
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 20 September 2018
Sec. Food Microbiology
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.02248
Gastrointestinal episodes associated with Vibrio species have been rising worldwide in the last few years. Consequently, it is important to comprehend how occurs the production of diarrhea, to establish new preventive and therapeutic measures. Besides the classical CT and TCP toxins, Zot, RTX, and Ace among others have been deeply studied in V. cholerae. However, in other Vibrio species of clinical interest, where some of these toxins have been reported, there is practically no information. Zot activates a cascade of signals inside of the cell that increase the permeability of epithelial barrier, while RTX causes depolymerization of the actin cytoskeleton and Ace increases the permeability of intestinal cell monolayers. The goal of this study is to acquire information about the distribution of these toxins in human pathogenic Vibrios and to review the progress in the study of their role in the intestinal epithelium during infection.
Gastrointestinal pathogens invade or disrupt the intestinal barrier by the action of secreted toxins. They can alter cell physiology by multiple mechanisms, being directly responsible for the pathology of the disease or favoring other processes such as manipulation of the host immune response, escape from the intracellular environment and penetration of host barriers, among others (Ugalde-Silva et al., 2016). The Vibrionaceae family includes several species of major importance in the clinical field that are able to cause gastroenteritis. Among these, Vibrio cholerae is the classical pathogen carrying an arsenal of diverse toxins that produce illness. This bacterium is the agent responsible for cholera, an infection of the small intestine whose classical symptoms are a watery diarrhea, vomiting and dehydration; it is associated with million cases and several deaths around the world each year. (Vezzulli et al., 2016; Plaza et al., 2018). Serotypes O1 and O139, mainly responsible for acute diarrheal disease, possess two main virulence genes, the cholera toxin (CT) and the toxin-coregulated pilus (TCP). However, other strains (non-O1/non-O139) that cause sporadic cases of diarrhea have toxigenic potential attributed to secretion systems (T3SS and T6SS) and other accessory toxins like zonula occludens toxin (Zot) (Chatterjee et al., 2009). Additionally, they have other genes, coding to hemolysins and repeats in toxin (MARTX) which have role helping to the colonization of the intestine (König et al., 2016). Additionally, accessory cholera enterotoxin (Ace) causes fluid secretion in ligated rabbit ileal loops (Trucksis et al., 1993) while cholix toxin (ChxA) is an exotoxin which has been characterized as a member of the eukaryotic elongation factor 2-specific ADP-ribosyltransferase toxins (König et al., 2016). V. cholerae is not the only Vibrio producing toxins; other members of the Vibrionaceae family also produce toxins that generate illness. Although it is not its primary pathology, V vulnificus is capable of producing gastroenteritis among its clinical manifestations. This pathogen secretes a number of exotoxins and enzymes (Liu et al., 2007), among which the cytolysin VvhA (Kim et al., 2017) and metalloprotease Vvp (Lee et al., 2008; Chen et al., 2017) are the most important join to RTX. This last toxin has been strongly associated with the virulence of this organism (Lee et al., 2007, 2008). It is suggested that this toxin might protect the bacterium from phagocytosis, promoting their survival. Surprisingly, sequencing of V. vulnificus MO6-24/O strain isolated from a septicemic patient showed that this strain possessed a phage-related gene cluster containing ace and zot genes (Park et al., 2011). Similarly, a recent study showed that some strains of Vibrio parahaemolyticus lacking the classical toxins TDH and TRH possess genomic islands and prophage elements containing RTX, Zot and Ace toxins. Zot has also been found in prophage f237 of the pandemic strain and in prophage-like elements in V. coralliilyticus and V. anguillarum, suggesting that the horizontal gene transfer (HGT) associated to phages coding zot occurs frequently among Vibrio species (Castillo et al., 2018). If pathogenicity genes are combined by HGT at high frequency the probability of generate new virulent species increases (Nishibuchi and Kaper, 1995), which is favored in estuaries and marine environments which represent a extensive pool of virulence genes associated to species of the genus Vibrio (Ceccarelli et al., 2013; Khouadja et al., 2014).
Martine Urtaza and their collaborators recently shown that the risk of infections associated to Vibrio species is rising in many parts of the world (Martinez-Urtaza et al., 2010), and as a consequence of global warming, it is expected that cases would be increasing in frequency and intensity. The broad spectrum of toxins found in the genomes of Vibrios draws attention to which toxins are essential in the development of gastroenteritis and which others are responsible for extra-intestinal infections. An unequivocal knowledge of the mechanisms responsible of the development of infection is crucial to establish new precautionary measures and treatments. The goal of this study is to gain understanding about the distribution of accessory toxins in human pathogenic Vibrios and to review the advancement in the study of the role of these toxins in the intestinal epithelium during infection by different Vibrio pathogens.
The intestines are organs in the digestive system tract involved in the uptake of nutrients and water (Farhadi et al., 2003). They also represent a barrier against pathogens of the outside environment. Due to the protection function, intestinal permeability is a highly regulated dynamic process. The intestinal barrier is mainly composed of three layers: mucus, epithelia, and the lamina propria (König et al., 2016). The epithelial layer is a single layer of cells, which selectively regulate the absorption of nutrients and prevent or modulate the access of microorganisms, toxins and other macromolecules from the intestinal lumen (Salvo-Romero et al., 2015). The epithelial cells that make up the barrier are maintained together by specialized intercellular junctions: desmosomes, adherens junctions (AJs) and tight junctions (TJs) (Citi et al., 2014; Ugalde-Silva et al., 2016). All of them, in addition to the intestinal microbiota and immunogenic mechanisms, possess a joint crucial role to maintain the appropriate function of the intestinal barrier. Consequently, the well-functioning of this barrier strongly depends on the normal function of the paracellular pathway (Farhadi et al., 2003). Tight junctions, also called zonulae occludentes, are the intercellular junctions most apically located (Figure 1; Gopalakrishnan et al., 2009) and interruption of these junctions contributes to the inflammatory response because of increased antigenic penetration (Al-Sadi et al., 2013). These junctions also regulate the selective paracellular permeability to solutes, ions, water and various macromolecule (Van Itallie and Anderson, 2014) and the entry of microorganisms inhabiting in the intestinal mucosa (Ugalde-Silva et al., 2016). This barrier function is conferred by a large and diverse group of transmembrane proteins mainly composed of proteins claudins, occludins, and the zonula occludens-associated (ZO) proteins 1, 2, and 3 (Lee et al., 2003) among others. The cytosolic scaffold protein ZO-1 directly and indirectly couples occludin and claudins to the other cytoplasmic TJ proteins (Turner et al., 2014) and the actin cytoskeleton (Figure 1).
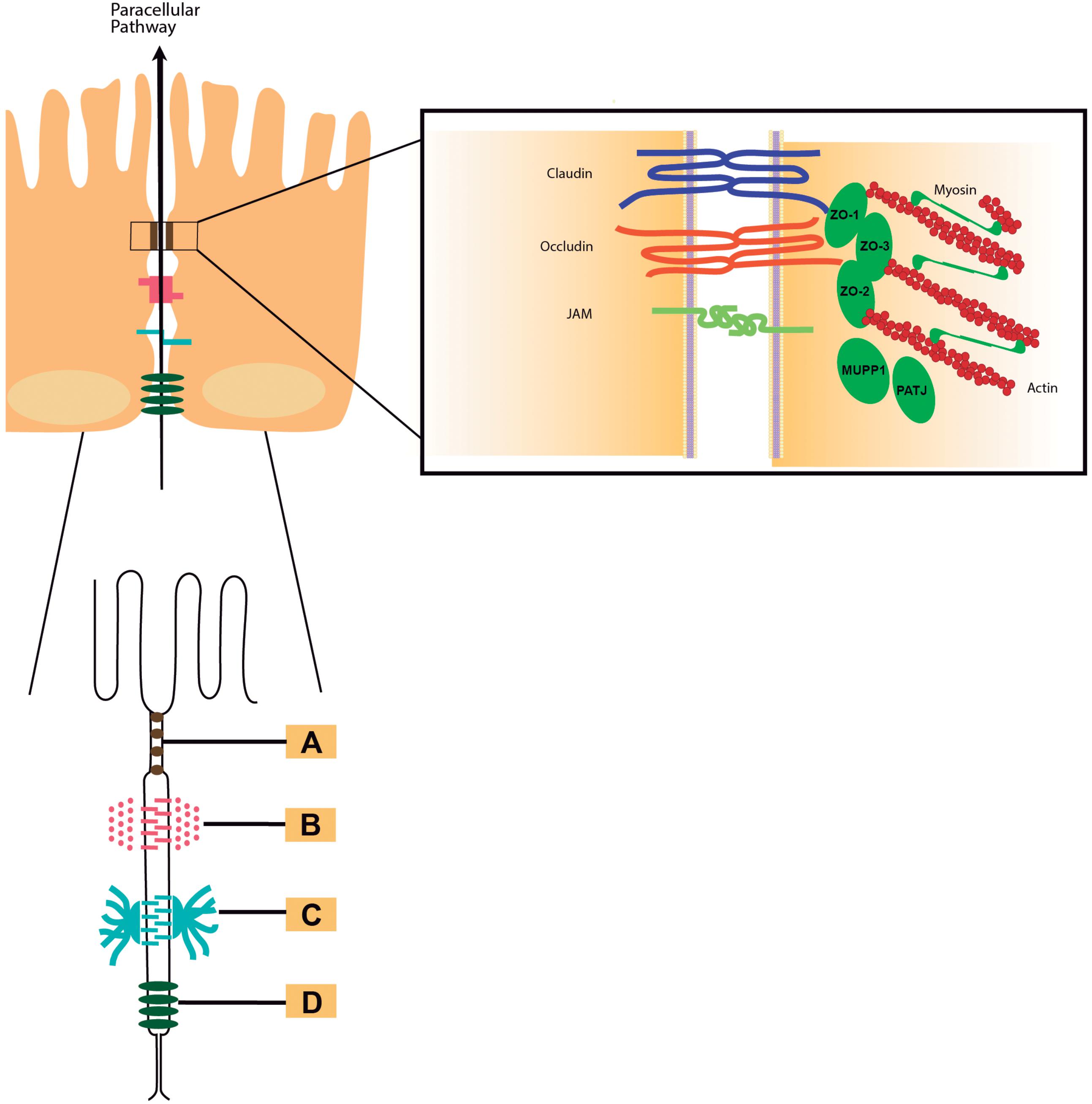
FIGURE 1. Intercellular junctions. Schematic draw of the intercellular junctions showing (A) tight junction, (B) adherens junction, (C) desmosome, and (D) gap junction. In detail to the right of the figure: Tight junctions are multiprotein complexes containing members of the claudins (in blue), occludin (in red), and junctional adhesion molecules (JAMs, in green color) families.
TJ assembly and disassembly is a dynamic process that involves endocytosis, migration and recycling in all epithelia. The regulation of TJs occurs by PKC activation affecting stability in the dynamic TJ complex. This is coincident with increases in paracellular permeability mediated by TJ (Turner et al., 2014). Several pro-inflammatory cytokines can also modulate TJ dynamics causing disruption of the intestinal TJ barrier and resulting in an increase of intestinal TJ permeability (Al-Sadi et al., 2009, 2013, 2016) while anti-inflammatory cytokines were shown to promote intestinal TJ barrier function (Al-Sadi et al., 2016). Other factors that impact TJ dynamics and assembly includes intracellular cAMP and calcium imbalance among others, which act through their varied effects on cellular kinases (König et al., 2016). Another structure indispensable for the integrity of paracellular pathway is the cytoskeleton. The cytoskeleton is the structure that maintains the shape and internal organization of the cells, besides giving mechanical support to carry on cellular movement and division. The interaction of TJ proteins with the actin maintain the structure of cytoskeletal, which is essential for the proper regulation of barrier functioning (González-Mariscal et al., 2013).
The epithelia comprise structures adapted to protect the tissues from pathogenic microorganisms, antigens and/or proinflammatory factors (Farhadi et al., 2003; Sousa et al., 2005). Conversely, pathogens have generated diverse strategies that disrupt the components that maintain the structure of epithelia and spread to various tissues (Sousa et al., 2005; Dubreuil, 2017). Pathogens can secrete enzymes that affect the extracellular part of junction components or toxins acting within the cell, disrupting intercellular junctions. Other can inject effector proteins into the host cell cytoplasm, altering cell functions by acting on cell signaling pathways (Ugalde-Silva et al., 2016). Invasive pathogens can destabilize the junctions by inducing a signaling cascade that lead to proinflammatory response or directly targeting the intercellular junction. The cytoskeleton is also a target for toxins by direct and indirect modifications through covalent or non-covalent mechanisms, respectively (Barbieri et al., 2002). The mechanism of action is different among toxins, some of them shift the equilibrium between F- (polymerized) and G-actin (monomeric) (Kudryashov et al., 2008), while other can affect directly actin.
The interactions between the enteric pathogenic microorganisms and their hosts is of great interest to try to understand several mechanisms of infectious diseases. The relationship between the toxic products of bacteria and diarrheal diseases has been studied extensively (Guttman and Finlay, 2009). Although one of the function of the epithelial barriers is to block the access of many organisms, certain pathogens have evolved to alter this barrier. In this context, most of gastrointestinal pathogens lead to intestinal secretion by elaboration of toxins or invasion (Fasano, 2012). They can use tight junction proteins as receptors for their internalization or destroy the junctions to enter to the underlying tissue. As a result, the altering of tight junctions elicit inflammatory cascades causing diarrhea as the ultimate goal (Dubreuil, 2017).
Since cholera is a global disease responsible for several cases of diarrhea and deaths around the world, the mechanisms of pathogenicity of this pathogen have been deeply studied (WHO (2015) Cholera: Fact Sheet No. 107). The cholera disease is an acute infectious diarrhea whose transmission occurs mainly through contaminated water or foods. Once this pathogen interacts with the epithelial cells of the human small intestine a massive watery efflux occurs characteristic of cholera diarrhea, which functions to disperse V. cholerae back into the environment. Therefore, the diarrhea causes severe dehydration and in many cases the death of infected people (Cordero et al., 2006).
The pathogenesis of cholera is a multifactorial process involving several genes that encode virulence factors that help the bacteria in colonization and the expression of the cholera toxin (CT). Each CT molecule is composed of one A subunit plus five B subunits. The B subunits bind to the ganglioside GM1 receptors in the epithelial cells of the intestinal mucosa. After binding, subunits A1 and A2 are separated, which facilitates the entry of component A1 into the cell. Component A1 of the CT stimulates the production of the enzyme adenyl cyclase, involved in the production of cyclic adenosine monophosphate (cAMP) (Bharati and Ganguly, 2011). The high intracellular concentrations of cAMP alter the transport of electrolytes through the cell membrane, activating the cystic fibrosis transmembrane conductance regulator (CFTR) and resulting in secretion of chloride ions into the lumen. The receptor for the CT is composed of flexible homopolymers of the monomeric form of the toxin-coregulated pili (TCP) pilin subunit TcpA that self-associates, holding cells together in microcolonies. All the ctxAB operon is part of the genome of the filamentous bacteriophage CTXf, lysogenized in the bacterium (Fasano et al., 1991; Waldor and Mekalanos, 1996; Olivier et al., 2007). Besides CT, it also carries the genes involved in the morphogenesis of the bacteriophage (psh, cep, orfU, and ace) and a gene that encodes a protein necessary for the assembly of the virion (zot). Both gene products, Zot and Ace, are also able to contribute to V. cholerae pathogenesis by inducing changes in the intestinal barrier (Fasano et al., 1991; Baudry et al., 1992; Fasano, 2012; Chatterjee et al., 2015)
Zonula occludens toxin (Zot) was discovered in V. cholerae when live oral vaccines, constructed by deletion of V. cholerae sequences encoding the A subunit of the CT, were applied to volunteers. These strains still provoked diarrhea (mild to moderate) in some volunteers due to the presence of another toxin that interacts with tight junctions affecting the paracellular pathway (Fasano et al., 1991). Zot is also encoded in the bacteriophage CTXf; its N-terminal side is involved in phage morphogenesis. In fact, a zot mutation impairs the release of phage particles into the culture supernatant (Uzzau et al., 1999). The C-terminal is secreted into the intestinal lumen after clivage (Fasano et al., 1991; Schmidt et al., 2007). Studies have shown that a smaller fragment of 12 kD is the fragment of Zot with biological activity (Di Pierro et al., 2001). The enterotoxic and permeabilizing effect of Zot on rabbit small intestine was first shown by Fasano and collaborators. They described that Zot has a regional effect which varies in the different segments of rabbit intestine (Fasano et al., 1997). Later, Uzzau and coworkers demonstrated that Zot induces a transitory reduction in transepithelial electrical resistance and an increase in transepithelial flux, increasing the permeability of TJs (Uzzau et al., 2001). They also identified the Zot region required for receptor binding. The Zot receptor is a protein that is located on the cell surface and acts by modulating the cytoskeleton and the tight junction complex inside of the cell (Uzzau et al., 2001). Currently it is known that Zot is positioned in the cell envelope of bacteria (Di Pierro et al., 2001; Salama et al., 2004) and that its action is mediated by intracellular signaling that leads to a reduces the actin filaments (changing the F- and G-actin pools, Figure 2). The change of actin microfilaments increases intestinal epithelial permeability by affecting the TJs (Lee et al., 2003; Goldblum et al., 2011). It has been demonstrated that Zot increases the transport of diverse macromolecules such as insulin, sucrose and acyclovir across several surfaces, including blood-brain barrier and mucosal (Gopalakrishnan et al., 2009).
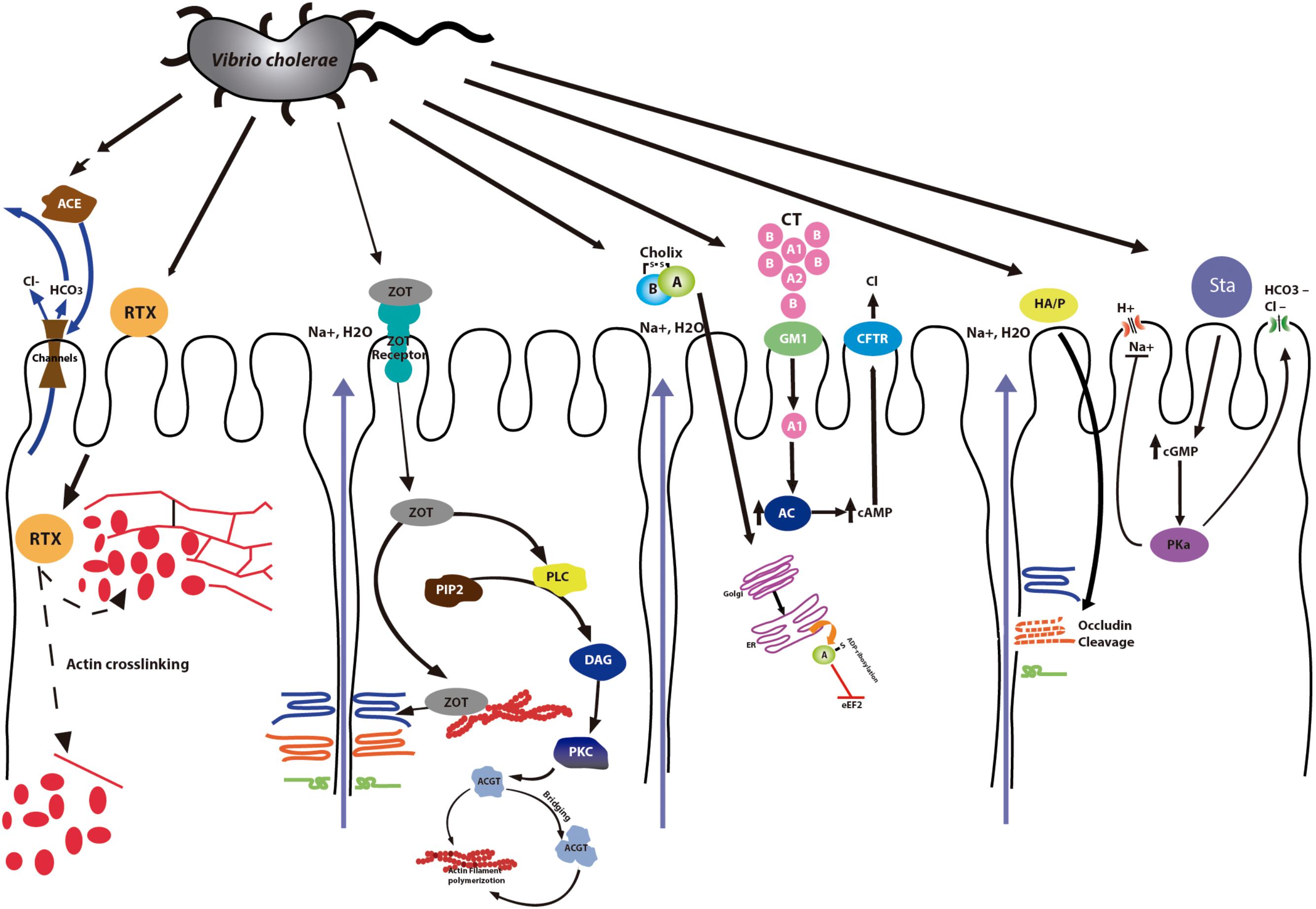
FIGURE 2. V. cholerae infection. Mechanism of action of V. cholerae toxins. The Cholera Toxin (CT) binds to the receptor (Ganglioside GM1) and enhances adenylate cyclase (Ac) activity, increasing cAMP. Elevated intracellular concentrations of cAMP activate the cystic fibrosis transmembrane conductance regulator (CFTR), resulting in secretion of chloride ions into the lumen. Zonula occludens toxin (Zot) affects the structure of the TJs increasing the permeability. Accesory cholera enterotoxin (ACE) stimulates Ca2+-dependent Cl-/HCO3- secretion in intestinal cells. Heat stable enterotoxin (Sta) leads to the increase of cGMP inhibiting the regulatory mechanism of Na+/Cl-. The Repeats in toxin (RTX) leads to the depolymerization of stress fibers. Cholix Toxin (ChT) inhibits protein synthesis. Finally, although Hemagglutinin (HA)/protease (HA/P) is not a toxin, it produces the cleavage of occludin into two distinct fragments, affecting the paracellular pathway of intestinal epithelial cells in culture. The accessory toxins Zot, Ace and RTX are also found in the genome of other Vibrio species.
The “virulence cassette” of V. cholerae includes genes encoding CT and Zot but also a third toxin called Ace (Trucksis et al., 1993; Anvari et al., 2012). This last is an integral membrane protein consisting of 96 amino acids which alters ion transport, causes accumulation in ligated rabbit ileal loops and is responsible for mild diarrhea (Chatterjee et al., 2011). Not much is known about this toxin because of the low amount produced by V. cholerae. Ace stimulates Ca2+ -dependent Cl- /HCO3- symporters in a colonic carcinoma T84 monolayer cell model, creating a potential difference across the membrane (Chatterjee et al., 2011, 2015).
Heat stable enterotoxin (ST) is a peptide composed of 17 aminoacids that induces Ca2+ release from the cell in response to IP3, leading to the activation of guanylyl cyclase and the production of cGMP (Al-Majali et al., 2007). The increase in intracellular cGMP inhibit the regulatory mechanism of Na +/Cl- eliciting secretory diarrhea (Al-Majali et al., 2000).
The repeats-in-toxin (RTX) proteins of Gram-negative bacteria have in common the mode of export via the Type I-Secretion System and classical C-terminal GD-rich repeats (Linhartová et al., 2010). The RTX family generally consists of hemolysins and cytolysins with molecular masses ranging between 40 and more than 600 kDa, that display a variety of activities (Linhartová et al., 2010; Dolores et al., 2015) and are activated by acylation post-translationally (Frey and Kuhnert, 2002). RTX pore-forming toxins involved in bacterial pathogenesis, characterized by repeats of a glycine and aspartate-rich, calcium-binding sequence motif are the members of RTX family most studied and known (Pei and Grishin, 2009; Satchell, 2011). The RTX toxins are four genes of two operons: rtxA encoding the toxin; rtxB/rtxE, an ATP-binding cassette transporter of RtxA; rtxC, an acylase of RtxA; and rtxD, with no clear function yet (Linhartová et al., 2010). They can act in a synergistic way, causing damage and triggering the liberation of inflammatory molecules (Boardman and Fullner Satchell, 2004). In vitro, these toxins show hemolytic and cytotoxic activity which induce damage to the membrane, osmotic changes and finally, cell death by lysis (Wiles and Mulvey, 2013). However, in host cells the cytotoxicity of RTX toxins produces also apoptosis, although the mechanisms is not understood (Wiles and Mulvey, 2013). The best characterized are the multifunctional-autoprocessing RTX (MARTX) toxins, a subgroup of very large RTX proteins (range from 3,500 to 5,300 aminoacid residues) with multiple activities and which constitute a combination of secreted toxins and multi-effector delivery systems (Woida and Satchell, 2018). These proteins are encoded by V. cholerae (VcRtxA), V. vulnificus and other pathogens (Lee et al., 2008). In V. cholerae the MARTXV c (MARTX of V. cholerae) is found in practically all strains (including environmental and clinical isolates and pandemic strains) (Menestrina et al., 1994; Chatterjee et al., 2008). This toxin acts by avoiding the elimination of V. cholerae from the intestine at the beginning of infection (Satchell, 2015). In this way, V. cholerae MARTXV c, contributes to the pathogenesis of cholera in model systems (Kudryashov et al., 2008) although not possess the cytolytic (or hemolytic) activities displayed by other RTX toxins. MARTXV c, like CT and pore-forming toxin hemolysin, is associated with the ability to establish a persistent intestinal infection by bacteria (Prochazkova and Satchell, 2008; Olivier et al., 2009). It has been shown that MARTXV c directly catalyzes a covalent cross-linking of G-actin (monomer) into oligomeric chains, causing cell rounding by disassembly of the actine stress fibers in culture (Sheahan et al., 2004; Cordero et al., 2006). Two distinct virulence activity domains in MARTXV c are responsible for the toxin effect. Actin cross-linking domain causes irreversible disassembly of the cytoskeleton by directly catalyzing the covalent cross-linking of monomeric G-actin. The Rho GTPase inactivation domain causes inactivation of small GTP-bound Rho, Rac, and Cdc42, resulting in depolymerization of actin (Sheahan et al., 2004; Kudryashov et al., 2008; Prochazkova and Satchell, 2008). In 2017, Chen and coworkers showed that MARTXs contain repeated motifs. In the C-terminus there are GD-rich repeats whereas the repeats at the N-terminus are required for toxin secretion and effector translocation (Chen et al., 2017).
Cholix toxin (Cholix, ChxA) is a newly identified virulence factor reported in non-pandemic strains (non O1/non O139 V. cholerae strains; Purdy et al., 2010). Cholix is a 70 kDa ADP-ribosyltransferase toxin that translocates into cells by receptor-mediated endocytosis (Ogura et al., 2011; Ogura et al., 2017) and utilizes eukaryotic elongation factor 2 (eEF2) as a substrate (Ogura et al., 2017). The transference of an ADP-ribose group from NAD + to a diphthamide in eEF2 mediated by cholix inhibit the synthesis of protein producing finally cell death (Jørgensen et al., 2008). Because of the similarity with exotoxin A, it is suggested that cholix toxin enters eukaryotic cells by endocytosis where it is cleaved and the catalytic domain is released to the cytosol where it exerts its effect. This toxin is active against mammal and crustacean cells (Fernandez and Alonso, 2009), suggesting that it plays a role in the survival in their natural environment. Interestingly, the study of Awasthi et al. (2013) showed that there are three types of cholix and none of them caused enterotoxicity in rabbits, however, two of them caused extensive damage in internal organs in mice, suggesting that cholix is associated with extraintestinal infections at least in one animal model (Awasthi et al., 2013)
Although HA/P, the V. cholerae hemagglutinin/protease, is not a toxin, it has been described that can play a role during colonization of the intestine (Lutfullah et al., 2008; Shinoda and Miyoshi, 2011). HA/P is Zn-dependent metalloprotease with mucinase activity, encoded by hapA (Silva et al., 2006; Lutfullah et al., 2008). It exhibit several activities including modification of toxins, degradation of the mucus barrier and acting on TJ-associated proteins, Cleavage of occludin by HA/P resulted in rearrangement of ZO-1, the F-actin cytoskeleton and disruption of paracellular barrier function (Silva et al., 2006; Benitez and Silva, 2016). HA/protease also shows homology to V. vulnificus elastase (VvpE) at aminoacidic level, which is important due to VvpE contributes to local tissue damage during infections produced by this Vibrio (Lee et al., 2015).
The first global spread of cholera disease, occurred from the early 1960s through the middle 1990s, was mainly associated to the El Tor strains of V. cholerae. However, since the late 1990s, a lineage known as the altered El Tor (AET) V. cholerae has come to predominate as the major cause of human cholera disease (Satchell et al., 2016) and it has been associated to severe cases of diarrhea. Interestingly, although V. cholerae possess diverse variants of MARTX toxin associated to environmental strains able to produce disease, the AET V. cholerae strains have an inactivated MARTX toxin gene. This inactivation is explained because the rtxA toxin gene possesses one SNP that introduces a stop codon, resulting in a truncated protein (Dolores and Satchell, 2013). Despite the absence of RTX, these strains are more virulent (Satchell et al., 2016), questioning if RTX is necessary for the pathogenicity of Vibrio. Some authors have proposed that this very large toxin is eliminated once it is not necessary, because it may be detrimental to growth due to energy costs (Dolores and Satchell, 2013). Besides, RTX is fully redundant in function with a pore-forming hemolysin (Olivier et al., 2009).
Interestingly, the null mutant of rtxA was the genetic background for following emergence of the ctxB7 allele, a point mutation in ctxB that created a CtxB with Asparagine at aminoacid 20 (H20 to N20 change) (Dolores and Satchell, 2013). The functional consequence of this change is unknown, but it is suggested that it may affect the maturation of the toxin (Satchell et al., 2016). This is significant considering that the severity of cholera is associated with the production of CT, as El Tor strains carrying ctxB-1 were associated with more severe symptoms (Siddique et al., 2010). Additional changes in the CTX gene resulted later in substitution of ctxB1 with ctxB7 (Rashid et al., 2016).
Strains carrying ctxB allela were first detected in Odisha, India, in 2007 (Kumar et al., 2009) and have been transmitted globally, reaching Cameroon (Africa) in 2009, Nepal in 2010 and Haiti in 2010 (Quilici et al., 2010; Hendriksen et al., 2011) where they produced a devastating outbreak.
Diarrhea associated with seafood consumption are mainly associated to pathogenic V. parahaemolyticus strains (García et al., 2009; Letchumanan et al., 2017). Several characteristic virulence associated factors of this pathogen such as hemolysins TDH (thermostable direct hemolysin), TRH (TDH-related hemolysin) and the secretion systems (Broberg et al., 2011; Ceccarelli et al., 2013; Raghunath, 2014) have been deeply studied. Nonetheless, the pathogenesis of V. parahaemolyticus is still not fully understood. Some studies have reported that environmental isolates of V. parahaemolyticus lacking most characteristic virulence factors (tdh, trh, and T3SS-2) are able to produce cellular damage (Mahoney et al., 2010; Castillo et al., 2018; Wagley et al., 2018) while others have reported clinical strains with absence of all principal virulence factors isolated with patients with gastroenteritis (García et al., 2013). These results indicate that classic virulence factors are not sufficient to explain the cytotoxicity and enterotoxicity of pathogenic V. parahaemolyticus strains and suggest that a novel virulence factor (or more than one) could be responsible for pathogenicity. A comparative genomic analysis of environmental and clinical strains of V. parahaemolyticus revealed the absence of most of the classical toxins and virulence factors described for V. parahaemolyticus in cytotoxic strains, but instead they had novel and uncharacterized toxins in the accessory genome, mainly associated with prophages and pathogenicity islands. Bioinformatics analysis revealed the presence of prophage-like elements which encoded a putative Zot-like enterotoxin (Castillo et al., 2018). Interestingly, three phages contained three different zot sequences, suggesting high diversity within the same species. However, although V. parahaemolyticus and V. cholerae Zot shared only 24% amino acid identity, they share some conserved regions (Castillo et al., 2018), suggesting that the structure acquired by Zot is more important than the sequence. Additionally, other V. parahaemolyticus strain, PMA 1.15, contained a prophage carrying a putative RTX toxin in addition a novel genomic island containing DNase and RTX toxin genes (see Figure 4 in Castillo et al., 2018). However, until this manuscript appeared, no studies about the function of Zot or RTX in V. parahaemolyticus were published.
V. vulnificus, another important human pathogen, is distributed worldwide in estuaries and marine environments, where is associated to food-borne and wound infections exhibiting high mortality (Ziolo et al., 2014; Kim et al., 2017), which exceeds 50%, and can increases to more than 90% in patients in serious condition (shock) (Horng-Ren et al., 2011; Chen et al., 2017). This bacterium produces several virulence factors that cause disease, including cytolysin VvhA, metalloprotease Vvp, flagella and RtxA toxin among others (Lee et al., 2008; Chen et al., 2017). RtxA1 toxin is the most potent cytotoxic virulence factor (Lee et al., 2007) of V. vulnificus (Gavin et al., 2017). It has been shown that it exerts dramatic effects on cytoskeletal rearrangement, contact cytotoxicity, hemolysis (inducing the apoptotic death of human epithelial cells (Kim et al., 2017)), invasion and lethality to mice, showing that it is a multifunctional virulence factor of V. vulnificus (Lee et al., 2007). Like that of V. cholerae, the RTX of V. vulnificus has multiple domains and it is autoprocessed (Jeong and Satchell, 2012; Gavin and Satchell, 2015; Chen et al., 2017; Kim et al., 2017). Mutants on this toxin are significantly attenuated for virulence (Liu et al., 2007; Kim et al., 2017). A V. vulnificus null mutant in the rtxA gene constructed by Lee et al. (2007) exhibited decreased cytotoxic activity, using NT-407 intestinal epithelial cells as model. MARTXV v (multifunctional-autoprocessing repeats-in-toxins toxin of V. vulnificus) and the cytolysin VvhA play a role in growth of bacteria in vivo, therefore the presence of both factors is directly correlated with mouse mortality (Jeong and Satchell, 2012). The importance of N-termini and C-termini of MARTXV v has been shown by Kim et al. (2015). A deletion in the C-terminal region blocked toxin secretion from the bacterium and consequently a reduction in the cytotoxicity of bacteria. In contrast, a deletion in the N-terminal domain completely abolished necrosis (Kim et al., 2015).
Interestingly, the complete genome sequence of V. vulnificus MO6-24/O isolated from a septicemic patient showed that this particular strain contains 272 specific genes, including phage-related genes. The gene cluster of the bacteriophage contains ace and zot, revealing genetic diversity resulting from extensive gene transfer (Park et al., 2011).
Finally, although it is not considered as an important human pathogen until today, recent observations have indicated that V. mimicus may cause epidemic diarrhea. Some of these strains contains the “cholera virulence cassette” containing genes encoding Zot, Ace and a core encoded pilus as well as CT (Shi et al., 1998, 2000).
Risk of gastrointestinal infections associated with Vibrio species has been rising worldwide as a consequence of global warming. However, to date the mechanisms involved in the production of diarrhea by some Vibrio species is not completely understood. V. cholerae has an arsenal of toxins, the most important being the classical CT, but also Zot, RTX and Ace contribute to the enterotoxicity of this pathogen, while cholix produces extraintestinal effects (Table 1). In Vibrio the HGT plays a significant role in the transmission of genes. In fact, bacteriophages and other mobile elements containing Zot, Ace and also RTX have been reported in the genomes of clinical and environmental strains of V. parahaemolyticus that exhibited high cytotoxicity. Also, a bacteriophage containing zot and ace genes was reported in a strain of V. vulnificus isolated from a patient with septicemia. Similarly, some strains of V. mimicus possess the virulence cassette of V. cholerae. Regrettably no studies of the characterization and function of these toxins were found until this manuscript was finished, and we do not know if they contribute to the pathogenicity of V. parahaemolyticus and V. vulnificus, and if they do, in what way. In the light of the facts, it seems urgent to know the molecular mechanisms behind the mode of action of these novel enterotoxins in Vibrios other than V. cholerae.
DP-R and KG conceived the idea. DP-R, KG and PN wrote the manuscript. LP and VJ made the figures. All authors read, discussed and approved the final version.
The authors acknowledge Fondecyt Iniciación 11140257, CONICYT, Chile; Fondecyt Regular 1181499, CONICYT, Chile; and competitive funds of Universidad de Las Américas PI2018026, Chile.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Claudia Carrasco for her support in preparing the figures.
Al-Majali, A. M., Asem, E. K., Lamar, C. H., Robinson, J. P., Freeman, M. J., and Saeed, A. M. (2000). Studies on the mechanism of diarrhea induced by Escherichia coli heat-stable enterotoxin (STa) in newborn calves. Vet. Res. Commun. 28, 327–338.
Al-Sadi, R., Boivin, M., and Ma, T. (2009). Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 14:2765–2778.
Al-Sadi, R., Guo, S., Ye, D., and Ma, T. Y. (2013). TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 183, 1871–1884. doi: 10.1016/j.ajpath.2013.09.001
Al-Sadi, R., Guo, S., Ye, D., Rawat, M., and Ma, T. Y. (2016). TNF-α modulation of intestinal tight junction permeability is mediated by NIK/IKK-α axis activation of the canonical NF-κB pathway. Am. J. Pathol. 186, 1151–1165. doi: 10.1016/j.ajpath.2015.12.016
Al-Majali, A. M., Ababneh, M. M., Shorman, M., and Saeed, A. M. (2007). Interaction of Escherichia coli heat-stable enterotoxin (STa) with its putative receptor on the intestinal tract of newborn kids. FEMS Inmunol. Med. Microbiol. 49, 35–40.
Anvari, S., Najar Peerayeh, S., Behmanesh, M., and Boustanshenas, M. (2012). Biological activity of recombinant accessory cholerae enterotoxin (Ace) on rabbit ileal loops and antibacterial assay. Cell J. 14, 209–214.
Awasthi, S. P., Asakura, M., Chowdhury, N., Neogi, S. B., Hinenoya, A., Golbar, H. M., et al. (2013). Novel cholix toxin variants, adp-ribosylating toxins in Vibrio cholerae Non-O1/Non-O139 strains, and their pathogenicity. Infect. Immun. 81, 531–541. doi: 10.1128/IAI.00982-12
Barbieri, J. T., Riese, M. J., and Aktories, K. (2002). Bacterial toxins that modify the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 18, 315–344.
Baudry, B., Fasano, A., Ketley, J., and Kaper, J. B. (1992). Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect. Immun. 60, 428–434.
Benitez, J. A., and Silva, A. J. (2016). Vibrio cholerae hemagglutinin(HA)/protease: an extracellular metalloprotease with multiple pathogenic activities. Toxicon 115, 55–62. doi: 10.1016/j.toxicon.2016.03.003
Bharati, K., and Ganguly, N. K. (2011). Cholera toxin: a paradigm of a multifunctional protein. Indian J. Med. Res. 133, 179–187.
Boardman, B. K., and Fullner Satchell, K. J. (2004). Vibrio cholerae strains with mutations in an atypical type i secretion system accumulate RTX toxin intracellularly. J. Bacteriol. 186, 8137–8143.
Broberg, C. A., Calder, T. J., and Orth, K. (2011). Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect. 13, 992–1001. doi: 10.1016/j.micinf.2011.06.013
Castillo, D., Pérez-Reytor, D., Plaza, N., Ramírez-Araya, S., Blondel, C. J., Corsini, G., et al. (2018). Exploring the genomic traits of non-toxigenic Vibrio parahaemolyticus strains isolated in Southern Chile. Front. Microbiol. 9:161. doi: 10.3389/fmicb.2018.00161
Ceccarelli, D., Hasan, N. A., Huq, A., and Colwell, R. R. (2013). Distribution and dynamics of epidemic and pandemic Vibrio parahaemolyticus virulence factors. Front. Cell. Infect. Microbiol. 3:97. doi: 10.3389/fcimb.2013.00097
Chatterjee, R., Nag, S., and Chaudhuri, K. (2008). Identification of a new RTX-like gene cluster in Vibrio cholerae. FEMS Microbiol. Lett. 284, 165–171. doi: 10.1111/j.1574-6968.2008.01199.x
Chatterjee, S., Ghosh, K., Raychoudhuri, A., Chowdhury, G., Bhattacharya, M. K., Mukhopadhyay, A. K., et al. (2009). Incidence, virulence factors, and clonality among clinical strains of Non-O1, non-O139 Vibrio cholerae isolates from hospitalized diarrheal patients in Kolkata, India. J. Clin. Microbiol. 47, 1087–1095. doi: 10.1128/JCM.02026-08
Chatterjee, T., Mukherjee, D., Dey, S., Pal, A., Hoque, K. M., and Chakrabarti, P. (2011). Accessory cholera enterotoxin, Ace, from Vibrio cholerae: structure, unfolding, and virstatin binding. Biochemistry 50, 2962–2972. doi: 10.1021/bi101673x
Chatterjee, T., Sheikh, I. A., Chakravarty, D., Chakrabarti, P., Sarkar, P., Saha, T., et al. (2015). Effects of small molecule calcium-activated chloride channel inhibitors on structure and function of accessory cholera enterotoxin (Ace) of Vibrio cholerae. PLoS One 10:e0141283. doi: 10.1371/journal.pone.0141283
Chen, C. L., Chien, S. C., Leu, T. H., Harn, H. I., Tang, M. J., and Hor, L. I. (2017). Vibrio vulnificus MARTX cytotoxin causes inactivation of phagocytosis-related signaling molecules in macrophages. J. Biomed. Sci. 24:58. doi: 10.1186/s12929-017-0368-2
Citi, S., Guerrera, D., Spadaro, D., and Shah, J. (2014). Epithelial junctions and Rho family GTPases: the zonular signalosome. Small GTPases 5:e973760. doi: 10.4161/21541248.2014.973760
Cordero, C. L., Kudryashov, D. S., Reisler, E., and Satchell, K. J. F. (2006). The actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin. J. Biol. Chem. 281, 32366–32374. doi: 10.1074/jbc.M605275200
Di Pierro, M., Lu, R., Uzzau, S., Wang, W., Margaretten, K., Pazzani, C., et al. (2001). Zonula occludens toxin structure-function analysis. identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. Biol. Chem. 276, 19160–19165.
Dolores, J., and Satchell, K. J. F. (2013). Analysis of Vibrio cholerae genome sequences reveals unique rtxA variants in environmental strains and an rtxA-null mutation in recent altered El Tor isolates. mBio 4:e624-12. doi: 10.1128/mBio.00624-12
Dolores, J. S., Agarwal, S., Egerer, M., and Satchell, K. J. F. (2015). Vibrio cholerae MARTX toxin heterologous translocation of beta-lactamase and roles of individual effector domains on cytoskeleton dynamics. Mol. Microbiol. 95, 590–604. doi: 10.1111/mmi.12879
Dubreuil, J. D. (2017). Enterotoxigenic Escherichia coli targeting intestinal epithelial tight junctions: an effective way to alter the barrier integrity. Microb. Pathog. 113, 129–134. doi: 10.1016/j.micpath.2017.10.037
Farhadi, A., Banan, A., Fields, J., and Keshavarzian, A. (2003). Intestinal barrier: an interface between health and disease. J. Gastroenterol. Hepatol. 18, 479–497. doi: 10.1046/j.1440-1746.2003.03032.x
Fasano, A. (2012). Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 10, 1096–1100. doi: 10.1016/j.cgh.2012.08.012
Fasano, A., Baudry, B., Pumplin, D. W., Wasserman, S. S., Tall, B. D., Ketley, J. M., et al. (1991). Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. U.S.A. 88, 5242–5246.
Fasano, A., Uzzau, S., Fiore, C., and Margaretten, K. (1997). The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology 112, 839–846. doi: 10.1053/gast.1997.v112.pm9041245
Fernandez, S., and Alonso, G. (2009). Colera y Vibrio cholerae. Rev. Inst. Nac. Hig. Rafael Rangel 40, 50–69.
Frey, J., and Kuhnert, P. (2002). RTX toxins in Pasteurellaceae. Int. J. Med. Microbiol. 292, 149–158. doi: 10.1078/1438-4221-00200
García, K., Bastías, R., Higuera, G., Torres, R., Mellado, A., Uribe, P., et al. (2013). Rise and fall of pandemic Vibrio parahaemolyticus serotype O3:K6 in southern Chile. Environ. Microbiol. 15, 527–534. doi: 10.1111/j.1462-2920.2012.02883.x
García, K., Torres, R., Uribe, P., Hernández, C., Rioseco, M. L., Romero, J., et al. (2009). Dynamics of clinical and environmental Vibrio parahaemolyticus strains during seafood-related summer diarrhea outbreaks in southern Chile. Appl. Environ. Microbiol. 75, 7482–7487. doi: 10.1128/AEM.01662-1669
Gavin, H. E., Beubier, N. T., and Satchell, K. J. F. (2017). The effector domain region of the Vibrio vulnificus MARTX toxin confers biphasic epithelial barrier disruption and is essential for systemic spread from the intestine. PLoS Pathog. 13:e1006119. doi: 10.1371/journal.ppat.1006119
Gavin, H. E., and Satchell, K. J. F. (2015). MARTX toxins as effector delivery platforms. Pathog. Dis. 73:ftv092. doi: 10.1093/femspd/ftv092
Goldblum, S. E., Rai, U., Tripathi, A., Thakar, M., De Leo, L., Di Toro, N., et al. (2011). The active Zot domain (aa 288–293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J. 25, 144–158. doi: 10.1096/fj.10-158972
González-Mariscal, L., Betanzos, A., Nava, P., and Jaramillo, B. E. (2013). Tight junction proteins. Prog. Biophys. Mol. Biol. 81, 1–44. doi: 10.1016/S0079-6107(02)00037-8
Gopalakrishnan, S., Pandey, N., Tamiz, A. P., Vere, J., Carrasco, R., Somerville, R., et al. (2009). Mechanism of action of ZOT-derived peptide AT-1002, a tight junction regulator and absorption enhancer. Int. J. Pharm. 365, 121–130. doi: 10.1016/j.ijpharm.2008.08.047
Guttman, J. A., and Finlay, B. B. (2009). Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 1788, 832–841. doi: 10.1016/j.bbamem.2008.10.028
Hendriksen, R. S., Price, L. B., Schupp, J. M., Gillece, J. D., Kaas, R. S., Engelthaler, D. M., et al. (2011). Population genetics of Vibrio cholerae from Nepal in 2010: evidence on the origin of the haitian outbreak. mBio 2:e157-11. doi: 10.1128/mBio.00157-11
Horng-Ren, L., Jen-Hsing, L., Yi-Hsuan, C., Chun-Liang, C., Chung-Ping, S., Yi-Chi, L., et al. (2011). RTX toxin enhances the survival of Vibrio vulnificus during infection by protecting the organism from phagocytosis. J. Infect. Dis. 203, 1866–1874. doi: 10.1093/infdis/jir070
Jeong, H. G., and Satchell, K. J. F. (2012). Additive function of Vibrio vulnificus MARTXVv and VvhA cytolysins promotes rapid growth and epithelial tissue necrosis during intestinal infection. PLoS Pathog. 8:e1002581. doi: 10.1371/journal.ppat.1002581
Jørgensen, R., Purdy, A. E., Fieldhouse, R. J., Kimber, M. S., Bartlett, D. H., and Merrill, A. R. (2008). Cholix toxin, a novel ADP-ribosylating factor from Vibrio cholerae. J. Biol. Chem. 283, 10671–10678. doi: 10.1074/jbc.M710008200
Khouadja, S., Suffredini, E., Baccouche, B., Croci, L., and Bakhrouf, A. (2014). Occurrence of virulence genes among Vibrio cholerae and Vibrio parahaemolyticus strains from treated wastewaters. Environ. Monit. Assess. 186, 6935–6945. doi: 10.1007/s10661-014-3900-9
Kim, B. S., Gavin, H. E., and Satchell, K. J. F. (2015). distinct roles of the repeat-containing regions and effector domains of the Vibrio vulnificus multifunctional-autoprocessing repeats-in-toxin (martx) toxin. mBio 6:e324-15. doi: 10.1128/mBio.00324-15
Kim, B. S., Gavin, H. E., and Satchell, K. J. F. (2017). Variable Virulence of Biotype 3 Vibrio vulnificus due to MARTX Toxin Effector Domain Composition. mSphere 2:e272-17. doi: 10.1128/mSphereDirect.00272-17
König, J., Wells, J., Cani, P. D., García-Ródenas, C. L., MacDonald, T., Mercenier, A., et al. (2016). human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 7:e196. doi: 10.1038/ctg.2016.54
Kudryashov, D. S., Durer, Z. A. O., Ytterberg, A. J., Sawaya, M. R., Pashkov, I., Prochazkova, K., et al. (2008). Connecting actin monomers by iso-peptide bond is a toxicity mechanism of the Vibrio cholerae MARTX toxin. Proc. Natl. Acad. Sci. U.S.A. 105, 18537–18542. doi: 10.1073/pnas.0808082105
Kumar, P., Jain, M., Goel, A. K., Bhadauria, S., Sharma, S. K., Kamboj, D. V., et al. (2009). A large cholera outbreak due to a new cholera toxin variant of the Vibrio cholerae O1 El Tor biotype in Orissa, Eastern India. J. Med. Microbiol. 58(Pt 2), 234–238. doi: 10.1099/jmm.0.002089-0
Lee, A., White, N., and van der Walle, C. F. (2003). The intestinal zonula occludens toxin (ZOT) receptor recognises non-native ZOT conformers and localises to the intercellular contacts. FEBS Lett. 555, 638–642.
Lee, B. C., Choi, S. H., and Kim, T. S. (2008). Vibrio vulnificus RTX toxin plays an important role in the apoptotic death of human intestinal epithelial cells exposed to Vibrio vulnificus. Microbes Infect. 10, 1504–1513. doi: 10.1016/j.micinf.2008.09.006
Lee, J. H., Kim, M. W., Kim, B. S., Kim, S. M., Lee, B. C., Kim, T. S., et al. (2007). Identification and characterization of the Vibrio vulnificus rtxA essential for cytotoxicity in vitro and virulence in mice. J. Microbiol. 45, 146–152.
Lee, S. J., Jung, Y. H., Song, E. J., Jang, K. K., Choi, S. H., and Han, H. J. (2015). Vibrio vulnificus VvpE stimulates IL-1β production by the hypomethylation of the IL-1β promoter and NF-κB activation via lipid raft–dependent ANXA2 recruitment and reactive oxygen species signaling in intestinal epithelial cells. J. Immunol. 195, 2282–2293. doi: 10.4049/jimmunol.1500951
Letchumanan, V., Chan, K. G., Khan, T. M., Bukhari, S. I., Ab Mutalib, N. S., Goh, B. H., et al. (2017). Bile sensing: the activation of Vibrio parahaemolyticus virulence. Front. Microbiol. 8:728. doi: 10.3389/fmicb.2017.00728
Linhartová, I., Bumba, L., Mašín, J., Basler, M., Osička, R., Kamanová, J., et al. (2010). RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 34, 1076–1112. doi: 10.1111/j.1574-6976.2010.00231.x
Liu, M., Alice, A. F., Naka, H., and Crosa, J. H. (2007). The HlyU protein is a positive regulator of rtxA1, a gene responsible for cytotoxicity and virulence in the human pathogen Vibrio vulnificus. Infect. Immun. 75, 3282–3289. doi: 10.1128/IAI.00045-07
Lutfullah, G., Amin, F., Khan, Z., Azhar, N., Azim, M. K., Noor, S., et al. (2008). Homology modeling of hemagglutinin/protease [HA/P (vibriolysin)] from Vibrio cholerae: sequence comparison, residue interactions and molecular mechanism. Protein J. 27, 105–114. doi: 10.1007/s10930-007-9113-0
Mahoney, J. C., Gerding, M. J., Jones, S. H., and Whistler, C. A. (2010). Comparison of the pathogenic potentials of environmental and clinical Vibrio parahaemolyticus strains indicates a role for temperature regulation in virulence. Appl. Environ. Microbiol. 76, 7459–7465. doi: 10.1128/AEM.01450-10
Martinez-Urtaza, J., Bowers, J. C., Trinanes, J., and De Paola, A. (2010). Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res. Int. 43, 1780–1790. doi: 10.1016/j.foodres.2010.04.001
Menestrina, G., Moser, C., Pellet, S., and Welch, R. (1994). Pore-formation by Escherichia coli hemolysin (HlyA) and other members of the RTX toxins family. Toxicology 87, 249–267. doi: 10.1016/0300-483X(94)90254-2
Nishibuchi, M., and Kaper, J. B. (1995). Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infect. Immun. 63, 2093–2099.
Ogura, K., Terasaki, Y., Miyoshi-Akiyama, T., Terasaki, M., Moss, J., Noda, M., et al. (2017). Vibrio cholerae cholix toxin-induced HepG2 cell death is enhanced by tumor necrosis factor-alpha through ROS and intracellular signal-regulated kinases. Toxicol. Sci. 156, 455–468. doi: 10.1093/toxsci/kfx009
Ogura, K., Yahiro, K., Tsutsuki, H., Nagasawa, S., Yamasaki, S., Moss, J., et al. (2011). Characterization of cholix toxin-induced apoptosis in HeLa cells. J. Biol. Chem. 286, 37207–37215. doi: 10.1074/jbc.M111.246504
Olivier, V., Queen, J., and Satchell, K. J. F. (2009). Successful small intestine colonization of adult mice by Vibrio cholerae requires ketamine anesthesia and accessory toxins. PLoS One 4:e7352. doi: 10.1371/journal.pone.0007352
Olivier, V., Salzman, N. H., and Satchell, K. J. F. (2007). Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect. Immun. 75, 5043–5051. doi: 10.1128/IAI.00508-07
Park, J. H., Cho, Y.-J., Chun, J., Seok, Y.-J., Lee, J. K., Kim, K.-S., et al. (2011). Complete genome sequence of Vibrio vulnificus MO6-24/O. J. Bacteriol. 193, 2062–2063. doi: 10.1128/JB.00110-11
Pei, J., and Grishin, N. V. (2009). The Rho GTPase inactivation domain in Vibrio cholerae MARTX toxin has a circularly permuted papain-like thiol protease fold. Proteins 77, 413–419. doi: 10.1002/prot.22447
Plaza, N., Castillo, D., Pérez-Reytor, D., Higuera, G., García, K., and Bastías, R. (2018). Bacteriophages in the control of pathogenic vibrios. Electron. J. Biotechnol. 31, 24–33. doi: 10.1016/j.ejbt.2017.10.012
Prochazkova, K., and Satchell, K. J. F. (2008). Structure-function analysis of inositol hexakisphosphate-induced autoprocessing of the Vibrio cholerae multifunctional autoprocessing RTX toxin. J. Biol. Chem. 283, 23656–23664. doi: 10.1074/jbc.M803334200
Purdy, A. E., Balch, D., Lizárraga-Partida, M. L., Islam, M. S., Martinez-Urtaza, J., Huq, A., et al. (2010). Diversity and distribution of cholix toxin, a novel ADP-ribosylating factor from Vibrio cholerae. Environ. Microbiol. Rep. 2, 198–207. doi: 10.1111/j.1758-2229.2010.00139.x
Quilici, M. L., Massenet, D., Gake, B., Bwalki, B., and Olson, D. M. (2010). Vibrio cholerae O1 variant with reduced susceptibility to ciprofloxacin, Western Africa. Emerg. Infect. Dis. 16, 1804–1805. doi: 10.3201/eid1611.100568
Raghunath, P. (2014). Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus. Front. Microbiol. 5:805. doi: 10.3389/fmicb.2014.00805
Rashid, M. U., Rashed, S. M., Islam, T., Johura, F. T., Watanabe, H., Ohnishi, M., et al. (2016). CtxB1 outcompetes CtxB7 in Vibrio cholerae O1, Bangladesh. J. Med. Microbiol. 65, 101–103. doi: 10.1099/jmm.0.000190
Salama, N. N., Fasano, A., Thakar, M., and Eddington, N. D. (2004). The effect of delta G on the transport and oral absorption of macromolecules. J. Pharm. Sci. 93, 1310–1319. doi: 10.1002/jps.20052
Salvo-Romero, E., Alonso-Cotoner, C., Pardo-Camacho, C., Casado-Bedmar, M., and Vicario, M. (2015). The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm. Dig. 107, 686–696. doi: 10.17235/reed.2015.3846/2015
Satchell, K. J. F. (2011). Structure and function of MARTX toxins and other large repetitive RTX proteins. Annu. Rev. Microbiol. 65, 71–90. doi: 10.1146/annurev-micro-090110-102943
Satchell, K. J. F. (2015). Multifunctional-autoprocessing repeats-in-toxin (MARTX) toxins of Vibrios. Microbiol. Spectr. 3, 1–22. doi: 10.1128/microbiolspec.VE-0002-2014
Satchell, K. J. F., Jones, C. J., Wong, J., Queen, J., Agarwal, S., and Yildiz, F. H. (2016). Phenotypic analysis reveals that the 2010 Haiti cholera epidemic is linked to a hypervirulent strain. Infect. Immun. 84, 2473–2481. doi: 10.1128/IAI.00189-16
Schmidt, E., Kelly, S. M., and van der Walle, C. F. (2007). Tight junction modulation and biochemical characterisation of the zonula occludens toxin C-and N-termini. FEBS Lett. 581, 2974–2980. doi: 10.1016/j.febslet.2007.05.051
Sheahan, K.-L., Cordero, C. L., and Fullner Satchell, K. J. (2004). Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin. Proc. Natl. Acad. Sci. U.S.A. 101, 9798–9803. doi: 10.1073/pnas.0401104101
Shi, L., Miyoshi, S., Bi, K., Nakamura, M., Hiura, M., Tomochika, K., et al. (2000). Presence of hemolysin genes (vmh, tdh and hlx) in Isolates of Vibrio mimicus determined by polymerase chain reaction. J. Health Sci. 46, 63–65. doi: 10.1248/jhs.46.63
Shi, L., Miyoshi, S., Hiura, M., Tomochika, K., Shimada, T., and Shinoda, S. (1998). Detection of genes encoding cholera toxin (CT), zonula occludens toxin (ZOT), accessory cholera enterotoxin (AE) and heat-stable enterotoxin (ST) in Vibrio mimicus clinical strains. Microbiol. Immunol. 42, 823–828. doi: 10.1111/j.1348-0421.1998.tb02357.x
Shinoda, S., and Miyoshi, S. (2011). Proteases produced by vibrios. Biocontrol Sci. 16, 1–11. doi: 10.4265/bio.16.1
Siddique, A. K., Nair, G. B., Alam, M., Sack, D. A., Huq, A., Nizam, A., et al. (2010). El Tor cholera with severe disease: a new threat to Asia and beyond. Epidemiol. Infect. 138, 347–352. doi: 10.1017/S0950268809990550
Silva, A. J., Leitch, G. J., Camilli, A., and Benitez, J. A. (2006). Contribution of hemagglutinin/protease and motility to the pathogenesis of El Tor biotype cholera. Infect. Immun. 74, 2072–2079. doi: 10.1128/IAI.74.4.2072-2079.2006
Sousa, S., Lecuit, M., and Cossart, P. (2005). Microbial strategies to target, cross or disrupt epithelia. Curr. Opin. Cell Biol. 17, 489–498. doi: 10.1016/j.ceb.2005.08.013
Trucksis, M., Galen, J. E., Michalski, J., Fasano, A., and Kaper, J. B. (1993). Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc. Natl. Acad. Sci. U.S.A. 90, 5267–5271. doi: 10.1073/pnas.90.11.5267
Turner, J. R., Buschmann, M. M., Sailer, A., Calvo, I. R., and Shen, L. (2014). The role of molecular remodeling in differential regulation of tight junction permeability. Semin. Cell Dev. Biol. 0, 204–212. doi: 10.1016/j.semcdb.2014.09.022
Ugalde-Silva, P., Gonzalez-Lugo, O., and Navarro-Garcia, F. (2016). Tight junction disruption induced by type 3 secretion system effectors injected by enteropathogenic and enterohemorrhagic Escherichia coli. Front. Cell. Infect. Microbiol. 6:87. doi: 10.3389/fcimb.2016.00087
Uzzau, S., Cappuccinelli, P., and Fasano, A. (1999). Expression of Vibrio cholerae zonula occludens toxin and analysis of its subcellular localization. Microb. Pathog. 27, 377–385. doi: 10.1006/mpat.1999.0312
Uzzau, S., Lu, R., Wang, W., Fiore, C., and Fasano, A. (2001). Purification and preliminary characterization of the zonula occludens toxin receptor from human (CaCo2) and murine (IEC6) intestinal cell lines. FEMS Microbiol. Lett. 194, 1–5. doi: 10.1111/j.1574-6968.2001.tb09437.x
Van Itallie, C. M., and Anderson, J. M. (2014). Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 0, 157–165. doi: 10.1016/j.semcdb.2014.08.011
Vezzulli, L., Grande, C., Reid, P. C., Hélaouët, P., Edwards, M., Höfle, M. G., et al. (2016). Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. U.S.A. 113, E5062–E5071. doi: 10.1073/pnas.1609157113
Wagley, S., Borne, R., Harrison, J., Baker-Austin, C., Ottaviani, D., Leoni, F., et al. (2018). Galleria mellonella as an infection model to investigate virulence of Vibrio parahaemolyticus. Virulence 9, 197–207. doi: 10.1080/21505594.2017.1384895
Waldor, M. K., and Mekalanos, J. J. (1996). Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272, 1910–1914. doi: 10.1126/science.272.5270.1910
Wiles, T. J., and Mulvey, M. A. (2013). The RTX pore-forming toxin α-hemolysin of uropathogenic Escherichia coli: progress and perspectives. Future Microbiol. 8, 73–84. doi: 10.2217/fmb.12.131
Woida, P. J., and Satchell, K. J. F. (2018). Coordinated delivery and function of bacterial MARTX toxin effectors. Mol. Microbiol. 107, 133–141. doi: 10.1111/mmi.13875
Keywords: Zot, RTX, Ace, toxins, tight junctions, intestinal epithelia, Vibrio
Citation: Pérez-Reytor D, Jaña V, Pavez L, Navarrete P and García K (2018) Accessory Toxins of Vibrio Pathogens and Their Role in Epithelial Disruption During Infection. Front. Microbiol. 9:2248. doi: 10.3389/fmicb.2018.02248
Received: 11 June 2018; Accepted: 03 September 2018;
Published: 20 September 2018.
Edited by:
Eugenia Bezirtzoglou, Democritus University of Thrace, GreeceReviewed by:
Christine Mielcarek, École de Biologie Industrielle, FranceCopyright © 2018 Pérez-Reytor, Jaña, Pavez, Navarrete and García. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine García, a2F0aGVyaW5lLmdhcmNpYUB1YXV0b25vbWEuY2w=; a2F0YS5nYXJjaWFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.