N. A., and Shafaie, A. (2000). A review of pathogenic Vibrio infections for clinicians. Infect. Med. 17, 665–685.
Das, B., Manna, S. K., Sarkar, P., and Batabyal, K. (2009). Occurrence of Vibrio parahaemolyticus in different finfish and shellfish species. J. Food Saf. 29, 118–125. doi: 10.1111/j.1745-4565.2008.00146.x
Davies, A. R., Capell, C., Jehanno, D., Nychas, G. J. E., and Kirby, R. M. (2001). Incidence of foodborne pathogens on European fish. Food Control 12, 67–71. doi: 10.1016/S0956-7135(00)00022-0
Deepanjali, A., Kumar, H. S., Karunasagar, I., and Karunasagar, I. (2005). Seasonal variation in abundance of total and pathogenic Vibrio parahaemolyticus bacteria in oysters along the Southwest Coast of India. Appl. Environ. Microbiol. 71, 3575–3580. doi: 10.1128/AEM.71.7.3575-3580.2005
Devi, R., Surendran, P. K., and Chakraborty, K. (2009). Antibiotic resistance and plasmid profiling of Vibrio parahaemolyticus isolated from shrimp farms along the southwest coast of India. World J. Microbiol. Biotechnol. 25, 2005–2012. doi: 10.1007/s11274-009-0101-8
Dileep, V., Kumar, H. S., Kumar, Y., Nishibuchi, M., Karunasagar, I., and Karunasagar, I. (2003). Application of polymerase chain reaction for detection of Vibrio parahaemolyticus associated with tropical seafoods and coastal environment. Lett. Appl. Microbiol. 36, 423–427. doi: 10.1046/j.1472-765X.2003.01333.x
Duran, G. M., and Marshall, D. L. (2005). Ready-to-eat shrimp as an international vehicle of antibiotic-resistant bacteria. J. Food Prot. 68, 2395–2401. doi: 10.4315/0362-028X-68.11.2395
Elamparithi, P., and Ramanathan, N. (2011). Identification of Vibrio parahaemolyticus isolates by PCR targeted to the toxR gene. Int. J. Pharm. Biol. Arch. 2, 1735–1737.
Fabbro, C., Cataletto, B., and Del Negro, P. (2010). Detection of pathogenic Vibrio parahaemolyticus through biochemical and molecular-based methodologies in coastal waters of the Gulf of Trieste (North Adriatic Sea). FEMS Microbiol. Lett. 307, 158–164. doi: 10.1111/j.1574-6968.2010.01969.x
Felsenstein, J. (1981). Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17, 368–376.
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791. doi: 10.2307/2408678
Food and Agriculture Organization [FAO] (2015). The FAO Action Plan on Antimicrobial Resistance 2016–2020. Rome: Food and Agriculture Organization of the United Nations.
Food and Agriculture Organization [FAO]. (2016). Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production. Available at: http://www.fao.org/3/a-i6209e.pdf
Food and Agriculture Organization/World Health Organization [FAO/WHO] (2011). Risk Assessment of Vibrio parahaemolyticus in Seafood: Interpretative Summary and Technical Report. Microbiological Risk Assessment Series. Available at: http://www.fao.org/3/a-i2225e.pdf
Fuenzalida, L., Hernandez, C., Toro, J., Rioseco, M. L., Romero, J., and Espejo, R. T. (2006). Vibrio parahaemolyticus in shellfish and clinical samples during two large epidemics of diarrhoea in southern Chile. Environ. Microbiol. 8, 675–683. doi: 10.1111/j.1462-2920.2005.00946.x
Gu, W., Yin, J., Yang, J., Li, C., Chen, Y., Yin, J., et al. (2014). Characterization of Vibrio cholerae from 1986 to 2012 in Yunnan Province, southwest China bordering Myanmar. Infect. Gen. Evol. 21, 1–7. doi: 10.1016/j.meegid.2013.10.015
Guglielmetti, E., Korhonen, J. M., Heikkinen, J., Morelli, L., and Von Wright, A. (2009). Transfer of plasmid-mediated resistance to tetracycline in pathogenic bacteria from fish and aquaculture environments. FEMS Microbiol. Lett. 293, 28–34. doi: 10.1111/j.1574-6968.2009.01512.x
Hajeb, P., Jinap, S., Ismail, A., Fatimah, A. B., Jamilah, B., and Abdul Rahim, M. (2009). Assessment of mercury level in commonly consumed marine fishes in Malaysia. Food Control 20, 79–84. doi: 10.1016/j.foodcont.2008.02.012
Han, F., Walker, R. D., Janes, M. E., Prinyawiwatkul, W., and Ge, B. (2007). Antimicrobial susceptibilities of Vibrio parahaemolyticus and Vibrio vulnificus isolates from Louisiana Gulf and retail raw oyster. Appl. Environ. Microbiol. 73, 7096–7098. doi: 10.1128/AEM.01116-07
Harikrishnan, R., Balasundaram, C., and Heo, M. S. (2011). Fish health aspects in grouper aquaculture. Aquaculture 320, 1–21. doi: 10.3347/kjp.2008.46.2.77
Heng, S.-P., Letchumanan, V., Deng, C.-Y., Ab Mutalib, N.-S., Khan, T. M., Chuah, L.-H., et al. (2017). Vibrio vulnificus: an environmental and clinical burden. Front. Microbiol. 8:997. doi: 10.3389/fmicb.2017.00997
Hoffmann, M., Brown, E. W., Feng, P. C., Keys, C. E., Fischer, M., and Monday, S. R. (2010). PCR-based method for targeting 16S-23S rRNA intergenic spacer regions among Vibrio species. BMC Microbiol. 10:90. doi: 10.1186/1471-2180-10-90
Huang, J. Y., Henao, O. L., Griffin, P. M., Vugia, D. J., Cronquist, A. B., Hurd, S., et al. (2016). Infection with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance – foodborne diseases active surveillance network, 10 U.S. sites, 2012-2015. Morb. Mortal. Wkly. Rep. 65, 368–371. doi: 10.15585/mmwr.mm6514a2
Iwamoto, M., Ayers, T., Mahon, B. E., and Swerdlow, D. L. (2010). Epidemiology if seafood-associated infections in the United States. Clin. Microbiol. Rev. 23, 399–411. doi: 10.1128/CMR.00059-09
Jerbi, M. A., Ouanes, Z., Besbes, R., Achour, L., and Kacem, A. (2011). Single and combined genotoxic and cytotoxic effects of two xenobiotics widely used in intensive aquaculture. Mutat. Res. 724, 22–27. doi: 10.1016/j.mrgentox.2011.04.010
Joseph, S. W., DeBell, R. M., and Brown, W. P. (1978). In vitro response to chloramphenicol, tetracycline, ampicillin, gentamicin, and beta-lactamase production by halophilic Vibrios from human and environmental sources. Antimicrob. Agents Chemother. 13, 244–248. doi: 10.1128/AAC.13.2.244
Jun, J. W., Kim, J. H., Choresca, C. H. Jr., Shin, S. P., Han, J. E., Han, S. Y., et al. (2012). Isolation, molecular characterization, and antibiotic susceptibility of Vibrio parahaemolyticus in Korean seafood. Foodborne Pathog. Dis. 9, 224–231. doi: 10.1089/fpd.2011.1018
Kaysner, C. A., and DePaola, A. Jr. (2004). Bacteriological Analytical Manual Chapter 9: Vibrio. Available at: https://www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm070830.htm [accessed June 6, 2009].
Kim, M., Kwon, T. H., Jung, S. M., Cho, S. H., Jin, S. Y., Park, N. H., et al. (2013). Antibiotic resistance of bacteria isolated from the internal organs of edible snow crabs. PLoS One 8:e70887. doi: 10.1371/journal.pone.0070887
Kim, Y. B., Okuda, J., Matsumoto, C., Takahashi, N., Hashimoto, S., and Nishibuchi, M. (1999). Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J. Clin. Microbiol. 37, 1173–1177.
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120. doi: 10.1007/BF01731581
Krumperman, P. H. (1983). Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 46, 165–170.
Kubota, K., Iwasaki, E., Inagaki, S., Nokubo, T., Sakurai, Y., Komatsu, M., et al. (2008). The human health burden of foodborne infections caused by Campylobacterm, Salmonella, and Vibrio parahaemolyticus in Miyagi Prefecture, Japan. Foodborne Pathog. Dis. 5, 641–648. doi: 10.1089/fpd.2008.0092
Kümmerer, K. (2009). Antibiotics in the aquatic environment – a review – part II. Chemosphere 75, 435–441. doi: 10.1016/j.chemosphere.2008.12.006
Law, J. W.-F., Ab Mutalib, N.-S., Chan, K.-G., and Lee, L.-H. (2015). Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front. Microbiol. 5:770. doi: 10.3389/fmicb.2014.00770
Lee, L.-H., and Raghunath, P. (2018). Editorial: vibrionaceae diversity, multidrug resistance and management. Front. Microbiol. 9:563. doi: 10.3389/fmicb.2018.00563
Lesley, M. B., Velnetti, L., Cheah, Y. K., Son, R., Kasing, A., Samuel, L., et al. (2011). Antibiotic resistance and plasmid profiling of Vibrio parahaemolyticus isolated from cockles (Anadara granosa) at Tanjung Karang, Kuala Selangor. Int. Food Res. J. 18, 1183–1188.
Lesmana, M., Subekti, D., Simanjuntak, C. H., Tjaniadi, P., Campbell, J. R., and Oyofo, B. A. (2001). Vibrio parahaemolyticus associated with Cholera-like diarrhea among patients in North Jakarta, Indonesia. Diagn. Microbiol. Infect. Dis. 39, 71–75. doi: 10.1016/S0732-8893(00)00232-7
Letchumanan, V., Chan, K.-G., Khan, T. M., Bukhari, S. I., Ab Mutalib, N.-S., Goh, B.-H., et al. (2017). Bile sensing: the activation of Vibrio parahaemolyticus virulence. Front. Microbiol. 8:728. doi: 10.3389/fmicb.2017.00728
Letchumanan, V., Chan, K.-G., and Lee, L.-H. (2014). Vibrio parahaemolyticus: a review on the pathogenesis, prevalence and advance molecular identification techniques. Front. Microbiol. 5:705. doi: 10.3389/fmicb.2014.00705
Letchumanan, V., Chan, K.-G., Pusparajah, P., Saokaew, S., Duangjai, A., Goh, B.-H., et al. (2016). Insights into bacteriophage application in controlling Vibrio species. Front. Microbiol. 7:1114. doi: 10.3389/fmicb.2016.01114
Letchumanan, V., Chan, K.-G., and Lee, L.-H. (2015a). An insight of traditional plasmid curing in Vibrio species. Front. Microbiol. 6:735. doi: 10.3389/fmicb.2015.00735
Letchumanan, V., Pusparajah, P., Loh, T. H. T., Yin, W.-F., Lee, L.-H., and Chan, K.-G. (2015b). Occurrence and antibiotic resistance of Vibrio parahaemolyticus from shell fish in Selangor, Malaysia. Front. Microbiol. 6:1417. doi: 10.3389/fmicb.2015.01417
Letchumanan, V., Yin, W.-F., Lee, L.-H., and Chan, K.-G. (2015c). Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 6:33. doi: 10.3389/fmicb.2015.00033
Lou, K., Ban, R., and Zhao, X. M. (2002). Curing of the Bacillus subtilis plasmid using sodium dodecyl sulfate. Trans. Tianjin Univ. 8, 148–151.
Ma, C., Deng, X., Ke, C., He, D., Liang, Z., Li, W., et al. (2014). Epidemiology and etiology characteristics of foodborne outbreaks caused by Vibrio parahaemolyticus during 2008-2010 in Guangdong Province, China. Foodborne Pathog. Dis. 11, 21–29. doi: 10.1089/fpd.2013.1522
Mandal, J., Sangeetha, V., Ganesan, V., Parveen, M., Preethi, V., Harish, B. N., et al. (2012). Third-generation cephalosporin-resistant Vibrio cholerae, India. Emerg. Infect. Dis. 18, 1326–1328. doi: 10.3201/eid1808.111686
Mandal, P. K., Biswas, A. K., Choi, K., and Pal, U. K. (2011). Methods for rapid detection of foodborne pathogens: an overview. Am. J. Food Tech. 6, 87–102. doi: 10.3923/ajft.2011.87.102
Manjusha, S., and Sarita, G. B. (2011). Plasmid associated antibiotic resistance in Vibrios isolated from coastal waters of Kerala. Int. Food Res. J. 18, 1171–1181.
Martin, A., Fahrbach, K., Zhao, Q., and Lodise, T. (2018). Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections dues to Enterobacteriaceae: results of systematic literature review and meta-analysis. Open Forum Infect. Dis. 5:ofy150. doi: 10.1093/ofid/ofy150
Meletis, G. (2016). Carbapenem resistance: overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 3, 15–21. doi: 10.1177/2049936115621709
Melo, L. M. R. D., Almeida, D., Hofer, E., Reis, C. M. F. D., Theophilo, G. N. D., Santos, A. F. D. M., et al. (2011). Antibiotic resistance of Vibrio parahaemolyticus isolated from pond-reared Litopenaeus vannamei marketed in Natal, Brazil. Braz. J. Microbiol. 42, 1463–1469. doi: 10.1590/S1517-838220110004000032
Molina-Aja, A., Garcia-Gasca, A., Abreu-Grobois, A., Bolan-Mejia, C., Roque, A., and Gomez-Gil, B. (2002). Plasmid profiling and antibiotic resistance of Vibrio strains isolated from cultured penaeid shrimp. FEMS Microbiol. Lett. 213, 7–12. doi: 10.1111/j.1574-6968.2002.tb11278.x
Noorlis, A., Ghazali, F. M., Cheah, Y. K., Tuan Zainazor, T. C., Wong, W. C., Tunung, R., et al. (2011). Antibiotic resistance and biosafety of Vibrio cholerae and Vibrio parahaemolyticus from freshwater fish at retail level. Int. Food Res. J. 18, 1523–1530.
Nordmann, P., Poirel, L., Toleman, M. A., and Walsh, T. R. (2011). Does broad-spectrum β-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J. Antimicrob. Chemother. 66, 689–692. doi: 10.1093/jac/dkq520
Nordstrom, J. L., Vickery, M. C. L., Blackstone, G. M., Murray, S. L., and DePaola, A. (2007). Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl. Environ. Microbiol. 73, 5840–5847. doi: 10.1128/AEM.00460-07
Oh, E. G., Son, K. T., Yu, H., Lee, T. S., Lee, H. J., Shin, S., et al. (2011). Antimicrobial resistance of Vibrio parahaemolyticus and Vibrio alginolyticus strains isolated from farmed fish in Korea from 2005 through 2007. J. Food Prot. 74, 380–386. doi: 10.4315/0362-028X.JFP-10-307
Osman, H., Suriah, A. R., and Law, E. C. (2001). Fatty acid composition and cholesterol content of selected marine fish in Malaysian waters. Food Chem. 73, 55–60. doi: 10.1016/S0308-8146(00)00277-6
Ottaviani, D., Bacchiocchi, I., Masini, L., Leoni, F., Carraturo, A., Giammarioli, M., et al. (2001). Antimicrobial susceptibility of potentially pathogenic halophilic Vibrios isolated from seafood. Int. J. Antimicrob. Agents 18, 135–140. doi: 10.1016/S0924-8579(01)00358-2
Ottaviani, D., Leoni, F., Talevi, G., Masini, L., Santarelli, S., Rocchegiani, E., et al. (2013). Extensive investigation of antimicrobial resistance in Vibrio parahaemolyticus from shellfish and clinical sources, Italy. Int. J. Antimicrob. Agents 42, 191–193. doi: 10.1016/j.ijantimicag.2013.05.003
Papp-Wallace, K. M., Endimiani, A., Taracila, M. A., and Bonomo, R. A. (2011). Carbapenems: past, present, and future. Antimicrob. Agents Chemother. 5, 4943–4960. doi: 10.1128/AAC.00296-11
Parveen, S., Hettiarachchi, K. A., Bowers, J. C., Jones, J. L., Tamplin, M. L., McKay, R., et al. (2008). Seasonal distribution of total and pathogenic Vibrio parahaemolyticus in Chesapeake Bay oysters and waters. Int. J. Food Microbiol. 128, 354–361. doi: 10.1016/j.ijfoodmicro.2008.09.019
Paydar, M., Teh, C. S. J., and Thong, K. L. (2013). Prevalence and characterization of potentially virulent Vibrio parahaemolyticus in seafood in Malaysia using conventional methods, PCR and REP-PCR. Food Control 32, 13–18. doi: 10.1016/j.foodcont.2012.11.034
Raghunath, P. (2015). Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus. Front. Microbiol. 5:805. doi: 10.3389/fmicb.2014.00805
Rico, A., Satapornvanit, K., Haque, M. M., Min, J., Nguyen, P. T., Telfer, T., et al. (2012). Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: a critical review. Rev. Aquac. 4, 75–93. doi: 10.1111/j.1753-5131.2012.01062.x
Romalde, J. L., Diéguez, A. L., Lasa, A., and Balboa, S. (2014). New Vibrio species associated to molluscan microbiota: a review. Front. Microbiol. 4:413. doi: 10.3389/fmicb.2013.00413
Saitou, N., and Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425.
Sawabe, T., Ogura, Y., Matsumura, Y., Gao, F., Amin, A. K. M., Mino, S., et al. (2013). Updating the Vibrio clades defined by multilocus sequence phylogeny: proposal of eight new clades, and the description of Vibrio tritonius sp. nov. Front. Microbiol. 4:414. doi: 10.3389/fmicb.2013.00414
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States–major pathogens. Emerg. Infect. Dis. 17, 7–15. doi: 10.3201/eid1701.P11101
Shaw, K. S., Goldstein, R. E. R., He, X., Jacobs, J. M., Crump, B. C., and Sapkota, A. R. (2014). Antimicrobial susceptibility of Vibrio vulnificus and Vibrio parahaemolyticus recovered from recreational and commercial areas of Chesapeake Bay and Maryland Coastal Bays. PLoS One 9:e89616. doi: 10.1371/journal.pone.0089616
Stepanauskas, R., Glenn, T. C., Jagoe, C. H., Tuckfield, R. C., Lindell, A. H., King, C. J., et al. (2006). Coselection for microbial resistance to metals and antibiotics in freshwater microcosms. Environ. Microbiol. 8, 1510–1514. doi: 10.1111/j.1462-2920.2006.01091.x
Su, Y. C., and Liu, C. (2007). Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol. 24, 549–558. doi: 10.1016/j.fm.2007.01.005
Subramani, S., and Vignesh, S. (2012). MAR index study and MDR character analysis of a few golden Staph isolates. Asian J. Pharm. Life Sci. 2, 151–154.
Sudha, S., Divya, P. S., Francis, B., and Hatha, A. A. M. (2012). ’Prevalence and distribution of Vibrio parahaemolyticus in finfish from Cochin (South India)’. Vet. Ital. 48, 269–281.
Suzita, R., Abu Bakar, F., Son, R., and Abdulamir, A. S. (2010). Detection of Vibrio cholerae in raw cockles (Anadara granosa) by polymerase chain reaction. Int. Food Res. J. 17, 675–680.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Tan, L. T.-H., Chan, K.-G., Lee, L.-H., and Goh, B.-H. (2016). Streptomyces bacteria as potential probiotics in aquaculture. Front. Microbiol. 79:79. doi: 10.3389/fmicb.2016.00079
Tan, T. L.-H., Chan, K.-G., and Lee, L.-H. (2014). Application of bacteriophage in biocontrol of major foodborne bacterial pathogens. J. Mol. Biol. Mol. Imaging 1, 1–9.
Tantillo, G. M., Fontanarosa, M., Di Pinto, A., and Musti, M. (2004). A review updated perspective on emerging Vibrios associated with human infections. Lett. Appl. Microbiol. 39, 117–139. doi: 10.1111/j.1472-765X.2004.01568.x
Taweel, A., Shuhaimi-Othman, M., and Ahmad, A. K. (2013). Assessment of heavy metals in tilapia fish (Oreochromis niloticus) from the Langat River and Engineering Lake in Bangi, Malaysia, and evaluation of the health risk from tilapia consumption. Ecotoxicol. Environ. Saf. 93, 45–51. doi: 10.1016/j.ecoenv.2013.03.031
Tendencia, E. A., and de la Peña, L. D. (2001). Antibiotic resistance of bacteria from shrimp ponds. Aquaculture 195, 193–204. doi: 10.1016/S0044-8486(00)00570-6
Thomas, S. K., Johney, J., and Ragunathan, R. (2018). Molecular characterization of Myroides gitamensis from fish samples and use bio preservatives. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 4, 568–578.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. doi: 10.1093/nar/25.24.4876
Thongjun, J., Mittraparp-Arthorn, P., Yingkajorn, M., Kongreung, J., Nishibuchi, M., and Vuddhakul, V. (2013). The trend of Vibrio parahaemolyticus infections in Southern Thailand from 2006 to 2010. Trop. Med. Health 41, 151–156. doi: 10.2149/tmh.2013-06
Tison, D. L., and Kelly, M. T. (1984). Vibrio species of medical importance. Diagn. Microbiol. Infect. Dis. 2, 263–276. doi: 10.1016/0732-8893(84)90057-9
Tsai, S. E., Jong, K. J., Tey, Y. H., Yu, W. T., Chiou, C. S., Lee, Y. S., et al. (2013). Molecular characterization of clinical and environmental Vibrio parahaemolyticus isolates in Taiwan. Int. J. Food Microbiol. 165, 18–26. doi: 10.1016/j.ijfoodmicro.2013.04.017
Vaseeharan, B., Ramasamy, P., Murugan, T., and Chen, J. C. (2005). In vitro susceptibility of antibiotics against Vibrio spp. and Aeromonas spp. isolated from Penaeus monodon hatcheries ponds. Int. J. Antimicrob. Agents 26, 285–291. doi: 10.1016/j.ijantimicag.2005.07.005
Vengadesh, L., Son, R., and Yoke-Kqueen, C. (2012). Molecular quantitation and characterization of Vibrio cholerae from different seafood obtained from wet market and supermarket. Int. Food Res. J. 19, 45–50.
Vimila, B., Thong, K. L., and Chong, V. C. (2010). Isolation, detection and genomic differentiation of Vibrio cholerae and Vibrio parahaemolyticus in Bachok, Kelantan. Malays. J. Sci. 29, 1–10. doi: 10.22452/mjs.vol29nosp.2
Vongxay, K., Wang, S., Zhang, X., Wu, B., Hu, H., Pan, Z., et al. (2008). Pathogenic characterization of Vibrio parahaemolyticus isolates from clinical and seafood sources. Int. J. Food Microbiol. 126, 71–75. doi: 10.1016/j.ijfoodmicro.2008.04.032
Walsh, T. R., Weeks, J., Livermore, D. M., and Toleman, M. A. (2011). Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect. Dis. 11, 355–362. doi: 10.1016/S1473-3099(11)70059-7
Wilson, B. A., and Salyers, A. A. (2003). Is the evolution of bacterial pathogens an out-of-body experience? Trends Microbiol. 11, 347–350. doi: 10.1016/S0966-842X(03)00179-3
Witus, I. W., and Vun, L. W. (2016). Aquaculture in Malaysia: a short review on current policy and legislation. Trans. Sci. Tech. 3, 150–154.
World Health Organization [WHO] (2014). Antimicrobial Resistance: Global Report on Surveillance. Geneva: World Health Organization. Available at: http://www.who.int/iris/handle/10665/112642
Xie, T., Wu, Q., Zhang, J., Xu, X., and Cheng, J. (2017). Comparison of Vibrio parahaemolyticus isolates from aquatic products and clinical by antibiotic susceptibility, virulence, and molecular characterization. Food Control 71, 315–321. doi: 10.1016/j.foodcont.2016.06.046
Xu, X., Cheng, J., Wu, Q., Zhang, J., and Xie, T. (2016). Prevalence, characterization, and antibiotic susceptibility of Vibrio parahaemolyticus isolated from retail aquatic products in North China. BMC Microbiol. 16:32. doi: 10.1186/s12866-016-0650-6
Yano, Y., Hamano, K., Satomi, M., Tsutsui, I., Ban, M., and Aue-umneoy, D. (2014). Prevalence and antimicrobial susceptibility of Vibrio species related to food safety isolated from shrimp cultured at inland ponds in Thailand. Food Control 38, 30–36. doi: 10.1016/j.foodcont.2013.09.019
Zarei, M., Borujeni, M. P., Jamnejad, A., and Khezrzadeh, M. (2012). Seasonal prevalence of Vibrio species in retail shrimps with an emphasis on Vibrio parahaemolyticus. Food Control 25, 107–109. doi: 10.1016/j.foodcont.2011.10.024
Keywords: Vibrio parahaemolyticus, carbapenem, freshwater, marine, antibiotic resistant, MAR index
Citation: Lee L-H, Ab Mutalib N-S, Law JW-F, Wong SH and Letchumanan V (2018) Discovery on Antibiotic Resistance Patterns of Vibrio parahaemolyticus in Selangor Reveals Carbapenemase Producing Vibrio parahaemolyticus in Marine and Freshwater Fish. Front. Microbiol. 9:2513. doi: 10.3389/fmicb.2018.02513
Received: 13 June 2018; Accepted: 02 October 2018;
Published: 25 October 2018.
Edited by:
Giovanna Suzzi, Università degli Studi di Teramo, ItalyReviewed by:
Lanming Chen, Shanghai Ocean University, ChinaDapeng Wang, Shanghai Jiao Tong University, China
Copyright © 2018 Lee, Ab Mutalib, Law, Wong and Letchumanan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Learn-Han Lee, bGVlLmxlYXJuLmhhbkBtb25hc2guZWR1; bGVlbGVhcm5oYW5AeWFob28uY29t Vengadesh Letchumanan, bHZlbmdhZGVzaEB5YWhvby5jb20=
†These authors have contributed equally to this work
 Learn-Han Lee
Learn-Han Lee Nurul-Syakima Ab Mutalib
Nurul-Syakima Ab Mutalib Jodi Woan-Fei Law
Jodi Woan-Fei Law Sunny Hei Wong
Sunny Hei Wong Vengadesh Letchumanan
Vengadesh Letchumanan